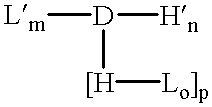Patents
Literature
5631results about "Peptide sources" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
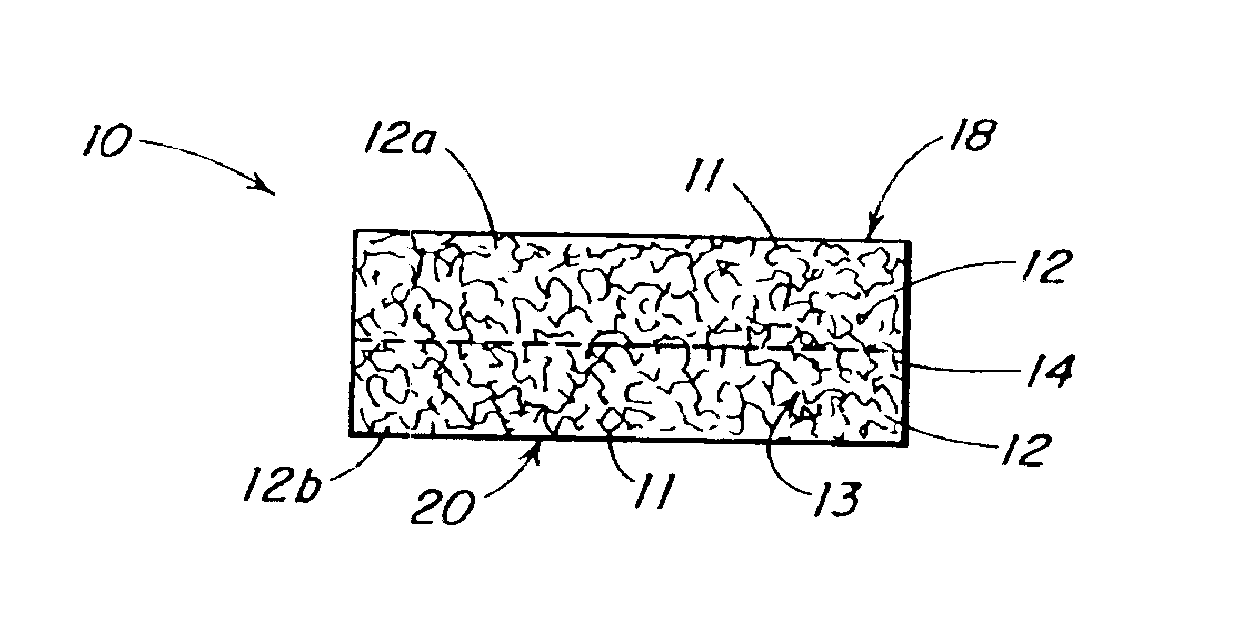
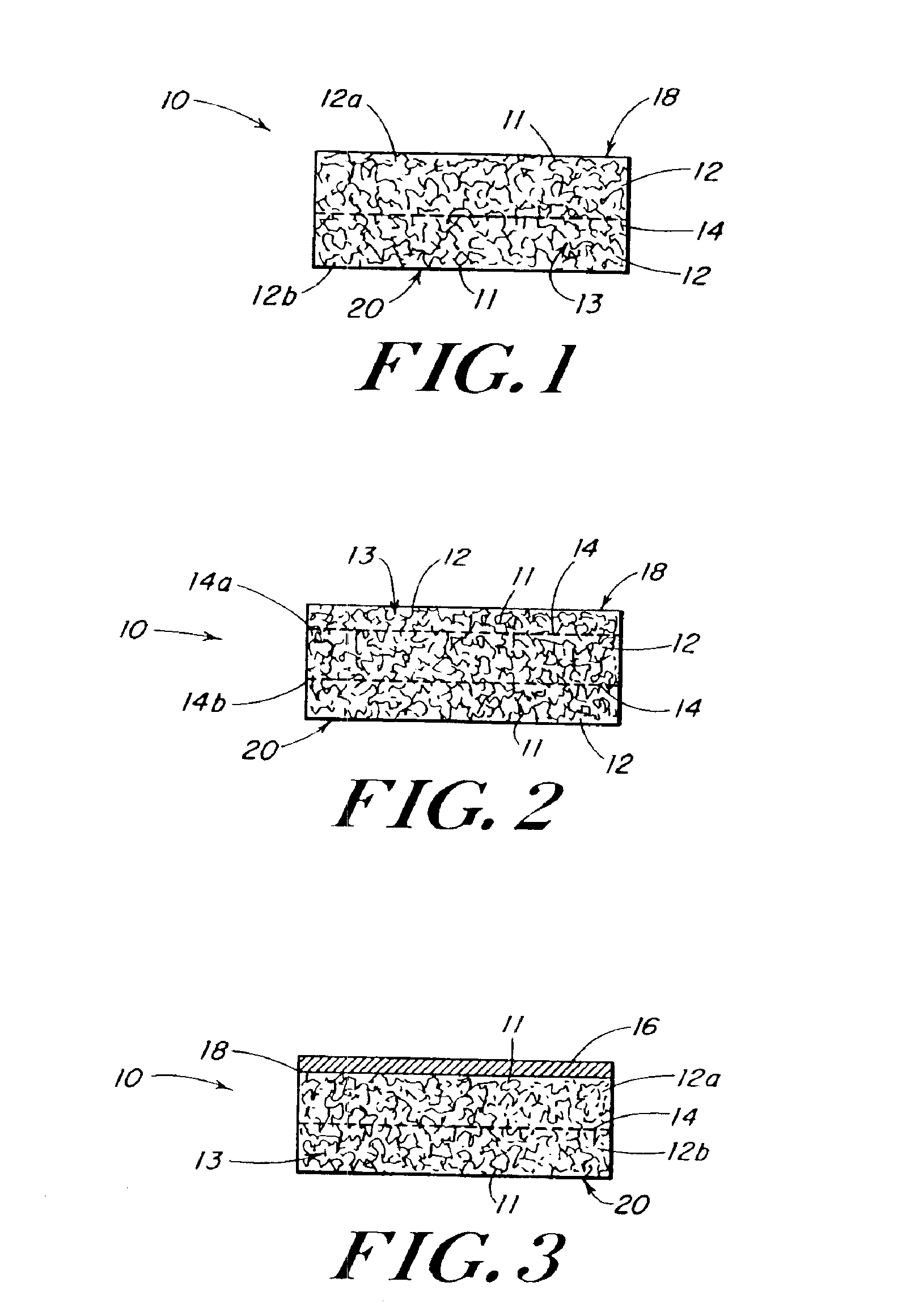
Porous tissue scaffoldings for the repair of regeneration of tissue
InactiveUS6333029B1Promote growthPromote regenerationPeptide/protein ingredientsAntipyreticRepair tissueOpen cell
The present patent describes a three-dimensional inter-connected open cell porous foams that have a gradient in composition and / or microstructure through one or more directions. These foams can be made from a blend of absorbable and biocompatible polymers that are formed into foams having a compositional gradient transitioning from predominately one polymeric material to predominately a second polymeric material. These gradient foams are particularly well suited to tissue engineering applications and can be designed to mimic tissue transition or interface zones.
Owner:ETHICON ENDO SURGERY INC
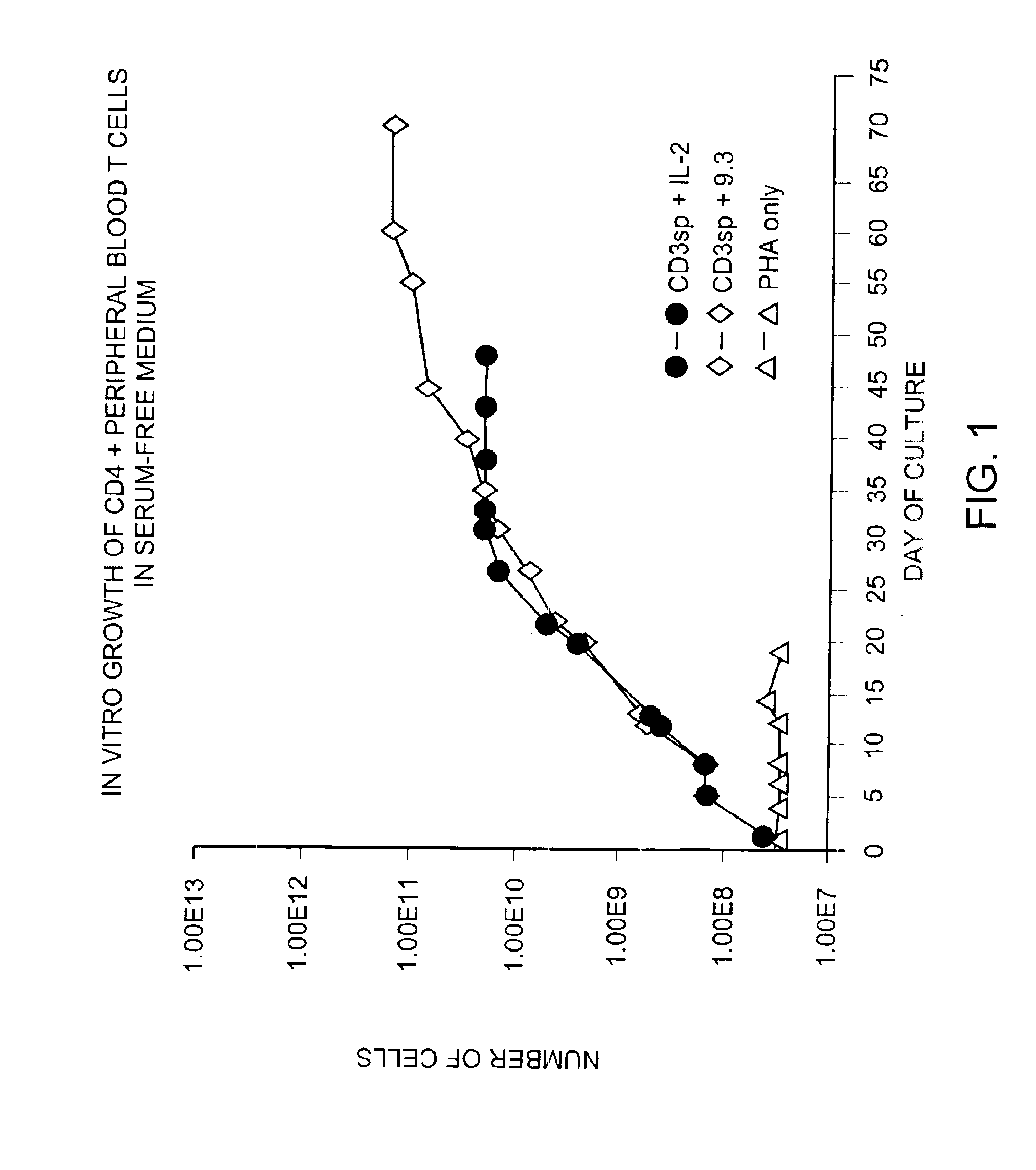
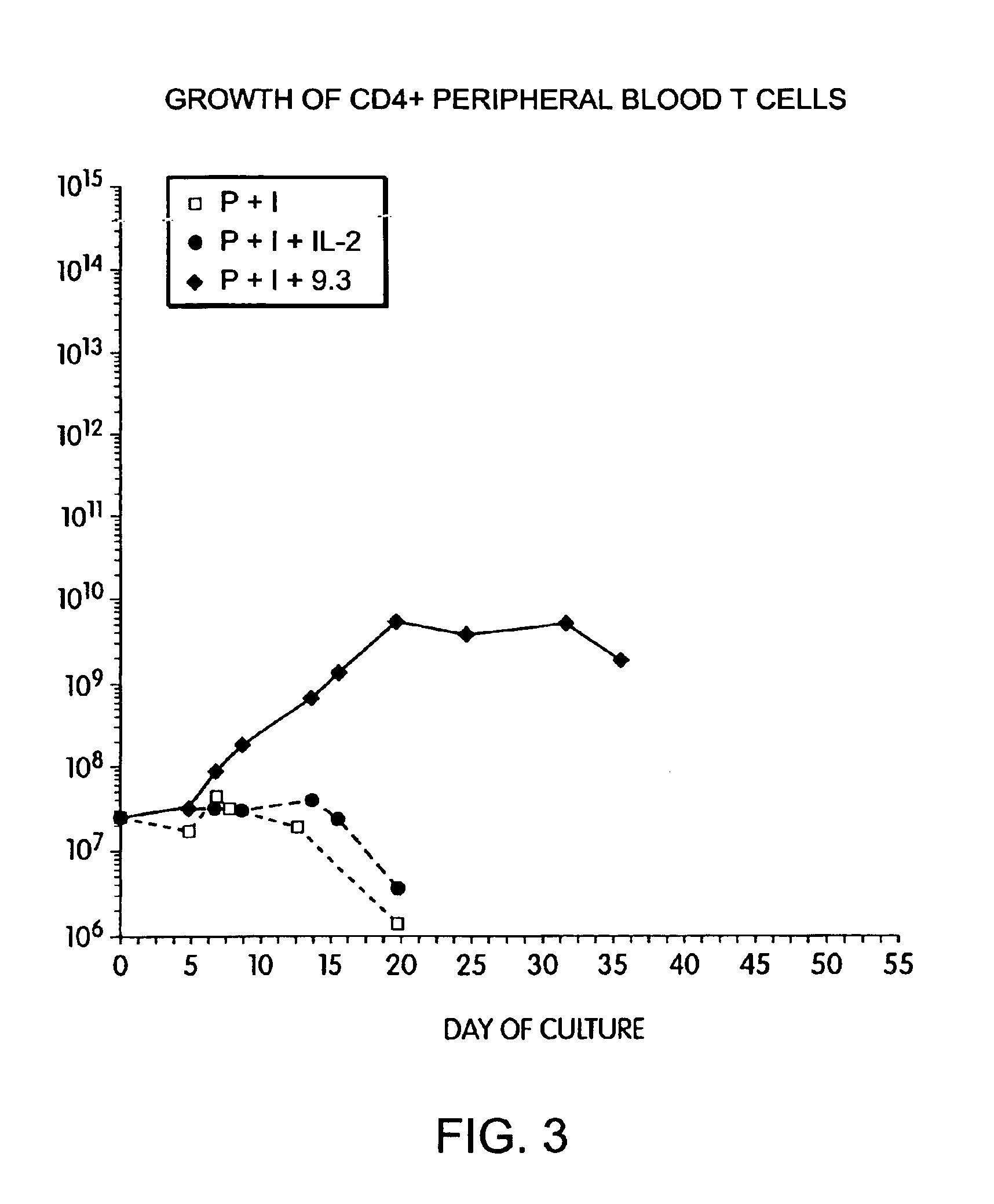
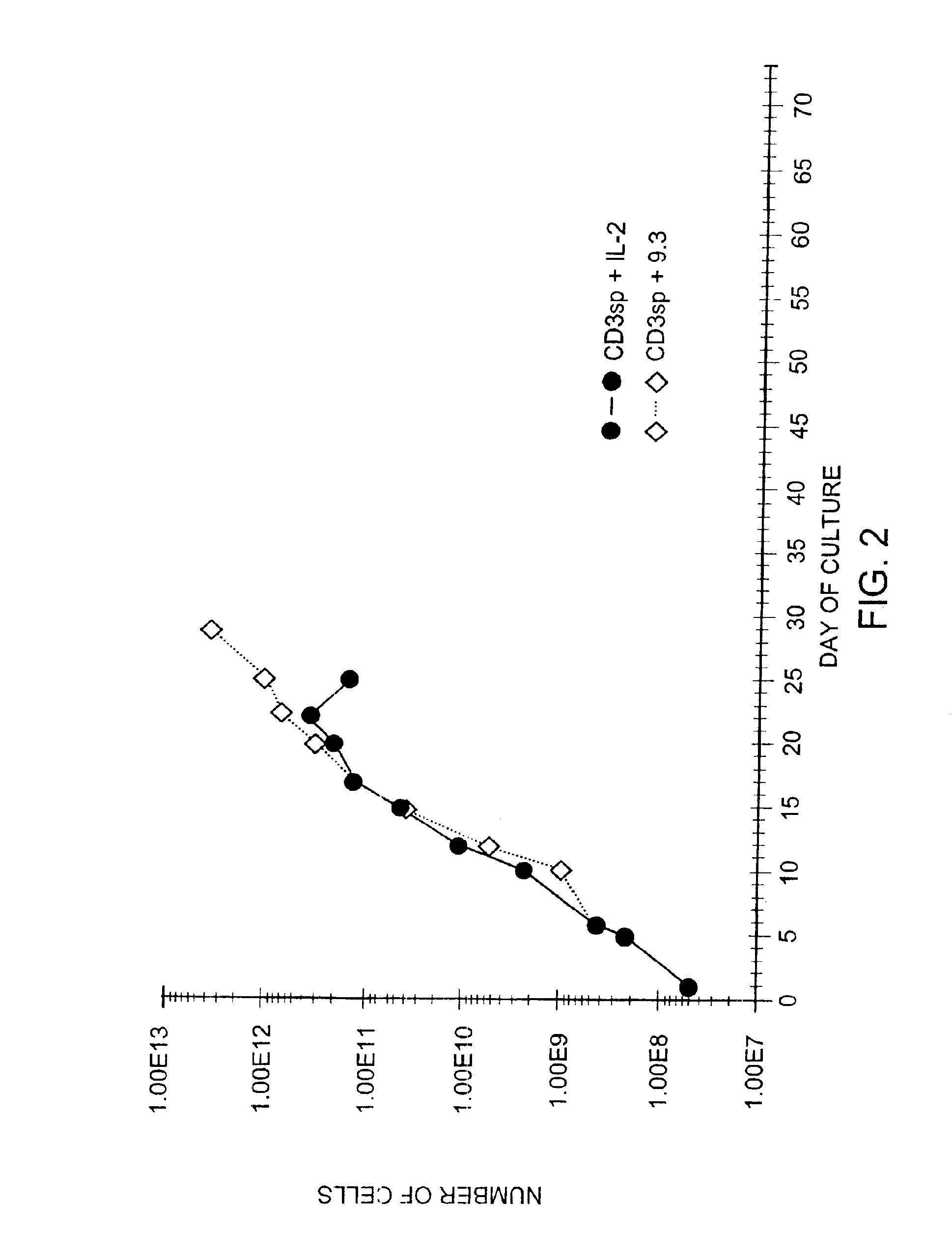
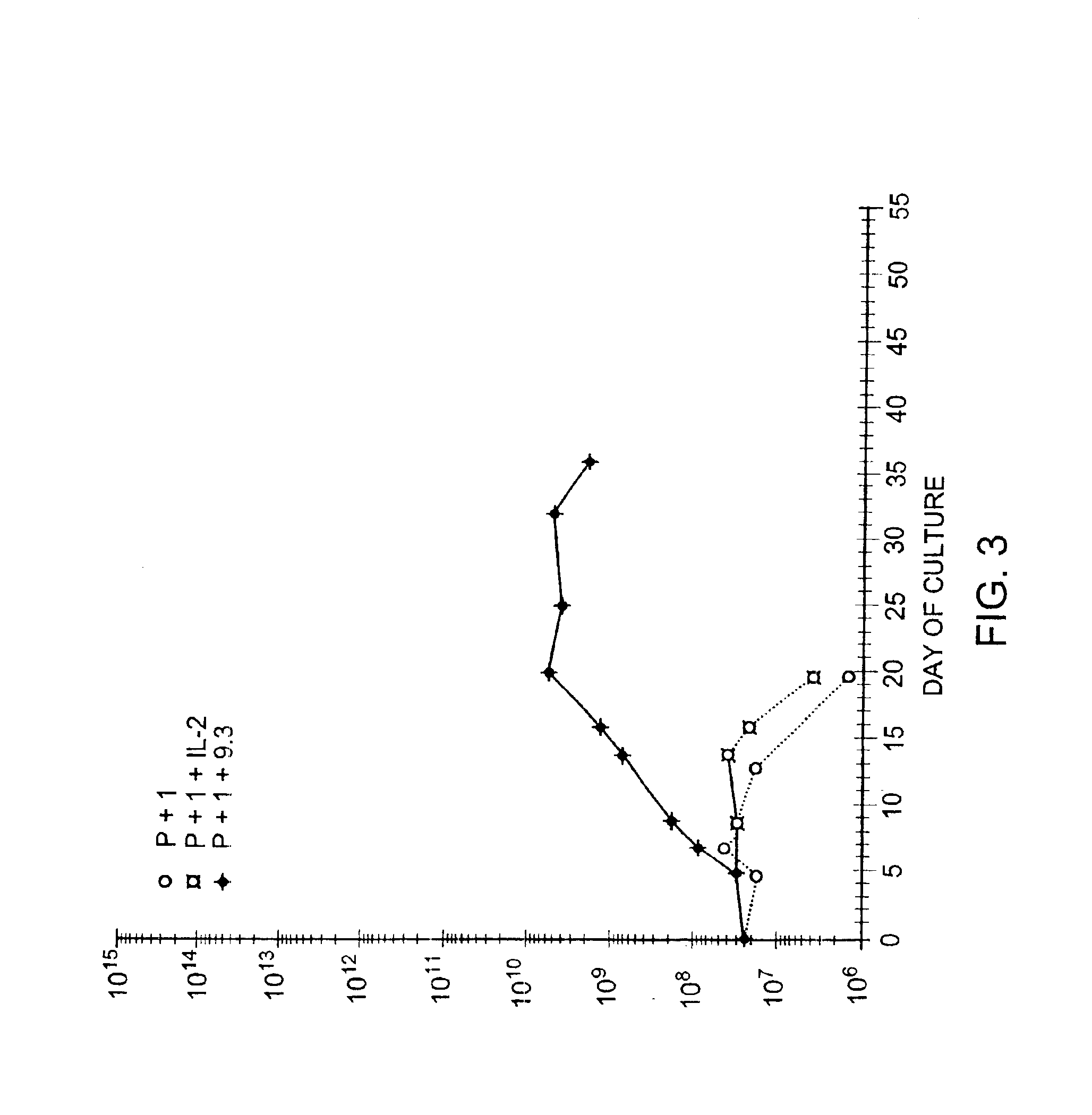
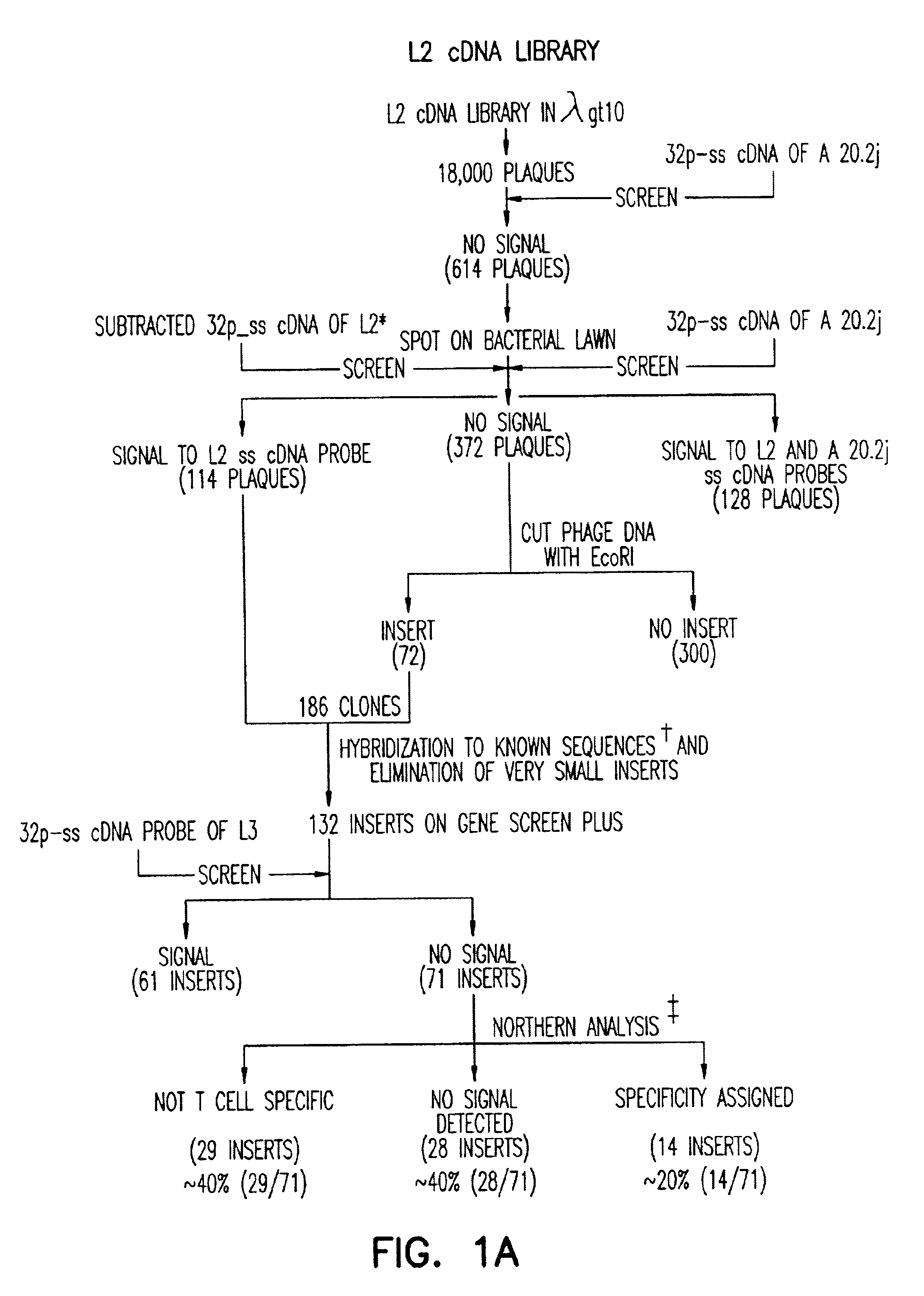
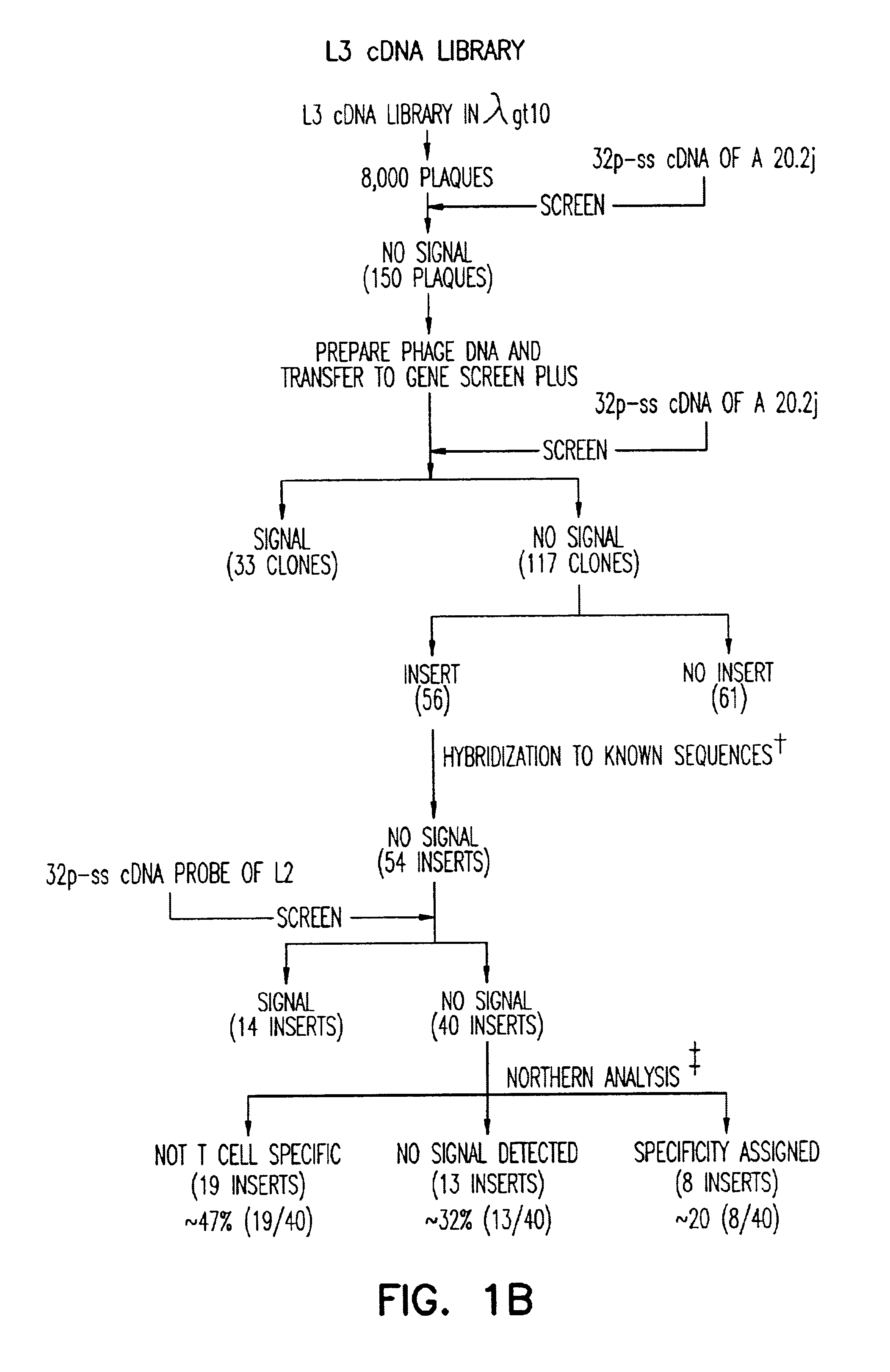
Methods for selectively stimulating proliferation of T cells
InactiveUS6905681B1Increase the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS6887466B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20050238649A1Improve bioavailabilityImprove compoundAntibacterial agentsBiocideD-norephedrineBiochemistry
Owner:SEAGEN INC
Neural regeneration peptides and methods for their use in treatment of brain damage
InactiveUS7563862B2High expressionEasy SurvivalPeptide/protein ingredientsGenetic material ingredientsNervous systemInjury brain
The invention discloses a family of peptides termed NRP compounds or NRPs that can promote neuronal migration, neurite outgrowth, neuronal proliferation, neural differentiation and / or neuronal survival, and provides compositions and methods for the use of NRPs in the treatment of brain injury and neurodegenerative disease. NRP compounds can induce neurons and neuroblasts to proliferate and migrate into areas of damage caused by acute brain injury or chronic neurodegenerative disease, such as exposure to toxins, stroke, trauma, nervous system infections, demyelinating diseases, dementias, and metabolic disorders. NRP compounds may be administered directly to a subject or to a subject's cells by a variety of means including orally, intraperitoneally, intravascularly, and directly into the nervous system of a patient. NRP compounds can be formulated into pharmaceutically acceptable dose forms for therapeutic use. Methods for detecting neural regeneration, neural proliferation, neural differentiation, neurite outgrowth and neural survival can be used to develop other neurally active agents.
Owner:CURONZ HLDG
Methods of treating HIV infected subjects
InactiveUS6905680B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
InactiveUS6852330B2Sufficient structural integritySufficient propertyPowder deliveryOrganic active ingredientsTissue repairSoft tissue repair
A biocompatible tissue repair stimulating implant or “scaffold” device, and methods for making and using such a device, are provided. The implant includes one or more layers of a bioabsorbable polymeric foam having pores with an open cell pore structure. A reinforcement component is also present within the implant to contribute enhanced mechanical and handling properties. The implant houses a biological component that may be released to tissue adjacent the location in which the implant is implanted to faciliate and / or expedite the healing of tissue. This biological component resides primarily within the foam component of the implant, being incorporated within pores formed within the foam.
Owner:DEPUY SYNTHES PROD INC
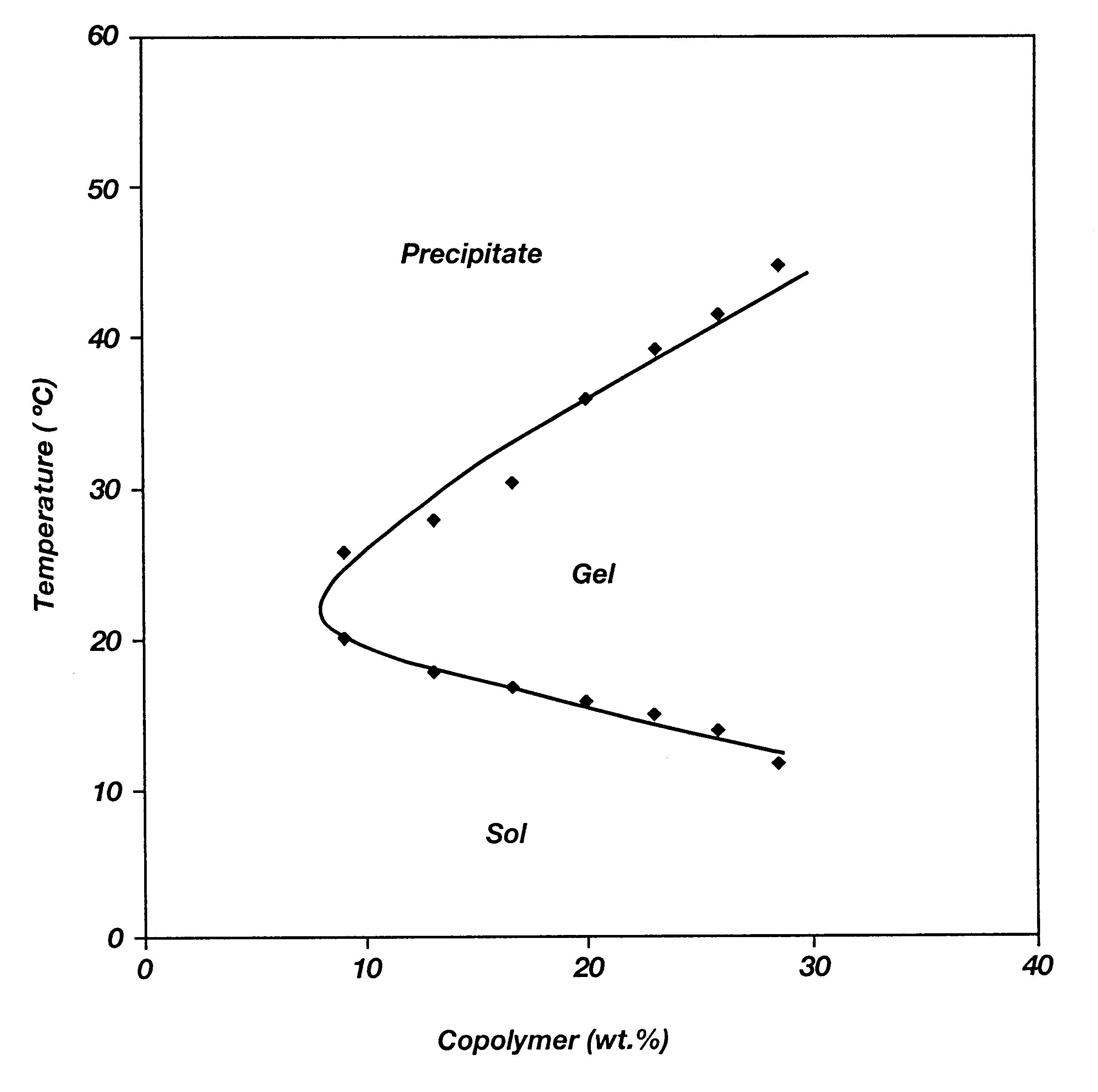
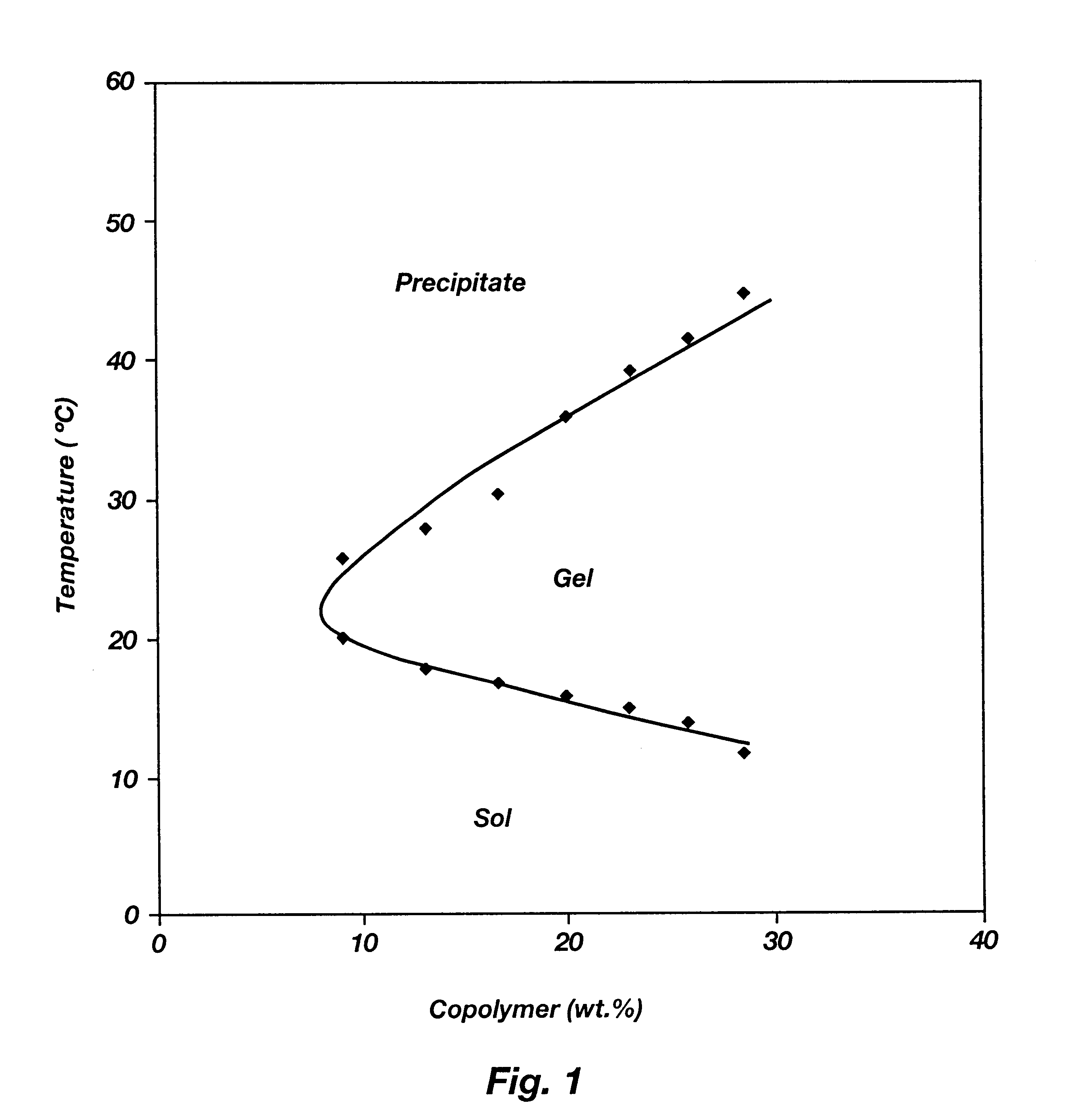
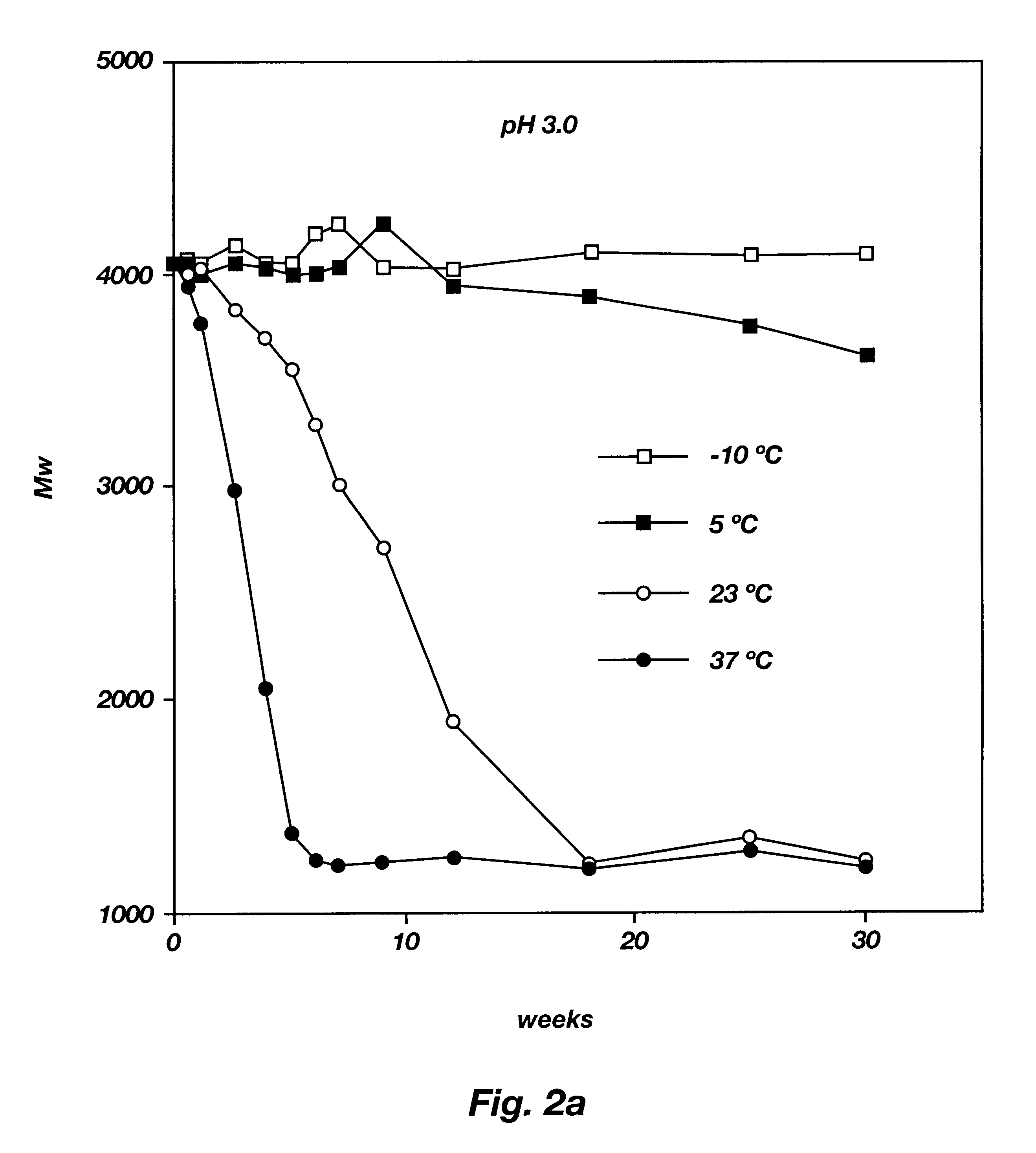
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Full-length plant cDNA and uses thereof
Owner:NAT INST OF AGROBIOLOGICAL SCI +1
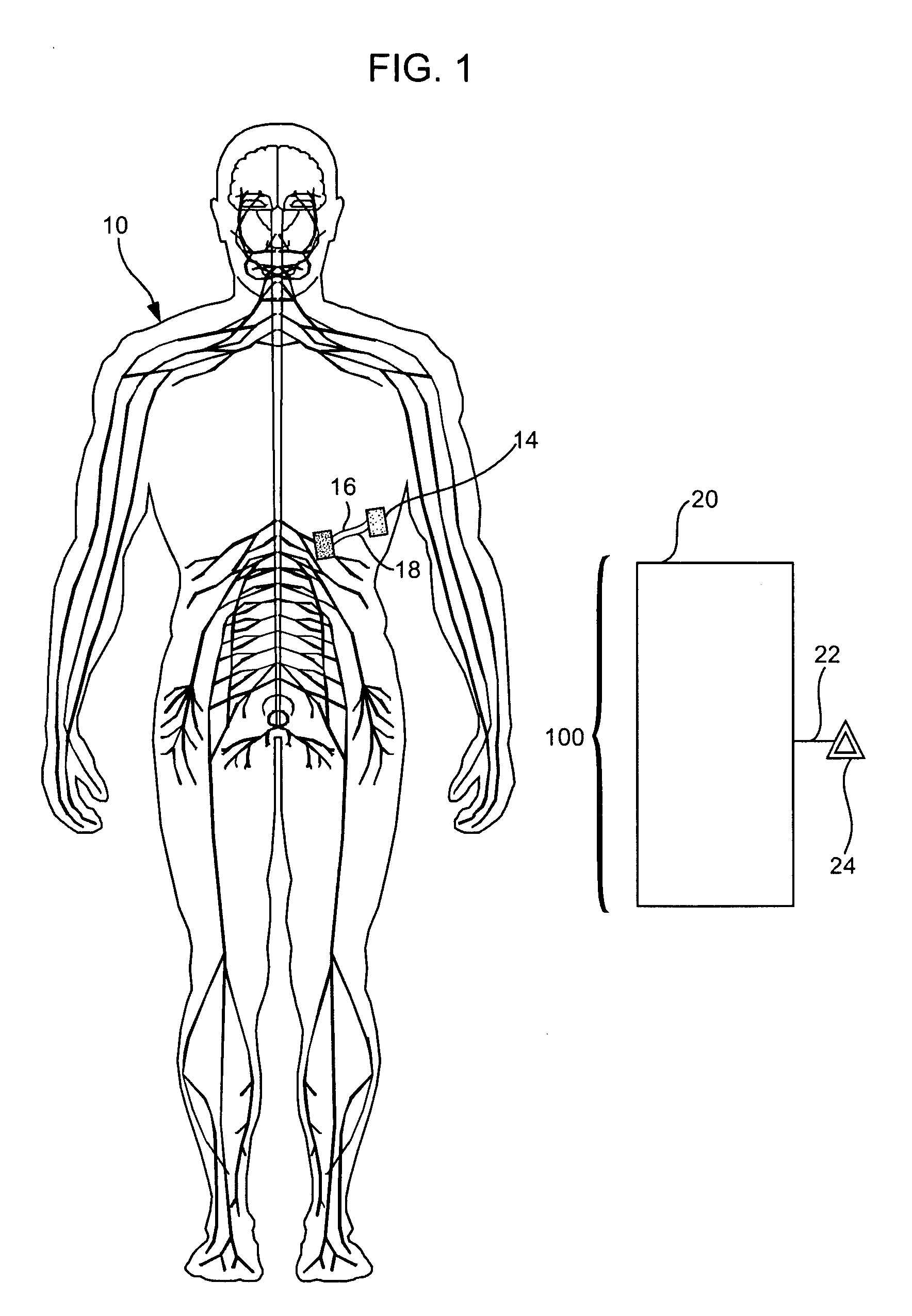
Treatment of conditions through modulation of the autonomic nervous system
InactiveUS20050153885A1Effective treatmentOrganic active ingredientsNervous disorderNervous systemMedicine
Methods are provided for treating a subject for a condition caused by an abnormality in the subject's autonomic nervous system. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is pharmacologically modulated with at least one aldosterone antagonist in a manner that is effective to treat the subject for the condition. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
Method for making heteromultimeric polypeptides
InactiveUS7642228B2Increase productionAntibacterial agentsPeptide/protein ingredientsCrystallographyAmino acid side chain
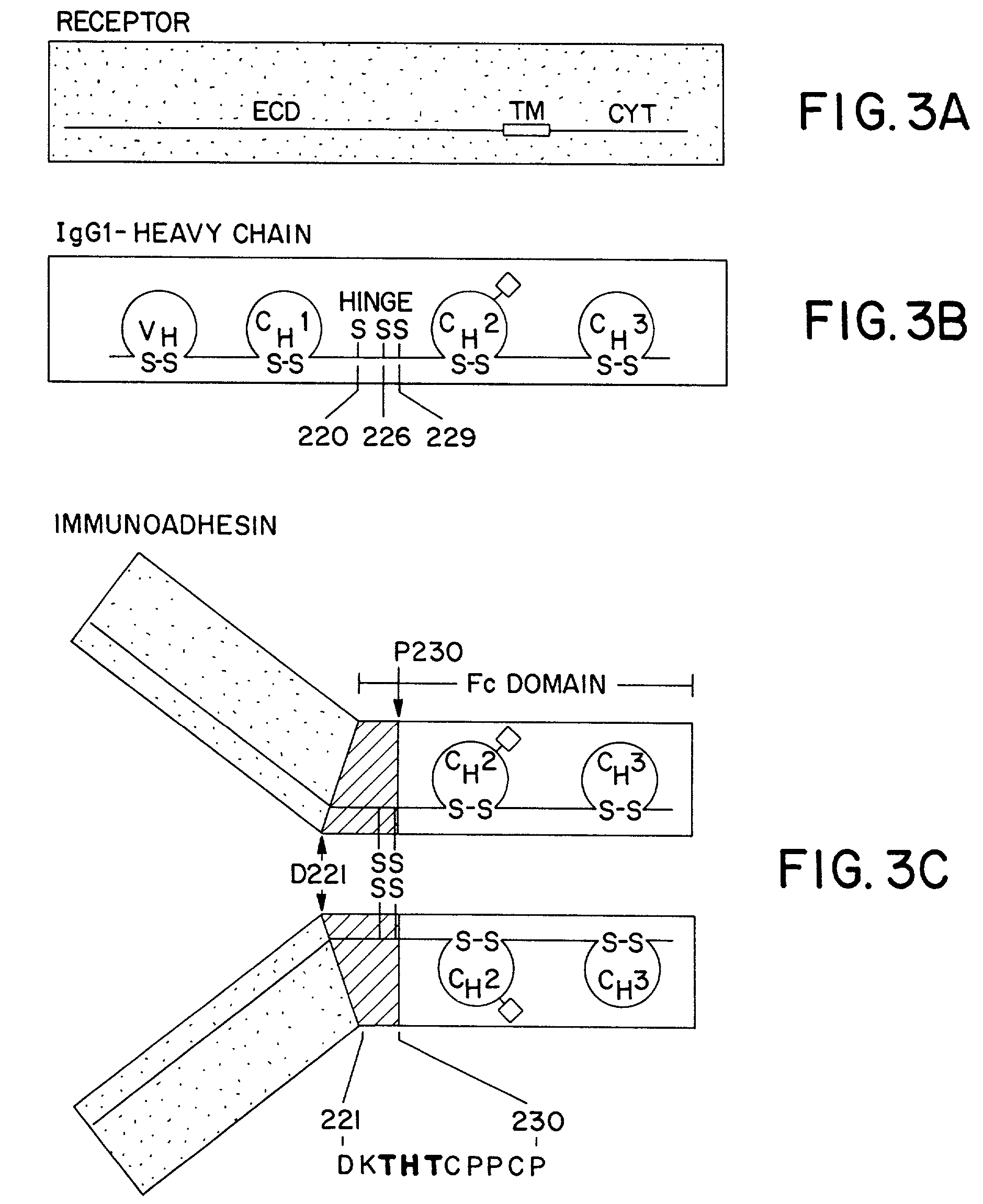
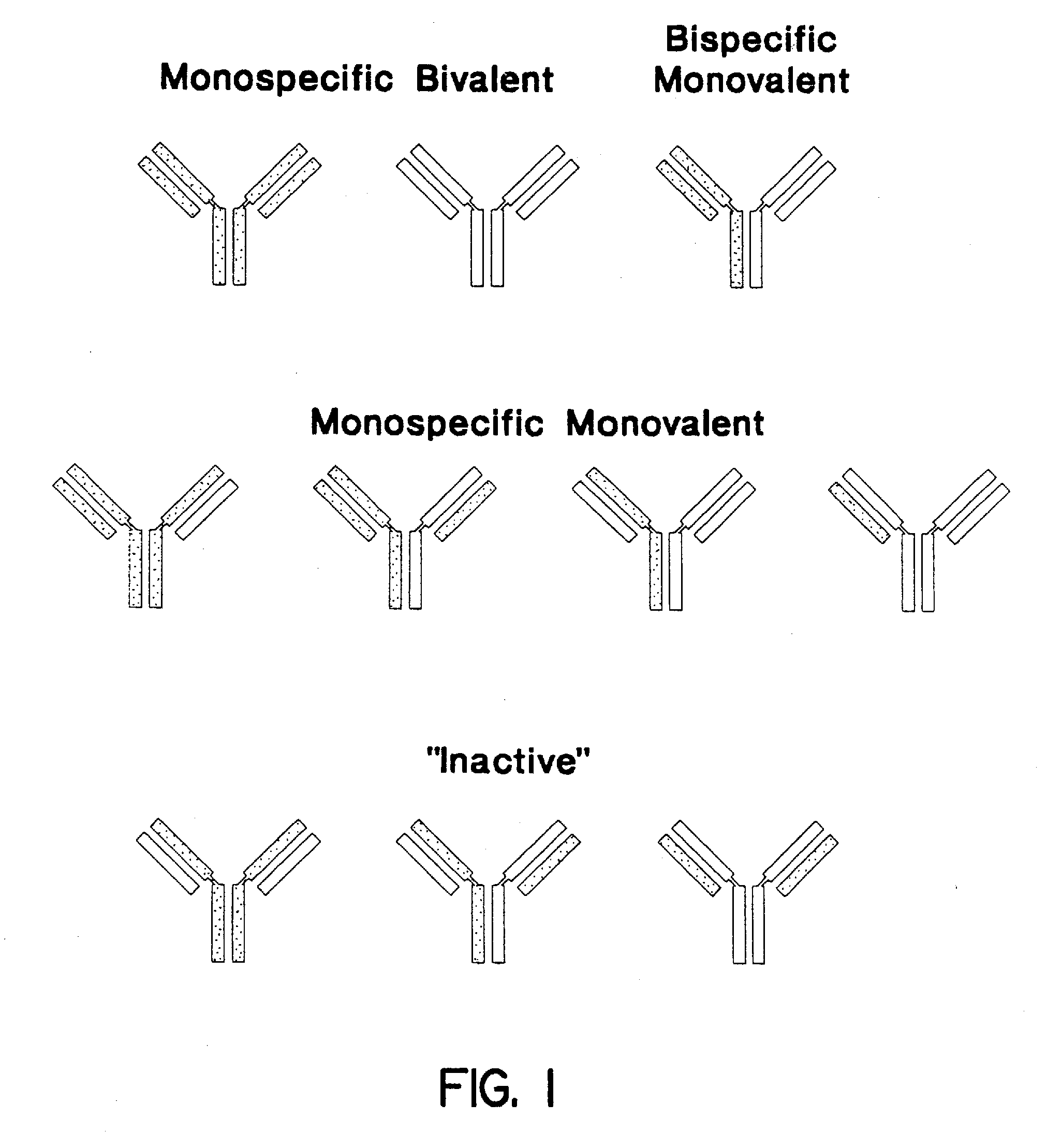
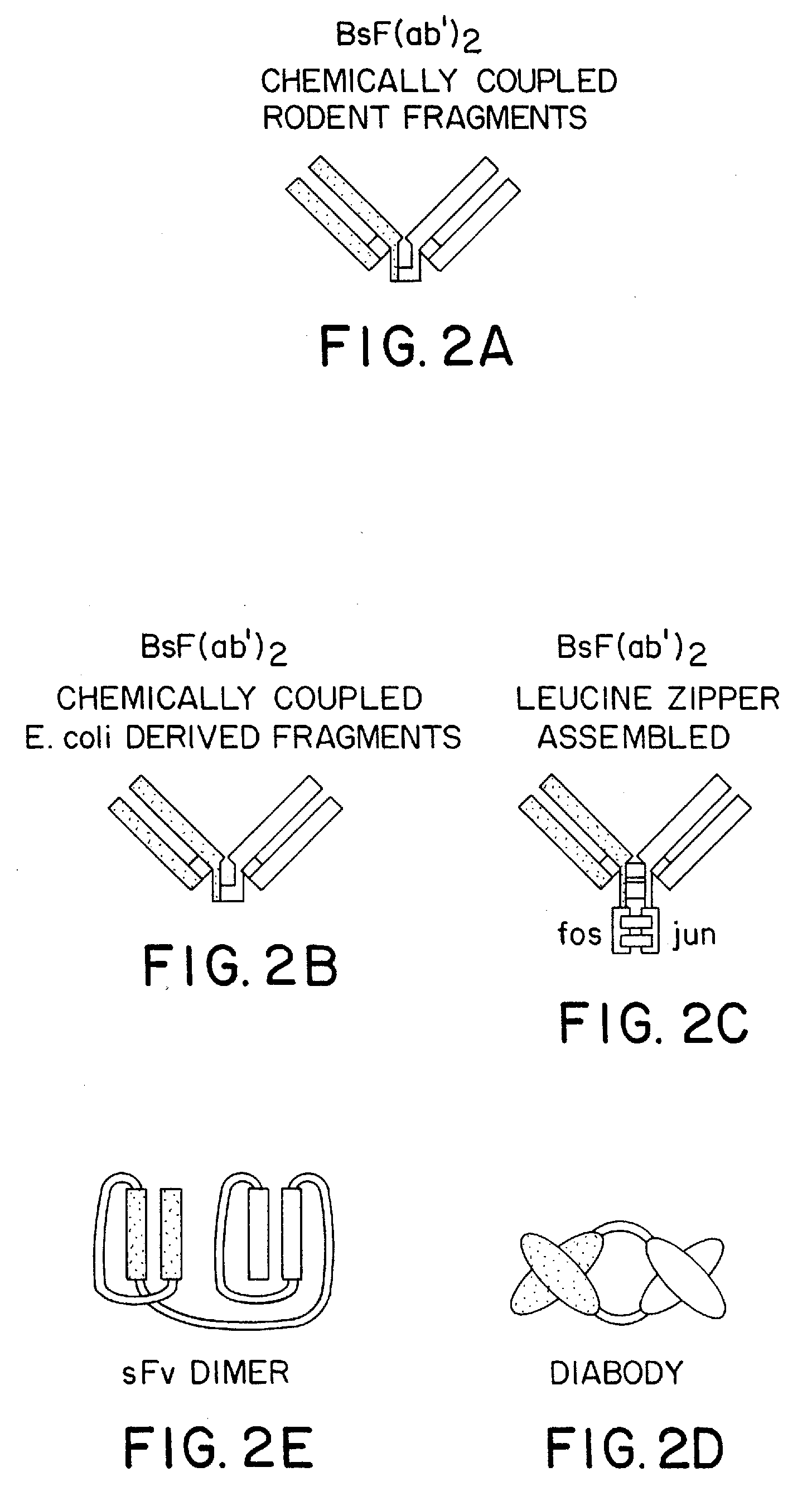
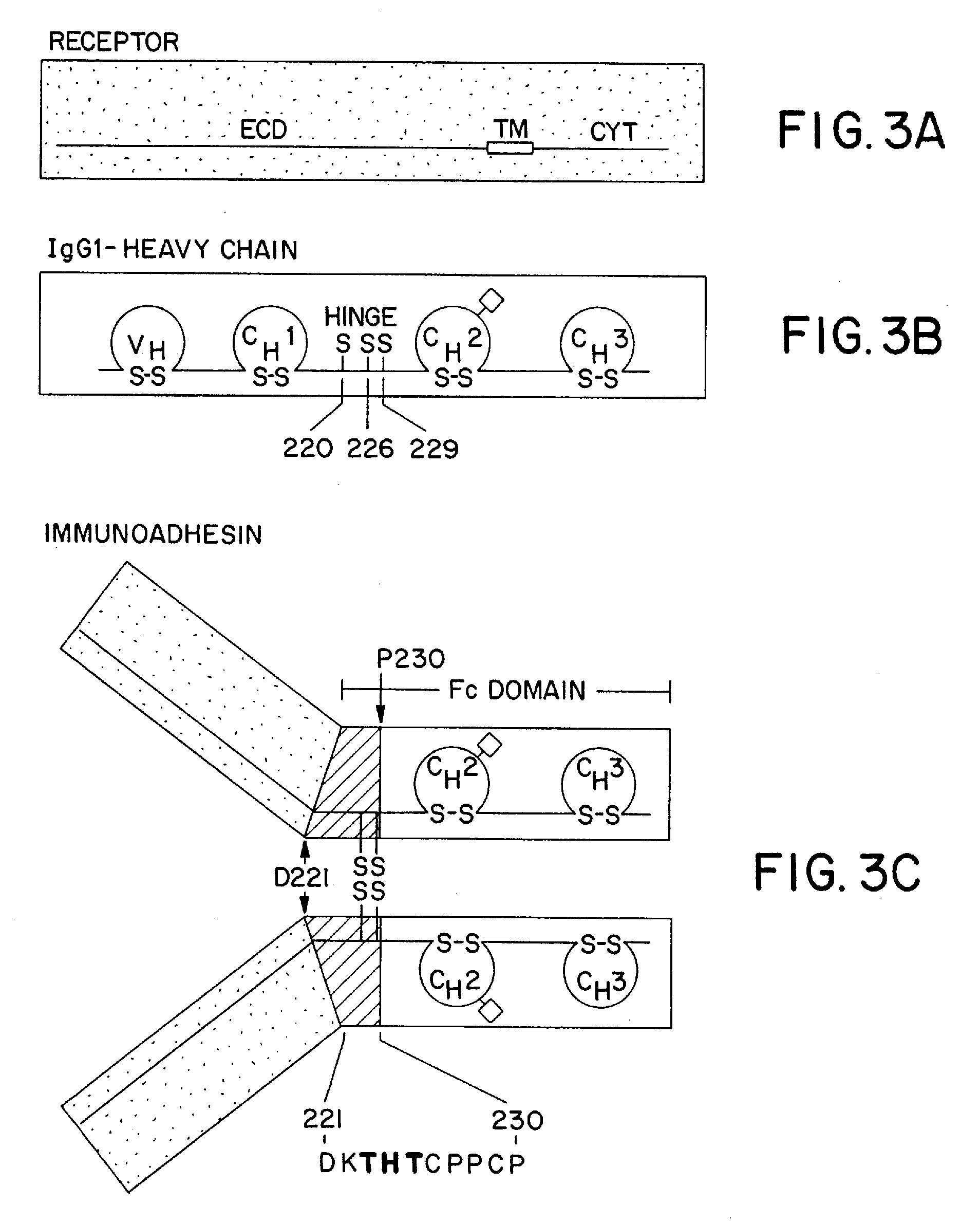
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method involves introducing a protuberance at the interface of a first polypeptide and a corresponding cavity in the interface of a second polypeptide, such that the protuberance can be positioned in the cavity so as to promote heteromultimer formation and hinder homomultimer formation. “Protuberances” are constructed by replacing small amino acid side chains from the interface of the first polypeptide with larger side chains (e.g. tyrosine or tryptophan). Compensatory “cavities” of identical or similar size to the protuberances are created in the interface of the second polypeptide by replacing large amino acid side chains with smaller ones (e.g. alanine or threonine). The protuberance and cavity can be made by synthetic means such as altering the nucleic acid encoding the polypeptides or by peptide synthesis.
Owner:GENENTECH INC
Pharmaceutical composition containing a stabilised mRNA optimised for translation in its coding regions
ActiveUS20050032730A1Overcome disadvantagesImprove efficiencyAntibacterial agentsVirusesTranslational efficiencyCoding region
The present invention relates to a pharmaceutical composition comprising a modified mRNA that is stabilised by sequence modifications and optimised for translation. The pharmaceutical composition according to the invention is particularly well suited for use as an inoculating agent, as well as a therapeutic agent for tissue regeneration. In addition, a process is described for determining sequence modifications that promote stabilisation and translational efficiency of modified mRNA of the invention.
Owner:CUREVAC SE
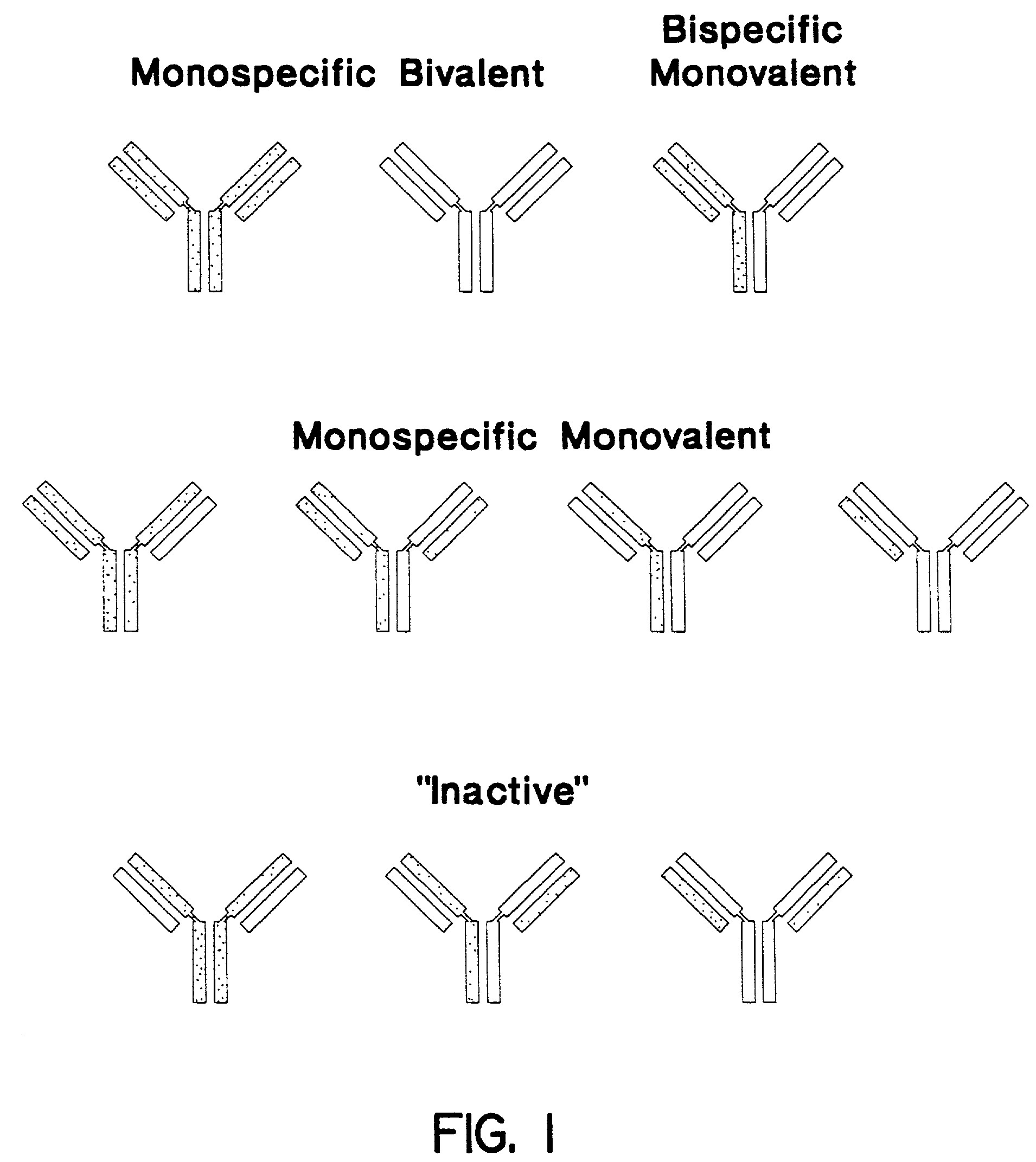
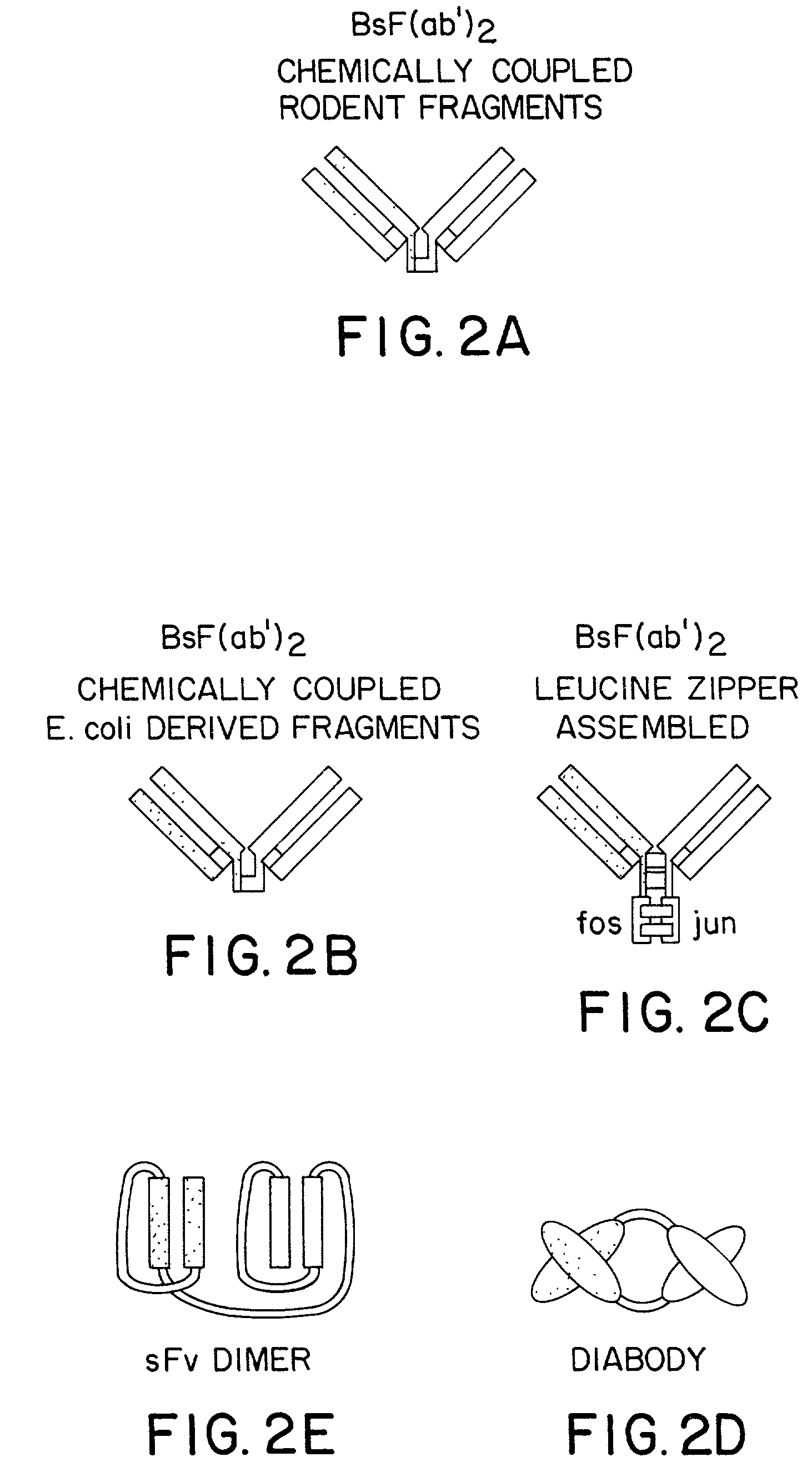
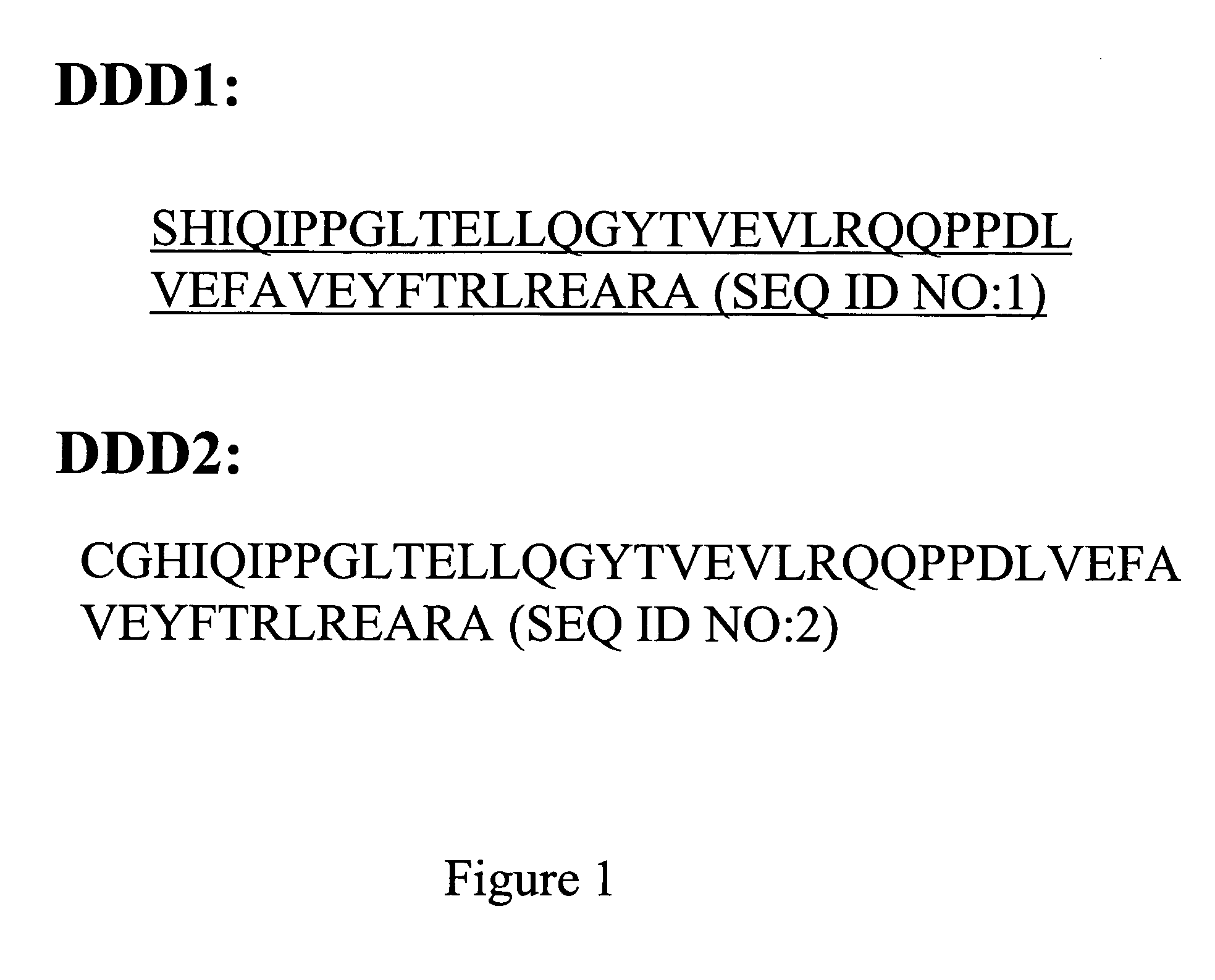
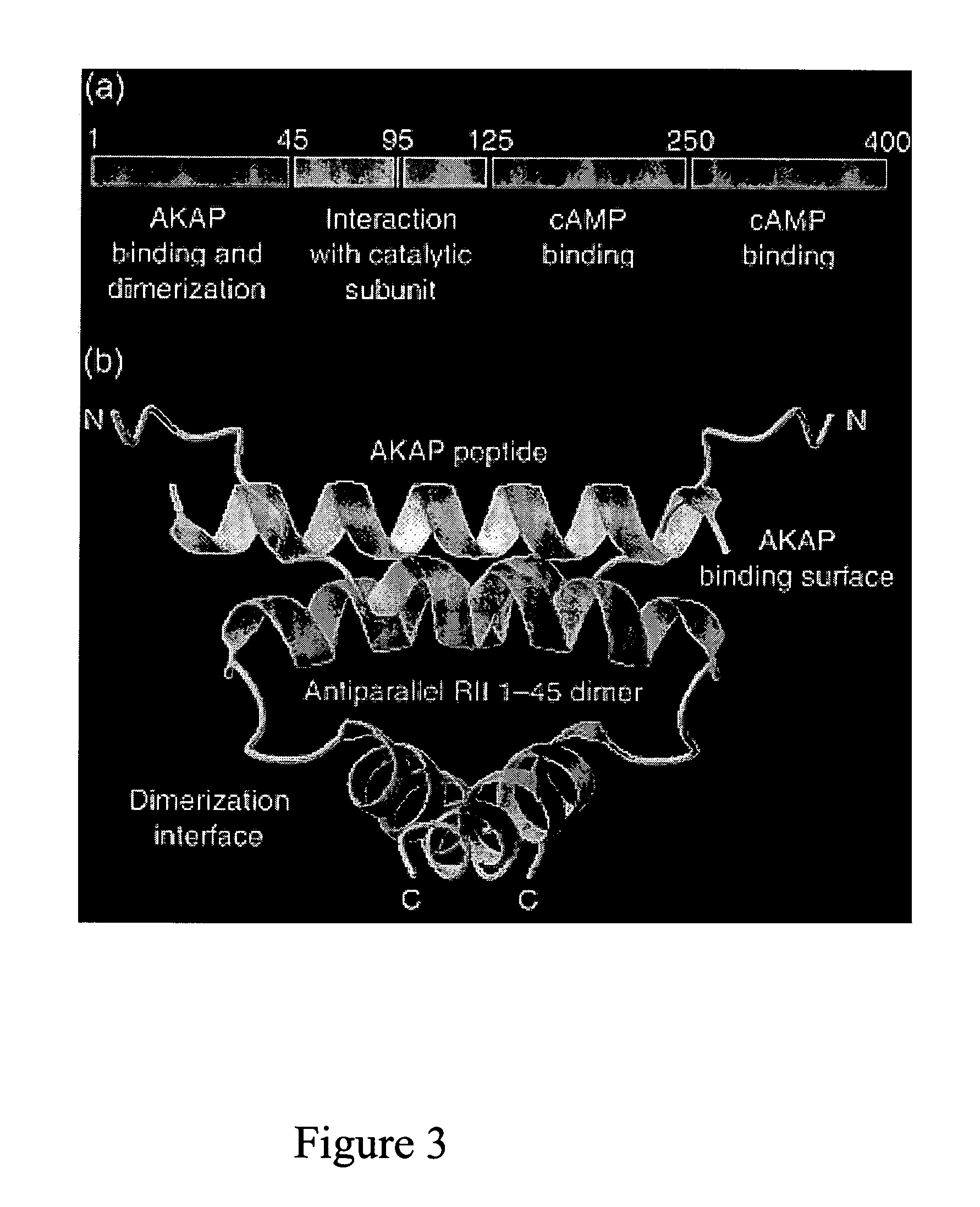
Stably tethered structures of defined compositions with multiple functions or binding specificities
Owner:IBC PHARMACEUTICALS INC
Method for making heteromultimeric polypeptides
InactiveUS20070014794A1Increase productionAntibacterial agentsPeptide/protein ingredientsCrystallographyAmino acid side chain
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method involves introducing a protuberance at the interface of a first polypeptide and a corresponding cavity in the interface of a second polypeptide, such that the protuberance can be positioned in the cavity so as to promote heteromultimer formation and hinder homomultimer formation. “Protuberances” are constructed by replacing small amino acid side chains from the interface of the first polypeptide with larger side chains (e.g. tyrosine or tryptophan). Compensatory “cavities” of identical or similar size to the protuberances are created in the interface of the second polypeptide by replacing large amino acid side chains with smaller ones (e.g. alanine or threonine). The protuberance and cavity can be made by synthetic means such as altering the nucleic acid encoding the polypeptides or by peptide synthesis.
Owner:GENENTECH INC
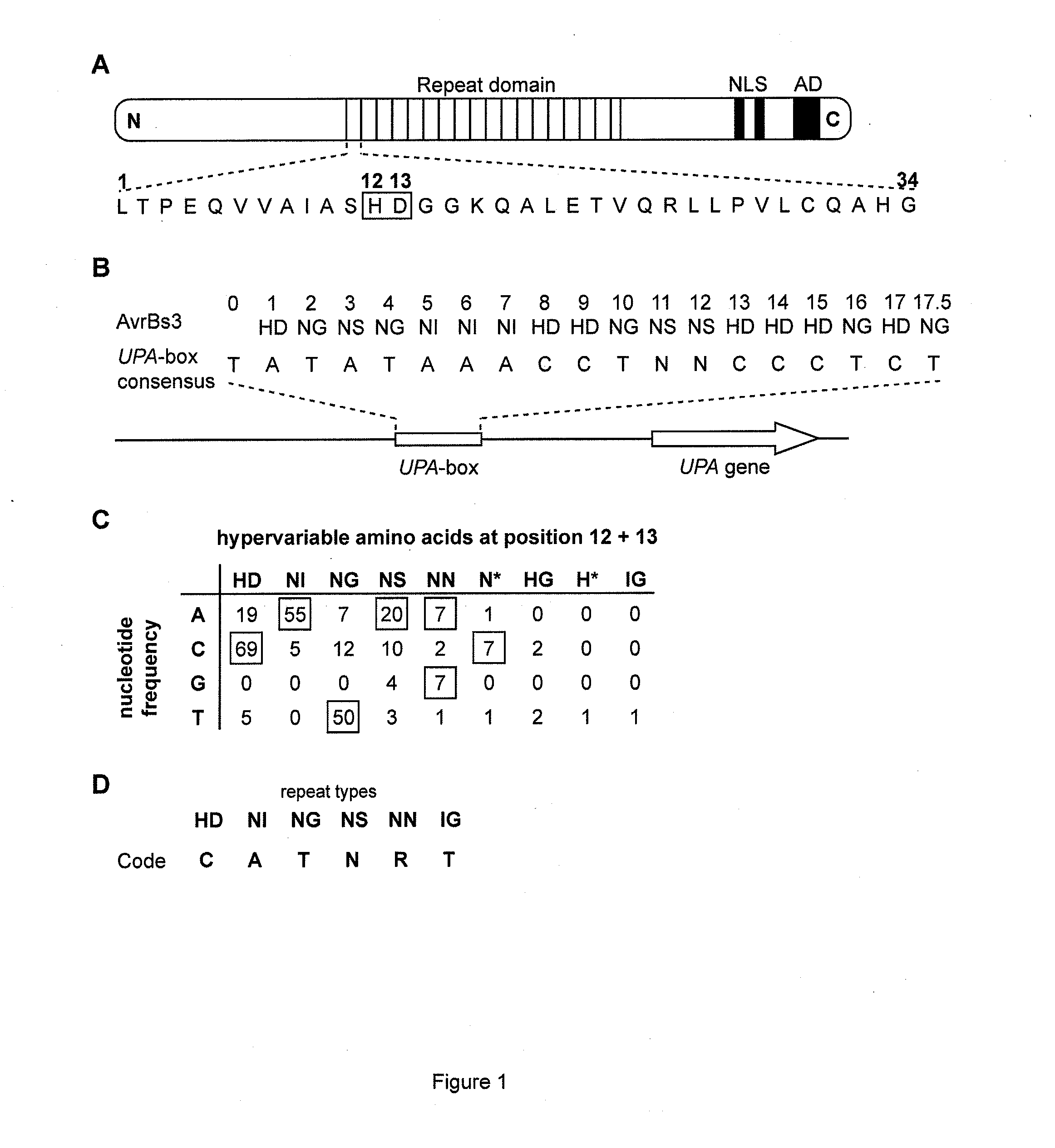
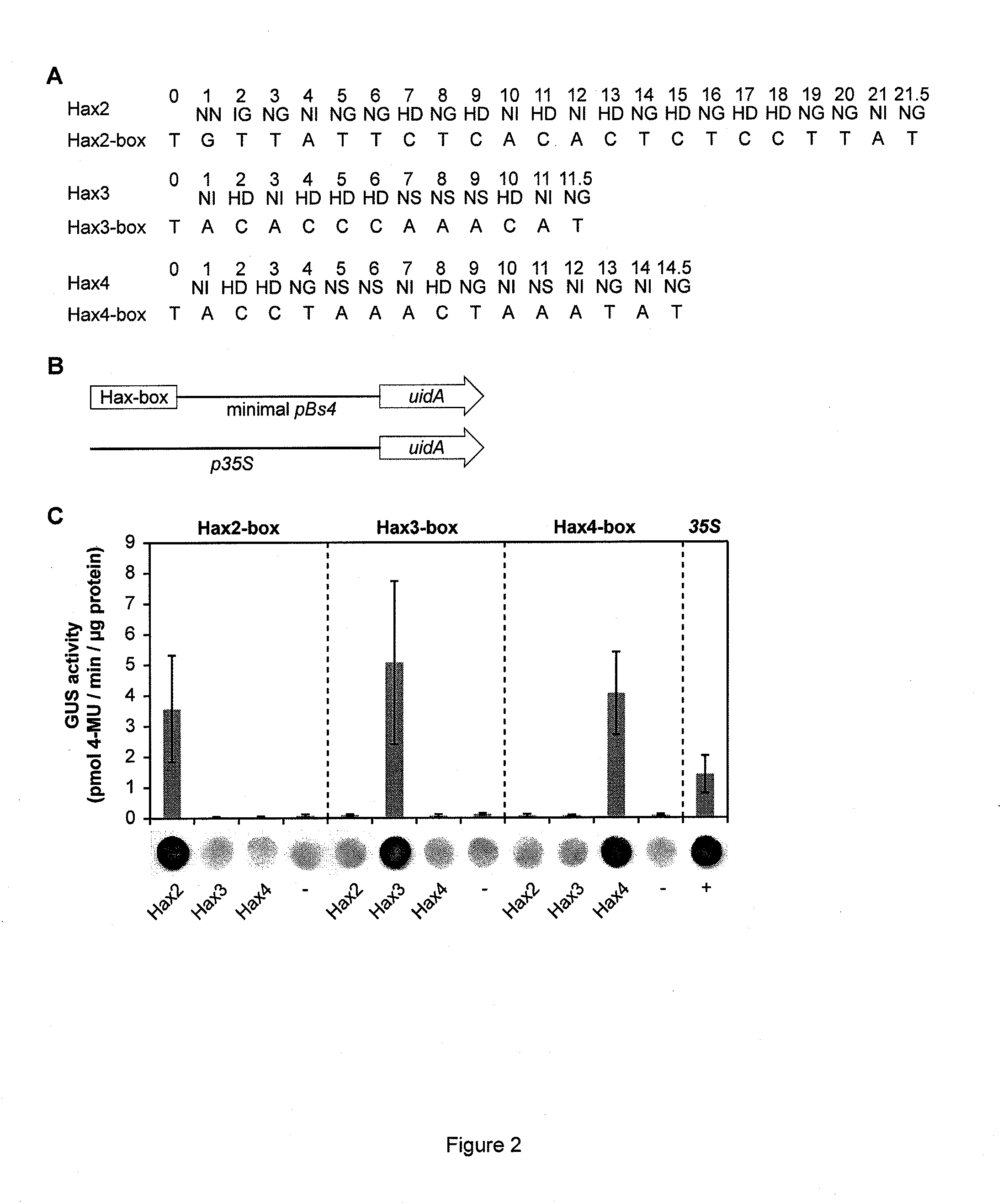
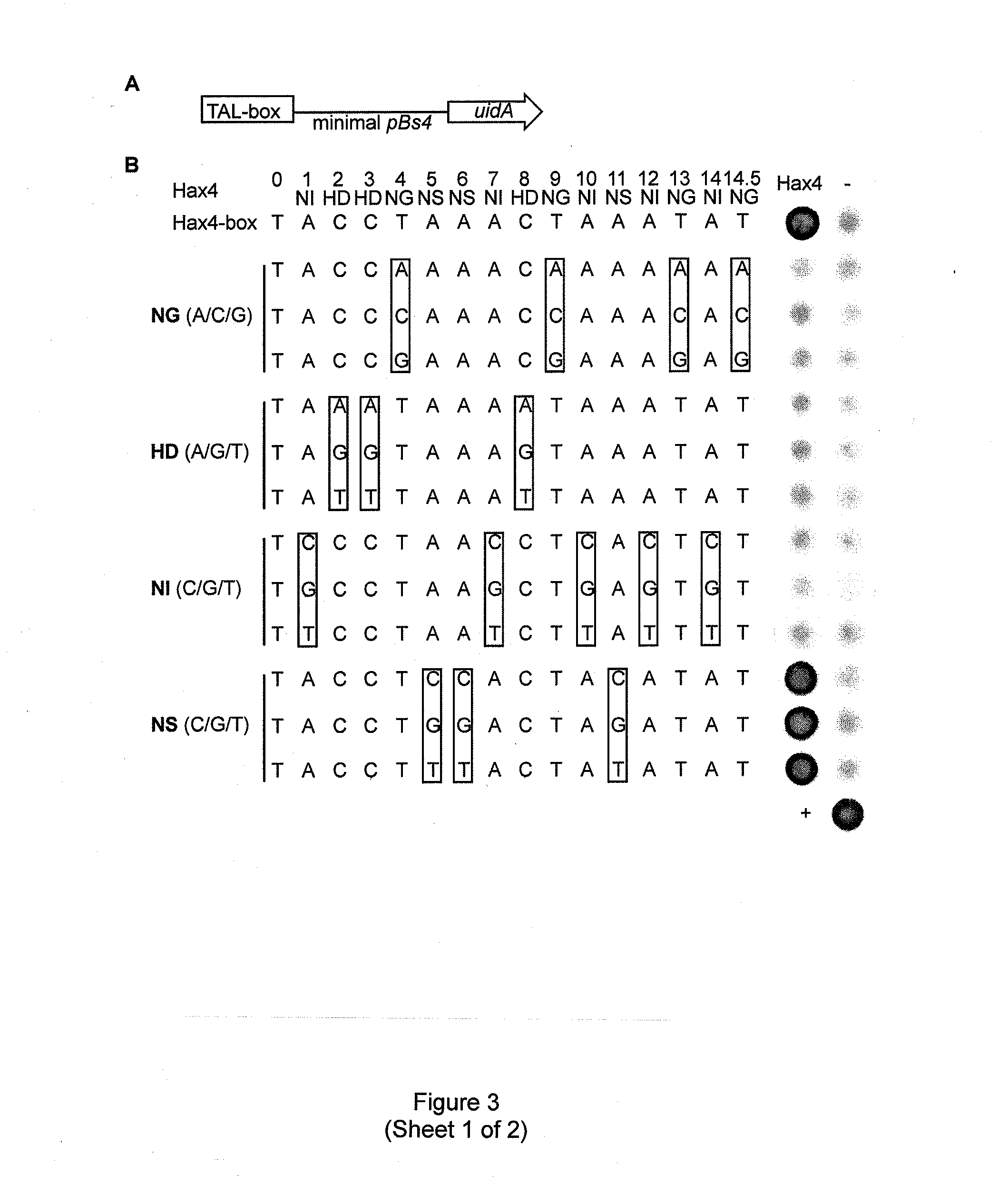
Modular dna-binding domains and methods of use
InactiveUS20110239315A1Enabling targeted DNA modificationFungiBacteriaDNA-binding domainDna targeting
The present invention refers to methods for selectively recognizing a base pair in a DNA sequence by a polypeptide, to modified polypeptides which specifically recognize one or more base pairs in a DNA sequence and, to DNA which is modified so that it can be specifically recognized by a polypeptide and to uses of the polypeptide and DNA in specific DNA targeting as well as to methods of modulating expression of target genes in a cell.
Owner:BONAS ULLA +2
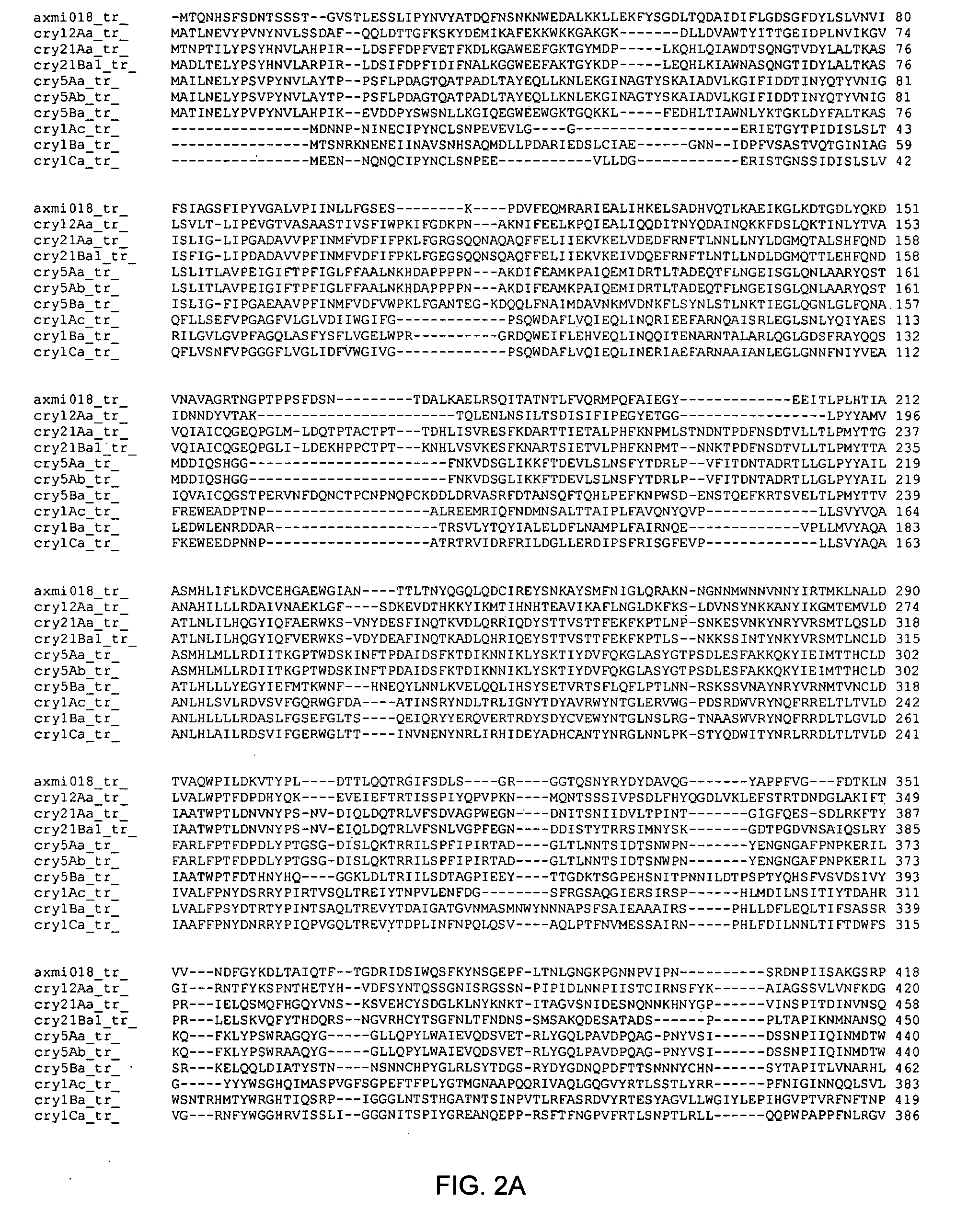
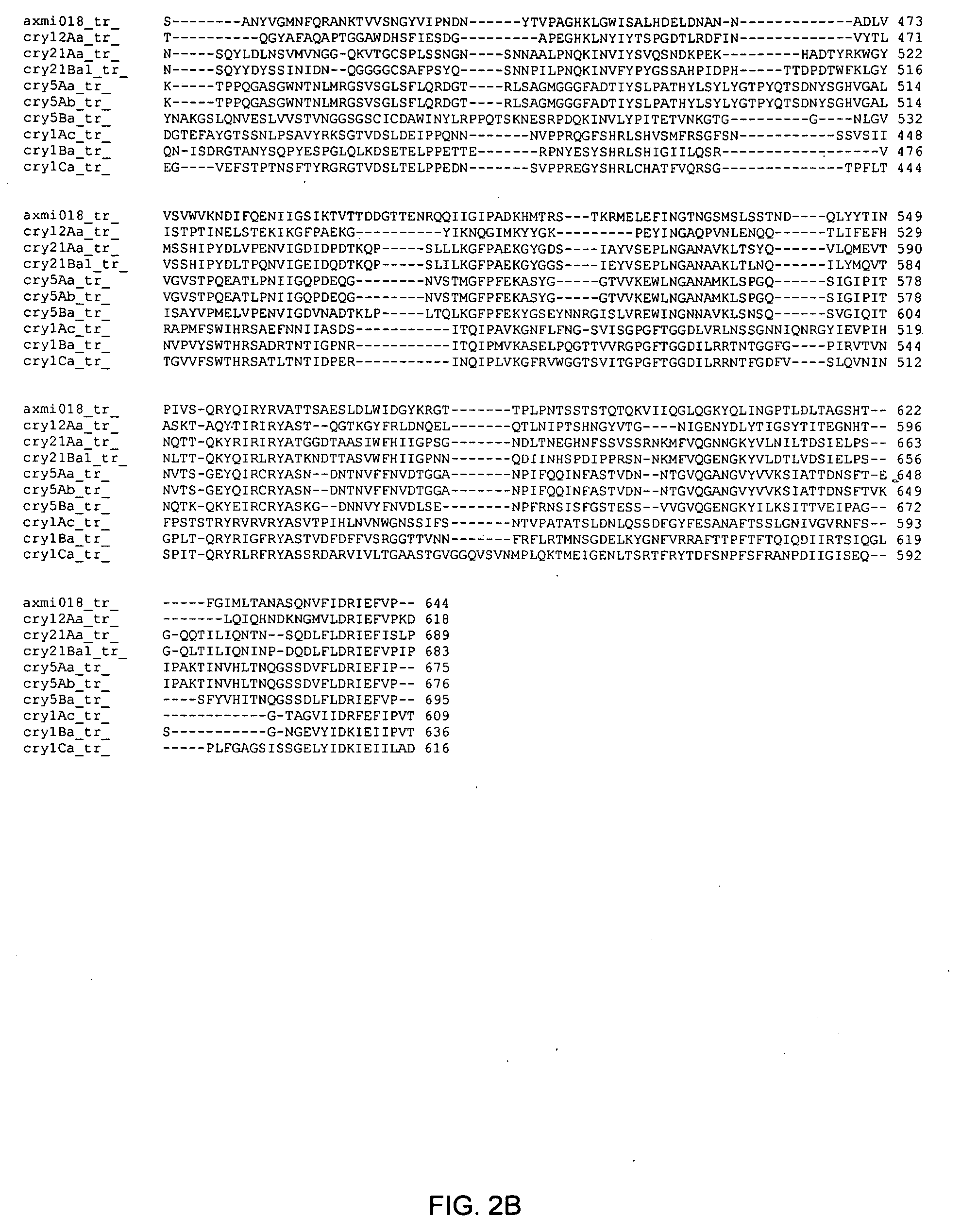
Axmi-018, axmi-020, and axmi-021, a family of delta-endotoxin genes and methods for their use
Compositions and methods for conferring pesticidal activity to bacteria, plants, plant cells, tissues and seeds are provided. Compositions comprising a coding sequence for a delta-endotoxin polypeptide are provided. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants and bacteria. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, isolated delta-endotoxin nucleic acid molecules are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequence shown in SEQ ID NO:2, 4, or 6, or the nucleotide sequence set forth in SEQ ID NO:1, 3, or 5, as well as variants and fragments thereof.
Owner:ATHENIX
Nucleotide sequences and corresponding polypeptides conferring modulated plant characteristics
ActiveUS20090094717A1Modulation characteristicLibrary member identificationVector-based foreign material introductionBiotechnologyNucleotide
The present invention relates to isolated nucleic acid molecules and their corresponding encoded polypeptides able confer the trait of modulated plant size, vegetative growth, organ number, plant architecture, sterility or seedling lethality in plants. The present invention further relates to the use of these nucleic acid molecules and polypeptides in making transgenic plants, plant cells, plant materials or seeds of a plant having such modulated growth or phenotype characteristics that are altered with respect to wild type plants grown under similar conditions.
Owner:CERES INC
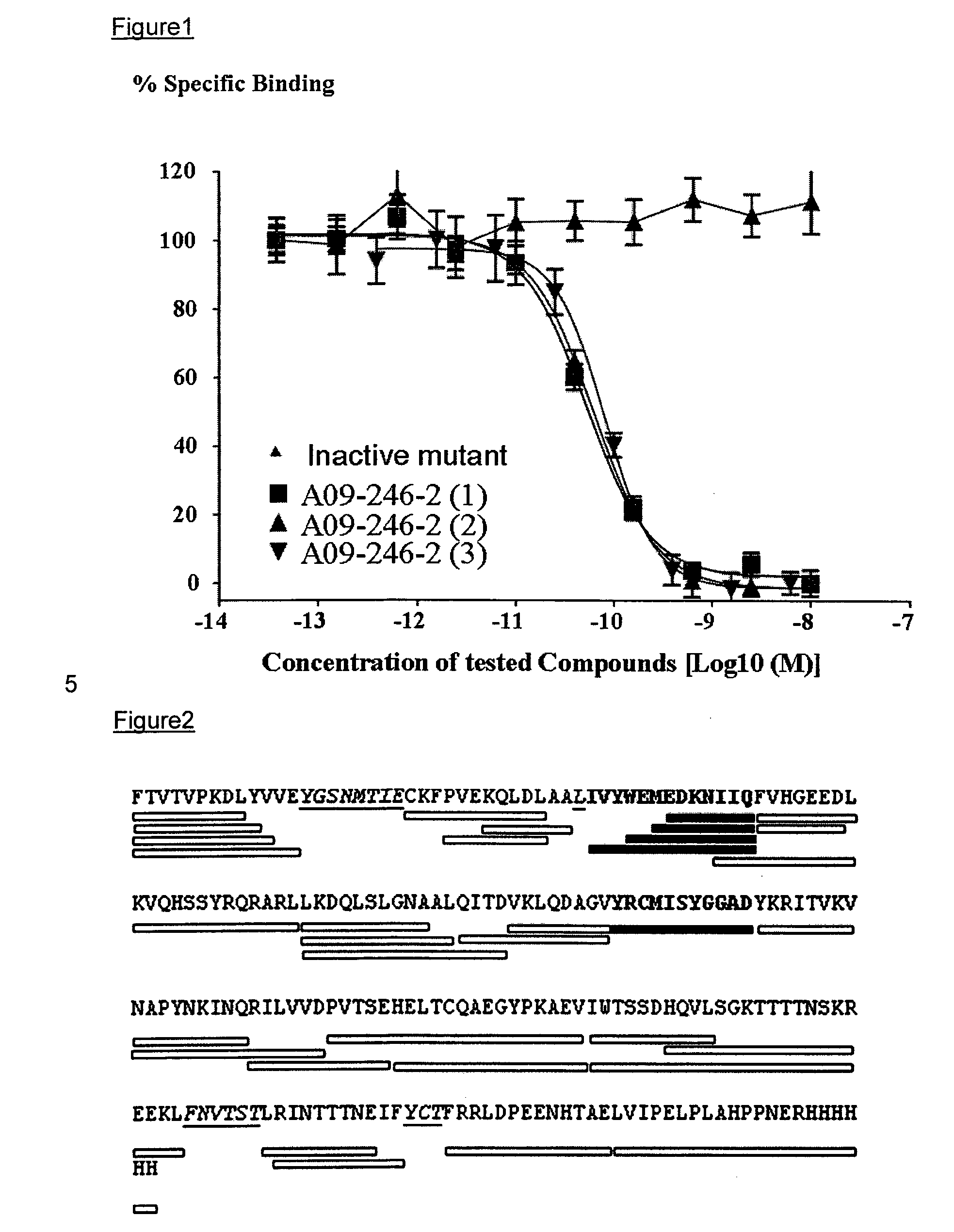
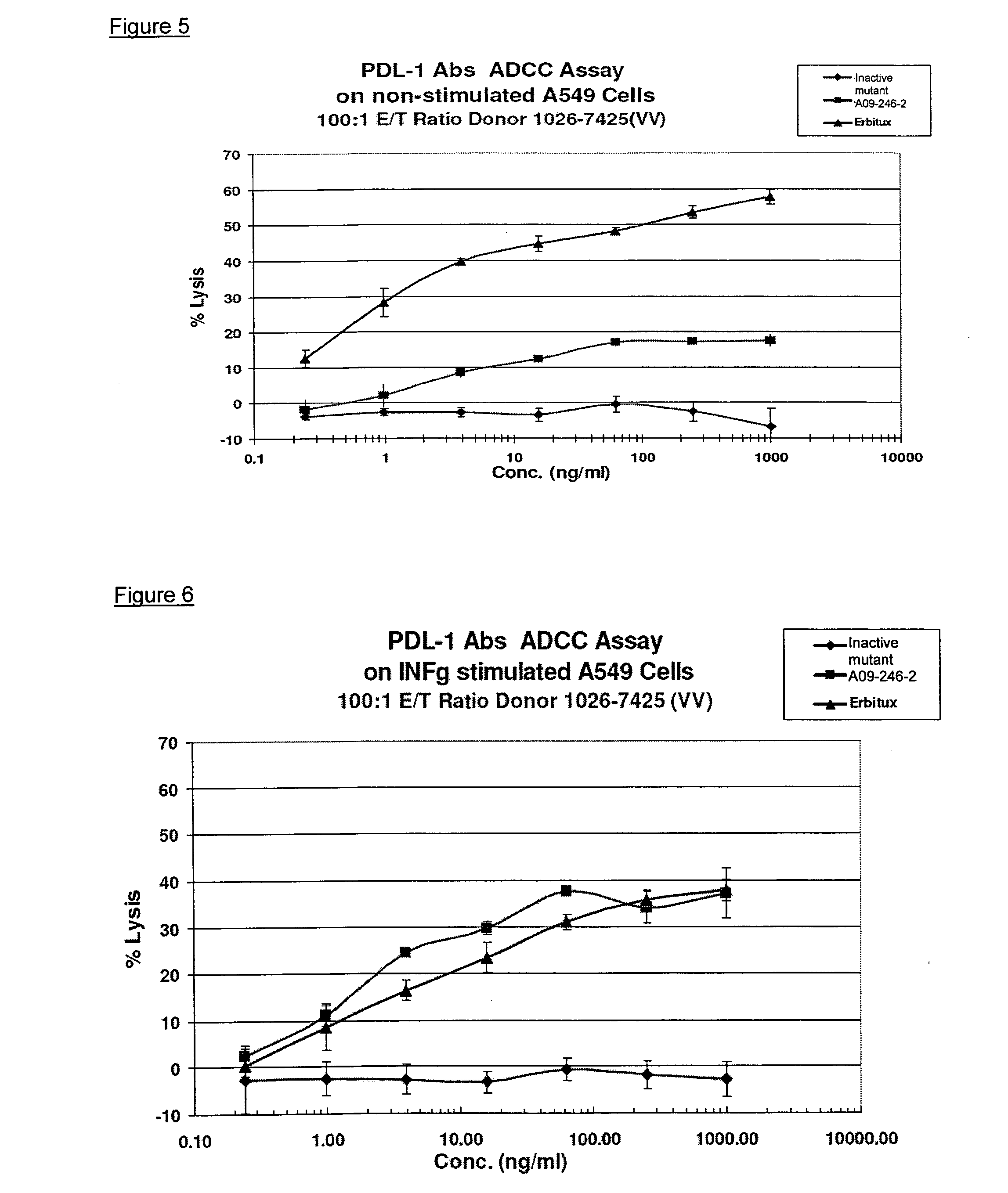
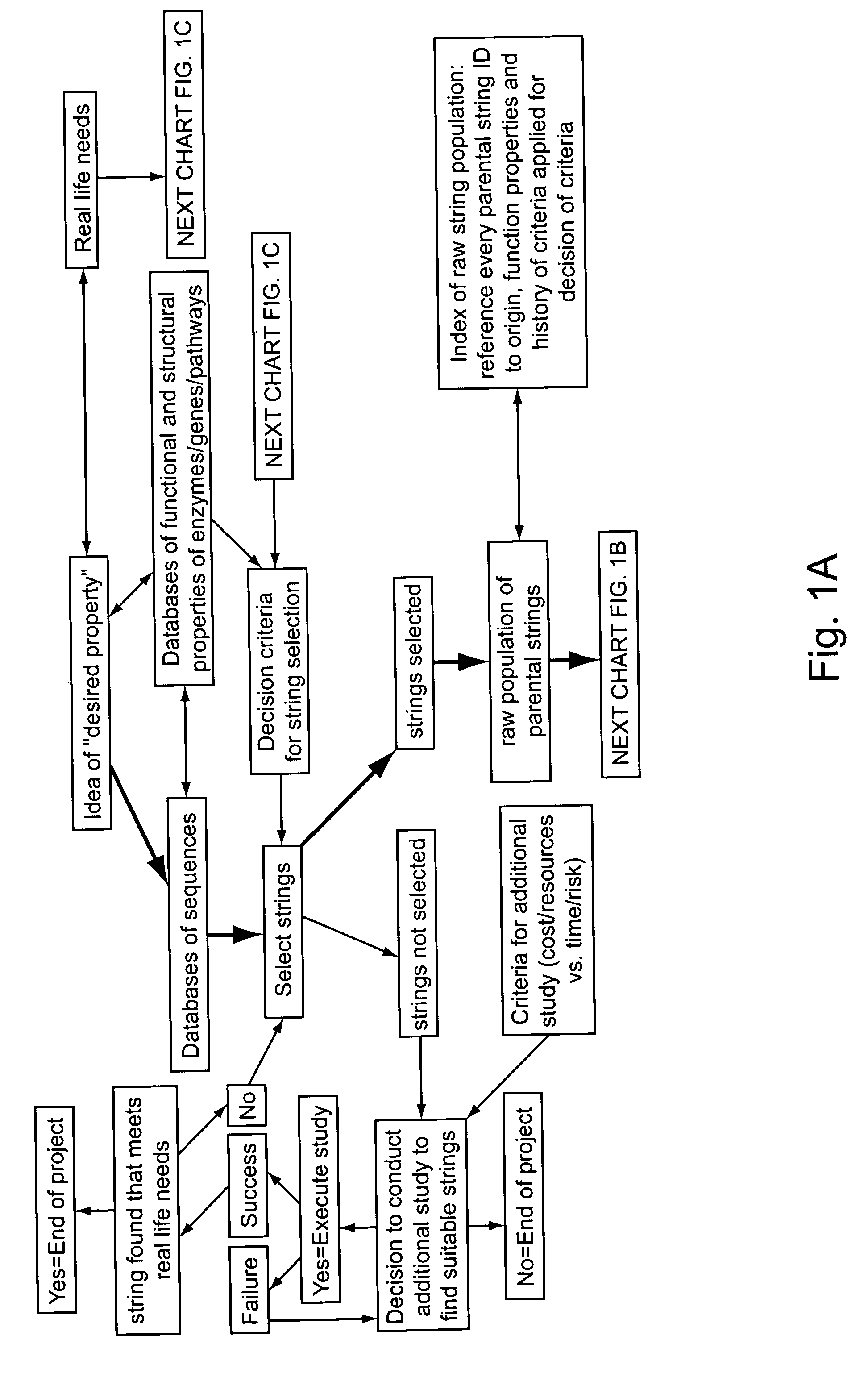
Anti-pd-l1 antibodies and uses thereof
ActiveUS20140341917A1Function increaseUpregulate cell-mediated immune responsesOrganic active ingredientsPeptide/protein ingredientsAntigen Binding FragmentAntigen binding
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
Methods for making character strings, polynucleotides and polypeptides having desired characteristics
InactiveUS7024312B1Simplifies overall synthesis strategyLow levelPeptide/protein ingredientsBiostatisticsPolynucleotideIn silico
“In silico” nucleic acid recombination methods, related integrated systems utilizing genetic operators and libraries made by in silico shuffling methods are provided.
Owner:CODEXIS MAYFLOWER HLDG LLC
Stabilized polypeptide compositions
ActiveUS20080050370A1Improved polypeptide compositionImprove methodPeptide/protein ingredientsAntibody mimetics/scaffoldsCrystallographyPolypeptide composition
Owner:BIOGEN MA INC
Anti-TIM-3 antibody
The present invention provides an anti-human TIM-3 antibody having high ADCC activity or antibody fragment thereof by screening a monoclonal antibody or antibody fragment thereof which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure and exhibits ADCC activity; a hybridoma which produces the antibody; a DNA encoding the antibody; a vector comprising the DNA; a transformant which is obtainable by introducing the vector; a method for producing the antibody or the antibody fragment thereof which comprises using the hybridoma or the transformant; a therapeutic agent and a diagnostic agent comprising the antibody or the antibody fragment thereof as an active ingredient.
Owner:KYUSHU UNIV +1
Compositions for regeneration and repair of cartilage lesions
InactiveUS6511958B1Increase ratingsImprove repair qualitySuture equipmentsPowder deliveryMedicineCartilage lesion
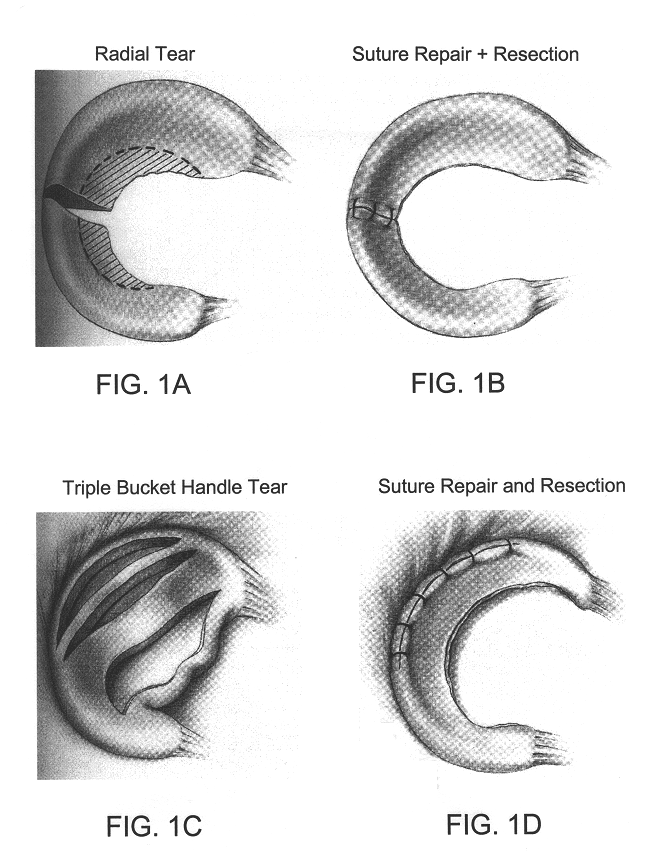
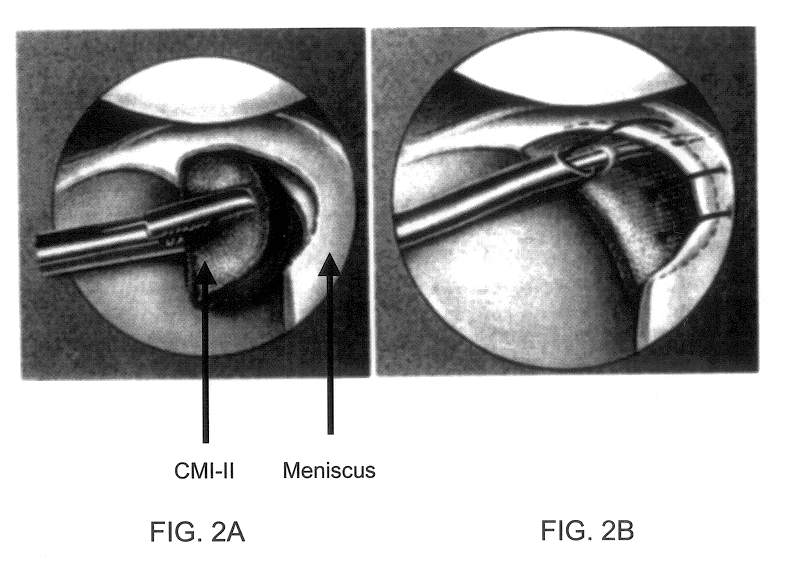
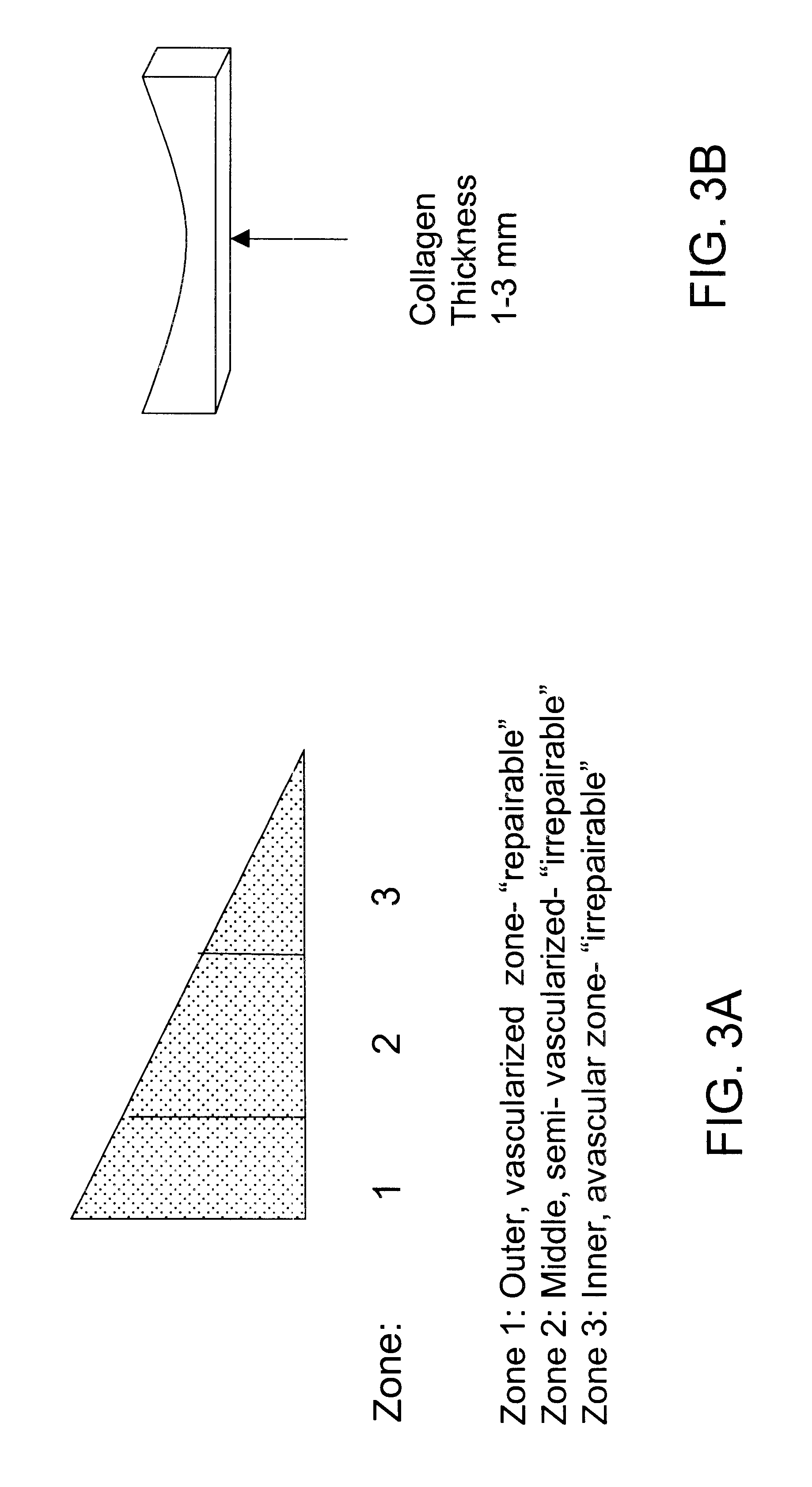
Disclosed is a cartilage repair product that induces both cell ingrowth into a bioresorbable material and cell differentiation into cartilage tissue. Such a product is useful for regenerating and / or repairing both vascular and avascular cartilage lesions, particularly articular cartilage lesions, and even more particularly mensical tissue lesions, including tears as well as segmental defects. Also disclosed is a method of regenerating and repairing cartilage lesions using such a product.
Owner:ZIMMER ORTHOBIOLOGICS
Bacillus thuringiensis CryET33 and CryET34 compositions and uses therefor
Disclosed are Bacillus thuringiensis strains comprising novel crystal proteins which exhibit insecticidal activity against coleopteran insects including red flour beetle larvae (Tribolium castaneum) and Japanese beetle larvae (Popillia japonica). Also disclosed are novel B. thuringiensis crystal toxin genes, designated cryET33 and cryET34, which encode respectively the coleopteran-toxic proteins, CryET33 (29-kDa) crystal protein, and CryET34 (14-kDa) crystal protein. Also disclosed are methods of making and using transgenic cells comprising the novel nucleic acid sequences of the invention.
Owner:MONSANTO TECH LLC
Modified polynucleotides for the production of secreted proteins
Owner:MODERNATX INC
Bacillus thuringiensis cryET33 and cryET34 compositions and uses therefor
Disclosed are Bacillus thuringiensis strains comprising novel crystal proteins which exhibit insecticidal activity against coleopteran insects including red flour beetle larvae (Tribolium castaneum) and Japanese beetle larvae (Popillia japonica). Also disclosed are novel B. thuringiensis crystal toxin genes, designated cryET33 and cryET34, which encode the colepteran-toxic crystal proteins, CryET33 (29-kDa) crystal protein, and the cryET34 gene encodes the 14-kDa CryET34 crystal protein. The CryET33 and CryET34 crystal proteins are toxic to red flour beetle larvae and Japanese beetle larvae. Also disclosed are methods of making and using transgenic cells comprising the novel nucleic acid sequences of the invention.
Owner:MONSANTO TECH LLC
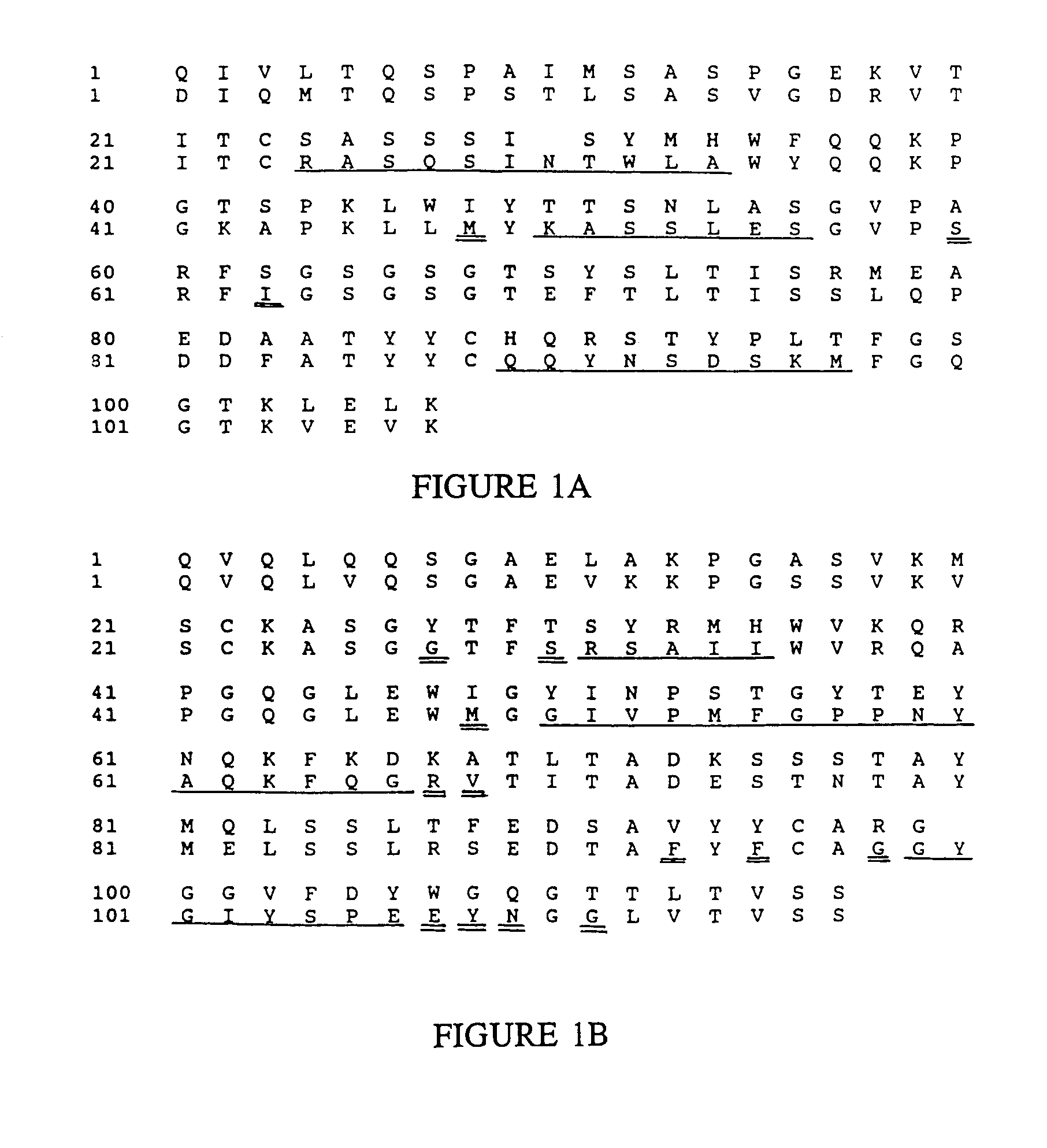
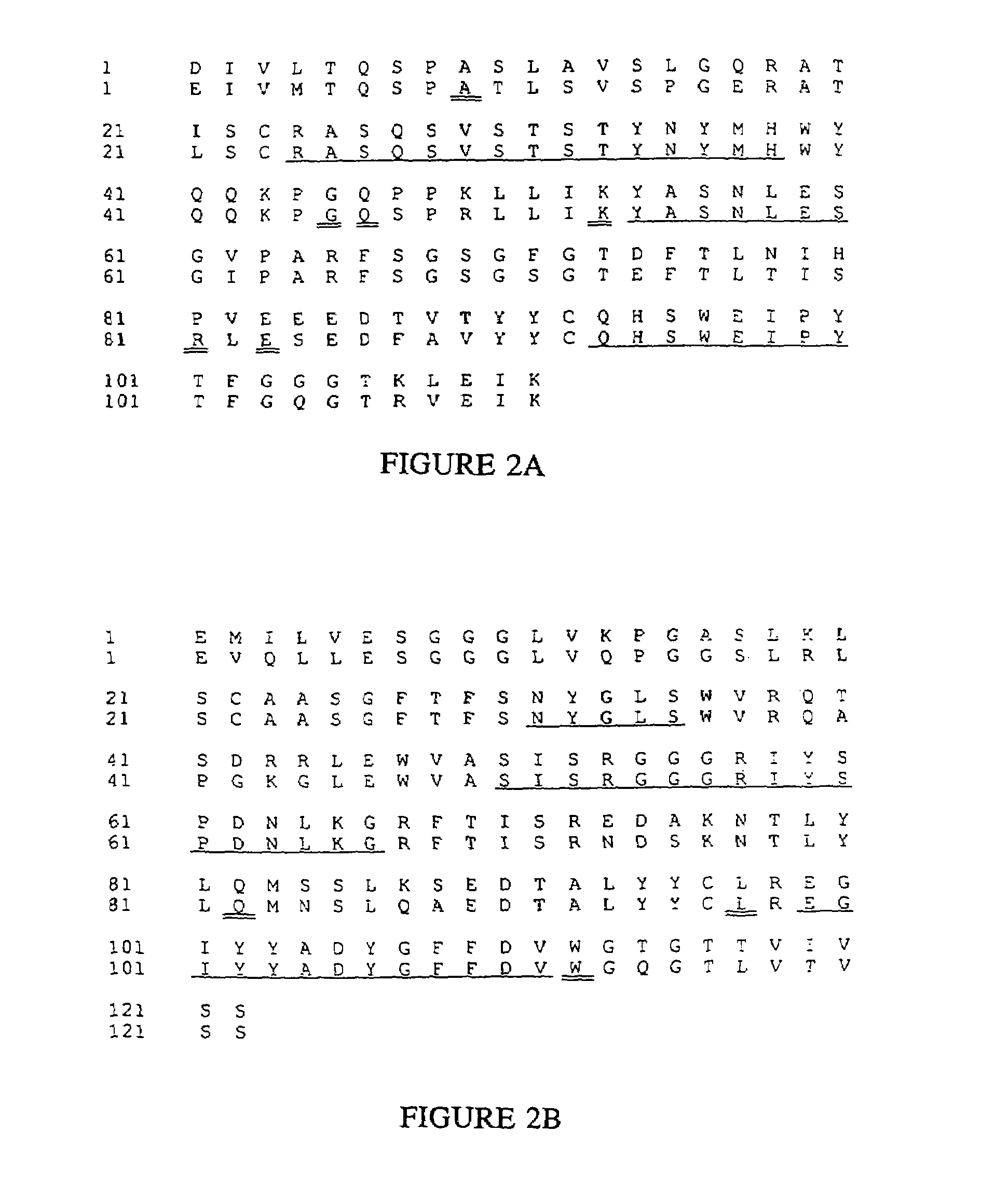
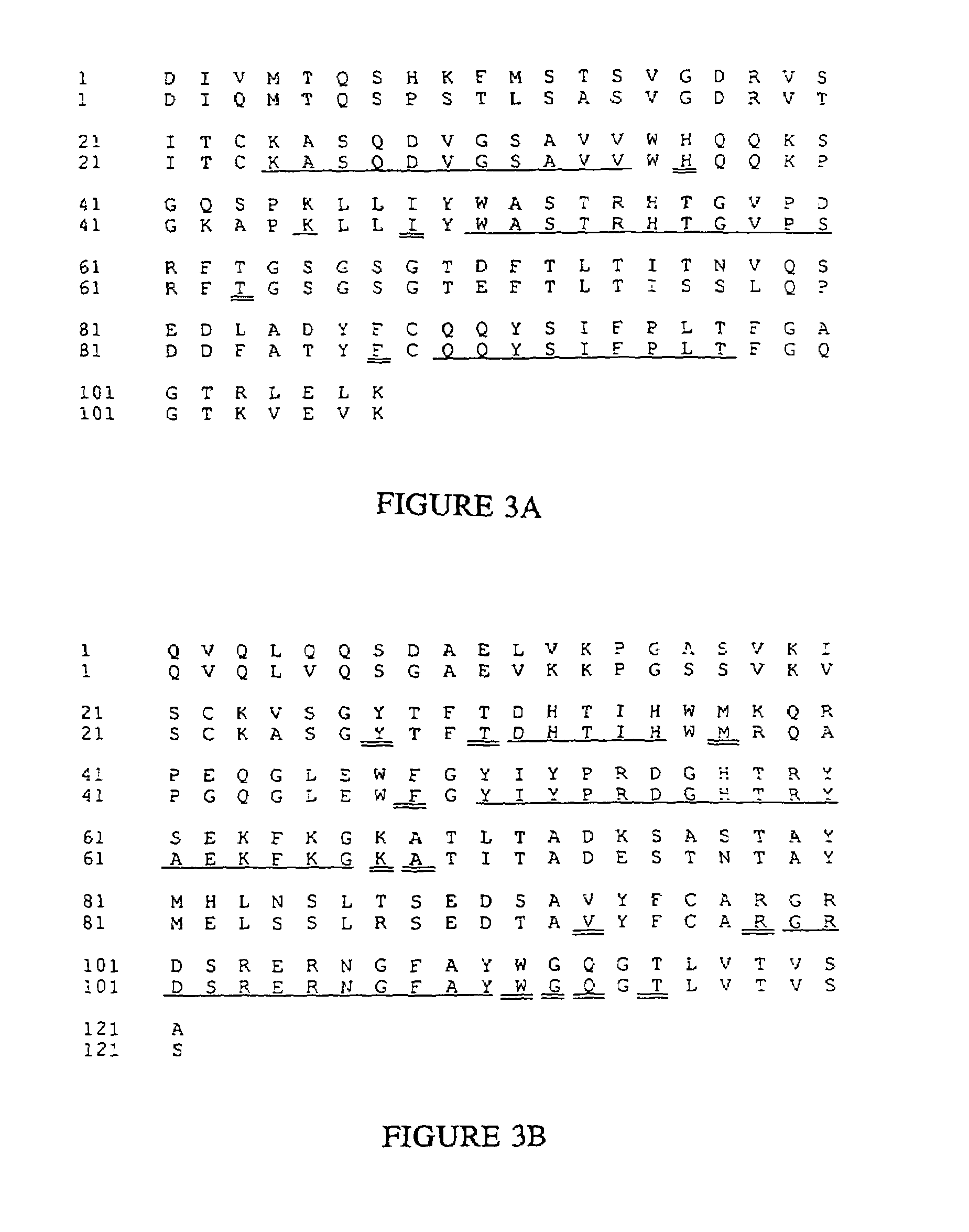
Humanized immunoglobulins
Novel methods for producing, and compositions of, humanized immunoglobulins having one or more complementarity determining regions (CDR's) and possible additional amino acids from a donor immunoglobulin and a framework region from an accepting human immunoglobulin are provided. Each humanized immunoglobulin chain will usually comprise, in addition to the CDR's, amino acids from the donor immunoglobulin framework that are, e.g., capable of interacting with the CDR's to effect binding affinity, such as one or more amino acids which are immediately adjacent to a CDR in the donor immunoglobulin or those within about about 3Å as predicted by molecular modeling. The heavy and light chains may each be designed by using any one or all of various position criteria. When combined into an intact antibody, the humanized immunoglobulins of the present invention will be substantially non-immunogenic in humans and retain substantially the same affinity as the donor immunoglobulin to the antigen, such as a protein or other compound containing an epitope.
Owner:PDL BIOPHARMA INCORPORATED
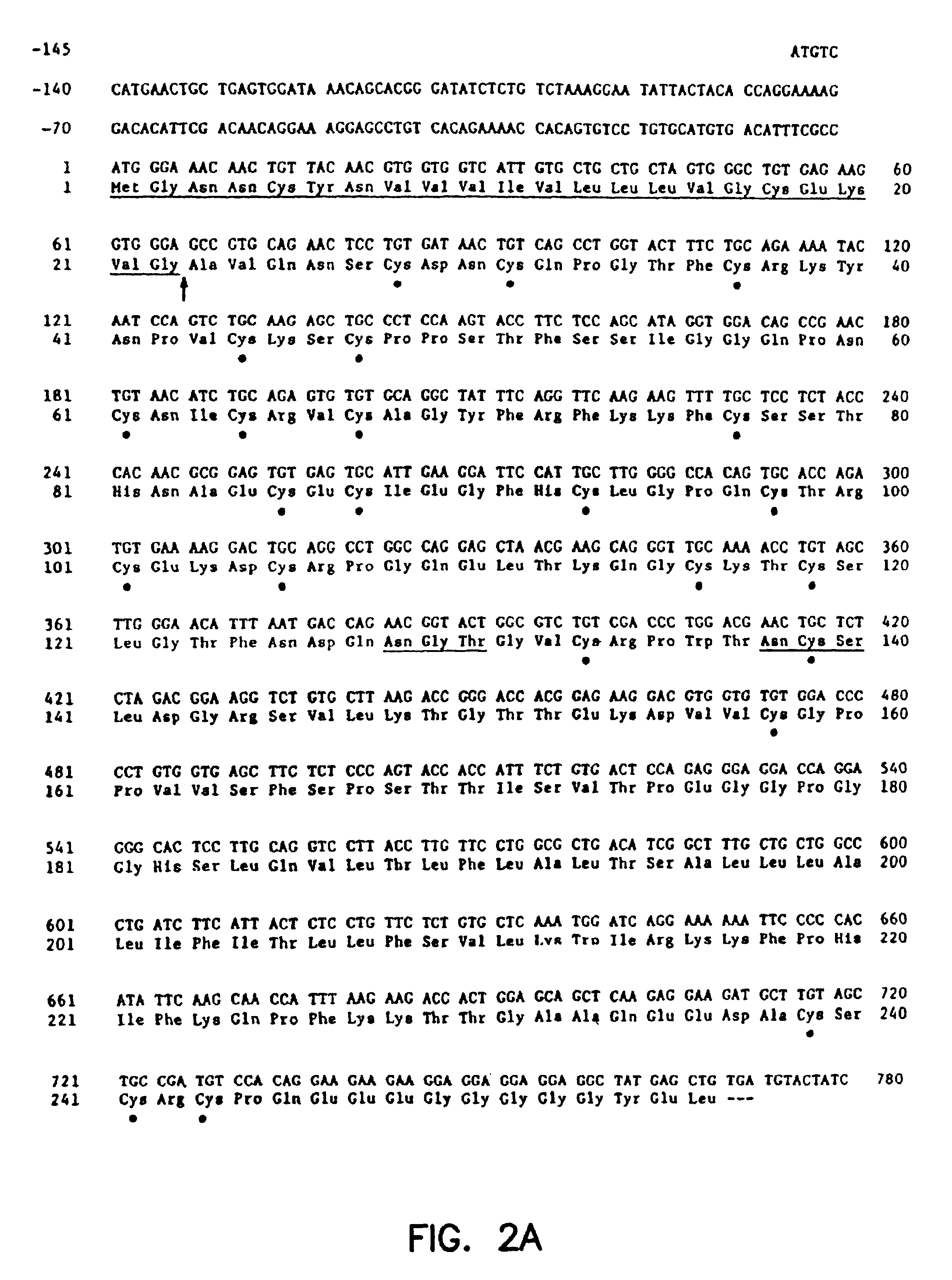
Antibody for 4-1BB
The present invention includes the receptor protein 4-1BB and the cDNA gene encoding for receptor protein 4-1BB. The nucleotide sequence of the isolated cDNA is disclosed herein along with the deduced amino acid sequence. The 4-1BB protein and fragments and derivatives can be used: 1) as a probe to isolate ligands to receptor protein 4-1BB, 2) to stimulate proliferation of B-cell's expressing 4-1BB, or 3) to block 4-1BB ligand binding. A monoclonal antibody against 4-1BB was developed which specifically recognizes an epitope on the extracellular domain of receptor protein 4-1BB. The monoclonal antibody can be used enhance T-cell proliferation and activation by treating T-cells that have expressed receptor protein 4-1BB with the monoclonal antibody. The effectiveness of the treatment was enhanced when conducted in the presence of protein tyrosinase kinase. A fusion protein for detecting cell membrane ligands to receptor protein 4-1BB was developed. It comprises the extracellular portion of the receptor protein 4-1BB and a detection protein bound to the portion of the receptor protein 4-1BB.
Owner:INDIANA UNIV RES & TECH CORP
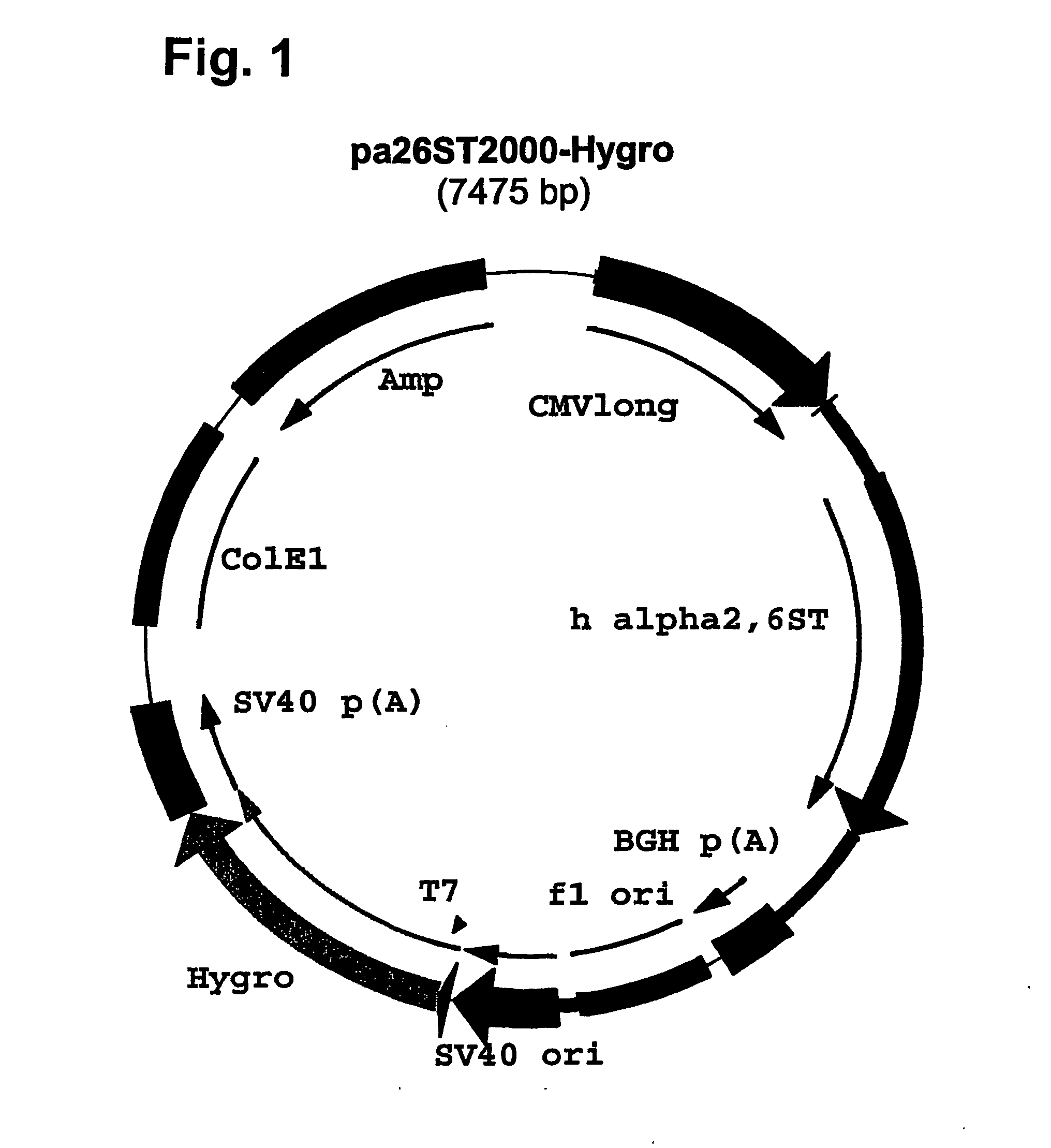
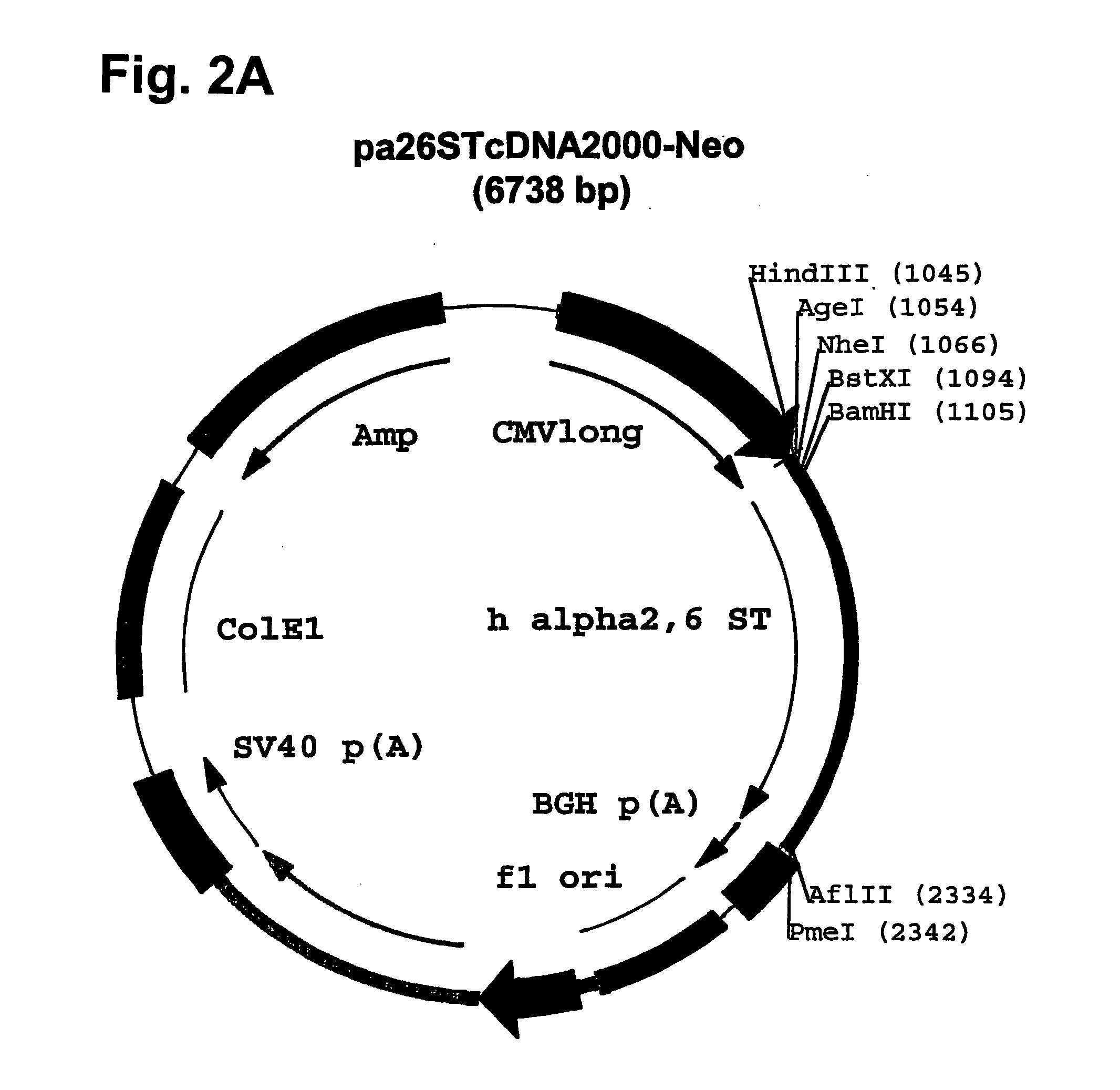
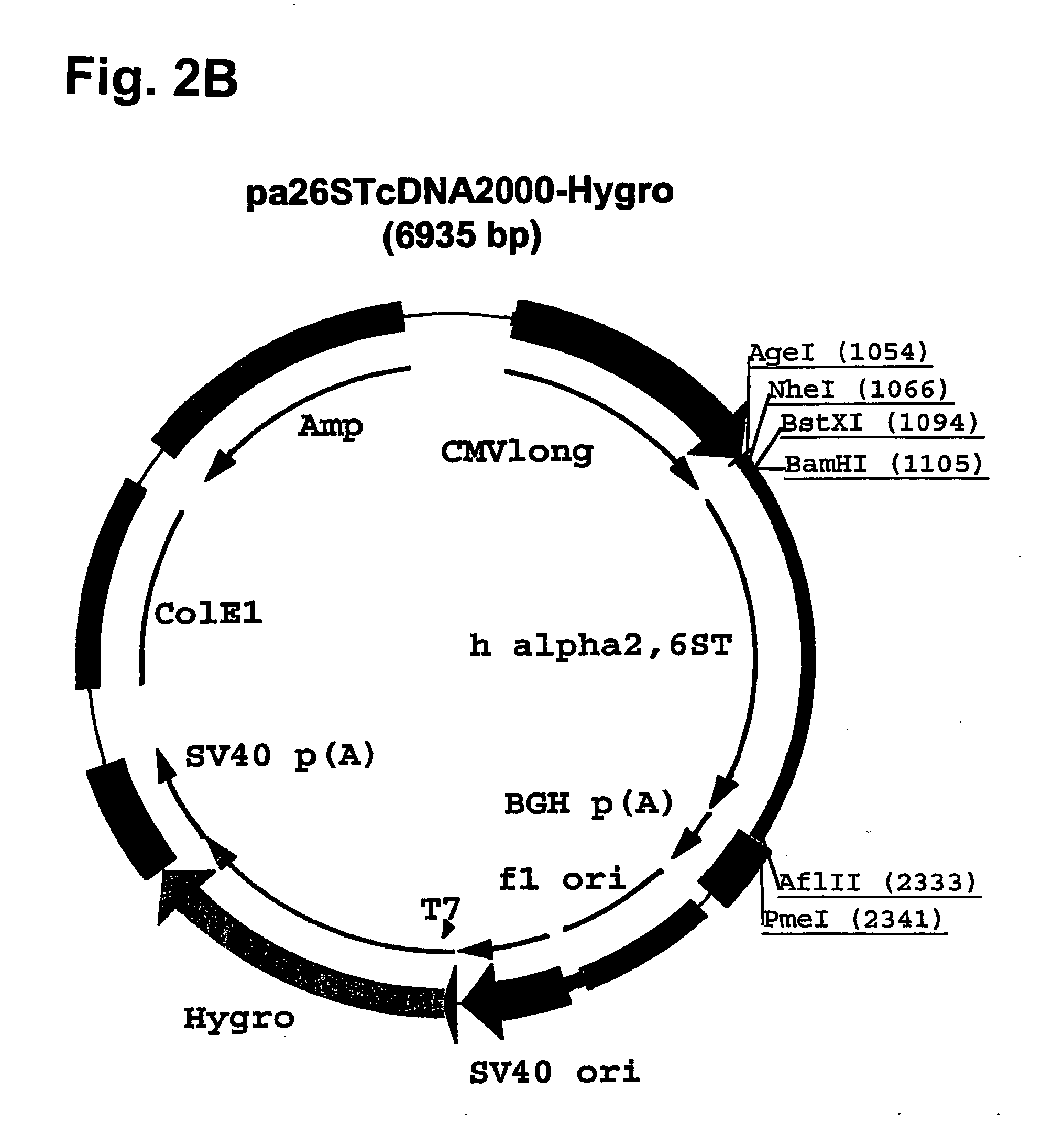
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com