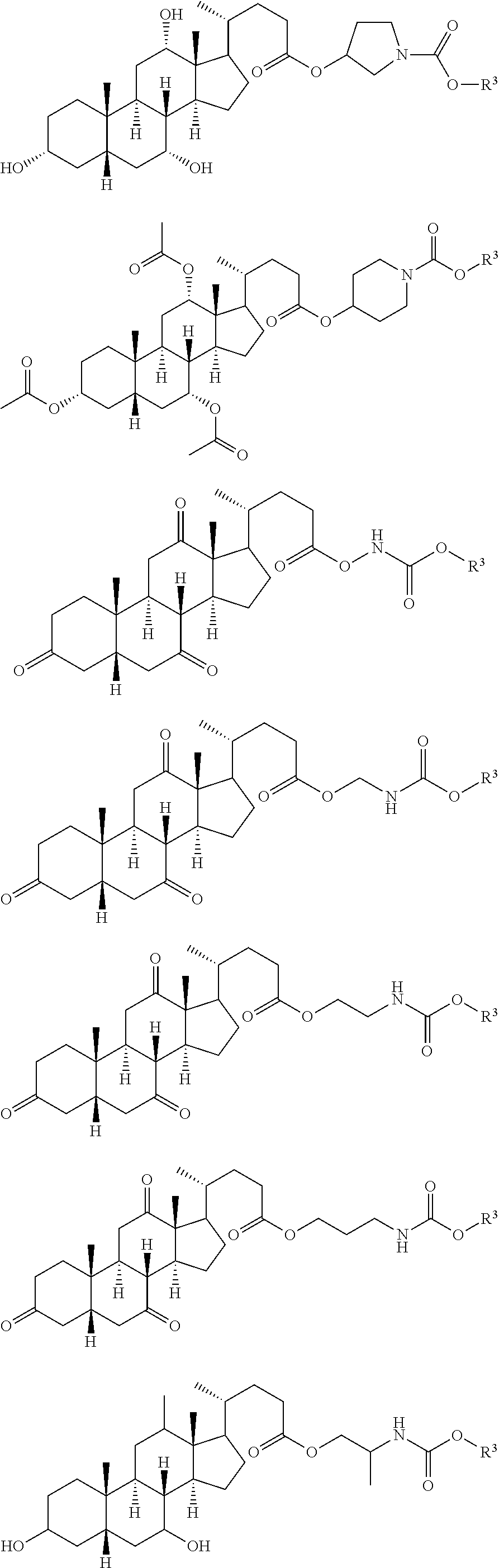
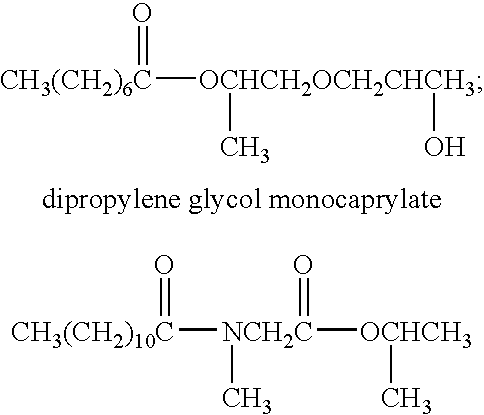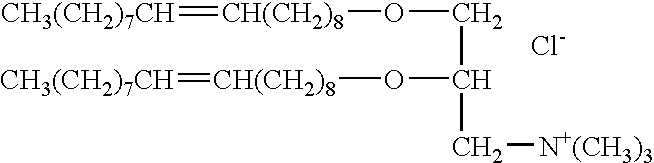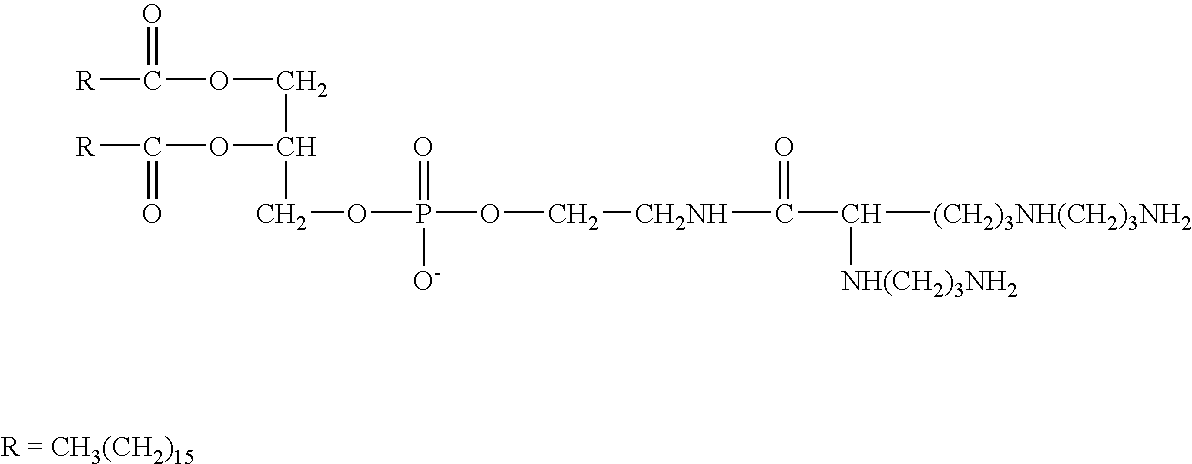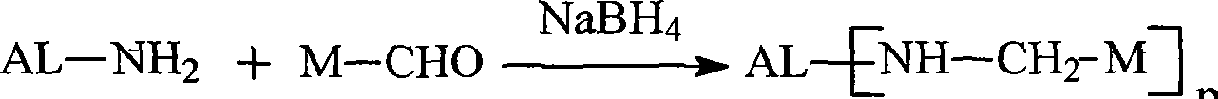Patents
Literature
907 results about "Cholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
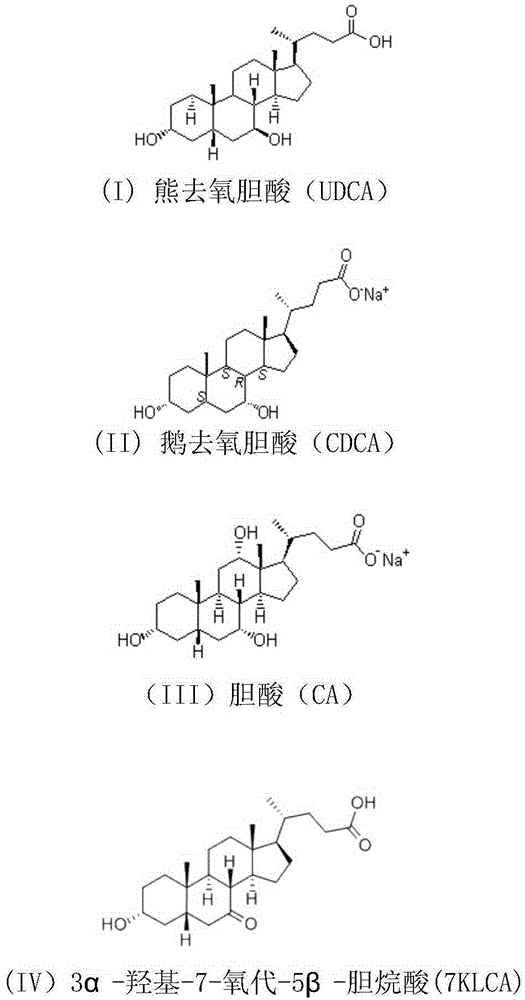
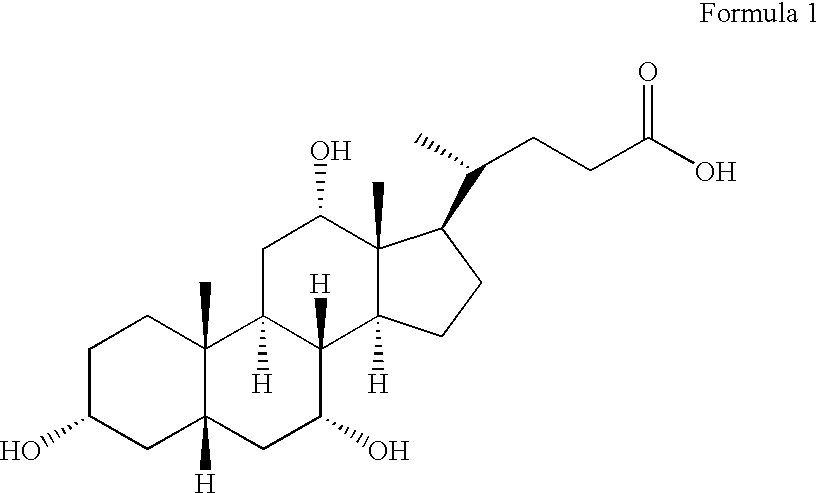
Cholic acid, also known as 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid is a primary bile acid that is insoluble in water (soluble in alcohol and acetic acid), it is a white crystalline substance. Salts of cholic acid are called cholates. Cholic acid, along with chenodeoxycholic acid, is one of the two major bile acids produced by the liver, where it is synthesized from cholesterol. These two major bile acids are roughly equal in concentration in humans. Derivatives are made from cholyl-CoA, which exchanges its CoA with either glycine, or taurine, yielding glycocholic and taurocholic acid, respectively.
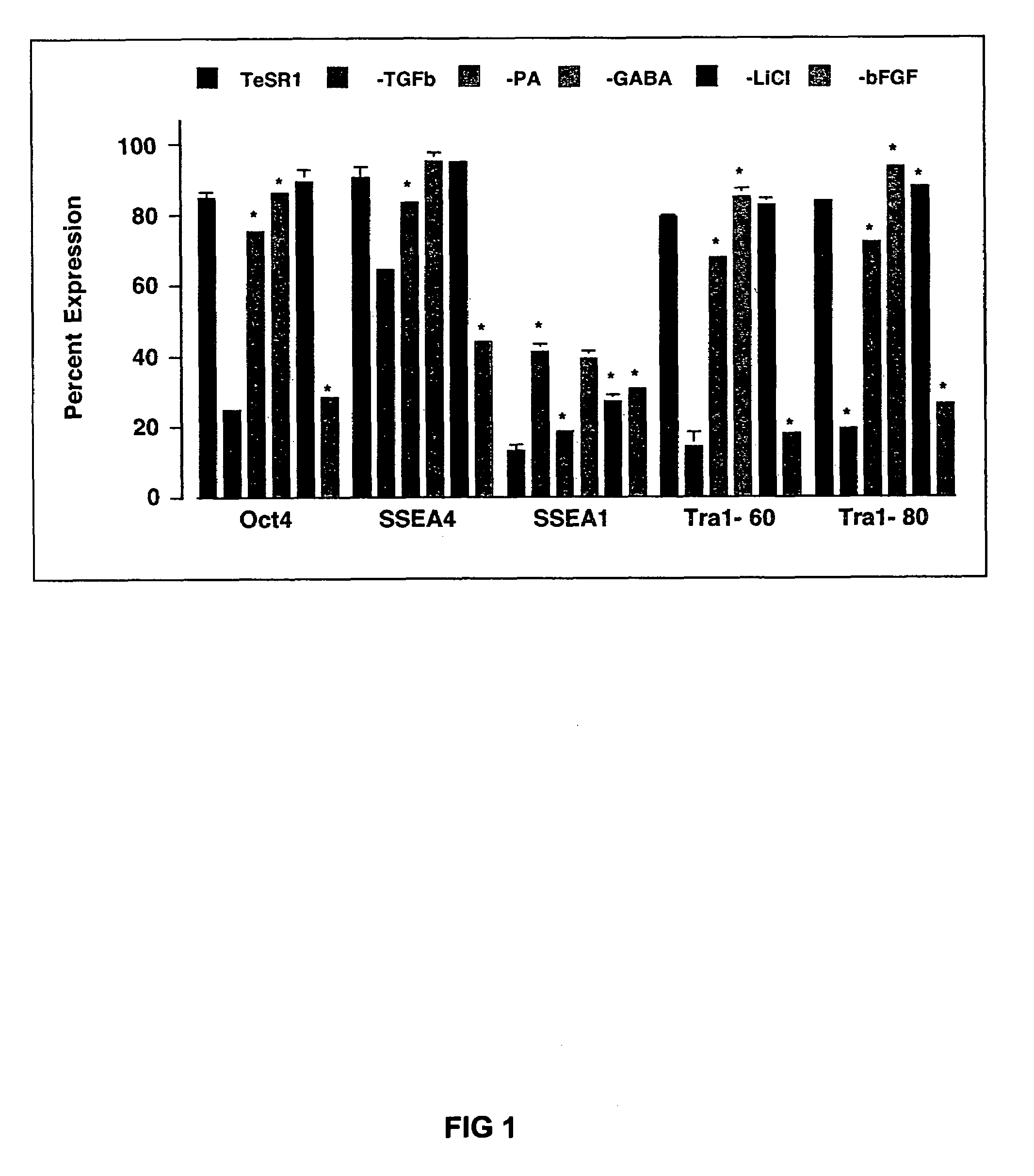
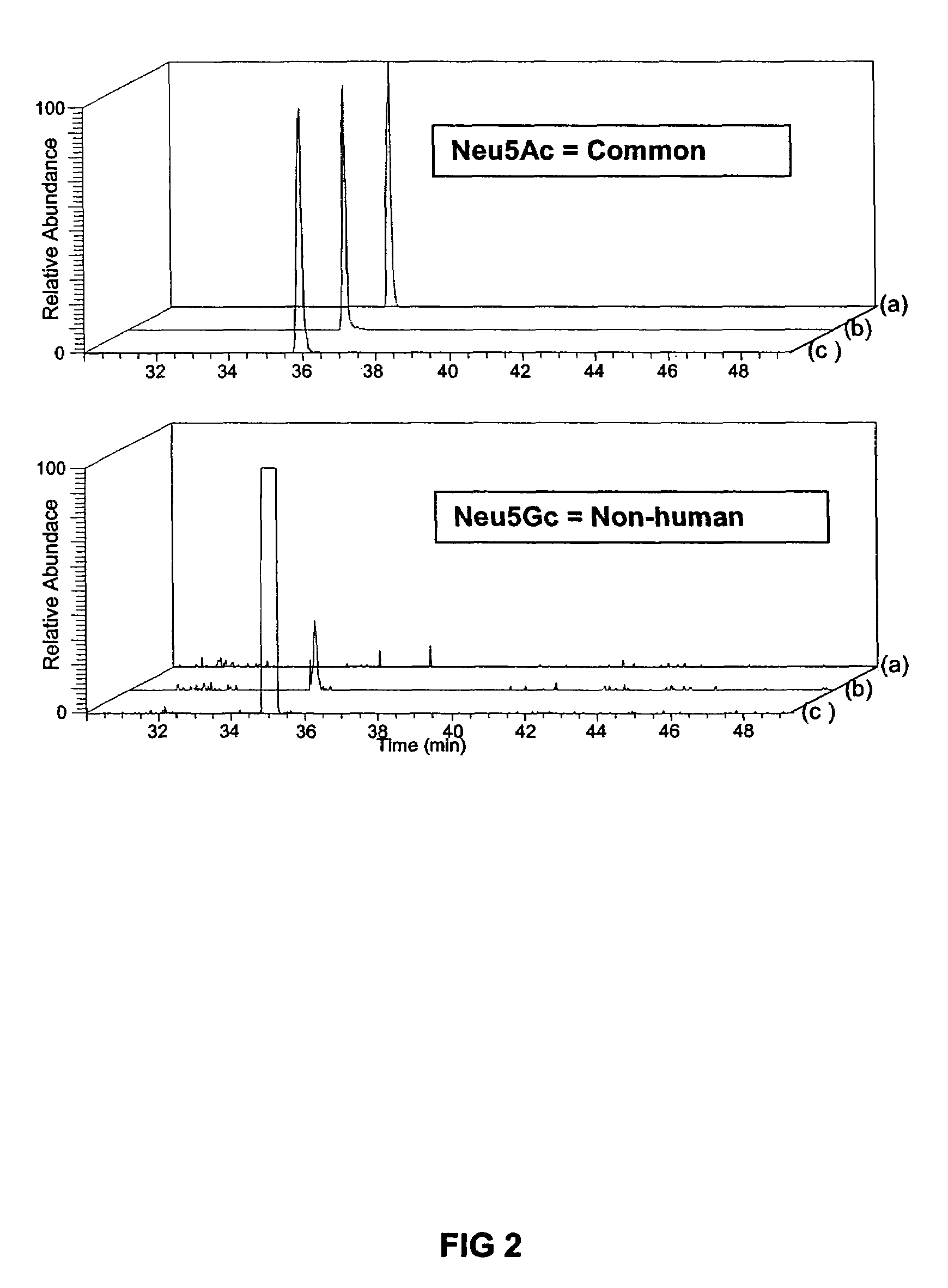
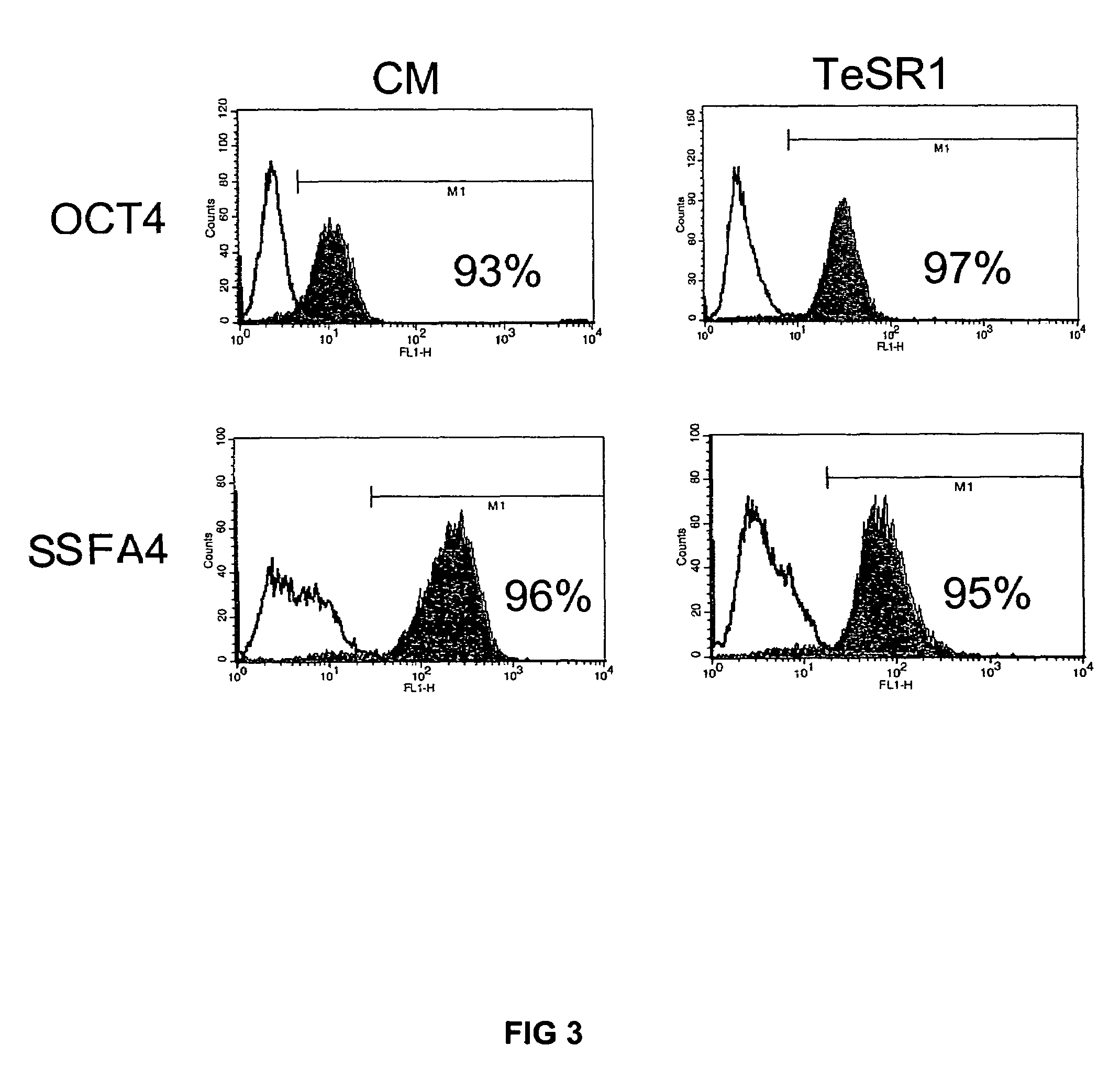
Medium containing pipecholic acid and gamma amino butyric acid and culture of embryonic stem cells
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor, gamma amino butyric acid, pipecholic acid, lithium and transforming growth factor beta are added to the medium in which the stem cells are cultured, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
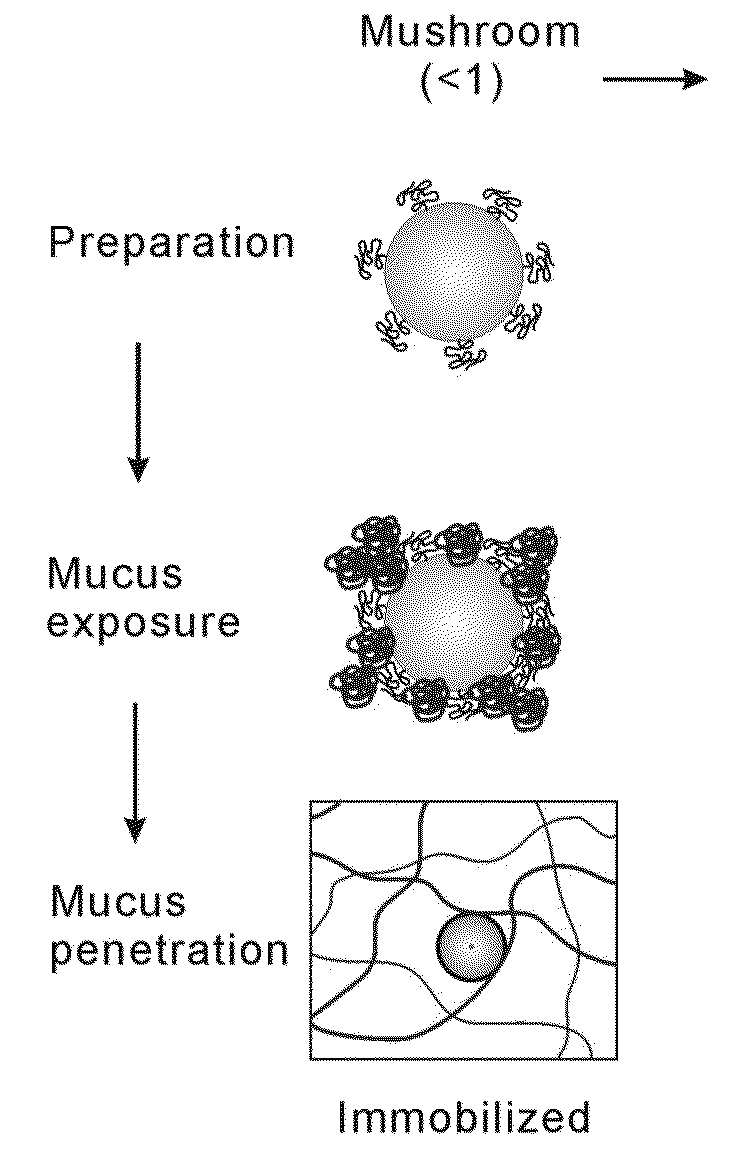
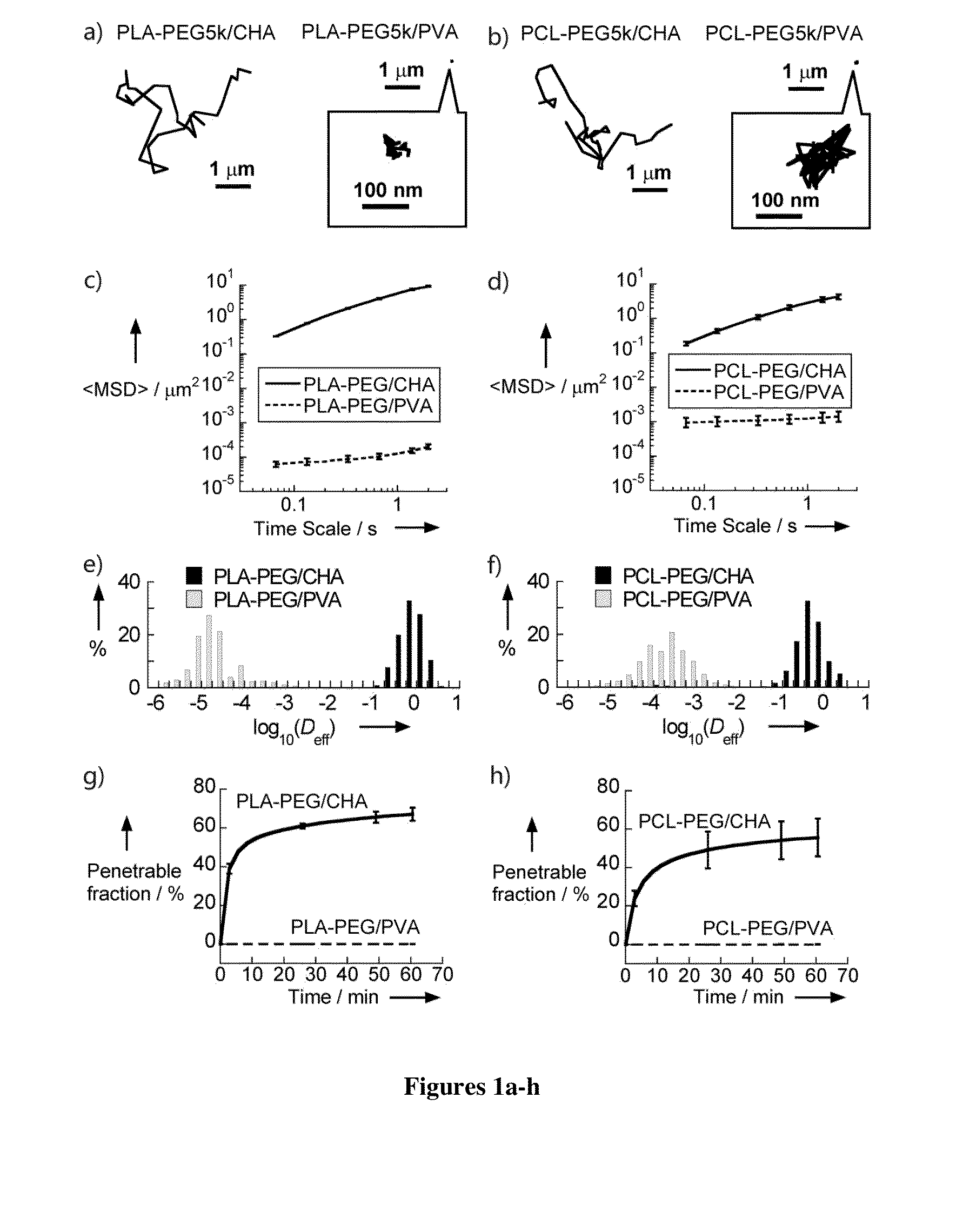
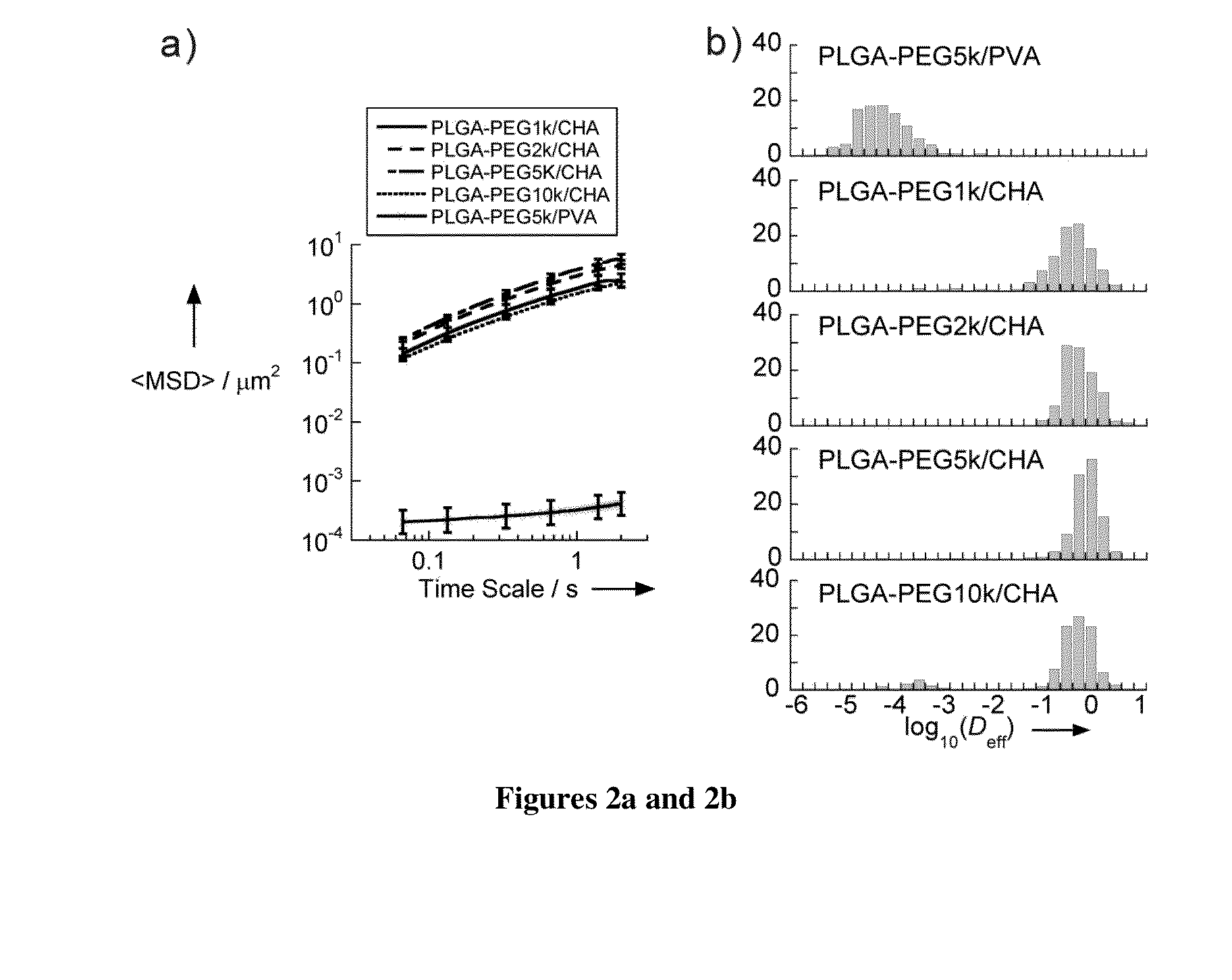
Nanoparticles with enhanced mucosal penetration or decreased inflammation
Nanoparticles formed by emulsion of one or more core polymers, one or more surface altering materials, and one or more low molecular weight emulsifiers have been developed. The particles are made by dissolving the one or more core polymers in an organic solvent, adding the solution of the one or more core polymers to an aqueous solution or suspension of the emulsifier to form an emulsion, and then adding the emulsion to a second solution or suspension of the emulsifier to effect formation of the nanoparticles. In the preferred embodiment, the molecular weight of the emulsifiers is less than 1500, 1300, 1200, 1000, 800, 600, or 500 amu. Preferred emulsifiers include cholic acid sodium salt, dioctyl sulfosuccinate sodium, hexadecyltrimethyl ammonium bromide, saponin, TWEEN® 20, TWEEN® 80, and sugar esters. The surface altering materials are present in an amount effective to make the surface charge of the particles neutral or essentially neutral when the one or more emulsifiers are charged. The emulsifiers have an emulsification capacity of at least about 50%, preferably at least 55, 60, 65, 70, 75, 80, 85, 90, or 95%.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
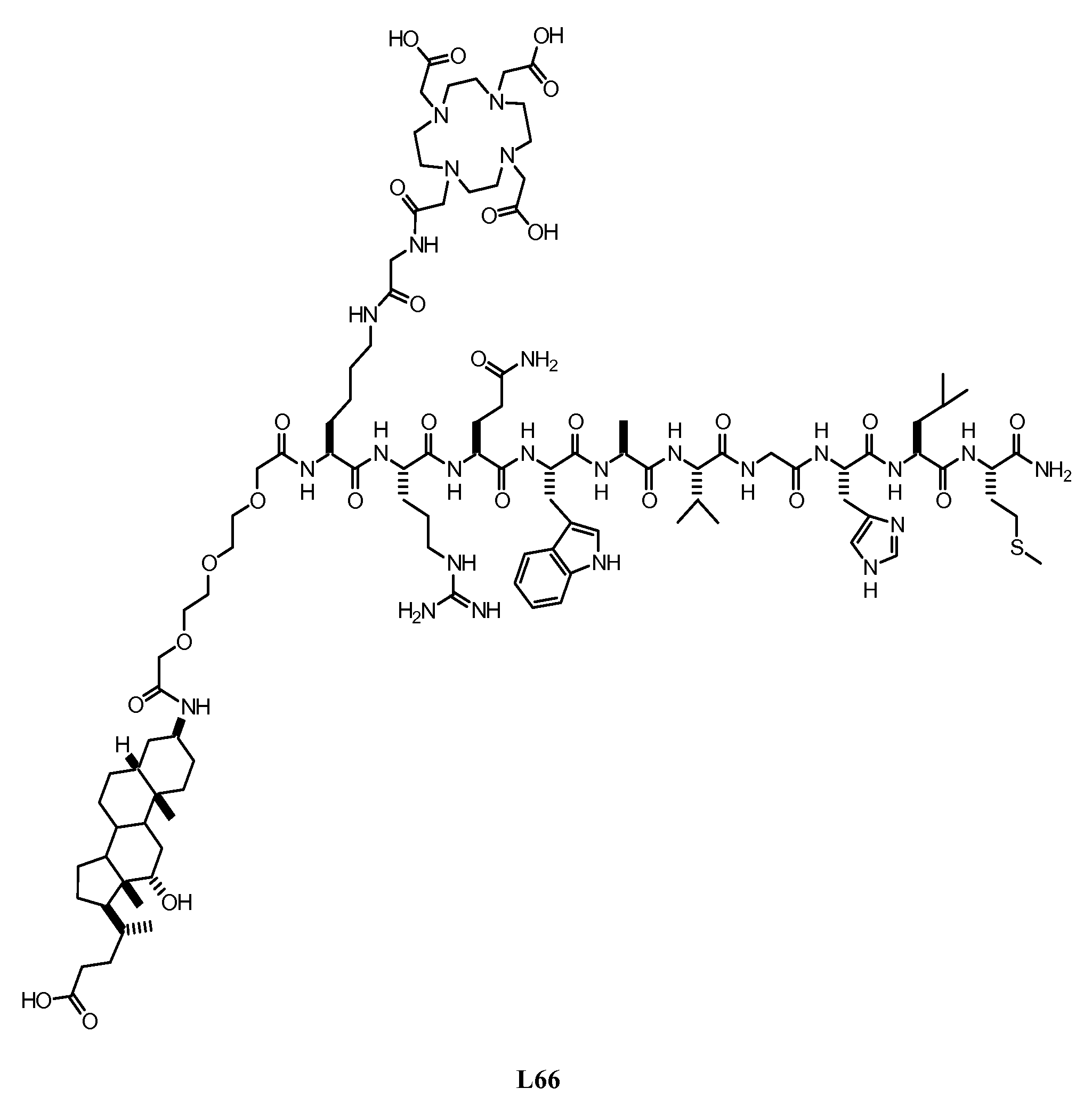
Gastrin Releasing Peptide Compounds
InactiveUS20080008649A1Improve targetingDecreasing aberrant vascular permeabilityRadioactive preparation carriersGastrin releasing peptideCholic acidTherapeutic Hormone
New and improved compounds for use in diagnostic imaging or therapy having the formula M-N—O—P-G, wherein M is a metal chelator having the structure: wherein R1-R5 and FG are as defined herein (in the form complexed with a metal radionuclide or not), N—O—P is the linker containing at least one non-alpha amino acid with a cyclic group, at least one substituted bile acid or at least one non-alpha amino acid, and G is the GRP receptor targeting peptide. In the preferred embodiment, M is an Aazta metal chelator or a derivative thereof. Methods for imaging a patient and / or providing radiotherapy or phototherapy to a patient using the compounds of the invention are also provided. Methods and kits for preparing a diagnostic imaging agent from the compound is further provided. Methods and kits for preparing a radiotherapeutic agent are further provided. Novel methods of treating prostate tumors or of delaying the progression of prostate tumors are also provided, including, methods of treating bone or soft tissue metastases of prostate cancer, methods for treating hormone sensitive and hormone refractory prostate cancer, methods for delaying the progression of hormone sensitive prostate cancer, for facilitating combination therapy in patients with hormone sensitive prostate cancer and for decreasing aberrant vascular permeability in patients with hormone sensitive prostate cancer.
Owner:BRACCO IMAGINIG SPA
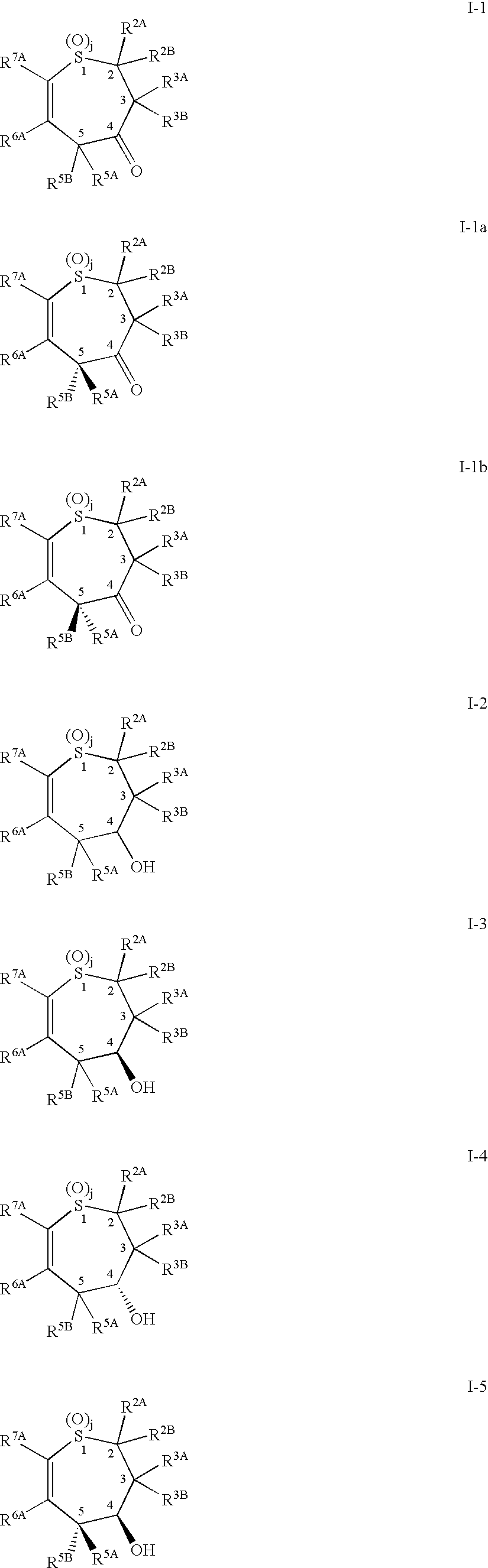
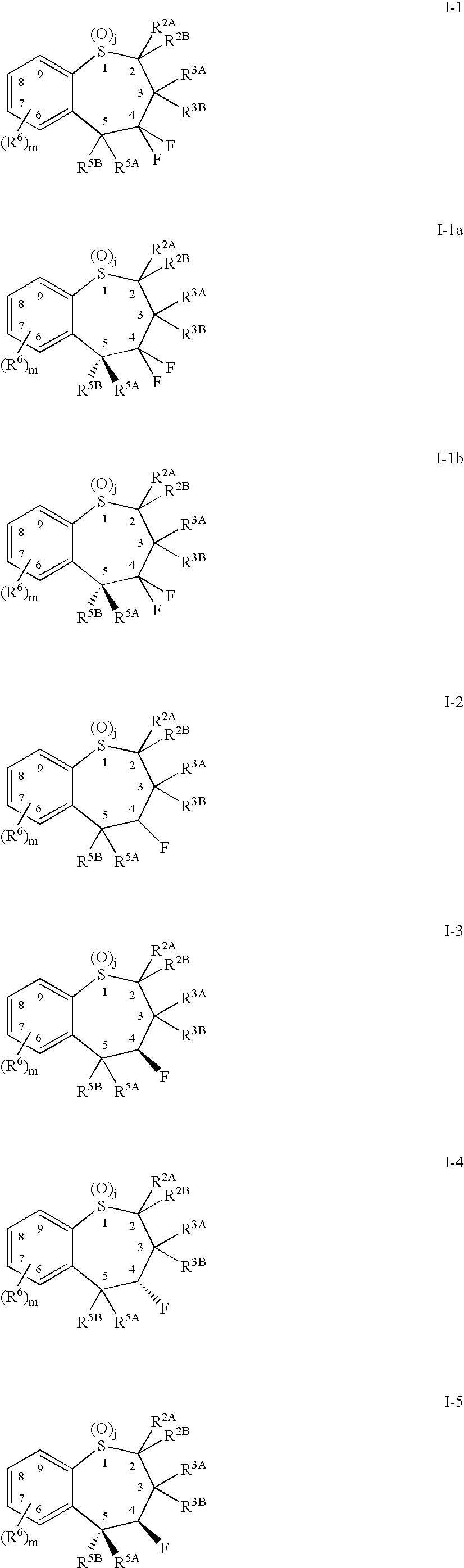
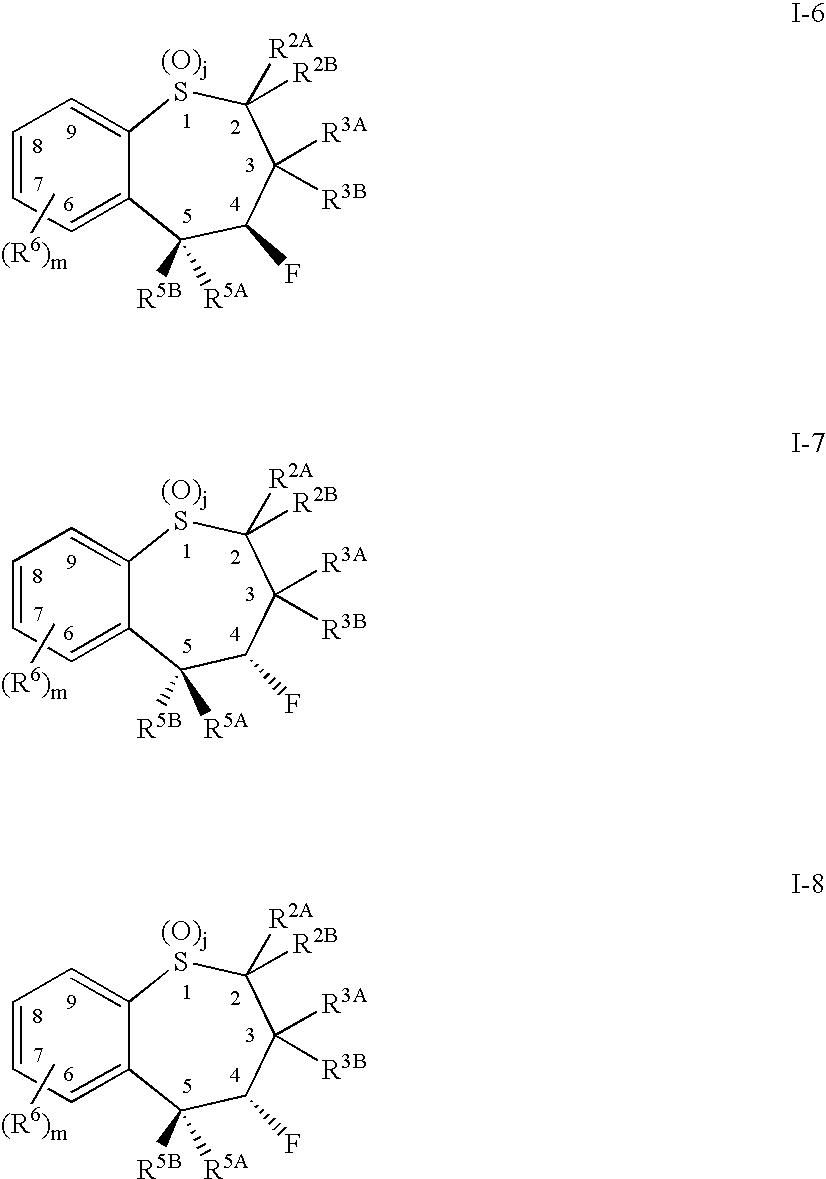
Alkyl/aryl hydroxy or keto thiepine compounds as inhibitors of apical sodium co-dependent bile acid transport (ASBT) and taurocholate uptake
Owner:PHARMACIA CORP
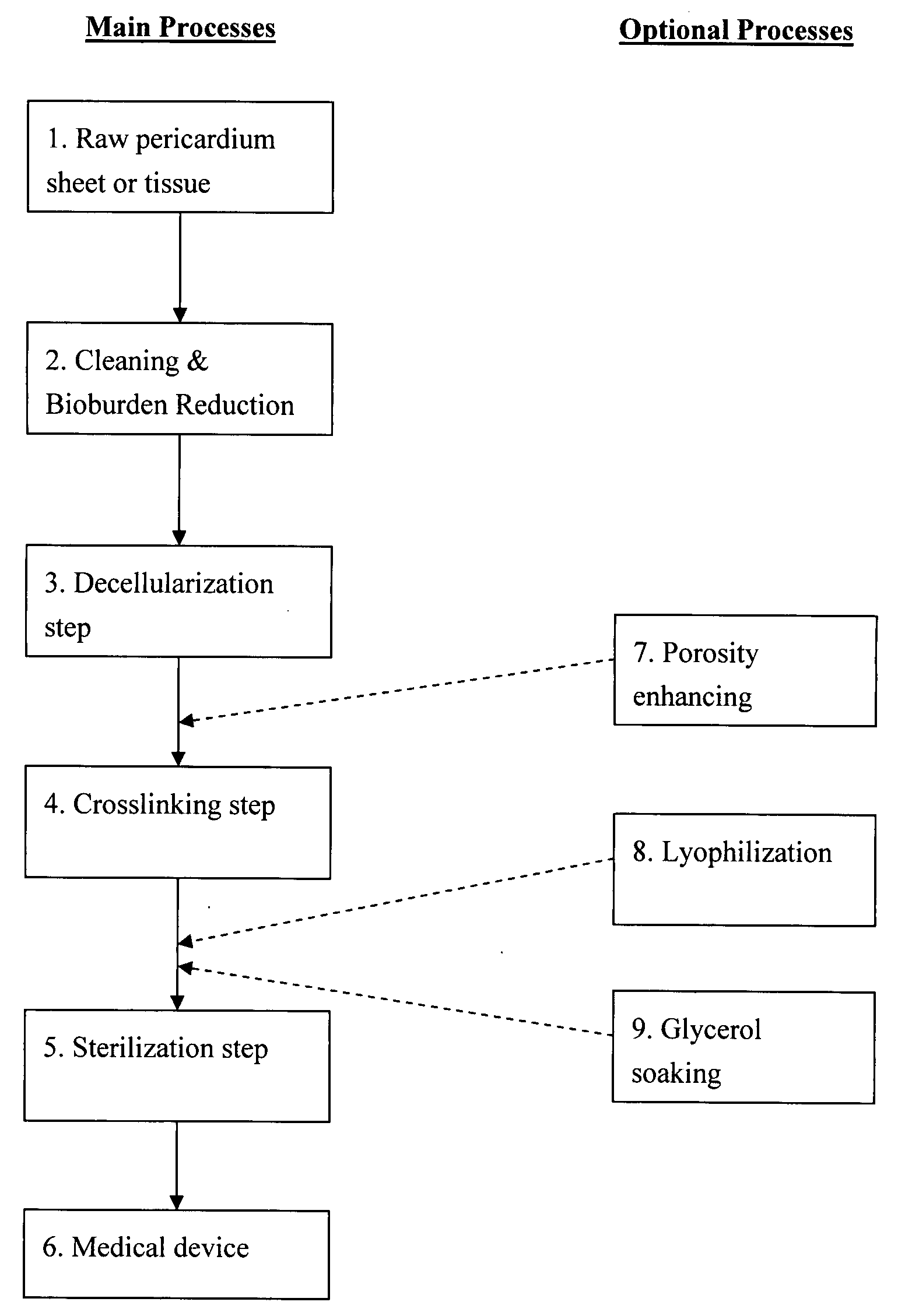
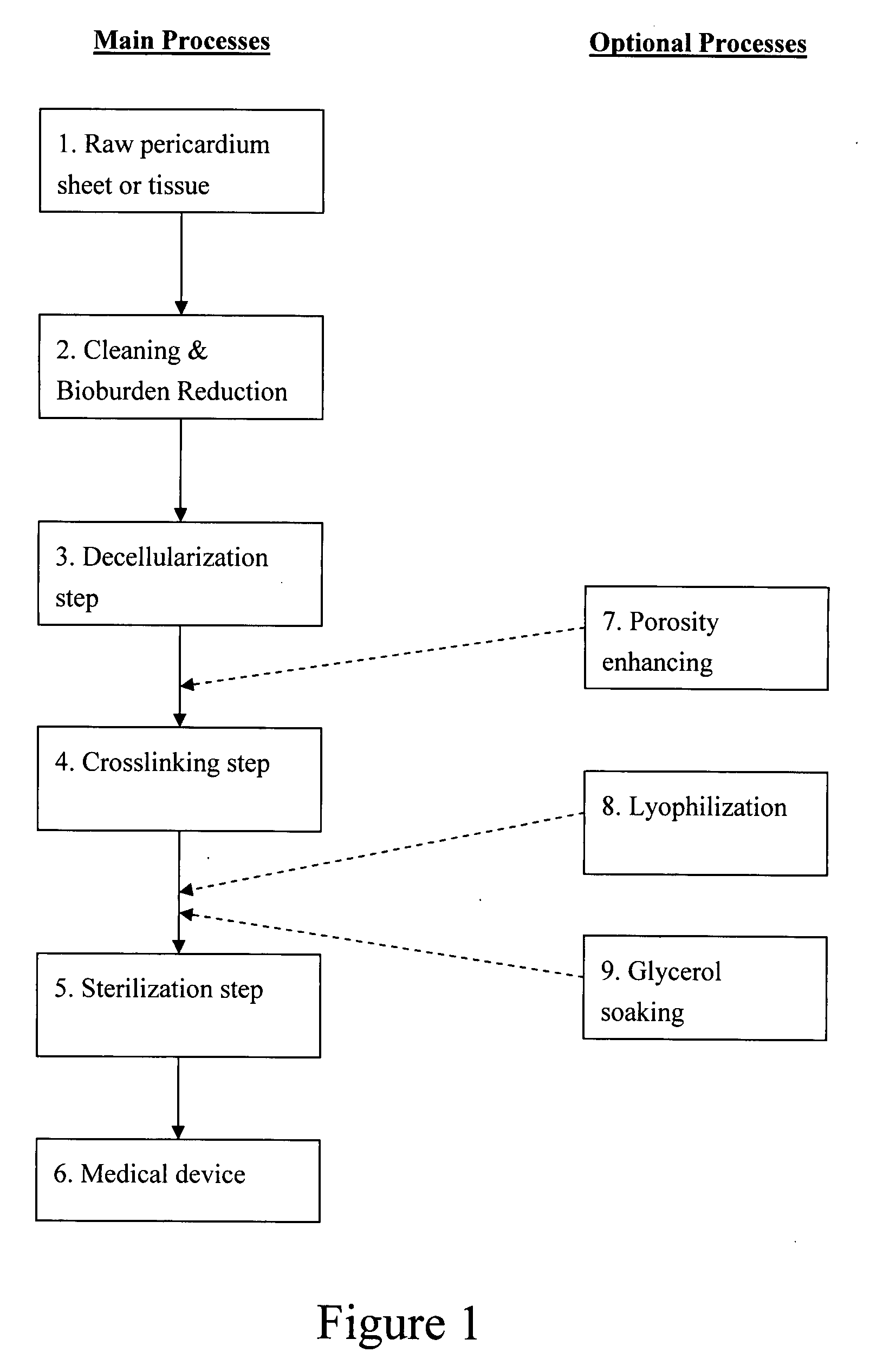
Decellularized pericardial tissue
InactiveUS20080195229A1Low antigenicityLow immunogenicityTissue regenerationProsthesisChemical treatmentPericardium
The invention discloses a decellularized pericardial tissue via chemical treatment with cholic acid or bile salts as a medical device and process of manufacture.
Owner:QUIJANO RODOLFO C +1
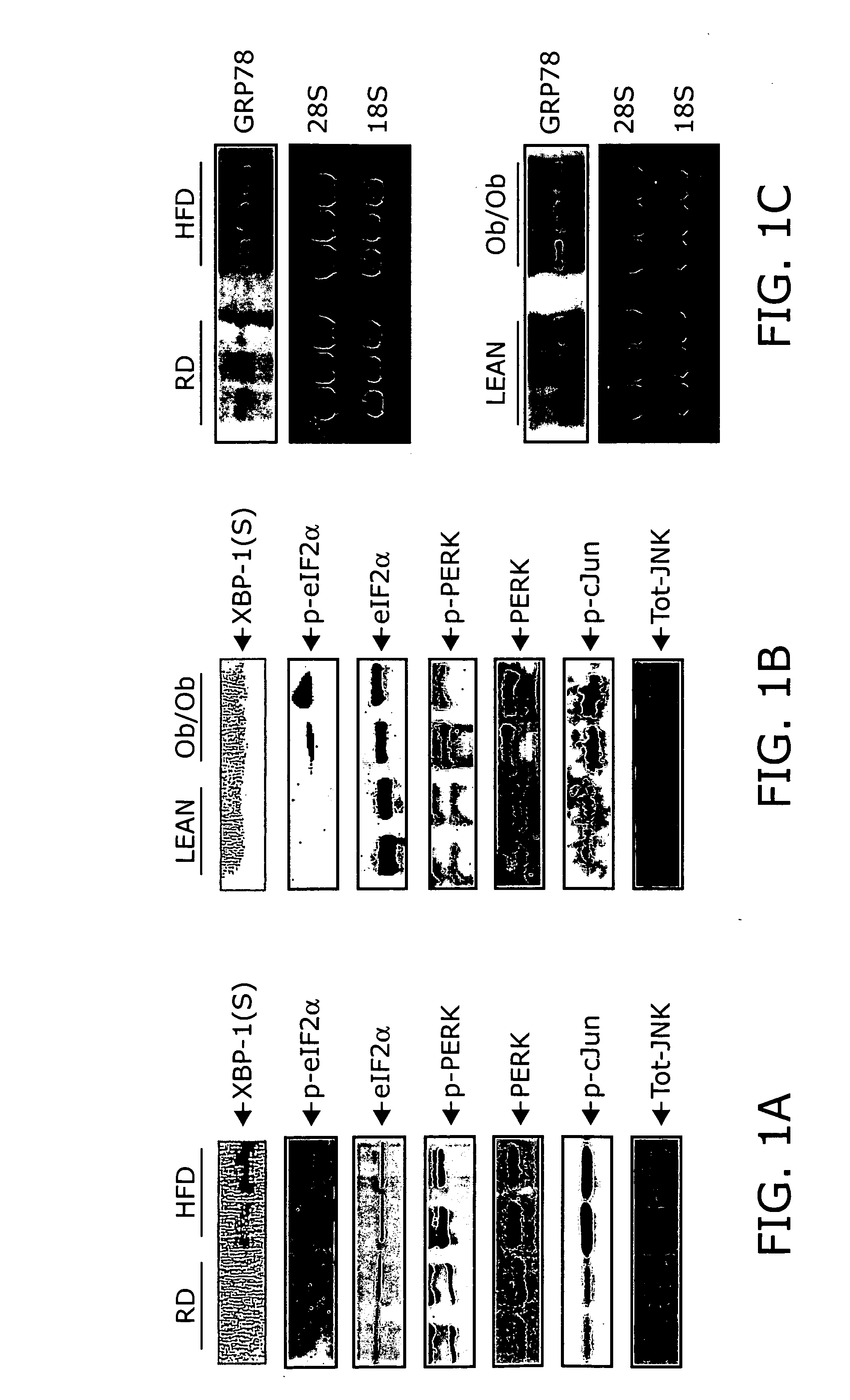
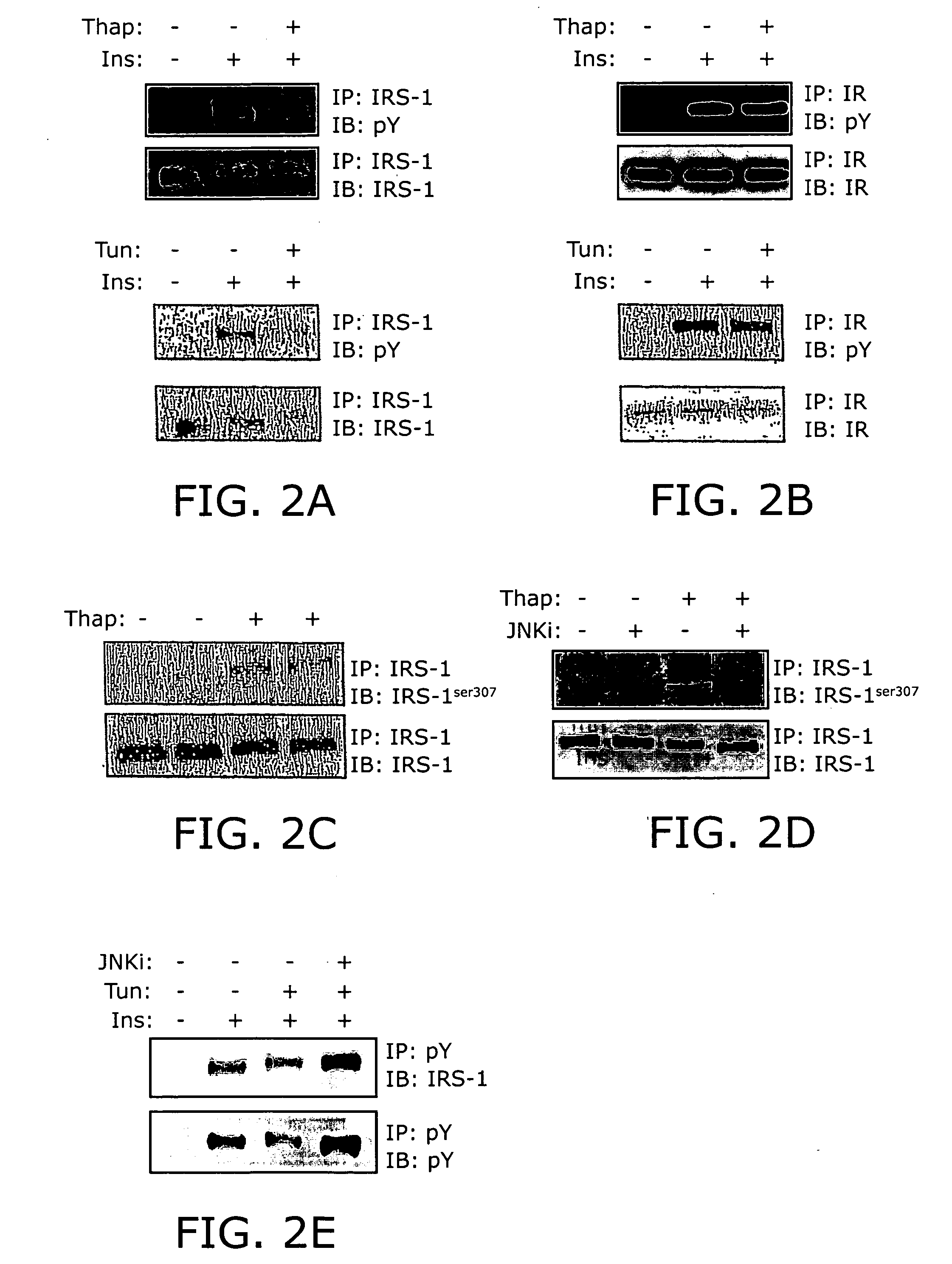
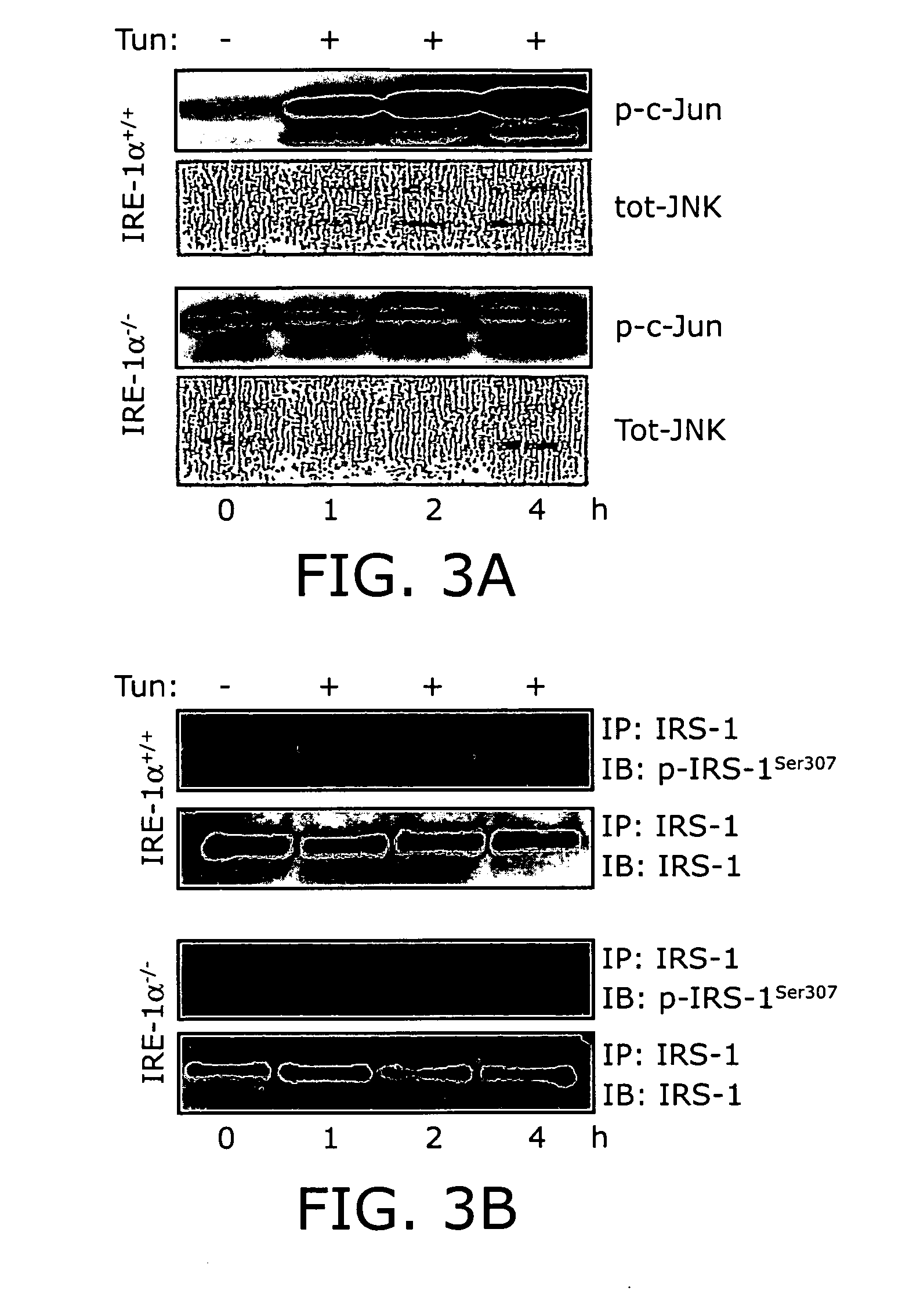
Reducing ER stress in the treatment of obesity and diabetes
InactiveUS20060073213A1Reduce ER stressIncrease insulin sensitivitySalicyclic acid active ingredientsBiocidePeripheral insulin resistanceTauroursodeoxycholic acid
Endoplasmic reticulum stress has been found to be associated with obesity. Therefore, agents that reduce or prevent ER stress may be used to treat diseases associated with obesity including peripheral insulin resistance, hypergylcemia, and type 2 diabetes. Two compounds which have been shown to reduce ER stress and to reduce blood glucose levels include 4-phenyl butyric acid (PBA), tauroursodeoxycholic acid (TUDCA), and trimethylamine N-oxide (TMAO). Other compounds useful in reducing ER stress are chemical chaperones such as trimethylamine N-oxide and glycerol. The present invention provides methods of treating a subject suffering from obesity, hyperglycemia, type 2 diabetes, or insulin resistance using ER stress reducers such as PBA, TUDCA, and TMAO. Methods of screening for ER stress reducers by identifying agents that reduce levels of ER stress markers in ER stressed cells are also provided. These agents may find use in methods and pharmaceutical compositions for treating obesity-associated diseases.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Mono- and di-fluorinated benzothiepine compounds as inhibitors of apical sodium co-dependent bile acid transport (ASBT) and taurocholate uptake
Owner:GD SEARLE & CO
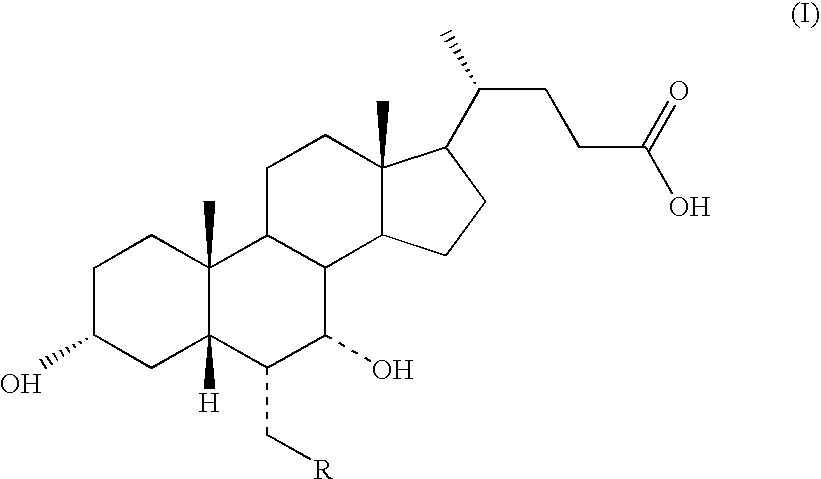
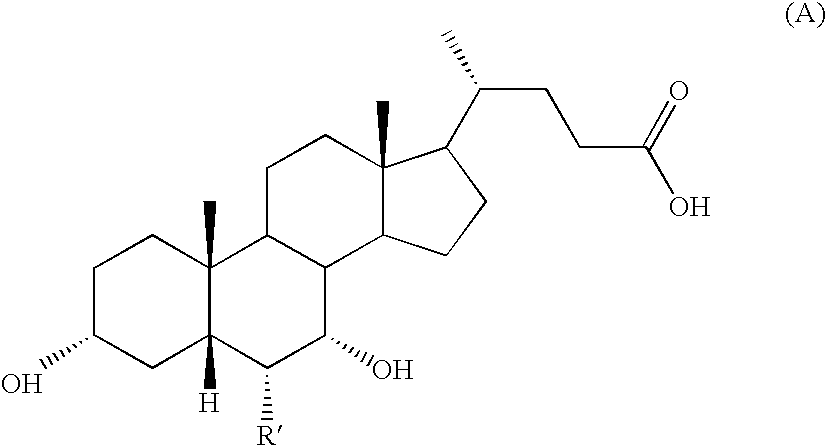
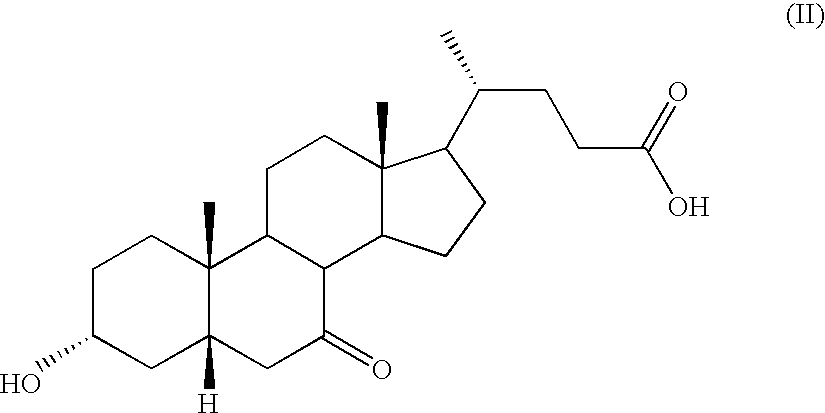
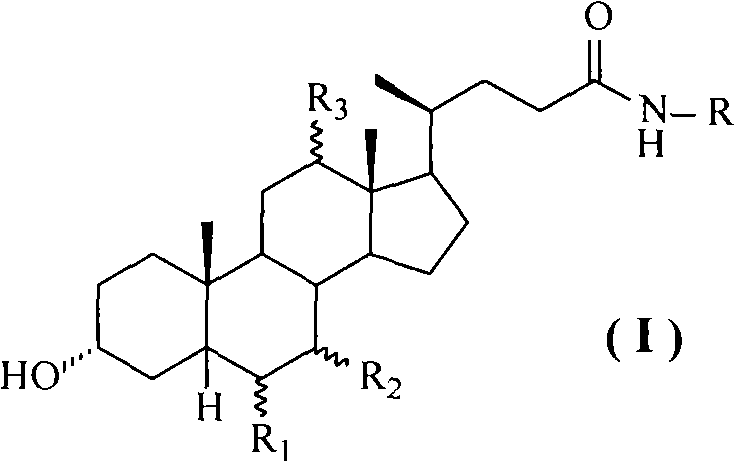
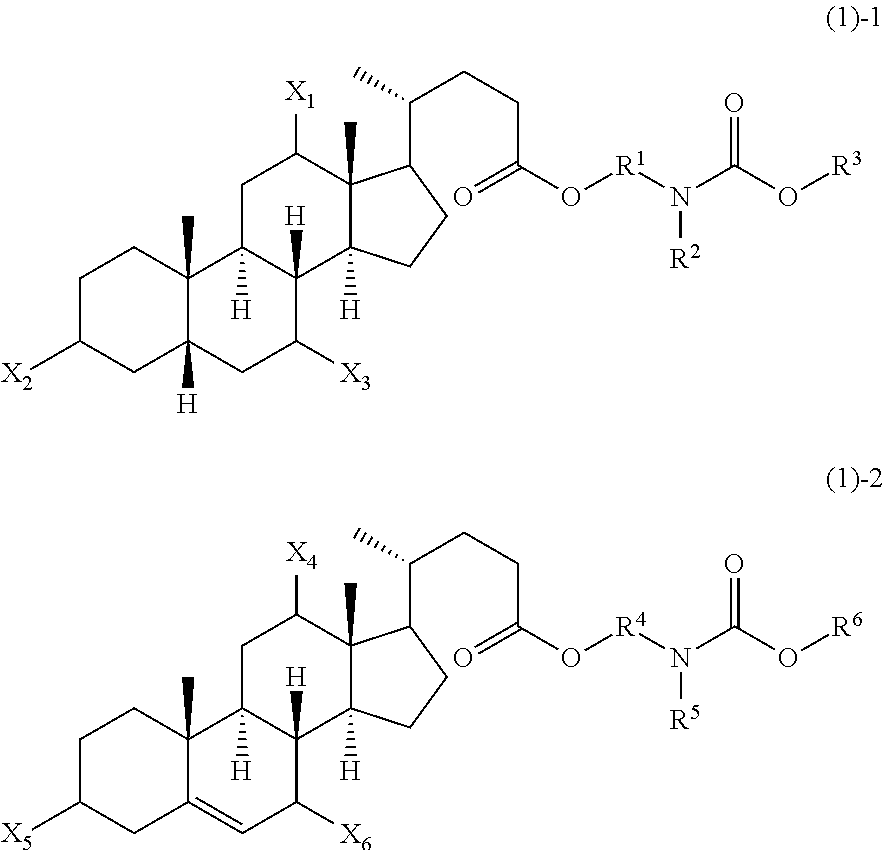
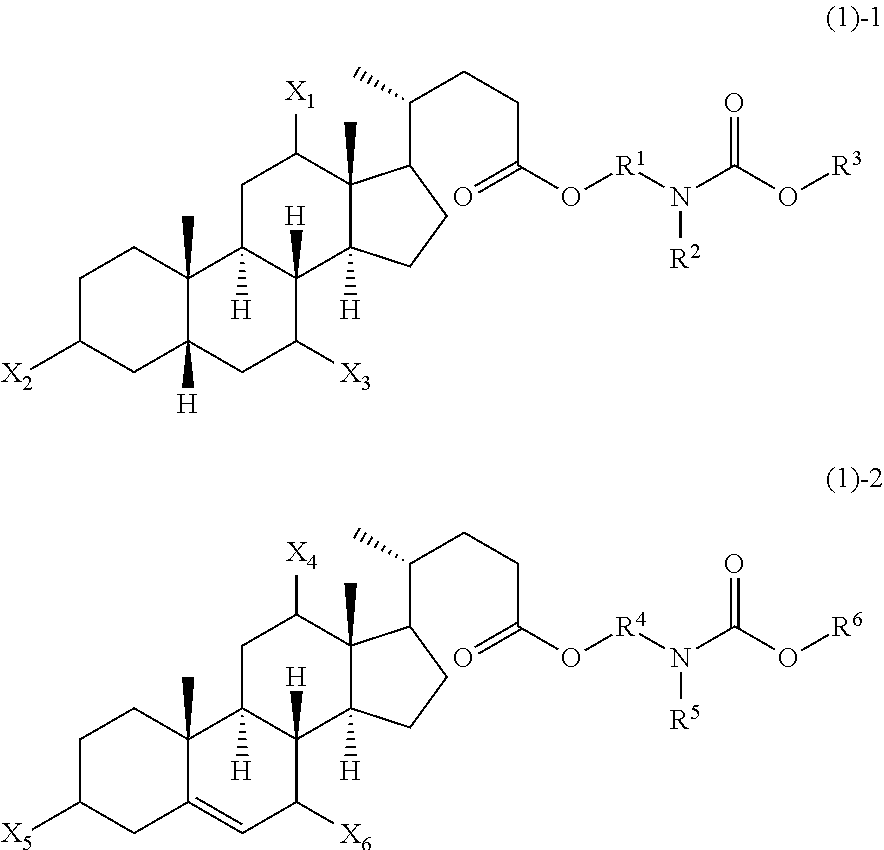
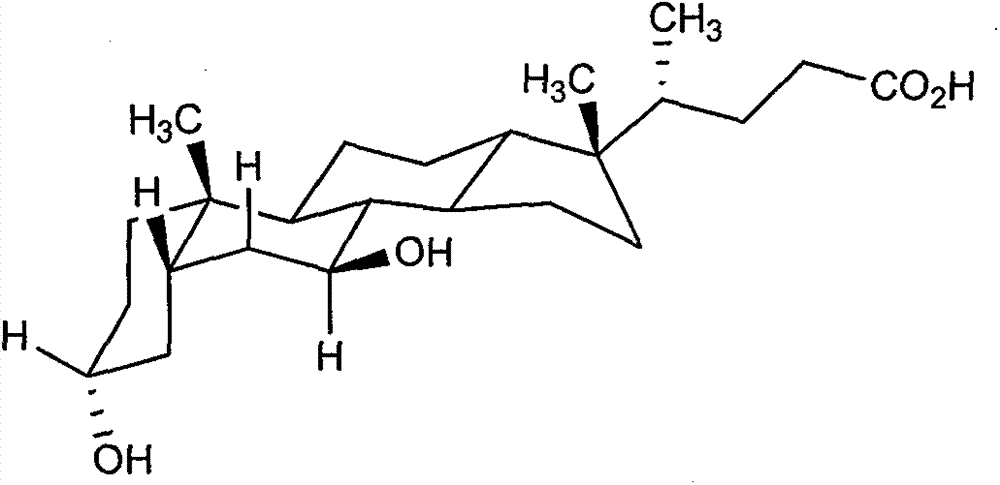
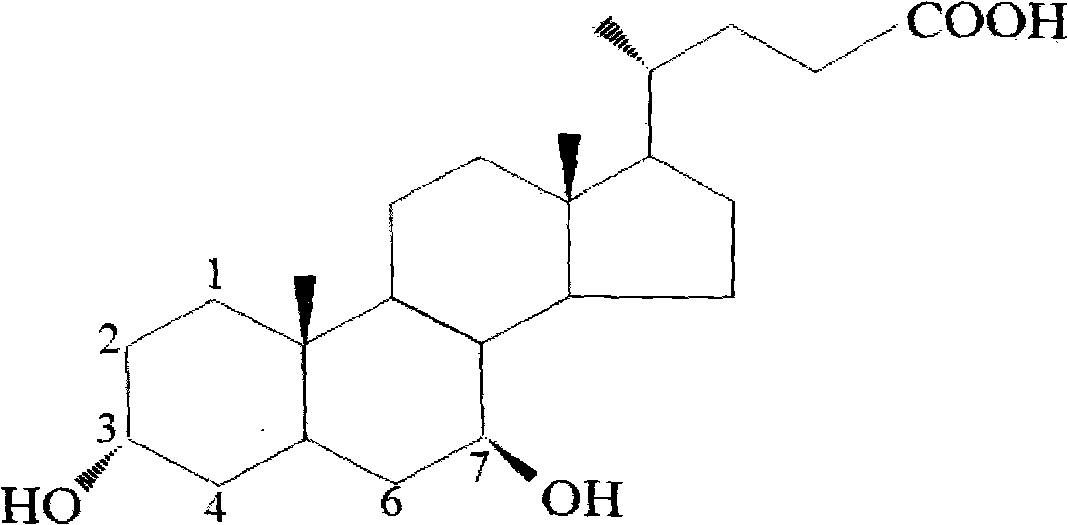
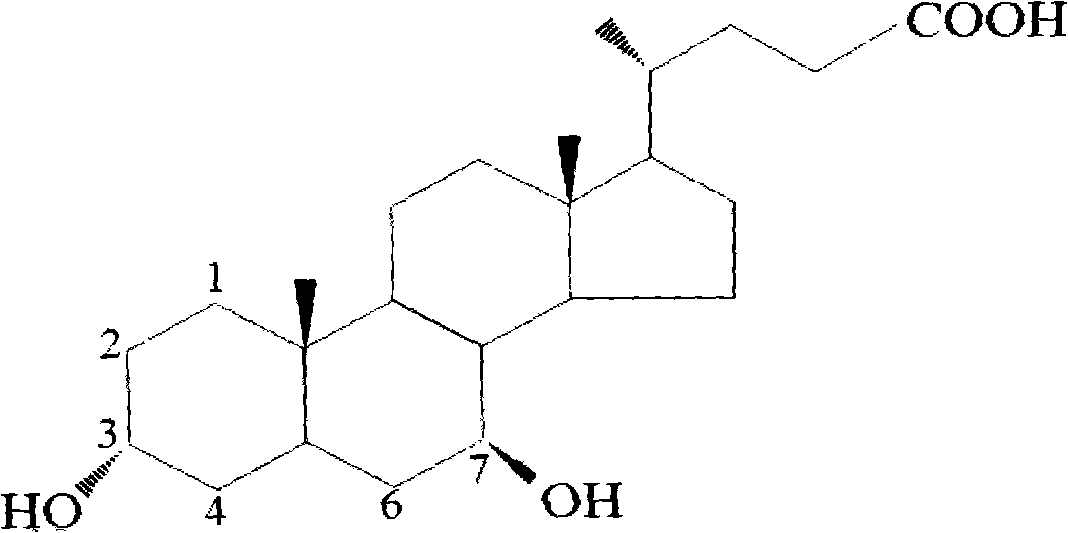
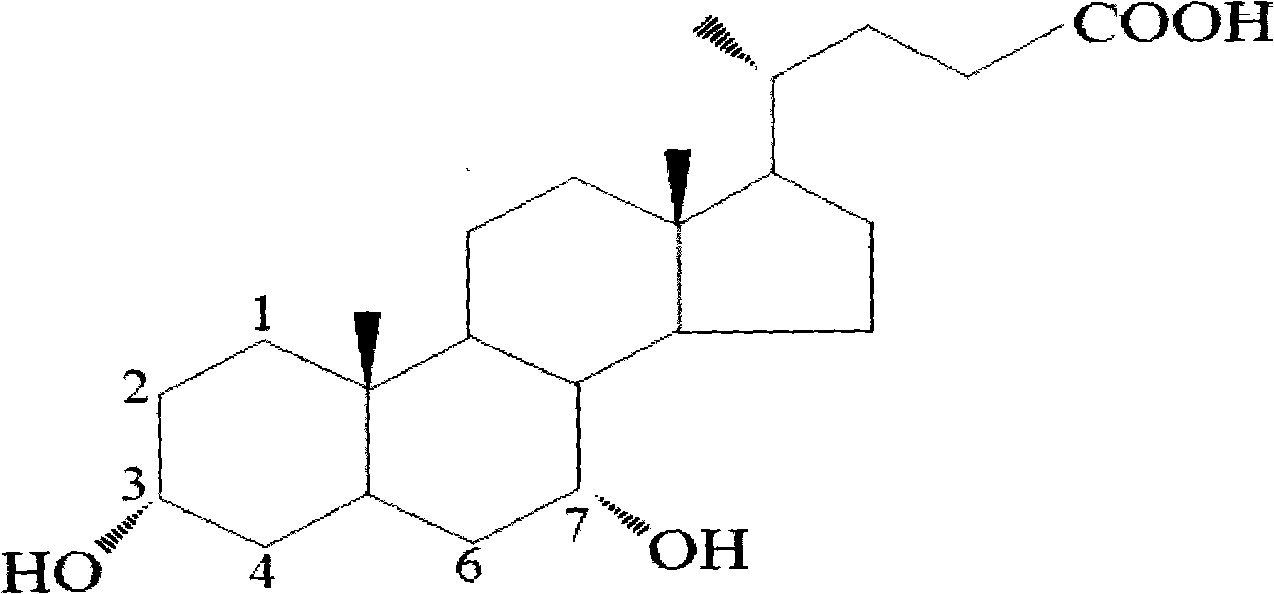
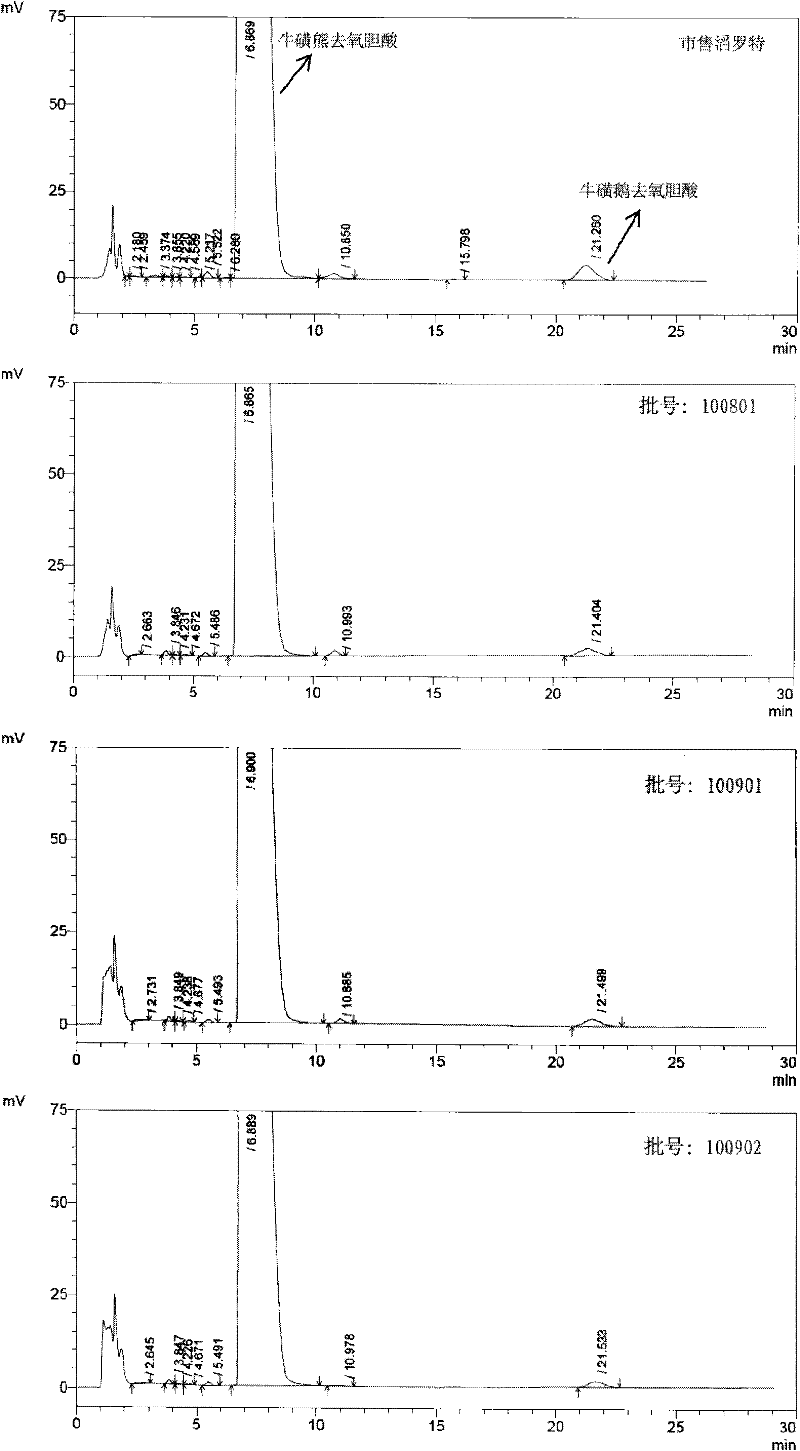
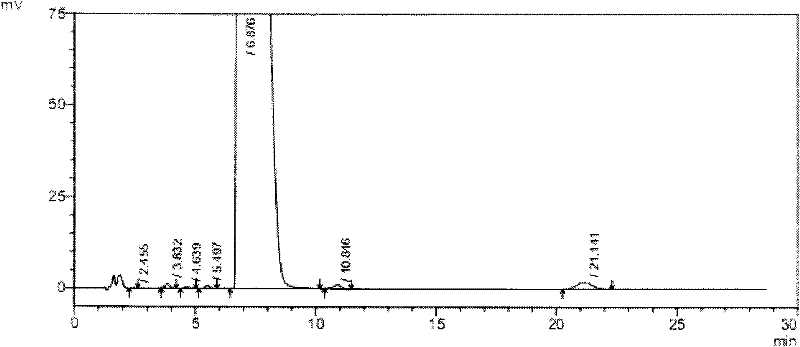
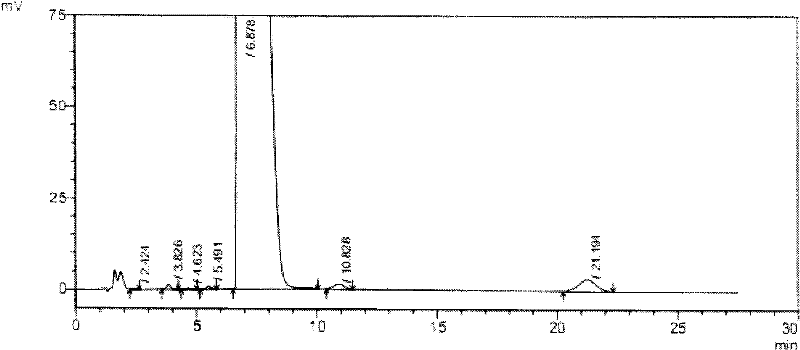
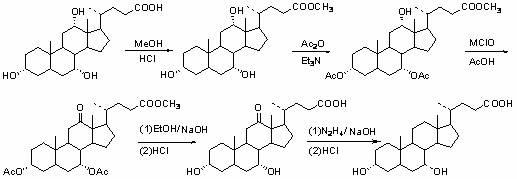
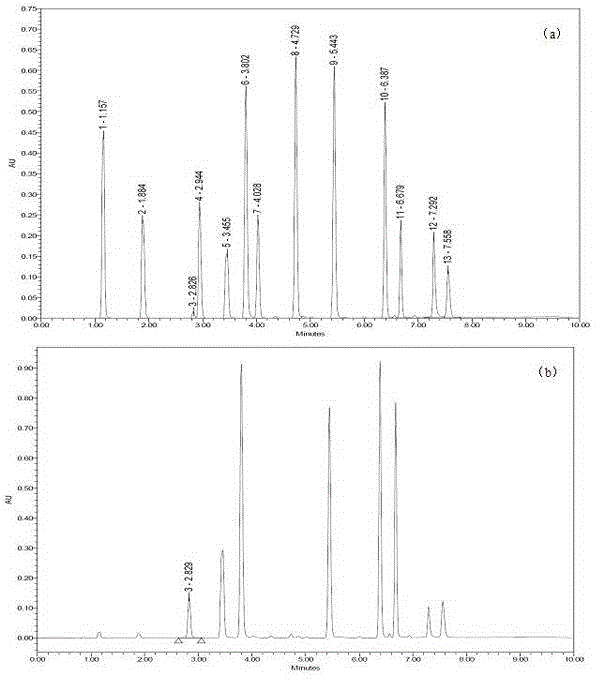
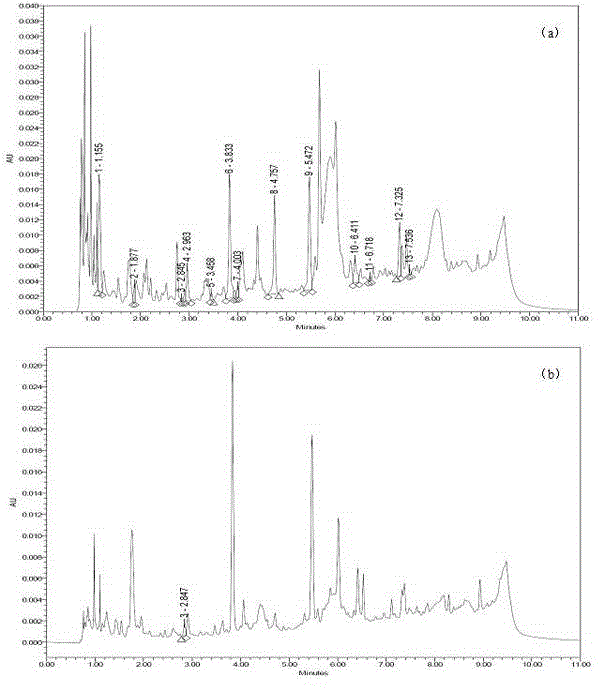
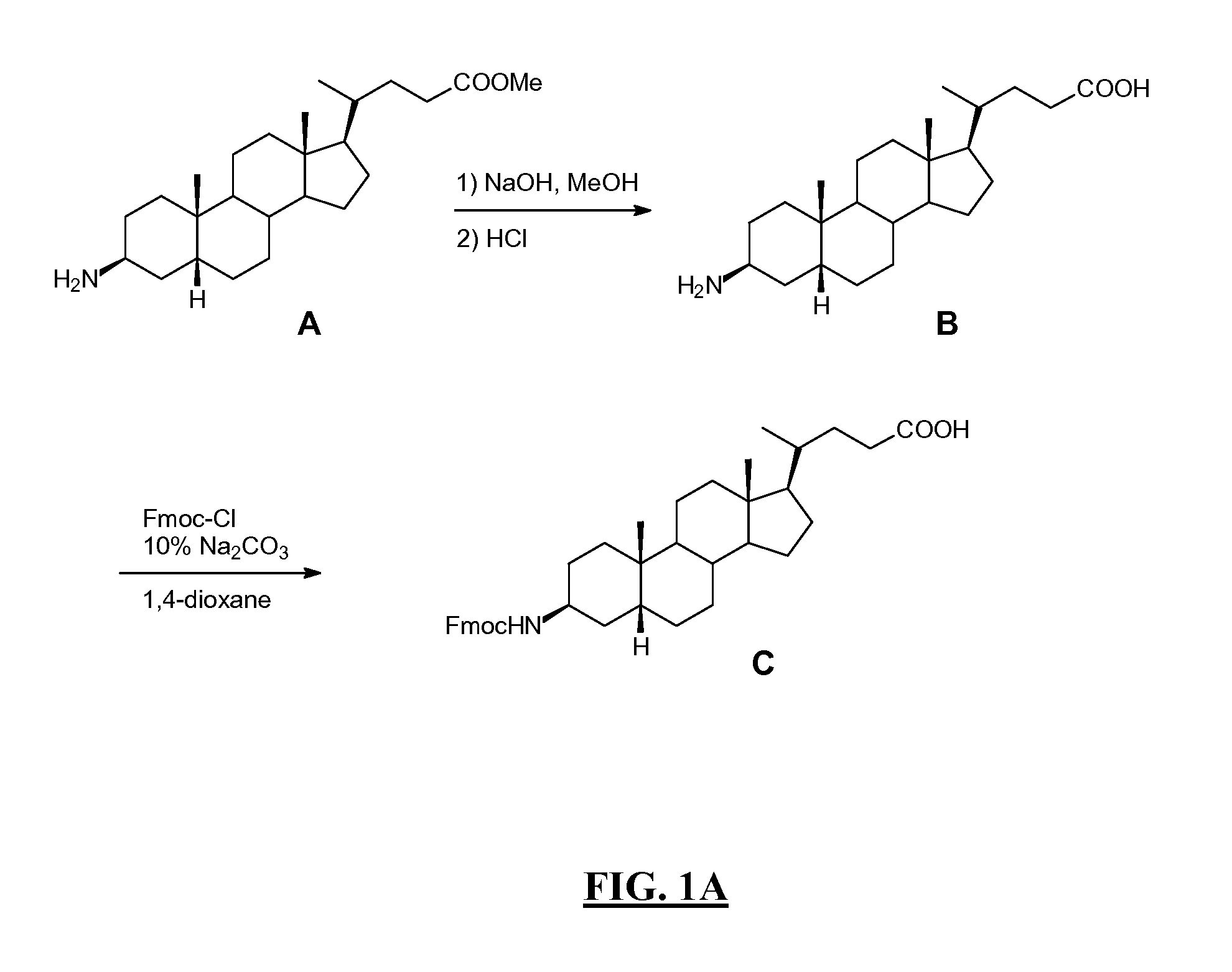
Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine
ActiveCN105669811ADifficult to purifyHigh purityMetabolism disorderSilicon compound active ingredientsKetoneMedicinal chemistry
The invention provides a preparation method of a 7-keto-6[alpha]-alkyl cholanic acid derivative. According to the preparation method provided by the invention, a 7-keto-6[beta]-alkyl cholanic acid derivative, as shown by a formula II, is used as a raw material, and the 7-keto-6[alpha]-alkyl cholanic acid derivative is prepared by converting a 6[beta] configuration into a 6[alpha] configuration under an acid or alkali condition. The invention also provides a 7-keto-6[beta]-alkyl cholanic acid derivative and an application thereof in preparation of 3[alpha],7[alpha]-dihydroxy-6[alpha]-alkyl-5[beta]-cholanic acid. The preparation method provided by the invention is simple and convenient, and is high in configuration conversion rate, and the product, the 7-keto-6[alpha]-alkyl cholanic acid derivative, is easy to purify, so that the purification difficulty for preparing the 3[alpha],7[alpha]-dihydroxy-6[alpha]-alkyl-5[beta]-cholanic acid is reduced.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Process for Preparing 3a(Beta)-7a(Beta)-Dihydroxy-6a(Beta)-Alkyl-5Beta-Cholanic Acid
ActiveUS20080214515A1Highly toxic hexamethylenphosphonamideOrganic active ingredientsMetabolism disorderCholic acidKetone
Process for preparing 3α-7α(β)-di-hydroxy-6α(β)-alkyl-5β-cholanic acid (I) in which R is a linear or branched C1-C5 alkyl and the relative intermediates 3α-hydroxy-6β-alkyl-7-keto-5β-cholanic (VIII) and 3α-hydroxy-6α-alkyl-7-keto-5β-cholanic (IX).
Owner:INTERCEPT PHARMA INC
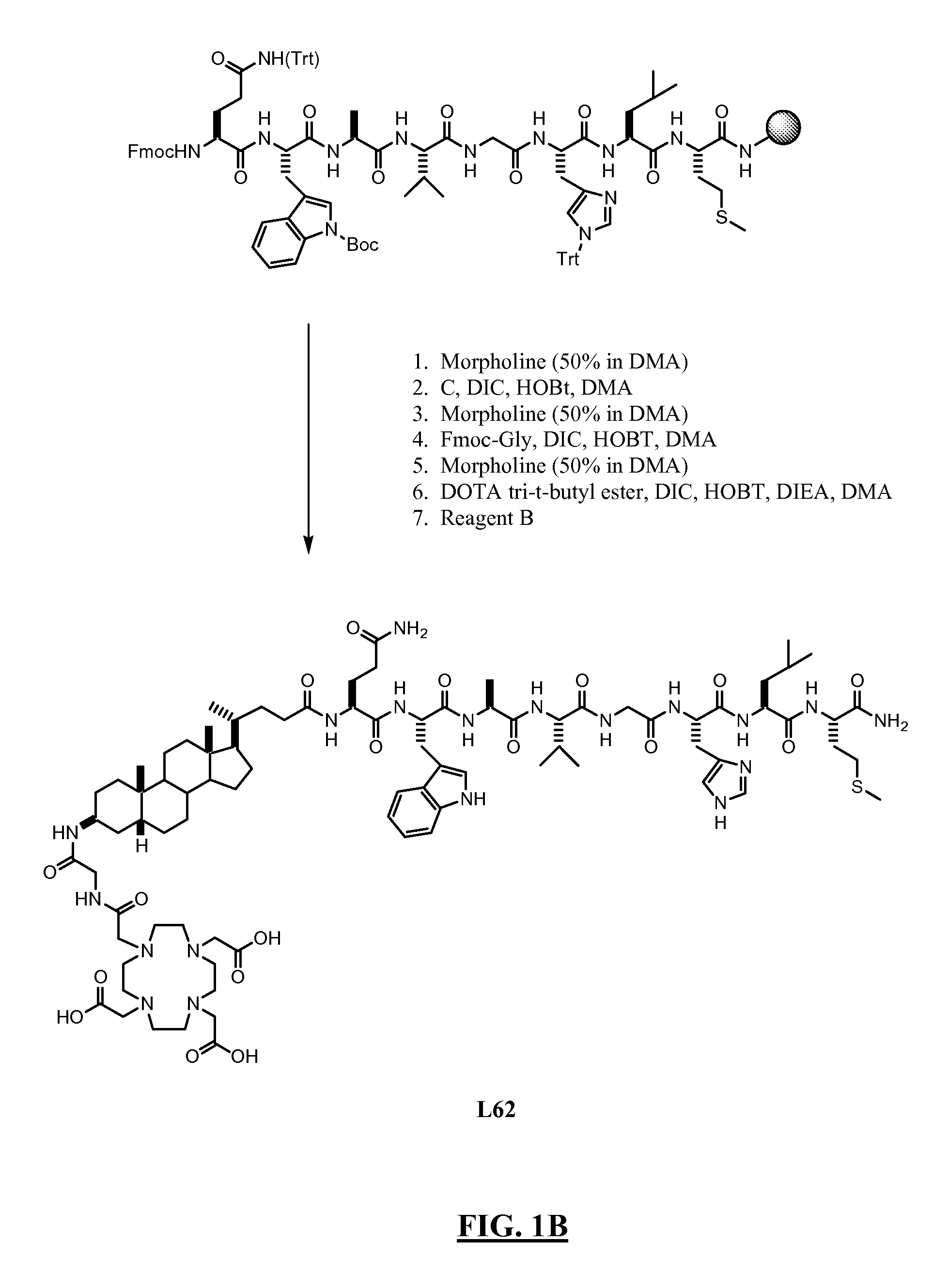
Method for preparing cholic acid conjugates
InactiveCN101307088AReduce pollutionRaw materials are cheap and easy to getPharmaceutical non-active ingredientsSteroidsCholic acidMorpholine
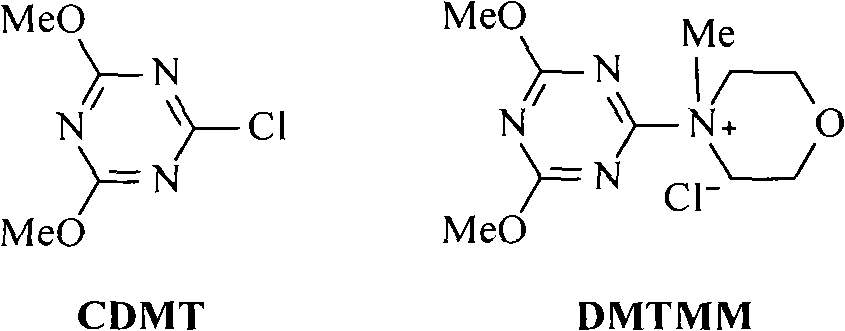
The invention provides a method for preparing a cholic acid coupler therapeutic medicine (I). The method adopts a taurine or glycinate compound as an initial material, and carries out a reaction to obtain the medicine (I) with the condensating agent of 2-chlorine-4,6-dimethoxy-1,3, 5-triazine or chloridized 4-(4,6- dimethoxy-1,3, 5-triazine-2-group)-4-methyl morpholine. The method has cheap and easily obtained raw material, gentle reaction conditions, solvent without water treatment, simple operation, high yield and friendly reaction environment. (in the formula, R1, R2, R3 represent H, alpha-OH, beta-OH, =O; R1, R2 and R3 can be the same or not the same; R represents CH2CH2SO3H, CH2COOH, CH2CH2SO3M, and CH2COOM; M represents metallic ions).
Owner:SICHUAN UNIV
Chemically amplified resist composition and patterning process
ActiveUS20160147150A1Increase contrastGood pattern profileElectric discharge tubesPhotosensitive materialsResistDissolution
A chemically amplified resist composition comprising a base polymer, an acid generator, and a basic compound which is a cholanoate having an acid labile group-protected amino group has a high contrast of alkaline dissolution rate before and after exposure and high resolution and forms a pattern of satisfactory profile with minimal roughness.
Owner:SHIN ETSU CHEM IND CO LTD
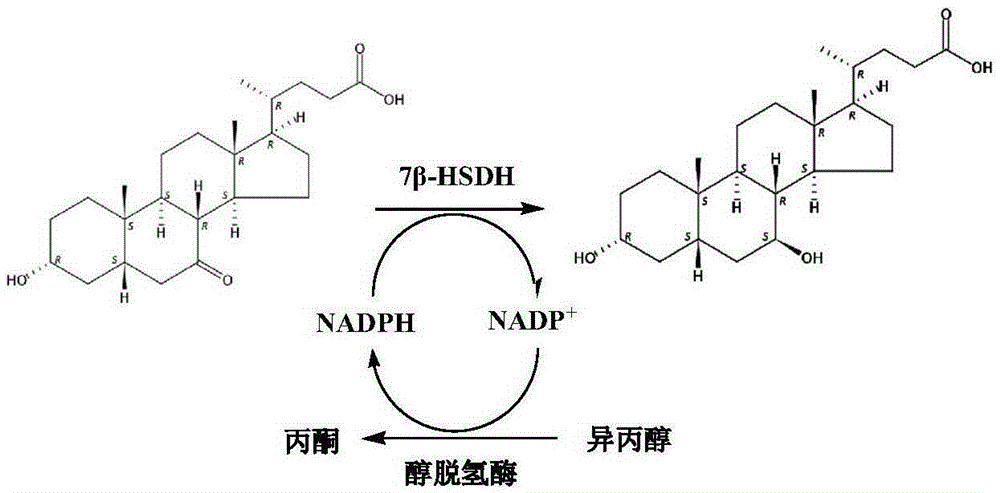
Mutant of 7 beta-hydroxyl steroid dehydrogenase, application of mutant and synthesis method
ActiveCN105274070ASuitable for industrial productionEasy to controlOxidoreductasesFermentationChemical synthesisCholic acid
The invention provides a mutant of 7 beta-hydroxyl steroid dehydrogenase, application of the mutant and a synthesis method. The mutant of the 7 beta-hydroxyl steroid dehydrogenase is characterized in that amino acid sequences of the mutant are Seq ID NO:4, and coded nucleotide sequences are Seq ID NO:3; or amino acid sequences of the mutant are Seq ID NO:6, and coded nucleotide sequences are Seq ID NO:5. The mutant, the application and the synthesis method have the advantages that cholic acid compounds, particularly ursodeoxycholic acid, can be catalytically synthesized by the efficient 7 beta-hydroxyl steroid dehydrogenase, mutant enzymes of the 7 beta-hydroxyl steroid dehydrogenase and coenzyme regeneration systems, accordingly, the substrate concentration can reach 100 g / L, the conversion rate is 99.2-99.5%, and the weight yield can reach 94-96%; and the enzymes can be inexpensively and easily obtained by the aid of a fermentation process, accordingly, the production cost and the product quality are superior to the production cost and the product quality of chemical synthesis methods, and the mutant and the synthesis method are applicable to industrial production.
Owner:苏州天绿生物制药有限公司
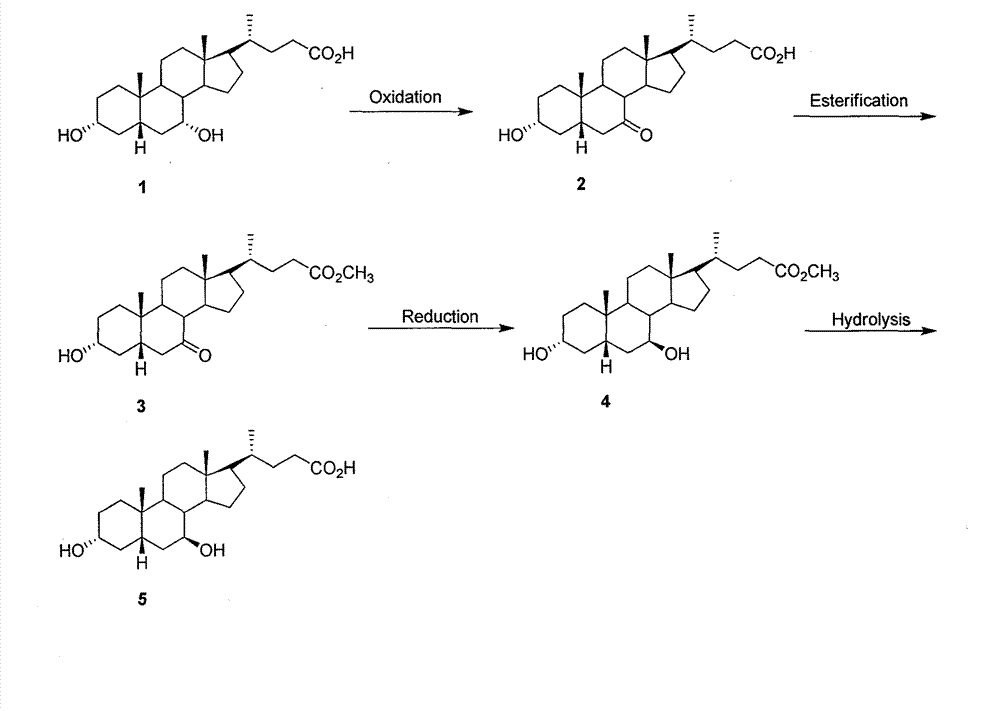
Preparation method of ursodeoxycholic acid
The invention provides a preparation method of ursodeoxycholic acid. Commercial available chenodeoxycholic acid is taken as raw materials, the ursodeoxycholic acid is obtained by four steps including selective oxidation, esterification, deoxidation and hydrolyzation, and the total yield is 85.7%. In a mixture of acetone and water, NBS is used for selective oxidation of hydroxy at C- 7 bit of the chenodeoxycholic acid, and the selective oxidation possesses excellent selectivity and high yield. NaBH14 / CeC13 may be used to deoxidize carbonyl at C-7 bit into hydroxy, and the ratio of alpha / beta is as high as 5 / 95.
Owner:EAST CHINA UNIV OF SCI & TECH
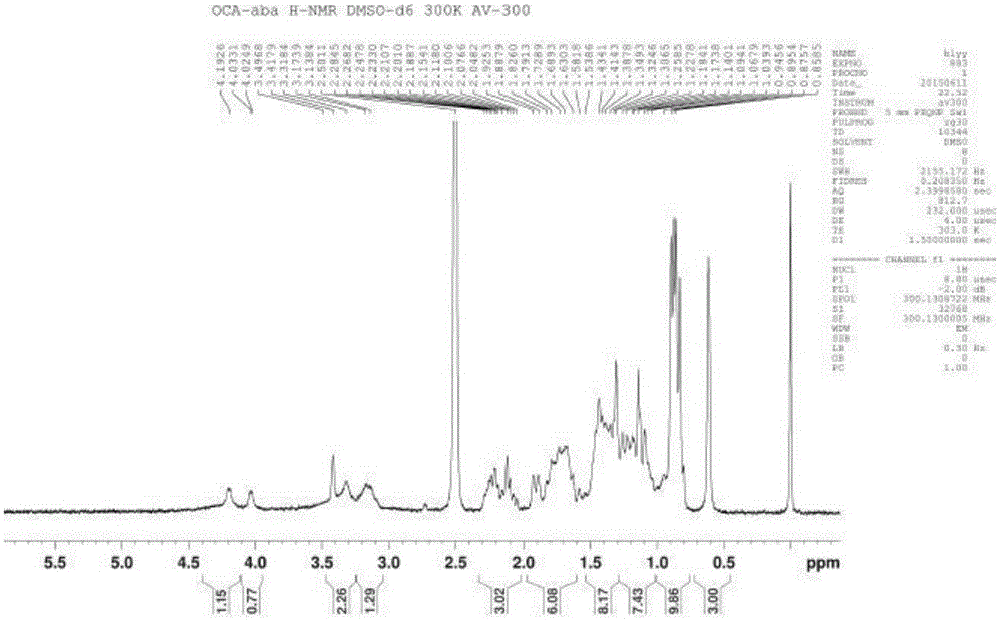
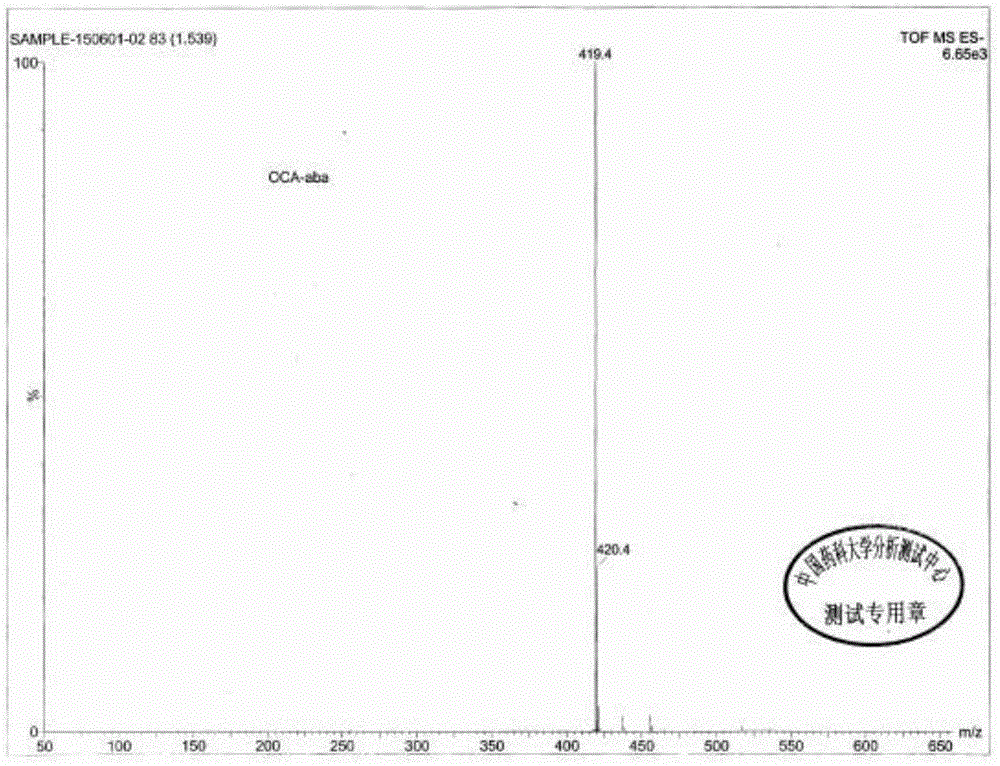
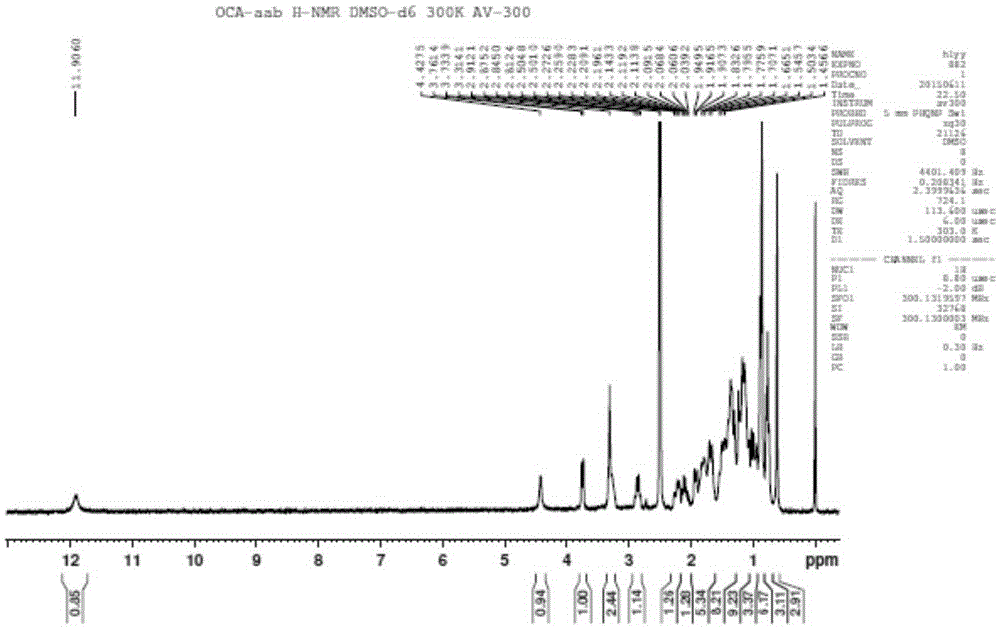
Method for synthesizing, separating and determining obeticholic acid (OCA) isomer
The invention relates to an obeticholic acid (OCA) isomer namely an OCA-alpha alpha beta body, a synthetic method of the OCA-alpha beta alpha body, and a method adopting the reversed phase liquid chromatography condition to separate the OCA isomer. With adoption of the technical scheme disclosed by the invention, the OCA-alpha alpha beta body and the OCA-alpha beta alpha body with the HPLC purity greater than 98% can be obtained to meet quality control of the isomer in OCA.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
Method of in vitro culturing medicine ox gallstone with natural ox gall
InactiveCN1347700AComplete process conditionsStable contentUnknown materialsDeoxycholic acidGallstones
Natural ox gall is first taurine regulated and bacteria fermented to prepare fermented ox gall. The fermented ox gall is gall is then mixed with clear saturated calcium salt solution through stirring, heated to boil, filtered to obtain red brown precipitate, which is stirred, washed, filtered to eliminate supernatant and mixed with bilirubin, cholalic acid, deoxycholic acid, inorganic salt and other effective components to obtain composite bilirubin calcium. Fermented ox gall and bilirubin calcium in certain proportion are mixed and the pH of mixture is regulated to below 6.8 and directional off axis rotated to form gallstone, and the gallstone is rest cultured, taken out and dried. The said process can obtain ox gallstone product with the same characteristics, structure, component and medicinal effect as high quality natural bezoar.
Owner:武汉键民大鹏药业有限公司
Preparation method of ursodesoxycholic acid
ActiveCN101987860AAvoid disadvantagesHigh puritySteroidsBulk chemical productionCholic acidChenodeoxycholic acid
The invention discloses a preparation method of ursodesoxycholic acid, which comprises the flowing steps of: a. carrying out 3-bit esterification on chenodeoxycholic acid to obtain 3-esterification protected chenodeoxycholic acid; b. carrying out 7-bit oxidation reaction on 3-bit ester to obtain 3-estergroup-7-oxocompounds; c. carrying out hydrolysis reaction or reduction reaction on the 3-estergroup-7-oxocompounds; d, carrying out 7-bit reduction reaction on 3a-hydroxy-7-oxo-5 beta-cholic acid or hydrolysis reaction on 3a-estergroup-7 beta- hydroxyl-5 beta-cholic acid to obtain crude products of the ursodesoxycholic acid; e. forming salt through the crude products of the ursodesoxycholic acid and organic base; and f. carrying out steps such as water adding dissolution, acid adding crystallization and the like on ursodesoxycholate to obtain pure ursodesoxycholic acid products with the purity higher than 99.0 percent. The invention aims at overcoming the defects in the prior art to provide a novel method for preparing the ursodesoxycholic acid, and the ursodesoxycholic acid prepared by the method has high yield and high purity.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
High efficiency technique for extracting bilirubin and bile acid by using animal bile as raw material
The present invention relates to process of extracting cholic acid, deoxycholic acid and bilirubin from bile of pig, ox and sheep. Bile of pig, ox and sheep consists of water in about 97 %, bile acid in about 2.5 % and bilirubin in about 0.4 %; and contains also phospholipid, cholesterol, Na, K, Ca, phosphate, carbonate, small amount of protein, and other components. Fresh bile is treated through cooling, filtering to defat, basic hydrolysis, acidification and organic solvent extracting to obtain bilirubin; the rest solution is further treated through deep saponification, acidification and organic solvent precipitation to obtain the mixture of cholic acid and deoxycholic acid; and the mixture is re-crystallization separated to obtain high purity cholic acid and deoxycholic acid.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Production method for extracting chenodeoxycholic acid using chicken gall
InactiveCN1850846AEasy to separateSimple processUnknown materialsSteroidsCholic acidChenodeoxycholic acid
The feature of the invention includes: adopting frozen chicken gallbladder slicing, heating to 70 degree centigrade, adding 10% weight of the bile boiling for 24 hours, reflux cooling, adjusting the pH value by hydrochloric acid to gain cream, washing to neutrality; adding 95% alcohol and 10% diatomite, reflux cooling and adding active carbon, reflux, cooling and filtering, adding petrol to the filtrate to take degreasing, reflux, standing, separating alcohol liquid and washing to neutrality in reaction kettle, adding 95% alcohol, after resolving, adding barium chloride and active carbon, reflux, hot filtering, concentrating white crystal from the filtrate, washing, and adding water and sodium carbonate water solution, heating and reflux, filtering, adjusting the pH value, drying. The invention has simple technology, low cost, and could drastically separate cholesterol, lithocholic acid, and cholic acid from gall, and improves the purity.
Owner:辽宁百隆生物工程有限公司
Stable freeze-dried formulation containing multiple kinds of vitamin and its preparation method
InactiveCN1843327AImprove stabilityPowder deliveryHydroxy compound active ingredientsFreeze-dryingWater soluble
The invention relates to a stable compound containing a plurality of vitamins and the method for preparing same. The compound comprises at least four kinds of fat-soluble vitamin, cholate, phosphatide and carriers or findings acceptable by pharmacy. The compound also comprises water soluble vitamins. The compound is a clear and transparent solution. The preparing method comprises: (1) dissolving the fat-soluble vitamin with liquid containing ethanol; (2) removing ethanol. The compound is used as nutriment for patients.
Owner:CHONGQING PHARMA RES INST +1
Regulation of mammalian keratinous tissue using skin and/or hair care actives
Personal care compositions containing an active selected from the group consisting of phlorogine, phlorgine BG, deoxyArbutin, sucrose dilaurate, bakuchiol, pyrenoine, millet, arlatone dioic acid, cinnamic acid, ferulic acid, achromaxyl, methyl nicotinamide, oil soluble licorice extract, folic acid, undecylenic acid, zinc undecylenate, L-tryptophan, thiamine HCl, hexylresorcinol, lipidami red vine, dragosine, methyl gentisate, inositol, symdiol 68, laminaine, their salts, their derivatives, their precursors, and / or combinations thereof. Methods for regulating the condition of mammalian keratinous tissue by topically applying the personal care compositions are also provided.
Owner:THE PROCTER & GAMBLE COMPANY
High-purity tauro ursodesoxy cholic acid and preparation method thereof
ActiveCN102477059AHigh purityImprove securityOrganic active ingredientsDigestive systemEthyl chloroformateCholic acid
The invention relates to high-purity tauro ursodesoxy cholic acid and a preparation method thereof. The content of taurochenodeoxycholic acid in the tauro ursodesoxy cholic acid is less than 0.7%. The tauro ursodesoxy cholic acid is safe and effective and does not have toxic and side effects in clinical application. The invention further provides a mixed anhydride reaction of ursodesoxycholic acid and ethyl chloroformate by taking acetone as a solvent. By means of control of a reaction condition and a reaction solvent, the tauro ursodesoxy cholic acid has the advantages of simple process, low cost, environmental friendlessness and industrial production; furthermore, the high-purity tauro ursodesoxy cholic acid can be obtained.
Owner:CHENGDU GUOHONG PHARMA
Reagents for intracellular delivery of macromolecules
InactiveUS6989434B1Increase rangeImprove delivery efficiencyBiocidePeptide/protein ingredientsCholic acidLipid formation
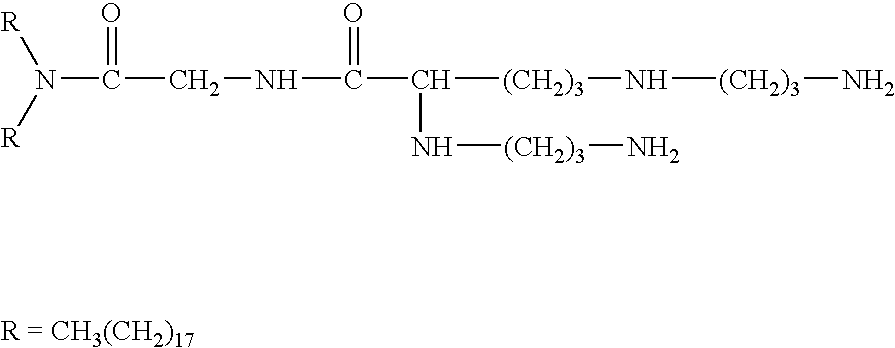
The present invention provides certain cationic lipids containing stigmasterol, ergosterol and cholic acid groups. Compounds of the invention are useful, either alone or in combination with other lipid aggregate-forming components (e.g., DOPE, DOSPA, DOTMA or cholesterol) for formulation into liposomes or other lipid aggregates. Such aggregates are cationic, and able to form stable complexes with anionic macromolecules, such as nucleic acids. The cationic lipids of the invention are useful in methods of transfecting cells, particularly to introduce nucleic acids into cells. The invention also related to kits for the preparation of lipid aggregates and to lipid aggregates and compositions for transfection of cells.
Owner:LIFE TECH CORP
Preparation and application of amphiphilic albumin derivative and pharmaceutical composition thereof
InactiveCN101543630AEvade captureHigh drug loadingPharmaceutical delivery mechanismMacromolecular non-active ingredientsCholic acidWater insoluble
The invention relates to a biodegradable amphiphilic albumin derivative and a pharmaceutical composition thereof. In the biodegradable amphiphilic albumin derivative, hydrophilic long-chain polyethyleneglycol and alkyl (acyl) group or (deoxidized) cholic acid are led to an albumin skeleton so that the amphiphilic albumin derivative is amphipathic and is self-assembled in water to form nano-micelle. The biodegradable amphiphilic albumin derivative is characterized in that medicament can be encapsulated through the double actions of a hydrophobic group and an albumin molecule chain with the medicament, and the capacity of the albumin encapsulating the medicament is markedly improved; in addition, a hydrophilic long-chain can reduce the immunogenicity of the albumin and improve the nano-micelle surface hydrophilicity so that the stability of the nano-micelle in aqueous medium can be improved and the nano-micelle has long-circulation characteristics in a body. The pharmaceutical composition of the biodegradable amphiphilic albumin derivative can be used as the carrier of organic medicament, water-insoluble or insoluble medicament and amphiphilic medicament, can be used for intravascular administration, intramuscular injection administration, oral administration, cavitary administration or external administration. The invention can be prepared with simple method and mature process and is suitable of large-scale continuous production.
Owner:CHINA PHARM UNIV
Method for producing high-purity chenodeoxy cholic acid from poultry and livestock bile
InactiveCN1775798ASimple extraction processSimple production processAntiinfectivesSteroids preparationCholic acidBile Juice
The invention discloses a method to manufacturing high purity chemocholic acid from fowl bile that belongs to refinement chemical and medicine field. It includes the following steps: a. saponification reaction: mixing the fowl bile and sodium hydroxide and reacting for 20-30 hours under the certain temperature; b. neutral reaction: adding dilute hydrochloric acid after saponification reaction and gaining raw cholic acid after filtering; c. column chromatography: adding dilute sodium hydroxide into raw cholic acid and injecting into chromatography column, uniting the eluent; super filtering: taking super filtering by super filtering film; e. gaining chemocholic acid that the purity is over 96% after adding dilute acid, filtering and drying. The invention has simple technology, high purity, low cost and environment protection.
Owner:SHANDONG BOERDE BIOLOGICAL SCI & TECH
Separation purification preparation method of chenodeoxycholic acid in pig's bile
InactiveCN1869044ASimple and fast operationLow costUnknown materialsSteroidsCholic acidChenodeoxycholic acid
A process for separating the chenodeoxycholic acid from pig's gall and purifying it includes such steps as preparing general cholic acid from the mother liquid generated by extracting the cholerythrin from pig's gall, saponifying, regulating pH value to obtain crude chenodeoxycholic acid, decoloring, defatting, preparing the deposit of barium chenodeoxycholate, reacting on potassium carbonate to remove Ba, regulating pH value, and purifying by silicon gel column.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Chenodeoxycholic acid synthesis method
The invention relates to a chenodeoxycholic acid (3 alpha, 7 alpha-dihydroxyl-5 belta-cholestane-24-acid) chemical synthesis method, belonging to the field of organic chemical synthesis. Chenodeoxycholic acid is prepared by the following steps of: (1) preparing cholate; (2) preparing 3 alpha, 7 alpha-acetyl-12 alpha-hydroxy cholate; (3) preparing 3 alpha, 7 alpha-acetyl-12-oxo-chenodeoxycholic acid ester; (4) preparing 12-oxo-chenodeoxycholic acid; and (5) preparing chenodeoxycholic acid. The application of the method for synthesizing chenodeoxycholic acid has the advantages of high yield rate, low cost and no pollution and is particularly convenient for industrial production.
Owner:ZHENGZHOU UNIV
Stabilised radiopharmaceutical compositions
InactiveUS20040057899A1Good water solubilityMaintain good propertiesPowder deliverySolution deliveryImaging agentAscorbic acid
The present invention relates to stabilised <99m>Tc radiopharmaceutical compositions, which include both a radioprotectant and one or more antimicrobial preservative(s), and hence have an extended lifetime of use. The radioprotectant is ascorbic acid, para-aminobenzoic acid, gentisic acid or a salt thereof with a biocompatible cation, and the antimicrobial preservative is one or more compound from the paraben series of preservatives. The invention is particularly useful for cationic, lipophilic <99m>Tc heart imaging agents such as Myoview(TM).
Owner:GE HEALTHCARE LTD
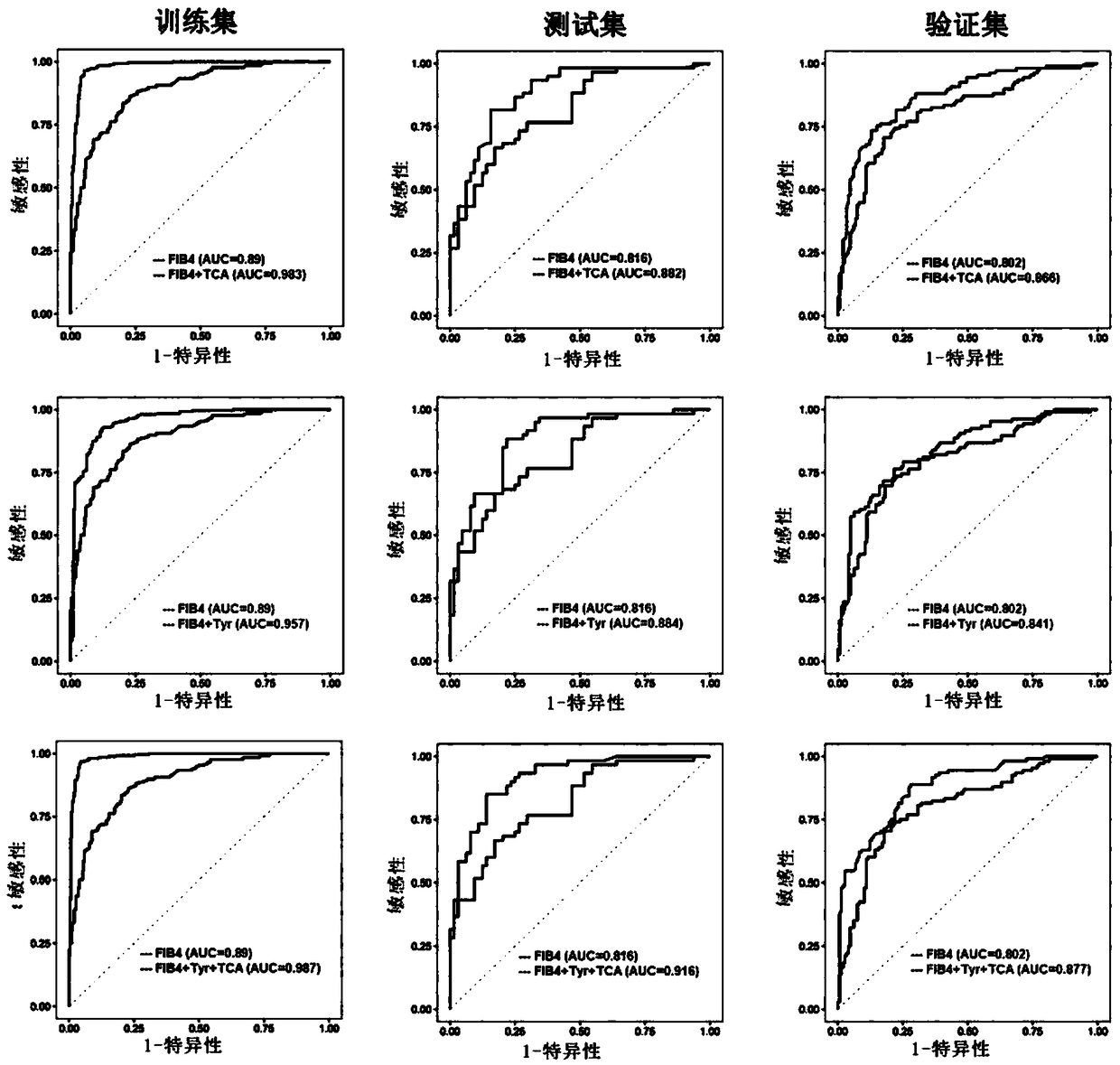
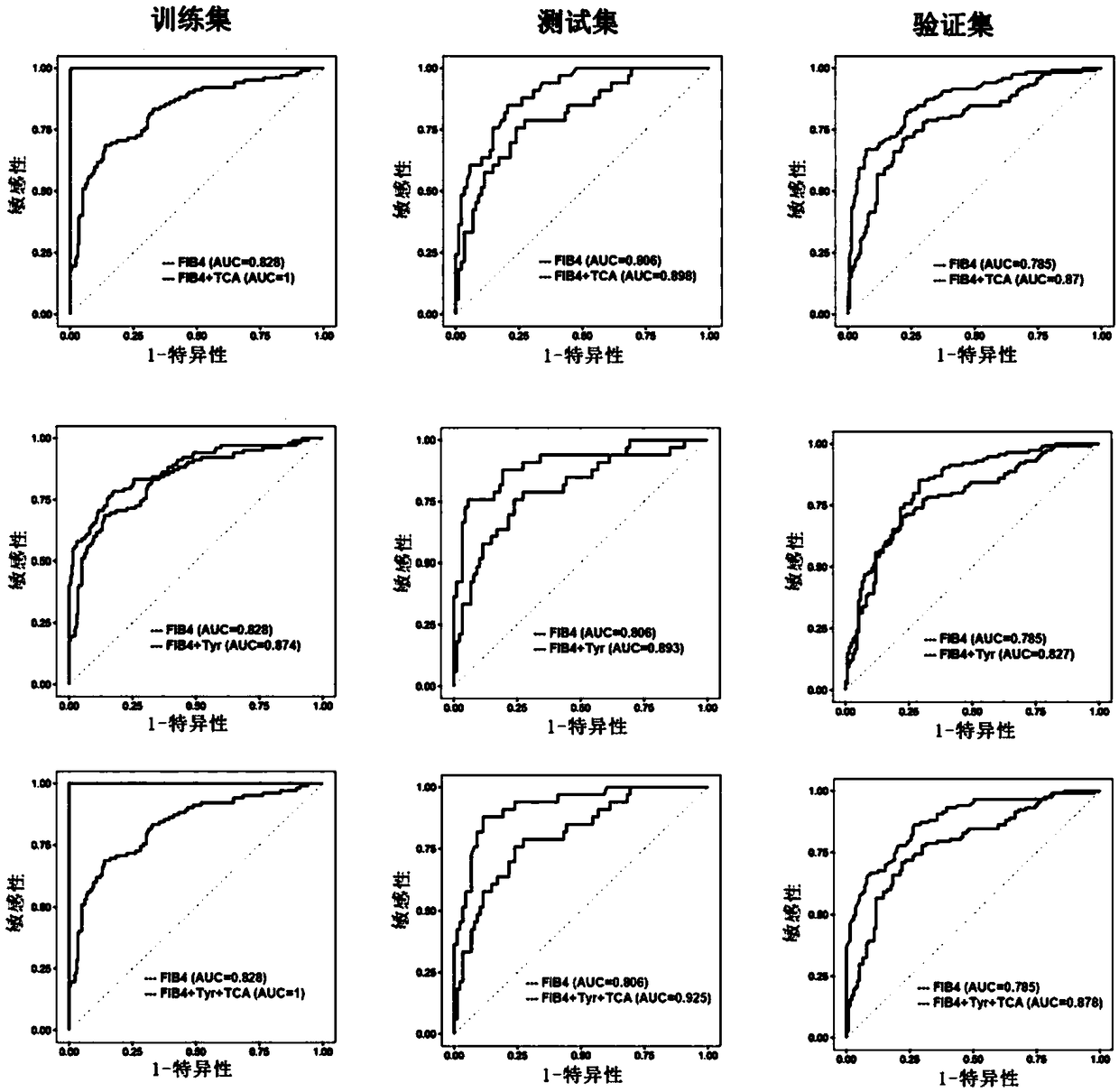
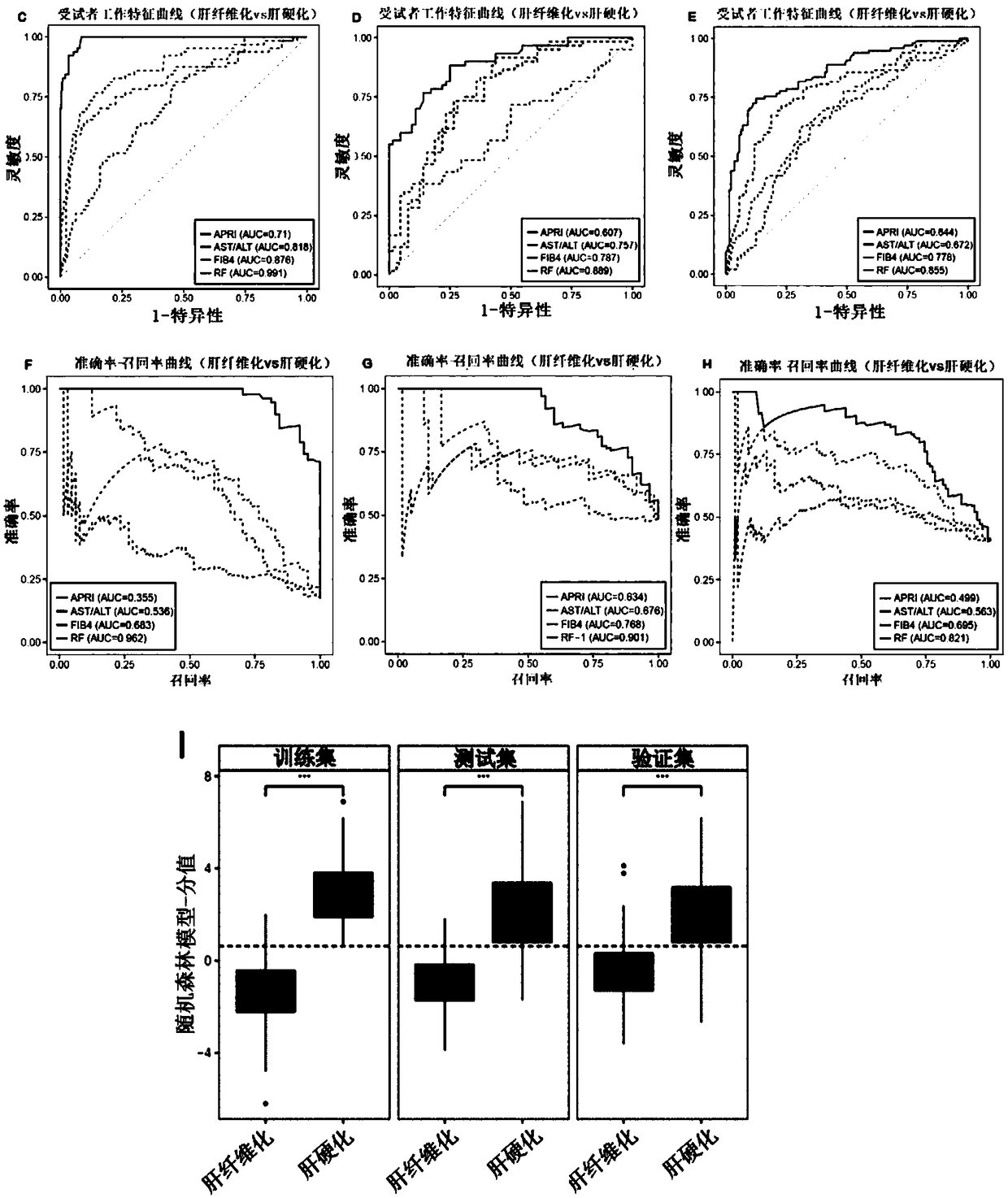
Biomarkers and kits for diagnosis of liver fibrosis and cirrhosis and use method
The invention provides a group of biomarkers which can be used for detecting liver fibrosis and cirrhosis. The biomarkers refer to metabolite components existing in a biological sample of a subject body and comprise a plurality of metabolites, wherein the metabolites are selected from cholic acid, amino acid and fatty acid. Metabolite combination comprises at least one amino acid, fatty acid and cholic acid, the metabolite combination is differentially expressed in at least one target plasma or serum and in a control plasma or serum. The biomarkers can optionally be combined with clinical indicators for diagnosis of liver fibrosis in the subject body. The biomarker composition has the characteristics of high sensitivity and specificity for pathological staging diagnosis of liver fibrosis patients, and can be clinically applied as a non-invasive means to improve clinical diagnosis and reduce puncture pain of the patients. The invention further provides a use method of the biomarkers andkits containing the biomarkers.
Owner:HUMAN METABOLOMICS INST INC
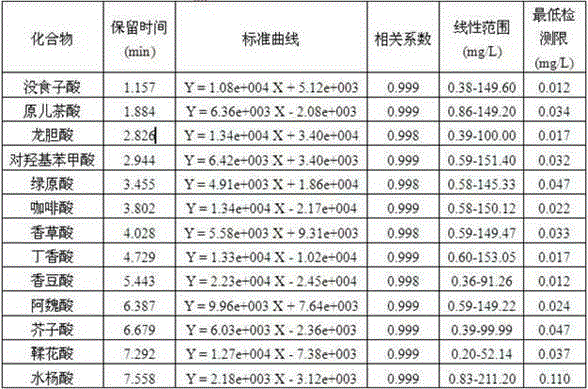
Method for fast and high-efficient determination of phenolic acids in grape wine
The invention relates to a method for fast and high-efficient determination of 13 phenolic acids in grape wine, belonging to the technical field of analysis of flavor substances in the grape wine. According to the method disclosed by the invention, ultra-performance liquid chromatography is utilized for performing qualitative and quantitative analysis on phenolic acid substances in the grape wine, and the method specifically comprises the following steps: selecting a BEHC18 chromatographic column, taking 2% acetic acid water and methanol as a mobile phase, setting the flow rate at 0.3mL / min, performing ultraviolet detection on wavelength to get the result that the wavelength of gentisic acid is 320nm and the wavelength of the remainder is 280nm, setting the column temperature at 50 DEG C, setting the sample injection quantity at 1 mu L, and quantifying by a peak area external reference method; and by adopting the method, the detection and the analysis can be simultaneously performed on the 13 phenolic acids in the grape wine within 10min. According to the method disclosed by the invention, any sample pretreatment is not required, and the loss caused by the pretreatment can be avoided; and compared with traditional HPLC (high-performance liquid chromatography), by adopting the method disclosed by the invention, more phenolic acid type compounds can be simultaneously detected, the separation effect is good, the analysis process is greatly shortened, convenient and easy to operate, the detection efficiency is greatly improved and the cost of the mobile phase is saved. The method disclosed by the invention has important significance for further system research of the flavor substances in the grape wine and improvement of the quality of the grape wine.
Owner:JIANGNAN UNIV
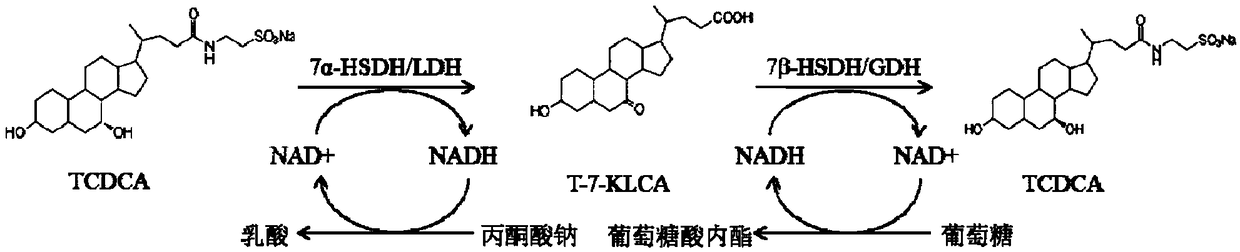
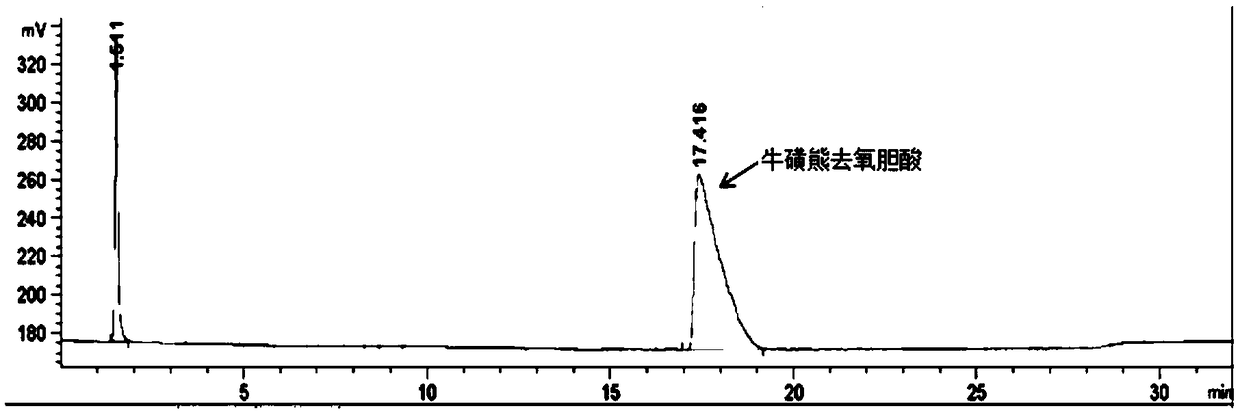
Method of preparing tauroursodeoxycholic acid by biotransformation and application of method
ActiveCN109402212AImprove conversion rateAvoid affinity purificationAntibody mimetics/scaffoldsNucleic acid vectorDrug biotransformationSubstrate concentration
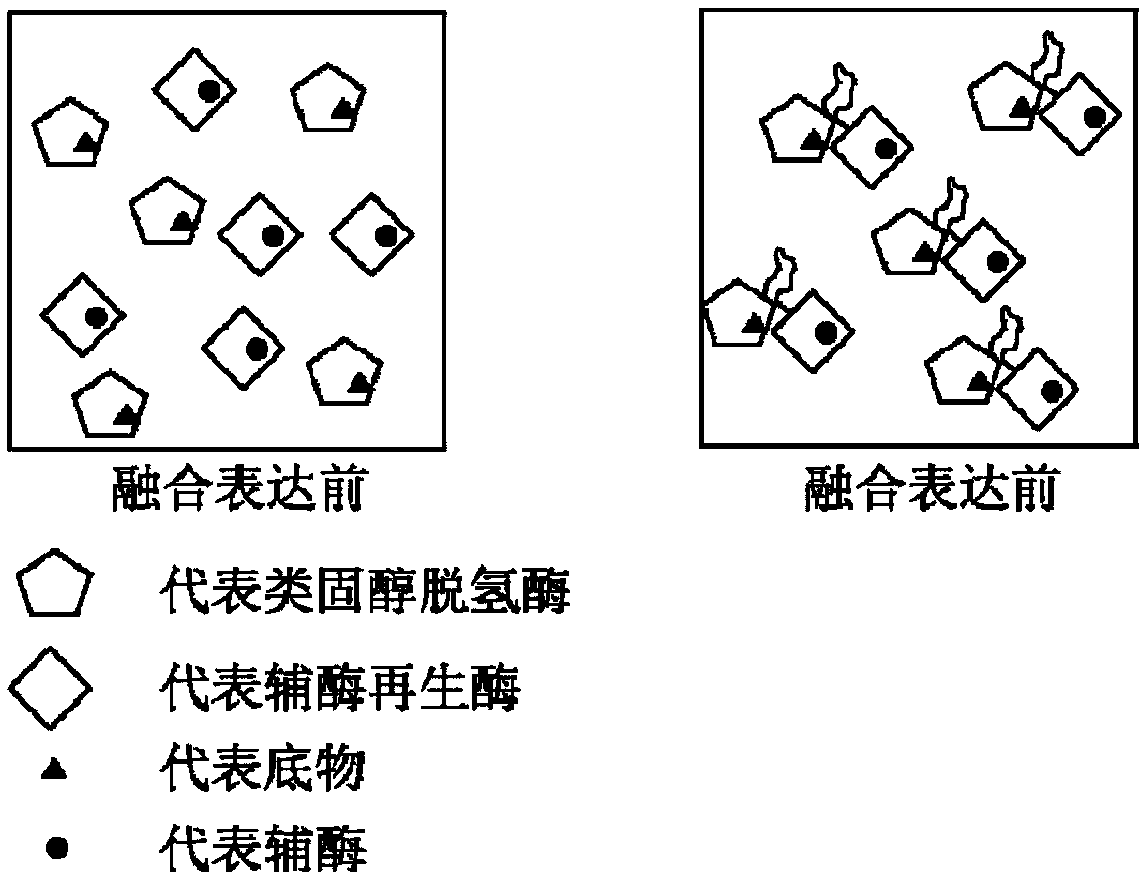
The invention discloses a method of preparing tauroursodeoxycholic acid by biotransformation and application of the method. Biotransformation includes genetic codon optimization, engineered bacteria construction, engineered bacteria cultivation, substrate transformation and product preparation. Tauroursodeoxycholic acid is prepared by transforming a substrate through direct fermentation of engineered bacteria; the substrate is taurochenodeoxycholic acid. The substrate may reach 250 g / L in concentration; the reaction time is short; substrate transformation rate reaches 98% and above; the obtained product reaches 99% and above in purity; cyclic regeneration of NAD+ (nicotinamide adenine dinucleotide +) in the reaction system helps greatly reduce the usage of the coenzyme NAD+; the cost of enzymic catalytic reaction is reduced; industrial amplification is benefited. hydroxysteroid dehydrogenase and the regenerated coenzyme are connected via a flexible polypeptide sequence to form a protein fusion polymer; binding distances to the substrate and coenzyme are shorter; transformation progress is more facilitated; the number of fermenting times in industrial production is decreased; the process is simplified; time cost and material cost are saved.
Owner:JIANGSU BANGZE BIOLOGICAL MEDICINE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka.patsnap.com/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000011.PNG)
![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka.patsnap.com/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000021.PNG)
![Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine Novel application of 7-keto-6[beta]-alkyl cholanic acid derivative in preparation of obeticholic acid and in field of medicine](https://images-eureka.patsnap.com/patent_img/56d80b07-fa92-4053-887a-acb6f82379a0/BDA0000609078430000022.PNG)