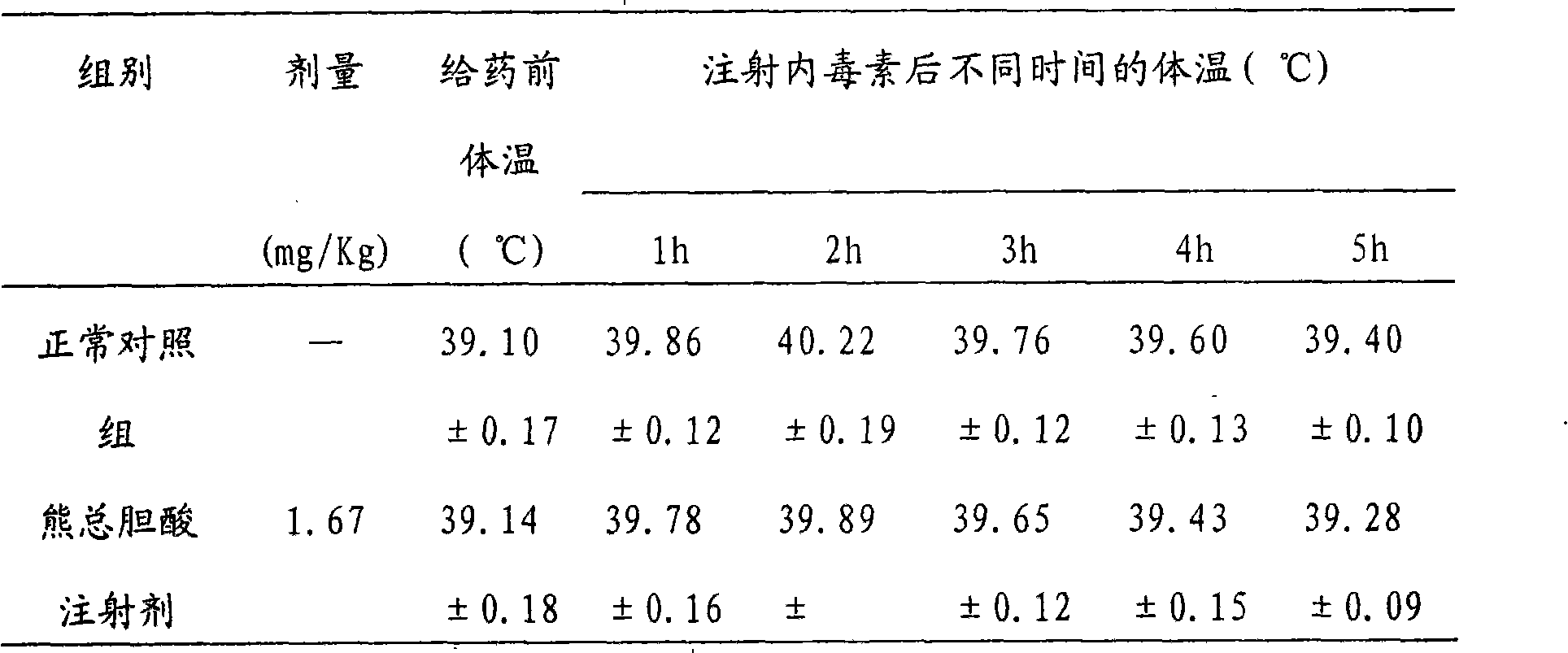
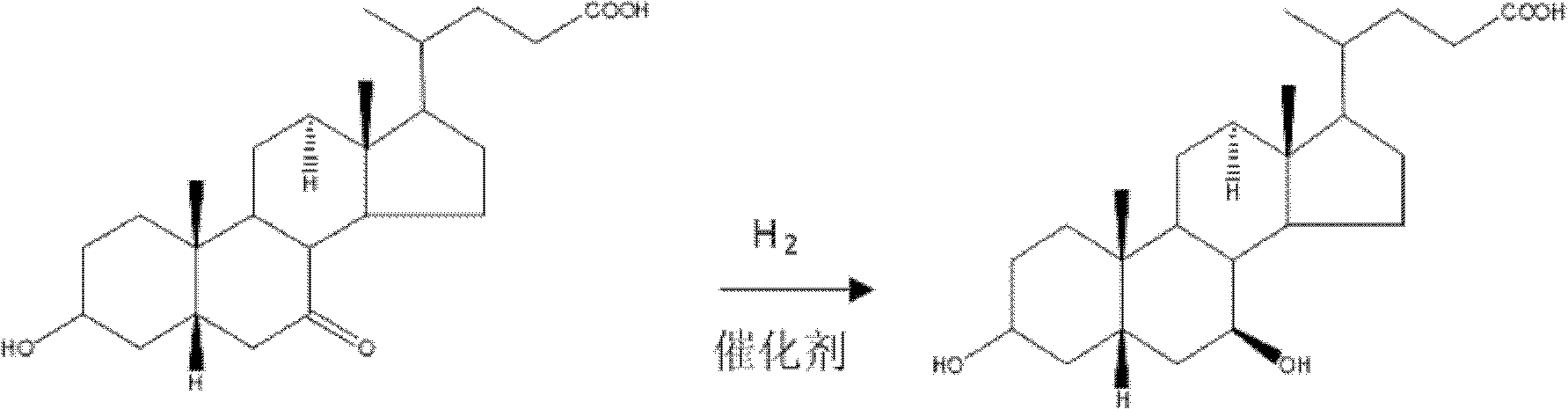
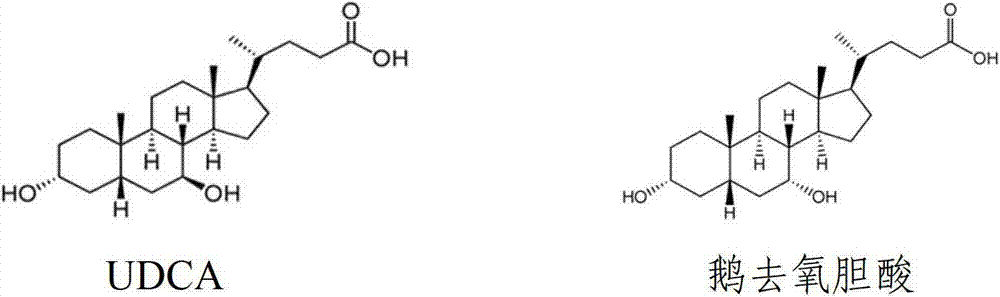
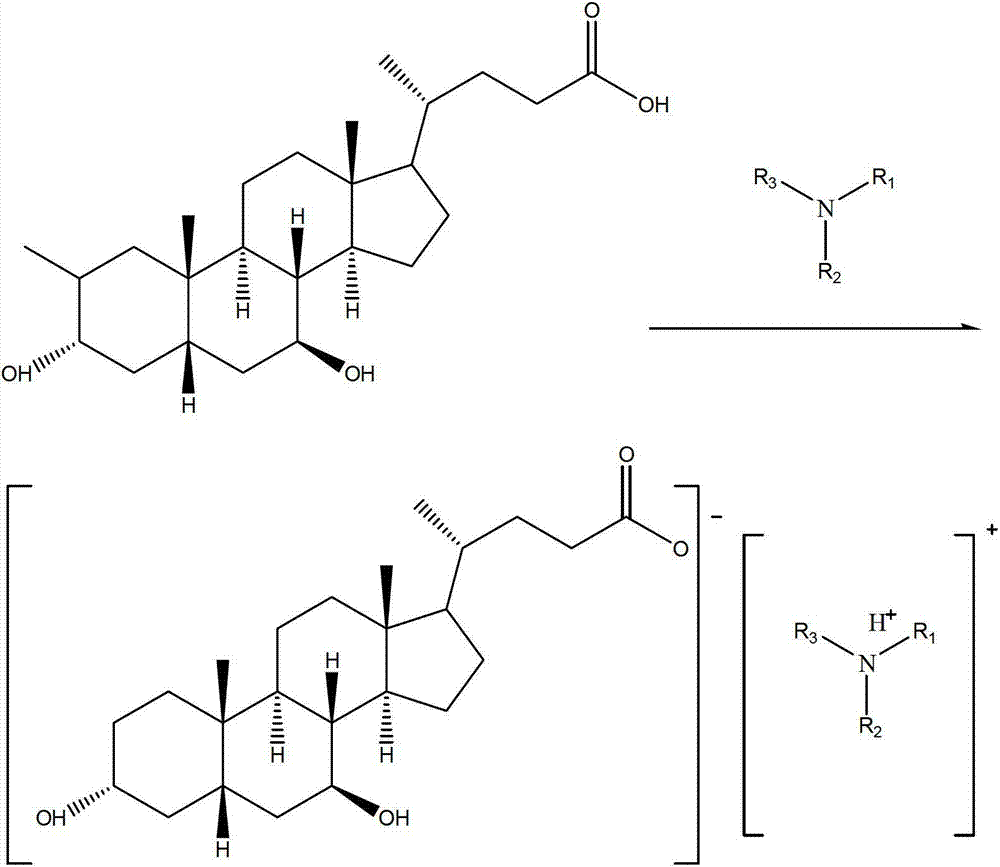
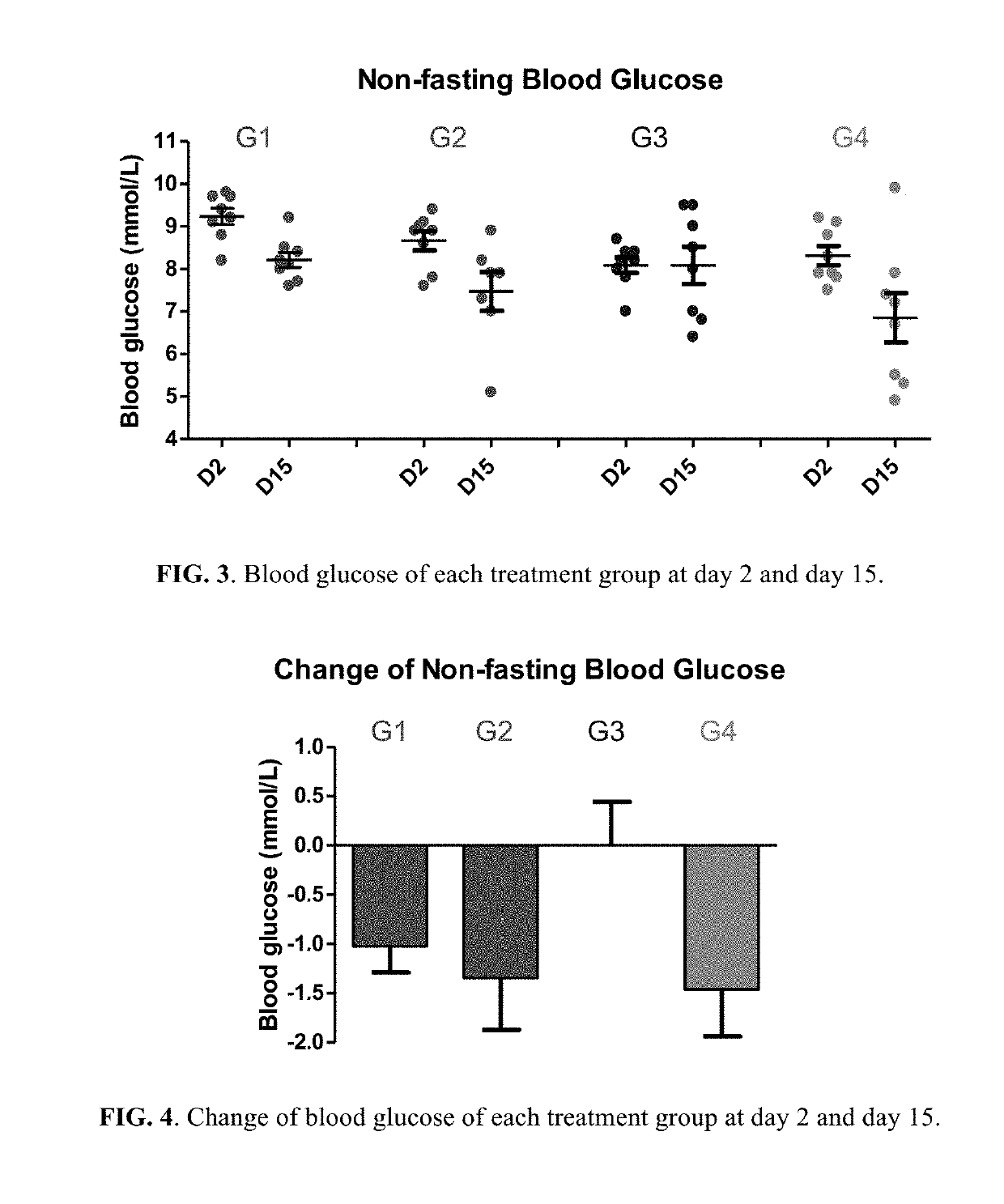
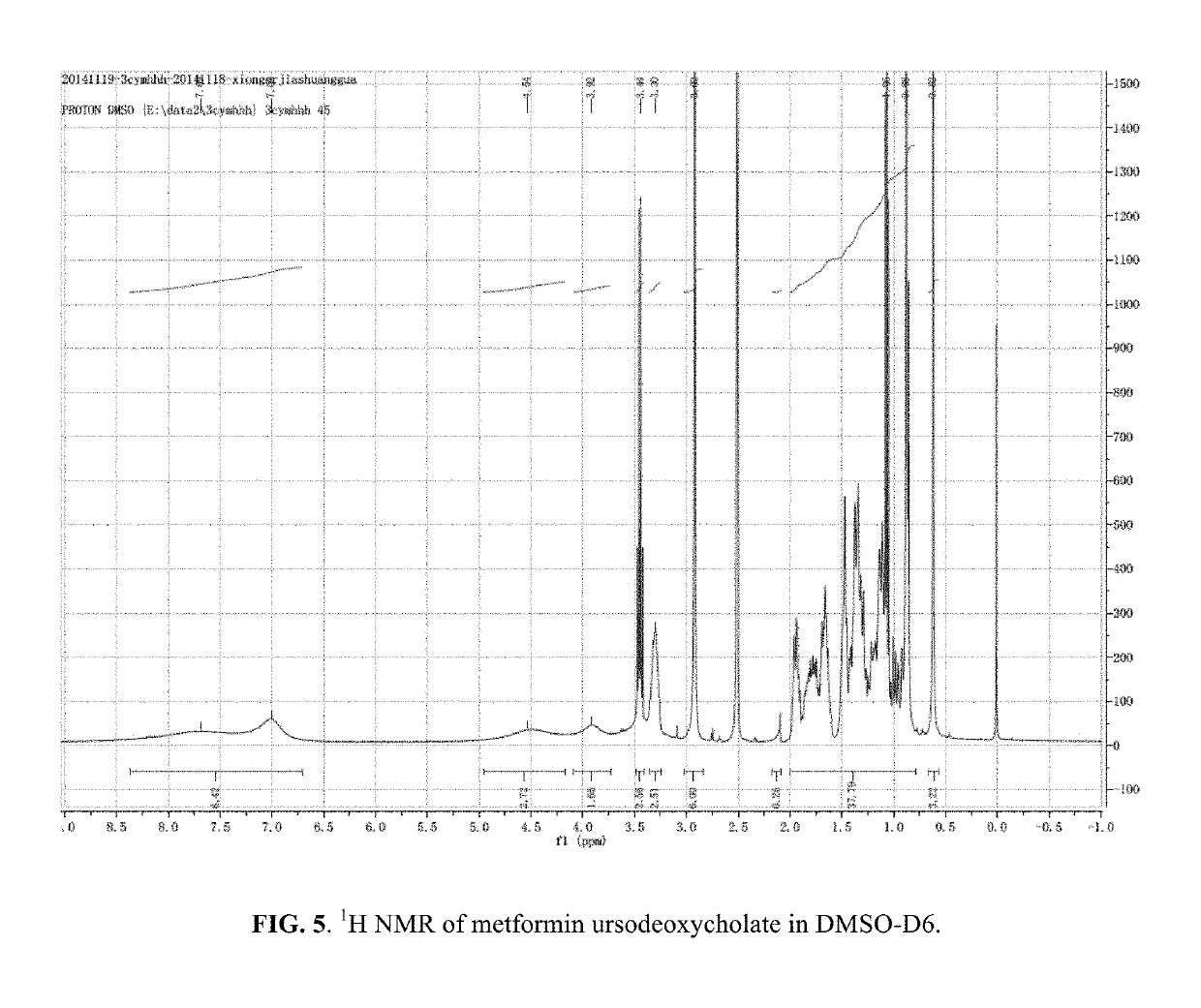
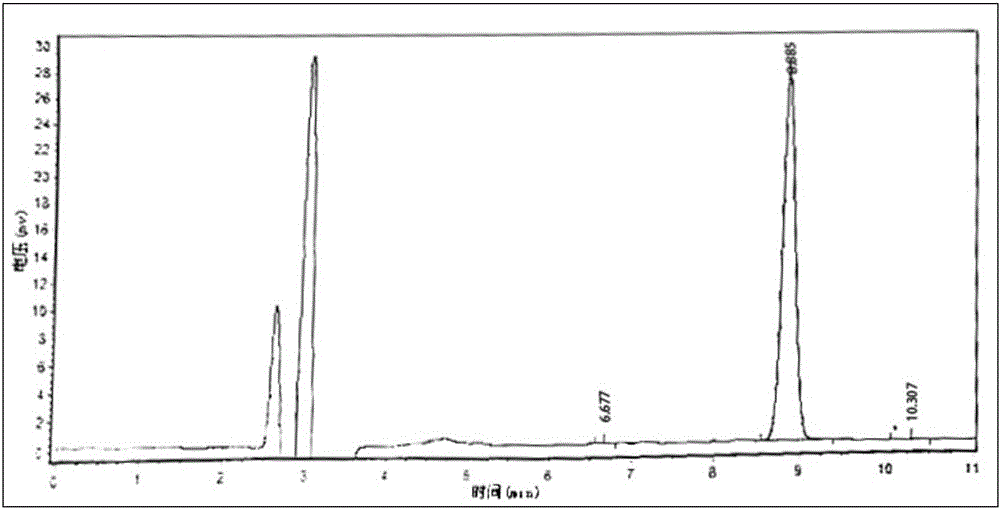
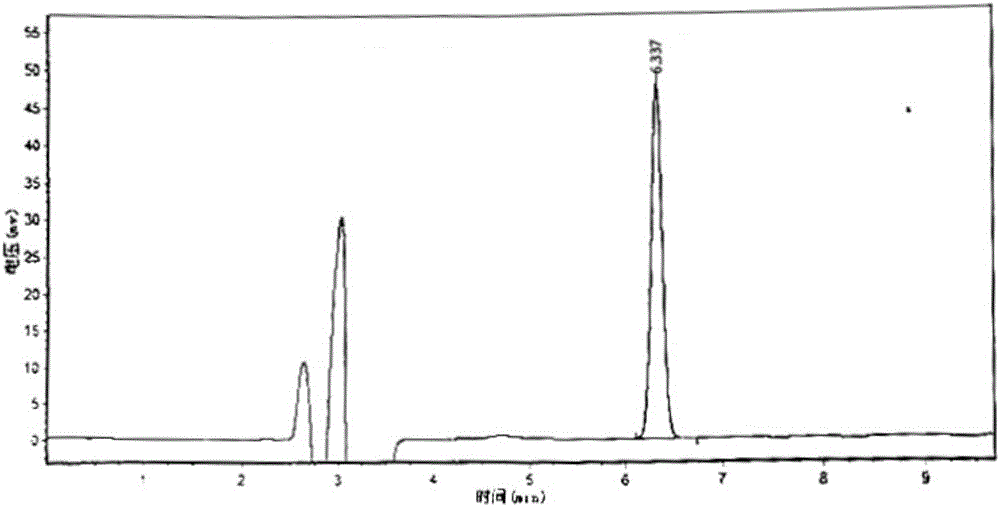
Patents
Literature
160 results about "Ursodesoxycholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
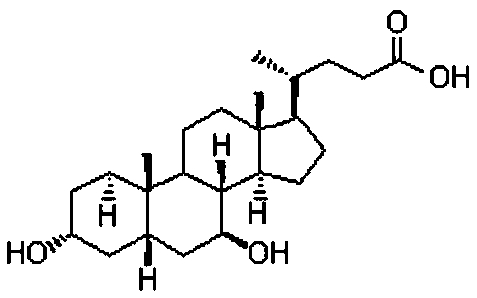
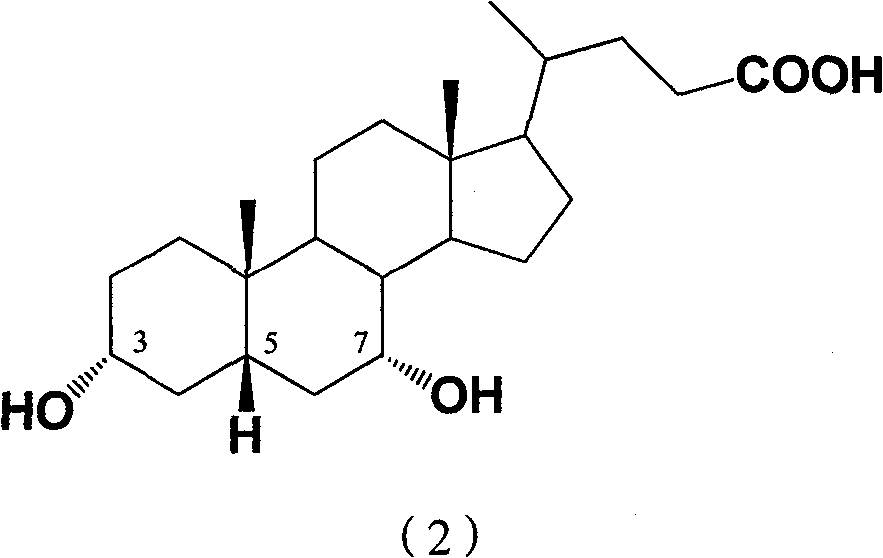
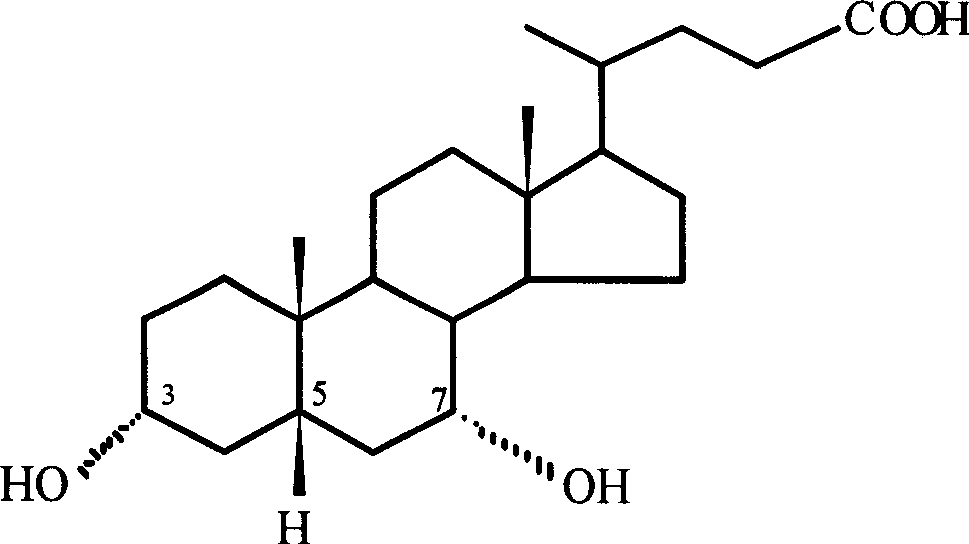
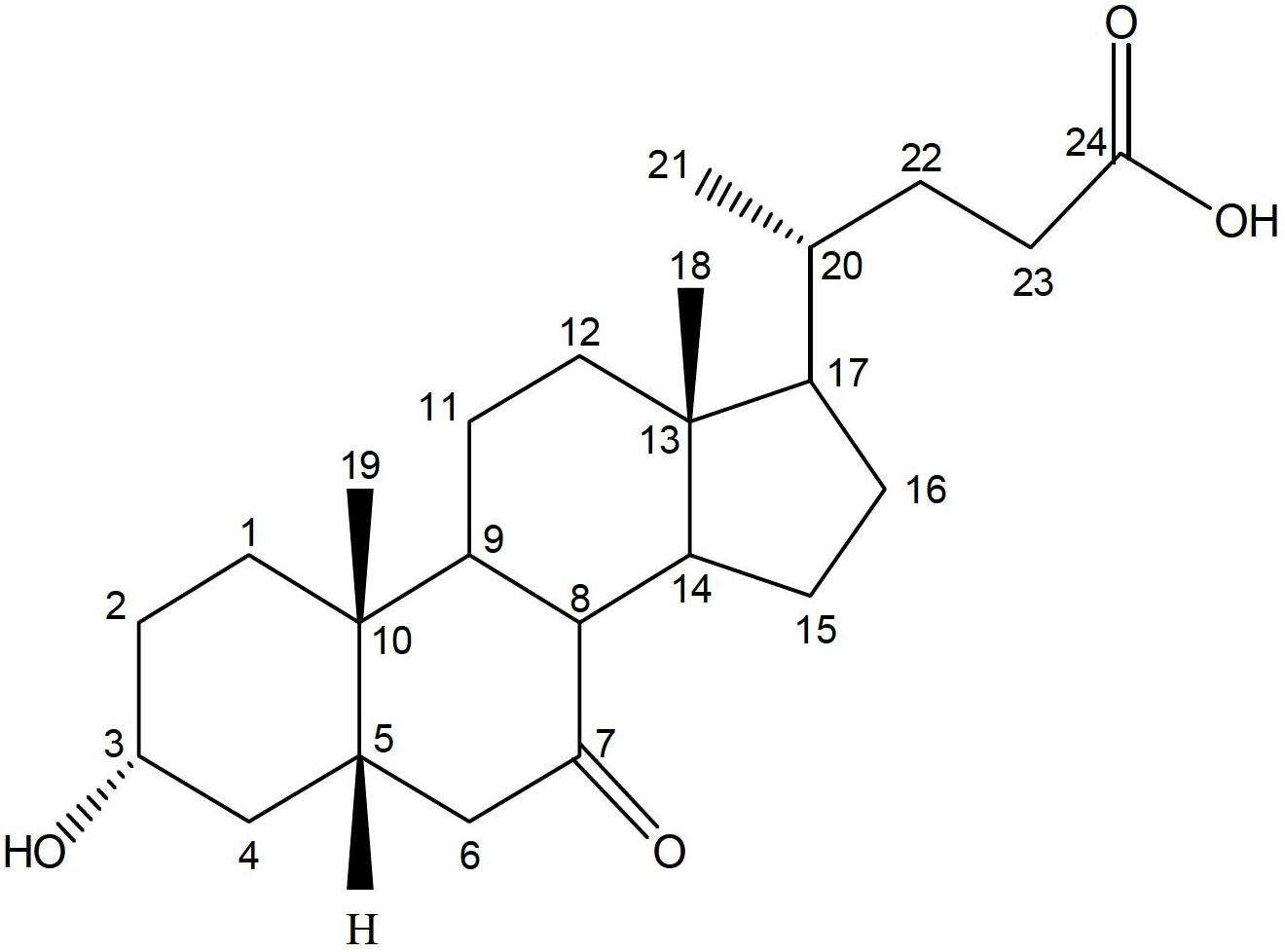
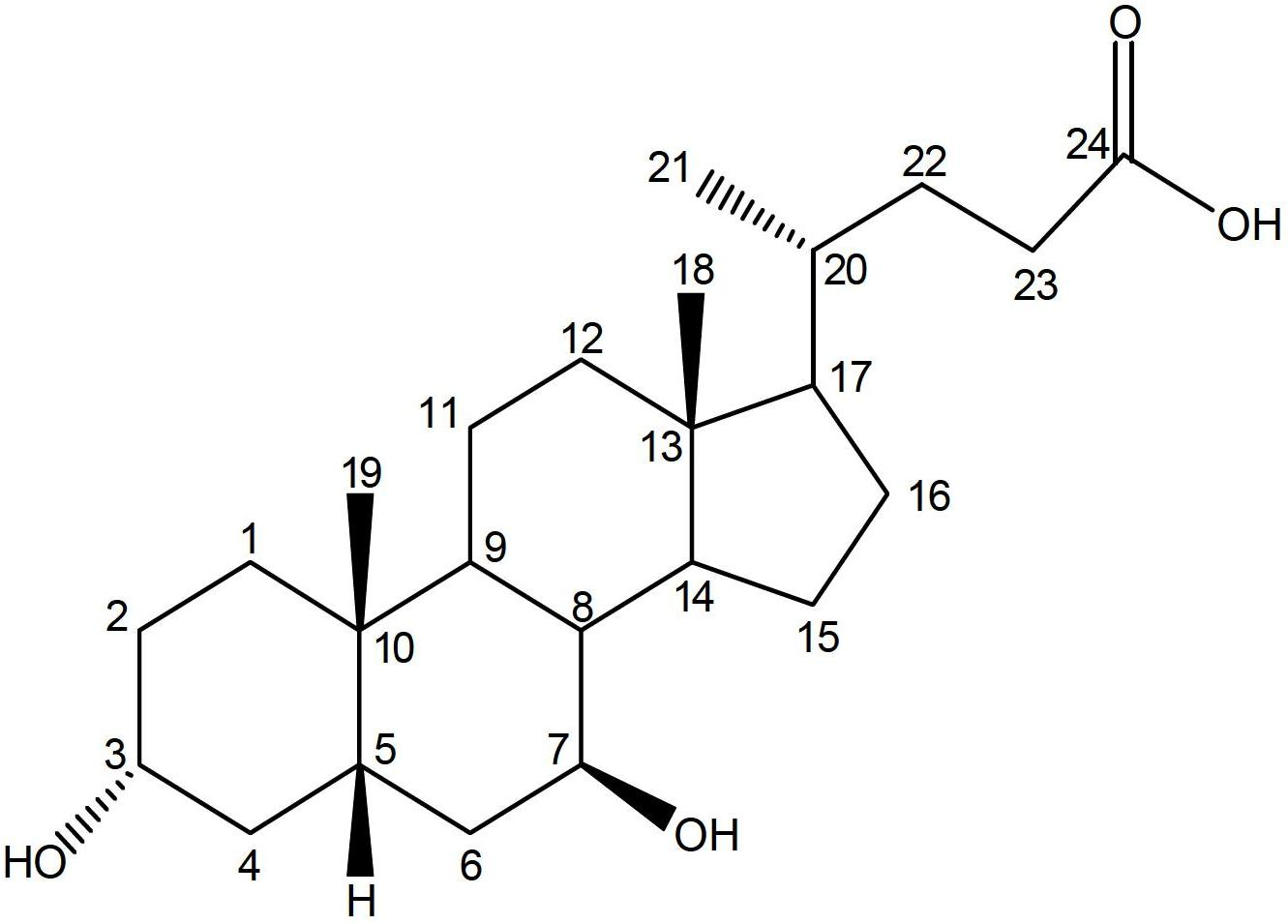
Ursodeoxycholic Acid is typically a bile acid which is used to treat gallstone problems. Gallstones can result in symptoms like jaundice, pain, inflammation of the pancreas and gall bladder too.
Method for preparing binding-form ursodesoxycholic acid by two-step enzymatic method
ActiveCN102994604ASimple preparation processImprove conversion rateFermentationChenodeoxycholic acidSolution state
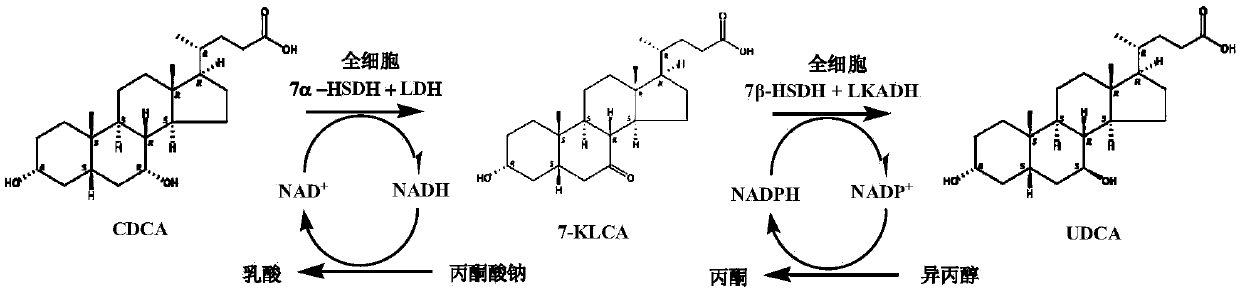
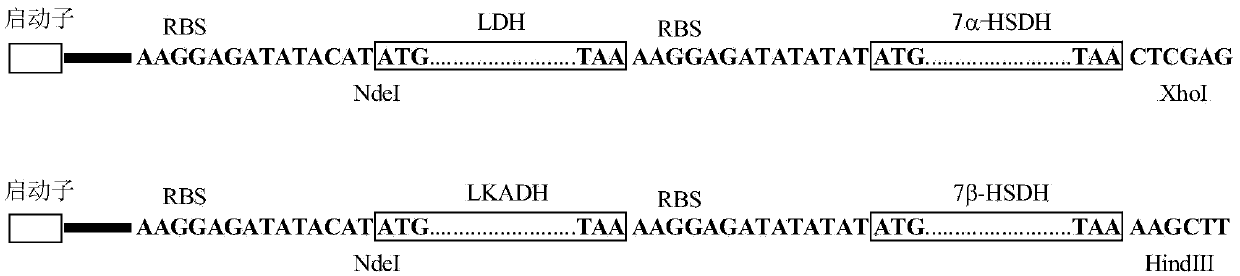
The invention relates to a method for preparing binding-form ursodesoxycholic acid by a two-step enzymatic method, belonging to the field of biotechnology. Under the water solution state, a substrate, namely binding-form chenodeoxycholic acid, is converted into the binding-form ursodesoxycholic acid in the presence of 7alpha-HSDH and 7beta-HSDH, the separation is not needed in the intermediate step, thus the method disclosed by the invention is very simple, and furthermore, the conversion efficiency is very high. Especially chicken bile, duck bile or goose bile in a mixture form can be subjected to reaction without separating and purifying the binding-form chenodeoxycholic acid. The preparation process is simple and easy to implement, and the new, simple and convenient preparation method is provided for the binding-form ursodesoxycholic acid.
Owner:SHANGHAI KAIBAO PHARMA
Method for catalyzing chenodeoxycholic acids to compound ursodesoxycholic acids through efficient whole-cells
ActiveCN105368828AFermentation methods are cheap and readily availableSuitable for industrial productionBacteriaMicroorganism based processesChemical synthesisLactate dehydrogenase
The invention provides a method for catalyzing chenodeoxycholic acids to compound ursodesoxycholic acids through efficient whole-cells. A 7a-hydroxysteroid dehydrogenase (7a-HSDH) and a lactic dehydrogenase (LDH) for regeneration of coenzyme nicotinamide adenine dinucleotide (NAD) are efficiently co-expressed in escherichia coli, escherichia coli whole cells are used to catalyze chenodeoxycholic acids (CDCA) to generate 3 alpha (Alpha)-hydroxyl-7-oxo-5bata (Beta)- cholanic acids (7-KLCA), and a reaction liquid which is obtained by catalyzing the chenodeoxycholic acids through whole cells is adjusted to be 7-KLCA crude products. Reconstitution cells can be easily obtained in low cost through a fermentation process, are better than a chemical synthesis method in production cost and product quality, and are suitable for commercial process.
Owner:苏州天绿生物制药有限公司
Preparation method of ursodeoxycholic acid
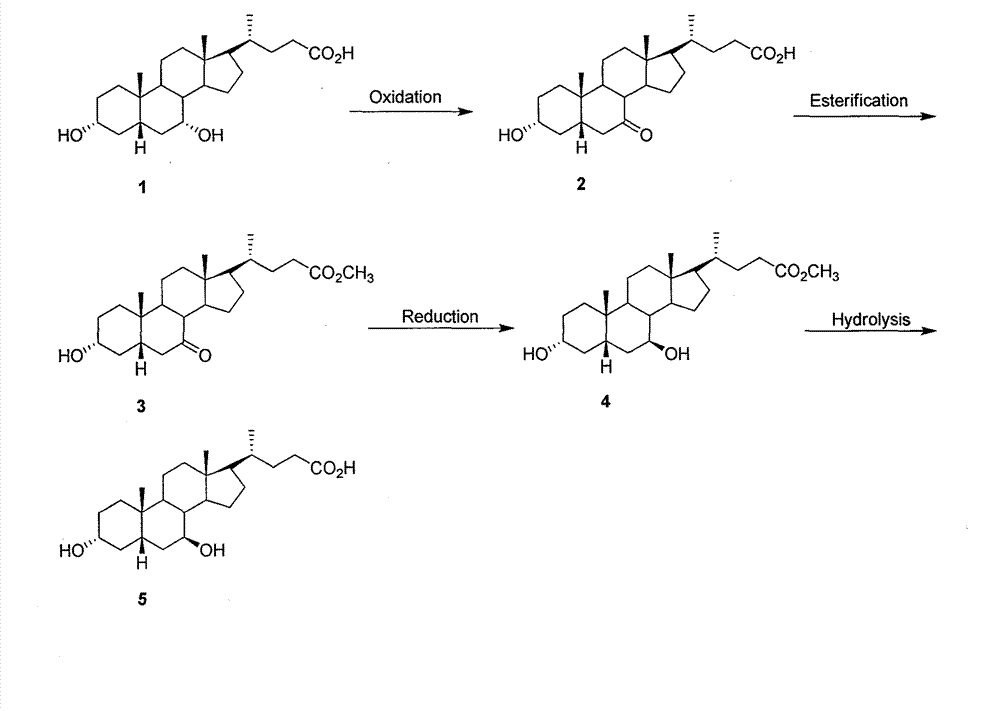
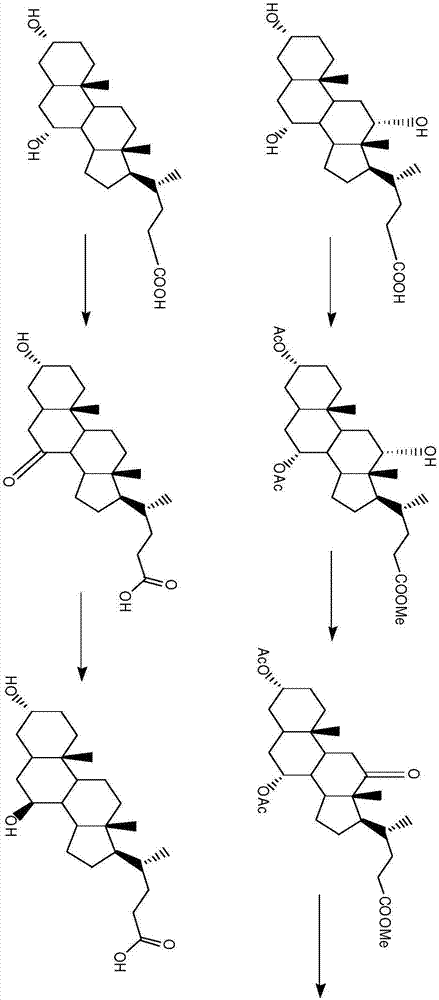
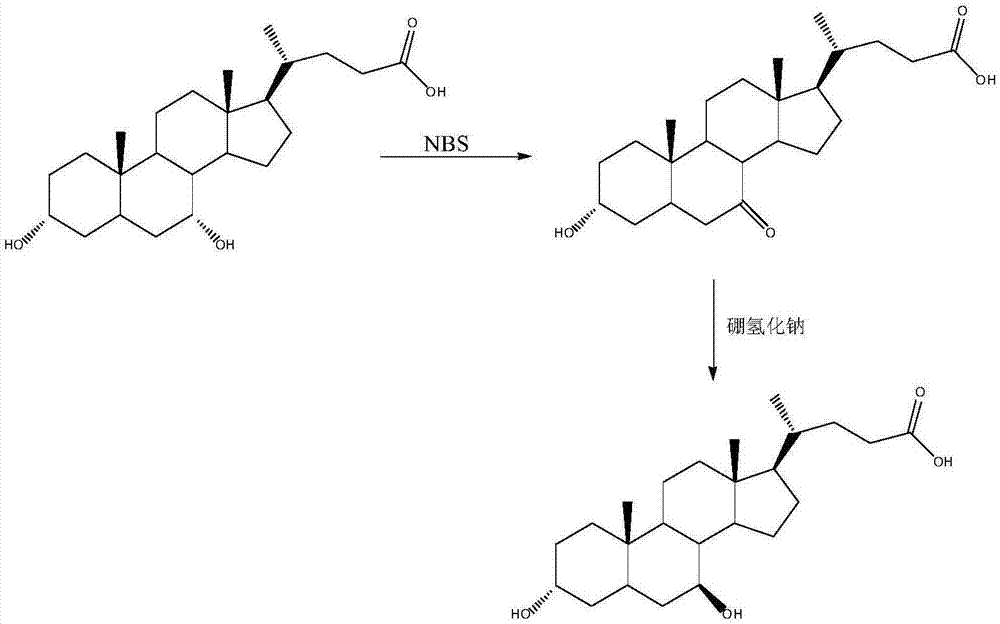
The invention provides a preparation method of ursodeoxycholic acid. Commercial available chenodeoxycholic acid is taken as raw materials, the ursodeoxycholic acid is obtained by four steps including selective oxidation, esterification, deoxidation and hydrolyzation, and the total yield is 85.7%. In a mixture of acetone and water, NBS is used for selective oxidation of hydroxy at C- 7 bit of the chenodeoxycholic acid, and the selective oxidation possesses excellent selectivity and high yield. NaBH14 / CeC13 may be used to deoxidize carbonyl at C-7 bit into hydroxy, and the ratio of alpha / beta is as high as 5 / 95.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of ursodesoxycholic acid
ActiveCN101987860AAvoid disadvantagesHigh puritySteroidsBulk chemical productionCholic acidChenodeoxycholic acid
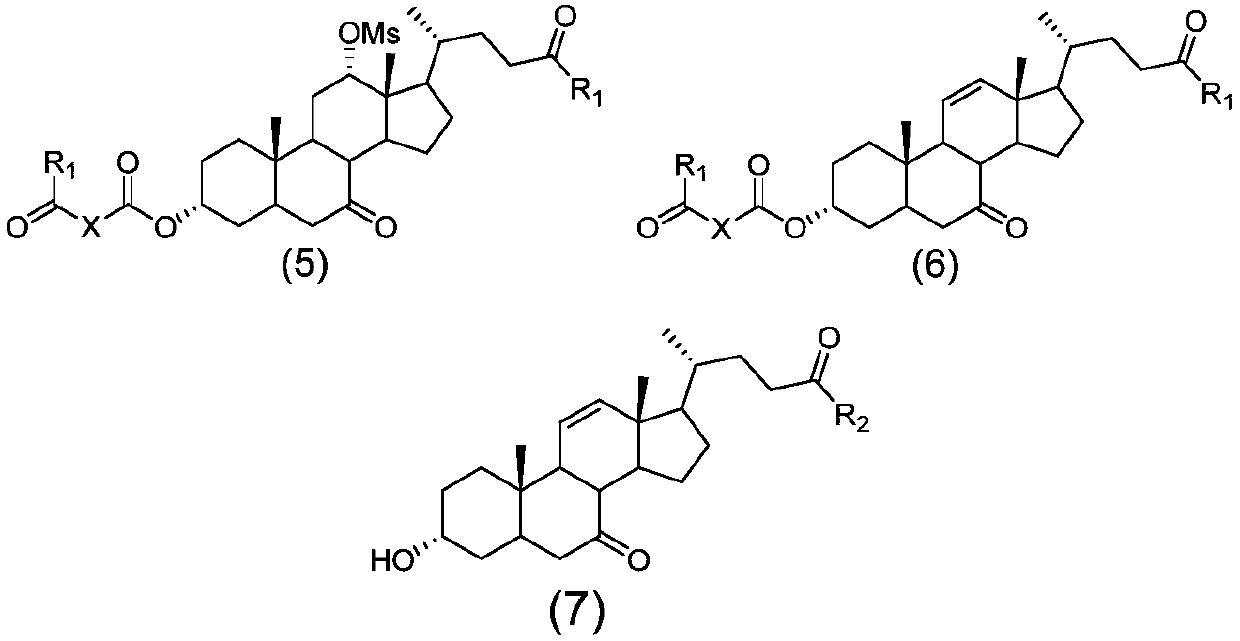
The invention discloses a preparation method of ursodesoxycholic acid, which comprises the flowing steps of: a. carrying out 3-bit esterification on chenodeoxycholic acid to obtain 3-esterification protected chenodeoxycholic acid; b. carrying out 7-bit oxidation reaction on 3-bit ester to obtain 3-estergroup-7-oxocompounds; c. carrying out hydrolysis reaction or reduction reaction on the 3-estergroup-7-oxocompounds; d, carrying out 7-bit reduction reaction on 3a-hydroxy-7-oxo-5 beta-cholic acid or hydrolysis reaction on 3a-estergroup-7 beta- hydroxyl-5 beta-cholic acid to obtain crude products of the ursodesoxycholic acid; e. forming salt through the crude products of the ursodesoxycholic acid and organic base; and f. carrying out steps such as water adding dissolution, acid adding crystallization and the like on ursodesoxycholate to obtain pure ursodesoxycholic acid products with the purity higher than 99.0 percent. The invention aims at overcoming the defects in the prior art to provide a novel method for preparing the ursodesoxycholic acid, and the ursodesoxycholic acid prepared by the method has high yield and high purity.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
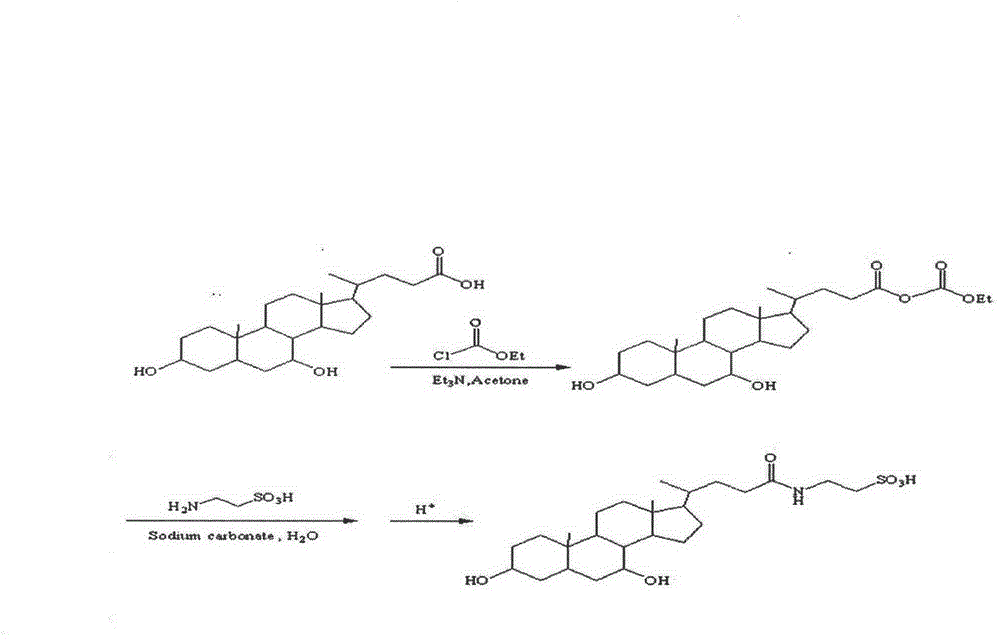
High-purity tauro ursodesoxy cholic acid and preparation method thereof
ActiveCN102477059AHigh purityImprove securityOrganic active ingredientsDigestive systemEthyl chloroformateCholic acid
The invention relates to high-purity tauro ursodesoxy cholic acid and a preparation method thereof. The content of taurochenodeoxycholic acid in the tauro ursodesoxy cholic acid is less than 0.7%. The tauro ursodesoxy cholic acid is safe and effective and does not have toxic and side effects in clinical application. The invention further provides a mixed anhydride reaction of ursodesoxycholic acid and ethyl chloroformate by taking acetone as a solvent. By means of control of a reaction condition and a reaction solvent, the tauro ursodesoxy cholic acid has the advantages of simple process, low cost, environmental friendlessness and industrial production; furthermore, the high-purity tauro ursodesoxy cholic acid can be obtained.
Owner:CHENGDU GUOHONG PHARMA
Total bile acid extract of bear bile powder and preparation method and application of injection thereof
ActiveCN101890048AAdd extraction stepEfficient removalAntibacterial agentsDigestive systemChenodeoxycholic acidAlkaline hydrolysis
The invention relates to a total bile acid extract of bear bile powder and a preparation method and application of injection thereof. The total bile acid extract of the bear bile powder is prepared by the following steps of: performing reflux extraction on the bear bile powder by using ethanol; recovering the ethanol from extracting solution, and concentrating the extracting solution; and performing alkaline hydrolysis, neutralization, acidification, ethyl acetate extraction, ethyl acetate crystallization and ethanol crystallization. The invention also provides a method for preparing the injection from the total bile acid extract of the bear bile powder. The prepared total bile acid extract of the bear bile powder and the injection thereof can be used for preparing medicaments for resisting bacteria and viruses, relieving cough and reducing sputum and protecting liver and gallbladder. The invention can improve the extraction yield and purity of ursodesoxycholic acid in the total bile acid extract of the bear bile powder and reduce the chenodeoxycholic acid content simultaneously, so the ursodesoxycholic acid content in the total bile acid extract of the bear bile powder is no less than 70 percent and the chenodeoxycholic acid content is no more than 20 percent. The ursodesoxycholic acid content in the total bile acid extract of the bear bile powder is higher, and the ursodesoxycholic acid yield is high and is over 18 percent generally.
Owner:SHANGHAI KAIBAO PHARMA
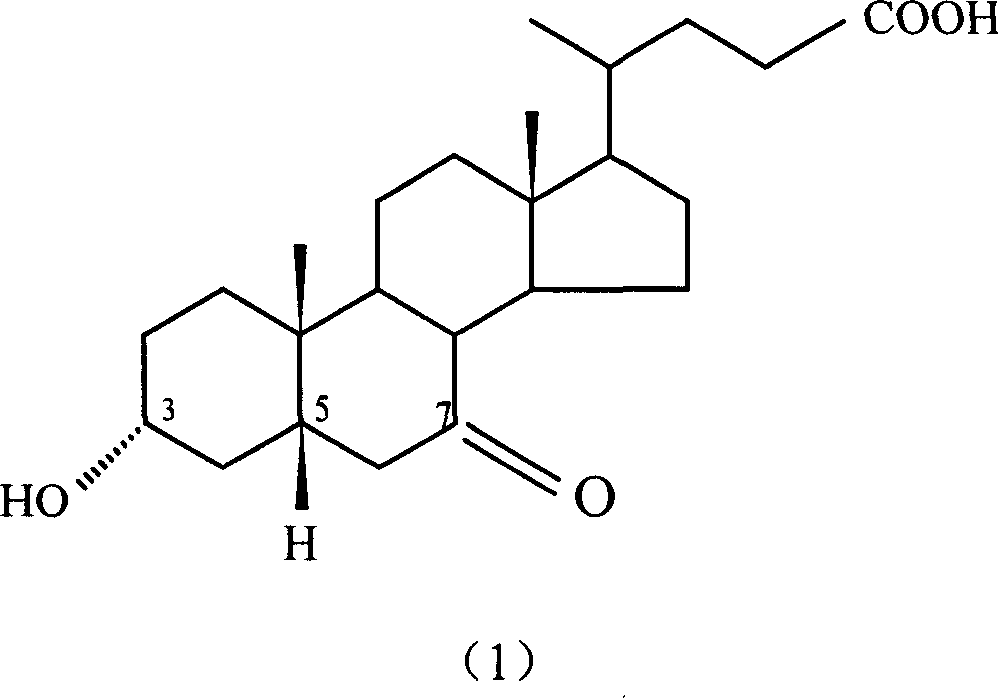
Method for preparing ursodesoxycholic acid by chiral catalytic hydrogenation of 7-ketodesoxycholic acid
InactiveCN102070693AReduce generationReduced purification stepsSteroidsChenodeoxycholic acidDistillation
The invention discloses a method for preparing an ursodesoxycholic acid by the chiral catalytic hydrogenation of a 7-ketodesoxycholic acid, which is characterized by comprising the following steps of: performing oxidation to prepare the 7-ketodesoxycholic acid from a chenodeoxycholic acid serving as an initiative raw material by using a common method; dissolving the 7-ketodesoxycholic acid into a solvent, adding a chiral catalyst, maintaining the pressure of 0 to 20 MPa under alkali condition, introducing nitrogen to perform hydrogenation reduction reaction at 10 to 80 DEG C, and performing distillation after the reaction is finished to remove the solvent; adding purified water in a volume which is 10 to 100 times that of a hydrogenation reduction reaction product, and adding acid liquor to crystallize the hydrogenation reduction reaction product; and separating solids from liquid, and performing washing and drying to obtain solid powder which is the ursodesoxycholic acid. The method for preparing the ursodesoxycholic acid by the chiral catalytic hydrogenation of the 7-ketodesoxycholic acid aims to overcome the shortcomings of the prior art, and ensures a short production flow, high yield and high quality.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Method for respectively recovering ursodesoxycholic acid and chenodeoxycholic acid from ursodesoxycholic acid waste mother liquor
ActiveCN102766185AEasy to operateMild reaction conditionsSteroidsOrganic solventChenodeoxycholic acid
The invention relates to a method for respectively recovering ursodesoxycholic acid and chenodeoxycholic acid from ursodesoxycholic acid waste mother liquor. Firstly waste mother liquor is dissolved in an inorganic base solution, and inorganic acid is added to form mixed sediment containing the ursodesoxycholic acid and the chenodeoxycholic acid; secondly, an organic solvent and an organic amine are added to separate ursodesoxycholic acid ammonium salt; and thirdly the ursodesoxycholic acid ammonium salt is acidized to recover the ursodesoxycholic acid, chenodeoxycholic acid ammonium salt left in the organic solvent is acidized and filtered to recover the chenodeoxycholic acid. The recovering method can effectively utilize the ursodesoxycholic acid waste mother liquor, recovers the ursodesoxycholic acid and the chenodeoxycholic acid contained in the ursodesoxycholic acid waste mother liquor, and is high in recover efficiency and product purity. The recovering method is simple to operate and mild in reaction conditions, used reagent is low in cost and wide in source, and the method is suitable for large-scale industrialized production.
Owner:苏州天绿生物制药有限公司 +1
Preparation method of ursodesoxycholic acid and enzyme for preparation
ActiveCN106636285AImprove conversion rateEasy to operateOxidoreductasesFermentationPhotochemistryUrsodesoxycholic acid
The invention relates to a method for preparing an ursodesoxycholic acid by using a biological enzyme catalysis technology and 7beta-hydroxysteroid dehydrogenase for preparation of the ursodesoxycholic acid. The method comprises the following steps: adopting a 3alpha-hydroxyl -7-oxo-5beta-cholanic acid as a substrate and catalyzing the 3alpha-hydroxyl -7-oxo-5beta-cholanic acid by using the 7beta-hydroxysteroid dehydrogenase to prepare the ursodesoxycholic acid in the presence of an NADP, glucose, glucose dehydrogenase and a buffer solution, wherein the 7beta-hydroxysteroid dehydrogenase is from Turneriella parva. The method is simple in operation, short in reaction time, the reaction conditions are mild and easy to control, the conversion rate of the substrate can reach 99.8% and the content of the obtained product is greater than 98.5%.
Owner:眉山市新功生物科技有限公司 +1
Preparation method of 7-keto lithocholic acid
InactiveCN1912192BSimple preparation stepsMild operating conditionsElectrolysis componentsElectrolytic organic productionPower flowOrganic solvent
The invention relates to 7-alkone lithocholic acid preparing method. It includes the following steps: dissolving the compound into the organic solvent to form electrolyte, electrolyzing the electrolyte by constant current at oxidizing medium condition to gain the object. The electrolyzing current density is 47.6A / m<2>-190.4A / m<2>. The invention has the advantages of moderate operation condition, green, simplifying ursodesoxycholic acid preparing steps.
Owner:EAST CHINA UNIV OF SCI & TECH
Ursodesoxycholic acid preparation method
The invention discloses an ursodesoxycholic acid preparation method. The method comprises the following steps: 1, adding chenodeoxycholic acid and a solvent A to a reaction container, stirring for dissolving, adding 7-alphaHSDH, 7-betaHSDH and a coenzyme II, and carrying out a reaction at a controlled temperature at a controlled pH value to convert chenodeoxycholic acid into ursodesoxycholic acid in order to obtain a conversion liquid; 2, heating the conversion liquid obtained in step 1 to denaturalize the 7-alphaHSDH and the 7-betaHSDH, centrifuging through a high speed centrifuge, removing proteins, adding a sodium hydroxide solution to the above obtained solution, distilling to remove the solvent A, adding water to dissolve obtained distillation residues, adding an acid, and crystallizing to obtain crude ursodesoxycholic acid; and 3, adding the crude ursodesoxycholic acid obtained in step 2 and a solvent B to the reaction container, heating and refluxing the crude ursodesoxycholic acid and the solvent B for 1h, cooling the obtained reaction product to normal temperature, and filtering the cooled product to obtain ursodesoxycholic acid with the purity being greater than 99%. The ursodesoxycholic acid preparation method has the advantages of simple technology, short synthesis route, high conversion rate, easy post-treatment and environmental protection.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Berberine salts, ursodeoxycholic salts and combinations, methods of preparation and application thereof
Owner:SHENZHEN HIGHTIDE BIOPHARM
Method for preparing ursodesoxycholic acid on basis of chemical oxidation and enzyme catalysis combination technology
ActiveCN106520888AReduce manufacturing costReduced activityFermentationEscherichia coliChenodeoxycholic acid
The invention discloses a new thought of generating 7-carbonyl-lithocholic acid by virtue of chenodeoxycholic acid dissolved by NBS acetone peroxide, and carrying out catalytic synthesis on the 7-carbonyl-lithocholic acid (7k-LCA) in combination with biological enzyme catalysis to obtain ursodesoxycholic acid (UDCA). The new thought is characterized by comprising the following steps: generating a catalytic enzyme (7 beta-HSDH) with high purity, high activity and high stability by virtue of the characteristics of high efficiency and low cost of the method of generating 7-carbonyl-lithocholic acid by virtue of chenodeoxycholic acid dissolved by NBS acetone peroxide and in combination with high-density fermentation of recombinant escherichia coli, and selectively catalyzing 7k-lithocholic acid in an aqueous phase to generate the ursodesoxycholic acid (UDCA). The synthesis process is simple, short in synthesis route, high in conversion rate and low in cost, and is a novel method for preparing ursodesoxycholic acid on the basis of a chemical oxidation and enzyme catalysis combination technology.
Owner:陕西岳达德馨生物制药有限公司
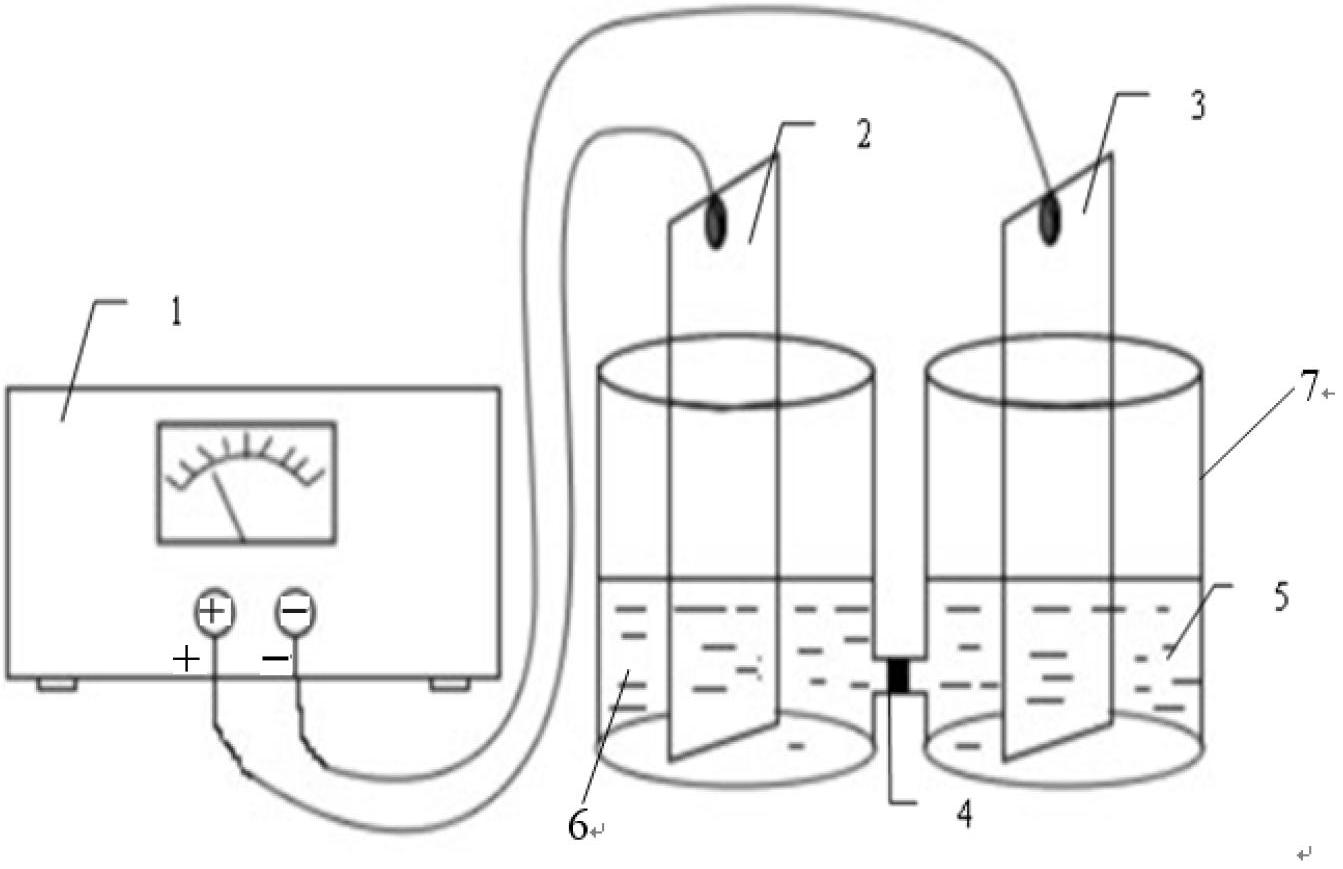
Method for preparing ursodesoxycholic acid by electro-reduction
ActiveCN102660755ANo pollution in the processSimple processCellsElectrolytic organic productionEnvironmental resistanceCholic acid
The invention relates to a method for preparing ursodesoxycholic acid by electro-reduction, which comprises the steps of: in a diaphragm electrolytic cell, evenly mixing 7-ketone liithocholic acid organic solution and potassium bromide water solution to be taken as catholyte, wherein in the catholyte, the concentration of 7-ketone liithocholic acid is within the range of 11.54-21.23mg / mL, the concentration of potassium bromide is within the range of 0.03-0.13mol / L, the volume ratio between an organic solvent in the 7-ketone liithocholic acid organic solution and water in the potassium bromide water solution is not less than 10: 3, acid solution is anolyte, the concentration of acid solution is within the range of 1-25%, and the active electrode area is 5*3 cm<2>; carrying out electrolysis by constant current, wherein the density of the constant current is within the range of 10-30A / m<2>, and the electrolytic temperature is 25-70 DEG C; and preferentially, after the electrolysis by the constant current, carrying out rotary evaporation on catholyte until that solid forms, adding alkali liquor for dissolving, adjusting the pH value to be 2.0, filtering and drying a filter cake. The method for preparing the ursodesoxycholic acid by the electro-reduction not only simplifies the process flow in the industrial production and reduces the production cost, but also is safe and environment-friendly, thus being suitable for large-scale popularization and application.
Owner:EAST CHINA UNIV OF SCI & TECH
Novel coupling compound of bile acid and anti-hepatitis virus medicament and medical use thereof
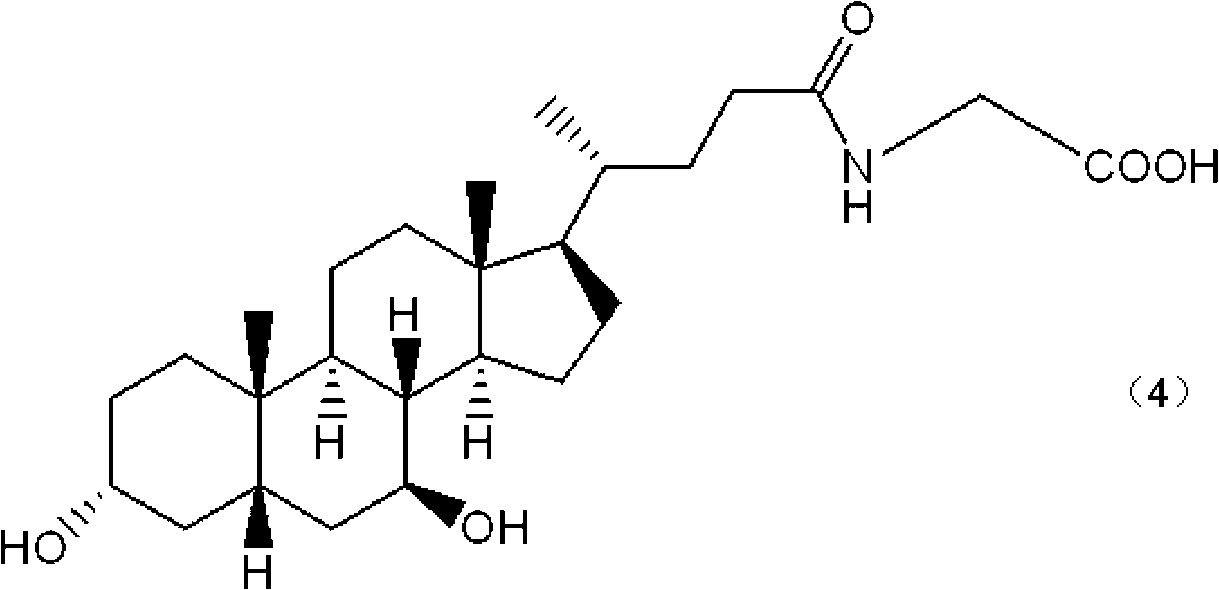
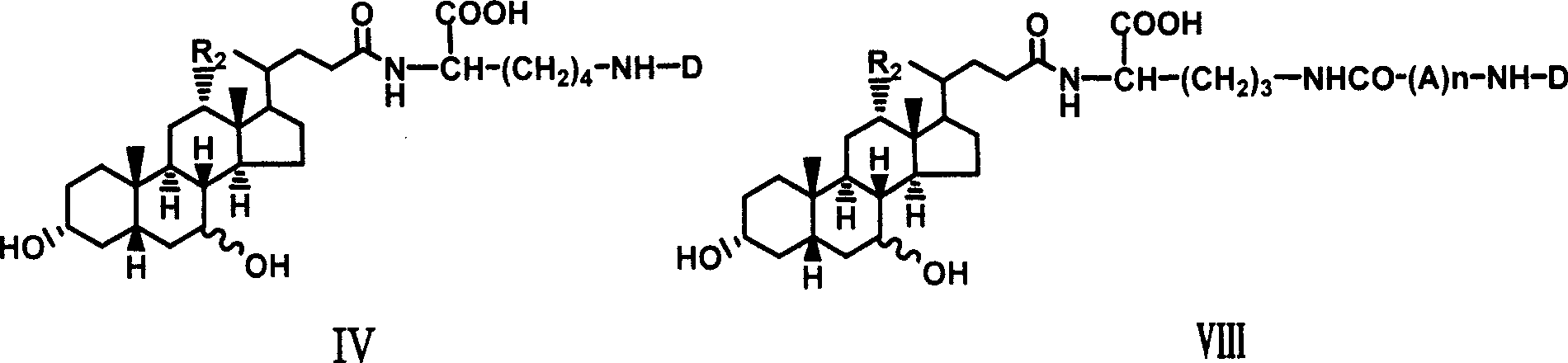
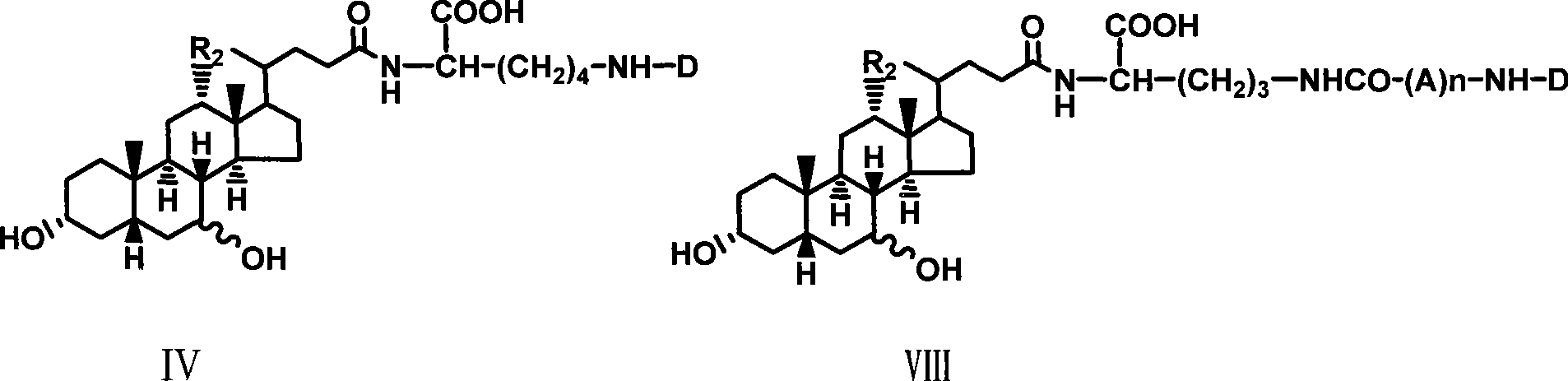
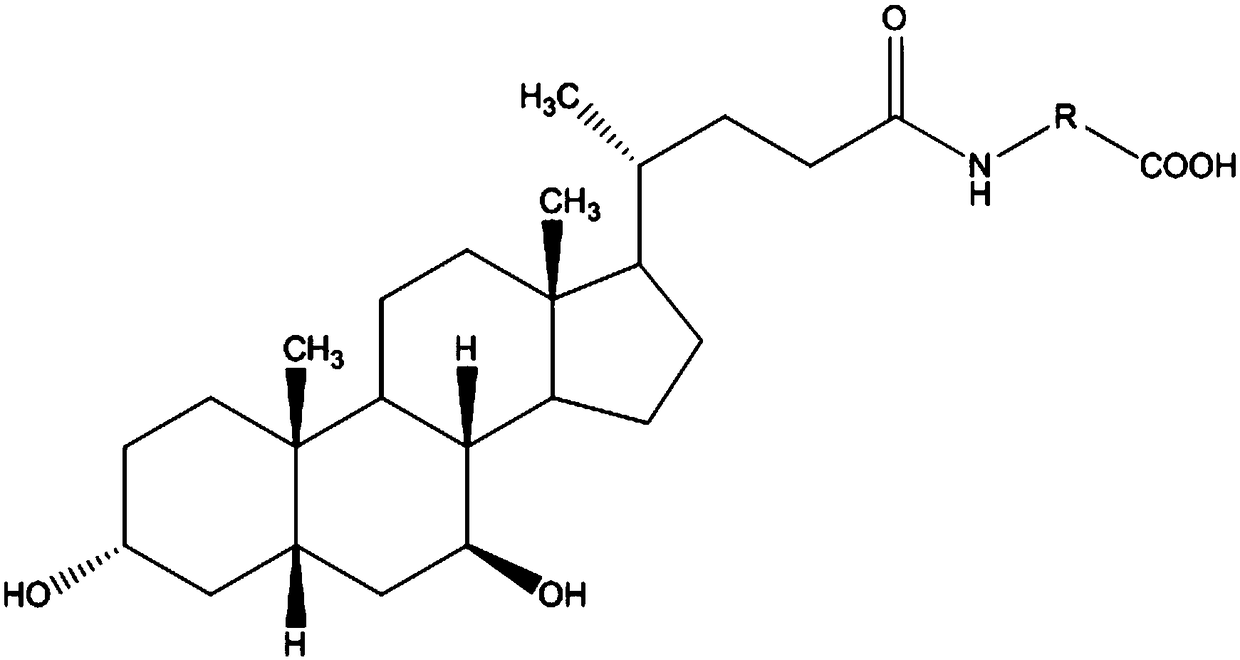
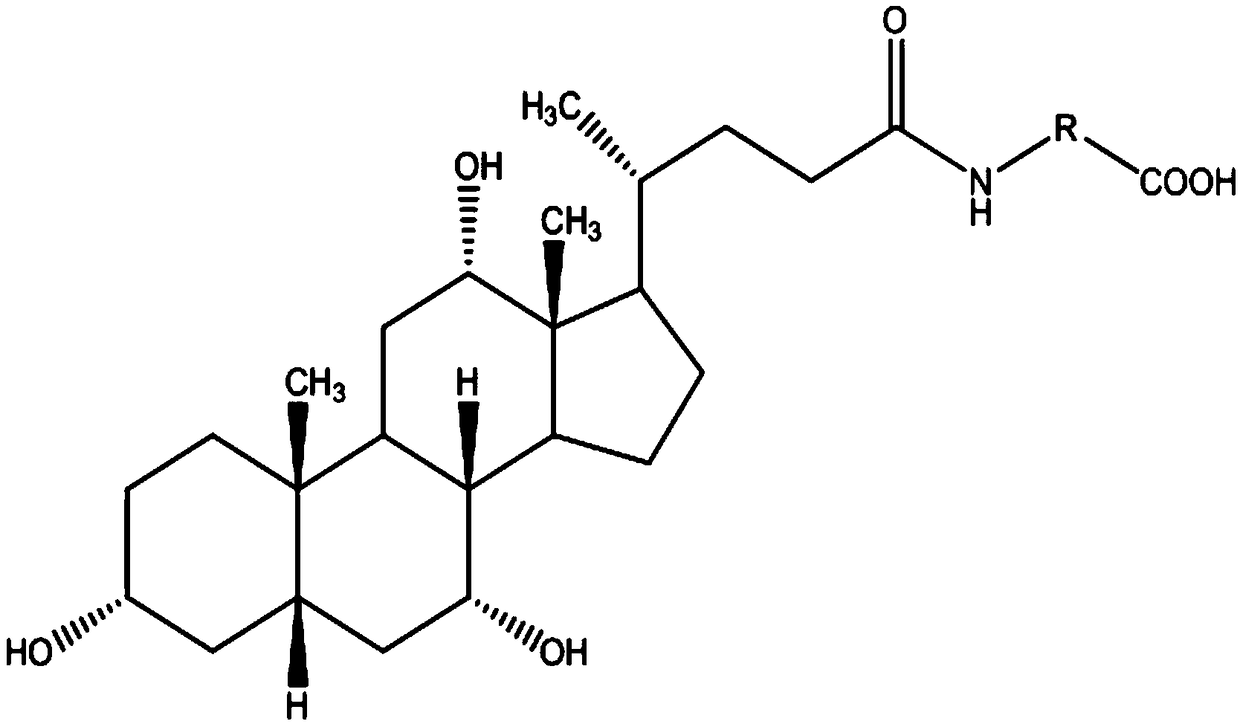
The invention relates to a bile acid-anti-hepatitis virus drug conjugate which is shown in the following formula, non-toxic pharmaceutically acceptable salts thereof, a preparation method, a pharmaceutical composition containing the compounds and a use. In the structural formula of figure (I), R represents OH (bile acid) or H (ursodeoxycholic acid), A represents amino acid, n is an integer from 1 to 3, and the structure of the amino acid is D type or L type. D represents the above structure (II).
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Drug for treatment of blood glucose, dyslipidemia and neurodegenerative diseases and preparation of drug
PendingCN109364084AAchieve healingInhibits receptor FXR activityNervous disorderHydroxy compound active ingredientsDyslipidemiaChenodeoxycholic acid
The invention provides a drug for treatment of blood glucose, dyslipidemia and neurodegenerative diseases and a preparation of the drug. The drug is characterized in that the drug inhibits the activity of an intestinal bacterial BSH enzyme and increases the level of conjugated bile acid in anthe intestinal tract, thus the activity of FXR of bile acid receptors FXR is inhibited, synthesis of liverbile acid is improved, and the level of liver cholesterol and triglyceride is decreased; and the drug comprises amide derivatives form by carboxyl in ursodesoxycholic acid (UDCA) or chenodeoxycholic acid (CDCA) steroid stem nucleus and amidogen in other amino acids in addition except for glycine and taurine, and thus blood glucose, dyslipidemia, fatty liver, hepatic encephalopathy and neurodegenerative diseases such as senile dementia can be treated.
Owner:SHENZHEN YUNHE PHARM TECH PARTNERSHIP LTD
Composition and preparation for resisting ageing and improving male energy, preparation method of preparation and application of composition
InactiveCN109045059AReasonable compositionAppropriate compatibilityOrganic active ingredientsInorganic active ingredientsSexual functionProtopanaxadiol
The invention discloses a composition for resisting ageing and improving male energy. The composition is prepared from a raw material and an auxiliary material; the raw material is prepared from the following components in parts by weight: 1-15 parts of nicotinamide mononucleotide, 1-10 parts of protopanoxadiol, 1-9 parts of icarisid I, 2-8 parts of baohuoside I, 3-7 parts of dehydroepiandrosterone and 2-10 parts of ursodesoxycholic acid. The composition is reasonable in composing prescription and proper in compatibility; in the composition, the four of the nicotinamide mononucleotide (NMN), the icarisid I, the baohuoside I and protopanoxadiol are used as monarch drugs together, the composition is capable of comprehensively regulating gonad axis and invigorating kidney-yang and liver, candelay ageing period of the gonad axis and improve the sexual function after being taken for a long time; through intercoordination and synergistic effect of various components, the composition is comprehensive in nutrition, has very good palatability, is particularly suitable for males to take and thus relieving the symptoms of male ageing syndrome and improving the male energy. The invention alsoprovides a preparation containing the composition and a preparation method of the preparation. The preparation method is simple, is moderate in condition and is suitable for industrial batch production.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
Novel 12 alpha-hydroxysteroid dehydrogenases, production and use thereof
The invention relates to 12 α-hydroxysteroid dehydrogenases, nucleic acid sequences coding for the same, expression cassettes and vectors, recombinant microorganisms containing the corresponding coding nucleic acid sequences, a method for producing said 12 α-hydroxysteroid dehydrogenases, a method for enzymatic oxidation of 12 α-hydroxysteroids using said enzyme, a method for enzymatic reduction of 12-ketosteroids using said enzyme, a method for qualitative or quantitative determination of 12-ketosteroids and / or 12α-hydroxysteroids using said 12α-hydroxysteroid dehydrogenases and a method for production of ursodesoxycholic acid, comprising the enzyme-catalysed cholic acid oxidation using said 12 α-hydroxysteroid dehydrogenases.
Owner:PHARMAZELL GMBH
Method for extracting chenodeoxycholic acid from goose and duck bile
The invention relates to a method for extracting chenodeoxycholic acid from goose and duck bile and belongs to the bioengineering field. According to the method, waste poultry bile in a factory undergoes steps of precipitation, filtering, purification and extraction and the like to extract chenodeoxycholic acid. The raw materials are abundant and low-priced. The extraction method provided by the invention has a similar effect with the extraction of ursodesoxycholic acid, can be used to utilize waste, reduce cost and enlarge medicinal resources.
Owner:曾科
Platinum (II) coordination complex and preparing method and application thereof
ActiveCN105713047AHas antitumor potentialGood in vitro inhibitory activityPlatinum organic compoundsAntineoplastic agentsPotassium tetrachloroplatinateOxygen
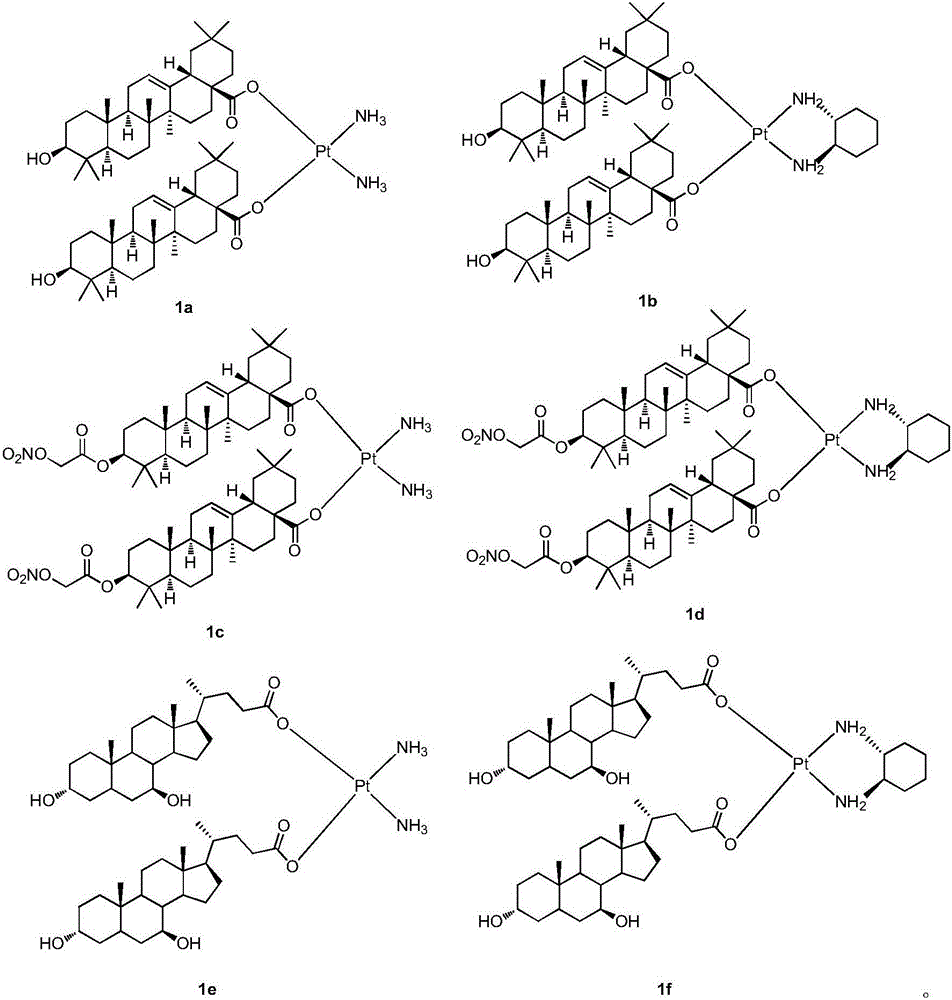
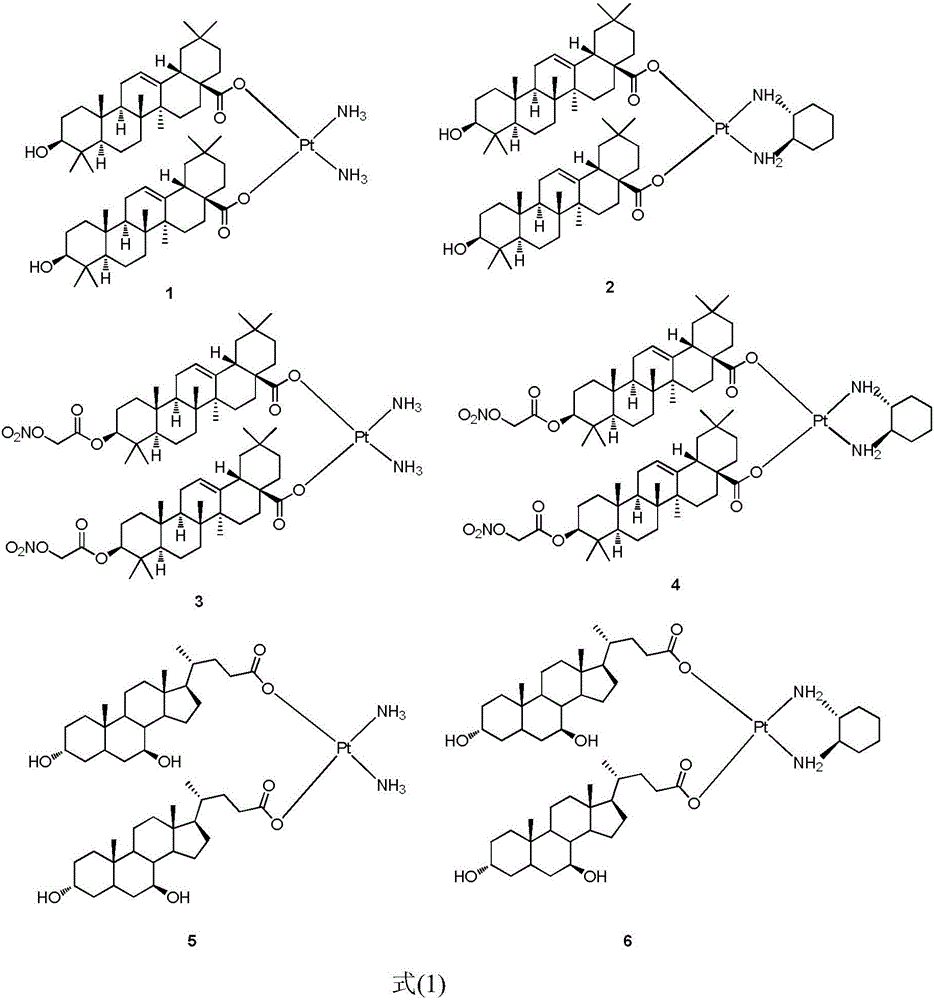
The invention discloses a platinum (II) coordination complex and a preparing method and application thereof. The preparing method includes the steps that active natural products such as oleanolic acid and ursodesoxycholic acid and derivatives of the active natural products serve as ligand; potassium tetrachloroplatinate(II) and ammonia or (R,R)-cyclohexanediamine are subjected to coordination reaction under the condition of potassium iodide, then iodide ions are removed through silver nitrate under the light shielding condition, and the mixture and sodium salt of ursodesoxycholic acid or oleanolic acid or the derivatives of oleanolic acid are reacted to obtain the product. The platinum (II) coordination complex has the good in-vitro antitumor activity, and can be applied to preparing medicine for treating malignant tumor.
Owner:SOUTHEAST UNIV
Method for preparing obeticholic acid, ursodeoxycholic acid and 7-ketolithocholicacid
InactiveCN108676049AEasy to manufactureHigh yieldSteroidsBulk chemical productionCholic acidSynthesis methods
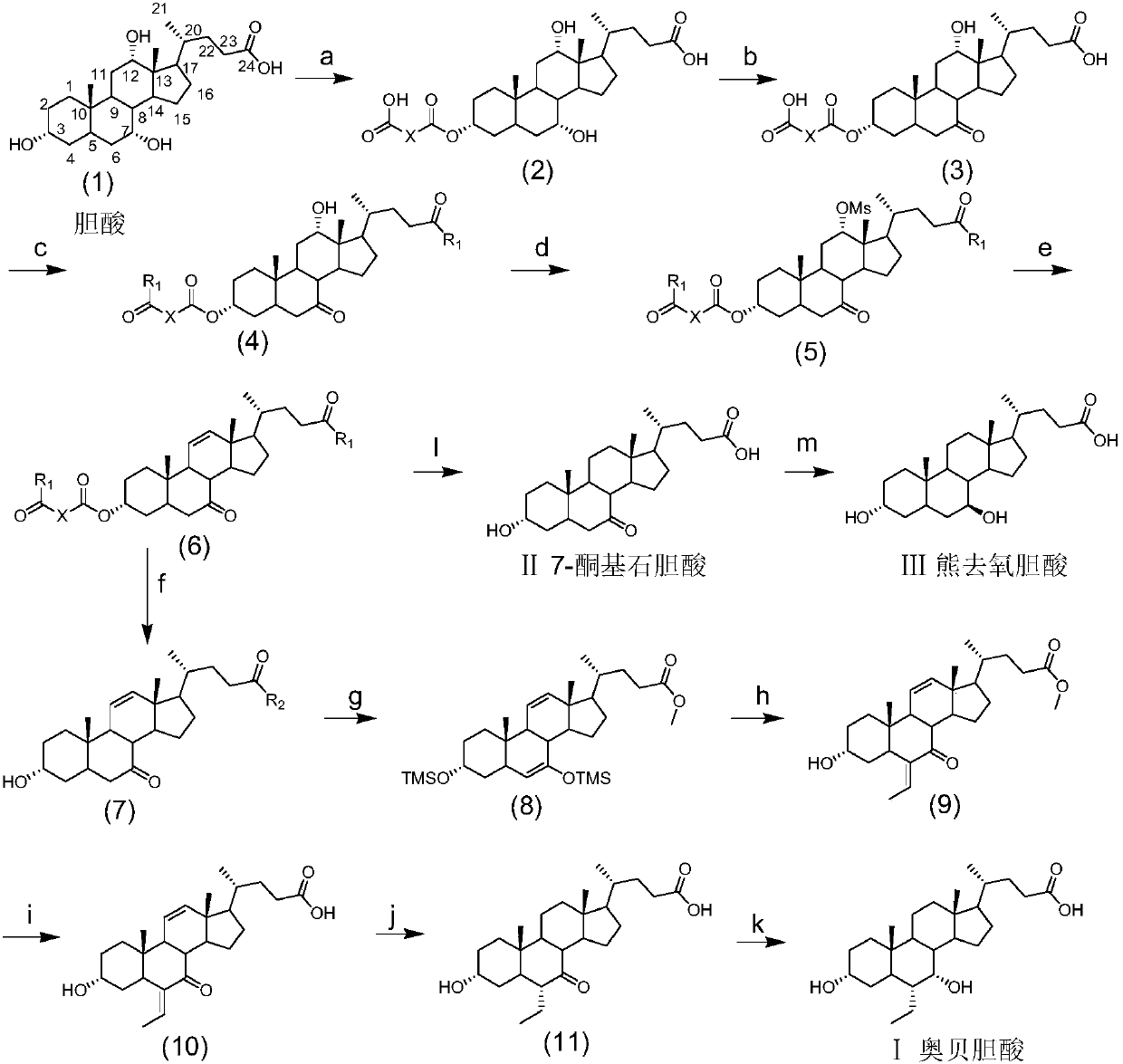
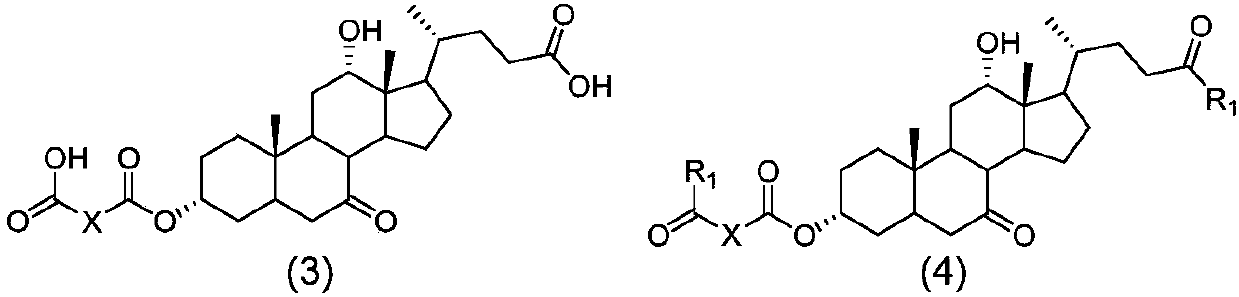
The invention discloses a method for preparing obeticholic acid, 7-ketolithocholicacid and ursodeoxycholic acid. Cholic acid is used as a raw material for preparing obeticholic acid through selectiveprotection by a 3-alpha-hydroxyl group, selective oxidation of a 7-alpha-hydroxyl group, esterification of a 24th carboxyl group, methanesulfonation of a 12-alpha-hydroxyl group, elimination, hydrolysis, silylation, condensation, hydrolysis, catalytic hydrogenation, carbonyl reduction and other reactions; an intermediate is subjected to catalytic hydrogenation to prepare the 7-ketolithocholicacidand then is reduced to prepare the ursodeoxycholic acid. The method provided by the invention uses cheap cholic acid as the raw material, and has advantages of novel synthesis method, low cost, high yield, mild reaction condition, high simplicity in operation, environmental friendliness and high convenience in industrial production.
Owner:EAST CHINA NORMAL UNIV
Purification method of ursodesoxycholic acid
The invention discloses a purification method of ursodesoxycholic acid. The method comprises the following steps: firstly, preparing a triethylammonium ursodesoxycholicate; secondly, hydrolyzing the triethylammonium ursodesoxycholicate; and finally, performing recrystallization and refining. The purification method of ursodesoxycholic acid disclosed by the invention is simple in step and easy to operate. The used solvent is simple and easily available, and small in energy consumption. The purity of the refined ursodesoxycholic acid obtained by the purification method reaches up to 99.8%.
Owner:眉山市新功生物科技有限公司
Method for synthesizing UDCA (ursodesoxycholic acid) by catalyzing CA (cholic acid) by chemical cells
InactiveCN107385006ASimplified catalytic stepsSimplify the conditionsFermentationEscherichia coliCholic acid
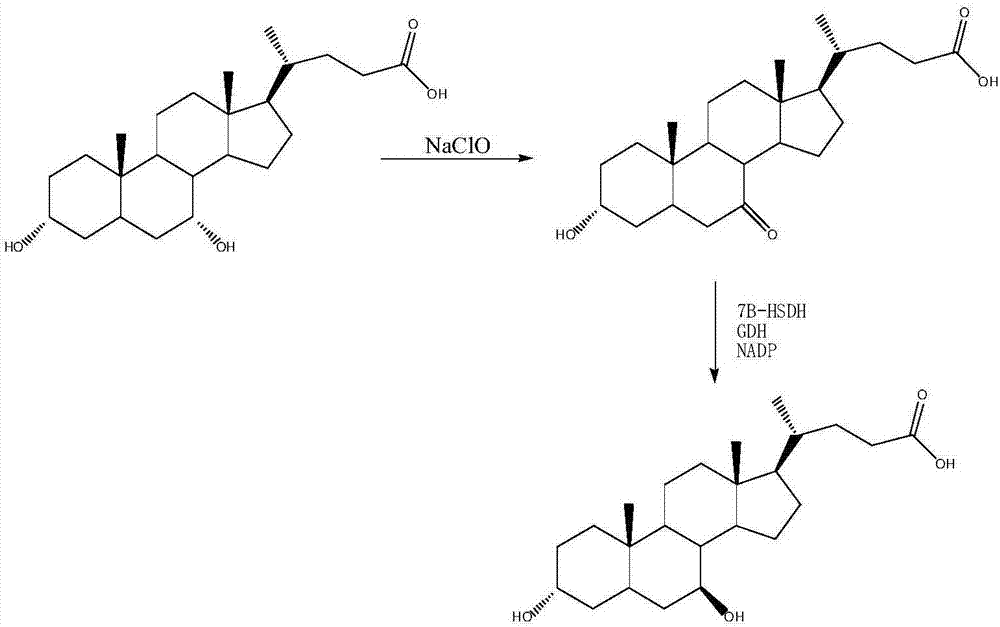
The invention provides a novel method for synthesizing UDCA (ursodesoxycholic acid) by catalyzing CA (cholic acid) by a whole-cell combined chemical method. According to the method, cholic acid is oxidized by the aid of sodium hypochlorite to obtain dehydrocholic acid, the dehydrocholic acid is catalyzed by the aid of a built Escherichia coli engineering bacterium cell to synthesize 12-keto-ursodesoxycholic acid, and the 12-keto-ursodesoxycholic acid is reduced into the ursodesoxycholic acid by a Wolff-Kishner method. The Escherichia coli engineering bacterium cell comprises a co-expression system of three foreign genes such as 7beta-hydroxysteroid dehydrogenase (7beta-HSDH), 3alpha-hydroxysteroid dehydrogenase (3alpha-HSDH) and coenzyme NADP (nicotinamide adenine dinucleotide phosphate) +regenerative glucose dehydrogenase (GDH). The method is cheap in chemical oxidation raw material, less in catalytic by-products and by-products, high in yield and cell catalyst yield and applicable to industrial production, and addition of coenzyme is omitted.
Owner:SUZHOU DUMEI BIOTECH CO LTD
Method for synthesizing ursodesoxycholic acid and tauroursodeoxycholic acid from swine bile
The invention relates to a method for synthesizing ursodesoxycholic acid and tauroursodeoxycholic acid from swine bile. The method comprises the following steps: extracting mixed cholic acid, separating the mixed cholic acid and synthesizing the ursodesoxycholic acid and the tauroursodeoxycholic acid; the method is characterized by utilizing the swine bile as a raw material, and carrying out front-end impurity removing by adopting enzymolysis, ultrafiltration and nanofiltration methods, thus extracting the mixed cholic acid; separating the extracted mixed cholic acid; then synthesizing the ursodesoxycholic acid and the tauroursodeoxycholic acid. By adopting the method disclosed by the invention, the raw material is wide and easy to obtain, the purity of the tauroursodeoxycholic acid is high, the conversion rate is high, the repeatability is good, the cost is lower, the operation is simple, the preparation technology is simple, environment friendliness is realized, the method is more suitable for industrial production and is beneficial for saving resources and reducing energy consumption, economic benefit and environmental benefit are obvious, and sustainable development of an industry can be promoted.
Owner:GUIZHOU HUIJING BIOTECH
Method for synthesizing ursodeoxycholic acid by taking BA as raw material
The invention discloses a synthesis method of ursodeoxycholic acid, and the method comprises the following steps: by using a botanical compound BA as a raw material, carrying out ethylene glycol protection, oxidation, side chain extension reaction, ethylene glycol removal protection, reduction, hydrolysis and the like to synthesize the ursodeoxycholic acid. Raw materials for synthesizing the ursodeoxycholic acid are cheap and easy to obtain, synthesis steps are easy and convenient to operate, the yield is high, environmental friendliness is achieved, and industrial production is facilitated.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
Injection for clearing heat, eliminating phlegm and removing toxicity
The invention relates to an injection for clearing heat, eliminating phlegm and removing toxicity. The injection comprises an active material, propylene glycol and injection water. The active material is prepared from bear gall powder or ursodesoxycholic acid, cornu gorais, scutellaria baicalensis, honeysuckle and fructus forsythia. The invention further relates to a preparing method and pharmaceutical application of the injection. The injection can be used for clearing heat, eliminating phlegm and removing toxicity, can be used for treating the wind-warm lung-heat disease and the syndrome of phlegm-heat obstructing lung, can be used for treating fever, coughing, expectoration, swollen sore throat, thirsty, reddened tongue and yellow tongue coating, and can also be used for treating the early stage of pneumonia, acute bronchitis, chronic bronchitis acute attack and upper respiratory infection with the mentioned syndromes.
Owner:李兴惠
Method for preparing chenodeoxycholic acid and ursodesoxycholic acid by directly extracting and synthetizing from porcine bile paste or leftovers
The invention provides a method for preparing chenodeoxycholic acid and ursodesoxycholic acid by directly extracting and synthetizing from porcine bile paste or leftovers, comprising the following steps: using lower content (15-35%) porcine bile paste or leftovers and pre-processing it to remove off non-cholic acids impurities; removing off cholic acid impurities below the chenodeoxycholic acid in the thin layer chromatography by bromine oxidation or chromium oxidation; removing off cholic acid impurities above the chenodeoxycholic acid or the ursodesoxycholic acid in the thin layer chromatography by conventional reduction method; directly preparing the chenodeoxycholic acid or the ursodesoxycholic acid.
Owner:郑州药凰中医药研究有限公司
Method for preparing sodium tauroursodeoxycholate
The invention provides a method for preparing sodium tauroursodeoxycholate. The method comprises (1) using ursodesoxycholic acid as raw materials and getting a mixed anhydride solution through acetylation and chloride reactions; (2) dissolving taurine into a sodium carbonate solution to get a taurine sodium carbonate solution which is then added in drops into the mixed anhydride solution of step (1) with stirring, and then filtering precipitate to get a filtrate; (3) adjusting pH of the filtrate of step (2) to 6 to 7, concentrating and drying to get crude dried products of tauro-ursodesoxycholic acid; and (4) desalting purifying and re-crystallizing the concentrated dried products of step (3) to get the sodium tauroursodeoxycholate. The method is low in cost and short in technology route, with a yield up to 78%, and is suitable for industrial scale production.
Owner:HANGZHOU BAOJI BIO TECH
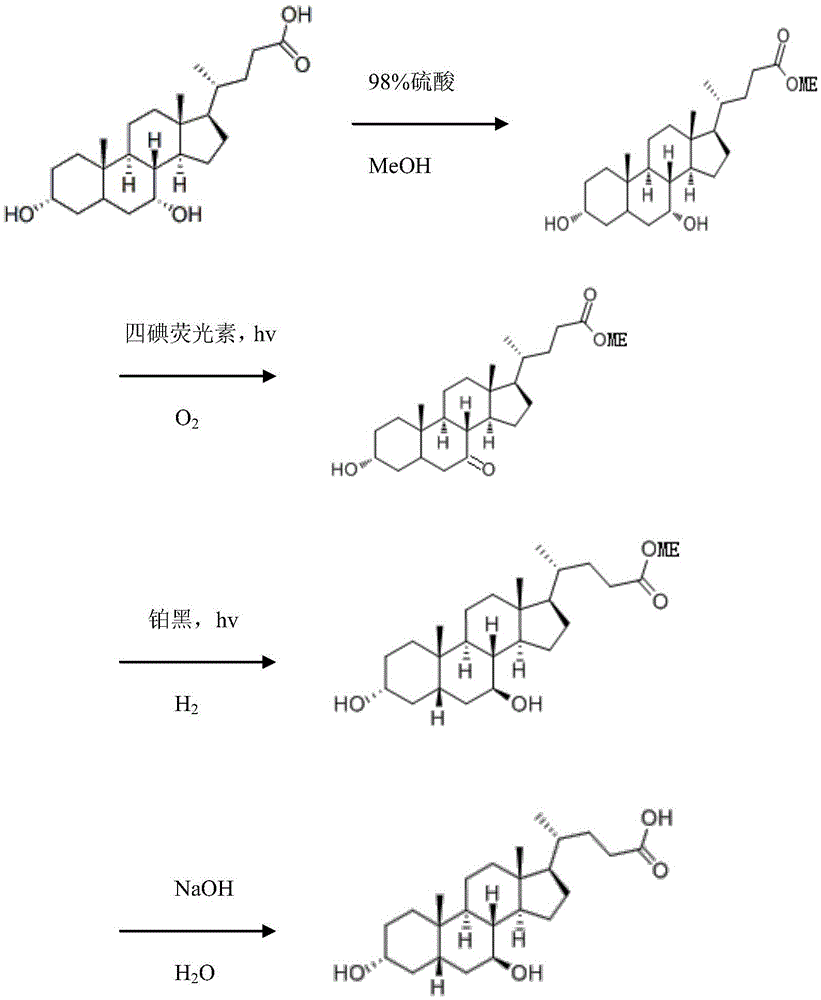
Method for synthesizing ursodesoxycholic acid with chenodeoxycholic acid by photochemical method
The invention discloses a method for synthesizing ursodesoxycholic acid with chenodeoxycholic acid by a photochemical method. The method comprises the following steps: preparing chenodeoxycholic acid methyl ester, preparing 3alpha-hydroxyl-7-keto-5beta-methyl cholanate by a photochemical oxidation process, preparing ursodesoxycholic acid methyl ester by a photochemical reduction method, and preparing ursodesoxycholic acid. The method mainly uses the photochemical method for converting chenodeoxycholic acid to ursodesoxycholic acid, the method has the advantages of mild reaction condition, high reaction efficiency and high selectivity; and the prepared ursodesoxycholic acid has the advantages of high yield, high purity and stable quality.
Owner:四川新功生物科技集团有限公司
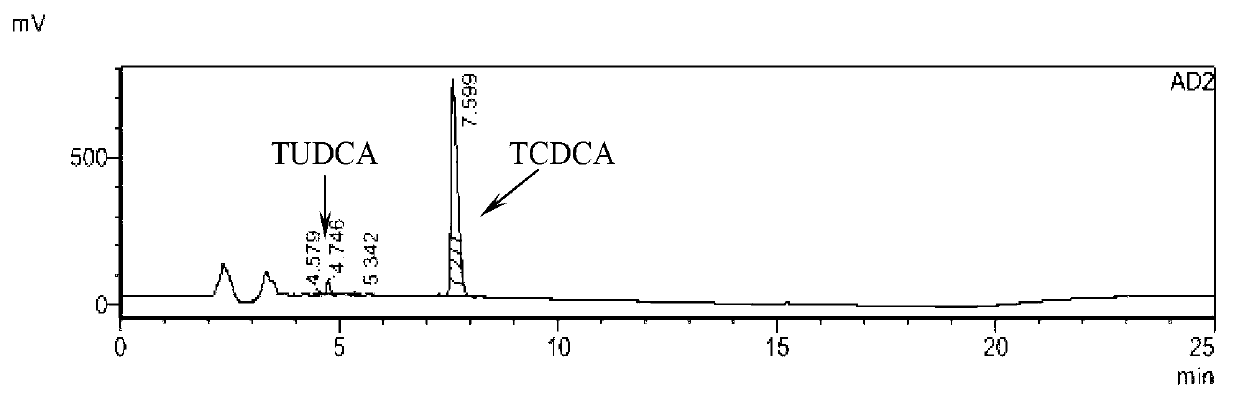
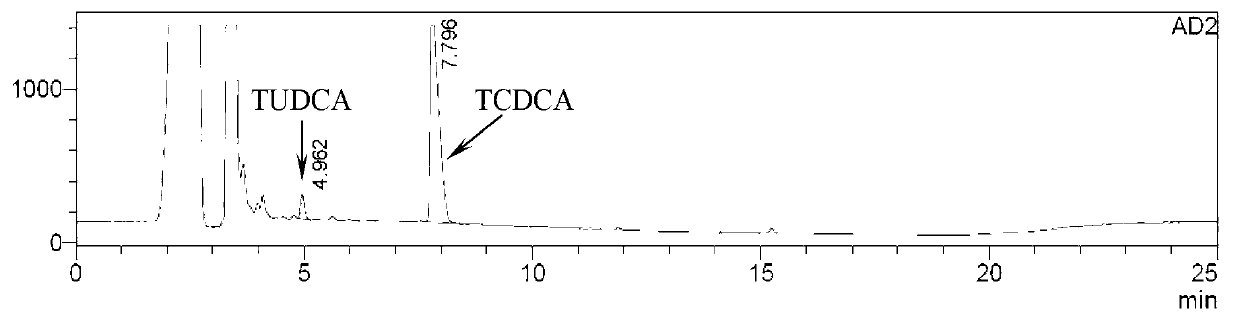
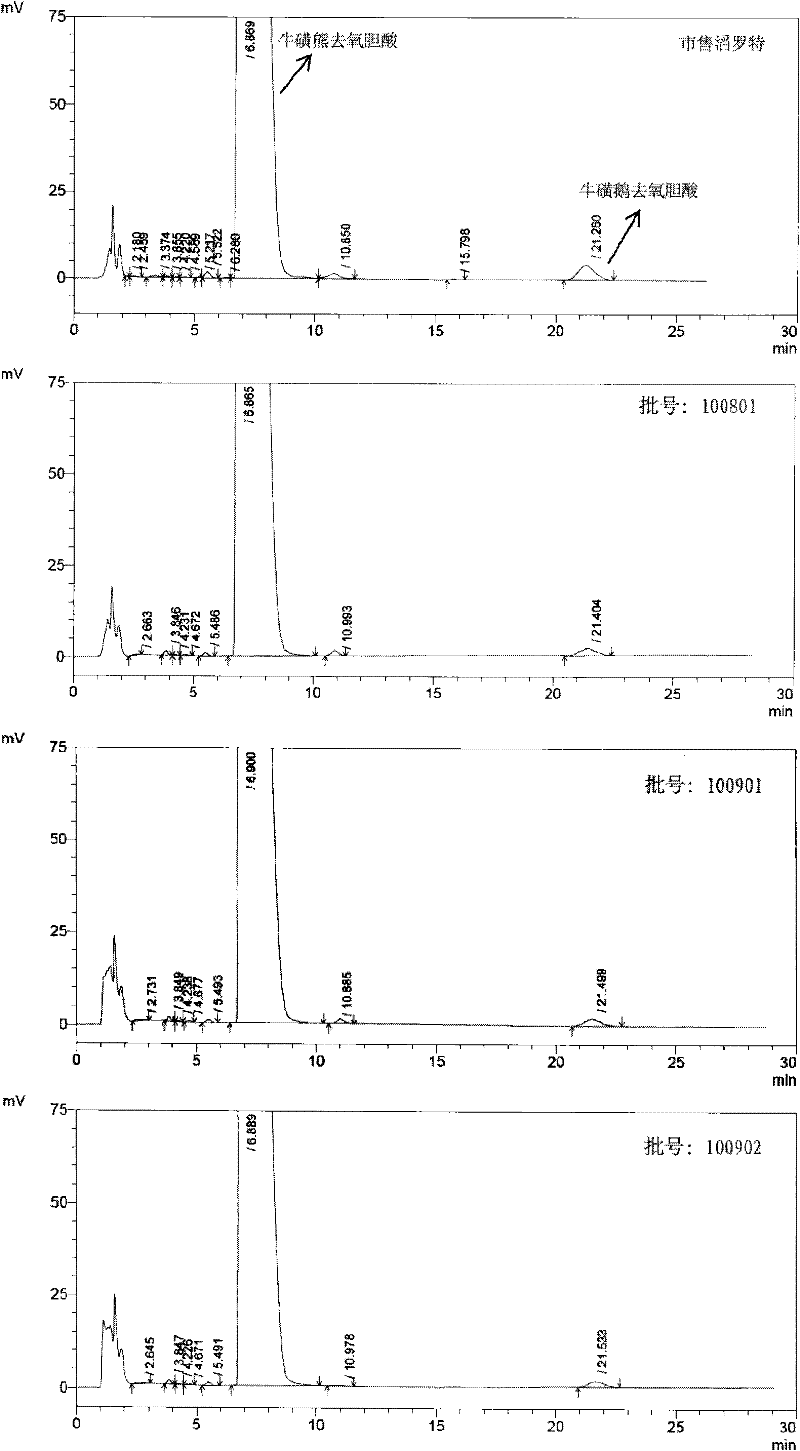
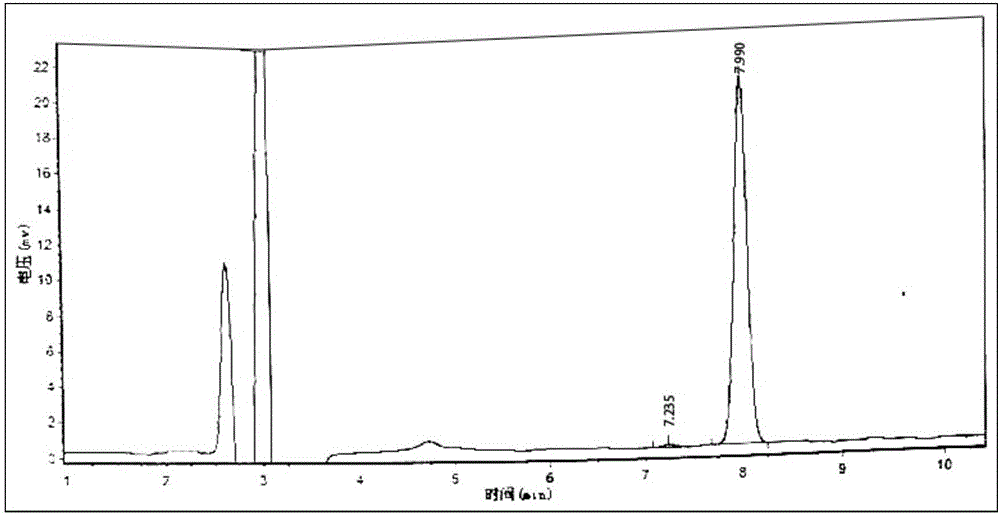
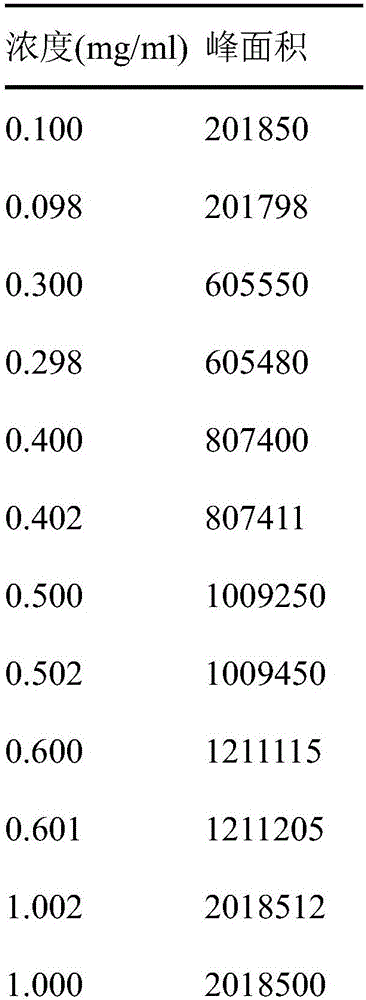
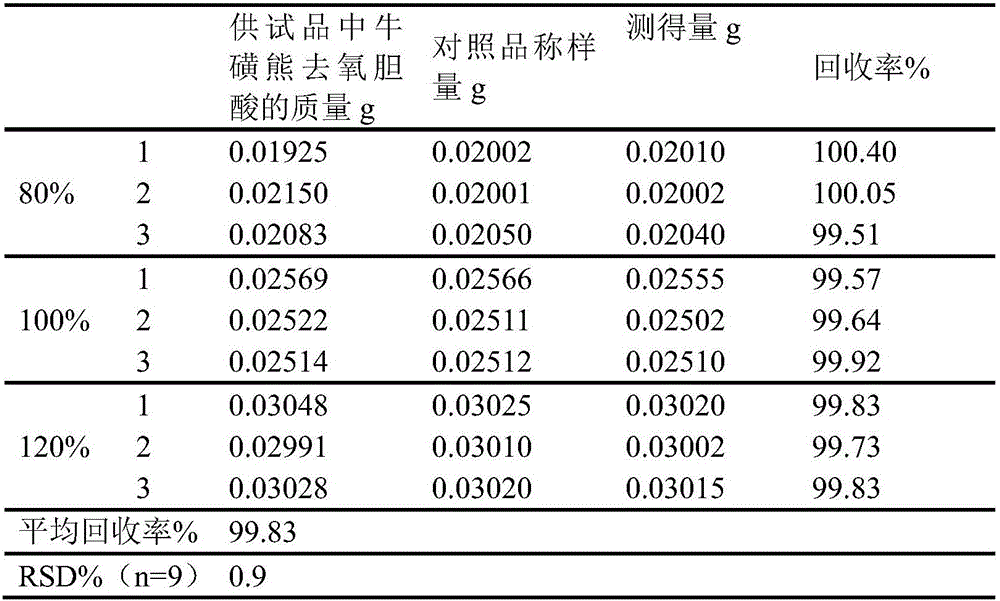
Novel detection method of tauroursodeoxycholic acid content and relevant substances
ActiveCN105891351AAccurate measurementReduce testing costsComponent separationCholic acidTest article
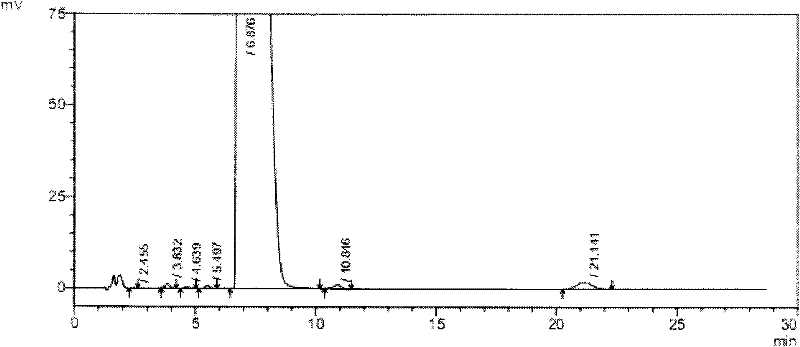
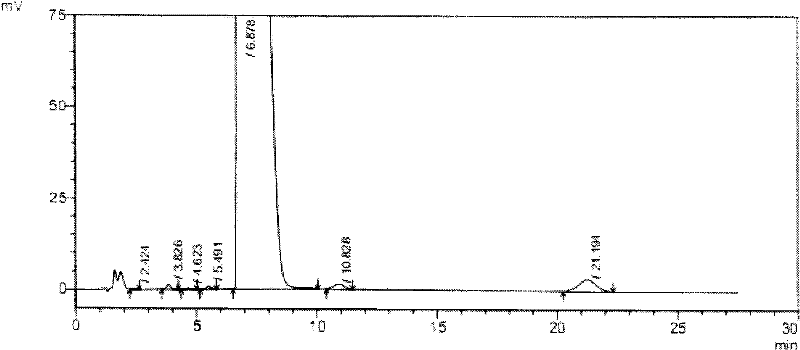
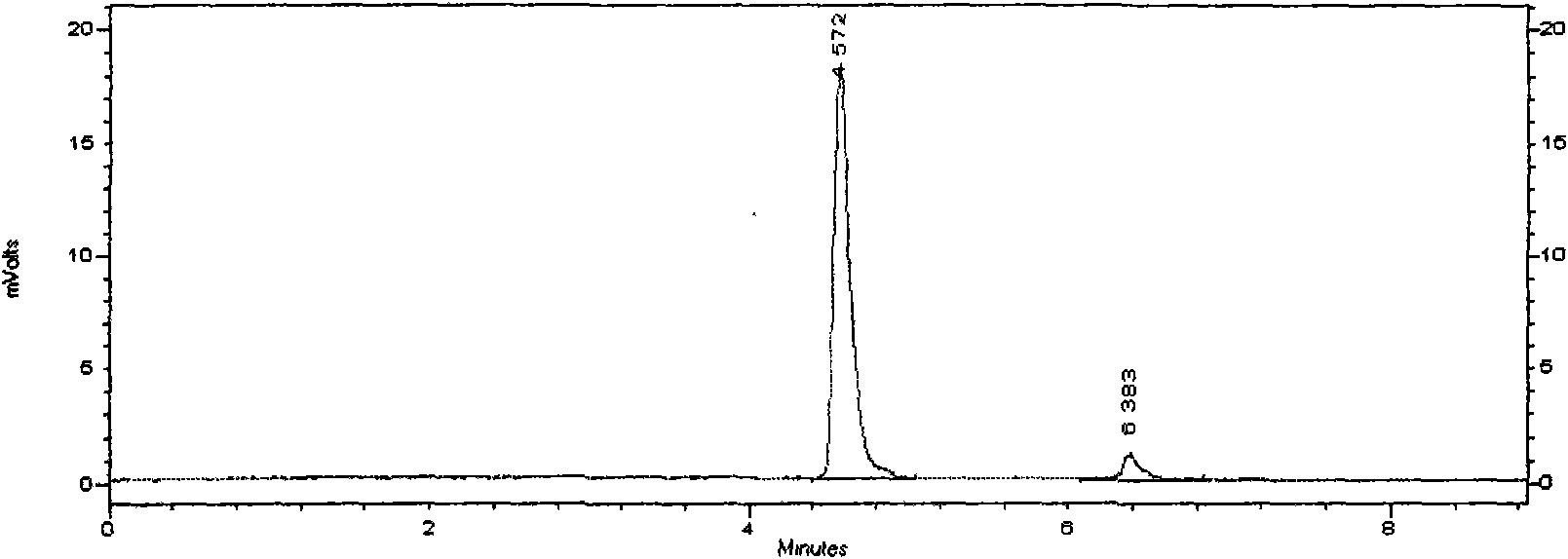
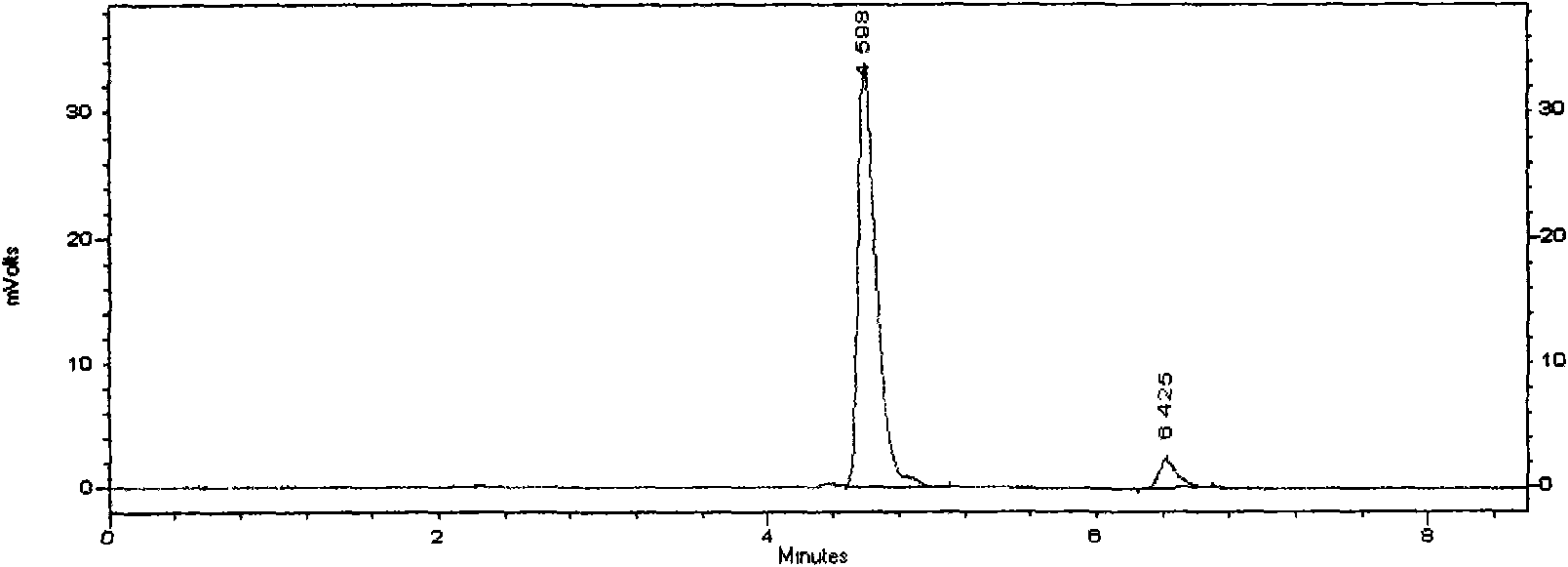
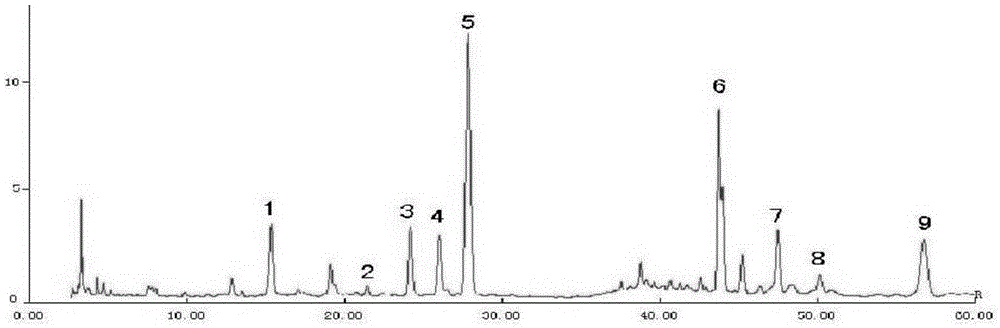
The invention discloses a novel detection method of the tauroursodeoxycholic acid content and relevant substances. The relevant substances are taurochenodeoxycholic acid and ursodesoxycholic acid. The method includes the steps of preparing a reference substance solution and a reference substance test article solution, injecting the solutions into a liquid phase chromatographic instrument, recording the peak area of tauroursodeoxycholic acid in a chromatograph, calculating the HPLC content through anhydrous tauroursodeoxycholic acid according to an external standard method, preparing an impurity reference substance solution and a sample solution, injecting the solutions into the liquid phase chromatographic instrument, recording the peak areas of taurochenodeoxycholic acid and ursodesoxycholic acid in the chromatogram, and calculating the amounts of the relevant substances in the tauroursodeoxycholic acid sample according to the external standard method. By means of the method, an RID is applied through the efficient liquid phase chromatography. Under the same liquid phase spectrum conditions, two cholic acid substances, namely tauroursodeoxycholic acid and ursodesoxycholic acid which are large in polarity difference, can be effectively separated and accurately and quantitatively detected.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com