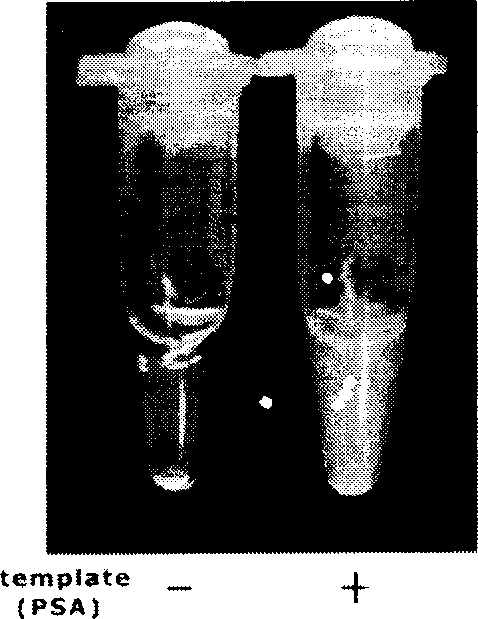
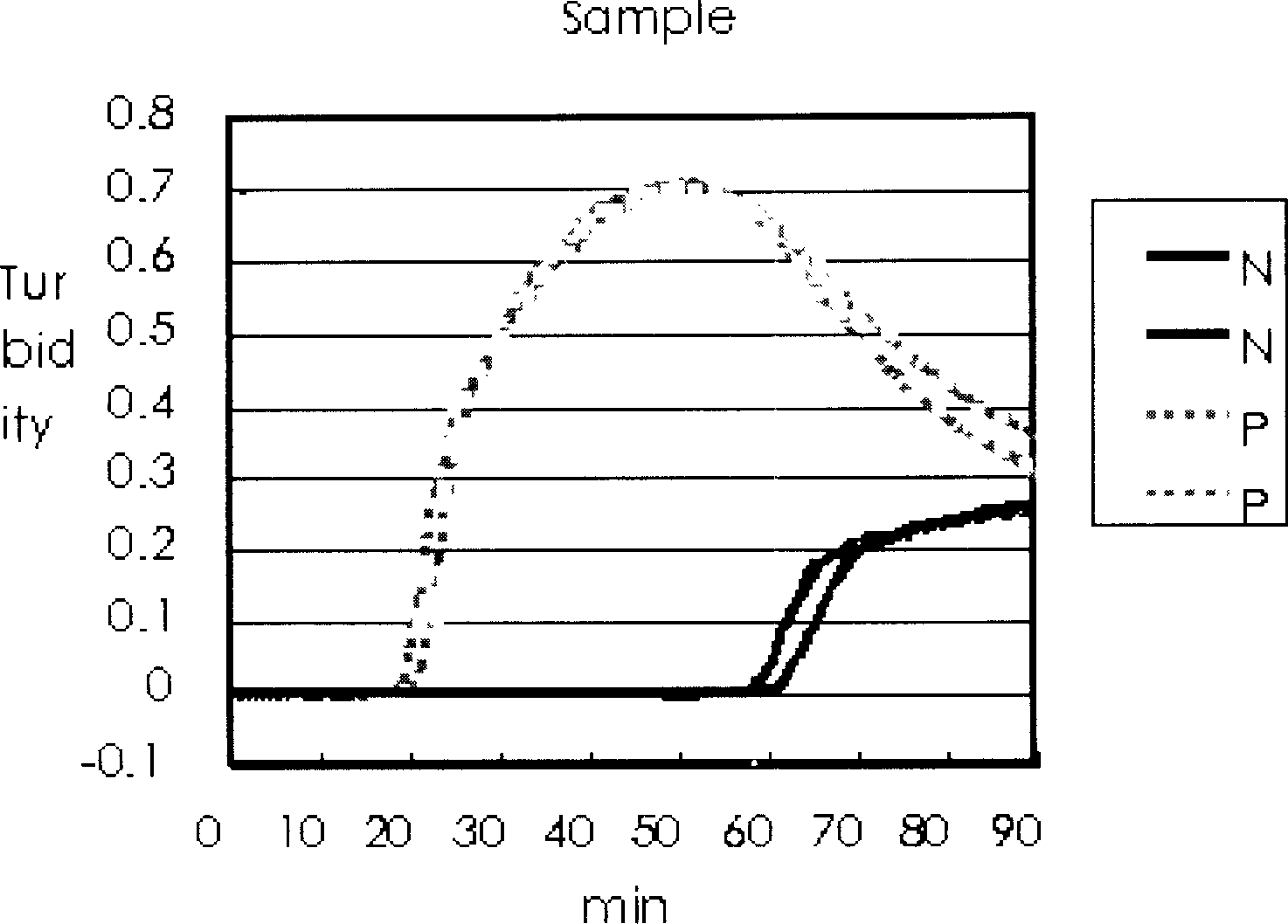
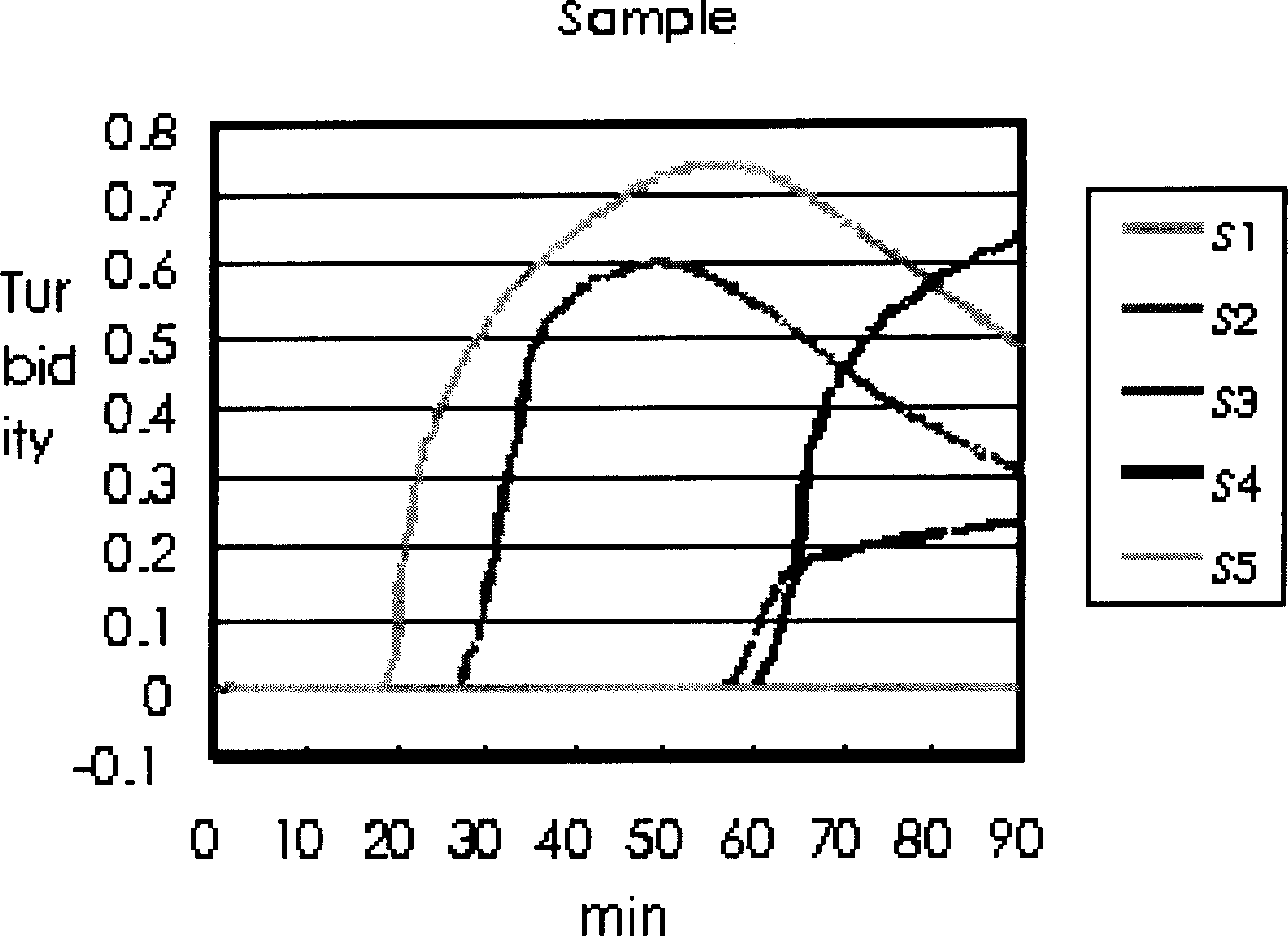
Method for sceening viral nucleic acid of blood through isothermal amplification based on loop mediated technique
A virus nucleic acid and blood screening technology, applied in the field of clinical medicine, can solve problems such as missed detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1 Application example of the operating method of the HBV blood screening and identification system
[0126] Reagent
Packing volume Storage conditions
nucleic acid
Extraction reagent
Solution 1
300μl Store at 4°C
Solution 2
400μl Store at 4°C
Solution 3
500μl Store at 4°C
LAMP test
agent
Master Mix.
5ml Store at -20°C
Primer Mix.
3ml Store at -20°C
2ml Store at -20°C
Analyzing Software
LA-200 Program Ver0.18 software
[0127] The specific operation method is as follows:
[0128] (1) Nucleic acid extraction
[0129] Follow the steps below for HBV nucleic acid extraction
[0130] a. Add 300μl of solution to a microcentrifuge tube of 1 to 1.5ml
[0131] b. Add 100μl serum or plasma to the centrifuge tube and vortex for 5 seconds
[0132] c. Incubate at room temperature (15-25°C) for 10 minutes, boil for 10 minutes, and cool to room ...
Embodiment 2
[0150] Example 2 HBV, HCV and HIV triple blood screening identification system operating method
[0151] Reagent
Packing volume Storage conditions
nucleic acid
Extraction reagent
Solution 1
300μl Store at 4°C
Solution 2
400μl Store at 4°C
Solution 3
500μl Store at 4°C
LAMP test
agent
Master Mix.
5ml Store at -20°C
Primer Mix.
3ml Store at -20°C
Enzyme Mix
2ml Store at -20°C
Analyzing Software
LA-200 Program Ver0.18 software
[0152] The specific operation method is as follows:
[0153] (1) Nucleic acid extraction
[0154] Follow the steps below for HBV, HCV and HIV nucleic acid extraction
[0155] Add 300 μl solution to 1.5 ml microcentrifuge tube
[0156] Add 100μl serum or plasma to the centrifuge tube and vortex for 5 seconds
[0157] Incubate at room temperature (15-25°C) for 10 minutes
[0158] Add 400μl solution 2 to the centrifuge tube, and vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com