Patents
Literature
230results about How to "Limit delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
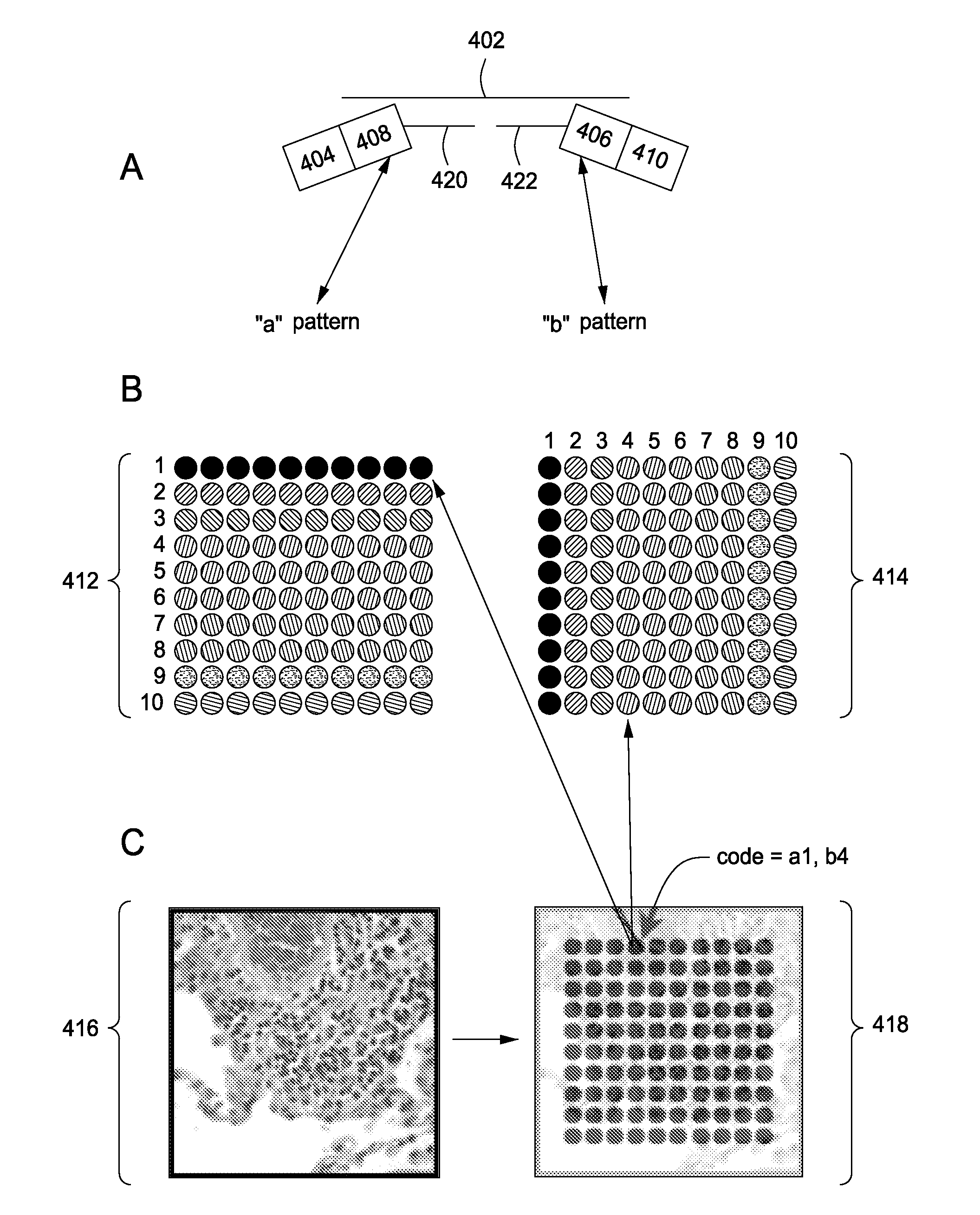
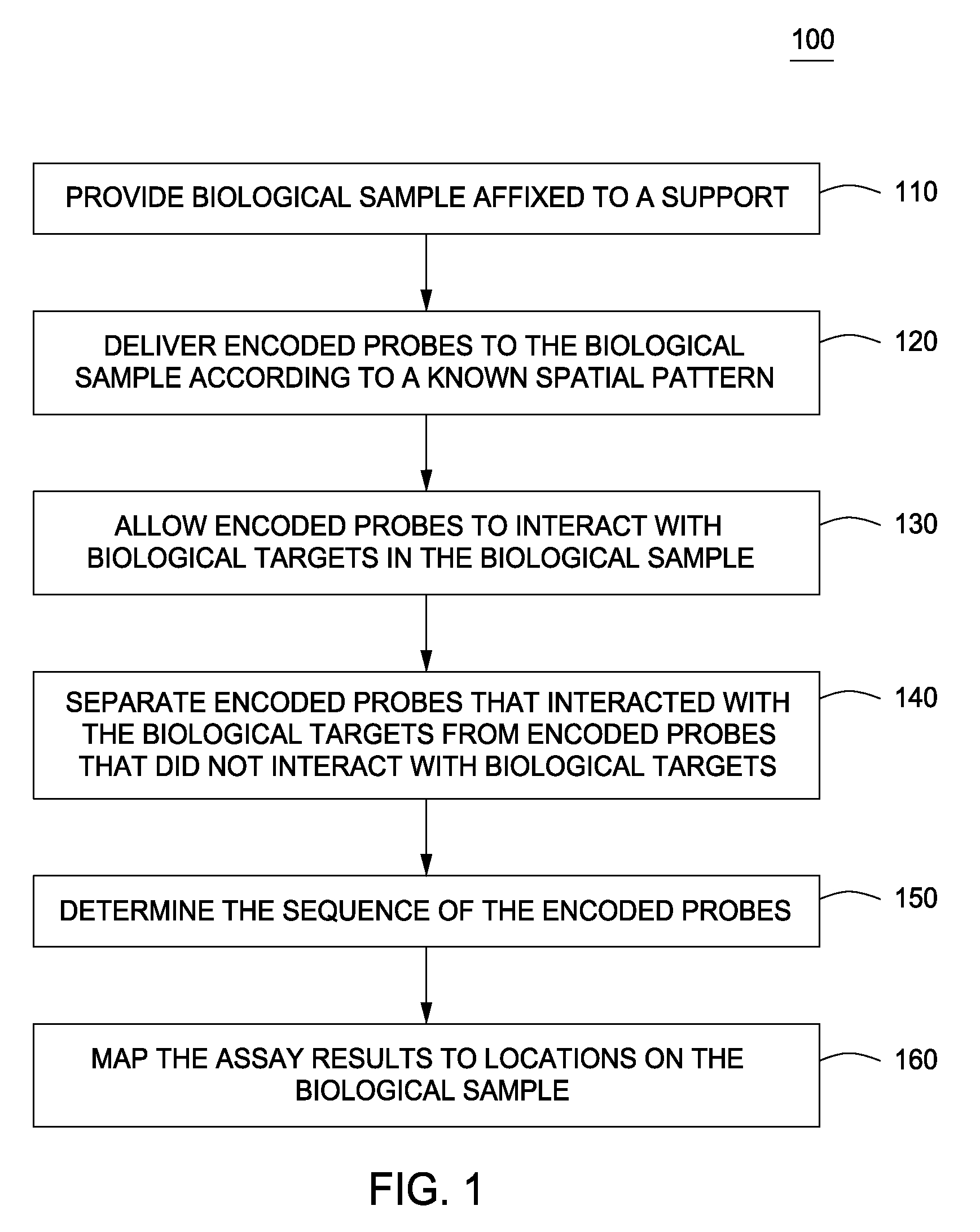
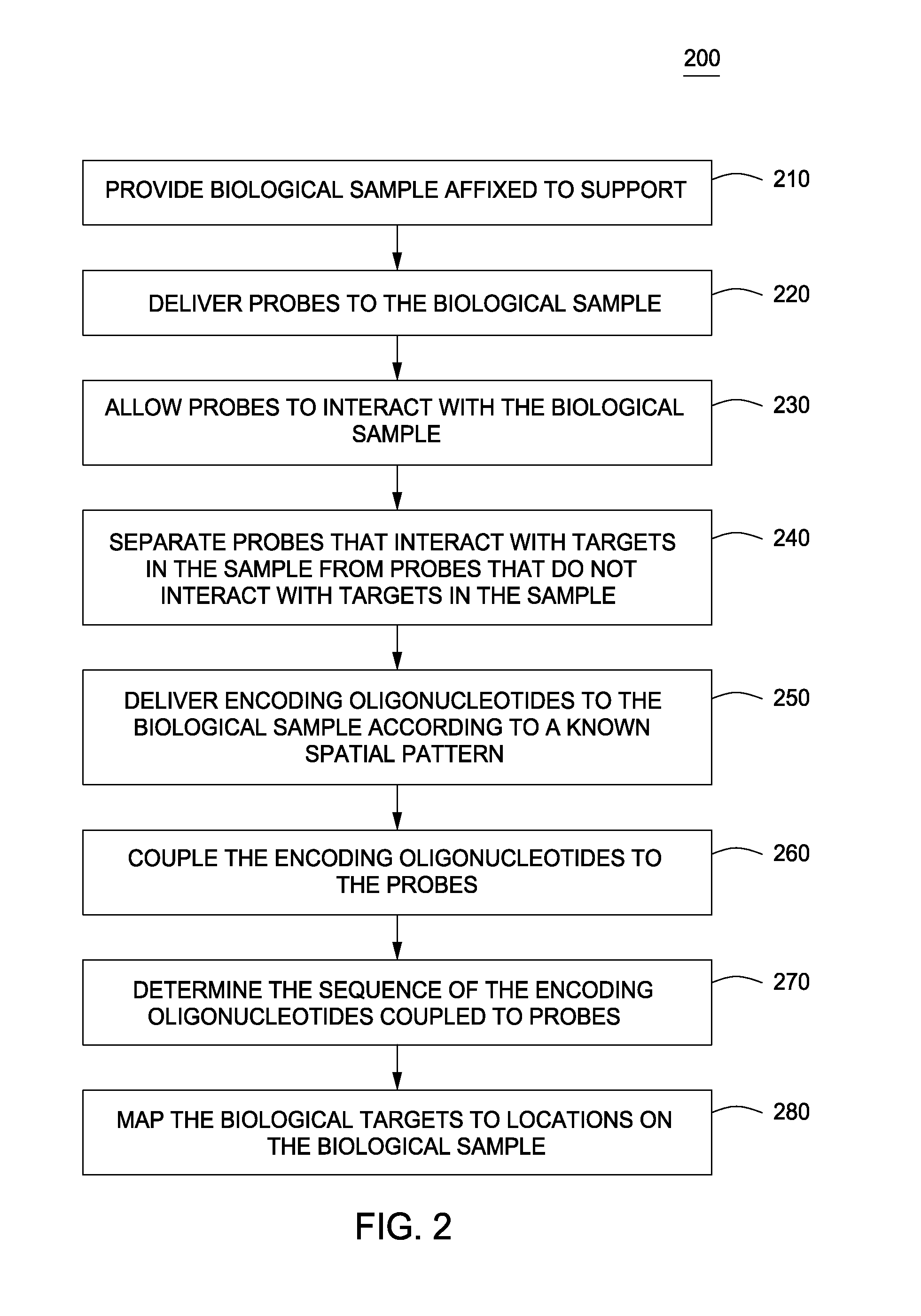
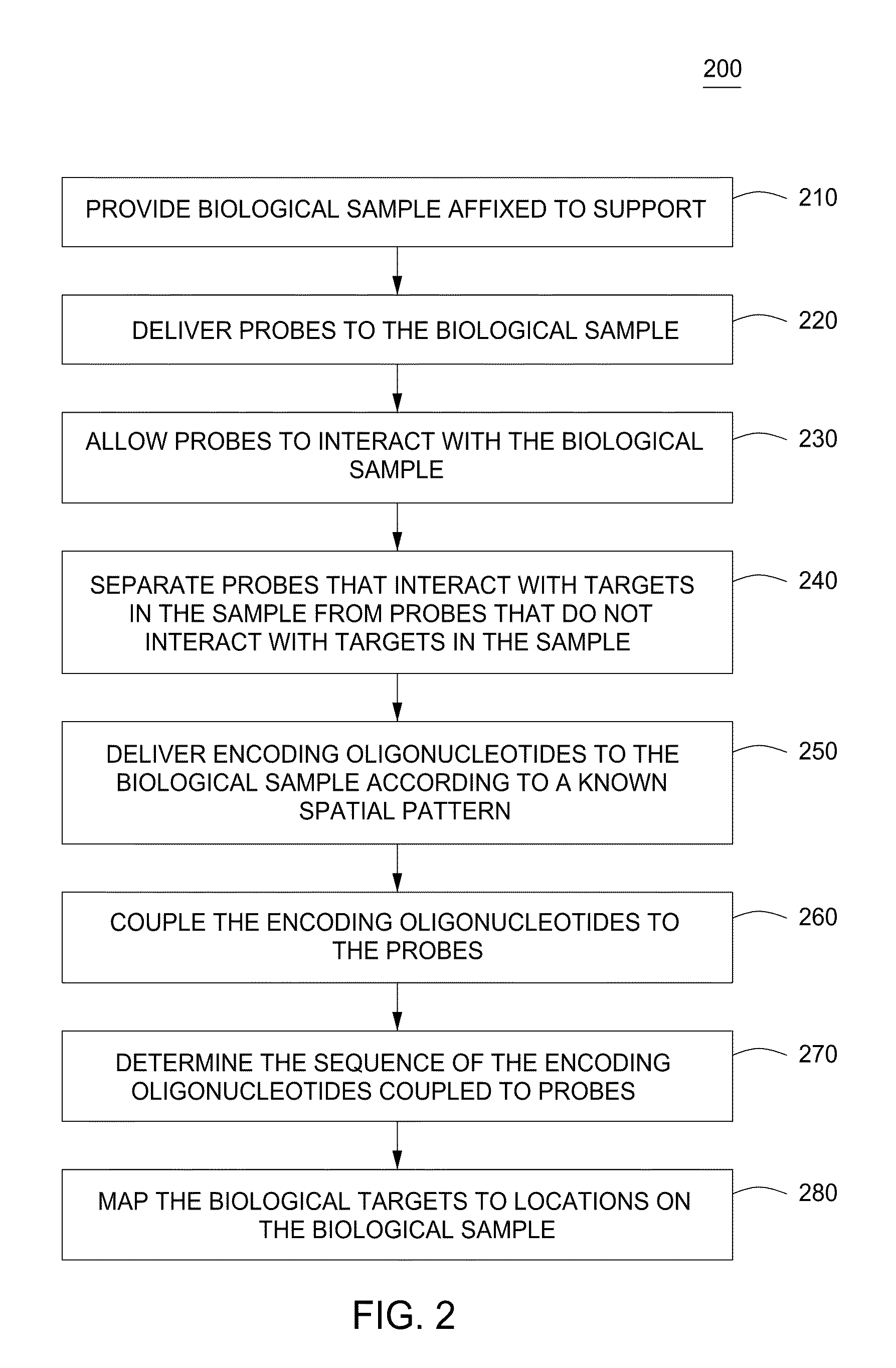
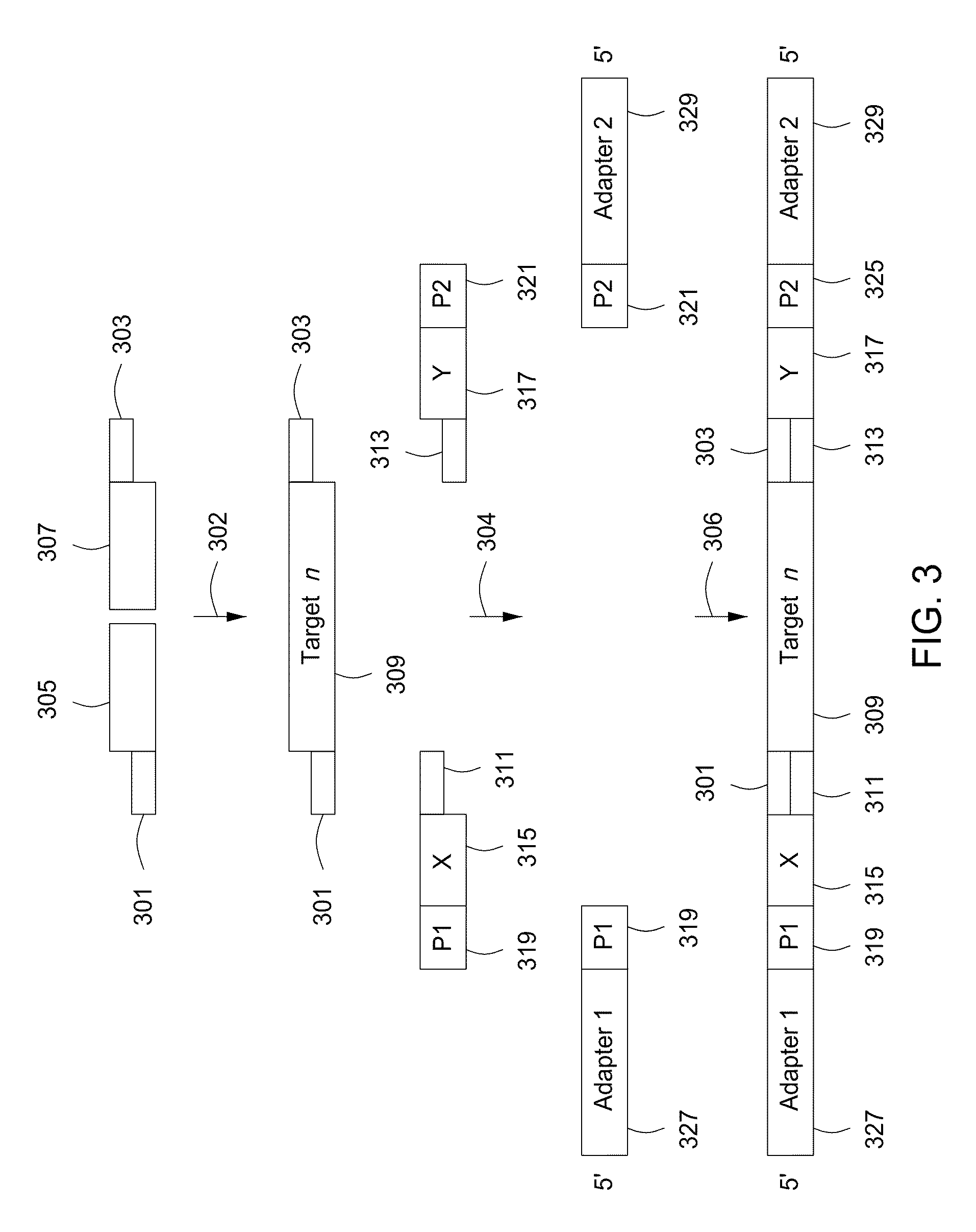
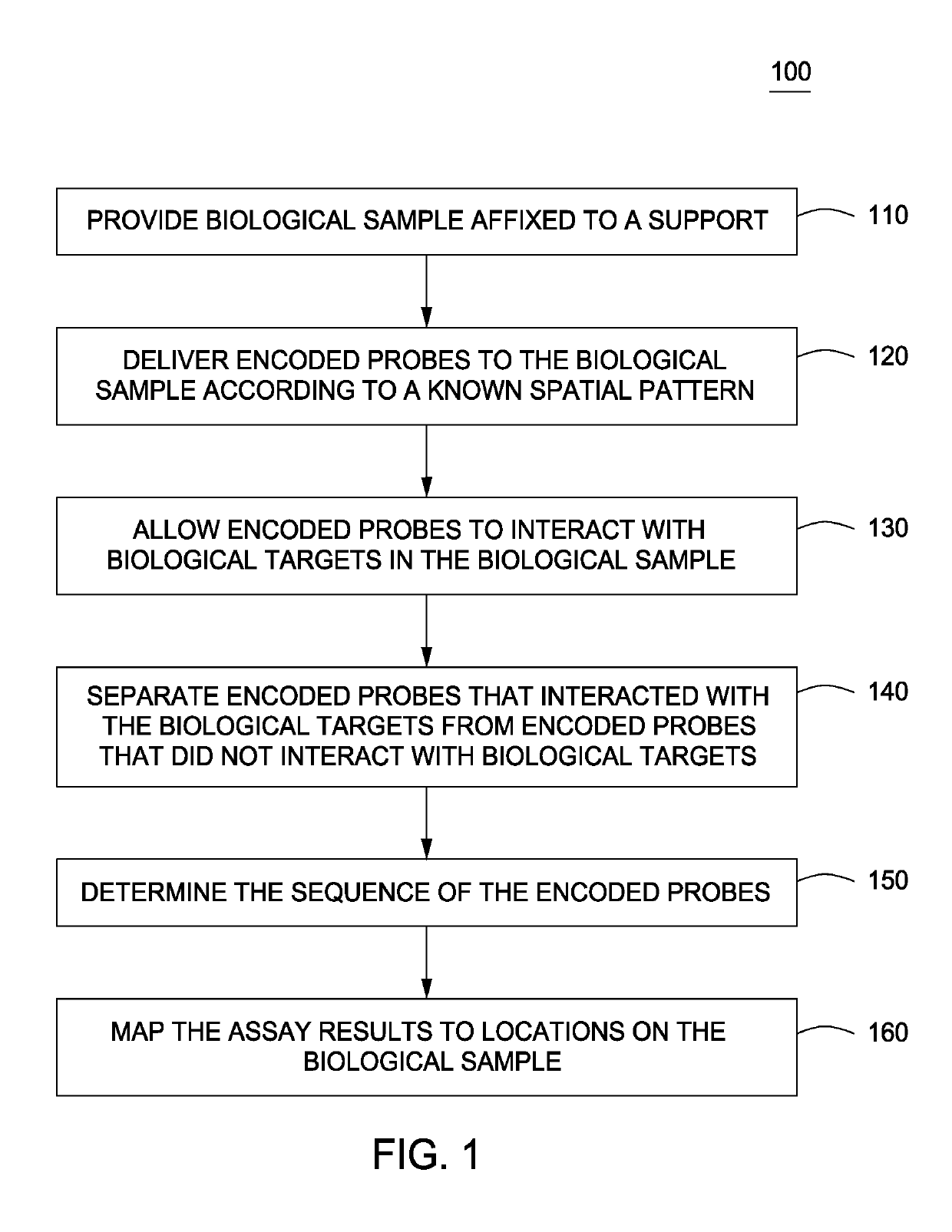
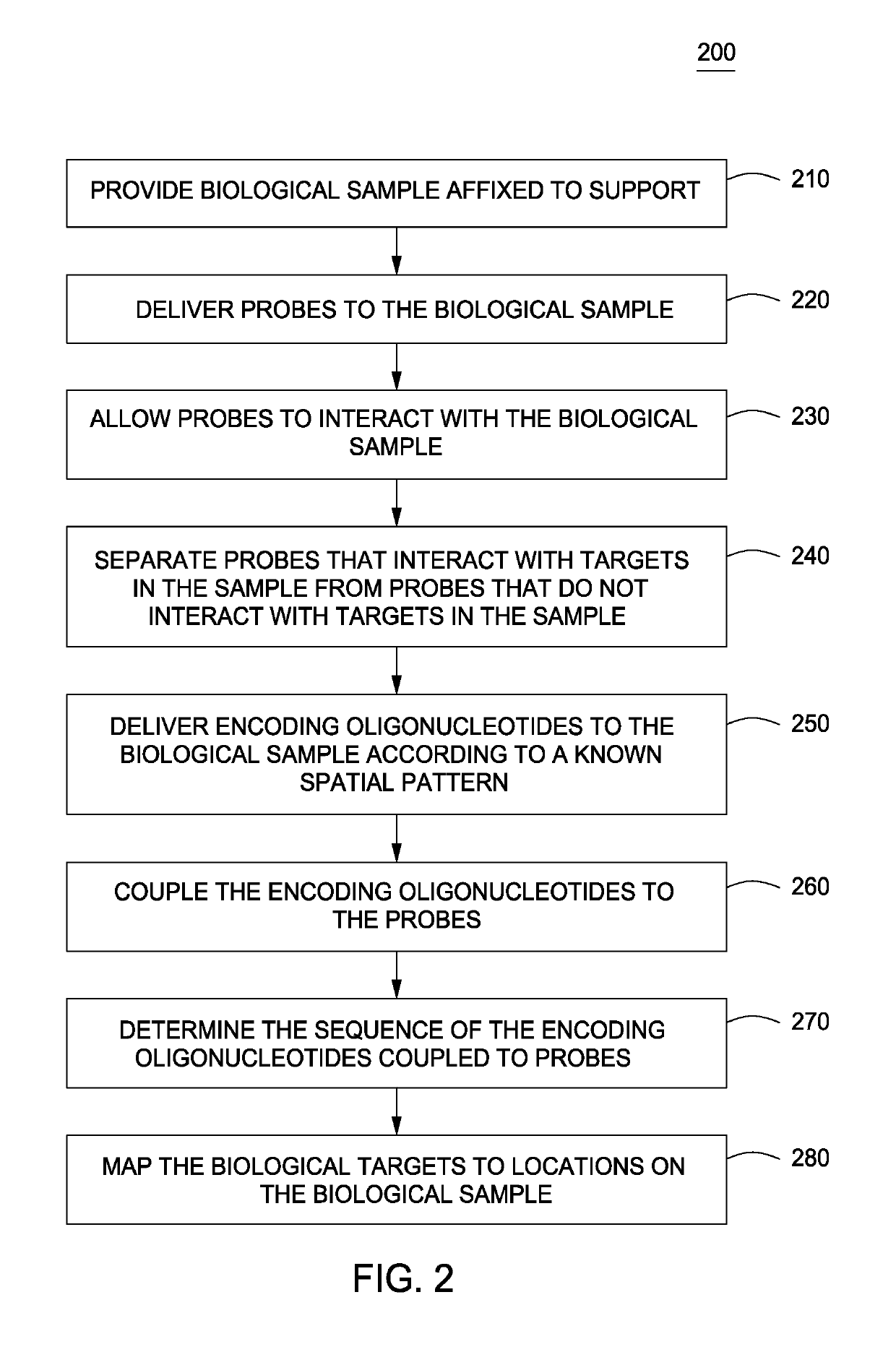
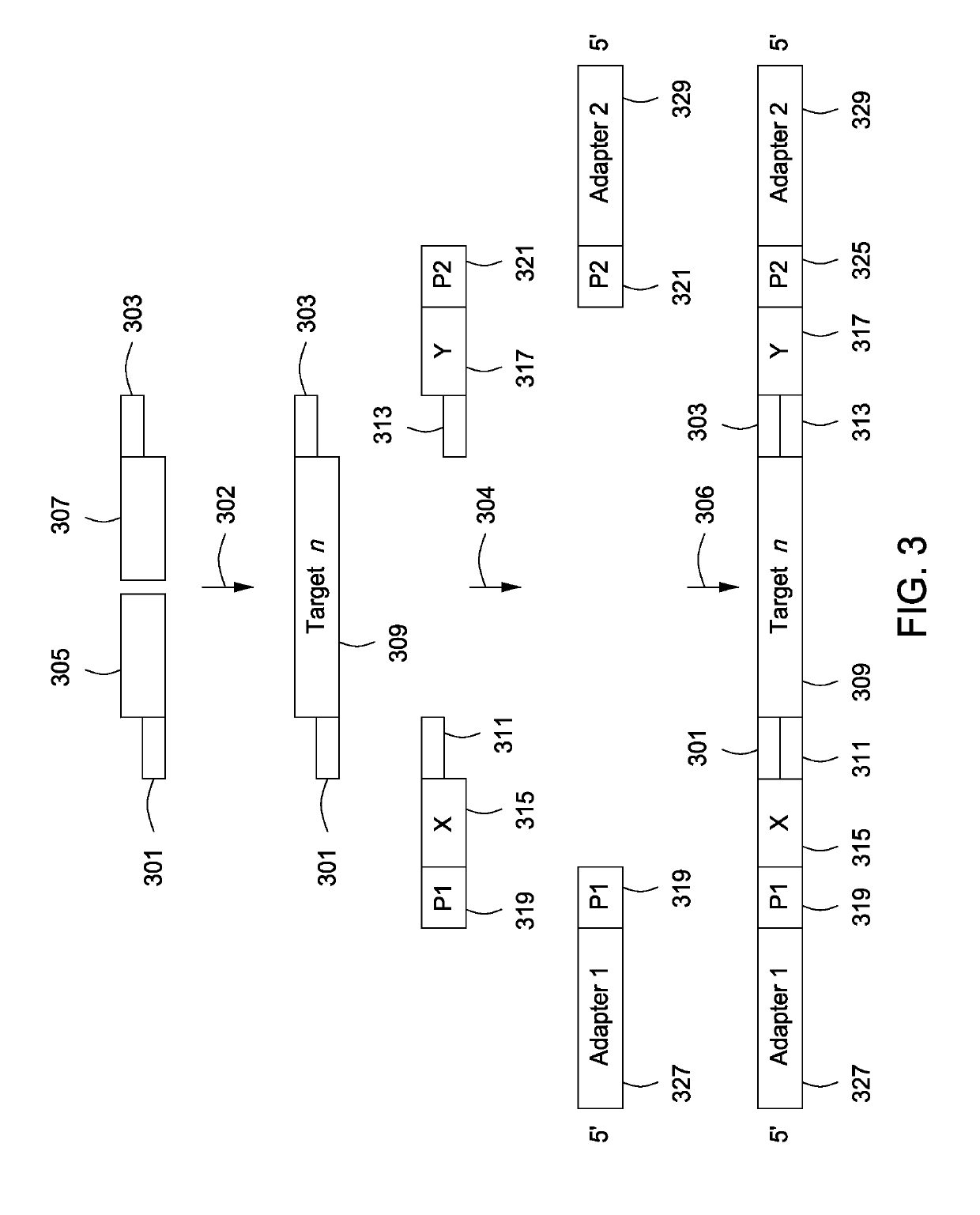
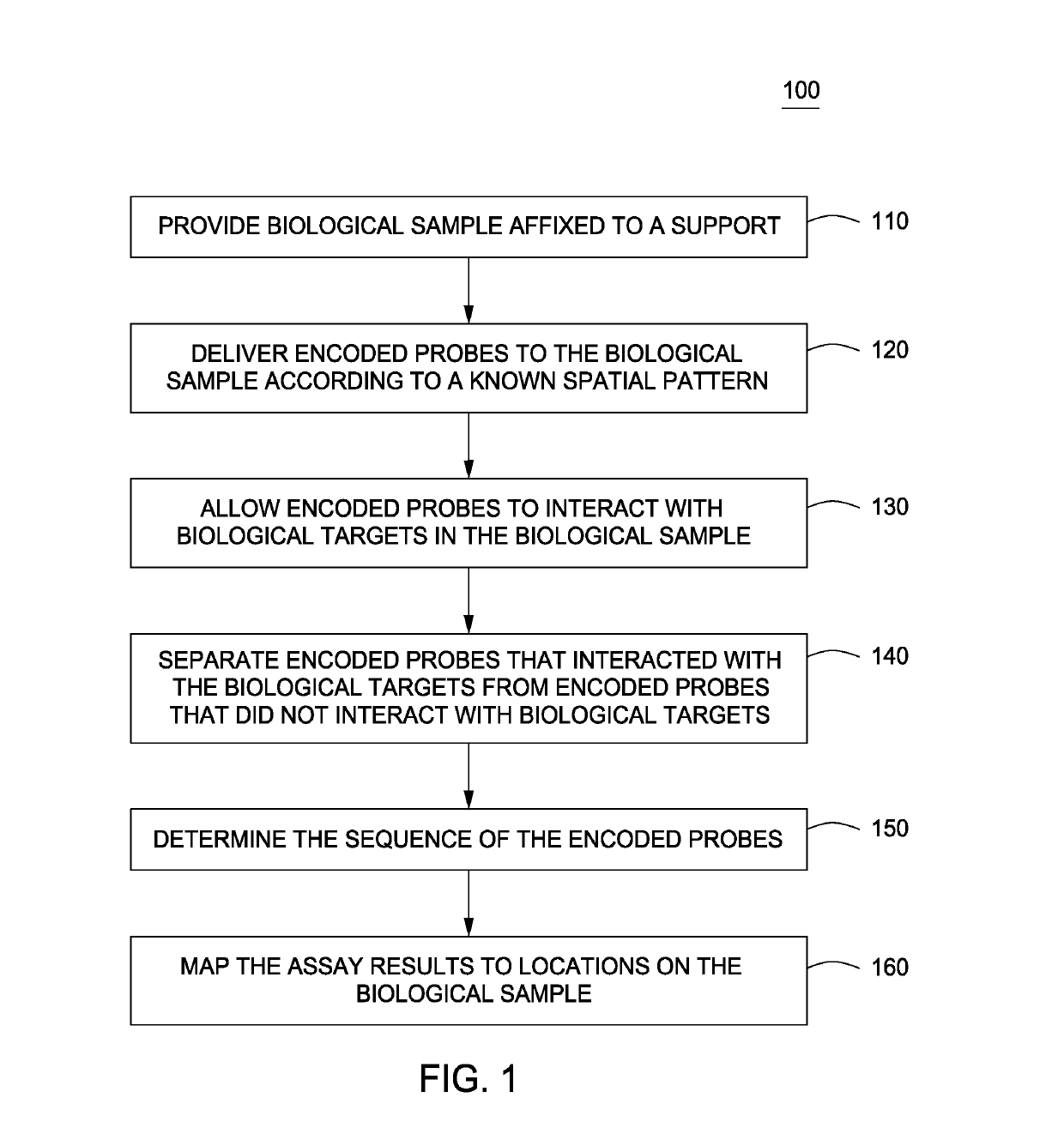
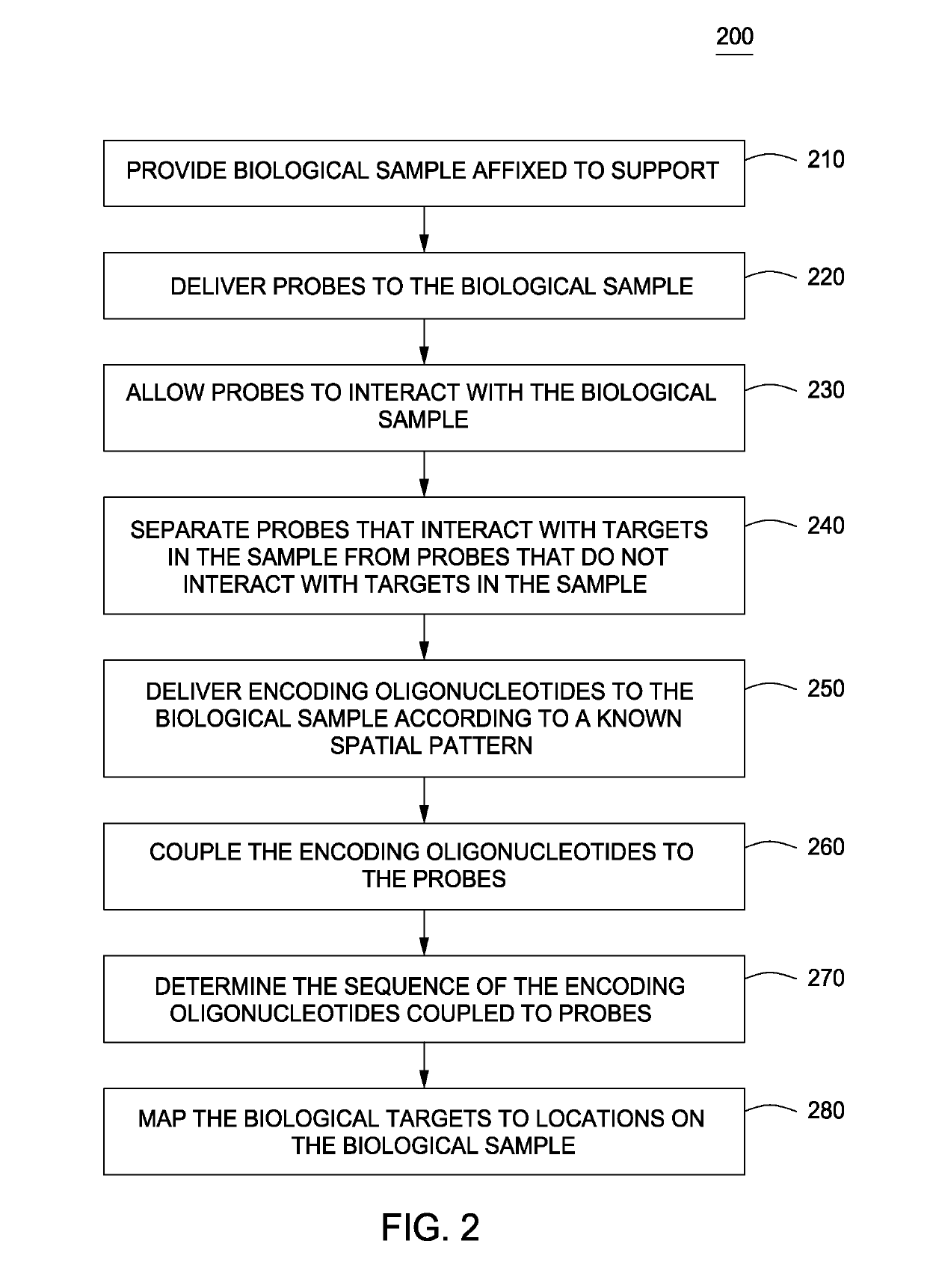
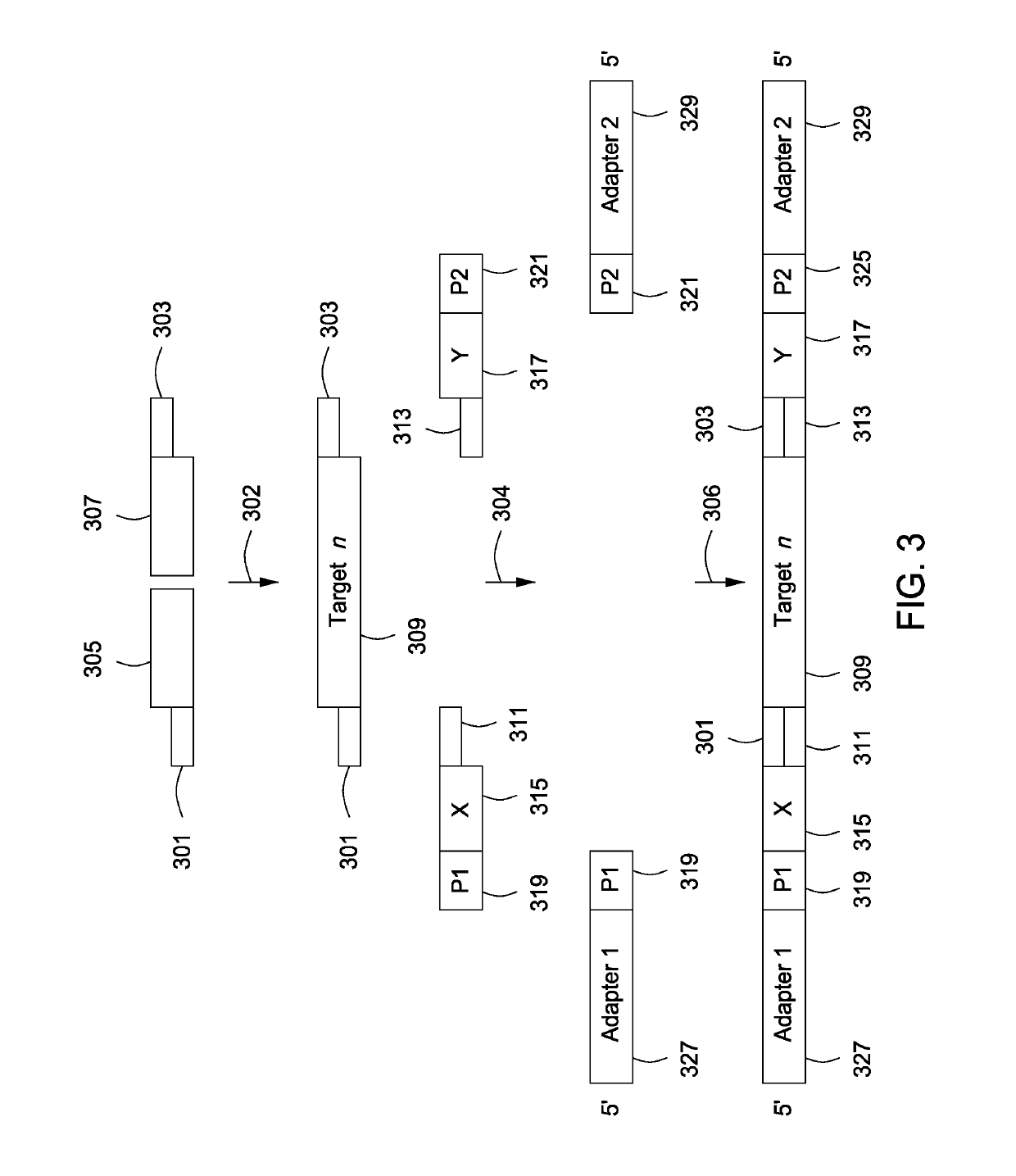
Spatially encoded biological assays
ActiveUS20110245111A1Improve the level ofLimit deliveryMicrobiological testing/measurementLibrary screeningReagentControlled delivery
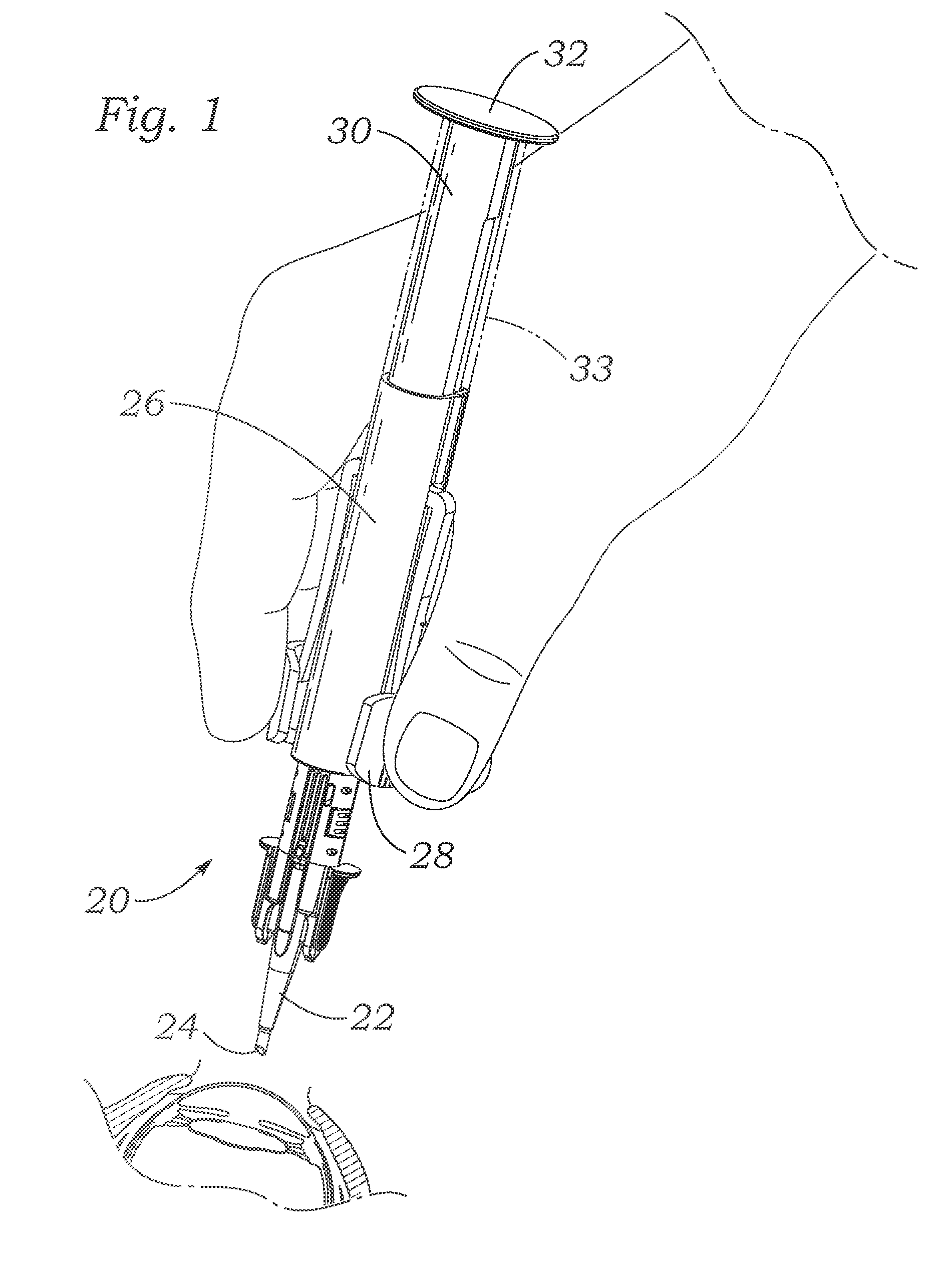
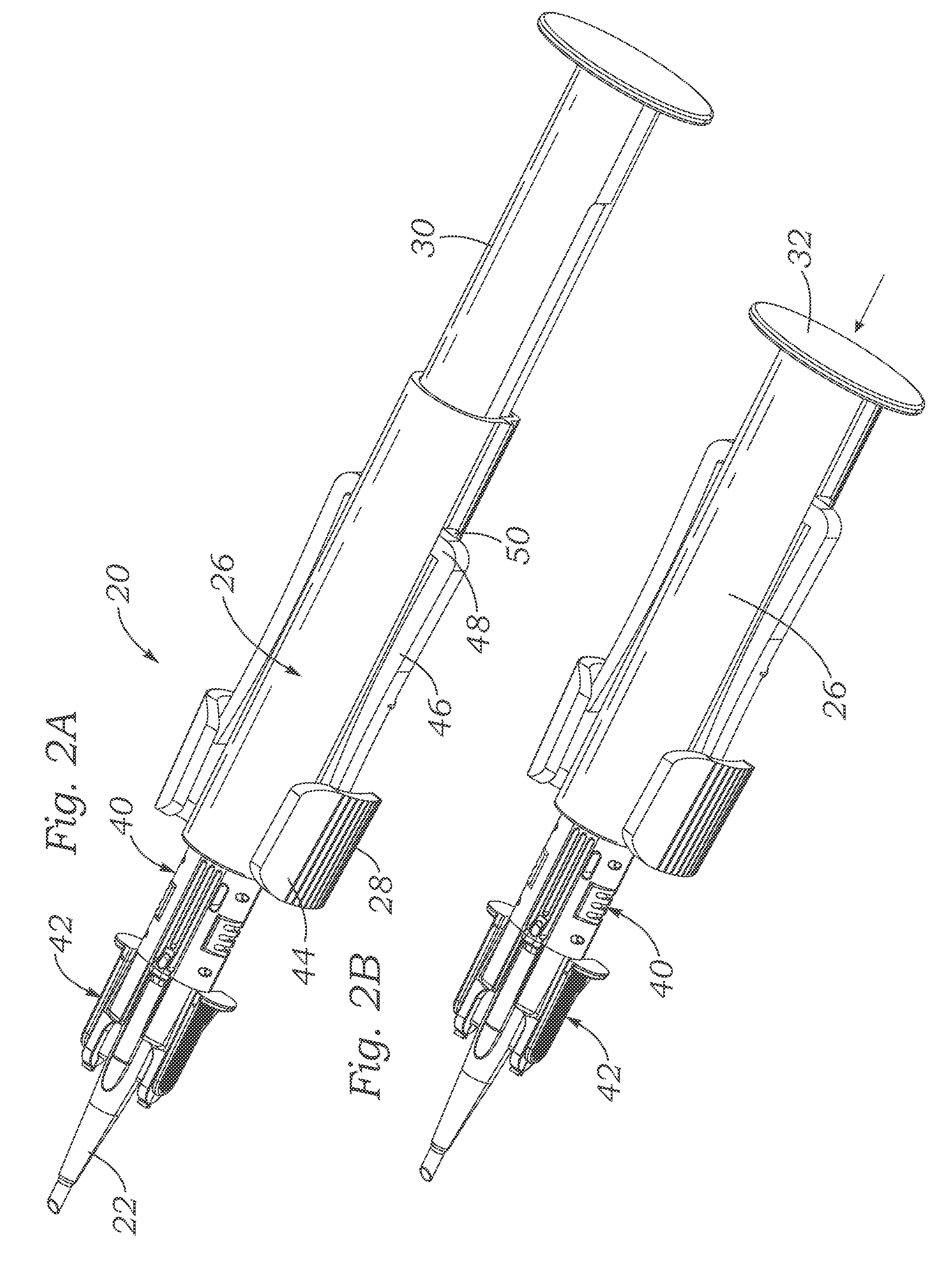
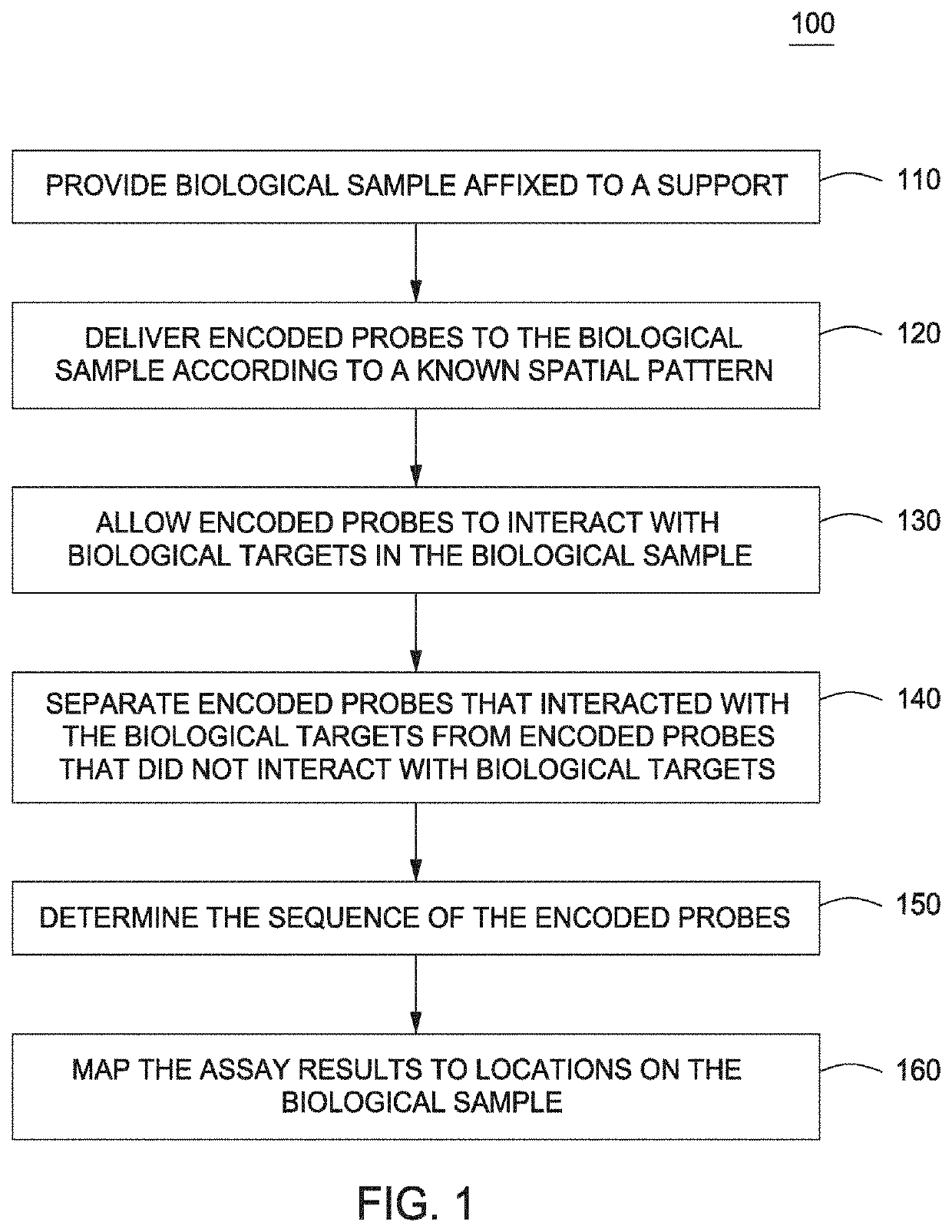
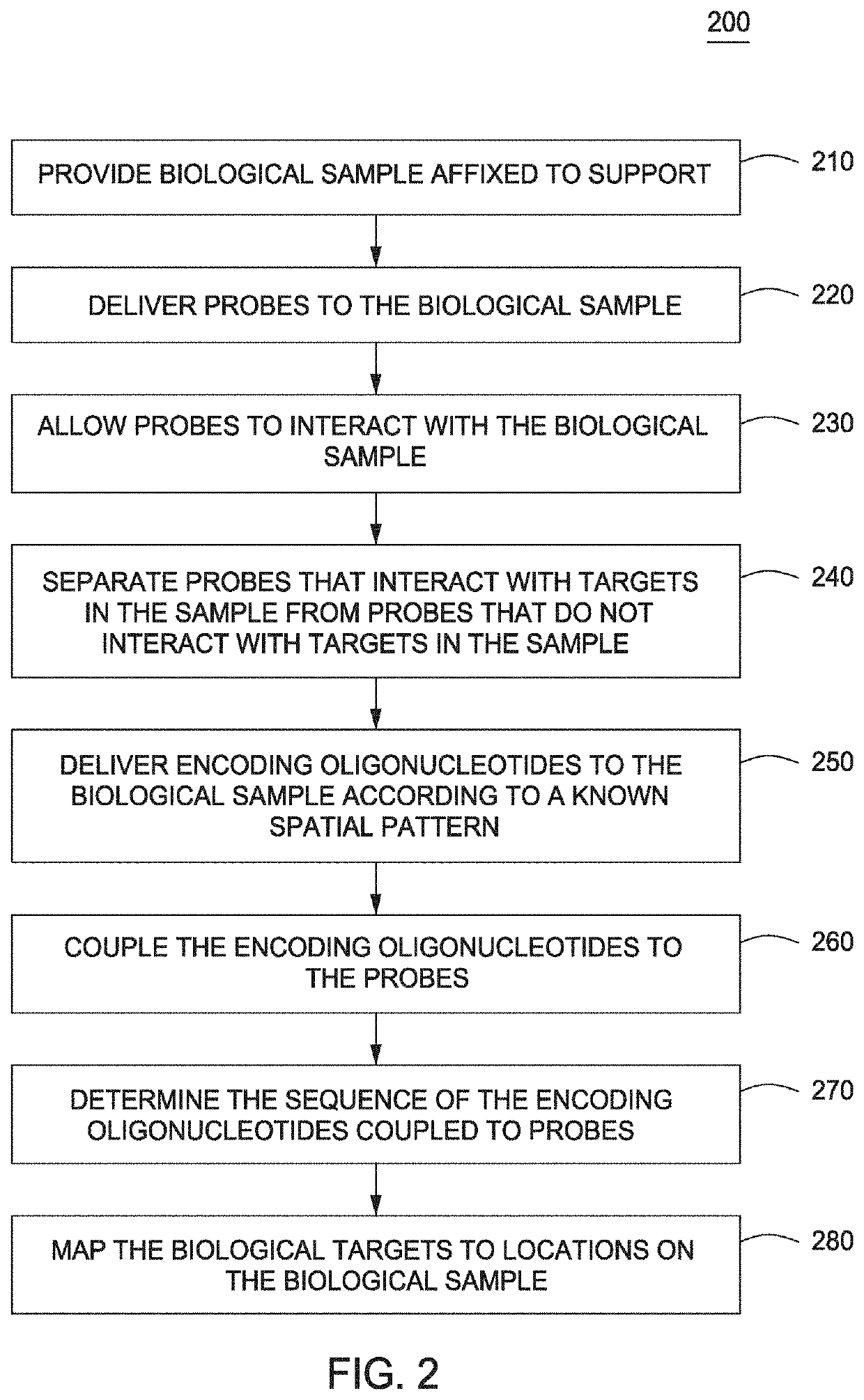
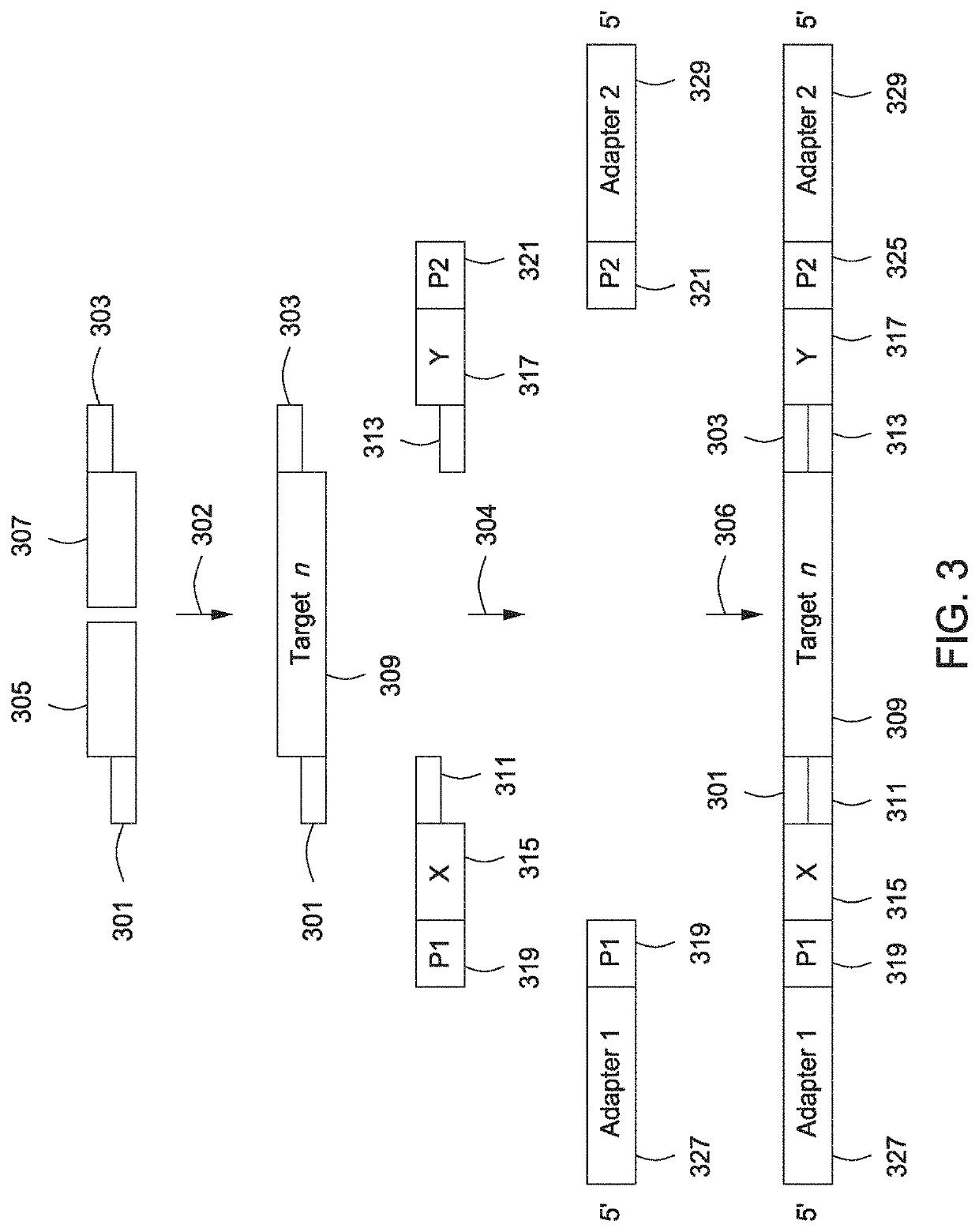
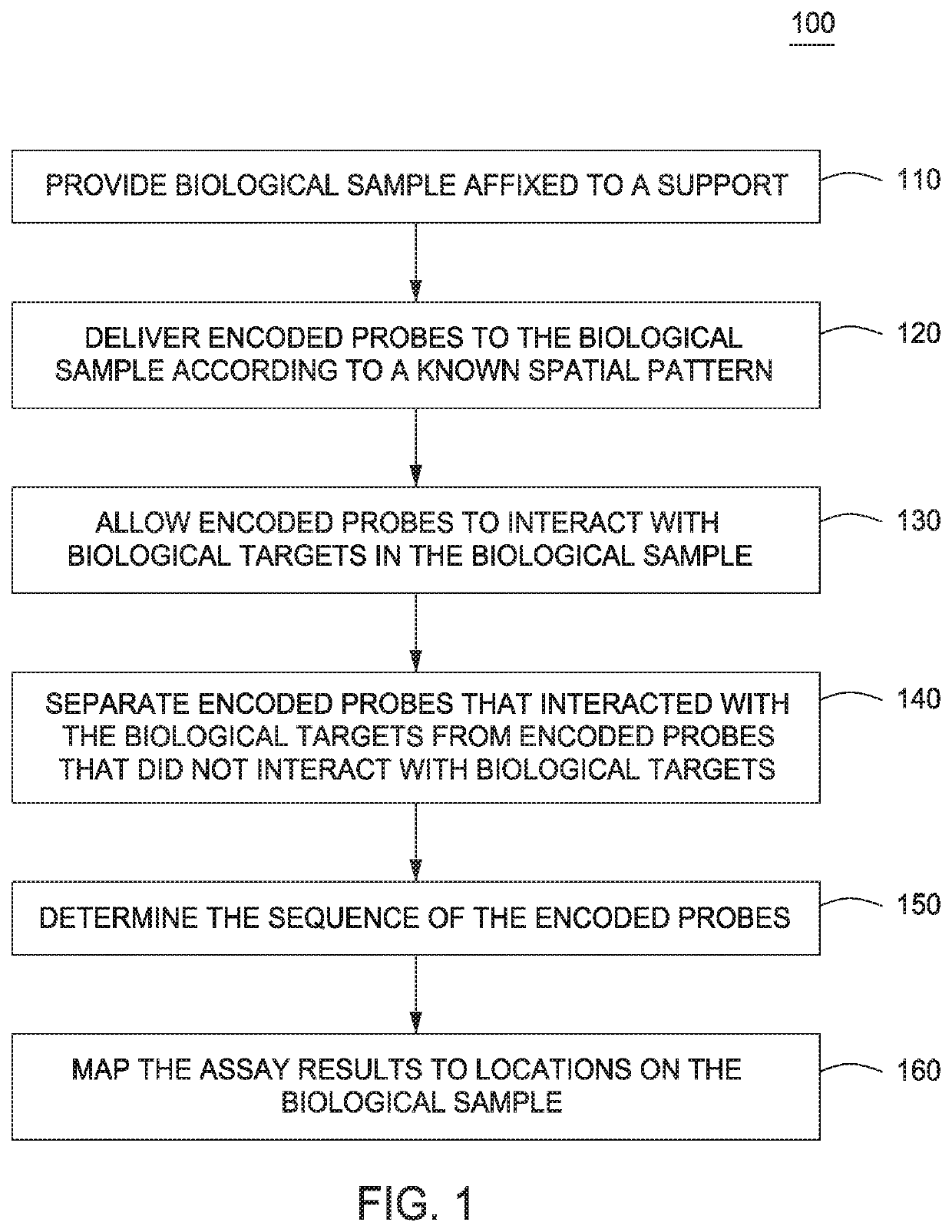
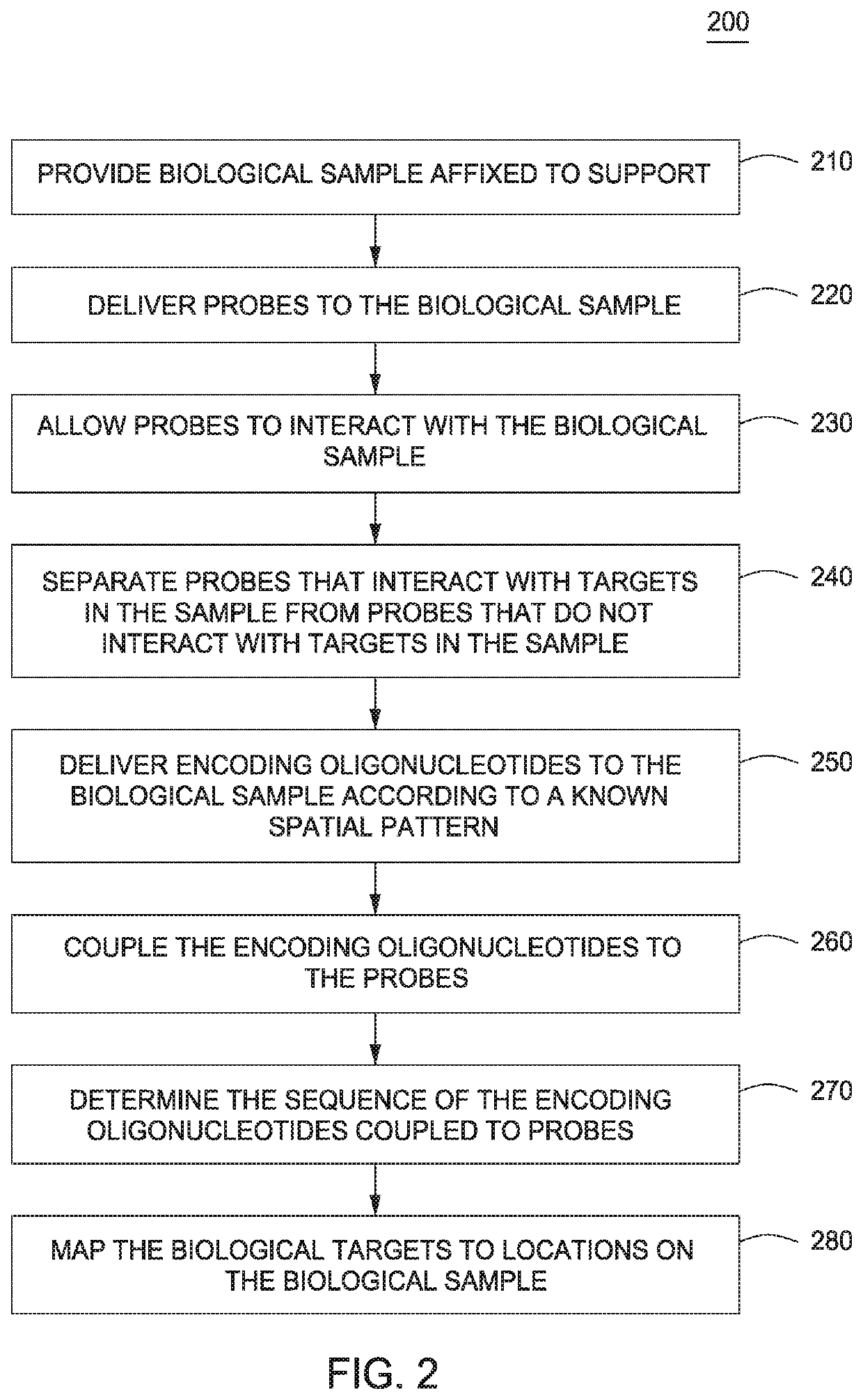
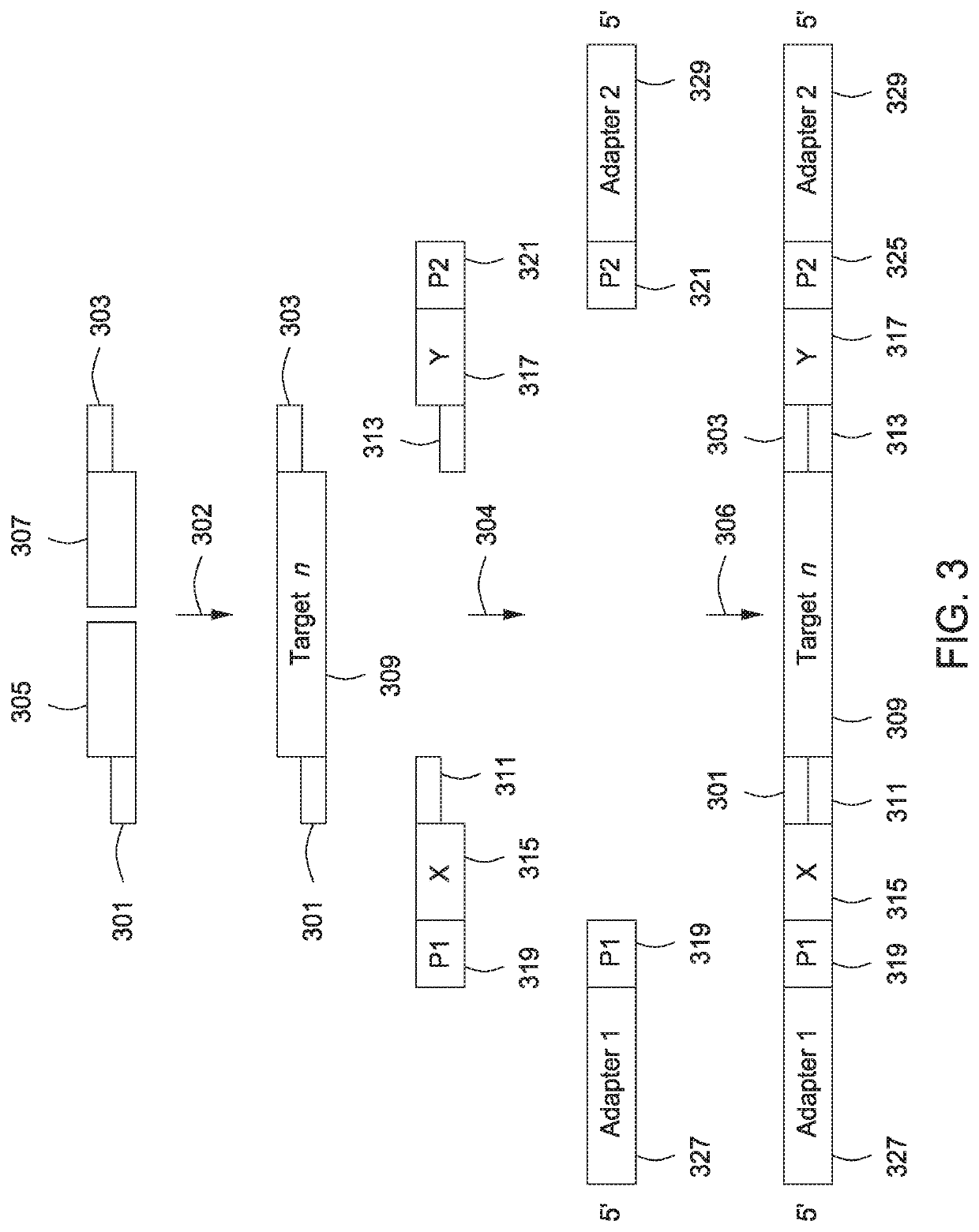
The present invention provides assays and assay systems for use in spatially encoded biological assays. The invention provides an assay system comprising an assay capable of high levels of multiplexing where reagents are provided to a biological sample in defined spatial patterns; instrumentation capable of controlled delivery of reagents according to the spatial patterns; and a decoding scheme providing a readout that is digital in nature.
Owner:PROGNOSYS BIOSCI
Spatially encoded biological assays
ActiveUS9371598B2Improve the level ofLimit deliveryMicrobiological testing/measurementLibrary screeningReagentInstrumentation
The present invention provides assays and assay systems for use in spatially encoded biological assays. The invention provides an assay system comprising an assay capable of high levels of multiplexing where reagents are provided to a biological sample in defined spatial patterns; instrumentation capable of controlled delivery of reagents according to the spatial patterns; and a decoding scheme providing a readout that is digital in nature.
Owner:PROGNOSYS BIOSCI
Battery recharge management for an implantable medical device
InactiveUS7167756B1Limit deliveryBatteries circuit arrangementsElectromagnetic wave systemMeasurement deviceElectrical battery
An implantable medical device having an implantable power source such as a rechargeable lithium ion battery. The implantable medical device includes a recharge module that regulates the recharging process of the implantable power source using closed-loop feedback control. The recharge module includes a recharge regulator, a recharge measurement device monitoring at least one recharge parameter, and a recharge regulation control unit for regulating the recharge energy delivered to the power source in response to the recharge measurement device. The recharge module adjusts the energy provided to the power source to ensure that the power source is being recharged under safe levels.
Owner:MEDTRONIC INC
Ablation in the Gastrointestinal Tract to Achieve Hemostasis and Eradicate Lesions With a Propensity for Bleeding
ActiveUS20090012513A1High failure rateReduced blood flowDilatorsSurgical instruments for heatingArteriovenous malformationGastric antral vascular ectasia
Devices and methods are provided for the ablation of regions of the digestive tract to achieve hemostasis and to eradicate chronically bleeding lesions as occur with gastric antral vascular ectasia (GAVE), portal hypertensive gastropathy (PHG), radiation proctopathy and colopathy, arteriovenous malformations, and angiodysplasia. Ablation is typically provided in a wide-field manner, and in conjunction with sufficient pressure to achieve coaptive coagulation. Ablation, as provided the invention, starts at the mucosa and penetrates deeper into the gastrointestinal wall in a controlled manner. Ablation control may be exerted by way of electrode design and size, energy density, power density, number of applications, pattern of applications, and pressure. Control may also be provided by a fractional ablation that ablates some tissue within a target region and leaves a portion substantially unaffected. Embodiments of the device include an ablational electrode array that spans 360 degrees and an array that spans an arc of less than 360 degrees.
Owner:TYCO HEALTHCARE GRP LP
Complement pathway modulators and uses thereof
ActiveUS20120295884A1Inhibition of catalytic activityImprove throughputAntibacterial agentsBiocideMedicineActive agent
Owner:NOVARTIS AG
Angle indexer for medical devices
InactiveUS6926713B2Reduce deliveryDelivery of ablation energy may be more controlledDiagnosticsSurgical needlesMedical deviceBiomedical engineering
Ablation device for creating a lesion within tissue includes an angle indexing apparatus. The angle indexing apparatus includes an index-key and an indexer. The index-key is secured to the ablation device. The indexer is secured in a position relative to which the index-key can move. The index-key is adapted to mate with the indexer in a plurality of positions, thereby allowing operation of the ablation device in a plurality of orientations.
Owner:BOSTON SCI SCIMED INC
Inhibitors of the 11-beta-hydroxysteroid dehydrogenase type 1 enzyme
ActiveUS20060149070A1Improve throughputLimit deliveryOrganic active ingredientsOrganic chemistryInsulin dependentEnzyme inhibitor
The present invention relates to compounds that are inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme. The present invention further relates to the use of inhibitors of 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme for the treatment of non-insulin dependent type 2 diabetes, insulin resistance, obesity, lipid disorders, metabolic syndrome and other diseases and conditions that are mediated by excessive glucocorticoid action.
Owner:ABBVIE INC
Rupturing controlled release device having a preformed passageway
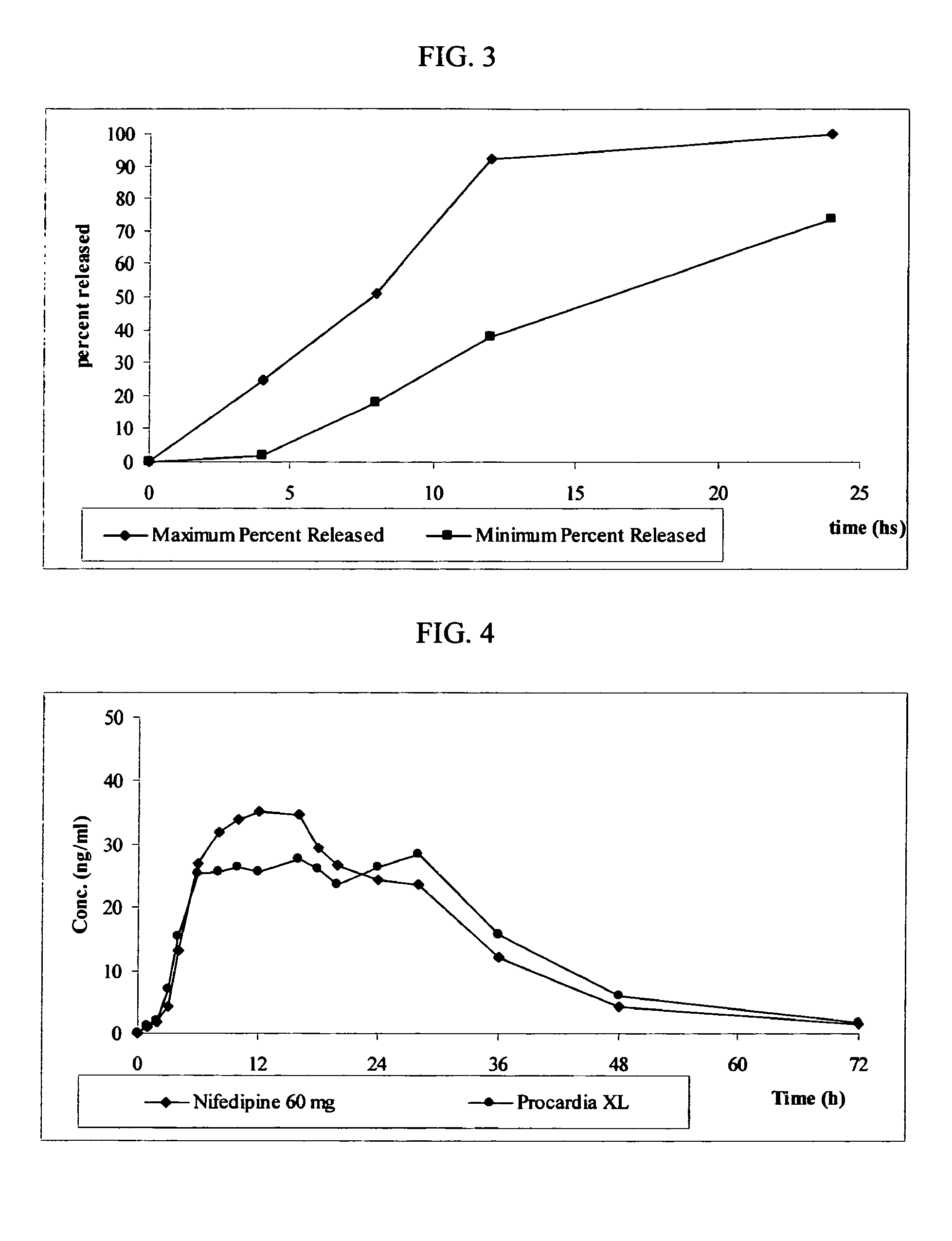
ActiveUS20050008702A1Increase release rateLarge particle sizePill deliveryCapsule deliveryControlled releaseMedicine
The present invention provides a simple and improved osmotic device that is capable of providing a controlled release of active agent contained in the core first through a preformed passageway and then through an in situ formed second passageway into an environment of use. One or both of the passageways optionally increases in size during use of the osmotic device. The preformed passageway and / or the second passageway increase the release rate of the active agent, enable the release of large particles containing active agent, and / or enable the release of active agents that are substantially insoluble in the environment of use. By virtue of the in situ formation of the second aperture, the device is able to release a greater overall percentage of active agent than it would release in absence of the second aperture.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Acoustic applicators for controlled thermal modification of tissue
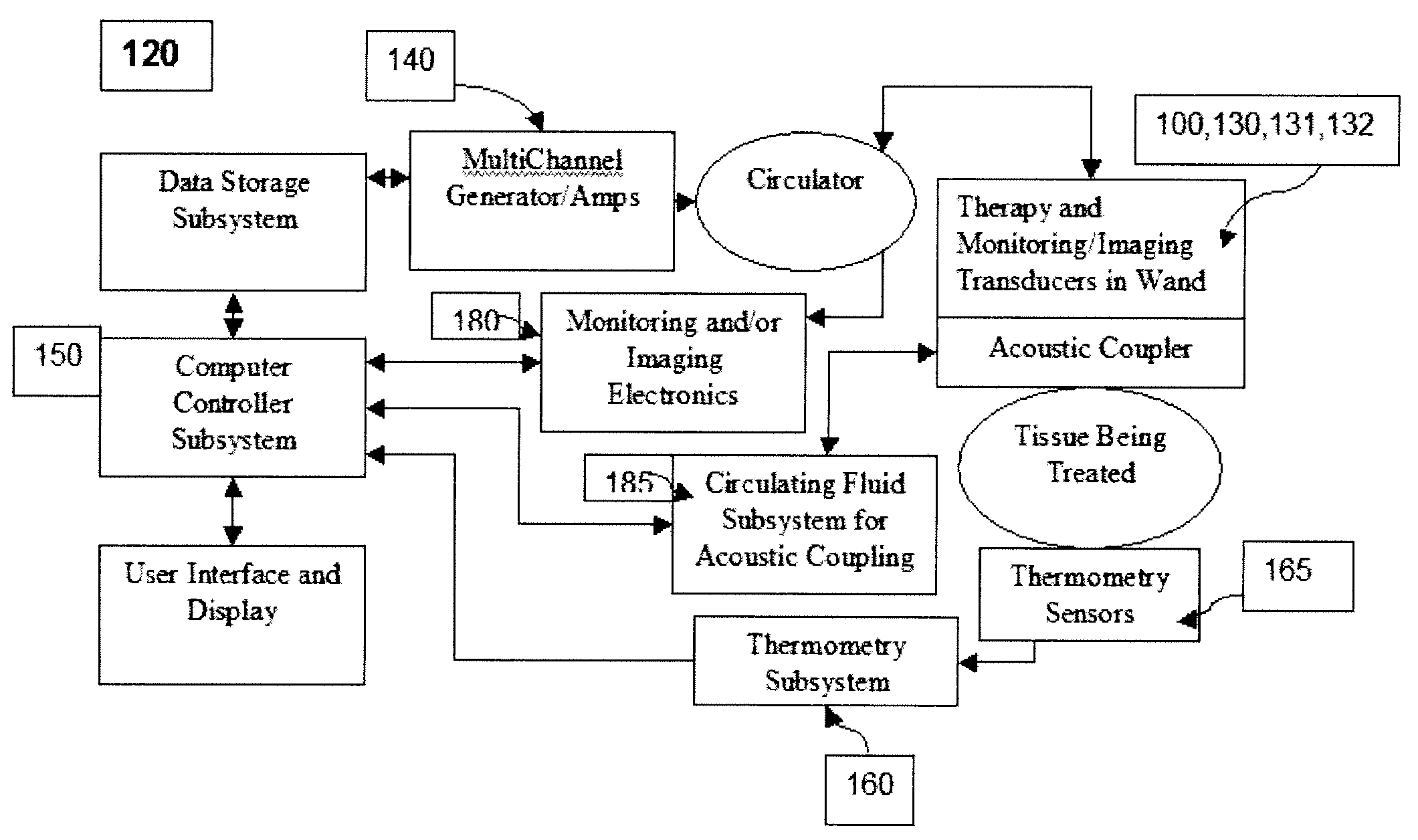
ActiveUS20080255478A1Limit deliveryShorten treatment timeUltrasound therapySurgeryMedicineTransducer
An apparatus and method for modifying collagen containing dermal tissue. The apparatus includes a source of ultrasound energy comprised of a plurality of curvilinear ultrasound transducers shaped to direct ultrasound energy to selected skin depths. The transducers have variable curvature, drive frequency, power level and geometries to effect precise control of collagen modification.
Owner:ACOUSTIC MEDSYST
Detection and protection of devices within a wireless power system
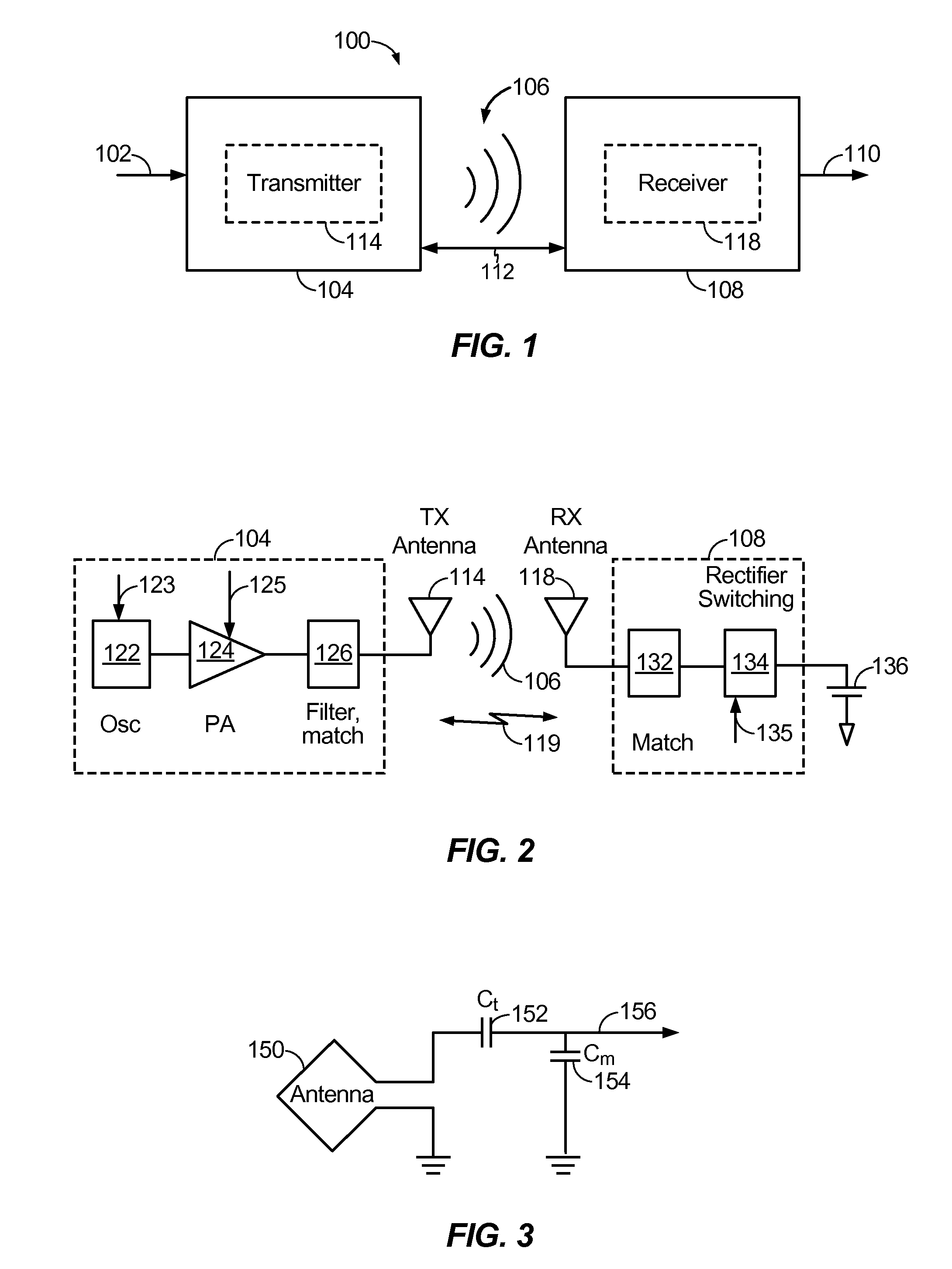
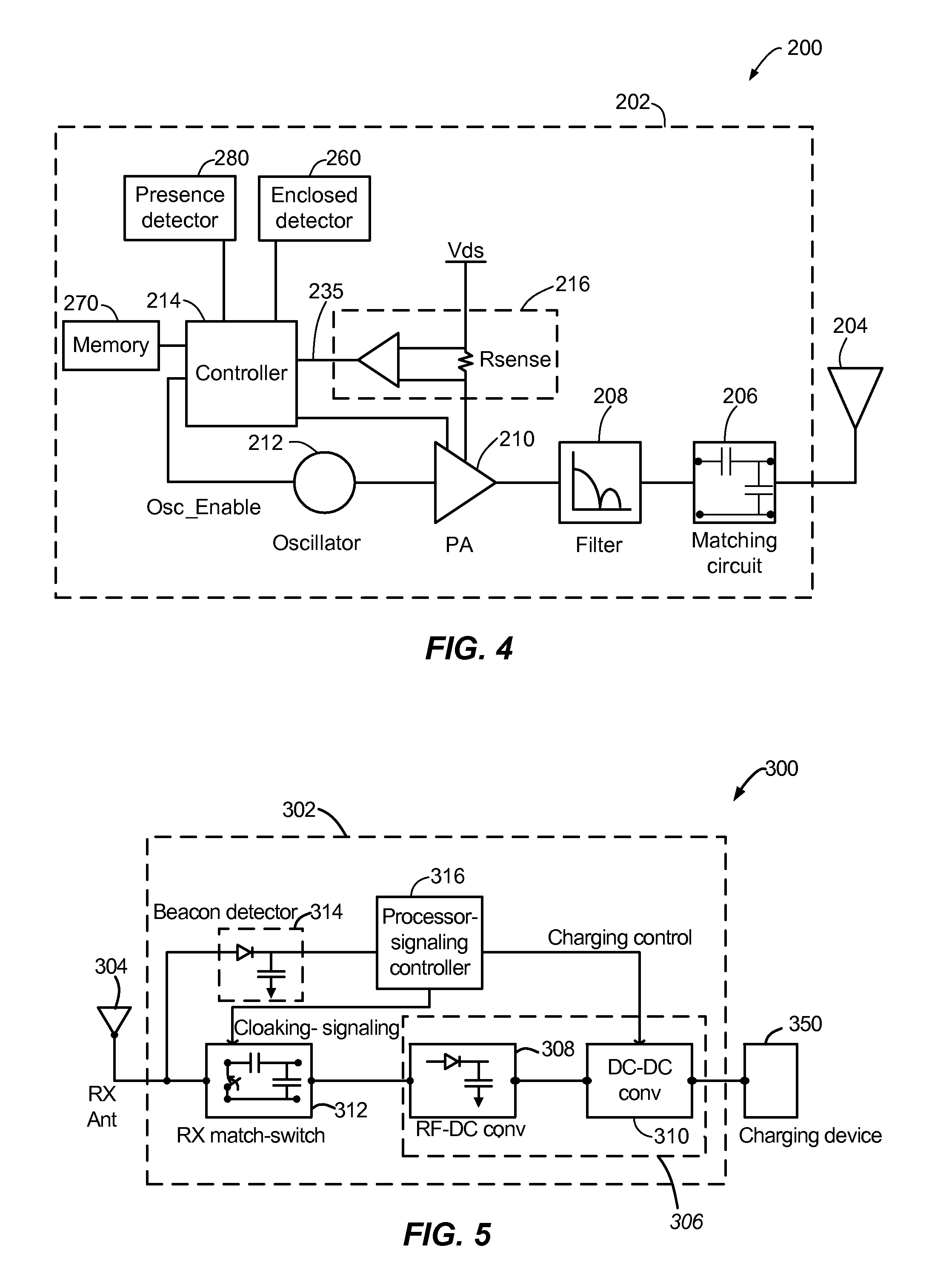
ActiveUS20110221388A1Limit deliveryNear-field transmissionElectric powerElectric power systemEngineering
Exemplary embodiments are directed to detecting and limiting power transfer to non-compliant devices. A method may include detecting one or more non-compliant devices positioned within a charging region of a wireless power transmitter. The method may further include limiting an amount of power delivered to at least one of the one or more non-compliant devices.
Owner:QUALCOMM INC
Fat accumulation-modulation compounds
InactiveUS20030144350A1Decreasing level of acid and triglyceride accumulationReducing weight gainBiocideMetabolism disorderDrugLine of therapy
The present invention pertains to compounds effective at modulating fatty acid or triglyceride ("fat") accumulation by cells, such compounds having therapeutic potential as regulators of body mass and for the treatment of overweight individuals, obesity, and metabolic disorders. Featured compounds are set forth and exemplified herein. Therapeutic methods and pharmaceutical compositions featuring these compounds are also provided.
Owner:ADIPOGENIX
Polymers for delivering nitric oxide in vivo
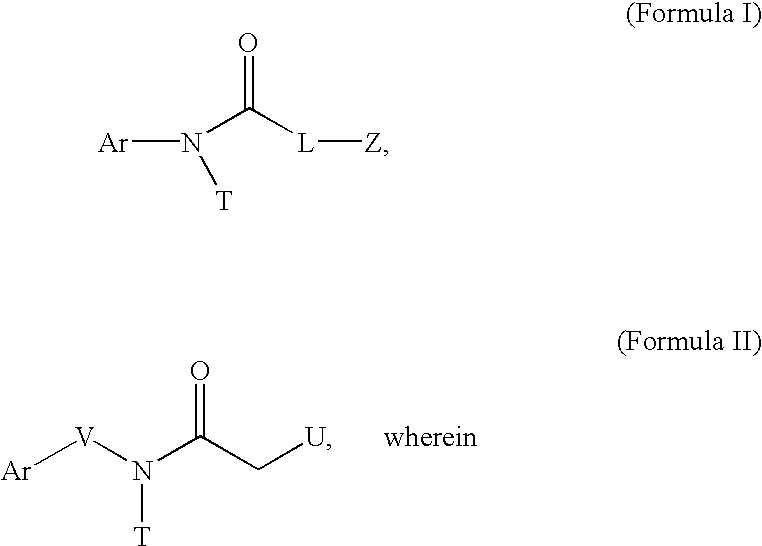
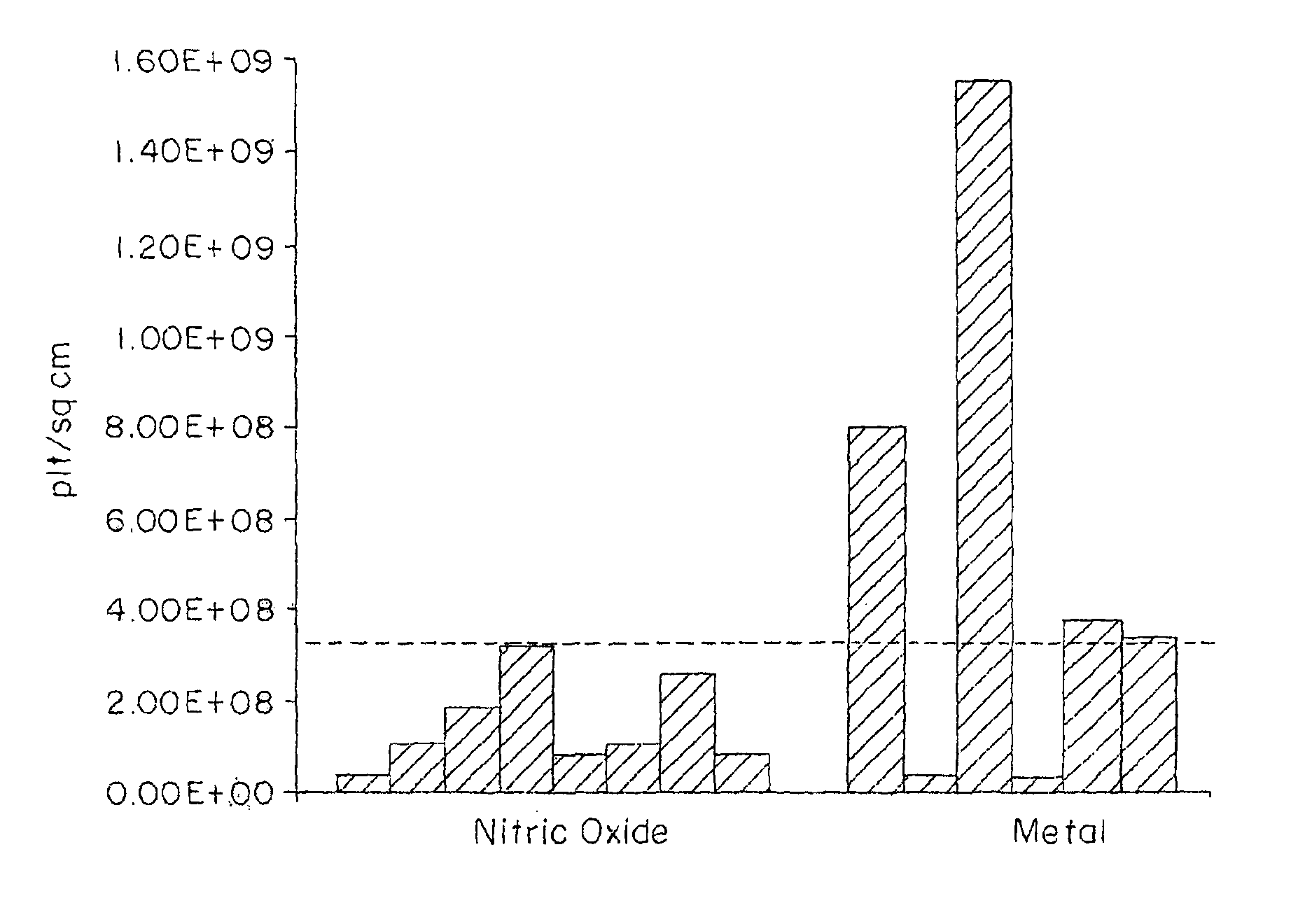
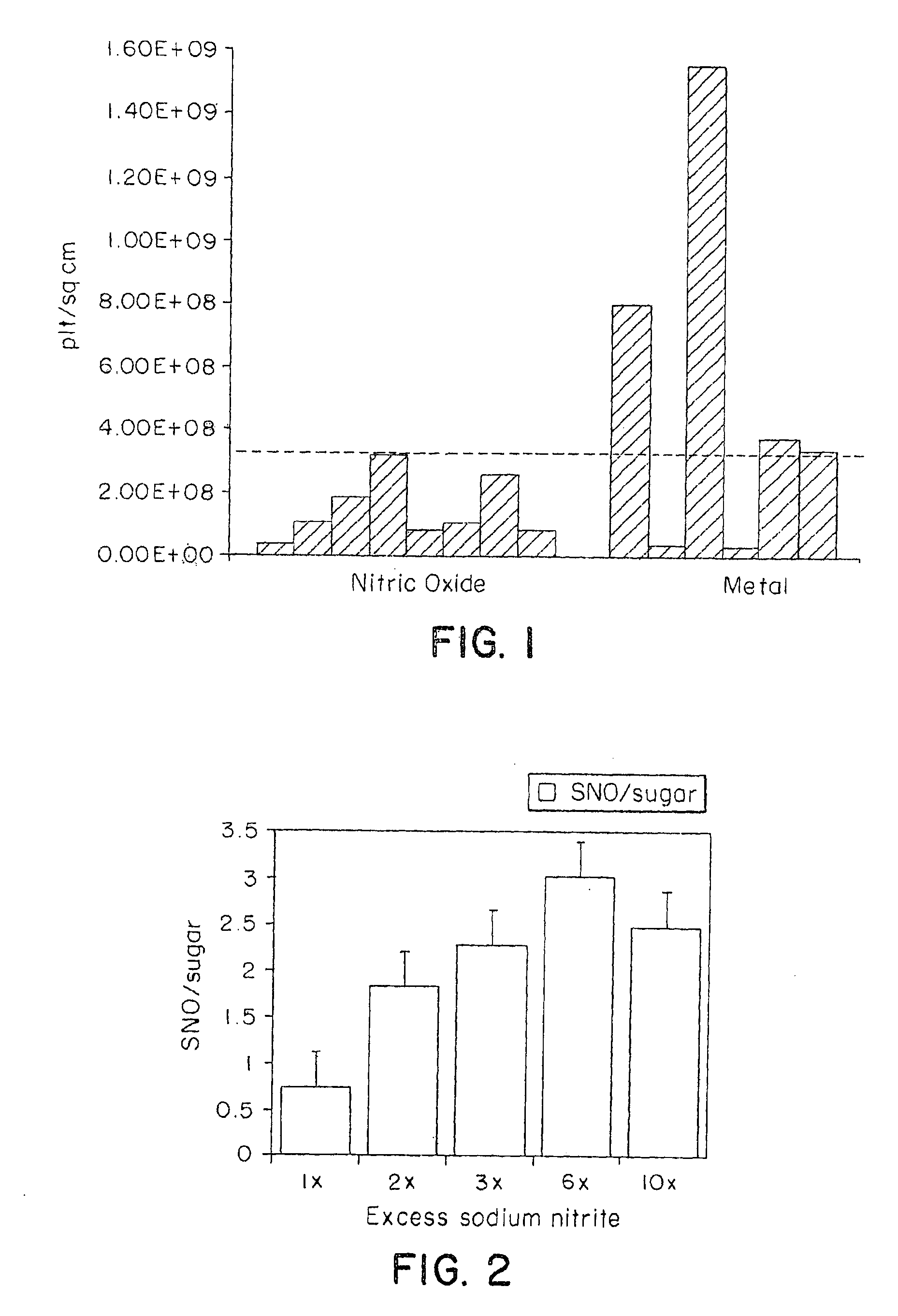
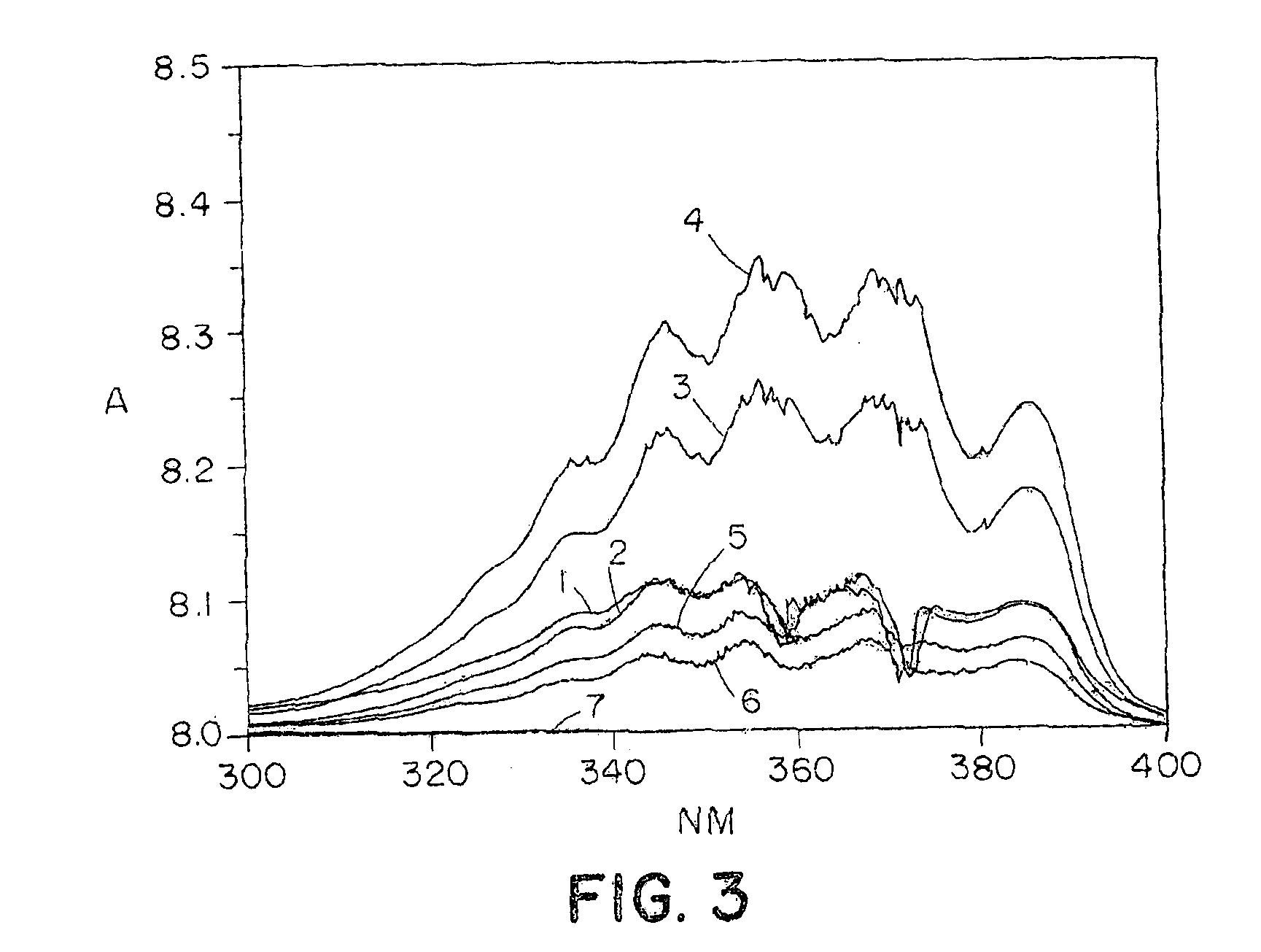
InactiveUS6875840B2Reducing platelet depositionReduce restenosisOrganic active ingredientsBiocideS-NitrosylationIn vivo
Disclosed are novel polymers derivatized with at least one —NOx group per 1200 atomic mass unit of the polymer. X is one or two. In one embodiment, the polymer is an S-nitrosylated polymer and is prepared by reacting a polythiolated polymer with a nitrosylating agent under conditions suitable for nitrosylating free thiol groups. The polymers of the present invention can be used to coat medical devices to deliver nitric oxide in vivo to treatment sites.
Owner:DUKE UNIV
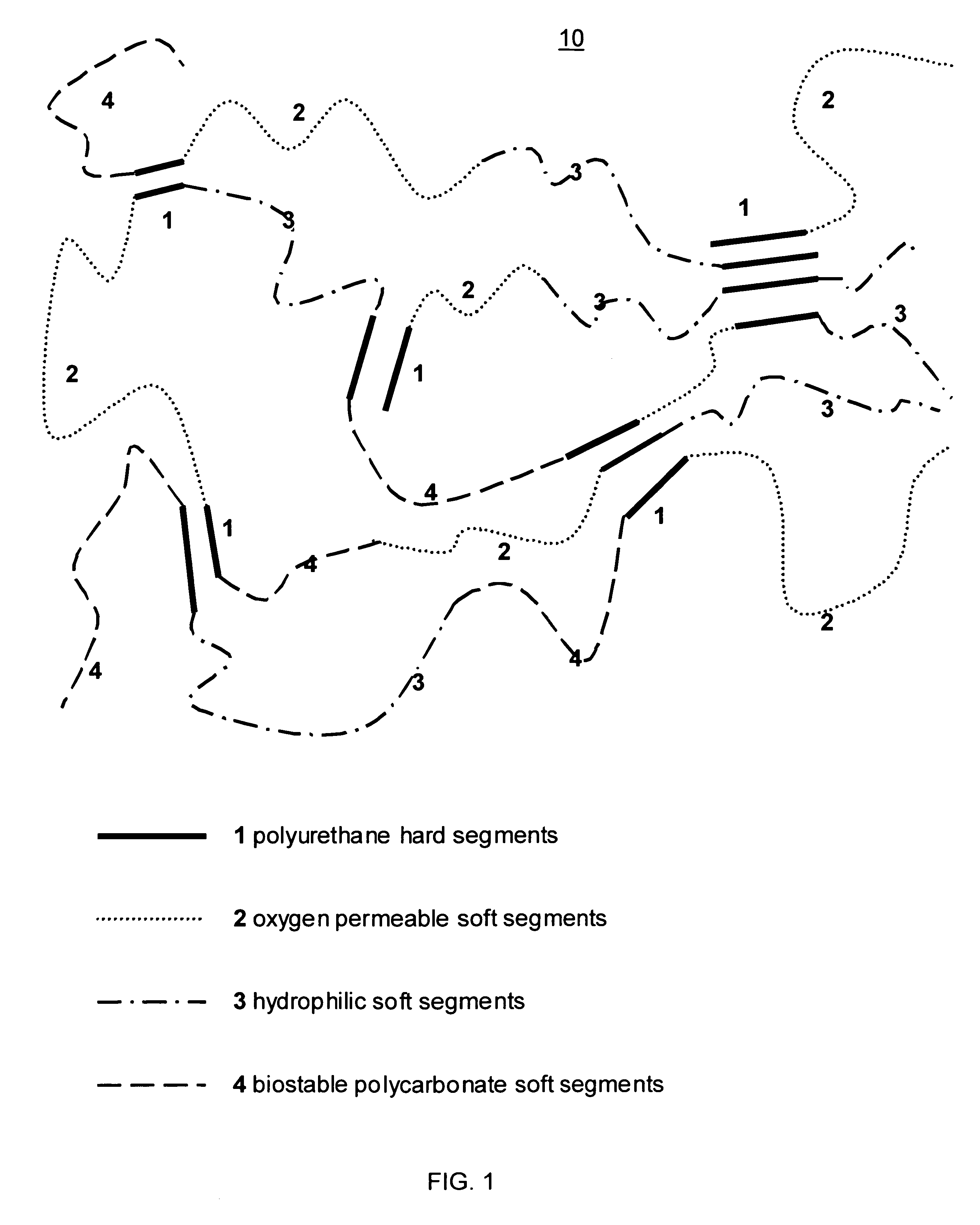
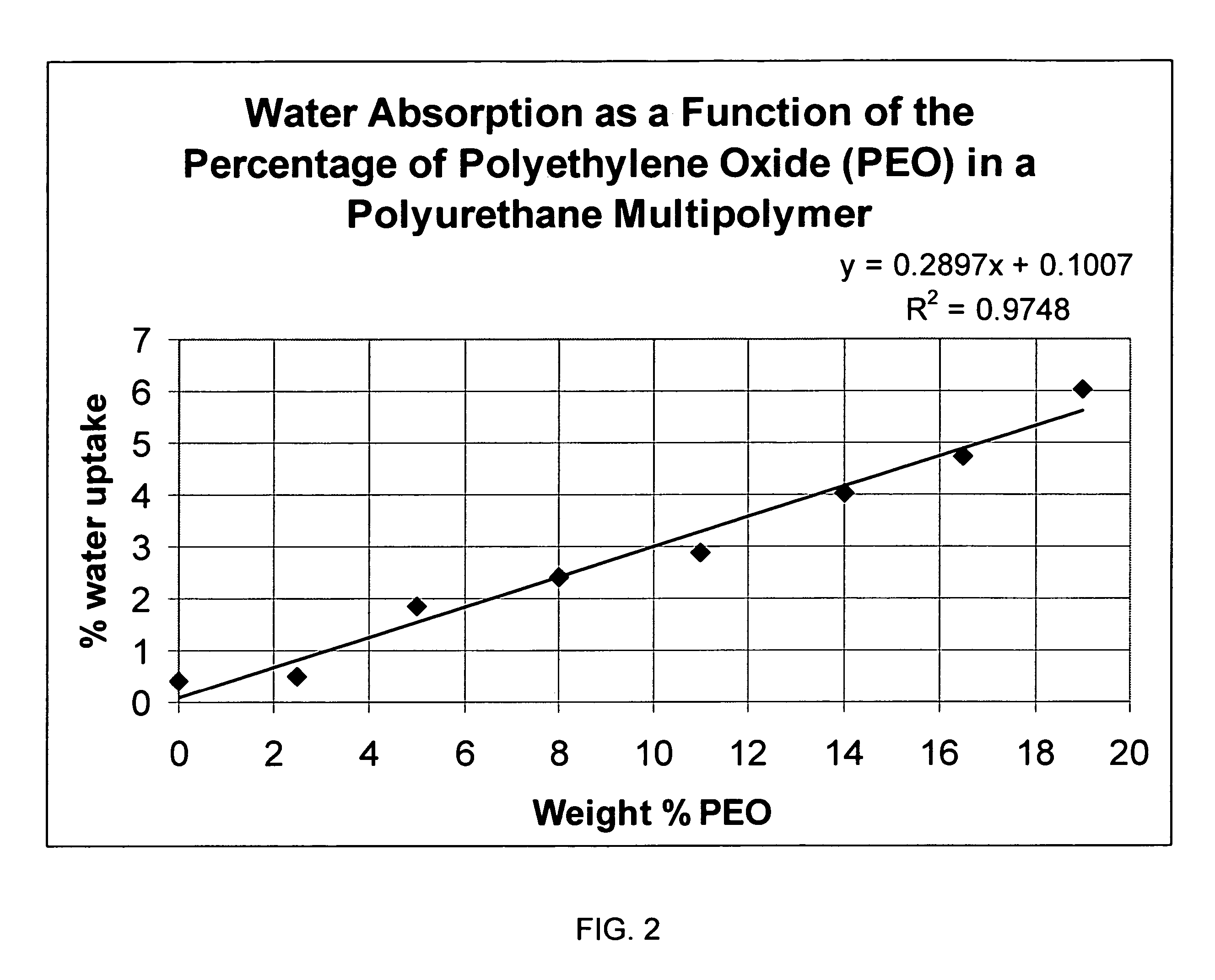
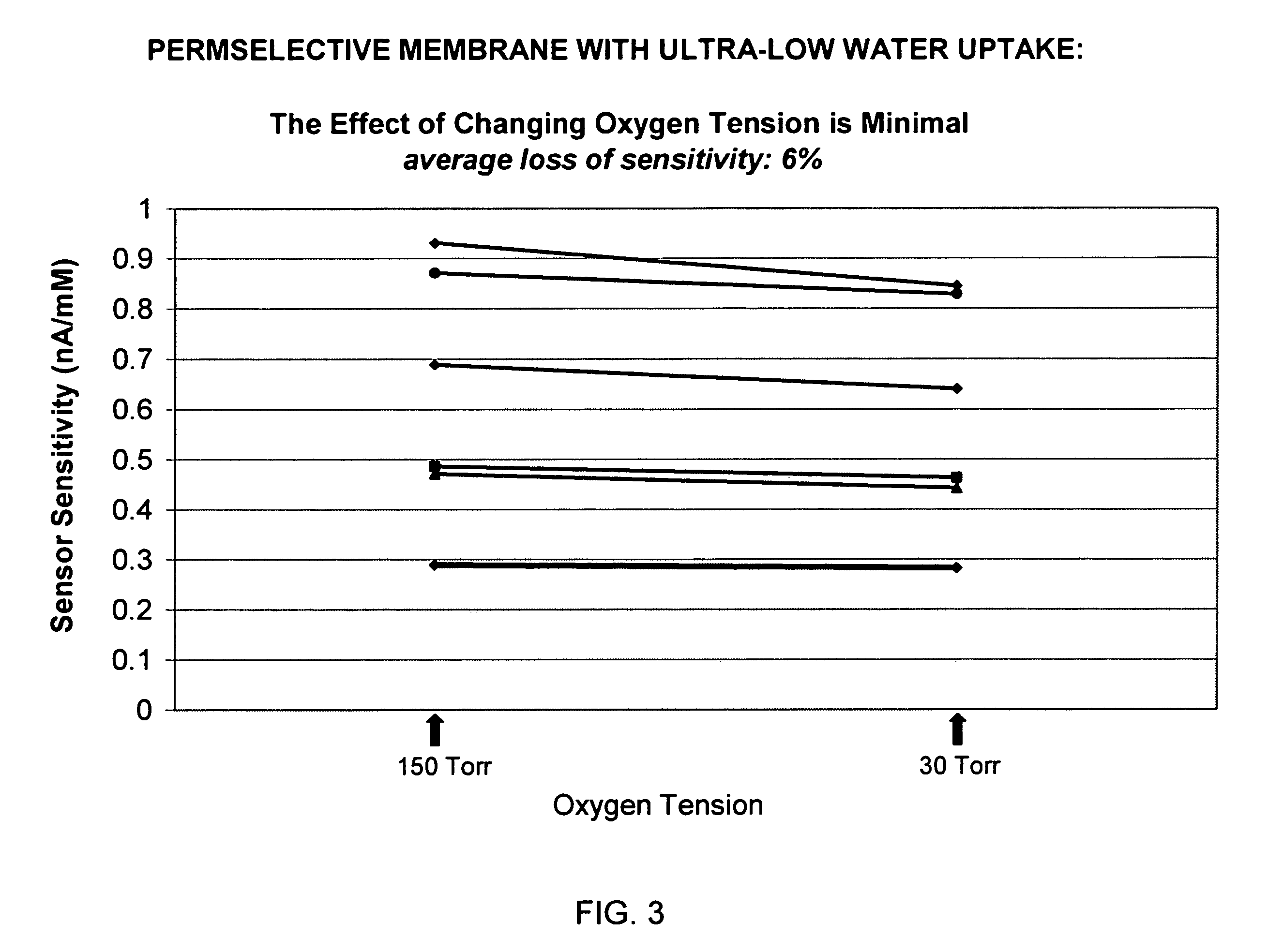
Biosensor membrane material
ActiveUS7687586B2Plateau in their responseLoss of integrityImmobilised enzymesBioreactor/fermenter combinationsProsthesisD-Glucose
Embodiments of the present invention provide various multipolymers and permselective membranes for use with biosensors and other implantable medical devices and prostheses. Embodiments of the present invention may provide structural strength and integrity, and further may control the influx of glucose, oxygen and / or water. Embodiments of the present invention may, for example, minimize or reduce the influx of glucose by minimizing the percentage of hydrophilic segments, which in turn minimizes the percentage of water uptake and the degree of glucose transport.
Owner:THE POLYMER TECH GROUP +1
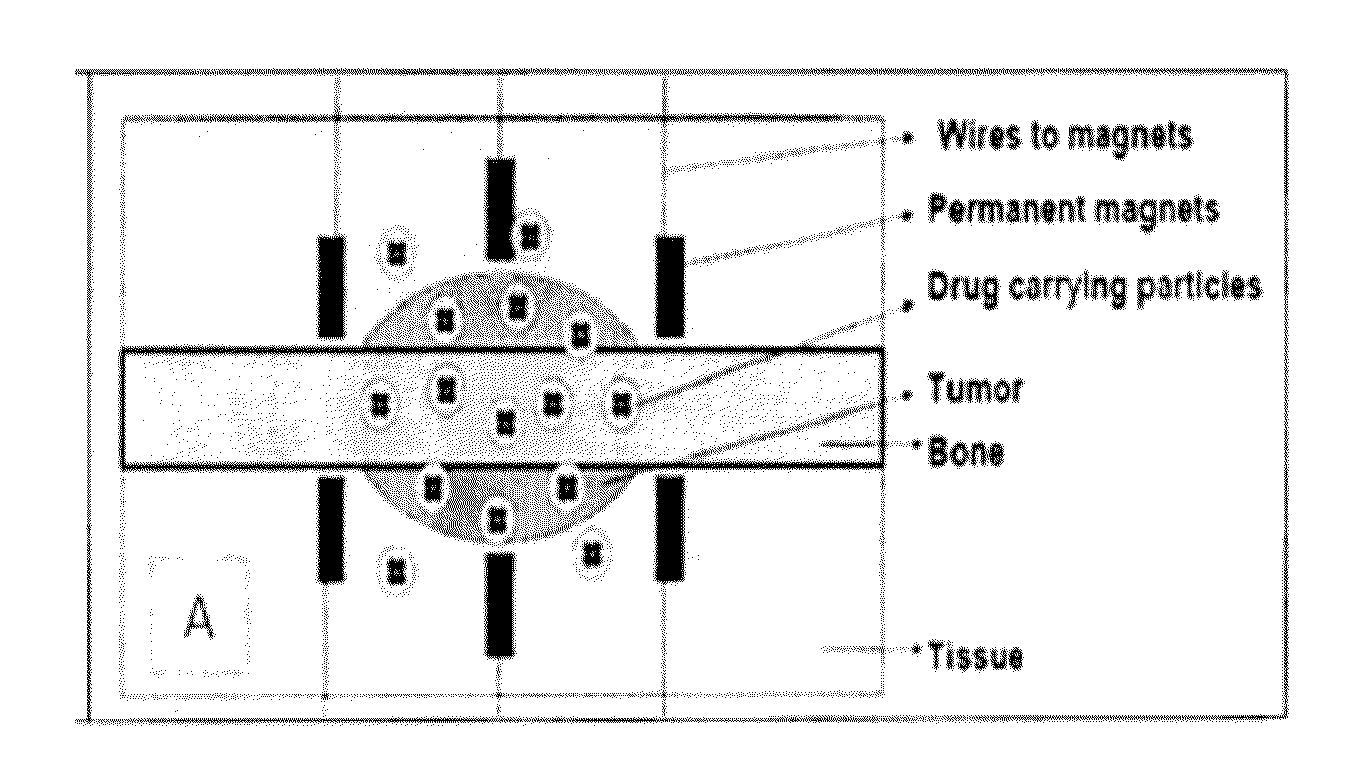
Composite magnetic nanoparticle drug delivery system
ActiveUS20120265001A1Accurate placementLimit deliveryBiocideHeavy metal active ingredientsDiseaseOil emulsion
A composite magnetic nanoparticle drug delivery system provides targeted controlled release chemotherapies for cancerous tumors and inflammatory diseases. The magnetic nanoparticle includes a biocompatible and biodegradable polymer, a magnetic nanoparticle, the biological targeting agent human serum albumin, and a therapeutic pharmaceutical composition. The composite nanoparticles are prepared by oil-in-oil emulsion / solvent evaporation and high shear mixing. An externally applied magnetic field draws the magnetic nanoparticles to affected areas. The biological targeting agent draws the nanoparticles into the affected tissues. Polymer degradation provides controlled time release delivery of the pharmaceutical agent.
Owner:WICHITA STATE UNIVERSITY
Biocompatible Coating of Medical Devices
A coated implantable medical device is described, wherein the coating comprises a coating matrix and particles of one or more molecular sieves, preferably zeolite of zeogrid particles, optionally loaded with one or more bioactive agents. The coating matrix itself can function as a second drug-carrying interface. The coating comprising the molecular sieve material has an excellent biocompatibility and allows suitable drug delivery into the body of an animal, preferably a mammal and most preferably a human.
Owner:KATHOLIEKE UNIV LEUVEN
Process for making functionalized films for cleaning products
InactiveUS7727946B2Difficult to handleLow costOrganic detergent compounding agentsOrganic/inorganic per-compounds compounding agentsWater solubleCleaning product
Owner:MONOSOL LLC +1
Monorail system
ActiveUS7615079B2Simple methodProtective structureSpinal implantsOsteosynthesis devicesImproved methodMonorail
Owner:K2M
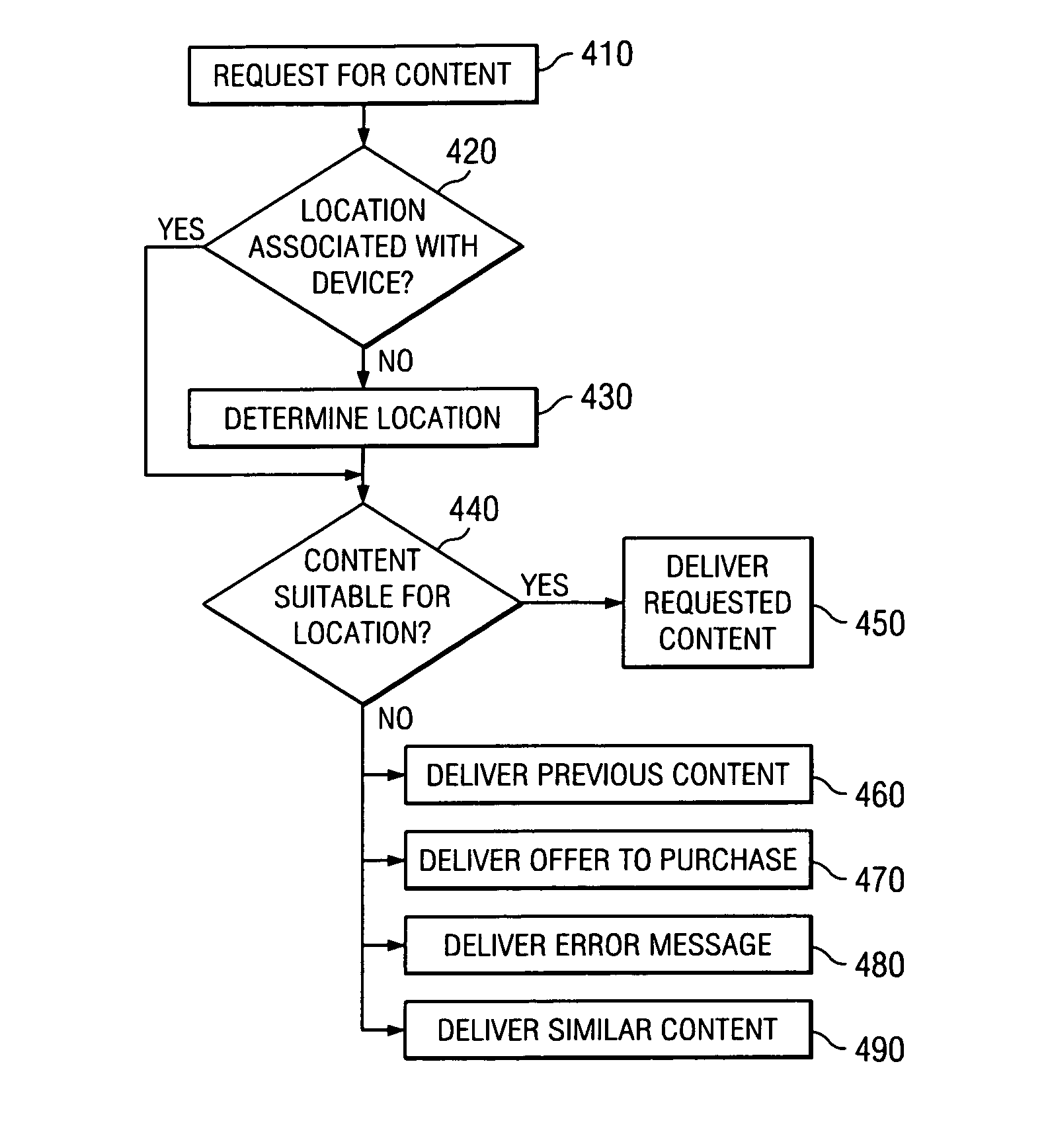
System and method for location based interaction with a device
ActiveUS8024186B1Limit deliveryMultiple digital computer combinationsSpeech recognitionLocation based systemsWorld Wide Web
Embodiments of these location-based systems and methods for device interaction may allow a content delivery system to provide certain content to a device, or restrict certain content from being delivered to the device, based on the location of the device. When a user requests certain content the location of the device may be determined and compared against an access control list defining a set or rules regarding that content to determine if the requested content may be accessed from that location. If the content may be accessed from this location the content may be delivered, otherwise an error message, or another option, may be delivered to the device. Similarly, the location of a device may be utilized to tailor the delivery of content to a device, such that content may be provided to a user based on the user's location, in certain cases with little or no stimulus from the user.
Owner:TIVO CORP
Spatially Encoded Biological Assays
ActiveUS20190271031A1Improve the level ofLimit deliveryMicrobiological testing/measurementLibrary screeningReagentControlled delivery
The present invention provides assays and assay systems for use in spatially encoded biological assays. The invention provides an assay system comprising an assay capable of high levels of multiplexing where reagents are provided to a biological sample in defined spatial patterns; instrumentation capable of controlled delivery of reagents according to the spatial patterns; and a decoding scheme providing a readout that is digital in nature.
Owner:PROGNOSYS BIOSCI
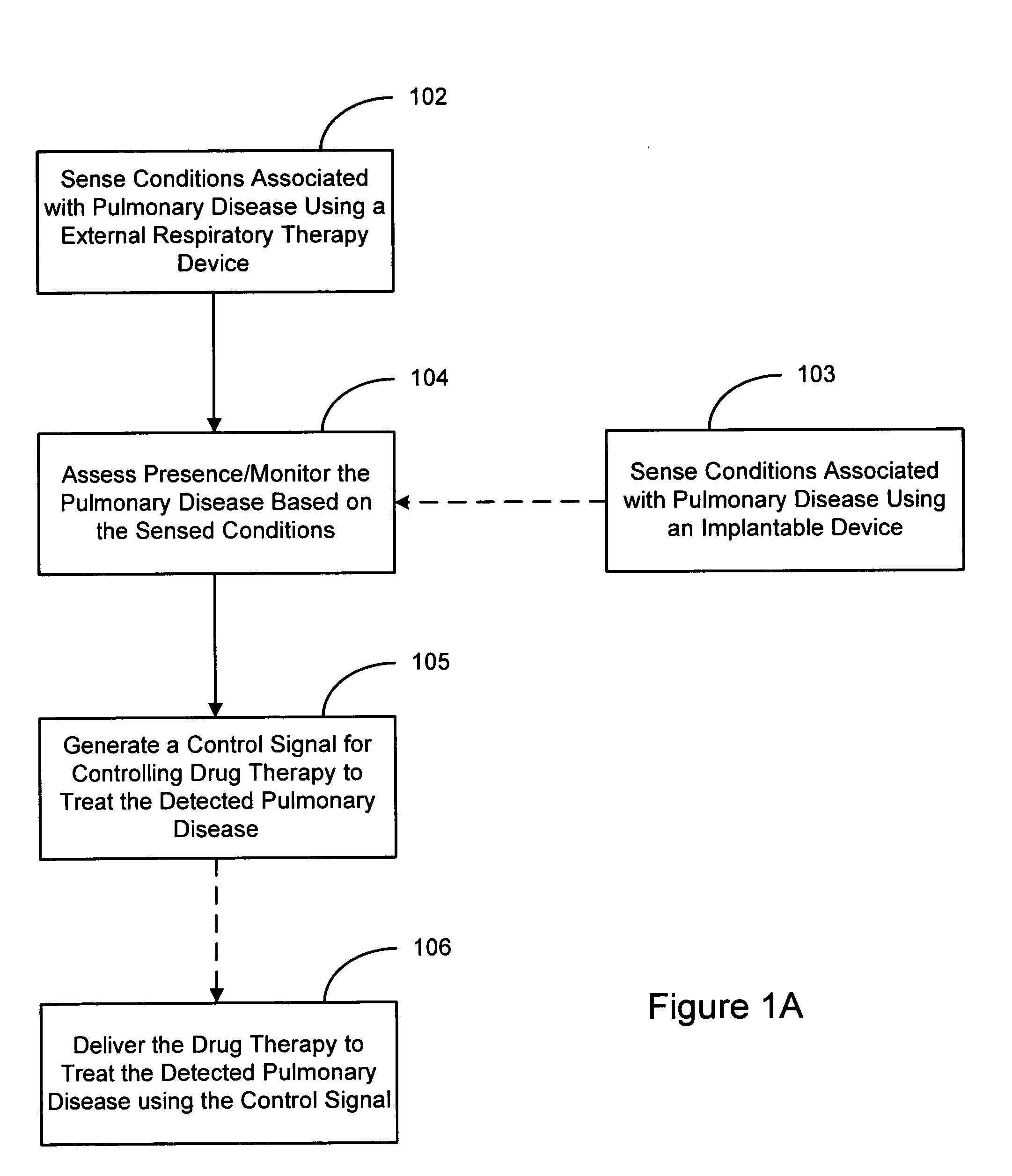
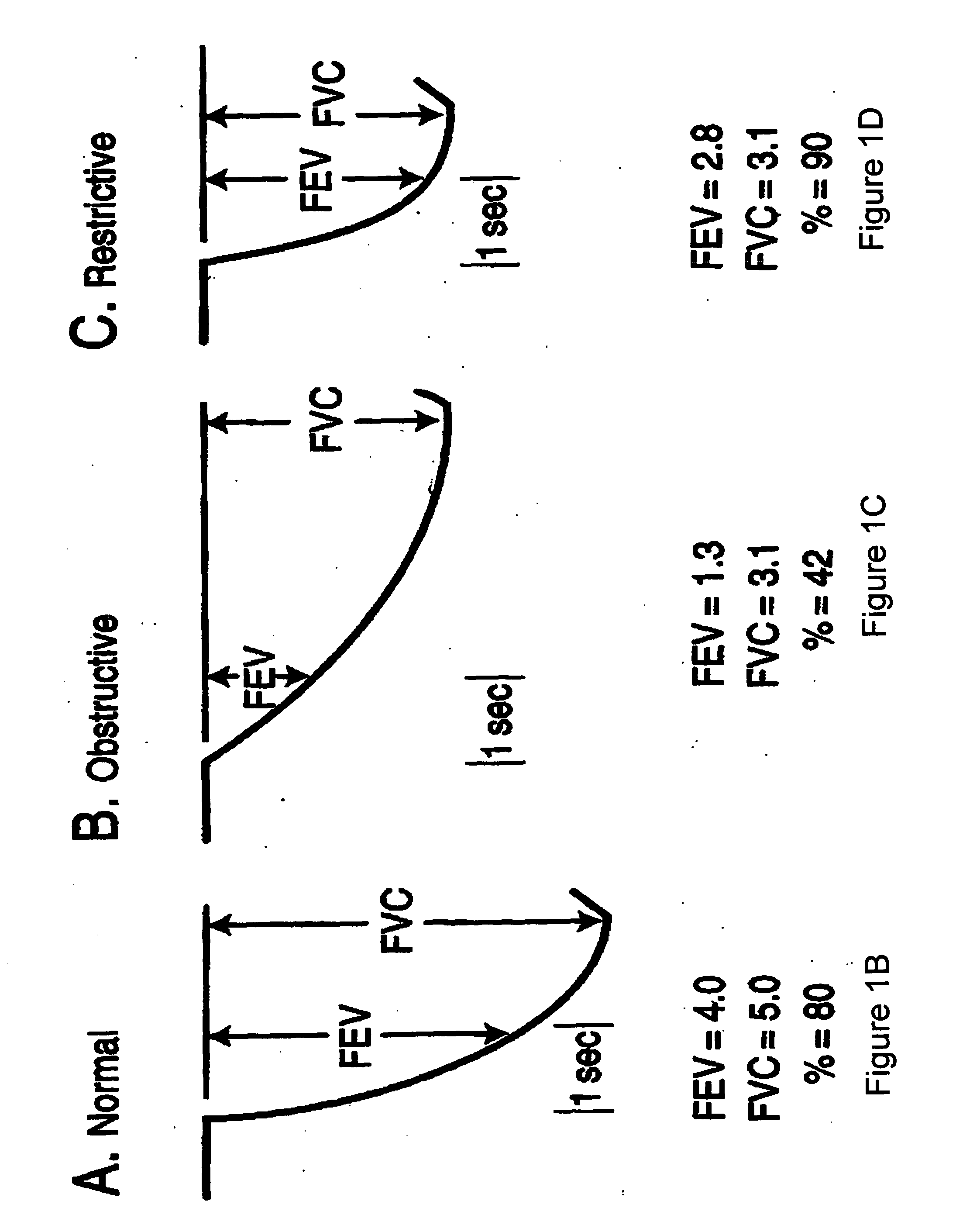
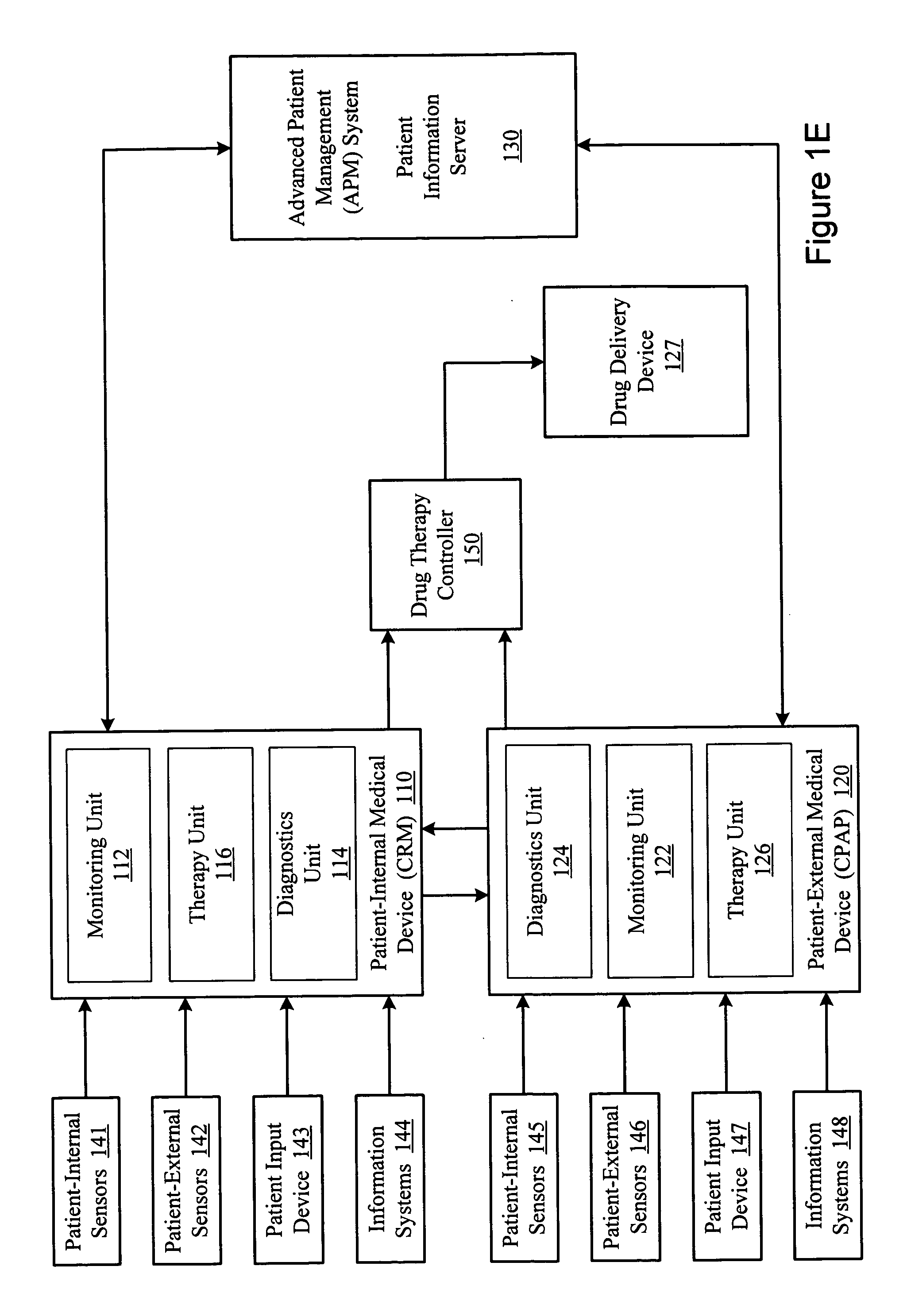
Methods and systems for assessing pulmonary disease with drug therapy control
An external respiratory therapy device incorporates sensors that may be used to sense physiological conditions or parameters associated with pulmonary disease. The sensed conditions may be used to detect and / or to assess a presence of various types of pulmonary diseases. The assessment of the pulmonary disease may be utilized to control a drug therapy delivered to the patient to treat the pulmonary disease.
Owner:CARDIAC PACEMAKERS INC
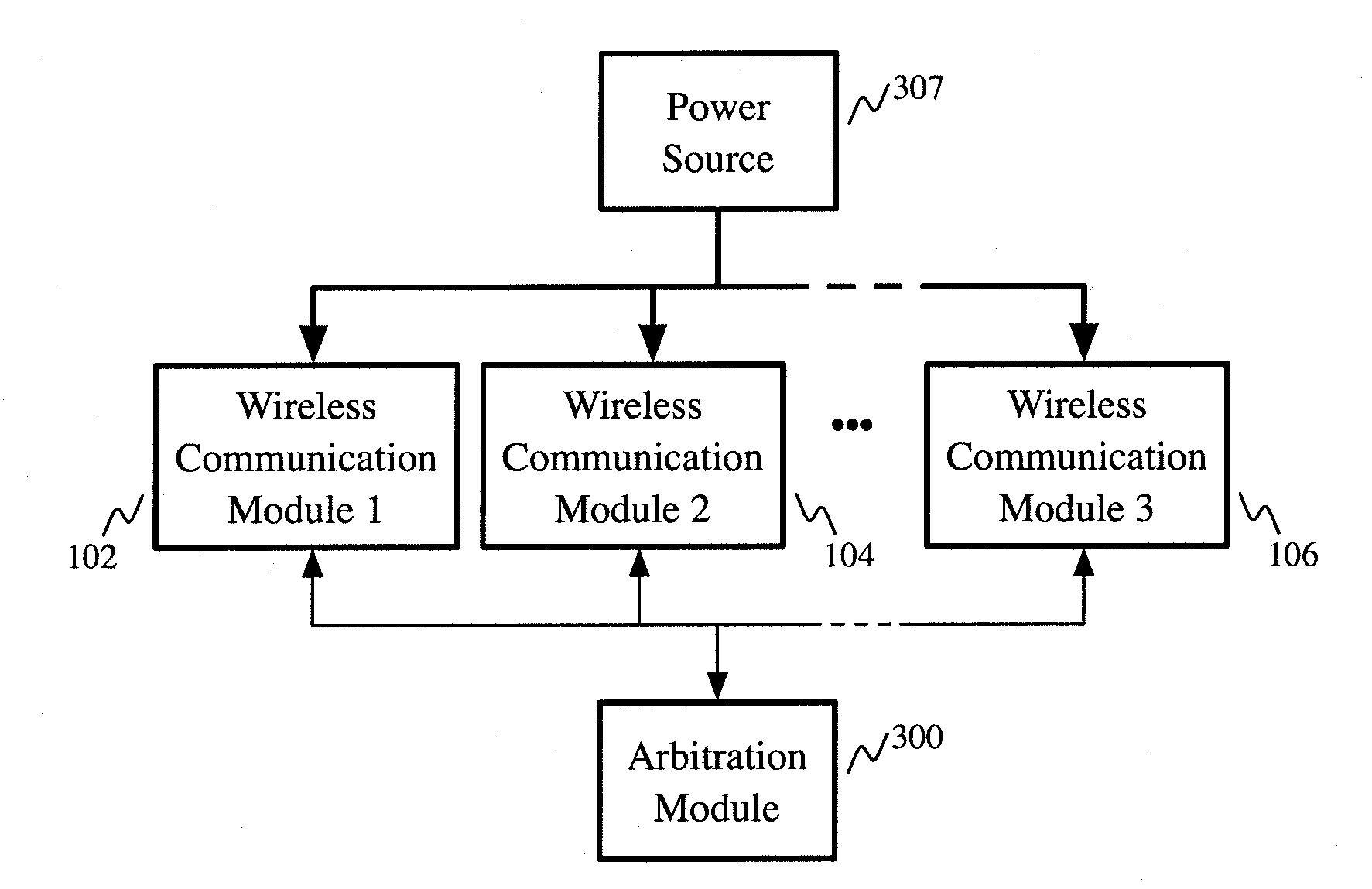
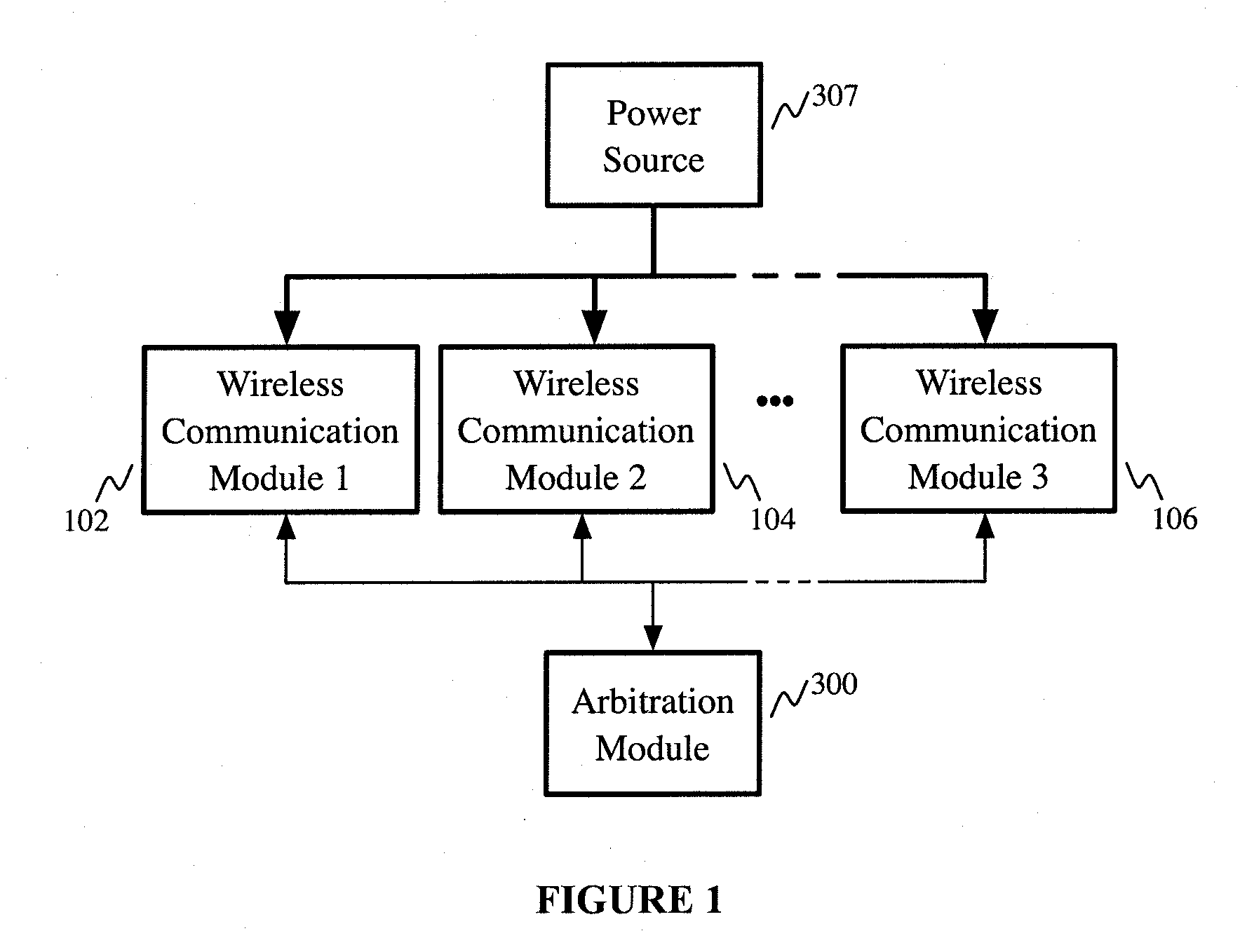
Apparatus providing plural wireless transceivers within a desired power budget and associated method
ActiveUS20100216524A1Limited powerLimit deliveryTelevision system detailsPower managementTransceiverWireless transceiver
The present invention provides an apparatus providing plural wireless transceivers within a desired power budget and associated methods. A plurality of wireless communication modules, each having a lower-power state and a higher-power state are provided, drawing power from a common source. Arbitration is performed to control which of the plurality of wireless communication modules are in the higher-power state, thereby controlling total power delivered by the power source to the plurality of wireless communication modules.
Owner:ROLLING WIRELESS S.À R L
Antisense antiviral compound and method for treating influenza viral infection
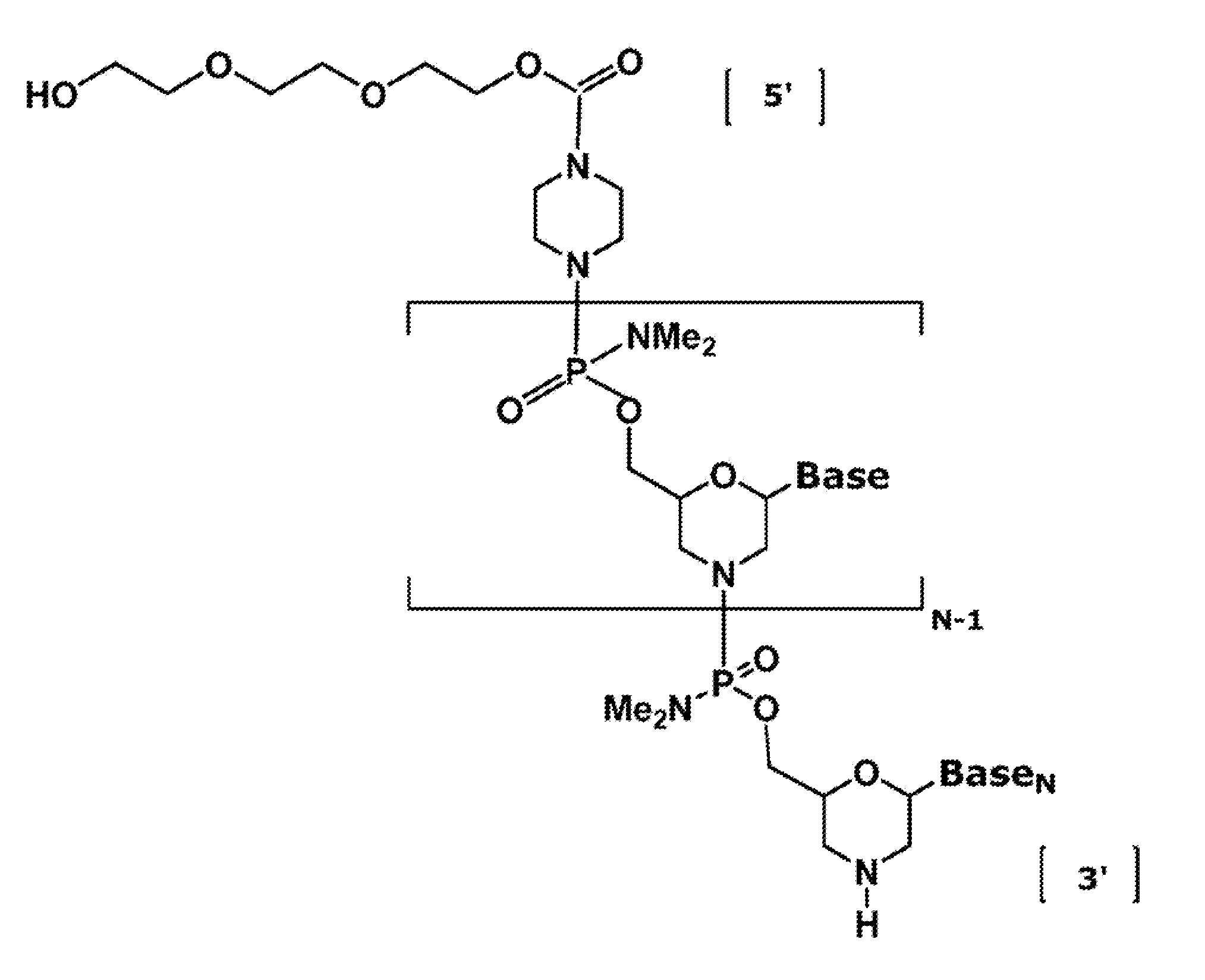
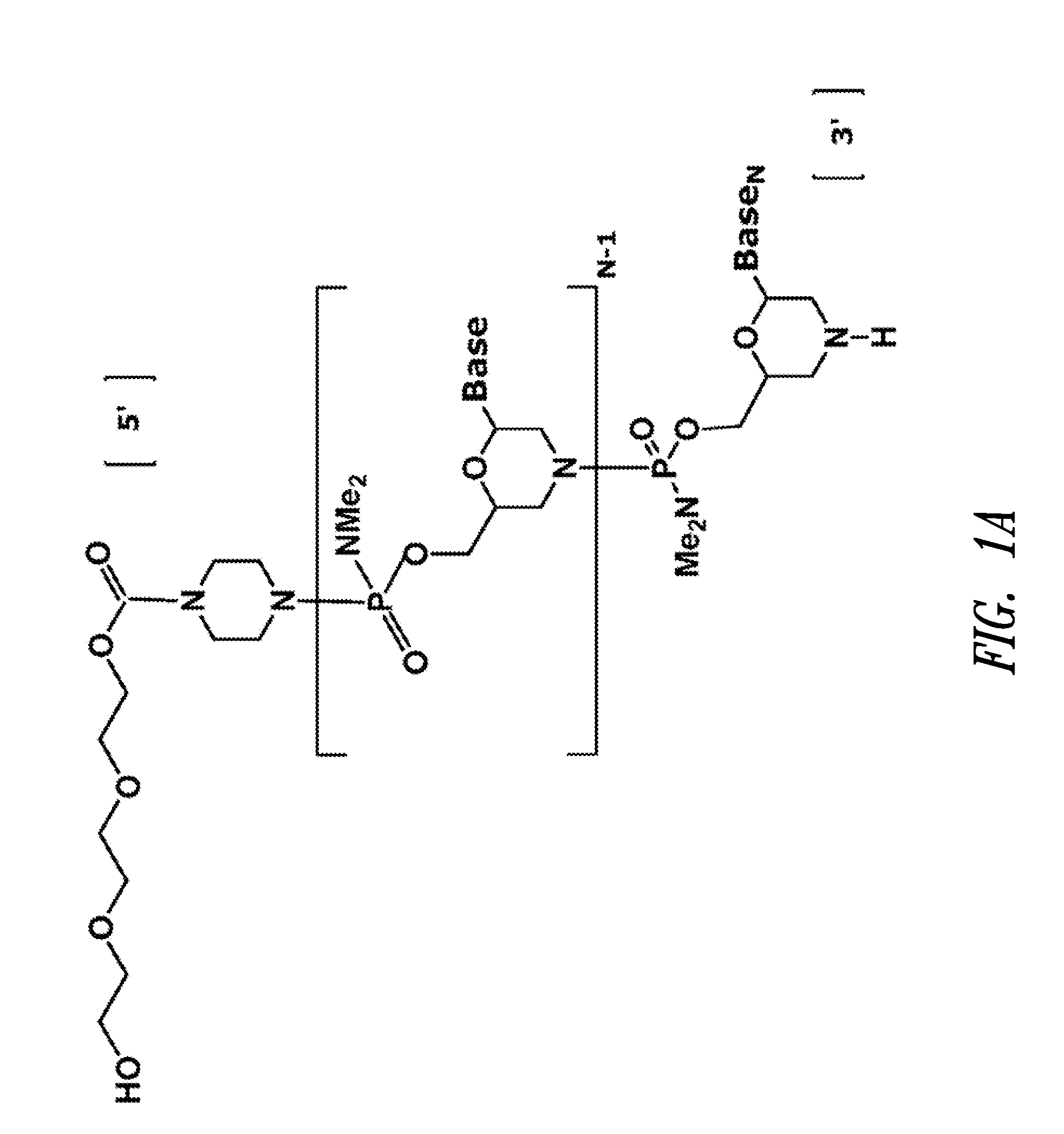
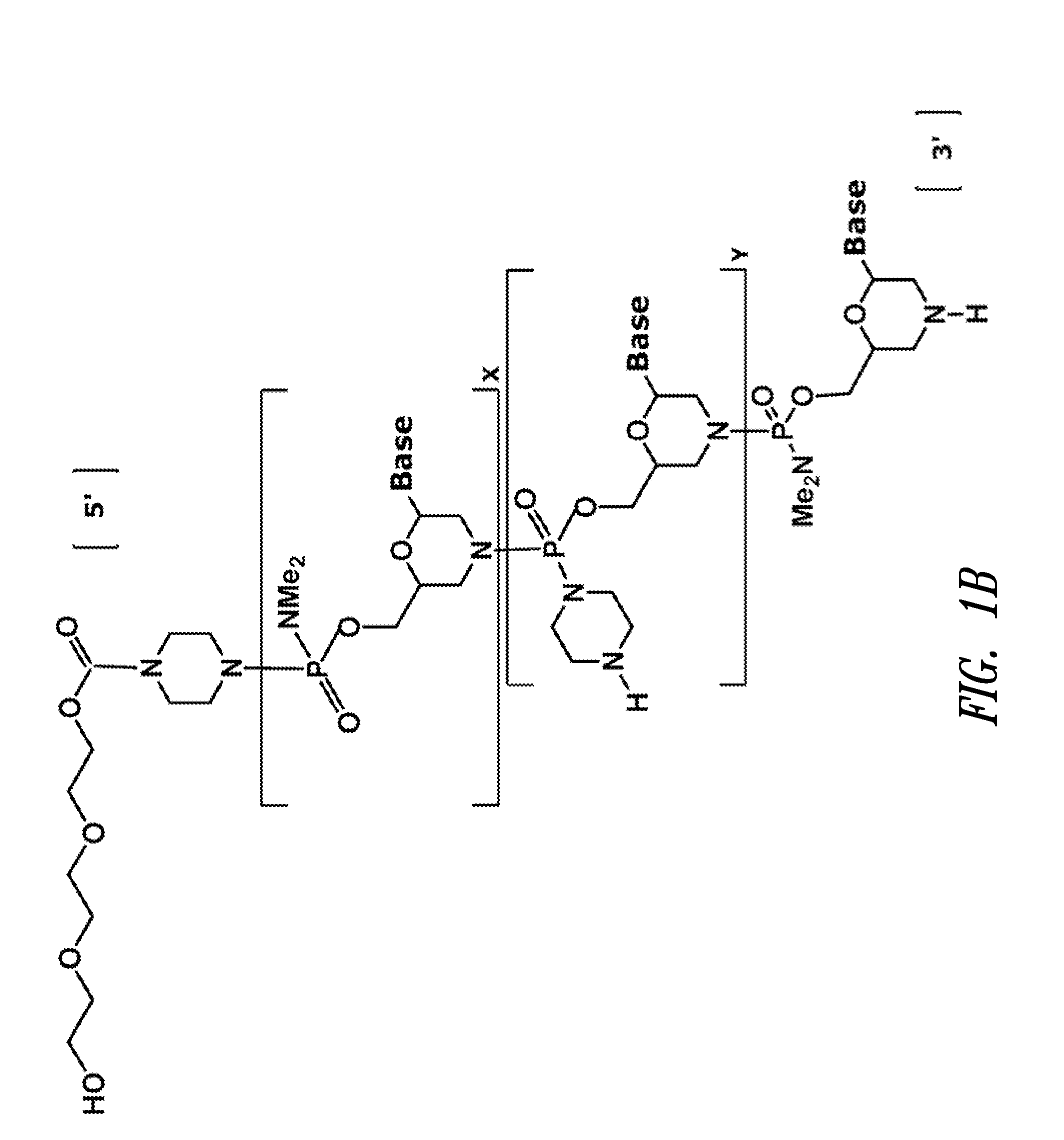
ActiveUS20110118334A1Improve throughputLimit deliveryAntibacterial agentsSugar derivativesInfluenzavirus CStart codon
The present invention relates to antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Orthomyxoviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of influenza virus infection in a mammal. Exemplary antisense antiviral compounds are substantially uncharged, or partially positively charged, morpholino oligonucleotides having 1) a nuclease resistant backbone, 2) 12-40 nucleotide bases, and 3) a targeting sequence of at least 12 bases in length that hybridizes to a target region selected from the following: a) the 5′ or 3′ terminal 25 bases of the negative sense viral RNA segment of Influenzavirus A, Influenzavirus B and Influenzavirus C; b) the terminal 30 bases of the 5′ or 3′ terminus of the positive sense vcRNA; c) the 45 bases surrounding the AUG start codon of an influenza viral mRNA and; d) 50 bases surrounding the splice donor or acceptor sites of influenza mRNAs subject to alternative splicing.
Owner:SAREPTA THERAPEUTICS INC
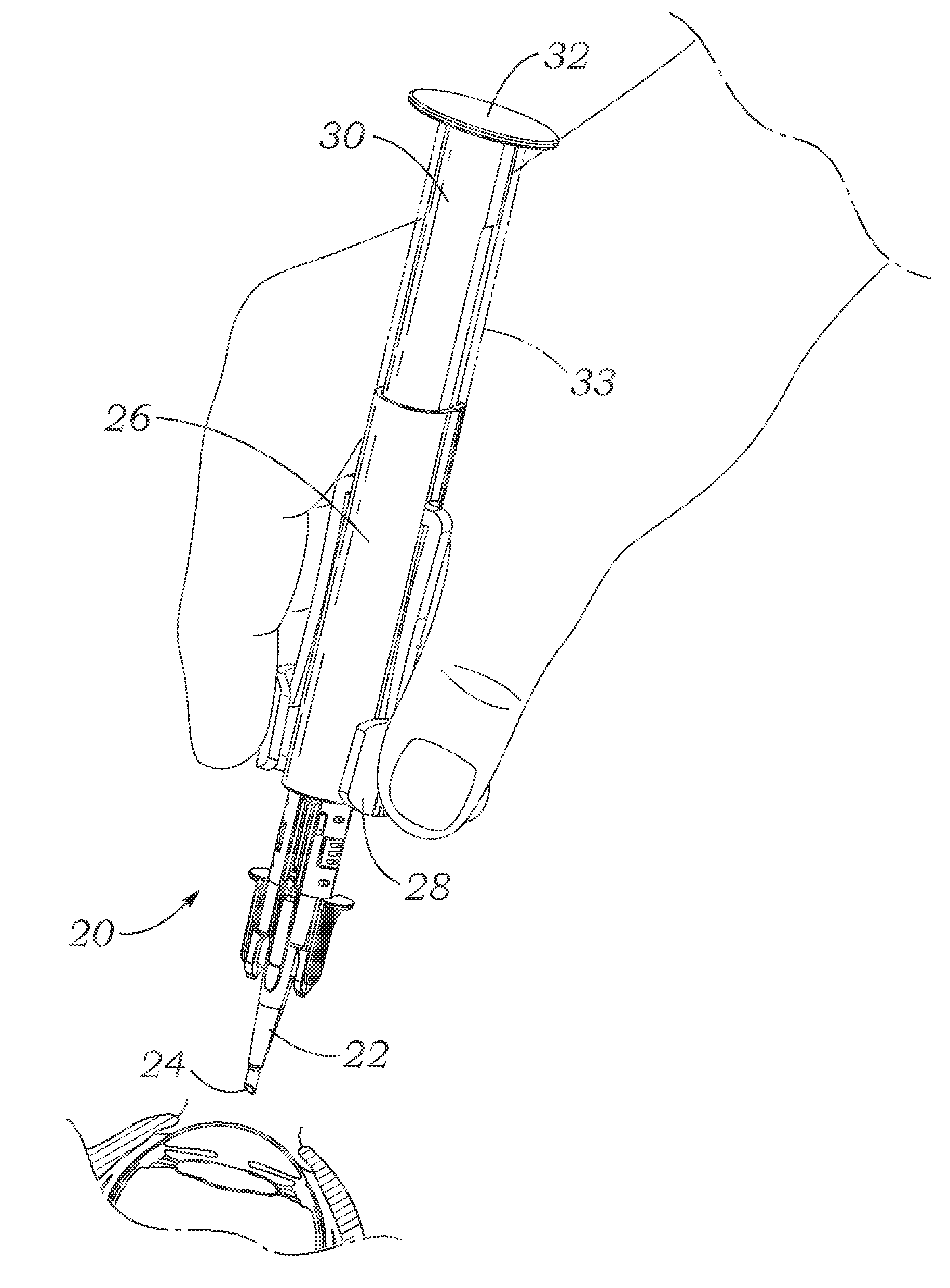
Automated preloaded intraocular lens injector
ActiveUS20140257317A1Easily holdPositive feedbackEye treatmentIntraocular lensIntraocular lensInjector
This intraocular lens (IOL) injector for delivering an IOL into an eye of a patient includes an IOL load chamber and connected delivery tube, and a spring-loaded push rod for urging the IOL through the delivery tube and out of a distal tip thereof. The injector includes an actuator that is cocked to compress an automatic delivery coil spring. Cocking the actuator also folds the IOL and may elongate a dual optic IOL. A braking mechanism may be provided to permit control of the spring-biased IOL advancement. The injector is in a pen style with finger plates on the side for better ergonomic control.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Spatially Encoded Biological Assays
ActiveUS20200299757A1Improve the level ofLimit deliveryMicrobiological testing/measurementLibrary screeningBiologic AssaysAssay
The present invention provides assays and assay systems for use in spatially encoded biological assays. The invention provides an assay system comprising an assay capable of high levels of multiplexing where reagents are provided to a biological sample in defined spatial patterns; instrumentation capable of controlled delivery of reagents according to the spatial patterns; and a decoding scheme providing a readout that is digital in nature.
Owner:PROGNOSYS BIOSCI
Spatially Encoded Biological Assays
ActiveUS20200325531A1Improve the level ofLimit deliveryMicrobiological testing/measurementLibrary screeningBiologic AssaysMultiplexing
Owner:PROGNOSYS BIOSCI
Spatially Encoded Biological Assays
InactiveUS20190300945A1Improve the level ofLimit deliveryMicrobiological testing/measurementLibrary screeningReagentControlled delivery
The present invention provides assays and assay systems for use in spatially encoded biological assays. The invention provides an assay system comprising an assay capable of high levels of multiplexing where reagents are provided to a biological sample in defined spatial patterns; instrumentation capable of controlled delivery of reagents according to the spatial patterns; and a decoding scheme providing a readout that is digital in nature.
Owner:PROGNOSYS BIOSCI
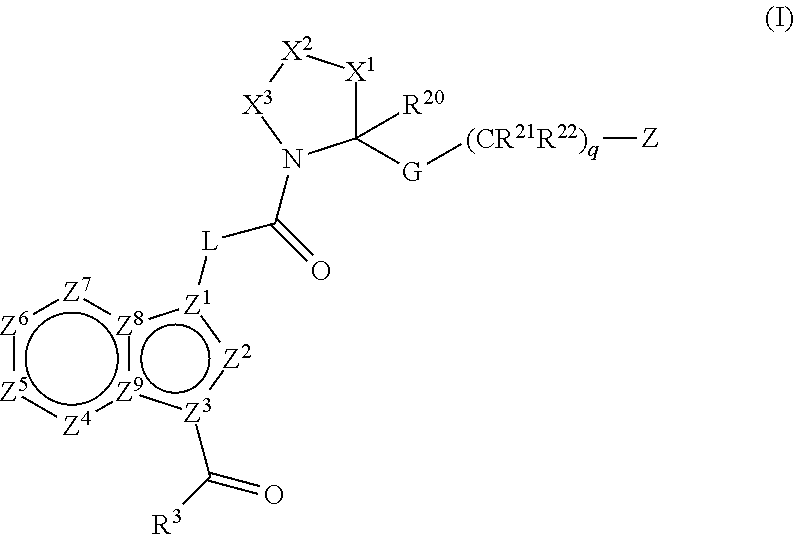
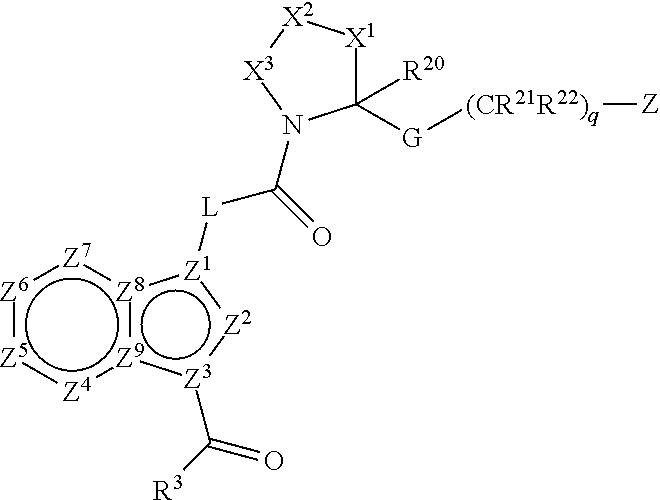
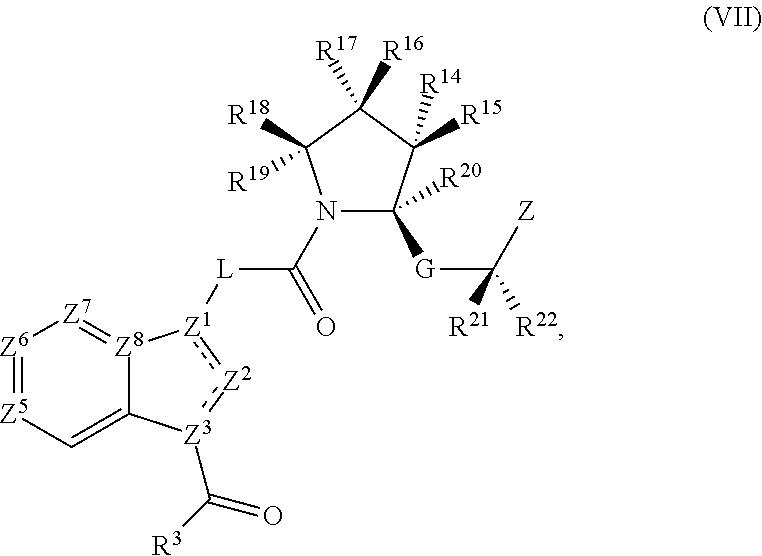
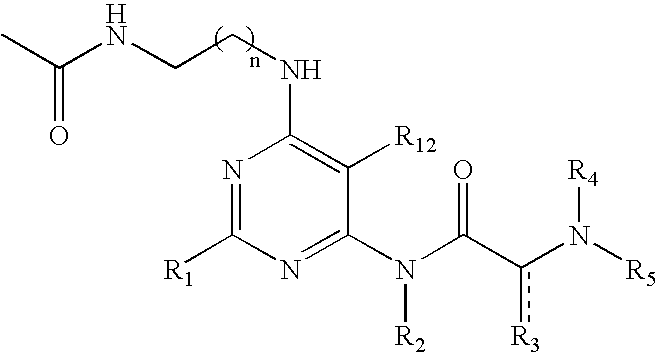
Pyrimidine A2b selective antagonist compounds, their synthesis and use
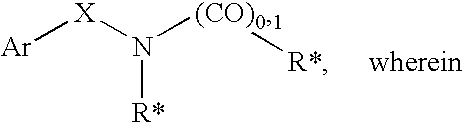
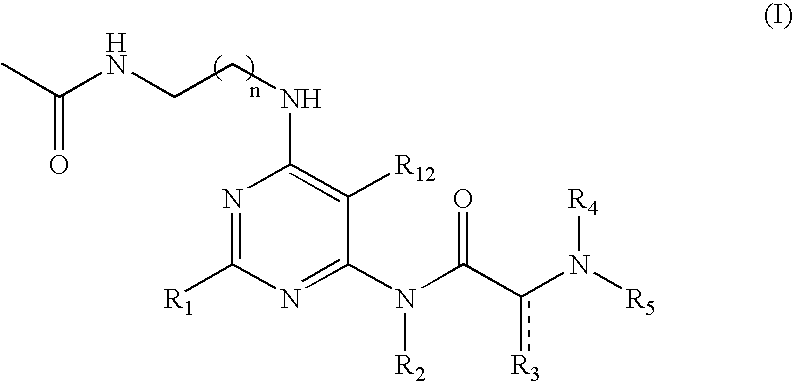
The subject invention provides compounds having the structure: wherein R1 is substituted or unsubstituted phenyl or a 5-6 membered heterocyclic or heteroaromatic ring containing from 1 to 5 heteroatoms; R2 is hydrogen, or a substituted or unsubstituted alkyl, —C(O)-alkyl, —C(O)—O-alkyl, alkoxy, cycloalkyl, alkenyl, monocyclic or bicyclic aryl, heteroaryl or heterocyclic moiety; R3 is hydrogen, or a substituted or unsubstituted alkyl, —C(O)-alkyl, —C(O)—O-alkyl, alkoxy, cycloalkyl, alkenyl, monocyclic or bicyclic aryl, heteroaryl or heterocyclic moiety, or R2 and R3 are joined to form a heterocyclic ring; wherein the dashed line represents a second bond which may be present or absent, and when present R3 is oxygen; R4 and R5 are each independently substituted or unsubstituted alkyl, —C(O)-alkyl, —C(O)—O-alkyl, alkoxy, cycloalkyl, alkenyl, monocyclic or bicyclic aryl, heteroaryl or heterocyclic moiety, or R4NR5 together form a substituted or unsubstituted monocyclic or bicyclic, heterocyclic or heteroaryl moiety containing from 1 to 6 heteroatoms; R12 is hydrogen, alkyl, halogen or cyano; and n is 0, 1, 2, 3 or 4, or an enantiomer, or a specific tautomer, or a pharmaceutically acceptable salt thereof and a method for treating a disease associated with the A2b adenosine receptor by administering a therapeutically effective amount of the compounds of the invention.
Owner:OSI PHARMA INC
Controlled release formulations using intelligent polymers
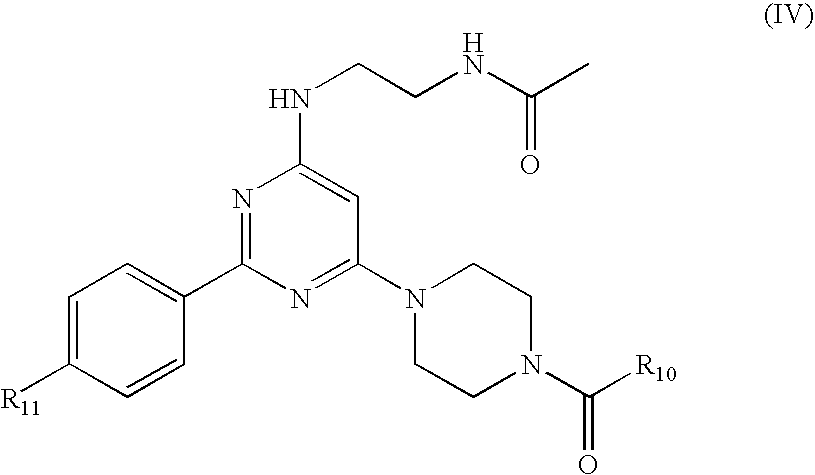
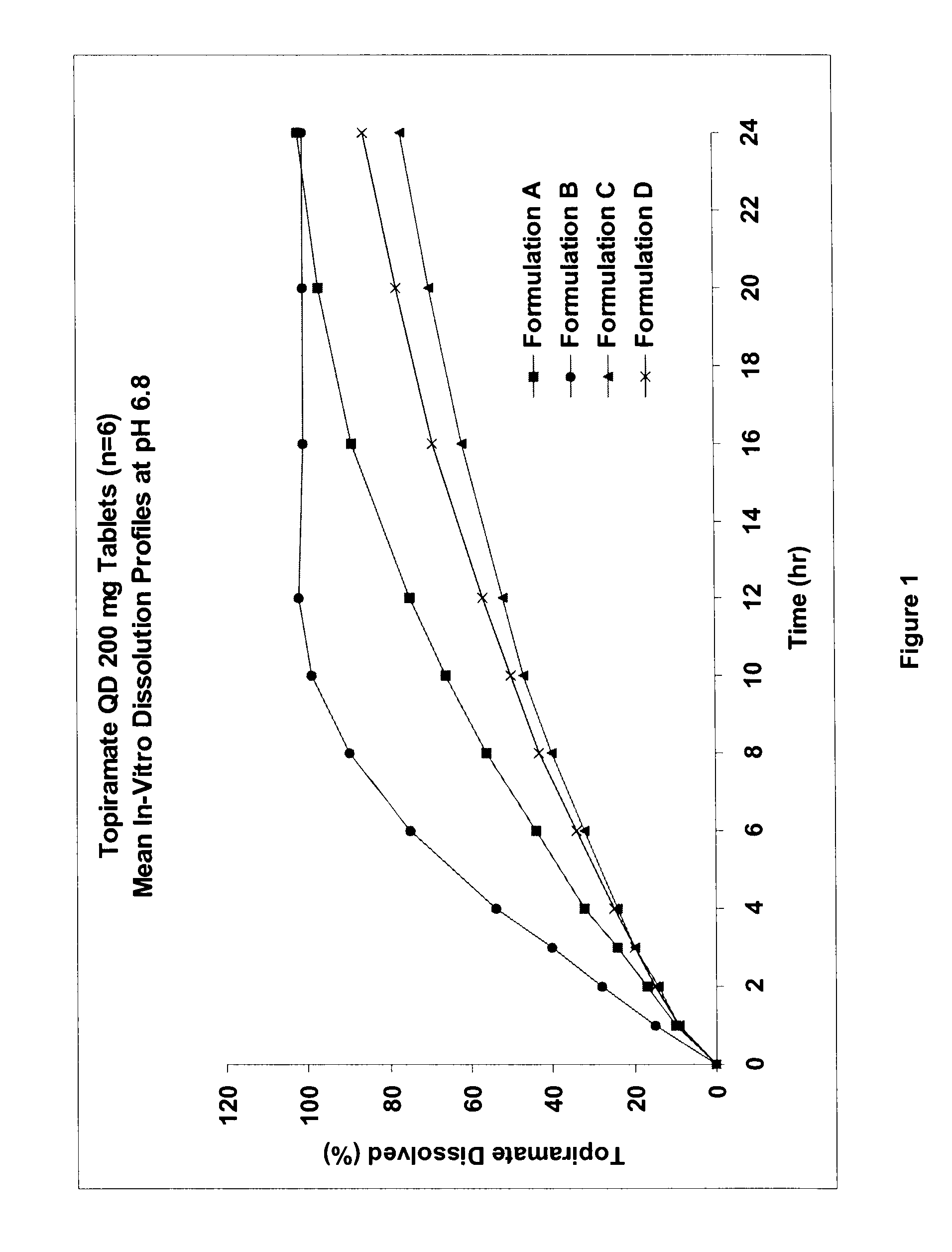
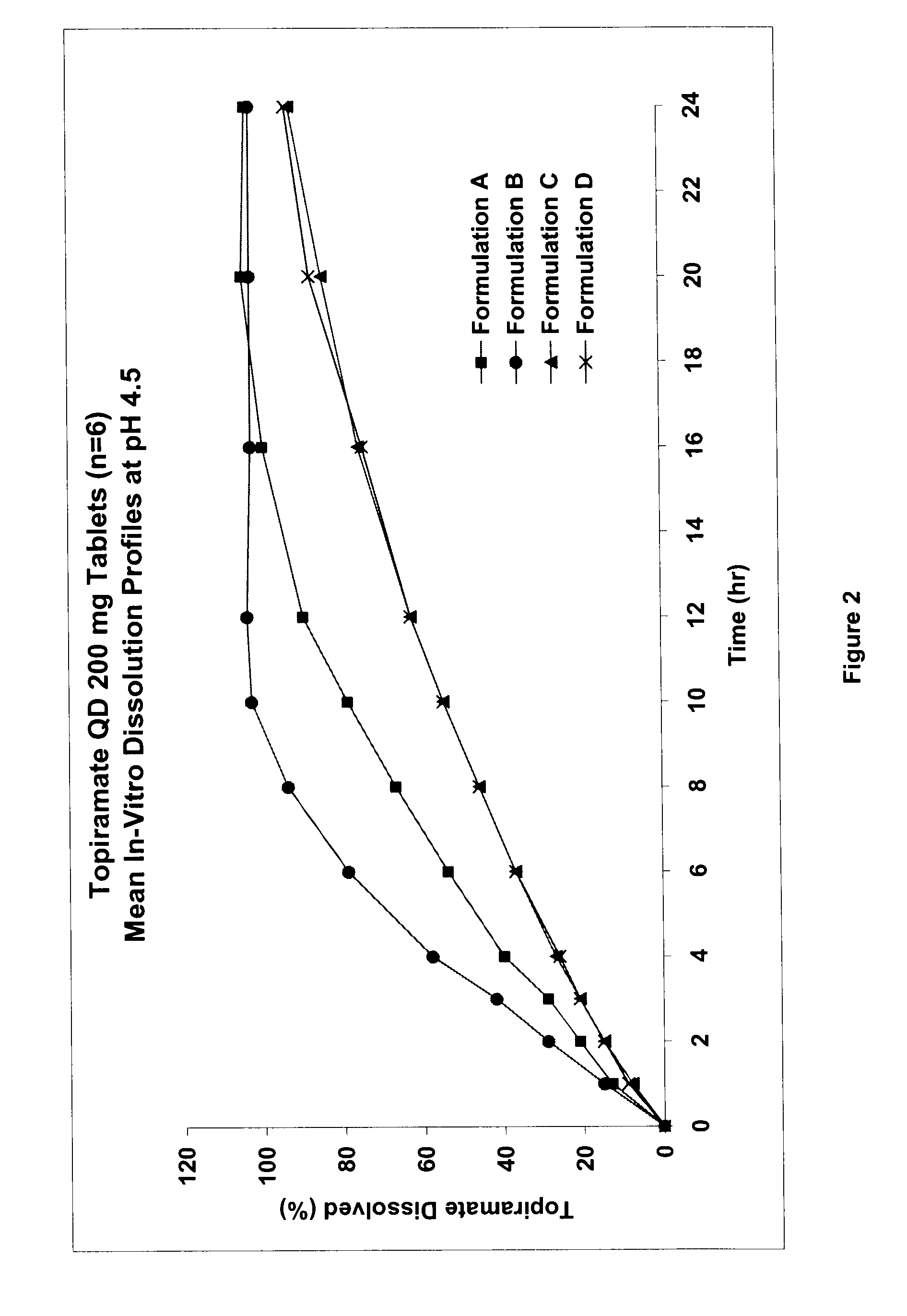
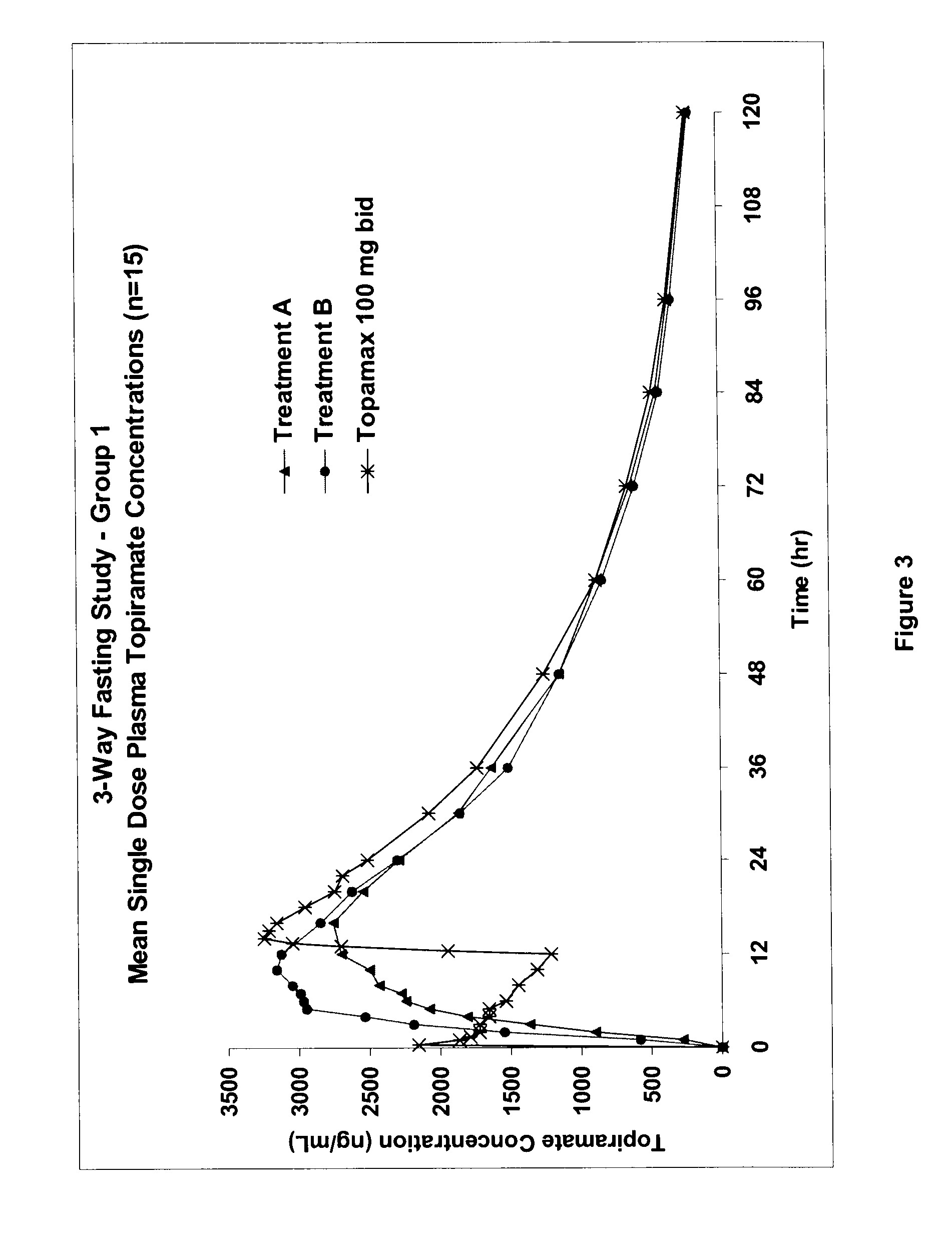
InactiveUS20080292700A1Prevent burstImparts gastrointestinal stealth characteristicNervous disorderMetabolism disorderSmart polymerTopiramate
A controlled release pharmaceutical composition comprises (a) topiramate or a pharmaceutically acceptable salt thereof, (b) a first intelligent polymer component; and (c) a second intelligent polymer component having opposite wettability characteristics to the first intelligent polymer component. The polymer components are effective for controlled release of the pharmaceutically active substance from the composition.
Owner:VALEANT INT BARBADOS
Functionalized films
InactiveUS20060257596A1Avoid contactMore economicallyEnvelopes/bags making machineryOrganic detergent compounding agentsWater solubleAqueous solution
Process for making a functionalized substrate in the form of a water-soluble film carrying a coating of a functional composition, the process comprising applying to at least one side of the film an aqueous solution comprising one or more functional materials to form the coating wherein the coating is formed from a plurality of layers in a stepwise manner and / or the aqueous solution comprises a film insolubilizer agent.
Owner:MONOSOL LLC +1
Rupturing controlled release device having a preformed passageway
ActiveUS8029822B2Increase release rateLarge particle sizePill deliveryCapsule deliveryControlled releaseMedicine
The present invention provides a simple and improved osmotic device that is capable of providing a controlled release of active agent contained in the core first through a preformed passageway and then through an in situ formed second passageway into an environment of use. One or both of the passageways optionally increases in size during use of the osmotic device. The preformed passageway and / or the second passageway increase the release rate of the active agent, enable the release of large particles containing active agent, and / or enable the release of active agents that are substantially insoluble in the environment of use. By virtue of the in situ formation of the second aperture, the device is able to release a greater overall percentage of active agent than it would release in absence of the second aperture.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com