Production method and applications of cyclic carbonate
A production method and technology of cyclocarbonate, which is applied in the field of cycloaddition reaction to prepare cyclocarbonate, can solve the problems of difficult product separation and purification, and achieve the effect of increasing capture capacity, not easy to lose, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
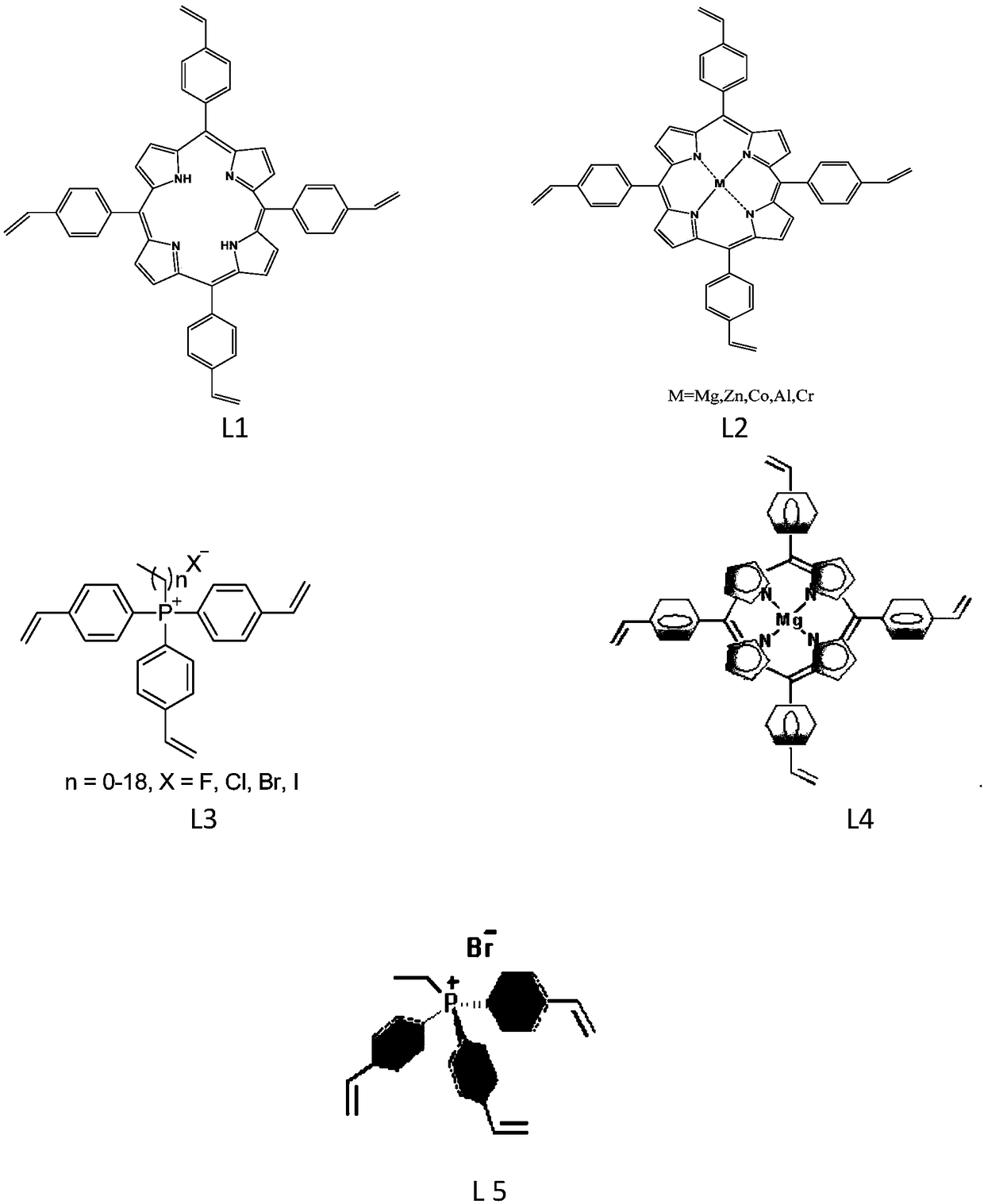
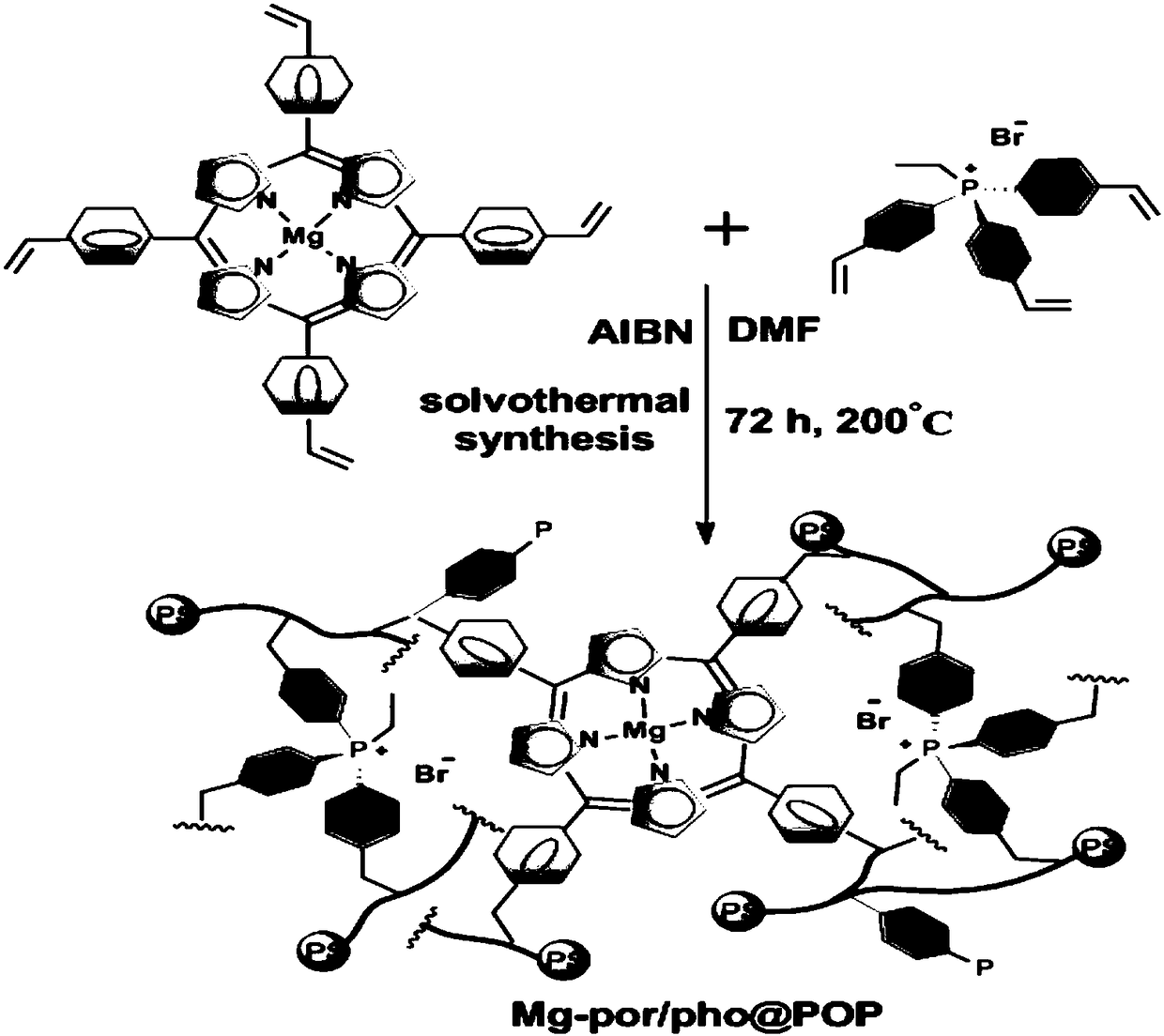
[0034] Under 298K and an inert gas protection atmosphere, 1.0g of tetrastyryl porphyrin monomer was dissolved in 150.0mL of dichloromethane solvent, and 18.1ml of triethylamine and 113mg of MgBr were added 2 ·Et 2 O, stirred for 12h, the mixture was filtered, washed with water, dried, extracted by Soxhlet for 72h, and then vacuum-dried at 40°C for 24h to obtain tetrastyryl functionalized magnesium porphyrin monomer (attached figure 1 , L4). Under 298K and an inert gas protection atmosphere, take 0.1 g of the above-mentioned vinyl functionalized magnesium porphyrin monomer and dissolve it in 12 ml of N,N-dimethylformamide, and then add 1.0 g of ethyl-tris(4-vinylbenzene ) phosphonium bromide (with figure 1 , L5), add 55mg free radical initiator azobisisobutyronitrile to the above solution, stir for 2 hours. The stirred solution was moved to an autoclave, and polymerized by solvothermal polymerization at 473K for 72h. After the above-mentioned polymerized solution is cooled ...
Embodiment 2
[0036] In Example 2, in addition to weighing 20.6mg CoCl 2 ·6H 2 O, alternative metal salt MgBr 2 ·Et 2 O, all the other catalyst synthesis processes are the same as in Example 1.
Embodiment 3
[0038] In embodiment 3, except taking by weighing 17.1mg Zn(OAc) 2 2H 2 O, alternative metal salt MgBr 2 ·Et 2 O, all the other catalyst synthesis processes are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com