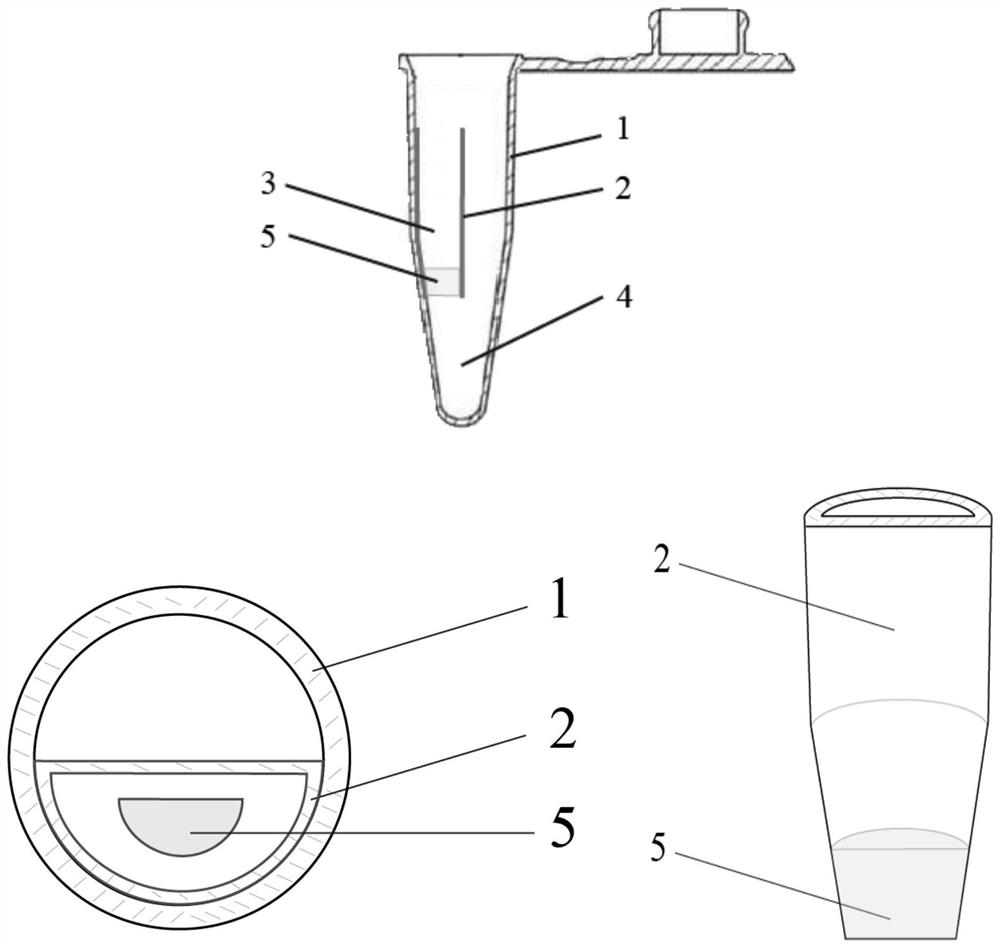
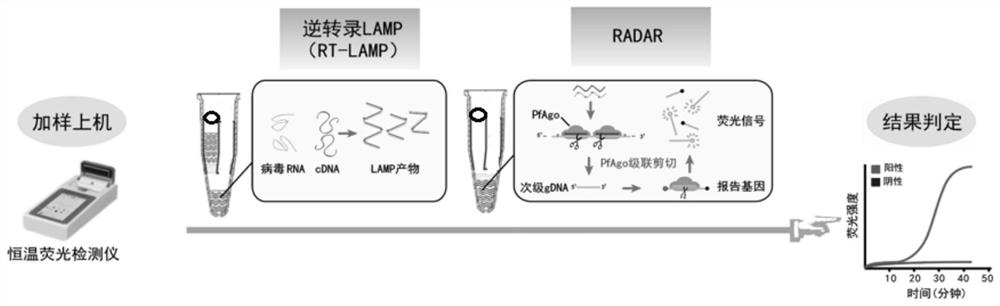
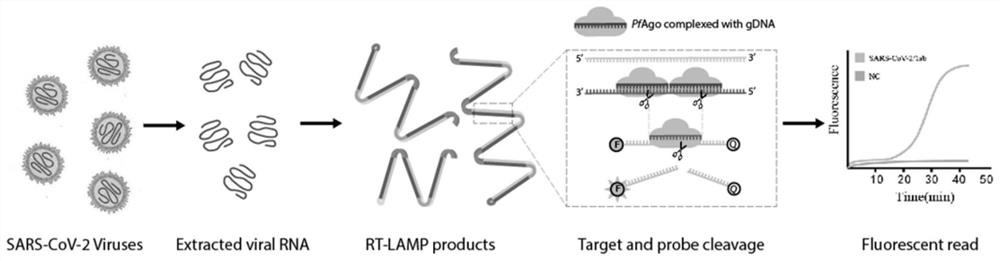
Kit and method for isothermal rapid detection of SARS-CoV-2 virus nucleic acid
A detection method and kit technology, applied in the direction of biochemical equipment and methods, microbial measurement/inspection, etc., can solve problems such as expensive synthesis, difficult to achieve multiple detection, and complicated crRNA design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] Preparation of detection reagent and detection method
[0170] In this embodiment, the SARS-CoV-2 viral nucleic acid isothermal rapid detection kit of the present invention and its use method are provided.
[0171] 1.1 Detection reagents and kits
[0172] In this embodiment, taking the detection of the ORF1b gene of SARS-CoV-2 virus as an example, the corresponding specific target nucleic acid sequence is:
[0173] 5'-ATGCACTTTTCGCATATACAAAACGTAATGTCATCCCTACTATAACTCAA ATGAATCTTAAGTATGCCATTAGTGCAAAGAATAGAGCTCGCACCGTAGCTGGT GTCTCTATCTGTAGTACTATGACCAATAGACAGTTTCATCAAAAATTATTGAAAT CAATAGCCGCCACTAGAGGAGCTACTGTAGTAATTGGAACAAGCAAATTCTATGGTGGTTGGCACAACATGTTAAAAACTGTTTATAGTGATGTAGAAAACCCTCACC TTATGGGTTGGGATTATCCTAAATGTGATAGAGCCATGCCTAACATGCTTAGAA TTATGGCCTCACTTGTTCTTGCTCGCAAACATACAACGTGTTGTAGCTTGTCACA CCGTTTCTATAGATTAGCTAATGAGTGTGCTCAAGTATTGAGTGAAATGGTCAT GTGTGGCGGTTCACTATATGTTAAACCAGGTGGAACCTCATCAGGAGATGCCAC AACTGCTTATGCTAATAGTGTTTTTAACATTTGTCAAGCTGTCACGGCCAATGTT AATGCACTTTTATC...
Embodiment 2
[0194] Preparation and detection method of detection reagent (without inner liner)
[0195] In this embodiment, the SARS-CoV-2 viral nucleic acid isothermal rapid detection kit of the present invention and its use method are provided.
[0196] 1.1 Detection reagents and kits
[0197] In this embodiment, the detection of the ORF1b gene of SARS-CoV-2 virus is taken as an example, and the corresponding specific target nucleic acid sequence refers to Example 1.
[0198] Based on the method of the present invention, the corresponding detection reagents include:
[0199] (1), amplification primer, specific sequence is as follows:
[0200]
[0201] (2) Specific guide ssDNA (gDNA), the specific sequence is as follows:
[0202] 2019-nCoV ORF 1b-gDNA 1 5'-P-TTGATGAGGTTCCACC-3' SEQ ID NO.51 2019-nCoV ORF 1b-gDNA 4 5'-P-TCAGTTGTGGCATCTC-3' SEQ ID NO.52
[0203] (3) Fluorescent reporter nucleic acid corresponding to specific guide ssDNA (gDNA), the specifi...
Embodiment 3
[0218] Test for different concentrations of standard substances to be tested
[0219] The standard substance to be tested (SEQ ID NO.: 65) was diluted according to the principle of 3-fold dilution, and diluted to 18000copies / mL, 6000copies / mL, 2000copies / mL, 670copies / mL, 220copies / mL, 70copies / mL As for the standard dilution, draw 140ul standard dilution respectively for nucleic acid extraction (QIAamp ViralRNA Mini Kit). Add 15ul nucleic acid extraction sample, negative control (H2O) and positive control (target fragment plasmid) to the amplification system in Example 2, make 3 groups for each concentration gradient, and do 7 repetitions for each group, and calculate the The detection rate of experiment is carried out by embodiment 3 steps.
[0220] The result is as Figure 4 As shown, 18000copies / mL, 6000copies / mL, 2000copies / mL, and 670copies / mL were all stably detected, and the negative control and positive control were established.
[0221] The results show that the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com