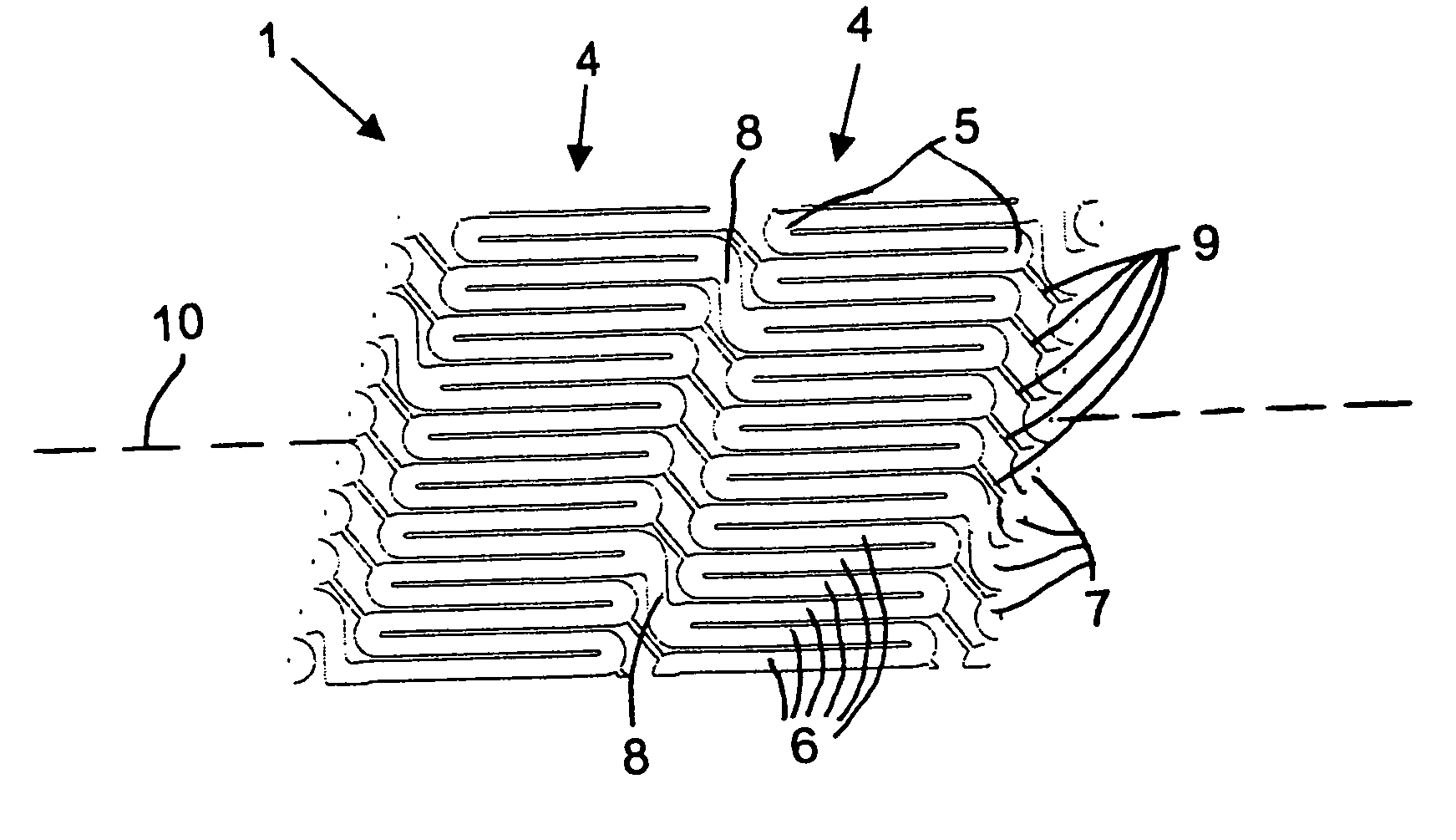
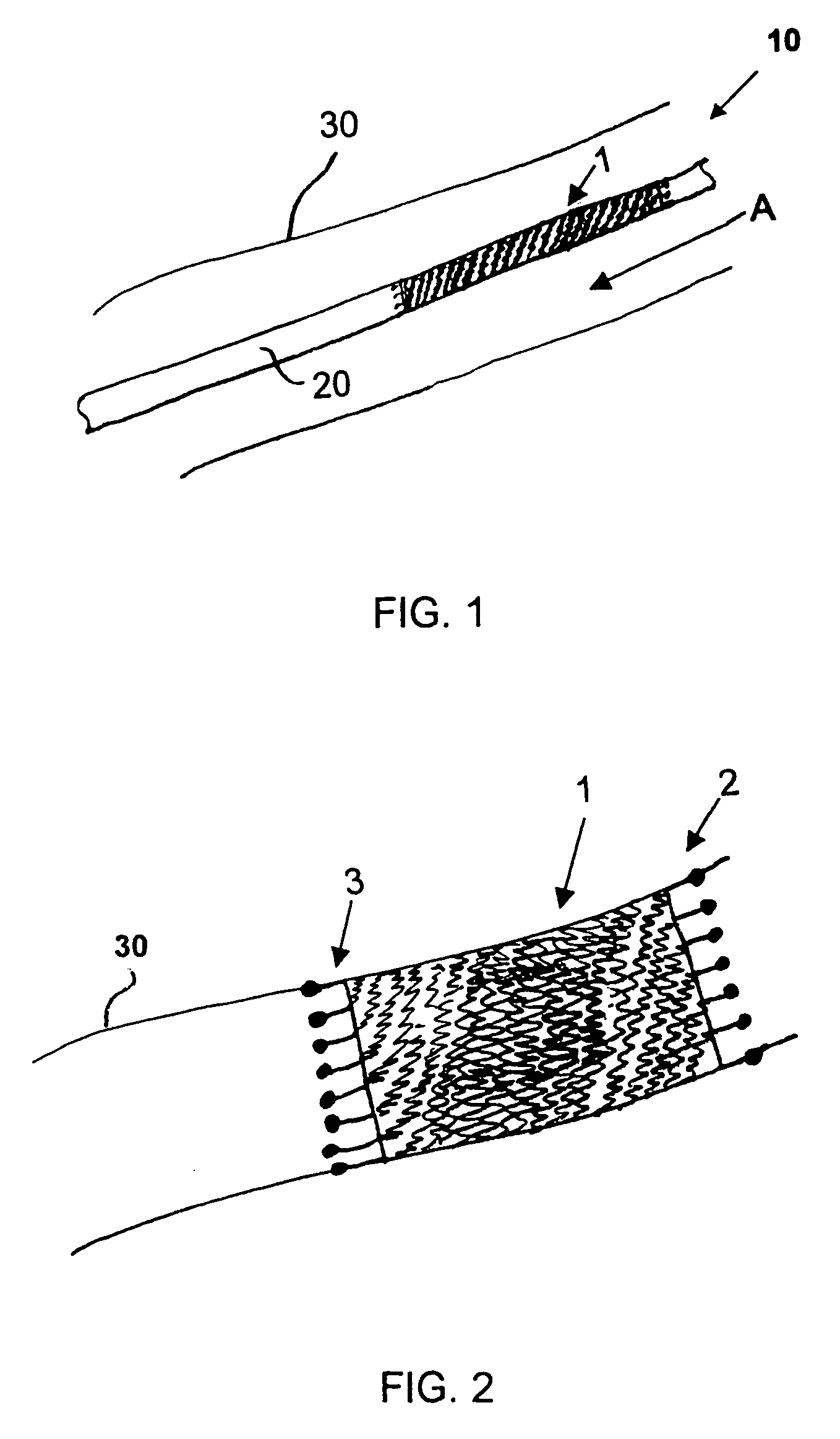
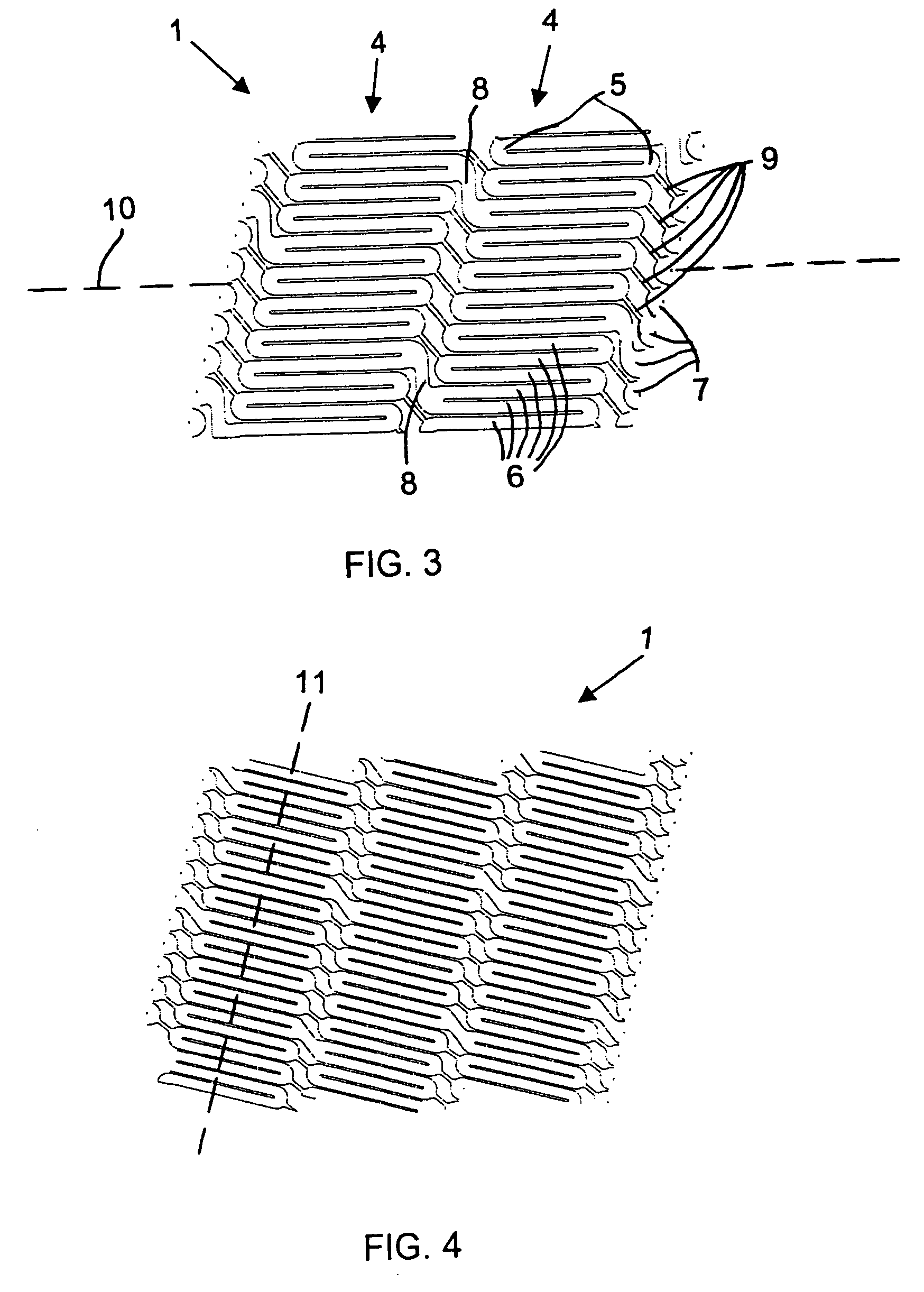
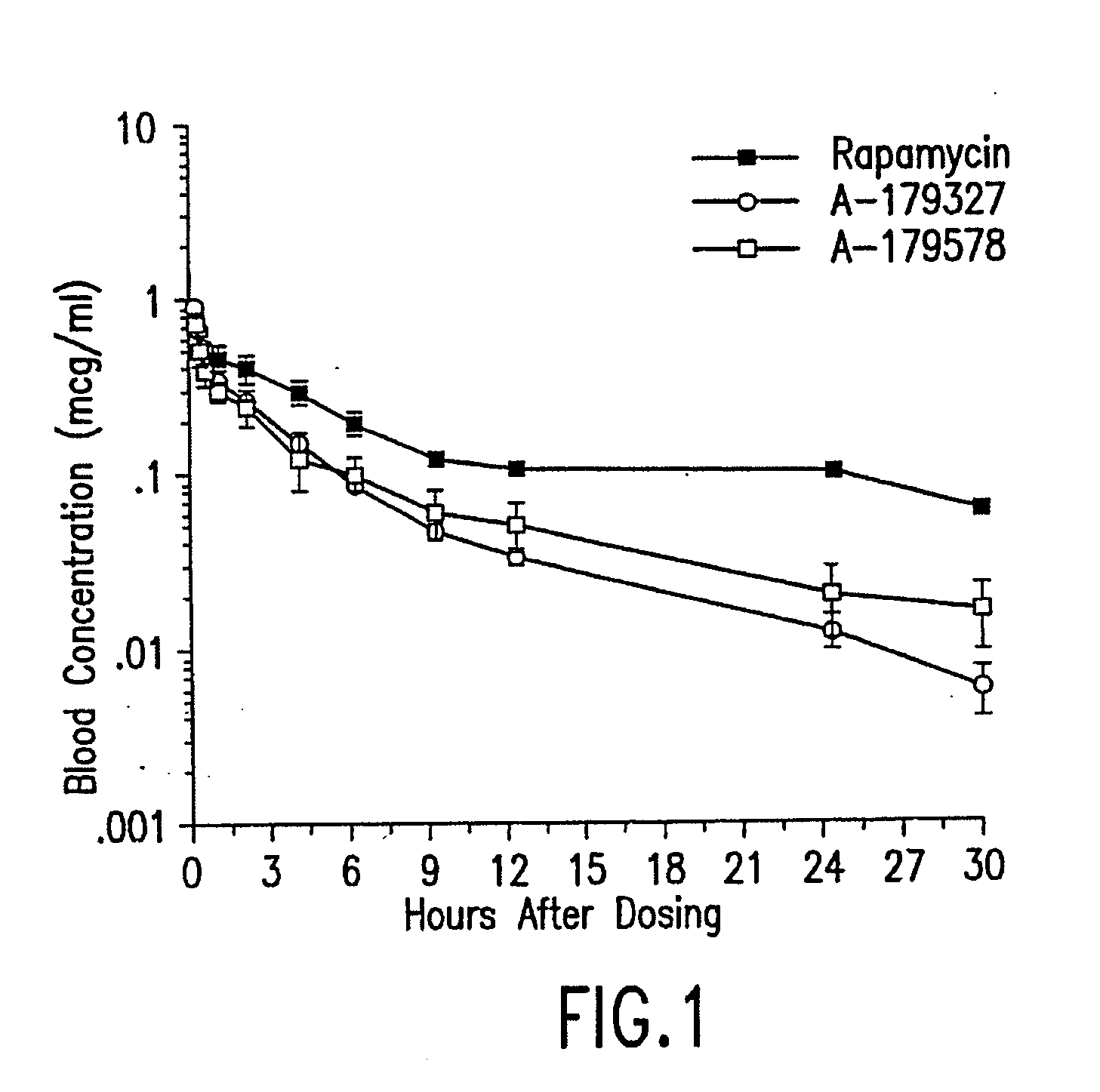
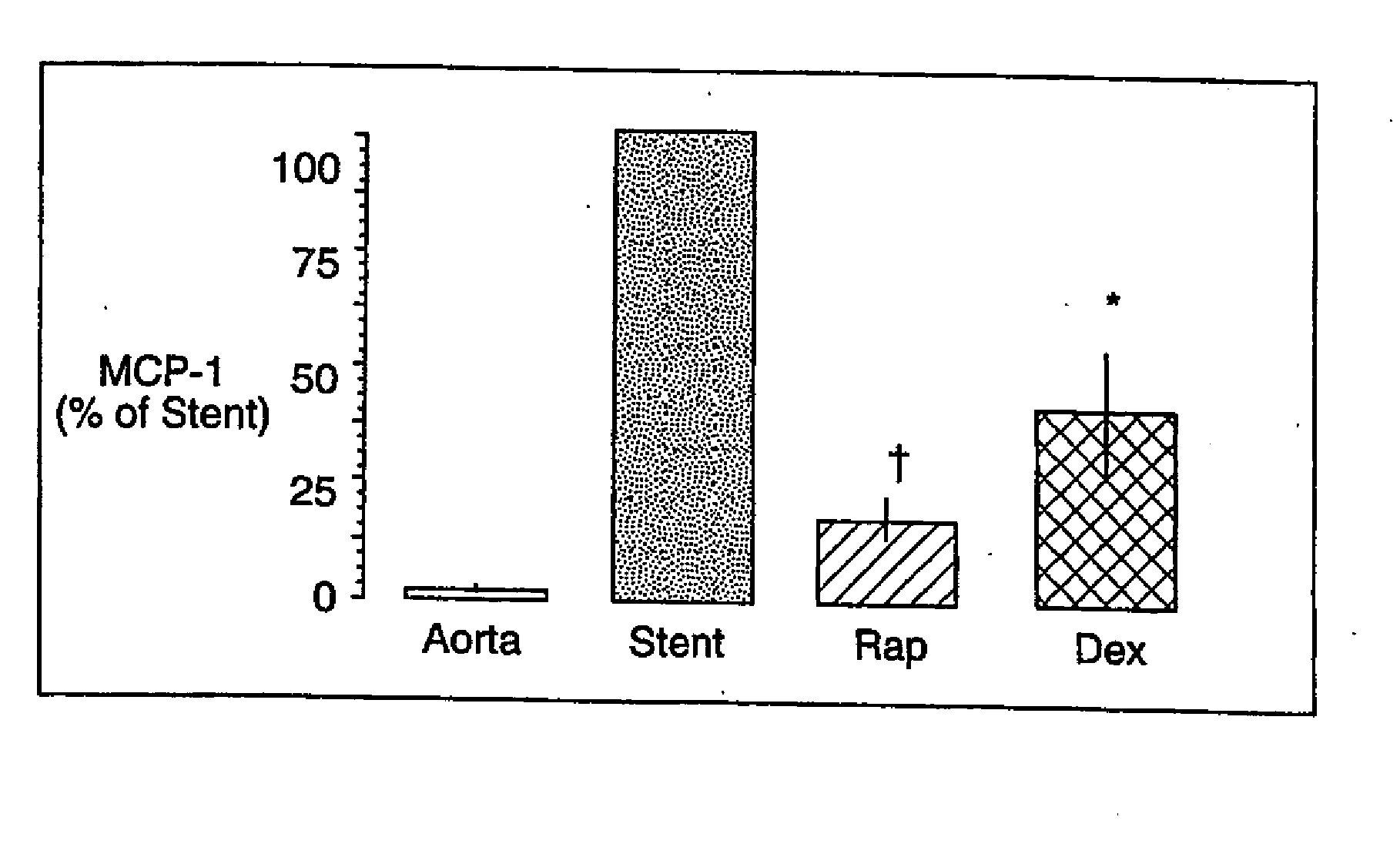
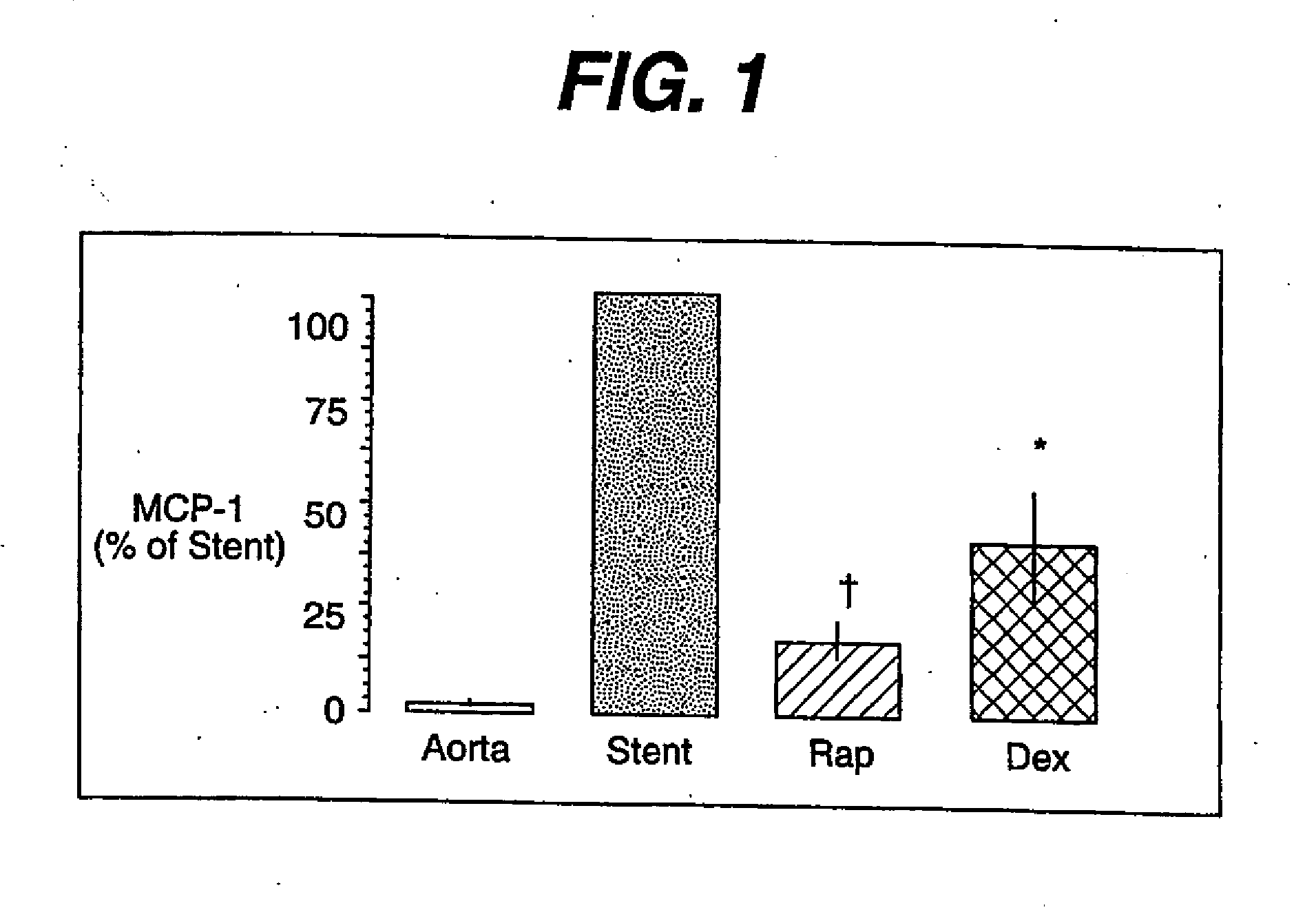
Patents
Literature
128results about How to "Reduce restenosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
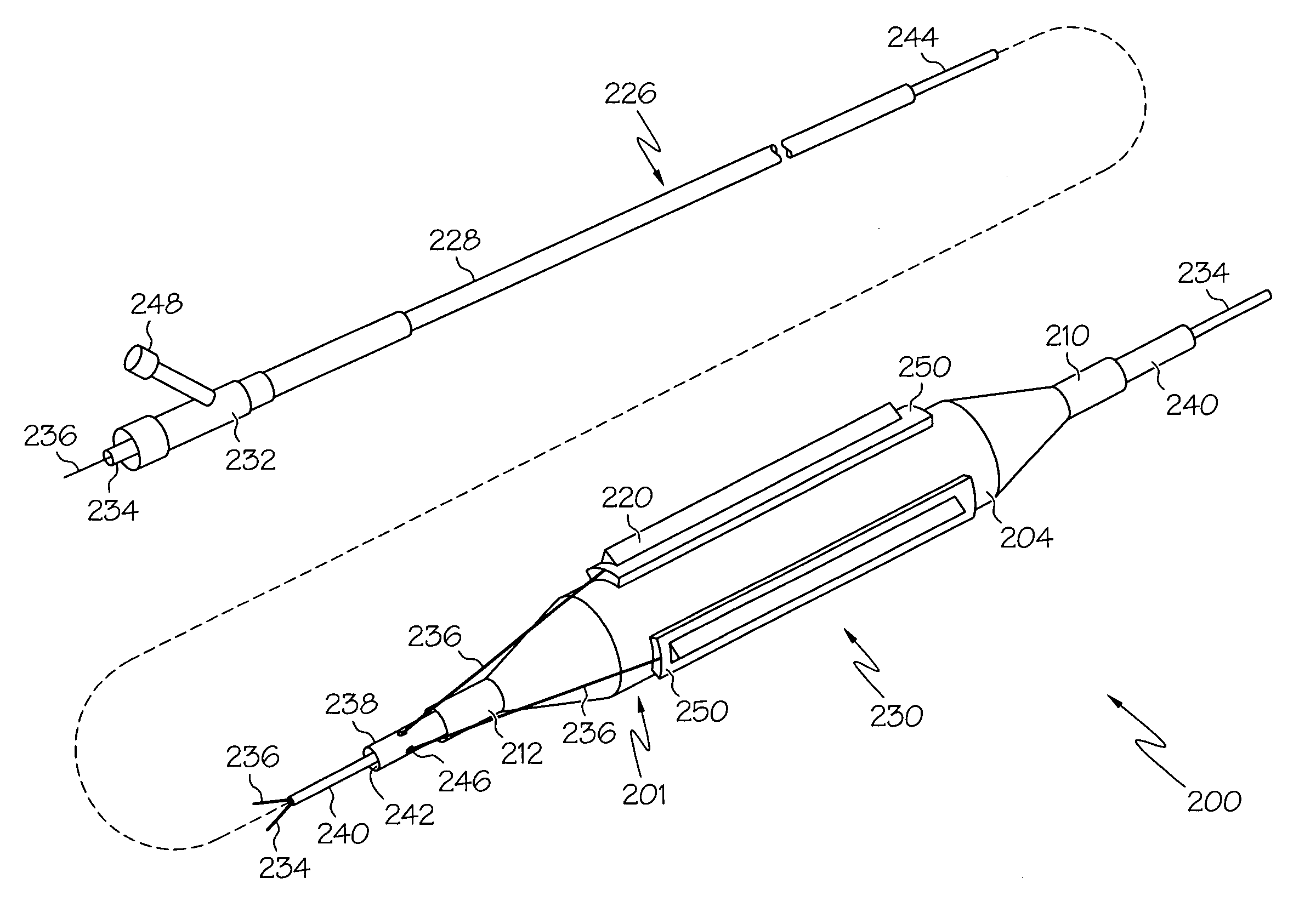
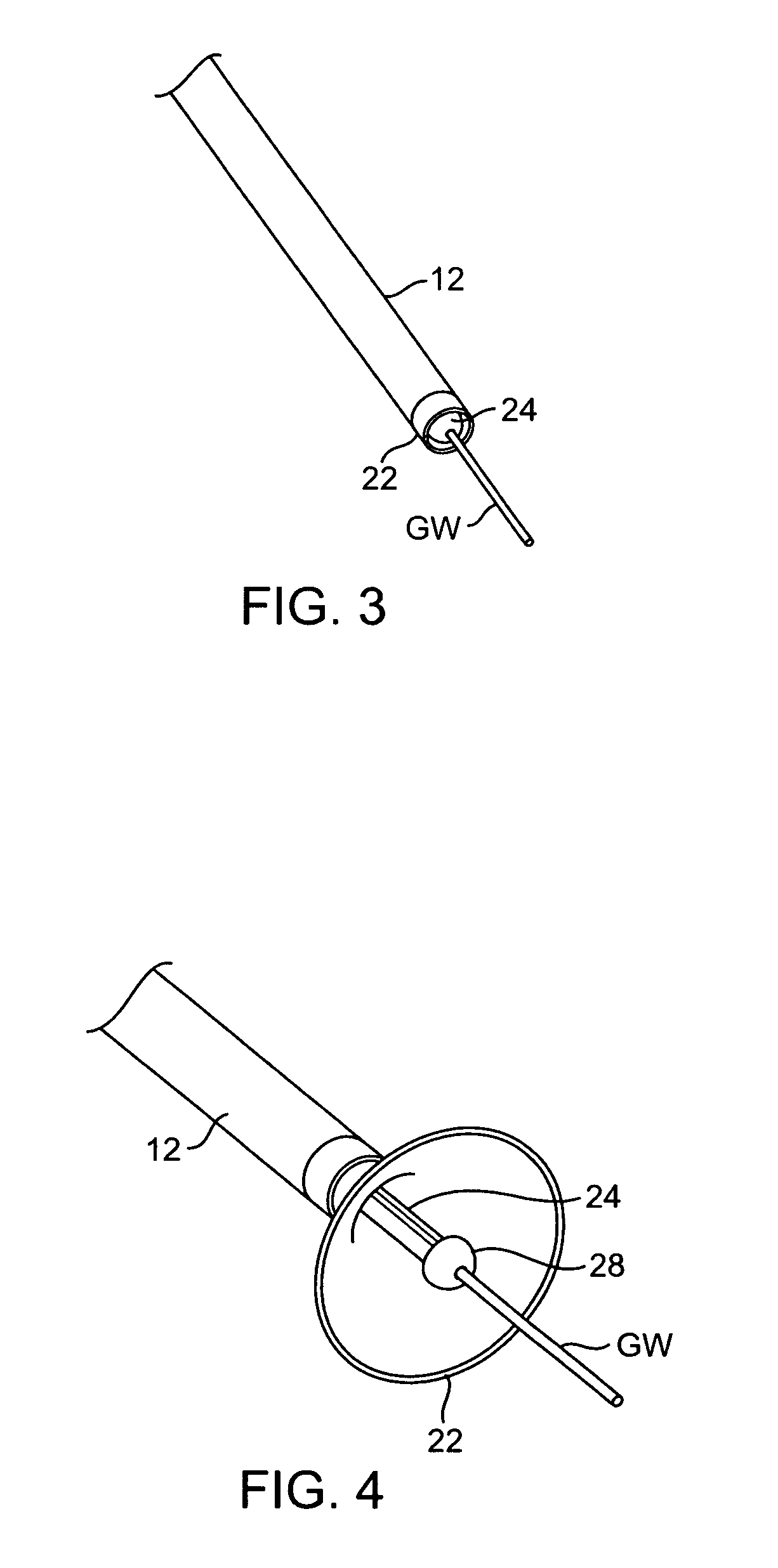
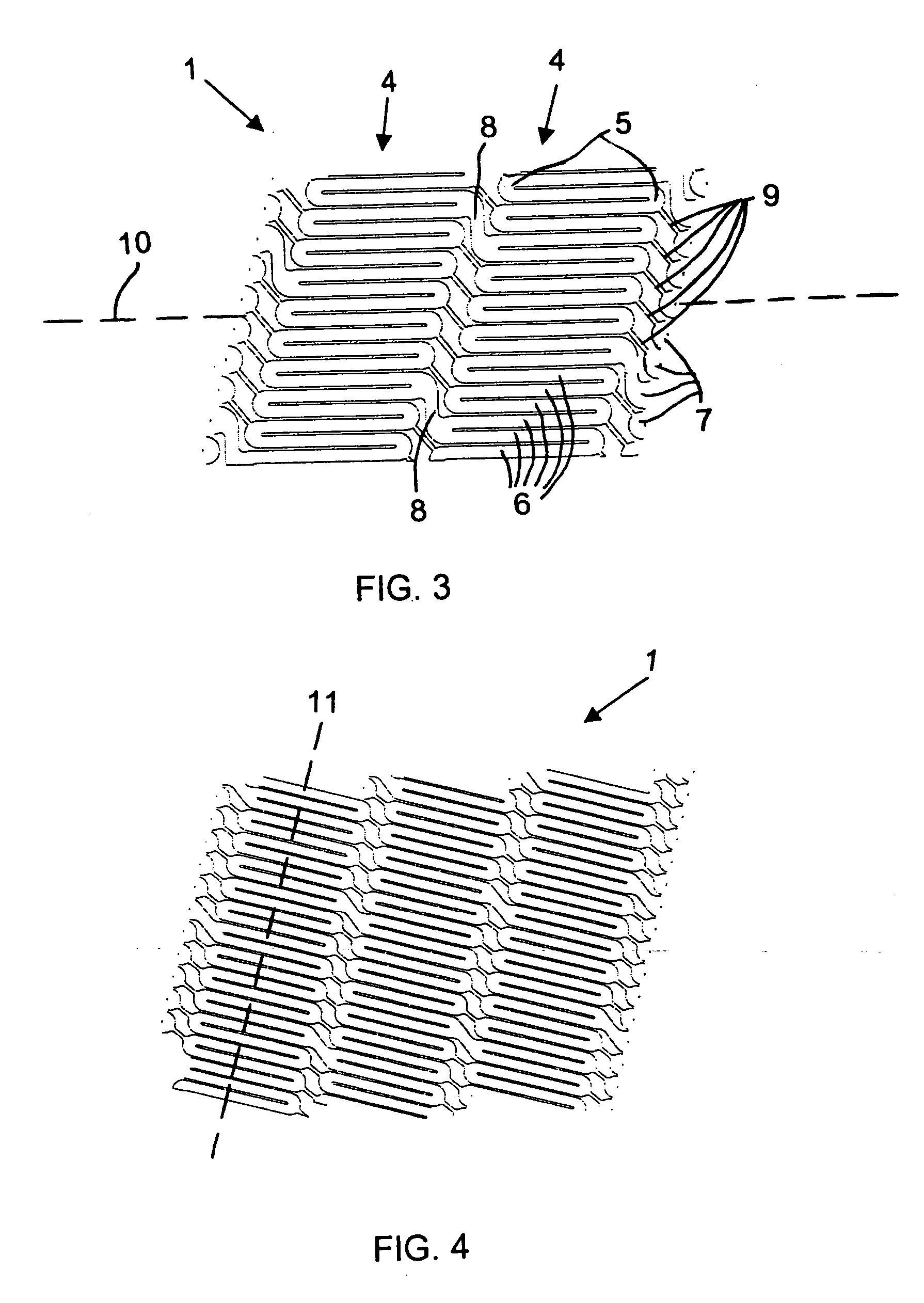
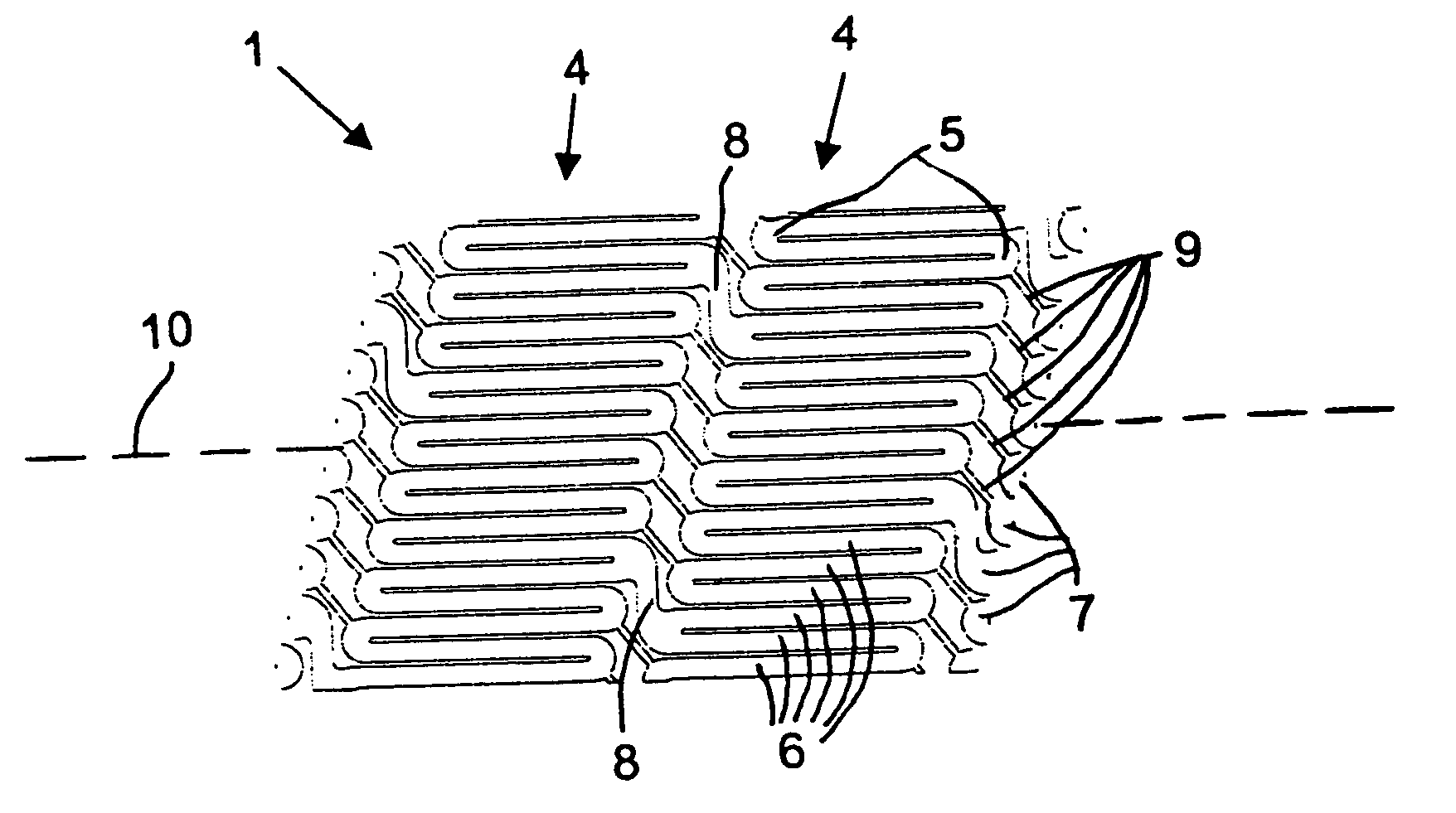
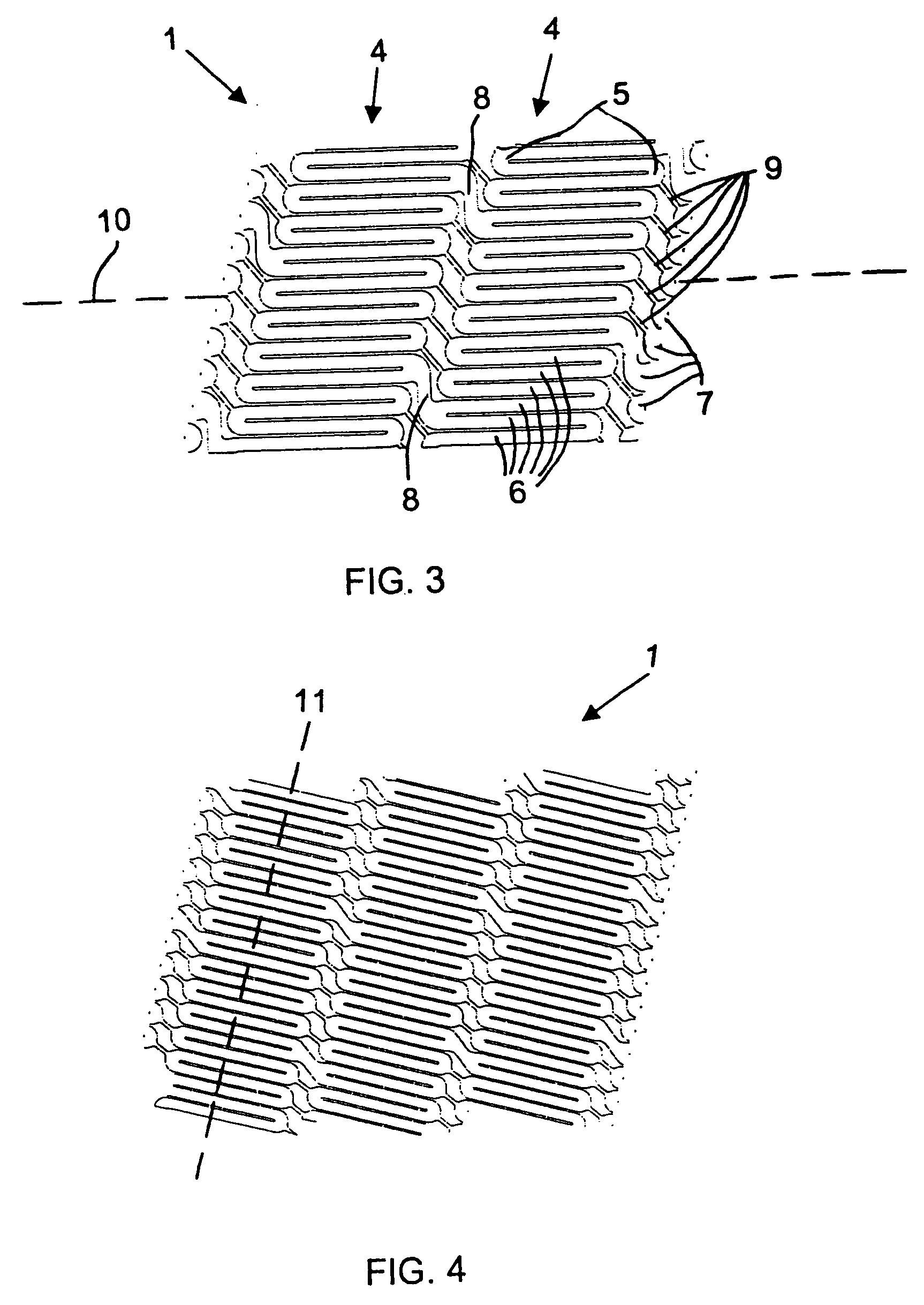
Catheter balloon with ultrasonic microscalpel blades
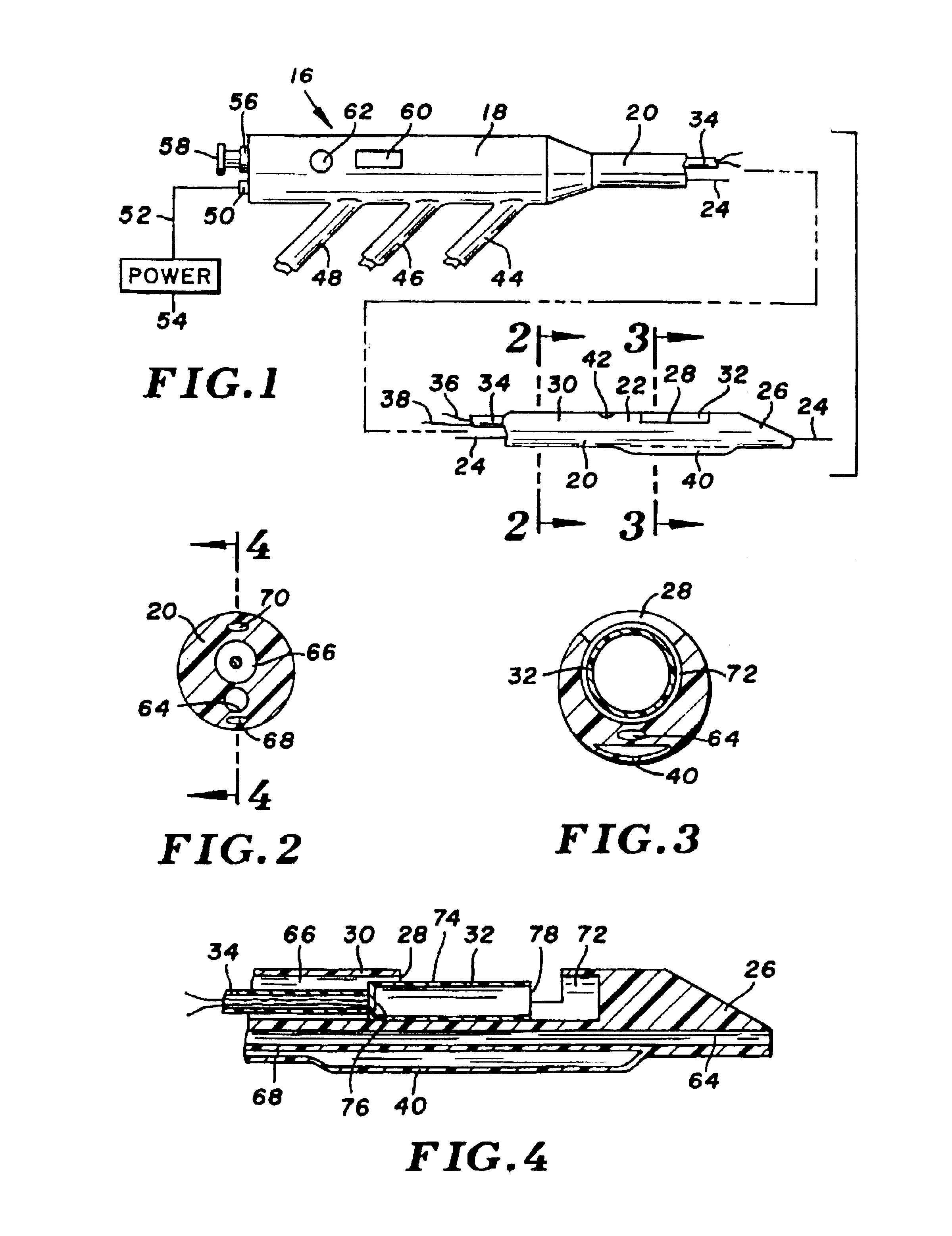
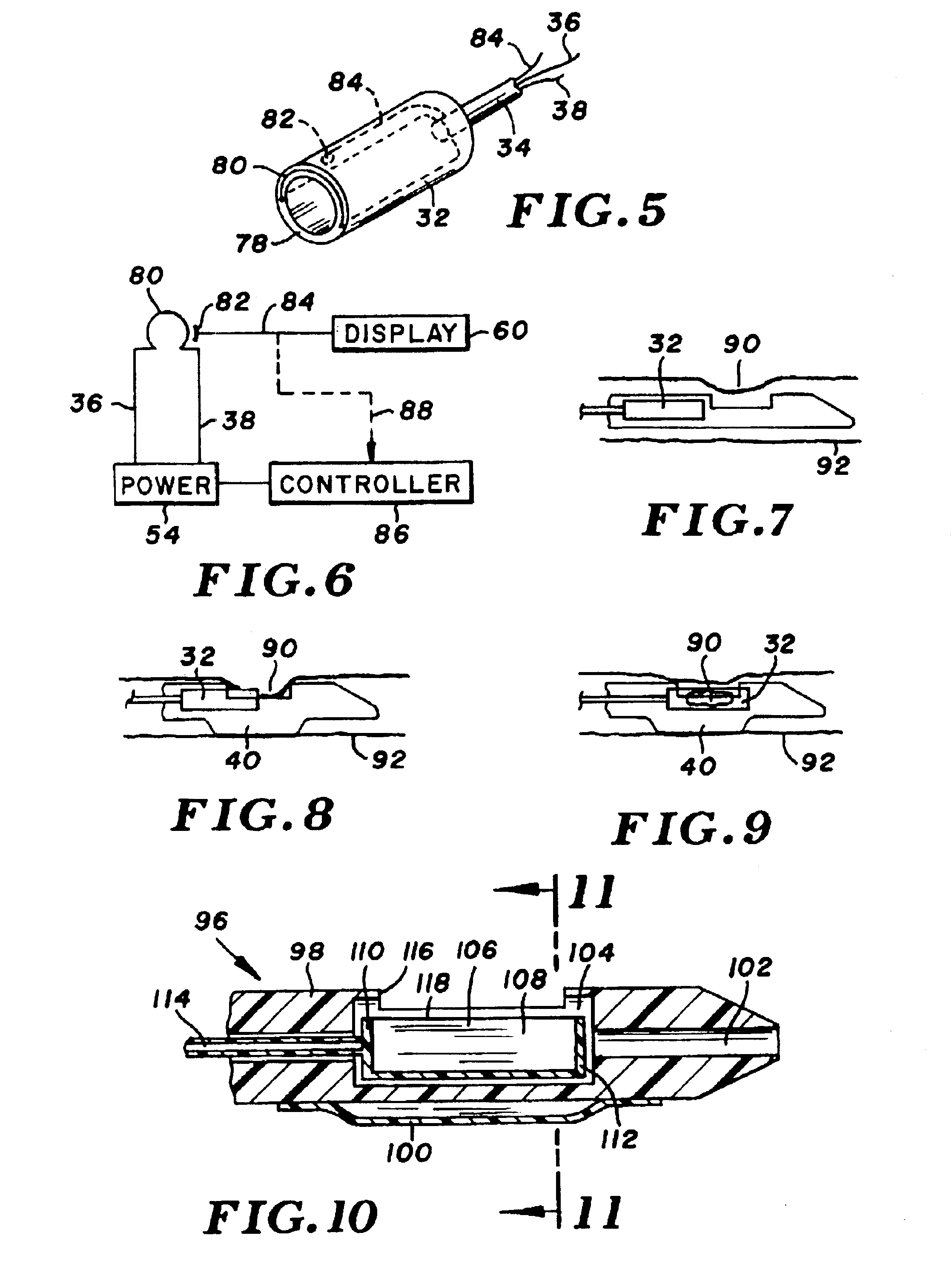
InactiveUS7153315B2Much sharper and cleaner incisionsCutting precision can be improvedCannulasSurgical instrument detailsBalloon catheterMedical treatment
The present invention provides a catheter balloon, and balloon catheter incorporating the catheter balloon, useful in medical dilation procedures. The catheter balloon includes at least one microscalpel operatively disposed on an outer surface thereof. The microscalpel may advantageously be operatively disposed relative to a power source so as to be controllably activatable. Also provided are methods of making the inventive balloon and / or catheter as well as methods of using the inventive catheter in a dilation / incising treatment.
Owner:BOSTON SCI SCIMED INC
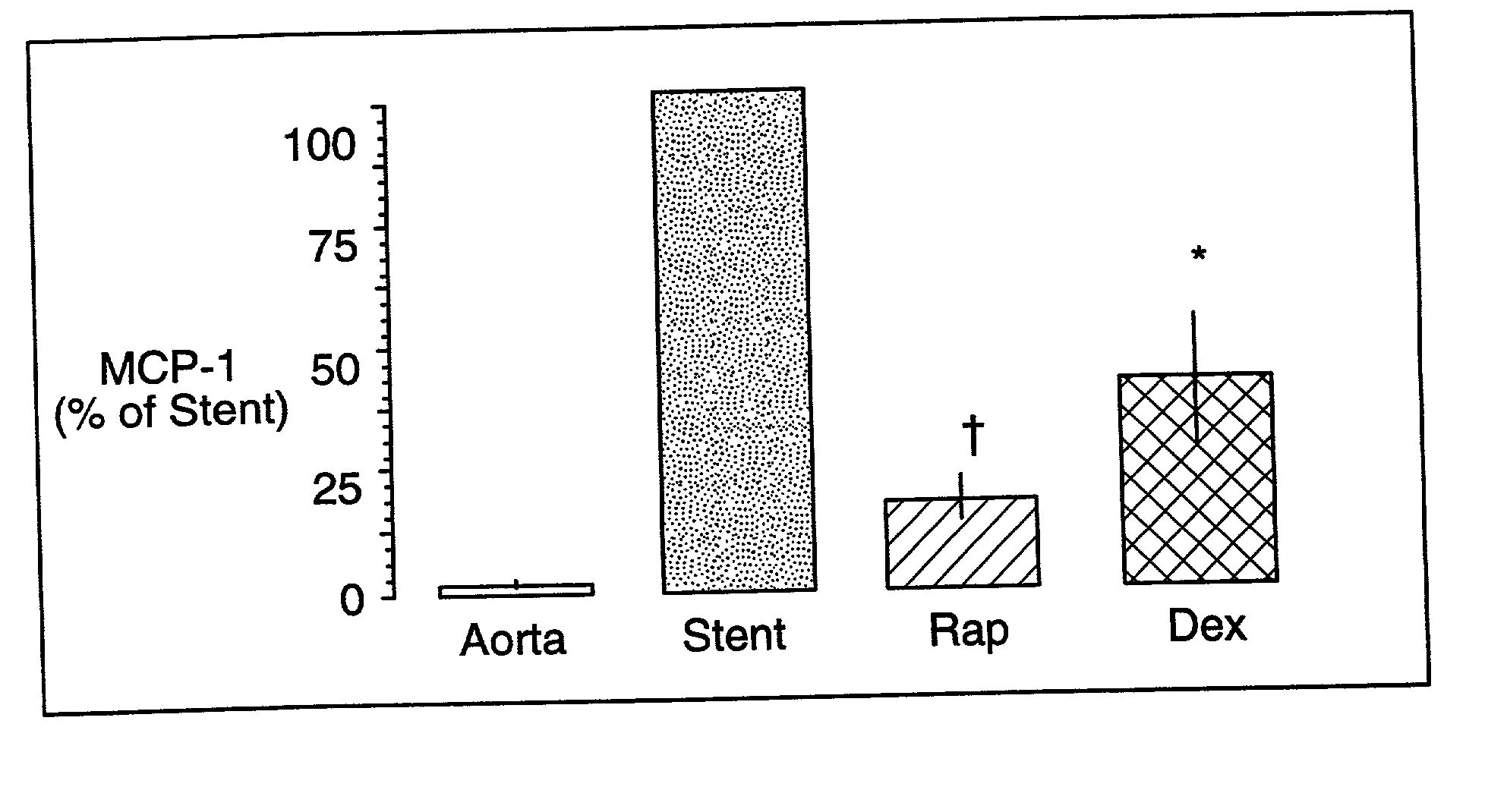
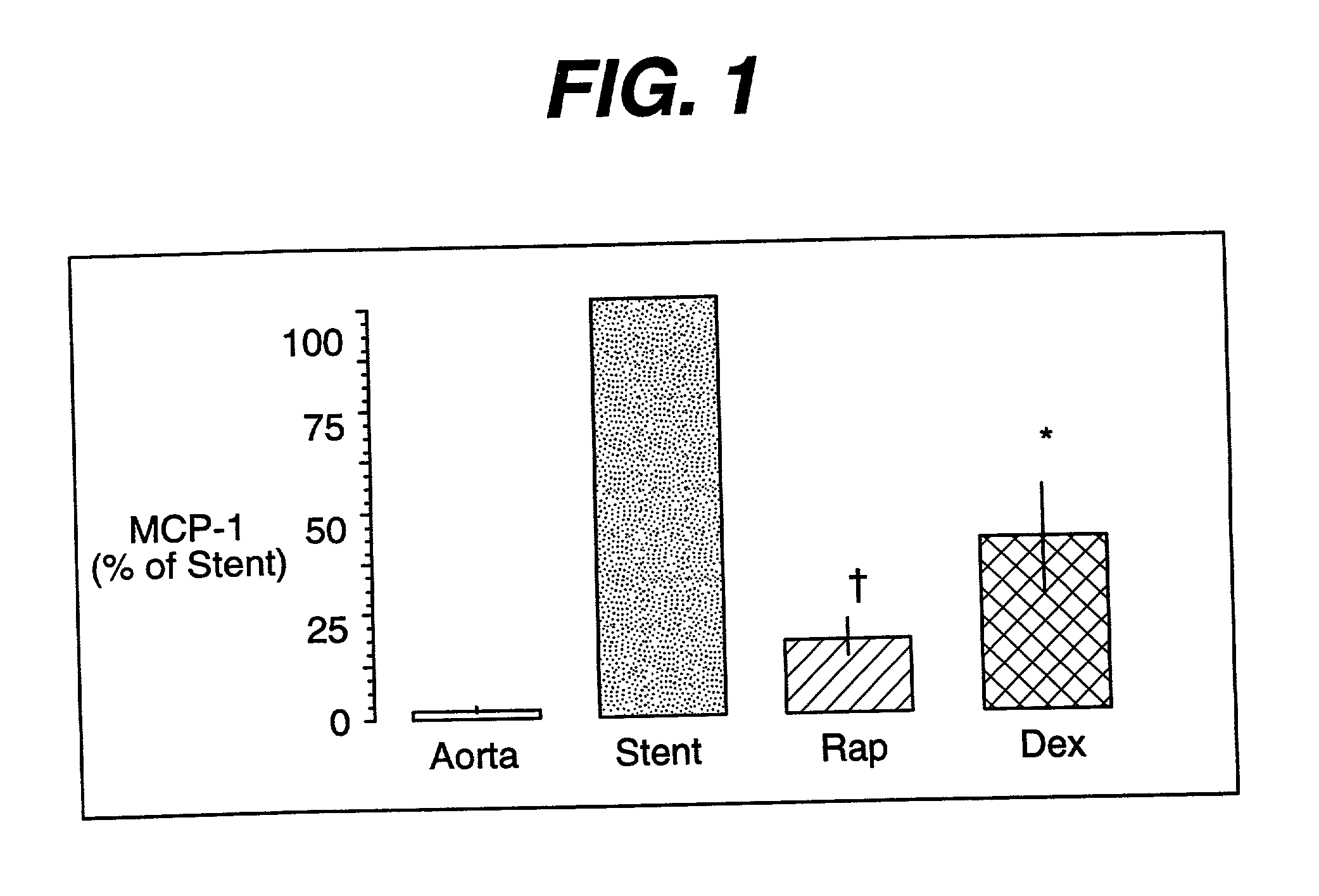
Drug/drug delivery systems for the prevention and treatment of vascular disease
InactiveUS20020007215A1Prevent proliferationGood effectOrganic active ingredientsStentsVascular diseaseWhole body
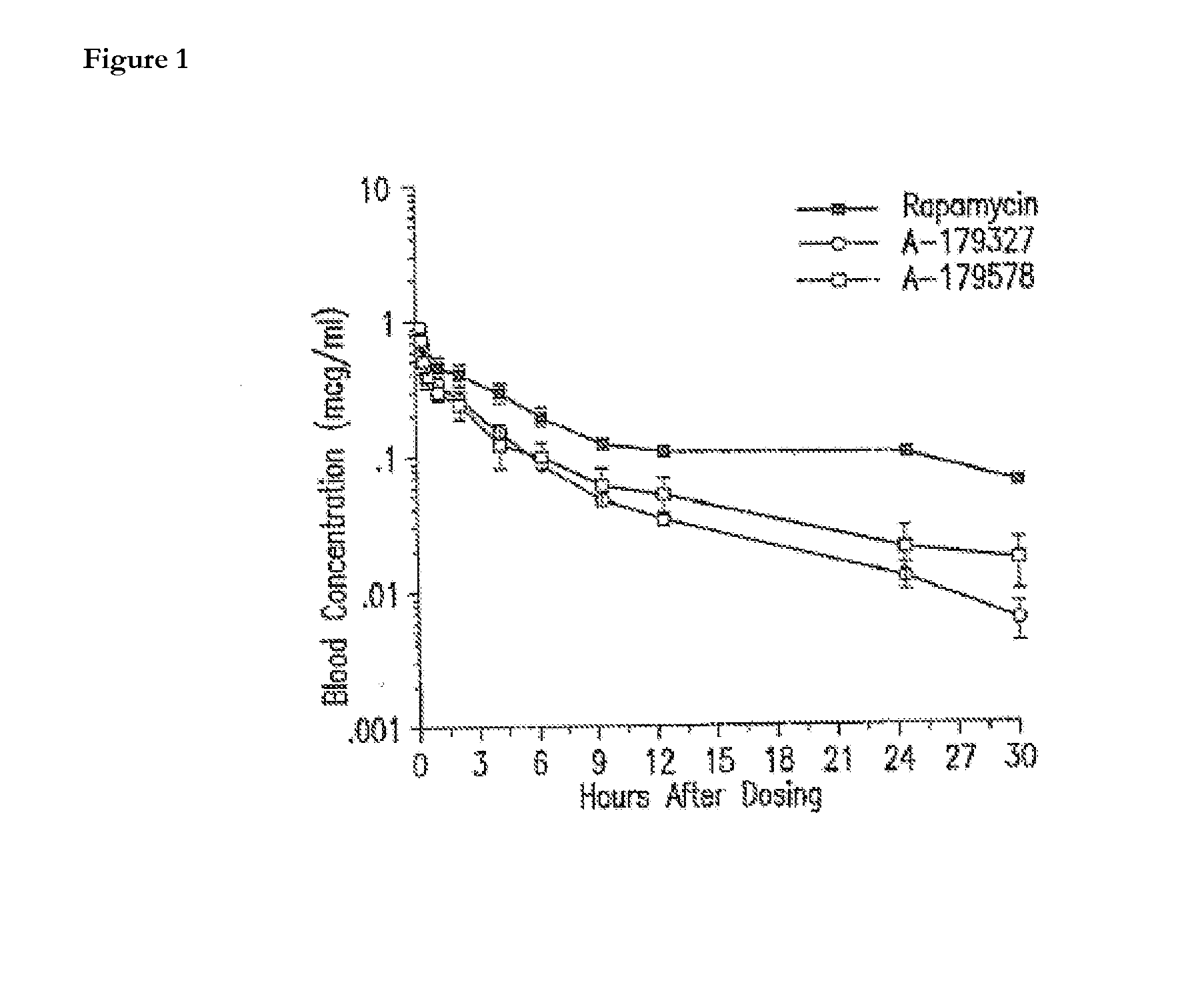
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH LLC
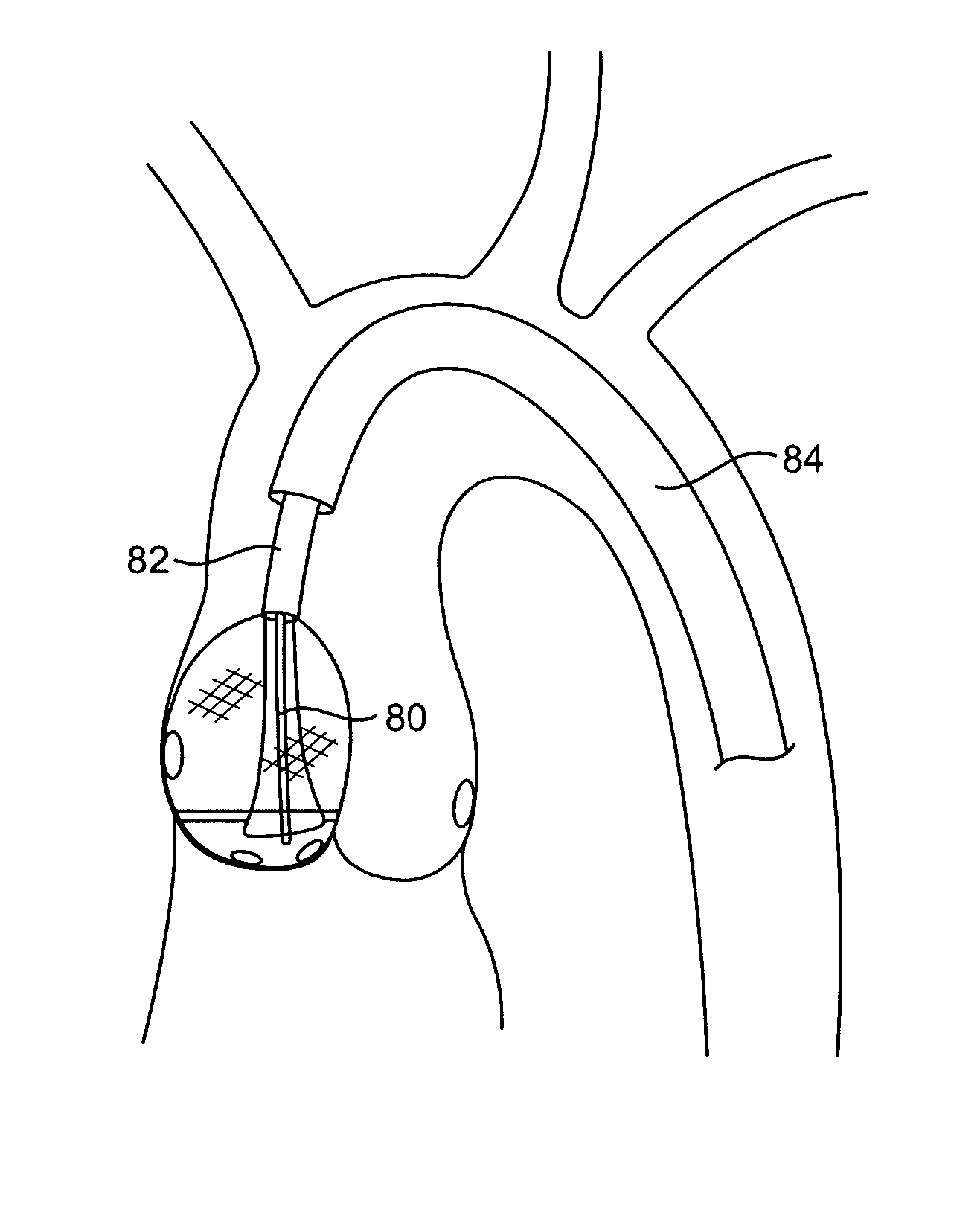
Aortic valve repair
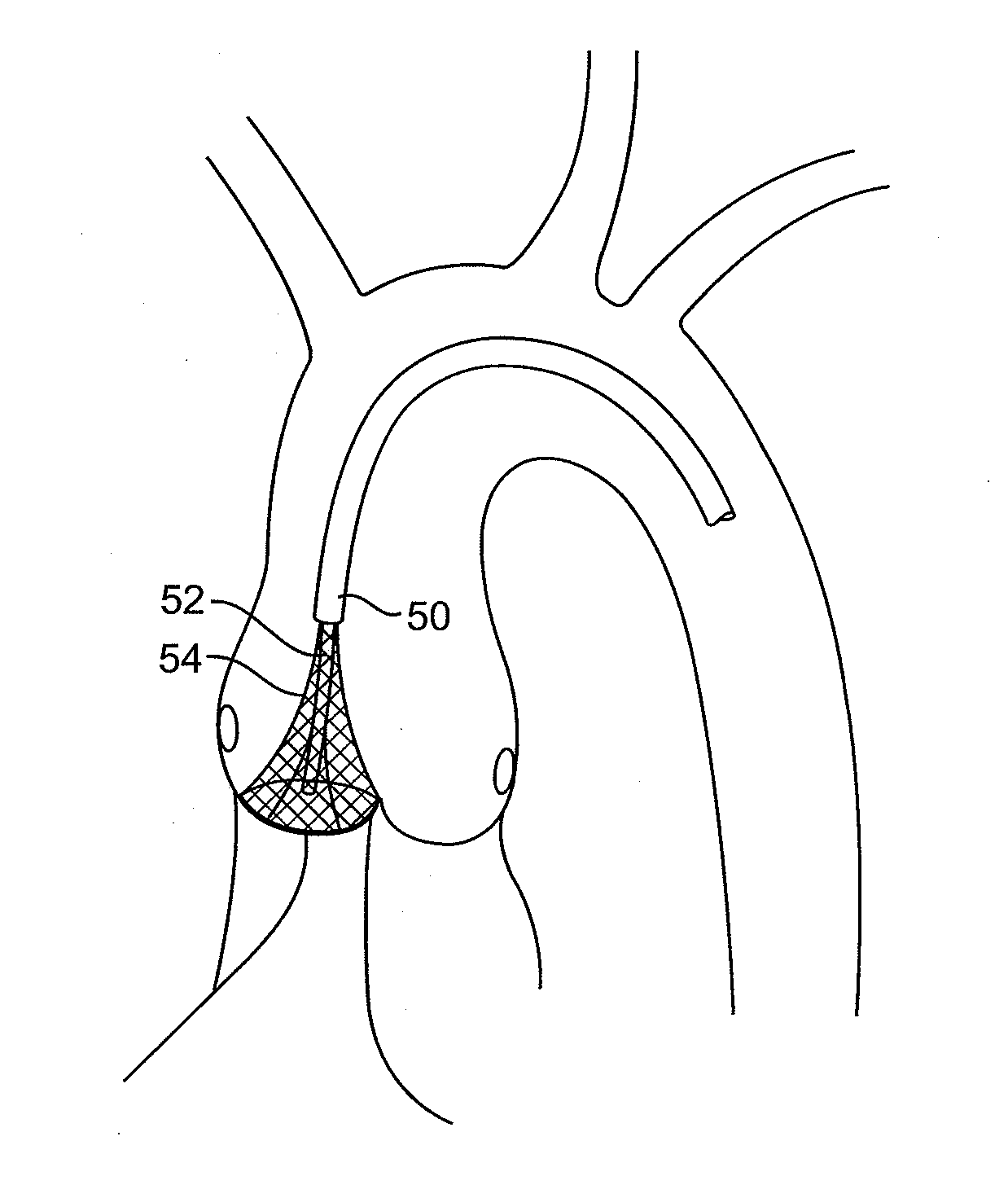
ActiveUS7803168B2Good curative effectReduce restenosisUltrasonic/sonic/infrasonic diagnosticsCannulasAortic valve repairNeoaortic valve
The present invention provides devices and methods for decalcifying an aortic valve. The methods and devices of the present invention break up or obliterate calcific deposits in and around the aortic valve through application or removal of heat energy from the calcific deposits.
Owner:TWELVE
Medical device
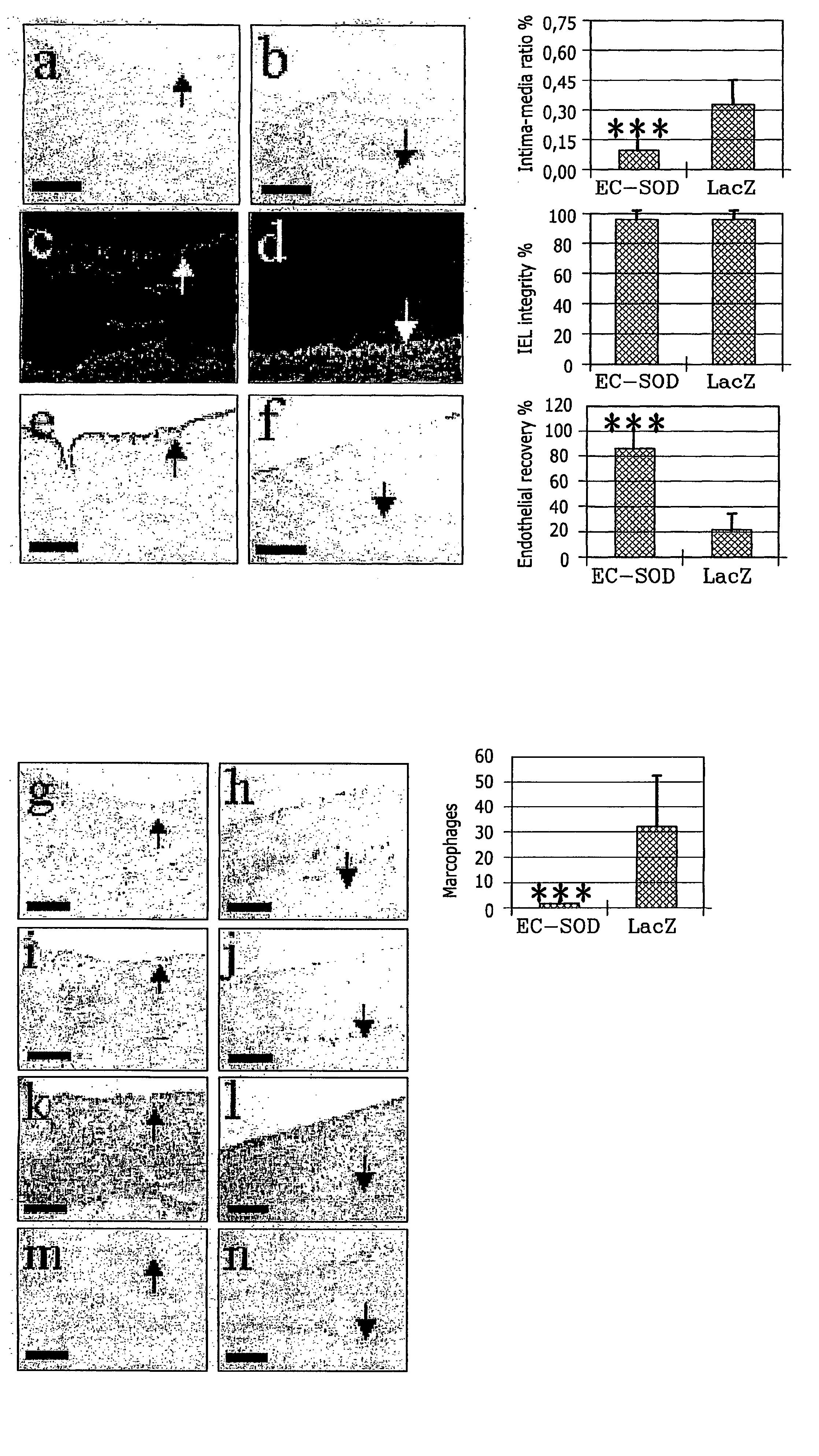
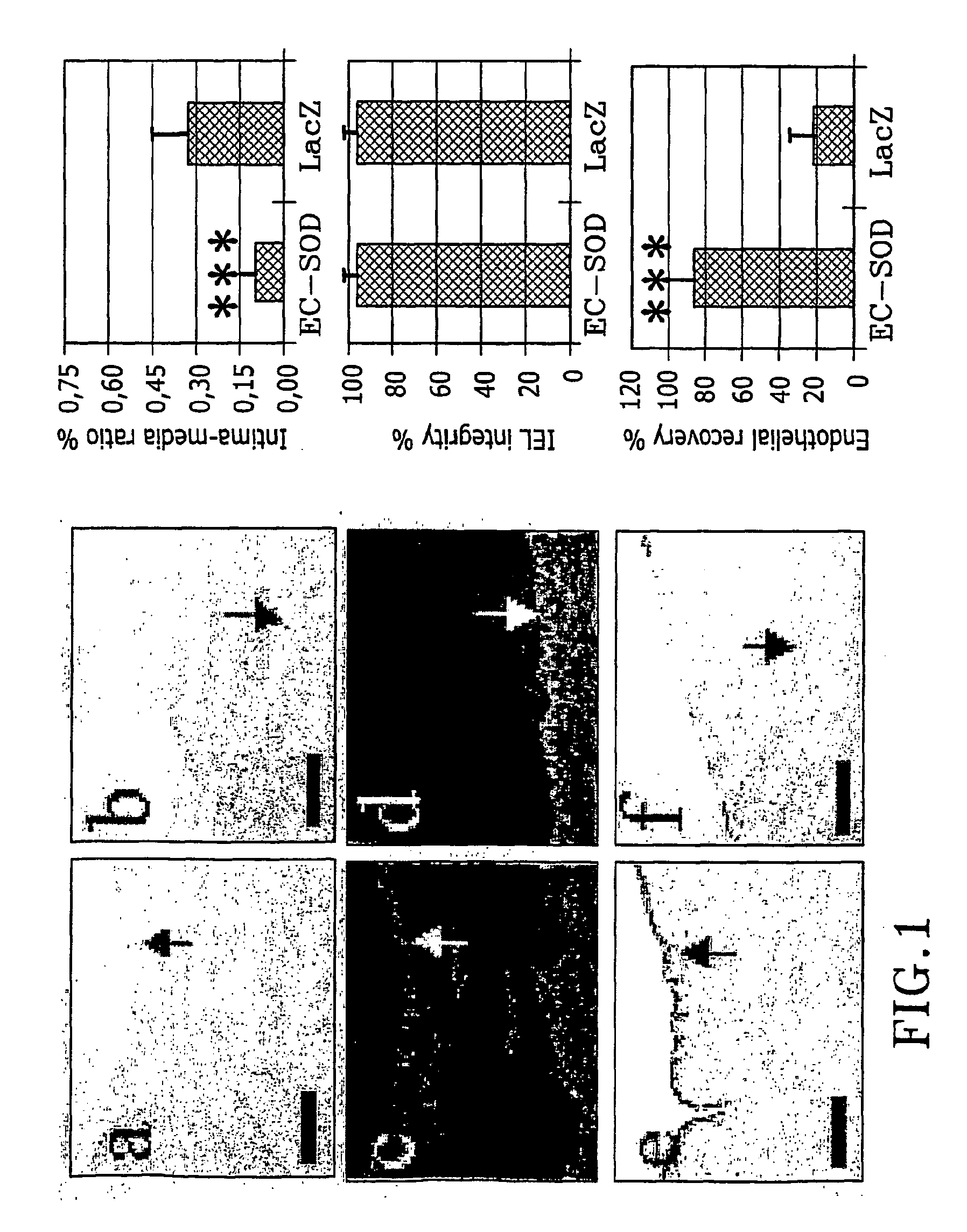
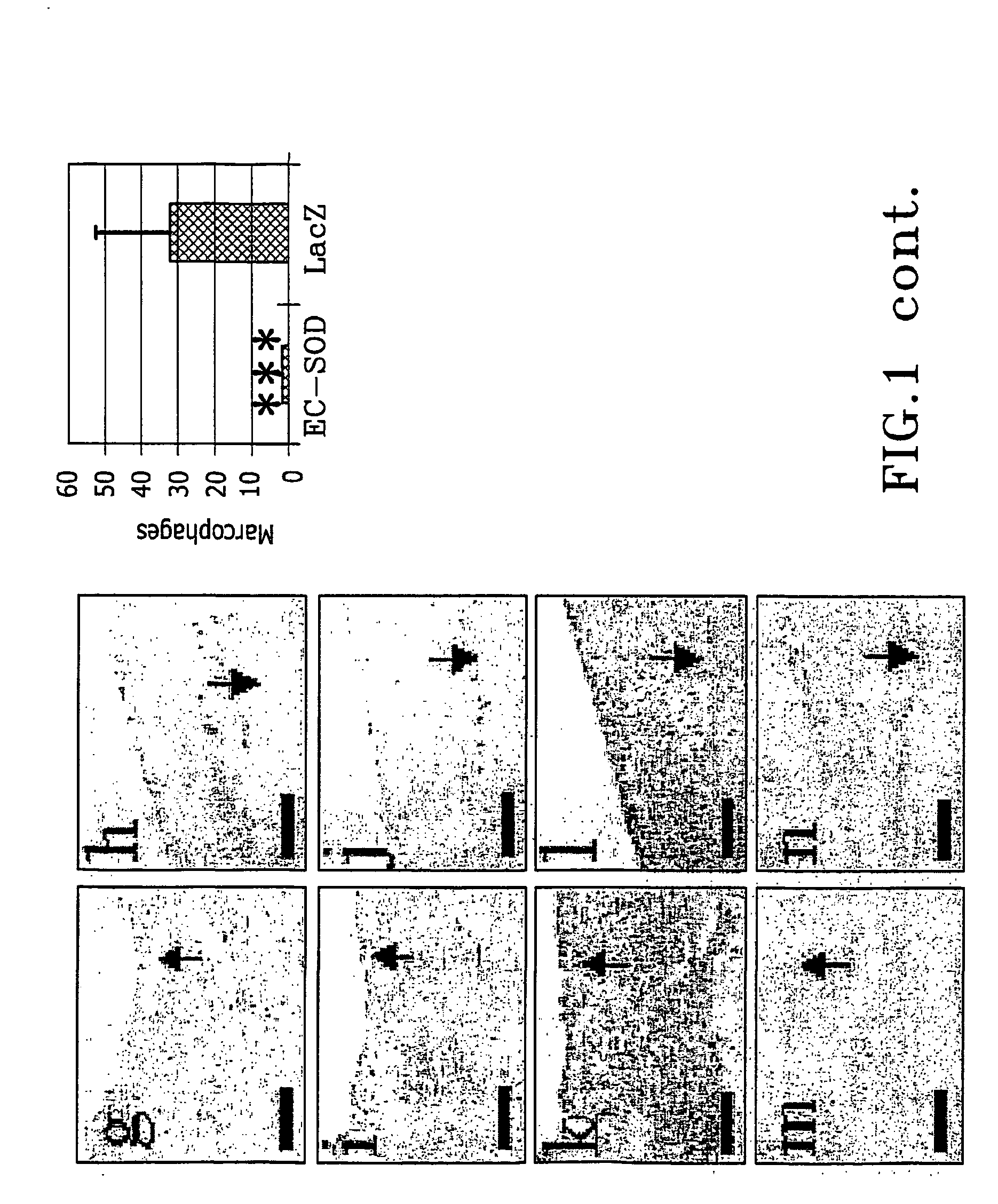
InactiveUS20050002981A1Reduce connective tissue hyperplasiaReduce restenosisStentsPeptide/protein ingredientsBiological propertyConnective tissue fiber
The present invention relates to the use of a gene transfer product to reduce hyperplastic connective tissue growth after tissue trauma or implantation of a medical device. The present invention also relates to a medical device with improved biological properties for an at least partial contact with blood, bodily fluids and / or tissues when introduced in a mammalian body, which device comprises a core and a nucleic acid, encoding a product capable of leading to production of extracellular superoxide dismutase present in a biologically compatible medium. Said nucleic acid encodes a translation or transcription product, which is capable of inhibiting hyperplastic connective tissue growth and promoting endothelialisation in vivo at least partially on a synthetic surface of said core. The present invention also relates to a method of producing a medical device according to the invention.
Owner:FIT BIOTECH OY PLC
Delivery of Highly Lipophilic Agents Via Medical Devices
InactiveUS20090216317A1Easy to transportIncrease drug retentionAntibacterial agentsBiocideBiomedical engineeringDisease
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT CARDIOVASCULAR +1
Delivery of highly lipophilic agents via medical devices
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
Delivery of highly lipophilic agents via medical devices
Owner:ABBOTT LAB INC
Delivery of highly lipophilic agents via medical devices
InactiveUS20060240070A1Easy to transportIncrease drug retentionBiocideFibrinogenMedicineMedical device
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
Cell seeded expandable body
InactiveUS20050096731A1Promote regenerationAugment tissue repairStentsSurgeryInsertion stentBody cavity use
Devices, systems and methods for treating medical conditions using cell therapy via body lumens. Localized delivery is achieved with the use of a stent-like expandable body seeded with cells. The expandable body is expanded to contact at least a portion of the inner walls of the body lumen and the cells, cellular products and / or other therapeutic agents are delivered to the surrounding tissue. The therapeutic benefit provided is dependent on the type of cells used and the features of the expandable body.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
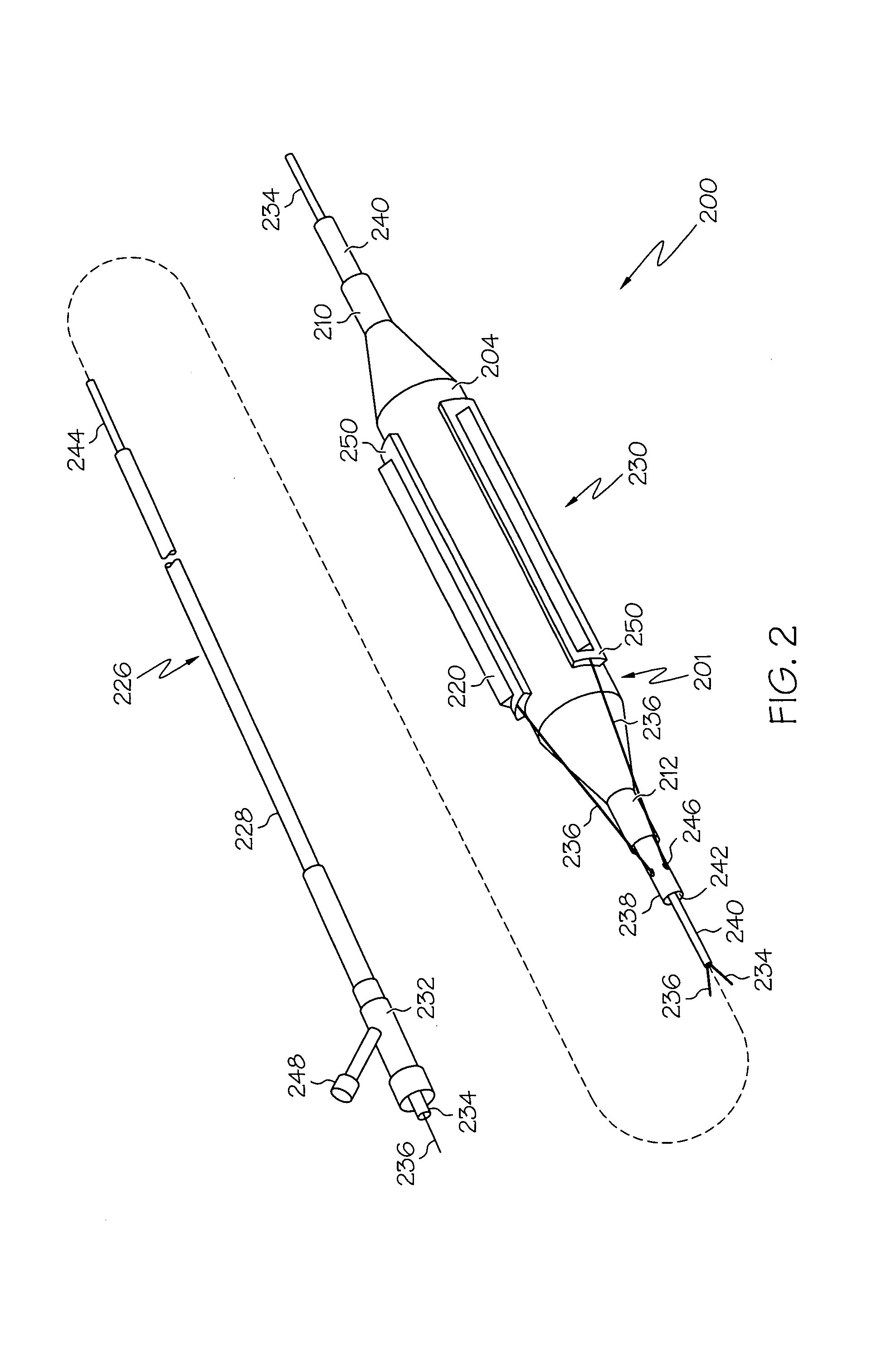
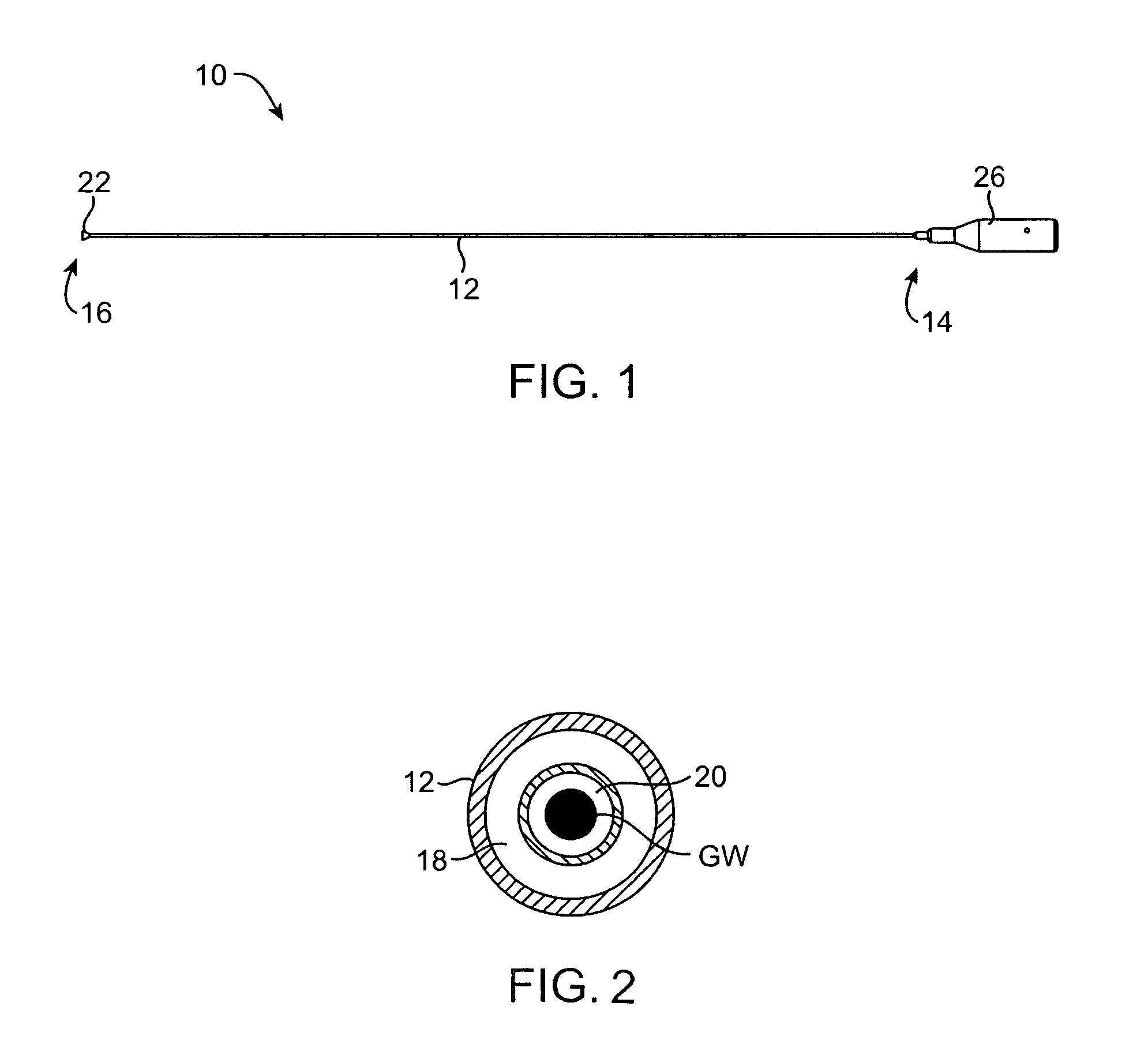
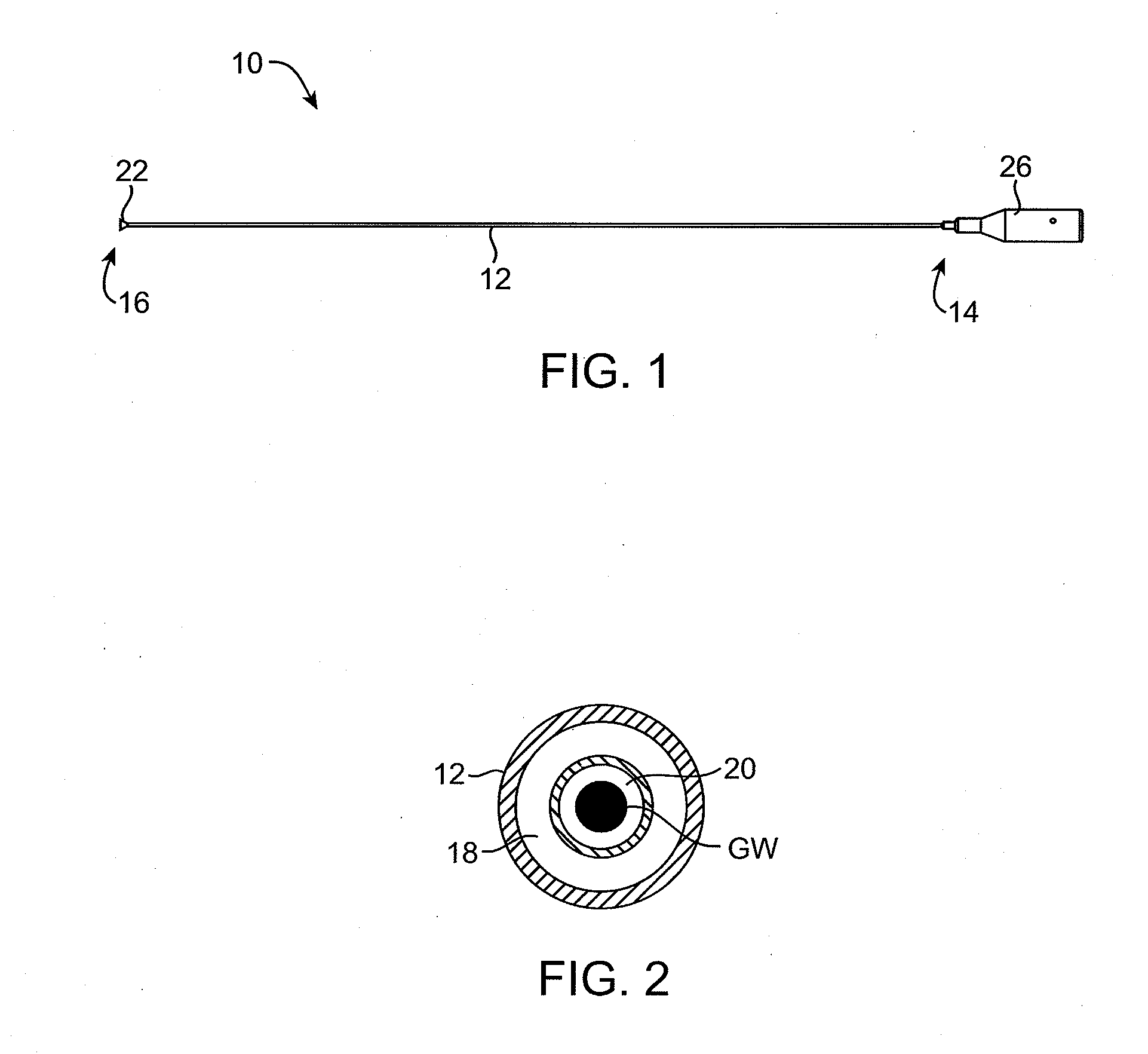
Tissue cutting catheter and RF cutting method
InactiveUS6872204B2Highly accurate and fine cutReduce pullingDiagnosticsCatheterElectrical resistance and conductanceTissue heating
A tissue cutting device includes a catheter with a window at its distal tip for admitting tissue into a catheter compartment. A cylindrical cartridge in the compartment has a cutting edge that supports an electrically conductive cutting element, e.g. a band or wire. The cutting element and adjacent tissue can be heated to a selected temperature by generating an electrical current through the cutting element. The catheter is maneuverable to position its distal end near the tissue to be cut. The catheter incorporates a dilatation balloon or other feature to urge the catheter against the tissue, so that at least part of the tissue may enter the compartment through the window. Then, the cartridge is manipulated from the catheter's proximal end to move the cutting edge across the window, cutting the tissue. According to alternative embodiments, the cartridge is either rotated or moved axially relative to the catheter and, in either event may be capable of closing the catheter window when the cut is complete. Further alternatives involve either placing an indifferent electrode on the patient and providing an RF signal via a single conductor to the cutting element for ohmic heating, or providing an RF (or a DC) current through the cutting element and two separate conductors for direct resistive heating of the cutting element.
Owner:CARDIOVASCULAR TECH INC
Aortic Valve Repair
InactiveUS20100324554A1Good curative effectReduce restenosisUltrasound therapyBalloon catheterAortic valve repairNeoaortic valve
The present invention provides devices and methods for decalcifying an aortic valve. The methods and devices of the present invention break up or obliterate calcific deposits in and around the aortic valve through application or removal of heat energy from the calcific deposits.
Owner:TWELVE INC
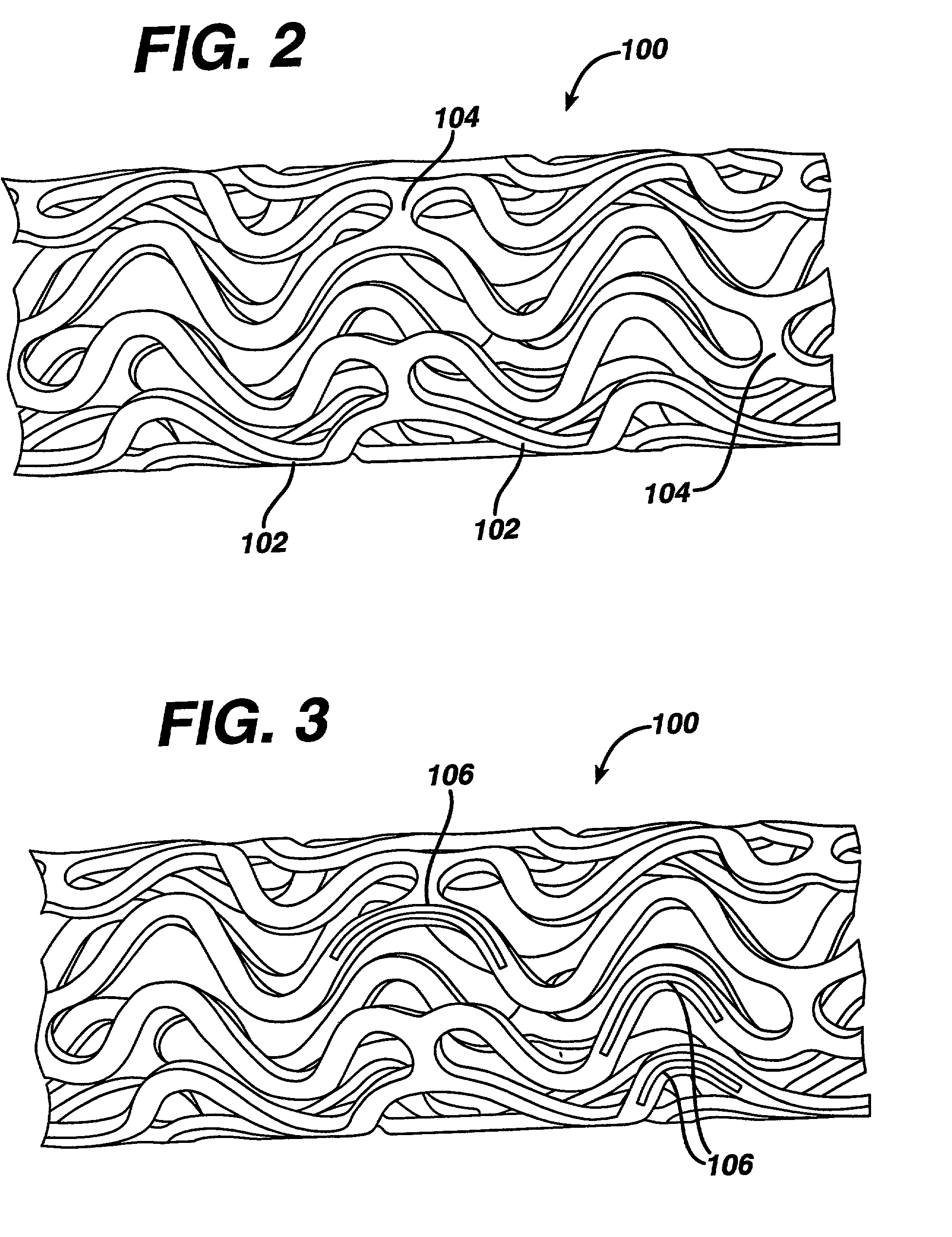
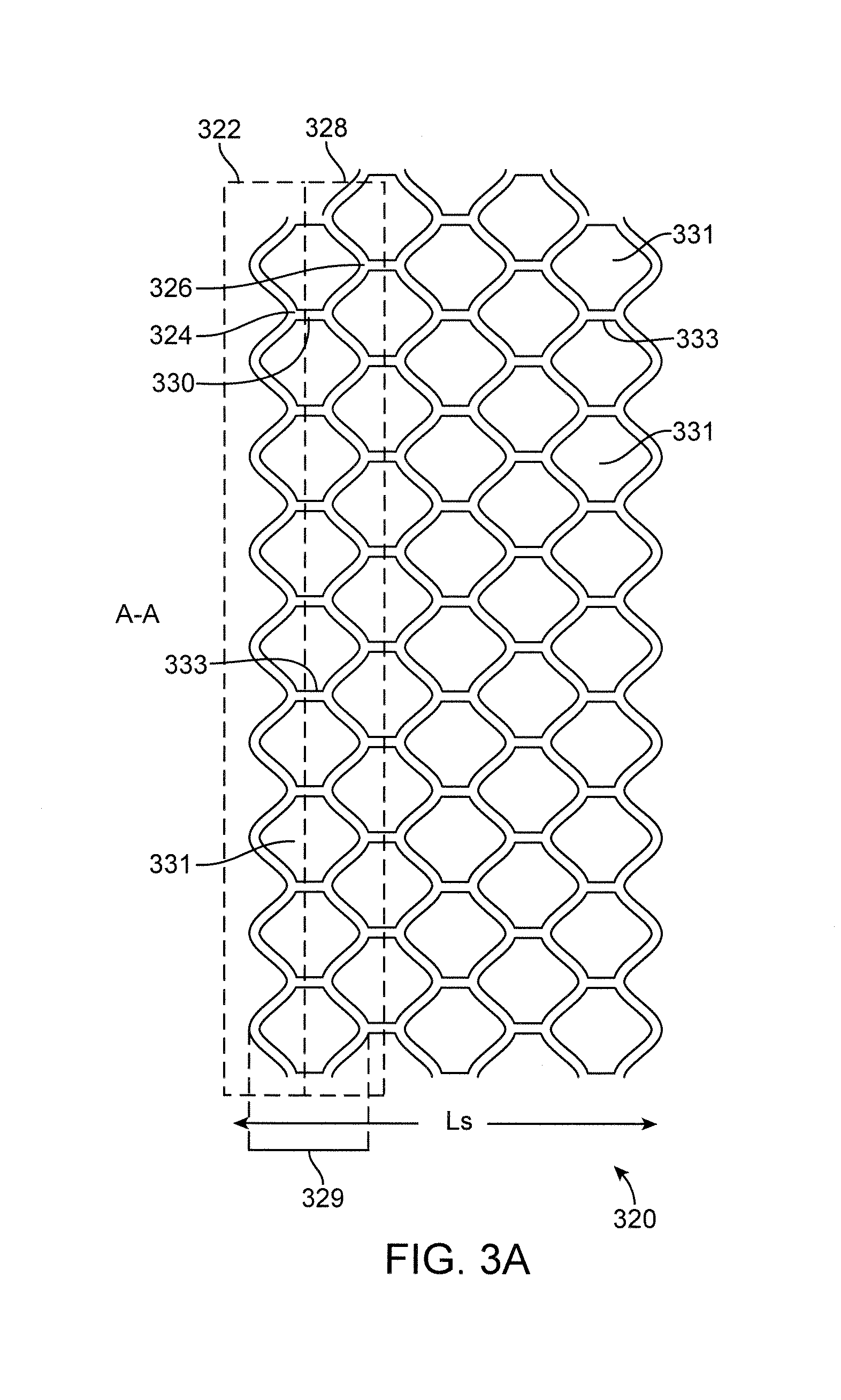
Stent and method for manufacturing the stent
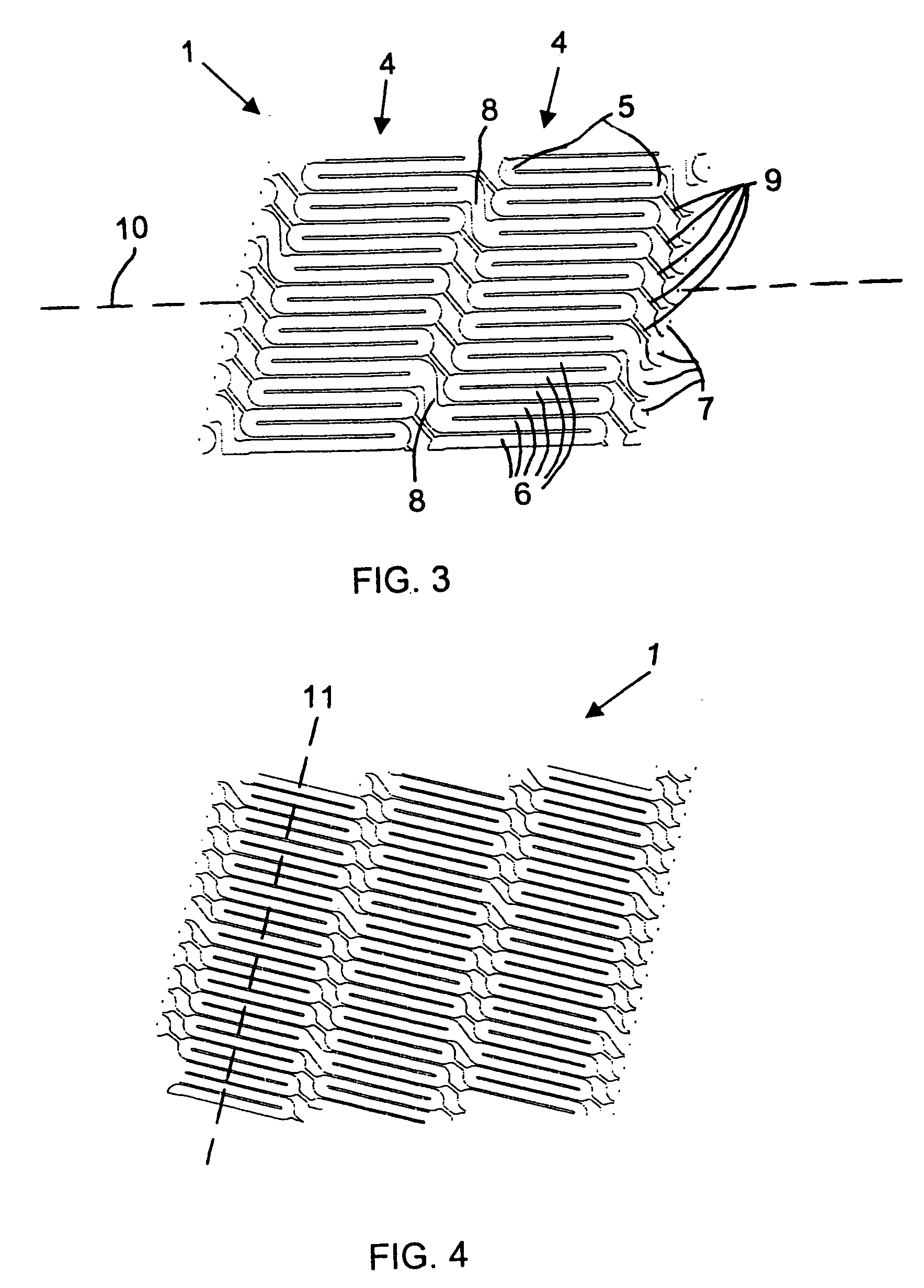
InactiveUS20060074480A1Improves helical machined-tube stentsReduce restenosisStentsLigamentsInsertion stentStent
A stent includes a stent body having a circumference and struts disposed helically about the circumference in turns. At least two of the struts have respective strut ends. At least two paddle-shaped markers extend away from a respective one of the strut ends. The markers have respective marker extreme ends and different overall longitudinal lengths substantially aligning the marker extreme ends approximately along a single circumference of the stent body. A method for manufacturing a helical stent includes the steps of providing a stent body with struts disposed about the circumference thereof in turns and with bridges connecting the struts in adjacent turns. The stent body is expanded and, thereafter, some of the bridges, in particular, sacrificial bridges, are removed.
Owner:ANGIOMED GMBH & CO MEDIZINTECHNIK KG
Hybrid amorphous metal alloy stent
InactiveUS20060178727A1Inhibit and decrease cell proliferationReduce restenosisStentsSurgeryMetal alloyBalloon dilatation
An expandable stent is provided, wherein the stent is advantageously formed of at least one amorphous metal alloy and a biocompatible material. The stent is formed from flat metal in a helical strip which is wound to form a tubular structure. The tubular structure is not welded but rather is wrapped or coated with a biocompatible material in order to maintain the amorphous metal in its tubular configuration. Said stent can be balloon expanded or self expanding.
Owner:MEDINOL LTD
Delivery of highly lipophilic agents via medical devices
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
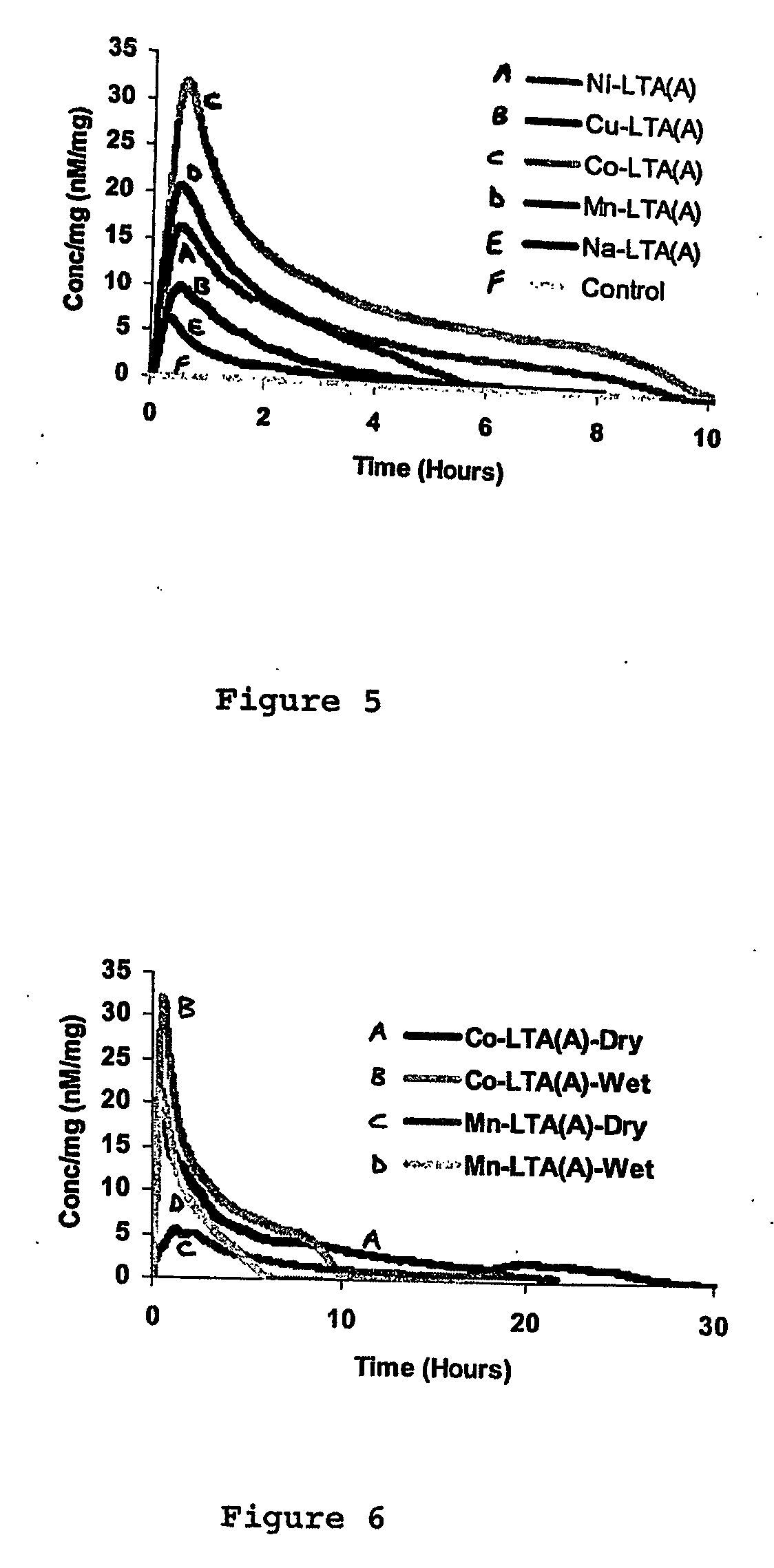
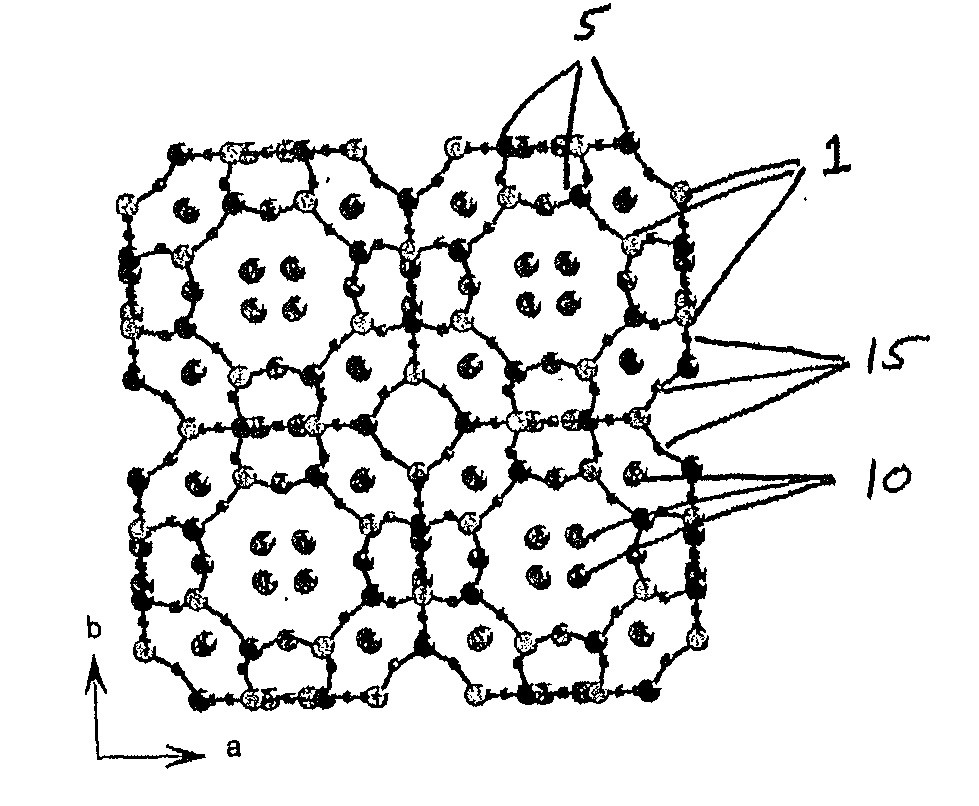
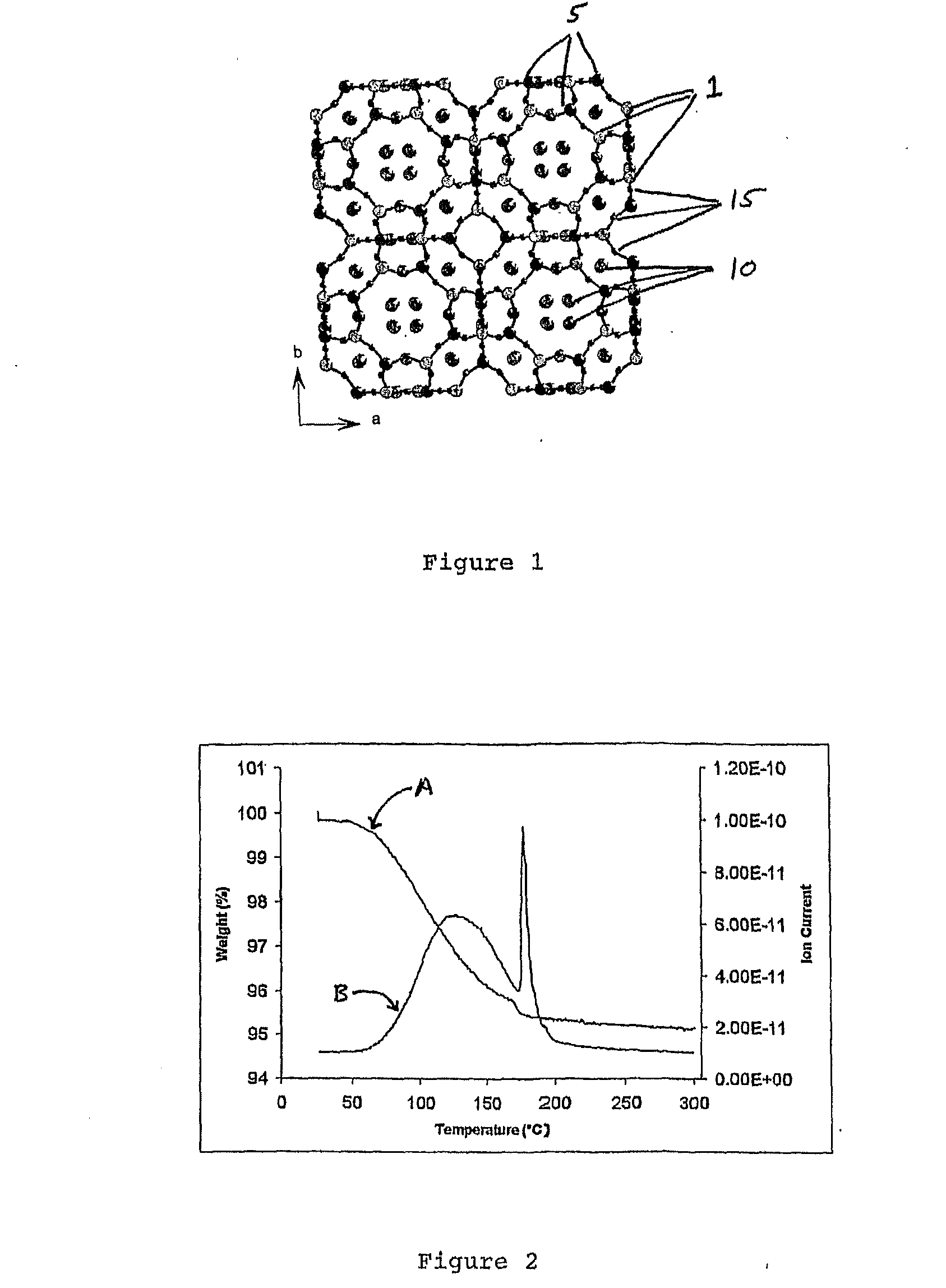
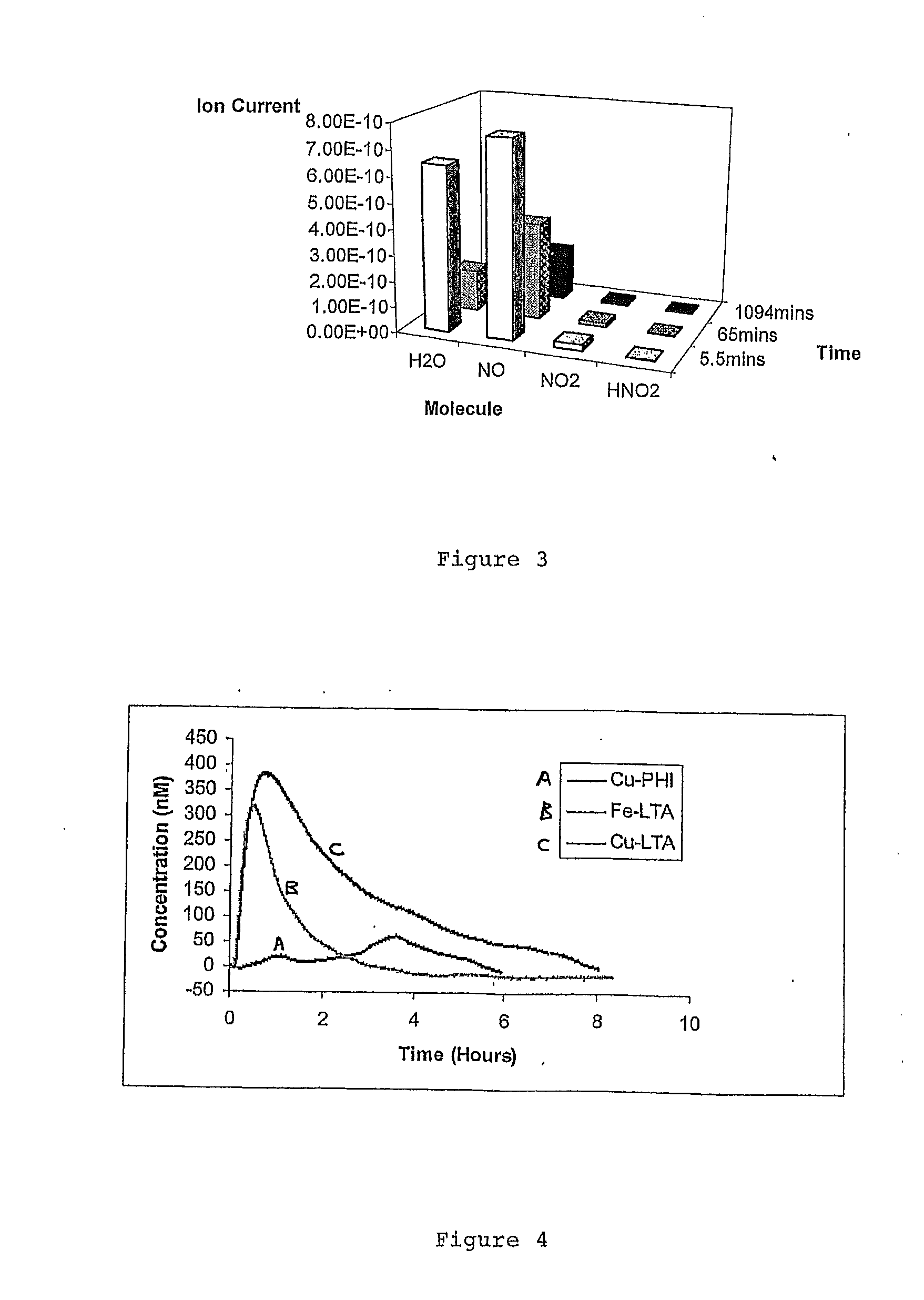
Zeolites for delivery of nitric oxide
ActiveUS20060269620A1Reduce restenosisAntibacterial agentsCosmetic preparationsNitric oxideChemistry
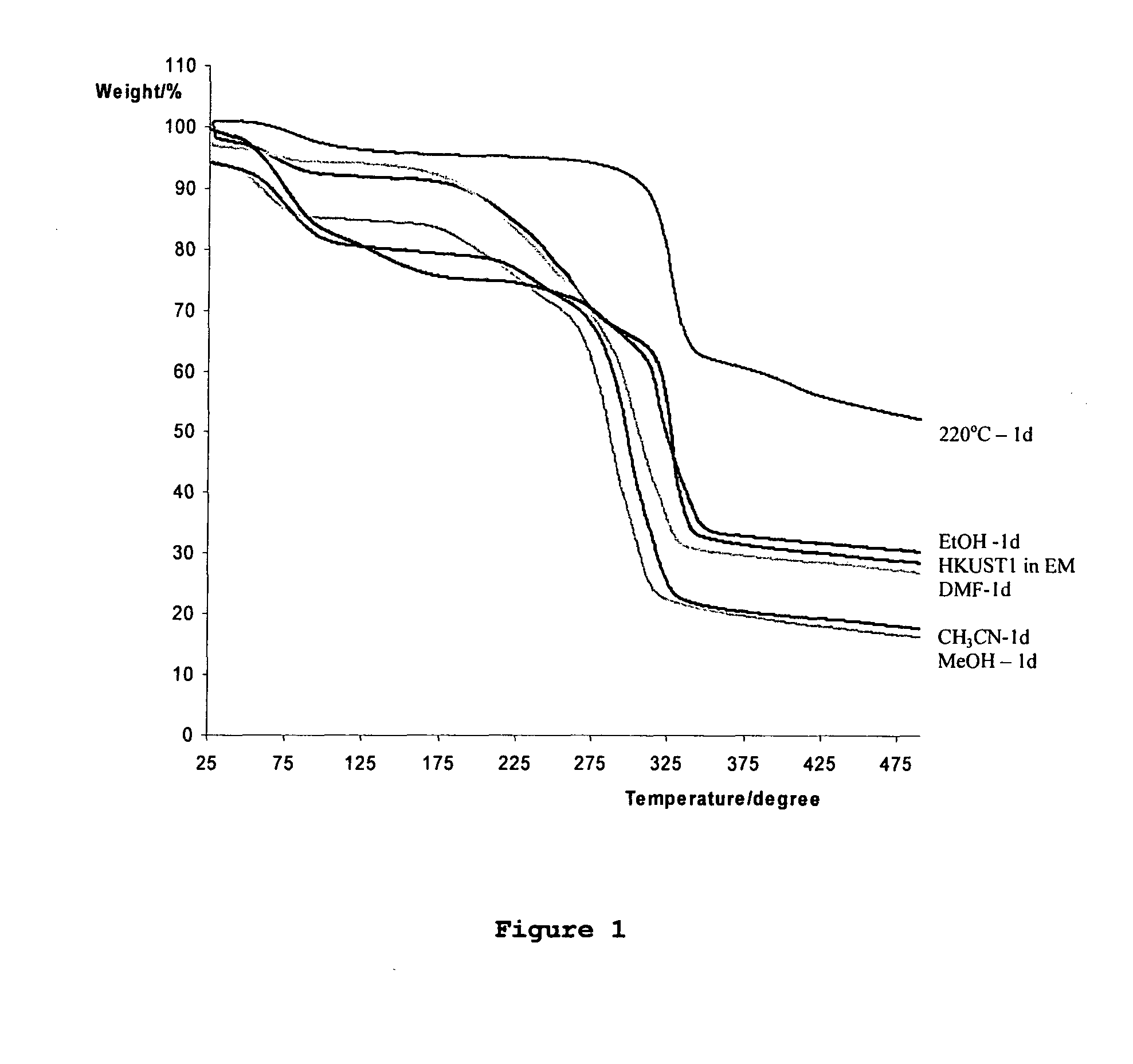
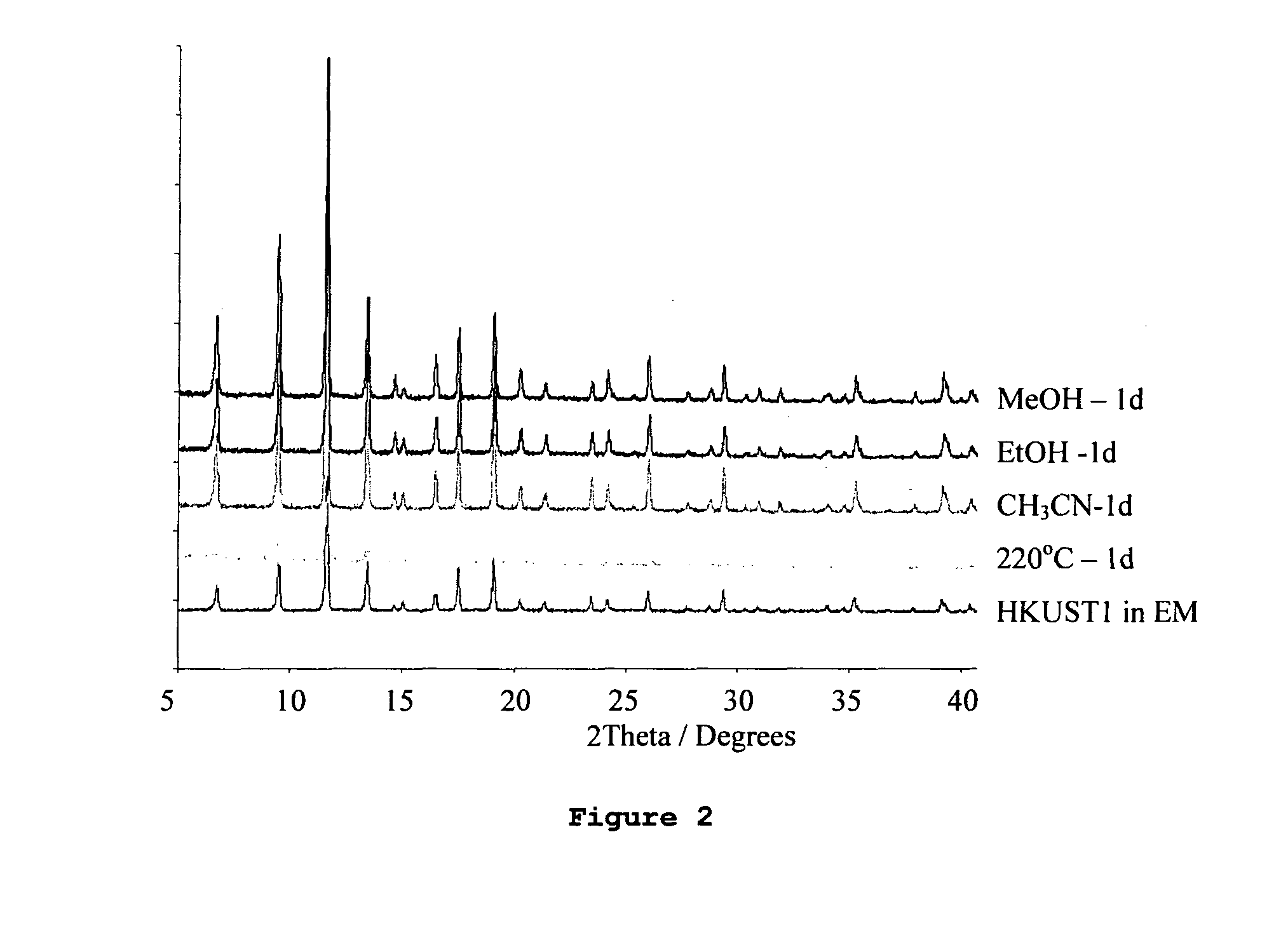
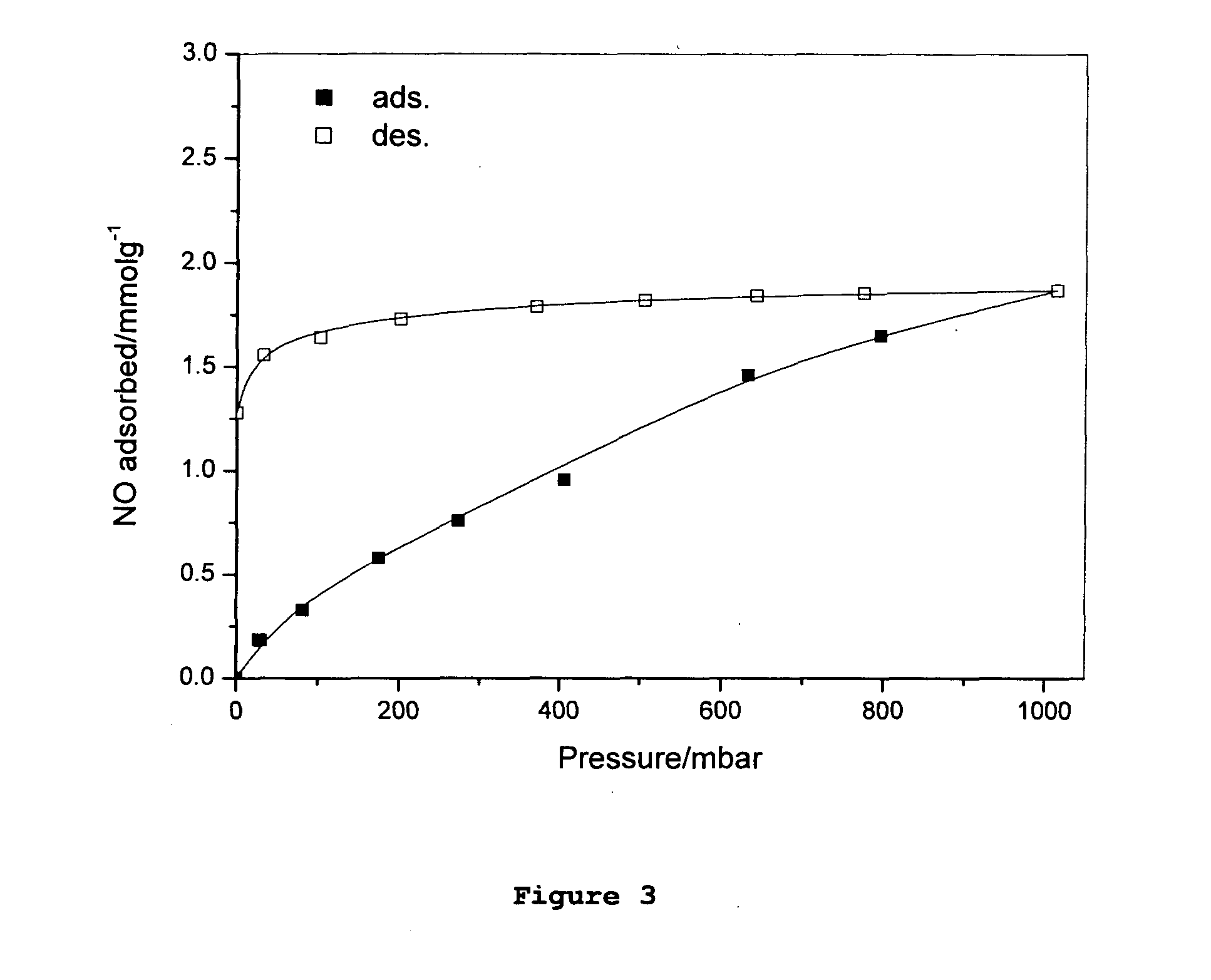
Adsorption and release of nitric oxide in metal organic frameworks
ActiveUS20100239512A1Reduce restenosisImprove adsorption capacityAntibacterial agentsCosmetic preparationsMetal-organic frameworkNitric oxide
Disclosed are metal organic frameworks that adsorb nitric oxide, NO-loaded metal organic frameworks, methods of preparing the NO-loaded metal organic frameworks, methods of releasing the nitric oxide into a solution or into air, and uses of the metal organic frameworks.
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
Zeolites for Delivery of Nitric Oxide
ActiveUS20100331968A1Reduce restenosisAntibacterial agentsCosmetic preparationsEngineeringNitric oxide
There is described zeolites containing releasably adsorbed nitric oxide, methods of preparing the zeolites, methods of releasing the nitric oxide into a solution or into air and uses of the zeolites in therapy.
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
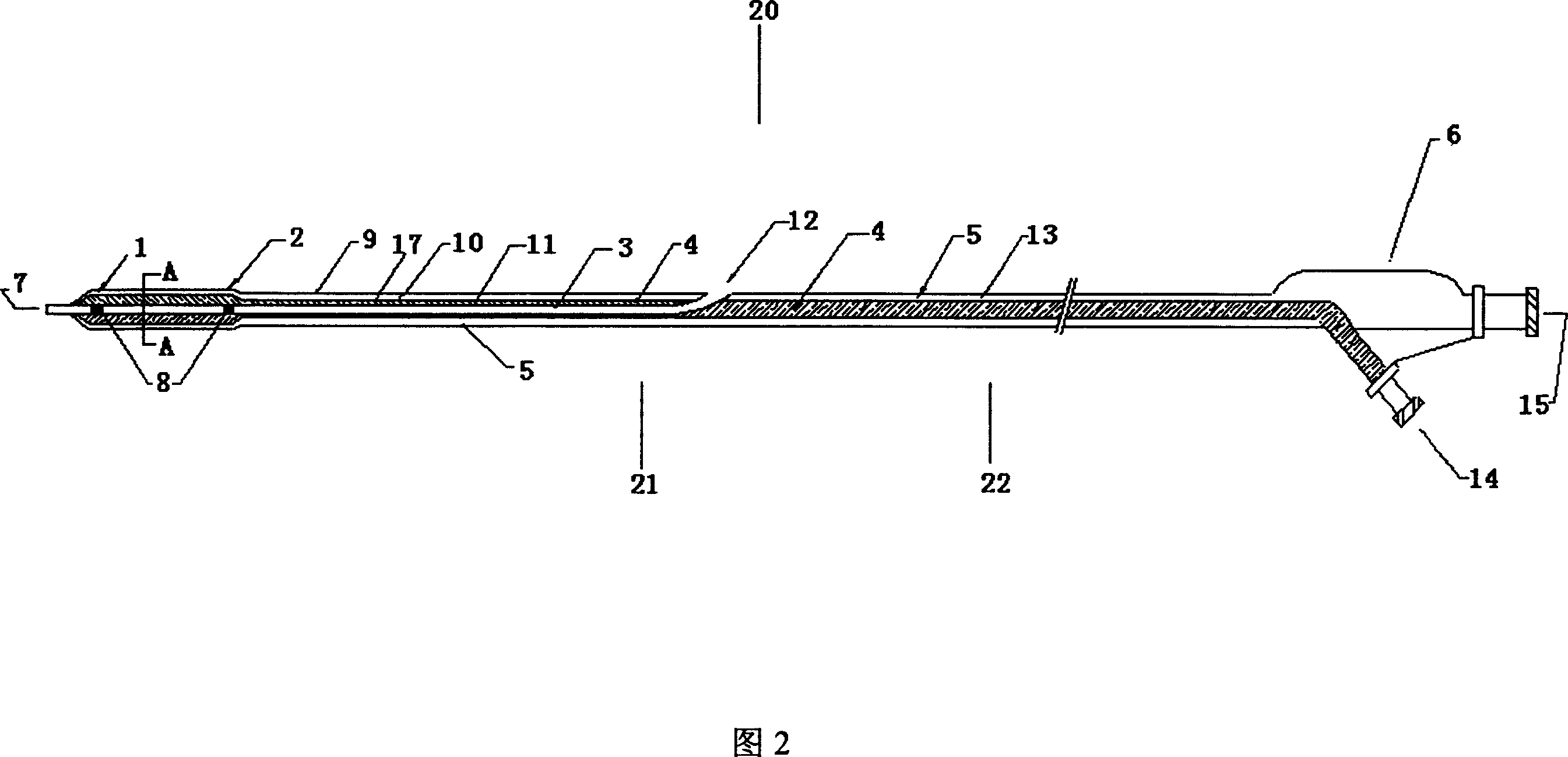
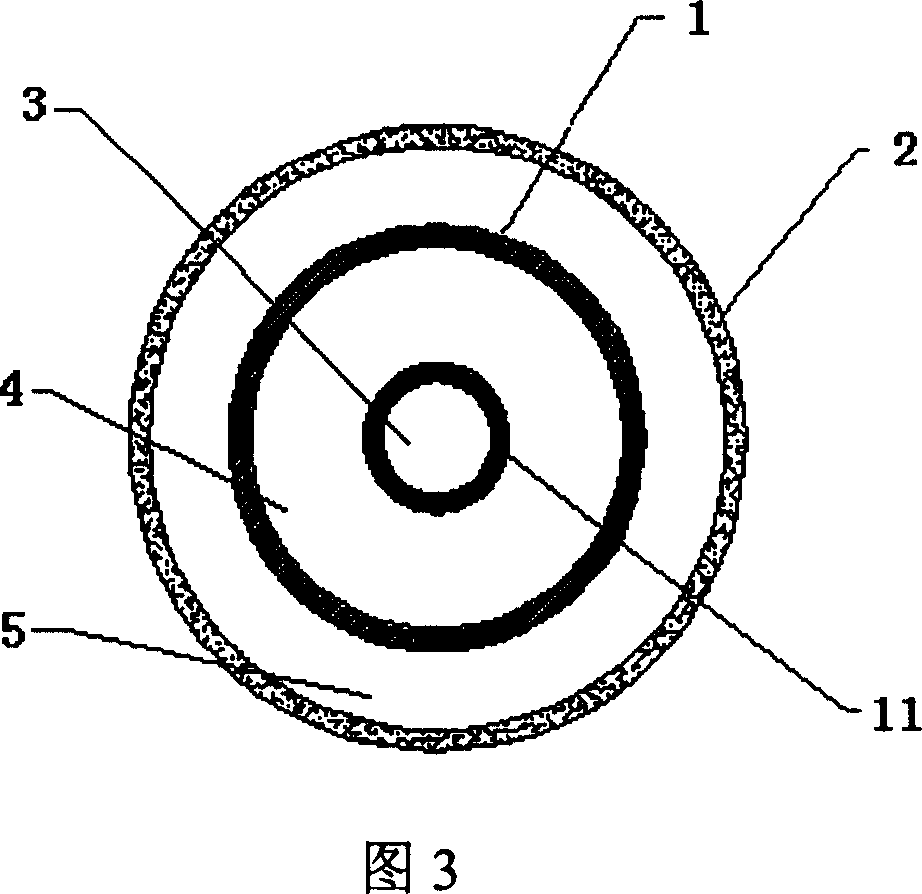
Double-layered balloon catheter
A dual-layer bag catheter is composed of two ball layers and three cavities. Its internal bag layer is used for expanding blood vessel. Its external bag layer made of unique millipore film is used for delivering the therapeutic genes or medicine to the wall of blood vessel or target organ.
Owner:SHANGHAI MICROPORT MEDICAL (GROUP) CO LTD
Stent and method for manufacturing the stent
InactiveUS20060060266A1Improves helical machined-tube stentsReduce restenosisStentsWelding/soldering/cutting articlesInsertion stentImplantation Site
A method for manufacturing a helical stent includes the steps of providing a stent body with struts disposed about the circumference thereof in turns and with bridges connecting the struts in adjacent turns. The stent body is at least partially expanded and, some time during expansion, some of the bridges, in particular, sacrificial bridges, are removed. A method for implanting this stent includes loading the stent at a stent delivery system, traversing the stent at the delivery system to an implantation site, and implanting the stent at the implantation site.
Owner:ANGIOMED GMBH & CO MEDIZINTECHNIK KG
Methods of administering rapamycin analogs with anti-inflammatories using medical devices
A medical device comprising a supporting structure capable of including or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may include one or more therapeutic agents or substances, with the carrier including a coating on the surface thereof, and the coating including the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to, This drug can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Stent and method for manufacturing the stent
A stent includes a stent body having a circumference and struts disposed helically about the circumference. Each of the struts has a strut length and a ratio of a number of the struts around the circumference to the strut length is greater than 800 per inch, in particular, over 1000 per inch. A method for manufacturing a helical stent includes the steps of providing a stent body with struts disposed about the circumference thereof in turns and with bridges connecting the struts in adjacent turns. The stent body is expanded and, thereafter, some of the bridges, in particular, sacrificial bridges, are removed.
Owner:ANGIOMED GMBH & CO MEDIZINTECHNIK KG
Medical devices containing rapamycin analogs
InactiveUS20050175660A1Reduce restenosisReduce restenosis rateBiocideOrganic chemistryTherapeutic effectAnti platelet
A medical device comprising a supporting structure capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating containing the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to, This drug can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Stent and method for manufacturing the stent
A stent includes a stent body with a diameter of between approximately 4 and 12 mm and a length of between approximately 10 and 250 mm. S-shaped struts are disposed helically about the circumference along helical turns. The struts have straight portions and curved portions connecting respectively adjacent ones of the straight portions. Bridges connect the struts in adjacent ones of the turns. The bridges include connecting bridges and a given number of sacrificial bridges before being expanded, and the connecting bridges and less than the given number of the sacrificial bridges after being at least partially expanded. A method for manufacturing a helical stent includes the steps of providing a stent body with struts disposed about the circumference thereof in turns and with bridges connecting the struts in adjacent turns. The stent body is expanded and, thereafter, some of the bridges, in particular, sacrificial bridges, are removed.
Owner:ANGIOMED GMBH & CO MEDIZINTECHNIK KG
Stent and method for manufacturing the stent
ActiveUS20060064158A1Improves helical machined-tube stentsReduce restenosisStentsLigamentsInsertion stentStent
A stent includes a stent body having a circumference, a diameter of between approximately 4 mm and approximately 12 mm, in particular, 8 mm, and a length of between approximately 10 mm and approximately 250 mm, in particular, 150 mm, and struts disposed helically about the circumference in turns. Substantially circumferentially oriented connecting bridges connect respectively adjacent ones of the turns. A method for manufacturing a helical stent includes the steps of providing a stent body with struts disposed about the circumference thereof in turns and with bridges connecting the struts in adjacent turns. The stent body is expanded and, thereafter, some of the bridges, in particular, sacrificial bridges, are removed.
Owner:ANGIOMED GMBH & CO MEDIZINTECHNIK KG
Drug/drug delivery systems for the prevention and treatment of vascular disease
InactiveUS7300662B2Prevent proliferationGood effectOrganic active ingredientsOrganic chemistryVascular diseaseWhole body
Owner:WYETH LLC
Delivery of highly lipophilic agents via medical devices
InactiveUS20060228452A1Easy to transportIncrease drug retentionBiocideSurgeryMedical deviceBiomedical engineering
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
Stand with self-deployable portion
InactiveCN101102728AReduce restenosisReduce inflammationStentsBlood vesselsInsertion stentThree vessels
The present invention provides methods and devices for placement of a stent in a bifurcation or ostial lesion. The stent comprises a main body and a flaring portion. The main body is designed to expand and support a main vessel while the flaring portion deploys at least partially in response to expansion of the main body and is designed to open into and support a side branch or bifurcation ostium area. The stent may also comprise a therapeutic agent which can be delivered to a blood vessel.
Owner:TRIREME MEDICAL
Segmented scaffolds and delivery thereof for peripheral applications
InactiveUS20130268045A1Reduce restenosisReduce the overall heightStentsBlood vesselsBiomedical engineering
Owner:ABBOTT CARDIOVASCULAR
Method for preparing blood vessel stent with polyester medicament eluting coating
ActiveCN101279111AReduce the occurrence of restenosisReduce the occurrence of subacute thrombosis and hemangiomaStentsSurgeryCarbon dioxidePercent Diameter Stenosis
The invention provides a preparation method of polyester drug-eluting coating intravascular stent. The method comprises the steps of the cleaning of the intravascular stent, the preparation of a coating solution, the coating of drug-eluting coating, the drying and disinfecting processes of drug-eluting coating, etc. The preparation method selects the polyester, which can be degraded into carbon dioxide and water in the human body in 3 to 6 months, metabolized by the human body and finally excreted out, as a polymer drug carrier. By adopting the polymer drug carrier, the stent with the coating can not only reduce the occurrence of restenosis, but also effectively reduce the occurrence of subacute thrombosis and hemangioma afterstent implantation, and guarantee the long-term clinic safety and efficiency after stent implantation. The polymer carrier with antiproliferative drugs is coated on the outer surface of the stent to achieve the function of reducing the intimal hyperplasia at one side of blood vessels and tissues. Meanwhile, fewer drugs are carried at the side facing to the blood vessel cavity to facilitate the curing of endothelium.
Owner:JIANGYIN BIODEGRADE MEDICAL TECH CO LTD
Drug/Drug Delivery Systems for the Prevention and Treatment of Vascular Disease
InactiveUS20070026036A1Reduce systemic toxicityEasy to manageBiocideOrganic chemistryVascular diseaseWhole body
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com