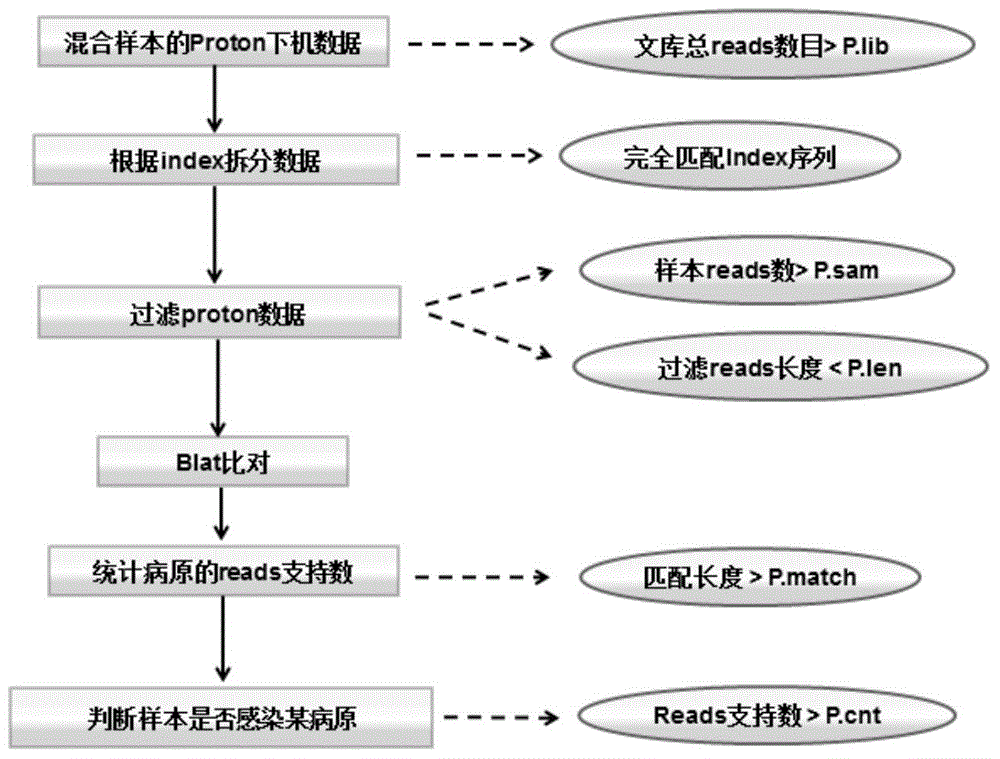
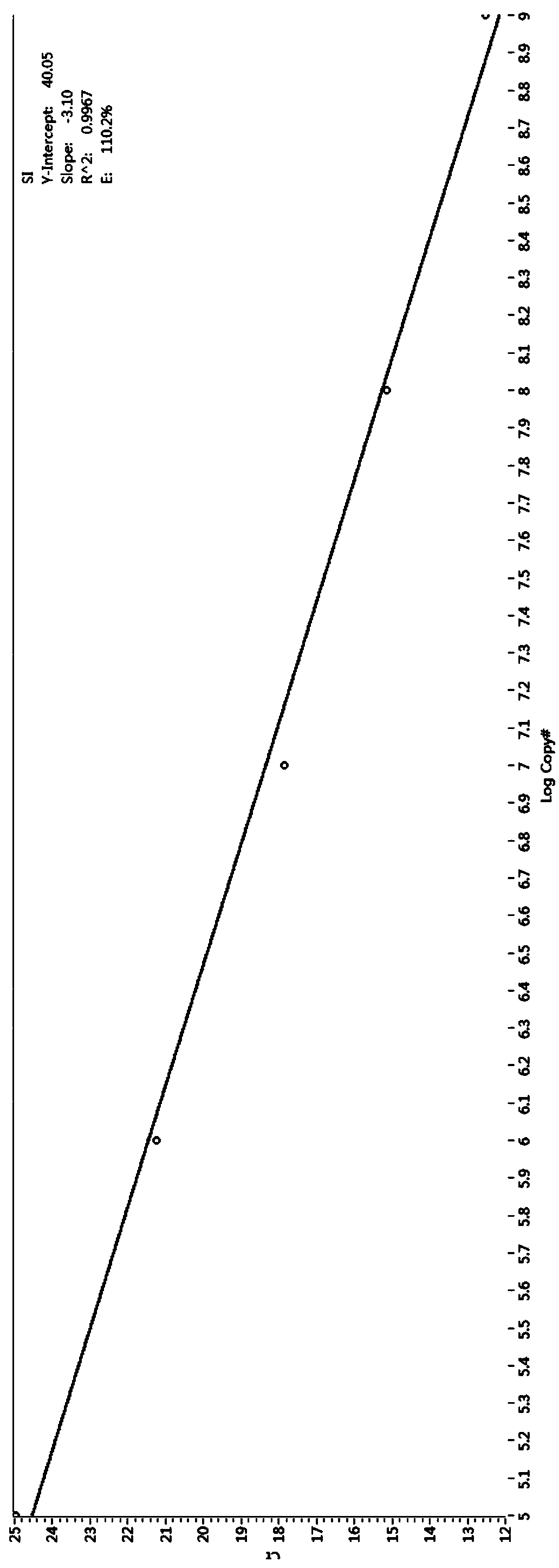
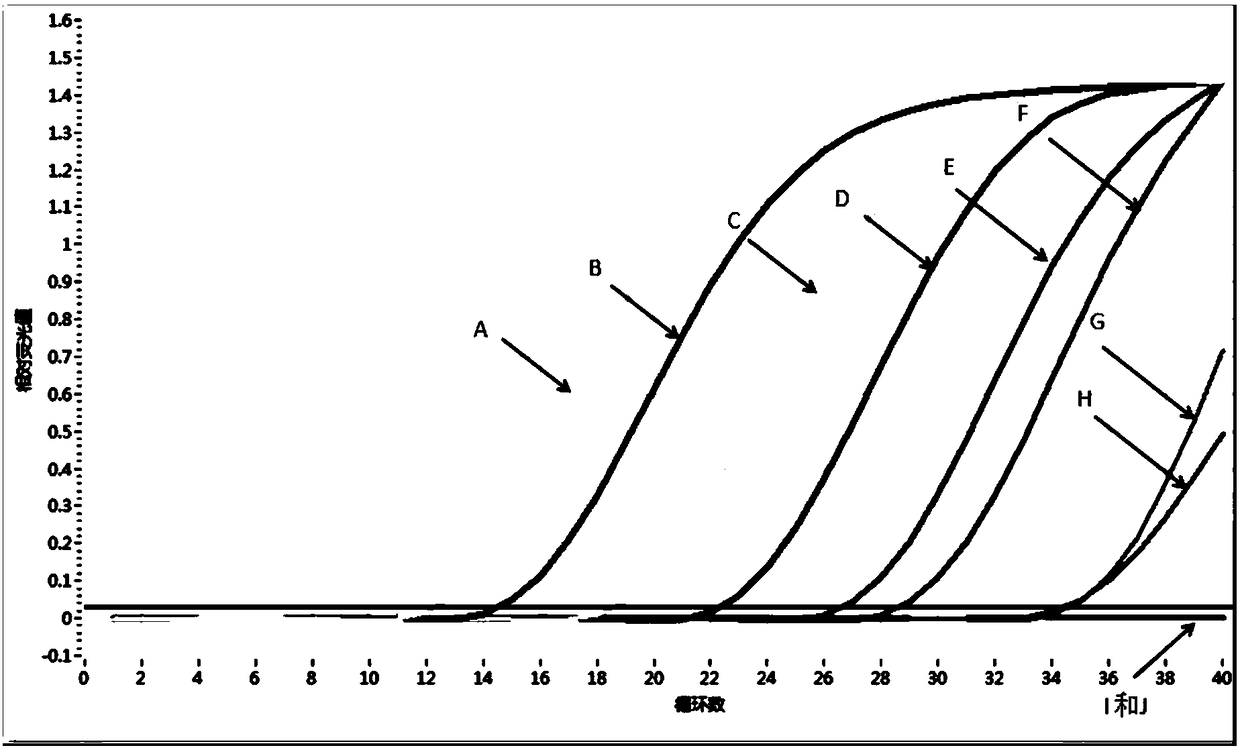
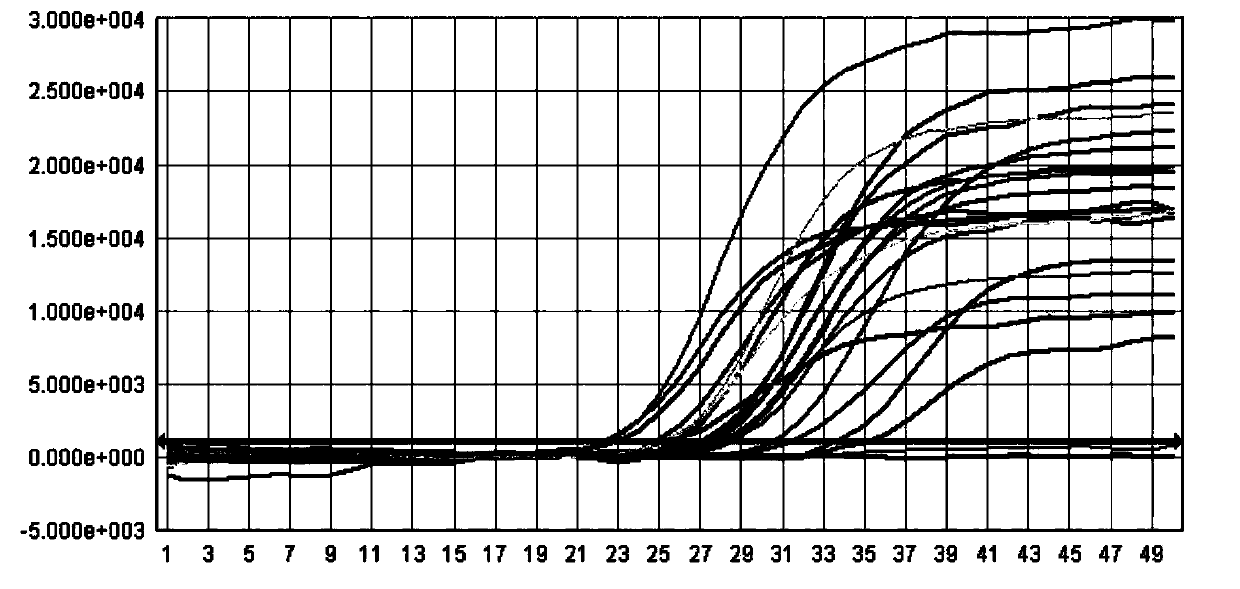
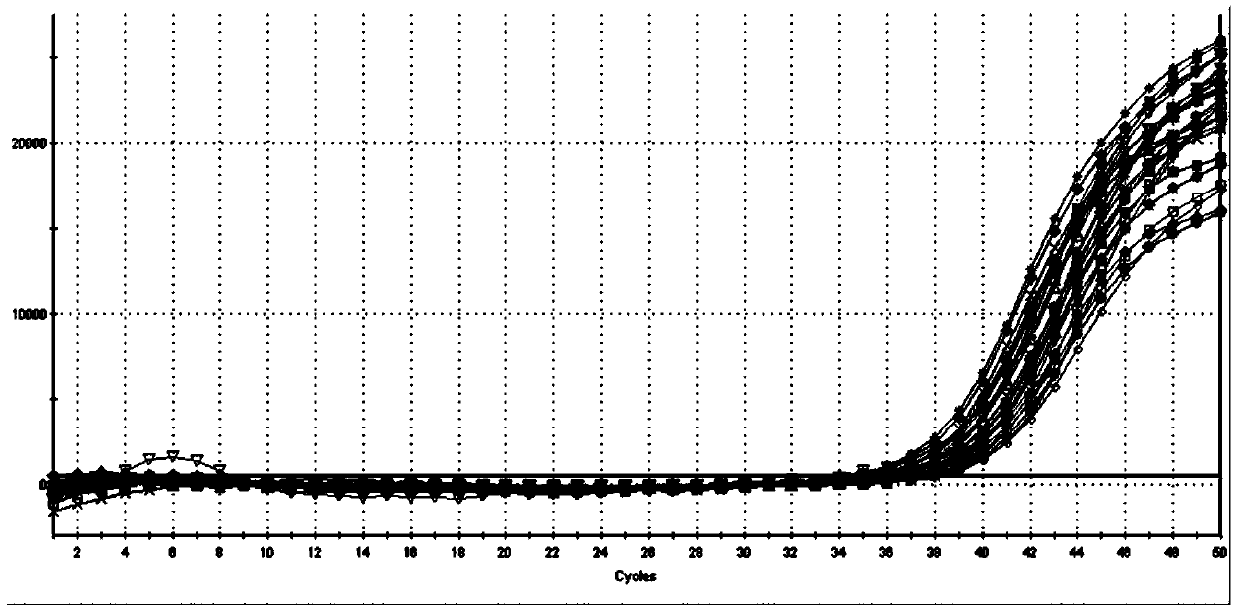
Patents
Literature
44 results about "Blood Screening" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Examination of the blood from a group of usually asymptomatic individuals to detect those with a high probability of having a given disease, typically by means of inexpensive diagnostic tests.
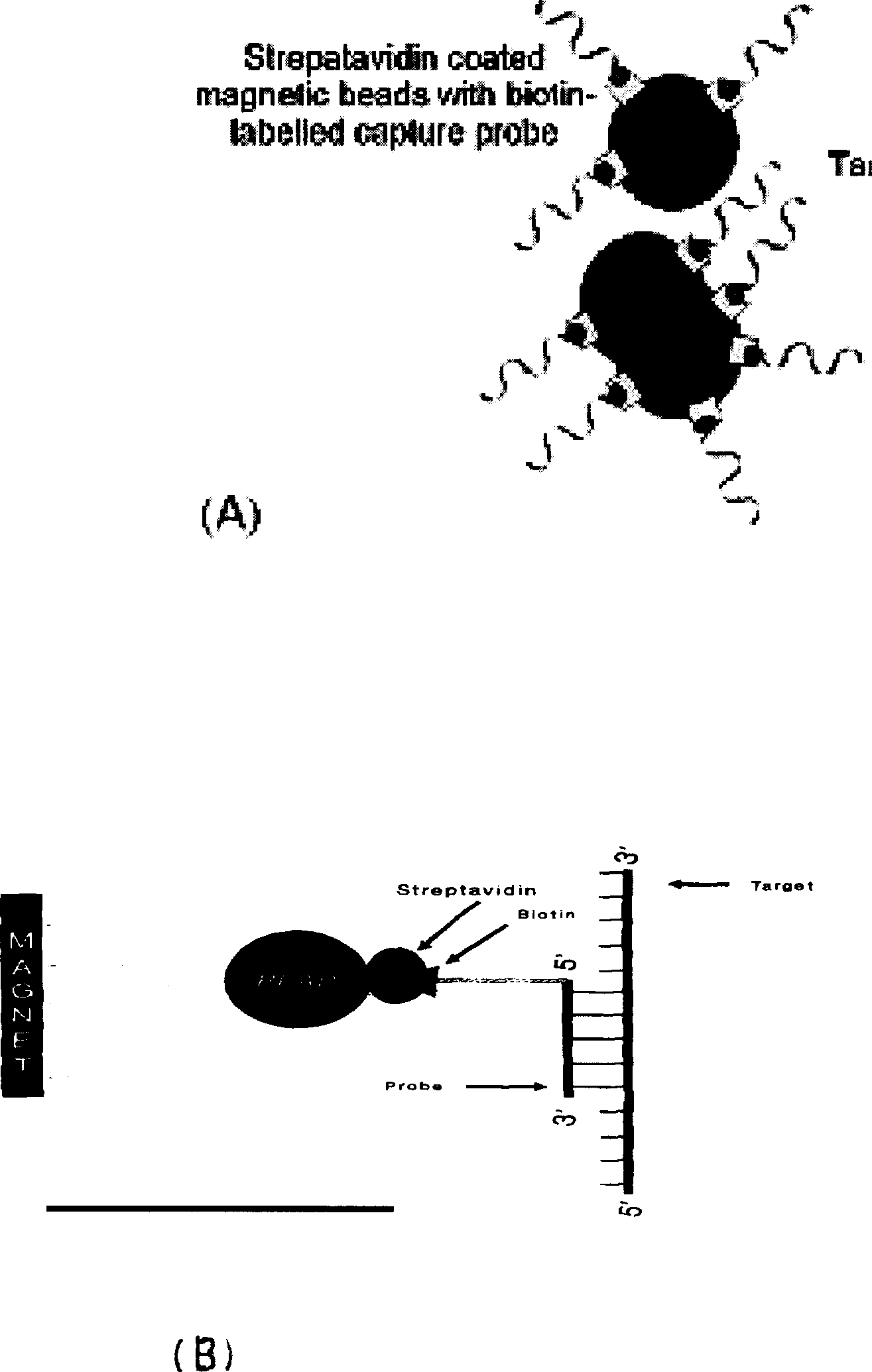
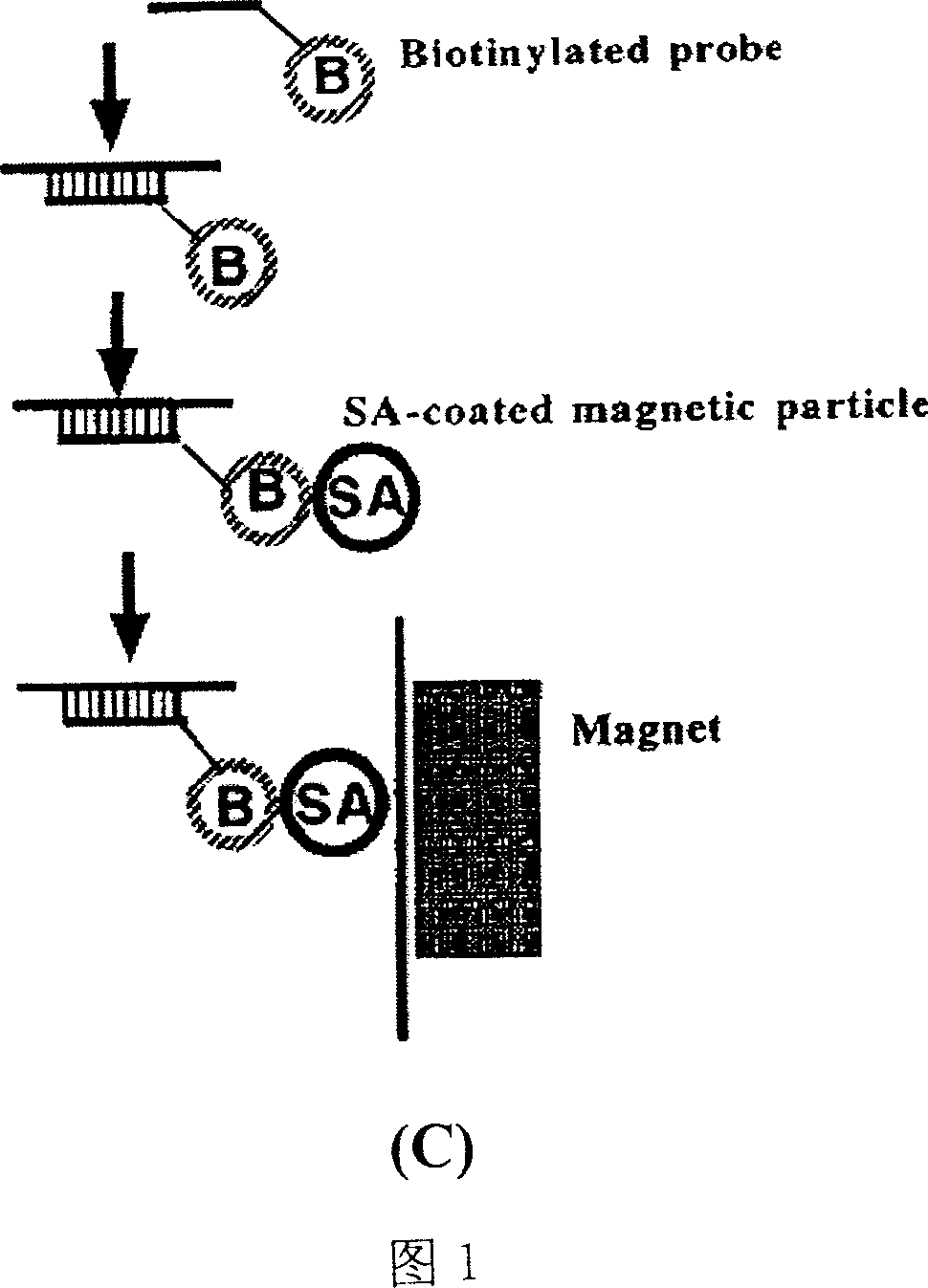
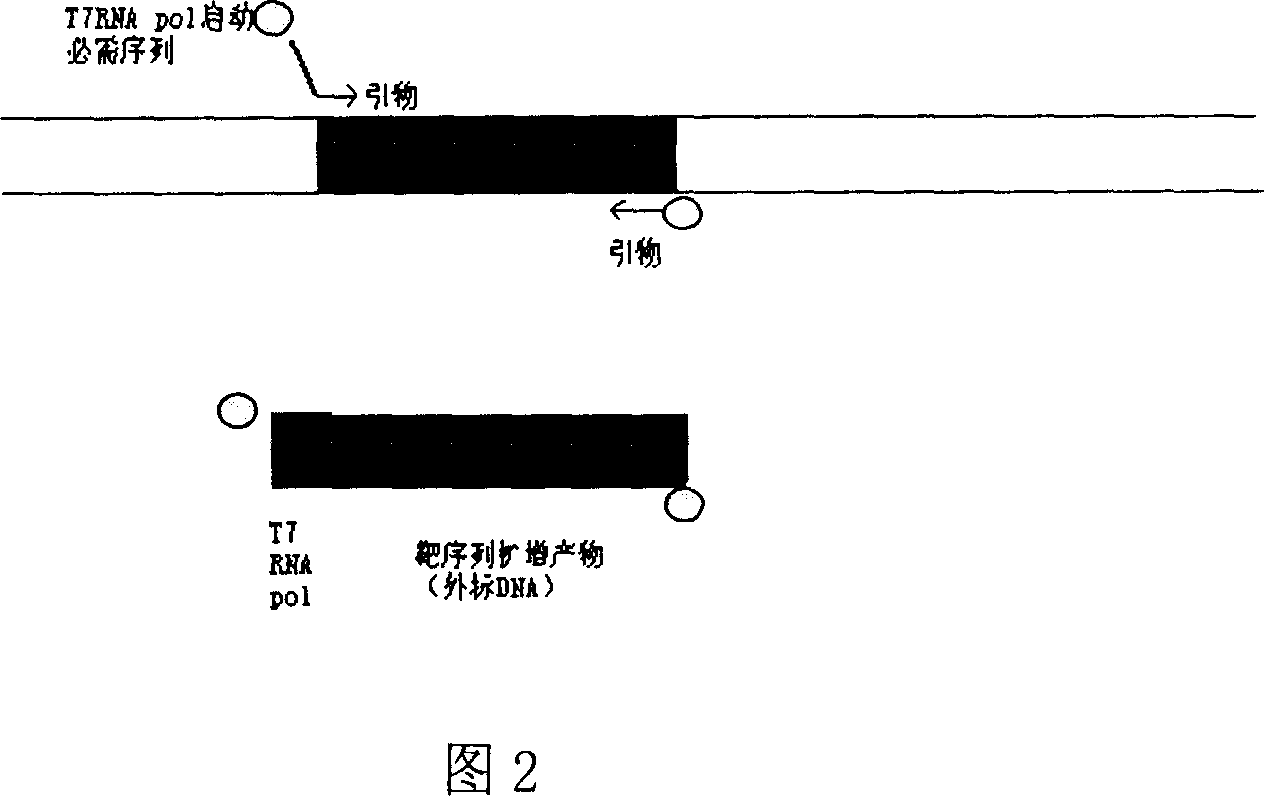
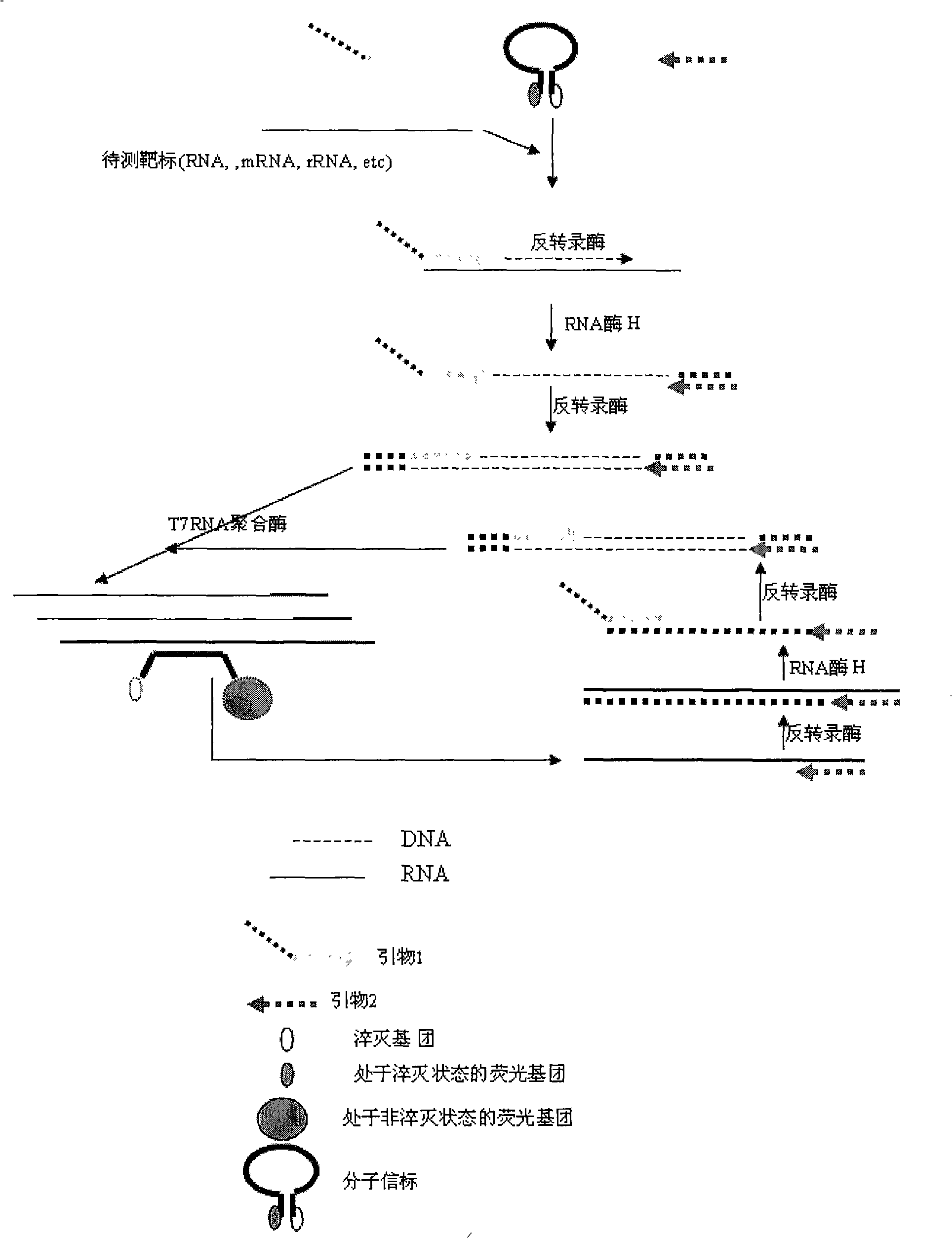
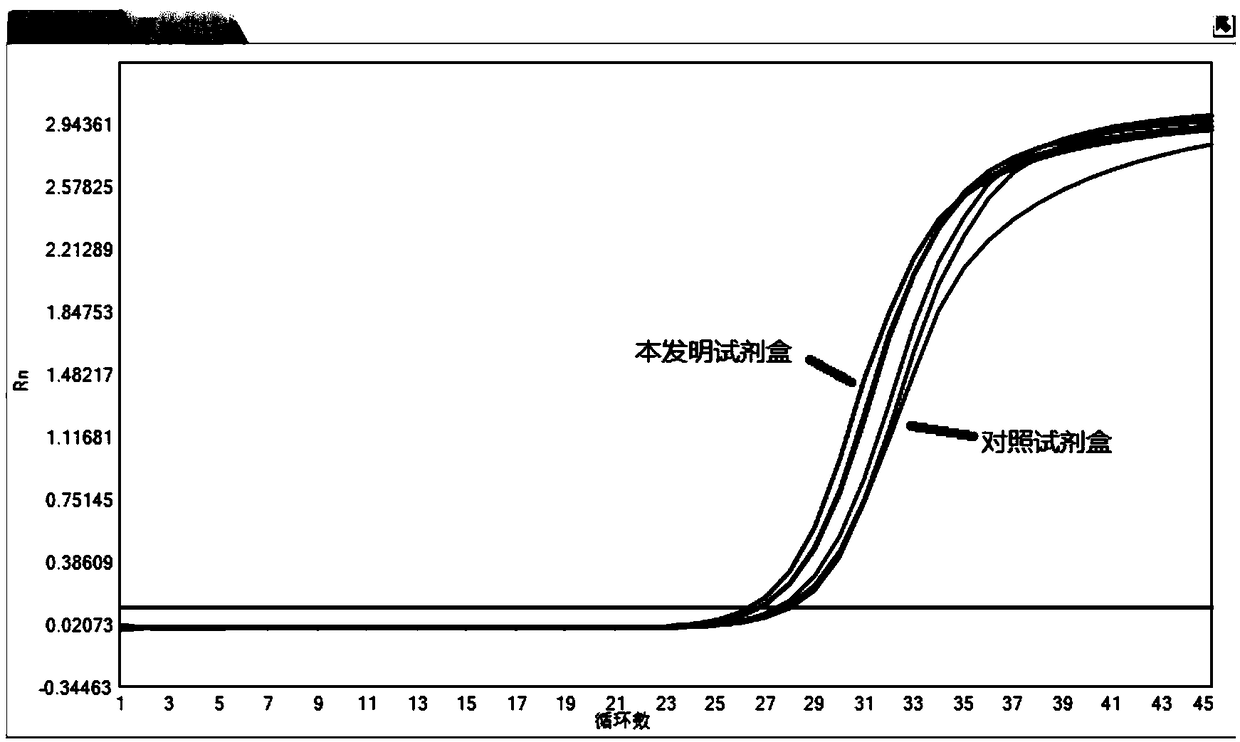
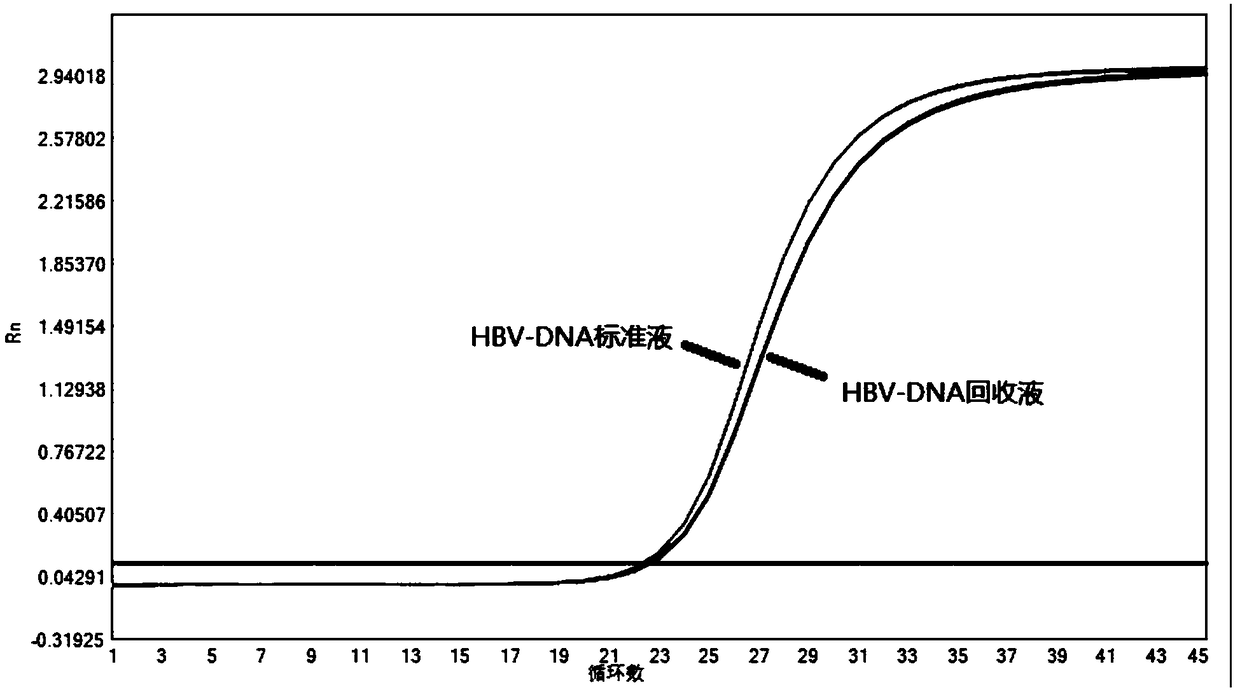
Method for inspecting hepatitis and AIDS virus nucleic acid by synchronous amplification and its reagent kit
ActiveCN1940087AHigh degree of automationHigh sensitivityMicrobiological testing/measurementMagnetic beadFluorescence
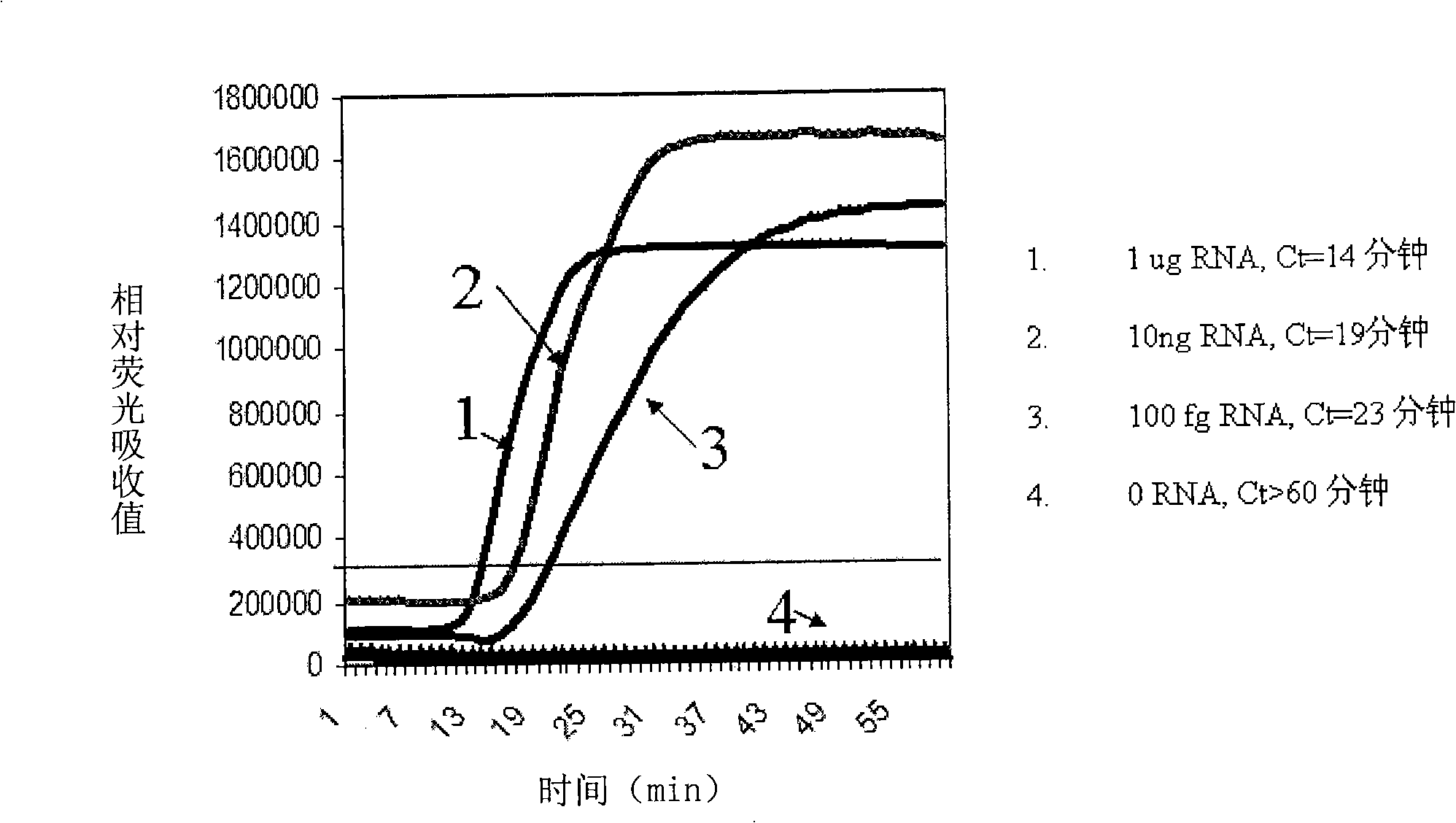
A method for inspecting hepatitis and AIDS nucleic acid by synchronized amplification and its reagent knit are disclosed. The process is carried out by taking magnetic ball as automatic medium, specific synchronized capturing HBV, CHV and HIV nucleic acid, accelerating biotin primer construction and purification by RNA external label and internal label, real-time synchronized inspecting and T-PCR amplifying based on Tagman probe. The reagent knit consists of dis-inhibitor, cracking liquid, magnetic ball suspension, washing liquor, internal check, RT-PCR reactive liquor, enzyme mixture, fluorescent mixture, positive check and negative check. It's accurate and automatic, has single-tube operation, closed inspection AND synchronized extraction, and it has better sensitivity and specific performance and can be used for large-scale blood screening and large-capacity clinical inspection.
Owner:SHANGHAI KEHUA BIO ENG
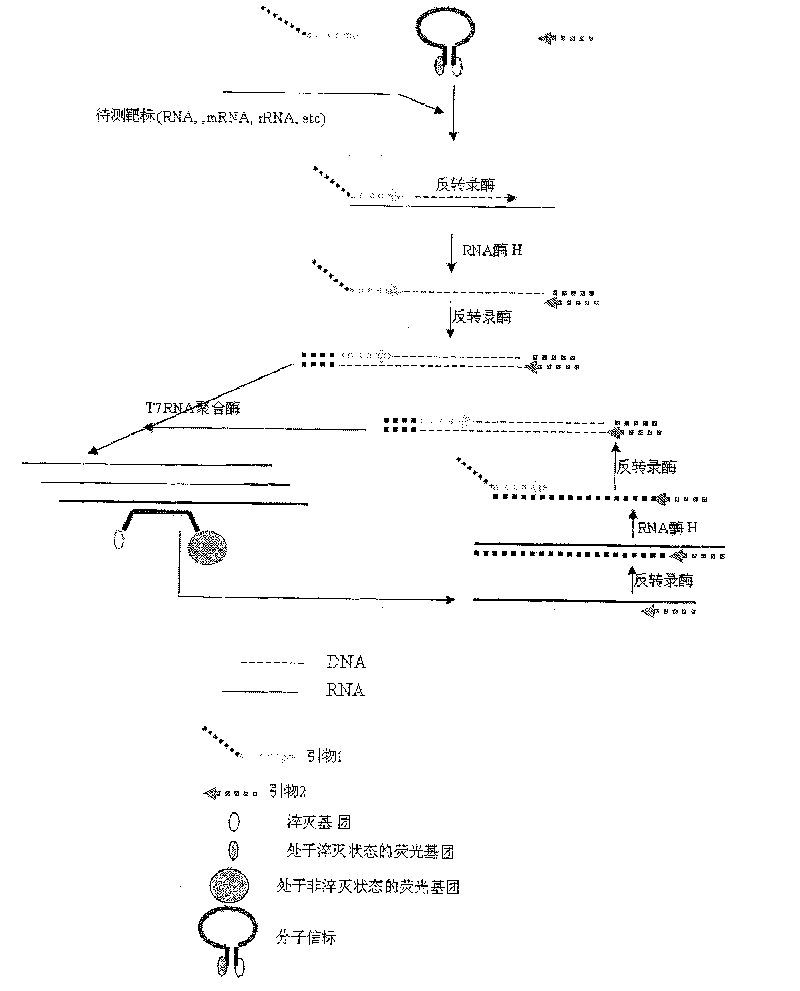
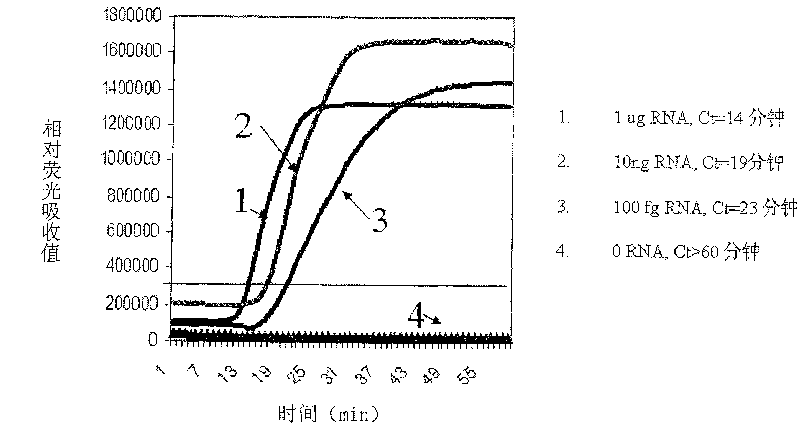
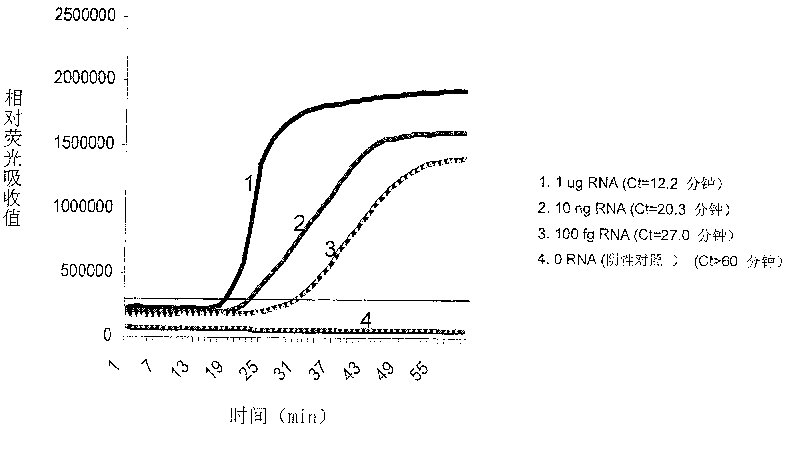
Constant temperature synchronous amplification detecting process for nucleic acid and use thereof
ActiveCN101333565AAvoid pollutionShorten the timeMicrobiological testing/measurementFluorescence/phosphorescenceNegative strandFluorescence
Owner:SHANGHAI RENDU BIOTECH
Method for extracting and purifying target nucleic acid and use thereof
ActiveCN101333564AImprove efficiencyEfficient captureMicrobiological testing/measurementDNA preparationBlood ScreeningOxygen
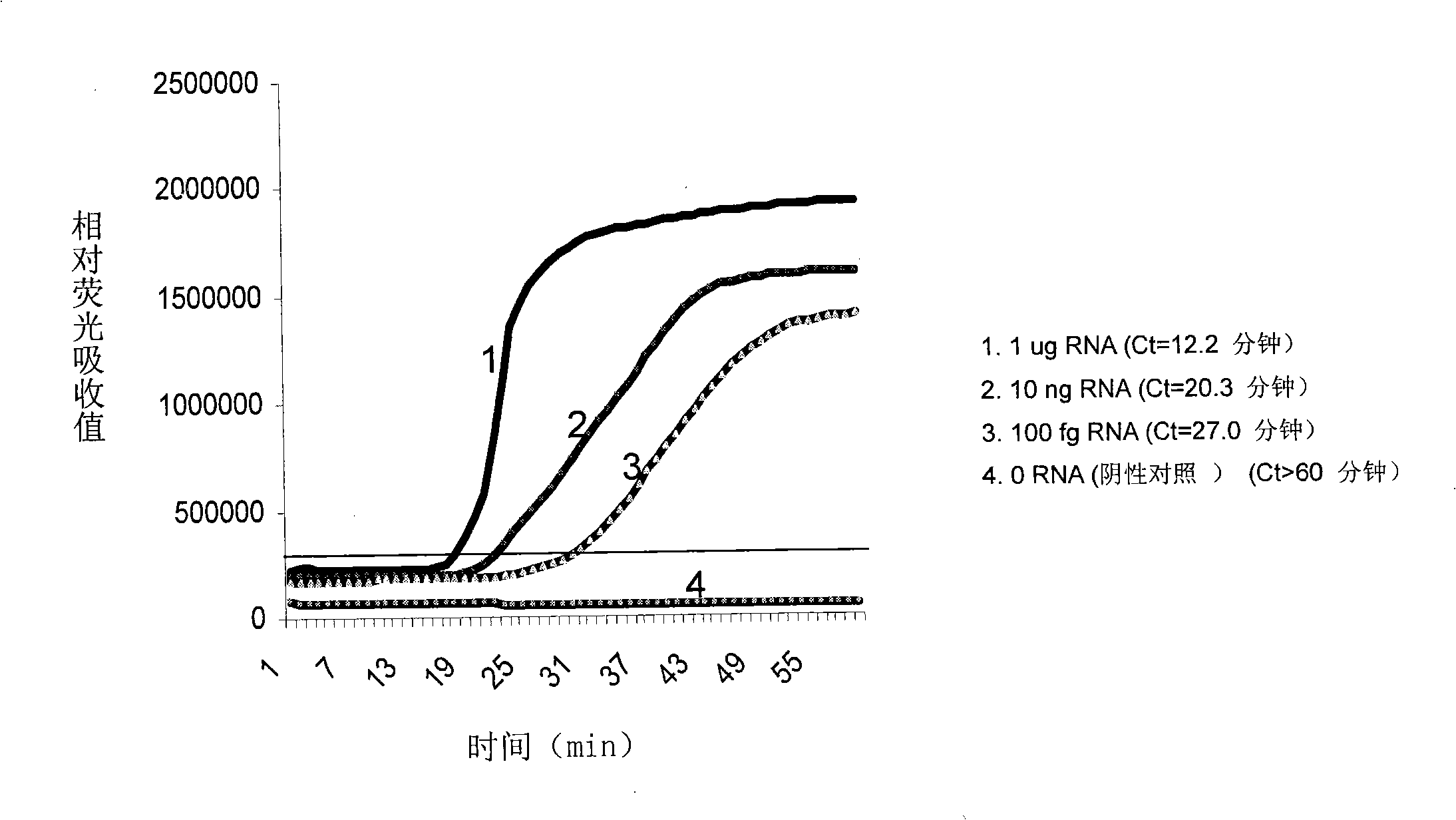
The invention discloses a method for extracting and purifying target nucleic acid and an application thereof. The extraction method comprises a marker B and a specific conjugate A, wherein, capturing nucleic acid marked by the marker B and target nucleic acid in sample are combined to a solid phase carrier combined with the marker B and the specific conjugate A to form a compound of capturing nucleic acid- target nucleic acid of solid phase carrier-A-B; and the capturing nucleic acid at least comprises a 2`-oxygen-methylation oligoclonal nucleic acid which is specifically combined with the target nucleic acid RNA and is marked by the marker B. The invention also provides a kit for extracting the target nucleic acid, which at least comprises a 2`-oxygen-methylation oligoclonal nucleic acid which is specifically combined with the target nucleic acid RNA and is marked by the marker B, and the 2`- oxygen- methylation oligoclonal nucleic acid is used as the capturing nucleic acid. The method has the advantages of high specificity and purity, less pollution, constant temperature in the reaction process, high sensitivity, fast detecting speed, low requirements on instruments and low cost. Thus, the method is particularly applicable to blood screening.
Owner:SHANGHAI RENDU BIOTECH
Integrated blood nucleic acid screening platform
InactiveCN101538612AEasy to operateSuitable for daily useMicrobiological testing/measurementFluorescence/phosphorescenceBlood centerWhole blood product
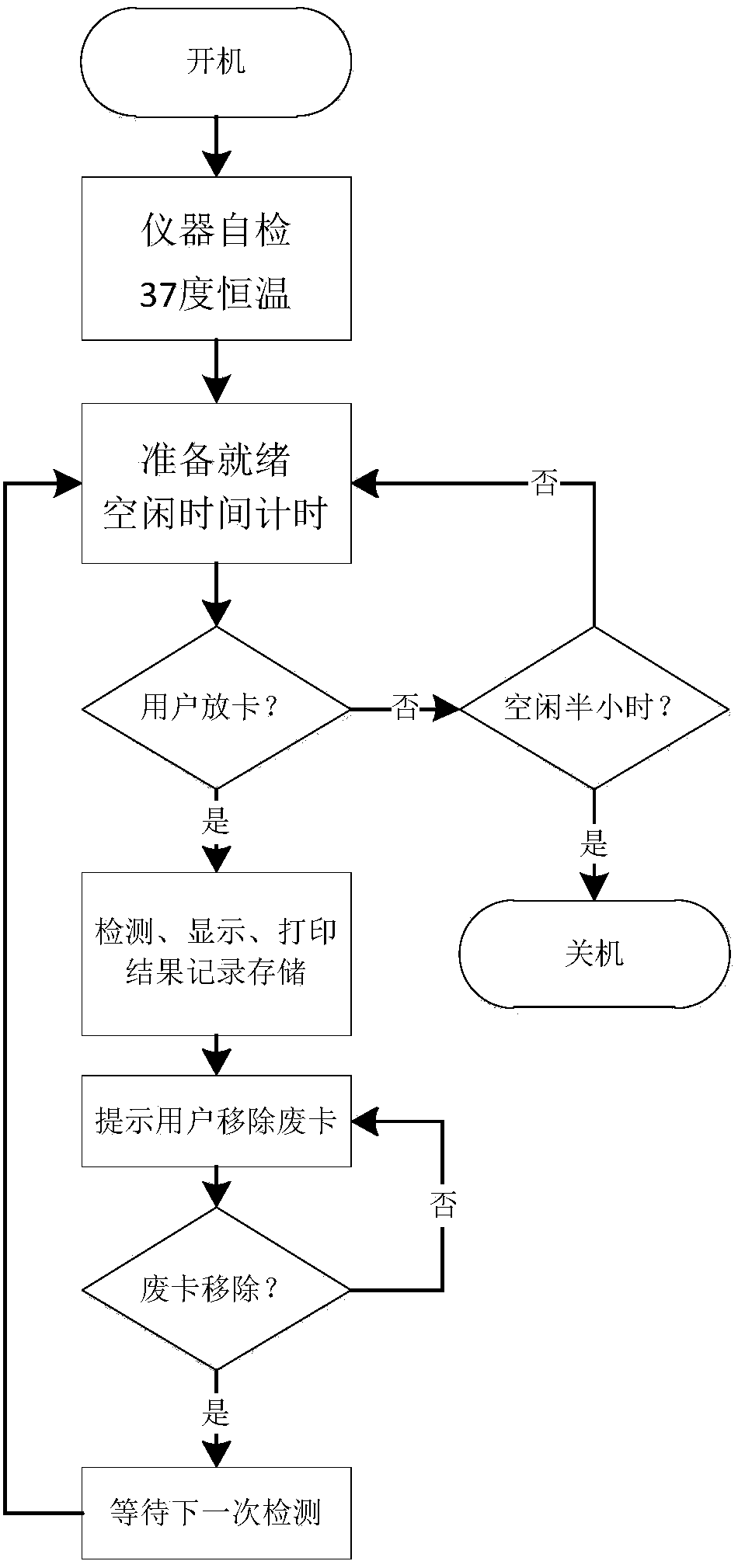
The invention belongs to the technical field of blood screening and specifically relates to an integrated blood nucleic acid screening platform; the tabletop of the screening platform is divided into five functional zones which are sequentially consumptive material zone, reagent zone, thermomagnetic operation zone, sampling zone and waste zone, wherein, the thermomagnetic operation zone is provided with a dedicated thermomagnetic swiveling mechanismAgowa7200 or a thermomagnetic device consisting of a discrete component deep-well multiwell plate magne, a deep-well multiwell plate bearing rack, a temperature control module and an oscillation module; the screening reagent comprises paramagnetic particle method nucleic acid extraction reagent for simultaneously extracting RNA and DNA and one-step method RT-PCRTaqMan fluorescent probe reagent for simultaneously augmenting HBV DNA, HCV RNA and HIV RNA; special functions such as sample scanning, mixed sample collection, extraction of nucleic acid, preparation of PCR reaction solution, split charging and loading and the like are controlled by programs. The screening platform features convenient operation, designed operating process, which greatly improves screening efficiency, therefore the screening platform is very suitable for daily use in blood centers at home and abroad, the detecting departments in enterprises producing blood products.
Owner:SHANGHAI KEHUA BIO ENG
Nucleic acid aptamer specifically combined with hepatitis C virus core protein and application thereof
ActiveCN102121009ASensitive highLow costMicrobiological testing/measurementDNA/RNA fragmentationAptamerMonoclonal antibody
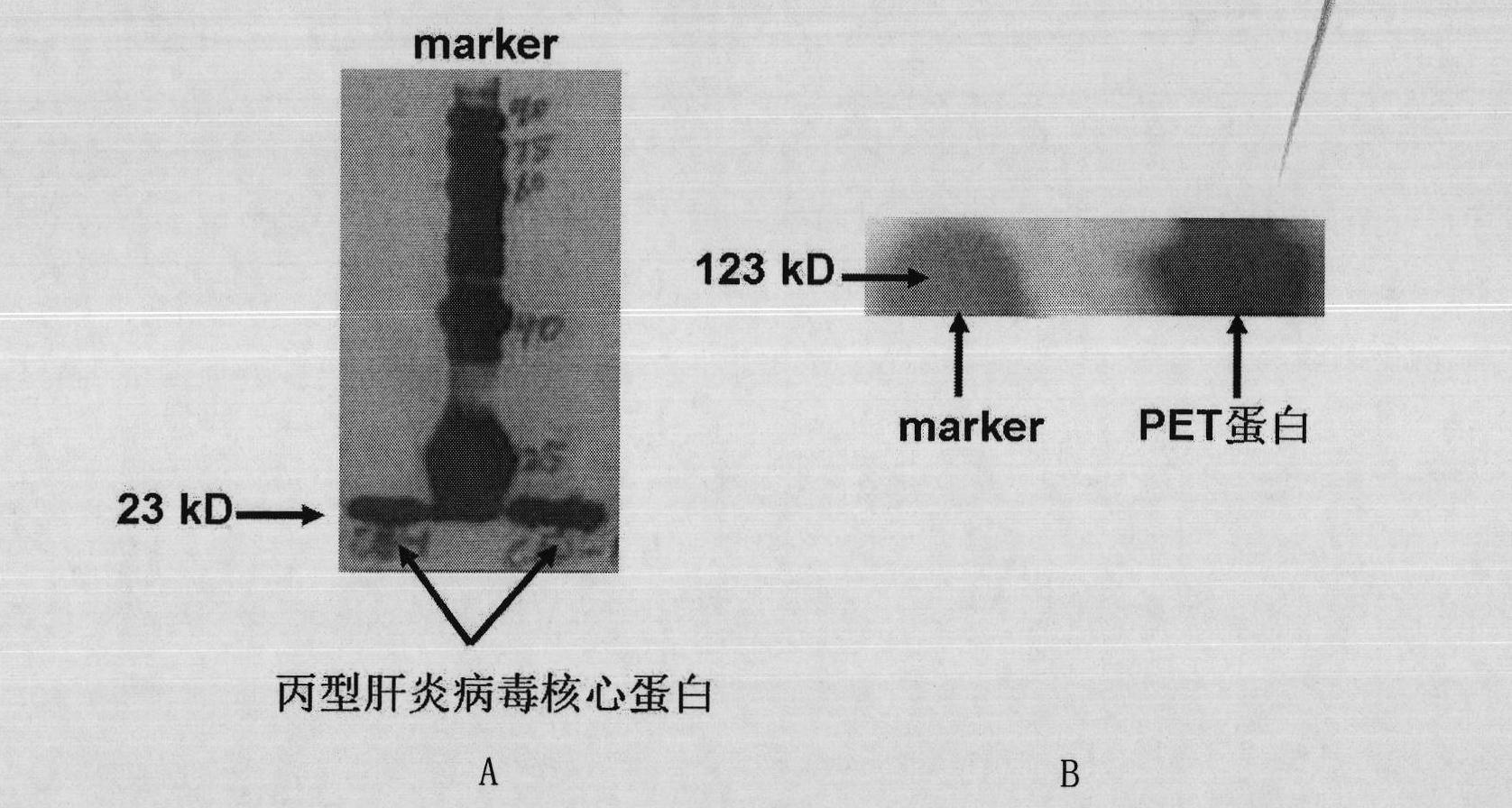
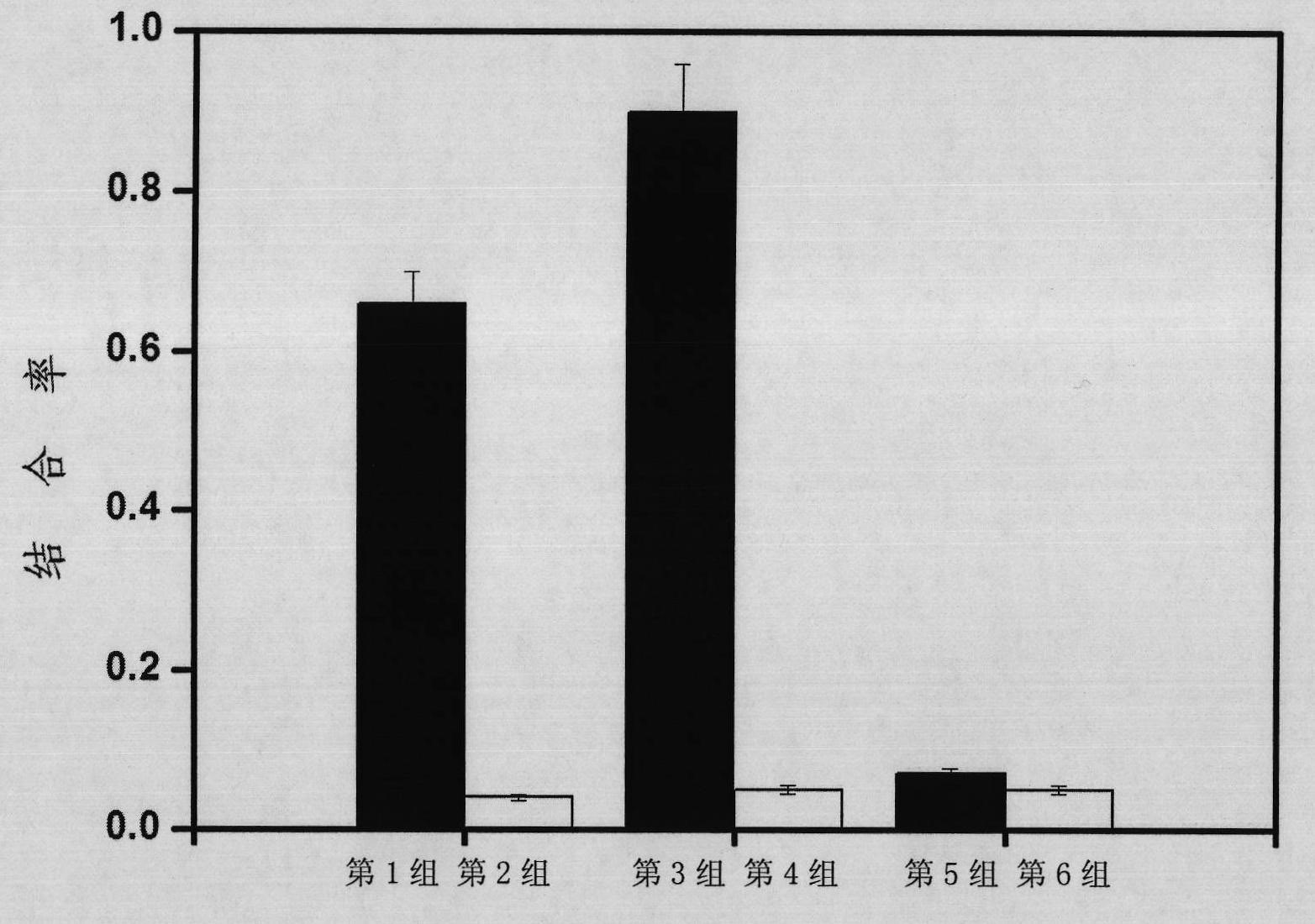
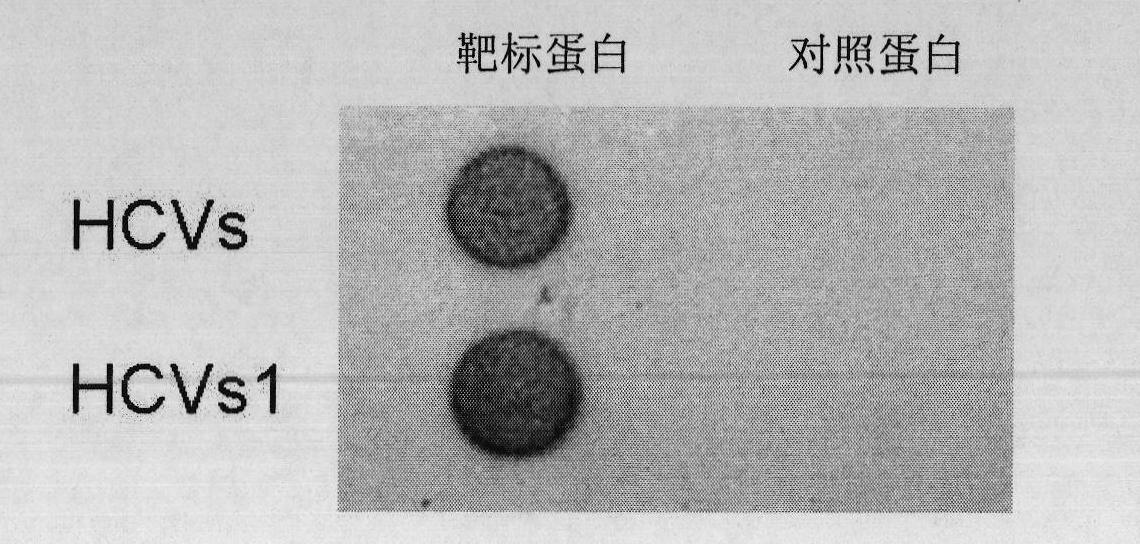
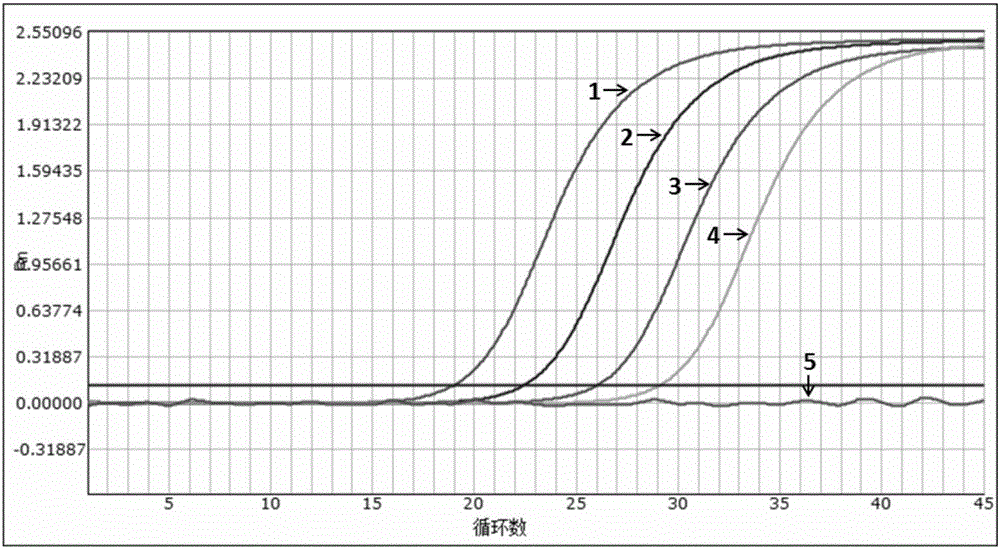
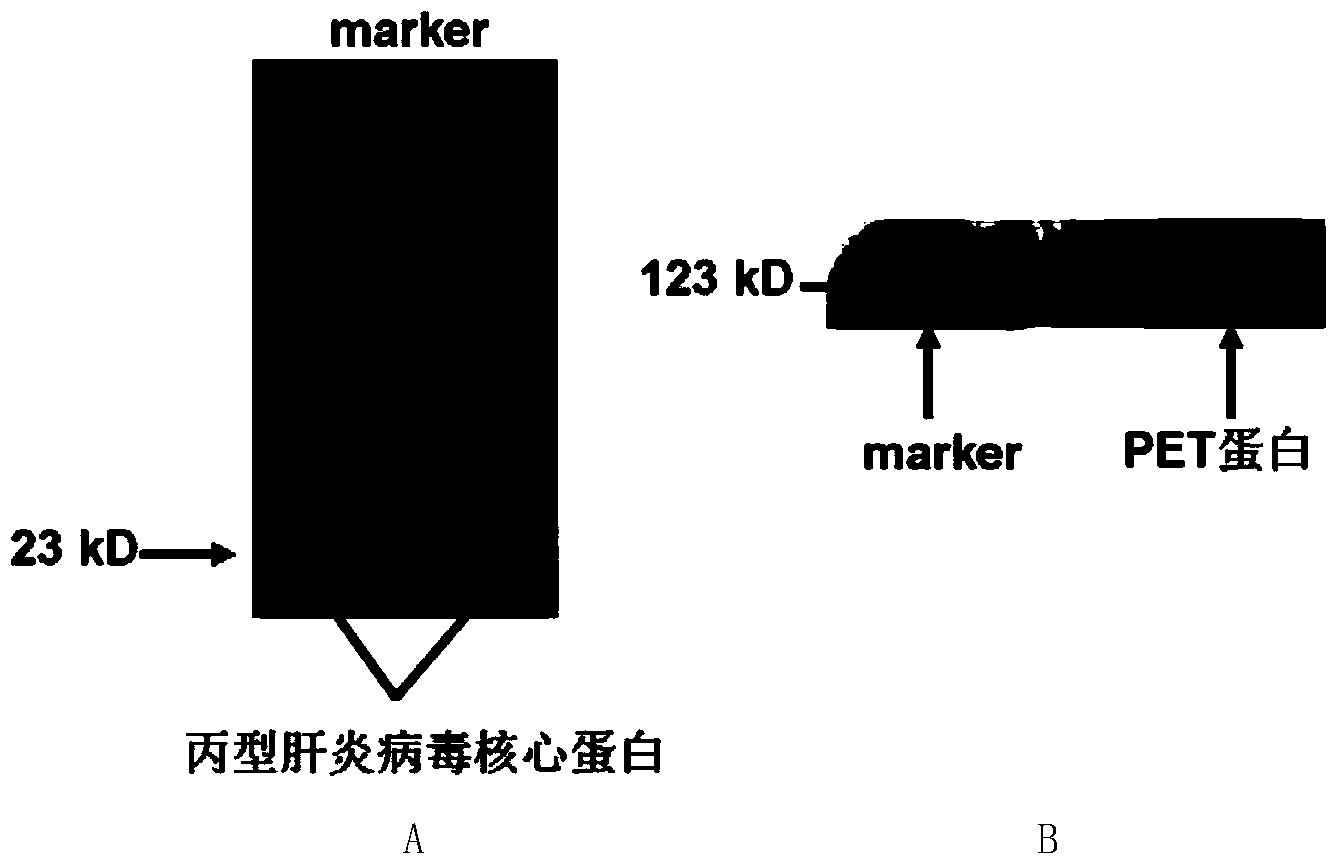
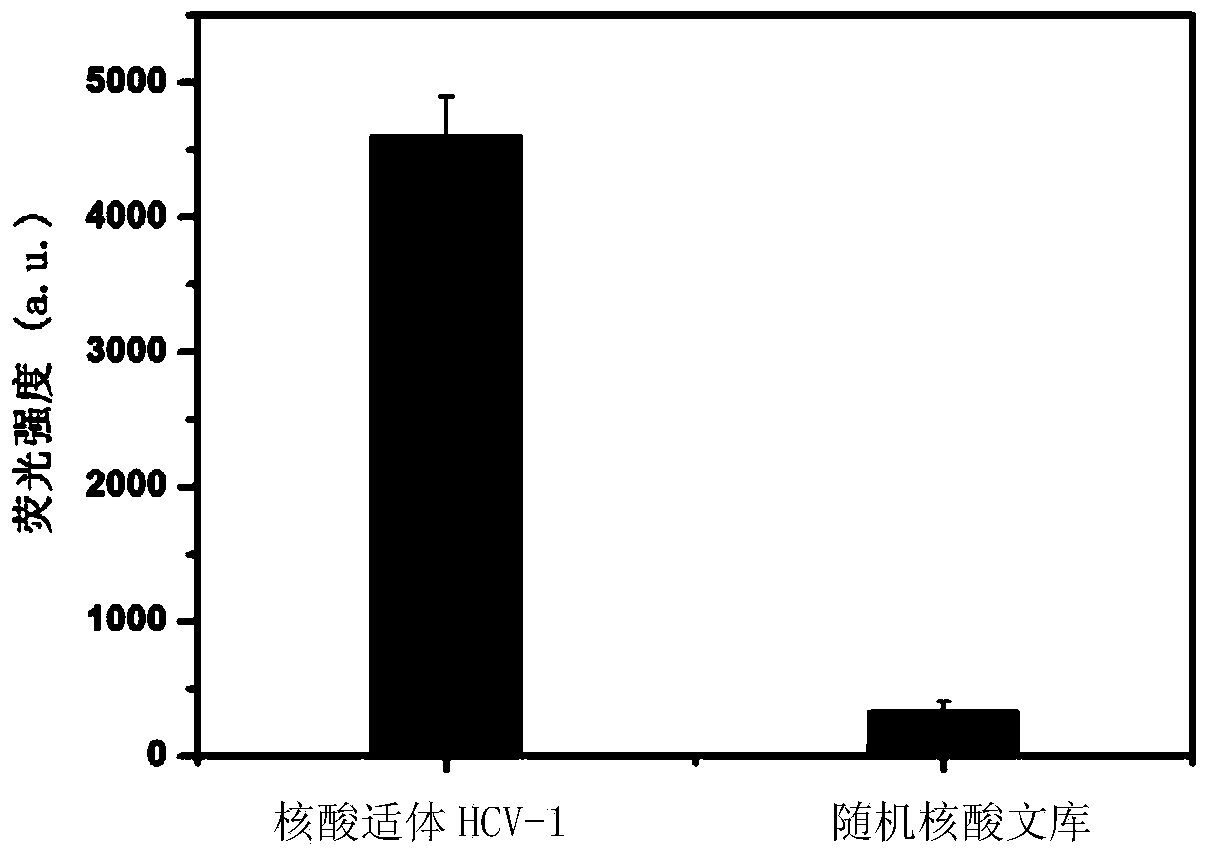
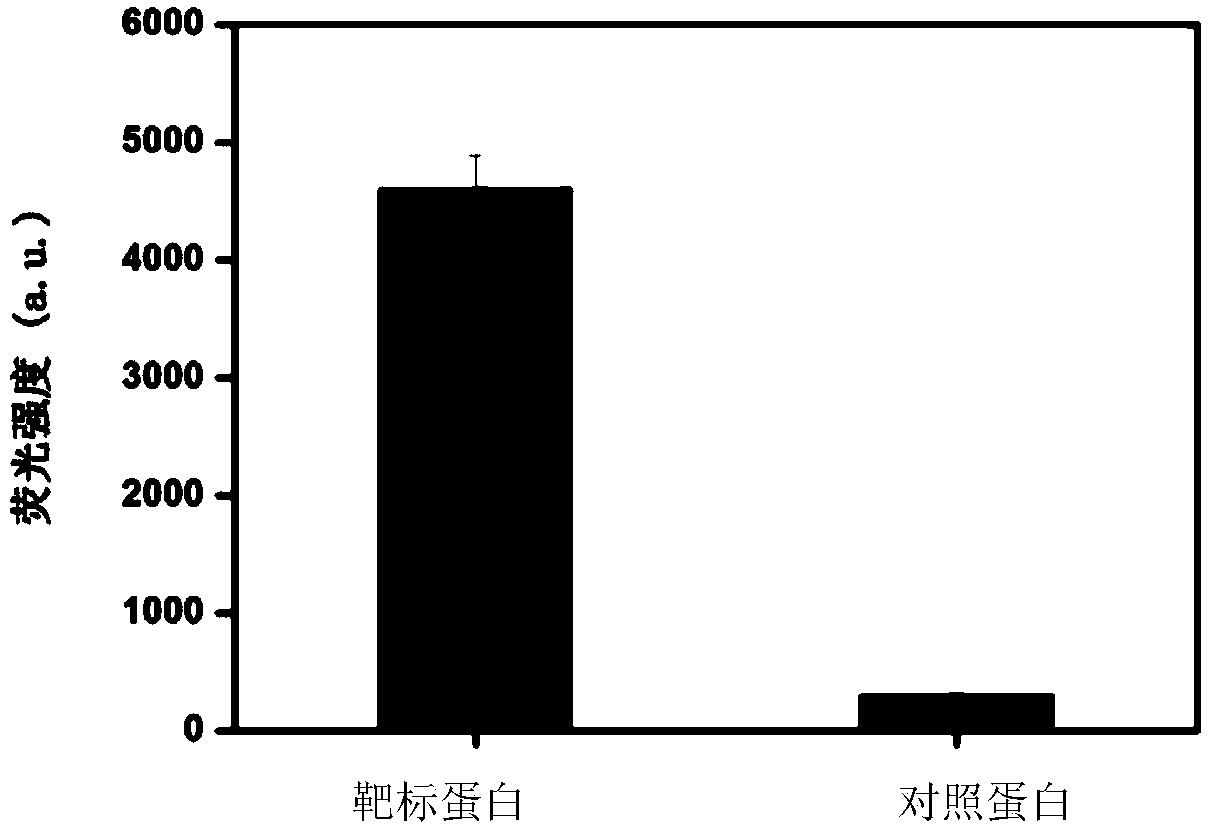
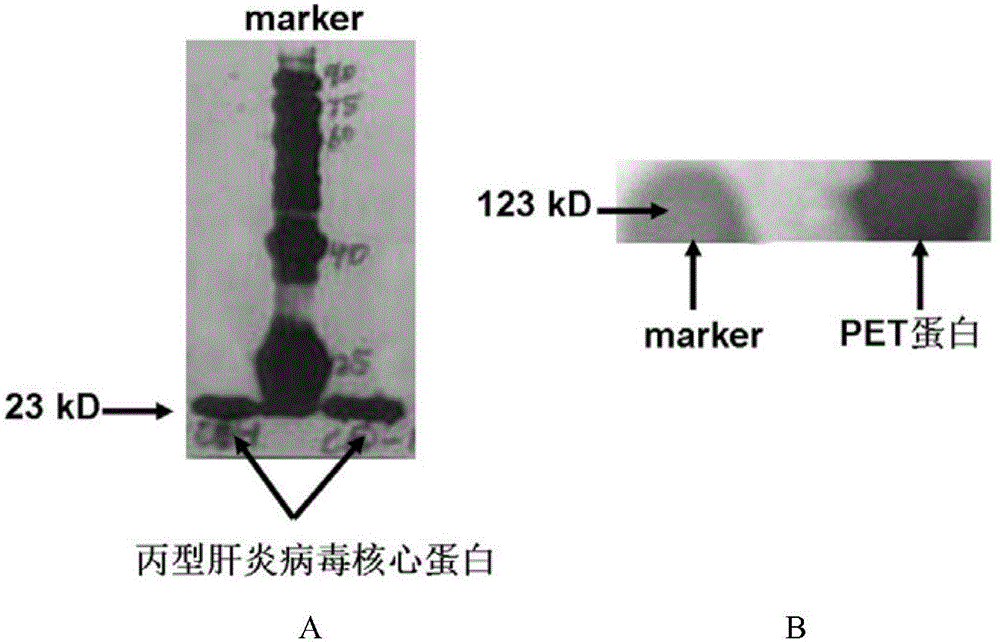
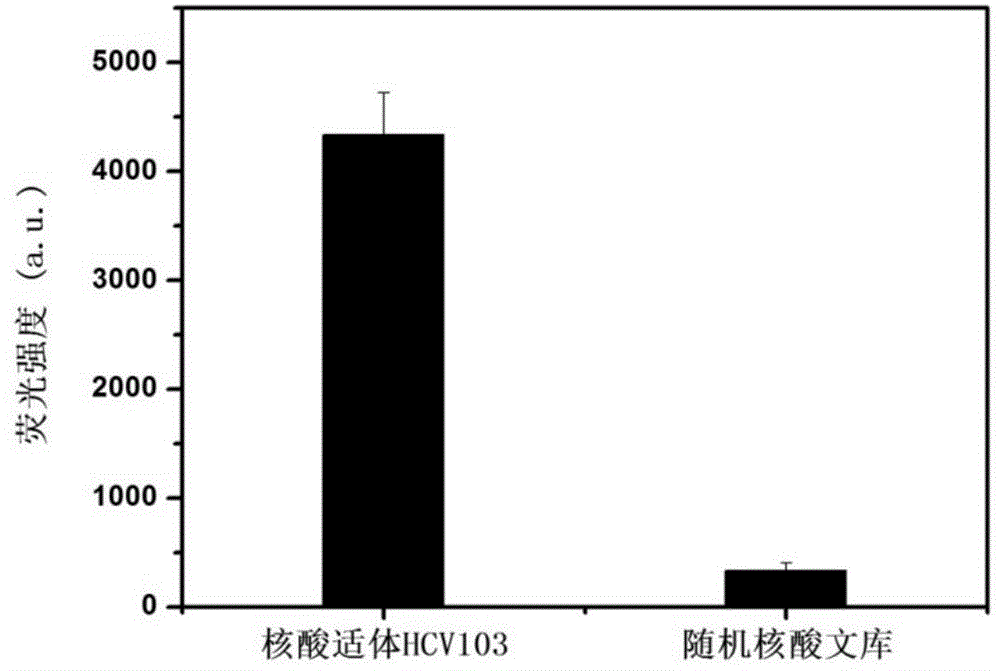
The invention discloses a nucleic acid aptamer specifically combined with a hepatitis C virus core protein and application thereof. The nucleic acid aptamer is a single stranded DNA shown in a sequence 2 of a sequence table or single stranded DNA containing the nucleic acid aptamer. The nucleic acid aptamer has better affinity with a hepatitis C virus core protein. By using the nucleic acid aptamer disclosed by the invention, the hepatitis C virus core protein in a solution can be captured and can be also detected, and hepatitis C serodiagnosis and blood screening can be realized. Part of thenucleic acid aptamer disclosed by the invention can be used to replace a monoclonal antibody to capture the core protein for hepatitis C detection. The invention has the advantages of high sensitivity, low cost, easiness of preparation and storage, and high application value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
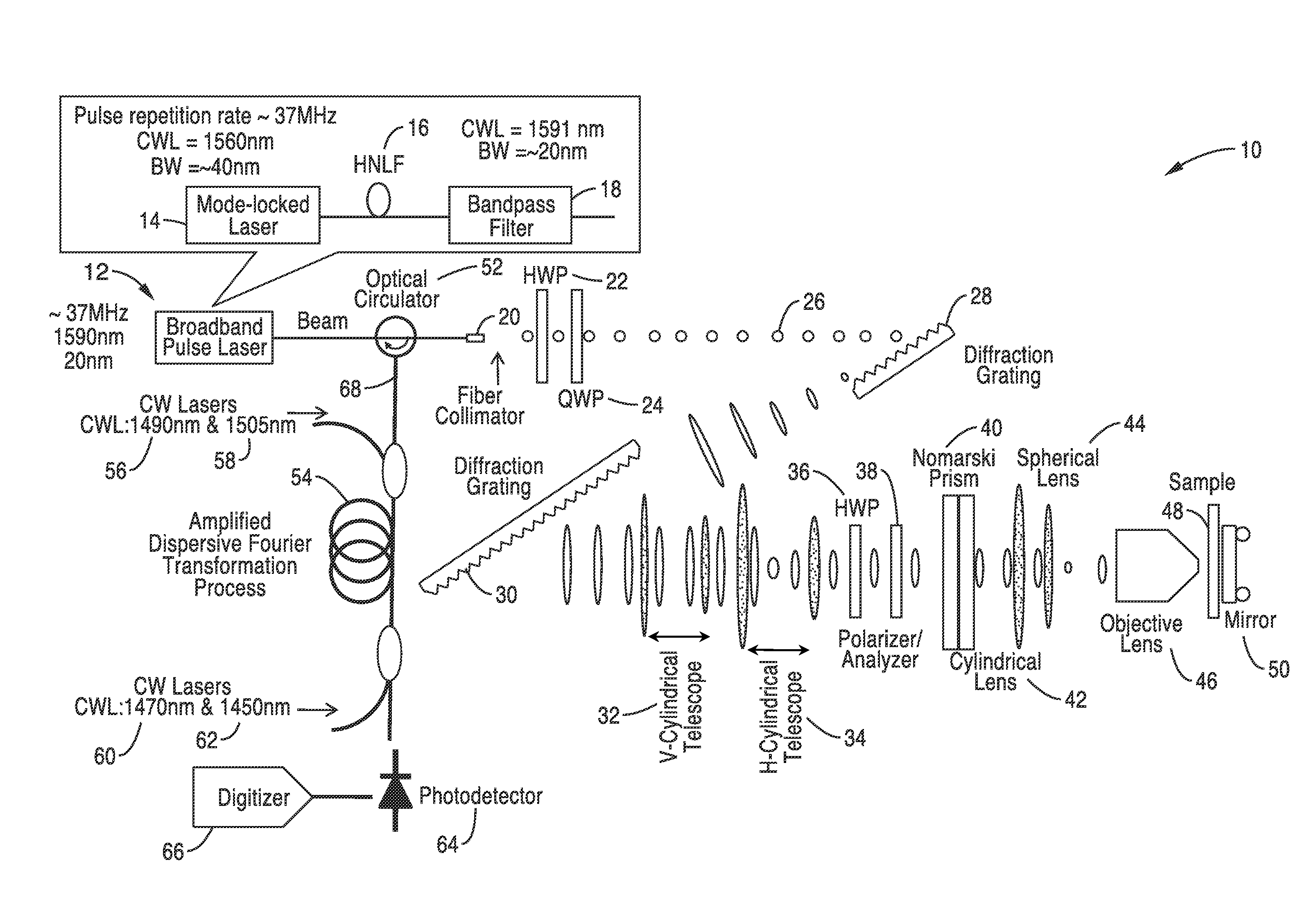
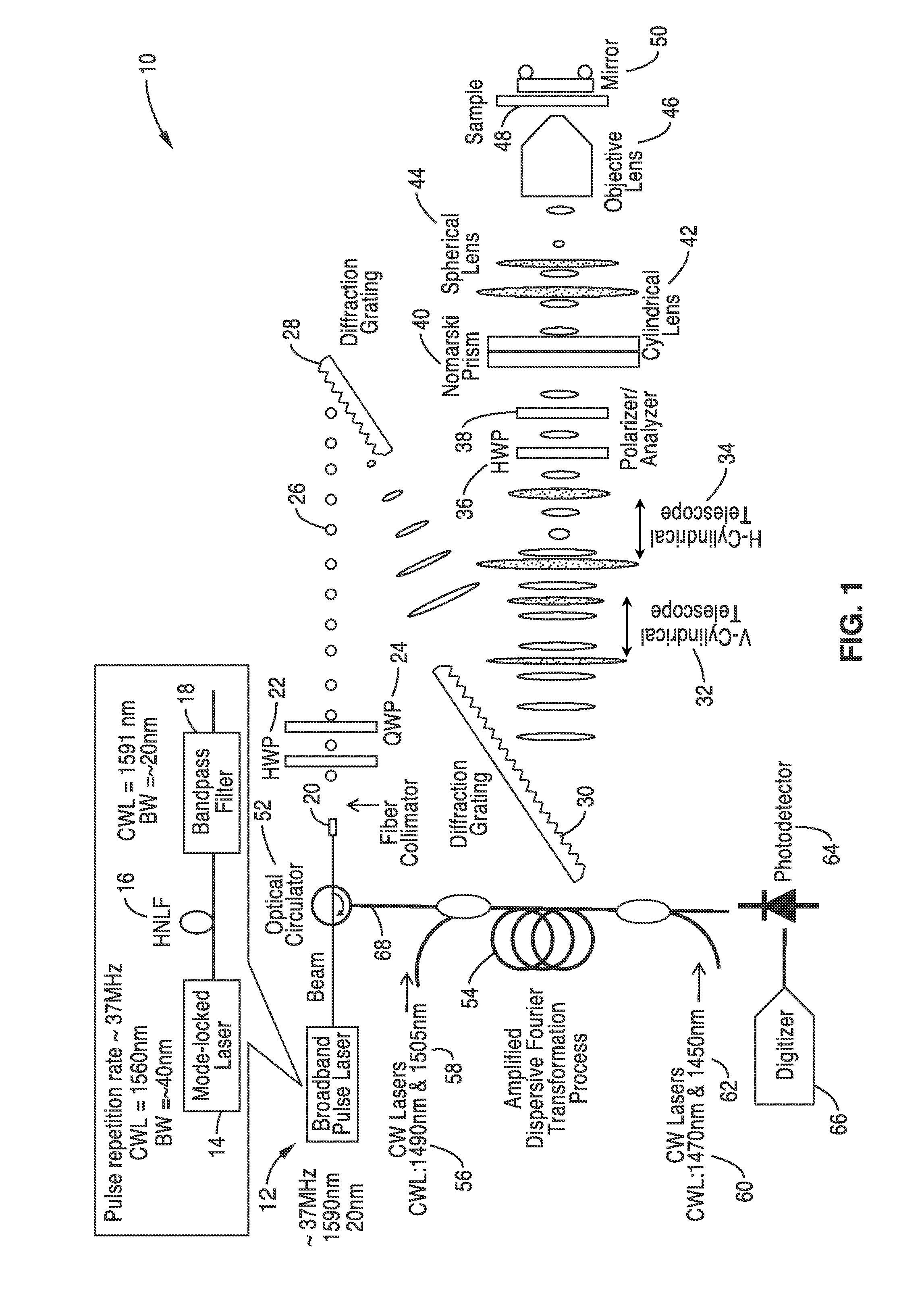
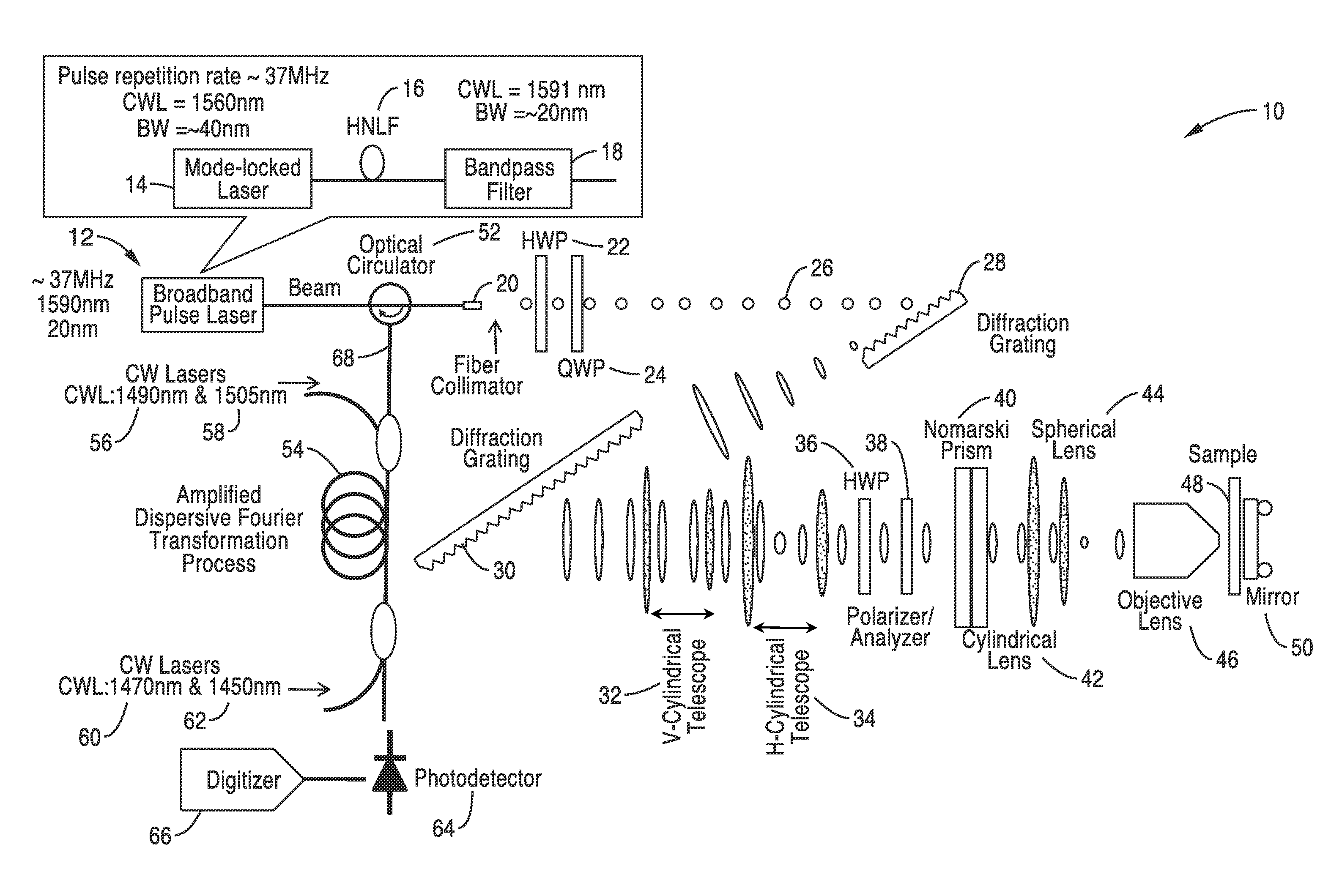
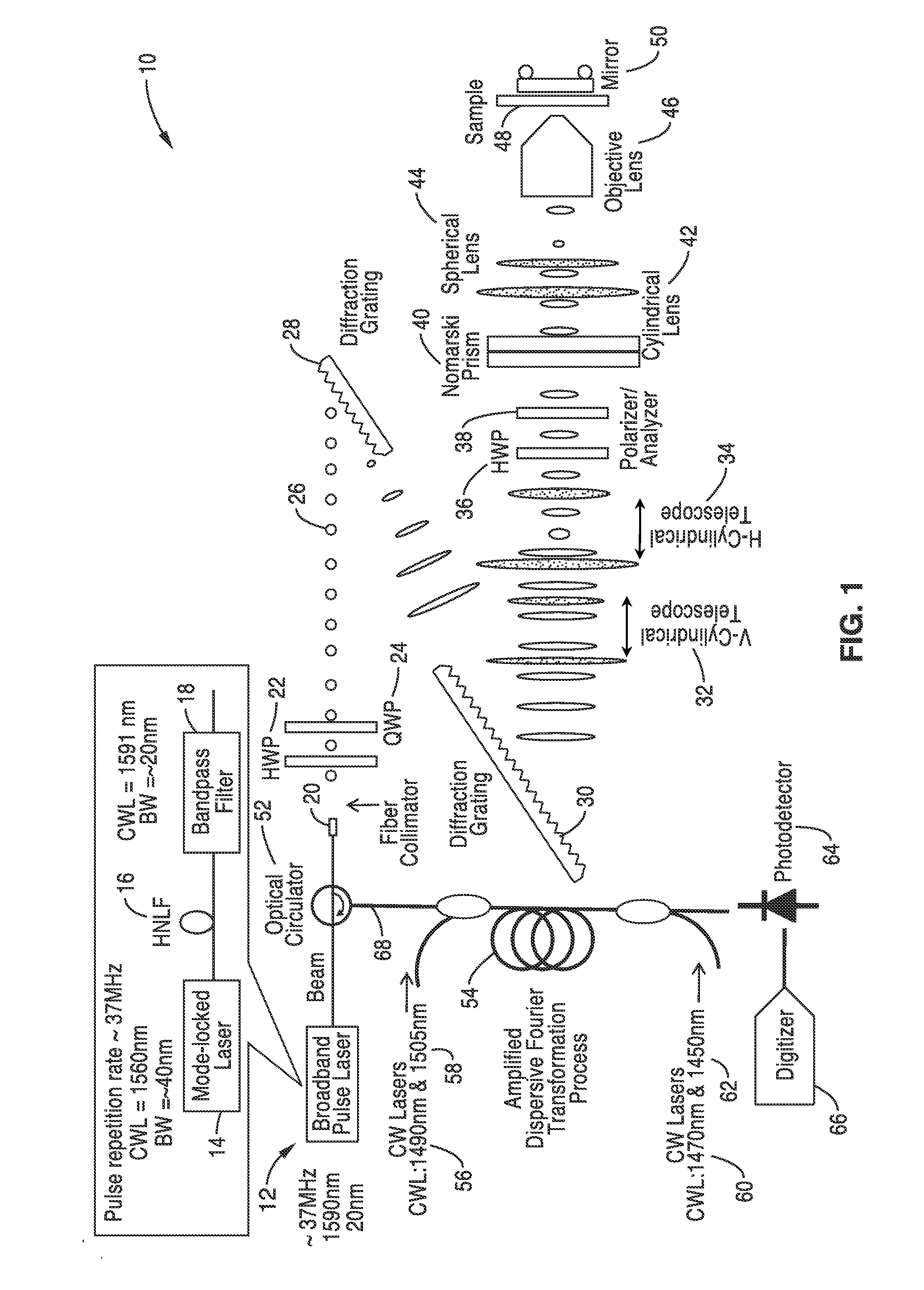
Differential interference contrast serial time encoded amplified microscopy
ActiveUS8654441B2Revolutionize blood analysisShort stayTelevision system detailsPrismsStainingSerial time-encoded amplified microscopy
Owner:RGT UNIV OF CALIFORNIA
Fast joint inspection kit for human immunodeficiency viruses, hepatitis B viruses and hepatitis C viruses and preparation and application thereof
ActiveCN105695631AReduced risk of cross-contaminationHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesMultiplexImmunodeficiency virus
The invention belongs to the field of biotechnology detection, and particularly relates to a fast joint inspection kit for human immunodeficiency viruses (HIV), hepatitis B viruses (HBV) and hepatitis C viruses (HCV) and preparation and application thereof. The kit comprises an HIV detecting primer and probe, an HBV detecting primer and probe and an HCV detecting primer and probe. A multiplex fluorescent PCR technology is adopted, three kinds of viral nucleic acid of the HIV, the HBV and the HCV are detected in a single PCR reaction tube at the same time, detecting sensitivity is high, good specificity is achieved, the human error rate is low, and time consumed in experiments is short. Fluorescence signals are detected in real time in the amplified reaction process, the whole process is conducted in a sealed mode, the risk of cross infection among samples is reduced, and the kit is suitable for being applied to large-scale blood screening and clinical examinations.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Nucleic acid aptamer of affinity viral hepatitis C core protein and application of nucleic acid aptamer
ActiveCN104004763ASensitive highLow costMaterial analysisDNA/RNA fragmentationAptamerMonoclonal antibody
The invention discloses a nucleic acid aptamer of an affinity viral hepatitis C core protein and an application of the nucleic acid aptamer. The nucleic acid aptamer is the following (a) or (b): (a) single-stranded DNA represented by sequence 2 in a sequence table; (b) single-stranded DNA containing the nucleic acid aptamer described in (a). Due to the adoption of the nucleic acid aptamer disclosed by the invention, the viral hepatitis C core protein in a solution can be captured and the viral hepatitis C core protein in a solution can also be detected, which are conductive to hepatitis C serology diagnosis and blood screening. By virtue of the nucleic acid aptamer disclosed by the invention, the monoclonal antibody can be partially replaced to capture core protein for detecting hepatitis C and the nucleic acid aptamer has the advantages of high sensitivity, low cost, and convenience in production and preservation. The nucleic acid aptamer has a relatively high application value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
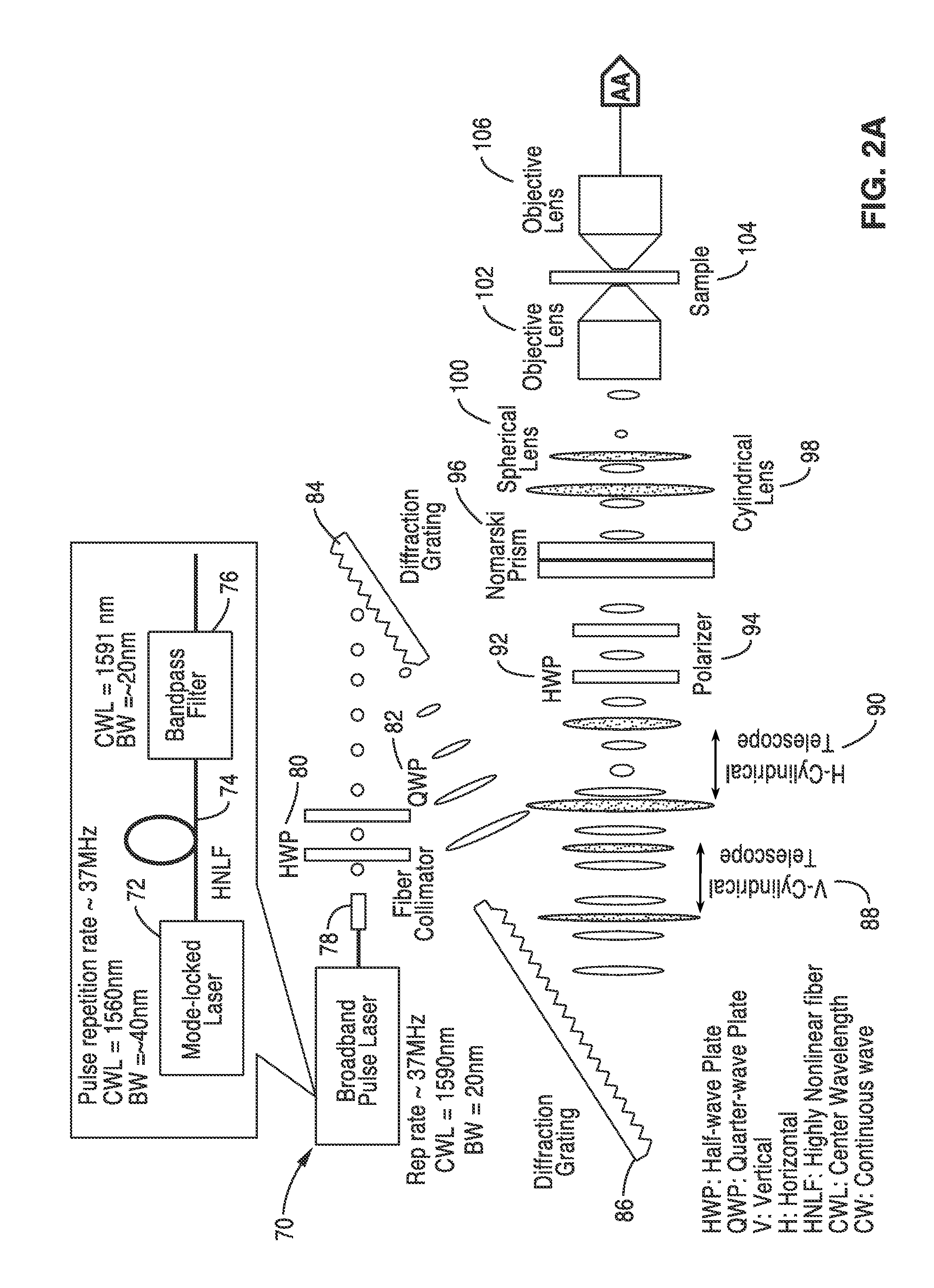
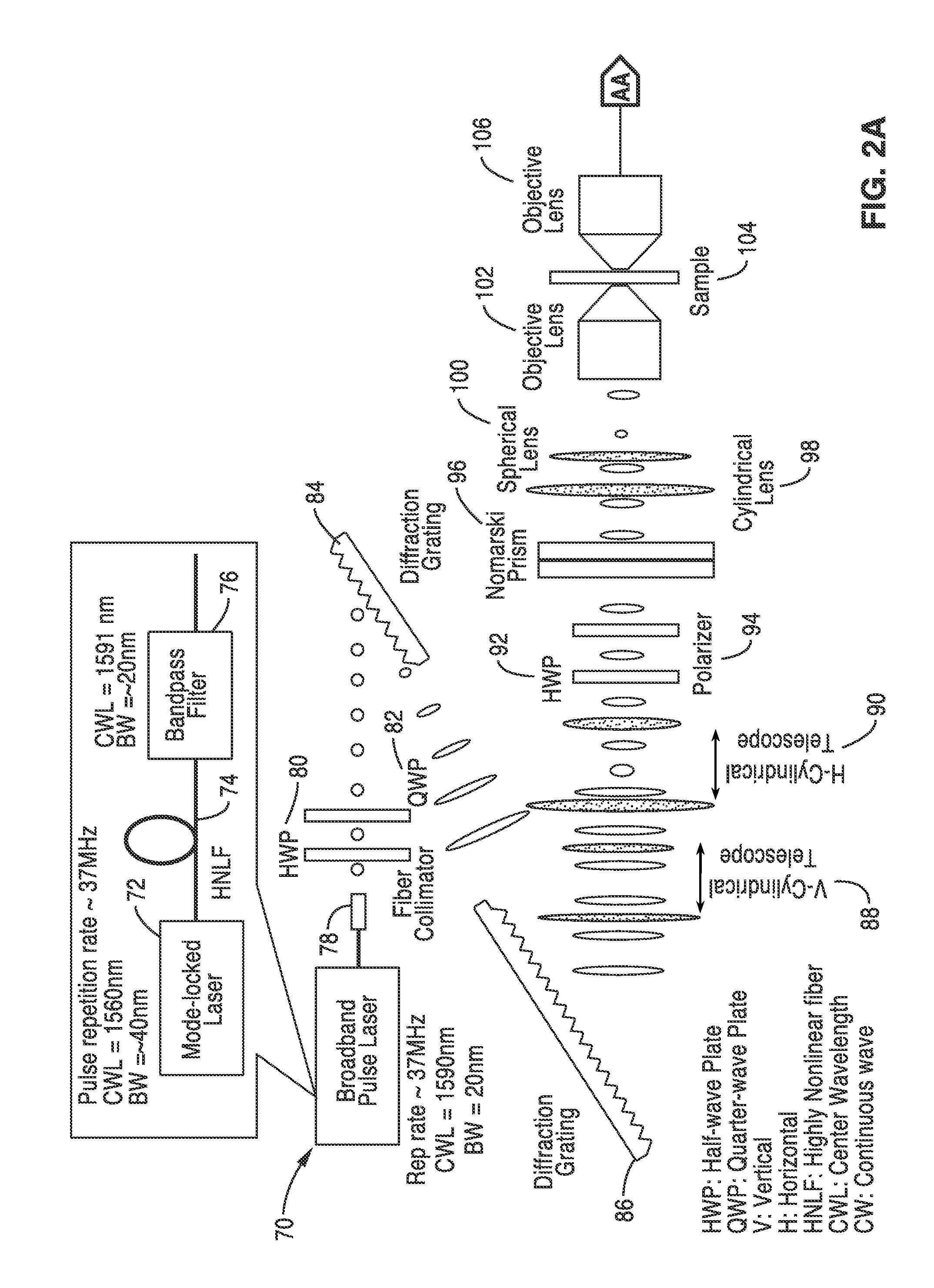
Differential interference contrast serial time encoded amplified microscopy
ActiveUS20130135529A1Revolutionize blood analysisShort stayTelevision system detailsColor signal processing circuitsStainingSerial time-encoded amplified microscopy
We describe methods and apparatus for high-speed high-contrast imaging one-, two- and three-dimensional imaging enabled by differential interference contrast time encoded amplified microscopy of transparent media without the need for chemical staining, that are suitable for a broad range of applications from semiconductor process monitoring to blood screening. Our methods and apparatus build on a unique combination of serial time-encoded amplified microscopy (STEAM) and differential interference contrast (DIC) microscopy. These methods and apparatus are ideally suited for identification of rare diseased cells in a large population of healthy cells and have the potential to revolutionize blood analysis and pathology including identification of cancer cells, such as Circulating Tumor Cells (CTC) in early stage disease.
Owner:RGT UNIV OF CALIFORNIA
Portable blood safety screening fluoroimmunassay system
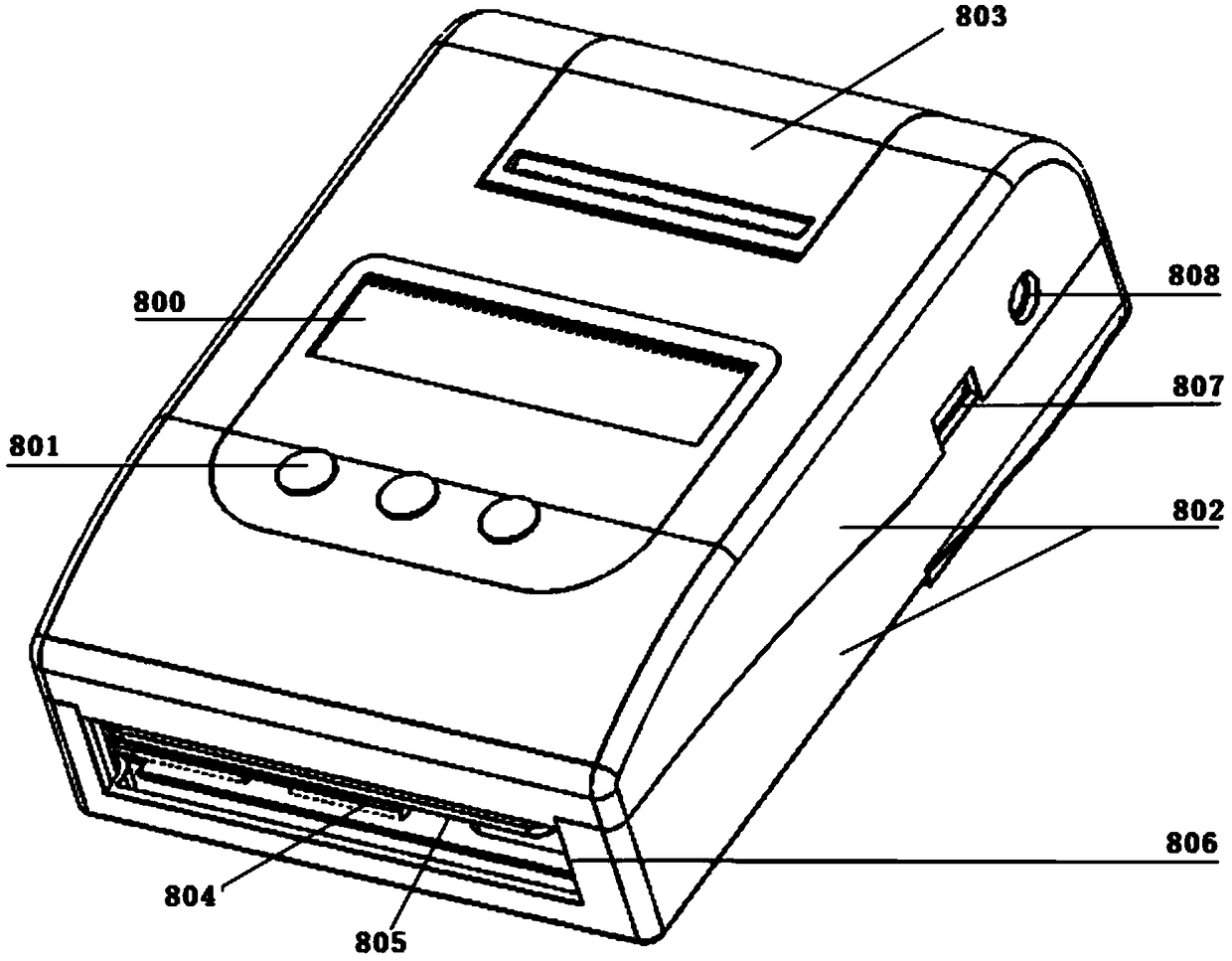
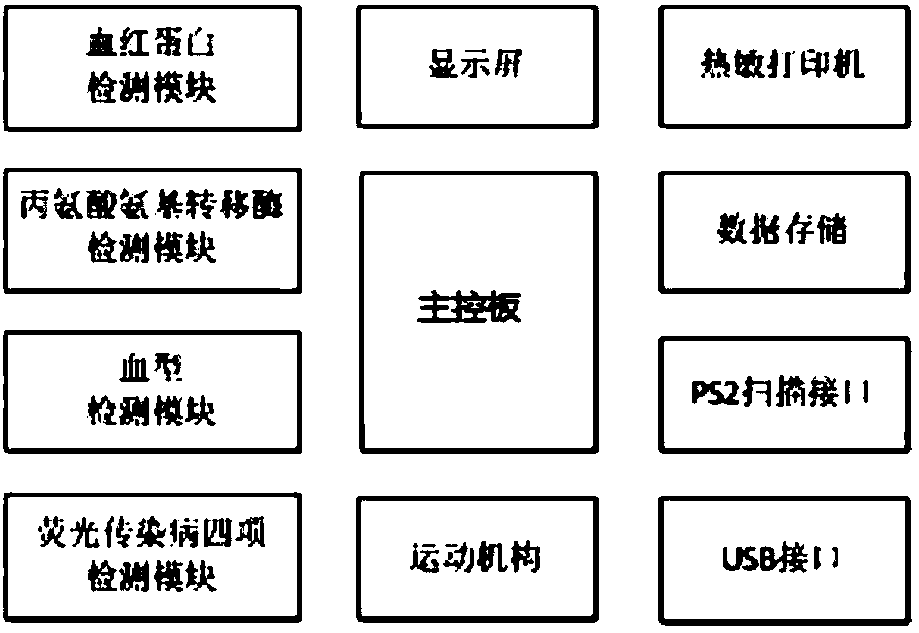
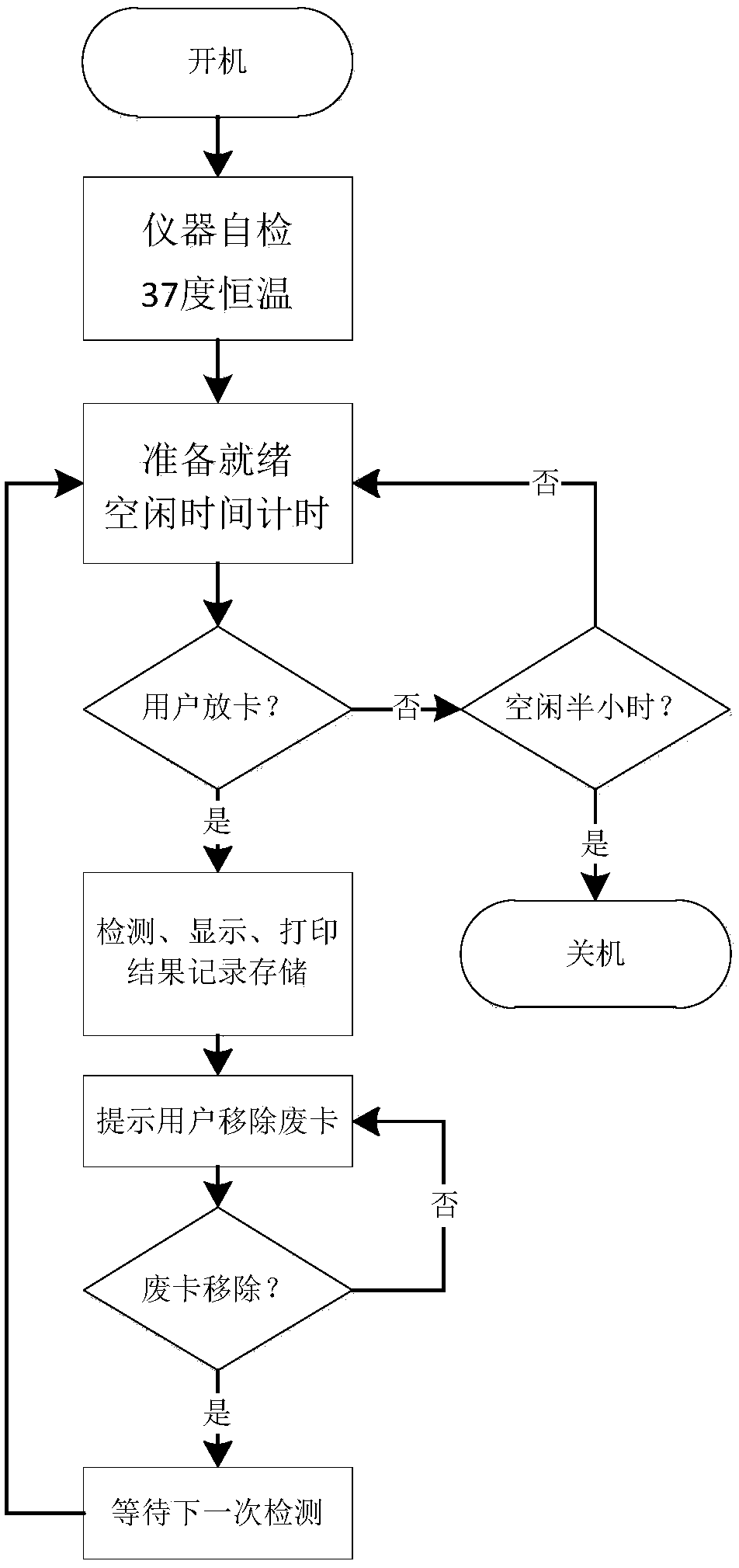
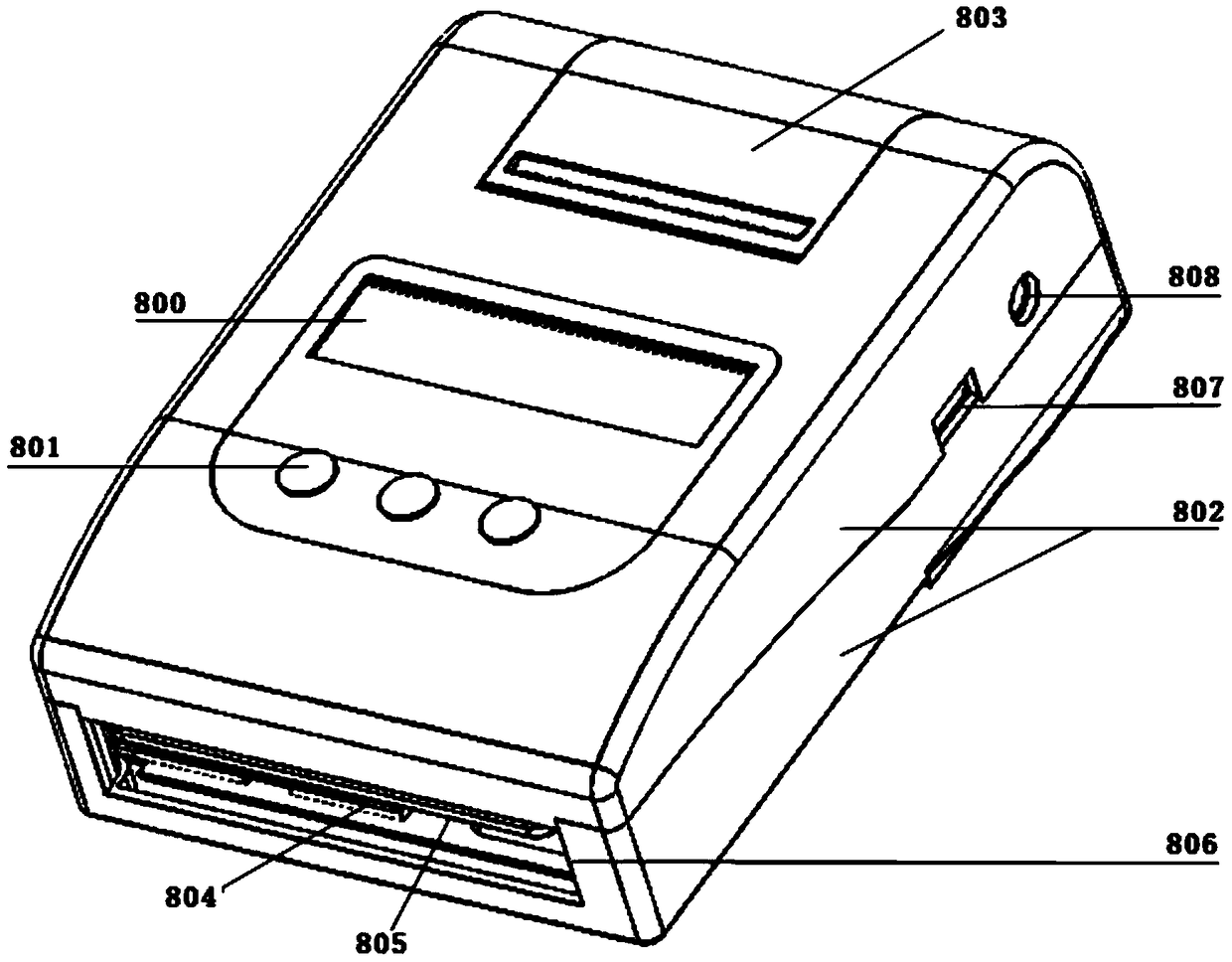
The invention discloses a portable blood safety screening fluoroimmunassay system. The portable blood safety screening fluoroimmunassay system comprises a portable blood fluoroimmunassay device and aportable blood detecting analysis card, wherein the portable blood fluoroimmunassay device is composed of a detecting mechanism, a master control panel, a data storage module and a detecting card trayfor conveying and carrying the portable blood detecting analysis card; the detecting mechanism comprises an alanine aminotransferase detecting module, a fluorescence infectious disease four test module, a blood type detecting module and a hemoglobin detecting module. For meeting the requirements on multiple tests during blood screening joint detection and by matching with a blood screening jointdetecting card, the portable blood safety screening fluoroimmunassay system can rapidly and accurately implement control over detecting reaction conditions and automatically complete interpretation and output of a number of detecting results.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +1
A blood screening combined detection card
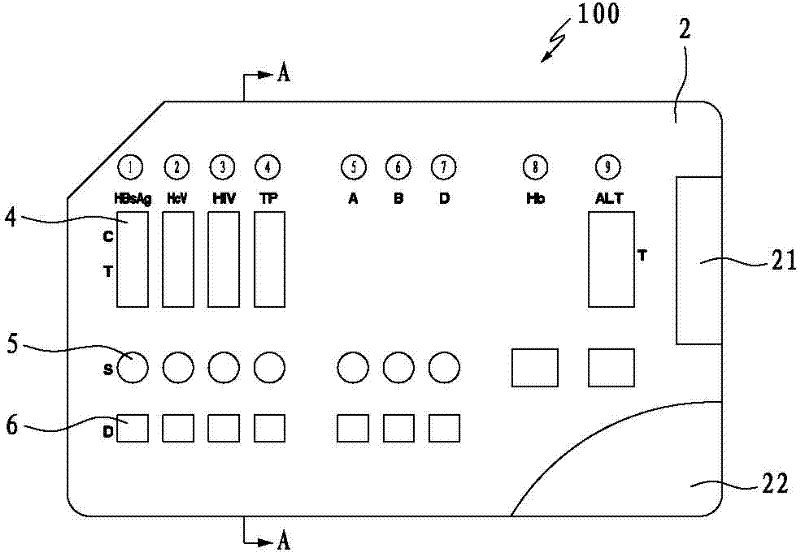
InactiveCN102288746AReduce the number of repetitions of the same operation stepsReduce the number of timesBiological testingMatching testMedicine
The invention discloses a combined detection card for blood screening, which includes a card base and a card cover fastened on the card base. The card base is provided with a plurality of card slots parallel to each other; the multiple card slots are divided into sequentially Multiple groups are arranged, and each group of card slots corresponds to the infectious disease detection area, blood type detection area, hemoglobin detection area and alanine aminotransferase detection area in sequence, and each card slot is equipped with a test strip matching its detection area; The card cover is sequentially provided with an observation window, a sampling hole and an auxiliary liquid hole at the positions corresponding to the test strips in the infectious disease detection area on the deck; There are sampling holes and auxiliary liquid holes in sequence on the position; the card cover is opened with a sampling hole at the position corresponding to the test strip in the hemoglobin detection area; the card cover is at the position corresponding to the test strip in the alanine aminotransferase detection area There are observation windows and sample injection holes on the top. The invention can realize the detection of multiple items in one card and can ensure the accuracy of detection.
Owner:INTEC PROD INC
Kit and method for extracting viral nucleic acid
InactiveCN108642044AHigh recovery rateEasy to operateMicrobiological testing/measurementDNA preparationMagnetic beadProteinase K
The invention discloses a kit and method for extracting viral nucleic acid from a blood screening mixed detection sample. The kit comprises a lysate, a binding solution, a first rinsing solution, a second rinsing solution, an eluent, silicon dioxide magnetic beads, proteinase K and nucleic acid settling agent, wherein the first rinsing solution contains the binding solution and sodium citrate. Theinvention further discloses a method for rapidly detecting viruses in plasma / serum. With the kit and the method provided by the invention, trace of viral nucleic acid can be easily captured from thesample under the specific pH, and the recovery rate of the obtained nucleic acid is high; the operation procedure is concise, the extraction and purification of viral nucleic acid can be completed within 30min, and thus the kit is more time-saving and labor-saving than the existing majority of commercial kits; the aimed sample volume dose is large, combined extraction can be carried out on small-volume samples, thus time is saved, and the reagent is saved.
Owner:GUANGZHOU YIXIN BIOTECH CO LTD
Blood ABD typing rapid comprehensive test card
ActiveCN103439518AShort detection timeEasy to carryMaterial analysis by observing effect on chemical indicatorBiological testingMedicineTyping
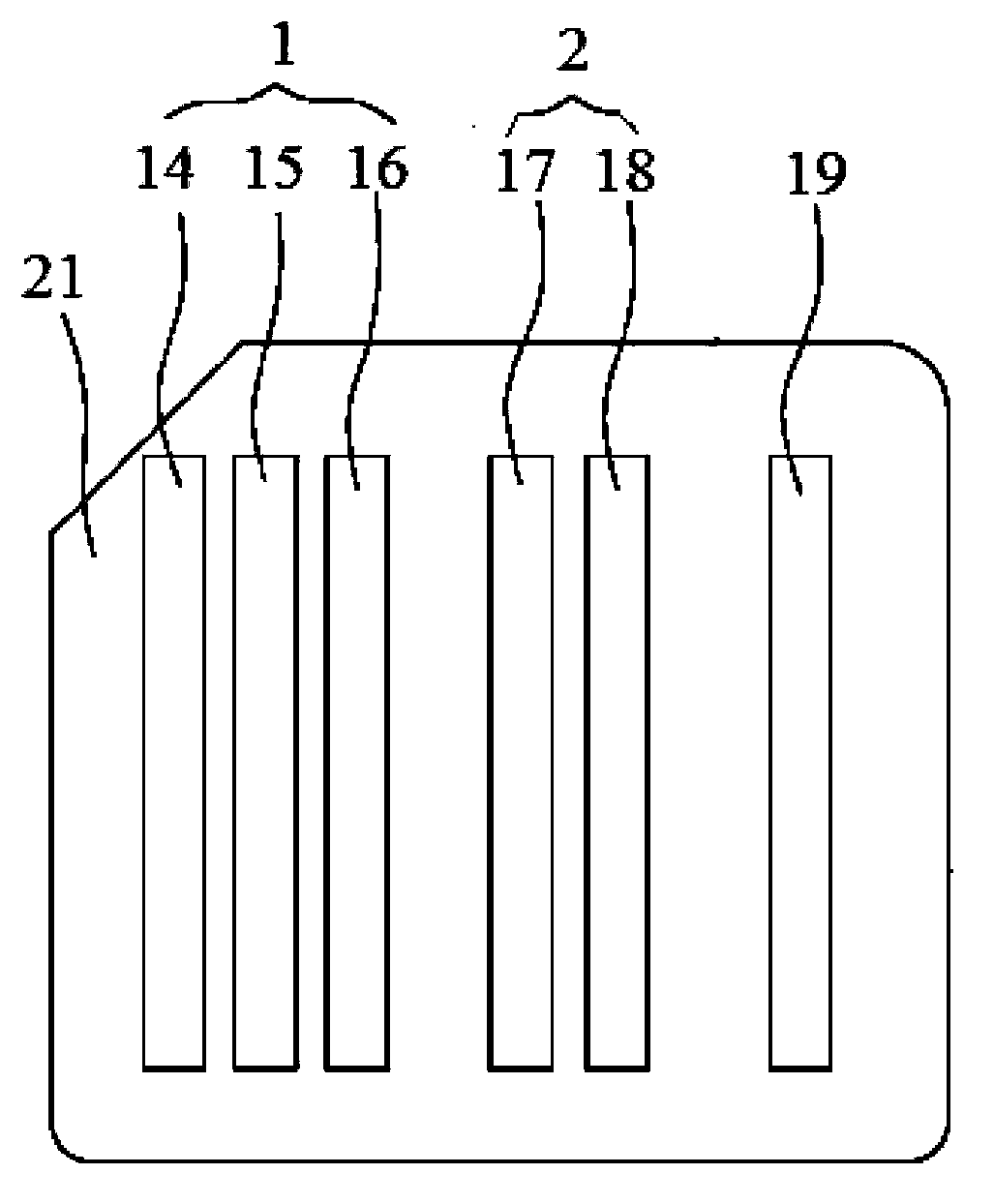
The invention discloses a blood ABD typing rapid comprehensive test card. A card seat (21) of the test card is provided with a plurality of card slots; the plurality of card slots are divided in to a plurality of groups arranged in order; the plurality of card slots are corresponding to a blood group forward typing test region (1), a blood group reverse typing test region (2) and a blood cross-matching test region (3) in sequence; each card slot is provided with a test strip matching the test region thereof; and a card cover is opened with a sampling hole (6) and a flushing hole (7) successively on the positions of each test strip corresponding to the blood group forward typing test region (1), the blood group reverse typing test region (2) and the blood cross-matching test region (3) on the card seat (21). The test card integrates blood group forward typing test, reverse typing test and blood cross-matching test on the same card for simultaneous tests, is short in test time, convenient for carrying, simple in operation, accurate and reliable in results, is suitable for being used in comprehensive tests of primary blood screening and blood group typing, and can also be applied in blood group typing test before transfusion in an emergency military period.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +1
Blood screening kit, blood screening method and blood screening device
InactiveCN105525035AReduced detection windowGuaranteed Screening Assay SensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsBlood ScreeningViral load
The invention provides a virus detecting kit, a blood screening method and a blood screening device. The virus detecting kit comprises at least two of six pairs of primers: SEQ ID NO:1 and SEQ ID NO:2, SEQ ID NO:3 and SEQ ID NO:4, SEQ ID NO:5 and SEQ ID NO:6, SEQ ID NO:7 and SEQ ID NO:8, SEQ ID NO:9 and SEQ ID NO:10 as well as SEQ ID NO:11 and SEQ ID NO:12. By the virus detecting kit, the blood screening method or the blood screening device, samples low in viral load can be detected, and accurate detecting results, low detection cost and short consumed time are achieved.
Owner:天津华大基因科技有限公司 +1
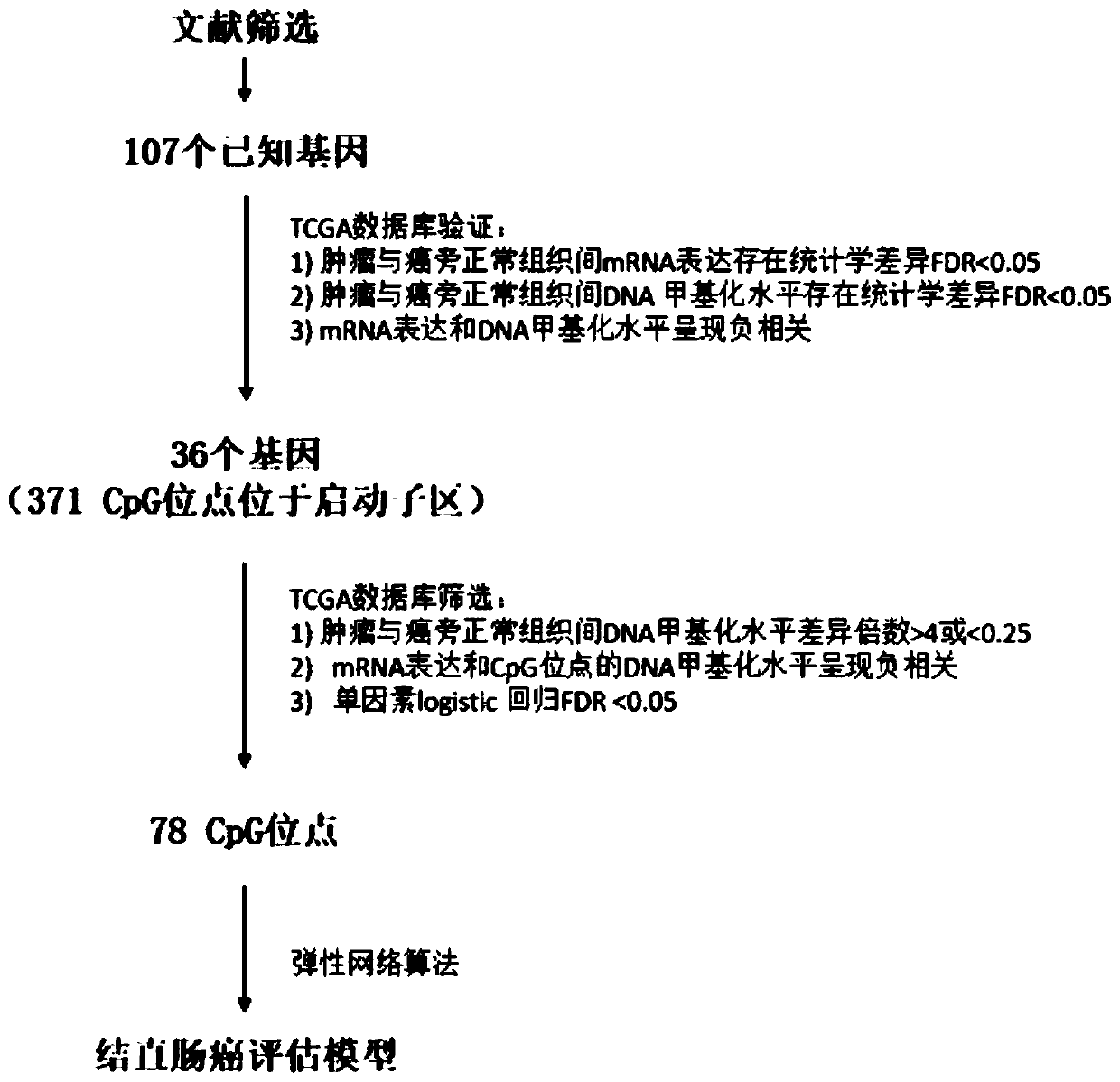
Colorectal cancer judgment model for distinguishing cancer tissue of colorectal cancer from paracancerous normal tissue and establishing method of colorectal cancer judgment model
The invention discloses a colorectal cancer judgment model for distinguishing cancer tissue of colorectal cancer from paracancerous normal tissue and an establishing method of the colorectal cancer judgment model. The method includes: selecting 107 genes related to colorectal cancer with methylation differences in the promoter region of the genes; verifying the expression of the 107 genes and the level of methylation in the promoter region by the aid of patients with both methylation and gene expression data in a TCGA (the cancer genome atlas) database, wherein a total of 37 genes are successfully verified, and 371 CpG sites are located in the promoter regions thereof; screening the 371 CpG sites by the aid of the data in the TCGA database, wherein a total of 78 CpG sites is selected; for the 78 CpG sites and on the basis of the data in the TCGA database, establishing the colorectal cancer judgment model by the aid of the elastic network algorithm. With the method, a theoretical basis is provided for blood screening of colorectal cancer in the future.
Owner:ZHEJIANG UNIV
Device and method for in-line blood testing using biochips
InactiveUS7785782B2Large fractionBioreactor/fermenter combinationsBiological substance pretreatmentsNucleic acid amplification techniqueDisease
A device for in-line blood screening and testing using biochips is disclosed. The screening methods include nucleic acid amplification techniques and antibody / antigen assays to detect target molecules and agents indicative of infectious diseases or metabolic diseases.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Real-time fluorescent quantitative PCR (polymerase chain reaction) detection method for three genotypes of human parvovirus B19, as well as universal detection primer, TaqMan probe and kit thereof
ActiveCN103451320AImprove accuracyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationWhole blood productGenotype
The invention discloses a real-time fluorescent quantitative PCR (polymerase chain reaction) detection method which can be used for simultaneously performing qualitative and quantitative detection on three genotypes of human parvovirus B19, as well as a universal detection primer, a TaqMan probe and a kit thereof. The universal detection primer and the TaqMan probe disclosed by the invention can be combined with real-time fluorescent quantitative PCR to realize the purpose of accurately, qualitatively and quantitatively detecting B19 virus DNA (deoxyribonucleic acid) of the three genotypes of a sample to be detected, a formed NAT (nucleic acid testing) detection method against the three genotypes of B19 virus on the basis is obviously better than the existing detection technology, and the real-time fluorescent quantitative PCR detection method has the advantages of high accuracy, low pollution, fast detection speed, low requirements on instruments and equipment, low cost and the like; in addition to clinical detection and blood screening, the real-time fluorescent quantitative PCR detection method can also be used for performing qualitative and quantitative analysis on pollution status of the B19 virus in raw material plasma and blood products in scientific research and production and further has important significance in ensuring virus safety of the blood products.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
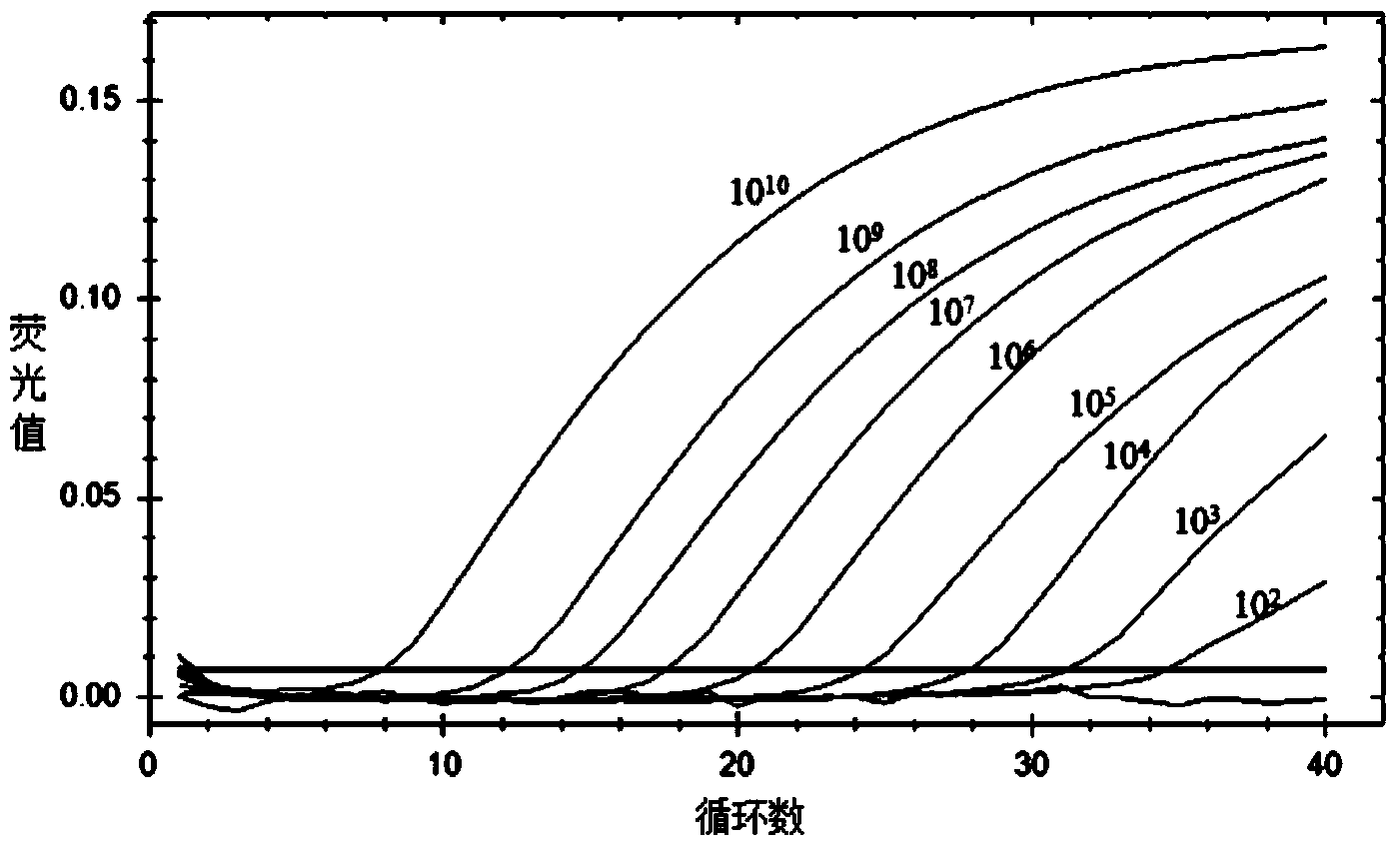
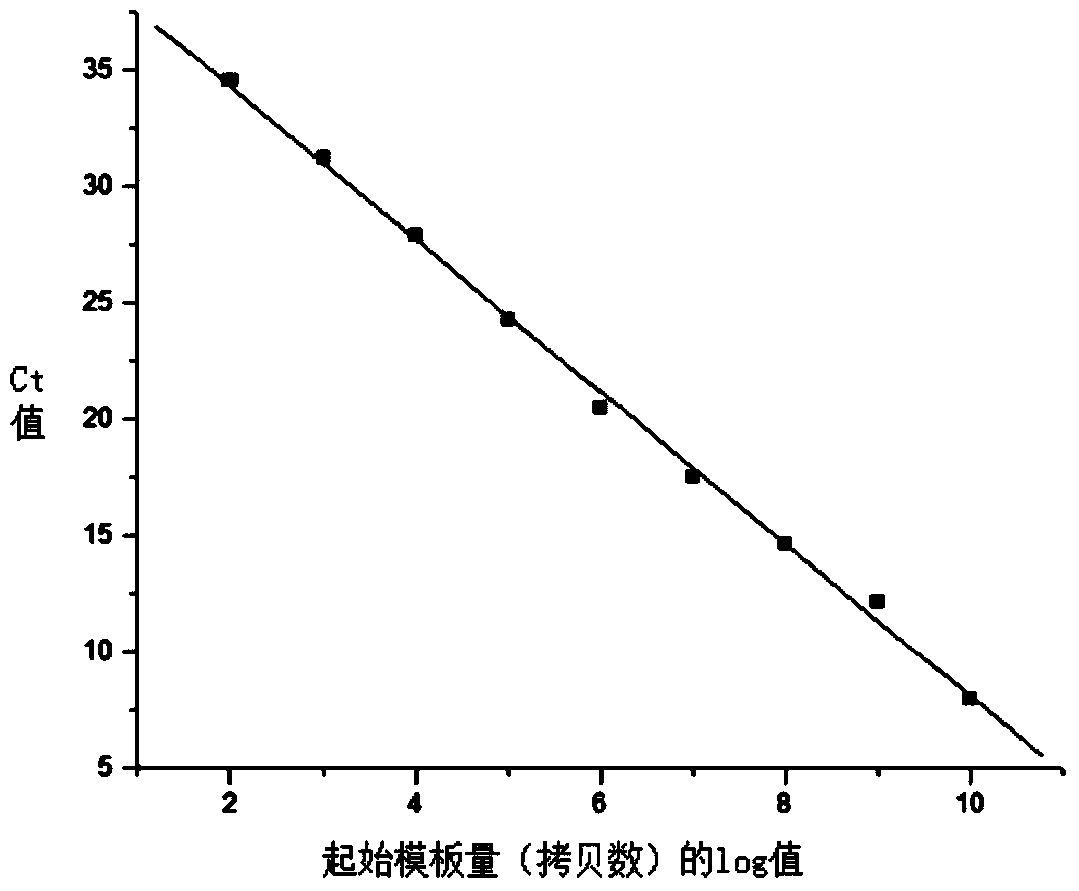
Gene detection kit for Thailand type alpha-thalassemia
ActiveCN102943116AEasy to useImprove accuracyMicrobiological testing/measurementForward primerBlood Screening
The invention relates to the field of biological medicine, and in particular relates to a gene detection kit for quickly detecting Thailand type alpha-thalassemia in a clinic sample. The technical scheme of the gene detection kit aims at providing the gene detection kit for the Thailand type alpha-thalassemia, wherein the gene detection kit comprises PCR (polymerase chain reaction) liquid, the PCR reaction liquid comprises a forward primer THAI-F and a reverse primer THAI-R, the primers are the forward 10724-10725 position and the reverse 1219-1220 position of the breaking point position aiming at the Thailand type alpha-thalassemia, and the primers are respectively designed at the forward 5'-end and the reverse 3'-end of the breaking point. According to the kit provided by the invention, the leak detection of the alpha-thalassemia caused by the common blood screening method can be reduced, the birth of the children who suffer from the heavy type alpha-thalassemia can be reduced or avoided, and a condition can be created for the more comprehensive thalassemia screening. The detection kit provided by the invention is convenient to use, high in accuracy, and good in social benefit and economic benefit in the high incidence area of the thalassemia.
Owner:亚能生物技术(深圳)有限公司
Constant temperature synchronous amplification detecting process for nucleic acid and use thereof
ActiveCN101333565BMicrobiological testing/measurementFluorescence/phosphorescenceNegative strandFluorescence
The invention discloses a constant temperature synchronous amplified detection method of nucleic acid as well as an application thereof. The method comprises the following steps of: 1) mixing reactants containing the following components: a. a nucleic acid sample under test; b. a primer 1: a 3`-terminal of the primer can be hybridized at a 3`-terminal or near the 3`-terminal of the nucleic acid sample under test, and a 5`-terminal is a promotor sequence; c. a primer 2: the primer can be hybridized with a 5`-terminal of a negative strand of the nucleic acid sample under test; d. one or a plurality of fluorescent probes; e. at least one DNA polymerase relied by RNA; and f. at least one RNA polymerase that can identify the promotor sequence; and 2) carrying out the constant temperature amplification reaction to the mixed reactants in a closed vessel, detecting the changes of fluorescence signals in a reaction system synchronously by a detector, and conducting the quantitative and qualitative detection to the nucleic acid sample according to the time and intensity of fluorescence signal changing. The method and the application have the advantages of low pollution, constant temperaturein the reaction process, high detection sensitivity, fast detection speed, low requirements on equipment and instrument and low cost, and are applied to nucleic acid testing in fields such as clinicalexamination and blood screening.
Owner:SHANGHAI RENDU BIOTECH
Specific primer and probe for detection of HTLV-I and HTLV-II and fluorescent quantitative PCR detection kit
InactiveCN108486281ARelieve painReduce the likelihood of infectionMicrobiological testing/measurementMicroorganism based processesBlood ScreeningDNA
The invention provides a specific primer and probe for simultaneous detection of HTLV-I and HTLV-II. The probe sequence is 5'-AGTGCCAAAGACCCTTCCTGGGCCTCT-3'. The specific primer is the following sequence or the complementary chain sequence of the following sequence, the upstream primer sequence is 5'-CCTTATATCAGAGGCCGAA-3', and the downstream primer sequence is 5'-TCTGGCAGCCCATTGTCAA-3'. Accordingto the specific primer and the probe, by extracting DNA in a to-be-detected sample and by combining the specific primer and the probe and a real-time fluorescent quantitative PCR detection technique,the purpose that HTLV-I and HTLV-II in the to-be-detected sample are accurately quantification can be achieved; and the infection states of HTLV-I and HTLV-II can be detected simultaneously only through one sample, and the specific primer and the probe are suitable for large-scale blood screening work in both cost and efficiency.
Owner:SUZHOU BAIYUAN GENT CO LTD
Viral nucleic acid extraction reagent
ActiveCN103695419AFix extraction flawsSimple methodMicrobiological testing/measurementDNA preparationViral nucleic acidBlood Screening
The invention firstly provides a biotin-labeled specific probe which is a biotin-labeled hepatitis c virus probe and has a sequence of biotin-(T)n-TGGTACTGCCTGATAGGGTGCTTGCG, wherein n is the number selected from 3 to 30, preferably 5 to 20. Correspondingly, the invention further provides a viral nucleic acid extraction reagent which comprises the biotin-labeled specific probe. The invention further provides the biotin-labeled hepatitis c virus probe and a biotin-labeled human immunodeficiency virus probe. The viral nucleic acid extraction reagent comprising the three biotin-labeled specific probes can be used for blood screening for three viruses.
Owner:SANSURE BIOTECH INC
Nucleic acid composition and test kit for blood screening
ActiveCN111206123AReduce the risk of missed detectionReduce distractionsMicrobiological testing/measurementAgainst vector-borne diseasesHematological testBlood Screening
The invention relates to the field of virus molecular inspection, and particularly provides a nucleic acid composition and a test kit for blood screening. The nucleic acid composition used in the blood screening provided by the invention comprises a nucleic acid composition for detecting HBV, HCV, HIV and COVID-2019. Researches of the inventor prove that the nucleic acid composition provided by the invention is strong in specificity, high in sensitivity and strong in interference resistance, and can realize rapid detection of HBV / HCV / HIV-1 / HIV-2 / SARS-Cov-2 in blood screening. Specific double-segment nucleic acid combination design is carried out on HIV-1 and HBV genomes, the situation of missing detection caused by virus gene mutation is greatly reduced, the detection rate is greatly increased, the residual risk of transfusion infection is reduced, screening is carried out on SARS-Cov-2 genomes, and the interference on the detection of HBV and HCV in the combination is obviously reduced. The blood screening detection test kit containing the nucleic acid combination provided by the invention has the advantages of high detection rate, good specificity and strong interference resistance.
Owner:ZHUHAI LIVZON DIAGNOSTICS
Aptamer with affinity to viral hepatitis C core antigen and application thereof
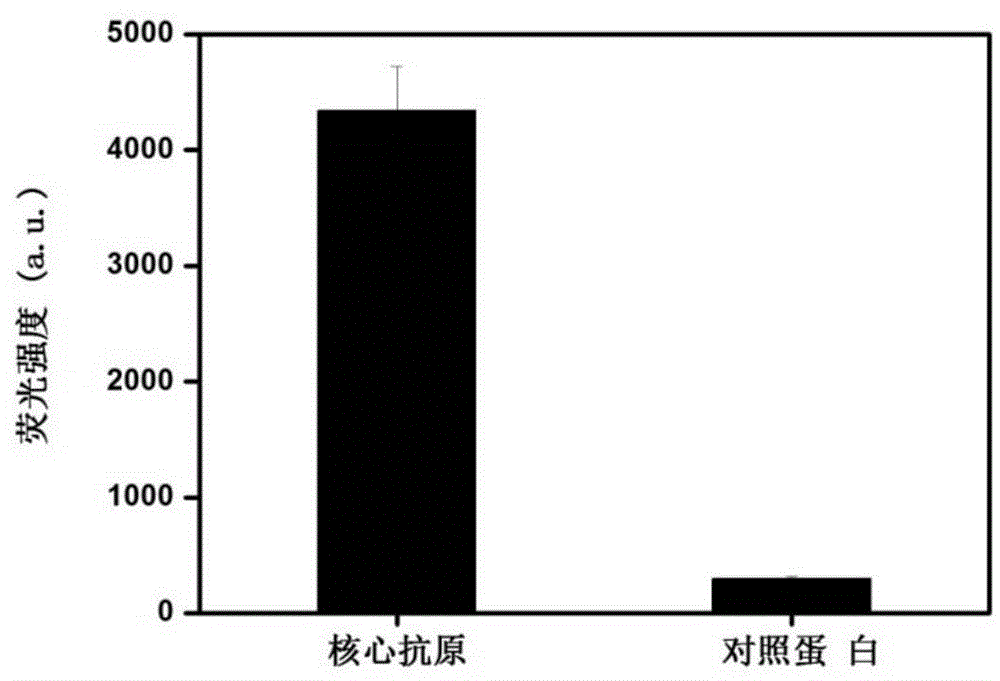
ActiveCN105483132ARealize detectionNew method for benefit development detectionMaterial analysisDNA/RNA fragmentationAntigenMonoclonal antibody
The invention discloses an aptamer with affinity to viral hepatitis C core antigen and application thereof. The aptamer provided by the invention is following (a) or (b): single-stranded DNA as shown in sequence 2 of following sequence table (a); single-stranded DNA containing the aptamer described in (a). The aptamer can be used to capture the viral hepatitis C core antigen in a solution and to detect the viral hepatitis C core antigen in the solution, facilitating serological diagnosis and blood screening of viral hepatitis. The aptamer can be used to partially replace a monoclonal antibody, capturing a core antigen to detect viral hepatitis C, and the aptamer has the advantages of high sensitivity, low cost, ease of preparation and ease of storage. The aptamer has high applicable value.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Immunoassay for plasmodium falciparum and assay device used therefor
InactiveUS20090197347A1Confirm presenceHigh sensitivity and specificityBiological material analysisAgainst vector-borne diseasesBlood ScreeningBlood plasma
Disclosed are an immunoassay of Plasmodium falciparum for determining the presence / absence of a specific and / or antibody thereof via label in conjugates bound to the specific antigen and / or antibody present in a sample, comprising immobilizing the specific antigen and antibody of Plasmodium falciparum on a solid phase, adding a sample obtained from a subject of interest to the solid phase so as to induce specific antibody-antigen reaction, adding a conjugate of the antigen and a label and a conjugate of the antibody and a label, separately prepared, so as to induce binding of at least one of the conjugates; and an assay device comprising the above-mentioned solid phase and conjugates.The present invention can effect specific detection of antigens and / or antibodies in patients with manifested malaria-symptoms as well as malaria carriers and can also be efficiently employed in samples at the early stage of malaria infection that is difficult to detect via conventional arts. Further, due to the capacity to utilize sera and blood plasma rather than whole blood, the present invention is well suited to large-scale examination such as blood screening.
Owner:LG LIFE SCI
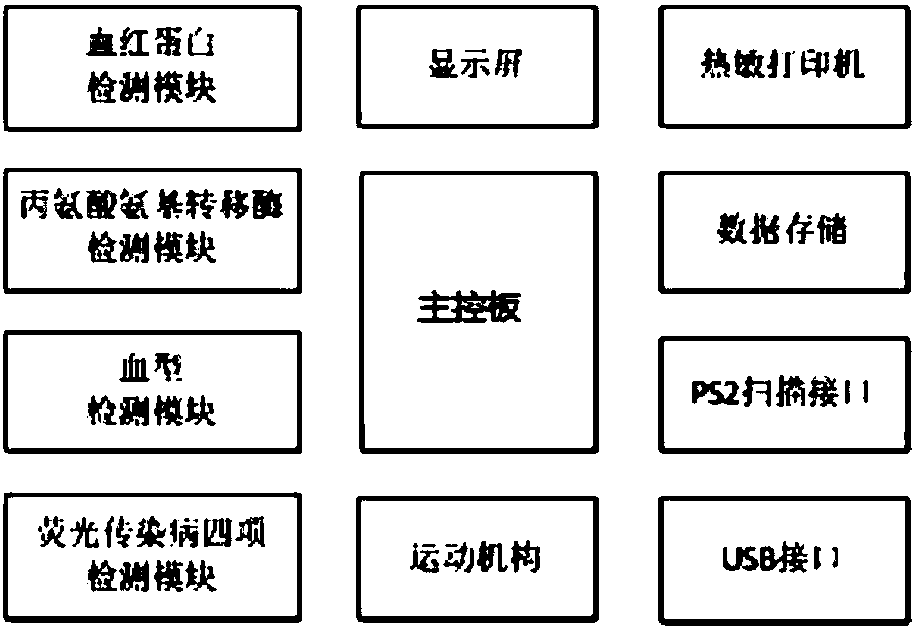
Portable type blood fluorescence immunoassay analyzer
PendingCN108362674AReduce volumeReduce weightBiological testingFluorescence/phosphorescenceGroup A - bloodAlanine aminotransferase
The invention discloses a portable type blood fluorescence immunoassay analyzer. The portable type blood fluorescence immunoassay analyzer comprises a detecting mechanism, a master control board, a data storage module and a detecting card tray used for transmitting a platform deck detecting card, wherein the detecting mechanism comprises an alanine aminotransferase detecting module, a fluorescentinfectious disease four-item detecting module, a blood type detecting module and a hemoglobin detecting module. The portable type blood fluorescence immunoassay analyzer is used for multi-item detection which is required in a blood screening joint detecting process, is matched with a blood screening joint detecting card, and can be used for quickly and accurately controlling reaction condition dection and automatically completing judgment and output of multi-item detected results.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +1
Reagent kit for synchronously detecting hepatitis, AIDS virus and syphilis helicoid nucleic acid
ActiveCN101487062BShort processing timeImprove energy efficiencyMicrobiological testing/measurementBlood centerBlood Screening
The invention discloses a kit which can synchronously diagnose hepatitis, AIDS virus and treponema pallidum nucleic acid, and relates to a gene diagnosis kit. The invention comprises: (1) pathogene concentrated solution and DNA and RNA nucleic acid simultaneous extraction liquid; (2) positive plasmid with the diagnose sequence of four pathogens comprising hepatitis b virus, hepatitis c virus, AIDS virus and treponema pallidum; and (3) TaqMan PCR amplified protocol diagnosed by the combination of one-step monosiphonous and single enzyme. The invention carries out simultaneous and real-time diagnosis, and has the advantages of having short flow time, high efficiency and high sensitivity, and avoiding the possible pollution and artificial errors owing to the simple operation and close diagnosis,. The kit is suitable to large-scale and high-flux blood screening, such as the nucleonic acid screening in blood center and biological product manufacturer, and is also suitable to high-capacity and high-efficient clinical nucleic acid examination.
Owner:WUHAN ZHENFU PHARMA CO LTD
Method for extracting specific nucleic acid in blood screening reagent
InactiveCN101497927AStrong specificityAvoid false positivesSugar derivativesMicrobiological testing/measurementMicro nanoFluorescence
The invention discloses an extraction method for specific nucleic acid in a blood screening reagent. The method comprises the following steps: 1.5 to 100 microns of micro-nano-magnetic beads containing chain avidin are coated with HBV, HCV and HIV specific nucleic acid sequences; the micro-nano-magnetic beads coated with the specific nucleic acid sequences are hybridized with the nucleic acid from the disrupted HBV, HCV and HIV viruses in blood samples; a nucleic acid purification instrument is used to separate the magnetic beads from the blood samples, the magnetic beads are washed with so that elute HBV, HCV and the HIV virus nucleic acids are washed off the magnetic beads; and the content of viruses is detected by fluorescence quantitative PCR. The adopted method is favorable for improving the specificity of nucleic acid extraction, avoids the false-positive detection results caused by the homology of all types of virus nucleic acid sequences so that to more accurate detection results are obtained, and can be widely used in blood screening.
Owner:HANGZHOU BIOER TECH CO LTD
Blood screening three-item multi-color single tube detection kit
InactiveCN107287348AIncrease the Tm valueSimple designMicrobiological testing/measurementMicroorganism based processesFluorescenceEnzyme system
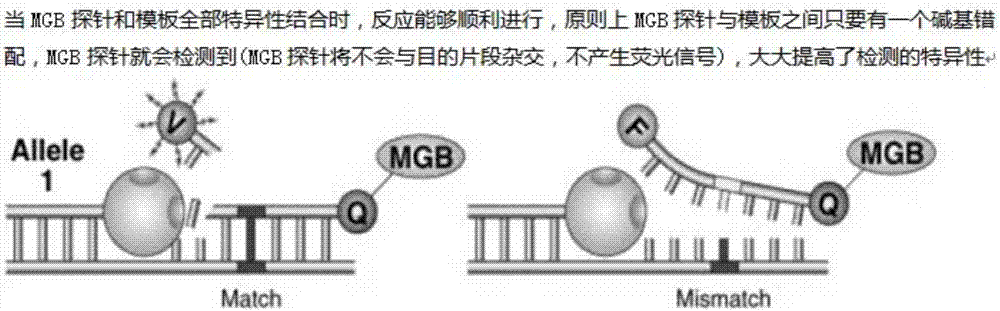
The invention discloses a blood screening three-item multi-color single tube detection kit. A blood screening three-item multi-color single tube detection reagent method comprises the steps of (1) using matched Guangzhou and real biological nucleic acid extraction reagents (a paramagnetic particle method), strictly according to the specification and carrying out automatic nucleic acid extraction; (2) designing a specific primer and a corresponding MGB fluorescent probe for an HBV, an HCV and an HIV (blood screening three items); (3) mixing the specific primer, the MGB probe and a fast enzyme system for detecting blood screening three items and carrying out amplification; and (4) detecting a fluorescent change of a reaction system and determining whether HBV, HCV and HIV infection exists or not. The method is simple in operation, and is capable of quickly detecting whether HBV, HCV and HIV infection exists or not at the same time. The detection method is multi-color single tube detection, three items of the HBV, the HCV and the HIV can be detected in PCR reaction of a same tube at the same time, and the three-item detection process is greatly simplified. The probe used for the detection method is the MGB probe, so that the detection sensitivity and specificity are greatly improved. The fast enzyme system is also applied to the detection method, so that the time required for reaction is greatly shortened and the work efficiency is improved.
Owner:GUANGZHOU HEAS BIOTECH CO LTD
Internet-based real-time shared blood screening indoor quality control system and method
ActiveCN111696655AEasy to distinguishSolve indoor quality control problemsHealthcare resources and facilitiesNucleic acid detectionQuality control system
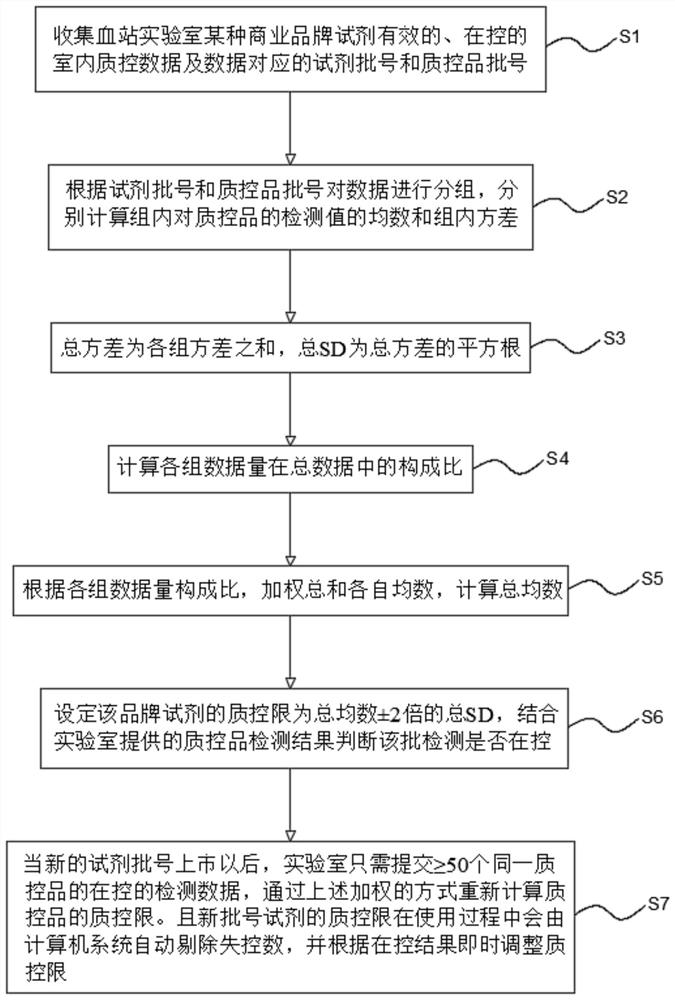
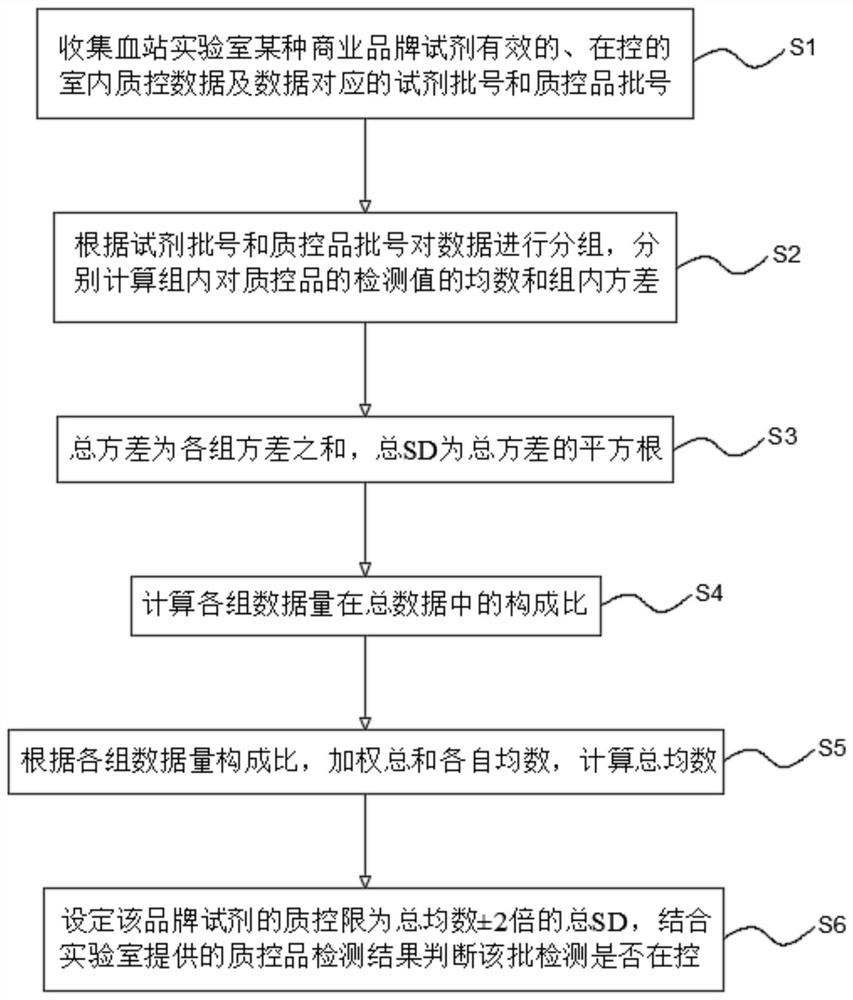
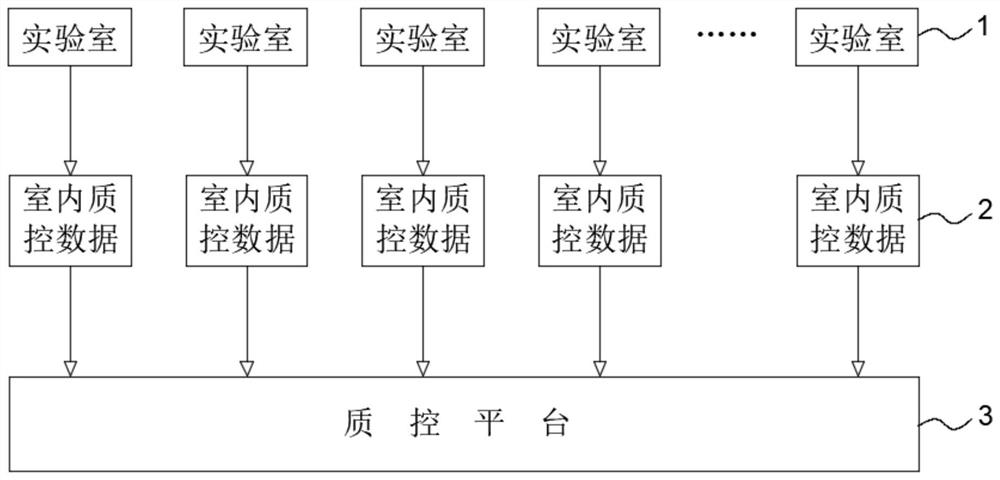
The invention relates to an internet-based real-time shared blood screening indoor quality control system and method. The method comprises the following steps: collecting effective and controlled indoor quality control data of a plurality of laboratory reagents of a certain commercial brand and reagent batch numbers and quality control product batch numbers corresponding to the data; grouping thedata according to the reagent batch number and the quality control product batch number; respectively calculating the mean number and the intra-group variance of the detection values of the intra-group quality control products; calculating the total SD; calculating the composition ratio of each group of data volume in the total data; calculating the total average number according to the data volume composition ratio of each group and the respective average number of the weighted sum; setting the quality control limit of the reagent to be + / -2 times of the total average number of total SD, wherein the detection result of the quality control product is in-control within the quality control limit, otherwise, the detection result is out of control. The system and the method provided by the invention can be used for serological detection of infectious disease markers and indoor quality control of nucleic acid detection in blood screening and clinical detection, can provide more accurate judgment results, and realizes real-time online analysis and shared analysis of indoor quality control results in reagent groups.
Owner:SHANGHAI BLOOD CENT
Method of extracting low-abundance viral nucleic acid
InactiveCN104073484AInteractive stabilityHigh affinityDNA preparationBiotin-streptavidin complexLysis
A method of extracting low-abundance viral nucleic acid is disclosed. The method includes steps of: adding a lysis liquid into blood to release viral nucleic acid into the solution; adding a biotin-labeled specific nucleic acid fragment capable of hybridization with the viral nucleic acid; adding streptavidin-labeled nanometer magnetic beads, wherein streptavidin on surfaces of the magnetic beads can be combined with biotin to form a magnetic bead-streptavidin-biotin-specific nucleic acid fragment-viral nucleic acid composite; and washing the magnetic beads with a washing liquid to remove the viral nucleic acid from the magnetic beads. By adoption of the method, the extraction efficiency and the yield of nucleic acid in low-titer viral samples can be increased so as to reduce false negative detection results due to the low extraction efficiency. The method is particularly suitable for clinic blood screening, and other applications.
Owner:SHANGHAI ENSURE BIOLOGICALS
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com