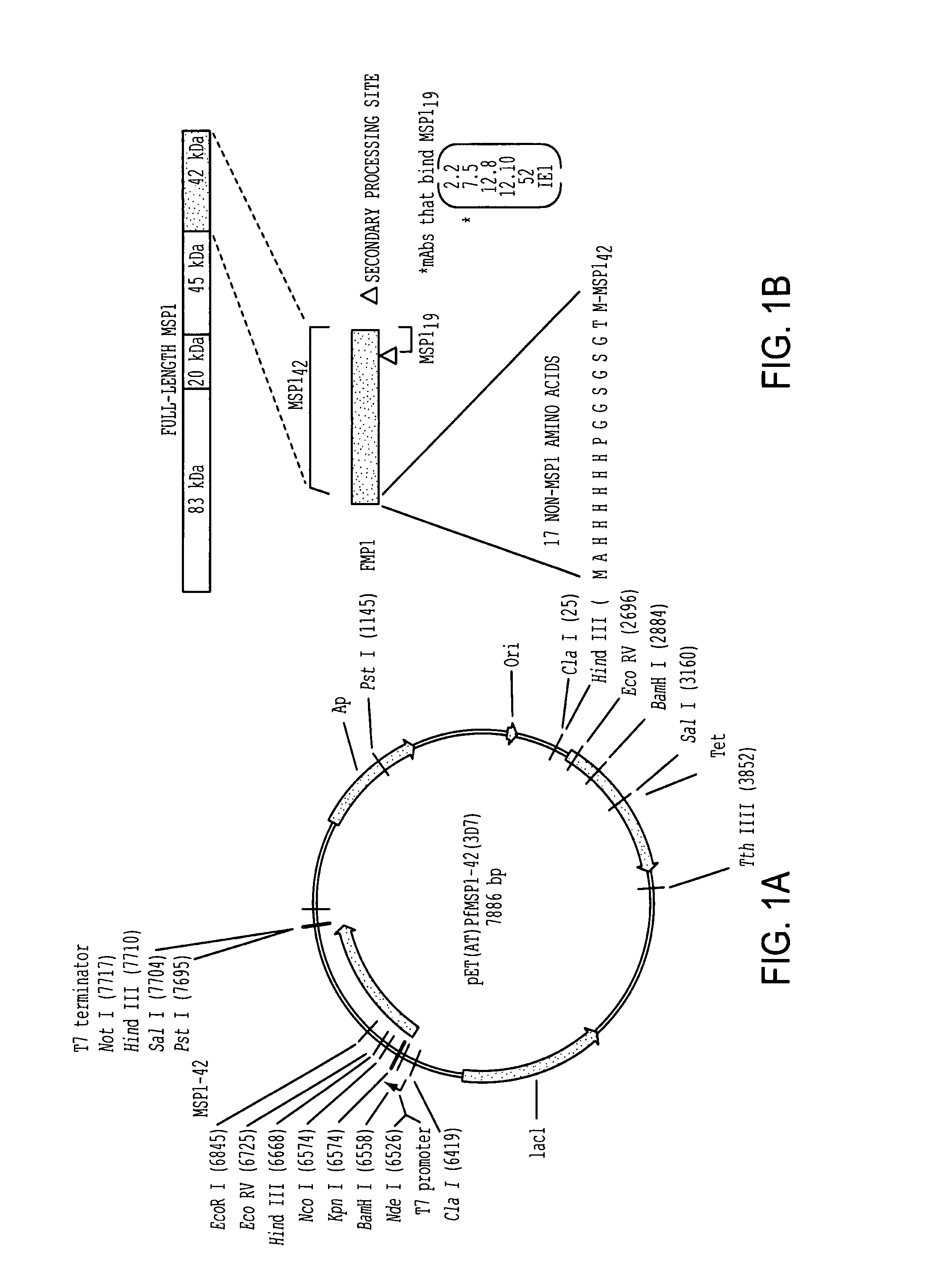
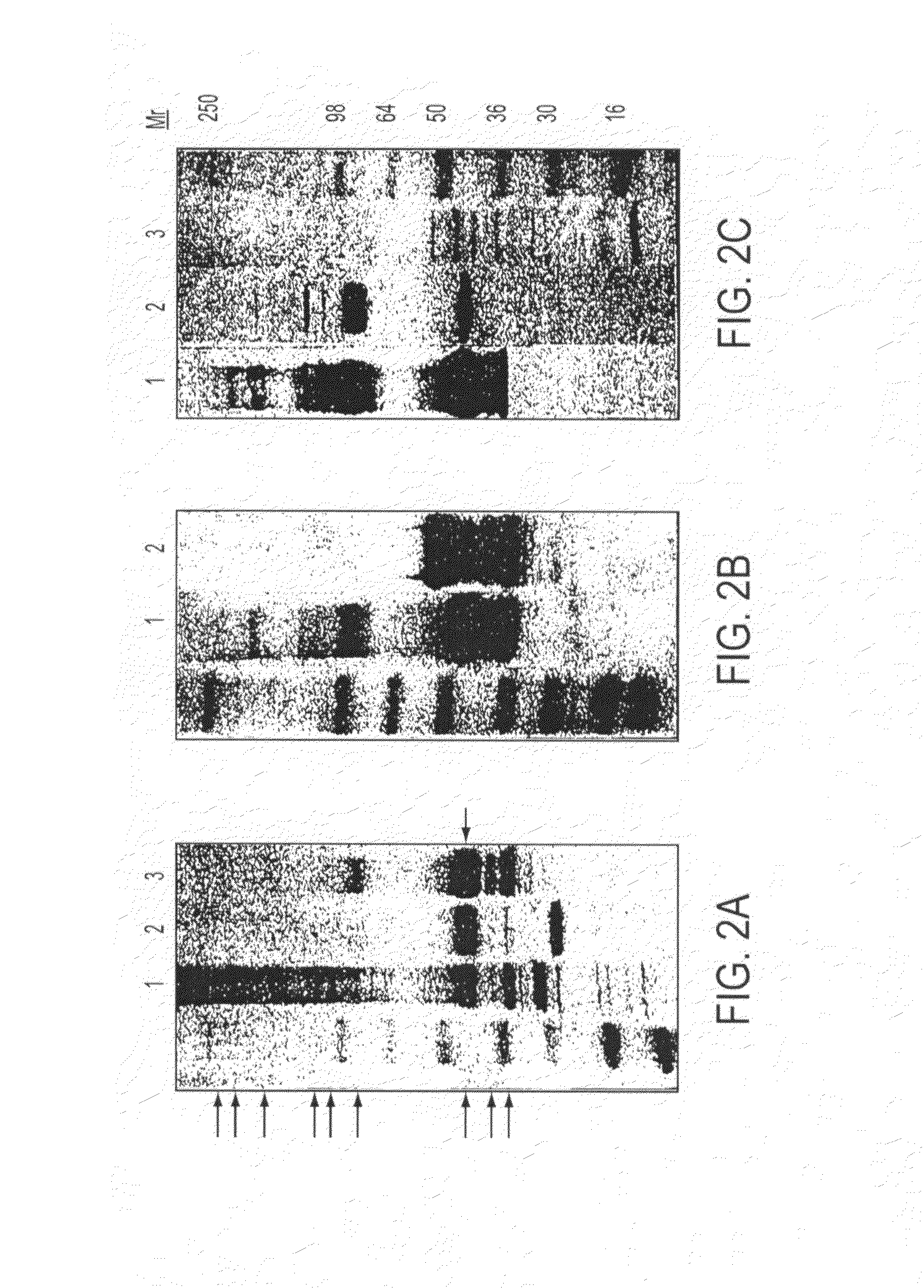
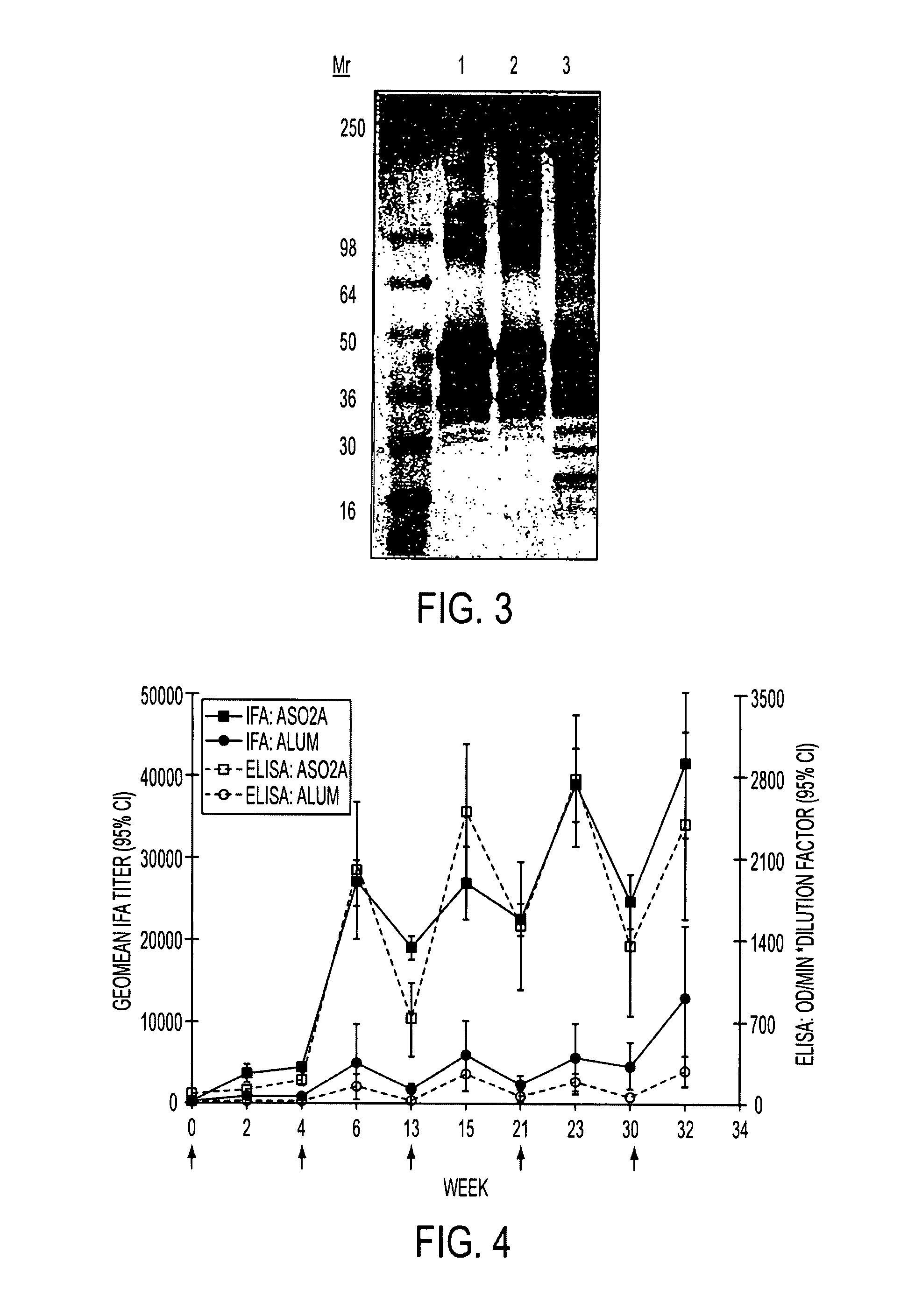
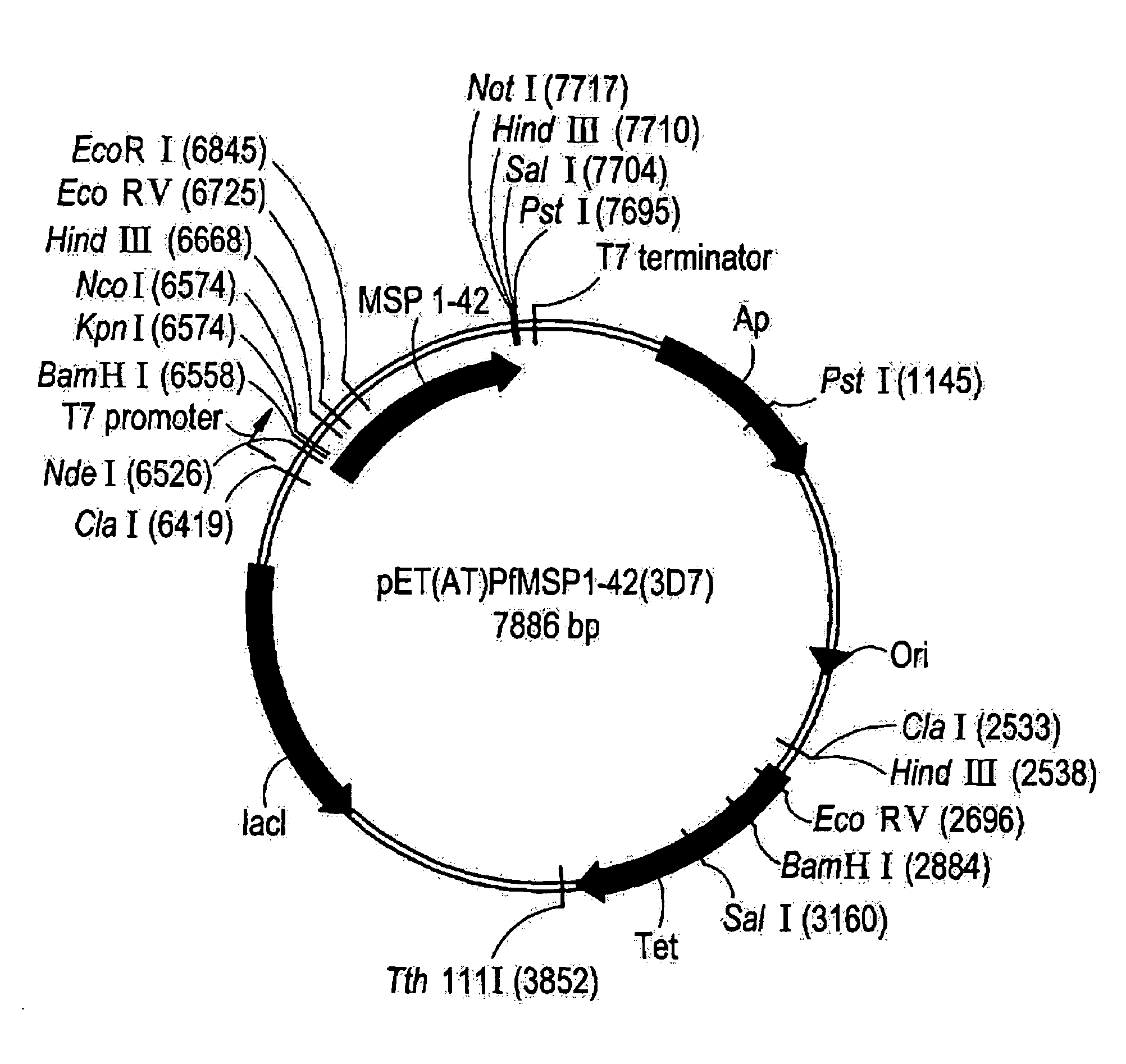
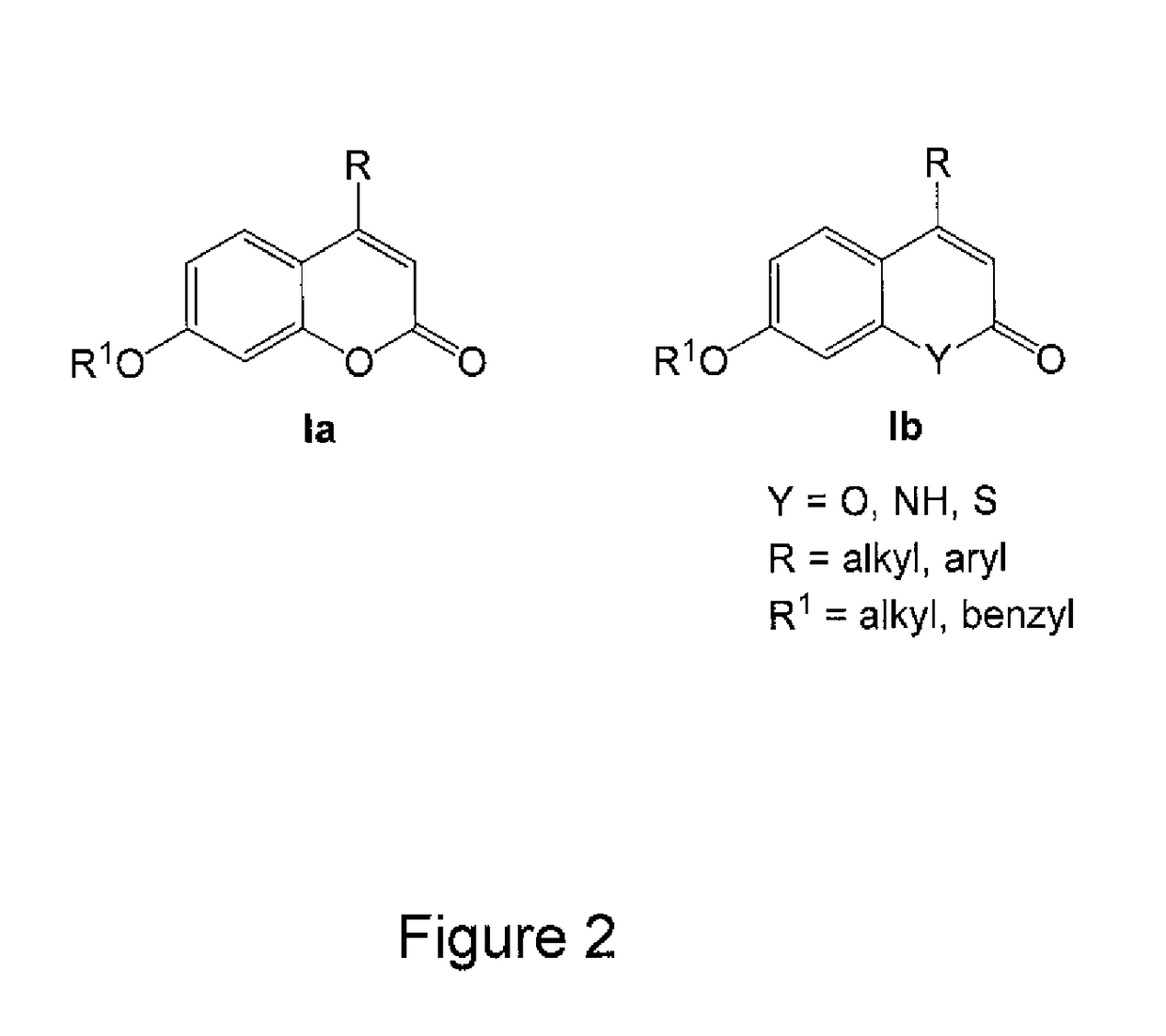
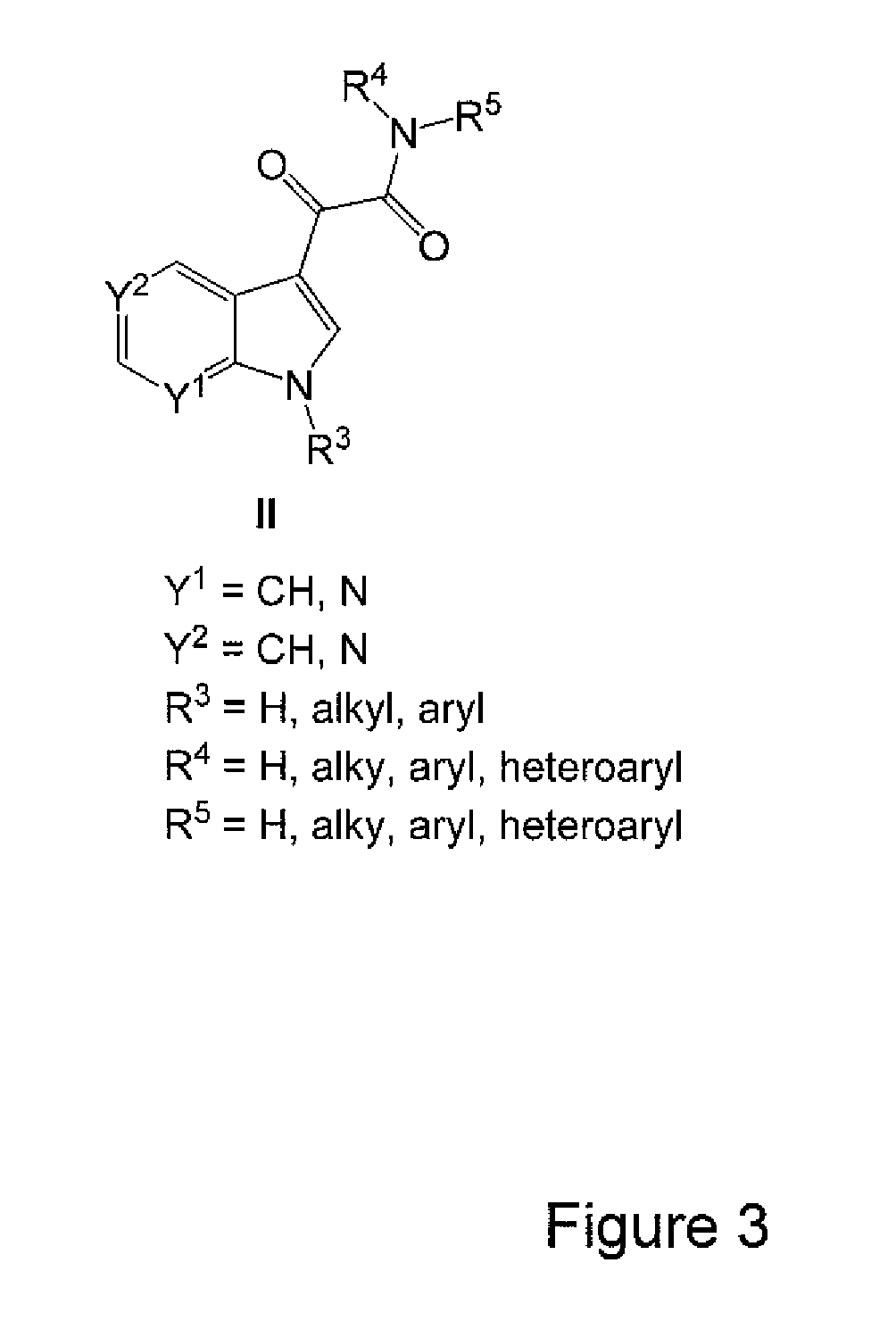
Patents
Literature
69 results about "Plasmodium falciparum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
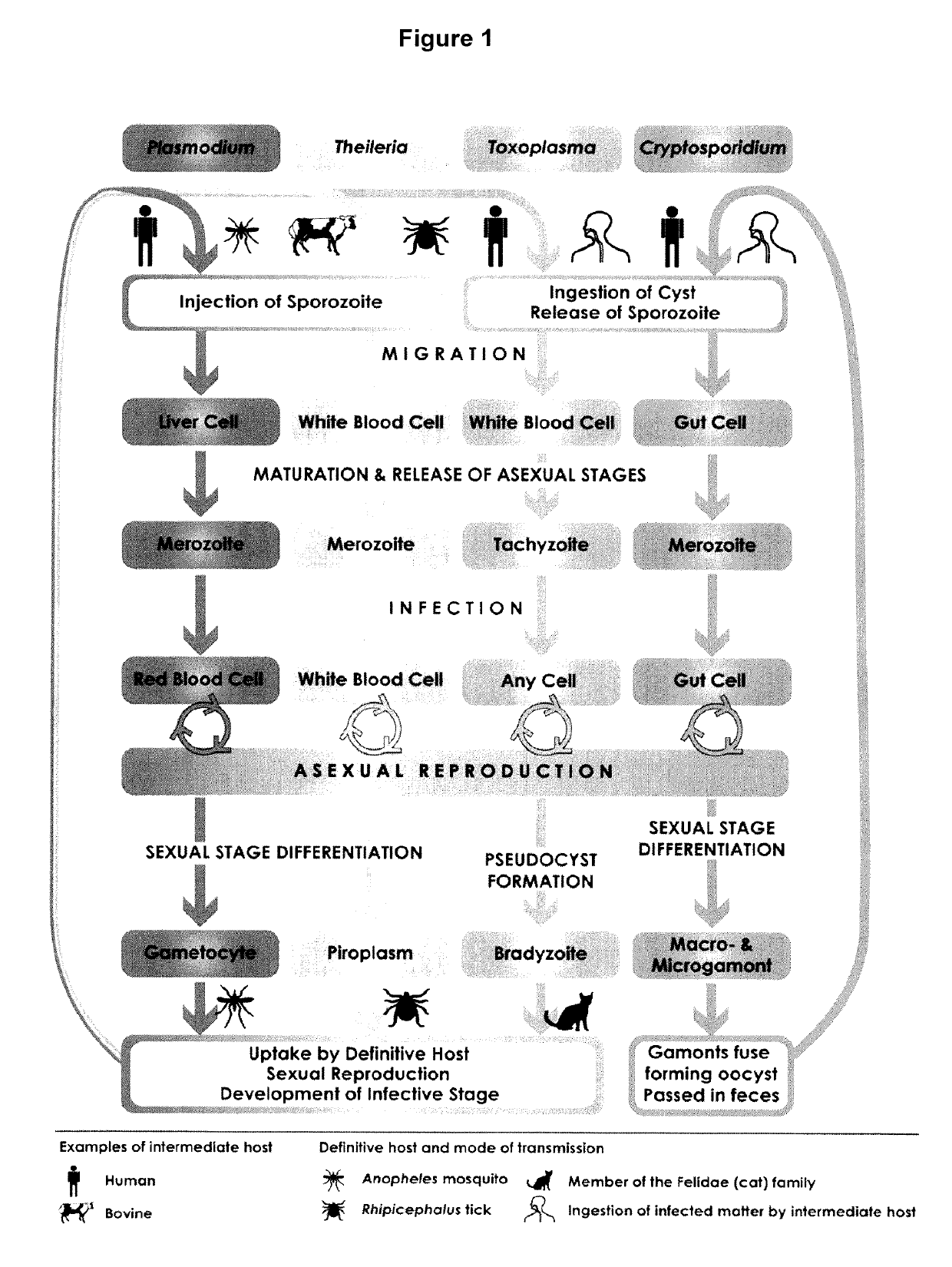
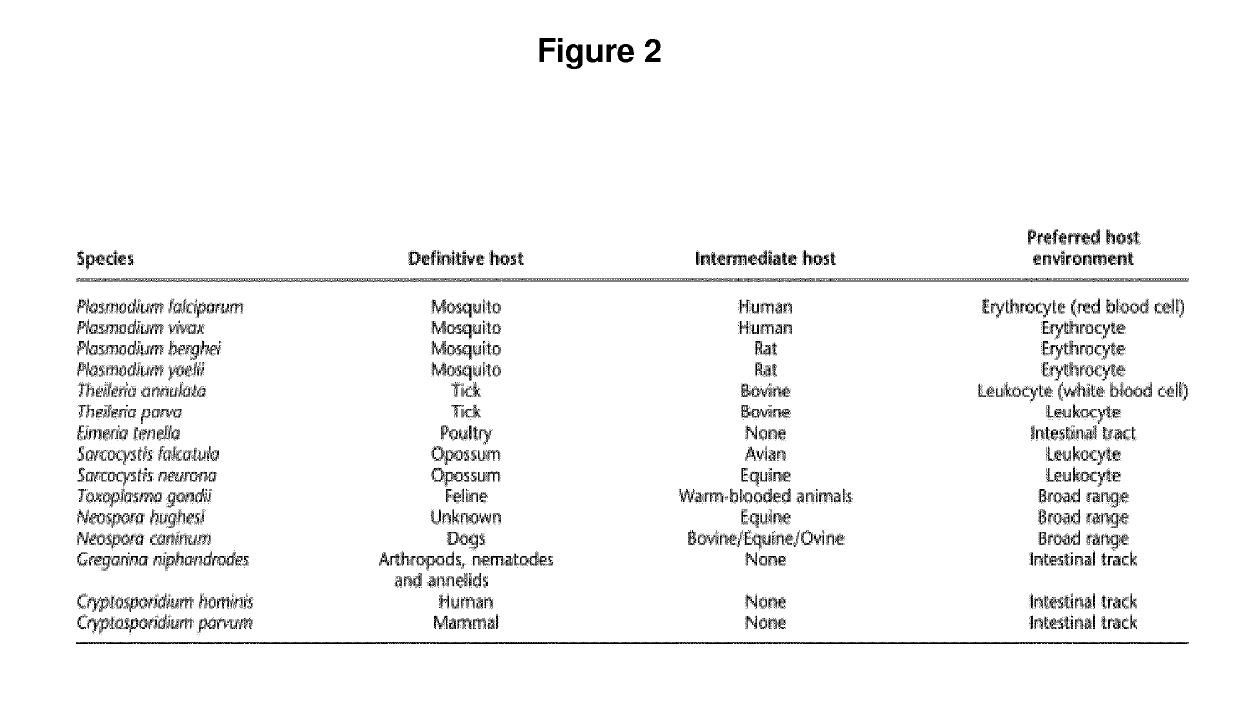
Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form called falciparum malaria which is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans, causing 435,000 deaths in 2017. It is also associated with the development of blood cancer (Burkitt's lymphoma) and is classified as Group 2A carcinogen.
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS7060276B2Eliminate the problemImprove responseSugar derivativesViral antigen ingredientsADAMTS ProteinsPlasmodium falciparum
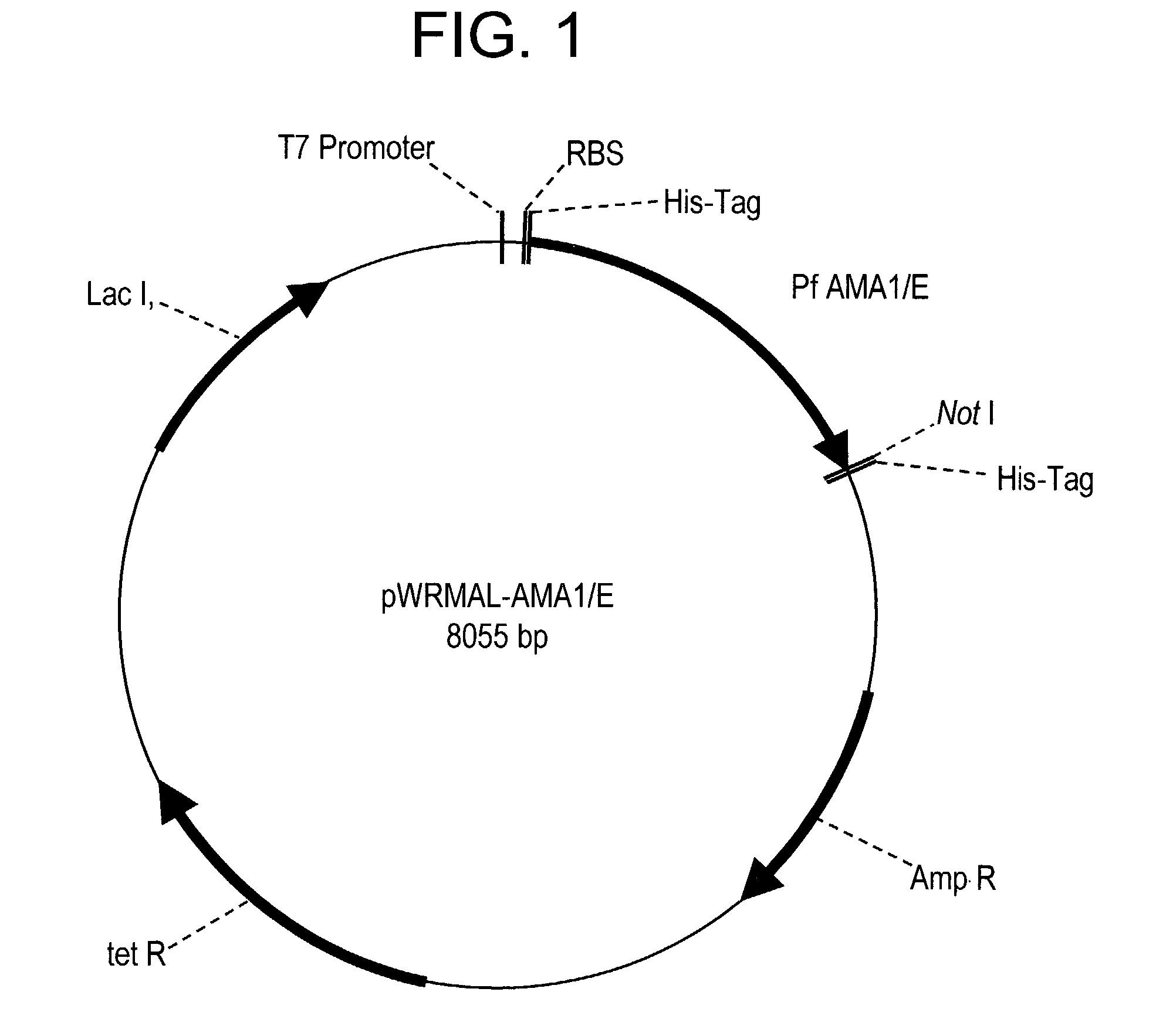
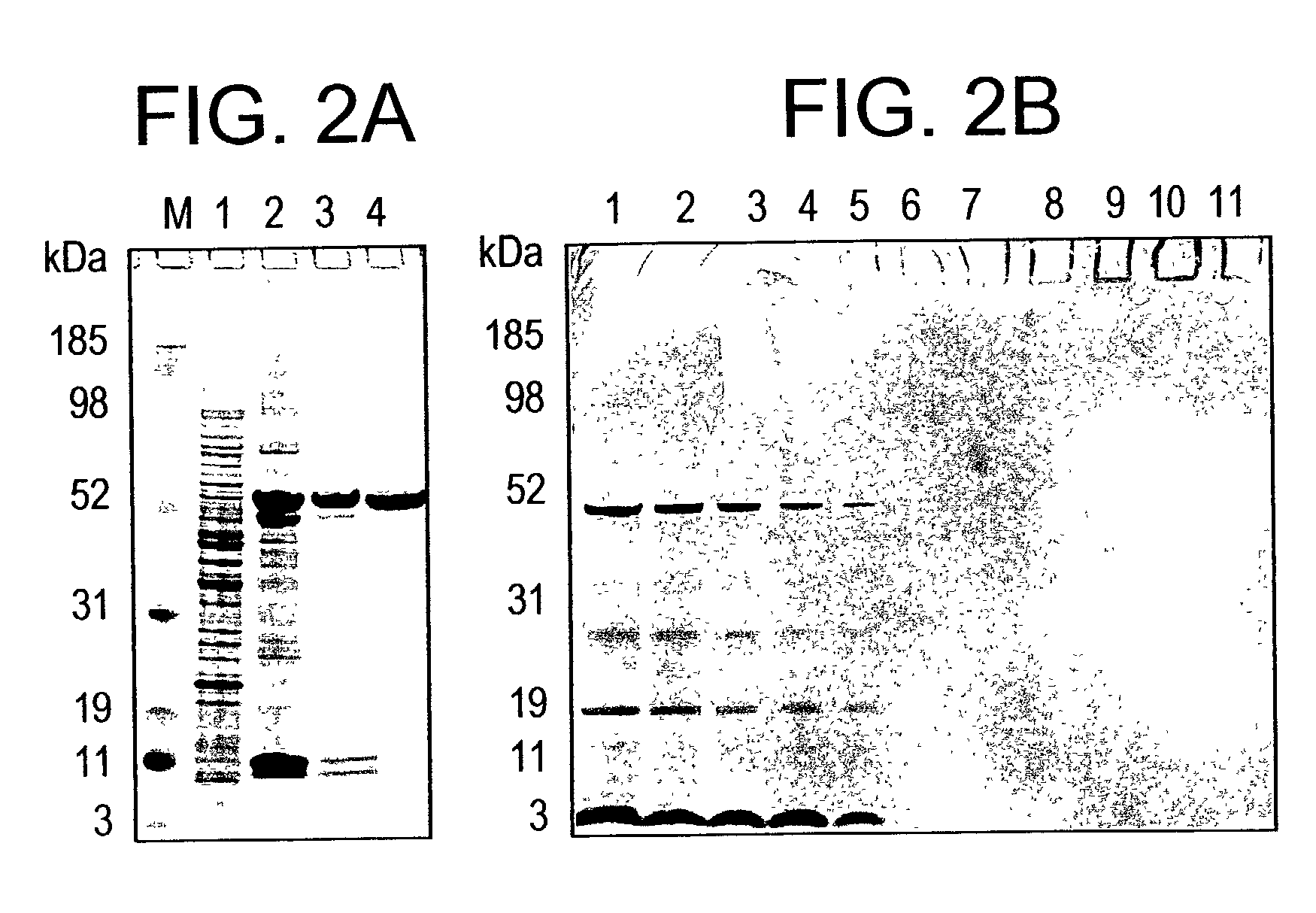
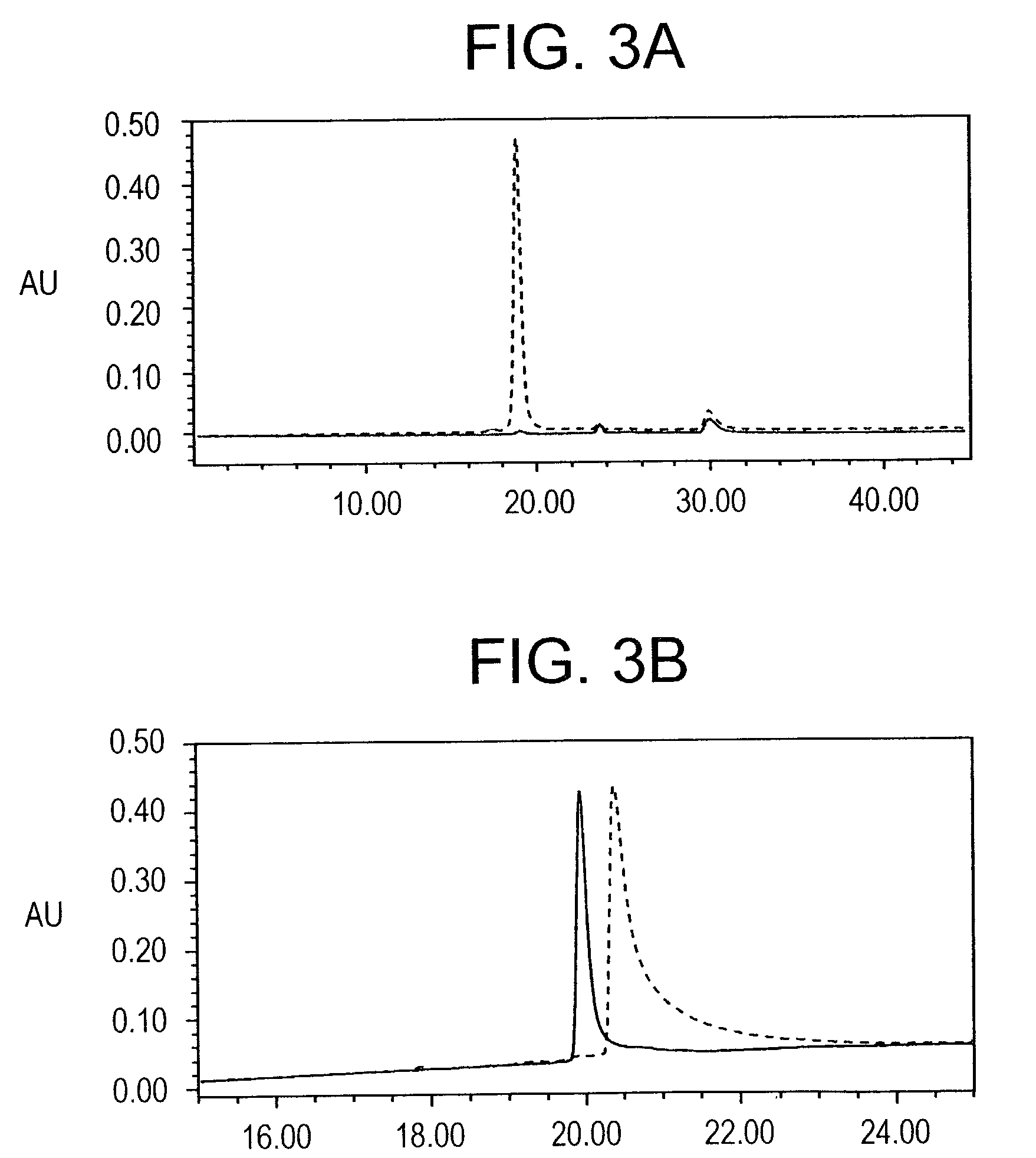
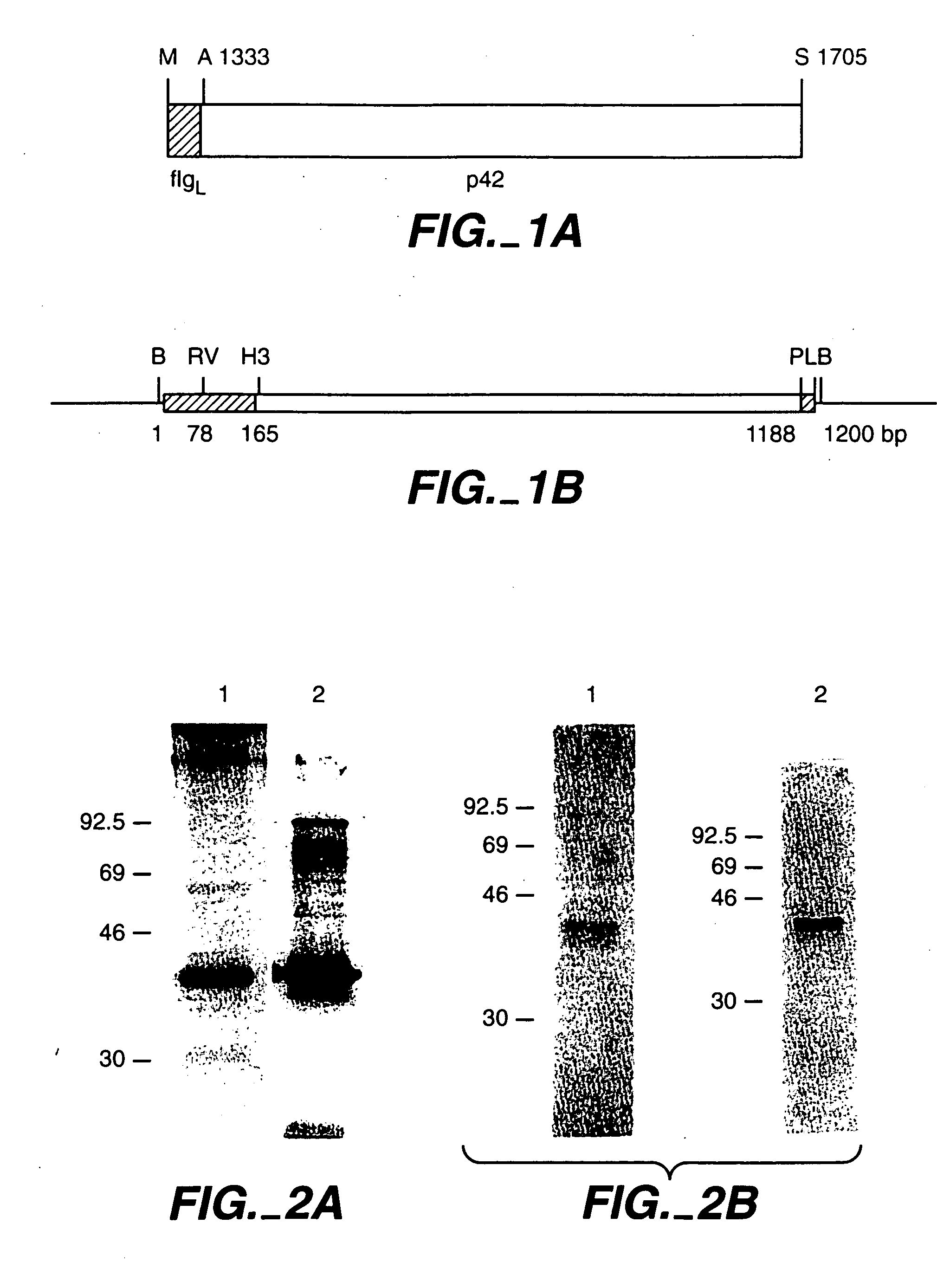
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a vaccine.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Baculovirus produced Plasmodium falciparum vaccine
Owner:UNIV OF HAWAII
Geldanamycin derivatives and method of use thereof
The present invention relates to novel geldanamycin derivatives which have antitumor and antiparasitic properties. The geldanamycin derivatives disclosed herein have antitumor properties in humans due to their interaction with human heat shock protein 90 (hsp90). The human parasites Plasmodium falciparum, Trypanosoma Cruzi, and Leishmania donovani are lethally susceptible to exposure to geldanamycin via complexation of geldanamycin with their homologs (Pfhsp90, hsp83, and hsp90, respectively) of the human hsp90. The geldanamycin derivatives disclosed herein also interact with these parasitic hsp90 homologs so as to have antiparasitic properties.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Preparation method of HRPII protein monoclonal antibody of plasmodium falciparum
ActiveCN101659975AGood repeatabilityAchieve serial expressionMicroorganism based processesFermentationChemical synthesisEscherichia coli
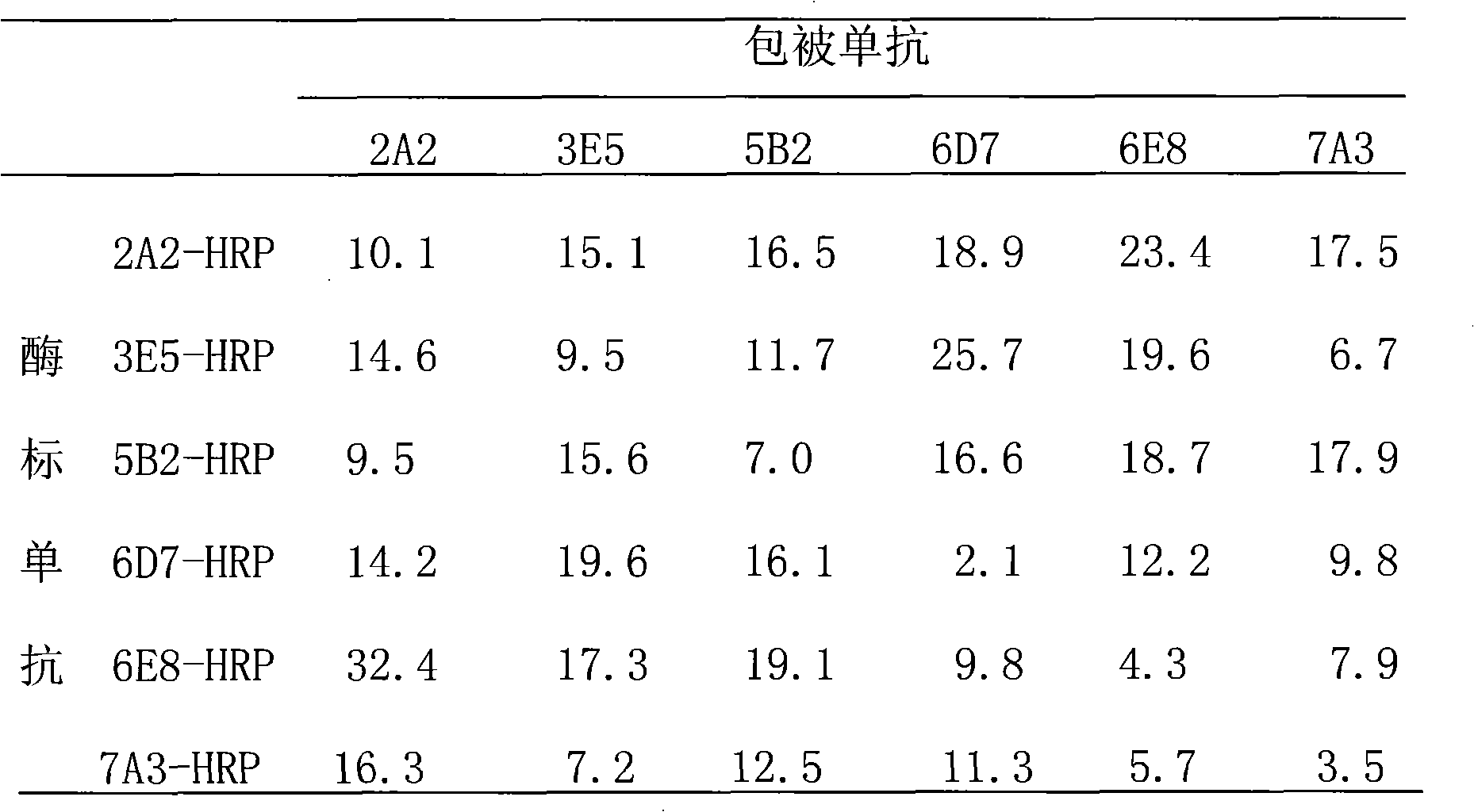
The invention relates to a preparation method of HRPII protein monoclonal antibody of plasmodium falciparum. The preparation method comprises the following steps of: adopting HRPII protein of plasmodium falciparum as target antigen and respectively analyzing and selecting two dominant antigen epitopes of A and B; respectively repeating the two dominant antigen epitopes of A and B, then continuously connecting four glycine and forming recombinant protein C; adopting most securest code of escherichia coli and converting the amino acid sequence of the recombinant protein C into corresponding nucleotide sequence; carrying out chemical synthesis to the former step to obtain the nucleotide sequence, and respectively adding enzyme cutting sites BamHI and EcoRI at the upstream and downstream thereof; inserting nucleotide fragment obtained by the former step into expression carrier PET-28a(+), constructing recombinant protein C expression carrier and inducing to express the recombinant proteinC in the escherichia coli BL21 (DE3); carrying out ultrasonic bacteria breaking and low-temperature centrifugation, then taking supernatant of the solution, affining a chromatographic column by nickel-agarose, eluting and obtaining purified recombinant protein C; after immunizing Balb / c mouse with the recombinant protein C for a plurality of times, taking and fusing spleen cells with sp2 / 0 myelomacells, and obtaining six hybridoma cell lines by multiple rounds of screening; and purifying monoclonal antibody, respectively marking horse radish peroxidase and prorating matching and combination of optimum monoclonal antibody by ELISA orthogonal experiment.
Owner:杭州新脉生物科技有限公司
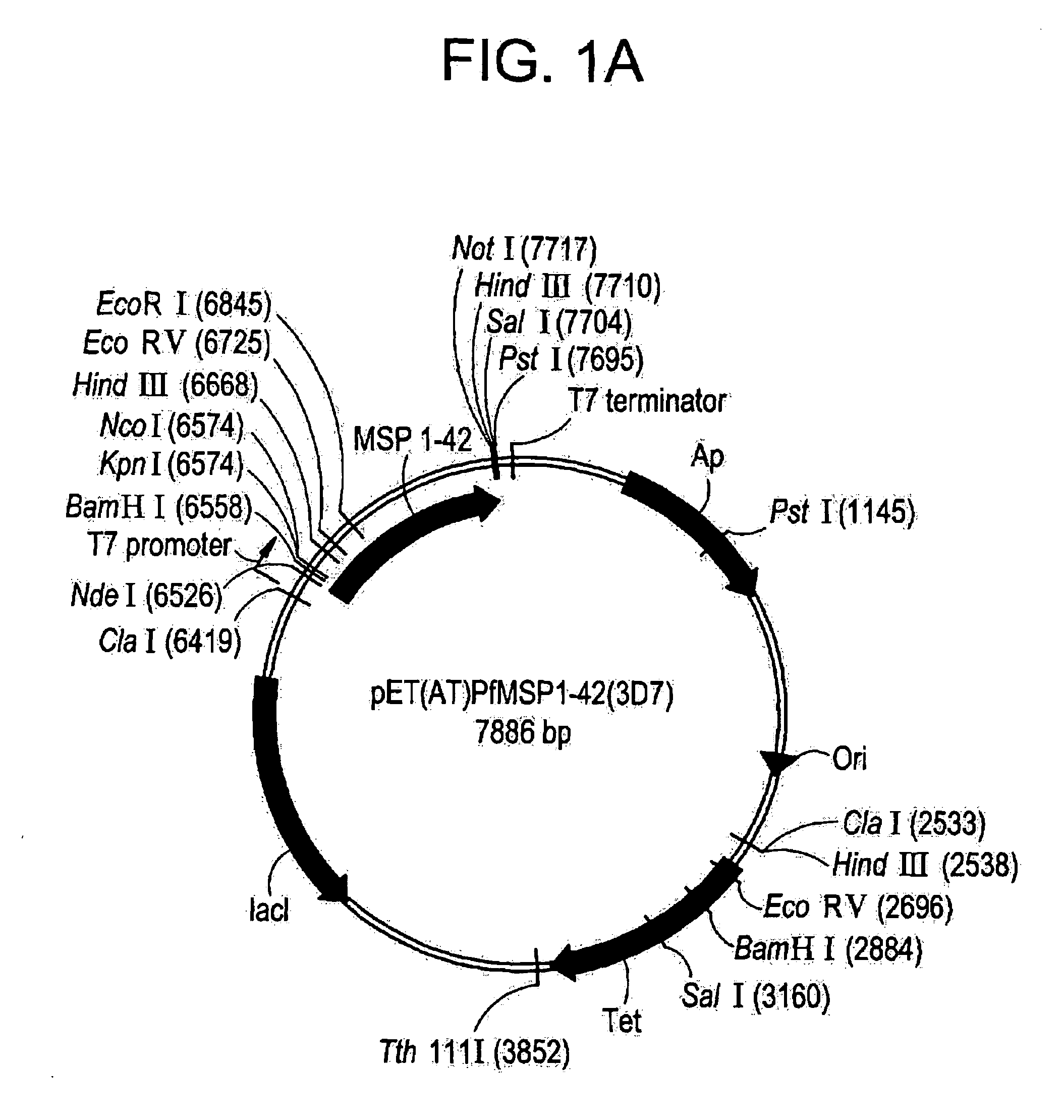
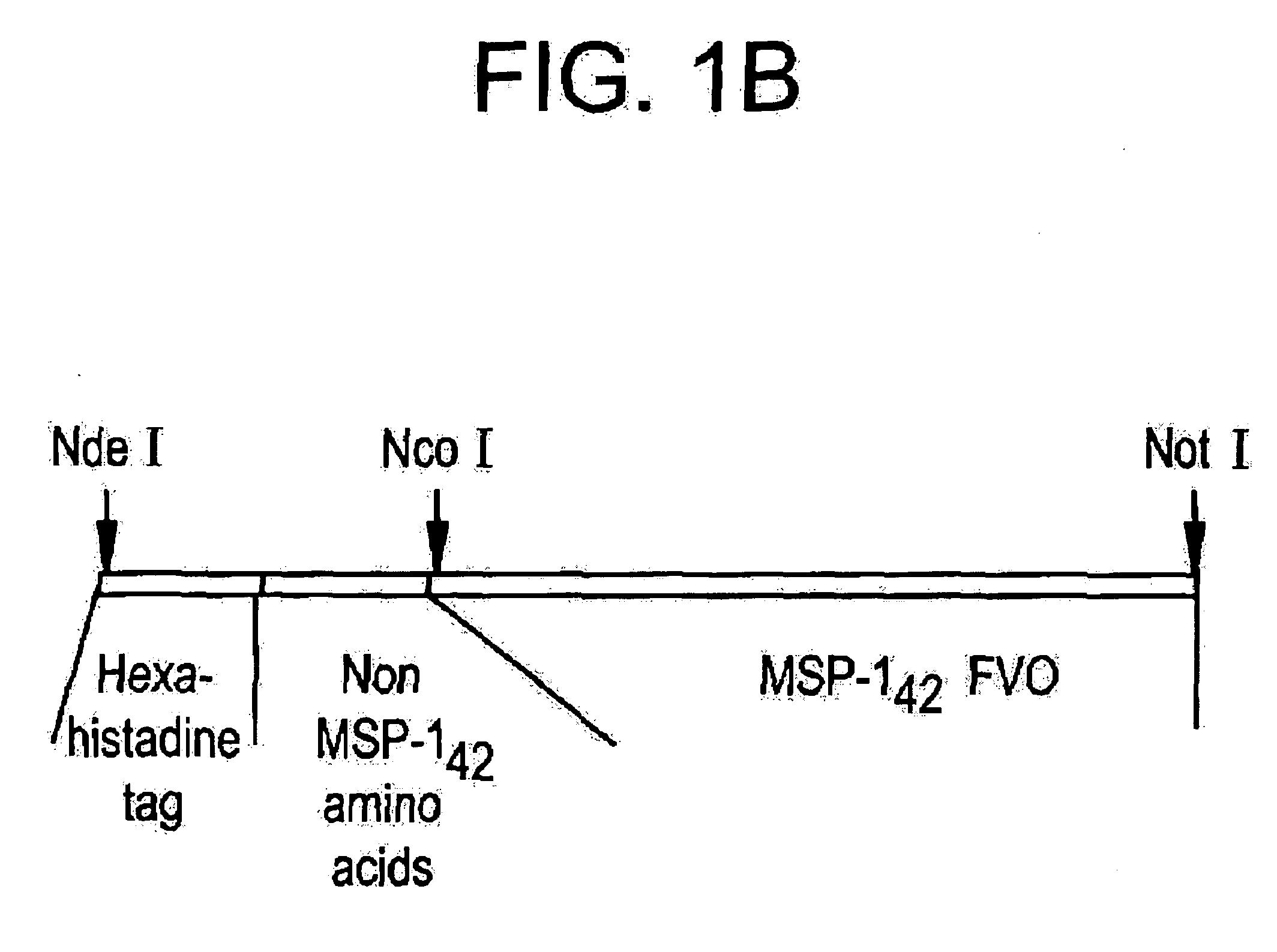
Method of purifying recombinant MSP 1-42 derived from Plasmodium falciparum
InactiveUS20060130159A1Minimize concentration polarizationReduce fluxDepsipeptidesPeptide preparation methodsBiotechnologySource material
Owner:GTC BIOTHERAPEUTICS INC
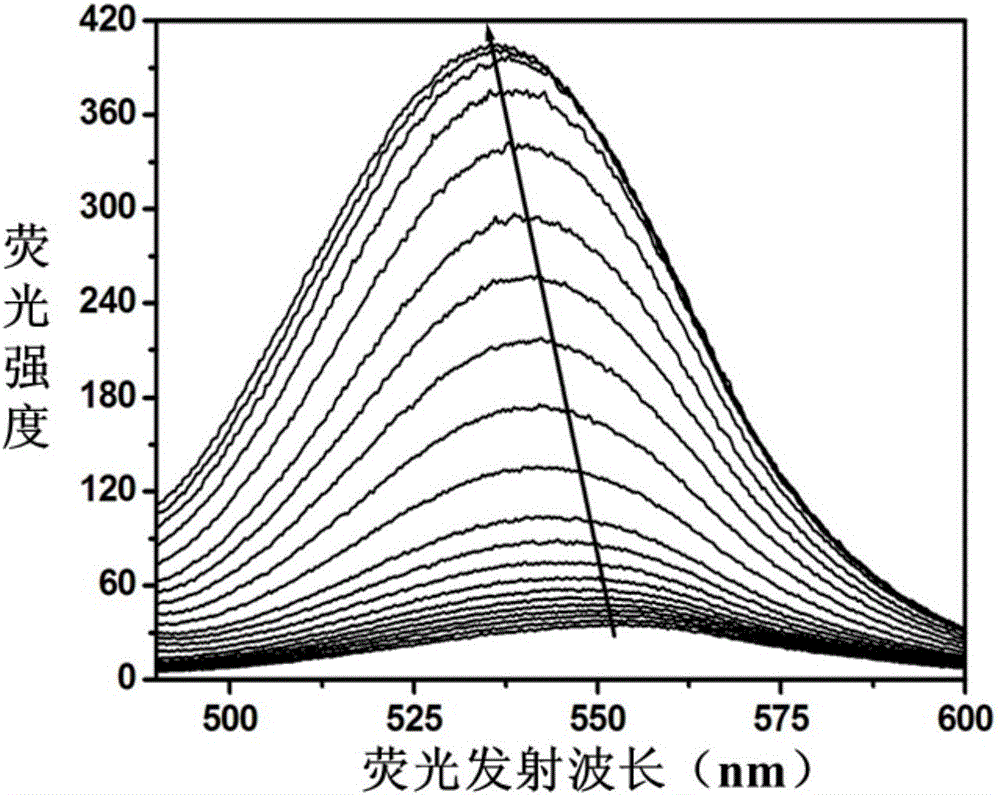
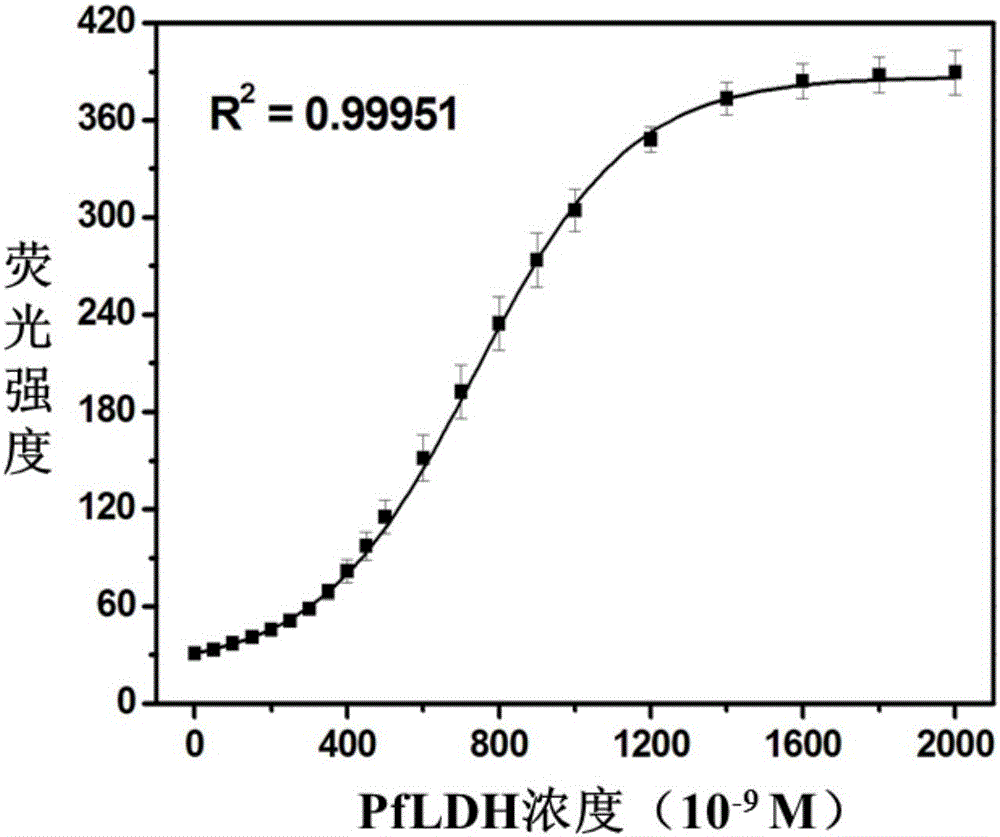
Fluorescent probe based on double-stranded DNA protection and application of same to preparation of drug used for detecting Plasmodium falciparum lactate dehydrogenase
InactiveCN106404726ASimple structureEasy to synthesizeFluorescence/phosphorescenceAgainst vector-borne diseasesLactate dehydrogenaseFluorescence
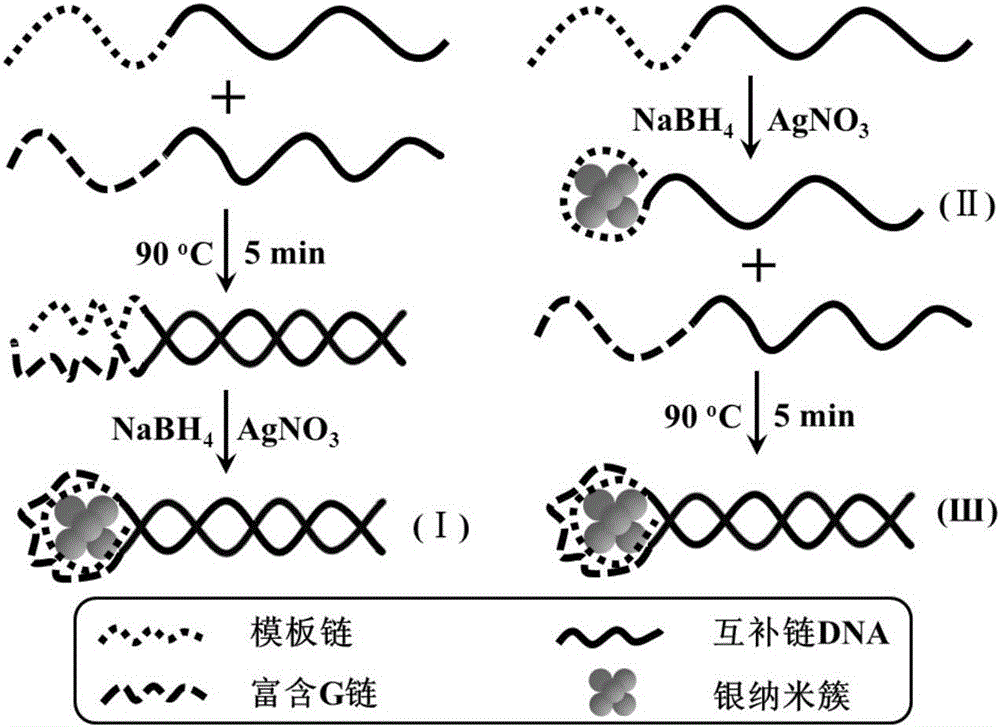
The invention provides a silver nano-cluster fluorescent probe based on double-stranded DNA protection and application of the same to preparation of a drug used for detecting Plasmodium falciparum lactate dehydrogenase, belonging to the technical field of fluorescent probes. DNA used in the invention is of a double strand structure, wherein one strand is composed of a complementary strand DNA and a template strand DNA, and the other strand is composed of a complementary strand DNA and G base-rich DNA; the template strand DNA is a protective group for synthesis of a silver nano-cluster and can be coordinated with the surface of the silver nano-cluster to prevent further expansion of the silver nano-cluster; the G base-rich DNA improves the fluorescence emission intensity of the silver nano-cluster by approaching the silver nano-cluster; the complementary strand DNA has a length of 10 to 30 bases and is rich in A (adenine) and T (thymine) bases; the template strand DNA has a length of 10 to 20 bases and is rich in C (cytosine) bases; and the G base-rich DNA has a length of 10 to 25 bases and is rich in G bases. A detection method provided by the invention is fast in detection speed, simple to operate, simple in system, stable in signal, high in sensitivity and free of any pretreatment and does not need any complex detection apparatuses.
Owner:JILIN UNIV
Pharmaceutical compounds
InactiveUS20090298818A1Reduce and even eliminate painNarrow downAntibacterial agentsBiocideDrug compoundMedicine
The invention provides the use of a compound for the manufacture of a medicament for the treatment of pain, wherein the compound is a compound of the formula (VI):or a salt, solvate, tautomer or N-oxide thereof;wherein the bicyclic group:is selected from the structures C1, C5 and C6:wherein n, R1, R2a, R3, R4a, R8 and R10 are as defined in the claims.The invention also provides the use of a compound of the formula (VI) for the manufacture of a medicament for the prophylaxis or treatment of a fungal, protozoal, viral or parasitic disease state or condition (other than a disease state or condition due to Plasmodium falciparum) or for use in the prophylaxis or treatment of Ewing's sarcoma, atherosclerosis or lupus erythematosus.
Owner:ASTEX THERAPEUTICS LTD
Expression, purification and uses of a plasmodium falciparum liver stage antigen 1 polypeptide
InactiveUS20100040640A1High protein yieldIncrease ratingsBacteriaPeptide/protein ingredientsAntigenPlasmodium falciparum
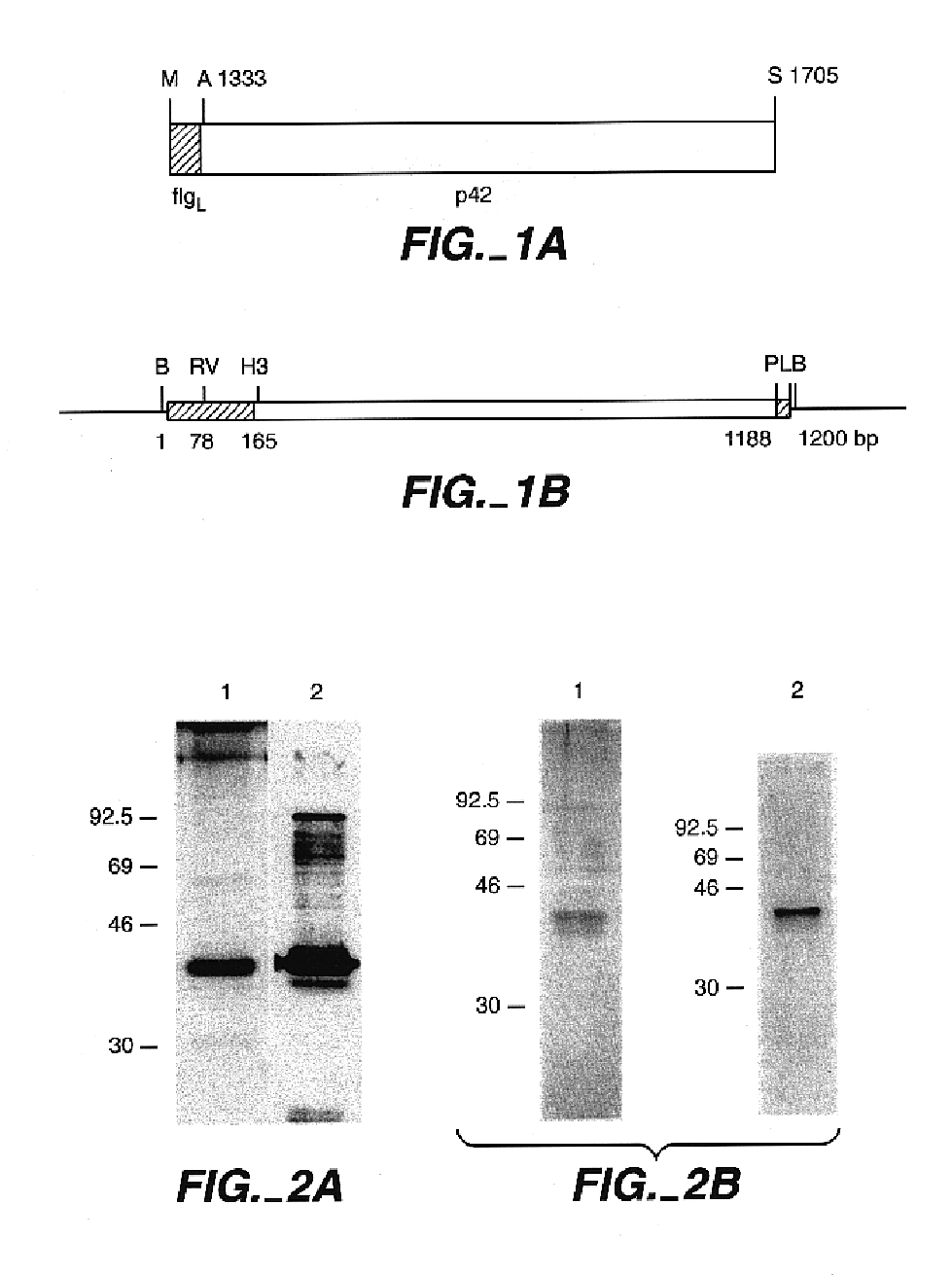
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) LSA-NRC polypeptide. The method of the present invention produces a highly purified polypeptide which is useful as a vaccine and as a diagnostic reagent.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
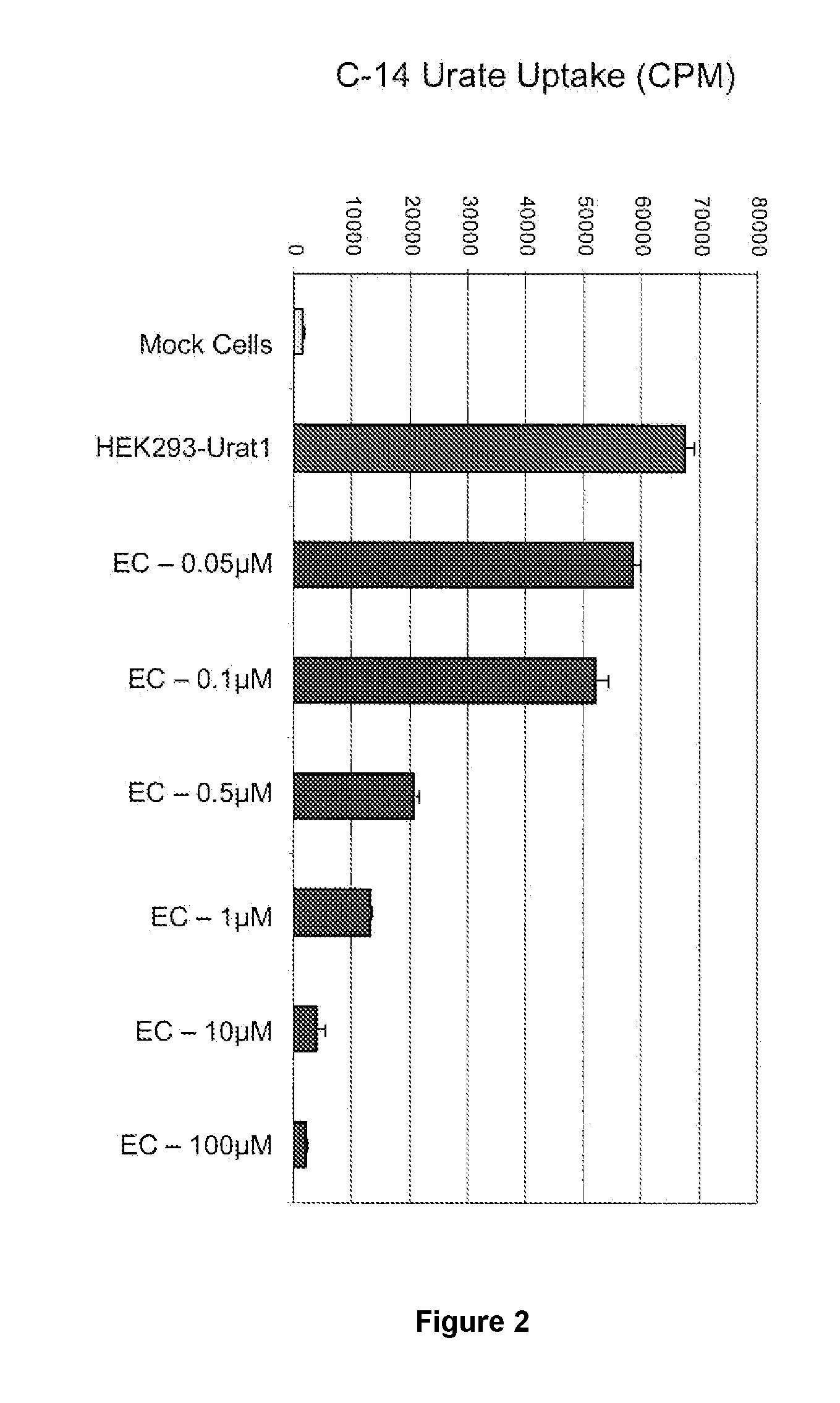
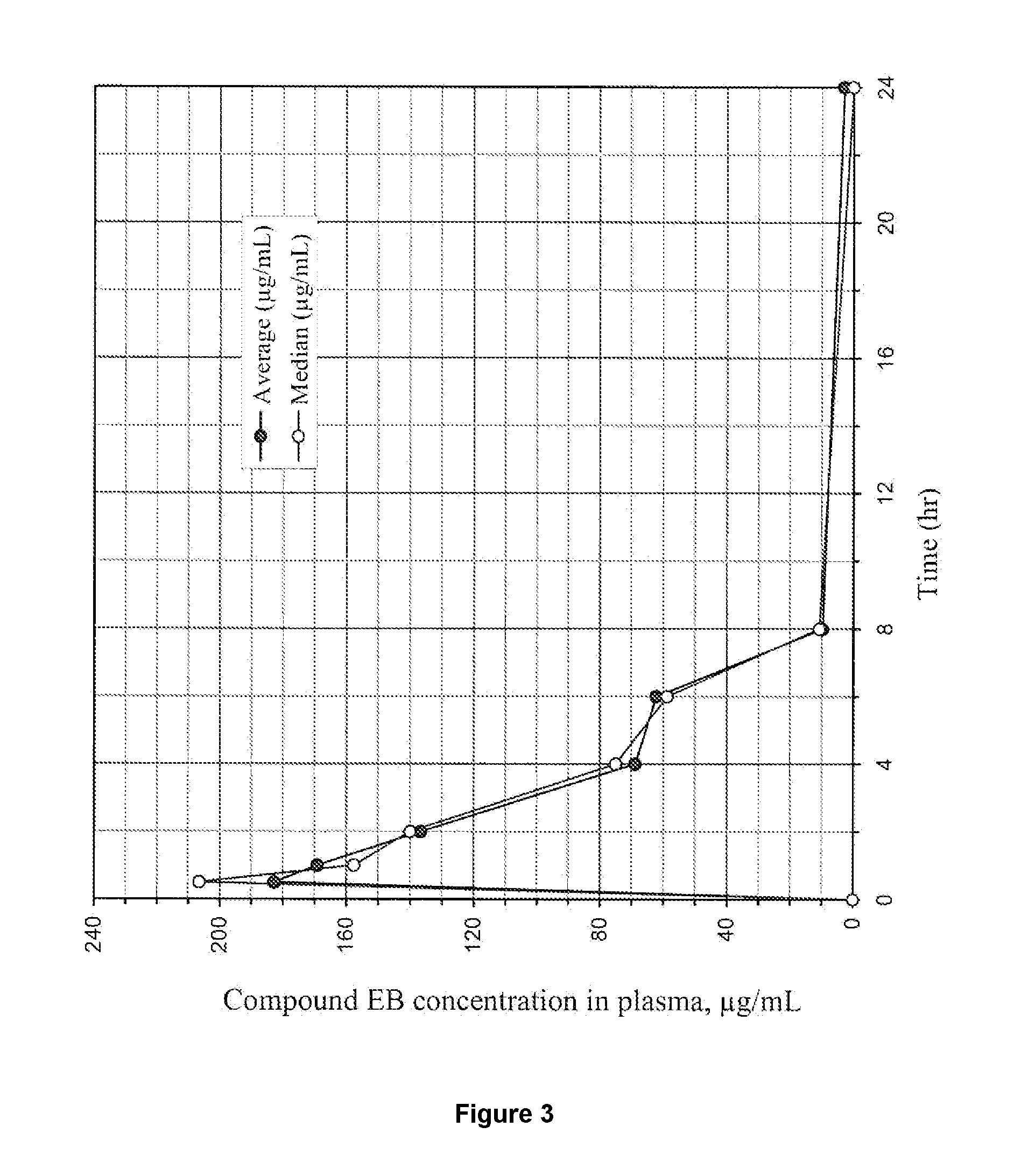
Tetrazole compounds for reducing uric acid
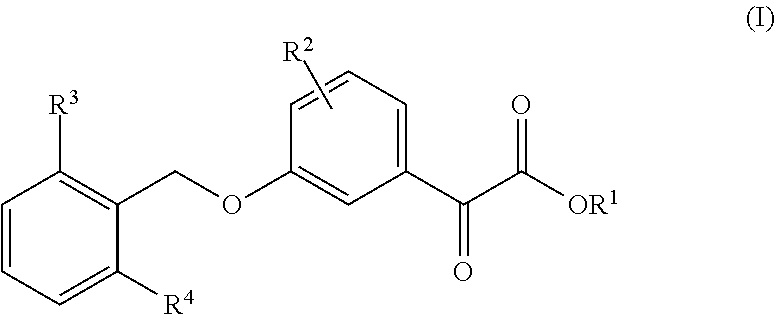
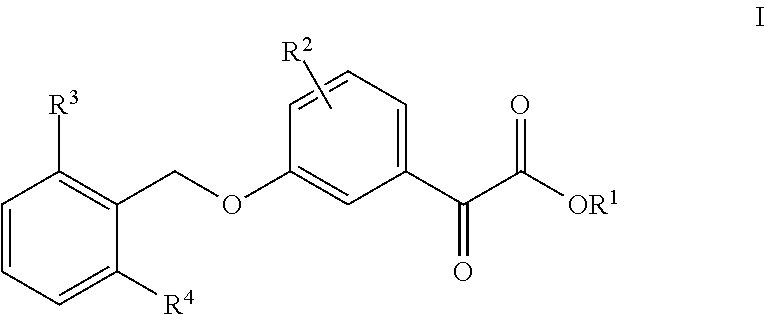
Uric acid in mammalian subjects is reduced and excretion of uric acid is increased by administering a compound of Formula I. The uric acid-lowering effects of the compounds of this invention are used to treat or prevent a variety of conditions including gout, hyperuricemia, elevated levels of uric acid that do not meet the levels customarily justifying a diagnosis of hyperuricemia, renal dysfunction, kidney stones, cardiovascular disease, risk for developing cardiovascular disease, tumor-lysis syndrome, cognitive impairment, early-onset essential hypertension, and Plasmodium falciparum-induced inflammation. In Formula 1, x is 1 or 2: y is O, 1, 2 or 3; and R1 is selected from the group consisting of hydrogen, alkyl having 1 or 2 carbon atoms, hydroxy, alkoxy having 1 or 2 carbon atoms, fluoro, chloro, bromo, and amino. A is phenyl unsubstituted or substituted by one, two or three groups selected from the group consisting of halo, alkyl having 1 or 2 carbon atoms, perfluoromethyL alkoxy having 1 or 2 carbon atoms, and perfluoromethoxy; or cycloalkyl having from 3 to 6 ring atoms wherein the cycloalky! is unsubstituted or one one two ring carbons are independently mono-substituted by methyl or ethyl; or a 5 or 6 membered heleraromatic ring having 1 or 2 ring heteroatoms selected from N, S and O and the heteroaromatic ring is covalently bound to the remainder of the compound by a ring carbon.
Owner:WELLSTAT THERAPEUTICS
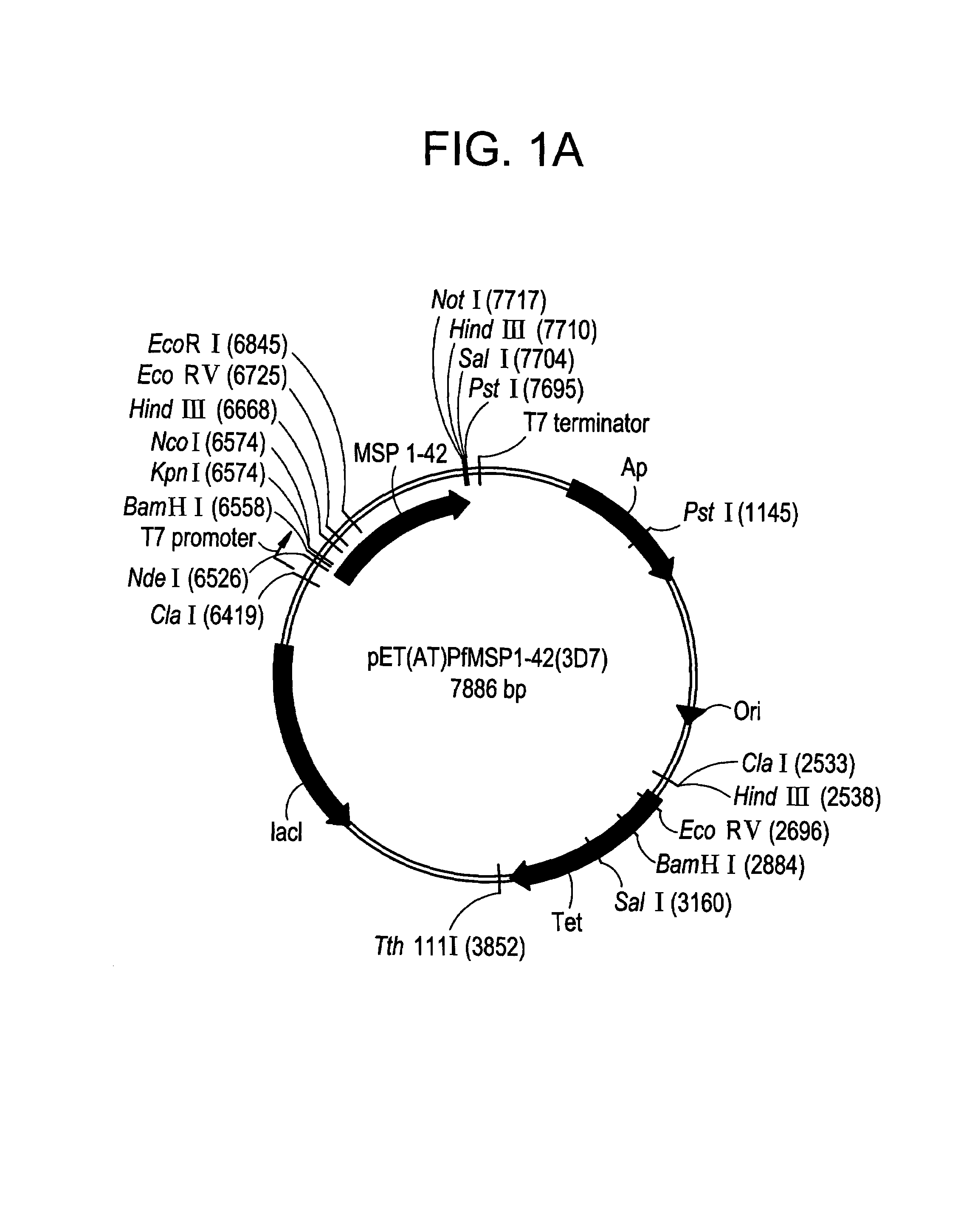
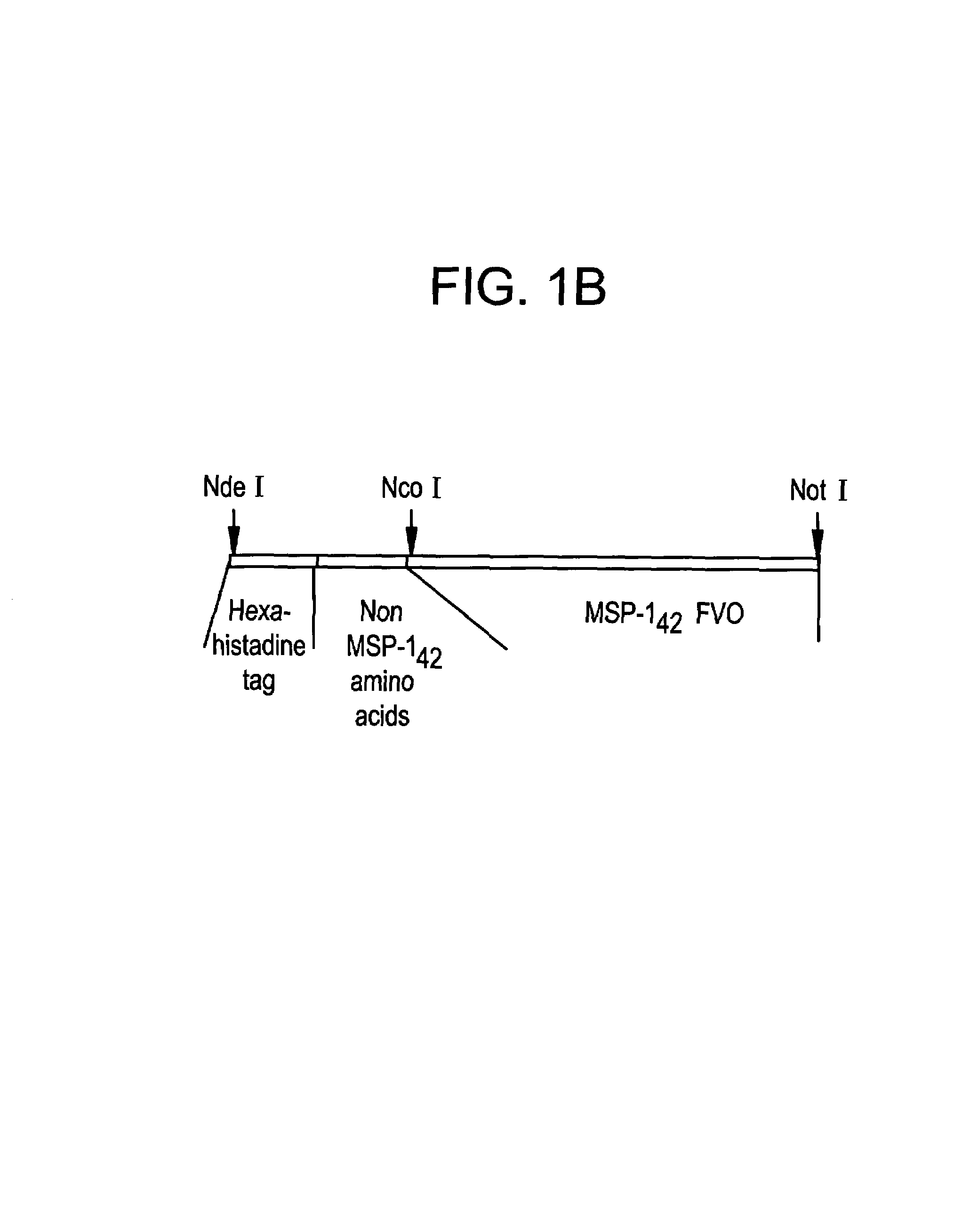
Recombinant P. falciparum merozoite protein-142 vaccine
InactiveUS7256281B2Not painful to administerIntrinsically safeAntibacterial agentsBacteriaPlasmodium falciparumAntibody production
In this application is described the expression and purification of a recombinant Plasmodium falciparum (FVO) MSP-142. The method of the present invention produces a highly purified protein that retains folding and disulfide bridging of the native molecule. The recombinant MSP-142 is useful as a diagnostic reagent, for use in antibody production, and as a vaccine.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Preparation method of plasmodium pf/pan detecting test paper
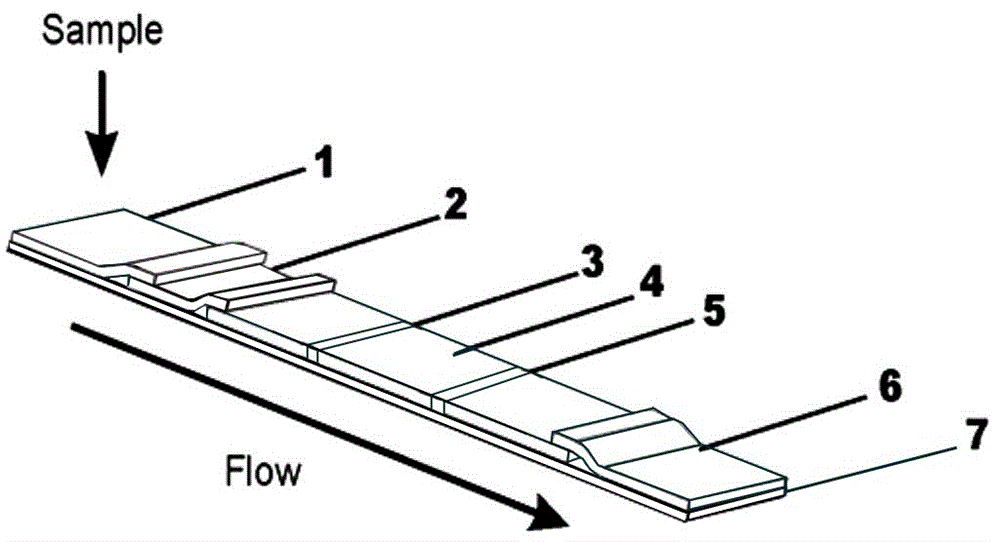
InactiveCN106908602ACause some damagesQuick judgmentBiological material analysisAgainst vector-borne diseasesGlass fiberCellulose
The invention discloses a preparation method of plasmodium pf / pan detecting test paper. The preparation method comprises the following steps of: separately performing metal spraying to a glass cellulose membrane by means of immunogold marked by a plasmodium falciparum lactic dehydrogenase monoclonal antibody and a plasmodium lactic dehydrogenase monoclonal antibody to obtain a pf gold pad and a pan gold pad; separately marking lines on a polyvinyl chloride bottom plate adhered with a nitrocellulose membrane by a plasmodium falciparum lactic dehydrogenase monoclonal antibody detection line coating liquid, a plasmodium lactic dehydrogenase monoclonal antibody detection line coating liquid and a control line coating liquid to obtain the polyvinyl chloride bottom plate with the dotted membrane; and assembling a sample pad, the gold pads, the polyvinyl chloride bottom plate with the dotted membrane and water absorbing paper together and performing cutting to obtain the detecting test paper. The detecting test paper obtained by the method disclosed by the invention can judge whether a blood sample contains plasmodium pf / pan or not by manual operation and reading by naked eyes by virtue of an immune directed flow chromatographic technique, is fast to diagnose and accurate in result, and does not damage the body of a testee.
Owner:SUZHOU WANMUCHUN BIOLOGICAL TECH
Tetrazole compounds for reducing uric acid
Uric acid in mammalian subjects is reduced and excretion of uric acid is increased by administering a compound of Formula I. The uric acid-lowering effects of the compounds of this invention are used to treat or prevent a variety of conditions including gout, hyperuricemia, elevated levels of uric acid that do not meet the levels customarily justifying a diagnosis of hyperuricemia, renal dysfunction, kidney stones, cardiovascular disease, risk for developing cardiovascular disease, tumor-lysis syndrome, cognitive impairment, early-onset essential hypertension, and Plasmodium falciparum-induced inflammation. In Formula 1, x is 1 or 2: y is O, 1, 2 or 3; and R1 is selected from the group consisting of hydrogen, alkyl having 1 or 2 carbon atoms, hydroxy, alkoxy having 1 or 2 carbon atoms, fluoro, chloro, bromo, and amino. A is phenyl unsubstituted or substituted by one, two or three groups selected from the group consisting of halo, alkyl having 1 or 2 carbon atoms, perfluoromethyL alkoxy having 1 or 2 carbon atoms, and perfluoromethoxy; or cycloalkyl having from 3 to 6 ring atoms wherein the cycloalky! is unsubstituted or one one two ring carbons are independently mono-substituted by methyl or ethyl; or a 5 or 6 membered heleraromatic ring having 1 or 2 ring heteroatoms selected from N, S and O and the heteroaromatic ring is covalently bound to the remainder of the compound by a ring carbon.
Owner:PHARMA CINQ LLC
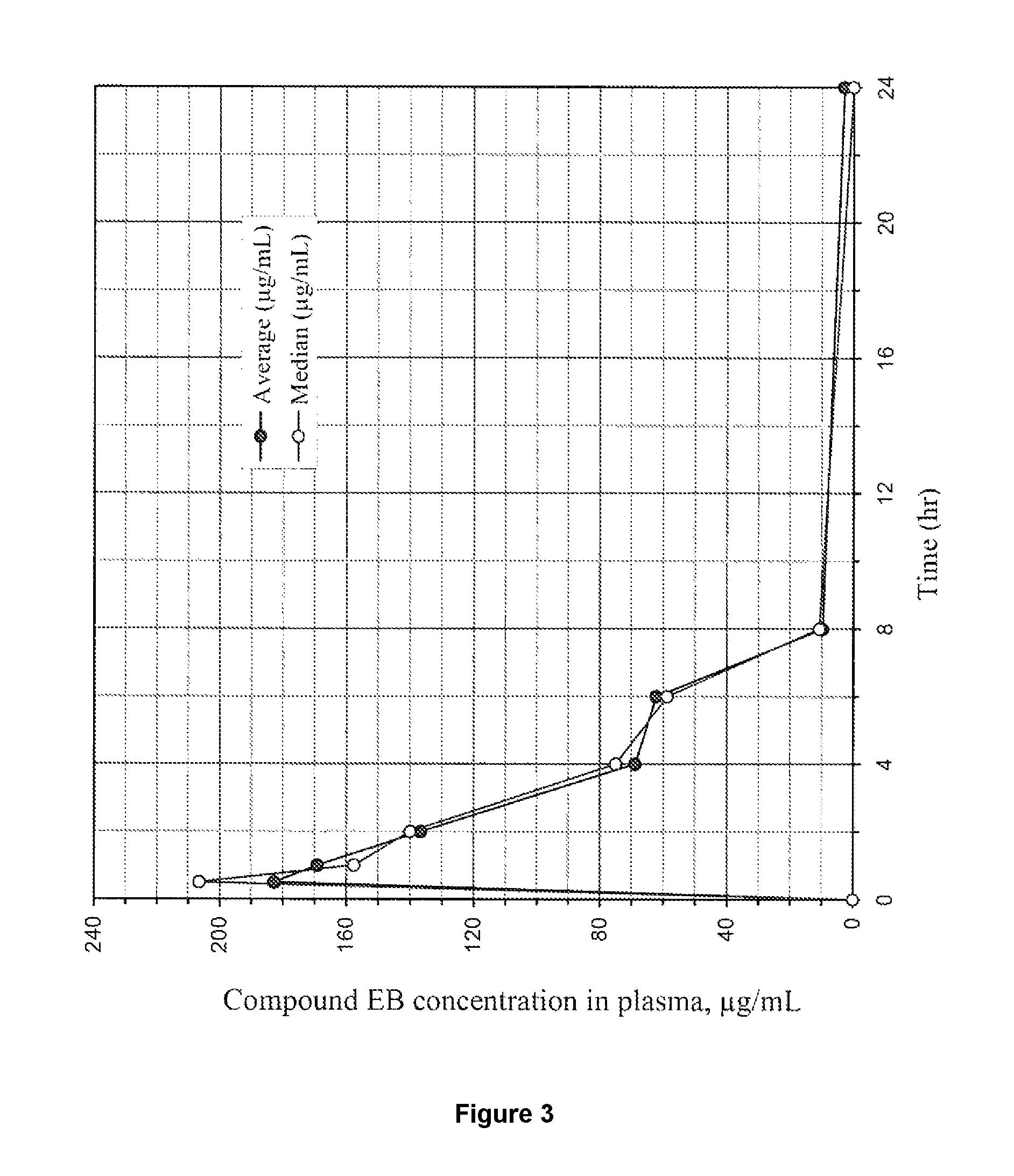
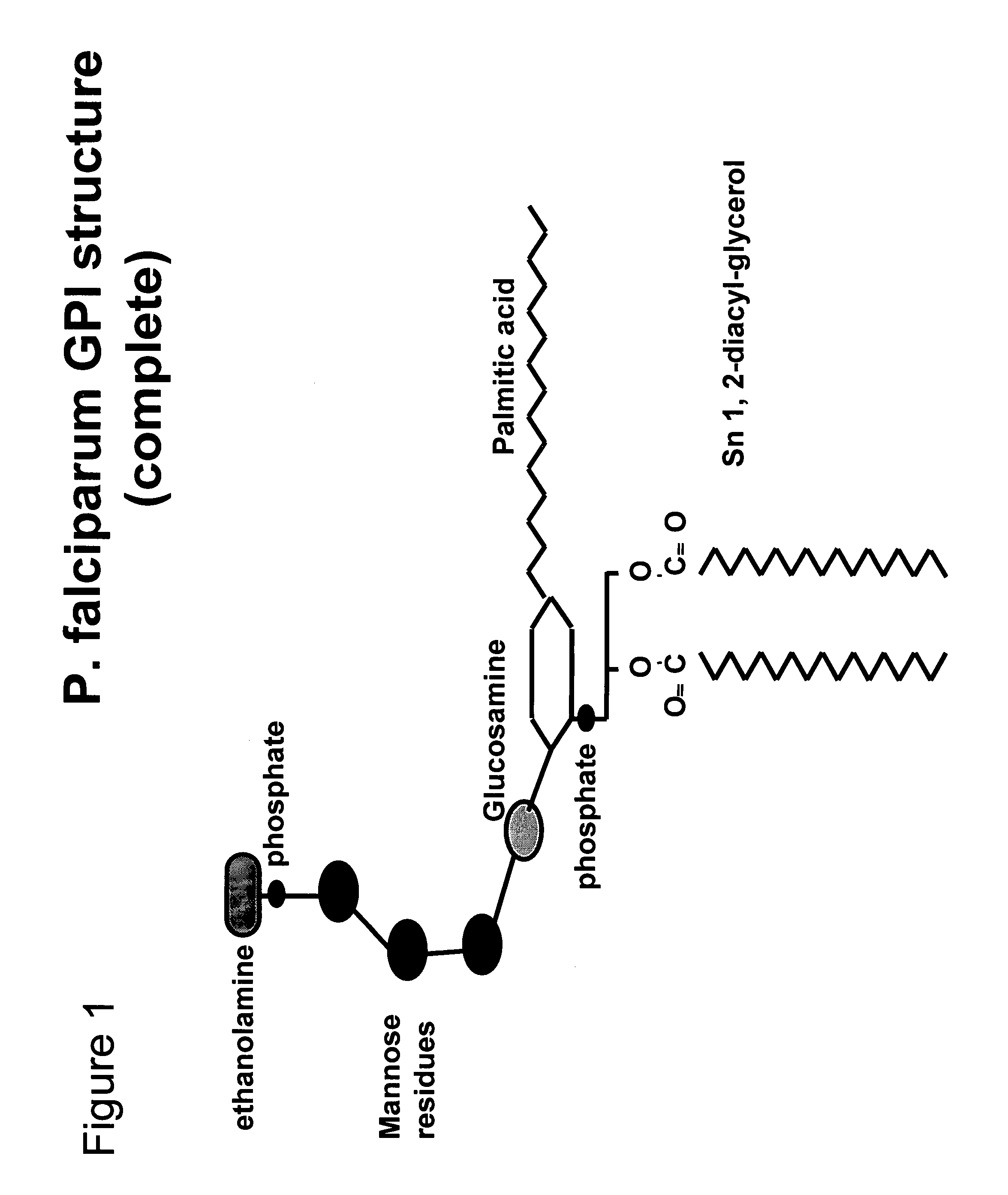
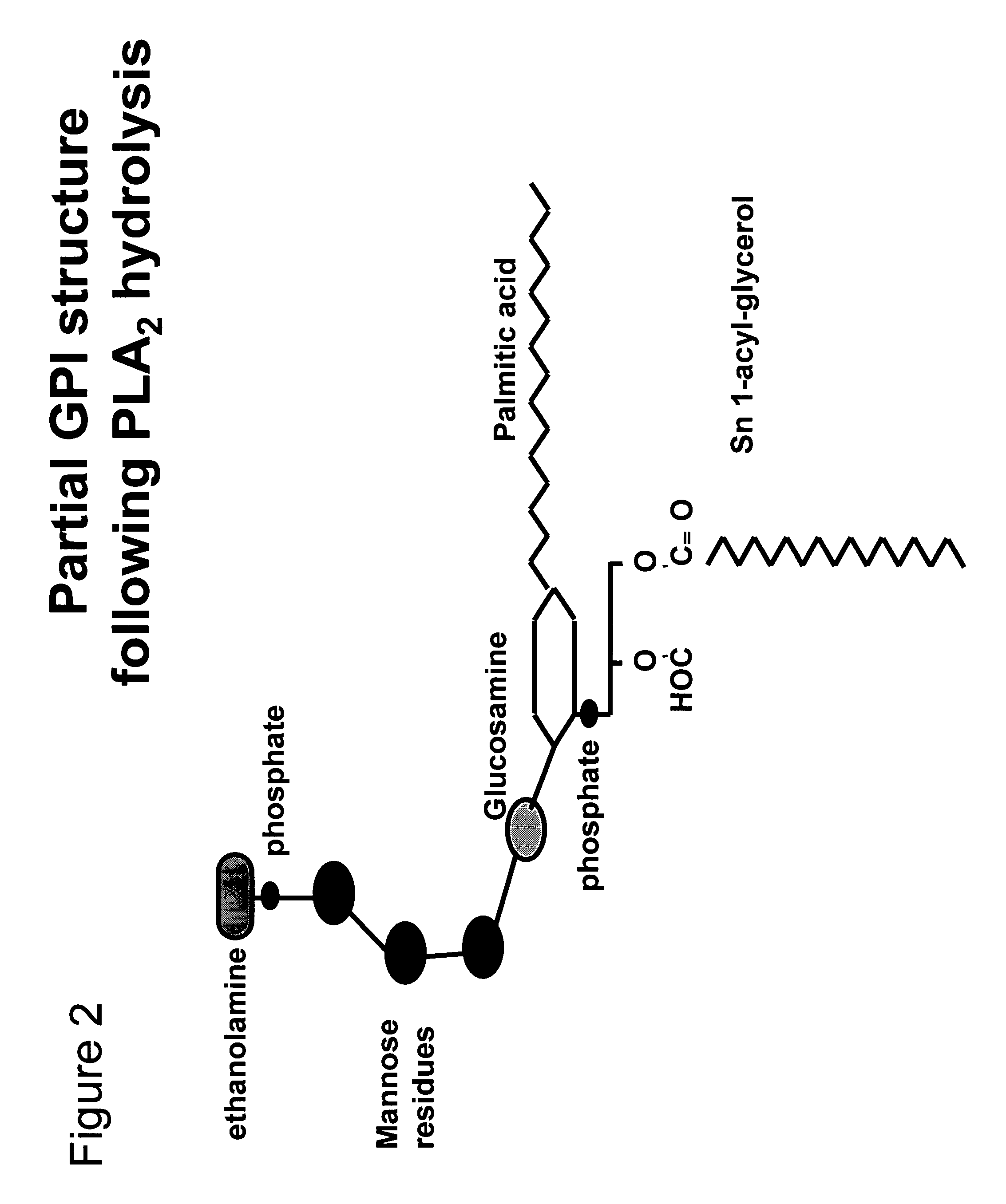
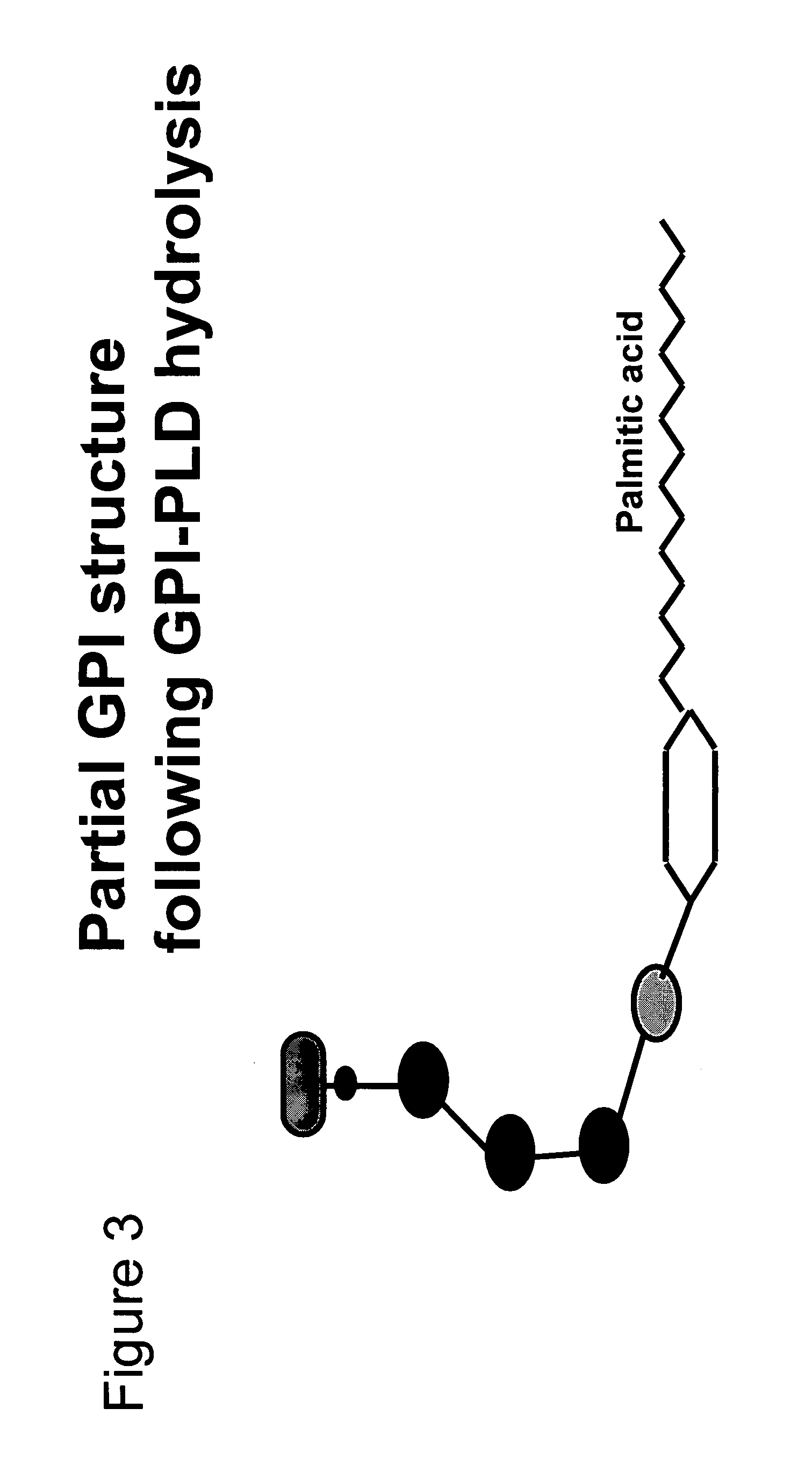
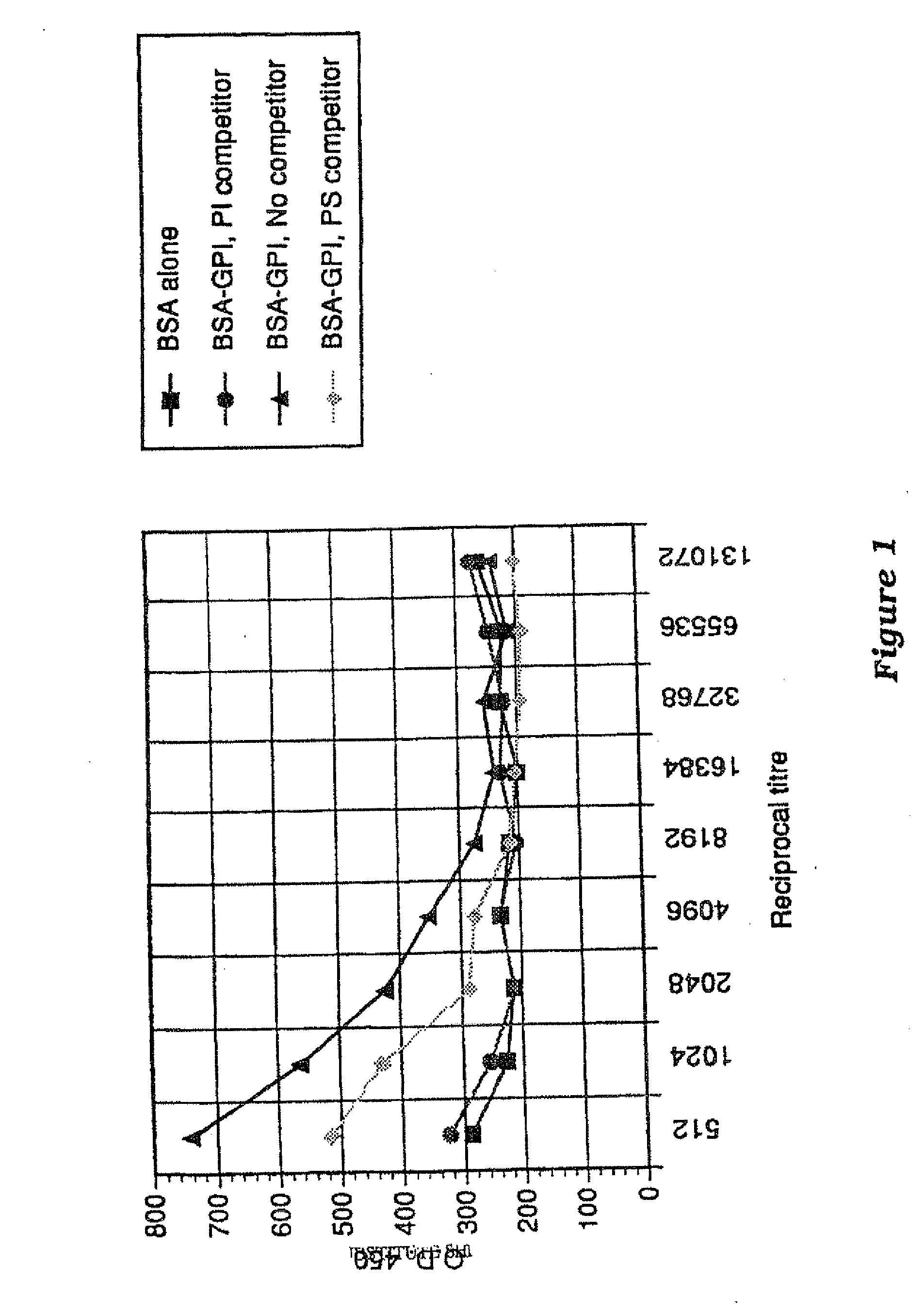
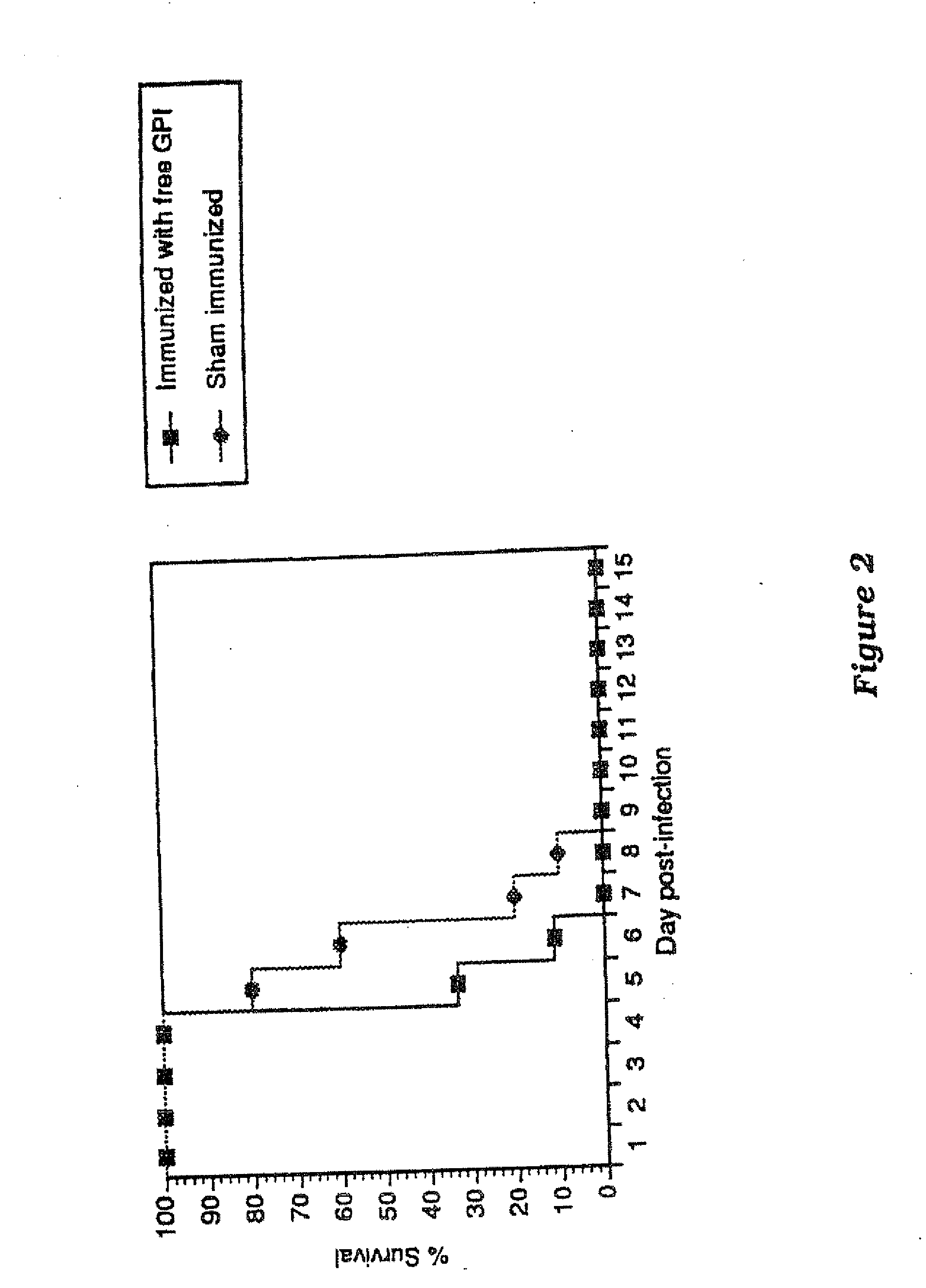
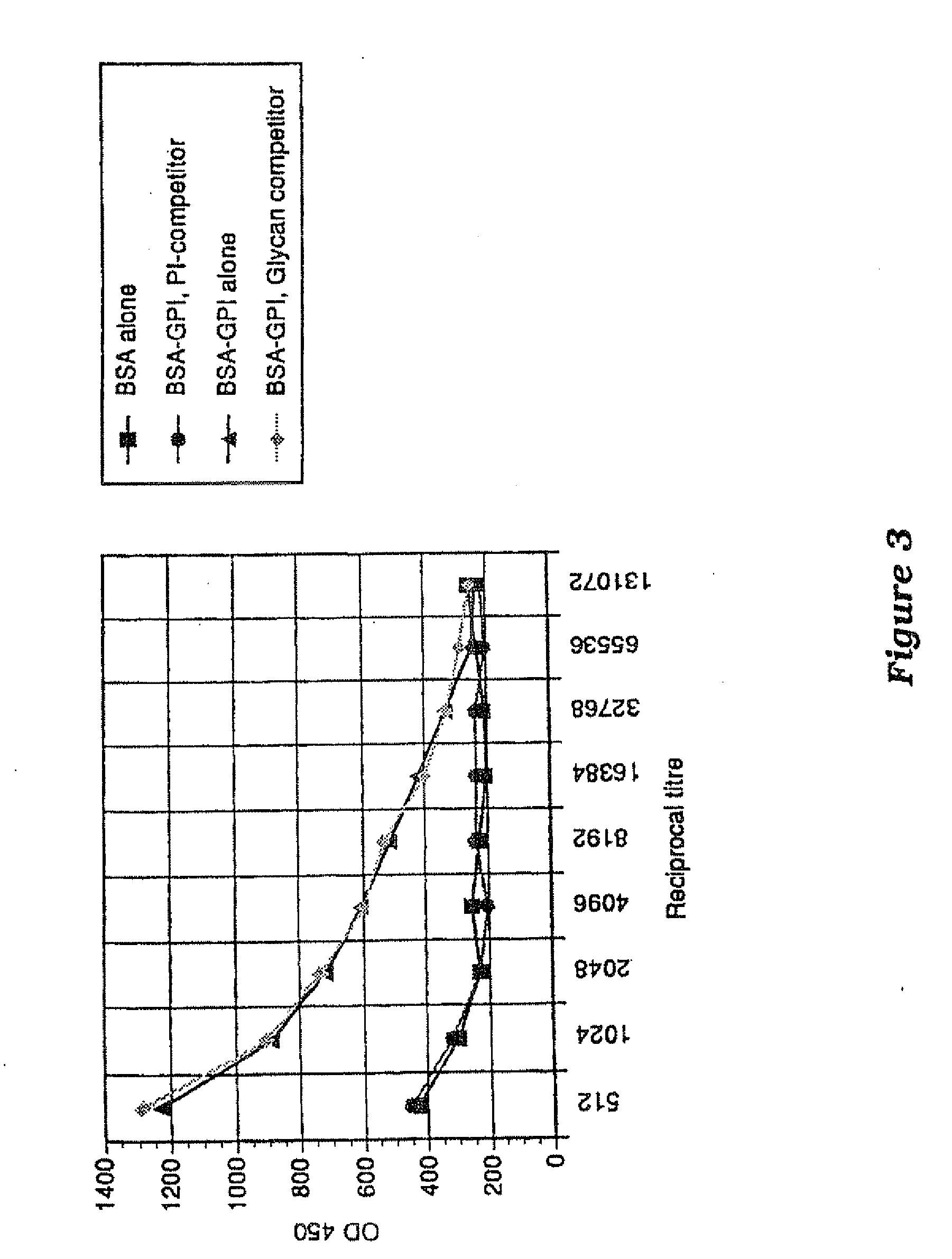
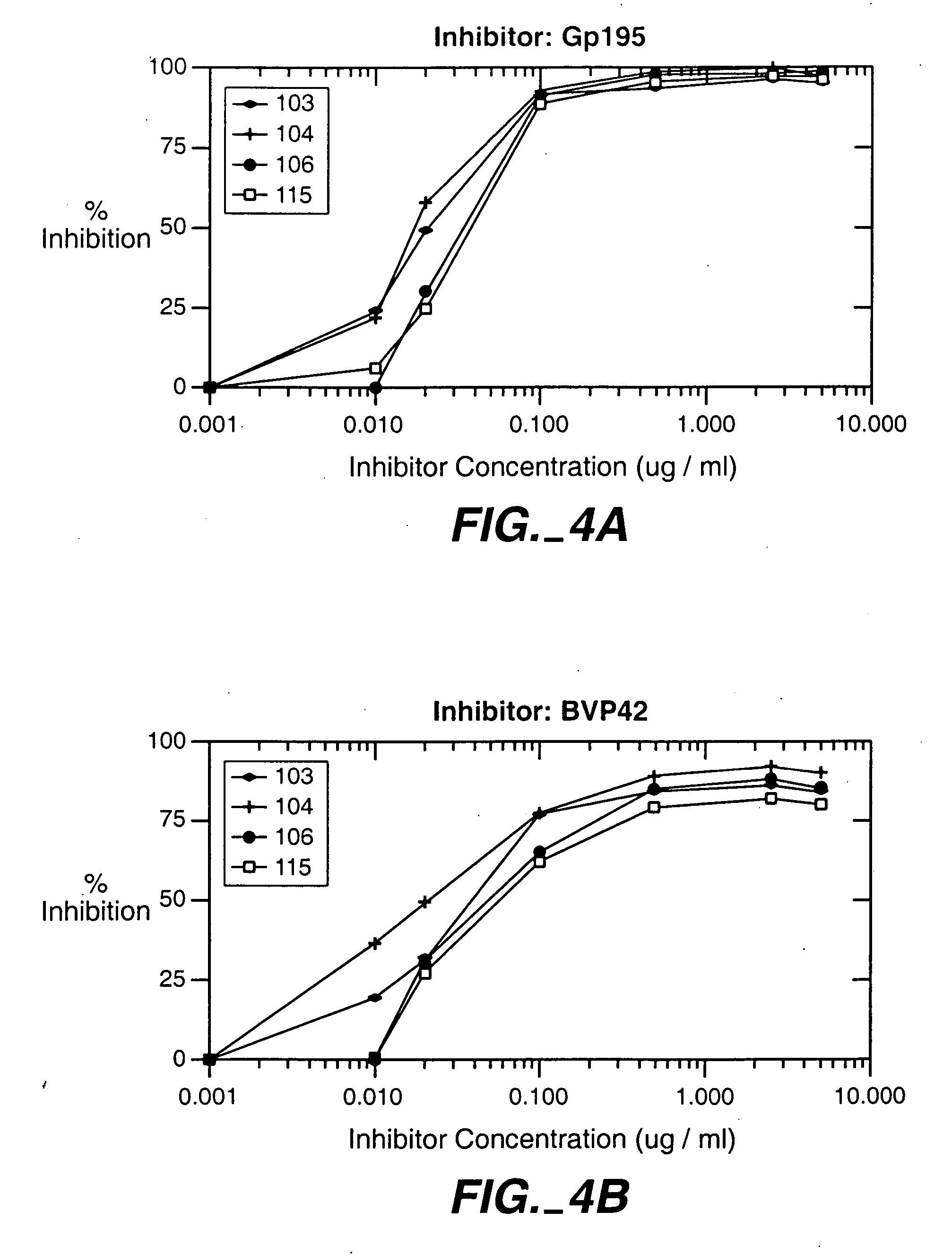
Immunogenic compositions and uses thereof
The present invention relates generally to a method of eliciting or otherwise inducing an effective immune response to a micro-organism and compositions for use therein. More particularly, the present invention relates to a method of inducing an immune response to a parasite utilising an immunogenic composition comprising a glycosylphosphatidylinositol (referred to herein as “GPI”) inositolglycan domain or its derivatives. Even more particularly, the present invention contemplates an immunogenic composition comprising the Plasmodium falciparum GPI inositolglycan domain or its derivatives. The present invention is useful, inter alia, as a prophylactic and / or therapeutic treatment for disease conditions such as, for example, infection by parasites and in particular infection by Plasmodium species.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
Recombinant P. falciparum merozoite protein-142 vaccine
InactiveUS7306806B2Eliminate the problemImprove responsePeptide/protein ingredientsImmunoglobulinsPlasmodium falciparumAntibody production
In this application is the expression and purification of a recombinant Plasmodium falciparum (3D7) MSP-142. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant MSP-142 is useful as a diagnostic reagent, for use in antibody production, and as a vaccine.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Recombinant P. falciparum merozoite protein-1 42 vaccine
InactiveUS20080075741A1Lower Level RequirementsReduce severityAntibacterial agentsBacteriaPlasmodium falciparumBiology
In this application is described the expression and purification of a recombinant Plasmodium falciparum (FVO) MSP-142. The method of the present invention produces a highly purified protein that retains folding and disulfide bridging of the native molecule. The recombinant MSP-142 is useful as a diagnostic reagent, for use in antibody production, and as a vaccine.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
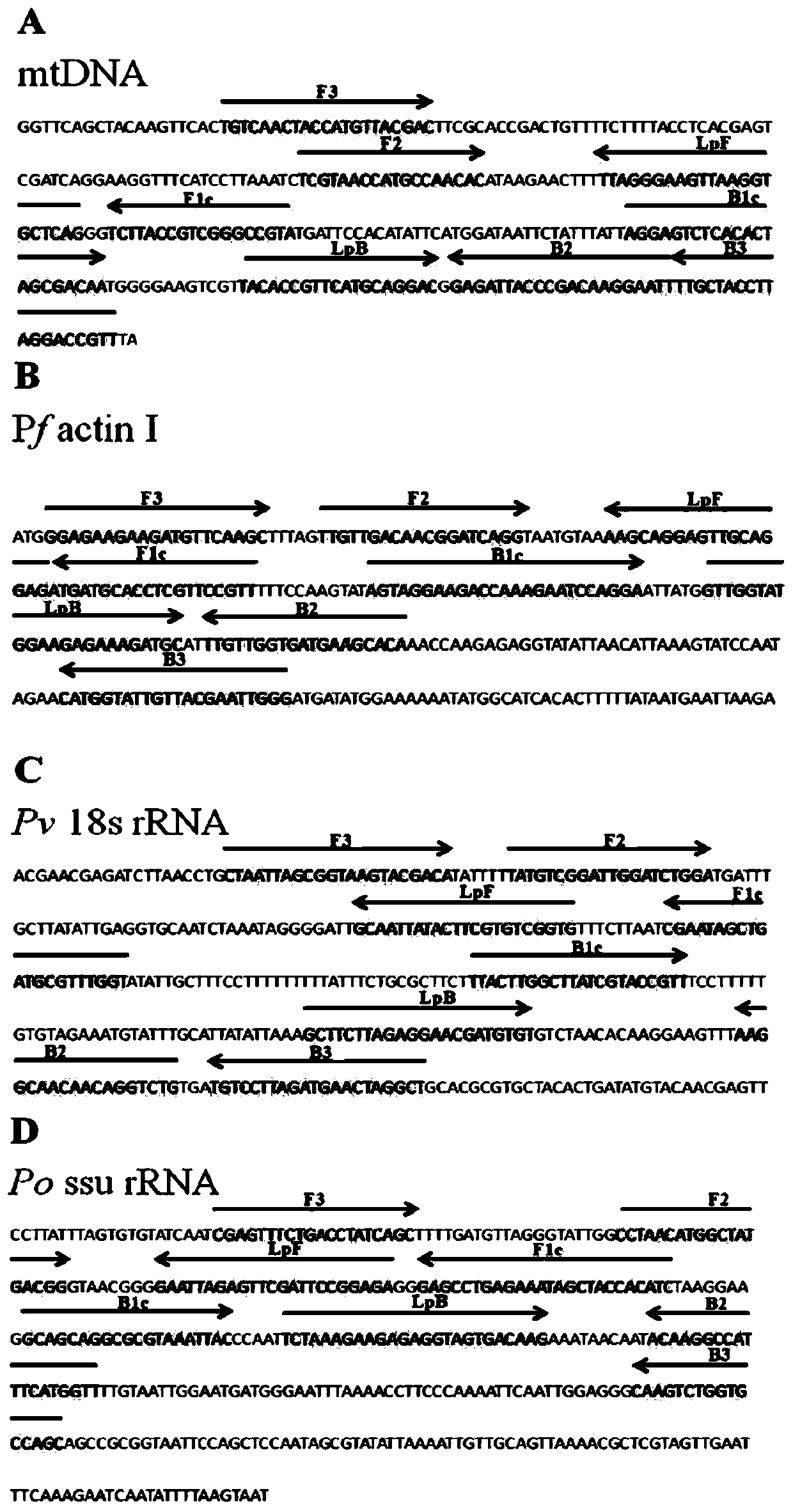
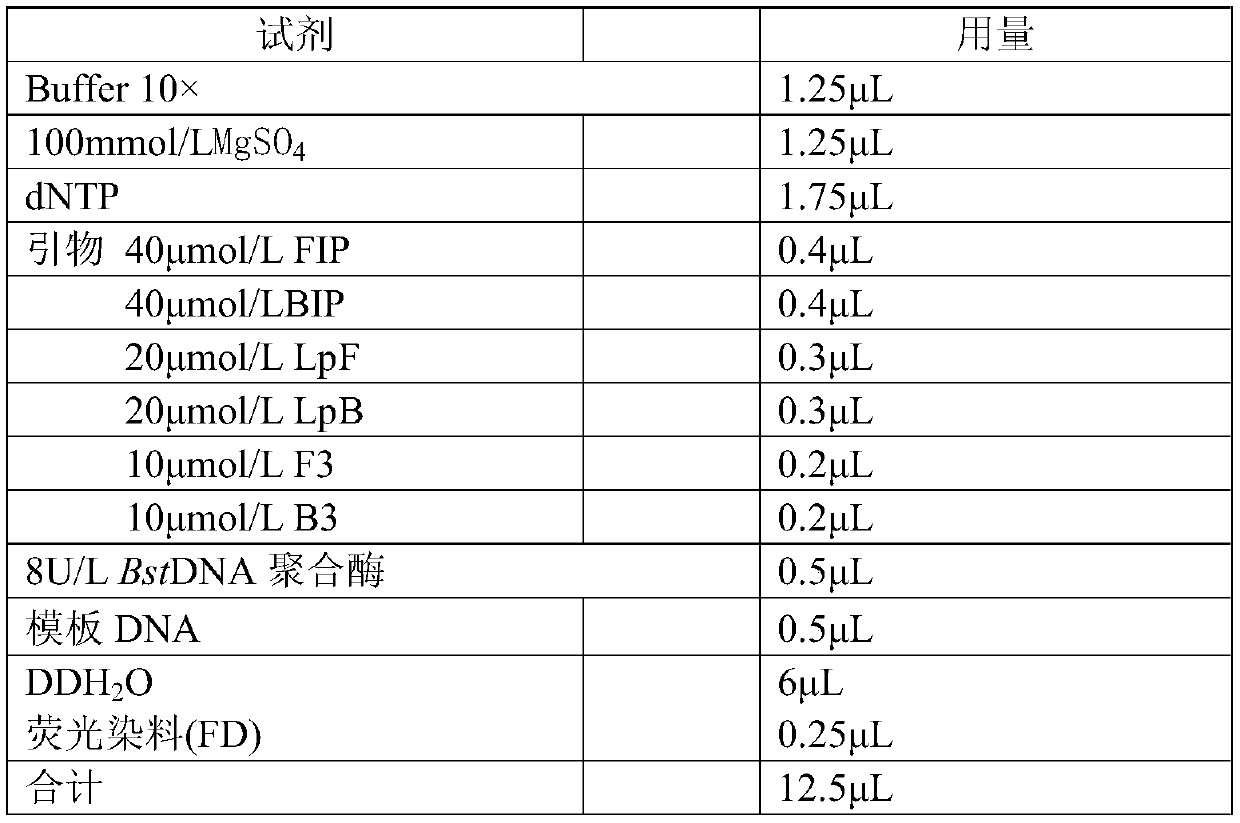
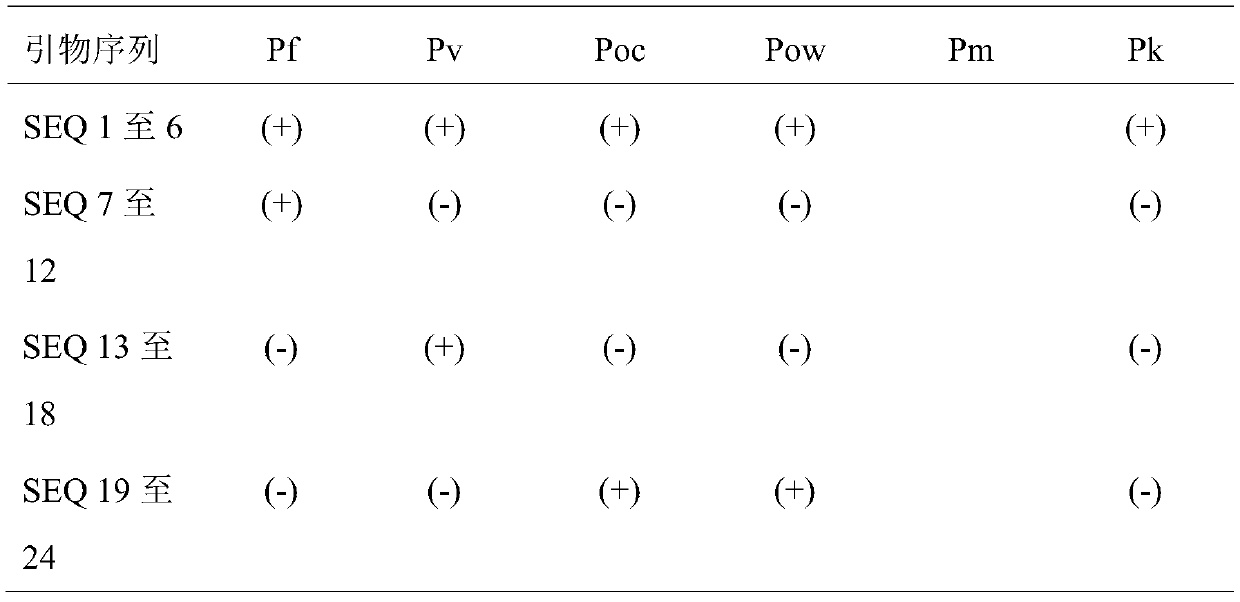
Plasmodium gene diagnosis primer
ActiveCN110331221ADetect the effect of treatmentMicrobiological testing/measurementAgainst vector-borne diseasesPlasmodium wenyoniLoop-mediated isothermal amplification
The present invention discloses a plasmodium gene diagnosis primer. The primer a loop-mediated isothermal amplification (LAMP) primer for detecting / identifying whether specific plasmodium and three species of plasmodium are present in samples. A use of the primer set can simultaneously or separately detect or identify infections of plasmodium and / or one or more of plasmodium falciparum, plasmodiumvivax and plasmodium ovale, gains precious time for diagnosis and treatment of malaria in China, and provides helps for preventing, controlling and eliminating prevalence of the malaria.
Owner:KUNMING MEDICAL UNIVERSITY
Immunogenic compositions and uses thereof
The present invention relates generally to a method of eliciting or otherwise inducing an effective immune response to a micro-organism and compositions for use therein. More particularly, the present invention relates to a method of inducing an immune response to a parasite utilising an immunogenic composition comprising a glycosylphosphatidylinositol (referred to herein as “GPIt”) inositolglycan domain or its derivatives. Even more particularly, the present invention contemplates an immunogenic composition comprising the Plasmodium falciparum GPI inositolglycan domain or its derivatives. The present invention is useful, inter alia, as a prophylactic and / or therapeutic treatment for disease conditions such as, for example, infection by parasites and in particular infection by Plasmodium species.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
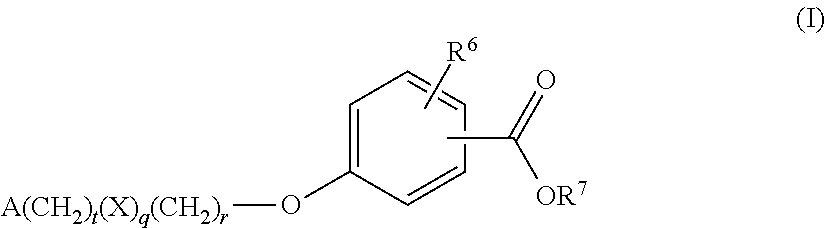
Benzoic acid compounds for reducing uric acid
Uric acid in mammalian subjects is reduced and excretion of uric acid is increased by administering a compound of Formula (I). The uric acid-lowering effects of the compounds of this invention are used to treat or prevent a variety of conditions including gout, hyperuricemia, elevated levels of uric acid that do not meet the levels customarily justifying a diagnosis of hyperuricemia, renal dysfunction, kidney stones, cardiovascular disease, risk for developing cardiovascular disease, tumor-lysis syndrome, cognitive impairment, early-onset essential hypertension, and Plasmodium falciparum-induced inflammation. In Formula I, t, q, r, R6, R7, X and A are as defined herein.
Owner:WELLSTAT THERAPEUTICS
Anti-parasitic complexes
InactiveUS10344082B2ImmunoglobulinsPharmaceutical non-active ingredientsCell surface structurePlasmodiidae
The technology provided herein relates to novel anti-parasitic complexes, in particular recombinant fusion proteins suitable as human and / or animal drugs against a parasite of the phylum Apicomplexa, in particular against Plasmodium falciparum (P. falciparum) comprising at least one component A and at least one component B, characterized in that component A has a binding activity for cellular surface structures presented on the surface of a parasite of the phylum Apicomplexa or for parasitic antigens presented on a parasitized host cell, and component B is a compound having anti-parasitic activity.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Molecular markers for five important pathogens and application thereof
InactiveCN106047993AAccurate identificationHigh resolutionMicrobiological testing/measurementAgainst vector-borne diseasesNucleotideYersinia pestis
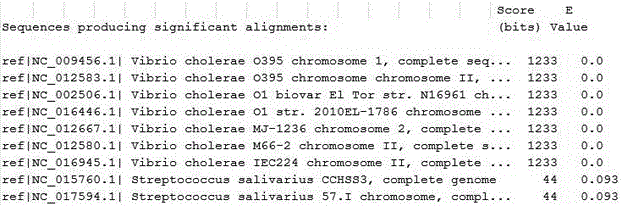
The invention discloses a method for using nucleotide DNA fragments as molecular markers for identification of five important pathogens, i.e., Yersinia pestis, Plasmodium falciparum, Vibrio cholera, Leishmania donovani and Trypanosoma brucei. The nucleotide DNA fragments can be used as effective genetic markers for rapid and accurate identification of pathogens, are beneficial for accurate and rapid discrimination of populations migrated from an original infection site and provide bases for molecular epidemiological study of pathogens.
Owner:SHANGHAI QY BIOTECH CO LTD
Fluorescent latex immunochromatography detection reagent for detecting falciparum malaria and preparation method thereof
InactiveCN105823892ASimple and fast operationLow costBiological material analysisBiological testingFluorescenceMonoclonal antibody
The invention provides a fluorescent latex immunochromatography detection reagent for detecting falciparum malaria and a preparation method thereof. The fluorescent latex immunochromatography detection reagent comprises a sample mat, a combining mat, a reaction film and a water absorption mat. The combining mat is coated with an anti-HRP-2 monoclonal antibody marked by fluorescent latex and anti-rabbit IgG, a detecting line and a quality control line are arranged on the reaction film, the detecting line is coated with an anti-HRP-2 monoclonal antibody, and the quality control line is coated with rabbit IgG. The fluorescent latex immunochromatography detection reagent for detecting falciparum malaria can fast detect plasmodium falciparum, and is fast to operate, low in cost and suitable for clinical use and field use.
Owner:GUANGZHOU WONDFO BIOTECH
3-Benzyloxyphenyloxoacetic Acid Compounds for Reducing Uric Acid
Uric acid in mammalian subjects is reduced and excretion of uric acid is increased by administering a compound of Formula (I) or its pharmaceutically acceptable salts. The uric acid-lowering effects of the compounds of this invention are used to treat or prevent a variety of conditions including gout, hyperuricemia, elevated levels of uric acid that do not meet the levels customarily justifying a diagnosis of hyperuricemia, renal dysfunction, kidney stones, cardiovascular disease, risk for developing cardiovascular disease, tumor-lysis syndrome, cognitive impairment, early-onset essential hypertension, and Plasmodium falciparum-induced inflammation. R1 is hydrogen or alkyl having from 1 to 3 carbon atoms. R2 is alkyl having from 1 to 3 carbon atoms, alkoxy having from 1 to 3 carbon atoms, hydroxy, nitro, halo, thio, alkylthio, or cyano. R3 and R4 are each independently hydrogen, methyl, ethyl, perfluoromethyl, methoxy, ethoxy, perfluoromethoxy, halo, hydroxy, nitro, or amino.
Owner:SHARMA SHALINI +1
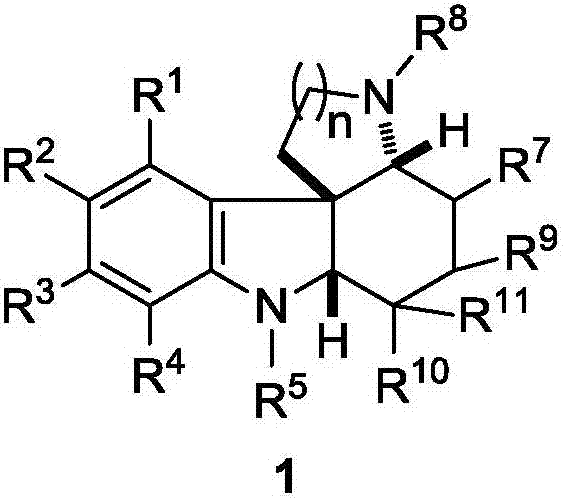
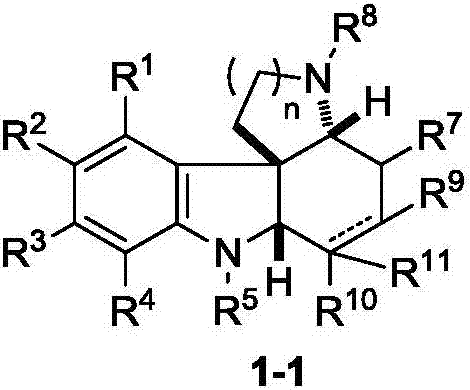
Fused polycyclic indoline compound and preparation method thereof as well as pharmaceutical composition and application
ActiveCN106957318AEnhanced inhibitory effectImprove biological activityOrganic active ingredientsOrganic chemistryPolycyclic compoundChemical compound
The invention discloses a fused polycyclic indoline compound and a preparation method thereof as well as a pharmaceutical composition and application. The compound provided by the invention has an obvious inhibiting effect on tumor cells Kyse-450, MDA-MB-231 and SKGT-4 and has remarkable bioactivity for plasmodium falciparum dd2 having drug resistance for chloroquine; meanwhile, the preparation method disclosed by the invention has the advantages of high reaction yield, short synthesis steps, wide range of suitable substituent group and simple operation, and is expected to realize an industrial application prospect. (The formula is shown in the description).
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Rapid and sensitive plasmodium falciparum detection method
InactiveCN104531840AMeet the requirements of safety testingMeet the testing requirementsMicrobiological testing/measurementMicroorganism based processesWorkloadPlasmodium falciparum
The invention relates to a rapid and sensitive plasmodium falciparum detection method. The detection method integrates two powerful molecular biology techniques of a polymerase chain reaction PCR and a microarray, PCR hybridized probes are directly fixed on a hybridization cabin in the microarray and are on a same chip with a PCR reaction chamber; the detection method includes the steps of extracting a blood sample DNA liquid, carrying out PCR amplification, hybridizing, cleaning, and reading and discriminating a result. The method can quickly and sensitively detect plasmodium falciparum, can greatly improve the detection efficiency of front line inspection and quarantine personnel of import and export ports, not only can reduce the workload, but also furthest solves a positive detection missing problem possibly existing in a traditional detection method, so as to furthest prevent generation of plasmodium epidemic situation.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Anti-malaria compositions and methods
Described herein are multilayer films that include modified polypeptide epitopes from Plasmodium falciparum, specifically a modified T* epitope. The multilayer films are capable of eliciting an immuneresponse in a host upon administration to the host. The multilayer films can include at least one designed peptide that includes the modified T* polypeptide epitope from a Plasmodium protozoan.
Owner:ARTIFICAL CELL TECH INC
Construction and pplication of thermostable yeast engineering strain for L-lactic acid production
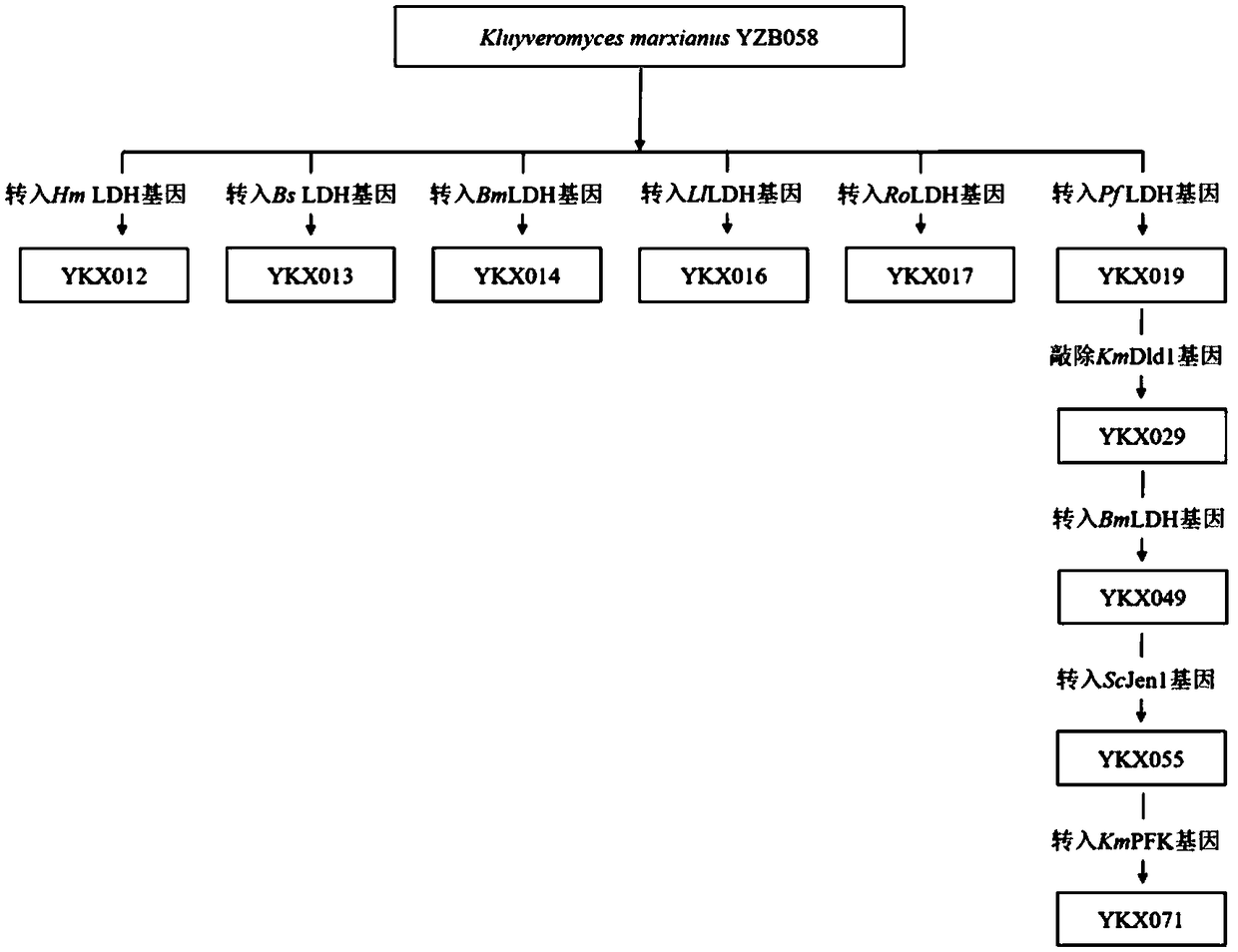
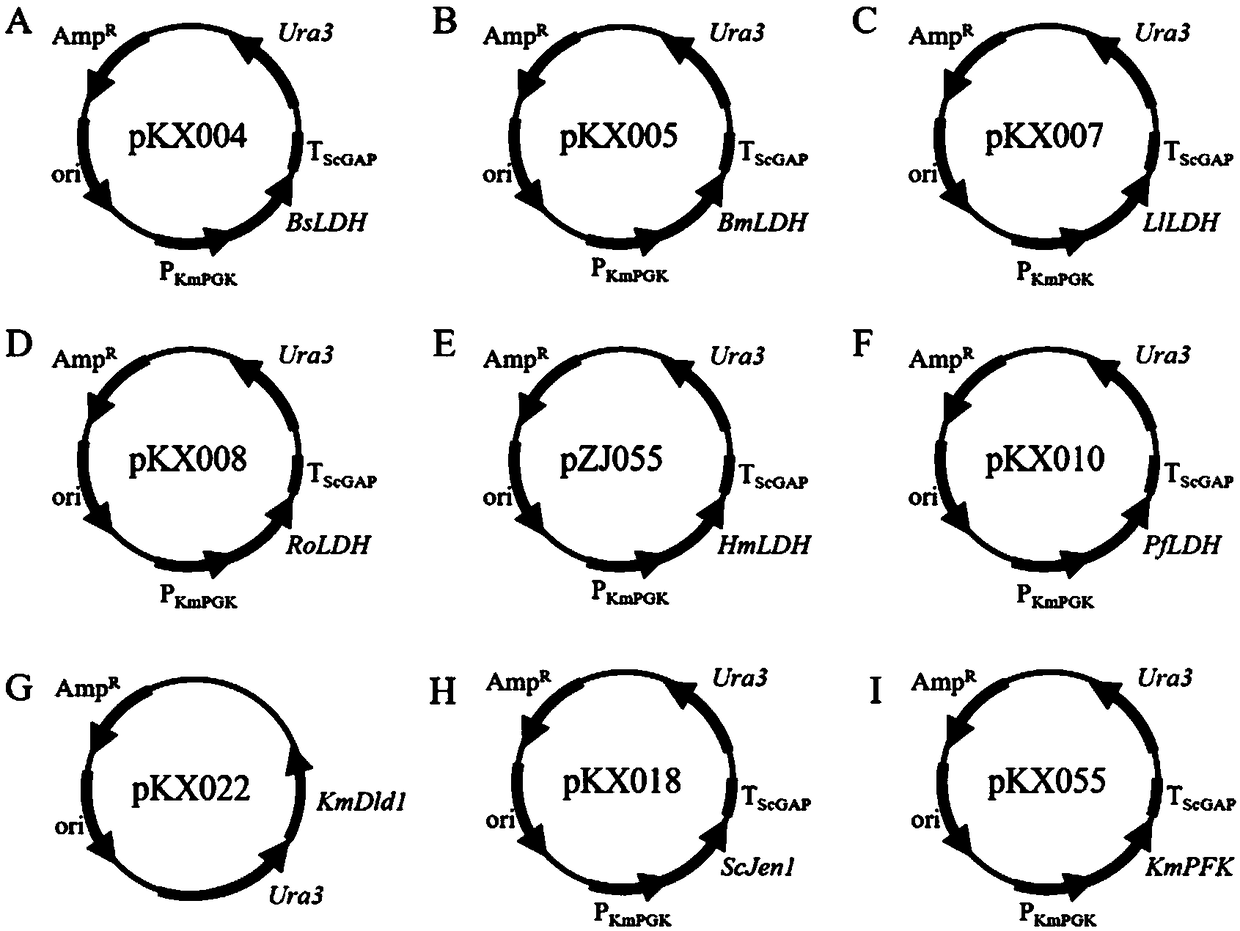
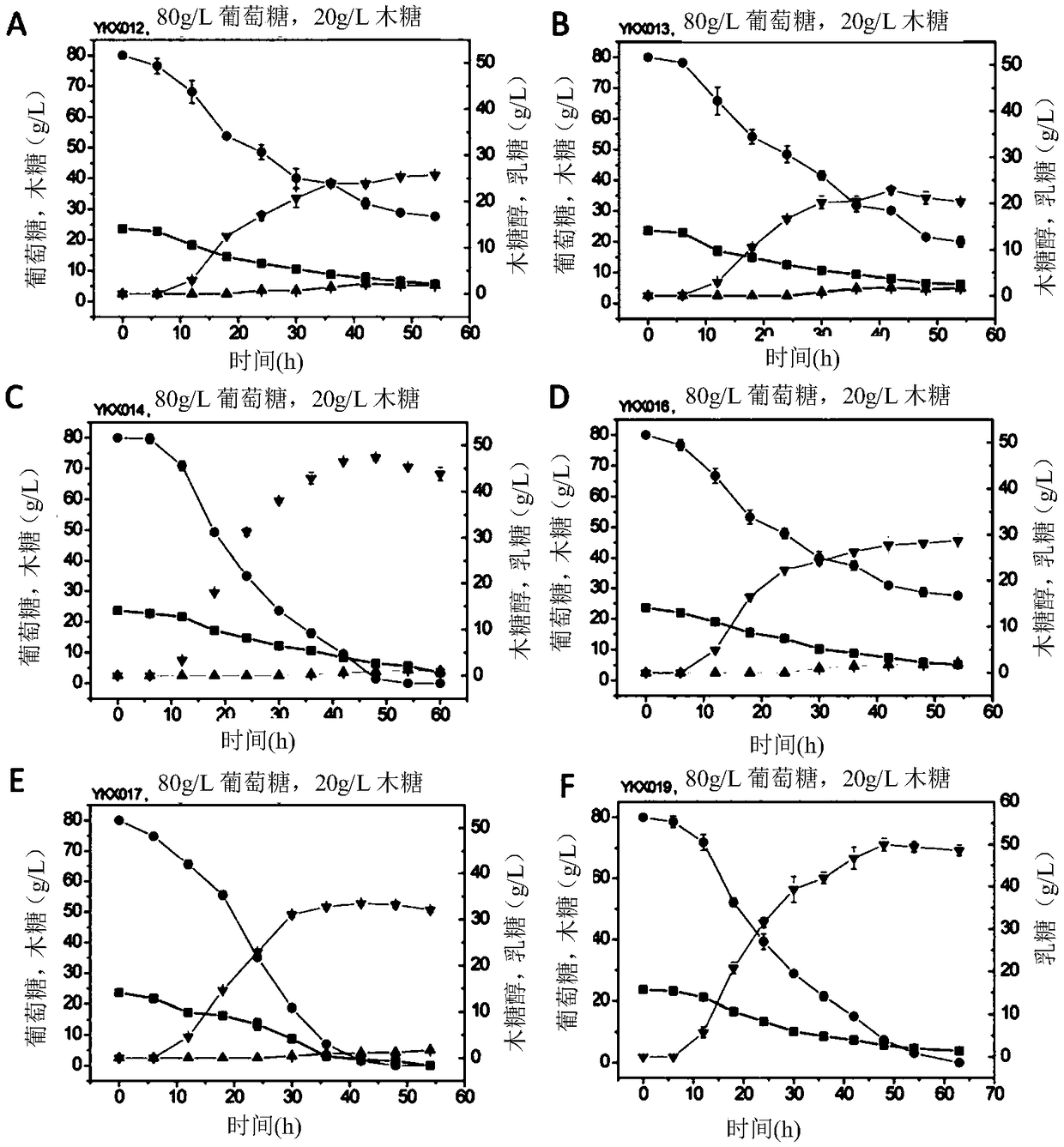
The present invention relates to construction and application of thermostable yeast engineering strain for L-lactic acid production. The invention provides an L-lactic acid production yeast engineering strain. The yeast engineering strain is Kluyveromyces marxianus engineering stain prepared by recombinant expression of Plasmodium falciparum L-lactate dehydrogenase gene PfLDH and / or Bacillus megaterium L-lactate dehydrogenase gene BmLDH, for example, the deposit number of the engineering strain is CGMCC No.16192. The invention also provides a method for constructing the engineering strain, which is used in L-lactic acid production and method for production of L-actic acid. The engineering strain of the invention can efficiently co-utilize glucose and xylose for fermentation at relatively high temperature (for example, 42 DEG C), and can rapidly produce large quantities of high optical purity L-lactic acid, can also be quickly and efficiently used as a representative of corncob lignocellulose fermentation production of L-lactic acid, is the first lignocellulosic substances can be used to produce lactic acid engineering yeast.
Owner:UNIV OF SCI & TECH OF CHINA
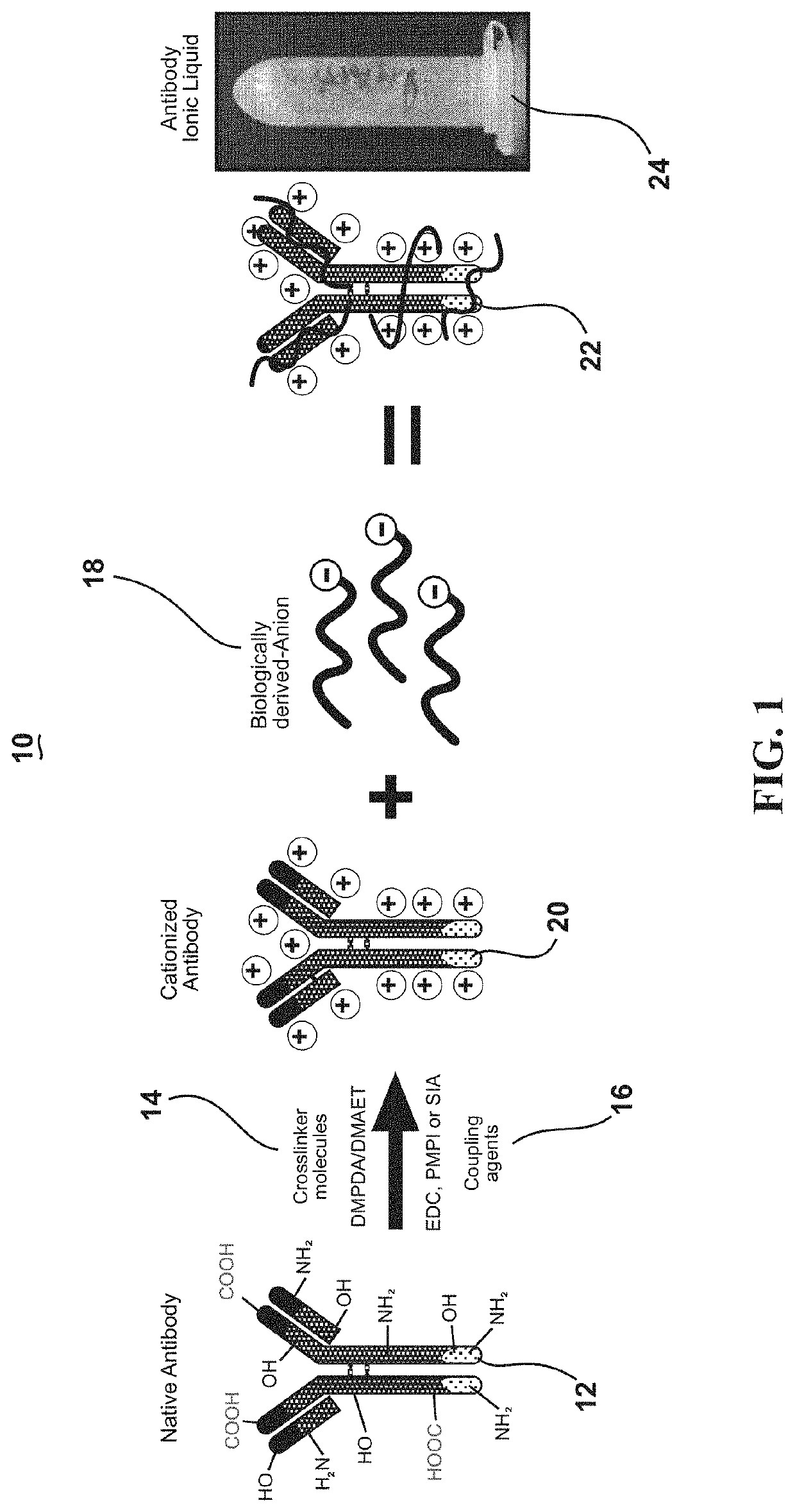
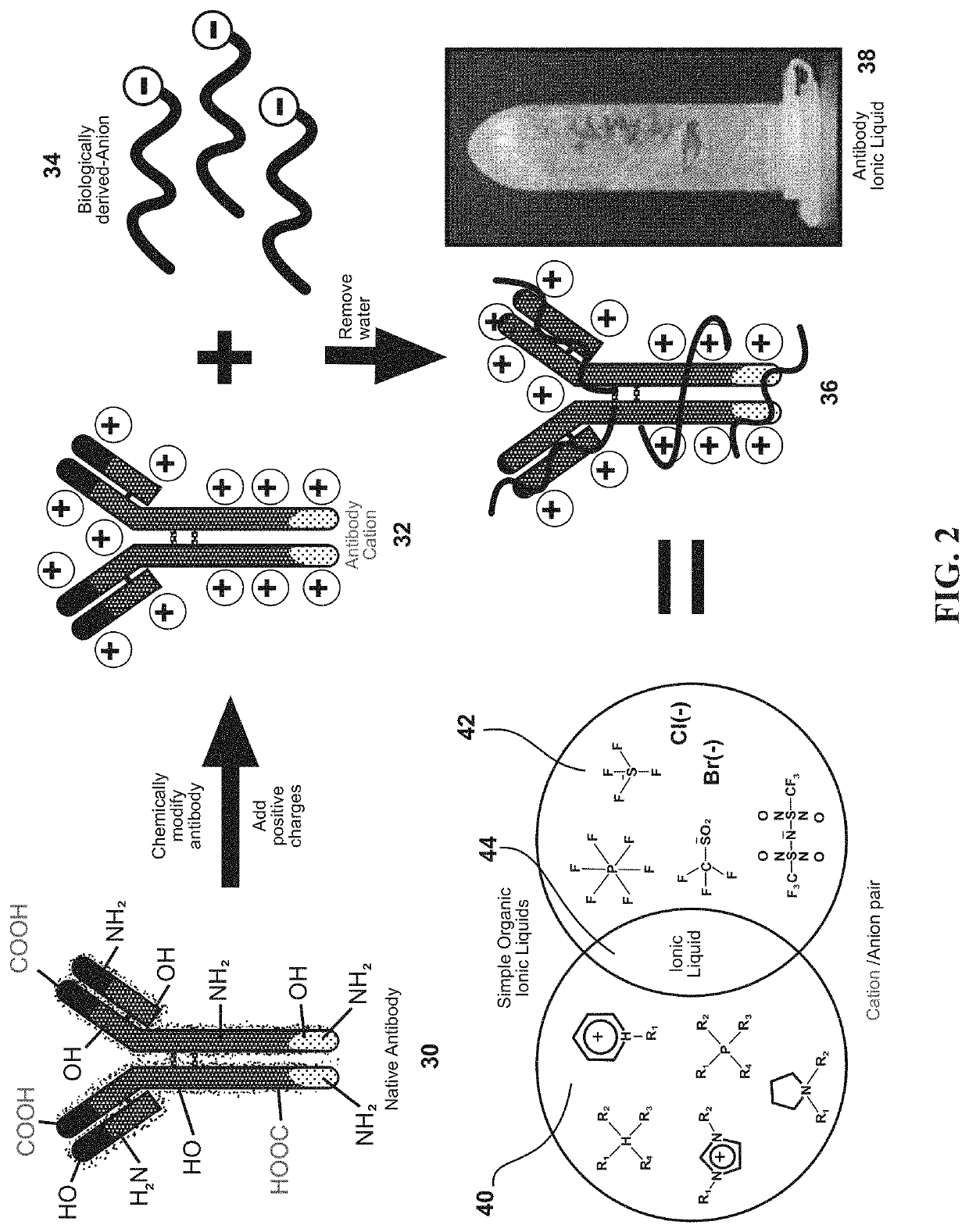
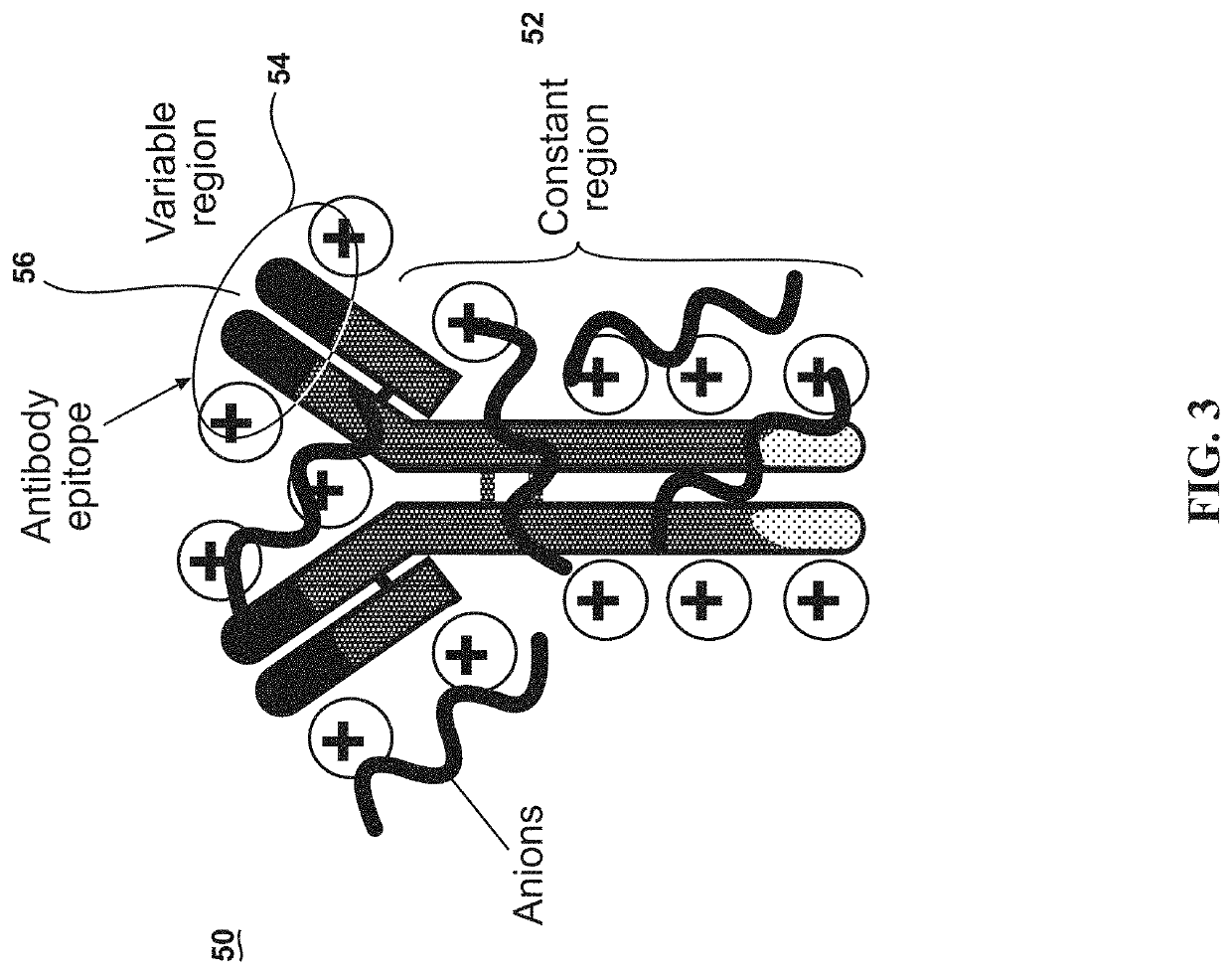
Ultrastable antibody ionic liquids
ActiveUS11058770B1Confirming cationizingLow melting pointAntibody ingredientsImmunoglobulinsAntiendomysial antibodiesSingle-Chain Antibodies
A stable protein ionic liquid, comprising an anti-hemoglobin cation / anion pair. The anti-hemoglobin cation / anion pair may be an anionic polymer of poly(ethylene glycol) 4-nonylphenyl 3-sulfopropyl ether. The anti-hemoglobin cation / anion pair may further comprise a cationized anti-hemoglobin antibody, single-chain antibodies from camelids, antibody fragments, polyclonal Anti-horse spleen ferritin antibodies, monoclonal Anti-Flag antibodies, monoclonal Anti-HRP2 to Plasmodium falciparum, polyclonal Anti-neuropeptide Y, polyclonal Anti-human troponin, isotypes of antibodies, or combinations of multiple antibodies.
Owner:THE UNITED STATES OF AMERICA AS REPRESETNED BY THE SEC OF THE AIR FORCE
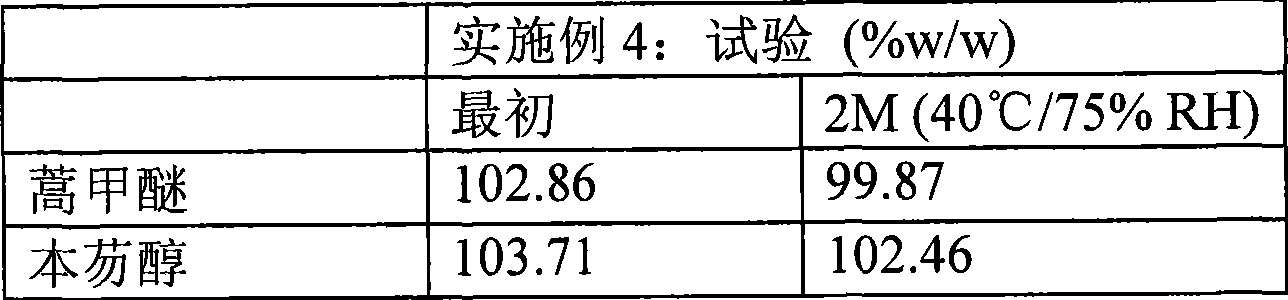
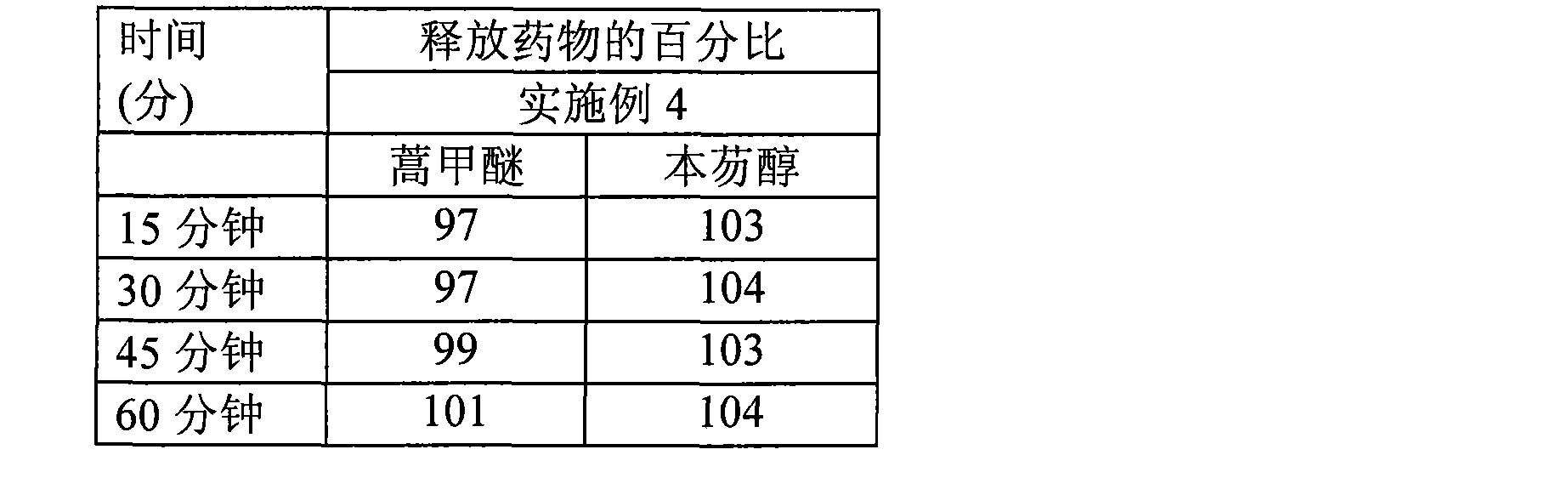
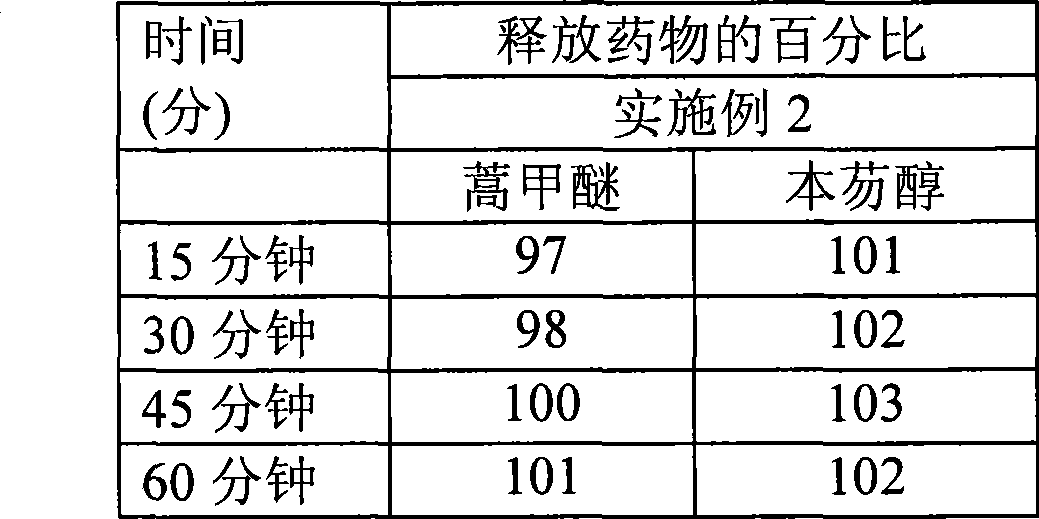
High dose oral pharmaceutical compositions of artemether and lumefantrine
InactiveCN101478965AAntiparasitic agentsHeterocyclic compound active ingredientsLumefantrineHigh doses
The present invention relates to high dose oral pharmaceutical compositions of artemether and lumefantrine, and process for preparation thereof. The compositions comprise of artemether and lumefantrine comprising artemether in an amount of from about 40 mg to about 80 mg, lumefantrine in an amount of from about 240 mg to about 480 mg. The compositions are useful for treatment of uncomplicated infections with Plasmodium falciparum, including strains from multi-drug-resistant areas.
Owner:RANBAXY LAB LTD
Baculovirus produced Plasmodium falciparum vaccine
Owner:UNIV OF HAWAII
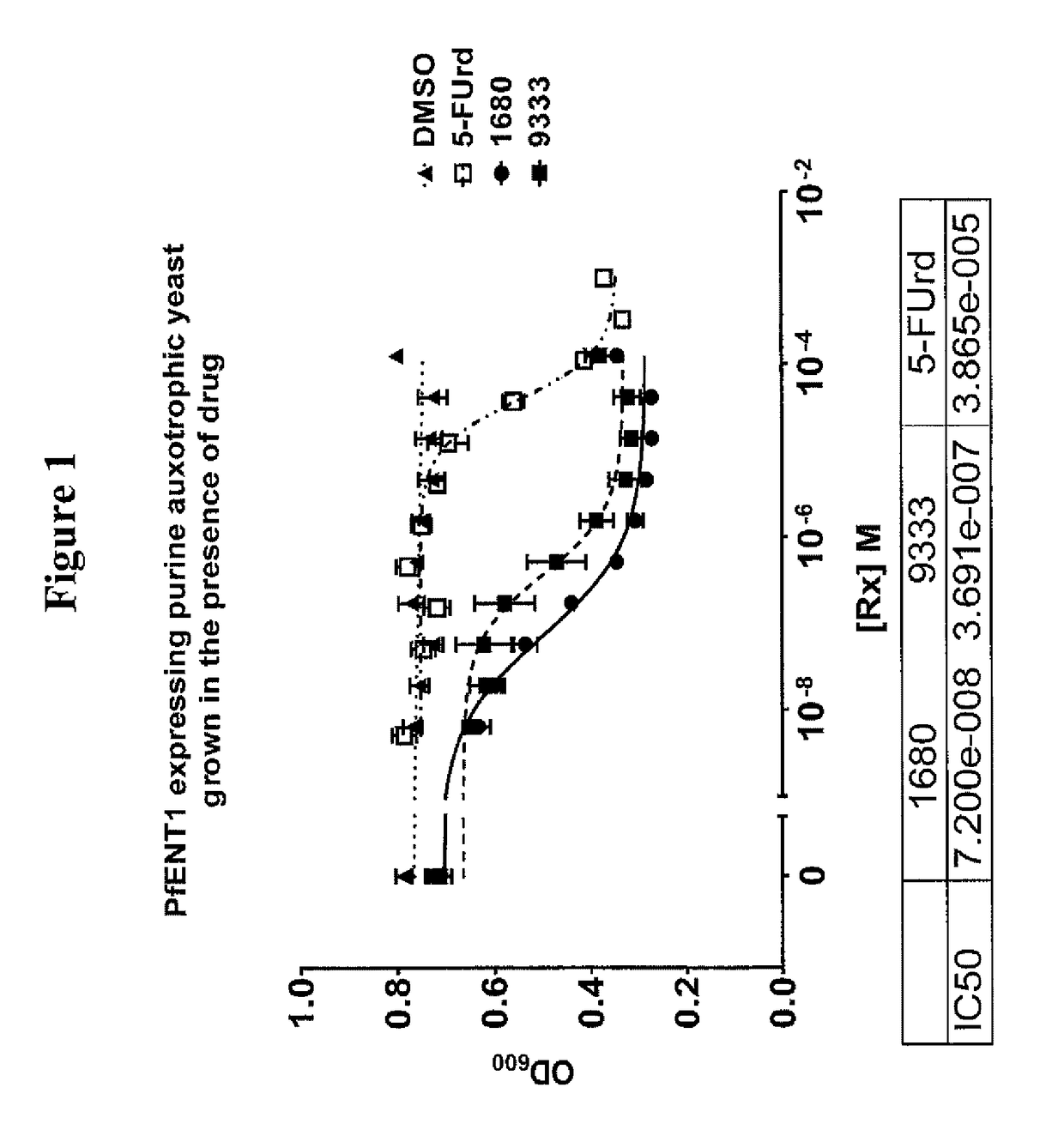
Inhibitors of Plasmodium falciparum equilibrative nucleoside transporter type I as anti-parasitic compounds
InactiveUS9695193B2Organic active ingredientsOrganic chemistryEquilibrative nucleoside transporter 1Equilibrative nucleoside transporter
Inhibitors of Plasmodium falciparum equilibrative nucleoside transporter type 1 are identified and methods of use as anti-parasitic compounds are provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com