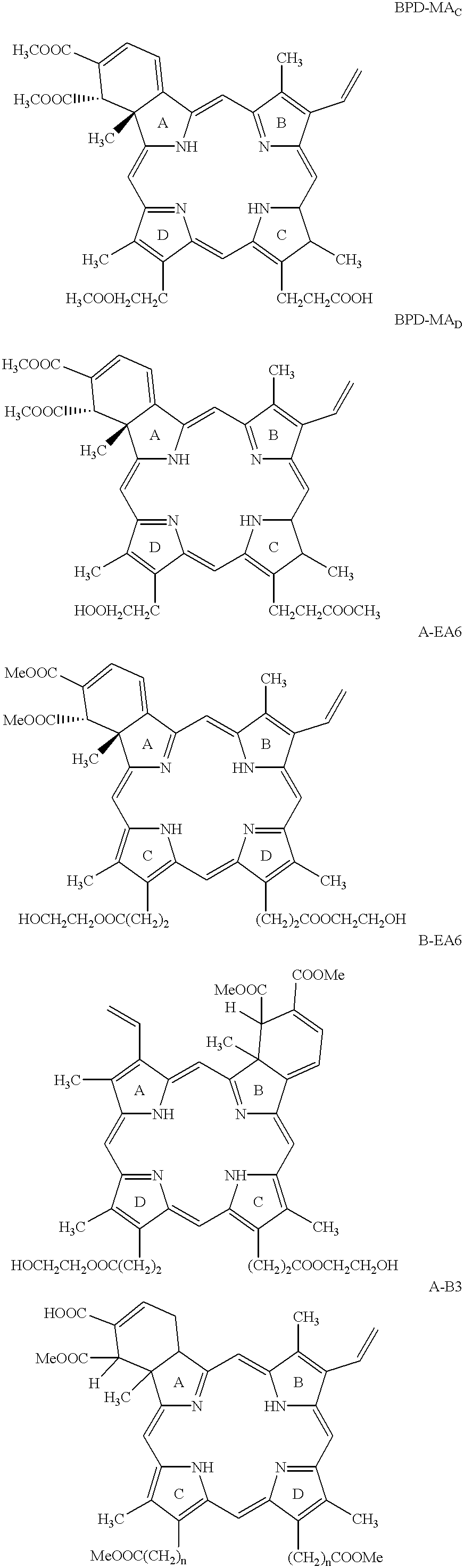
Patents
Literature
185 results about "Immunoadjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adjuvants are mostly pharmacological agents of drug or biological origin used to modify the antigenicity of immunization components, i.e., to stimulate, potentiate, or depress the immune response or to inhibit or enhance specific subclasses of immunocytes. Adjuvants augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. Classical agents (Freund's adjuvant, BCG, Corynebacterium parvum) contain bacterial antigens. Some adjuvants are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a variety of antigens, or antigen-specific, affecting a restricted type of immune response to a narrow group of antigens. Since adjuvants enhance the body's immune response, they can be considered a type of immune modulator.
Vaccine composition containing synthetic adjuvant
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
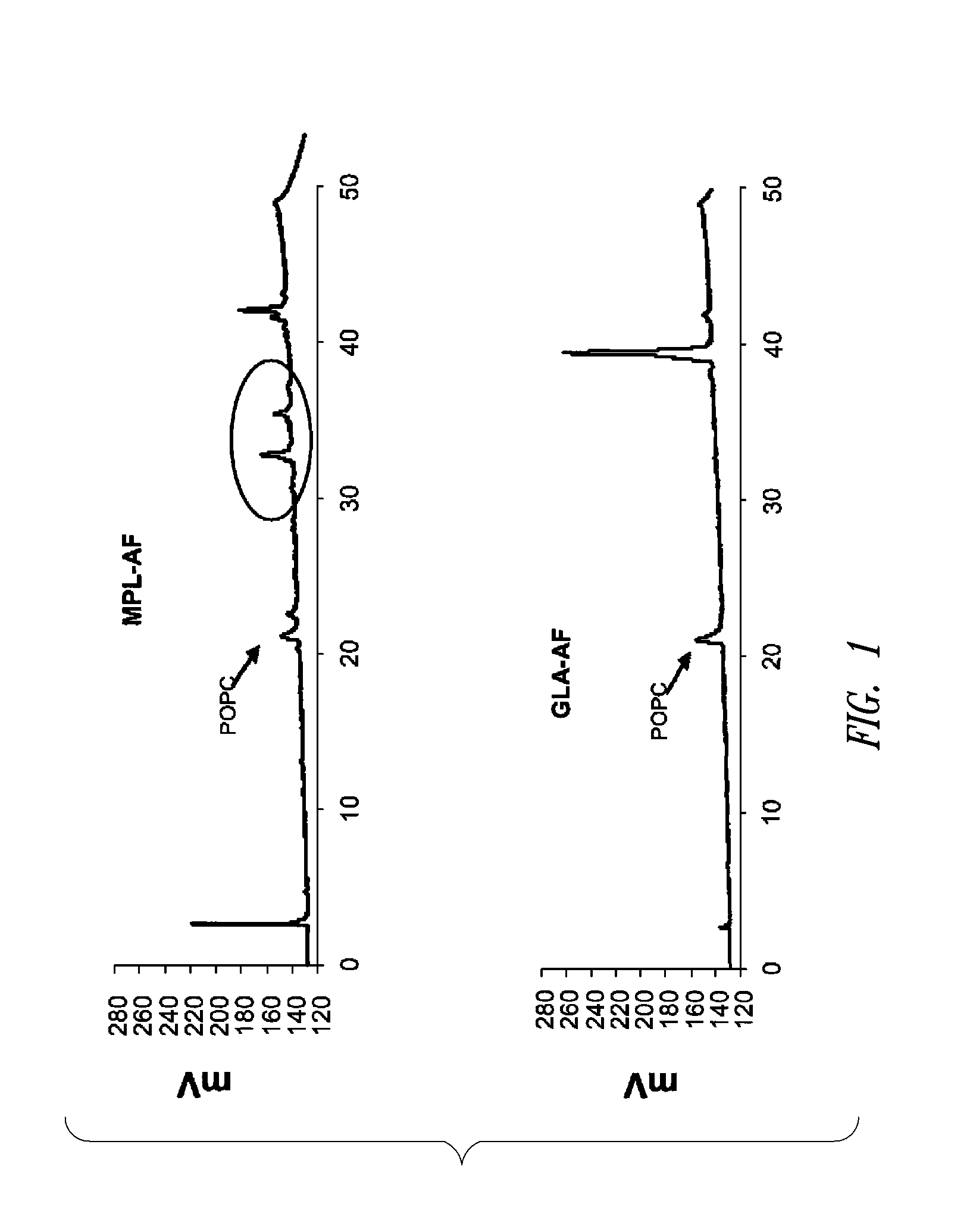
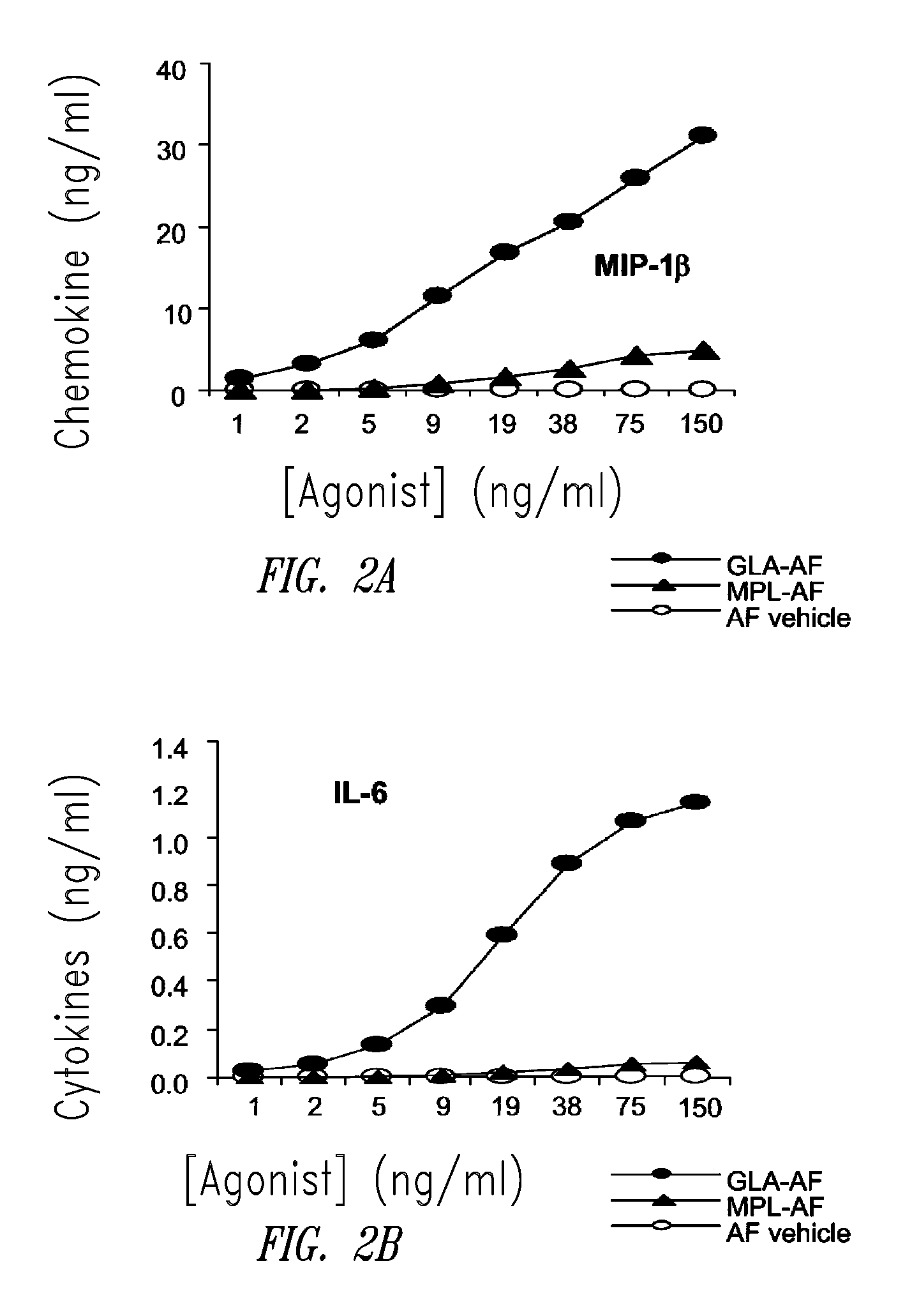
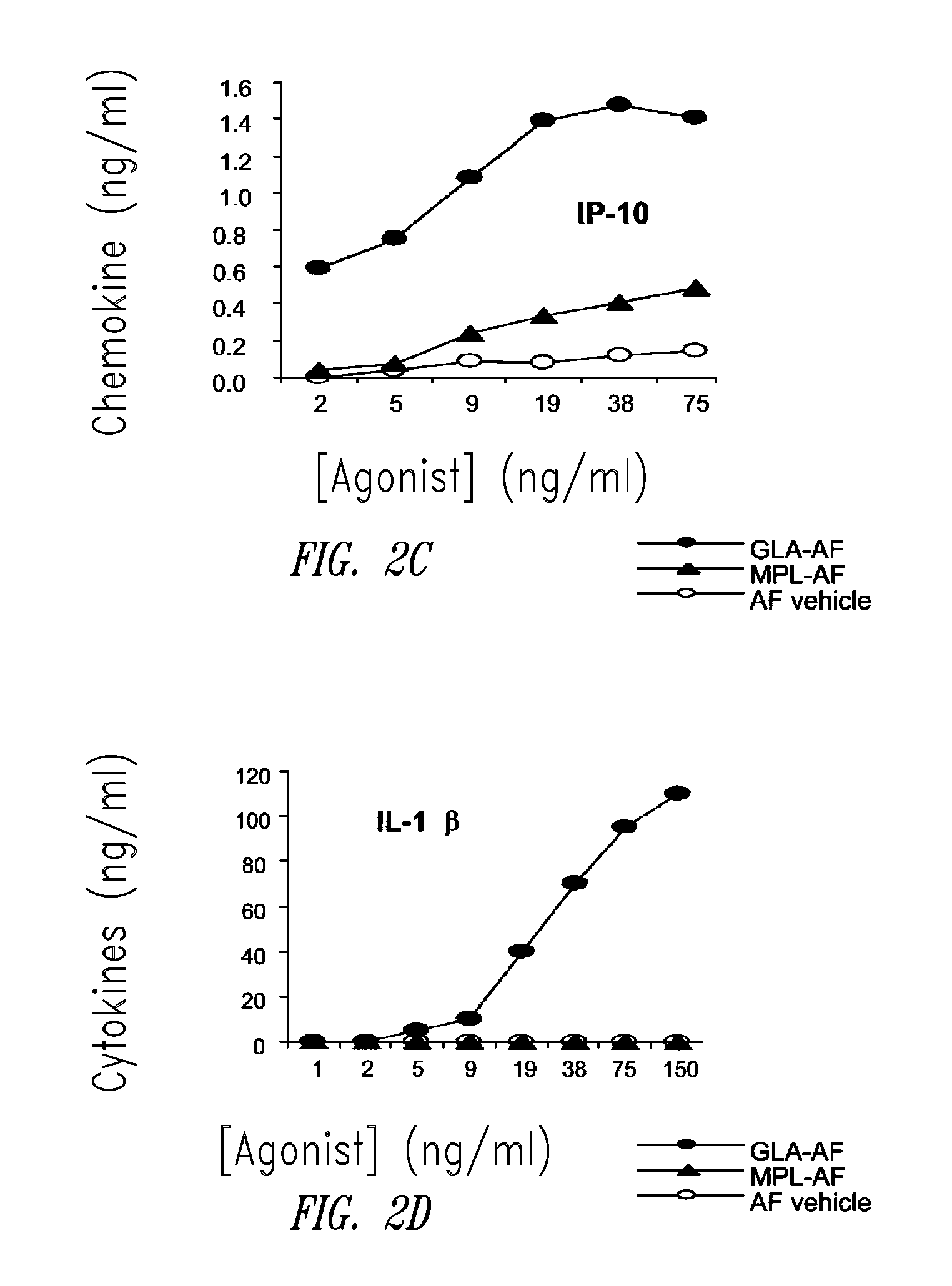
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Formyl methionyl peptide vaccine adjuvant
InactiveUS6017537AConvenient easy to formulateStimulate immune responseBiocideNanotechPeptide vaccinePeptide
The present invention relates to immunological adjuvants comprised of the N-formyl methionyl peptide fMLP. FMLP, when used as an adjuvant in accordance with the present invention, provides for an immune response to suboptimal doses of recombinant antigens.
Owner:VIROGENETICS
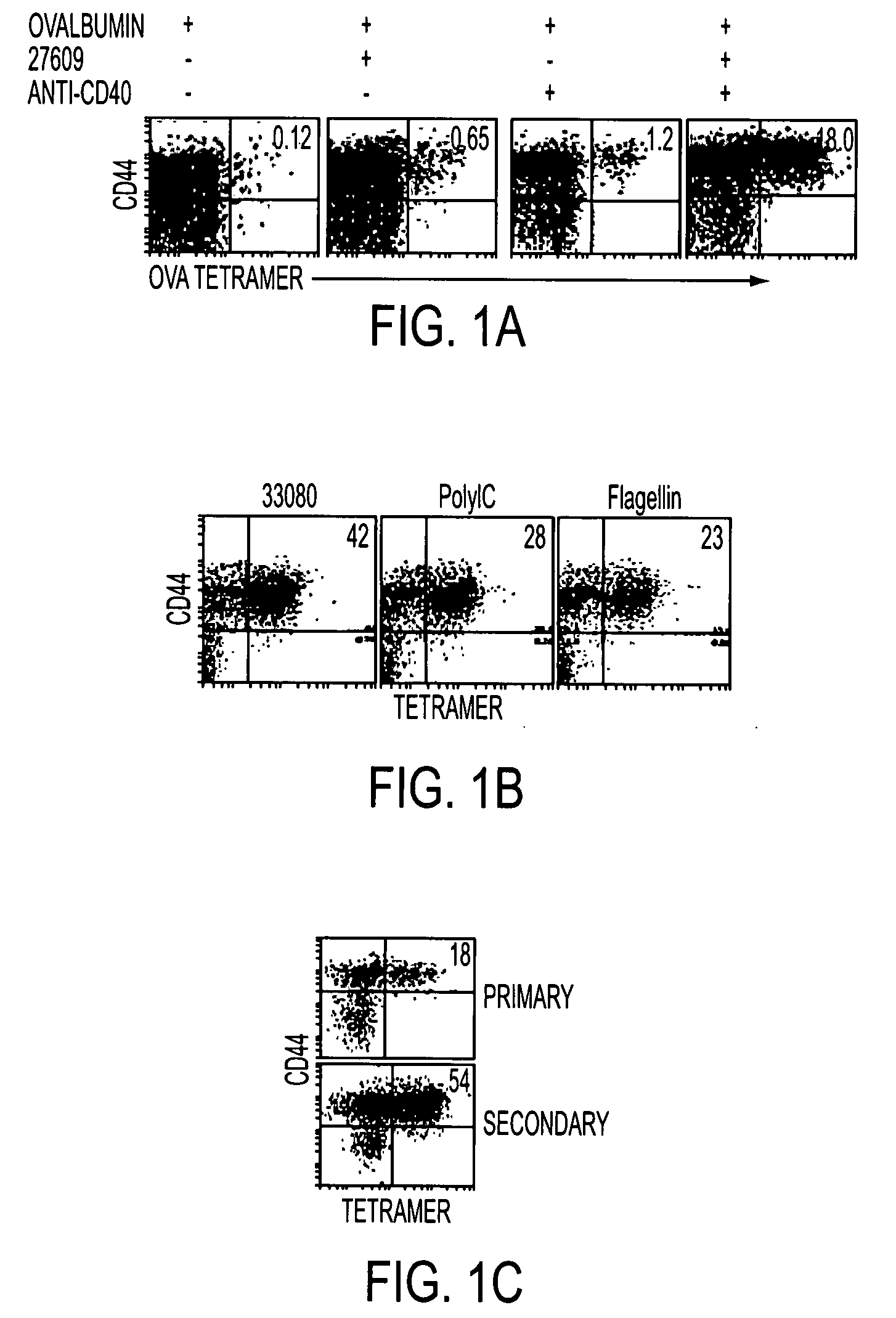
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
InactiveUS20080241139A1Enhanced T cell responseImprove responseAntibacterial agentsAntimycoticsDiseaseYeast
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
Tlr agonist (flagellin)/cd40 agonist/antigen protein and DNA conjugates and use thereof for inducing synergistic enhancement in immunity
InactiveUS20090004194A1Improve immunityEnhance cellular immune responseAntibacterial agentsFungiDiseaseTlr agonists
Owner:UNIV OF COLORADO THE REGENTS OF
Recombinant multiple domain fusion protein mitogens and use thereof for inducing enhancement or repression of antigen-specific immunity.
ActiveUS20100303811A1Increase heightVirusesPeptide/protein ingredientsIMMUNE SUPPRESSANTSAutoimmune responses
The invention relates to cell stimulatory fusion proteins and DNA sequences, vectors comprising at least two agonists of TNF / TNFR super family, immunoglobulin super family, cytokine family proteins and optional antigen combination. Instructions for use of these proteins and DNA constructs as immune adjuvants and vaccines for treatment of various chronic diseases such as viral infection are also provided. Additionally, the use of these protein and DNA constructs as immune suppressant for treatment of various chronic diseases, such as autoimmunity and organ transplant rejection, is also illustrated.
Owner:OCHI ATSUO
Vaccine composition containing synthetic adjuvant
Owner:ACCESS TO ADVANCED HEALTH INST
GM1 binding deficient exotoxins for use as immunoadjuvants
InactiveUS20060002960A1Low toxicityRetaining adjuvant activityBacterial antigen ingredientsPharmaceutical delivery mechanismBacterial exotoxinGanglioside binding
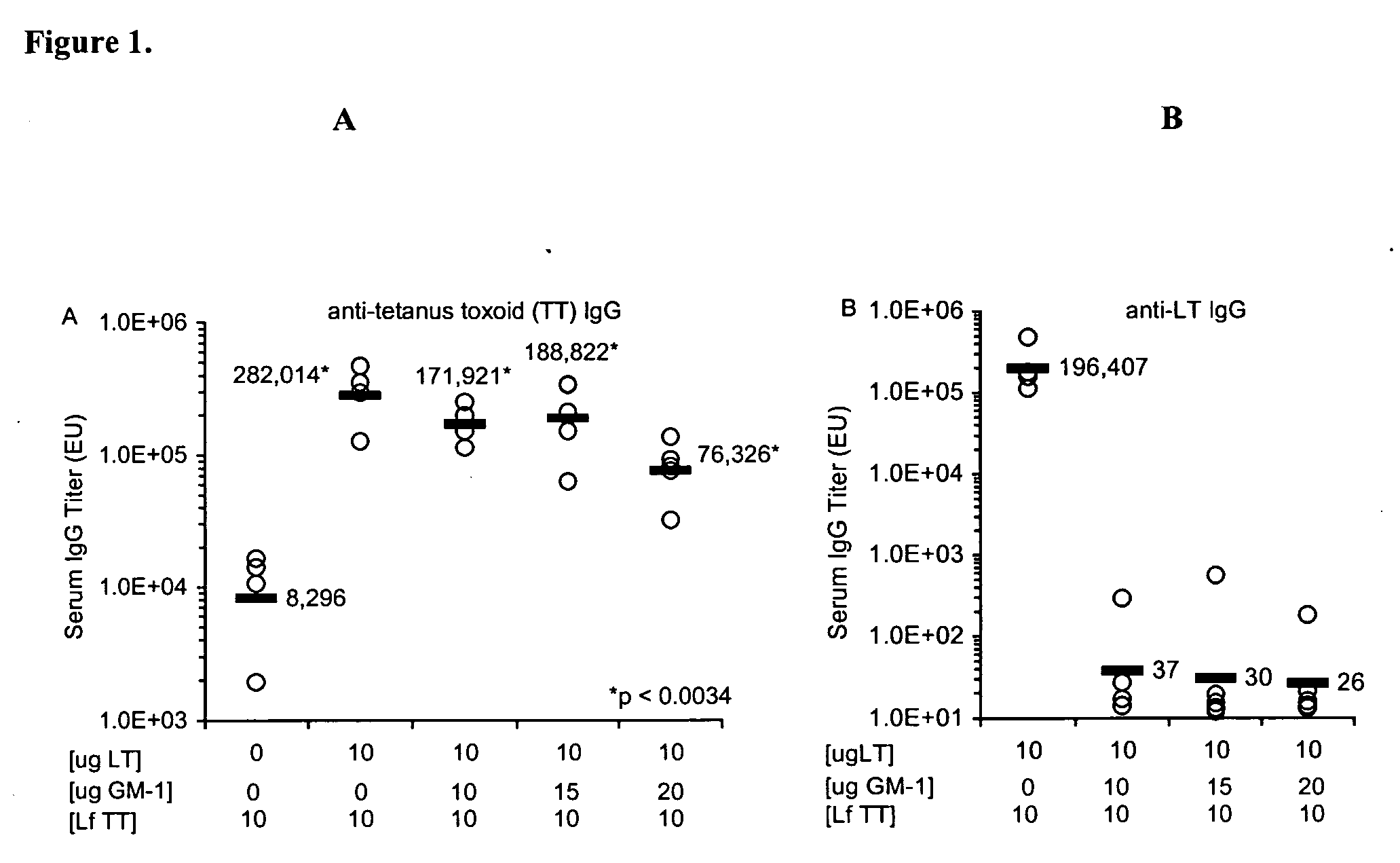
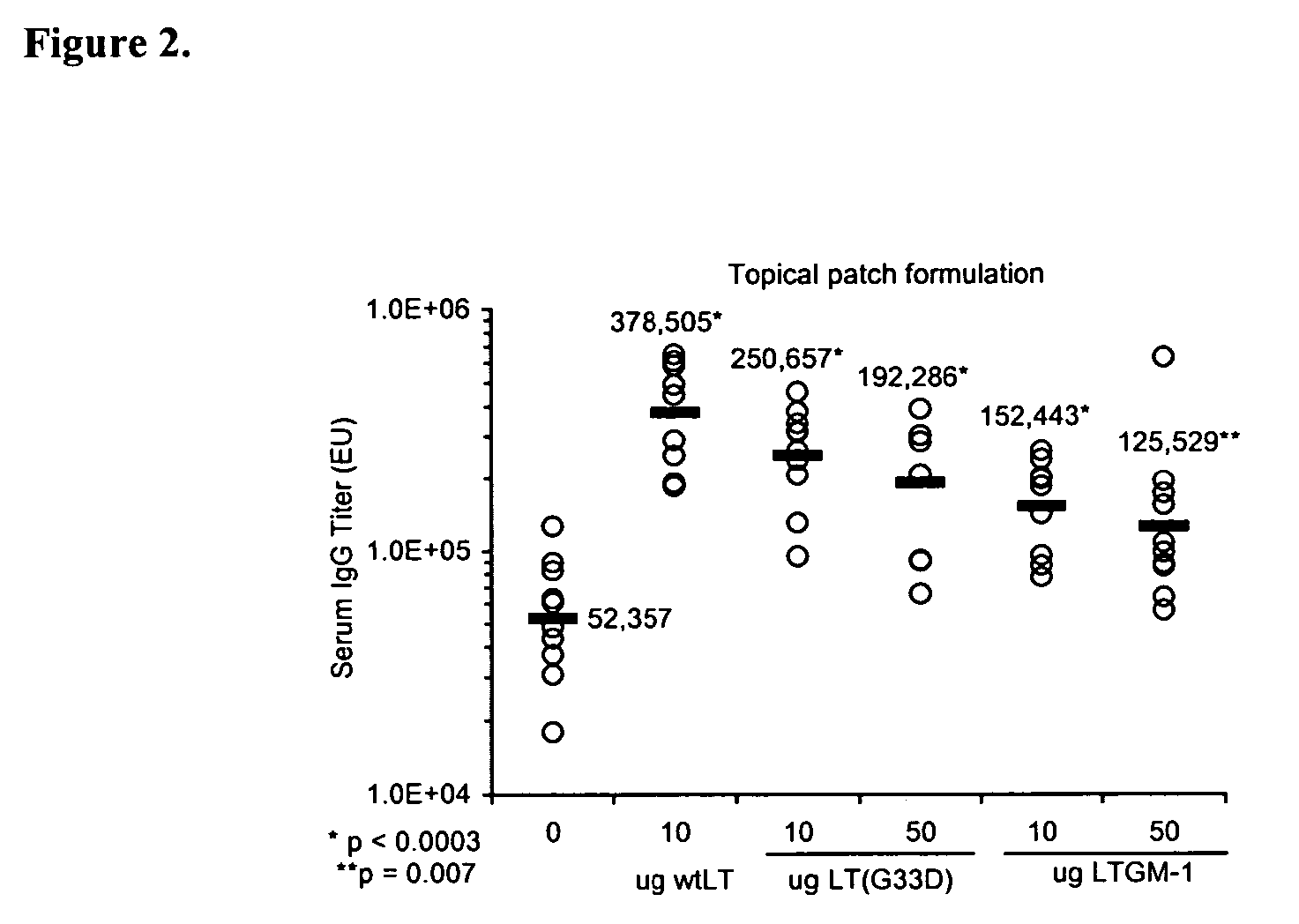
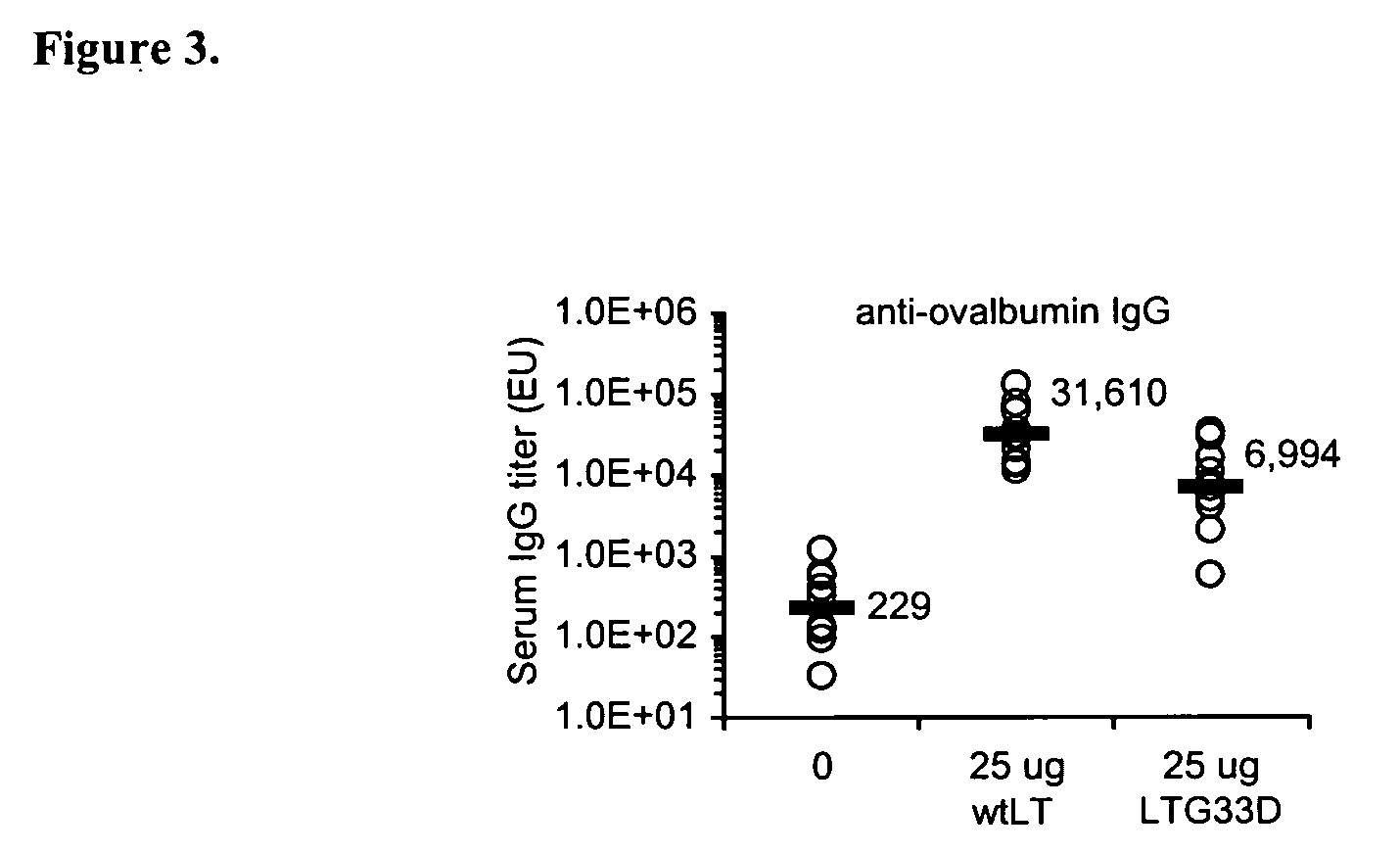
Addition of a bacterial ADP-ribosylating exotoxin (bARE) to a formulation (e.g., immunogen or vaccine) or a system (e.g., patch or kit) for immunization enhances the immune response in a subject to one or more components of the formulation. Binding of the B subunit of a bARE to ganglioside GM1 of the subject in vivo, however, mediates toxicity and limits the use of native bARE as adjuvants. Mutation or in vitro coupling of the B subunit to ligands such as GM1 inhibits binding to GM1 in vivo, thereby eliminating toxicity but retaining desired adjuvant activity. The use of such detoxified, GM-1 binding deficient exotoxins provides a safe and potent new strategy for development of effective formulation for immunization.
Owner:INTERCELL USA
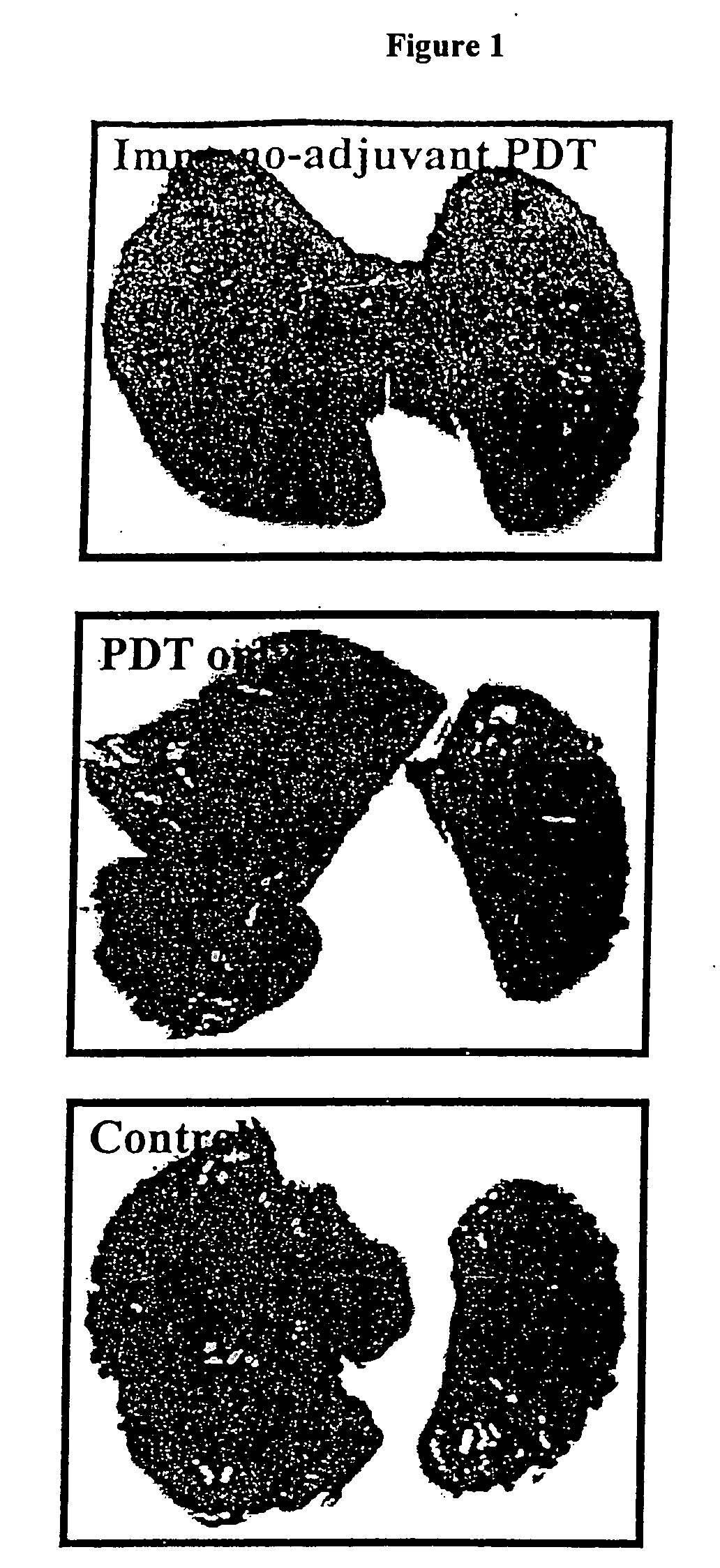
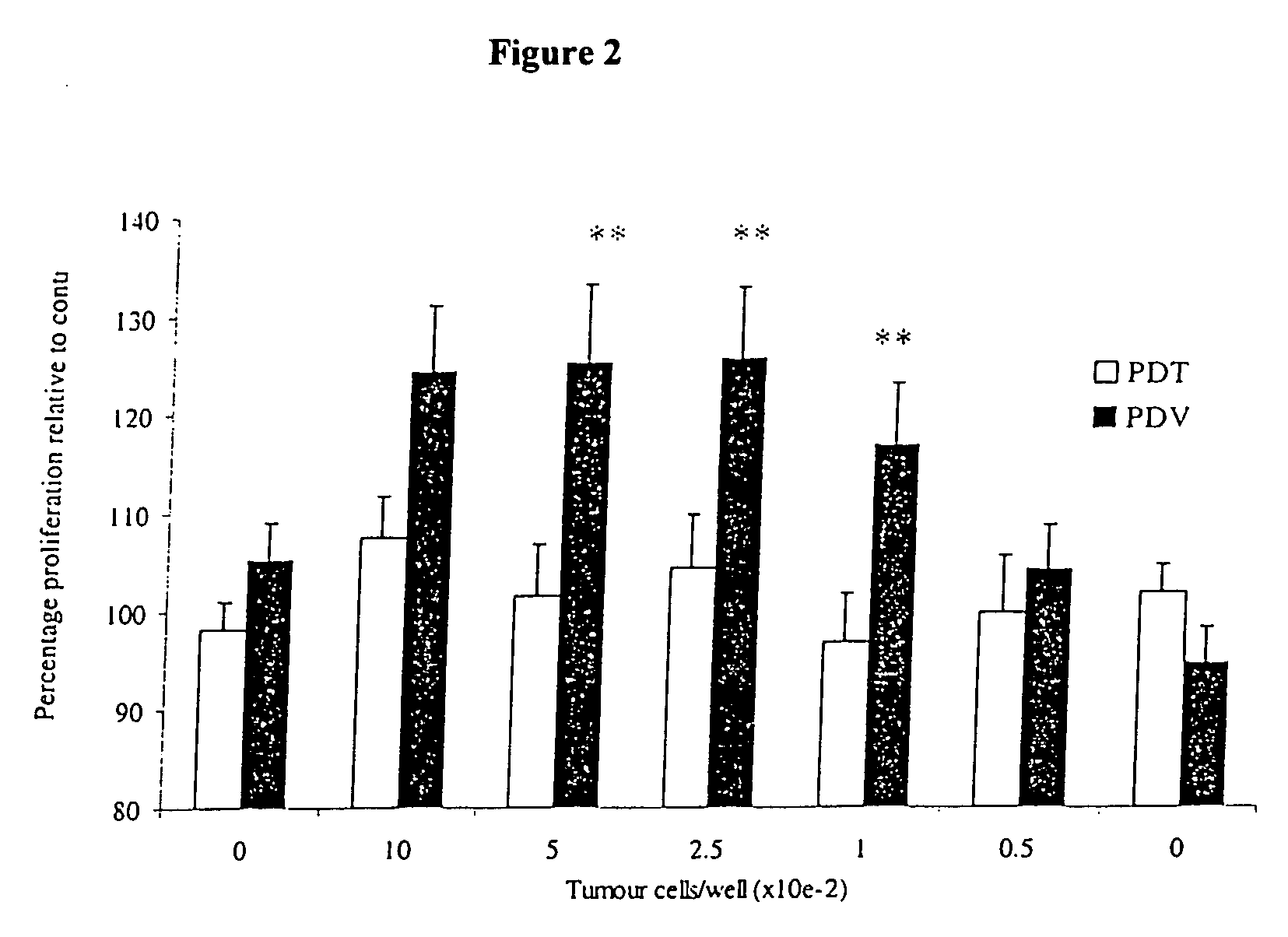
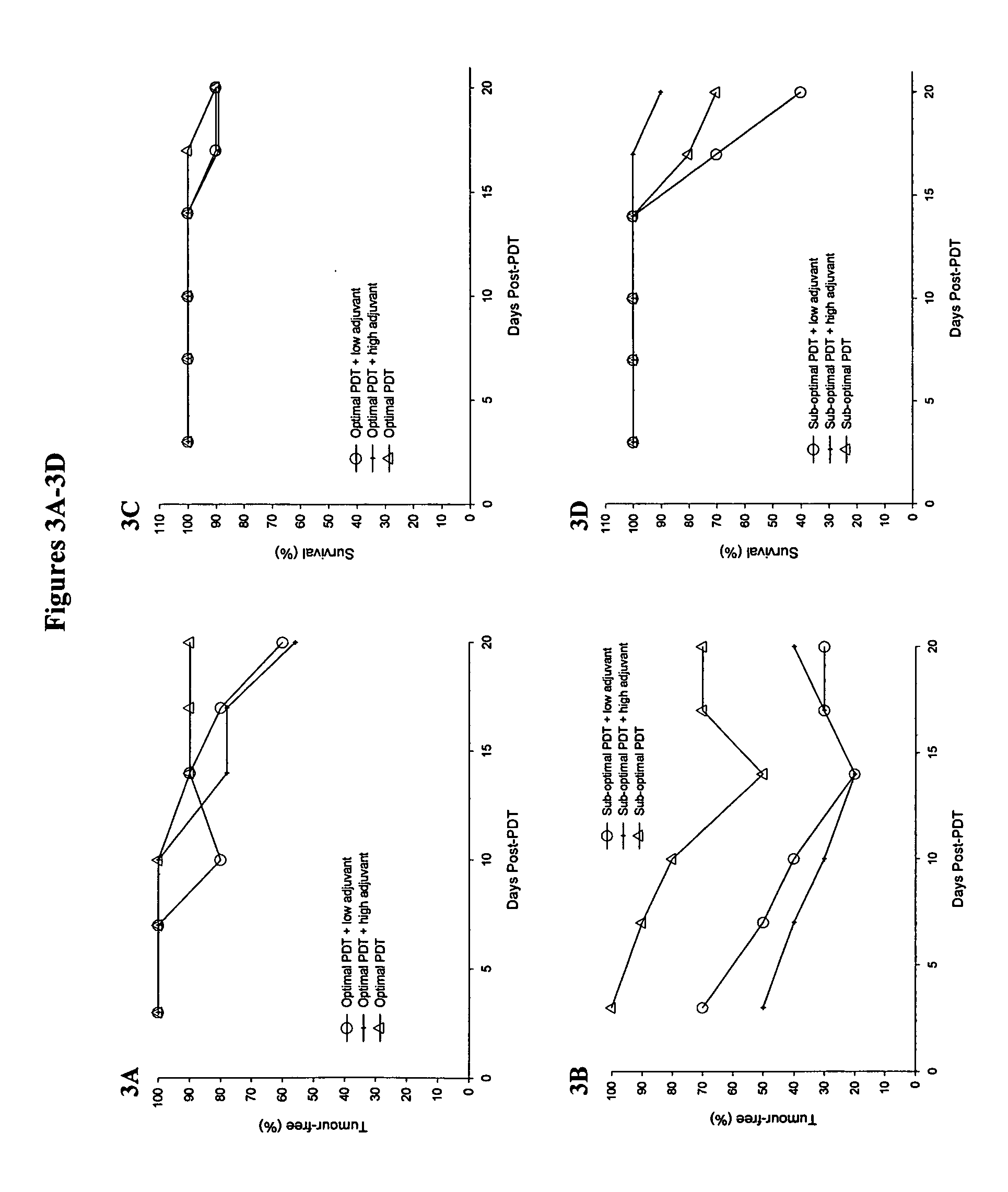
Immuno-adjuvant PDT treatment of metastatic tumors
Immuno-adjuvant photodynamic therapy to treat and prevent metastatic cancer is effected using photosensitizers in combination with immuno-adjuvants to destroy metastatic tumor cells.
Owner:QLT INC +1
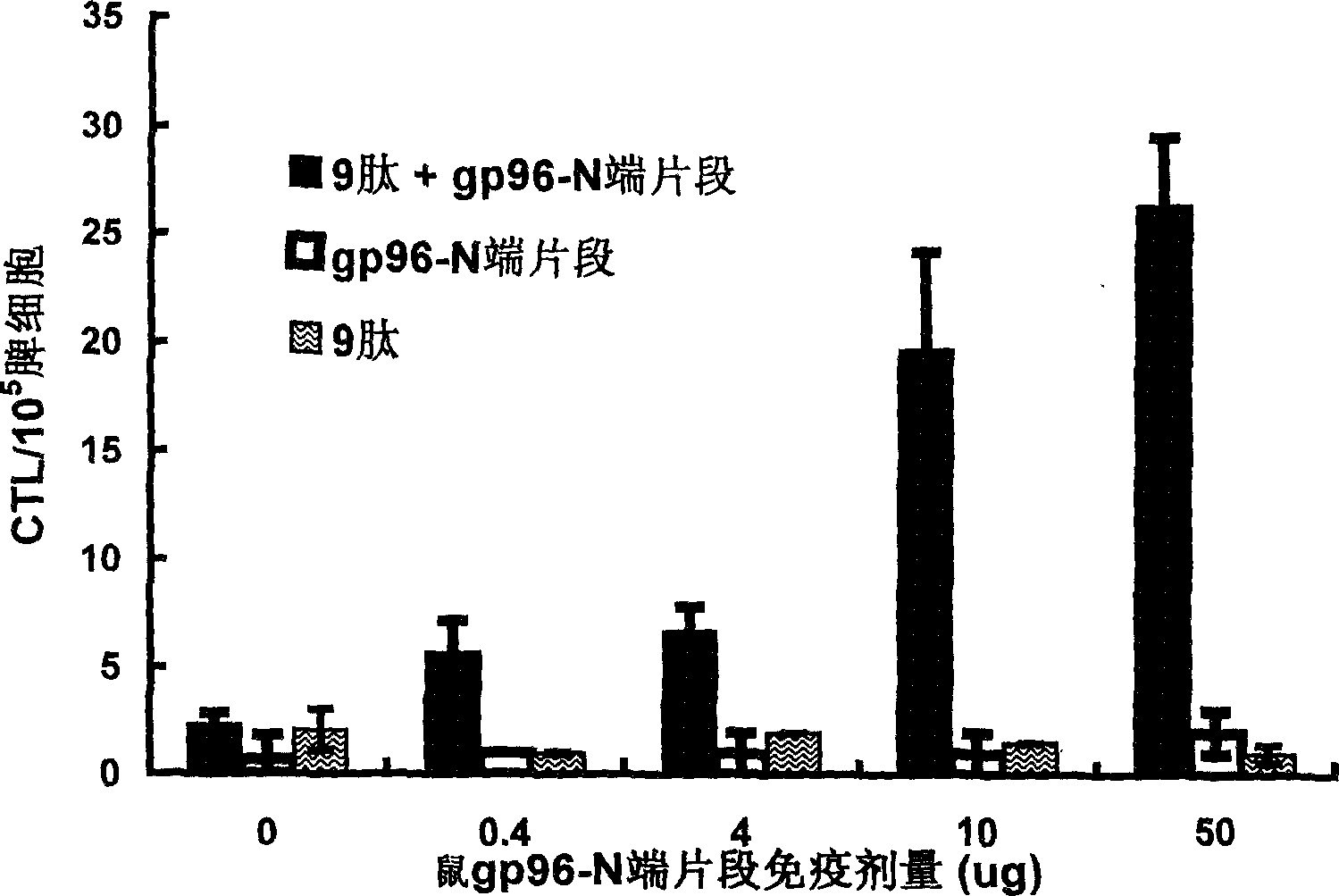
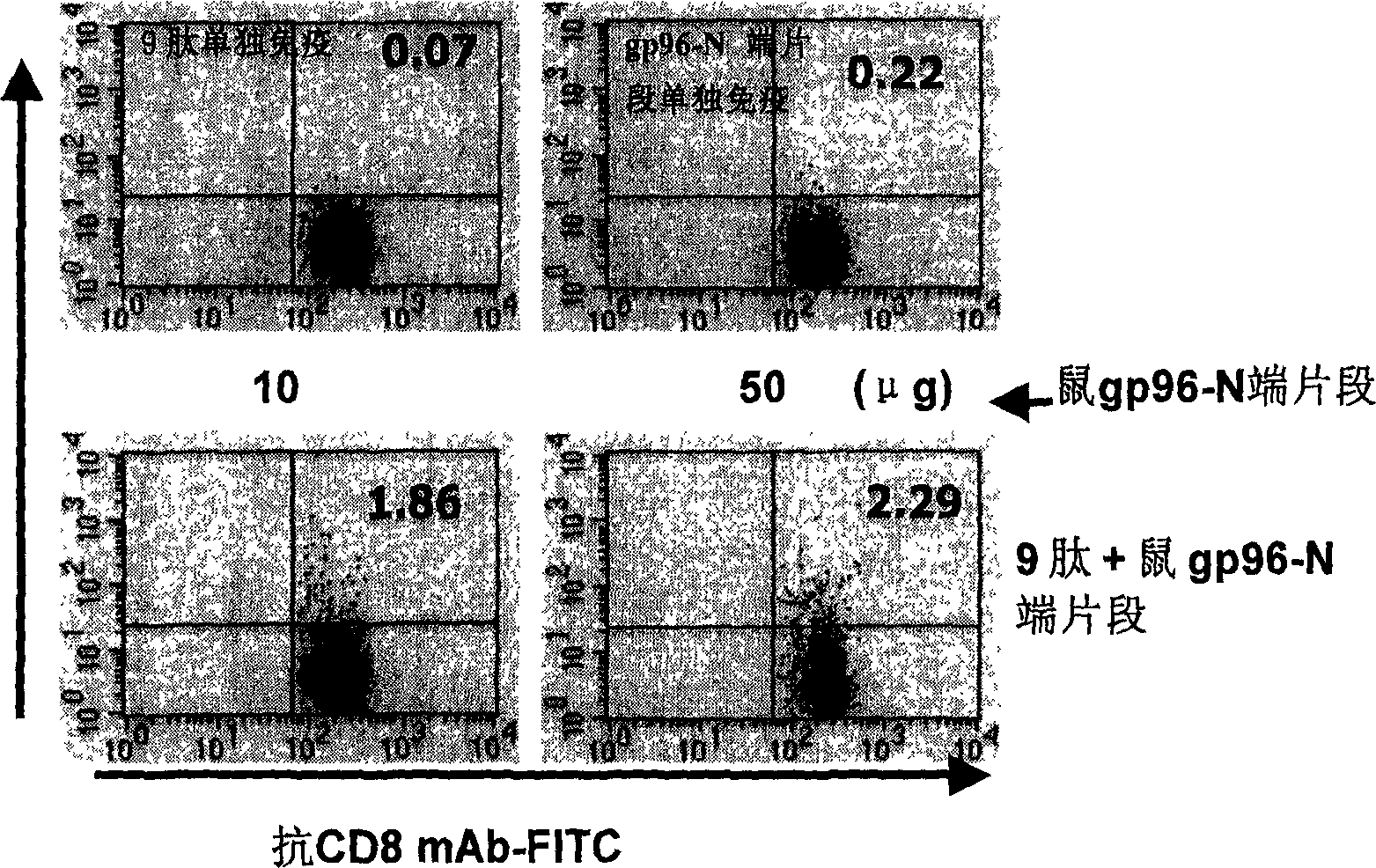
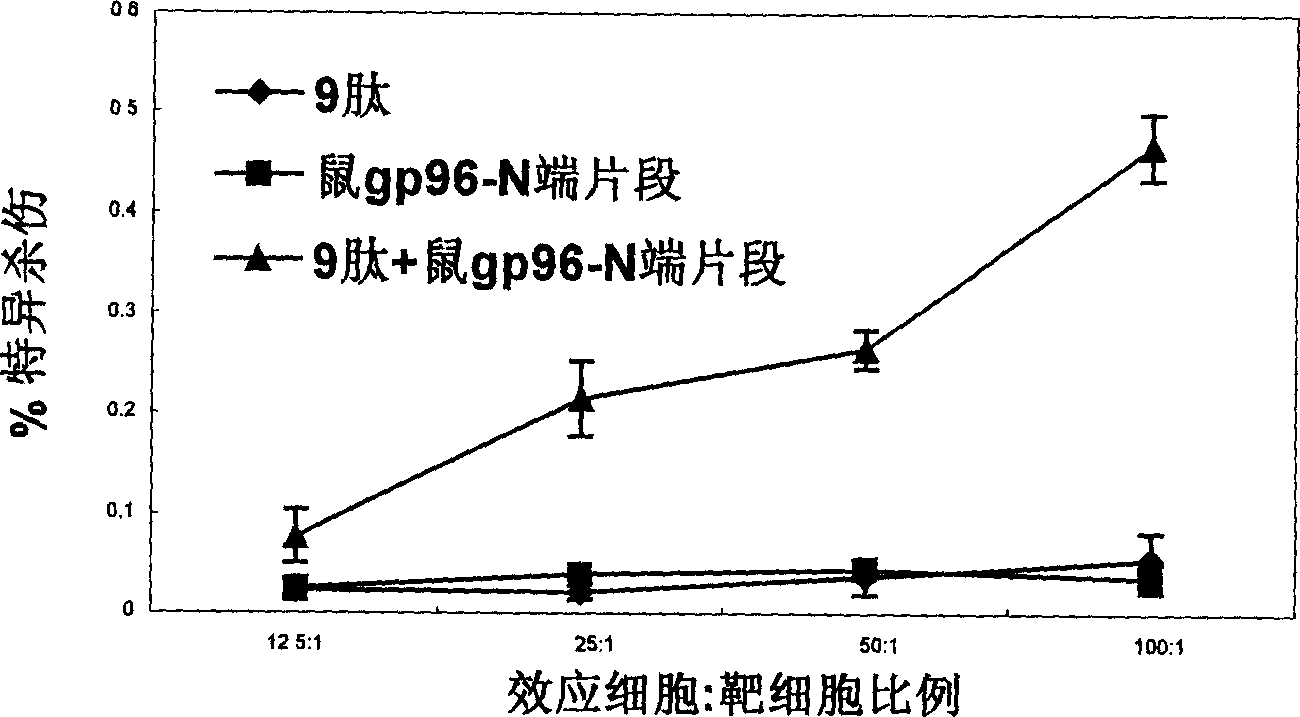
Immunological adjuvant, and its application in preparing vaccine and medicine for anti-virus
InactiveCN1718243AImprove immune activityReach clearAntiviralsAntibody medical ingredientsAnti virusDisease
An immunoadjuvant used to prepare the antiviral vaccine or medicine for increasing the immune activity of the antigens for HBV, HCV, SARS coronavirus, fowl influenza virus, etc is a kind of human or animal's novel heat shock proteins gp96, hsp108 and hsp70.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
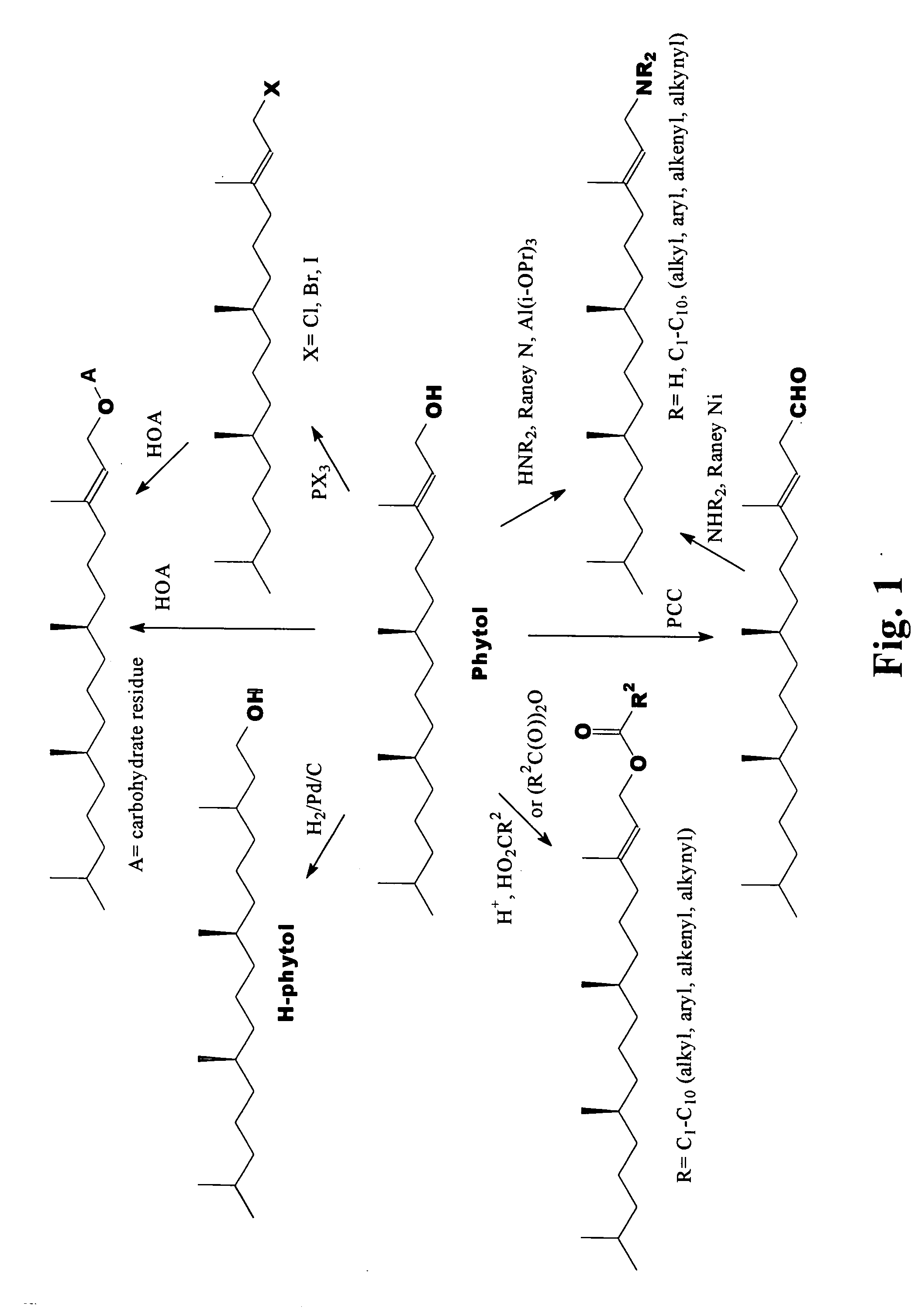
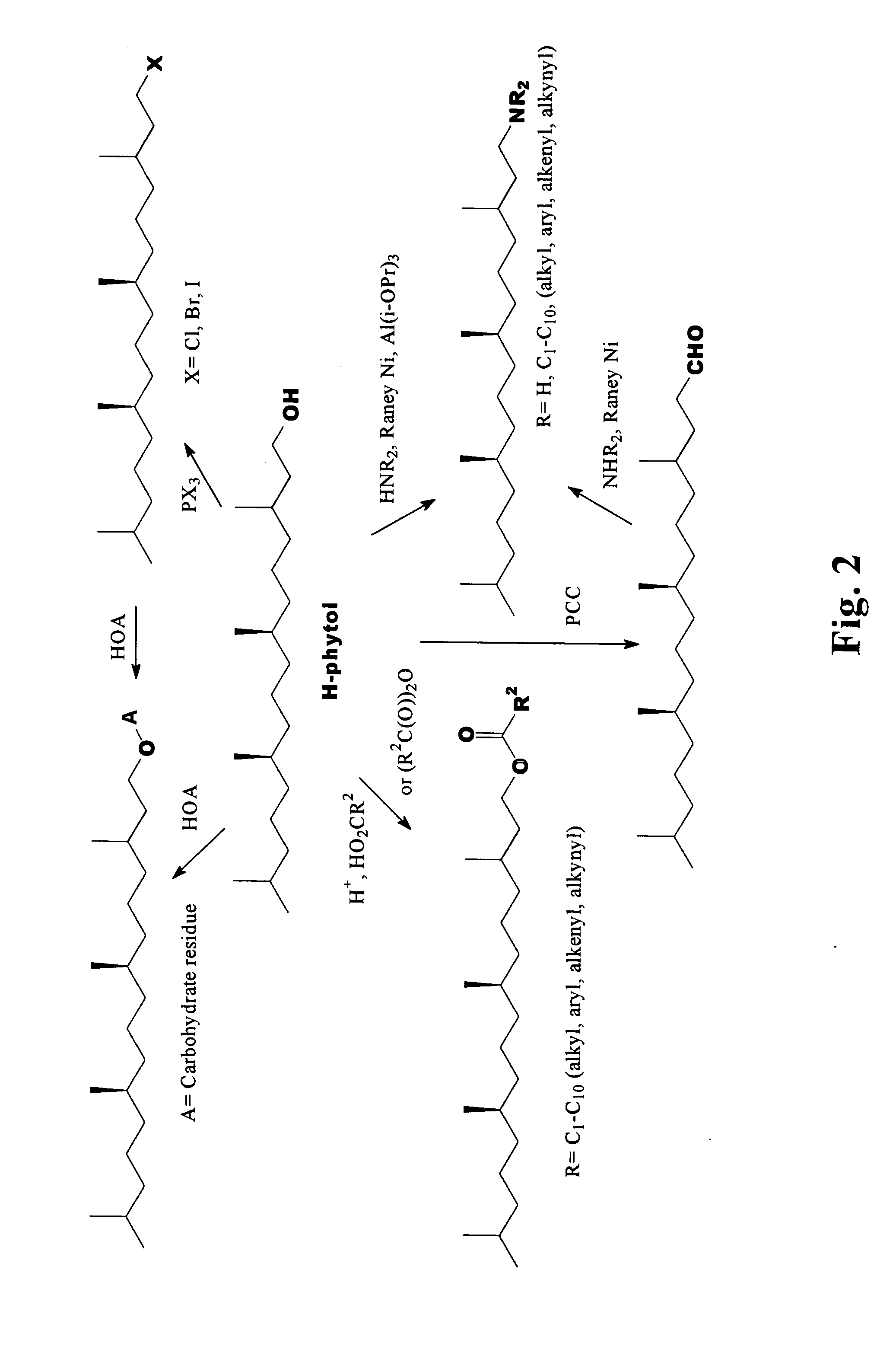
Novel phytol derived immunoadjuvants and their use in vaccine formulations
InactiveUS20050158329A1Improving immunogenicityInduce immunogenic responseBiocideHydroxy compound active ingredientsSide effectParticulate antigen
This invention relates to a novel immunoadjuvant, an adjuvant component, and vaccines containing the adjuvant component. The adjuvant includes phytol or a phytol derivative. The adjuvant component, when combined with a soluble or particulate antigen, provides a vaccine with an enhanced ability to induce both humoral and cytotoxic immune responses while displaying reduced toxicity and / or adverse side effects over vaccines that include the antigen but without the benefit of this adjuvant component.
Owner:GHOSH SWAPAN K
Immuno-adjuvant PDT treatment of metastatic tumors
InactiveUS20050187207A1Prevent and inhibit developmentAvoid spreadingBiocidePhotodynamic therapyPhotodynamic therapyPhotosensitizer
Immuno-adjuvant photodynamic therapy to treat and prevent metastatic cancer is effected using photosensitizers in combination with immuno-adjuvants to destroy metastatic tumor cells.
Owner:VALEANT PHARMA INT +1
Virus preparation or vaccine production method by culturing cells with polyester fiber carrier
InactiveCN102406926AHigh culture specific surface areaReduce the chance of infectionAnimal cellsViral antigen ingredientsPolyesterCulture cell
The invention relates to a virus preparation or vaccine production method by culturing cells with a polyester fiber carrier. The method particularly comprises the following steps: 1) preparing a polyester fiber cell culturing carrier by adopting a processing method; 2) in a cell culturing system, culturing proliferation cells in a cell proliferation culture solution with the treated polyester fiber cell culturing carrier; 3) changing a cell maintaining culture solution after the cells grow to a certain density or number, and inoculating virus to infect cells; 4) amplifying virus; 5) harvesting virus fluid; 6) inactivating or implementing living vaccine treatment according to the required preparation type; 7) concentrating, purifying and cracking the virus fluid; 8) adding a stabilizing agent and an immunoadjuvant; and 9) packaging vaccine. According to the method, a material which has low price and is suitable for wall attaching growth of the cells is used to make the cell culturing carrier, carry out cell culture and prepare virus or produce vaccine, so that the cost of the cell carrier is greatly reduced.
Owner:上海泰因生物技术有限公司
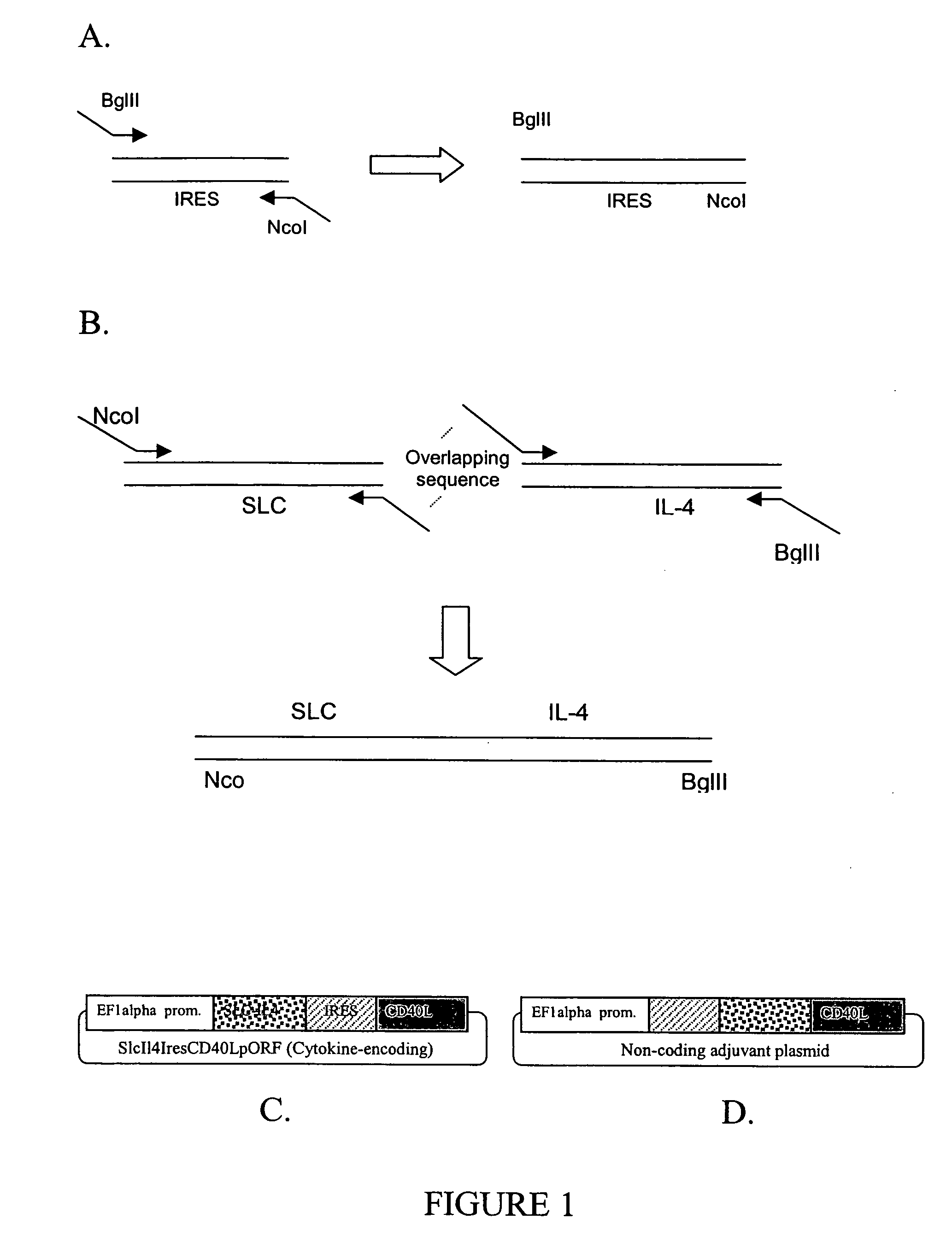
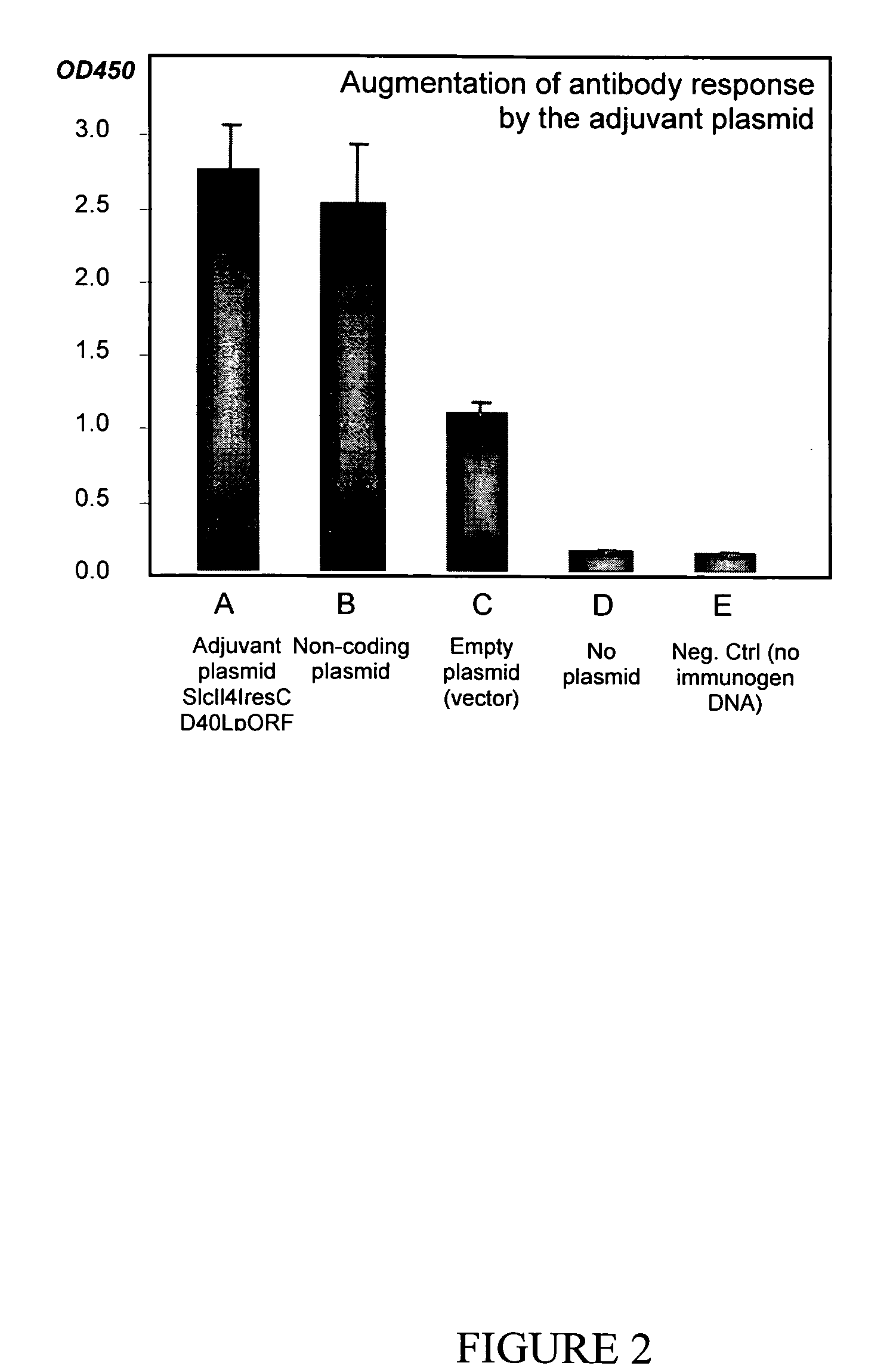
Bacterial plasmid with immunological adjuvant function and uses thereof
InactiveUS20050287118A1Easy to produceBiocideGenetic material ingredientsEukaryotic plasmidsCytokine
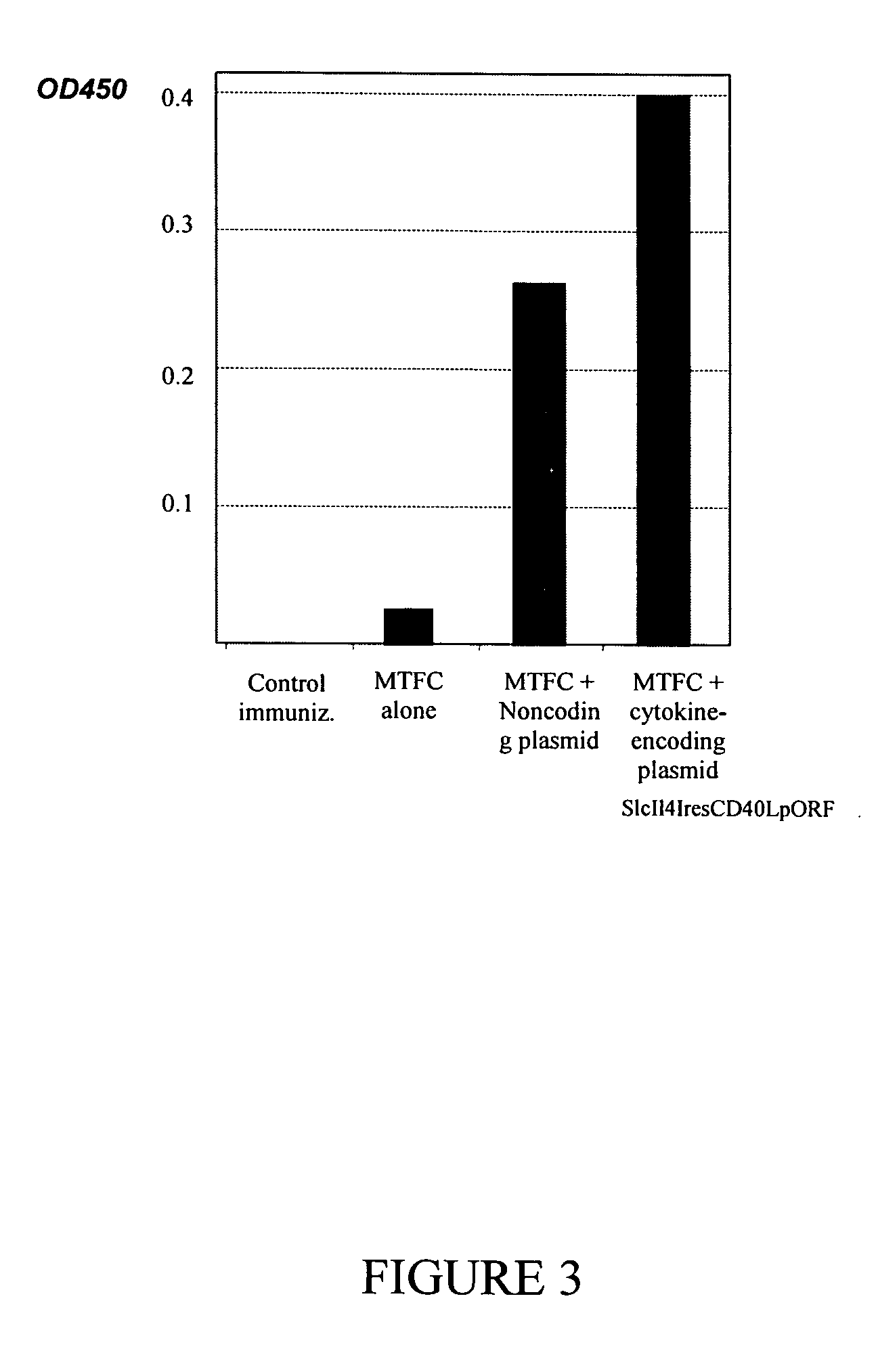
Plasmid adjuvant compositions and methods for enhancing an immune response to a coadministered immunogen are described. The plasmid adjuvants include a combination of cytokines and chemokines designed to elicit an enhanced immune response. Particular combinations can be provided to generate a Th1 and / or a Th2 immune response.
Owner:EPITOMICS INC
Immunogen by using mutant CRM197 of diphtheria toxin as carrier, preparation method, and application
ActiveCN101050236AGood effectHigh recovery ratePeptide/protein ingredientsPeptide preparation methodsN-HydroxysuccinimideCarrier protein
This invention relates to a method for preparing immunogen G17CRM197 containing diphtherin mutant CRM197 as carrier. The method comprises: crosslinking diphtherin mutant CRM197 with gastrin G17 through epsiv-maleimidyl caproic acid N-hydroxysuccinimide eater (EMCS). Diphtherin mutant CRM197 is used as a carrier, and has such advantages as high recovery rate and easy purification. The method has such advantages as high product crosslinking rate, short preparation time, and low cost. Immunogen G17CRM197 can be used as an effective component in therapeutic vaccines, or used in anti-tumor drugs with appropriate immunoadjuvants.
Owner:QILU PHARMA HAINAN
Saponin with immunoadjuvant function, preparation method, vaccine preparation containing the saponin as adjuvant and uses thereof
InactiveCN101402666ASimple preparation processSimple methodAntiinfectivesSteroidsDiseaseCell immune response
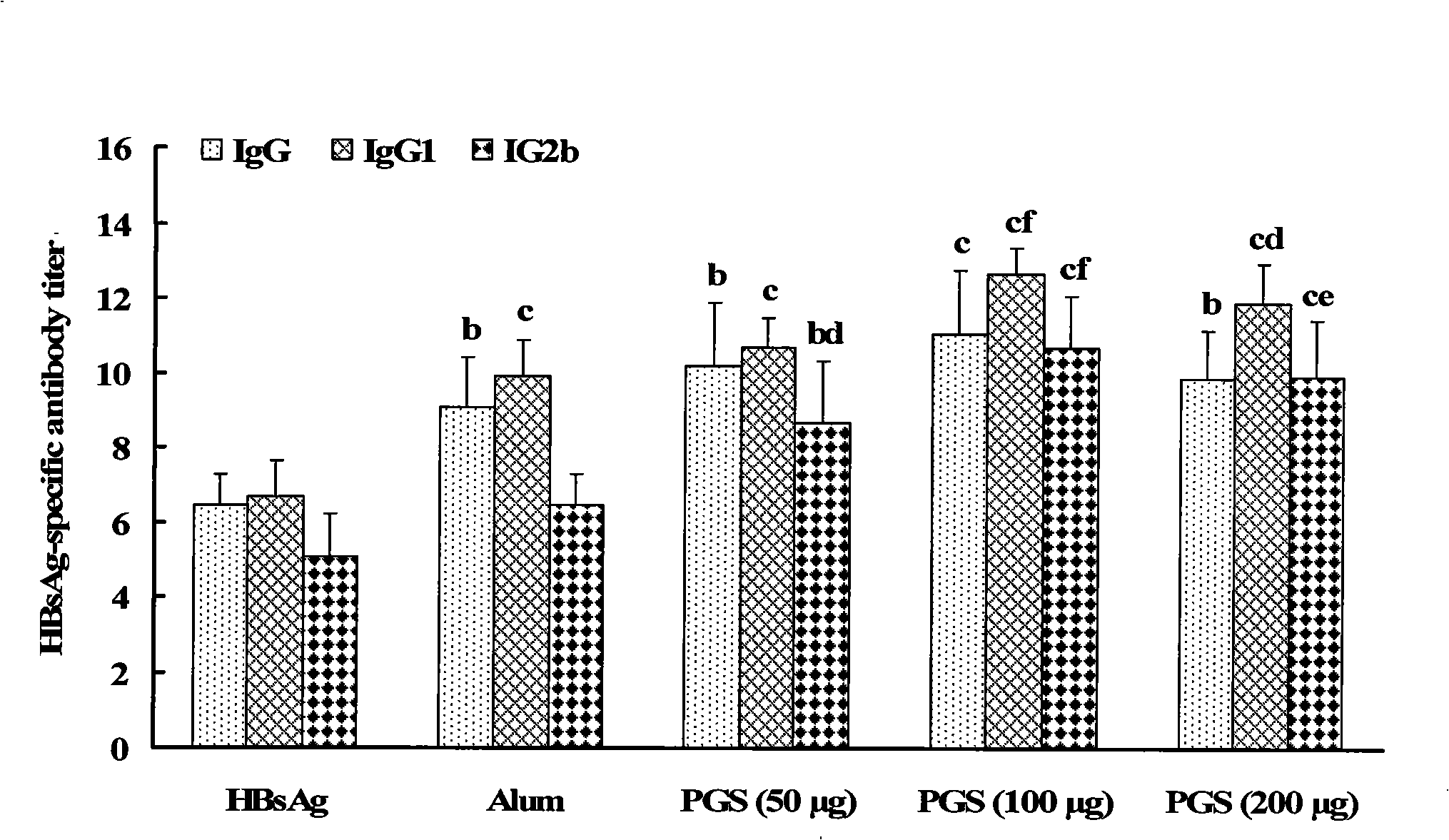
The invention relates to a saponin with immune adjuvant function and a preparation method thereof, a vaccine preparation containing the saponin as an adjuvant, and applications of the saponin and the vaccine preparation in the prevention and treatment of infectious diseases and cancers of human and animals. The saponin is platycodin D, platycodin D2, or a total-saponin containing the two saponin compounds. The platycodin D and platycodin D2 are both extracted and separated from balloonflower, a Chinese medicine. The saponin can induce an organism to generate Th1-type and Th2-type immune responses, show the capability of inducing the organism to generate stronger cell immune response and humoral immune response to a vaccine than the alhydrogel adjuvant known in the prior art, and can be taken as the immune adjuvant for a plurality of vaccines and achieve an ideal immunity effect. The vaccine which takes the saponin as the adjuvant has simple preparation technology and simple and convenient method, and the quality is easy to control and the saponin can be reserved by freezing.
Owner:ZHEJIANG UNIV
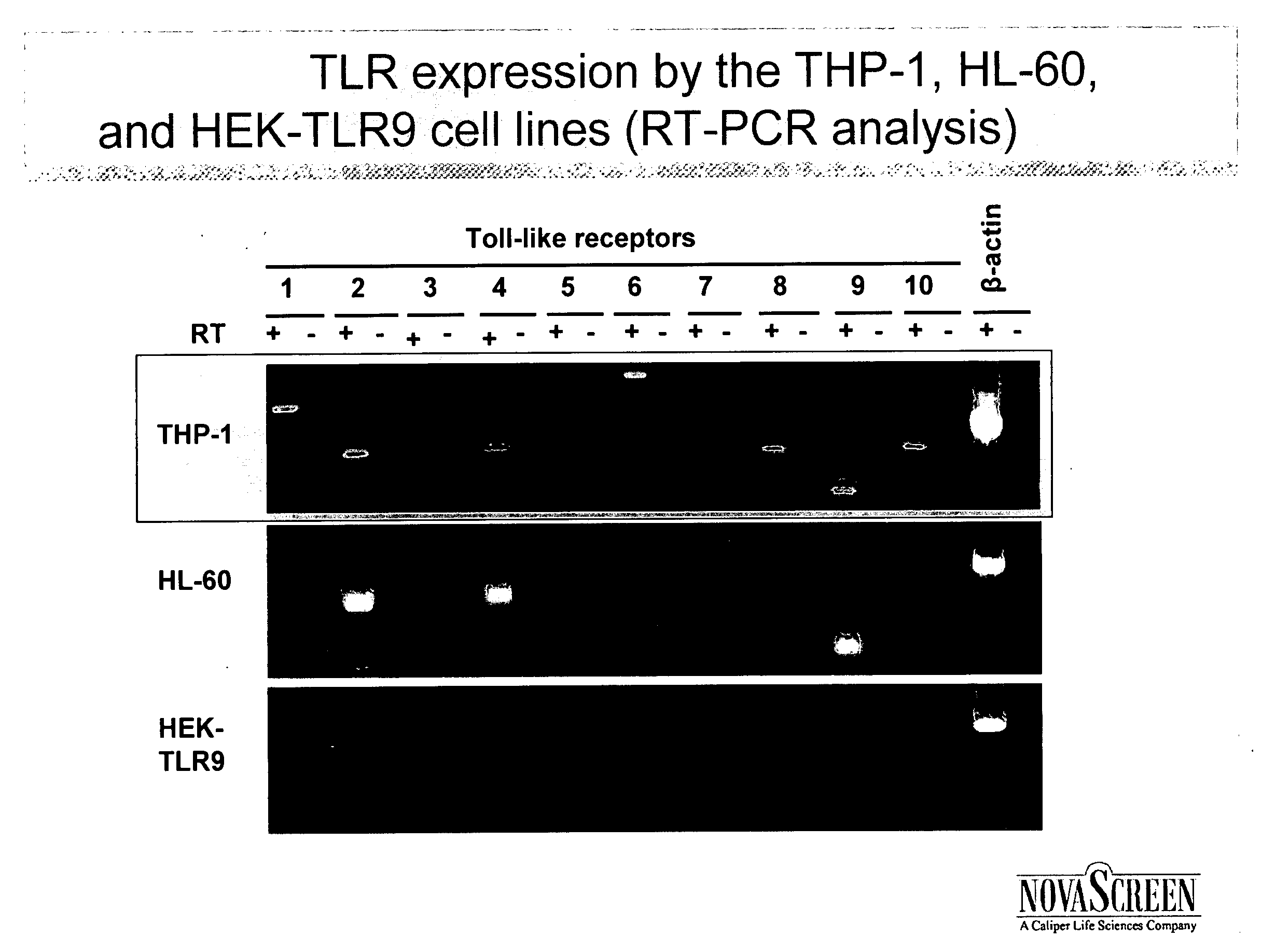
Methods of screening for immuno-adjuvants and vaccines comprising anti-microtubule immuno-adjuvants
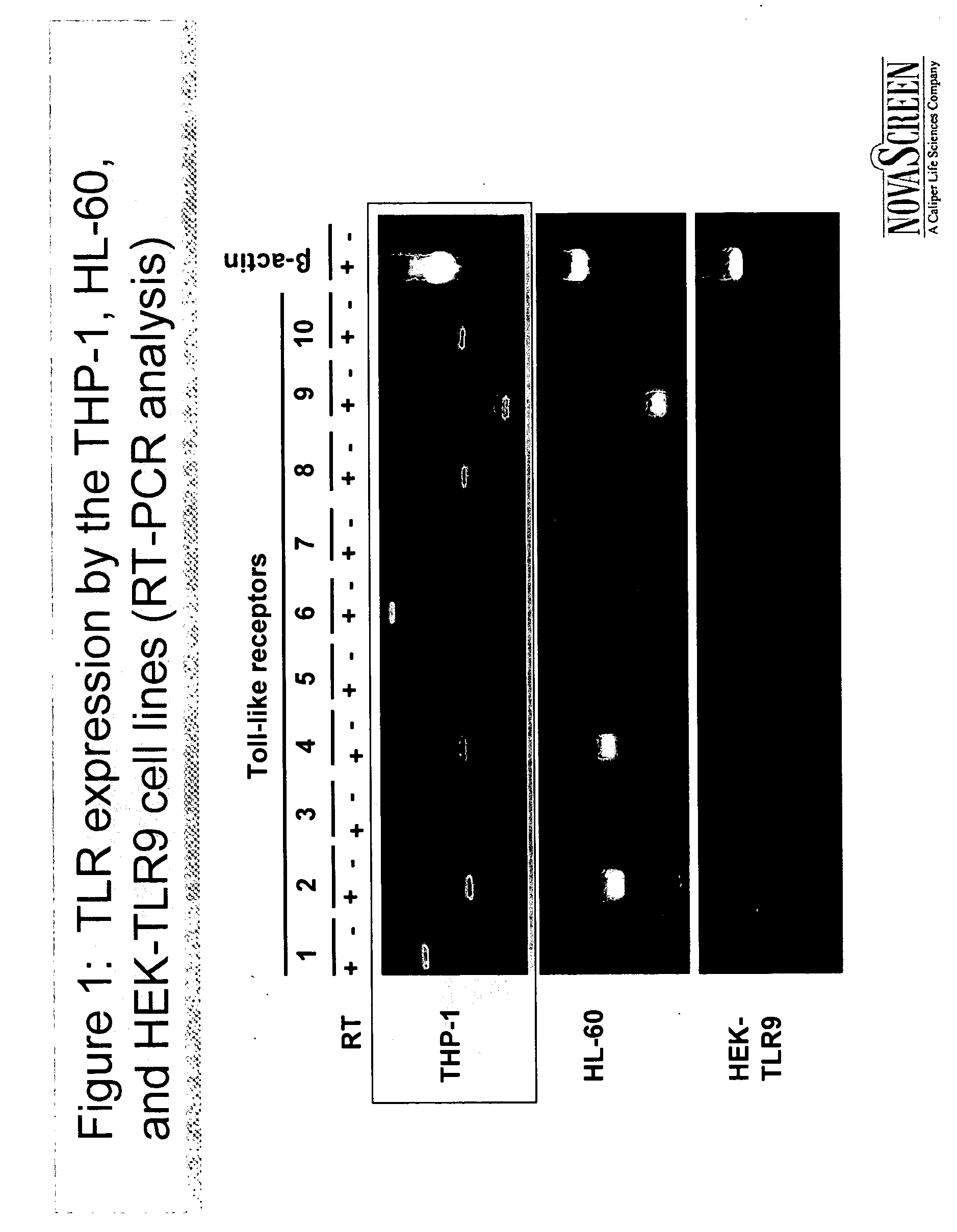
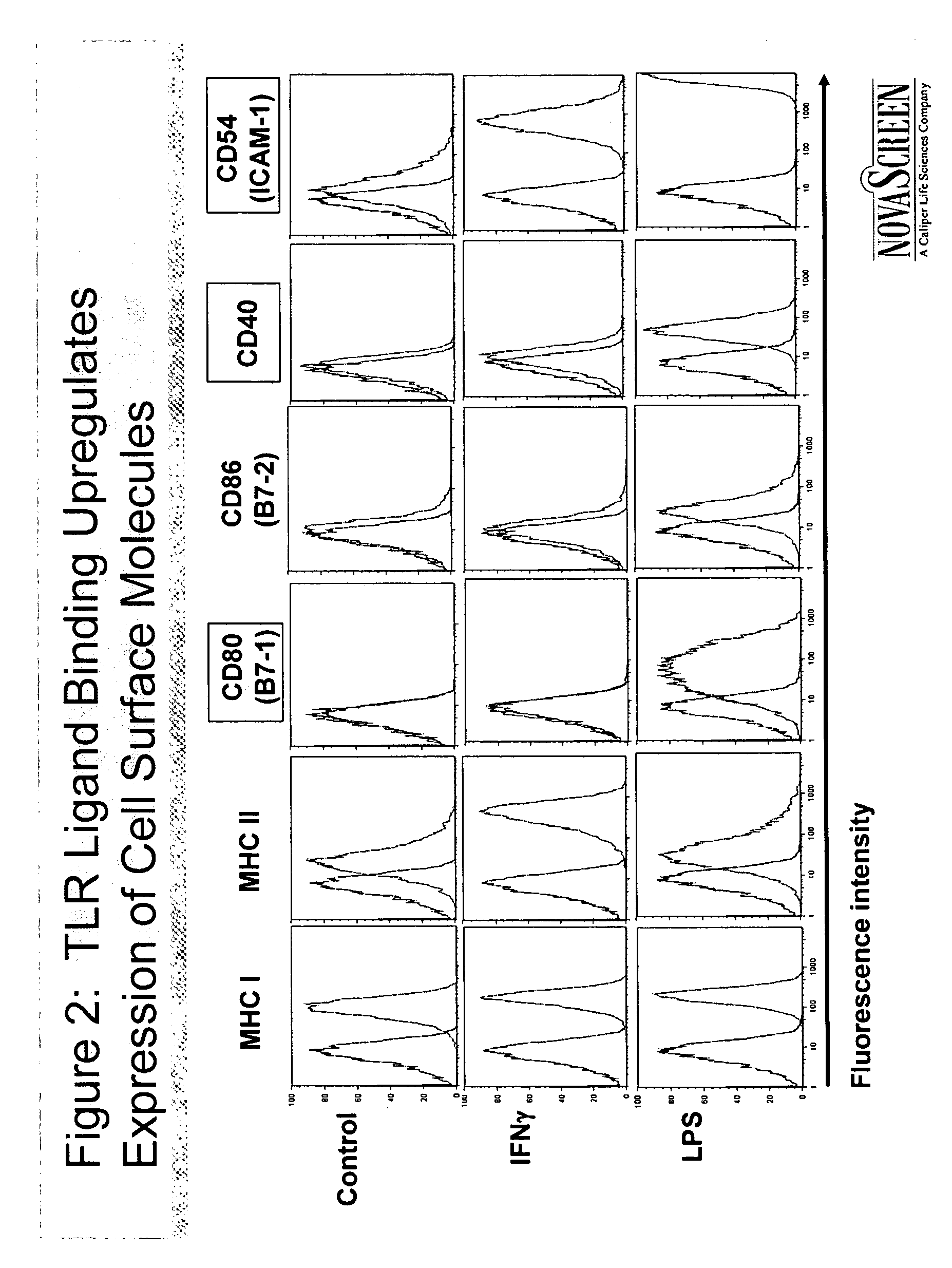
InactiveUS20070059319A1Stimulating innate immune systemEnhancing innate immunityBiocideMicrobiological testing/measurementInnate immune systemToll-like receptor binding
A method of screening for agents that stimulate the innate immune system in mammals employs markers that respond to Toll-like receptor binding. Agents identified in the assay boost both innate and adaptive immune responses, when administered alone or in combination with vaccines.
Owner:NOVASCREEN BIOSCI
Short peptide, application of short peptide as vaccine adjuvant, and vaccine
The invention provides a short peptide, application of the short peptide as a vaccine adjuvant, and a vaccine using the short peptide as the vaccine adjuvant. The short peptide is simple to prepare and can improve immune response of antigen after simple physical mixing with the antigen. The sequence of the short peptide is X-GFFYK, wherein X is a terminating group. Preferably, the short peptide is of D configuration. The short peptide can be used as an immunoadjuvant to improve immunogenicity of antigen and allows a main body to undergo intense antigen-specific cellular immune and humoral immune response; the short peptide is applicable to a variety of antigens; and the short peptide is easy to prepare and has a single and controllable component.
Owner:NANKAI UNIV
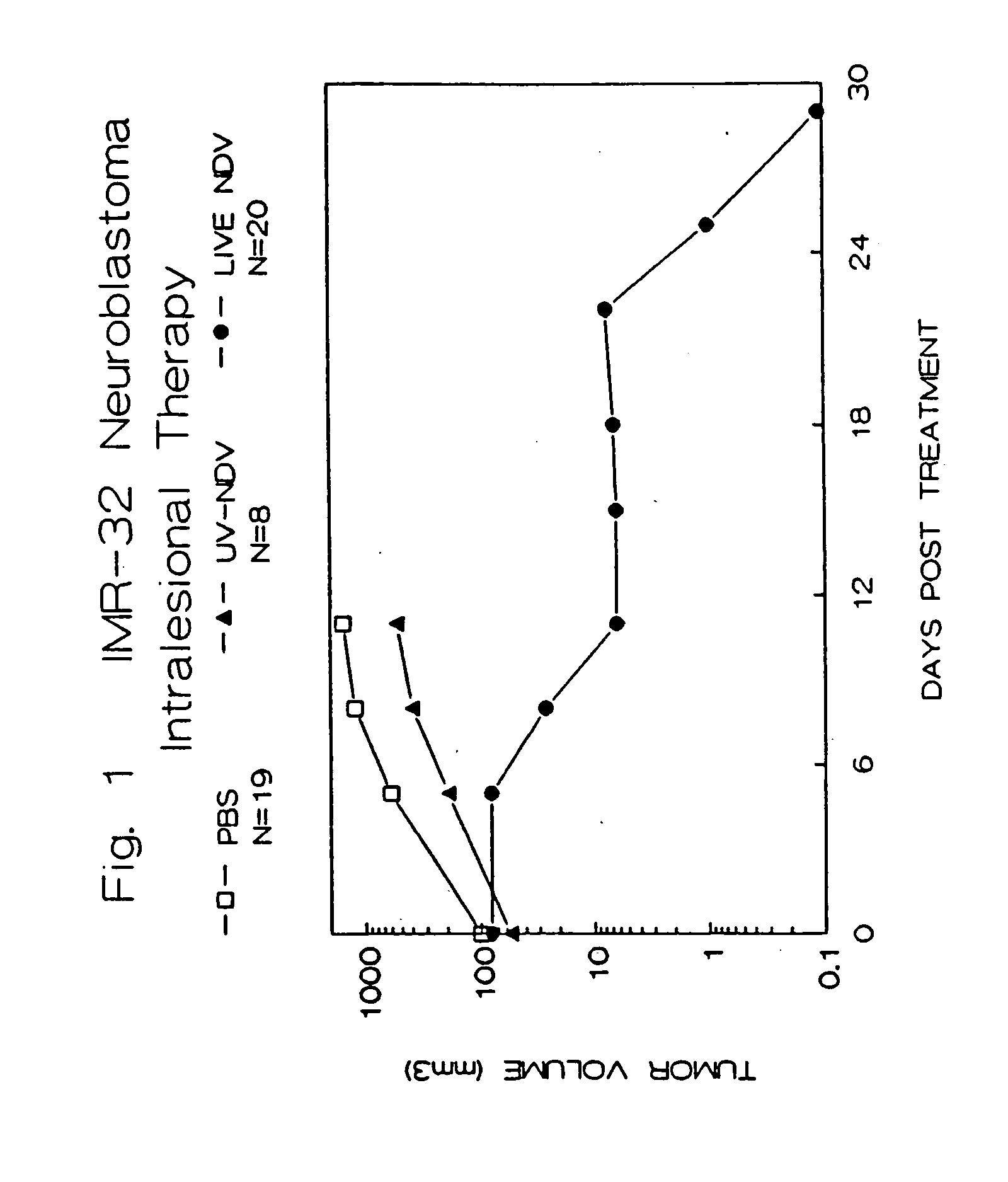
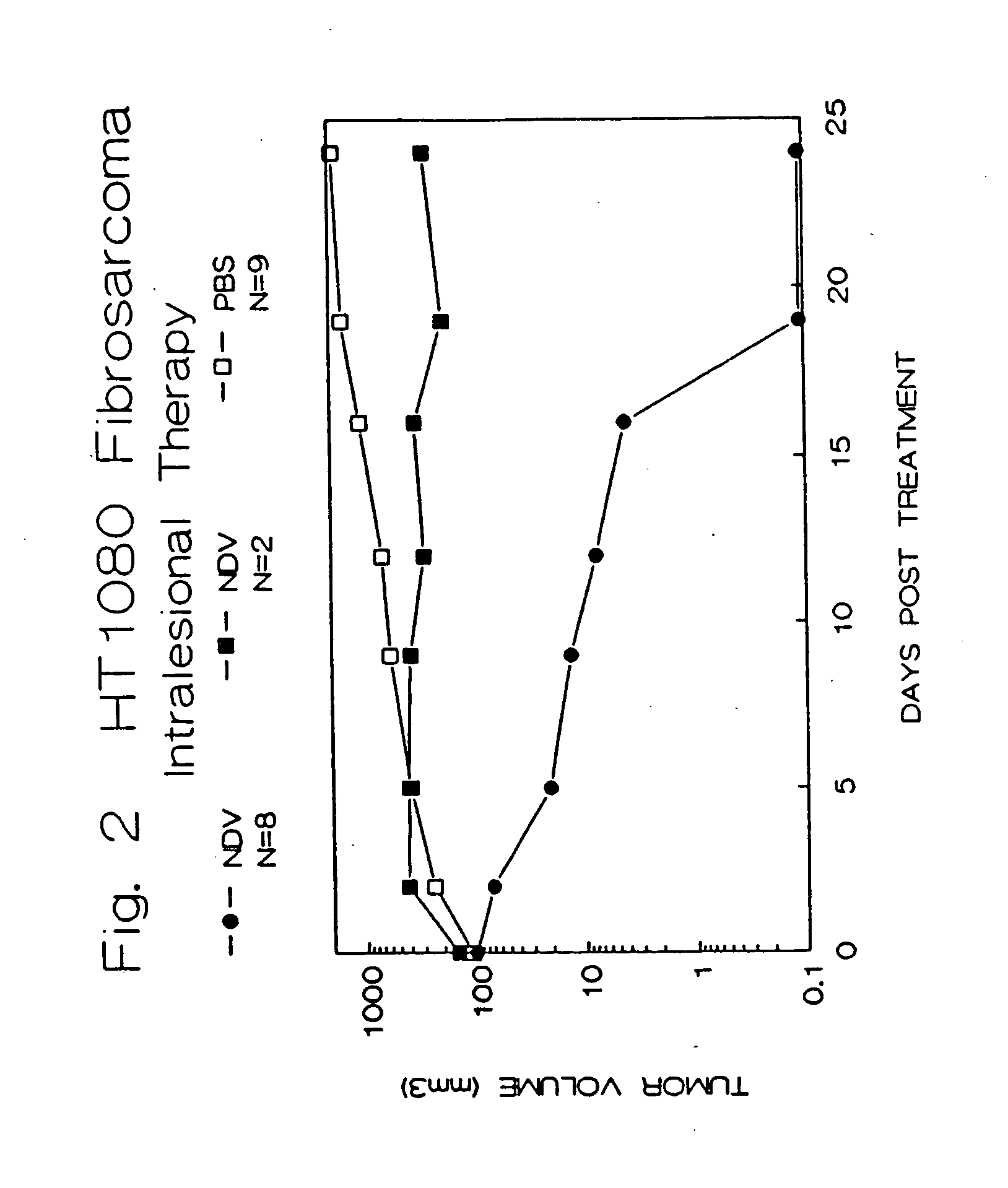
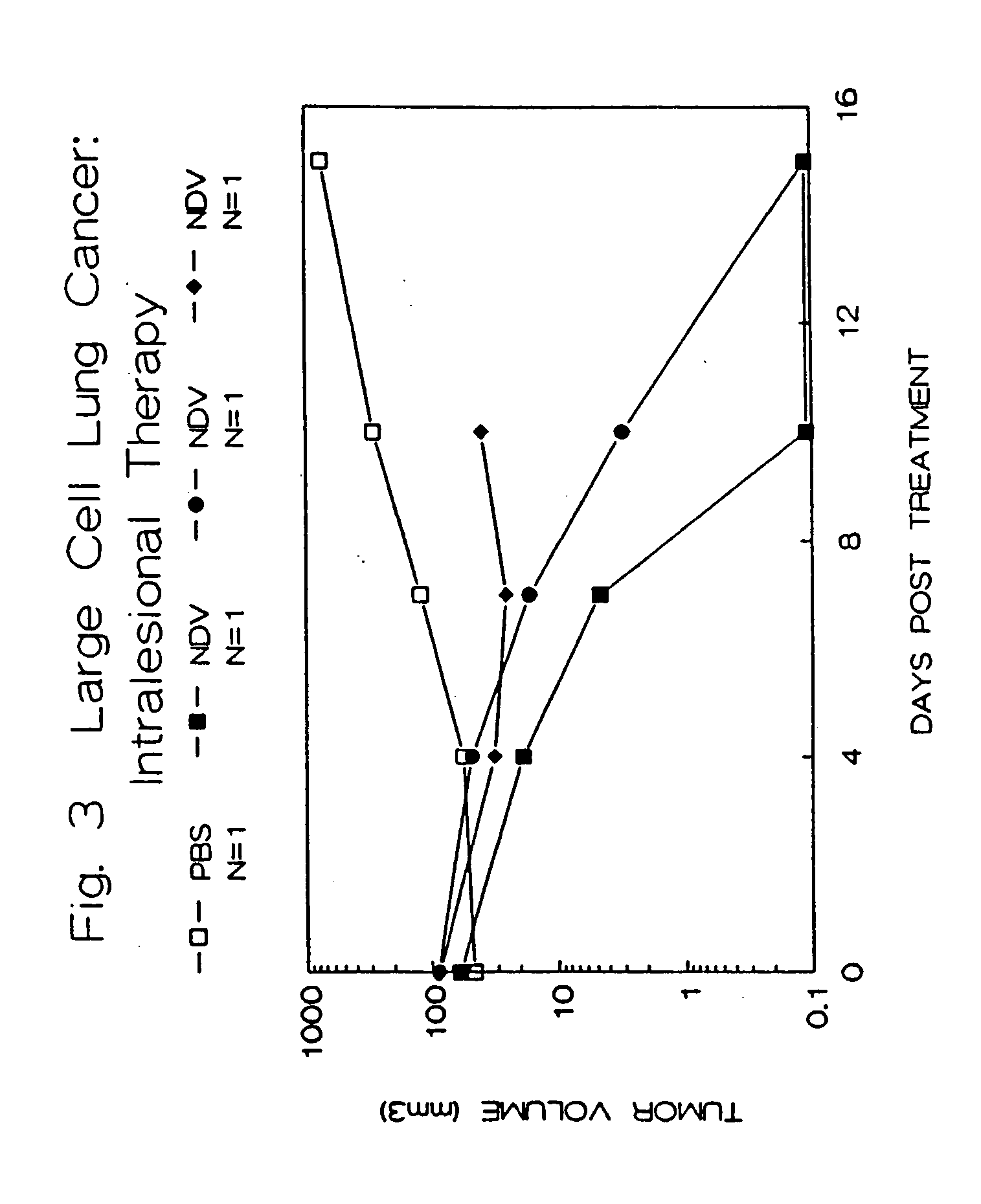
Methods of treating and detecting cancer using viruses
The invention provides a method of treating cancer in a mammal comprising administering to the mammal an effective amount of virus, particularly Newcastle Disease Virus or other Paramyxovirus. The invention also provides a method of treating cancer in a mammal comprising administering such viruses to the mammal in combination with another agent such as a chemotherapeutic compound, immunoadjuvant, cytokine, or immunosuppressive agent. The invention further provides a method of detecting cancer cells in a mammal using Paramyxovirus as an imaging agent and as an indicator of cancer cell growth in the mammal. The invention further provides genetically engineered Paramyxoviruses, and kits containing the viral compositions disclosed by the invention.
Owner:WELLSTAT BIOLOGICS CORP
Immunoadjuvant
InactiveUS20060008478A1Prevent bad situationsEfficiently exhibit potent adjuvant activityPeptide/protein ingredientsCancer antigen ingredientsBiological bodyLiving body
An immunoadjuvant which can efficiently exhibit potent adjuvant activity and avoid conditions undesirable for living bodies, and comprises precipitates formed by coacervation of (a) a soluble protein (provided that a soluble protein contained in tuberculin is excluded), and (b) a mucopolysaccharide, and further comprises (c) a soluble protein contained in tuberculin wherein said (c) is coprecipitated with the precipitates.
Owner:RIKEN +1
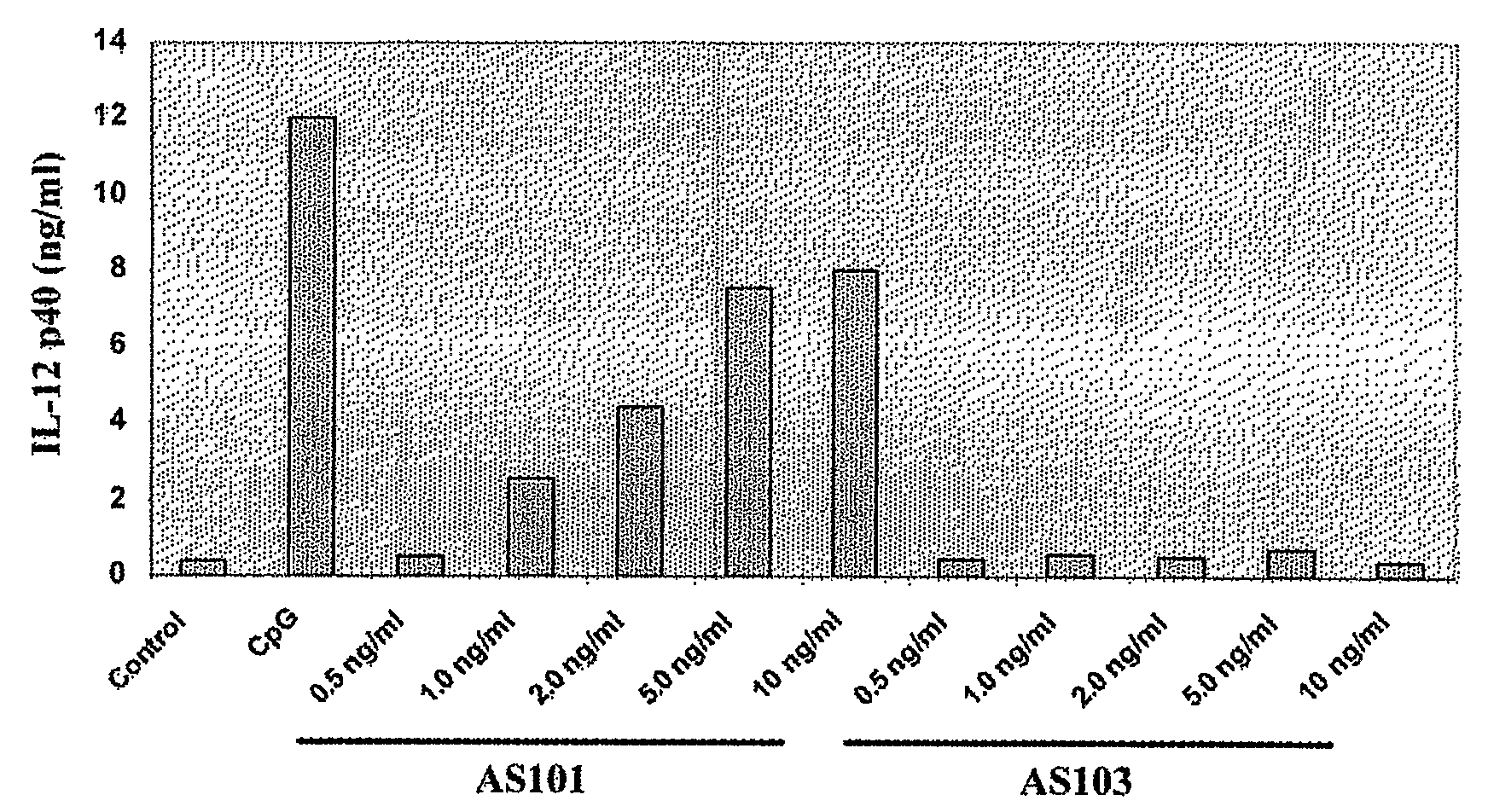
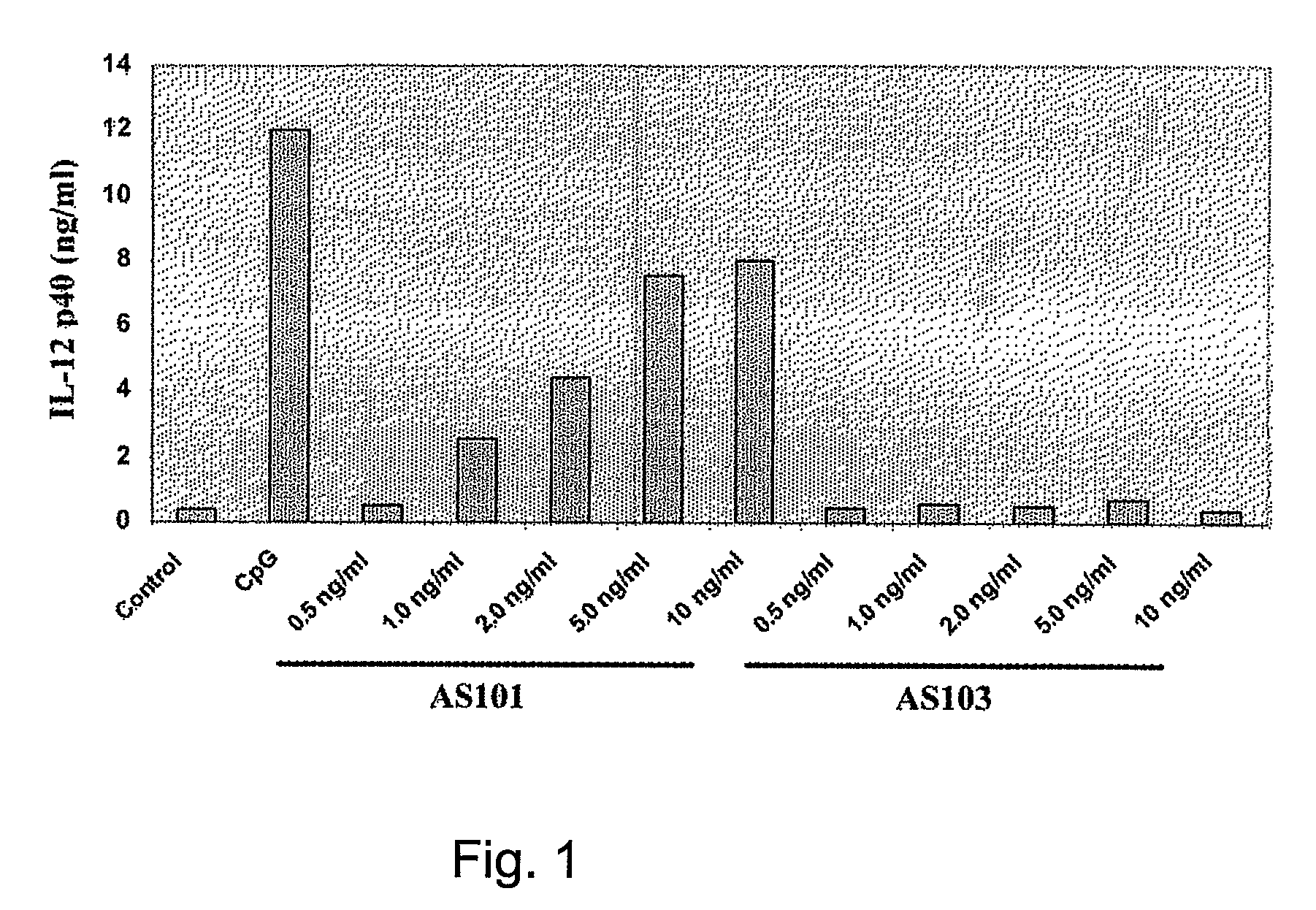
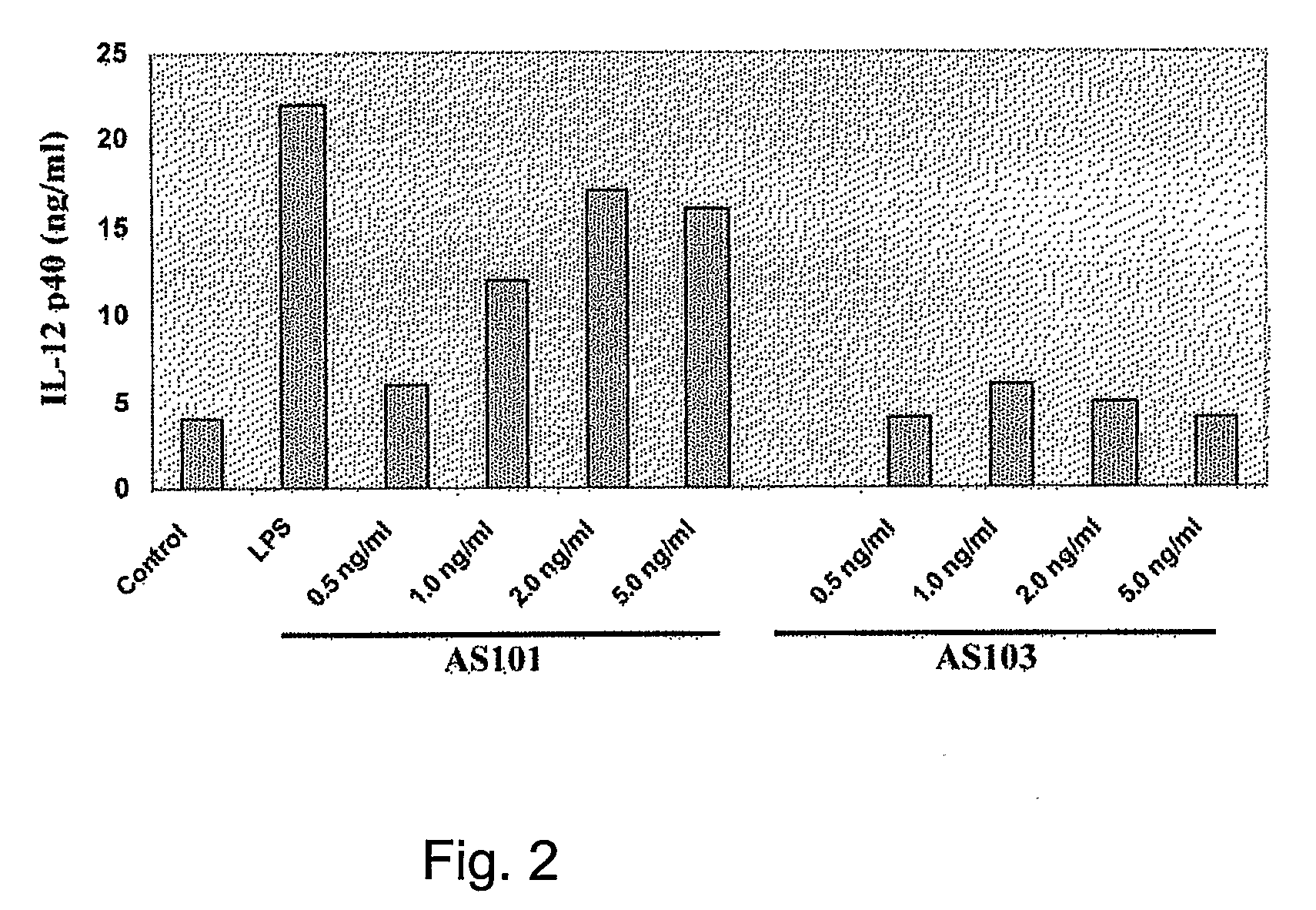
Use of Tellurium Compounds as Adjuvants
InactiveUS20080260770A1Enhance immune responseEffective adjuvanting amountOrganic non-active ingredientsCarrier-bound antigen/hapten ingredientsWhite blood cellTellurium compounds
Methods for enhancing the immune response of a subject to an immunoeffector, and methods of enhancing interleukin-12 production, which are effected by administering an amount of the immunoeffector and an effective adjuvanting amount of a tellurium-containing compound are provided. The enhanced immune response may be a cell-mediated or a humoral immune response. A pharmaceutical composition, which comprises the tellurium-containing compound, the immunoeffector and a pharmaceutically acceptable carrier is also provided. Use of a tellurium-containing compound as an adjuvant for immunization is also provided.
Owner:BIOMAS
Application of chitosan serving as immuno-adjuvant in preparing mouse allergic asthma model
InactiveCN102727887ASmall side effectsStrong immunostimulatory effectAntibody medical ingredientsEosinophilic inflammationAirway responsiveness
The invention belongs to the technical field of biology, and relates to an allergic asthma model, in particular to application of chitosan serving as immuno-adjuvant in preparing a mouse allergic asthma model. According to the application, the mouse allergic asthma model prepared by applying chitosan serving as immuno-adjuvant is subjected to airway responsiveness, lung tissue pathological examination and inflammation marker inspection, and results indicate that the mouse allergic asthma model prepared by applying chitosan serving as immuno-adjuvant is remarkably superior to a conventional immuno-adjuvant prepared asthma model in airway eosinophilic inflammation, airway responsiveness and airway inflammatory cytolines and other aspects. The chitosan has low price and rich sources; and the mouse asthma model by using chitosan as immuno-adjuvant is remarkably superior to a traditional adjuvant, and has the advantages of simple method, low cost, good reproducibility and the like.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Method for synthesizing swainsonine antigen
InactiveCN102250238AAvoid poisoningThe reaction system is stableSerum albuminFreeze-dryingBovine serum albumin
The invention discloses a method for synthesizing a swainsonine antigen, comprising the following steps of: firstly performing a reaction between halo alkyl aryl fatty acid and alcohol to generate halo alkyl aryl fatty acid ester, followed by a reaction between halo alkyl aryl fatty acid ester and swainsonine to generate a quaternary ammonium salt with a structure of aromatic nucleus, performing a reaction between the quaternary ammonium salt and sodium hydroxide for deesterification to generate a sodium salt, adjusting the pH value by the use of hydrochloric acid to generate SW-halo alkyl aryl fatty acid; condensing SW-halo alkyl aryl fatty acid with bovine serum albumin (BSA) to produce SW-BSA, sieving through a glucan gel column chromatography for desalination, freeze drying, determining the proportion of BSA and SW, and adding an immunoadjuvant for emulsification to obtain the SW immune antigen. The antigen is white and oily with the effective content being 5mg / mL and the effective immune period being 12 months. The effective protection rate reaches more than 90%.
Owner:NORTHWEST A & F UNIV
Immunogenic composition comprising peptides derived from cytomegalovirus and the use thereof
InactiveUS20120093848A1Effective immune responseEfficient activationViral antigen ingredientsAntiviralsSpecific immunityTrue positive rate
The present invention provides (poly)peptides, which are recognized by human cytomegalovirus (CMV)-specific immune cells. The present invention further provides a combination of multiple CMV (poly)peptides, comprising at least two different groups of (poly)peptides according to the invention as well as conjugates, comprising said (poly)peptides and / or immune adjuvants thereof. Furthermore, this invention provides mixtures, comprising said (poly)peptides and / or immune cells thereof, which are used to generate CMV-specific immune effector cells with high sensitivity and specificity. In addition, the present invention provides a preparation method of CMV-specific immune effector cells, by using said (poly)peptides, adjuvants, immune cells and / or mixtures thereof to generate anti-CMV immune response.
Owner:VECTORITE BIOMEDICA +1
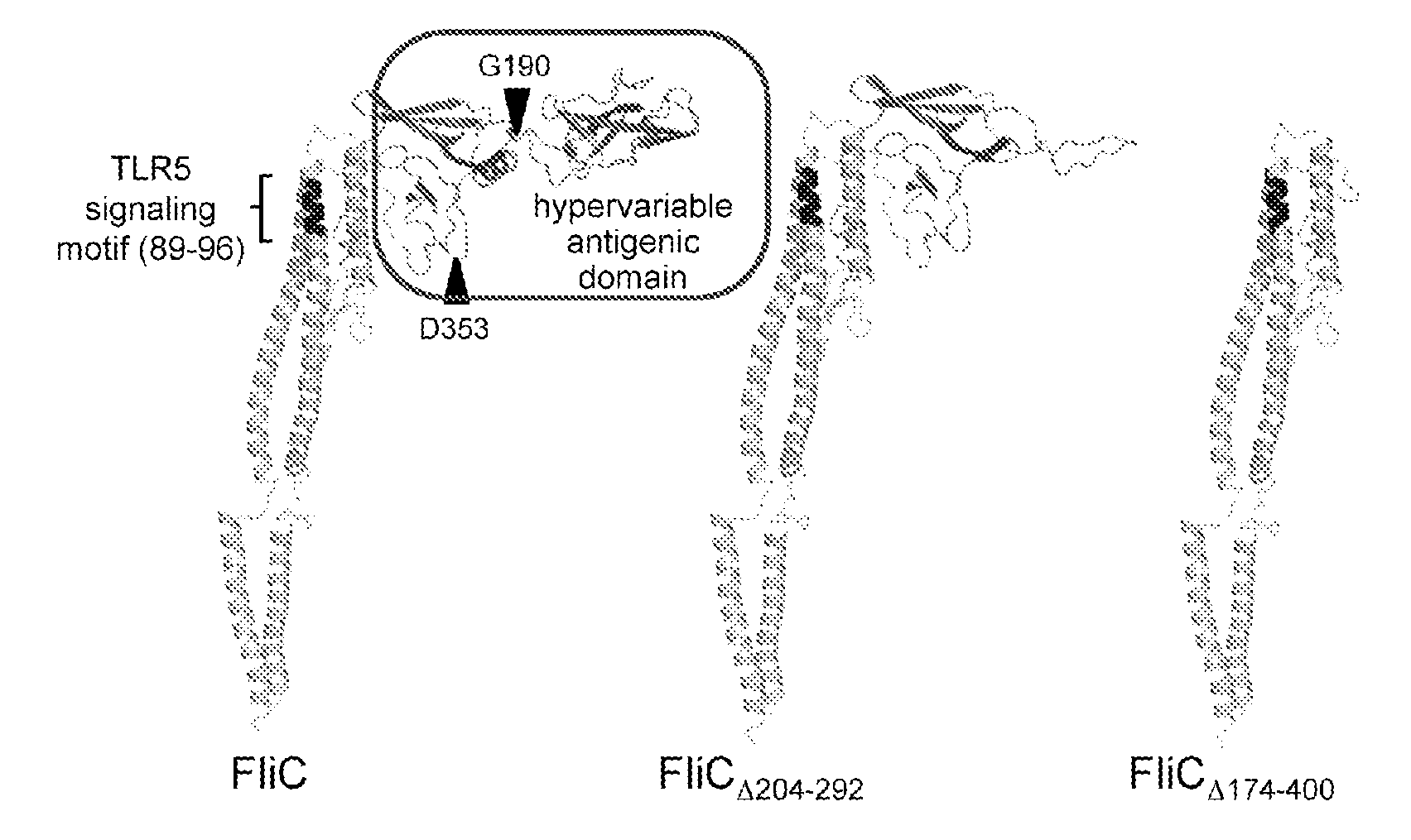
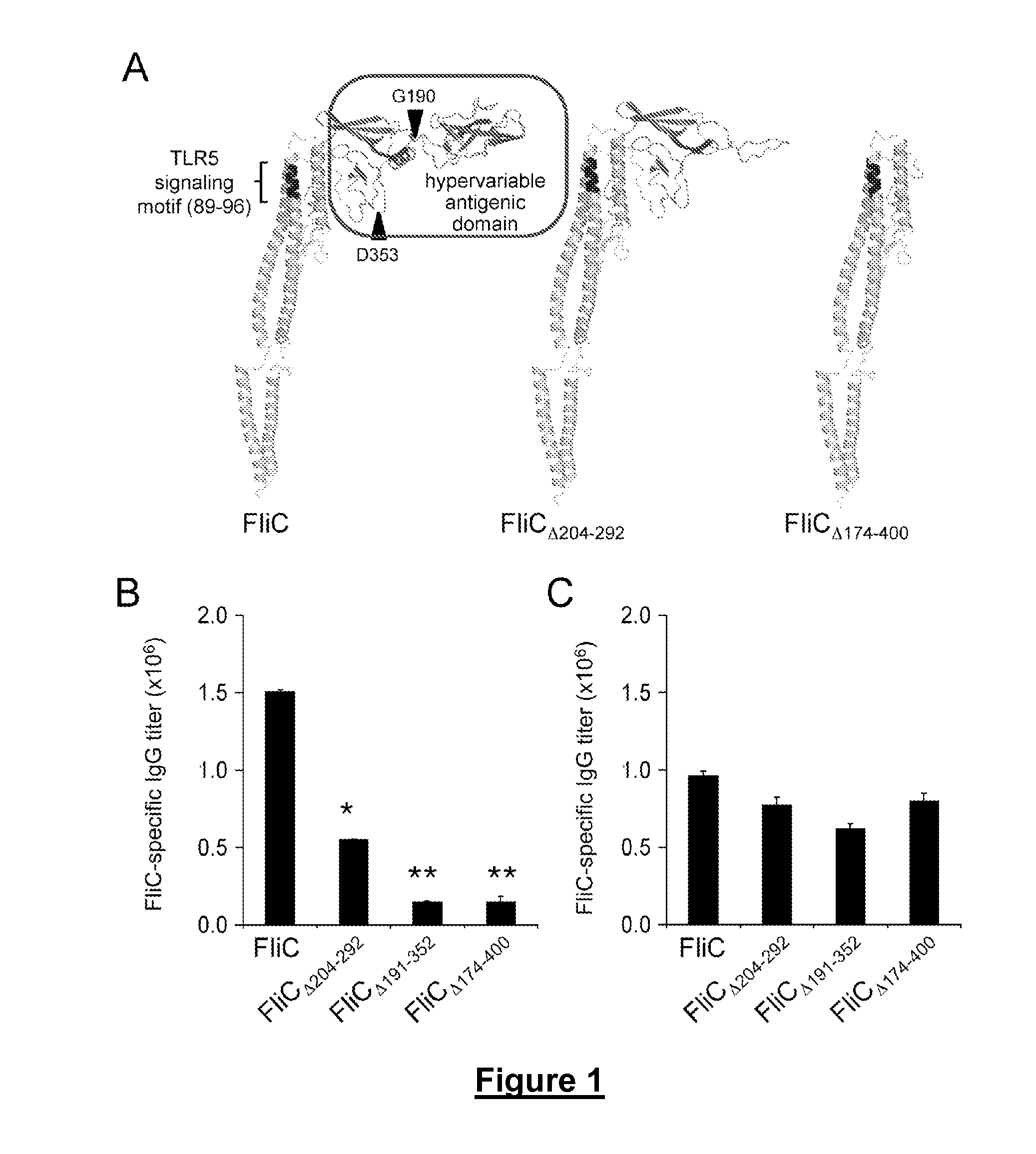
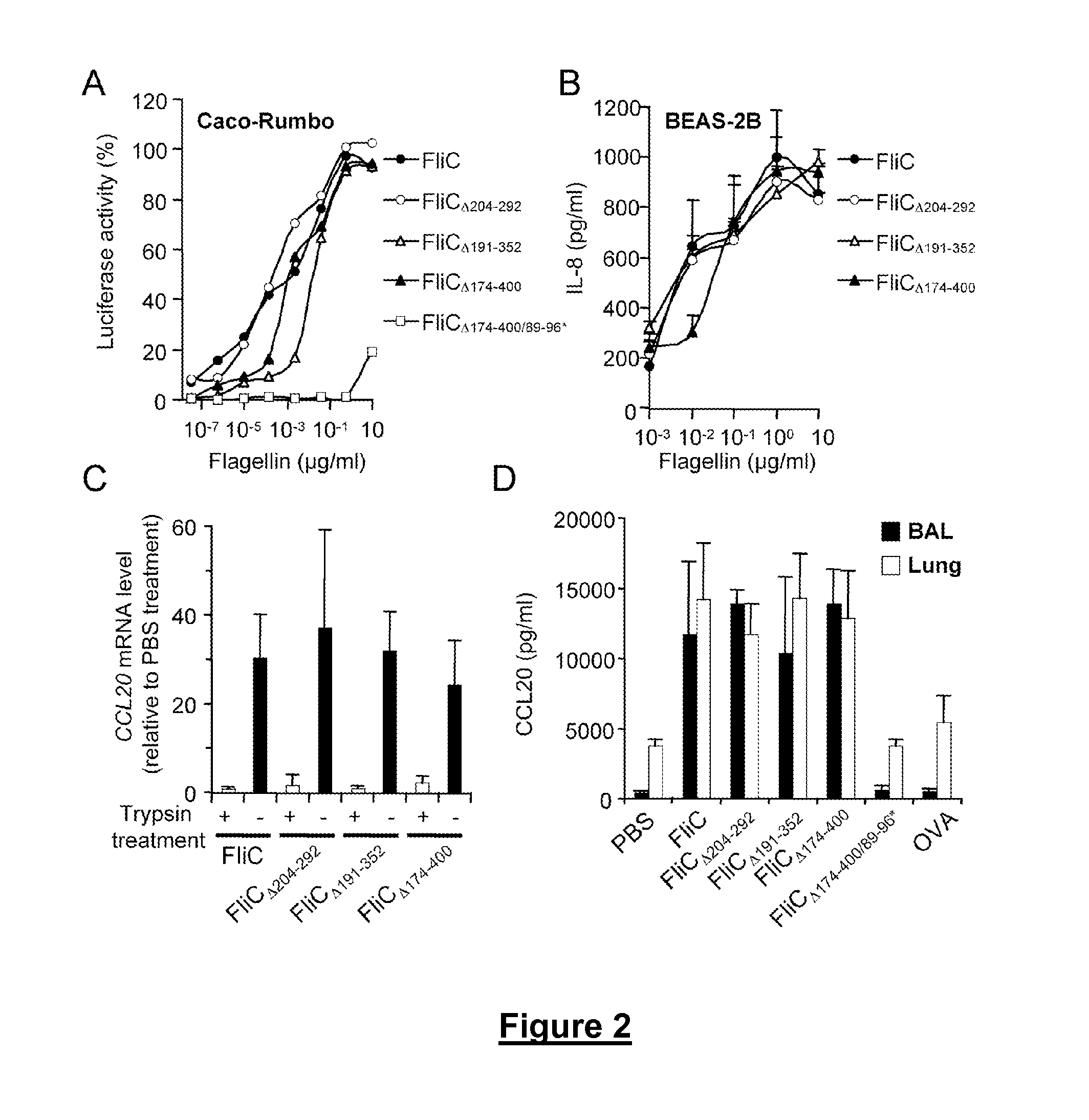
Novel Immunoadjuvant Flagellin-Based Compounds and Use Thereof
The present invention relates to novel peptide compounds derived from flagellin originating from Salmonella enterica that exhibit an in vivo immune adjuvant activity.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Interleukin-12 as a veterinary vaccine adjuvant
InactiveCN1555271AStimulate immune responseImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsWhite blood cellImmunomodulating Agent
Owner:WYETH LLC
Novel water soluble composite immune adjuvant and porcine circovirus disease vaccine
ActiveCN107375922AGood immune protectionStrong immune adjuvant effectViral antigen ingredientsAntiviralsDiseaseBaculovirus expression
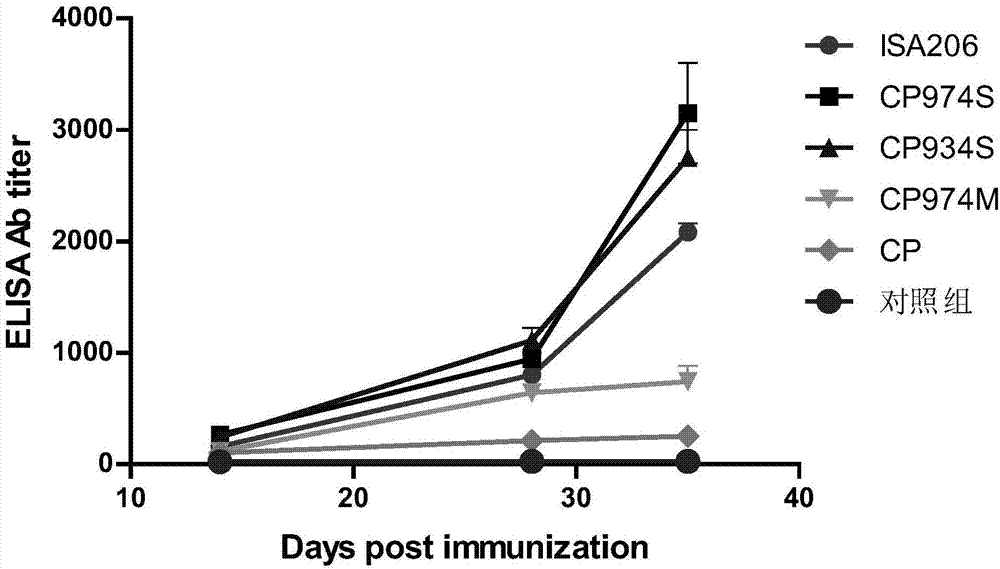
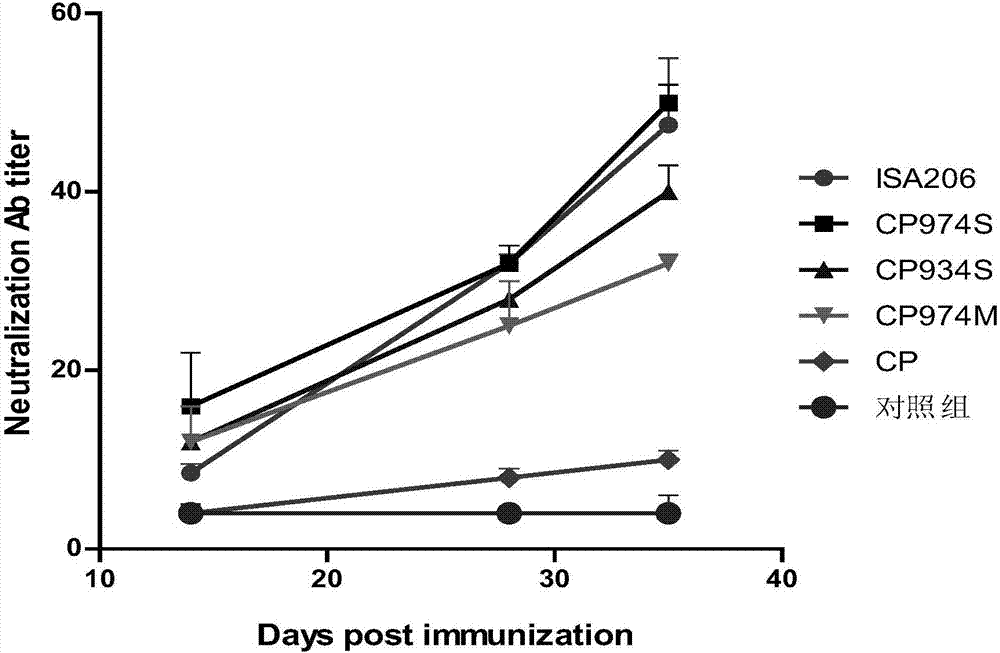
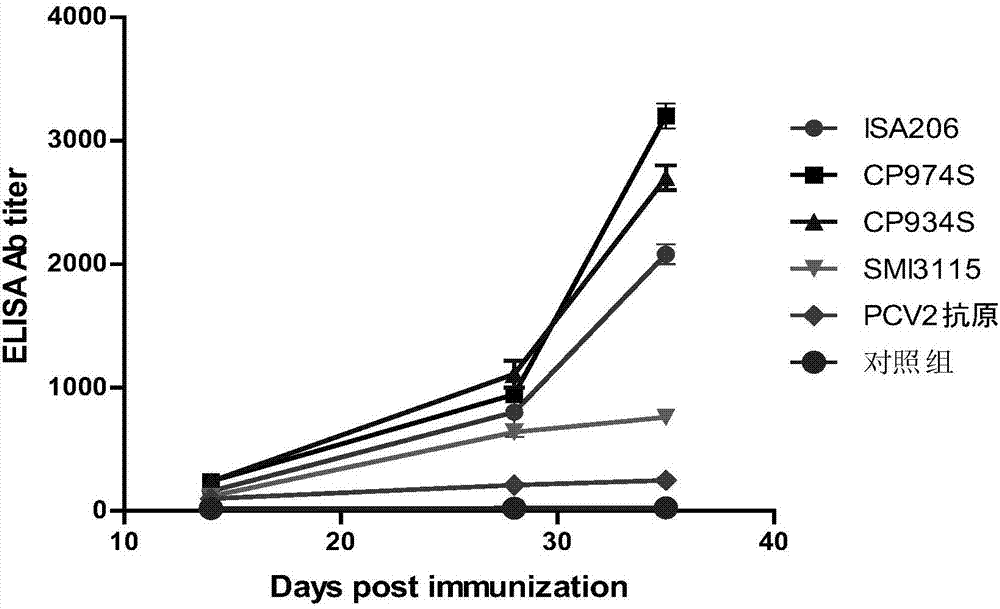
The invention discloses a novel water soluble composite immune adjuvant and a porcine circovirus disease vaccine. Four aqueous adjuvants (CP974S, CP934S, CP974M, and CP) are screened and designed to prepare a PCV2 inactivated vaccine. The results of mouse immunity tests and pig immunity tests show that CP974S and CP934S have a strong immune adjuvant effect on the PCV2 inactivated vaccine, and the pig immunity protective effect of CP974S aqueous adjuvant is better than the PCV2 imported ISA206 adjuvant inactivated vaccine. A baculovirus expressed PCV2Cap recombinant protein is taken as the basis to prepare different adjuvant vaccines. The results of mouse immunity tests and pig immunity tests show that two aqueous adjuvants (CP974S and CP934S) also have a strong immunity adjuvant effect on the PCV2Cap recombinant protein and the pig immunity effect of CP974S adjuvant vaccine is better than imported PCV2Cap subunit vaccine. The CP974S aqueous adjuvant fills the gap of water soluble adjuvant for pigs in China. The developed PCV2 vaccine is safe and effect and has an important application prospect.
Owner:NANJING AGRICULTURAL UNIVERSITY
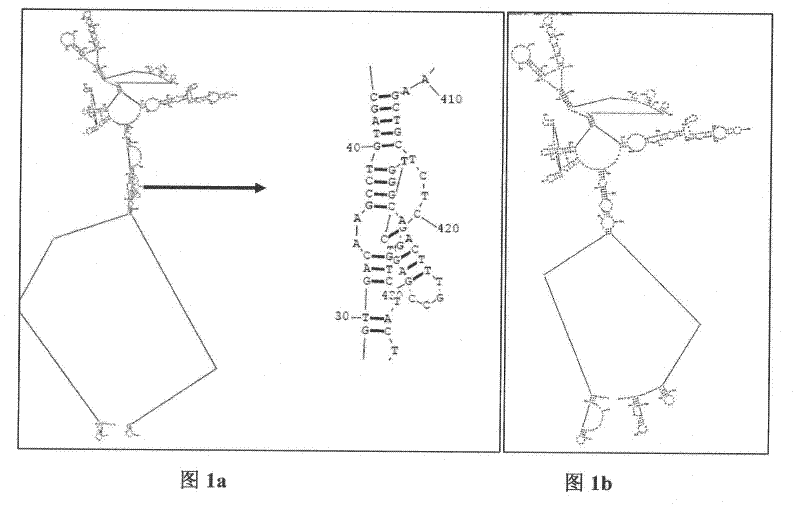
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979AMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticL929 cellEscherichia coli
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for preparing recombinant goose interleukin-2 protein and its application
InactiveCN1544629AImprove stabilityImprove biological activityAntiviralsDepsipeptidesEscherichia coliBiotechnology
The invention discloses a recombinant goose interleukin-2(gsIL-2) protein preparing method and its use. The DNA fraction of RT-PCR amplified Zhedong white goose interleukin-2, prokaryotic expression carrier pBAD / HisB, and eukaryotic expression carriers pMET alpha B and pMETB compose expression particles pBAD / HisB / gsIL-2, pMET alpha B / gsIL-2 and pMETA / gsIL-2, which are converted to Escherichia coli LMG194, microzyme PMAD11 and PMAD16, respectively, then inducing expression. After inducing, making ultrasonic wave cracking and deposit elimination by centrifugation on Escherichia coli and microzyme PMAD16 to obtain crude products of gsIL-2 protein, PMAD11 expressed gsIL-2 is mainly separated in the culture medium, centrifugating and taking supernatant to obtain gsIL-2 protein crude products. Using Ni-NTAArgarrose protein purifying system, thus able to faster obtain gsIL-2 pure product, and the crude and pure products can act as immune assistant and disease-curing drugs. It applied genetic engineering technique to prepare recombinant protein gsIL-2, and has simple technical flow, low production cost, good stability, high bioactivity and other characters.
Owner:ZHEJIANG UNIV
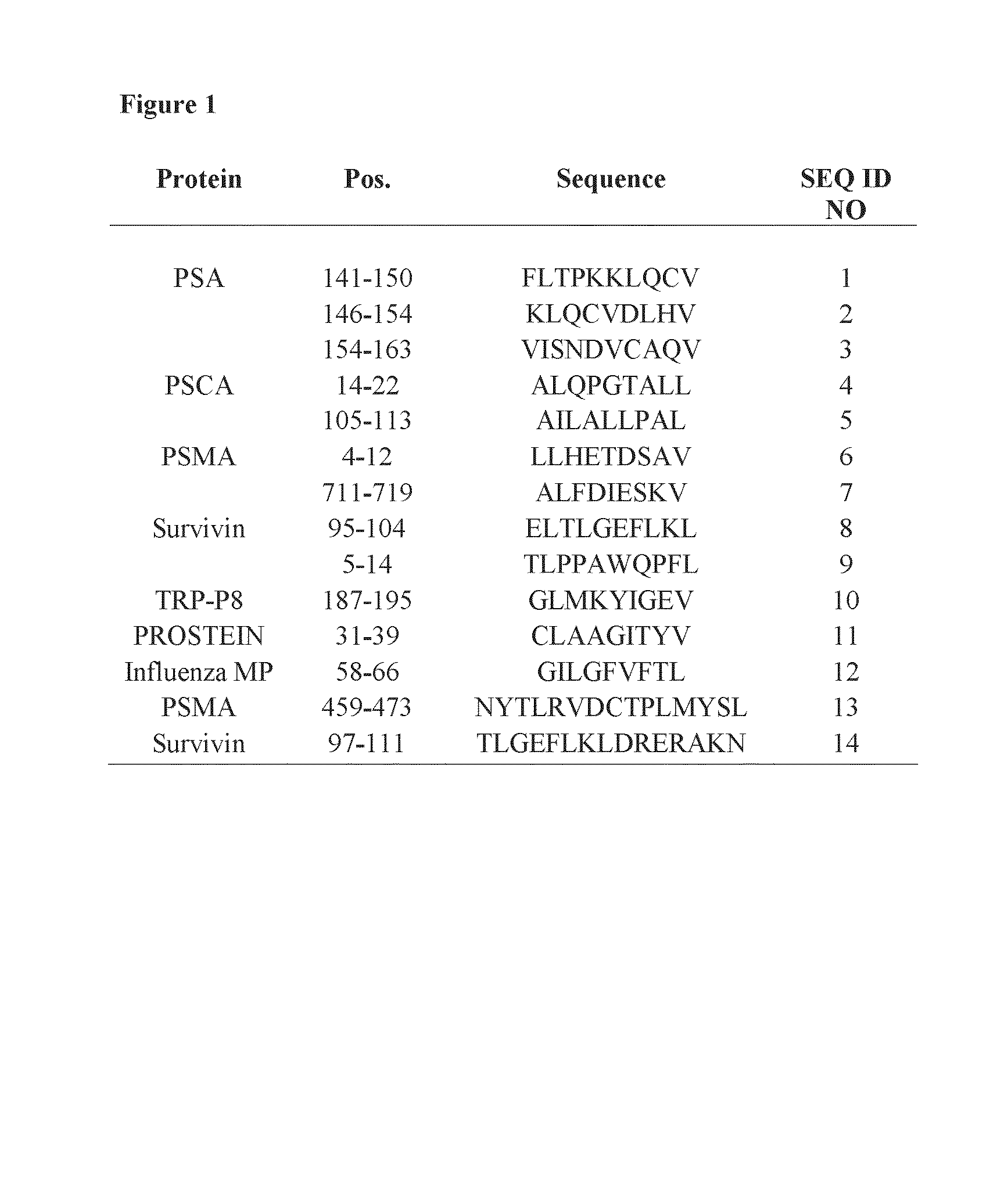
HLA-binding peptides derived from prostate-associated antigenic molecules and methods of use thereof
Methods and compositions for immunotherapeutic treatment of prostate cancer are disclosed. More specifically methods of treating patients with prostate cancer comprising administering compositions comprising HLA-binding peptides derived from prostate-associated antigenic molecules, either with or without immunological adjuvants, are disclosed.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Immunoadjuvant composition and use thereof
InactiveUS20120070452A1Activate immunityImprove securityAntibacterial agentsOrganic active ingredientsBULK ACTIVE INGREDIENTActive ingredient
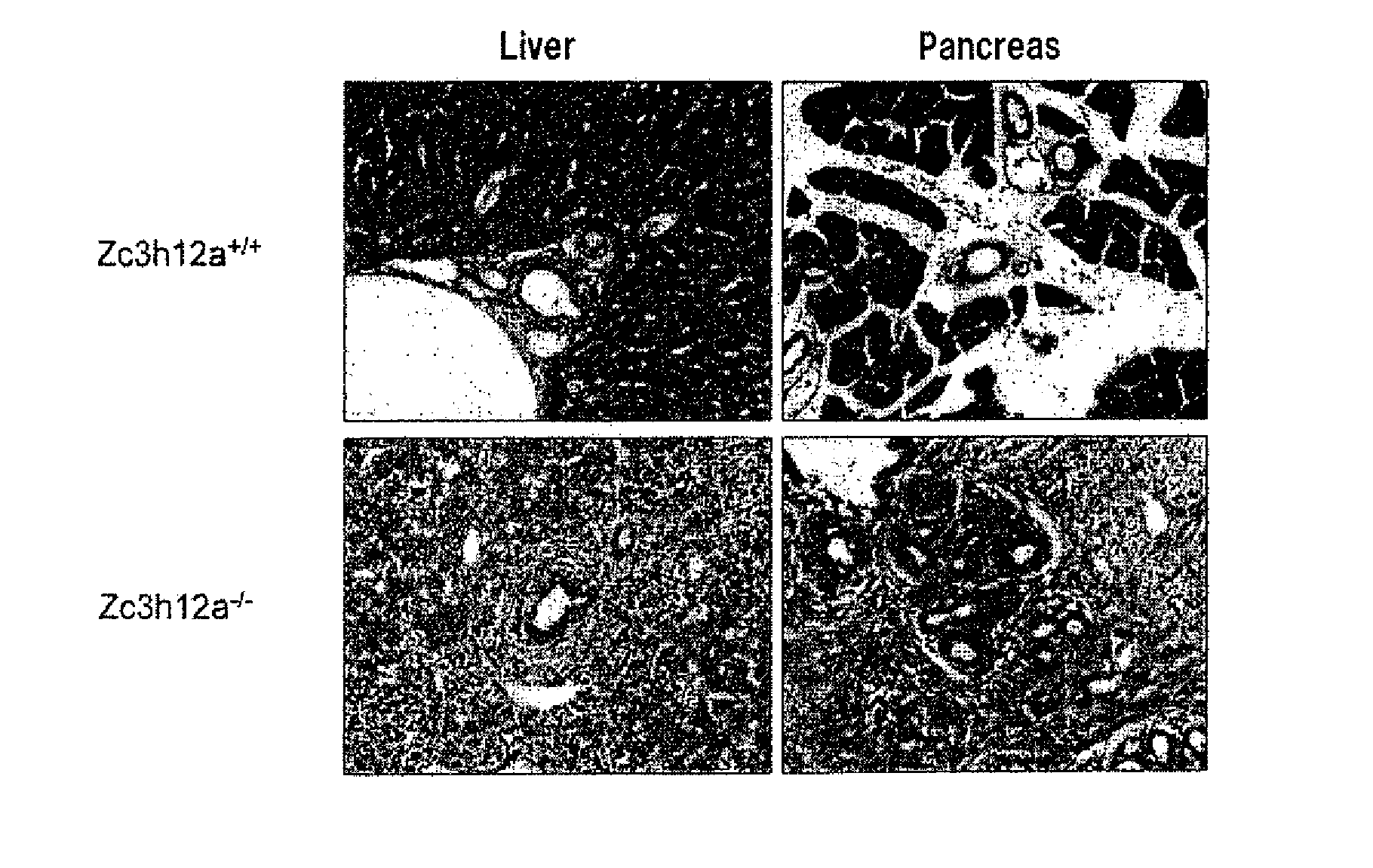
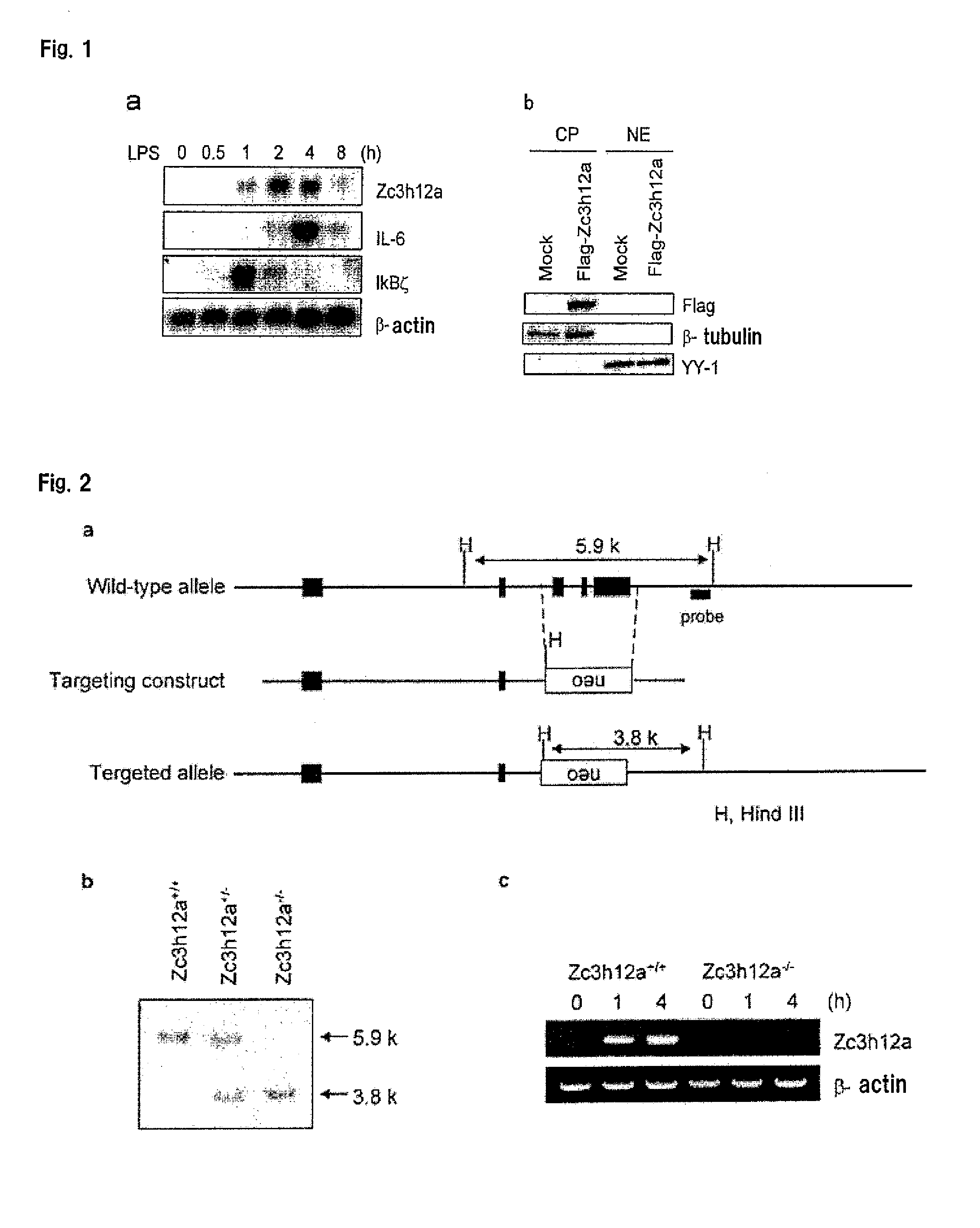
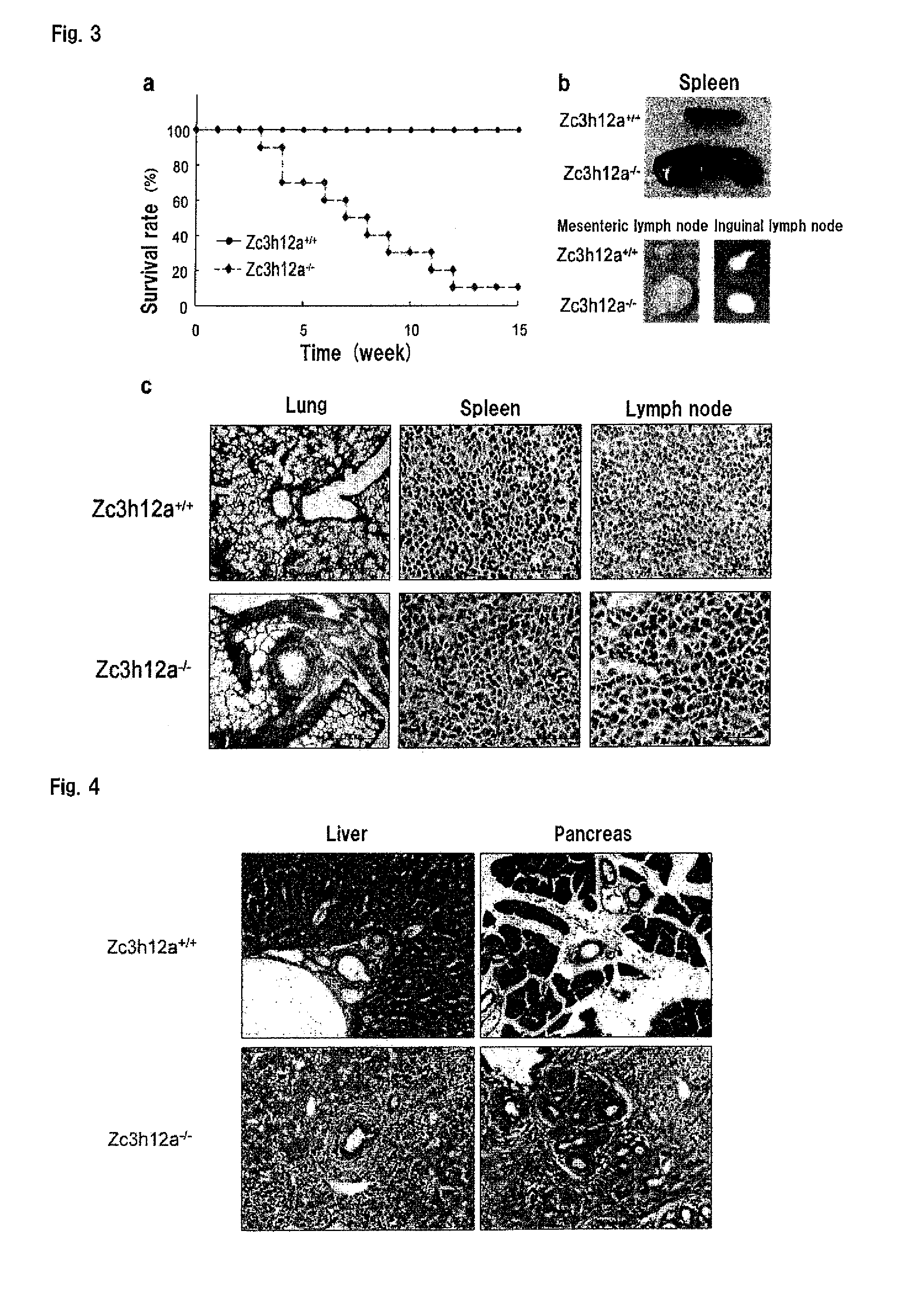
Disclosed is a composition comprising, as an active ingredient, at least one selected from the group consisting of a Zc3h12a gene inhibitor and a Zc3h12a protein inhibitor. This composition can be used as an immunoadjuvant.
Owner:OSAKA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com