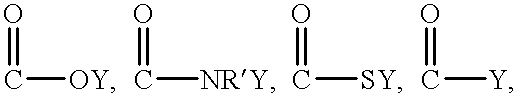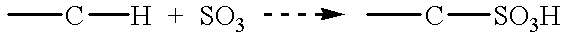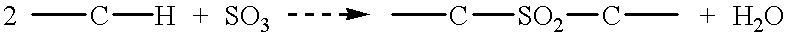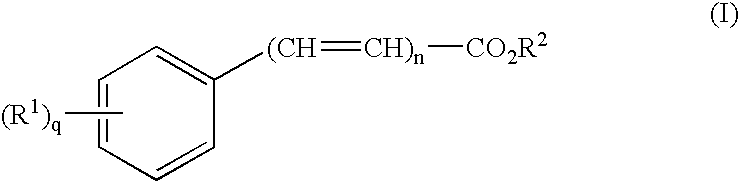Patents
Literature
15687results about "Sheet delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
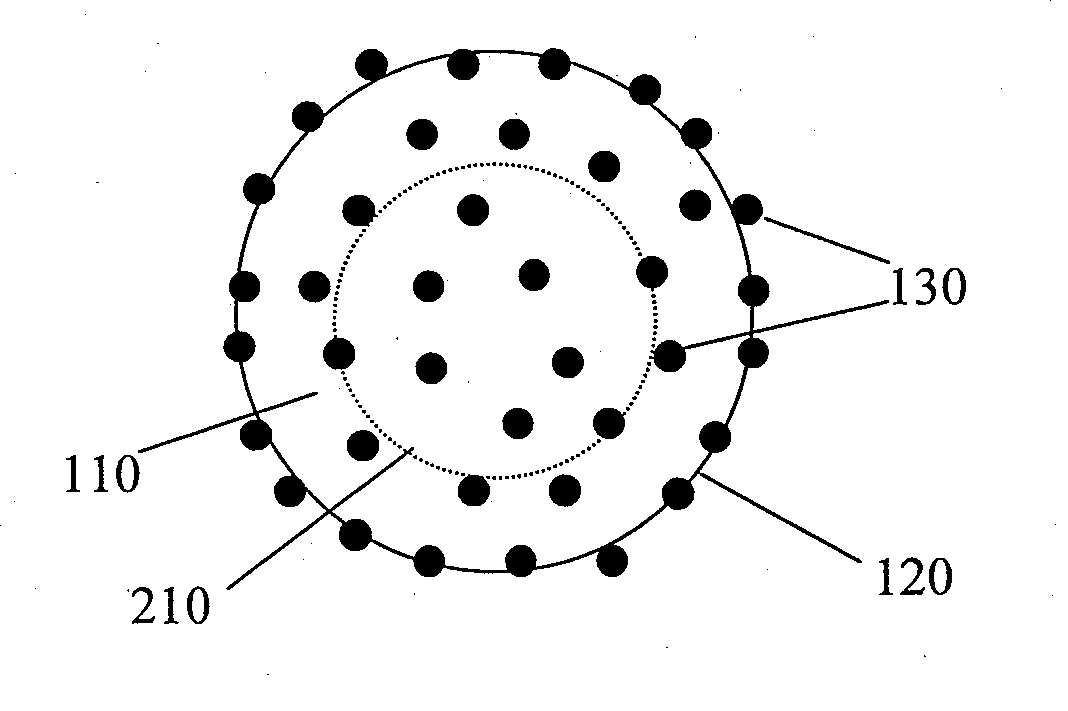
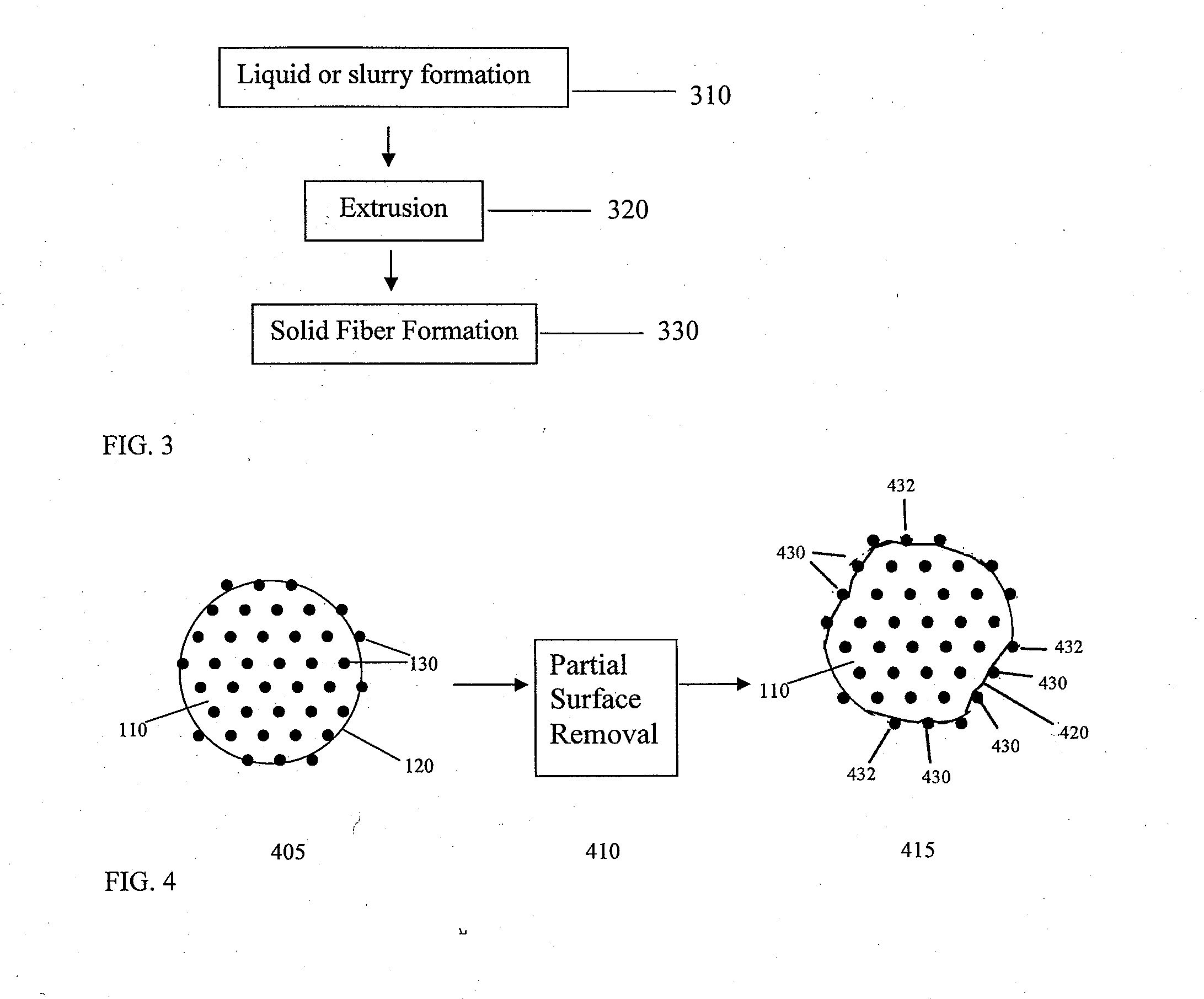
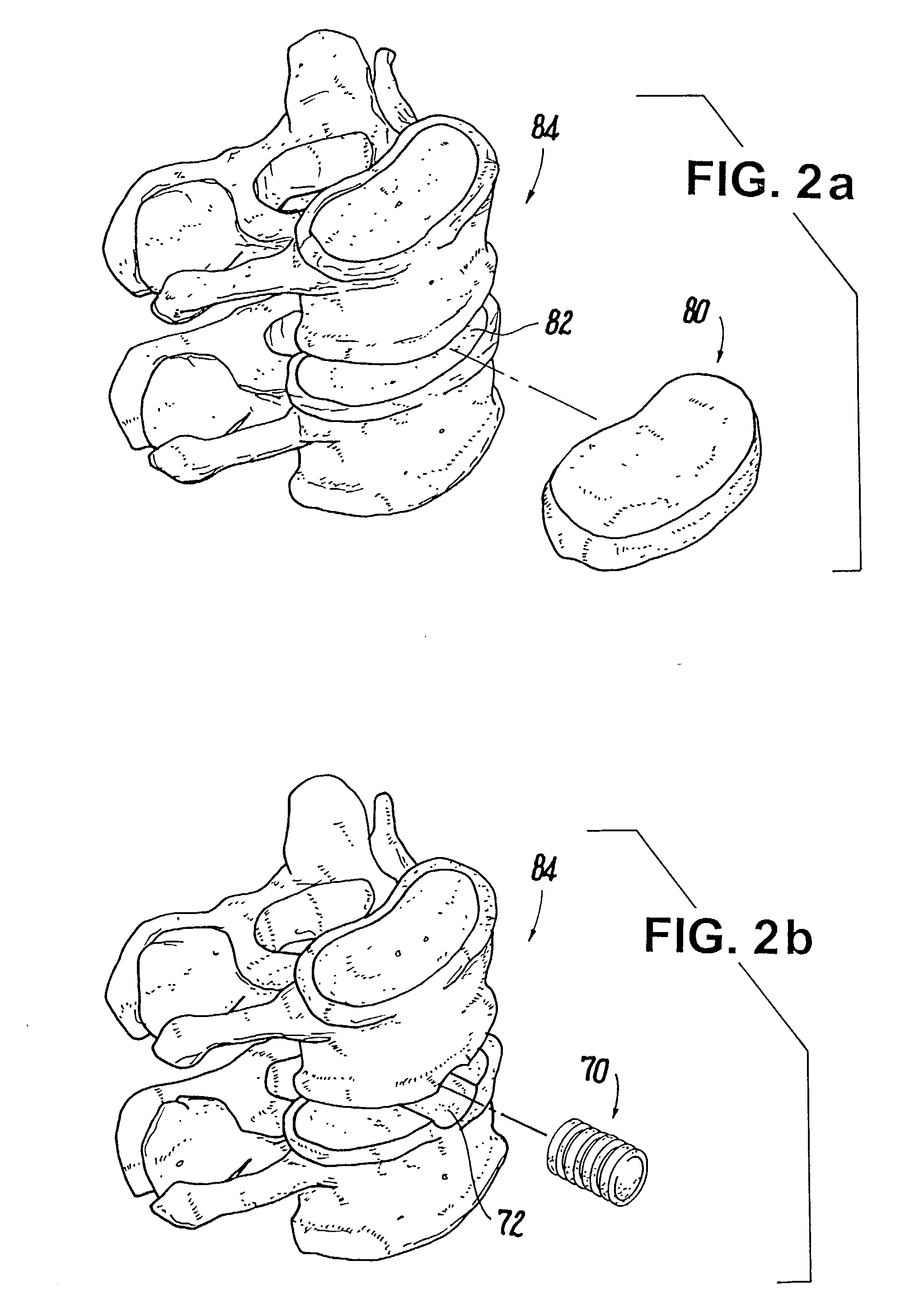
Porous tissue scaffoldings for the repair of regeneration of tissue
InactiveUS6333029B1Promote growthPromote regenerationPeptide/protein ingredientsAntipyreticRepair tissueOpen cell
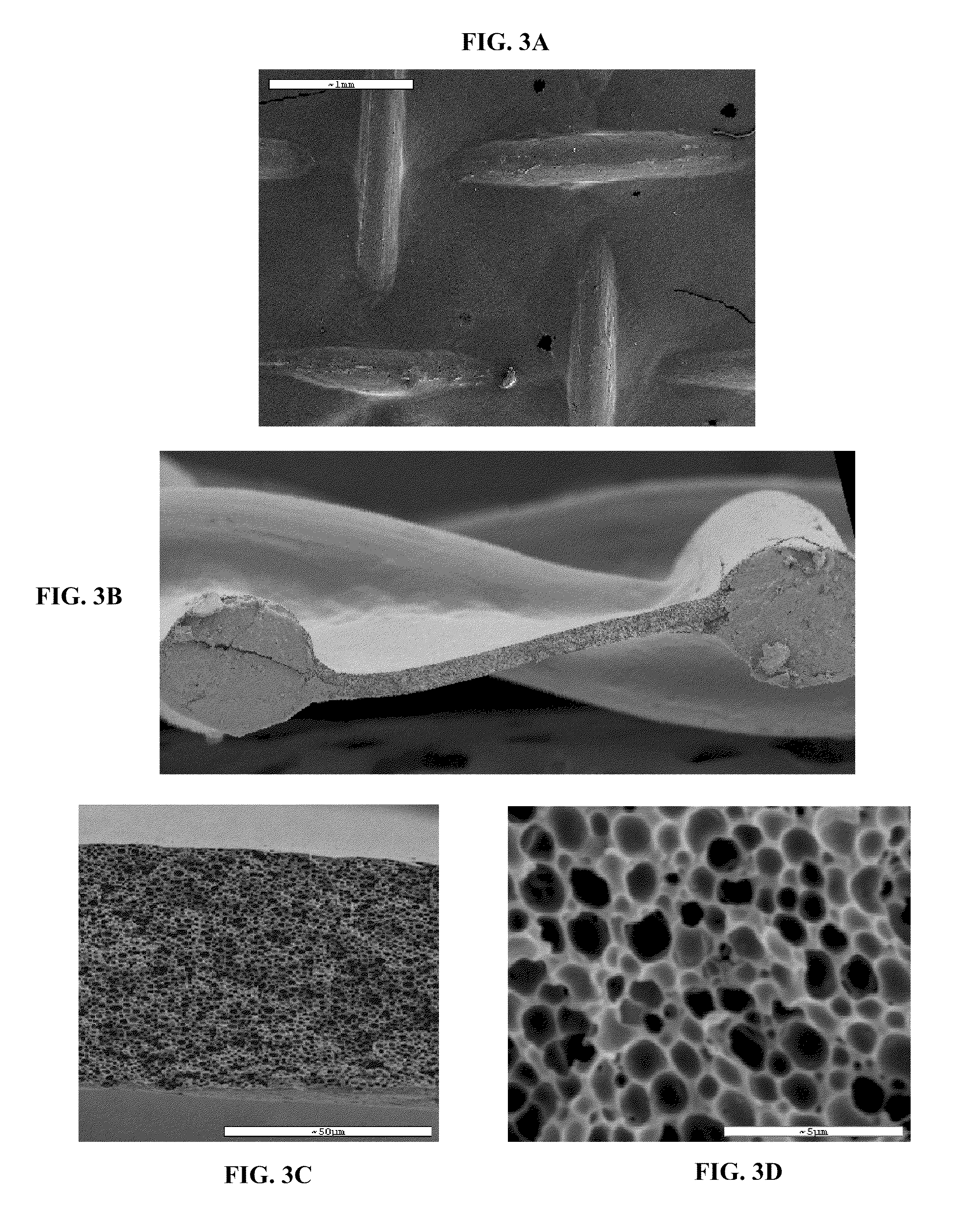
The present patent describes a three-dimensional inter-connected open cell porous foams that have a gradient in composition and / or microstructure through one or more directions. These foams can be made from a blend of absorbable and biocompatible polymers that are formed into foams having a compositional gradient transitioning from predominately one polymeric material to predominately a second polymeric material. These gradient foams are particularly well suited to tissue engineering applications and can be designed to mimic tissue transition or interface zones.
Owner:ETHICON ENDO SURGERY INC
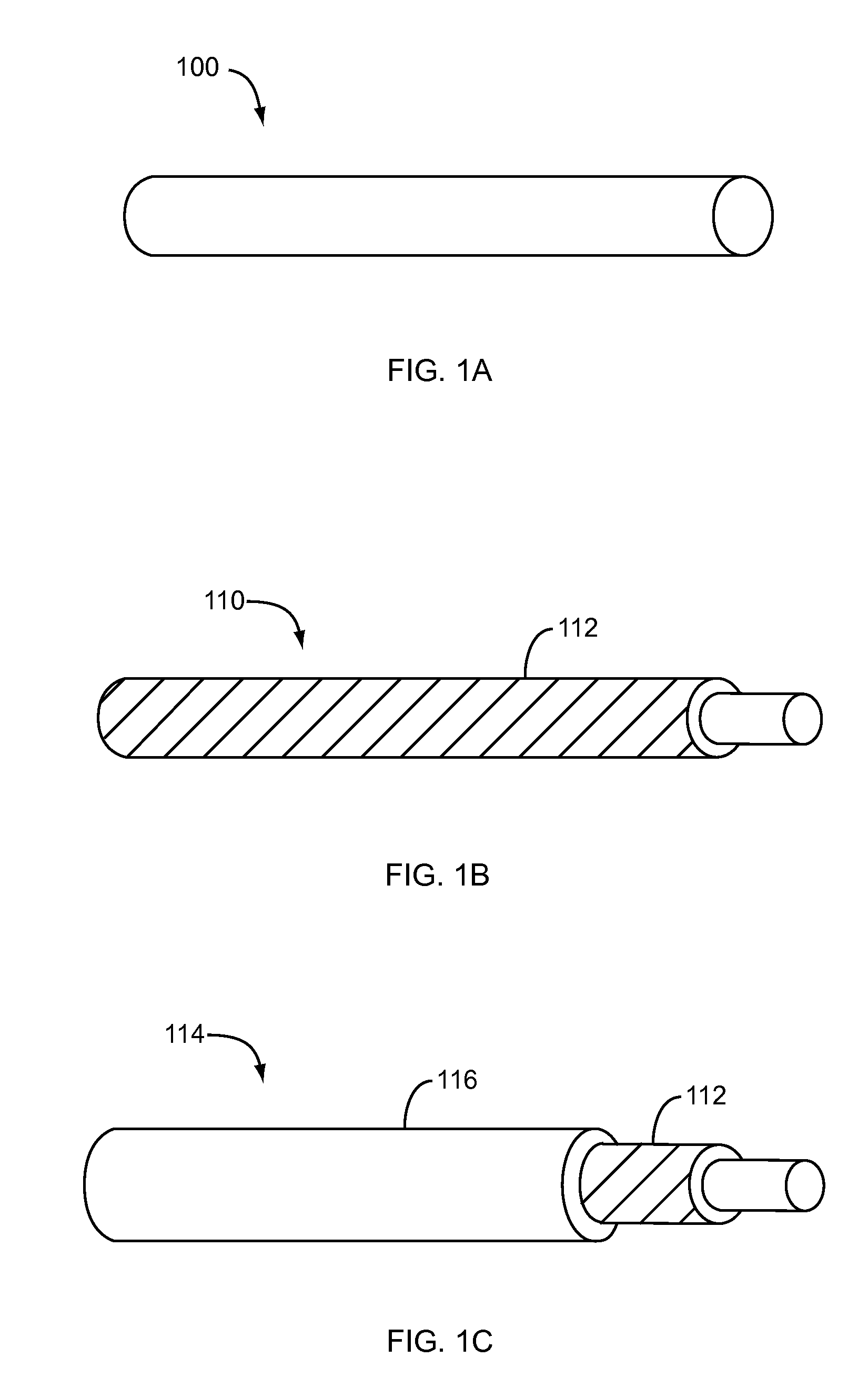
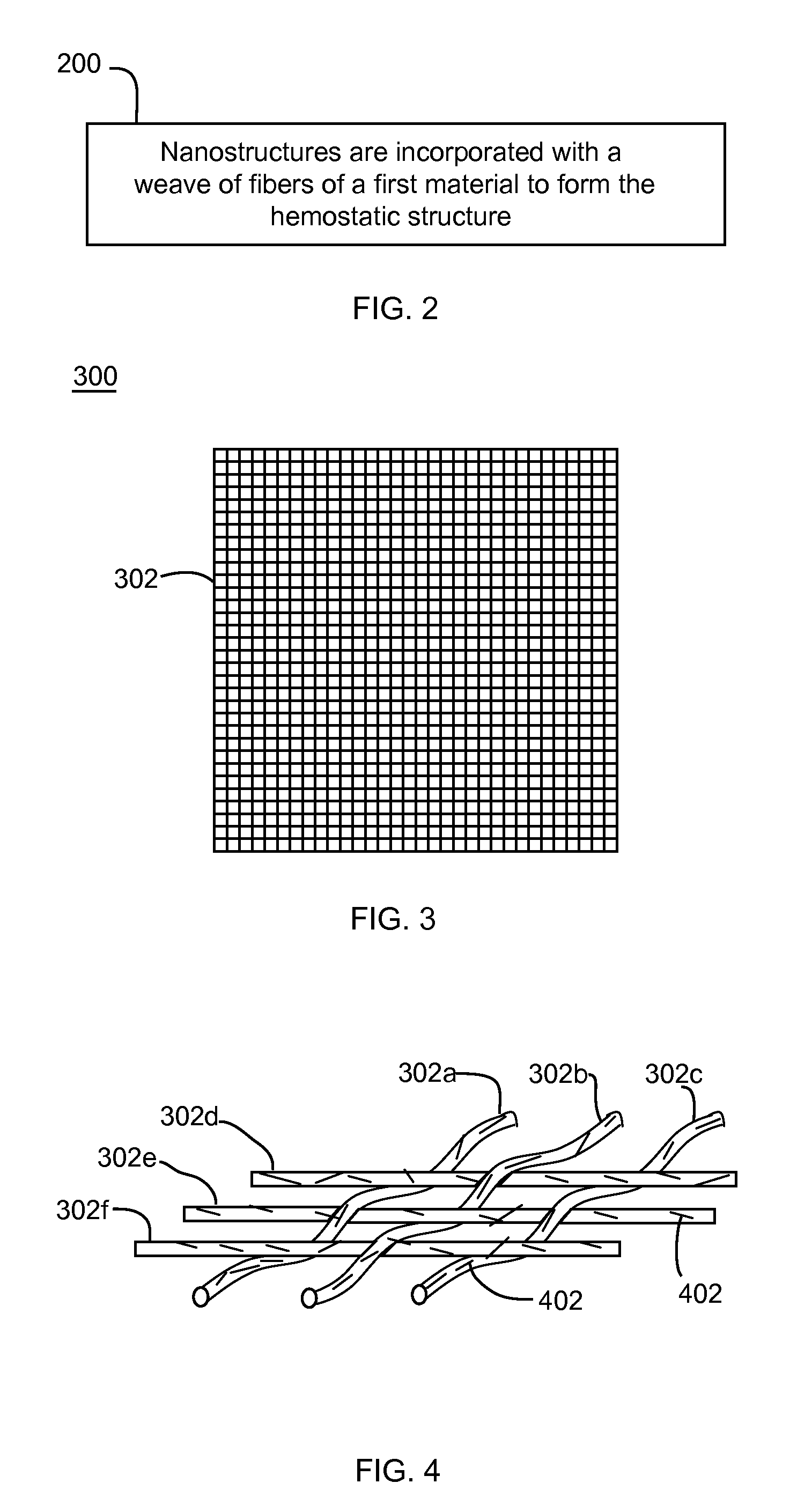
Nanostructure-enhanced platelet binding and hemostatic structures
InactiveUS8319002B2Enhancing overall rate and strengthInduce platelet binding and efficient hemostasisBiocideSurgical adhesivesPlateletNanofiber
Methods, systems, and apparatuses for nanomaterial-enhanced platelet binding and hemostatic medical devices are provided. Hemostatic materials and structures are provided that induce platelet binding, including platelet binding and the coagulation of blood at a wound / opening caused by trauma, a surgical procedure, ulceration, or other cause. Example embodiments include platelet binding devices, hemostatic bandages, hemostatic plugs, and hemostatic formulations. The hemostatic materials and structures may incorporate nanostructures and / or further hemostatic elements such as polymers, silicon nanofibers, silicon dioxide nanofibers, and / or glass beads into a highly absorbent, gelling scaffold. The hemostatic materials and structures may be resorbable.
Owner:NANOSYS INC
Hemostatic fibrous material
InactiveUS20120004636A1Promote blood clottingPromoting blood clottingBiocideDiagnosticsFiberMolecular materials
Owner:TELEFLEX LIFE SCI LTD
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Polymer-based, sustained release drug delivery system
InactiveUS20120016467A1Reduce interactionSuture equipmentsAntibacterial agentsRate limitingSolubility
Disclosed is a sustained release system that includes a polymer and a prodrug having a solubility less than about 1 mg / ml dispersed in the polymer. Advantageously, the polymer is permeable to the prodrug and may be non-release rate limiting with respect to the rate of release of the prodrug from the polymer. This permits improved drug delivery within a body in the vicinity of a surgery via sustained release rate kinetics over a prolonged period of time, while not requiring complicated manufacturing processes.
Owner:PSIVIDA INC
Drug-eluting medical devices
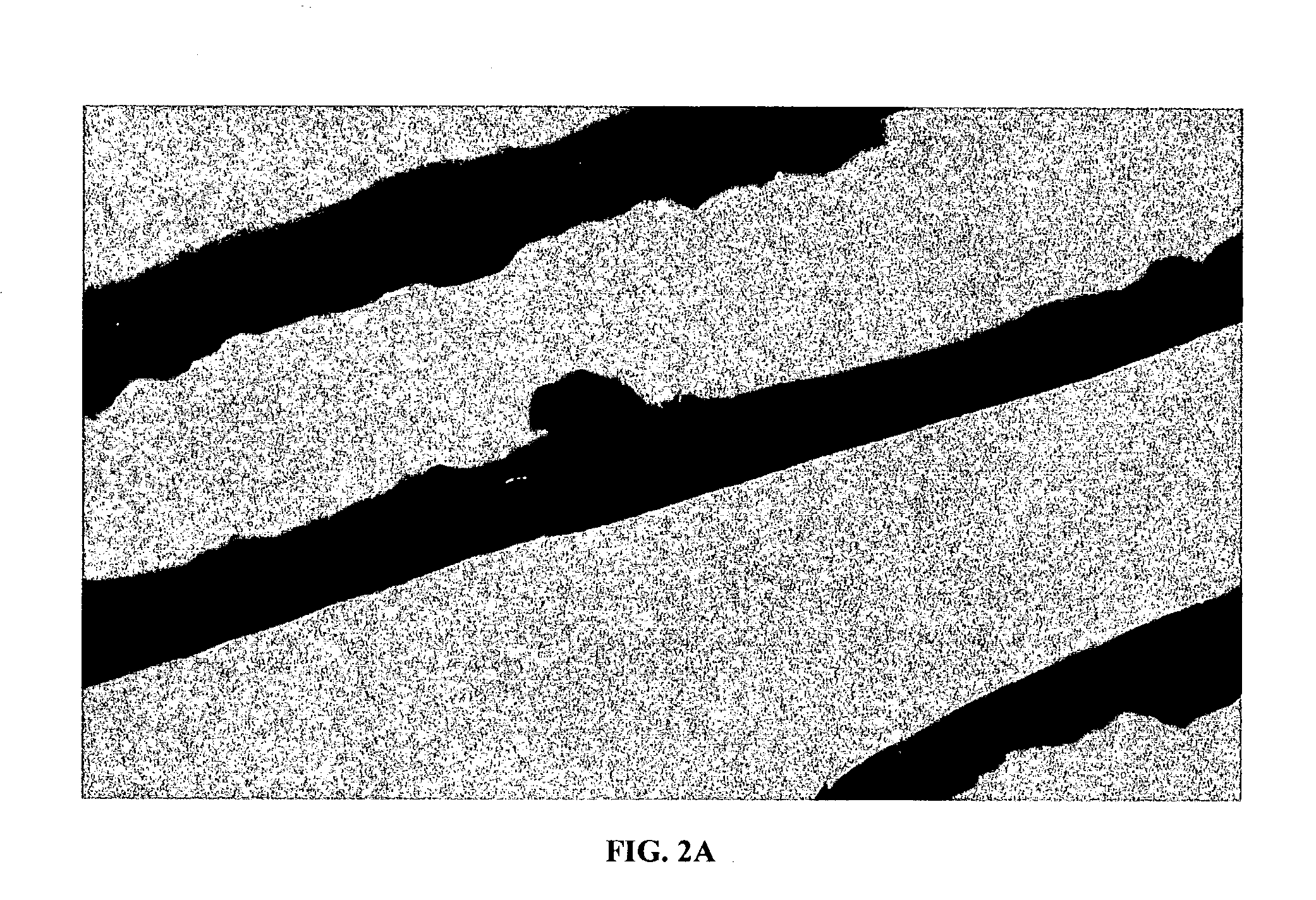
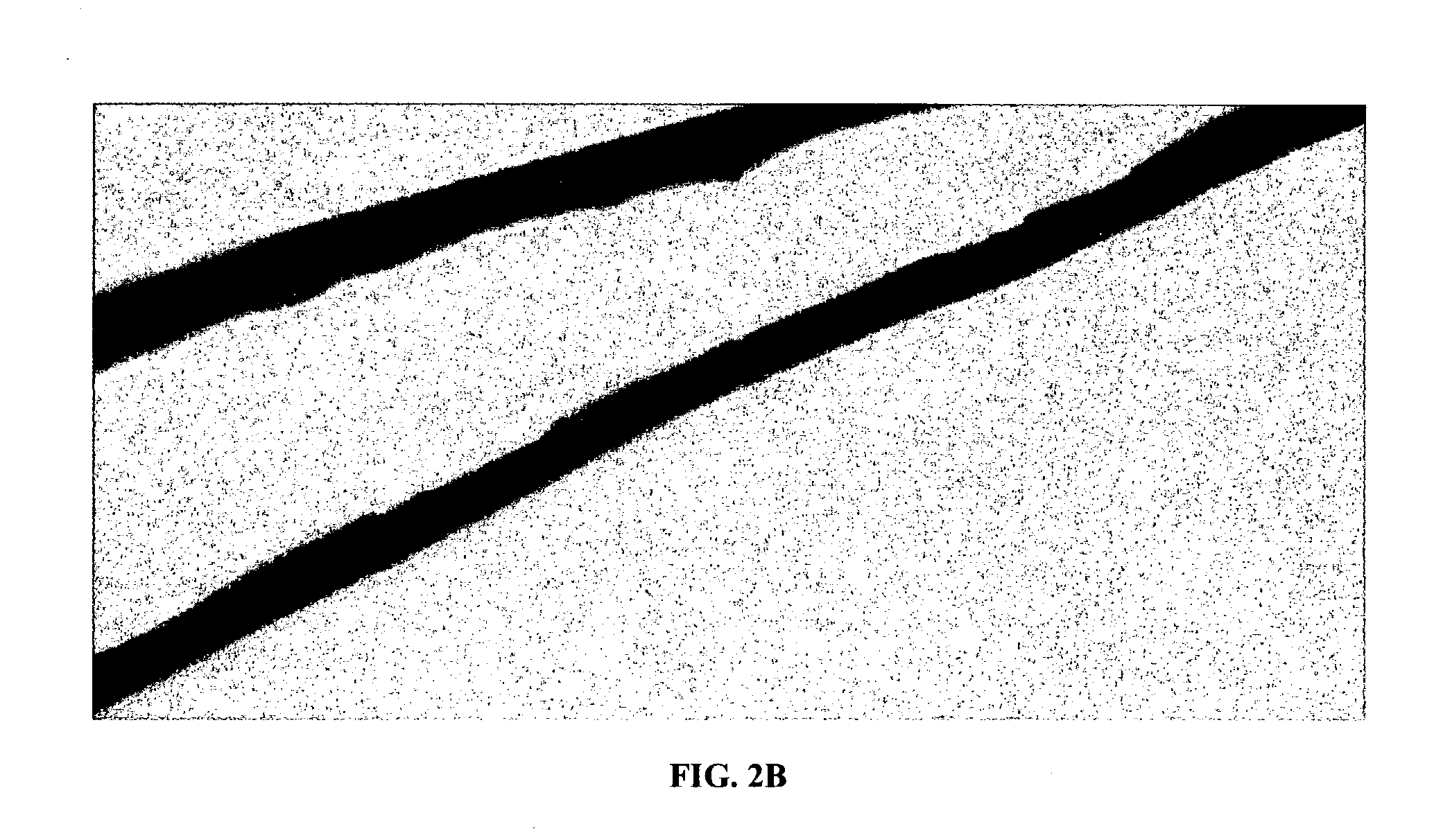
Composite structures composed of a device as a core structure, being a medical device or article, and a porous polymeric coat and designed capable of encapsulating bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures.
Owner:RAMOT AT TEL AVIV UNIV LTD
Antimicrobial polymer compositions and the use thereof
An antimicrobial composition comprising: a complex of an anionic polyester with an antimicrobial cationic surfactant, wherein the anionic polyester has at least one carboxylic group. A medical device having an antimicrobial composition comprising: a complex of an anionic polyester with an antimicrobial cationic surfactant wherein the anionic polyester has at least one carboxylic group.
Owner:ETHICON INC
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
Drug-delivering composite structures
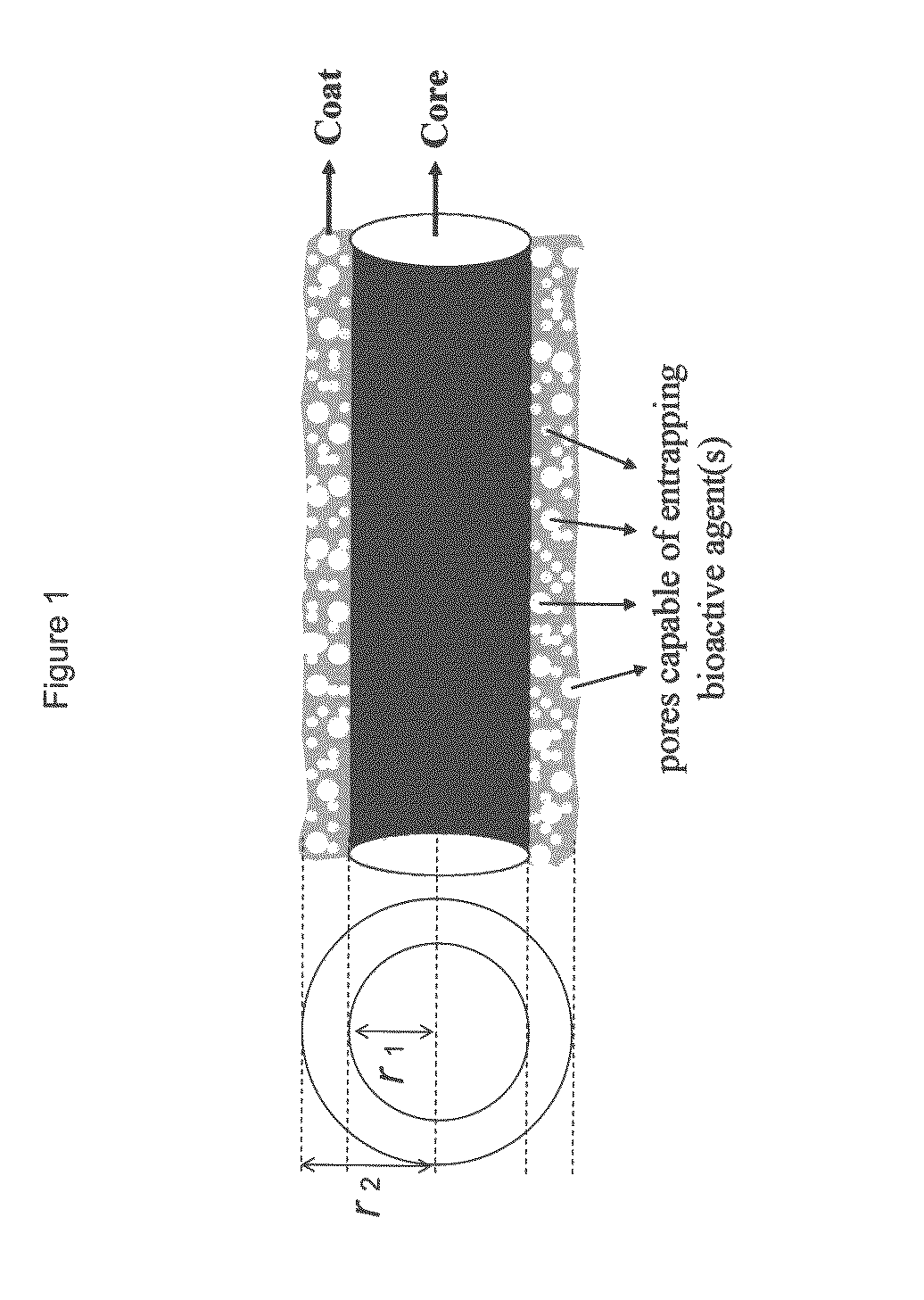
Composite structures composed of a fibril core and a polymeric coat and designed capable of encapsulating both hydrophobic and hydrophilic bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures, and medical devices and disposable articles made therefrom.
Owner:ZILBERMAN MEITAL
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
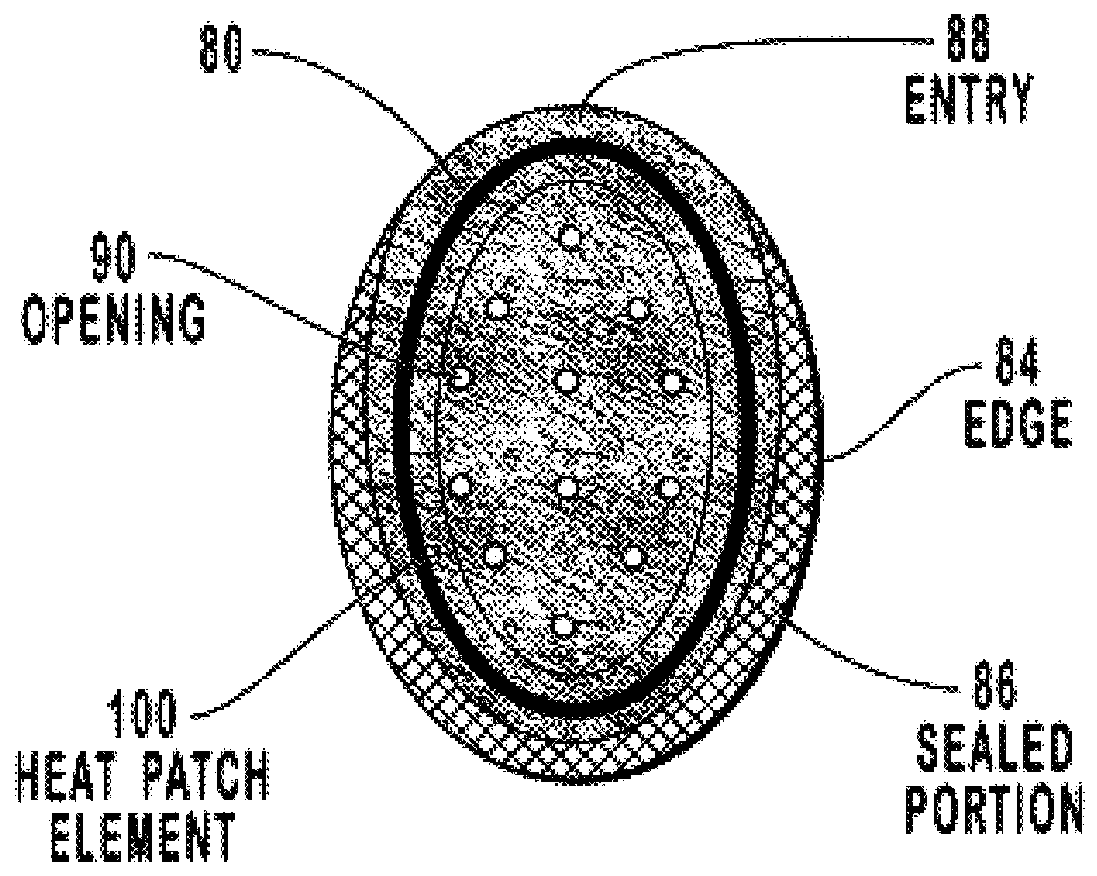
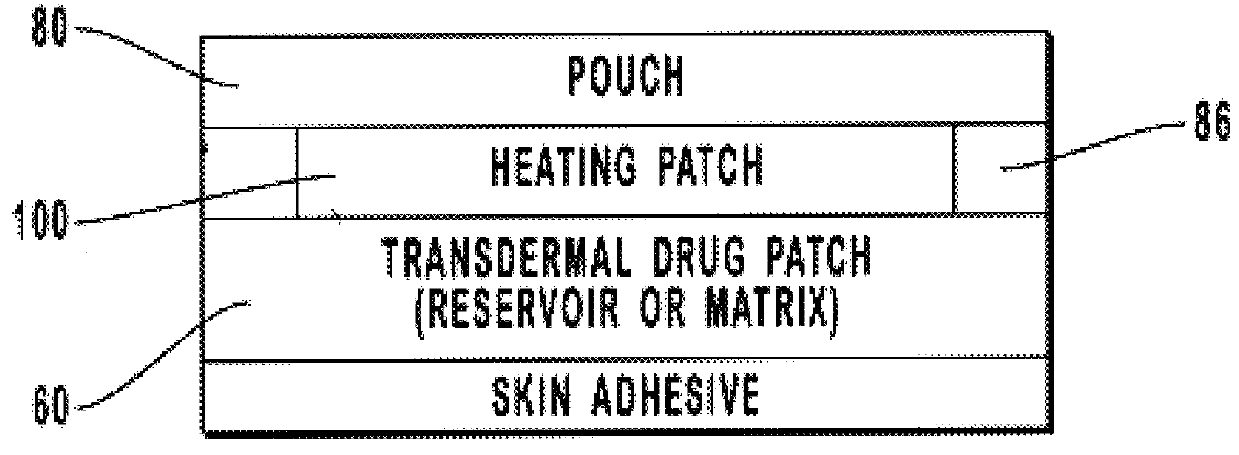
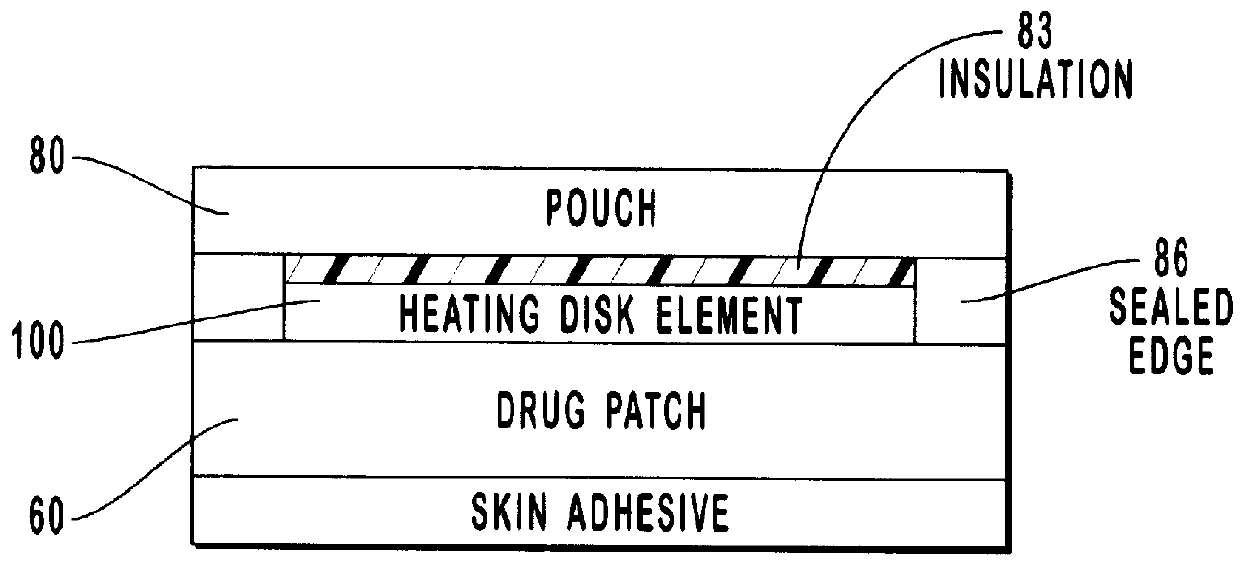
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS6261595B1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin aid thereby improves dermal drug administration.
Owner:ZARS INC
Medical device applications of nanostructured surfaces
InactiveUS20060204738A1Improve adhesionIncrease frictionBiocideMaterial nanotechnologyOsteoblastNanofiber
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, methods for enhancing cellular functions on a surface of a medical device implant are disclosed which generally comprise providing a medical device implant comprising a plurality of nanofibers (e.g., nanowires) thereon and exposing the medical device implant to cells such as osteoblasts.
Owner:GLO TECH LLC
Shaped load-bearing osteoimplant and methods of making same
InactiveUS20030039676A1Promotes new host bone tissue formationPermit of mechanical propertySuture equipmentsDental implantsMedicineHard tissue
A load-bearing osteoimplant, methods of making the osteoimplant and method for repairing hard tissue such as bone and teeth employing the osteoimplant are provided. The osteoimplant comprises a shaped, coherent mass of bone particles which may exhibit osteogenic properties. In addition, the osteoimplant may possess one or more optional components which modify its mechanical and / or bioactive properties, e.g., binders, fillers, reinforcing components, etc.
Owner:WARSAW ORTHOPEDIC INC
Method of making functionalized nanotubes
InactiveUS6203814B1Good dispersionImprove adhesionMaterial nanotechnologyCarbon compoundsChemical MoietyFiber
Owner:HYPERION CATALYSIS INT
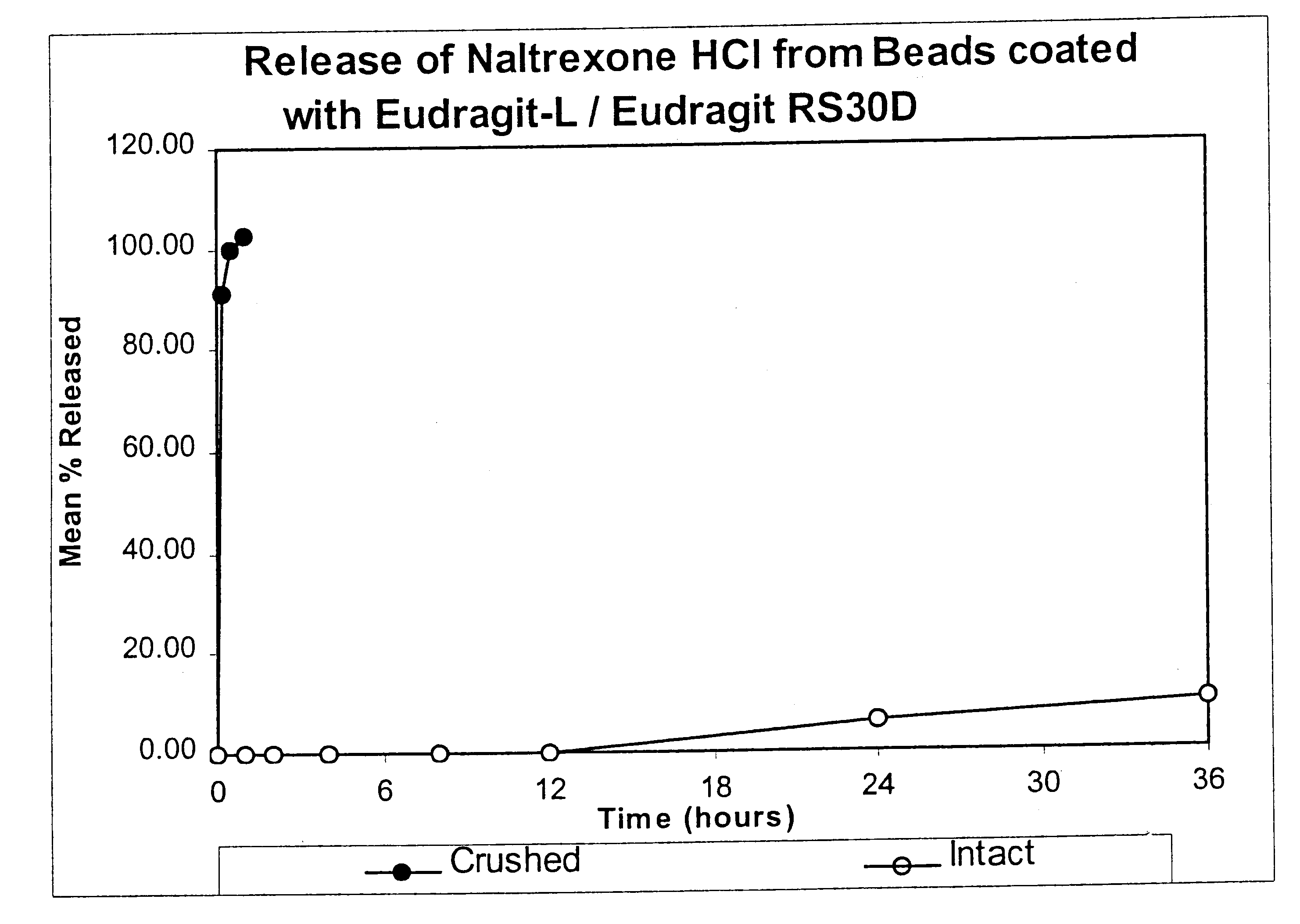
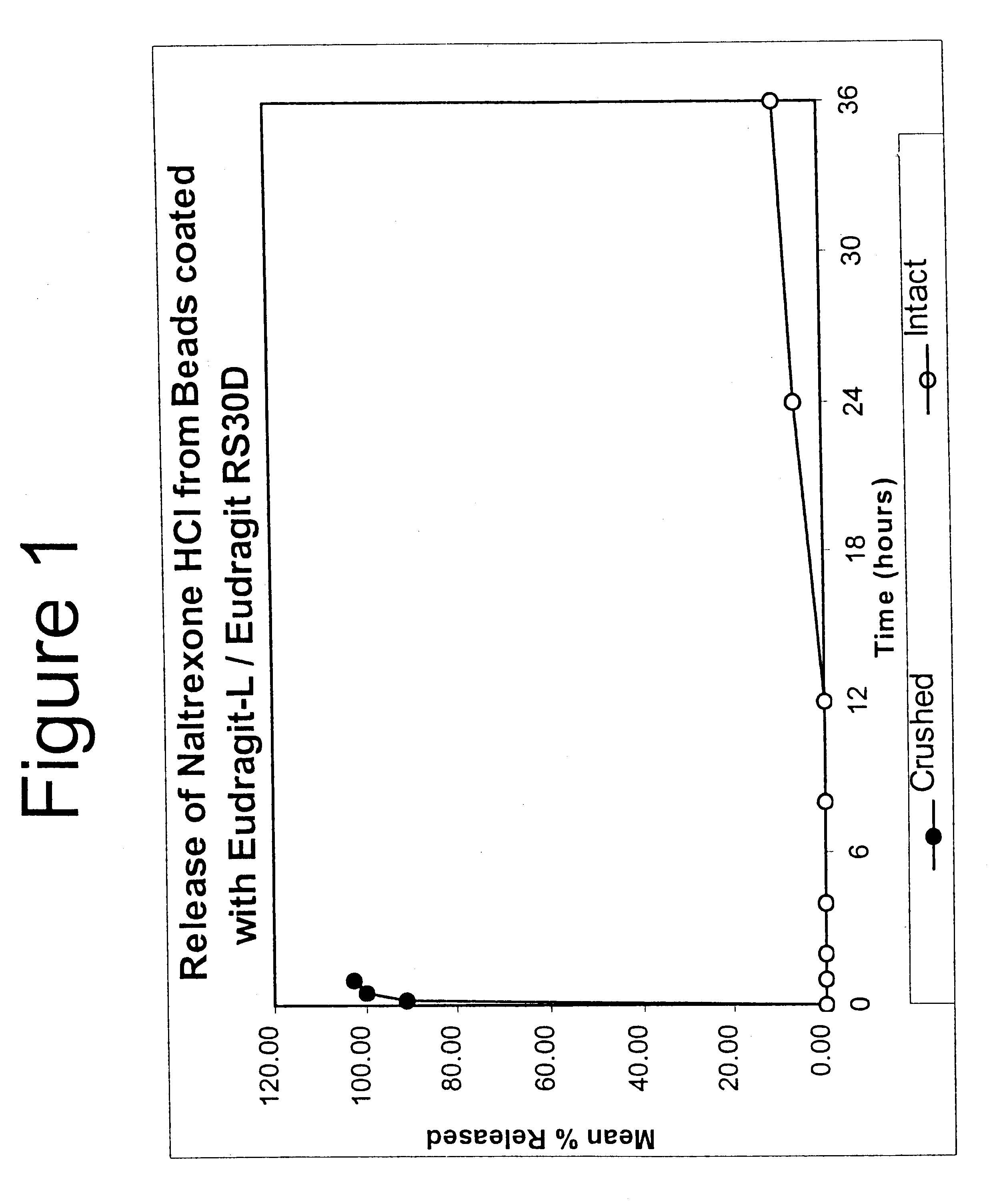
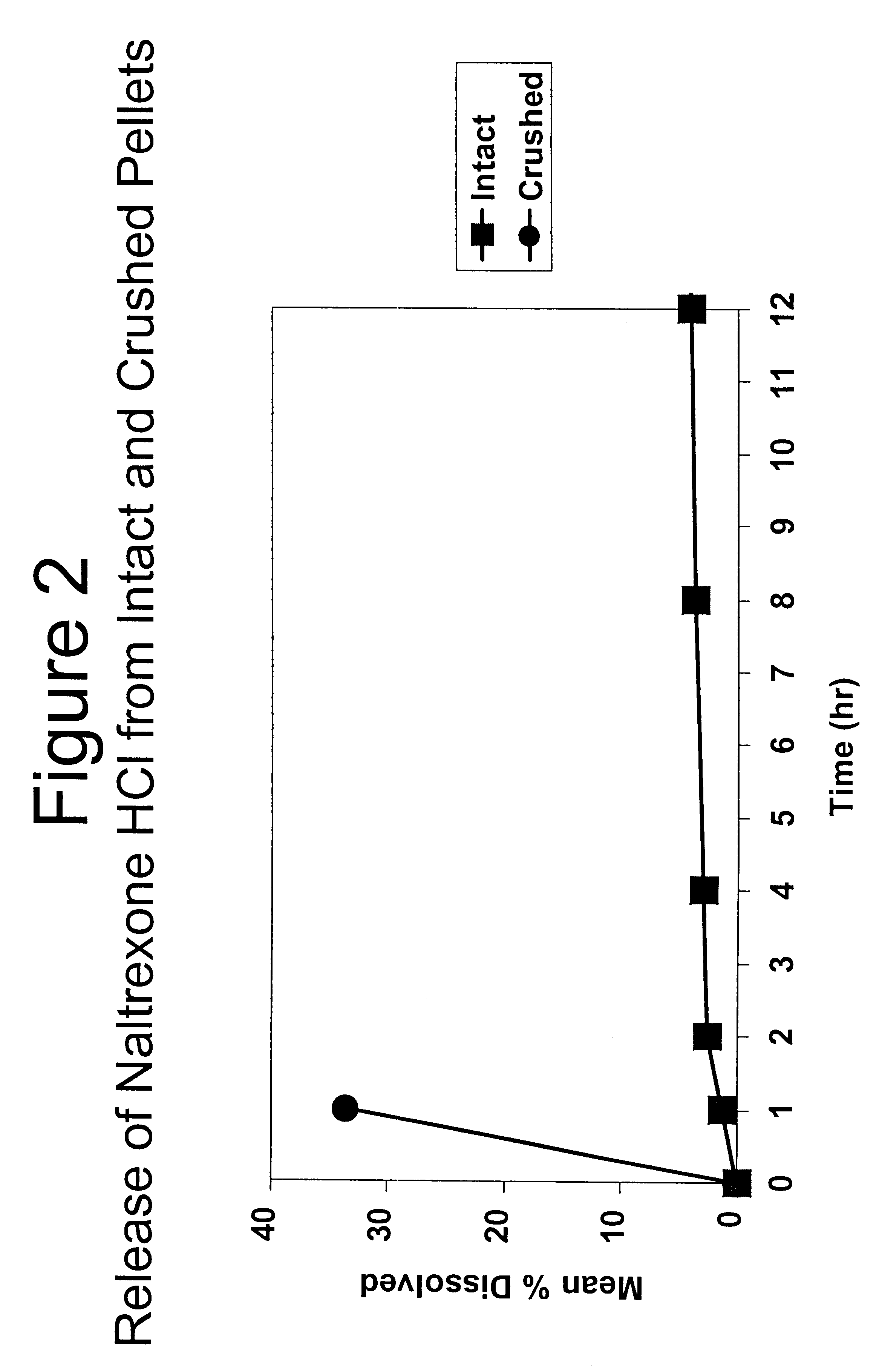
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Article having a lotioned topsheet
InactiveUS6861571B1Reduce adhesionImprove usabilityCosmetic preparationsToilet preparationsMedicineLotion
An article containing a liquid pervious topsheet coated with a lotion composition is disclosed. The lotion composition provides a skin benefit and / or reduces the adherence of BM to the skin of the wearer, thereby improving the case of BM clean up. The lotion composition applied to the article in a nonuniform manner, preferably such there are regions on the article's topsheet that are not coated with lotion.
Owner:THE PROCTER & GAMBLE COMPANY
Infusion device and an adhesive sheet material and a release liner
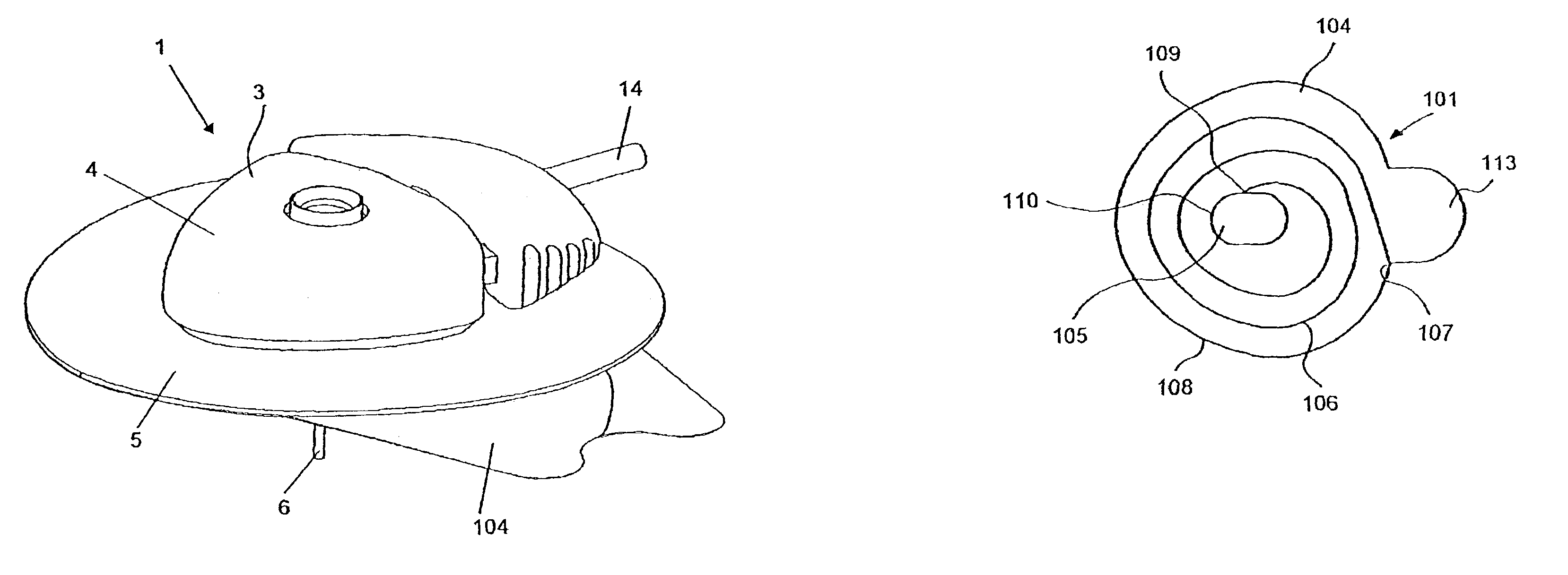
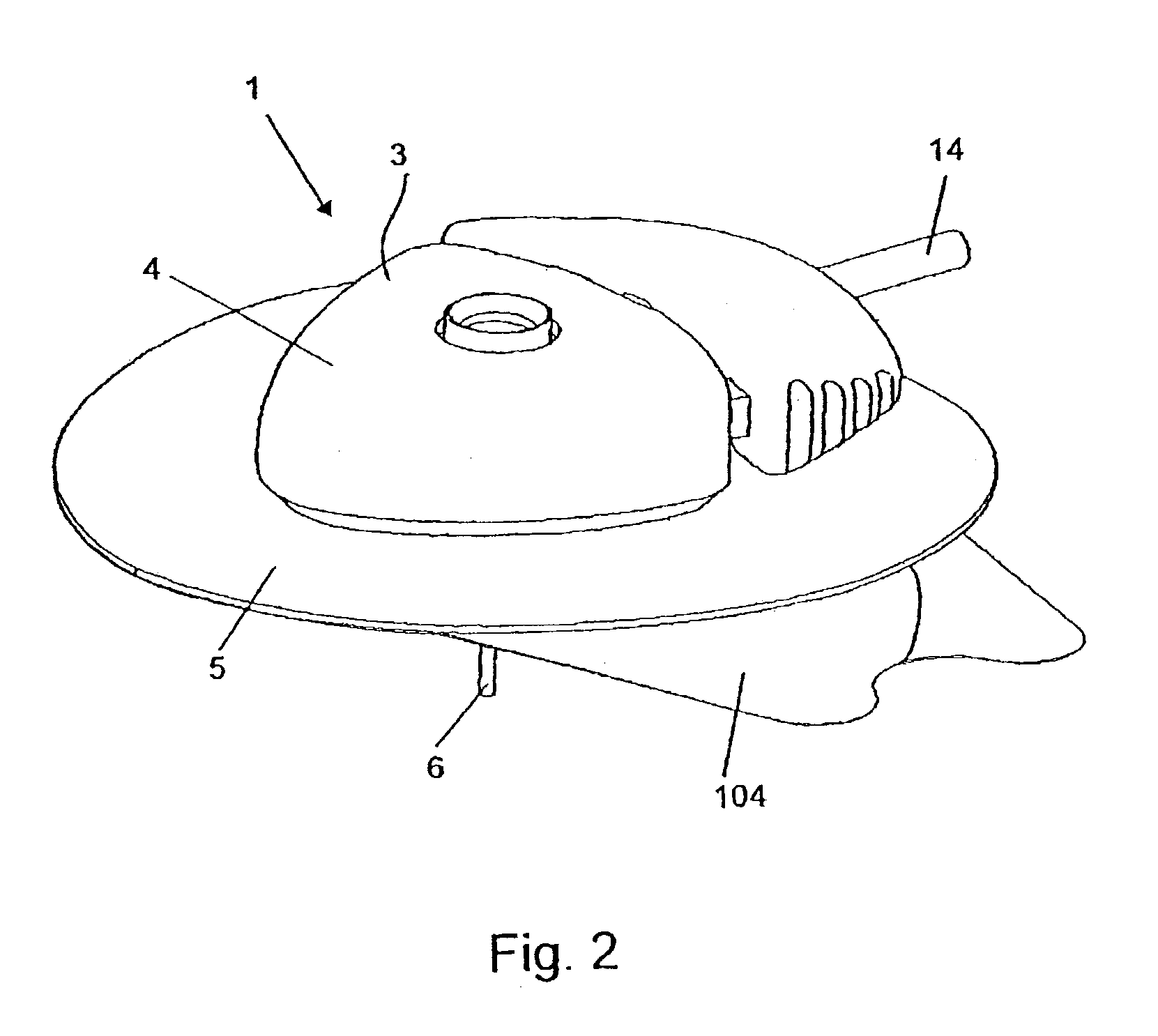
ActiveUS7070580B2Easy to handleForce is smallPressure infusionInfusion needlesLower faceBiomedical engineering
An infusion device (1) comprising a housing (3) with an upper face plate (4) and a lower face plate (5) and an adhesive sheet material (101) placed on the lower face plate (5) for securing the infusion device (1) to the skin, the adhesive sheet material (101) comprising a backing layer (102) which has an adhesive layer (103) on one surface, the adhesive layer (103) being covered by a removable release liner (104), and wherein the release liner (104) comprising at least one score line (106) comprising a spiral or helix, wherein the starting point (107) for the score line (106) is placed in the periphery (108) of the release liner (104) and wherein the score line (106) continues to an end point placed on the border of the periphery (110) of a central aperture (105) of the release liner.
Owner:UNOMEDICAL AS
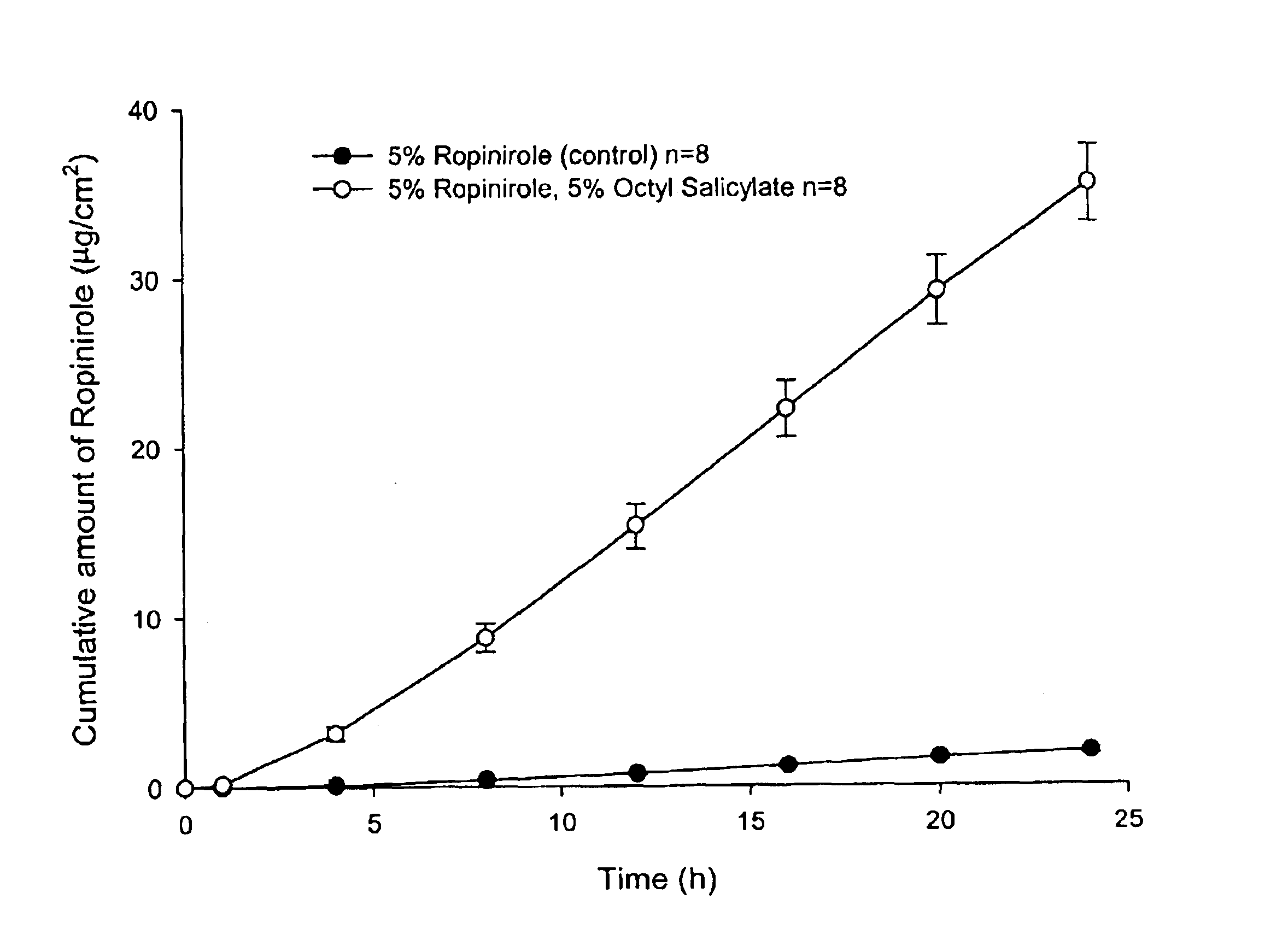
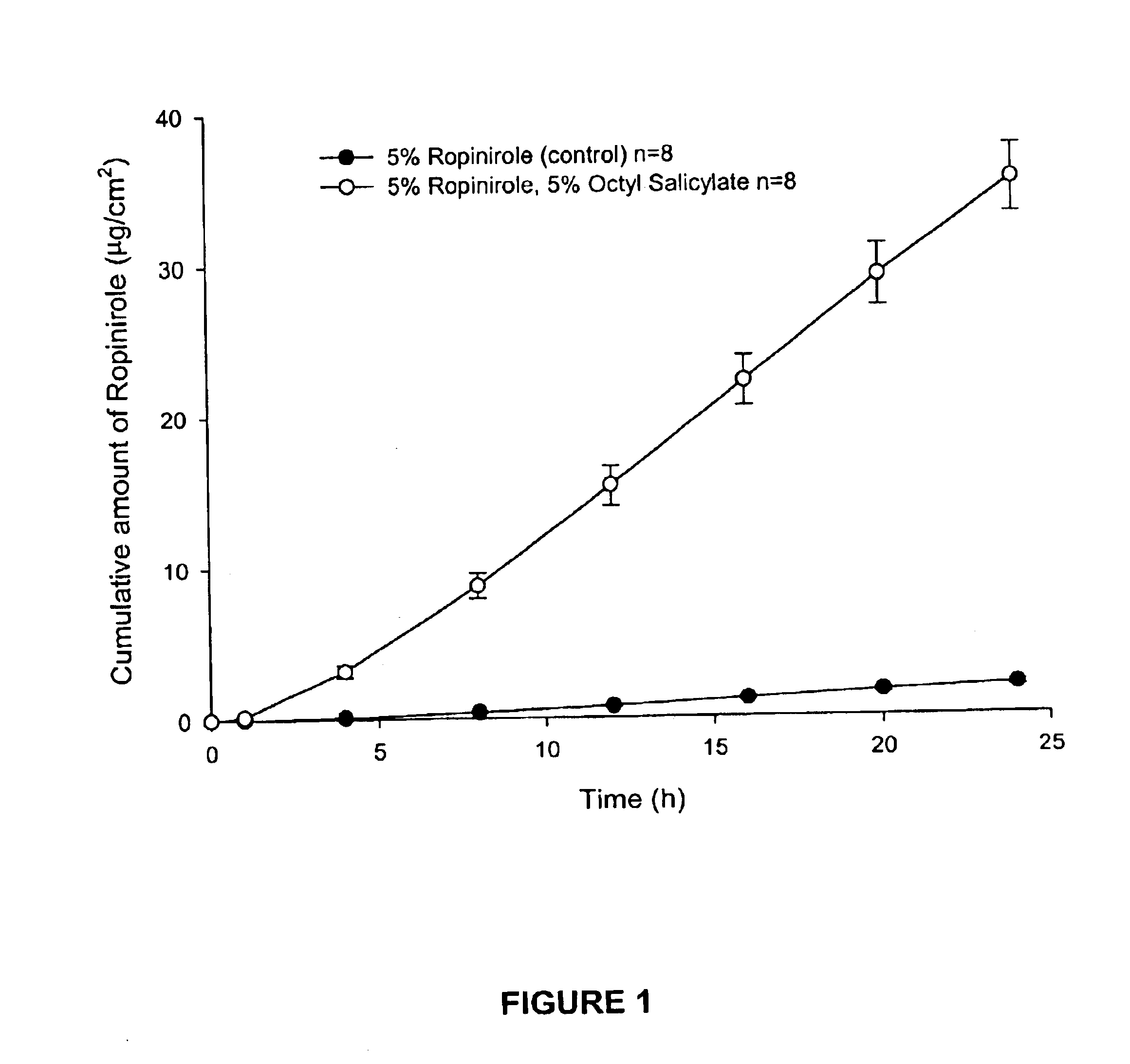
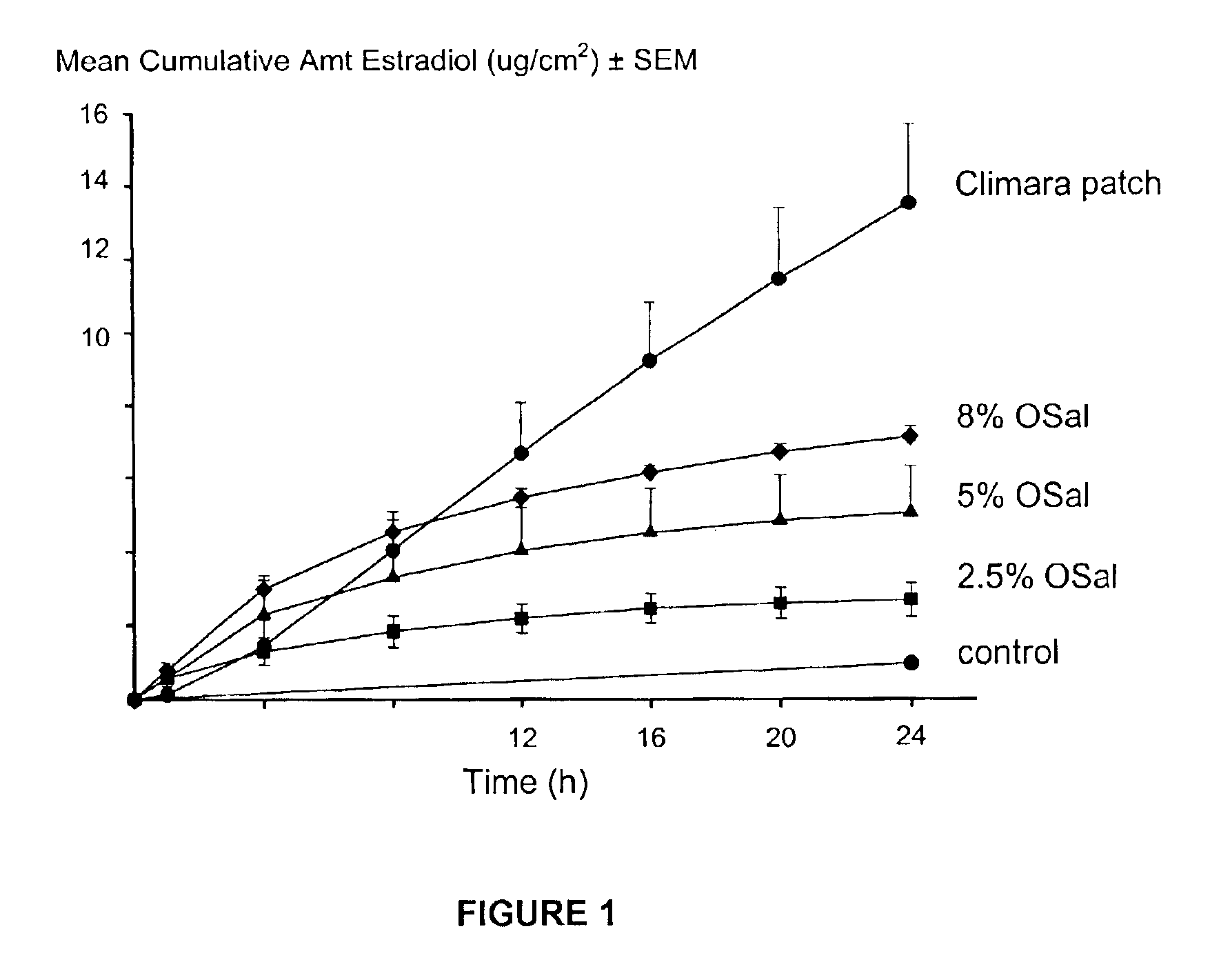
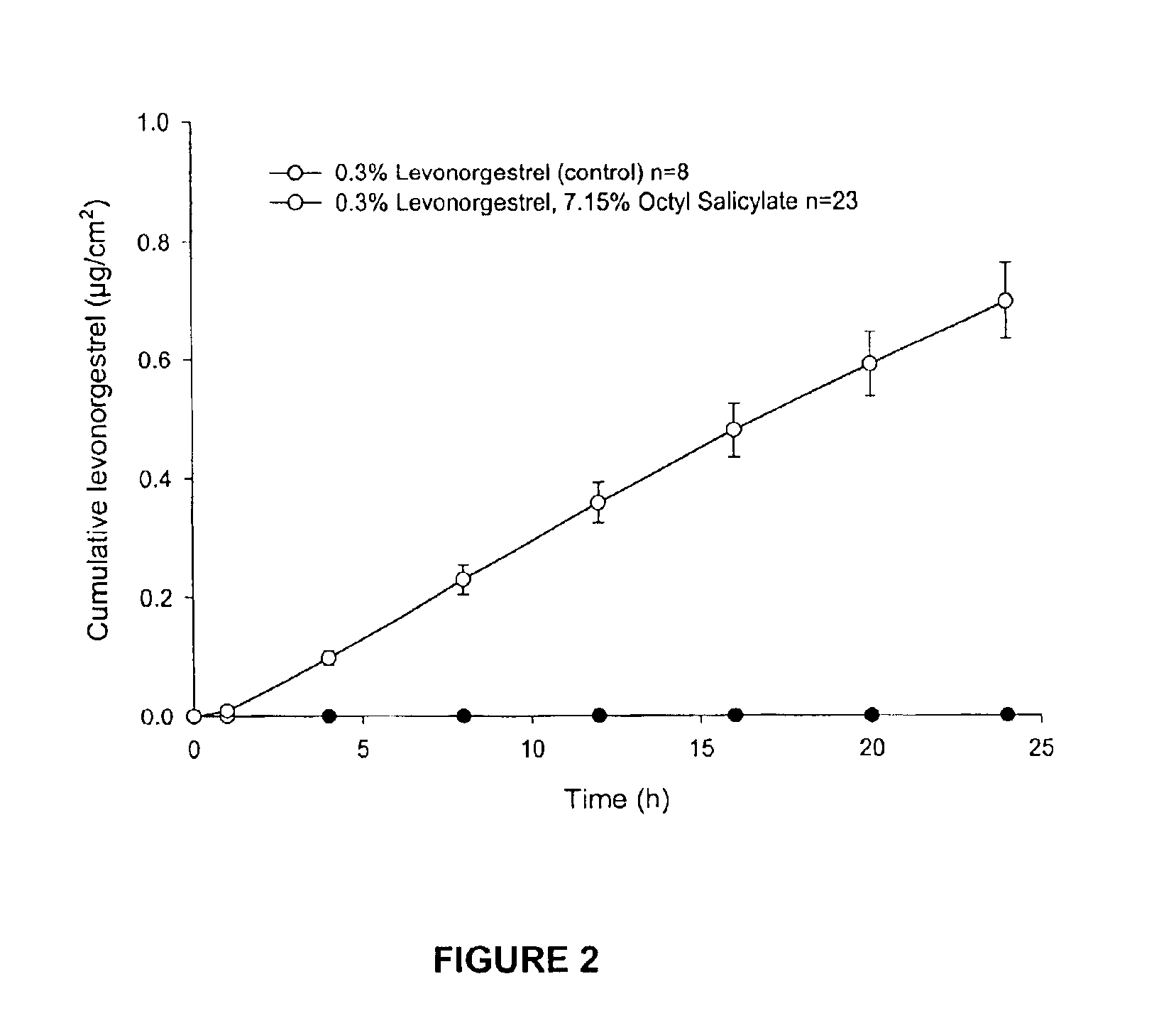
Transdermal delivery of antiparkinson agents
InactiveUS6929801B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyMedicine
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of an antiParkinson agent; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting antiParkinson agent to an animal which comprises applying an effective amount of the antiParkinson agent in the form of the drug delivery system of the present invention.
Owner:ACRUX DDS
Surface topographies for non-toxic bioadhesion control
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Transdermal delivery of hormones
InactiveUS6923983B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyHormones regulation
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of a hormone; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting hormone to an animal which comprises applying an effective amount of the hormone in the form of the drug delivery system of the present invention
Owner:ACRUX DDS
Antimicrobial silver compositions
The present invention comprises methods and compositions for antimicrobial silver compositions comprising silver nanoparticles. The present invention further comprises compositions for preparing silver nanoparticles comprising at least one stabilizing agent, one or more silver compounds, at least one reducing agent and a solvent. In one aspect, the stabilizing agent comprises a surfactant or a polymer. The polymer may comprise polymers such as polyacrylamides, polyurethanes, and polyamides. In one aspect, the silver compound comprises a salt comprising a silver cation and an anion. The anion may comprise saccharinate derivatives, long chain fatty acids, and alkyl dicarboxylates. The methods of the present invention comprise treating devices with the silver nanoparticle compositions, including, but not limited to, such devices as woven wound care materials, catheters, patient care devices, and collagen matrices. The present invention further comprises treatment of humans and animals wacr6ith the antimicrobial devices described herein.
Owner:AVENT INC
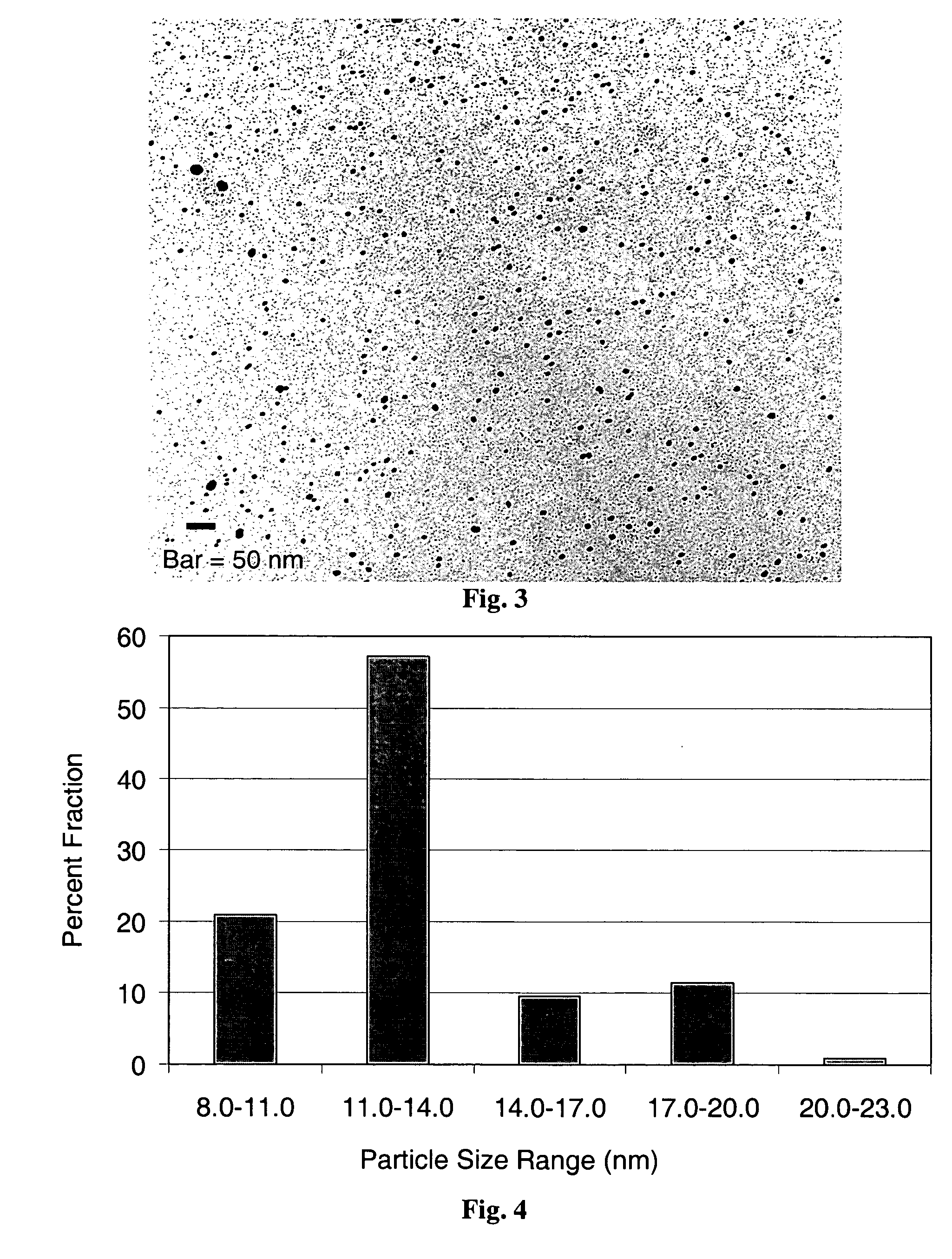
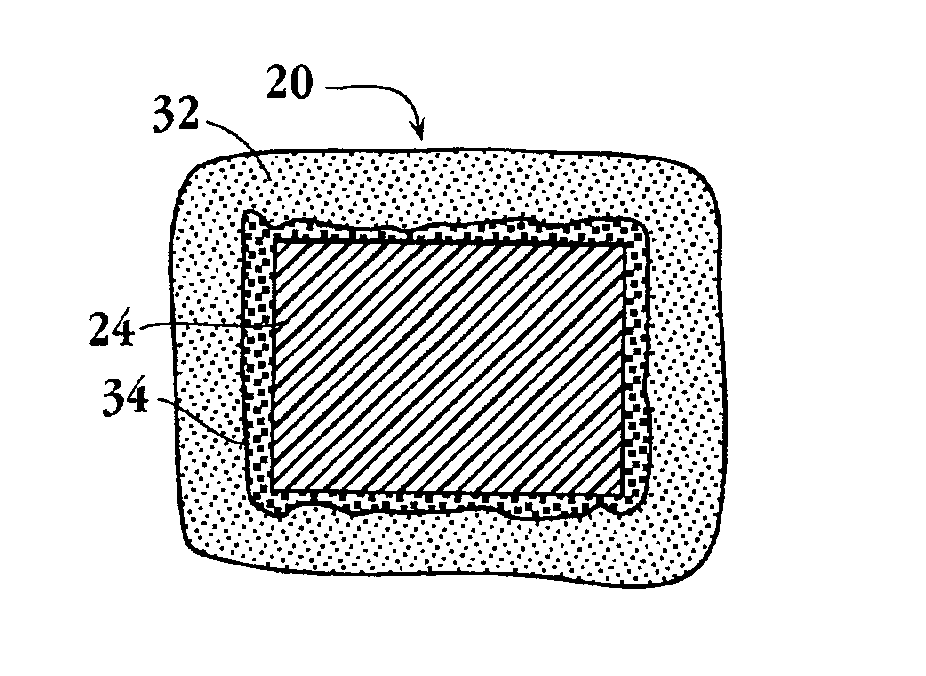
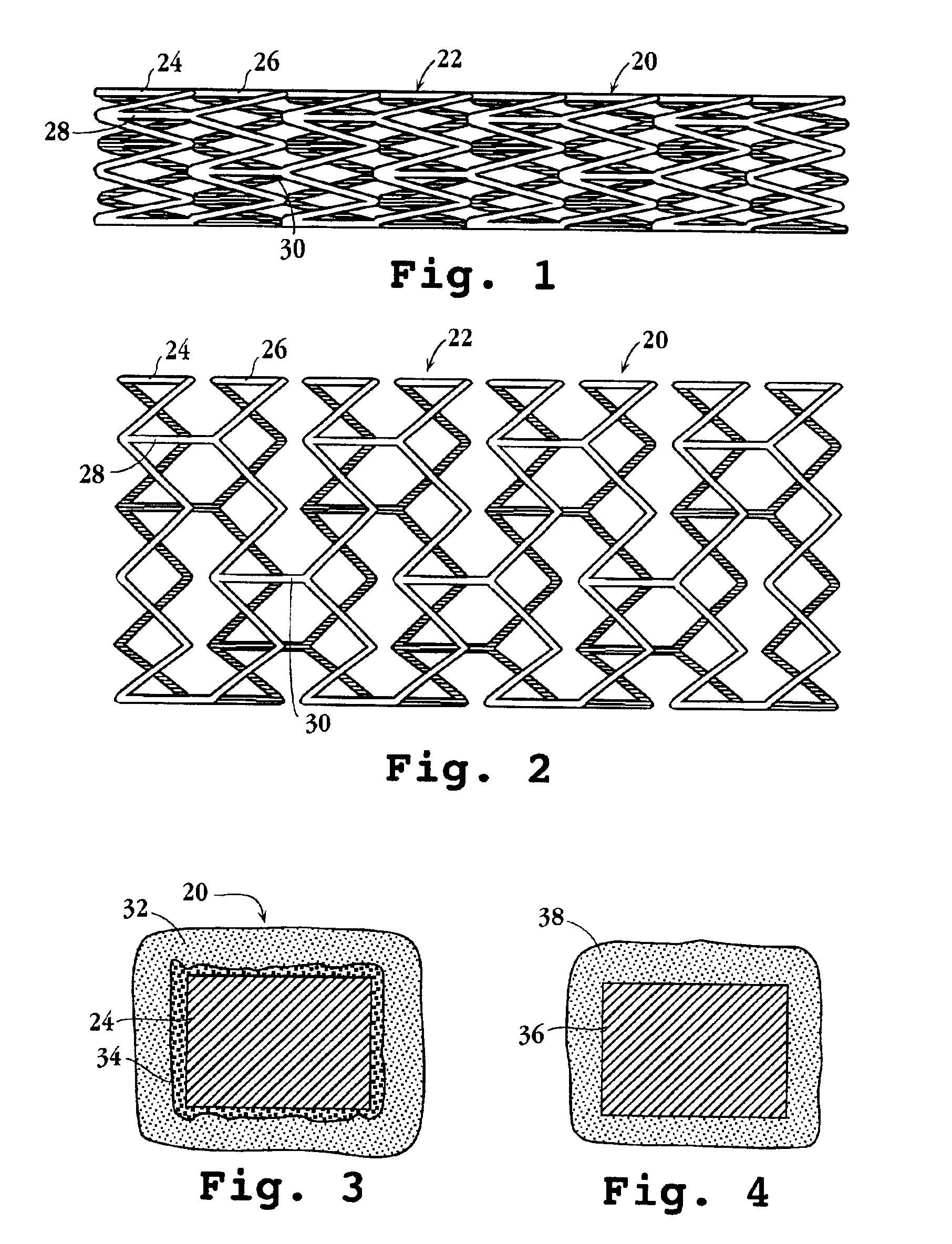
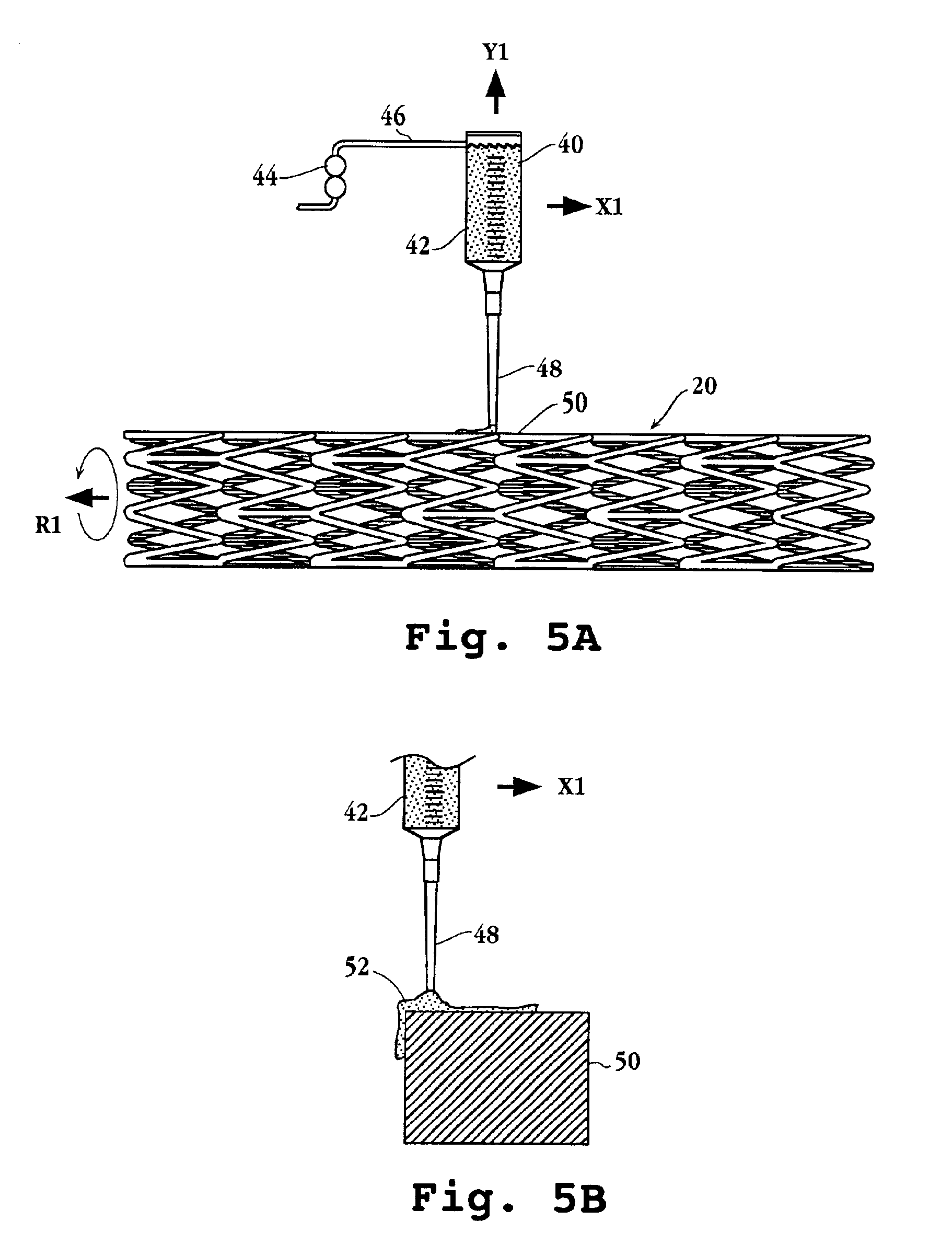
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Transdermal delivery system
The present invention relates to the discovery of a transdermal delivery system that can deliver high molecular weight pharmaceuticals and cosmetic agents to skin cells. A novel transdermal delivery system with therapeutic and cosmetic application and methods of use of the foregoing is disclosed.
Owner:ORIX +1
Process for making an ingestible film
The invention relates to film products containing desired levels of active components and methods of their preparation. Desirably, the films disintegrate in water and may be formed by a controlled drying process, or other process that maintains the required uniformity of the film. Desirably, the films may be exposed to temperatures above that at which the active components typically degrade without concern for loss of the desired activity.
Owner:AQUESTIVE THERAPEUTICS INC
Solid solution perforator for drug delivery and other applications
InactiveUS6945952B2Fast biodegradationBarrier property can be diminished and controlledSurgeryMicroneedlesDrug reservoirDrugs solution
A solid drug solution perforator (SSP) system and an associated drug reservoir are provided for delivering therapeutic, prophylactic and / or cosmetic compounds, for nutrient delivery and for drug targeting. For drug delivery, the SSP system includes an active drug ingredient and a matrix of perforator material that biodegrades or dissolves quickly upon contact with a patient's body. The SSP system provides a skin barrier perforator and a controller for prompt initiation and cut-off drug delivery. In a preferred method of transdermal drug delivery, an SSP system containing a selected drug penetrates into an epidermis or dermis, and the drug is promptly released from the (dissolving) SSP system perforator. An additional drug is optionally delivered from a patch reservoir through skin pores created by insertion of the perforator. Formulation and fabrication procedures for the SSP and associated reservoir are also provided. An SSP system can be fabricated with variety of shapes and dimensions.
Owner:THERAJECT INC.
Microneedle devices and microneedle delivery apparatus
InactiveUS20050261631A1Reduces tip fractureEffective perforationSurgical needlesMicroneedlesStratum corneumPain experience
Microneedle devices with microneedles having a truncated tapered shape are disclosed. The microneedles of microneedle devices may also have a controlled aspect ratio. Microneedle delivery apparatus are disclosed that include drivers designed to deliver microneedles at velocities that may enhance perforation of the stratum corneum while limiting the sensation of pain experienced at the delivery site.
Owner:3M INNOVATIVE PROPERTIES CO
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Wound healing device
ActiveUS20080275409A1Promote growthPromote woundWound drainsVaccination/ovulation diagnosticsMicron scaleVacuum pressure
Methods and devices transmit micromechanical forces locally on the millimeter to micron scale for promoting wound healing. Micromechanical forces can selectively be applied directly to tissue, in some embodiments, by using microchambers fluidically connected to microchannels. Each chamber, or in some cases, group of chambers, may be associated with a valve to control vacuum pressure, positive pressure, liquid delivery, and / or liquid removal from each chamber or group of chambers. Application of embodiments of the invention may shorten wound-healing time, reduce costs of therapy, enable restoration of functional tissue, and reduce the need for more invasive therapies, including surgery.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
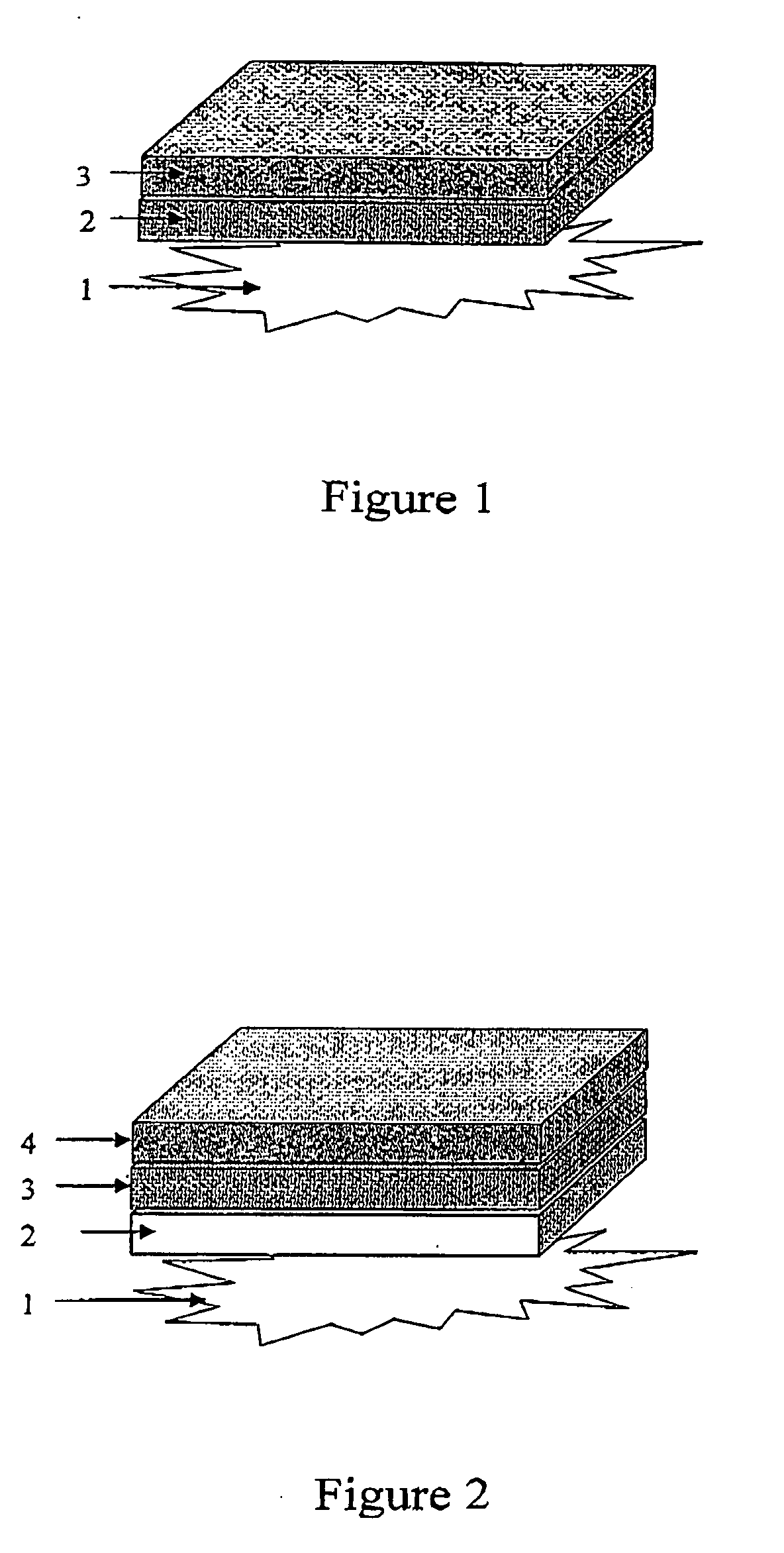
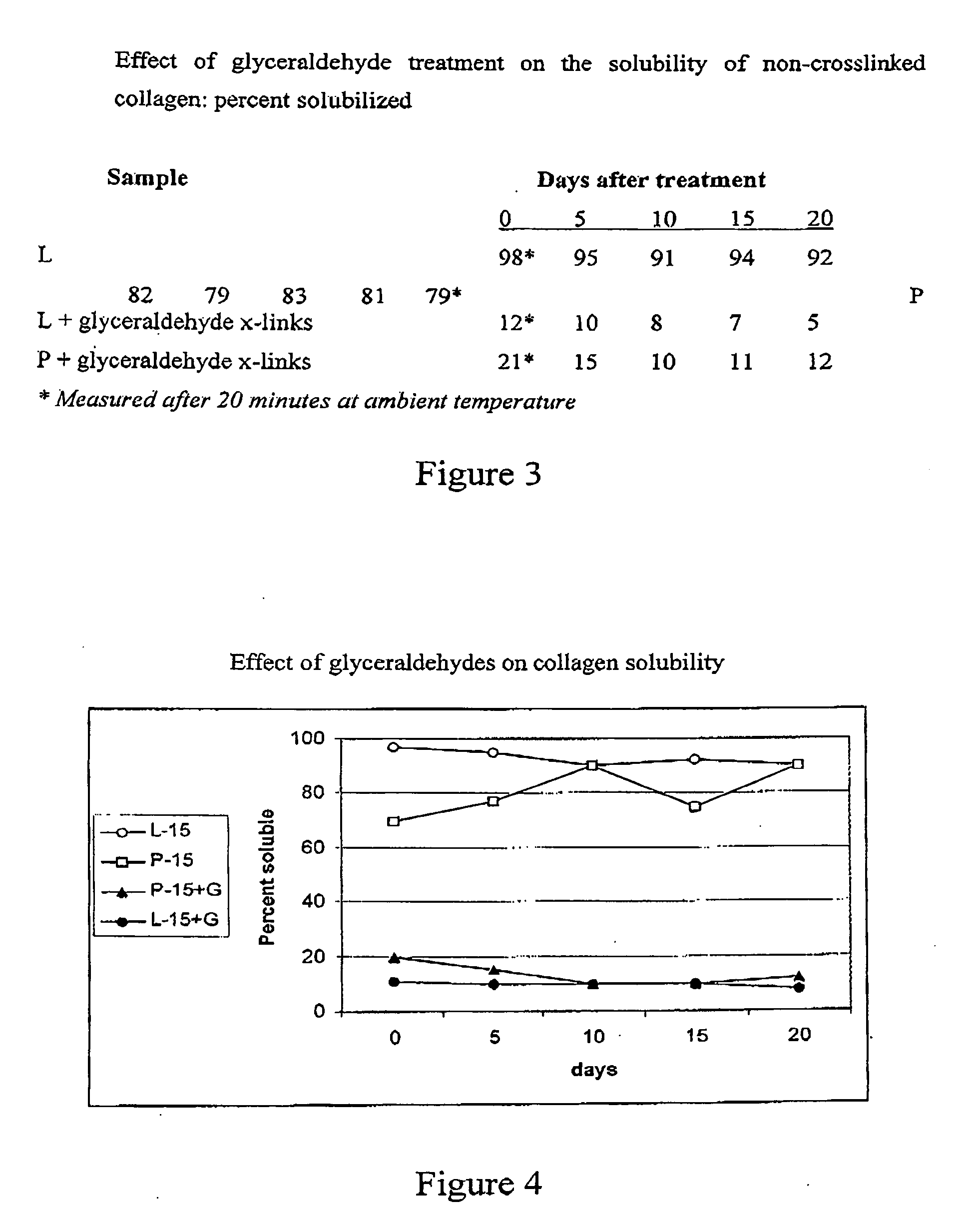
Multi-layer collagenic article useful for wounds healing and a method for its production thereof
InactiveUS20060159731A1Minimize side effectsEliminate dangerPeptide/protein ingredientsPharmaceutical non-active ingredientsCross-linkCollagen matrices
A multi-layer collagen article useful for wound healing includes at least two layers; wherein at least one layer, facing the wound side, has an effective amount of non or partially cross-linked collagen; and at least one layer having an effective amount of highly cross-linked collagen matrices. A method for the production of the collagen article and a method of enhancing wound healing by means of administering the multi-layer collagen are provided.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Topically applied clotting material
A composition, system, articles and method for the enhancement of clotting in wounds with extravascular blood flow, especially where the surface of the tissue has been broken is described. The system consists of biotolerable, porous particulates applied to the surface of a wound with liquid blood thereon. The porous nature of the particulate material, either free-flowing or packaged or restrained on or in a surface, enhances clotting. Chemical or biochemical agents, such as additional clotting agents, therapeutic agents, antibiotics, clot strengthening agents (such as fibrous structural materials), and the like may optionally be included on, with or within the porous particles. The particles may comprise such diverse materials as organics, metallics, inorganics, ceramics, and the like, both natural and artificial. It is generally preferred that the pore size distribution lies within a general range, and this range may vary from animal to animal and condition to condition, but generally falls within about 0.5 to 1000 nanometers or 3,000 to 200,000 Daltons.
Owner:MEDAFOR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com