Collagen biofabric and methods of preparation and use therefor
a biofabric and collagen technology, applied in the field of collagenous membranes, can solve the problems of scarce sources of biological raw materials, less desirable tissues, scarcity of human donor tissues for grafting, etc., and achieve the effects of improving physical and biophysical properties, superior characteristics, and improving tensile strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
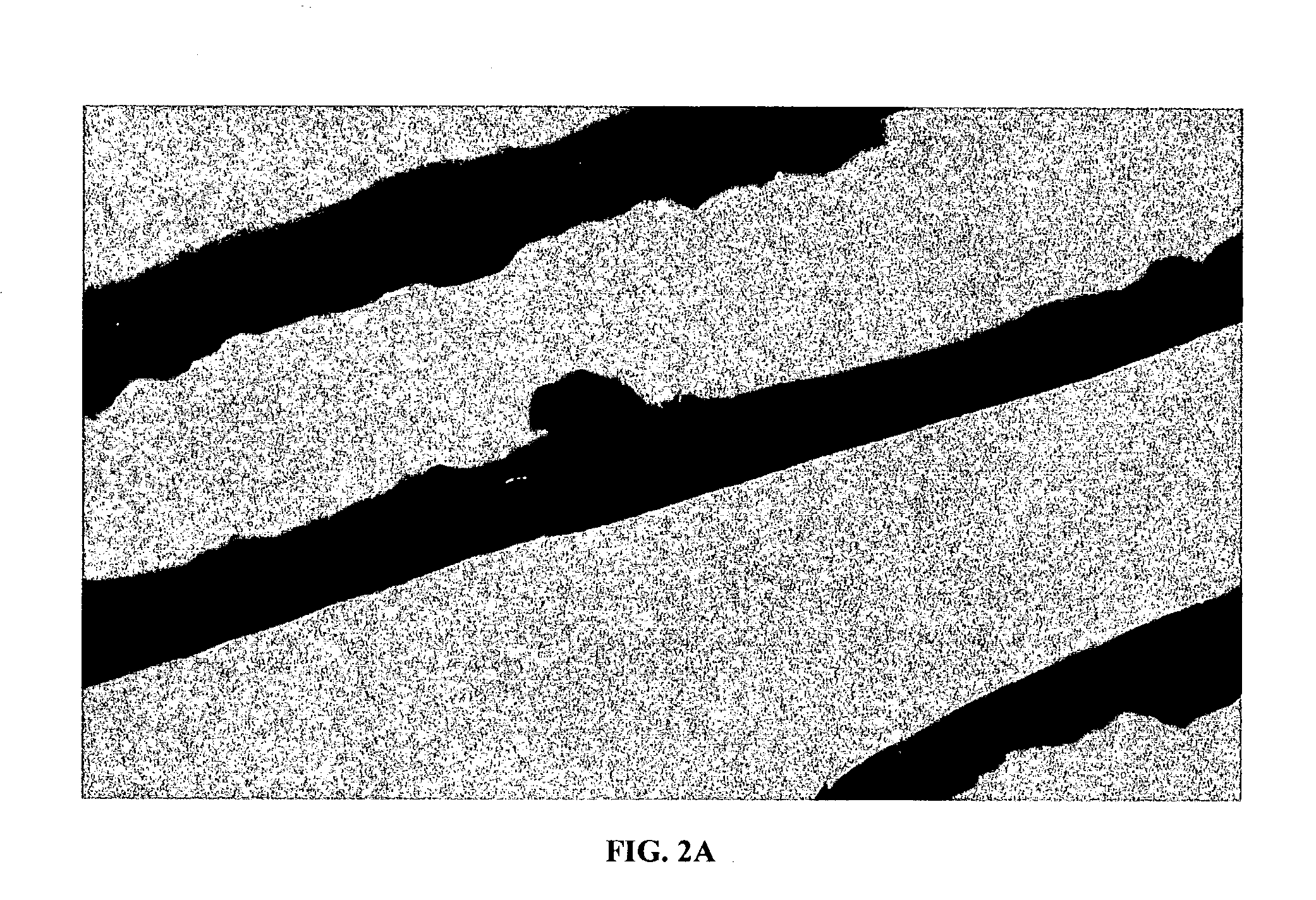
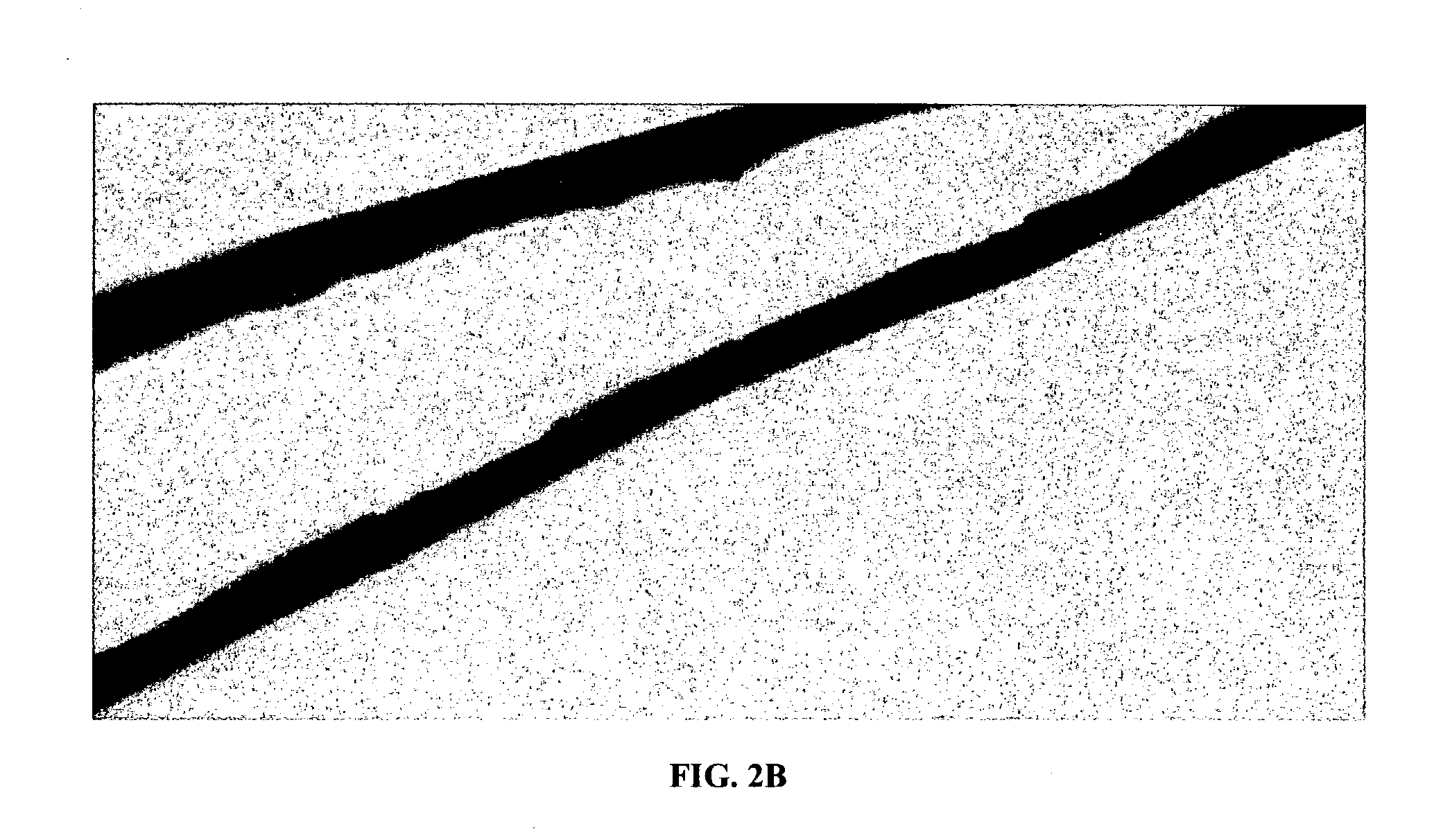
[0037] The present invention provides a collageneous membrane or biofabric derived from the placenta of a mammal, preferably of a human. The collagen biofabric is prepared so as to retain the native collagen conformation, i.e., the native tertiary and quaternary conformation, in the final product. In addition to the collagen biofabric, the present invention also provides methods of making the collagen biofabric, and of using the biofabric in a medical setting.
[0038] The present invention provides a collagen biofabric comprising a dehydrated, decellularized and substrate-free amniotic membrane so that the amniotic membrane has a native tertiary and quaternary structure. In some embodiments the invention provides a decellularized and substrate-free collagen biofabric comprising of collagen, elastin, and fibronectin.
[0039] In some embodiments, the invention provides an amniotic membrane laminate comprising a collagen biofabric of the invention. The amniotic membrane laminate prepared i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com