Anti-malaria compositions and methods
A compositional and antigenic technology, applied in drug combinations, resistance to vector-borne diseases, anti-infectives, etc., can solve problems such as low efficacy, controversial efficacy, and inability to expand the actual number.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1: Exemplary Peptide Design and Synthesis
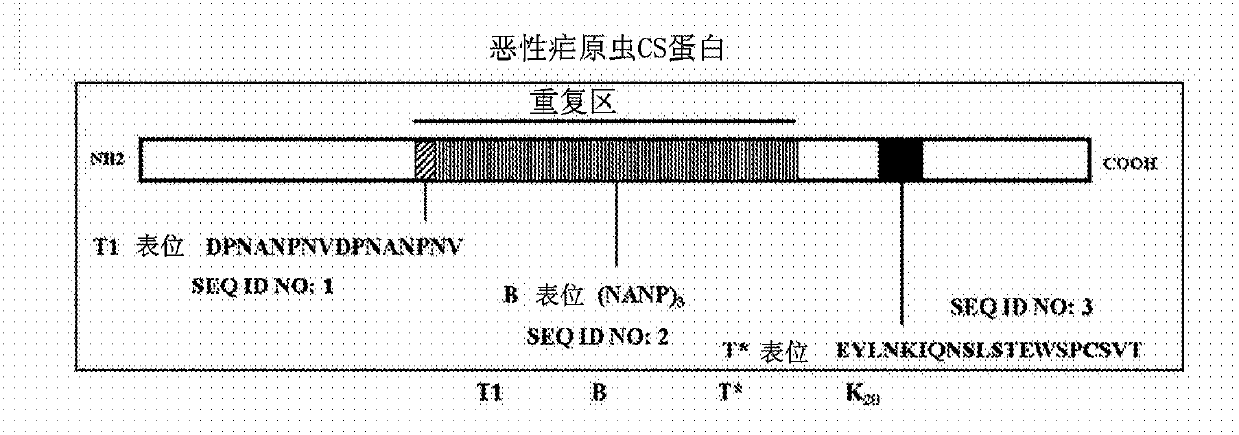
[0132] The designed peptides are based on the T1BT* multivalent peptide of Plasmodium falciparum CS. The surface adsorption area K 20 (SEQ ID NO: 9) or K 20 Y (SEQ ID NO: 16) was added to the C-terminus to generate a designed polypeptide (DP) for incorporation into LbL particles ( figure 1 ). When Pam3Cys was conjugated to DP, the linker sequence SKKKK was also added. Peptides were synthesized and prepared as C-terminal amides using standard solid-phase peptide chemistry methods. Briefly, fluorenylmethoxycarbonyl (Fmoc) amino acids were double-coupled to Rink MBHA amide resin with a CEM Liberty microwave peptide synthesizer using the manufacturer's synthetic protocol with minor changes in the coupling temperature. After peptide synthesis, the Pam3Cys group was added to the resin by manual coupling of Pam3Cys-OH or automatic coupling of Fmoc-Pam2Cys-OH, followed by Fmoc removal and final capping step with palmitic ...
Embodiment 2
[0147] Example 2: LBL manufacture of vaccine microparticles
[0148] 20cm is equipped for LBL 2 ,500kD(MWCO) mPES filter module from SpectrumLabs (Rancho Dominiguez, CA) Research IIi tangential flow filtration system. Poly-L-glutamic acid sodium salt (PGA) and poly-L-lysine hydrobromide (PLL) were obtained from Sigma-Aldrich, USA (catalog numbers P4636 and P6516, respectively). Using a modified version of the method reported by Volodkin et al., from 0.33MCaCl 2 and 0.33M Na 2 CO 3 Co-precipitated spherical, mesoporous CaCO 3 Core (2 to 5 μm). (D.V. Volodkin et al. Adv. Funct. Mater. 2012, 1). All steps were performed at room temperature.
[0149] TFF unit filled with 20 mL of 3% CaCO 3 (dry weight) microparticle suspension that was kept in constant circulation at 40 mL / min during the treatment. The particles were washed by infiltration with 100 mL of 10 mM HEPES buffer pH 7.4. The permeation valve was closed and a 5.0 mL aliquot of 6.3 mg / mL PLL was added in a si...
Embodiment 3
[0152] Example 3: Immunophenotype induced by immunization with T1BT* microparticles.
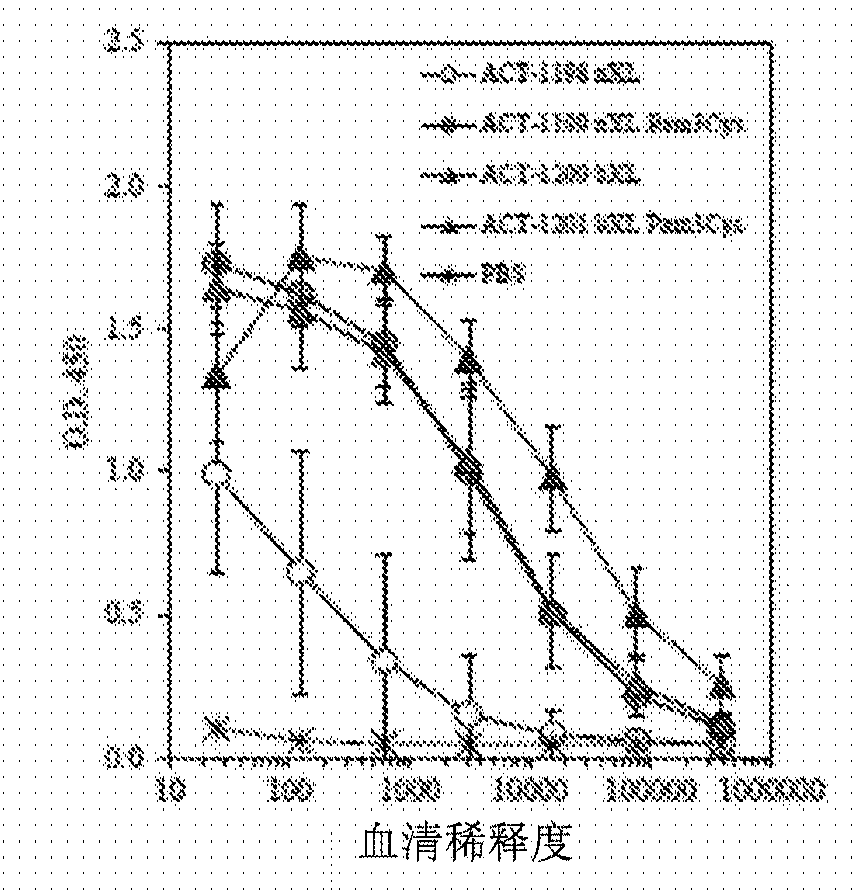
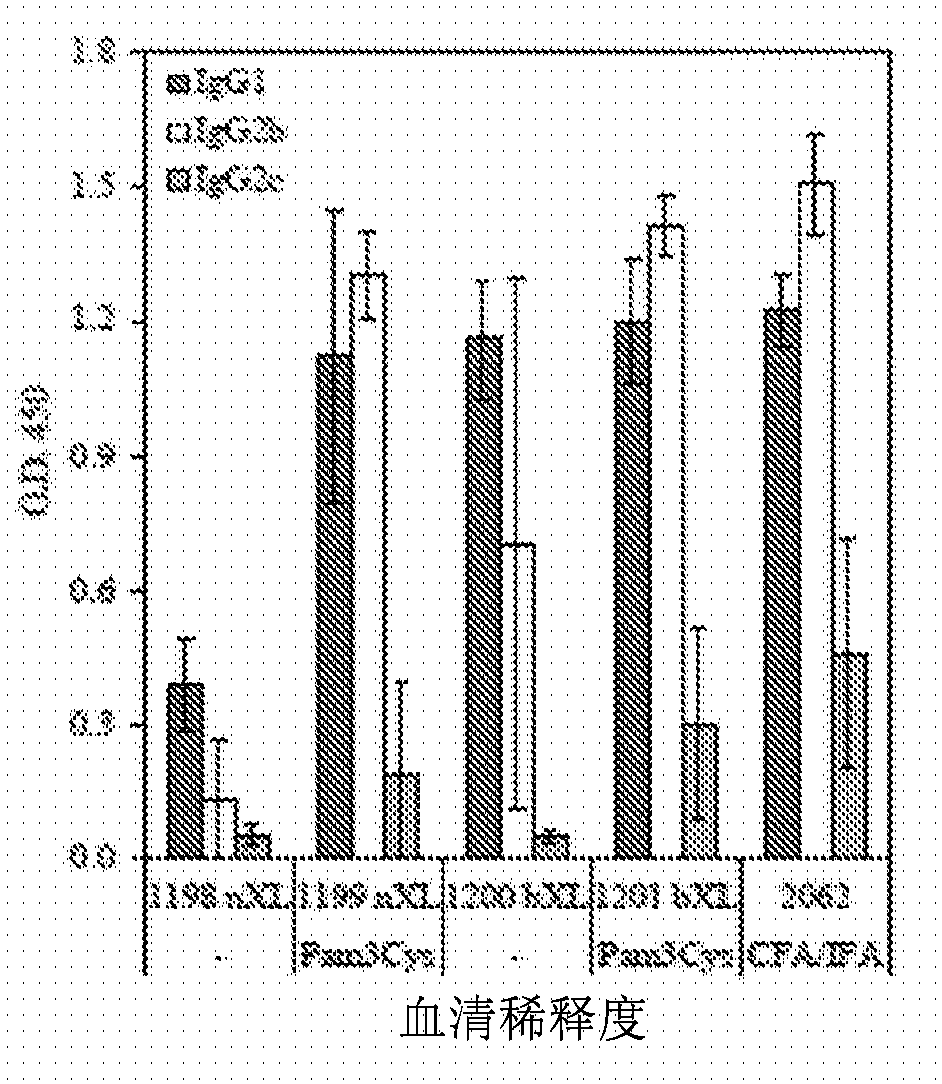
[0153] C57BL / 6J mice were immunized with the indicated constructs on days 0, 21 and 42. like figure 2 Sera collected on day 49 were tested in an ELISA against T1B peptide as indicated in . Results show mean ± SD of 10 mice per group. like image 3 Sera were tested at 1:250 and plates were probed with isotype-specific detection antibodies as indicated in . Results show mean ± SD of 10 mice per group. Splenocytes were harvested on day 49 and restimulated with T1B peptide in IFNγ and IL-5 ELISPOT plates as Figure 4 shown in . Data depict mean ± SD of 3 mice per group. nXL = no crosslinking, bXL = base layer crosslinking.
[0154] These results demonstrate that LBL microparticles loaded with designed peptides comprising the T1BT* subunit peptide of the P. falciparum circumsporozoite protein elicit both humoral and cellular immune responses against the contained antigenic epitopes. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com