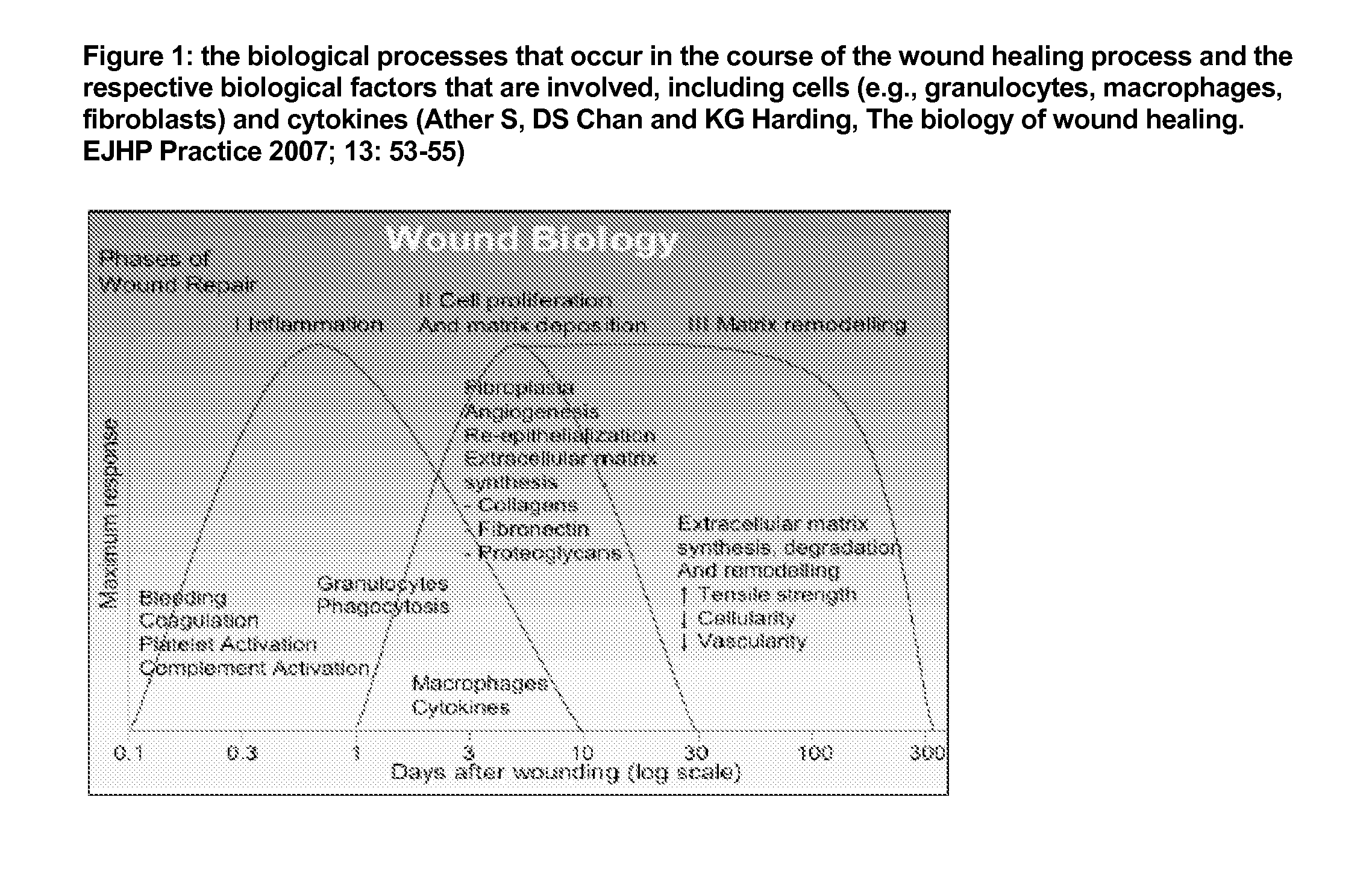
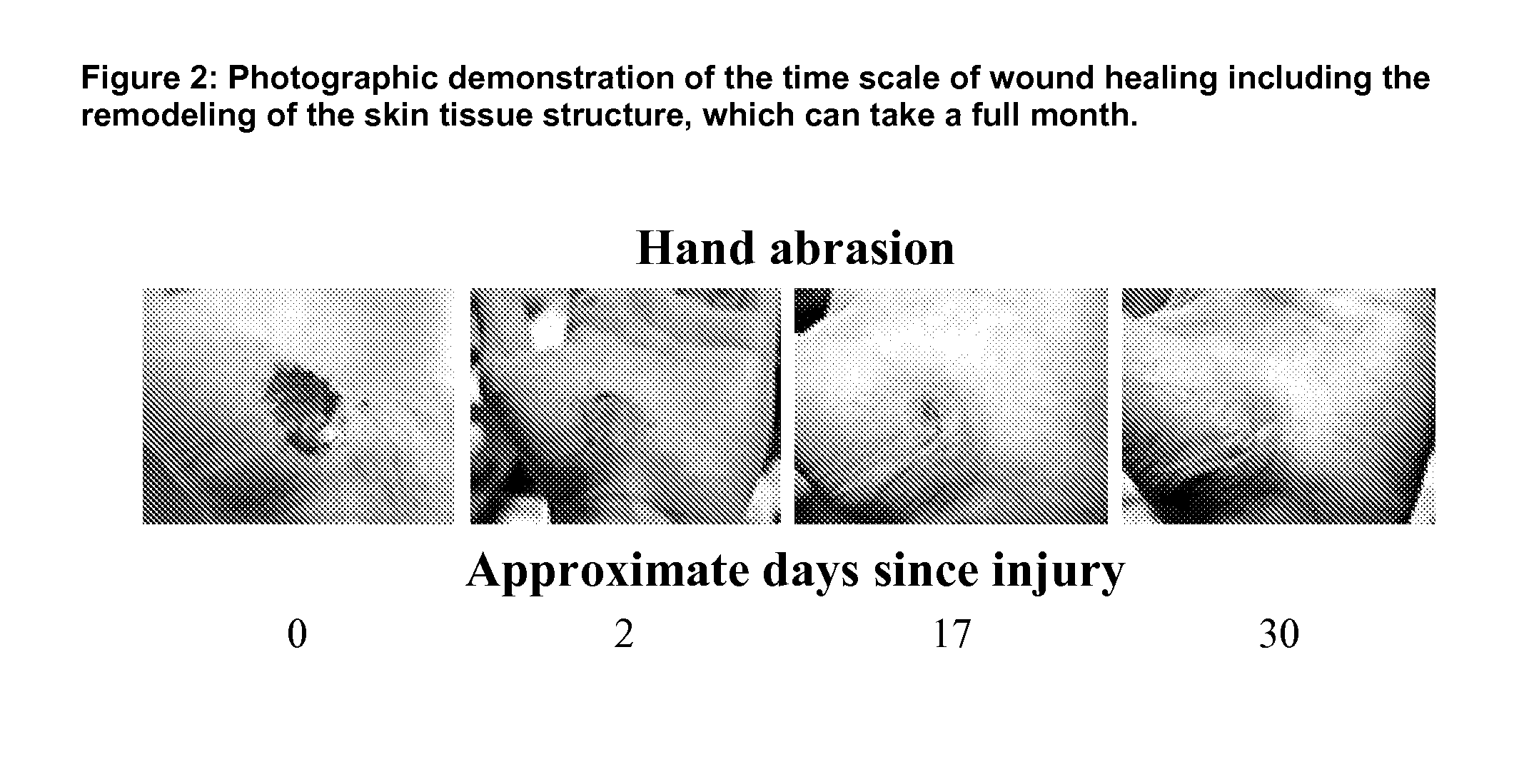
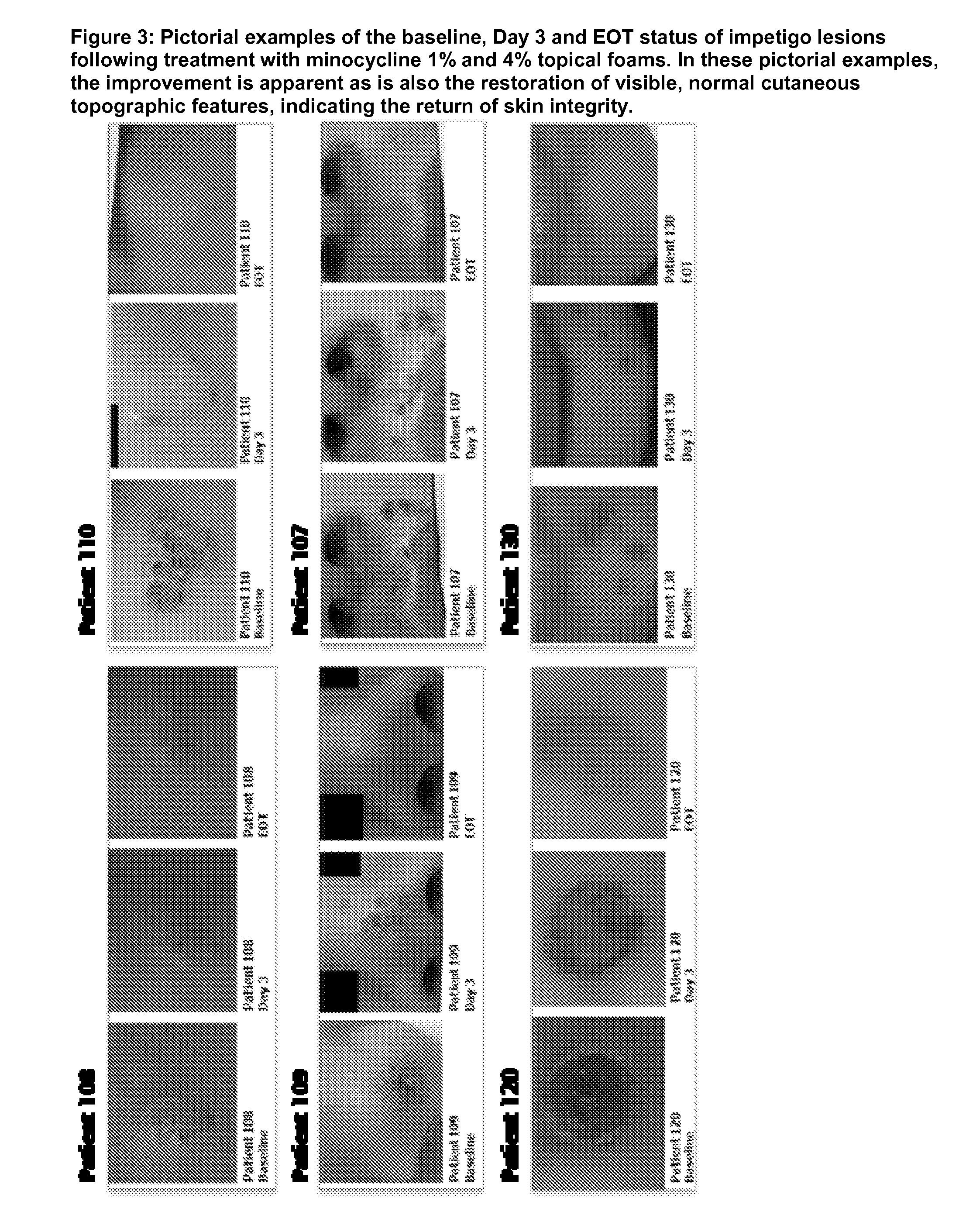
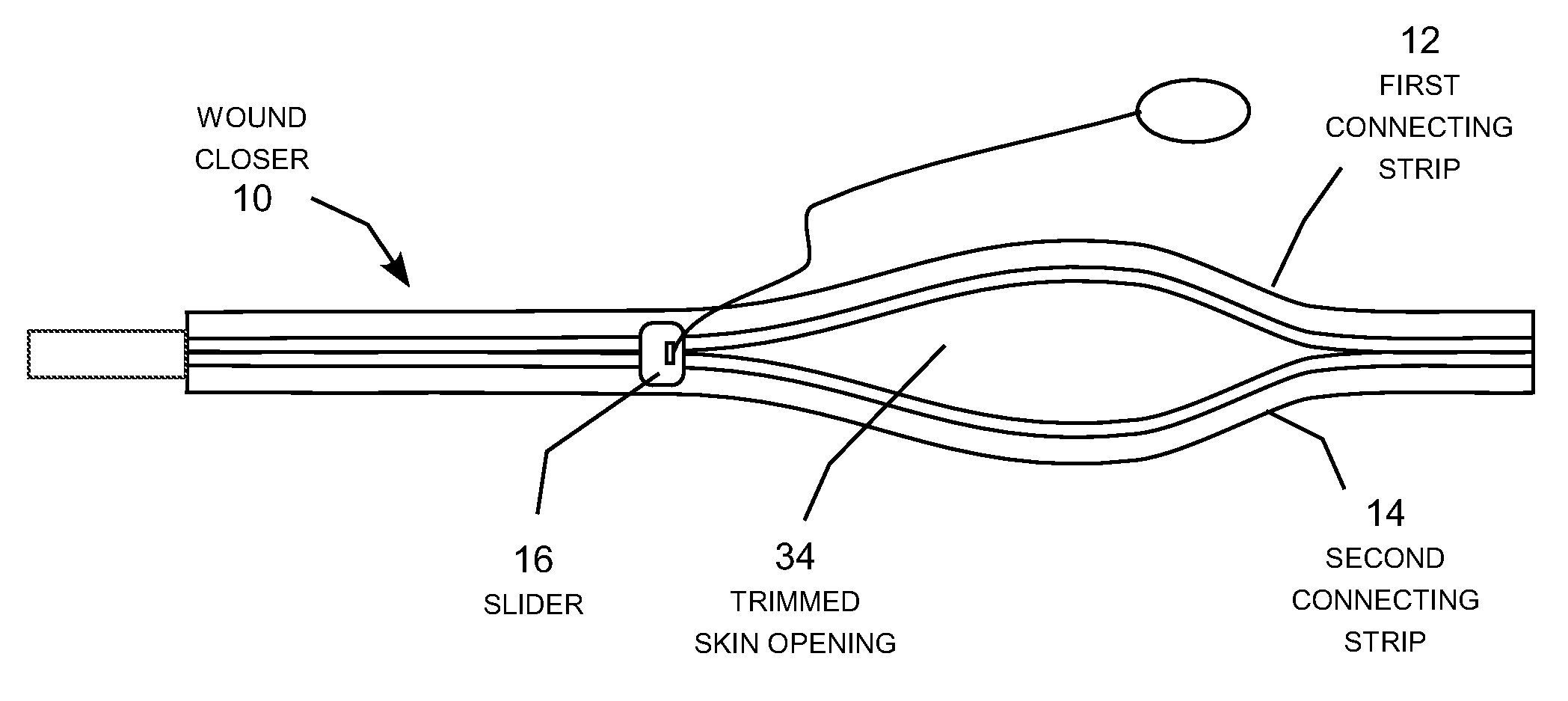
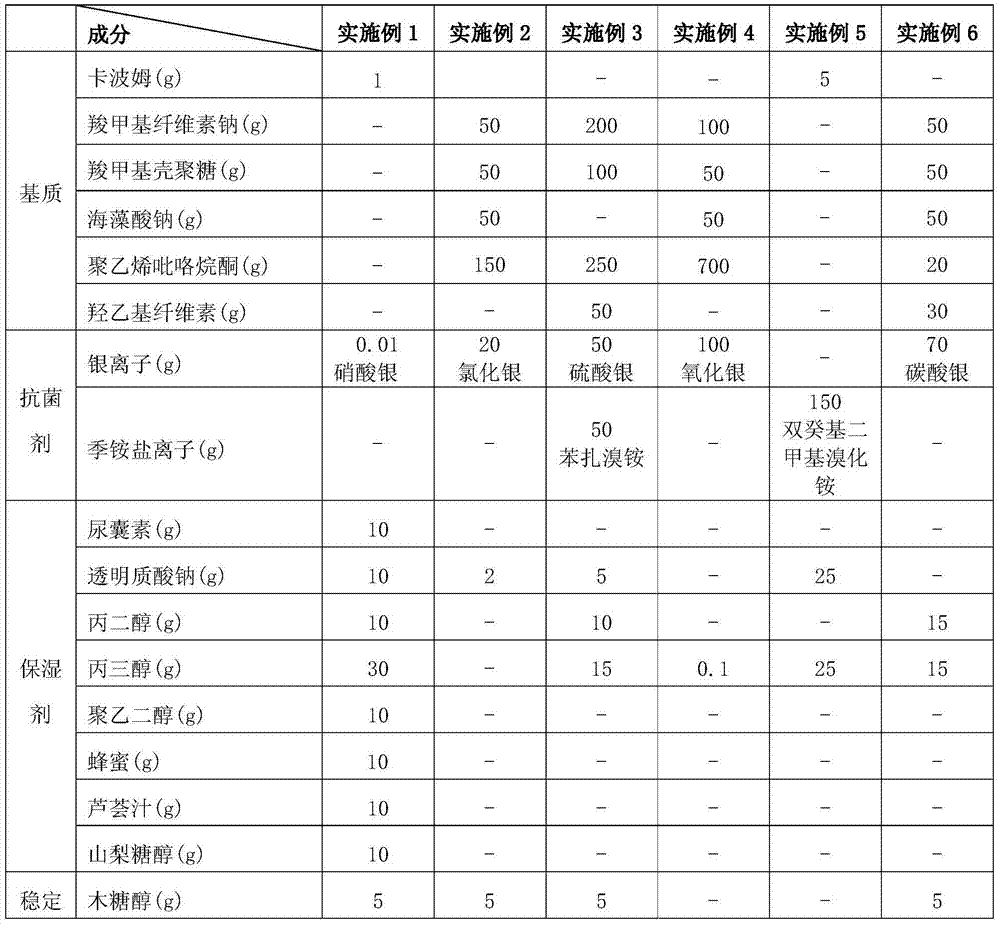
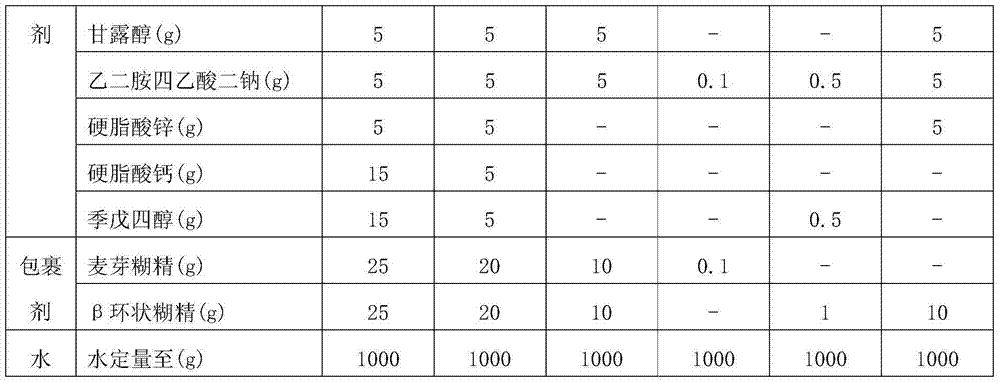
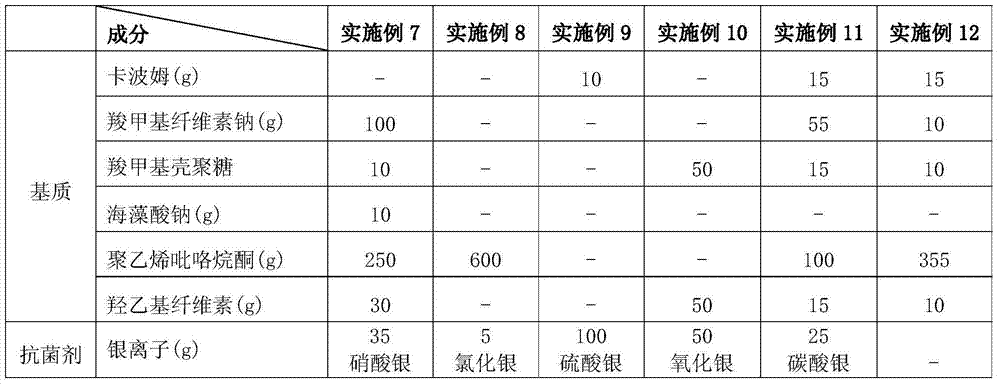
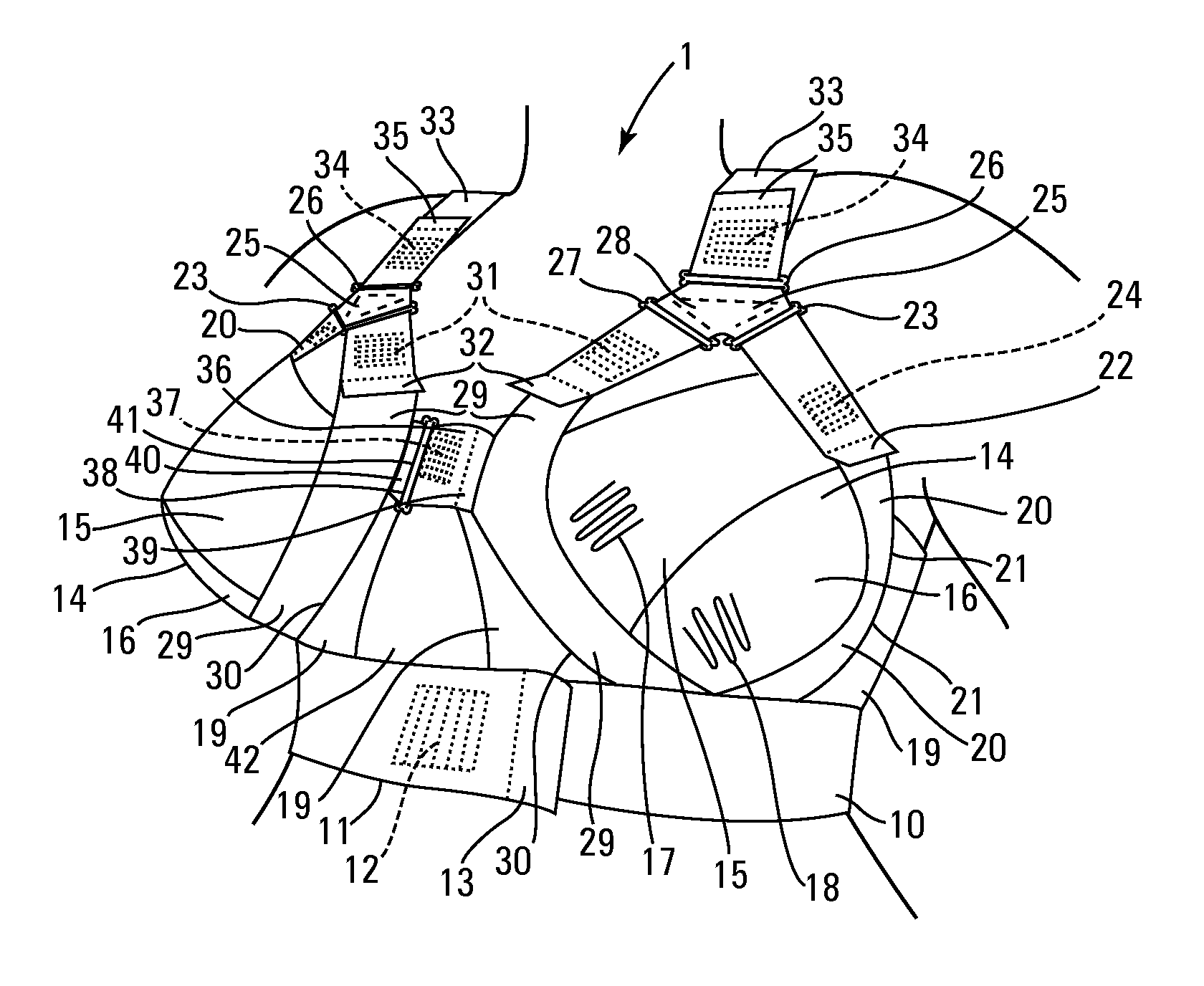
Patents
Literature
823 results about "Injury mouth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mouth injuries can happen to anyone, but are very common in children. Damage done to the cavity of the mouth or surrounding area is considered a mouth injury. This includes: Lips. Frenulum (flap of skin under the lips) Inner cheek. Tongue.
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
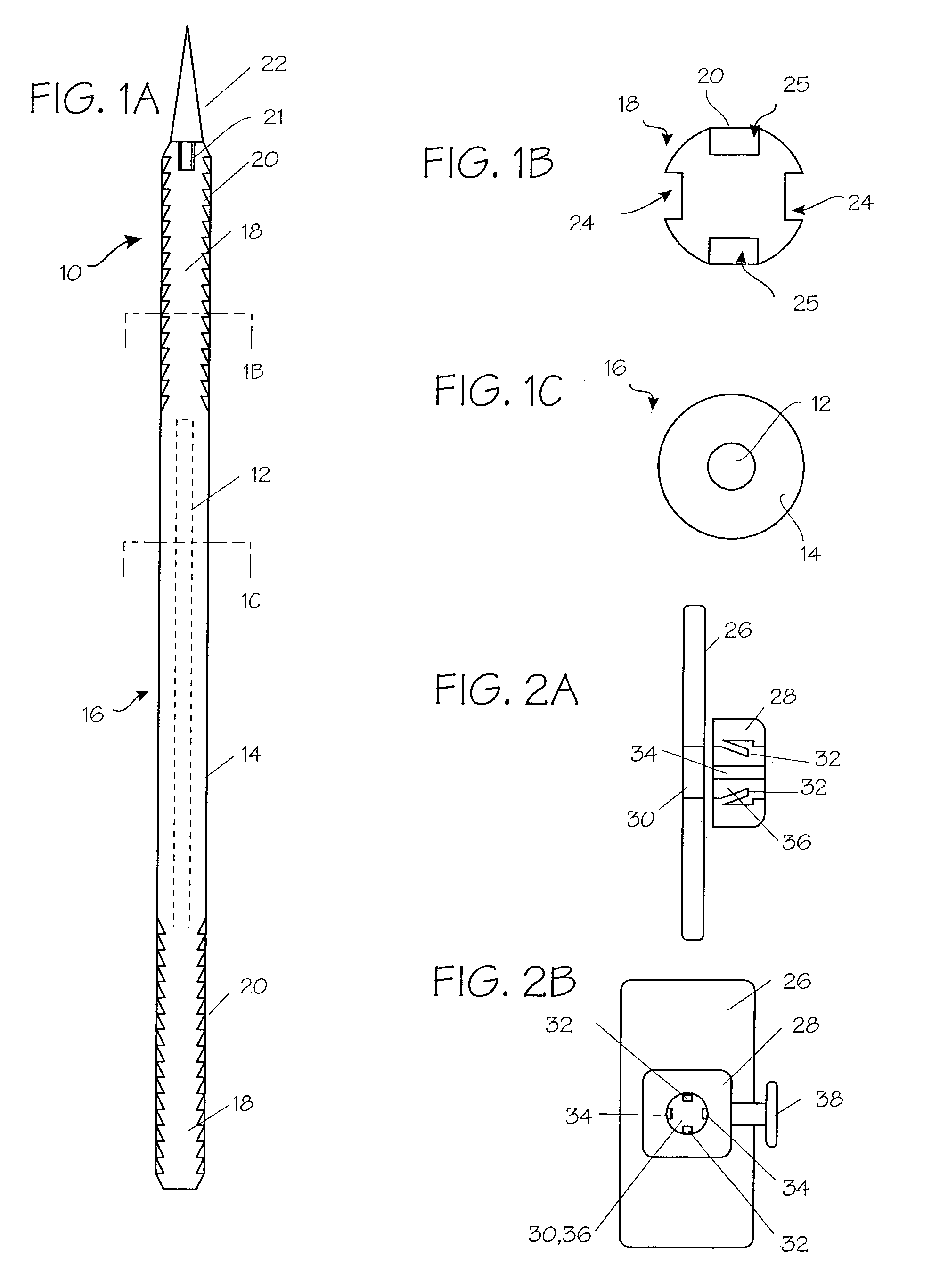
Suture method
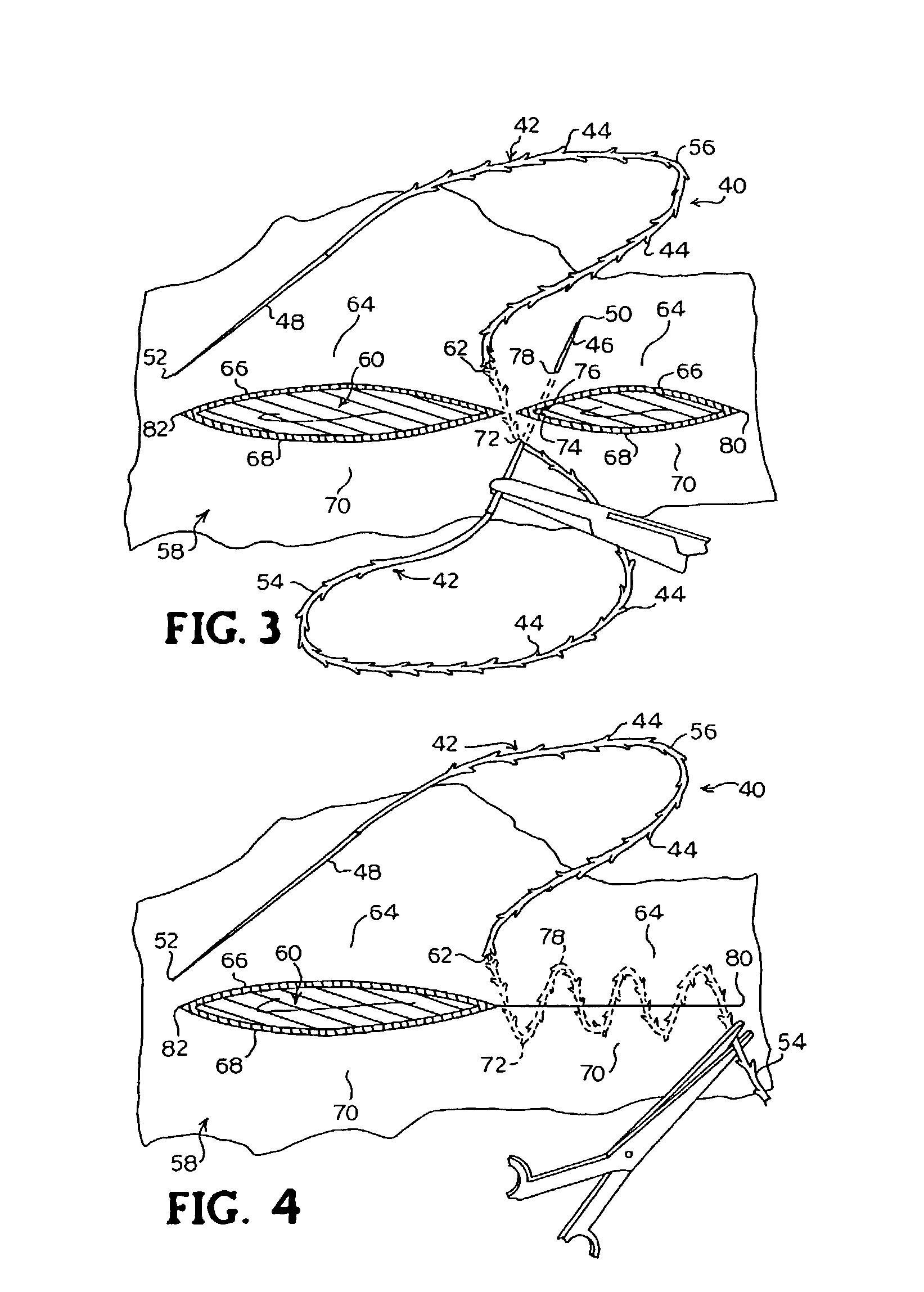
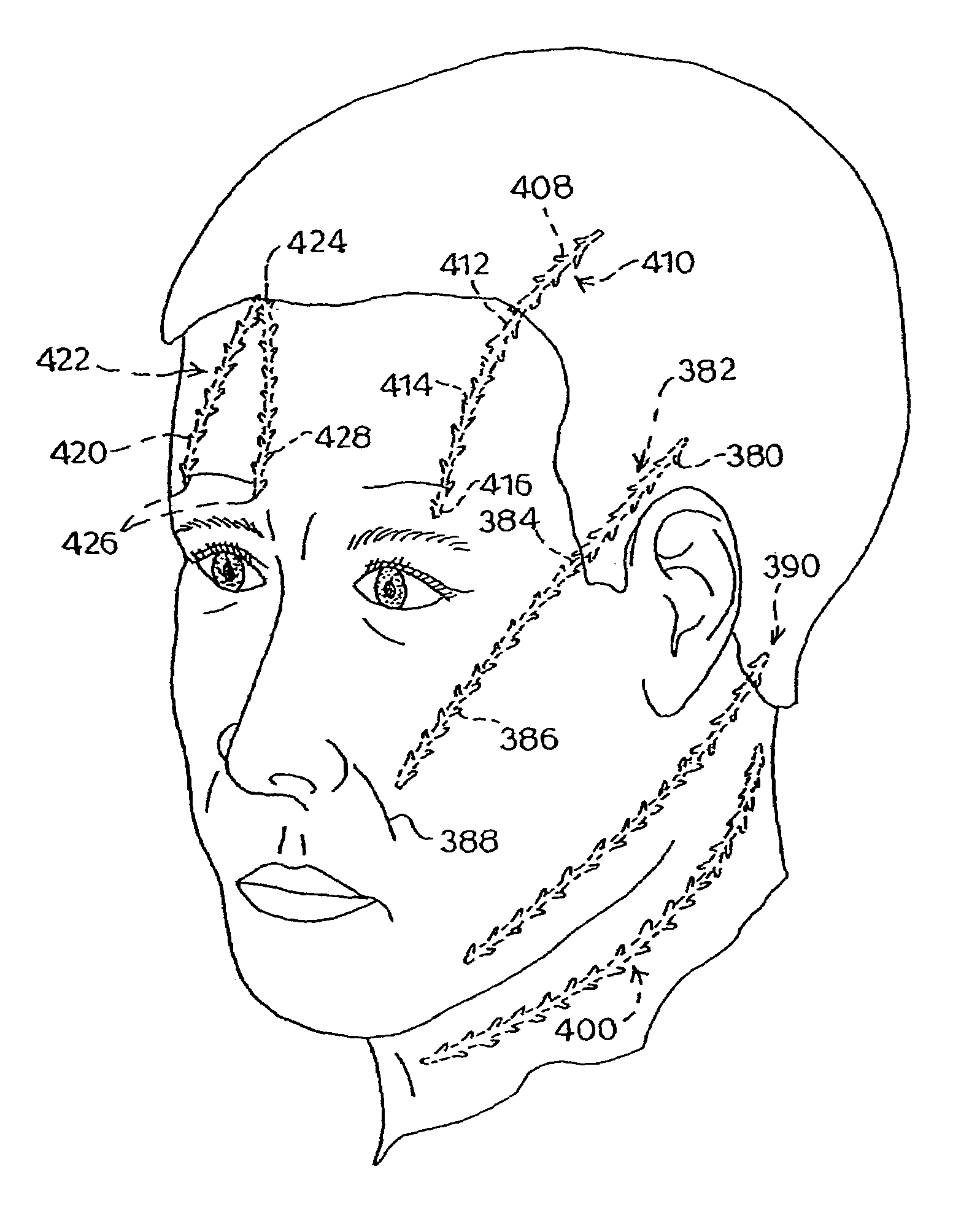
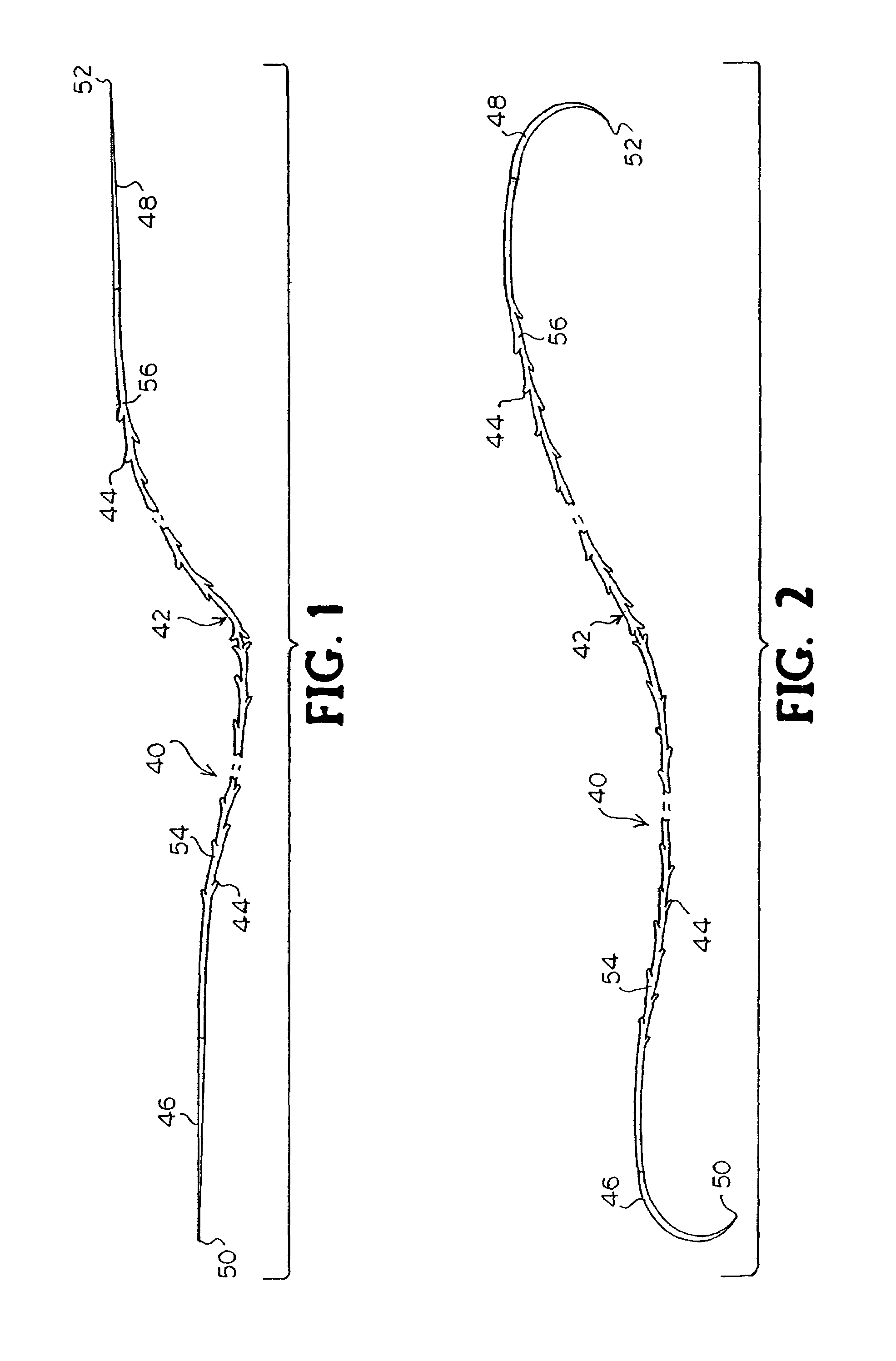
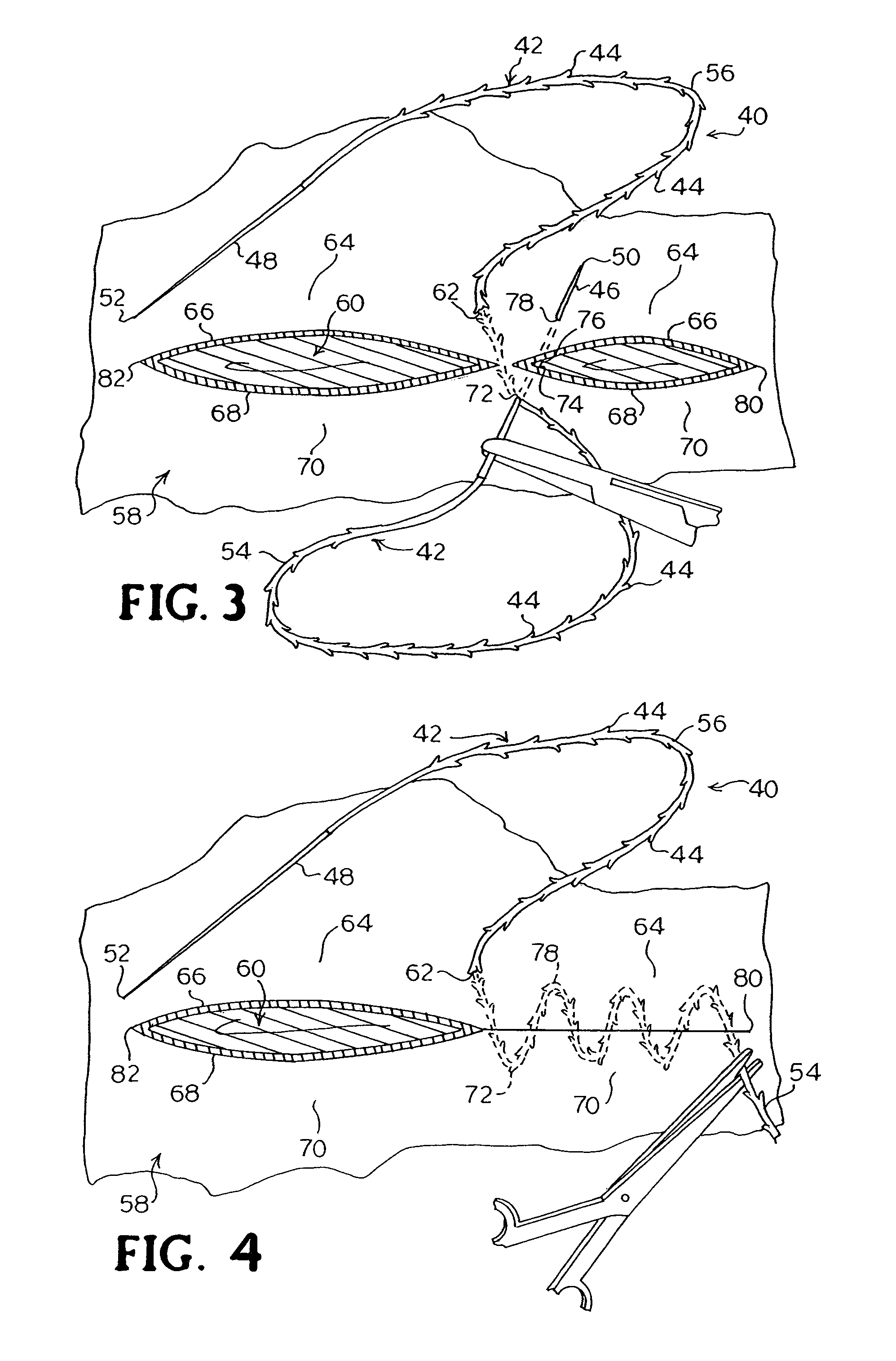
A method for joining and holding closed a wound in bodily tissue, fastening junctions of wounds, tying off wounds, joining a foreign element to tissue, and altering the position of tissue using a barbed suture including sharp pointed ends. Each end of the suture includes barbs on that permit movement in an opposing direction to the barbs on the other end of the suture. This two-way barbed suture is used by the method of the present invention in applications including abdominal surgeries such as a Nissen fundoplication, laparoscopic uses such as stabilizing a bowel structure and performing a closure of a cystostomy, liver to bowel anastomosis, closure of an orifice of a Zenker's Diverticulum, endoscopic uses such as closure of ulcerative lesions or and post-procedural tissue defects, bladder wound closure, valve replacement surgery, device attachment, cosmetic surgery, and blood vessel wound closure.
Owner:ETHICON INC
Suture method
A method for joining and holding closed a wound in bodily tissue, fastening junctions of wounds, tying off wounds, joining a foreign element to tissue, and altering the position of tissue using a barbed suture including sharp pointed ends. Each end of the suture includes barbs on that permit movement in an opposing direction to the barbs on the other end of the suture. This two-way barbed suture is used by the method of the present invention in applications including abdominal surgeries such as a Nissen fundoplication, laparoscopic uses such as stabilizing a bowel structure and performing a closure of a cystostomy, liver to bowel anastomosis, closure of an orifice of a Zenker's Diverticulum, endoscopic uses such as closure of ulcerative lesions or and post-procedural tissue defects, bladder wound closure, valve replacement surgery, device attachment, cosmetic surgery, and blood vessel wound closure.
Owner:ETHICON INC
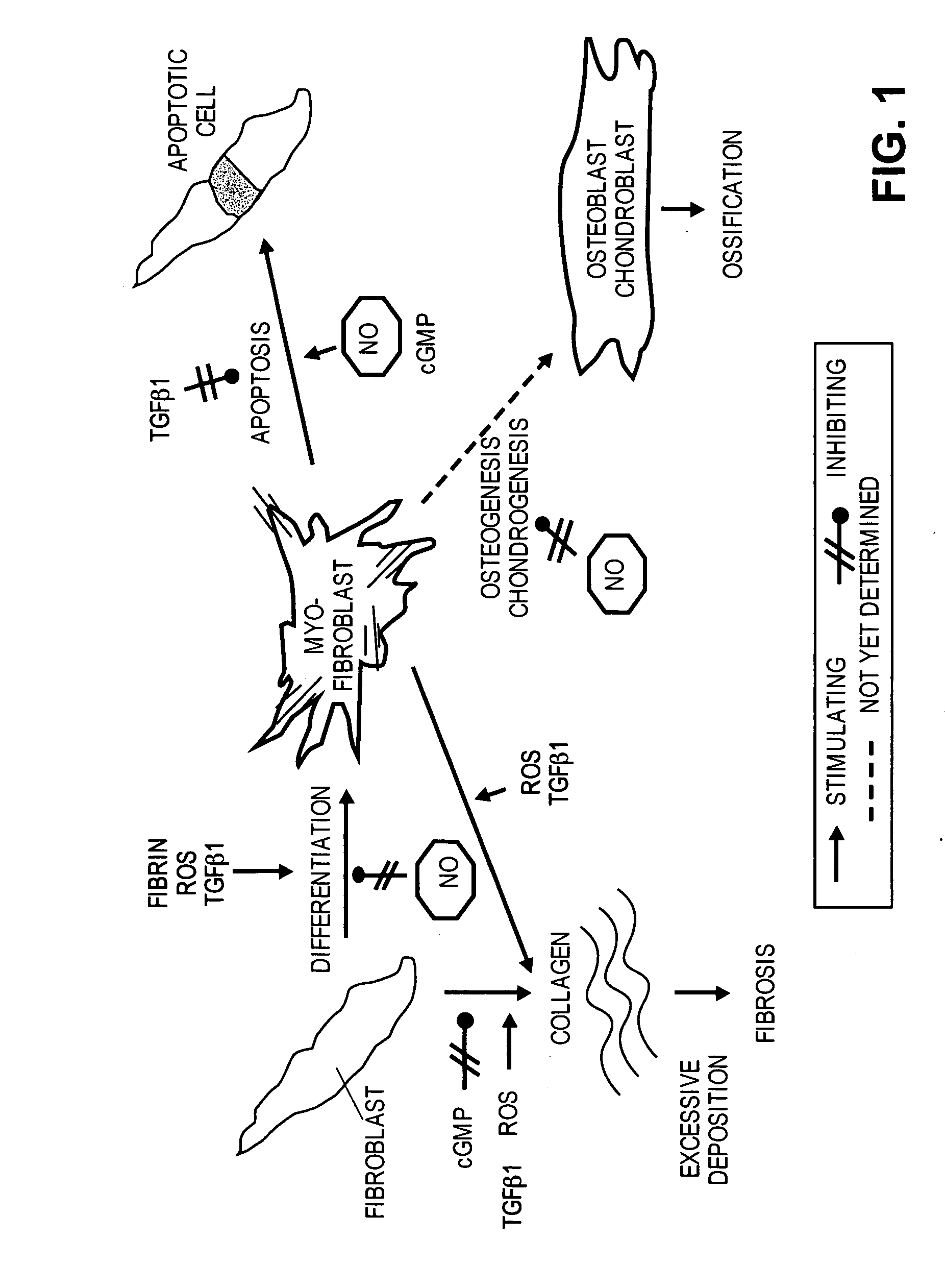
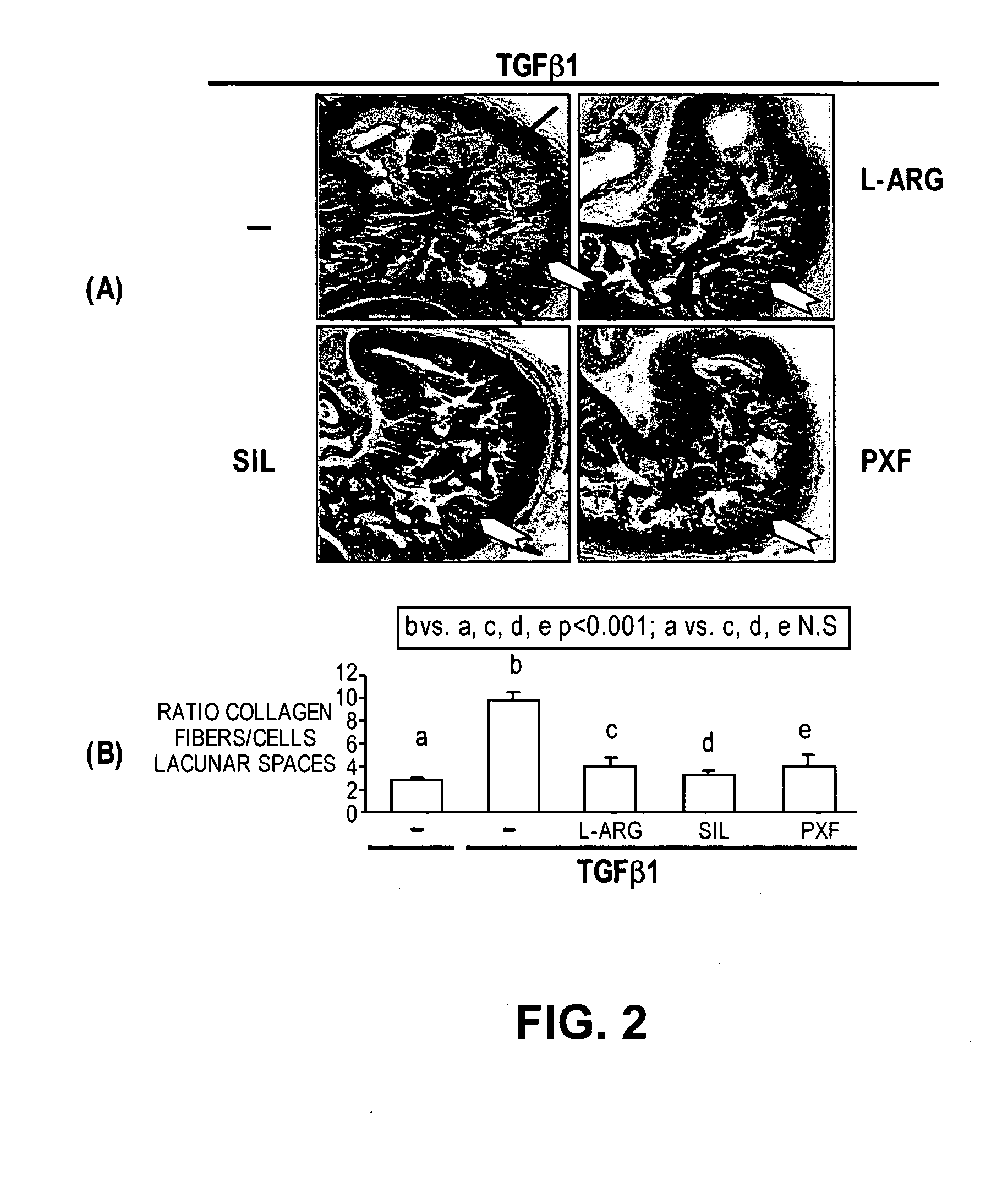
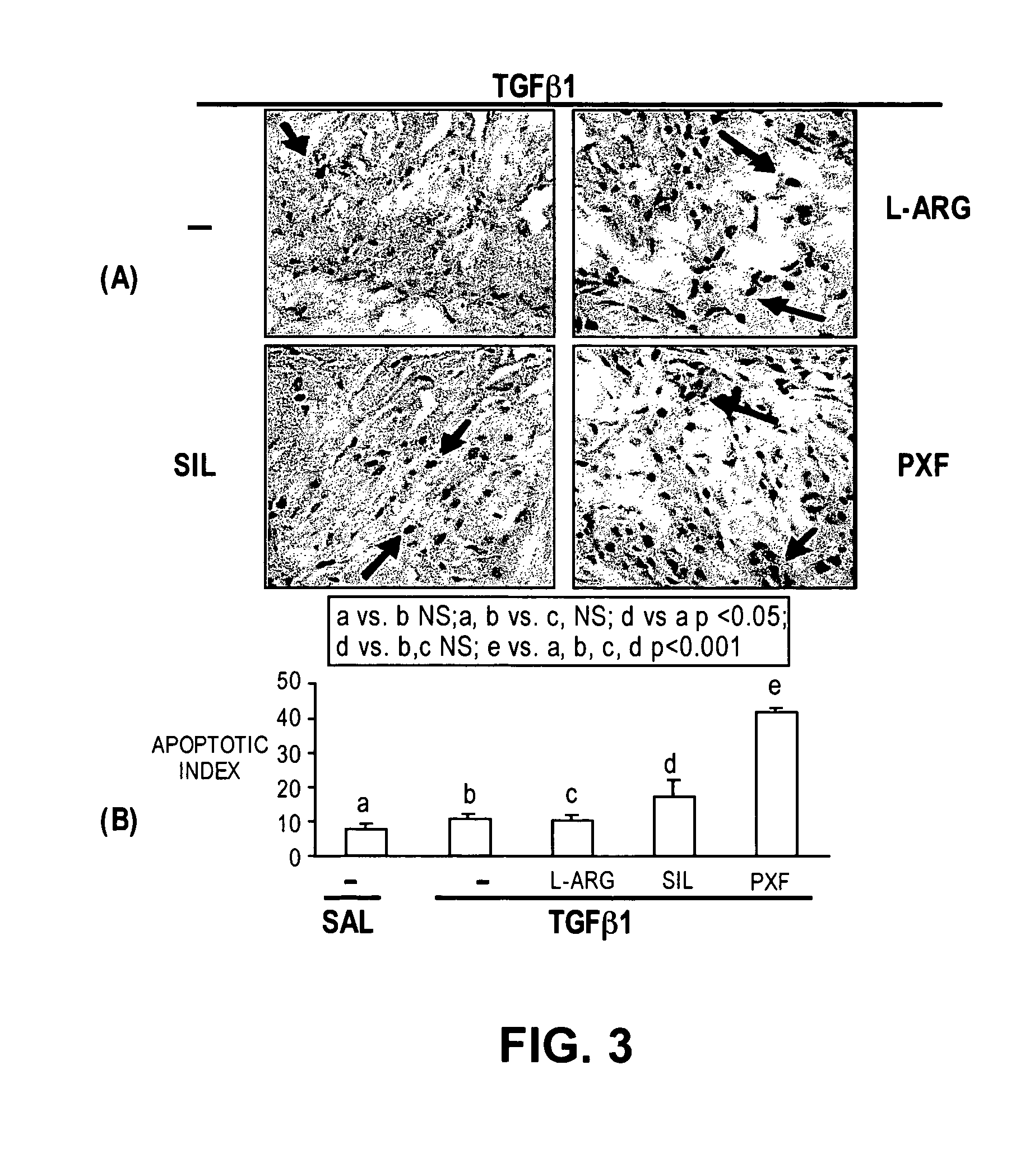
Methods of use of inhibitors of phosphodiesterases and modulators of nitric oxide, reactive oxygen species, and metalloproteinases in the treatment of peyronie's disease, arteriosclerosis and other fibrotic diseases
ActiveUS20050085486A1Increasing NO levelReduce expressionBiocidePharmaceutical delivery mechanismFemale Sexual Arousal DisorderCyclase
The present methods and compositions are of use for treatment of conditions involving fibrosis, such as Peyronie's disease plaque, penile corporal fibrosis, penile veno-occlusive dysfunction, Dupuytren's disease nodules, vaginal fibrosis, clitoral fibrosis, female sexual arousal disorder, abnormal wound healing, keloid formation, general fibrosis of the kidney, bladder, prostate, skin, liver, lung, heart, intestines or any other localized or generalized fibrotic condition, vascular fibrosis, arterial intima hyperplasia, atherosclerosis, arteriosclerosis, restenosis, cardiac hypertrophy, hypertension or any condition characterized by excessive fibroblast or smooth muscle cell proliferation or deposition of collagen and extracellular matrix in the blood vessels and / or heart. In certain embodiments, the compositions may comprise a PDE-4 inhibitor, a PDE-5 inhibitor, a compound that elevates cGMP and / or PKG, a stimulator of guanylyl cyclase and / or PKG, a combination of a compound that elevates cGMP, PKG or NO with an antioxidant that decreases ROS, or a compound that increases MMP activity.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
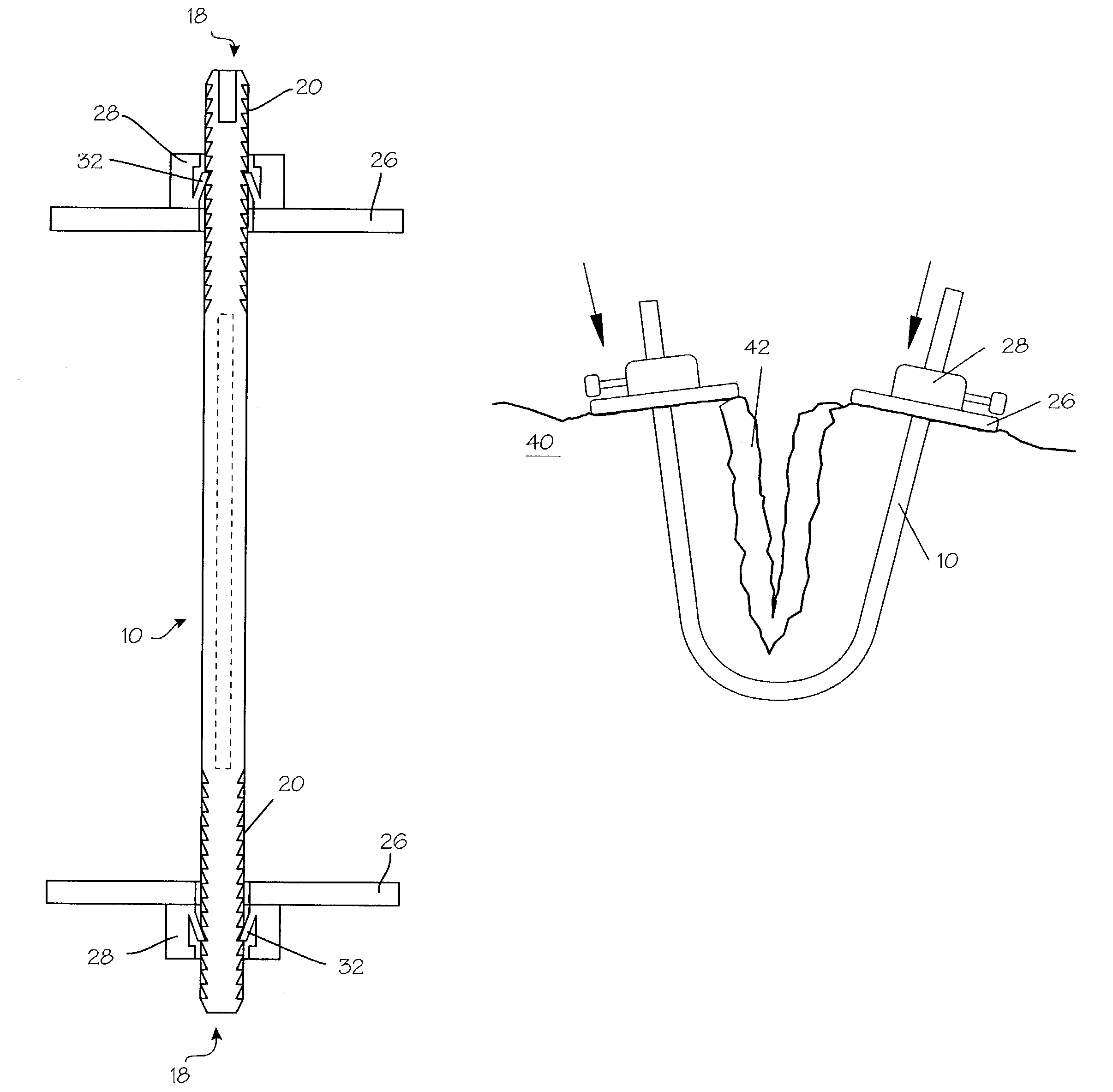
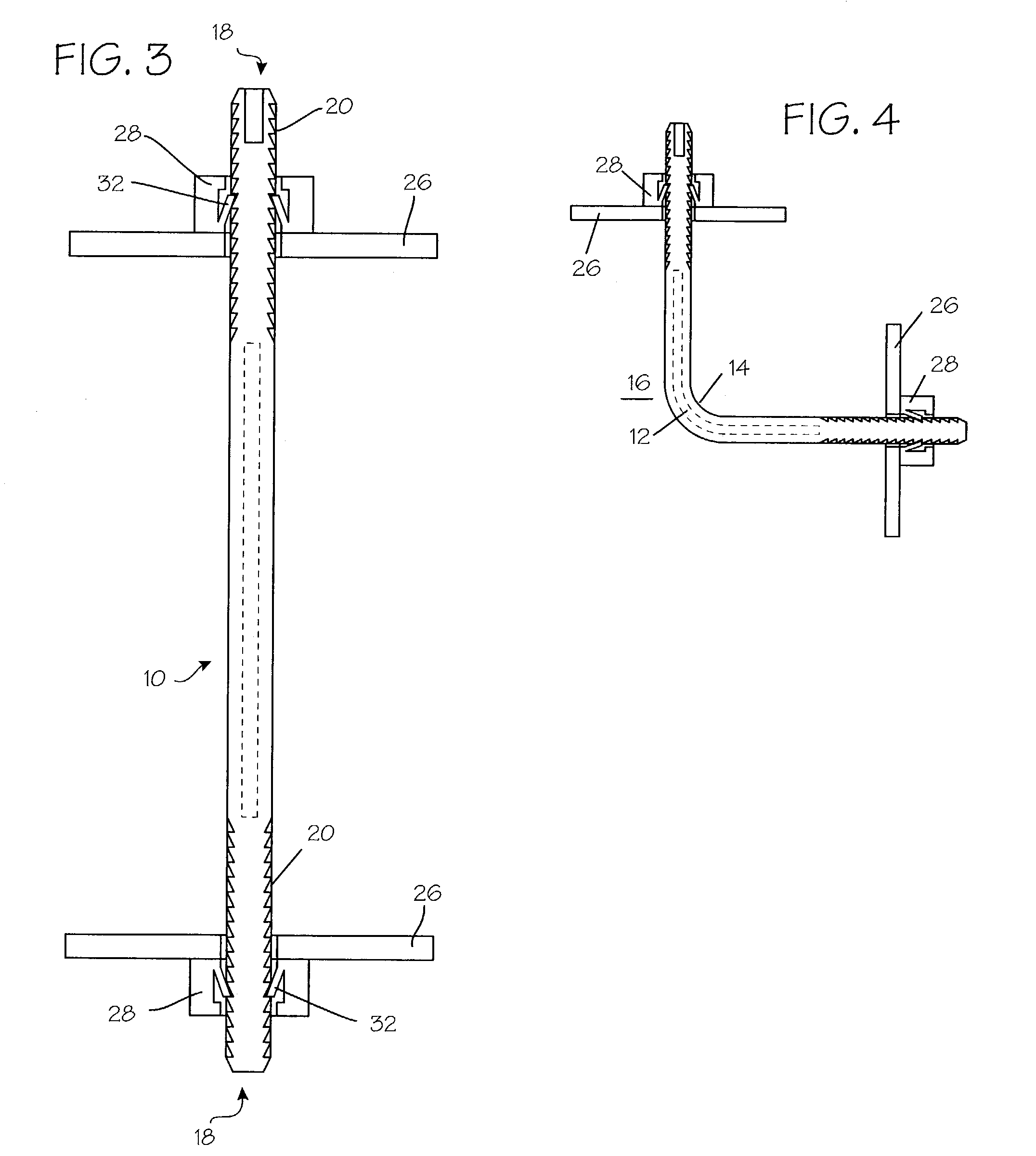
Method and apparatus for solid organ tissue approximation
InactiveUS7235090B2Improved haemostatic tissue appositionEasy and fast applicationIntravenous devicesSurgical veterinaryTrauma surgeryParenchyma
Devices and methods are disclosed for achieving hemostasis in solid visceral wounds. Such devices and methods are especially useful in the emergency, trauma surgery or military setting. In such cases, the patient may have received trauma to the abdominal viscera. The devices utilize flexible, variable depth transfixing bolts that penetrate the viscera. These bolts are pulled tight to bring the tissue into apposition and hold said tissue in apposition while the wound heals. These bolts overcome the limitations of sutures that are currently used for the same purposes. The bolts come in a variety of lengths and diameters. Since the bolts are flexible, the curvature may be adjusted by the surgeon. The devices are flexible, bendable, and conformable in their wet or dry state. They can be used either straight or through a broad range of curvatures to suit the needs of various pathologies. The bolts include pressure plates that are capable of exerting compressive pressure over broad areas of visceral wounds without causing tearing of the friable parenchyma. The bolts may be placed and removed by open surgery or laparoscopic access.
Owner:DAMAGE CONTROL SURGICAL TECH
Dissection handpiece with aspiration means for reducing the appearance of cellulite
ActiveUS20120165725A1Prevent removalIncrease kinetic energyIncision instrumentsExcision instrumentsWound healingSkin treatments
A dermatological skin treatment device is provided. The device comprises a handpiece and a cutting tool, wherein the tool is inserted through the conduit and percutaneously inserted into a tissue disposed within a recessed area of the handpiece. The device and method cut the fibrous structures under the skin that cause cellulite at an angle substantially parallel to the surface of the skin and replace these structures with a non-cellulite forming structure by deploying a highly fibrous mesh through a single needle hole to create a highly fibrous layer directly or through wound healing processes. A tool is provided to aspirate excess fluid and tissue from the treatment area.
Owner:ULTHERA INC
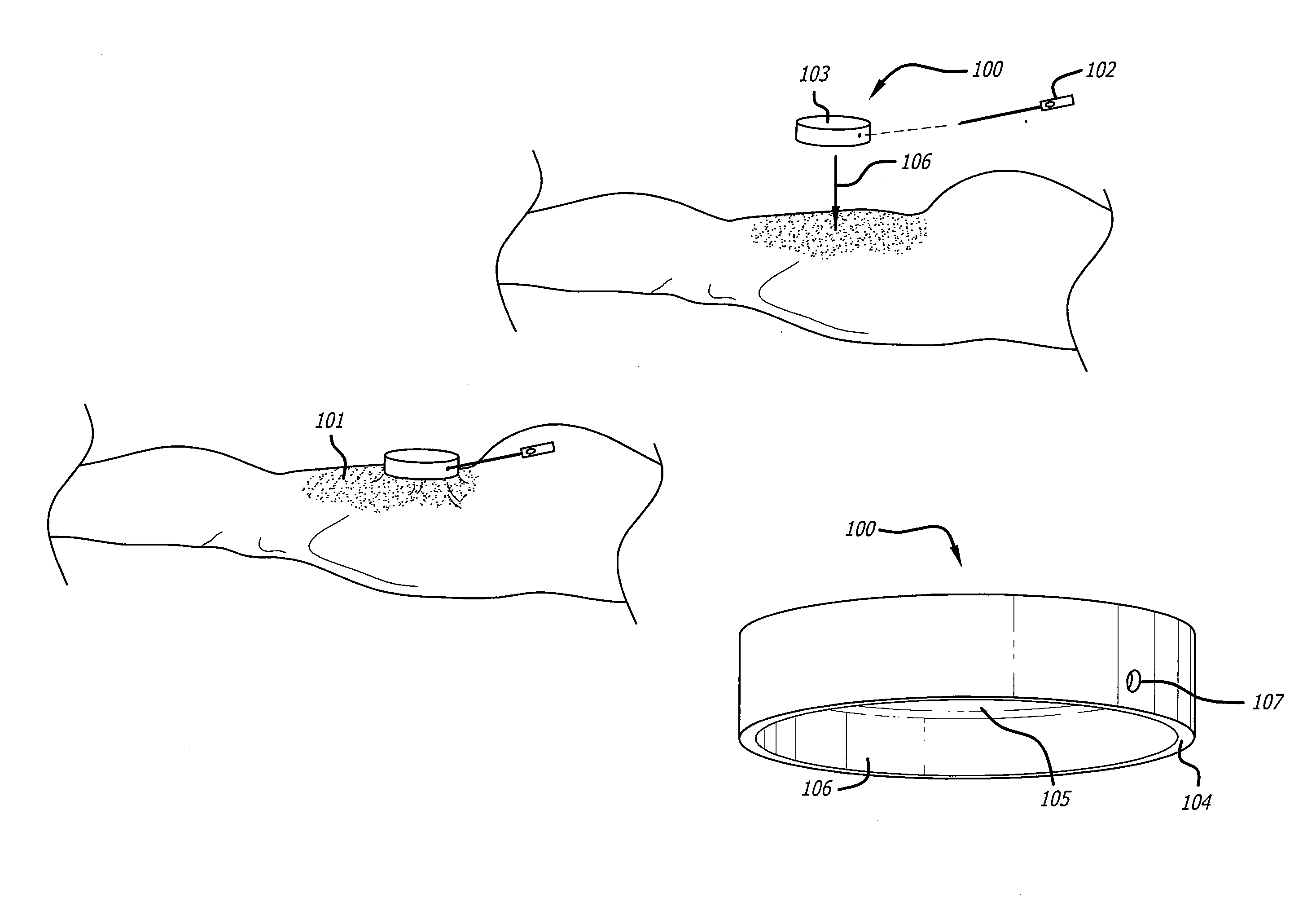
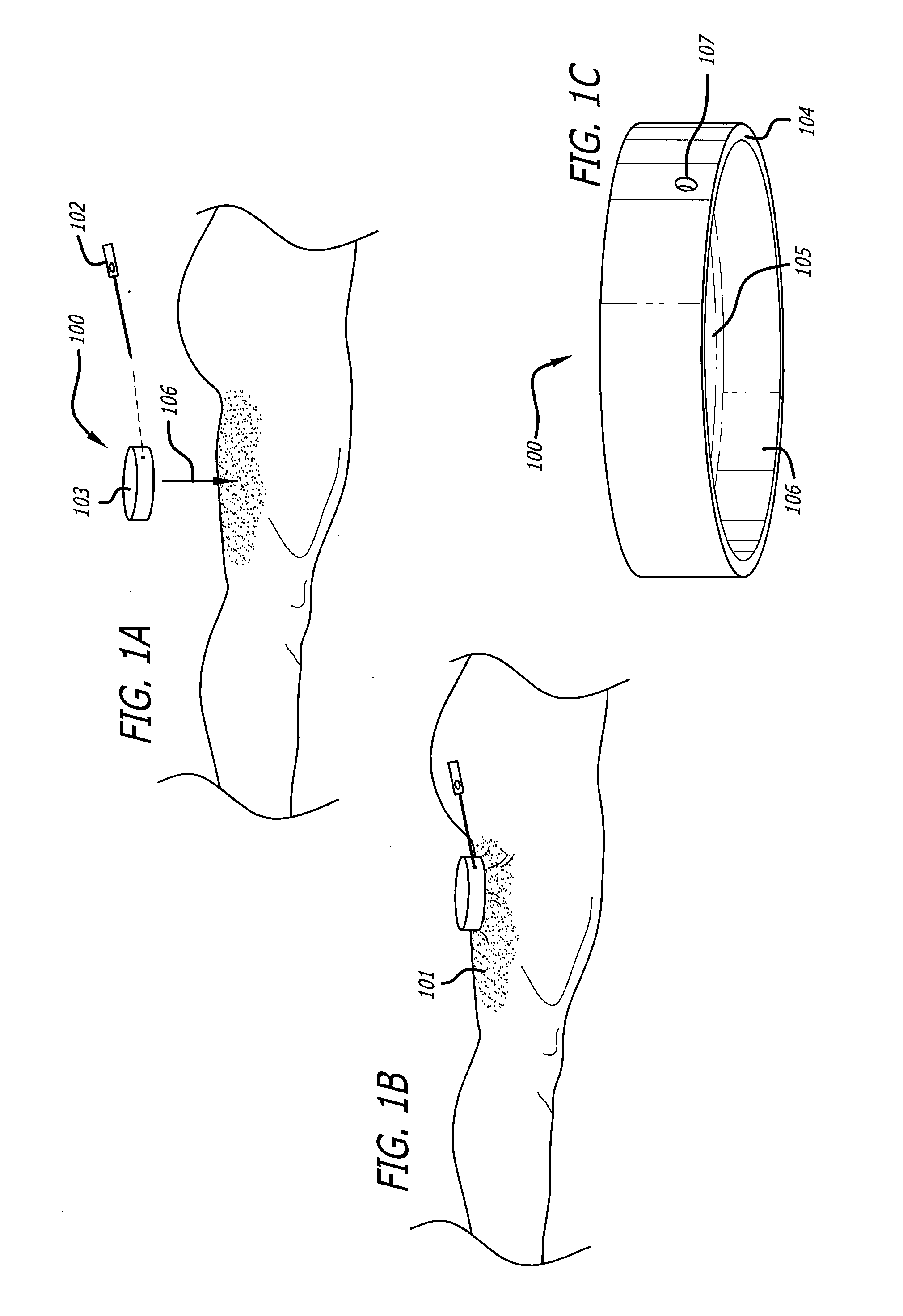
Surgical packing devices
InactiveUS20110028934A1Avoid lossImproved haemostatic packing devicesPlastersTourniquetsTrauma surgeryInjury mouth
Devices and methods are disclosed for achieving hemostasis in patients who have received skin-penetrating wounds to the body in regions such as the shoulder, pelvis, neck, or groin, where standard bandages are difficult to apply and where large blood vessels exist which can hemorrhage severely. Such haemostatic packing devices and methods are especially useful in the emergency, trauma surgery, or military setting. The devices utilize packing pillows that are held in place by rigid structures that can cause the packing pillows to be brought into the wounds and be held in place while the packing pillows are inflated to fill the wounds, prevent bleeding, and tamponade hemorrhaging large blood vessels exposed therein.
Owner:DAMAGE CONTROL SURGICAL TECH
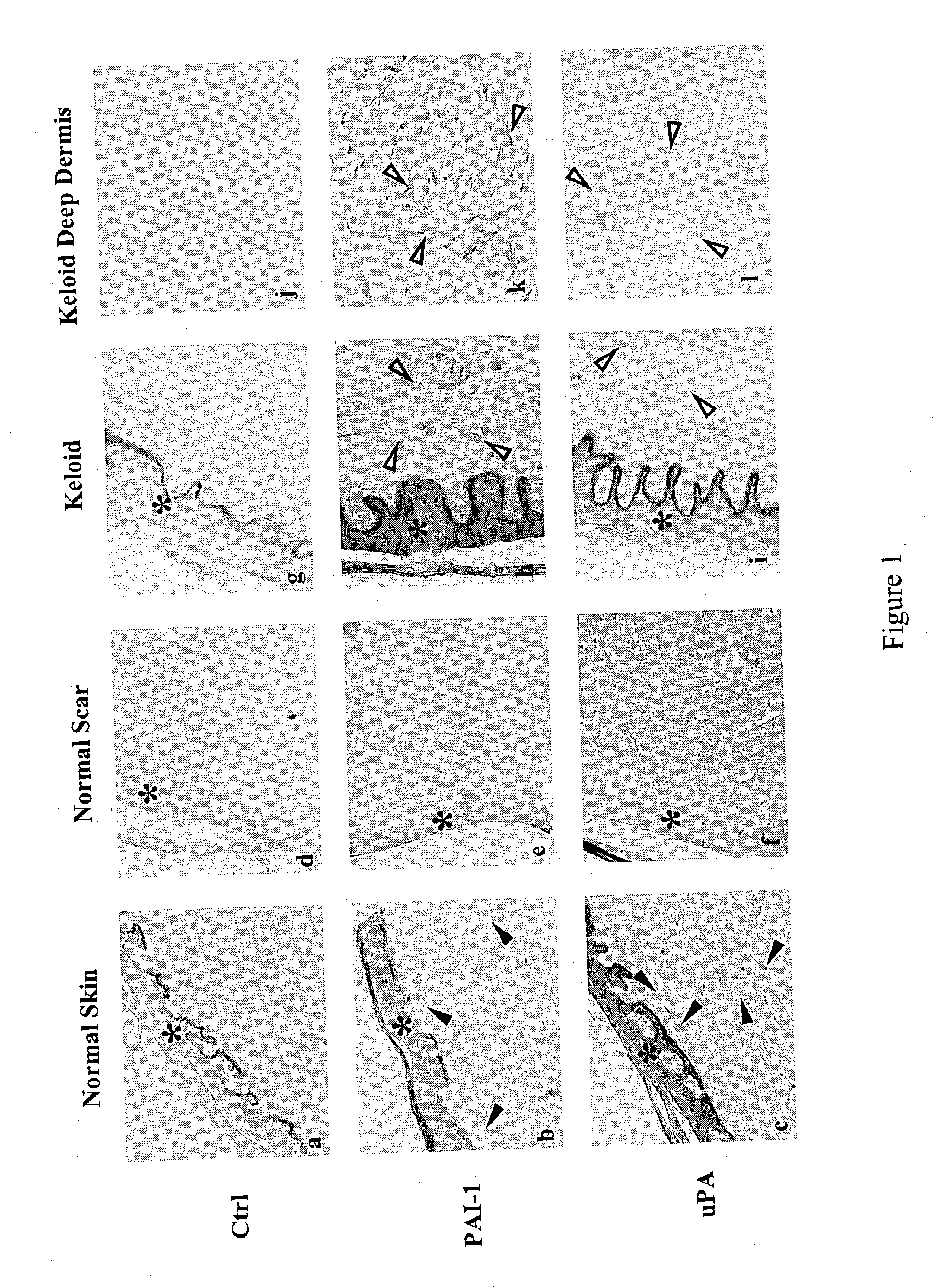
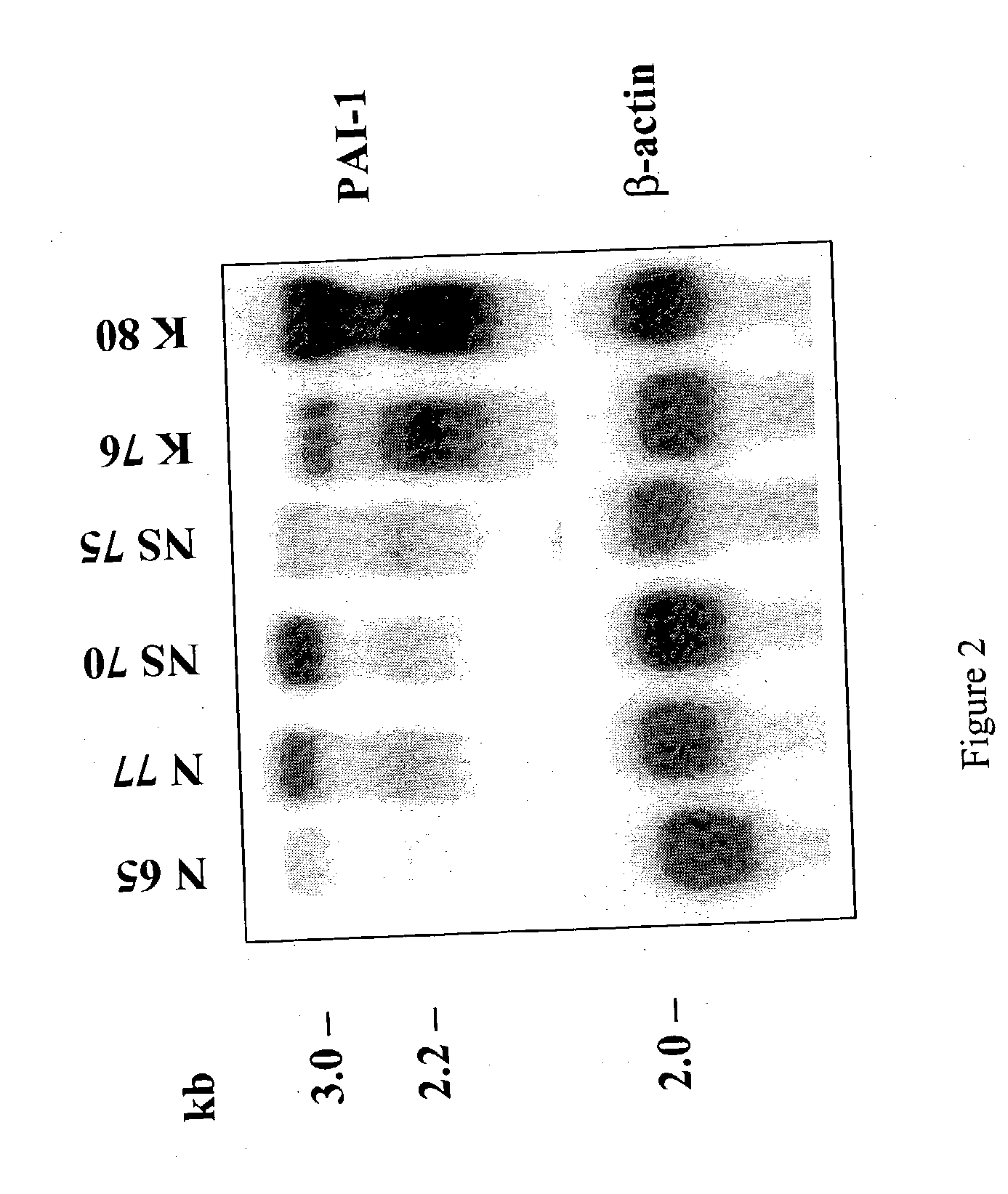
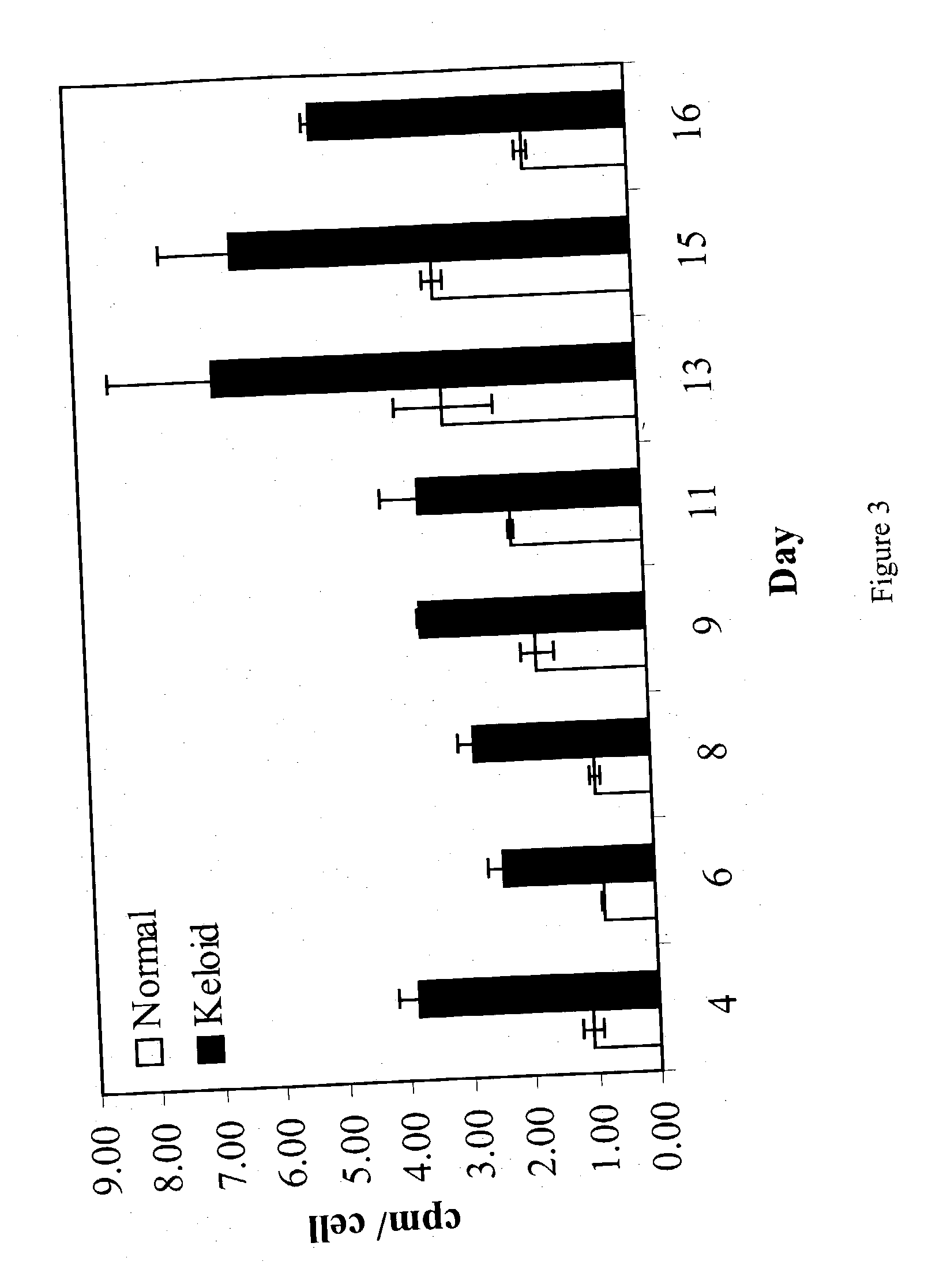
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
The present invention relates to findings that reducing the activity of Plasminogen Activator Inhibitor-1 (PAI-1) suppresses an excessive deposition of collagen which is known as a cause for the formation of abnormal scars. These abnormal scars include but are not limited to keloids, adhesions, hypertrophic scars, skin disfiguring conditions, fibrosis, fibrocystic conditions, contractures, and scleroderma, all of which are associated with or caused by an excessive deposit of collagen in a wound healing process. Accordingly, aspects of the present invention are directed to the reduction of PAI-1 activity to decrease an excessive accumulation of collagen, prevent the formation of an abnormal scar, and / or treat abnormal scars that result from an excessive accumulation of collagen. The PAI-1 activity can be reduced by PAI-1 inhibitors which include but are not limited to PAI-1 neutralizing antibodies, diketopiperazine based compounds, tetramic acid based compounds, hydroxyquinolinone based compounds, Enalapril, Eprosartan, Troglitazone, Vitamin C, Vitamin E, Mifepristone (RU486), and Spironolactone to name a few. Another aspect of the present invention is directed to methods of measuring PAI-1 activity in a wound healing process and determining the propensity of the formation of an abnormal scar.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Expandable temporary abdominal closure
InactiveUS20050085757A1Easy to manufactureSimple methodWound clampsBandagesInjury mouthThoracic cavity
A system for temporary abdominal or thoracic closure provides an expanded folded section allowing shielding of the wound from contamination and visual inspection while relieving abdominal pressure.
Owner:SANTANELLO STEVEN
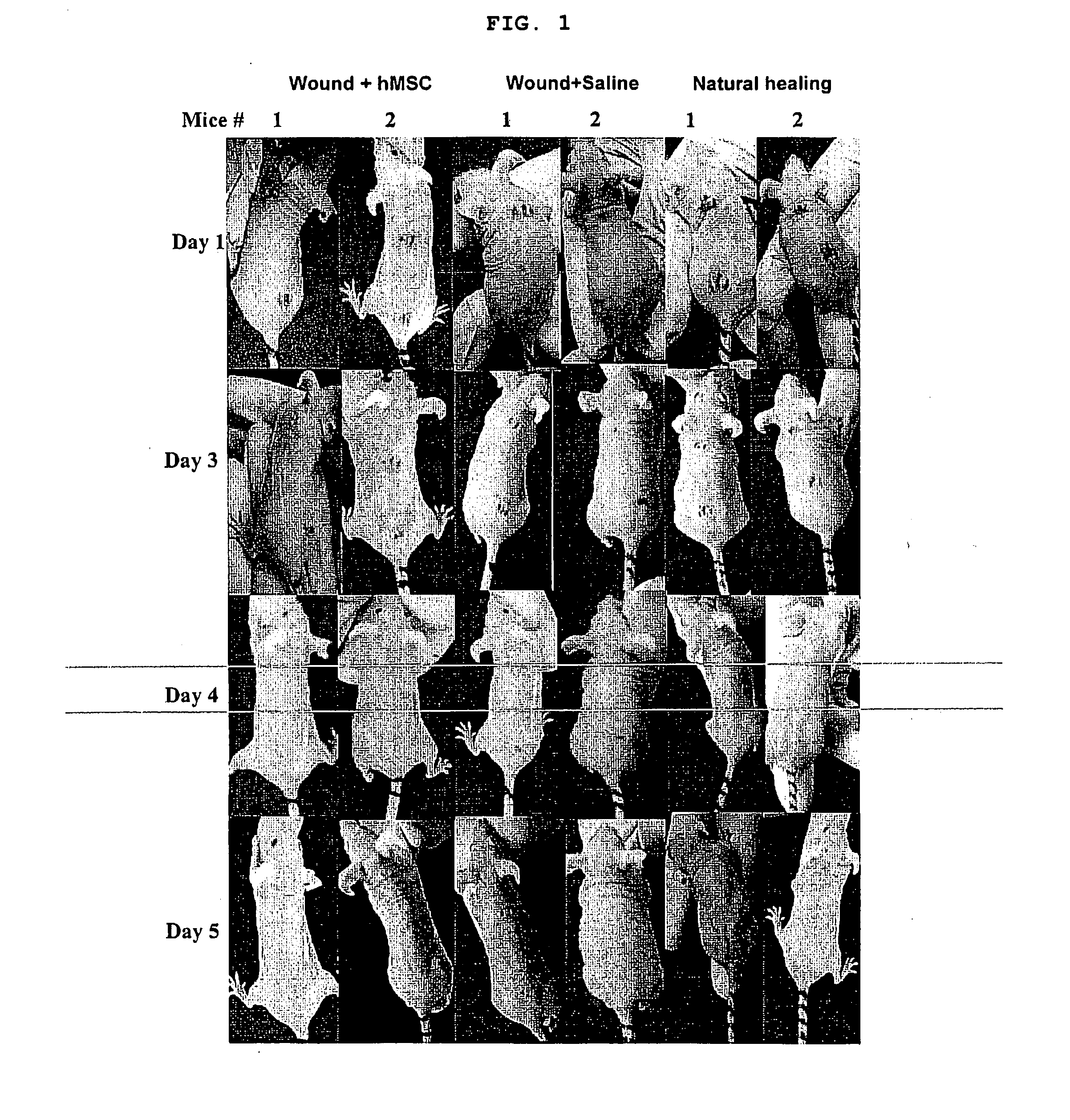
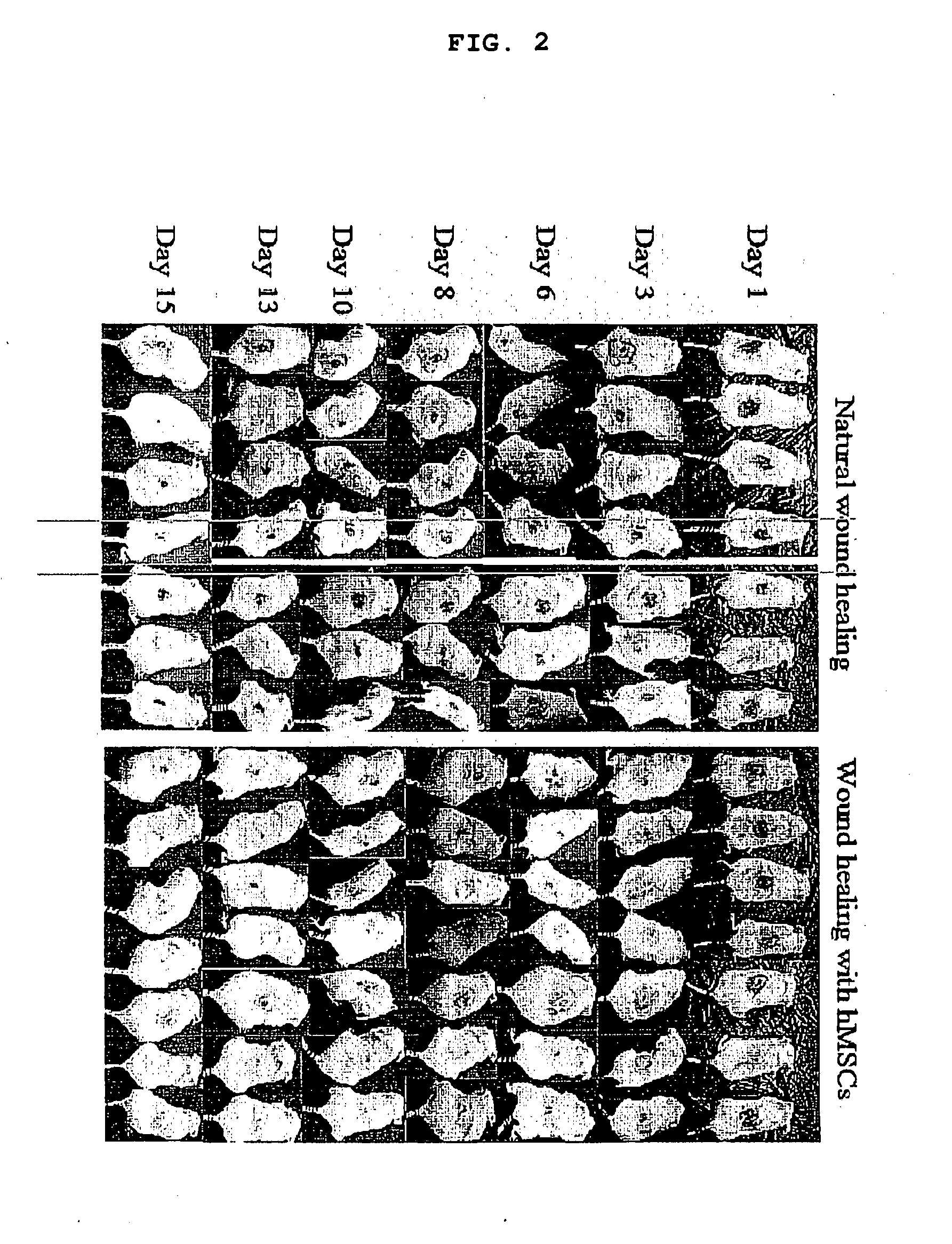
Use of stem cells for wound healing
InactiveUS20110020291A1Promote wound healingMinimizing formation of scar tissueBiocideEpidermal cells/skin cellsInjury mouthChronic wound
Cells, compositions, and methods of cell therapy for administering a therapeutically effective amount of stem cells or cell concentrate to achieve accelerated wound healing of normal and chronic wounds, while minimizing the formation of scar tissue.
Owner:RUTGERS THE STATE UNIV
Use of tetracycline compositions for wound treatment and skin restoration
InactiveUS20150164922A1Effective drug deliveryRapid clinical improvementBiocideTetracycline active ingredientsDiseaseSkin integrity
Methods of treatment and dosage regimes using hydrophobic gel or foam compositions comprising a tetracycline antibiotic for accelerating the return of skin integrity and or in treating or alleviating a disorder including a wound, burn, impetigo, acne, rosacea, a skin disease caused by a bacteria or a tetracycline antibiotic responsive disease, wherein the foam composition or gel is administered topically to a target area on a subject having the disorder and wherein the target area comprises an area of skin, or mucosa or an eye.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Method and device for mending skin openings
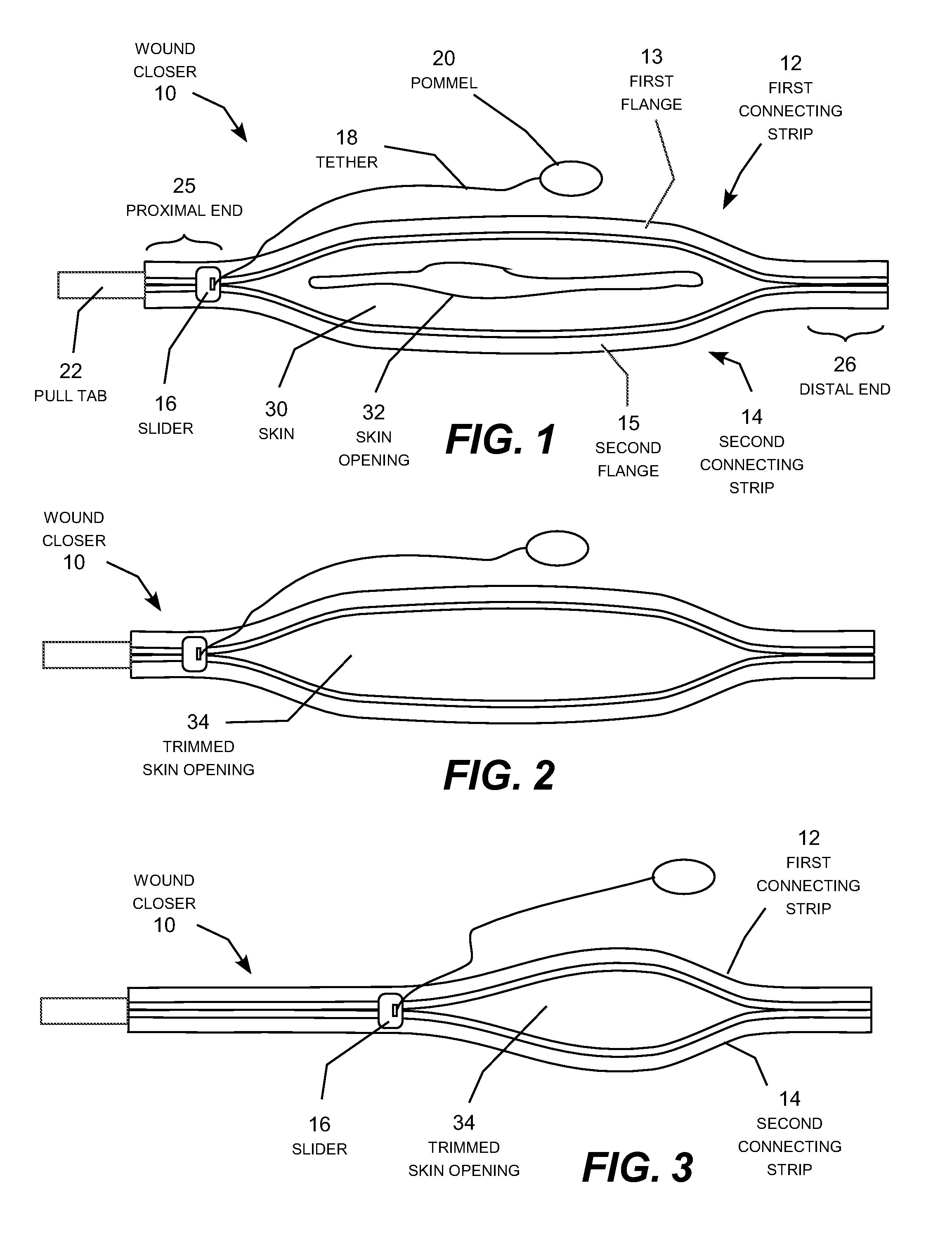
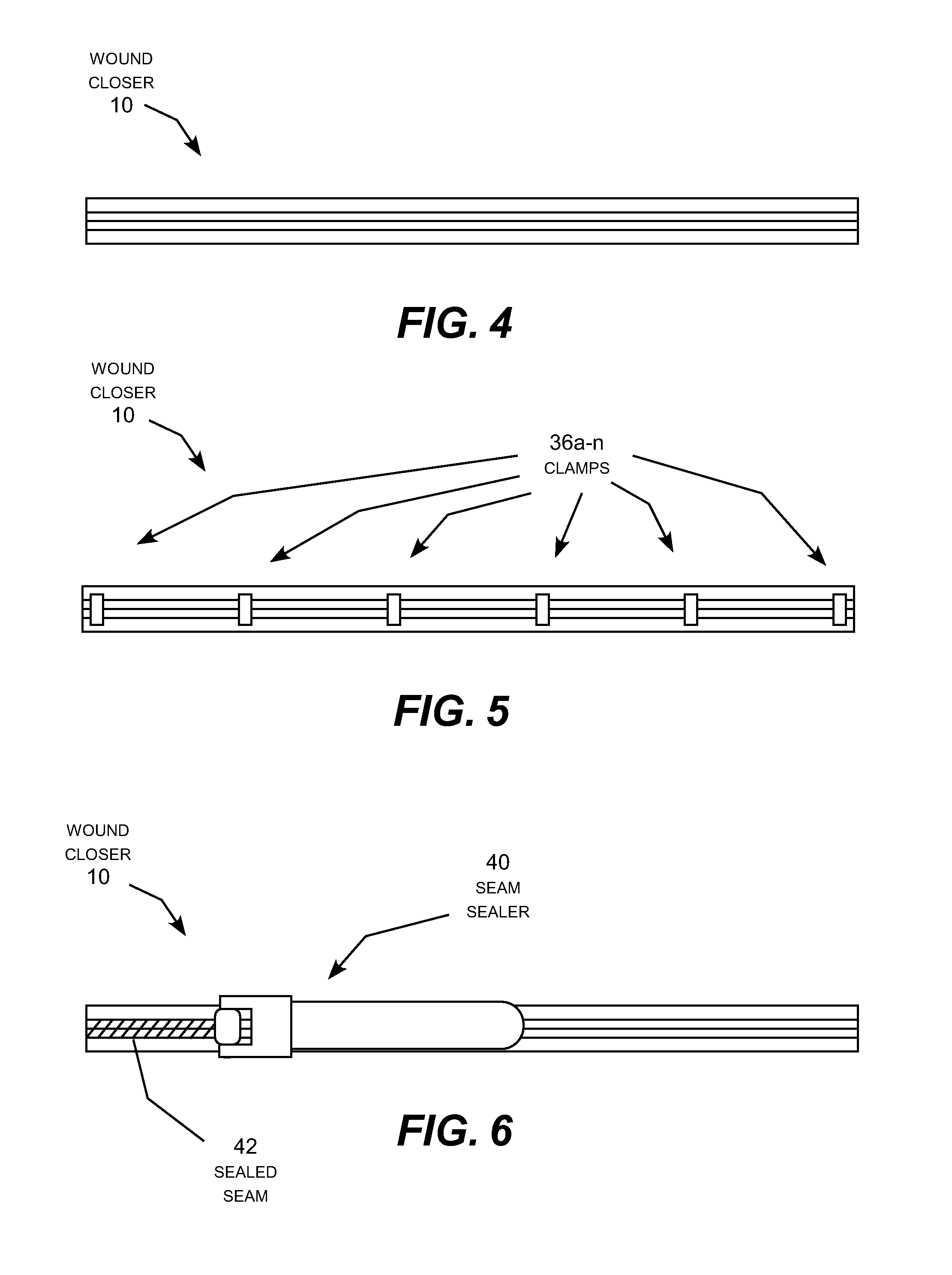
A zipper-type wound closer attachable to the skin around a skin opening and zipped closed to pull the skin opening closed. The wound closer includes two elongated connecting strips whose feet are pre-coated with adhesive and attached to the skin around the skin opening. The edges of the wound are trimmed flush with the interior sides of connecting strips, and the strips are then zipped together by moving a slider along the connecting strips to join the strips together like a familiar zipper. The wound zipper forms a continuous, toothless seam to prevent seepage of fluid through the seam. By holding the freshly trimmed edges of the skin opening together immobile in a clean, firm, closely aligned connection, the wound closer recreates the conditions under which scar-free healing of the skin can occur naturally; useful for repairing wounds and removing existing scars.
Owner:WARREN PETER D WARREN D
Compositions and methods for treating lacerations, abrasions, avulsions, burns, ulcers, and cases of excessive bleeding
InactiveUS20090148502A1Reducing and ameliorating excessive bleedingReduce controlAntibacterial agentsPowder deliveryExcessive BleedingMedicine
Described herein are compositions and methods related to wound treatment. Compositions are multi-components admixed in amounts and ratios to meet specific objectives for optimally treating various types of wound injury.
Owner:HEMO NANOSCI
Methods of treating wounds using IL-23
InactiveUS7332156B2Enhanced wound healing responseImprove responseAntibacterial agentsOrganic active ingredientsWound healingInjury mouth
Provided are methods of treatment for skin disorders. In particular, treatment, the skin disorders are generally inflammatory skin disorders, including improper wound healing. Provided are methods of using of a cytokine molecule.
Owner:MERCK SHARP & DOHME CORP
Dressing
A wound dressing for application to a protruding part of a joint of the body, in particular the heel. The dressing includes an elongated first part for placing on one side of the protruding joint part, and a second part for placing on another side of the joint, and two ear parts extending laterally from the second part. At least the central parts of the first part, the second part and the ear parts of the dressing are covered with an absorbent element. A portion of each laterally extending ear part between its outermost lateral point and a longitudinal tip of the second part is concave to facilitate wrapping of the ear parts around the heel without overlapping of the absorbent element.
Owner:COLOPLAST AS
Disposable finger sleeve for appendages
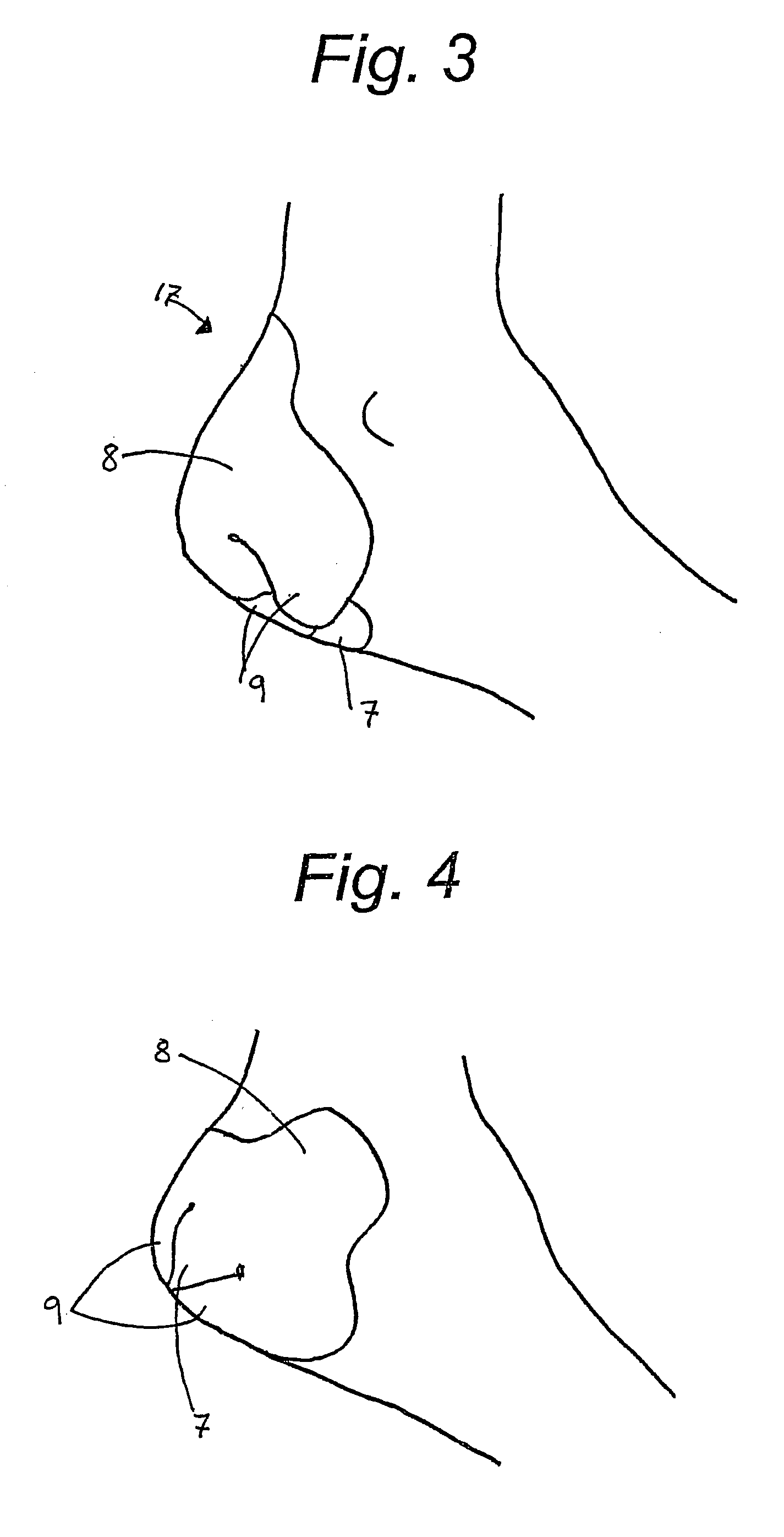
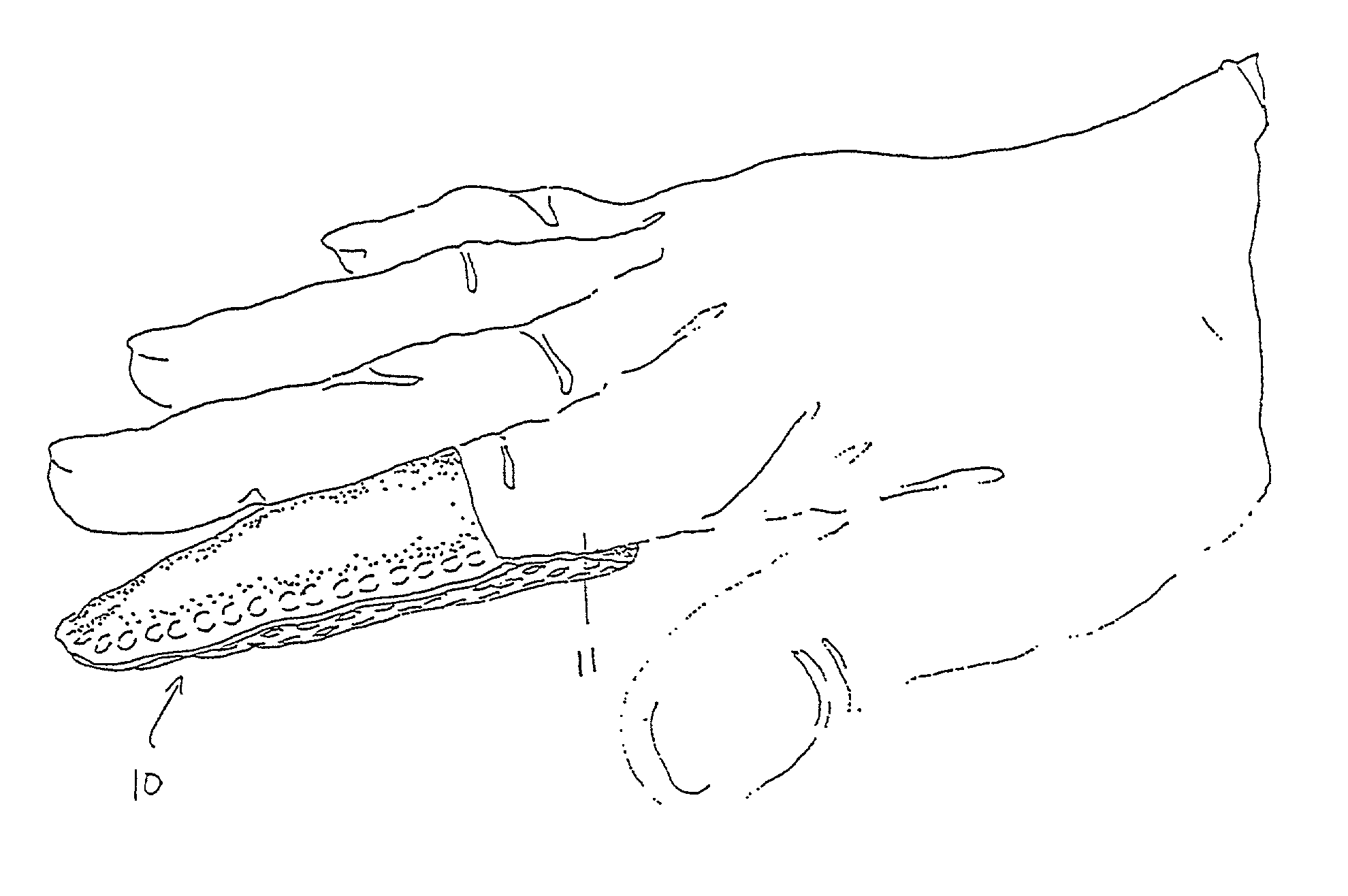
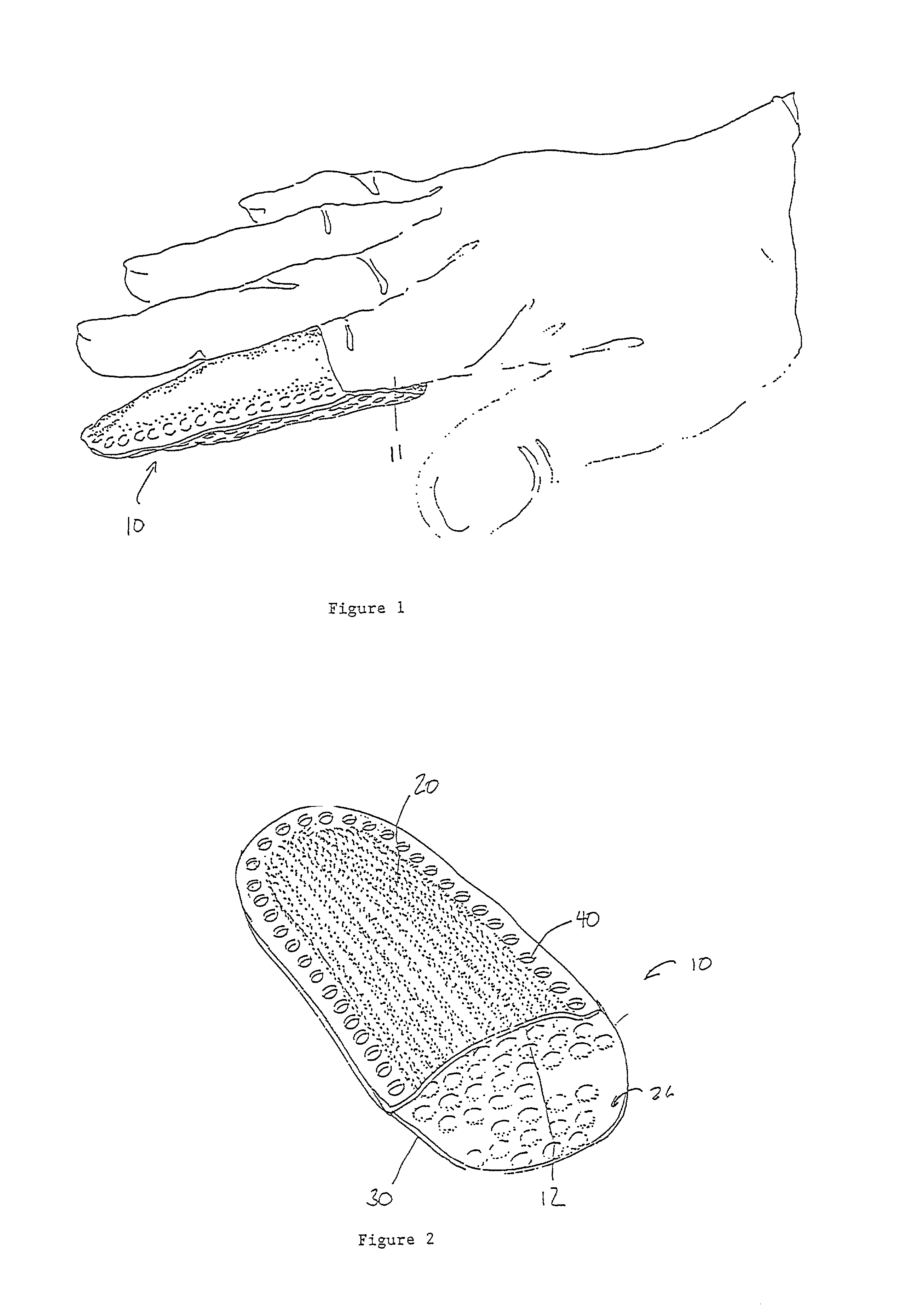
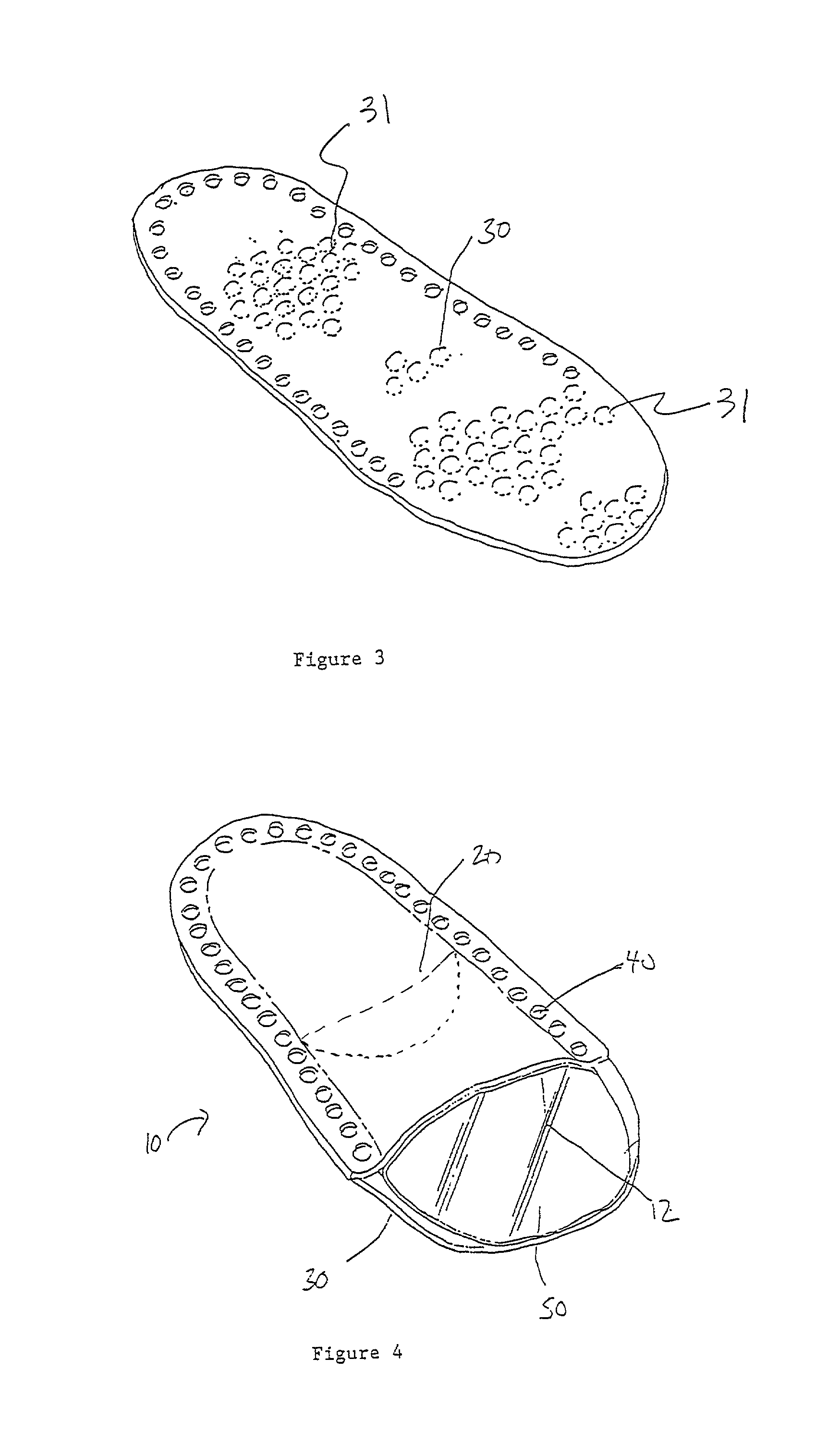
InactiveUS7012169B2Avoid flowStay breathableFinger bandagesBrush bodiesElastomerCTS - Carpal tunnel syndrome
A device that can be used to treat appendage ailments is provided. The device, or appendage sleeve, can be used for wounds, cuts, and blisters, as well as joint related ailments, such as arthritis and carpal tunnel syndrome. In some instances, the appendage sleeve can at least partially made from an elastomeric material, such as an elastomeric nonwoven, so that the sleeve can more aptly fit onto a finger or toe. Furthermore, the sleeve can also possess a barrier that is liquid impermeable, but vapor permeable so that the finger or toe of a user is more comfortable. Various additives can be applied to the sleeve to aid for therapeutic purposes.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Hydrogel wound dressing for treating laser cauma and burns and scalds as well as preparation method thereof
ActiveCN103495199APromote wound healingImprove antibacterial propertiesAbsorbent padsBandagesBurned skinLaser burn
The invention relates to hydrogel wound dressing for treating laser cauma and burns and scalds. The active components of the hydrogel wound dressing include an antibacterial agent, a humectant and a stabilizer, wherein the antibacterial agent inhibits adhesion and growth of the wound of laser cauma and surrounding bacteria and can kill various pathogenic bacteria at the cauma part, so as to effectively promote union of the laser cauma. The antibacterial agent adopted by the invention does not generate drug resistance, the humectant can maintain moisture of the wound, promote union of the wound surface, prevent formation of scars and alleviate wound pain. With the adoption of the stabilizer, the hydrogel maintains long-time antibacterial effectiveness. Experiments verify that the hydrogel dressing provided by the invention has the effects of effectively relieving wound pain, controlling local infection, maintaining moisture of wound skins, protecting skins, accelerating and promoting union of wound surface and wound and preventing formation of scars, and can be used for healing laser-burned skin wound surfaces (such as laser mole removal and the like) and wound surfaces of burns and scalds.
Owner:SAIKE SAISI BIOTECH CO LTD
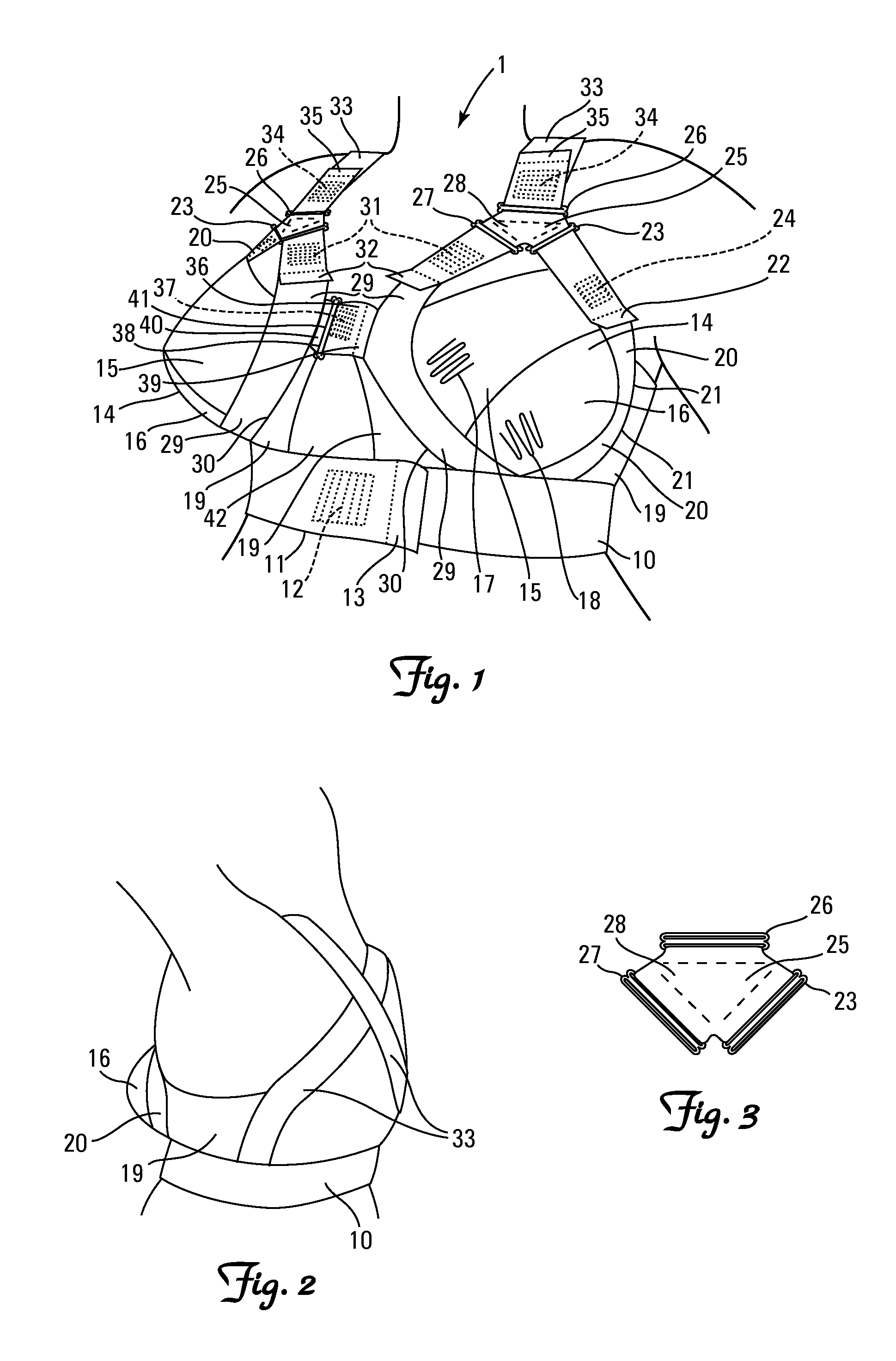
Post-operative brassiere
A breast-supportive and breast-positioning brassiere designed to be used postoperatively by patients including obese patients and fuller-sized women who have undergone cardiothoracic surgery that requires a mid-sternal incision (sternotomy). The brassiere is also for other interventions in the thoracic region, when a comfortable and efficient individual positioning and support of the breast(s) would be desirable; an example being to prevent symmastia after breast augmentation surgery. The brassiere prevents gravitation of the breast tissue to the lateral sides, keeps the breast tissue away from the mid center, and supports the weight of the breasts. The brassiere is designed to promote less pain, less wound complications, esthetically improved wound healing, less heat generation, improved wound inspection and access for wound care, while maintaining support and dignity.
Owner:QUALITEAM SRL
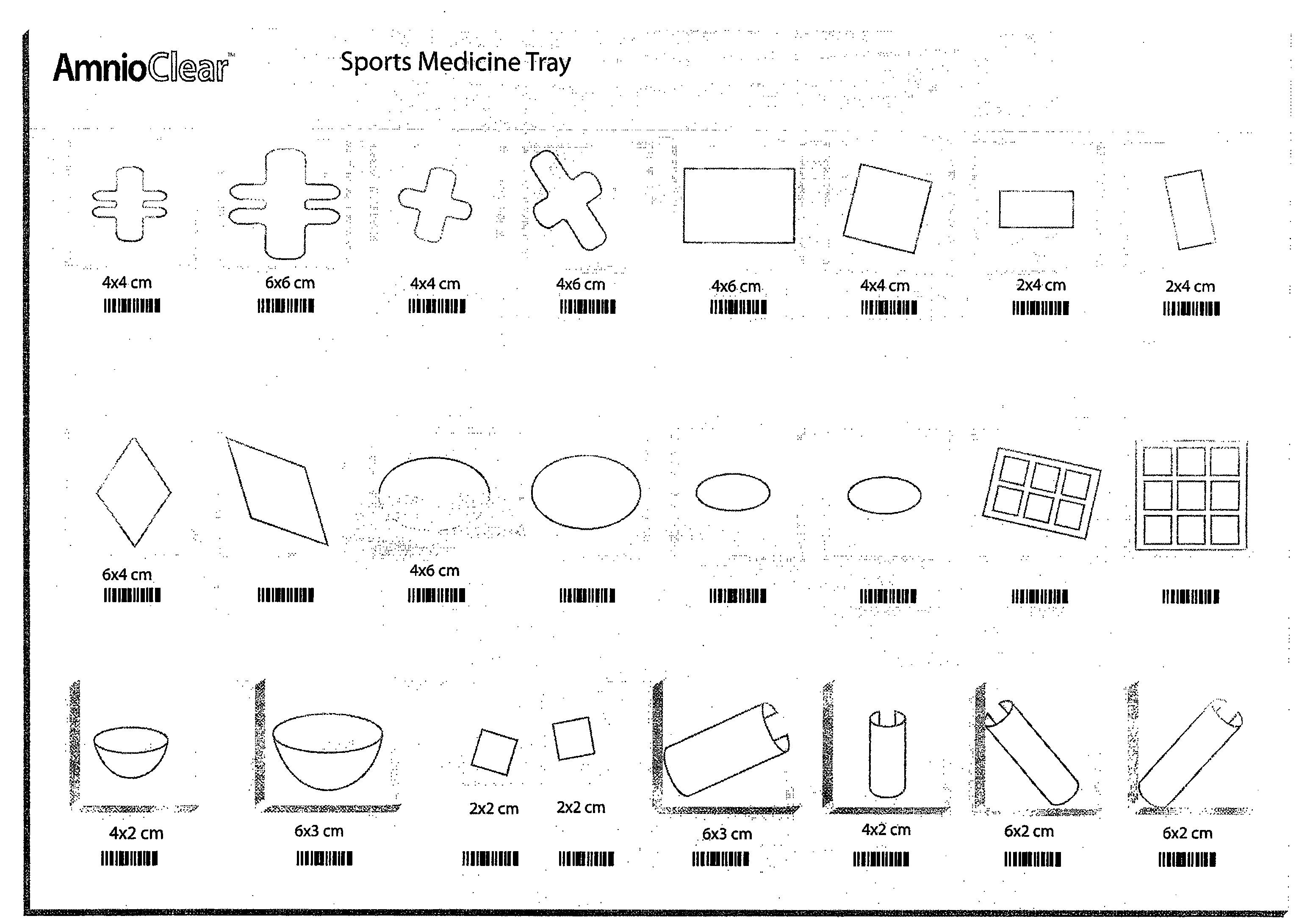
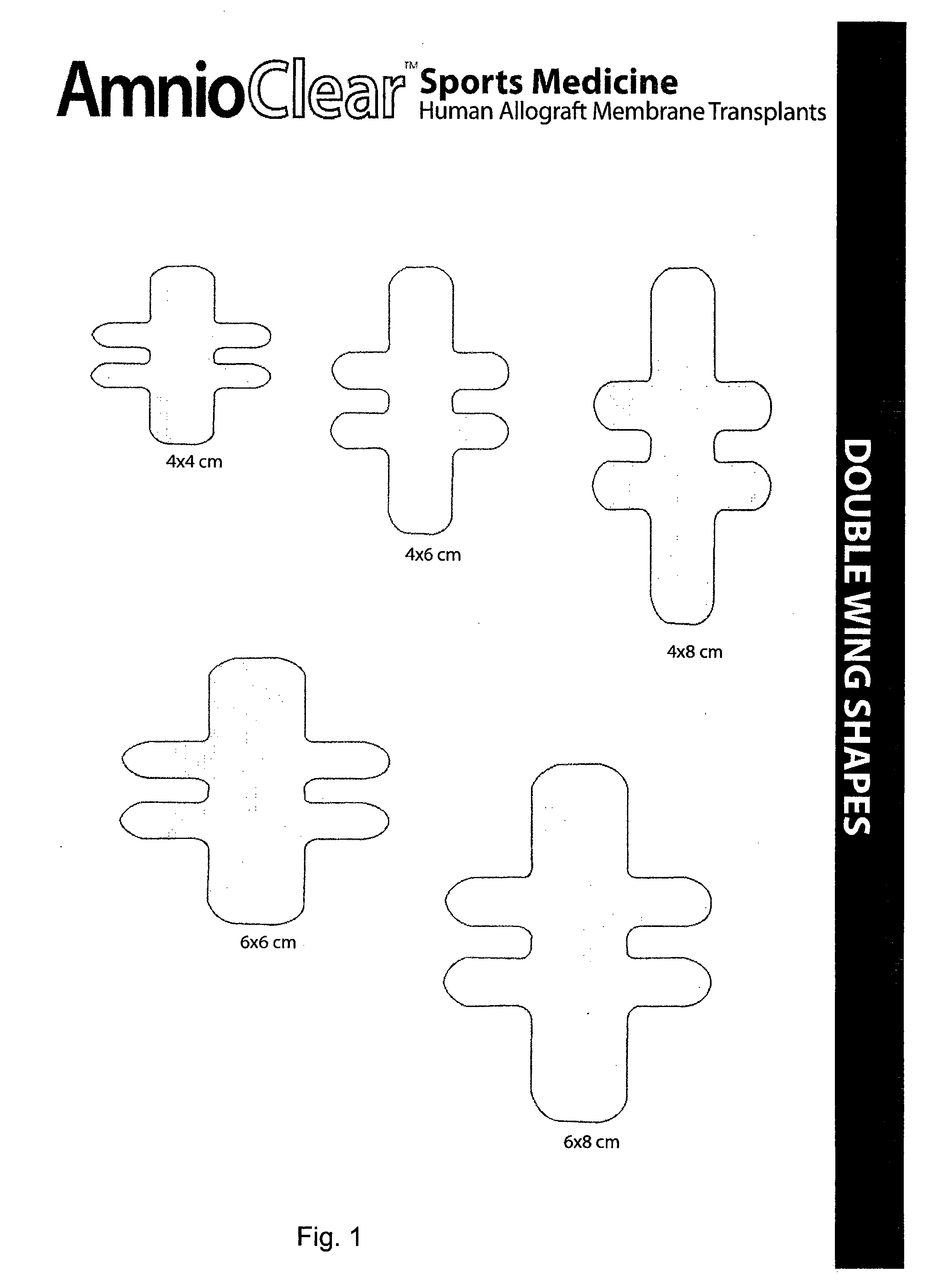
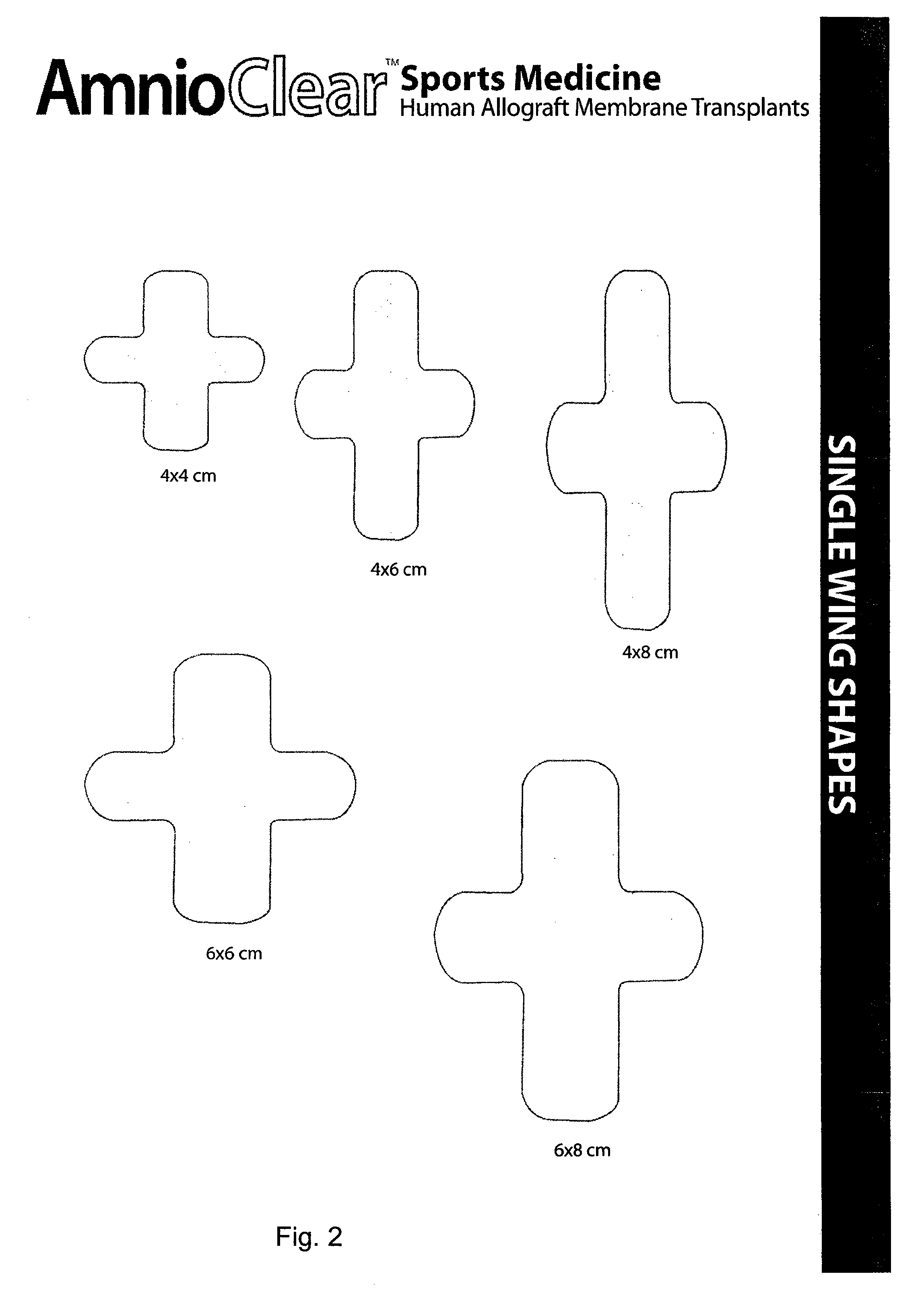
Amnion and chorion constructs and uses thereof in sport injury surgeries
Improved methods for sport injury surgeries are described. The improvement includes covering a damaged site of fascia with at least one of an amniotic fluid and a construct for use in surgical repair of the sport injury prior to wound closing. The construct contains an allograft comprising at least one layer of human amnion and chorion tissues and the construct has a size and shape suitable for covering the damaged site of fascia. The method improves fascial membrane repair, reduces complications and recovery time of sport injury surgeries.
Owner:LIVENTA BIOSCI
Wearable partial task surgical simulator
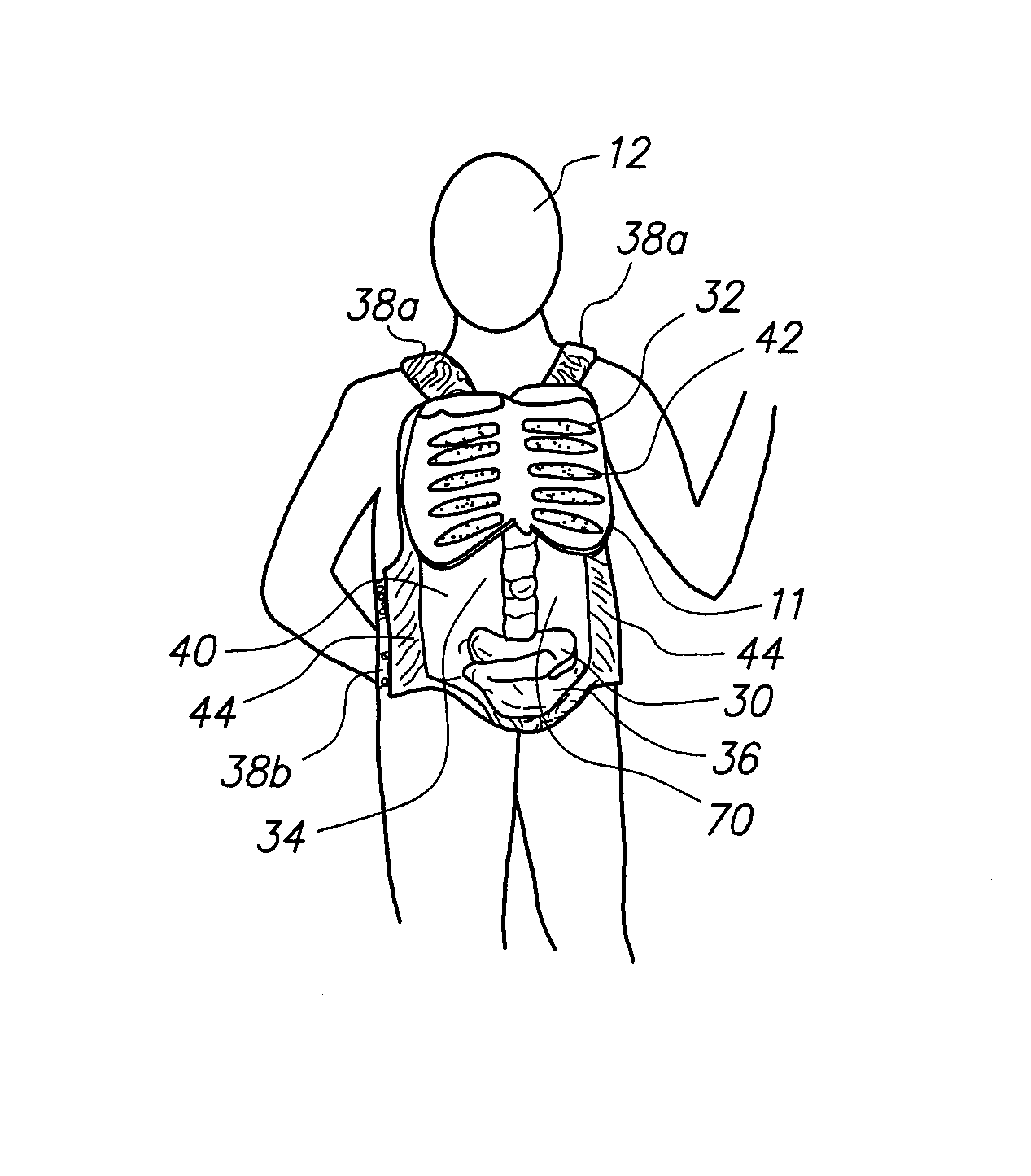
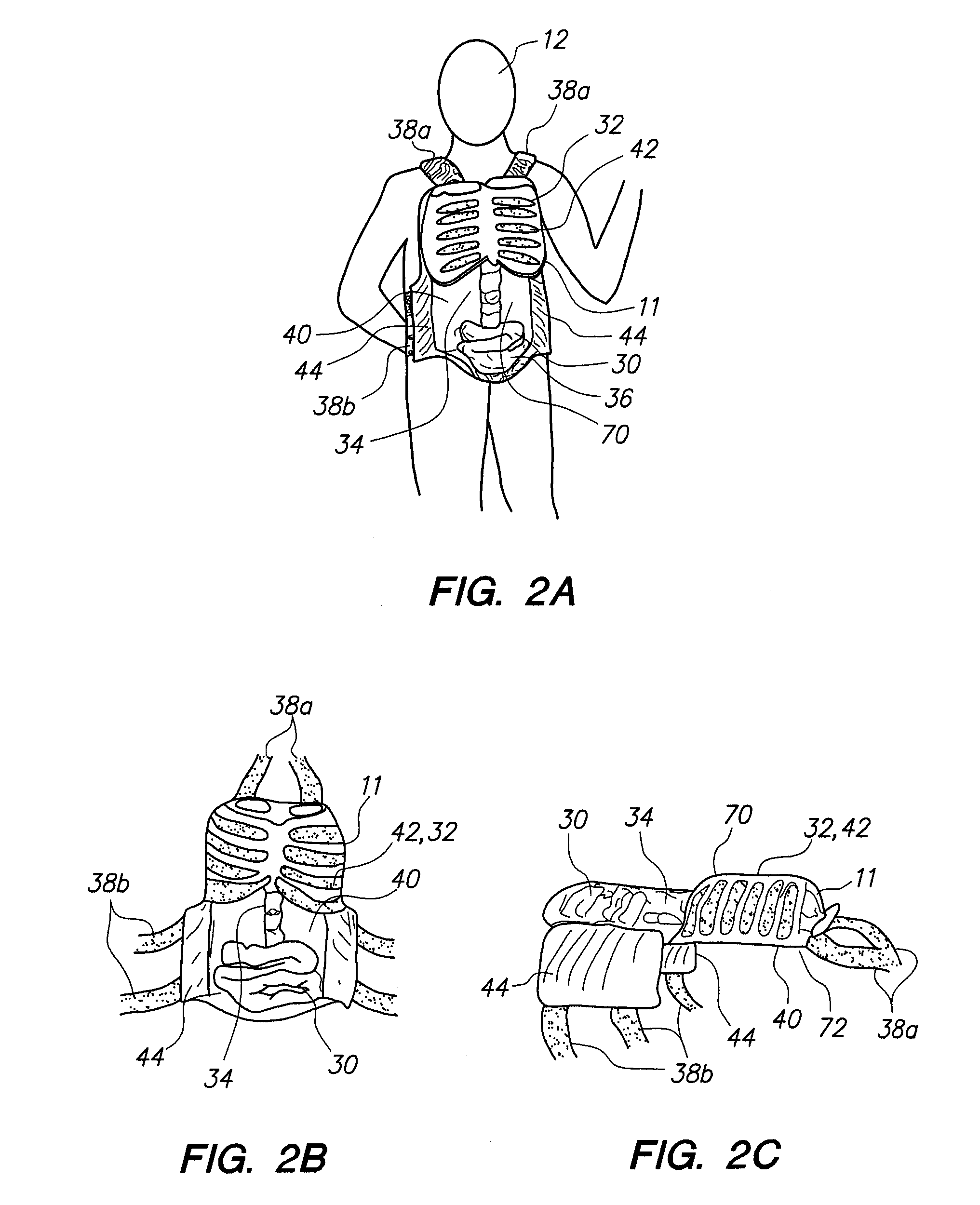
A wearable device for simulating wounds and injuries received during a trauma event includes a raiment and vest for covering the torso of a person. The raiment has an outer surface with a color and a texture comparable to human skin. Mounted on the outer surface is at least one wound simulator formed with an orifice that is in fluid communication with a fluid reservoir. Thus, the person can selectively expel a blood-like fluid from the reservoir, and through the wound simulator orifice, to simulate a trauma event. The vest includes an artificial rib cage and prosthetic internal organs juxtaposed with at least one wound simulator to simulate internal effects of a trauma event.
Owner:STRATEGIC OPERATIONS INC
Formulations for the prevention or the treatment of diseases affecting mucosae or skin, or for pregnancy prevention, and an applicator for the delivery of topical formulations into mucosal cavities
This invention relates to formulations for the prevention of infection and / or abnormal conditions of mucosae and / or skin caused by any pathogen and / or any disease, and more particularly for the prevention of sexually transmitted infections specially HIV and HSV. This invention also relates to formulations for the treatment of infection and / or abnormal conditions of skin and / or mucosae and more particularly for the treatment of herpetic lesions. The formulations could be used as a prophylactic agent to prevent accidental infection of health care workers. The formulations could be used for the healing and / or treatment of burn wounds and prevention of further infection. This invention also relates to the development of a unique vaginal / ano-rectal applicator for the uniform delivery of any topical formulations to treat and / or prevent any infection and / or abnormal conditions of mucosa cavity caused by any pathogen and / or disease.
Owner:UNIV LAVAL
Post-cesarean section scar management undergarment
The present invention comprises a modified woman's undergarment that includes a peri-pubic light compression panel allowing for compression at the incision site and a treatment dressing means for exposing the wound to a medicament. The combination of light compression and treatment with applicable medicament functions to minimize the formation of scars following cesarean section incisions performed in the lower transverse uterine or peri-pubic area. The undergarment is not limited in style and can include various styles such as a bikini, thong, low-rise, support style, full or high cut undergarment. Considering the long phase of wound healing, this design flexibility allows for the use of the garment under a variety of street clothes. Unlike a girdle or abdominal support device, the crux of the invention does not require heavy or high abdominal support, although for those applications requiring girdle-type support, the invention could also be fabricated in a style that allows abdominal support along with the focused incision peri-pubic compression and scar management. In addition, the size, thickness and dimensions of the present invention undergarment are not limited.
Owner:BROOKS CATHERINE
Vascular suturing device with needle capture
A surgical device of suturing vascular vessels is described, as well as methods for suturing tissue employing the surgical device. The device includes at least one needle advanceable through tissue and into a needle capture element within a distal end of the surgical device to draw lengths of suture material which can then be used to close various puncture wounds, particularly in vascular tissue.
Owner:ABBOTT LAB INC
Surgical after-care garment
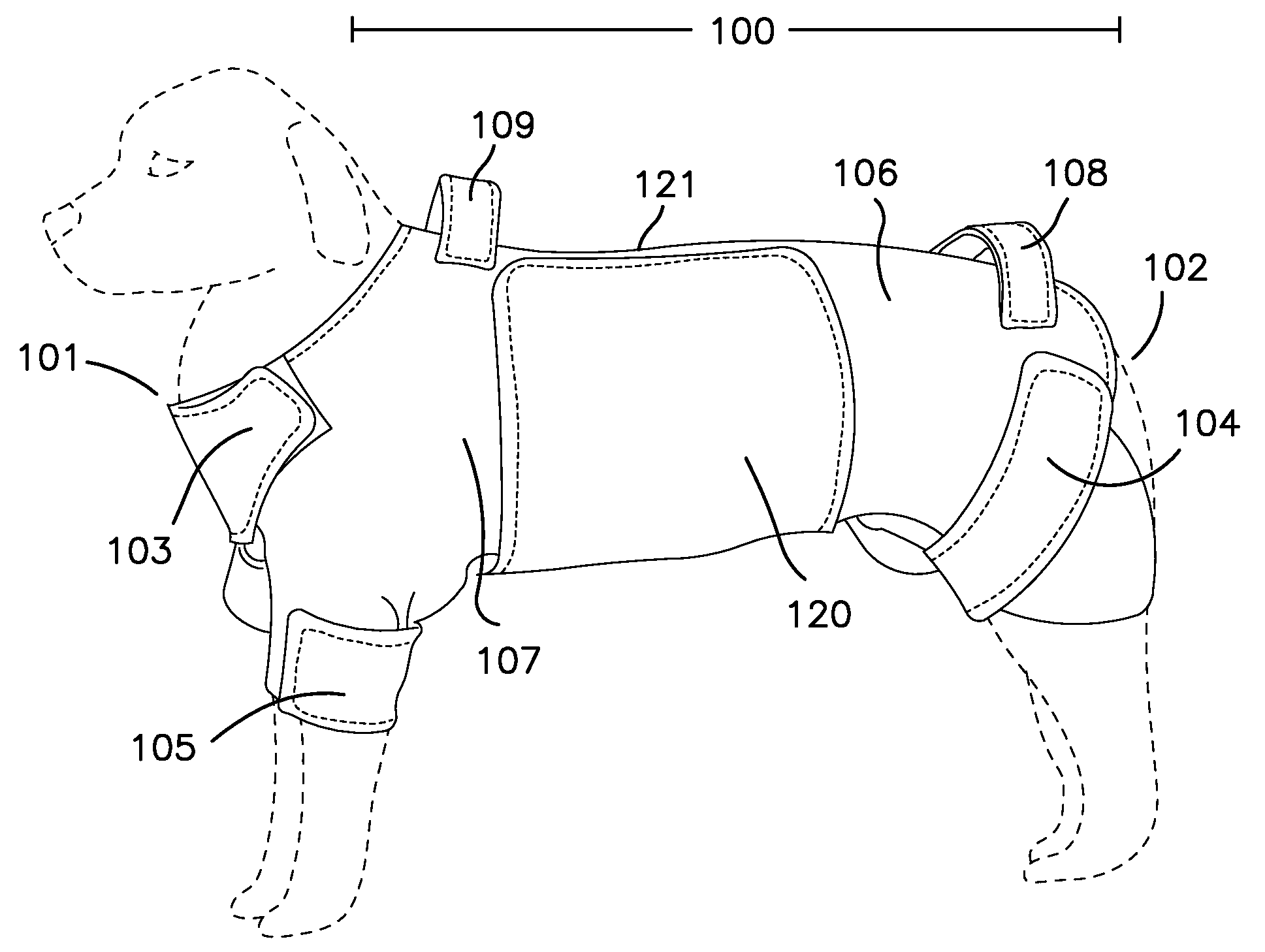
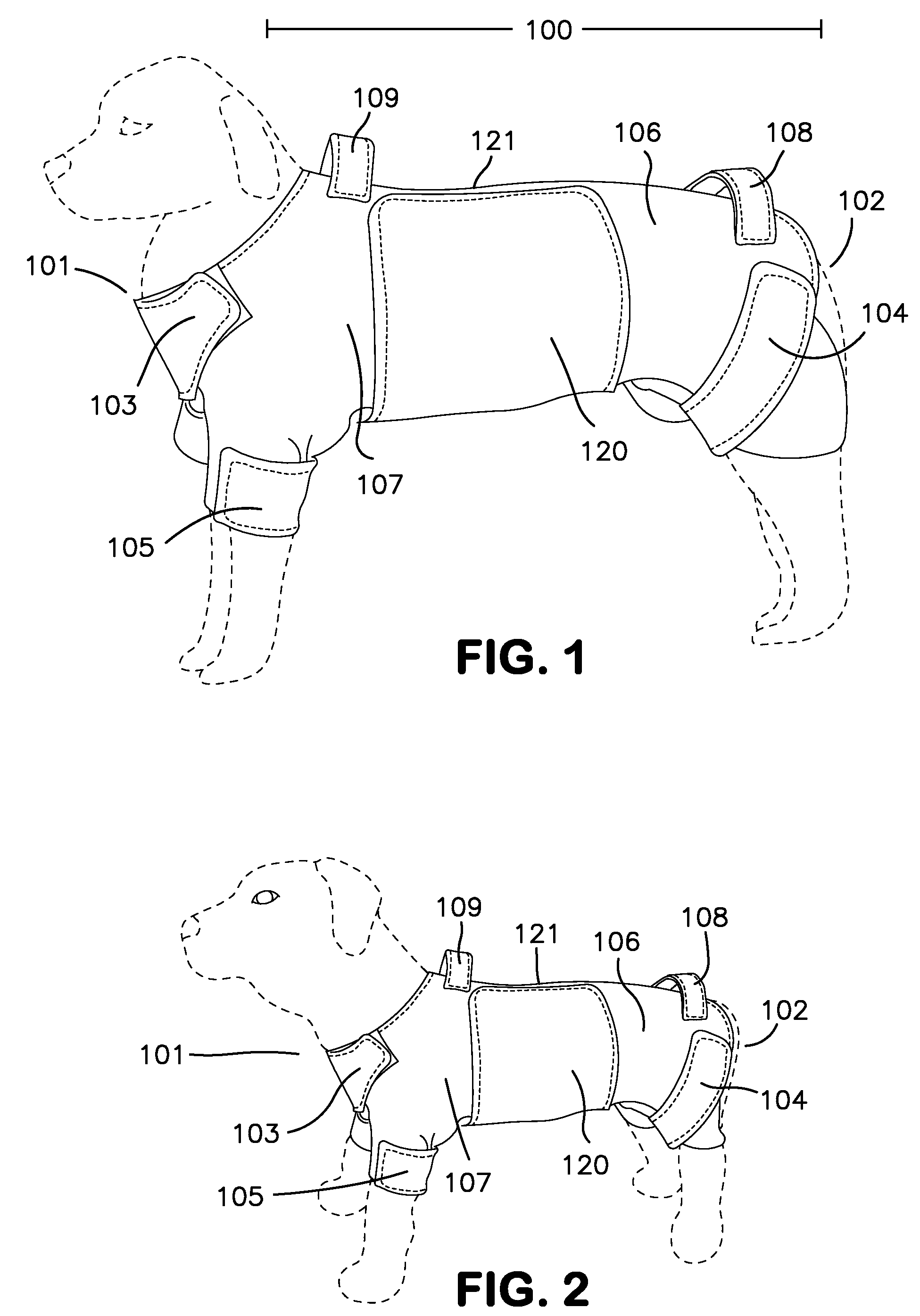
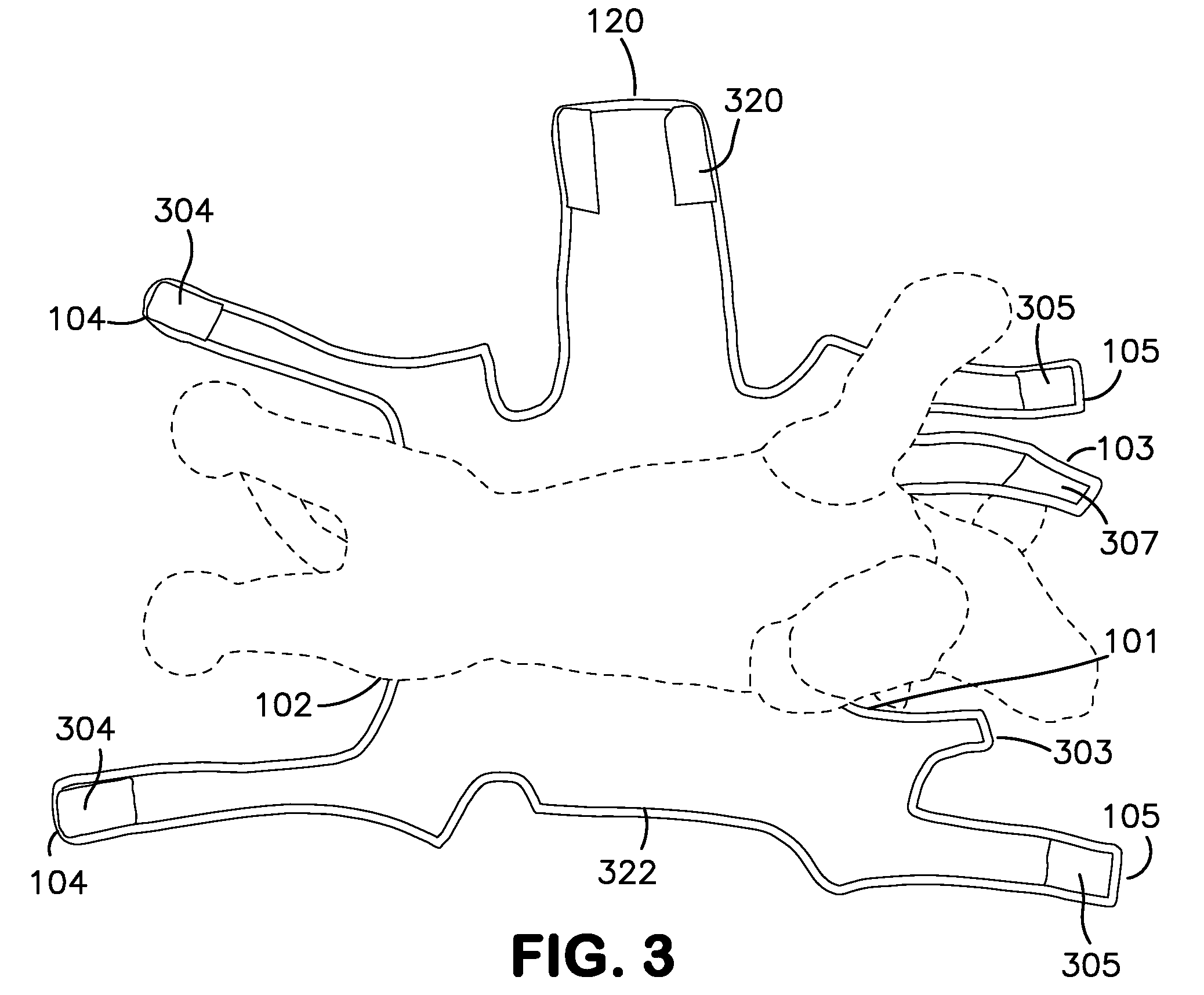
ActiveUS8733296B1Promote healingLimit motionOther apparatusTaming and training devicesInjury mouthWound site
A removable garment for animals to cover and protect an abdominal surgical or wound site. Generally, the removable garment is comprised of a body wrap with front leg, rear leg, and neck closures, and an abdominal panel, extending distal the body wrap. Each leg and neck closure, as well as the abdominal panel, contains an attachment designed to removeably secure the closure, and ultimately the garment itself, to an animal.
Owner:PET PERILS
Methods and compositions for tissue regeneration
InactiveUS20060121002A1Reestablish integrityInhibit excessive scar formationPeptide/protein ingredientsGenetic material ingredientsCell-Extracellular MatrixInjury mouth
The present invention provides the use and composition of matter of angiogenic or other growth / cytokine factors expressed by mixtures of allogeneic human cell strains or lines of various types and stages of differentiation. Also provided are unencapsulated preparations (mixed with or applied to extracellular matrix material or synthetic biocompatible substances) for the purpose of temporary application to wounds or defects in the skin or other tissues for the restoration of blood supplying connective tissue to enable organ-specific cells to reestablish organ integrity as well as to inhibit excessive scar formation.
Owner:SMITH & NEPHEW INC
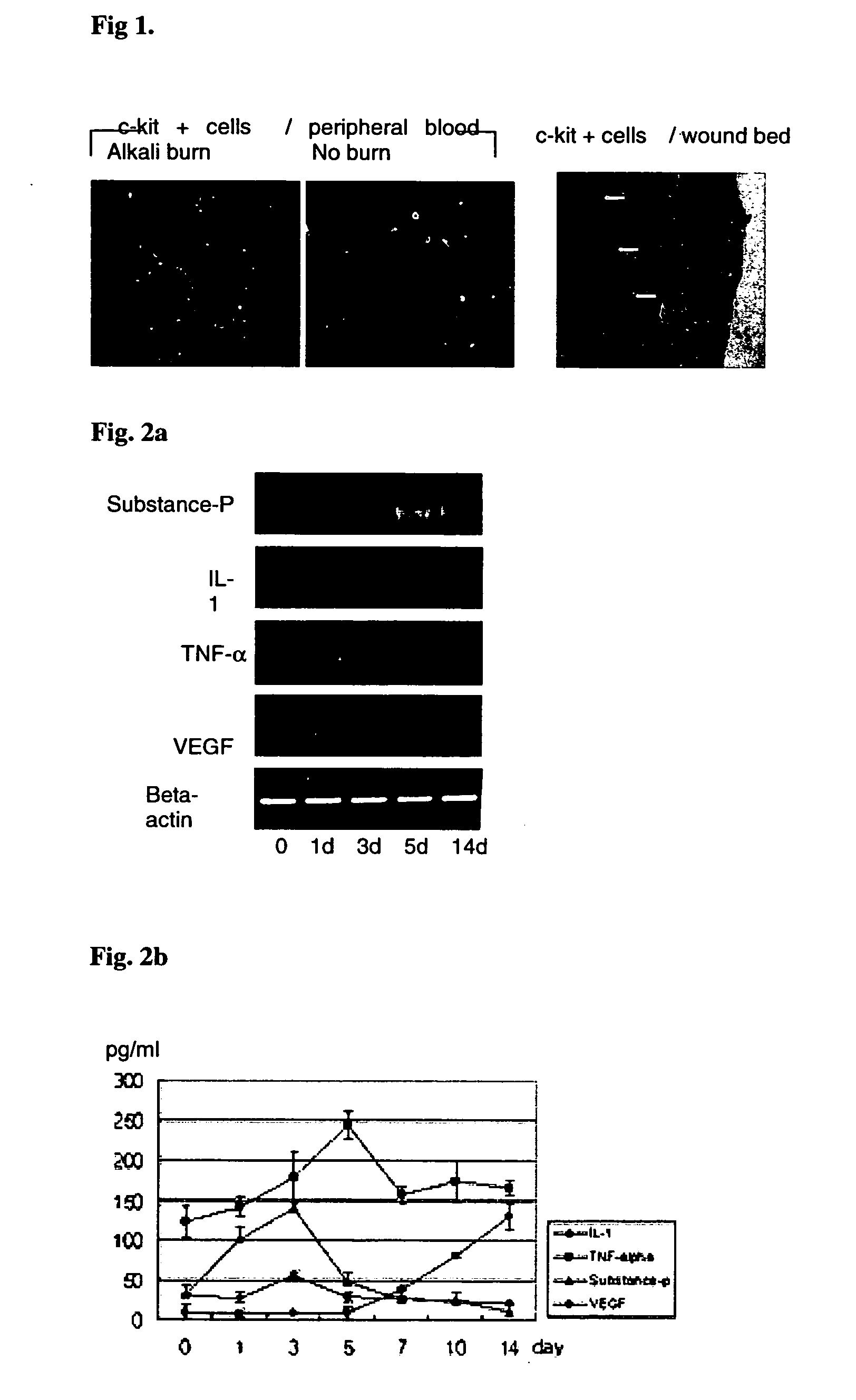
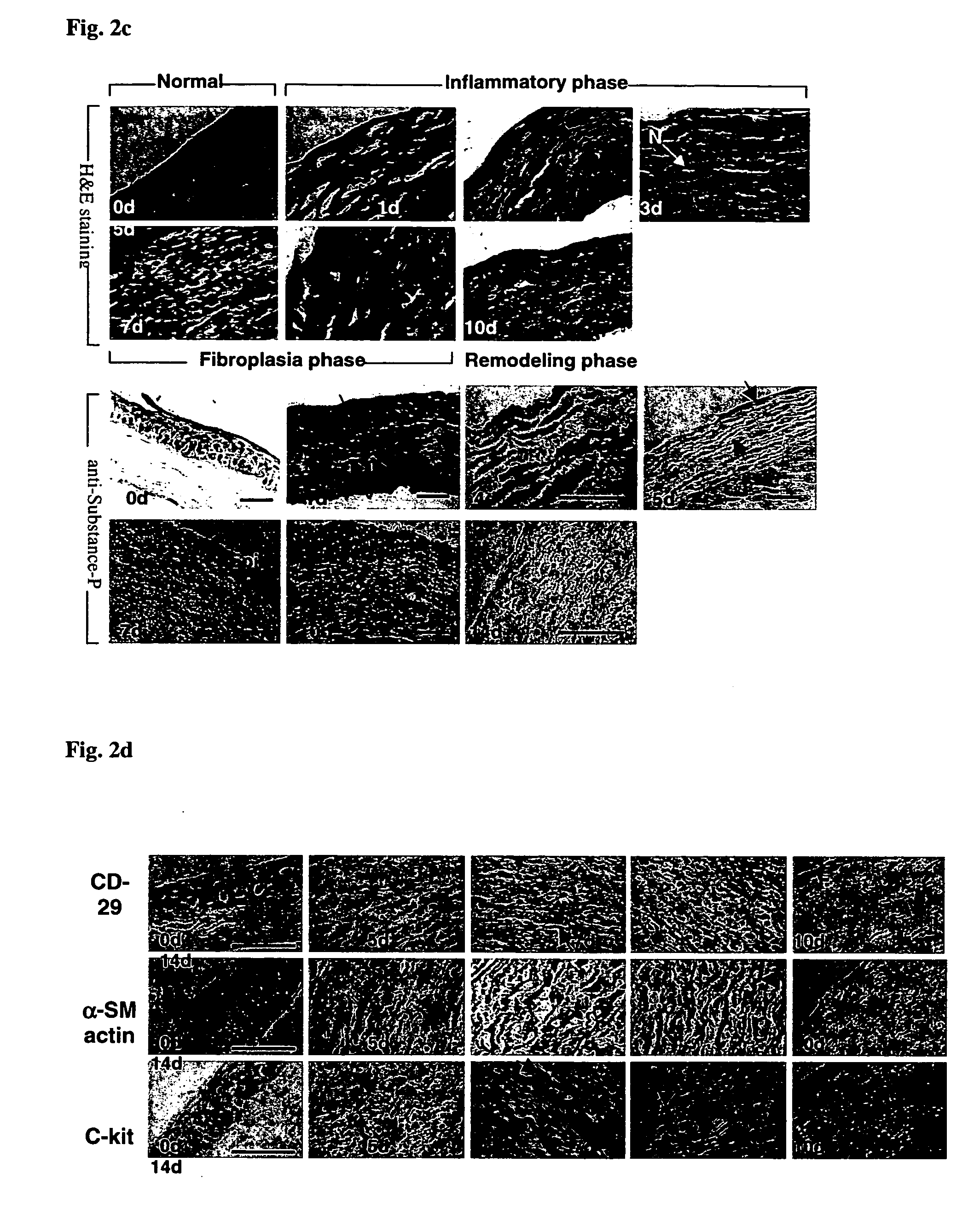
Use of substance P for mobilization of Mesenchymal stem cells or proliferation of Mesenchymal stem cells and for wound healing or facilitating wound healing
ActiveUS20060127373A1Promote migrationFacilitates cornea wound healingBiocideTachykinin ingredientsWound healingInjury mouth
The present invention relates to a use of Substance-P for the manufacture of a medicament for mobilization or proliferation of Mesenchymal stem cells (MSCs) from the bone marrow, or facilitating said mobilization or proliferation, and use of Substance-P for the manufacture of a medicament for wound-healing or facilitating wound-healing.
Owner:KOREA INST OF RADIOLOGICAL & MEDICAL SCI +1
Wearable device
The invention features methods and devices for controlling bleeding from blood vessels that may be damaged as a result of trauma or impact with an object, such as a bullet or shrapnel. The device may be wearable by a user and include one or more components, such as wound sealant and multiple inflatable balloons / bladders. The device may be integrated into a garment, e.g., a vest, jacket, trousers, or full body suit. Once triggered (automatically or manually), the device may be used to deliver wound sealant to a wound site and / or pressure to the wound site by selective inflation of one or more balloons over exsanguinating blood vessels that may be damaged, thereby stopping or minimizing the bleeding. Alternatively, or in addition, the device may be used to stabilize a wounded wearer for, e.g., transportation purposes, or to provide buoyancy. Devices of the invention may also be used as a blood pressure monitor, as a massaging device, and as a breast pump. Devices and methods of the invention may also be used for repairing or stabilizing machines, such as vehicles (e.g., automobiles and boats).
Owner:LEGIONARIUS LLC
Devices and methods for treatment of fistulas and complex wounds
ActiveUS9078990B1Easy constructionReduce needElectrotherapyWound drainsIntestinal structureEnteric fistula
An enteric fistula healing device, configured for application over a fistula or other wound to physically separate the fistula from the remainder of the wound area, such that any effluent from the intestine or bowel, or other enteric substances, that pass through the fistula are prevented from communicating with the wound area. The device is configured to collapse from a first height to a shorter second height.
Owner:FISTULA SOLUTION
Abdominal postoperative binder and method of use
InactiveUS6270469B1Promote healingDiscourages deep breathingBreast bandagesSuspensory bandagesInjury mouthPeritoneum
The invention is a postoperative binder and method of use. The binder is made of relatively inelastic material that is cut to fit the patient and held in place by a plurality of tails fastened with Velcro(R) binders. The present invention uses mechanical, rather than elastic, compression. Mechanical loads are carried over the iliac crest, by hooking the tails of the binder over the iliac crest and then bifurcating the tails for attachment to the abdominal portion of the binder. The present invention provides support of lower abdominal tissue, especially near the genitals and in the area of the peritoneum. The invention is also a method of preventing post-operative wound infection, reducing incidence of seroma and hematoma formation and wound separation while reducing pain and the need for pain medication by passing a relatively inelastic abdominal binder over the patient's iliac crest and abdomen to place the binder in tension so as to provide a greater than 180 degree radius of compression to the wound.
Owner:MOTT GEORGE E
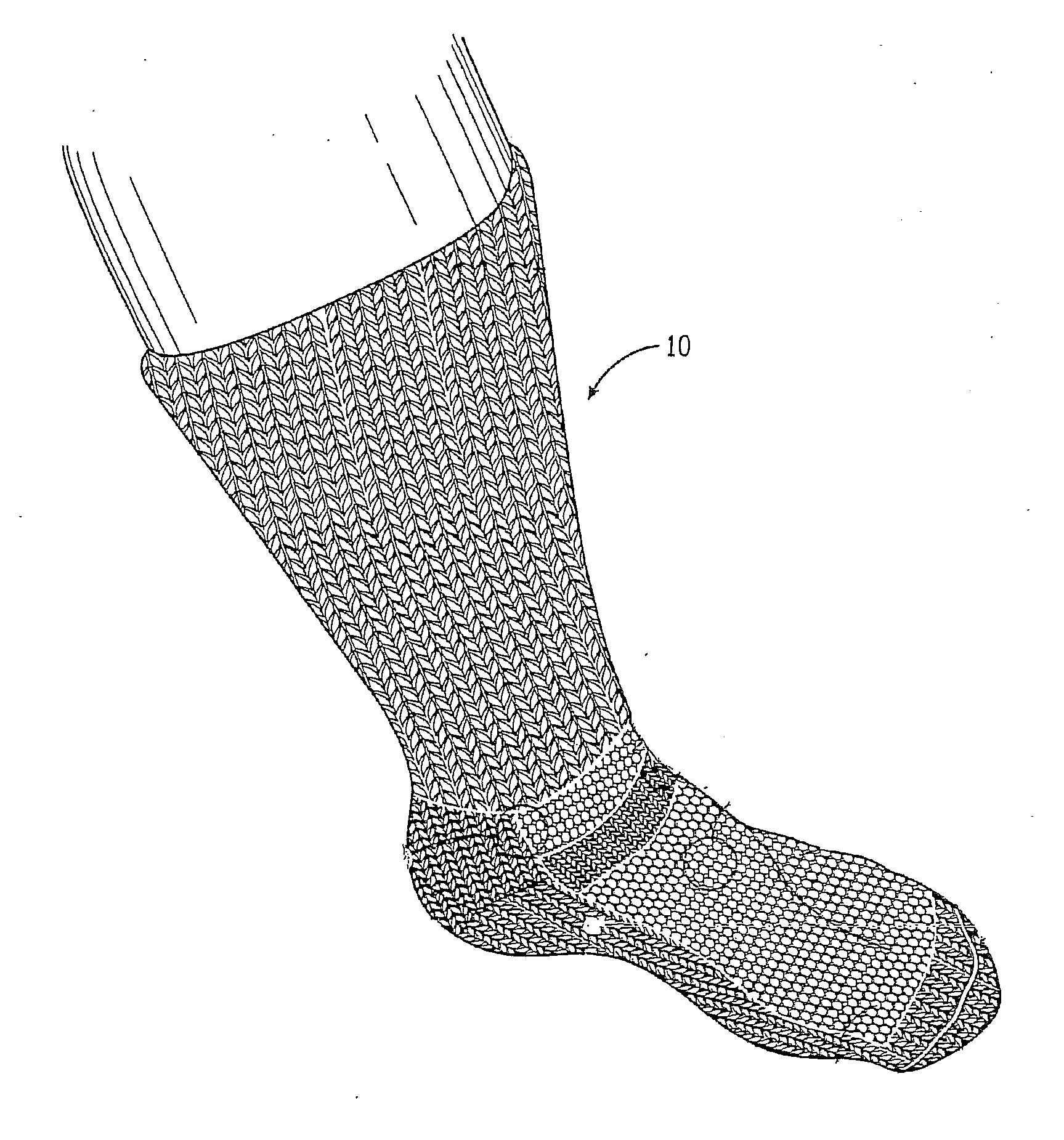
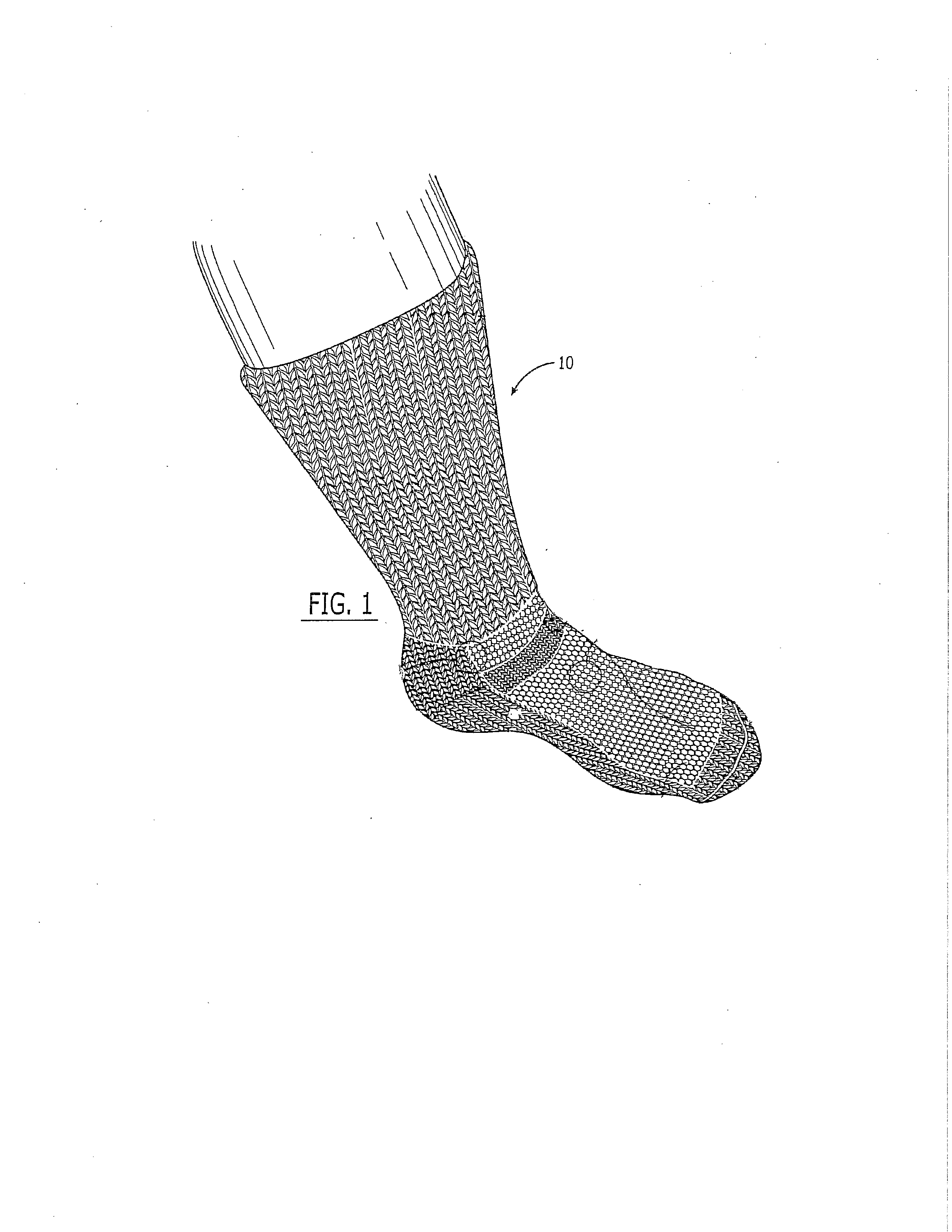
Sock for treatment of foot and leg wounds, methods of use and manufacture
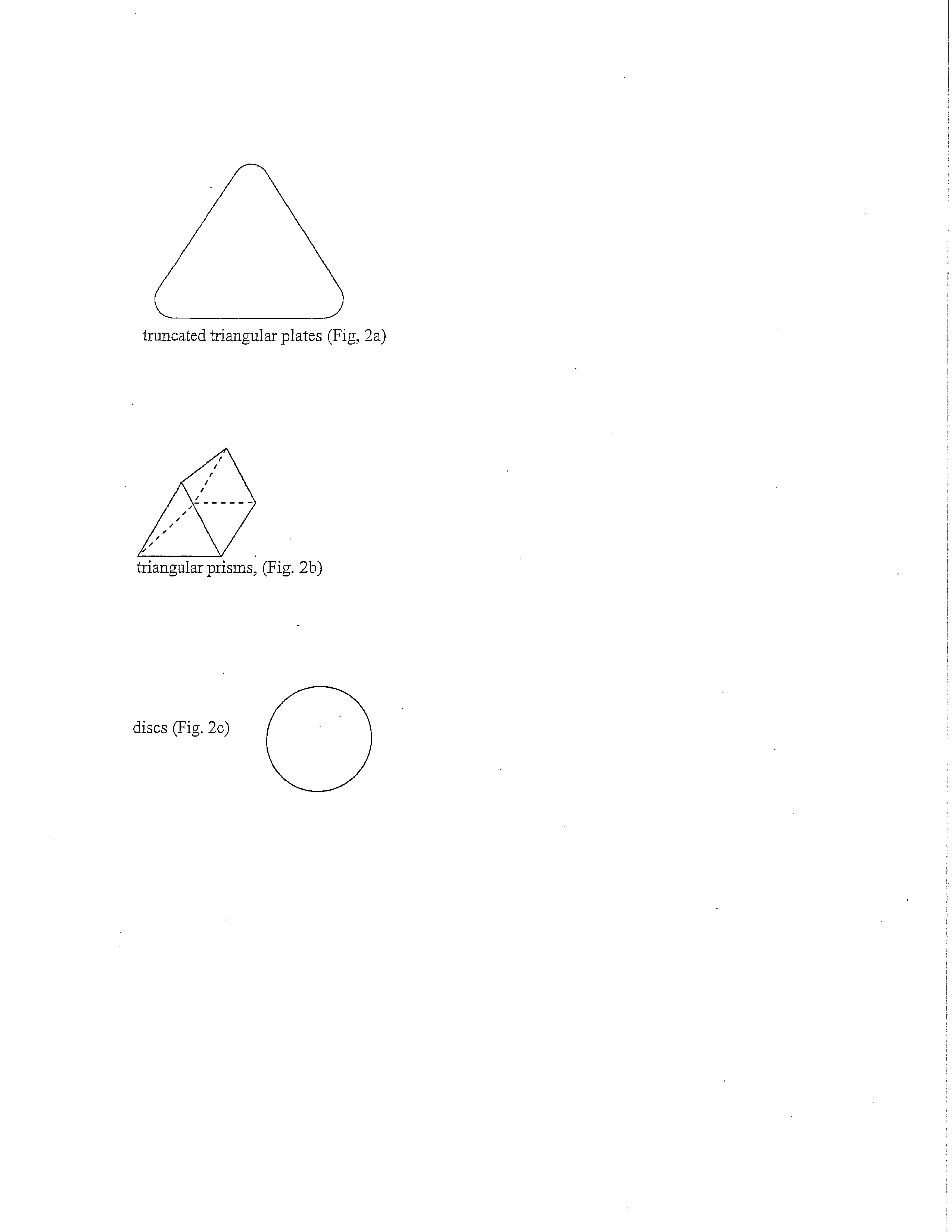
The Improved Sock is made of yarns knitted into a foot and calf, with graduated compression on an individual's foot from the foot to the calf. The yarns can include wool and alpaca fibers. A substantial proportion of wool and / or alpaca are on the inside of the sock so as to be in direct contact with the skin and wound. The Improved Sock provides absorption and wicking of inflammatory mediators, bacteria and biofilm and necrotic exudate from the foot and leg. The Improved Sock has AgNP shapes electrostatically bonded to the yarn. At least 30% of the mass of the AgNP shapes attached to the fibers have a shape selected from the group consisting of truncated triangular plates (a triangle with the corners rounded off), triangular prisms, discs and combinations of two or more of them. The Improved Sock functions as a unique wound dressing with the sock in direct contact with the wound.
Owner:VIVE WEAR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com