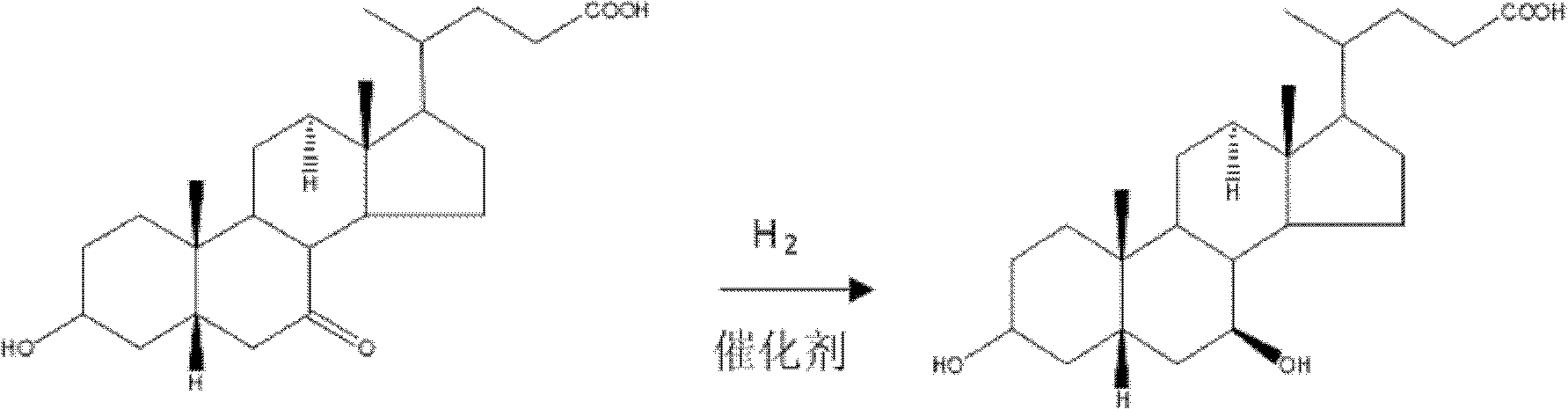
Method for preparing ursodesoxycholic acid by chiral catalytic hydrogenation of 7-ketodesoxycholic acid
A technology of ketodeoxycholic acid and ursodeoxycholic acid, which is applied in the field of chiral catalytic hydrogenation of 7-ketodeoxycholic acid to generate ursodeoxycholic acid, can solve problems such as poor stereoselectivity, and achieves easy operation control, The effect of high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Replace the air in the hydrogenation reactor with nitrogen, add 100L of methanol, stir and dissolve, add 10Kg of 7-ketodeoxycholic acid, 4Kg of potassium ethoxide, and 0.2 grams of 1# catalyst. After replacing the nitrogen in the reactor with hydrogen, keep the hydrogen pressure in the reactor at 15Mpa, and react at room temperature for 5 hours. Distill to remove the solvent in the reaction kettle, add 200L purified water, stir to dissolve, adjust PH4 with 1:1 hydrochloric acid, centrifuge, wash the filter cake with purified water until the pH of the washing water is greater than 5. Vacuum dry at 75°C until the water content is less than 0.5%, and obtain Xiongqu Oxycholic acid 9.5Kg.
Embodiment 2
[0034] Replace the air in the hydrogenation reactor with nitrogen, add 100L of ethanol, stir and dissolve, then add 10Kg of 7-ketodeoxycholic acid, 4Kg of sodium bicarbonate, and 0.5g of 2# catalyst. After replacing the nitrogen in the reactor with hydrogen, keep the hydrogen pressure in the reactor at 10Mpa, and react at room temperature for 3 hours. Distill to remove the solvent in the reaction kettle, add 150L purified water, stir to dissolve, adjust PH3 with 1:2 sulfuric acid, centrifuge, wash the filter cake with purified water until the pH of the washing water is greater than 5. Vacuum dry at 75°C until the water content is less than 0.5%, and get Xiongqu Oxycholic acid 9.4Kg.
Embodiment 3
[0036] Replace the air in the hydrogenation reactor with nitrogen, add 200L of tert-butanol, stir and dissolve, then add 10Kg of 7-ketodeoxycholic acid, 4Kg of sodium carbonate, and 1g of 3# catalyst. After replacing the nitrogen in the reactor with hydrogen, keep the hydrogen pressure in the reactor at 5Mpa, and react at room temperature for 4 hours. Distill to remove the solvent in the reaction kettle, add 300L purified water, stir to dissolve, adjust PH3 with 1:3 hydrochloric acid, centrifuge, wash the filter cake with purified water until the pH of the washing water is greater than 5. Vacuum dry at 75°C until the water content is less than 0.5%, and obtain Xiongqu Oxycholic acid 9.6Kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com