Pen device for administration of parathyroid hormone
A parathyroid hormone, pen-type technology, applied in the direction of drug devices, infusion sets, other medical devices, etc., can solve problems such as syringe extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] Detailed description of the preferred embodiment
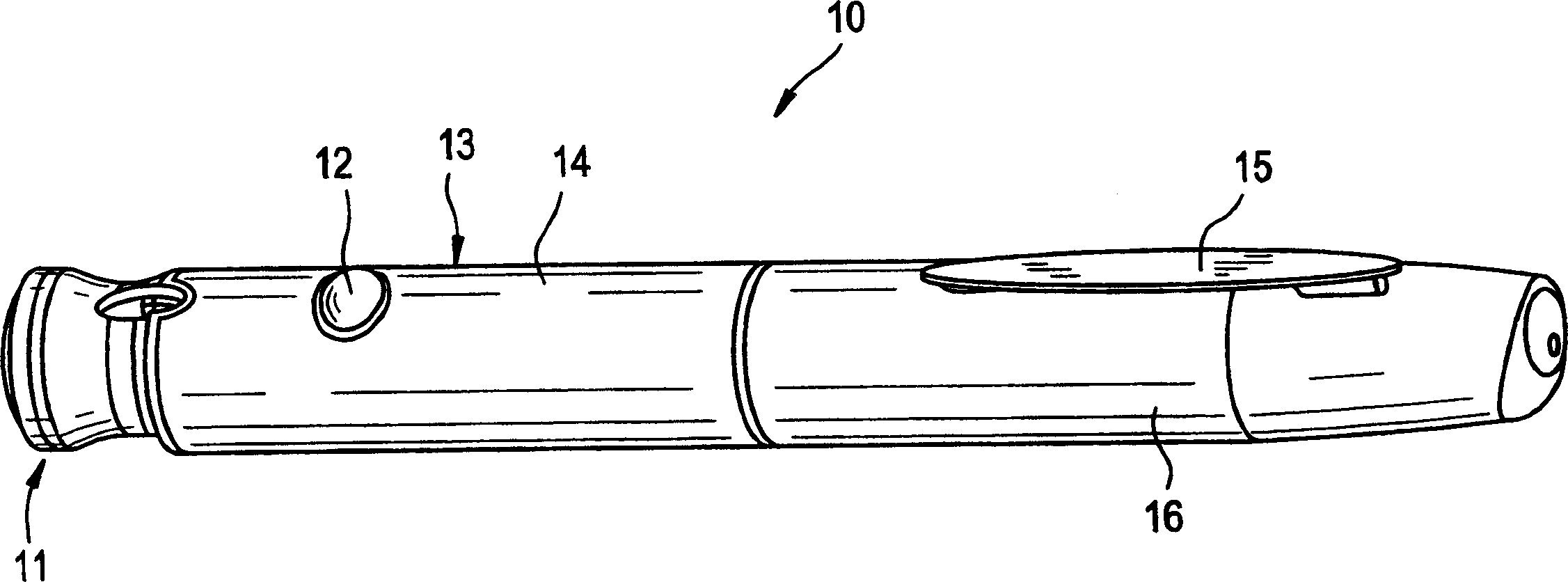
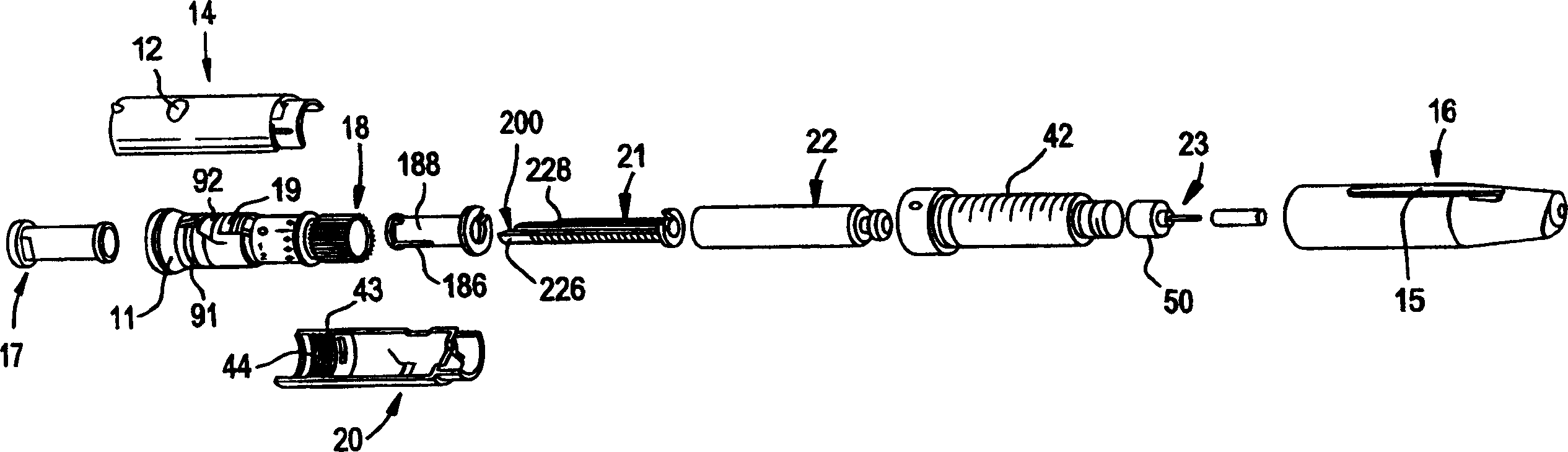
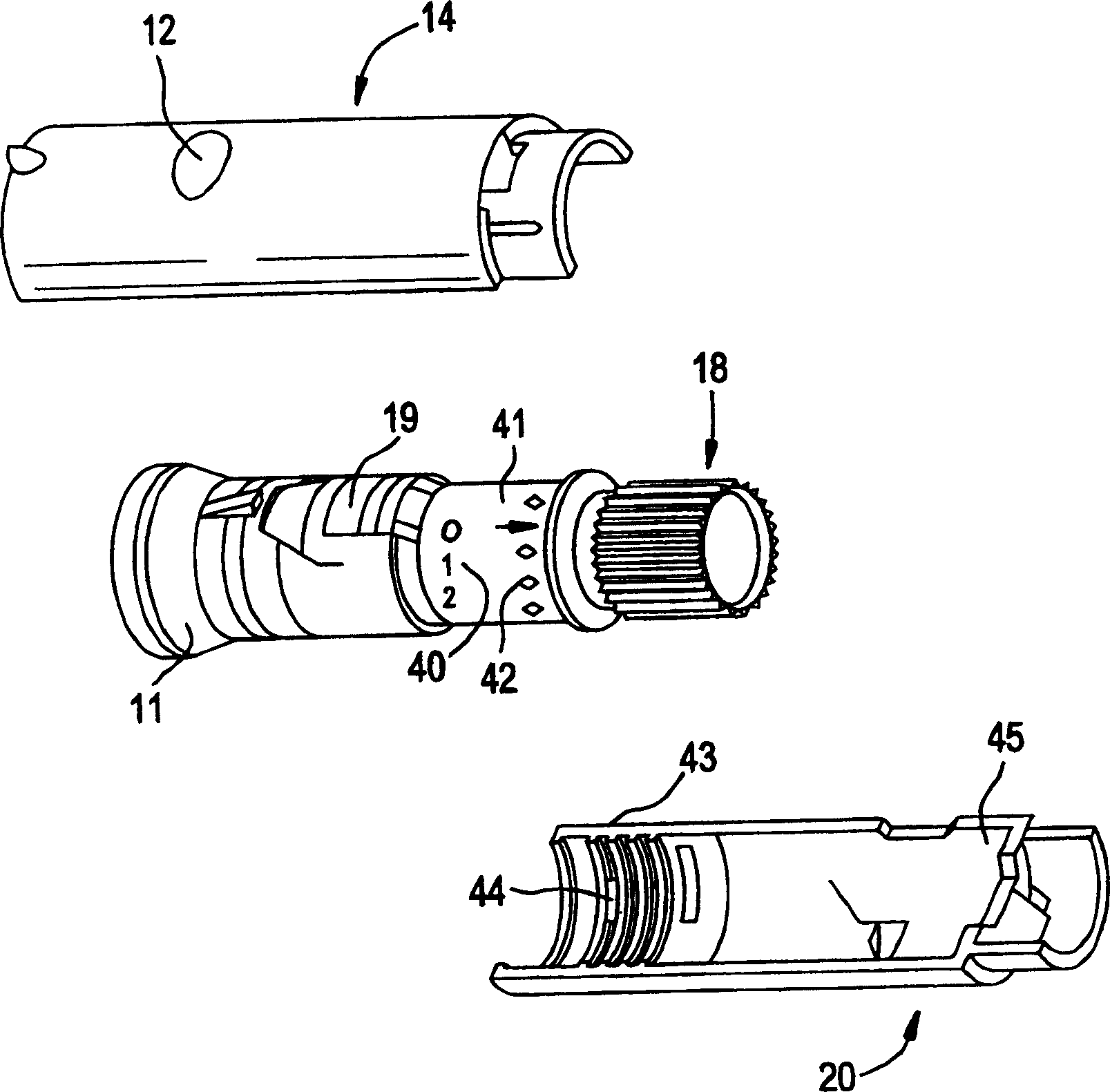
[0027] figure 1 The pen device of the present invention is shown in . The pen device 10 takes the shape of a standard writing pen and has a clip 15 attached to the cap 16 which allows the pen to be securely secured in a pocket or other carrying device. Such as figure 2 As shown in , the cap 16 protects the pen needle 23 and provides access to a reservoir or cartridge 22 . To achieve the single dose feature of the present invention, the injector body of the pen device is provided with a hard stop. Although the position of the hard stop in the pen is not critical, it must be positioned in such a way that the dose adjustment knob 11 cannot be rotated outwards so that the The location where the pen delivers more than a predetermined maximum single dose. In cases where PTH is prescribed for the treatment of osteoporosis, a single daily dose of 80 μL has been established to be effective. Preferably, the hard stop is lo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com