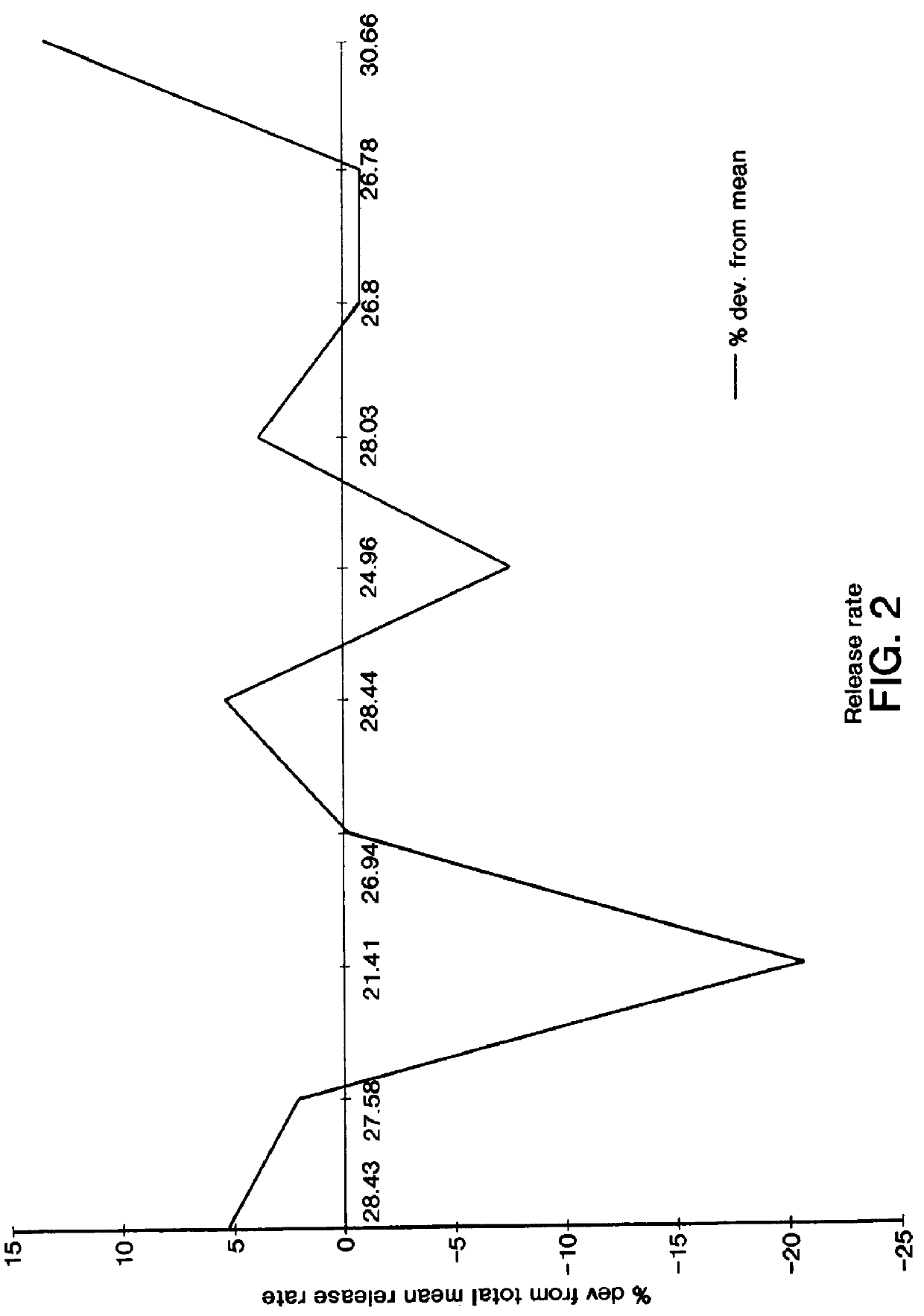
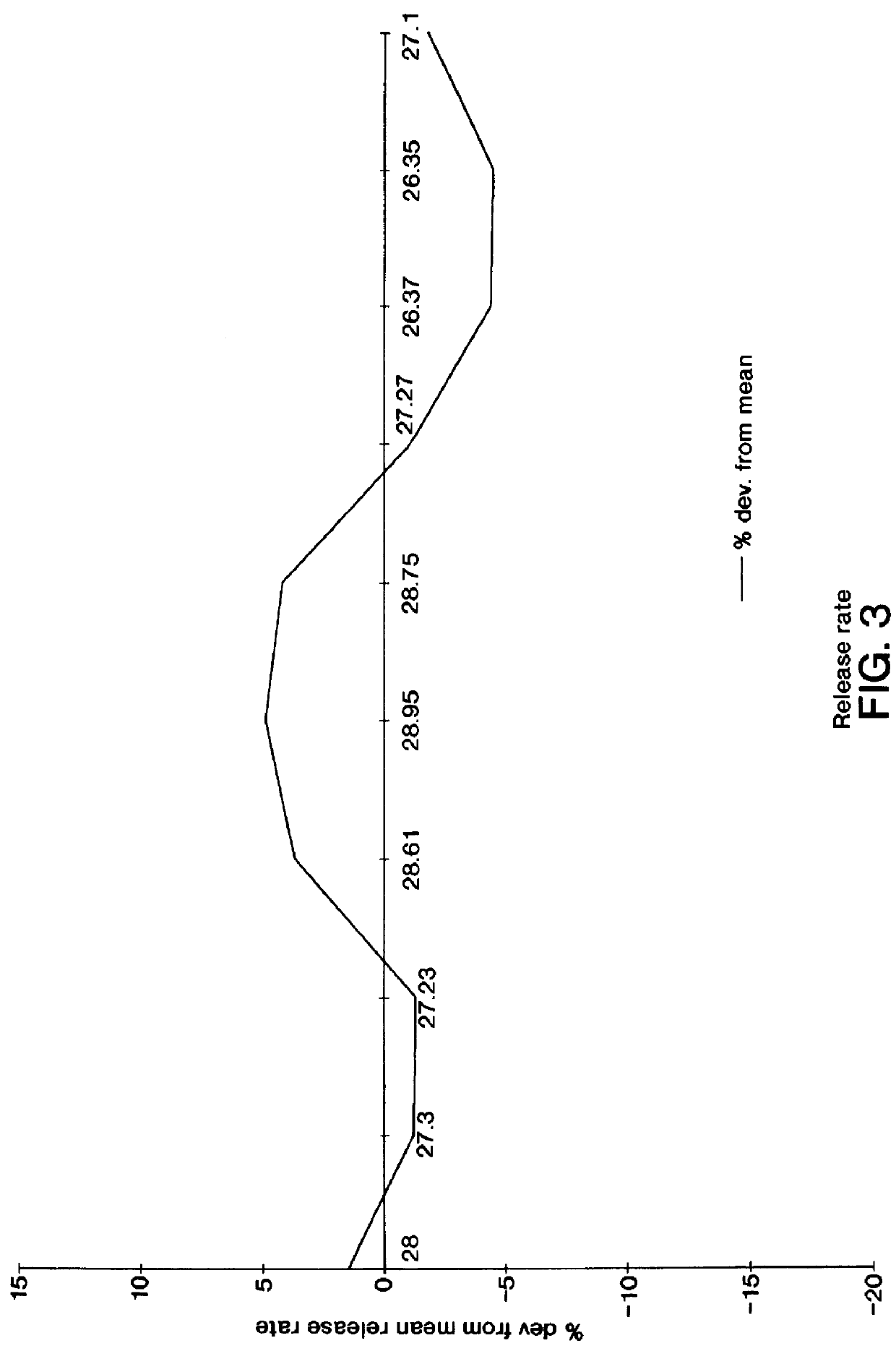
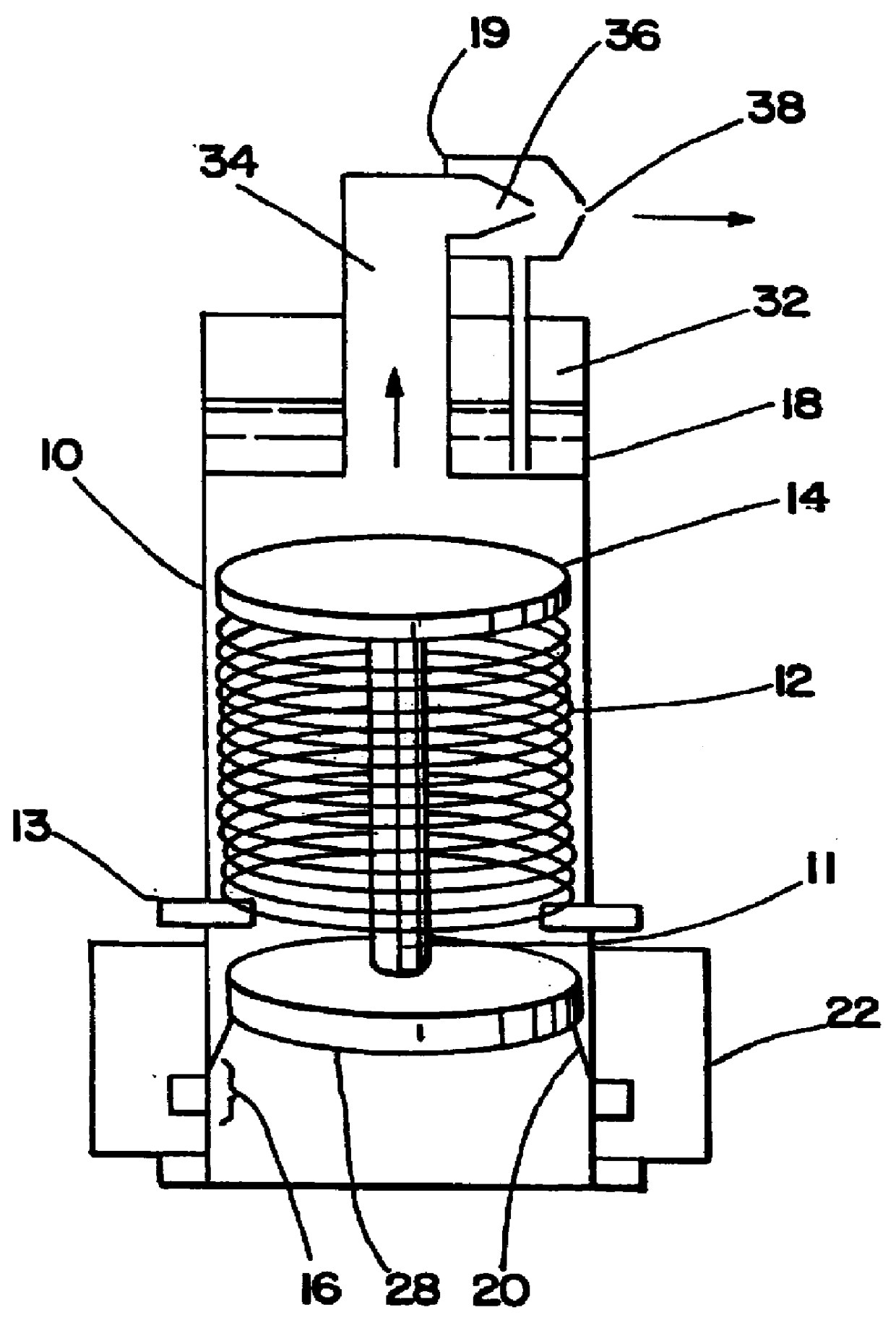
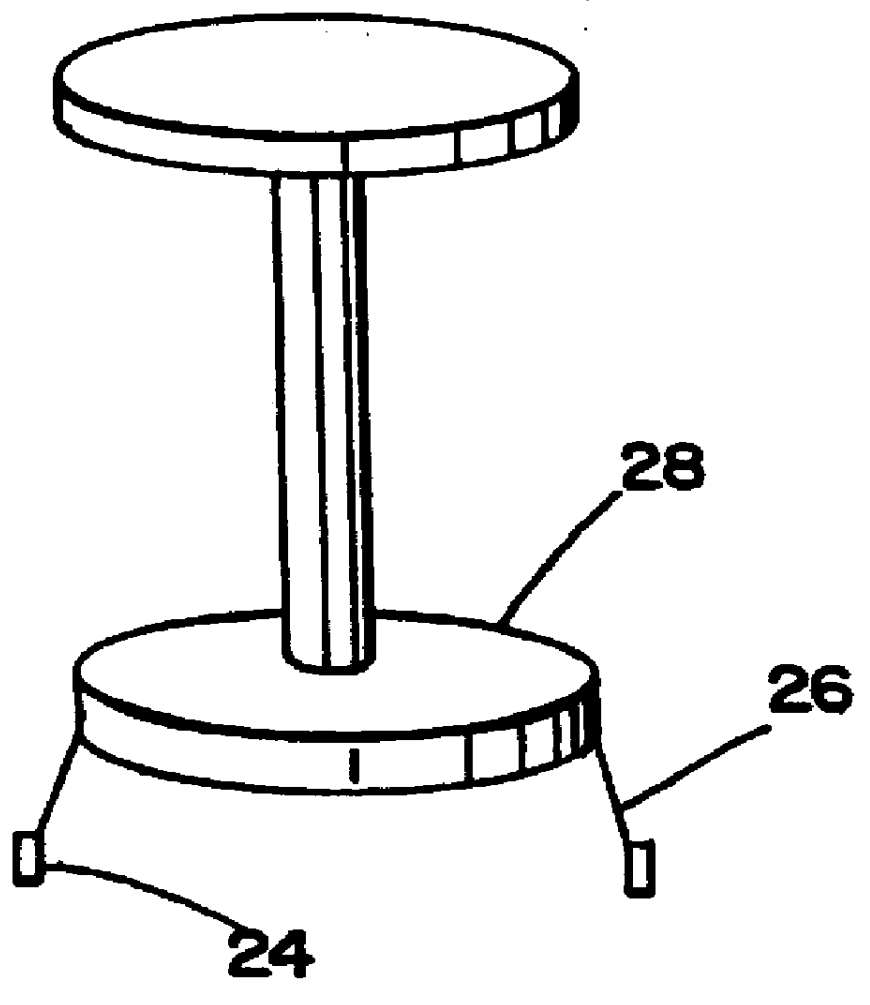
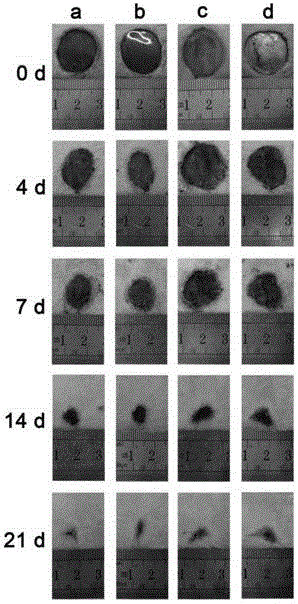
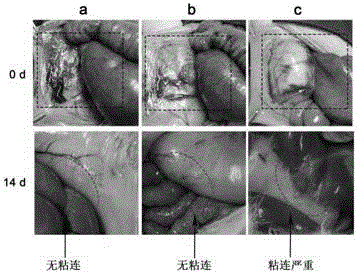
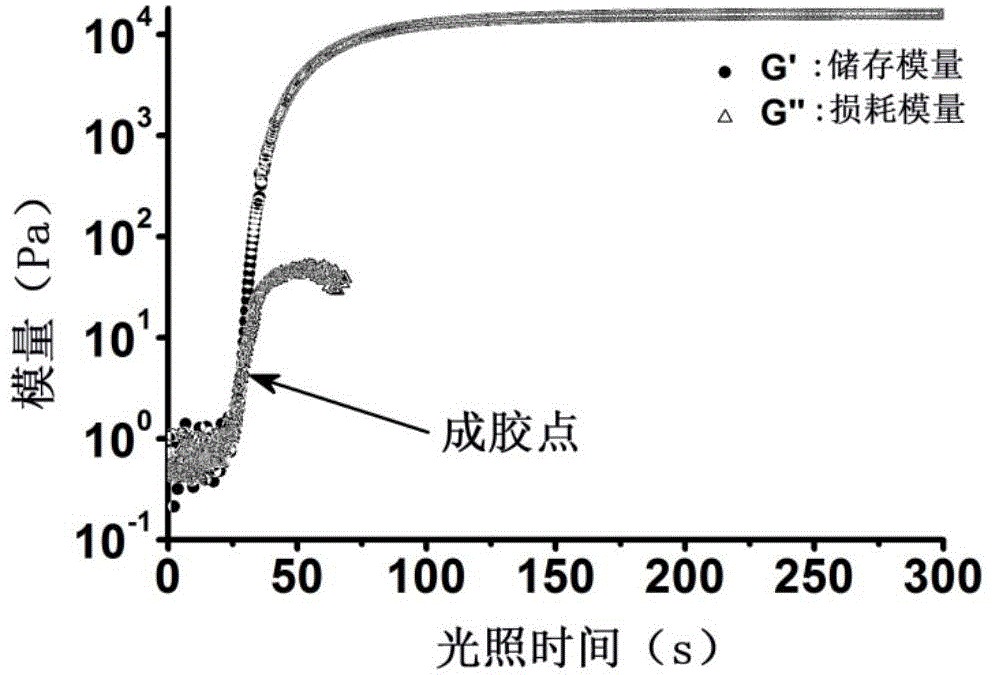
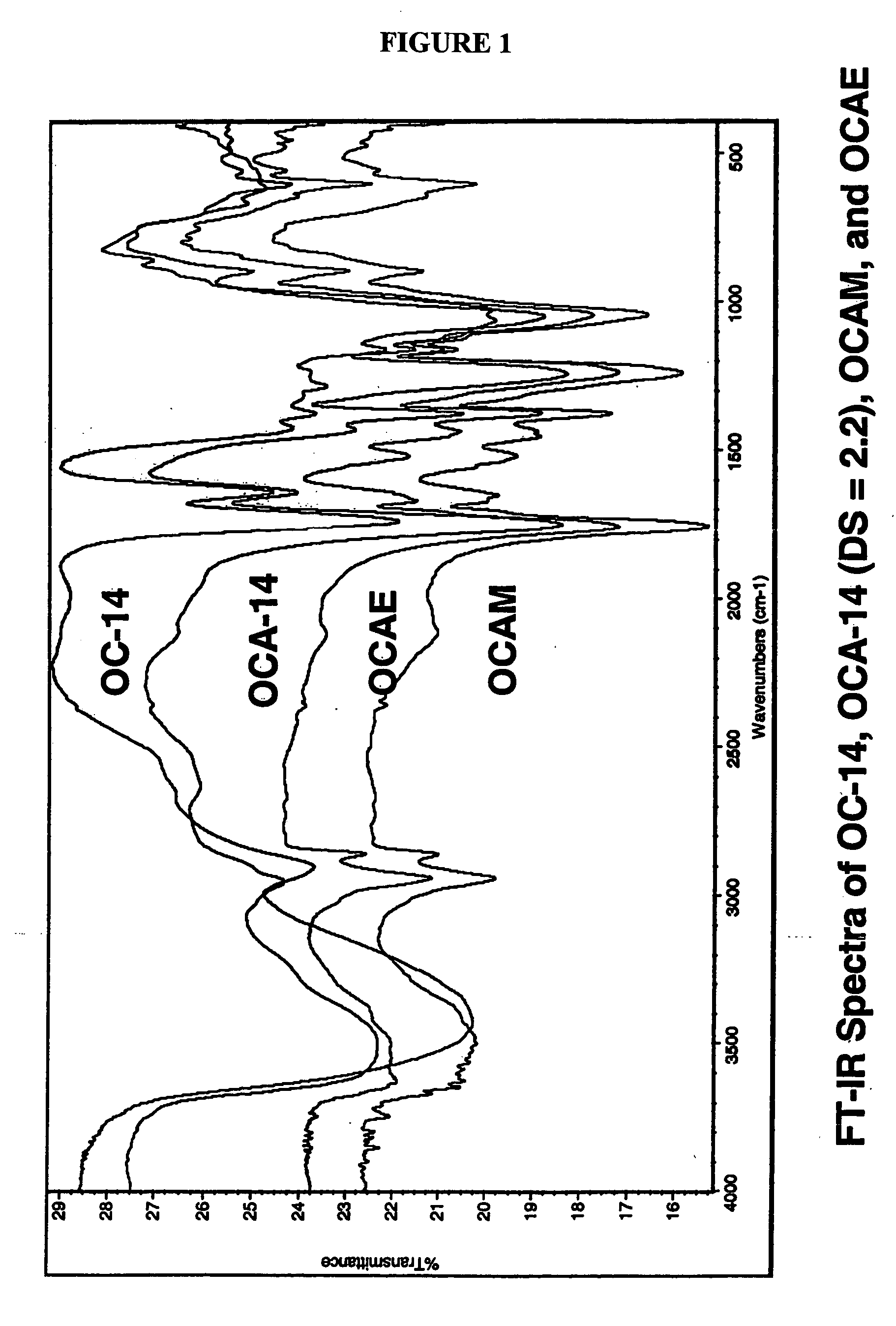
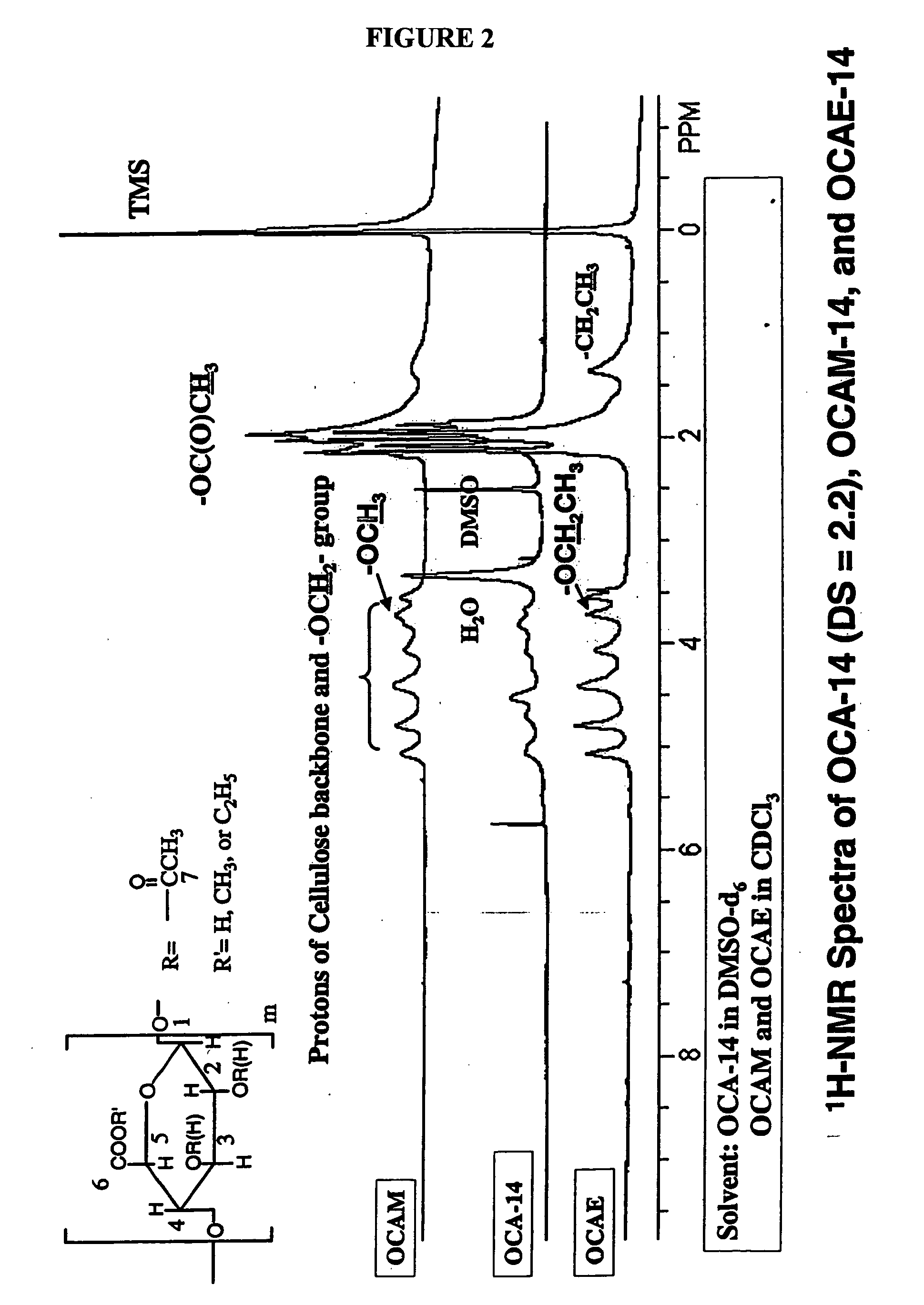
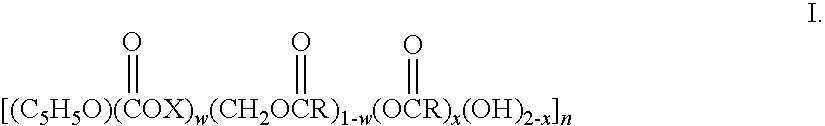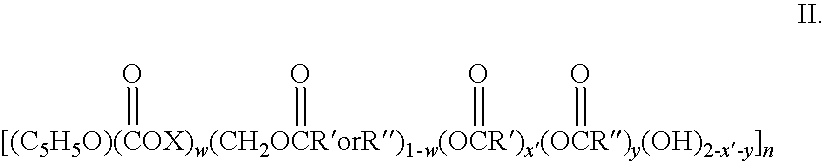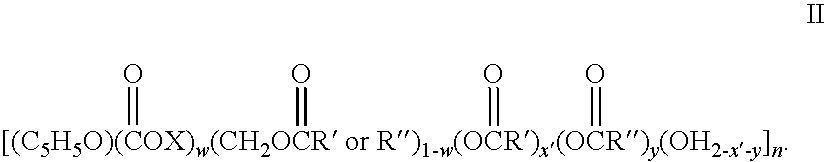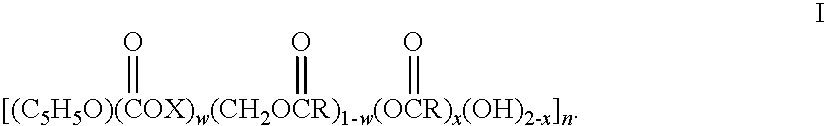Patents
Literature
4014 results about "Drug carrier" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A drug carrier is any substrate used in the process of drug delivery which serves to improve the selectivity, effectiveness, and/or safety of drug administration. Drug carriers are primarily used to control the release of a drug into systemic circulation. This can be accomplished either by slow release of the drug over a long period of time (typically diffusion) or by triggered release at the drug's target by some stimulus, such as changes in pH, application of heat, and activation by light. Drug carriers are also used to improve the pharmacokinetic properties, specifically the bioavailability, of many drugs with poor water solubility and/or membrane permeability.
Vaccine composition containing synthetic adjuvant
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
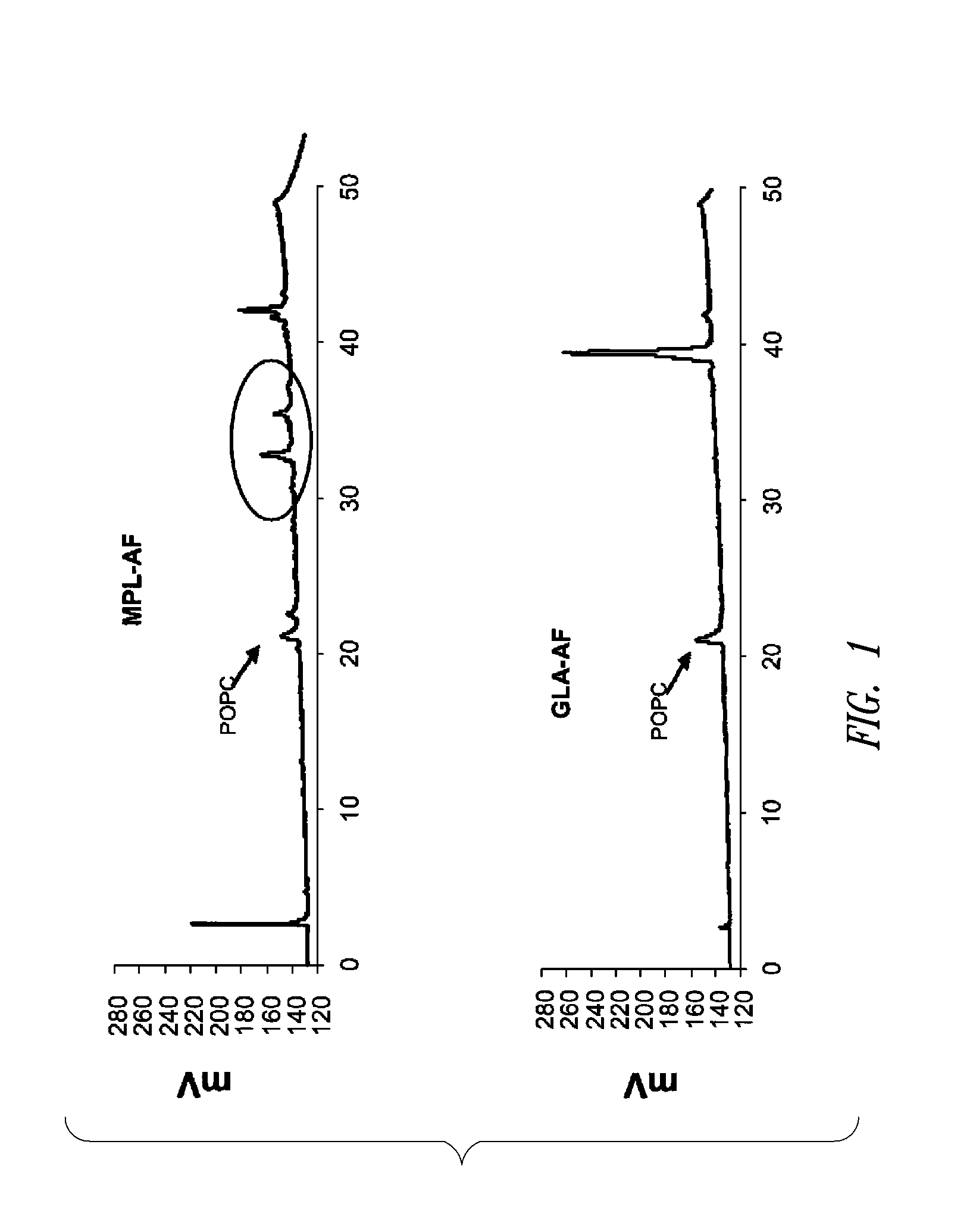
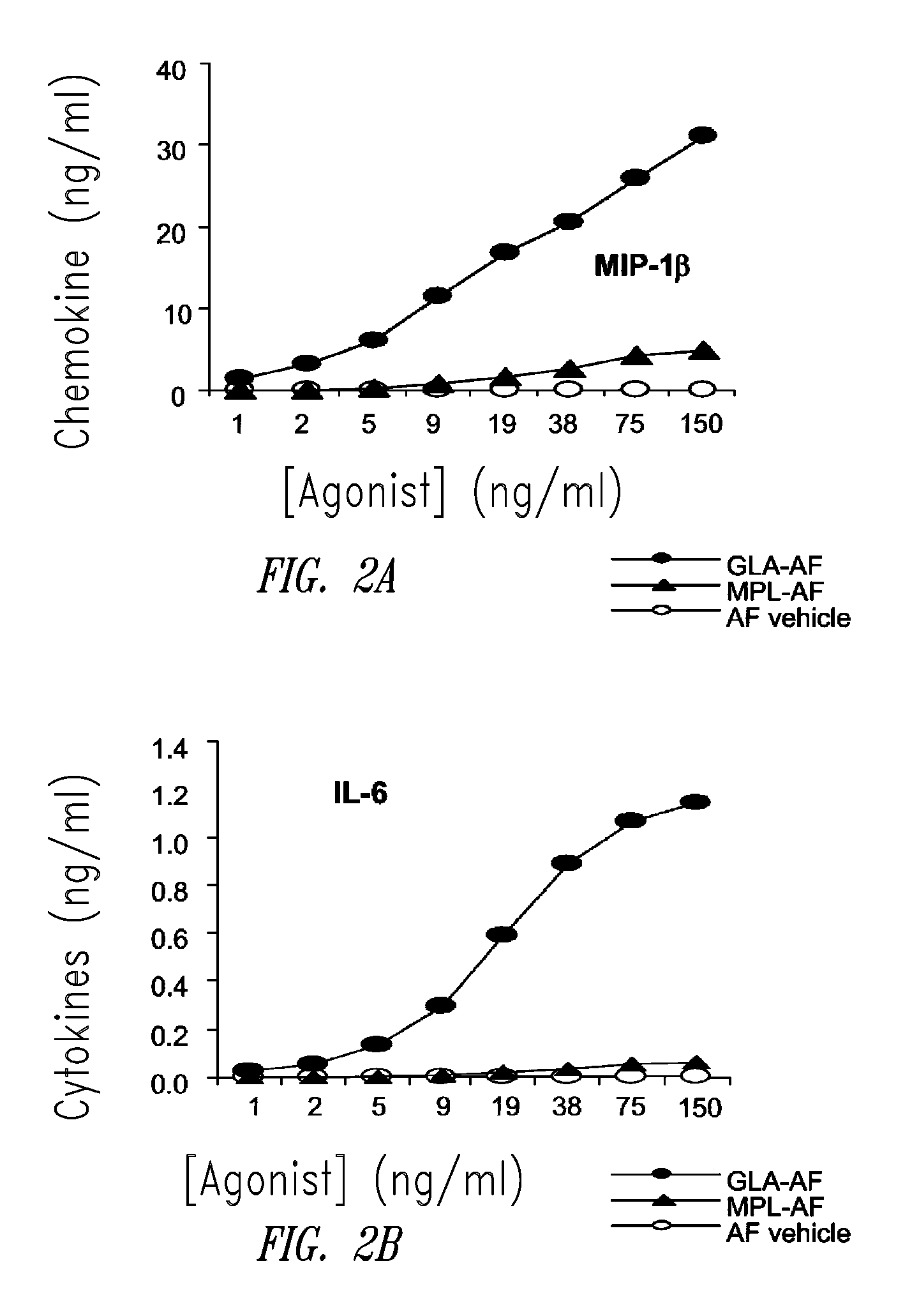
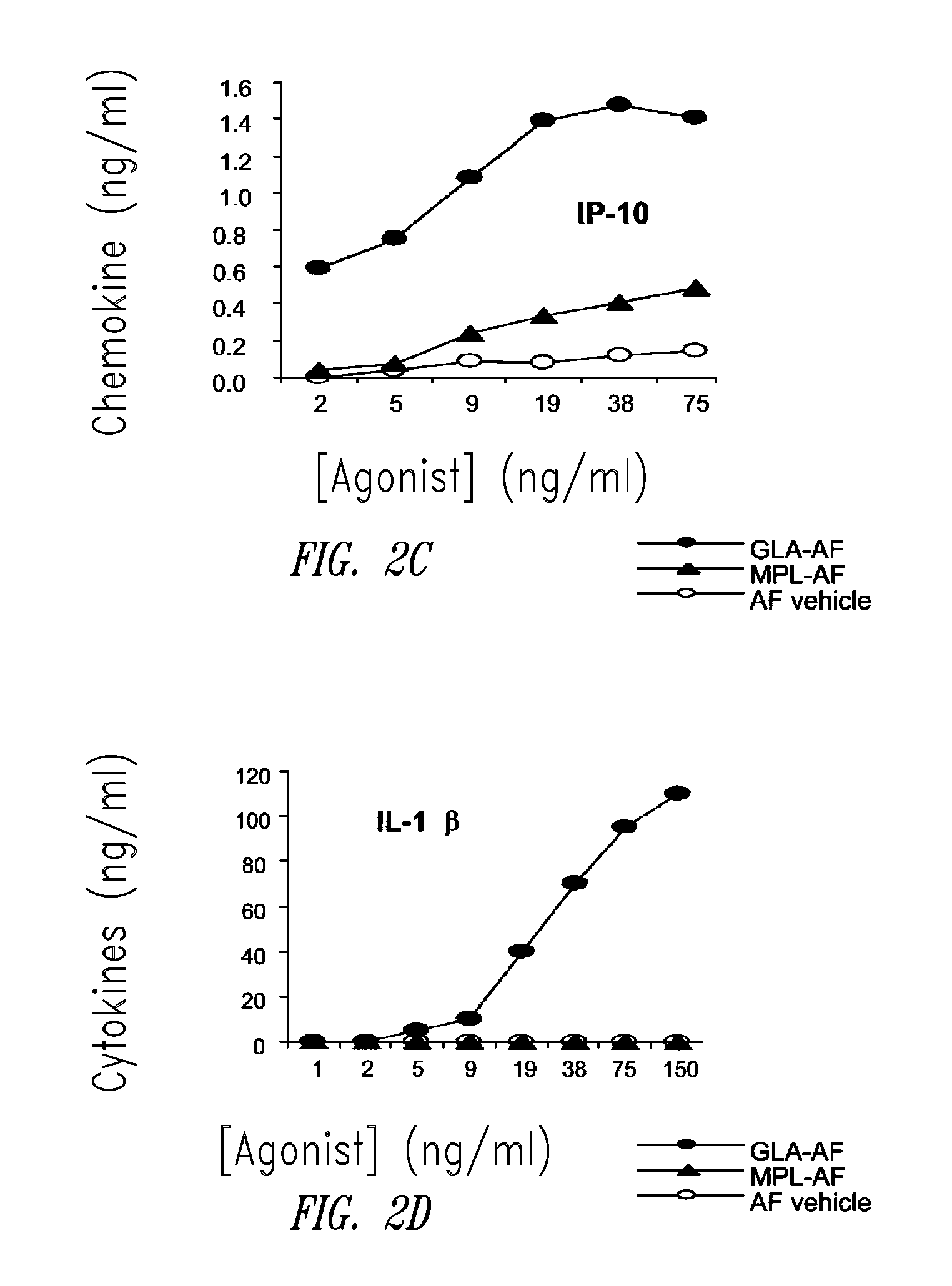
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Dosage form, process of making and using same
The invention disclosed pertains to a dosage form comprising an agent formulation comprising drug and pharmaceutical carrier of cooperating particle size and means for dispensing the agent formulation from the dosage form.
Owner:ALZA CORP
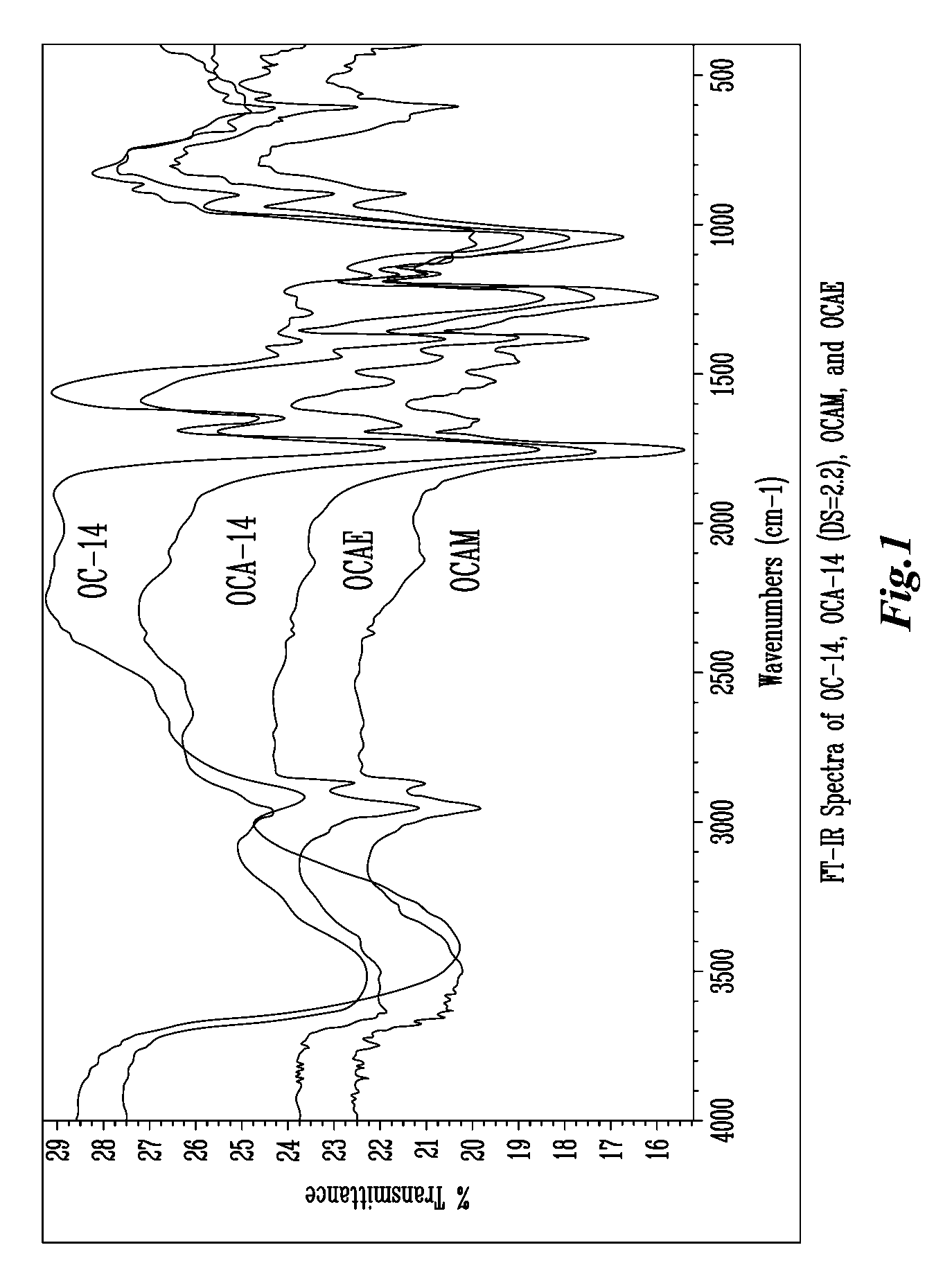
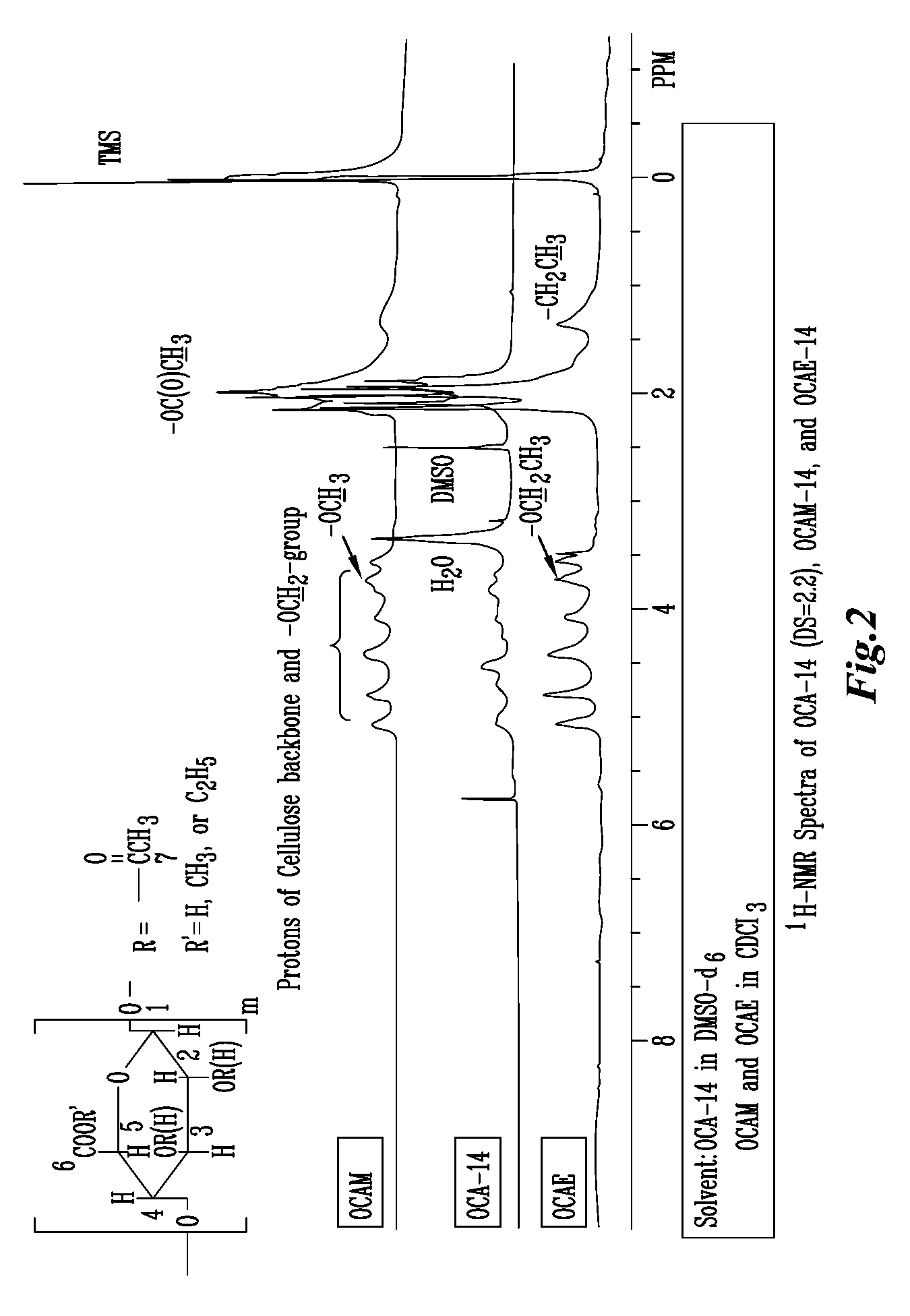
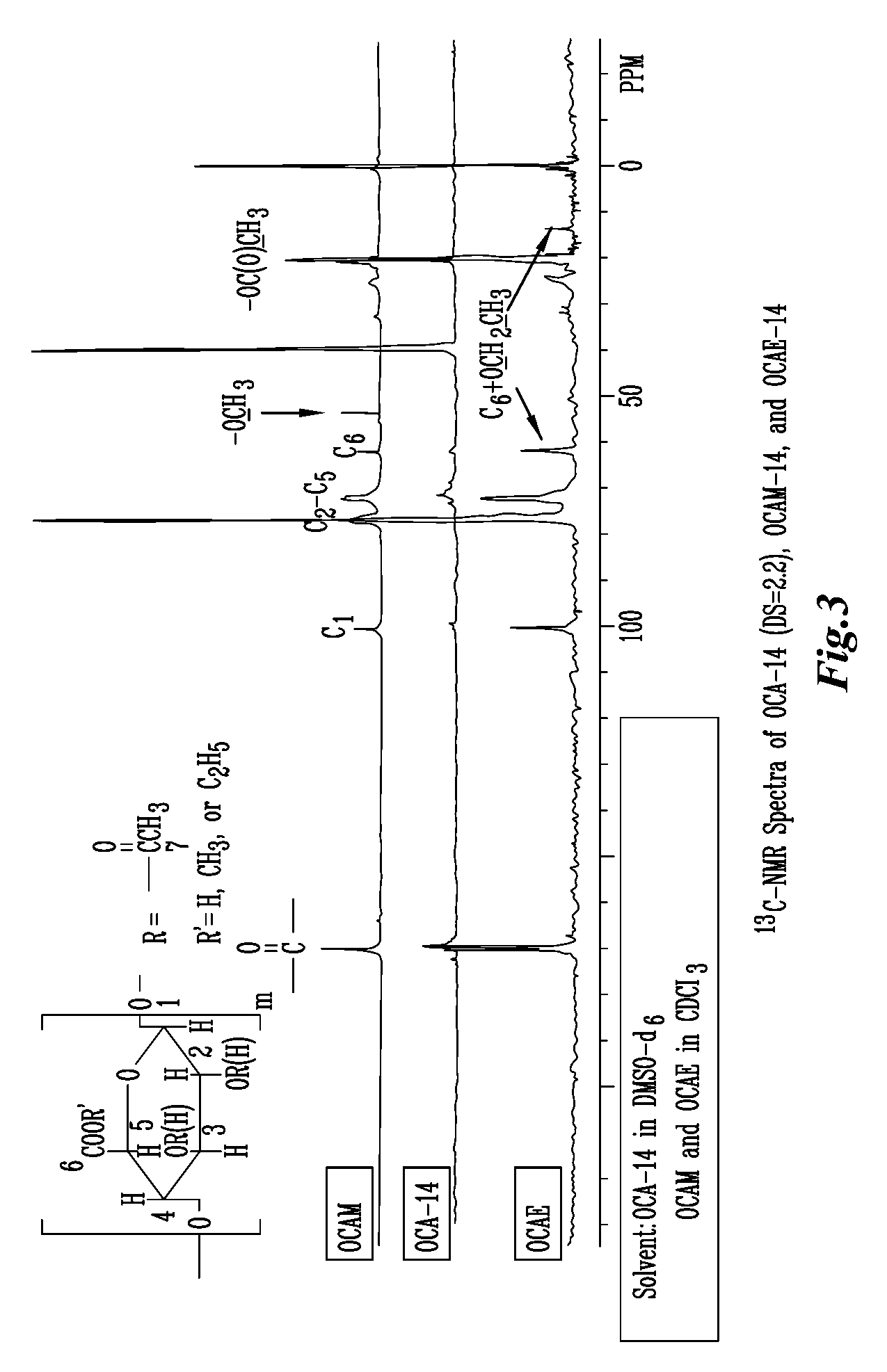
Biodegradable oxidized cellulose esters
Owner:UNIV OF IOWA RES FOUND
Chloroquine coupled antibodies and other proteins with methods for their synthesis
InactiveUS20070166281A1Improve efficacyImprove transportBiocidePeptide/protein ingredientsDrug conjugationTreatment effect
This invention discloses compositions of chloroquine-coupled active agents such as therapeutic antibodies or insulin, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the drug is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a drug directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing the drug for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and antibody or insulin to their site of action.
Owner:KOSAK KENNETH M
Biodegradable oxidized cellulose esters
The present invention relates to the preparation of a series of oxidized cellulose esters suitable for use as a drug carrier in the development of biodegradable controlled and / or sustained release pharmaceutical, agricultural, and veterinary compositions, such as films, compacts, microspheres, and pellets. The esters are prepared by acylation of oxidized cellulose having at least 3% carboxyl groups. The resulting oxidized cellulose esters are soluble in aqueous alkaline solutions, water, and a variety of organic solvents.
Owner:UNIV OF IOWA RES FOUND
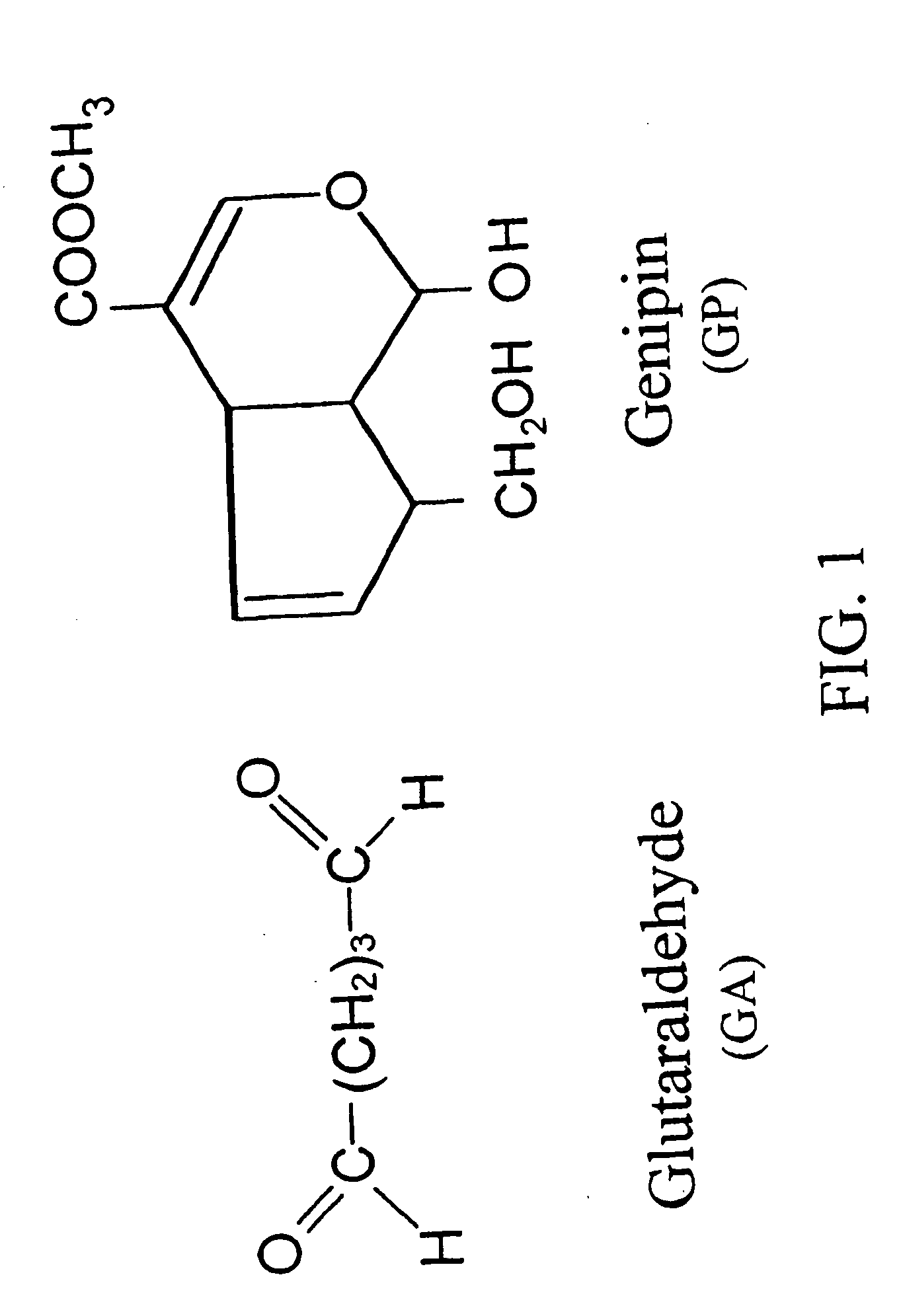
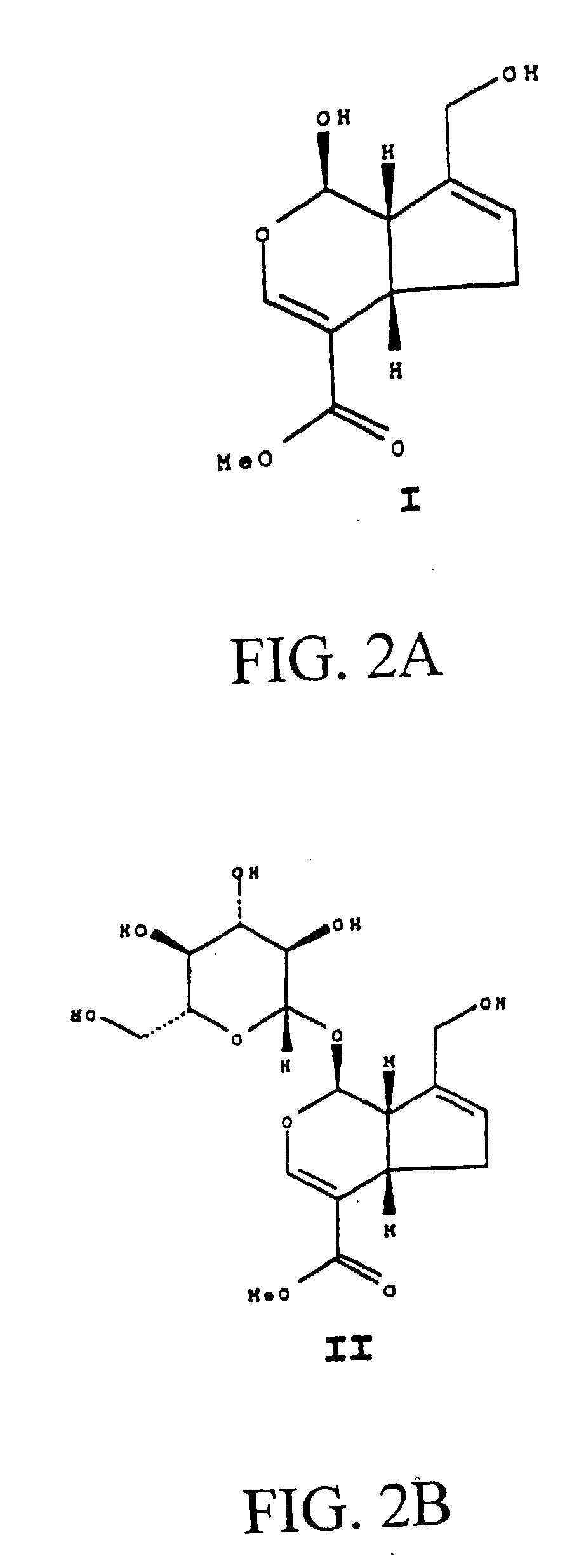
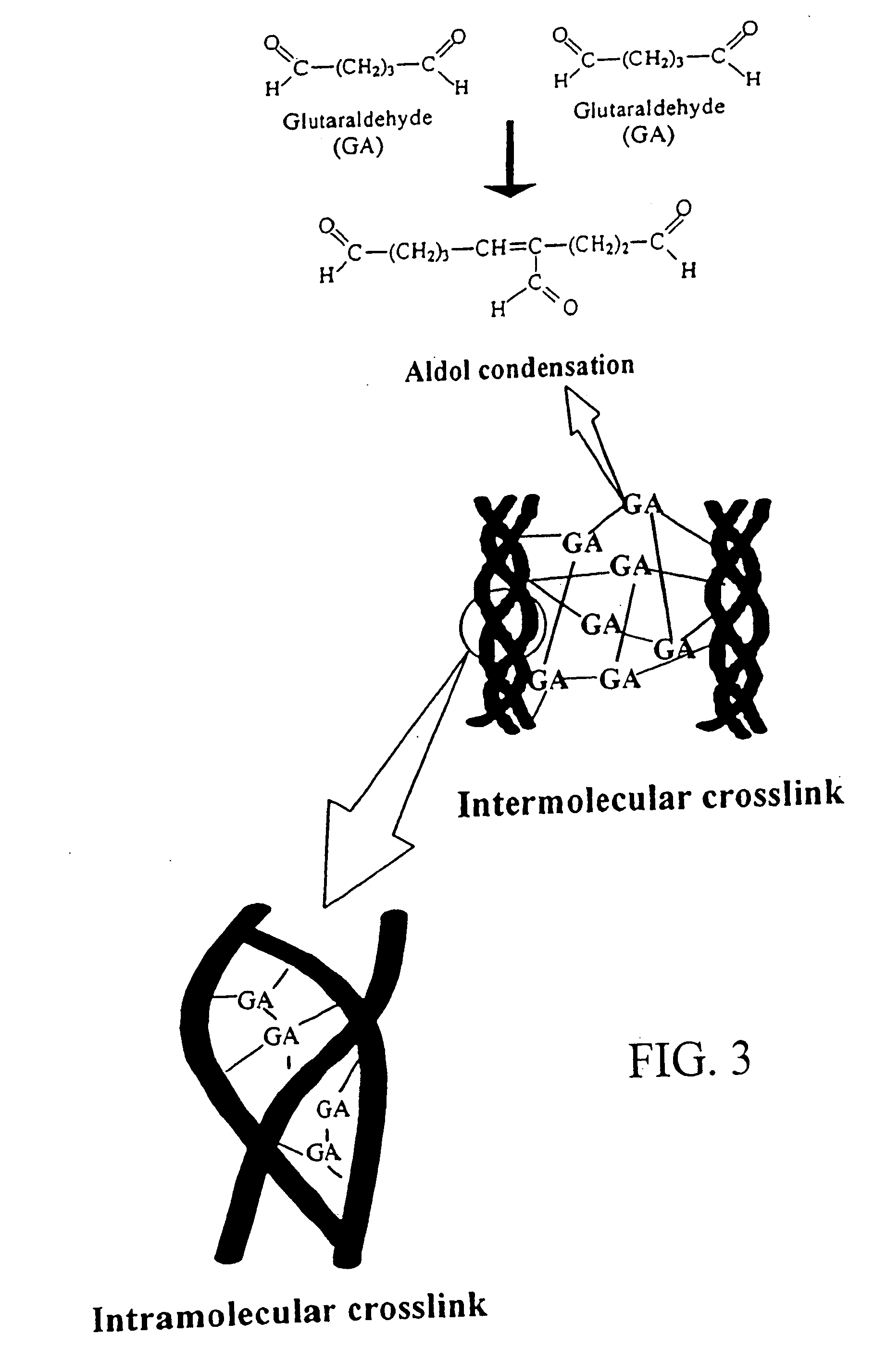
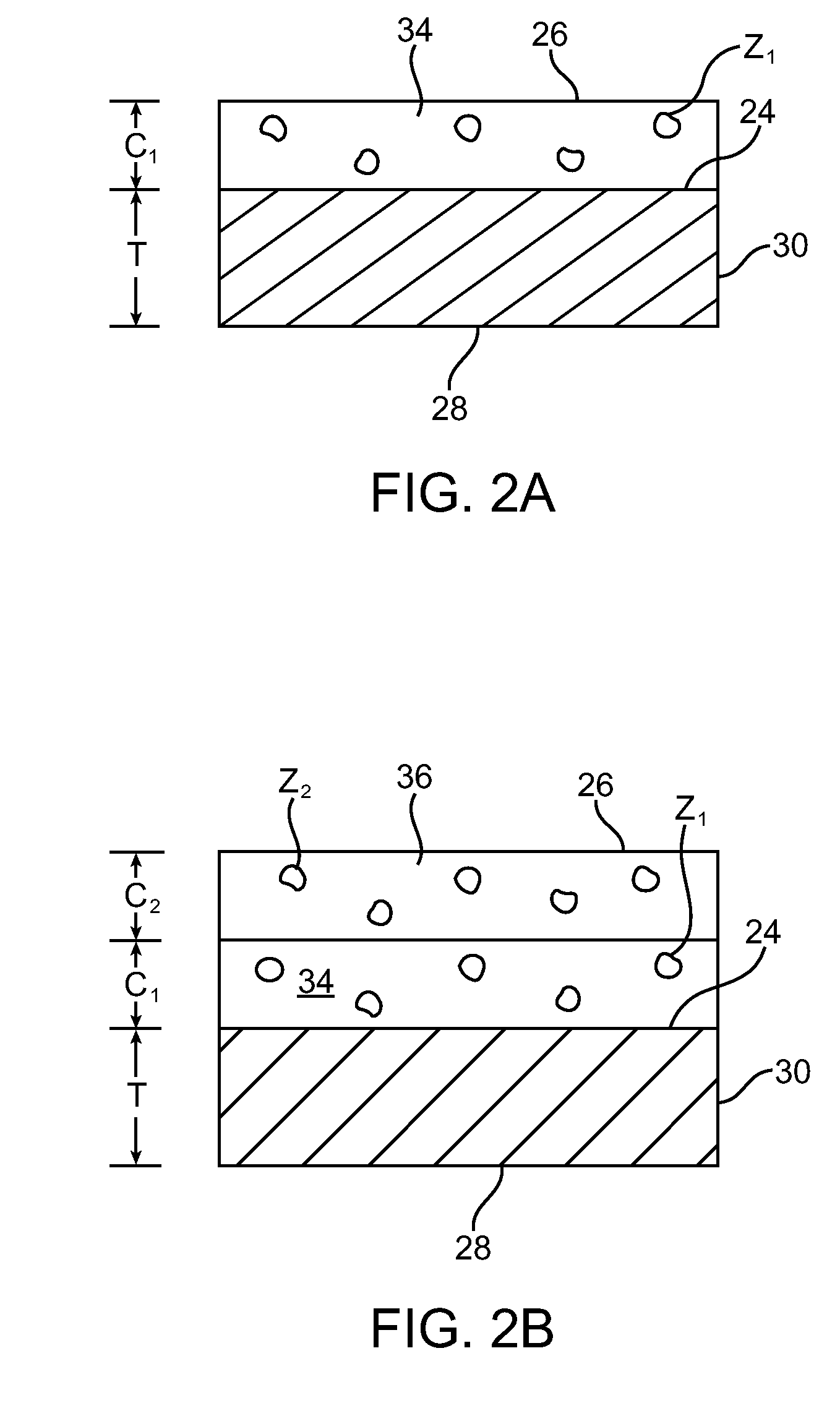
Drug-eluting stent having collagen drug carrier chemically treated with genipin
InactiveUS20050123582A1Reduce releaseAntibacterial agentsOrganic active ingredientsVulnerable plaqueInsertion stent
A method for treating vulnerable plaques of a patient, comprising: providing a biodegradable stent comprising a first supporting zone made of a first biodegradable material, wherein the supporting zone comprises at least a portion of continuous circumference of the stent; and a second therapeutic zone made of a second biodegradable material, wherein the therapeutic zone comprises at least one bioactive agent; delivering the biodegradable stent to the vulnerable plaques; orienting the therapeutic zone at about the luminal surface of the vulnerable plaque; and releasing the at least one bioactive agent for treating the vulnerable plaques.
Owner:GP MEDICAL
Cyclodextrin polymer compositions for use as drug carriers
Owner:KOSAK KENNETH M
Biodegradable oxidized cellulose esters and their uses as microspheres
InactiveUS7649089B2Extended durationOrganic active ingredientsGranular deliveryControlled releaseAlcohol
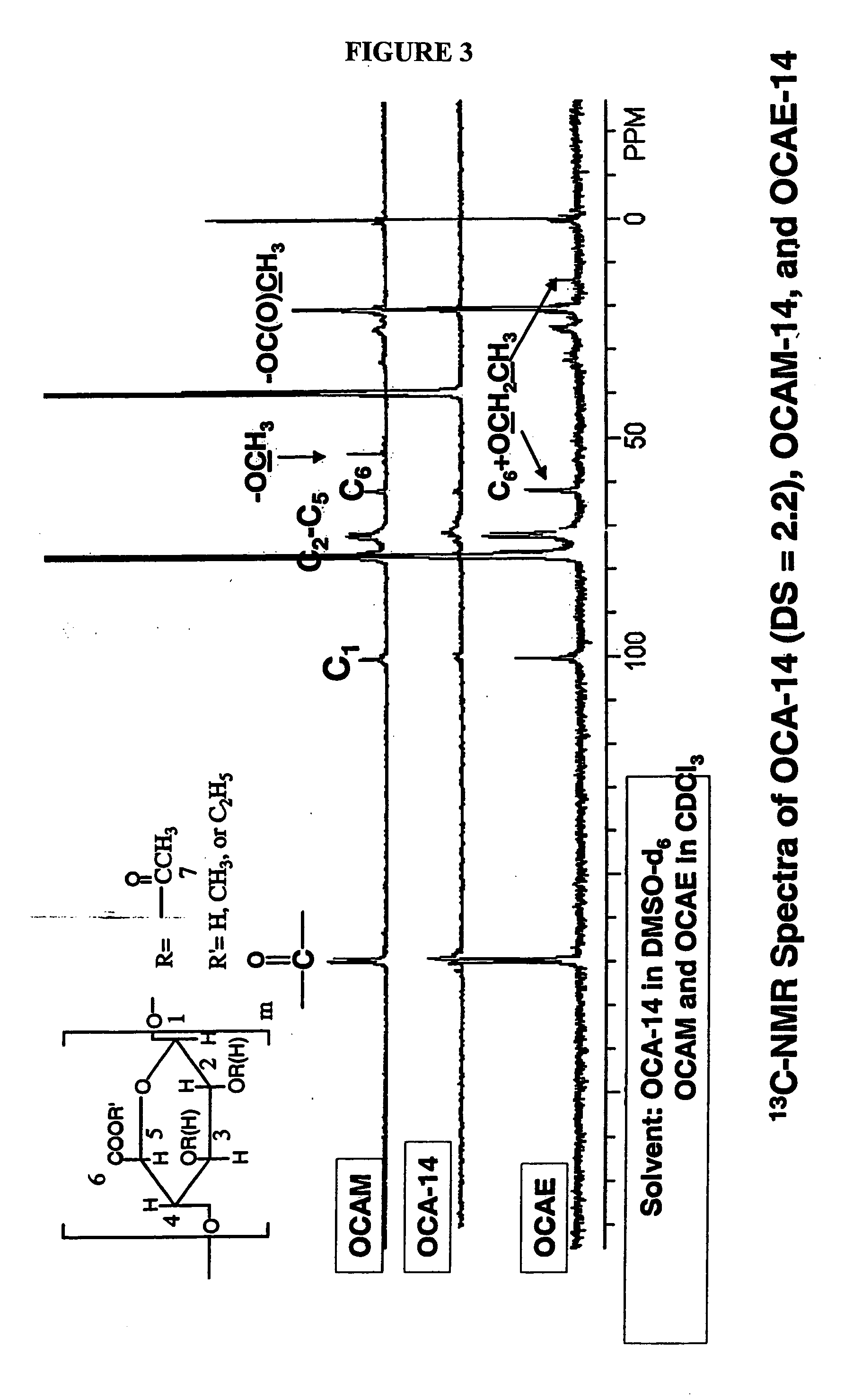
A new cellulose excipient, OCCAE, suitable for use as a binder, filler, and / or disintegrant in the development of solid dosage forms and as a bodying agent or a drug carrier in the preparation of topical formulations is described. The cellulose excipient is formed by reacting an oxidized cellulose ester with an alcohol in the presence of a catalyst. The invention also describes the formation of controlled release microspheres using OCCAE and / or oxidized cellulose esters that may be used to control the release of drug in a patient over a time period of several hours to several days.
Owner:UNIV OF IOWA RES FOUND
Pharmaceutical carrier device suitable for delivery of pharmaceutical compounds to mucosal surfaces
InactiveUS7579019B2Improve the situationPromote healing, asepty, scarificationOrganic active ingredientsPowder deliveryDrug compoundDrug carrier
The present invention relates to a pharmaceutical delivery device for application of a pharmaceutical to mucosal surfaces. The device comprises an adhesive layer and a non-adhesive backing layer, and the pharmaceutical may be provided in either or both layers. Upon application, the device adheres to the mucosal surface, providing localized drug delivery and protection to the treatment site. The kinetics of erodability are easily adjusted by varying the number of layers and / or the components.
Owner:ARIUS TWO
Pharmaceutical carrier device suitable for delivery of pharmaceutical compounds to mucosal surfaces
InactiveUS20050048102A1Improve the situationPromote healing, asepty, scarificationPowder deliveryAdhesive dressingsDrug compoundDrug carrier
The present invention relates to a pharmaceutical delivery device for application of a pharmaceutical to mucosal surfaces. The device comprises an adhesive layer and a non-adhesive backing layer, and the pharmaceutical may be provided in either or both layers. Upon application, the device adheres to the mucosal surface, providing localized drug delivery and protection to the treatment site. The kinetics of erodability are easily adjusted by varying the number of layers and / or the components.
Owner:ARIUS TWO
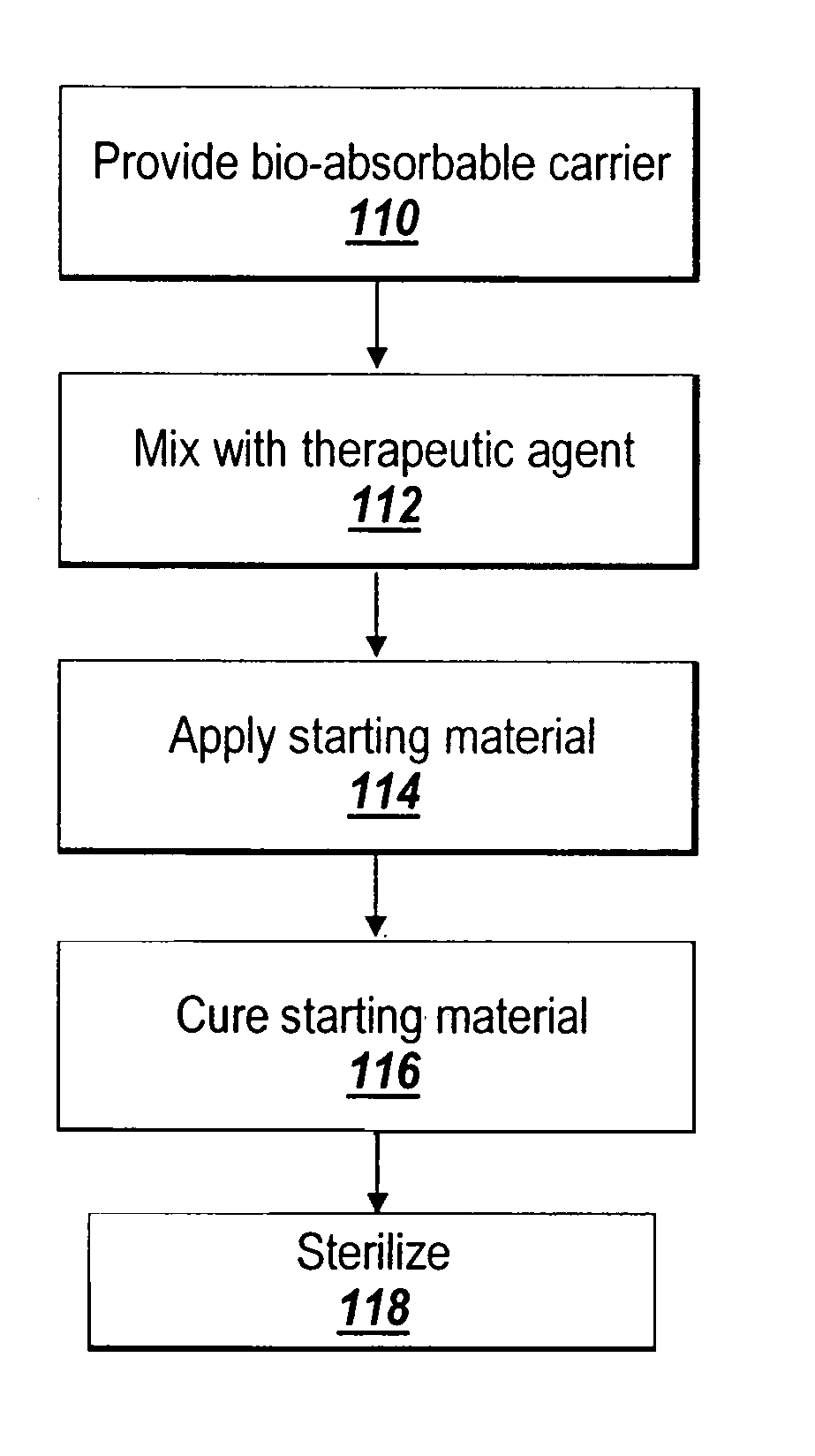
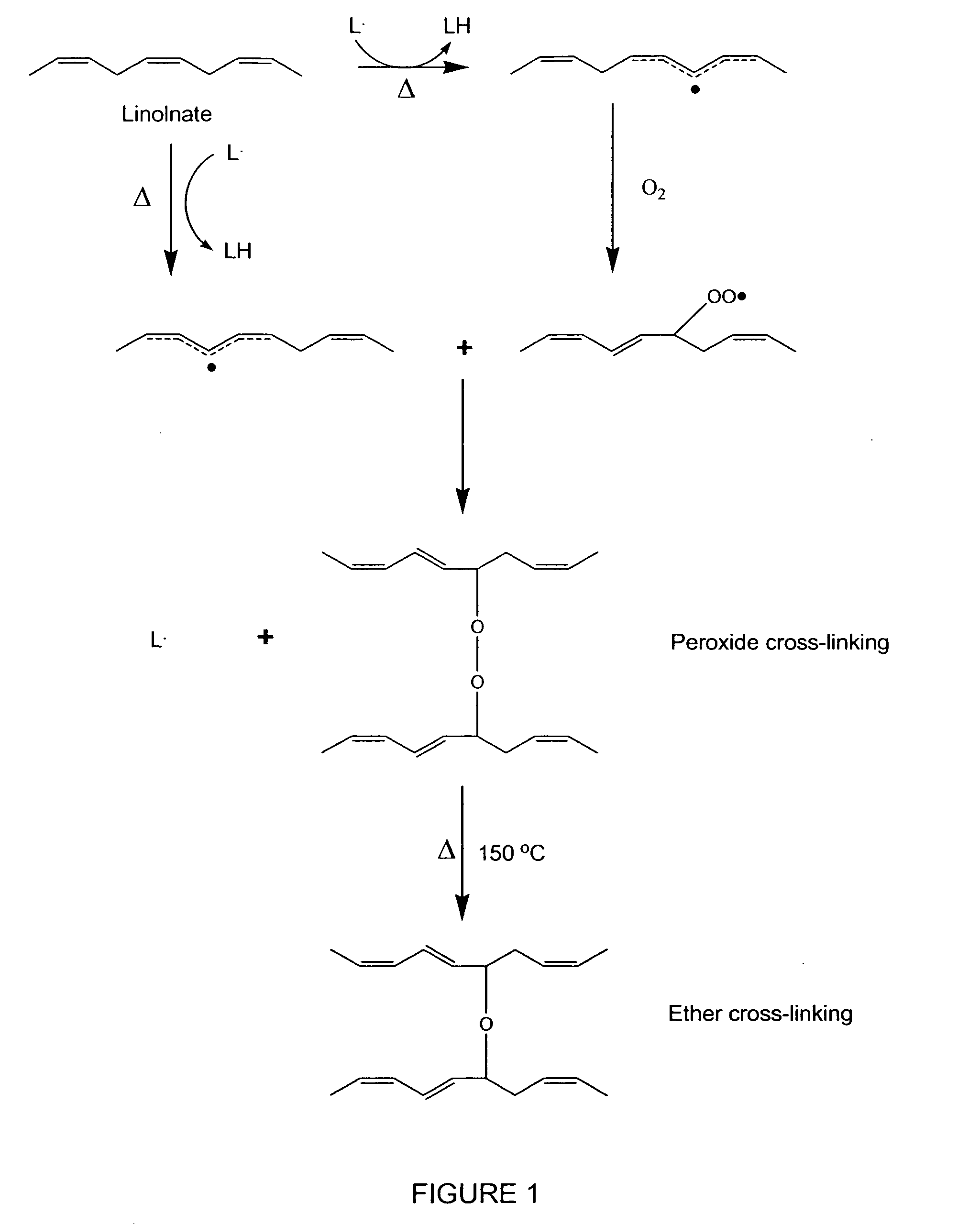
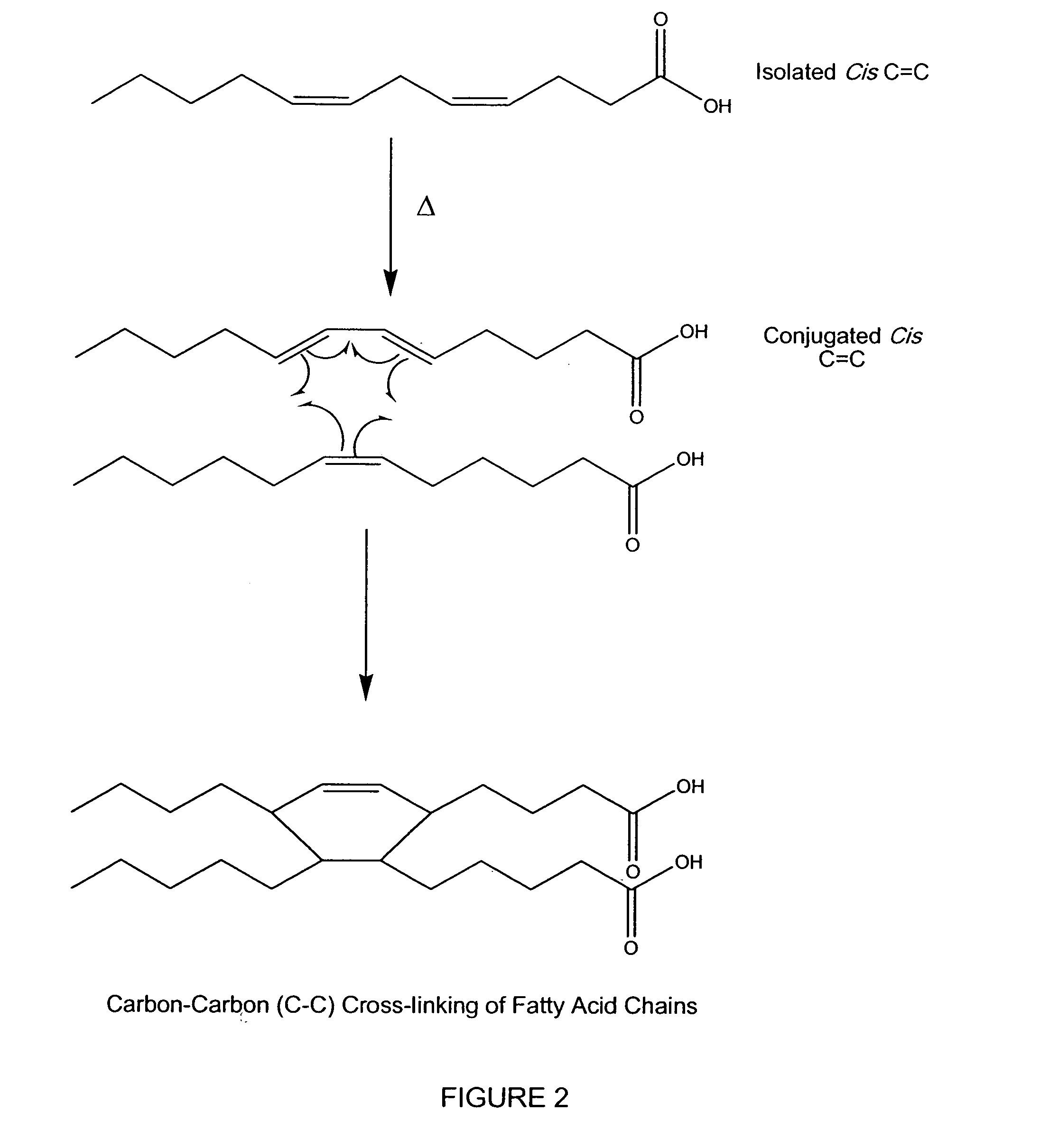
Hydrophobic cross-linked gels for bioabsorbable drug carrier coatings
ActiveUS20070202149A1Need can be addressedEasy to controlPowder deliveryAntipyreticCross-linkDrug carrier
Owner:ATRIUM MEDICAL
Preparation method of core-shell structured synthetic polymer-natural polymer composite fiber
InactiveCN102817105ASimple and fast operationMild reaction conditionsConjugated cellulose/protein artificial filamentsFilament/thread formingFiberPolymer science
The invention discloses a preparation method of a core-shell structured synthetic polymer-natural polymer composite fiber, and the method comprises the steps of: (1) selecting one or several of synthetic polymers to dissolve in a solvent, and conducting stirring until complete dissolution; (2) selecting a natural polymer to dissolve in a solvent, or adding a spinning assistant, and carrying out stirring until complete dissolution; and (3) taking the solution prepared in step (1) as an outer tube spinning solution, adopting the solution prepared in step (2) as an inner tube spinning solution, injecting them into the inner tube and the outer tube of a coaxial spinneret, and performing coaxial electrospinning at room temperature. The core-shell structured nano-fiber prepared by the invention selects the synthetic polymer as the shell layer, and can inhibit water molecules from penetrating the natural polymer as the core layer. The natural polymer as the core layer can more effectively encapsulate active substances to avoid inactivation of the active substances in the presence of an organic solvent, so that the core-shell structured composite fiber can play a good drug sustained release role in the drug carrier field, and an integral activity can be maintained.
Owner:SHANGHAI JIAO TONG UNIV +1
Apparatus and method for delivery of micro and submicro quantities of materials
A process and apparatus (invention) permitting the distribution of micro or submicro liter or gram (exceptionally small) quantities of liquids and powders, (hereafter materials) in patterns and quantities that are especially well-suited for ophthalmologic patient self-application of drugs to his own eye(s) with substantially no overdose. The invention is thus also highly beneficial in those many topical drug applications where overdoses should be avoided. This is particularly applicable for the eye, where overdoses can have many deleterious medical consequences. Use of the invention permits exceptionally small doses of ophthalmologic drugs. Moreover, it also permits the repeatability of the quantity size of the materials chosen for delivery. In the case of drugs, particularly opthomologic drugs, this means that the invention permits the repeated multiple distribution of the same chosen drug / carrier quantity resulting in a uniform repeatable dosage. Variation of dose from one application to another is very unacceptable. The preferred apparatus of the invention employs a venturi configuration inventively modified which uses gas under pressure to pump exceptionally small quantities of material from a reservoir for delivery. In especially preferred embodiments of the invention, said reservoir is pressurized by at least a quantity of gas at somewhat above atmospheric. prior to the removal of any material by pumping.
Owner:ROGERS ROBERT L +1
Method of treating degenerative spinal disorders
InactiveUS20080045949A1Reduce loadPromotes extracellular matrix productionInternal osteosythesisAntipyreticDevice implantDisease
The present invention describes methods for treating a degenerative intervertebral disc disorder comprising implanting a disc stabilization device into a subject and administering at least one therapeutic agent which promotes healing of the disc to the subject. The invention also includes methods of promoting healing of damaged or degenerated intervertebral discs comprising decreasing the load of the disc through a disc stabilization device and inhibiting the inflammatory process. Also described are hydrogels for use in combination with extradiscal stabilization devices, as intradiscal stabilization devices, as drug carriers, and as combinations thereof.
Owner:ABBOTT LAB INC +1
Non-radical photochemical crosslinked hydrogel material preparation method, product and application
ActiveCN105131315APrecise and controllable time and spaceEasy to operateOrganic active ingredientsCosmetic preparationsPolymer scienceHydroxylamine
The present invention provides a non-radical photo-crosslinked hydrogel preparation method, comprising the following steps: a component A is dissolved in a biocompatible medium to obtain a solution A, component B-hydrazide, hydroxylamine or primary amine high molecular derivative is dissolved in a biocompatible medium to obtain a solution B; the solution A and the solution B are evenly mixed to obtain a hydrogel precursor solution; under illumination, aldehyde group produced by light excitation of o-nitrobenzyl in the component A of the hydrogel precursor solution is crosslinked with hydrazone, hydroxylamine or primary amine group in the component B in the form of respective formation of oxime and Schiff base to produce the hydrogel. The present invention also provides a kit for the hydrogel preparation, and application of the hydrogel in tissue repair, beauty and as a cell, protein or drug carrier. The tissue surface light-situ gel can be achieved by the hydrogel, in particular, wound surface in-situ thin glue formation can be achieved, and the hydrogel is especially suitable for clinical wound surface tissue repair and isolation.
Owner:上海戴云化工科技有限公司 +2
Biodegradable oxidized cellulose esters and their uses as microspheres
A new cellulose excipient, OCCAE, suitable for use as a binder, filler, and / or disintegrant in the development of solid dosage forms and as a bodying agent or a drug carrier in the preparation of topical formulations is described. The cellulose excipient is formed by reacting an oxidized cellulose ester with an alcohol in the presence of a catalyst. The invention also describes the formation of controlled release microspheres using OCCAE and / or oxidized cellulose esters that may be used to control the release of drug in a patient over a time period of several hours to several days.
Owner:UNIV OF IOWA RES FOUND
Patch and method for transdermal delivery of bupropion base
InactiveUS6312716B1Good for weight lossRelieve symptomsOrganic active ingredientsBiocideTransdermal patchDrug withdrawal symptoms
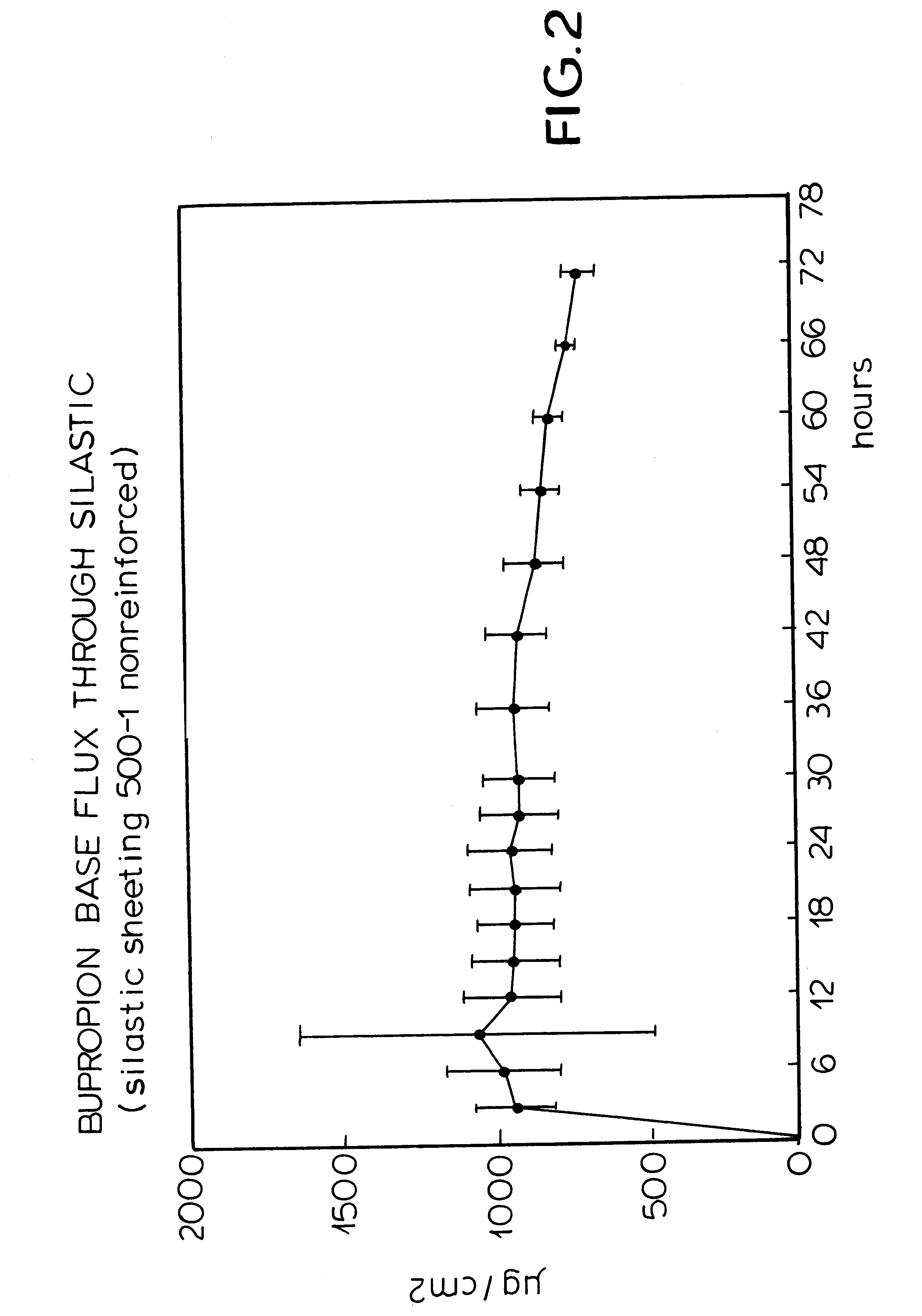
The invention includes a patch and method for transdermal delivery of bupropion base. In the method of this invention, a patient is administered a bupropion base in an amount effective to alleviate withdrawal symptoms and to prevent or reduce craving of nicotine in said patient. Alternatively, an effective amount of bupropion base is delivered to alleviate depression in a patient or to treat obesity. A transdermal patch includes a bupropion base. The bupropion base can be mixed with an acceptable pharmaceutical carrier.
Owner:COLLEGIUM PHARMA INC
Aggregates with increased deformability, comprising at least three amphipats, for improved transport through semi-permeable barriers and for the non-invasive drug application in vivo, especially through the skin
InactiveUS20040105881A1Increase drug concentrationImprove distributionOrganic active ingredientsAntipyreticBiological bodyDrug carrier
Owner:IDEA AG
Preparation of polysaccharide beads
The present invention relates a method of preparing agarose beads, which method results in a population of beads which are of relatively uniform particle size. In an advantageous embodiment, the beads are of a particle size less than 10 μm, and the coefficient of variation C.V. of the population is less than 15%. The beads according to the invention are advantageously used in biological separation methods, such as in the production of chromatographic packing materials; drug carriers; or in any method of biological engineering.
Owner:CYTIVA BIOPROCESS R&D AB
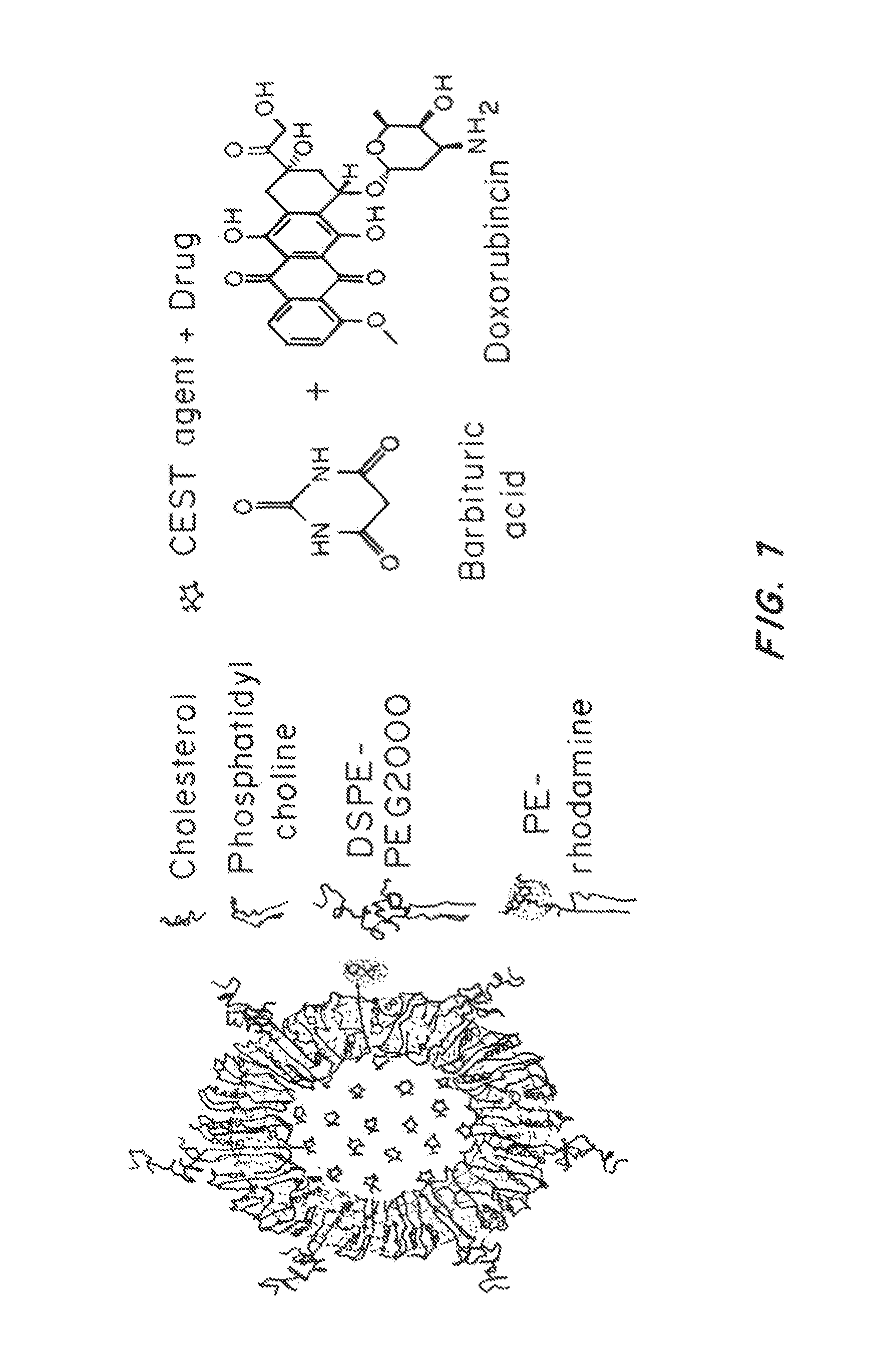
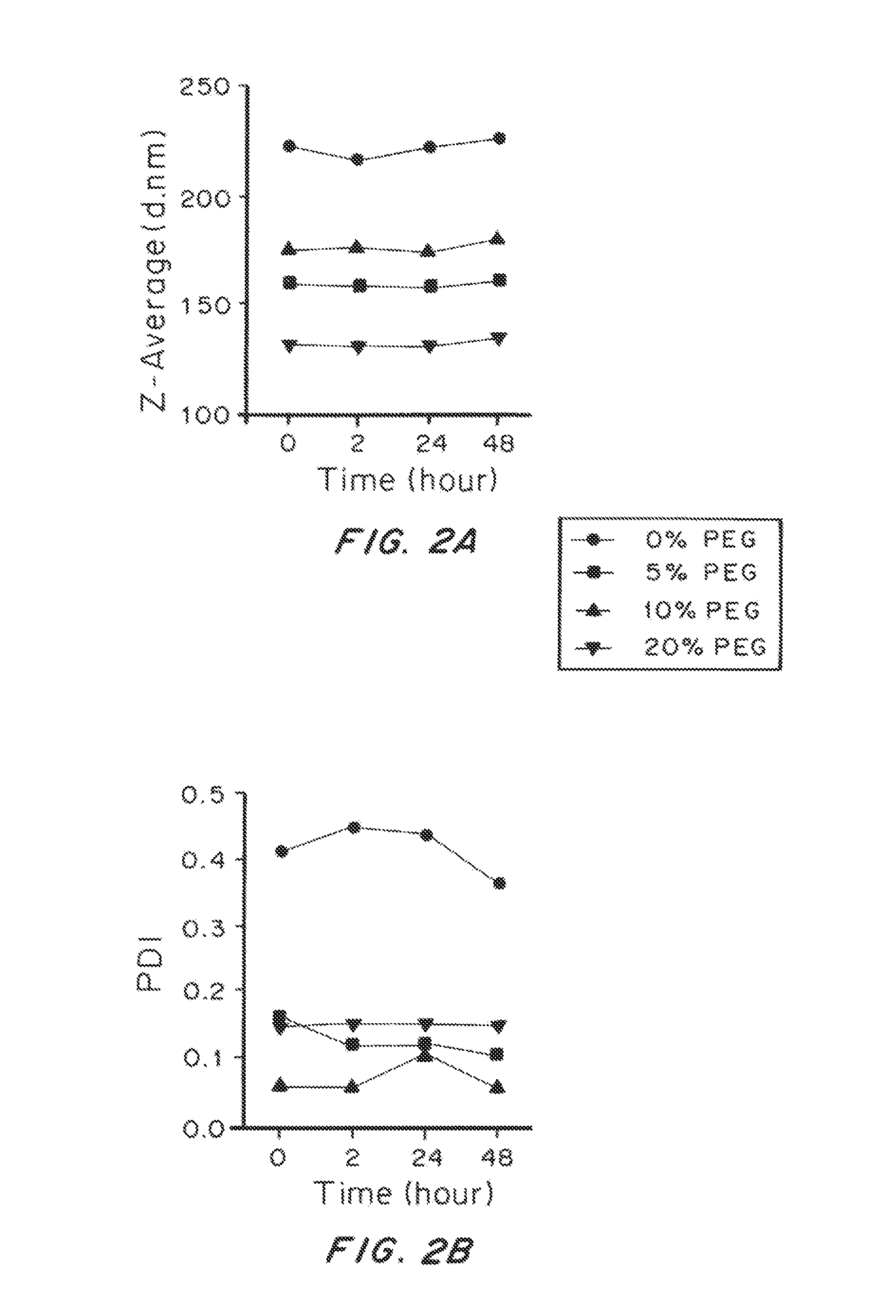
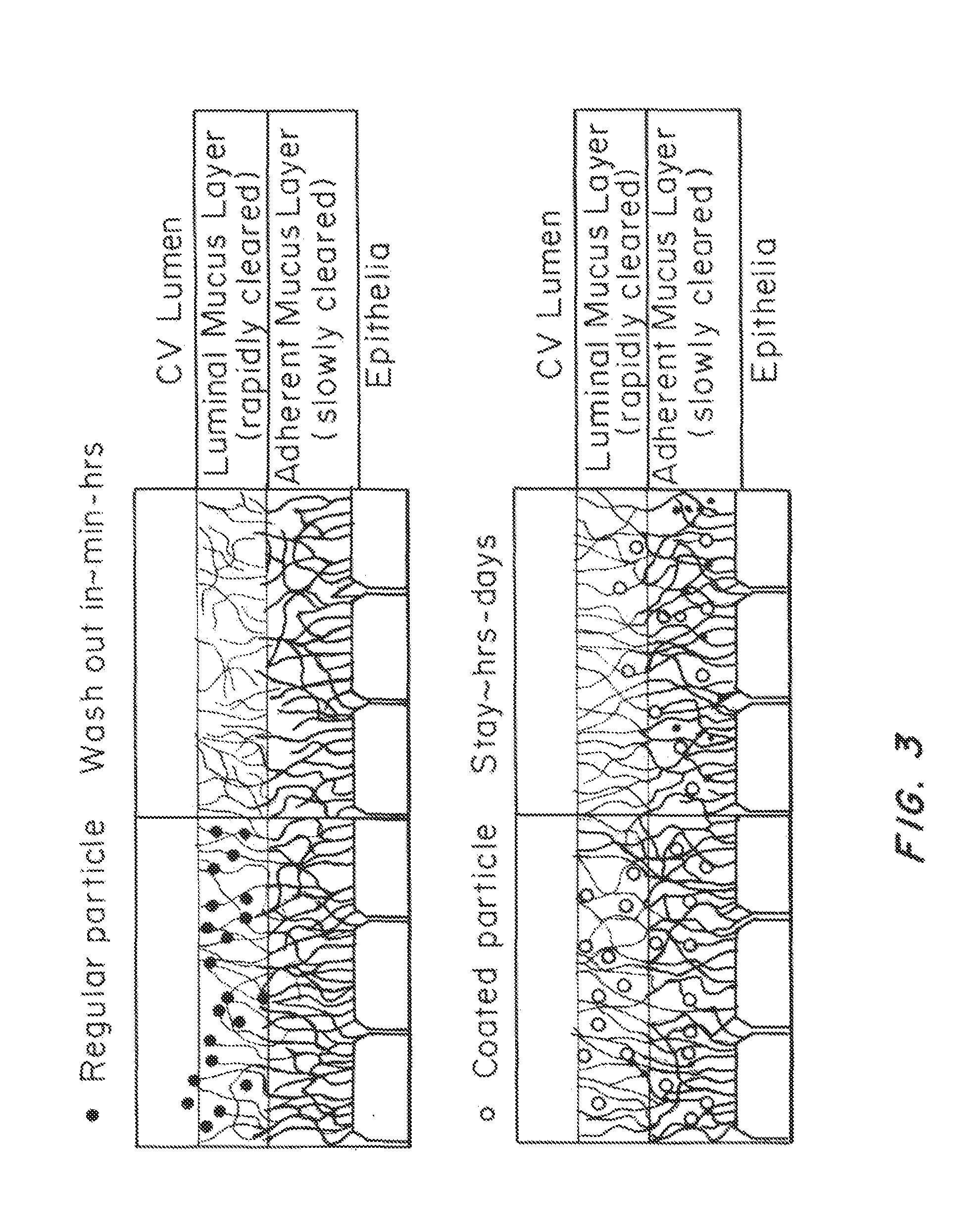
Lipid-Based Drug Carriers for Rapid Penetration Through Mucus Linings
Mucus-penetrating liposomal nanoparticles and methods of making and using thereof are described herein. The nanoparticles contain one or more lipids, one or more PEG-conjugated lipids, and optionally one or more additional materials that physically and / or chemically stabilize the particles. The nanoparticle have an average diameter of about 100 nm to about 300 nm, preferably from about 100 nm to about 250 nm, more preferably from about 100 nm to about 200 nm. The particles are mobile in mucus. The liposomes can further contain one or more therapeutic, prophylactic, and / or diagnostic agent to be delivered to a mucosal surface, such as the CV tract, the colon, the nose, the lungs, and / or the eyes. The liposomes can further contain one or more CEST agents to allow real time imaging of the particles in a live animal. The particles may also further contain an imaging agent, such as a fluorescent label.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Medical Device Having Coating With Zeolite Drug Reservoirs
A medical device having a drug-eluting coating that includes a pharmaceutical compound or, more generally, a therapeutic material housed within pores of a zeolite carrier. The zeolite carrier has an open porous structure with reservoirs for holding the therapeutic material. The therapeutic material loaded zeolites may be suspended or dispersed within a bioerodible polymer matrix to provide controlled delivery of the therapeutic material. Zeolite drug carriers may have enhanced or optimally engineered pore sizes for a particular therapeutic material and release profile. Along with a therapeutic material, reservoirs of a zeolite drug delivery system may include a release agent. The release agent may be used to entrap the therapeutic material until such time as a triggering condition is met that prompts the release agent to activate and thereby release the therapeutic material from the zeolite reservoir.
Owner:MEDTRONIC VASCULAR INC
Stent with eccentric coating
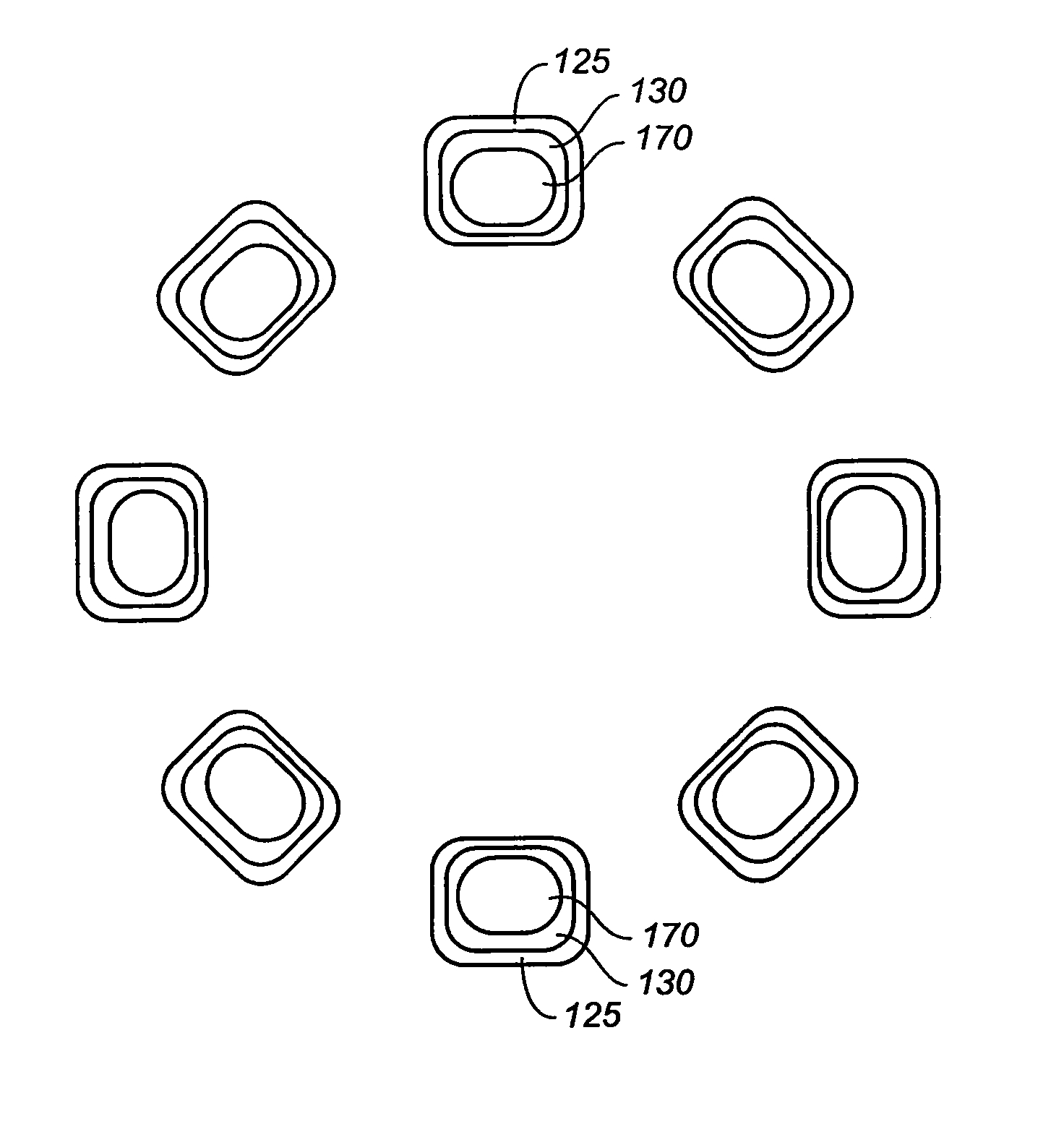
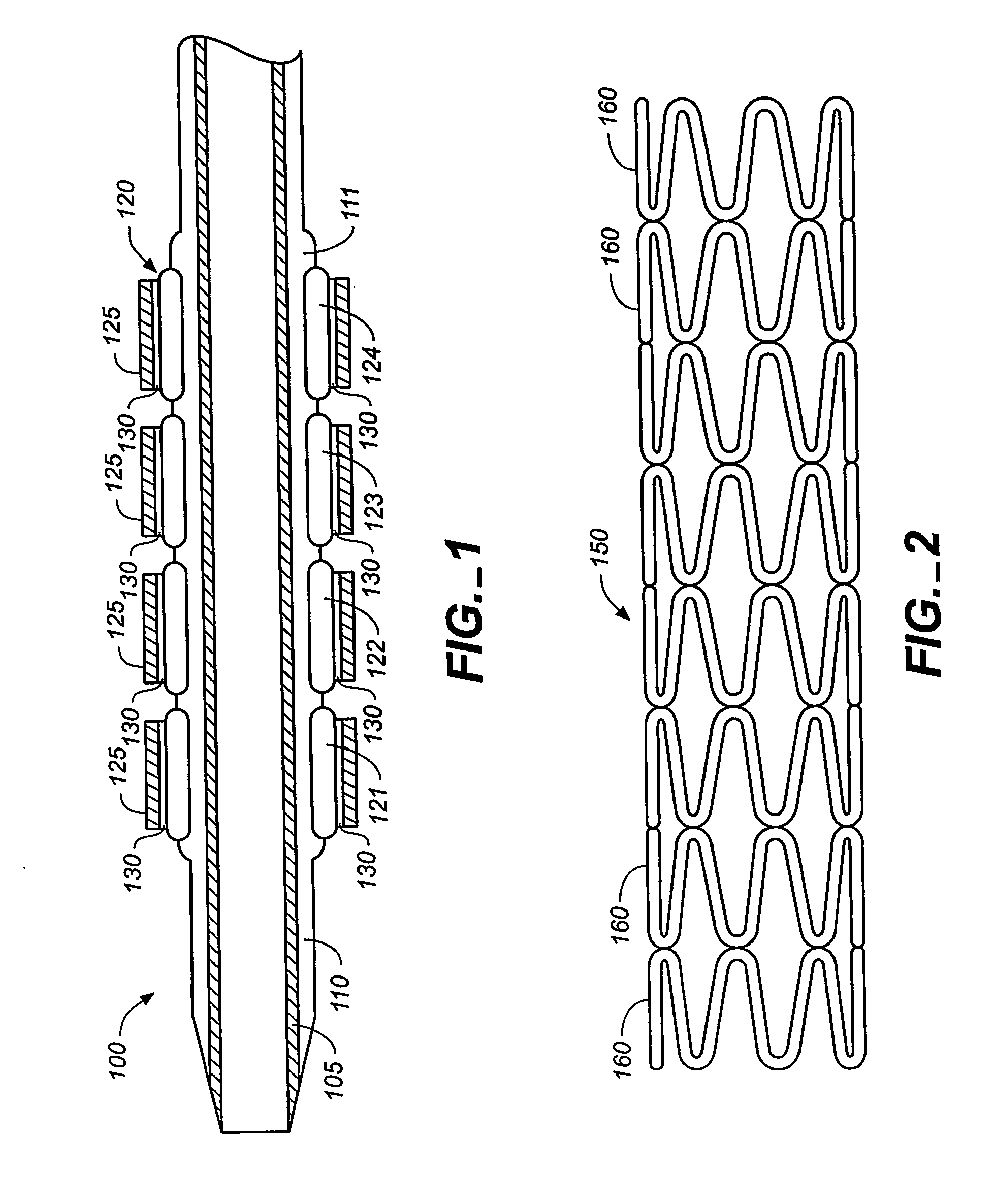
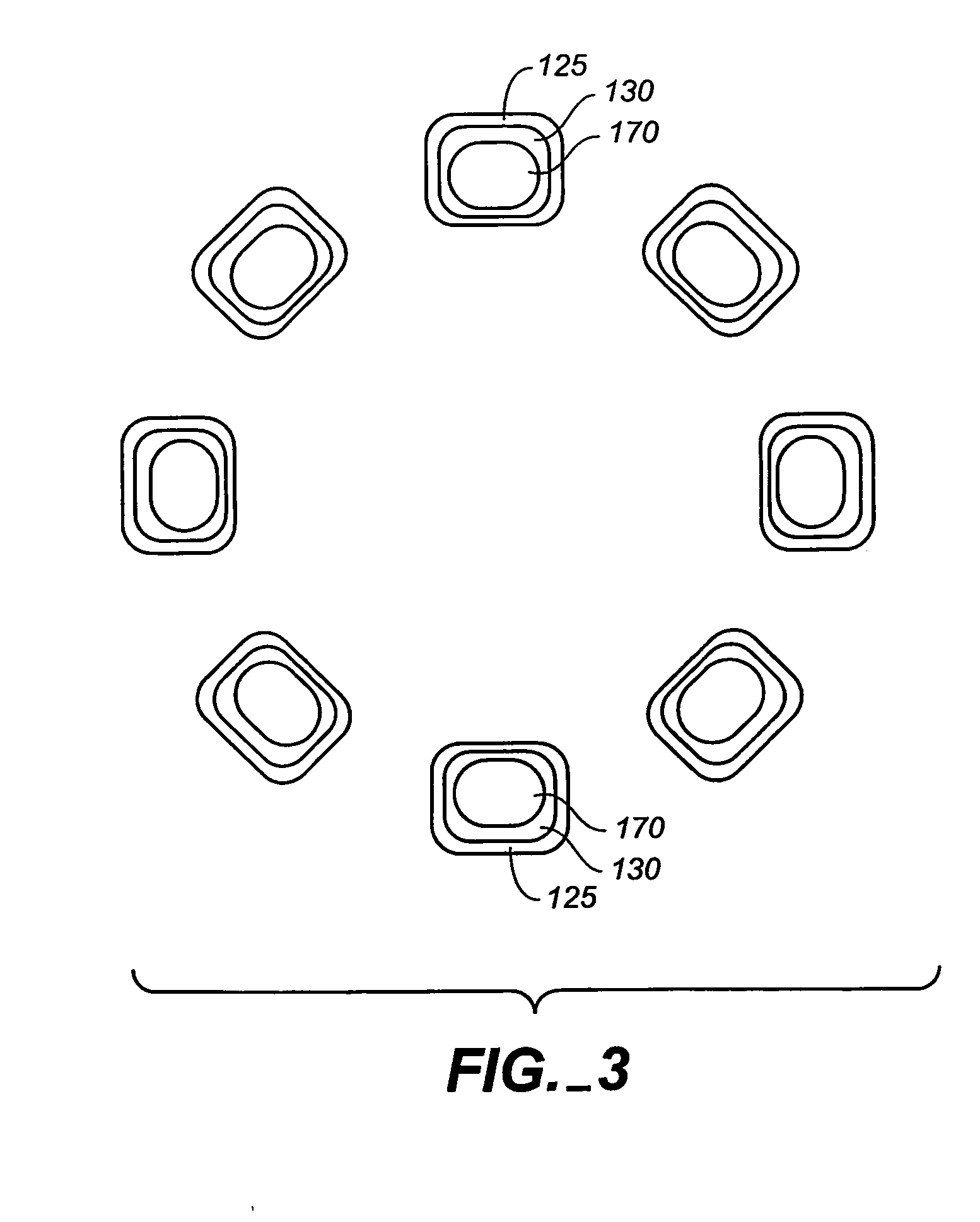
InactiveUS20060149365A1Avoid wastingReduce drug deliveryStentsLiquid surface applicatorsInsertion stentDrug carrier
The stent (120) with an eccentric coating (130) of the present invention provides a coating having a different coating thickness on the stent outer diameter and stent inner diameter, i.e., an eccentric coating. The eccentric coating can be the primary carrier for a drug or other therapeutic agent. The eccentric coating can be thicker on the stent outer diameter to supply more drug to the vessel wall in which the coated stent is deployed and less drug to the vessel lumen. In one embodiment, a cap coating (125) can be disposed on the eccentric coating to protect the eccentric coating, control the elution rate from the eccentric coating, provide an additional drug carrier, or provide combinations thereof. The eccentric coating can be applied by spraying a coating liquid on the stent outer diameter with a fixture mandrel interior to the stent to regulate the spray to the stent inner diameter.
Owner:MEDTRONIC VASCULAR INC
Hydrophobic cross-linked gels for bioabsorbable drug carrier coatings
ActiveUS8124127B2Need can be addressedEasy to controlPowder deliveryAntipyreticCross-linkDrug carrier
Owner:ATRIUM MEDICAL
Nucleic acid carriers for delivery of therapeutic agents
InactiveUS20070225213A1Improve solubilityLow biological effectHeavy metal active ingredientsBiocideIn vivoProliferation rate
Nucleic acid drug carriers comprise a nucleic acid carrier complexed with a drug, wherein the nucleic acid carrier and the drug are associated non-covalently, and optionally other agents such as spacer, transfection agents, and targeting agents. The nucleic acid drug complex are discovered to have permissive or refractory uptake depending on many factors including cell type, proliferation rate, among others. The refractive uptake of the nucleic acid drug complex are shown to be useful in the nucleic acid targeting of drugs, both in vitro and in vivo. Novel drug compositions are disclosed that effectively reduce the toxicity of drugs while maintaining drug activity and enhancing a drug's therapeutic index.
Owner:KOSAK MATTHEW K
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
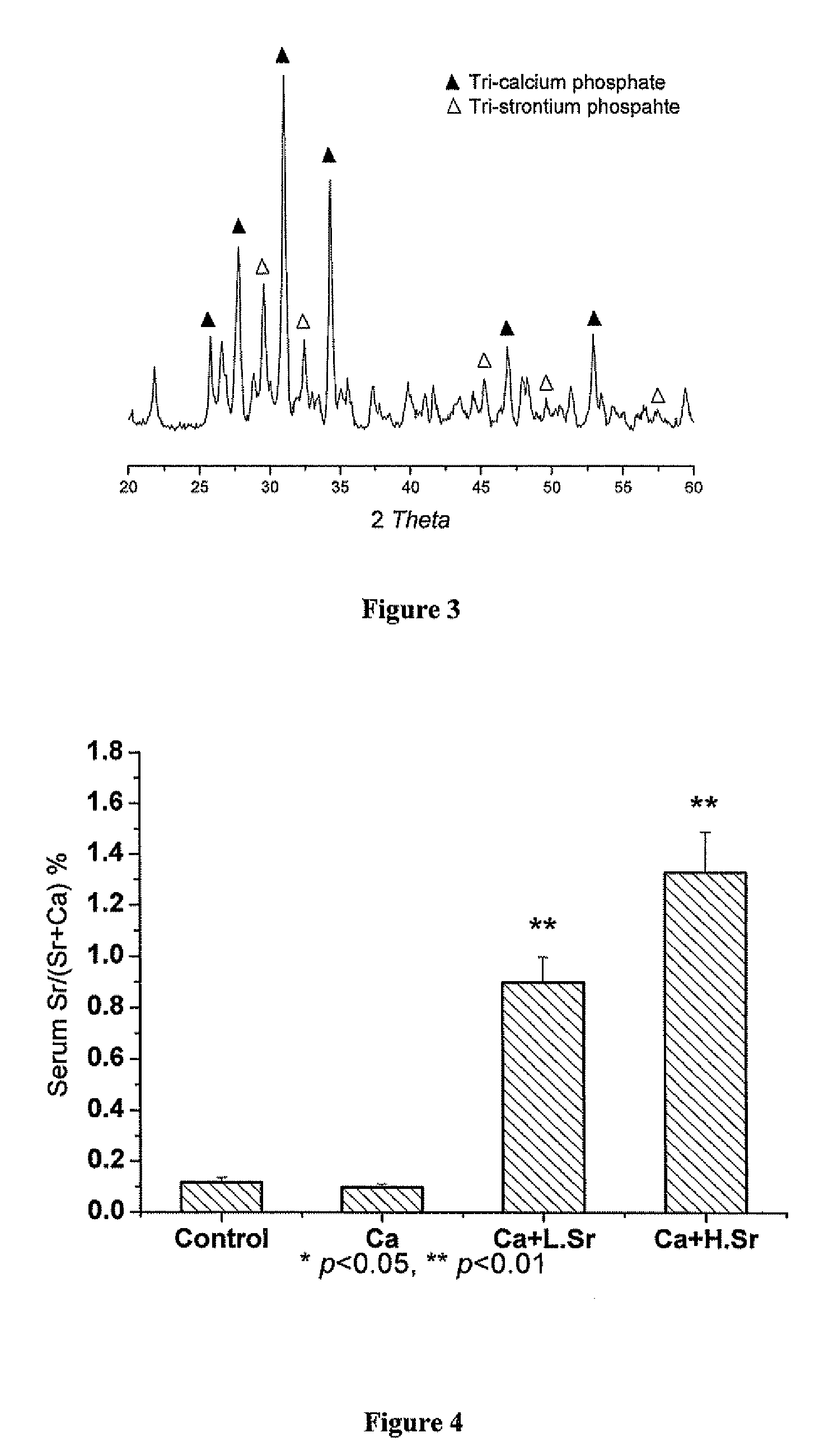
Strontium fortified calcium nano-and microparticle compositions and methods of making and using thereof
InactiveUS20080317807A1Good biocompatibilityPrevent looseningHeavy metal active ingredientsBiocideDiseasePhosphate
Compositions containing strontium fortified calcium nanoparticles and / or microparticles, and methods of making and using thereof are described herein. The strontium fortified calcium compounds contain calcium ions, calcium atoms, strontium ions, strontium atoms, and combinations thereof and one or more anions. Exemplary anions include, but are not limited to, citrate, phosphate, carbonate, and combinations thereof. The particles can be formulated for enteral or parenteral administration by incorporating the particles into a pharmaceutically carrier. The compositions can further contain one or more active agents useful for bone diseases or disorders, such as vitamin D, growth factors, and combinations thereof. The compositions can be used to treat or prevent one or more bone diseases or disorders of the bone, such as osteoporosis. Alternatively, the particles can be coated onto a substrate, such as the surface of an implant. The coatings can be used to improved biocompatibility of the implant, prevent loosening of the implant, reducing leaching of metal ions from metallic implants, and reduce corrosion. The coatings can be applied to the substrate using a variety of techniques well known in the art. In one embodiment, the coating is applied using electrophoretic deposition. The use of nano- and / or microparticles that provide high surface area helps to improve interfacial strength between the coating and the implant, which allows for the use of lower sintering temperatures. Lowering sintering temperatures minimizes or prevents thermal decomposition of the coating material and / or degradation of the implant material.
Owner:THE UNIVERSITY OF HONG KONG
Biodegradable oxidized cellulose esters
InactiveUS20060093672A1Less-expensive to produceOrganic active ingredientsBiocideOrganic solventMicrosphere
The present invention relates to the preparation of a series of oxidized cellulose esters suitable for use as a drug carrier in the development of biodegradable controlled and / or sustained release pharmaceutical, agricultural, and veterinary compositions, such as films, compacts, microspheres, and pellets. The esters are prepared by acylation of oxidized cellulose having at least 3% carboxyl groups. The resulting oxidized cellulose esters are soluble in aqueous alkaline solutions, water, and a variety of organic solvents.
Owner:UNIV OF IOWA RES FOUND
Drug Carrier and Drug Carrier Kit for Inhibiting Fibrosis
ActiveUS20080193512A1Minimize side effectsPreventing and suppressing and improving fibrosisBiocidePowder deliveryDiseaseSide effect
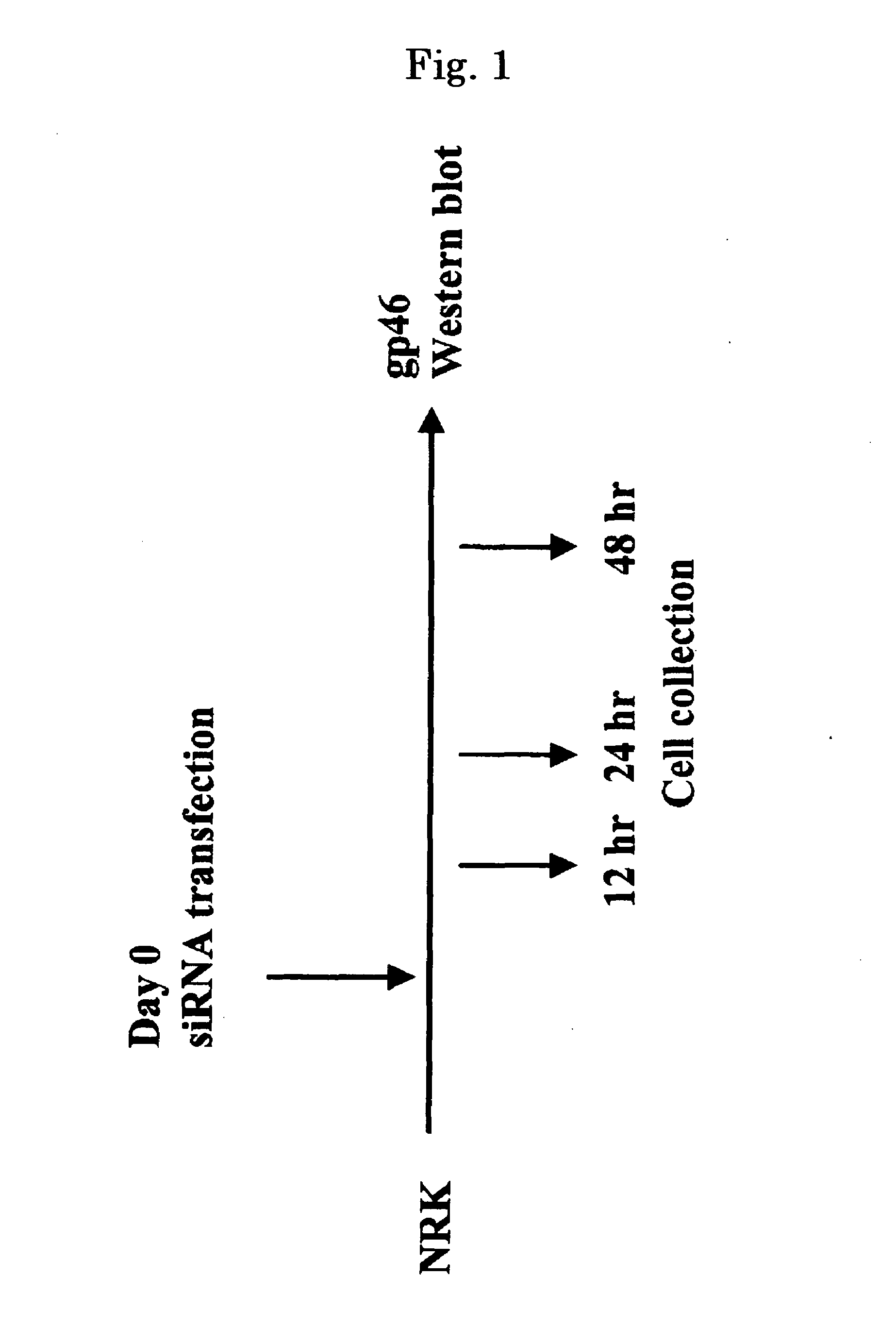
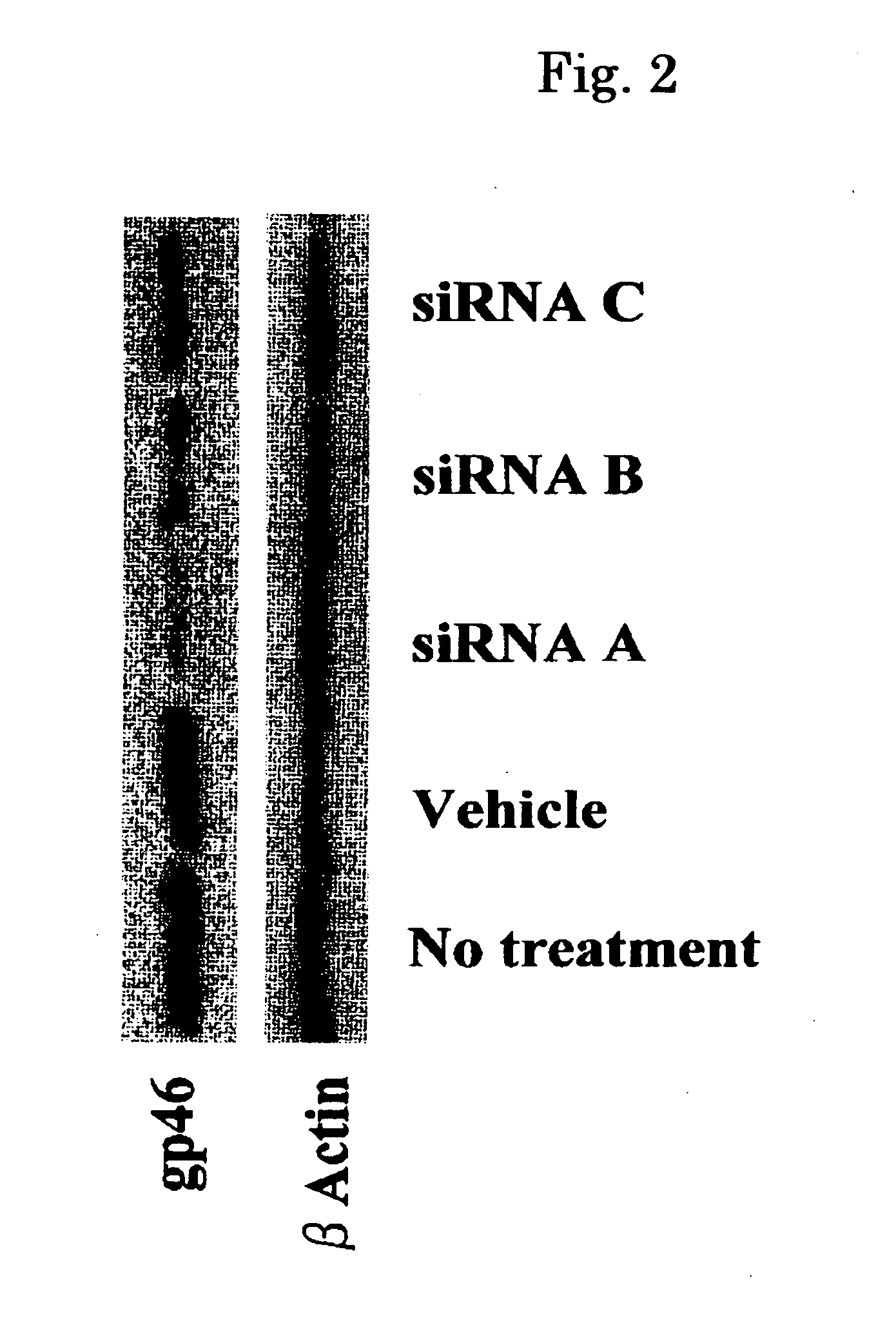
An astrocyte-specific drug carrier containing a retinoid derivative and / or a vitamin A analog as a constituent; a drug delivery method with the use of the same; a drug containing the same; and a therapeutic method with the use of the drug. By binding a drug carrier to a retinoid derivative such as vitamin A or a vitamin A analog or encapsulating the same in the drug carrier, a drug for therapeutic use can be delivered specifically to astrocytes. As a result, an astrocyte-related disease can be efficiently and effectively inhibited or prevented while minimizing side effects. As the drug inhibiting the activity or growth of astrocytes, for example, a siRNA against HSP47 which is a collagen-specific molecule chaperone may be encapsulated in the drug carrier. Thus, the secretion of type I to type IV collagens can be inhibited at the same time and, in its turn, fibrosis can be effectively inhibited.
Owner:NITTO DENKO CORP
Method for synthesizing BTC (1,3,5-benzenetricarboxylic acid)-based nanoscale organometallic framework material
InactiveCN102336774AAvoid pollutionAvoid wastingCopper organic compoundsNickel organic compoundsMetal-organic frameworkNano devices
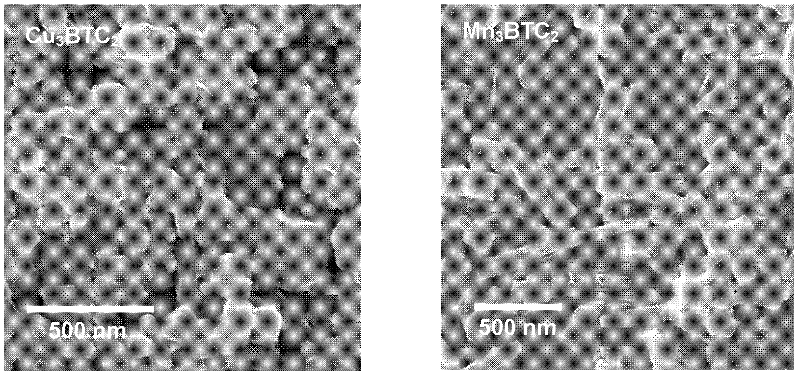
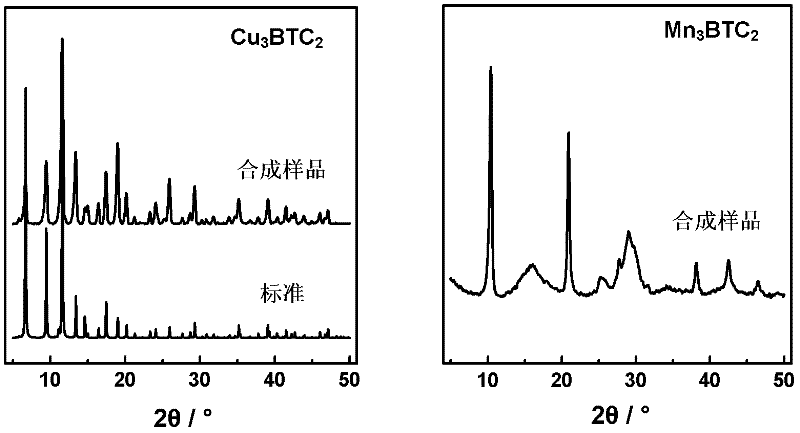
The invention discloses a method for rapidly synthesizing a BTC (1,3,5-benzenetricarboxylic acid)-based nanoscale organometallic framework material at room temperature. The nanomaterial is prepared by the following steps: mixing metal acetate aqueous solution with BTC solution at room temperature, and reacting to obtain BTC-based organometallic framework nanoparticles. The method is rapid, simple and energy-saving; the yield is high; the used raw materials and solvents are inexpensive and easily available; the safety problem of commonly used nitrate is not caused; the method is favorable to the industrial production and has wide application prospects in the fields of catalysis, adsorption, drug carriers, building of nano devices and the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com