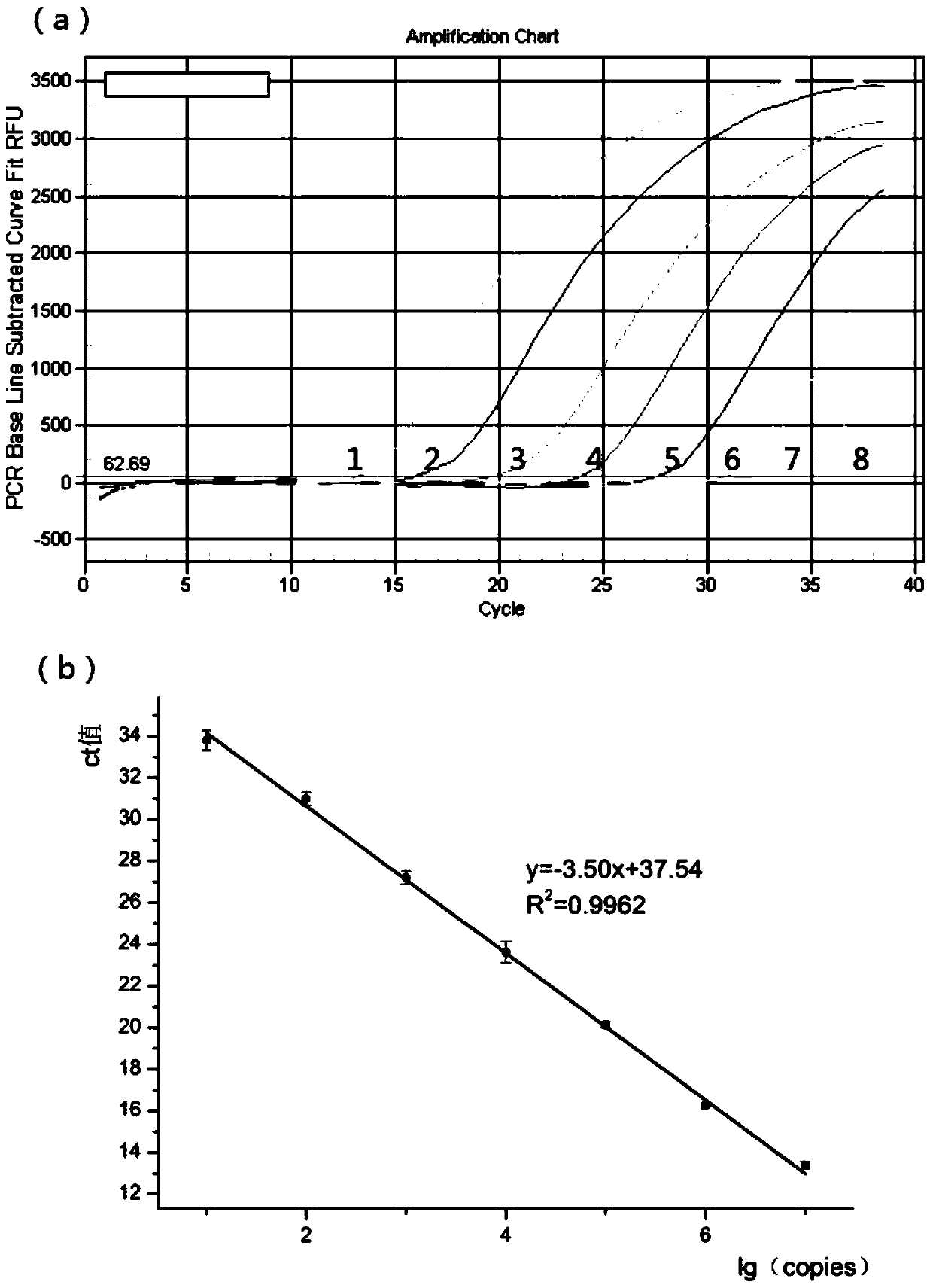
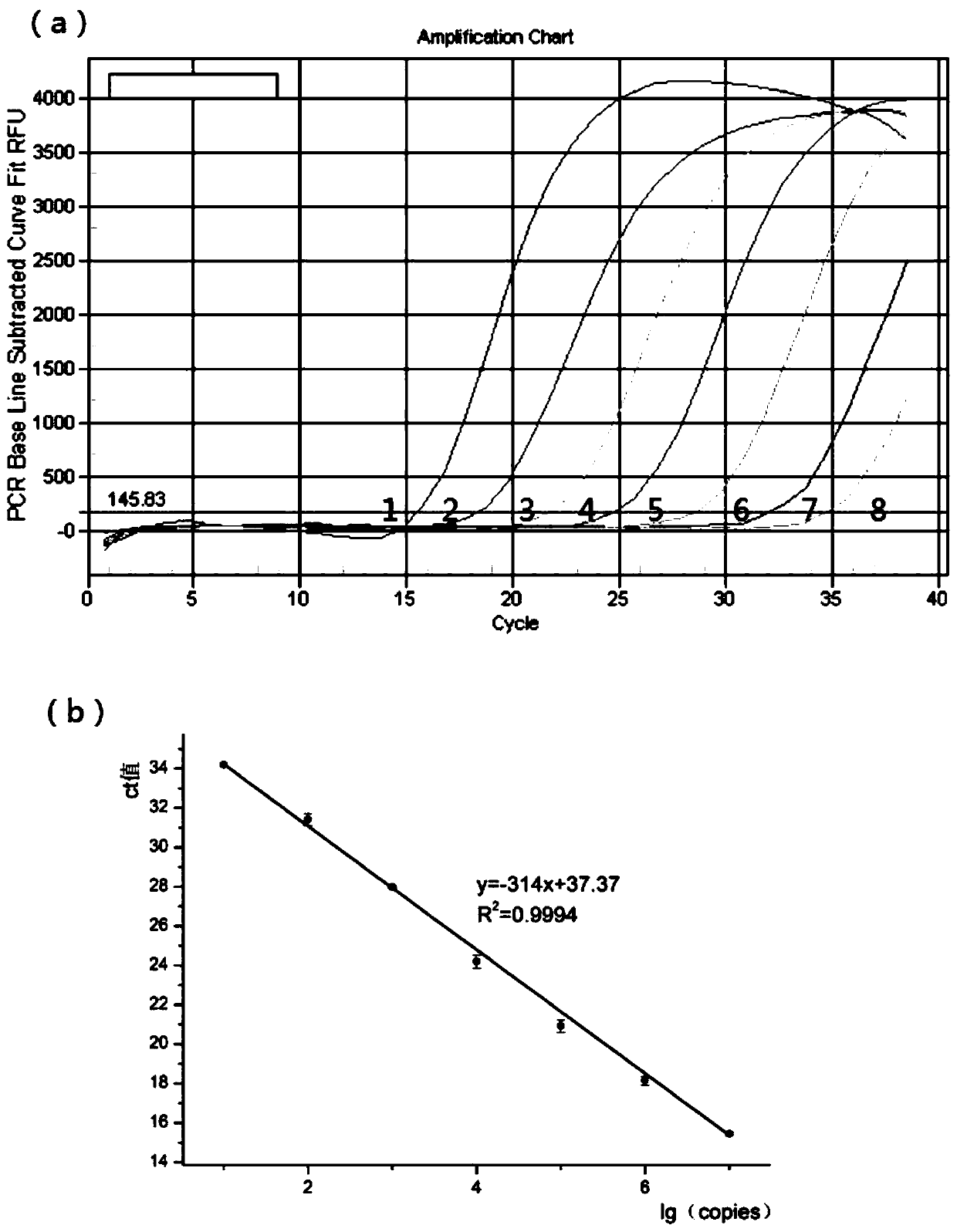
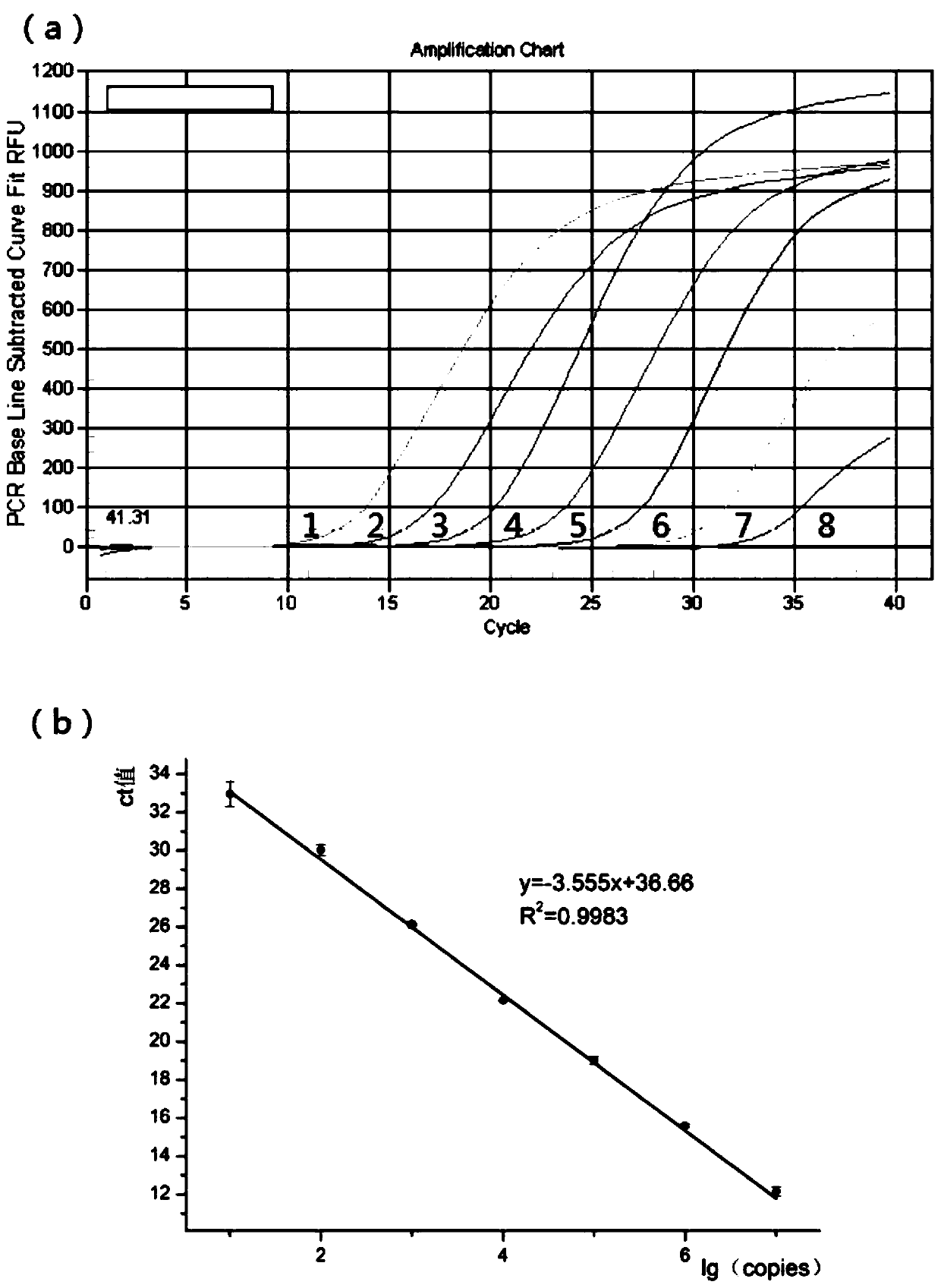
Patents
Literature
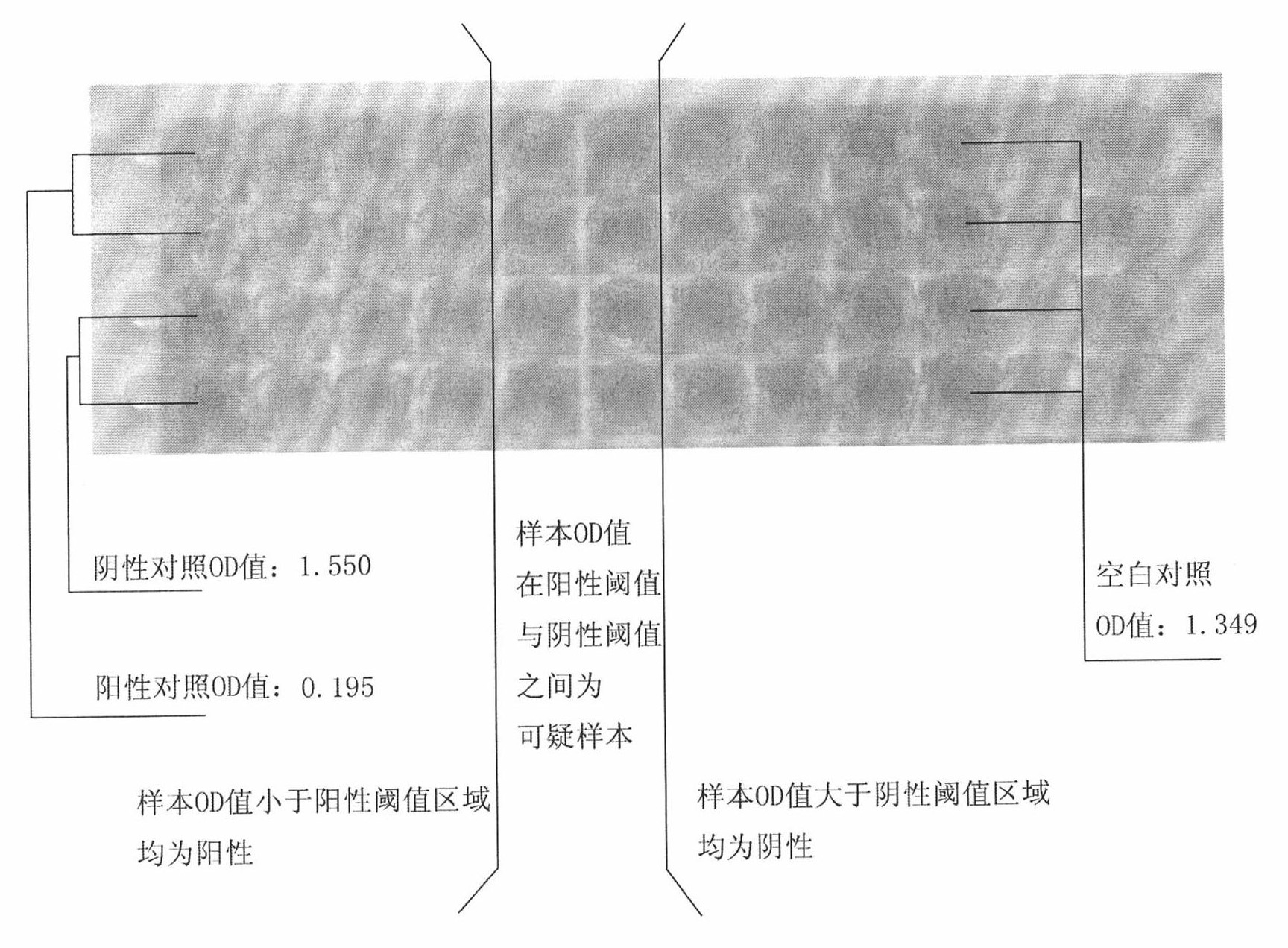
460 results about "African swine fever virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
African swine fever virus (ASFV) is a large, double-stranded DNA virus in the Asfarviridae family. It is the causative agent of African swine fever (ASF). The virus causes a haemorrhagic fever with high mortality rates in domestic pigs; some isolates can cause death of animals as quickly as a week after infection. It persistently infects its natural hosts, warthogs, bushpigs, and soft ticks of the genus Ornithodoros, which likely act as a vector, with no disease signs. It does not cause disease in humans. ASFV is endemic to sub-Saharan Africa and exists in the wild through a cycle of infection between ticks and wild pigs, bushpigs, and warthogs. The disease was first described after European settlers brought pigs into areas endemic with ASFV, and as such, is an example of an emerging infectious disease.
Gene deletion attenuated African swine fever virus and application thereof as vaccine
ActiveCN110093324AGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
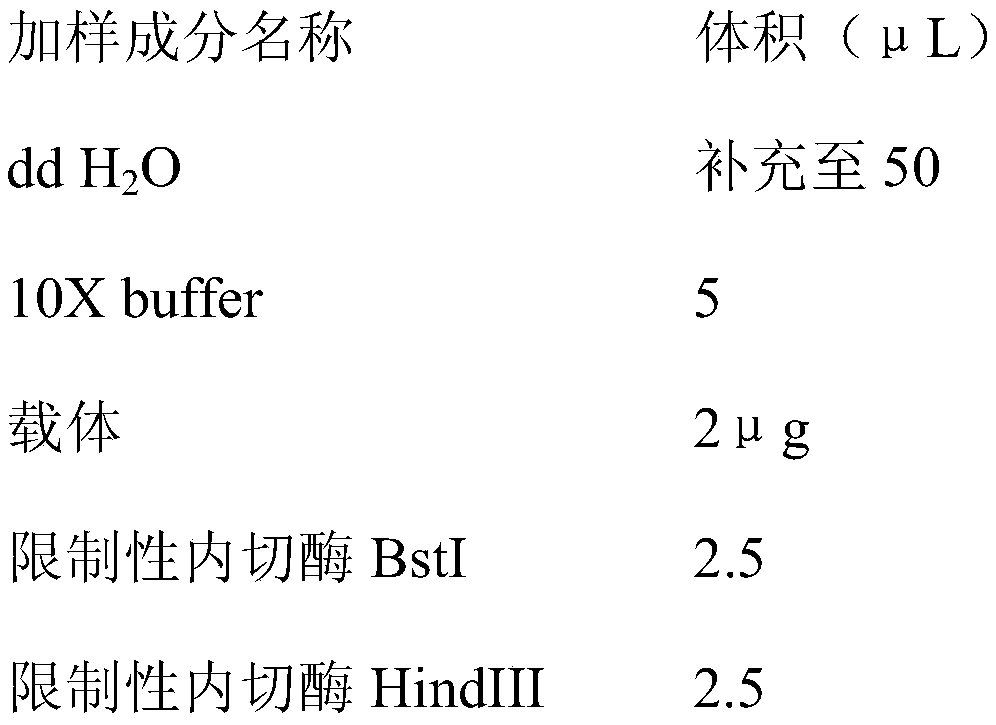
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Rapid fluorescence PCR detection kit for ASFV (African swine fever virus)
PendingCN109593893ADetection fitReduce manual operationsMicrobiological testing/measurementDNA/RNA fragmentationSerum igeFluorescence
The invention discloses a rapid fluorescence PCR detection kit for ASFV (African swine fever virus). The kit comprises specific primers ASFV-F and ASFV-R as well as a TaqMan probe ASFV-P, the 5' end of the probe labels fluorescence dye as FAM and the 3' end labels a fluorescence quenching group as BHQ-1. The kit can be used for detecting the ASFV in nasal swabs, blood, serum, plasma and tissue ofswine to realize rapid detection of the ASFV. In the whole ASFV detection process, only 40 min is taken from DNA extraction to obtaining of detection results, so that the detection time is greatly shortened, and the detection efficiency is improved.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD +2
Four-gene-deletion weak-toxin strain for African swine fever viruses and application of four-gene-deletion weak-toxin strain
InactiveCN110551695AEasy to solveViral antigen ingredientsMicrobiological testing/measurementAfrican swine feverToxin
The invention discloses a four-gene-deletion weak-toxin strain for African swine fever viruses. The weak-toxin strain is the four-gene-deletion weak-toxin strain for an African swine fever virus SY18separation strain, and the following gene function protein is deleted: CD2v gene coding products and three multigene family genes( MGF360-12L, MGF360-13L and MGF360-14L ) coding products. The invention further discloses an application of the weak-toxin strain of the African swine fever viruses to preparation of vaccines for preventing or treating African swine fever. The weak-toxin strain of the African swine fever viruses can provide complete immunoprotection effect on attack of ASFV parent toxin strains, is high in safety, and is suitable for being used as vaccine candidate strains for preventing the African swine fever.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Multi-epitope fusion diagnosis antigen for African swine fever virus as well as preparation method and application thereof
InactiveCN108148138AImprove featuresIncreased sensitivityAntibody mimetics/scaffoldsVirus peptidesAntigenBacillus coli
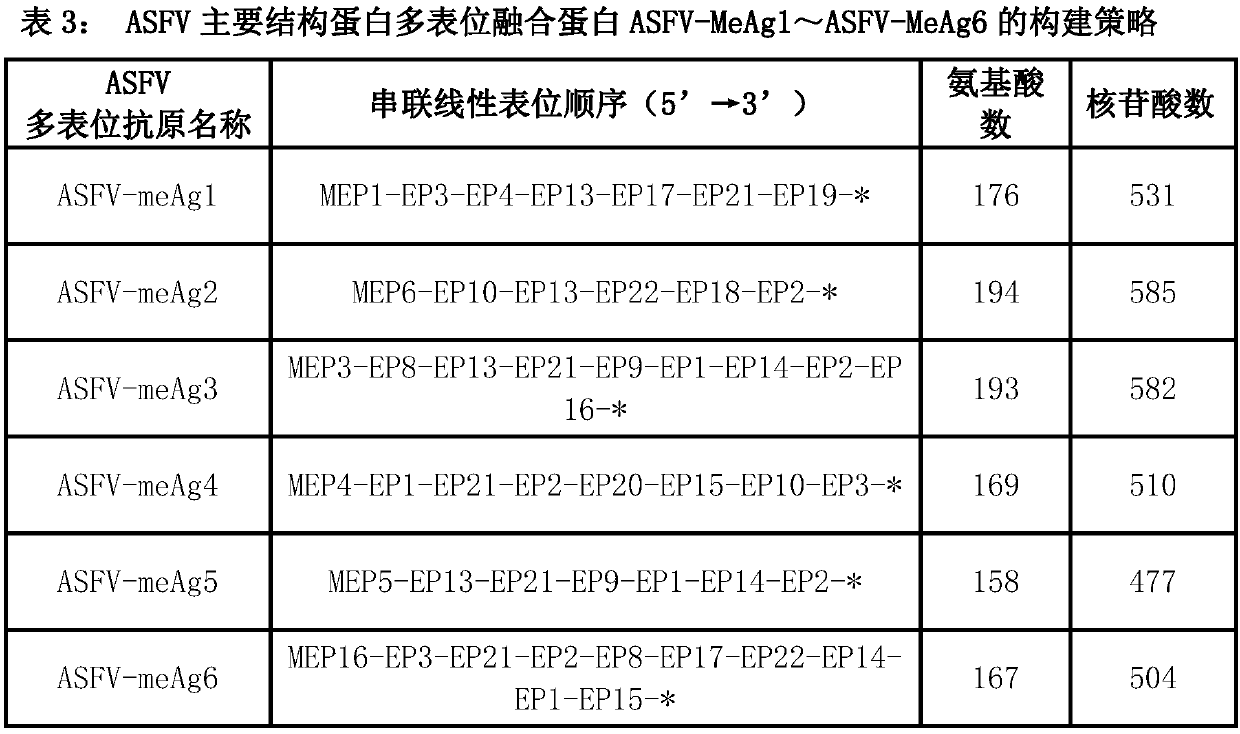
The invention discloses a multi-epitope fusion diagnosis antigen for African swine fever virus as well as a preparation method and application thereof. An ASFV (African swine fever virus) important structural protein gene encoding amino acid sequence is analyzed, screened and recombined through bioinformatics software, a multi-epitope fusion antigen gene is built and synthesized and is expressed in bacillus coli; through screening, the recombinant multi-epitope fusion antigen ASFV-meAg6 is obtained, so that diagnosis antigen protein with strong specificity and high sensitivity is provided foran ASFV serological diagnosis method.
Owner:SHIHEZI UNIVERSITY
CHO (Chinese hamster ovary) cell strain with high-efficiency expression of CD2V protein of African swine fever (ASF)
ActiveCN110078801AHigh expressionEasy to purifyVirus peptidesMicroorganism based processesAfrican swine feverChinese hamster
The invention provides CD2V protein of African swine fever (ASF) capable of being expressed with high-efficiency in a CHO (Chinese hamster ovary) cell strain. The amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; a recombinant plasmid constructed by the invention is used for expressing the CD2V protein of an African swine fever virus in CHO cells; the invention further provides a recombinant CHO cell strain prepared by transfecting the CHO cells through the recombinant plasmid, the recombinant CHO cell strain can be used to prepare the CD2V protein, and the prepared protein canbe used for differential diagnosis of the African swine fever. According to the cell strain with the expression of the CD2V protein of the African swine fever, the expression quantity is high, purification is easy, the cell strain can be used for the differential diagnosis, and a solid foundation is laid for the production of subunit vaccines and diagnostic reagents of the African swine fever.
Owner:YEBIO BIOENG OF QINGDAO
Attenuated African Swine Fever Virus Vaccine Based in the Deletion of MGF Genes
ActiveUS20160130562A1Viral antigen ingredientsMicrobiological testing/measurementVirulent characteristicsDomestic pig
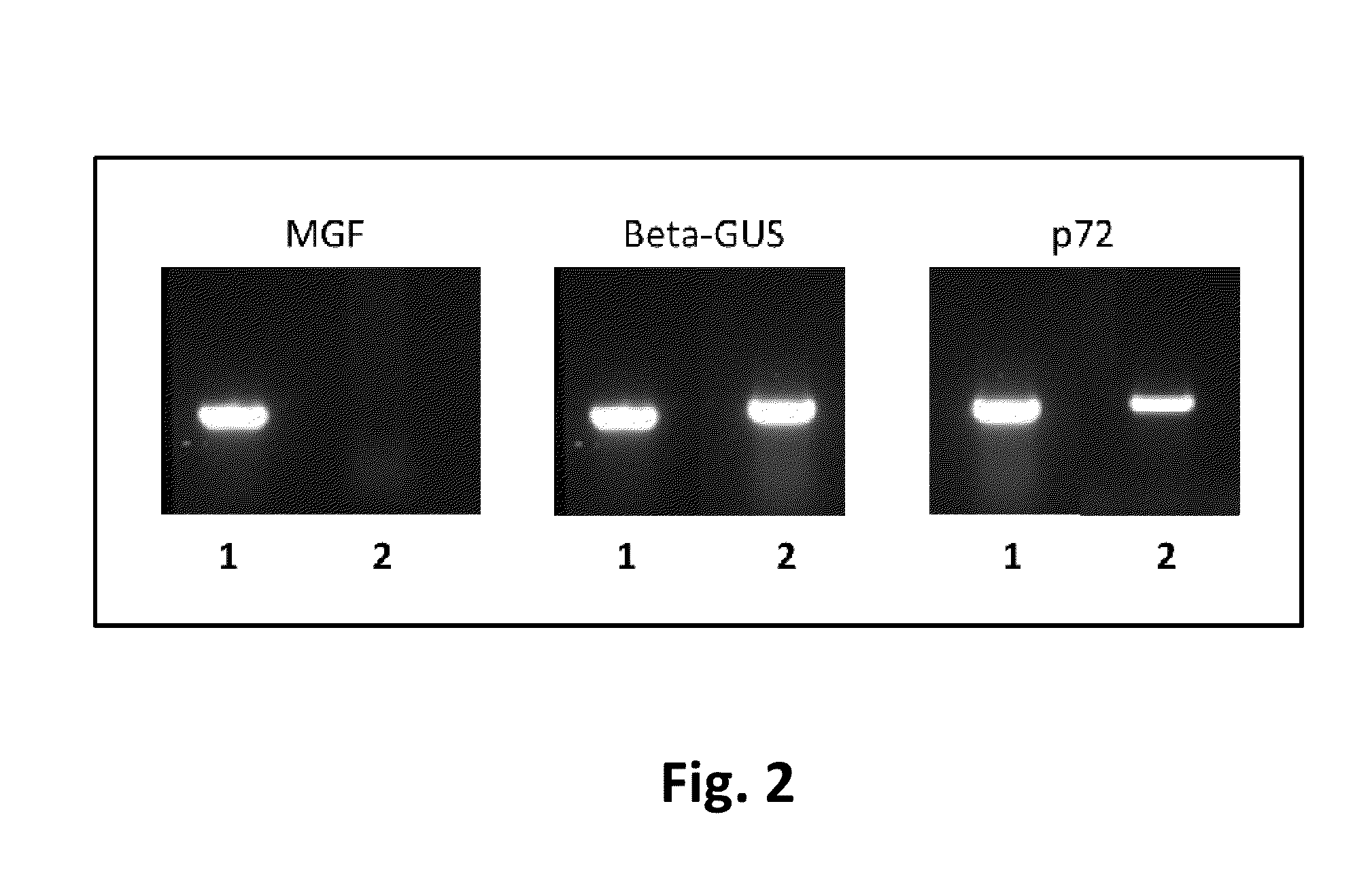
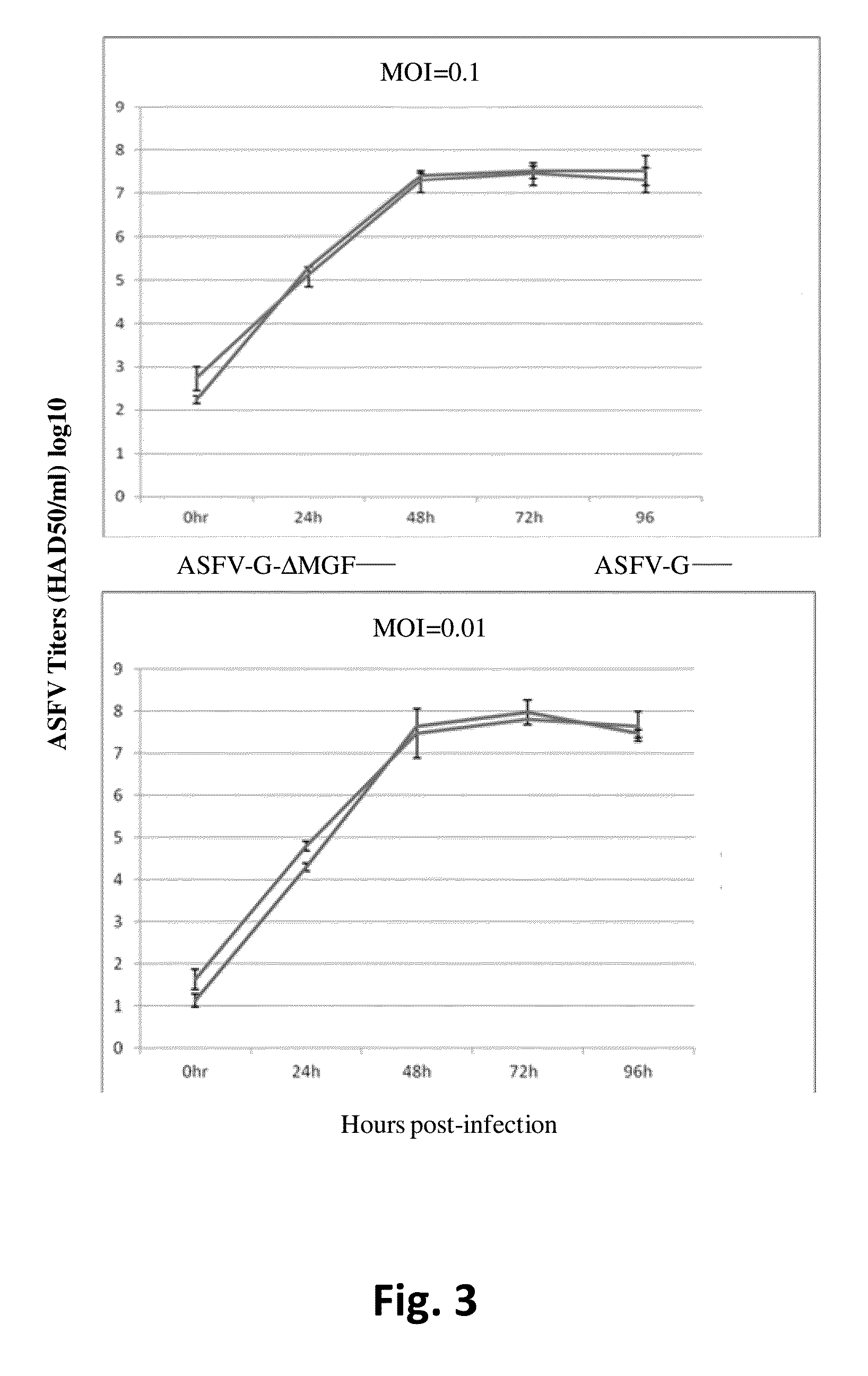
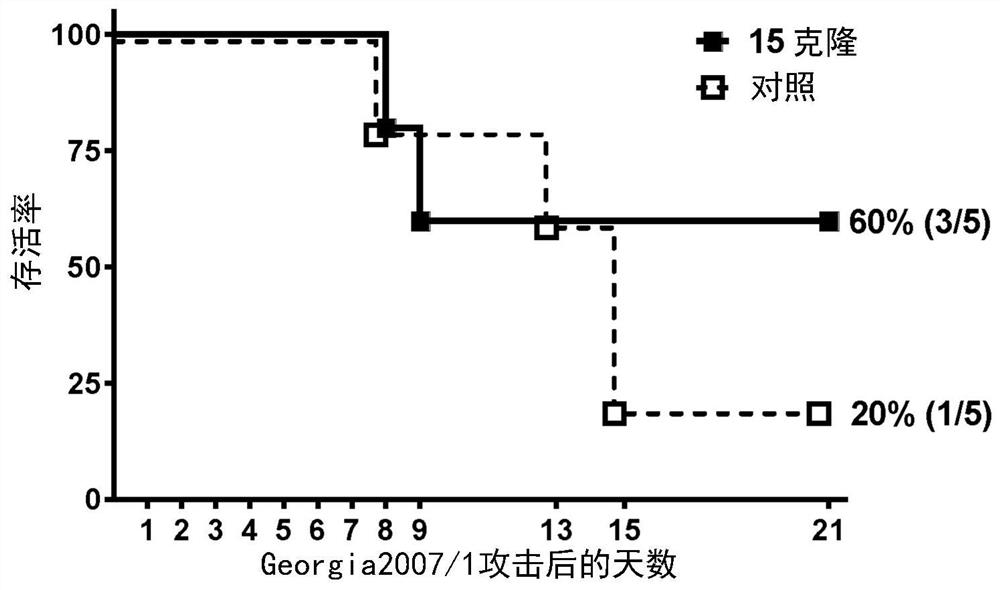
African swine fever virus (ASFV) is the etiological agent of a contagious and often lethal viral disease of domestic pigs. Control of ASF has been hampered by the unavailability of vaccines. Experimental vaccines have been derived from naturally occurring, cell culture-adapted, or genetically modified live attenuated ASFVs; however, these vaccines are only successful when protecting against homologous viruses. Among viral genes reported to be involved in virulence are components of the multi gene family (MGF). Here we report the construction of a recombinant ΔMGF virus derived from the highly virulent ASFV Georgia 2007 (ASFV-G) isolate. In vivo, ASFV-G ΔMGF administered intramuscularly (IM) to swine at either 102 or 104 HAD50 are completely attenuated; the inoculated animals are completely asymptomatic. Animals infected with 102 or 104 HAD50 of ASFV-G ΔMGF are protected against the presentation of clinical disease when challenged at 28 days post infection with the virulent parental strain Georgia 2007.
Owner:US SEC AGRI +1
A real-time isothermal recombinase-polymerase amplification detection kit for African swine fever viruses
ActiveCN106521027AQuick checkRapid diagnosisMicrobiological testing/measurementMicroorganism based processesForward primerAfrican swine fever
A real-time isothermal recombinase-polymerase amplification detection kit for African swine fever viruses is disclosed. A forward primer sequence for detecting the African swine fever viruses through a method provided by the kit is shown as SEQ ID NO:1, a reverse primer sequence is shown as SEQ ID NO:2 and a probe sequence is shown as SEQ ID NO:3. A real-time isothermal recombinase-polymerase amplification method provided by the invention for ASFV detection is simple and convenient in operation, rapid in reaction and low in detection cost, can be used for ASFV detection in a laboratory and on site, particularly ASFV detection in quarantine ports, airports and epidemic disease outbreak sites, and provides a novel and reliable technique support for ASF control in China.
Owner:HANGZHOU ZHONGCE BIO SCI&TECH CO LTD
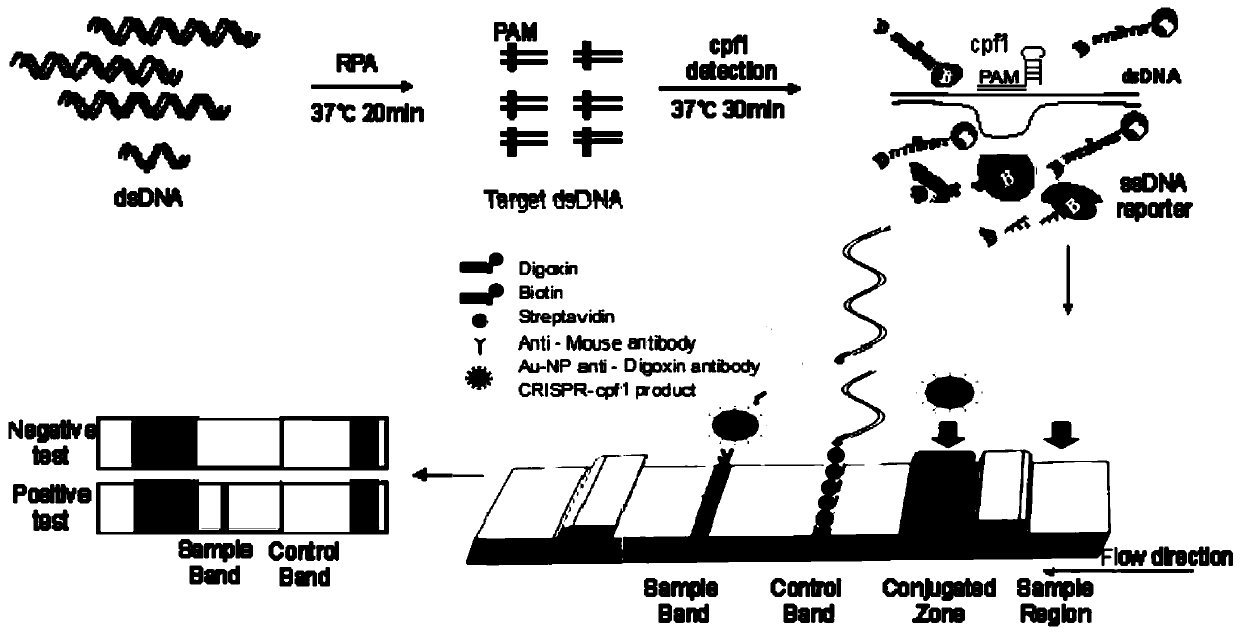
Cpf1 reagent kit and detection method for quickly detecting nucleic acid of African swine fever virus
ActiveCN110551846AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverFluorescence
The invention discloses a Cpf1 reagent kit for quickly detecting nucleic acid of an African swine fever virus. The Cpf1 reagent kit comprises a Cpf1 detection system suitable for quickly detecting theAfrican swine fever virus, and an immune colloidal gold test strip, wherein the Cpf1 detection system comprises specific crRNA protein, specific Cpf1 protein and a single-chain DNA(ssDNA) reporting system in accordance with a p72 gene of the African swine fever virus, the specific crRNA is one or more of crRNAs from ASFV P72 crRNA1 to ASFV P72 crRNA10, and the sequence of the specific crRNA is SEQ NO.4 to SEQ NO.13; and the single-chain DNA(ssDNA) reporting system comprises ssDNA FQreporter for fluorescence detection of a microplate reader and / or ssDNA DB reporter for detecting the immune colloidal gold test strip. According to the Cpf1 reagent kit disclosed by the invention, for the first time, the Cpf1 is used for detecting the African swine fever virus, and has the advantages of beinghigh in sensitivity, high in specificity, short in time consumption, high in flux, independent of large-scale experiment equipment and the like. The advantages enable a detection method based on the immune colloidal gold test strip developed by the invention to be conveniently used in basic laboratories and breeding enterprises to be used for performing detection, identification and diagnosis on basic quick detection of the African swine fever.
Owner:SHANGHAI TECH UNIV
African swine fever P30 protein recombinant baculovirus expression vector and preparation method thereof
The invention provides African swine fever P30 protein recombinant baculovirus expression vector and a preparation method thereof. The method comprises: amplifying in plasmid PCR-4TOPO-P30 of ASFV (African swine fever virus) P30 full-length gene to obtain P39 gene, linking the amplified P30 gene to a baculovirus vector pFastBac 1 to construct recombinant baculovirus vector pFastBac1-ASFV-P30, converting into competent Escherichia coli cells DH10Bac to obtain recombinant shuttle bacmid rBacmid-ASFV-P30, transfecting to insect cells Sf9 after verification is correct to obtain recombinant baculovirus, and passage amplifying the recombinant baculovirus, linking baculovirus high in titer and containing ASFV P30 gene to High Five insect cells for eukaryotic expression of ASFV P30. The African swine fever P30 protein recombinant baculovirus expression vector is constructed by using the method, a recombinant baculovirus expression system is used to express African swine fever P30 protein in insect cells, and basis is laid for African swine fever ELISA (enzyme-linked-immunosorbent serologic assay) detections.
Owner:QINGDAO AGRI UNIV
PCR primer for detecting African swine fever virus, kit and application thereof
ActiveCN105695634AAccurate diagnosisEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverNucleotide sequencing
The invention discloses a PCR primer for detecting an African swine fever virus, a kit and application thereof, and belongs to the detection field of the African swine fever virus. According to a conserved region of an ASFV p72 gene on the GenBank, four pairs of primers are designed, the primer with strong specificity and high sensitivity is screened out from the four pairs of primers, and the primer is composed of nucleotide sequences as shown in SEQ ID No.1 and SEQ ID No.2. The invention further discloses a kit prepared from the primer and used for detecting the African swine fever virus, and a corresponding PCR detection method is established. The detection method established by the invention is more specific and sensitive in comparison with two African swine fever virus PCR detection methods recommended by OIE; and the clinical sample detection result shows that the PCR detection method disclosed by the invention is simple in operation, low in cost, good in specificity and high in sensitivity, and can be effectively applied to the screening and fast diagnosis of the African swine fever.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains
PendingCN110184390ARapid identificationImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationTonsilAfrican swine fever
The invention provides an amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains. A P72 gene of ASFV and a 5'UTR noncoding region of CSFV arerespectively used as an amplification target area, a pair of specific primers and a TaqMan MGB probe (SEQ ID NO:1-6) are designed, a real-time fluorescent quantitation PCR(FQ-PCR) technique is used, and identification and detection of ASFV and CSFV are realized. The detection reagent kit provided by the invention is suitable for detecting viral nucleic acid in samples of serum, spleen, lymph nodes, tonsil, kidney and the like of suspected ASFV or CSFV infected pigs, the sensitivity can reach 1.0*10<1>copy / [mu]L, and the detection reagent kit does not have any cross reactions with other pathogens which are liable to be in mixed infection with the ASFV and the CSFV or of which the infection symptoms are similar such as PRRSV, PRV, PCV2, PPV, JEV and HPS.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
African swine fever virus p72 recombinant protein, monoclonal antibody and test paper
ActiveCN110642926AImproving immunogenicityStrong specificityVirus peptidesBiological material analysisClassical swine fever virus CSFVNucleotide
The invention provides an African swine fever virus p72 recombinant protein, a monoclonal antibody and test paper. An amino acid sequence of the African swine fever virus p72 recombinant protein is obtained by linking amino acid sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2 through flexible amino acid fragments, and has strong immunogenicity and specificity. The invention also provides a nucleotide sequence for encoding the recombinant protein. The invention also provides the monoclonal antibody against the African swine fever virus p72 recombinant protein obtained by immunizing animals byusing the recombinant protein. The invention also provides hybridoma cells 7A7 and 3E5 with preservation numbers of CGMCC No. 18540 and CGMCC No. 18539, respectively, and the monoclonal antibody secreted by the hybridoma cells has high sensitivity and specificity to African swine fever virus p72. The invention also provides the latex microsphere test paper containing the recombinant protein antibody and the colloidal gold test paper containing the recombinant protein antibody, and the test paper has the advantages of small batch difference, high detection sensitivity and simple operation.
Owner:北京纳百生物科技有限公司 +1
Real-time fluorescent LAMP detection primer group, kit and detection method of African swine fever virus non-structural gene
InactiveCN106947838ASensitiveImprove featuresMicrobiological testing/measurementMicroorganism based processesGenotypeFluorescent pcr
The invention provides a real-time fluorescent LAMP detection primer group, a kit and a detection method of an African swine fever virus (ASFV) non-structural gene. The primer group is designed based on a non-structural DNA polymerase G1211R gene and comprises an FIP primer, a BIP primer, an F3 primer and a B3 primer. A detection result shows that a typical S-shaped nucleic acid amplification curve, and an amplification product has a specific melting curve. An ASFV70 strain virus nucleic acid is taken as a template, and LAMP detection is better than a fluorescent PCR (Polymerase Chain Reaction) method in sensitivity. Intra-assay and inter-assay variable factors of repetitive testing LAMP detection are both smaller than 5 percent. An ASFVArm07 stain is used for preparing various clinic simulated samples, and detected positive rate is up to 17.31 percent. The detection method provided by the invention can provide a new technological means for preventing and controlling African swine fever virus, and detection on different genetic stains and quick screening for exit and entry are facilitated.
Owner:TECH CENT OF GUANGZHOU CUSTOMS
Immunogenic compositions and vaccines comprising african swine fever virus peptides and proteins and uses thereof
The present invention relates to African swine fever virus (ASFV) peptides and / or polypeptides as well as immunogenic fragments thereof, corresponding encoding AFSV oligonucleotides and / or polynucleotides as well as immunogenic fragments thereof, immunogenic compositions, vaccines and uses thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
African hog cholera virus fluorescent quantitative PCR detecting reagent and preparation and use thereof
InactiveCN101463396AFast detection methodSensitive highMicrobiological testing/measurementLower limitFluorescence
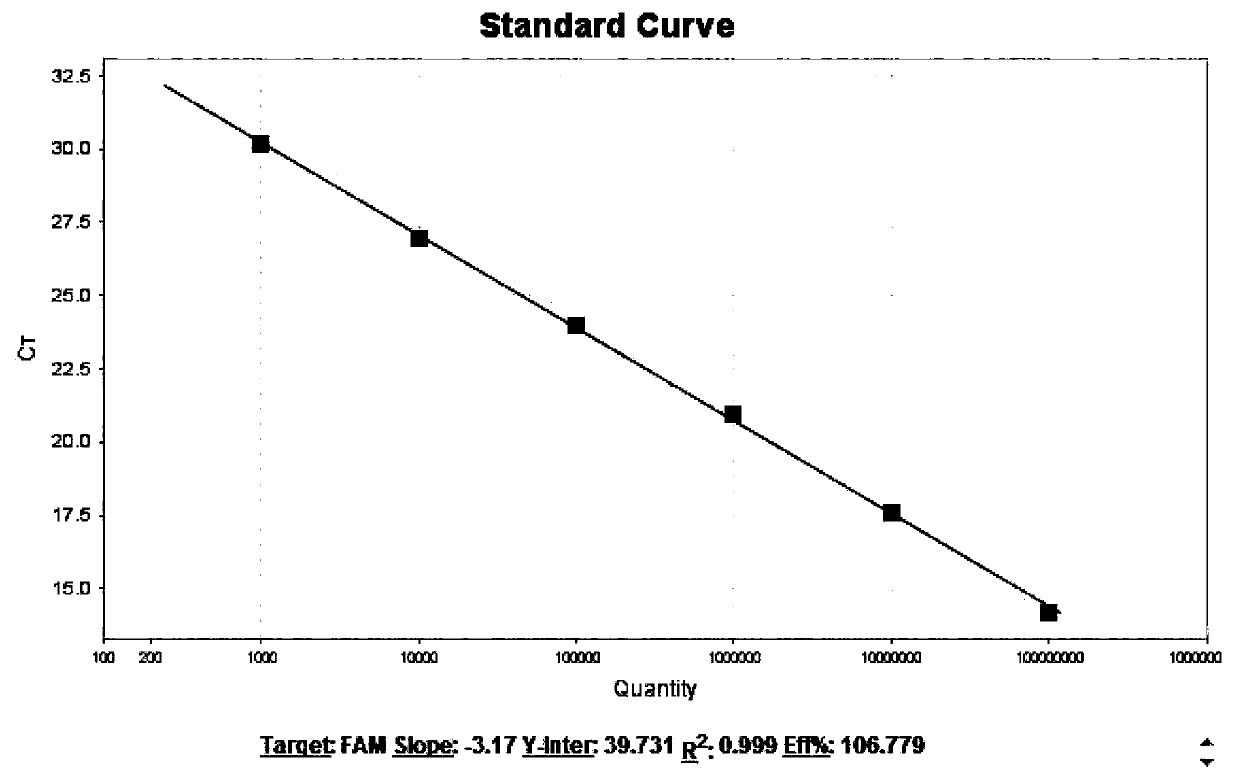
The invention discloses a fluorescence quantitative PCR detection reagent for African swine fever virus, and a preparation method and the application thereof. A set of specific primers and Taqman probes are designed and synthesized to be used for detecting ASFV P54 in relevant porcine products. A standard curve drawn in the invention provides a standard for the quantitative detection of ASFV P54. The invention establishes a fast and simple real-time fluorescence quantitative PCR detection system with strong specificity and high flexibility. The detection time is only several hours, and the detection lower limit can be 15 copies. The invention can be applied to the diagnosis and quarantine technology towards the imported relevant porcine products at port, and the invention provides reliable and effective technical condition for the import quarantine work of the country without ASF.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Reagent, detection method and application for detection of African hog cholera virus
InactiveCN109735657AShort detection timeRapid prevention and controlMicrobiological testing/measurementMicroorganism based processesAgricultural scienceRecombinase Polymerase Amplification
The invention discloses a reagent, detection method and application for the detection of African hog cholera virus. The reagent comprises a specific primer pair and a probe, upstream and downstream primers of the primer pair are respectively sequences shown in Seq ID No. 1 and 2, and the probe is a sequence shown in Seq ID No. 3 or its reverse complement sequence; in the probe sequence shown in the SEQ ID No.3, a 28th base modifies fluorescent quenching group-dT, a 31st base is replaced with a base analog, a 33rd base modifies a fluorescent group-dT, and a 3' end modifies C3 Spacer. The reagent is sensitive, specific and efficient for detection of the African hog cholera virus by recombinase polymerase amplification; compared with conventional conventional or real-time fluorescent PCR, thereagent and the detection method have short detection time and simple operation, are especially suitable for on-site testing, and are of great significance for the rapid prevention and control of theAfrican hog cholera virus and guarantee of production safety.
Owner:SHENZHEN AUDAQUE DATA TECH
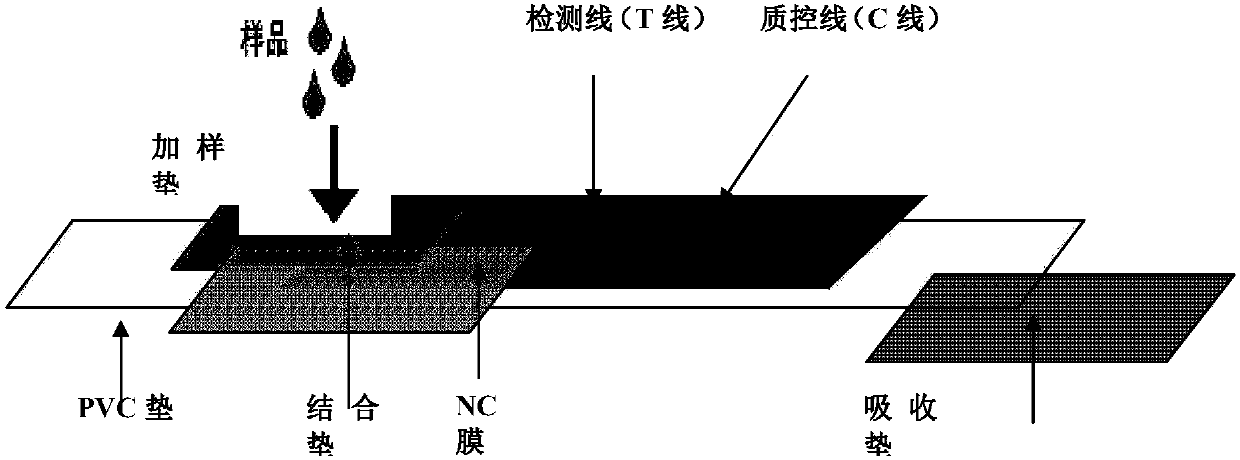
Quick African swine fever virus detection card and application thereof
InactiveCN109781980AStrong specificityIncreased sensitivityBiological testingStaphylococcus aureusPolyclonal antibodies
The invention discloses a quick African swine fever virus detection card and application thereof, so as to solve the technical problem of hard African swine fever virus detection. Polyclonal antibodies PoAbI against African swine fever virus p30, P54 and p72 protein labeled with colloidal gold are absorbed on a colloidal gold labeling pad of the detection card; a detection membrane is provided with goat or rabbit anti-mouse IgG antibodies or a quality control line C printed by staphylococcus aureus SPA; and a detection line T printed by polyclonal antibodies PoAbII against African swine fevervirus p30, P54 and p72 protein is also arranged. The invention also discloses a using method for the quick African swine fever virus detection card. The method comprises steps: a to-be-detected sampleis acquired, and PBS or water is added for suspension or grinding; the sample is dripped to the sample loading end of the quick African swine fever virus detection card; and horizontal placing is carried out to observe a result. The quick African swine fever virus detection card adopts a double antibody sandwich method, and has the advantages of strong specificity, high sensitivity, high detection efficiency, high efficiency and practicability, and important practical application value.
Owner:河南中泽生物工程有限公司 +1
African swine fever virus nucleic acid amplification primer, detection method and kit
InactiveCN101921878AQuick checkEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationGold particlesHybrid compound
The invention relates to an African swine fever virus nucleic acid amplification primer, a detection method and a kit. The primer pair and the specific nucleic acid probe are selected from SEQ ID NO: 1-12. The detection method comprises the following steps: (a) amplifying nucleic acid in the sample to be detected by using a PCR primer; (b) labeling the detection probe labeled by alkyl sulfydryl group on nano gold particles; (c) hybridizing the capture probe labeled by biotin and the detection probe labeled by nano gold in step (b) with a metamorphic PCR product; (d) adding the hybrid system in step (c) to a Streptavidin-coated ELISA plate to capture the hybrid compound; (e) carrying out silver enhancement to capture nano gold; (f) and stoping the silver enhancement reaction, and visually inspecting the grey scale judgment result. The invention has the characteristics of high detection sensitivity, strong detection specificity and low detection cost, can effectively eliminate false positive or false negative result when the PCR method is used for detecting African swine fever virus, and quickly detect the African swine fever virus nucleic acid.
Owner:YANGZHOU UNIV
Immunogenic compositions and vaccines comprising african swine fever virus peptides and proteins and uses thereof
PendingCN113543801AViral antigen ingredientsVirus peptidesClassical swine fever virus CSFVPolynucleotide
The present invention relates to African swine fever virus (ASFV) peptides and / or polypeptides as well as immunogenic fragments thereof, corresponding encoding AFSV oligonucleotides and / or polynucleotides as well as immunogenic fragments thereof, immunogenic compositions, vaccines and uses thereof.
Owner:BOEHRINGER INGELHEIM VETMEDICA GMBH
Recombinase polymerase application (RPA) primer for rapidly detecting African swine fever virus (ASFV) nucleic acid, preparation method of RPA primer, and kit
InactiveCN107937624AQuick checkStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyWater baths
The invention discloses a recombinase polymerase application (RPA) primer for rapidly detecting African swine fever virus (ASFV) nucleic acid, a preparation method of the RPA primer, and a kit, belonging to the technical field of biology. A pair of RPA primers, i.e., an upstream primer <210>2 and a downstream primer <210>3 which have high specificity and strong sensibility are screened out; an RPAdetection system for rapidly detecting the ASFV nucleic acid is further established by means of the primers. Compared with the common polymerase chain reaction (PCR) method, RPA-lateral flow assay (LFA) has the advantages that firstly, the RPA-LFA belongs to an isothermal amplification technology and is low in requirements for instruments and equipment, and reactions can be completed only by means of a constant temperature water bath kettle; secondly, the detection speed of the RPA-LFA is fast, and the reaction time is 40 minutes and is shorter than the conventional PCR reaction time; thirdly, the visualization of detection results can be realized. Due to the characteristics, the RPA-LFA method established by the invention can be used for rapid detection and differential diagnosis of ASFVin common laboratories of grassroots units.
Owner:SHIHEZI UNIVERSITY
Recombinant adenovirus vector for expressing African swine fever virus (ASFV) EP402R gene, construction method of recombinant adenovirus vector and preparation method of recombinant adenovirus
InactiveCN108504687AAchieve normal expressionEasy to filterVirus peptidesMicroorganism based processesMultiple cloning siteAfrican swine fever virus
The invention provides a recombinant adenovirus vector for expressing an African swine fever virus (ASFV) EP402R gene, a construction method of the recombinant adenovirus vector and a preparation method of a recombinant adenovirus, belonging to the technical field of genetic engineering. The method provides recombinant adenovirus plasmids pAD-EP402R for expressing the EP402R gene, a pAD-EF1alpha-GFP adenovirus expression vector is used as basis, and the EP402R gene is introduced at a multiple cloning site. The invention also provides the preparation method of the recombinant adenovirus for expressing the EP402R gene; the contracted plasmids pAD-EP402R are mixed with an adenovirus packaging system PEI so as to realize the packaging process of the adenovirus, so that the recombinant adenovirus capable of directly infecting eukaryotic cells is obtained; therefore, the aim of the normal expression of the EP402R gene in the eukaryotic cells is realized, and a foundation is laid for the study of a recombinant adenoviral vector candidate vaccine based on expression of the EP402R.
Owner:YANGZHOU UNIV
Competitive ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting antibody of African swine fever virus and application thereof
The invention discloses a competitive ELISA (Enzyme-Linked Immuno Sorbent Assay)kit for detecting an antibody of an African swine fever virus and application thereof, belonging to the technical field of organisms. The kit is used for detecting an antibody of the African swine fever virus in pig serum by adopting prokaryotically expressed recombinant P54 protein as an envelope antigen according toa competitive ELISA principle. The envelope antigen in a 96 pore plate in the kit is prokaryotically expressed recombinant P54 protein and has favorable antigenicity. The enzyme-linked immuno kit provided by the invention comprises the P54 protein enveloped 96 pore plate, positive control, negative control, a horseradish peroxidase marked monoclonal antibody, a concentrated cleaning solution, serum diluent, a TMB substrate and a stopping solution. The kit can be used for screening samples in bulk, main reagents in the kit are provided in a working solution way, and the use is convenient.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Attenuated African swine fever virus with gene deletion and its application as a vaccine
ActiveCN110093324BGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
African swine fever virus georgia strain adapted to efficiently grow in the vero cell line
We have developed an ASFV Georgia strain adapted to grow in Vero cell line. The resulting virus, ASF-GVAV, efficiently grows in Vero cells although it still is able to significantly replicate in primary cell cultures of swine macrophages. ASF-GVAV virus was successfully used as parental virus to develop several recombinant ASF viruses. The development of an ASFV adapted to grow in an established cell line is a significant advance for research and development of vaccine candidate strains using genetic manipulation based in the process of homologous recombination. The GVAVS can be utilized as a basis for large scale production of ASF vaccines.
Owner:US SEC AGRI +1
Triple PCR detection primer and kit for rapidly distinguishing African swine fever virus wild strains from gene deletion strains
ActiveCN110551853AReduce testing costsShorten detection timeMicrobiological testing/measurementMicroorganism based processesAgricultural scienceAfrican swine fever
The invention discloses a triple PCR detection primer set for rapidly distinguishing African swine fever virus wild strains from CD2V and / or 360-505R gene deletion strains. Nucleotide sequences of three pairs of detection primers are as shown in SEQ ID NO: 1-6. Three pairs of primers are utilized to amplify three genes of African swine fever viruses CD2V, P72 and 360-505R at a time, so that the detection cost for identifying different genes is reduced, and the detection time for identifying different genes is shortened; and moreover, three genes with different lengths can be amplified only byone-time PCR reaction, and whether gene deletion exists in the strains or not can be distinguished. The three genes can be identified by one-time PCR amplification only, the cost is reduced by about 2 / 3 for a traditional method for respectively amplifying and detecting the samples of the three genes, and the triple PCR detection primer set for rapidly distinguishing the African swine fever virus wild strains from the CD2V and / or 360-505R gene deletion strains has wide market prospect.
Owner:SOUTH CHINA AGRI UNIV +1
Triple real-time fluorescent quantitative PCR kit for detecting African swine fever wild strains and gene deletion strain
InactiveCN111020062AHigh detection sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVAfrican swine fever
The invention provides a triple real-time fluorescent quantitative PCR detection primer for detecting an African swine fever wild strain and a gene deletion strain, and a kit and a detection method thereof. The triple fluorescent quantitative detection kit is developed and researched for three genes CD2V, VP72 and MGF-360 14L of the African swine fever virus by utilizing a multiple fluorescent PCRtest means, and whether a sample is infected with the African swine fever virus and whether gene deletion exists in the infected virus or not can be determined at the same time. The method can detecta large number of samples at the same time, provides an effective tool for scientifically and reasonably preventing and controlling African swine fever, guarantees the healthy development of the pigindustry, and has the advantages of convenience in operation, high sensitivity, strong specificity, short detection time and the like.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and preparation method and application thereof
InactiveCN104745731AMicrobiological testing/measurementMicroorganism based processesPositive controlAfrican swine fever
The invention discloses a triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and a preparation method and application thereof. Three sets of specific primers and Taqman probes as well as positive controls specific to African swine fever virus CP530R genes, swine fever virus 5 minute-UTR genes and swine respiratory syndrome virus NSP2 genes respectively are designed and synthesized, and a rapid, easy and convenient triple fluorescent RT-PCR detection system with high specificity and high sensitivity is established by using the three sets of primers and probes, so that nucleic acids of the African swine fever viruses, the swine fever viruses and the respiratory syndrome viruses can be detected synchronously from a detected sample within 3-4 hours in a rapid, accurate, specific, safe, easy and convenient way. The detection reagent can be applied to synchronous detection of the nucleic acids of trace African swine fever viruses, swine fever viruses and respiratory syndrome viruses in hogs and relevant samples thereof.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Dual-fluorescence PCR detection reagent, kit and detection method for classical swine fever virus and African swine fever virus
PendingCN110760620AReduce workloadLow costMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVSwine Fever Virus
The invention discloses a dual fluorescence PCR detection reagent, a kit and a detection method for swine fever virus and African swine fever virus, and belongs to the field of animal pathogen detection. Aiming at a 5'-UTR gene of the swine fever virus and a P72 gene of the African swine fever virus, primers and probes capable of covering all strains and suitable for double detection are separately designed and screened, including two pairs of specific primers and two specific probes; the invention also describes a kit containing the primers and the probes and a PCR detection method using theprimers and the probes; the invention, through the design of the primers and the adjustment of each component of PCR, realizes purposes of one-time analysis and simultaneous detection and differentiation of the swine fever virus and the African swine fever virus on the premise of no reduction in sensitivity and specificity, which not only reduces the workload and cost of detection, but also greatly saves the detection time, thus gaining valuable time for epidemic disease prevention and treatment.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY +1
African swine fever polymerase chain reaction (PCR) detection method and oligonucleotide primer pair
ActiveCN103320536AStrong specificityGuaranteed to be correctMicrobiological testing/measurementMicroorganism based processesPig farmsDisease
The invention belongs to the technical field of biological detection and relates to an African swine fever polymerase chain reaction (PCR) detection method and an oligonucleotide primer pair. The method comprises the following steps: designing a pair of specific primers by referring to a complete genome sequence of 23 ASFVP72 protein strains recorded in GenBank, exploring the optimal reaction system and reaction conditions according to the designed primers, performing sensitivity test, specificity test and reproducibility test on the established PCR reaction method, and performing PCR method detection on clinical samples of sows suffered from reproductive disturbance or piglets with respiratory disturbance symptoms and aborted fetuses from 14 pig farms. The test results prove that the established PCR method can sensitively and rapidly detect the African swine fever virus. The PCR detection method established in the research has high applicability and can be used for detecting the African swine fever disease.
Owner:QINGDAO AGRI UNIV
African swine fever virus vaccine and preparation method thereof
ActiveCN112876570AAntibody mimetics/scaffoldsVirus peptidesAntiendomysial antibodiesAfrican swine fever
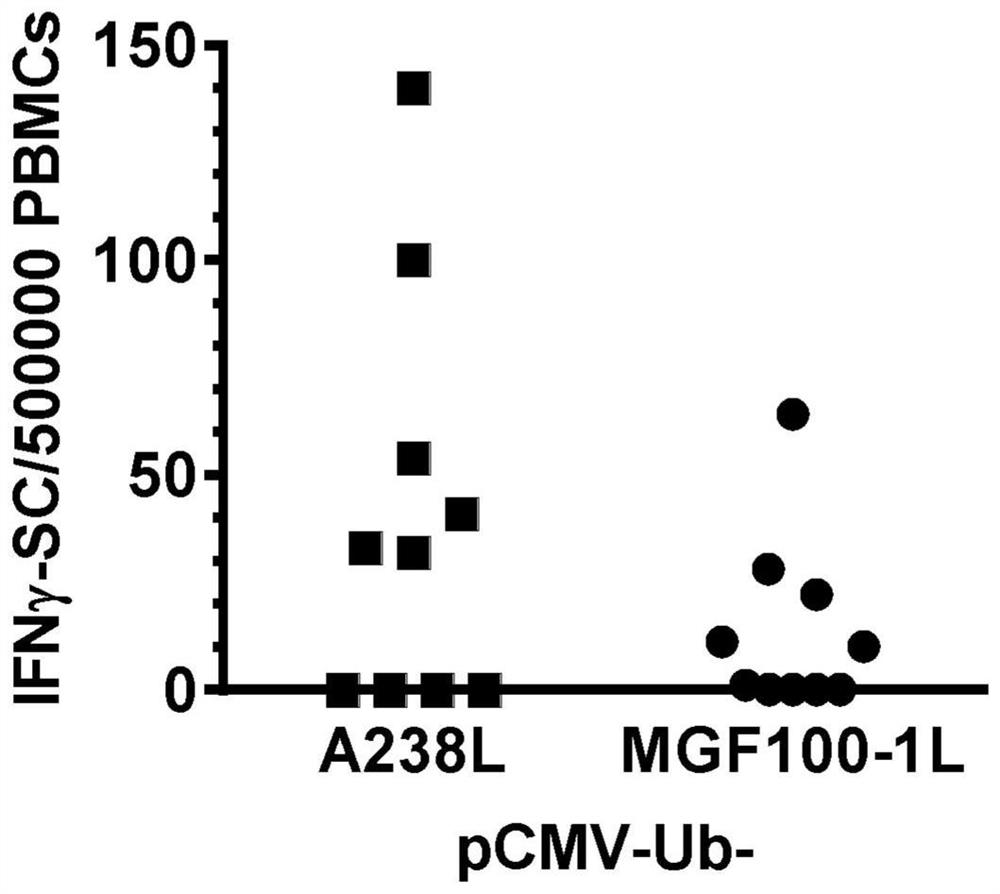
The invention discloses an African swine fever vaccine and a preparation method thereof. According to the invention, the structural proteins P72, P30, P54 or CD2v-AC of the African swine fever virus are respectively displayed on the surface of the cage-shaped structure of the self-assembled ferritin, so that the humoral immune efficacy and width of the vaccine are improved, and the immunogenicity of the structural proteins of the African swine fever virus is improved. Structural proteins P30, P54 and CD2v are recombined to obtain recombinant proteins, and the recombinant proteins are connected with ubiquitin to obtain two recombinant proteins, so that the cellular immune effect of the structural proteins of the African swine fever virus is further improved, and better immune protection is provided for animals. The invention also provides a method for preparing the recombinant protein or the African swine fever vaccine. The African swine fever vaccine provided by the invention can initiate a wide neutralizing anti-African swine fever antibody, not only improves the immune efficacy, but also expands the immune range, provides effective immune protection for virulent infection, and has the potential of becoming a universal safe vaccine with multiple protection effects.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com