Short peptide, application of short peptide as vaccine adjuvant, and vaccine
A vaccine adjuvant and short peptide technology, which is applied to the short peptide, its application as a vaccine adjuvant and the field of vaccines, can solve the problems of high cost, poor safety, difficult synthesis and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
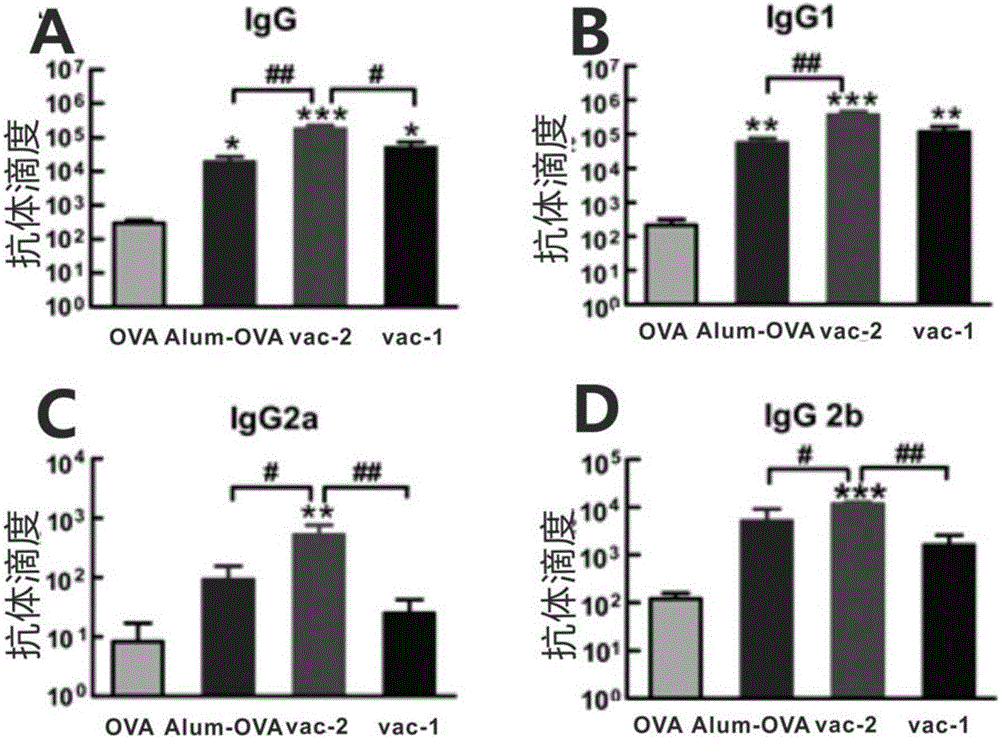
[0050] Preparation of vaccine vac-1 loaded with OVA protein in short peptide hydrogel at pH 7.0 and room temperature 20°C
[0051] (1) Synthesize the L-configuration short peptide Nap-GFFY by FMOC-solid-phase synthesis method, the structural formula is as follows:
[0052]
[0053] Specific steps are as follows:
[0054] 1) Weigh 0.5mmol 2-cl-Trt resin into a solid-phase synthesizer, add 10mL of anhydrous dichloromethane (hereinafter referred to as DCM), place on a shaker and shake for 5min to fully swell the 2-cl-Trt resin ;
[0055] 2) Remove DCM from the solid-phase synthesizer equipped with 2-cl-Trt resin with ear washing ball;
[0056] 3) Dissolve 0.75 mmol of Fmoc-protected amino acid in 10 mL of anhydrous DCM, add 0.75 mmol of DIEPA, then transfer to the above-mentioned solid-phase synthesizer, add 0.75 mmol of DIEPA, and react at room temperature for 1 h;
[0057] 4) Sealing: Remove the reaction solution in the solid-phase synthesizer with ear wash balls, and the...
preparation Embodiment 2
[0067] Short peptide hydrogel Nap-G at pH 7.0 and room temperature 20°C D f D f D Preparation of vaccine vac-2 loaded with OVA protein
[0068] (1) Synthesis of Nap-G by FMOC-solid phase synthesis method D f D f D Y, the structural formula is as follows:
[0069]
[0070] Specific steps are as follows:
[0071] 1) Weigh 0.5 mmol 2-cl-Trt resin into a solid-phase synthesizer, add 10 mL of DCM, place on a shaker and shake for 5 minutes to fully swell the 2-cl-Trt resin;
[0072] 2) Remove DCM from the solid-phase synthesizer equipped with 2-cl-Trt resin with ear washing ball;
[0073] 3) Dissolve 0.75 mmol of Fmoc-protected amino acid in 10 mL of anhydrous DCM, add 0.75 mmol of DIEPA, then transfer to the above-mentioned solid-phase synthesizer, add 0.75 mmol of DIEPA, and react at room temperature for 1 h;
[0074] 4) Sealing: Remove the reaction solution in the solid-phase synthesizer with ear wash balls, and then wash with anhydrous DCM, each time using 10 mL of DC...
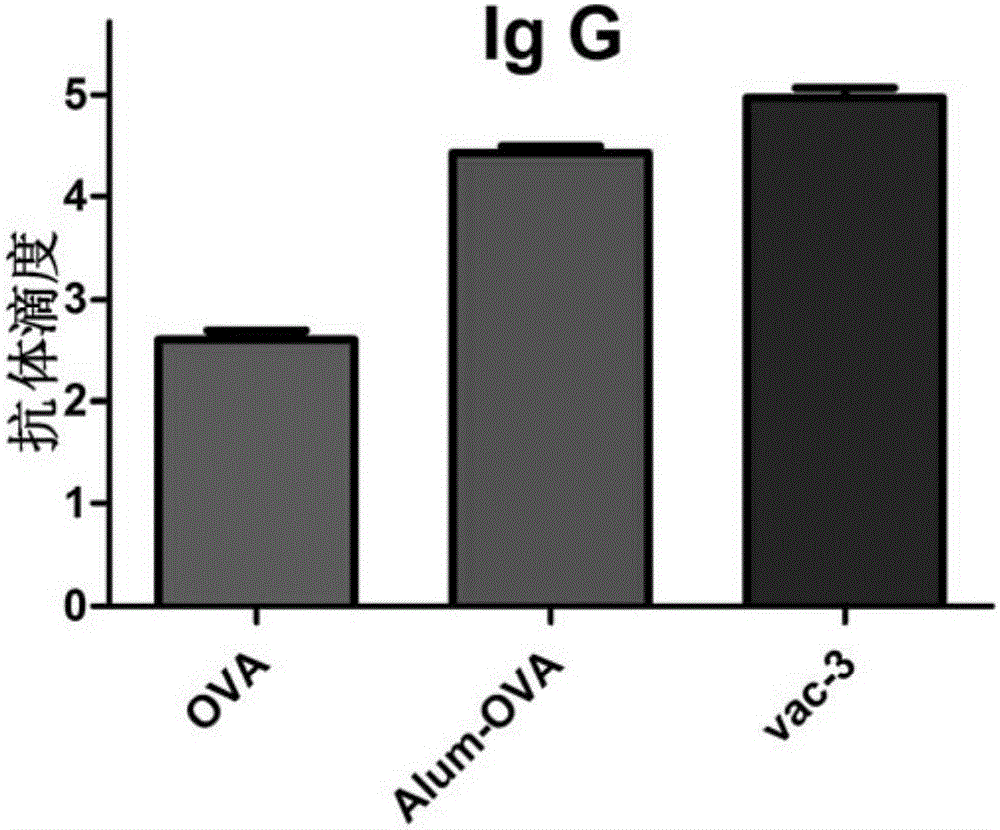
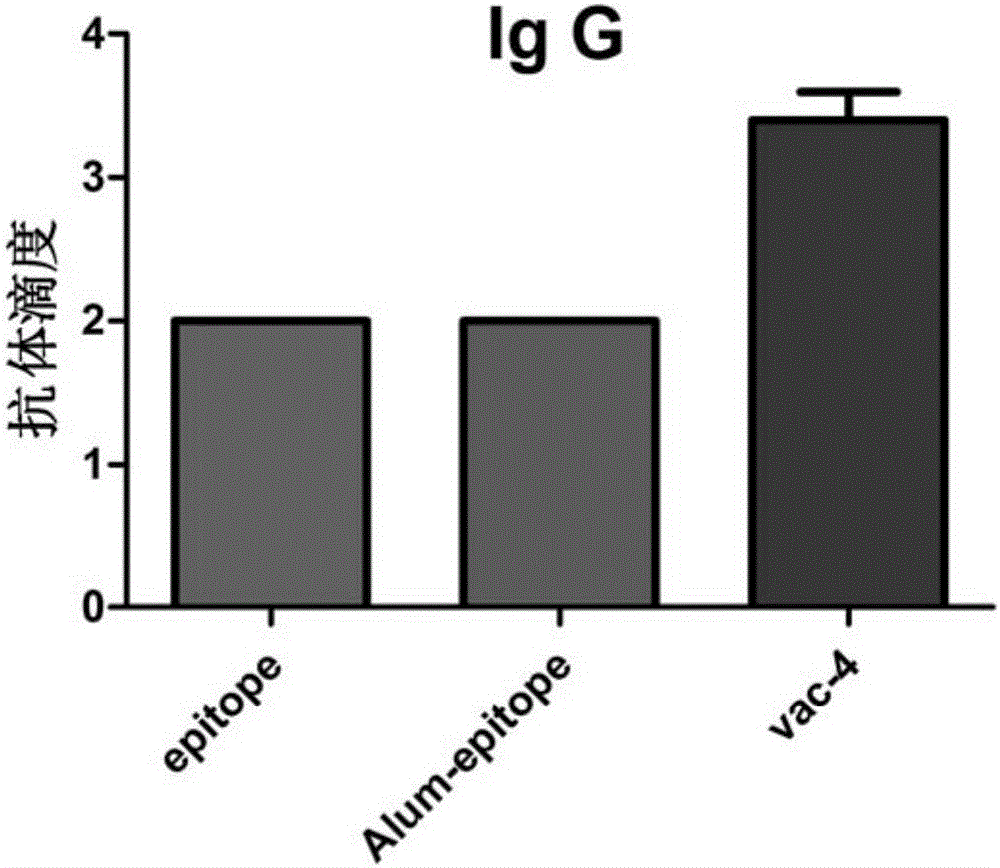
preparation Embodiment 3
[0084] Synthesis of Short Peptide Nap-G by FMOC-Solid Phase Synthesis D f D f D Y D K, the specific steps are as follows:
[0085] 1) Weigh 0.5 mmol 2-cl-Trt resin into a solid-phase synthesizer, add 10 mL of DCM, place on a shaker and shake for 5 minutes to fully swell the 2-cl-Trt resin;
[0086] 2) Remove DCM from the solid-phase synthesizer equipped with 2-cl-Trt resin with ear washing ball;
[0087] 3) Dissolve 0.75 mmol of Fmoc-protected amino acid in 10 mL of anhydrous DCM, add 0.75 mmol of DIEPA, then transfer to the above-mentioned solid-phase synthesizer, add 0.75 mmol of DIEPA, and react at room temperature for 1 h;
[0088]4) Sealing: Remove the reaction solution in the solid-phase synthesizer with the ear washing ball, and then wash with anhydrous DCM, each time the amount of DCM is 10mL, and the washing time is 1min. : 20mL of DIEPA:methanol=17:1:2 solution, reacted at room temperature for 10min;
[0089] 5) Remove the reaction liquid in the solid-phase syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com