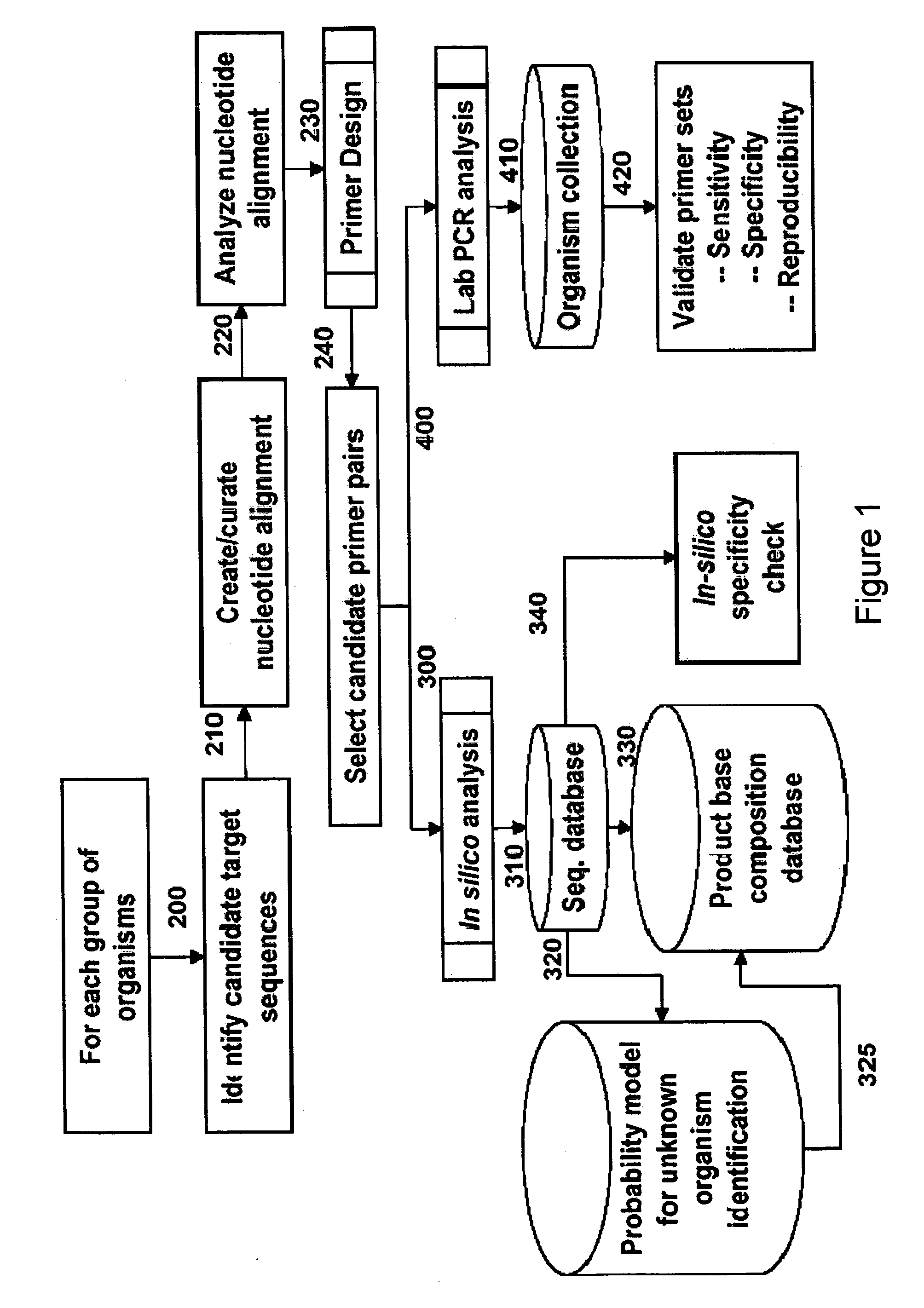
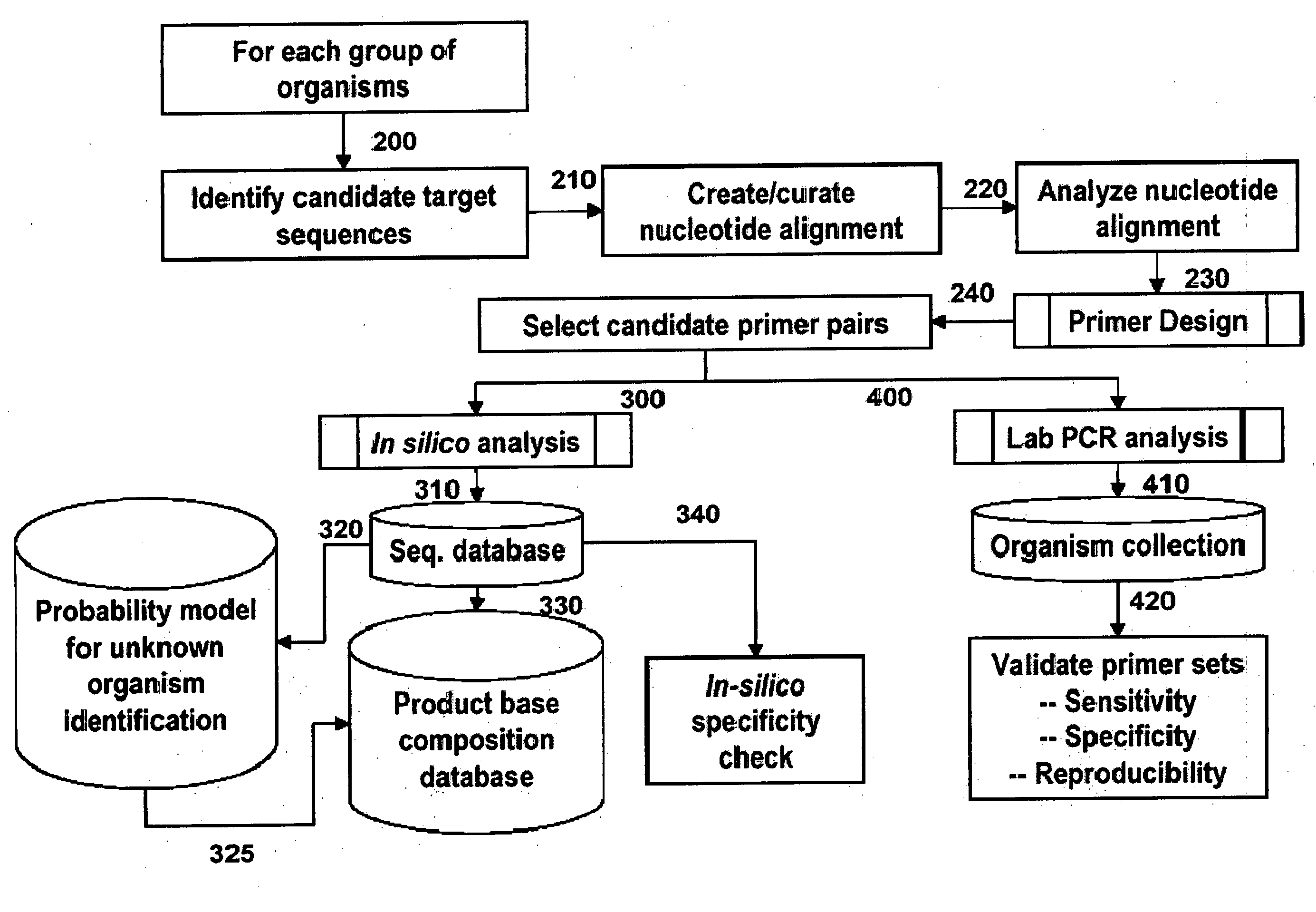
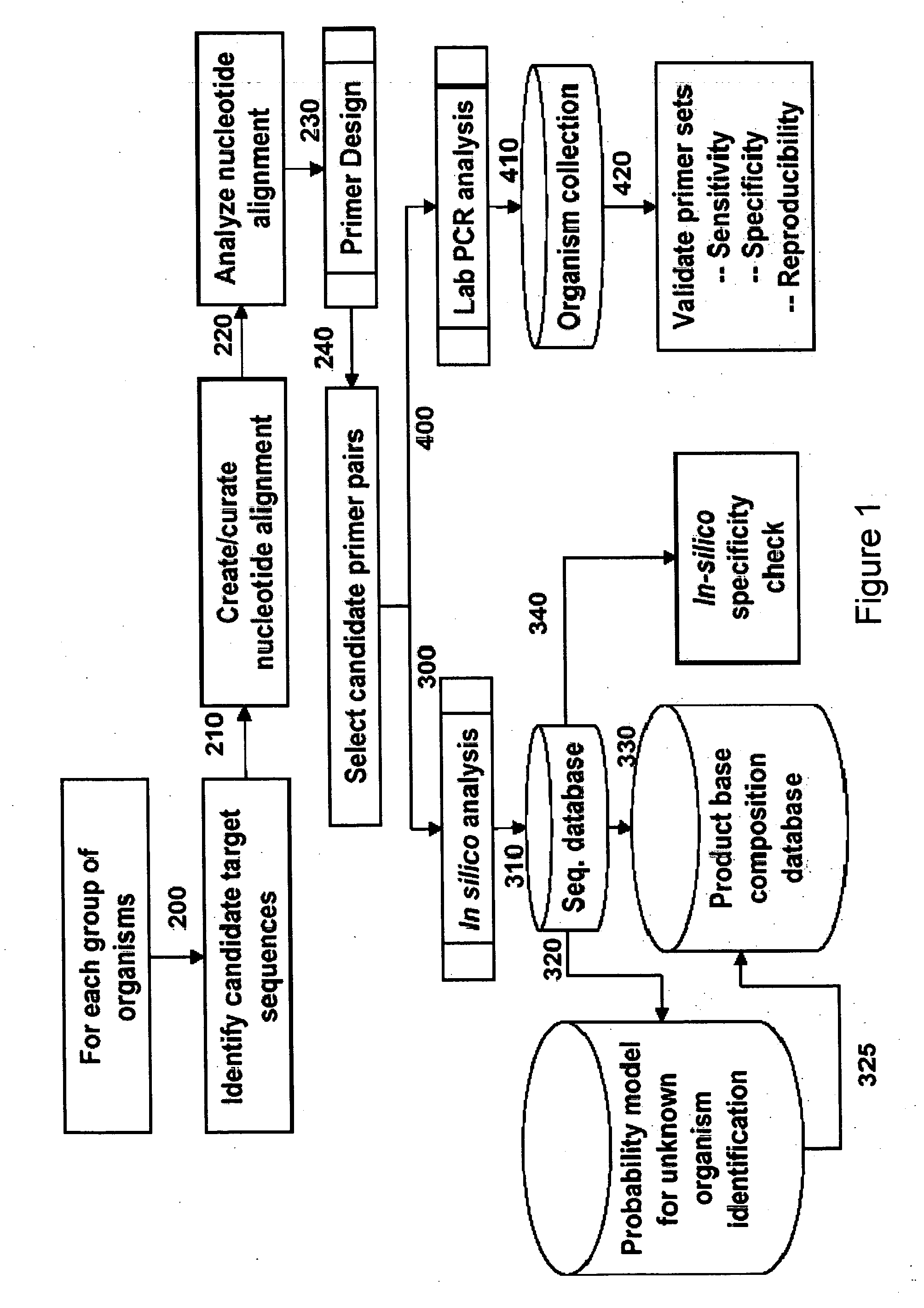
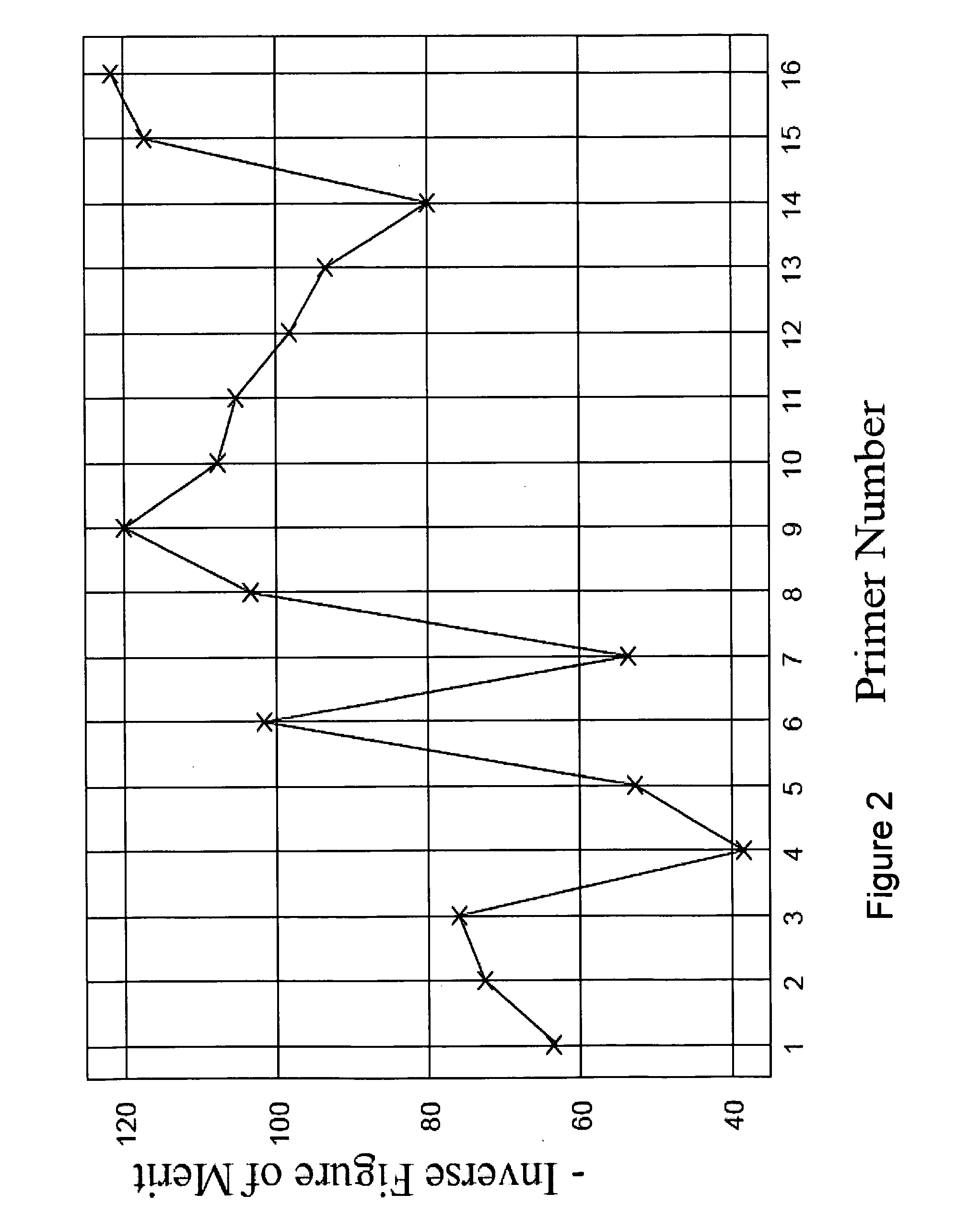
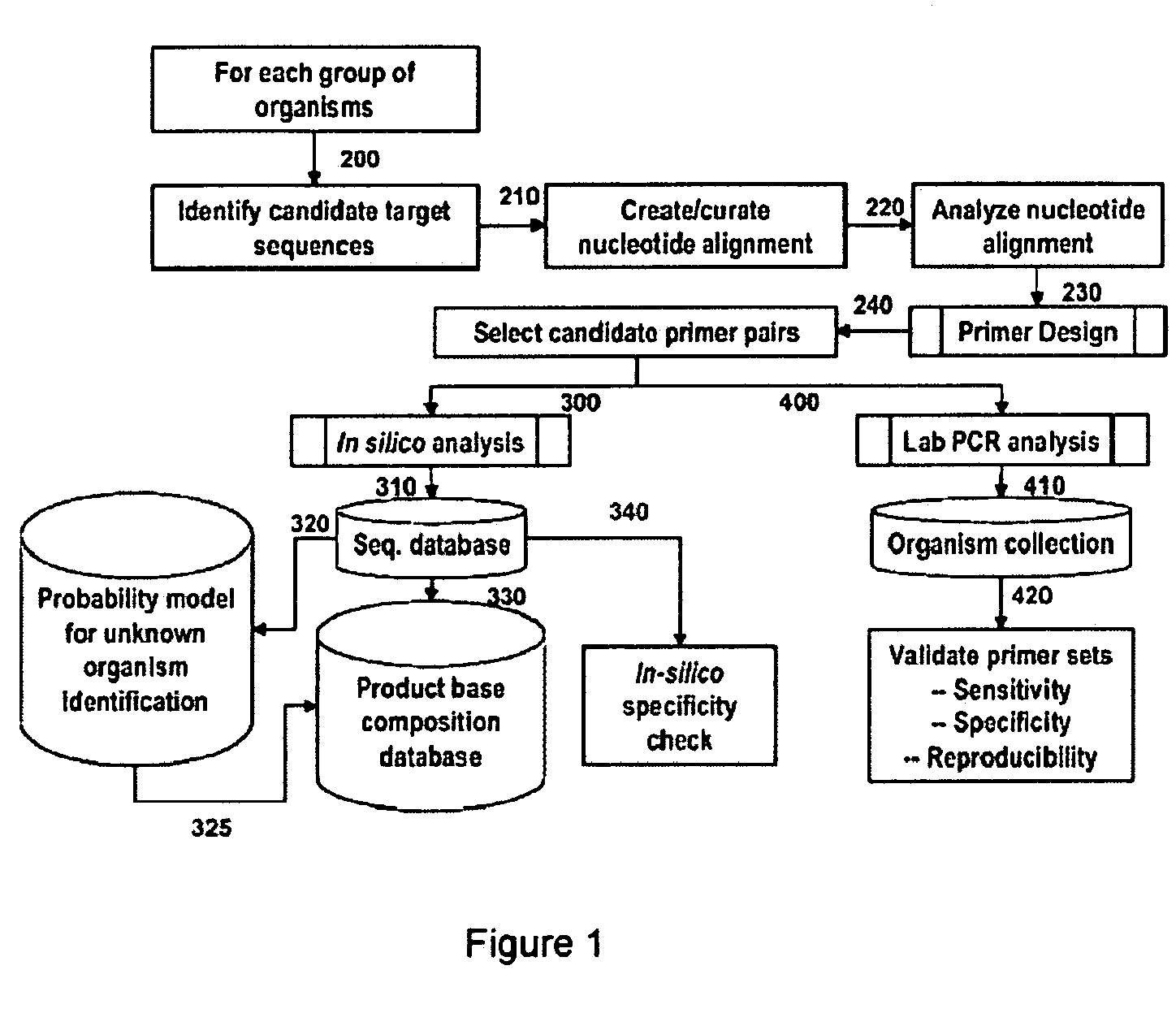
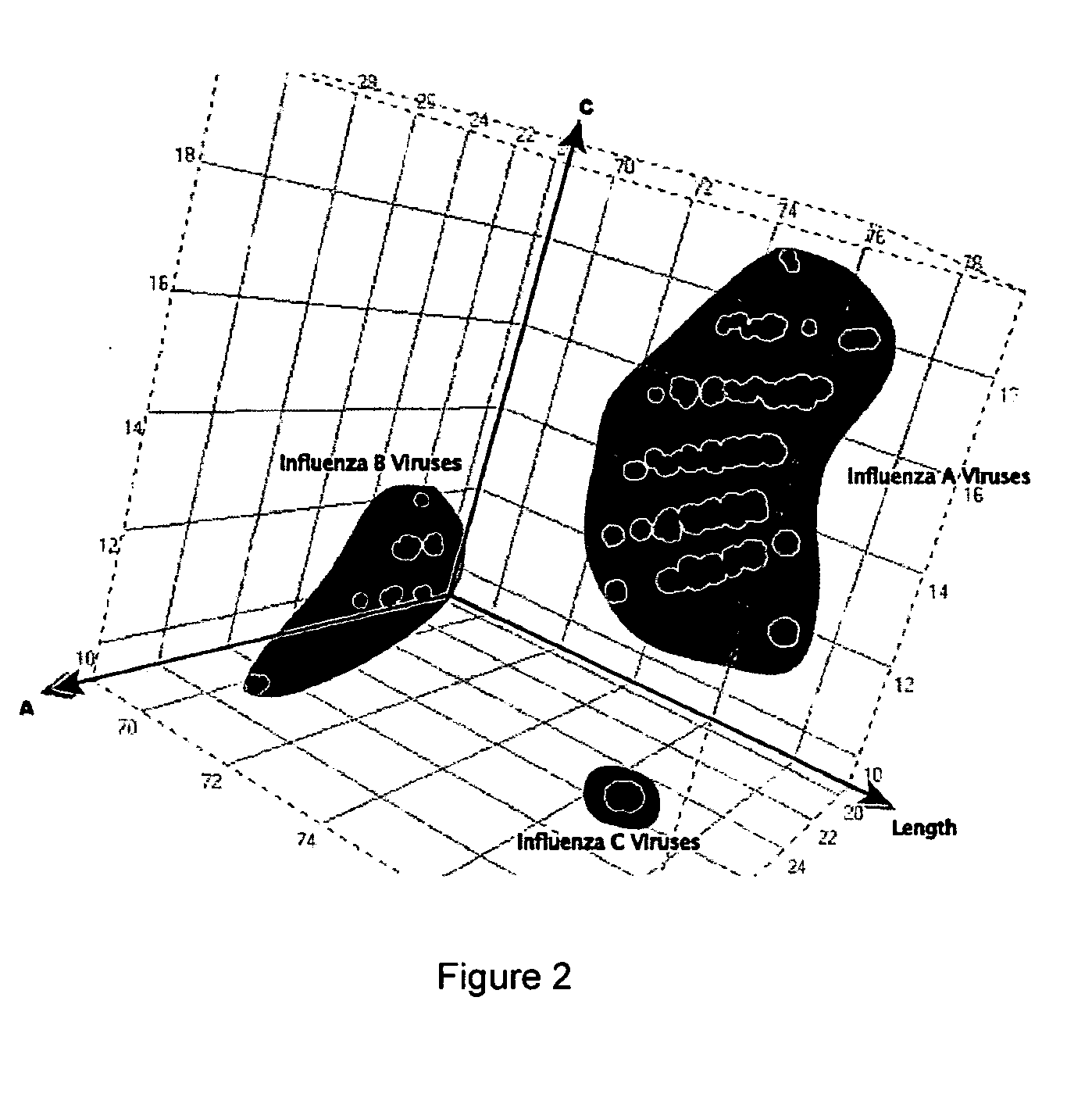
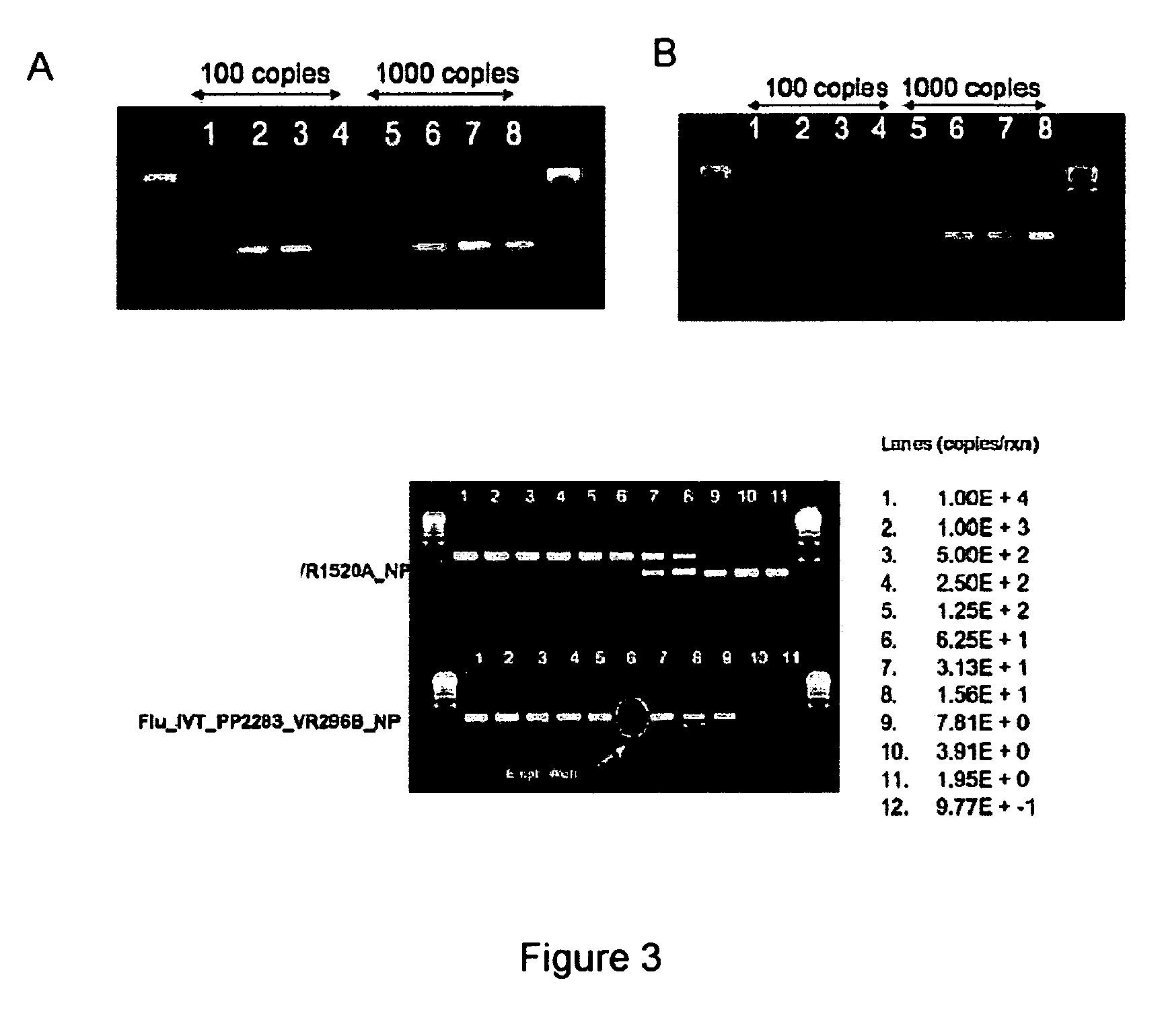
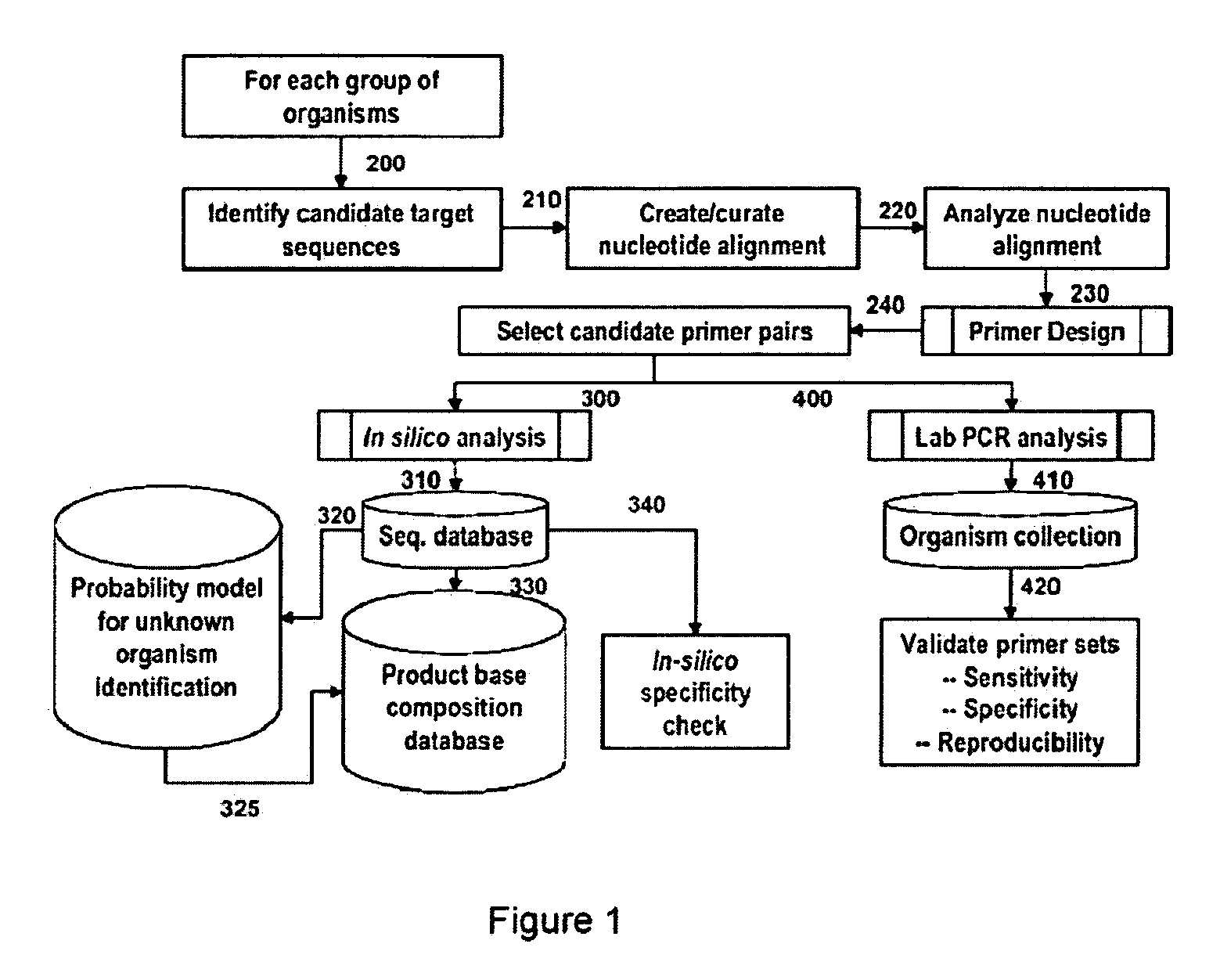
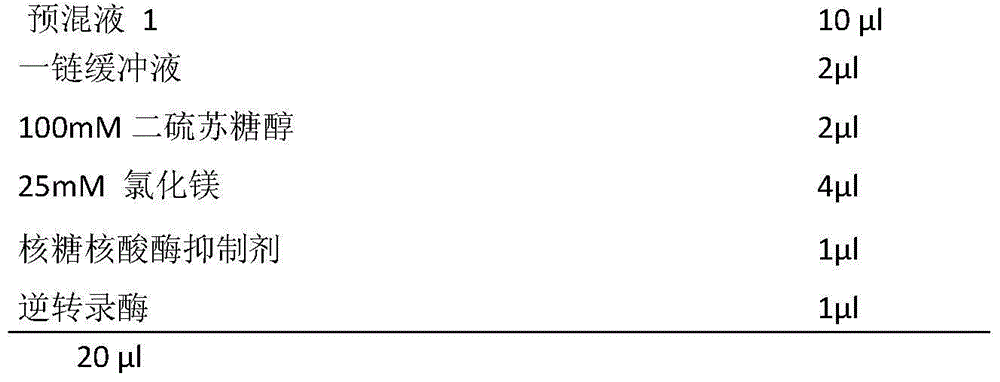Patents
Literature
512 results about "Viral nucleic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
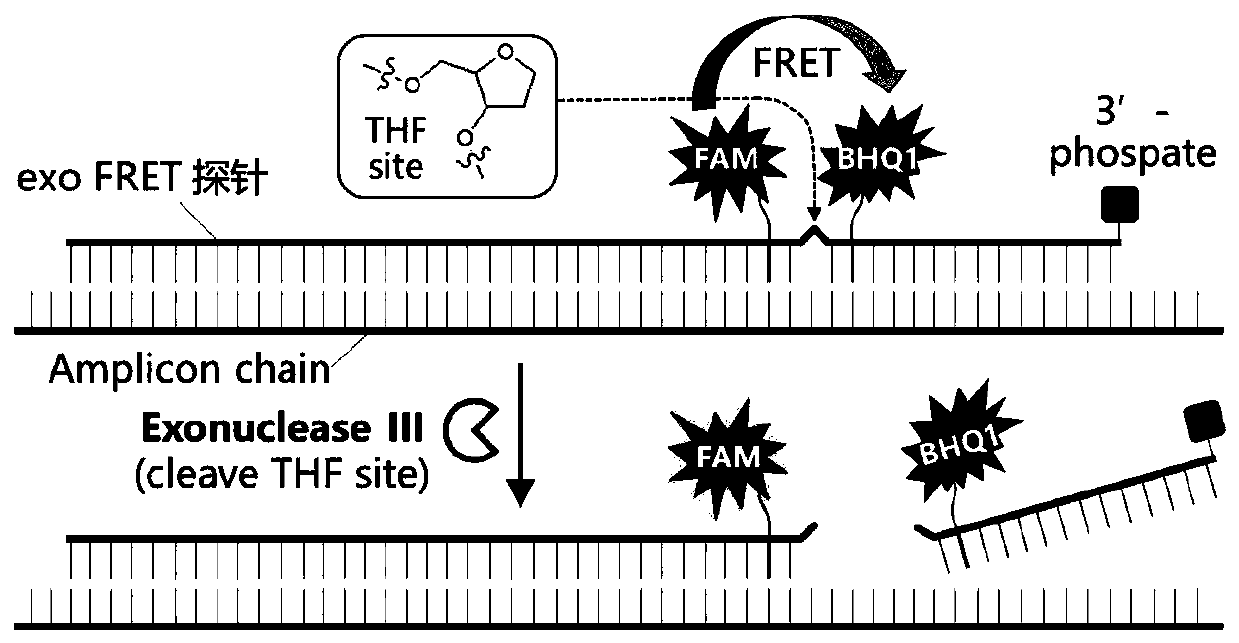
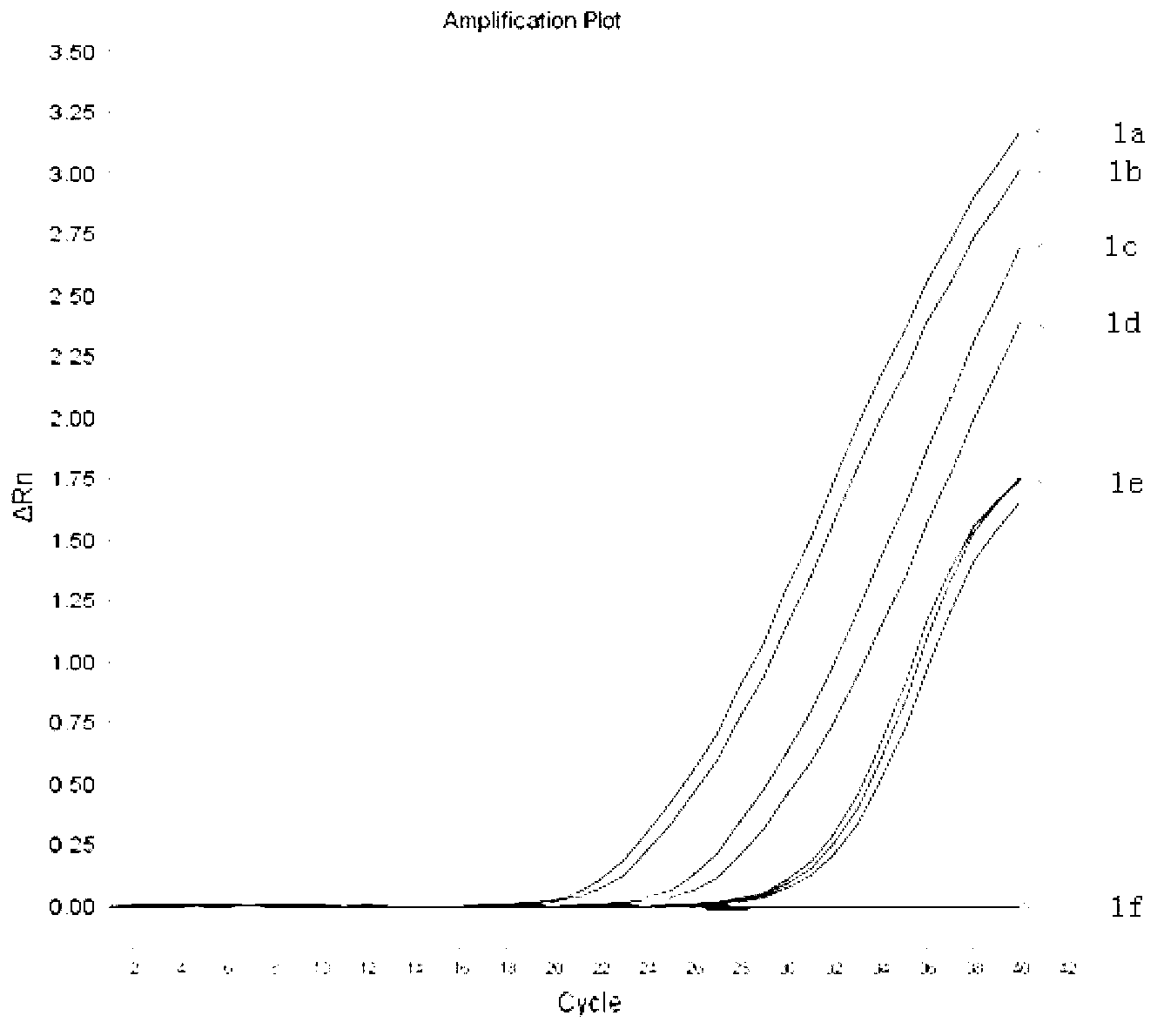
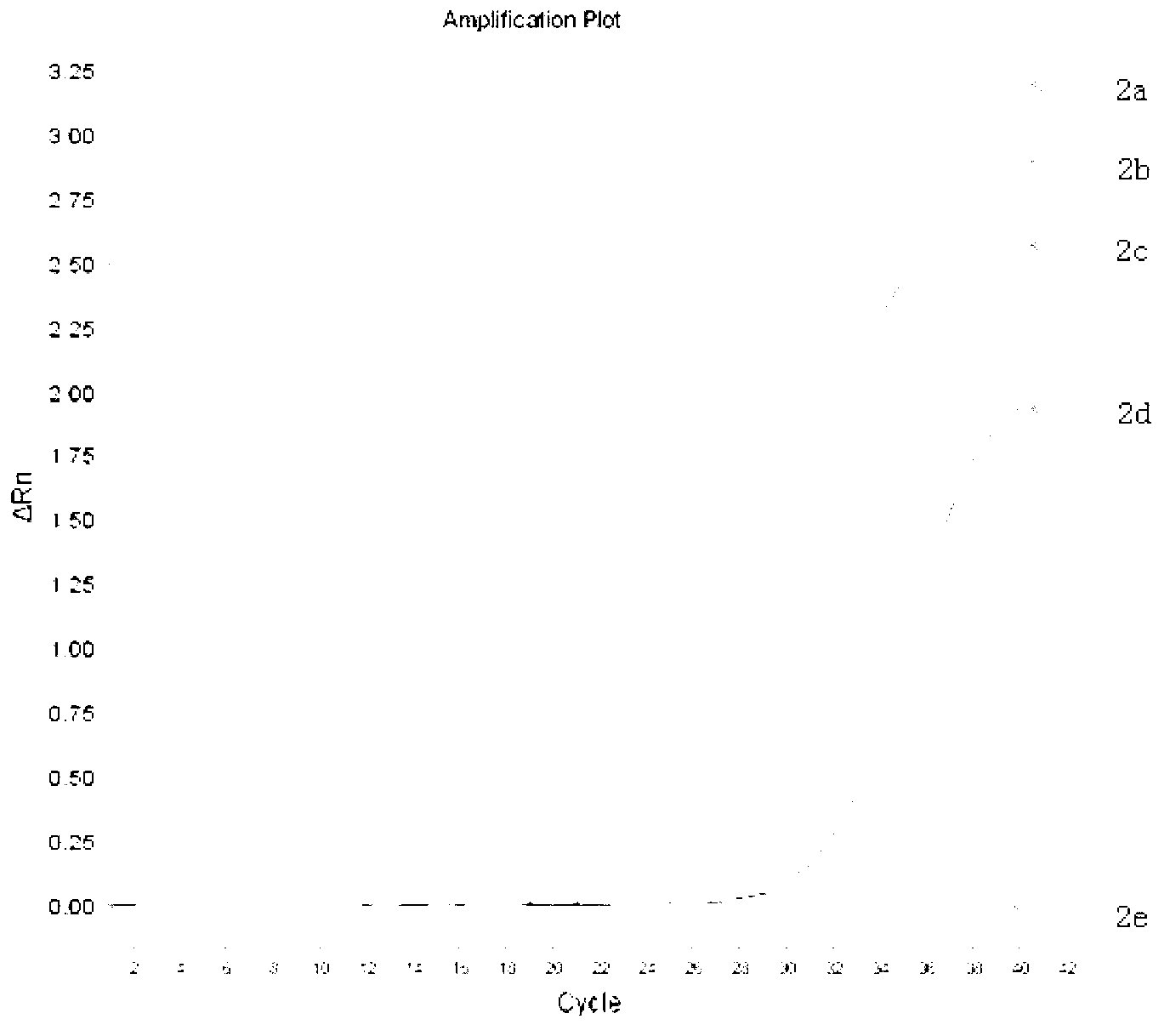
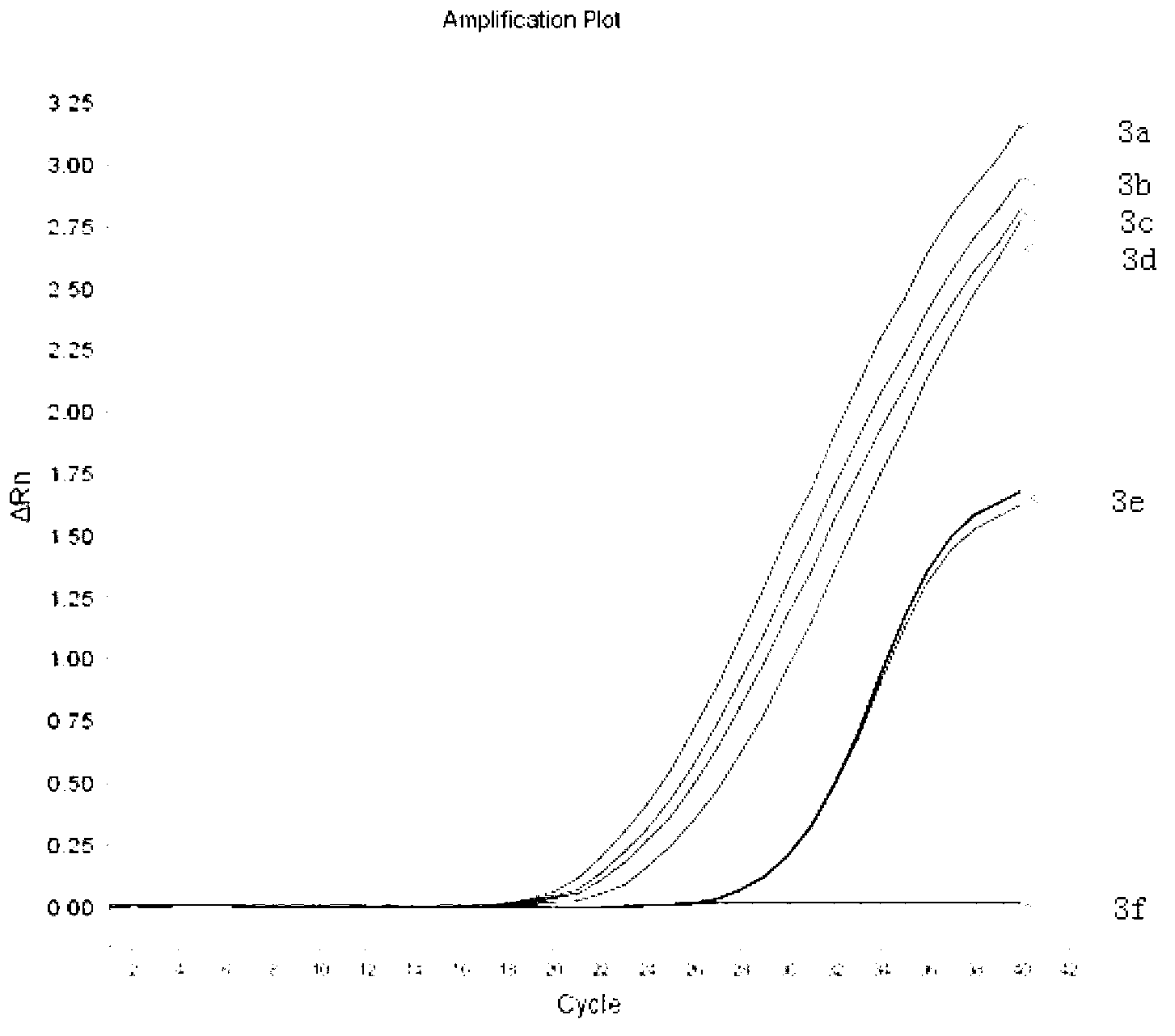
The viral nucleic acid (Either DNA or RNA) has the genetic codes for the synthesis of proteins to produce new viruses, i.e, virus' s genome. When a virus finds a host cell, the nucleic acid is injected into the host cell.
Compositions for use in identification of viral hemorrhagic fever viruses
InactiveUS7312036B2Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
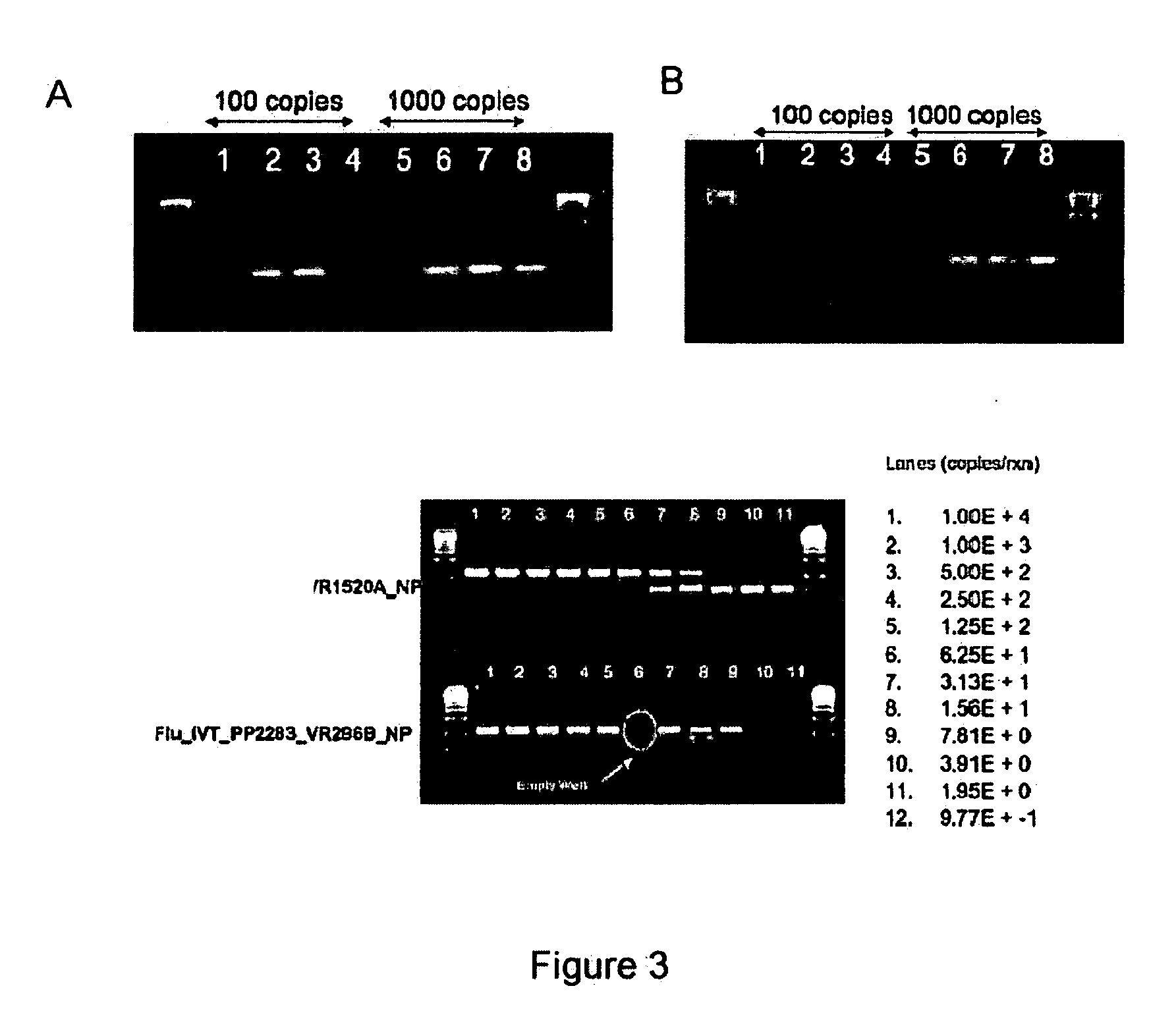
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses that cause viral hemorrhagic fevers by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Virus infection detection and identification method based on metagenomics
ActiveCN105112569AHigh detection sensitivityLow initial sample size requirementMicrobiological testing/measurementViral nucleic acidNucleic acid sequence
The invention provides a virus infection detection and identification technology based on metagenomics. The virus infection detection and identification technology based on the metagenomics comprises the four portions of sample preparation, high-throughput sequencing, bioinformatic analysis and result re-checking. In the sample preparation portion, viral nucleic acid is effectively extracted or enriched from detection samples according to the requirements of the virus infection detection and identification technology based on the metagenomics and characteristics of different types of detection samples, and a nucleic acid library which can be used for a next-generation sequencing instrument is established. In the high-throughput sequencing portion, the nucleic acid library established in the sample preparation step is sequenced so to obtain sufficient high-quality nucleic acid sequence information. In the bioinformatic analysis portion, a large number of high-quality nucleic acid sequences obtained in the high-throughput sequencing step is analyzed to further obtain viral component information prompted by the nucleic acid of the samples. In the result re-checking portion, a bioinformatic analysis result and other information, such as technical contrast, are integrated to perform comprehensive study and judgment, finally alternative infection virus is determined, and other technologies, such as PCR, are utilized to perform re-checking.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Compositions for use in identification of viral hemorrhagic fever viruses
InactiveUS20060057605A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses that cause viral hemorrhagic fevers by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Compositions with Modified Nucleases Targeted to Viral Nucleic Acids and Methods of Use for Prevention and Treatment of Viral Diseases
ActiveUS20090047272A1Good antiviral effectPreventing and treatingHydrolasesPeptide/protein ingredientsViral diseaseViral nucleic acid
Antiviral compositions comprising a modified nuclease, or a plurality of such modified nucleases having at least one non-natural amino acid residue substituted for a naturally occurring amino acid in a parent nuclease are provided, as are methods of use and kits providing unit dosages of such compositions.
Owner:NANONASE INC
Compositions for use in identification of influenza viruses
InactiveUS20070087340A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
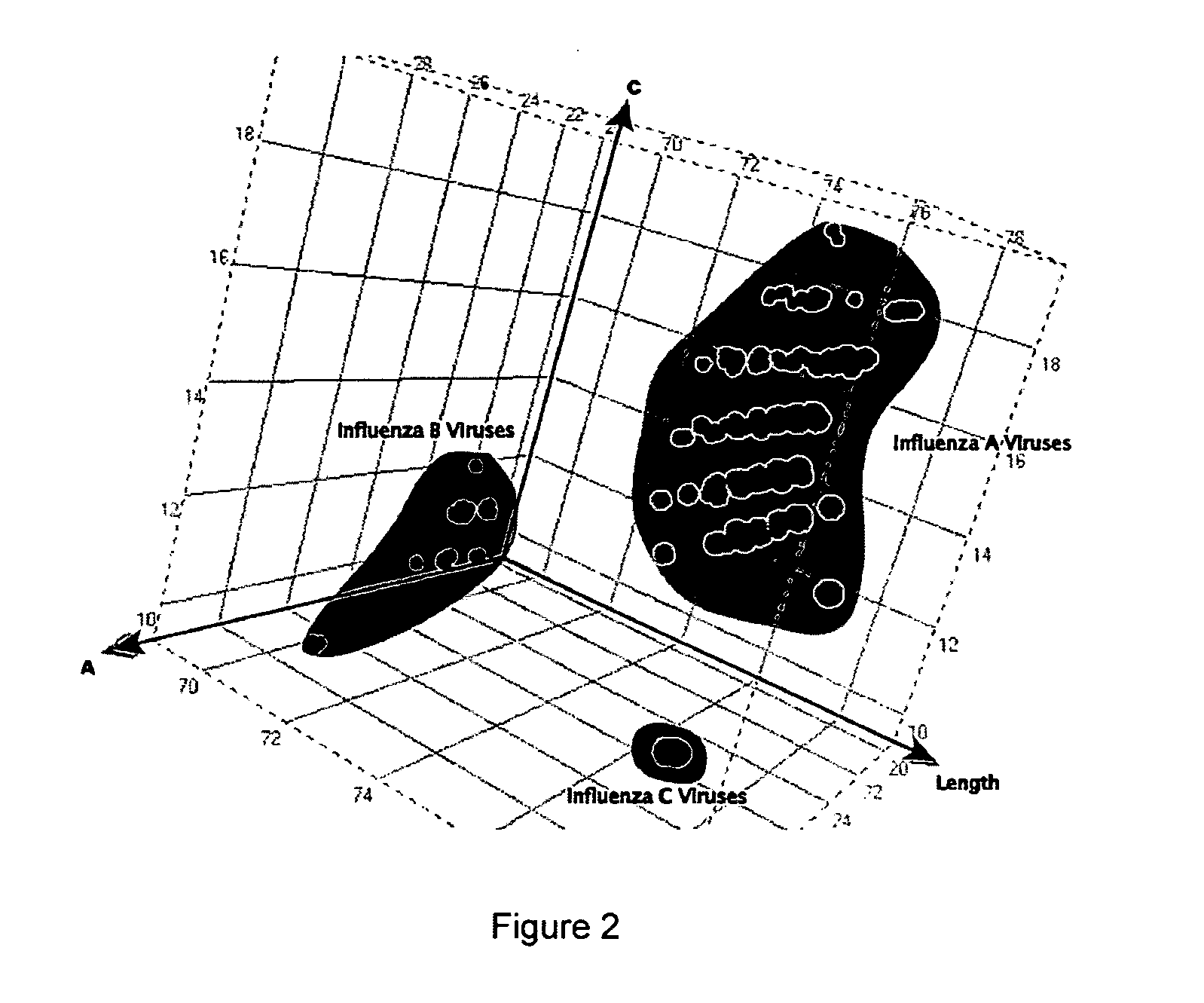
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses which are members of the influenza virus family by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Compositions for use in identification of influenza viruses
InactiveUS20070087336A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses which are members of the influenza virus family by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Compositions for use in identification of influenza viruses
InactiveUS20070087341A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses which are members of the influenza virus family by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Compositions for use in identification of orthopoxviruses
InactiveUS20060275749A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
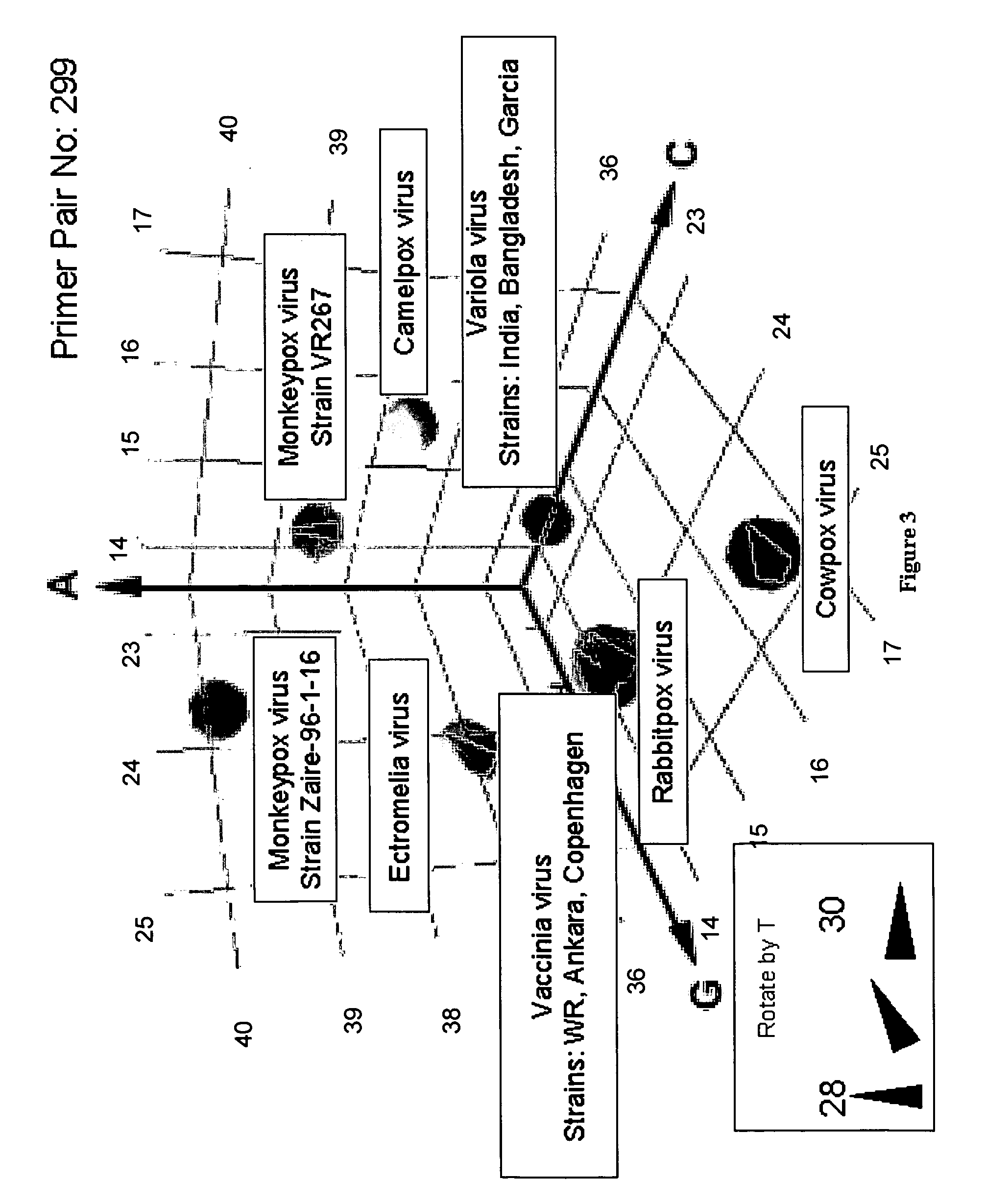
Oligonucleotide primers and compositions and kits containing the same for rapid identification of orthopoxviruses by amplification of a segment of viral nucleic acid followed by molecular mass analysis are provided.
Owner:IBIS BIOSCI
Compositions for use in identification of influenza viruses
InactiveUS20070184434A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses which are members of the influenza virus family by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
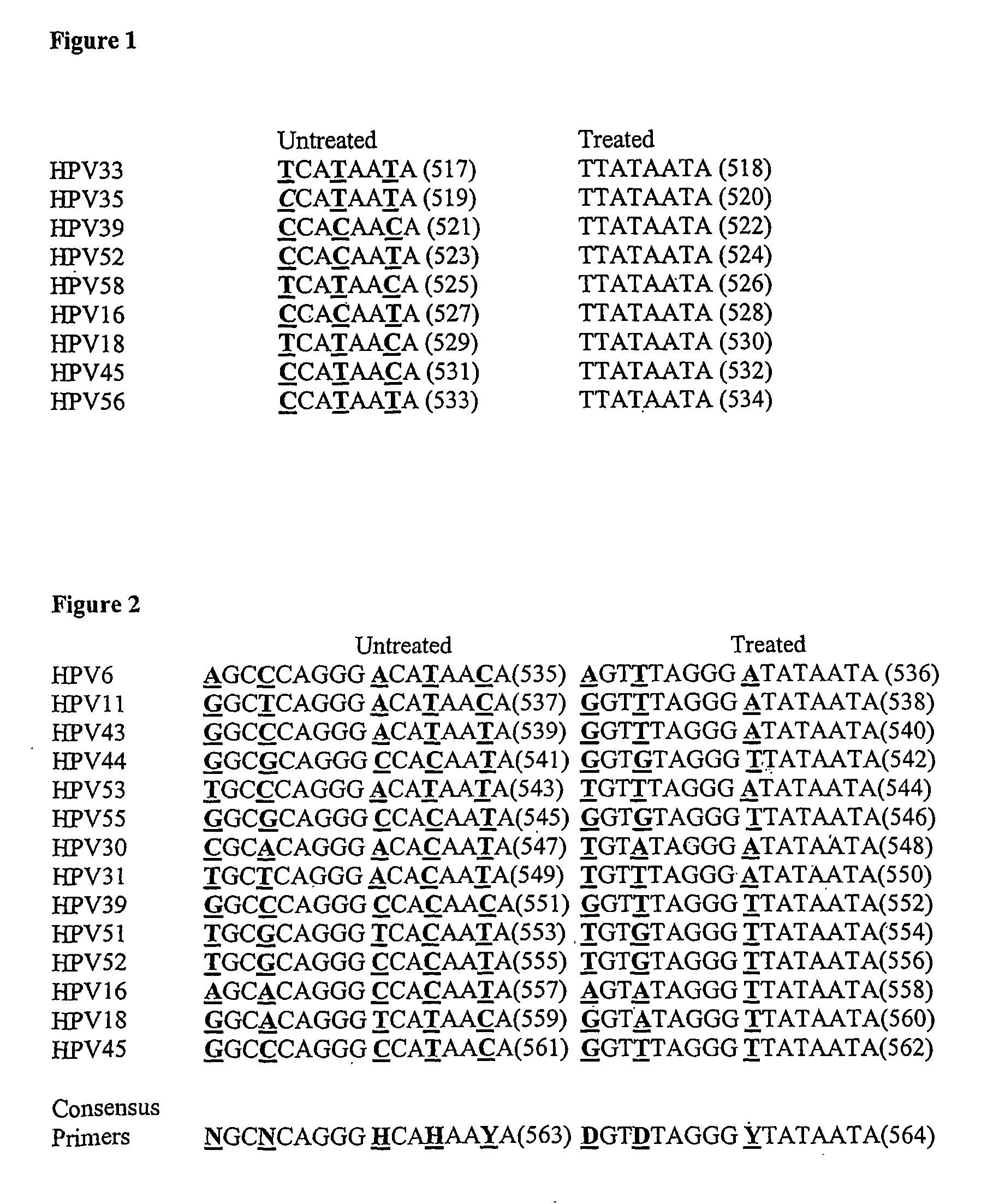
Detection of human papilloma virus
InactiveUS20090029346A1Reduce in quantityMicrobiological testing/measurementCytosineAlphapapillomavirus
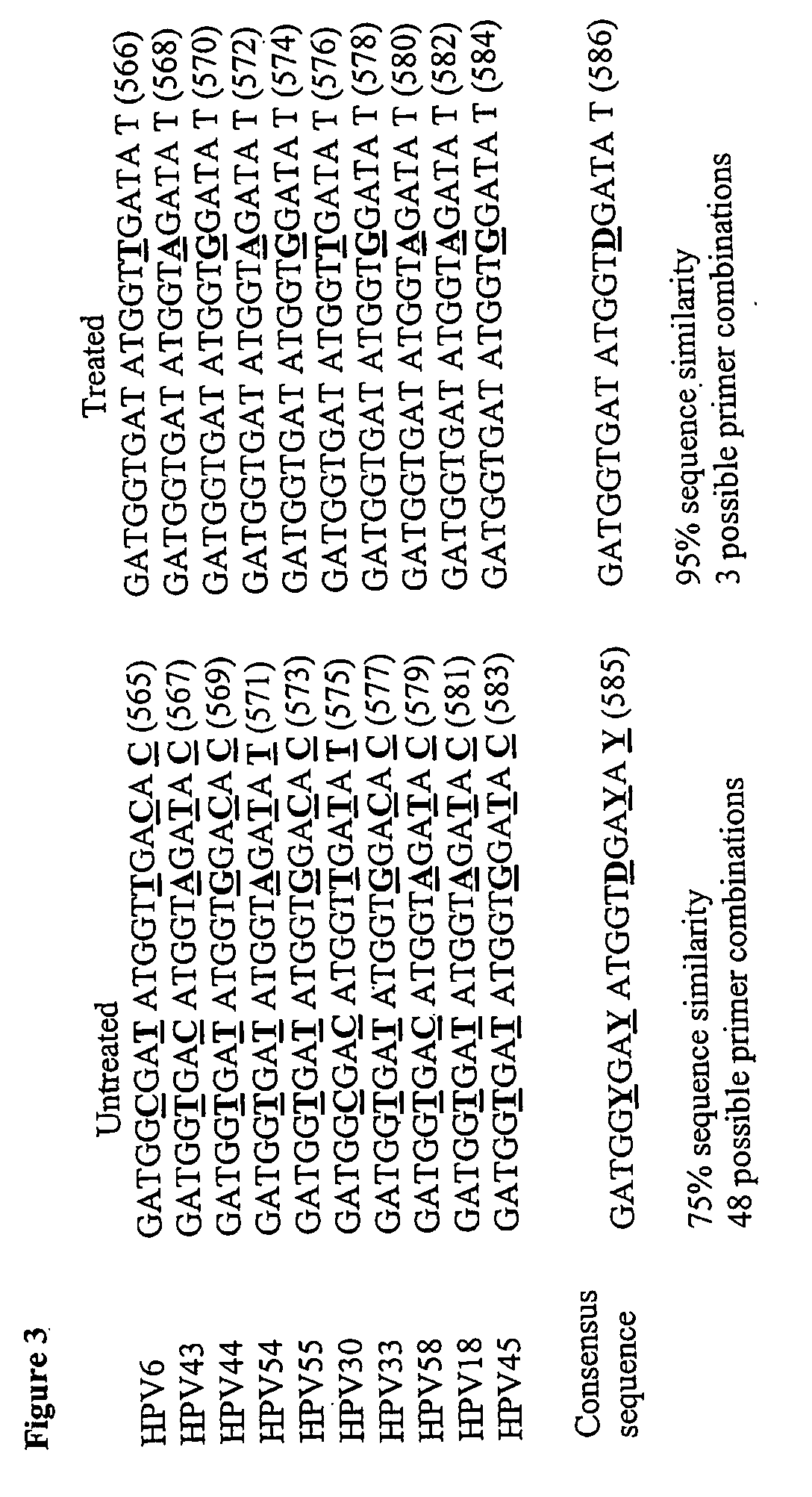
An assay for detecting HPV comprising treating the viral nucleic acid with an agent that modifies cytosine to form derivative viral nucleic acid, amplifying at least a part of the derivative viral nucleic acid to form an HPV-specific nucleic acid molecule, and looking for the presence of an HPV-specific nucleic acid molecule, wherein detection of the HPV-specific nucleic acid molecule is indicative HPV.
Owner:HUMAN GENETIC SIGNATURES PTY LTD
Simian adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof
ActiveUS20120027788A1Low cross-reactivityReduce adverse effectsFungiVirusesDiseaseSimian Adenoviruses
The present invention relates to novel adenovirus strains with an improved sero-prevalence. In one aspect, the present invention relates to isolated polypeptides of adenoviral capsid proteins such as hexon, penton and fiber protein and fragments thereof and polynucleotides encoding the same. Also provided is a vector comprising the isolated polynucleotide according to the invention and adenoviruses comprising the isolated polynucleotides or polypeptides according to the invention and a pharmaceutical composition comprising said vector, adenovirus, polypeptide and / or polynucleotide. The invention also relates to the use of the isolated polynucleotides, the isolated polypeptides, the vector, the adenoviruses and / or the pharmaceutical composition for the therapy or prophylaxis of a disease.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions for use in identification of influenza viruses
InactiveUS20070087339A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses which are members of the influenza virus family by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Compositions for use in identification of influenza viruses
InactiveUS20070087337A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses which are members of the influenza virus family by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Compositions for use in identification of influenza viruses
InactiveUS20070087338A1Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
Isothermal amplification kit for detecting SARS-COV-2 and primer probe set
PendingCN111074007AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationViral nucleic acidBiotin
The invention discloses an isothermal amplification kit for detecting SARS-COV-2 and a primer probe set. The kit comprises (1) inactivated lysate; and (2) an isothermal amplification system: a reaction buffer solution Buffer A, magnesium acetate Buffer B, negative control, nuclease-free water, a primer probe set and RAA enzyme. The lysate is used for rapid inactivation and release of viral nucleicacid, an isothermal amplification technology is utilized for rapidly enriching and amplifying a target area, whether a specific amplified product exists is rapidly determined through combination of aprobe with modification groups and a product with biotin, by a lateral chromatography technology and by use of colloidal gold developing. The kit is simple to operate, professional extraction reagents and detection instruments are not required limitation of detection laboratories and professionals is freed, the kit can perform rapid detection in an instrument-free state outdoors, the whole process only needs 30 min, and reading is very convenient.
Owner:上海世和医学检验实验室有限公司
Virus sample preservation solution, nucleic acid extraction reagent and viral nucleic acid extraction method
InactiveCN111172239AReduce detection stepsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionLysis
The invention discloses a virus sample preservation solution with a lysis function and a method for extracting viral nucleic acid. Since sample preservation and lysis are completed in one step, no additional lysis solution is needed, further diluting of a virus sample is prevented, the adding amount of the sample during the extraction process of viral nucleic acid can be increased, and the sensitivity of subsequent detection is improved. Meanwhile, because the preservation and lysis of the virus sample are completed in one step, the step of nucleic acid detection is removed, the process is optimized, and the efficiency of sample detection is improved. Compared with a conventional virus sample preservation solution such as a PBS buffer solution, the virus sample preservation solution has good preservation effect, more detection samples can be obtained, the detection sensitivity is improved, and the processing flow is simplified.
Owner:上海思路迪医学检验所有限公司
Matrix and System for Preserving Biological Specimens for Qualitative and Quantitative Analysis
InactiveUS20140038172A1Accurate and reproducible quantificationSafe and convenient and simple deviceBioreactor/fermenter combinationsBiological substance pretreatmentsTarget analysisPolyolefin
The present invention provides a device, system, and methods of use comprising an absorbent hydrophobic polyolefin matrix, and methods of use thereof, for storage, preserving, and recovering liquid suspension of biological specimens containing analytes of interest in a dry state. The dried biological specimens containing analytes of interest absorbed on the polyolefin matrix are reconstituted such as with molecular-grade water and released by compressing the polyolefin matrix. The reconstituted biological analytes are qualified for subsequent analysis, such as for qualitative and quantitative analysis of viral nucleic acids, such a viral load testing, genotyping, and sequencing. Also provided are kits with instructions, and methods of use thereof, for storage, preserving, and recovering biological specimens containing analytes of interest using the compression device of the invention.
Owner:VIVEBIO
Kits for diagnosis and monitoring of viral infection by analysis of viral transrenal nucleic acids in urine
Owner:INST NAT PER LE MALATTIE INFETTIVE LAZZARO SPALLANZANI IRCCS
Viral core protein-cationic lipid-nucleic acid-delivery complexes
InactiveUS20090209037A1Improve liposome based gene transferImproved cationic liposome mediated gene transferNervous disorderPeptide/protein ingredientsHeterologousViral Core Proteins
A nucleic acid delivery complex is provided which comprises a condensed polypeptide / nucleic acid complex and a cationic lipid wherein the complex comprises (a) a nucleic acid sequence of interest (NOI); and (b) one or more viral nucleic acid packaging polypeptides, or derivatives thereof, said polypeptides or derivatives thereof being (i) capable of binding to the NOI; and (ii) capable of condensing the NOI; and wherein the NOI is heterologous to the polypeptide. Also provided is a method of introducing an NOI into a cell using the delivery vector.
Owner:TAGAWA TOSHIAKI +7
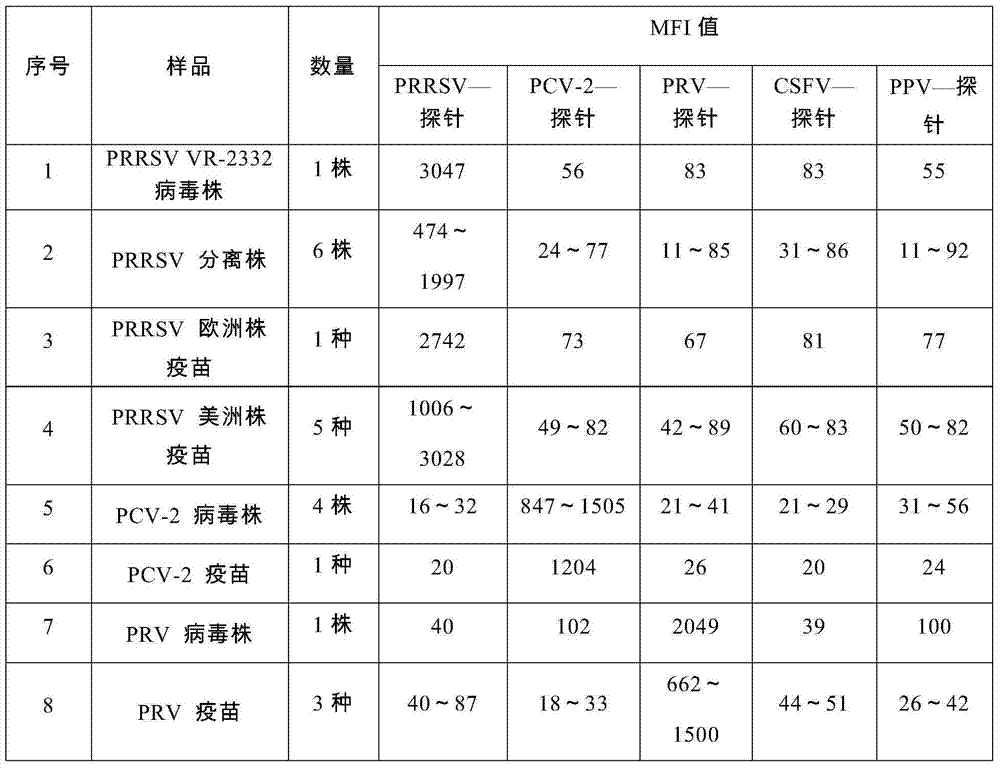
Nucleic acids of liquid-phase gene chip for synchronously detecting five porcine viruses and detection method thereof
ActiveCN104328218AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVMultiplex
The invention provides a set of nucleic acids of a liquid-phase gene chip for synchronously detecting five porcine viruses, which comprise forward and reverse primers and hybrid probes for porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus type 2 (PCV2), porcine pseudorabies virus (PRV), classical swine fever virus (CSFV) and porcine parvovirus (PPV). The invention also provides a multiplex liquid-phase chip high-flux molecular biology detection method of the five porcine viruses. According to the method, porcine virus nucleic acids in the sample to be detected are extracted to perform multiplex unsymmetric nucleic acid amplification / multiplex liquid-phase gene chip (suspension chip) combined detection, thereby synchronously and accurately detecting and identifying the five porcine viruses in the sample to be detected. The method has the advantages of high specificity, high sensitivity, high stability, high flux and high detection speed, and is simple to operate.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains
PendingCN110184390ARapid identificationImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationTonsilAfrican swine fever
The invention provides an amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains. A P72 gene of ASFV and a 5'UTR noncoding region of CSFV arerespectively used as an amplification target area, a pair of specific primers and a TaqMan MGB probe (SEQ ID NO:1-6) are designed, a real-time fluorescent quantitation PCR(FQ-PCR) technique is used, and identification and detection of ASFV and CSFV are realized. The detection reagent kit provided by the invention is suitable for detecting viral nucleic acid in samples of serum, spleen, lymph nodes, tonsil, kidney and the like of suspected ASFV or CSFV infected pigs, the sensitivity can reach 1.0*10<1>copy / [mu]L, and the detection reagent kit does not have any cross reactions with other pathogens which are liable to be in mixed infection with the ASFV and the CSFV or of which the infection symptoms are similar such as PRRSV, PRV, PCV2, PPV, JEV and HPS.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
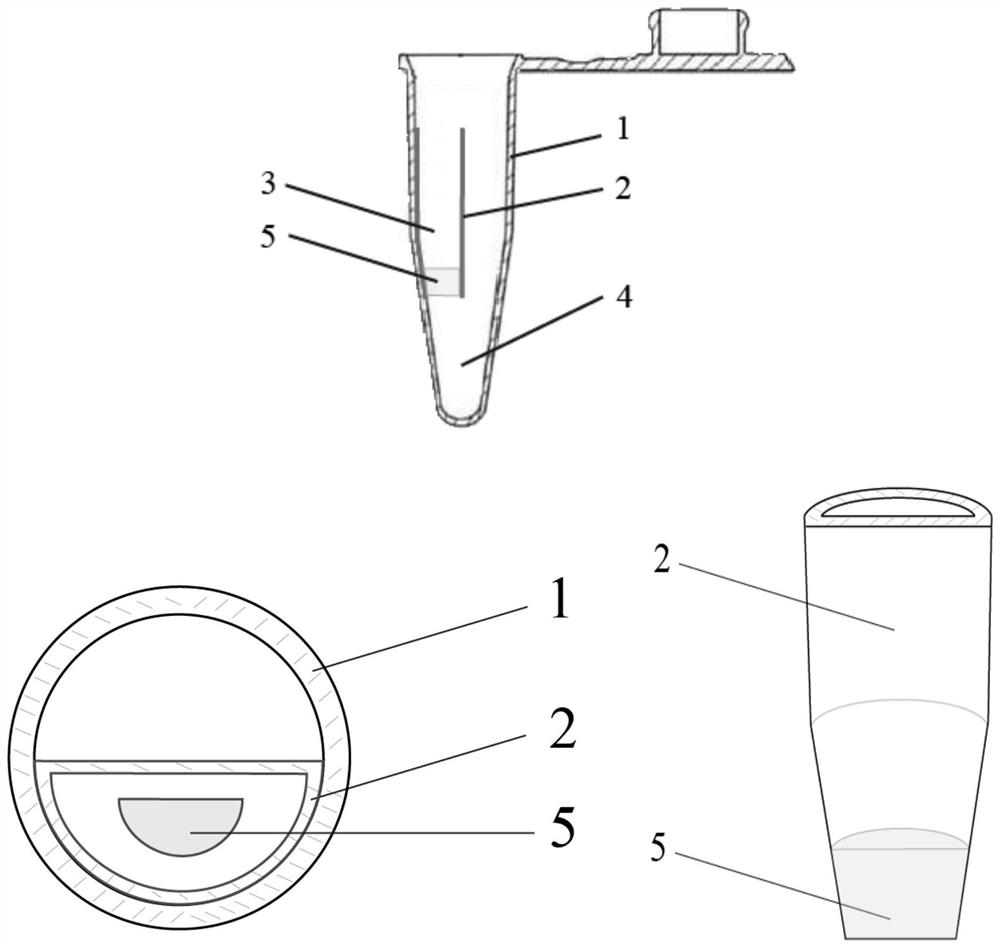
Extracting method of viral nucleic acid capable of being used for high flux full automation extraction by magnetic bead method
ActiveCN101851617ASimple and fast operationEasy to realize high-throughput automationDNA preparationHigh fluxMagnetic bead
The invention provides an extracting method of viral nucleic acid capable of being used for high flux full automation extraction by a magnetic bead method, which is characterized by comprising the steps of separating viruses via a magnetic bead I: absorbing the viruses to the magnetic bead I from a biological sample to obtain the magnetic bead I with the viruses which is called as a virus-magnetic bead I composite for short, additionally adding a magnetic field to separate the virus-magnetic bead I composite from the sample, suspending the virus-magnetic bead I composite in a lysis buffer I, dissociating the virus from the magnetic bead I in the lysis buffer, additionally adding a magnetic field to remove the magnetic bead I, and heating to crack the virus; and separating a viral nucleic acid via a magnetic bead II: absorbing the viral nucleic acid in a viral lysate via the magnetic bead II, additionally adding a magnetic field to separate the magnetic bead II with the viral nucleic acid from the viral lysate, washing the magnetic bead II with the viral nucleic acid by using a washing solution, and eluting the viral nucleic acid from the magnetic bead II by using a solution to obtain a purified viral nucleic acid. In the invention, cracking cells and separating viruses are completed at one step in one tube by using the magnetic beads; and extraction of the viruses and the viral nucleic acid can be achieved by using two magnetic beads so that the extraction process is simple and rapid without a complicated device, and high pass automatic operation is easy to be realized.
Owner:宁波市博坤生物科技有限公司
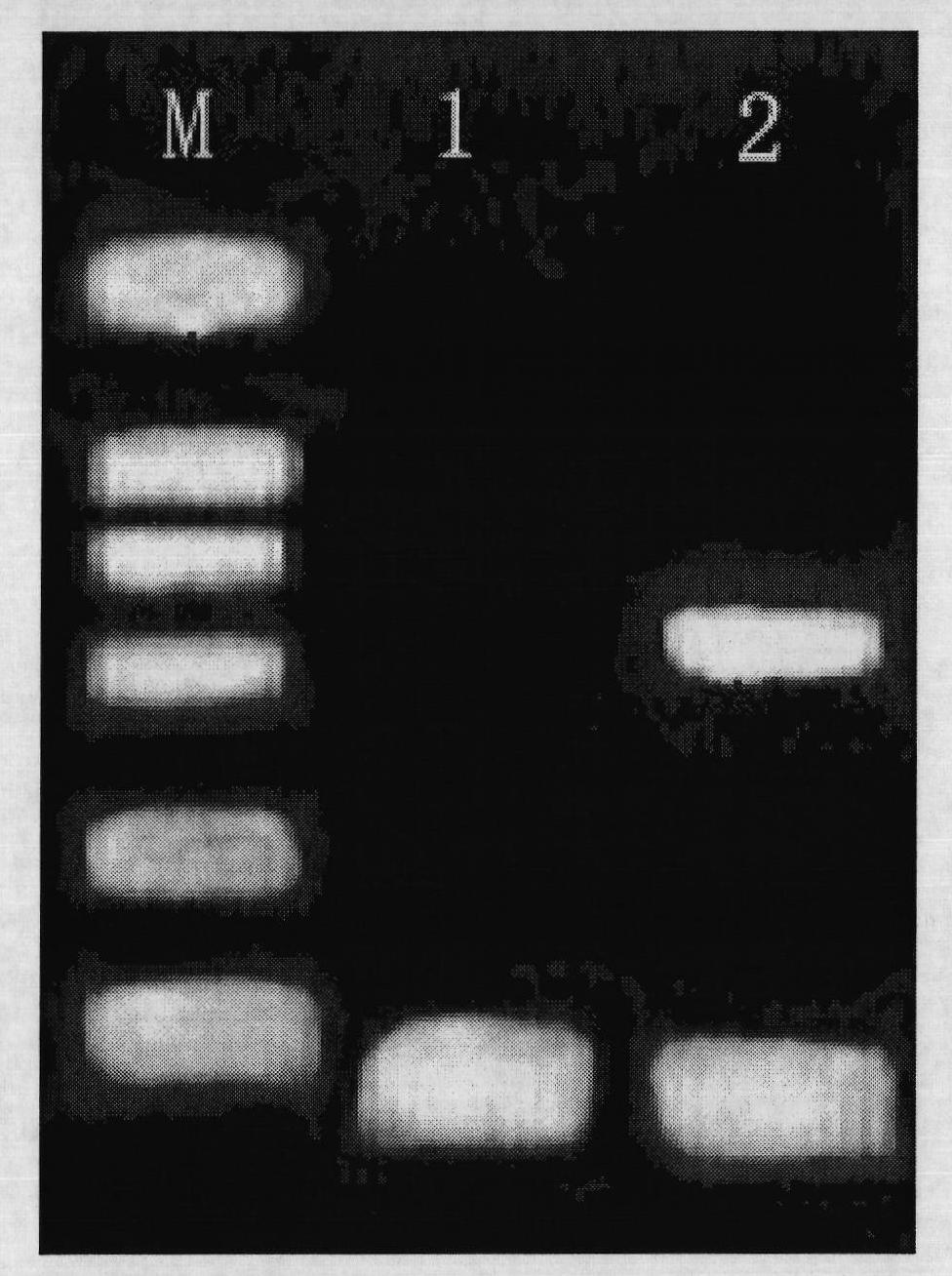
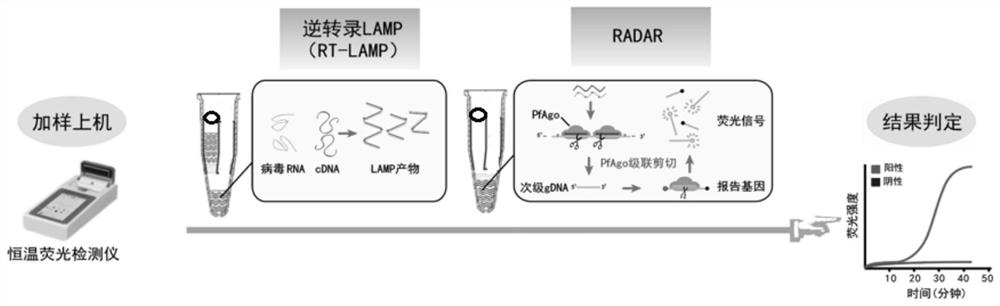
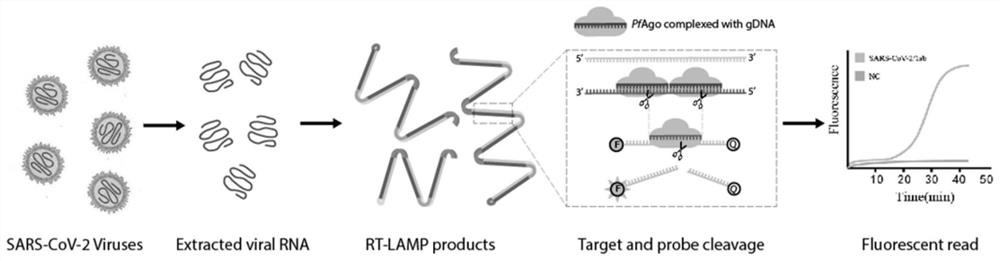
Kit and method for isothermal rapid detection of SARS-CoV-2 virus nucleic acid
ActiveCN111926117AMicrobiological testing/measurementAgainst vector-borne diseasesViral nucleic acidGene
The invention provides a kit and a method for isothermal rapid detection of SARS-CoV-2 virus nucleic acid. The detection method comprises the following steps: (a) providing a to-be-detected sample containing a target nucleic acid molecule; (b) mixing the to-be-detected sample with a cleavage reagent or a cleavage buffer solution containing the cleavage reagent to form a detection system, wherein the cleavage reagent comprises two guide ssDNAs, a gene editing enzyme (Ago) and a first reporter nucleic acid molecule, the first reporter nucleic acid molecule is provided with a fluorescent group and a quenching group, and the two guide ssDNAs are adjacent to each other; and (c) carrying out fluorescence detection on the detection system so as to obtain a fluorescence signal value, and if the fluorescence signal value is detected in the detection system, indicating that the target nucleic acid molecule exists in the sample, and if the fluorescence signal value is not detected in the detection system, indicating that the target nucleic acid molecule does not exist in the sample. The method provided by the invention can detect the target nucleic acid molecule with high sensitivity and accuracy.
Owner:JIAOHONG BIOTECHNOLOGY (SHANGHAI) CO LTD
Methods for purifying adeno-associated virus particles
The present invention provides methods of purifying encapsidated virus, e.g., viral particles comprising viral nucleic acid, from compositions comprising encapsidated viral nucleic acid and viral particles that lack viral nucleic acid; methods for reducing the particle:genome ratio in a preparation of encapsidated viral nucleic acid; and methods for selectively inactivating viral particles that lack viral nucleic acid in a liquid composition comprising encapsidated viral nucleic acid and the viral particles that lack viral nucleic acid. The methods generally involve subjecting the composition to hydrostatic pressure such that the viral particles lacking viral nucleic acid are selectively inactivated.
Owner:RGT UNIV OF CALIFORNIA
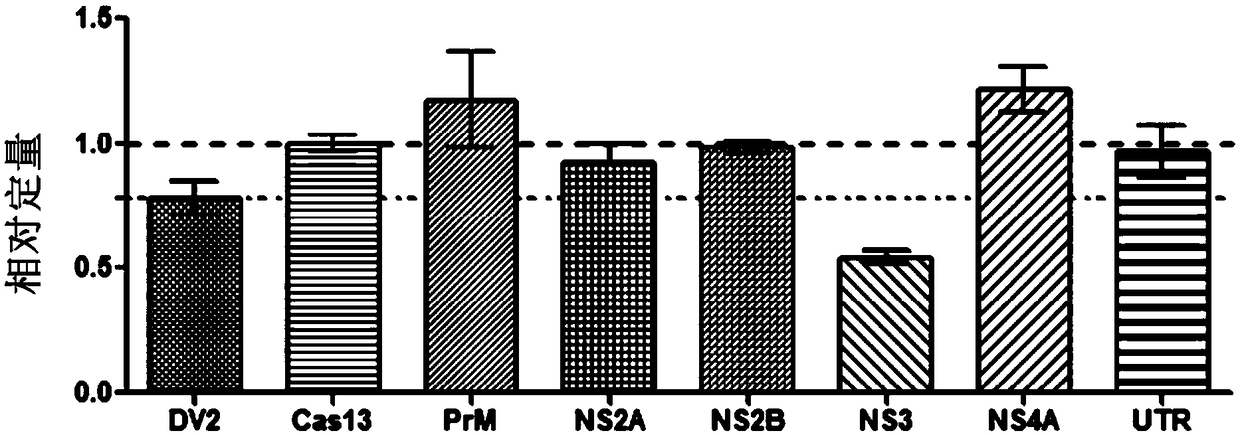
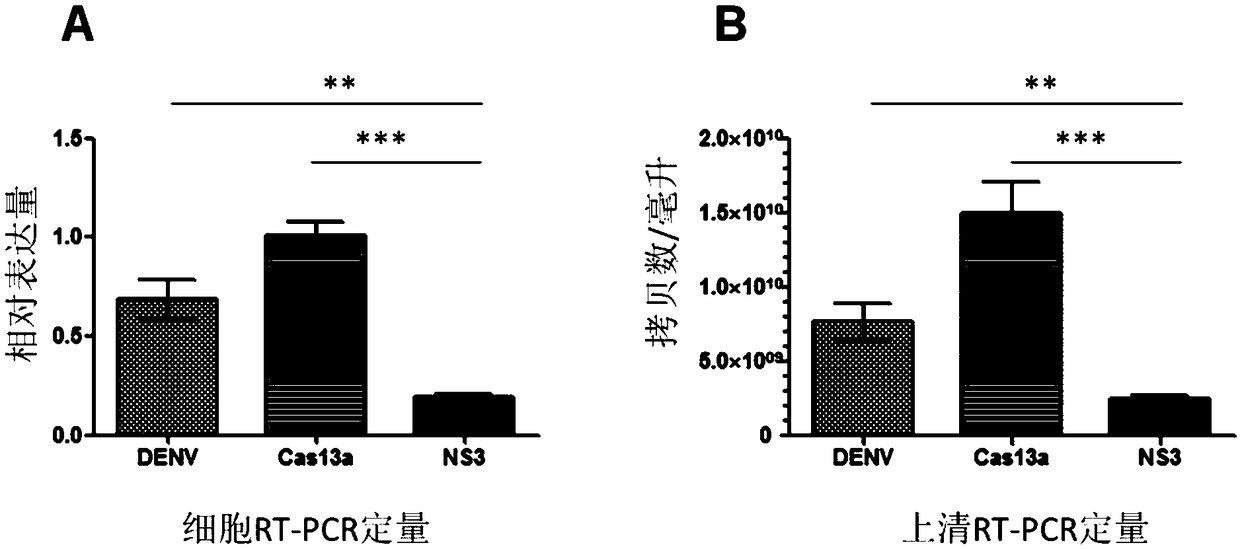
Effective Cas13a-based anti-dengue virus nucleic acid target and application thereof
ActiveCN108715849ALower resistanceImprove efficiencyOrganic active ingredientsSsRNA viruses positive-senseAnti virusGene targets
The invention discloses an effective Cas13a-based anti-dengue virus nucleic acid target and application thereof. The invention provides a CRISPR-Cas13a system for inhibiting dengue virus, wherein theCRISPR-Cas13a system comprises a Cas13a protein and a crRNA corresponding to an NS3 gene target of the dengue virus, or a complex formed by the Cas13a protein and crRNA; and NS3 gene targets of the dengue virus are 4657th-4685th nucleotide sequences of the NS3 gene. In the invention, a novel anti-dengue virus method is found, is different from a traditional anti-virus method, directly targets a viral nucleic acid, specifically degrades a target gene, and makes the virus lose the replication ability; therefore, the effective Cas13a-based anti-dengue virus nucleic acid target has characteristicsof high efficiency, high specificity and programmability, is not easy to produce drug resistance generated by traditional antiviral drugs, and may become a novel antiviral drug in the future.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
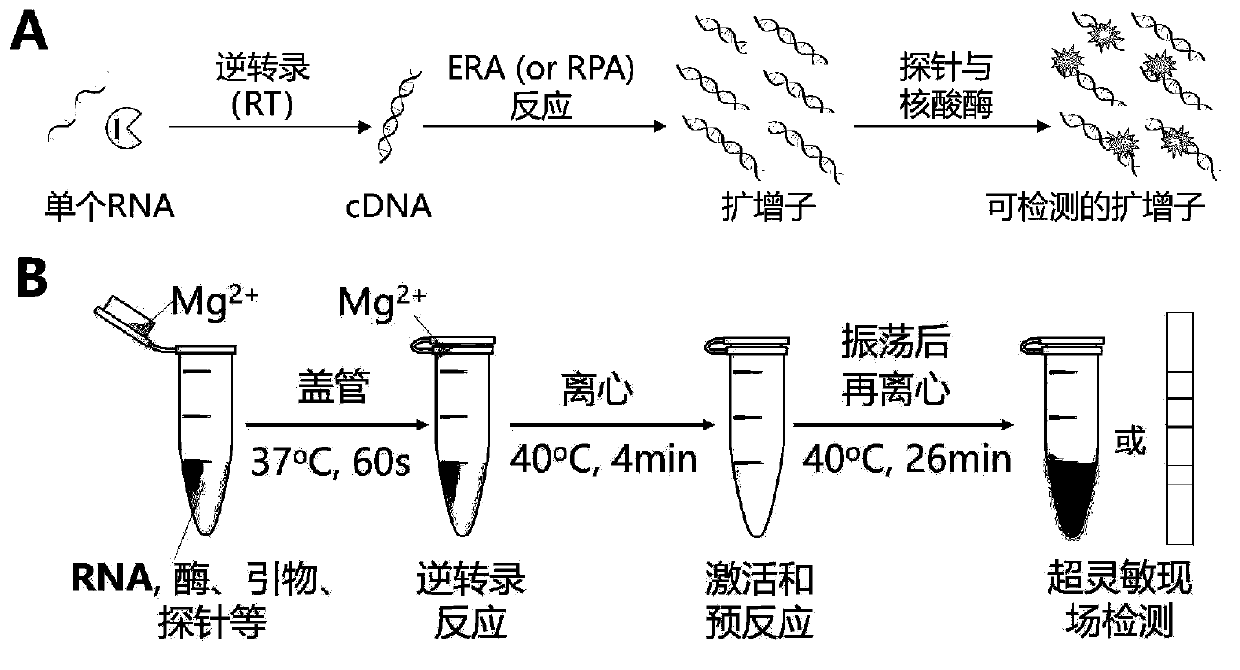
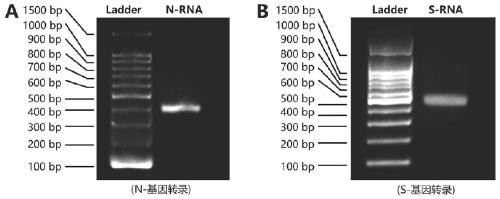
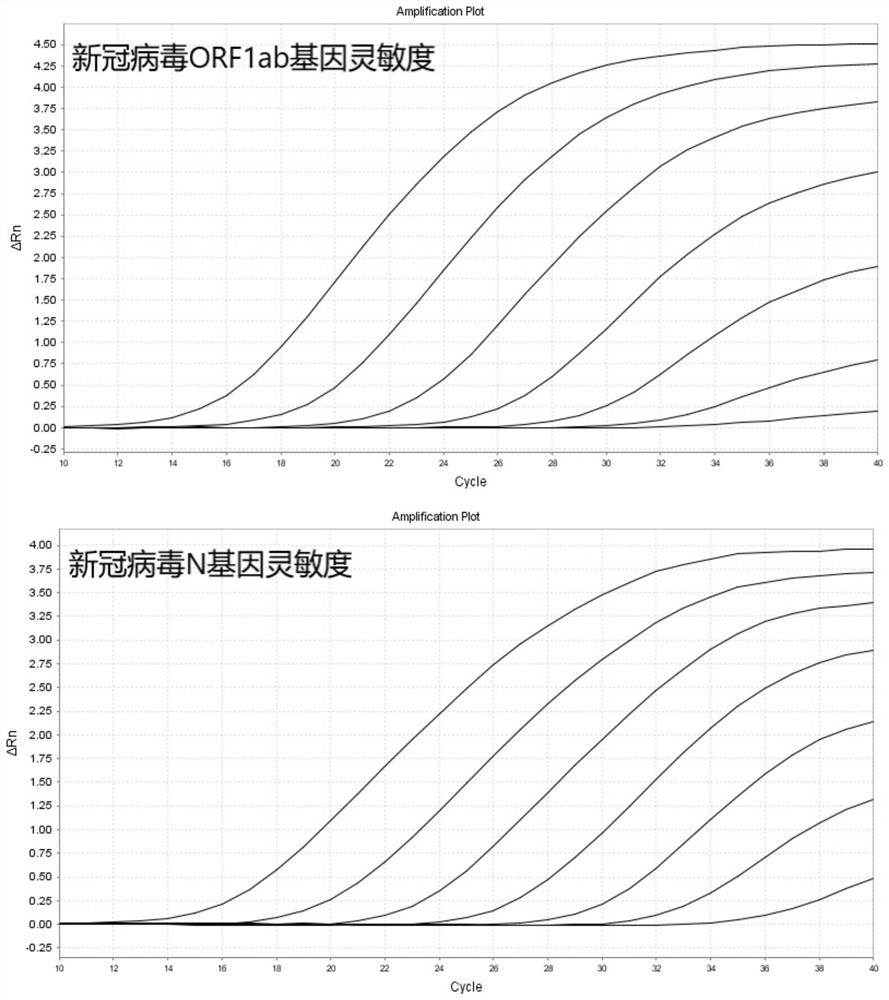
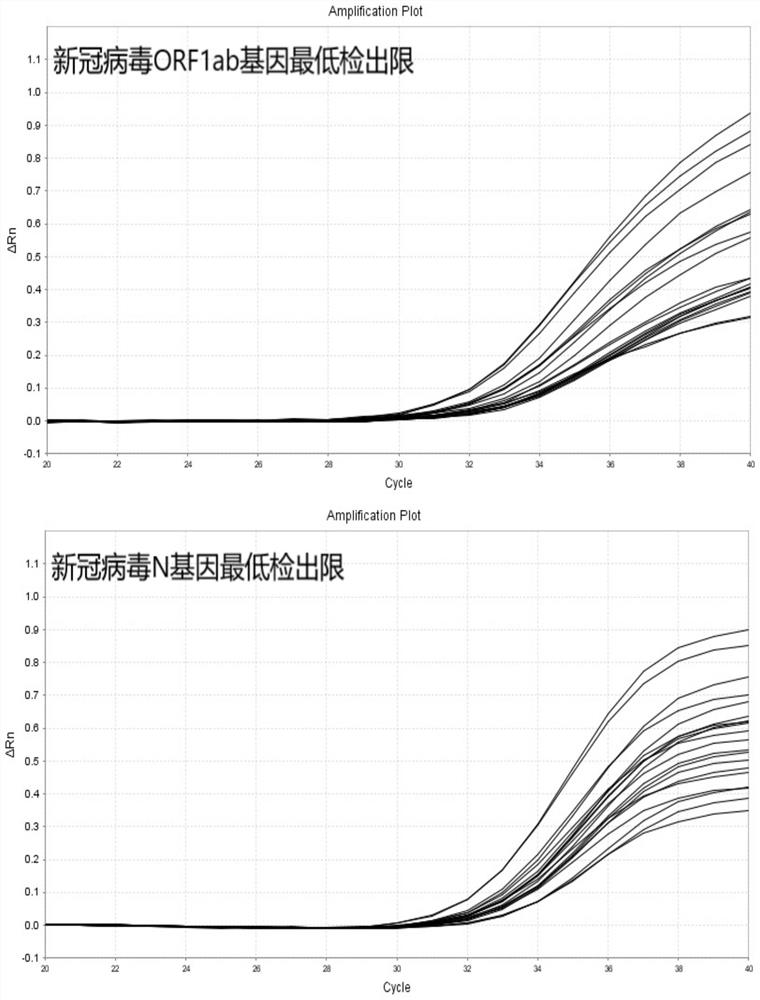
Probe and primer for detecting novel coronavirus SARS-CoV-2, kit, detection method and application
ActiveCN111560472AOutbreak controlGuaranteed reliabilityMicrobiological testing/measurementBiological testingNucleic acid detectionViral nucleic acid
The invention discloses a probe, a primer, a kit, and a detection method for detecting novel coronavirus SARS-CoV-2, and application, belongs to the technical field of molecular biology nucleic acid detection technology, and aims to solve problems in field detection, reduce pollution caused in detection process, increase virus RNA detection sensitivity and specificity. The invention provides the probe and the primer for detecting novel coronavirus SARS-CoV-2, and the kit containing the probe and the primer. The invention further provides a whole-course encapsulated procedure for exponential amplification from RNA (WEPEAR method). According to the invention, the detection sensitivity on N gene as low as 1ag (about 4 copies) is realized; the detection sensitivity on S gene as low as 1 copy is achieved, meanwhile, double genes of virus nucleic acid RNA based on RT-RPA (or RT-ERA) are detected at the same time so as to further improve the reliability of the detection result, and the kit can be used for field detection or household detection.
Owner:HARBIN INST OF TECH
Adenovirus multi-fluorescent quantitative PCR (polymerase chain reaction) detection kit and using method thereof
InactiveCN103160619AEasy to manufactureLow cost and stableMicrobiological testing/measurementFluorescence/phosphorescenceQuality controlViral nucleic acid
The invention discloses an adenovirus multi-fluorescent quantitative PCR (polymerase chain reaction) detection kit, comprising PCR liquid, a primer probe combination, a positive quality control sample, and a negative quality control sample. The adenovirus multi-fluorescent quantitative PCR detection kit also comprises liquid for nucleic acid extraction and an inner reference substance for nucleic acid extraction, wherein the inner reference substance is inactivated T4 bacteriophage; the primer probe combination comprises a primer probe (general: SEQ ID NO4-6; subtype SEQ ID NO7-9) for adenovirus nucleic acid and a primer probe (SEQ ID NO1-3) for the inner reference substance; the 5' terminals of the SEQ ID NO3, 6, 9 are connected to a fluorescent report group; and the 3' terminals are connected to a fluorescent quenching group. A using method of the kit is also disclosed by the invention. Viral nucleic acid extraction is carried out by the liquid for nucleic acid extraction before the fluorescent quantitative PCR detection; and the inner reference substance is added in the extraction process of the viral nucleic acid. The kit and the using method thereof disclosed by the invention have the characteristics of being convenient to use, and accurate in detection result; all adenoviruses can be detected; and B and E subgroups can be parted.
Owner:北京海斯凯生物科技有限公司
Detection method for respiratory tract viruses
ActiveCN110093455AHigh detection sensitivityLow initial sample size requirementMicrobiological testing/measurementMicroorganism based processesInformation analysisRespiratory tract disease
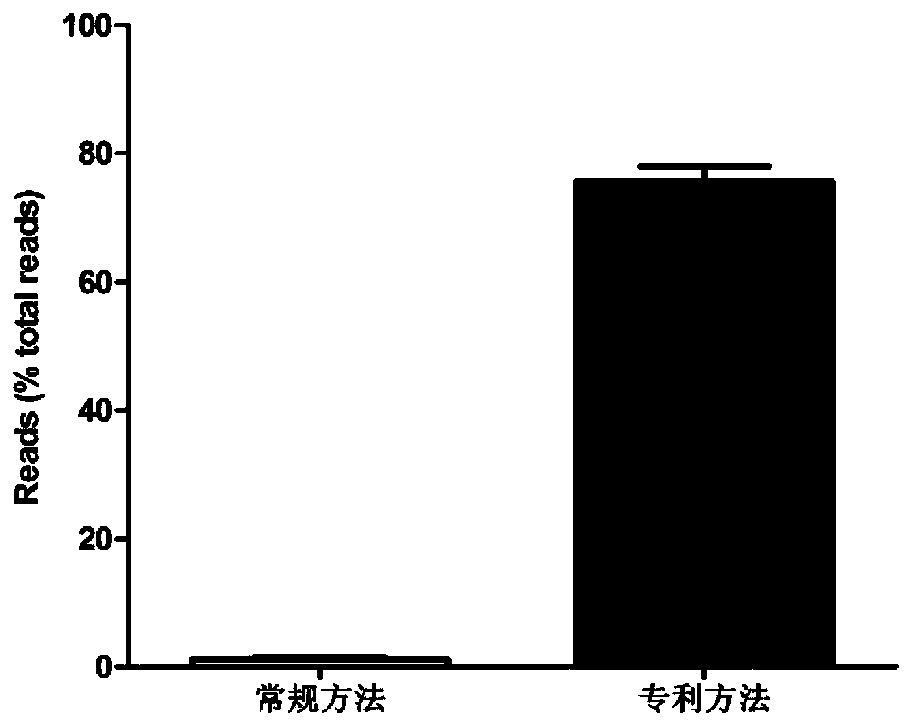
The invention provides a high-throughput detection method for efficient and rapid enrichment of respiratory tract viruses. The high-throughput detection and identification technology for efficient andrapid enrichment of the respiratory tract viruses includes the following four parts: enrichment of respiratory tract sample viruses, library building, high-throughput sequencing and biological information analysis. Enrichment of the respiratory tract sample viruses is a core part of the technology. Firstly, nucleic acids of hosts except viruses in the samples are removed by a biophysical method,and then sample viral nucleic acids are extracted. With the help of detection of an Agilent 2100 instrument, only 200 pg nucleic acids are used for building a library. The results of high-energy sequencing and biological information analysis show that the reads number of the viruses is increased from less than 0.1% with treatment by conventional methods to 84% or more, and the detection technologynot only can detect sample infected viruses quickly, increases the coverage rate (95-100% or more) of a whole genome sequence of the viruses, and greatly reduces the cost of sequencing.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Method for sceening viral nucleic acid of blood through isothermal amplification based on loop mediated technique
This invention belongs to clinic domain, discloses a detecting method for screening viral nucleic acid of blood through isothermal amplification based on loop mediated technique, which is used to detecting nucleic acid virus selection of blood. We detect or select the nucleic acid consisting of HBV, HCV and HV by technology of molecular domain, this technology process is that: using special primer, using LMAP tech platform to amplify target gene under assistant of a series control system and inner comparison. This invent has the virtue of simple and convenient, economical and fast, delicacy and special, so that it has a expansive application.
Owner:SHANGHAI BLOOD CENT +1
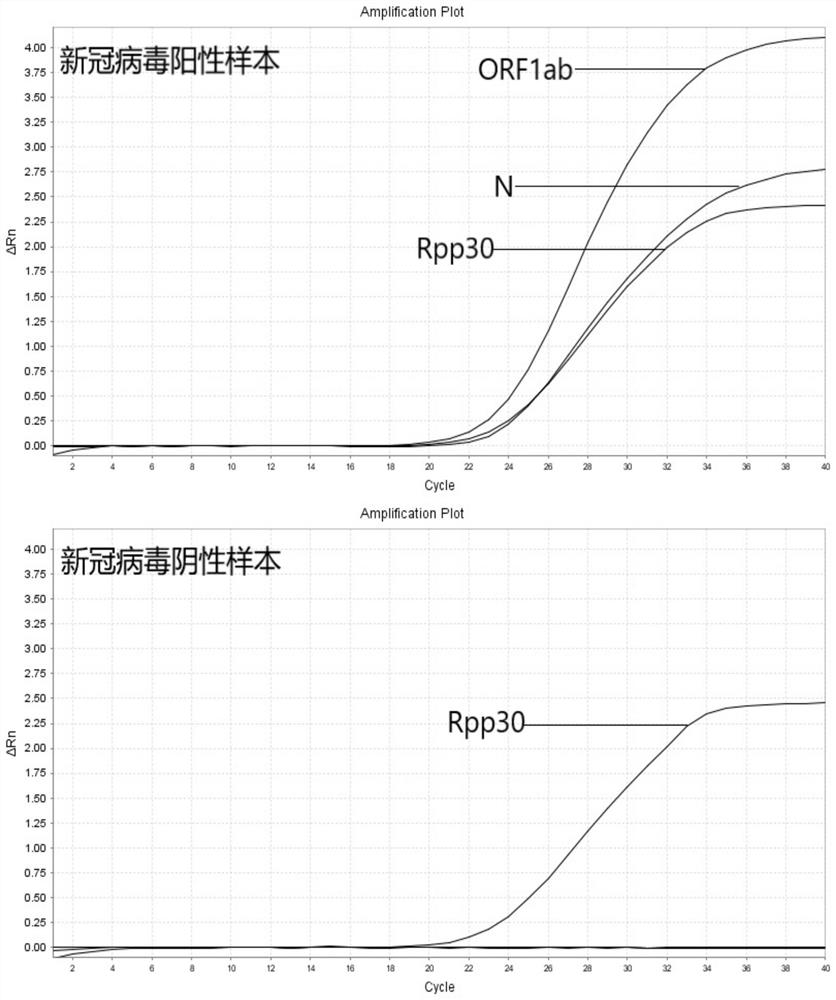
COVID-19 nucleic acid testing primer probe composition, COVID-19 nucleic acid testing kit and COVID-19 nucleic acid testing method
PendingCN111621604AAvoid mutual interferenceStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesForward primerViral nucleic acid
The invention relates to the field of the biological technology and the medical examination technology, and provides a COVID-19 nucleic acid testing primer probe composition, a COVID-19 nucleic acid testing kit and a COVID-19 nucleic acid testing method for solving the problems of low accuracy, poor specificity and low sensitivity of a traditional virus nucleic acid testing method. The COVID-19 nucleic acid testing primer probe composition comprises a first primer probe group, a second primer probe group and a third primer probe group, wherein the first primer probe group comprises a forward primer ORF1ab-F, a probe ORF1ab-P and a reverse primer ORF1ab-R; the second primer probe group comprises a forward primer N-F, a probe N-P and a reverse primer N-R; and the third primer probe group comprises a forward primer Rpp30-F, a probe Rpp30-P and a reverse primer Rpp30-R. According to the primer probe composition disclosed by the invention, through artful design of an amplification primer pair and a detection probe, mutual interference between a plurality of primer pairs and corresponding detection probes is avoided. The kit has a simple structure, an interior label is used for monitoring a collection, transportation and extraction process of a sample to be detected, and the false negative of a detection result is avoided.
Owner:SHANGHAI CHROMYSKY MEDICAL RES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com