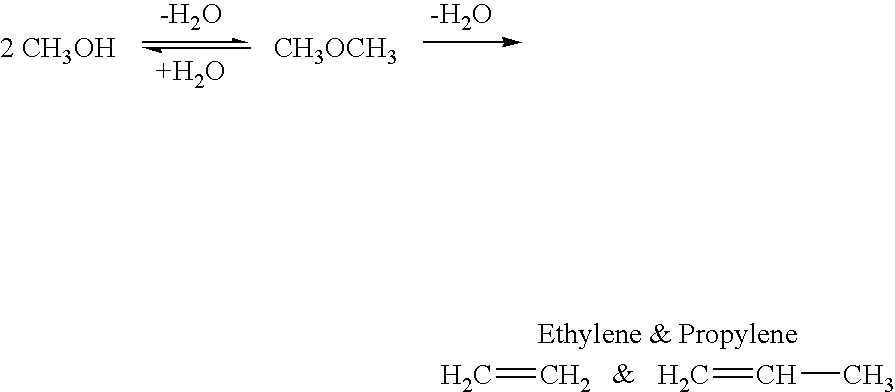Patents
Literature
192 results about "Carbon dioxide production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
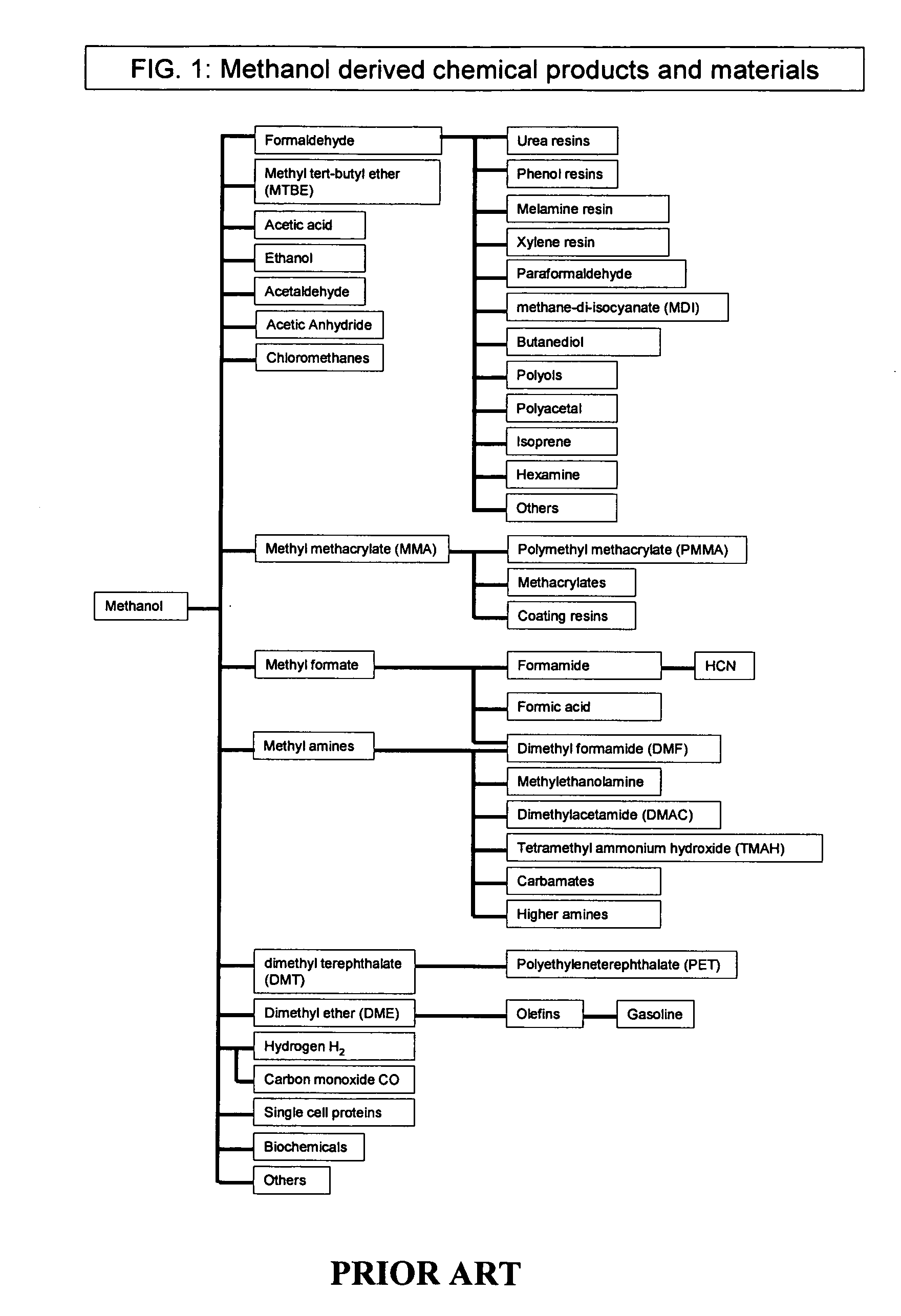
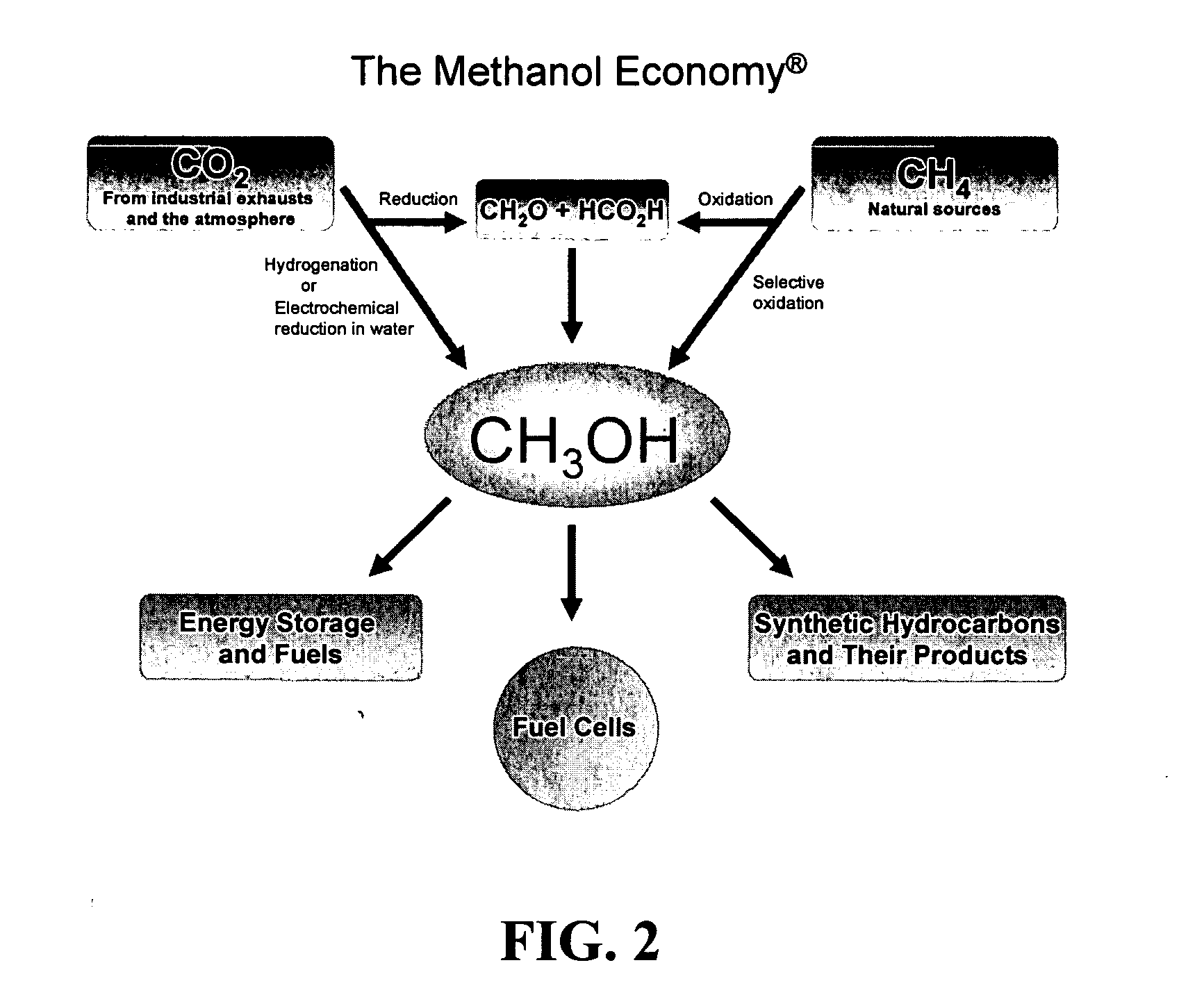
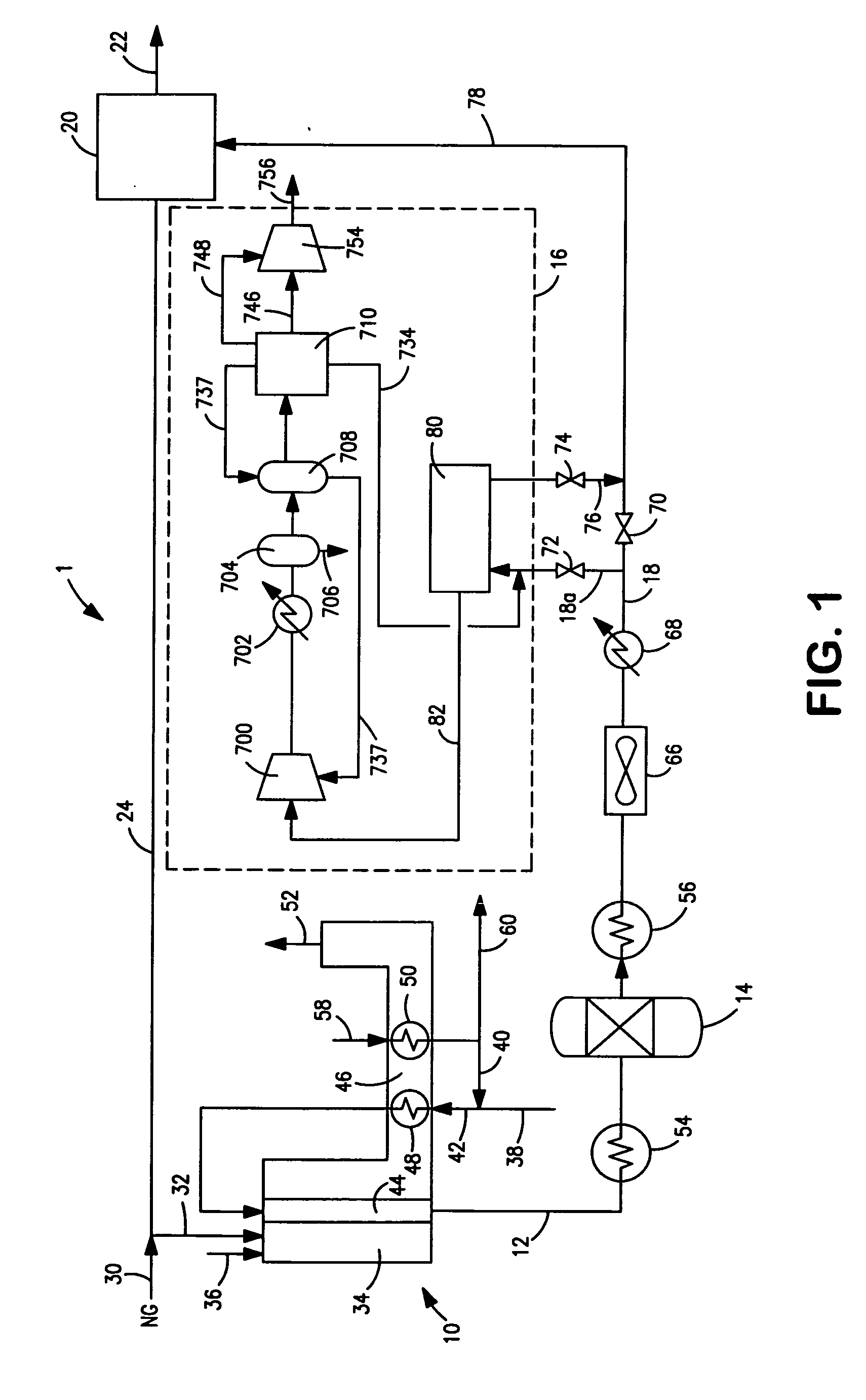
Efficient and selective conversion of carbon dioxide to methanol, dimethyl ether and derived products
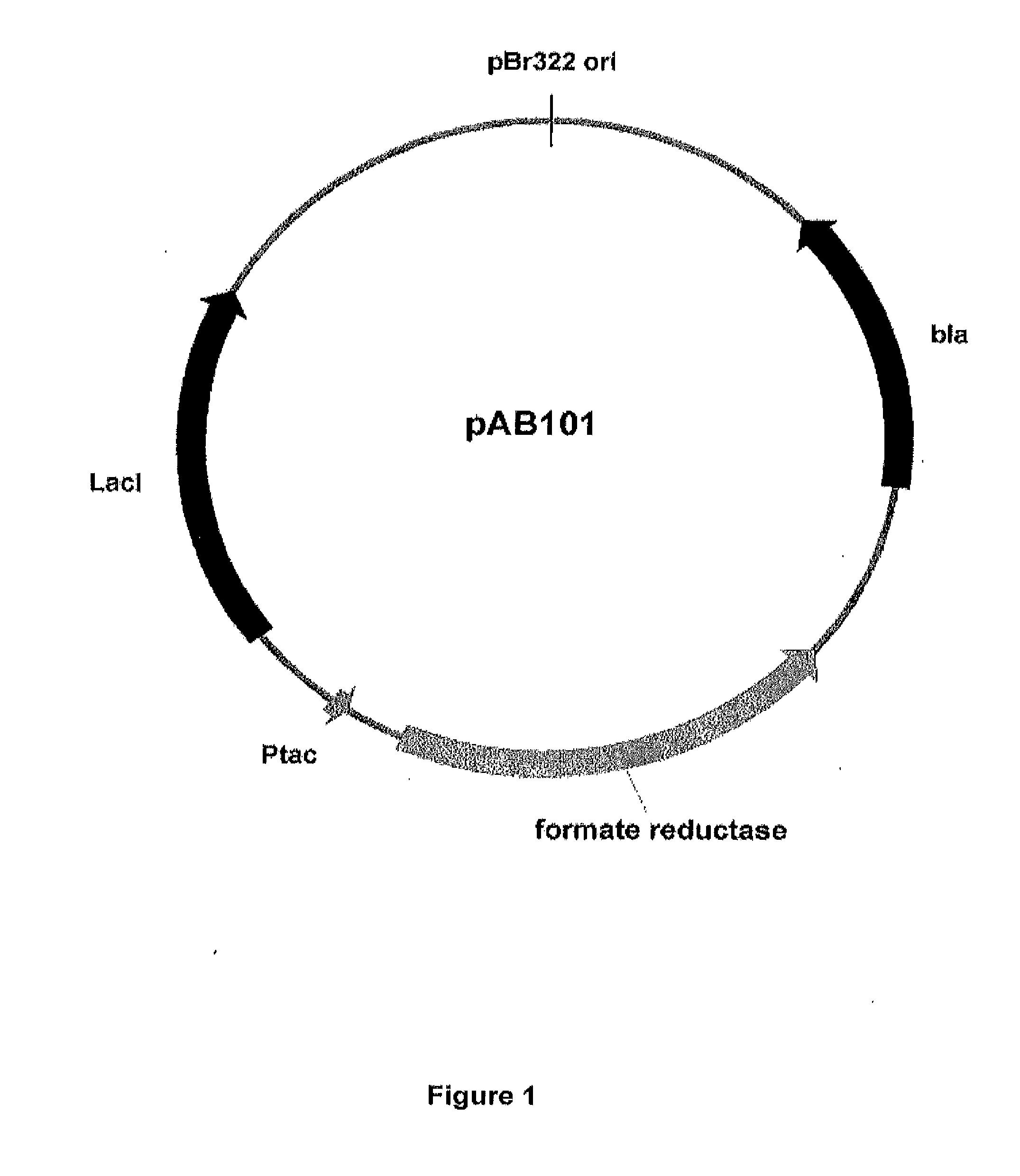
ActiveUS20060235091A1Minimize or eliminate the disadvantages or dangers inherentElectrolysis componentsCarbon compoundsHydrogenFlue gas
An environmentally beneficial method of producing methanol from varied sources of carbon dioxide including flue gases of fossil fuel burning powerplants, industrial exhaust gases or the atmosphere itself. Converting carbon dioxide by electrochemical reduction produces formic acid acid and some formaldehyde and methanol mixtures. The formic acid can be used as source of carbon as well as hydrogen to produce methanol, dimethyl ether and other products.
Owner:UNIV OF SOUTHERN CALIFORNIA
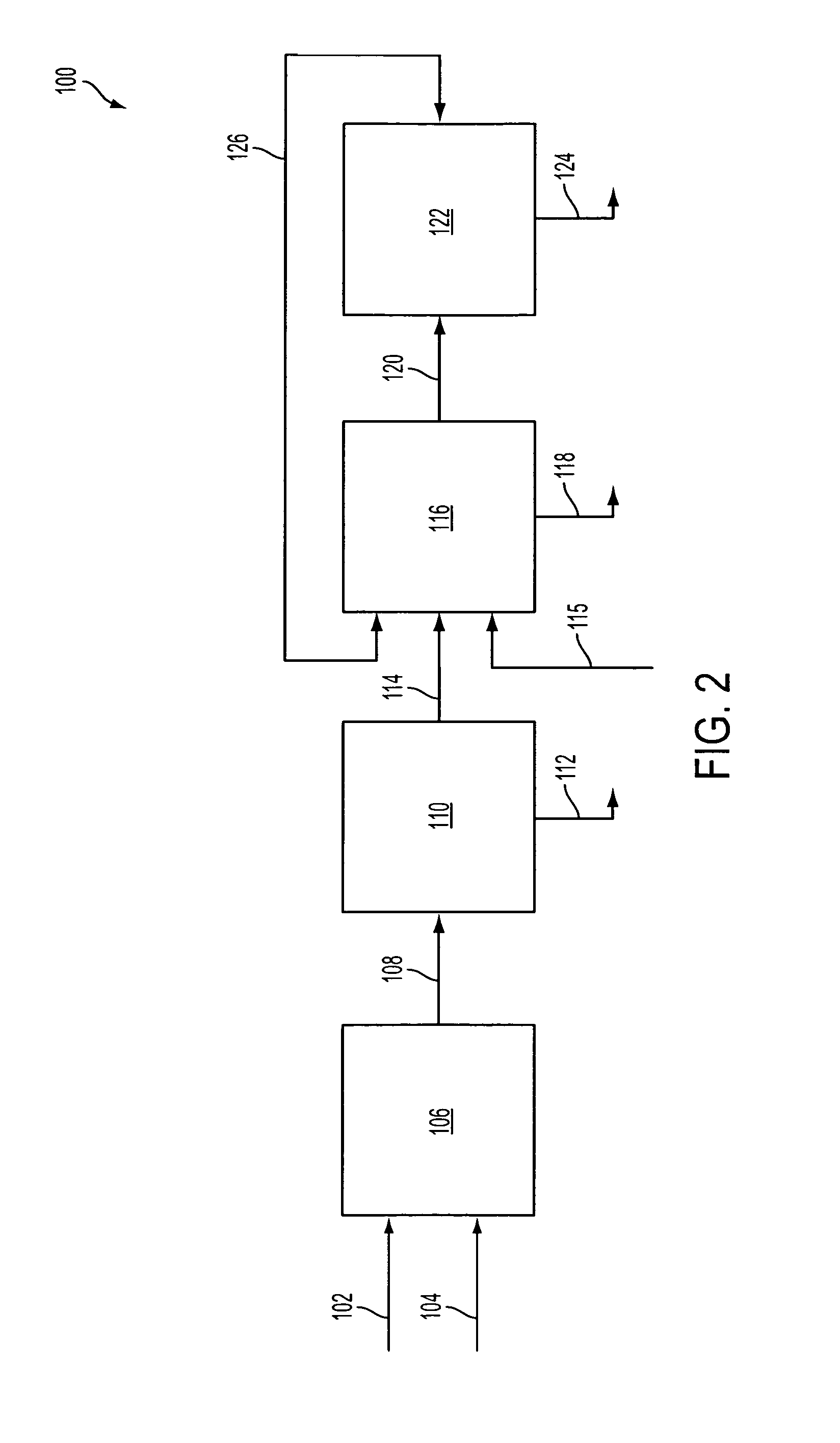
Carbon dioxide production method
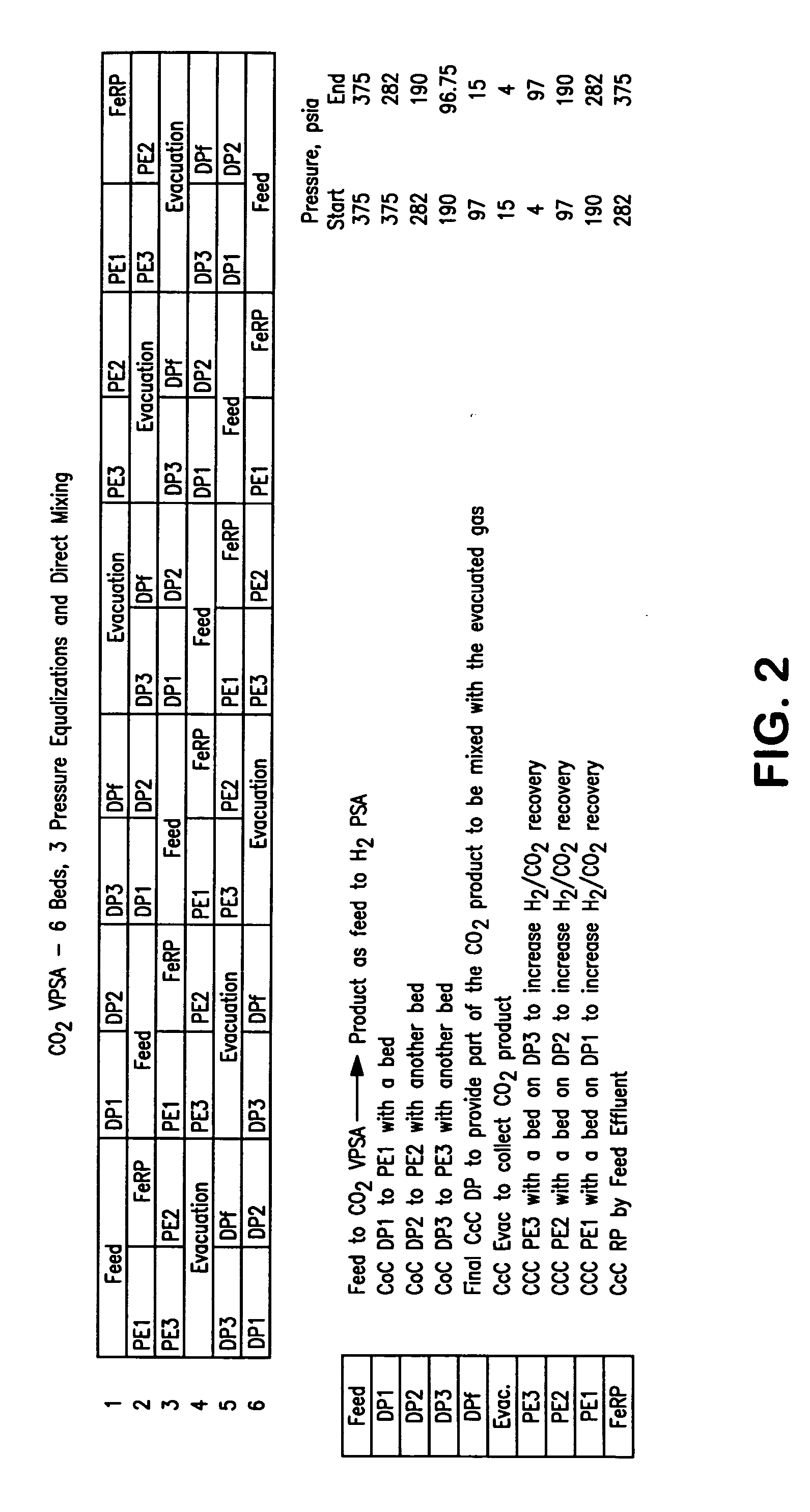
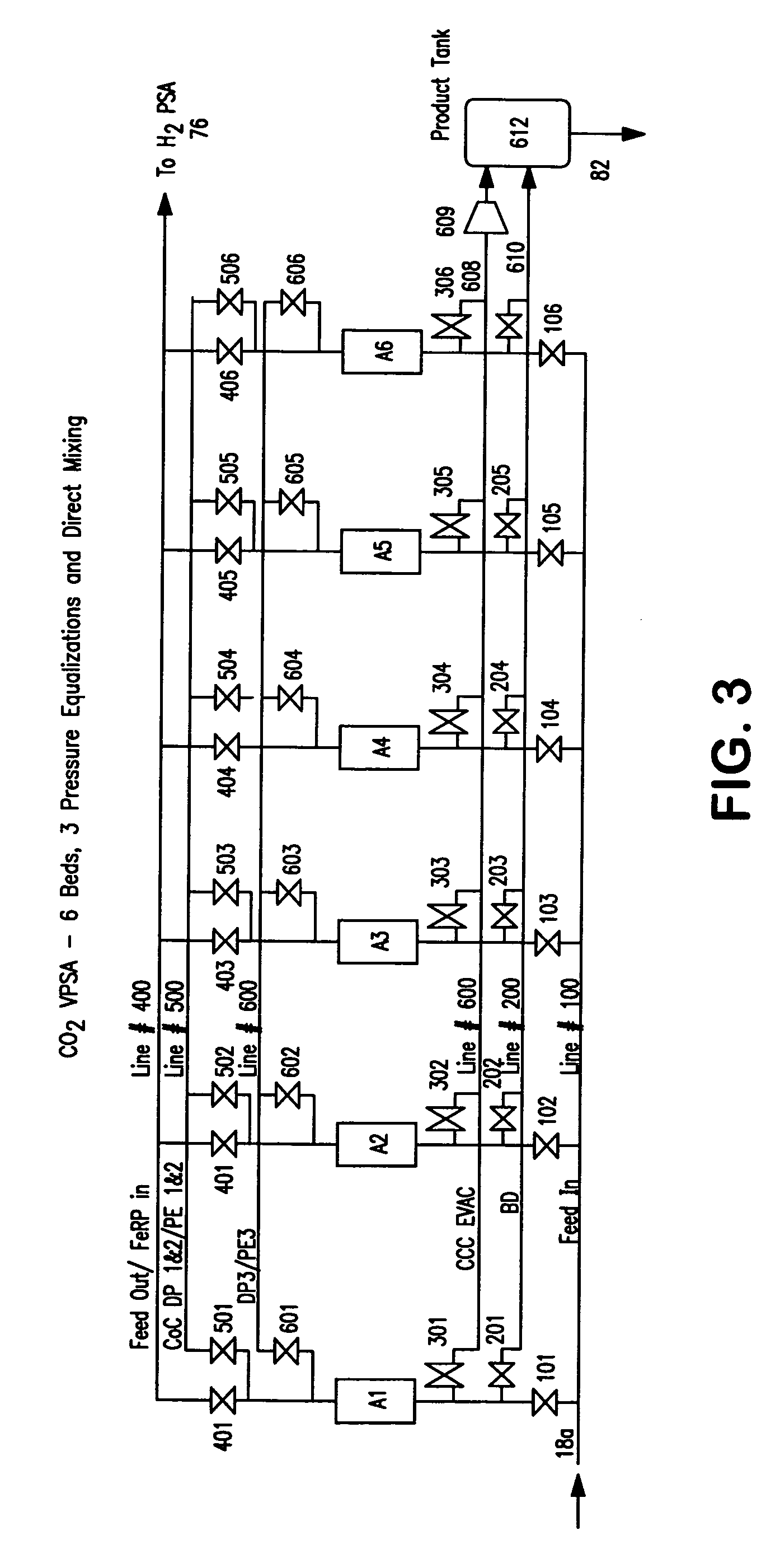
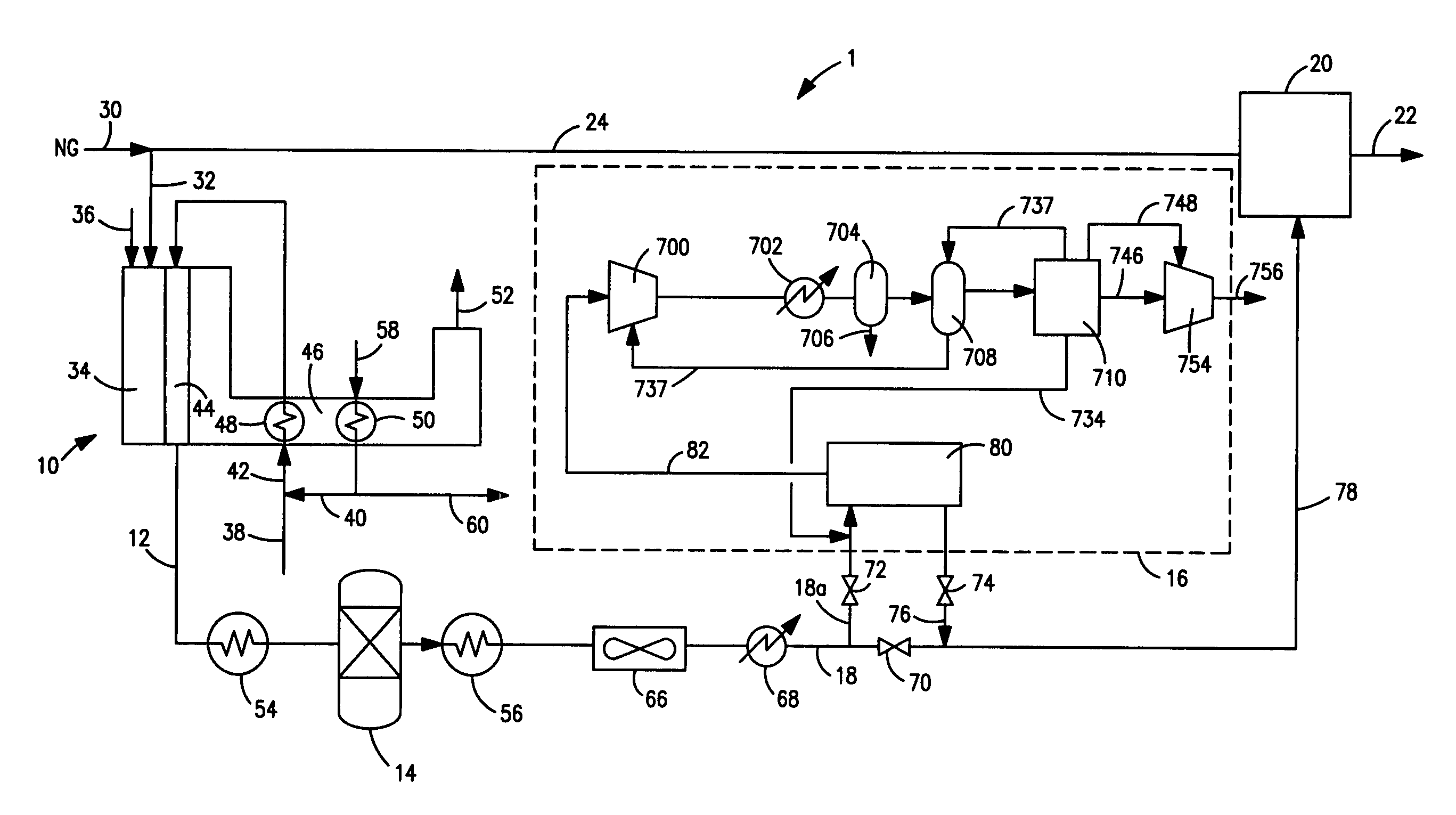
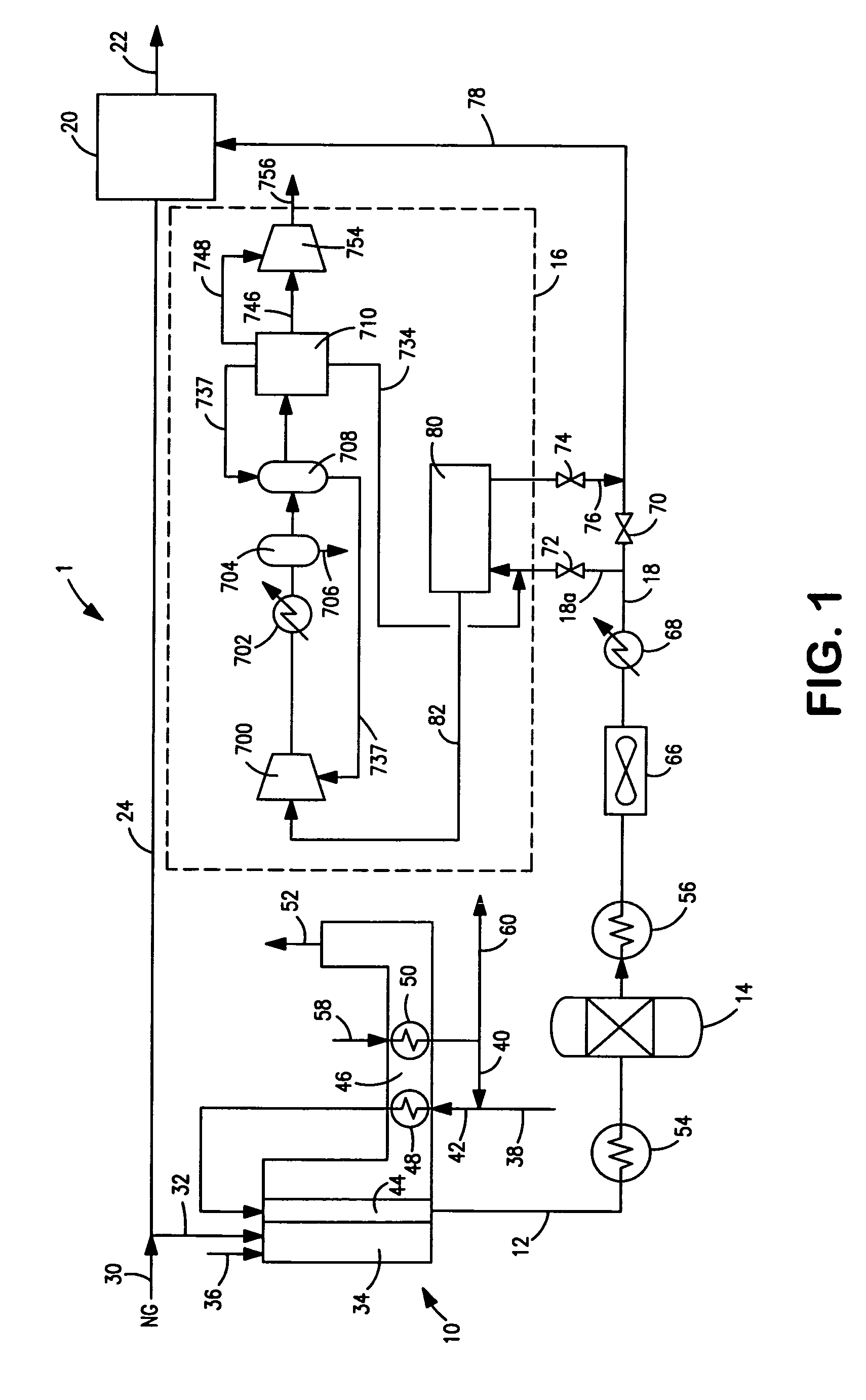
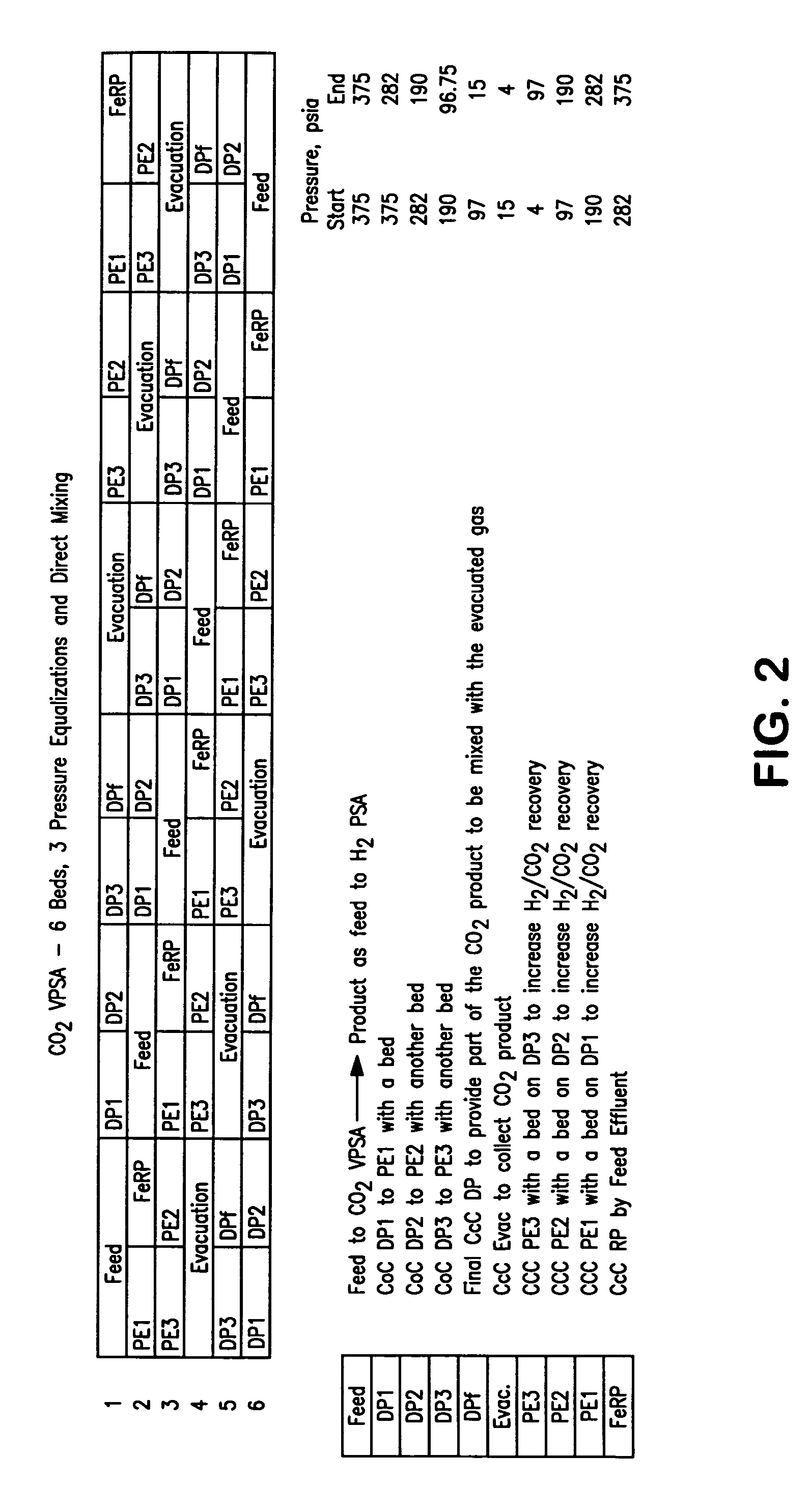
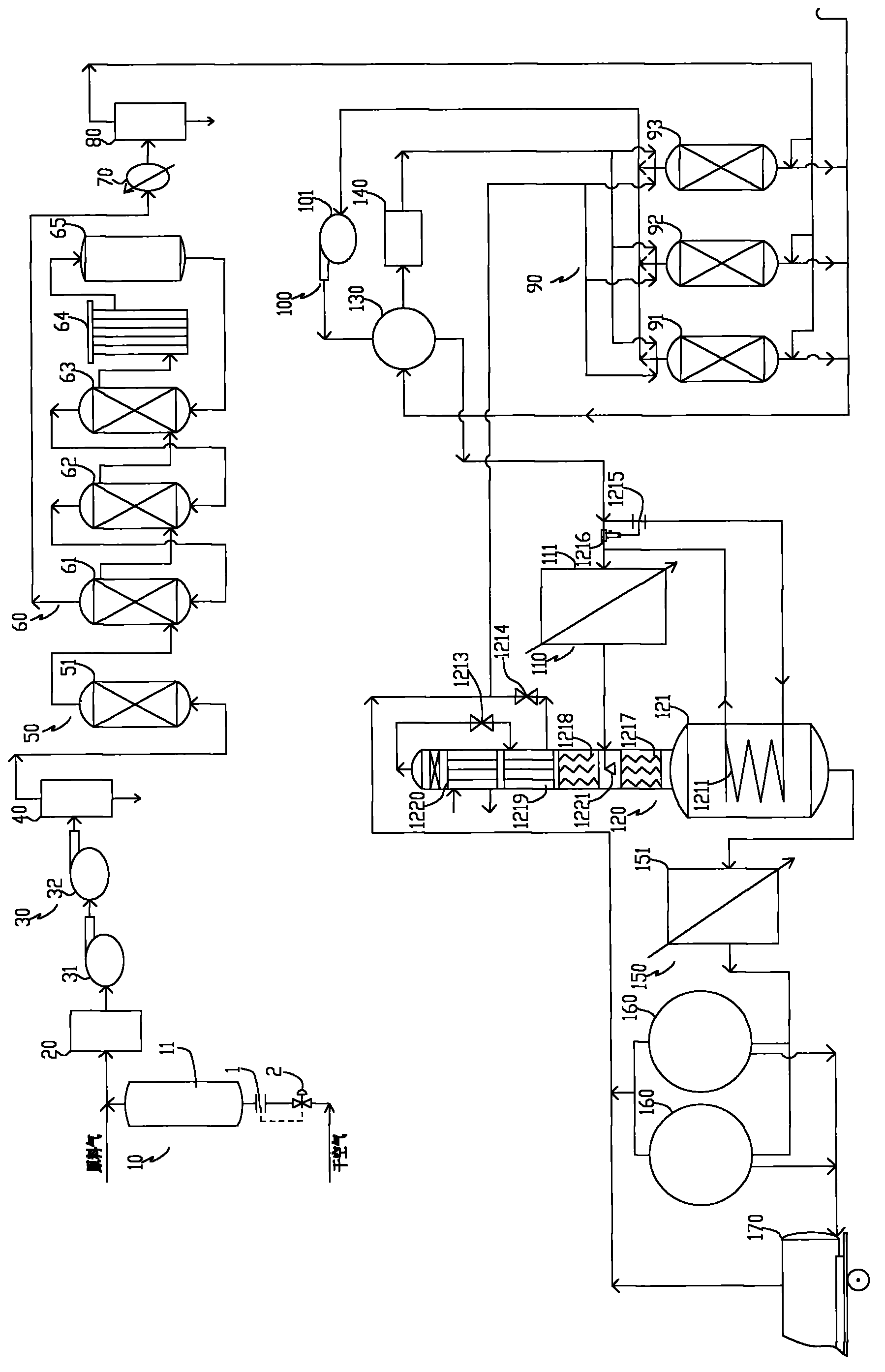
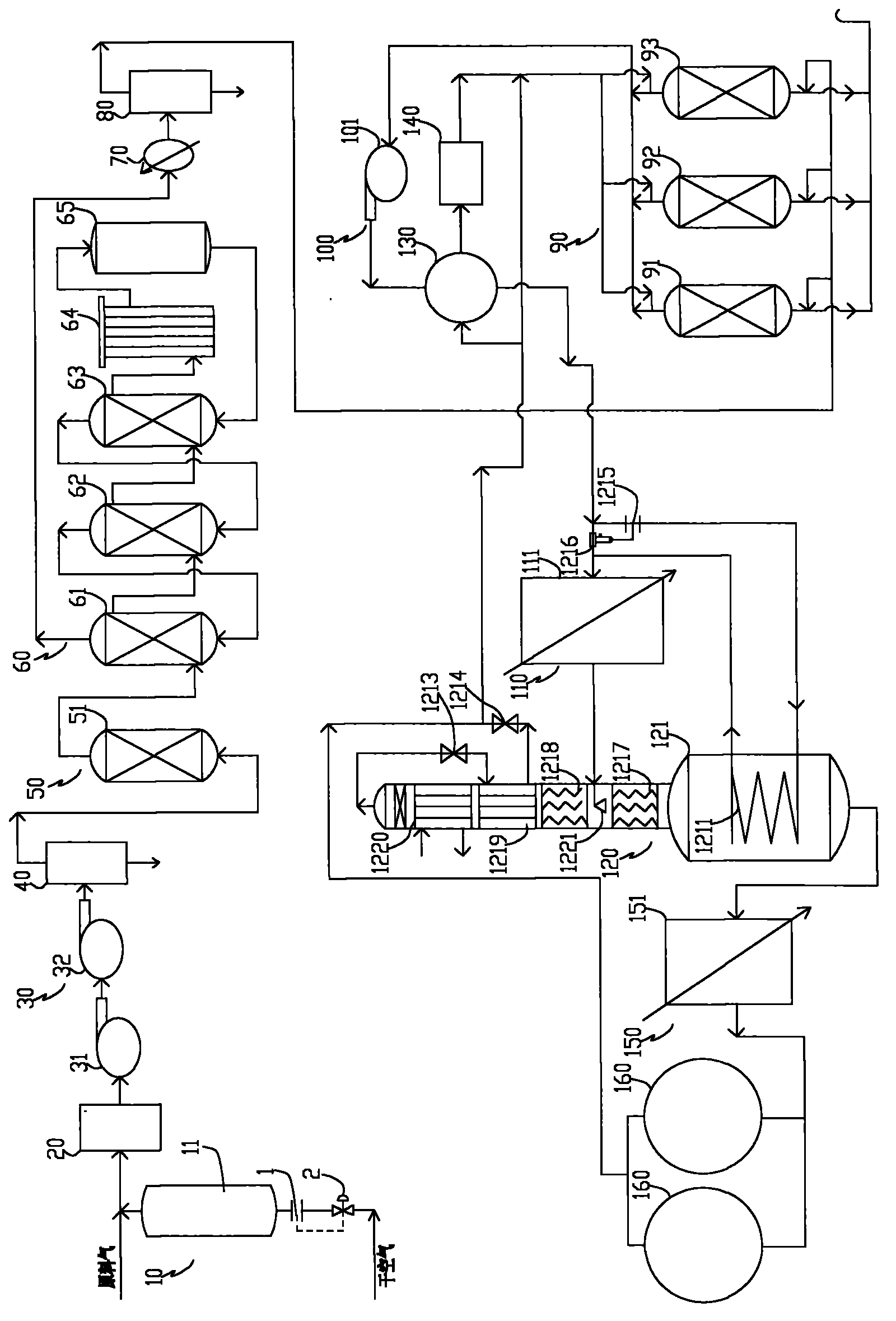
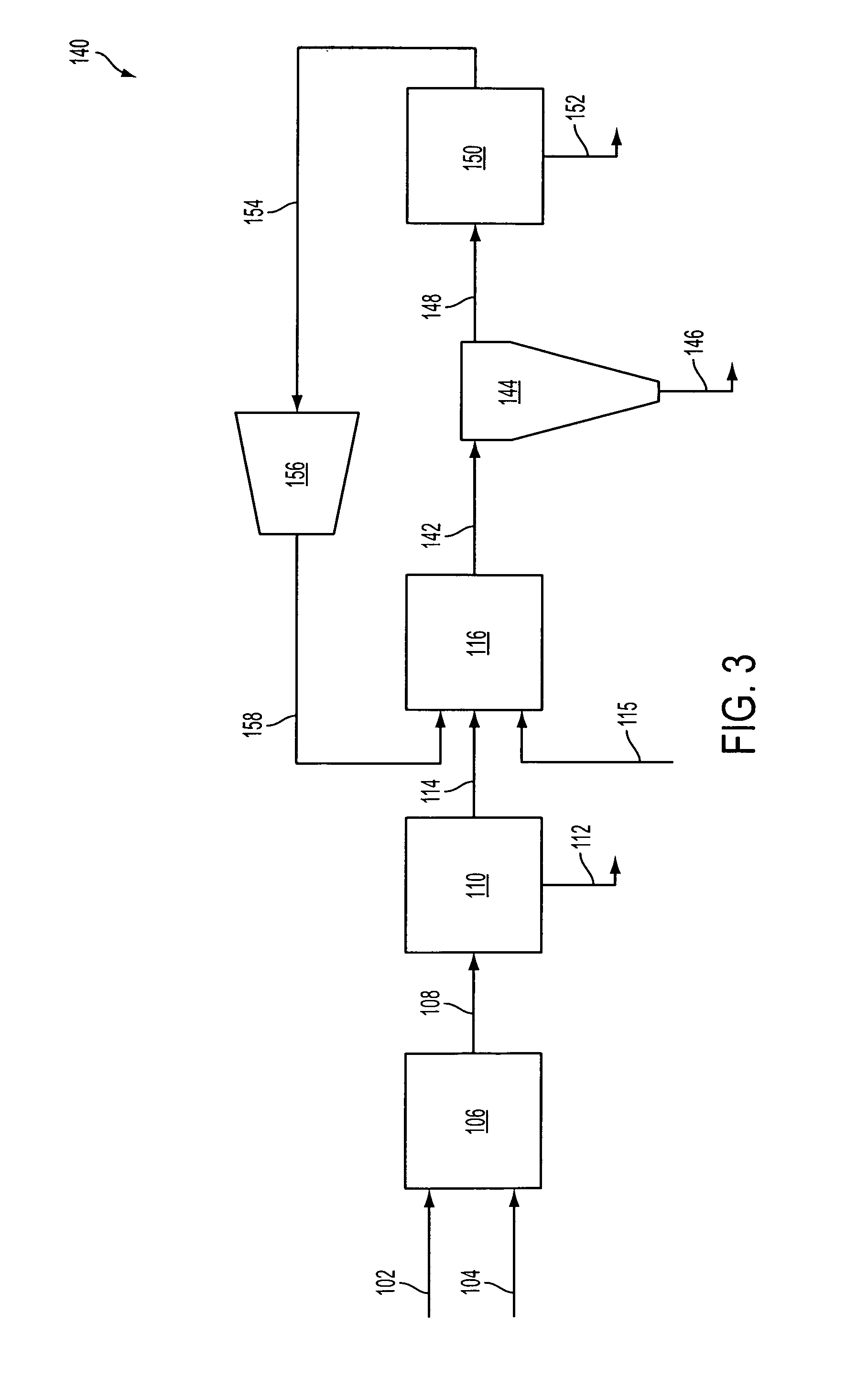
ActiveUS20070232706A1Increased hydrogen recoveryPromote recoverySolidificationLiquefactionVacuum pressureCarbon dioxide production
A method of producing a carbon dioxide product stream from a synthesis gas stream formed within a hydrogen plant having a synthesis gas reactor, a water-gas shift reactor, located downstream of the synthesis gas reactor to form the synthesis gas stream and a hydrogen pressure swing adsorption unit to produce a hydrogen product recovered from the synthesis gas stream. In accordance with the method the carbon dioxide from the synthesis gas stream by separating the carbon dioxide from the synthesis gas stream in a vacuum pressure swing adsorption system, thereby to produce a hydrogen-rich synthesis gas stream and a crude carbon dioxide stream and then purifying the crude carbon dioxide stream by a sub-ambient temperature distillation process thereby to produce the carbon dioxide product. A hydrogen synthesis gas feed stream to the hydrogen pressure swing adsorption unit is formed at least in part from the hydrogen rich stream.
Owner:PRAXAIR TECH INC
Carbon sequestration and dry reforming process and catalysts to produce same
ActiveUS7794690B2Reduce gas emissionsCatalyst carriersHydrogen productionCarbon dioxide productionCarbon sequestration
A carbon sequestration and dry reforming process for the production of synthesis gas and sequestered carbon from carbon dioxide. Two-dimension catalysts for sequestering carbon and a process to produce same. A method for activating two dimension catalysts.
Owner:SCOPRA SCI & GENIE SEC
System for the production of methane from co2
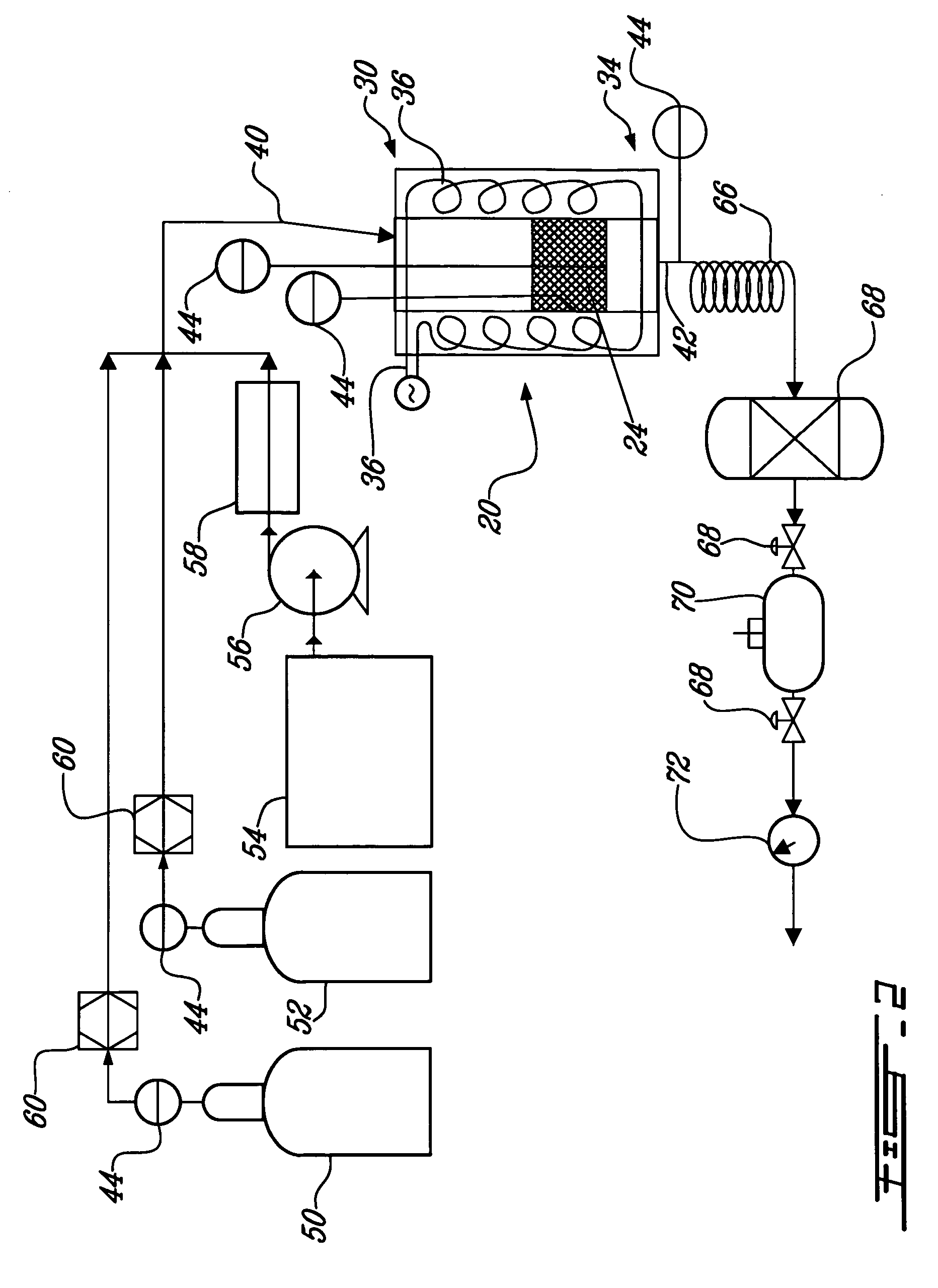
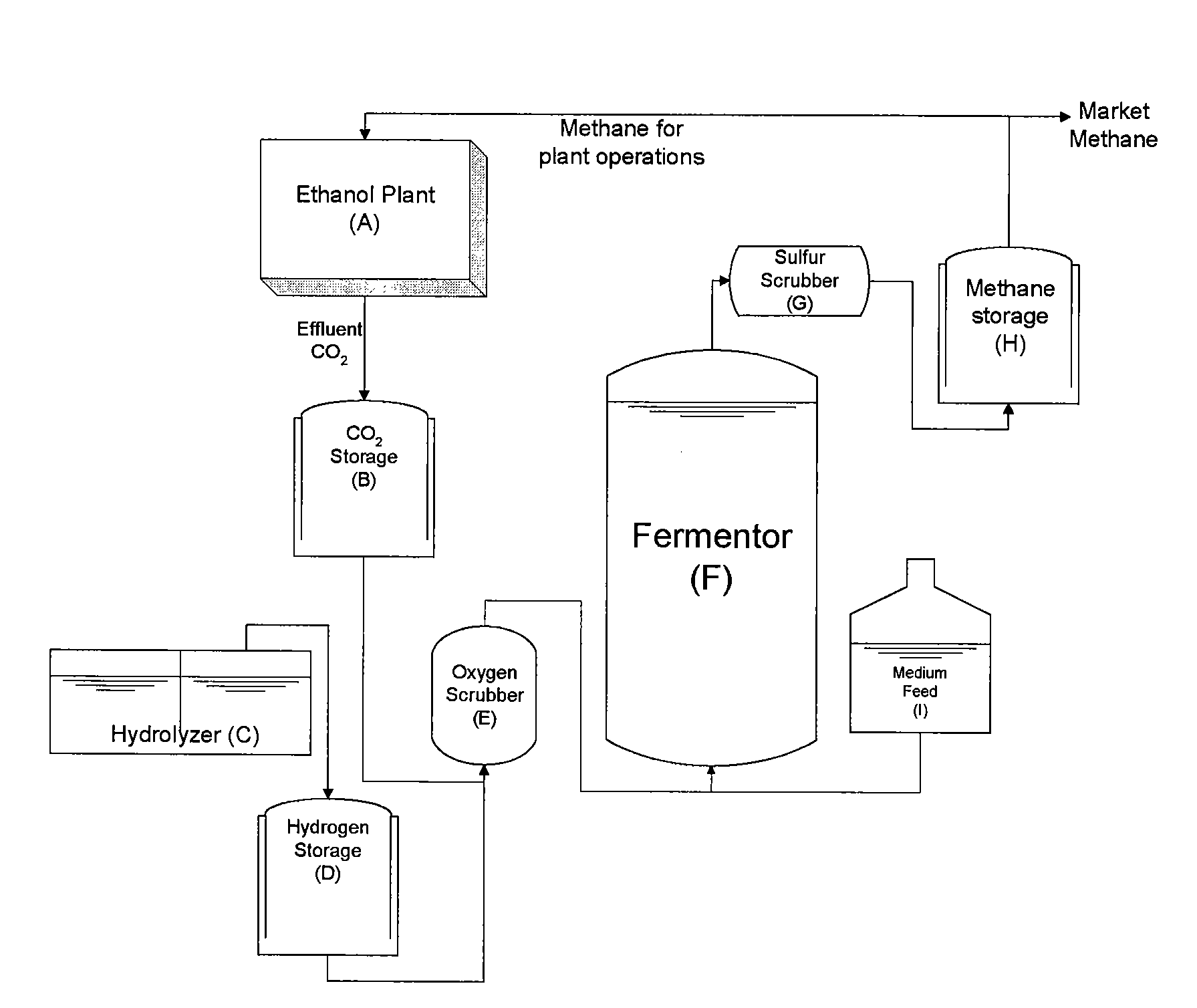
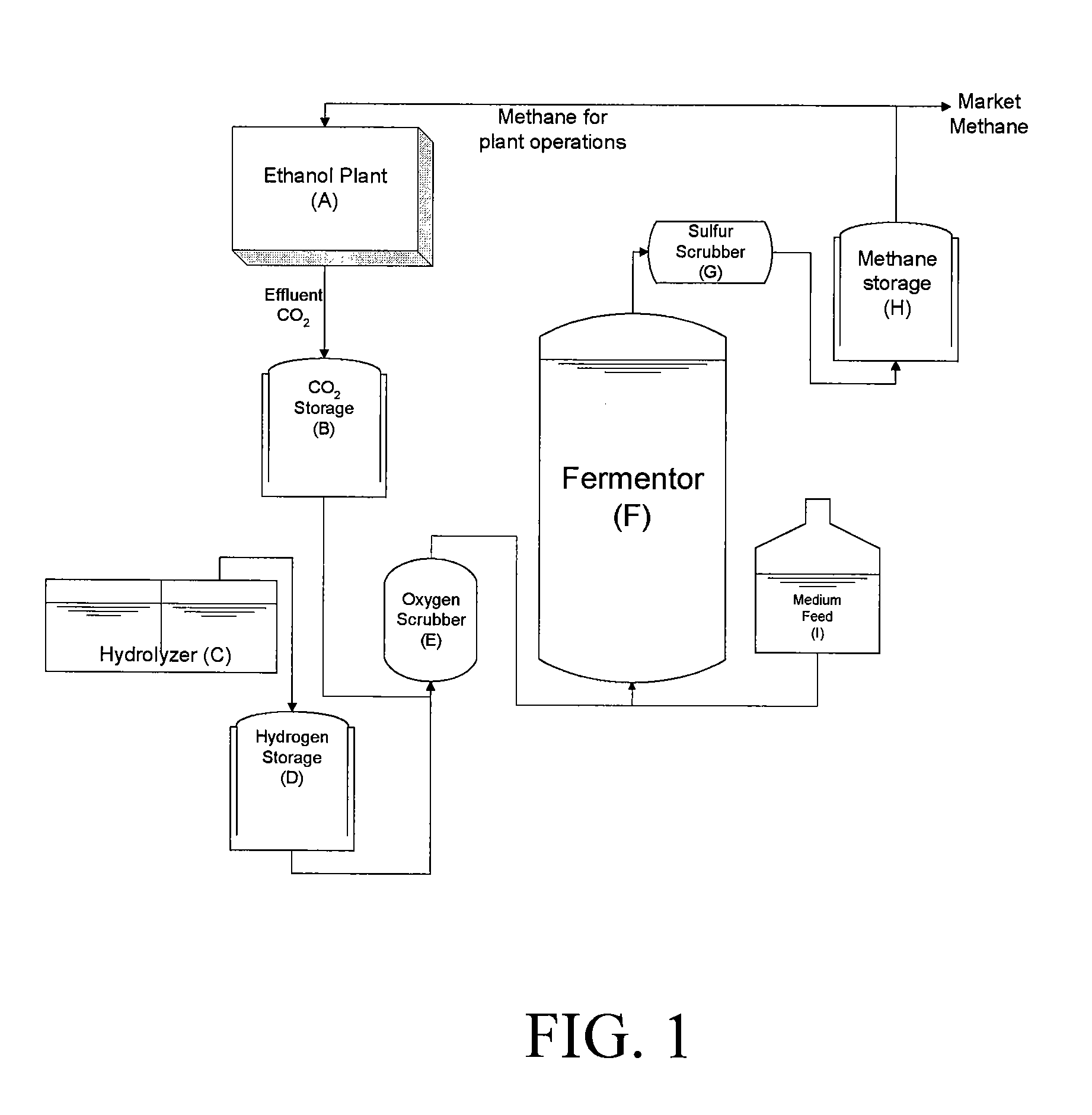
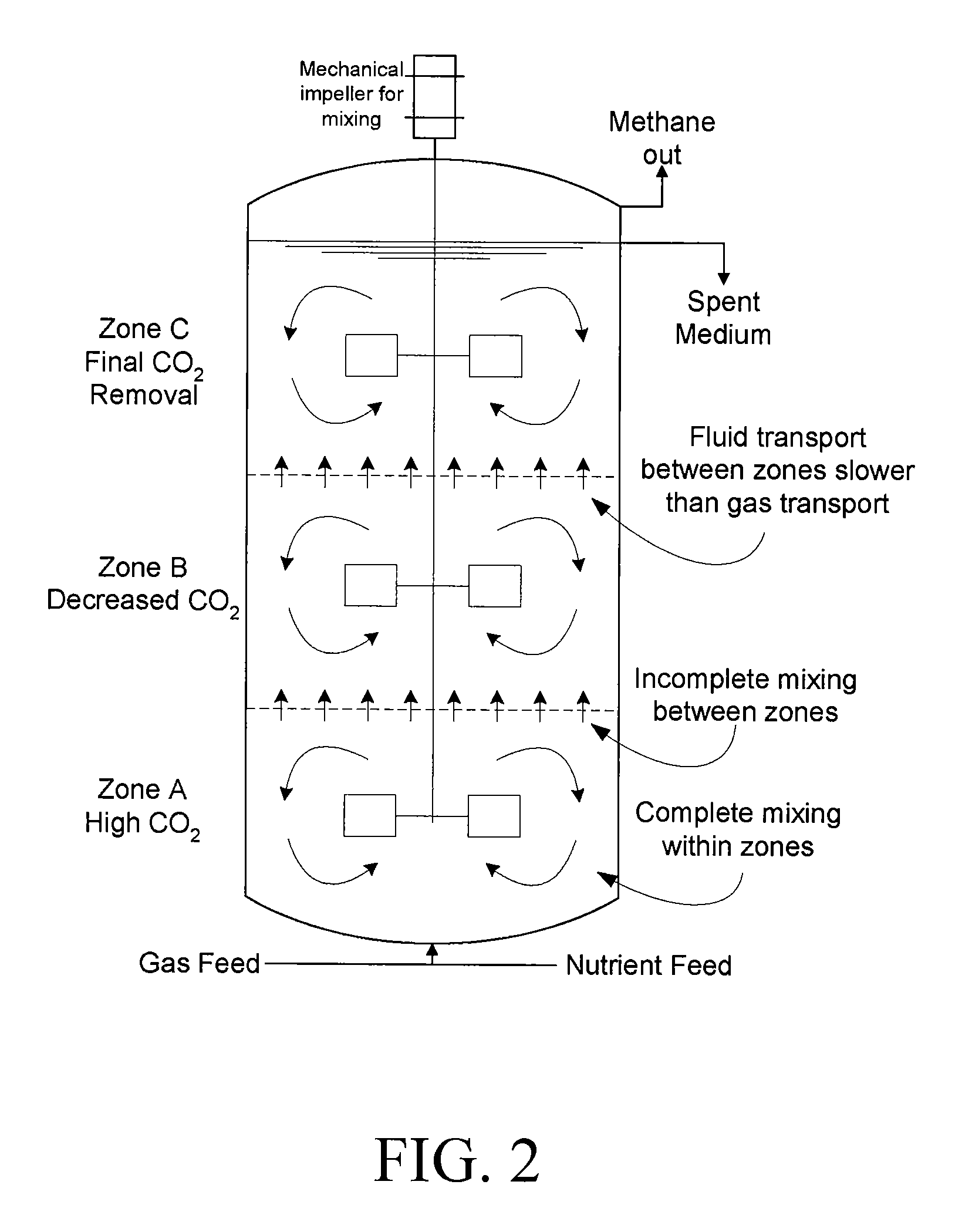
InactiveUS20090130734A1Improve efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsCarbon dioxide productionMethanogenic archaeon
Owner:UNIVERSITY OF CHICAGO
Non-porous catalysts for co2 sequestration through dry reforming
InactiveUS20090203519A1Reduce gas emissionsMaterial nanotechnologyHydrogenPorous catalystCarbon dioxide production
A carbon sequestration and dry reforming process for the production of synthesis gas and sequestered carbon from carbon dioxide. Two-dimension (non-porous) catalysts for sequestering carbon are also disclosed and a process to produce same as well as a method for activating two dimension catalysts.
Owner:UNIV DE SHERBROOKE +1
Method for preparing aromatic hydrocarbons through hydrogenation of carbon dioxide
ActiveCN107840778AEasy to prepareRaw materials are cheap and easy to getHydrocarbon from carbon oxidesMolecular sieve catalystsMolecular sieveCarbon dioxide production
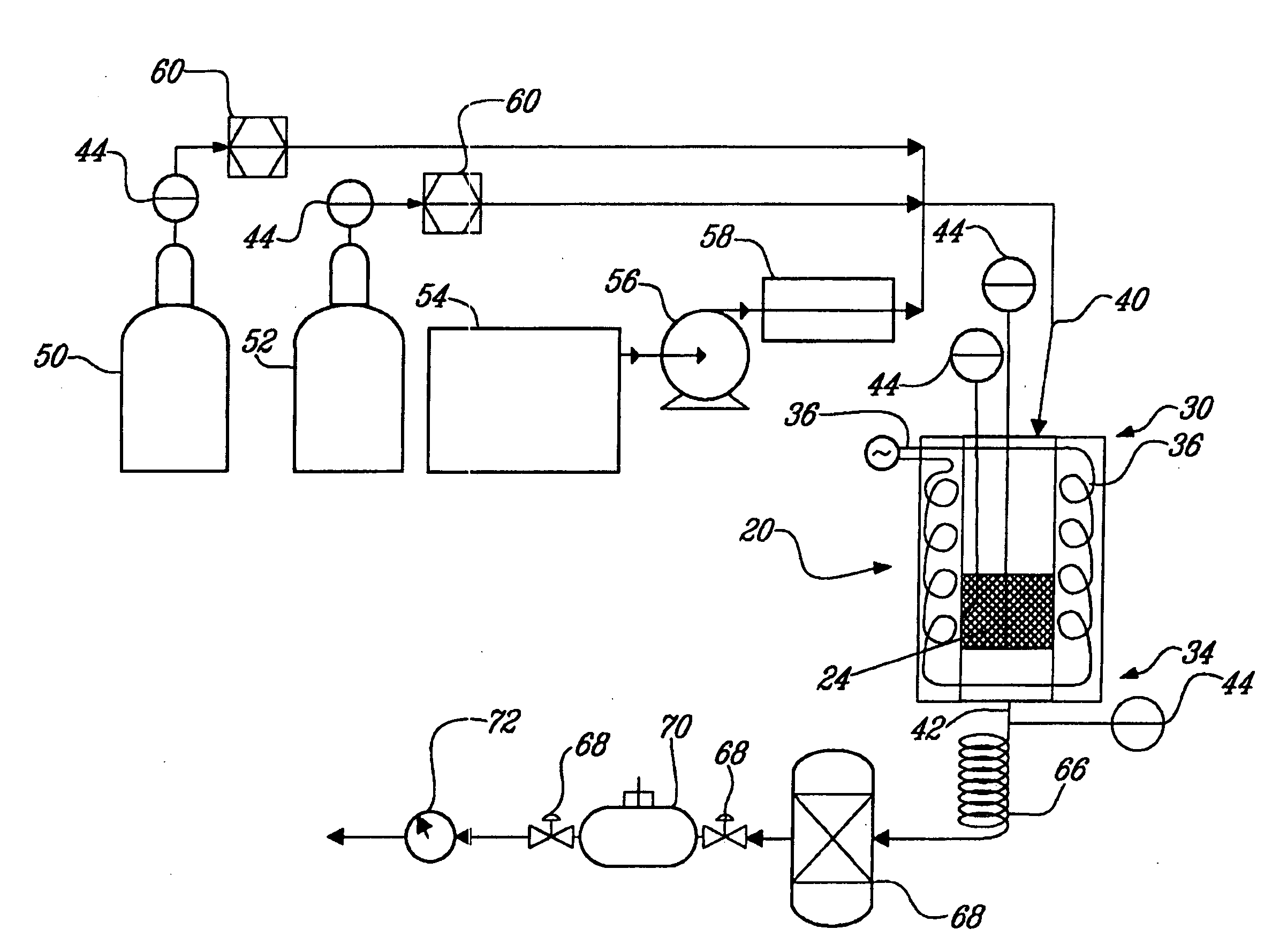
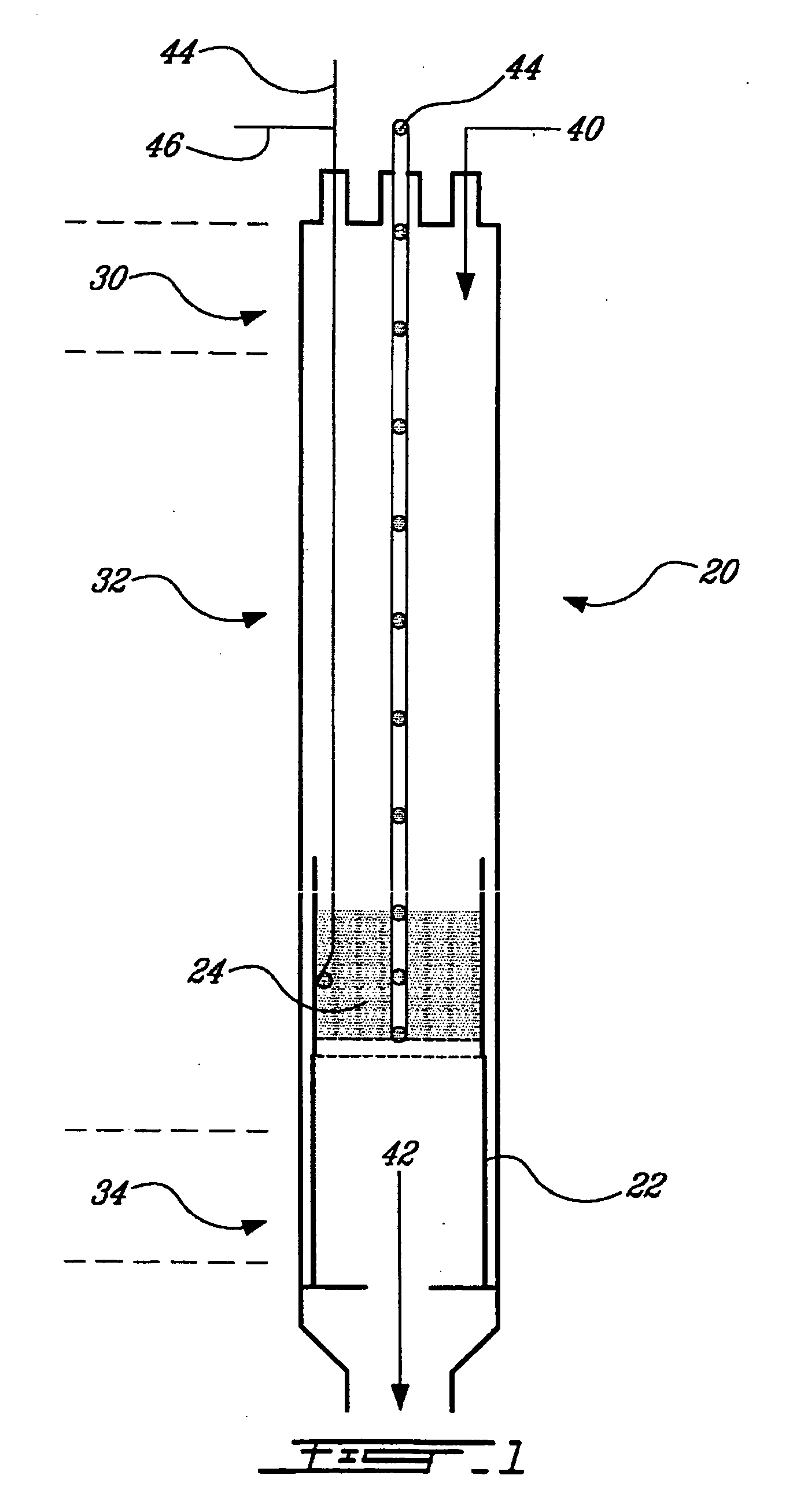
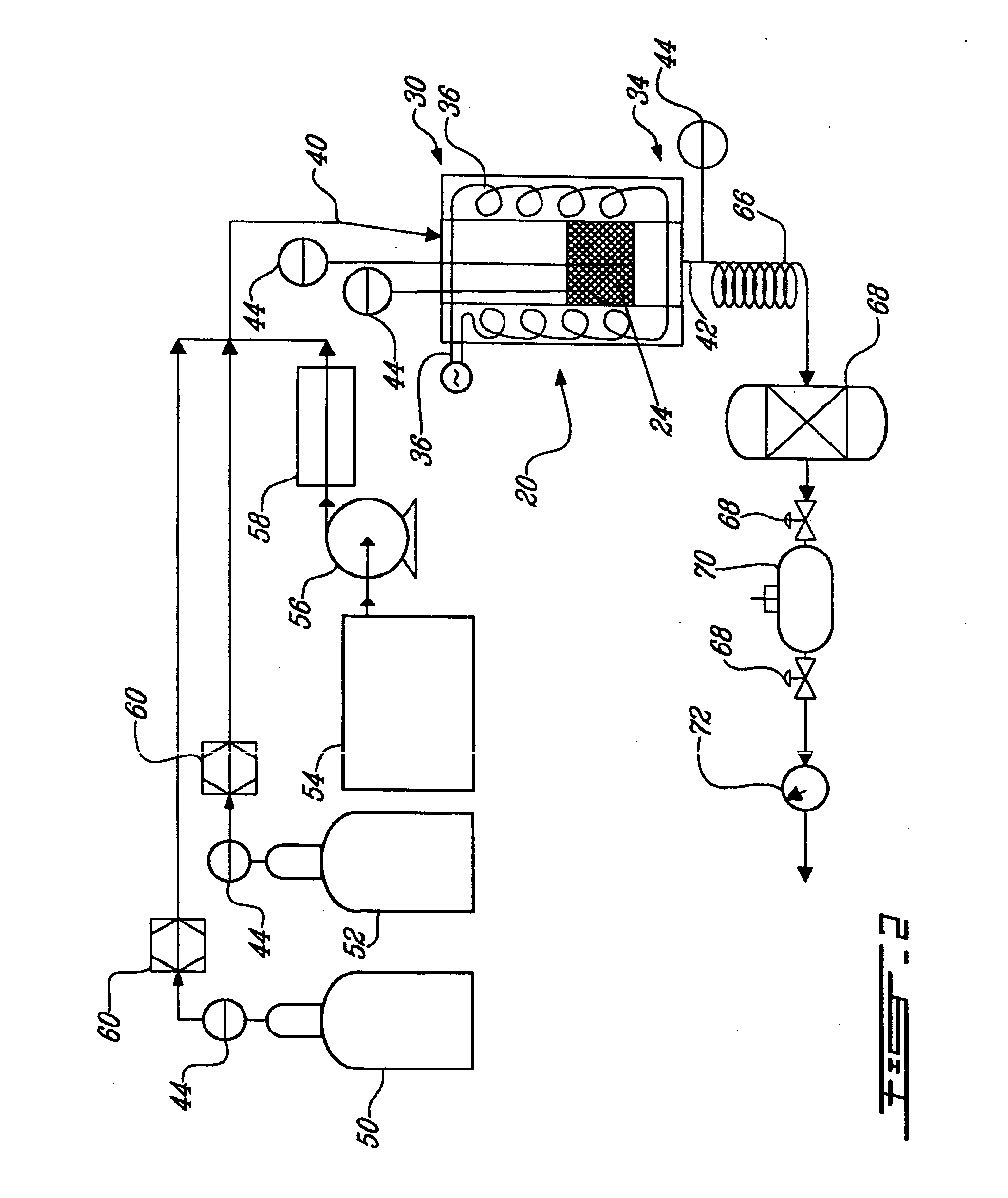
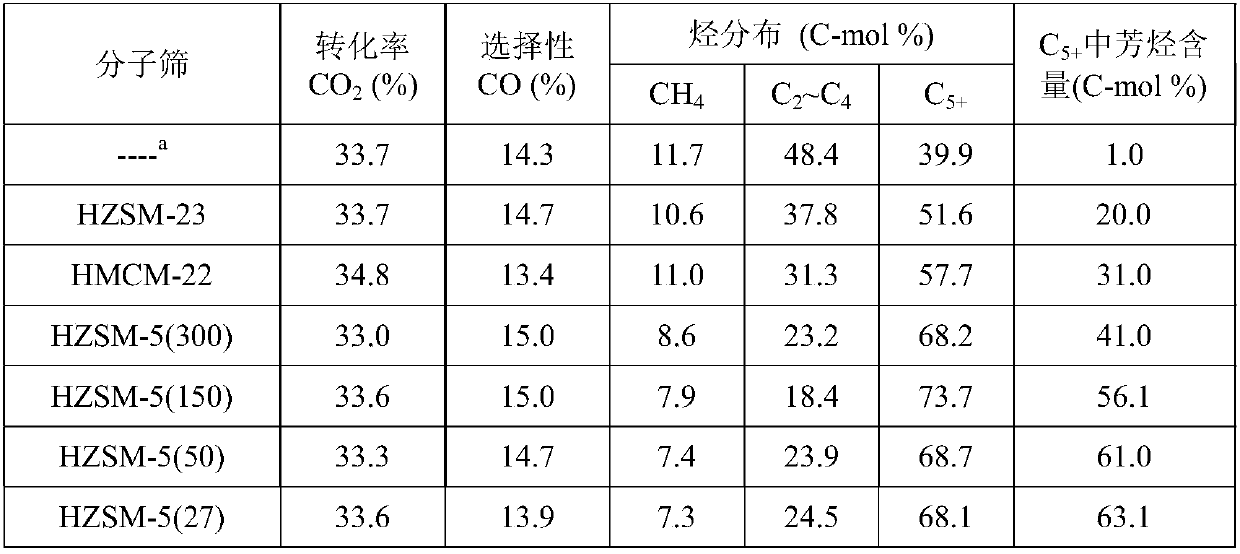
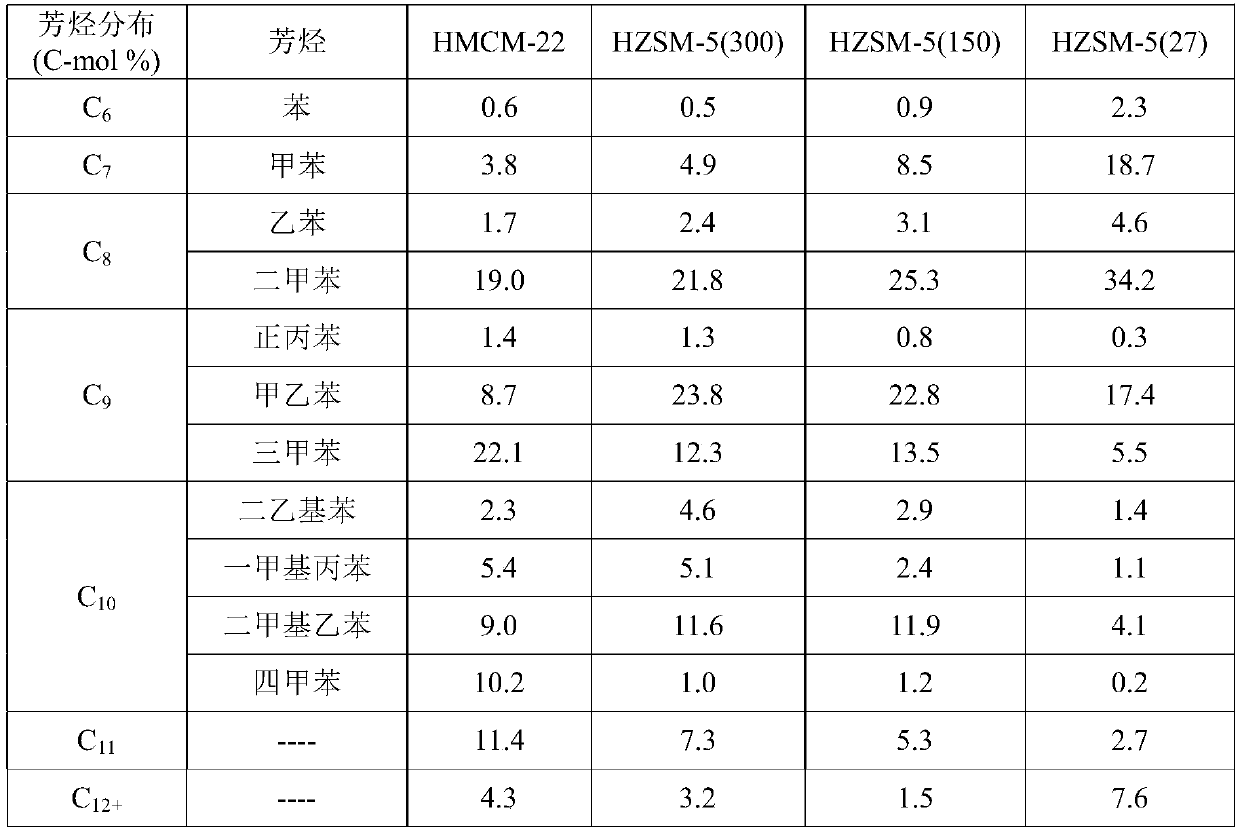
The invention provides a method for preparing aromatic hydrocarbons through hydrogenation of carbon dioxide. A gas mixture composed of carbon dioxide and hydrogen is directly converted under the catalysis of a multifunctional composite catalyst under the reaction conditions of a temperature of 250 to 450 DEG C, a pressure of 0.01 to 10.0 MPa, space velocity of 500 to 50000 mL / (h.g<cat>) and a H<2> / CO<2> mol ratio of 0.5 to 8.0 so as to produce the aromatic hydrocarbons. The composite catalyst is prepared by mixing a first component with a second component, wherein the first component is a Fe-based catalyst for preparation of low-carbon olefins through hydrogenation of carbon dioxide, and the second component is one or more than two selected from a group consisting of molecular sieves modified or unmodified by metals and mainly exerting aromatization effect on olefins. According to the method, the one-way CO2 conversion rate can reach 33% or above; the selectivity of hydrocarbon products can be controlled to be 80% or above; the content of methane in hydrocarbon products is lower than 8%; the content of C<5+> hydrocarbons is higher than 65%; and the aromatic hydrocarbons account for63% or more of the C<5+> hydrocarbons. The method opens up a novel route for production of aromatic hydrocarbons from carbon dioxide.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
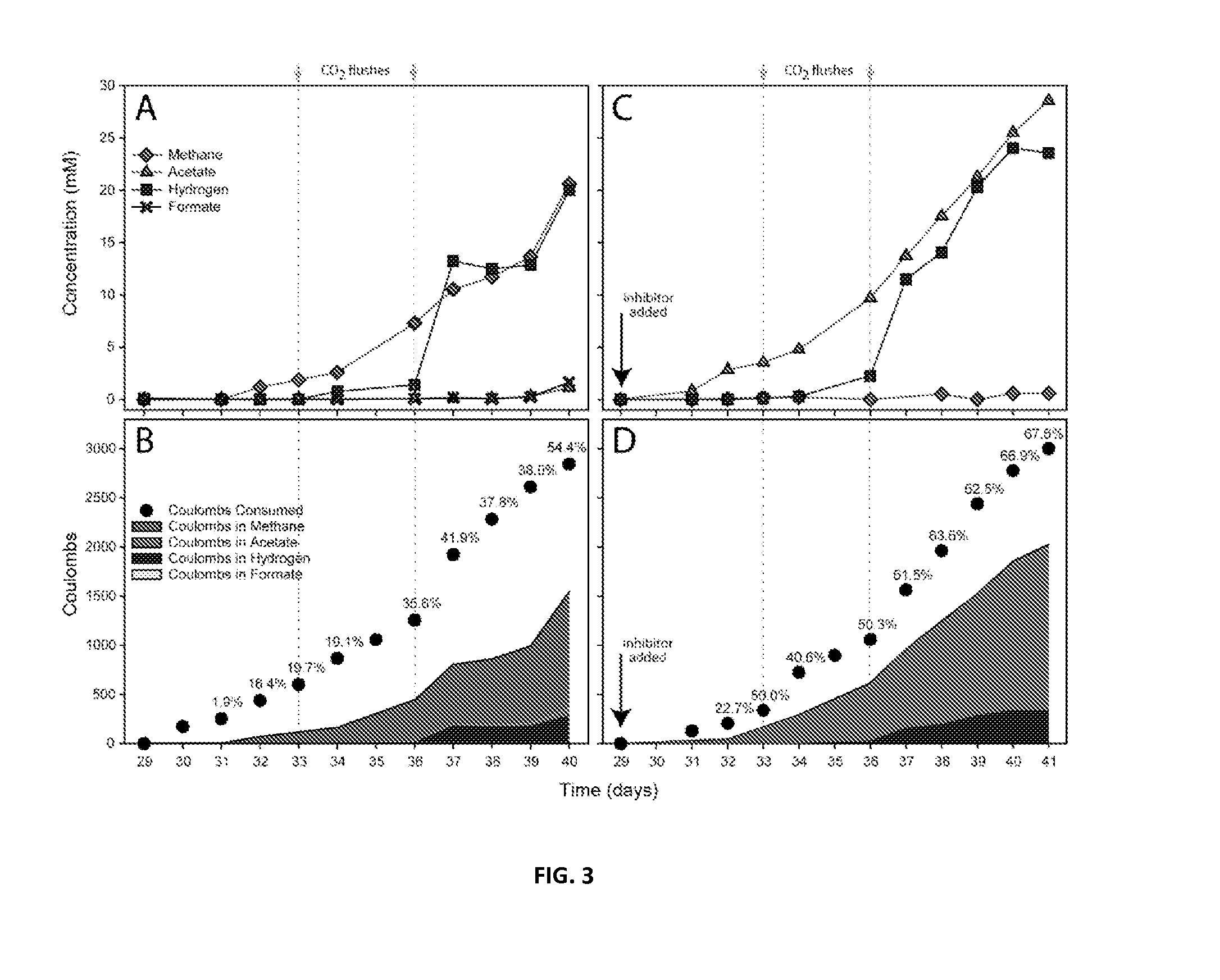
Method for restoring carbon dioxide to produce methane and acetic acid by utilizing biological electrochemical system
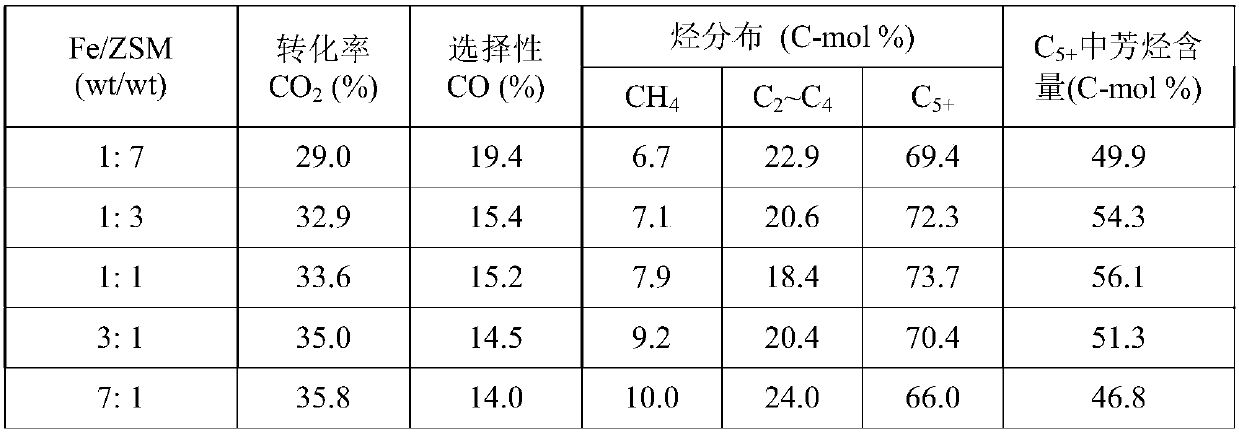
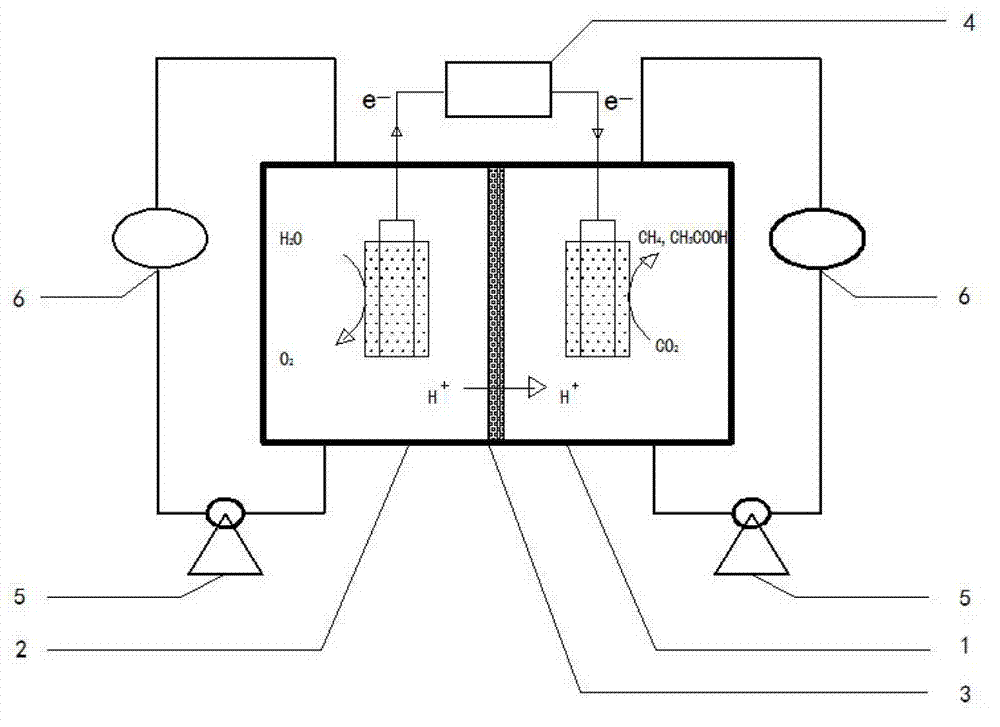
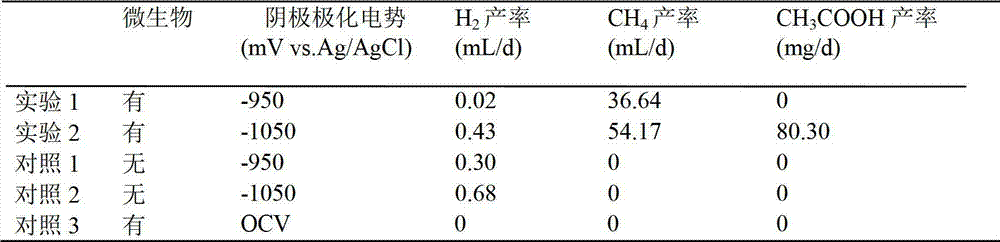
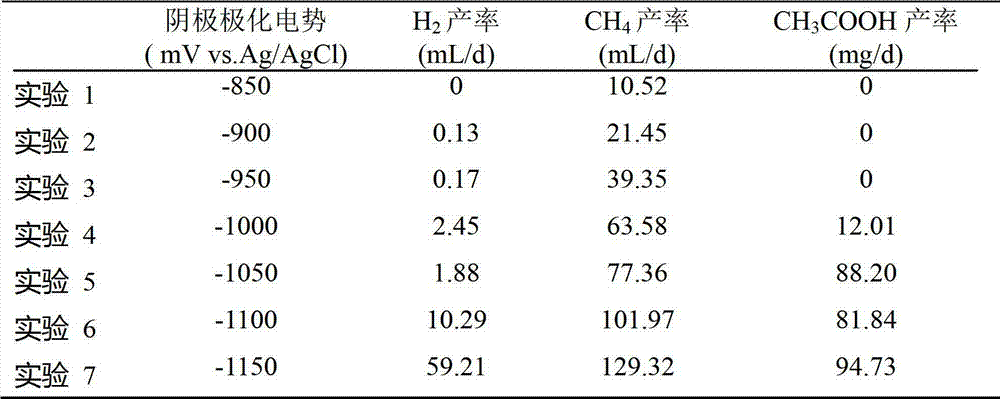
The invention provides a method for restoring carbon dioxide to produce methane and acetic acid by utilizing a biological electrochemical system and regulating and controlling microorganism metabolites by utilizing cathodic polarization potential. A bio-cathode is prepared in the biological electrochemical system, carbon dioxide (CO2) is fed into a cathode chamber and an anode chamber to circularly aerate, the cathodic polarization potential is set from -850mV to -1150mV (vs.Ag / AgCl), and microorganism on a cathode can directly obtain electrons from electrodes or from hydrogen produced by the electrodes to restore the carbon dioxide and produce the methane and the acetic acid. The methane and the acetic acid which are microbial synthesis products can be regulated and controlled by setting different cathodic polarization potentials. The electrodes do not need to use expensive catalyst, and are low in cost. The method for restoring the carbon dioxide to produce the methane and the acetic acid by utilizing the biological electrochemical system is rapid in production rate of the methane and the acetic by restoring the carbon dioxide, and has important application prospect for fixedly converting the carbon dioxide and synthetizing organic chemicals.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Integration of pressure swing adsorption with a power plant for co2 capture/utilization and n2 production
ActiveUS20130333391A1Reduce pressureGas treatmentCarbon compoundsCarbon dioxide productionCombustion
Systems and methods are provided for combined cycle power generation while reducing or mitigating emissions during power generation. Recycled exhaust gas from a power generation combustion reaction can be separated using a swing adsorption process so as to generate a high purity CO2 stream while reducing / minimizing the energy required for the separation and without having to reduce the temperature of the exhaust gas. This can allow for improved energy recovery while also generating high purity streams of carbon dioxide and nitrogen.
Owner:EXXONMOBIL UPSTREAM RES CO
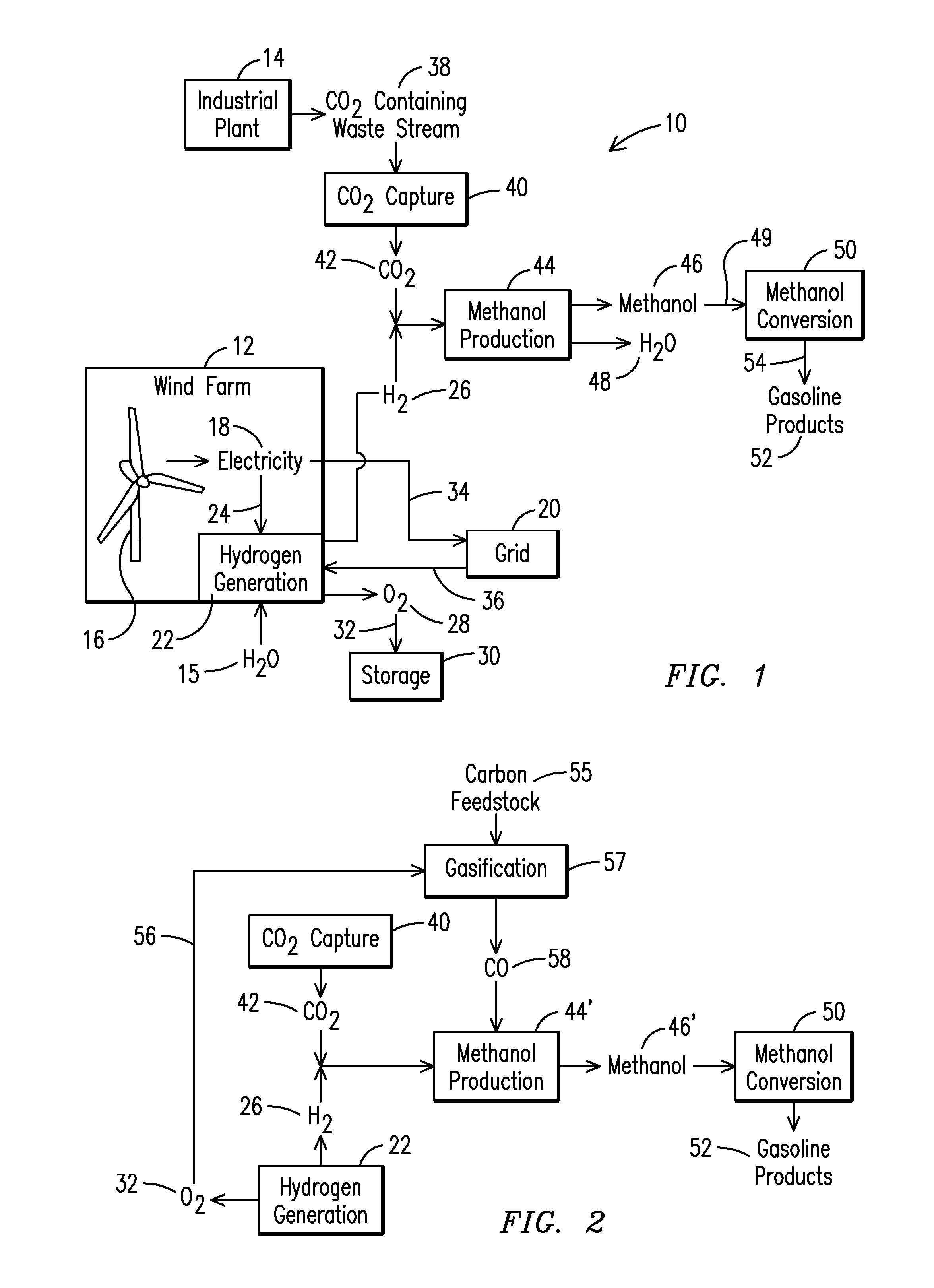
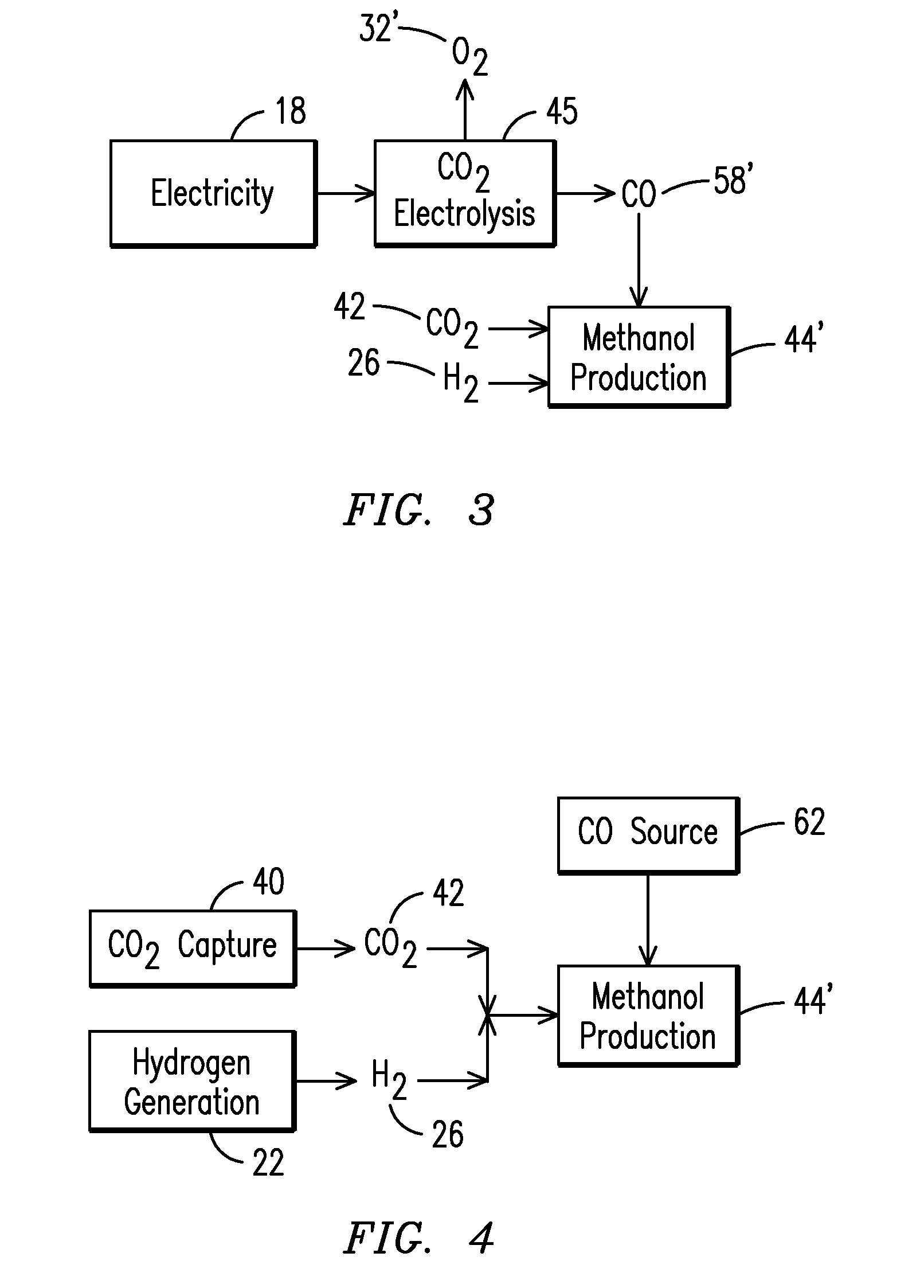
Method for Producing Fuel from Captured Carbon Dioxide
InactiveUS20080072496A1Electrolysis componentsHydrocarbon from carbon oxidesHydrogenCarbon dioxide production
The invention provides a method for producing combustible fuels from a gaseous mixture containing carbon dioxide, which comprises: (i) capturing CO2 from said gaseous mixture by means of K2CO3, thus forming KHCO3; (ii) releasing the CO2 from said KHCO3; and (iii) subsequently producing fuel from the released CO2 by reaction with hydrogen.
Owner:ENGINEUITY RES & DEV +1
Carbon dioxide production method
A method of producing a carbon dioxide product stream from a synthesis gas stream formed within a hydrogen plant having a synthesis gas reactor, a water-gas shift reactor, located downstream of the synthesis gas reactor to form the synthesis gas stream and a hydrogen pressure swing adsorption unit to produce a hydrogen product recovered from the synthesis gas stream. In accordance with the method the carbon dioxide from the synthesis gas stream by separating the carbon dioxide from the synthesis gas stream in a vacuum pressure swing adsorption system, thereby to produce a hydrogen-rich synthesis gas stream and a crude carbon dioxide stream and then purifying the crude carbon dioxide stream by a sub-ambient temperature distillation process thereby to produce the carbon dioxide product. A hydrogen synthesis gas feed stream to the hydrogen pressure swing adsorption unit is formed at least in part from the hydrogen rich stream.
Owner:PRAXAIR TECH INC
Method for producing viscous hydrocarbon using steam and carbon dioxide
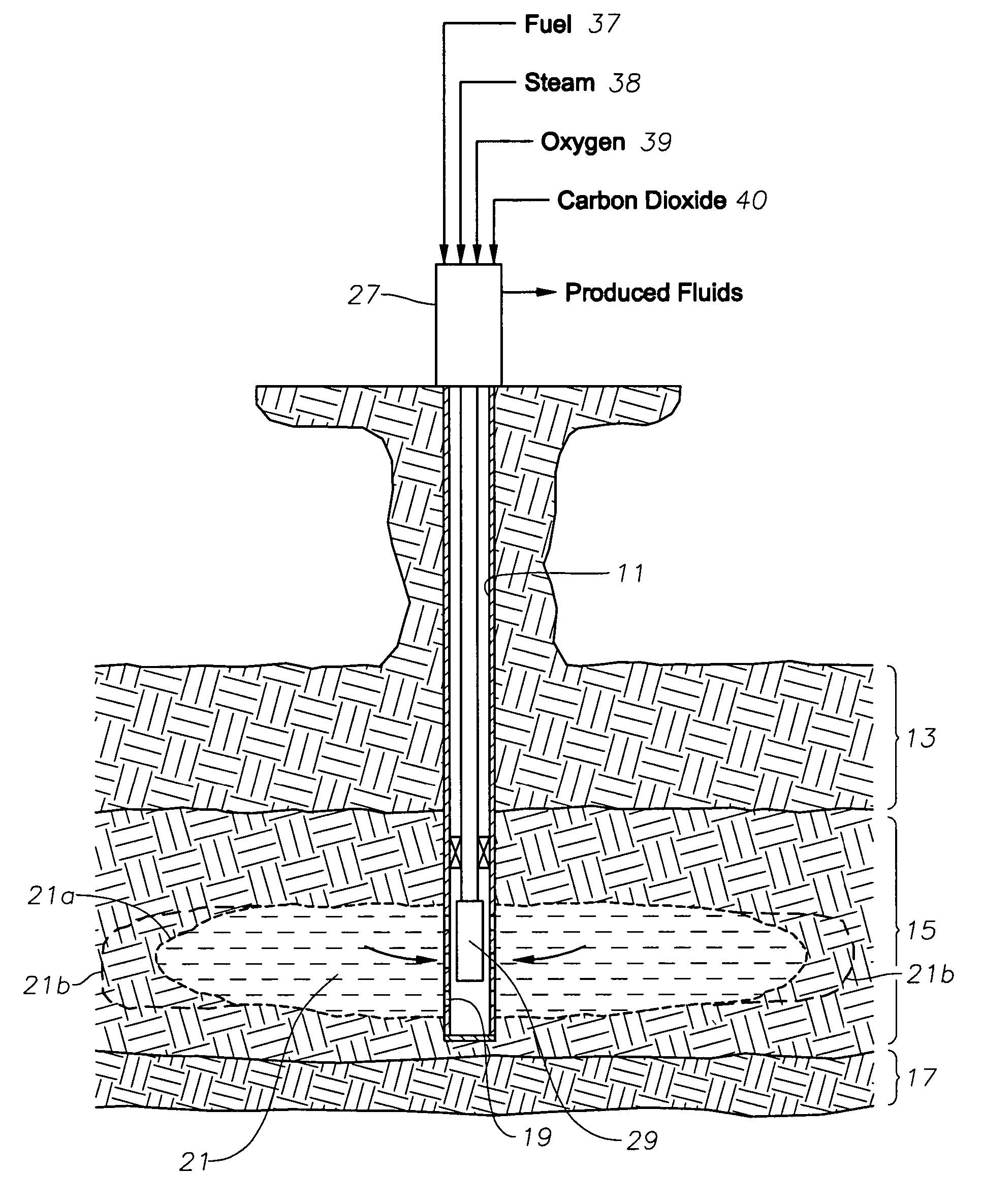
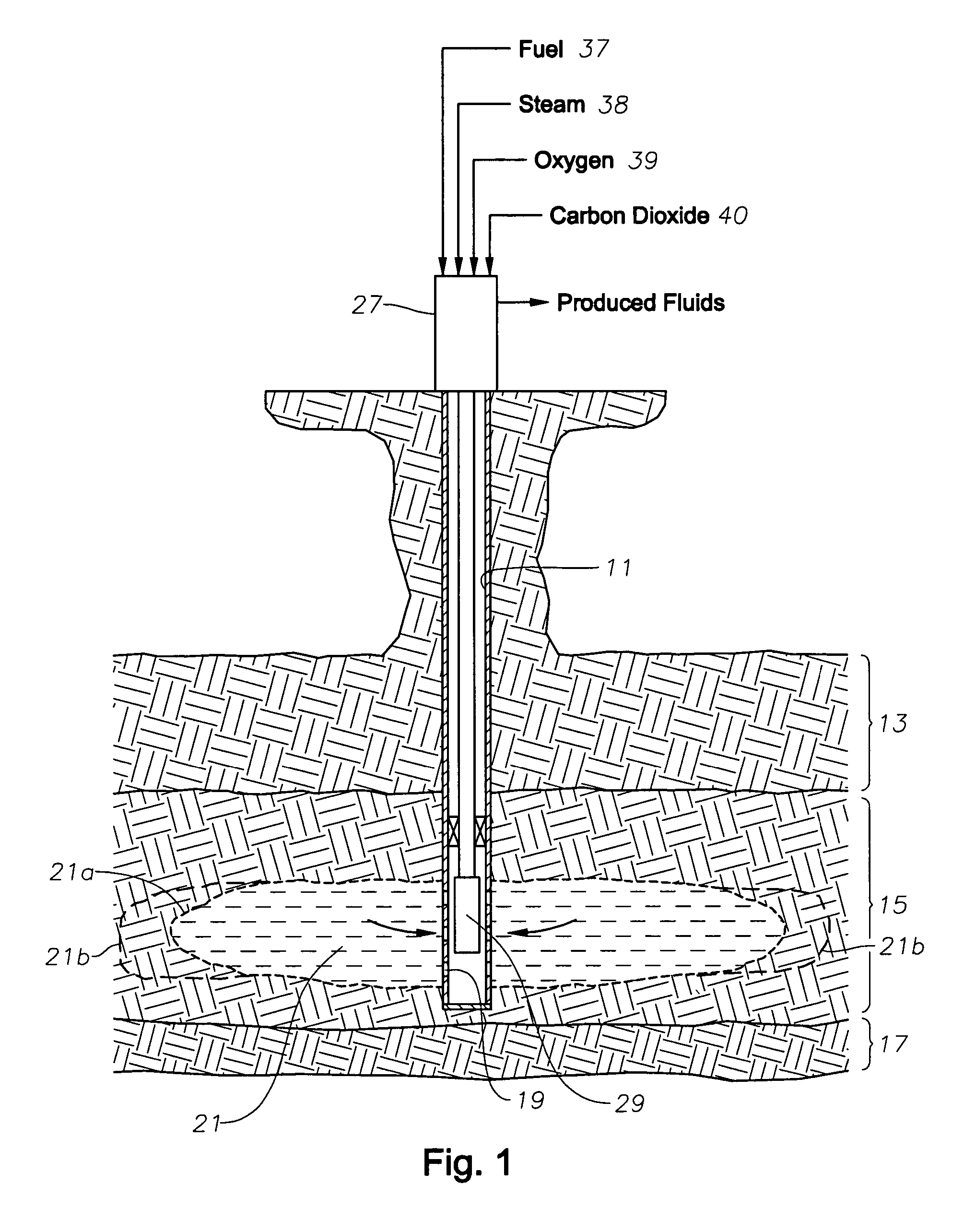
ActiveUS8091625B2Low viscosityIncrease productionInsulationFluid removalCombustorCarbon dioxide production
A downhole burner is used for producing heavy-oil formations. Hydrogen, oxygen, and steam are pumped by separate conduits to the burner, which burns at least part of the hydrogen and forces the combustion products out into the earth formation. The steam cools the burner and becomes superheated steam, which is injected along with the combustion products into the earth formation. Carbon dioxide is also pumped down the well and injected into the formation.
Owner:WORLD ENERGY SYST
Production of fuel materials utilizing waste carbon dioxide and hydrogen from renewable resources
ActiveUS7989507B2Hydroxy compound preparationEmission reduction for energy storageHydrogenCarbon dioxide production
Owner:SIEMENS ENERGY GLOBAL GMBH & CO KG
Method for manufacturing a tube sheet and heat exchanger assembly for a pool reactor or pool condenser; corresponding tube sheet and heat exchanger assembly
ActiveUS20150086440A1Firmly connectedMinimal thermal stressChemical/physical/physico-chemical reactor detailsHeat exchanger casingsCarbon dioxide productionEngineering
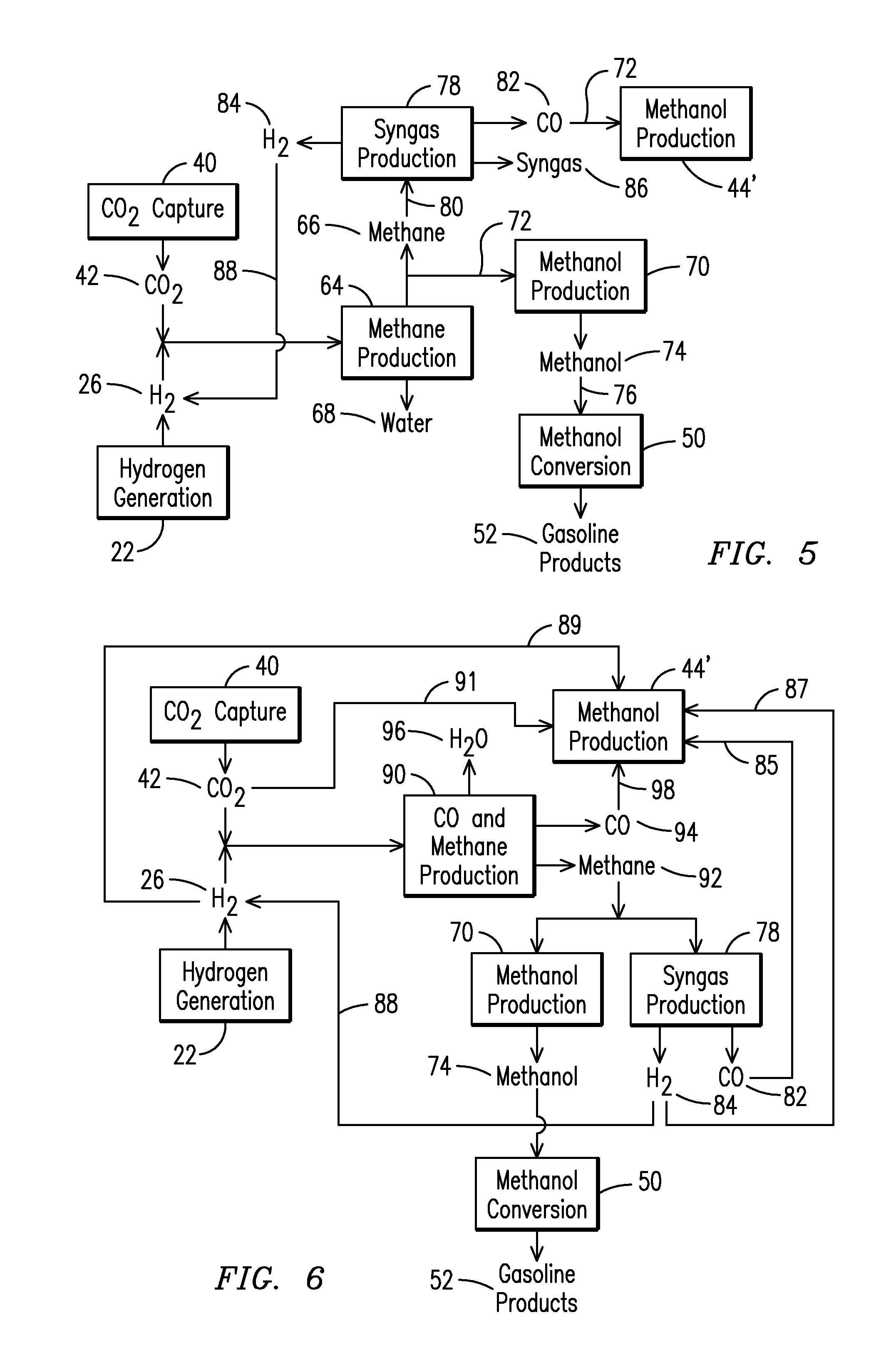
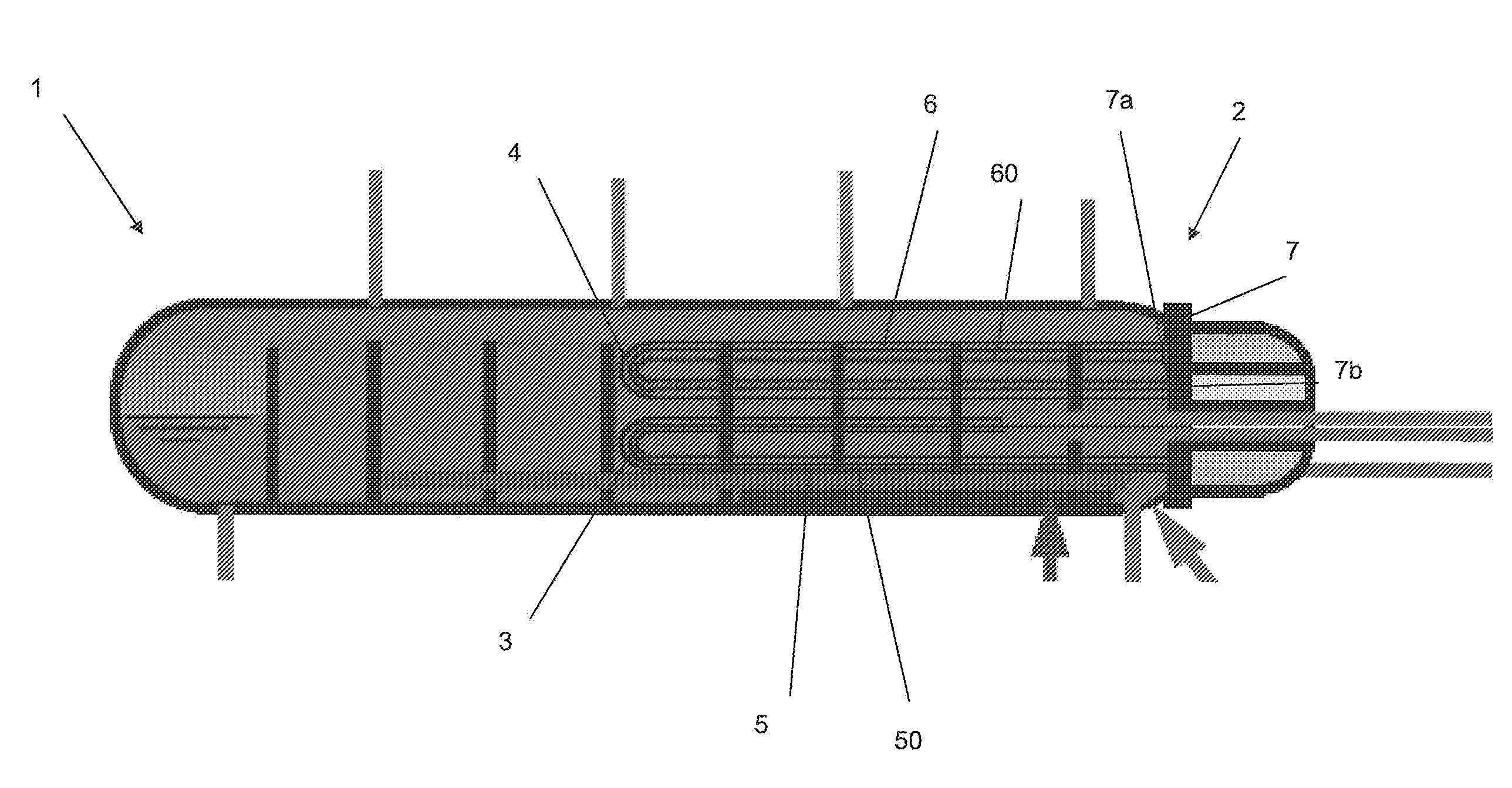
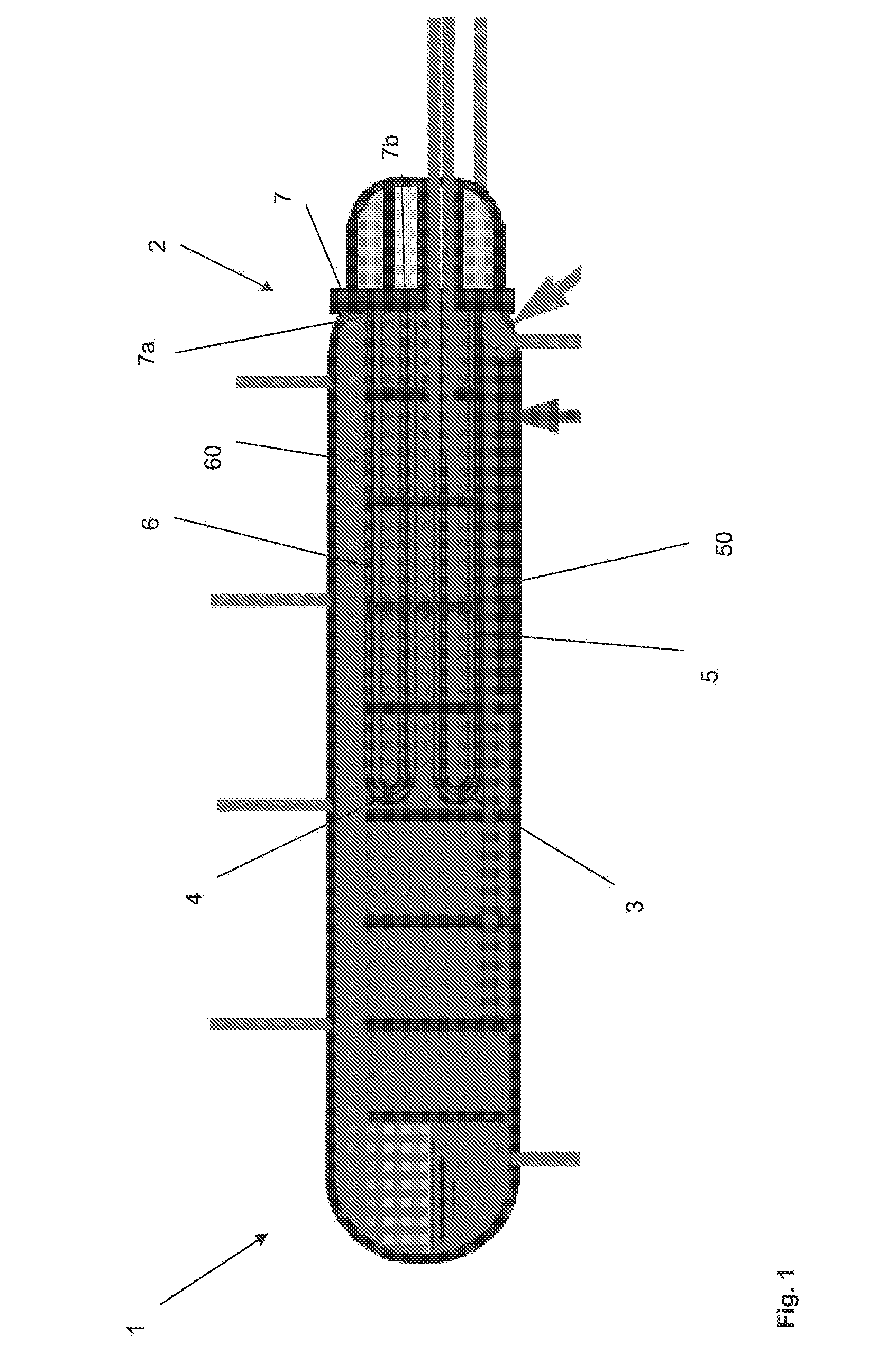
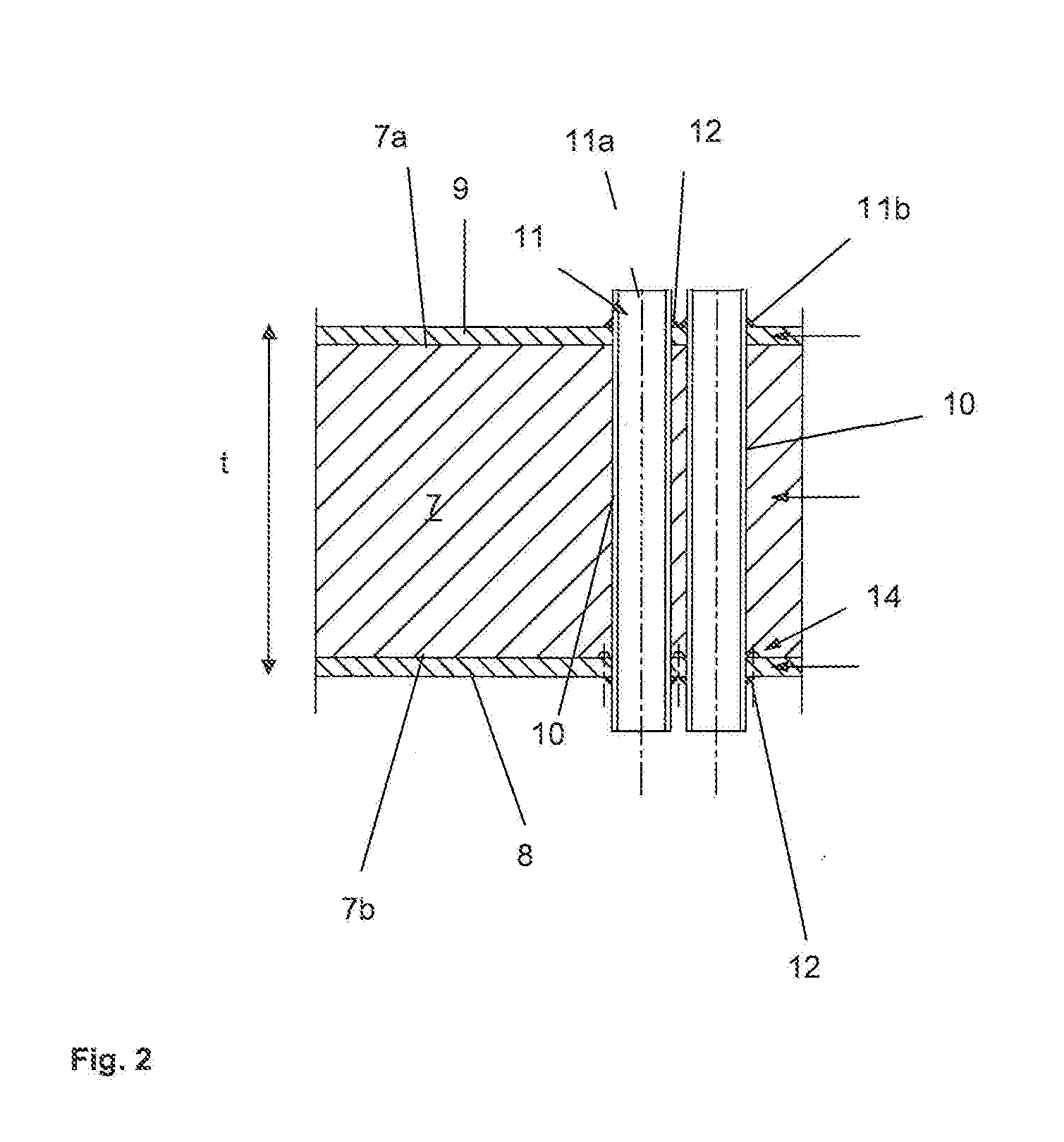
The present application relates to a method of manufacturing a tube sheet (7) and heat exchanger assembly for a pool reactor or pool condenser for use in the production of urea from ammonia and carbon dioxide, wherein the method comprises manufacturing of the tube sheet (7) from a carbon steel material grade and providing said tube sheet (7) with corrosion protective layers (8, 9) of an austenitic- ferritic duplex stainless steel grade, wherein the heat exchanger comprises at least one U-shaped tube (13) of an austenitic-ferritic duplex stainless steel grade, the method further comprises inserting at least two sleeves (11) of an austenitic-ferritic duplex stainless steel grade through the tube sheet (7) such that both ends of the sleeve (11) extend in a direction away from the tube sheet (7), the method further comprises connecting the sleeves (11), at least the opposing ends thereof, to at least the protective layers (8,9) of the tube sheet (7) and finally, connecting both ends of the at least one U-shaped tube (13) to the respective sleeves (11).
Owner:STAMICARBON BV
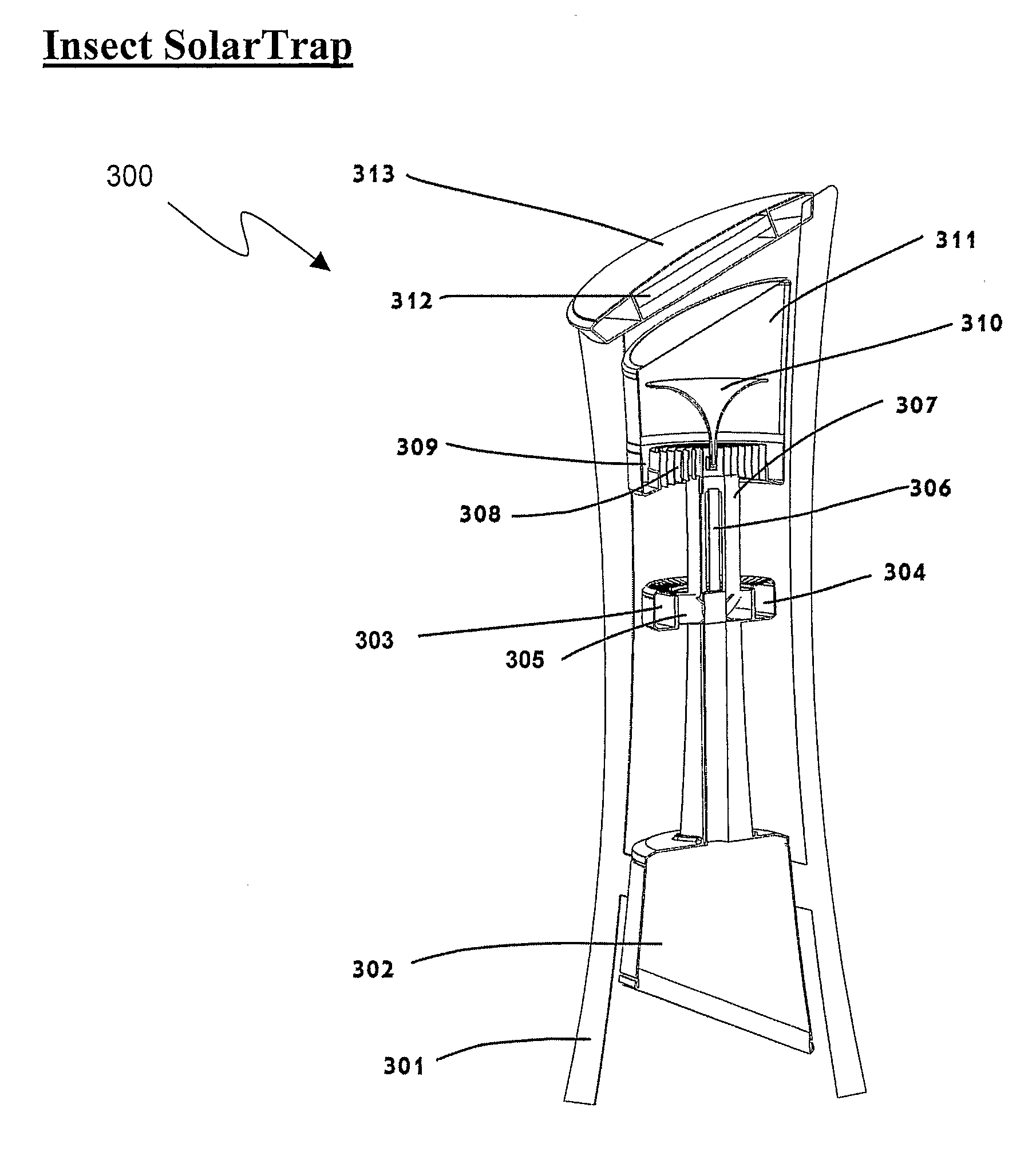
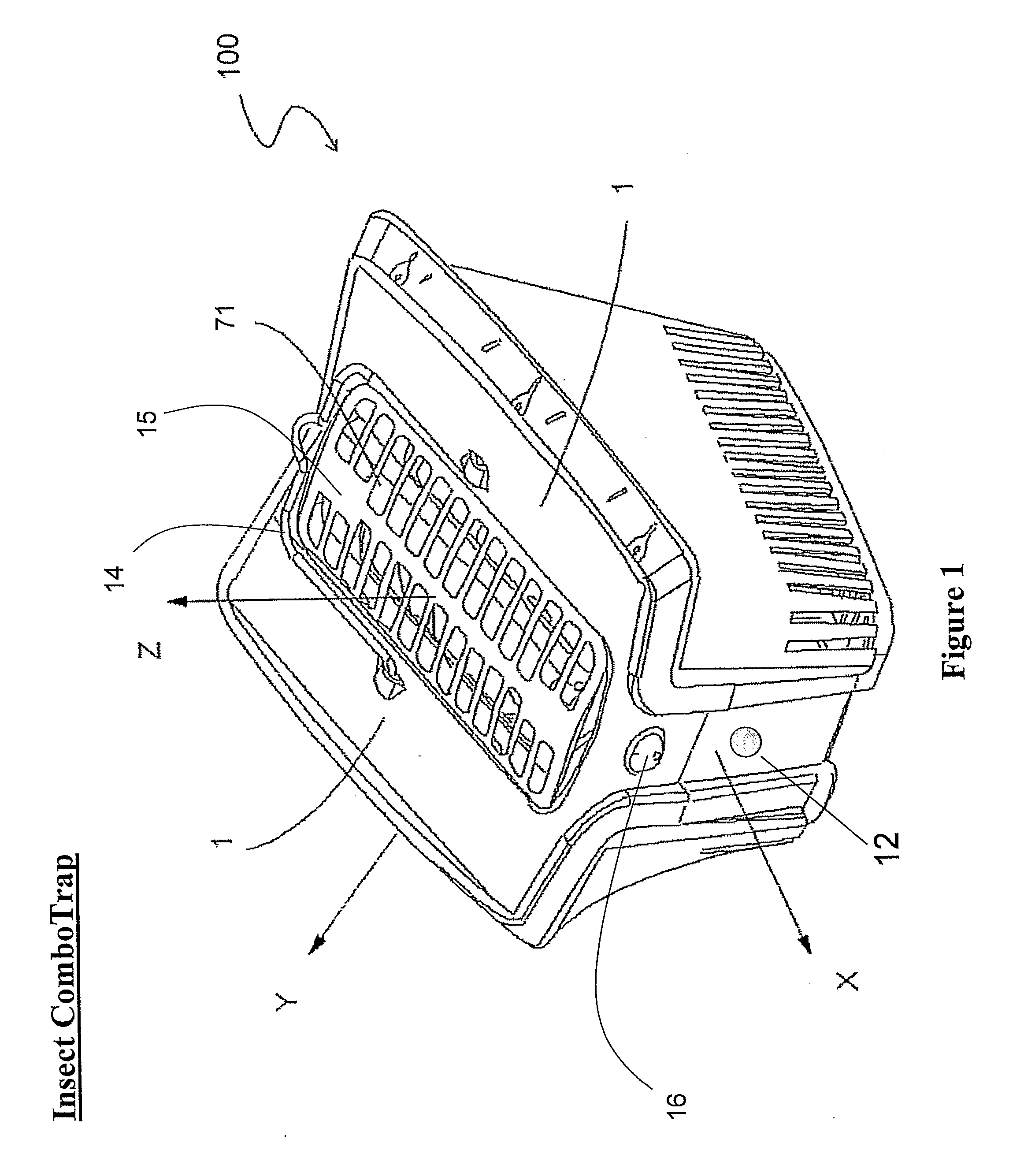
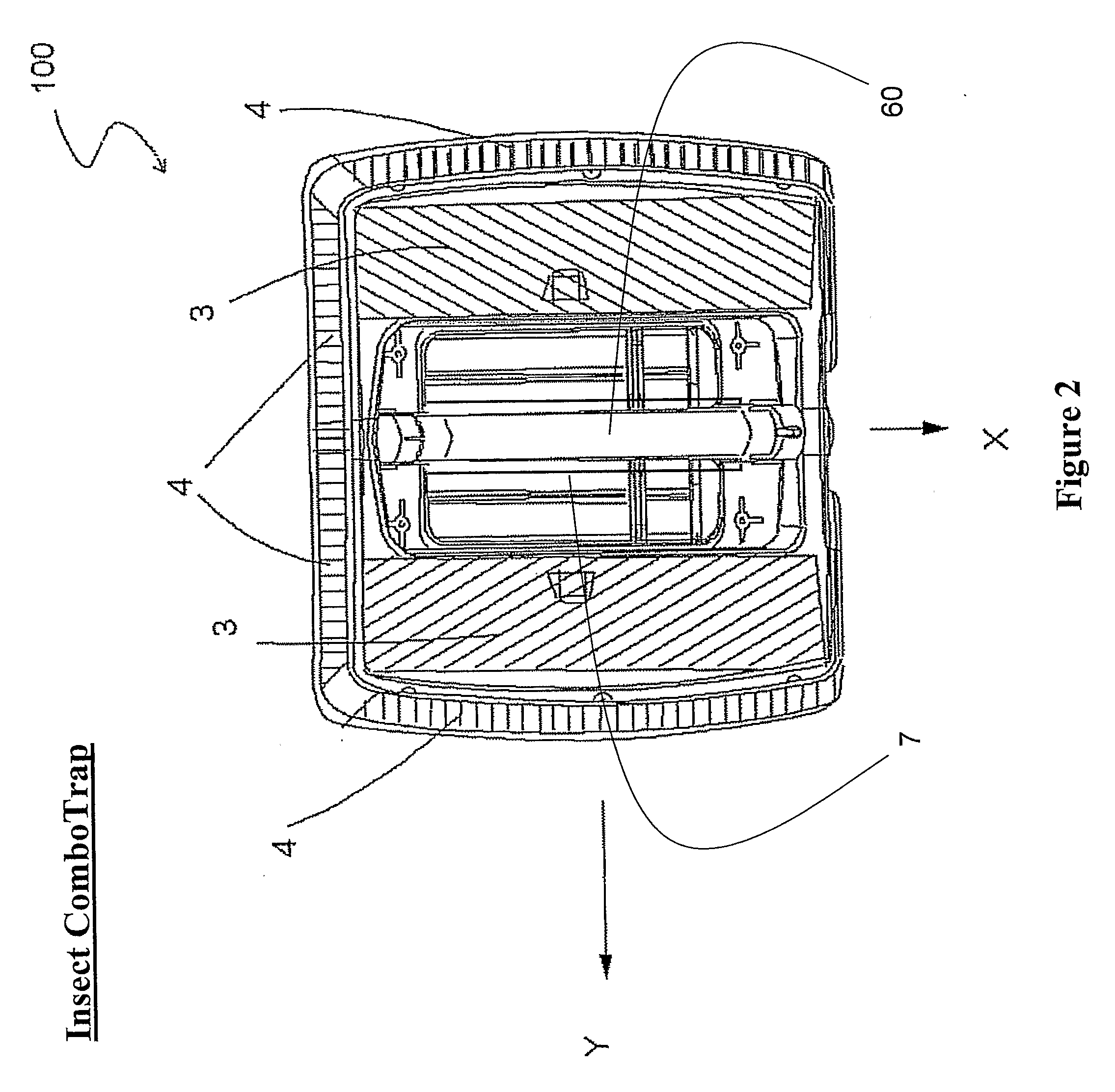
Devices for trapping insects
InactiveUS20100229459A1Eliminate flyImprove abilitiesBiocideElectric shock equipmentsCarbon dioxide productionTrapping
The present invention discloses trapping devices for biting (e.g. mosquitoes, sand flies, black flies, and biting midges) and nuisance flies (e.g. houseflies, filth flies, and fruit flies). The outdoor solution for biting flies includes a solar panel, a housing, a bag, and a ventilator located in the housing. The ventilator creates a capture zone having an airflow toward the bag. A CO2 generator, chemical attractants, a heat source, and a UV light attract mosquitoes to the capture zone. The chemical attractants are released continuously, the CO2 is released in pulses, and the ventilator and UV are operated in independent programmable intervals. The device is efficient in energy consumption and CO2 production. Other trapping devices are disclosed having a combination of insect-attracting mechanisms including a ventilator. Novel insect zappers including a ventilator are disclosed as well. Indoor and outdoor solutions for insects including biting and nuisance flies.
Owner:WESTHAM LTD
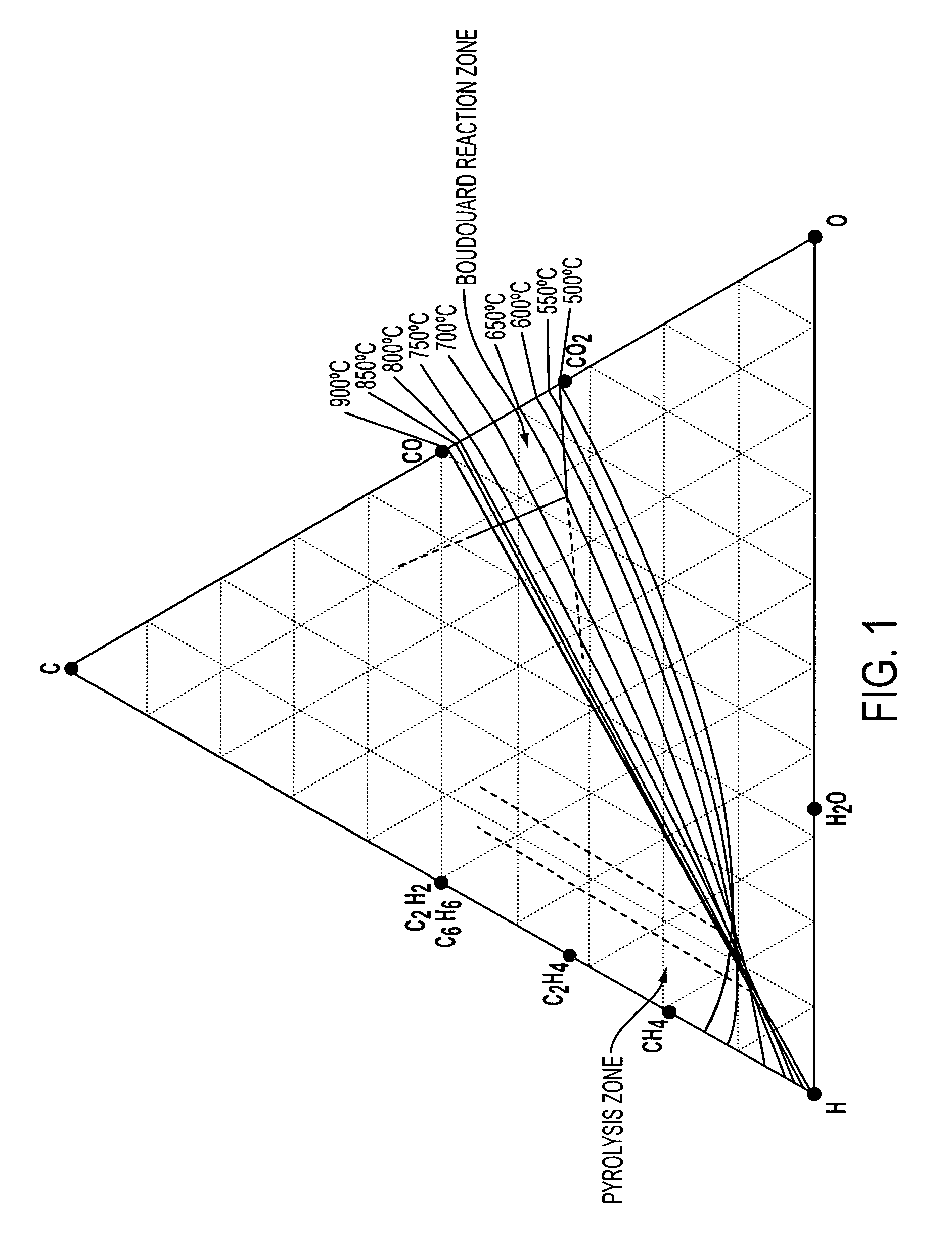
Methods for producing solid carbon by reducing carbon dioxide
ActiveUS20150071846A1Easy to set upLow costMaterial nanotechnologyCarbon nanotubesSolid carbonCarbon dioxide production
A two-stage reaction process includes reacting gaseous carbon dioxide with a reducing agent to form carbon monoxide and water. At least a portion of the water is condensed to form a dry tail gas. The dry tail gas, with the possible addition of a reducing agent, reacts to convert at least a portion of the carbon monoxide to solid carbon and water. Other methods include reacting a feed gas mixture to form a reaction mixture, condensing water from the reaction mixture to form a dried reaction mixture, mixing the dried reaction mixture with a recirculating gas to form a catalytic converter feed gas mixture, flowing the catalytic converter feed gas mixture through a catalytic converter to form solid carbon and a tail gas mixture containing water, and flowing the tail gas mixture through a heat exchanger.
Owner:SEERSTONE
Carbon dioxide sequestration and capture
InactiveUS20100084283A1Calcium/strontium/barium carbonatesCellsCarbon dioxide productionAtmospheric air
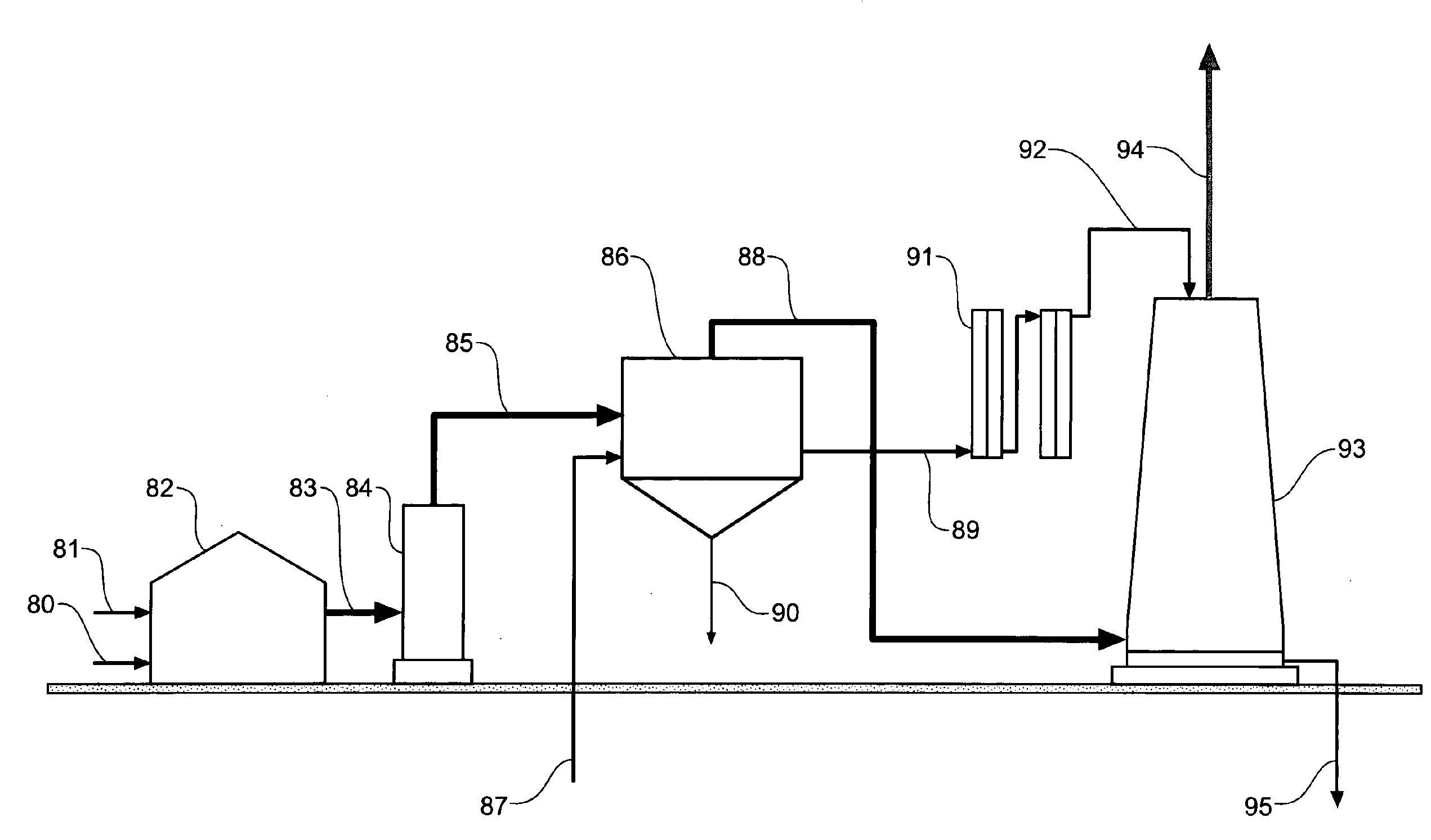
A process to convert carbon dioxide into a stable substance with electrolytically activated seawater and use this process to sequester carbon dioxide from coal power plants (82) and similar carbon dioxide producing equipment, and capture and sequester carbon dioxide from the atmosphere. Electrolytically activated seawater (92) is produced using a unipolar electrolytic cell (91) and is sprayed into a contacting tower (93) or into the air.
Owner:GOMEZ RODOLFO ANTONIO M
Carbon nano-tube production from carbon dioxide
InactiveUS20160023905A1Increase conversion rateMulti-walled nanotubesFibre chemical featuresCarbon dioxide productionCarbon nanotube
Disclosed is a method for making carbon nanotubes comprising (a) reducing a nickel containing catalyst with a reducing agent in a first reaction chamber, (b) contacting the nickel containing catalyst with carbon dioxide under conditions sufficient to produce a reaction product, (c) transferring the reaction product to a second reaction chamber, wherein the second reaction chamber comprises a Group VIII metal containing catalyst, and (d) contacting the Group VIII metal containing catalyst with the reaction product under conditions sufficient to produce carbon nanotubes, wherein the first and second reaction chambers are in flow connection during the transfer step (c), wherein the only source of carbon used to form the carbon nanotubes is from the carbon dioxide used in step (b), and wherein at least 20% of the carbon from the carbon dioxide used in step (b) is converted into carbon nanotubes.
Owner:SAUDI BASIC IND CORP SA
Method for reducing carbon dioxide discharge amount in food-grade liquid carbon dioxide product production
The invention relates to a carbon dioxide production method and especially relates to a method for reducing a carbon dioxide discharge amount in food-grade liquid carbon dioxide product production. The method comprises the following steps of 1, a denitrification process, 2, a first compression process, 3, a desulfuration process, 4, a dealkylation process, 5, a purification process, 6, a second compression process, 7, a liquefaction process, and 8, a distillation purification process. The method can greatly reduce a carbon dioxide discharge amount.
Owner:ANQING KAIMEITE GAS CO LTD
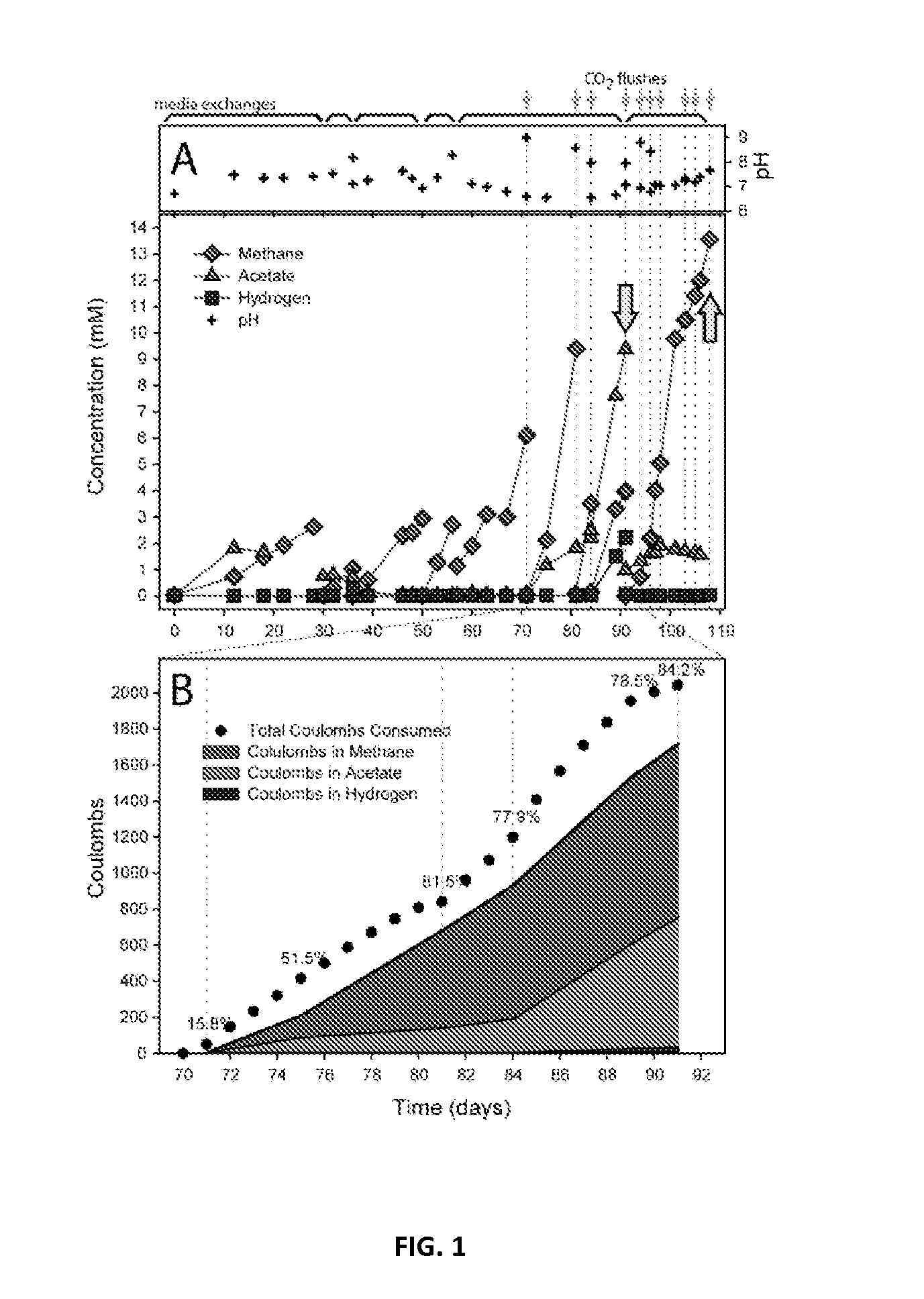
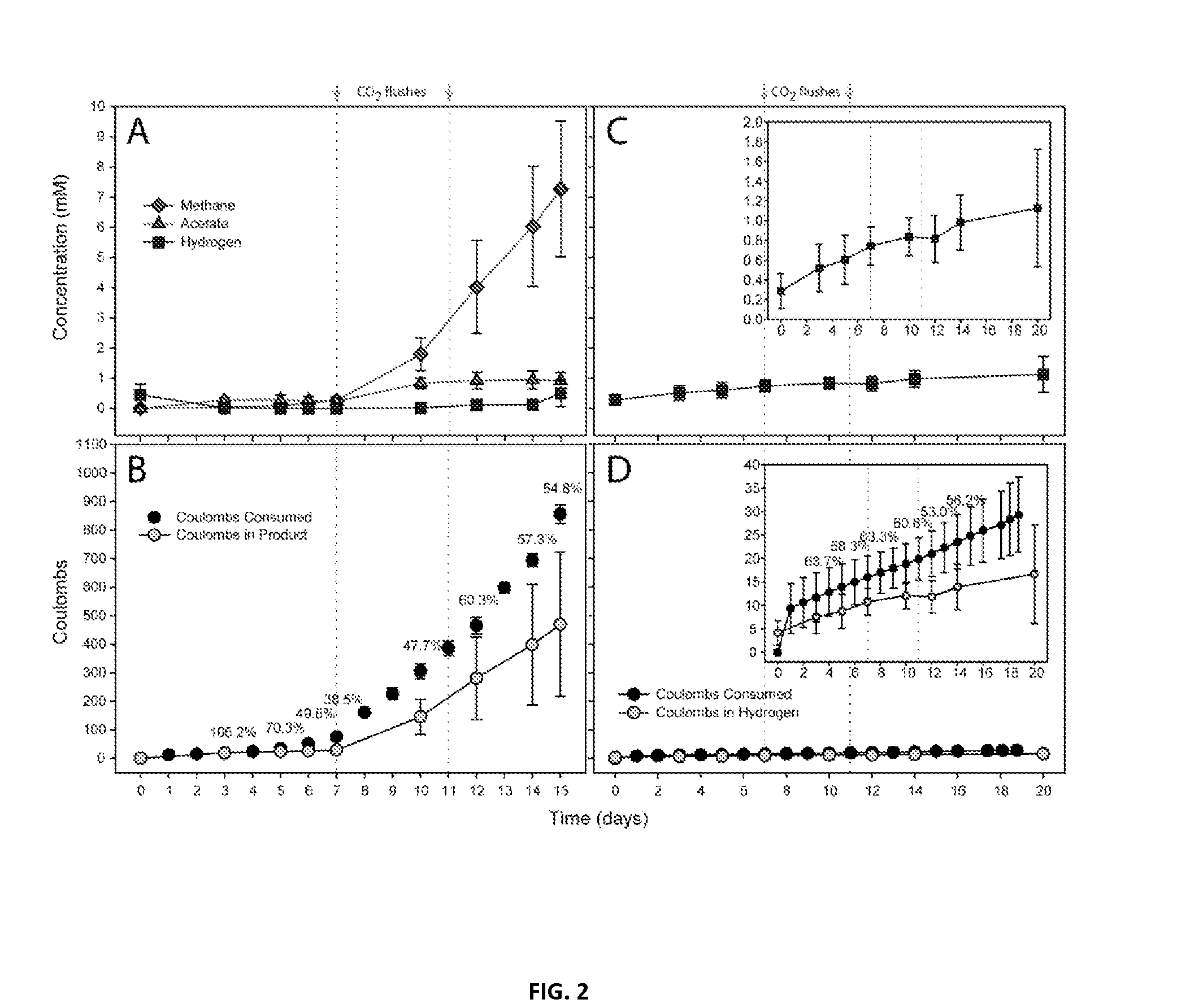
Microbial electrosynthetic cells
InactiveUS20150259669A1Increase productionReduce methane productionElectrolysis componentsBacteriaCarbon dioxide productionMicrobial electrosynthesis
Methods are provided for microbial electrosynthesis of H2 and organic compounds such as methane and acetate. Method of producing mature electrosynthetic microbial populations by continuous culture is also provided. Microbial populations produced in accordance with the embodiments as shown to efficiently synthesize H2, methane and acetate in the presence of CO2 and a voltage potential. The production of biodegradable and renewable plastics from electricity and carbon dioxide is also disclosed.
Owner:MUSC FOUND FOR RES DEV
Combined production device of hydrocarbon production through methanol dehydration and hydrogen and carbon dioxide production through methanol reforming
InactiveCN101712883ALow calorific valueHigh calorific valueHydrogenCarbon compoundsGeneration processCarbon dioxide production
The invention relates to a combined production device of hydrocarbon production through methanol dehydration and hydrogen and carbon dioxide production through methanol reforming, which is characterized in that methanol is utilized as raw materials to co-produce petroleum, liquefying gas, hydrogen and carbon dioxide; heat generated during hydrocarbon production through methanol dehydration is used for a methanol reforming process; water generated during the hydrocarbon production through the methanol dehydration is used for the methanol reforming process; hydrogen generated by reforming can be used for a thermoelectric device and the like, and generated carbon dioxide is recovered and can be used for producing products such as food grade carbon dioxide and the like. The raw material methanol has rich sources. Petroleum and liquefying gas products satisfy the standards of clean fuel and has large product market demands. The hydrogen generated by a methanol reforming device is used as fuel and can realize near zero carbon dioxide emission in a thermoelectricity generation process.
Owner:国科瑞德(北京)能源科技发展有限公司
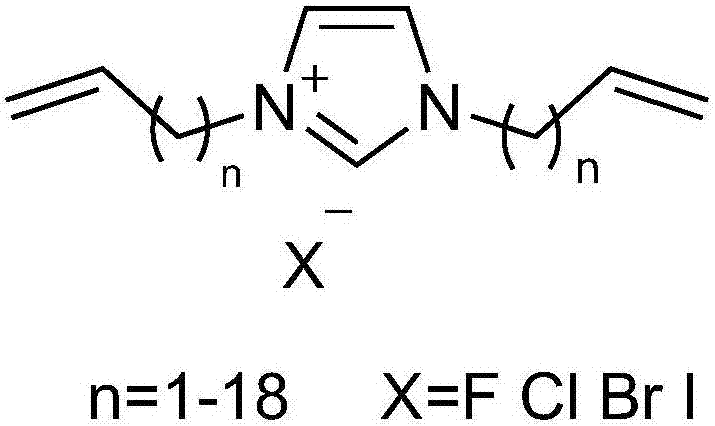
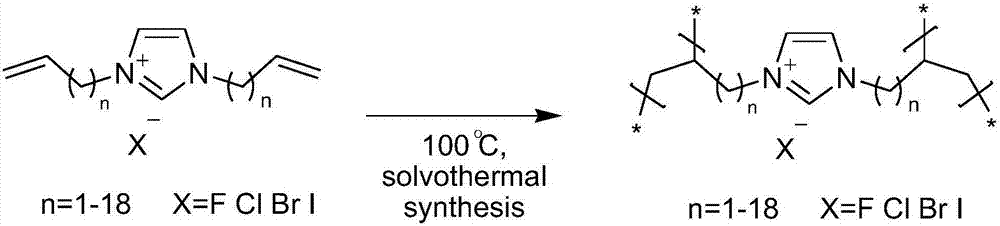
Imidazole salt organic polymer catalyst, preparation method and applications thereof
ActiveCN107537575AImprove stabilityImprove applicabilityProductsOrganic chemistryCross-linkCarbon dioxide production
The present invention relates to an imidazole salt organic polymer catalyst, a preparation method and applications thereof, wherein an alkylene functionalized imidazole salt compound is subjected to self-polymerization or is subjected mixed polymerization with a cross-linking agent to form an imidazole salt organic polymer, a Lewis acid is added or is not added to the obtained imidazole salt organic polymer, and when the Lewis acid is added, the metal ions in the acid and the N and / or P in the organic polymer are subjected to coordination so as to obtain the imidazole salt organic polymer catalyst. According to the present invention, the catalyst can be used in fixed beds, slurry beds, tank reactors, trickle beds and other reactors; with the application of the catalyst of the present invention in the cyclic carbonate production reaction of epoxy compounds and carbon dioxide, the catalyst has high activity, the generated cyclic carbonate has good selectivity, and the applicability of the substrate is strong; and the catalyst has good stability, and the separation of the catalyst from the and the reactant and the product is simple and efficient.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Methods for producing solid carbon by reducing carbon dioxide
ActiveUS9090472B2Easy to set upLow costMaterial nanotechnologyCarbon nanotubesSolid carbonCarbon dioxide production
Owner:SEERSTONE
Process and catalyst system for the production of high quality syngas from light hydrocarbons and carbon dioxide
ActiveUS20150031922A1Improve thermal stabilityImprove the immunityHydrocarbon distillationHydroxy compound preparationSyngasCarbon dioxide production
The present invention describes a process and catalysts for the conversion of a light hydrocarbon and carbon dioxide input stream into high quality syngas with the subsequent conversion of the syngas into fuels or chemicals. In one aspect, the present invention provides an efficient, solid solution catalyst for the production of a carbon containing gas from carbon dioxide and light hydrocarbons. The catalyst comprises a single transition metal, and the transition metal is nickel.
Owner:INFINIUM TECH LLC
Production method and applications of cyclic carbonate
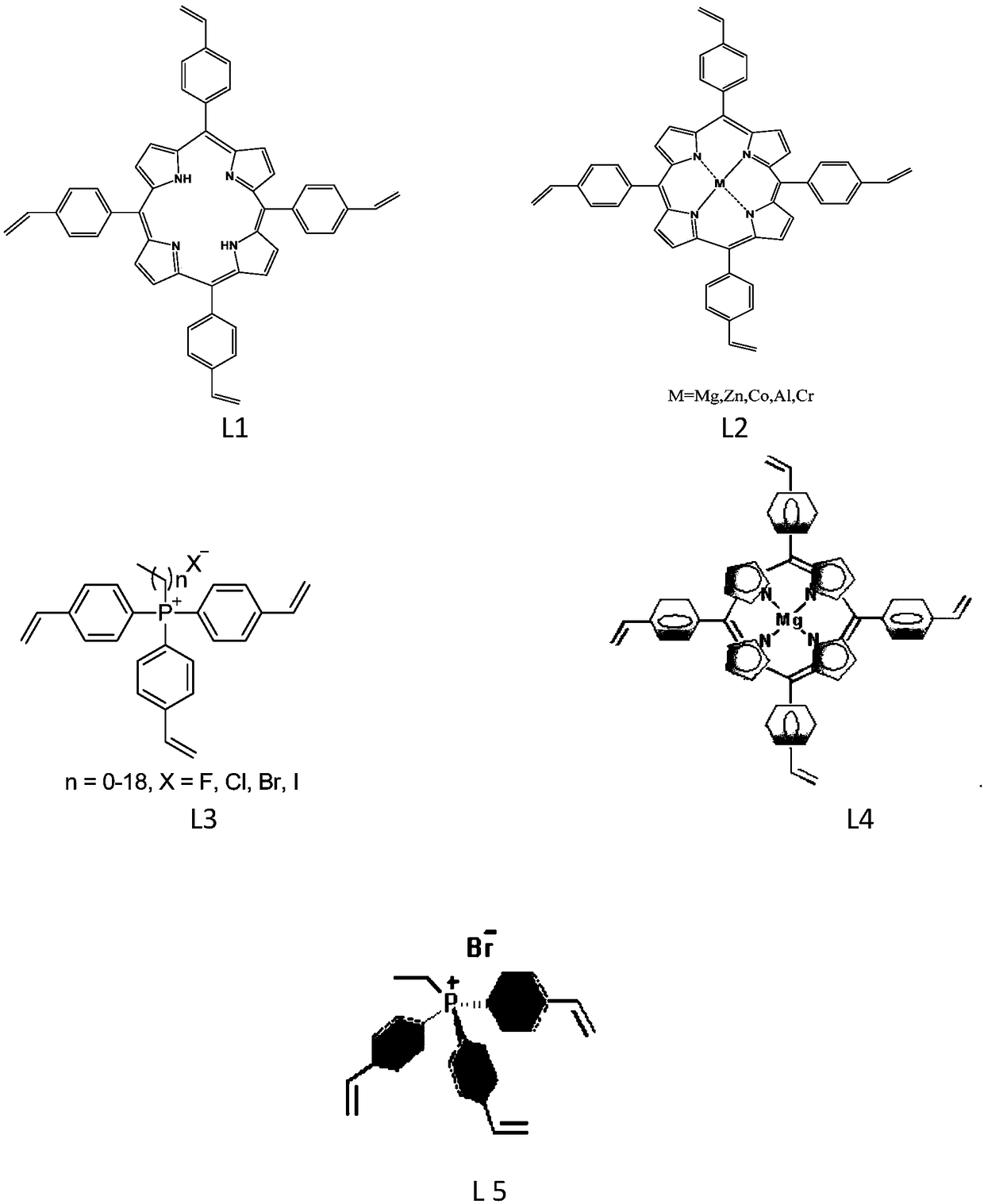
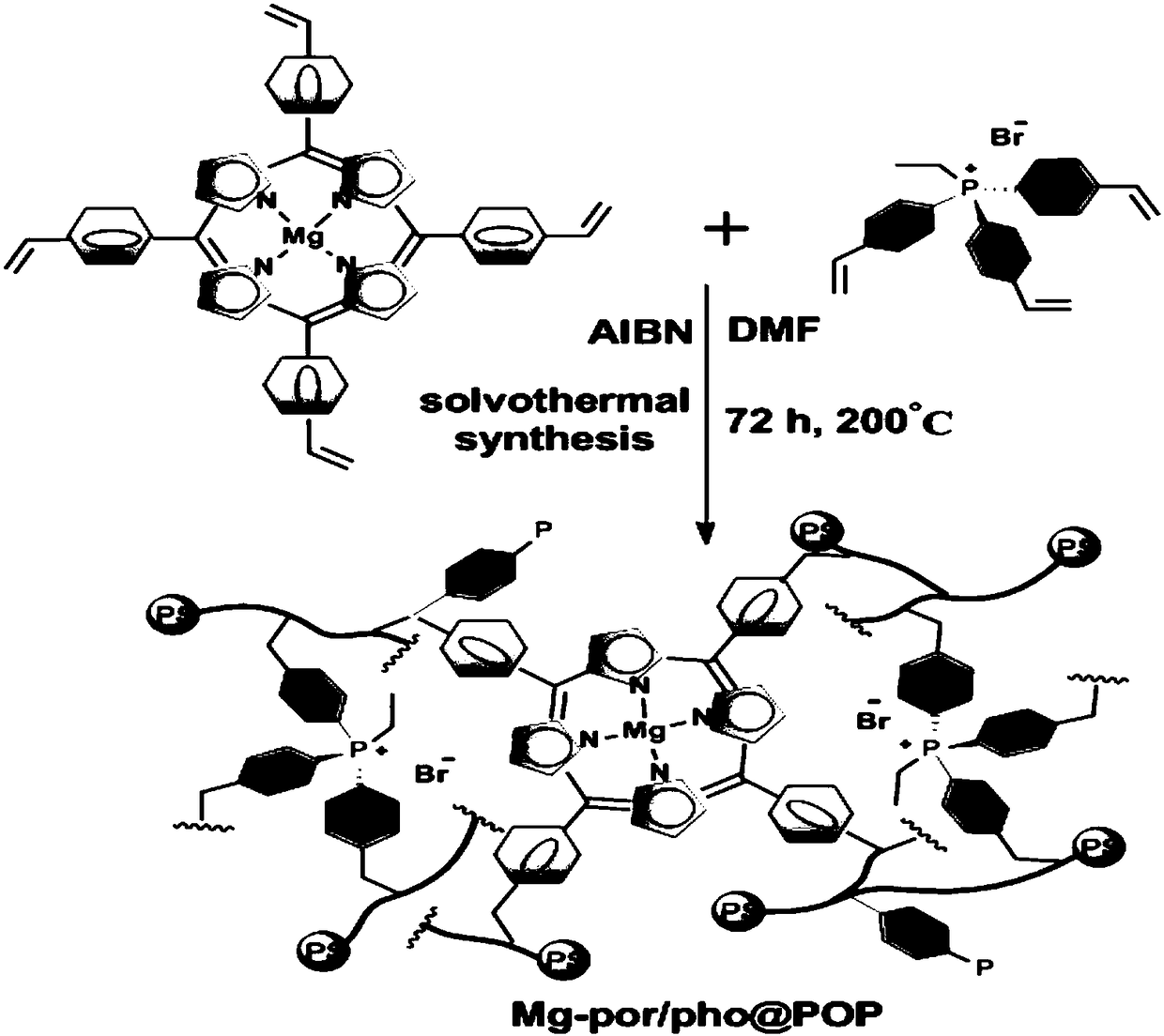
ActiveCN108440485AGood swelling propertiesImprove stabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEpoxyPhosphonium salt
The invention relates to a production method and applications of cyclic carbonate. According to the present invention, the used catalyst is a multifunctional biomimetic heterogeneous catalyst formed by an organic porous copolymer based on metalloporphyrin and a quaternary phosphonium salt, the catalyst carrier is formed by carrying out mixed polymerization on alkylene-functionalized metalloporphyrin and a quaternary phosphonium salt, and the active component of the catalyst comprises metalloporphyrin (as a Lewis acid activation epoxy compound) and a quaternary phosphonium salt (as a ring-opening nucleophilic reagent) functional group; the obtained multifunctional biomimetic heterogeneous catalyst can be used for fixed beds, slurry beds, kettle type reactors, trickle beds and other reactors; the obtained multifunctional biomimetic heterogeneous catalyst has good performance, extremely high activity (2000-6000 h<-1>), good selectivity (more than 99%) of the formed cyclic carbonate and strong substrate applicability in the cyclic carbonate production reaction between the epoxy compound and the carbon dioxide, further has good stability, and can be simply and efficiently separated fromthe substrate and the product.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Reducing Carbon Dioxide Production and Increasing Ethanol Yield During Microbial Ethanol Fermentation
InactiveUS20120052542A1Reduce productionMinimization requirementsFungiBacteriaMicroorganismCarbon dioxide production
The present invention provides compositions and methods for producing ethanol wherein the amount of CO2 by-product is reduced during the fermentation process. The invention includes the use of oxidized lignin during the fermentation process.
Owner:ATHENA BIOTECH INC
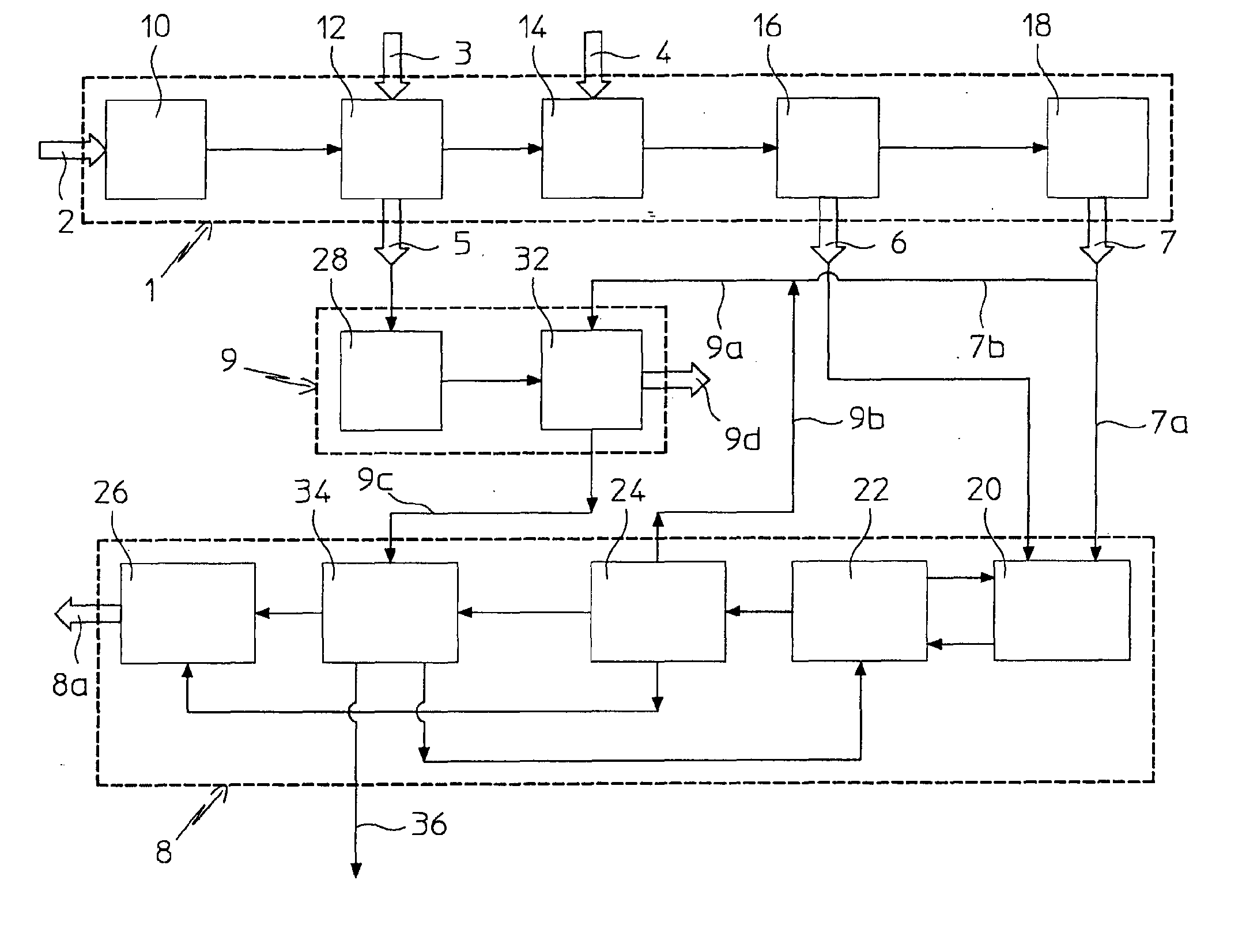
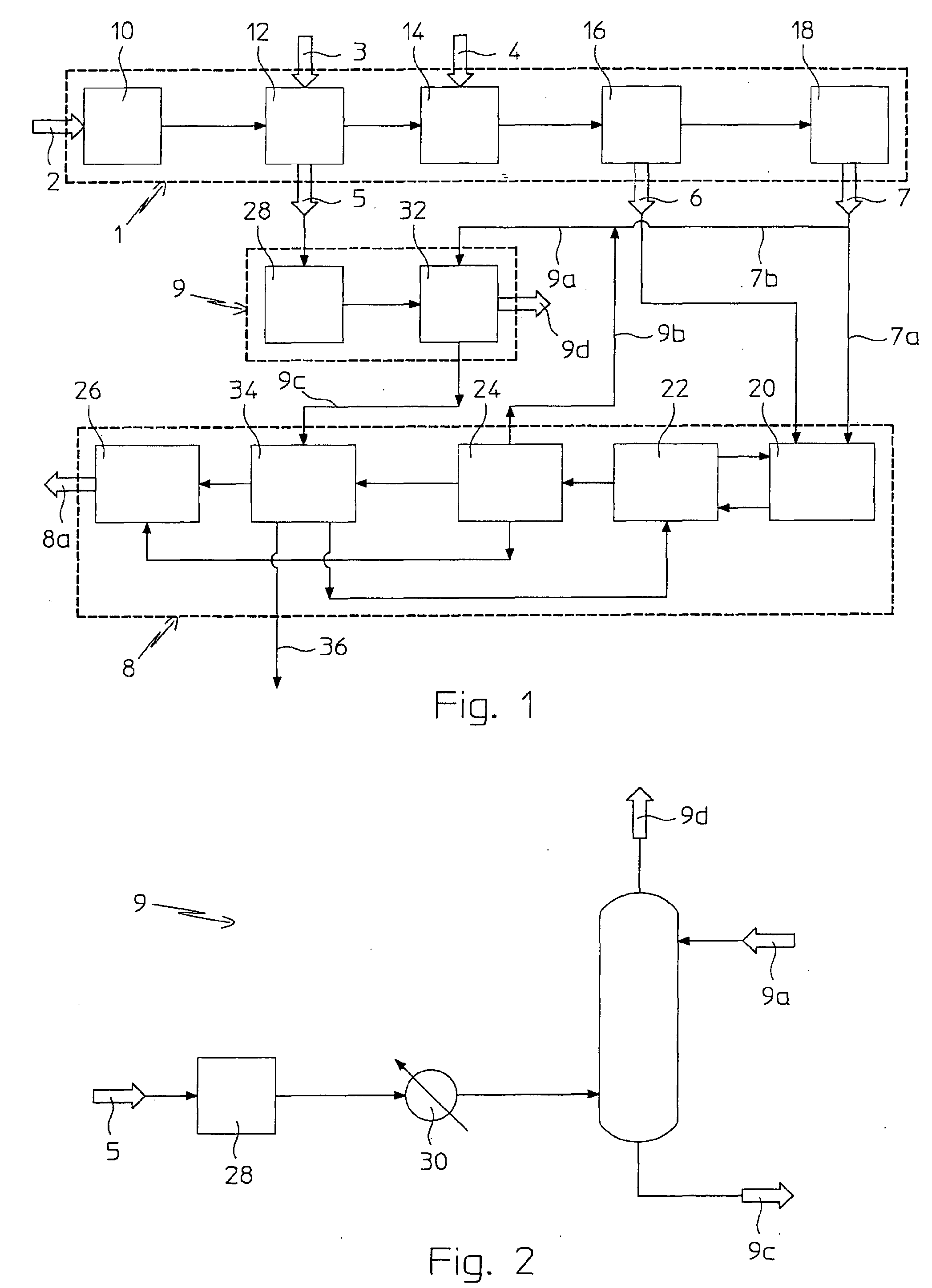
Process for Urea Production from Ammonia and Carbon Dioxide
ActiveUS20070287863A1Urea derivatives preparationOrganic compound preparationCombustionCarbon dioxide production
A process for urea production comprises a first process step in which ammonia (7) and carbon dioxide (6) are obtained, subjecting natural gas (1) to reforming treatments (12, 14), and a second step of urea (8a) production from such ammonia (7) and from carbon dioxide, through a formation of a solution comprising urea and ammonium carbamate in a urea synthesis reactor (20) and a subsequent decomposition of the ammonium carbamate and. urea recovery, the process comprises the steps of:—treating combustion smokes (5) comprising carbon dioxide with an aqueous solution (9a) comprising a part (7b) of such ammonia (7), obtaining an aqueous ammonium carbamate solution (9c);—supplying the solution (9c) thus obtained to the second process step.
Owner:CASALE SA
Production of crystalline sodium bicarbonate using CO2 recovered from another alkali production process
InactiveUS20150175434A1Produced cost-effectivelyCost-effective processBicarbonate preparationAlkali metal sulfite preparationSodium bicarbonateSurge tank
A process for the joint production of crystalline sodium bicarbonate and another alkali compound, in which the step for producing such alkali compound generates CO2 as a byproduct, at least a portion of which is used as a feed to the sodium bicarbonate production step. The produced alkali compound is preferably crystalline sodium sulfite. The joint production process preferably employs as feedstock one or more sodium carbonate liquors derived from trona ore. A gas feed which contains CO2 byproduct is subjected to a gas treatment which may include water removal and / or compression before it is used to produce sodium bicarbonate crystals from a sodium carbonate liquor. Such gas feed may comprise a reactor offgas exiting a sulfite reactor; a vent gas exiting a feed or surge tank; a decarbonation gas exiting a decarbonation unit; a vent gas vented from a crystallizer heater; or combinations of two or more thereof.
Owner:SOLVAY CHEM INC
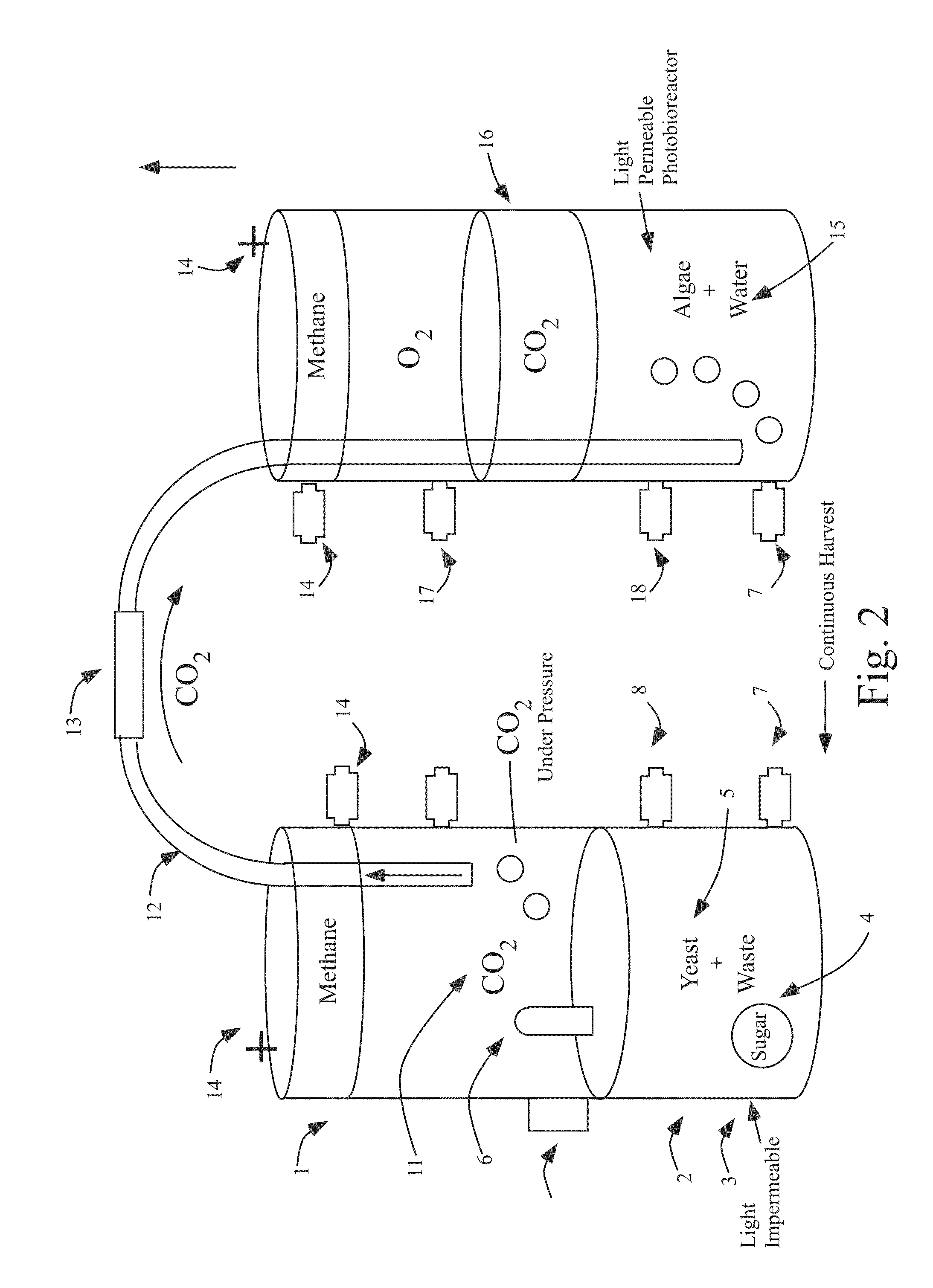
Systems and methods for production of algal biomass
InactiveUS20140199759A1Reduce carbon footprintEasy to set upBioreactor/fermenter combinationsBiological substance pretreatmentsBrickCarbon dioxide production
Self-sustaining, self-contained systems and methods for producing biofuels and for producing biofuel feedstock from algae. The system is carbon neutral or may be carbon positive, fixing more carbon than it releases to the atmosphere. In various embodiments, the system may be coupled to an existing carbon dioxide producing process to reduce or completely eliminate carbon dioxide output, making the existing system carbon neutral, and providing valuable and tradable carbon credits. The system may also comprise modular tiles comprising a biomass sandwiched between two panels and use a combination of microbes, nutrients, water, and sunlight to generate biological hydrocarbon compounds that can be used in almost any type of engine.
Owner:TANTILLUS SYNERGY LTD
Catalysts supported on natural polymers for the production of carbonates from co2
InactiveUS20190193063A1Improve responseImprove efficiencyOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCarbon dioxide productionOrganic base
The present invention describes a process to prepare catalyst systems based on metal salts, supported on natural polymers and co-catalyzed by organic bases, for the catalytic transformation of carbon dioxide to organic carbonates through cycloaddition reactions to epoxides. The advantages of the presented system can be summarized on the use of raw materials of low cost for the preparation of the catalyst system, minimal environmental risk due to the low toxicity of the materials used, in some cases biodegradable such as the natural polymers, as well as high catalytic efficiency, reaching selectivities up to 100% and in some cases quantitative yields.
Owner:INST MEXICANO DEL GASOLINEEO
Method for producing methane by converting carbon dioxide through methanobacterium thermaggregans in activated reservoir
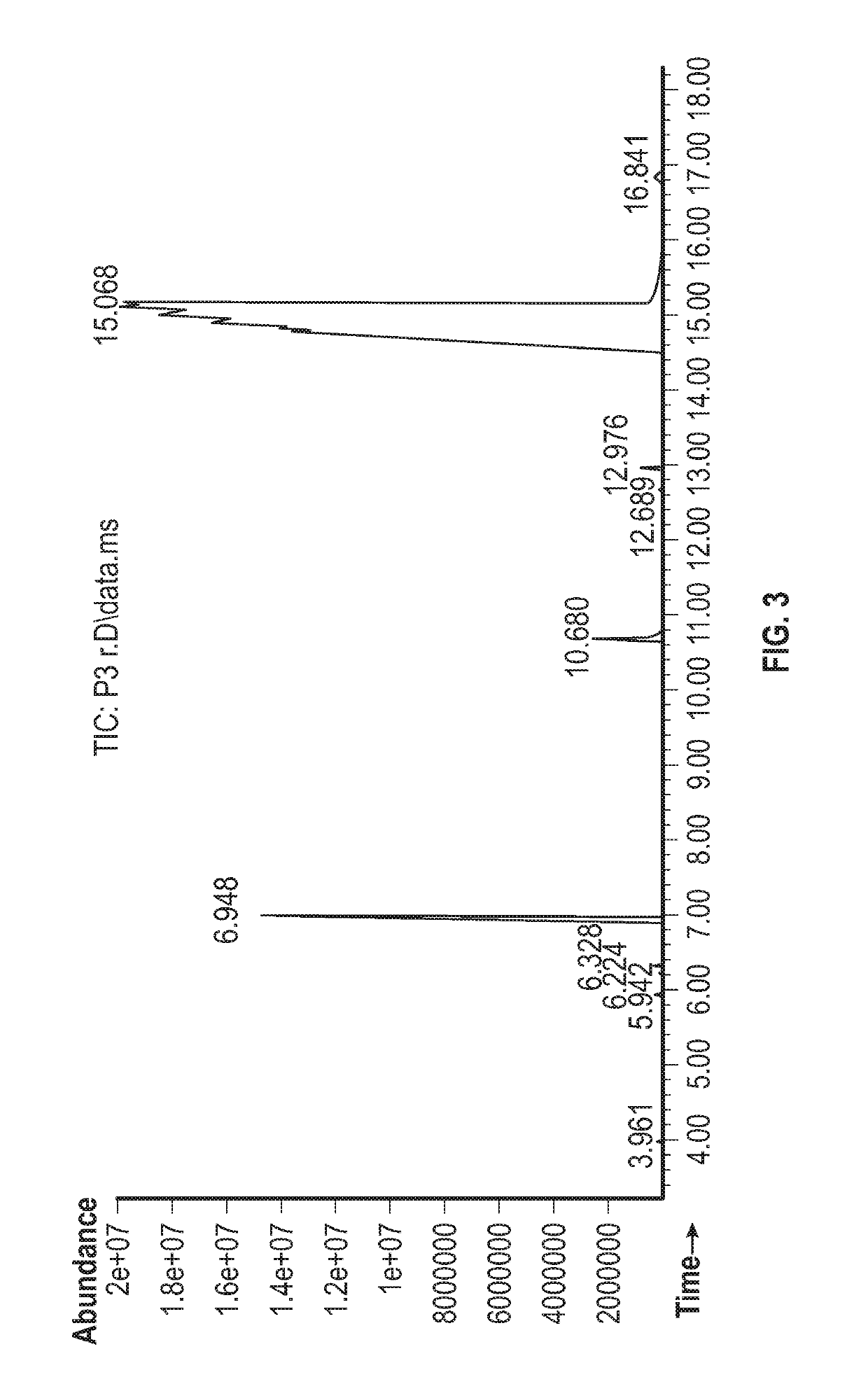
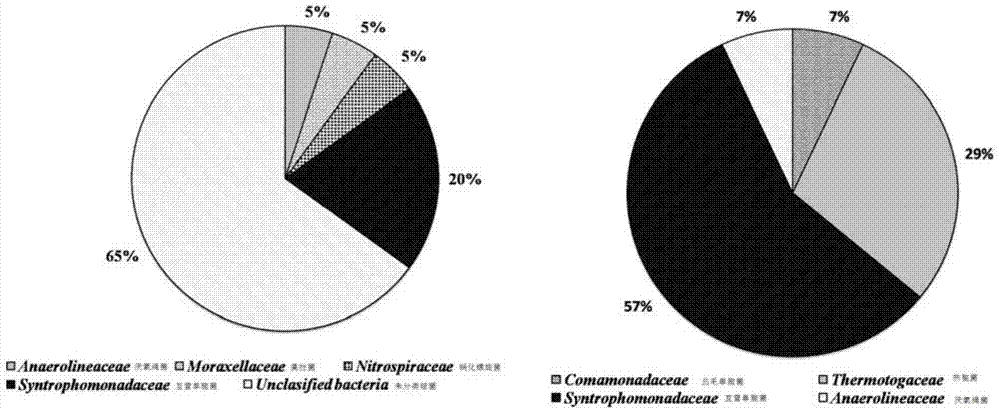
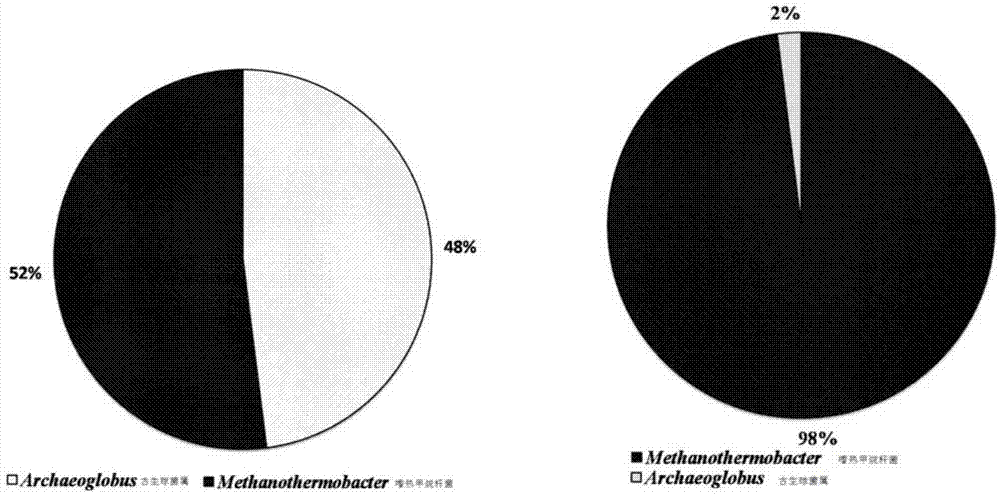
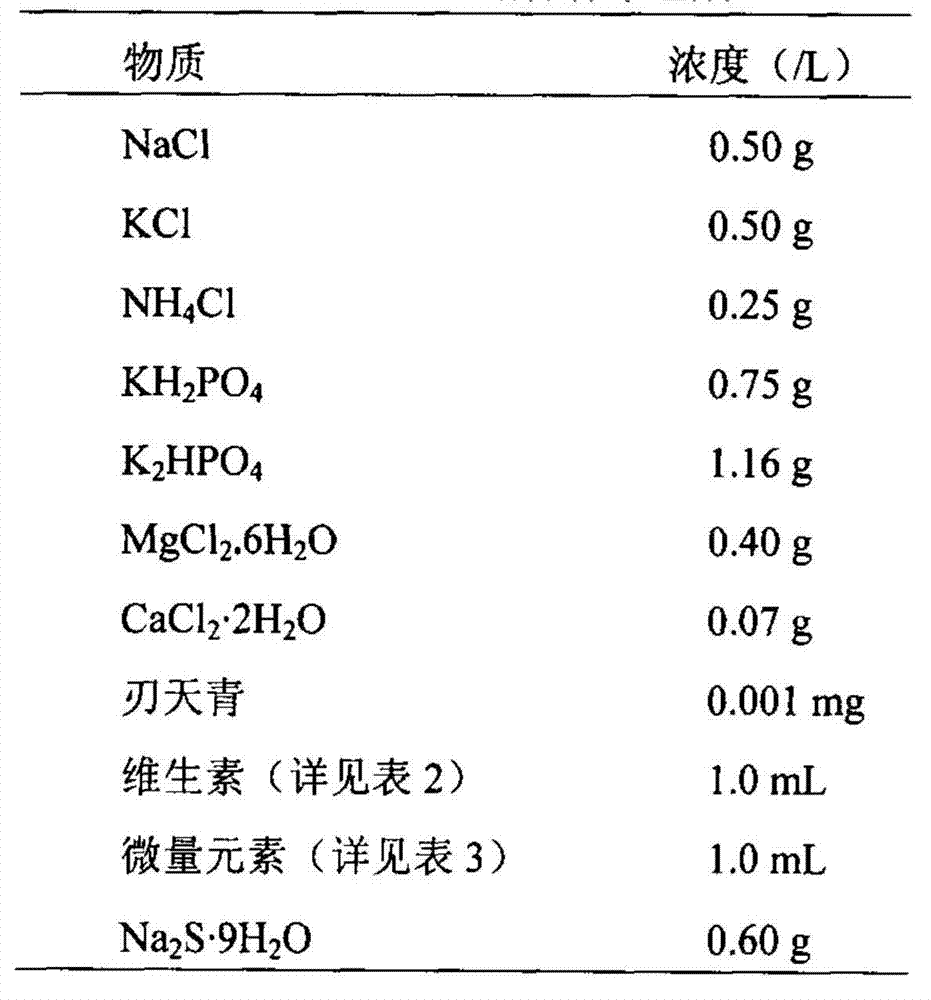
Owner:DAQING HUALI ENERGY BIOLOGICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com