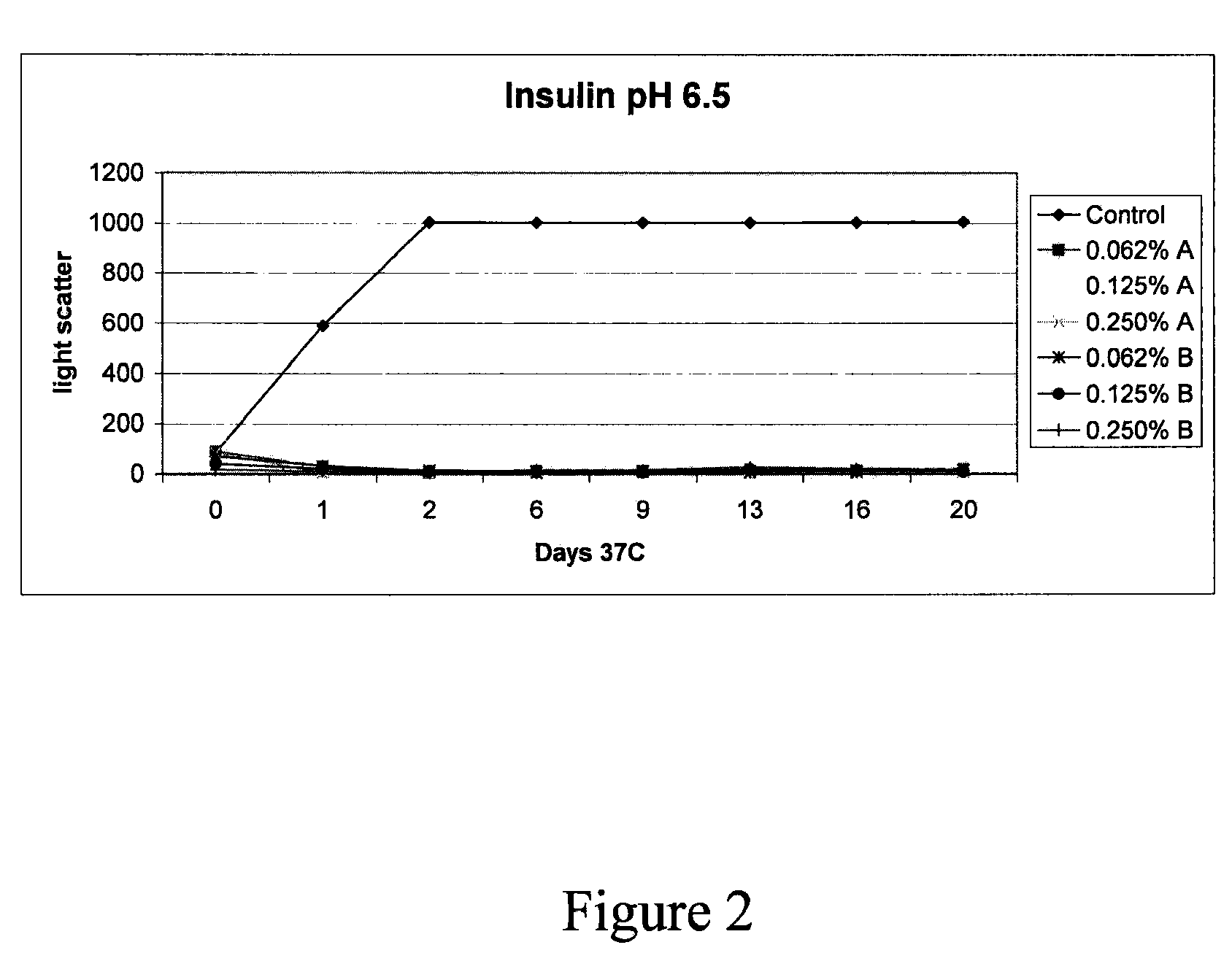
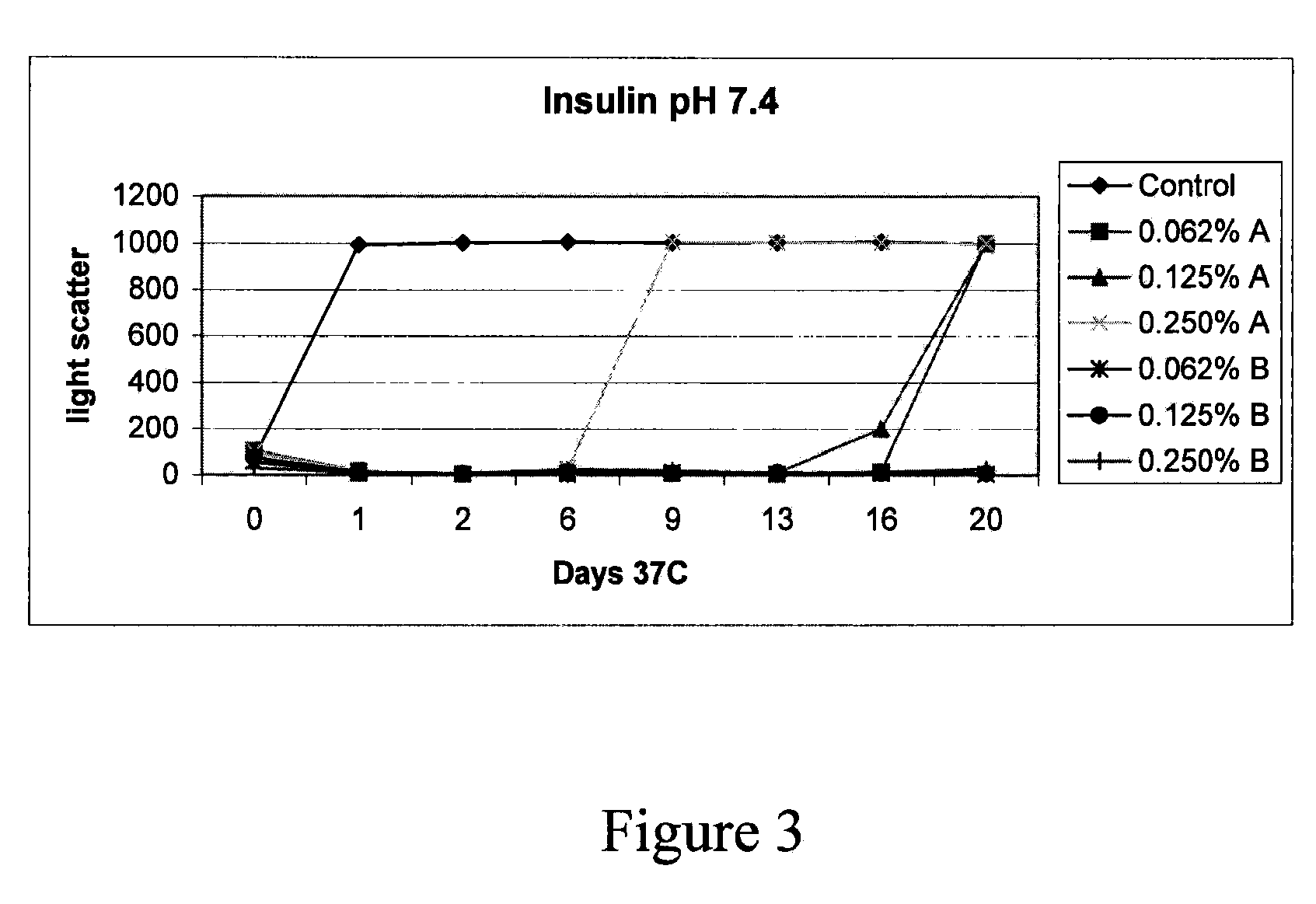
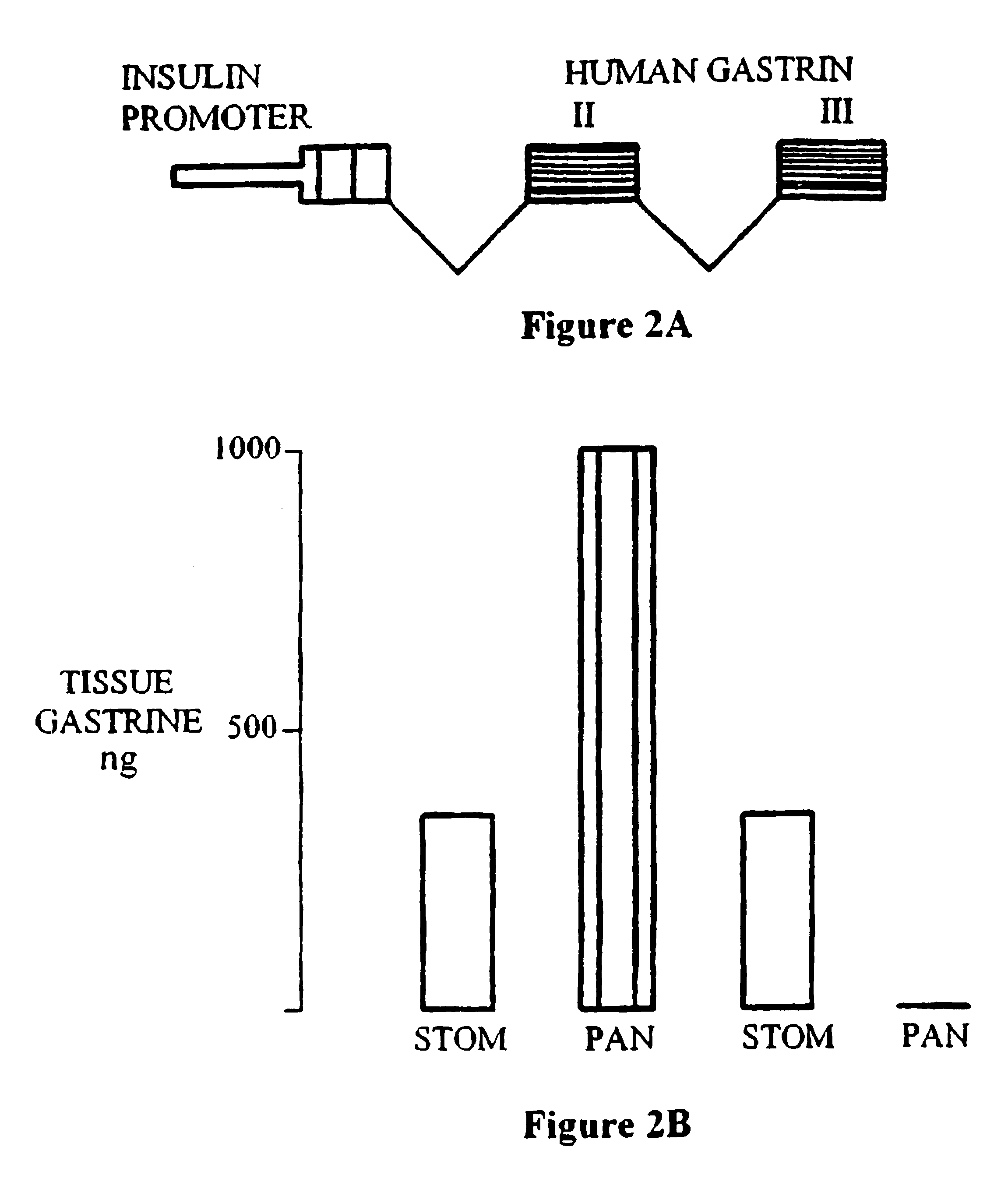
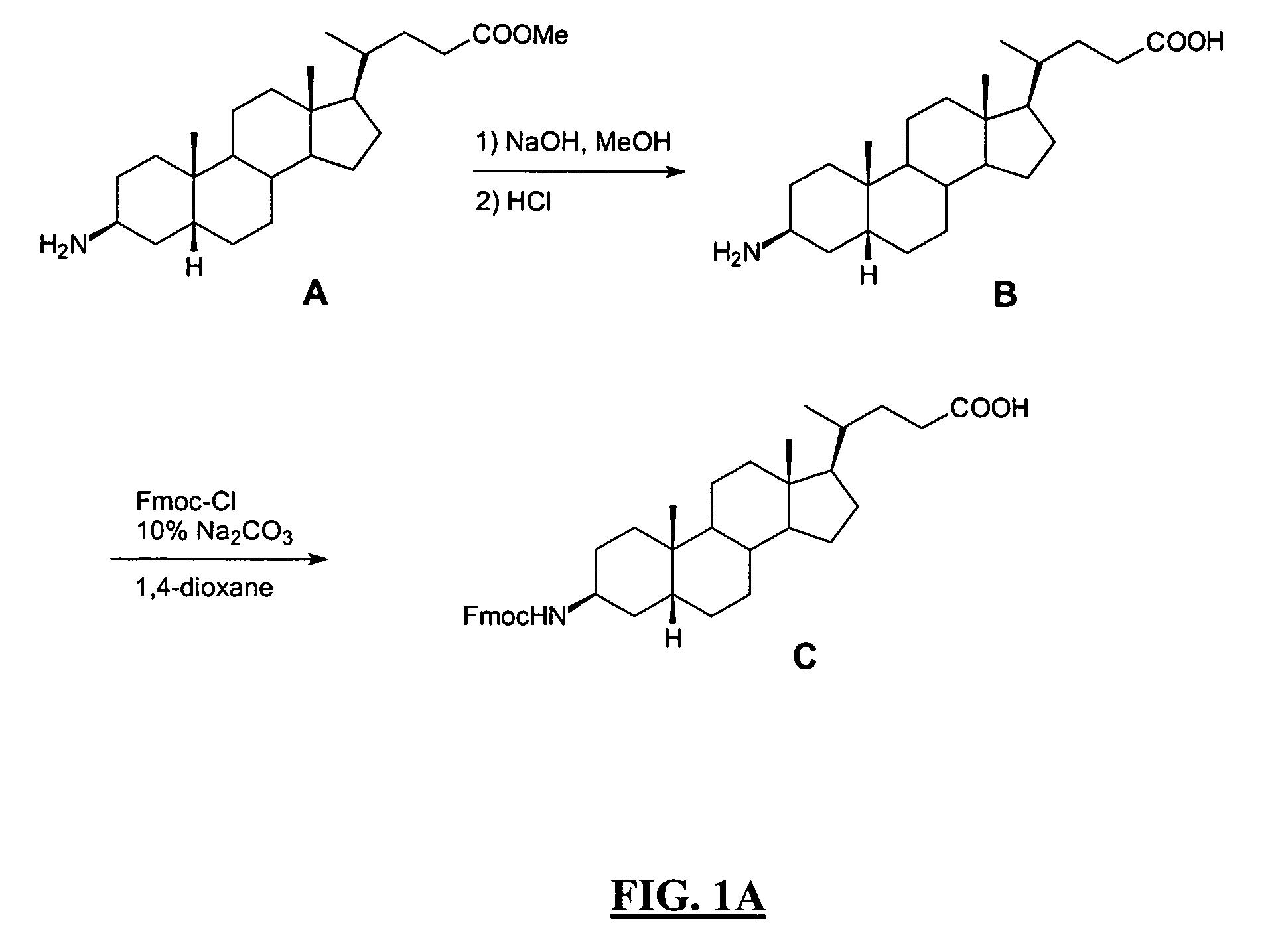
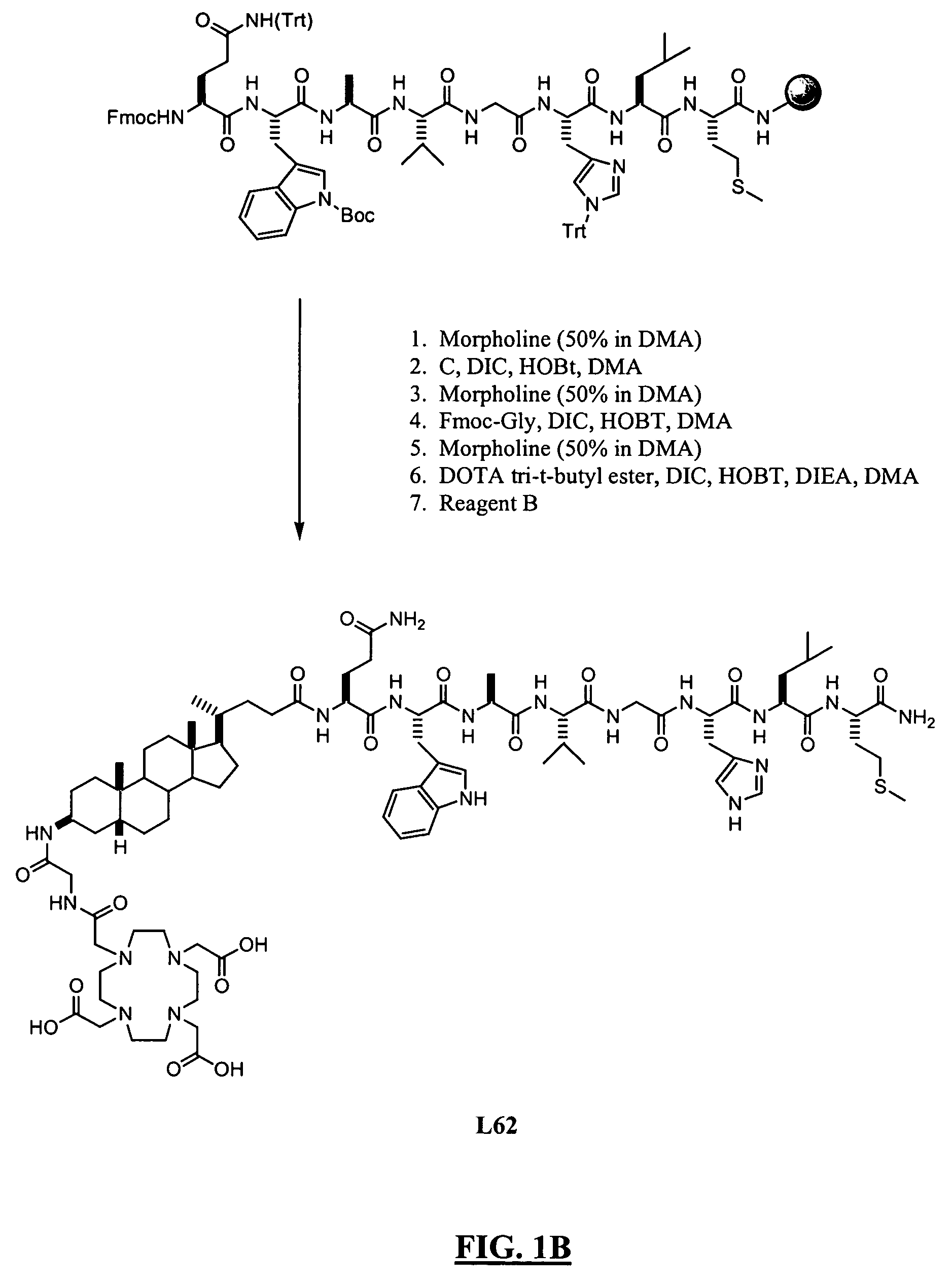
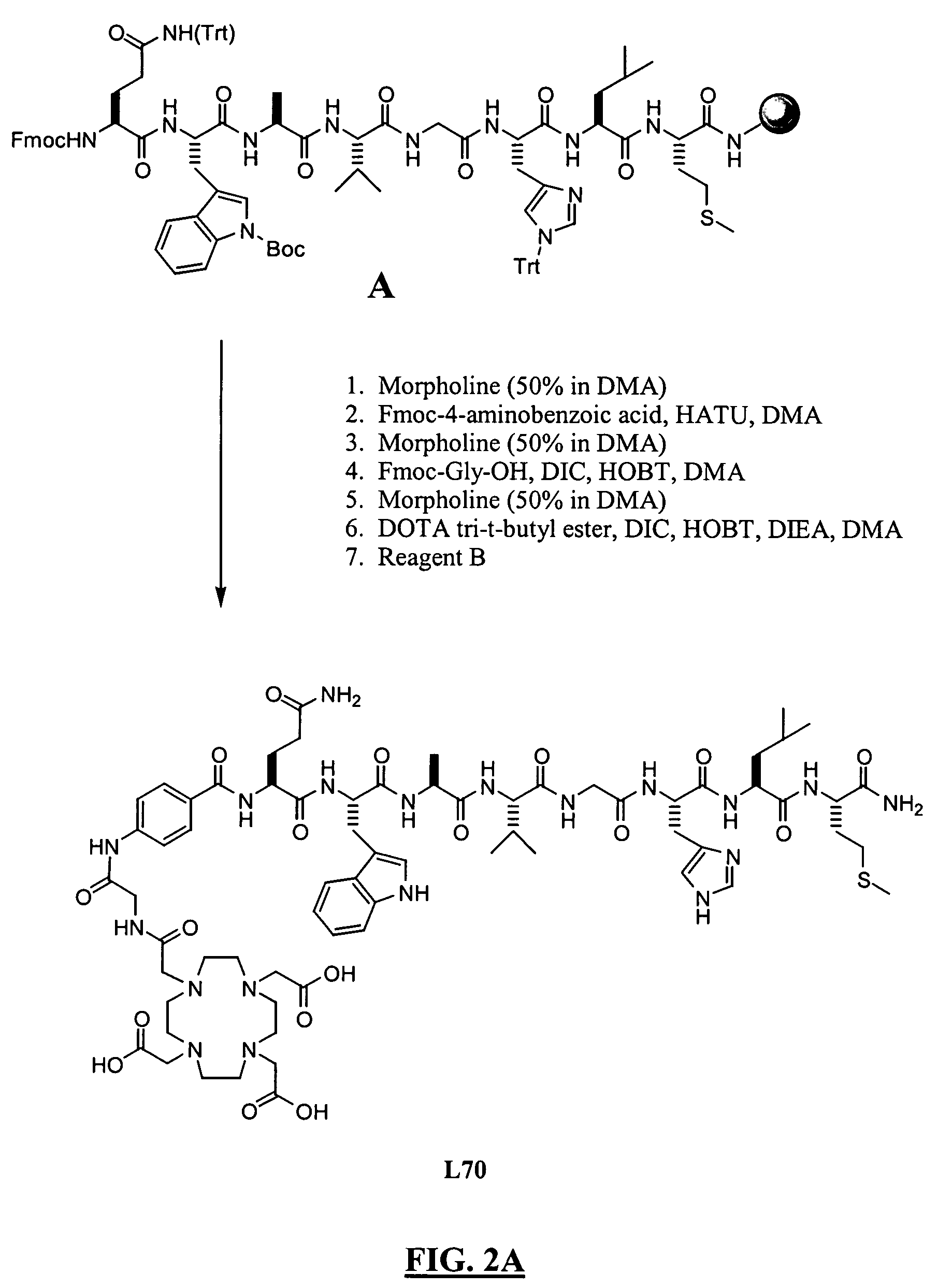
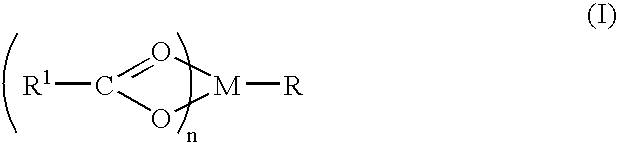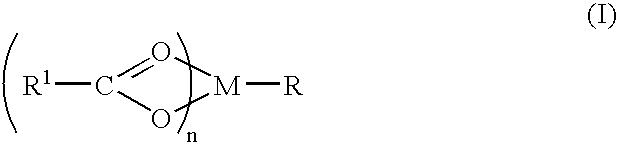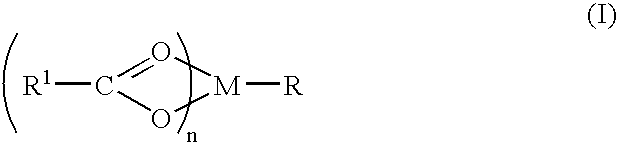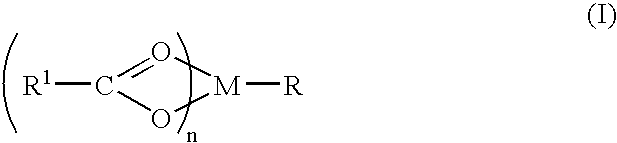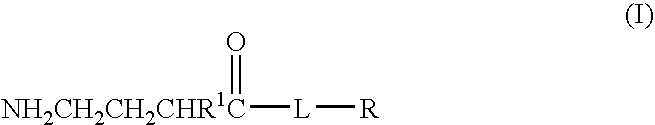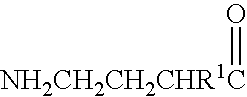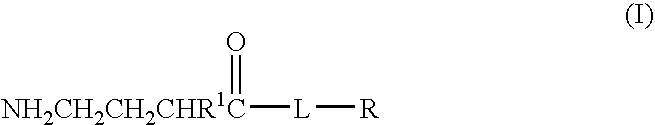Patents
Literature
252 results about "Gastrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
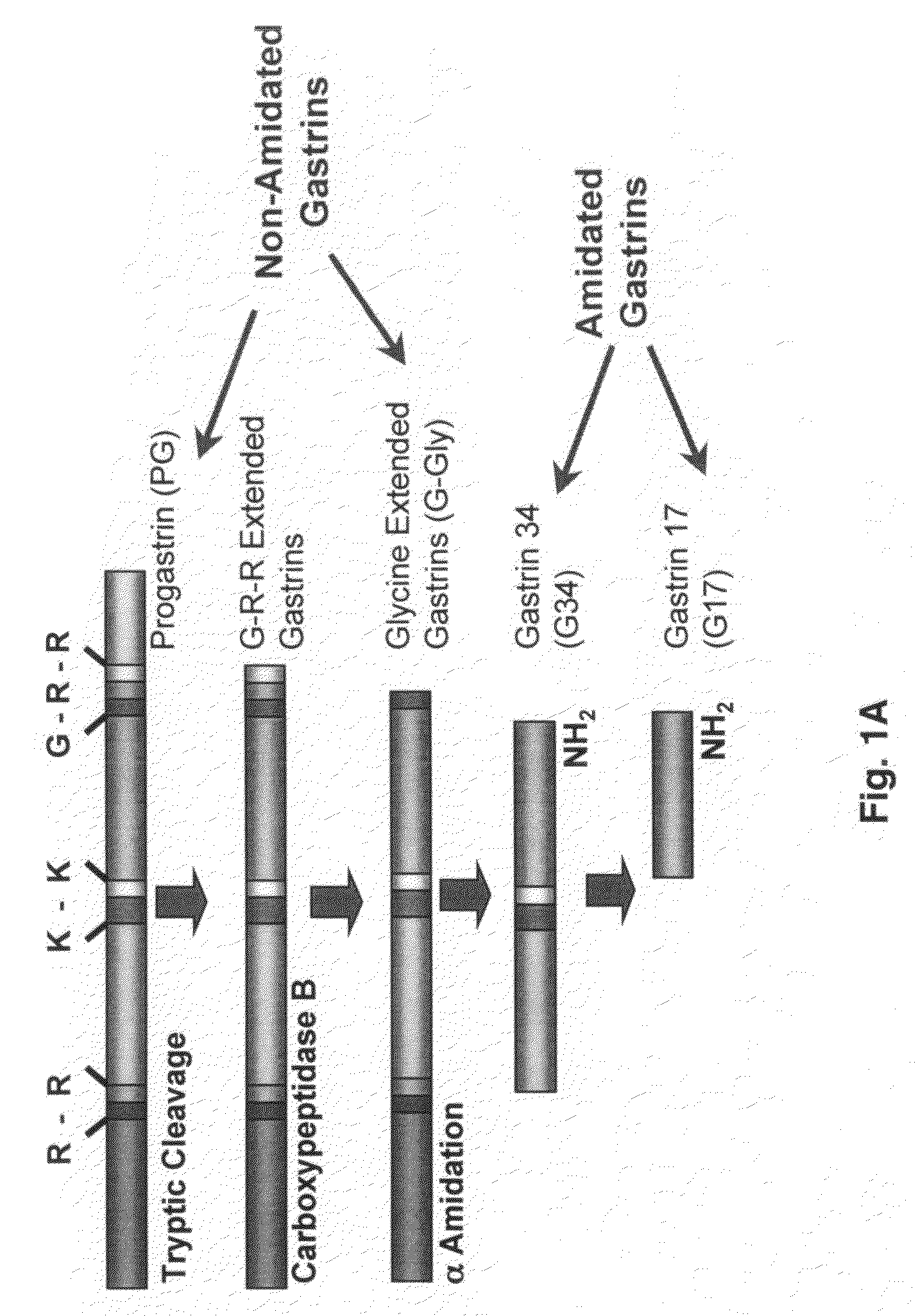
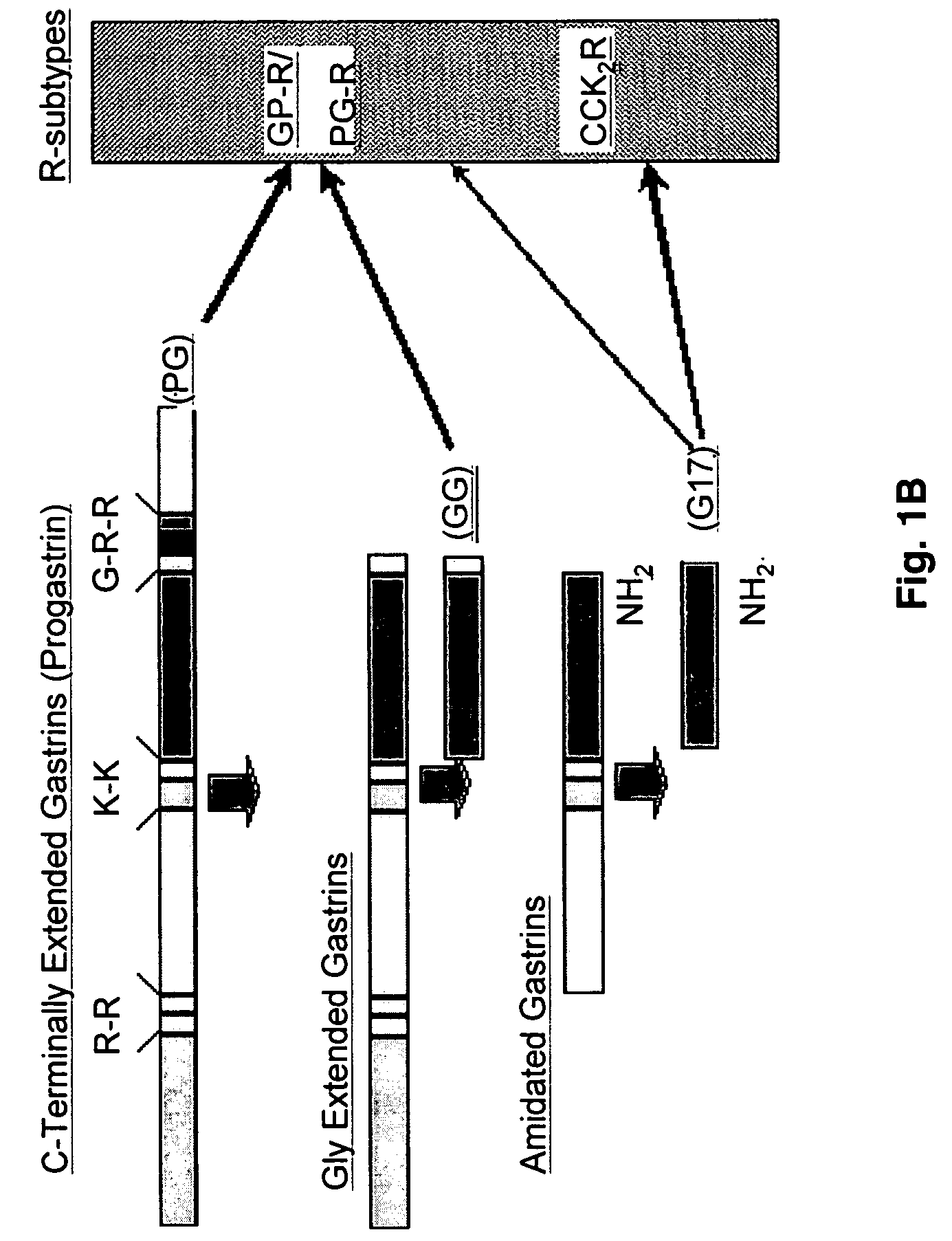
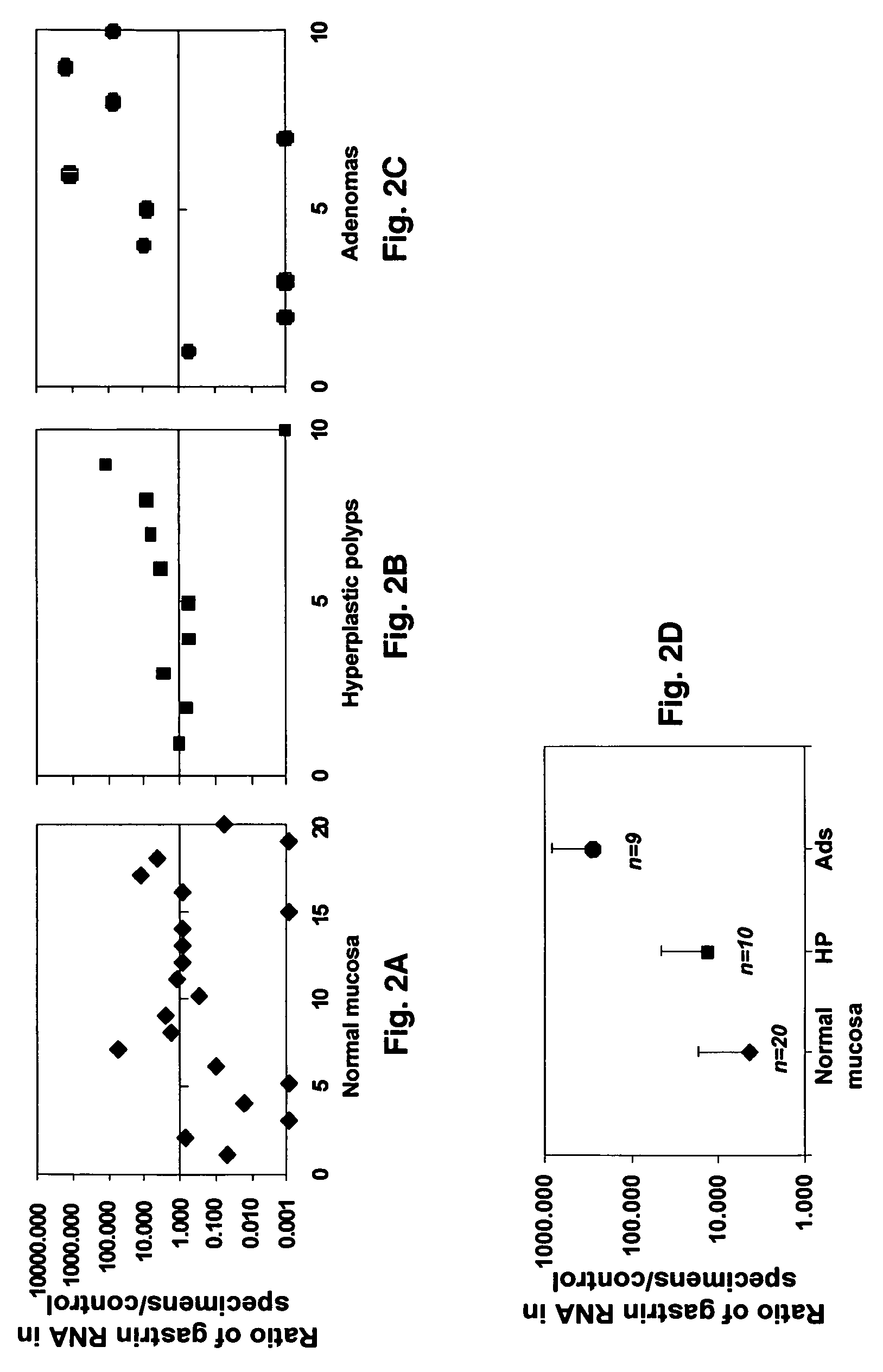
Gastrin is a peptide hormone that stimulates secretion of gastric acid (HCl) by the parietal cells of the stomach and aids in gastric motility. It is released by G cells in the pyloric antrum of the stomach, duodenum, and the pancreas.
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
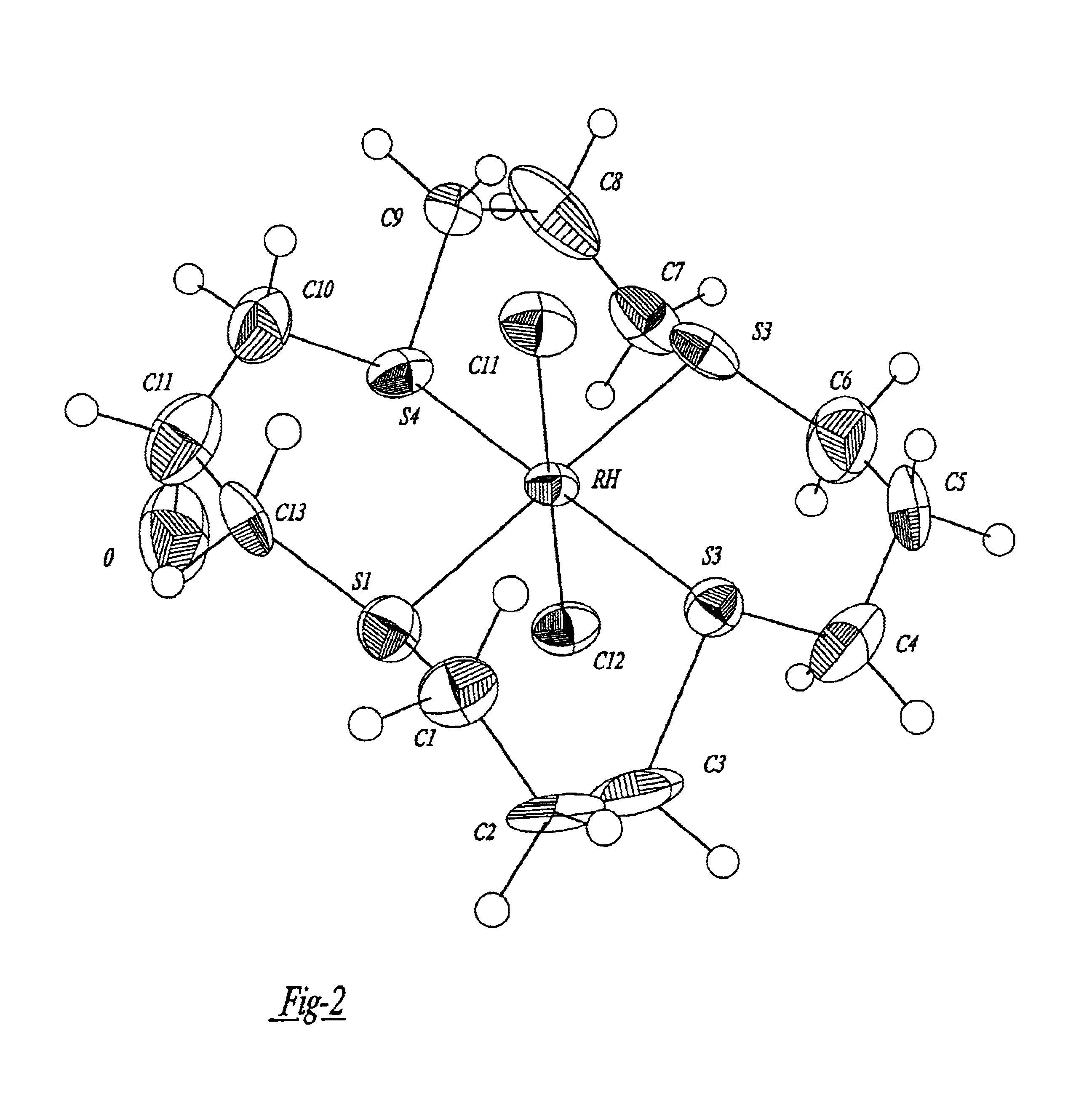
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
Immunogenic compositions to the CCK-B/gastrin receptor and methods for the treatment of tumors
InactiveUS6548066B1Inhibiting autocrine growth-stimulatory pathwayEffectively prevent the binding of the peptide hormones to the receptorsPeptide/protein ingredientsReceptors for hormonesTissue biopsyPassive Immunizations
The invention concerns immunogens, immunogenic compositions and method for the treatment of gastrin-dependent tumors. The immunogens comprise a peptide from the CCK-B / gastrin-receptor conjugated to a spacer and to an immunogenic carrier. The immunogens are capable of inducing antibodies in vivo which bind to the CCK-B / gastrin-receptor in tumor cells, thereby preventing growth stimulating peptide hormones from binding to the receptors, and inhibiting tumor cell growth. The immunogens also comprise antibodies against the CCK-B / gastrin-receptor for passive immunization. The invention also concerns diagnostic methods for detecting gastrin-dependent tumors in vivo or from a tissue biopsy using the antibodies of the invention.
Owner:CANCER ADVANCES INC
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS20060058219A1Improve efficiencyEliminate side effectsBiocidePeptide/protein ingredientsTryptophanSaccharin
A compound is provided that has the formula NH2CH2CH2CH2C(O)N—R (I) where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
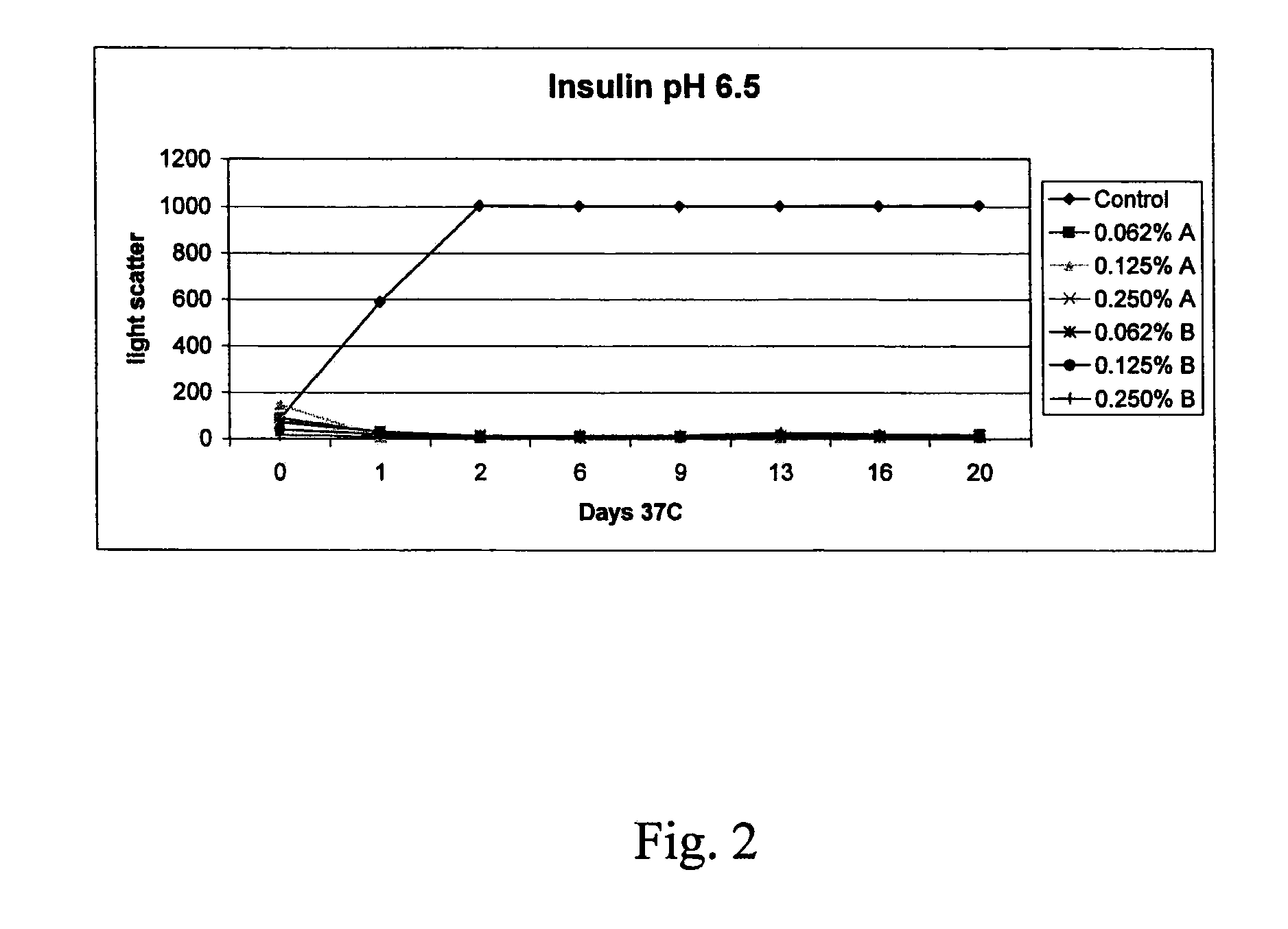
Stabilizing Alkylglycoside Compositions and Methods Thereof
ActiveUS20080268032A1Convenient treatmentReduce aggregationBiocidePeptide/protein ingredientsGastrin-releasing peptidePeptide T
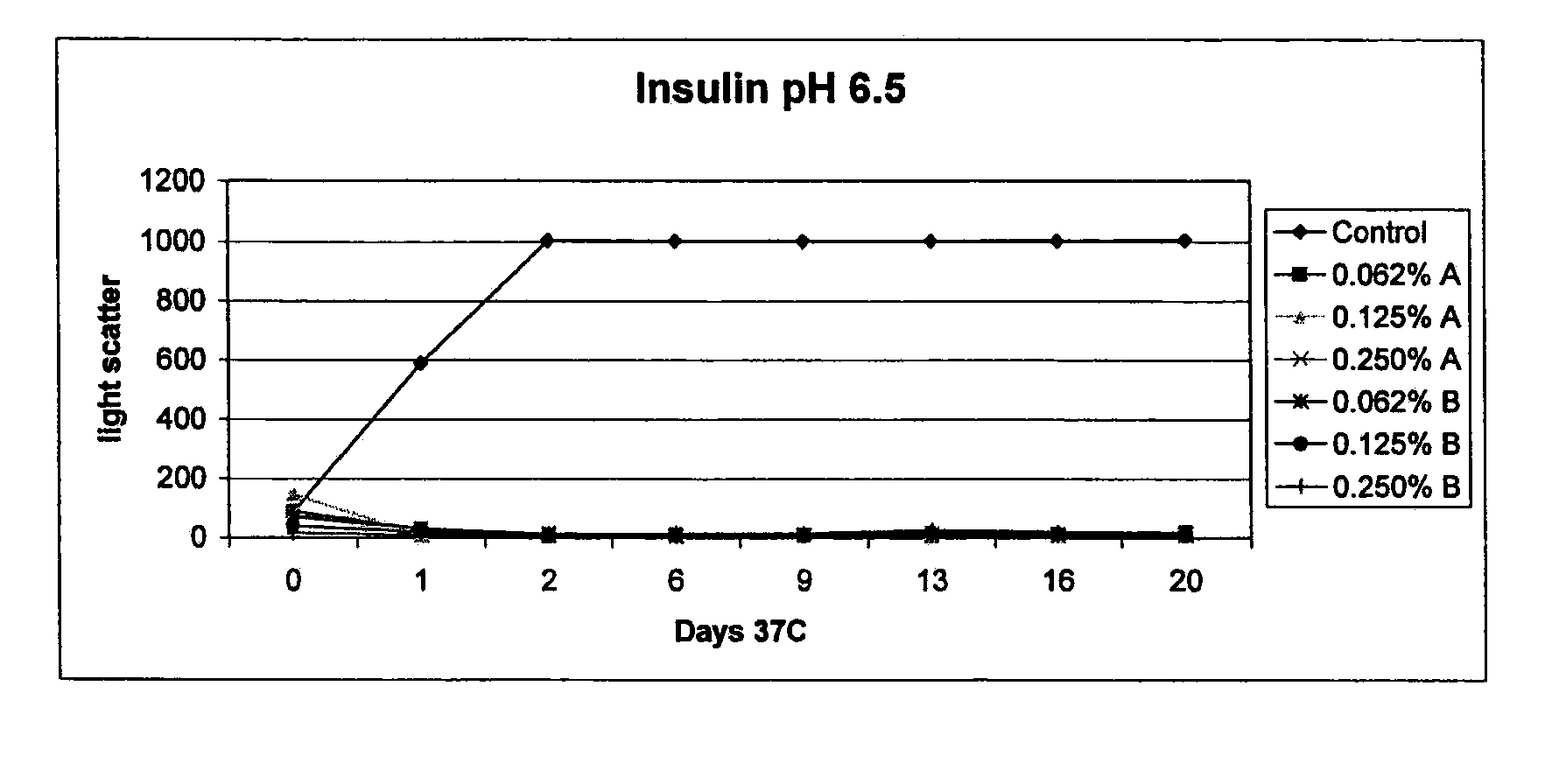
The present invention relates to alkylglycoside-containing compositions and methods for increasing the stability, reducing the aggregation and immunogenicity, increasing the biological activity, and reducing or preventing fibrillar formation of a peptide, polypeptide, or variant thereof, for example amylin, a monoclonal antibody, insulin, Peptide T or analog thereof, gastrin, gastrin releasing peptides, gastrin releasing peptide-like (GRP) proteins, epidermal growth factor or analog thereof.
Owner:AEGIS THERAPEUTICS LLC
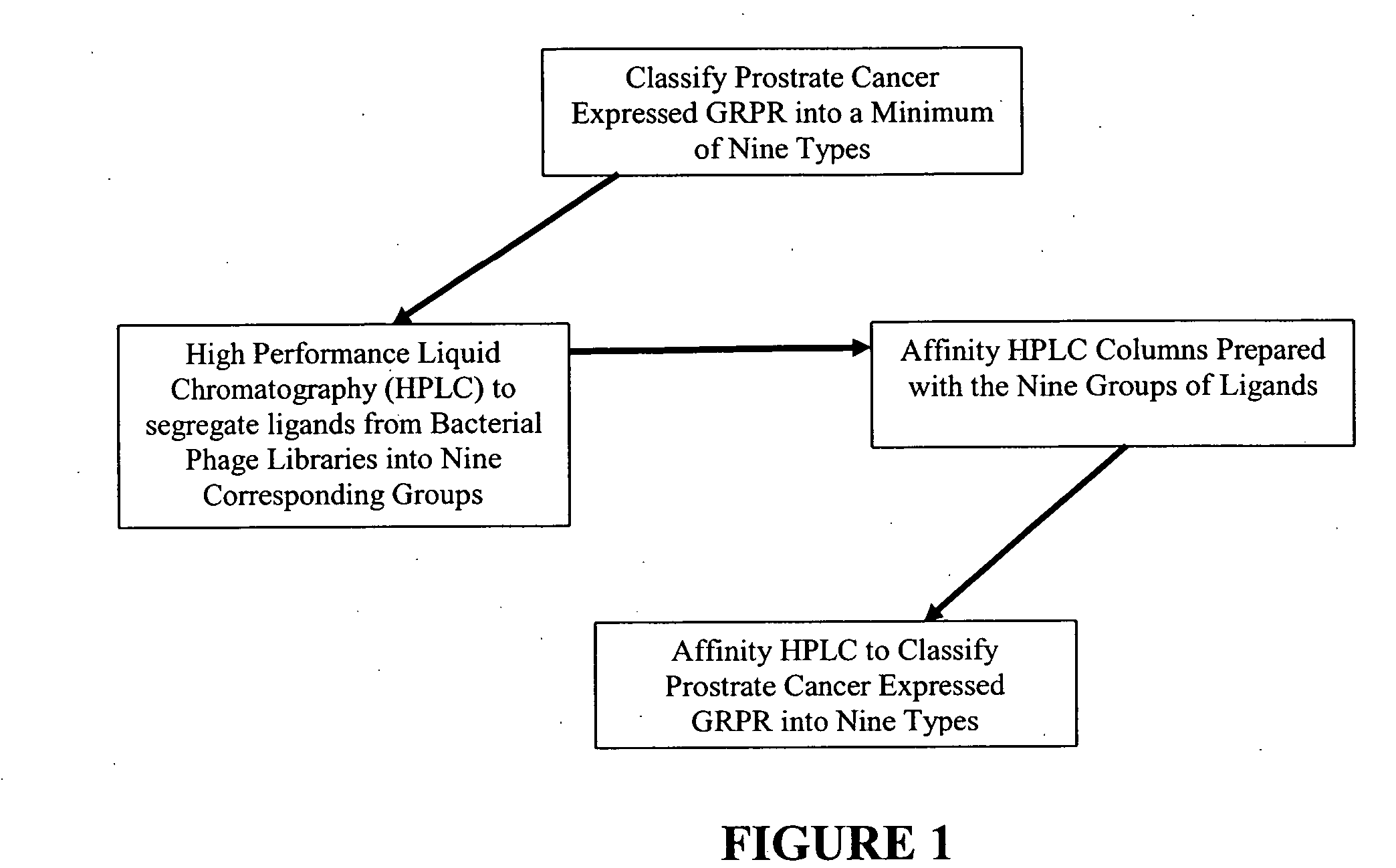
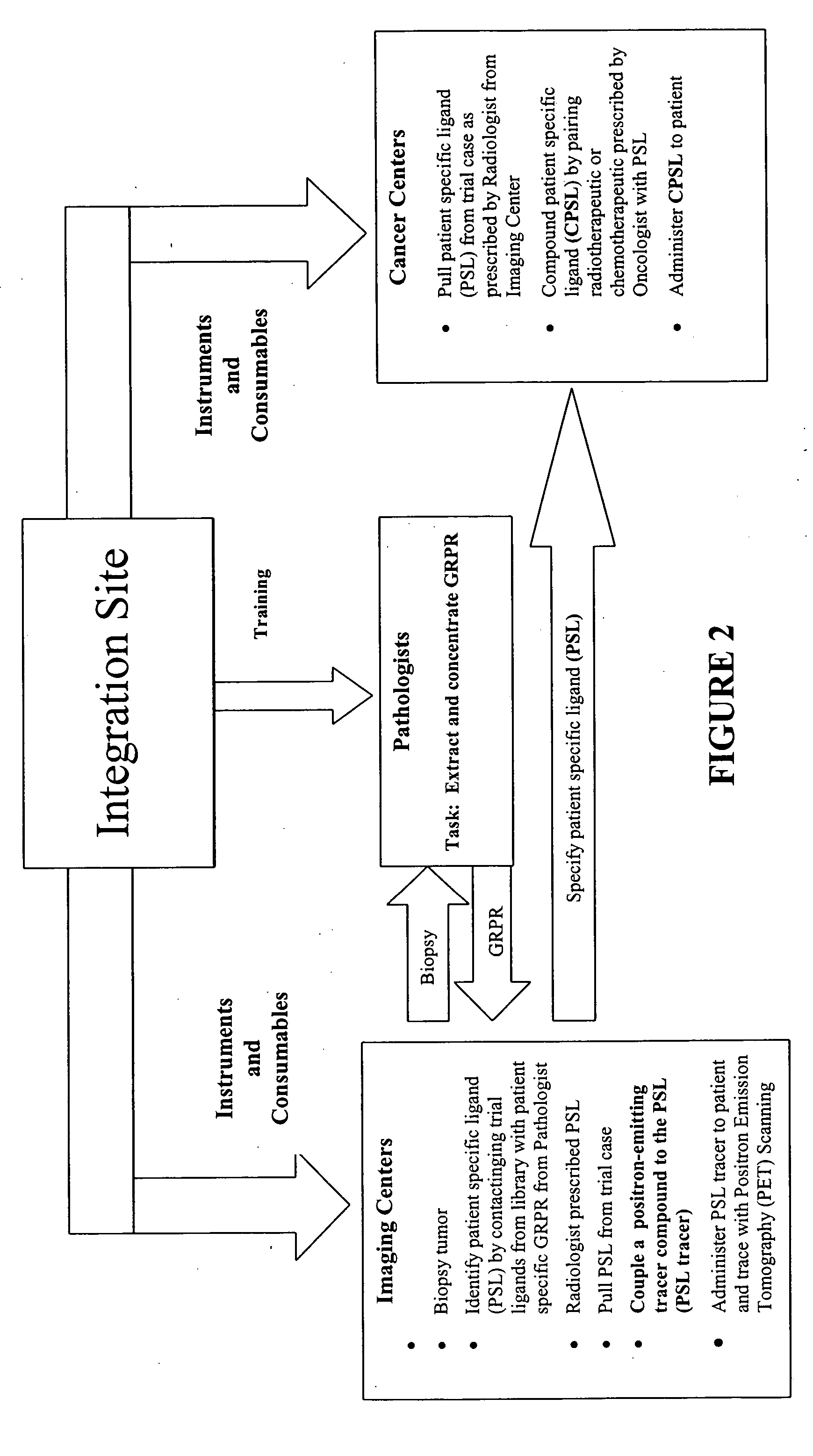
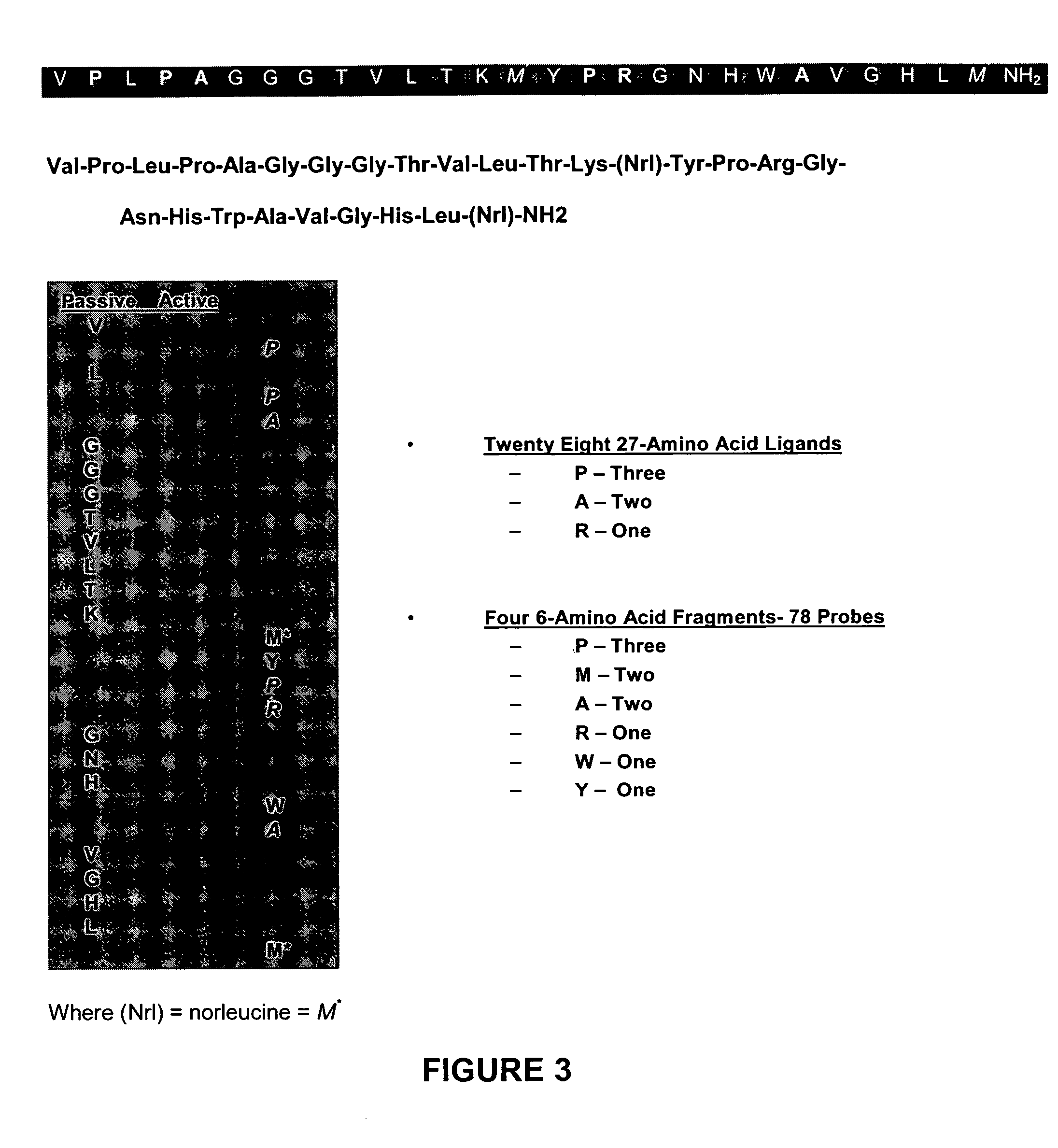
Method, compositions and classification for tumor diagnostics and treatment
InactiveUS20060160157A1Quick and easy determinationImprove survivalCompound screeningApoptosis detectionAbnormal tissue growthSide effect
The present invention is directed towards classifying tumor biomarkers, particularly membrane receptors, and more particularly the gastrin-releasing peptide (GPR) receptors, identified in patient samples, then linking therapeutic agents (chemical, radiological, or biological) to patient-specific ligands that bind to such receptors, clinicians can produce diagnostic and treatment compositions and implement treatment regimens which, by using the classified and identified biomarkers, and due to their improved accuracy, increase success and decrease undesired side effects from such treatments.
Owner:ZUCKERMAN MATHEW MARK
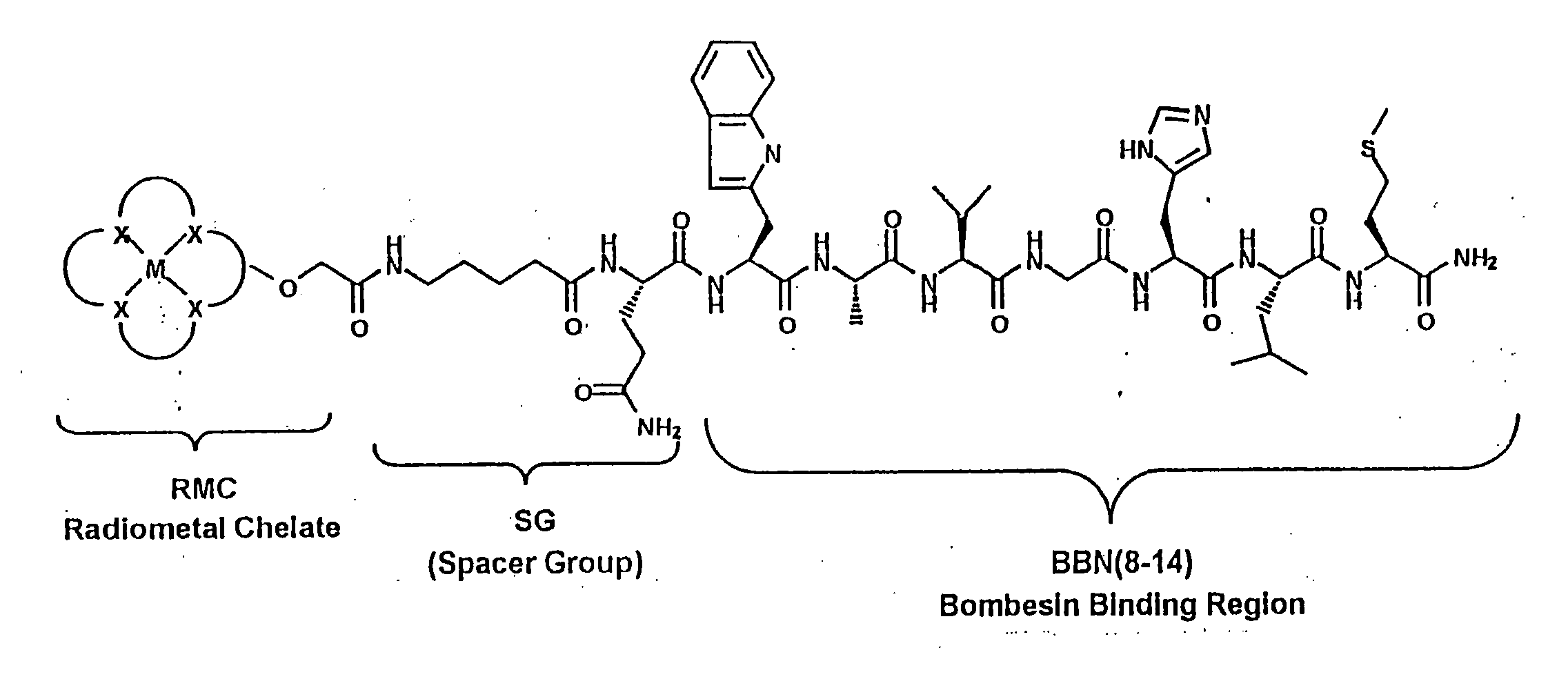
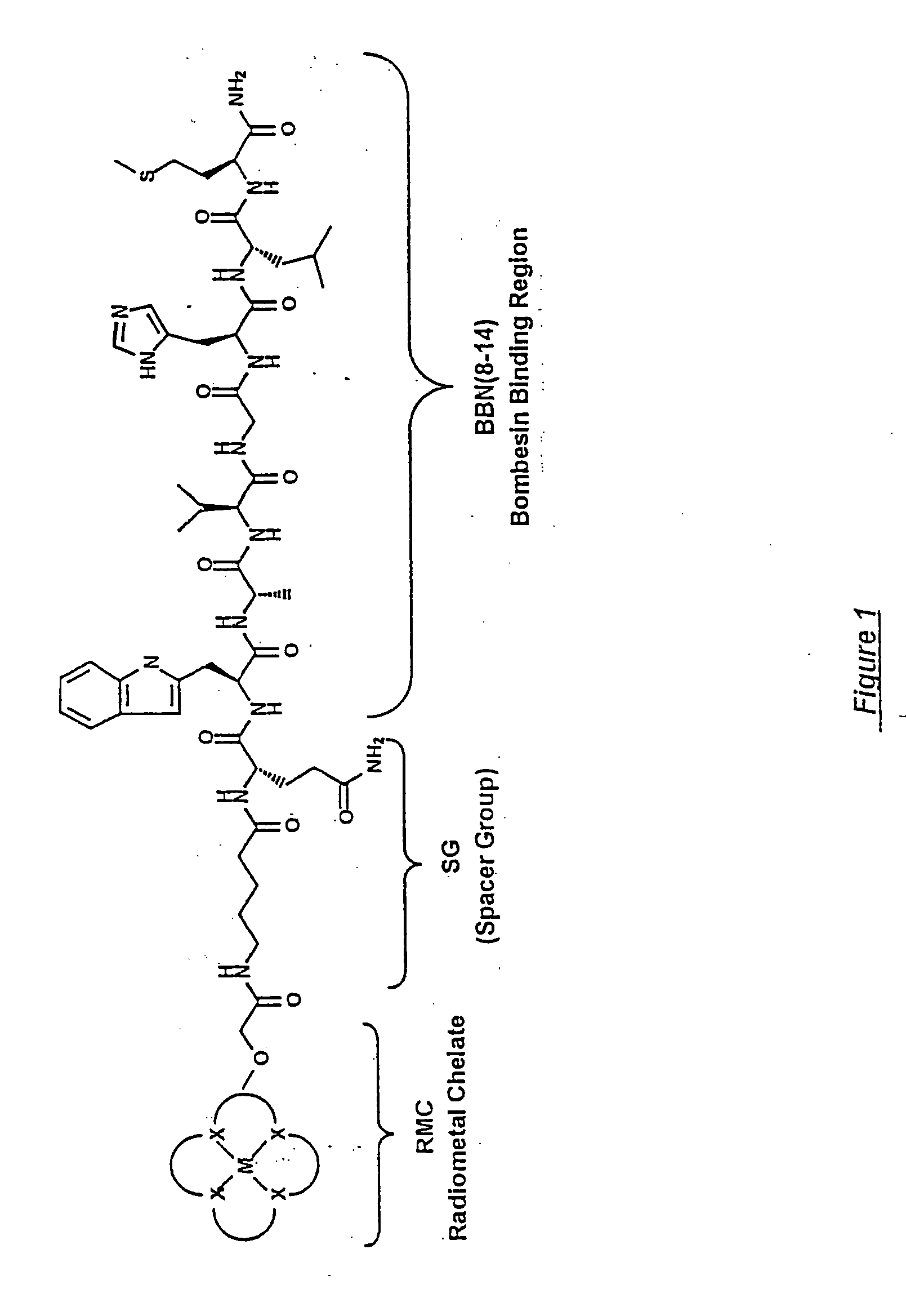
Gastrin Releasing Peptide Compounds
InactiveUS20080008649A1Improve targetingDecreasing aberrant vascular permeabilityRadioactive preparation carriersGastrin releasing peptideCholic acidTherapeutic Hormone
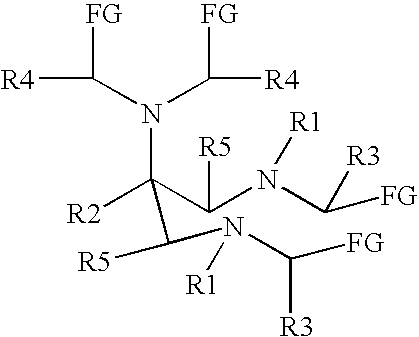
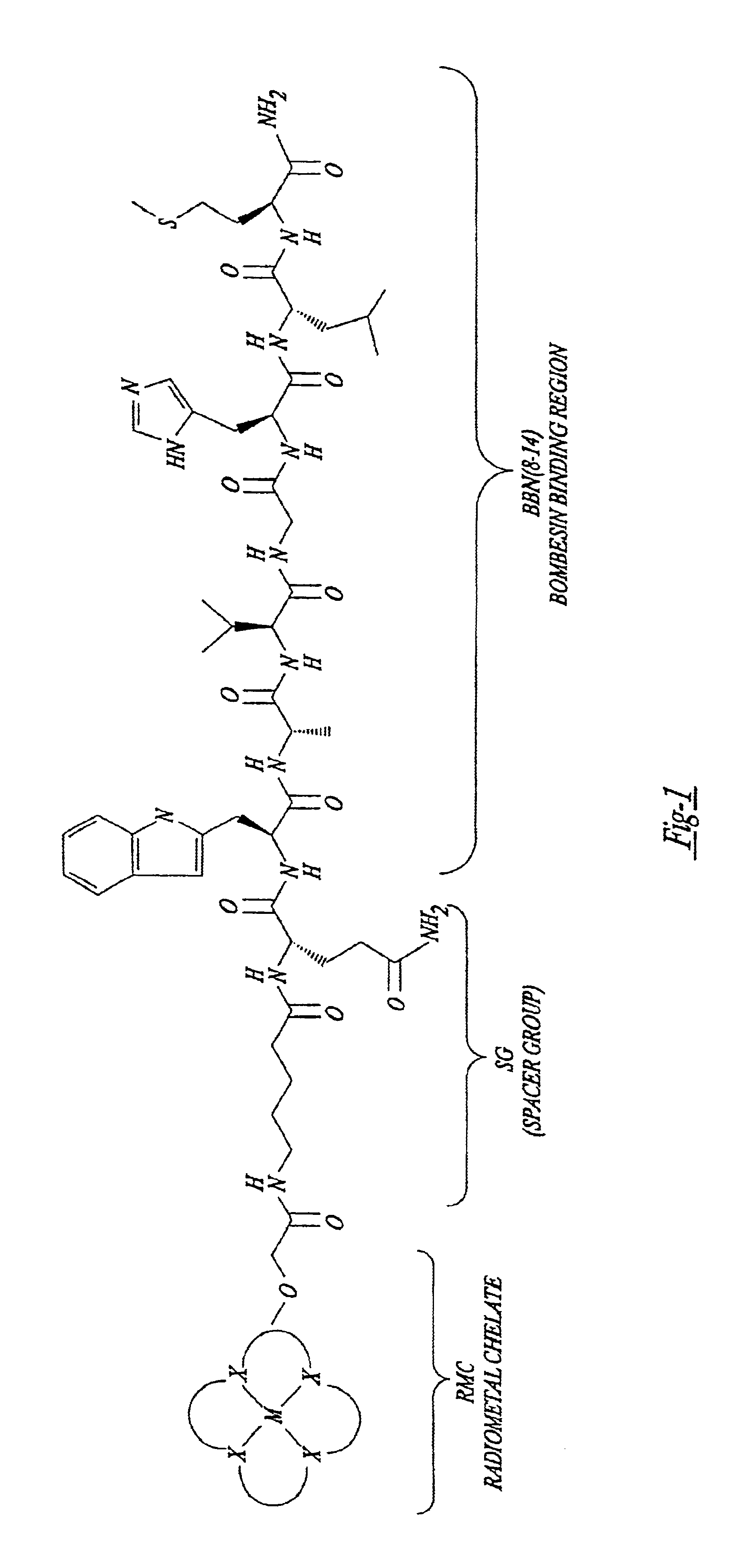
New and improved compounds for use in diagnostic imaging or therapy having the formula M-N—O—P-G, wherein M is a metal chelator having the structure: wherein R1-R5 and FG are as defined herein (in the form complexed with a metal radionuclide or not), N—O—P is the linker containing at least one non-alpha amino acid with a cyclic group, at least one substituted bile acid or at least one non-alpha amino acid, and G is the GRP receptor targeting peptide. In the preferred embodiment, M is an Aazta metal chelator or a derivative thereof. Methods for imaging a patient and / or providing radiotherapy or phototherapy to a patient using the compounds of the invention are also provided. Methods and kits for preparing a diagnostic imaging agent from the compound is further provided. Methods and kits for preparing a radiotherapeutic agent are further provided. Novel methods of treating prostate tumors or of delaying the progression of prostate tumors are also provided, including, methods of treating bone or soft tissue metastases of prostate cancer, methods for treating hormone sensitive and hormone refractory prostate cancer, methods for delaying the progression of hormone sensitive prostate cancer, for facilitating combination therapy in patients with hormone sensitive prostate cancer and for decreasing aberrant vascular permeability in patients with hormone sensitive prostate cancer.
Owner:BRACCO IMAGINIG SPA
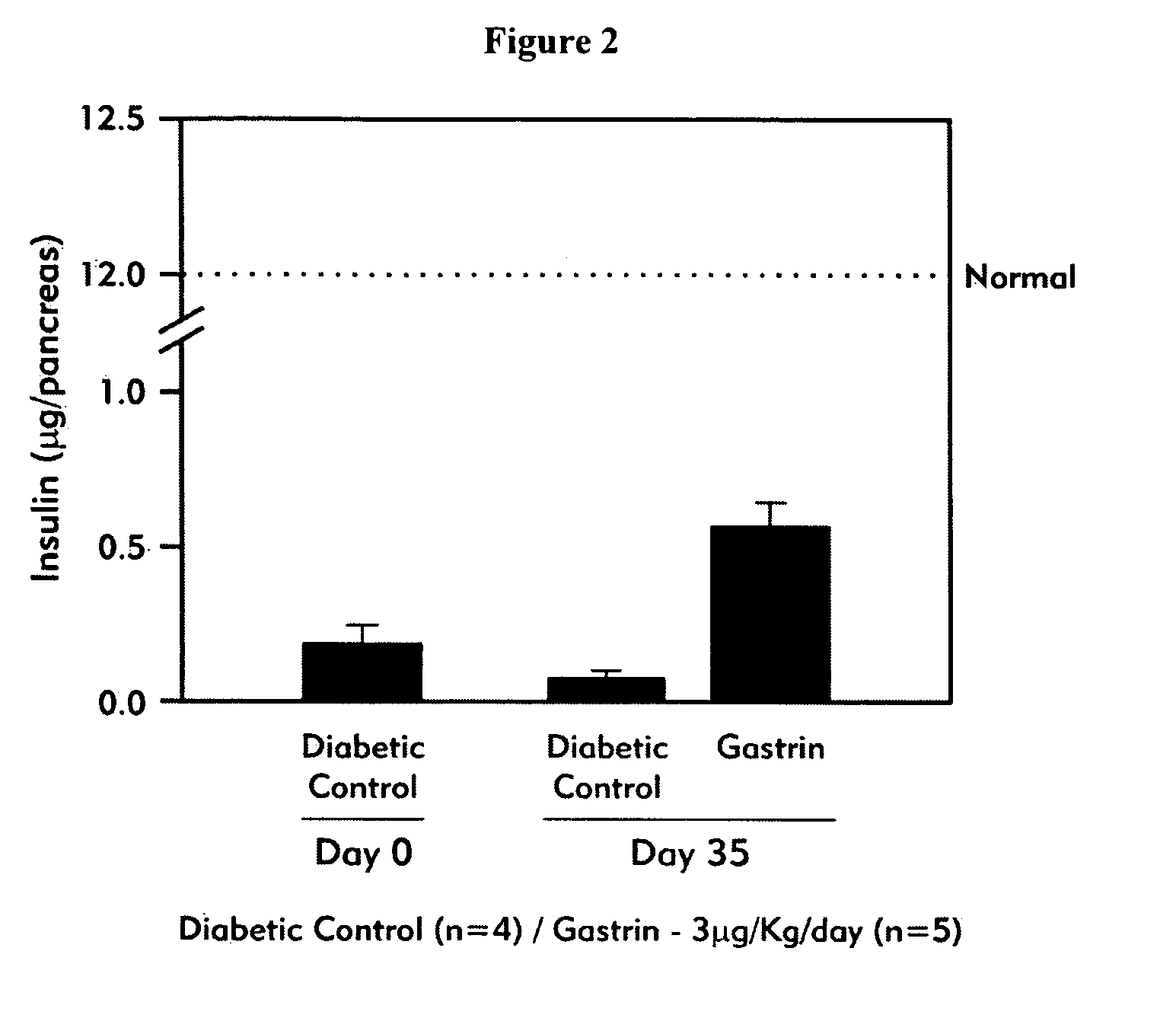
Compositions and methods for treating diabetes
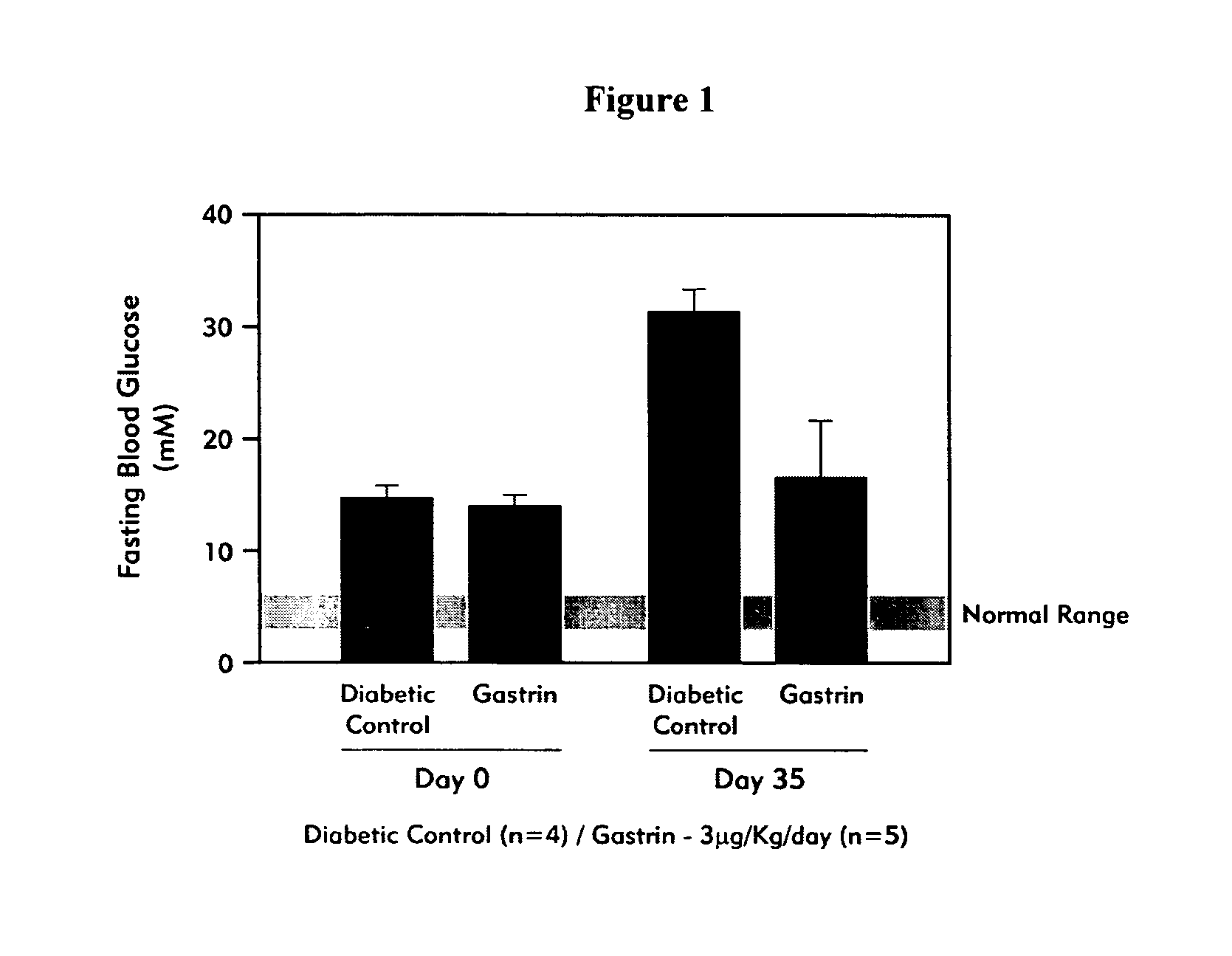
Compositions and methods for islet neogenesis therapy comprising an EGF and a gastrin in combination with immune suppression, and for treating or preventing early stage diabetes with a gastrin / CCK receptor ligand and an immunosuppressant are provided.
Owner:WARATAH PHARMA INC
Liposomal vaccine
InactiveUS20050169979A1High weight ratioHigh encapsulation efficiencyPeptide/protein ingredientsMicroencapsulation basedHormones regulationLiposome
The invention provides liposomal vehicles for encapsulating relatively high levels of immunogenic protein substances including immunogens directed against hormones and hormone receptors, such as gastrin and gonadotropin releasing hormone and their receptors. The liposome encapsulating large amounts of immunogens can be injected parenterally to induce effective immune responses without exhibiting significant adverse tissue reactogenicity. Methods for production of the liposomal vaccines and methods of their administration for treatment of diseases and conditions associated with the cognate hormones are also provided.
Owner:RECEPTOR BIOLOGIX +1
Gastrin releasing peptide compounds
InactiveUS7226577B2Peptide/protein ingredientsDigestive systemGastrin-releasing peptideImaging agent
New and improved compounds for use in radiodiagnostic imaging or radiotherapy having the formula M-N-O-P-G, wherein M is the metal chelator (in the form complexed with a metal radionuclide or not), N-O-P is the linker, and G is the GRP receptor targeting peptide. Methods for imaging a patient and / or providing radiotherapy to a patient using the compounds of the invention are also provided. A method for preparing a diagnostic imaging agent from the compound is further provided. A method for preparing a radiotherapeutic agent is further provided.
Owner:BRACCO IMAGINIG SPA
Gastrin releasing peptide compounds
InactiveUS20060018830A1Minimizing interference signalHigh quantum yieldBiocideCarbamic acid derivatives preparationGastrin-releasing peptideImaging agent
New and improved compounds for use in diagnostic imaging or therapy having the formula M-N-O-P-G, wherein M is a metal chelator having the structure: wherein R1-R5 and FG are as defined herein (in the form complexed with a metal radionuclide or not), N-O-P is the linker containing at least one non-alpha amino acid with a cyclic group, at least one substituted bile acid or at least one non-alpha amino acid, and G is the GRP receptor targeting peptide. In the preferred embodiment, M is an Aazta metal chelator or a derivative thereof. Methods for imaging a patient and / or providing radiotherapy or phototherapy to a patient using the compounds of the invention are also provided. Methods and kits for preparing a diagnostic imaging agent from the compound is further provided. Methods and kits for preparing a radiotherapeutic agent are further provided.
Owner:BRACCO IMAGINIG SPA
Treatment of diabetes
InactiveUS20060189520A1Increased proliferationReduce the amount requiredPeptide/protein ingredientsTissue cultureDiabetes mellitusIslet neogenesis
Compositions and methods are provided for islet neogenesis therapy comprising a member of a group of factors that complement a gastrin / CCK receptor ligand, with formulations, devices and methods for sustained release delivery and for local delivery to target organs.
Owner:WARATAH PHARMA INC
Gastrin compositions and formulations, and methods of use and preparation
InactiveUS20040266682A1Peptide/protein ingredientsGenetic material ingredientsFunctional abilitiesDiabetic patient
An embodiment of the invention provided herein is a pharmaceutical composition comprising a gastrin compound having an extended activity upon administration to a subject in comparison with native gastrin. Methods are provided of conjugating portions of the amino acid sequence of gastrin having functional ability to bind to the gastrin / CCK receptor, to various carrier moieties, including the use of amino acid spacer regions, and use of bifunctional cross-linking reagents. Methods of treating a diabetes patient with the compositions are provided.
Owner:WARATAH PHARMA INC
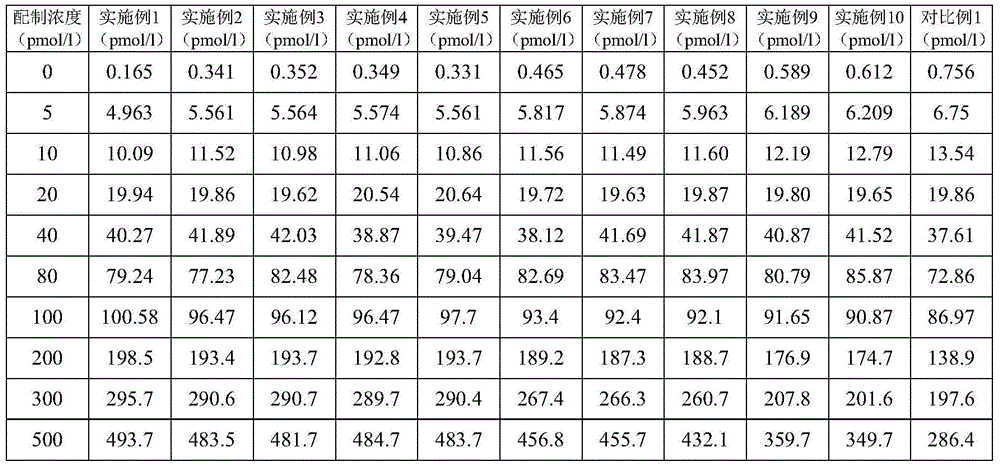
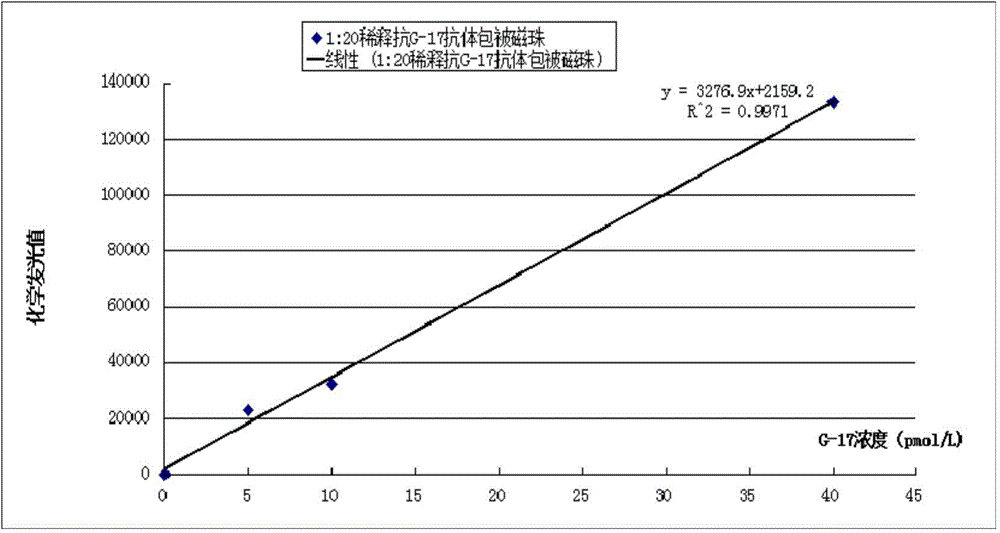
Kit for detecting gastrin-17 and preparation method as well as application thereof
ActiveCN104614536AAccurate measurementSensitive assayChemiluminescene/bioluminescenceBiological material analysisBinding siteAntibody
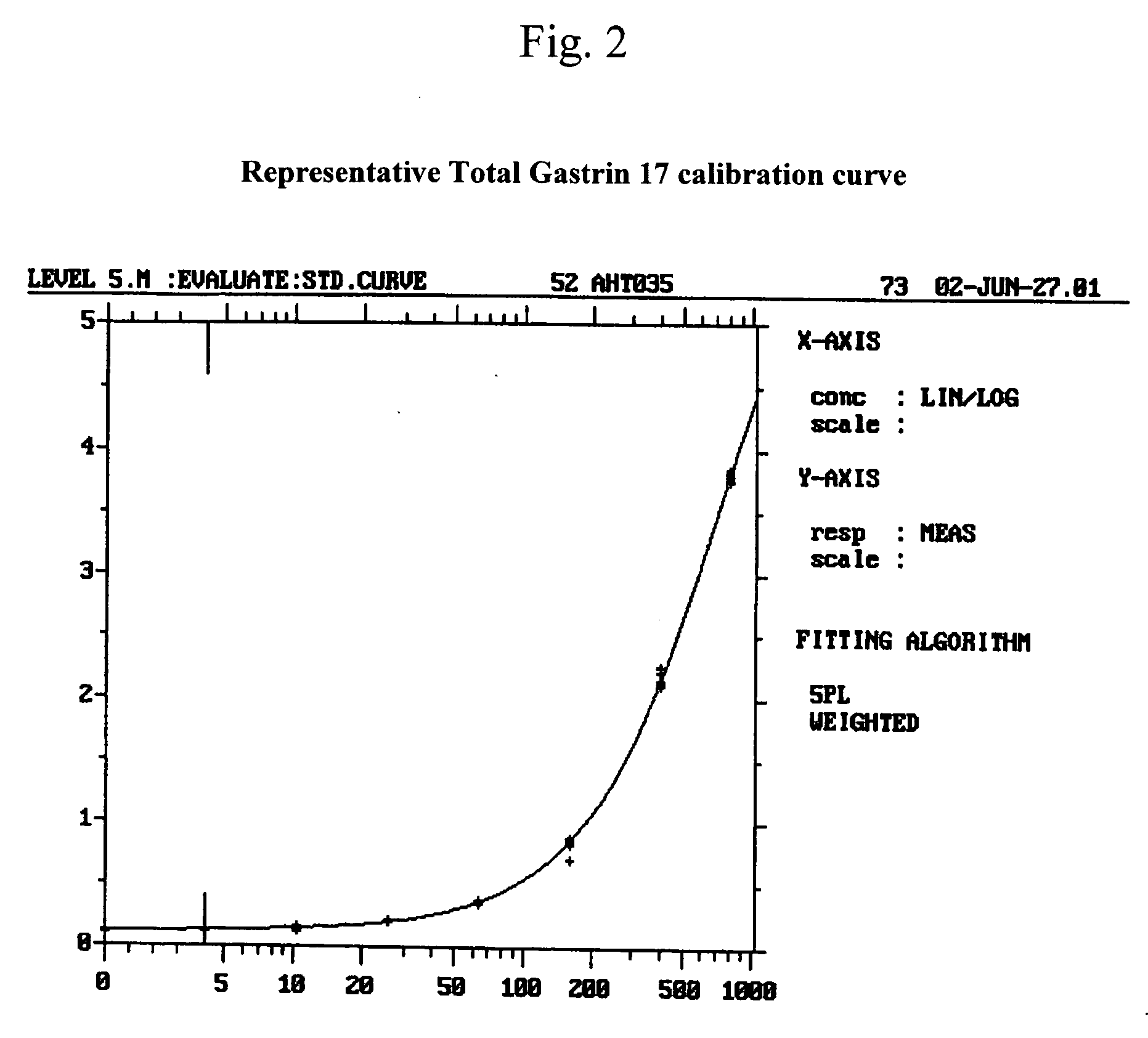
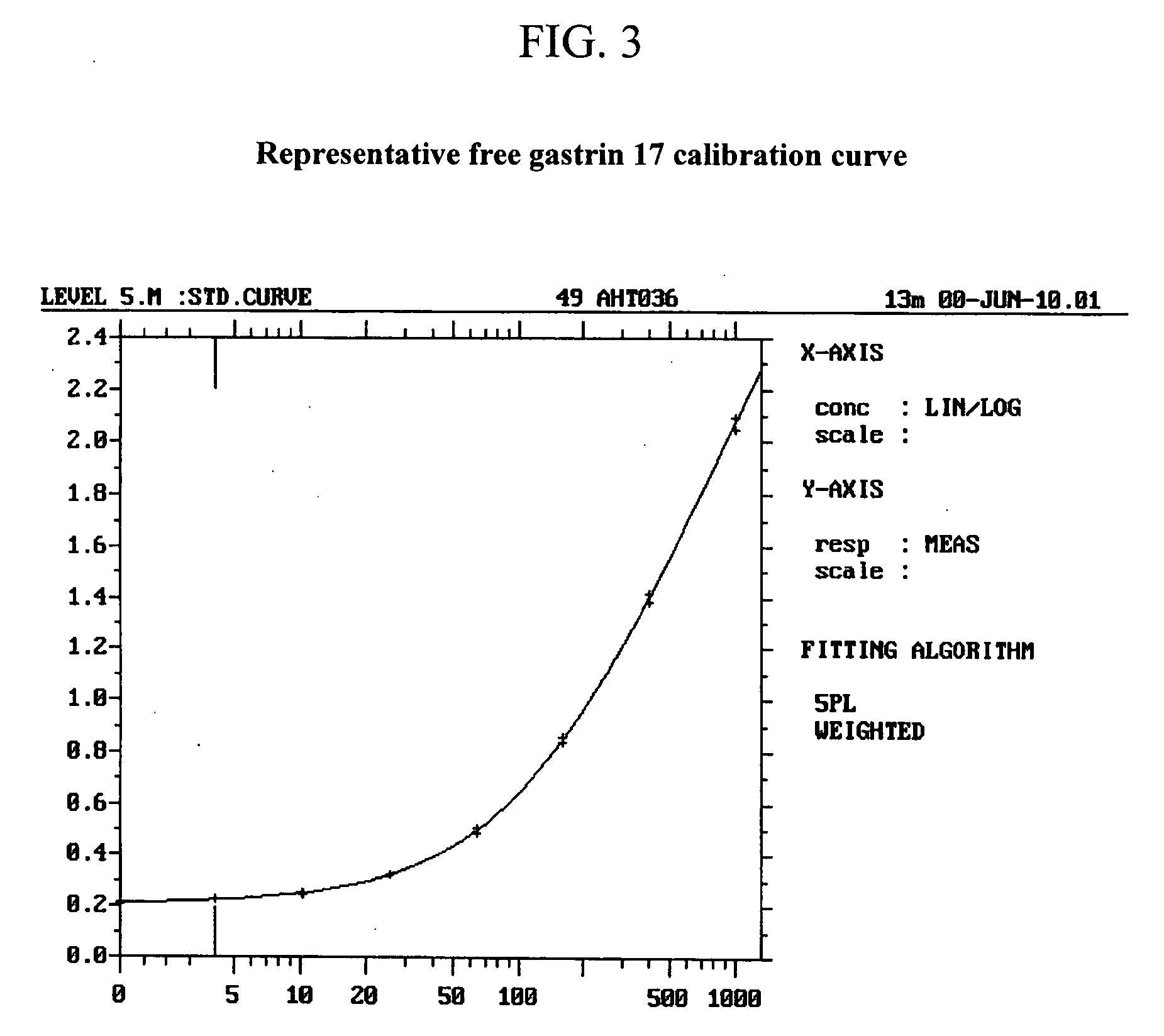
The invention provides a kit for detecting gastrin-17 and a preparation method as well as application thereof. The kit comprises components A and B, wherein the component A is a first anti-gastrin-17 antibody marked with a tracing marker or coated with a magnetic ball, and the component B is a second anti-gastrin-17 antibody coated with the magnetic ball or marked with the tracing marker, or the gastrin-17; moreover, any one of the components A and B is marked with the tracing marker, the other one is coated with the magnetic ball; and the first anti-gastrin-17 antibody and the second anti-gastrin-17 antibody have different binding sites with the gastrin-17. The invention provides a method for detecting the gastrin-17, with the kit provided by the invention, content of the gastrin-17 in a sample can be detected accurately and sensitively according to a double antibody sandwich method principle or a competition method principle.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Stabilizing alkylglycoside compositions and methods thereof
ActiveUS8226949B2Convenient treatmentReduce aggregationBiocideAntipyreticPeptide TGastrin-releasing peptide
The present invention relates to alkylglycoside-containing compositions and methods for increasing the stability, reducing the aggregation and immunogenicity, increasing the biological activity, and reducing or preventing fibrillar formation of a peptide, polypeptide, or variant thereof, for example parathyroid hormone (PTH) or PTH analogs, amylin, a monoclonal antibody, insulin, Peptide T or analog thereof, gastrin, gastrin releasing peptides, gastrin releasing peptide-like (GRP) proteins, epidermal growth factor or analog thereof.
Owner:AEGIS THERAPEUTICS LLC
Treatment for diabetes
InactiveUS6558952B1Not prevent and decrease progressionMore complicatedOrganic active ingredientsPeptide/protein ingredientsInsulin Secreting CellEGF Receptors
Methods and compositions for treating diabetes mellitus in a patient in need thereof are provided. The methods include administering to a patient a composition providing a gastrin / CCK receptor ligand, e.g., a gastrin, and / or an epidermal growth factor (EGF) receptor ligand, e.g., TGF-alpha, in an amount sufficient to effect differentiation of pancreatic islet precursor cells to mature insulin-secreting cells. The composition can be administered systemically or expressed in situ by cells transgenically supplemented with one or both of a gastrin / CCK receptor ligand gene, e.g., a preprogastrin peptide precursor gene and an EGF receptor ligand gene, e.g., a TGF-alpha gene. The methods also include transplanting into a patient cultured pancreatic islets in which mature insulin-secreting beta cells are proliferated by exposure to a gastrin / CCK receptor ligand and an EGF receptor ligand.
Owner:THE GENERAL HOSPITAL CORP +1
Monoclonal antibodies to gastrin hormone
InactiveUS20060020119A1Microbiological testing/measurementDigestive systemImmunofluorometric AssaysGlycine extended gastrin
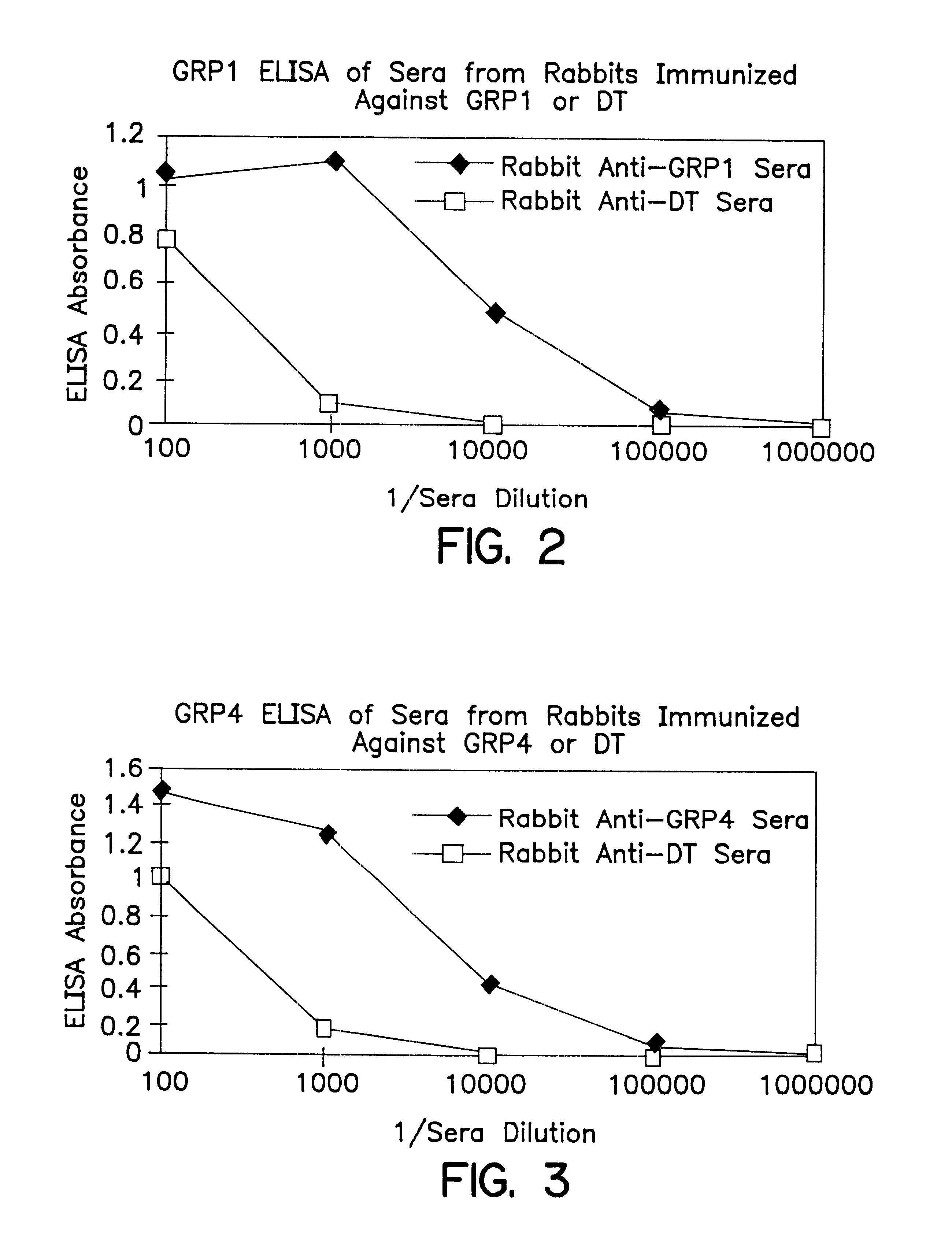
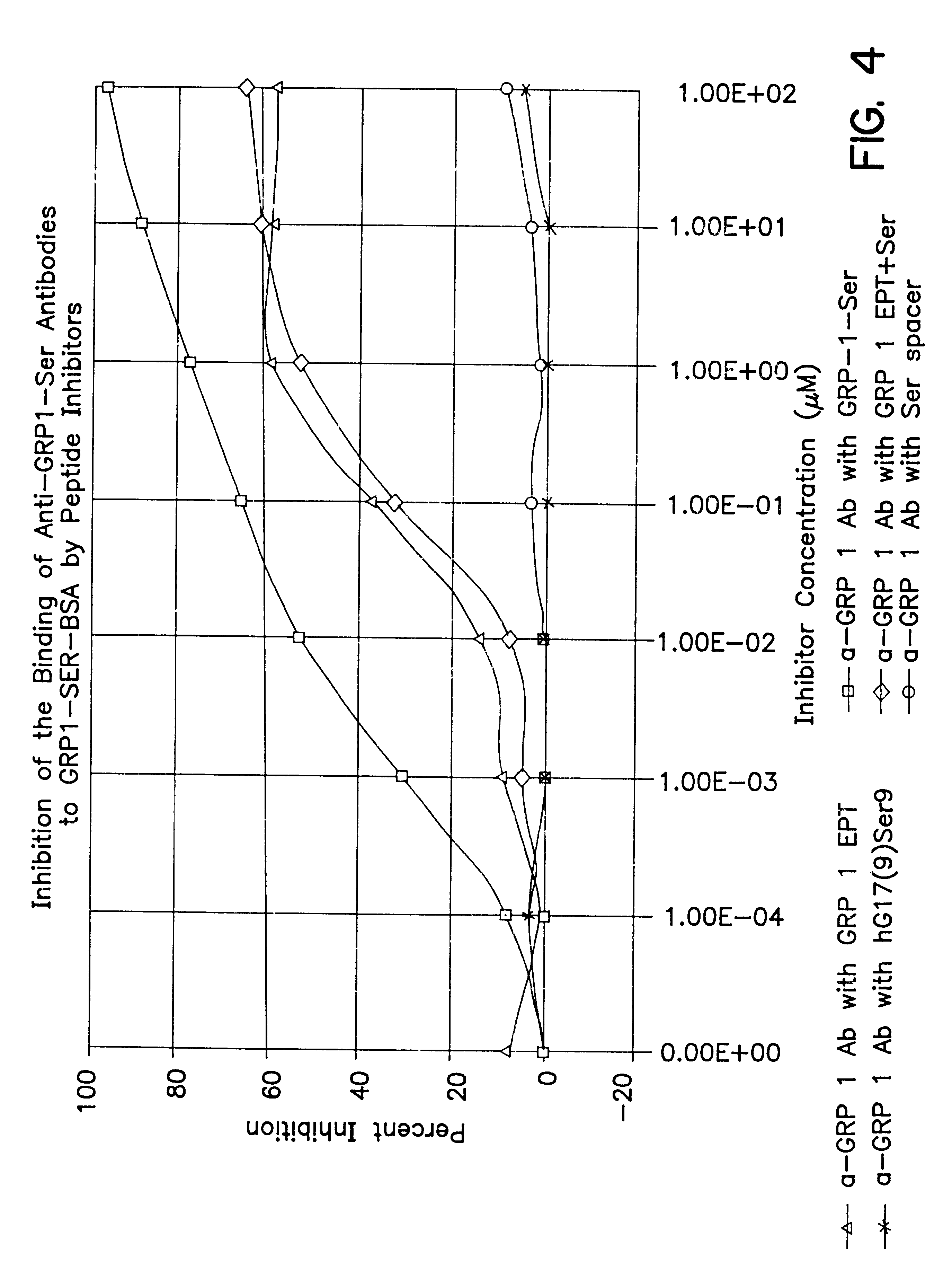
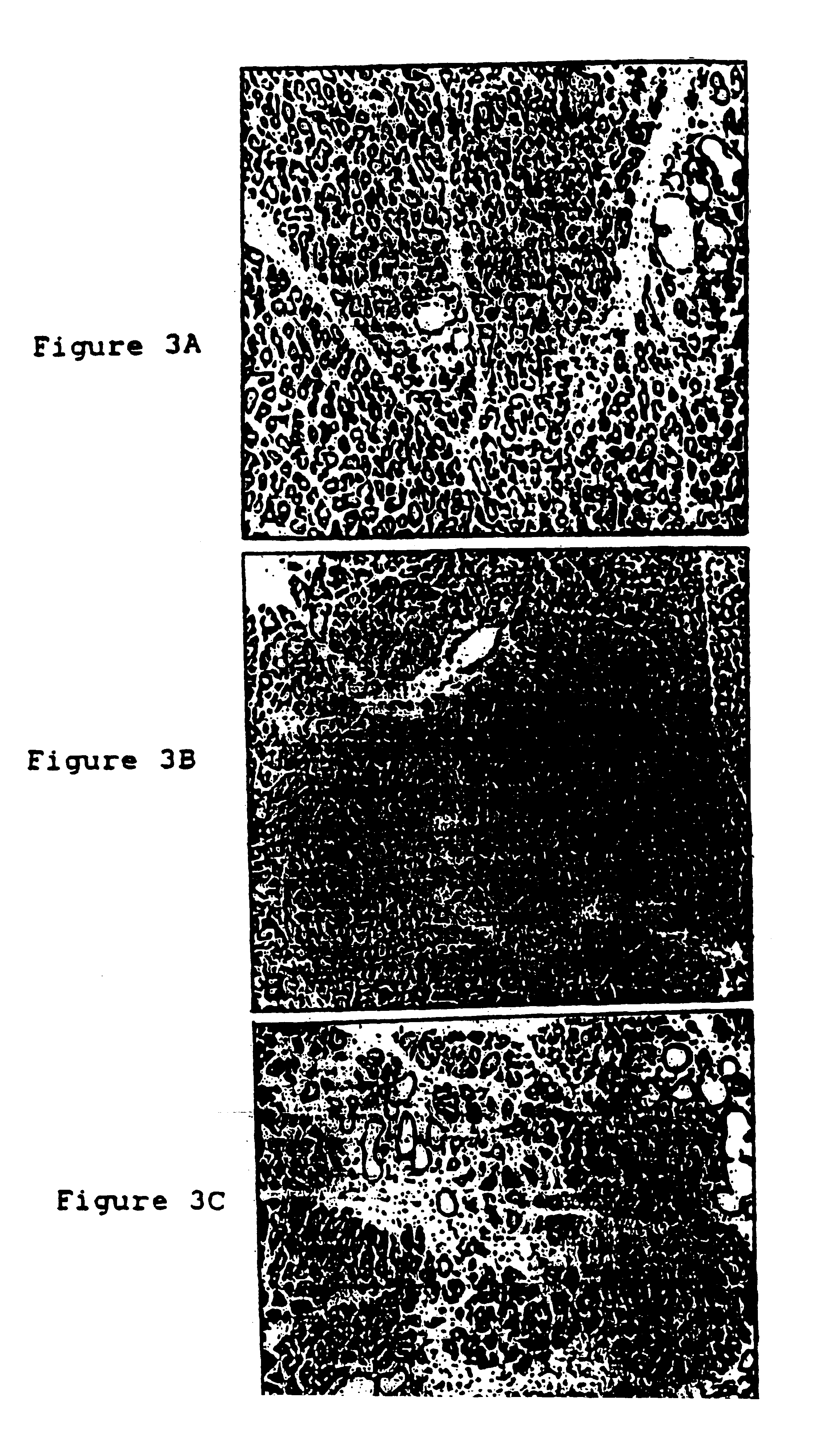
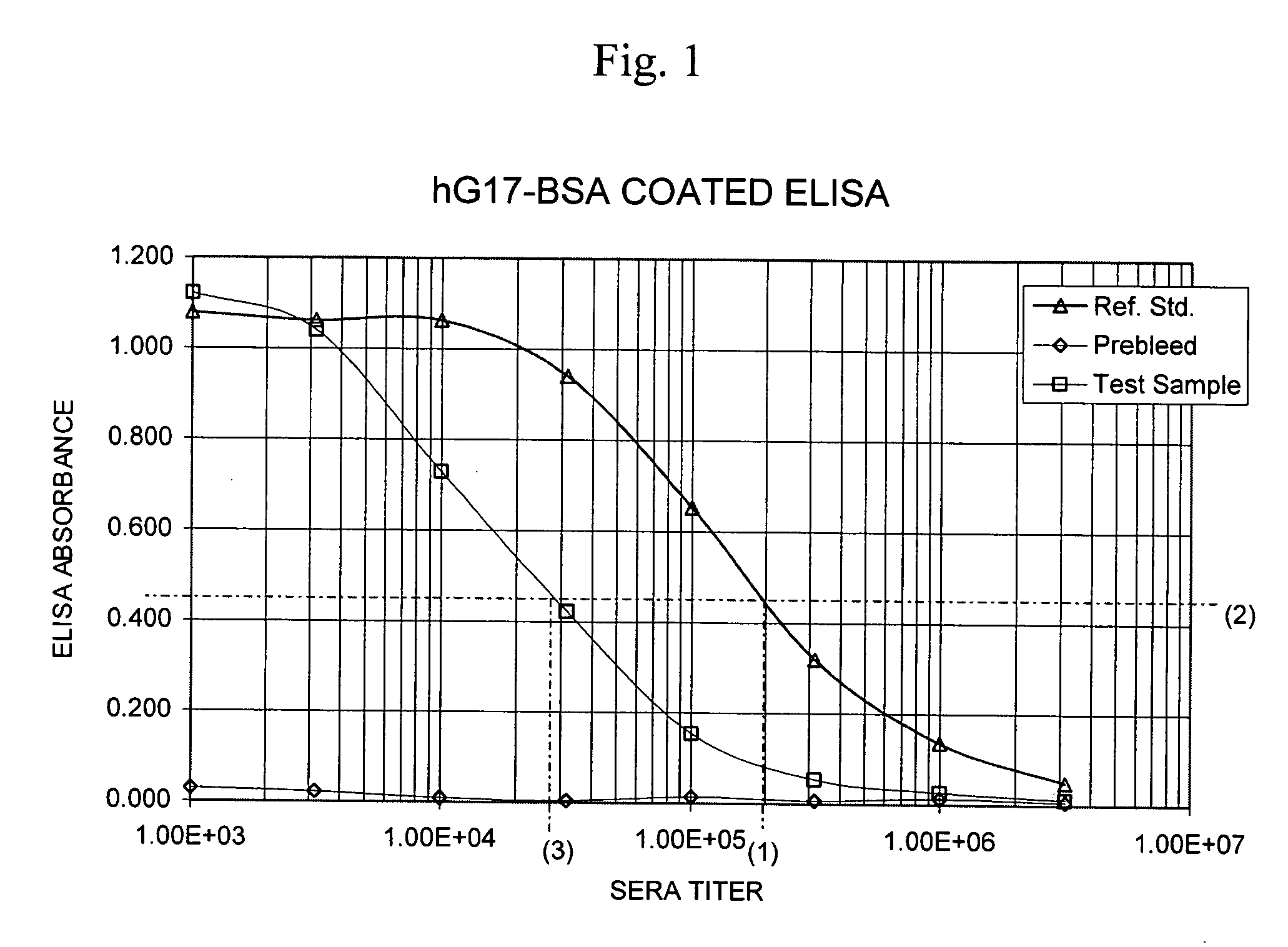
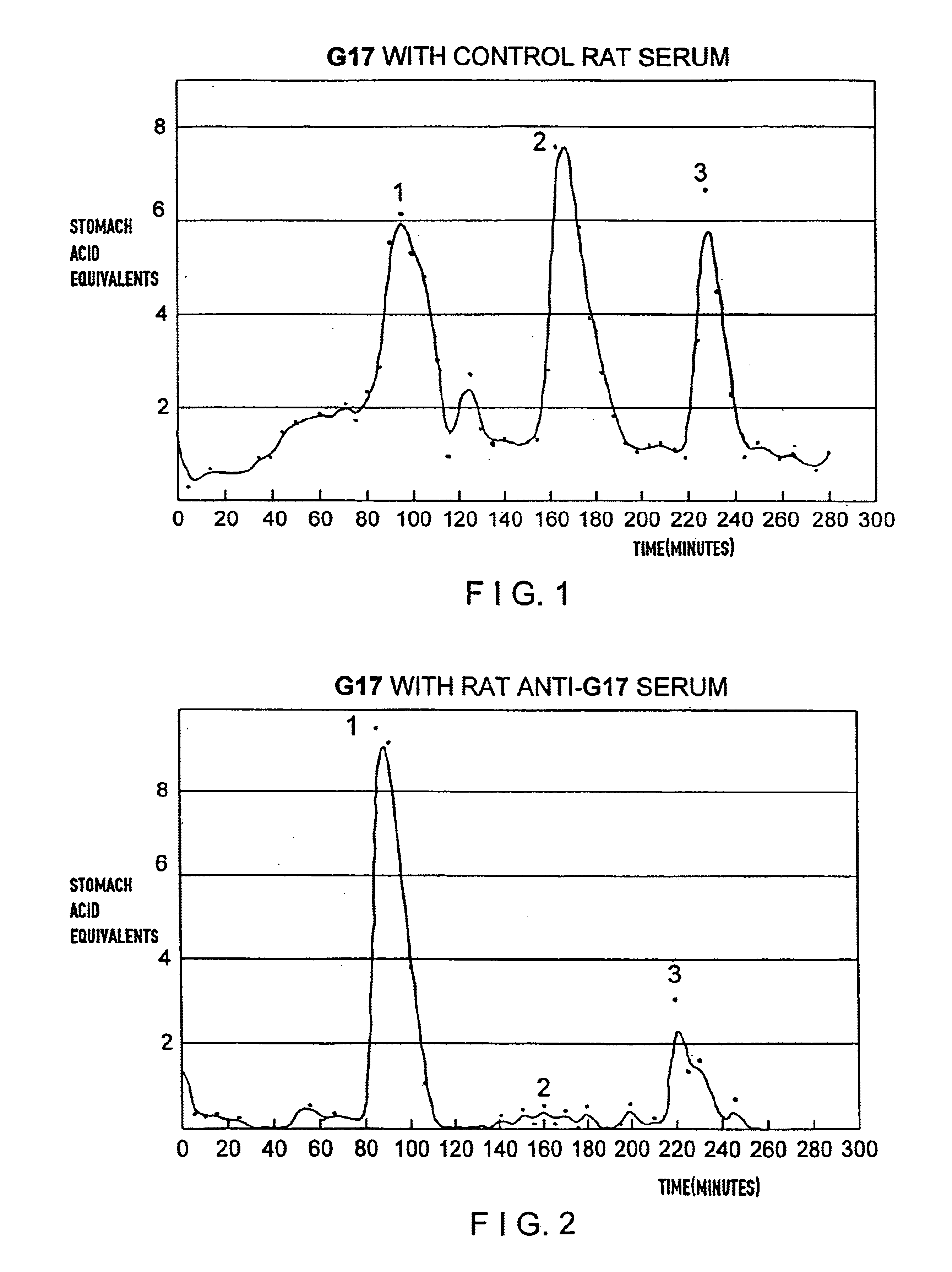
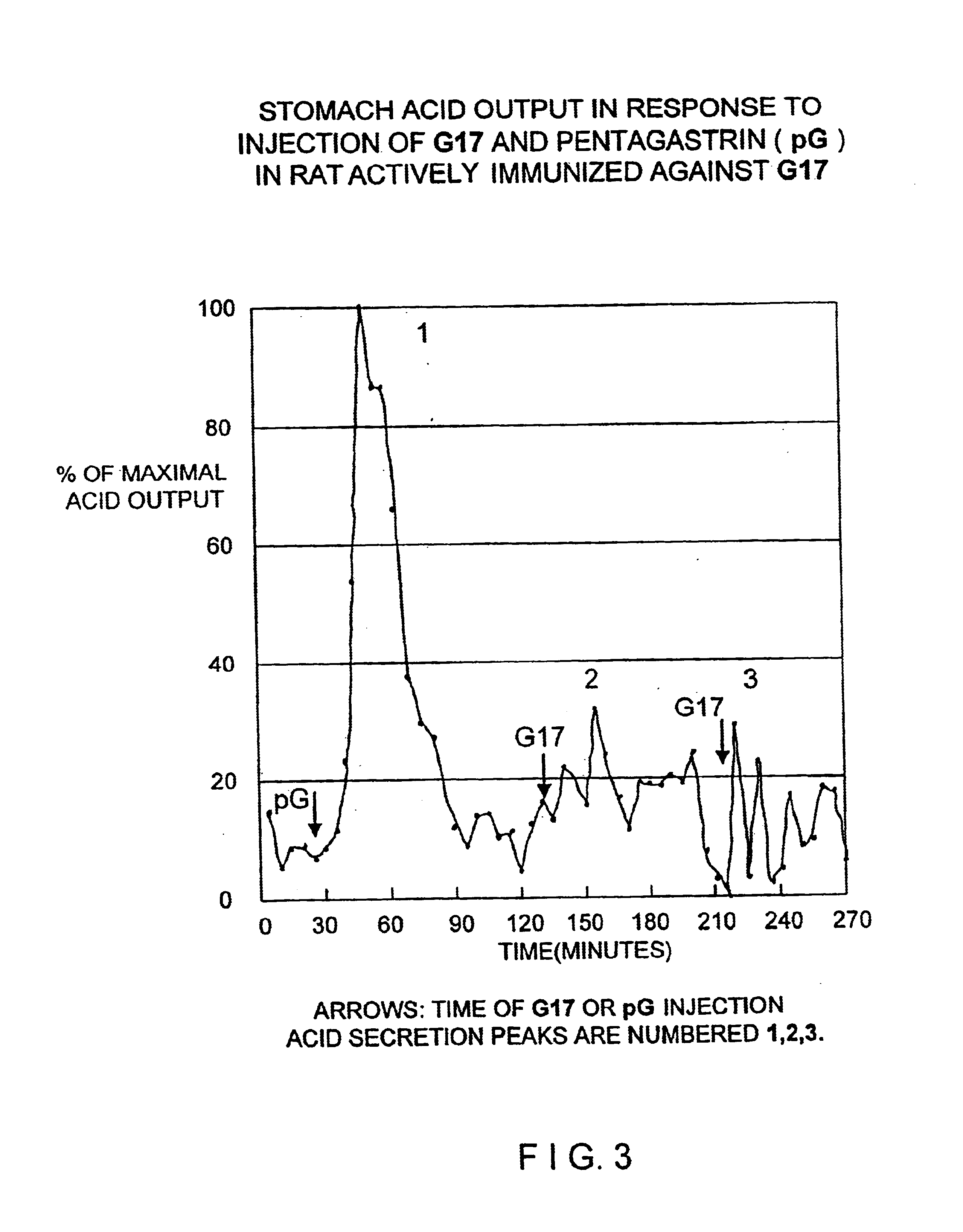
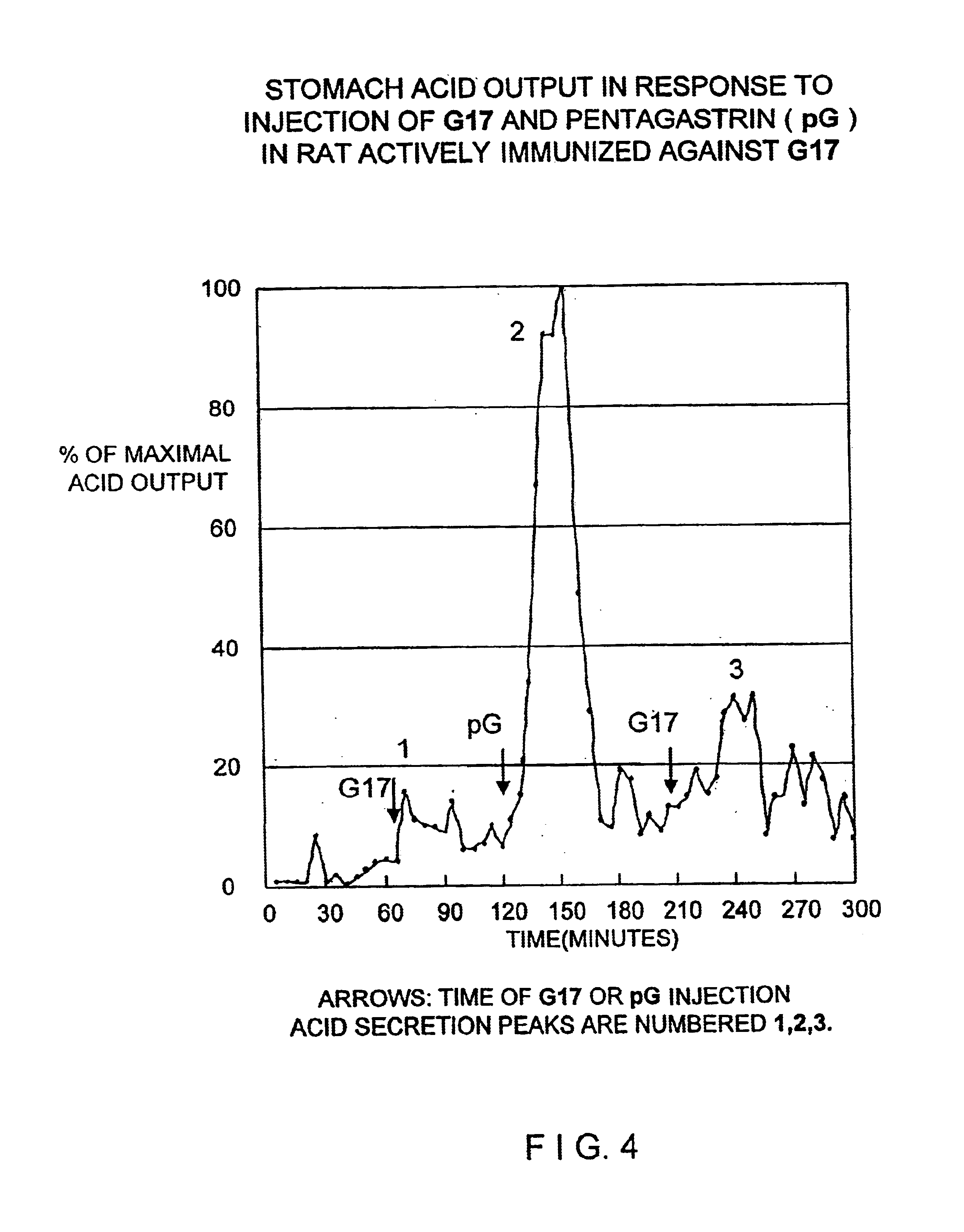
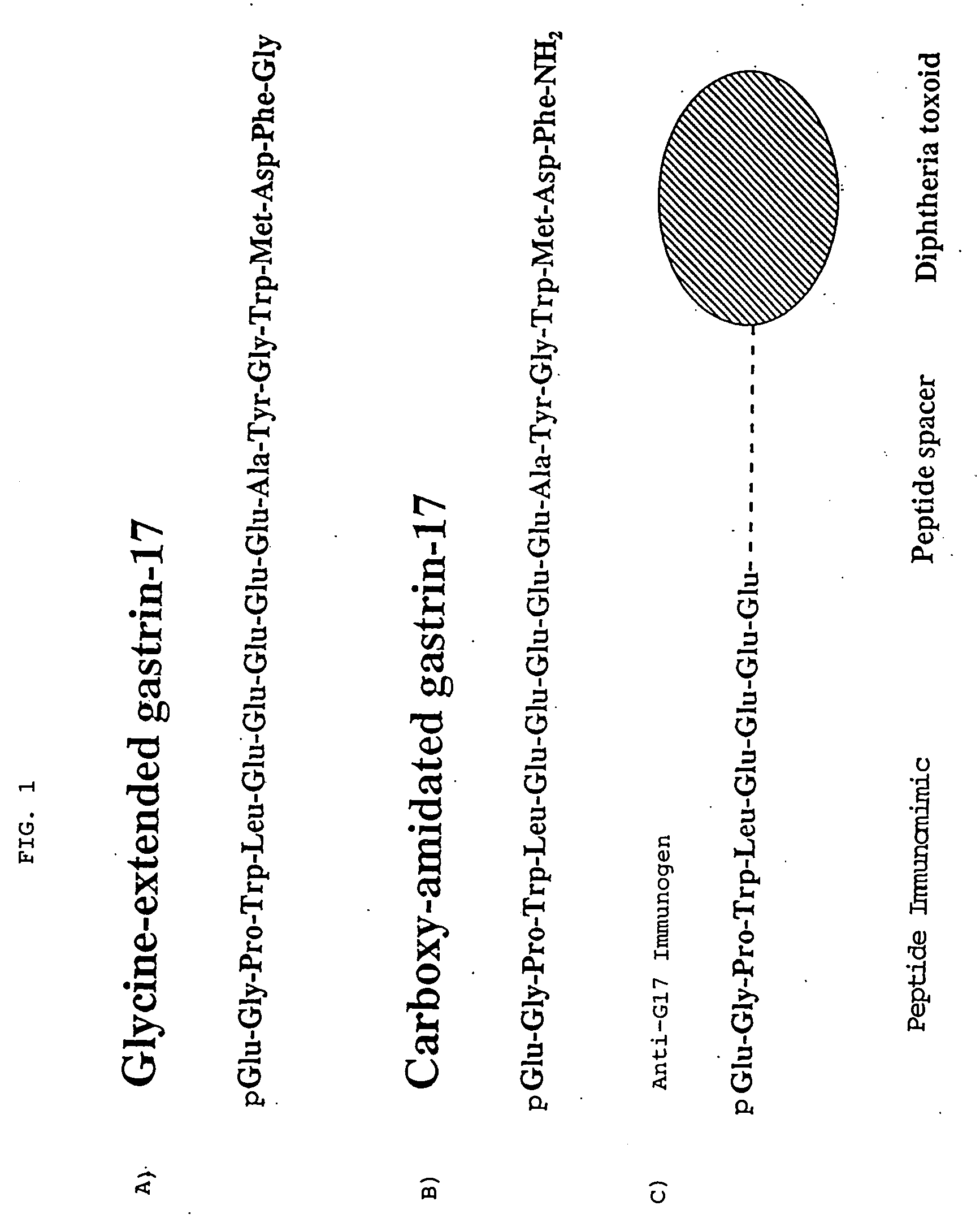
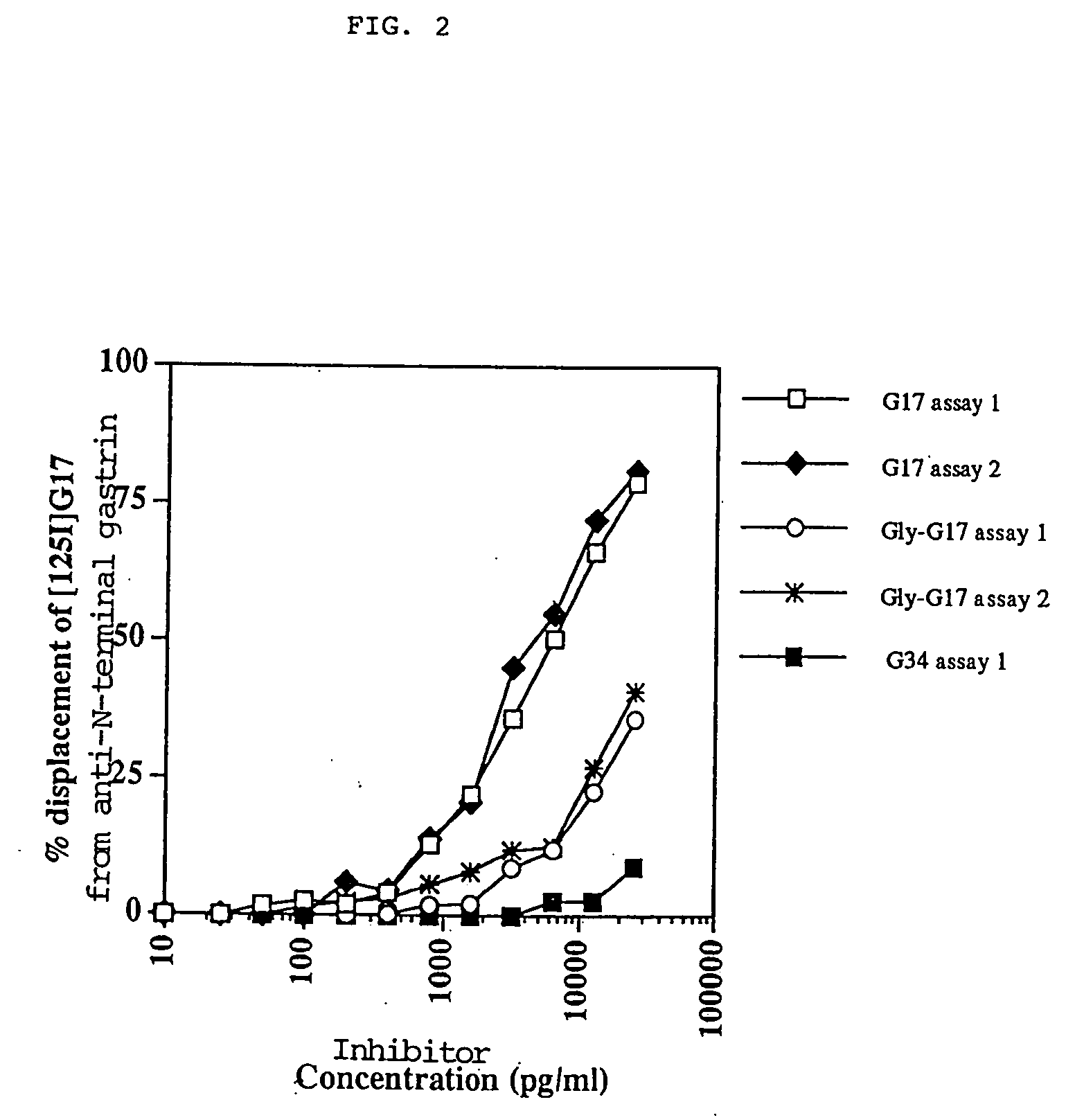
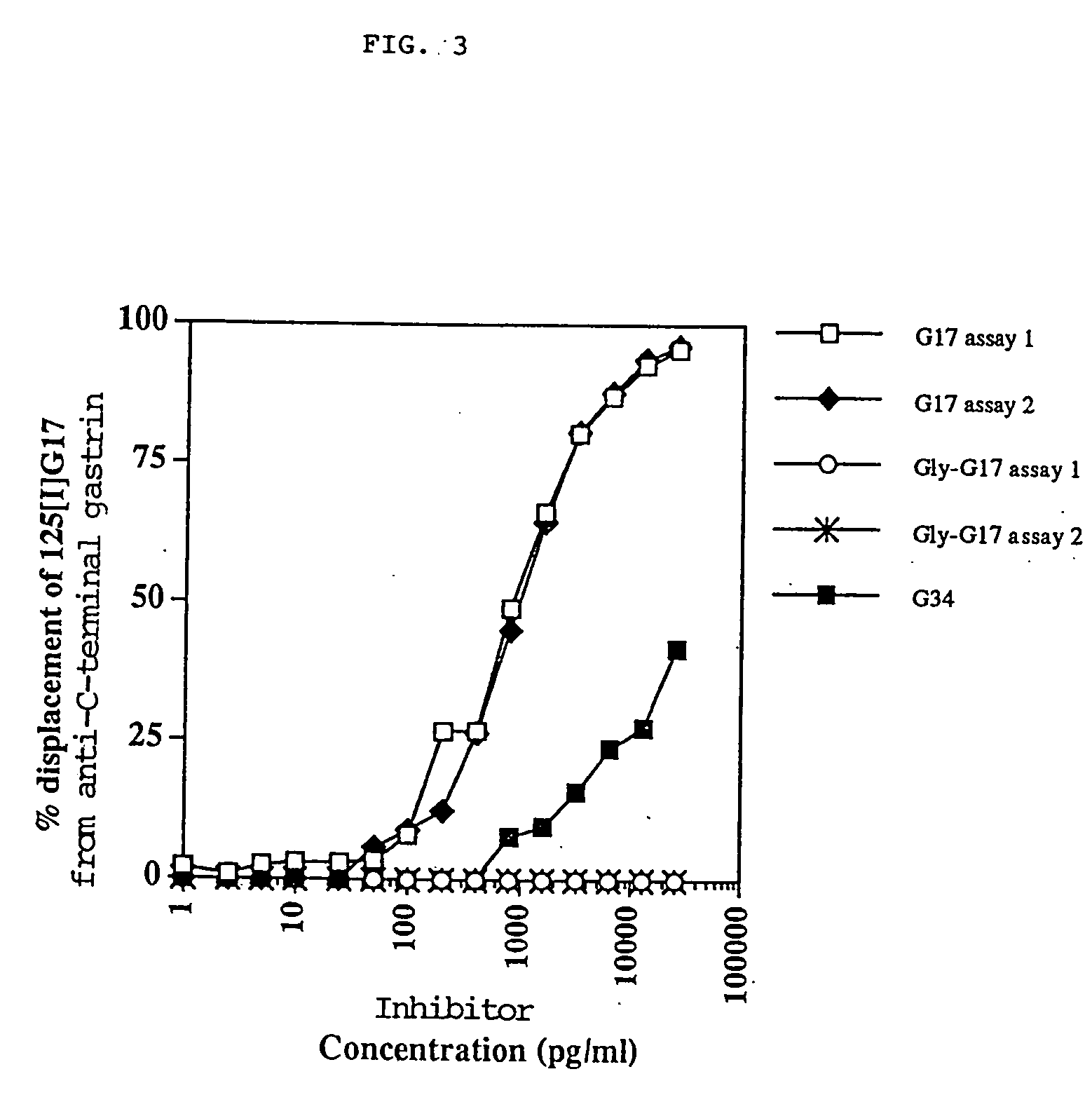
The present invention provides monoclonal antibodies (MAbs) selective for the N-termini and C-termini of the gastrin hormone forms, gastrin-17 (G17), glycine-extended gastrin-17 (G17-Gly), gastrin-34 (G34) and glycine-extended gastrin-34 (G34-Gly); and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of gastrin-17 (G17), glycine-extended gastrin-17 (G17-Gly), gastrin-34 (G34) and glycine-extended gastrin-34 (G34-Gly). These assays are useful for monitoring a gastrin-mediated disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. Pharmaceutical compositions of the MAbs of the invention are also provided, along with methods of diagnosis, prevention and treatment of gastrin-mediated diseases or conditions. Methods of evaluating a gastrin hormone-blocking treatment are described. The course of a gastrin-mediated disease or condition may be monitored in a patient by means of assay methods provided.
Owner:CANCER ADVANCES INC
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula NH2CH2CH2CHR1C(O)N—R (I) where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Immunogenic compositions against gastrin peptides
InactiveUS6861510B1Effective mean of controlling and preventingEliminate side effectsPeptide/protein ingredientsSnake antigen ingredientsHormones regulationIn vivo
Immmunogenic compositions useful for the treatment of ulcers or tumors whose growth is dependent on or stimulated by gastrin hormones are disclosed. The immunogenic compositions induce antibodies in a subject which selectively neutralize the specific hormones. Pharmaceutical compositions comprising effective amounts of the immunogenic compositions and methods of treatment using the compositions are disclosed. A method of reversing the inventive treatments by neutralizing the antibodies induced in vivo is also disclosed.
Owner:CANCER ADVANCES INC
Liposomal vaccine
InactiveUS20070082043A1High weight ratioHigh encapsulation efficiencyPeptide/protein ingredientsMicroencapsulation basedWater solubleLiposome
The invention provides liposomal vehicles for encapsulating relatively high levels of water-soluble substances including immunogens directed against gastrin and gonadotropin releasing hormone. The liposome encapsulating large amounts of immunogens can be injected parentally to induce effective immune responses without exhibiting significant adverse tissue reactogenicity.
Owner:RECEPTOR BIOLOGIX
Immunogenic compositions comprising progastrin and uses thereof
ActiveUS7854932B2Inhibits gastrin-induced proliferationTreat cancerPowder deliveryPeptide/protein ingredientsOncologyAnnexin
The present invention is drawn to immunotherapeutic methods to treat tumors / cancers that produce progastrin ectopically or are dependent on progastrin for their growth. Disclosed herein are immunogenic compositions comprising agents that target progastrin, agents that target the progastrin receptor, annexin II, or both. Such a composition may be administered in combination with chemotherapy or to an individual who had been previously subjected to chemotherapy or radiation therapy. The cancers that may be treated using such a composition may include but are not limited to colon cancer, breast cancer, lung cancer or pancreatic cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
InactiveUS7151084B2Nervous disorderPeptide/protein ingredientsMembrane TransportersPancreatic hormone
A complex is provided for the treatment of neurogenic conditions having the formula:where R1 isM is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium(II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium(II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium(II):transporter ligand.
Owner:MILLER LANDON C G
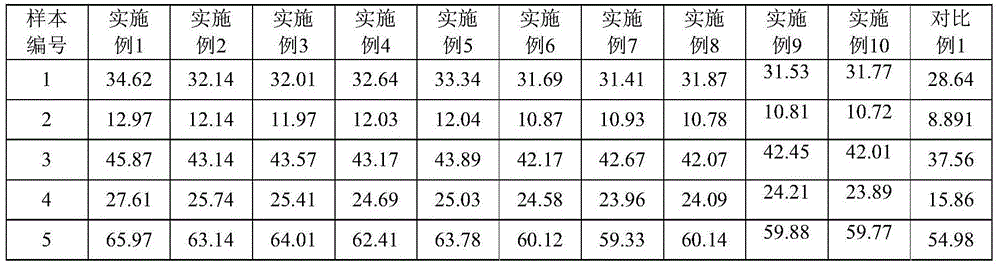
Gastrin-17 enzymatic chemiluminescence immunoassay kit
InactiveCN104914251ALittle variance between production batchesHigh affinityDisease diagnosisBiological testingMicrosphereImmune complex deposition
The invention discloses a gastrin-17 enzymatic chemiluminescence immunoassay kit and belongs to the technical field of chemiluminescence immunoassay analysis. The kit comprises an enzyme label liquid, a gastrin-17 standard, gastrin-17 monoclonal antibody-coated immunomagnetic beads, a sample diluent, a chemiluminescent substrate liquid and a washing liquid. The principle of the gastrin-17 enzymatic chemiluminescence immunoassay kit comprises that a gastrin-17 monoclonal antibody is connected to the surface of a magnetic bead so that a solid phase agent is obtained, and through capture of gastrin-17 in a sample and use of an enzyme-labeled anti-gastrin-17 monoclonal antibody, a solid phase-antibody-antigen-enzyme-labeled antibody sandwiched immune complex is formed. Through combination of a chemiluminescence technology and an immunomagnetic bead technology, the prepared kit has the advantages of high sensitivity, good specificity, wide linearity range and good stability and can satisfy clinical requirements on stomach function detection.
Owner:BIOHIT BIOTECH HEFEI +1
Monoclonal Antibodies to Progastrin
The present invention provides progastrin-binding molecules specific for progastrin that do not bind gastrin-17(G17), gastrin-34(G34), glycine-extended gastrin-17(G17-Gly), or glycine-extended gastrin-34(G34-Gly). Further, the invention provides monoclonal antibodies (MAbs) selective for sequences at the N-terminus and the C-terminus of the gastrin precursor molecule, progastrin and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of progastrin and gastrin hormone species in immuno-detection and quantitation assays. These assays are useful for diagnosing and monitoring a gastrin-promoted disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. The progastrin-binding molecules are useful therapeutically for passive immunization against progastrin in progastrin-promoted diseases or conditions. Also provided are surrogate reference standard (SRS) molecules that are peptide chains of from about 10 to about 35 amino acids, wherein the SRS molecule comprises at least two epitopes found in a protein of interest of greater than about 50 amino acids. Such SRS molecules are useful as standards in place of authentic proteins of interest.
Owner:CANCER ADVANCES INC
Immunological methods for the treatment of gastrointestinal cancer
InactiveUS20050025770A1Limit the cancer-trophic hormone levels producedGrowth inhibitionPeptide/protein ingredientsDigestive systemGlycine extended gastrinGlycine
Owner:CANCER ADVANCES INC
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS7074775B2Improve efficiencyEliminate side effectsBiocideDipeptide ingredientsTryptophanSecretin
A compound is provided that has the formulaNH2CH2CH2CH2C(O)N—R (I)where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
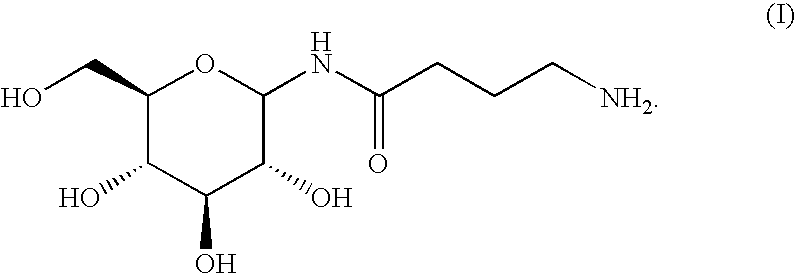
Gastrin receptor-avid peptide conjugates
InactiveUS20060067886A1Peptide/protein ingredientsRadioactive preparation carriersDiseaseGastrin-releasing peptide receptor
A compound for use as a therapeutic or diagnostic radiopharmaceutical includes a group capable of complexing a medically useful metal attached to a moiety which is capable of binding to a gastrin releasing peptide receptor. A method for treating a subject having a neoplastic disease includes administering to the subject an effective amount of a radiopharmaceutical having a metal chelated with a chelating group attached to a moiety capable of binding to a gastrin releasing peptide receptor expressed on tumor cells with subsequent internalization inside of the cell. A method of forming a therapeutic or diagnostic compound includes reacting a metal synthon with a chelating group covalently linked with a moiety capable of binding a gastrin releasing peptide receptor.
Owner:HOFFMAN TIMOTHY J +4
Gastrin releasing peptide compounds
InactiveUS7611692B2Ultrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsGastrin-releasing peptideImaging agent
New and improved compounds for use in diagnostic imaging or therapy having the formula M—N—O—P—G, wherein M is an optical label or a metal chelator (in the form complexed with a metal radionuclide or not), N—O—P is the linker, and G is the GRP receptor targeting peptide. Methods for imaging a patient and / or providing radiotherapy or phototherapy to a patient using the compounds of the invention are also provided. Methods and kits for preparing a diagnostic imaging agent from the compound is further provided. Methods and kits for preparing a radiotherapeutic agent are further provided.
Owner:BRACCO IMAGINIG SPA
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formulaNH2CH2CH2CHR1C(O)N—R (I)where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Monoclonal antibodies to progastrin
The present invention provides progastrin-binding molecules specific for progastrin that do not bind gastrin-17(G17), gastrin-34(G34), glycine-extended gastrin-17(G17-Gly), or glycine-extended gastrin-34(G34-Gly). Further, the invention provides monoclonal antibodies (MAbs) selective for sequences at the N-terminus and the C-terminus of the gastrin precursor molecule, progastrin and the hybridomas that produce these MAbs. Also provided are panels of Mabs useful for the detection and quantitation of progastrin and gastrin hormone species in immuno-detection and quantitation assays. These assays are useful for diagnosing and monitoring a gastrin-promoted disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. The progastrin-binding molecules are useful therapeutically for passive immunization against progastrin in progastrin-promoted diseases or conditions. Also provided are surrogate reference standard (SRS) molecules that are peptide chains of from about 10 to about 35 amino acids, wherein the SRS molecule comprises at least two epitopes found in a protein of interest of greater than about 50 amino acids. Such SRS molecules are useful as standards in place of authentic proteins of interest.
Owner:CANCER ADVANCES INC
Gastrin receptor-avid peptide conjugates
InactiveUS6921526B2Peptide/protein ingredientsGastrin releasing peptideSynthonGastrin-releasing peptide receptor
A compound for use as a therapeutic or diagnostic radiopharmaceutical includes a group capable of complexing a medically useful metal attached to a moiety which is capable of binding to a gastrin releasing peptide receptor. A method for treating a subject having a neoplastic disease includes administering to the subject an effective amount of a radiopharmaceutical having a metal chelated with a chelating group attached to a moiety capable of binding to a gastrin releasing peptide receptor expressed on tumor cells with subsequent internalization inside of the cell. A method of forming a therapeutic or diagnostic compound includes reacting a metal synthon with a chelating group covalently linked with a moiety capable of binding a gastrin releasing peptide receptor.
Owner:UNIVERSITY OF MISSOURI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com