Catalyst for producing 1,2-cyclohexane dicarboxylic acid diesters
A technology of cyclohexanedicarboxylic acid dibasic ester and phthalic acid dibasic ester, which is applied in the field of hydrogenation of phthalic acid dibasic ester and can solve problems such as harm to human health and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
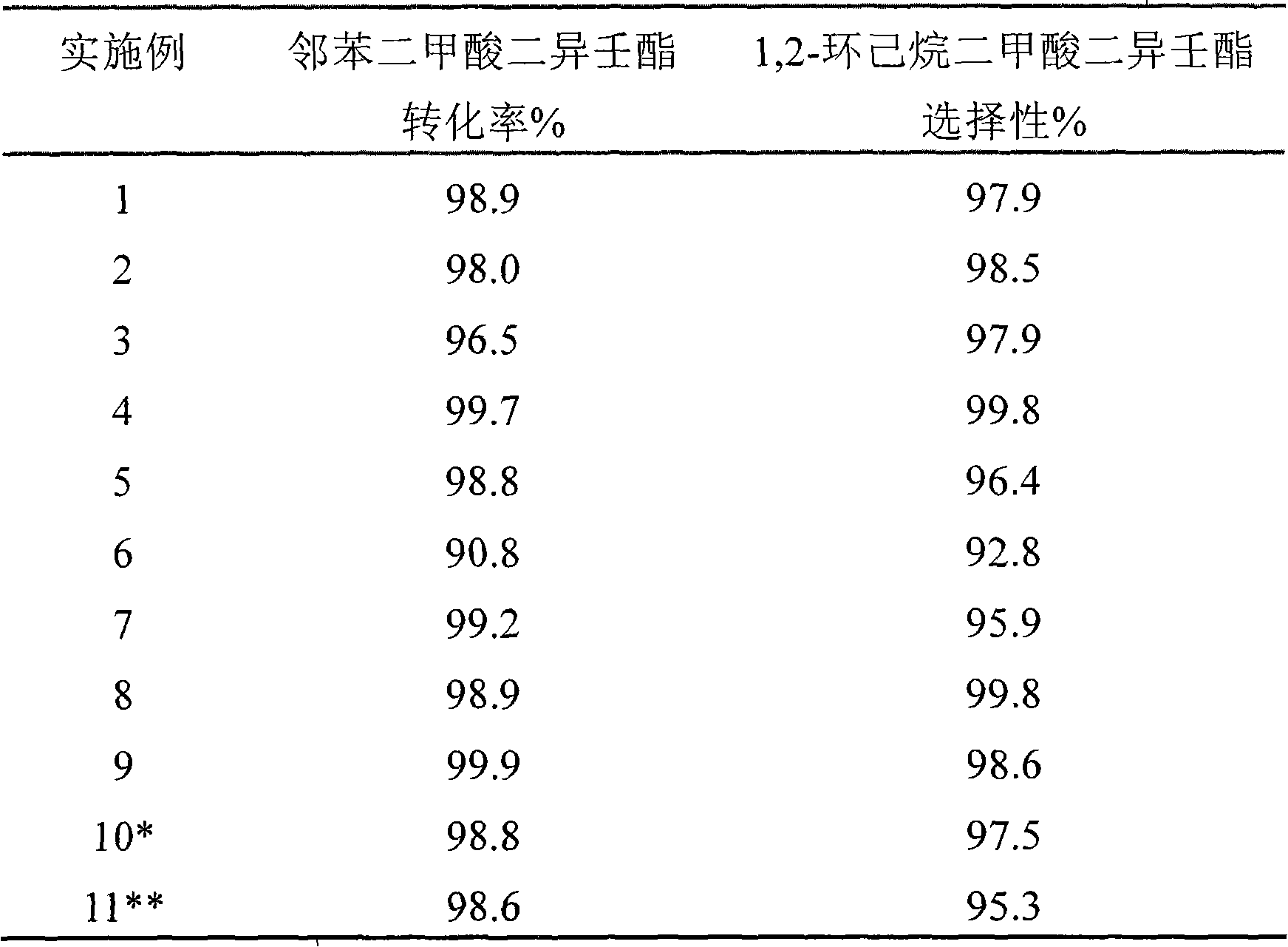
Examples
Embodiment 1
[0021] The catalyst of embodiment 1 is 1.0%Pd-0.03%Ru / Al 2 o 3 . Weigh 100 grams of macroporous Al 2 o 3 (BET: 56m 2 / g, 37.9nm), configuration 101ml contains 1.683 grams of PdCl 2 and 0.0624 g RhCl 3 Hydrochloric acid aqueous solution, impregnated with this aqueous solution the above-mentioned macroporous Al 2 o 3 Carrier, air-dried, oven-dried at 120°C for 4 hours, calcined at 300°C for 5 hours, hydrogen at 300°C (atmospheric pressure, 2400h -1 ) reduction activation for 5 hours.
Embodiment 2
[0023] Catalyst 1.0%Pd-0.03%Ru-1%Cu / Al of embodiment 2 2 o 3 . Weigh 100 grams of macroporous Al 2 o 3 (BET: 56m 2 / g, 37.9nm), weighed 3.880 grams of Cu (NO 3 ) 2 .3H 2 O was dissolved in 100ml of distilled water, and the above-mentioned macroporous Al was impregnated with this aqueous solution 2 o 3 The carrier was dried at 120°C for 4 hours and calcined at 500°C for 5 hours. 101ml of configuration contains 1.700 grams of PdCl 2 and 0.0623 g RhCl 3 Aqueous hydrochloric acid, impregnating the above Cu / Al with this aqueous solution 2 o 3 Samples were air-dried, oven-dried at 120°C for 4 hours, baked at 300°C for 5 hours, and in hydrogen at 300°C (atmospheric pressure, 2400h -1 ) reduction activation for 5 hours.
Embodiment 3
[0025] Catalyst 1.0%Pd-0.03%Ru-1%Fe / Al of embodiment 3 2 o 3 . Weigh 100 grams of macroporous Al 2 o 3 (BET: 56m 2 / g, 37.9nm), weighed 7.382 grams of Fe (NO 3 ) 3 .9H 2 O was dissolved in 100ml of distilled water, and the above-mentioned macroporous Al was impregnated with this aqueous solution 2 o 3 The carrier was dried at 120°C for 4 hours and calcined at 500°C for 5 hours. 101ml of configuration contains 1.700 grams of PdCl 2 and 0.0623 g RhCl 3 Hydrochloric acid aqueous solution, impregnate the above Fe / Al with this aqueous solution 2 o 3 Samples were air-dried, oven-dried at 120°C for 4 hours, baked at 300°C for 5 hours, and in hydrogen at 300°C (atmospheric pressure, 2400h -1 ) reduction activation for 5 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com