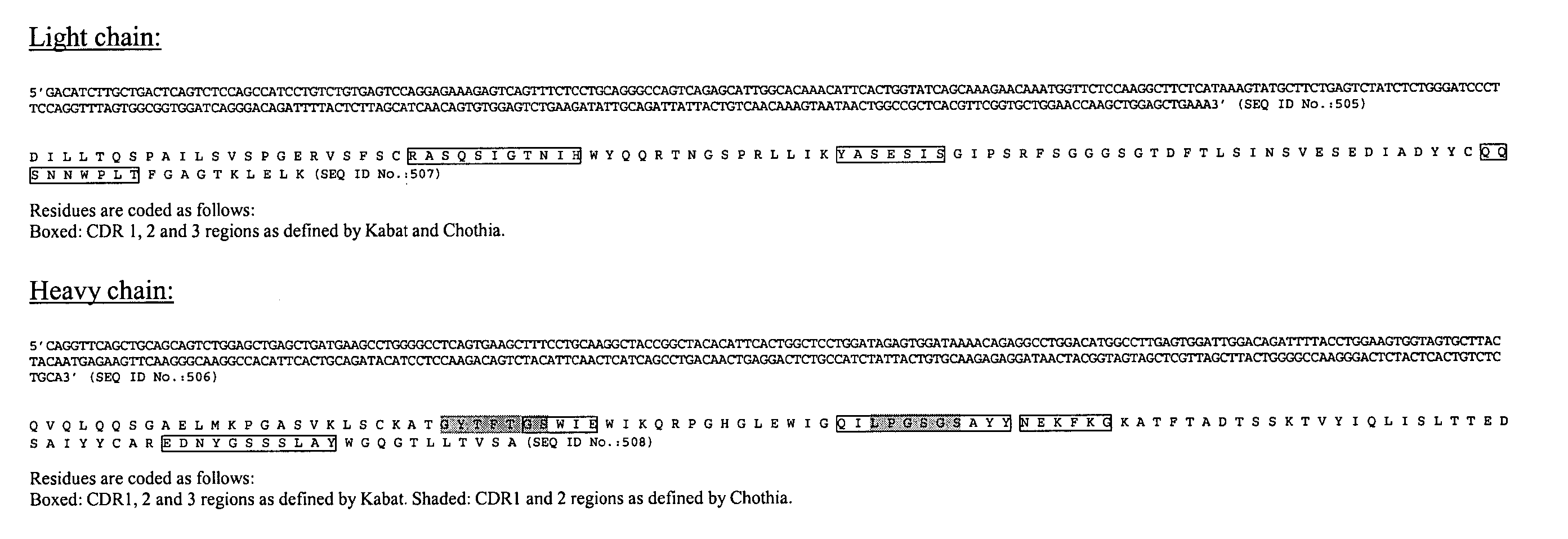
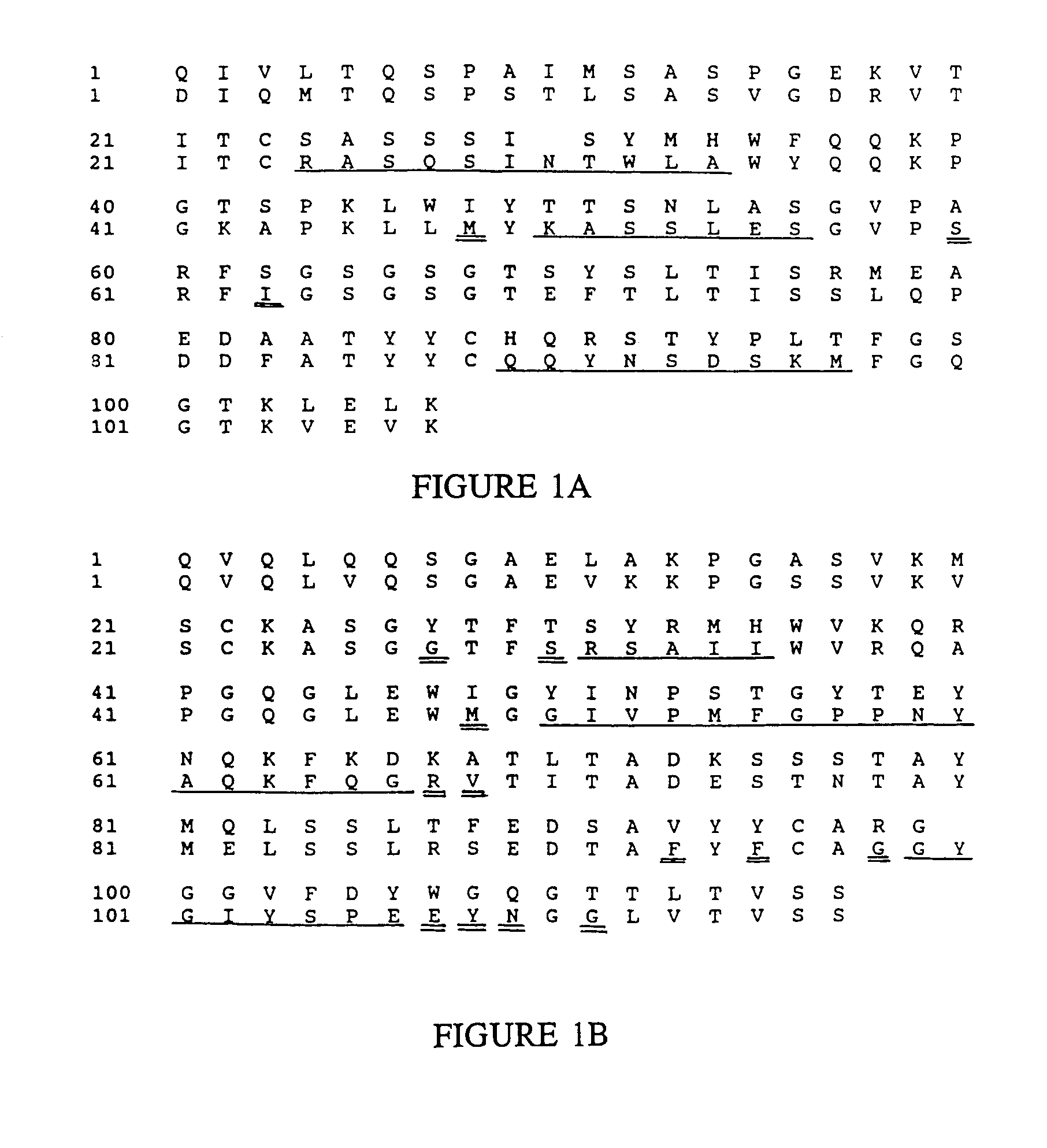
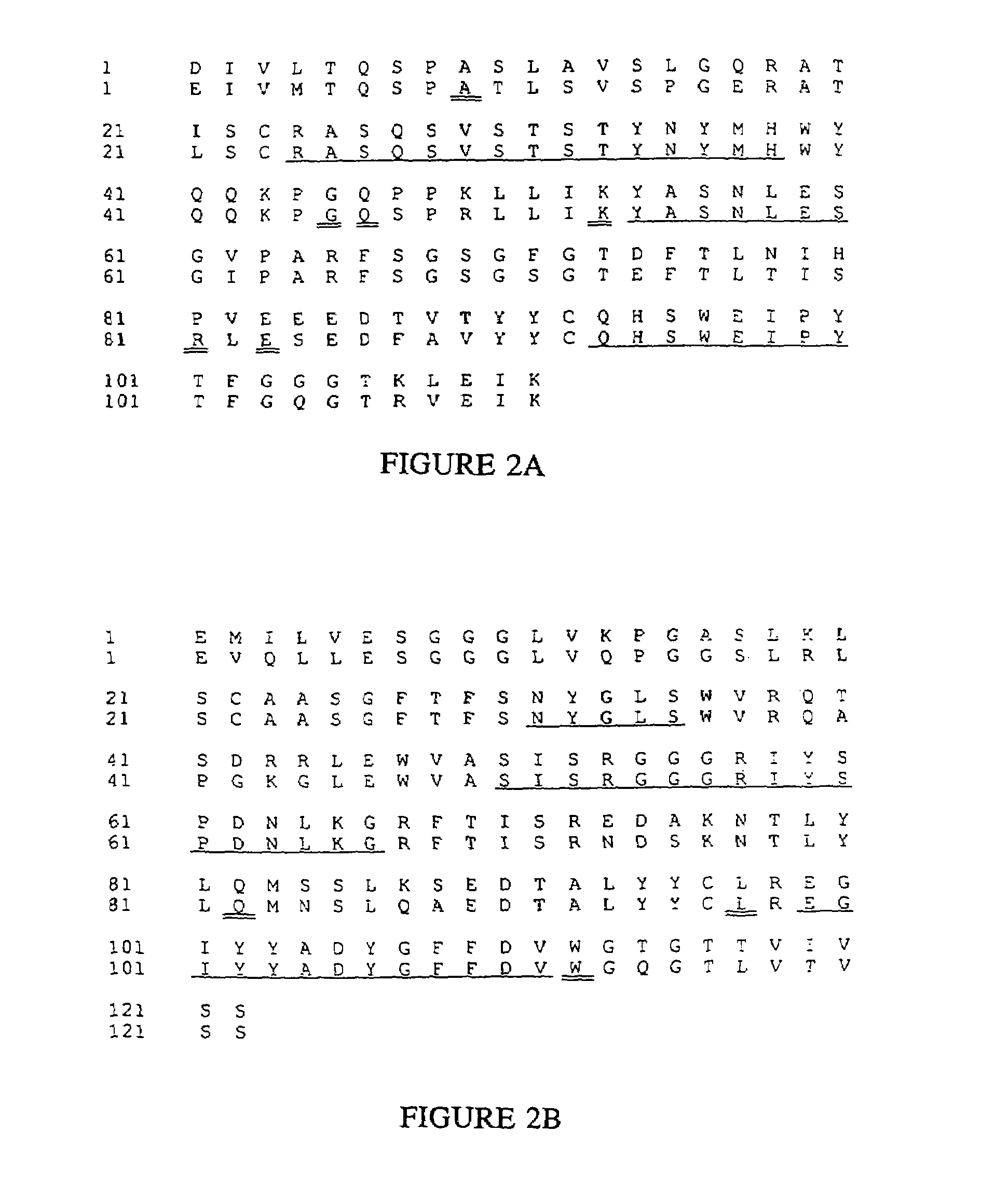
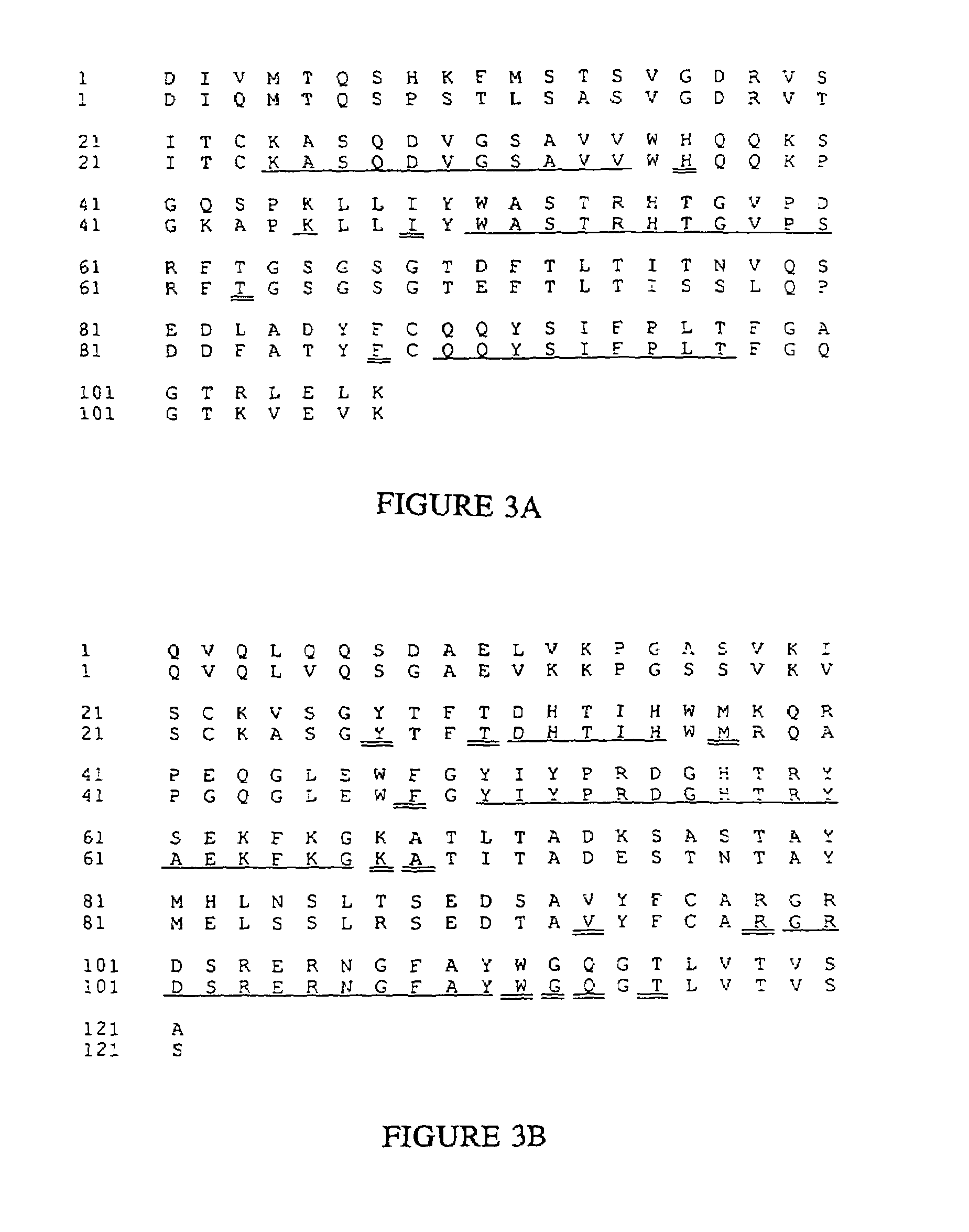
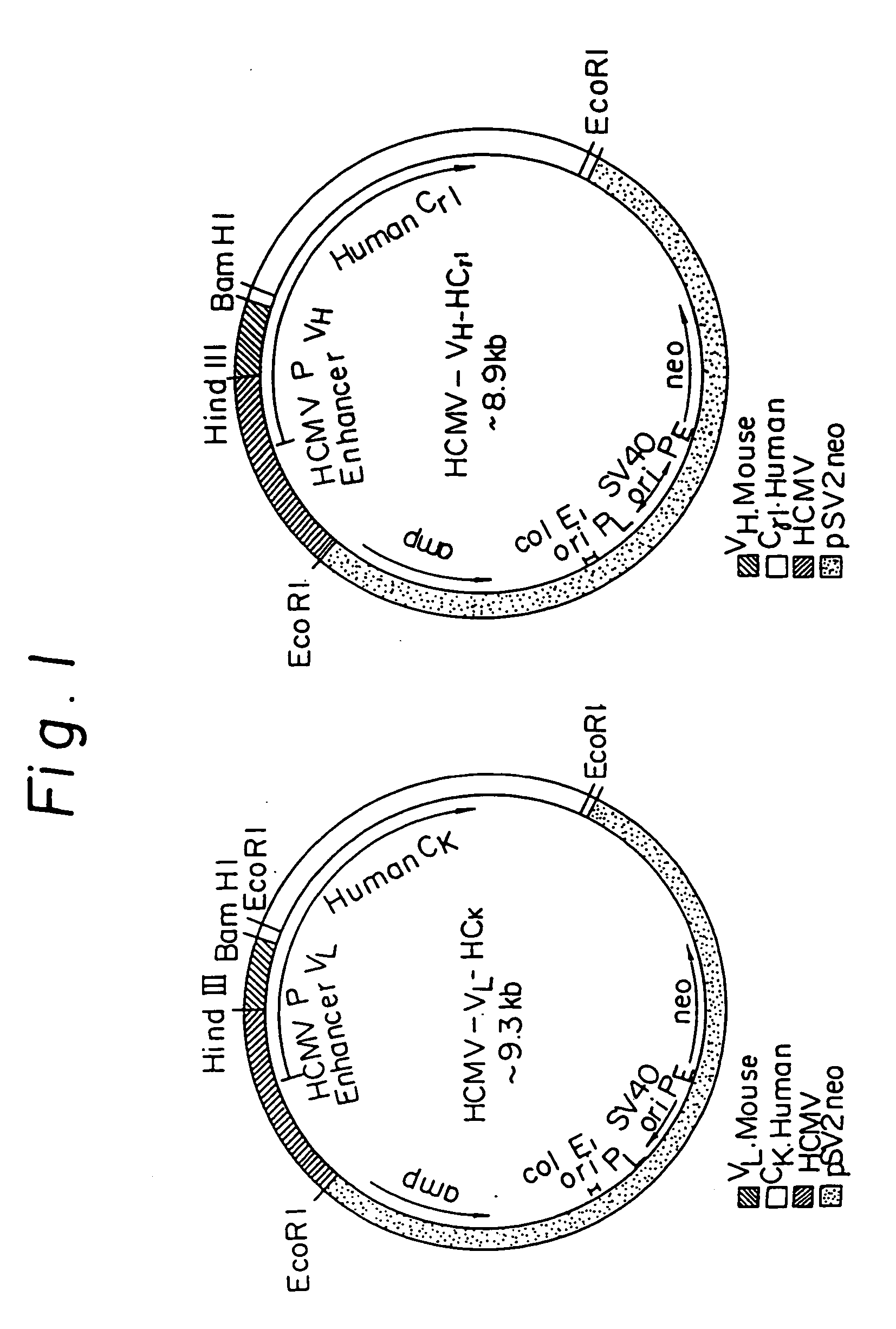
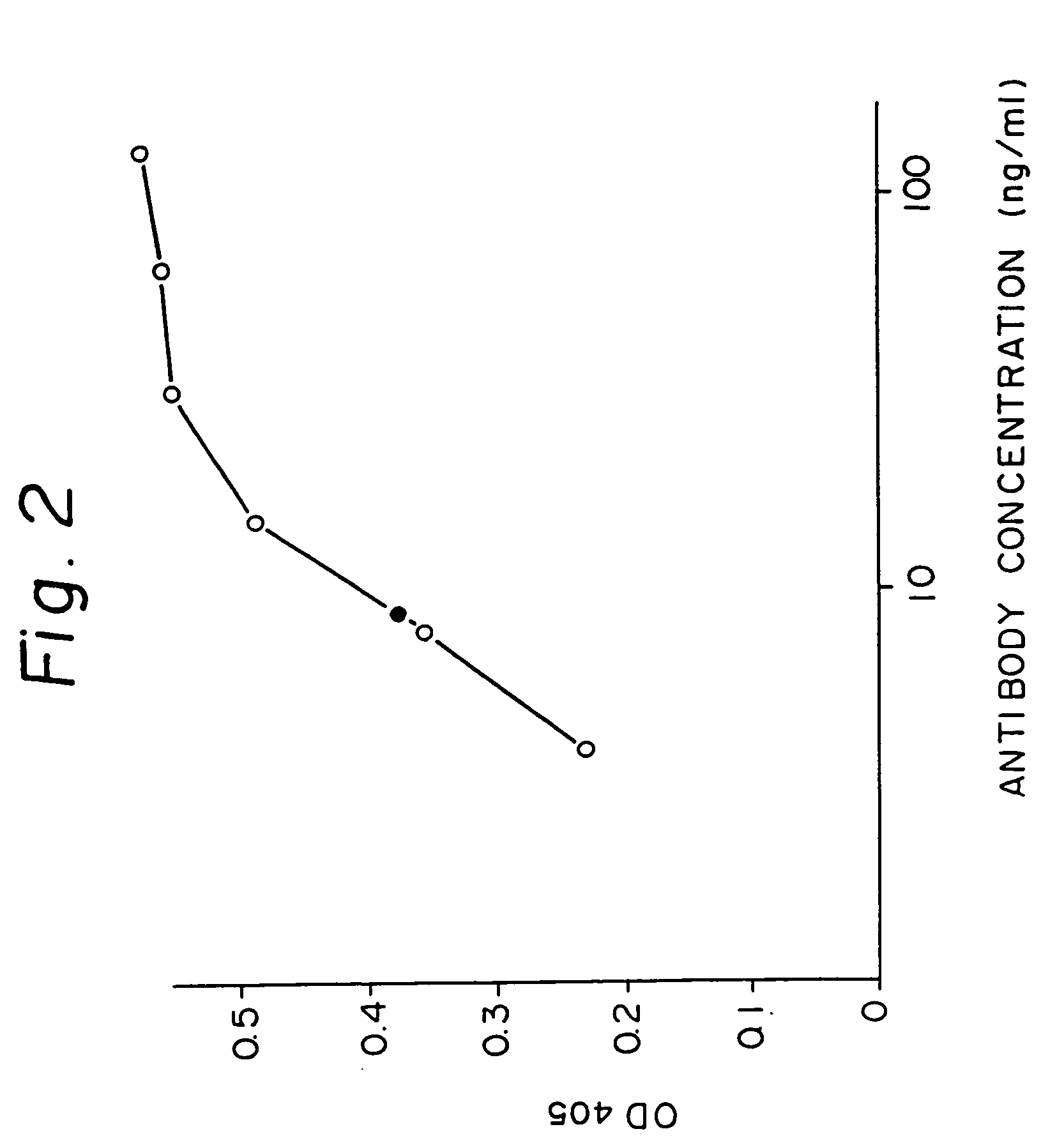
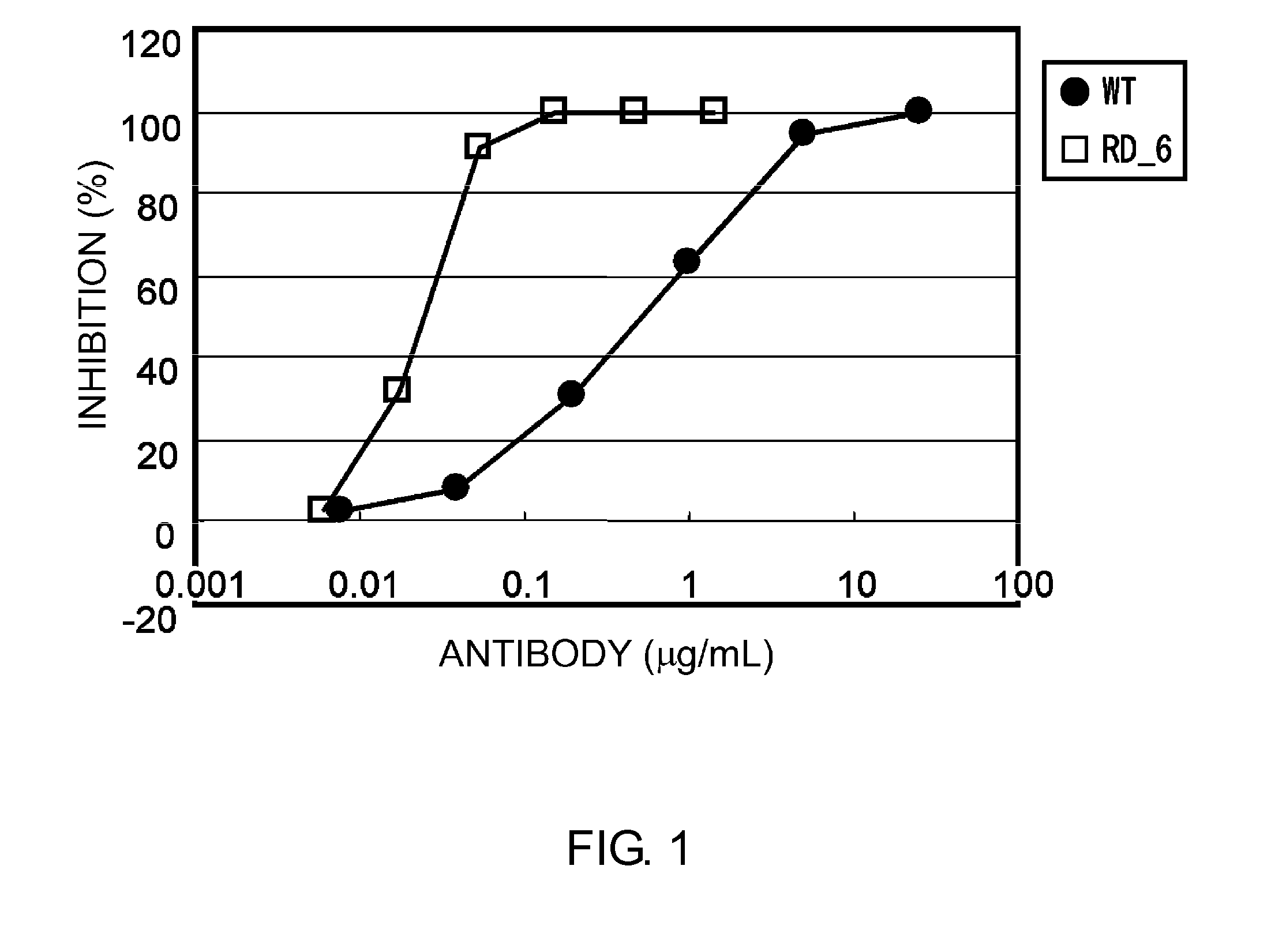
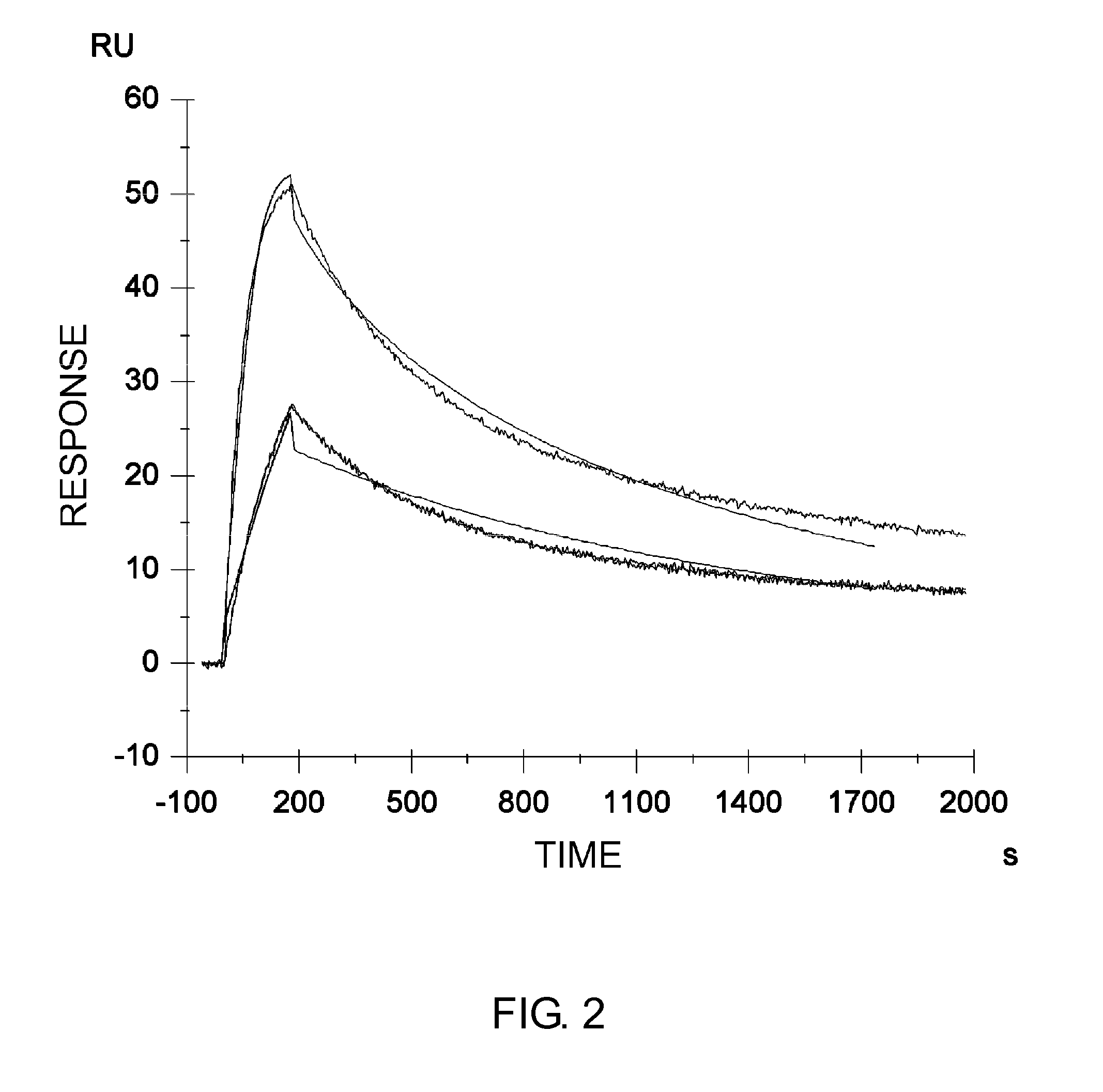
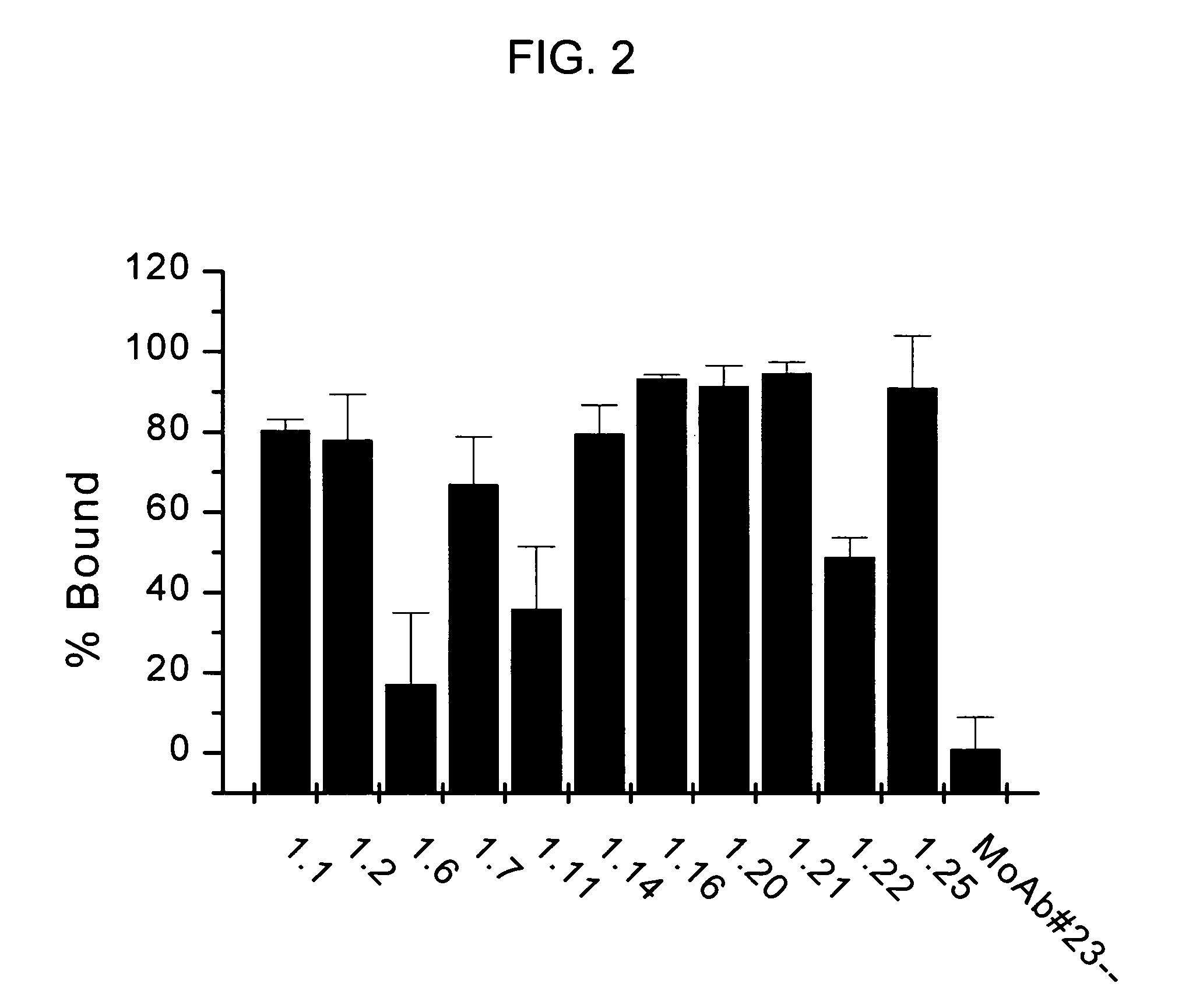
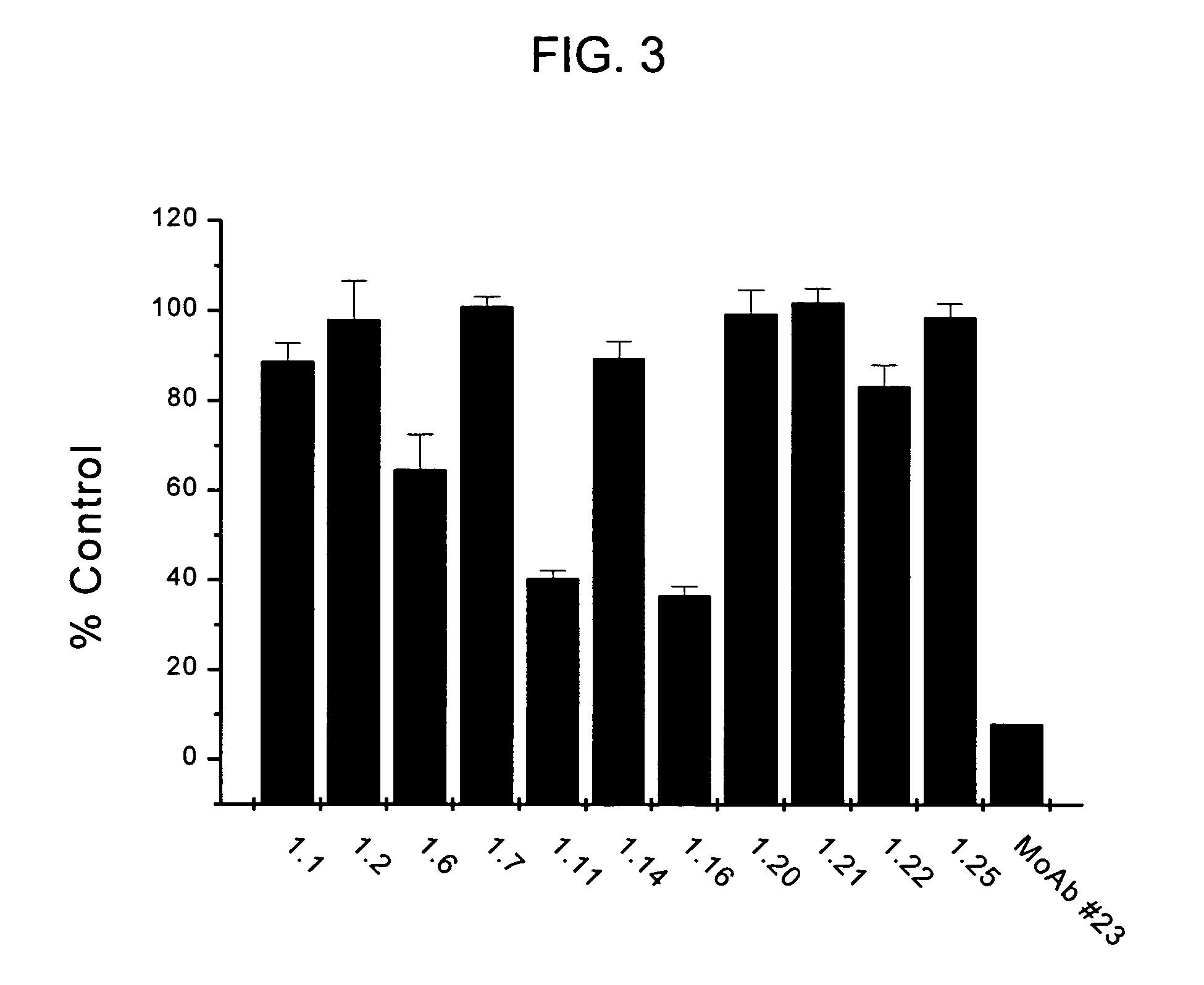
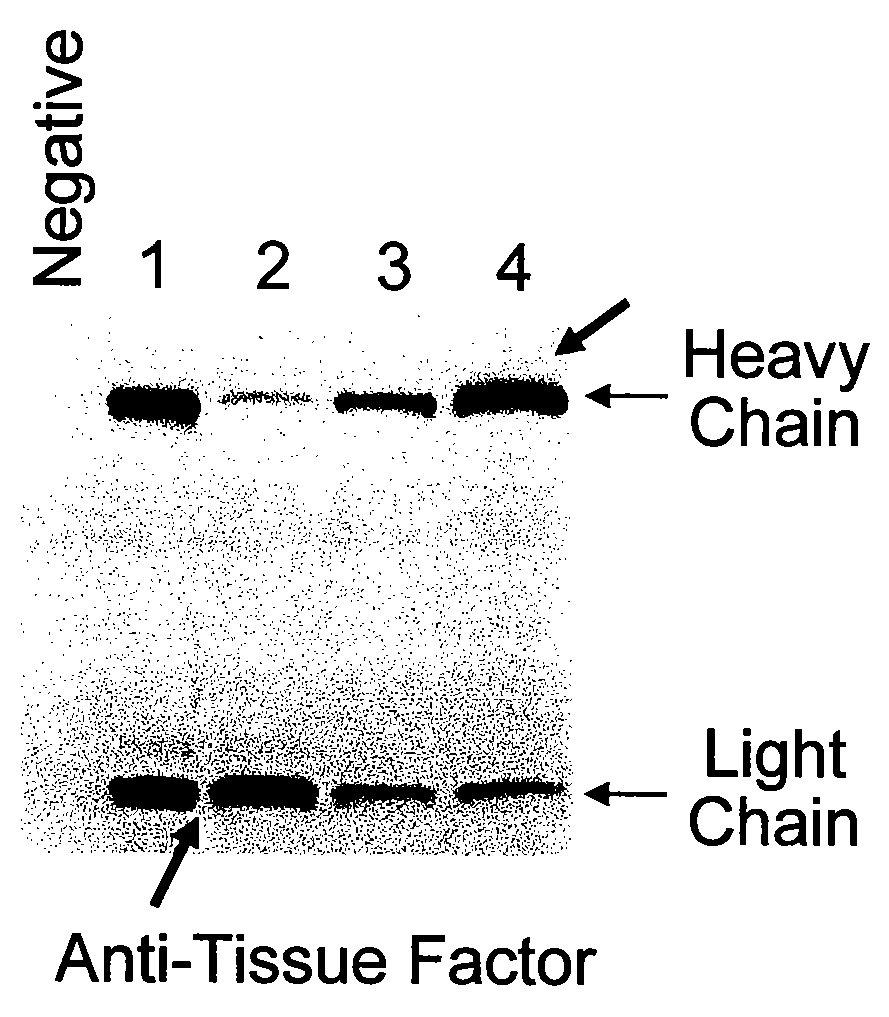
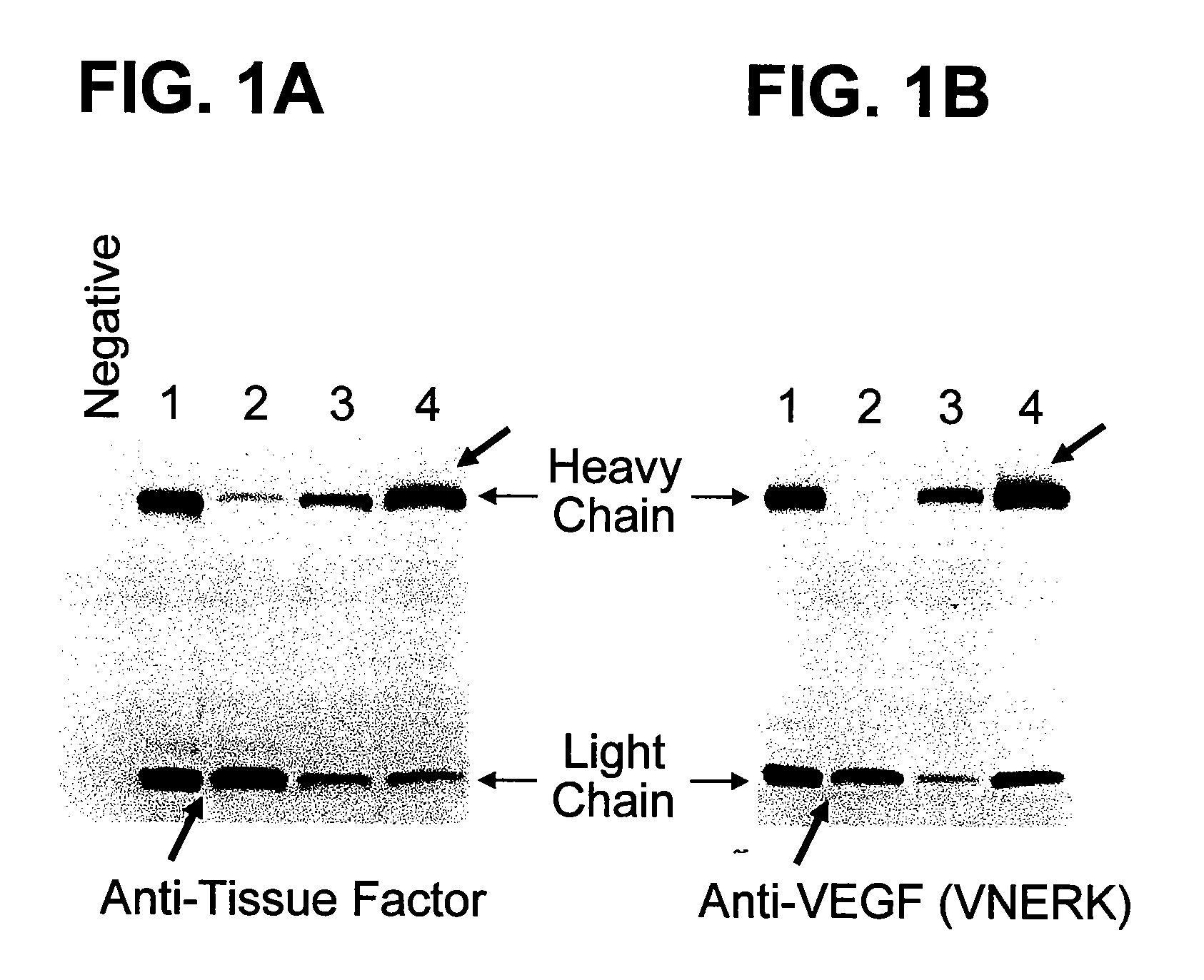
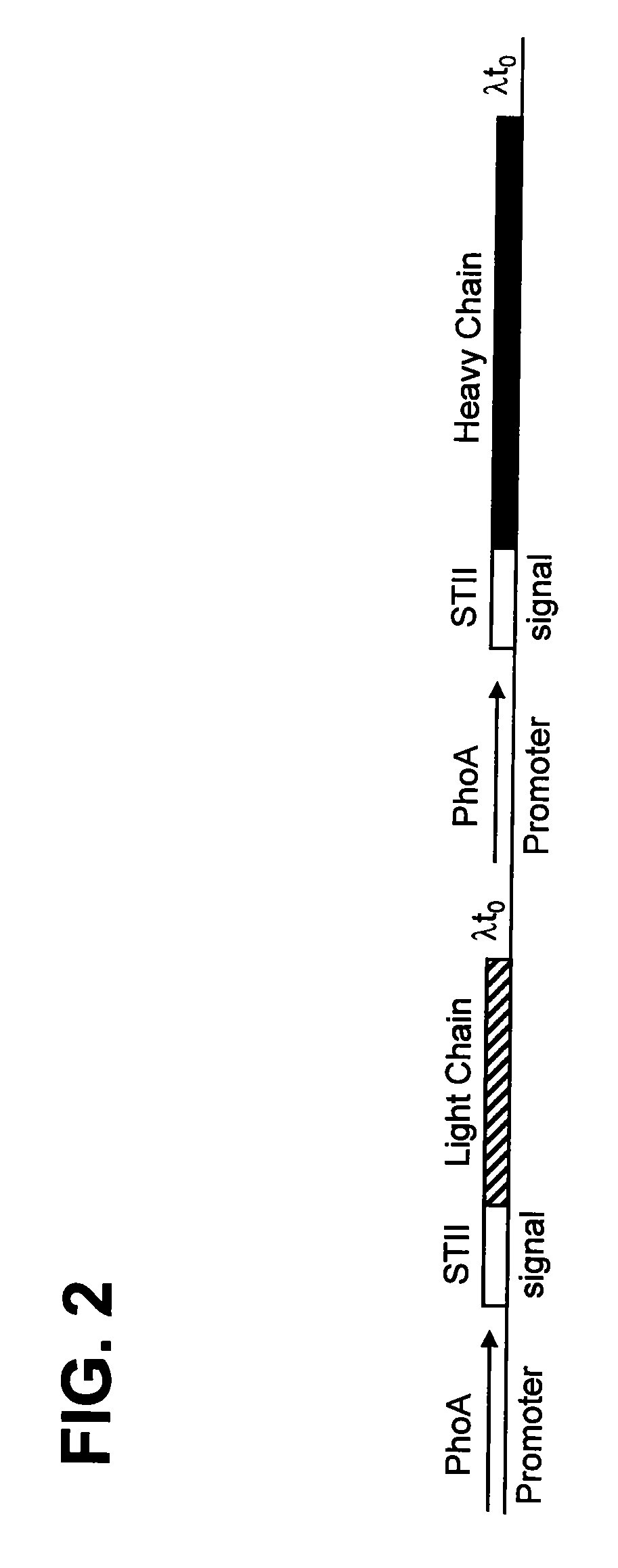
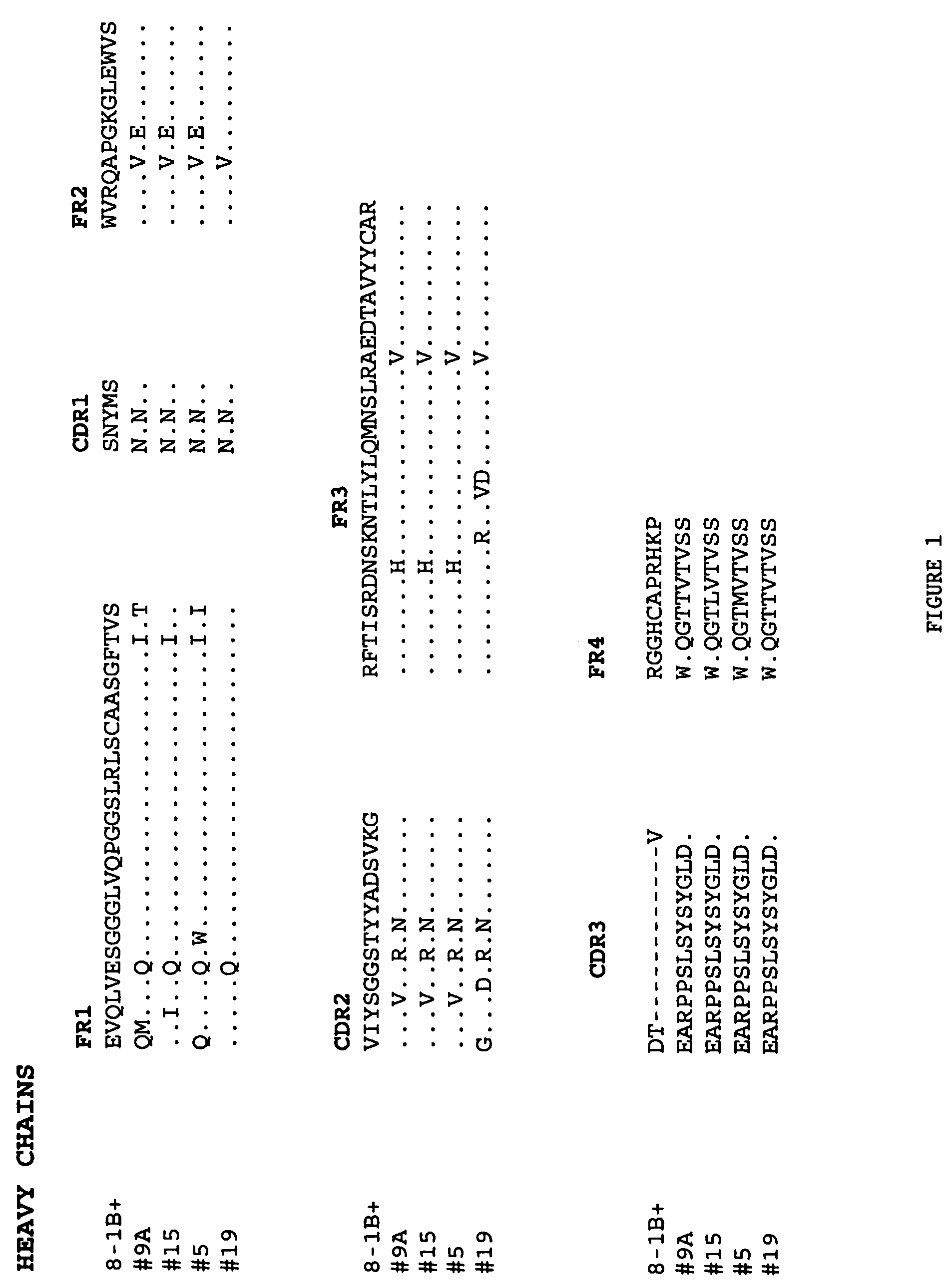
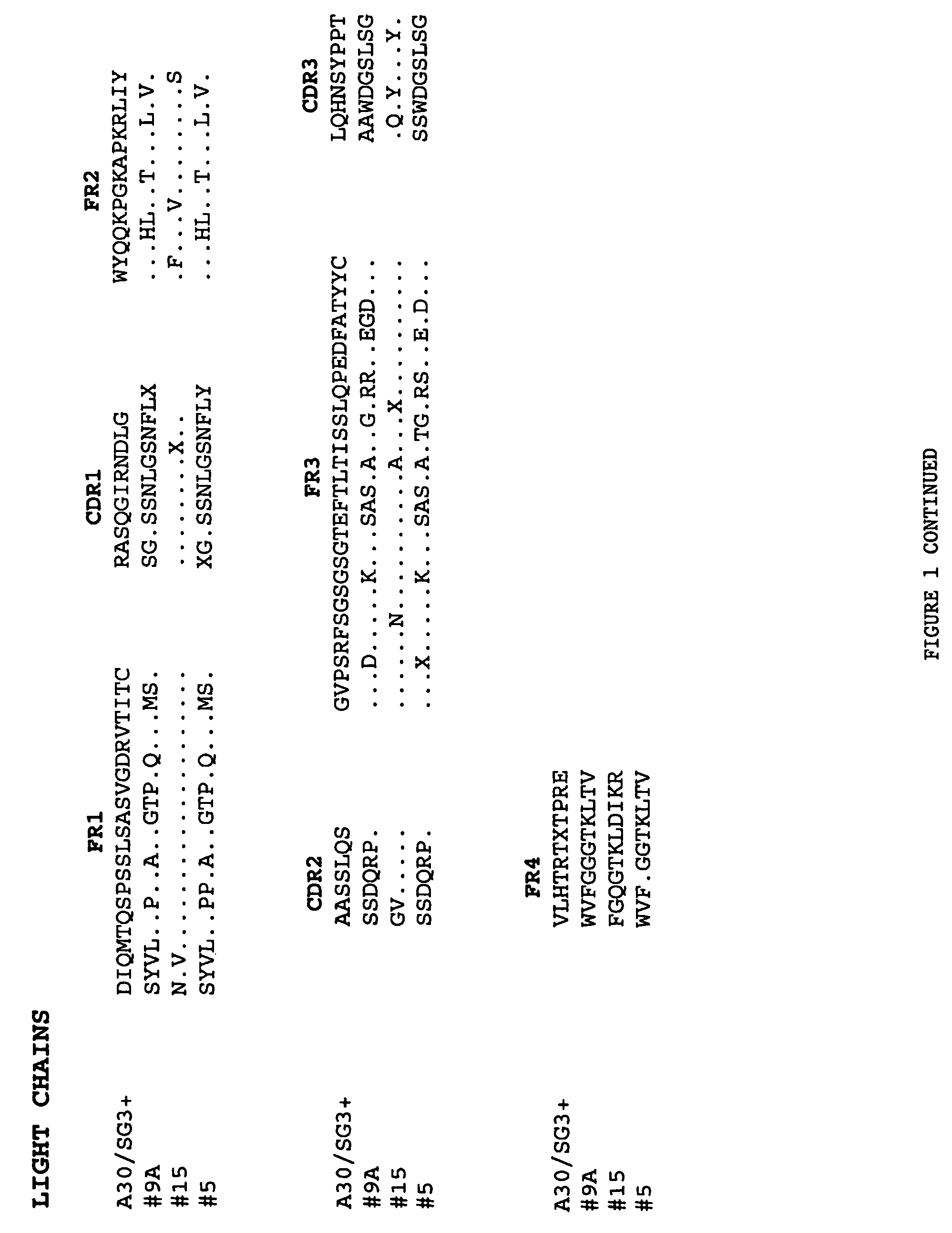
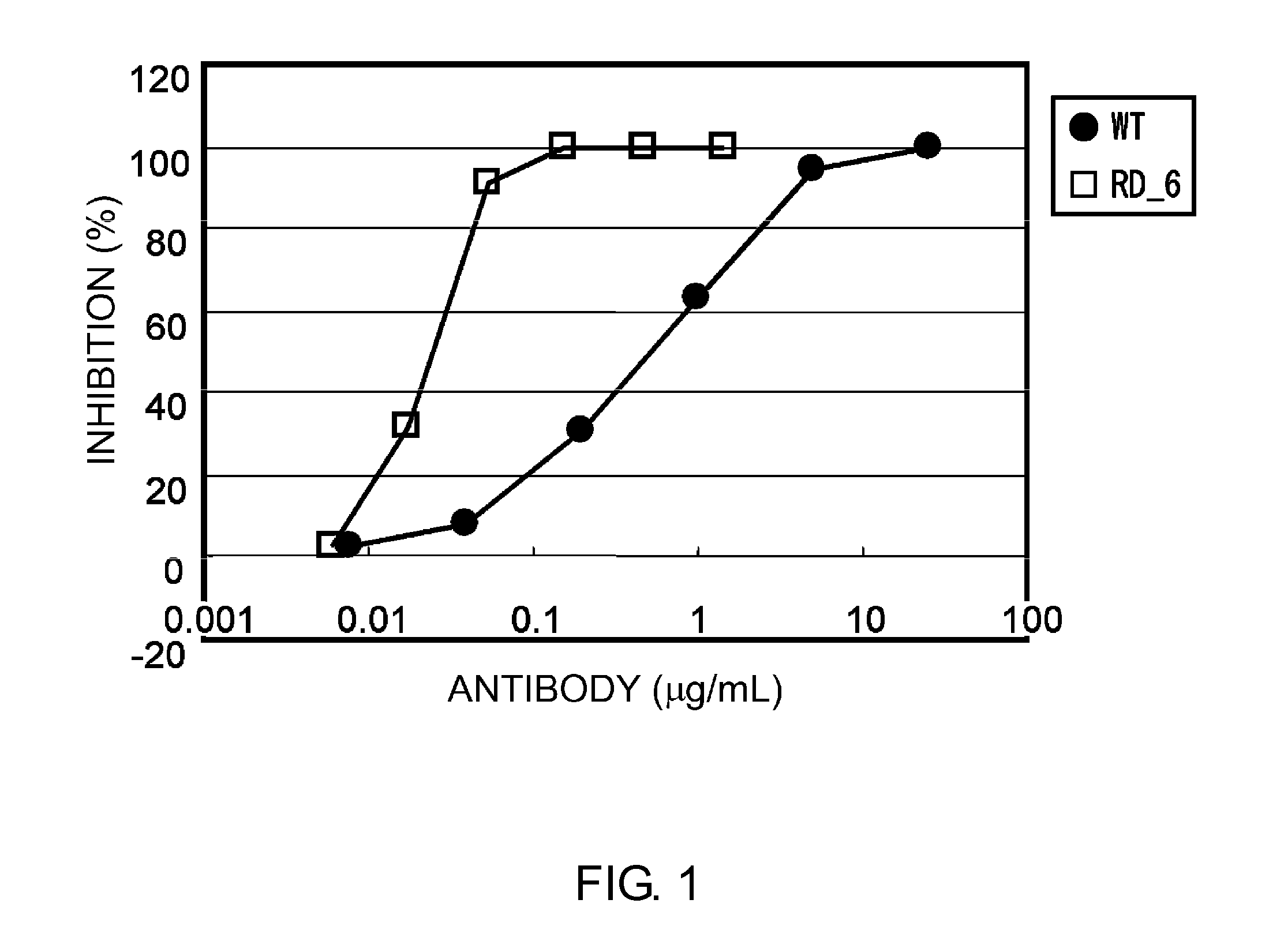
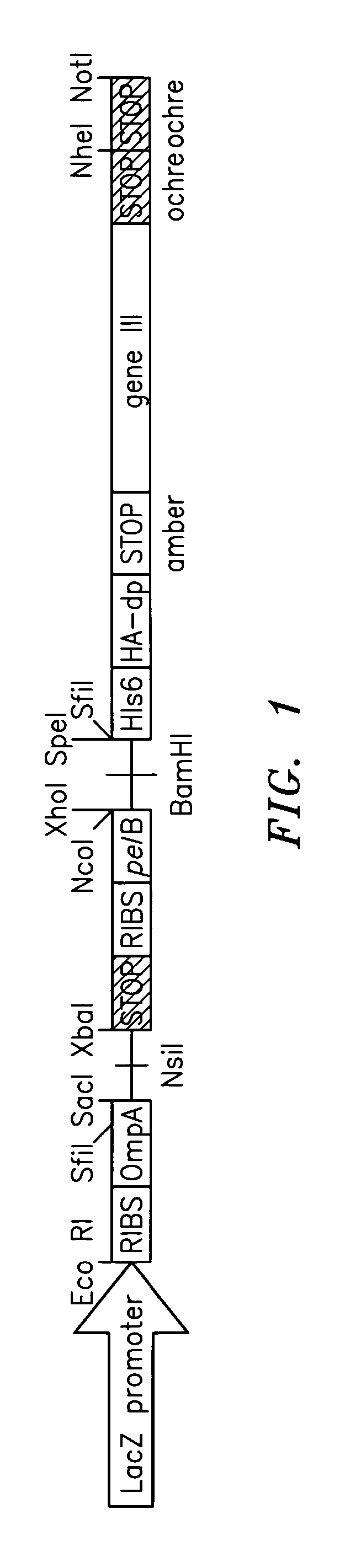
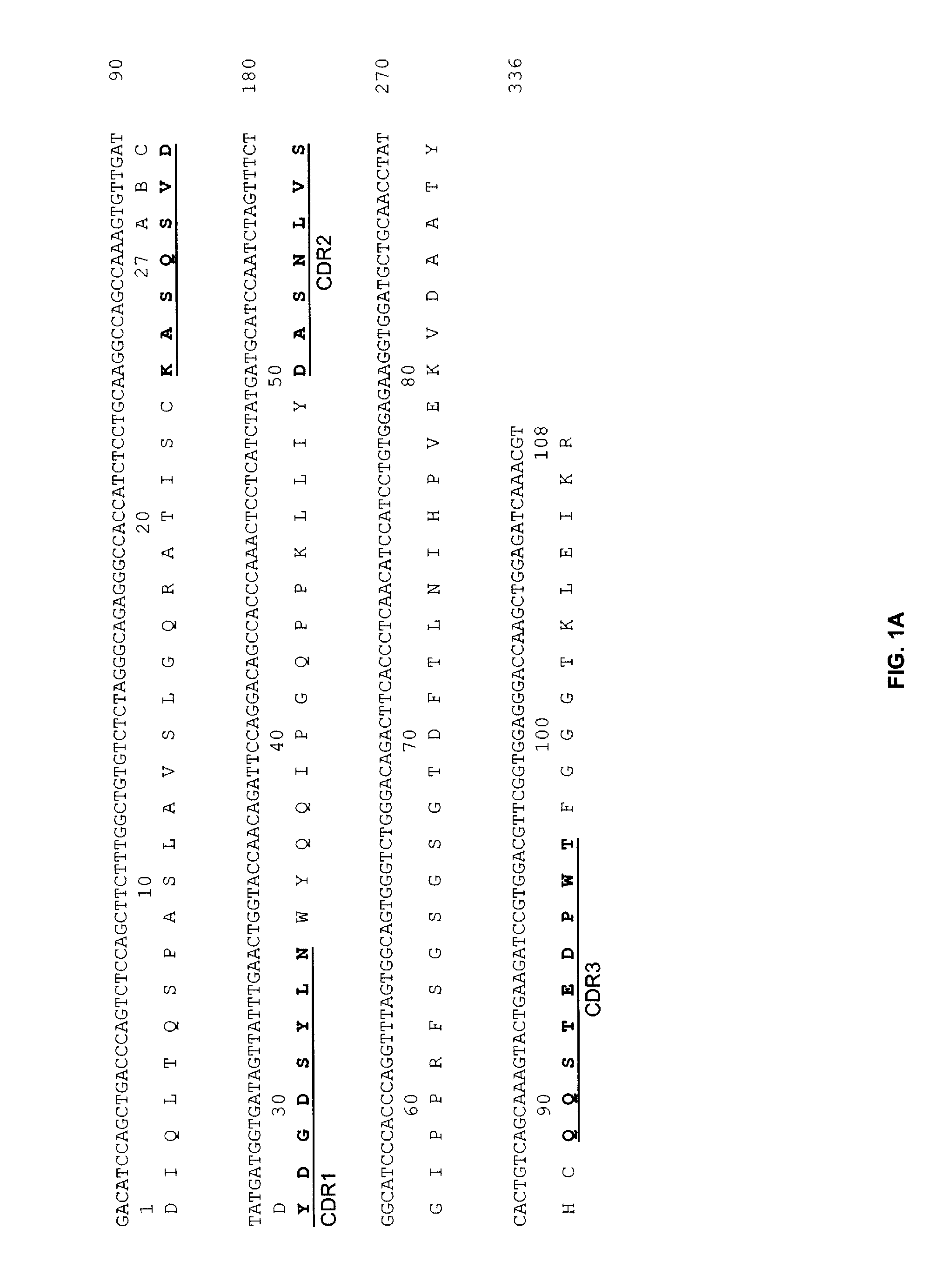
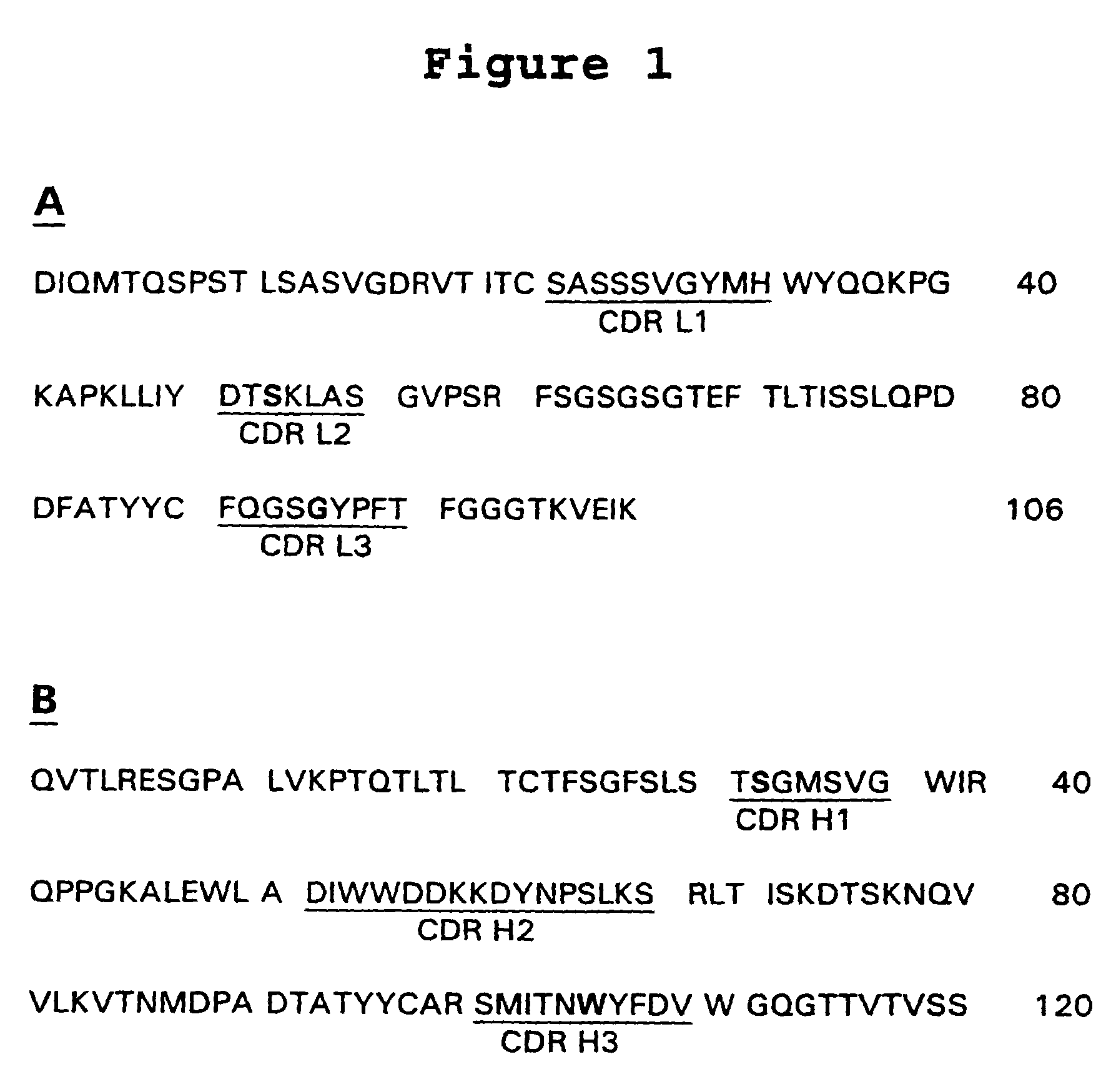
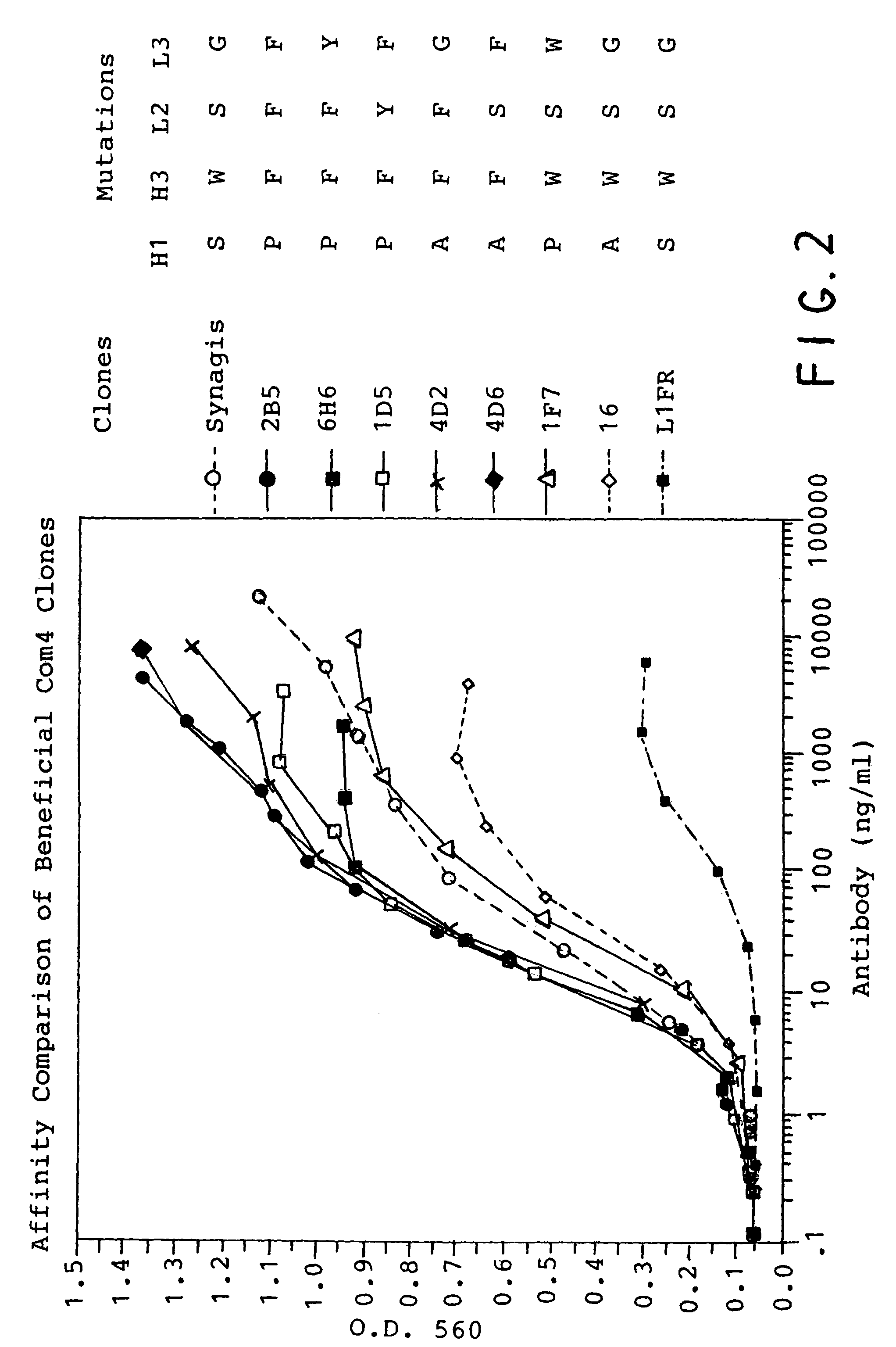
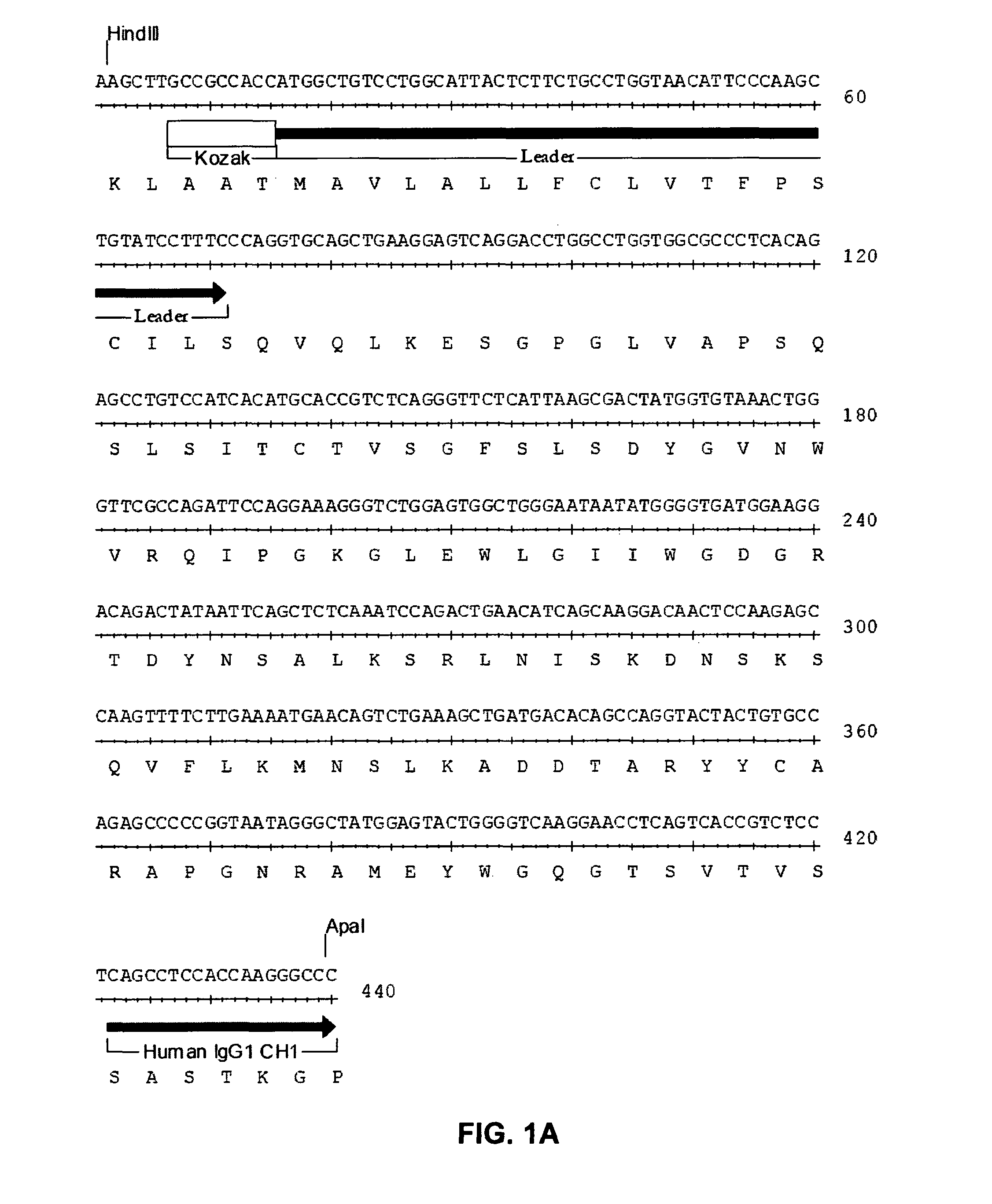
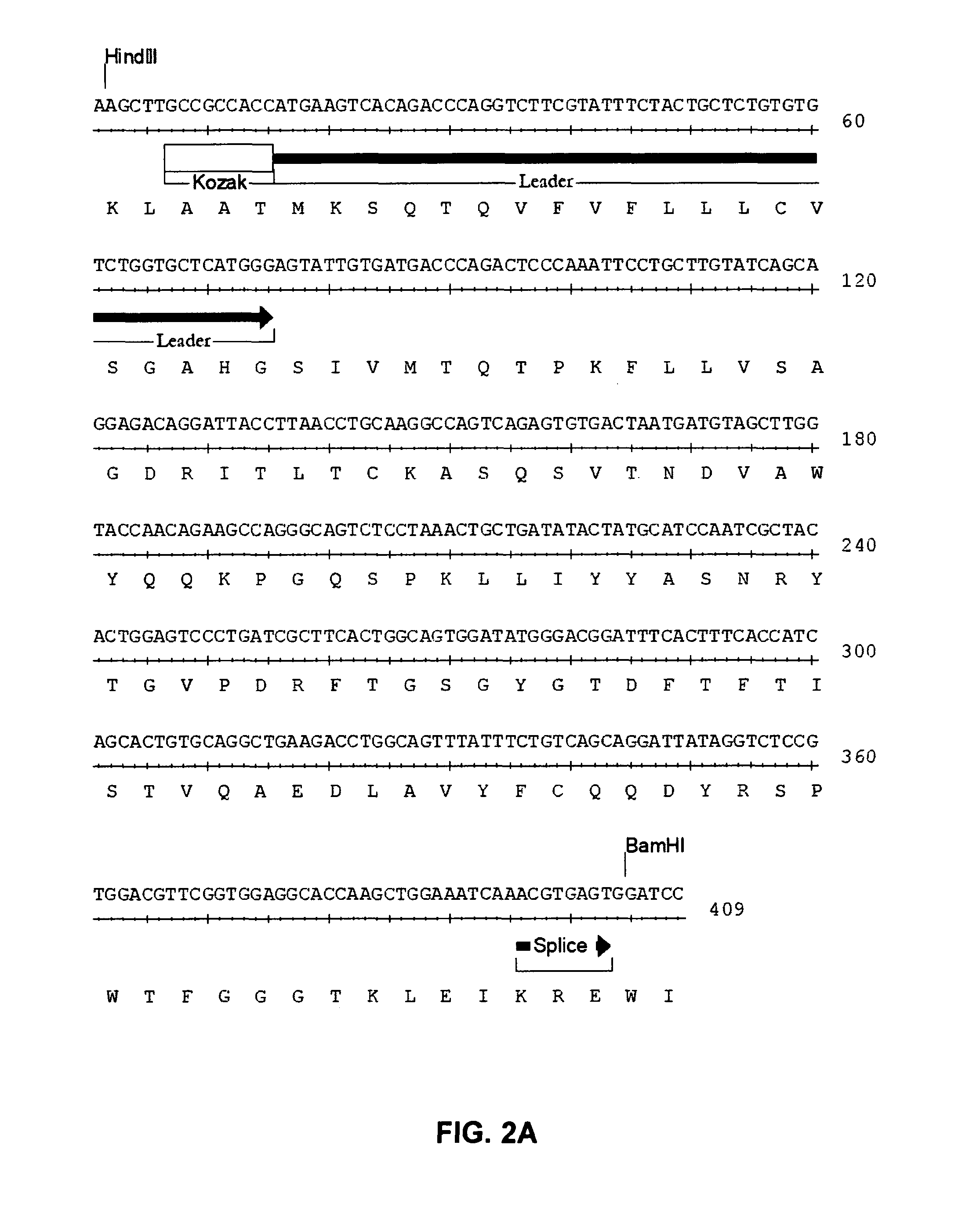
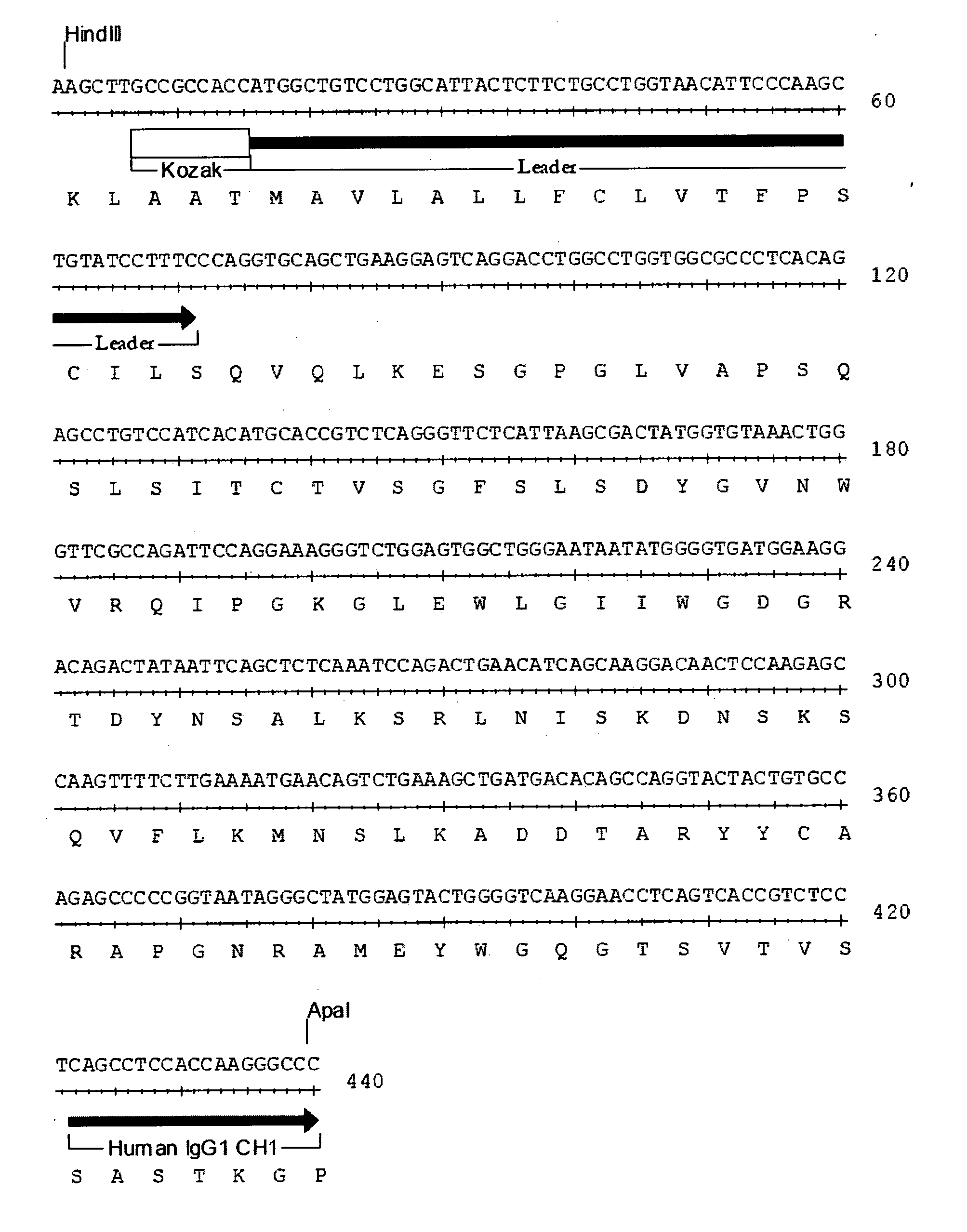
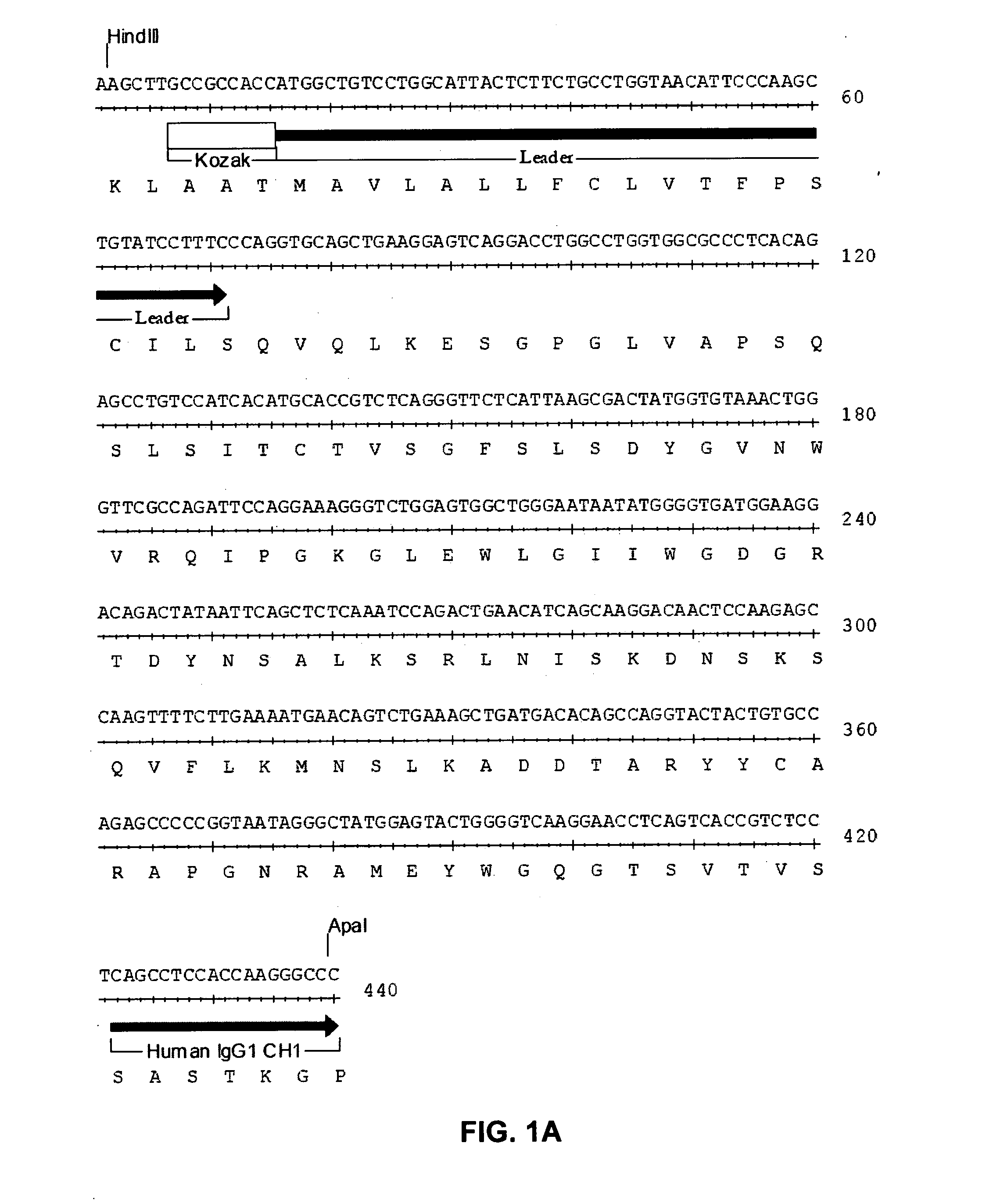
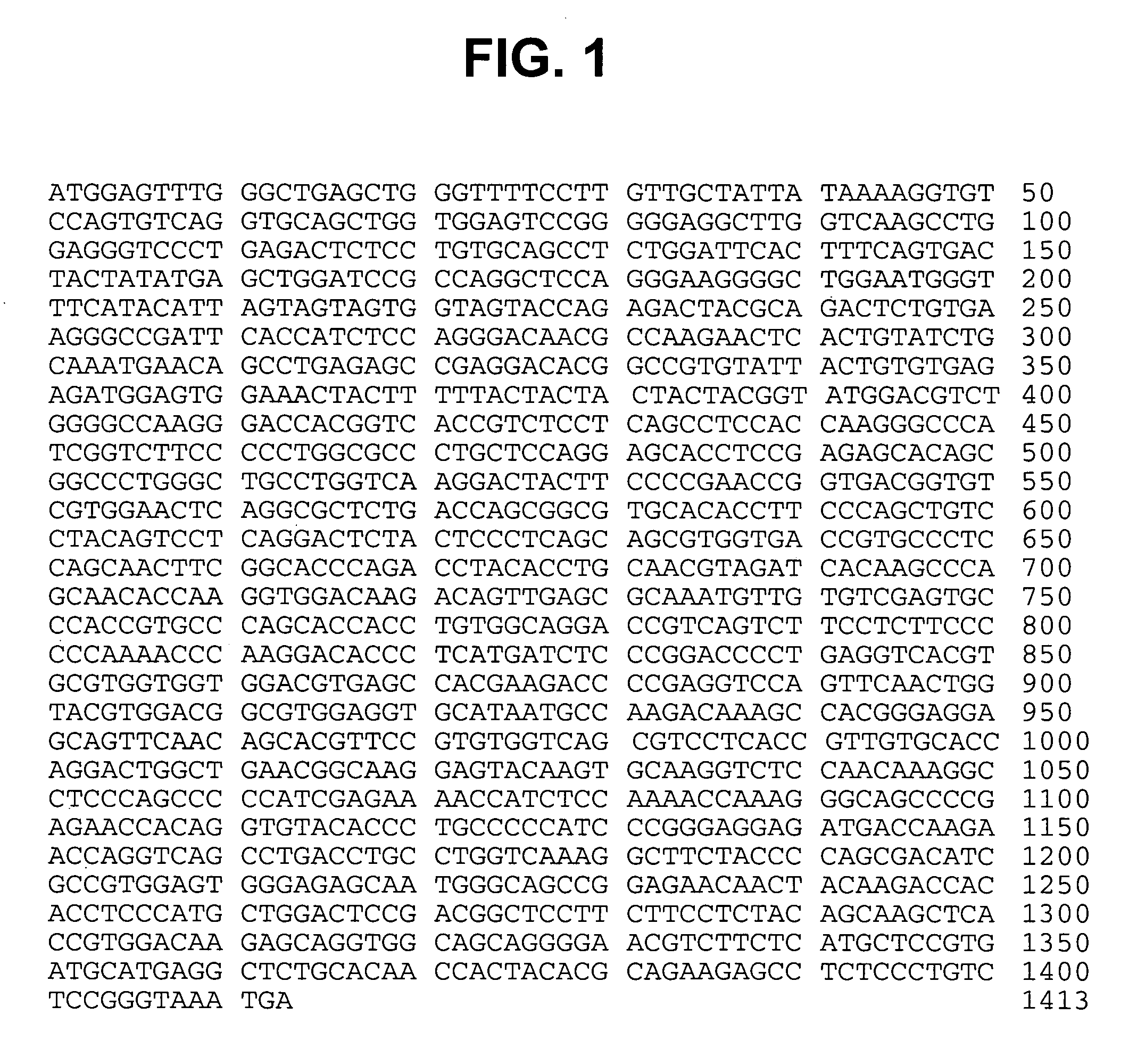
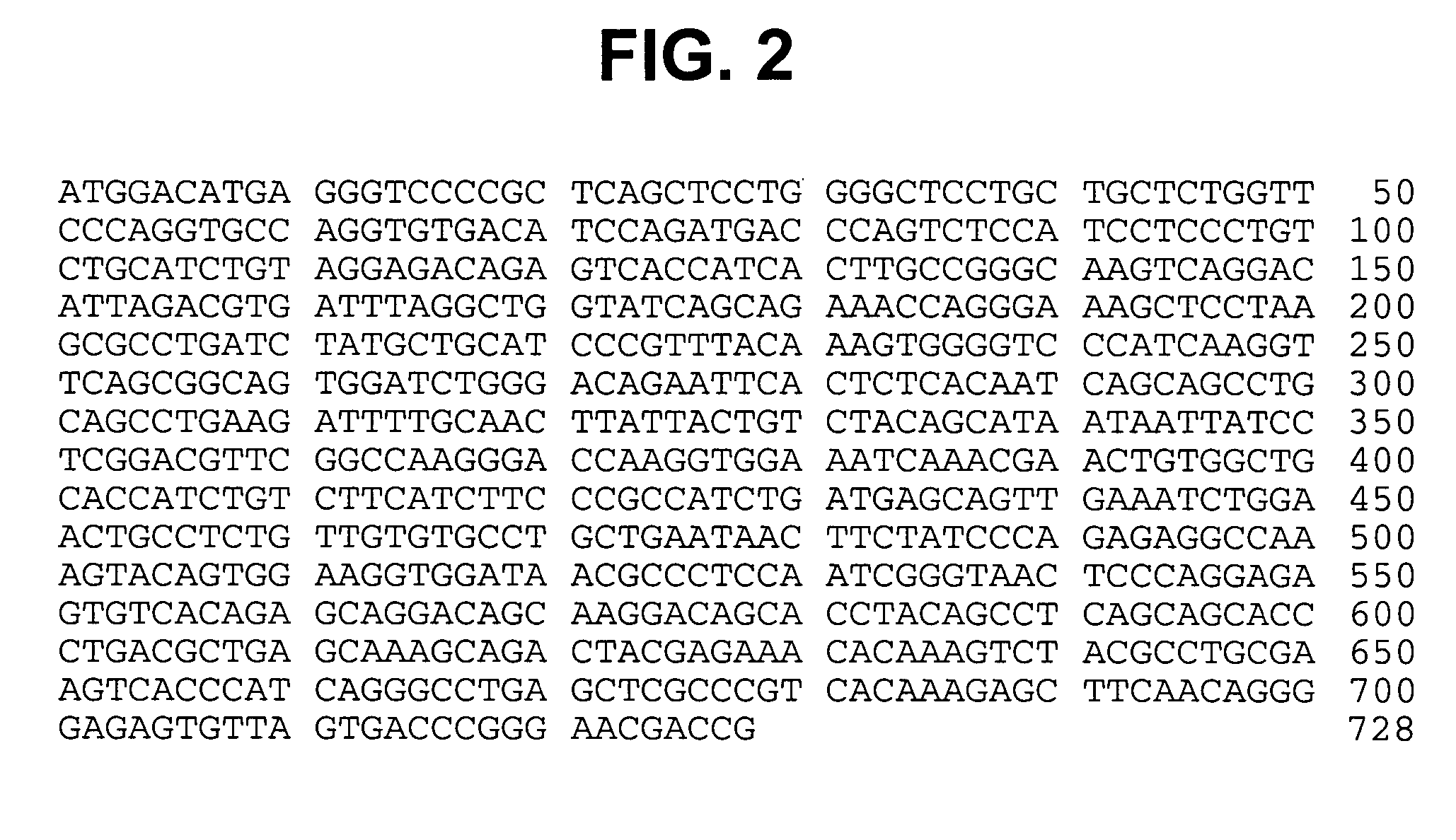
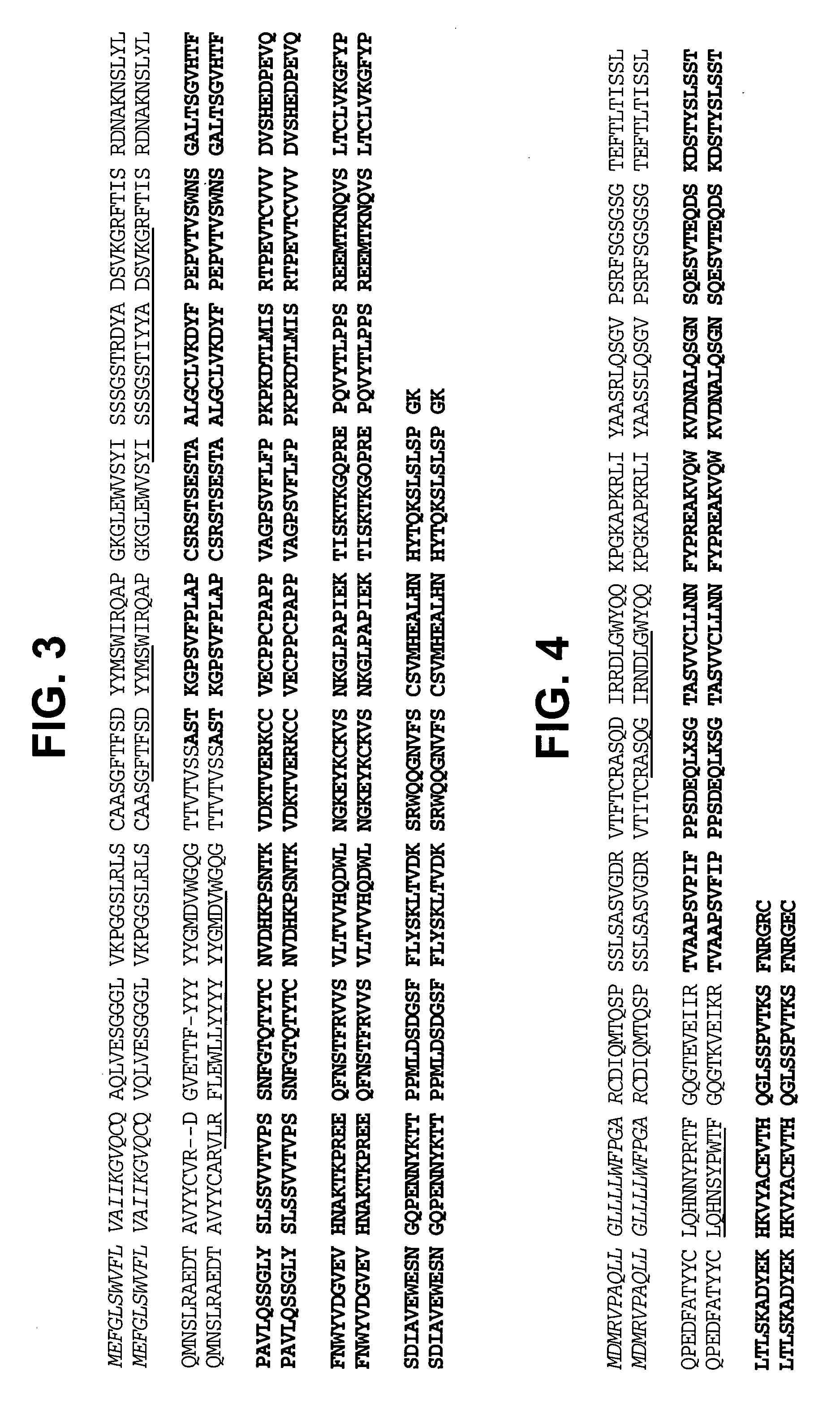
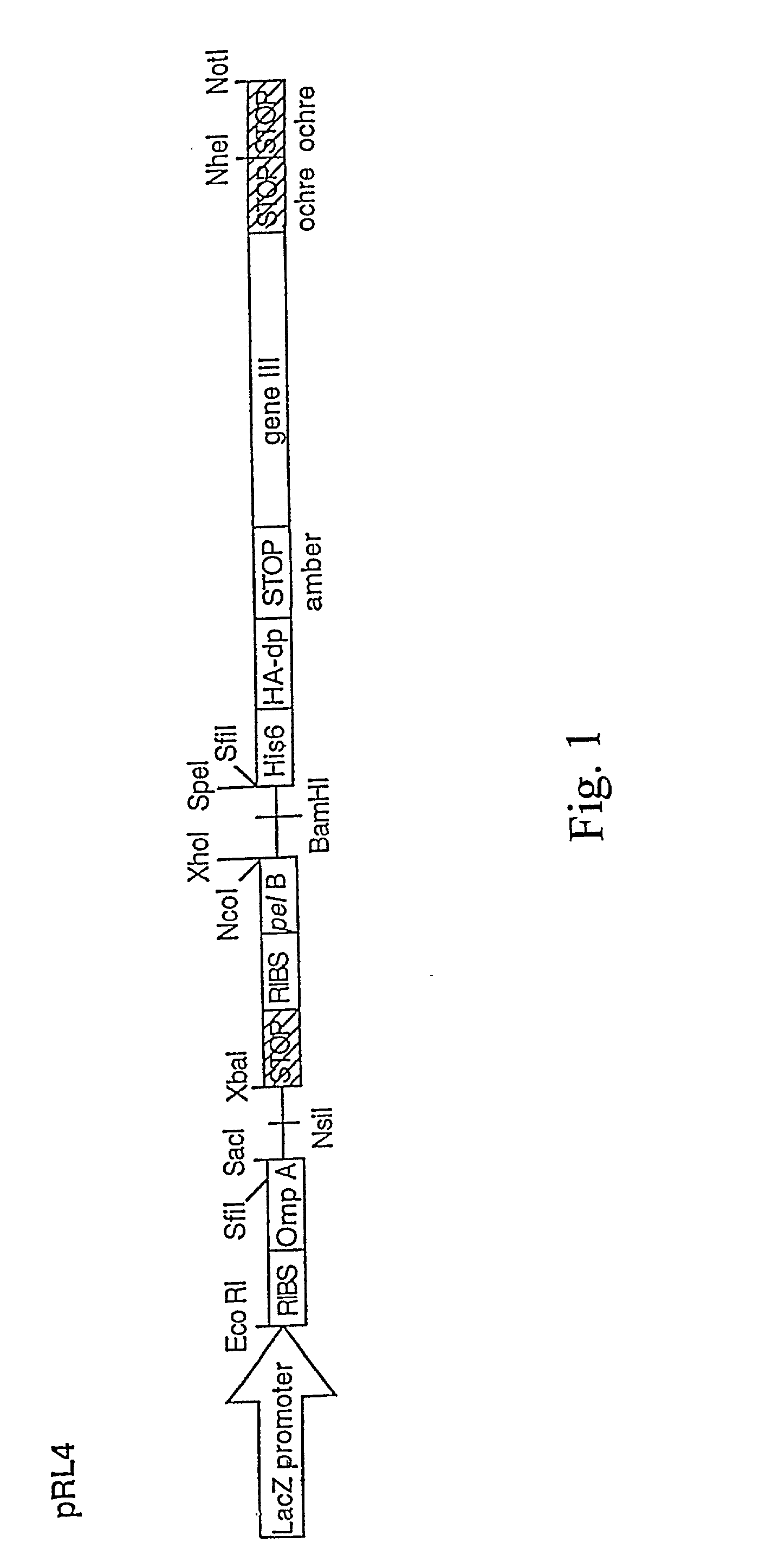
Patents
Literature
293 results about "Framework region" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
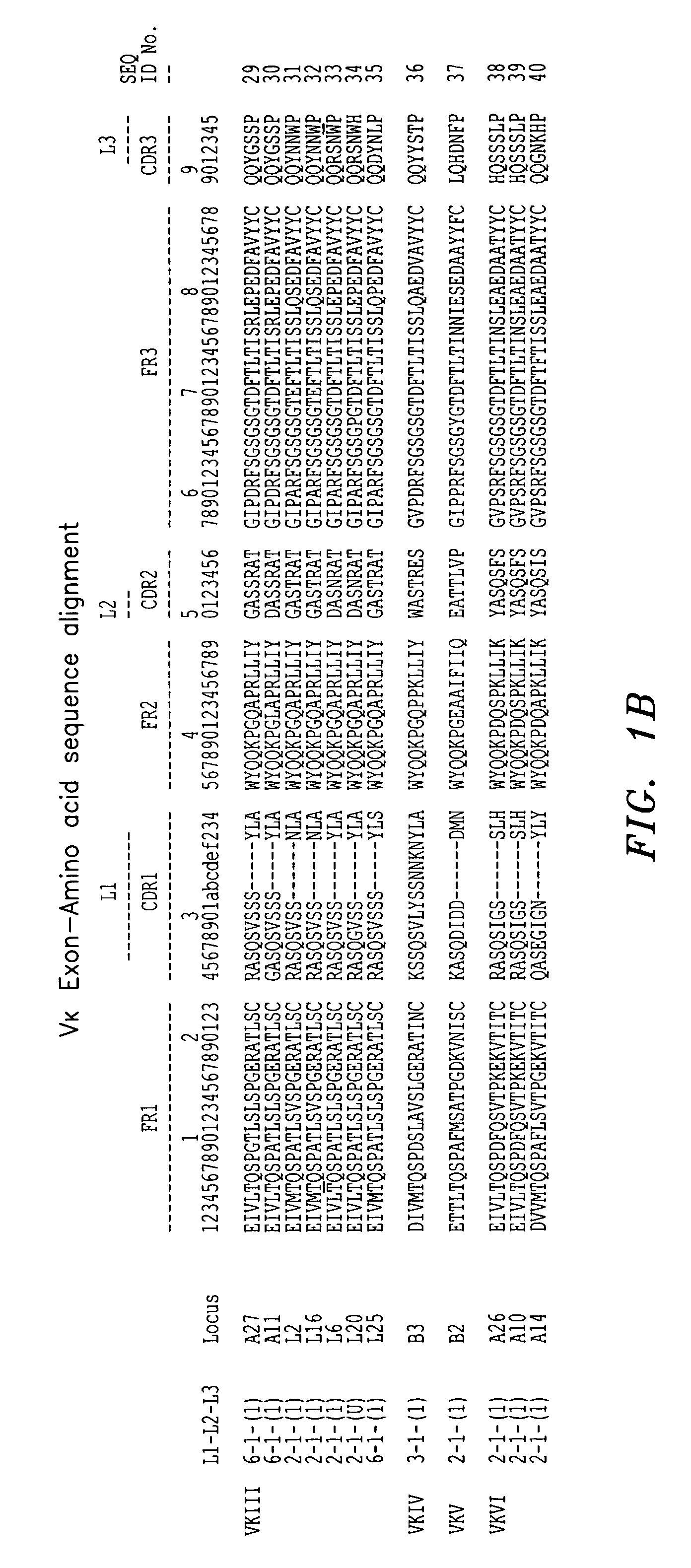
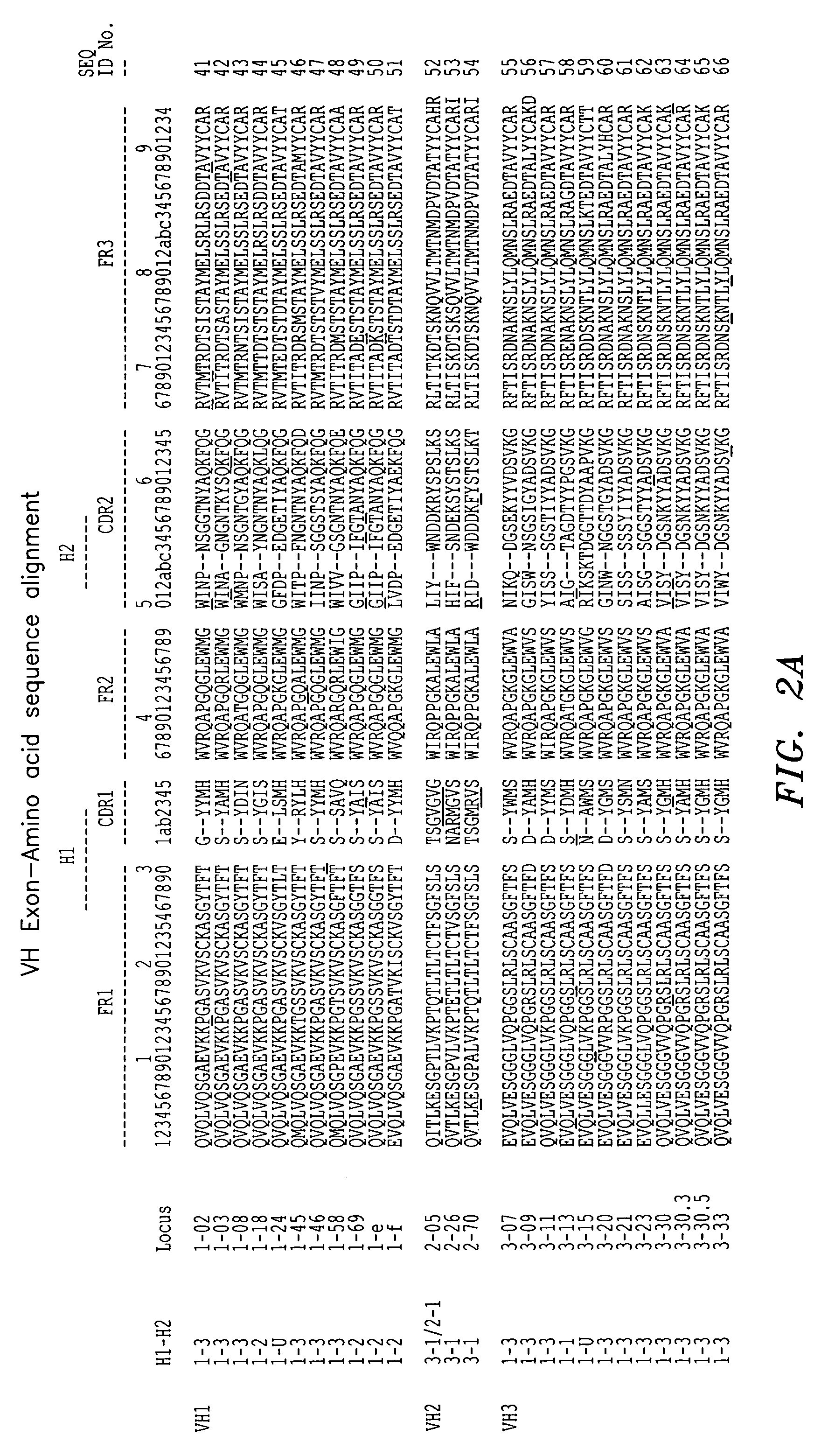
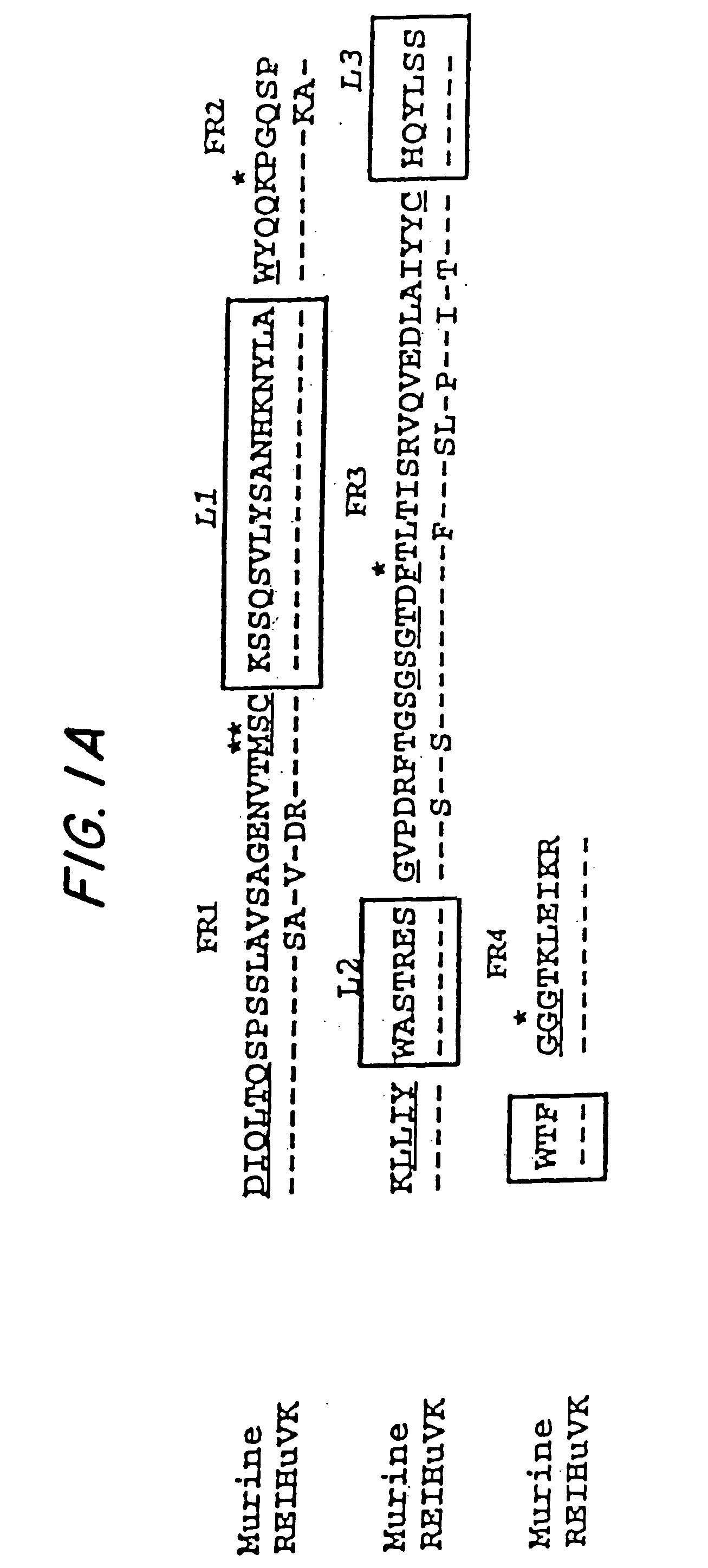
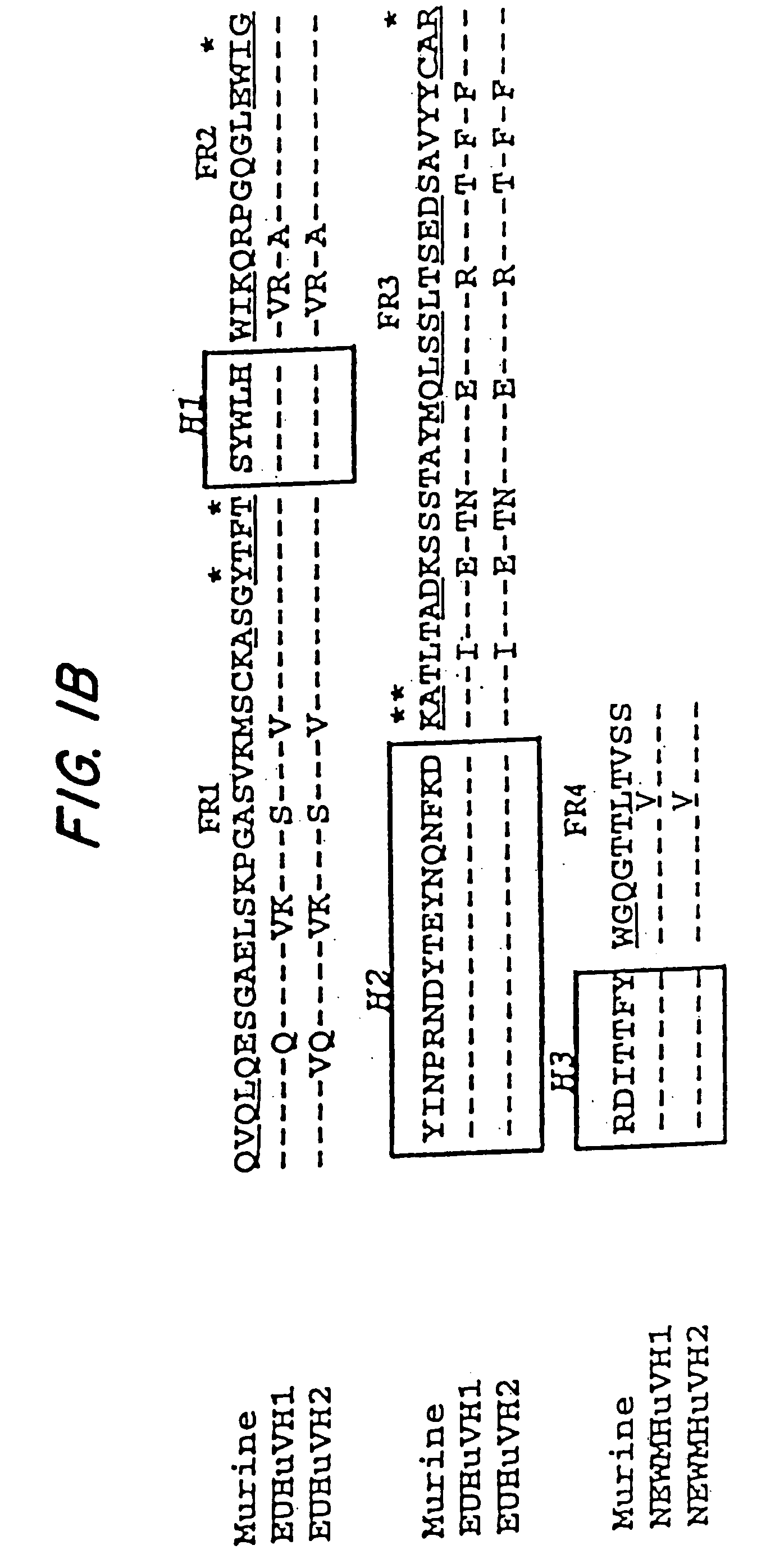
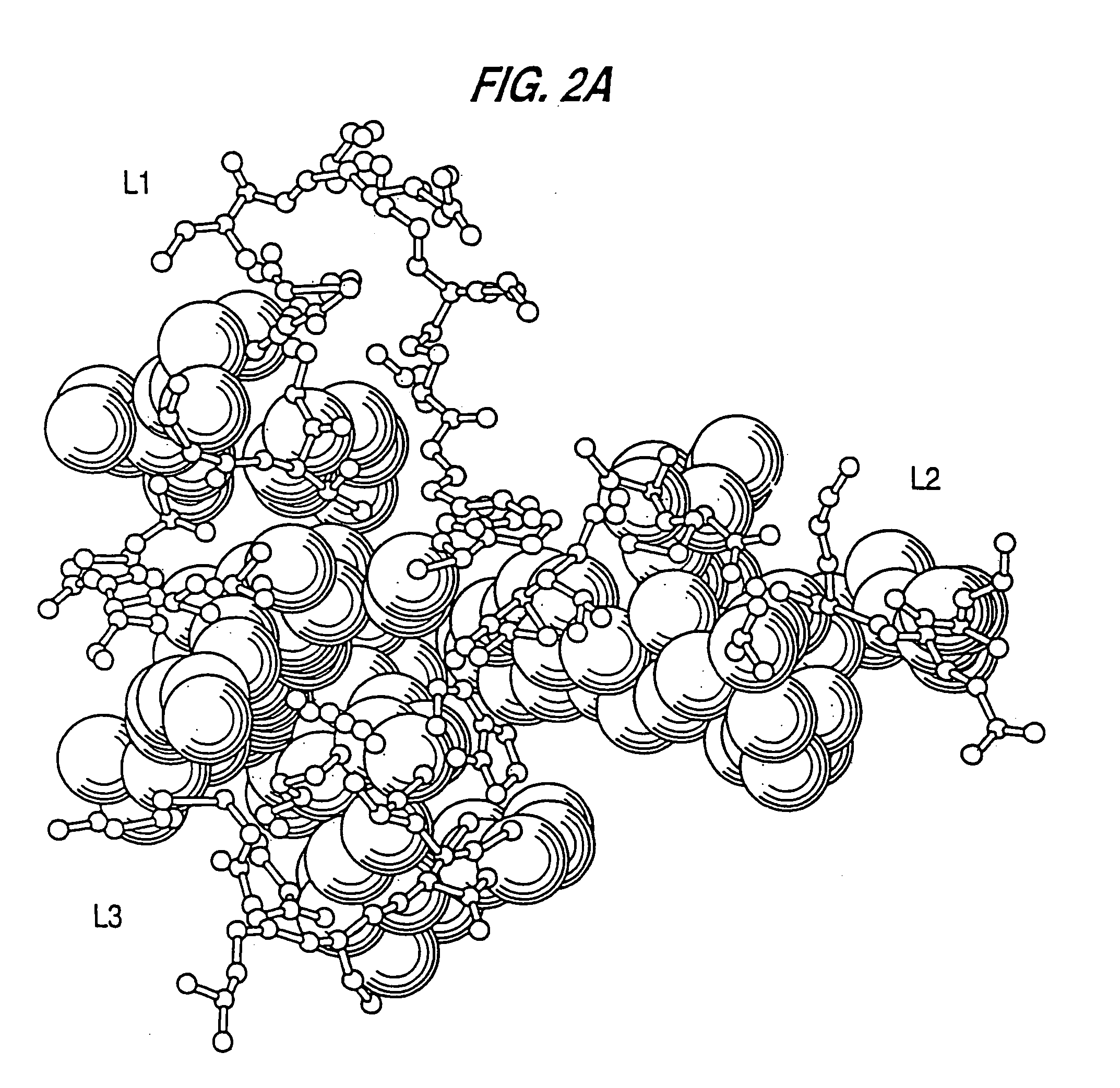
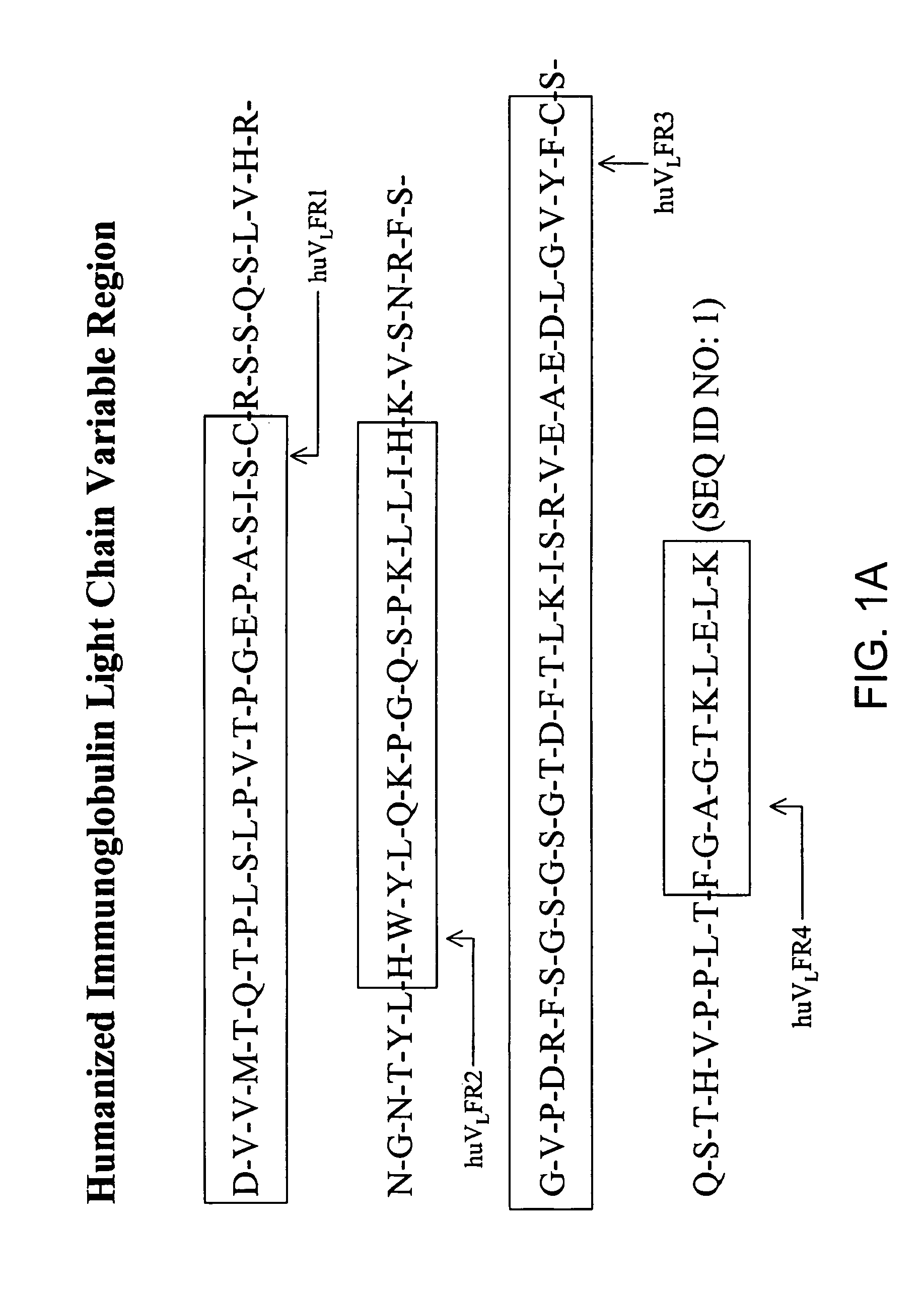
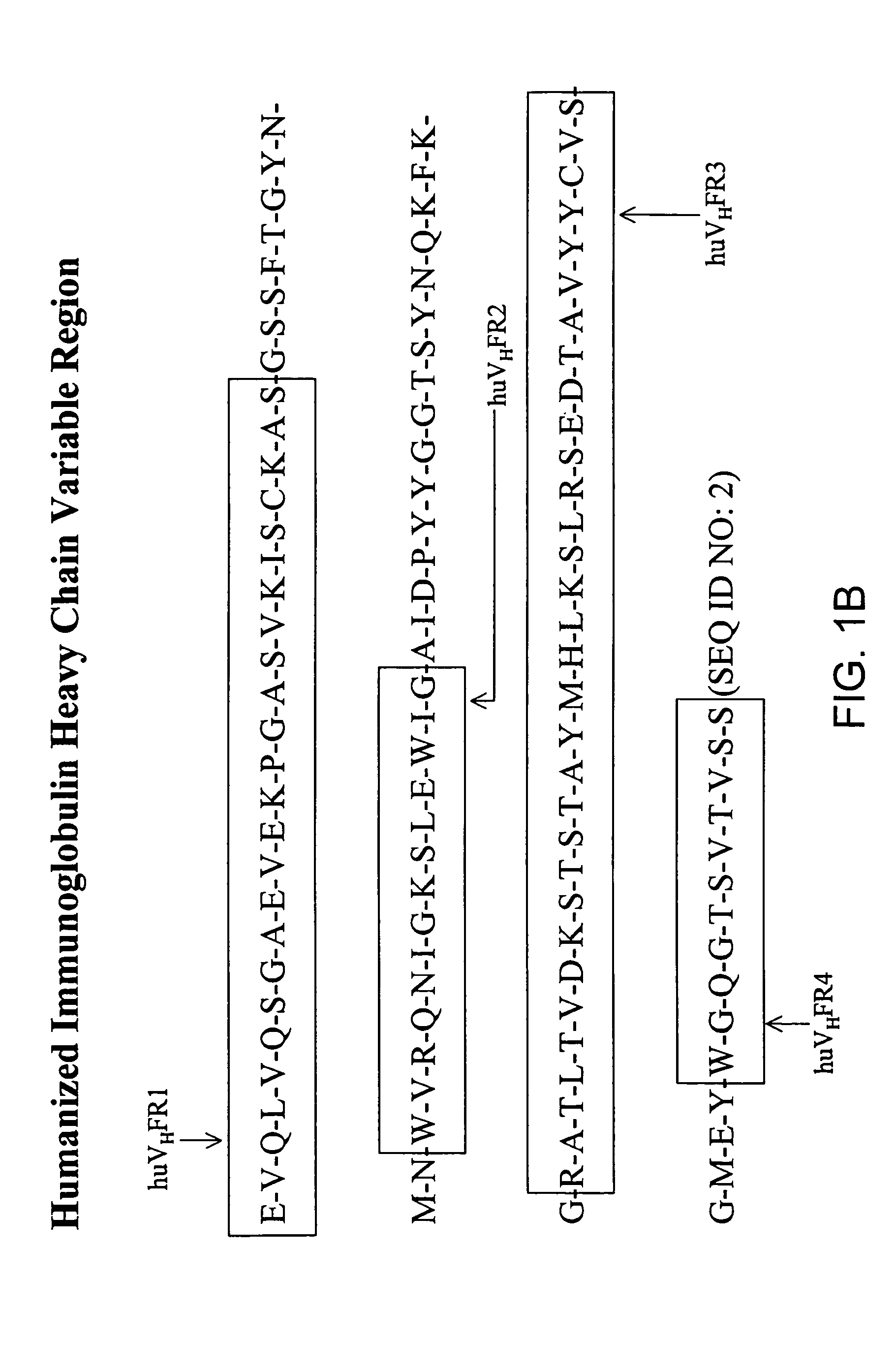
In molecular biology, a framework region is a subdivision of the variable region (Fab) of the antibody. The variable region is composed of seven amino acid regions, four of which are framework regions and three of which are hypervariable regions. The framework region makes up about 85% of the variable region. Located on the tips of the Y-shaped molecule, the framework regions are responsible for acting as a scaffold for the complementarity determining regions (CDR), also referred to as hypervariable regions, of the Fab. These CDRs are in direct contact with the antigen and are involved in binding antigen, while the framework regions support the binding of the CDR to the antigen and aid in maintaining the overall structure of the four variable domains on the antibody. To increase its stability, the framework region has less variability in its amino acid sequences compared to the CDR.
Humanization of antibodies
InactiveUS20050042664A1Limited diversityFast and less labor intensive productionHybrid immunoglobulinsMicrobiological testing/measurementAntigen bindingHumanized antibody
The present invention provides methods of re-engineering or re-shaping an antibody from a first species, wherein the re-engineered or re-shaped antibody does not elicit undesired immune response in a second species, and the re-engineered or re-shaped antibody retains substantially the same antigen binding-ability of the antibody from the first species. In accordance with the present invention, a combinatorial library comprising the CDRs of the antibody from the first species fused in frame with framework regions derived from a second species can be constructed and screened for the desired modified antibody. In particular, the present invention provides methods utilizing low homology acceptor antibody frameworks for efficiently humanizing an antibody or a fragment thereof. The present invention also provides antibodies produced by the methods of the invention.
Owner:MEDIMMUNE LLC
Humanized immunoglobulins
Novel methods for producing, and compositions of, humanized immunoglobulins having one or more complementarity determining regions (CDR's) and possible additional amino acids from a donor immunoglobulin and a framework region from an accepting human immunoglobulin are provided. Each humanized immunoglobulin chain will usually comprise, in addition to the CDR's, amino acids from the donor immunoglobulin framework that are, e.g., capable of interacting with the CDR's to effect binding affinity, such as one or more amino acids which are immediately adjacent to a CDR in the donor immunoglobulin or those within about about 3Å as predicted by molecular modeling. The heavy and light chains may each be designed by using any one or all of various position criteria. When combined into an intact antibody, the humanized immunoglobulins of the present invention will be substantially non-immunogenic in humans and retain substantially the same affinity as the donor immunoglobulin to the antigen, such as a protein or other compound containing an epitope.
Owner:PDL BIOPHARMA INCORPORATED
Reshaped human antibody to human interleukin-6 receptor
InactiveUS7479543B2Peptide/protein ingredientsAntibody mimetics/scaffoldsComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising:(A) an L chain comprising,(1) a human L chain C region, and(2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and(B) an H chain comprising,(1) a human H chain C region, and(2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R.Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
Reshaped human antibody to human interleukin-6 receptor
InactiveUS20050142635A1Less immunogenicPeptide/protein ingredientsHydrolasesComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising: (A) an L chain comprising, (1) a human L chain C region, and (2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and (B) an H chain comprising, (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R. Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
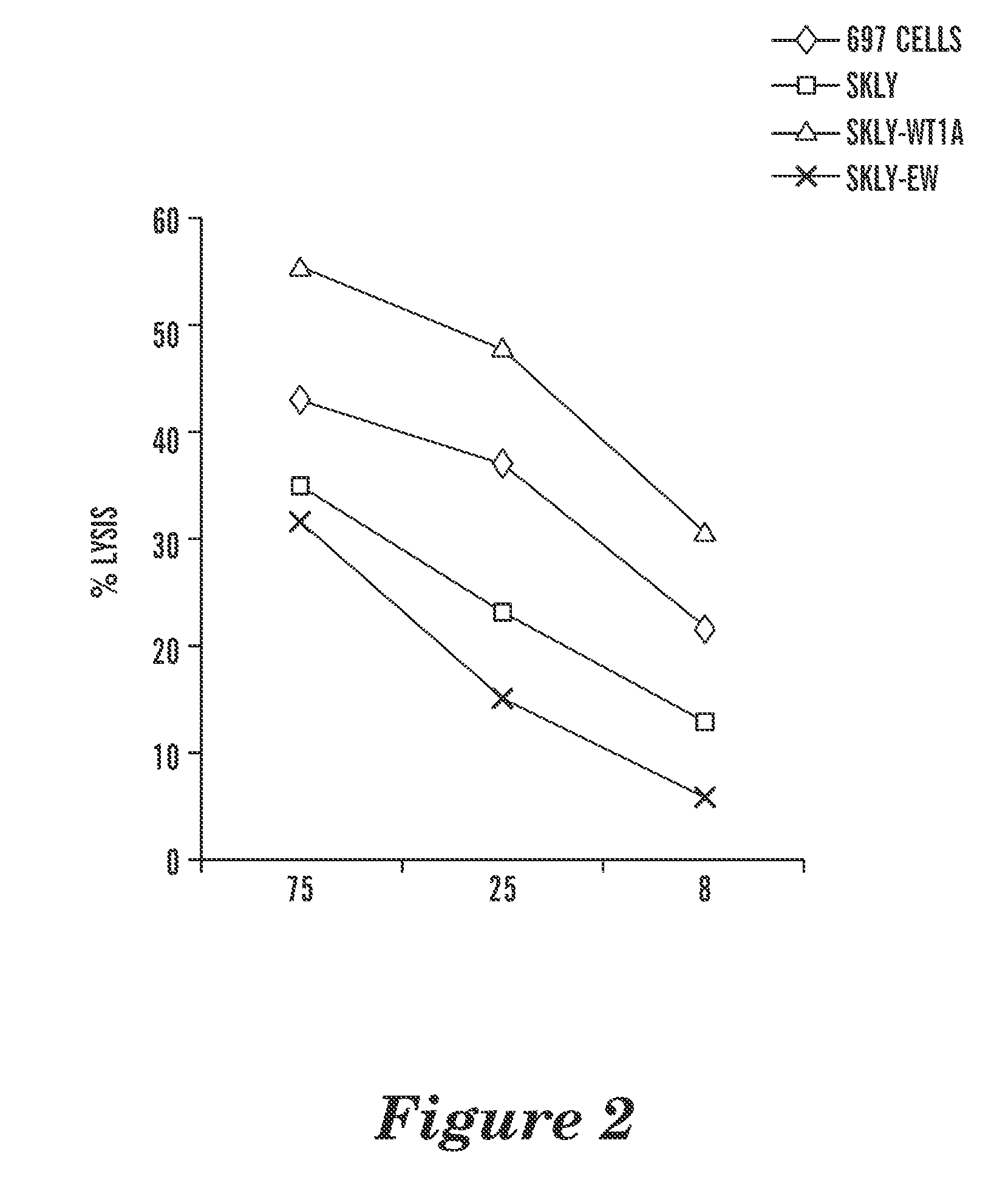
T cell receptor-like antibodies specific for a wti peptide presented by hla-a2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
Hybrid antibodies
Hybrid antibodies and / or hybrid antibody fragments and methods of making them are provided. In one embodiment the hybrid antibodies and / or hybrid antibody fragments contain heavy and / or light variable regions that contain two or more framework regions derived from at least two antibodies. In another embodiment, at least two of the framework regions are classified in the same germline gene family. In one embodiment, at least two framework regions are classified in the same germline gene family member. The hybrid antibodies or hybrid antibody fragments may contain human framework regions and nonhuman CDRs.
Owner:ALEXION PHARMA INC
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Humanized immunoglobulin reactive with α4β7 integrin
InactiveUS7147851B1Reduce inflammationLess immunogenicFungiBacteriaComplementarity determining regionAntigen binding
The present invention relates to humanized immunoglobulins having binding specificity for α4β7 integrin, comprising an antigen binding region of nonhuman origin (e.g., rodent) and at least a portion of an immunoglobulin of human origin (e.g., a human framework region, a human constant region). In one embodiment, the humanized immunoglobulin can compete with murine Act-1 for binding to human α4β7 integrin. In a preferred embodiment, the antigen binding region of the humanized immunoglobulin comprises each of the complementarity determining regions of the light and heavy chains of the murine Act-1 antibody.
Owner:MILLENNIUM PHARMA INC
Reduction of the nonspecific animal toxicity of immunotoxins by mutating the framework regions of the Fv to lower the isoelectric point
InactiveUS7521054B2Growth inhibitionPolypeptide with localisation/targeting motifAnimal cellsNon specificFramework region
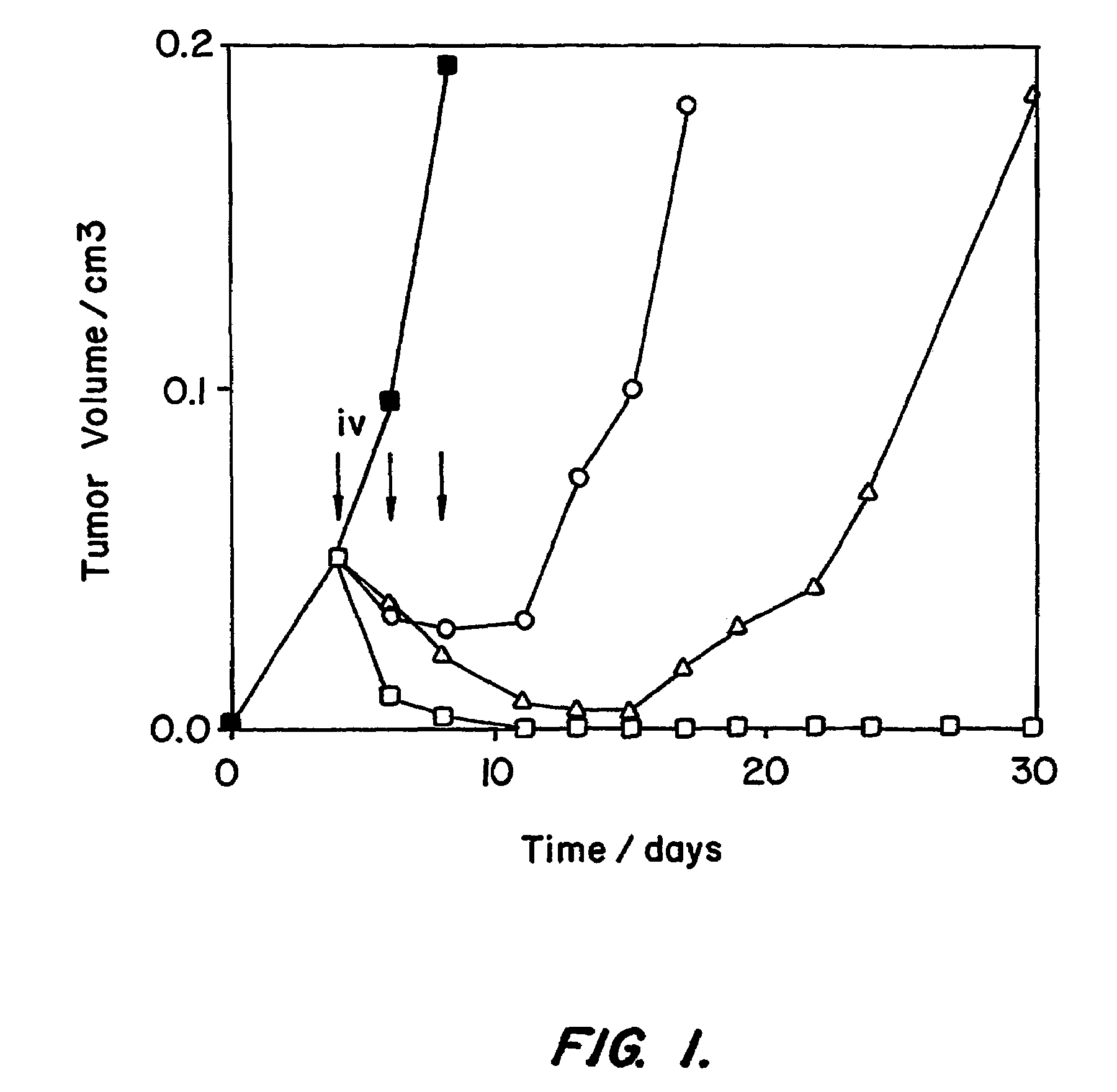
The invention provides recombinant immunotoxins that have been modified from a parental immunotoxin to lower liver toxicity. The immunotoxins are created by specifically mutating charged residues in the framework regions of the heavy chain, the light chain, or both, of the antibody portion or antigen-binding fragment thereof of the parental immunotoxin to reduce the pI of the antibody or fragment. In preferred forms, the antibody portion of the parental is an anti-Tac, anti-mesothelin, or anti-LewisY antigen antibody or antigen-binding fragment, and in particularly preferred forms the antibody portion is an M16 dsFv, a St6 dsFv or a Mt9 dsFv, or a sequence that has at least 90% sequence identity to one of these molecules but retain the particular mutations that lower pI without affecting antibody activity. The invention further provides nucleic acids encoding the recombinant immunotoxins of the invention, expression cassettes comprising the nucleic acids, and host cells comprising the expression cassettes. The invention also provides a method for killing a cell comprising an antigen on the surface of the cell, the method comprising contacting the cell with a recombinant immunotoxin of the invention that has an antibody or antigen-binding fragment thereof that binds specifically to the antigen on the surface of the cell, and uses of immunotoxins of the invention for the manufacture of medicaments.
Owner:HEALTH & HUMAN RESOURCES GOVERNMENT OF THE UNITED STATES THE AS REPRESENTED BY THE SEC OF THE DEPT OF
Anti-properdin antibodies, and methods for making and using same
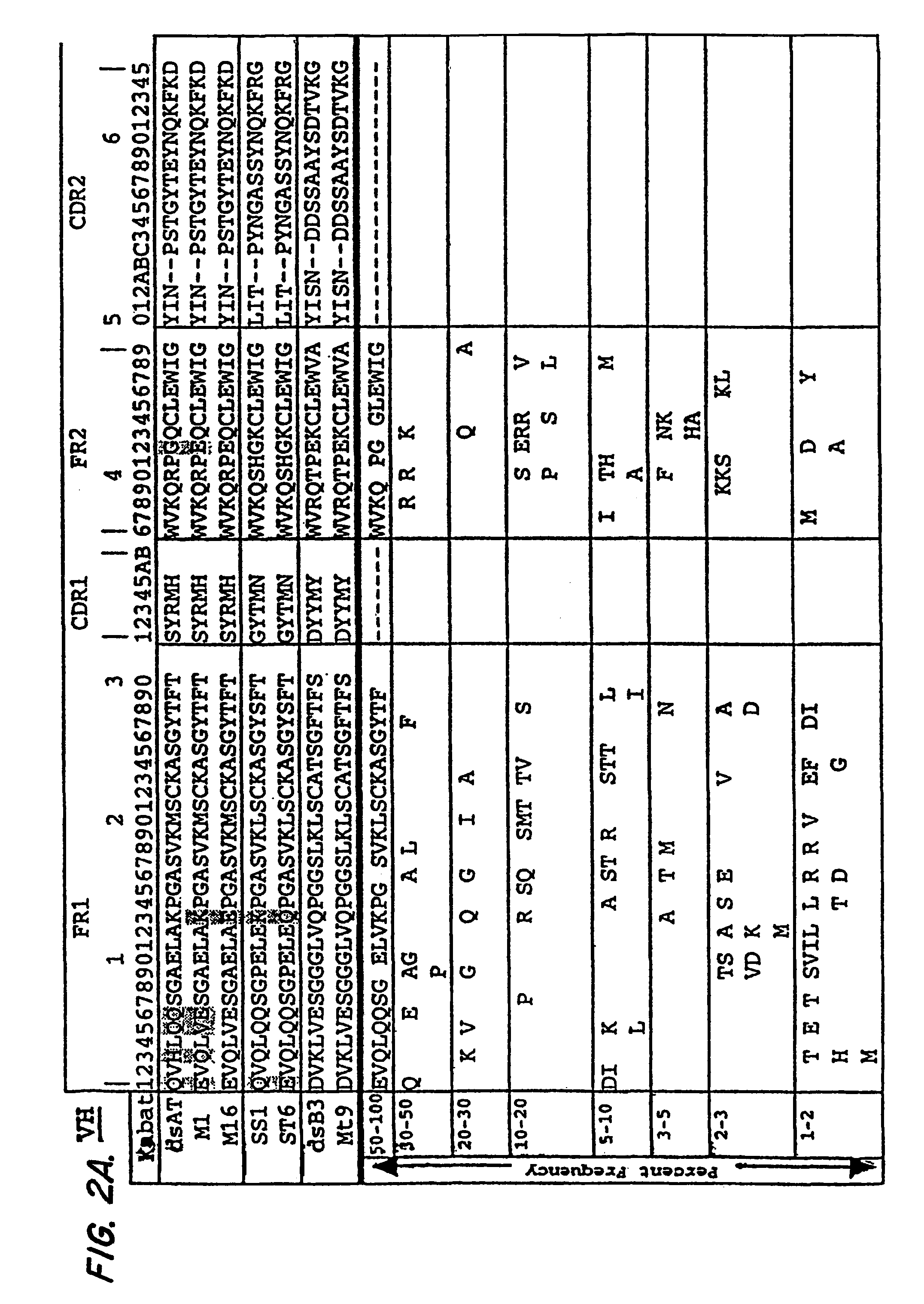
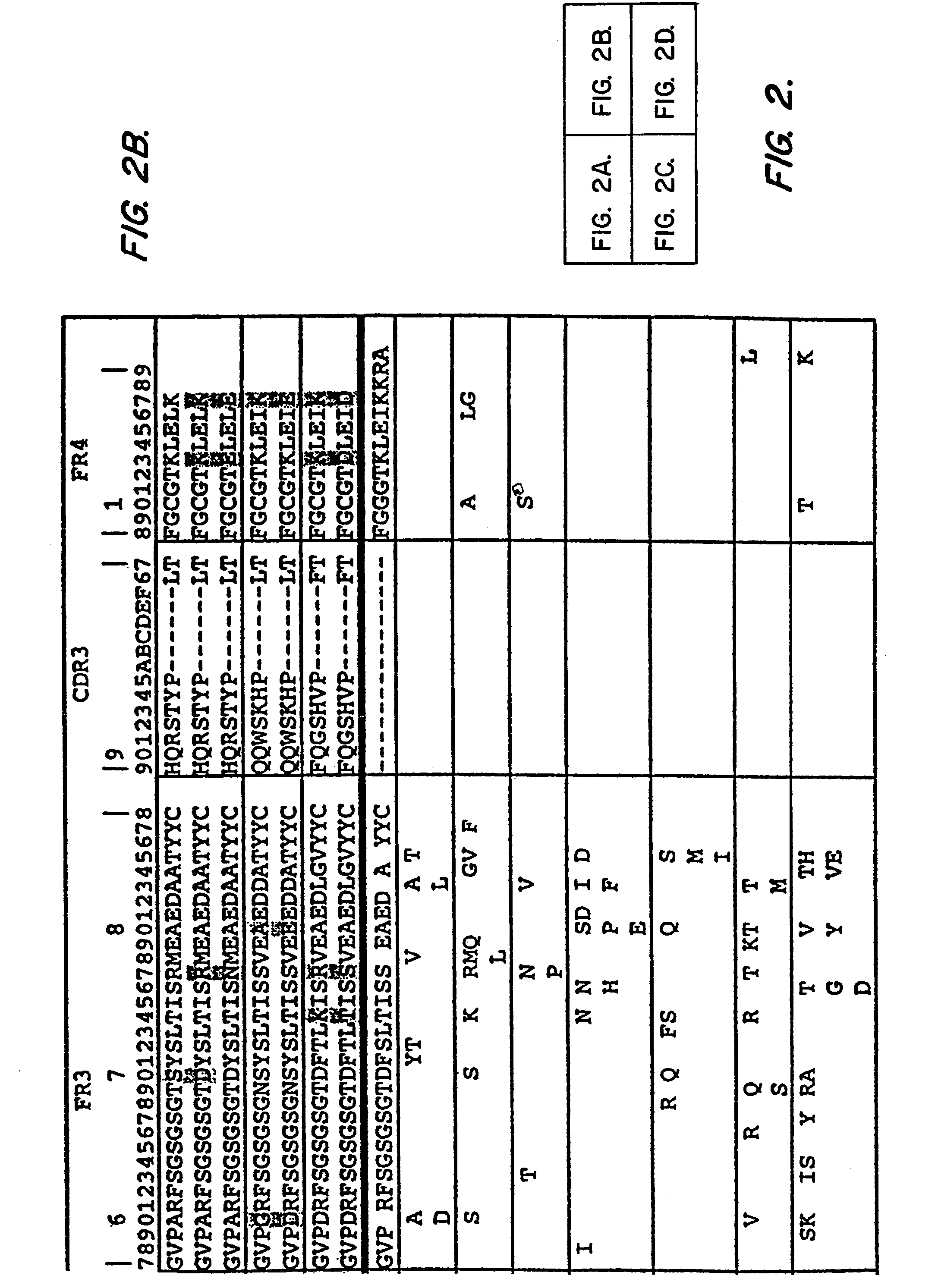
The present invention is related to antibodies directed to the antigen properdin and uses of such antibodies. In particular, in accordance with the present invention, there are provided fully human monoclonal antibodies directed to the antigen properdin. Nucleotide sequences encoding, and polypeptides comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDR's), specifically from FR1 through FR4 or CDR1 through CDR3, are provided. Hybridomas or other cell lines expressing such immunoglobulin molecules and monoclonal antibodies are also provided.
Owner:AMGEN FREMONT INC
Antibodies and methods for making and using them
InactiveUS20080187966A1Speed up the processIncrease productionImmunoglobulins against growth factorsRecombinant DNA-technologyDisulfide bondingAntigen Binding Fragment
The present invention provides methods for producing humanized antibodies and increasing the yield of antibodies and / or antigen binding fragments when produced in cell culture. In one aspect of the invention, at least one framework region amino acid residue of the variable domain is substituted by a corresponding amino acid from a variable domain consensus sequence subgroup that has the most sequence identity with the HVR1 and / or HVR2 amino acid sequence of the variable domain. In another aspect, an amino acid is placed at a position proximal to a cys residue that participates in an intrachain variable domain disulfide bond that corresponds to an amino acid found at that position in a variable domain consensus sequence subgroup that has the most sequence identity with the HVR1 and / or HVR2 amino acid sequence of the variable domain.
Owner:GENENTECH INC
Humanized antibody
InactiveUS7456260B2Hybrid immunoglobulinsSugar derivativesHumanized antibodyReceptor for activated C kinase 1
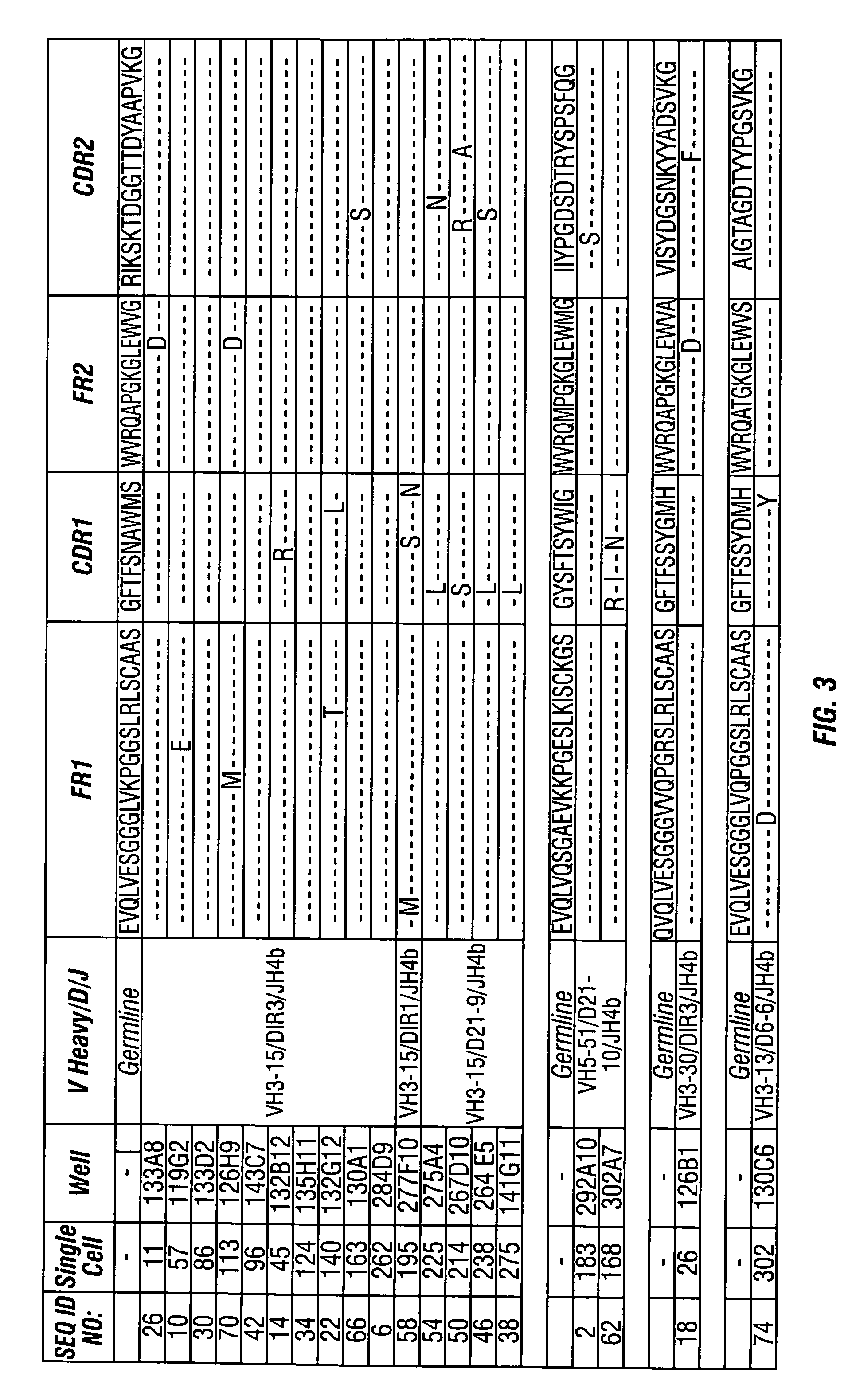
The current invention provides new human variable chain framework regions and humanized antibodies comprising the framework regions. The invention also provides a new method of identifying framework acceptor regions in framework sequences for backmutation to graft a donor sequence to the human framework.
Owner:UNITED STATES OF AMERICA +1
Anti-IL-6 Receptor Antibody
InactiveUS20130317203A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionDisulfide bondingAntiendomysial antibodies
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Anti-NKG2A antibodies and uses thereof
ActiveUS8206709B2Improved propertyLow immunogenicityHybrid immunoglobulinsAntipyreticComplementarity determining regionAmino acid
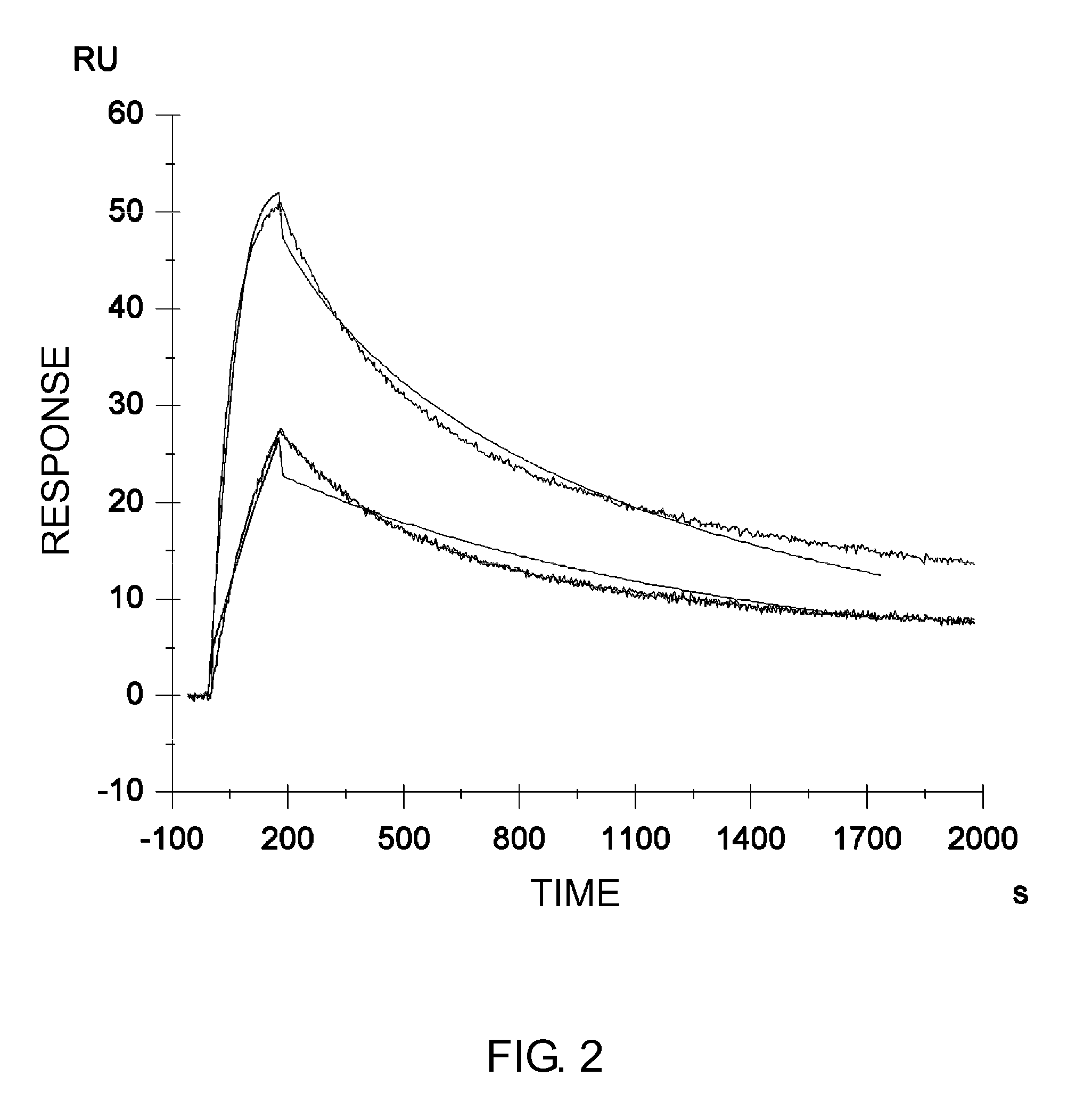
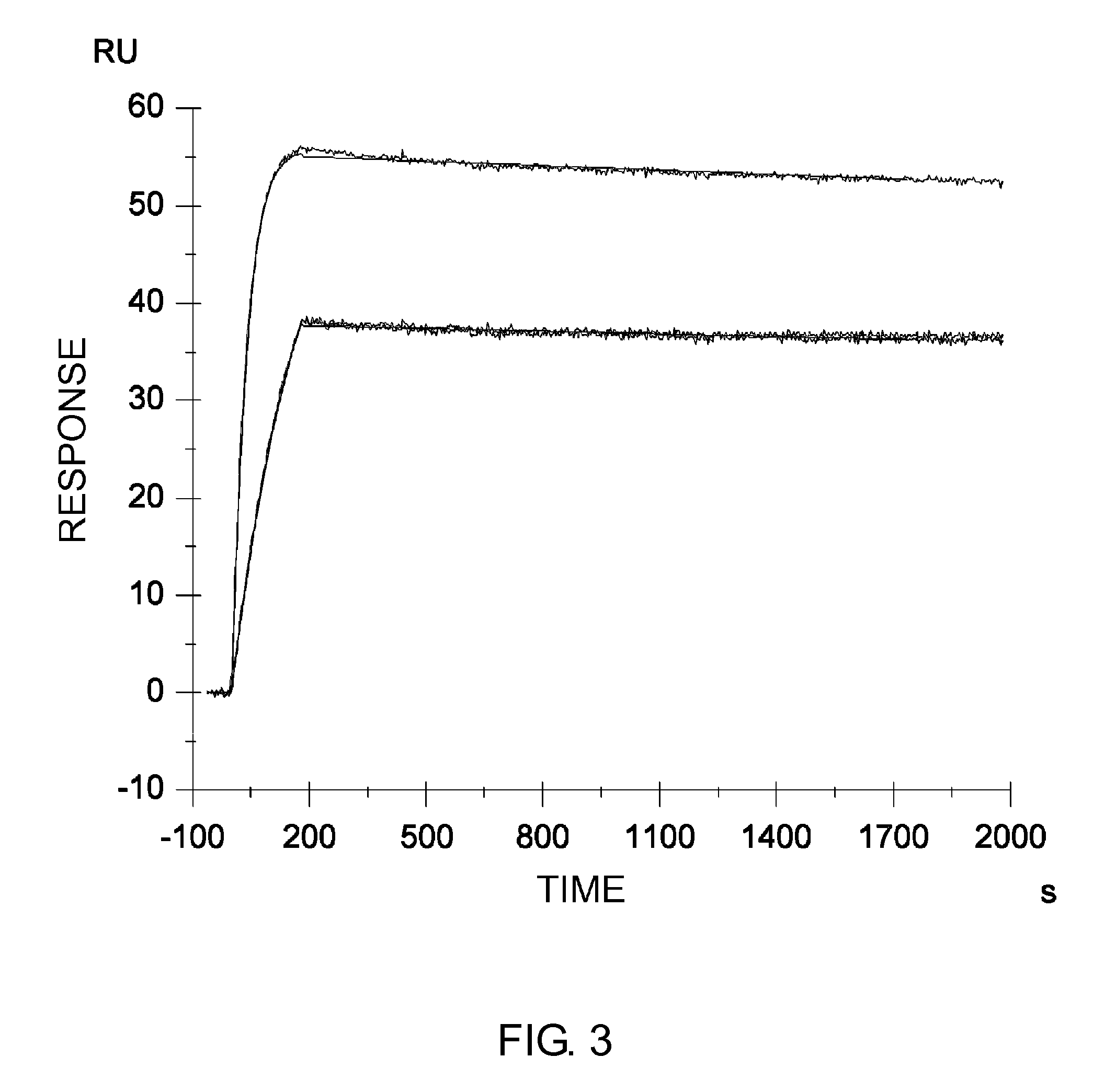
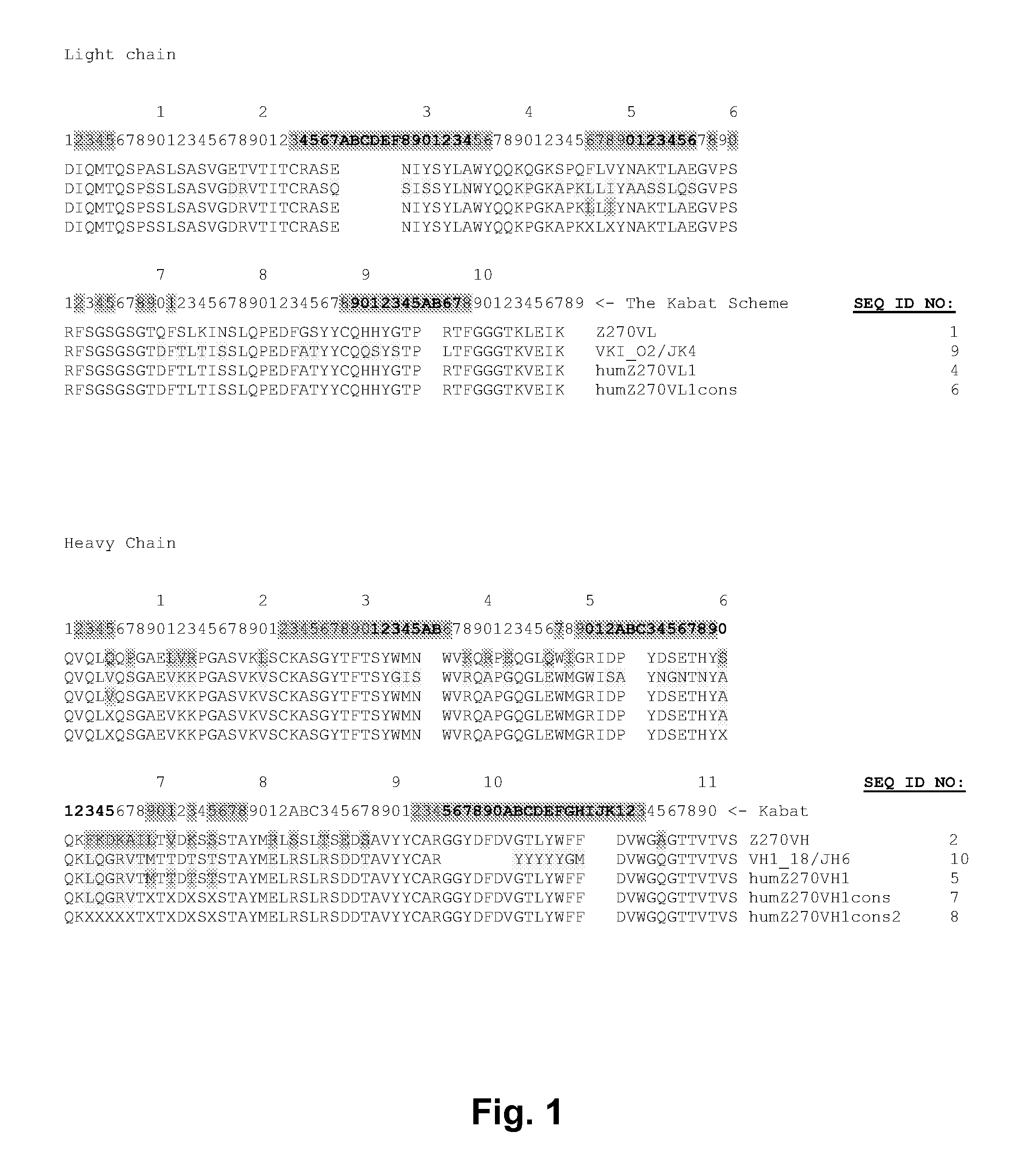
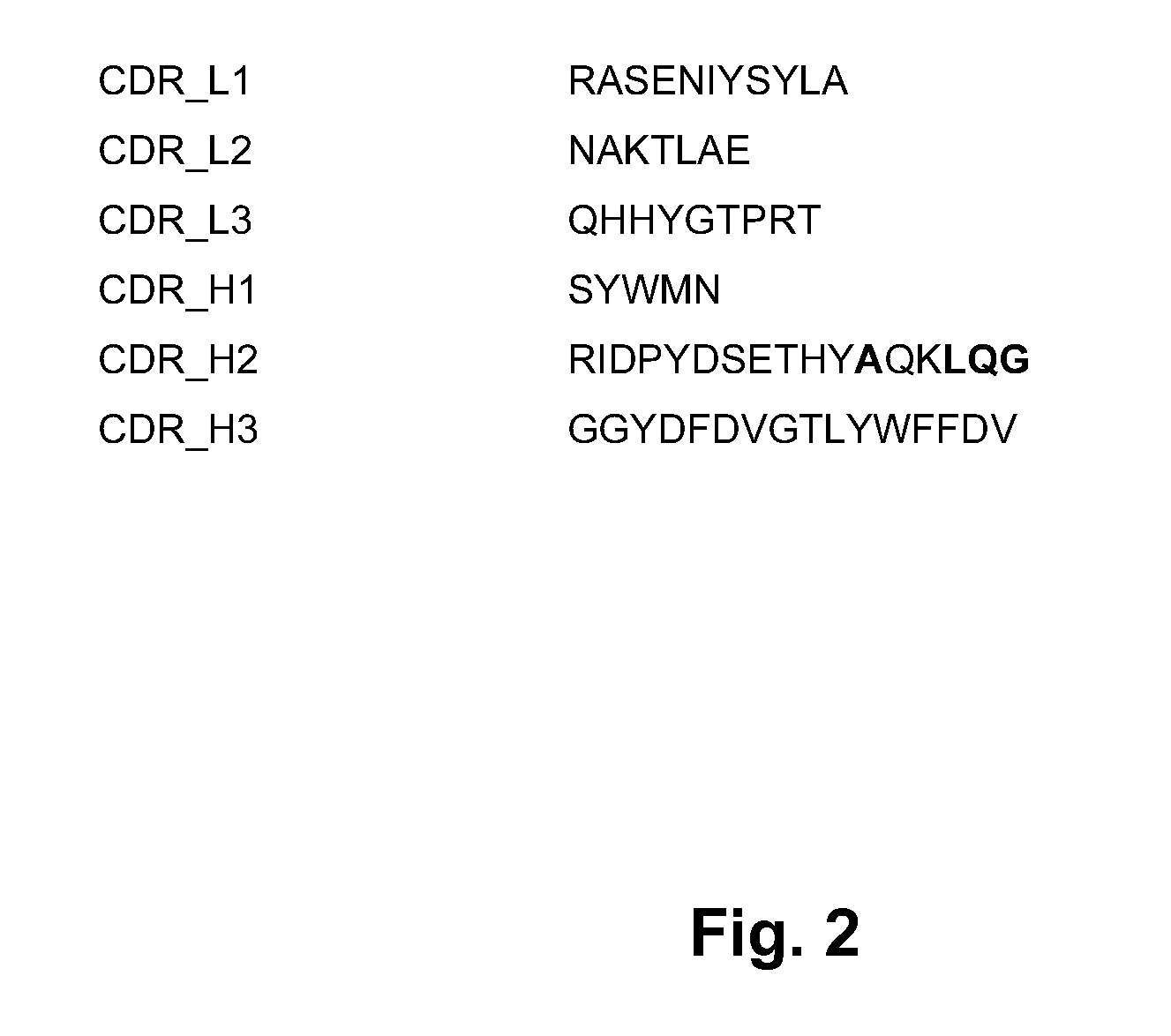
Described herein are anti-NKG2A antibodies suitable for human therapy, including humanized versions of murine anti-NKG2A antibody Z270, as well as related methods and materials for producing and using such antibodies. Exemplary complementarity-determining regions (CDRs) sequences and sites for optional amino acid back-substitutions in framework region (FR) and / or CDRs of such antibodies are also described.
Owner:NOVO NORDISK AS
Anti-properdin antibodies, and methods for making and using same
InactiveUS20060093599A1Antibacterial agentsNervous disorderComplementarity determining regionHeavy chain
The present invention is related to antibodies directed to the antigen properdin and uses of such antibodies. In particular, in accordance with the present invention, there are provided fully human monoclonal antibodies directed to the antigen properdin. Nucleotide sequences encoding, and polypeptides comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDR's), specifically from FR1 through FR4 or CDR1 through CDR3, are provided. Hybridomas or other cell lines expressing such immunoglobulin molecules and monoclonal antibodies are also provided.
Owner:AMGEN FREMONT INC
Rationally designed antibodies
Antibodies or fragments thereof having CDR regions replaced or fused with biologically active peptides are described. Flanking sequences may optionally be attached at one or both the carboxy-terminal and amino-terminal ends of the peptide in covalent association with adjacent framework regions. Compositions containing such antibodies or fragments thereof are useful in therapeutic and diagnostic modalities.
Owner:ALEXION PHARMA INC
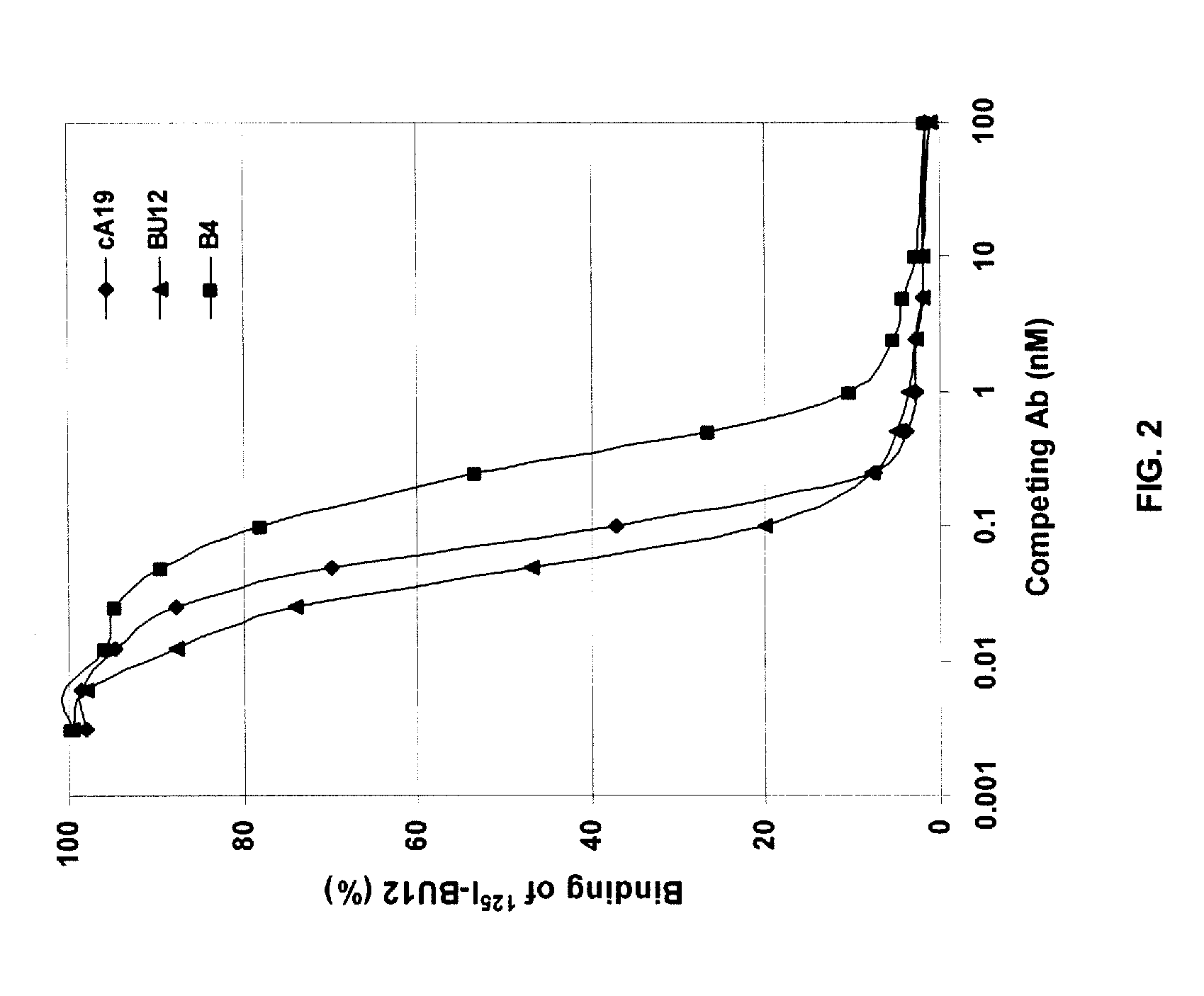
Anti-CD19 Antibodies
The present invention provides humanized, chimeric and human anti-CD19 antibodies, anti-CD19 antibody fusion proteins, and fragments thereof that bind to a human B cell marker. Such antibodies, fusion proteins and fragments thereof are useful for the treatment and diagnosis of various B-cell disorders, including B-cell malignancies and autoimmune diseases. In more particular embodiments, the humanized anti-CD19 antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels. In a particularly preferred embodiment, the substitutions comprise a Ser91Phe substitution in the hA19 VH sequence.
Owner:IMMUNOMEDICS INC
Ultra high affinity neutralizing antibodies
InactiveUS7740851B2Low costReduce efficacyHybrid immunoglobulinsAntibody mimetics/scaffoldsDiseaseComplementarity determining region
Ultra high affinity antibodies with binding affinities in the range of 1010 M−1, and even 1011 M−1 are disclosed. Such antibodies include antibodies having novel high affinity complementarity determining regions (CDRs), especially those with framework and constant regions derived from either humans or mice. Methods of preparing and screening such antibodies, as well as methods of using them to prevent and / or treat disease, especially virus-induced diseases, are also disclosed.
Owner:MEDIMMUNE LLC
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
Modified human IGF-IR antibodies
InactiveUS20050069539A1Nervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsVaccine ImmunogenicityFramework region
The present invention relates to antibodies, which are directed to the human IGF-1 receptor (IGF-1R) and are to be administered for the treatment of cancer. The antibodies of the present invention have been altered to comprise antibodies with one or more selected germlne framework amino acid residues which replace one or more corresponding somatically mutated residues in the variable region of the unaltered antibody. The modification results in the framework region mutations converted to germline. The modification results in a reduced propensity for the antibody to elicit an immune response (reduced immunogenicity) following administration to a human subject.
Owner:PFIZER INC +1
Immunocytokine sequences and uses thereof
ActiveUS7169904B2Low immunogenicityGood effectGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman cellGlycosphingolipid
The invention provides a family of antibodies that specifically bind the human cell surface glycosphingolipid GD2. The antibodies comprise modified variable regions, more specially, modified framework regions, which reduce their immunogenicity when administered to a human. The antibodies may be coupled to a therapeutic agent and used in the treatment of cancer.
Owner:MERCK PATENT GMBH
Immuno-conjugates and methods for producing them
ActiveUS20120171115A1Reducing and preventing residuePermit productionFungiBacteriaImmunoglobulin Heavy Chain Variable RegionDisulphide bonds
The present invention provides an isolated protein comprising an immunoglobulin variable region comprising at least two cysteine residues positioned within framework region (FR) 2 and / or at least two cysteine residues positioned within framework region (FR3), wherein if at least two of the cysteine residues in FR2 and / or FR3 are not conjugated to a compound then an intra-framework disulphide bond is capable of forming between the cysteine residues. Preferably the protein comprises an immunoglobulin heavy chain variable region (VH) and an immunoglobulin light chain variable region (VL), wherein at least one of the variable regions comprises the two cysteine residues. The present invention also provides conjugates of the protein and another compound.
Owner:AVIPEP
Engineered antibodies with new world primate framework regions
InactiveUS20080095767A1Low immunogenicityAntibacterial agentsNervous disorderPrimateComplementarity determining region
The present invention provides an antibody or antigen-binding portion thereof having a variable region comprising at least two complementarity determining regions (CDRs) and at least three framework regions. The the framework regions are, or are derived from New World primate framework regions, and at least one of the CDRs is a non-New World primate CDR.
Owner:CEPHALON AUSTRALIA
T cell receptor-like antibodies specific for a WT1 peptide presented by HLA-A2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
Methods of use for antibodies against parathyroid hormone
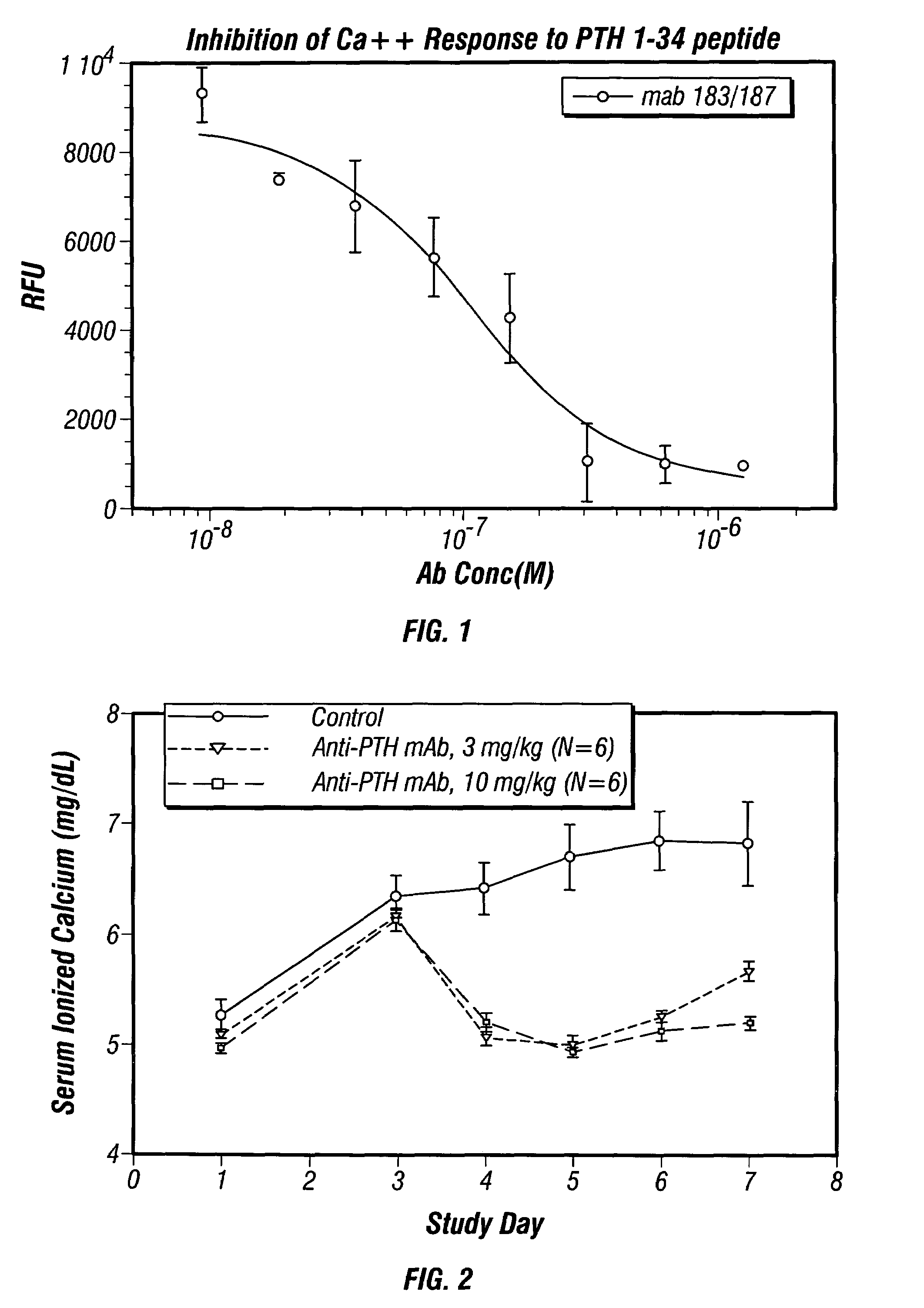
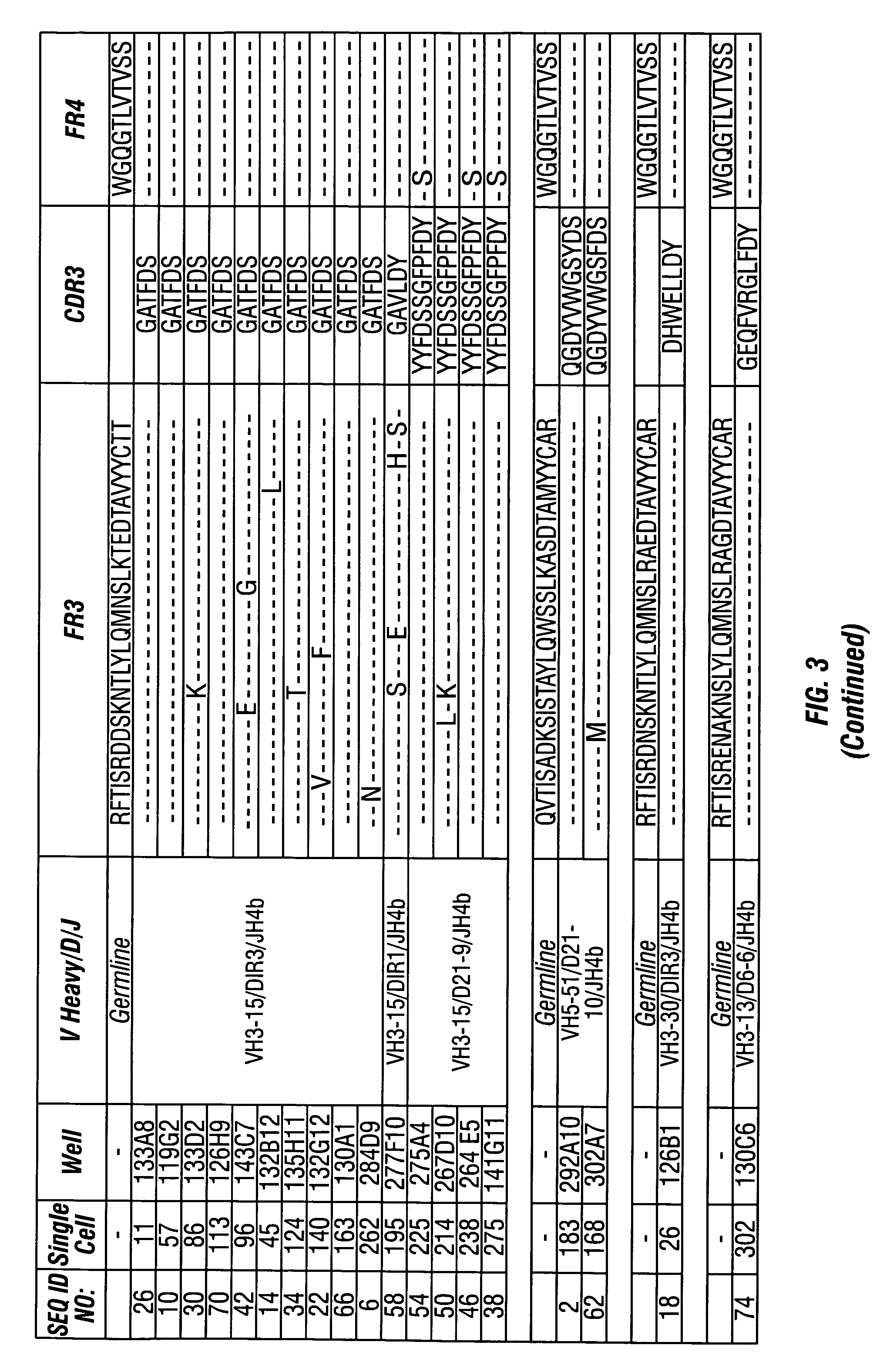
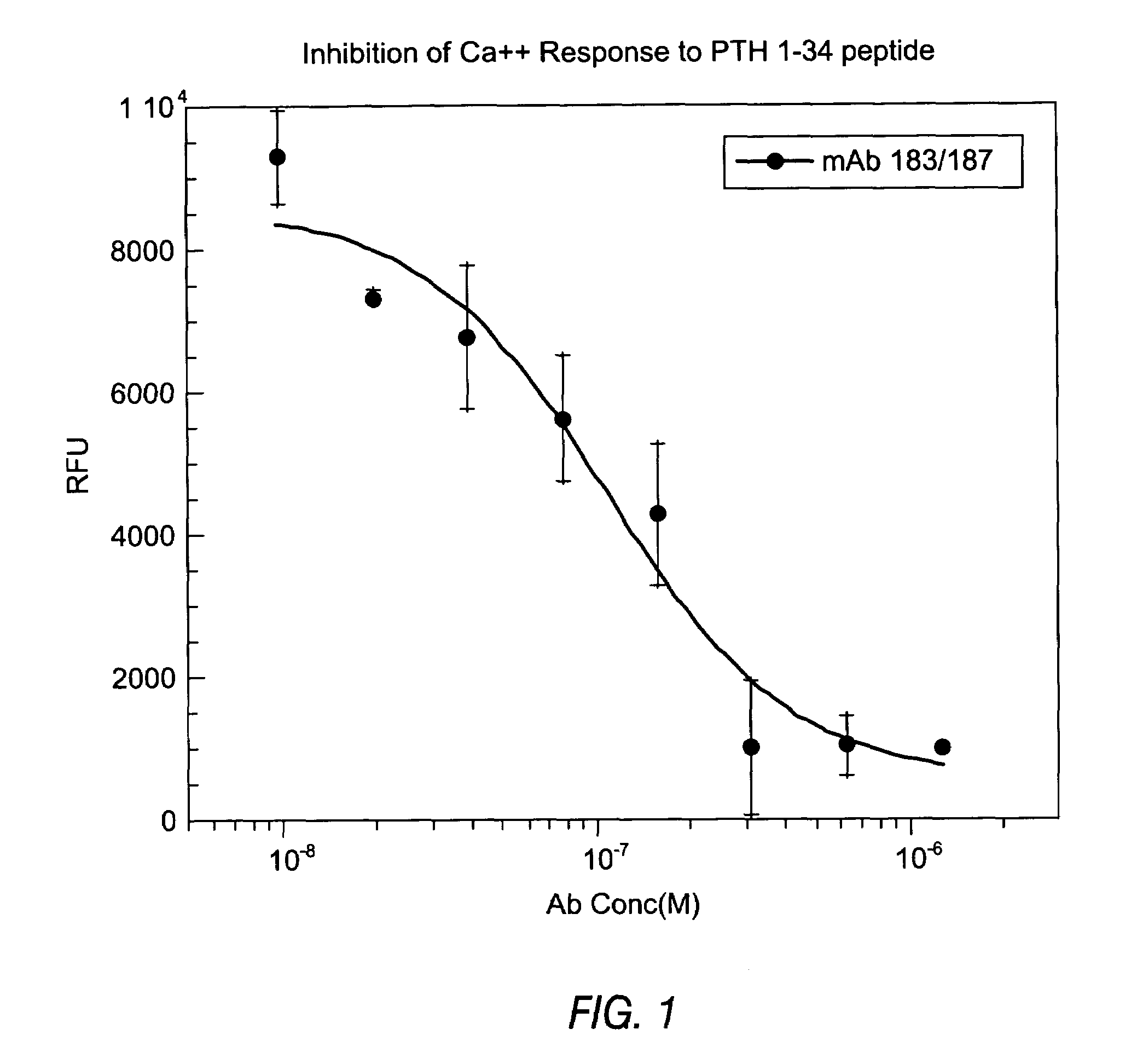
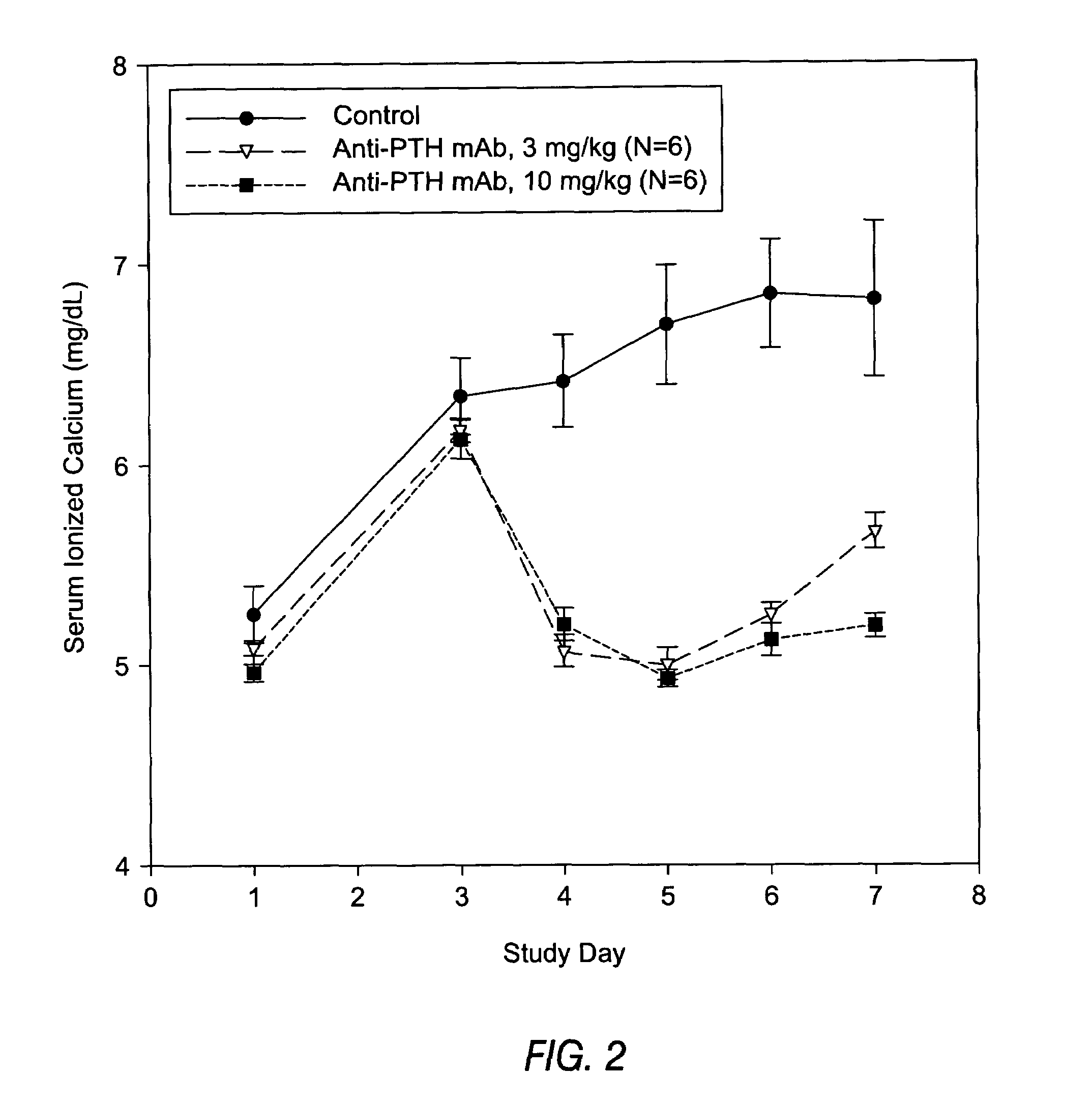
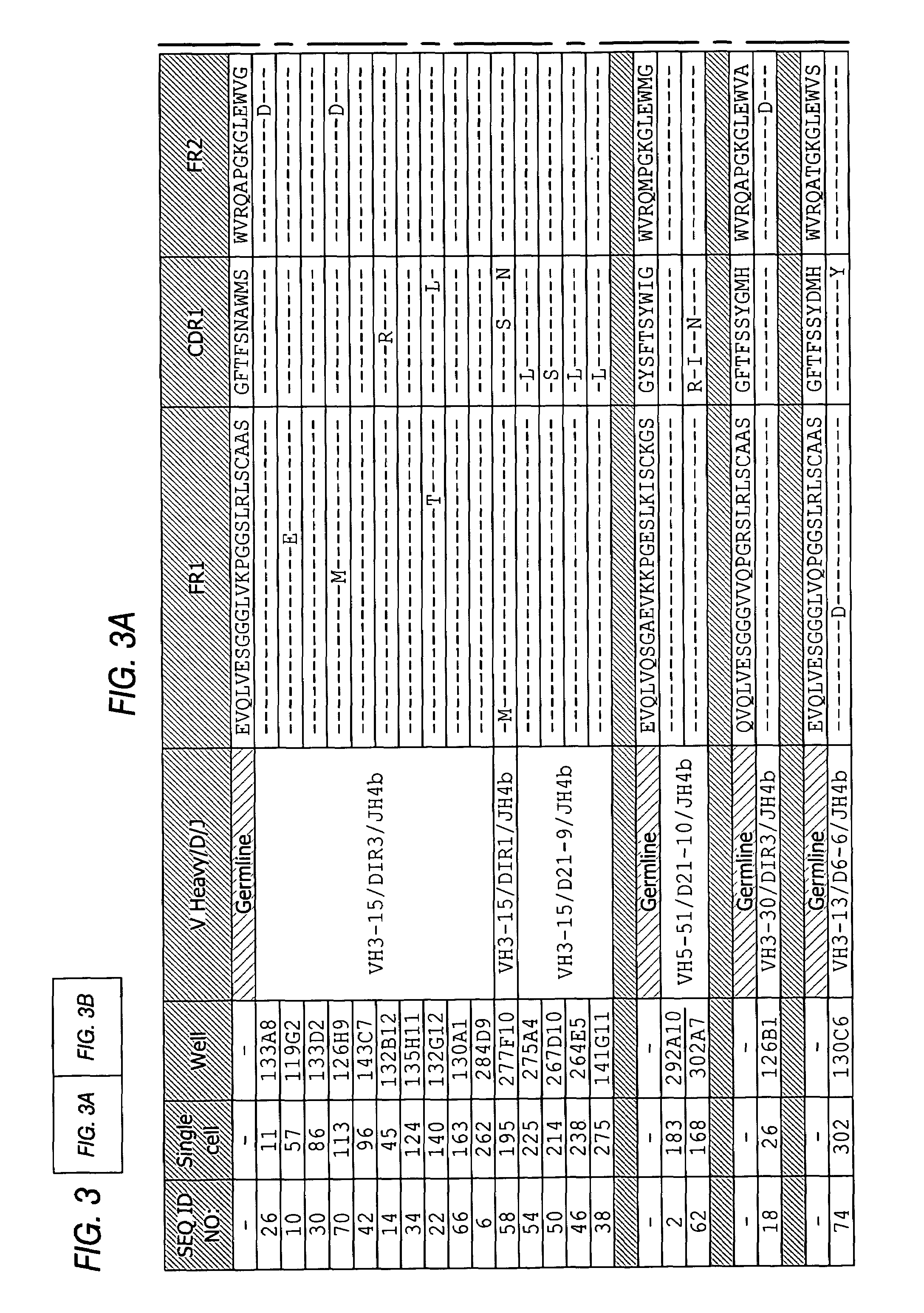
InactiveUS7318925B2Easy to neutralizeHigh affinityAntibody ingredientsImmunoglobulins against hormonesAntigenComplementarity determining region
Embodiments of the invention described herein relate to antibodies directed to the antigen parathyroid hormone (PTH) and uses of such antibodies. In particular, in some embodiments, there are provided fully human monoclonal antibodies directed to the antigen PTH. In further embodiments, nucleotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDRs), specifically from FR1 through FR4 or CDR1 through CDR3, are provided.
Owner:ABQENIX INC
Antibodies directed to parathyroid hormone (PTH) and uses thereof
InactiveUS7288253B2Increase the gapReduce clearanceBiocidePeptide/protein ingredientsAntigenComplementarity determining region
Embodiments of the invention described herein relate to antibodies directed to the antigen parathyroid hormone (PTH) and uses of such antibodies. In particular, in some embodiments, there are provided fully human monoclonal antibodies directed to the antigen PTH. In further embodiments, nucleotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDRs), specifically from FR1 through FR4 or CDR1 through CDR3, are provided.
Owner:ABQENIX INC
Rationally designed antibodies
Antibodies or fragments thereof having CDR regions replaced or fused with biologically active peptides are described. Flanking sequences may optionally be attached at one or both the carboxy-terminal and amino-terminal ends of the peptide in covalent association with adjacent framework regions. Compositions containing such antibodies or fragments thereof are useful in therapeutic and diagnostic modalities.
Owner:ALEXION PHARMA INC
Designing method for gradient coil of self-shielding superconductive nuclear magnetic resonance imaging system
InactiveCN105718677AReduce inductanceLarge line spacingSpecial data processing applicationsNMR - Nuclear magnetic resonanceIntensity coefficient
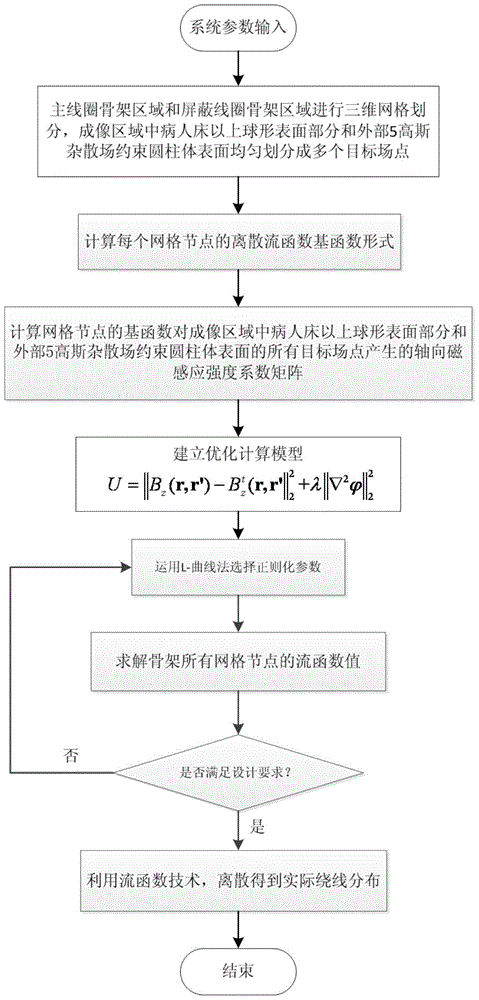
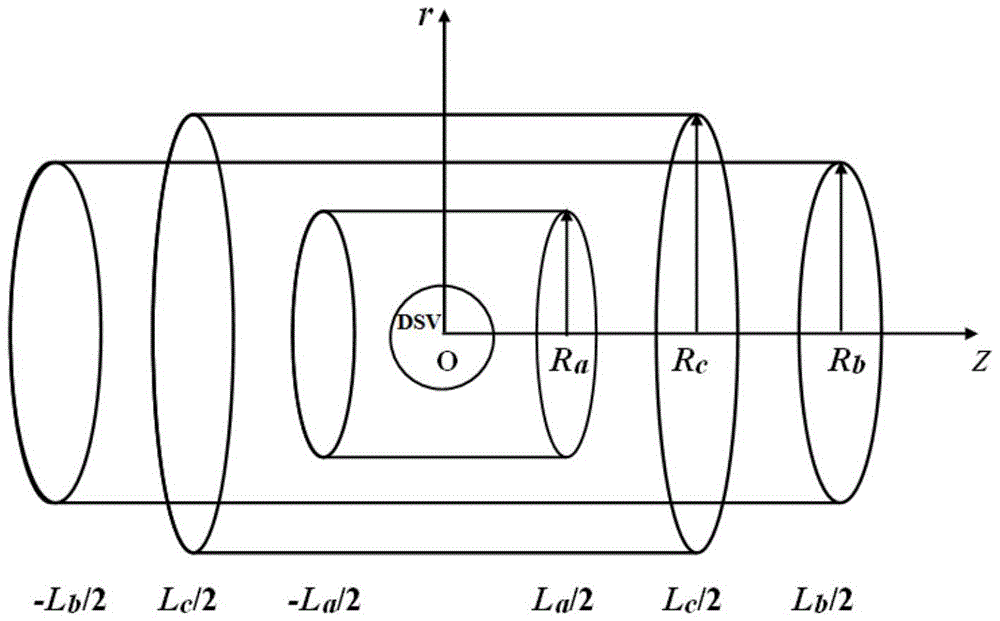
The invention discloses a designing method for a gradient coil of a self-shielding superconductive nuclear magnetic resonance imaging system.The designing method comprises the steps that firstly, three-dimensional continuous triangular mesh dividing is performed on a main coil framework region and a shielding coil framework region, and an axial magnetic induction intensity coefficient matrix generated by mesh nodes in the main coil framework region and the shielding coil framework region on a target field point is calculated according to a boundary elements method; secondly, an optimization calculation model is built through a regularization method, wherein the optimization calculation model comprises two parts, the first part is deviation between the axial magnetic induction intensity generated by the mesh nodes in the main coil framework region and the shielding coil framework region on a target field point and the expected target magnetic induction intensity, the second part is the quadratic sum of the flow function curvature of the mesh nodes, and flow function values of the mesh nodes in the main coil framework region and the shielding coil framework region are obtained through solution; lastly, actual winding distribution of the gradient coil is obtained through a flow function method.
Owner:INST OF ELECTRICAL ENG CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com