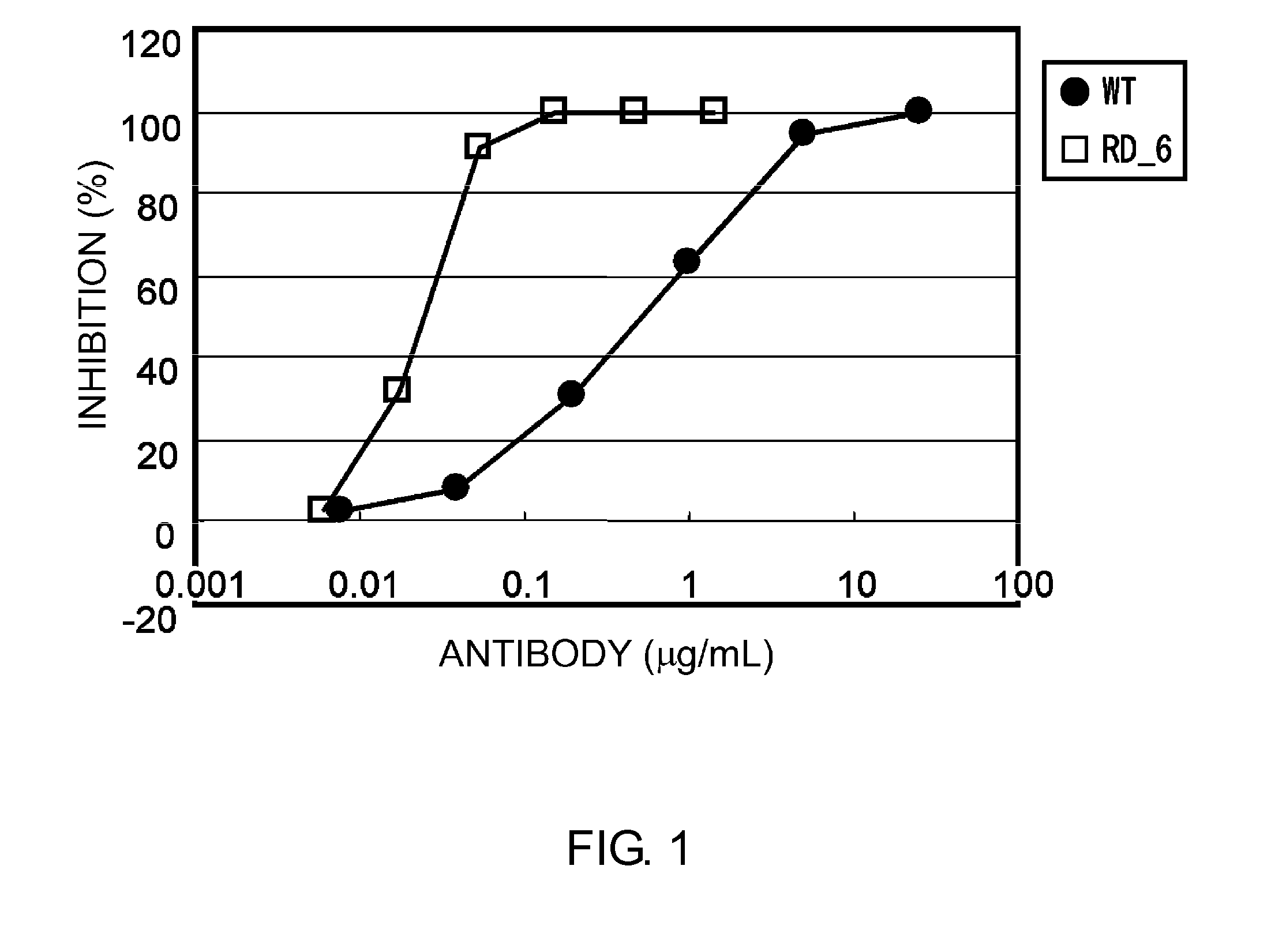
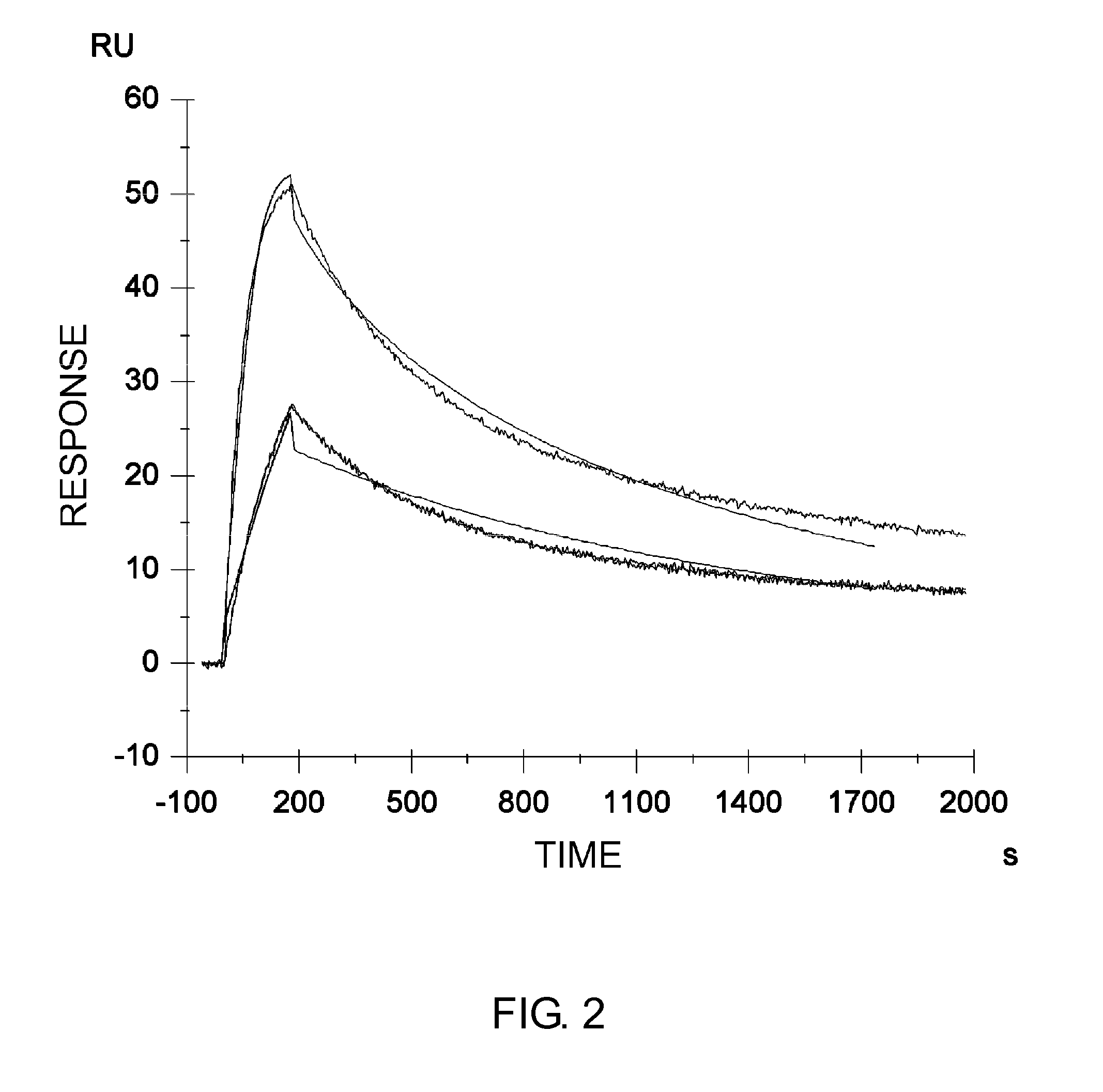
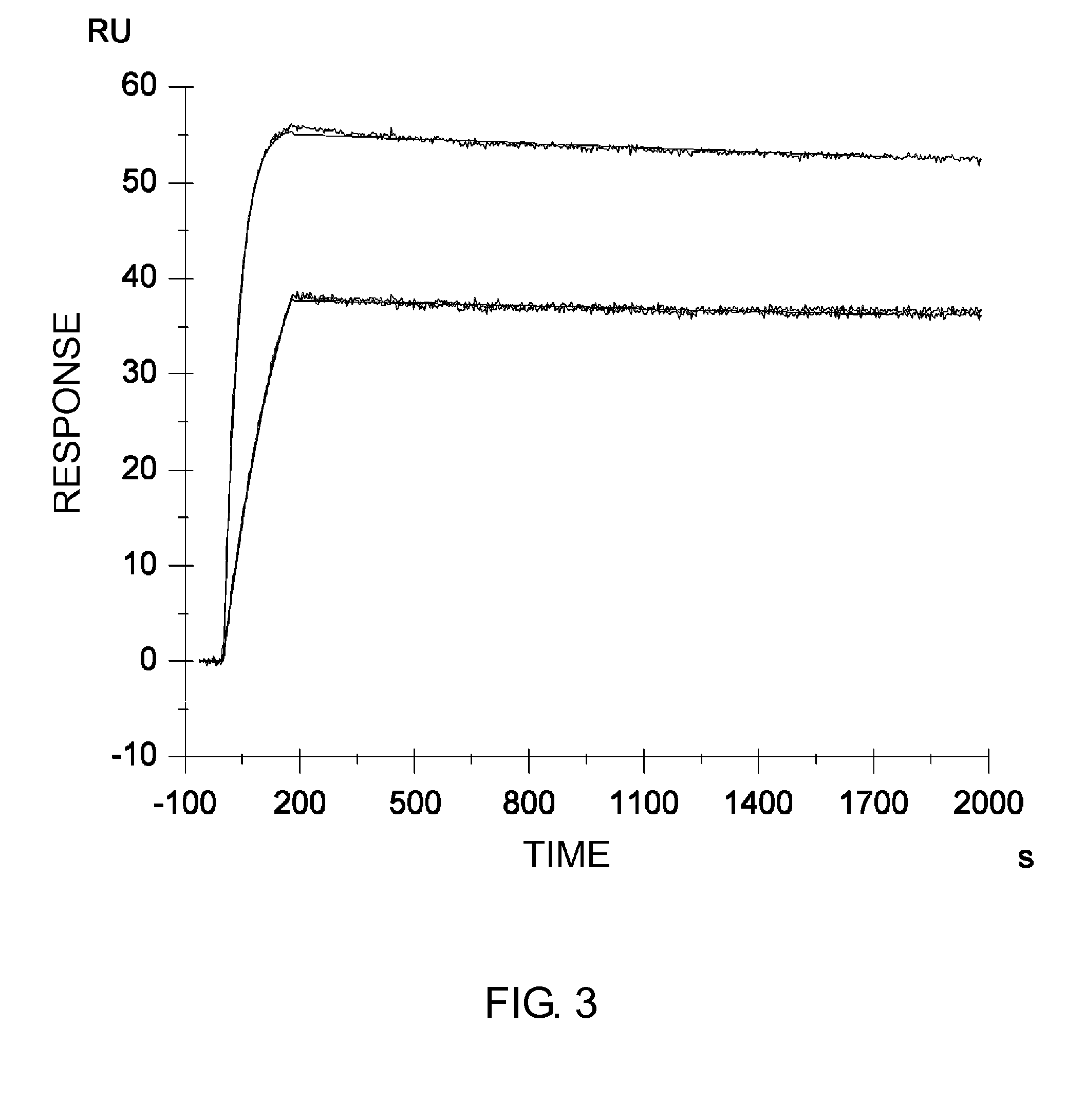
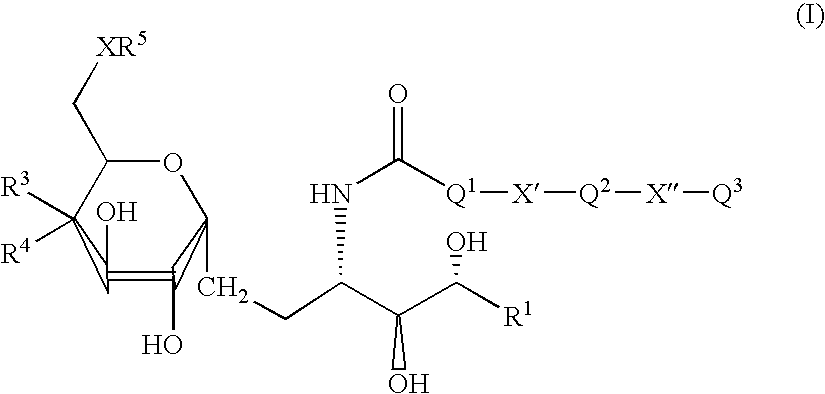
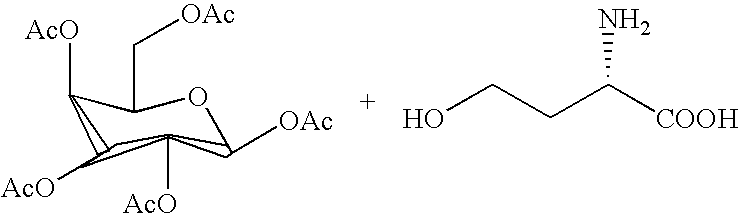Patents
Literature
1573 results about "Vaccine Immunogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Wanted immunogenicity is typically related with vaccines, where the injection of an antigen (the vaccine) provokes an immune response against the pathogen (virus, bacteria...) aiming at protecting the organism.
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
Super humanized antibodies
InactiveUS6881557B2Antibody mimetics/scaffoldsAnalogue computers for chemical processesHuman sequenceHumanized antibody
Disclosed herein are methods for humanizing antibodies based on selecting variable region framework sequences from human antibody genes by comparing canonical CDR structure types for CDR sequences of the variable region of a non-human antibody to canonical CDR structure types for corresponding CDRs from a library of human antibody sequences, preferably germline antibody gene segments. Human antibody variable regions having similar canonical CDR structure types to the non-human CDRs form a subset of member human antibody sequences from which to select human framework sequences. The subset members may be further ranked by amino acid similarity between the human and the non-human CDR sequences. Top ranking human sequences are selected to provide the framework sequences for constructing a chimeric antibody that functionally replaces human CDR sequences with the non-human CDR counterparts using the selected subset member human frameworks, thereby providing a humanized antibody of high affinity and low immunogenicity without need for comparing framework sequences between the non-human and human antibodies. Chimeric antibodies made according to the method are also disclosed.
Owner:ARROWSMITH TECH
Structure-based selection and affinity maturation of antibody library
InactiveUS7117096B2High affinityImprove throughputPeptide librariesPeptide/protein ingredientsProtein insertionIn vivo
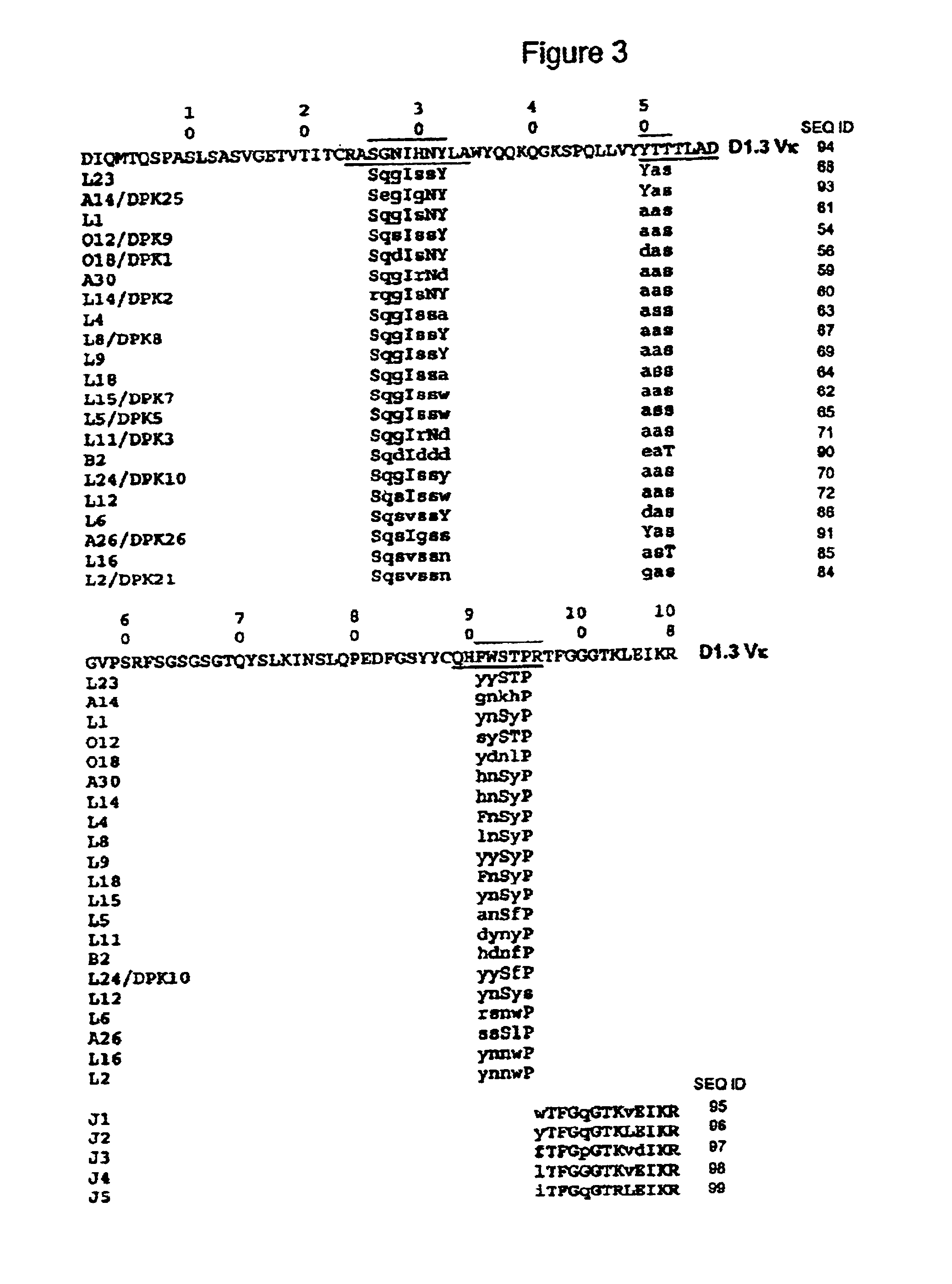
The present invention provides a structure-based methodology for efficiently generating and screening protein libraries for optimized proteins with desirable biological functions, such as antibodies with high binding affinity and low immunogenicity in humans. In one embodiment, a method is provided for constructing a library of antibody sequences based on a three dimensional structure of a lead antibody. The method comprises: providing an amino acid sequence of the variable region of the heavy chain (VH) or light chain (VL) of a lead antibody, the lead antibody having a known three dimensional structure which is defined as a lead structural template; identifying the amino acid sequences in the CDRs of the lead antibody; selecting one of the CDRs in the VH or VL region of the lead antibody; providing an amino acid sequence that comprises at least 3 consecutive amino acid residues in the selected CDR, the selected amino acid sequence being a lead sequence; comparing the lead sequence profile with a plurality of tester protein sequences; selecting from the plurality of tester protein sequences at least two peptide segments that have at least 10% sequence identity with lead sequence, the selected peptide segments forming a hit library; determining if a member of the hit library is structurally compatible with the lead structural template using a scoring function; and selecting the members of the hit library that score equal to or better than or equal to the lead sequence. The selected members of the hit library can be expressed in vitro or in vivo to produce a library of recombinant antibodies that can be screened for novel or improved function(s) over the lead antibody.
Owner:ABMAXIS
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Use of synthetic glycolipids as universal adjuvants for vaccines against cancer and infectious diseases
InactiveUS20050192248A1Enhancement and extension of durationBiocideOrganic active ingredientsDiseaseAdjuvant
The present invention relates to methods and compositions for augmenting an immunogenicity of an antigen in a mammal, comprising administering said antigen together with an adjuvant composition that includes a synthetic glycolipid compound of Formula I, as described herein. According to the present invention, the use of a compound of Formula I as an adjuvant is attributed at least in part to the enhancement and / or extension of antigen-specific Th1-type responses, in particular, CD8+ T cell responses. The methods and compositions of the present invention can be useful for prophylaxis and treatment of various infectious and neoplastic diseases.
Owner:NEW YORK UNIV +1
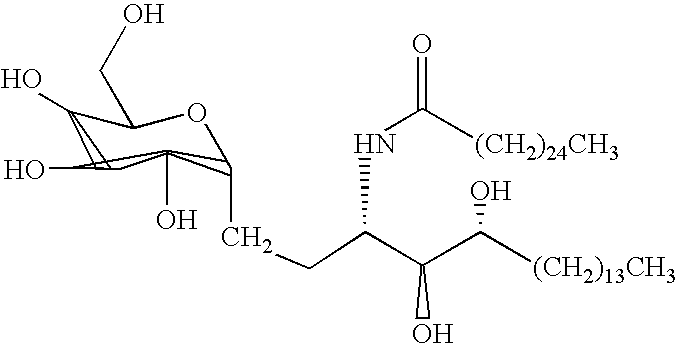
Saponin compositions and uses thereof
Owner:ANTIGENICS
Compositions and methods for administering Borrelia DNA
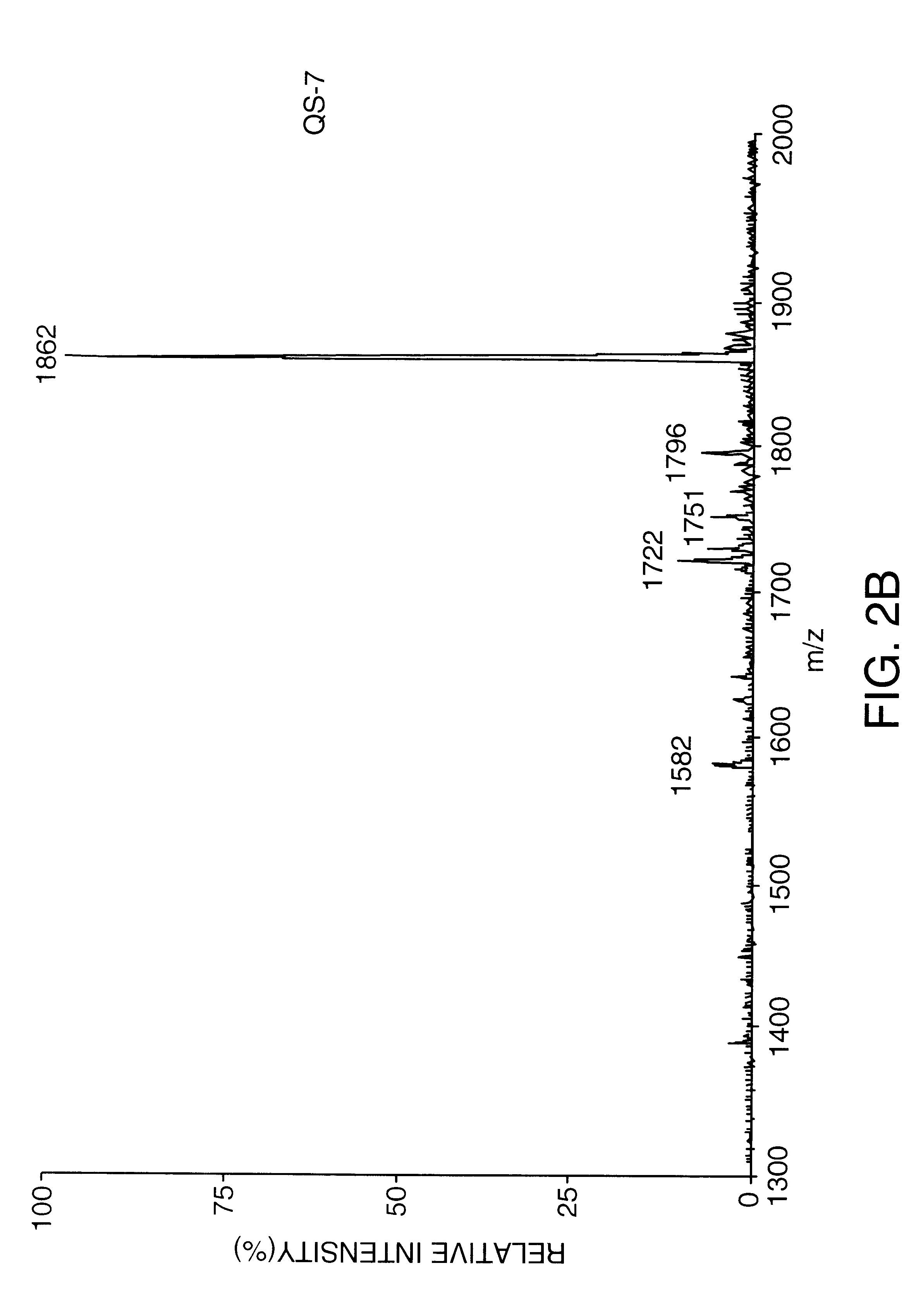
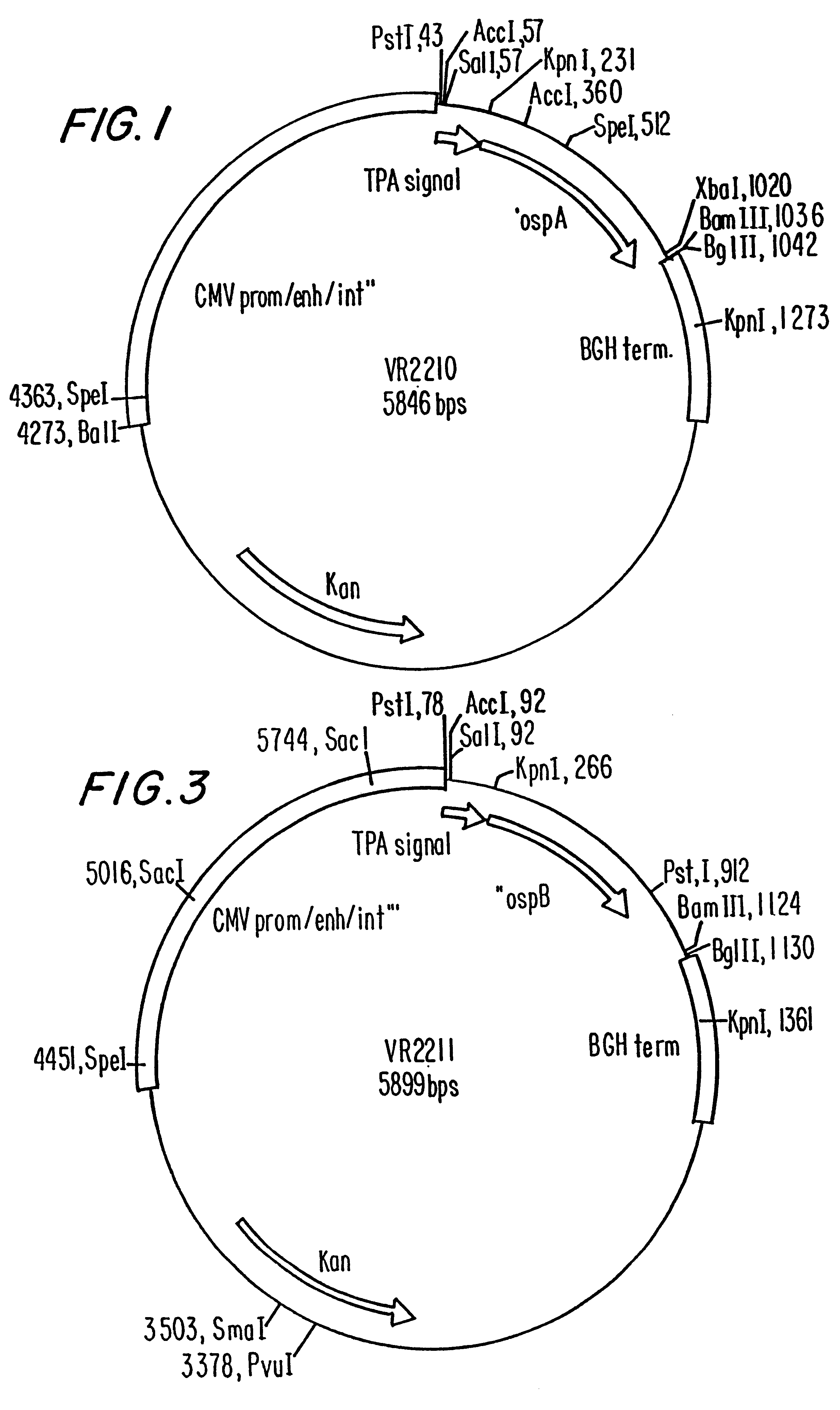
Disclosed is a vaccine against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding Borrelia OspA or OspB, and a DNA encoding a terminator. Disclosed too is an immunogenic composition against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid comprising a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding a Borrelia OspC, and a DNA encoding a terminator. And, methods for making and using such vaccines and the immunogenic composition are also disclosed.
Owner:PASTEUR MERIEUX SERUMS & VACCINS SA
Novel proteins with altered immunogenicity
The present invention provides methods for combining computational methods for modulating protein immunogenicity with computational methods for identifying sequences with desired structural and functional properties. More specifically, the methods of the present invention may be used to identify modifications that increase or decrease the immunogenicity of a protein by affecting antigen uptake, MHC binding, T-cell binding, or antibody binding, while retaining or enhancing functional properties.
Owner:XENCOR
Reducing the immunogenicity of fusion proteins
InactiveUS6992174B2Low immunogenicityHigh expressionPeptide/protein ingredientsAntibody mimetics/scaffoldsMHC class IIVaccine Immunogenicity
Disclosed are compositions and methods for producing fusion proteins with reduced immunogenicity. Fusion proteins of the invention include a junction region having an amino acid change that reduces the ability of a junctional epitope to bind to MHC Class II, thereby reducing its interaction with a T-cell receptor. Methods of the invention involve analyzing, changing, or modifying one or more amino acids in the junction region of a fusion protein in order to identify a T-cell epitope and reduce its ability to interact with a T cell receptor. Compositions and methods of the invention are useful in therapy.
Owner:MERCK PATENT GMBH
Antibodies and Fc fusion proteins with altered immunogenicity
InactiveUS20060275282A1Low affinityMaintain activityAntibody mimetics/scaffoldsImmunoglobulins against animals/humansVaccine ImmunogenicityAntibody
Variant antibodies and Fc fusion proteins with reduced immunogenicity are described. In particular, the variants of antibodies and Fc fusion proteins have reduced ability to bind one or more human class II MHC molecules are described.
Owner:XENCOR INC
Compositions of prokaryotic phenylalanine ammonia-lyase and methods of using compositions thereof
ActiveUS20080008695A1Reduce protein aggregationReduce enzyme activityNervous disorderPeptide/protein ingredientsWild typeVaccine Immunogenicity
The present invention is directed to phenylalanine ammonia-lyase (PAL) produced by prokaryotes, wherein such prokaryotic PAL wherein the PAL variant has a greater phenylalanine-converting activity and / or a reduced immunogenicity as compared to a wild-type PAL. The invention thus provides compositions of bacterial PAL and biologically active fragments, mutants, variants and analogs thereof, as well as methods for the production, purification, and use of such compositions for therapeutic and industrial purposes.
Owner:BIOMARIN PHARMA INC
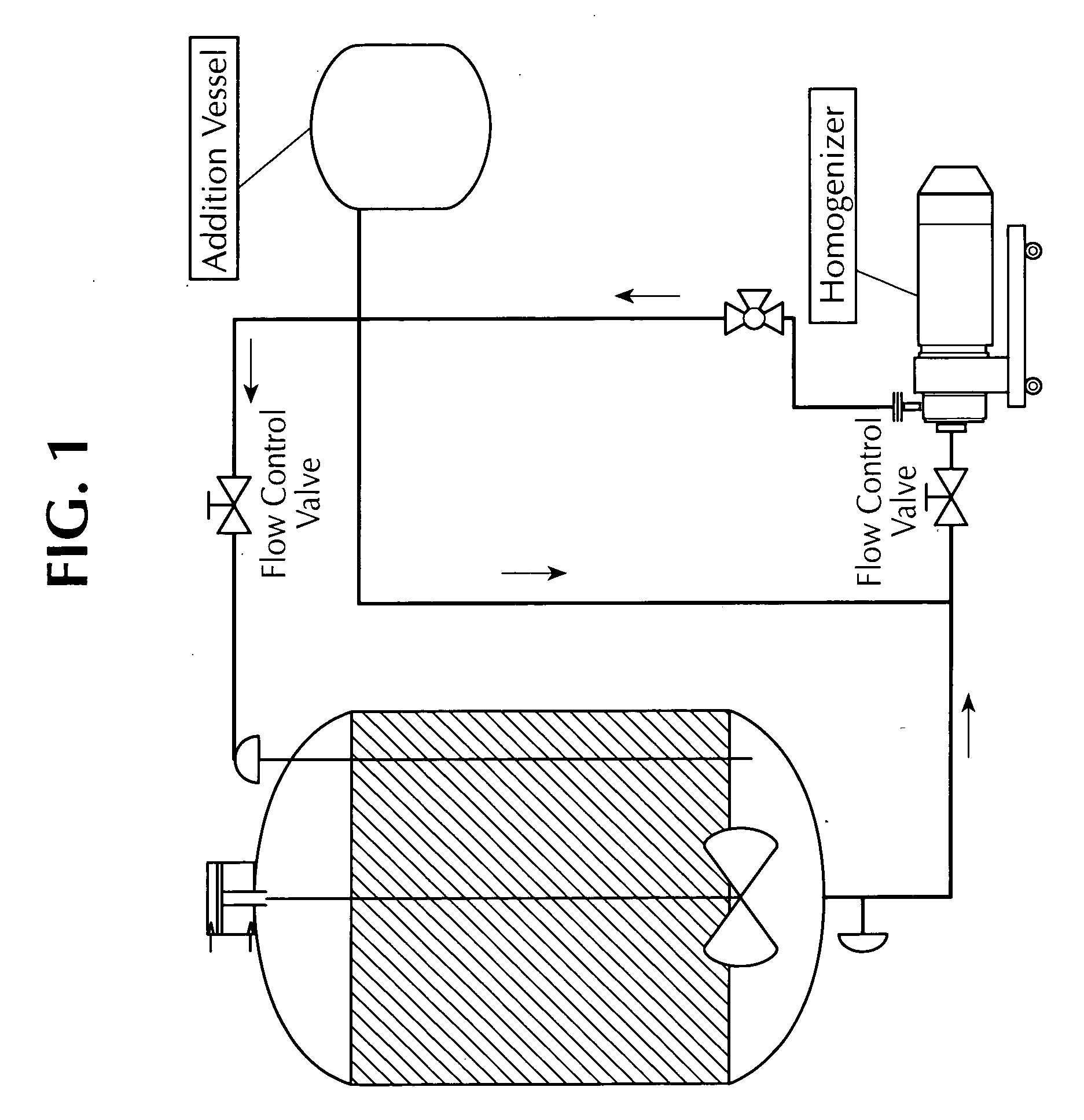
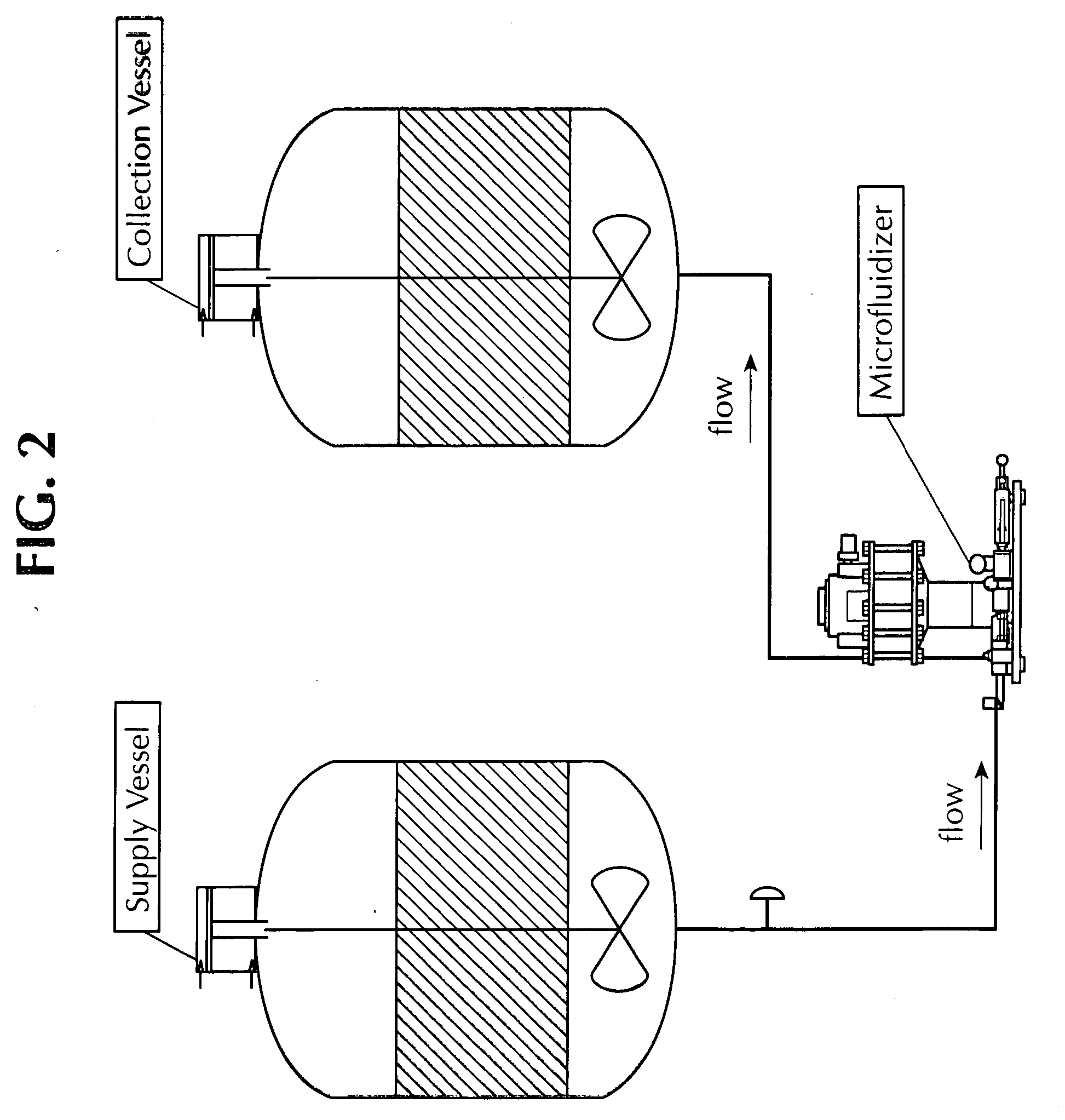
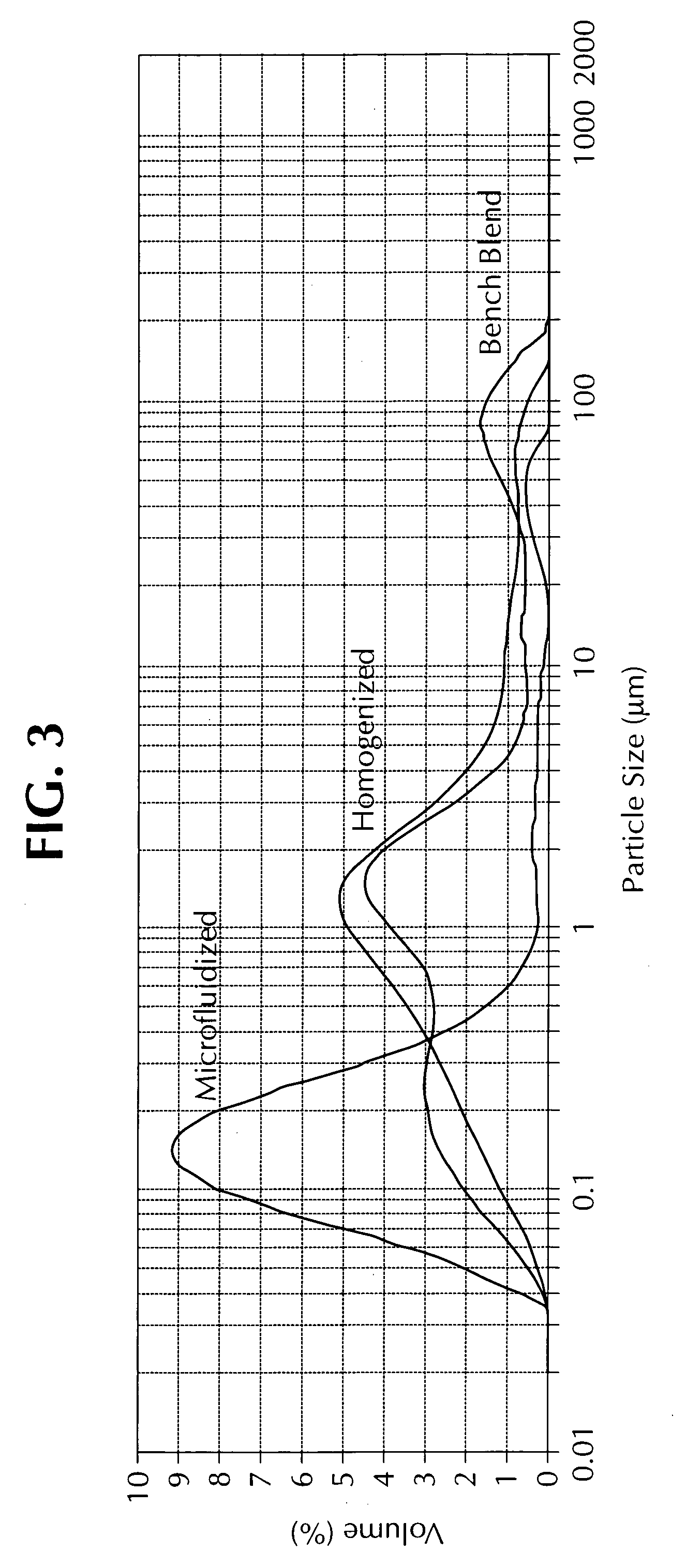
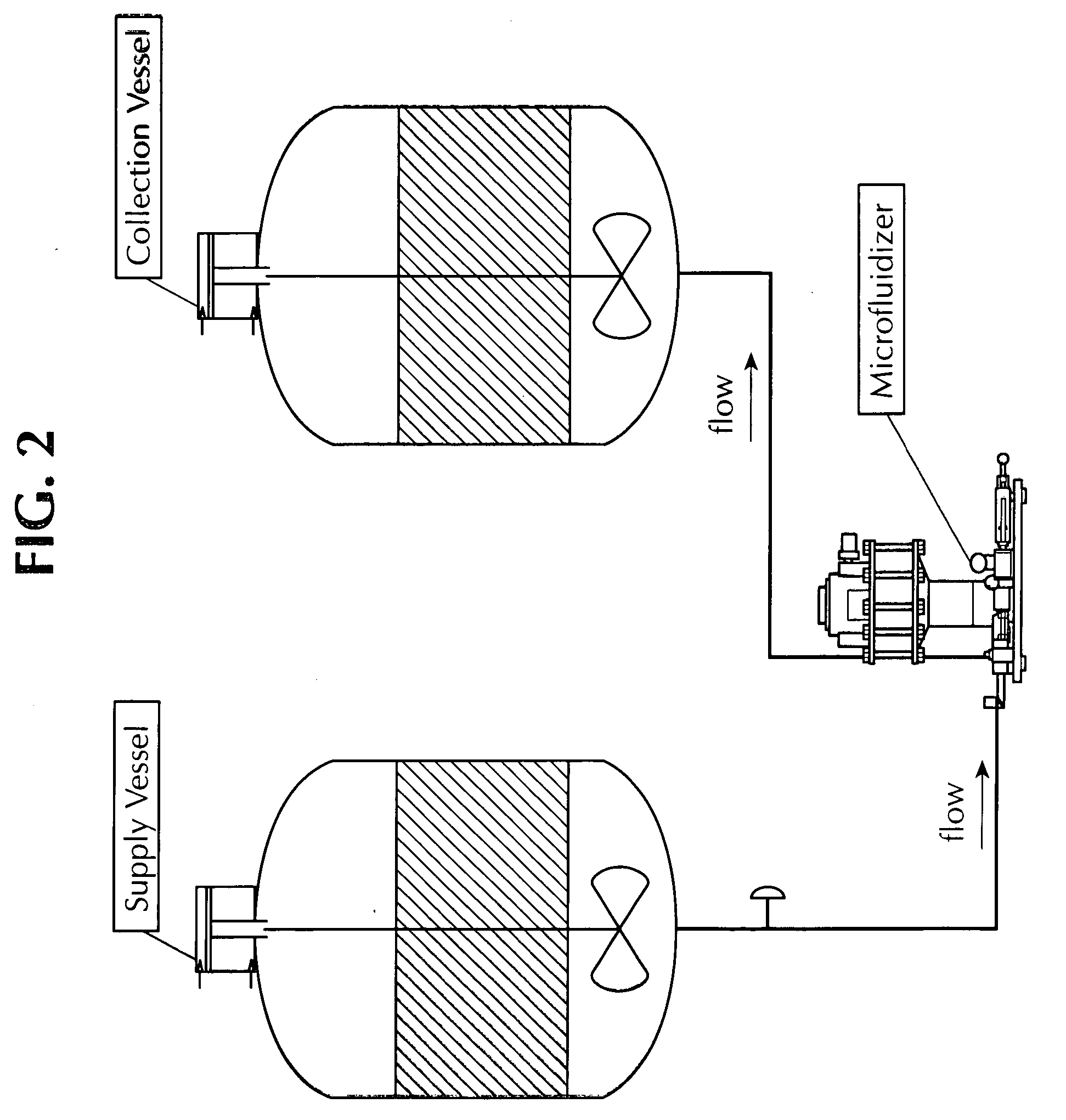
Microfluidized oil-in-water emulsions and vaccine compositions
This invention provides submicron oil-in-water emulsions useful as a vaccine adjuvant for enhancing the immunogenicity of antigens. The present invention also provides vaccine compositions containing an antigen combined with such emulsions intrinsically or extrinsically. Methods of preparing the emulsions and vaccines are also provided by the present invention.
Owner:ZOETIS SERVICE LLC
Novel multi-oligosaccharide glycoconjugate bacterial meningitis vaccines
InactiveUS20010048929A1Inhibition effectWeight increaseAntibacterial agentsPeptide/protein ingredientsSerotypeTumor antigen
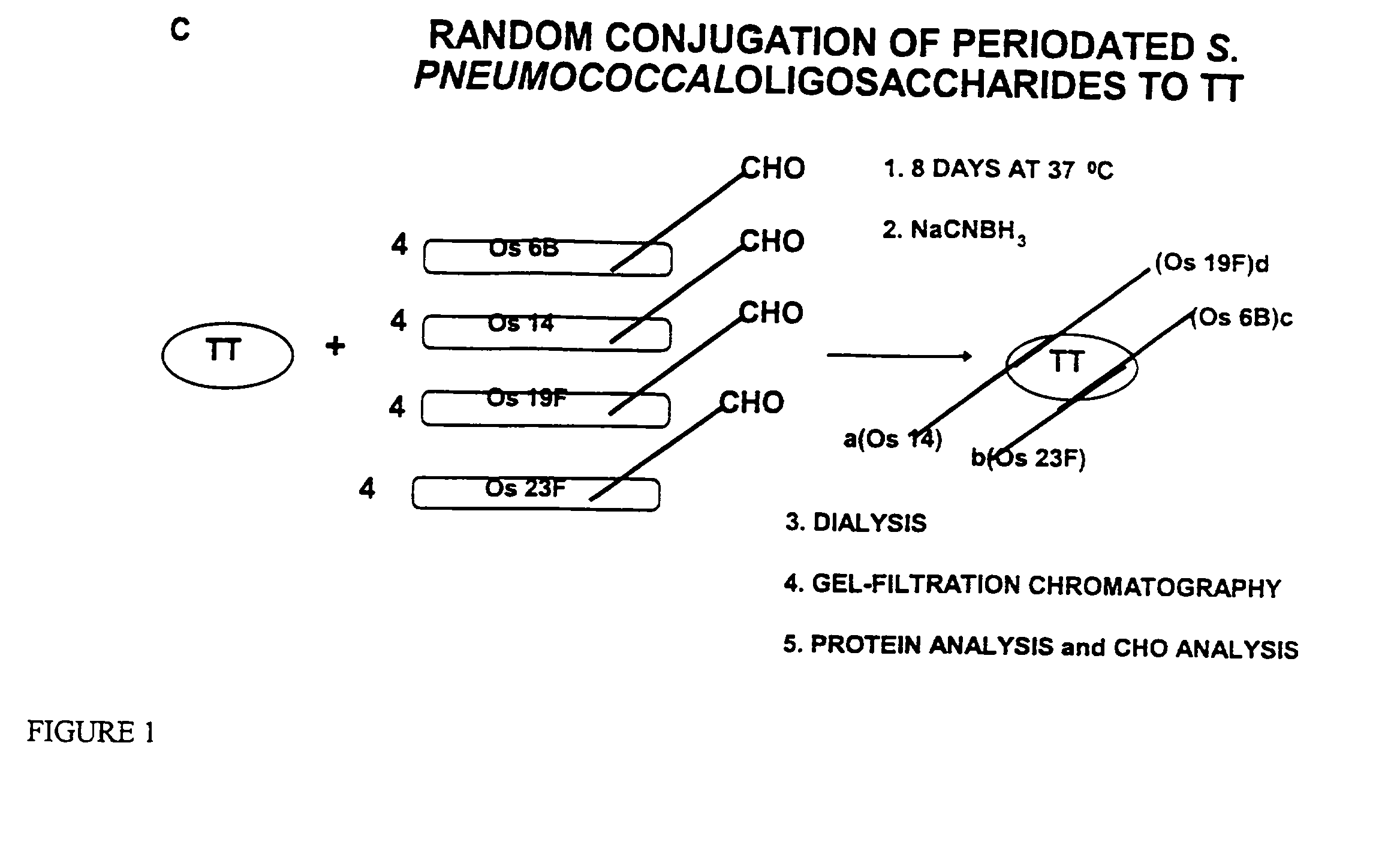
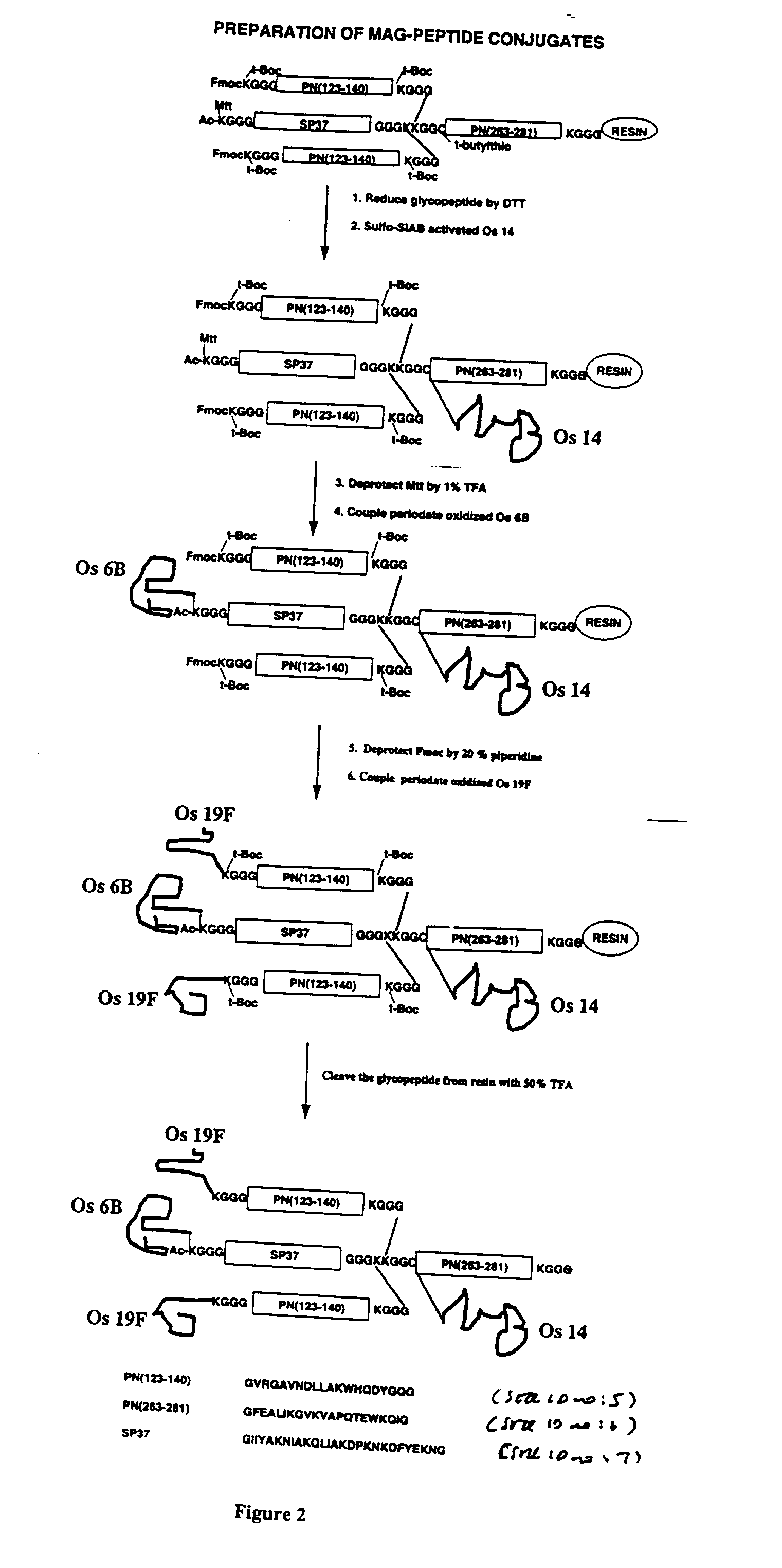
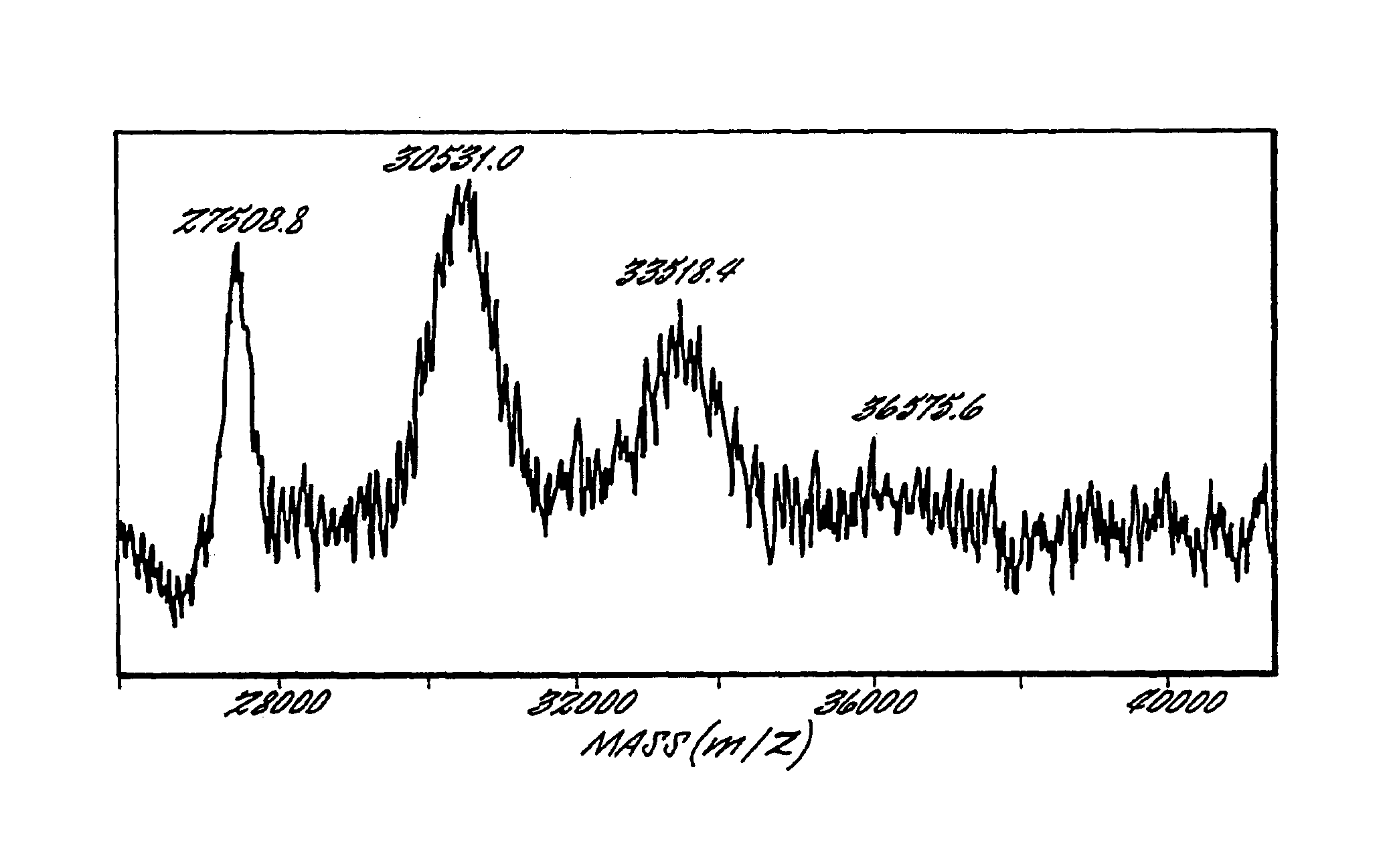
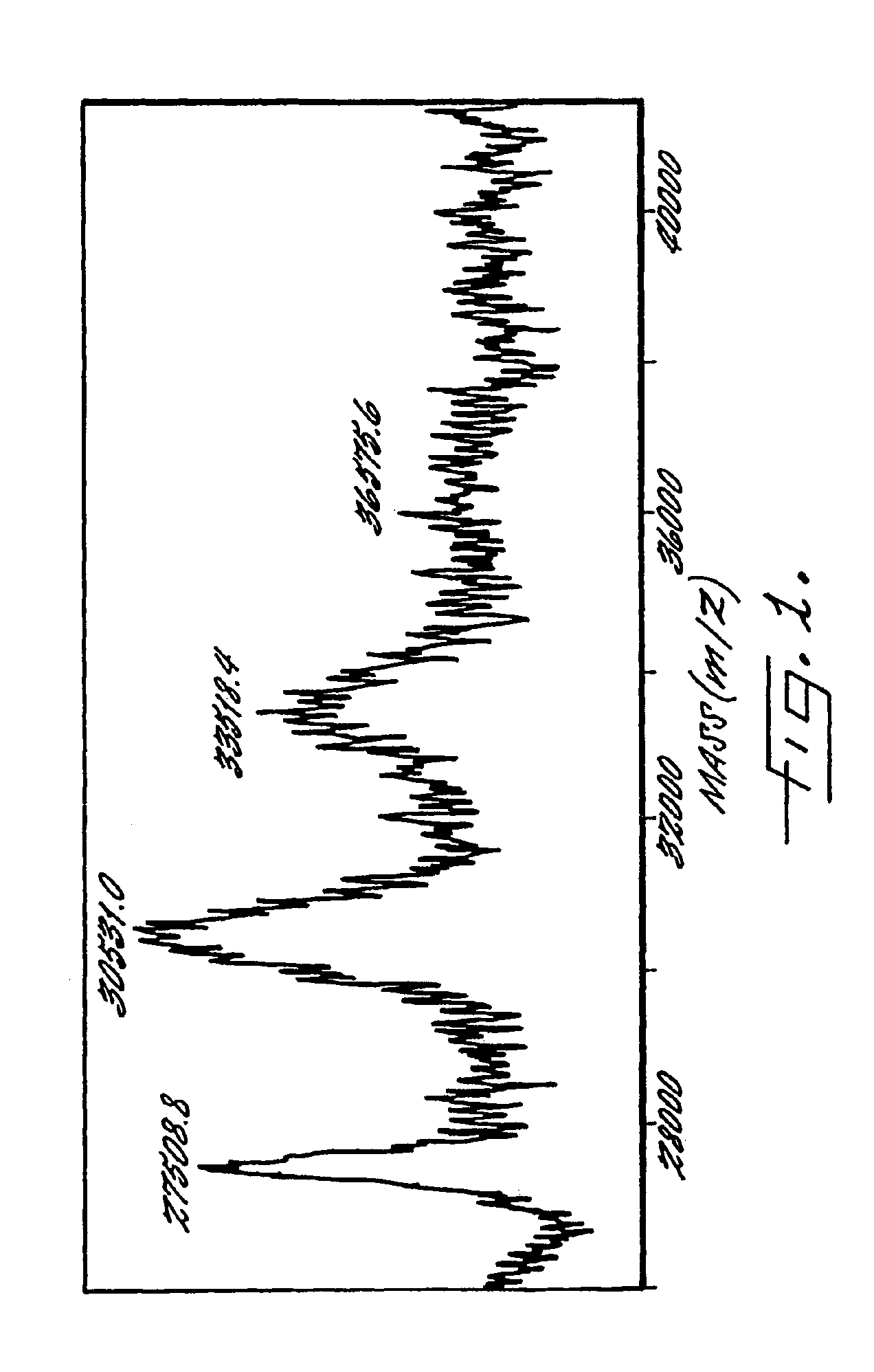
Multivalent immunogenic molecules comprise a carrier molecule containing at least one functional T-cell epitope and multiple different carbohydrate fragments each linker to the carrier molecule and each containing at least one functional B-cell epitope. The carrier molecule inputs enhanced immunogenicity to the multiple carbohydrate fragments. The carbohydrate fragments may be capsular oligosaccharide fragments from Streptococcus pneumoniae, which may be serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F or 23F, or Neisseria meningitidis, which may be serotype A, B, C, W-135 or Y. Such oligosaccharide fragments may be sized from 2 to 5 kDa. Alternatively, the carbohydrate fragments may be fragments of carbohydrate-based tumor antigens, such as Globo H, LeY or STn. The multivalent molecules may be produced by random conjugation or site-directed conjugation of the carbohydrate fragments to the carrier molecule. The multivalent molecules may be employed in vaccines or in the generation of antibodies for diagnostic application.
Owner:CONNAUGHT LAB
Soluble, degradable poly (ethylene glycol) derivatives for controllable release of bound molecules into solution
InactiveUS6864350B2Limit deliveryImparting sizePharmaceutical non-active ingredientsSynthetic polymeric active ingredientsSolubilityKidney
PEG and related polymer derivatives having weak, hydrolytically unstable linkages near the reactive end of the polymer are provided for conjugation to drugs, including proteins, enzymes, small molecules, and others. These derivatives provide a sufficient circulation period for a drug-PEG conjugate and then for hydrolytic breakdown of the conjugate and release of the bound molecule. In some cases, drugs that previously had reduced activity when permanently coupled to PEG can have therapeutically suitable activity when coupled to a degradable PEG in accordance with the invention. The PEG of the invention can be used to impart water solubility, size, slow rate of kidney clearance, and reduced immunogenicity to the conjugate. Controlled hydrolytic release of the bound molecule in the aqueous environment can then enhance the drug delivery system.
Owner:NEKTAR THERAPEUTICS INC
Compositions of prokaryotic phenylalanine ammonia-lyase and methods of using compositions thereof
ActiveUS7534595B2High catalytic activityExtended half-lifeNervous disorderPeptide/protein ingredientsWild typeDrug biological activity
Owner:BIOMARIN PHARMA INC
Modified RNA with decreased immunostimulatory properties
ActiveUS20160235864A1Reduce innate immune responseIncrease mRNA levelsOrganic active ingredientsPeptide/protein ingredientsVaccine ImmunogenicityRNA
The present invention provides a method for providing modified mRNAs of reduced immunogenicity and / or immunostimulatory capacity for use in protein replacement therapy. The invention further provides modified mRNAs and pharmaceutical compositions comprising the modified mRNAs according to the invention for use in protein replacement therapy.
Owner:CUREVAC SE
Isolating biological modulators from biodiverse gene fragment libraries
InactiveUS6994982B1Increase diversityEasy to controlPeptide-nucleic acidsPeptide librariesExpression LibraryNucleotide
The present invention provides a method for identifying a modulator or mediator of a biological activity, which activity includes antigenicity and or immunogenicity, said method comprising the step of:(i) producing a gene fragment expression library derived from defined nucleotide sequence fragments; and(ii) assaying the expression library for at least an amino acid sequence derived from step (i) for a biological activity wherein that activity is different from any activity the amino acid sequence may have in its native environment.
Owner:PHYLOGICA
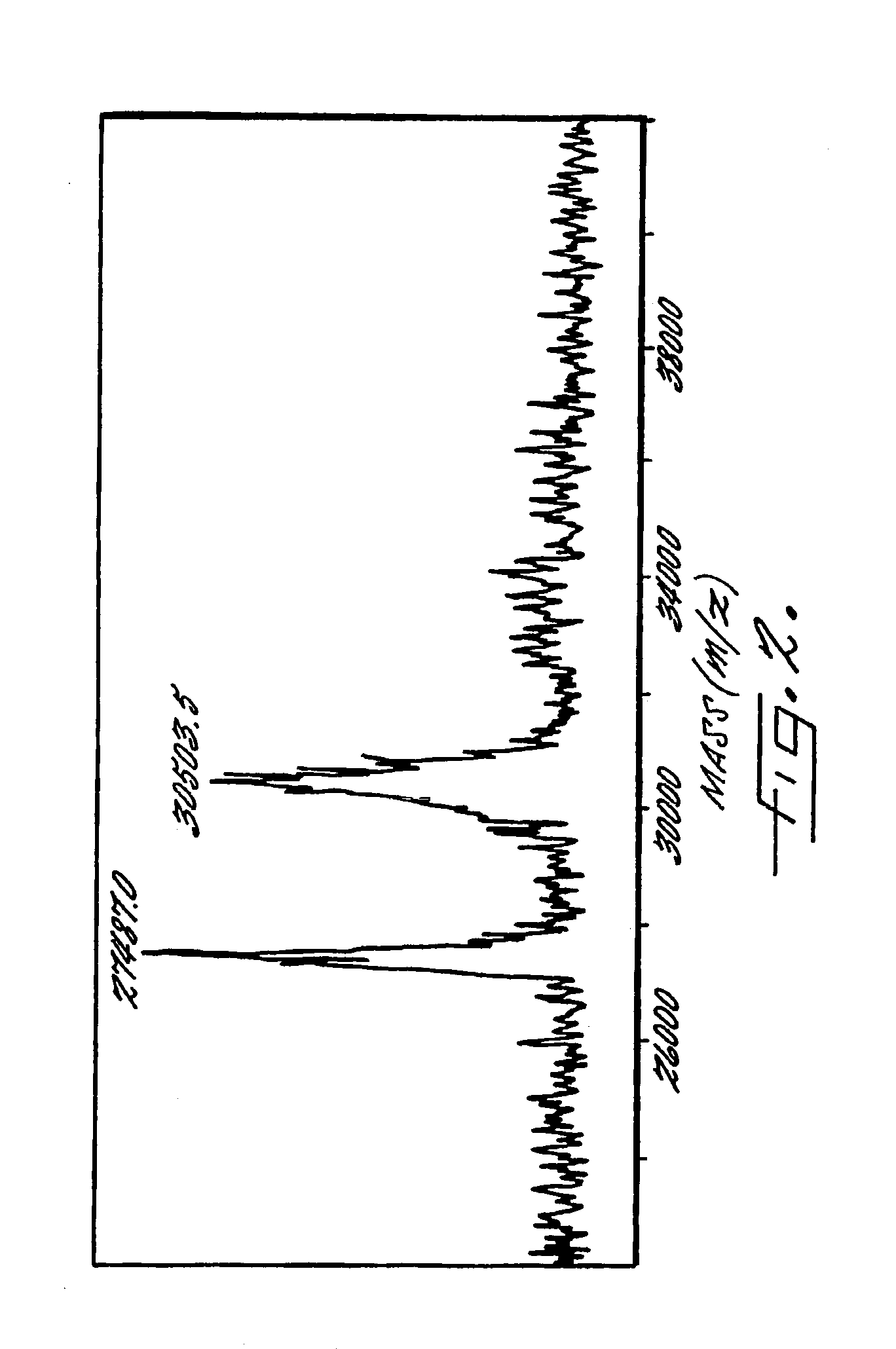
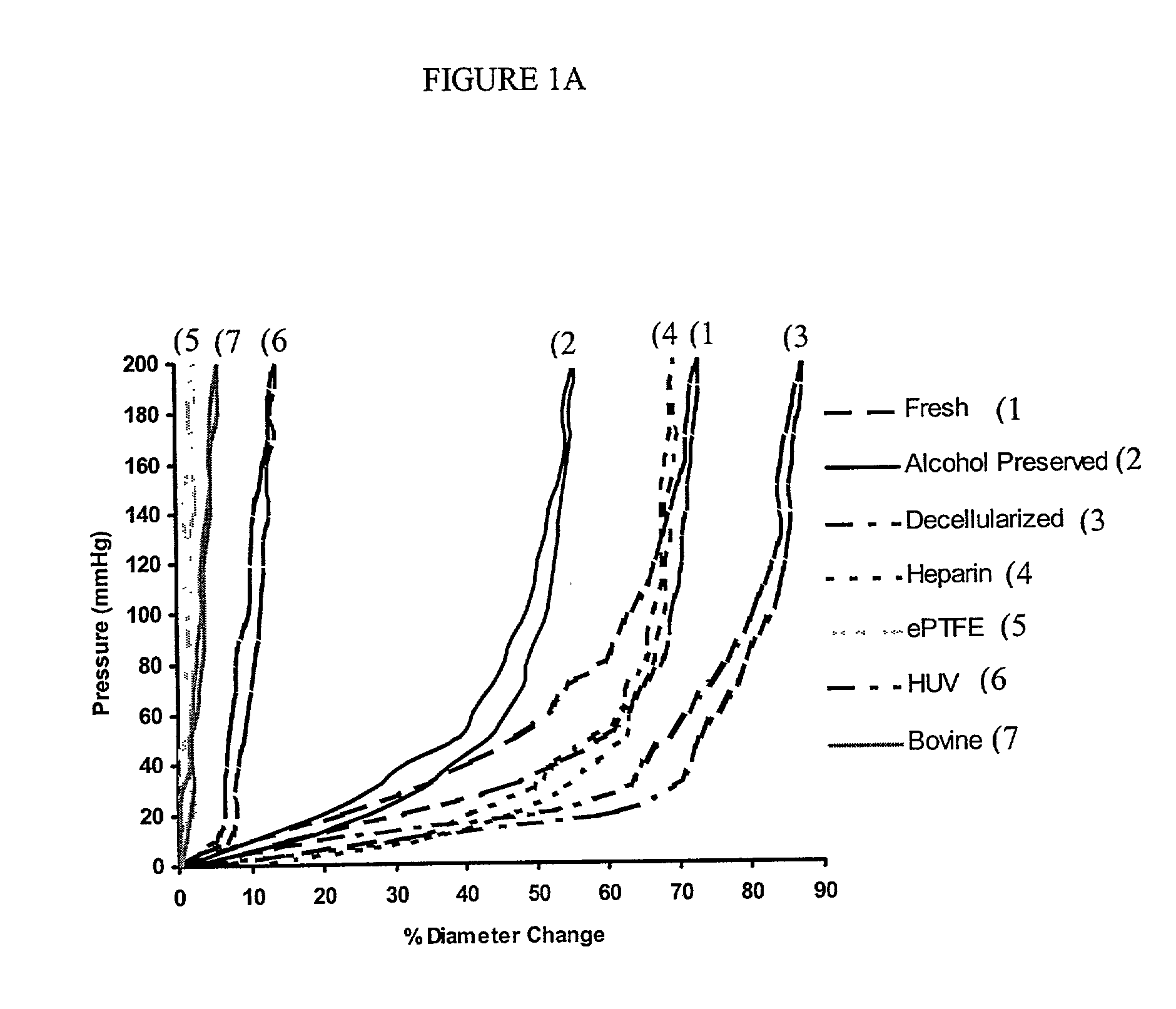
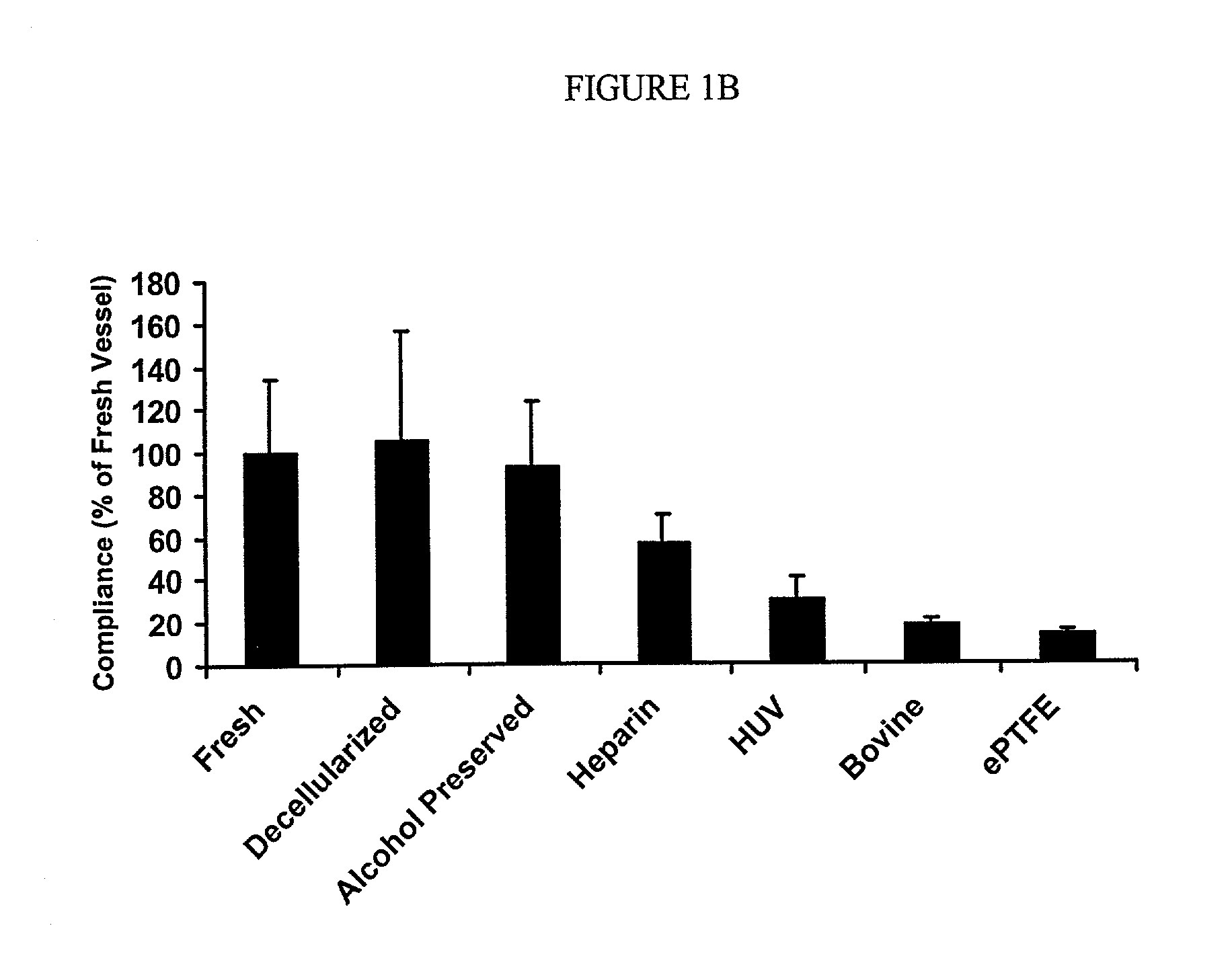
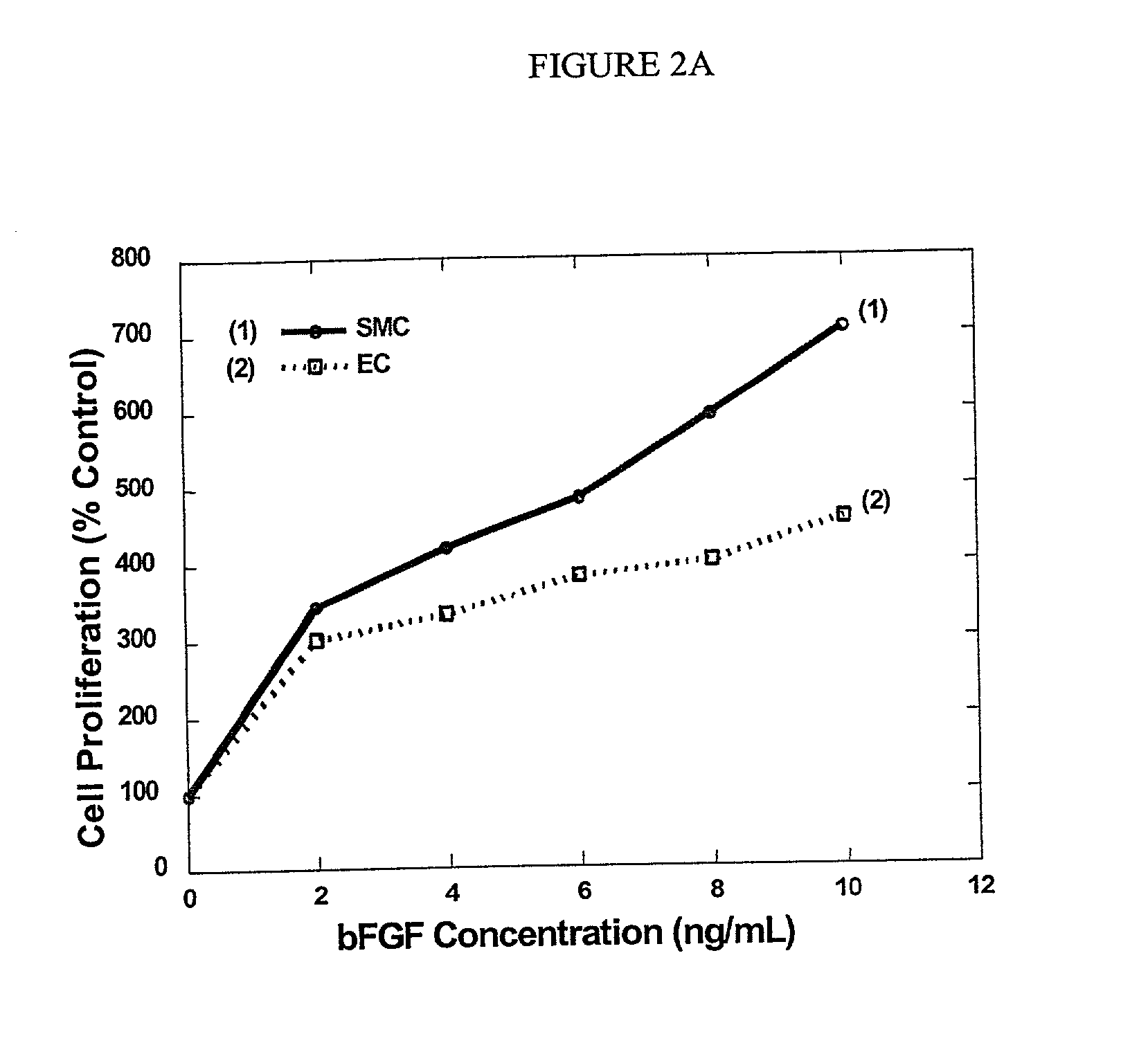
Decellularized vascular prostheses resistant to thrombus occlusion and immunologic rejection
The present invention relates to decellularized vascular prostheses that are resistant to thrombus occlusion and have a low level of immunogenicity. The vascular prostheses are denuded of cells, and coated with an anti-thrombogenic agent and a growth factor that promotes recellularization and further reduces the immunogenicity. The prostheses have high mechanical strength, resist aneurysm rupture, and allow for secure surgical sutures while maintaining structural integrity. The present invention provides vascular prostheses that are blood vessels, valves or portions of vessels containing valves. The present invention is also useful for coating synthetic vascular stents.
Owner:BAYLOR COLLEGE OF MEDICINE
Peptide pharmaceutical compositions
ActiveUS20070111938A1Improve biological activityReducing and preventing aggregationBiocidePeptide/protein ingredientsMedicinePeptide T
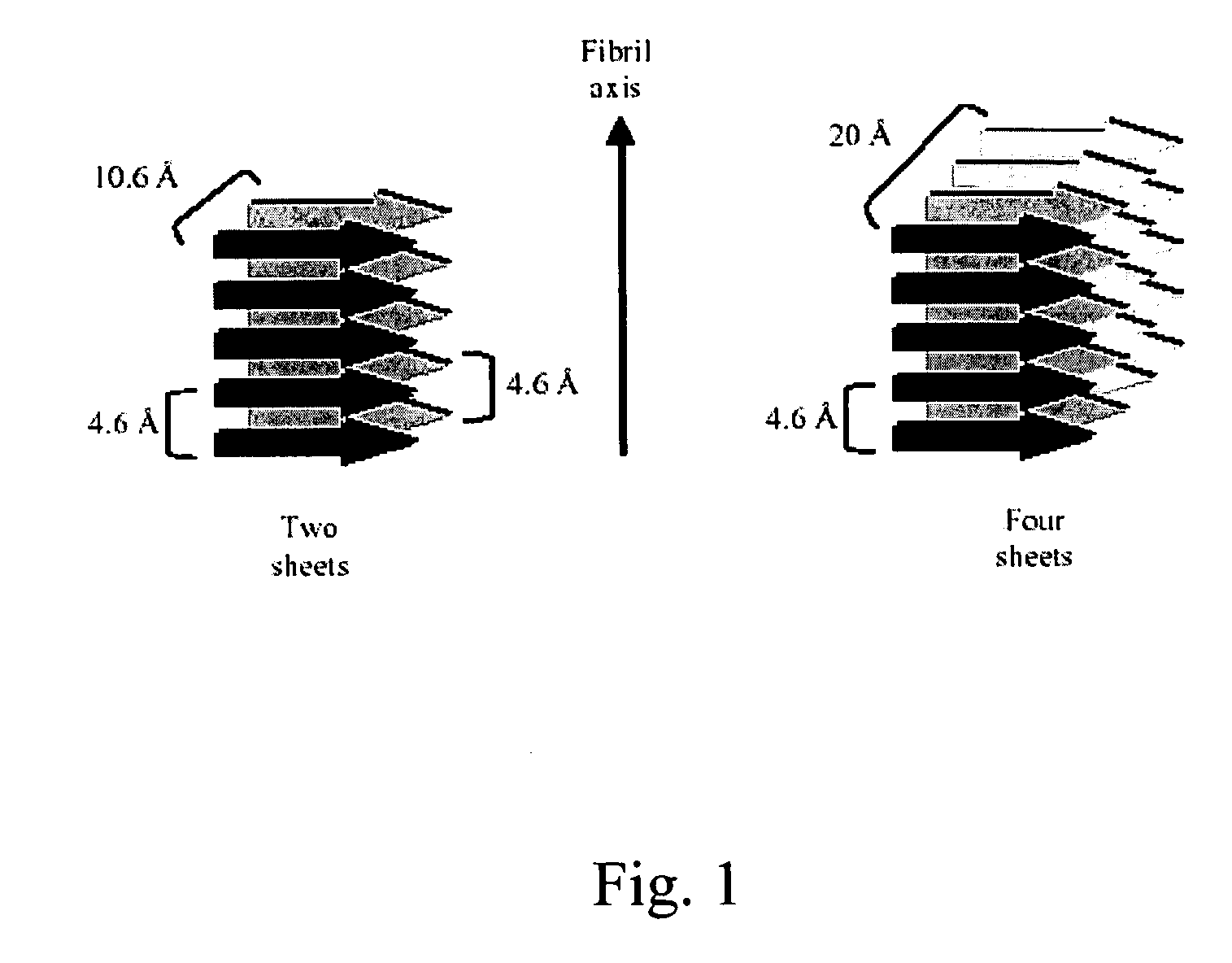
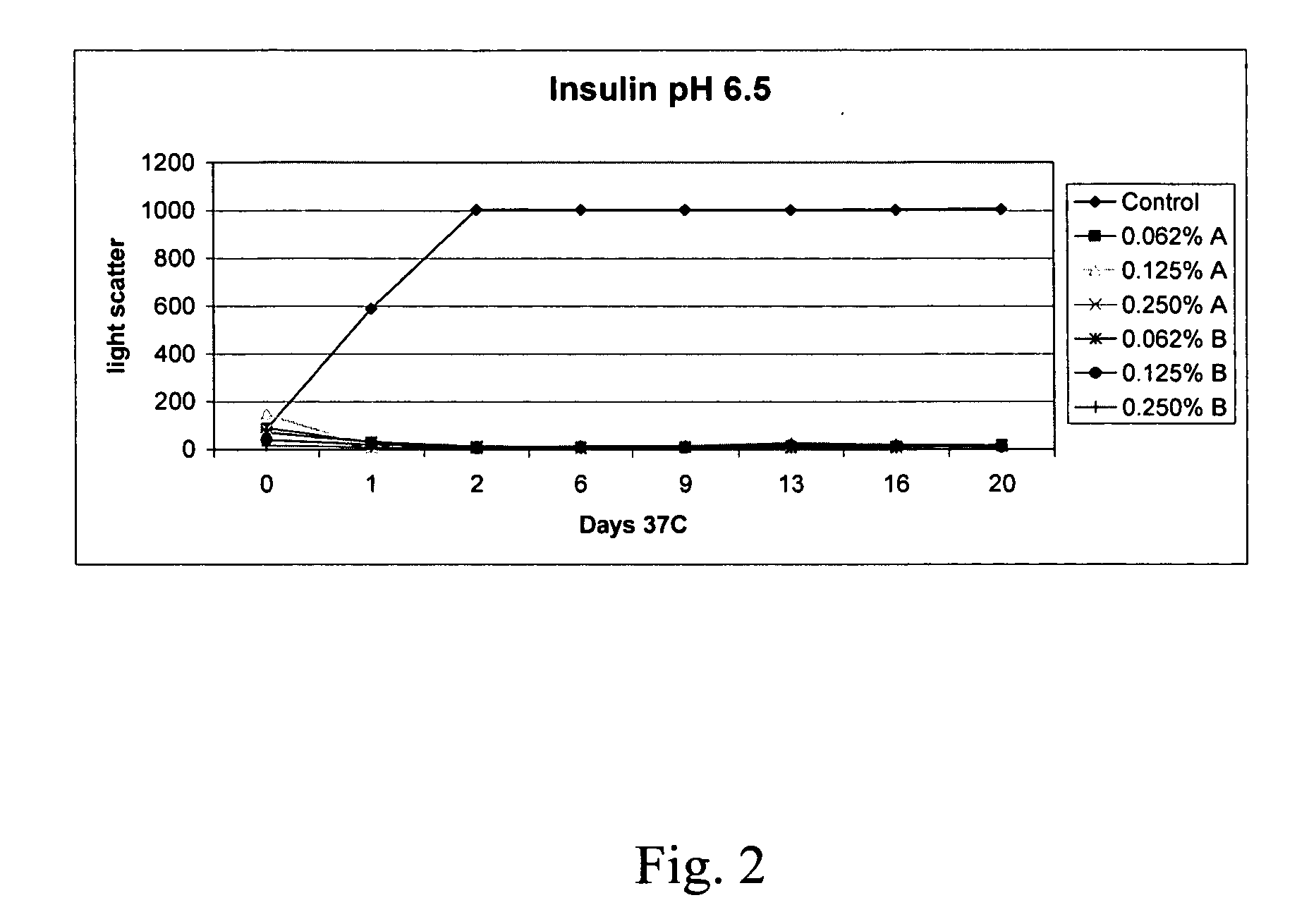
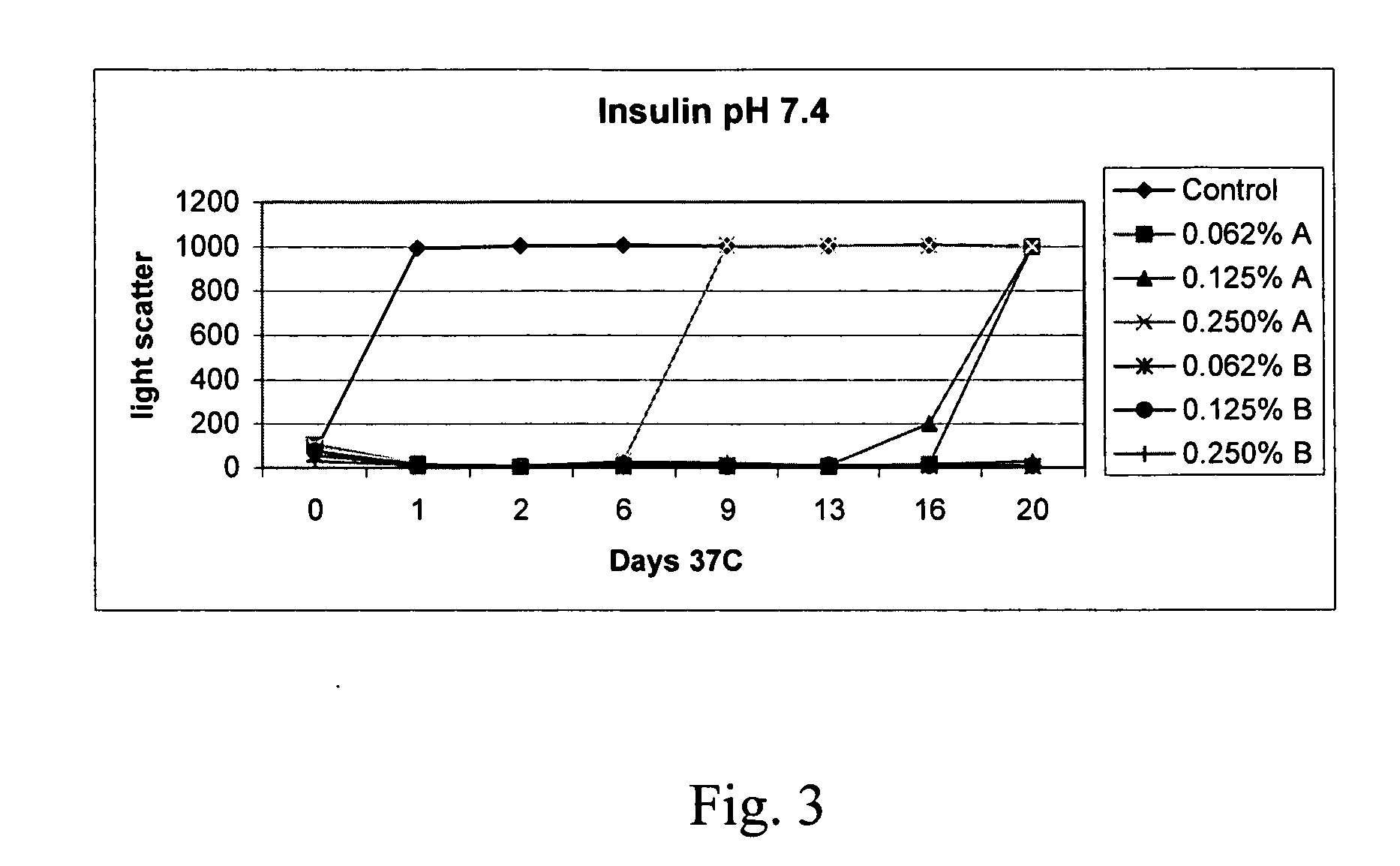
The present invention relates to alkylglycoside-containing compositions and methods for increasing the stability, reducing the aggregation and immunogenicity, increasing the biological activity, and reducing or preventing fibrillar formation of a peptide, polypeptide, or variant thereof, for example insulin and Peptide T or analog thereof.
Owner:PEPTIDE ACCOUNT LLC
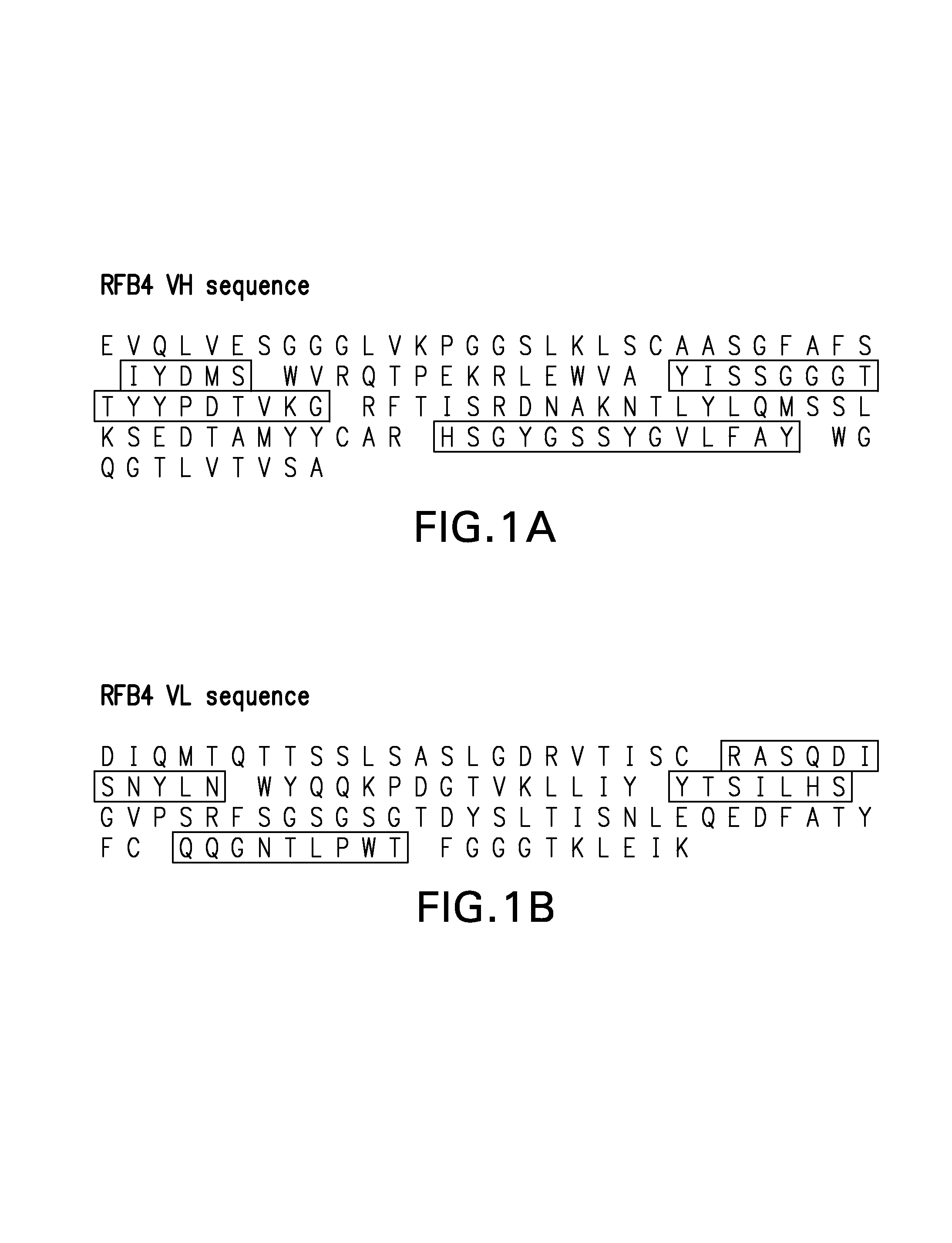
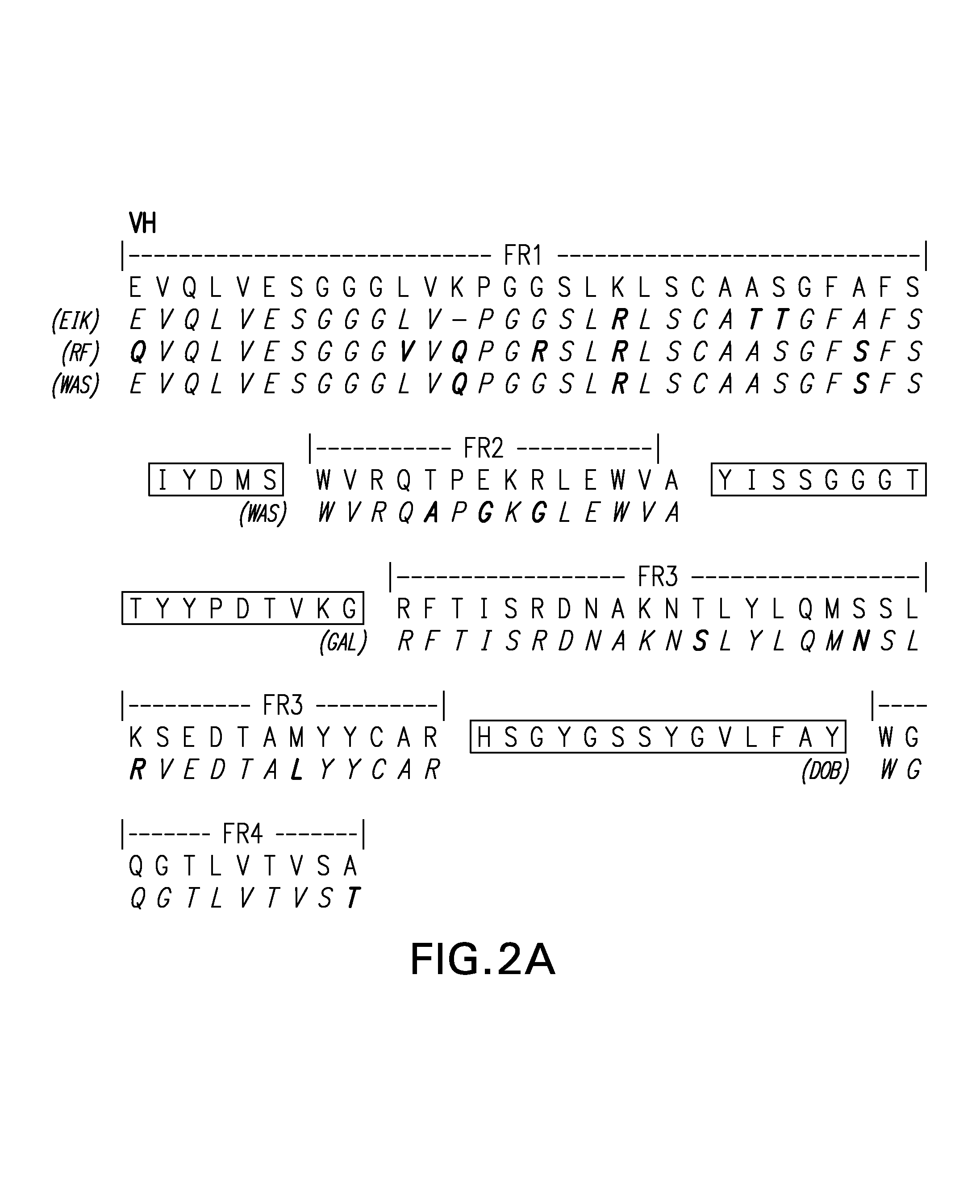
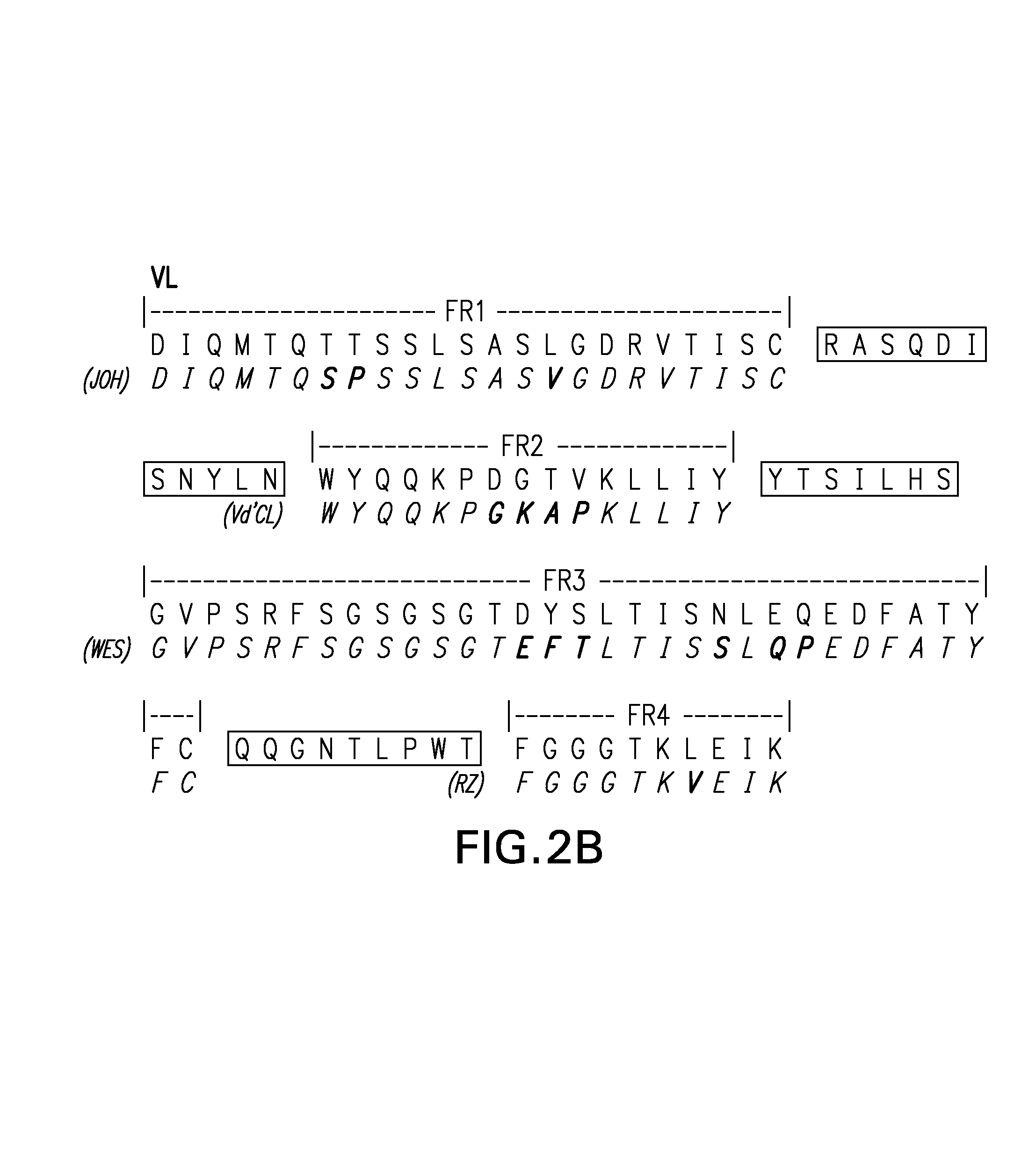
Framework-patched immunoglobulins
InactiveUS7321026B2Reduced and eliminated immunogenicityIncrease flexibilityAntipyreticAnalgesicsB-Cell EpitopesVaccine Immunogenicity
Framework (FR)-patching is a novel approach to modify immunoglobulin for reducing potential immunogenicity without significant alterations in specificity and affinity. Unlike previous described methods of humanization, which graft CDRs from a donor onto the frameworks of a single acceptor immunoglobulin, we patch segments of framework (FR1, FR2, FR3, and FR4), or FRs, to replace the corresponding FRs of the parent immunoglobulin. Free assortment of these FRs from different immunoglobulins and from different species can be mixed and matched into forming the final immunoglobulin chain. A set of criteria in the choice of these FRs to minimize or eliminate the need to reintroduce framework amino acids from the parent immunoglobulin for patching is described. The approach gives greater flexibility in the choice of framework sequences, minimizes the need to include parent framework amino acids, and, most importantly, reduces the chances of creating new T- and B-cell epitopes in the resultant immunoglobulin.
Owner:SKYTECH TECH
Nucleic acid library design and assembly
InactiveUS20080064610A1Reduce solubilityImproving immunogenicityVector-based foreign material introductionLibrary creationSolubilityENCODE
Owner:CODON DEVICES
Generating protein pro-drugs using reversible PPG linkages
InactiveUS20050079155A1Reduce and block activityEliminate side effectsPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectProtecting group
The invention relates to methods for modulating the side effects, including immunogenicity, of a protein therapeutic via derivatization with a protein protecting group using reversible or labile linkages.
Owner:XENCOR
Microfluidized oil-in-water emulsions and vaccine compositions
Owner:ZOETIS SERVICE LLC
Method for preparing modified polypeptides
InactiveUS20030165996A1Reduced and increased immunogenicityImprove throughputSugar derivativesMicrobiological testing/measurementHalf-lifeNucleotide sequencing
Methods for producing polypeptide with altered immunogenicity or improved stability properties are disclosed. The methods involve a) expressing a diversified population of nucleotide sequences encoding a polypeptide of interest, b) screening the polypeptides expressed in step a) for function, immunogenicity and / or stability, c) selecting functional polypeptides having altered immunogenicity and / or increased stability, e.g. functional in vivo half-life as compared to the polypeptide of interest, and d) optionally subjecting the nucleotide sequence encoding the polypeptide selected in step c) to one or more repeated cycles of steps a)-c). In a further step the expressed polypeptides of step a) or c) can be conjugated to at least one non-polypeptide moiety.
Owner:MAXYGEN
Regulated attenuation of live vaccines to enhance cross-protective immunogenicity
ActiveUS20060233829A1Improve food safetyImprove abilitiesBacterial antigen ingredientsBacteriaUltrasound attenuationVaccine Immunogenicity
Owner:WASHINGTON UNIV IN SAINT LOUIS
Adeno-associated virus vector for boosting immunogenicity of cells
InactiveUS7001765B2Improving immunogenicityBiocideGenetic material ingredientsAdeno associate virusVaccine Immunogenicity
The present invention provides an Adeno-Associated Virus (AAV) vector having a foreign DNA coding for a protein that boosts immunogenicity of cells. The invention also provides a vaccine containing such a vector and the use of both.
Owner:MEDIGENE
Uniform fluorescent microsphere with hydrophobic surfaces
InactiveUS20080019921A1Maximize plasma half-lifeMinimize interactionUltrasonic/sonic/infrasonic diagnosticsCompounds screening/testingBlood flowBioaccumulation
Fluorescent microspheres for the measurement of blood flow are provided. The microspheres are substantially uniform in diameter and have a hydrophobic surface, which allows them to circulate more freely throughout bloodstream, while reducing immunogenicity, particle aggregation and bioaccumulation. The hydrophobic surface on each microsphere is generally comprised of polymeric material having a limited surface charge.
Owner:LIFE TECH CORP
Stabilizing alkylglycoside compositions and methods thereof
ActiveUS7425542B2Convenient treatmentReduce aggregationBiocidePeptide/protein ingredientsPeptide TDrug biological activity
The present invention relates to alkylglycoside-containing compositions and methods for increasing the stability, reducing the aggregation and immunogenicity, increasing the biological activity, and reducing or preventing fibrillar formation of a peptide, polypeptide, or variant thereof, for example insulin and Peptide T or analog thereof.
Owner:RAPID PHARMA +1
Viruses and virus-like particles for multiple antigen and target display
InactiveUS20060121468A1Improve effectivenessImprove responseSsRNA viruses positive-senseVectorsTissue targetingVaccine Immunogenicity
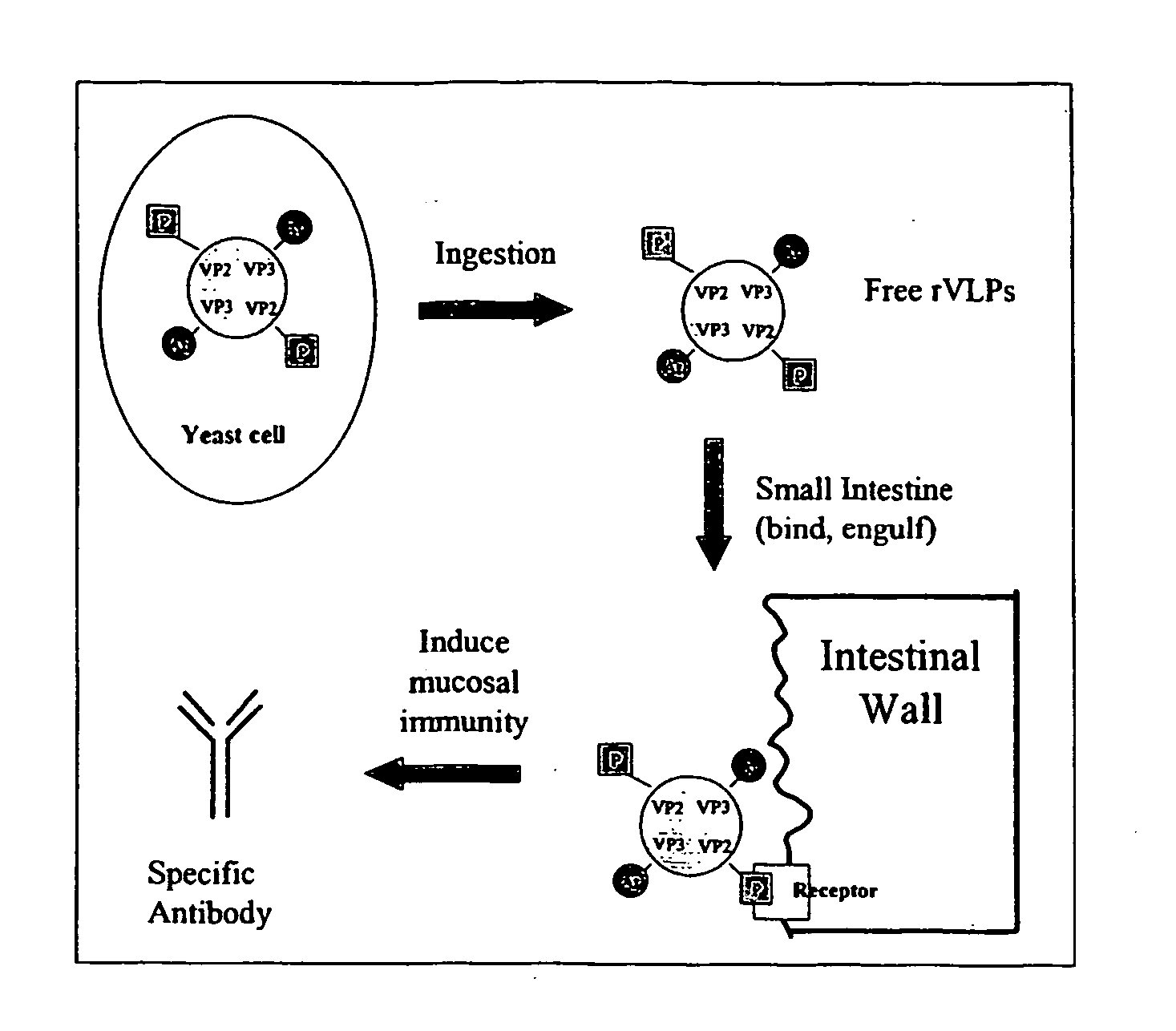
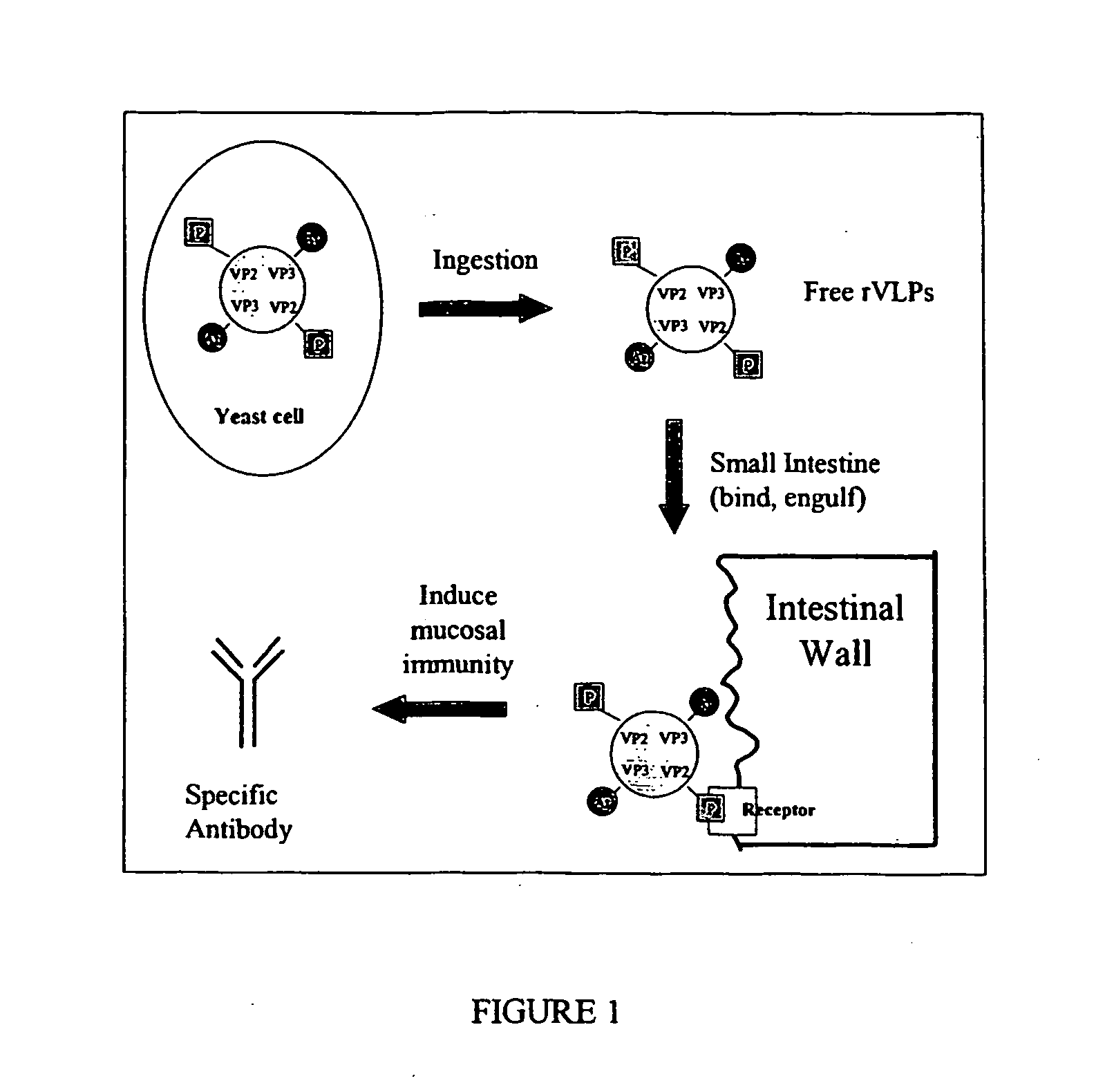
The present invention relates to the display of antigenic or allergenic components along with a tissue-targeting component on viruses or virus-like particles. Capsid protein genes are recombinantly modified to contain the specified components then expressed within a host organism, such as yeasts, bacteria, or algae, and allowed to spontaneously form active virus particles or virus-like particles. The recombinant complexes (virus or virus-like particle) can then be purified or used in situ as a therapeutic tool for disease or allergy prevention. The expression of multivalent and multifunctional components to increase the immunogenicity of the recombinant complexes, especially on oral administration, is provided.
Owner:ADVANCED BIONUTRITION CORP
Compositions of prokaryotic phenylalanine ammonia-lyase and methods of treating cancer using compositions thereof
ActiveUS7537923B2High catalytic activityImprove stabilityNervous disorderPeptide/protein ingredientsWild typeCancer therapy
The present invention is directed to phenylalanine ammonia-lyase (PAL) variants produced by prokaryotes, wherein such prokaryotic PAL variant has a greater phenylalanine-converting activity and / or a reduced immunogenicity as compared to a wild-type PAL. The invention provides compositions of prokaryotic PAL and biologically active fragments, mutants, variants or analogs thereof, as well as methods for the production, purification, and use of such compositions for therapeutic purposes, including the treatment of cancer.
Owner:BIOMARIN PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com