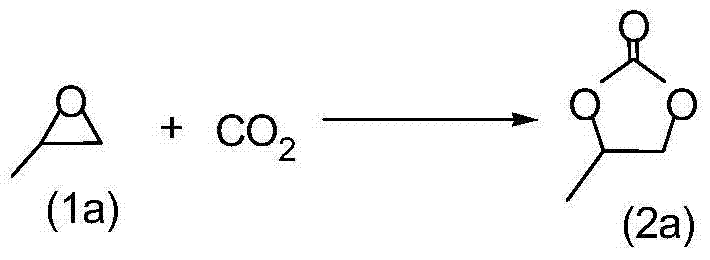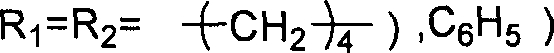Patents
Literature
1183 results about "Cycloaddition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
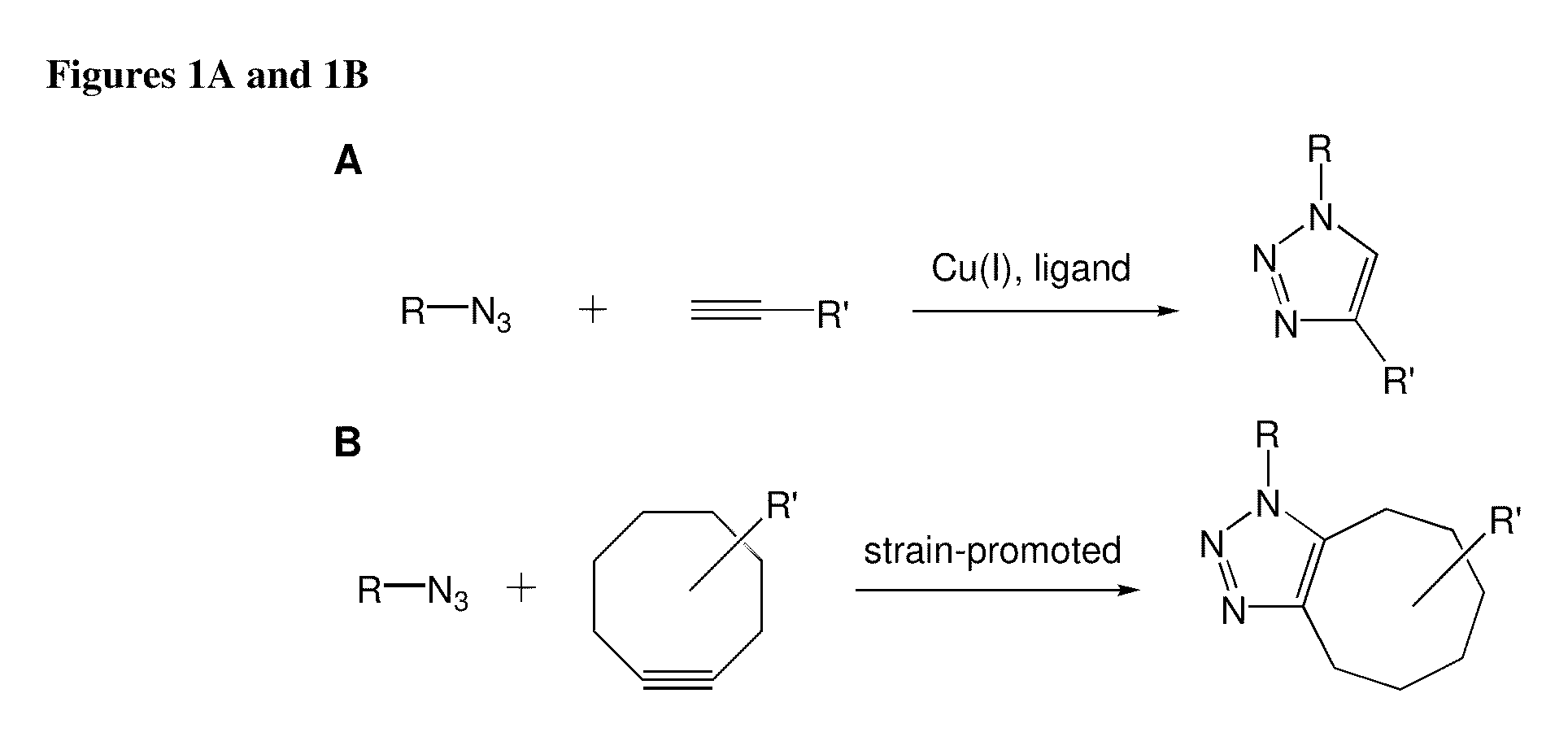
A cycloaddition is a chemical reaction, in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile.
Biomolecular coupling methods using 1,3-dipolar cycloaddition chemistry
InactiveUS20050032081A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBio moleculesCycloaddition
This invention provides methods for covalently affixing a biomolecule to either a second molecule or a solid surface using 1,3-dipolar cycloaddition chemistry. This invention also provides related methods and compositions.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
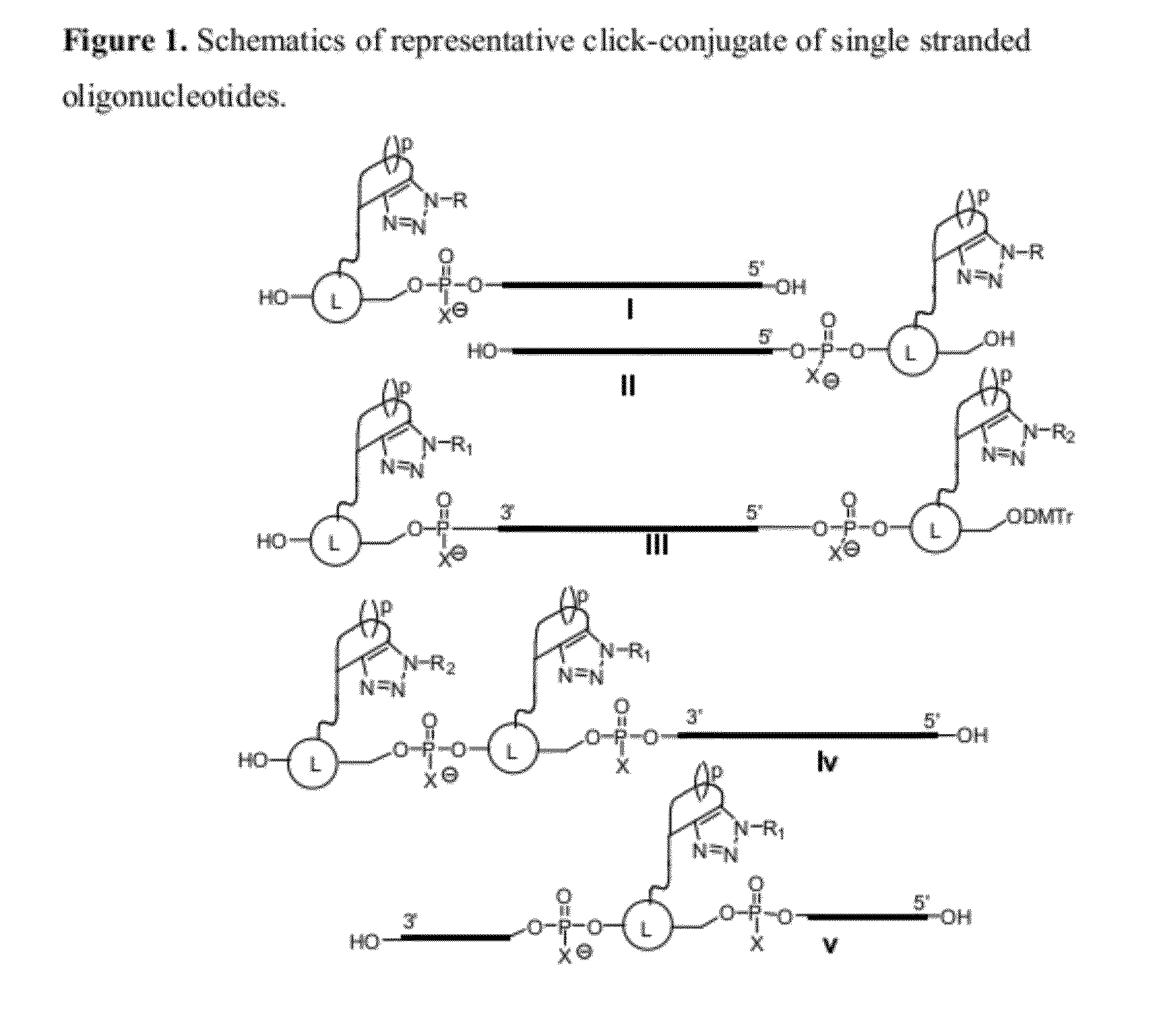
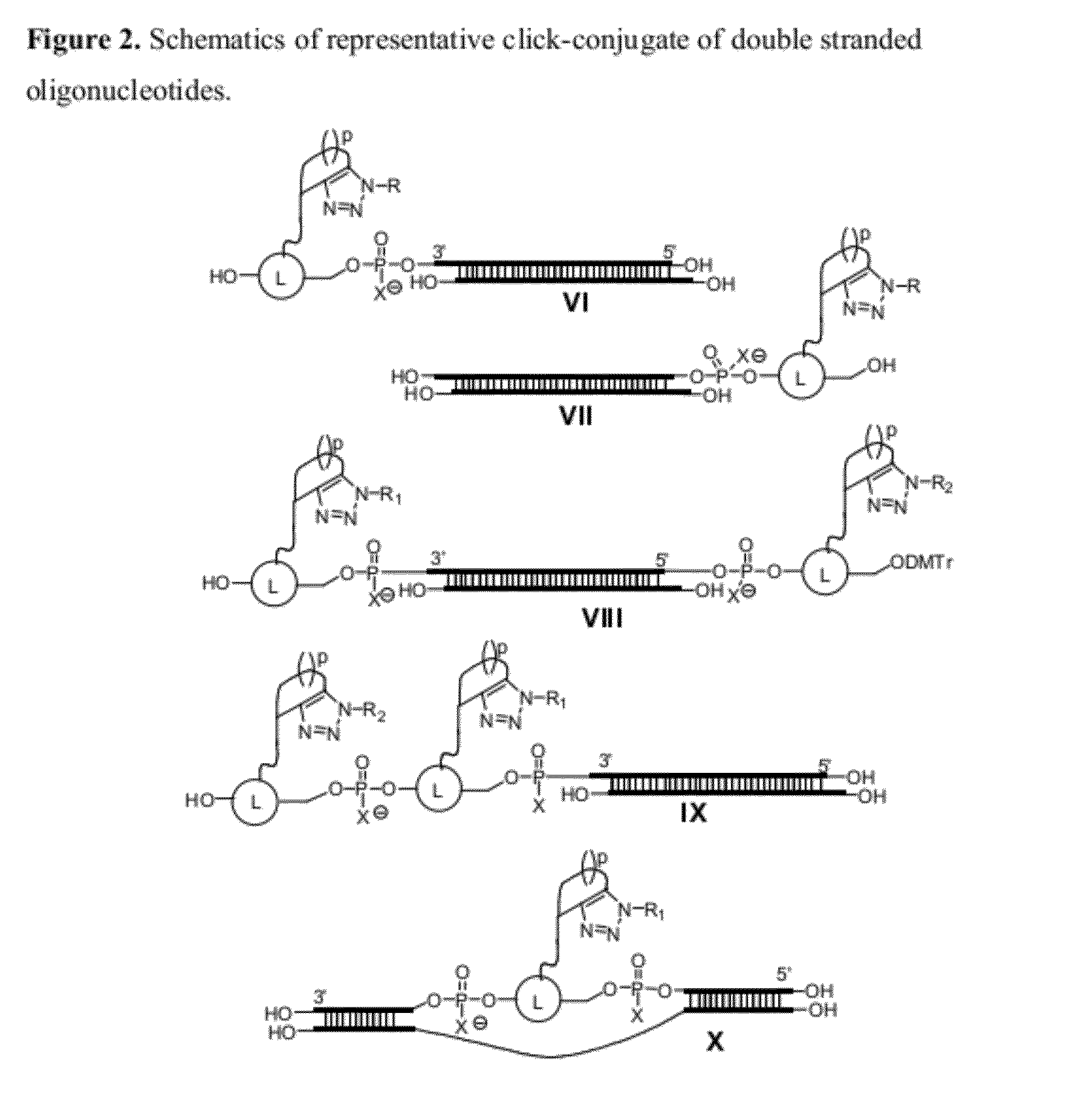
Chemical modifications of monomers and oligonucleotides with cycloaddition
The invention features compounds of formula I or II:In one embodiment, the invention relates compounds and processes for conjugating ligand to oligonucleotide. The invention further relates to methods for treating various disorders and diseases such as viral infections, bacterial infections, parasitic infections, cancers, allergies, autoimmune diseases, immunodeficiencies and immunosuppression.
Owner:ALNYLAM PHARMA INC
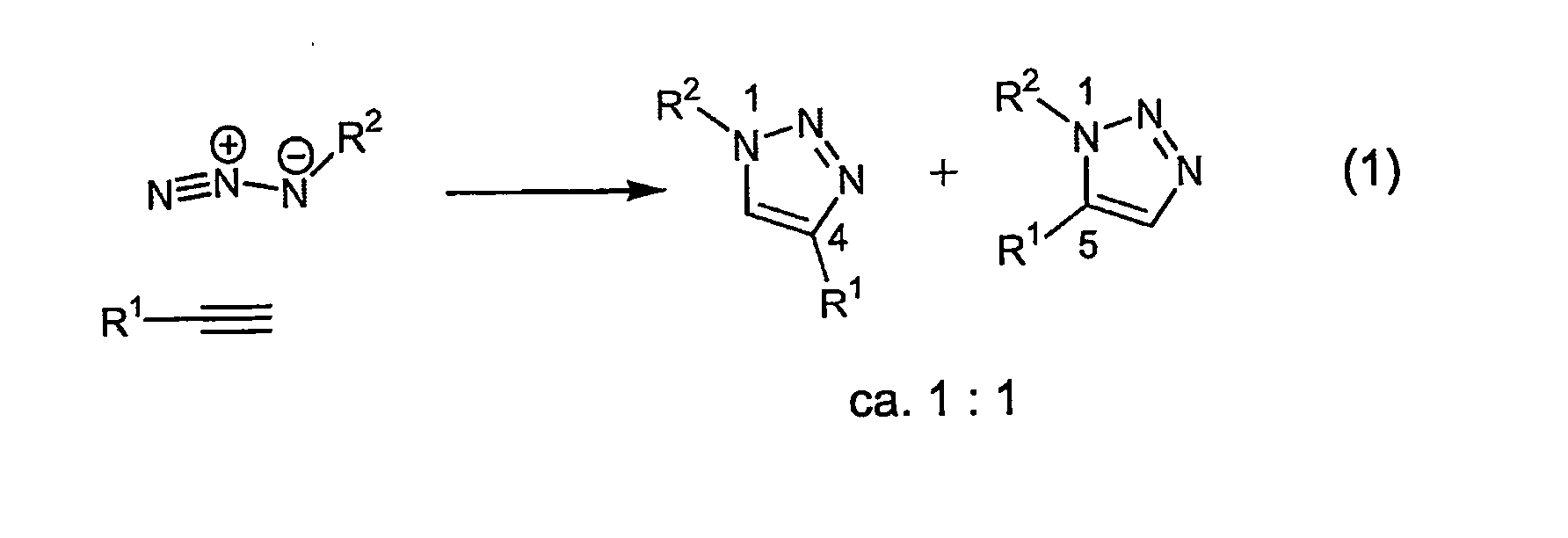
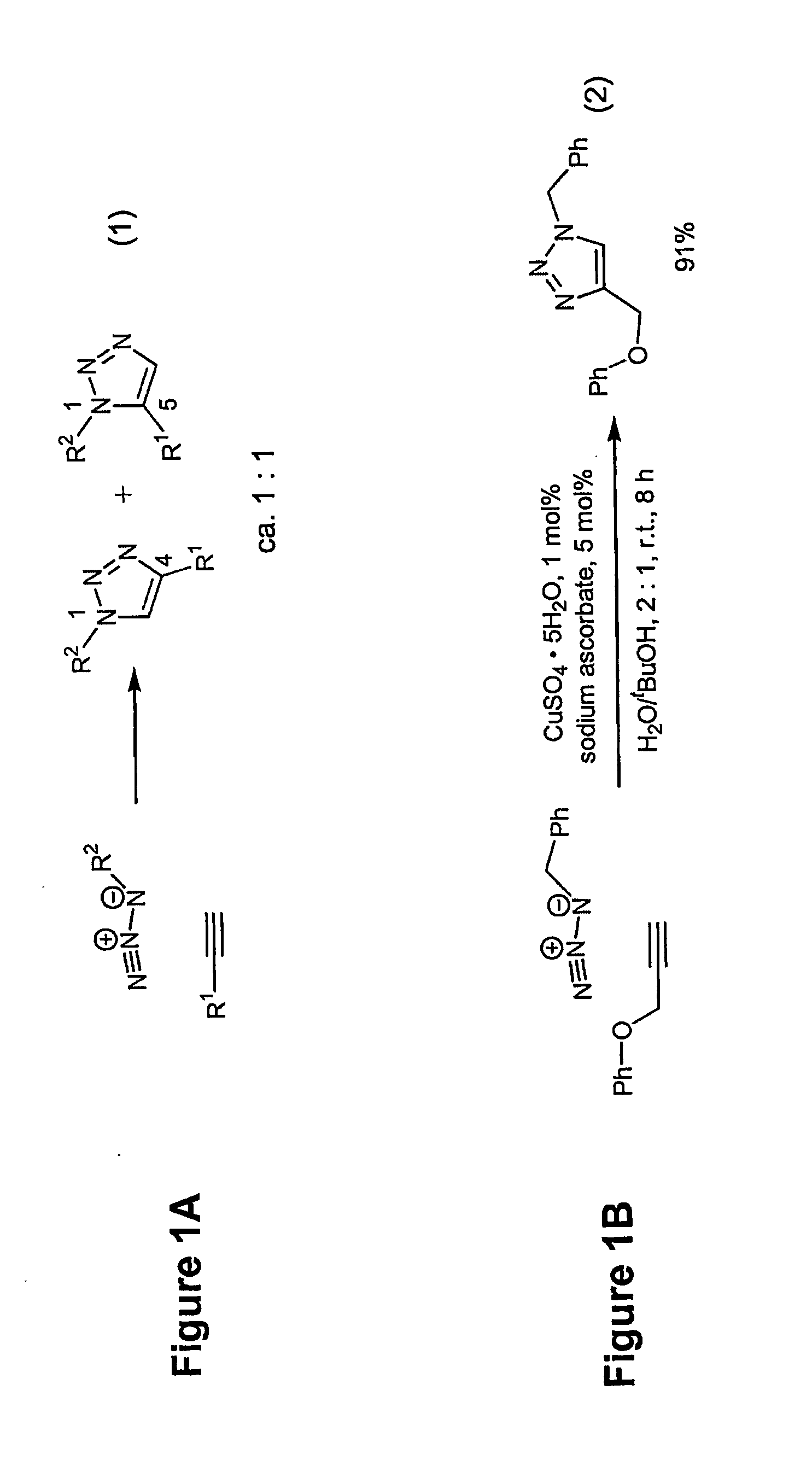
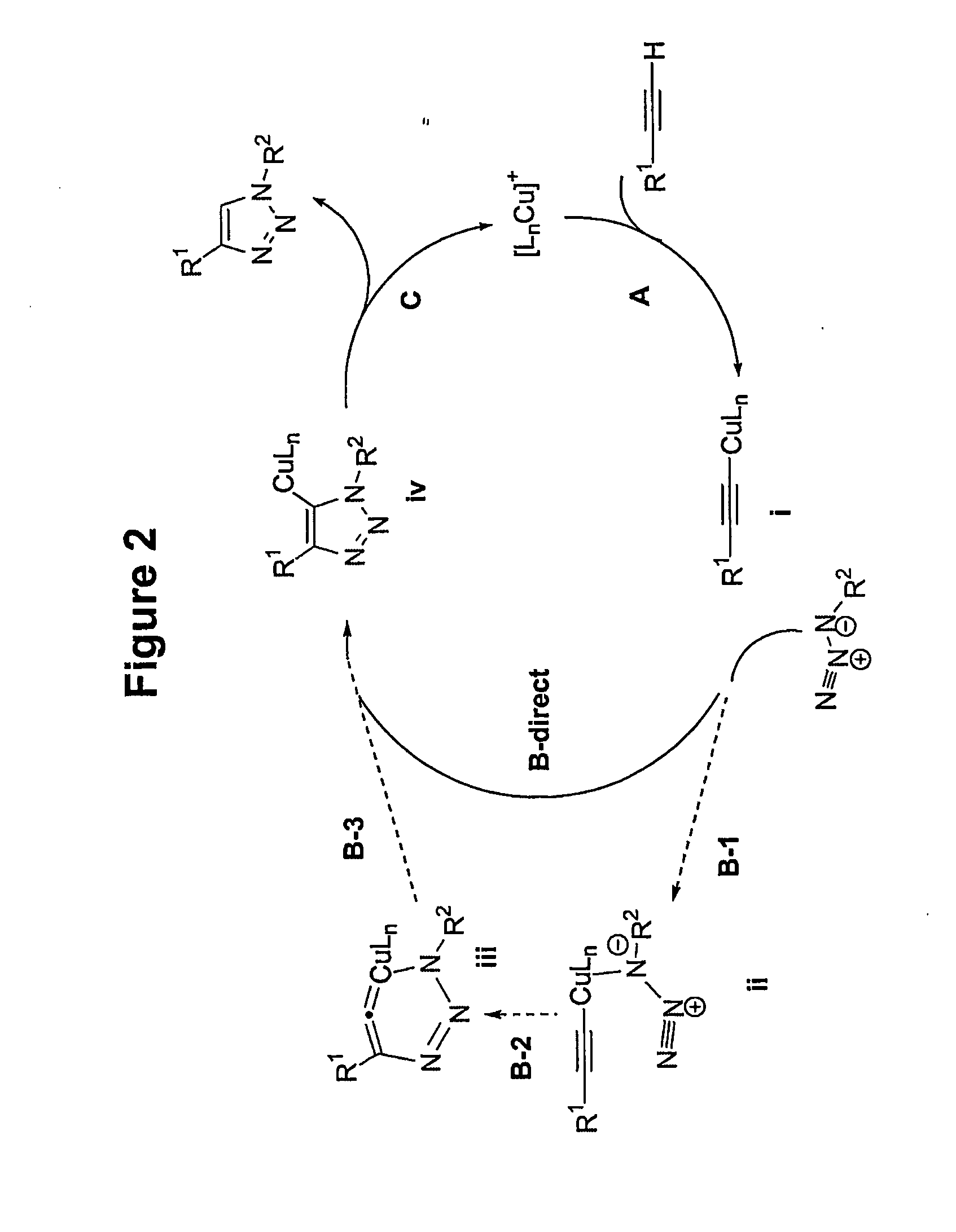
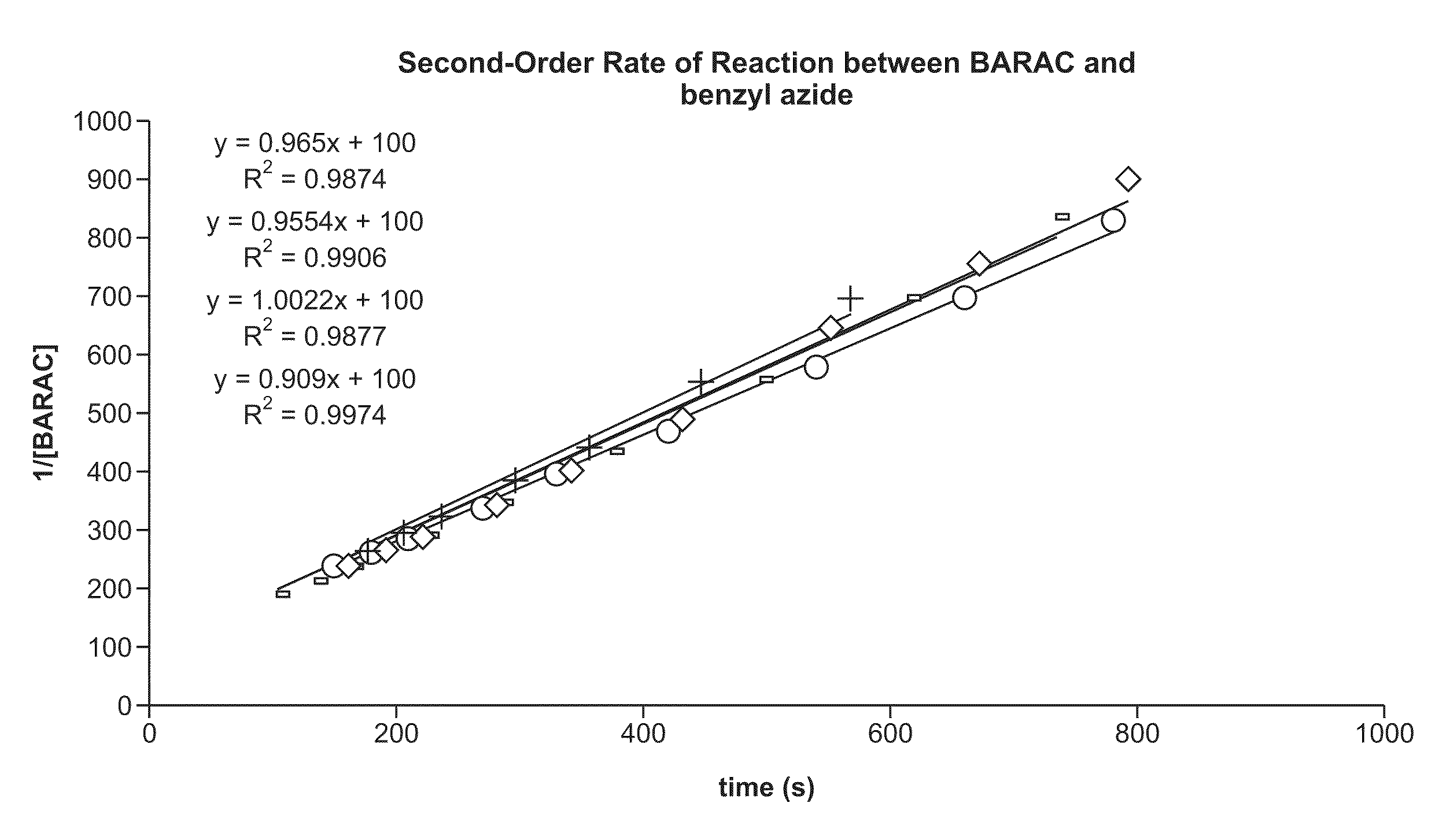
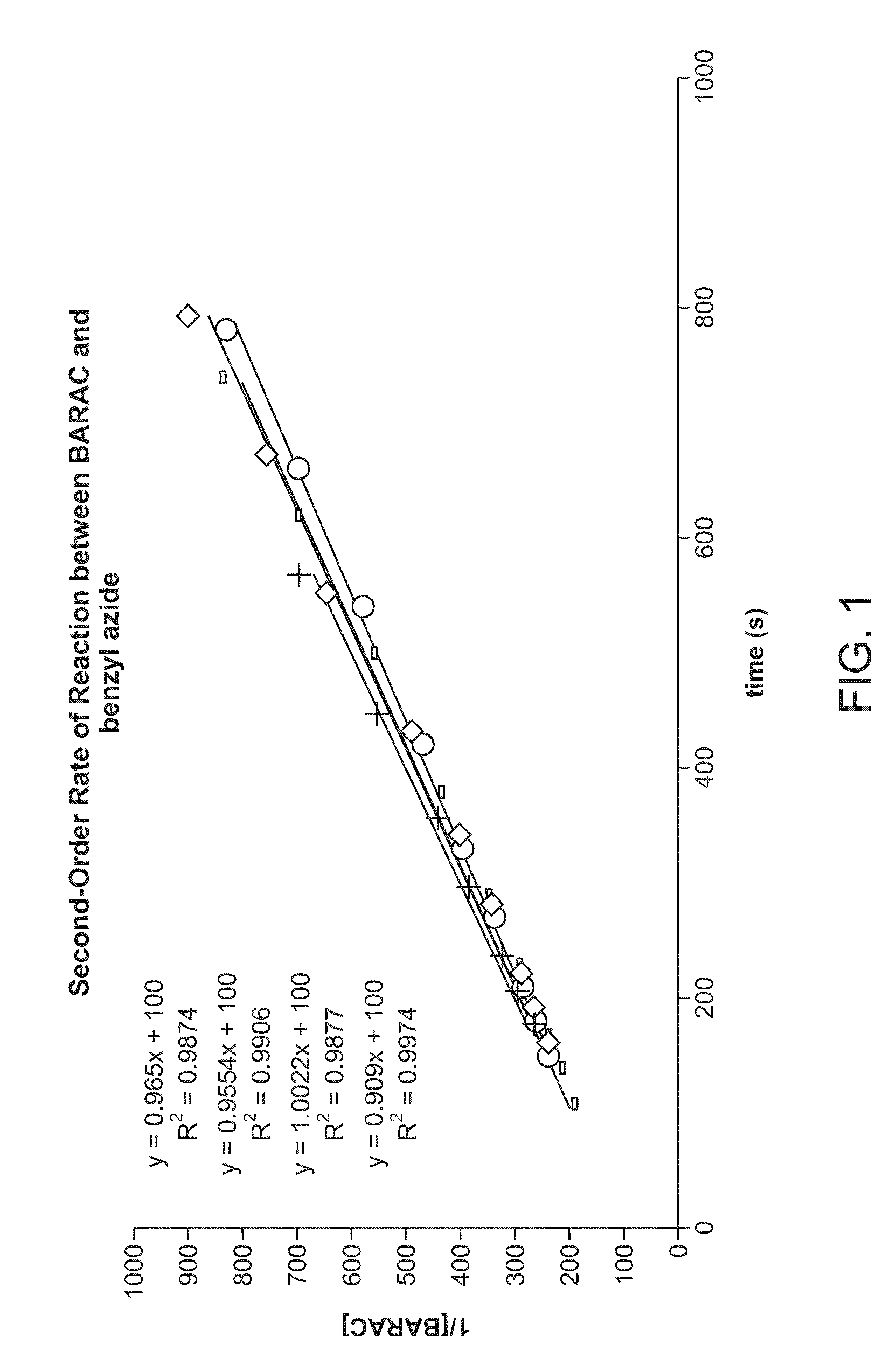
Copper-catalysed ligation of azides and acetylenes
InactiveUS7763736B2Organic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical ligationAqueous alcohol
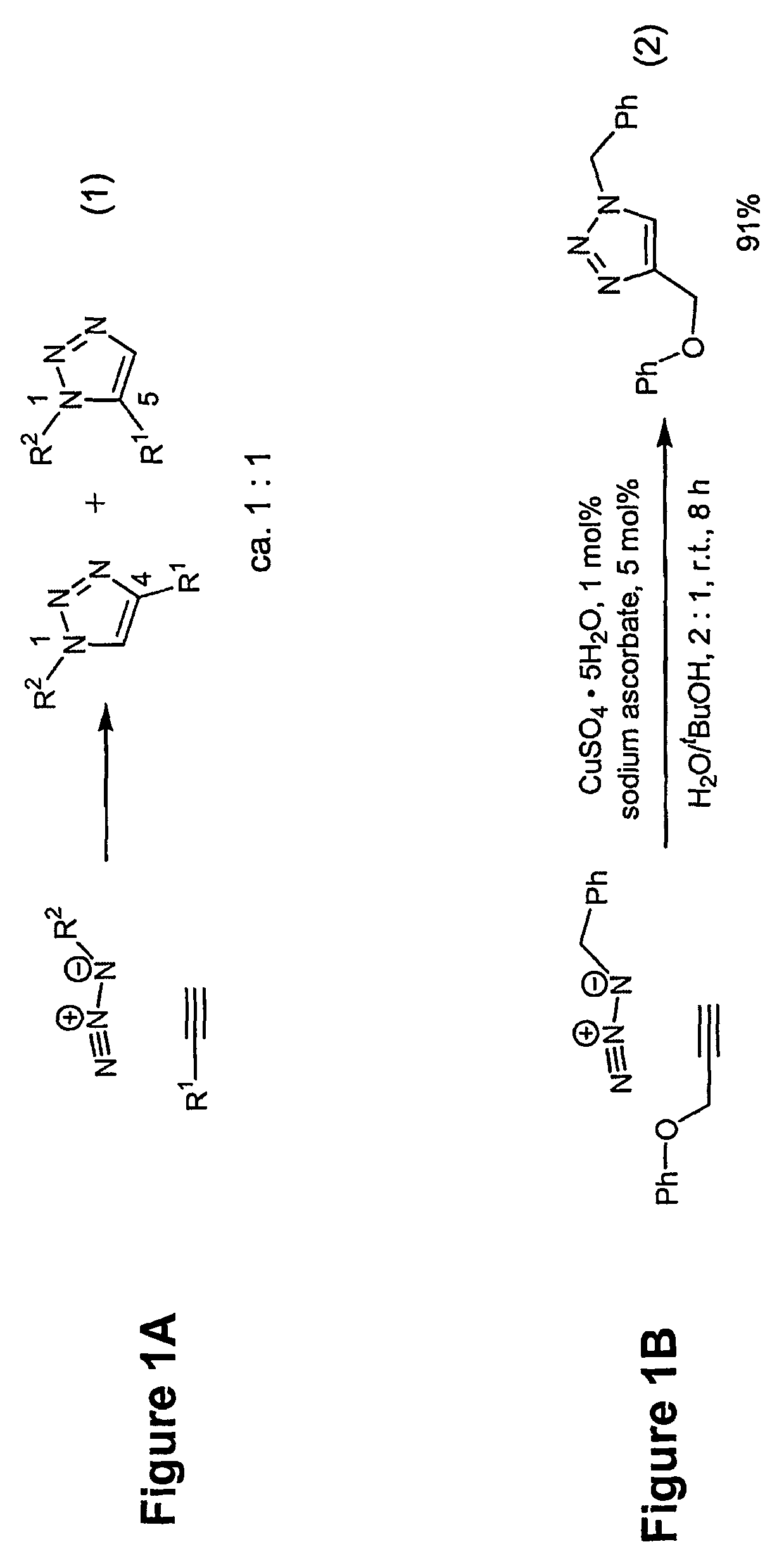
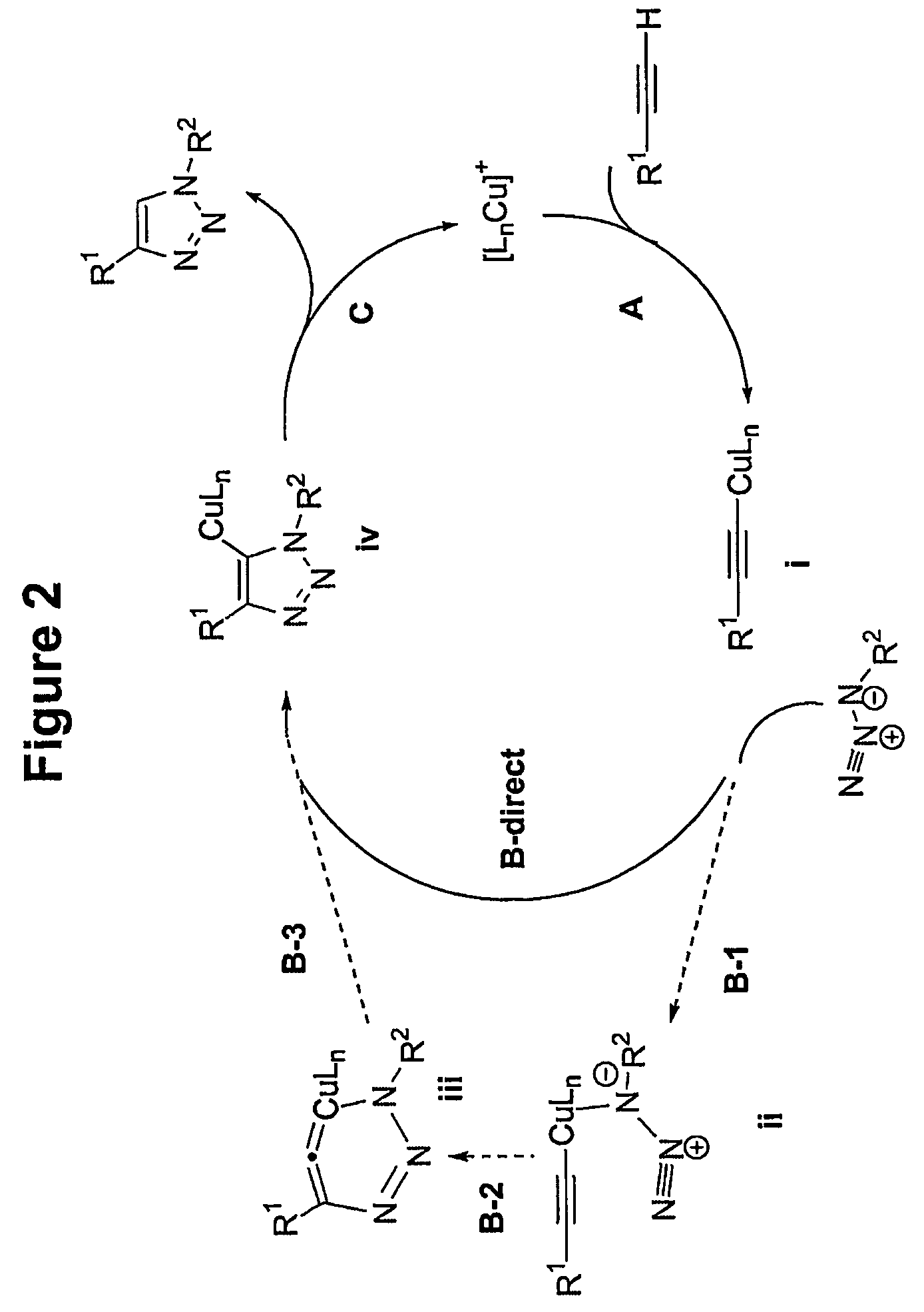
A copper catalyzed click chemistry ligation process is employed to bind azides and terminal acetylenes to provide 1,4-disubstituted 1,2,3-triazole triazoles. The process comprises contacting an organic azide and a terminal alkyne with a source of reactive Cu(I) ion for a time sufficient to form by cycloaddition a 1,4-disubstituted 1,2,3-triazole. The source of reactive Cu(I) ion can be, for example, a Cu(I) salt or copper metal. The process is preferably carried out in a solvent, such as an aqueous alcohol. Optionally, the process can be performed in a solvent that comprises a ligand for Cu(I) and an amine.
Owner:TETARD INC
Compositions and methods for modification of biomolecules
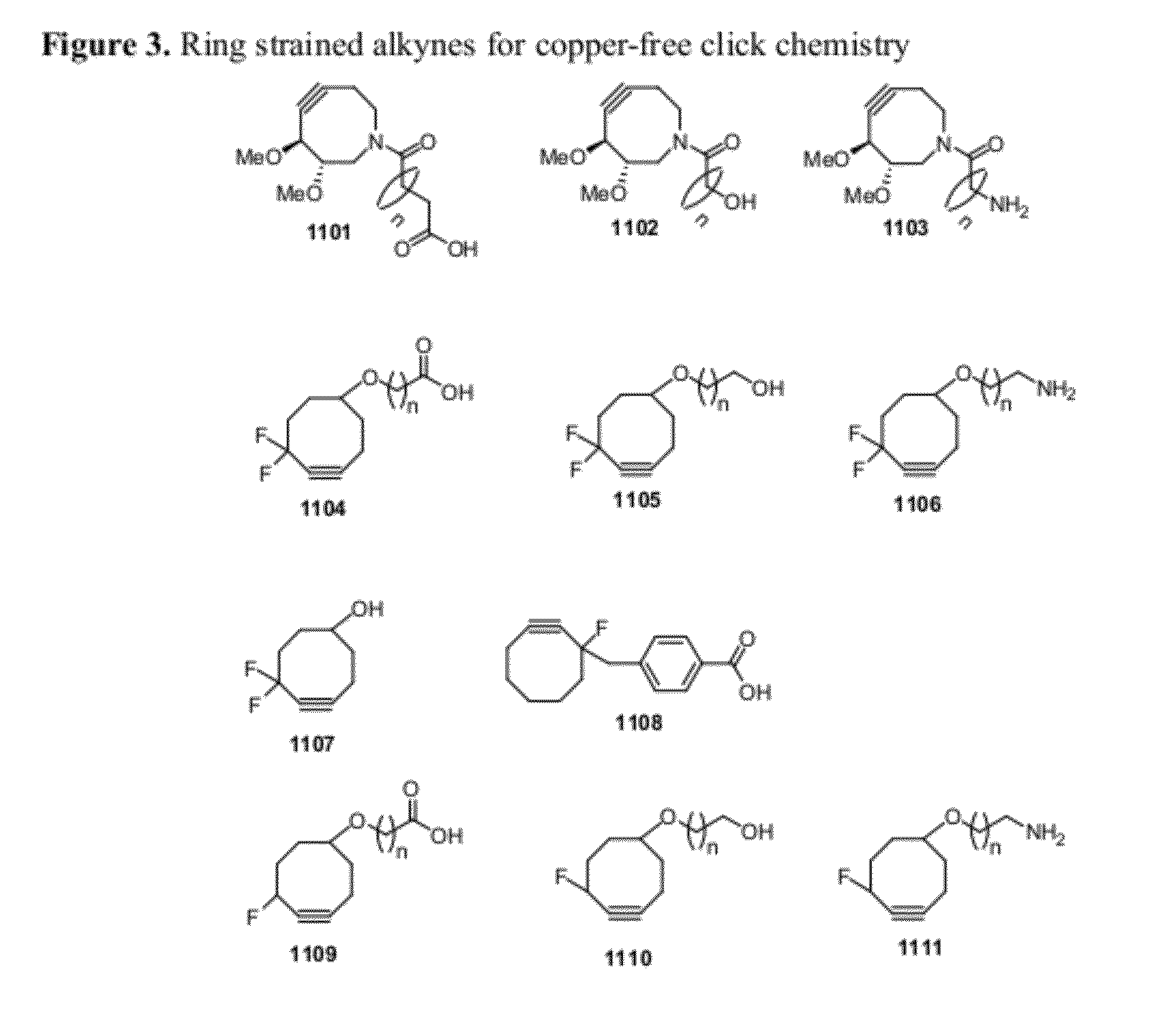
The present invention provides modified cycloalkyne compounds; and method of use of such compounds in modifying biomolecules. The present invention features a cycloaddition reaction that can be carried out under physiological conditions. In general, the invention involves reacting a modified cycloalkyne with an azide moiety on a target biomolecule, generating a covalently modified biomolecule. The selectivity of the reaction and its compatibility with aqueous environments provide for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
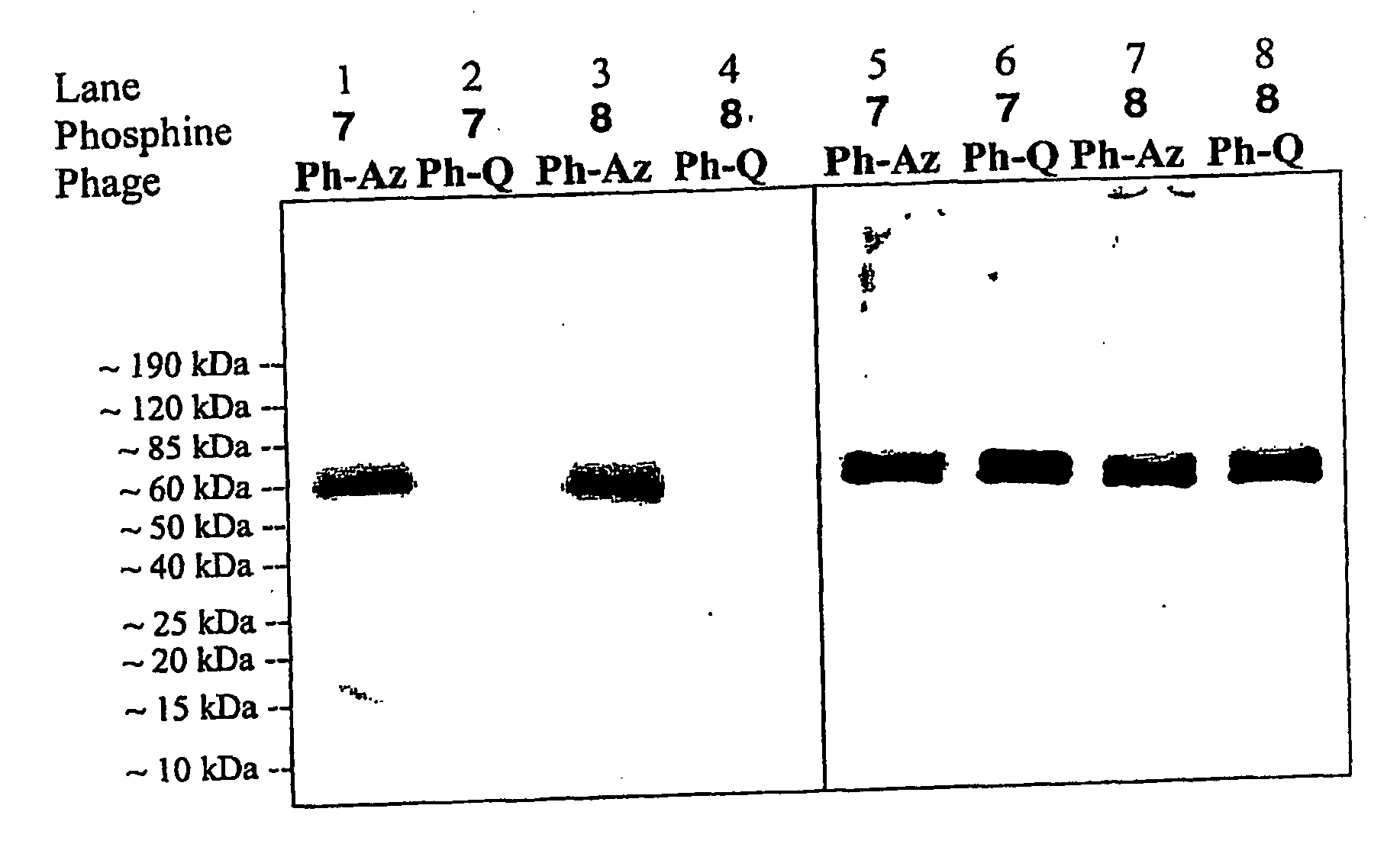
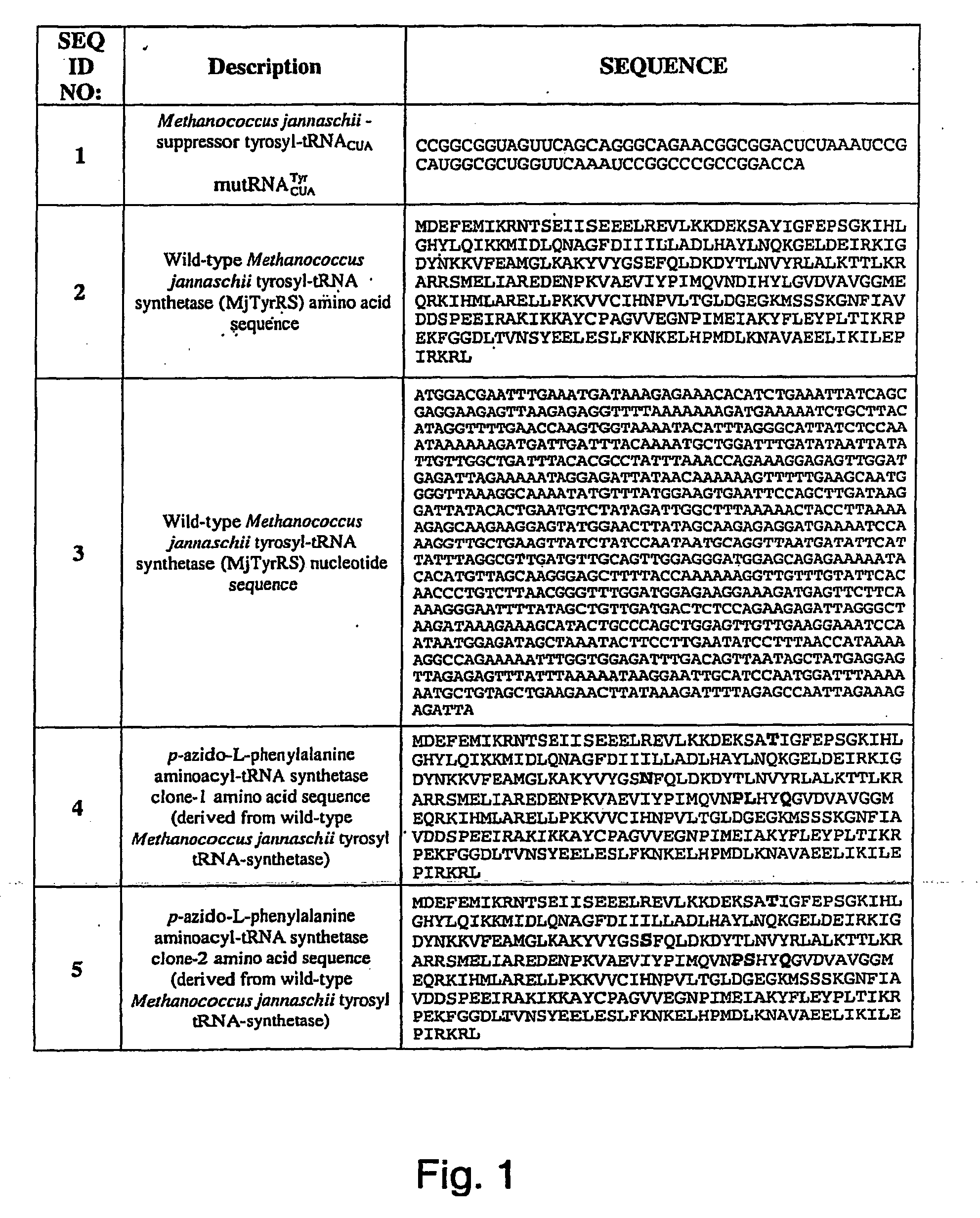
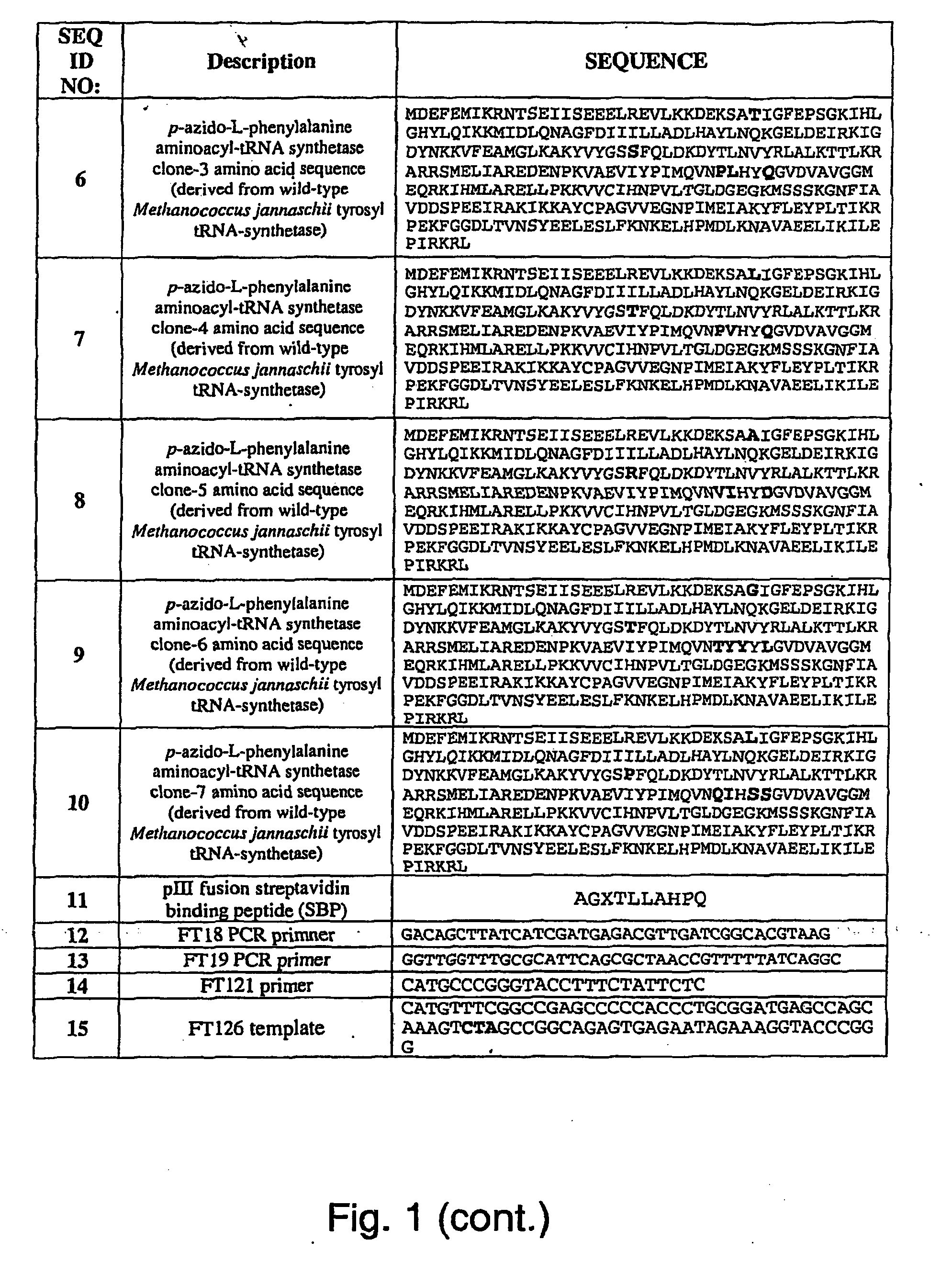
Selective posttranslational modification of phage-displayed polypeptides
ActiveUS20070178448A1Easy to detectEasy to quantifyAntibody mimetics/scaffoldsVirus peptidesArylCycloaddition
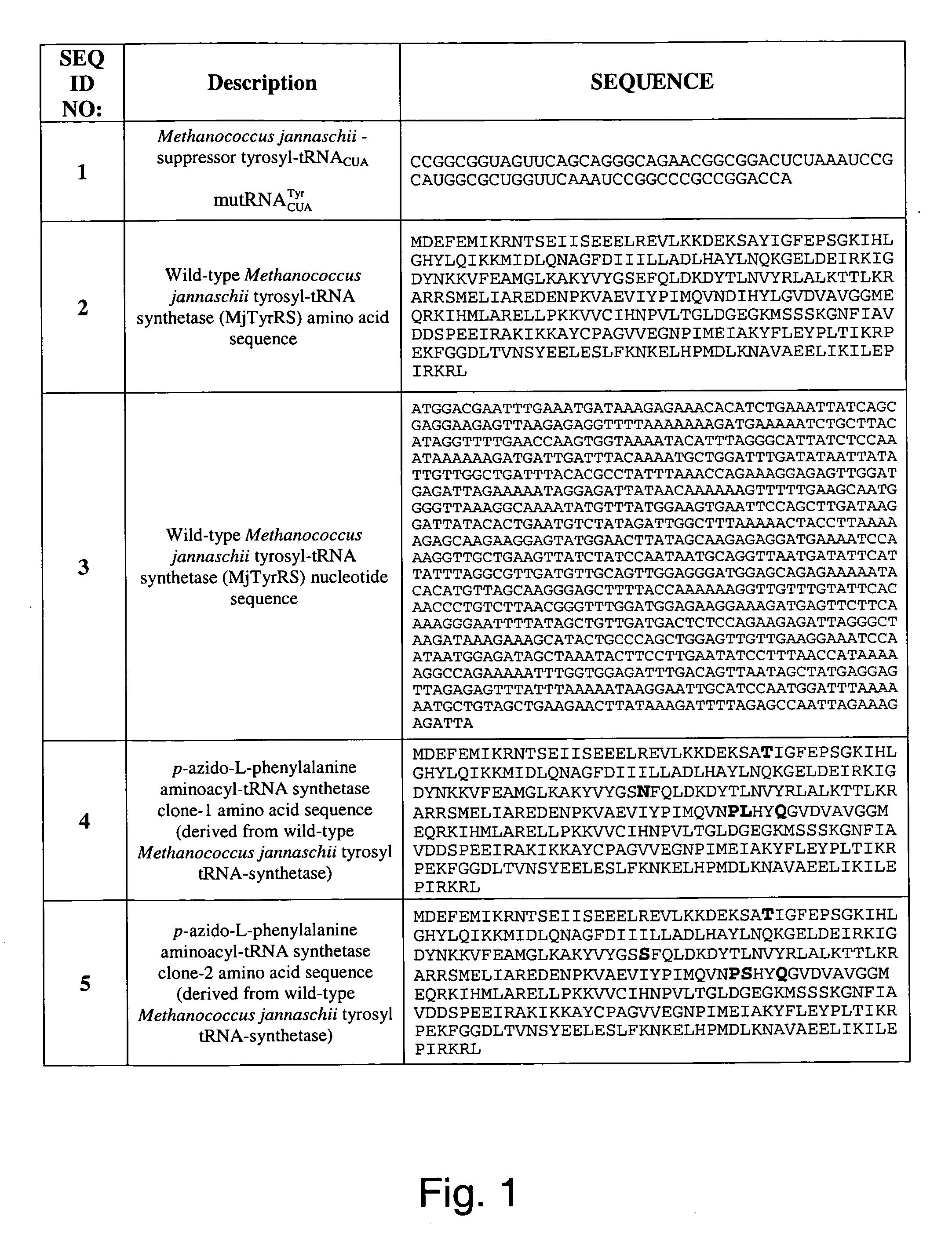
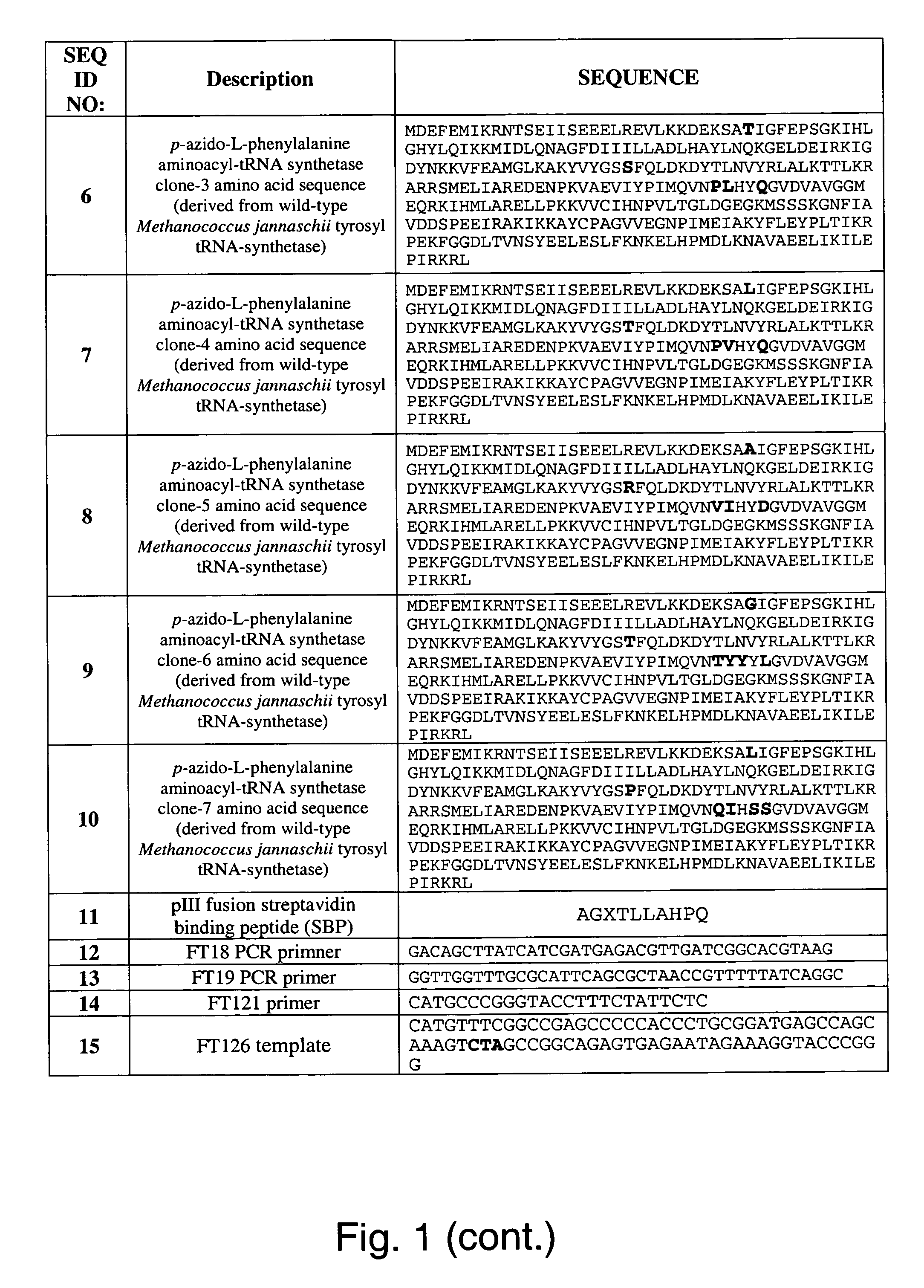
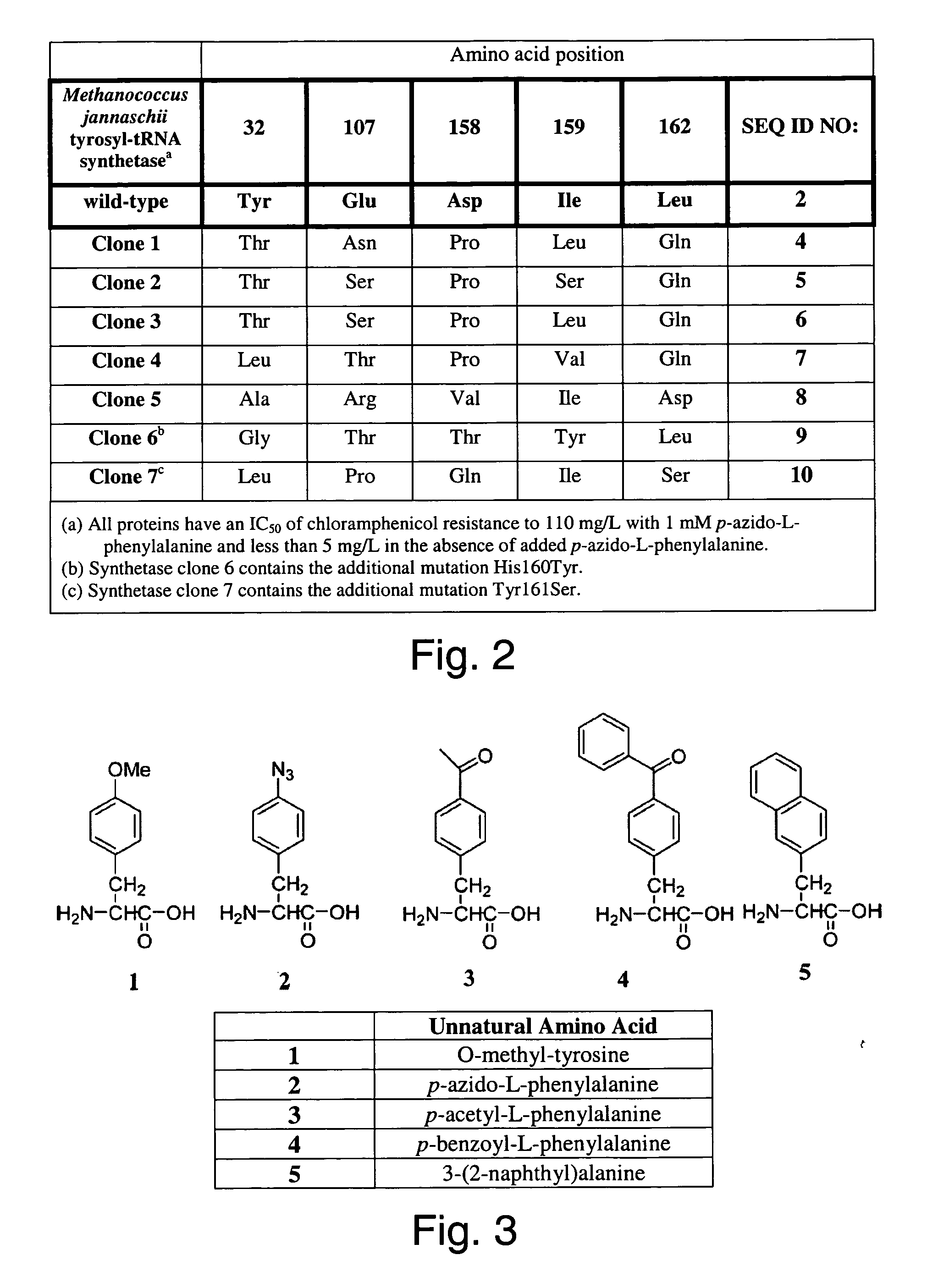
The invention relates to posttranslational modification of phage-displayed polypeptides. These displayed polypeptides comprise at least one unnatural amino acid, e.g., an aryl-azide amino acid such as p-azido-L-phenylalanine, or an alkynyl-amino acid such as para-propargyloxyphenylalanine, which are incorporated into the phage-displayed fusion polypeptide at a selected position by using an in vivo orthogonal translation system comprising a suitable orthogonal aminoacyl-tRNA synthetase and a suitable orthogonal tRNA species. These unnatural amino acids advantageously provide targets for posttranslational modifications such as azide-alkyne [3+2] cycloaddition reactions and Staudinger modifications.
Owner:THE SCRIPPS RES INST
Compositions and methods for modification of biomolecules
The present invention provides modified cycloalkyne compounds; and method of use of such compounds in modifying biomolecules. The present invention features a cycloaddition reaction that can be carried out under physiological conditions. In general, the invention involves reacting a modified cycloalkyne with an azide moiety on a target biomolecule, generating a covalently modified biomolecule. The selectivity of the reaction and its compatibility with aqueous environments provide for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for modification of biomolecules
The present invention provides modified cycloalkyne compounds; and method of use of such compounds in modifying biomolecules. The present invention features a cycloaddition reaction that can be carried out under physiological conditions. In general, the invention involves reacting a modified cycloalkyne with an azide moiety on a target biomolecule, generating a covalently modified biomolecule. The selectivity of the reaction and its compatibility with aqueous environments provide for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
Biomolecular coupling methods using 1,3-dipolar cycloaddition chemistry
InactiveUS20090240030A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCouplingCycloaddition
This invention provides methods for covalently affixing a biomolecule to either a second molecule or a solid surface using 1,3-dipolar cycloaddition chemistry. This invention also provides related methods and compositions.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Selective Posttranslational Modification of Phage-Displayed Polypeptides
ActiveUS20090137424A1High selectivityPreserves viral infectivityAntibody mimetics/scaffoldsMicrobiological testing/measurementArylCycloaddition
The invention relates to posttranslational modification of phage-displayed polypeptides. These displayed polypeptides comprise at least one unnatural amino acid, e.g., an aryl-azide amino acid such asp-azido-L-phenylalanine, or an alkynyl-amino acid such as para-propargyloxyphenylalanine, which are incorporated into the phage-displayed fusion polypeptide at a selected position by using an in vivo orthogonal translation system comprising a suitable orthogonal aminoacyl-tRNA synthetase and a suitable orthogonal tRNA species. These unnatural amino acids advantageously provide targets for posttranslational modifications such as azide-alkyne [3+2]cycloaddition reactions and Staudinger modifications.
Owner:THE SCRIPPS RES INST
Toner, developer, image forming method, and toner container
ActiveUS20070015077A1Quality improvementHigh color reproductionDevelopersElectrographic processes using charge patternPolyesterParticulates
A toner is provided manufactured by a method having the steps: dispersing toner constituents including a resin, in an aqueous medium containing a particulate resin, wherein the resin has a polyester skeleton formed by a ring-opening addition reaction of a cyclic ester with a first compound having an active hydrogen group; and a developer and an image forming method using the toner, and a toner container containing the toner.
Owner:RICOH KK
Processes for the preparation of a monodisperse polymers, processes for the continuous polymerization of cyclic monomers, and polymers prepared thereby
The present invention relates to a method for preparing lactone polymers, carbonate polymers, lactone-carbonate block copolymers and lactone-arbonate random copolymers via a ring-opening addition reaction of a lactone monomer, a cyclic carbonate monomer or a mixture thereof using an initiator and in the presence of a specific titanium-type Lewis acid catalyst. The resulting polymers have a molecular weight distribution (Mw / Mn) approximately equal to 1 or a extremely high purity of single-structure components. The polymer molecules range in an oligomer region to a molecular weight of approximately 200,000. The present invention also relates to a method for preparing lactone polymers, carbonate polymers and lactone-carbonate copolymers having a narrow molecular weight distribution and which can be obtained via a continuous polymerisation of a lactone monomer and / or a cyclic carbonate monomer using an initiator in an extruder in the presence of a specific titanium-type Lewis acid catalyst or an aluminium-type Lewis acid catalyst. The present invention also relates to these resulting polymers.
Owner:DAICEL CHEM IND LTD
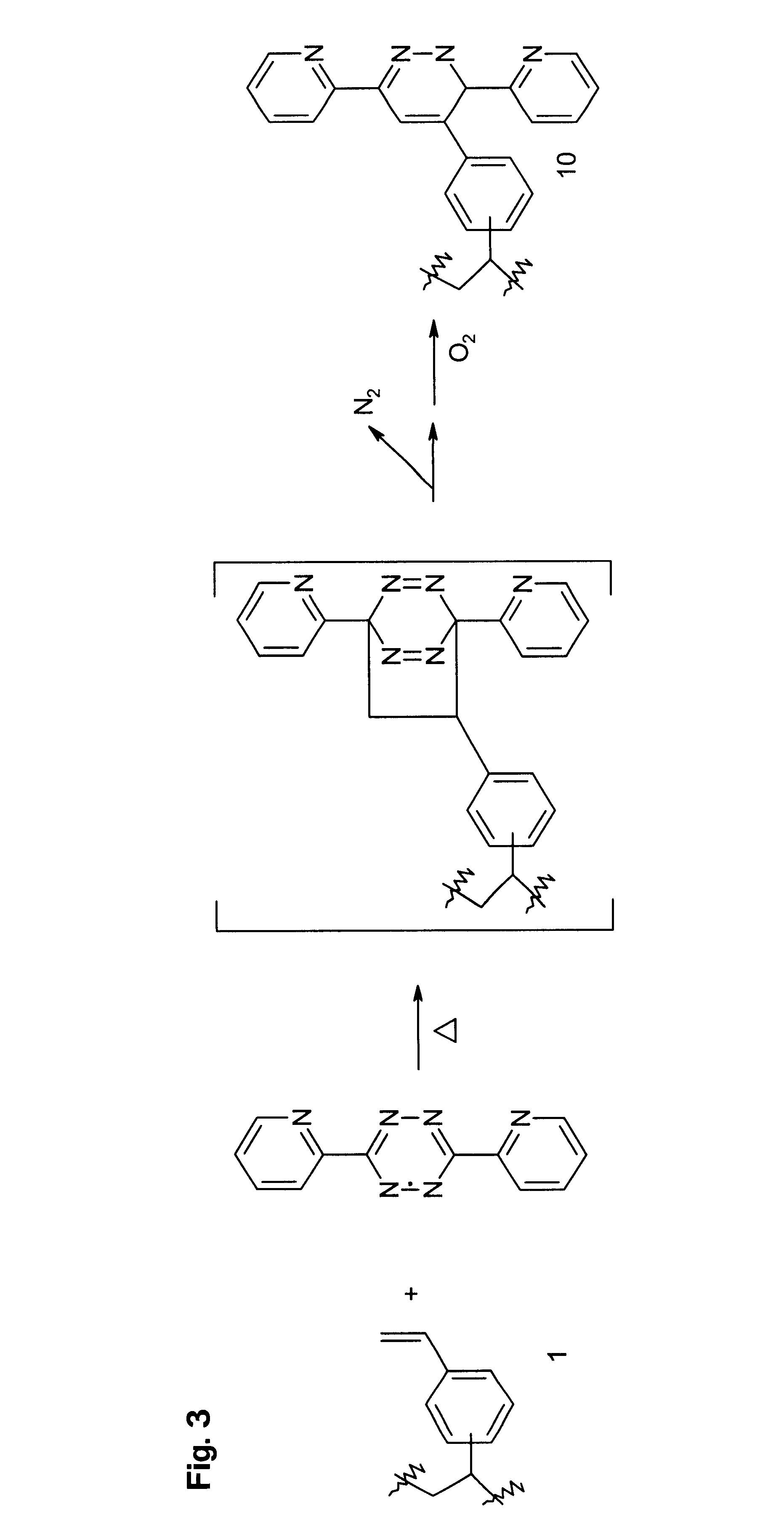
Cycloaddition functional polymers from (vinyl) polystyrene
A functional polymer having active and stable functional groups useful for separation or reactive processes in chemical manufacture or analysis is disclosed. The polymer comprises repeat units having structures corresponding to cycloaddition products of polymeric 1-(vinylphenyl)ethylene with electron-poor alkenes. A process for preparing the functional polymer does not require radical reactions or the exclusion of oxygen. The properties of the polymeric matrix produced can be adjusted by modifying the polymer. For example, the polymer particle size and shape, porosity, swellability, surface area, and number, type and distribution of functional groups may be controlled.
Owner:ACTIVE MATERIALS
Bioadhesive Composition Formed Using Click Chemistry
The present disclosure provides compositions which may be utilized as adhesives or sealants in medical and surgical applications. The compositions may, in embodiments, be formed from the cycloaddition reaction of a first component possessing at least one azide group with a second component possessing at least one alkyne group.
Owner:TYCO HEALTHCARE GRP LP
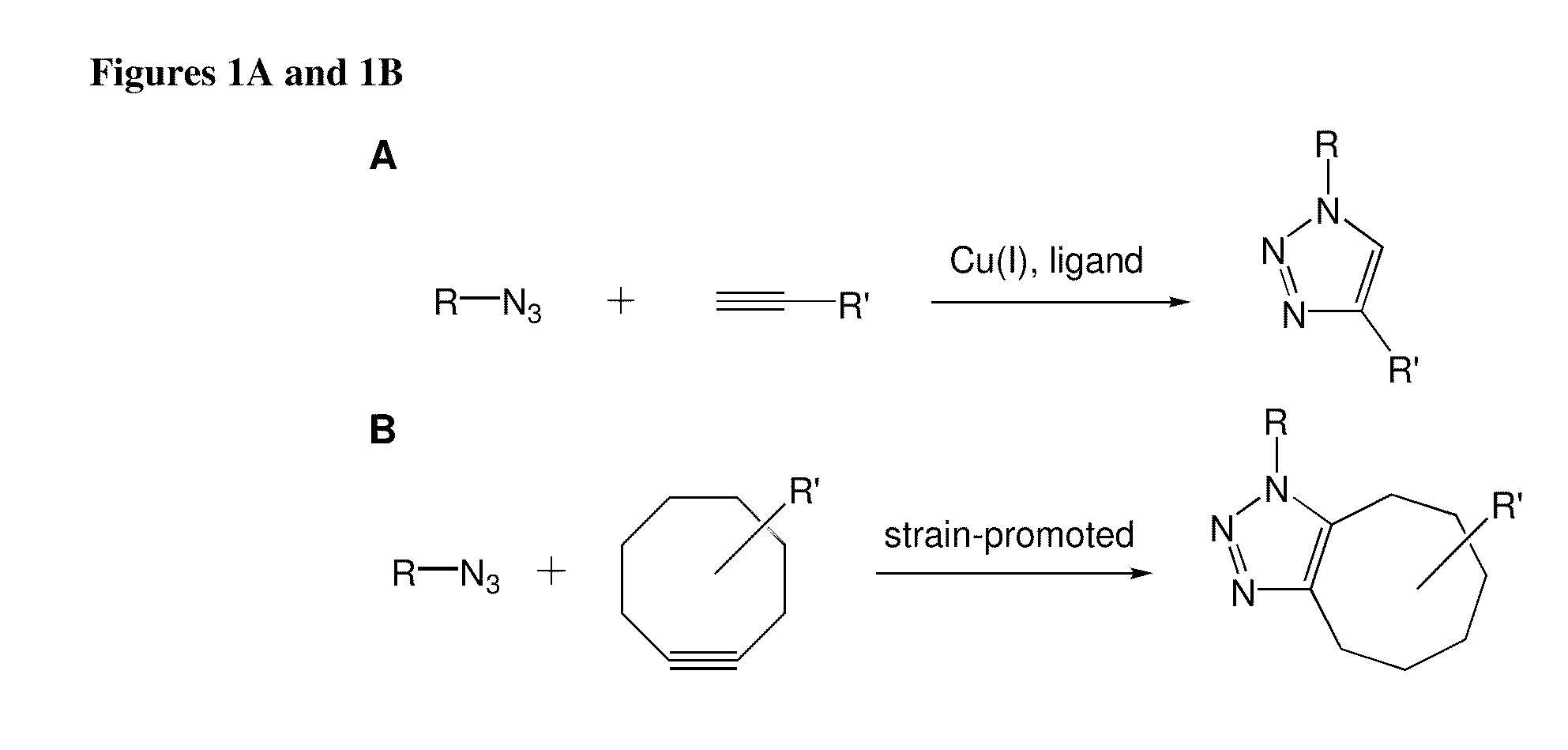
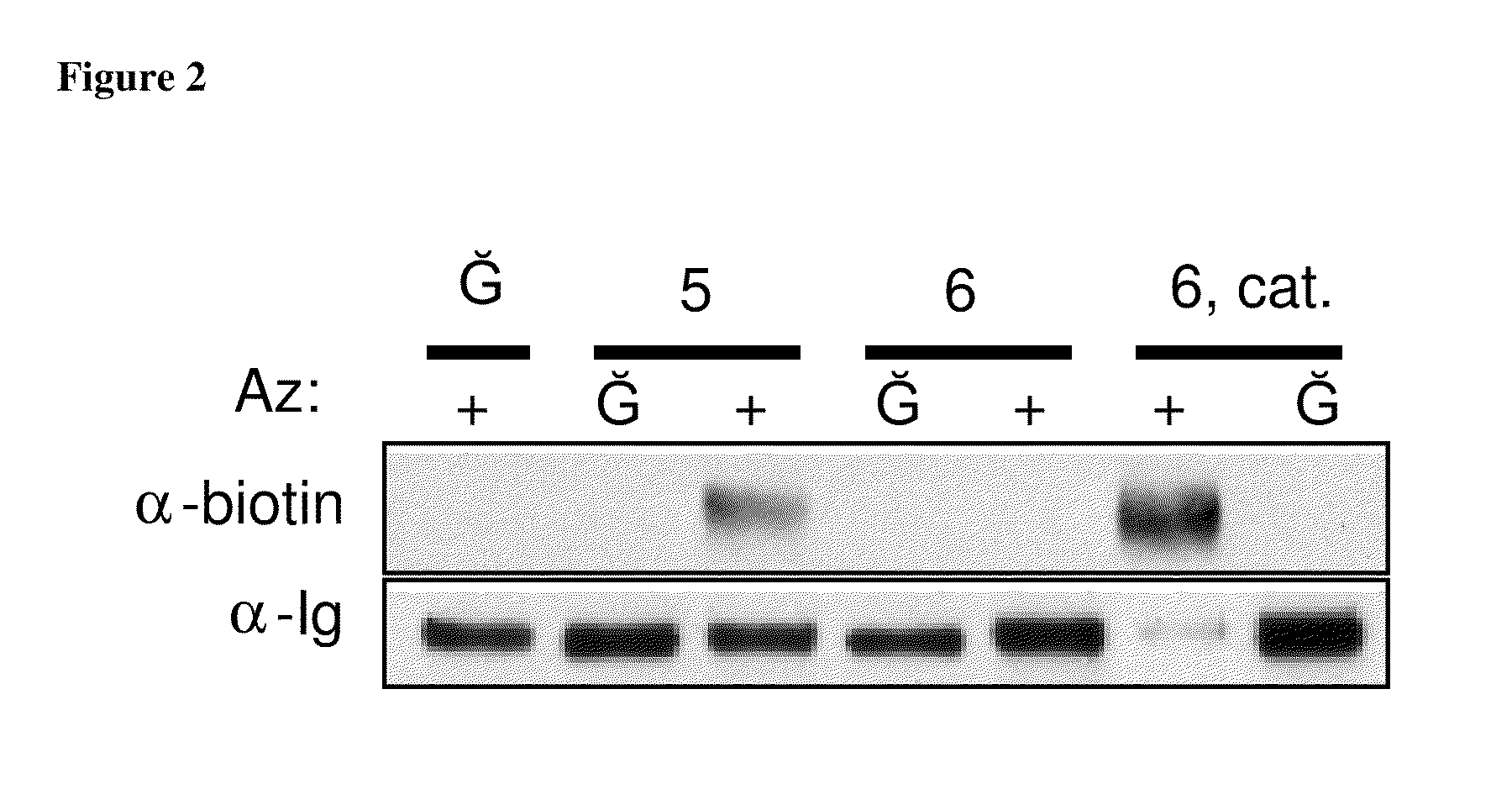
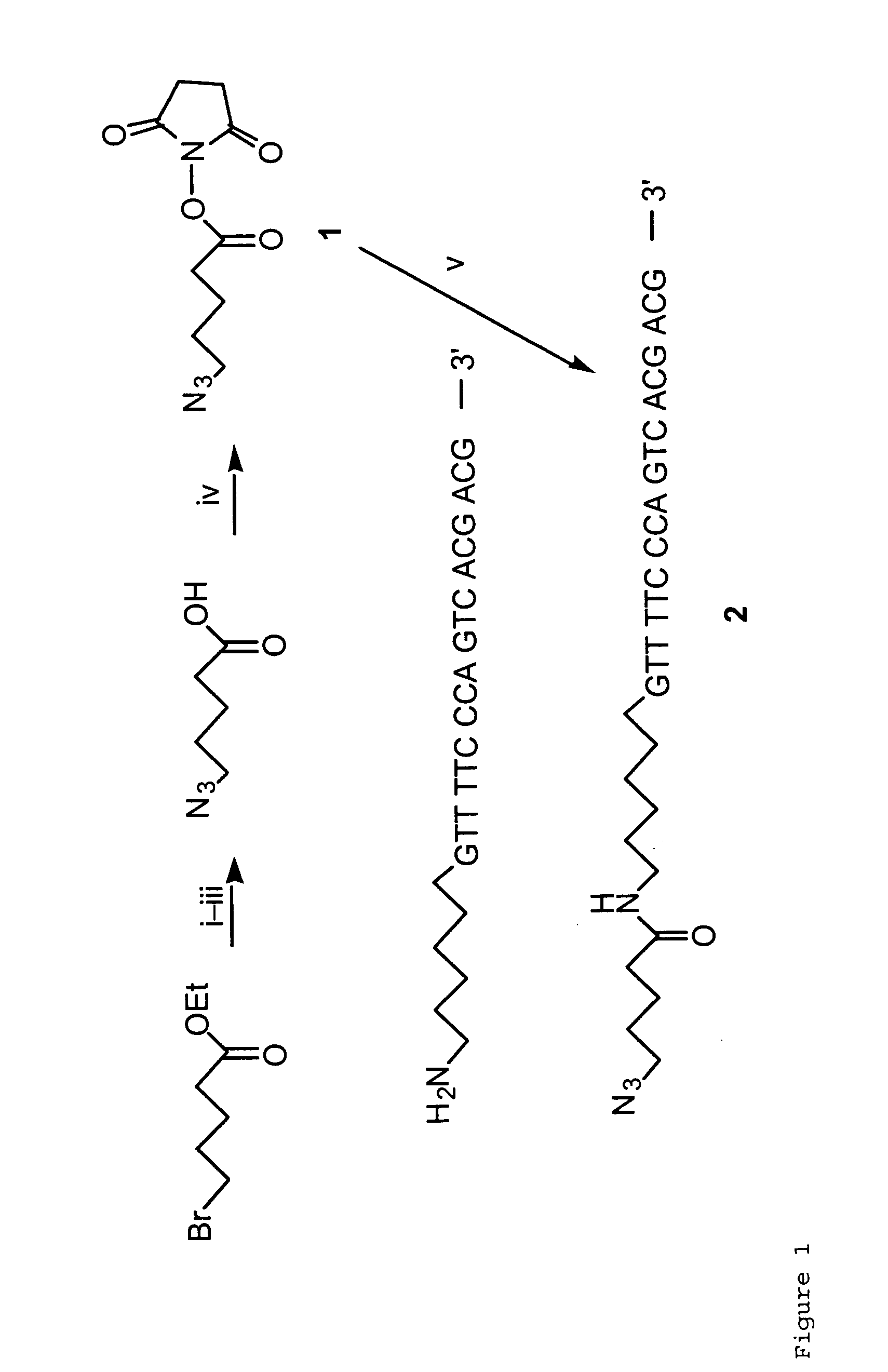
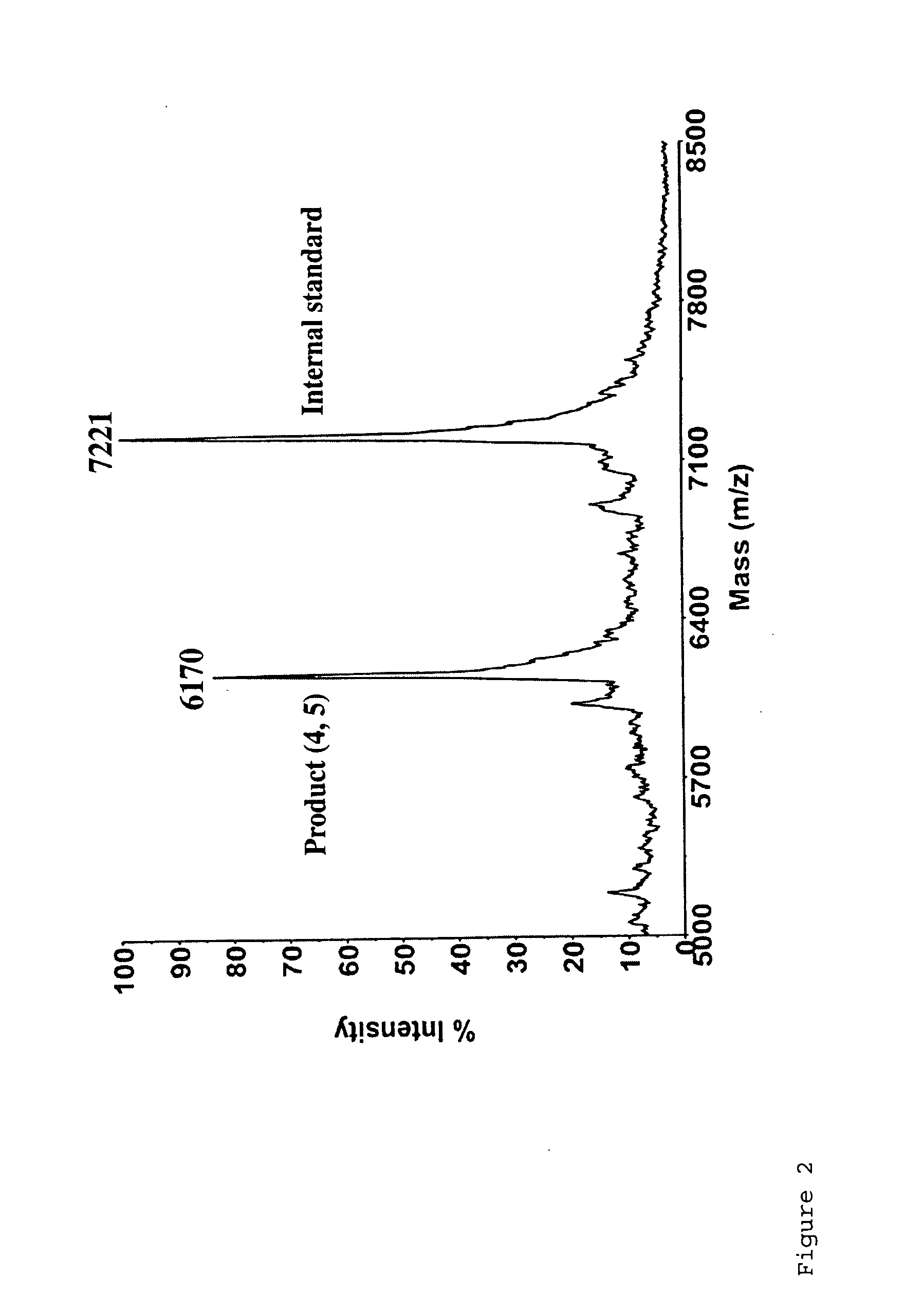
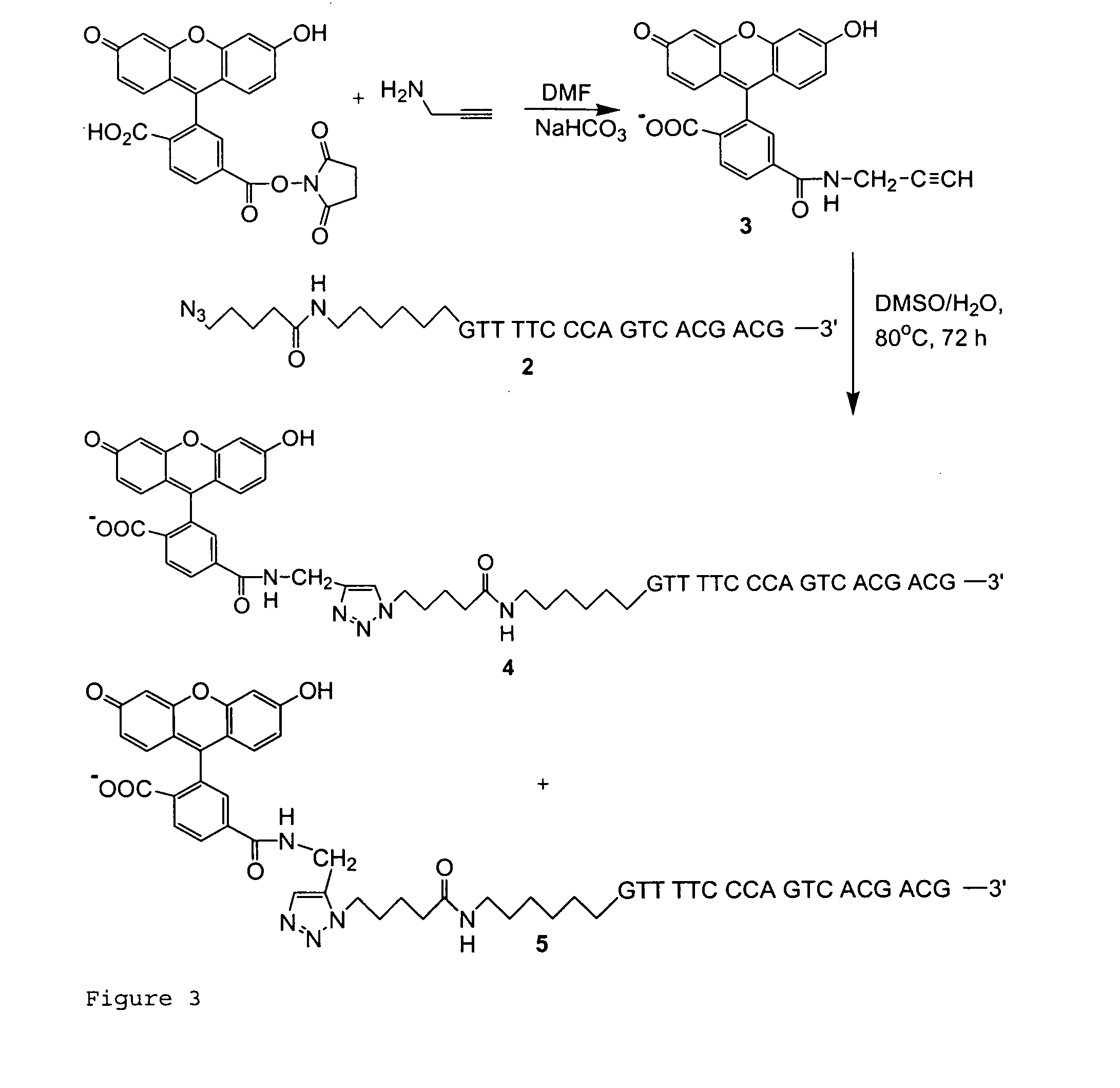
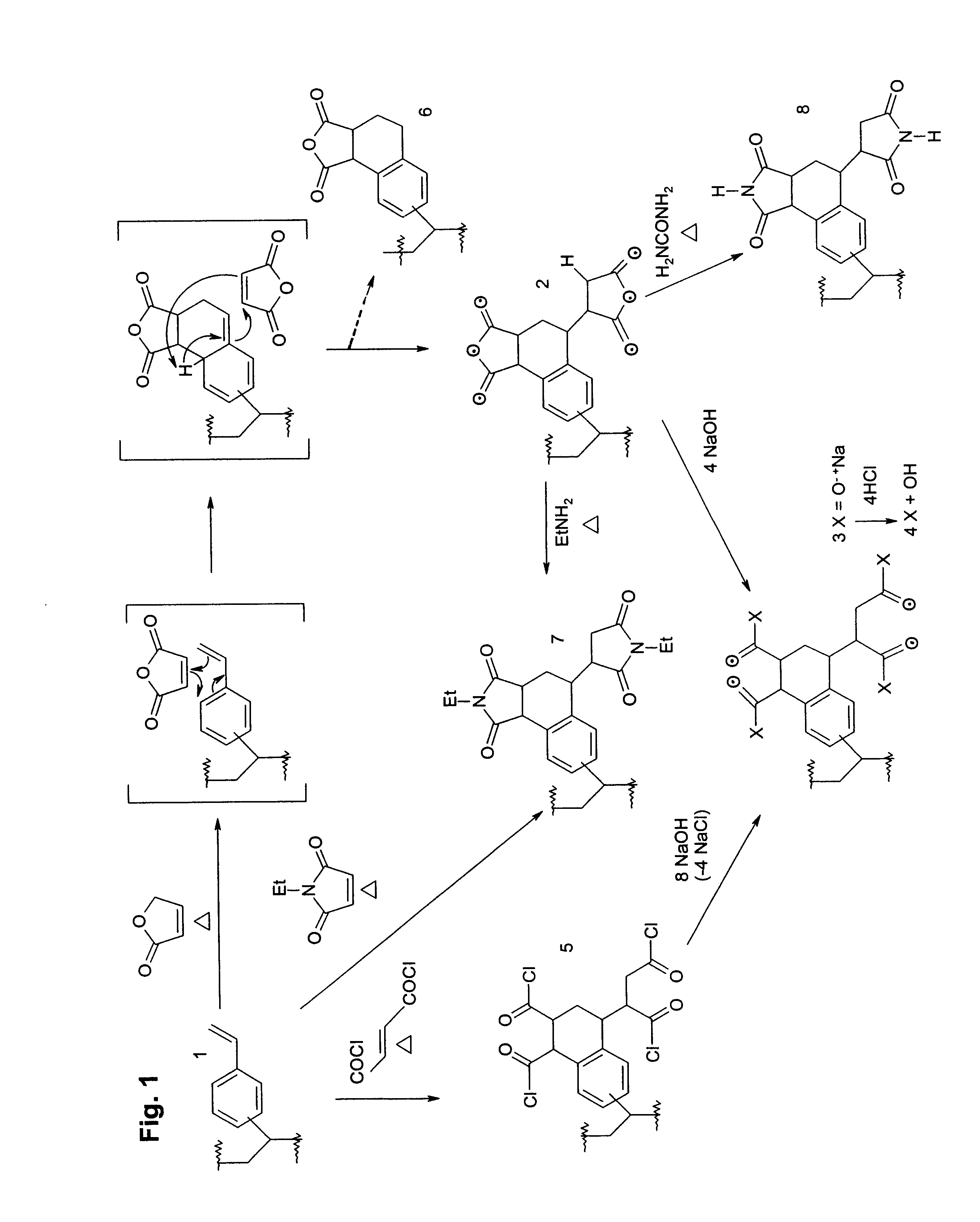
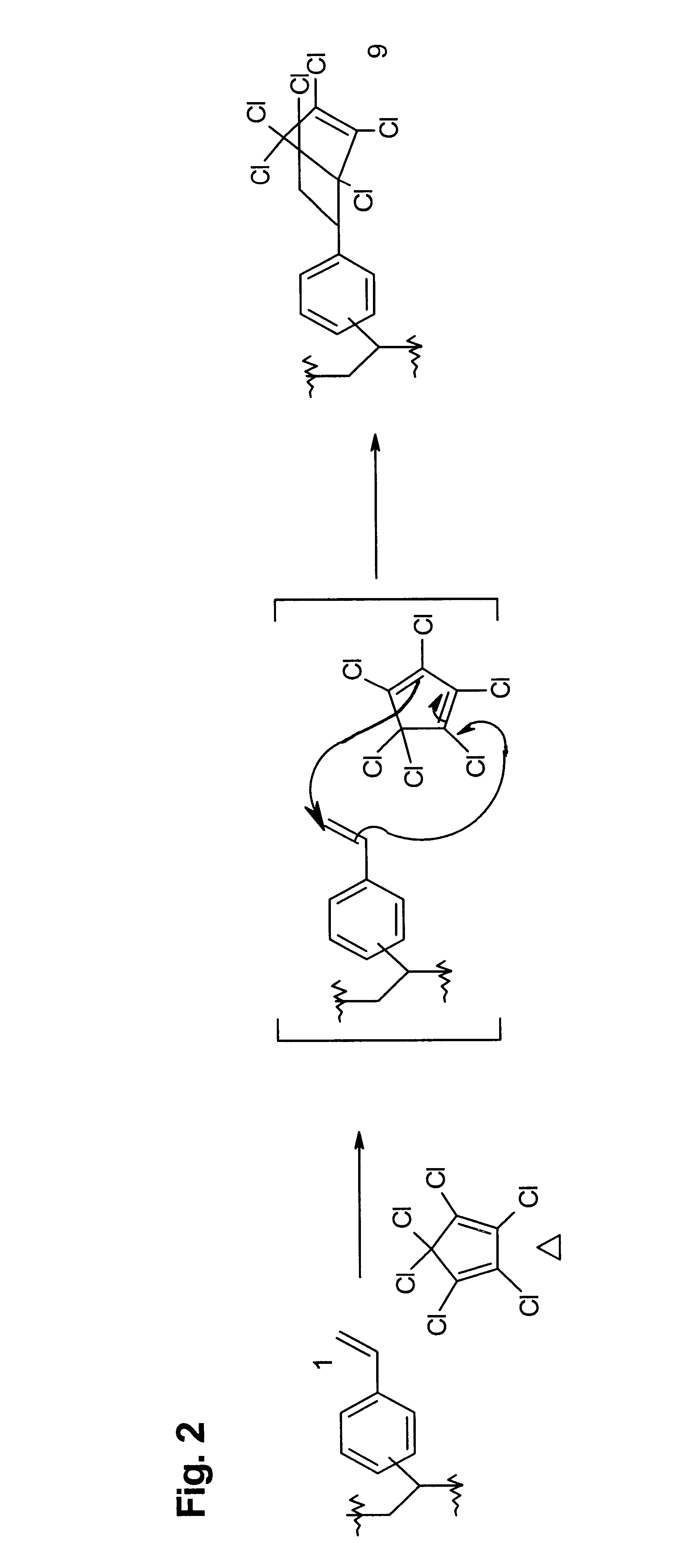
Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition
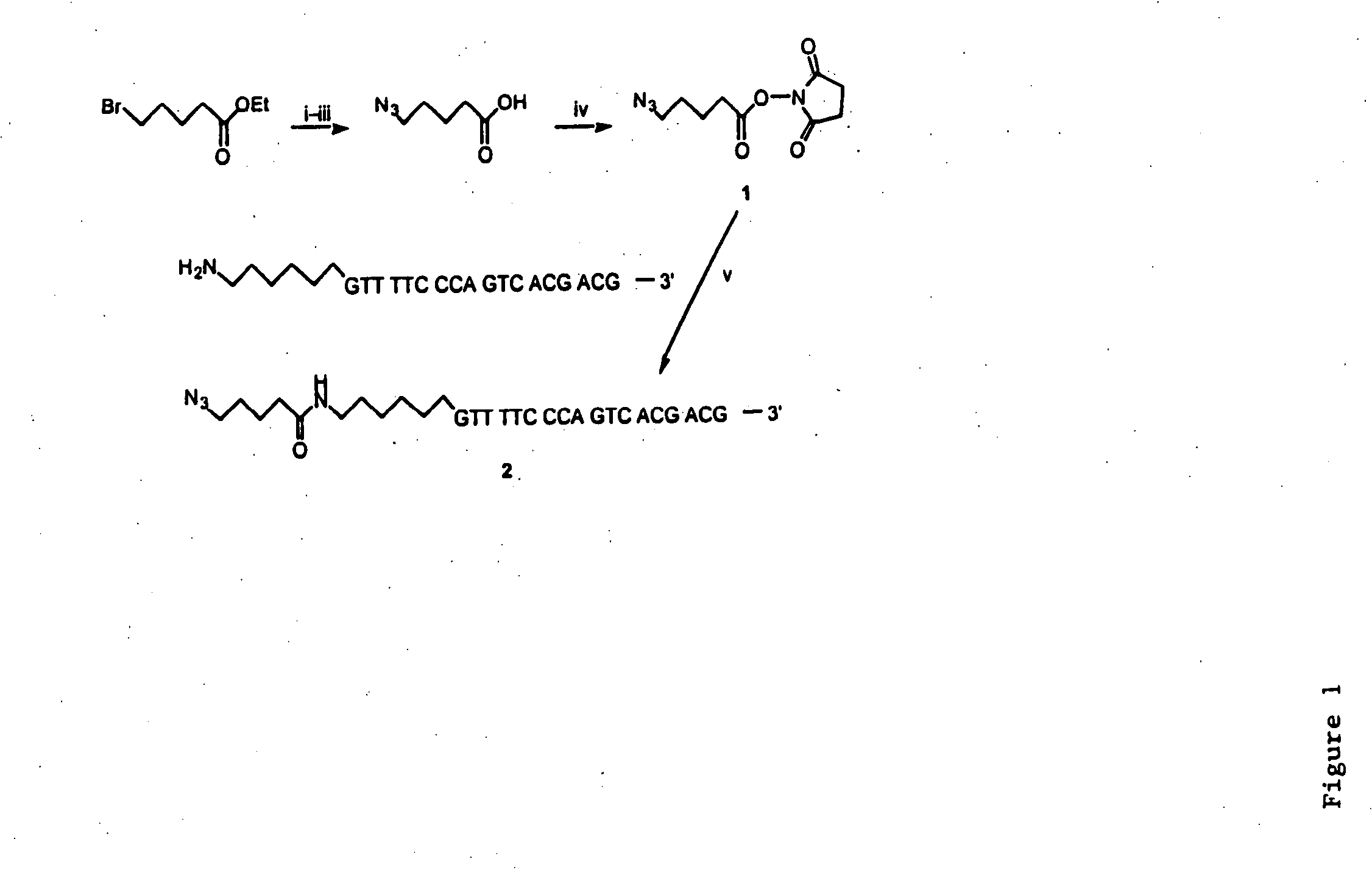
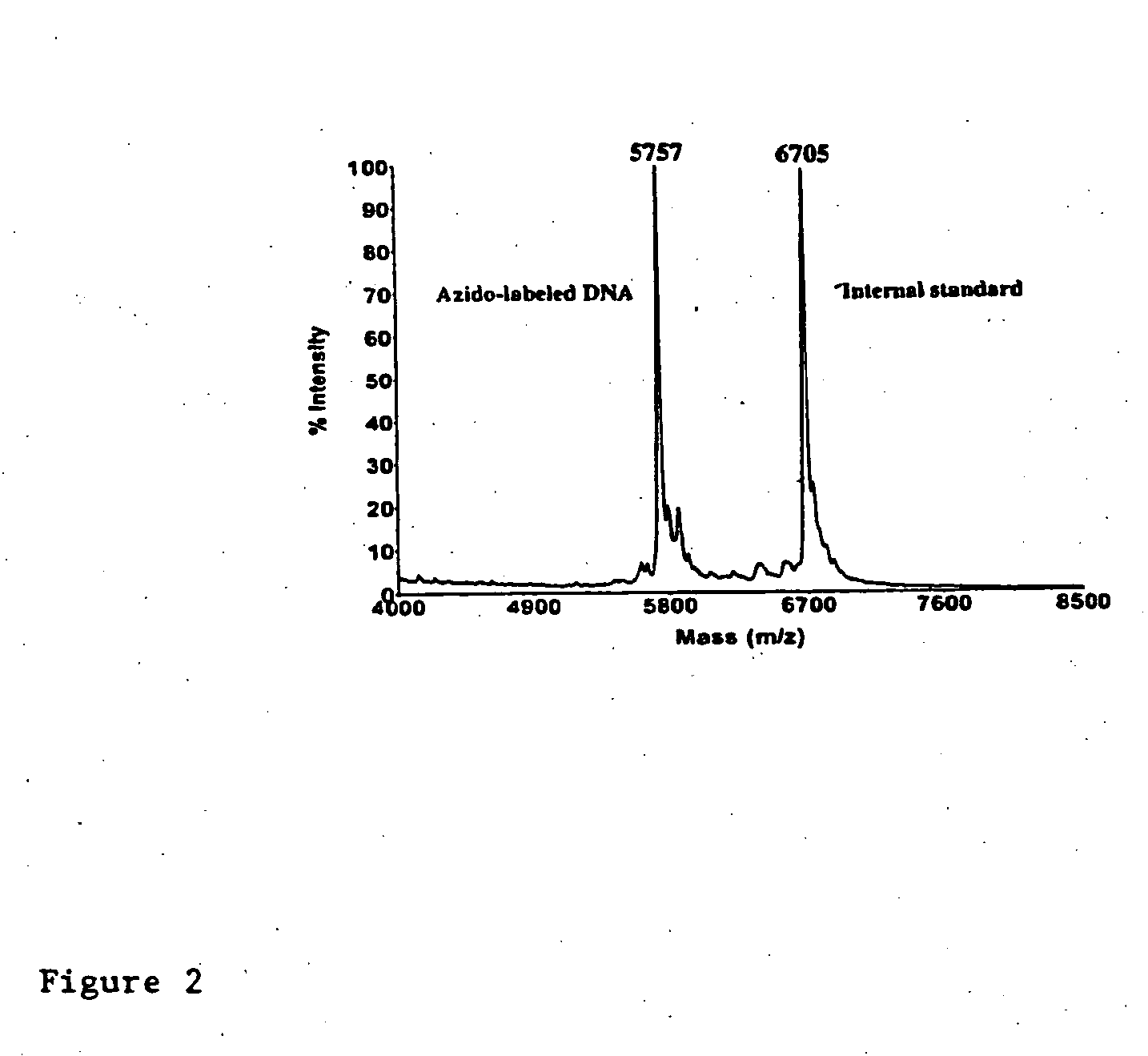
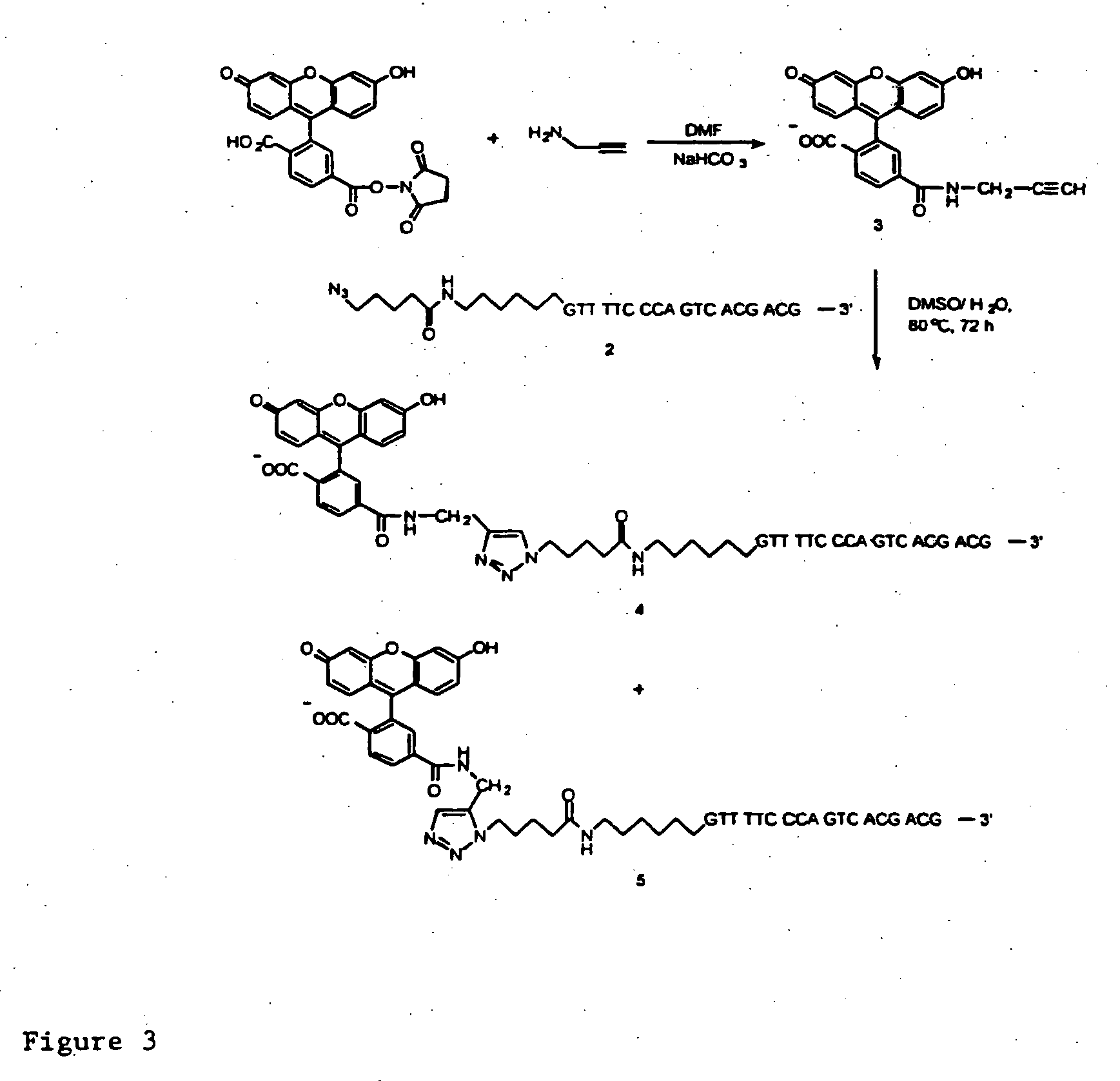
InactiveUS20080267878A1NanomedicineRadioactive preparation carriersBiotin-streptavidin complexMolecular imaging
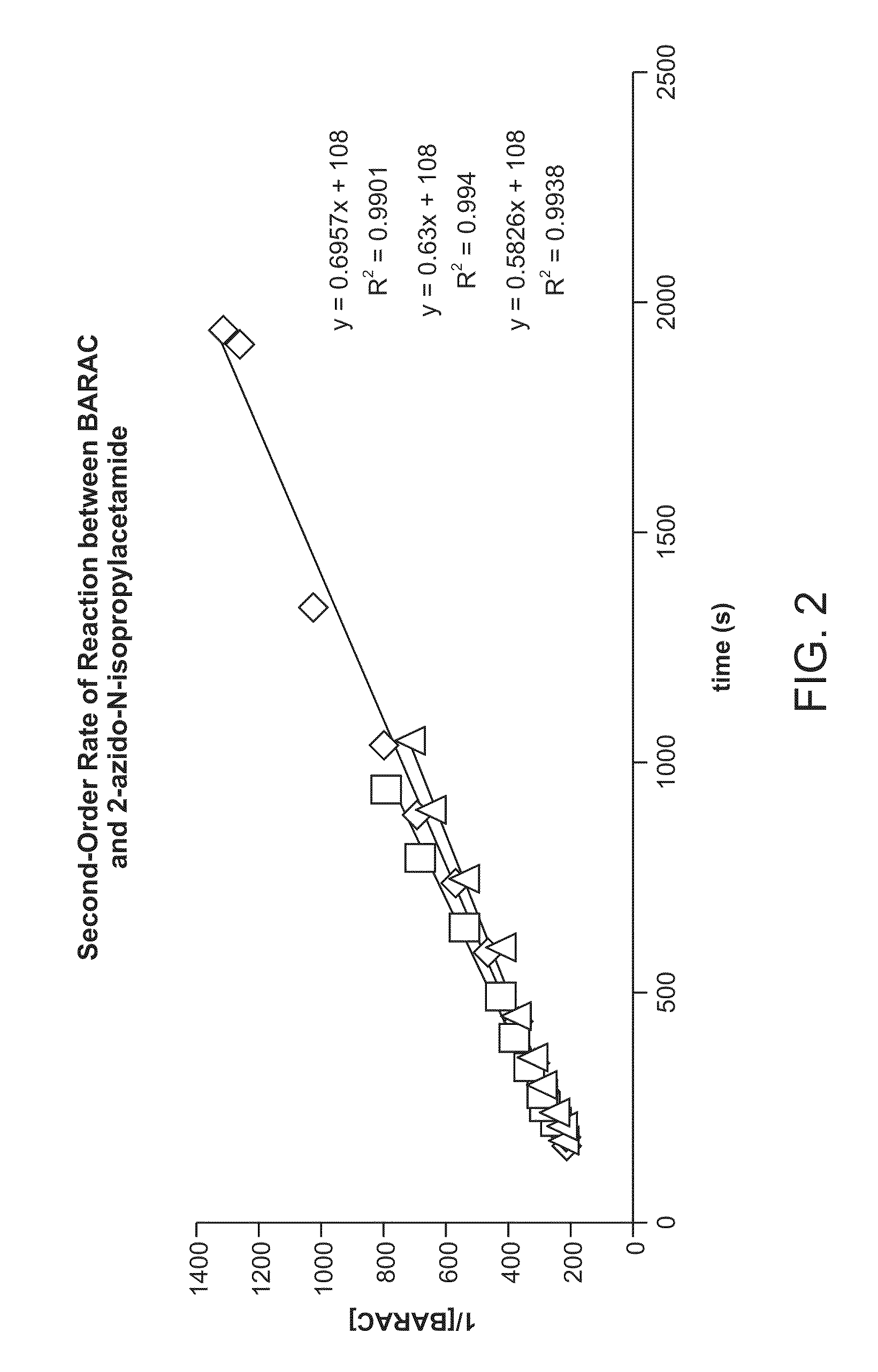
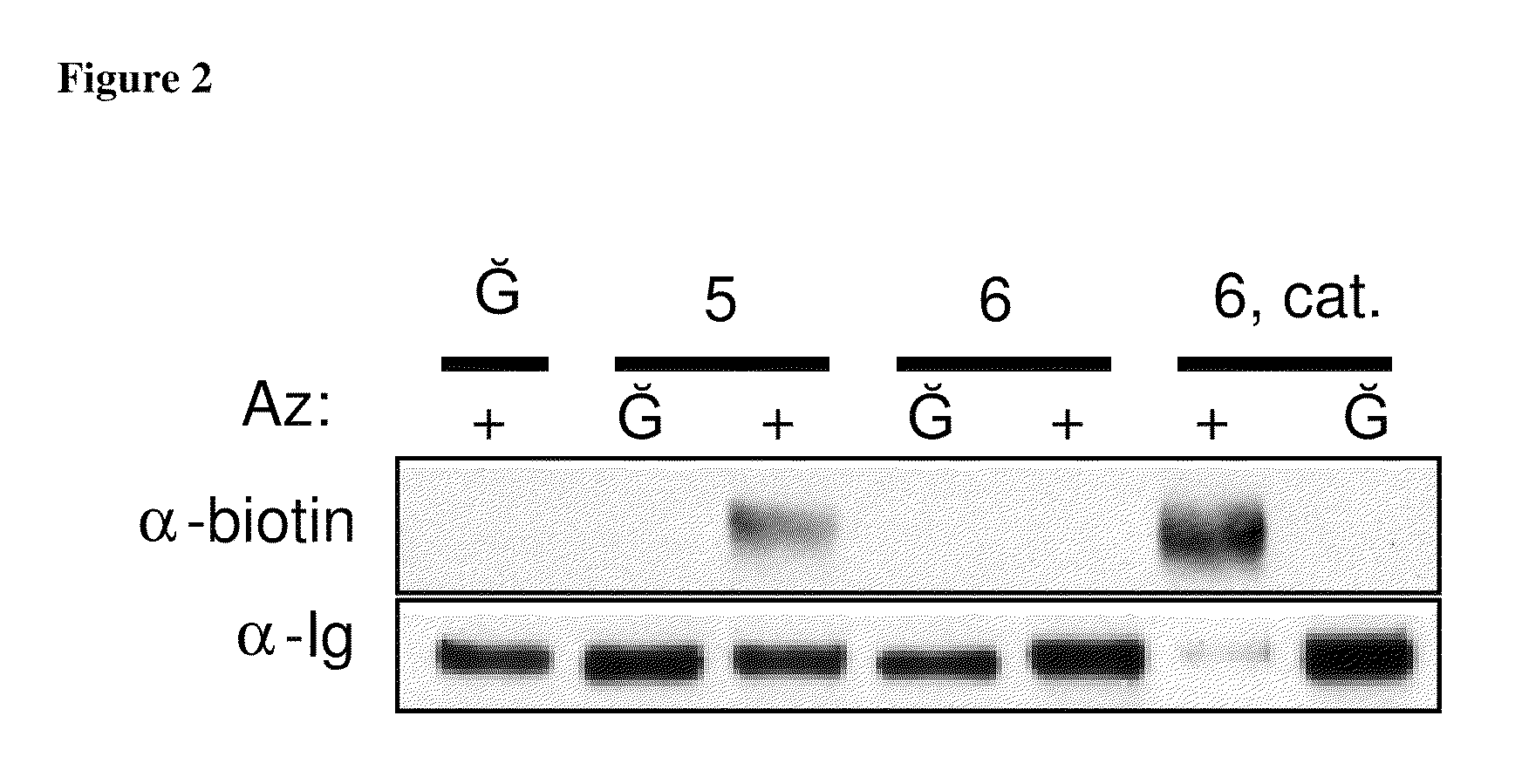
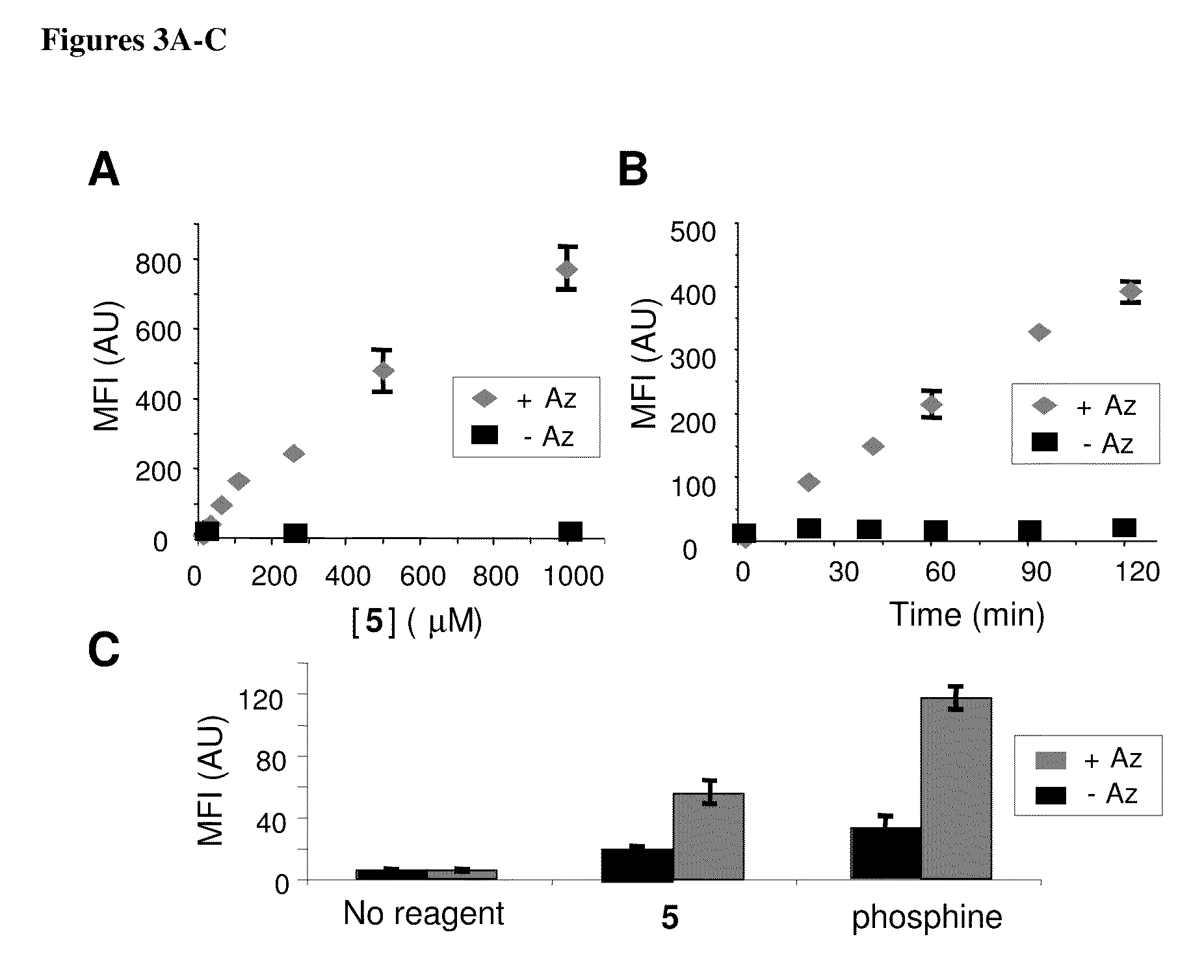
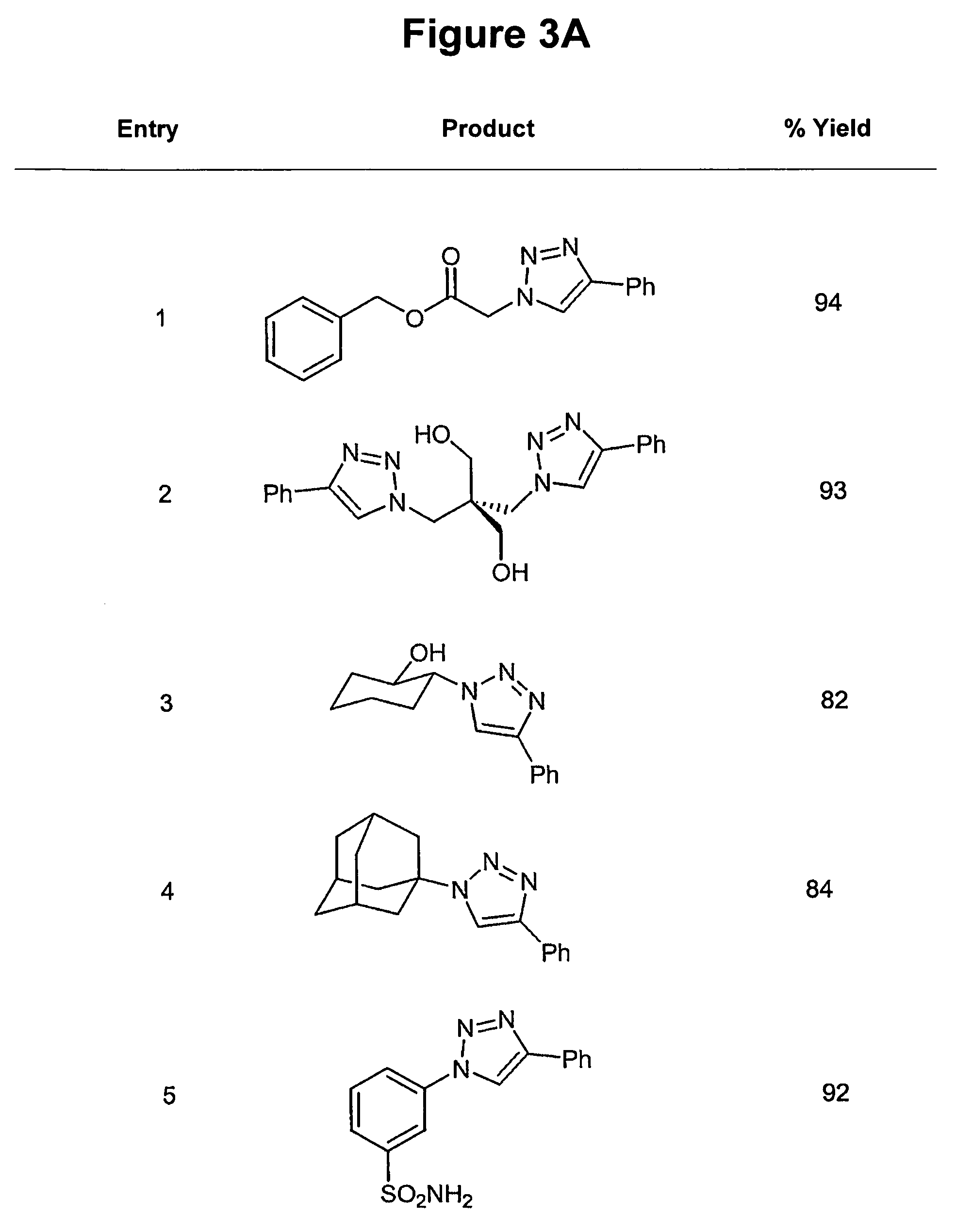
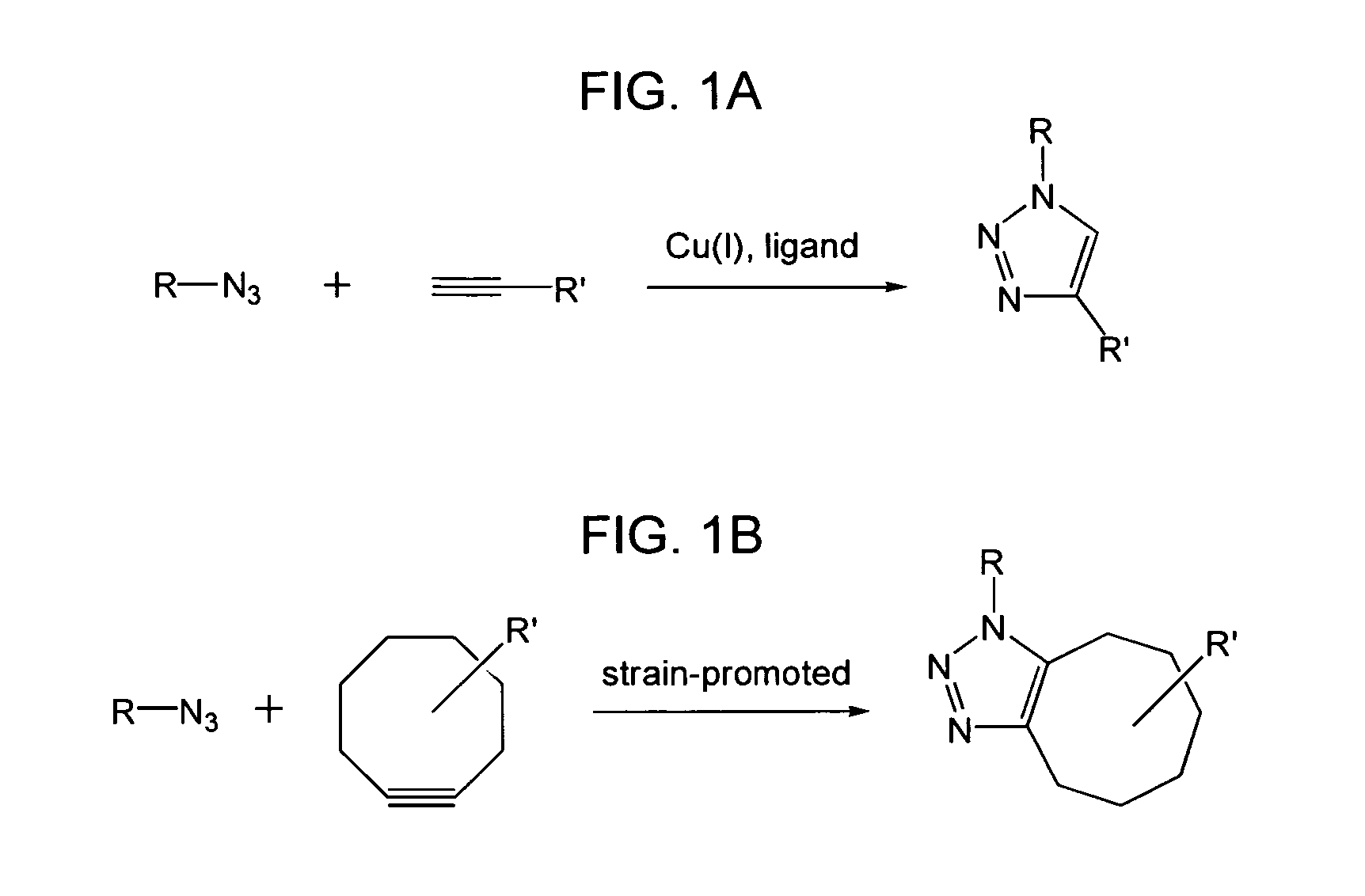
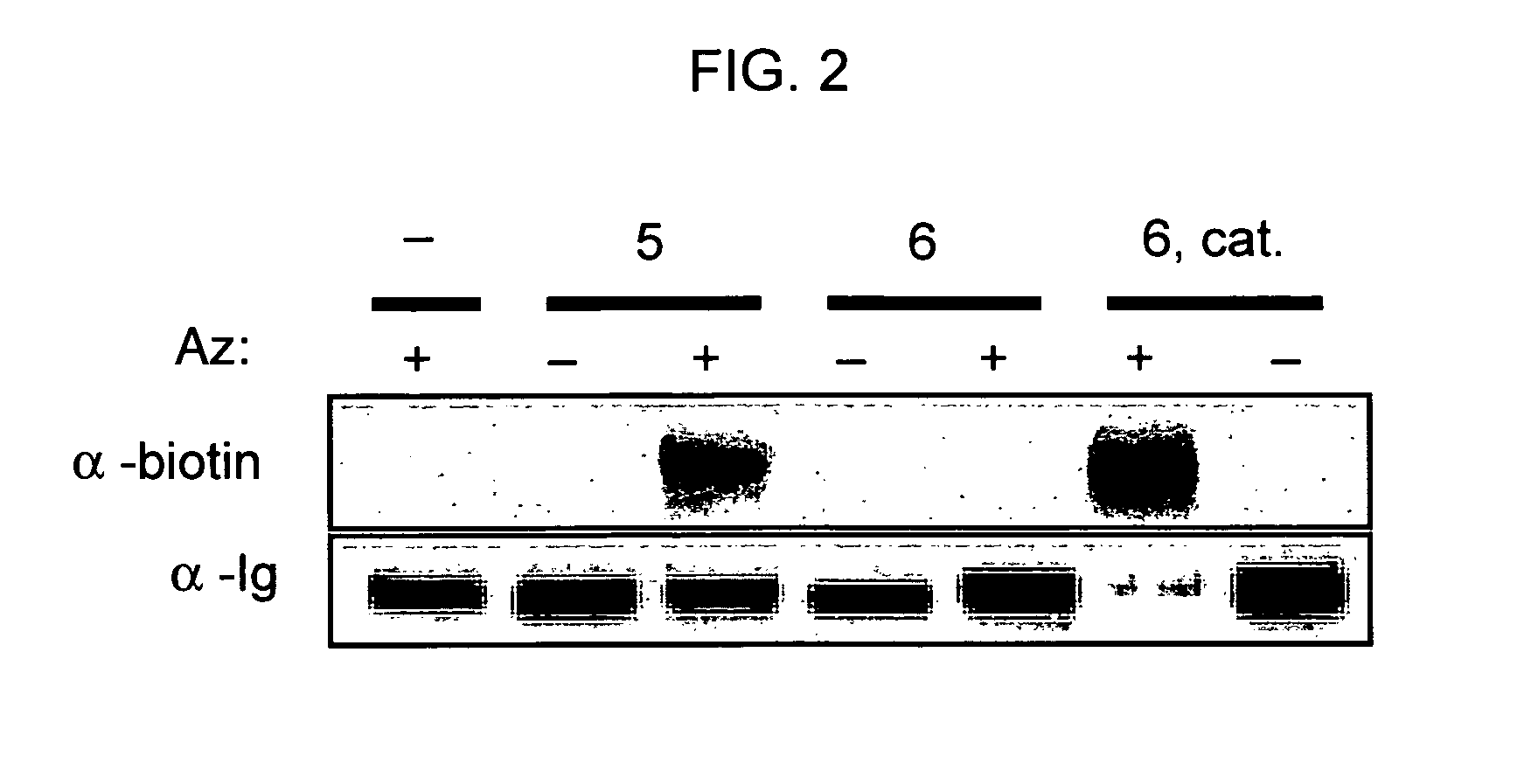
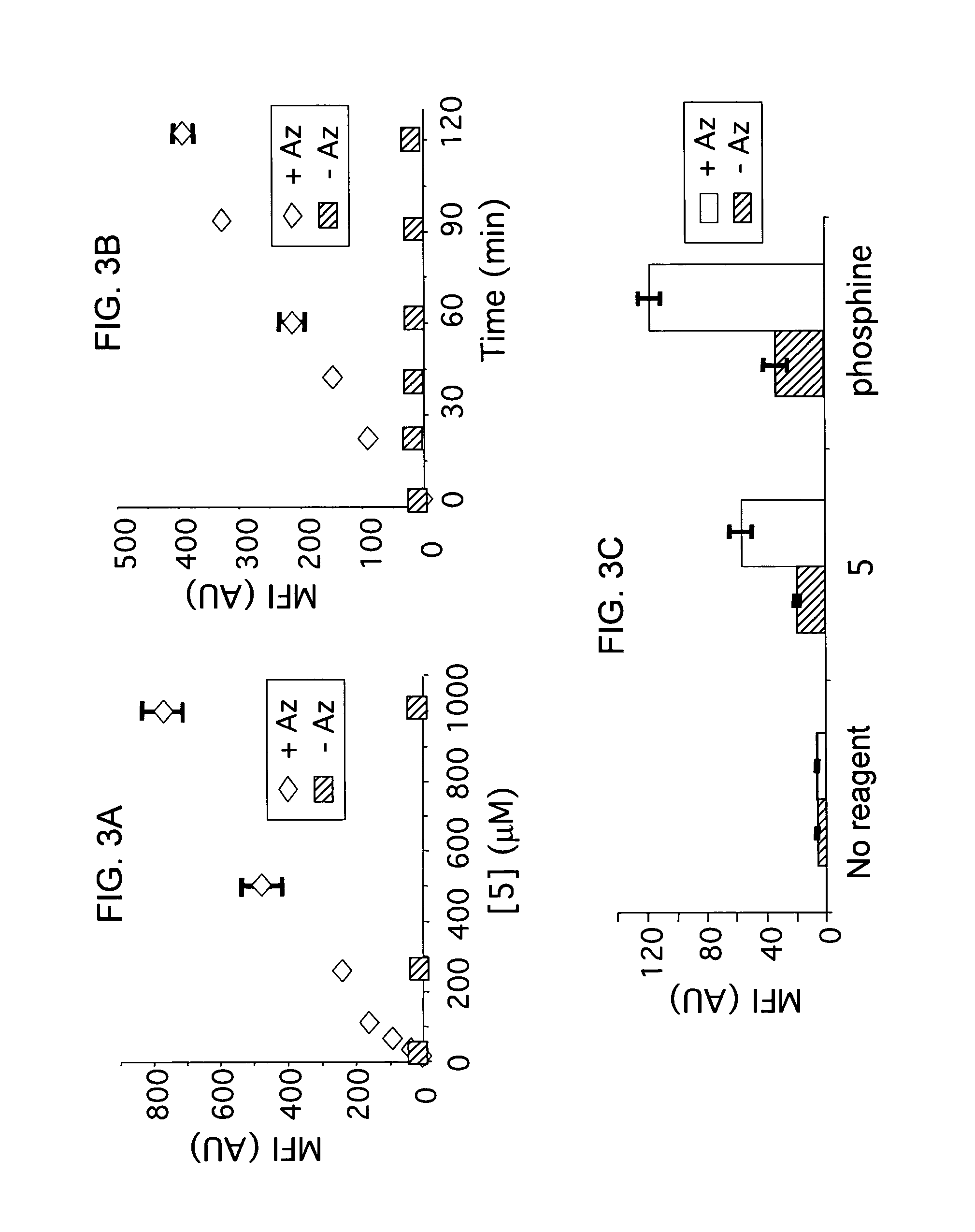
The use of a selective chemical and bioorthogonal reaction providing a covalent ligation such as the [3+2] cycloaddition, in targeted molecular imaging and therapy is presented, more specifically with interesting applications for pre-targeted imaging or therapy. Current pre-targeted imaging is hampered by the fact that it relies solely on natural / biological targeting constructs (biotin / streptavidin). Size considerations and limitations associated with their endogenous nature severely limit the number of applications. The present invention describes how the use of an abiotic, bio-orthogonal reaction which forms a stable adduct under physiological conditions, by way of a small or undetectable bond, can overcome these limitations.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Methods and compositions for labeling nucleic acids
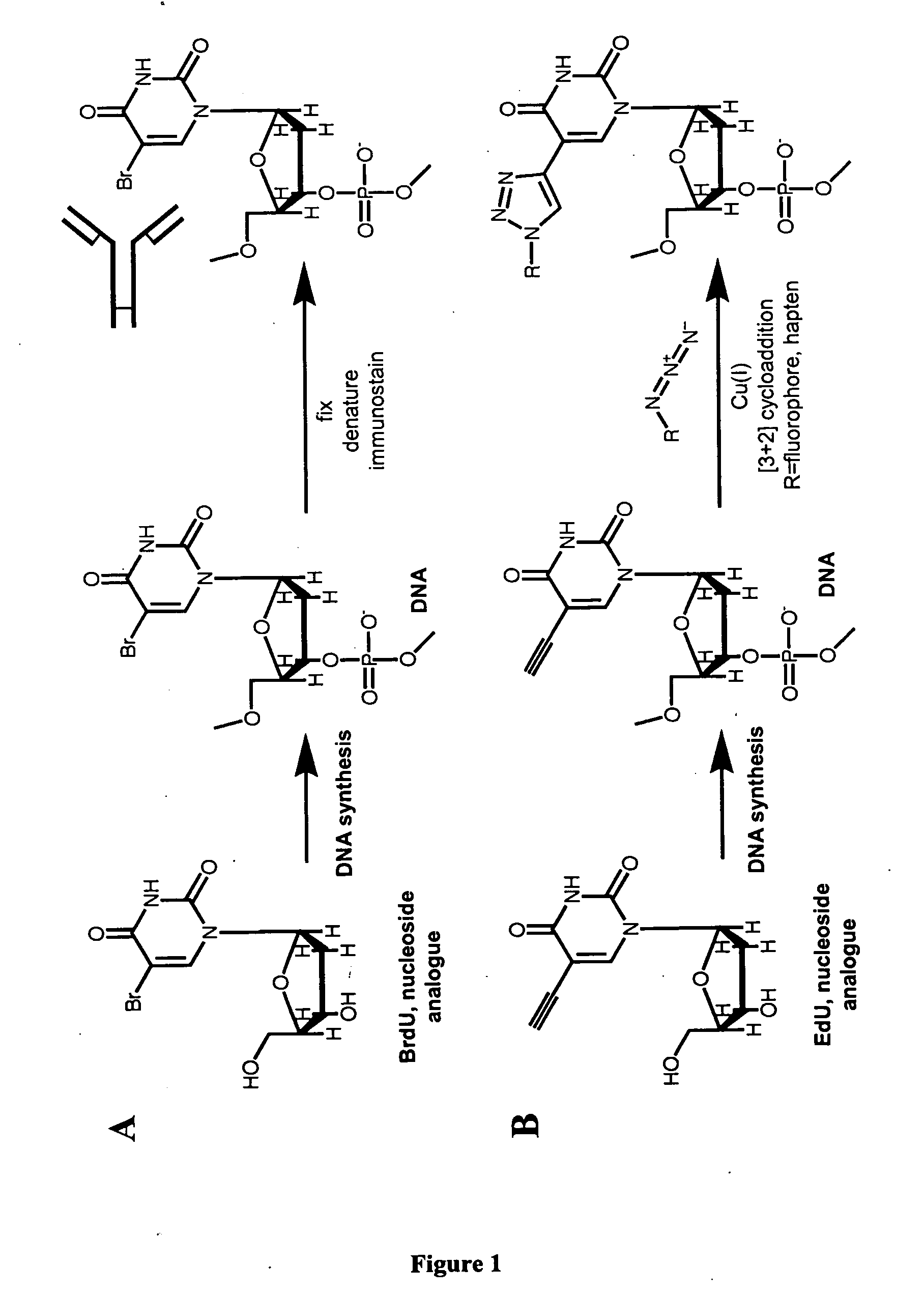
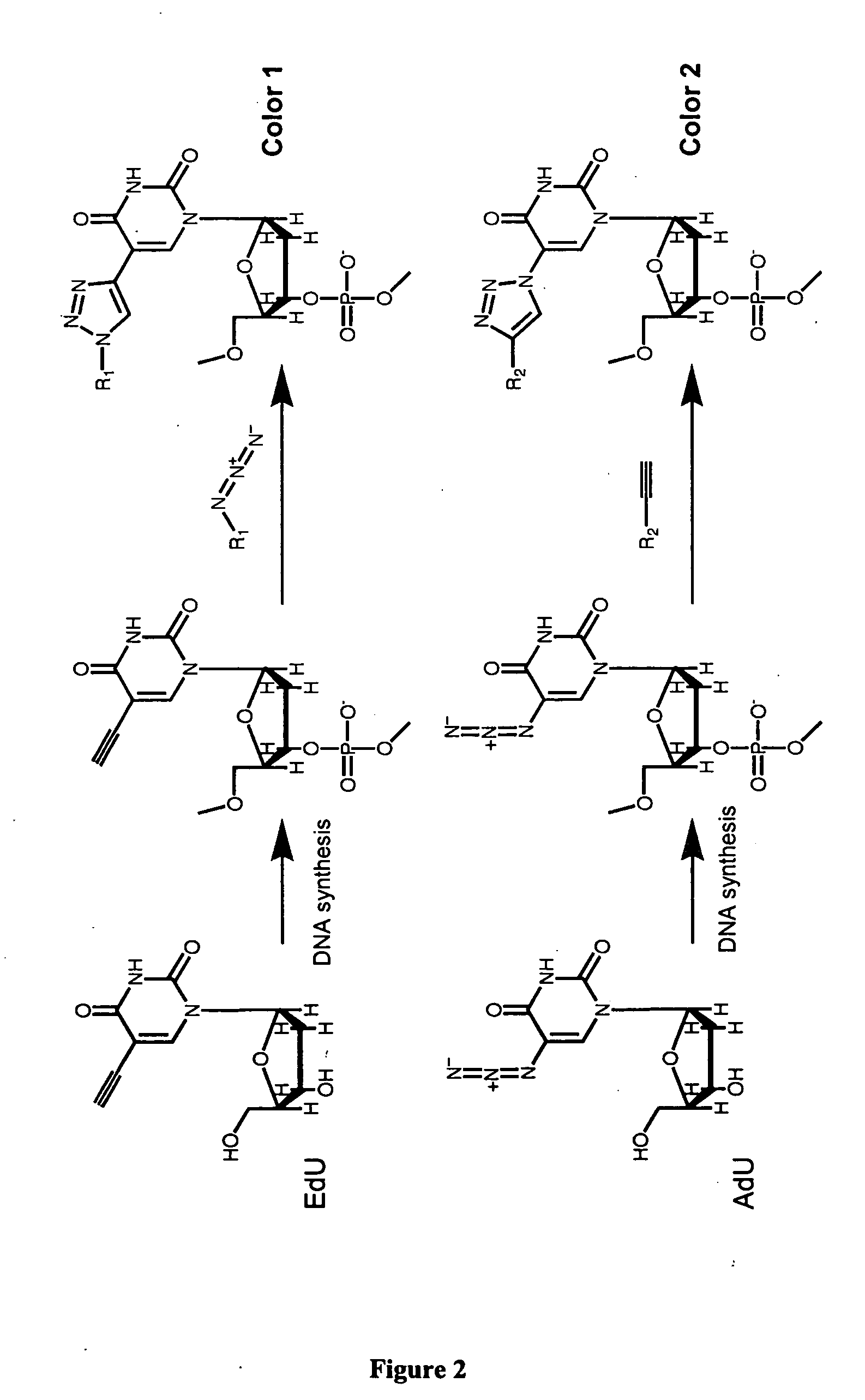
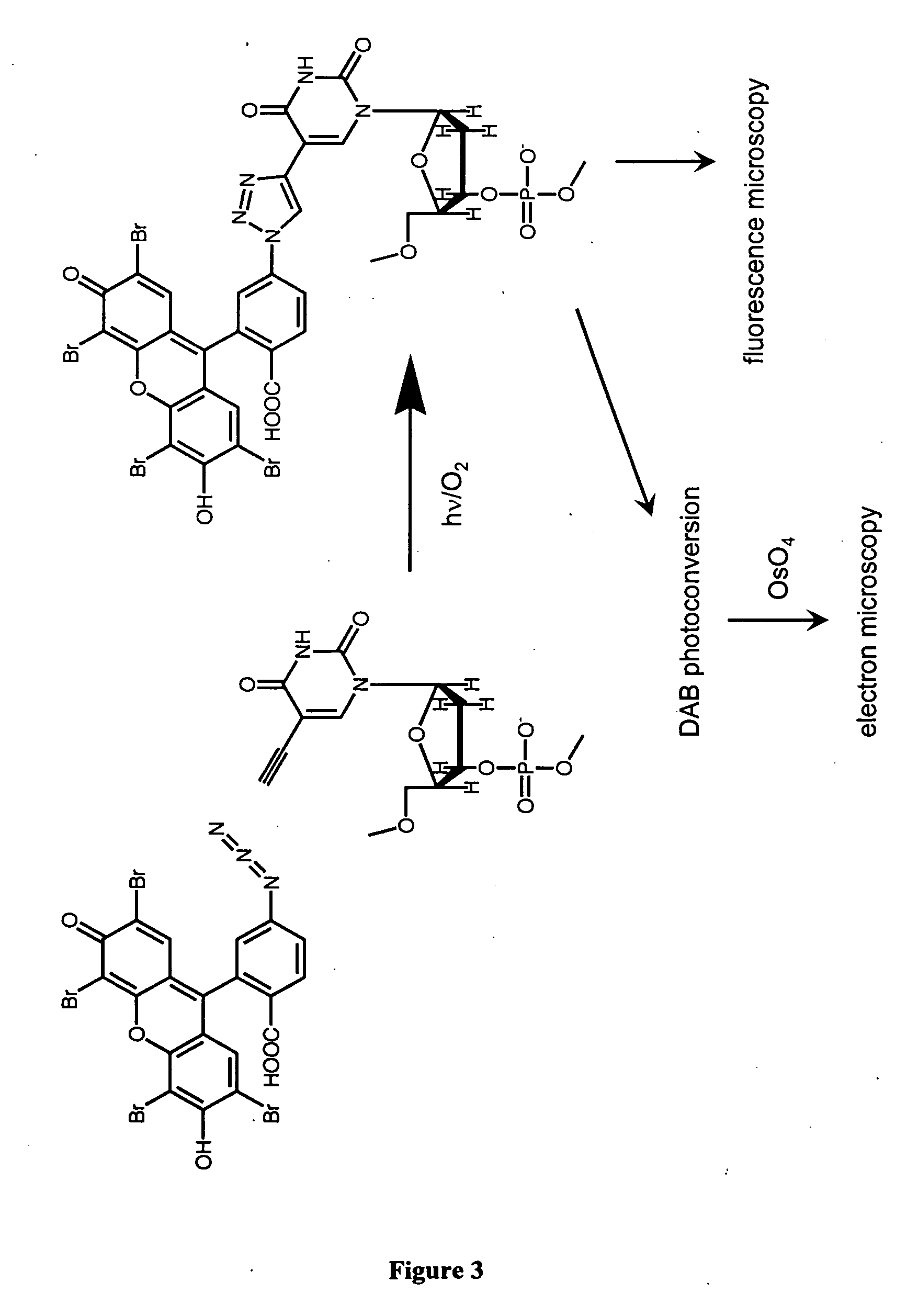
The present invention relates to methods for the labeling of nucleic acid polymers in vitro and in vivo. Certain methods are provided that include a [3+2] cycloaddition between a nucleotide analogue incorporated into a nucleic acid polymer and a reagent attached to a label. Other methods are provided that include a Staudinger ligation between a nucleotide analogue incorporated into a nucleic acid polymer and a reagent comprising a substituted triarylphosphine attached to a label. Such methods do not require fixation and denaturation and therefore can be applied to the labeling of nucleic acid polymers in living cells and in organisms. Also provided are methods for measuring cellular proliferation. In these methods, the amount of label incorporated into the DNA is measured as an indication of cellular proliferation. The methods of the invention can be used in a wide variety of applications including clinical diagnosis of diseases and disorders in which cellular proliferation is involved, toxicity assays, and as a tool for the study of chromosomes' ultrastructures.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
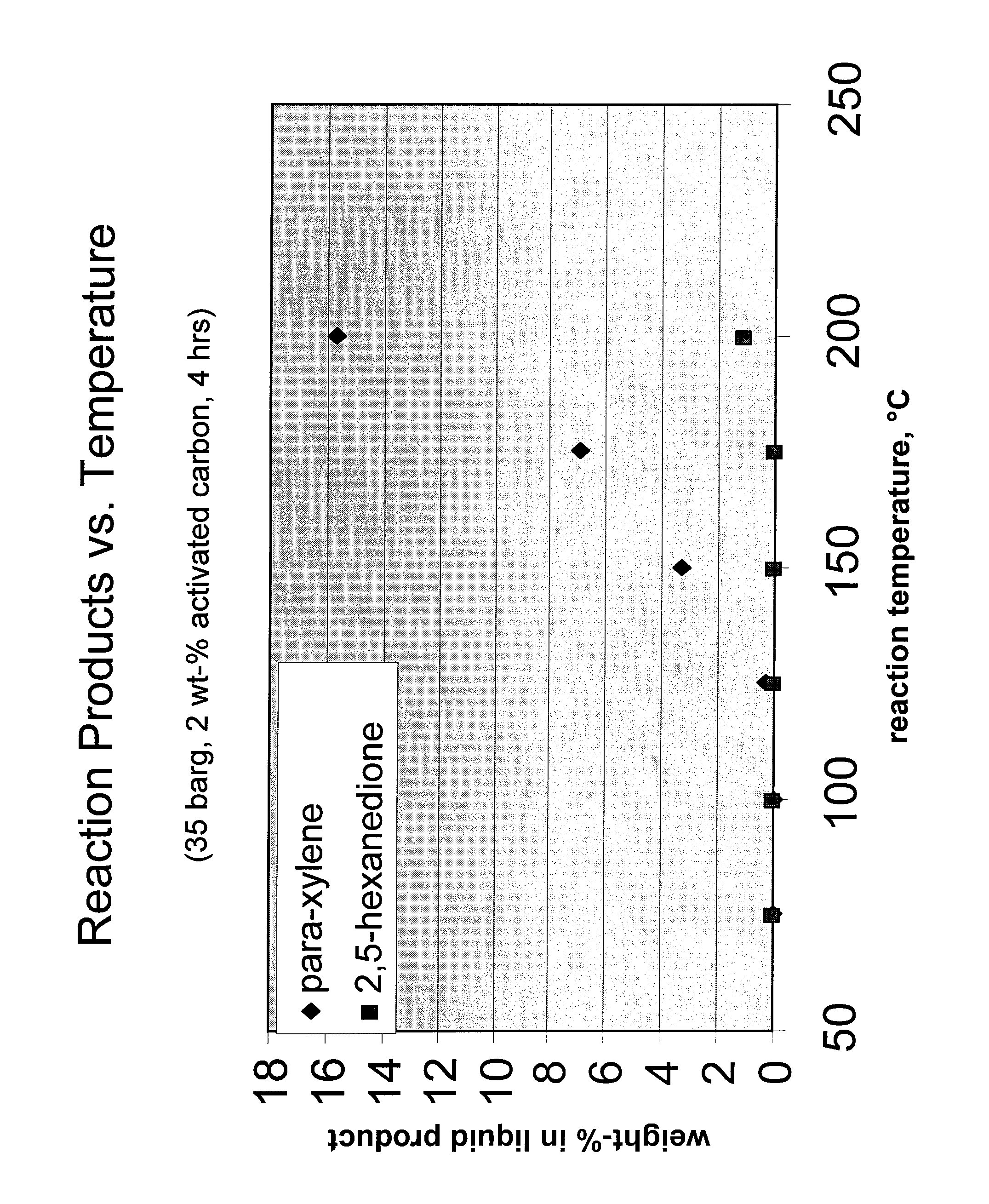
Carbohydrate route to para-xylene and terephthalic acid
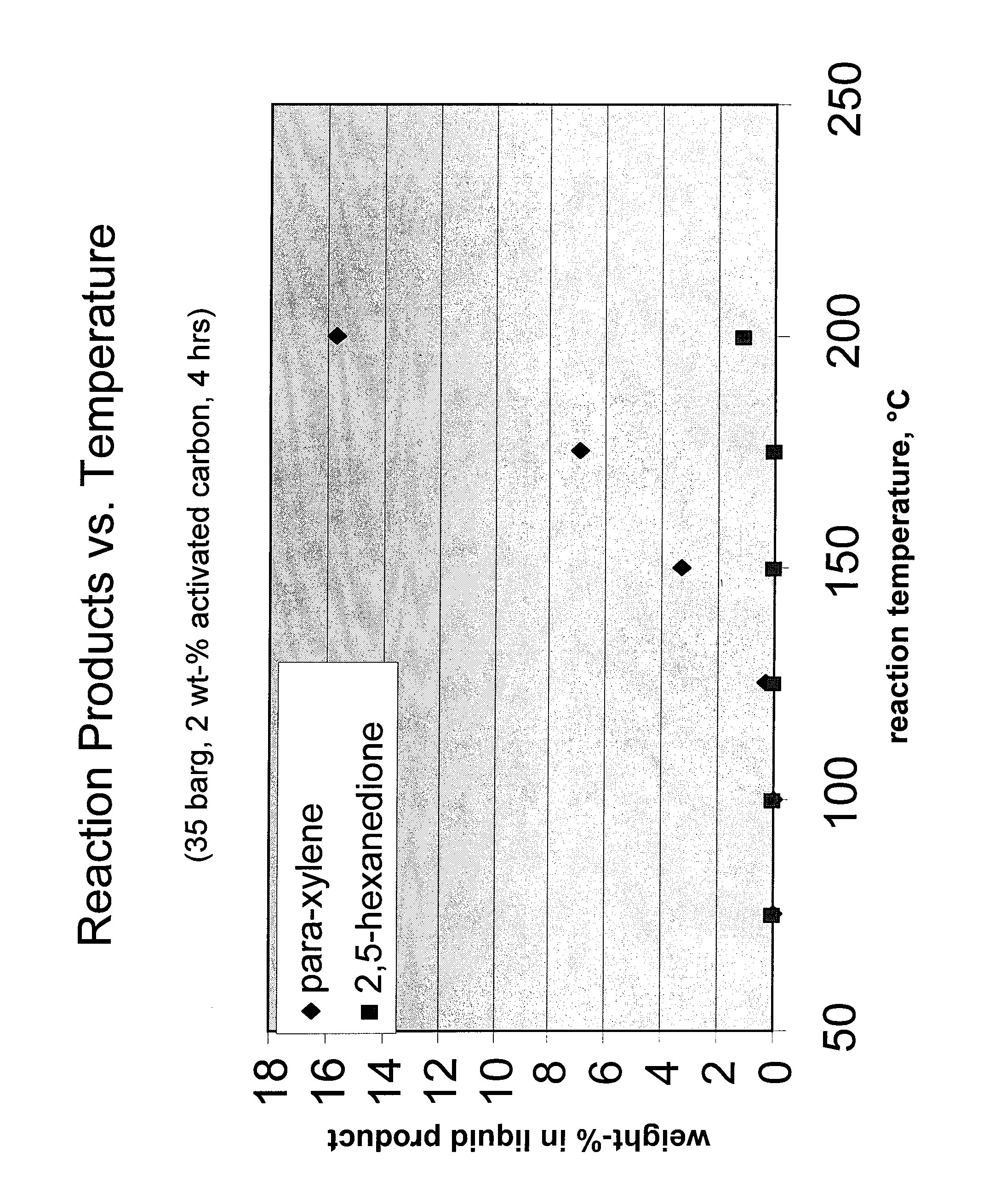
Catalytic processes for the conversion of 2,5-dimethyl furan (DMF) to para-xylene are described. Para-xylene is a key product that is currently obtained commercially from petroleum sources. However, it has now been determined that the cycloaddition of ethylene to DMF provides an alternative route to para-xylene. Advantageously, the DMF starting material for the processes may be synthesized from carbohydrates (e.g., glucose or fructose), thereby providing a pathway that relies at least partly, if not completely, on renewable feedstocks.
Owner:UOP LLC
Method for synthesizing cricoid carbonate by addition reaction of carbon dioxide and epoxy compound ring
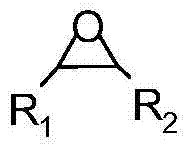
InactiveCN101037431AHigh activityHigh selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEpoxyCycloaddition
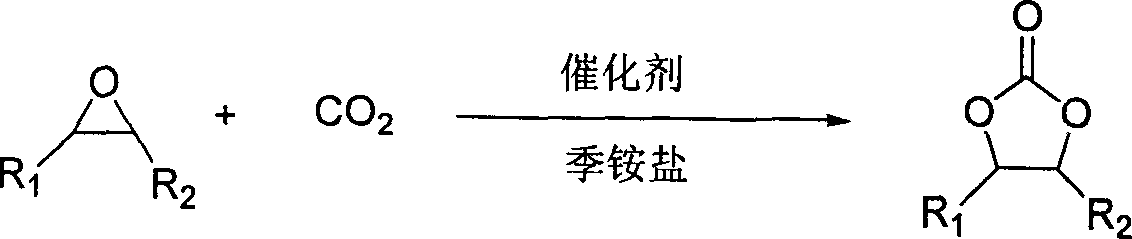
The invention discloses a method for synthesizing cyclic carbonate by a cycloaddition reaction of carbon dioxide and epoxy-compound. The catalyzer is composed of metal-salt, ion liquid and quaternary ammonium salt. The cyclic carbonate is produced by catalyzing the carbon dioxide and epoxy-compound for a cycloaddition reaction under a reaction temperature of 30-200 DEG C, and a carbon dioxide pressure of 0.5-10 MPa. The reaction progress doesn't add any organic solvent. The purified cyclic carbonate by a simple purification may achieve a high purity more than 98%. The catalyzer can be reused and keeps an invariable activity. The catalyzer system has a high activity and low cost. The method can synthesize the cyclic carbonate quickly and highly selectively in a simple operation so than it will be a perfect industrial prospect.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
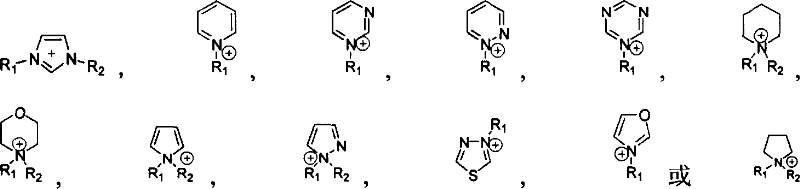
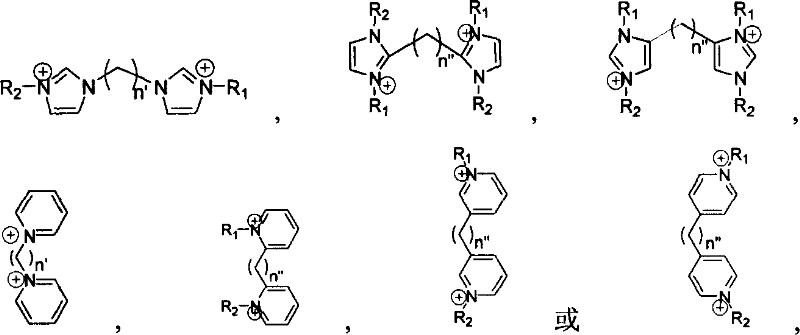
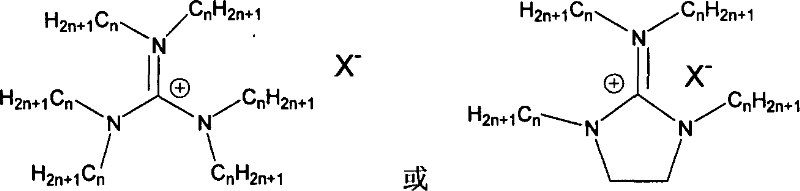
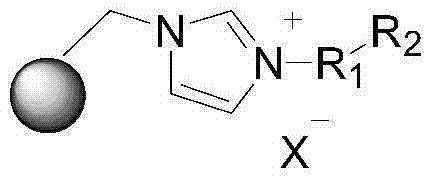
Supported ionic liquid catalyst, as well as preparation and application thereof
InactiveCN103495437AChemical choice goodMild reaction conditionsProductsOrganic chemistryEpoxyCycloaddition
The invention discloses a supported ionic liquid catalyst. The supported ionic liquid catalyst is characterized in that imidazole is immobilized onto chloromethylated FDU mesoporous phenolic resin by nucleophilic reaction and reacts with halides containing different functional groups to prepare a mesoporous material supported functional imidazole type ionic liquid catalyst. The preparation of the catalyst comprises the following steps: chloromethylation of a mesoporous material, imidazolation and supporting of ionic liquid. The catalyst is used for synthesizing a cyclic carbonate by cyclic addition reaction of carbon dioxide and an epoxy compound, and the using quantity of the catalyst is 0.1-2mol% of the liquid content of the epoxy compound. Compared with the prior art, a large number of hydroxyl groups exist in the mesoporous material, and the supported ionic liquid catalyst has the double advantages of an inorganic mesoporous material and an organic polymer, as well as large specific surface area, adjustable pore structure, good hydrophobicity and chemical stability, high catalytic activity, good chemical selectivity, easiness in separation and recovery, mild reaction conditions, environmental friendliness and easiness in industrial implementation.
Owner:EAST CHINA NORMAL UNIV
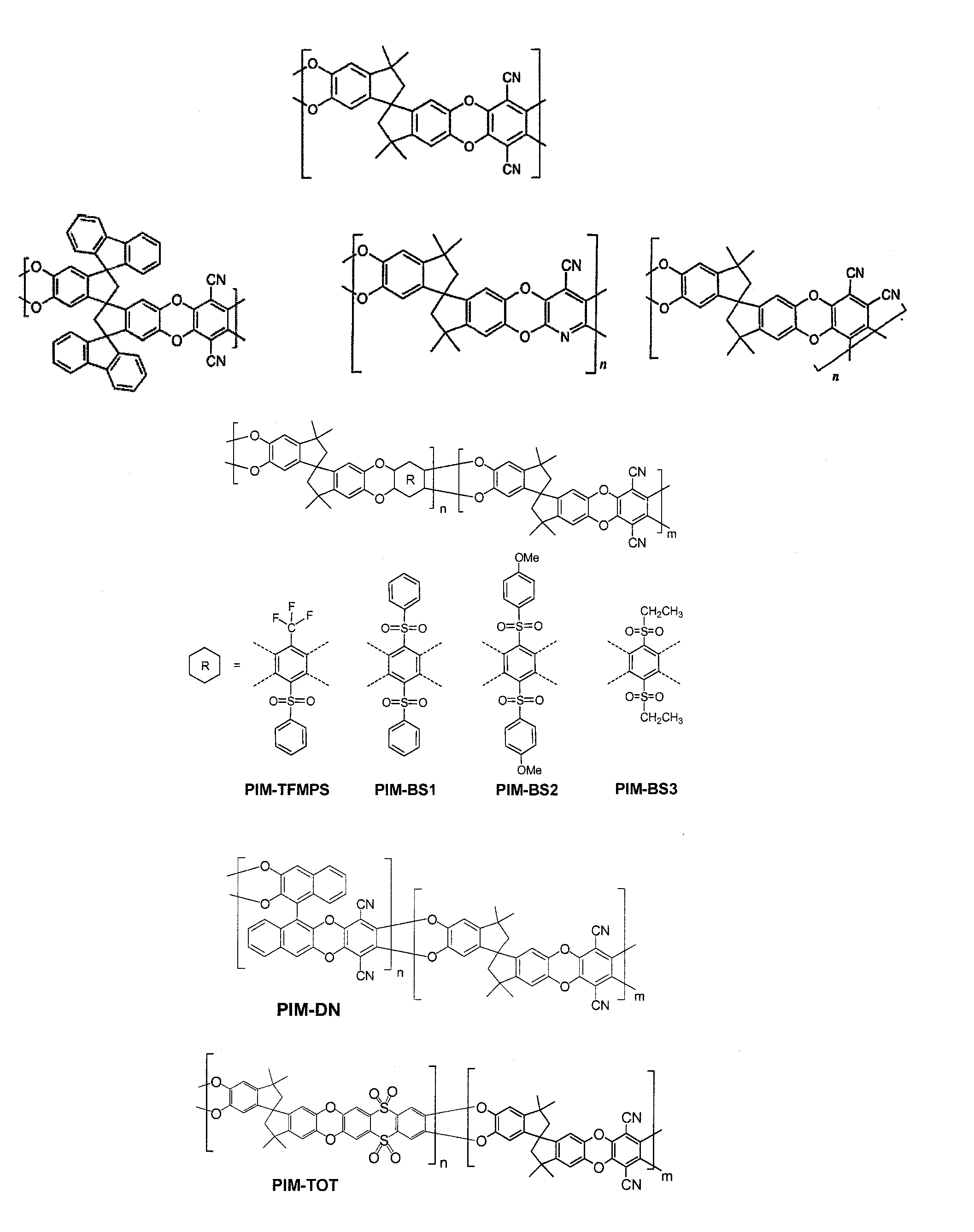
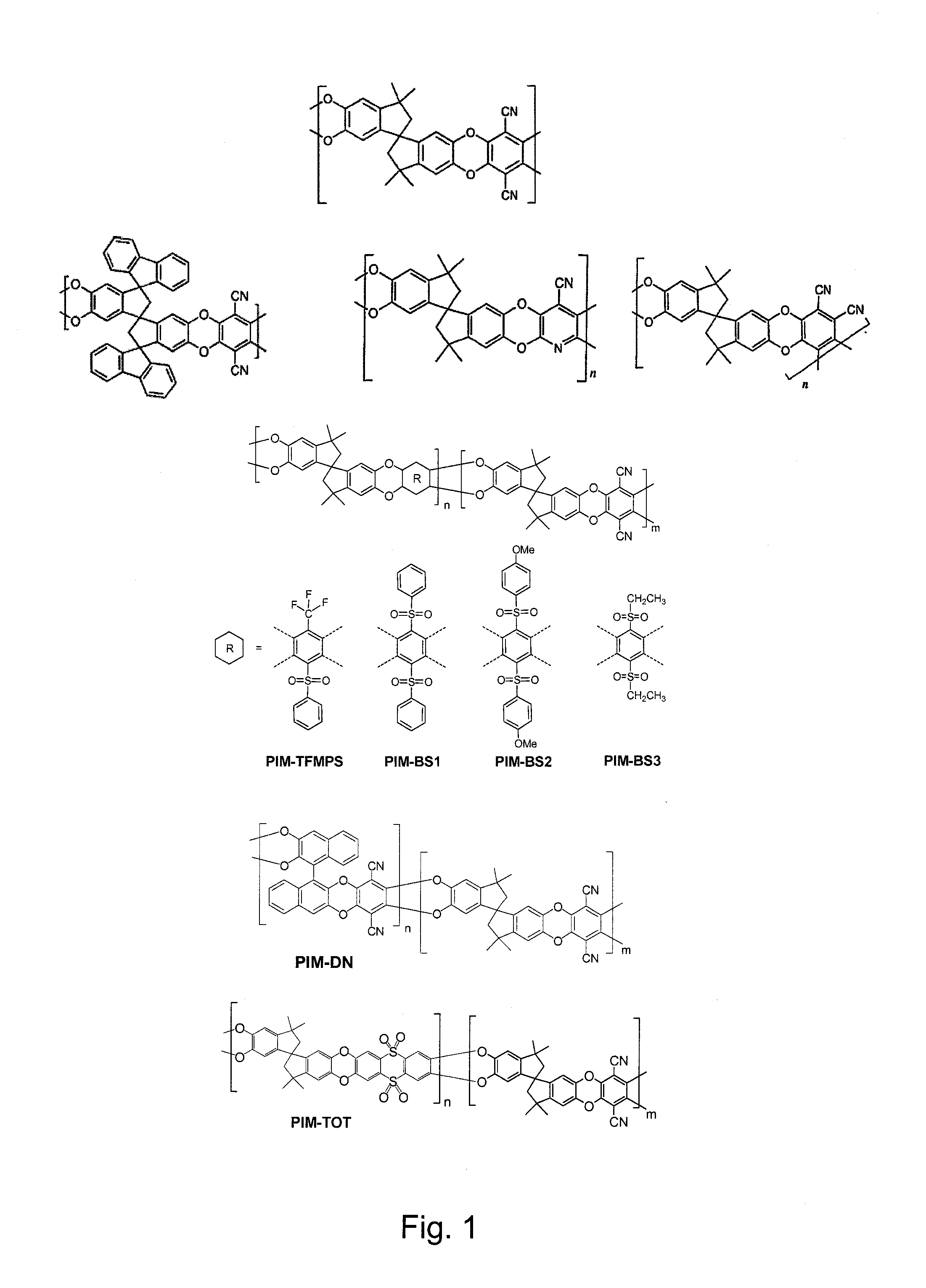
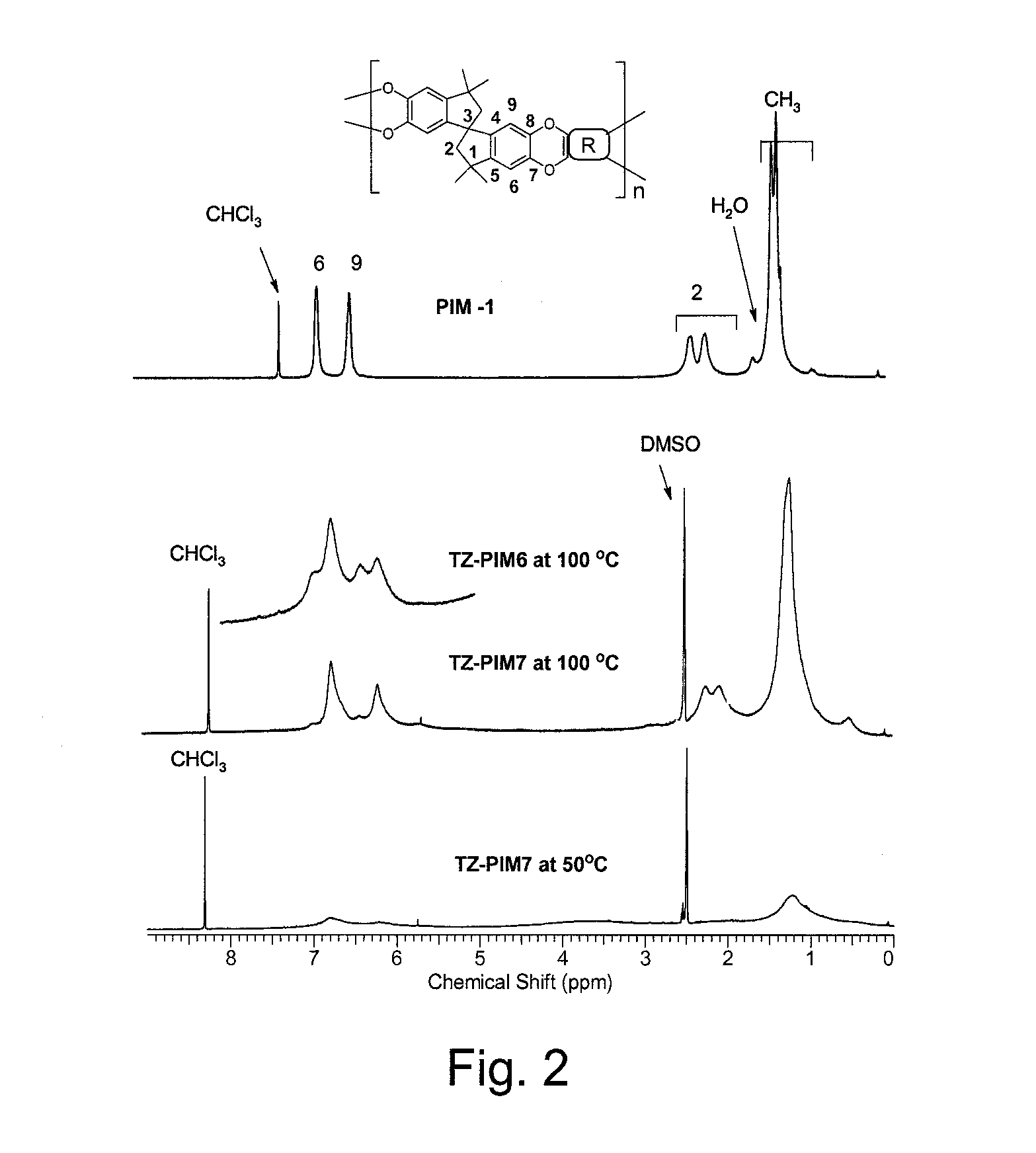
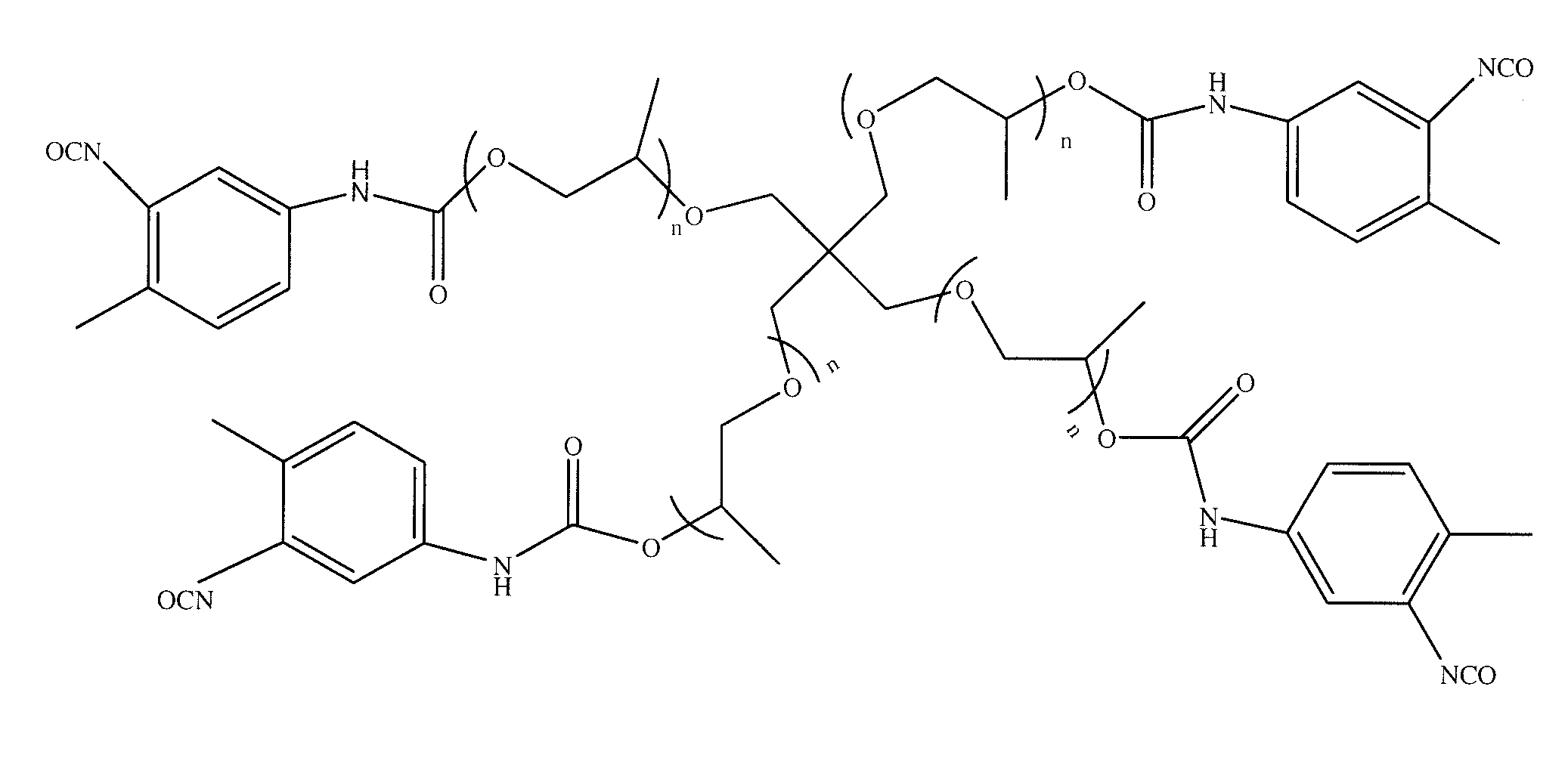
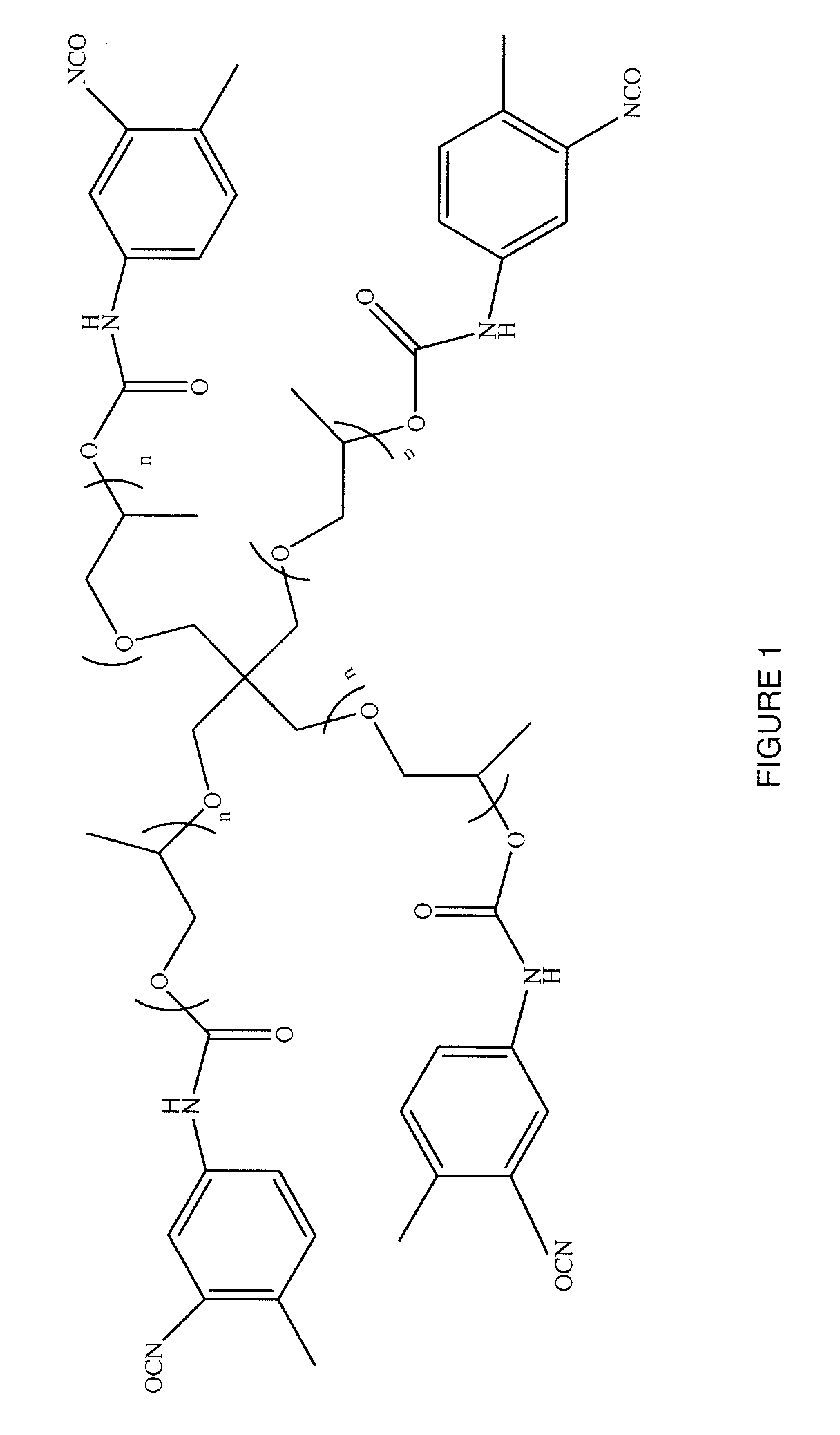
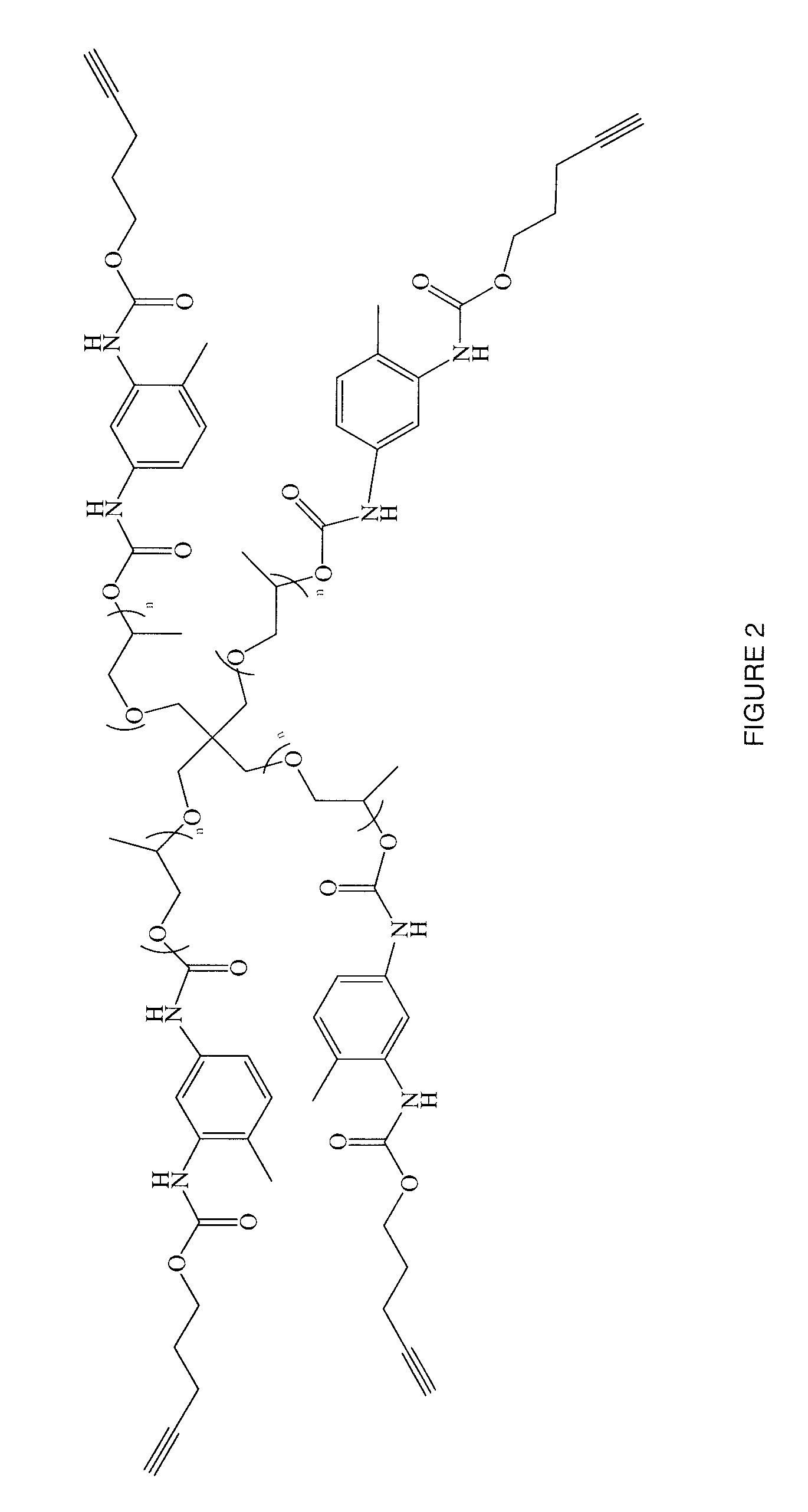
Polymers of intrinsic microporosity containing tetrazole groups
InactiveUS8623928B2Extending possible structureIncrease rangeOrganic chemistryOther chemical processesTetrazoleClick chemistry
The invention provides a tetrazole-containing polymer of intrinsic microporosity comprising (10) or more subunits, wherein one or more of the subunits comprise one or more tetrazolyl moieties. In one embodiment, a polymer of intrinsic microporosity (PIM-1) was modified using a “click chemistry” [2+3] cycloaddition reaction with sodium azide and zinc chloride to yield new PIMs containing tetrazole units. Polymers of the present invention are useful as high-performance materials for membrane-based gas separation, materials for ion exchange resins, materials for chelating resins, materials for superabsorbents, materials for ion conductive matrixes, materials for catalyst supports or materials for nanoparticle stabilizers.
Owner:NAT RES COUNCIL OF CANADA
Compositions and methods for delivering a substance to a biological target
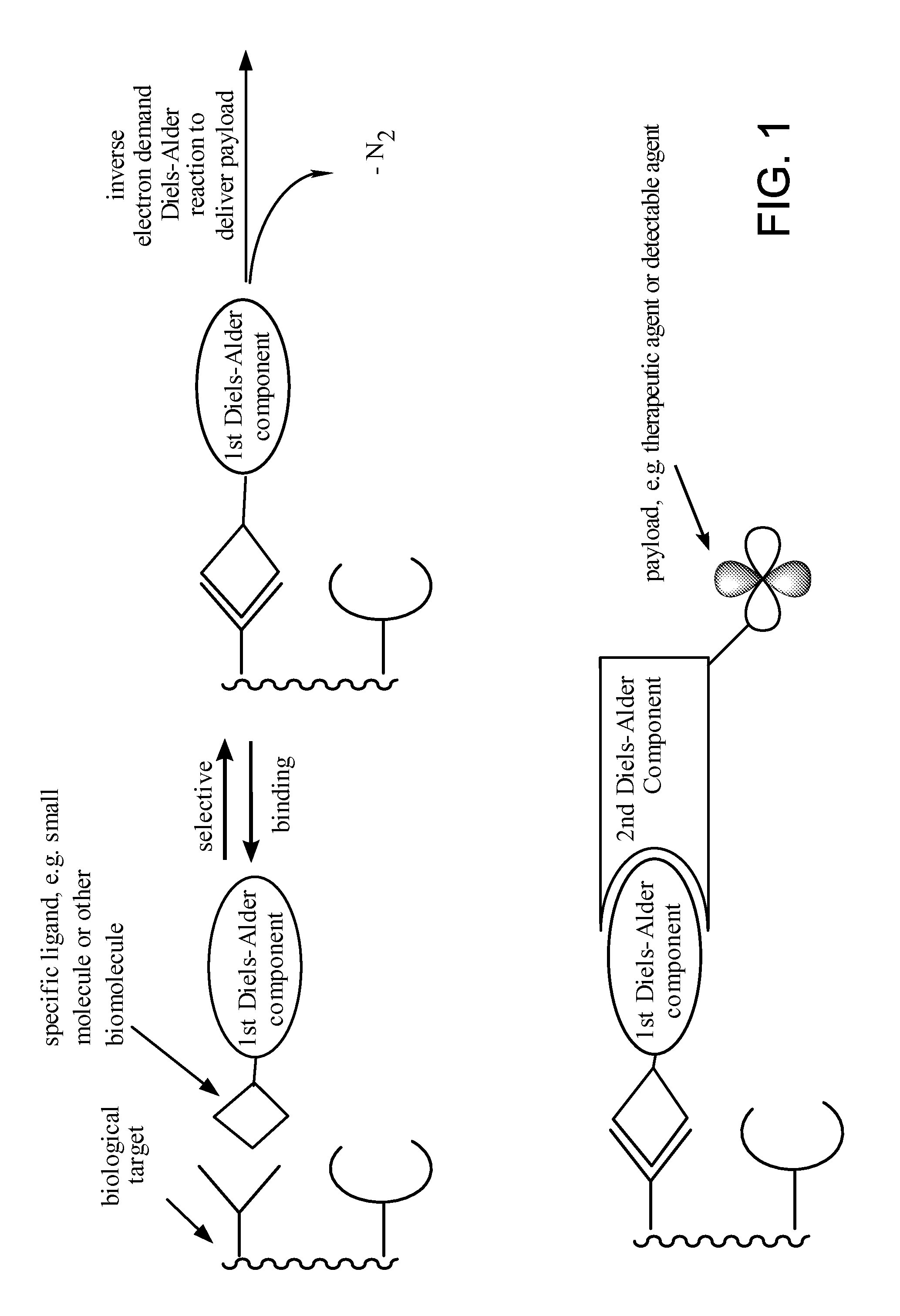
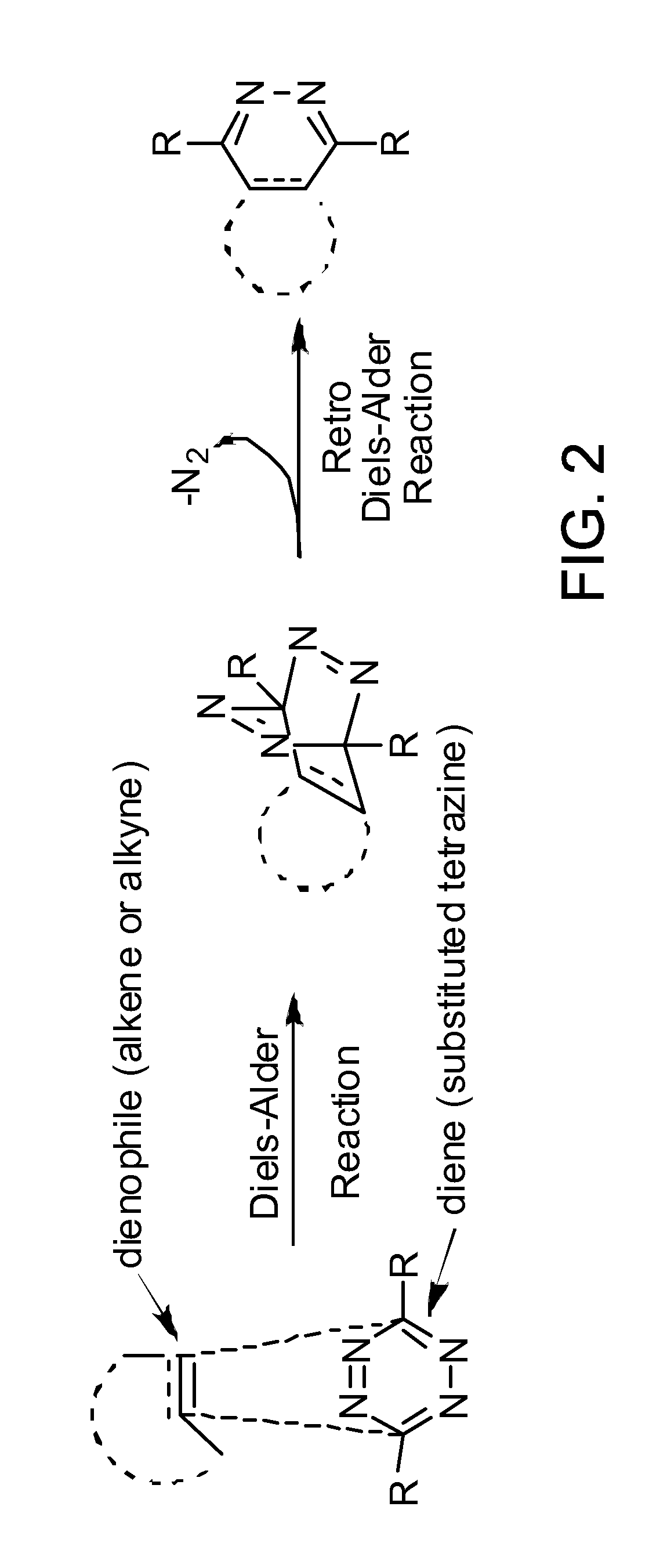
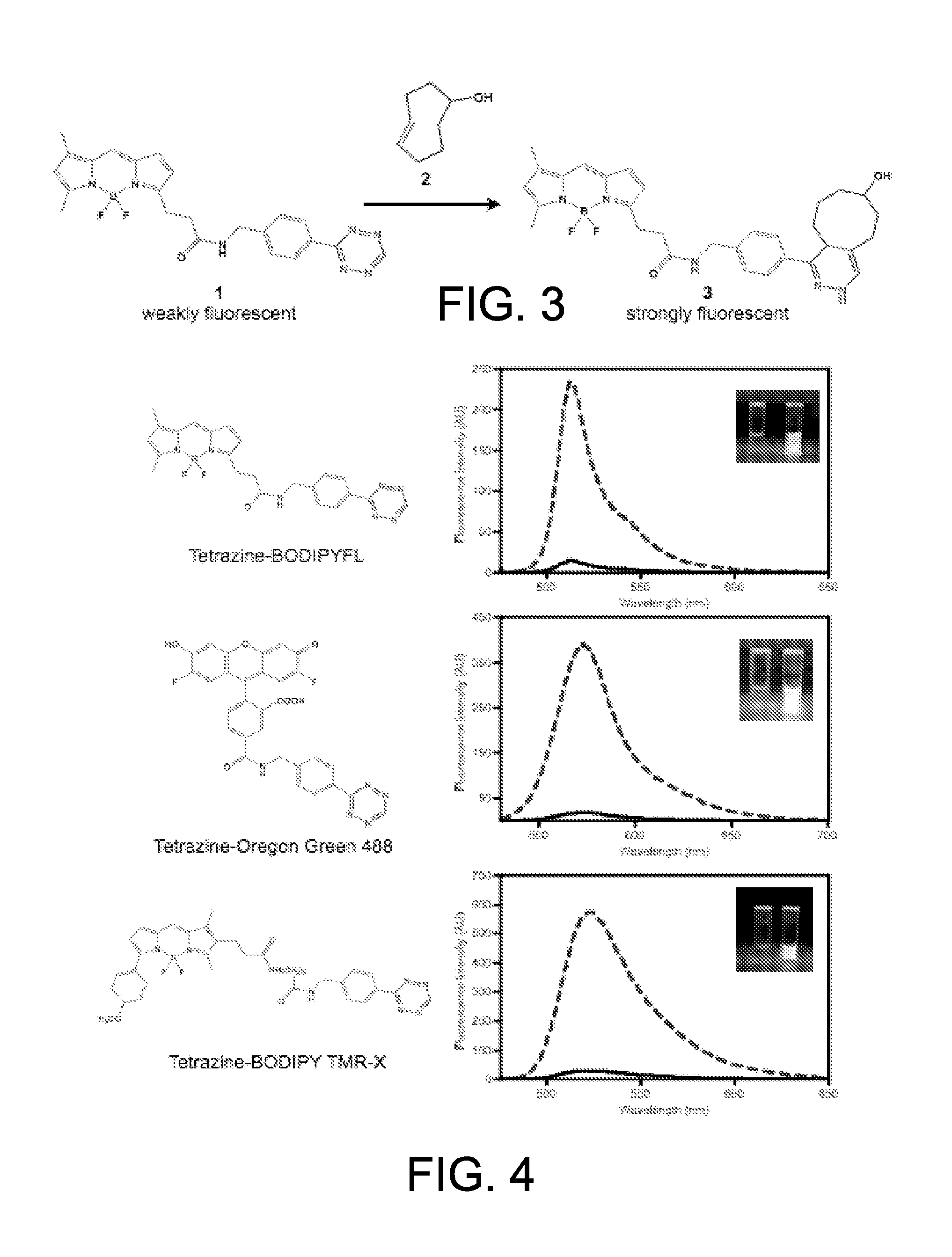
ActiveUS20110268654A1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsBiological targetCycloaddition
The present application provides compositions and methods using bioorthogonal inverse electron demand Diels-Alder cycloaddition reaction for rapid and specific covalent delivery of a “payload” to a ligand bound to a biological target.
Owner:THE GENERAL HOSPITAL CORP
Carbohydrate route to para-xylene and terephthalic acid
ActiveUS8314267B2Organic compound preparationHydrocarbon by hydrocarbon and non-hydrocarbon condensationFuranCycloaddition
Catalytic processes for the conversion of 2,5-dimethyl furan (DMF) to para-xylene are described. Para-xylene is a key product that is currently obtained commercially from petroleum sources. However, it has now been determined that the cycloaddition of ethylene to DMF provides an alternative route to para-xylene. Advantageously, the DMF starting material for the processes may be synthesized from carbohydrates (e.g., glucose or fructose), thereby providing a pathway that relies at least partly, if not completely, on renewable feedstocks.
Owner:UOP LLC
Copper-catalysed ligation of azides and acetylenes
InactiveUS20080214831A1Organic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleSufficient time
A copper catalyzed click chemistry ligation process is employed to bind azides and terminal acetylenes to provide 1,4-disubstituted 1,2,3-triazole triazoles. The process comprises contacting an organic azide and a terminal alkyne with a source of reactive Cu(I) ion for a time sufficient to form by cycloaddition a 1,4-disubstituted 1,2,3-triazole. The source of reactive Cu(I) ion can be, for example, a Cu(I) salt or copper metal. The process is preferably carried out in a solvent, such as an aqueous alcohol. Optionally, the process can be performed in a solvent that comprises a ligand for Cu(I) and an amine.
Owner:TETARD INC
Method for synthesizing cyclic carbonate from carbon dioxide and epoxy compound through reaction of cycloaddition
ActiveCN1796384AMild reaction conditionsSimple processOrganic chemistryChemical recyclingOrganic solventSynthesis methods
This invention relates to a cycloaddition synthesis method for cyclic carbonates from carbon dioxide and epoxides. In this method, both supported catalysts and cocatalysts are adopted and the cycloaddition reaction is kept at a temperature of 50~200 deg.C and a carbon dioxide pressure of 0.1~10MPa for 0.5~10 hours. Qualitative and quantitative analysis with 6890 / 5973 chromatography-MS and NMR shows that the product purity is over 99% and the highest separation yield can be up to 95%. Compared to current methods, no organic solvents are adopted in this invention and therefore it is free from the toxicity of bezene, dichloromethane, and so on.
Owner:江苏华昌新材料技术研究有限公司
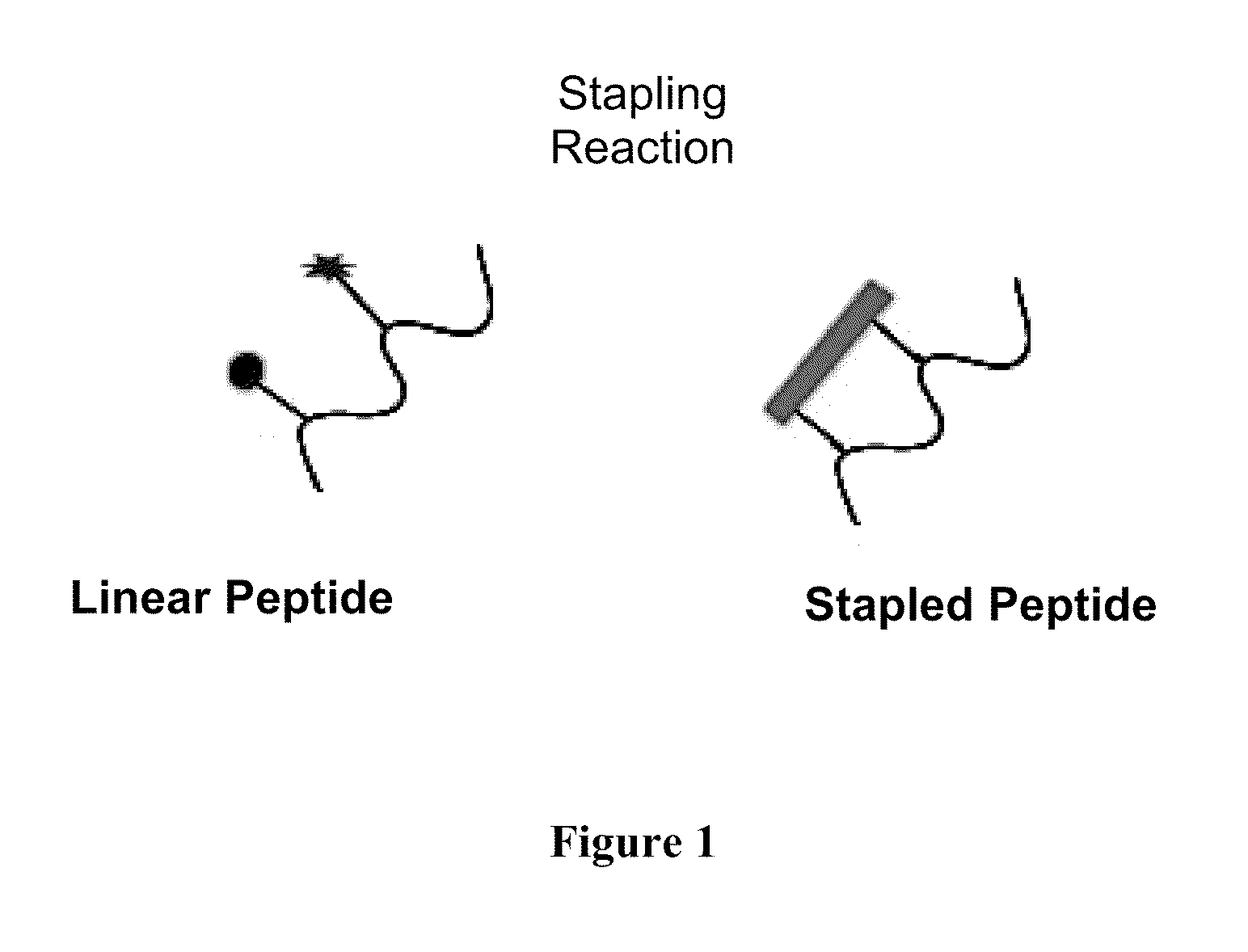
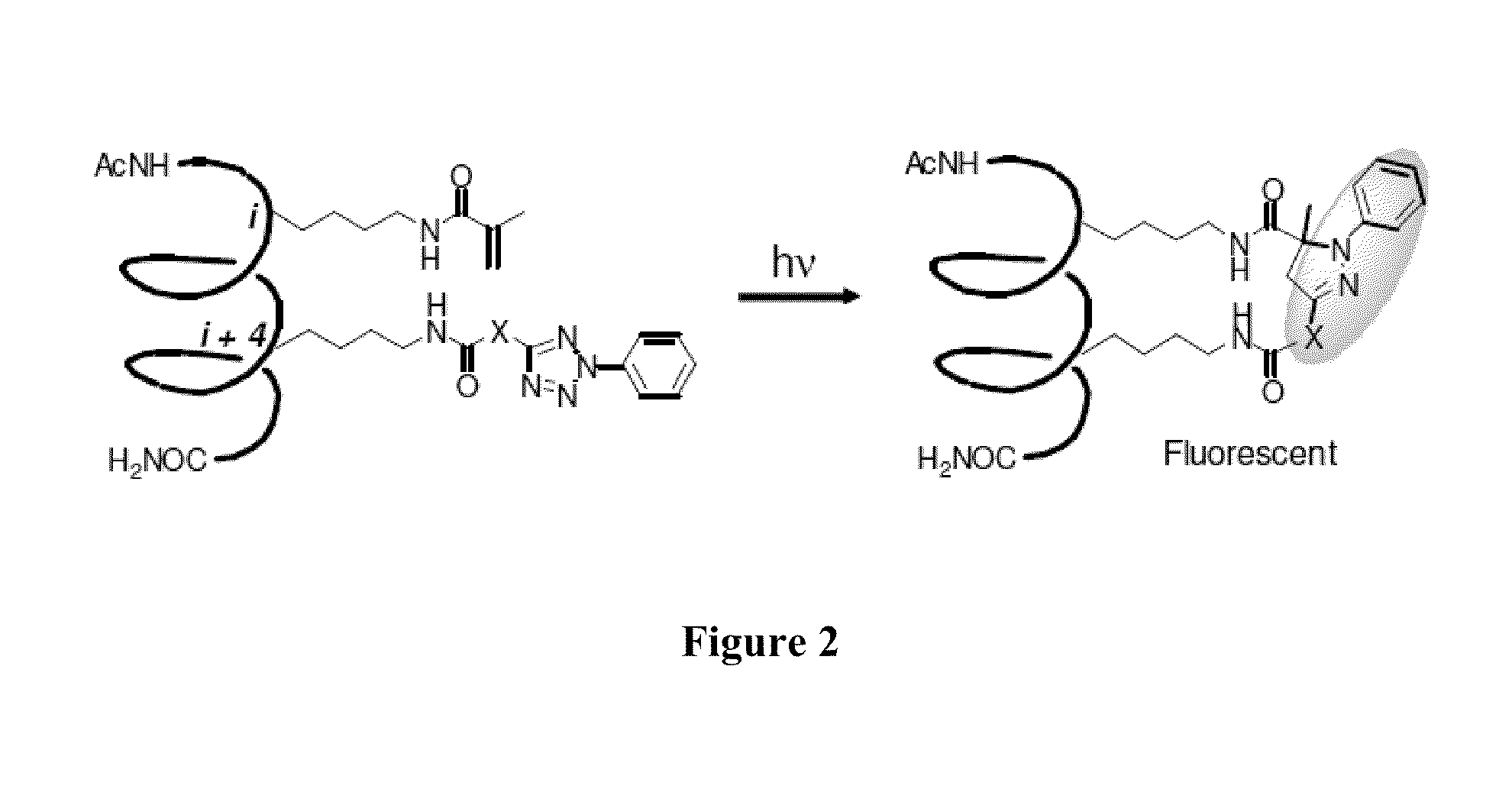
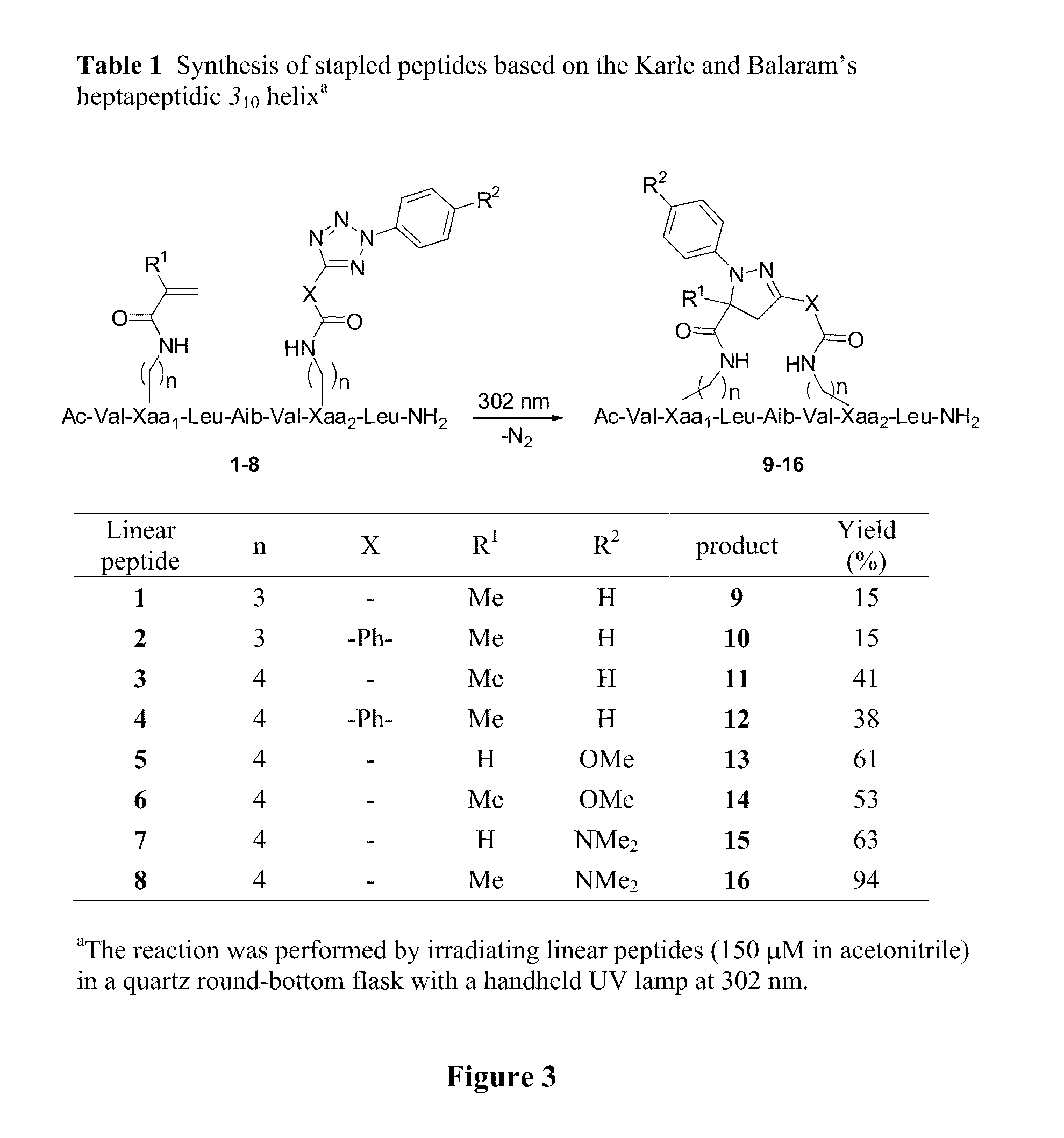
Stapled Peptides and Method of Synthesis
ActiveUS20100093086A1Reasonable reaction yieldSimple procedurePeptide/protein ingredientsPeptide sourcesCycloadditionStapled peptide
A method for preparing stapled peptides. The stapled peptides, including helical stapled peptides, are prepared according to a photochemically-based method, a [3+2] cycloaddition reaction. The helical stapled peptides exhibit increased helicity, thermal stability and cell permeability.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Process for synthesizing cyclic carbonate
InactiveCN1343668AEasy to separateHigh purityOrganic chemistryEpoxyNitrogenous heterocyclic compound
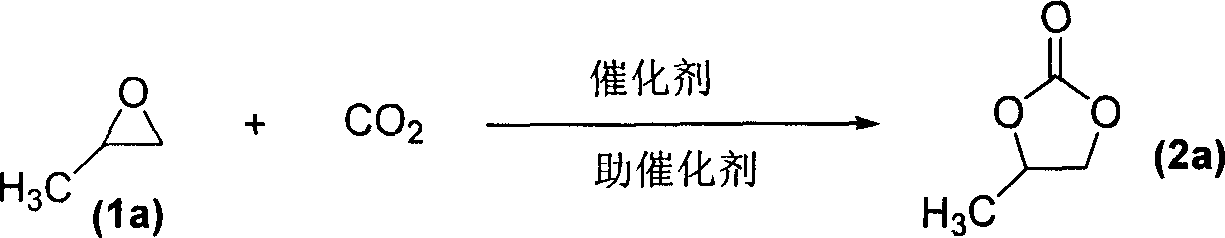
A process for preparing cyclic carbonate features that catalytic cycloaddition reaction of CO2 on epoxy compound at 100-140 deg.C and 1.5-4.5 MPa for 4-8 hrs under the existance of the catalyst whichis composed of azacyclic compound (halogenated alkylpyridine or halogenated 1,3-dialkyl imidazole) and non-metal halide, and the cocatalyst which is alkali-metal halide or ammonium tetrebutyl bromide. Its advantages are high catalytic activity, smooth reaction condition, easy separation of resultant from catalyst, and cyclic use of catalyst.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Compositions and Methods for Modification of Biomolecules
InactiveUS20110207147A1Sugar derivativesArtificial cell constructsCycloadditionCombinatorial chemistry
Provided are modified cycloalkyne compounds; and methods of use of such compounds in modifying biomolecules. Embodiments include a cycloaddition reaction that can be carried out under physiological conditions. The cycloaddition reaction involves reacting a modified cycloalkyne with an azide moiety on a target biomolecule, generating a covalently modified biomolecule. The selectivity of the reaction and its compatibility with aqueous environments provide for its application in vivo and in vitro.
Owner:RGT UNIV OF CALIFORNIA
Bioadhesive composition formed using click chemistry
Owner:TYCO HEALTHCARE GRP LP
Compositions and methods for modification of biomolecules
The present invention provides modified cycloalkyne compounds; and method of use of such compounds in modifying biomolecules. The present invention features a cycloaddition reaction that can be carried out under physiological conditions. In general, the invention involves reacting a modified cycloalkyne with an azide moiety on a target biomolecule, generating a covalently modified biomolecule. The selectivity of the reaction and its compatibility with aqueous environments provide for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
Hetero diels-alder adducts of pentacene as soluble precursors of pentacene
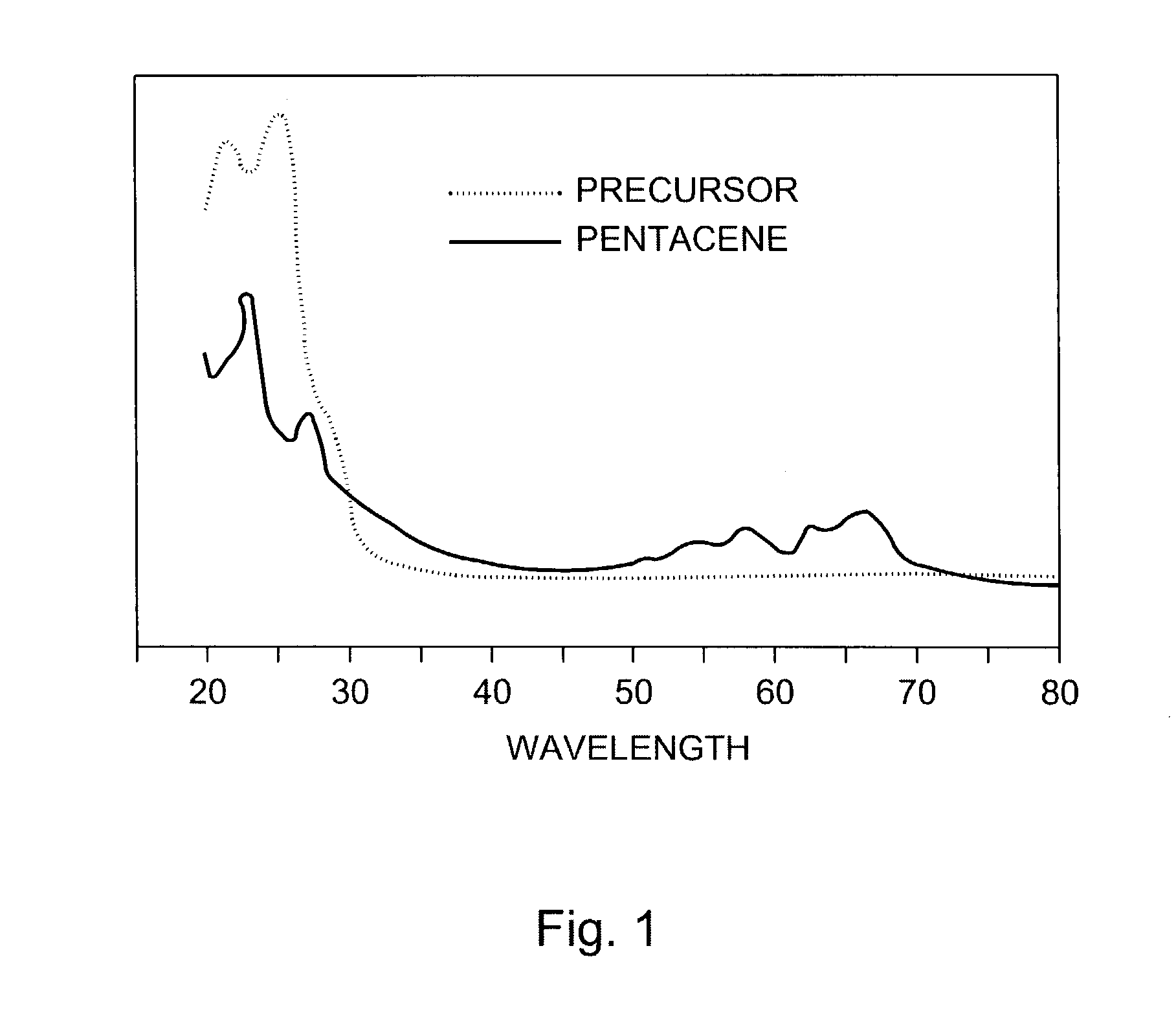
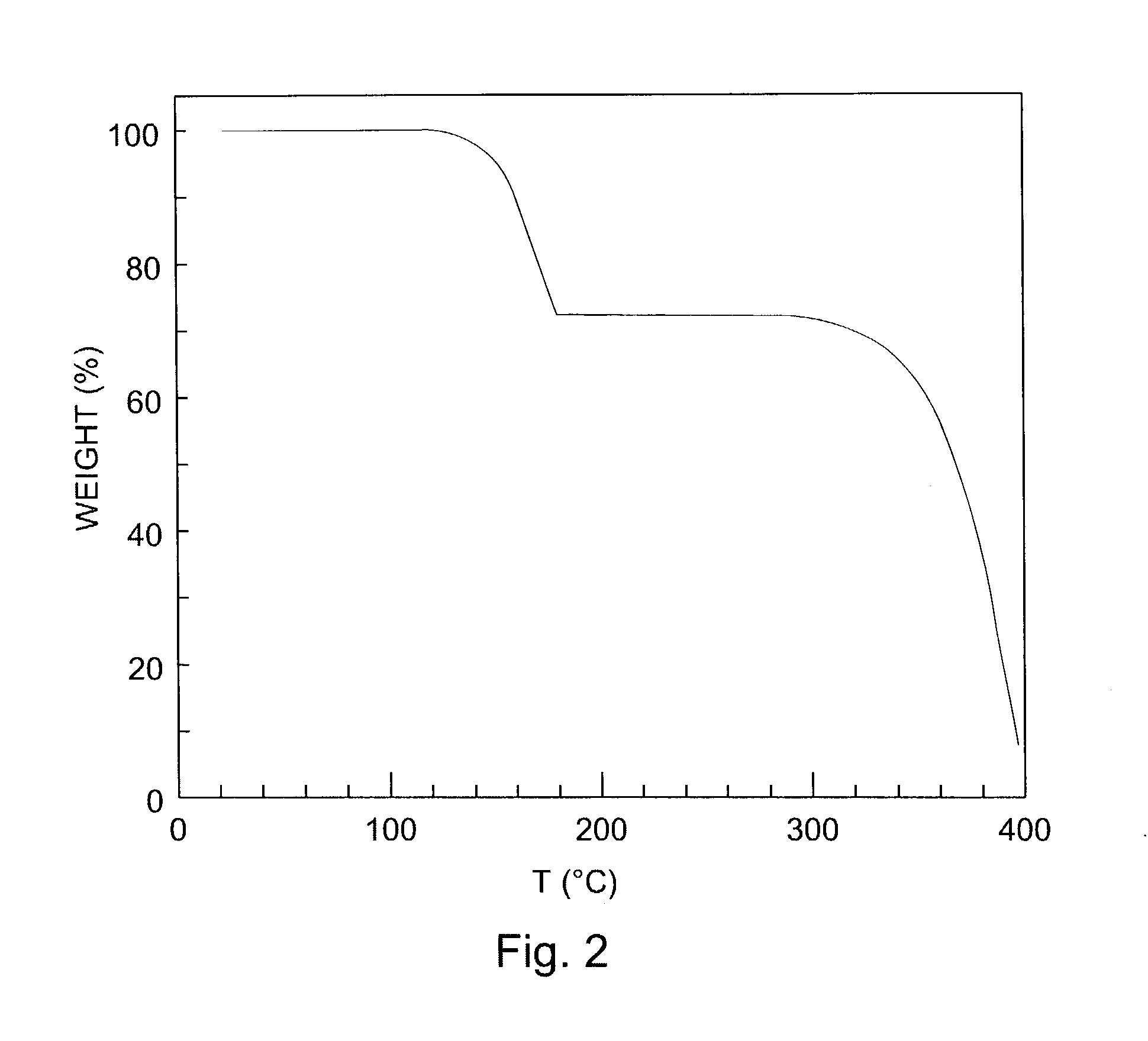
The present invention describes organic solvent-soluble Diels-Alder adducts of polycyclic aromatic compounds, such as, oligothiophene, perylene, benzo[ghi]perylene, coronene and polyacenes, with variety of dienophiles containing at least one heteroatom and in some cases two heteroatoms bonded to aromatic moiety, such as, thioxomalonates, azodicarboxylates, thialdehyde, acylnitroso and N-sulfinylamides. The Diels-Alder adducts are prepared by a simple, one step cycloaddition reaction of the polycyclic aromatic compounds, such as, pentacene, or other fused aromatic compounds, with heterodienophiles. The Diels-Alder adducts according to the present invention all form soluble adducts with pentacene and can be converted back to pentacene by retro-Diels-Alder reaction at moderate (60–250° C.) temperatures both in bulk, in solution or as thin-films.
Owner:GLOBALFOUNDRIES INC
Water-soluble hyperbranched epoxy resin and preparation method thereof
The invention relates to a preparation method of water-soluble hyperbranched epoxy resin. The method comprises the following steps of: (1) making a polyol serving as a core react with an acid to generate a water-soluble hyperbranched polymer under an appropriate condition; (2) functionally modifying the terminal group of the water-soluble hyperbranched polymer to generate a part of terminal carboxyl group water-soluble hyperbranched polymers; (3) undergoing a ring opening addition reaction on the water-soluble hyperbranched polymer and epoxy chloropropane to generate an addition product; (4) undergoing a ring closing reaction on the addition product under the action of catalyst to generate an epoxy terminal group, and generating a sodium salt through the terminal carboxyl group simultaneously to obtain the water-soluble hyperbranched epoxy resin. The hyperbranched epoxy resin prepared with the method can be dissolved into water to form uniform dispersed system, and has flowing viscosity less than 10 Pa.S, high temperature resistance and high adhesive force.
Owner:ZHEJIANG RONGTAI TECH ENTERPRISE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



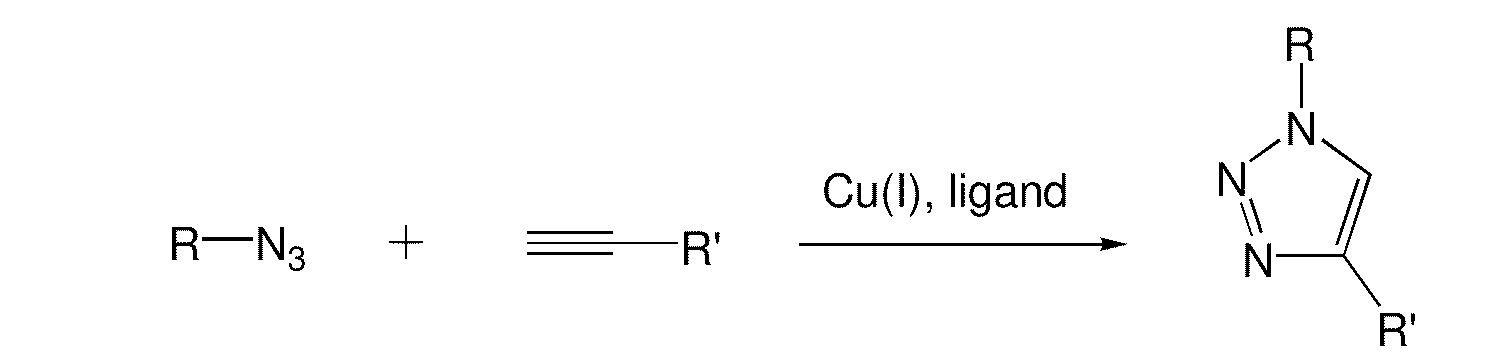
![Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition](https://images-eureka.patsnap.com/patent_img/f254bdb1-9433-4d65-8b75-909a30cbb9ed/US20080267878A1-20081030-D00000.png)
![Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition](https://images-eureka.patsnap.com/patent_img/f254bdb1-9433-4d65-8b75-909a30cbb9ed/US20080267878A1-20081030-D00001.png)
![Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition](https://images-eureka.patsnap.com/patent_img/f254bdb1-9433-4d65-8b75-909a30cbb9ed/US20080267878A1-20081030-D00002.png)