System and process for preparing aromatic hydrocarbon by converting methanol or dimethyl ether
A technology for dimethyl ether and aromatic hydrocarbons, which is applied in the field of aromatic hydrocarbon production and can solve the problems of not considering recycling methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
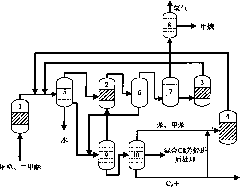
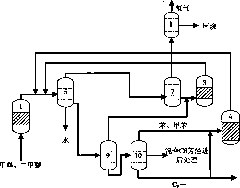
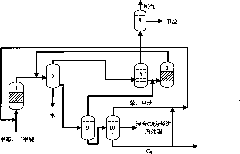
[0119] Methanol enters the aromatization reactor 1 (the aromatization reactor adopts a fixed bed) to participate in the reaction under the action of Zn-ZSM-5 catalyst, the reaction temperature is 470°C, the reaction pressure is 0.05MPa, and the weight space velocity is 0.6hr -1 . The reacted material enters the gas-liquid-liquid separator 5 and is divided into gas phase products, oil phase products and water, and the gas phase products enter the light olefin aromatization reactor 2, the reactor is a fixed bed reactor, and the catalyst is Zn-ZSM -5 molecular sieve catalyst, the reaction temperature is 450°C, the reaction pressure is 0.1MPa, and the weight space velocity is 1hr -1 . The reacted material enters the gas-liquid separator 6 after being condensed and is divided into a gas phase product and an oil phase product, and the gas phase product enters the gas phase separator 7, and adopts the method of absorption and desorption to separate hydrogen, methane and other C 2 +...
Embodiment 2
[0121] Methanol enters the aromatization reactor 1 (the aromatization reactor adopts a fixed bed) to participate in the reaction under the action of Ag-ZSM-5 catalyst, the reaction temperature is 450°C, the operating pressure is 0.14MPa, and the weight space velocity is 0.7hr -1 . The reacted material enters the gas-liquid-liquid separator 5 and is divided into gas phase product, oil phase product and water, and the gas phase product enters directly into the gas phase separator 7. The mixture of benzene and toluene generated by the reaction is used as the absorbent, and the absorption temperature is 40 ℃, the absorption pressure is 2MPa. Hydrogen and methane are separated by membrane separation. Low-carbon hydrocarbons without hydrogen enter low-carbon hydrocarbon aromatization reactor 3 for aromatization reaction. The reactor is a fixed-bed reactor, the catalyst is Mo-ZSM-5 molecular sieve catalyst, and the reaction temperature is 600°C. Pressure is 1MPa, weight space veloc...
Embodiment 3
[0123] Methanol enters the aromatization reactor 1 (the aromatization reactor adopts a fluidized bed) and participates in the reaction under the action of Ga-P-ZSM-5 catalyst. The reaction temperature is 550°C, the reaction pressure is 0.5MPa, and the weight space velocity is 5 hours -1 . The reacted material enters the gas-liquid-liquid separator 5 and is divided into gas phase product, oil phase product and water. The gas-phase product that comes out from gas-liquid-liquid separator 5 directly enters gas-phase separator 7 to combine hydrogen and methane with C 2 +Low-carbon hydrocarbons are separated, and the gas phase separator 7 adopts a rectification method for separation. Hydrogen and methane are separated by pressure swing adsorption. C without hydrogen 2 + The low-carbon hydrocarbon stream enters the low-carbon hydrocarbon reactor 3 for aromatization. Low-carbon hydrocarbon aromatization reactor 3 is a fixed-bed reactor, the catalyst is Zn-P-ZSM-35 molecular sieve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com