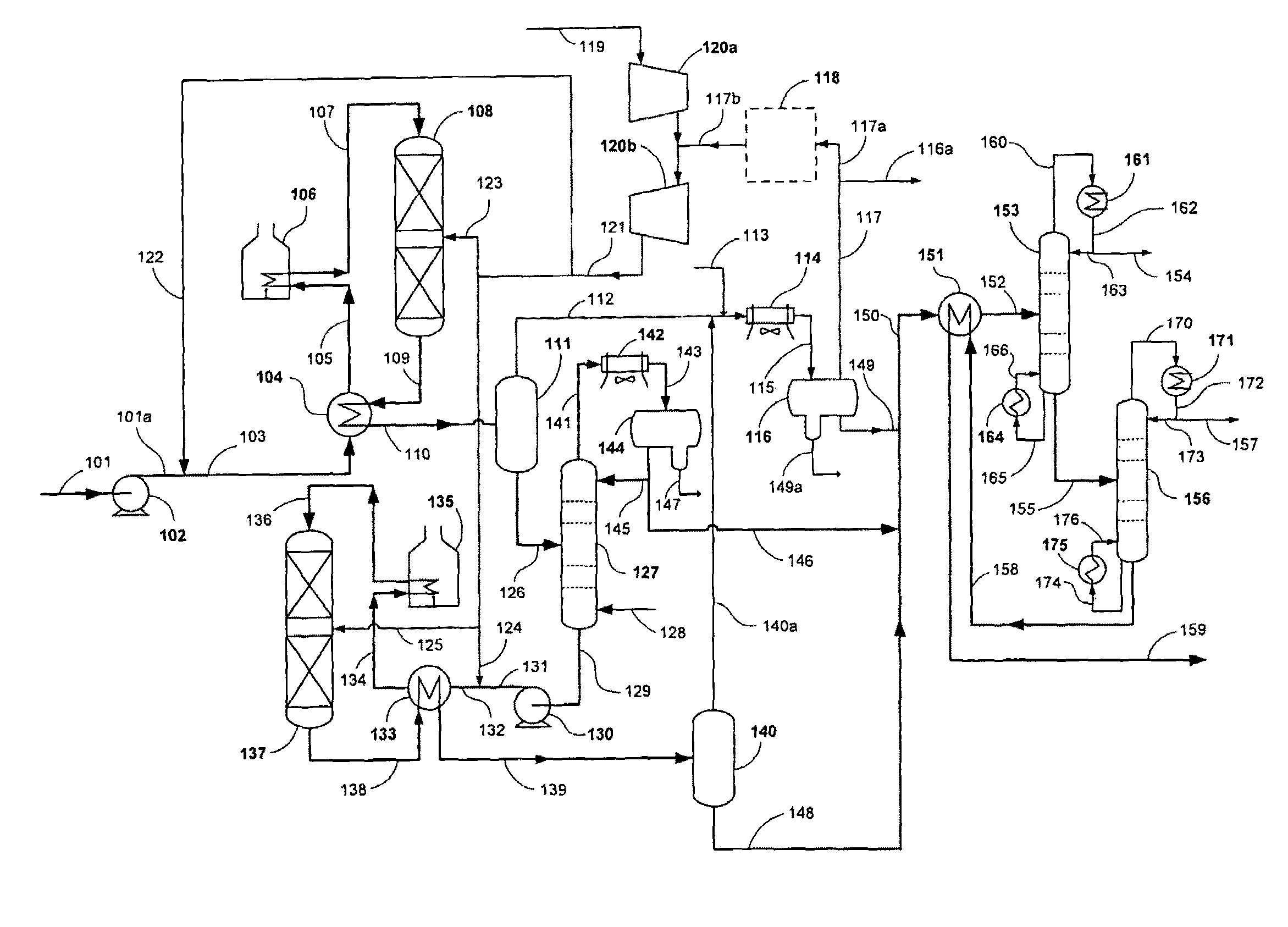
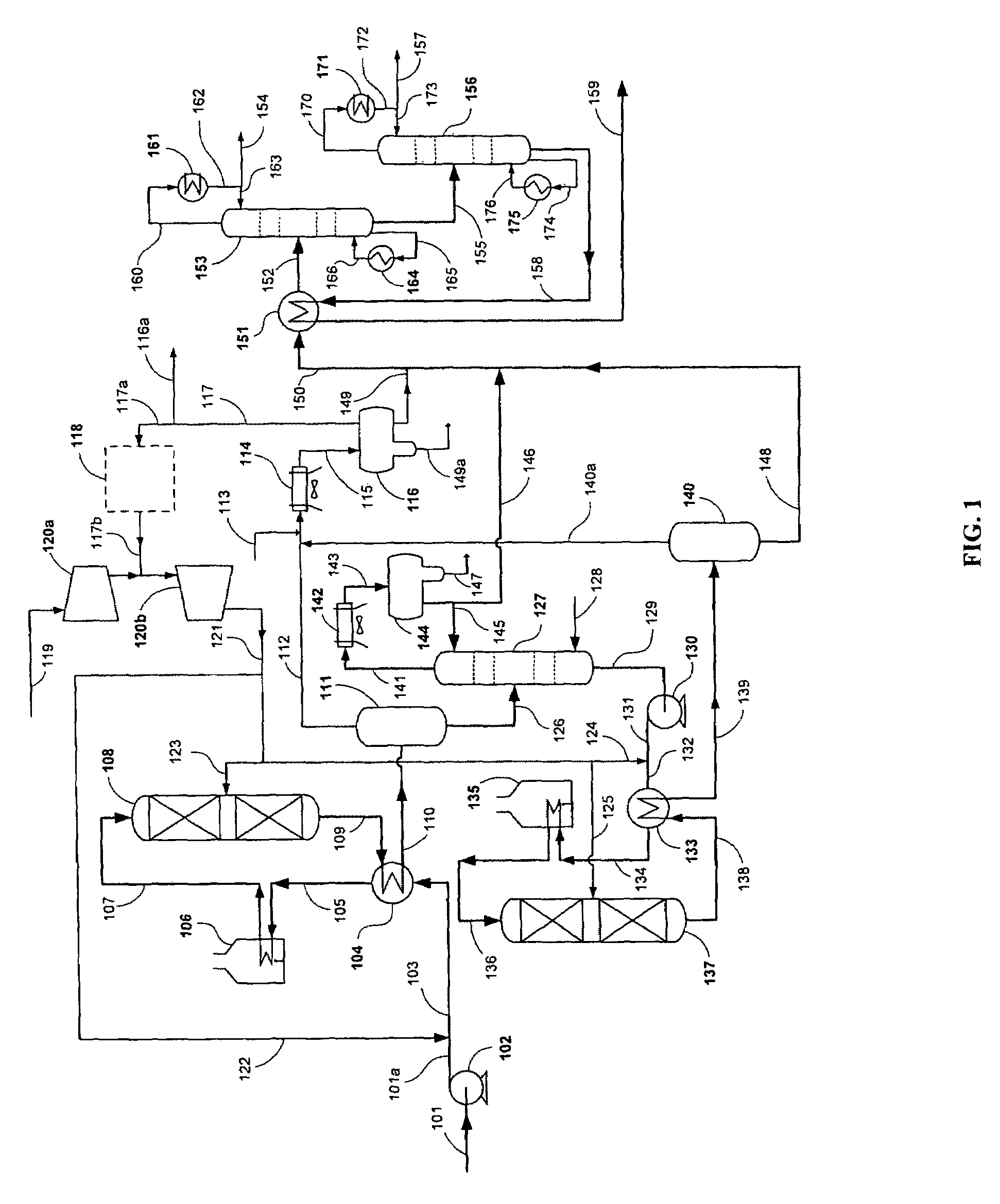
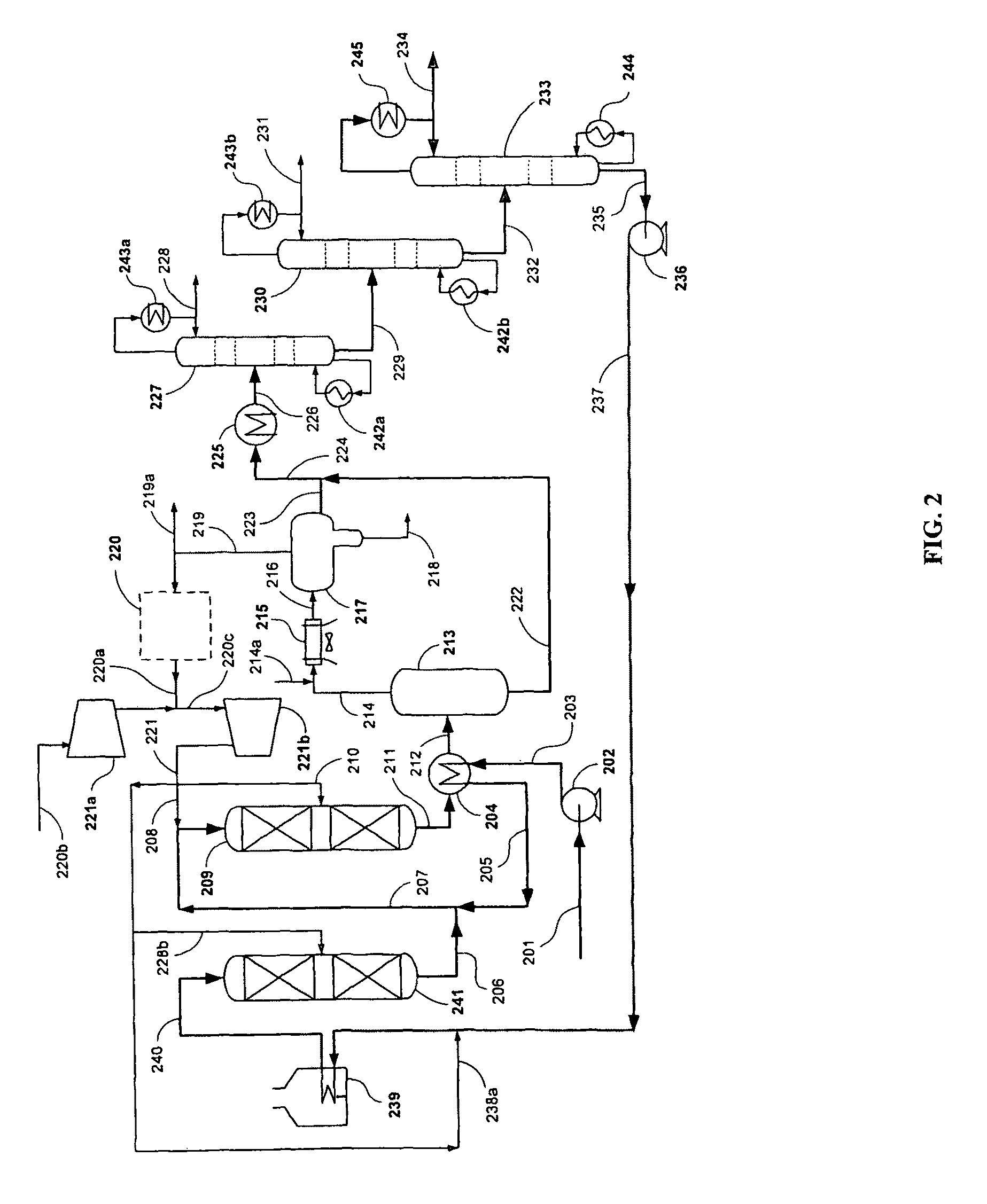
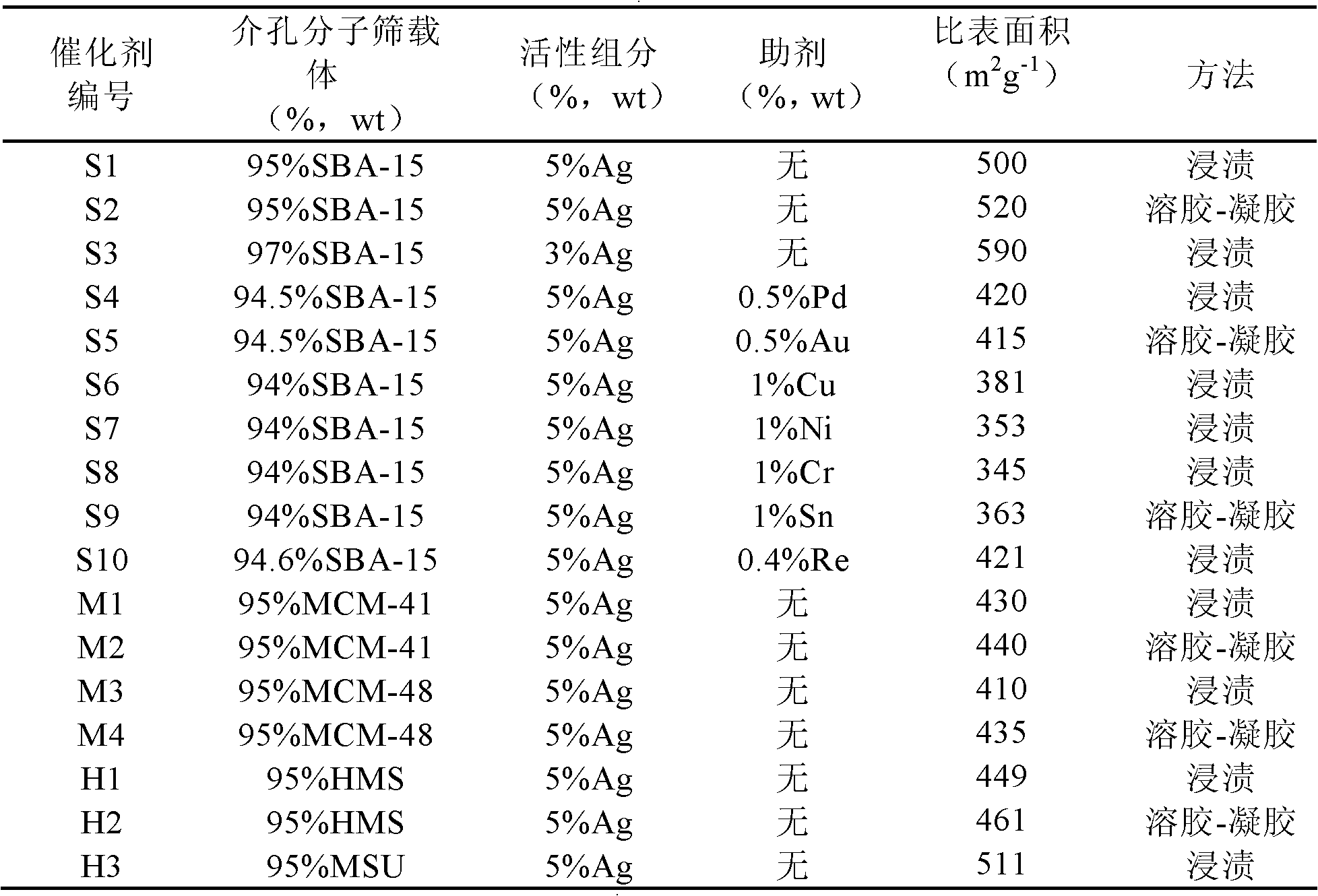
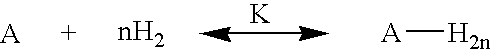Patents
Literature
1453 results about "Hydrogen pressure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
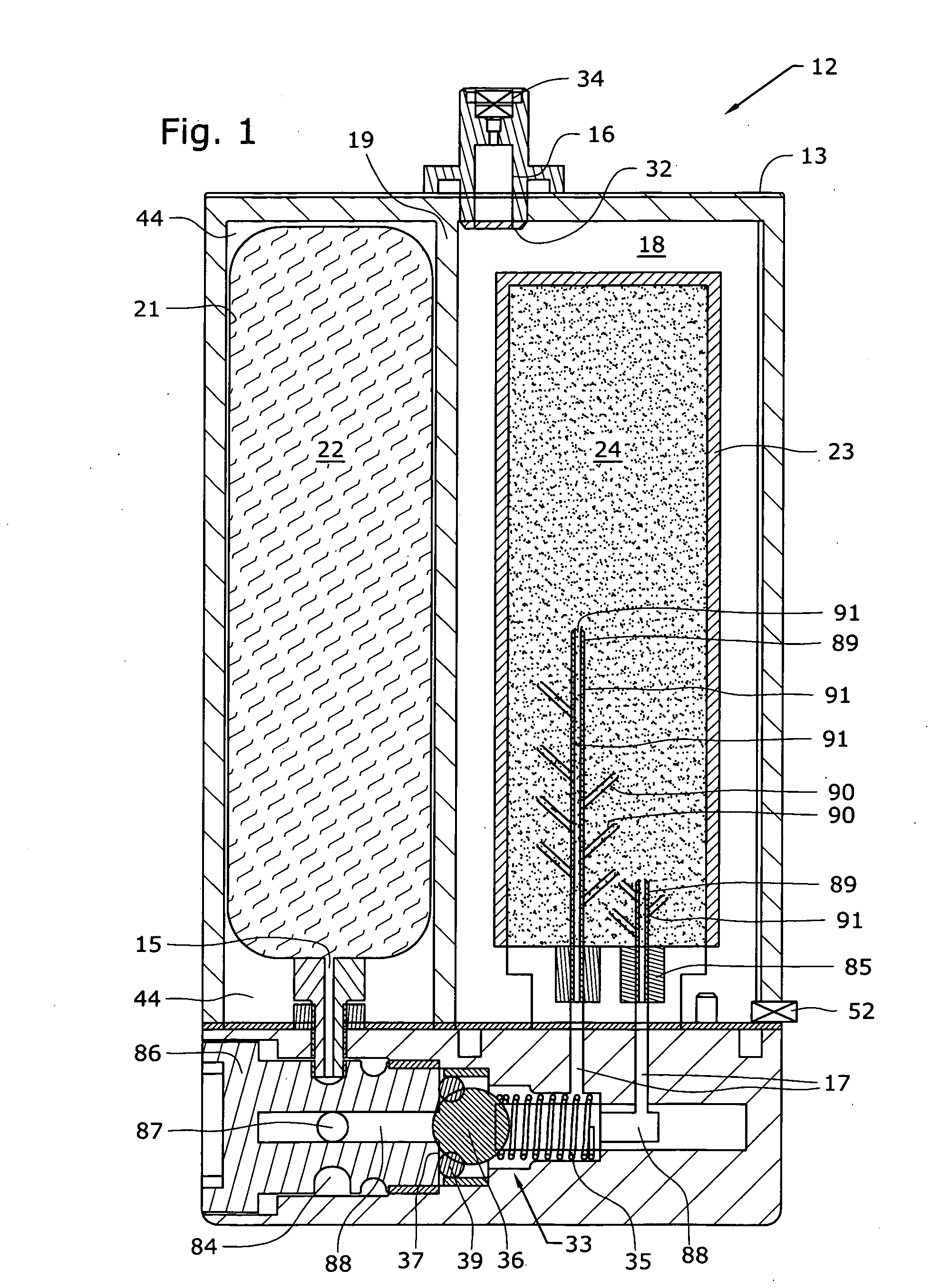
At room temperature and under standard pressure conditions, hydrogen is a gas that is tasteless, odorless and colorless. Hydrogen can exist as a liquid under high pressure and an extremely low temperature of 20.28 kelvin (−252.87°C, −423.17 °F).
Carbon dioxide production method
ActiveUS20070232706A1Increased hydrogen recoveryPromote recoverySolidificationLiquefactionVacuum pressureCarbon dioxide production
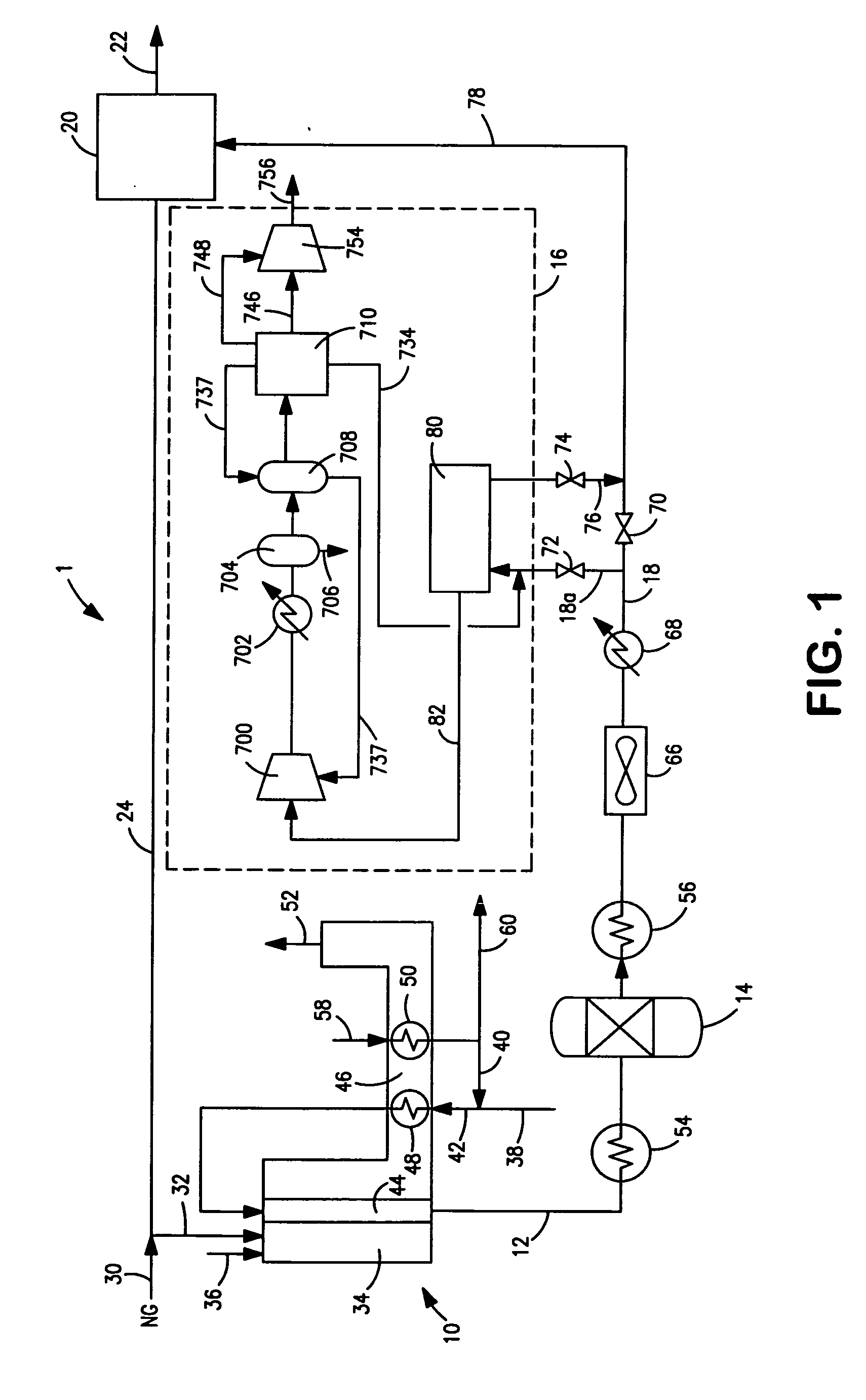
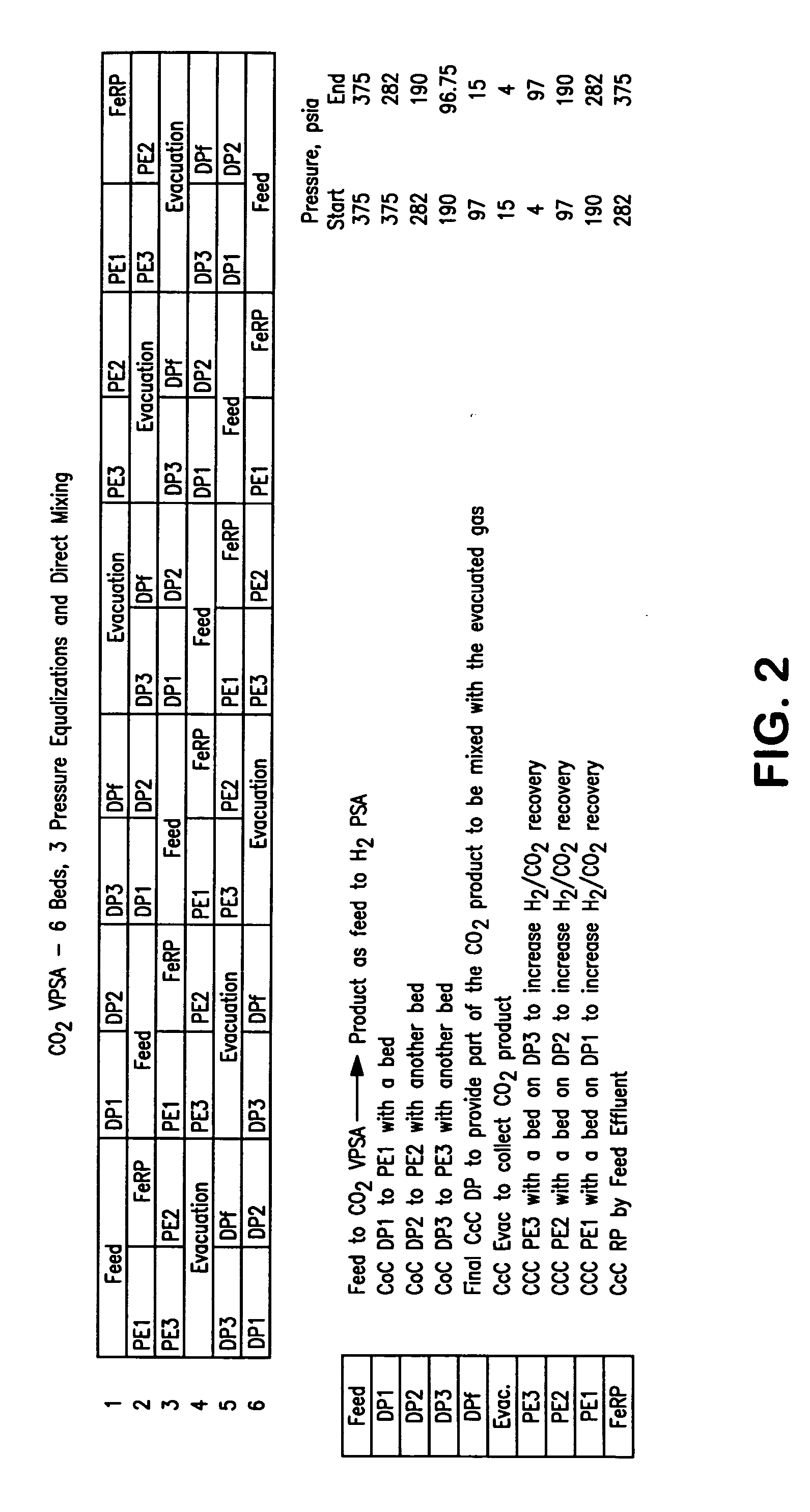
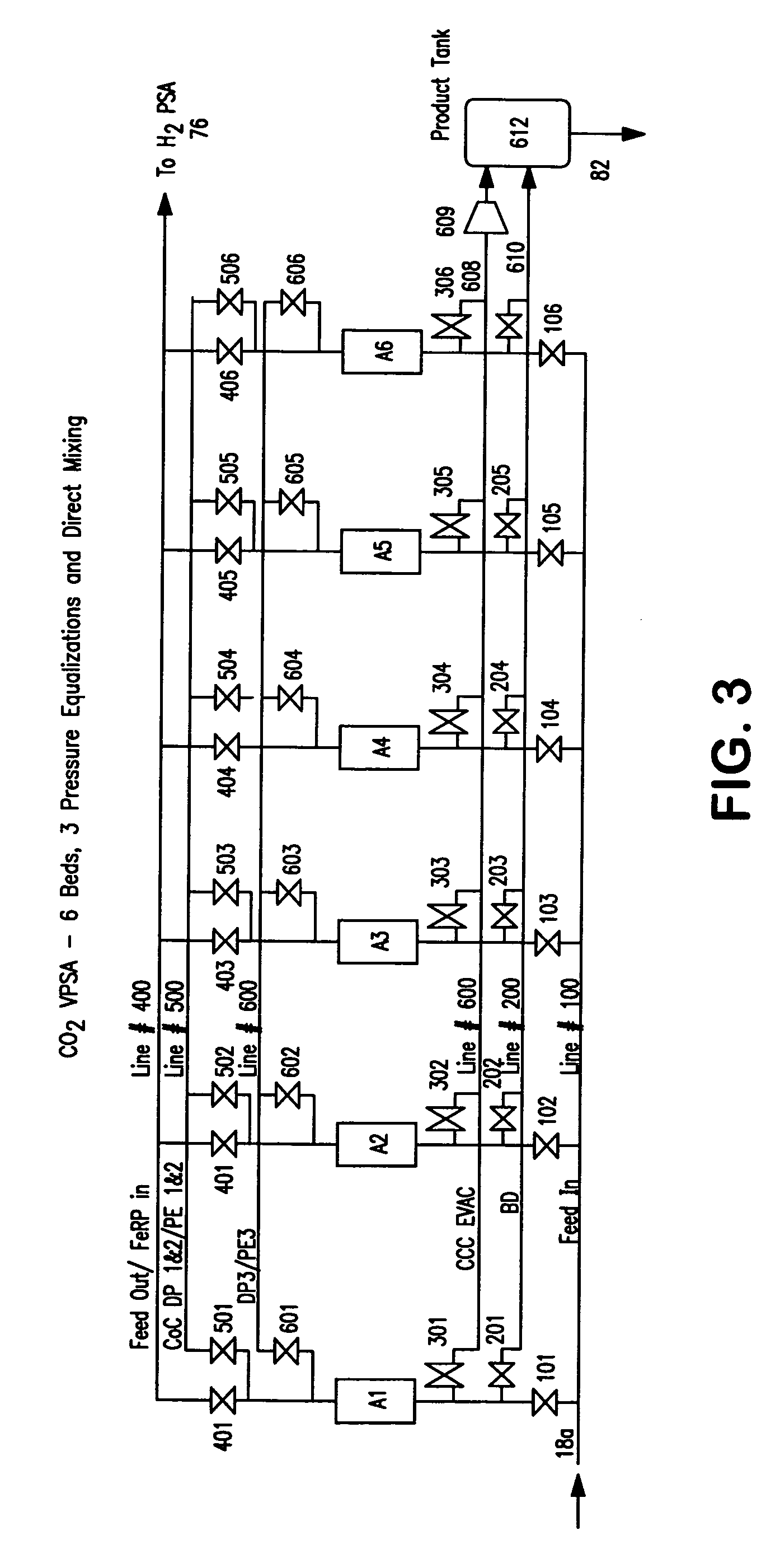
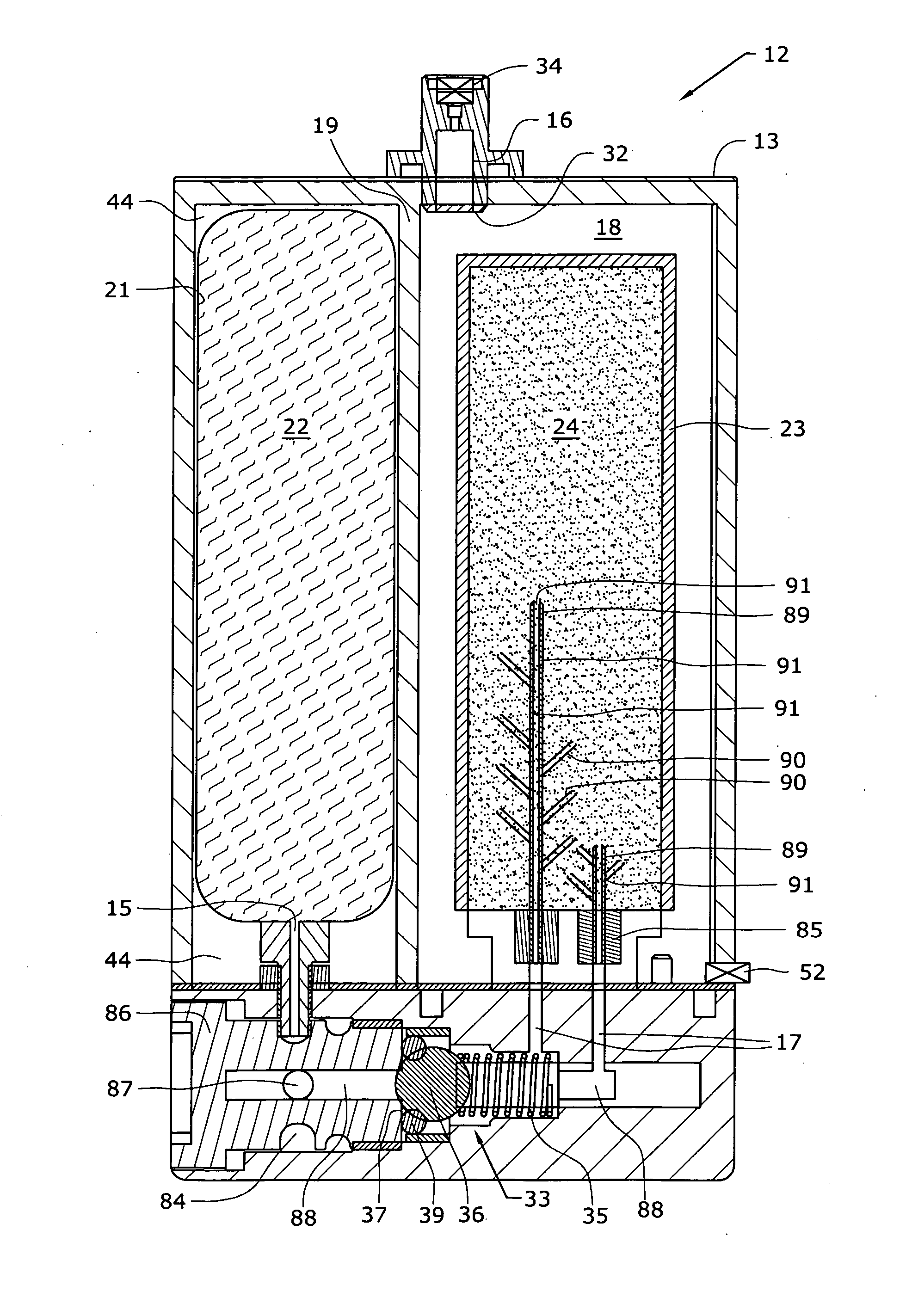
A method of producing a carbon dioxide product stream from a synthesis gas stream formed within a hydrogen plant having a synthesis gas reactor, a water-gas shift reactor, located downstream of the synthesis gas reactor to form the synthesis gas stream and a hydrogen pressure swing adsorption unit to produce a hydrogen product recovered from the synthesis gas stream. In accordance with the method the carbon dioxide from the synthesis gas stream by separating the carbon dioxide from the synthesis gas stream in a vacuum pressure swing adsorption system, thereby to produce a hydrogen-rich synthesis gas stream and a crude carbon dioxide stream and then purifying the crude carbon dioxide stream by a sub-ambient temperature distillation process thereby to produce the carbon dioxide product. A hydrogen synthesis gas feed stream to the hydrogen pressure swing adsorption unit is formed at least in part from the hydrogen rich stream.
Owner:PRAXAIR TECH INC
Hydrogen-generating fuel cell cartridges
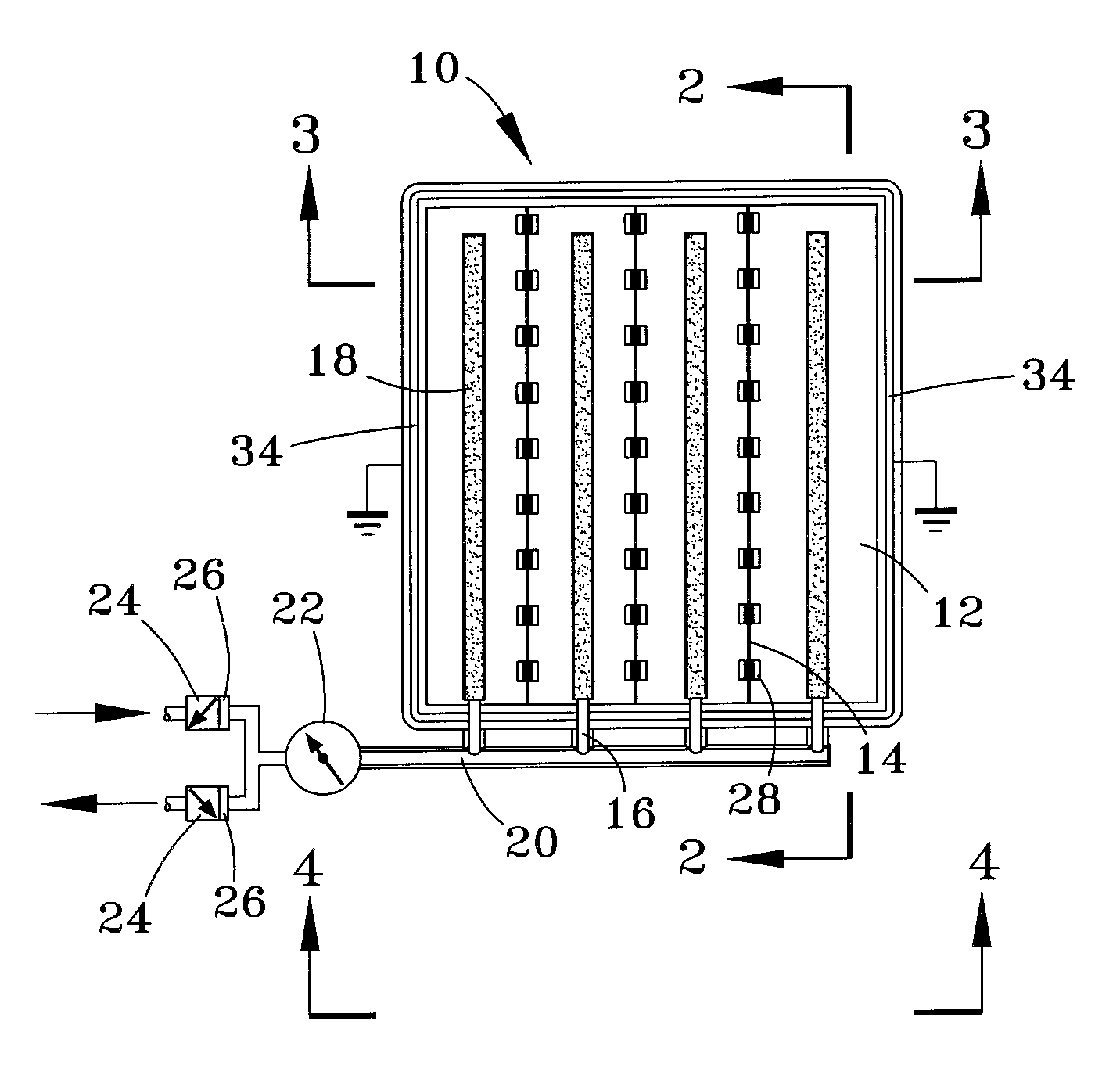
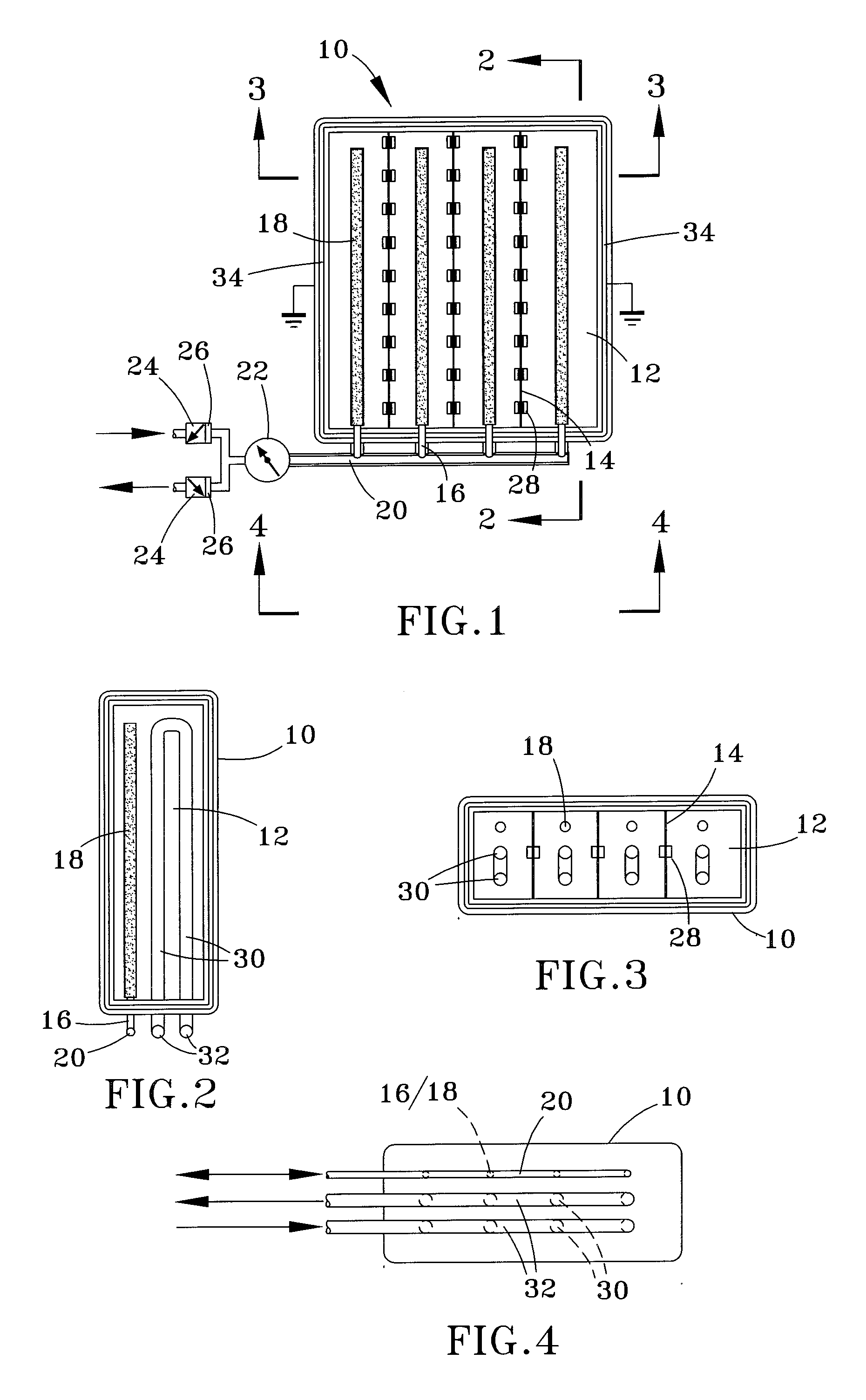
ActiveUS20060174952A1Minimize fluctuationDiaphragm valvesReactant parameters controlHydrogen pressureNuclear engineering
The present application is directed to a gas-generating apparatus and various pressure regulators or pressure-regulating valves. Hydrogen is generated within the gas-generating apparatus and is transported to a fuel cell. The transportation of a first fuel component to a second fuel component to generate of hydrogen occurs automatically depending on the pressure of a reaction chamber within the gas-generating apparatus. The pressure regulators and flow orifices are provided to regulate the hydrogen pressure and to minimize the fluctuation in pressure of the hydrogen received by the fuel cell. Connecting valves to connect the gas-generating apparatus to the fuel cell are also provided.
Owner:INTELLIGENT ENERGY LTD
Direct biomass hydroliquefaction process comprising two ebullated bed hydroconversion steps
ActiveCN102127462AExtend your lifeEasy to operateBiofuelsWaste based fuelHydrogen pressureLignocellulosic biomass
A process for direct hydroliquefaction of biomass selected from algae, lignocellulosic biomass and / or of one or more constituents of lignocellulosic biomass selected from the group comprising cellulose, hemicellulose and / or lignin for producing fuel bases comprising two successive hydroconversion stages under high hydrogen pressure in ebullating bed reactors. Hydroconversion takes place in the presence of a supported catalyst of the type for hydroconversion of petroleum residue and a suspension composed of the biomass and a solvent, preferably a hydrogen donor solvent and preferably recycled from the process. The biomass can undergo a pretreatment of drying and / or roasting and / or grinding and / or demineralization prior to hydroliquefaction.
Owner:INST FR DU PETROLE
Hydrogen-Generating Fuel Cell Cartridges
ActiveUS20080233462A1Minimize fluctuationDiaphragm valvesOperating means/releasing devices for valvesFuel cellsNuclear engineering
Owner:INTELLIGENT ENERGY LTD
Method for preparing ethanediol from polyhydroxy compounds
ActiveCN101735014ARaw material resources are renewableMeet the requirements of sustainable developmentOrganic compound preparationCatalyst activation/preparationHydrogen pressureCobalt
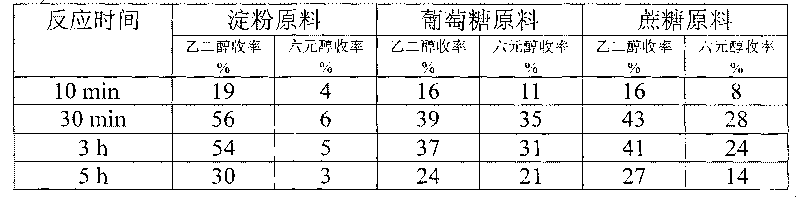
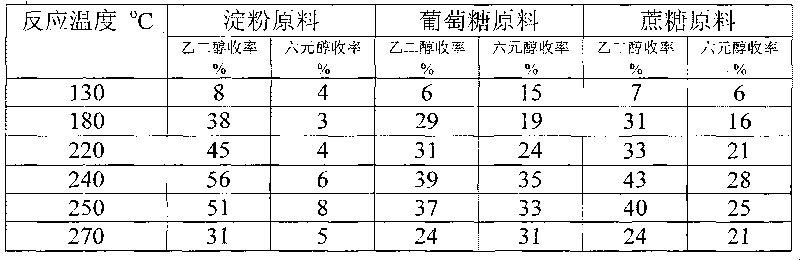
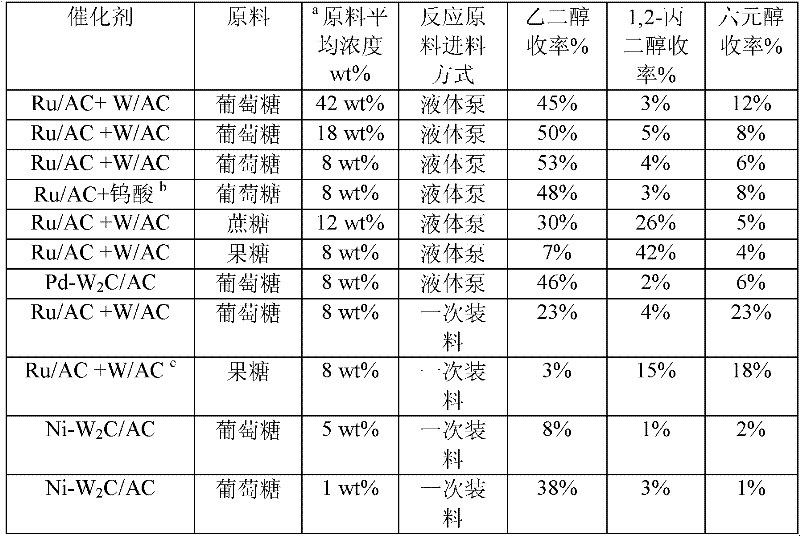
The invention provides a method for preparing ethanediol from polyhydroxy compounds comprising starch, hemicellulose, cane sugar, glucose, fructose and fructosan. The method comprises the following steps of: taking the polyhydroxy compounds as reaction raw materials, and taking metals, carbides, nitrides, and phosphides of transition metals in families VIII, IX and X, such as ferrum, cobalt, nickel, ruthenium, rhodium, palladium, iridium, platinum, molybdenum and tungsten as catalytic active components to form a polymetallic catalyst; and performing further catalytic conversion under a hydrothermal condition that the temperature is 120 to 300 DEG C and the hydrogen pressure is 1 to 13 MPa to prepare the ethanediol from the polyhydroxy compounds with high efficiency, high selectivity and high yield. The method for preparing the ethanediol from the polyhydroxy compounds has the outstanding advantages of renewable raw materials, environment-friendly reaction process, and atom economical efficiency. Simultaneously, compared with other techniques taking biomasses as the raw materials, the method has the advantages of simple process and high yield.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing ethylene glycol from cellulose
ActiveCN101723802ALow costWide variety of sourcesOrganic compound preparationCatalyst activation/preparationHydrogen pressurePolyethylene glycol
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
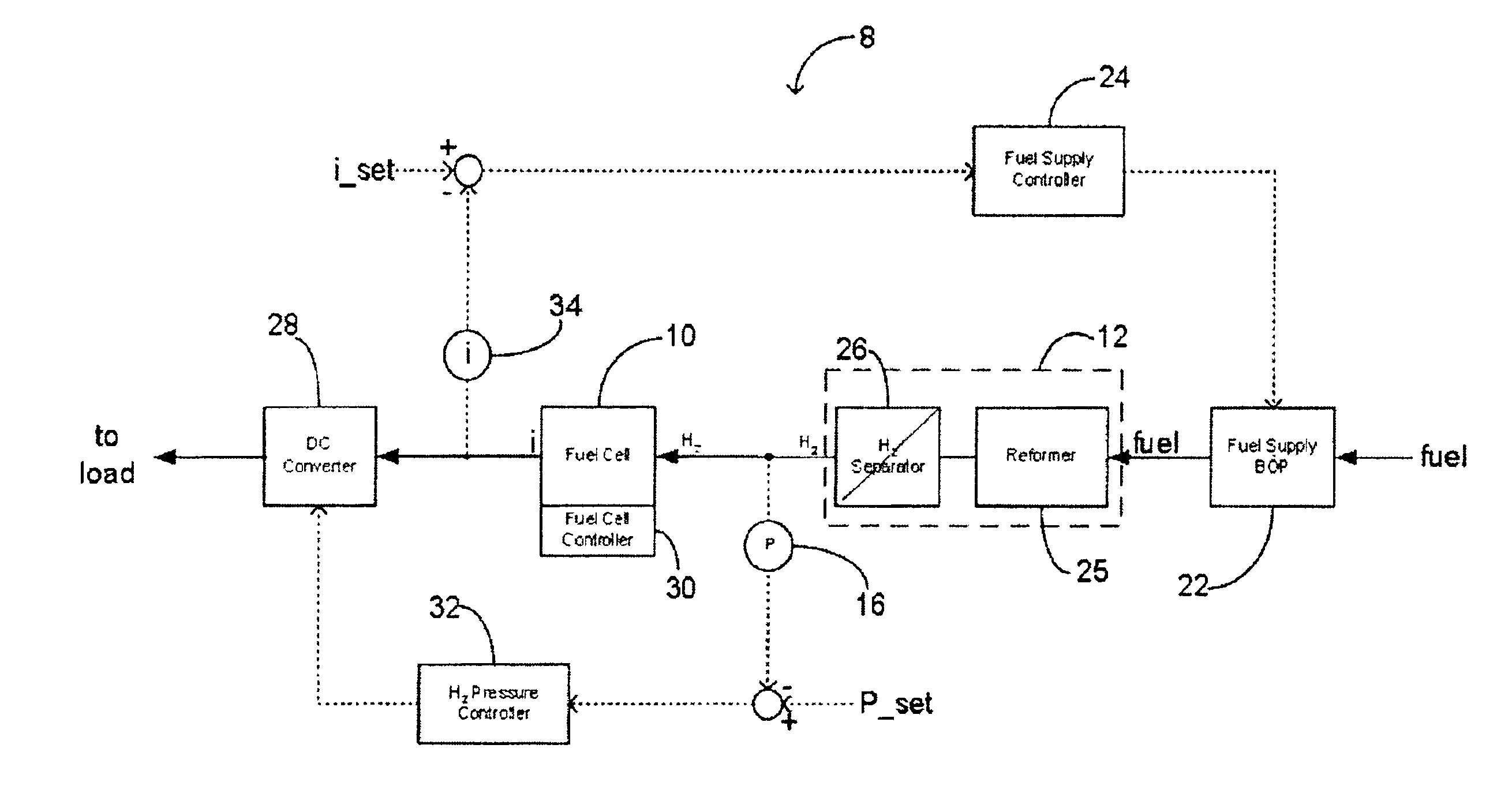
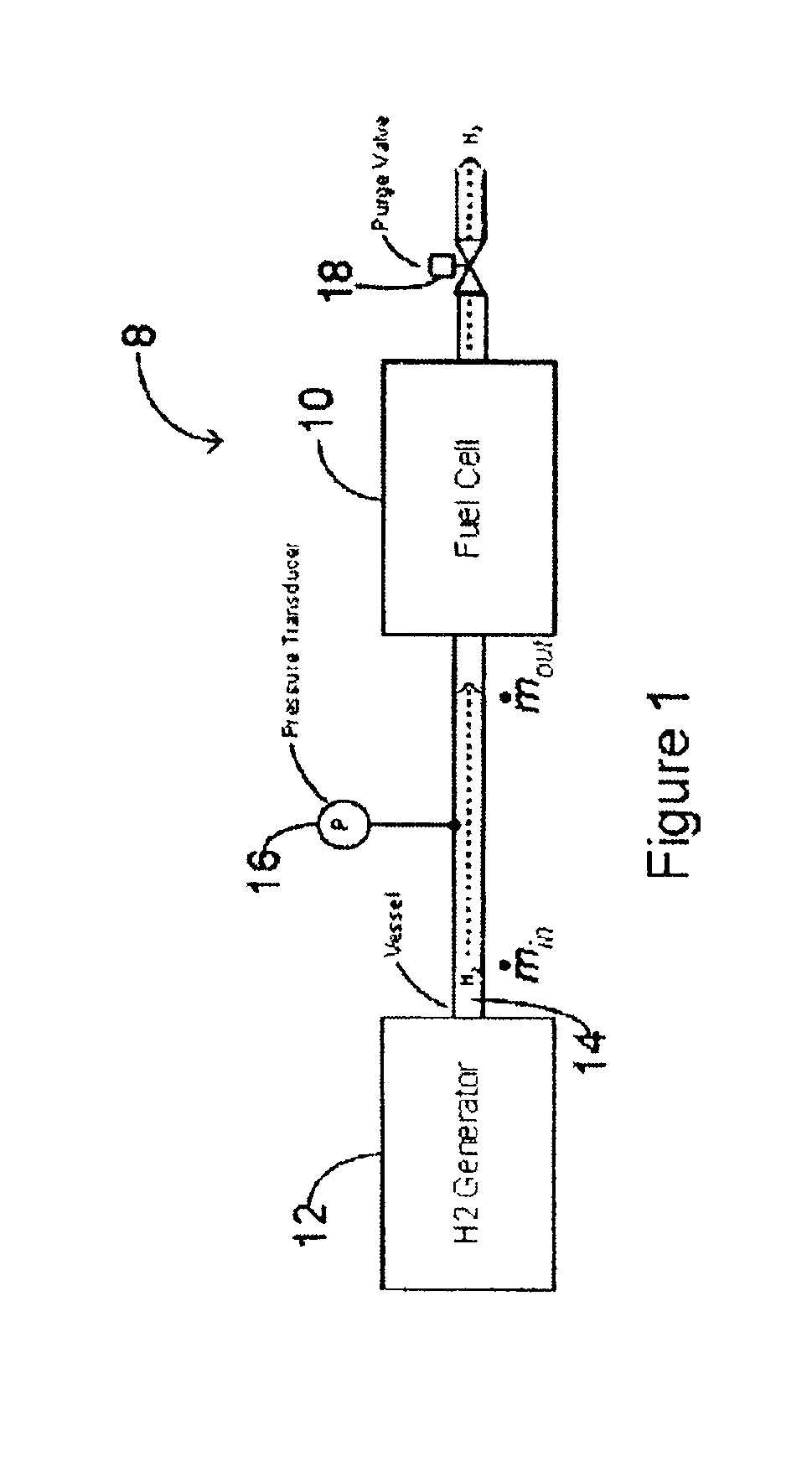
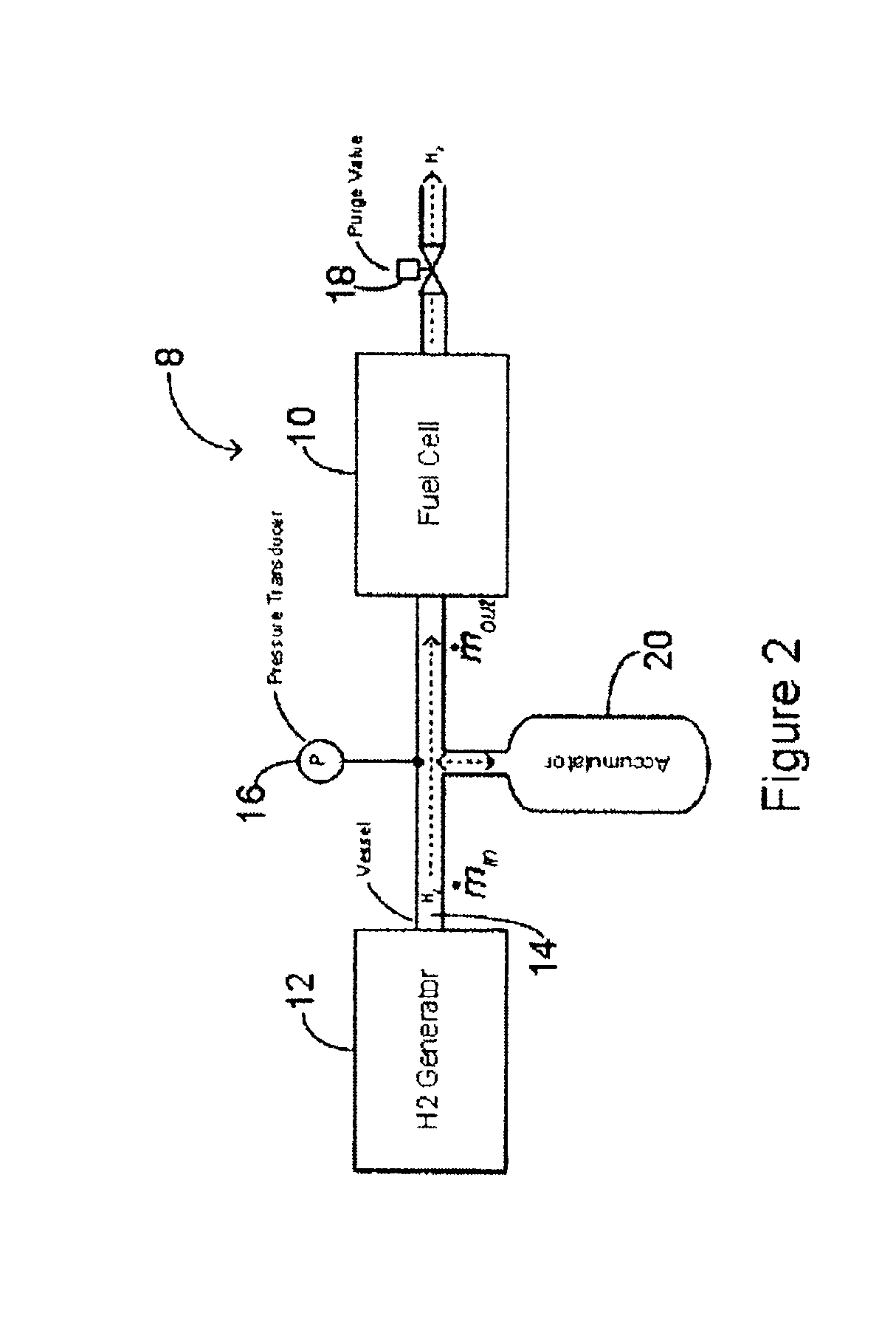
Method and system for controlling the operation of a hydrogen generator and a fuel cell
InactiveUS6893755B2Batteries circuit arrangementsReactant parameters controlThermodynamicsHydrogen pressure
This application relates to a method and system for controlling the supply of fuel to a dead-ended hydrogen fuel cell. The invention may be utilized, for example, to more efficiently integrate the operation of a hydrogen fuel cell and a hydrogen generator, such as a reformer coupled with a hydrogen separation unit. The invention ensures that the production and consumption of hydrogen are effectively balanced to avoid negative feed line pressure fluctuations. The fuel supply control subsystem and hydrogen consumption control subsystems are, however, “decoupled” and hence independently operable. The invention may include an accumulator disposed in a flow path between the hydrogen generator and the fuel cell for storing hydrogen under pressure. The accumulator is sufficiently large in volume such that the pressure of hydrogen in the flow path does not deviate substantially from a target pressure, even during the waste purging sessions. The system enables the use of low-cost pressure transducers in place of mass flow meters. In one aspect of the invention raffinate flow from the hydrogen separator can be controllably adjusted to regulate the temperature or other operating parameters of the reformer.
Owner:CELLEX POWER PRODS
Method for preparing ethylene glycol and 1,2-propylene glycol by using saccharide solution
ActiveCN102675045AIncrease concentrationReduce distillation energy consumptionOrganic compound preparationHydroxy compound preparationHydrogen pressurePolyethylene glycol
The invention provides a method for preparing ethylene glycol and 1,2-propylene glycol by using a high-concentration saccharide solution. Reaction raw materials comprise cane sugar, glucose, fructose, fructosan, xylose, soluble lower polyxylose and soluble starch. According to the method, high-concentration saccharide is used as a reaction raw material, and a high-pressure pump feeding mode is used in a reaction process which is performed in a high-pressure reaction kettle; iron, cobalt, nickel, ruthenium, rhodium, palladium, iridium and platinum which serve as transition metal in eighth, ninth and tenth groups are used as hydrogenation active ingredients; the hydrogenation active ingredients form a composite catalyst together with metal tungsten, tungsten carbide, tungsten nitride, tungsten phosphide, tungsten oxide, tungsten sulfide, tungsten chloride, tungsten hydroxide, tungsten bronze, tungstic acid, tungstate, metatungstic acid, metatungstate, paratungstic acid, paratungstate, peroxotungstic acid, peroxotungstate and tungsten-containing heteropolyacid which serve as catalytic active ingredients; and the high-concentration saccharide solution can be efficiently prepared into the ethylene glycol and the propylene glycol at high selectivity and high yield in a one-step catalytic conversion process under the hydrothermal condition that the temperature is 120 to 300 DEG C and the hydrogen pressure is 1 to 13MPa. By the method, the problem of coking of the high-concentration saccharide in the catalytic conversion process can be effectively solved, and high-concentration ethylene glycol and propylene glycol can be prepared by the high-concentration saccharide.
Owner:中科柏易金(郑州)新能源科技有限责任公司
Catalyst for producing 1,2-cyclohexane dicarboxylic acid diesters
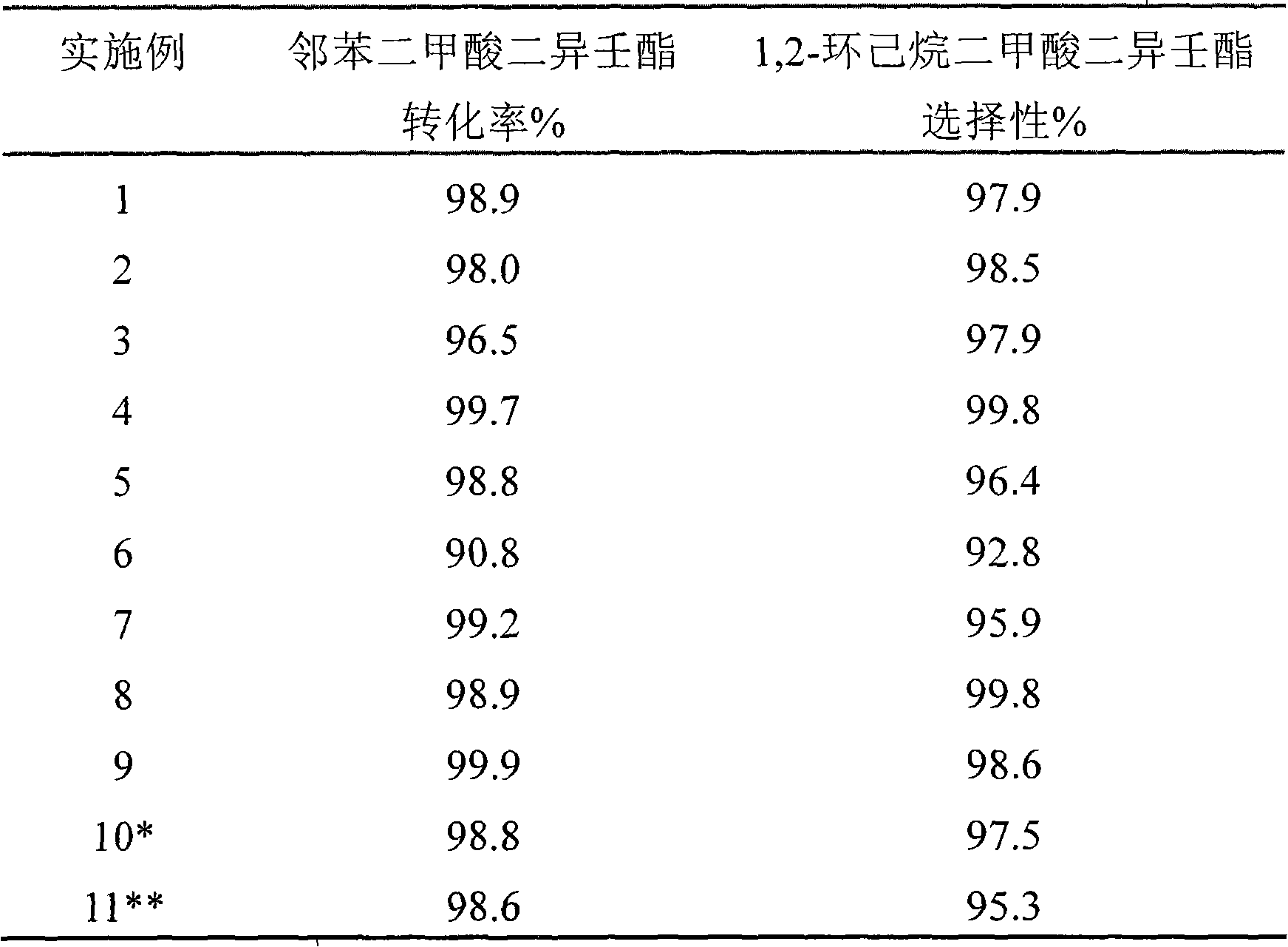
InactiveCN101406840AHigh selectivityLow reaction pressureOrganic compound preparationCarboxylic acid esters preparationHydrogen pressureBULK ACTIVE INGREDIENT
The invention provides a catalyst for converting diisononyl phthalate, diisooctyl phthalate, dibutyl phthalate and other long-chain esters into corresponding 1, 2-cyclohexane dicarboxylic acid binary ester through hydrogenation. The catalyst for converting the diisononyl phthalate, the diisooctyl phthalate, the dibutyl phthalate and other long-chain esters into corresponding the 1,2-cyclohexane dicarboxylic acid binary ester through the hydrogenation consists of main active ingredients, additives and carriers, wherein the main active ingredients are noble metal Ru and Pd; the additives are Fe, Co, Ni, Cu and other metals or oxides; and macroporous Al2O3, ZrO2, TiO2 and the like are selected as the carriers. Under certain temperature, certain hydrogen pressure and the action of the catalyst, the diisononyl phthalate, the diisooctyl phthalate, the dibutyl phthalate and other long-chain esters in a trickle bed reactor can be converted into the corresponding the 1, 2-cyclohexane dicarboxylic acid binary ester with high activity and high selectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
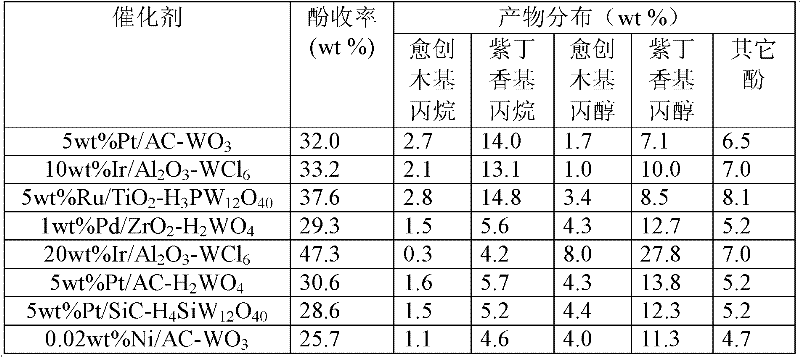
Application of tungsten-based catalyst in lignin catalytic hydrogenation for producing aromatic compound
ActiveCN102476980AWide variety of sourcesLow costCatalyst carriersOrganic compound preparationIridiumHydrogen pressure
The invention relates to hydrocracking of lignin, and specifically relates to a method for applying a tungsten-based catalyst to catalyze lignin hydrocracking for producing an aromatic compound. The catalyst comprises a main active component of non-zero-valent tungsten, and a second metal component of a small amount of one or more transition metals selected from zero-valent nickel, cobalt, ruthenium, iridium, palladium, platinum, iron, and copper. According to the method, raw materials such as lignin, biomass hydrolysis residue, lignosulfonate, and alkaline lignin are subject to catalytic hydrogenation under a hydrothermal condition with a temperature of 120 to 450 DEG C and a hydrogen pressure of 1 to 20MPa; the raw materials are cracked into C6-C9 phenolic compounds with high selectivity. A maximal phenol yield reaches 55.6%. Compared to existing technologies, according to the invention, renewable natural biomasses are adopted as raw materials, such that the raw materials are cheap, and have wide sources; inorganic acid and alkali are not required, such the production of a large amount of alkaline solution in traditional lignin catalysis is avoided; the tungsten-based catalyst is cheap; the reaction process is green, and has atom economical characteristics.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
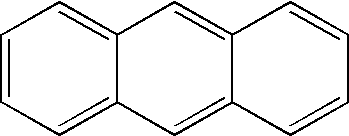
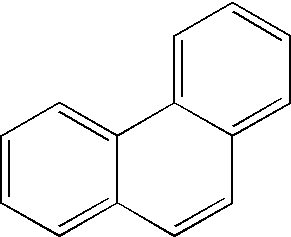
Hydrogen storage by reversible hydrogenation of pi-conjugated substrates
Processes are provided for the storage and release of hydrogen by means of a substantially reversible catalytic hydrogenation of extended pi-conjugated substrates which include large polycyclic aromatic hydrocarbons, polycyclic aromatic hydrocarbons with nitrogen heteroatoms, polycyclic aromatic hydrocarbons with oxygen heteroatoms, polycyclic aromatic hydrocarbons with alkyl, alkoxy, ketone, ether or polyether substituents, pi-conjugated molecules comprising 5 membered rings, pi-conjugated molecules comprising six and five membered rings with nitrogen or oxygen hetero atoms, and extended pi-conjugated organic polymers. The hydrogen, contained in the at least partially hydrogenated form of the extended pi-conjugated system, can be facilely released for use by a catalytic dehydrogenation of the latter in the presence of a dehydrogenation catalyst which can be effected by lowering the hydrogen gas pressure, generally to pressures greater than 0.1 bar or raising the temperature to less than 250° C. or less, or by a combination of these two process parameters.
Owner:AIR PROD & CHEM INC
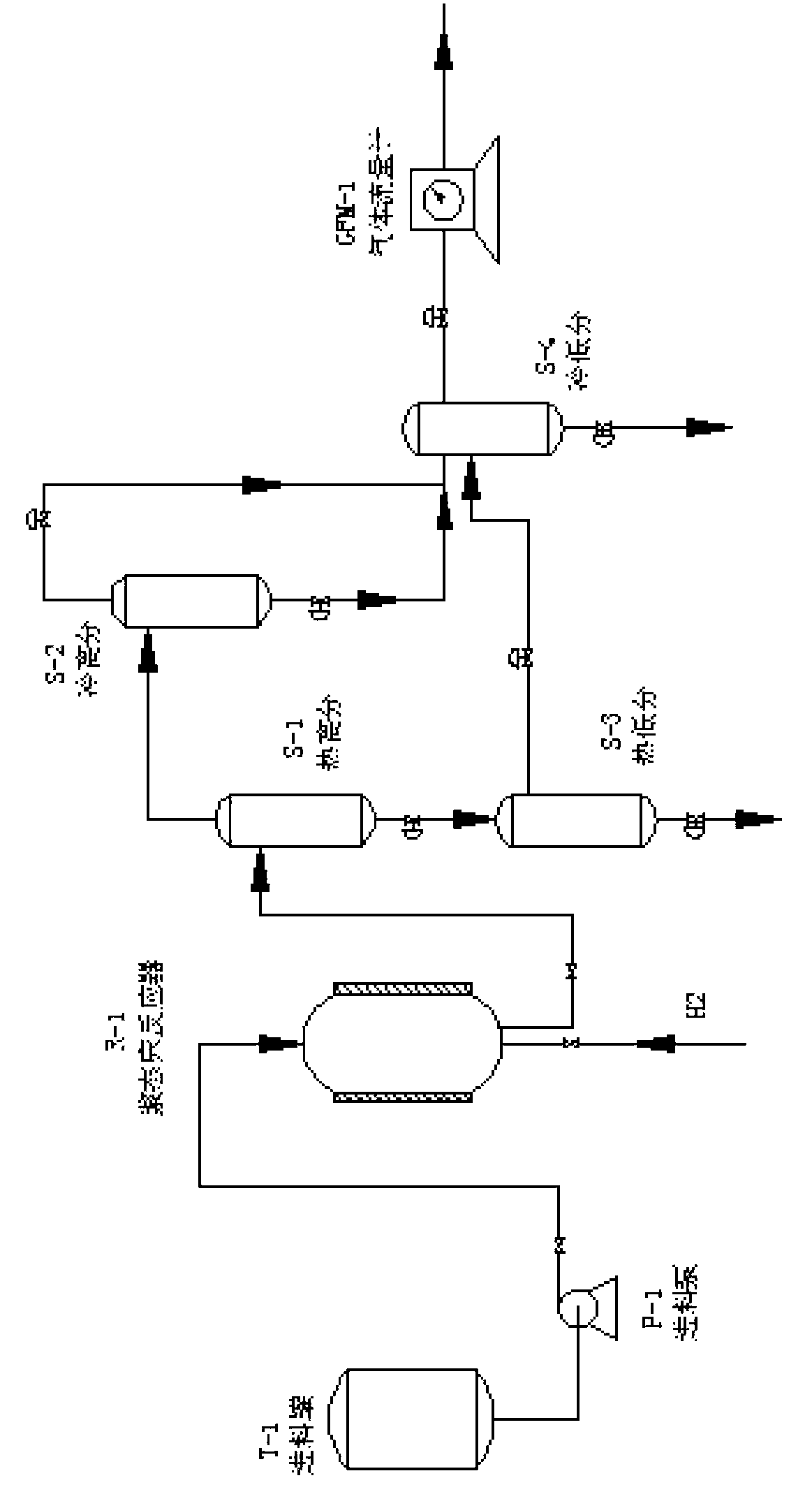
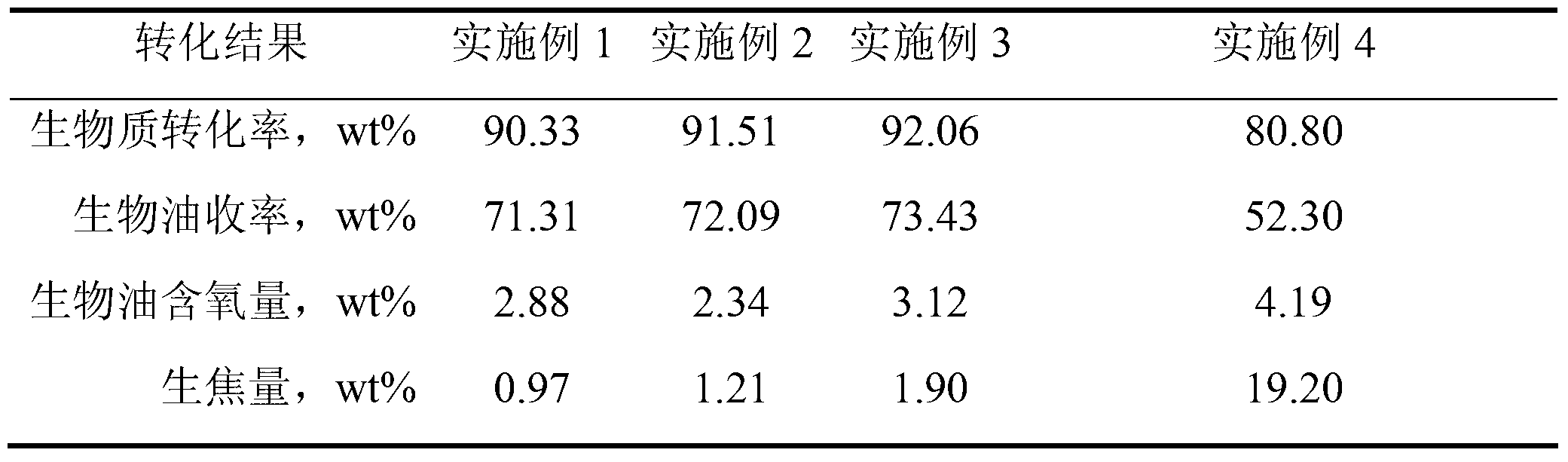
Heavy oil and biomass hydrogenation coliquefaction treatment process
ActiveCN103242871AGood deoxidation effectUniform reaction temperatureBiofuelsHydrocarbon oil crackingDistillationHydrogen pressure
The invention relates to a heavy oil and biomass hydrogenation coliquefaction treatment process in which one or two of heavy oil and biomasses are used as raw materials in vacuum gas oil with the distillation range of 360-540 DEG C and FCC (Fluid Catalytic Cracking) oil slurry. In the process, an oil-soluble transition metal organic compound with hydrogenation activity is used as a catalyst, dimethyl sulfide is used as a vulcanizing agent, a full-back-mixing type empty barrel reactor is used as a slurry column hydrogenation reactor, the reaction temperature is uniform, and the structure is simple. By using the process, the conversion rate of the biomasses can be up to over 90wt% at lower hydrogen pressure of 4-8MPa and the temperature of 370-430 DEGC, and the yield of oil phases is up to over 70wt%. A dispersive hydrogenation catalyst adopted in the process can be used for greatly reducing the quantity of green cokes and taking a better deoxidization effect on the biomasses.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Methods for dehydration of sugars
The invention includes a method of dehydration of a sugar using a dehydration catalyst and a co-catalyst within a reactor. A sugar is introduced and H2 is flowed through the reactor at a pressure of less than or equal to about 300 psig to convert at least some of the sugar into an anhydrosugar product. The invention includes a process for producing isosorbide. A starting material comprising sorbitol is flowed into a reactor. H2 is counter flowed through the reactor. The starting material is exposed to a catalyst in the presence of a co-catalyst which comprises at least one metal. The exposing is conducted at a hydrogen pressure of less than or equal to 300 psig within the reactor and the hydrogen removes at least some of any water present during the exposing and inhibits formation of colored byproducts.
Owner:BATTELLE MEMORIAL INST
Method of rapidly carrying out a hydrogenation of a hydrogen storage material
InactiveUS6680042B1Alkali/alkaline-earth/beryllium/magnesium hydridesReversible hydrogen uptakeHydrogen pressureRoom temperature
Disclosed is a method for rapidly carrying out a hydrogenation of a material capable of absorbing hydrogen. It was discovered that when a powder of a material capable of absorbing hydrogen is ground under a hydrogen pressure, not at room temperature but at a higher temperature (about 300° C. in the case of magnesium) and in the presence of a hydrogenation activator such as graphite and optionally a catalyst, it is possible to transform completely the powder of this material into a hydride. Such a transformation is achieved in a period of time less than 1 hour whereas the known methods call for periods of time as much as 10 times longer. This is an unexpected result which gives rise to a considerable reduction in the cost of manufacture of an hydride, particularly MgH2.
Owner:HYDRO QUEBEC CORP
Hydrocracking process for biological feedstocks and hydrocarbons produced therefrom
A process for hydrocracking biomass, and the hydrocarbons produced therefrom. A feed stream having free fatty acids, fatty acid esters, or combinations thereof is contacted with a first catalyst under hydrogen pressure and heat. The hydrocarbon product stream which is comprised predominantly of n-paraffins is separated into heavy and light fractions. The heavy fraction is contacted with a second catalyst under hydrogen pressure and heat to produce an effluent stream which is combined with the light n-paraffin fraction to form a unique middle distillate product useful as a diesel or jet fuel.
Owner:REG SYNTHETIC FUELS LLC
Method for producing ethylene glycol from polyhydroxy compound
ActiveUS20110046419A1High yieldHigh selectivityOxygen-containing compound preparationOrganic compound preparationIridiumHydrogen pressure
A method for producing ethylene glycol, including (a) adding a polyhydroxy compound and water to a sealed high-pressure reactor, (b) removing air and introducing hydrogen, and (c) allowing the polyhydroxy compound to react in the presence of a catalyst while stiffing. The catalyst includes a first active ingredient and a second active ingredient. The first active ingredient includes a transition metal of Group 8, 9, or 10 selected from iron, cobalt, nickel, ruthenium, rhodium, palladium, iridium, and platinum, and / or a mixture thereof. The second active ingredient includes a metallic state of molybdenum and / or tungsten, or a carbide, nitride, or phosphide thereof. The method is carried out at a hydrogen pressure of 1-12 MPa, at a temperature of 120-300° C. for not less than 5 min in a one-step catalytic reaction. The efficiency, selectivity, and the yield of ethylene glycol are high. The preparation process is simple and the materials used are renewable.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method of preparing ethylene glycol from cellulose
ActiveUS7960594B2High yieldHigh selectivityOxygen-containing compound preparationOrganic compound preparationCelluloseIridium
A method for preparing ethylene glycol from cellulose uses the cellulose as the feed for the reaction. The cellulose conversion is performed over catalysts which are composed of the metallic state, carbides, nitrides, or phosiphides of molybdenum or tungsten, and metallic cobalt, nickel, ruthenium, rhodium, palladium, iridium, and platinum of the group 8, 9, or 10 transition metals. The catalytic conversion of cellulose is conducted at 120 to 300° C. and hydrogen pressure 1 to 12 MPa under the hydrothermal conditions to achieve the high efficiency, high selectivity, and high yield of ethylene glycol. Compared to the existing method of preparing ethylene glycol from ethylene, the method, using the renewable raw material for the reaction, is friendly to the environment, and has high atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing aromatic hydrocarbon by carrying out catalytic hydrodeoxygenation on lignin
InactiveCN104744204AWide variety of sourcesRaw materials are renewableHydrocarbon from oxygen organic compoundsEthylene productionIridiumPtru catalyst
The invention relates to a method for preparing aromatic hydrocarbon by carrying out catalytic hydrodeoxygenation on lignin. A catalyst used in the method provided by the invention comprises two active components, namely an acid site being one or combination of more than one of transition metal elements niobium, tantalum, zirconium, molybdenum, tungsten and rhenium and a hydrogenation or hydrogen transfer active site being one or more than one of ruthenium, platinum, palladium, iridium, iron, cobalt, nickel and copper. According to the method provided by the invention, a phenol group, a guaiacol group, a syringa phenolic group compound, natural lignin and industrial lignin are taken as raw materials, water is taken as a solvent, high selectivity catalytic hydrodeoxygenation is carried out at the temperature of 180-350 DEG C and hydrogen pressure of 0.1-5MPa or with methyl alcohol, isopropyl alcohol and formic acid as hydrogen sources, so that C6-C9 aromatic hydrocarbon is obtained, the highest mass yield of aromatic hydrocarbon is 10%, and content of aromatic hydrocarbon in product oil can be up to more than 75%. The method provided by the invention has the advantages that reproducible natural biomass can be used as a raw material, and the raw material is cheap and available; the water is taken as the solvent, so that a reaction process is environment-friendly; and content of aromatic hydrocarbon in the product is high, and reaction conditions are mild.
Owner:EAST CHINA UNIV OF SCI & TECH
Methods for preparing ethylene glycol from polyhydroxy compounds
ActiveUS20120172633A1High yieldHigh selectivityOxygen-containing compound preparationOrganic compound preparationHydrogen pressureHeteropoly acid
This invention provides methods for producing ethylene glycol from polyhydroxy compounds such as cellulose, starch, hemicellulose, glucose, sucrose, fructose, fructan, xylose and soluble xylooligosaccharides. The methods uses polyhydroxy compounds as the reactant, a composite catalyst having active components comprising one or more transition metals of Groups 8, 9, or 10, including iron, cobalt, nickel, ruthenium, rhodium, palladium, iridium, and platinum, as well as tungsten oxide, tungsten sulfide, tungsten hydroxide, tungsten chloride, tungsten bronze oxide, tungsten acid, tungstate, metatungstate acid, metatungstate, paratungstate acid, paratungstate, peroxotungstic acid, pertungstate, heteropoly acid containing tungsten. Reacting at a temperature of 120-300° C. and a hydrogen pressure of 1-13 MPa under hydrothermal conditions to accomplish one-step catalytic conversion. It realizes efficient, highly selective, high yield preparation of ethylene glycol and propylene glycol from polyhydroxy compounds. The advantage of processes disclosed in this invention include renewable raw material and high atom economy. At the same time, compared with other technologies that converts biomass raw materials into polyols, methods disclosed herein enjoy advantages including simple reaction process, high yield of targeted products, as well as easy preparation and low cost for the catalysts.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Tungsten carbide catalysts, their preparation and application in synthesis of ethylene glycol from cellulose
ActiveUS20100255983A1Improve efficiencyHigh selectivityOxygen-containing compound preparationOrganic compound preparationHydrogen pressureCobalt
Tungsten carbide catalysts are used in preparation of ethylene glycol by hydrogenating degradation of cellulose. The catalyst includes tungsten carbide as main catalytic active component, added with small amount of one or more transition metals such as nickel, cobalt, iron, ruthenium, rhodium, palladium, osmium, iridium, platinum, and copper as the second metal, supported on one or more porous complex supports such as active carbon, alumina, silica, titanium dioxide, silicon carbide, zirconium oxide, for conversion of cellulose to ethylene glycol. The catalyst realizes high efficiency, high selectivity, and high yield in the conversion of cellulose to ethylene glycol at the temperature of 120-300° C., hydrogen pressure of 1-10 MPa, and hydrothermal conditions. Compared to the existing industrial synthetic method of ethylene glycol using ethylene as feedstock, the invention has the advantages of using renewable raw material resources, environment friendly process, and excellent atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing single benzene ring phenolic compound from alkali lignin
InactiveCN102372607AEasy to separateMild reaction conditionsOrganic compound preparationEther preparationBenzeneHydrogen pressure
A method for preparing single benzene ring phenolic compounds by catalytic conversion of alkali lignin. Hydrogen is added into water phase to degrade alkali lignin into single benzene ring phenolic compound, under a reaction temperature of 90-180 DEG C, a hydrogen pressure of 1.0-10.0MPa and under effect of a metal nanoparticle catalyst; and alkali compound is added into the reaction. The metal nanoparticle catalyst is one or more selected from Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag or Au in any ratio. The method has mild reaction conditions, low energy consumption, low costs, high operating safety and easily separated products.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method of preparing ethylene glycol cellulose
ActiveUS20100256424A1High yieldHigh selectivityOxygen-containing compound preparationOrganic compound preparationCelluloseIridium
A method for preparing ethylene glycol from cellulose uses the cellulose as the feed for the reaction. The cellulose conversion is performed over catalysts which are composed of the metallic state, carbides, nitrides, or phosiphides of molybdenum or tungsten, and metallic cobalt, nickel, ruthenium, rhodium, palladium, iridium, and platinum of the group 8, 9, or 10 transition metals. The catalytic conversion of cellulose is conducted at 120 to 300° C. and hydrogen pressure 1 to 12 MPa under the hydrothermal conditions to achieve the high efficiency, high selectivity, and high yield of ethylene glycol. Compared to the existing method of preparing ethylene glycol from ethylene, the method, using the renewable raw material for the reaction, is friendly to the environment, and has high atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Polyethylene processes and compositions thereof
Embodiments of the present disclosure relate to a method of preparing polyethylene compositions comprising polymerizing ethylene in a first gas-phase reactor and polymerizing ethylene in a second gas-phase reactor in the presence of hydrogen; wherein at least one of the first or second gas-phase reactors comprises a first and second polymerization zone; wherein a hydrogen pressure of the first and second polymerization zones are different such that at least a portion of the second ethylene cycles through the first and second polymerization zones and a gas mixture of each polymerization zone is partially or totally prevented from entering the other zone.
Owner:BASELL POLYOLEFINE GMBH +1
Hydrogen storage by reversible hydrogenation of pi-conjugated substrates
InactiveUS7351395B1Easily reversibleNot volatileMultiple metal hydridesHydrogen productionPartial hydrogenationDehydrogenation
Processes are provided for the storage and release of hydrogen by means of a substantially reversible catalytic hydrogenation of extended pi-conjugated substrates which include large polycyclic aromatic hydrocarbons, polycyclic aromatic hydrocarbons with nitrogen heteroatoms, polycyclic aromatic hydrocarbons with oxygen heteroatoms, polycyclic aromatic hydrocarbons with alkyl, alkoxy, nitrile, ketone, ether or polyether substituents, pi-conjugated molecules comprising 5 membered rings, pi-conjugated molecules comprising six and five membered rings with nitrogen or oxygen hetero atoms, and extended pi-conjugated organic polymers. The hydrogen, contained in the at least partially hydrogenated form of the extended pi-conjugated system, can be facilely released for use by a catalytic dehydrogenation of the latter in the presence of a dehydrogenation catalyst which can be effected by lowering the hydrogen gas pressure, generally to pressures greater than 0.1 bar or raising the temperature to less than 250° C. or less, or by a combination of these two process parameters.
Owner:AIR PROD & CHEM INC
Hydrocracking process for biological feedstocks and hydrocarbons produced therefrom
A process for hydrocracking biomass, and the hydrocarbons produced therefrom. A feed stream having free fatty acids, fatty acid esters, or combinations thereof is contacted with a first catalyst under hydrogen pressure and heat. The hydrocarbon product stream which is comprised predominantly of n-paraffins is separated into heavy and light fractions. The heavy fraction is contacted with a second catalyst under hydrogen pressure and heat to produce an effluent stream which is combined with the light n-paraffin fraction to form a unique middle distillate product useful as a diesel or jet fuel.
Owner:REG SYNTHETIC FUELS LLC
Method for synthesizing methyl glycollate and ethylene glycol by dimethyl oxalate hydrogenation
ActiveCN102336666AEasy to makeImprove performanceMolecular sieve catalystsOrganic compound preparationHydrogen pressureHydrogenation reaction
The invention discloses a method for synthesizing methyl glycollate and ethylene glycol by dimethyl oxalate hydrogenation. The method comprises the following steps of: performing hydrogenation reaction on dimethyl oxalate serving as a raw material under the action of a catalyst at the temperature of between 120 and 300 DEG C under the hydrogen pressure of 0.2 to 10.0MPa, performing fixed bed continuous reaction, and thus obtaining target products, namely the methyl glycollate and the ethylene glycol. The method is mild in reaction conditions and environmentally-friendly, the conversion rate of the dimethyl oxalate reaches over 99 percent, the total selectivity of the methyl glycollate and the ethylene glycol reaches over 98 percent, the selectivity of the methyl glycollate serving as a main product reaches 95 percent, and a new effective path is provided for preparing the methyl glycollate and the ethylene glycol by dimethyl oxalate hydrogenation.
Owner:SHANGHAI HUAYI GRP CO
Hydrogen-Generating Fuel Cell Cartridges
ActiveUS20110212374A1Extended shelf lifeEfficient productionReactant parameters controlHydrogen/synthetic gas productionFuel cellsNuclear engineering
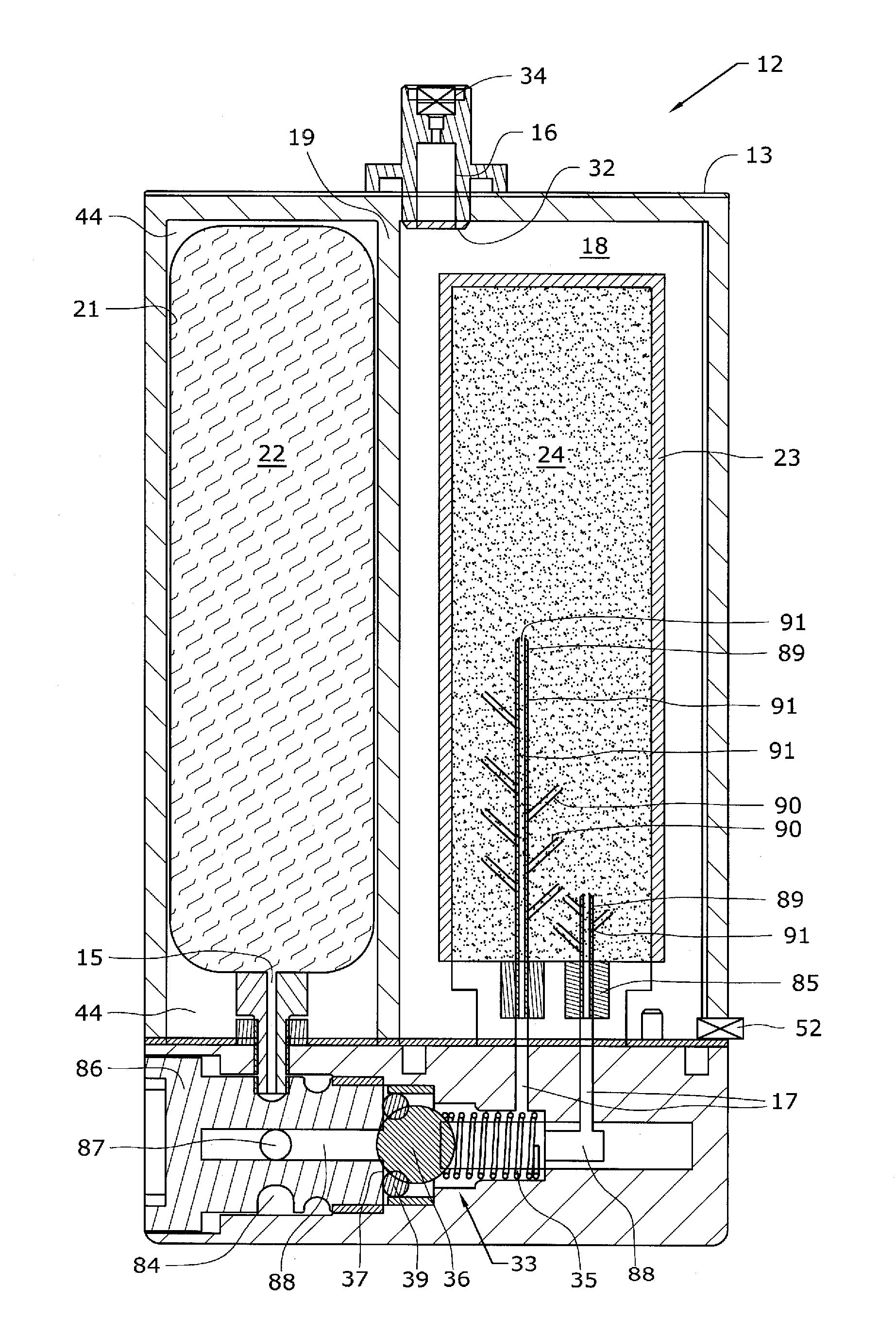
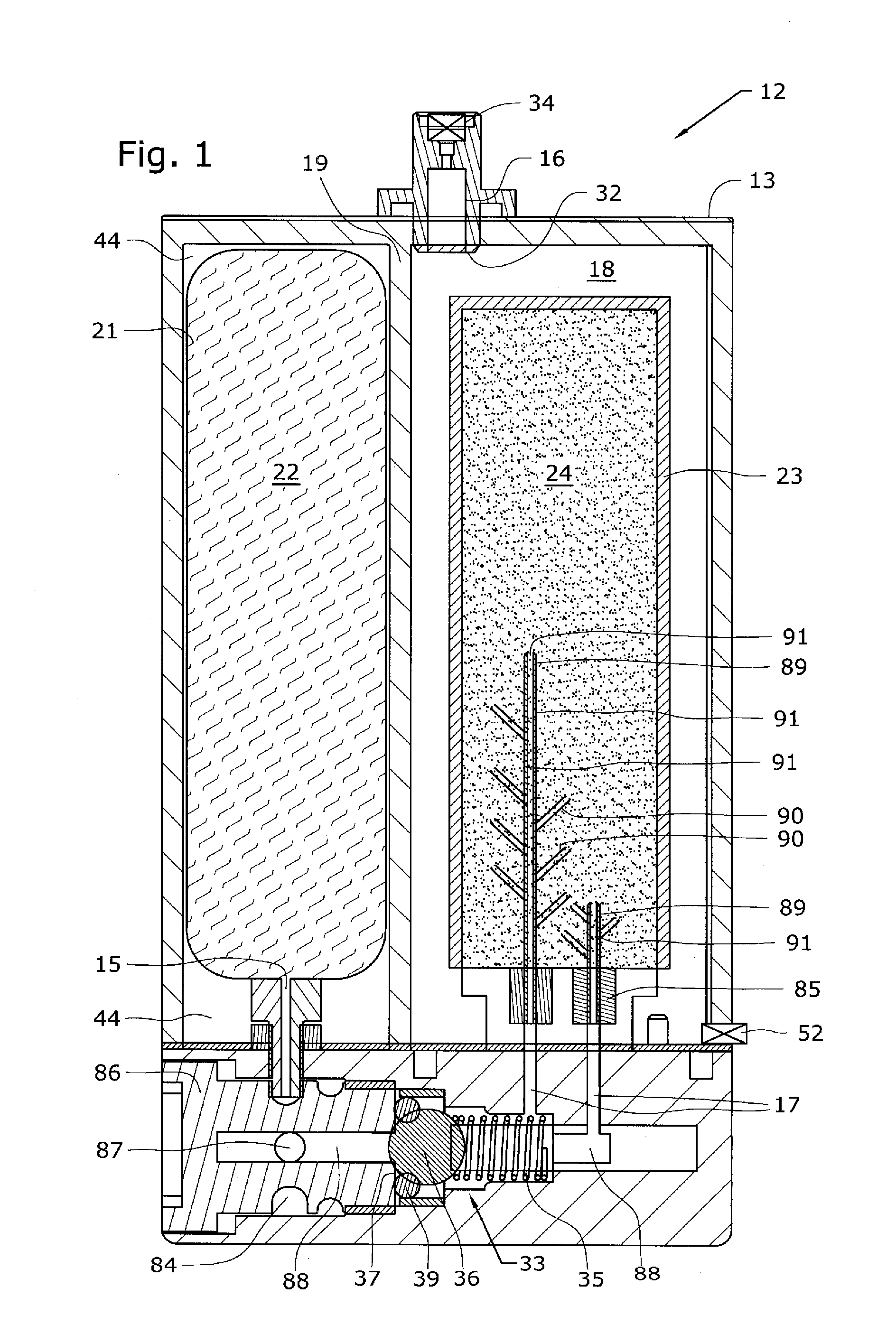
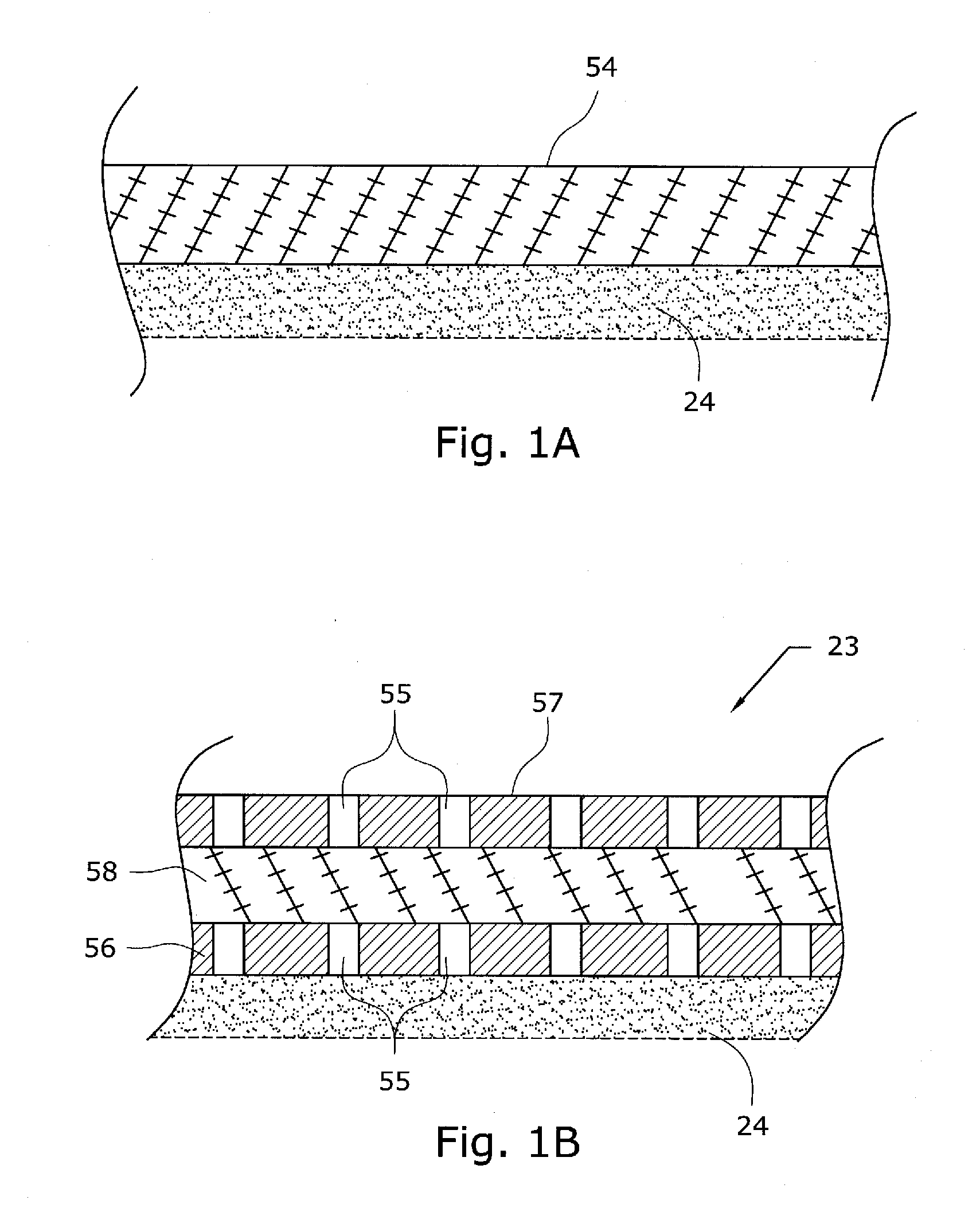
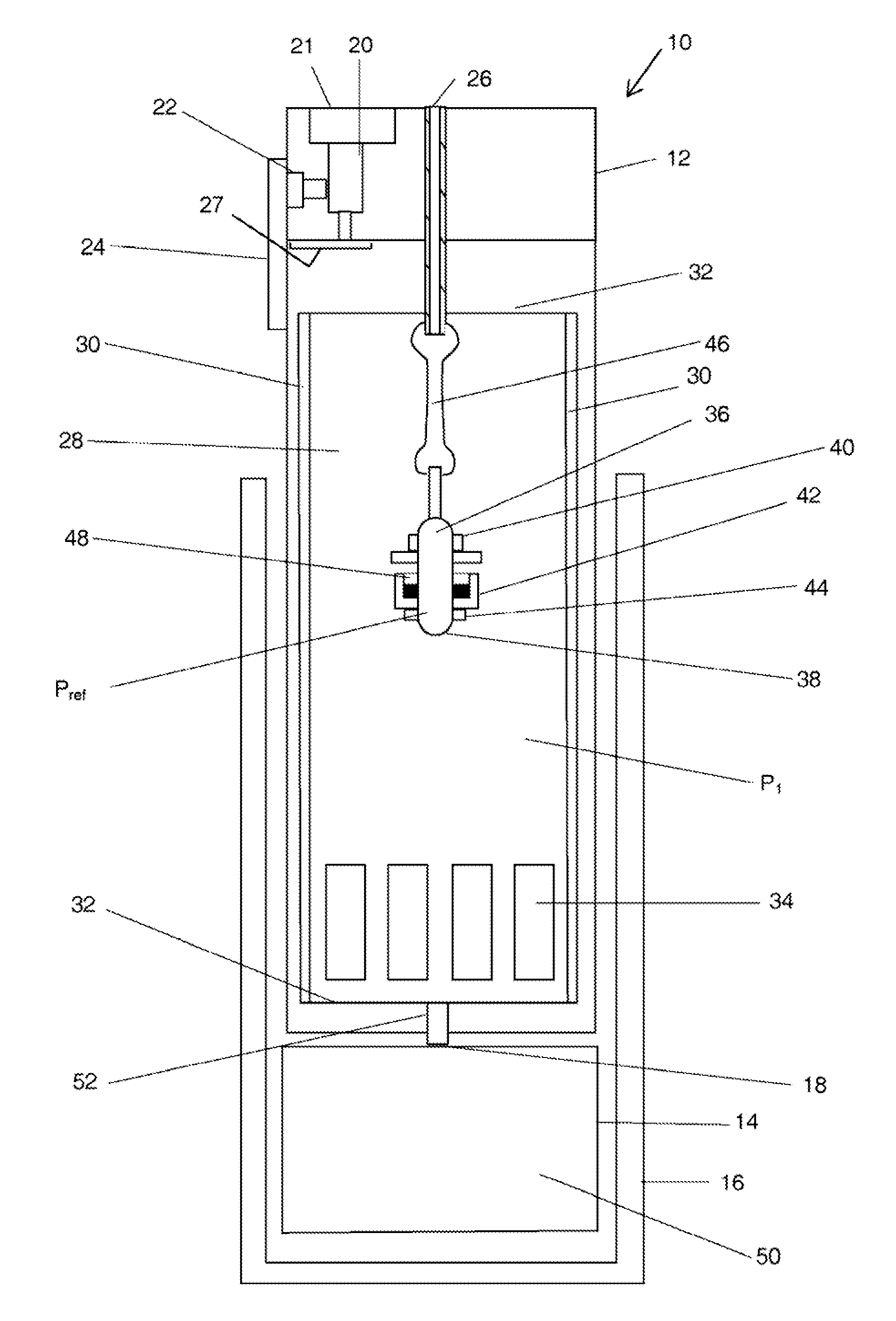
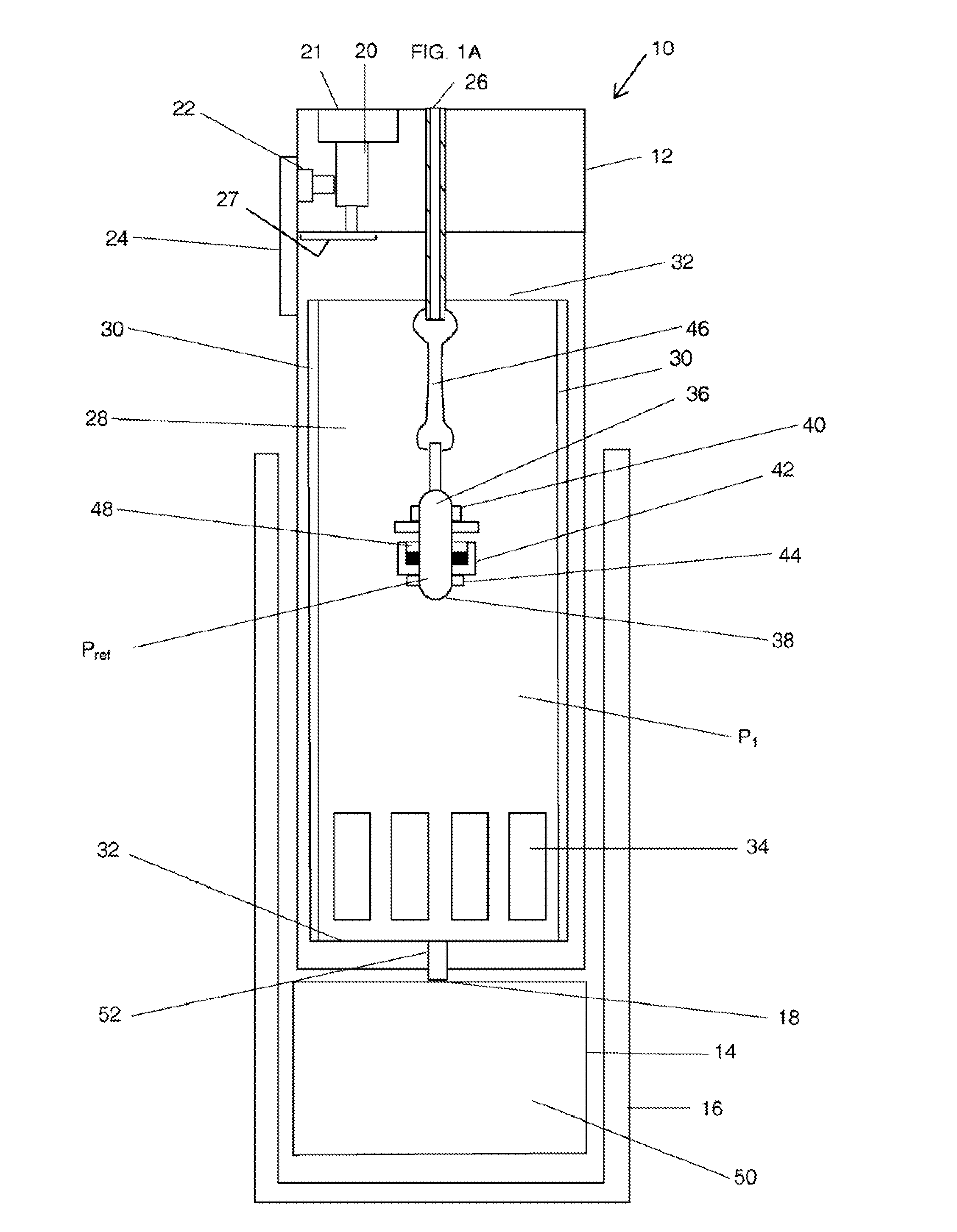
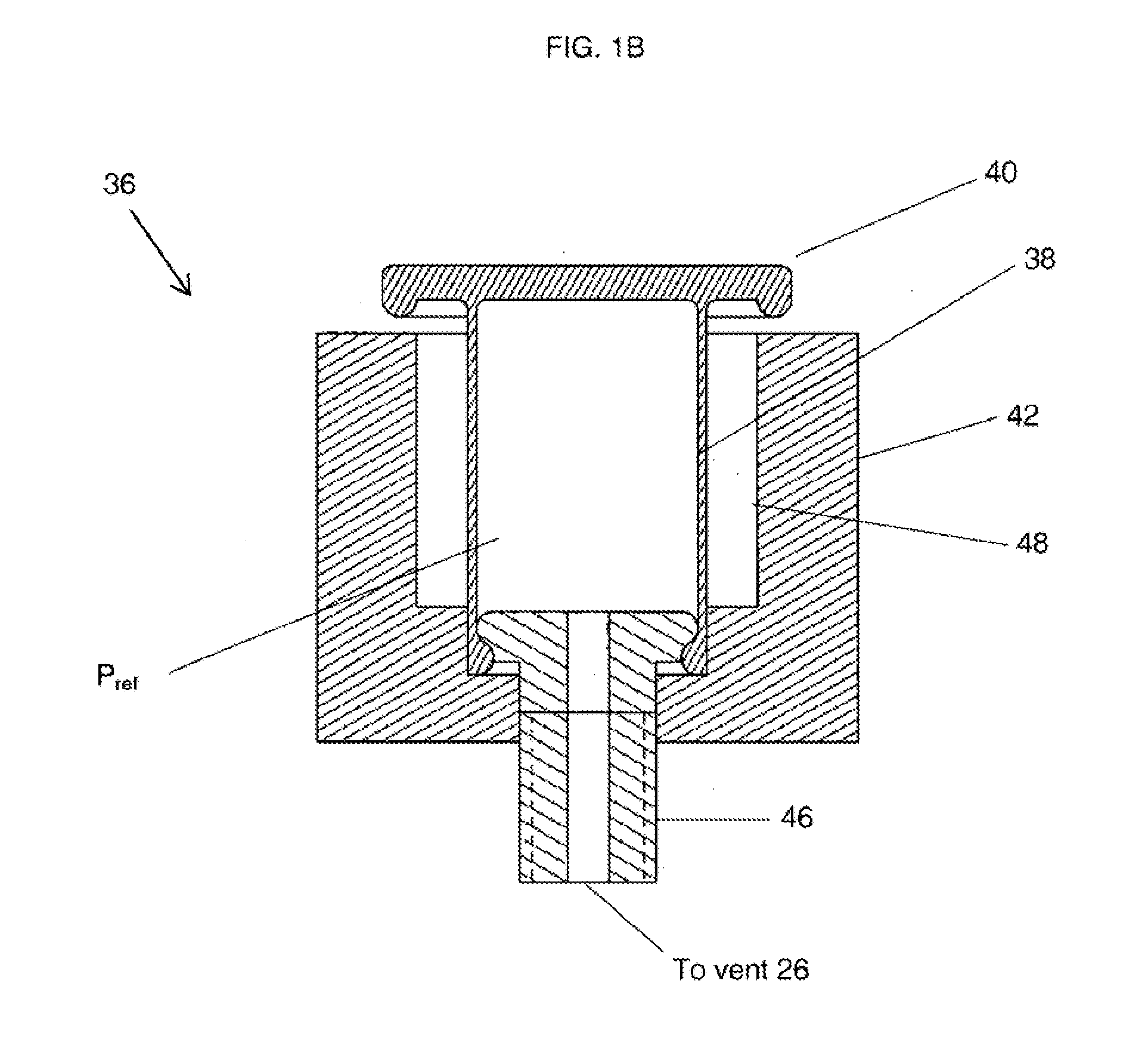
The present application is directed to a gas-generating apparatus (10). Hydrogen is generated within the gas-generating apparatus and is transported to a fuel cell. The generation of hydrogen is regulated automatically by the selective exposure of a catalyst (48) to the fuel mixture depending on the pressure inside the reaction chamber (28) of the gas-generating apparatus. Catalyst sealing mechanisms (40, 42) are provided at least partially within the reaction chamber to regulate the hydrogen pressure and to minimize the fluctuations in pressure of the hydrogen received by the fuel cell.
Owner:INTELLIGENT ENERGY LTD
Hydrogen storage tank and method of using
A hydrogen storage tank for containing solid-state hydrogen storage media, and method for determining the hydrogen fill level in the tank. The tank has at least one compartment for storing the storage media, passages for transporting hydrogen gas to and from the compartment, and a heat distribution system for establishing a substantially uniform temperature in the storage media. A look-up table is generated for the tank that relates hydrogen pressure changes in the tank versus hydrogen fill levels in the tank as a function of temperature. The tank is filled to full capacity by adsorbing hydrogen on the storage media. Thereafter, hydrogen is released from the storage media for a time period during which there is a substantially constant demand on the tank, the change in pressure of the released hydrogen gas is measured, and the look-up table is applied to determine the hydrogen fill level in the tank.
Owner:INDIANA UNIV RES & TECH CORP
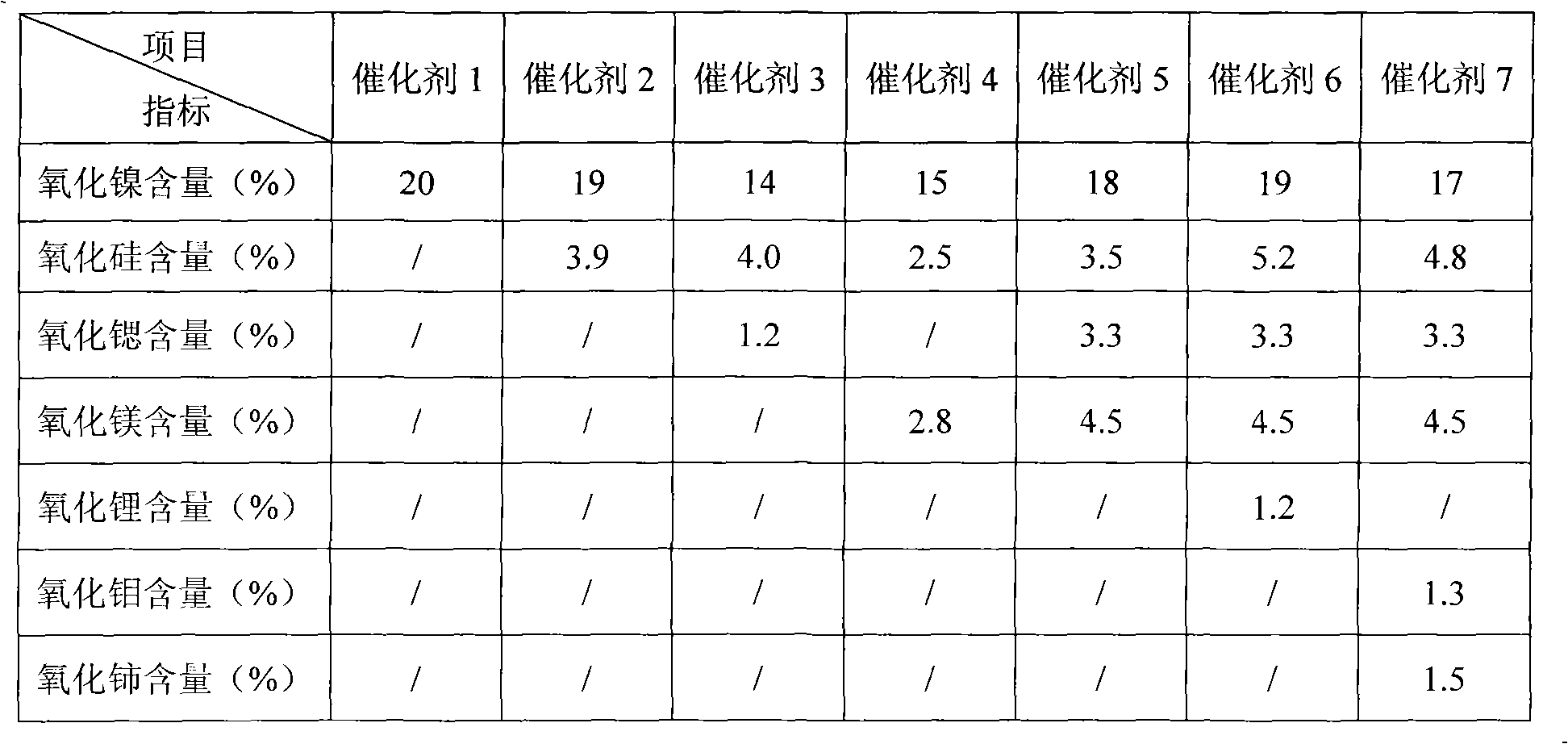
Pre-treatment method of nickel hydrogenation catalyst
ActiveCN101822985AShorten the timeControllable temperature riseHydrocarbon by hydrogenationCatalyst activation/preparationPretreatment methodHydrogen pressure
The invention relates to a pre-treatment method of a nickel hydrogenation catalyst, which mainly comprises two steps that: (1) when reducing gas exists, the catalyst is reduced on a fixed bed reactor or a converter through a segmentation method, the volume ratio of the reducing gas to the catalyst is 500 to 1000Nm3 / m3 / h, and the process conditions are as follows: the pressure is 0.1 to 0.8MPa, the temperature goes up to 230 to 250 DEG C at the temperature rise speed of 15 to 50DEG C / h and is maintained for 3 to 5h, and then goes up to 390 to 400DEG C at the temperature rise speed of 10 to 20DEG C / h and is maintained for 4 to 5h, and the reduction is over; and (2) catalyst passivation: the hydrogen pressure is 2.0 to 3.0MPa, the temperature of a catalyst bed layer is 20 to 30DEG C, the passivator airspeed is 5 to 20h-1, and the passivator and the catalyst contact for 3 to 5h; and the passivator is an oil product which contains olefin, the olefin mass content is 0.1 to 40 percent, and the sulfur content is 0 to 500mu g / g.
Owner:PETROCHINA CO LTD
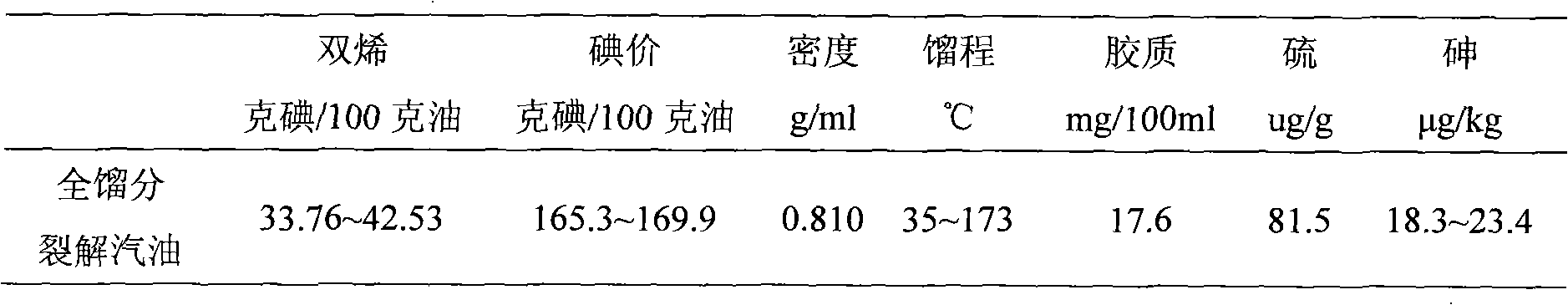
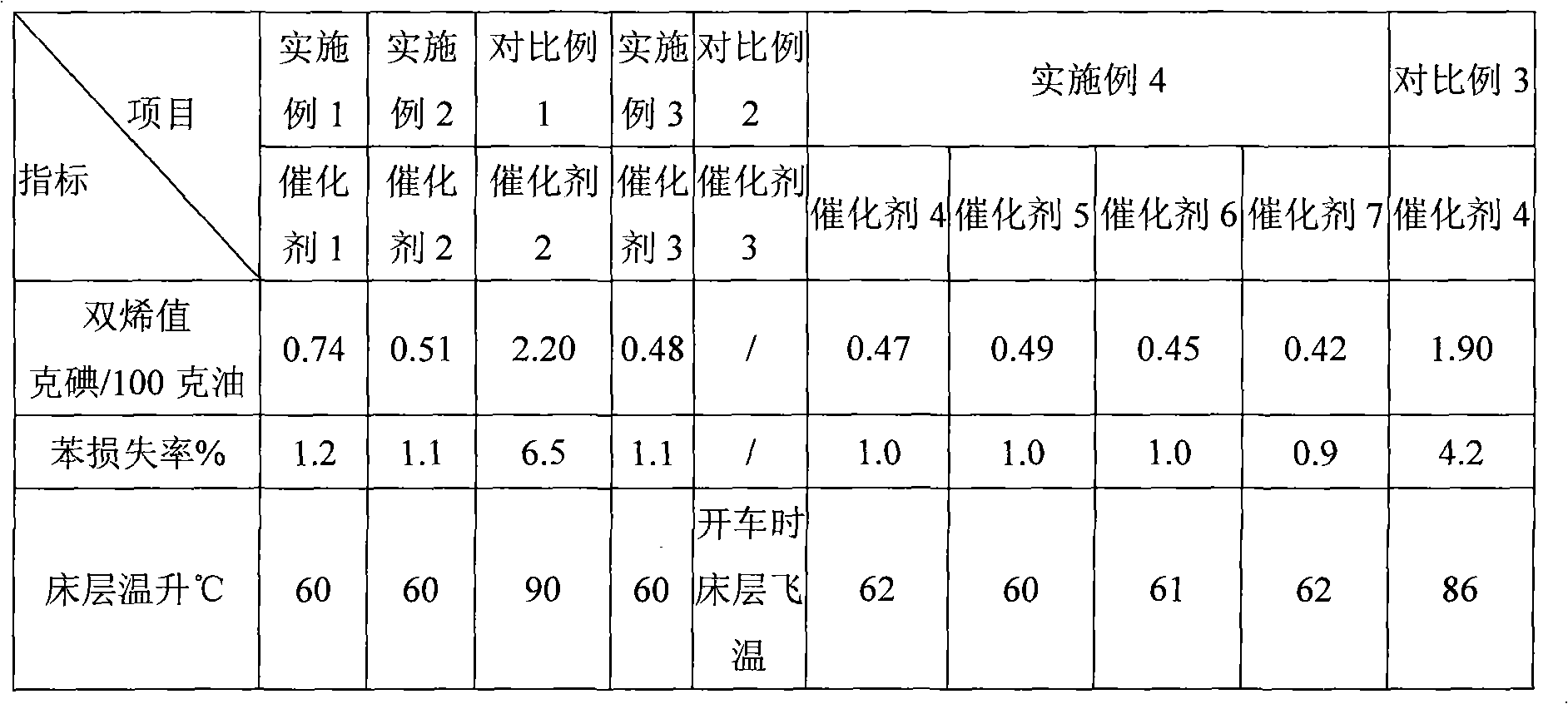
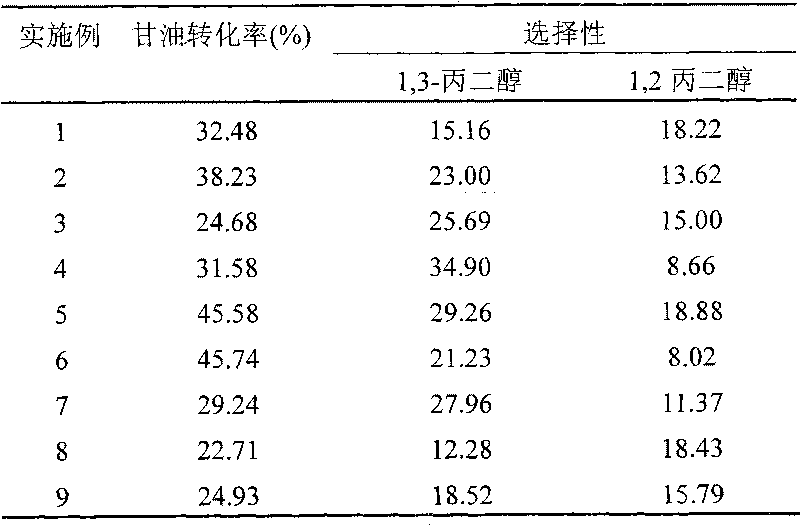
Method for preparing 1,3-propylene glycol by directly hydrogenizing glycerol
ActiveCN101723801AHigh selectivityImprove conversion ratePreparation by OH group eliminationMetal/metal-oxides/metal-hydroxide catalystsHydrogen pressureReaction temperature
The invention relates to a method for preparing 1,3-propylene glycol by directly hydrogenizing glycerol. A reaction system comprises glycerol, hydrogen, catalyzer and two or more of dissolvants. In the reaction system, the weight ratio of the dissolvants to the glycerol is 0.2-9.8:1, and the weight ratio of the catalyzer to the glycerol is 0.2-2:1; the reaction conditions are shown as follows: the reaction temperature is 80-300 DEC G, the hydrogen pressure is 0.1-18.0MPa, and the contact time of the glycerol and the catalyzer in the reaction process is 0.3-50h; the catalyzer contains a carrier and active components carried on the carrier, wherein the carrier is ZrO2, SiO2-Al2O3 or Al2O3, and the active components are one or more of Ru, Pt, Pd and Rh. The invention can obviously increase the selectivity of generating 1,3-propylene glycol by hydrogenizing glycerol or increase the percent conversion of glycerol and reduce the generation of the byproduct of 1,2-propylene.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com