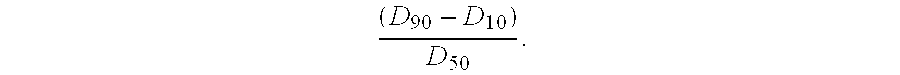Patents
Literature
124 results about "Parathyroid hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
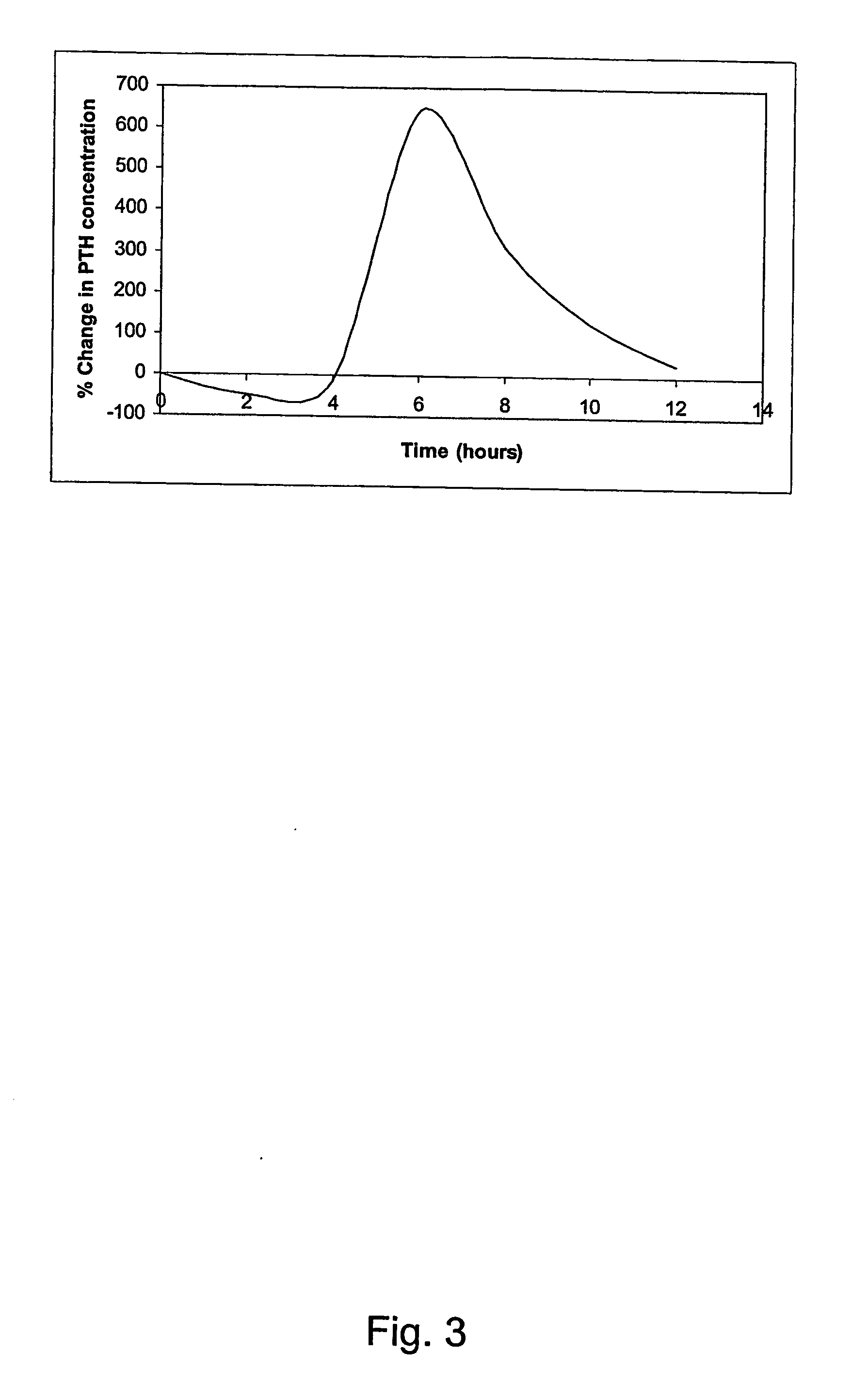
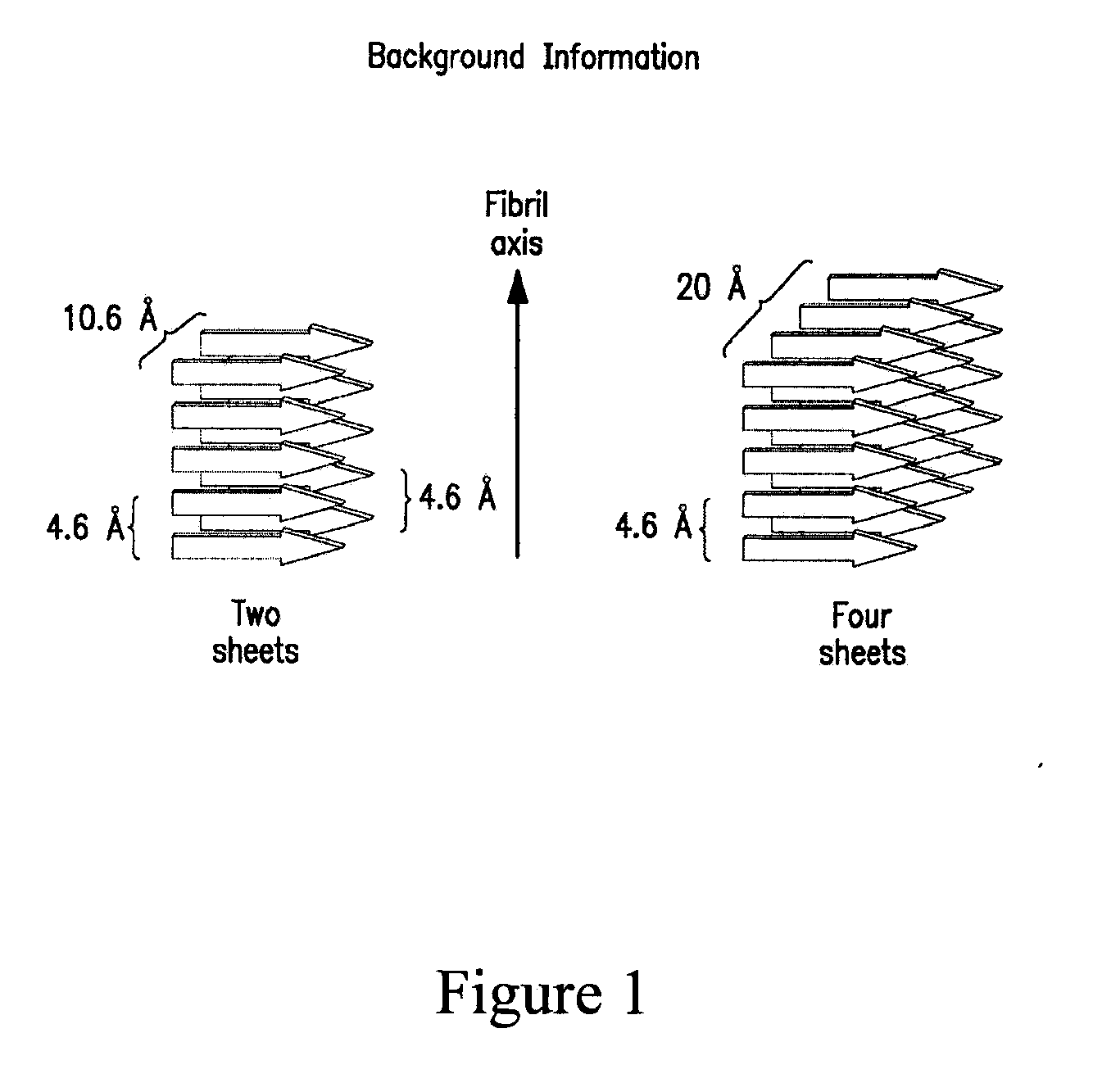
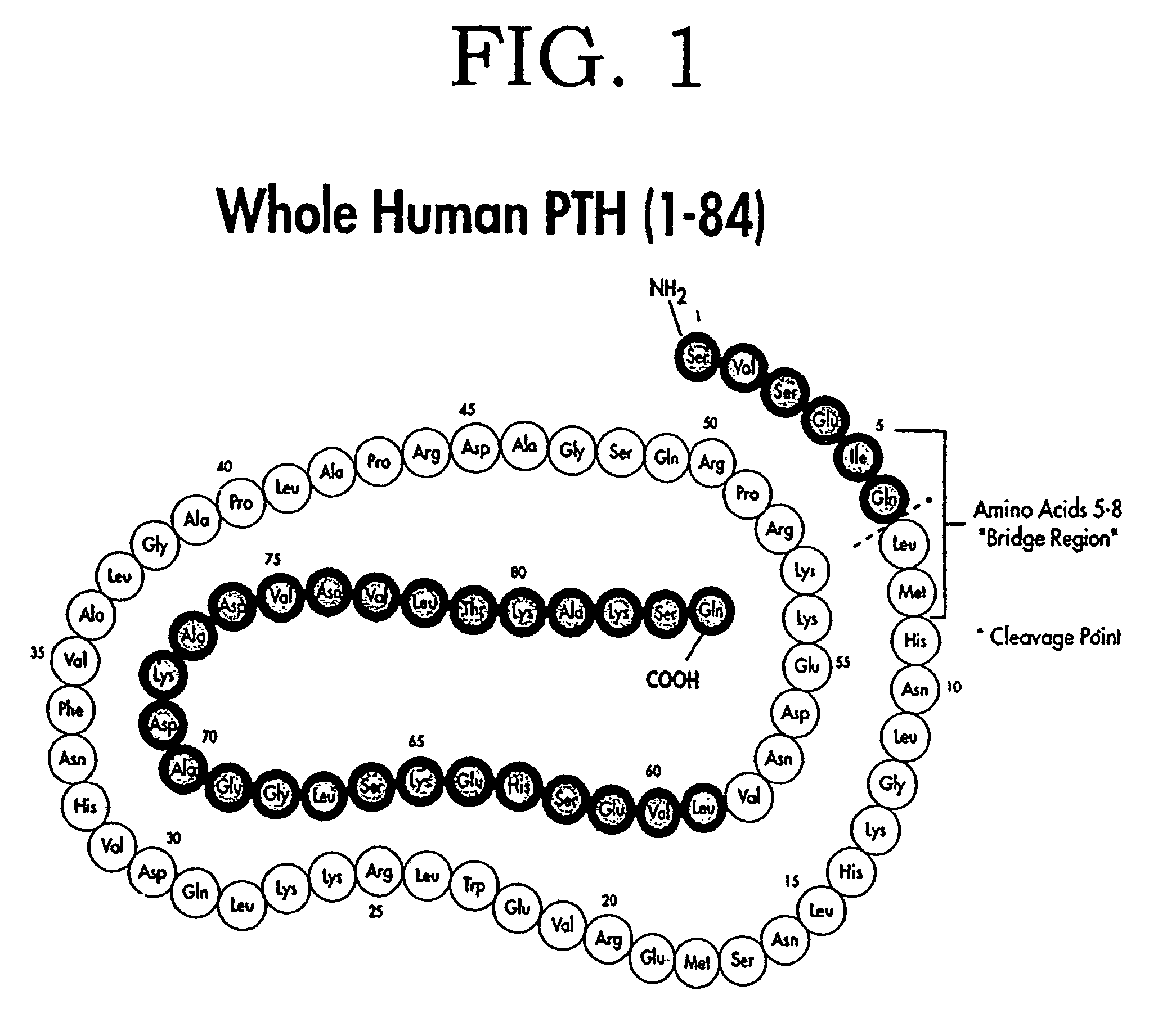
Parathyroid hormone (PTH), also called parathormone or parathyrin, is a hormone secreted by the parathyroid glands that regulates the serum calcium through its effects on bone, kidney, and intestine. PTH influences bone remodeling, which is an ongoing process in which bone tissue is alternately resorbed and rebuilt over time. PTH is secreted in response to low blood serum calcium (Ca²⁺) levels. PTH indirectly stimulates osteoclast activity within the bone matrix (osteon), in an effort to release more ionic calcium (Ca²⁺) into the blood to elevate a low serum calcium level. The bones act as a (metaphorical) "bank of calcium" from which the body can make "withdrawals" as needed to keep the amount of calcium in the blood at appropriate levels despite the ever-present challenges of metabolism, stress, and nutritional variations. PTH is "a key that unlocks the bank vault" to remove the calcium.
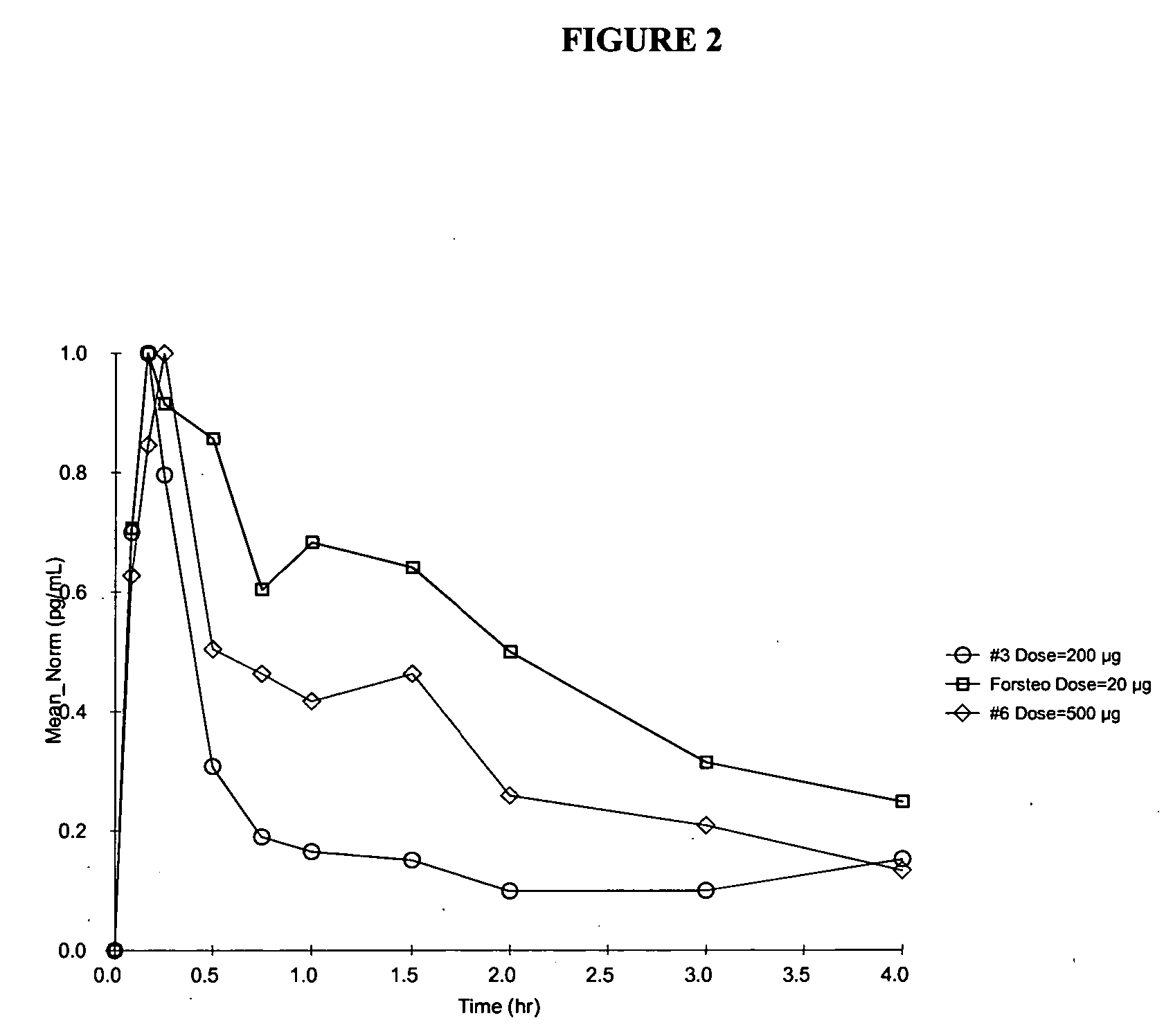
Method and device for the controlled delivery of parathyroid hormone
InactiveUS7497855B2Efficiently providePeptide/protein ingredientsMicromachined deliveryOsteopoikilosisMedical device
Method and devices are provided for extended and controlled delivery of parathyroid hormone to a patient. The method includes implanting a medical device into the patient, the medical device comprising a substrate, a plurality of reservoirs in the substrate, a release system contained in each of the reservoirs, wherein the release system comprises parathyroid hormone; and controllably releasing a pharmaceutically effective amount of the parathyroid hormone from the reservoirs. The parathyroid hormone can be released intermittently, such as once daily over an extended period (e.g., two months, ten months, or more.). The device can further include reservoirs containing a bone resorption inhibitor or other drug for release. The devices are useful in delivering PTH for the treatment and prevention of bone loss, such as associated with osteoporosis.
Owner:MICROCHIPS BIOTECH INC
Parathyroid hormone (pth) containing pharmaceutical compositions for oral use
InactiveUS20070155664A1Quick releaseSufficient amountOrganic active ingredientsPeptide/protein ingredientsRegimenBlood plasma
A pharmaceutical composition for oral administration comprising PTH, wherein the in vitro release of PTH-when tested in a dissolution test of pharmacopoeia standard-is delayed with at least 2 hours and once the release starts, at least 90% w / w such as, e.g., at least 95% or at least 99% of all PTH contained in the composition is released within at the most 2 hours. The composition may also comprises a calcium containing compound and / or a vitamin, D. In particular, PTH is administered in combination with a calcium-containing compound for the treatment or prevention of bone-related diseases, so that I) an effective amount of a calcium-containing compound is administered to lower the plasma level of endogenous PTH, and II) an effective amount of PTH is administered to obtain a peak concentration of Pm once the endogeneous PTH level is lowered. This present a potential therapeutic or prophylactic regimen for bone-related disorders including osteoporosis.
Owner:NYCOMED DANMARK AS
Method for inhibiting bone resorption
ActiveUS20090074763A1Inhibiting bone resorptionLower Level RequirementsAntibacterial agentsNervous disorderBone densityIncreased bone mineral density
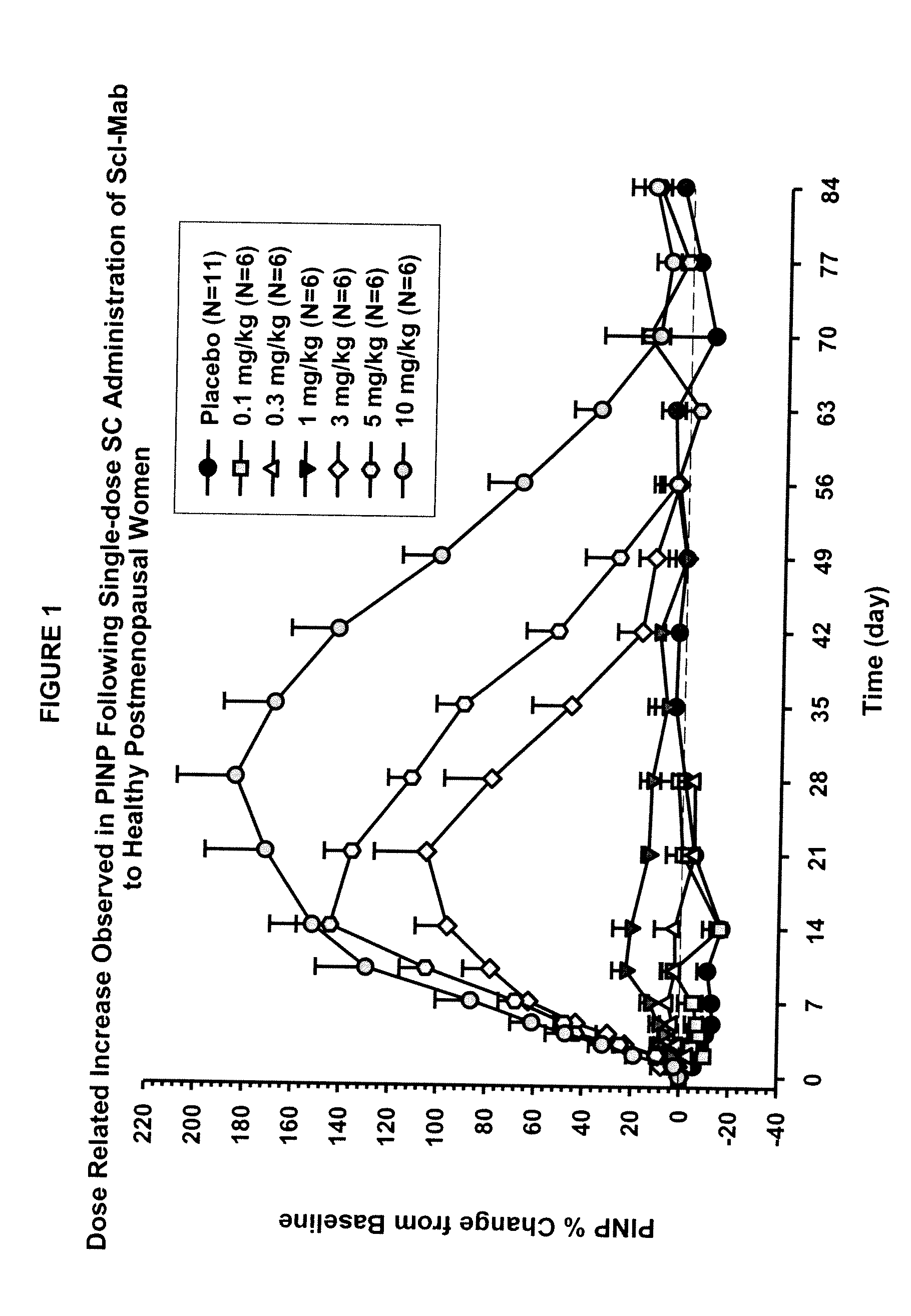
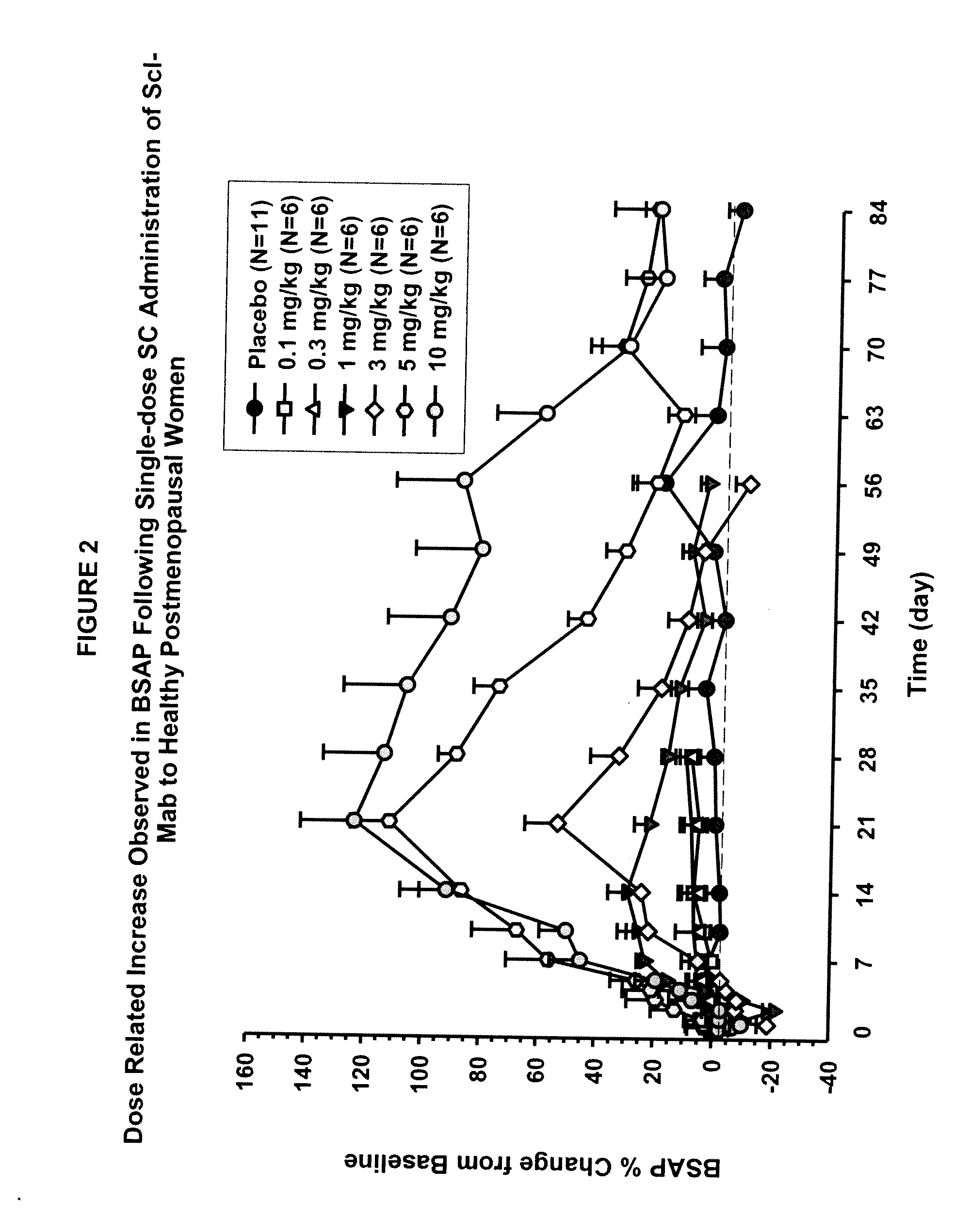
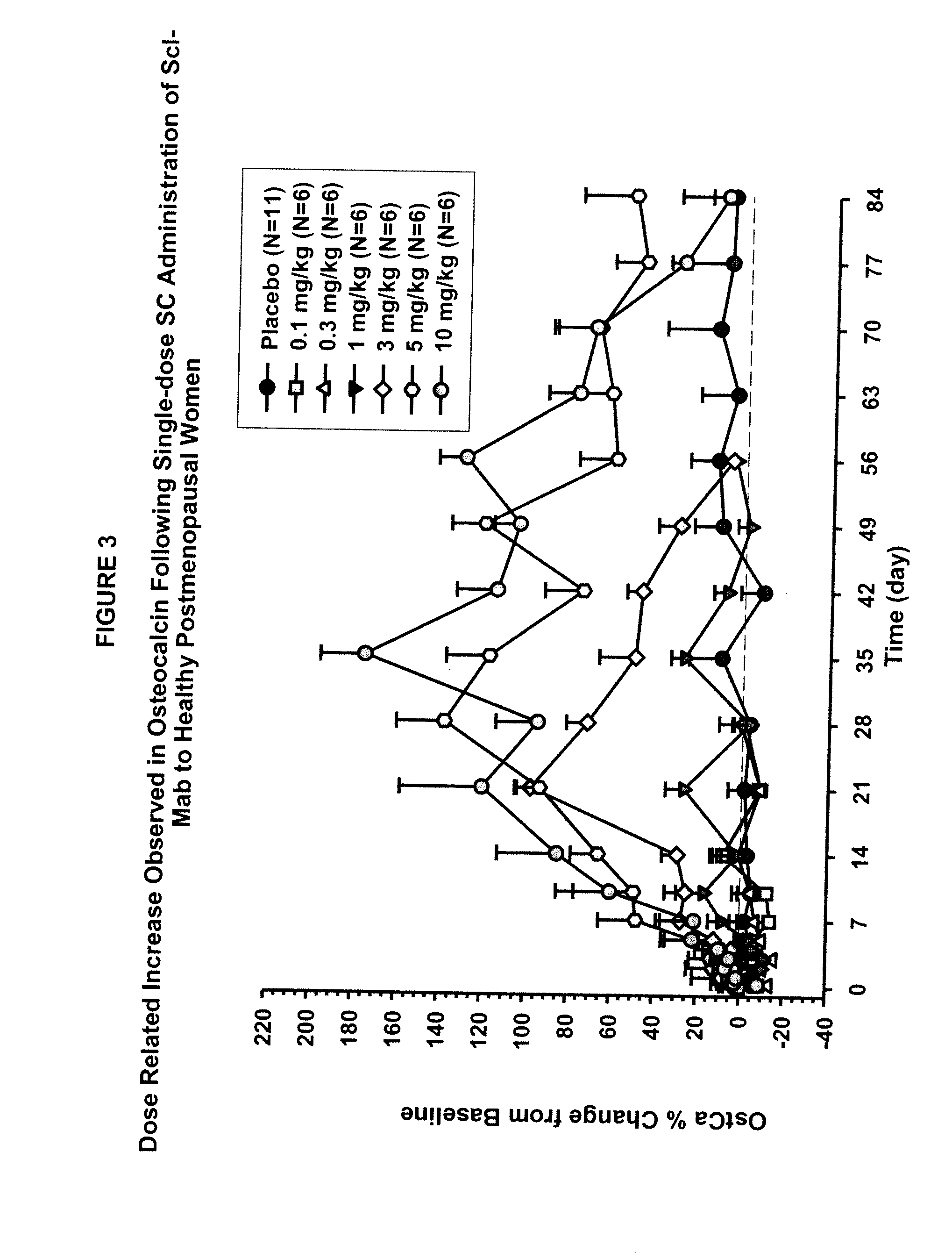
The invention is directed to a method of inhibiting bone resorption. The method comprises administering to a human an amount of sclerostin inhibitor that reduces a bone resorption marker level for at least 2 weeks. The invention also provides a method of monitoring anti-sclerostin therapy comprising measuring one or more bone resorption marker levels, administering a sclerostin binding agent, then measuring the bone resorption marker levels. Also provided is a method of increasing bone mineral density; a method of ameliorating the effects of an osteoclast-related disorder; a method of treating a bone-related disorder by maintaining bone density; and a method of treating a bone-related disorder in a human suffering from or at risk of hypocalcemia or hypercalcemia, a human in which treatment with a parathyroid hormone or analog thereof is contraindicated, or a human in which treatment with a bisphosphonate is contraindicated.
Owner:AMGEN INC
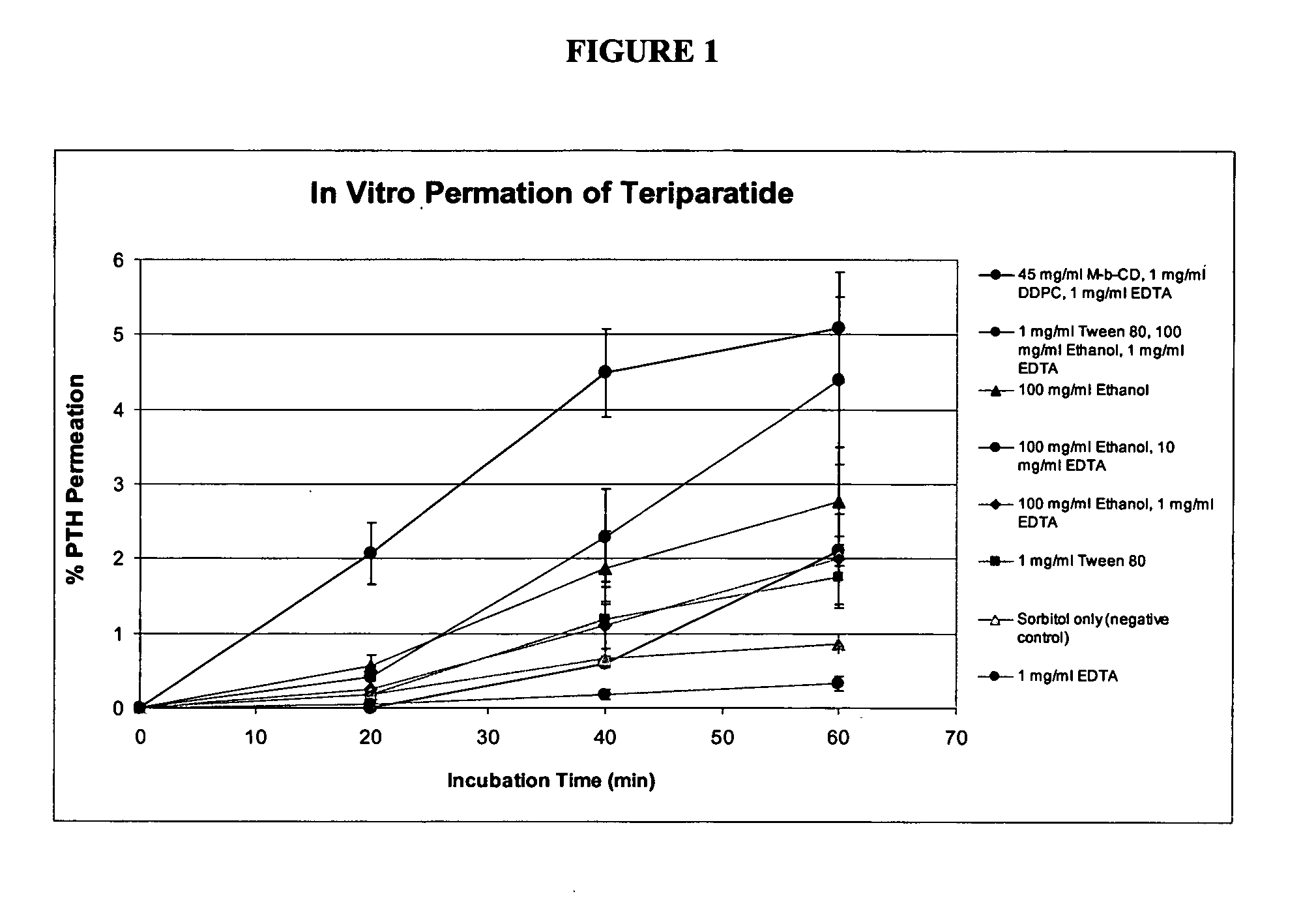
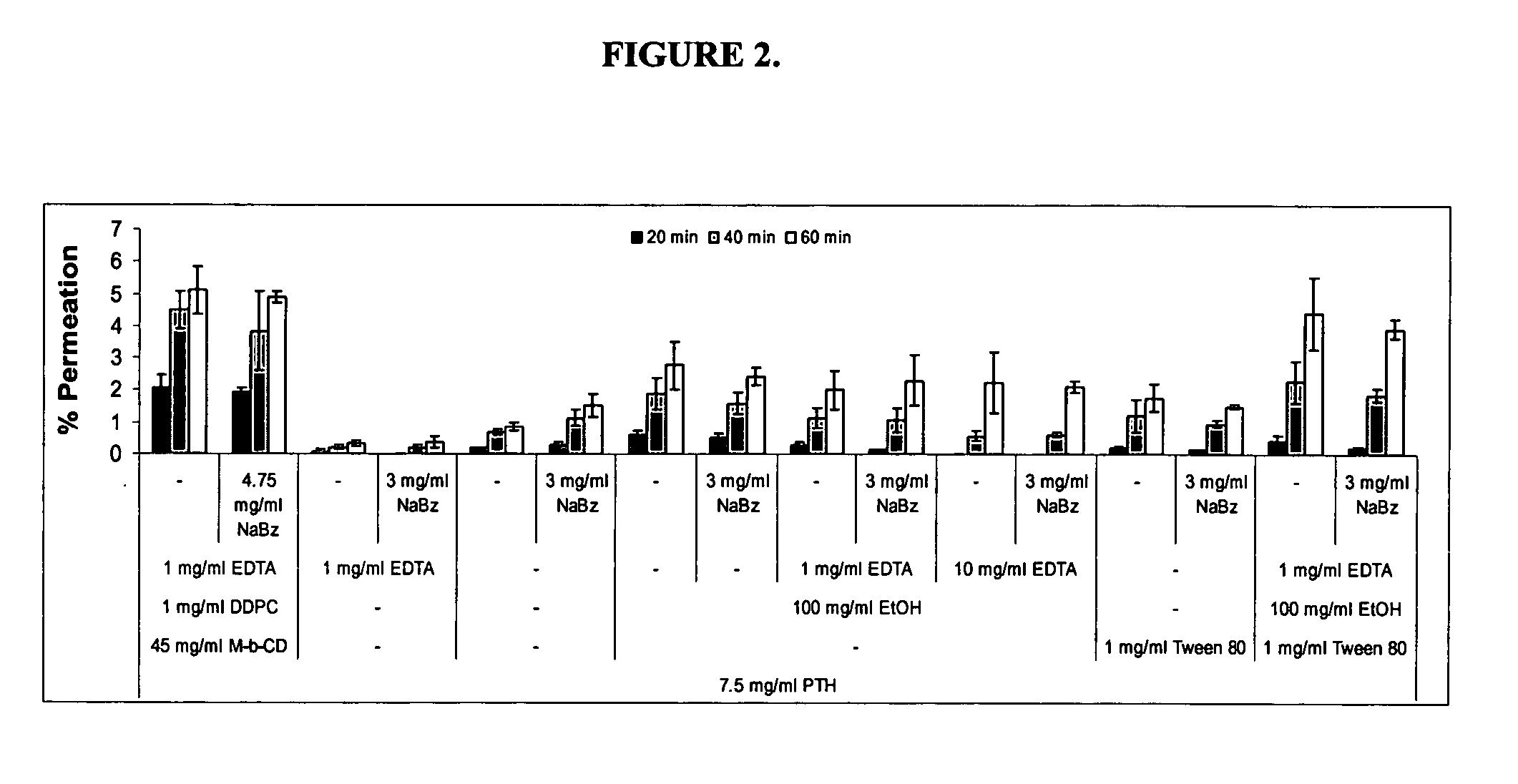
Compositions and methods for enhanced mucosal delivery of parathyroid hormone
InactiveUS20050276843A1Reduce damage-causing attritionHigh bonding strengthBiocideOrganic active ingredientsOsteoporosisPrevention breast cancer
Pharmaceutical compositions and methods are described comprising at least a parathyroid hormone peptide (PTH) preferably PTH1-34 and one or more mucosal delivery-enhancing agents for enhanced nasal mucosal delivery of PTH, for treating or preventing osteoporosis or osteopenia in a mammalian subject, preferably a human.
Owner:MARINA BIOTECH INC
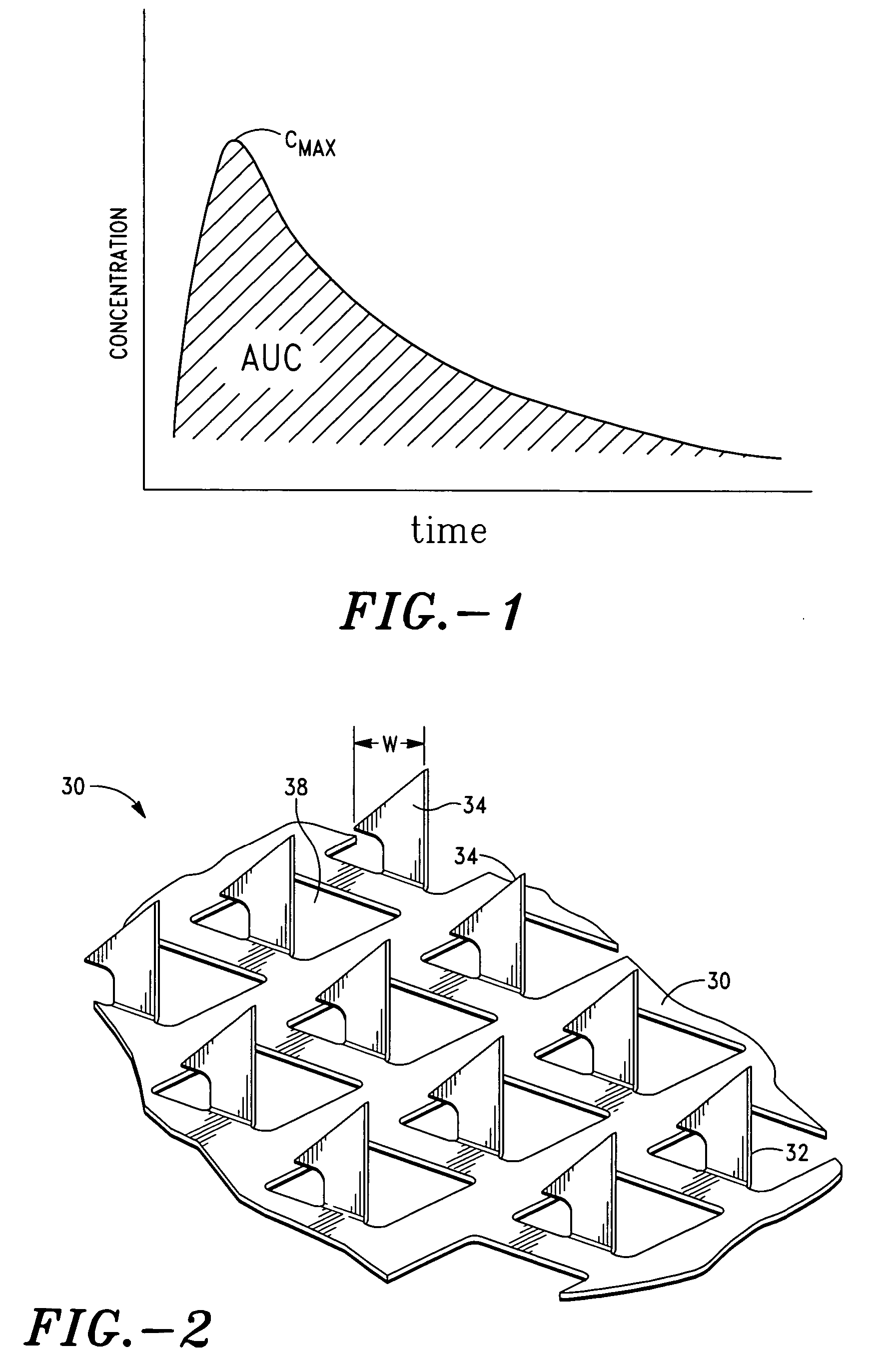
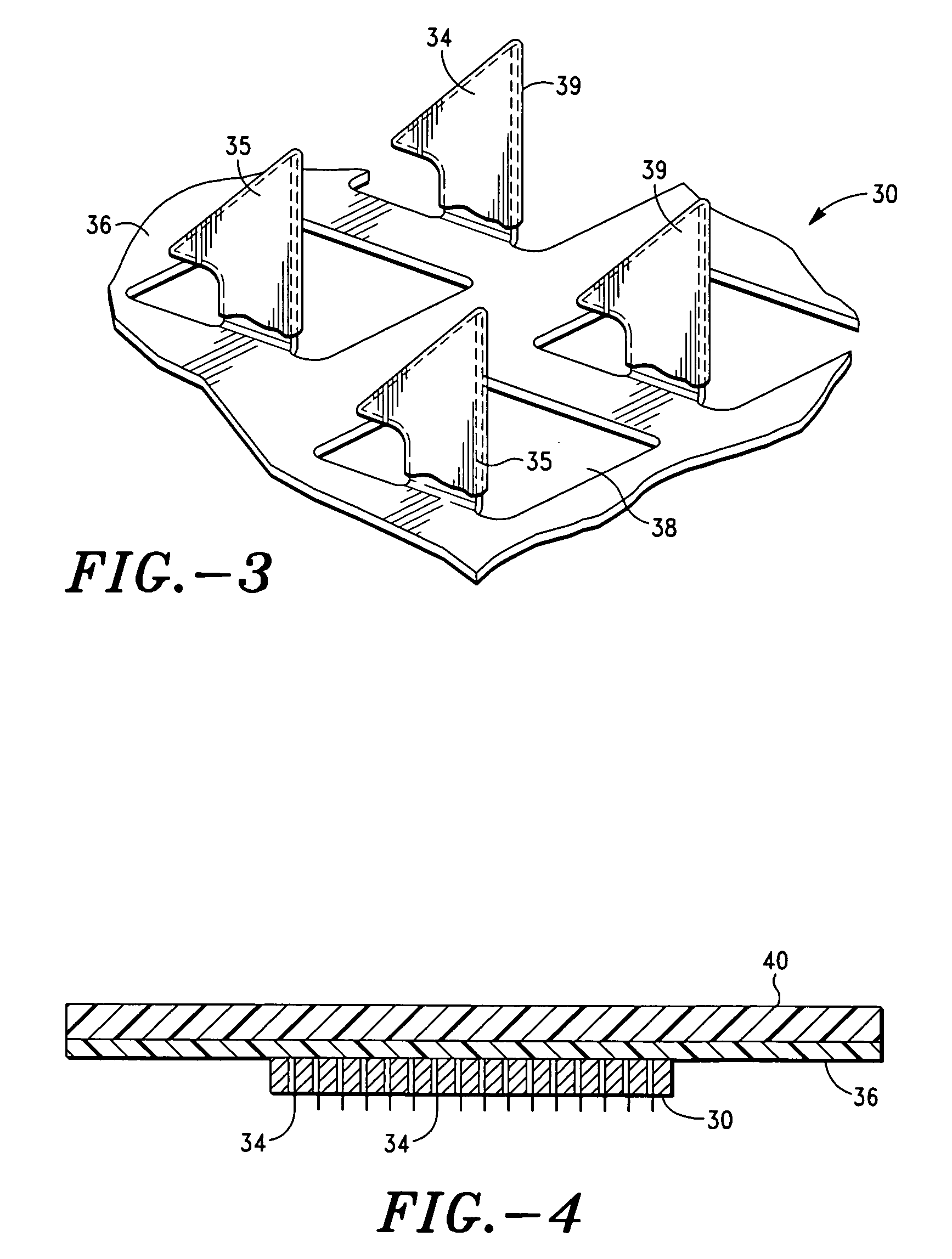
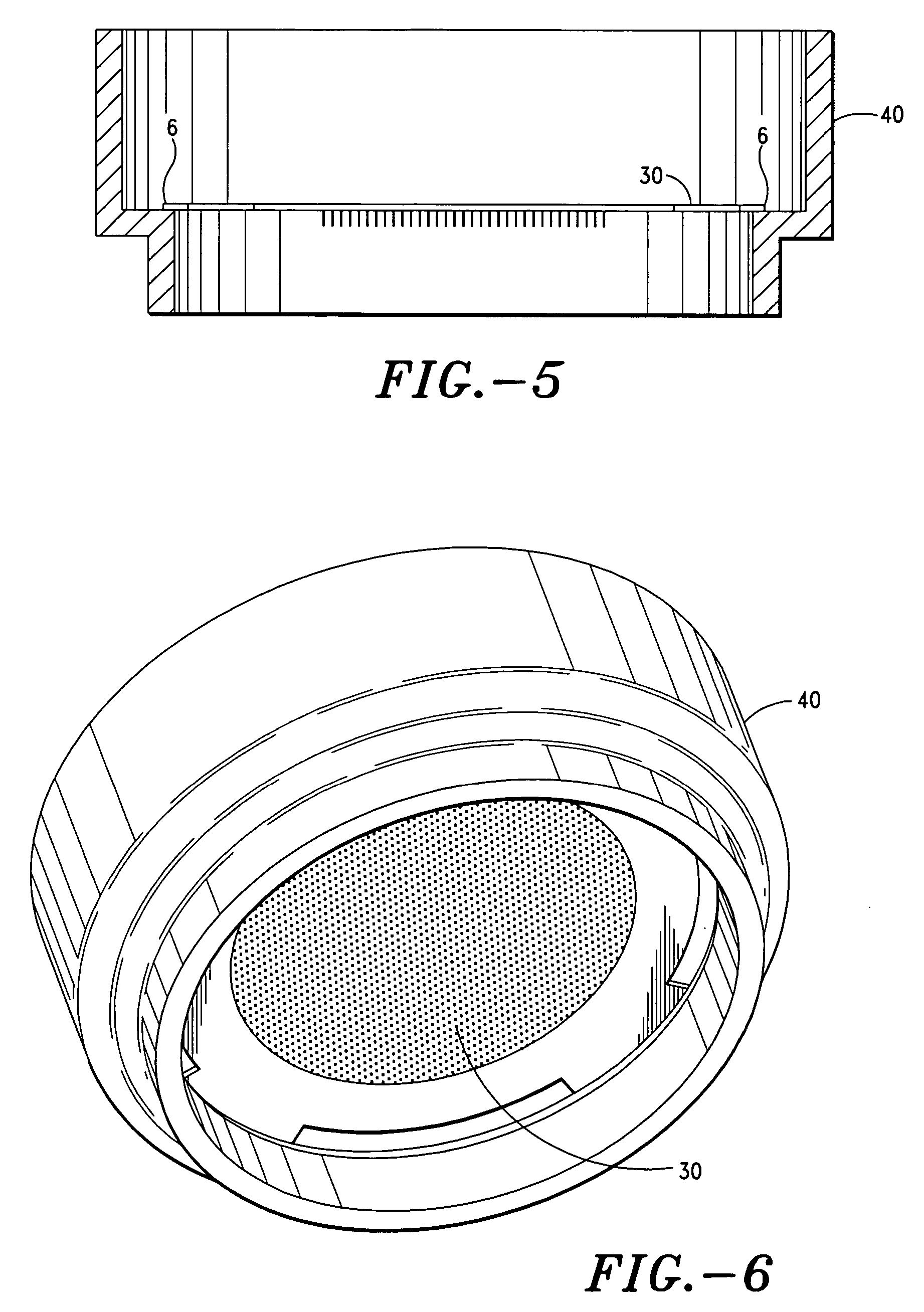
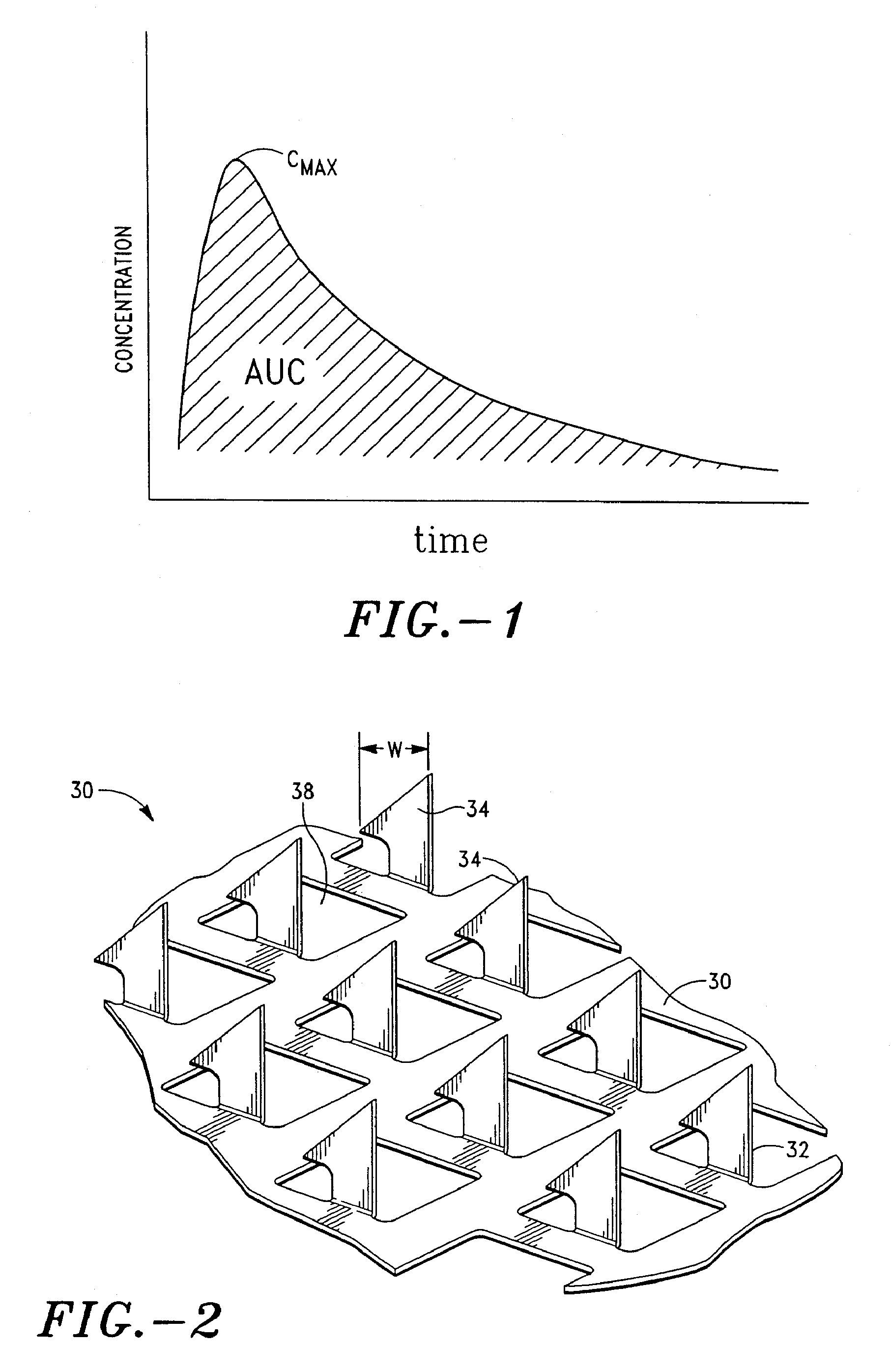
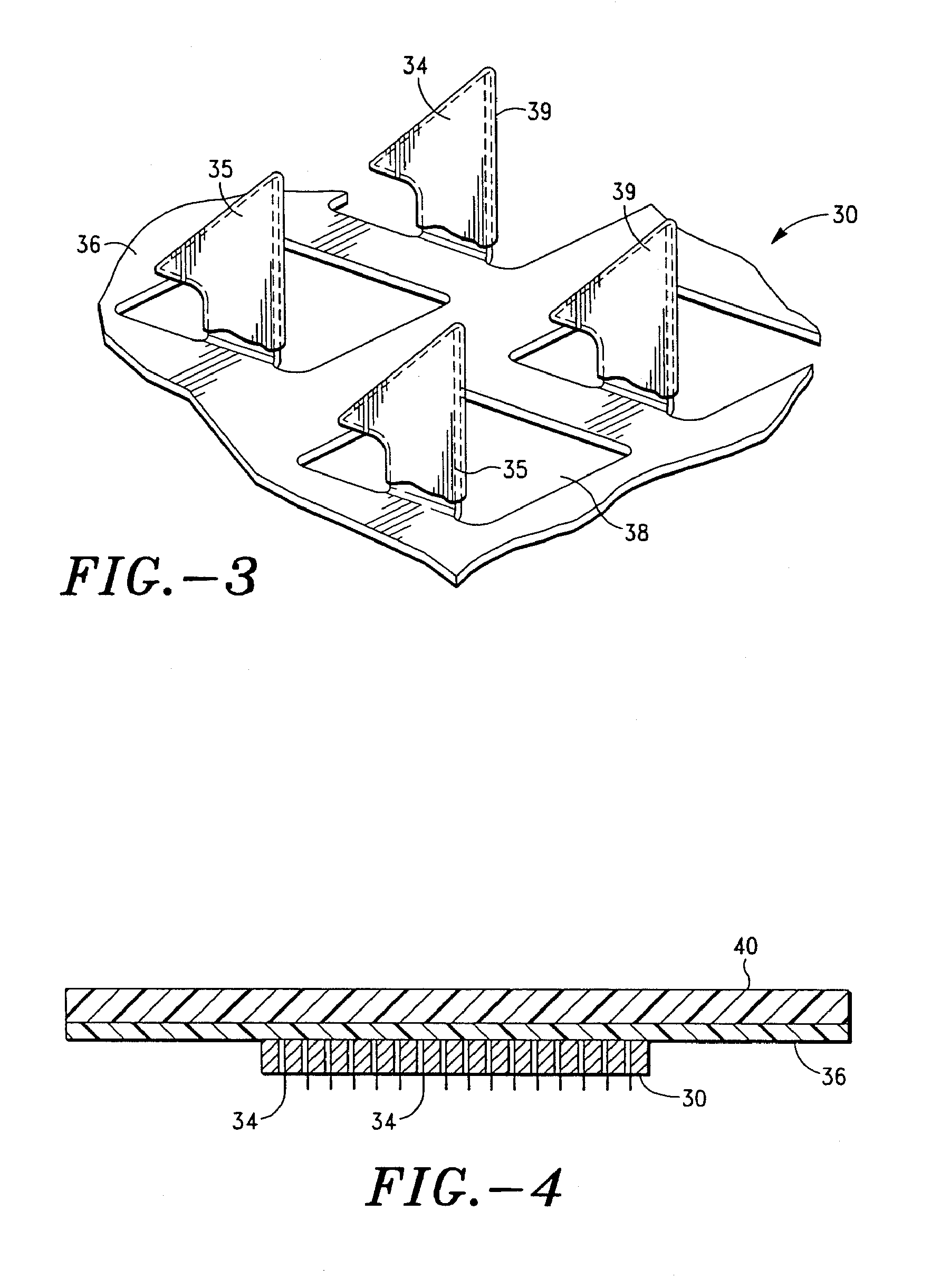
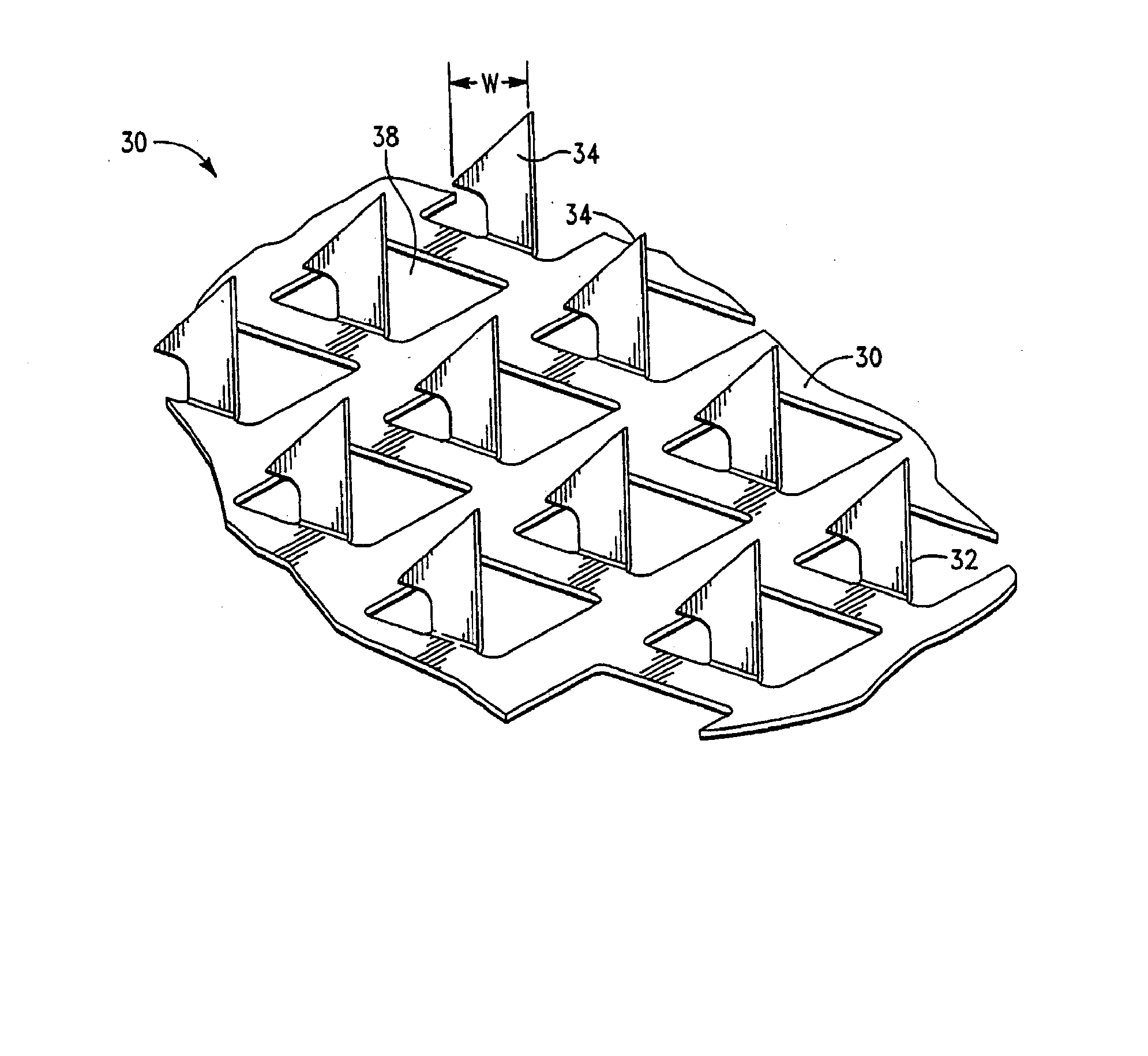
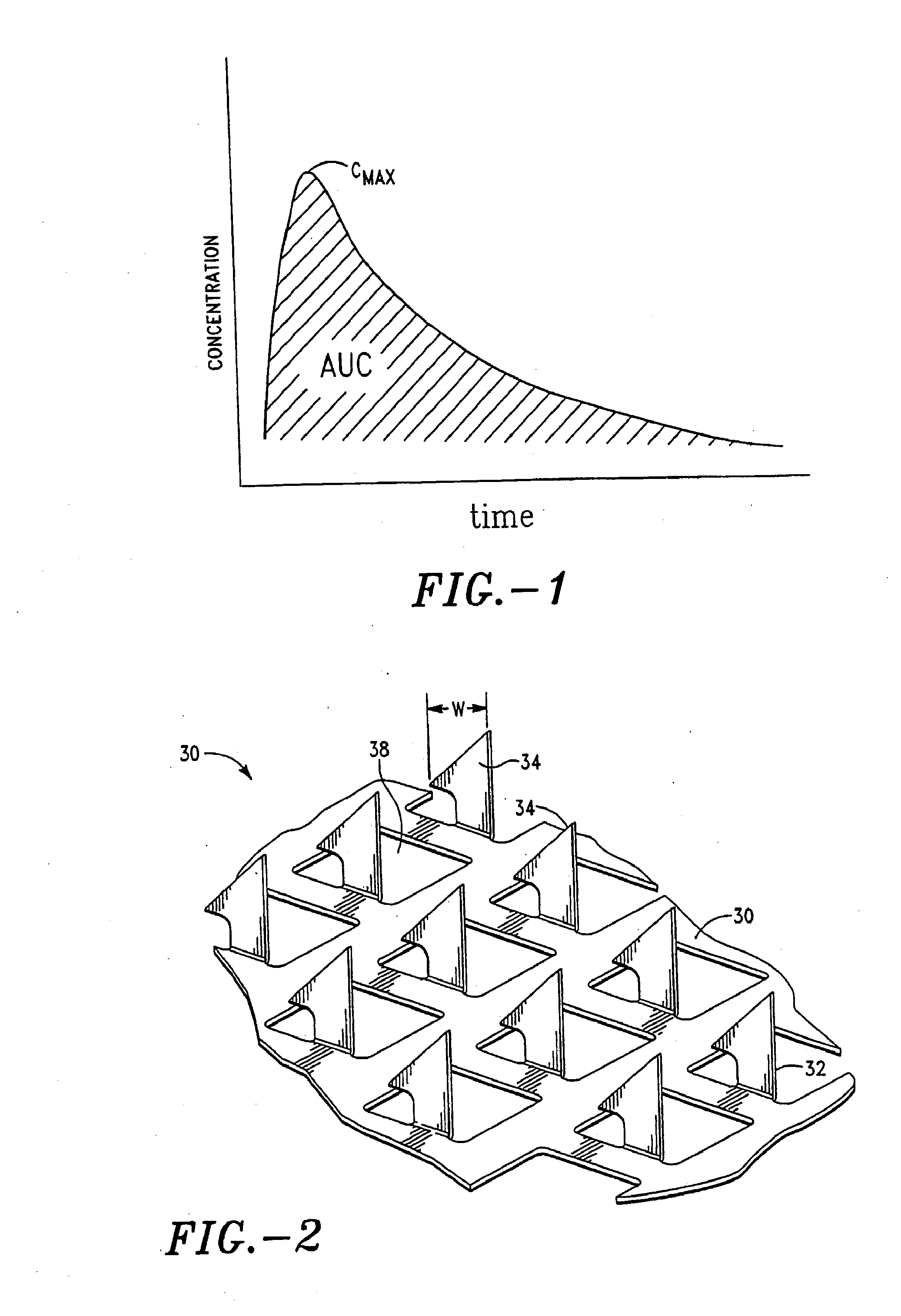
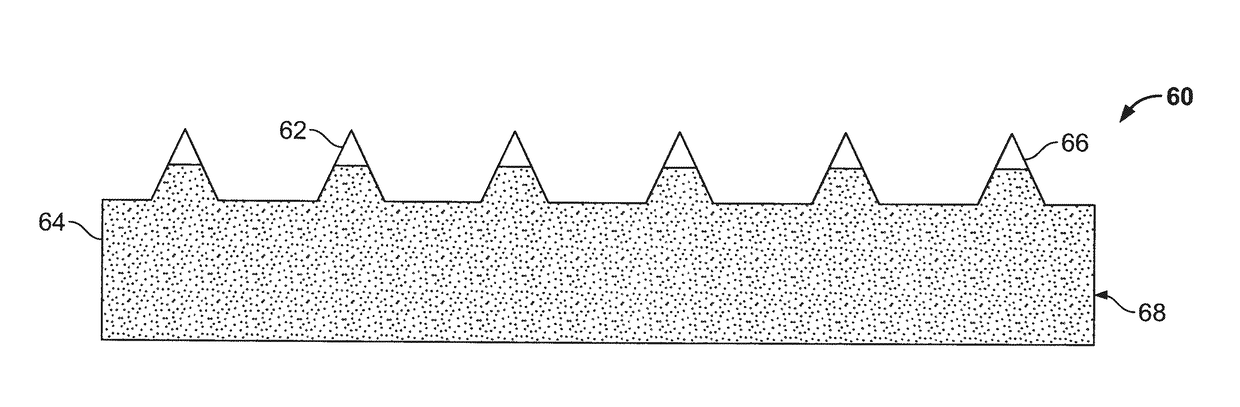
Apparatus and method for transdermal delivery of parathyroid hormone agents
ActiveUS20050256045A1Minimize and eliminate bleedingMinimize and eliminate and irritationPeptide/protein ingredientsPharmaceutical delivery mechanismActive agentBiocompatible coating
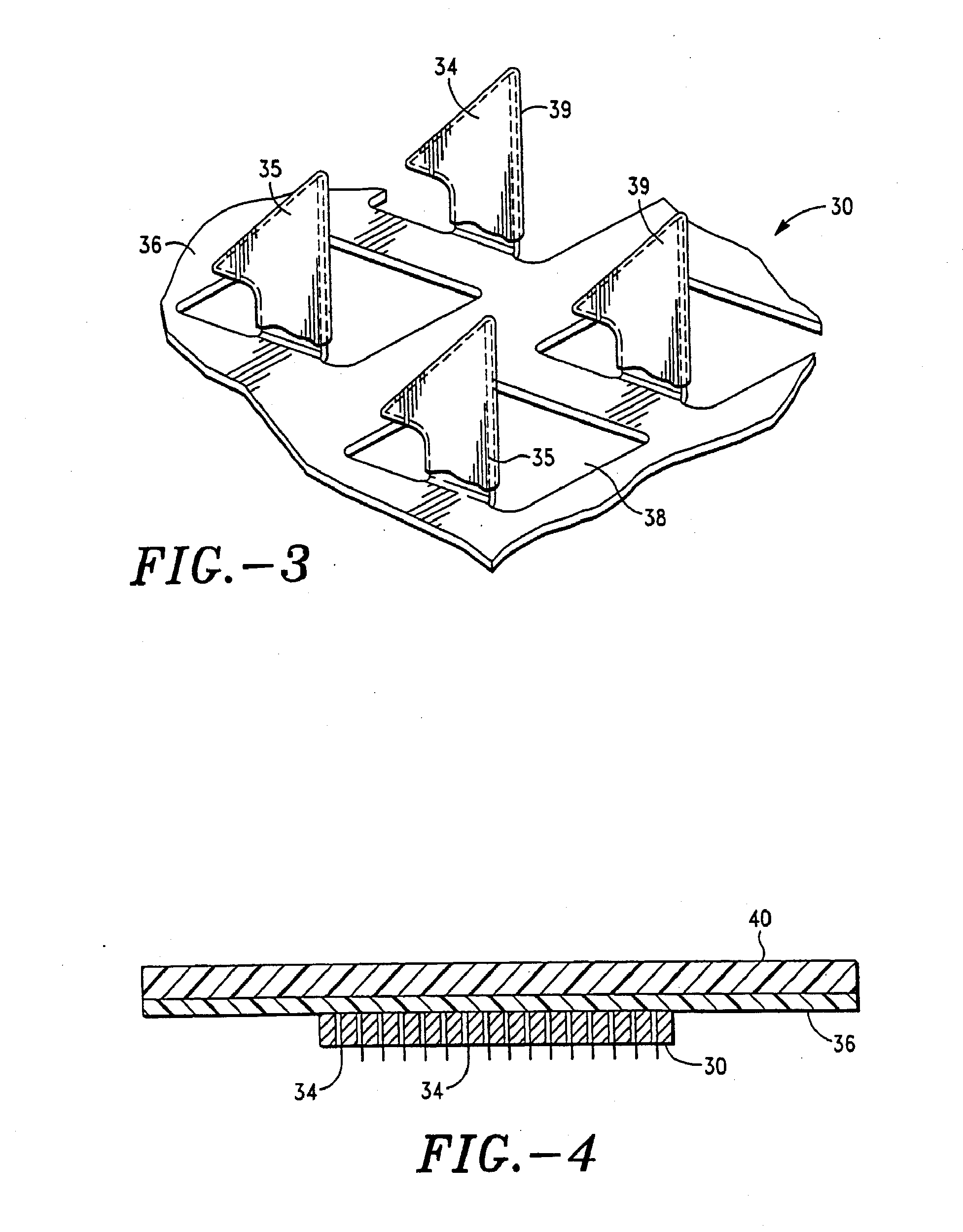
An apparatus and method for transdermally delivering a biologically active agent comprising a delivery system having a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers. In one embodiment, the PTH-based agent is contained in a biocompatible coating that is applied to the microprojection member.
Owner:ALZA CORP
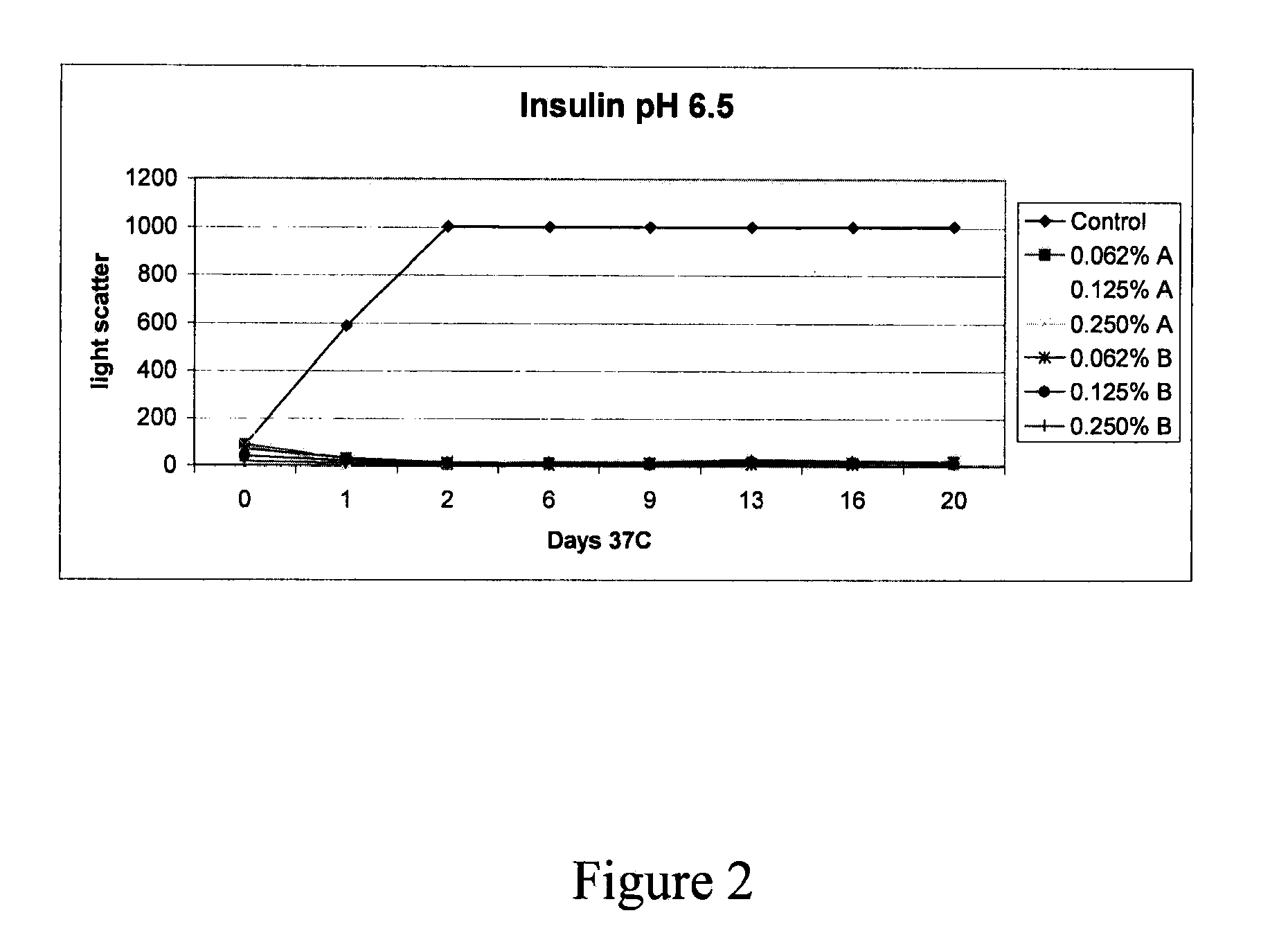
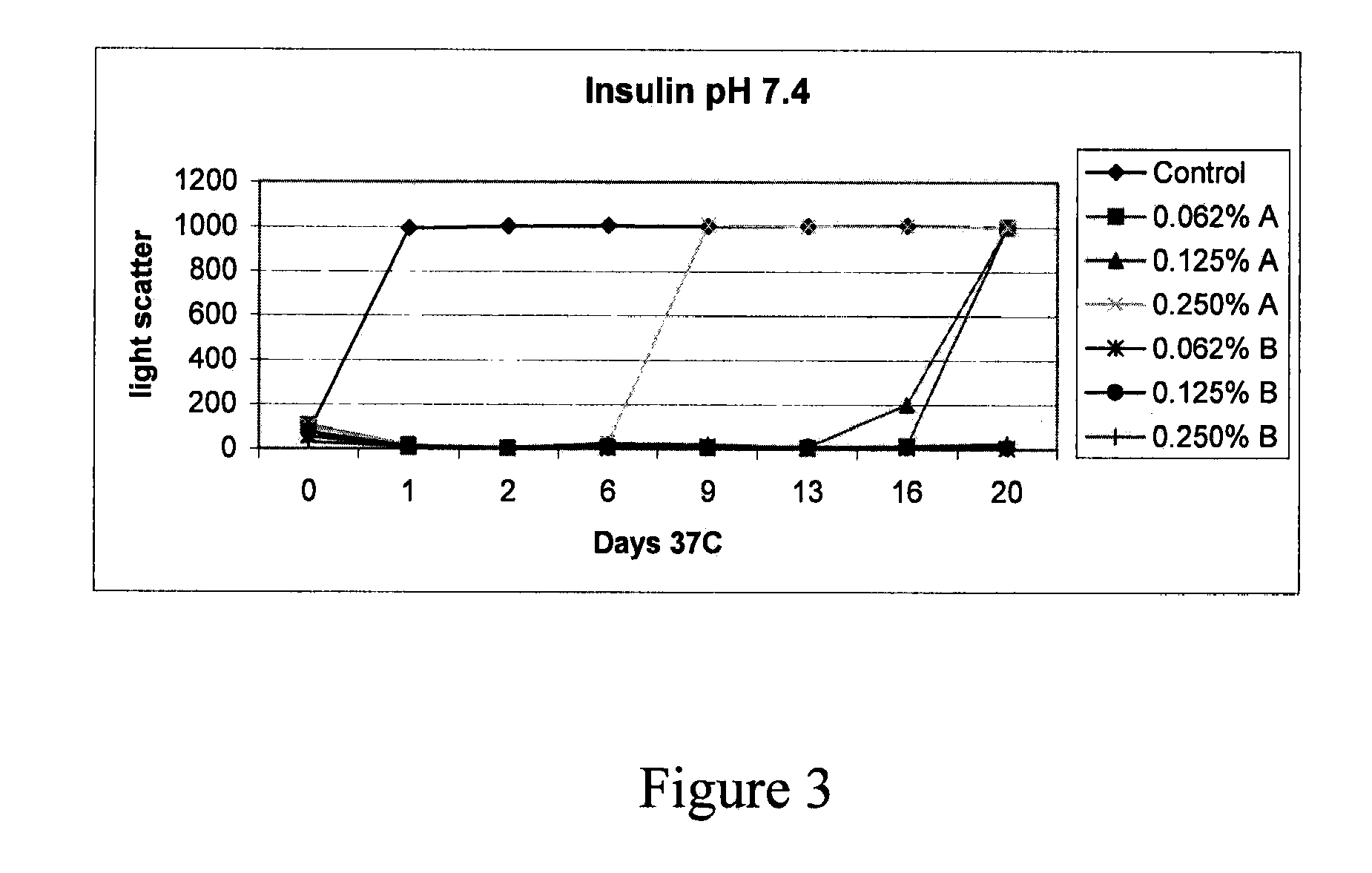
Stabilizing alkylglycoside compositions and methods thereof
InactiveUS20090326193A1Convenient treatmentReduce aggregationHormone peptidesCarbohydrate active ingredientsGlycosideMedicine
The present invention relates to alkylglycoside-containing compositions and methods for preventing loss of a parathyroid hormone (PTH) analog or octreotide via denaturation due to adherence upon contact with glass.
Owner:ZELOS THERAPEUTICS +1
Stable pharmaceutical dosage forms of teriparatide
InactiveUS20060189533A1Low viscosityReduce adhesionPeptide/protein ingredientsPharmaceutical delivery mechanismTeriparatideBioavailability
A parathyroid hormone (1-34) (PTH) dosage form is described that is suitable for multi-use administration. A dosage form of parathyroid hormone (1-34) (PTH) comprising an aqueous pharmaceutical formulation for aerosolized intranasal delivery of PTH having a bioavailability of about 5% or greater, wherein the formulation comprises a therapeutically effective amount of PTH and polysorbate, and wherein least 90% of the PTH can be recovered after storage for 24 weeks at 5° C.
Owner:NASTECH PHARMA
Apparatus and method for transdermal delivery of parathyroid hormone agents
ActiveUS7556821B2Good biocompatibilityPeptide/protein ingredientsSkeletal disorderBiocompatible coatingActive agent
An apparatus and method for transdermally delivering a biologically active agent comprising a delivery system having a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers. In one embodiment, the PTH-based agent is contained in a biocompatible coating that is applied to the microprojection member.
Owner:ALZA CORP
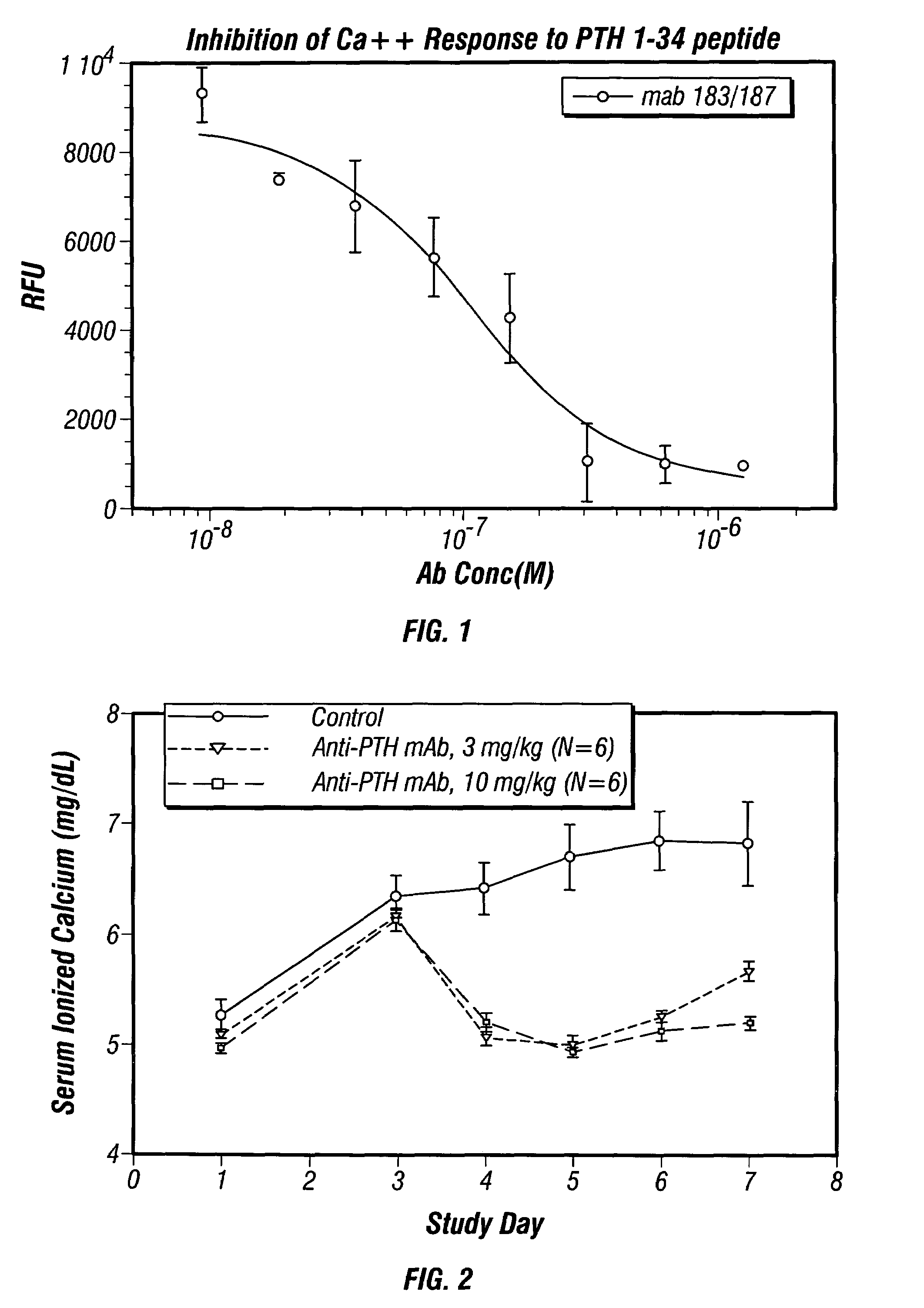
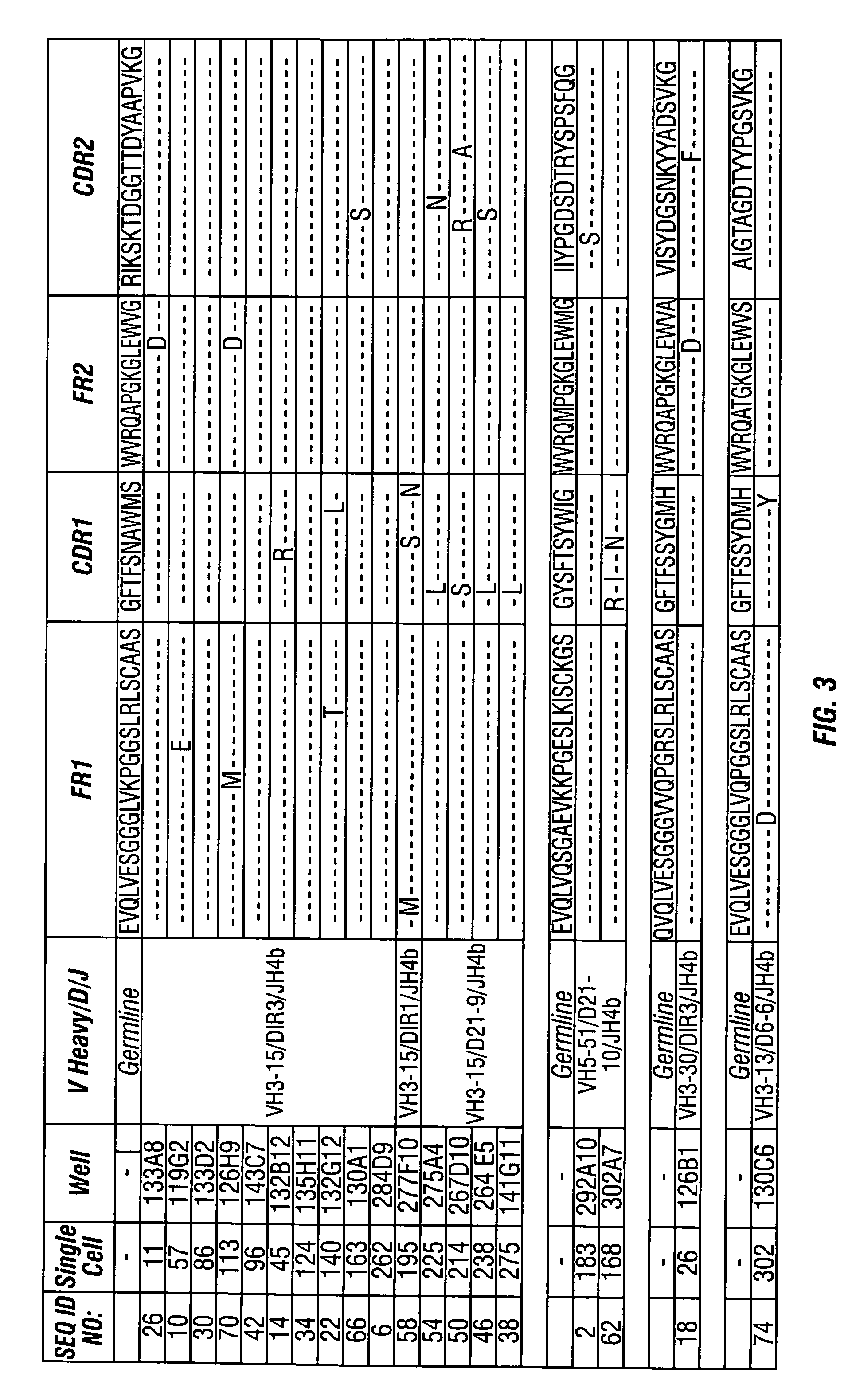
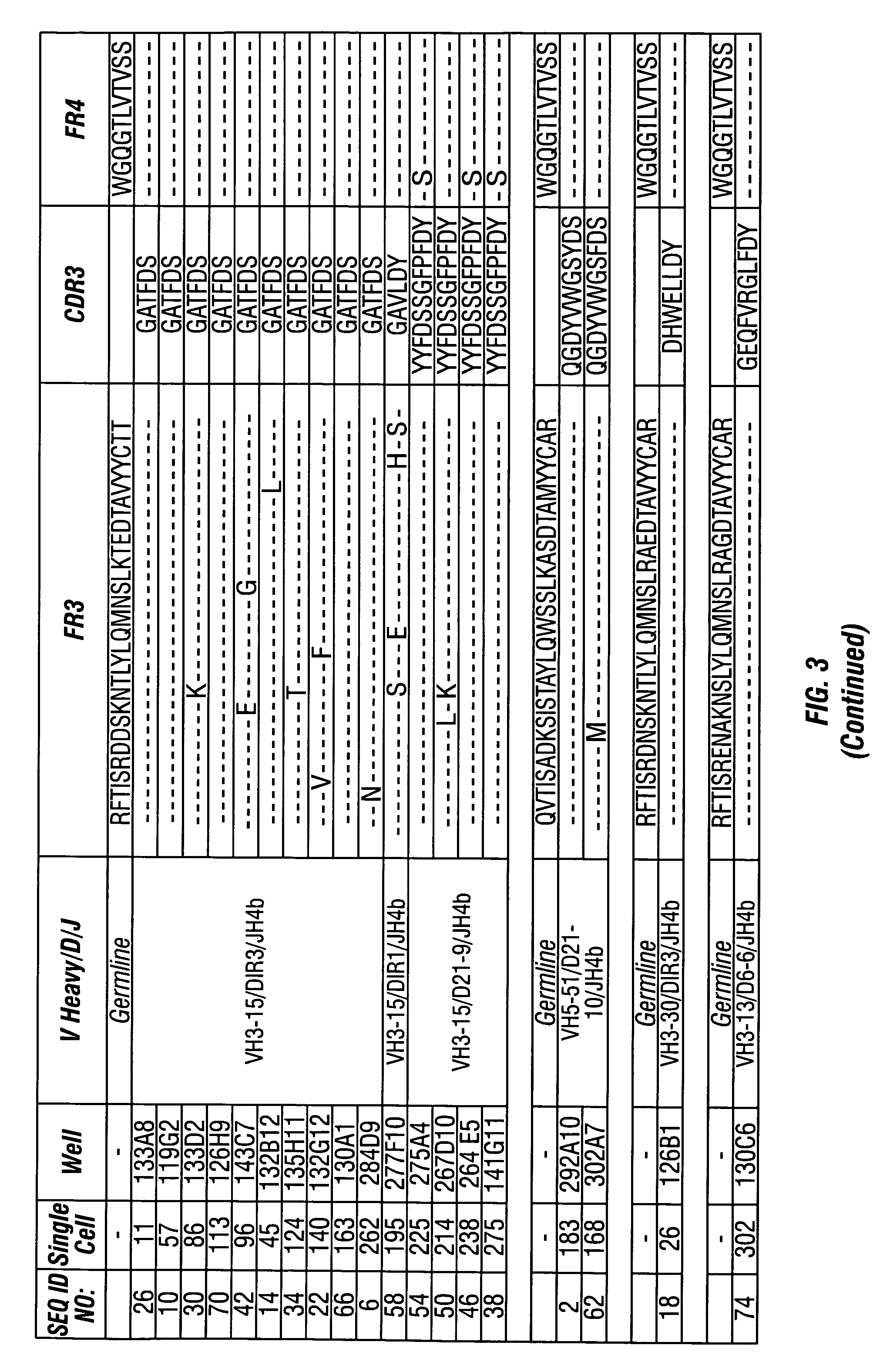
Methods of use for antibodies against parathyroid hormone
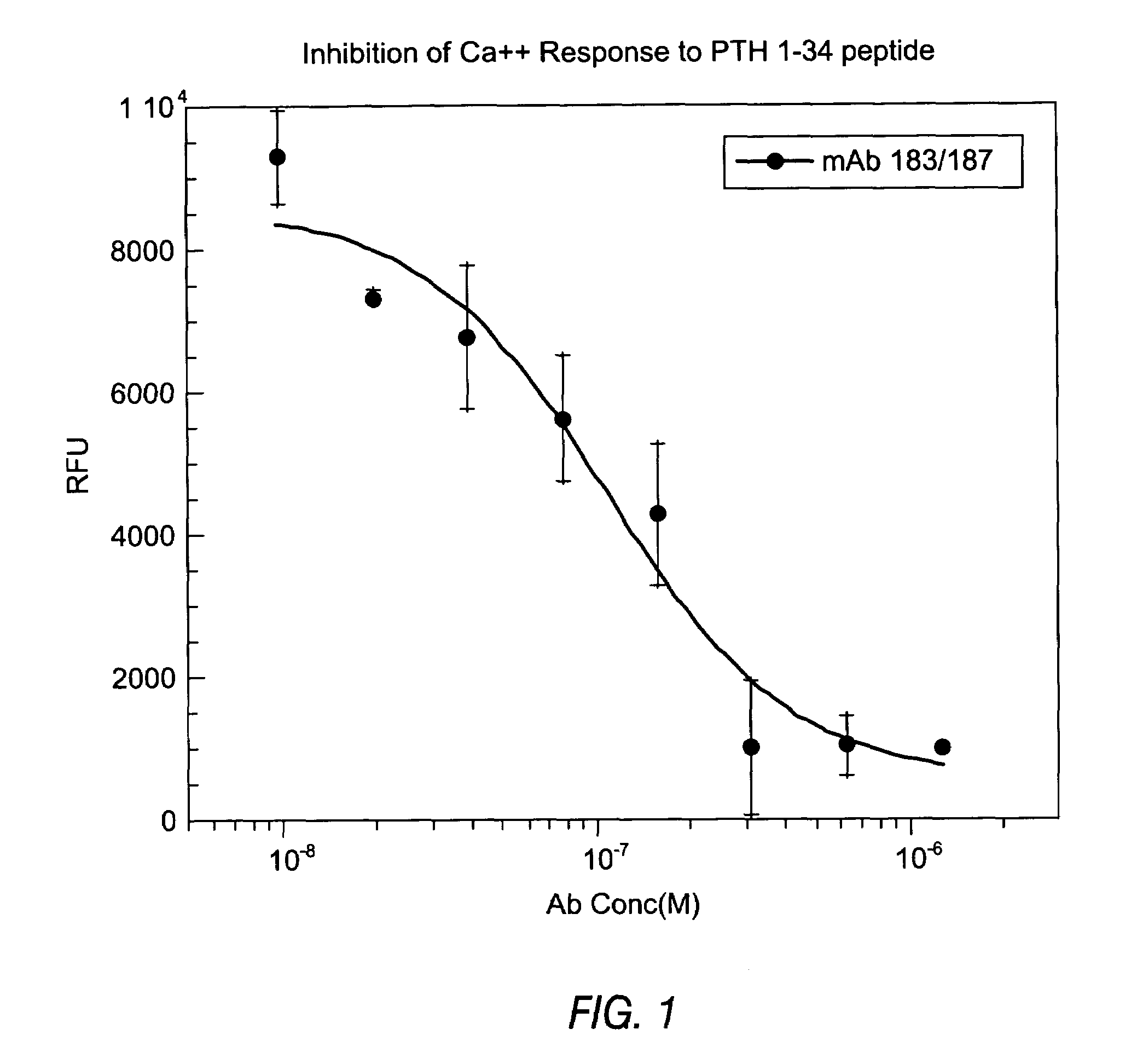
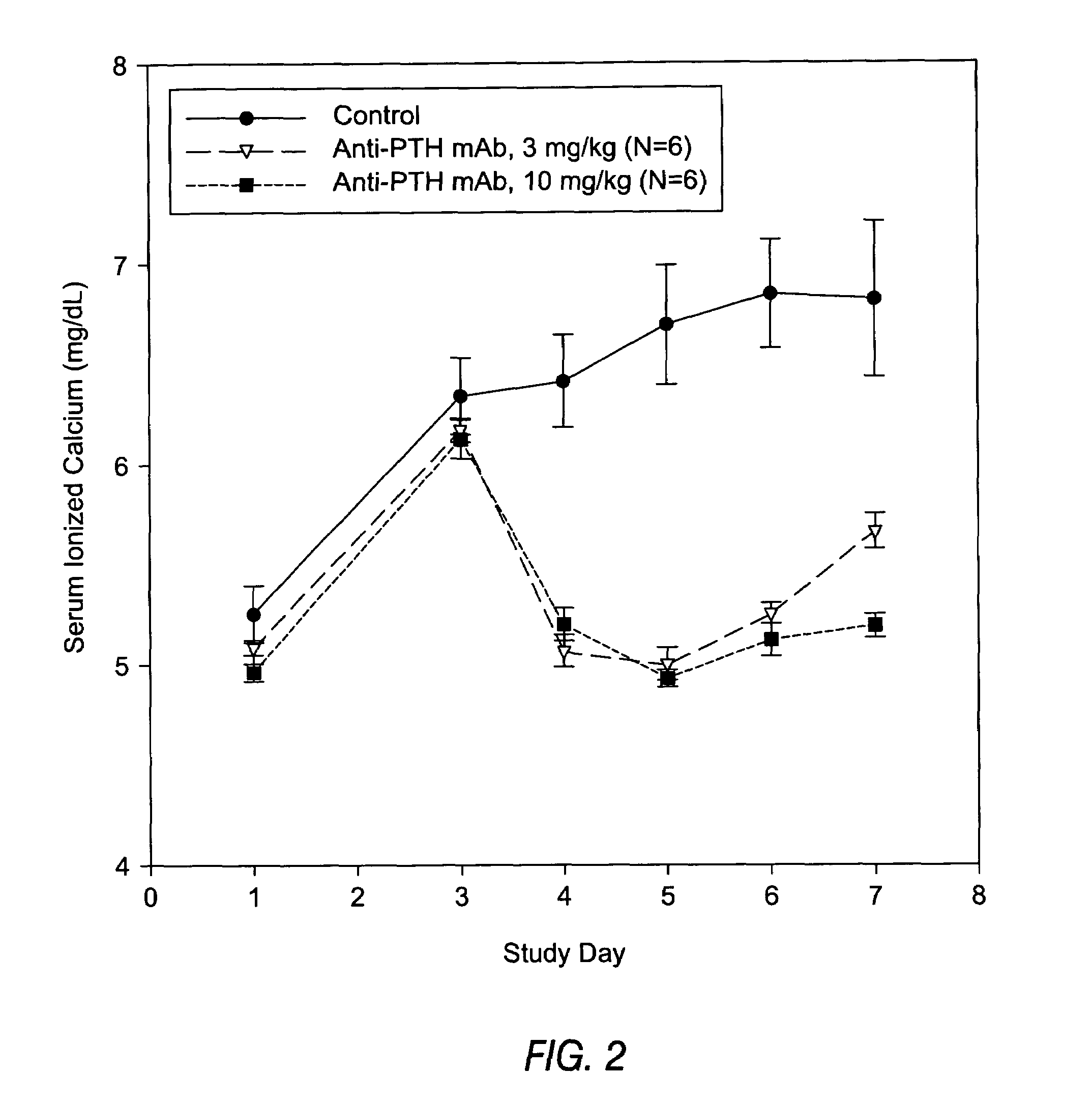
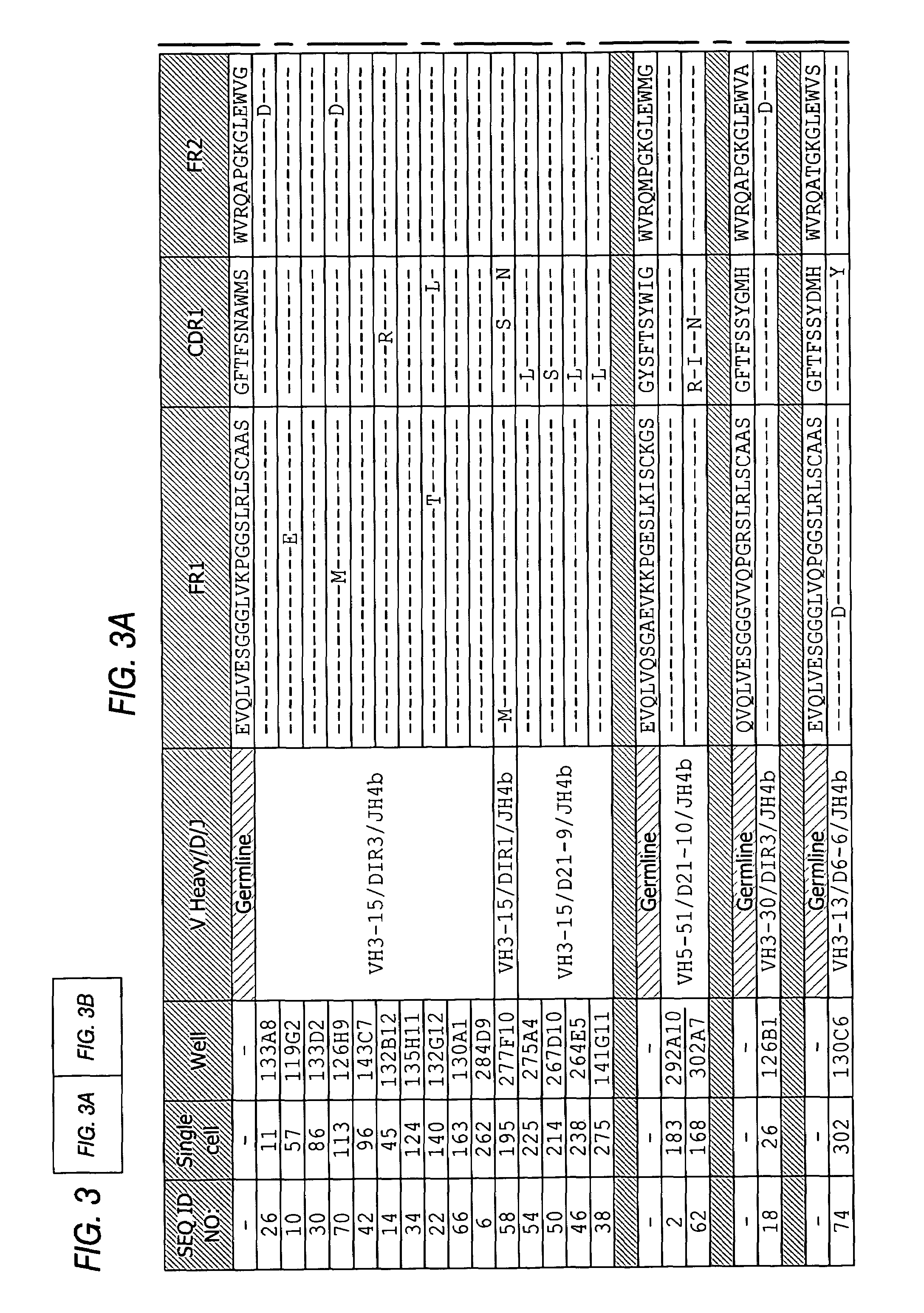
InactiveUS7318925B2Easy to neutralizeHigh affinityAntibody ingredientsImmunoglobulins against hormonesAntigenComplementarity determining region
Embodiments of the invention described herein relate to antibodies directed to the antigen parathyroid hormone (PTH) and uses of such antibodies. In particular, in some embodiments, there are provided fully human monoclonal antibodies directed to the antigen PTH. In further embodiments, nucleotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDRs), specifically from FR1 through FR4 or CDR1 through CDR3, are provided.
Owner:ABQENIX INC
Antibodies directed to parathyroid hormone (PTH) and uses thereof
InactiveUS7288253B2Increase the gapReduce clearanceBiocidePeptide/protein ingredientsAntigenComplementarity determining region
Embodiments of the invention described herein relate to antibodies directed to the antigen parathyroid hormone (PTH) and uses of such antibodies. In particular, in some embodiments, there are provided fully human monoclonal antibodies directed to the antigen PTH. In further embodiments, nucleotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDRs), specifically from FR1 through FR4 or CDR1 through CDR3, are provided.
Owner:ABQENIX INC
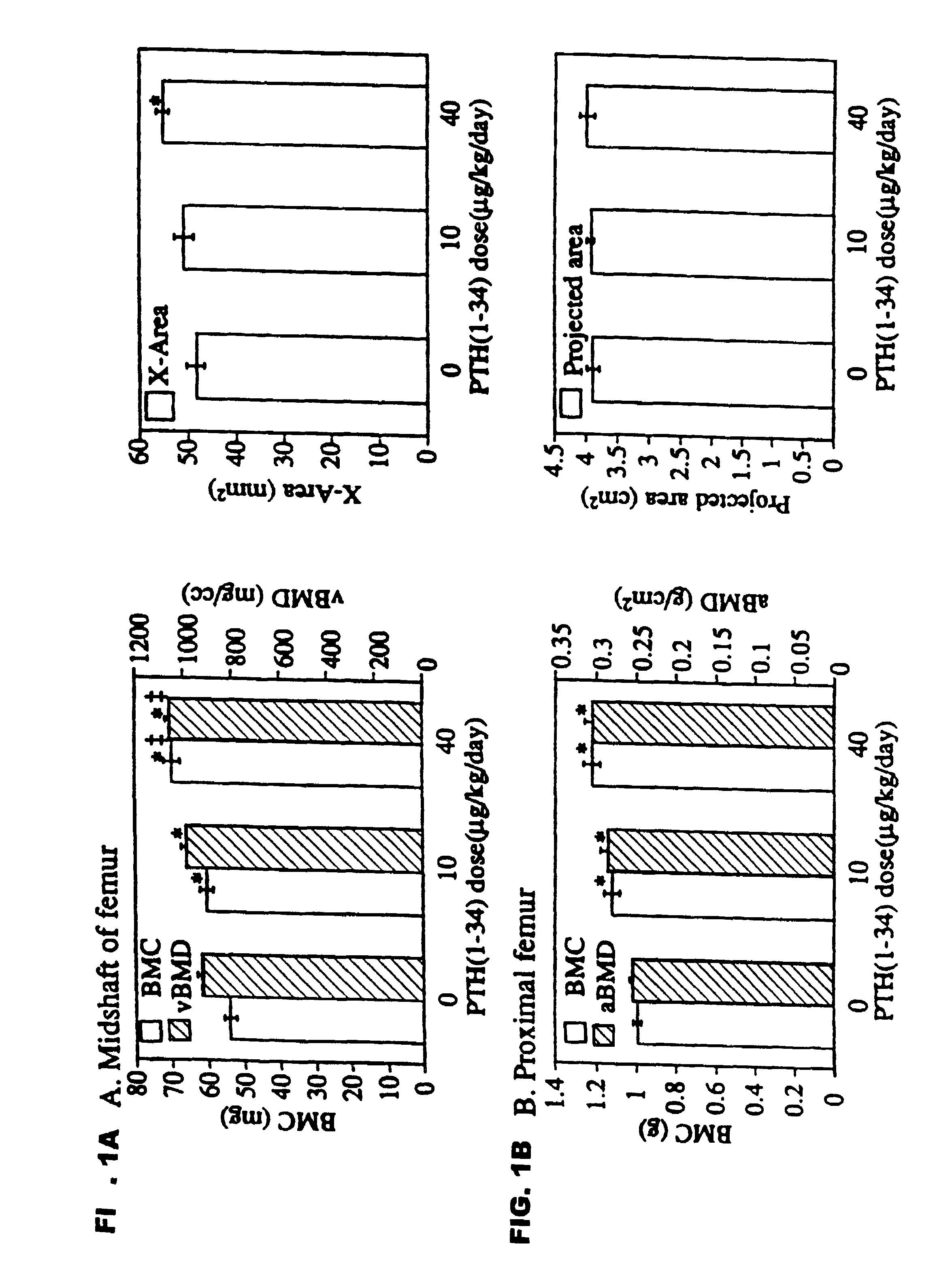
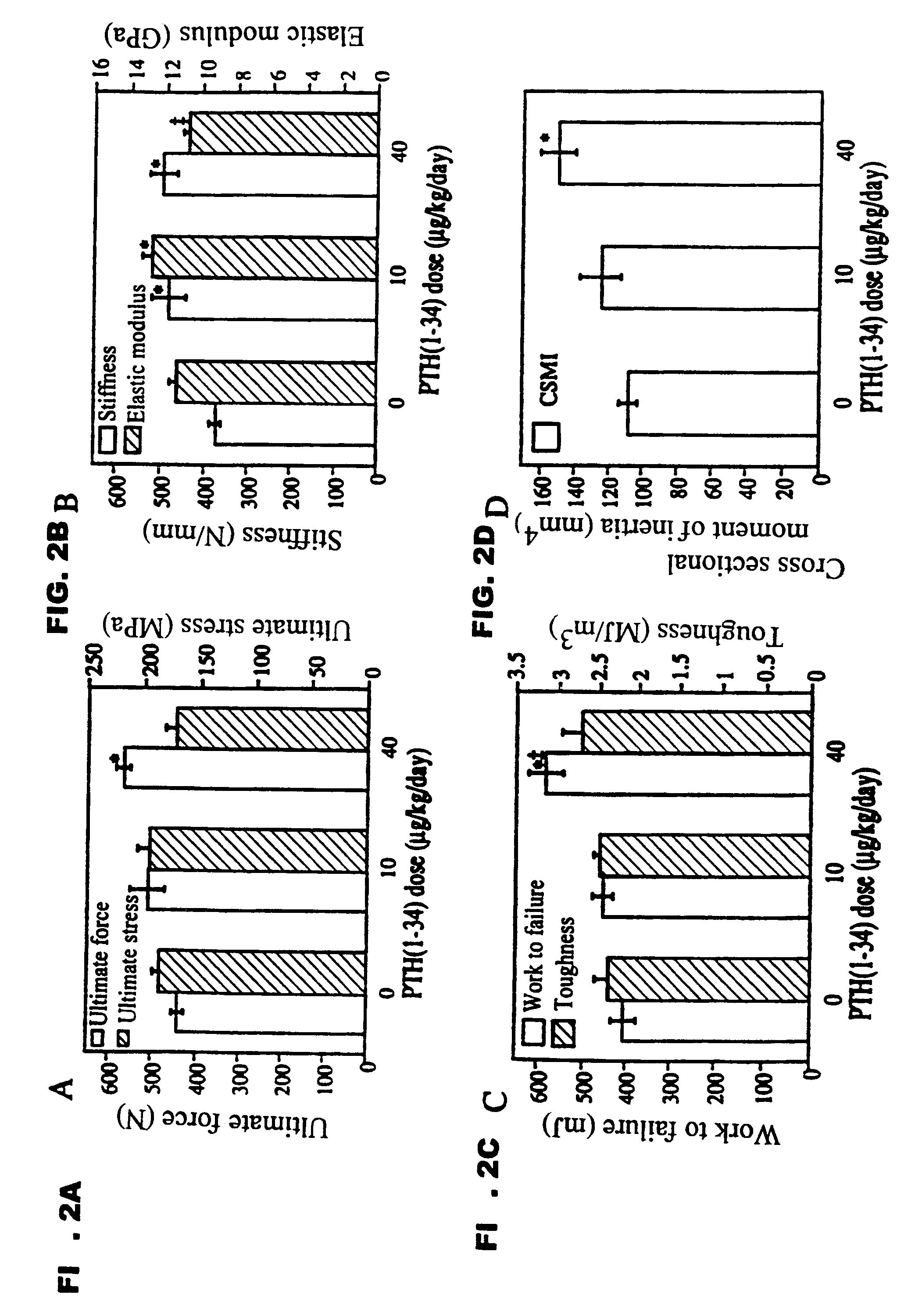
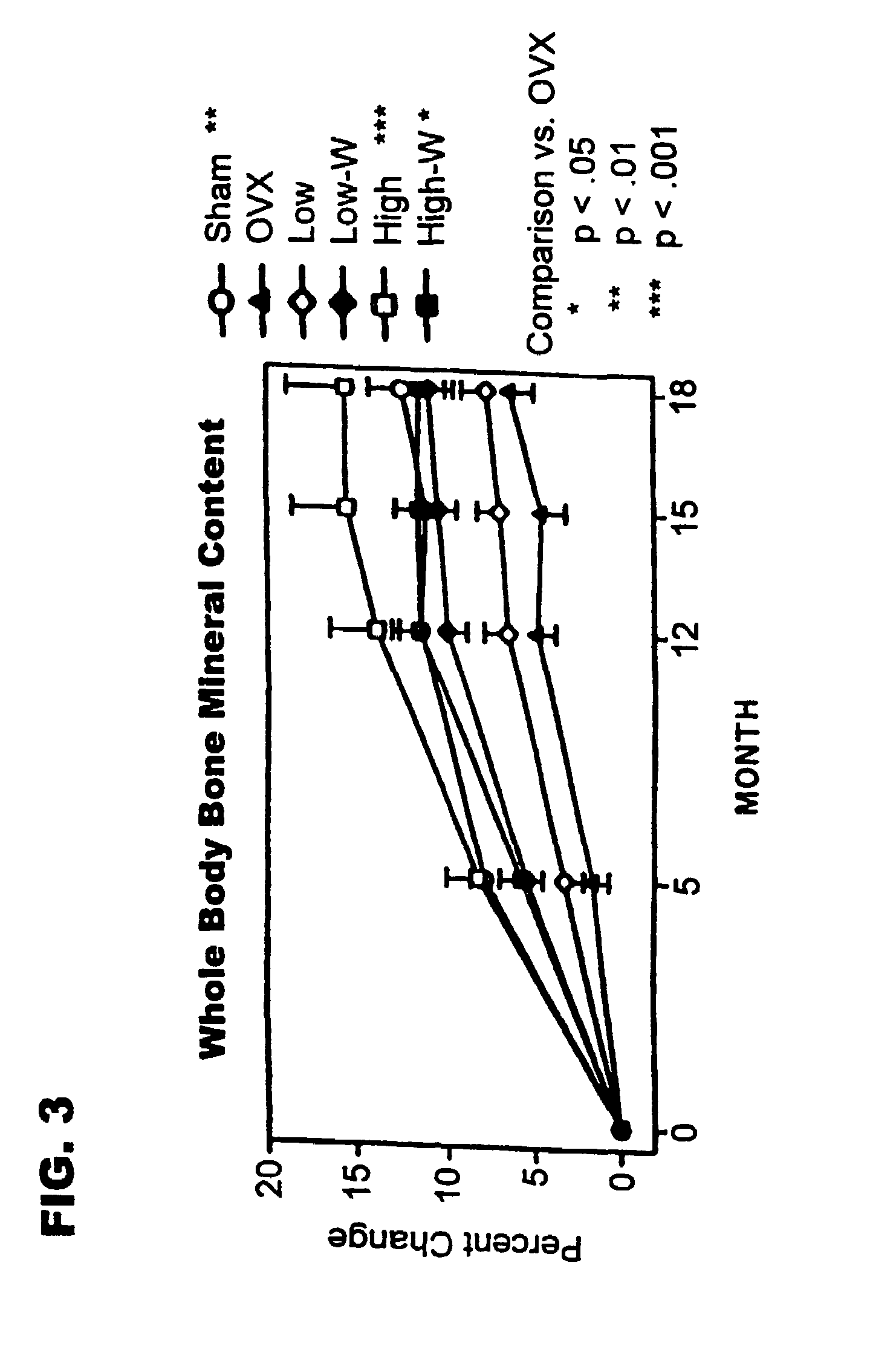
Method of increasing bone toughness and stiffness and reducing fractures
InactiveUS6977077B1Increasing toughness and stiffnessReduced incidence and severityOrganic active ingredientsPeptide/protein ingredientsDysostosisFracture reduction
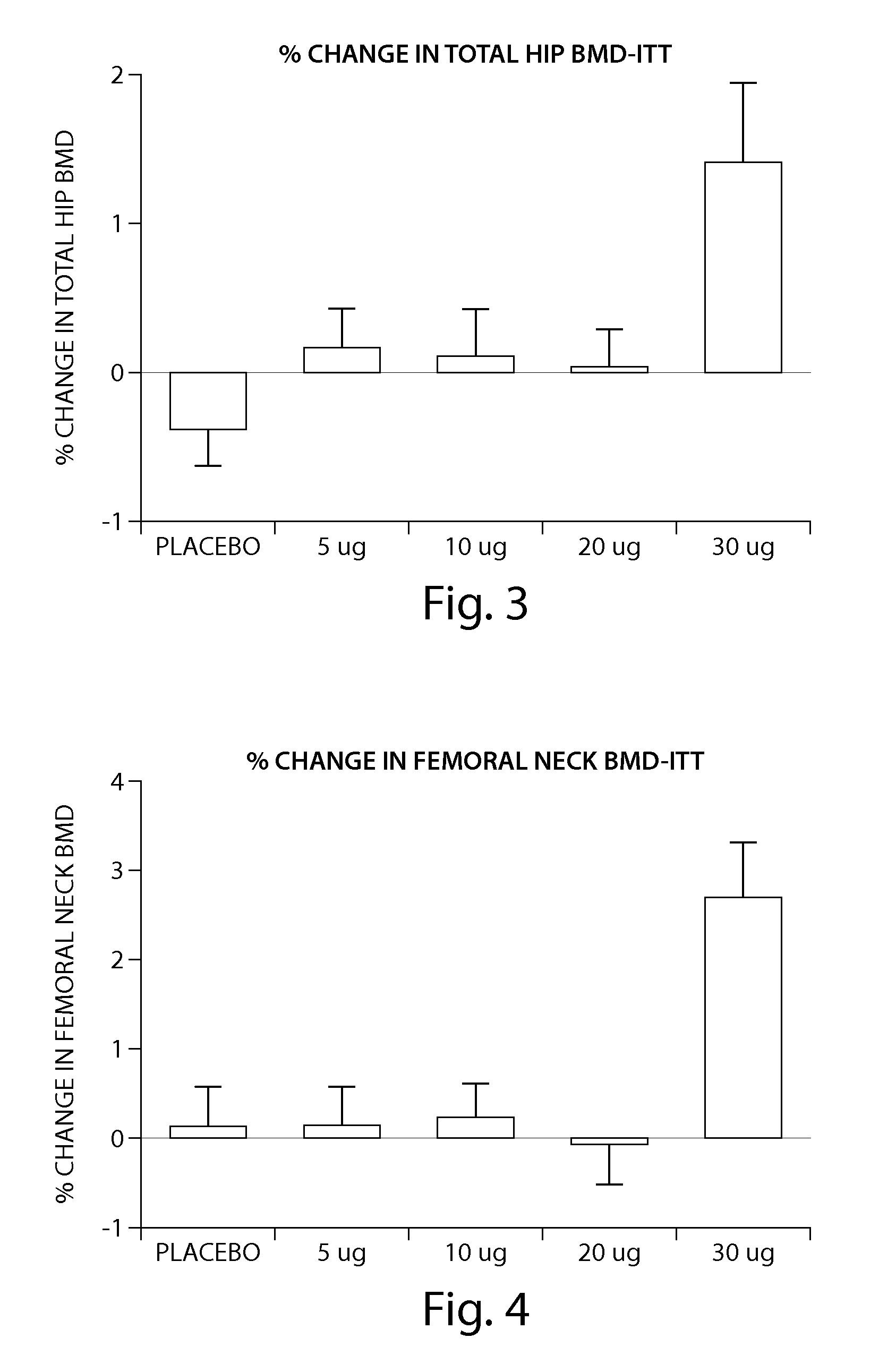
The invention relates to a method for increasing the toughness and / or stiffness of bone and / or reducing the likelihood and / or severity of bone fracture by administering a parathyroid hormone. The method can be employed to increase toughness or stiffness of bone at a site of a potential or actual trauma, such as the hip or spine of a person at risk of or suffering from osteoporosis. The method of the invention can reduce the incidence of vertebral fracure, reduce the incidence of multiple vertebral fractures, reduce the severity of vertebral fracture, and / or reduce the incidence of non-vertebral fracture.
Owner:ELI LILLY & CO
Apparatus and Method for Transdermal Delivery of Parathyroid Hormone Agents to Prevent or Treat Osteopenia
InactiveUS20080039775A1Improve pharmacokineticsImprove bioavailabilityPeptide/protein ingredientsMicroneedlesActive agentBiocompatible coating
An apparatus and method for transdermally delivering a biologically active agent to prevent or treat osteopenia, comprising a delivery system having a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers. In one embodiment, the PTH-based agent is contained in a biocompatible coating that is applied to the microprojection member.
Owner:ALZA CORP
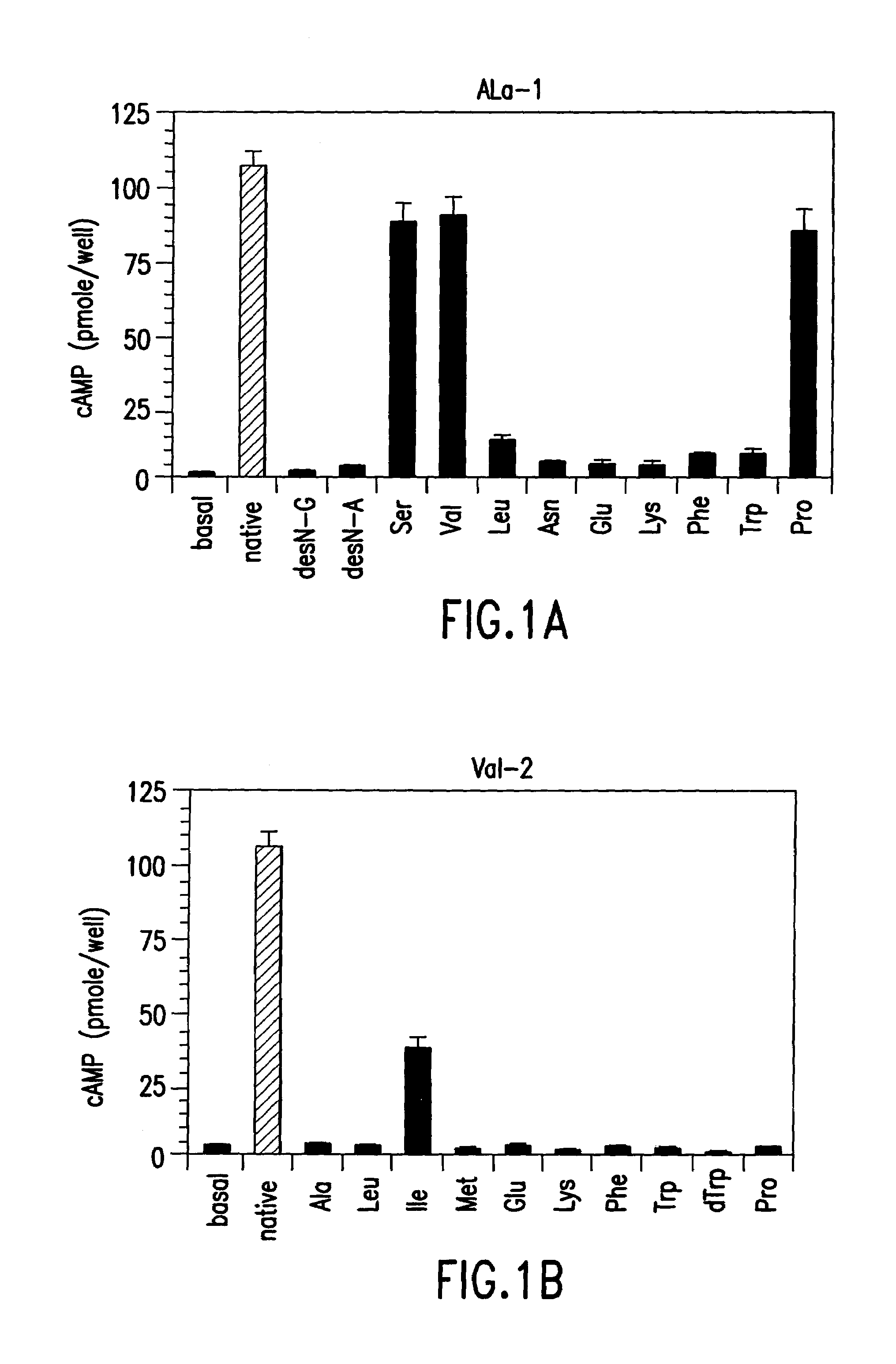
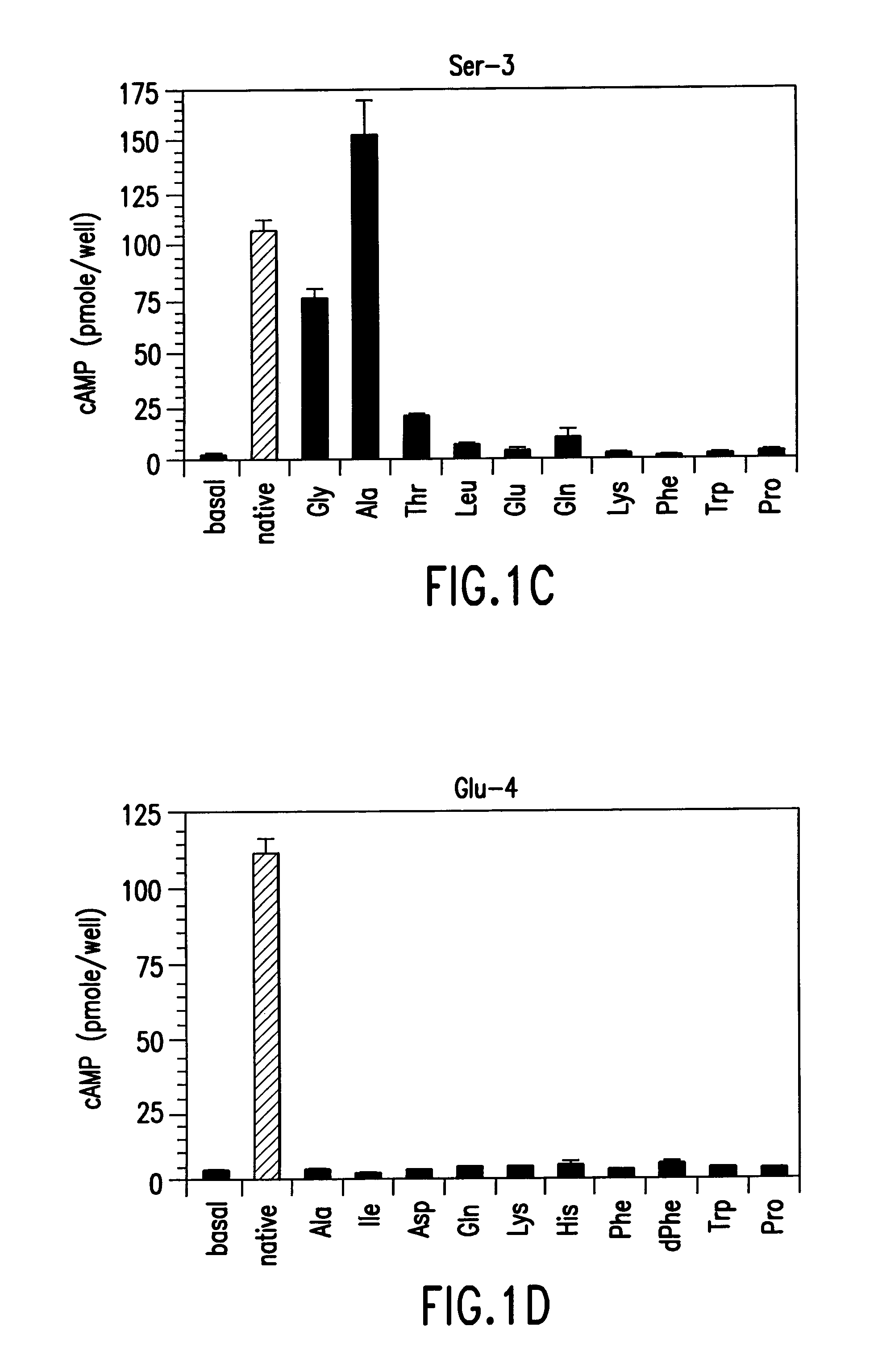
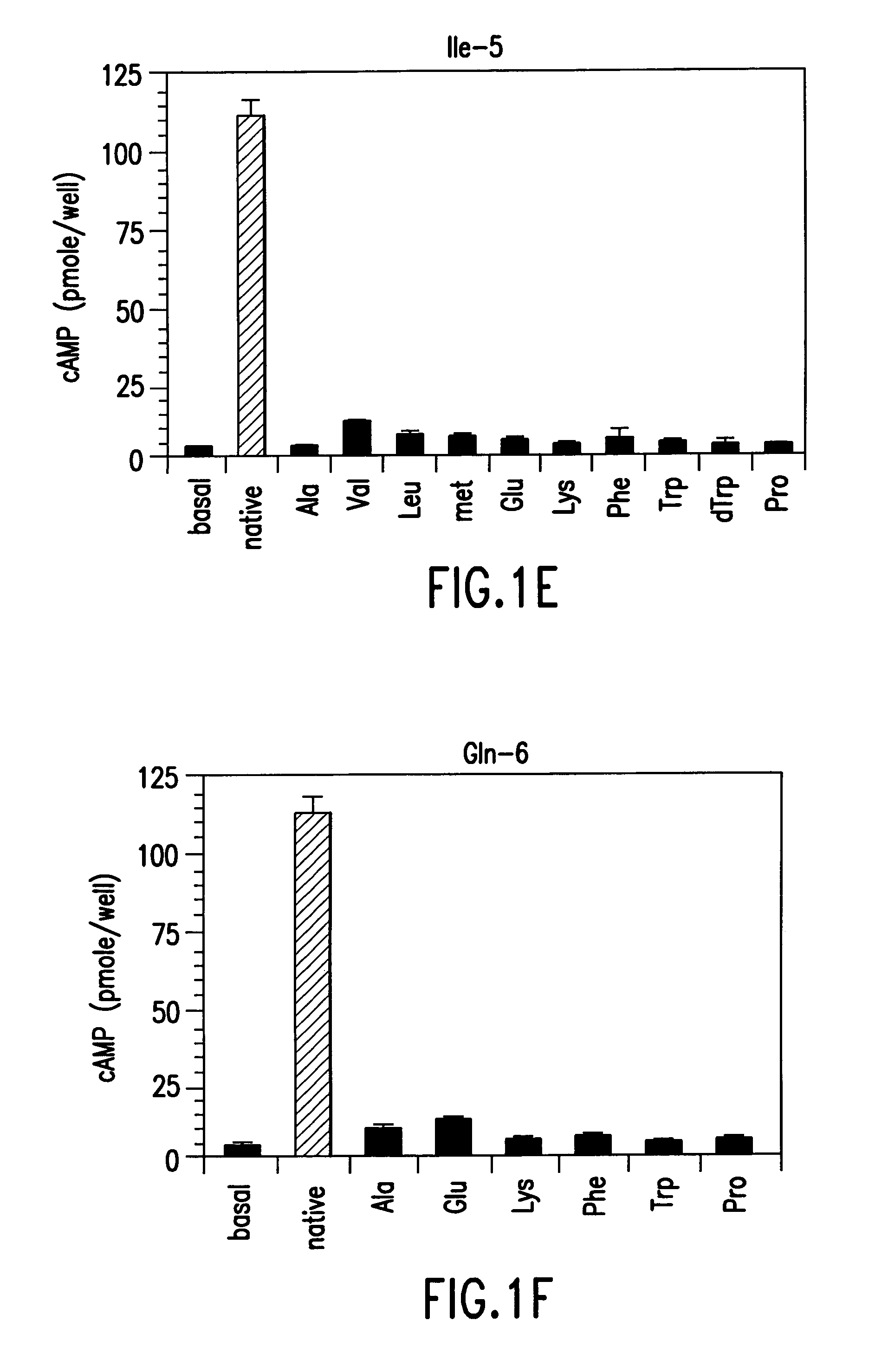
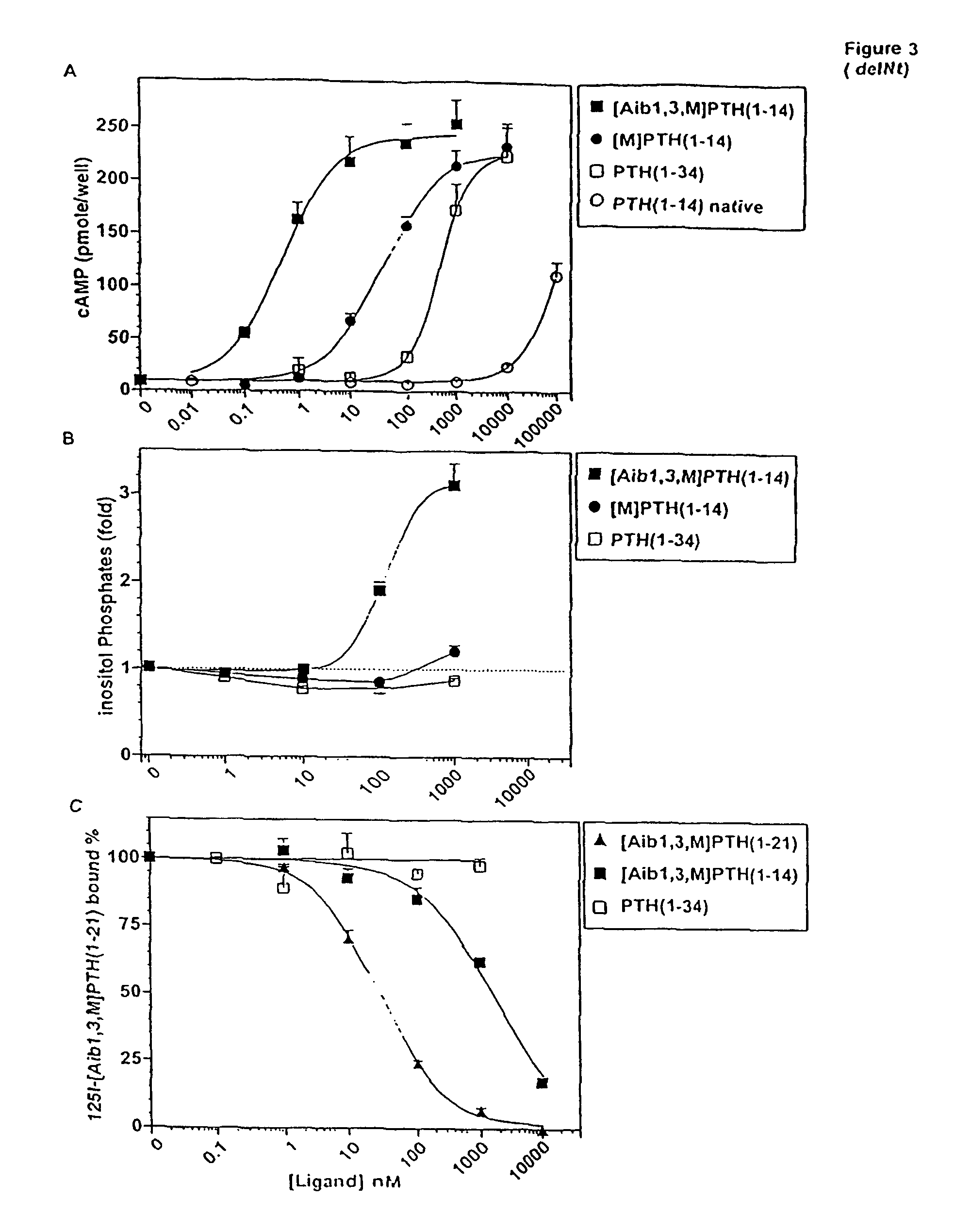
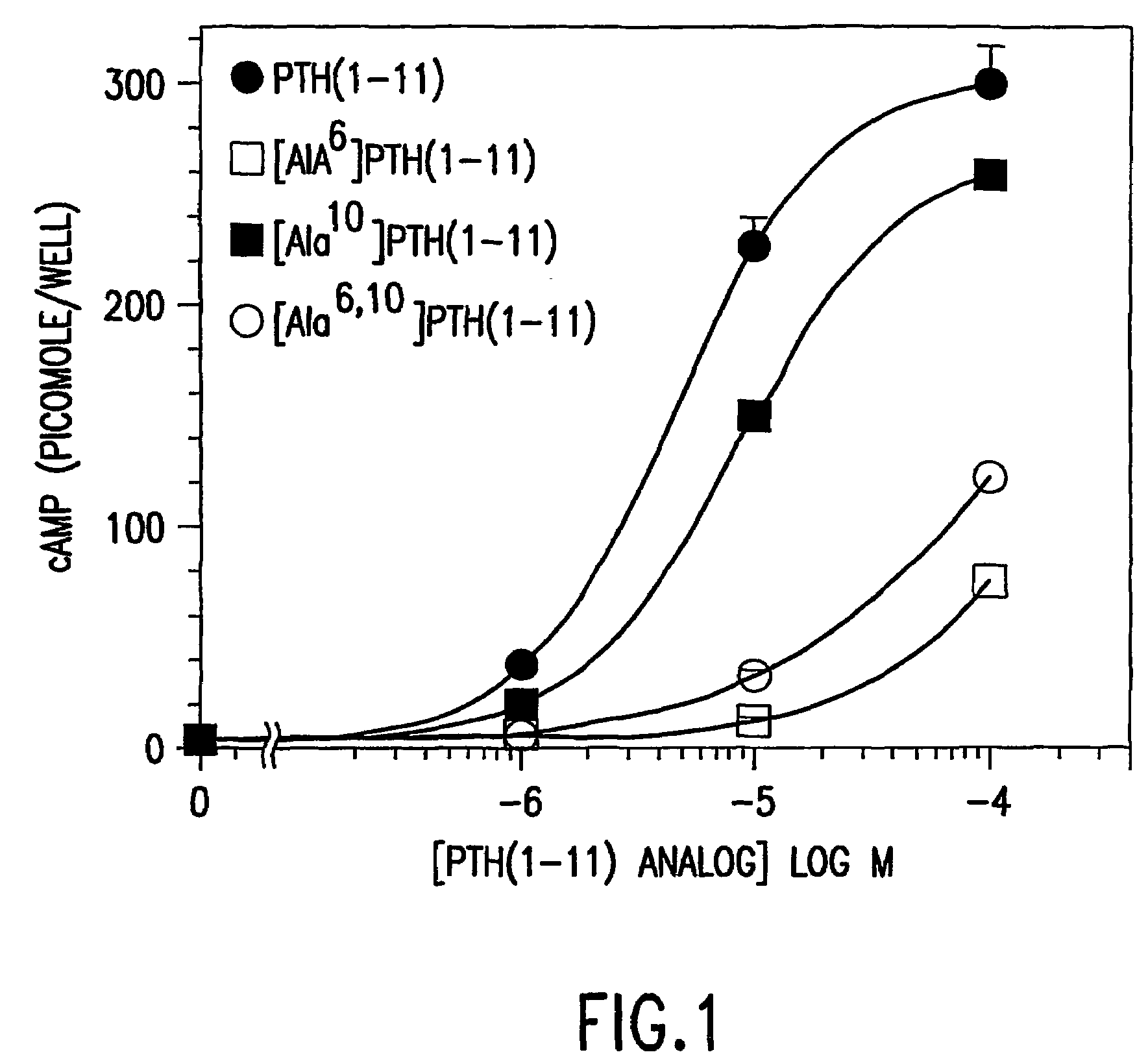
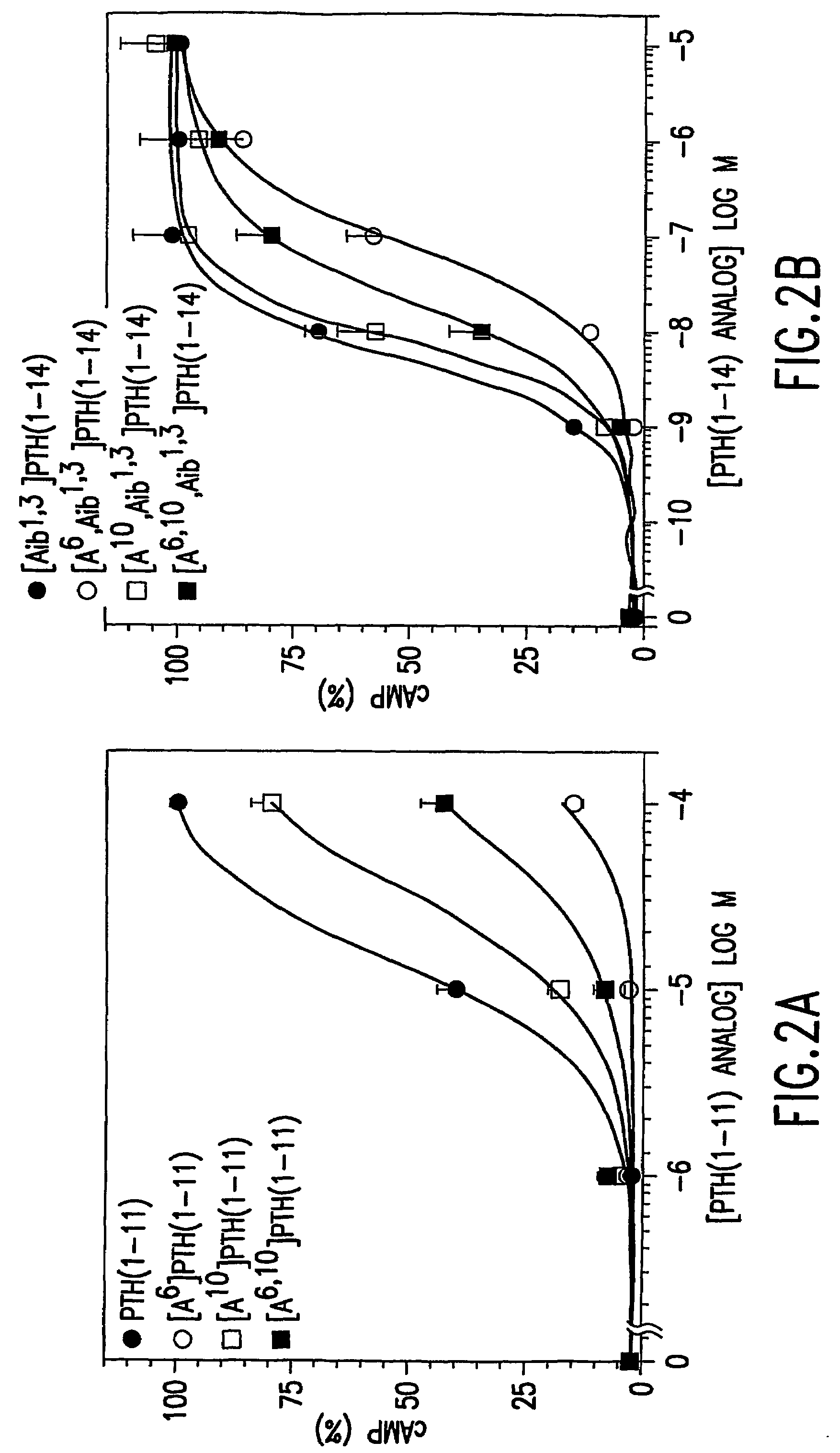
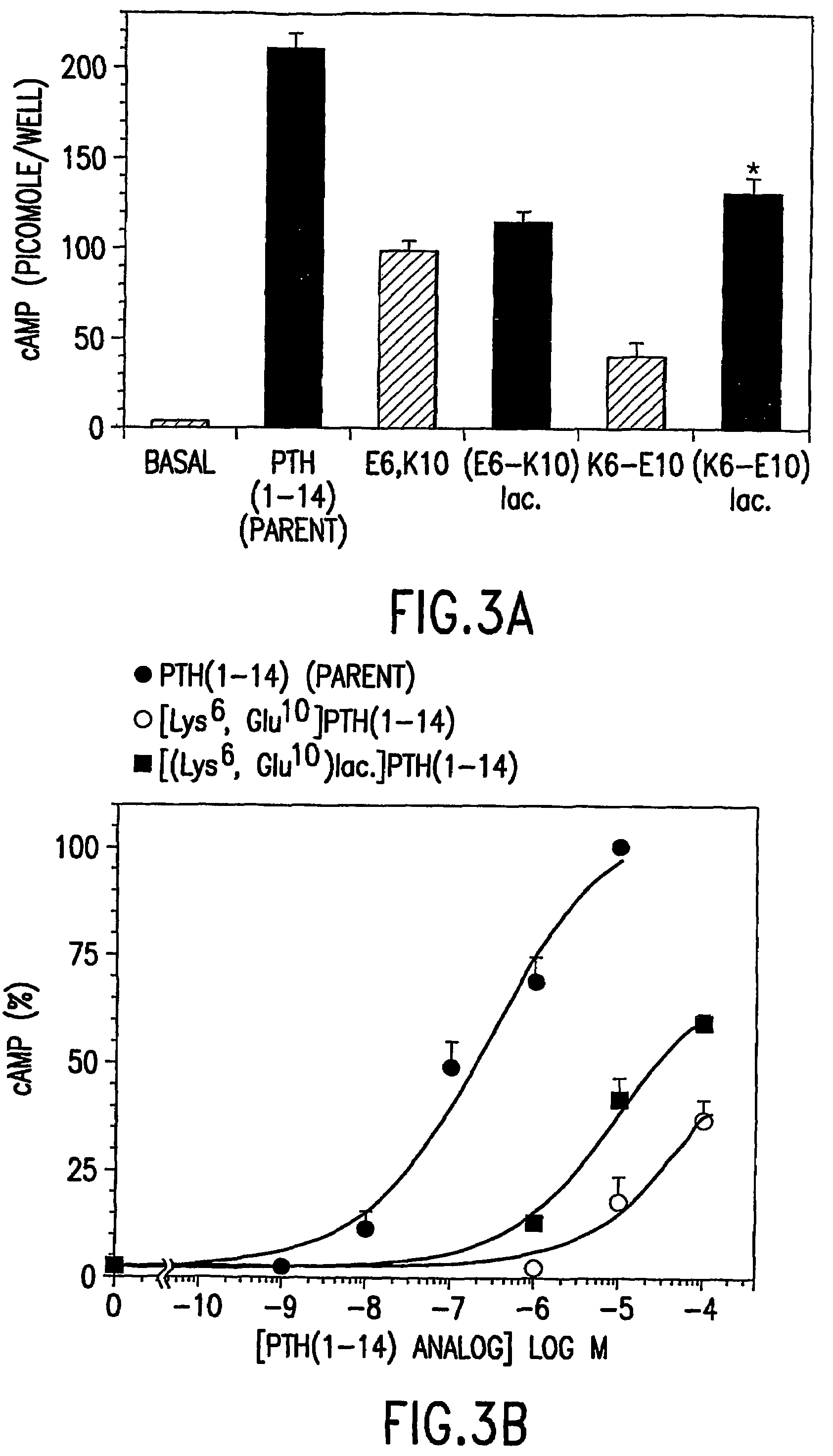
Conformationally constrained parathyroid hormone (pth) analogs
ActiveUS20050026839A1Decrease in bone massQuality improvementIn-vivo radioactive preparationsPeptide/protein ingredientsDiseaseAmino acid substitution
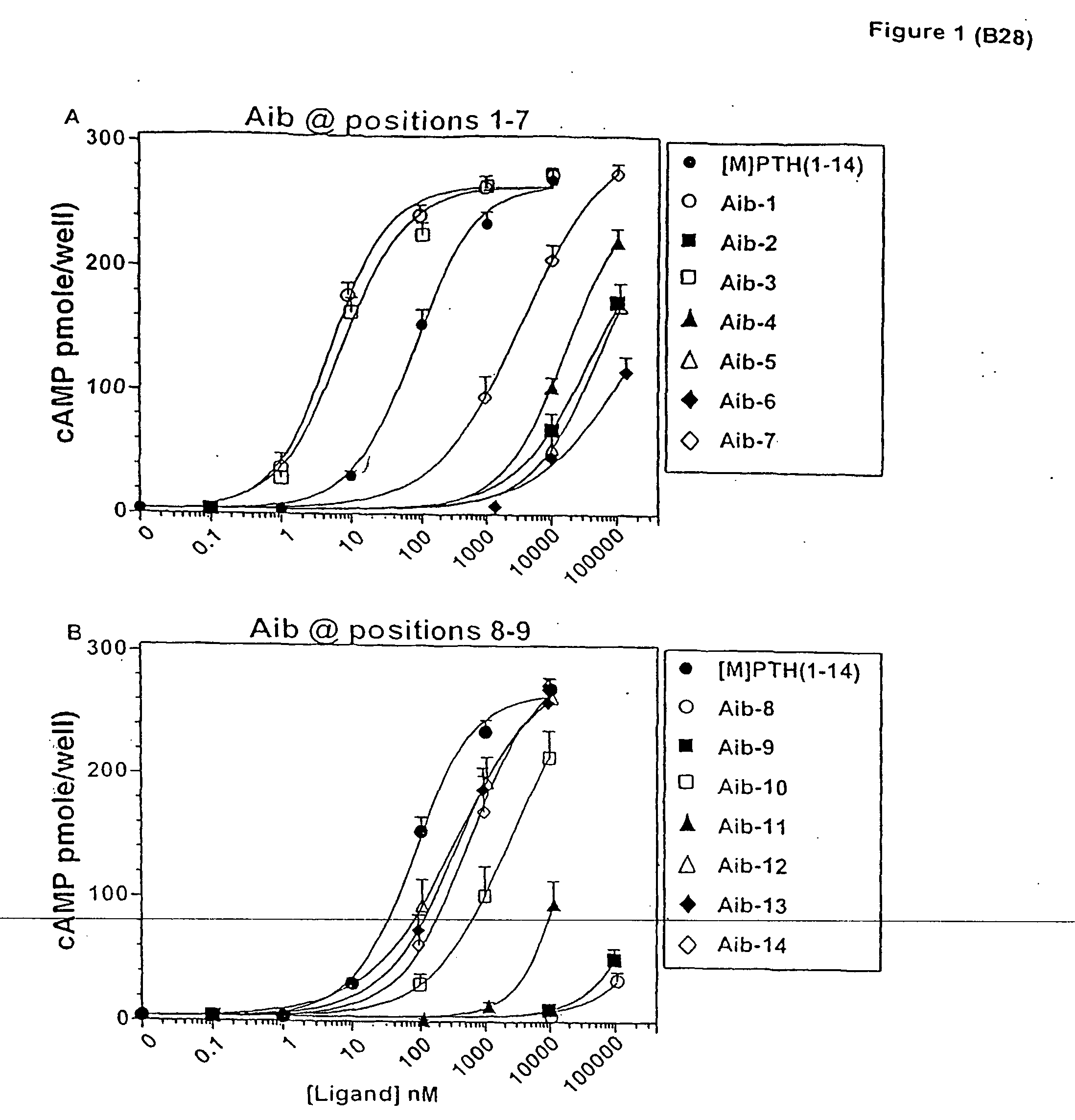
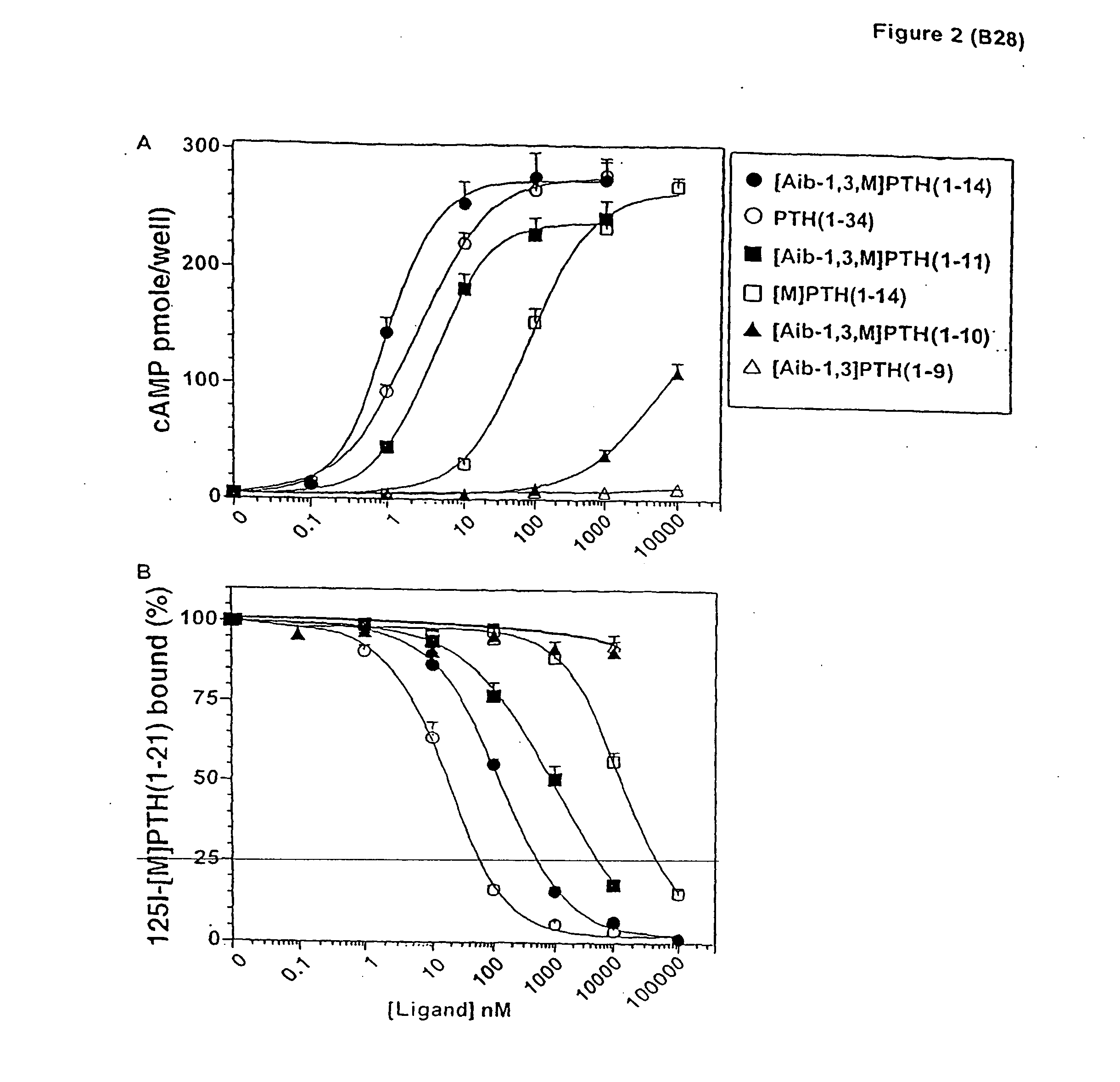
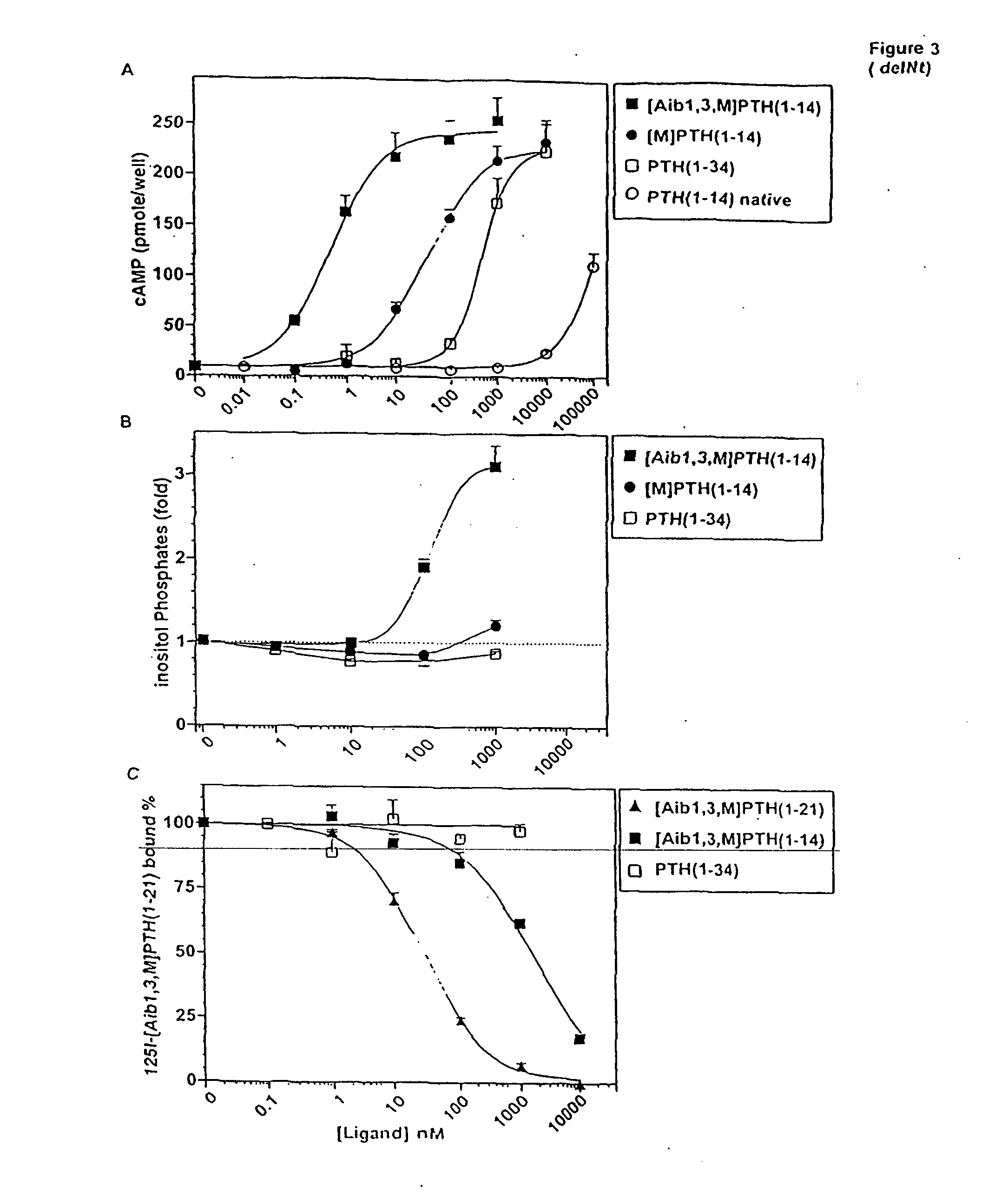
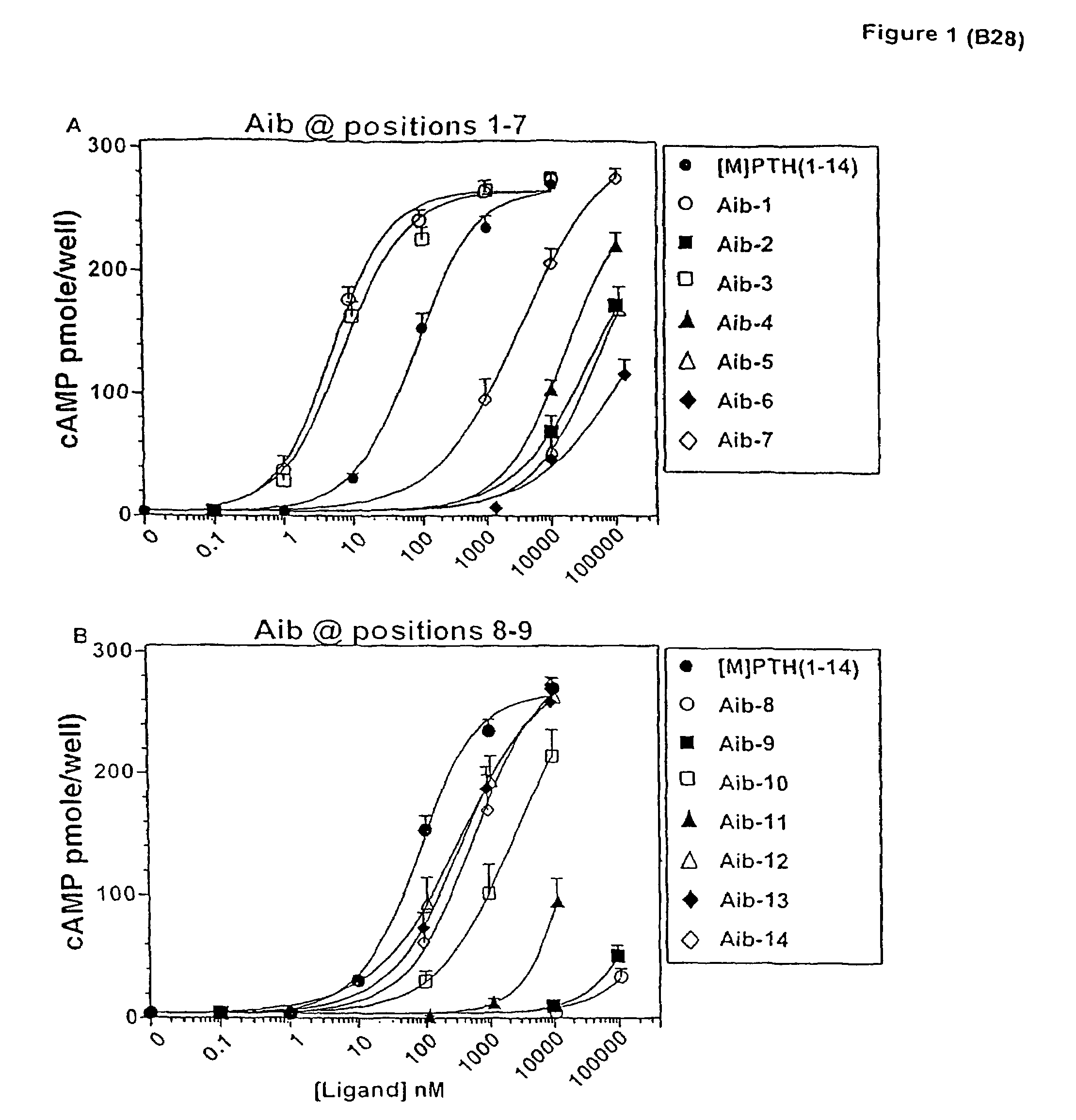
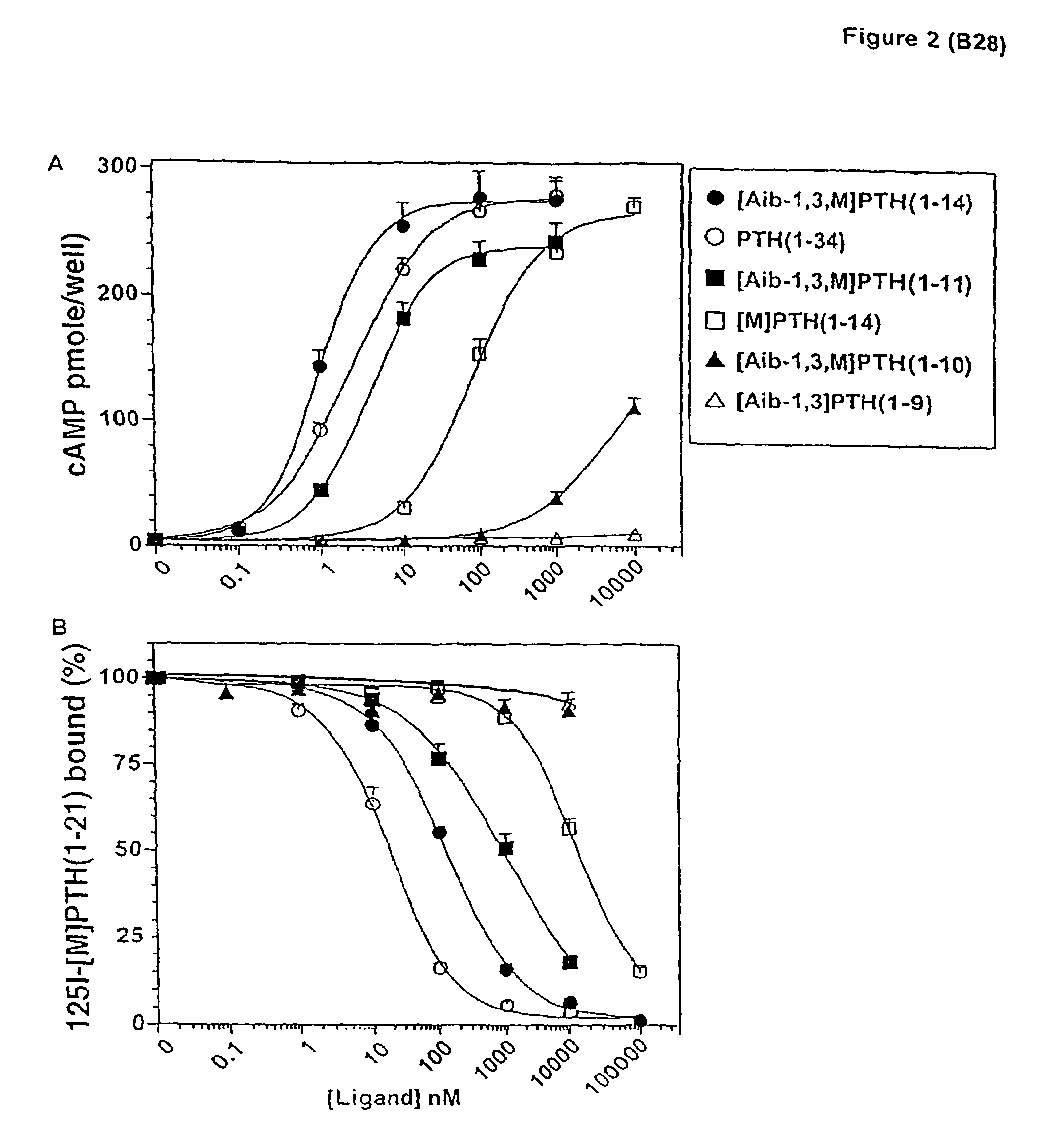
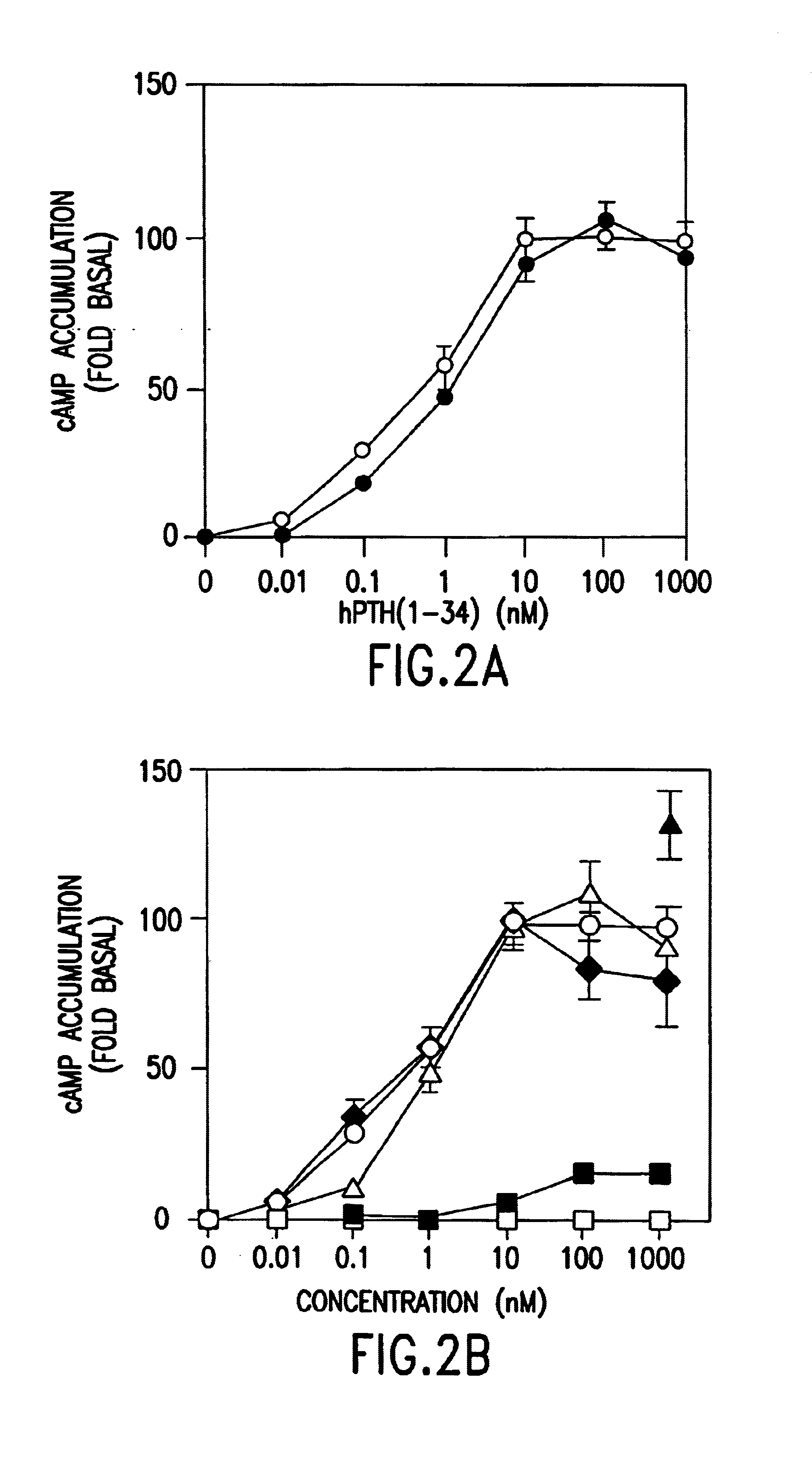
The present invention relates to conformationally constrained parathyroid hormone (PTH) analogs, and methods of preparing and using the PTH analogs. The invention provides novel PTH polypeptide derivatives containing amino acid substitutions at selected positions in the polypeptides. The invention provides derivatives of PTH (1-34), PTH(1-21), PTH(1-20), PTH(1-19), PTH(1-18), PTH(1-17), PTH(1-16), PTH(1-15), PTH(1-14), PTH(1-13), PTH(1-12), PTH(1-11) and PTH(1-1 0) polypeptides, wherein at least one residue in each polypeptide is a helix, preferably an a-helix, stabilizing residue. The invention also provides methods of making such peptides. Further, the invention encompasses compositions and methods for use in limiting undesired bone loss in a vertebrate at risk of such bone loss, in treating conditions that are characterized by undesired bone loss or by the need for bone growth, e.g. in treating fractures or cartilage disorders and for raising cAMP levels in cells where deemed necessary.
Owner:THE GENERAL HOSPITAL CORP
Polypeptide derivatives of parathyroid hormone (PTH)
InactiveUS7022815B1Increase in cAMPPeptide/protein ingredientsPeptide sourcesBiochemistryParathyroid hormone
Novel parathyroid hormone(PTH) polypeptide derivatives are disclosed, as are pharmaceutical compositions containing said polypeptides, and synthetic and recombinant methods for producing said polypeptides. Also disclosed are methods for treating mammalian conditions characterized by decreases in bone mass using therapeutically effective pharmaceutical compositions containing said polypeptides. Also disclosed are methods for screening candidate compounds of the invention for antagonistic or agonistic effects on parathyroid hormone receptor action. Also disclosed are diagnostic and therapeutic methods of said compounds.
Owner:THE GENERAL HOSPITAL CORP
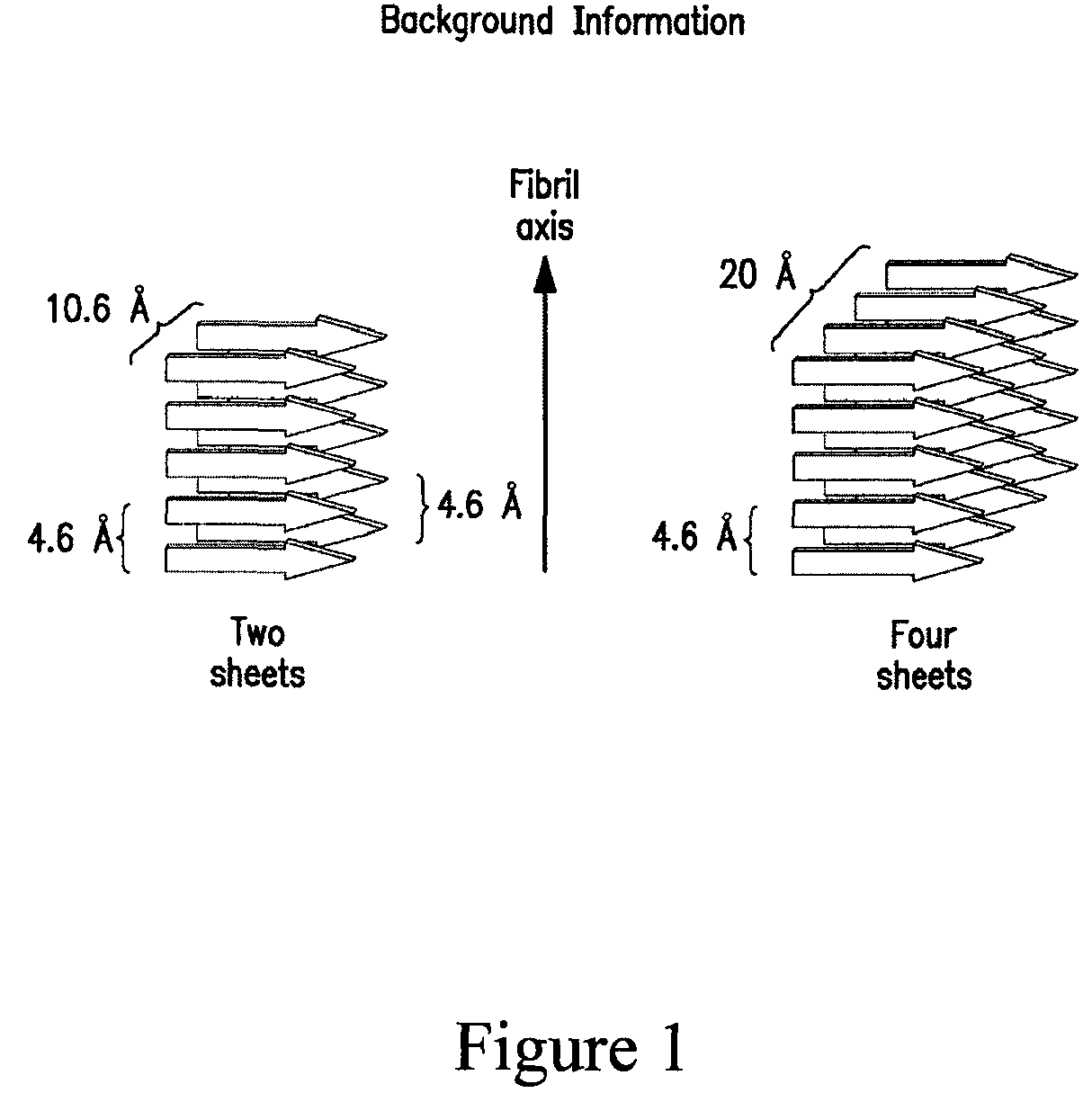
Stabilizing alkylglycoside compositions and methods thereof
ActiveUS8226949B2Convenient treatmentReduce aggregationBiocideAntipyreticPeptide TGastrin-releasing peptide
The present invention relates to alkylglycoside-containing compositions and methods for increasing the stability, reducing the aggregation and immunogenicity, increasing the biological activity, and reducing or preventing fibrillar formation of a peptide, polypeptide, or variant thereof, for example parathyroid hormone (PTH) or PTH analogs, amylin, a monoclonal antibody, insulin, Peptide T or analog thereof, gastrin, gastrin releasing peptides, gastrin releasing peptide-like (GRP) proteins, epidermal growth factor or analog thereof.
Owner:AEGIS THERAPEUTICS LLC
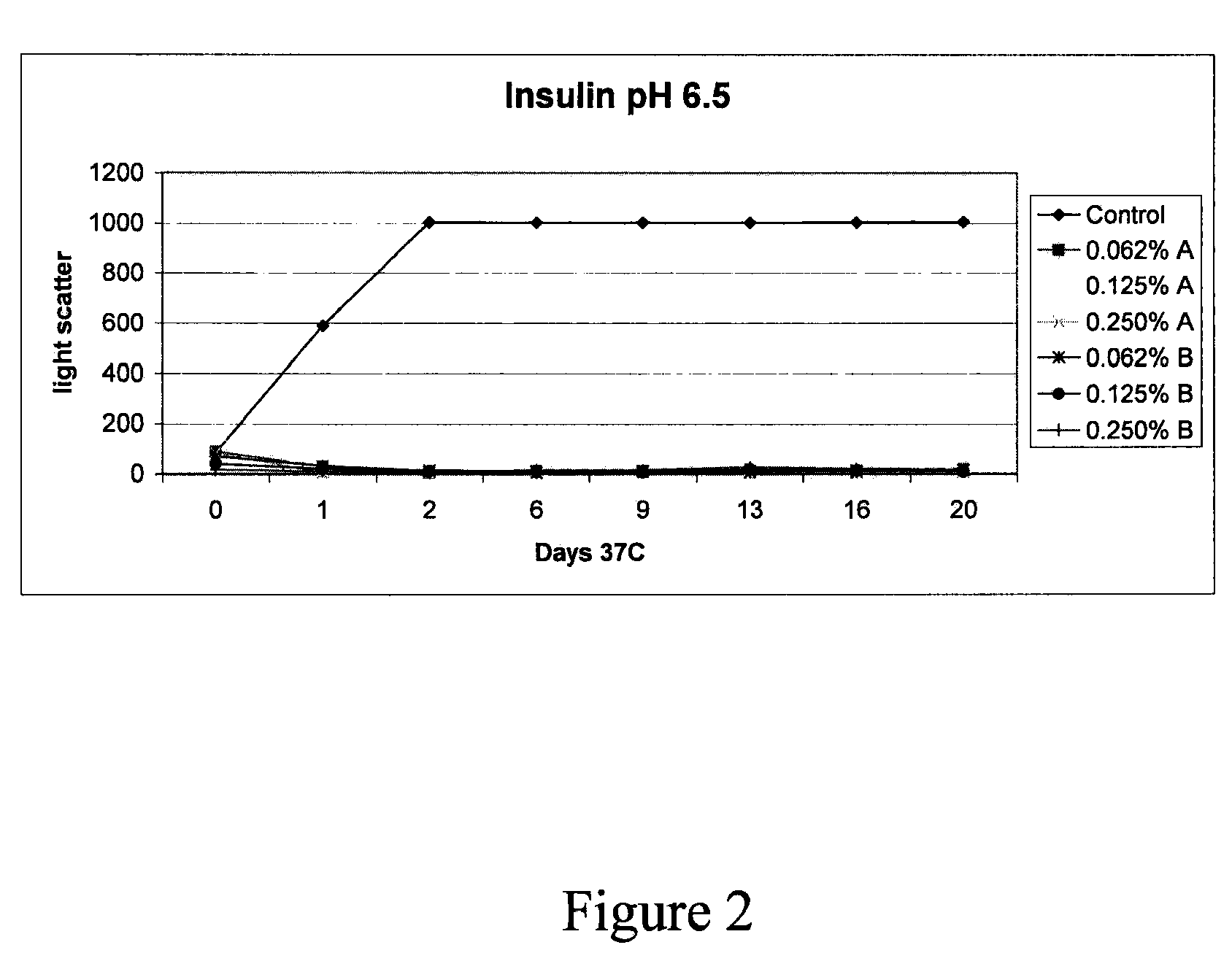
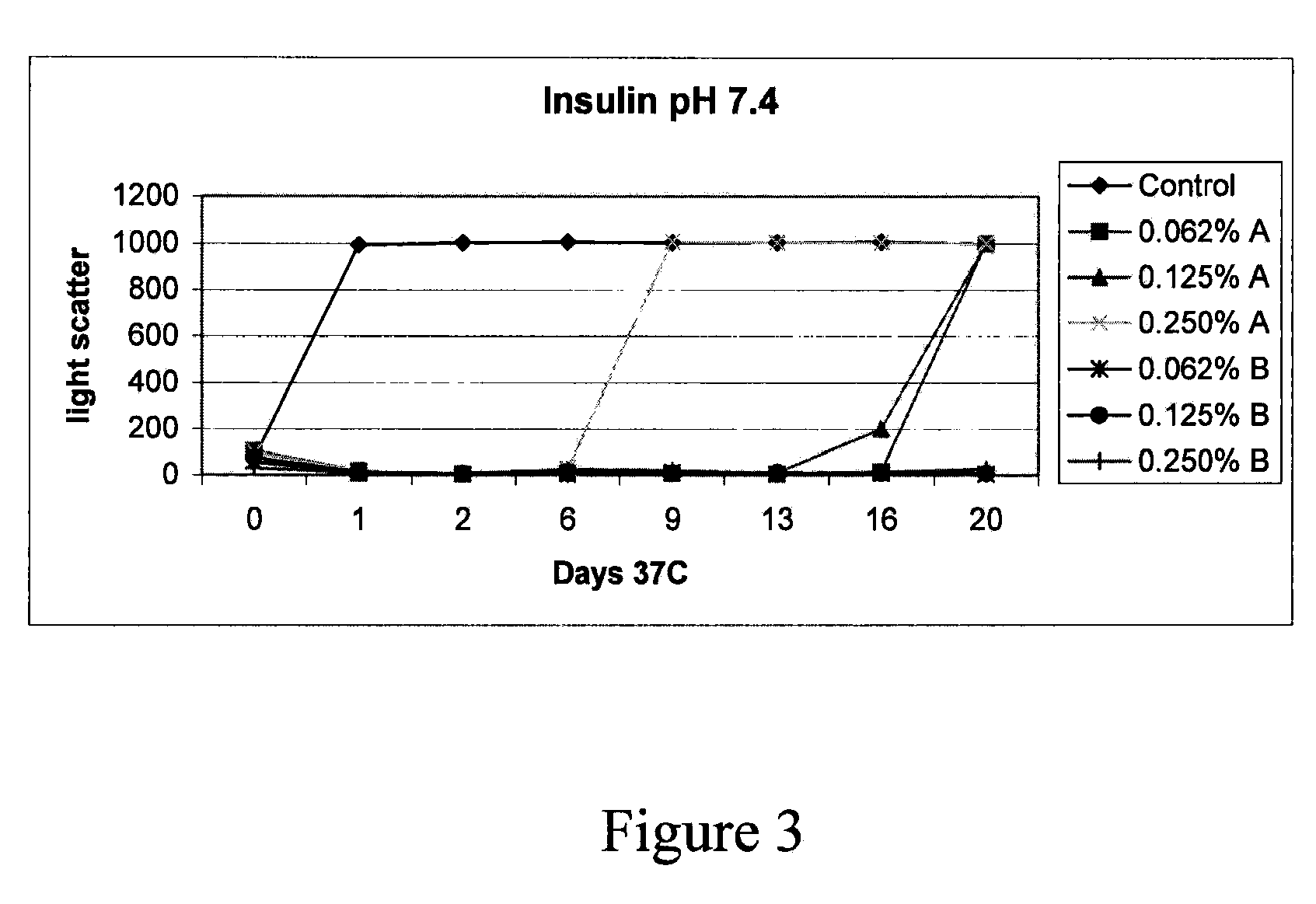
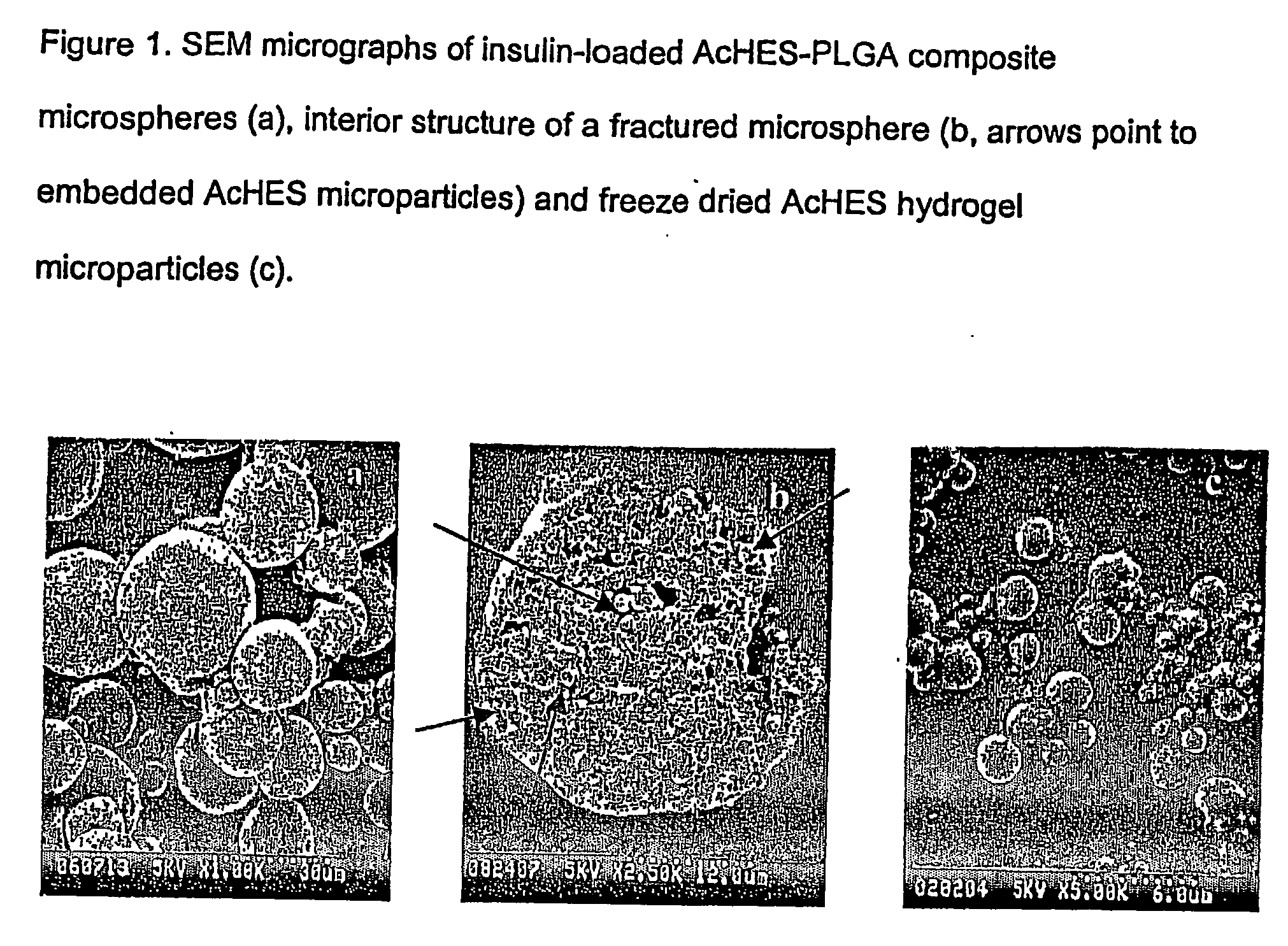
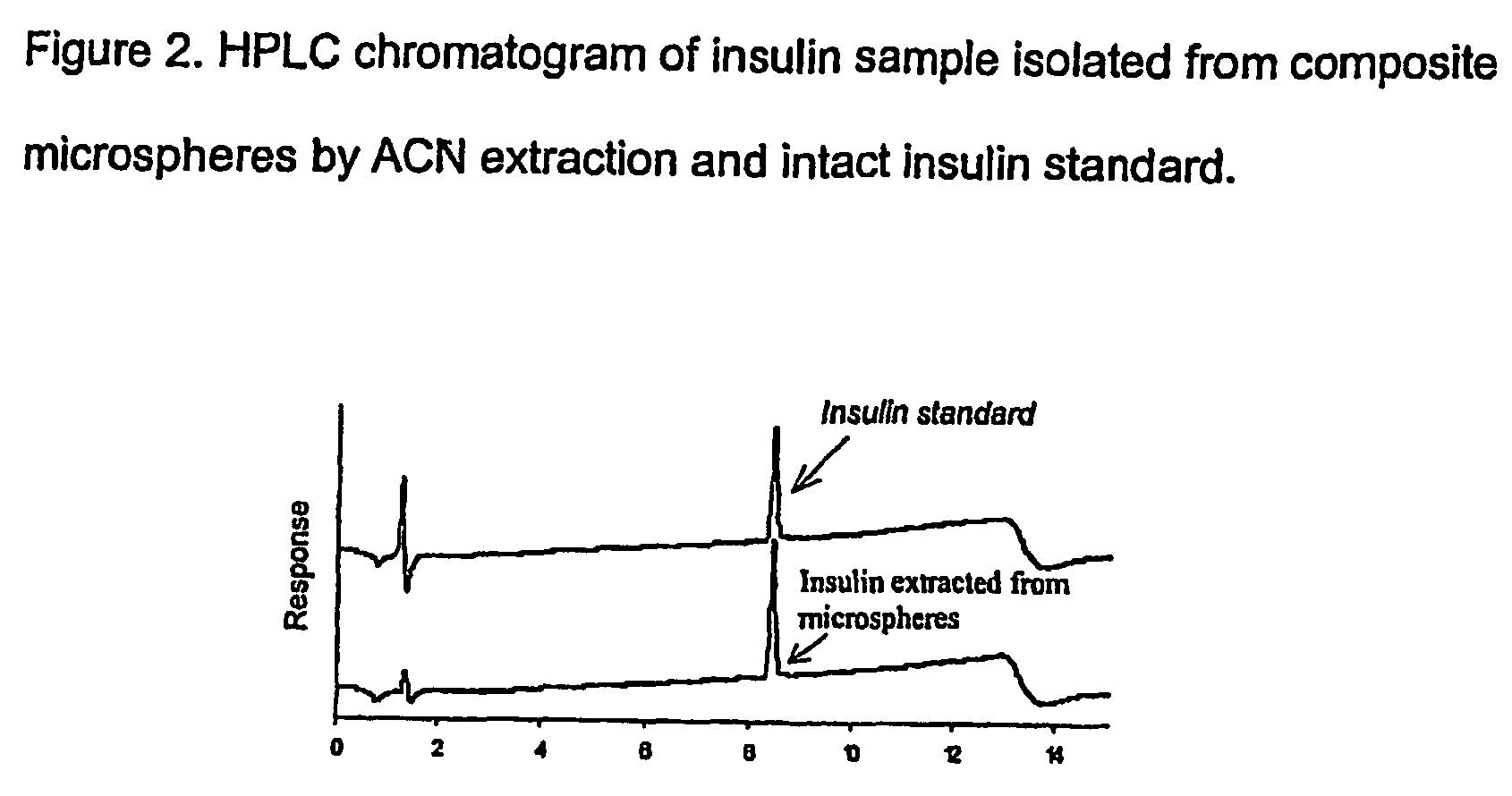
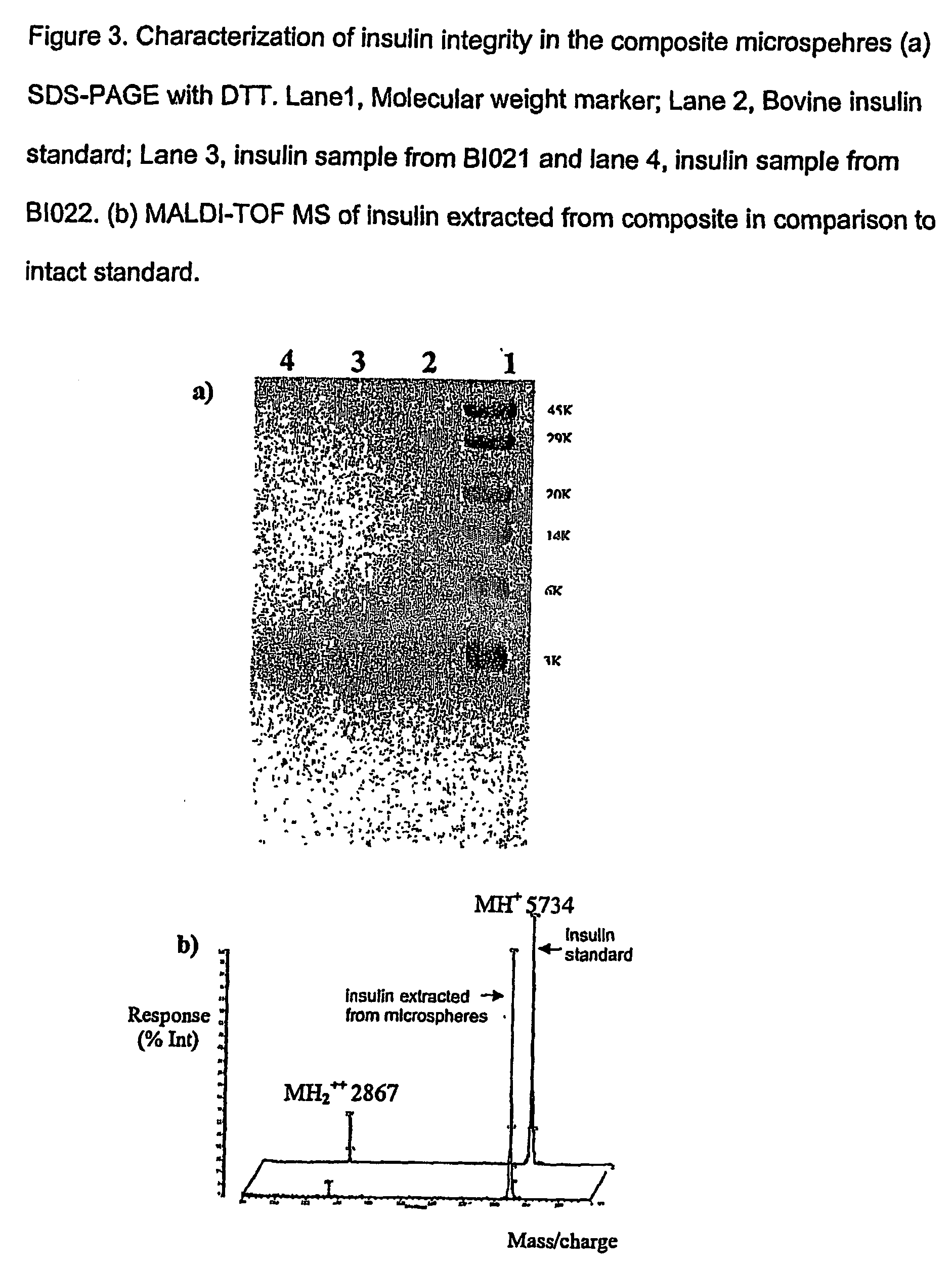
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
Nucleic acids encoding PTH functional domain conjugate peptides
InactiveUS7244834B2Good curative effectSugar derivativesPeptide/protein ingredientsC-terminal bindingBinding domain
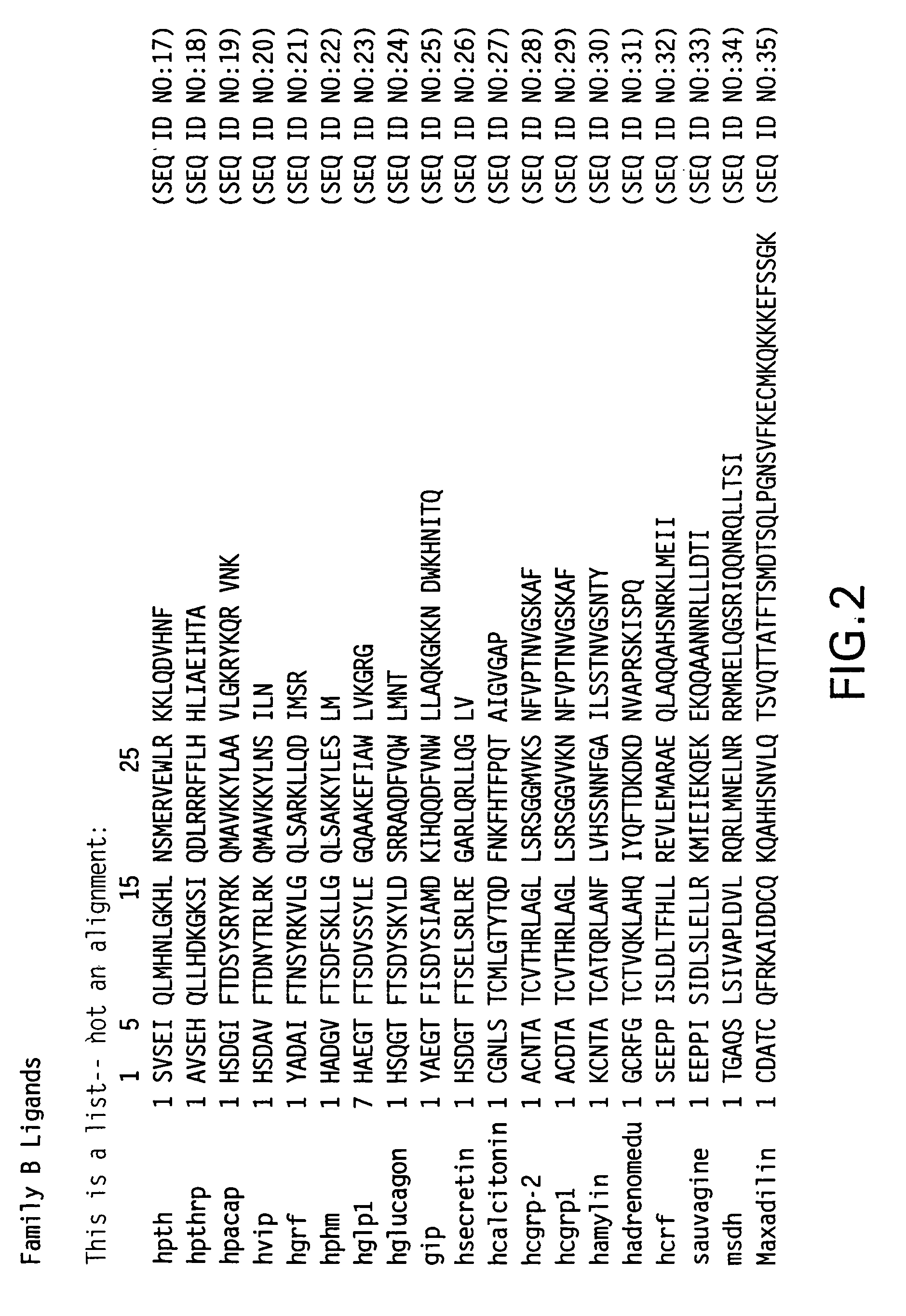
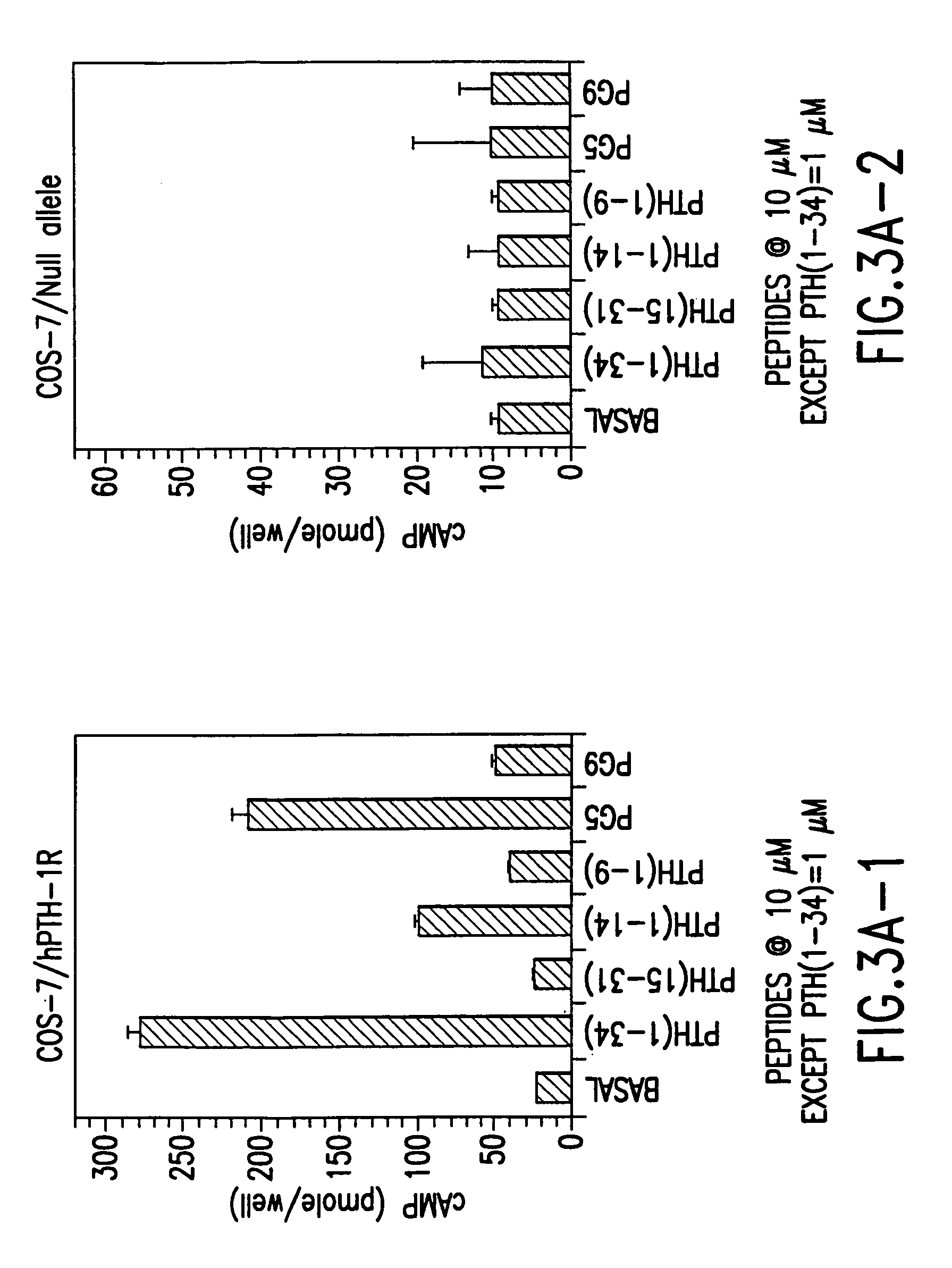
Novel parathyroid hormone (PTH) peptides and analogs thereof of the PTH(1–34) fragment are disclosed that combine the N-terminal signaling domain (residues 1–9) and the C-terminal binding domain (residues 15–31) via a linker. Nucleic acid molecules and peptides for PTH(1–9)-(Gly)5-PTH(15–31) (PG5) and PTH(1–9)-(Gly)7-PTH(15–31) and a novel PTH receptor are disclosed. Additionally, methods of screening for PTH agonists, pharmaceutical compositions and methods of treatment are disclosed.
Owner:THE GENERAL HOSPITAL CORP
Regulation of mineral and skeletal metabolism
InactiveUS20070066514A1Reduce the possibilityRelieve symptomsPeptide/protein ingredientsMetabolism disorderPhosphatePhosphorylation
A method is disclosed whereby levels of calcium, phosphate and parathyroid hormone are measured in a patient. The patient is treated with a formulation comprising a compound having phosphotonin activity and thereafter measurements are made again. Dosing of the formulation is adjusted based on measurements with measuring, administering and adjusting dosing continually repeated as needed.
Owner:ACOLOGIX
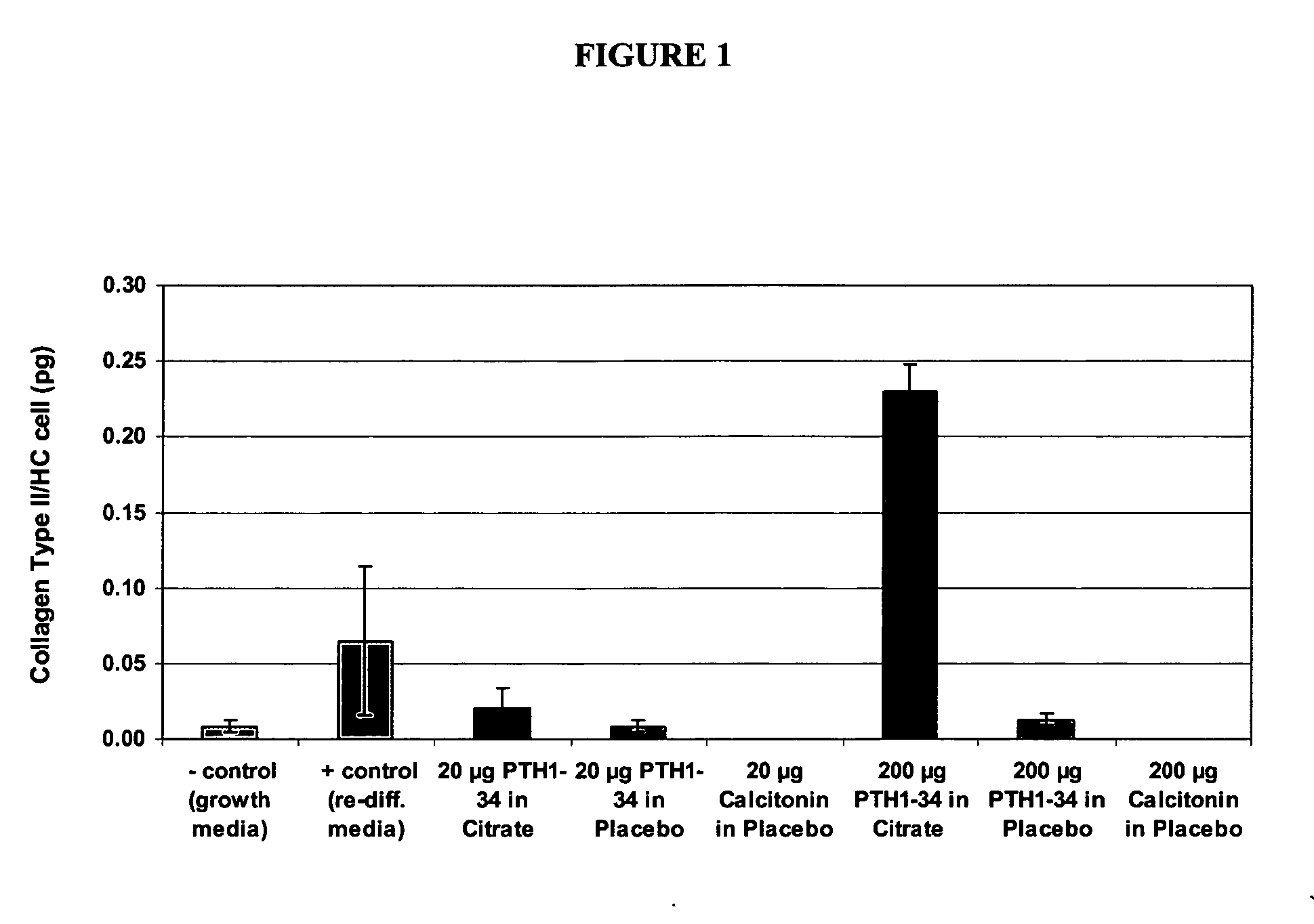
Stabilized teriparatide solutions
A stabilized pharmaceutical composition in the form of a solution for parenteral administration of a parathyroid hormone is described wherein the therapeutically active ingredient is stabilized with a buffer and a polyol. Preferred preparations contain in an aqueous solution human PTH(1-34), mannitol, an acetate or tartrate buffering agent and m-cresol or benzyl alcohol as a preservative.
Owner:ELI LILLY & CO
Conformationally constrained parathyroid hormone (PTH) analogs
ActiveUS7572765B2In-vivo radioactive preparationsPeptide/protein ingredientsAmino acid substitutionCvd risk
The present invention relates to conformationally constrained parathyroid hormone (PTH) analogs, and methods of preparing and using the PTH analogs. The invention provides novel PTH polypeptide derivatives containing amino acid substitutions at selected positions in the polypeptides. The invention provides derivatives of PTH (1-34), PTH(1-21), PTH(1-20), PTH(1-19), PTH(1-18), PTH(1-17), PTH(1-16), PTH(1-15), PTH(1-14), PTH(1-13), PTH(1-12), PTH(1-11) and PTH(1-1 0) polypeptides, wherein at least one residue in each polypeptide is a helix, preferably an a-helix, stabilizing residue. The invention also provides methods of making such peptides. Further, the invention encompasses compositions and methods for use in limiting undesired bone loss in a vertebrate at risk of such bone loss, in treating conditions that are characterized by undesired bone loss or by the need for bone growth, e.g. in treating fractures or cartilage disorders and for raising cAMP levels in cells where deemed necessary.
Owner:THE GENERAL HOSPITAL CORP
Compositions and methods for intranasal administration of inactive analogs of PTH or inactivated preparations of PTH or PTH analogs
Pharmaceutical compositions and methods are described comprising at inactive forms or parathyroid hormone peptide (PTH) or PTH analogs wherein the inactive forms are activated upon administration into the systemic circulation. Also described is a method of preventing local reaction to a biologically active agent, preparing a formulation comprising said biologically active agent, a solubilizing agent and a surfactant, and administering such formulation by contacting said formulation with a mucosal surface.
Owner:NASTECH PHARMA
Conformationally constrained parathyroid hormone (PTH) analogs with lactam bridges
Owner:THE GENERAL HOSPITAL CORP
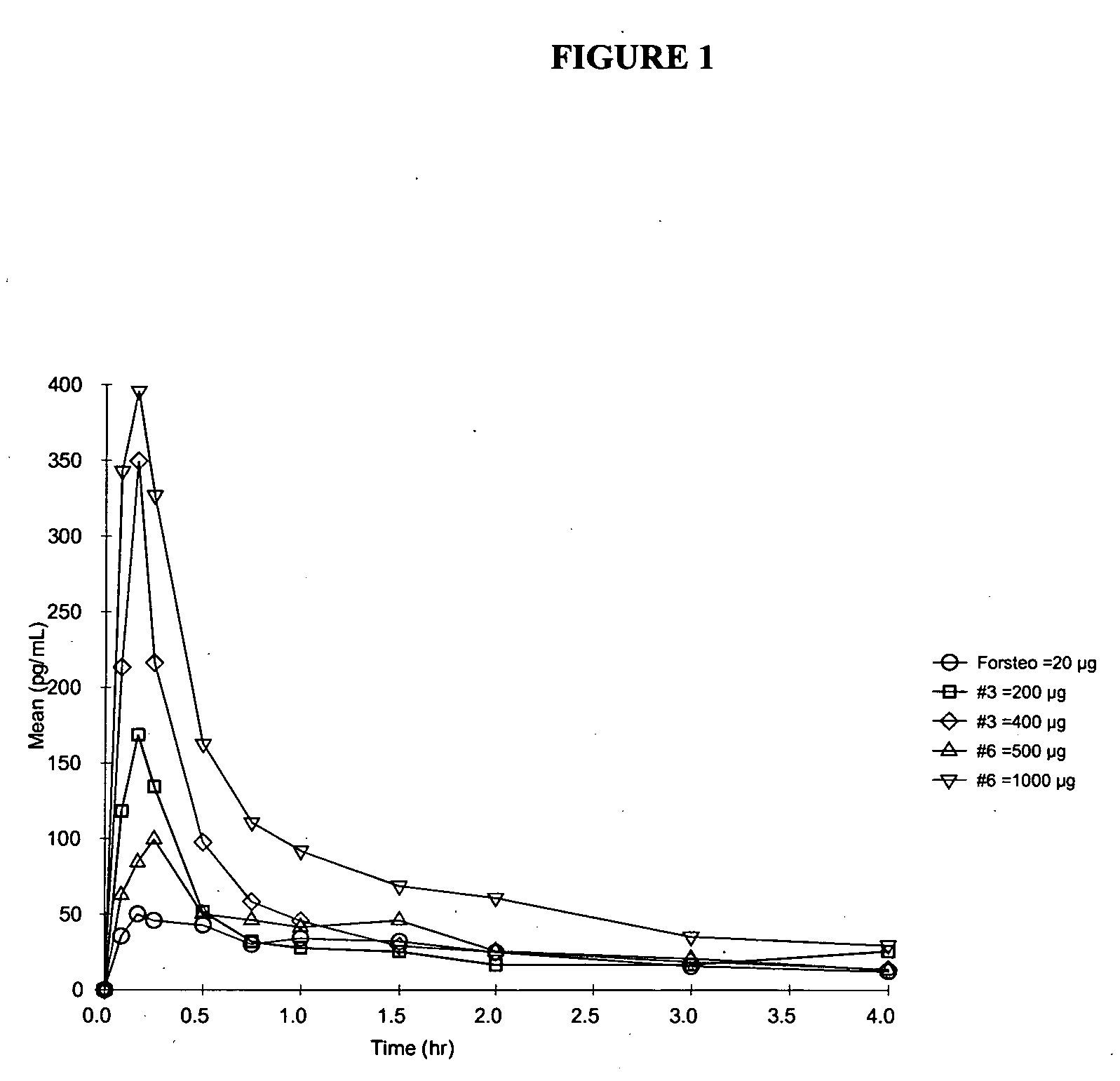
Parathyroid hormone analogues and methods of use
InactiveUS20070270341A1High densityRestore bonePeptide/protein ingredientsSkeletal disorderSide effectParathyroid Hormone Analogue
The present invention is directed to novel methods of treating a subject with a bone deficit disorder. The methods generally include administering to a subject in need thereof a pharmaceutically acceptable formulation comprising a parathyroid hormone (PTH) peptide analogue in a daily dose sufficient to result in an effective pharmacokinetic profile and maintained adenylate cyclase activity, while simultaneously reducing undesirable side effects.
Owner:MORLEY PAUL +2
GRAS composition for enhanced mucosal delivery of parathyroid hormone
What is described is an aqueous pharmaceutical composition for intranasal delivery of PTH, comprising a PTH molecule, and one or more excipients selected from the group consisting of a chelating agent, an alcohol, and a surface active agent, wherein the PTH molecule selected from the group consisting of SEQ NO: 1, SEQ NO: 2, and SEQ NO: 3.
Owner:MARINA BIOTECH INC
Method and device for transdermal delivery of parathyroid hormone using a microprojection array
A method and a drug delivery system for transdermally administering parathyroid hormone (PTH) in a pulsatile fashion are provided, where the drug delivery system comprises an array of microprojections each comprising PTH.
Owner:CORIUM PHARMA SOLUTIONS INC
Screening assays for G protein coupled receptor agonists and antagonists
InactiveUS7033773B1Compound screeningApoptosis detectionG protein-coupled receptorRenal epithelial cell
Parathyroid hormone (PTH) and its closely related peptide, PTHrP, share the same receptor, PTHR. LLC-PK1 cells are porcine renal epithelial cells which do not normally express PTHR. The present-invention provides stably transfected LLC-PK1 cells which express human PTHR. Also provided are methods for determining whether a compound of interest is an agonist or antagonist of a Gs or Gq protein coupled receptor.
Owner:THE GENERAL HOSPITAL CORP
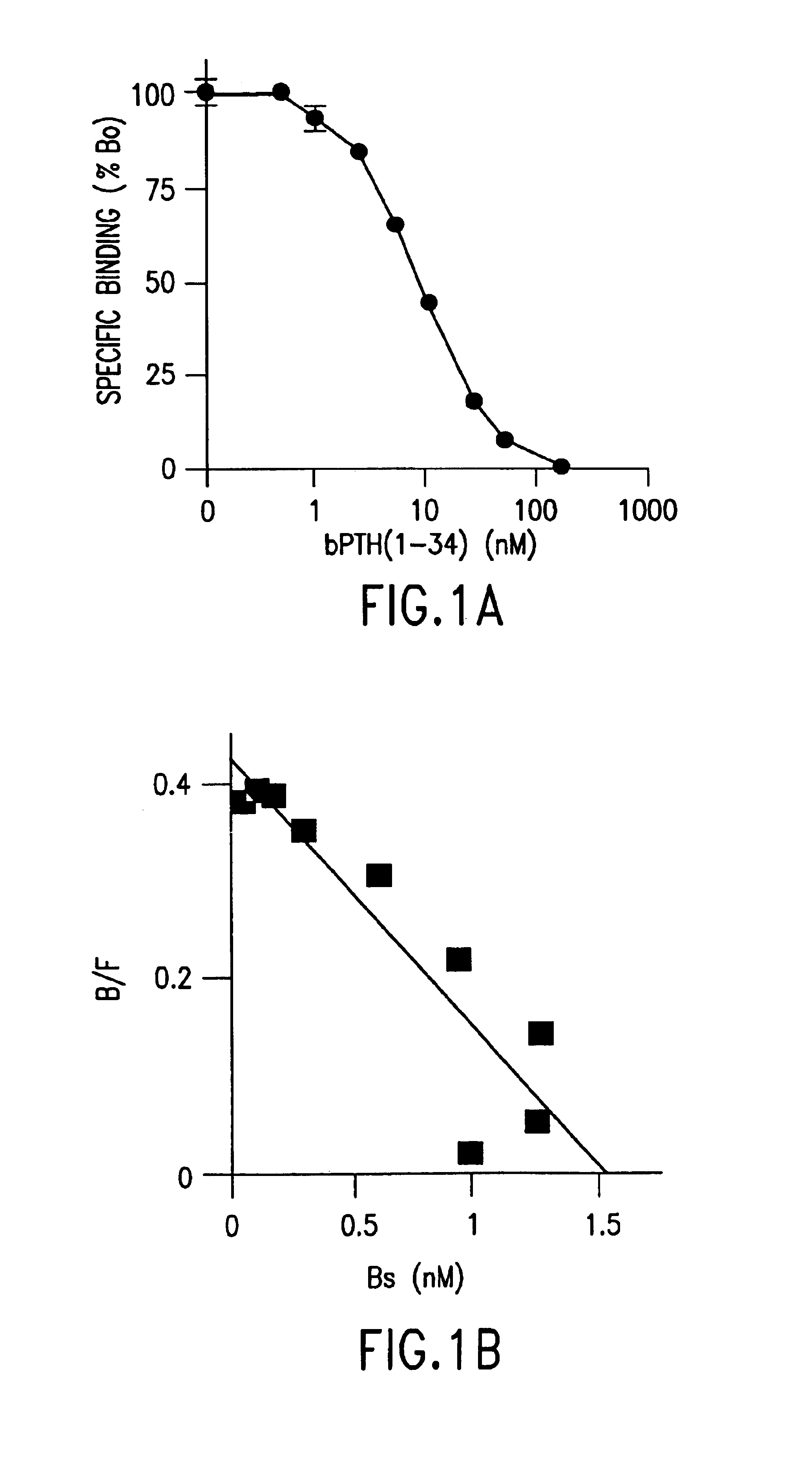
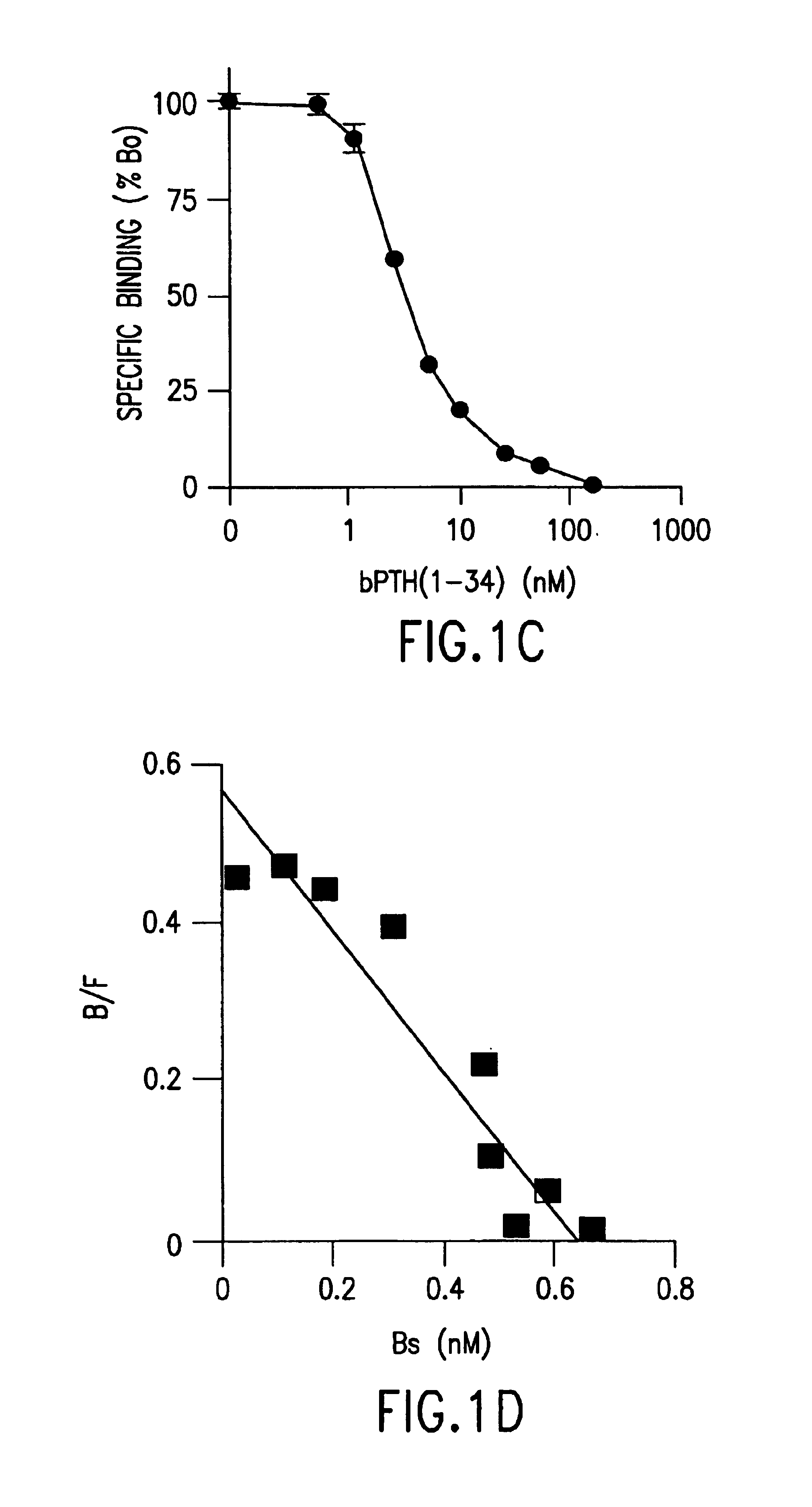
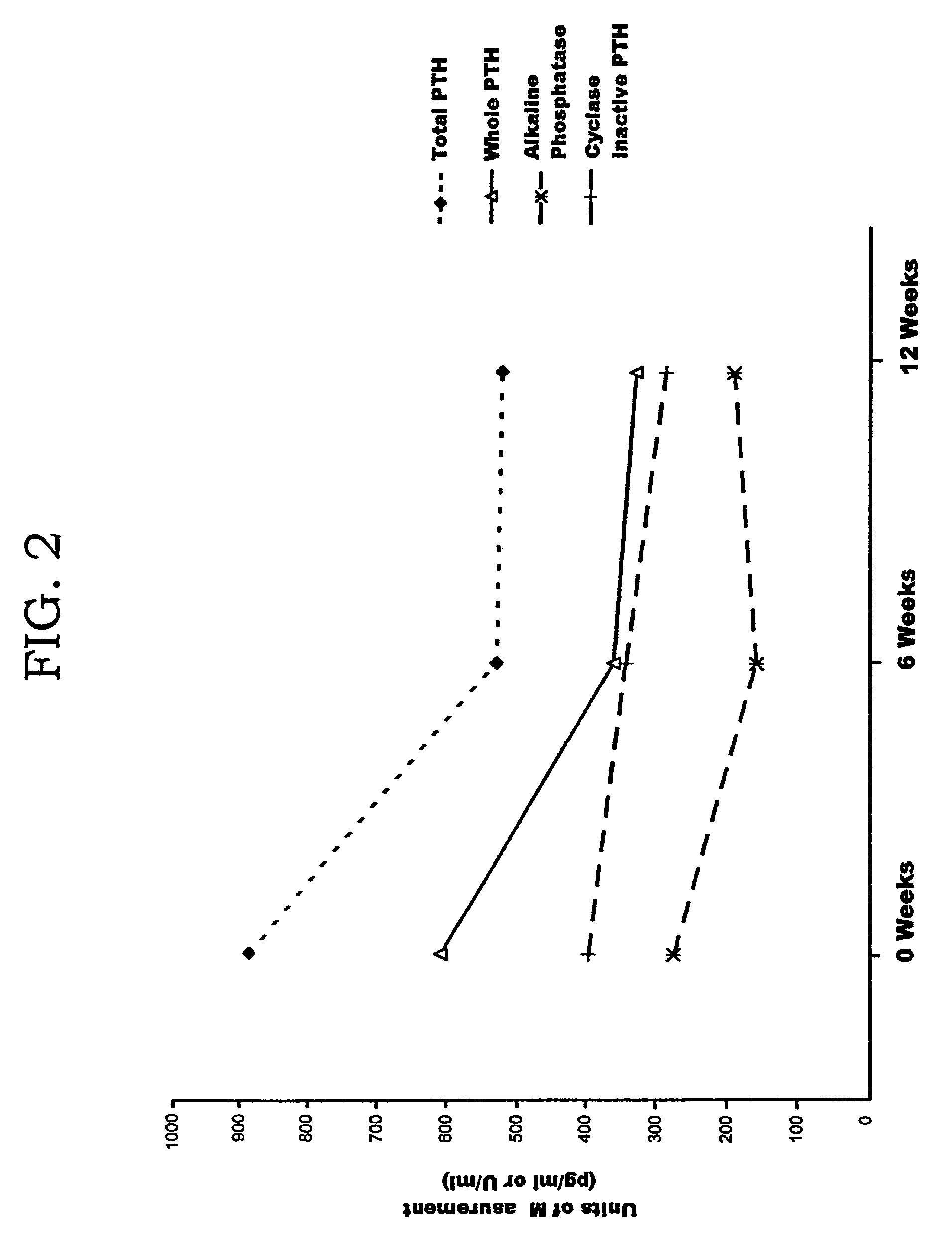
Methods and controls for monitoring assay quality and accuracy in parathyroid hormone measurement
InactiveUS7459276B2Extended shelf/storageImprove accuracyBiological material analysisBiological testingPhysiologyParathyroid hormone measurement
The present invention relates to the use of control compositions and kits comprising such to evaluate and monitor the consistency of assays utilized to determine parathyroid hormone levels.
Owner:SCANTIBODIES LAB
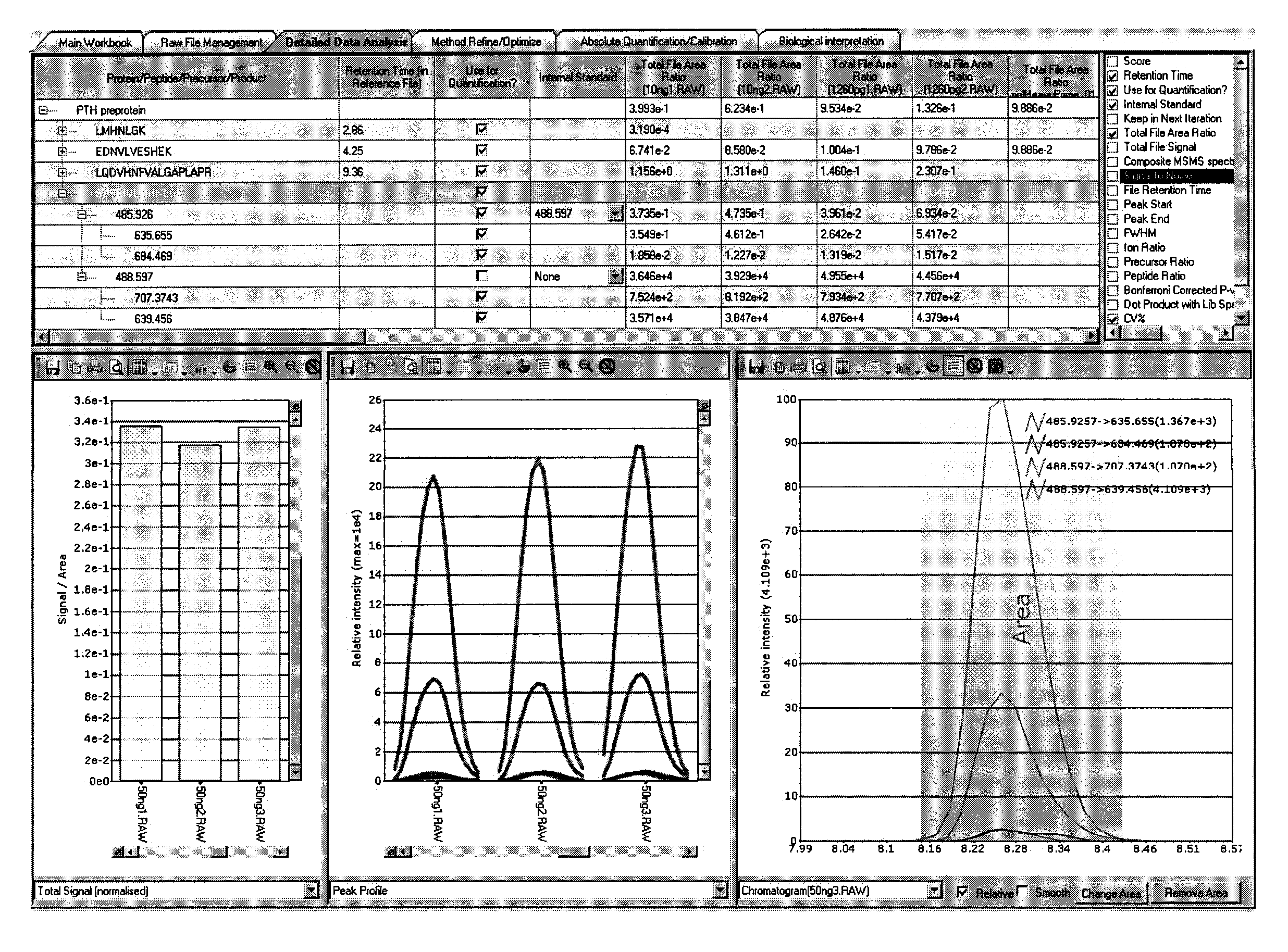
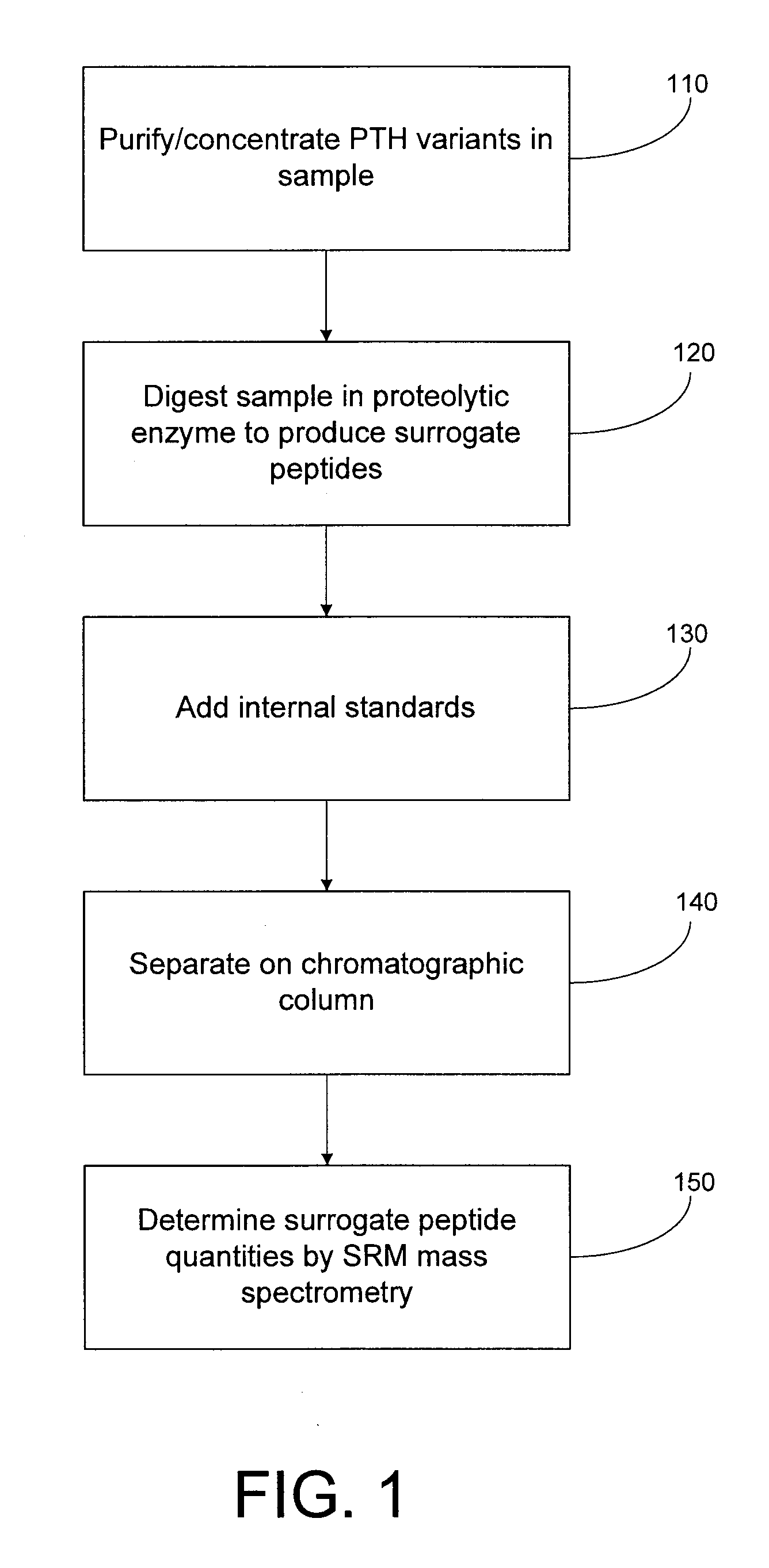
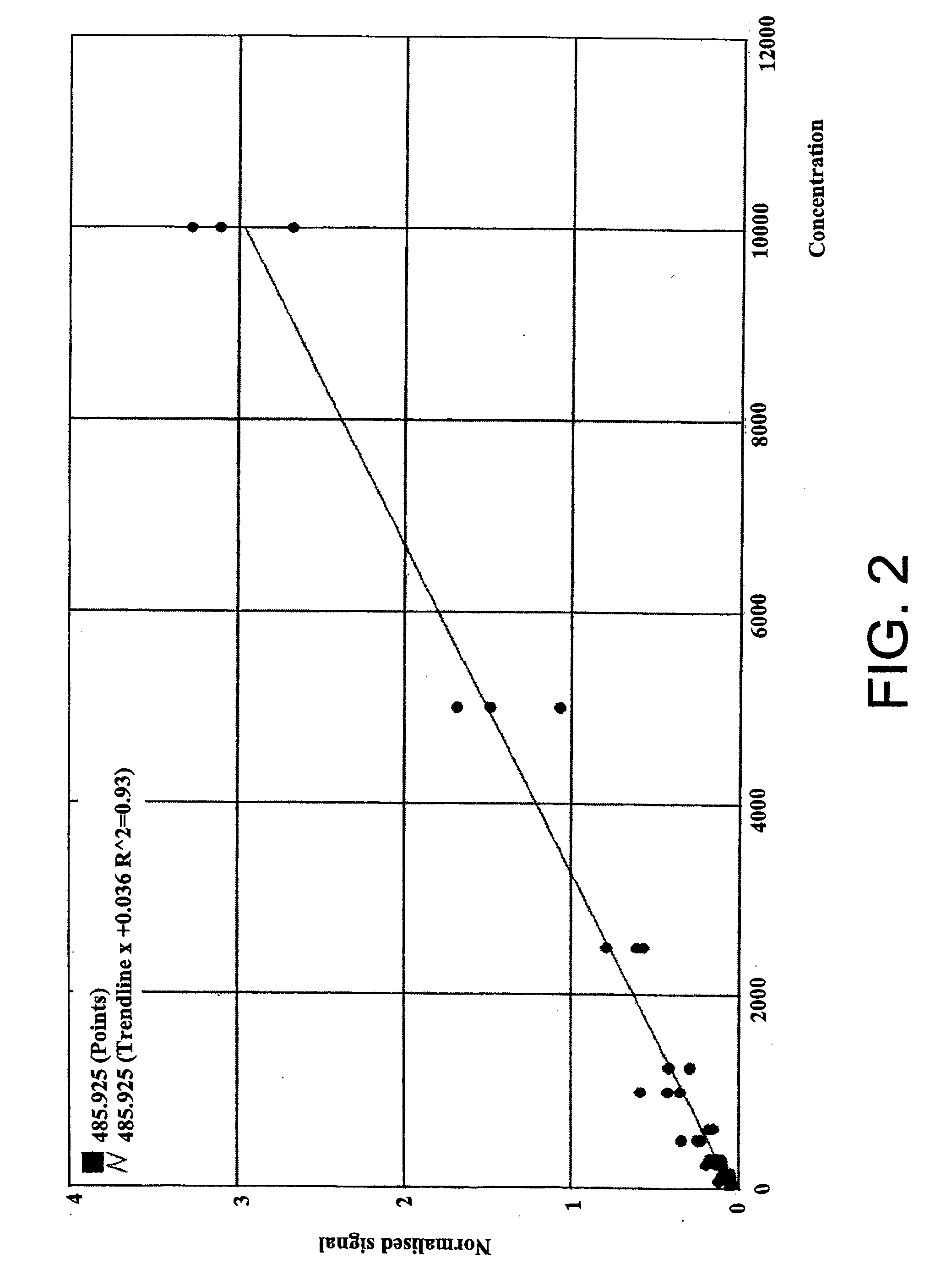
Assay for monitoring parathyroid hormone (PTH) variants by tandem mass spectrometry
InactiveUS8383417B2Mass spectrometric analysisMaterial analysis by electric/magnetic meansMass Spectrometry-Mass SpectrometryProtein mass spectrometry
Methods are described for monitoring the amounts of PTH variants in a biological sample by digesting the sample to produce surrogate peptides specific to the targeted PTH variants, and detecting and quantifying the surrogate peptides by selective reaction monitoring (SRM) mass spectrometry, using a set of precursor-to-product ion transitions optimized for sensitivity and selectivity. The PTH variants, or a portion thereof, may be concentrated in the sample by means of immunoaffinity capture or other suitable technique. The mass spectrometric method described herein enables the concurrent measurement of peptides representative of a plurality of targeted PTH variants in a single assay.
Owner:INTRINSIC BIOPROBES +1
Preparation of mesenchymal stem cells (MSCs) derived exosomes and application of the same in acute lung injury
InactiveCN104694466AImprove securityIncrease concentrationSkeletal/connective tissue cellsUnknown materialsFicollPurification methods
The invention relates to preparation and a purification production process of mesenchymal stem cells (MSCs) derived exosomes, and application of the exosomes as a non-cell therapy in the treatment of acute lung injury. The MSCs deriving the exosomes belong to the wall adhesion type of stem cells, and the preparation and purification process have the characteristics that (1) parathyroid hormone is added specially for the preparation of MSCs condition medium, and (2) discontinuous Ficoll density gradient method and immunomagnetic beads separation and purification method are combined for separation and purification so as to obtain the exosomes. Rat acute lung injury (ALI) model experiments confirm that the exosomes can be used for treatment of ALI, so as to lay the foundation for the realization of non-stem cell therapies to substitute stem cells therapies.
Owner:SHANXI MEDICAL UNIV
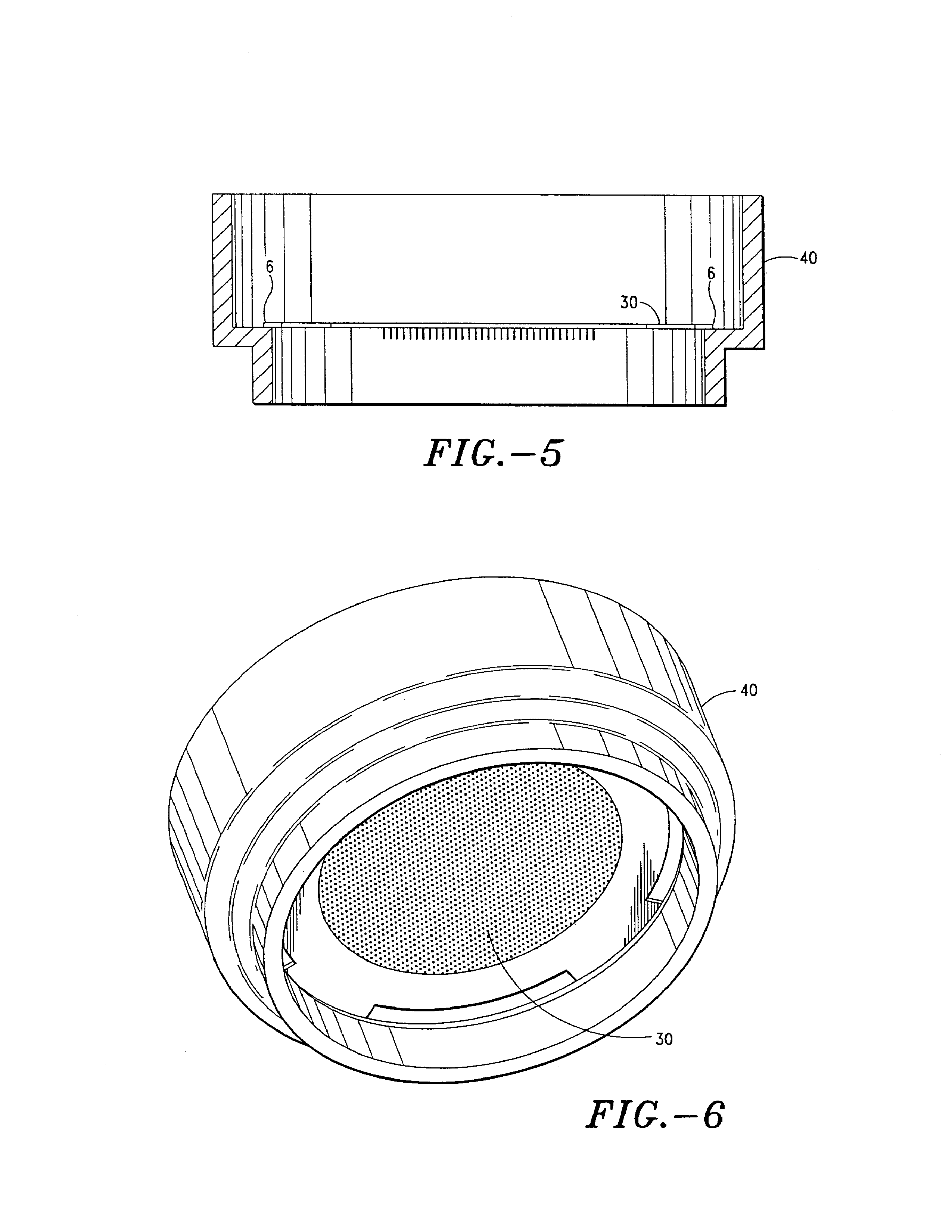
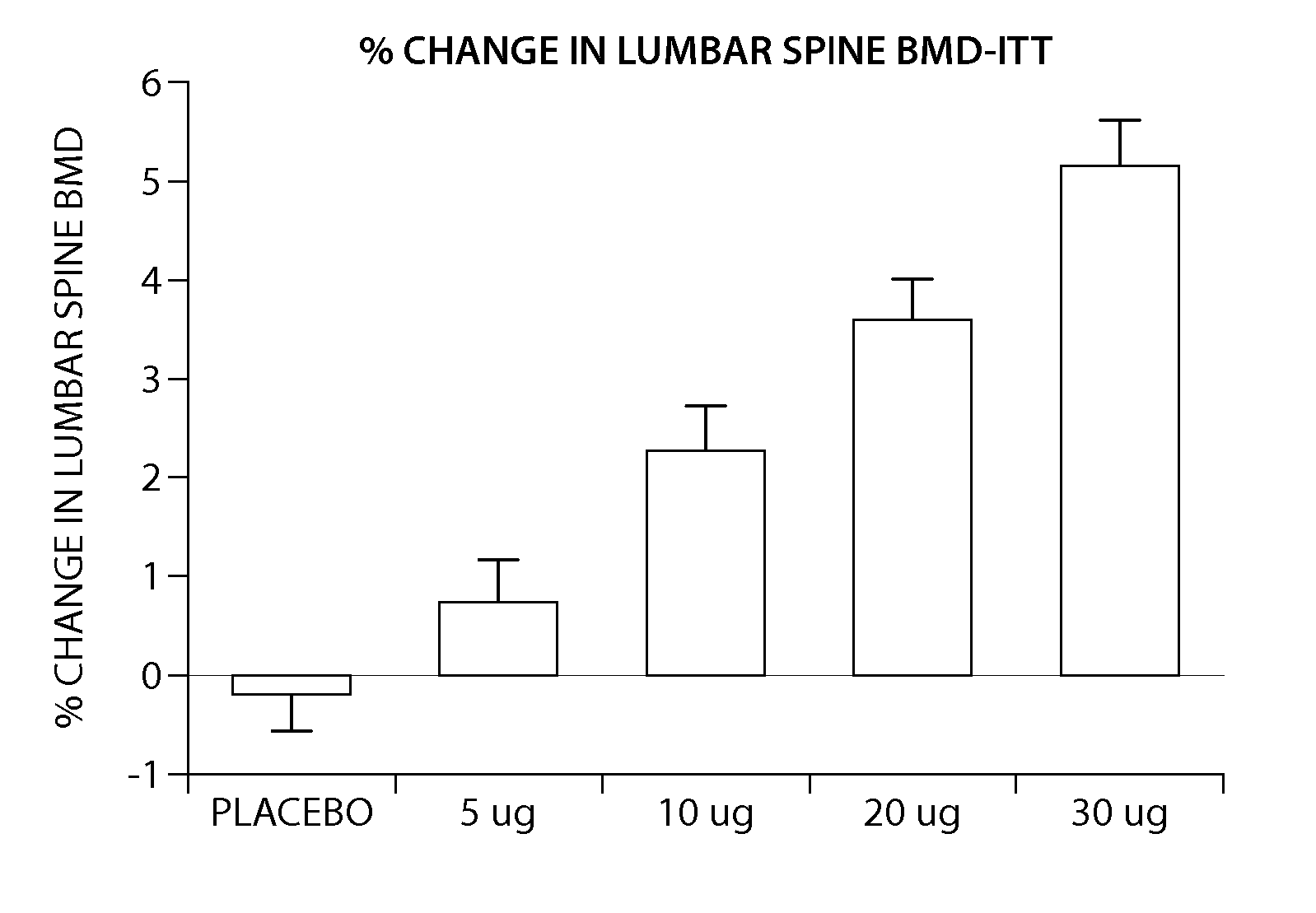
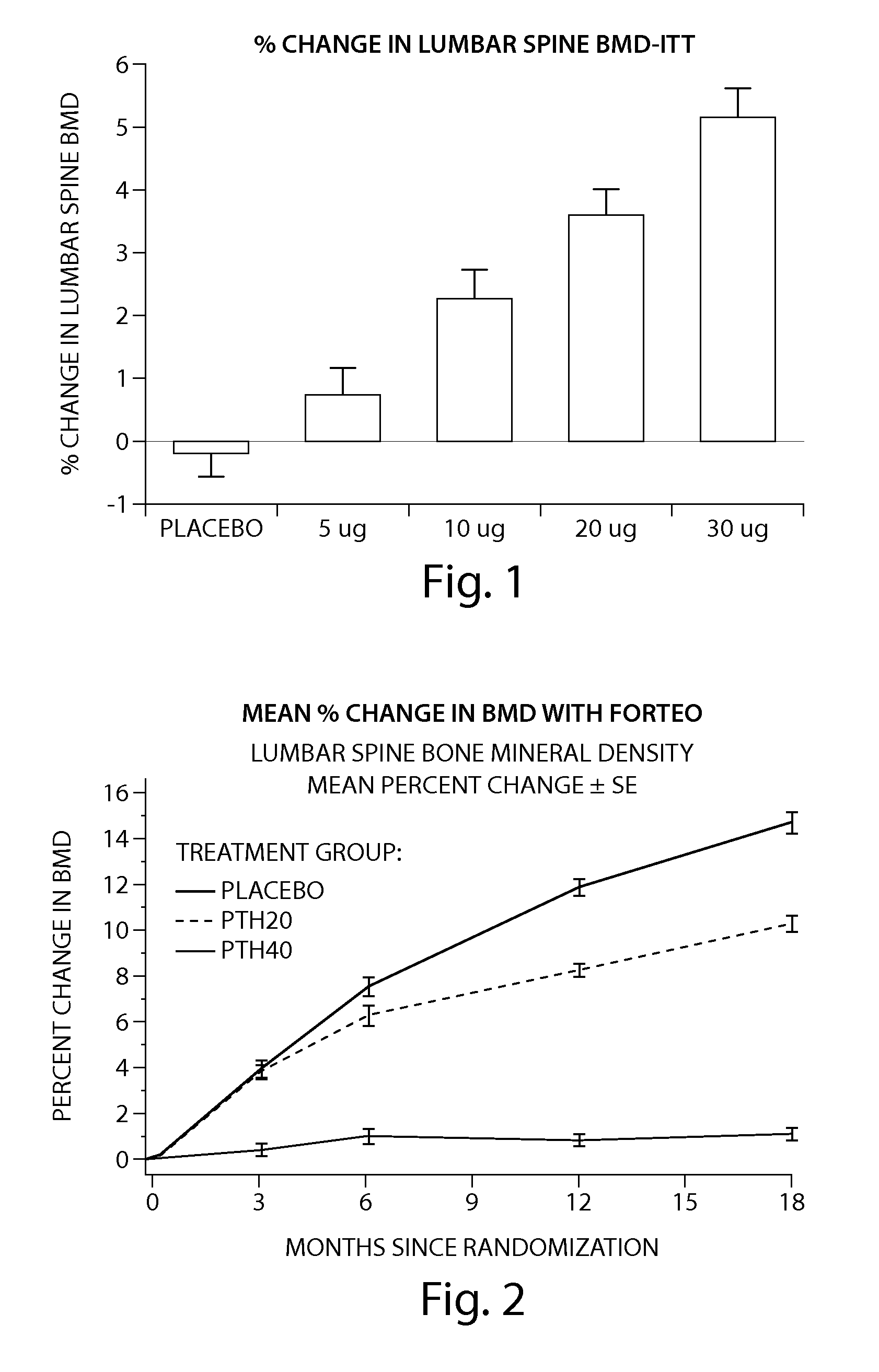
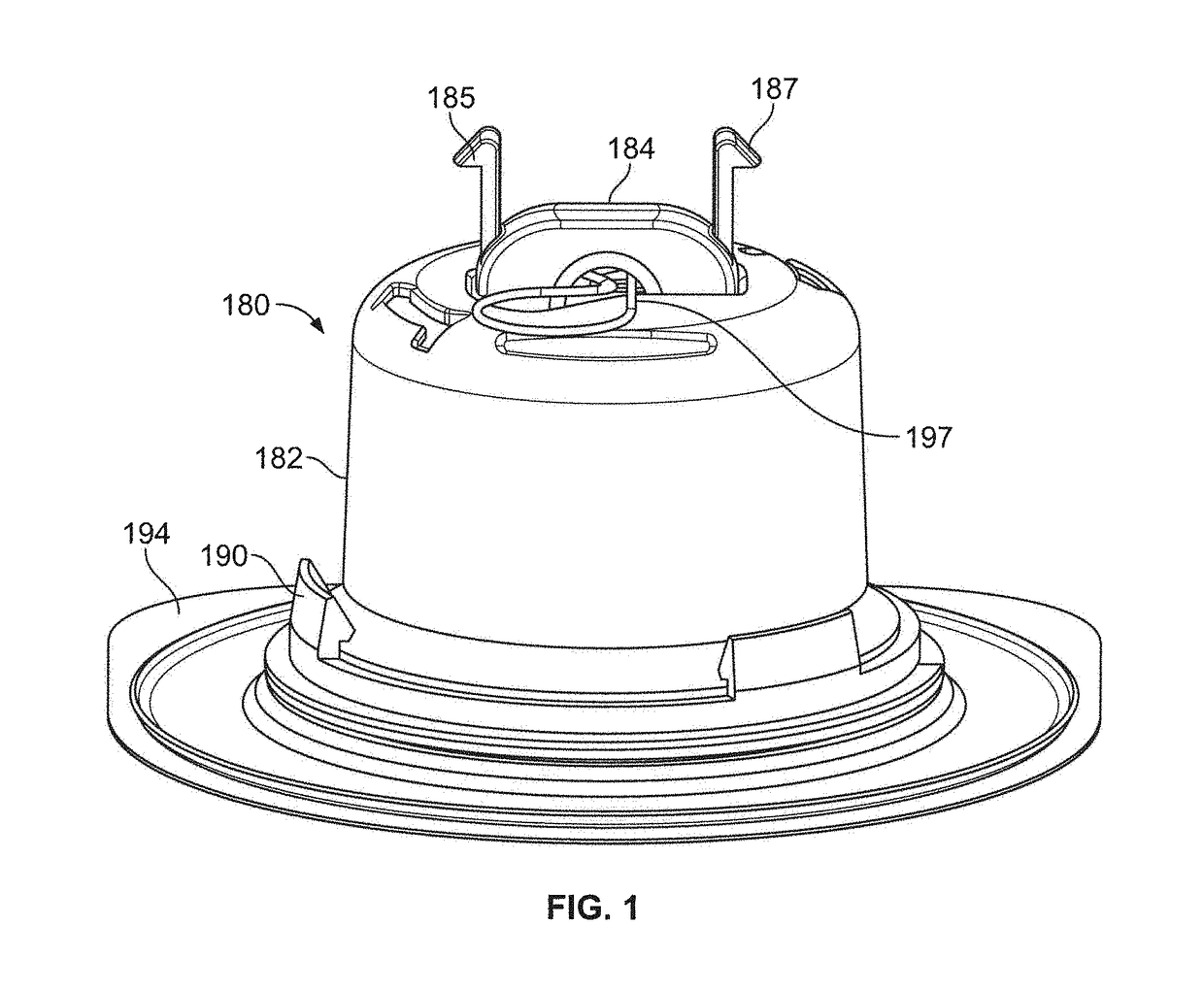
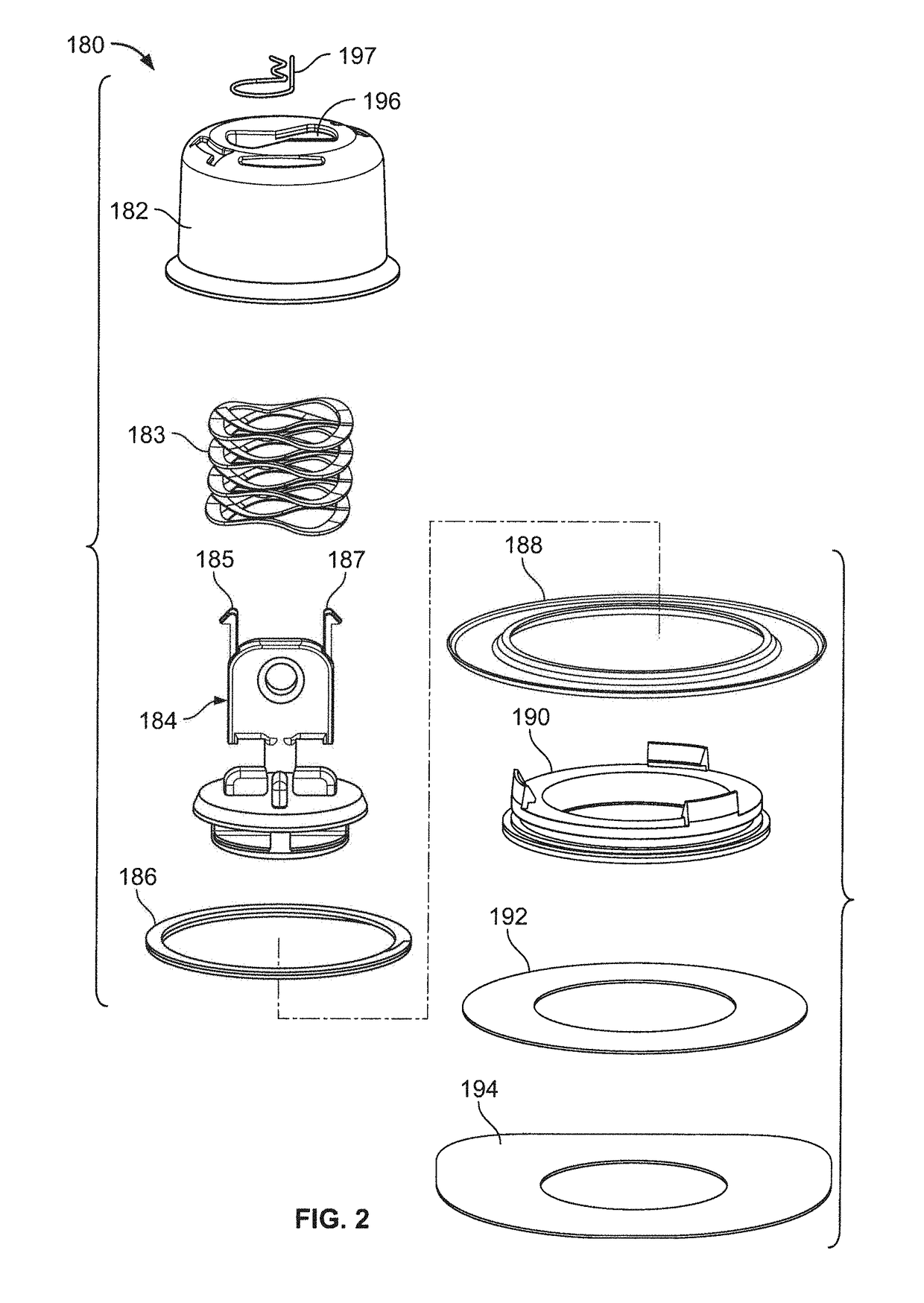
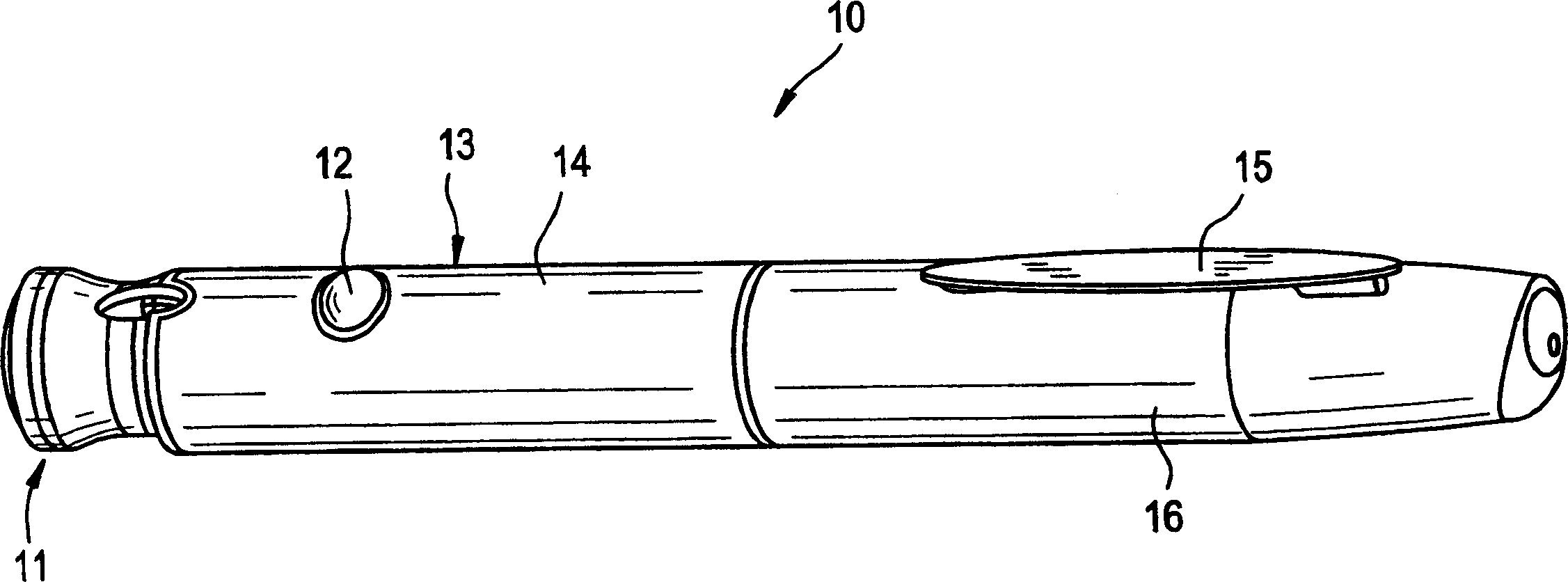
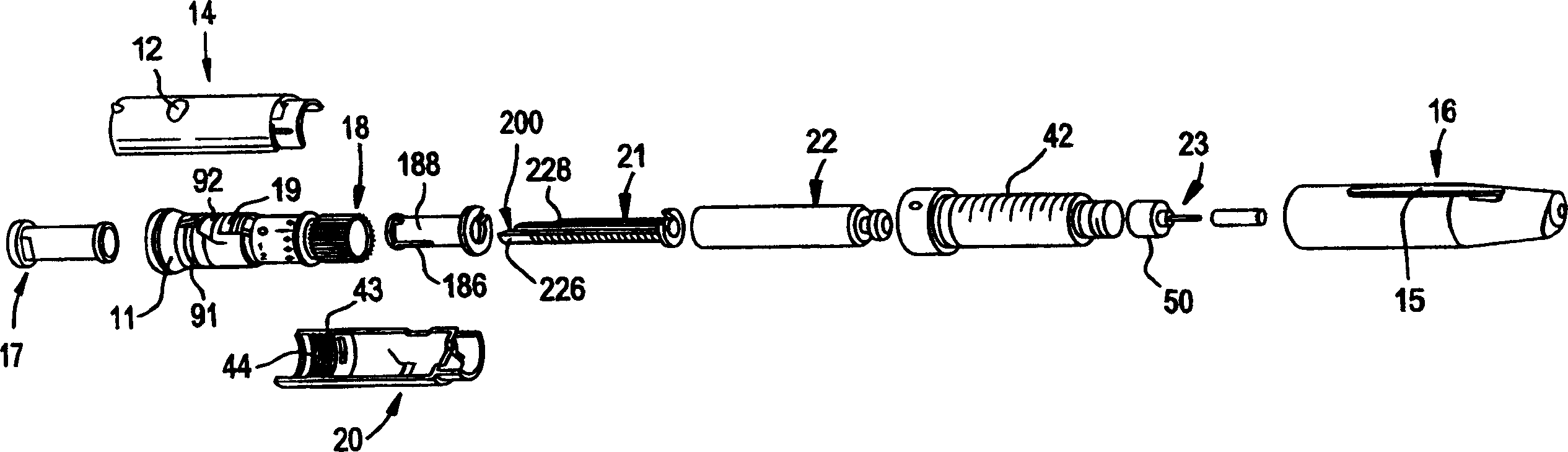
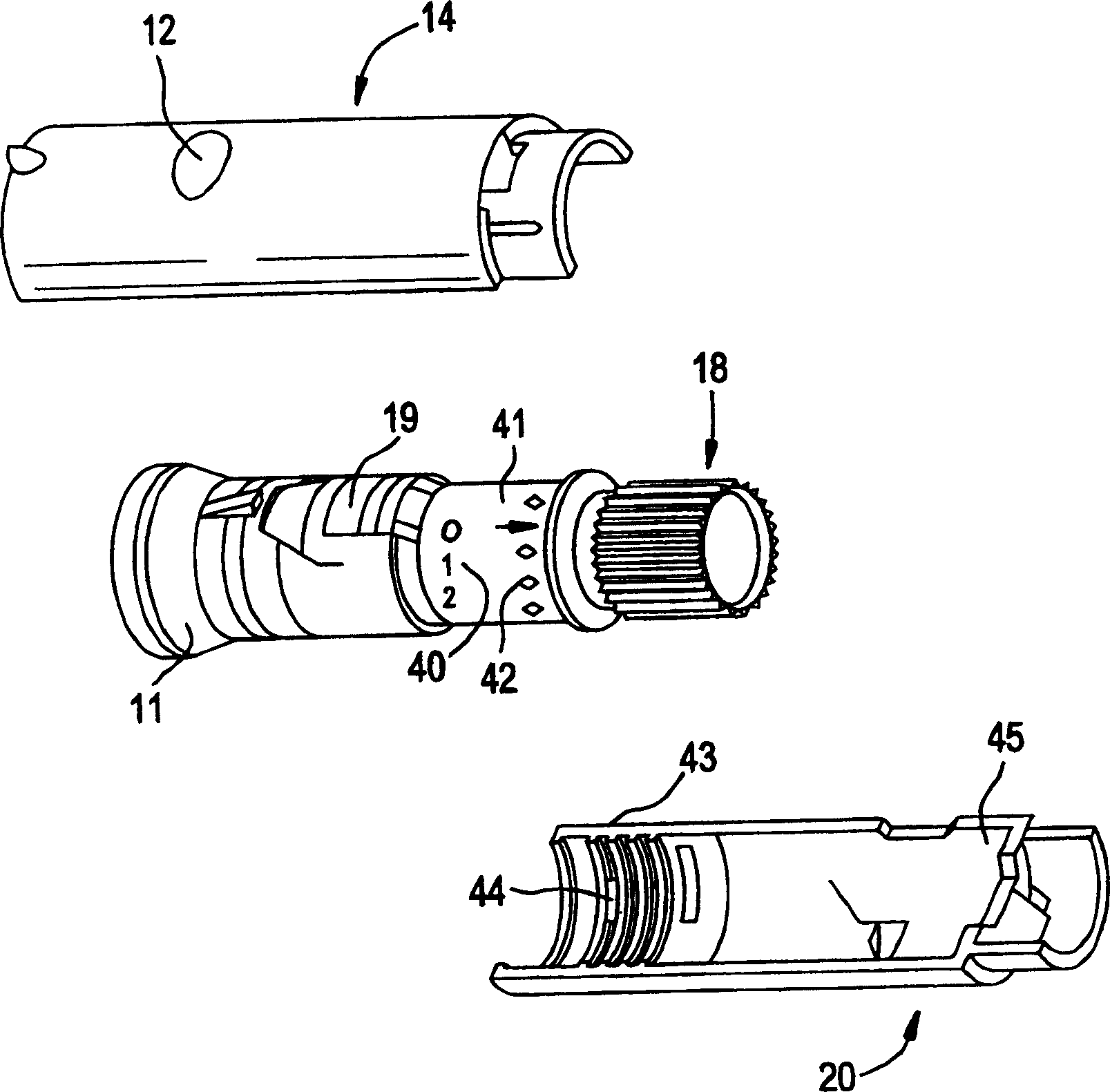
Pen device for administration of parathyroid hormone
A pen delivery device and a procedure for administering parathyroid hormone is provided to allow for the priming and injection of a single predetermined dose. The method is a three-step method using visual indicators to set the priming and injectable dose deliveries. The device is made of a minimal number of parts, which include a housing, a dose knob, a generally cylindrical disengaging assembly located within the proximal end of the dose knob, and an externally threaded drive stem. The pen device further includes a mechanism that prevents the user from dialing up a dosage greater than that remaining in the cartridge.
Owner:ELI LILLY & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com