Method for preparing single benzene ring phenolic compound from alkali lignin
A technology for monophenol and alkali lignin, which is applied in the field of catalytic conversion of lignin to prepare monophenol compounds, can solve the problems of inability to realize continuous flow reaction, increase the difficulty of catalyst recycling and product separation, etc. Low, mild reaction conditions, low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
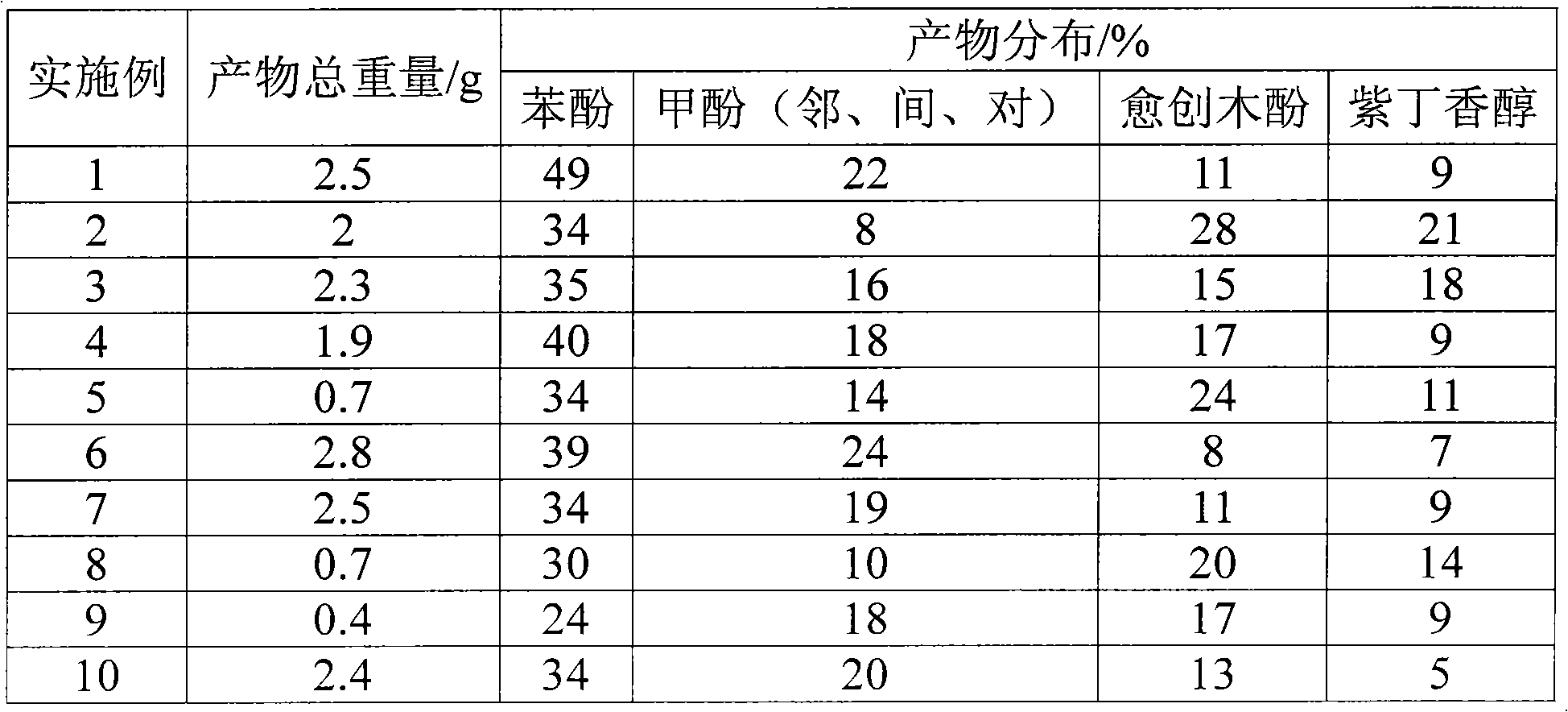
[0018] Preparation of Au nanoparticle catalysts. Add 1 mL of 1% by weight HAuCl hydrate to 100 mL of water with constant stirring 4 ·3H 2 O. After 10 minutes, 1 ml of 1% by weight sodium citrate hydrate was added. After 10 minutes of addition, an additional 1 mL of 0.075% by weight NaBH was added 4 , the solution was stirred continuously for 5 minutes, and stored for future use.
[0019] Transfer the prepared catalyst into a 300 ml reaction kettle, add 5.0 grams of alkali lignin (kraft lignin) and 3.0 grams of potassium carbonate, feed hydrogen replacement three times, heat to 120 ° C, open the hydrogen inlet valve, and the pressure stabilization of the inlet The valve is adjusted to 6.0MPa. After reacting for 10 hours, stop the reaction, cool, adjust the pH value to 7, extract three times with ethyl acetate, 50 mL each time, after combining, use gas chromatography to analyze the product distribution, remove ethyl acetate with rotary evaporation, and weigh it as the total...
Embodiment 2
[0021] Preparation of Ni nanoparticle catalysts. (1) The preparation of hydrazine hydrate / NaOH solution is as follows: 1.0M NaOH solution is added dropwise to 100 mL of 85% hydrazine hydrate solution until the pH value is 13. (2) Mix 150mL of n-hexane, 30g of surfactant CTAB, and 40mL of n-hexanol, and add the hydrazine hydrate / NaOH solution dropwise under stirring until clear, forming a hydrazine hydrate microemulsion. (3) Preparation of 0.5M NiCl 2 Solution 100mL. (4) Mix 150mL n-hexane, 30g surfactant CTAB, and 30mL n-hexanol, and add 0.5M NiCl dropwise under stirring 2 solution until clear as NiCl 2 Microemulsion. (5) Put the NiCl2 microemulsion bottle into an oil bath with a temperature of 70° C., quickly add the hydrazine hydrate microemulsion, continue stirring for 1 hour, cool down, and set aside. Measure 100mL of catalyst solution, adopt the reaction method of Example 1. The reaction results are shown in Table 1.
Embodiment 3
[0023] Preparation of Ru nanoparticle catalysts. Weigh 1.8g of PVP K-30, dissolve it in 100ml of water, and stir for 15 minutes. Weigh RuCl 3 ·xH 2 O 120mg was dissolved in 4mL water, and mixed with PVP solution, the reaction temperature was 90°C, the reaction time was 12 hours, the hydrogen flow rate was 30mL / min, cooled, and set aside. Adopt the reaction method of embodiment 1. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com