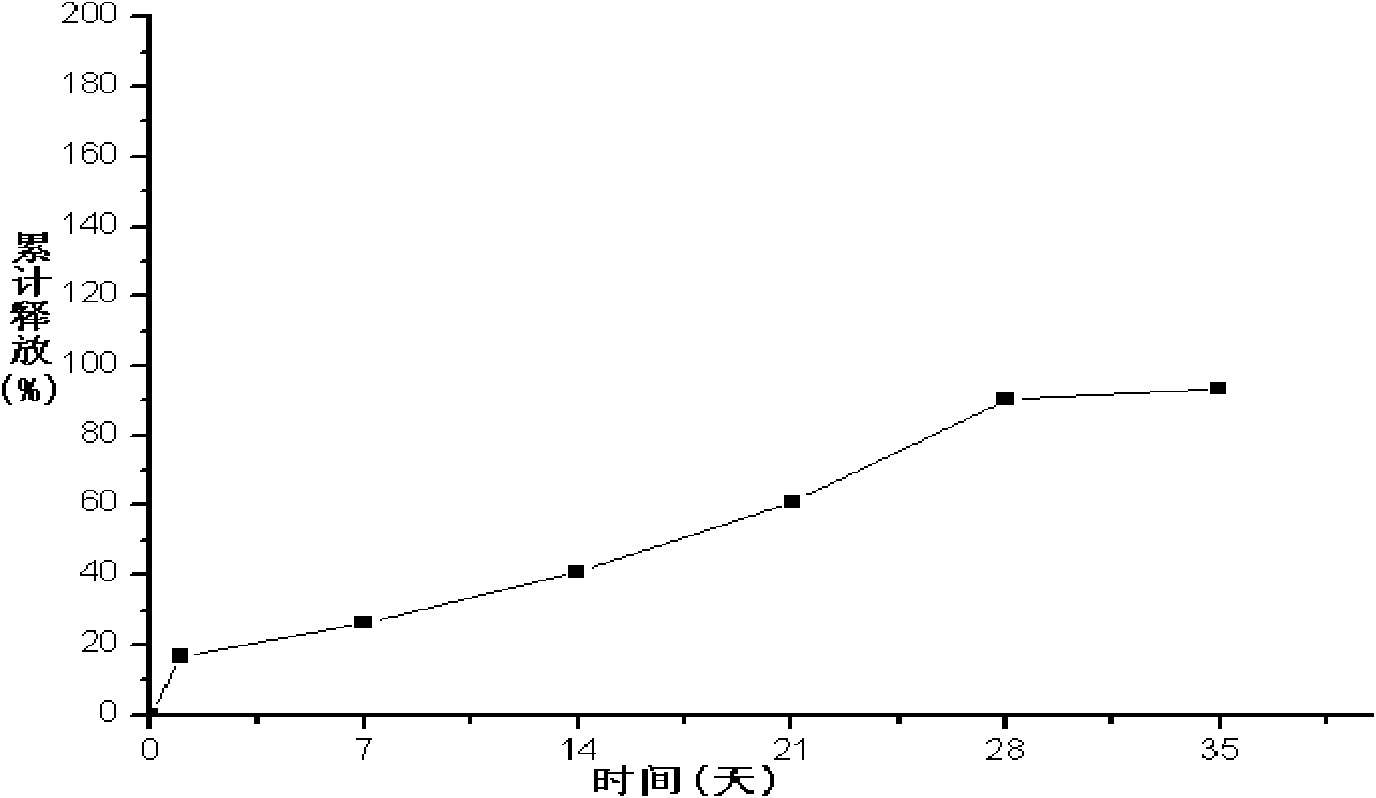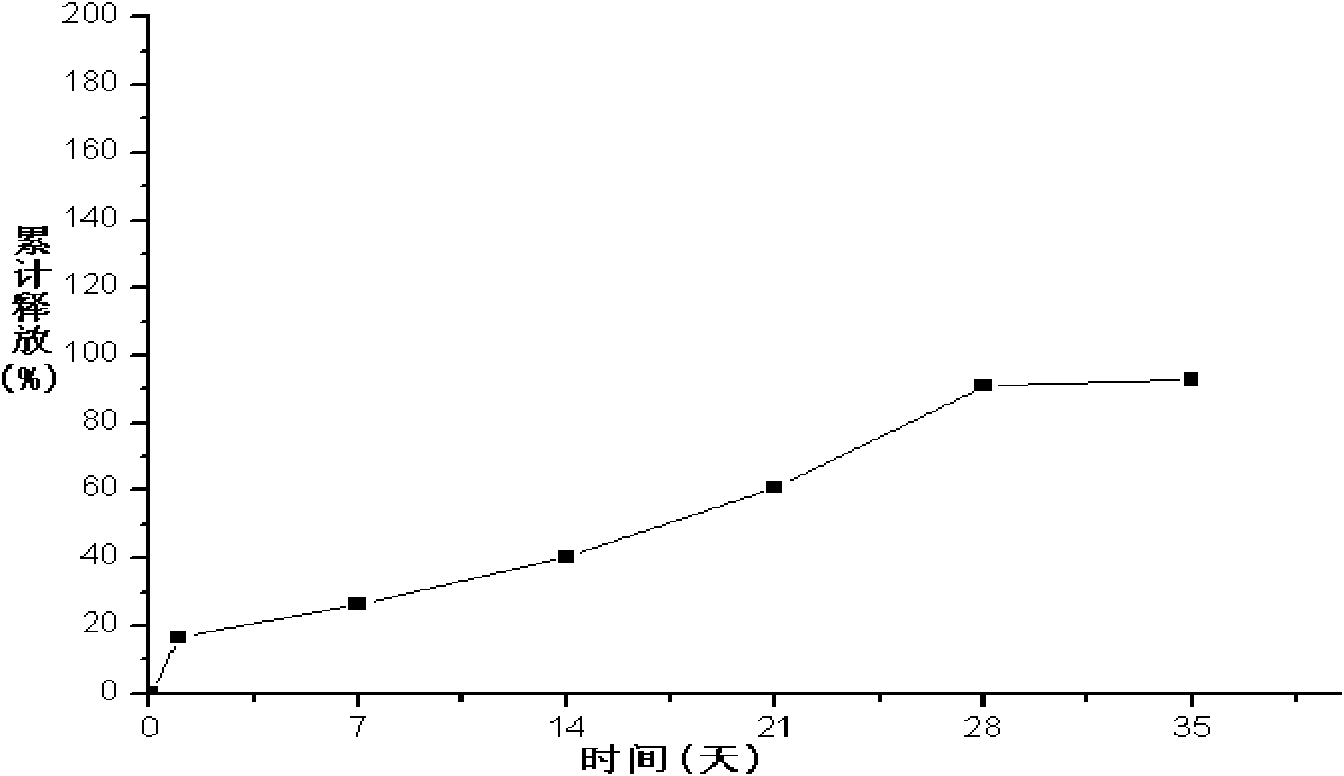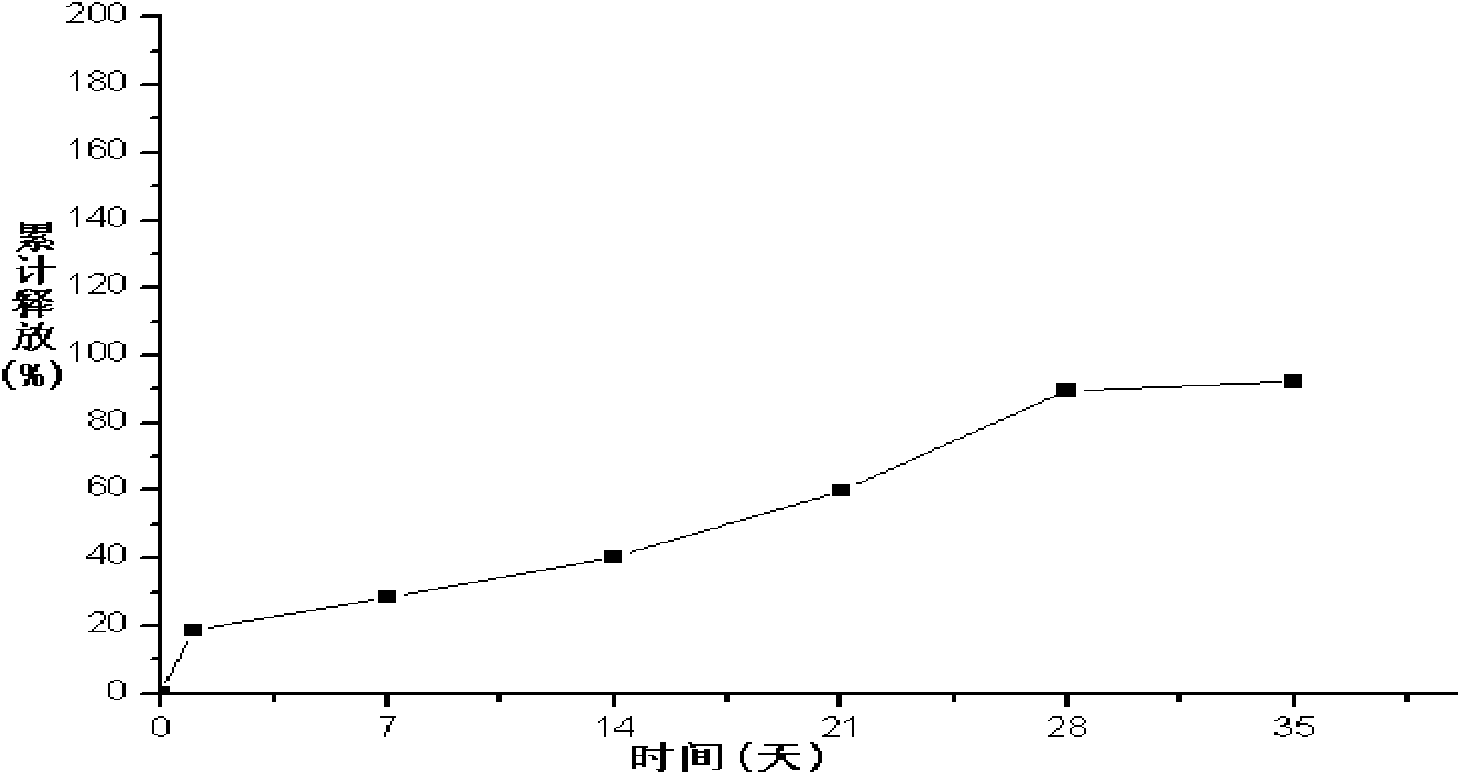Patents
Literature
513 results about "Subcutaneous injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A subcutaneous injection is administered as a bolus into the subcutis, the layer of skin directly below the dermis and epidermis, collectively referred to as the cutis. Subcutaneous injections are highly effective in administering medications such as insulin, morphine, diacetylmorphine and goserelin. Subcutaneous (as opposed to intravenous) injection of recreational drugs is referred to as "skin popping." Subcutaneous administration may be abbreviated as SC, SQ, sub-cu, sub-Q, SubQ, or subcut. Subcut is the preferred abbreviation for patient safety.
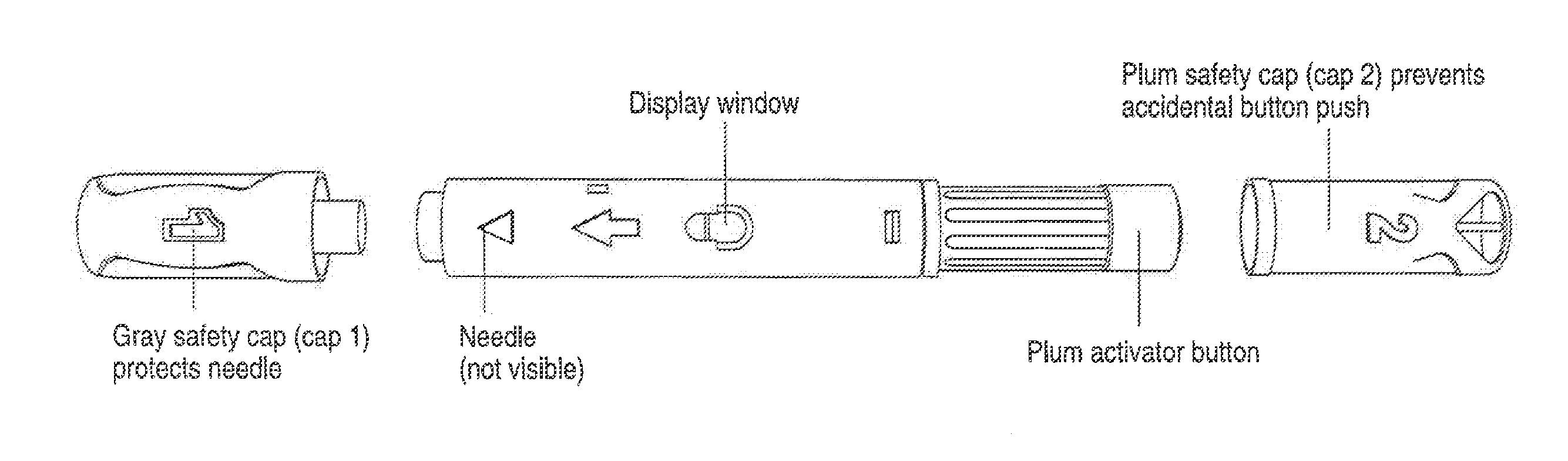
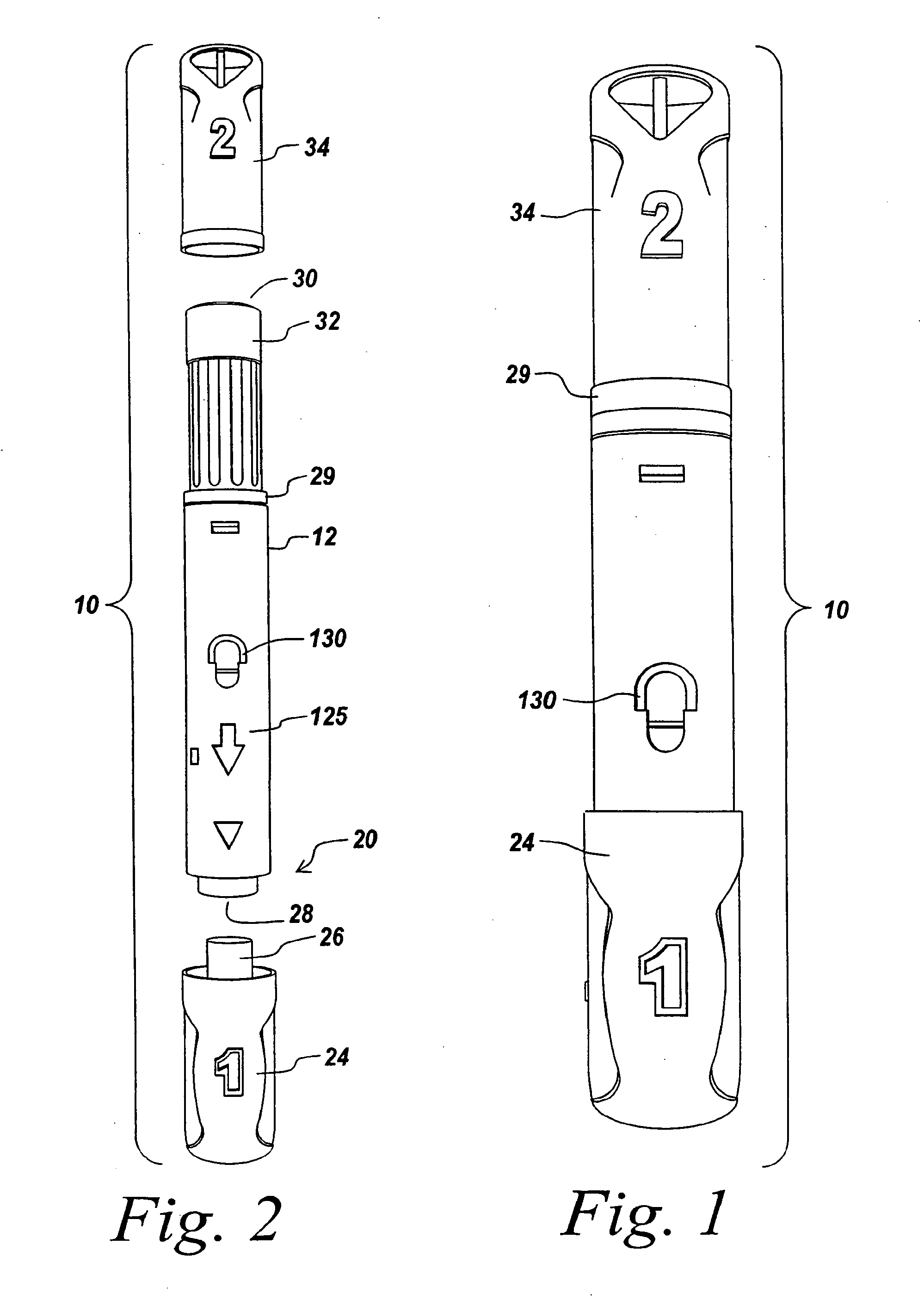
Automatic injection device
ActiveUS20100160894A1Easy to useReduce anxietyPeptide/protein ingredientsAntipyreticHypodermoclysisSubcutaneous injection
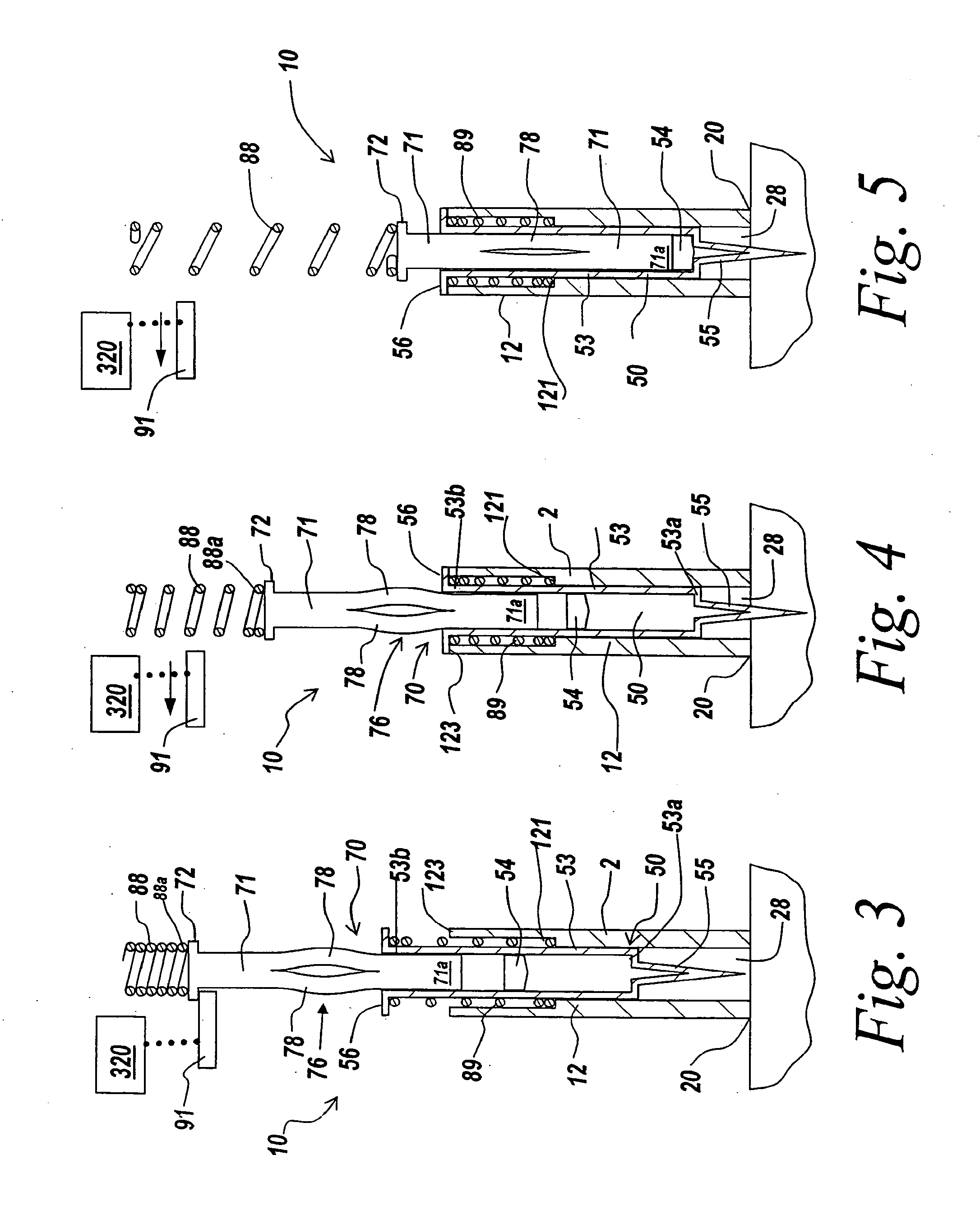
The invention provides an automatic injection device for providing a subcutaneous injection of a substance into a user, comprising: a housing having an open first end and a second end; a syringe movably disposed in the housing, the syringe including a barrel portion for holding the substance, a hollow needle in fluid communication with the barrel portion for ejecting the substance from the syringe, and a bung for sealing the barrel portion and selectively applying pressure to the substance to force the substance through the hollow needle; a plunger for first moving the syringe towards the first end such that the needle projects from the first end and subsequently applying pressure to the bung, the plunger including a rod connected at a first end to the bung, a compressible expanded central portion and a flange between a second end of the rod and the compressible expanded central portion; and a biasing mechanism for biasing the plunger towards the first open end of the housing, the biasing mechanism disposed about the second end of the rod between the flange and the second end of the housing. The present invention also provides methods and kits for using an automatic injection device, and methods and kits for promoting an automatic injection device comprising a medication based on advantageous properties of the device as compared to a pre-filled syringe. The invention also provides methods and kits for training a recipient on use of the automatic injection device.
Owner:ABBVIE BIOTECHNOLOGY LTD
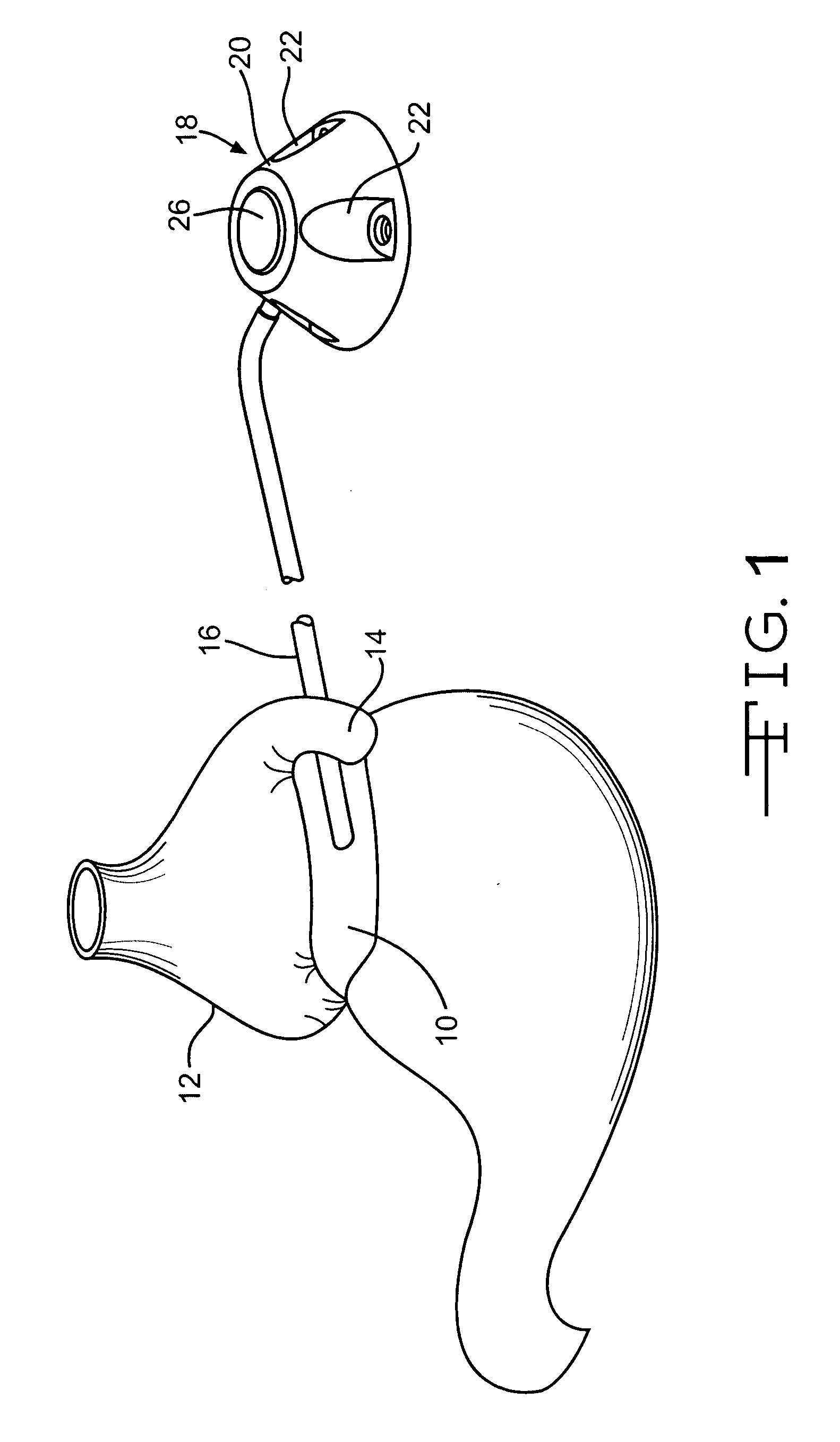
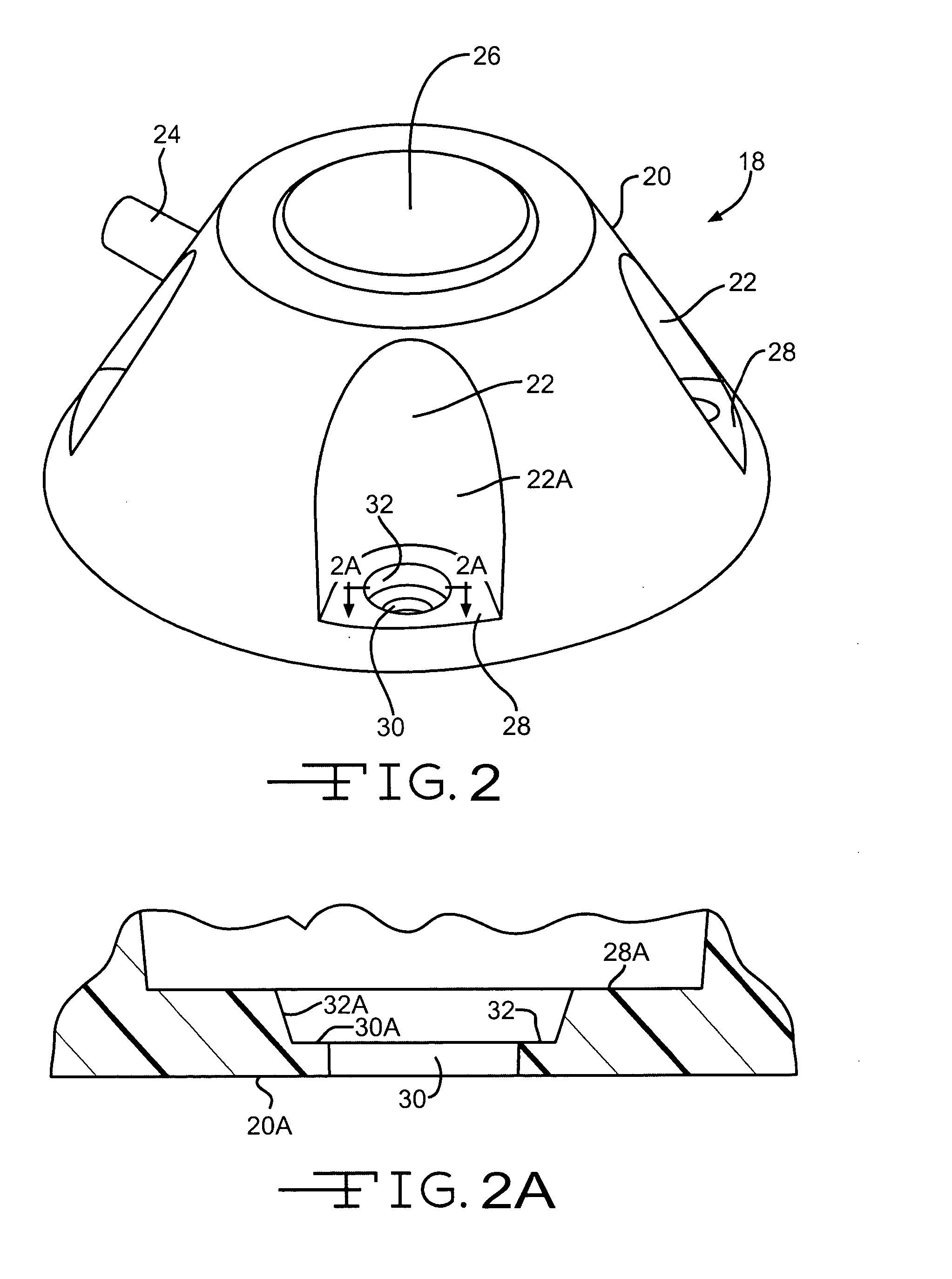
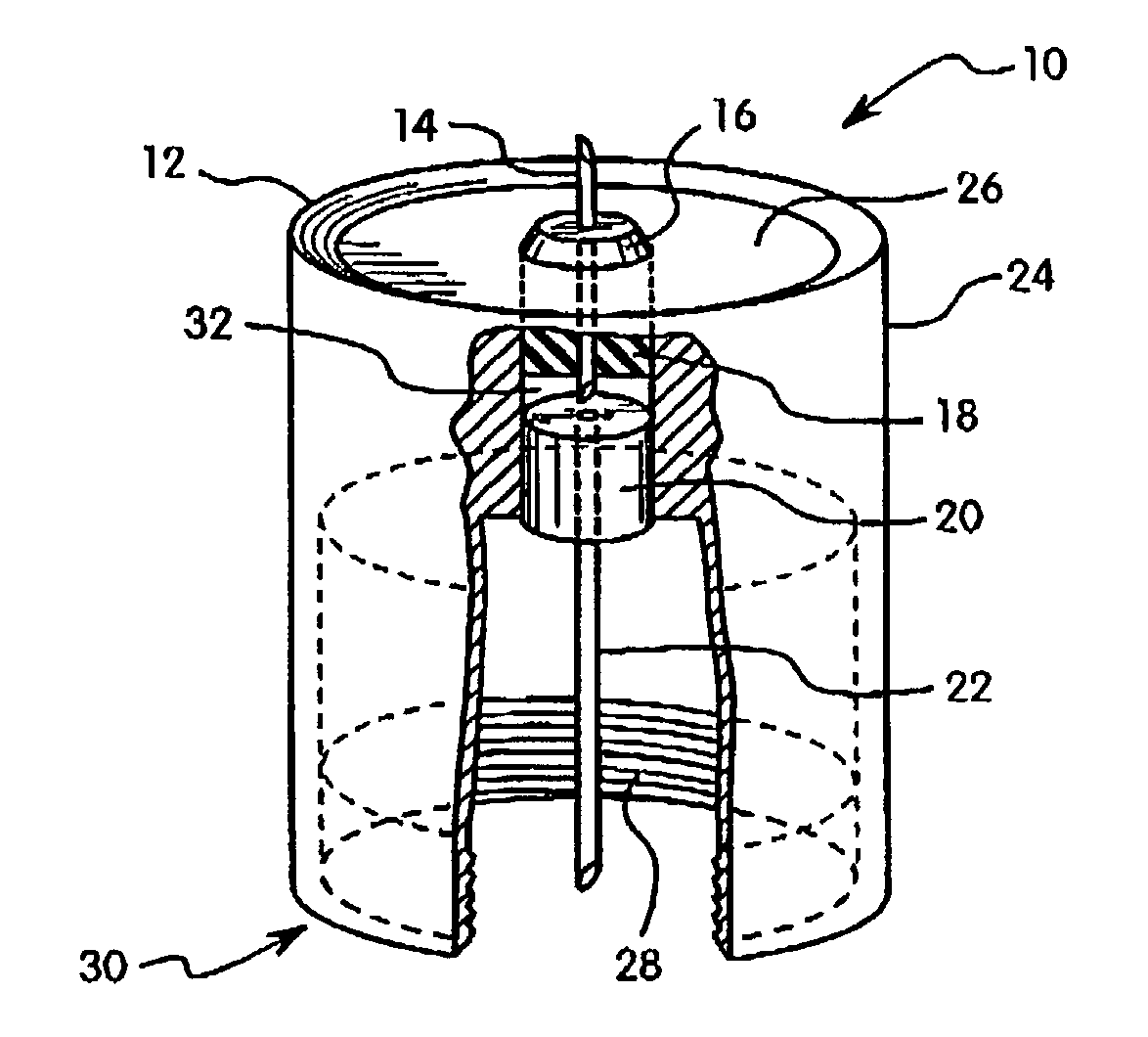
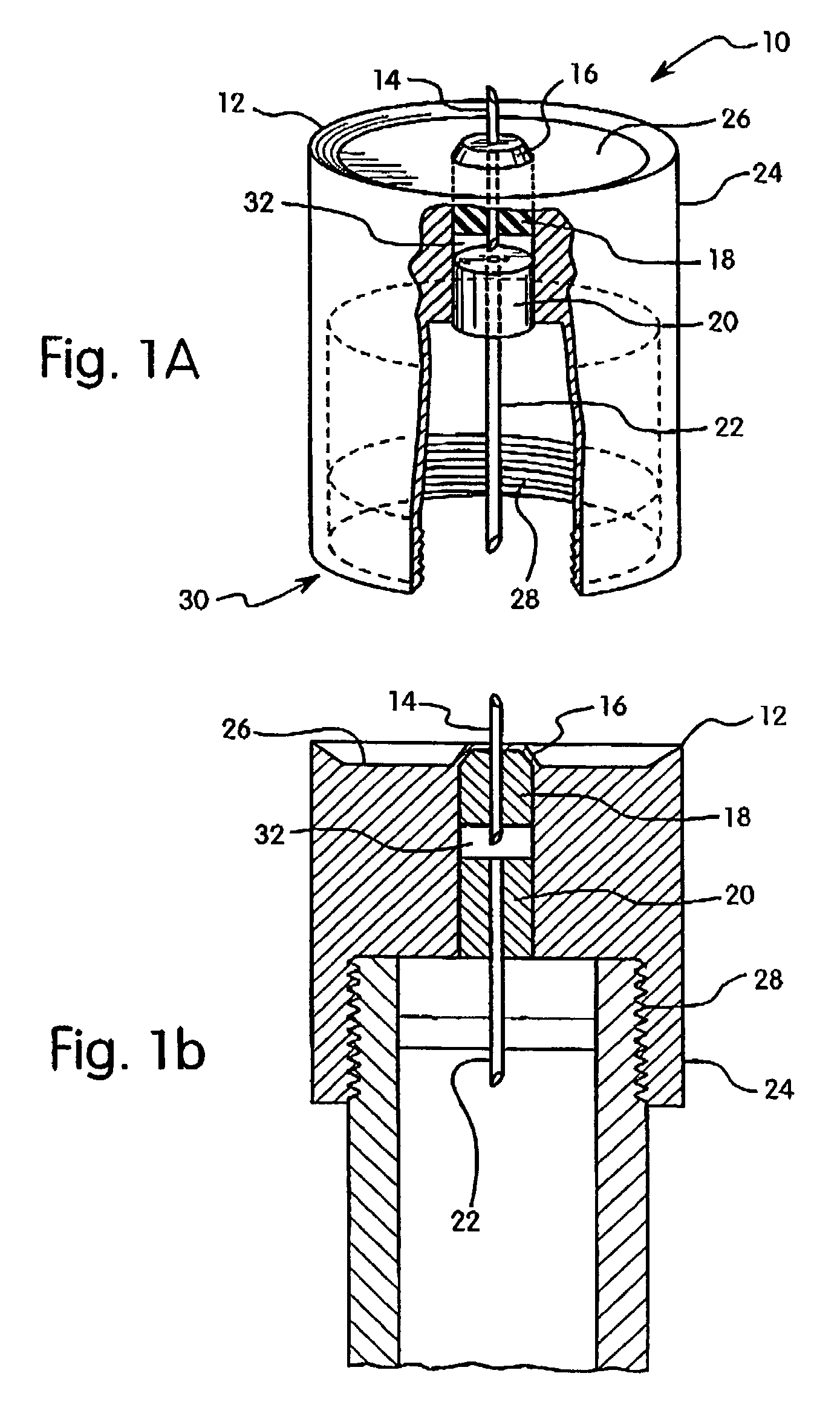
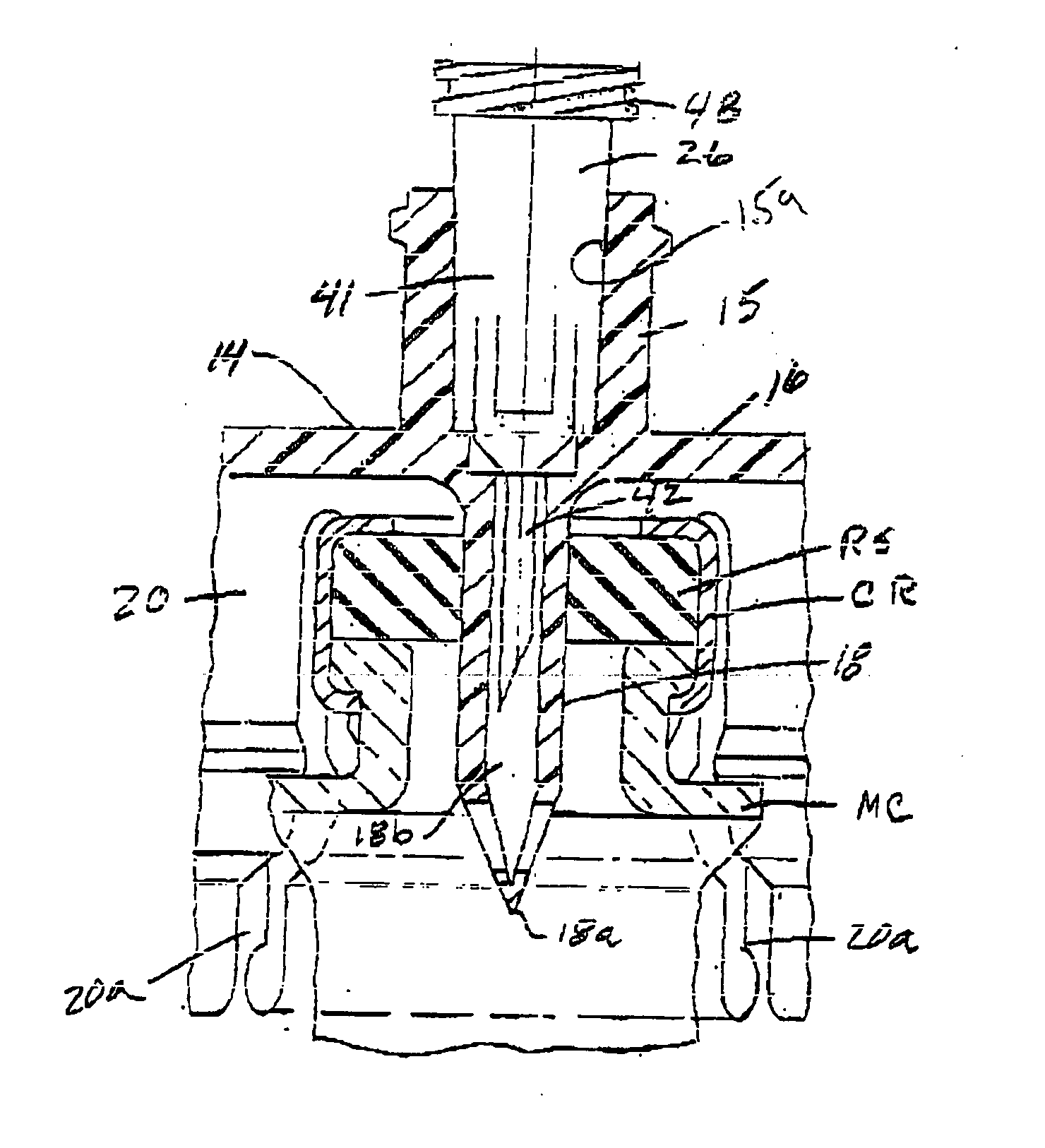
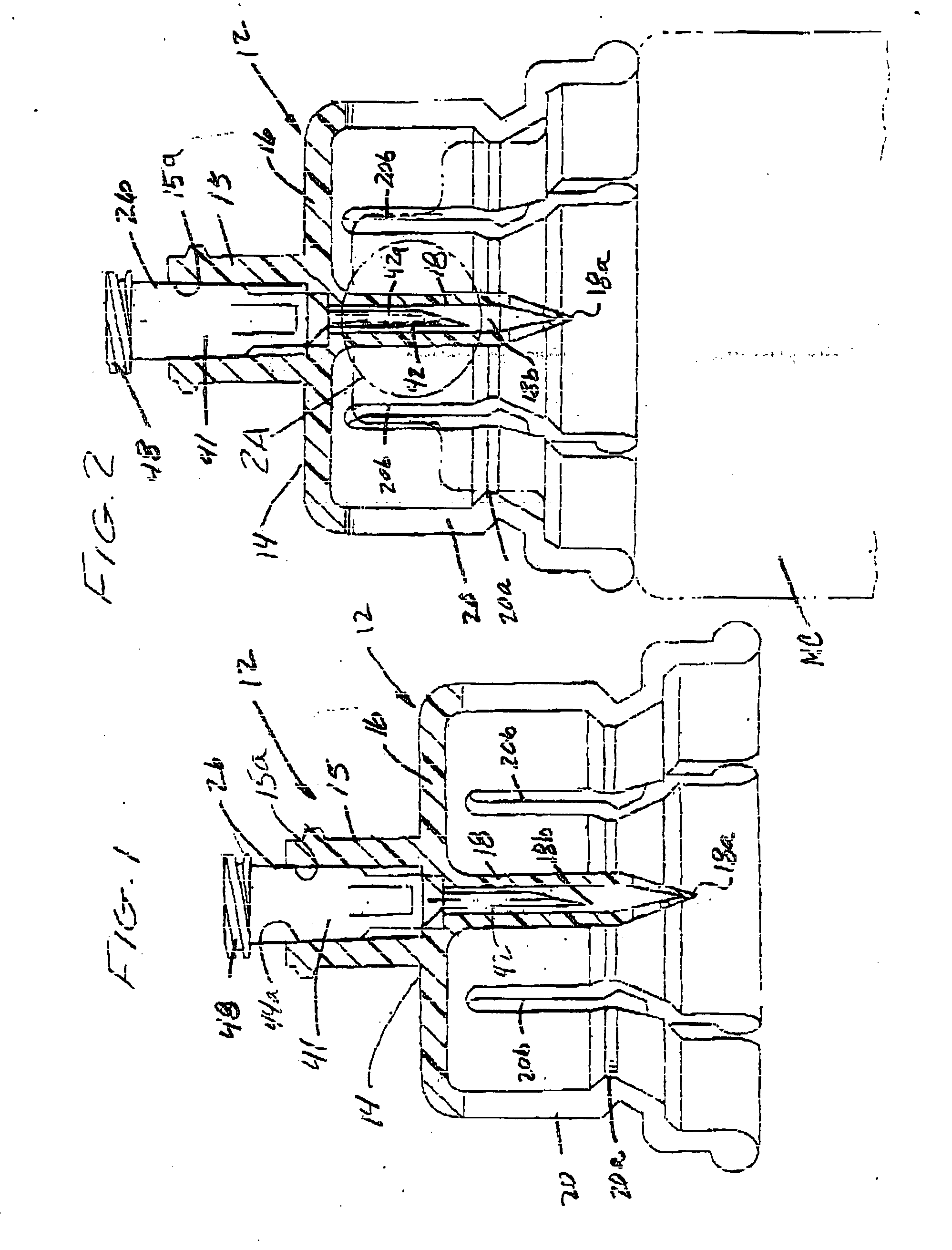
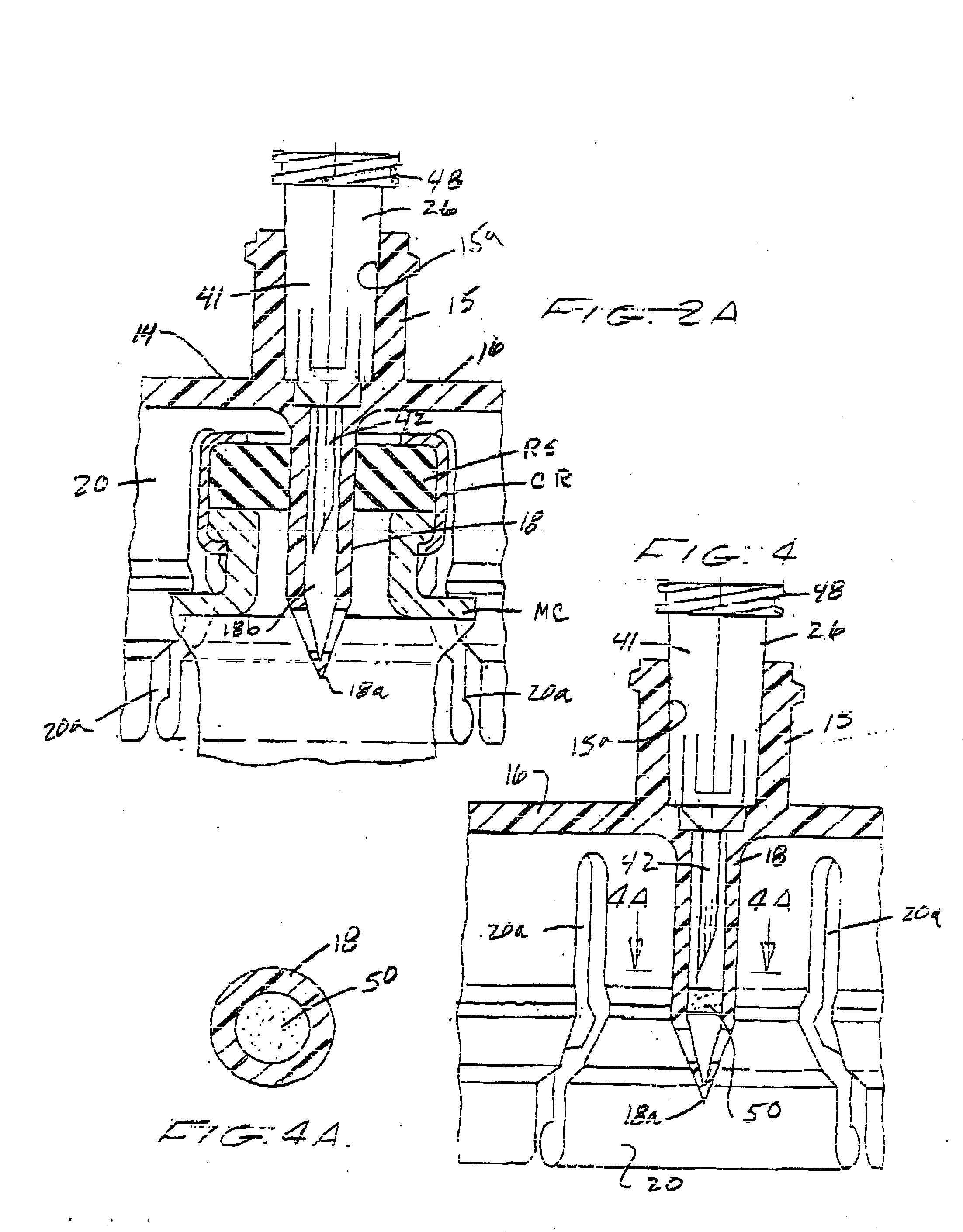
Subcutaneous injection port for applied fasteners
A injection port includes a delivery device guidance configuration which cooperates with a separate fastener delivery device. The delivery device guidance configuration guides and receives the delivery end of the device to locate it in the proper position to apply the fastener. Although not limited thereto, a particular device and fastener are shown in use with the injection port in which a guidance bore guides and receives the delivery end of the device to locate it appropriately to deliver the fastener in the appropriate position.
Owner:ETHICON INC +1
Automatic injection device
ActiveUS20120107783A1Easy to useReduce anxietyAntipyreticAutomatic syringesSubcutaneous injectionInjection device
Owner:ABBVIE BIOTECHNOLOGY LTD
Disposable double pointed injection needle, and an insulin injection system comprising a disposable double pointed injection needle
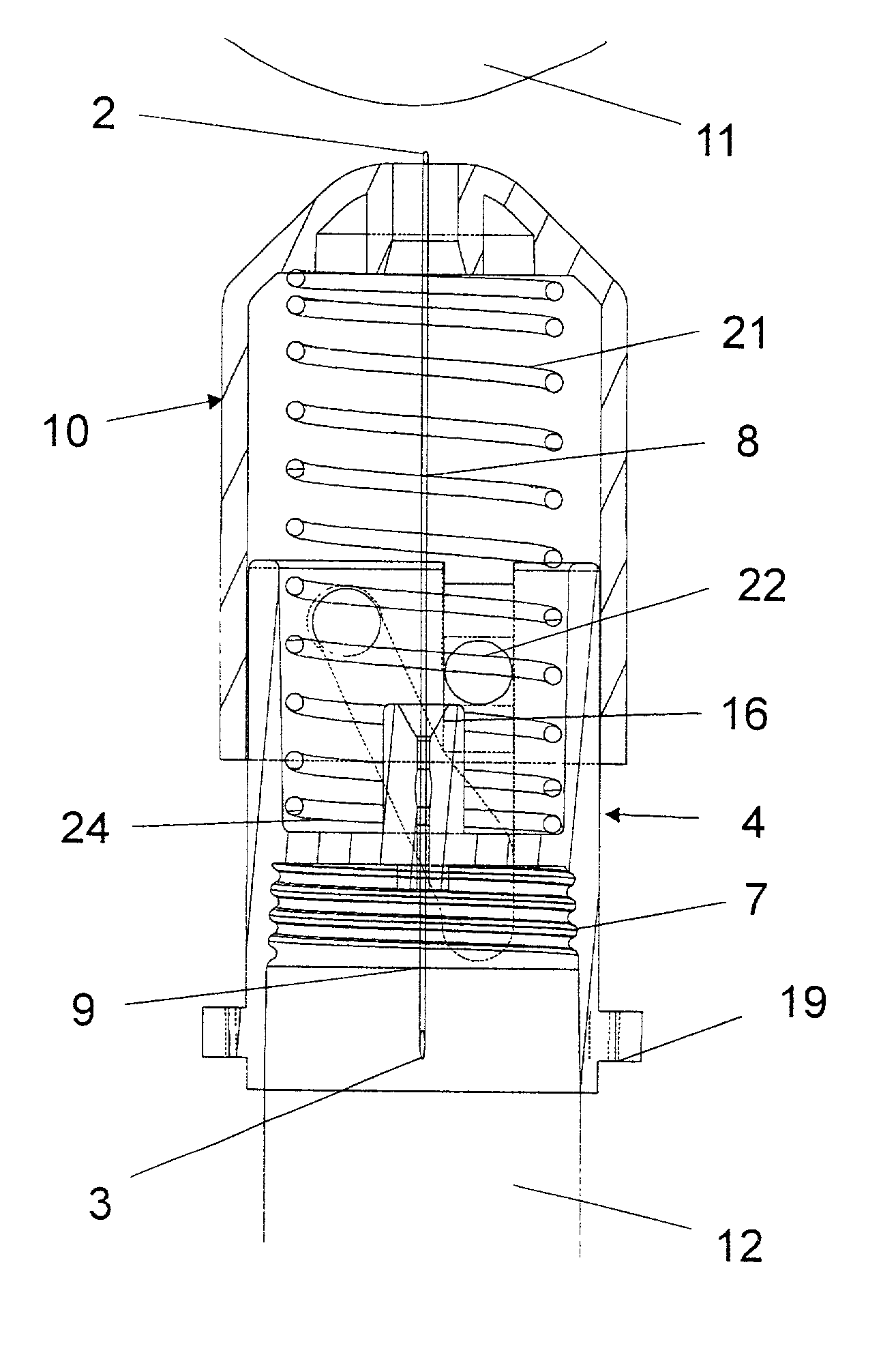
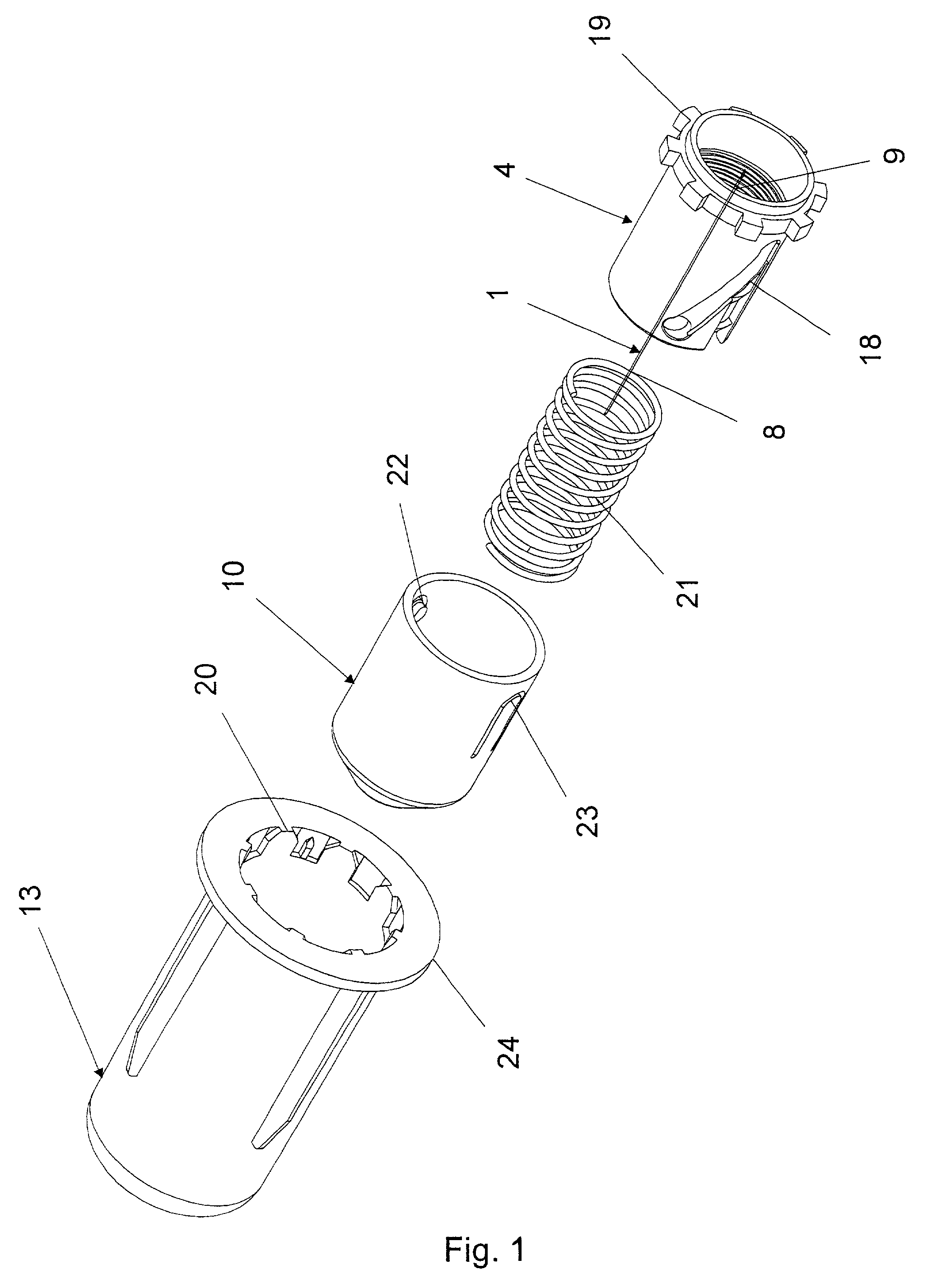
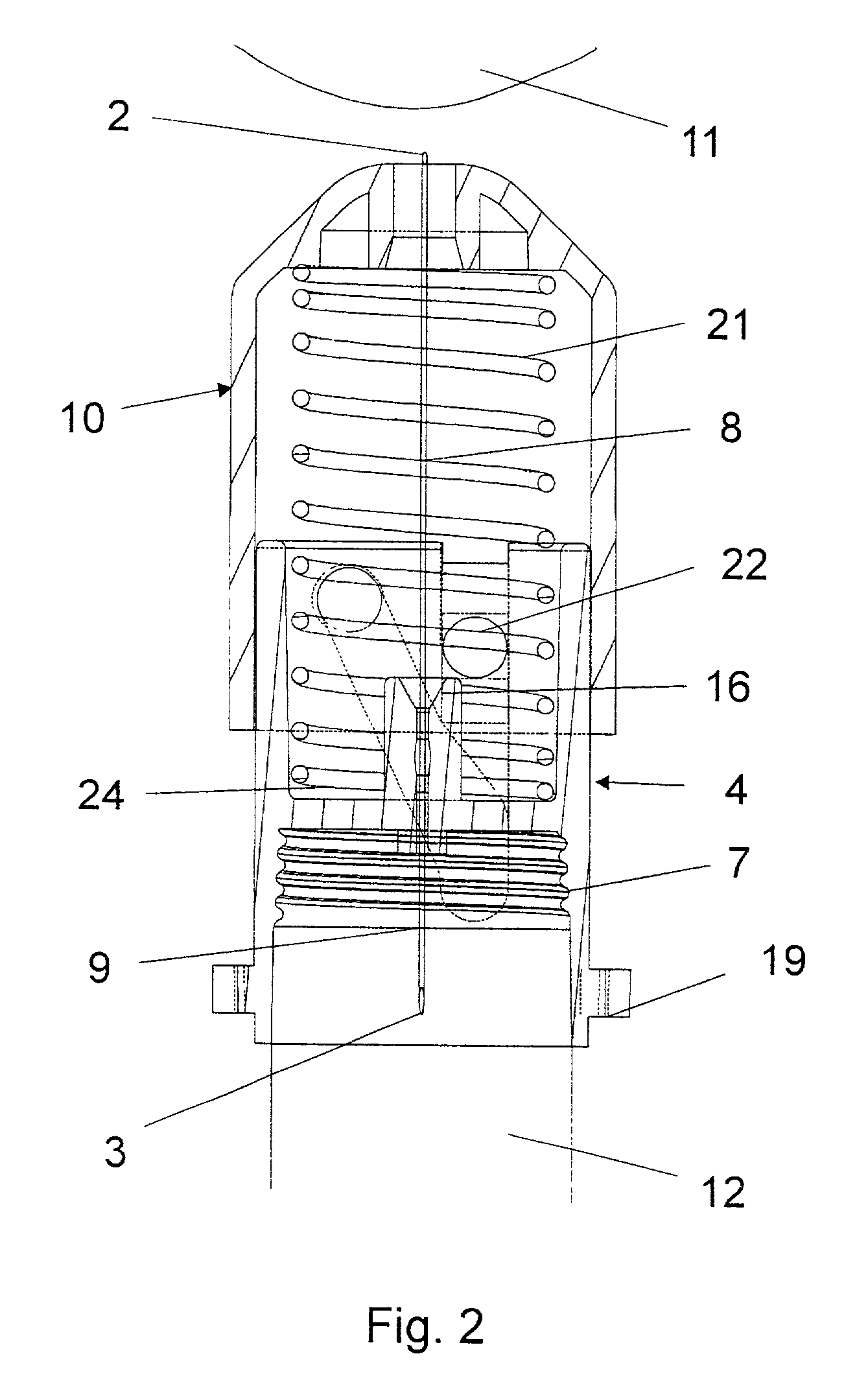
A disposable double pointed injection needle has a needle hub to which a thin needle cannula is permanently fastened and which needle hub can be mounted on to a syringe comprising a dose setting and injection mechanism and a cartridge containing a liquid medicine to be injected subcutaneously into a human body. The needle hub is provided with a safety shield guided on the outside surface of the needle hub. The safety shield is urged in a direction away from the needle hub by a spring located between the needle hub and the safety shield. The safety shield has a number of protrusions guided in guiding tracks on the outside surface of the needle hub. The guiding tracks are designed such that the safety shield during injection is moved towards the needle hub, and after injection is moved away from the needle hub by the spring and locked in an irreversible position where the safety shield covers the needle cannula and prevents accidental needle stick injuries.
Owner:NOVO NORDISK AS
Microneedle-based pen device for drug delivery and method for using same
ActiveUS7556615B2Accurate accessAccurate transmissionAmpoule syringesAutomatic syringesHypodermoclysisCompressible material
A system and method is provided for an injectable substance delivery pen comprising a microneedle hub assembly removably engaged with a pen device body which includes a cartridge, a plunger, and a drive mechanism. The hub assembly includes at least one microneedle for intradermal or shallow subcutaneous injection of the contents of the cartridge. The cartridge, plunger and drive mechanism components of the pen body are fabricated of non-compliant and non-compressible materials to allow effective communication of the cartridge contents via the microneedle patient interface.
Owner:BECTON DICKINSON & CO
Subcutaneous anti-HER2 antibody formulations and uses thereof
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
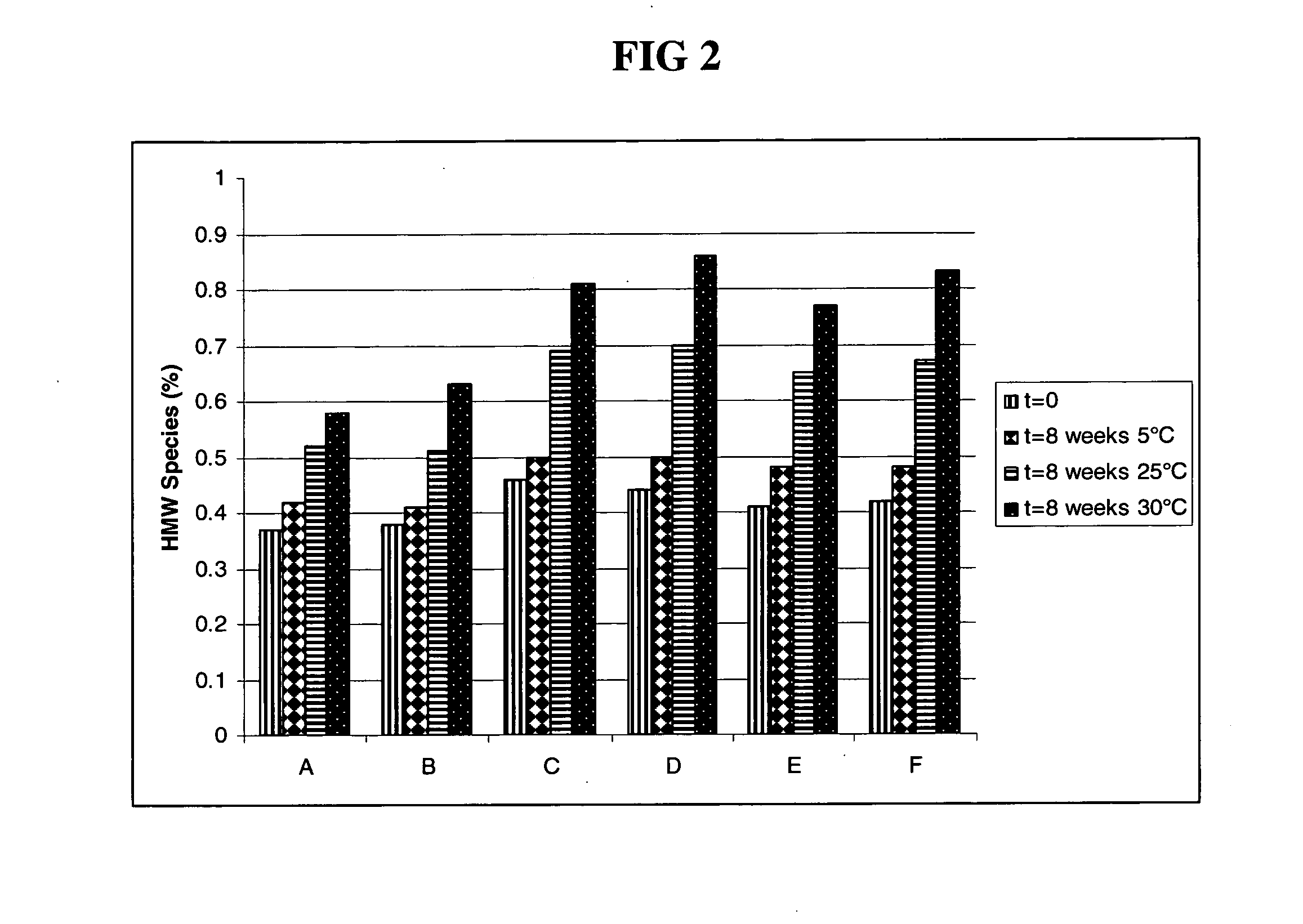
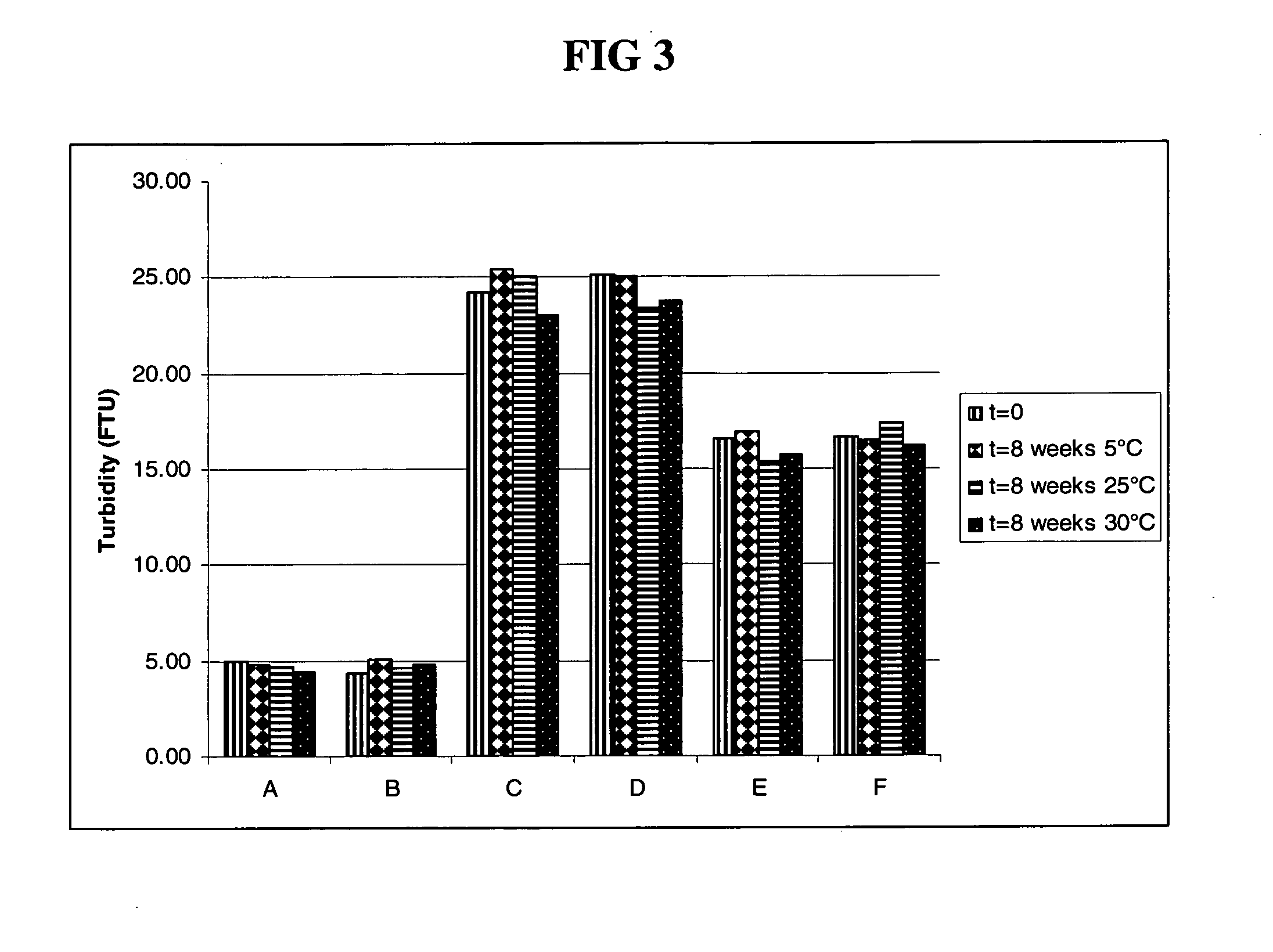
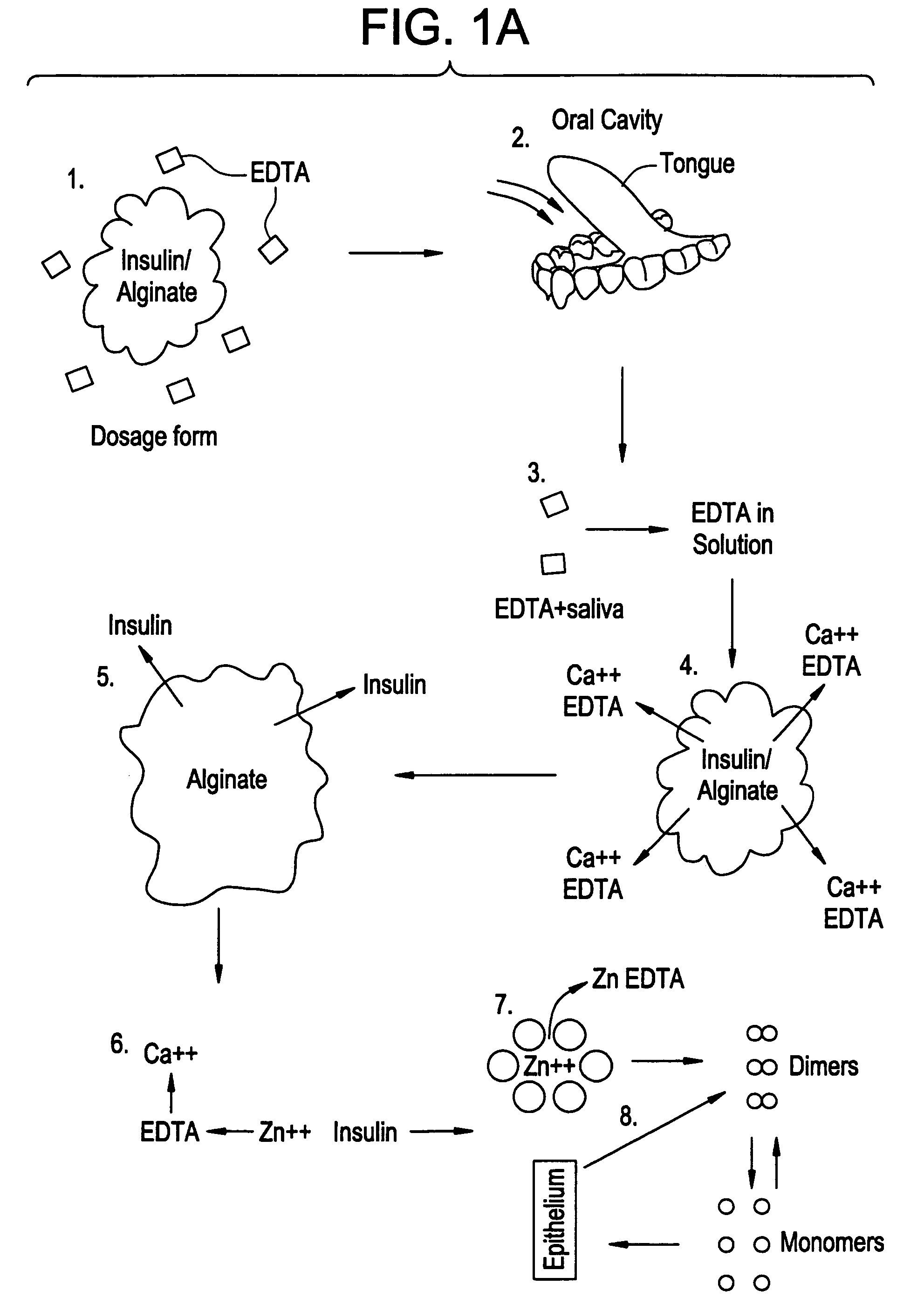
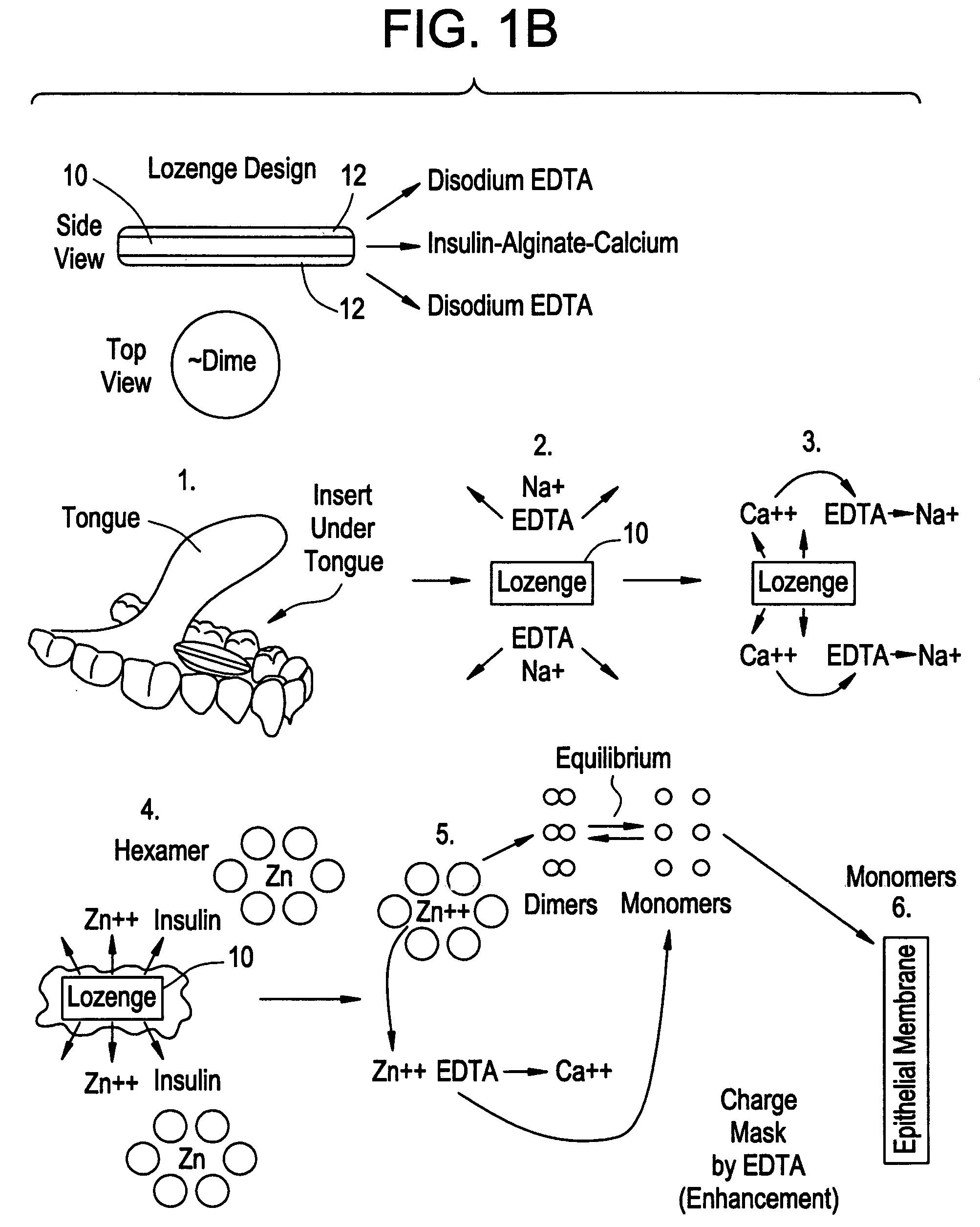
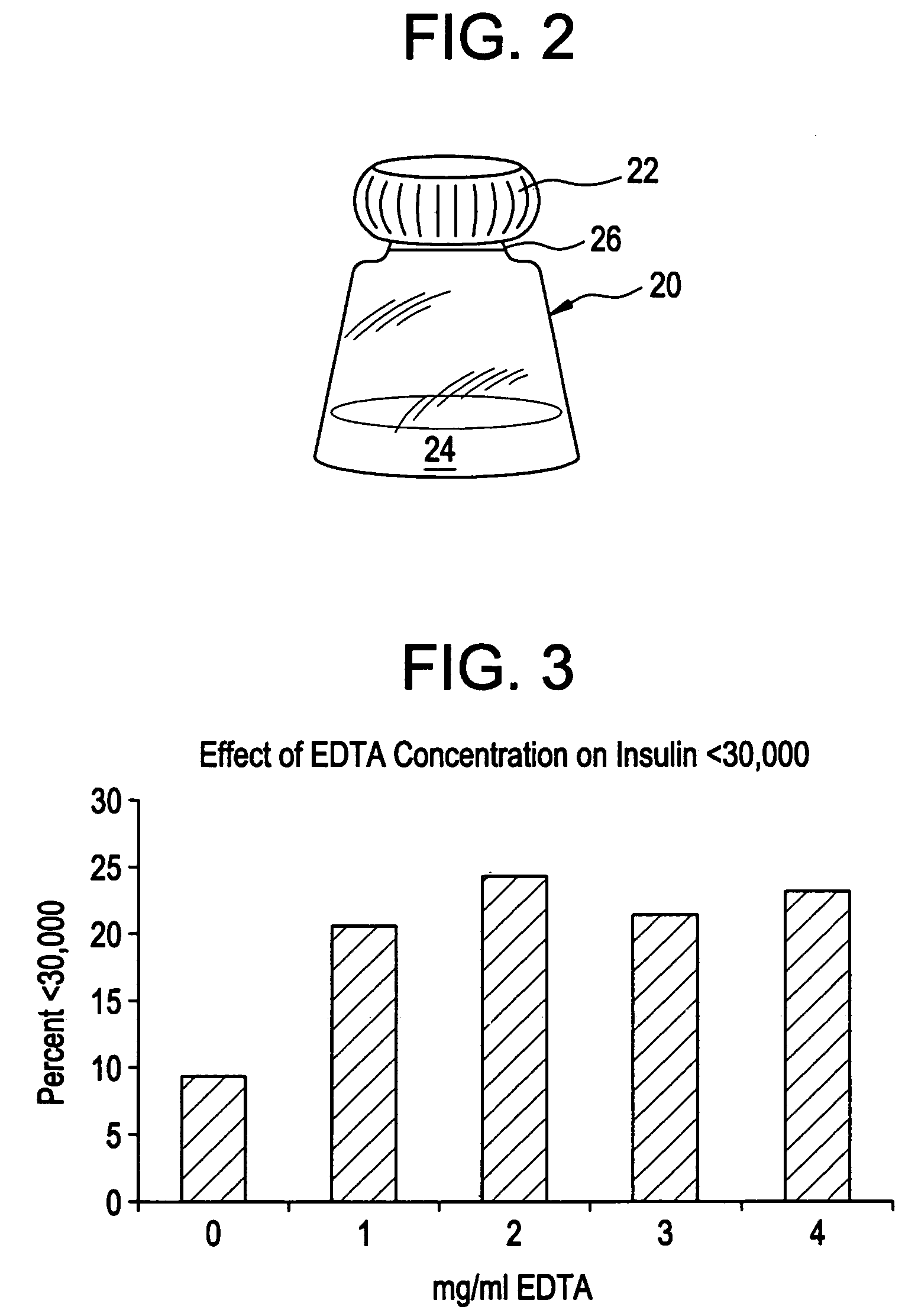
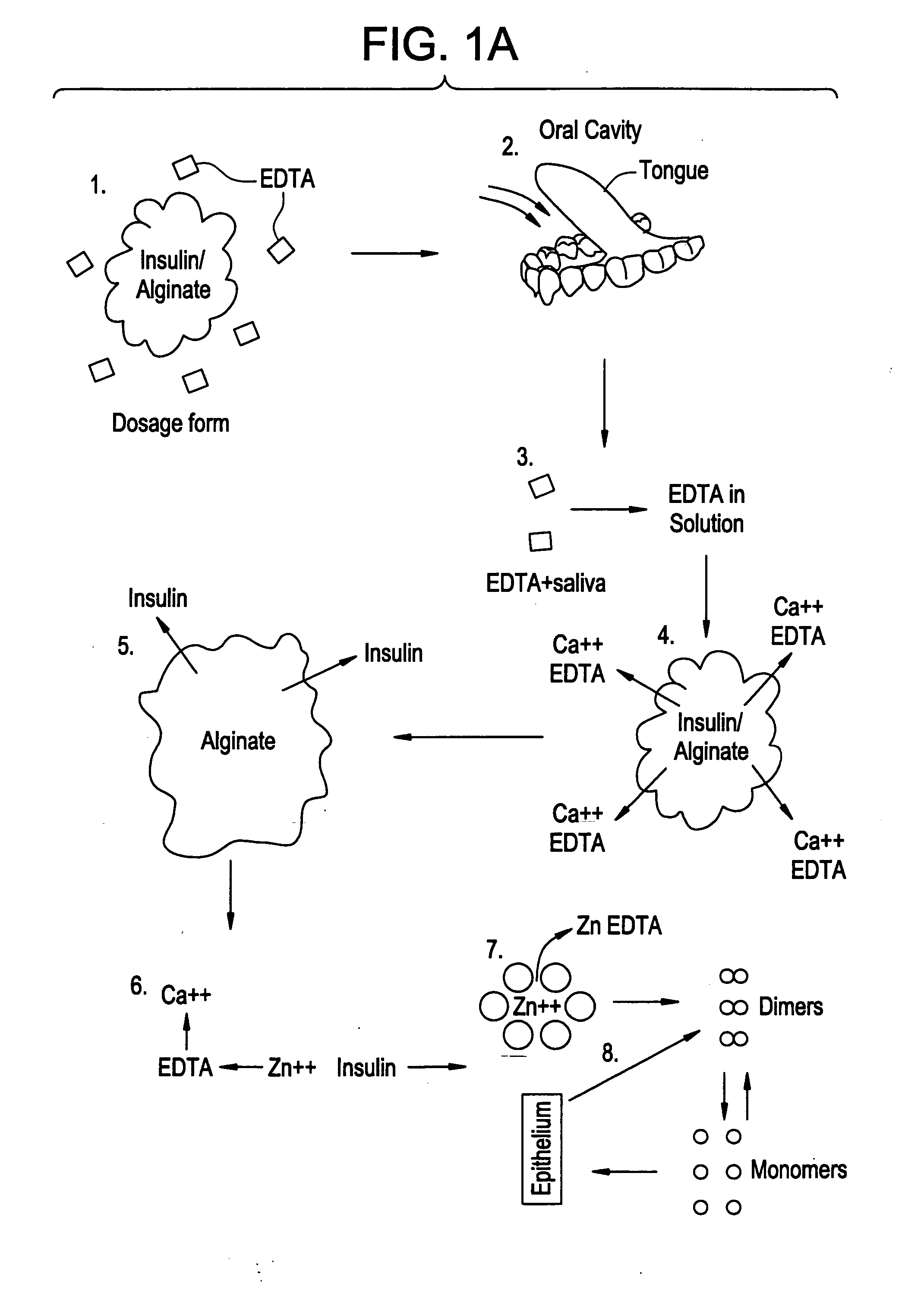
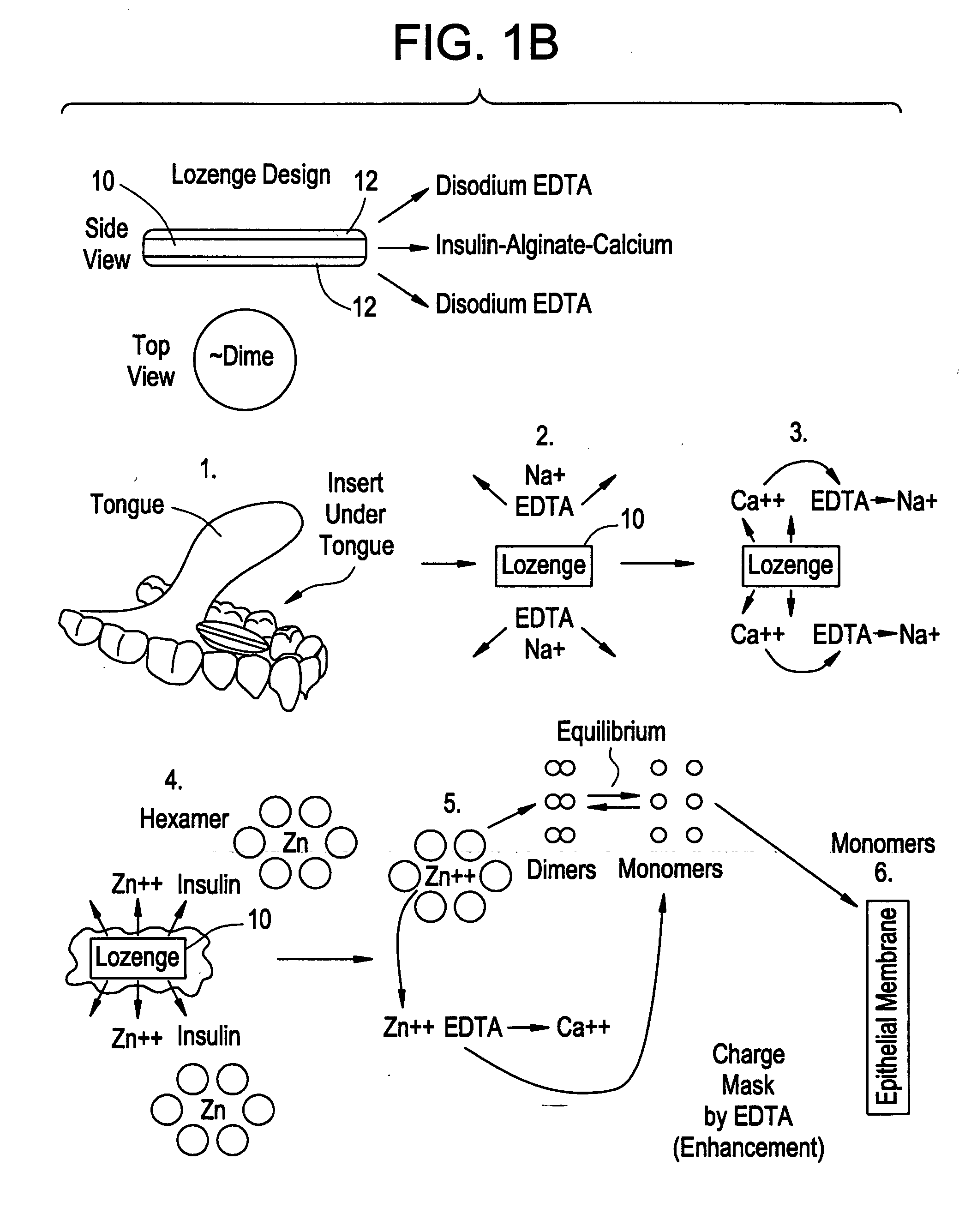
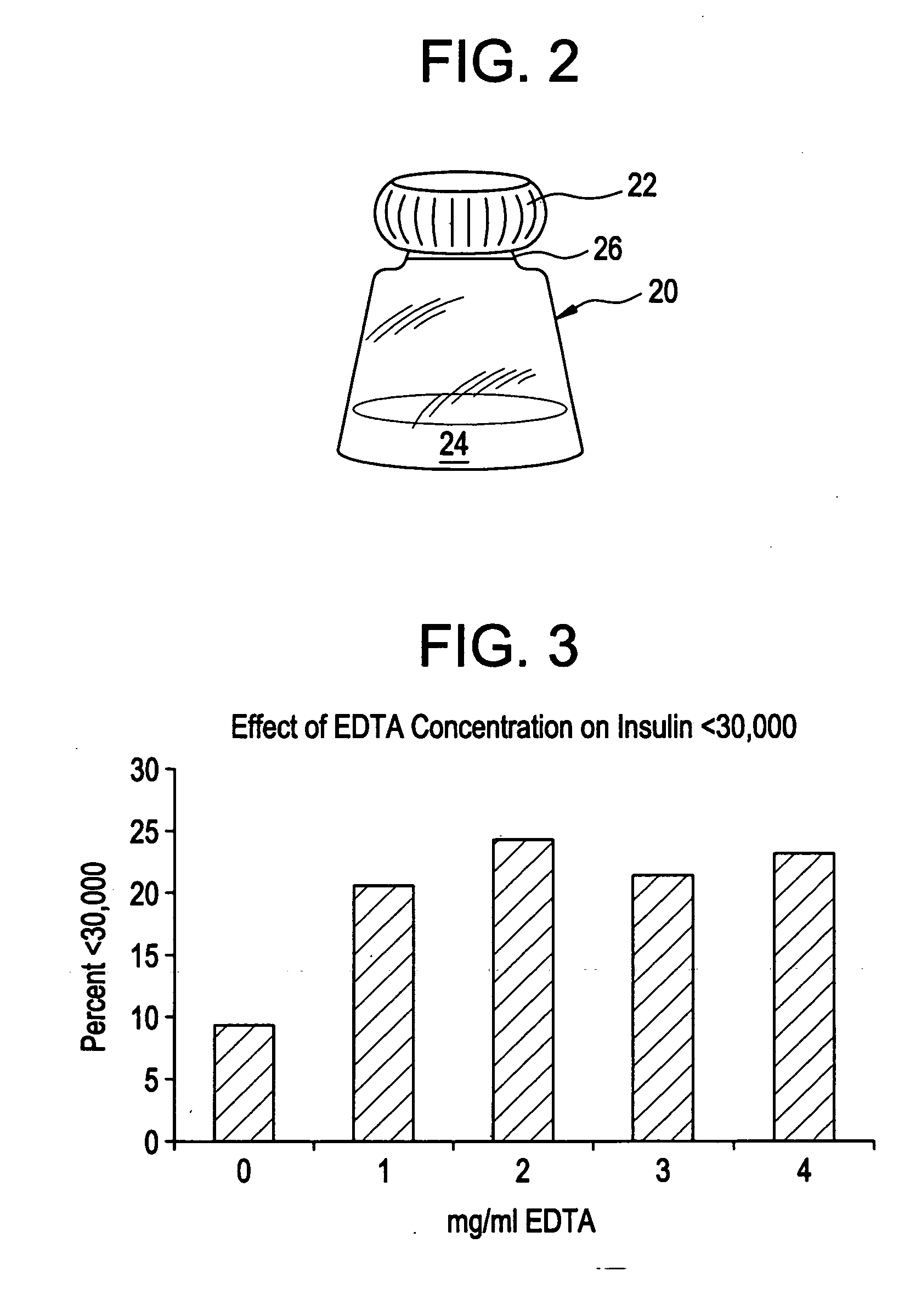
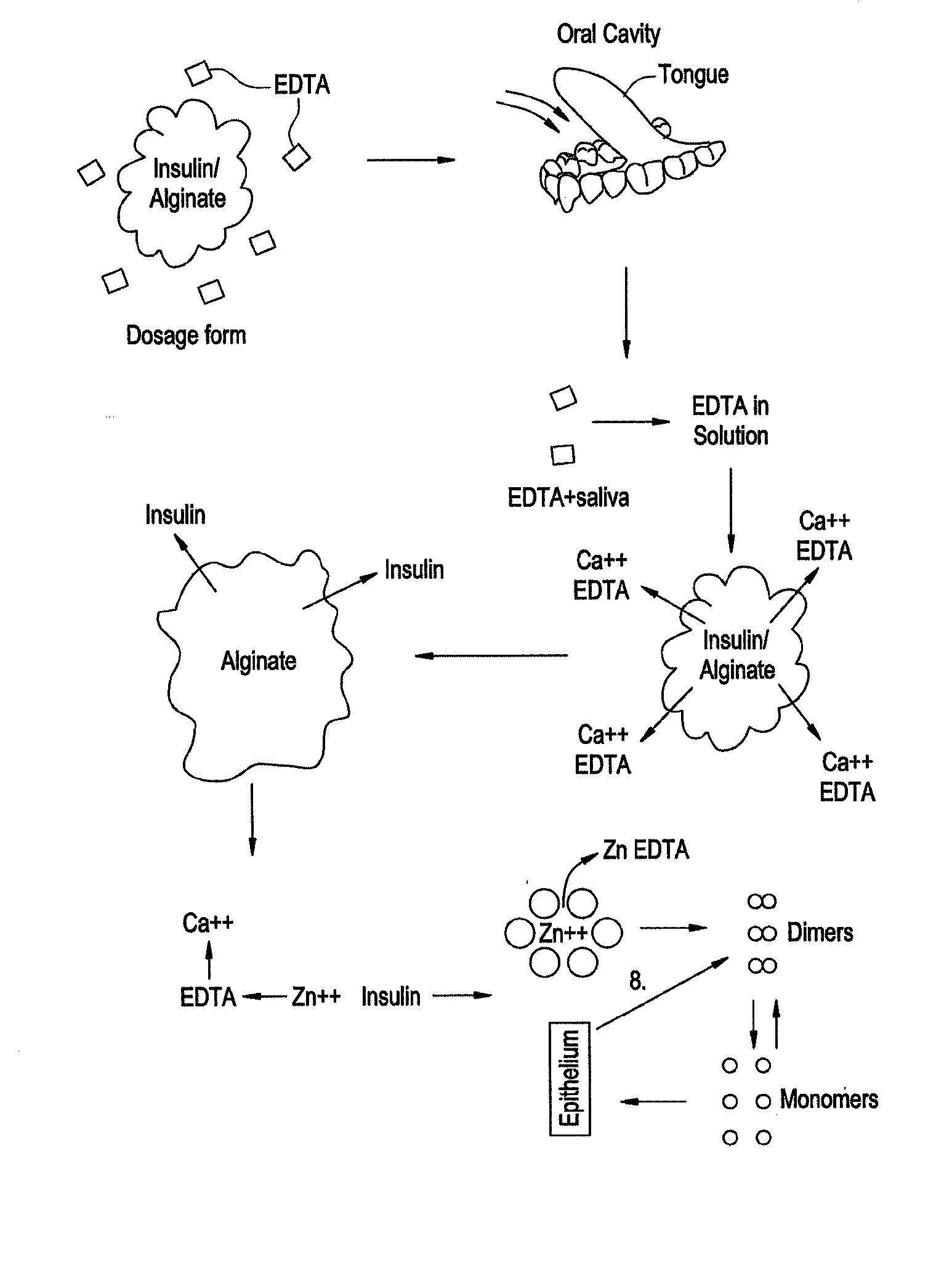
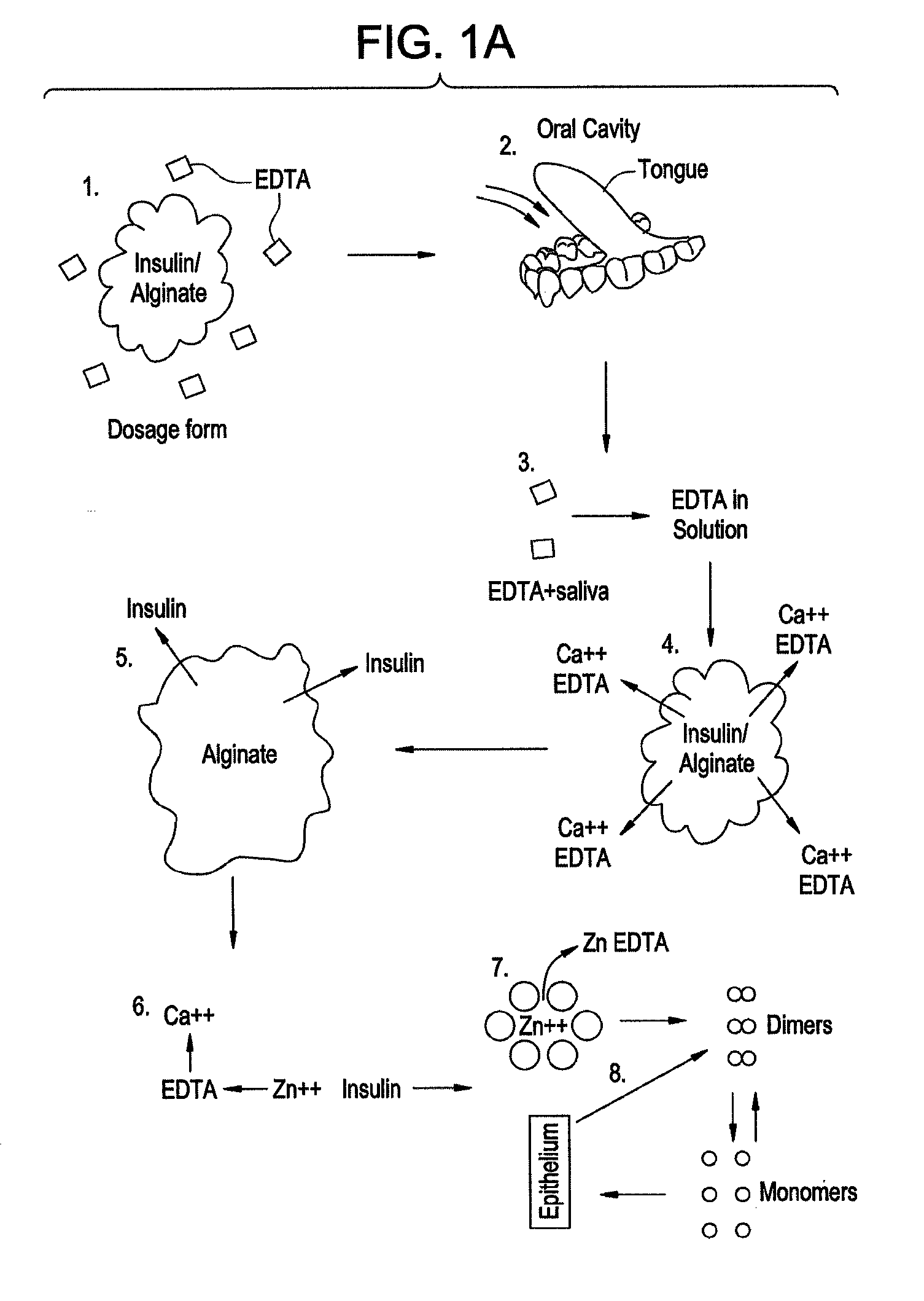
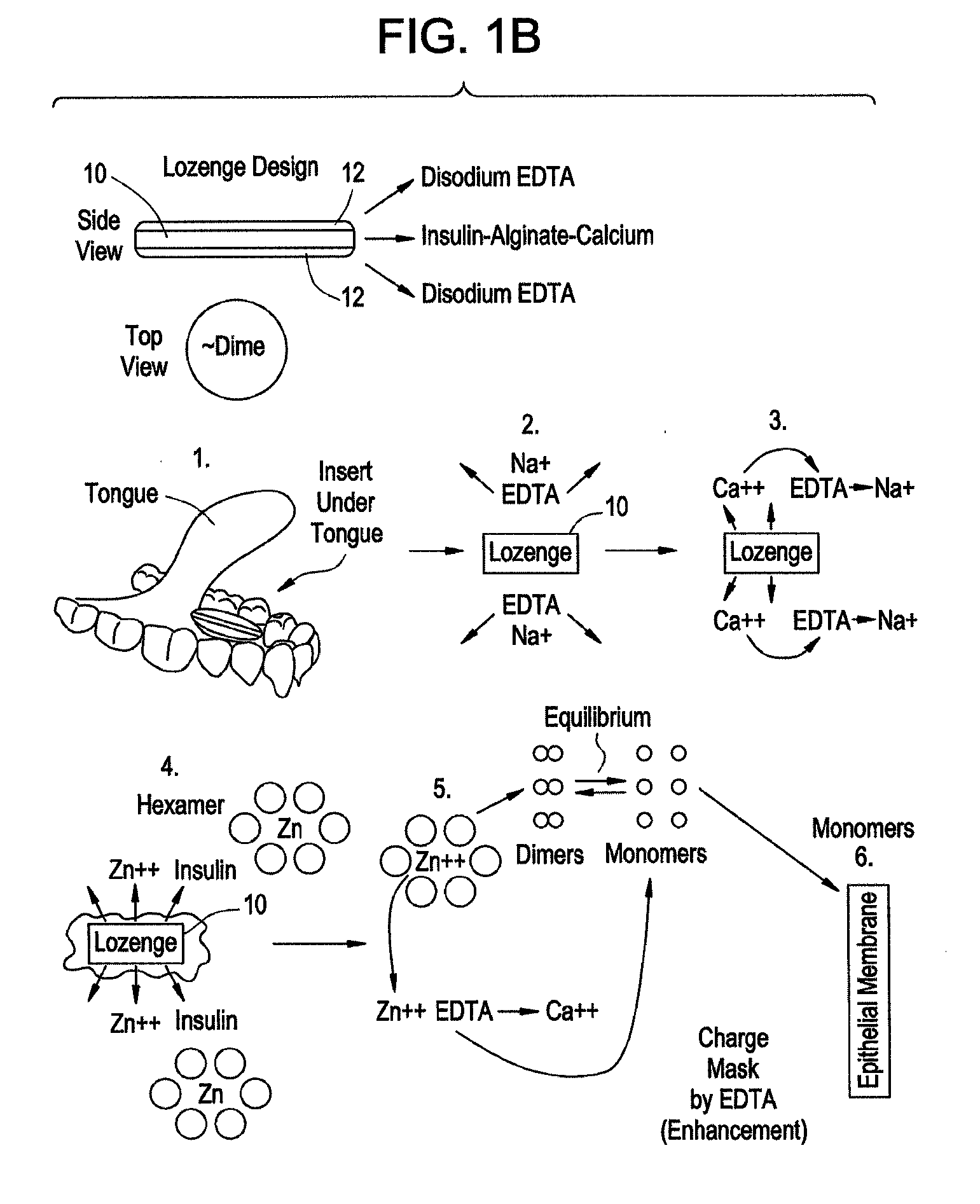
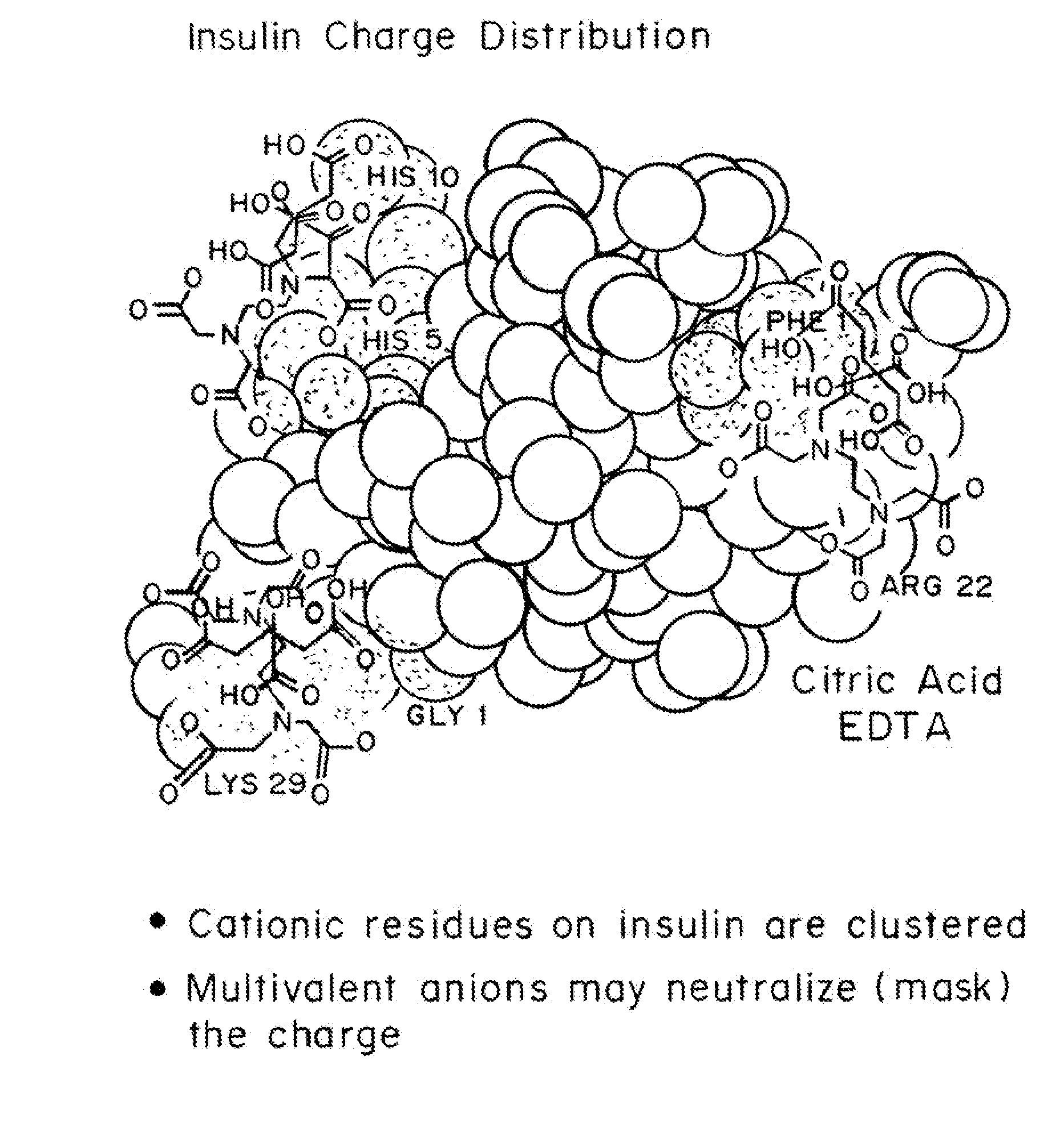
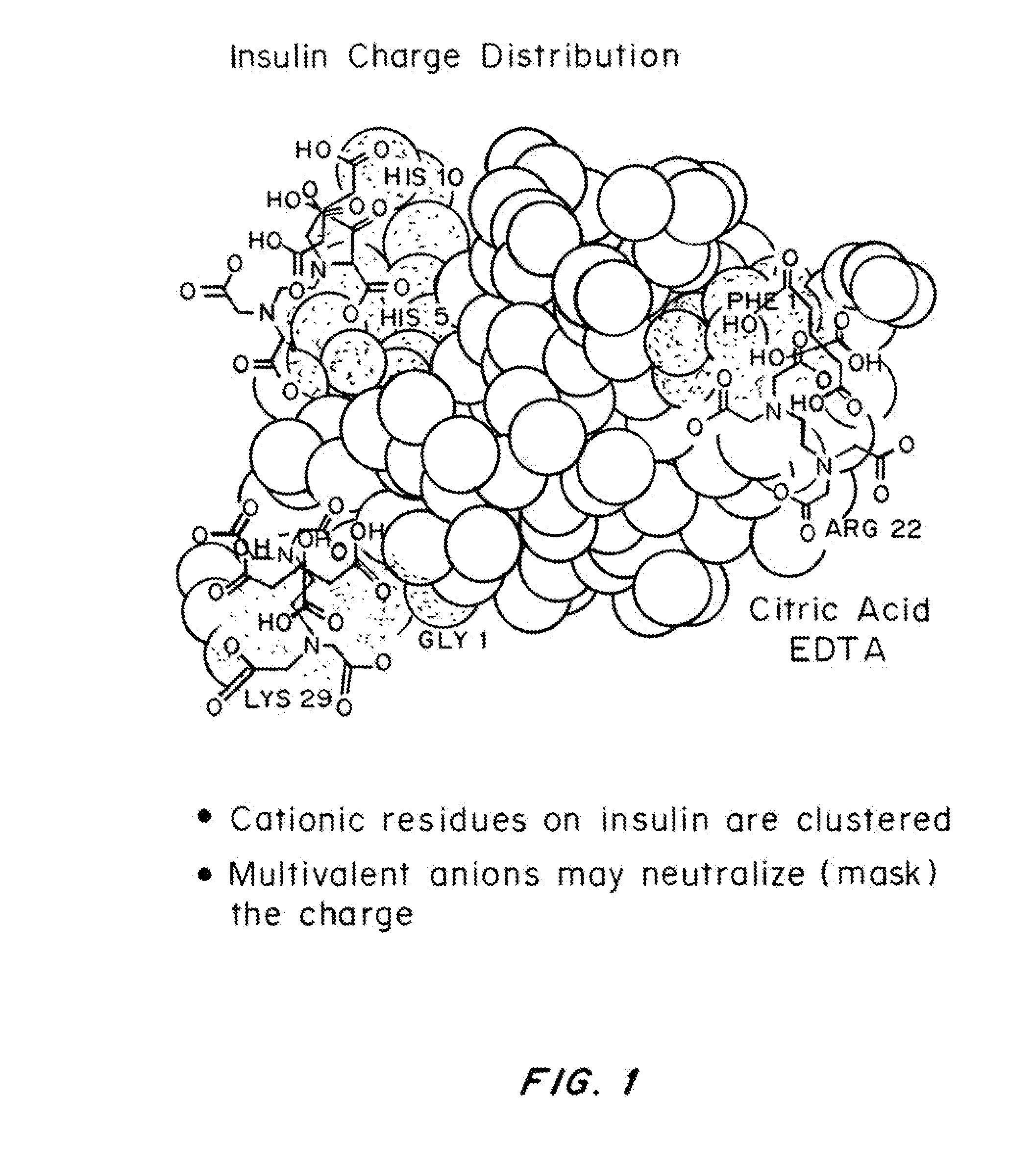
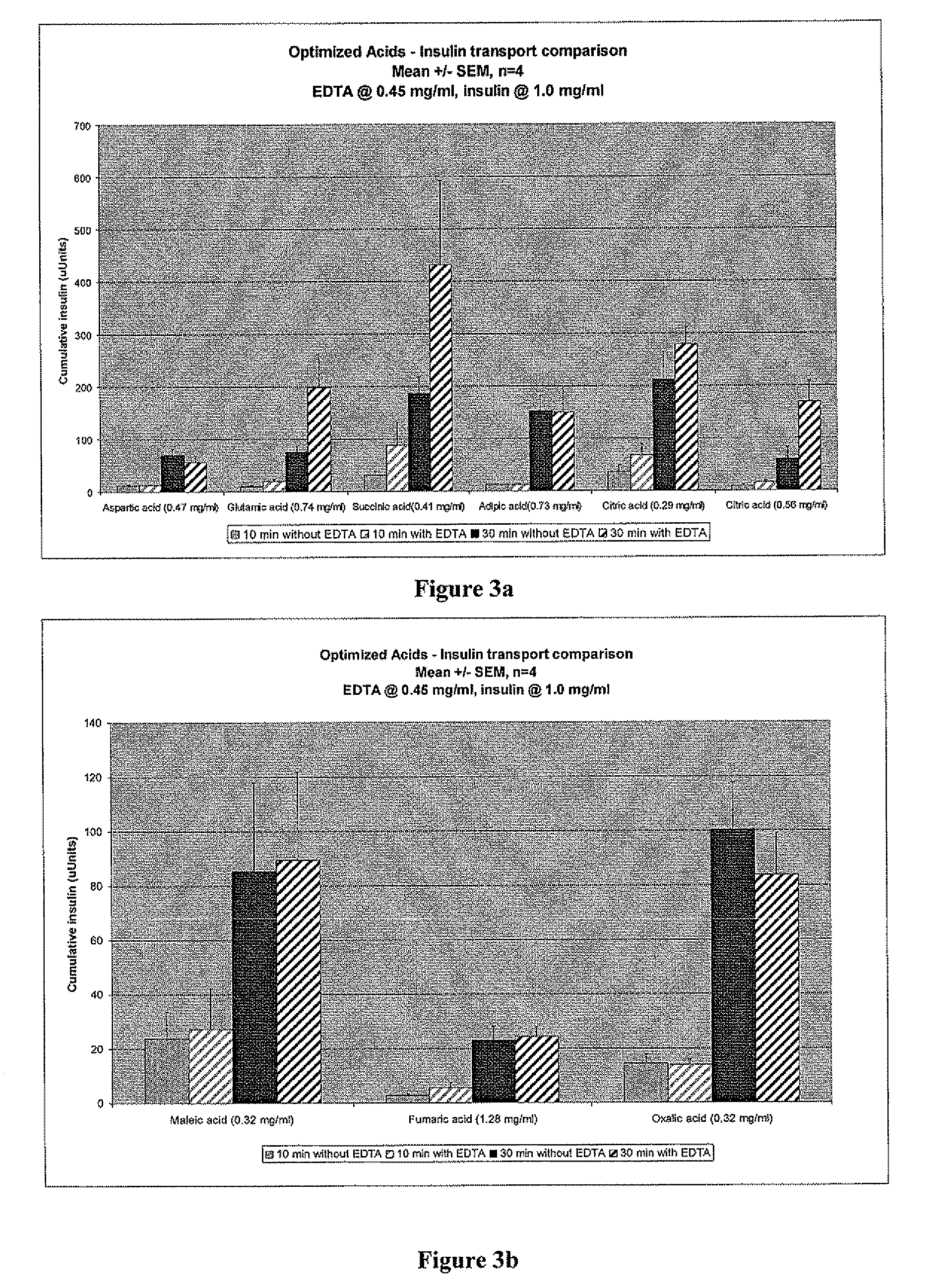
Rapid acting drug delivery compositions
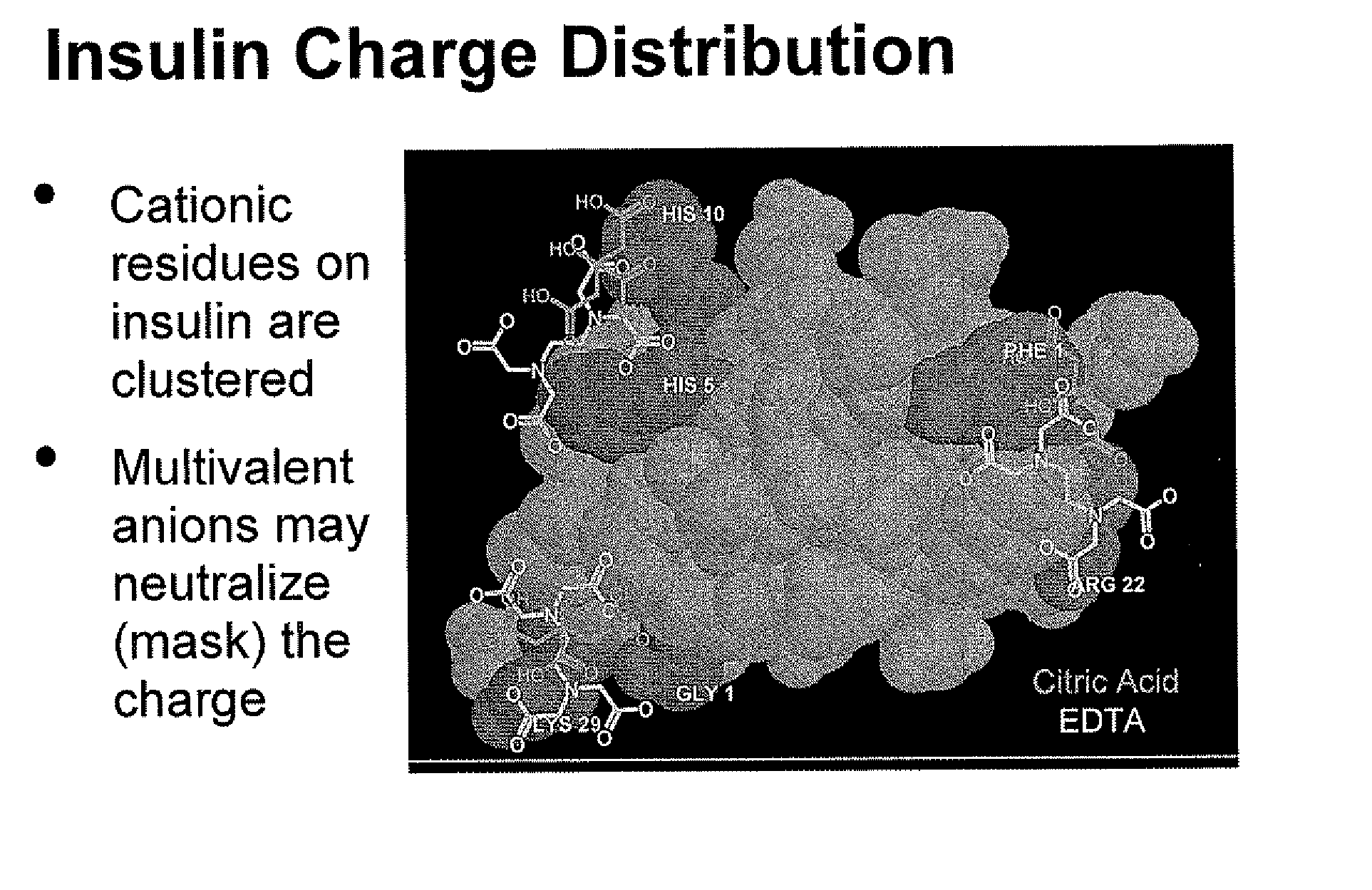
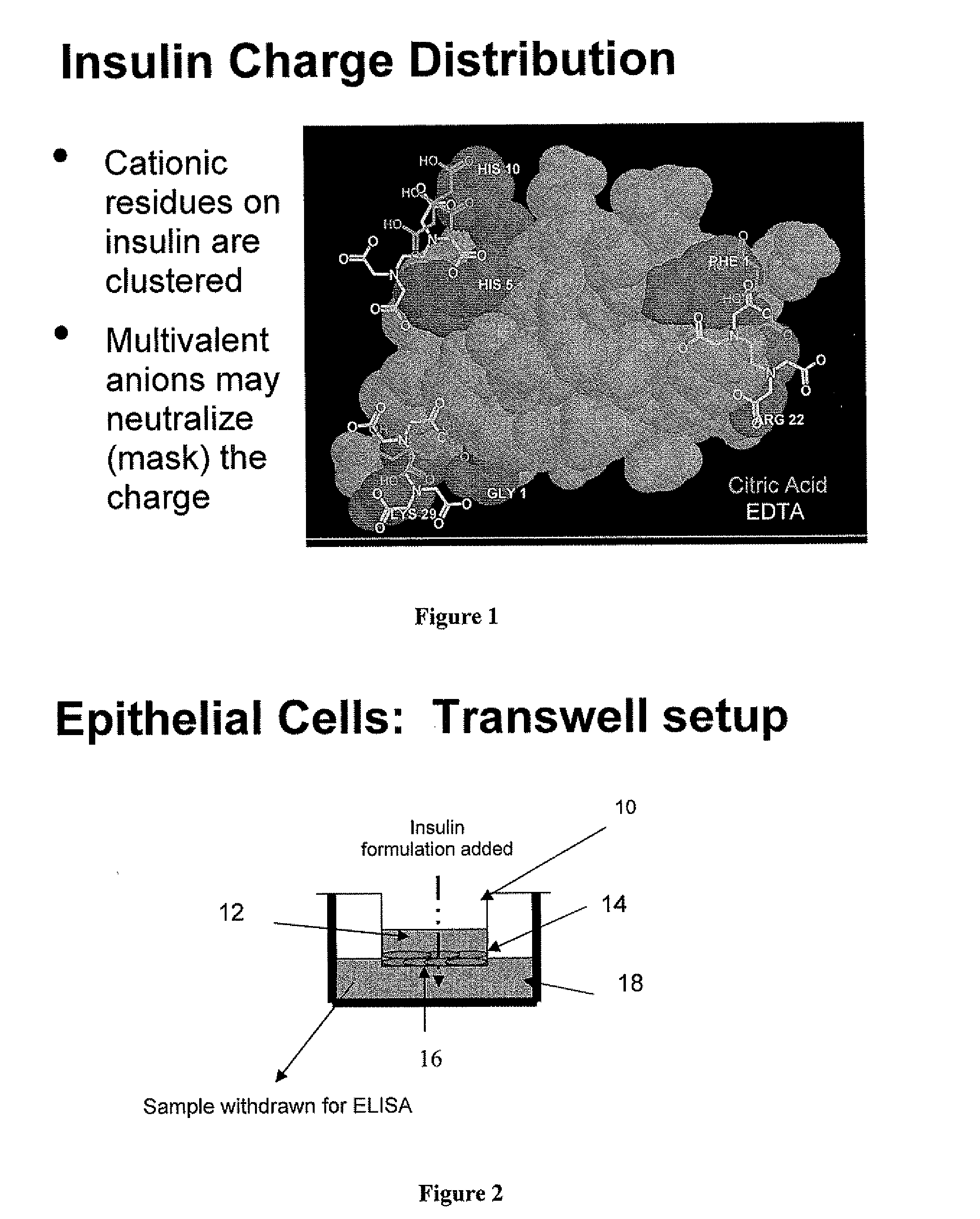
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Method of implanting a subcutaneous injection port having stabilizing elements
A method of implanting an injection port using the step of providing an injection port having a housing having a body, a fluid reservoir, a needle penetrable septum, and at least one stabilizing element mounted to the housing comprising a member having an undeployed position and a deployed position, wherein the stability element extends radially from the body. The method further involves the step of creating a surgical incision through the skin and subcutaneous fat layers of the patient to expose the fascia, and placing the injection port between the subcutaneous fat layer and the fascia tissue. The method even further involves the step of deploying the stability element.
Owner:ETHICON ENDO SURGERY INC
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Subcutaneous injection port with stabilizing elements
An implantable surgical injection port including a housing having a body, a closed distal end, a open proximal end and a fluid reservoir therebetween. The housing includes a needle penetrable septum attached to the housing about the opening. The injection port further includes at least one stabilizing element mounted to the housing for stabilizing the port within tissue. The stabilizing element is a member having an undeployed position and a deployed position. Wherein the element extends radially from the body in the deployed position.
Owner:ETHICON ENDO SURGERY INC
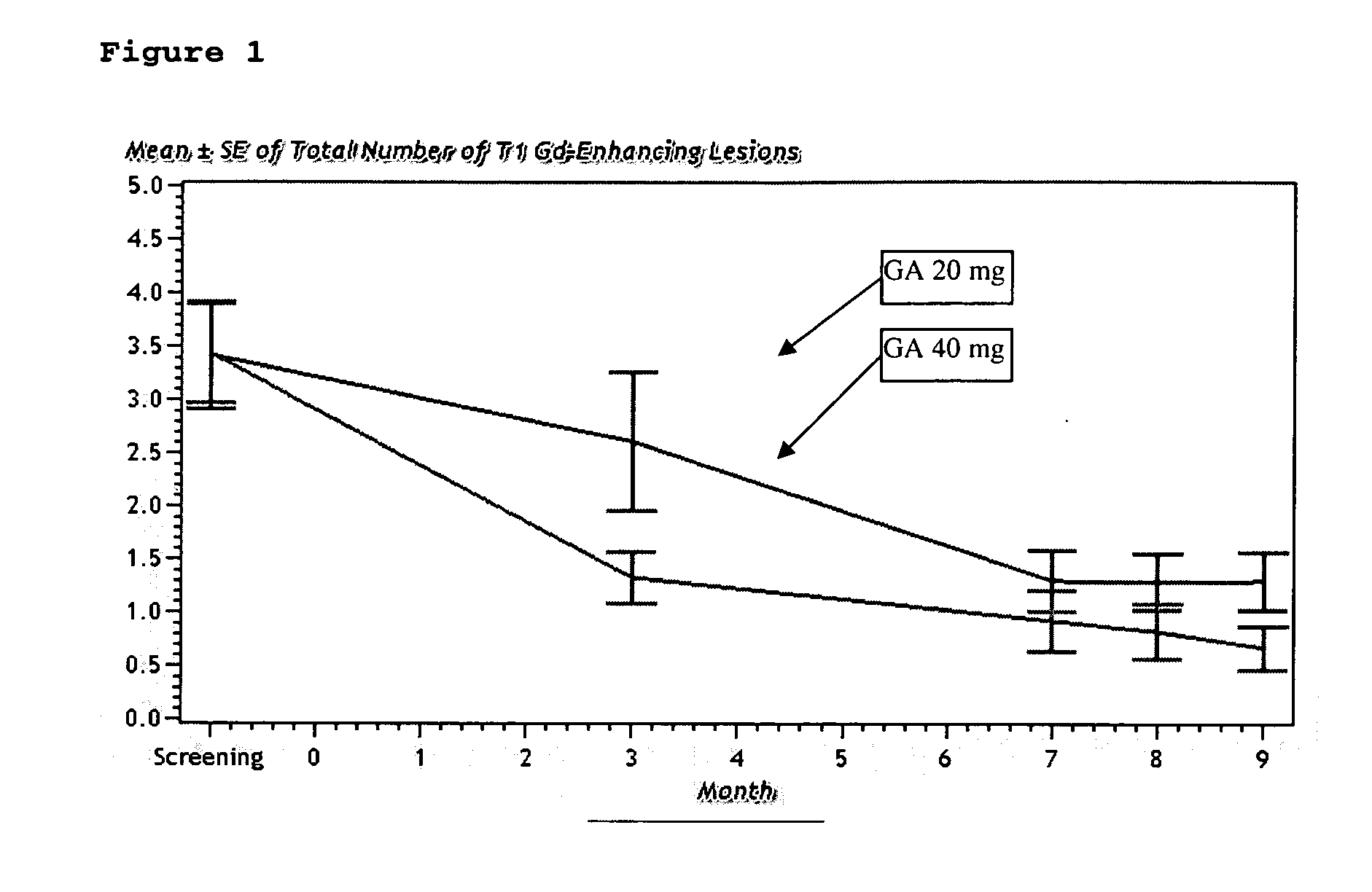
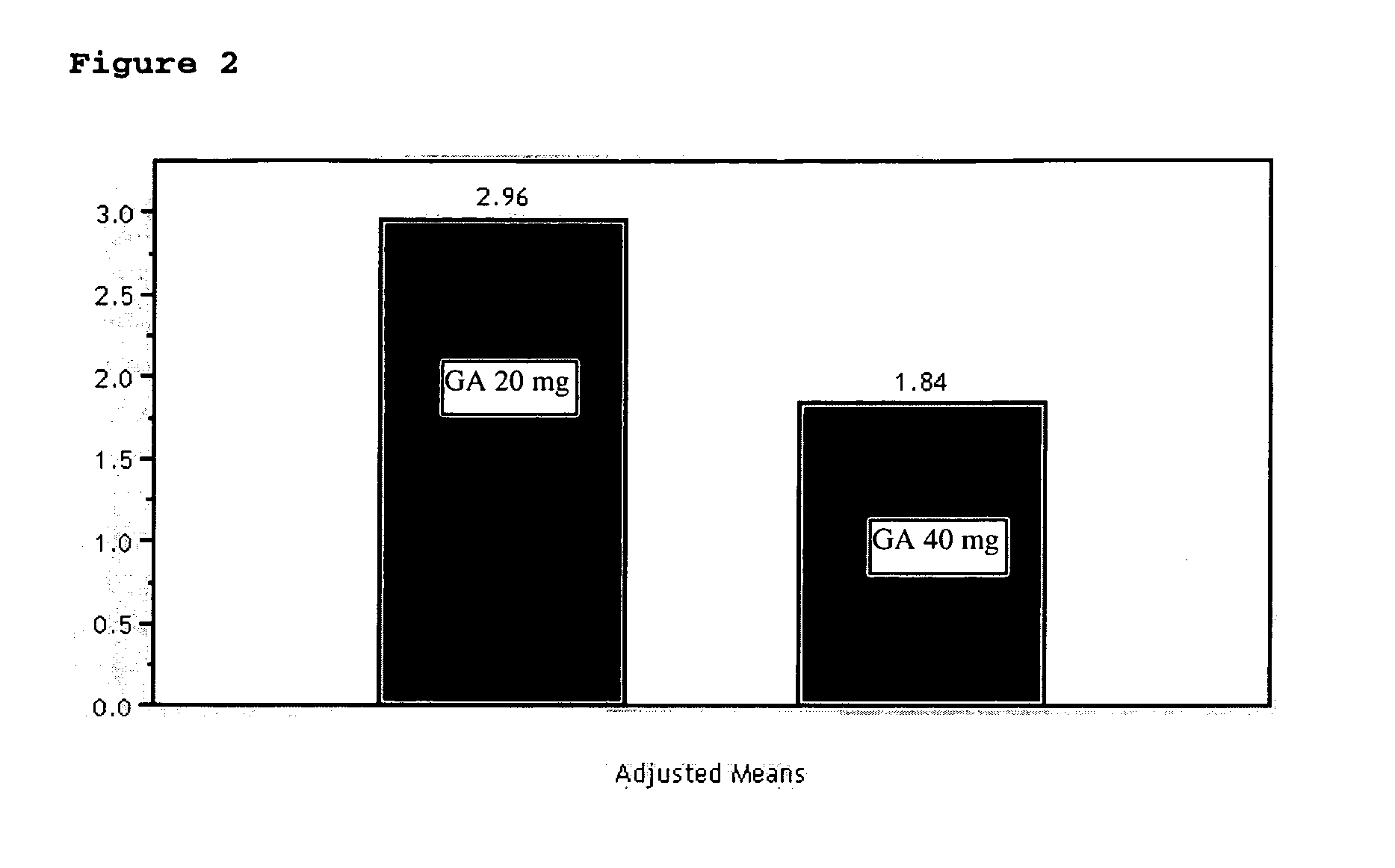
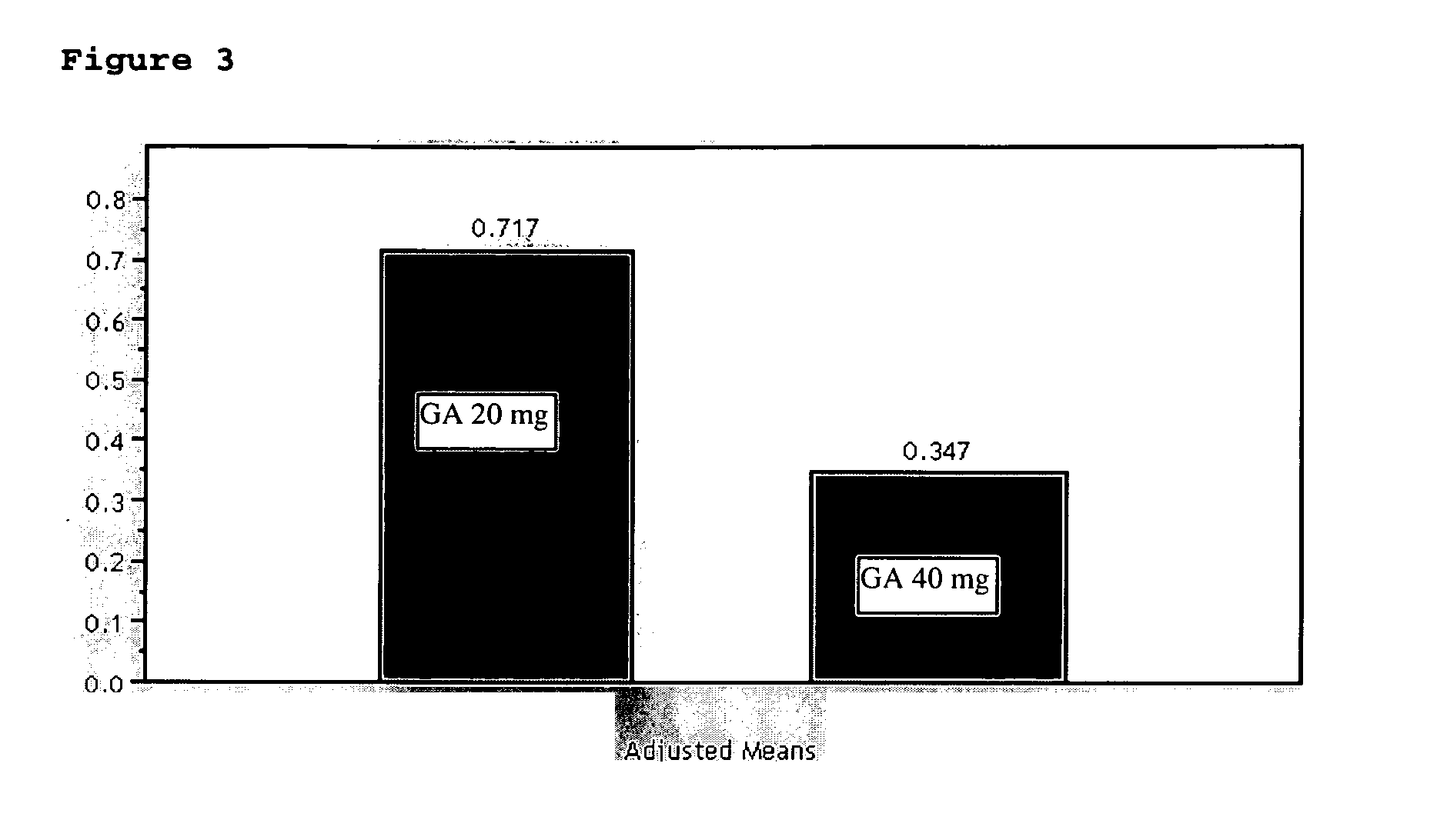
Method of treating multiple sclerosis
InactiveUS20070161566A1Relieve symptomsReducing MRI-monitored disease activity and burden of a patientTetrapeptide ingredientsDepsipeptidesEnhancing LesionSubcutaneous injection
This invention provides a method of alleviating a symptom of a patient suffering from a relapsing form of multiple sclerosis which comprises periodically administering to the patient by subcutaneous injection a single dose of a pharmaceutical composition comprising 40 mg of glatiramer acetate so as to thereby alleviate the symptom of the patient. This invention also provides a method of reducing Gd-enhancing lesions in the brain and a pharmaceutical composition in a unit dosage.
Owner:TEVA PHARMA IND LTD
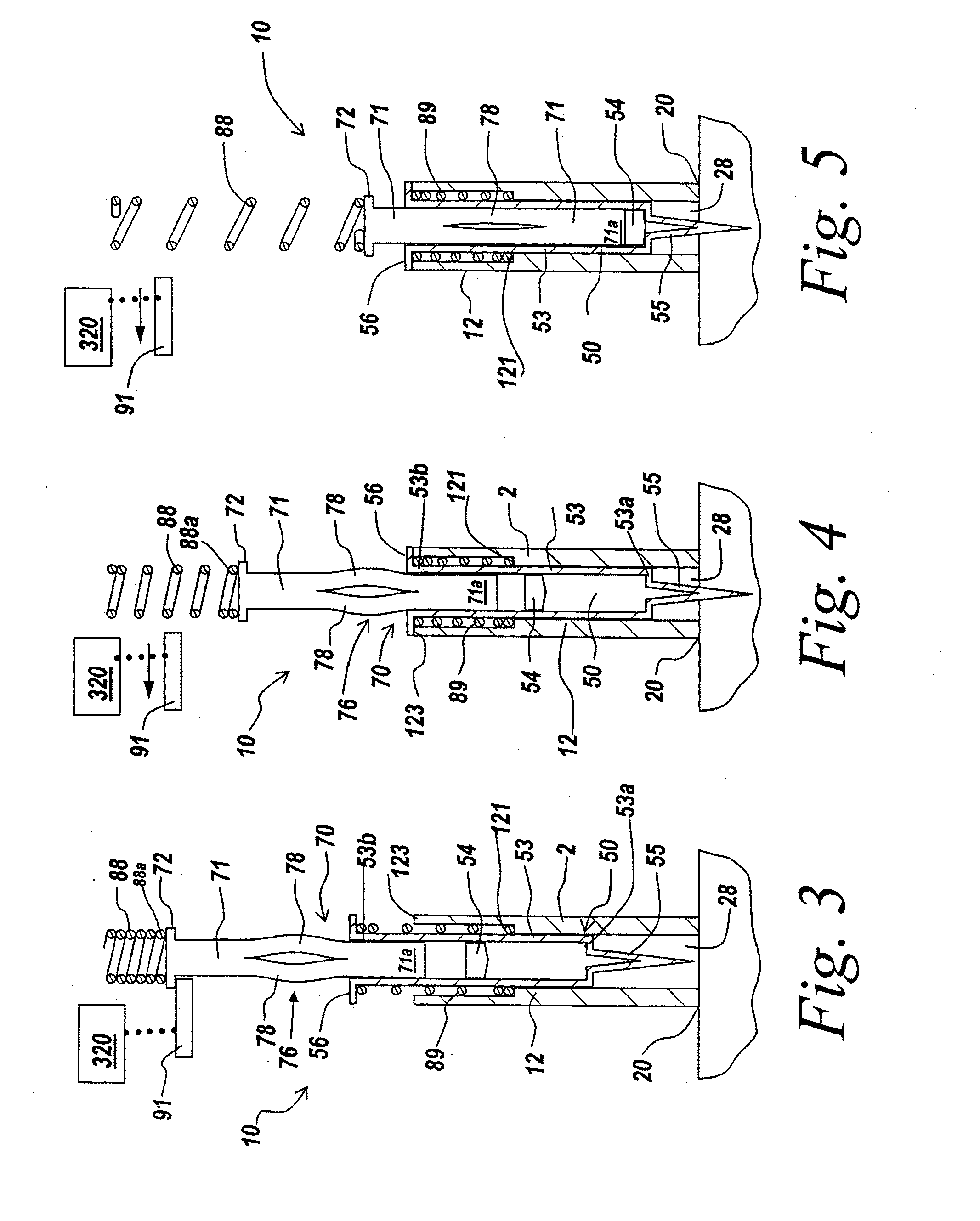
Medicament administration apparatus
InactiveUS20050137523A1Simple designReduce manufacturing costInfusion syringesPharmaceutical containersHypodermic needleSurgery
An apparatus for removal of premixed drugs or reconstitution of lyophilized drugs and for the injection of the reconstituted drug into the patient. The apparatus includes a syringe assembly and an adapter assembly that can be removably connected to a medicament container containing a premixed drug or lyophilized medicament. The syringe assembly of the apparatus includes a body portion to form a liquid chamber between the forward end of the body portion and the piston and a syringe cannula assembly. The syringe cannula assembly, which can be removably interconnected with the body portion, comprises a cannula support and a hypodermic needle sealably connected to the cannula support. The adapter assembly comprises an adapter preferably molded from a moldable plastic that includes a top wall, an adapter cannula connected to and extending from the top wall and a variety of connectors connected to the top wall for removably interconnecting the adapter with the medicament container. The adapter assembly further includes syringe connector member connected to the top wall for removably interconnecting the syringe with the adapter in a manner to uniquely position the syringe cannula within the lumen of the adapter cannula wherein it is completely shielded from external contamination and prevent print damage and injury to the user.
Owner:WYATT PHILIP W +1
Compositions and methods for enhanced mucosal delivery of peptide YY and methods for treating and preventing obesity
InactiveUS7166575B2Improved pharmacokineticImproved pharmacodynamic resultPowder deliveryPeptide/protein ingredientsDiseaseHypodermoclysis
Pharmaceutical compositions and methods are described comprising at least one peptide YY compound and one or more intranasal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity. In one aspect, the intranasal delivery formulations and methods provide enhanced delivery of peptide YY to the blood plasma or central nervous system (CNS) tissue or fluid, for example, by yielding a peak concentration (Cmax) of the peptide YY in the blood plasma or CNS tissue or fluid of the subject that is 20% or greater compared to a peak concentration of the peptide YY in the blood plasma or CNS tissue or fluid of the subject following administration to the subject of a same concentration or dose of the peptide YY to the subject by subcutaneous injection.
Owner:MDRNA
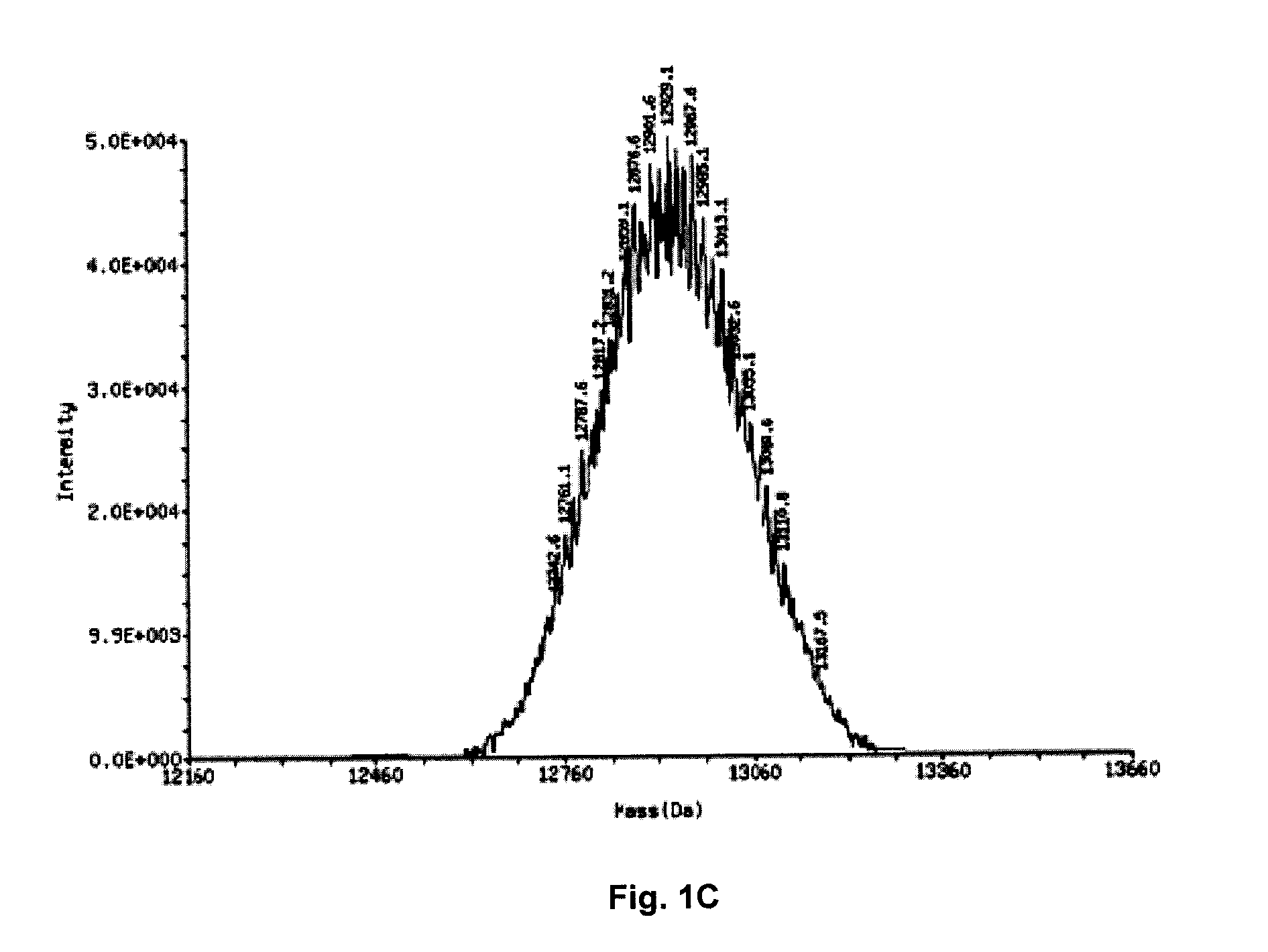
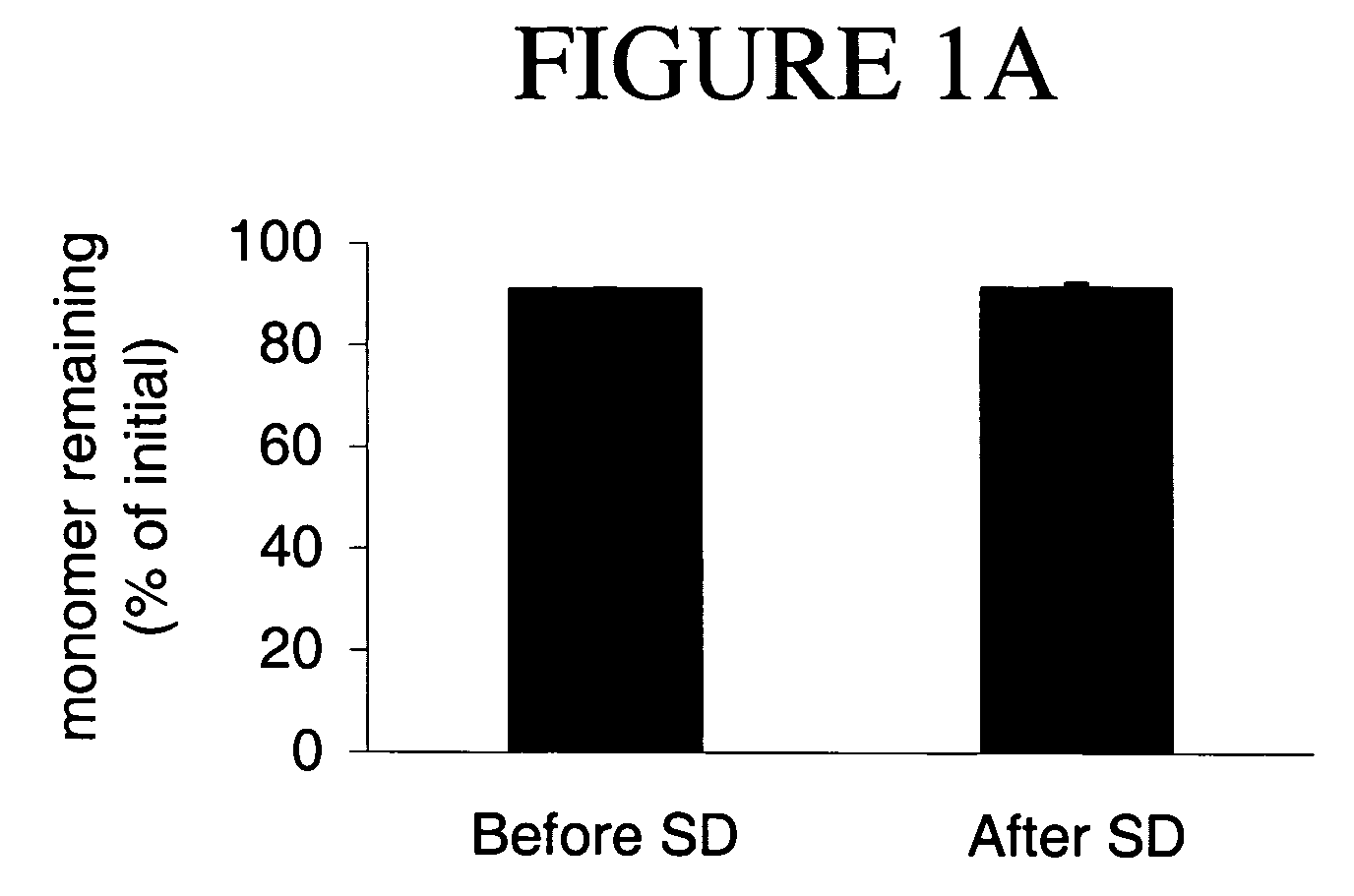
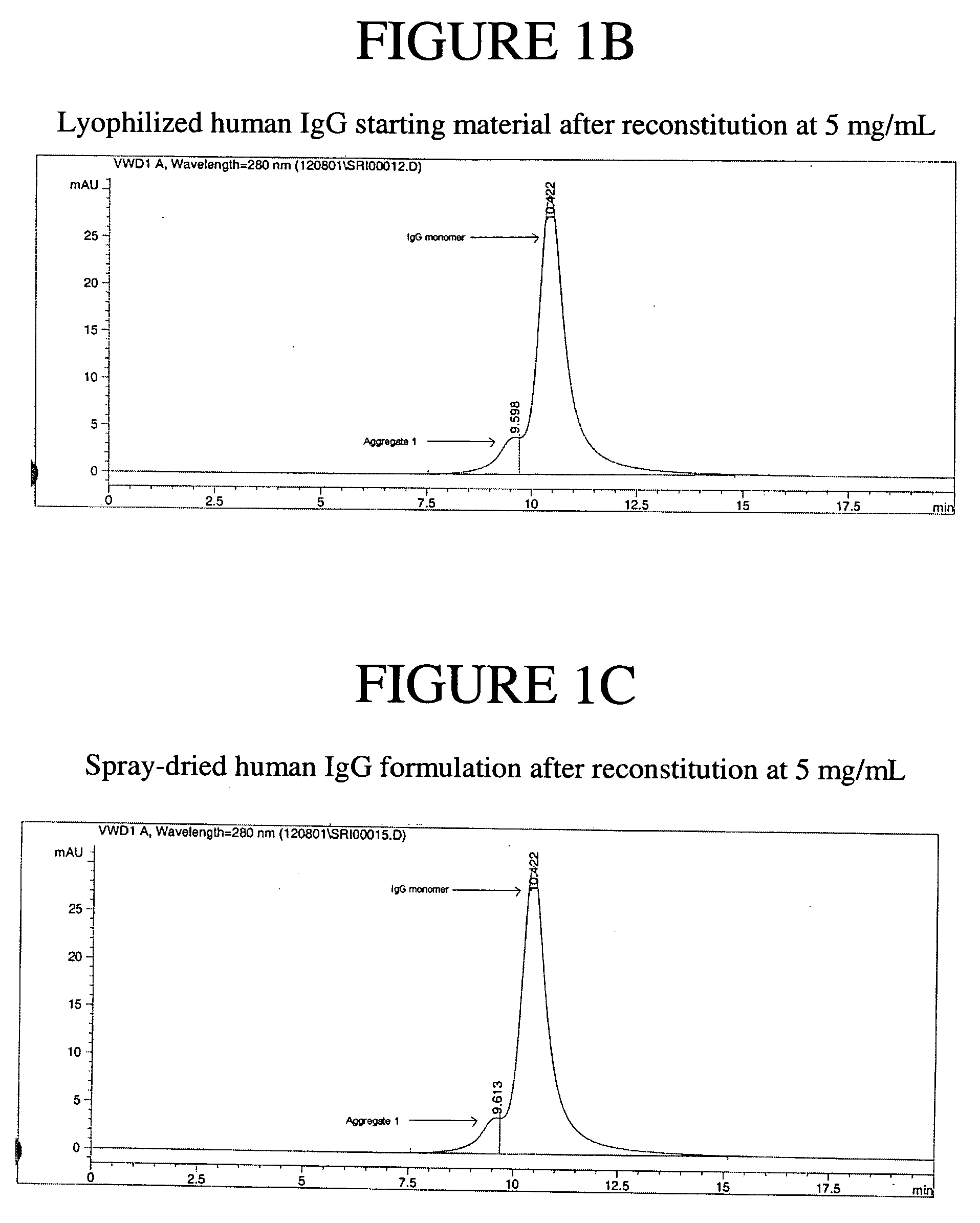
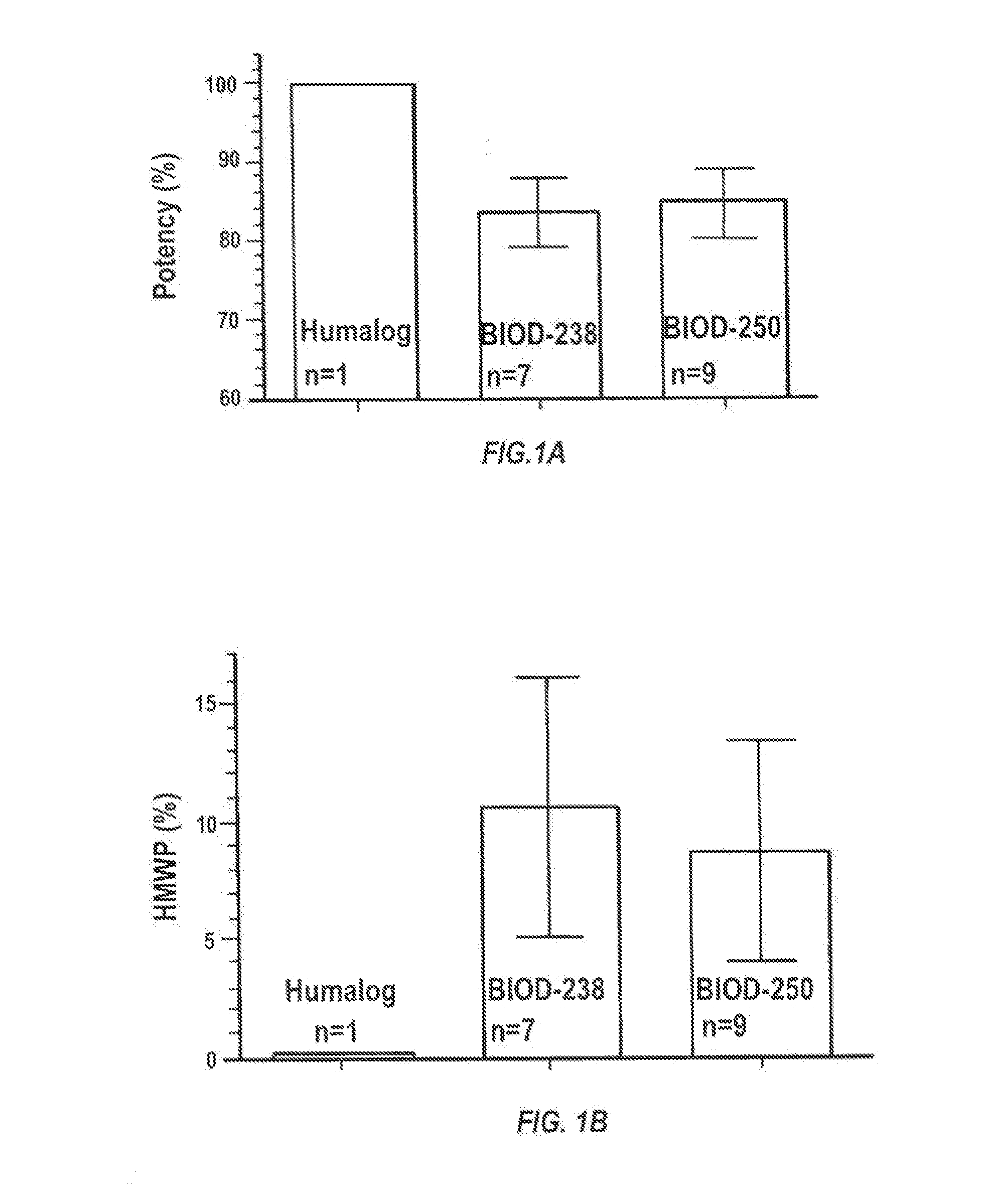
Stable lyophilized pharmaceutical formulation of IgG antibodies
InactiveUS7592004B2Process stabilityAvoid formingPowder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-IL2 ReceptorHigh concentration
Owner:ABBVIE BIOTHERAPEUTICS
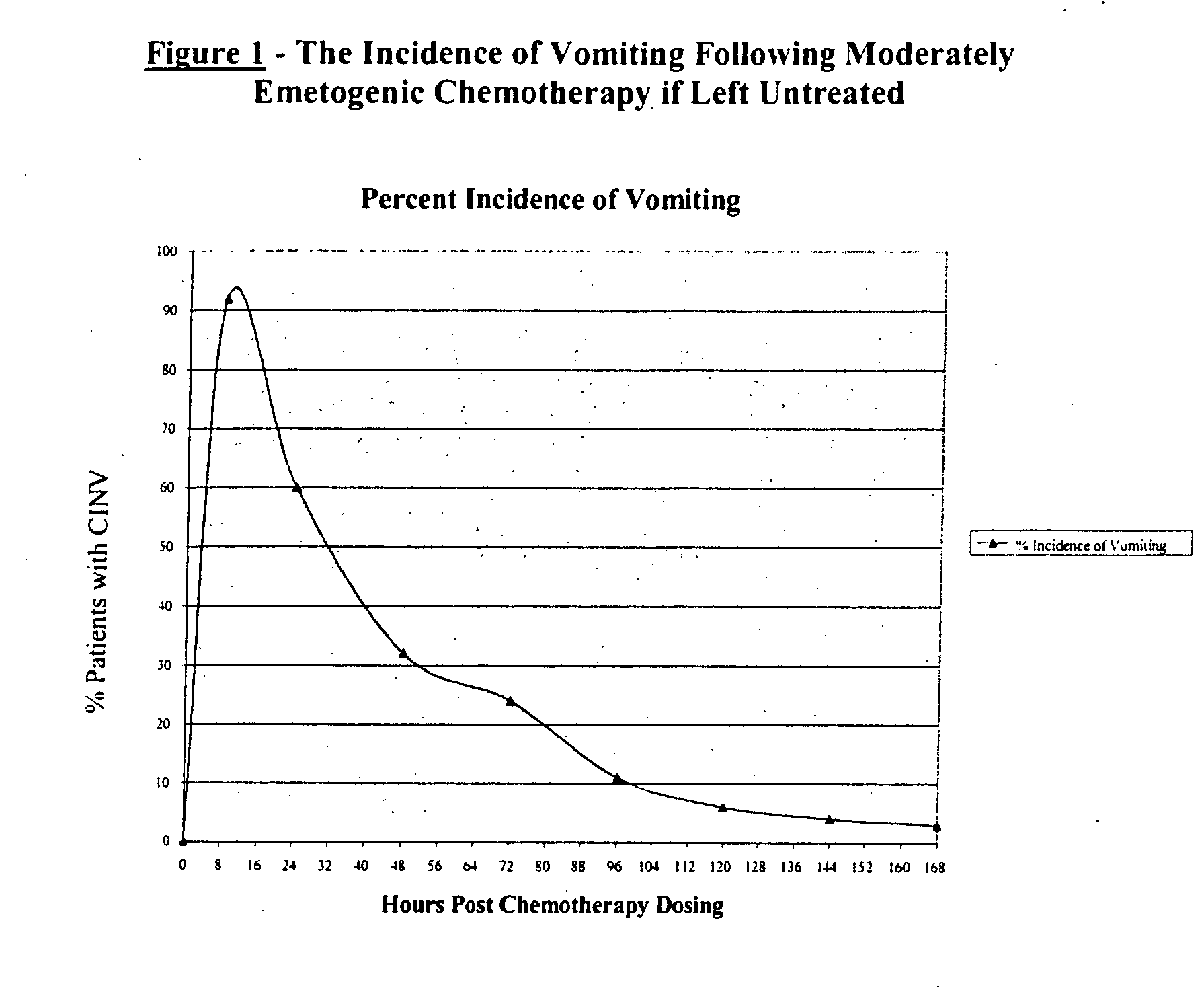
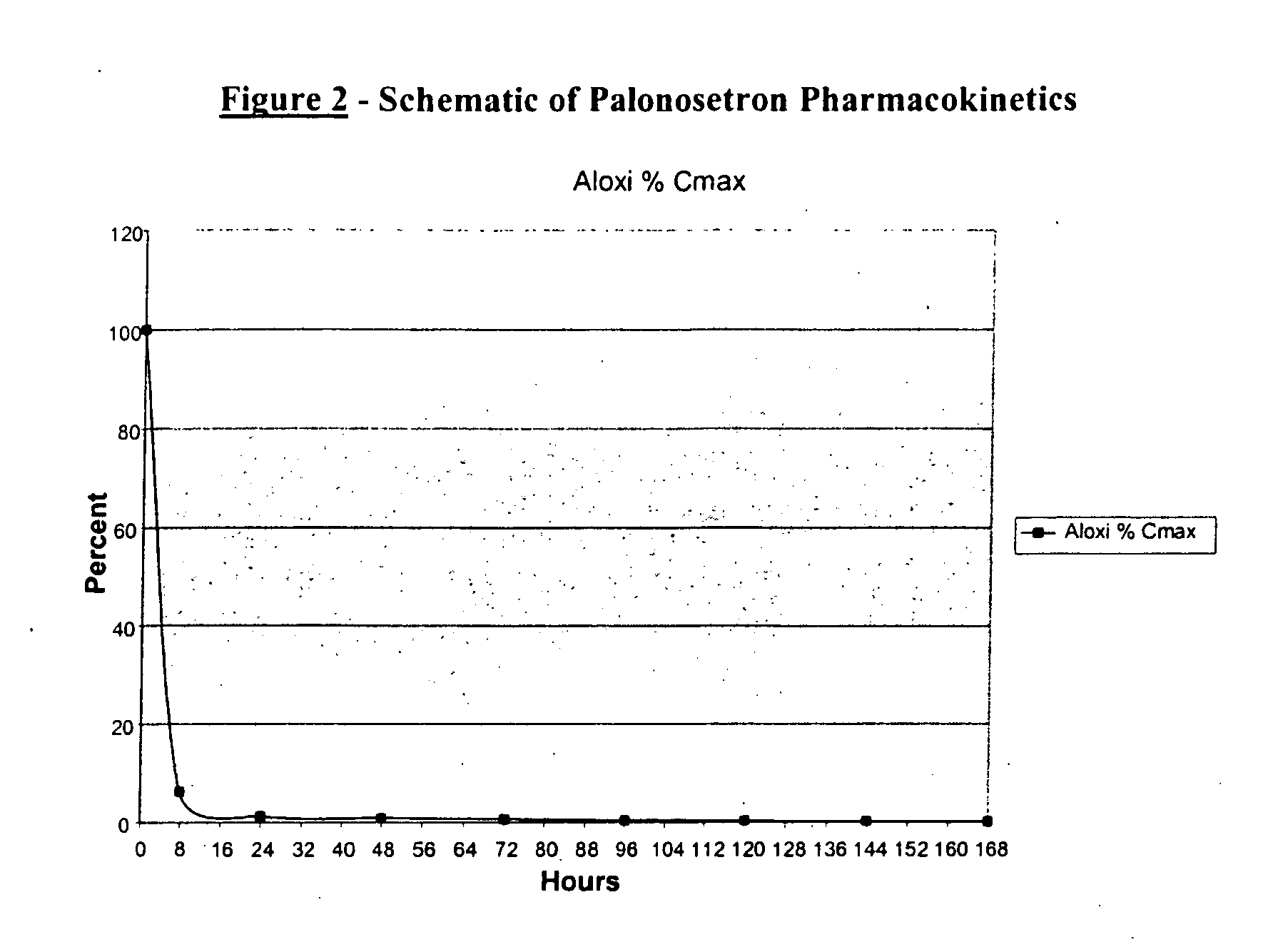
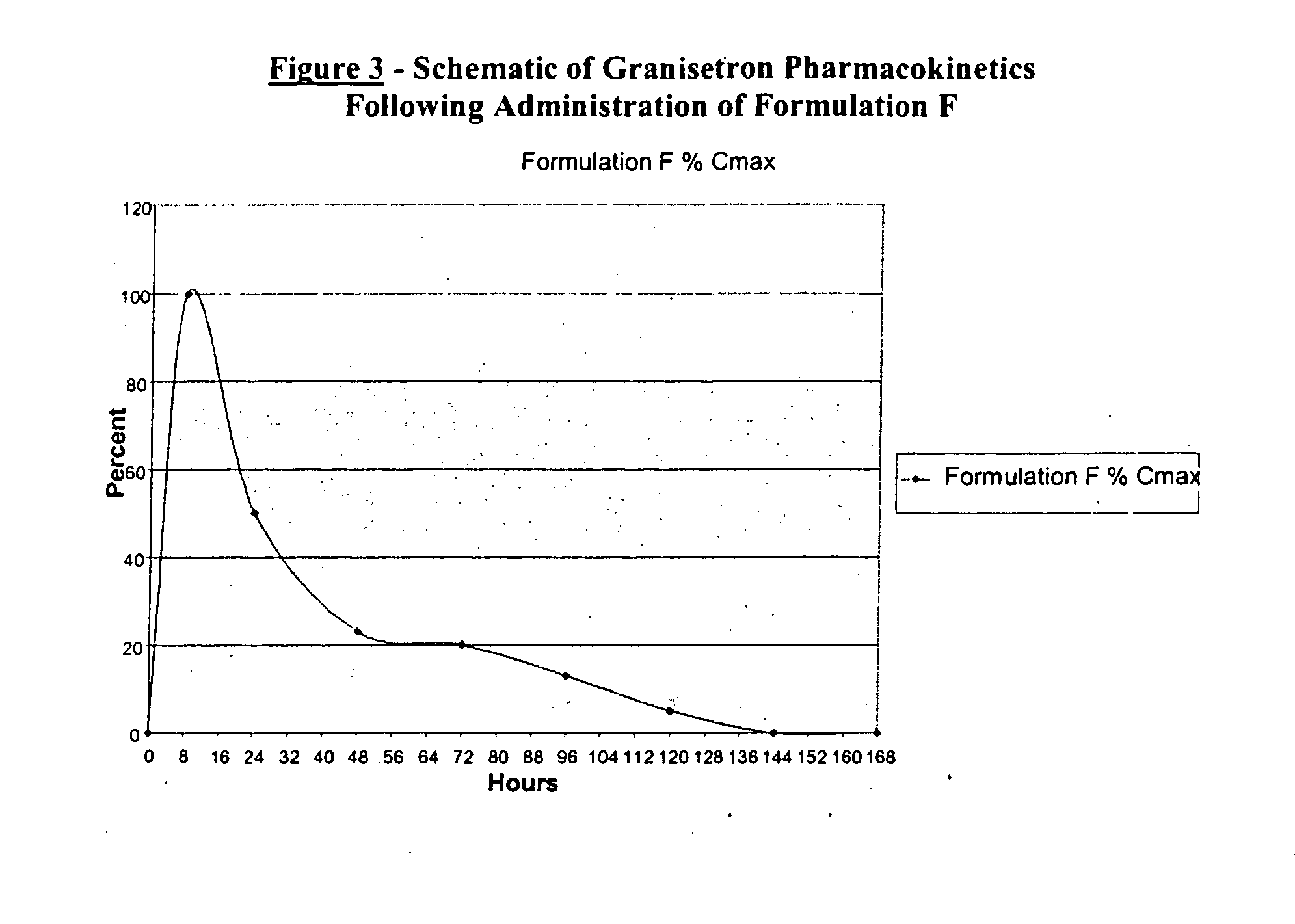
Methods for the prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV)
InactiveUS20070265329A1Eliminate the effects ofProlong the action timeBiocideDigestive systemControl releaseEmetogenic chemotherapy
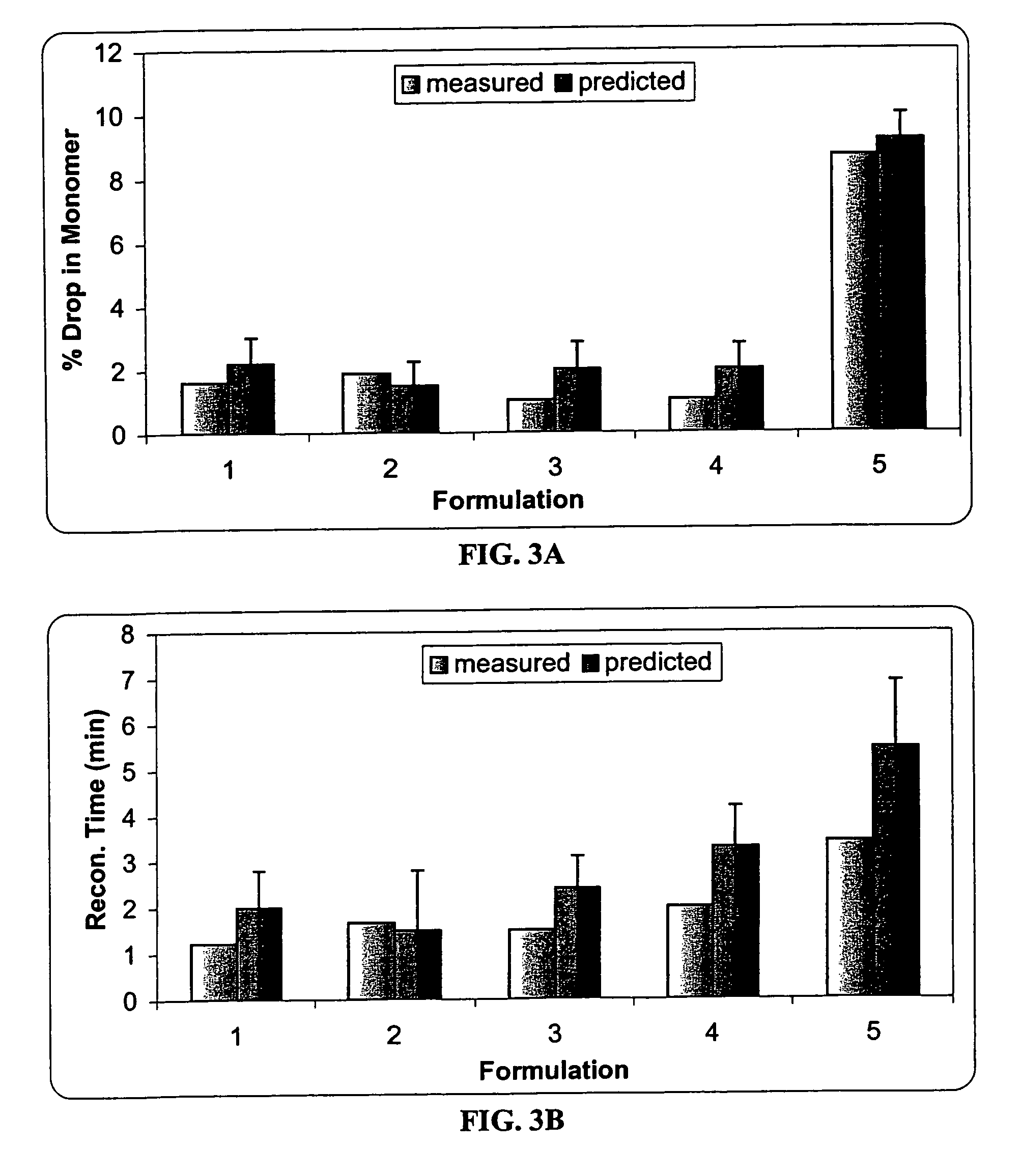
A pharmaceutical composition for the sustained release of an effective amount of a selective 5-hydroxytryptamine 3 (5-HT3) receptor antagonist for the prevention, reduction or alleviation of acute and delayed chemotherapy-induced nausea and vomiting (CINV) following a course of emetogenic chemotherapy, wherein the composition is administered by subcutaneous injection, the composition comprising a 5-HT3 receptor antagonist, a semi-solid delivery vehicle and a pharmaceutically acceptable liquid excipient; wherein the composition, when administered in a single dosage, provides a controlled release of the 5-HT3 receptor antagonist and prolonging the release of the 5-HT3 receptor antagonist that tracks the profile of the incidence of vomiting.
Owner:AP PHARMA INC
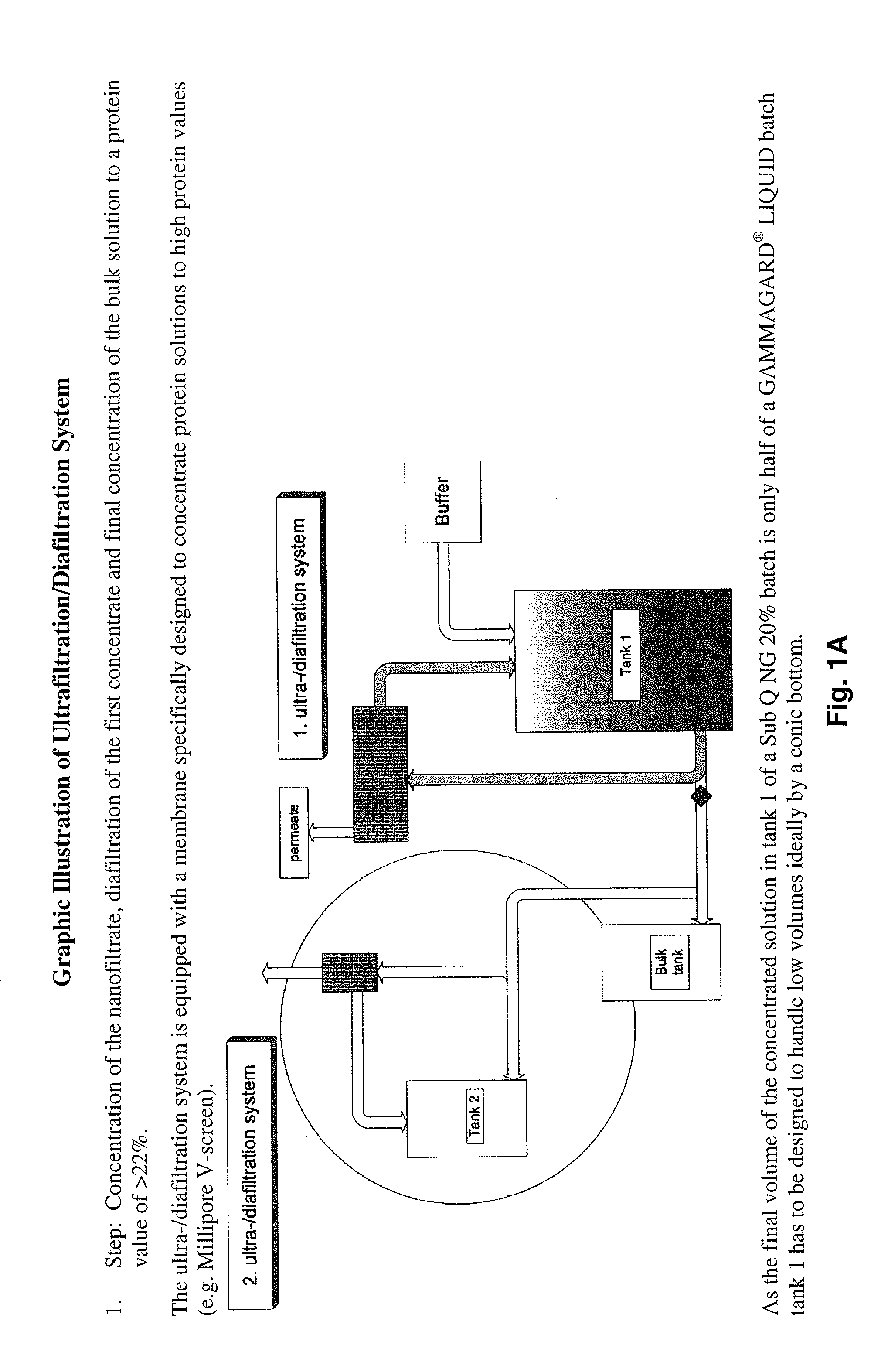
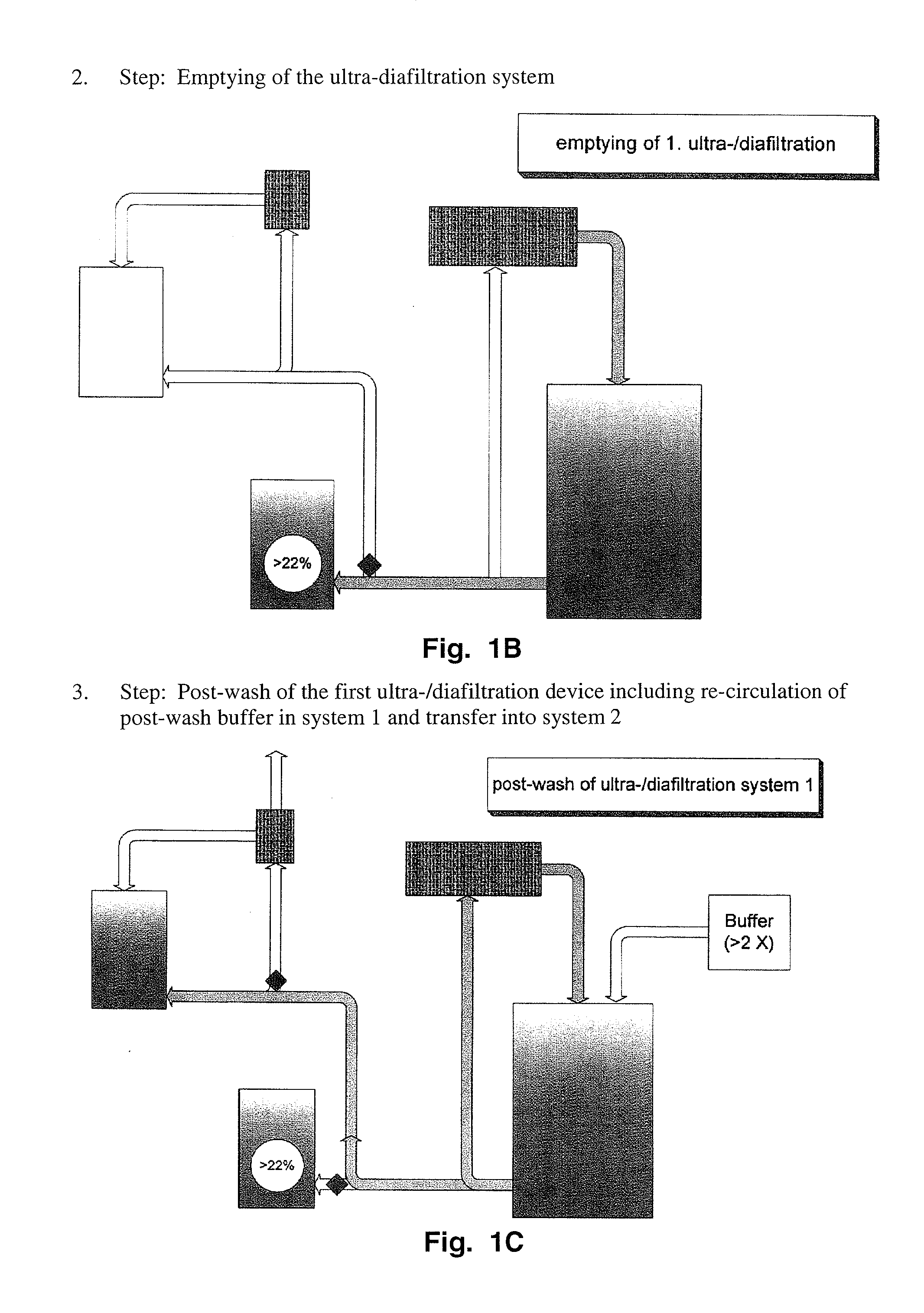
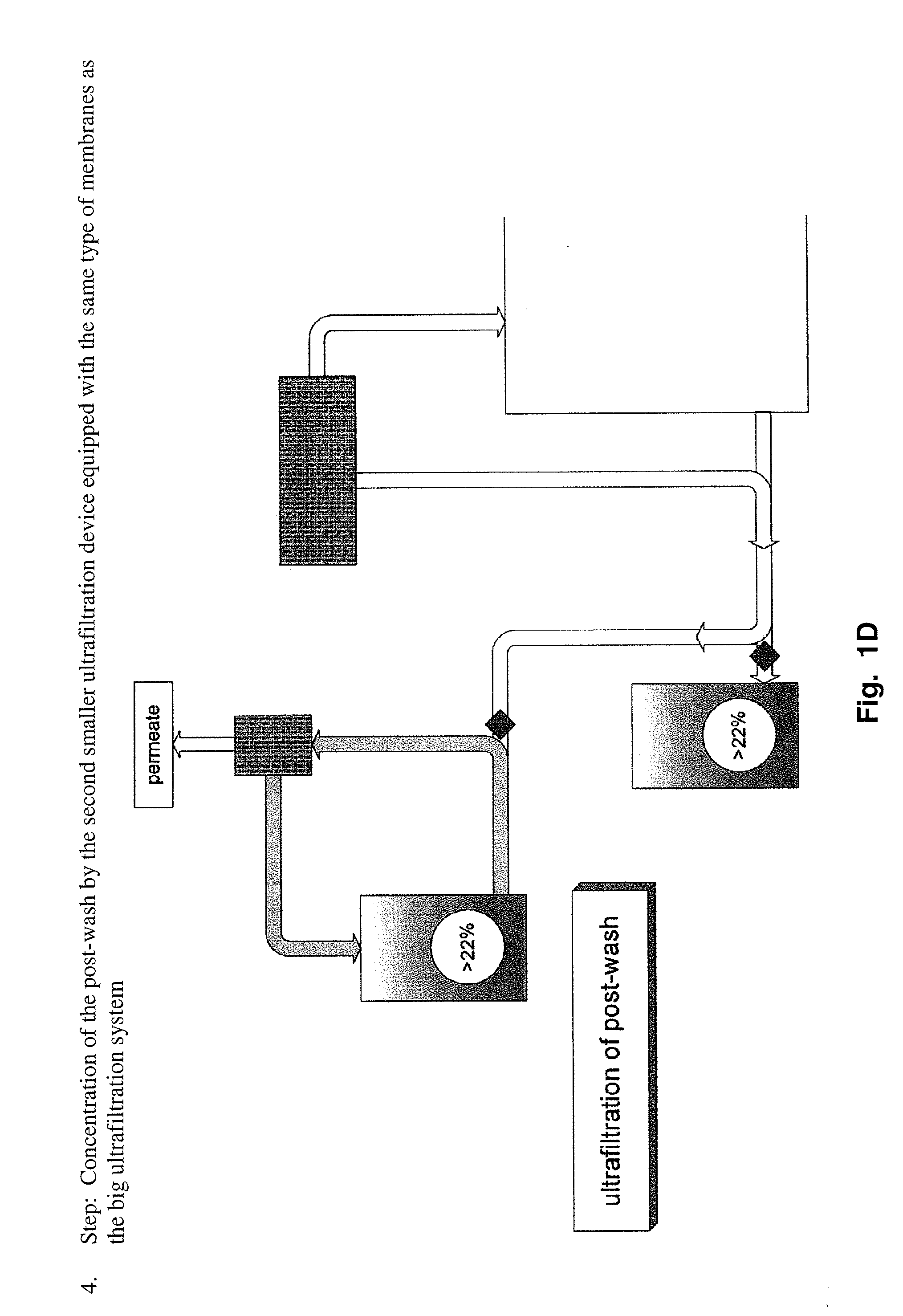
Method to produce a highly concentrated immunoglobulin preparation for subcutaneous use
The present invention relates to a new and improved method for preparing a highly concentrated immunoglobulin composition from pooled plasma for subcutaneous injection. A composition comprising 20% or more immunoglobulin suitable for subcutaneous use is also described.
Owner:TAKEDA PHARMA CO LTD
Rapid Acting Drug Delivery Compositions
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
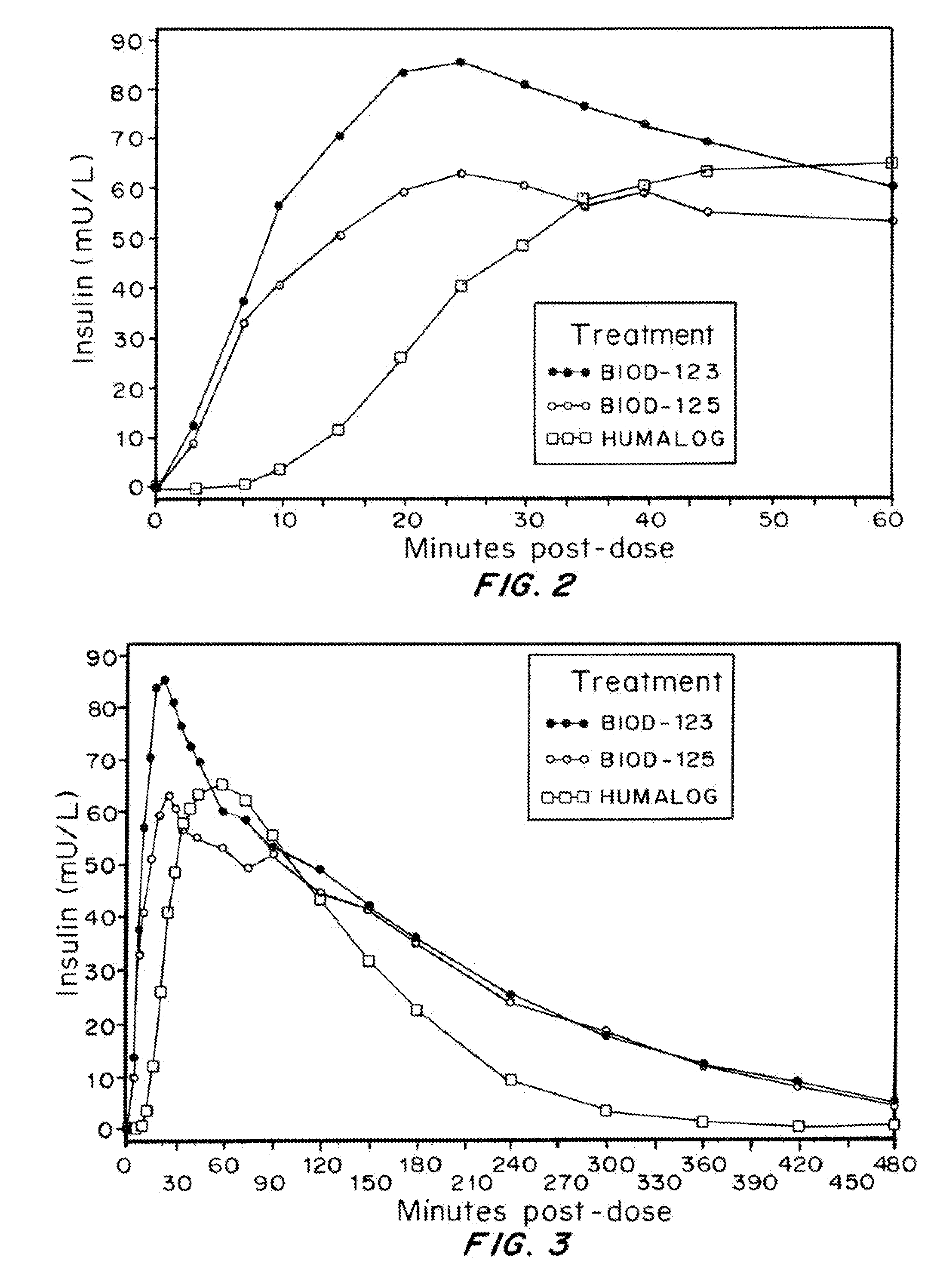
Owner:BIODEL INC
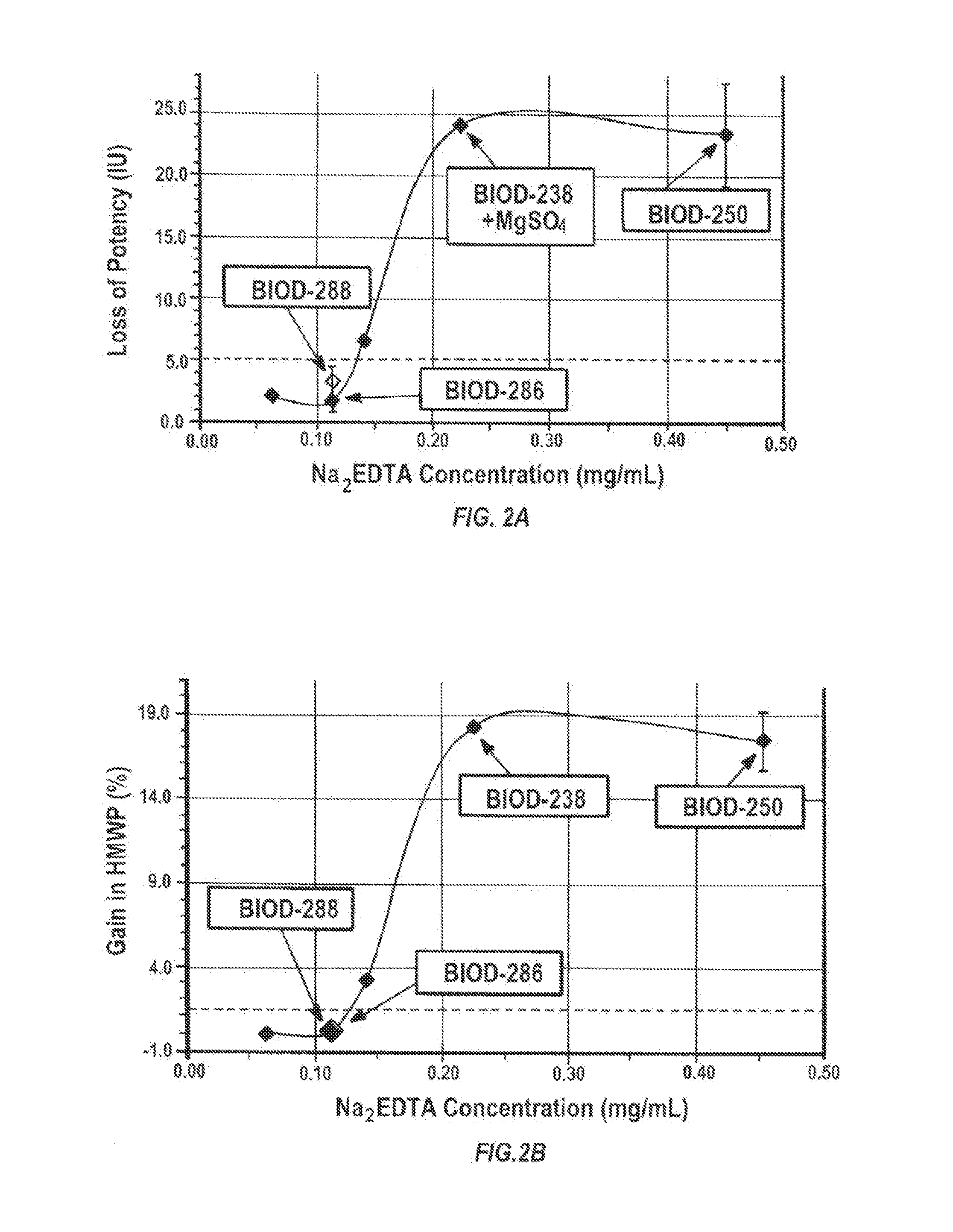
Magnesium Compositions for Modulating the Pharmacokinetics and Pharmacodynamics of Insulin and Insulin Analogs, and Injection Site Pain
ActiveUS20140113856A1Improved injection site tolerabilityPeptide/protein ingredientsMetabolism disorderEthylenediamineMagnesium salt
Compositions and methods for modulating injection site pain associated with rapid acting injectable insulin formulations have been developed for subcutaneous injection. The formulations contain insulin in combination with a zinc chelator such as ethylenediaminetetraacetic acid (“EDTA”), a dissolution / stabilization agent such as citric acid, a magnesium salt, and, optionally, additional excipients. New presentations include rapid acting concentrated insulin formulations and a way to enhance the absorption of commercially available rapid acting analog formulations by mixing them with a vial containing dry powder excipients that accelerate their absorption. Devices for mixing excipient and insulin together at the time of administration, while minimizing residence time of the mixture, are also described.
Owner:ELI LILLY & CO
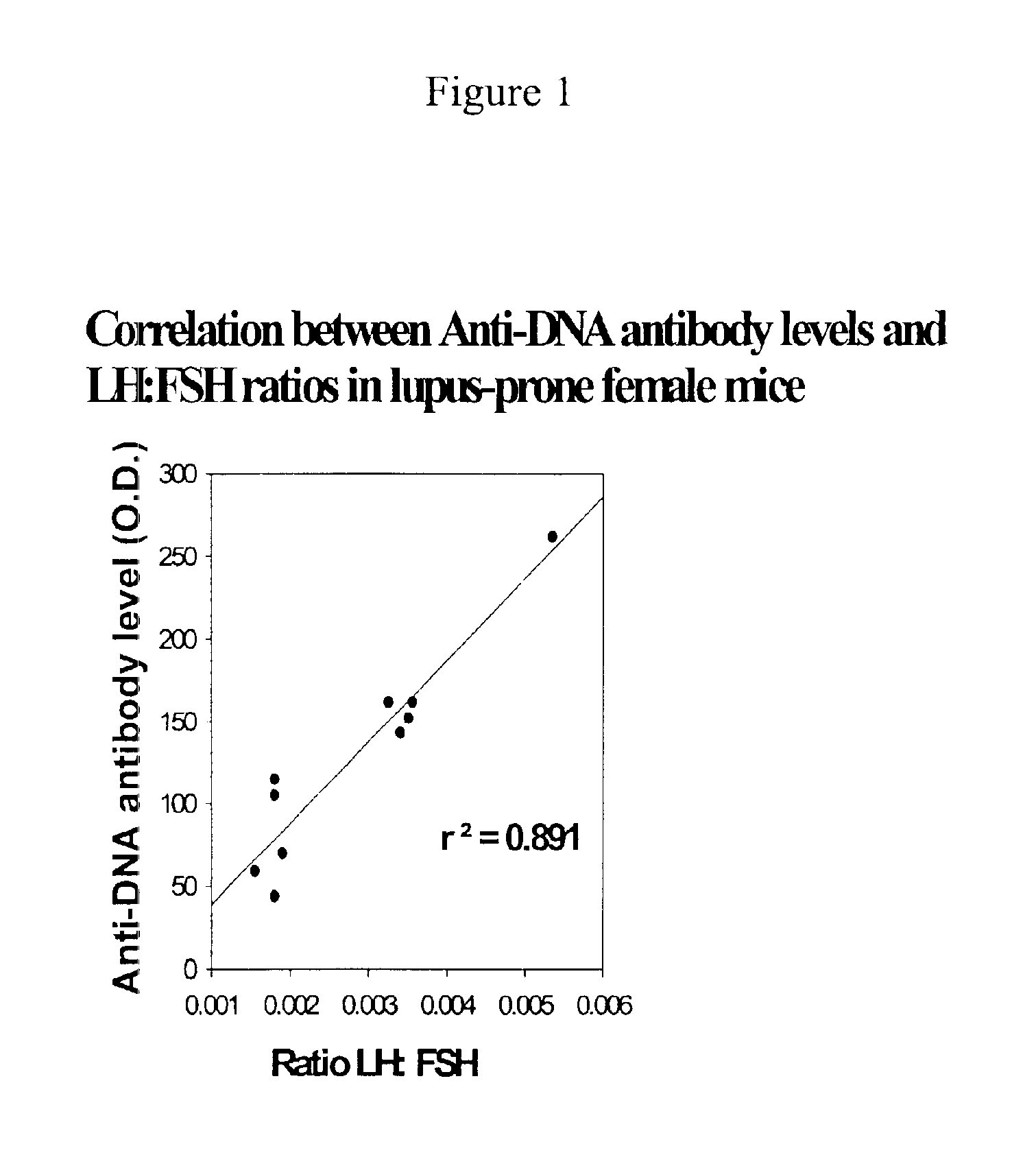
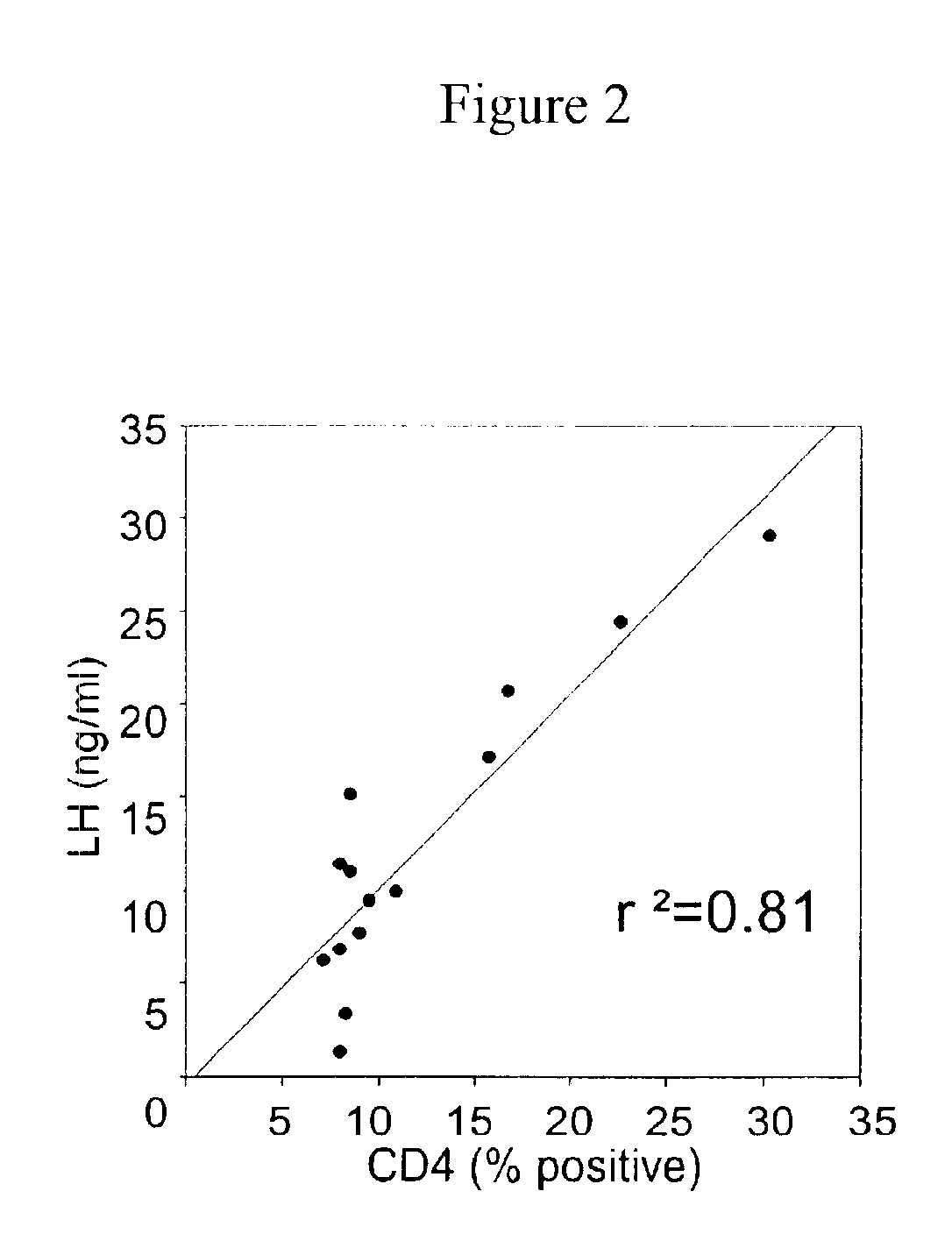
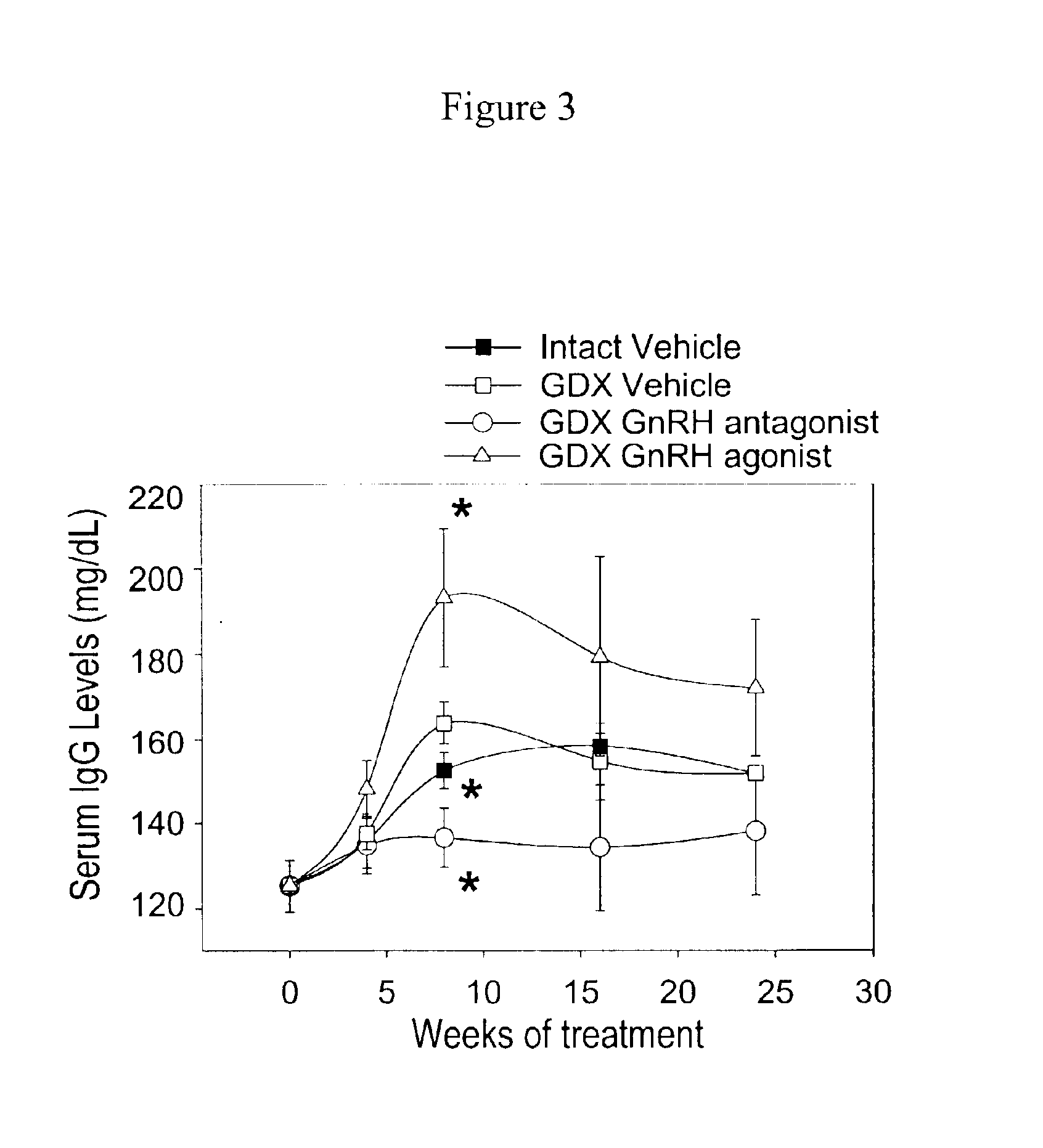
Prevention of diabetes and prolongation of the honeymoon phase of diabetes by administration of GnRH antagonists
InactiveUS6875843B2Reduce morbidityProlong honeymoon phase of diabetesPeptide/protein ingredientsMetabolism disorderDiabetes mellitusMammal
Owner:CHILDRENS MERCY HOSPITAL
Insulin formulations for insulin release as a function of tissue glucose levels
InactiveUS20090175840A1Reduce productionRaise the pHPeptide/protein ingredientsMetabolism disorderInjections insulinReducing agent
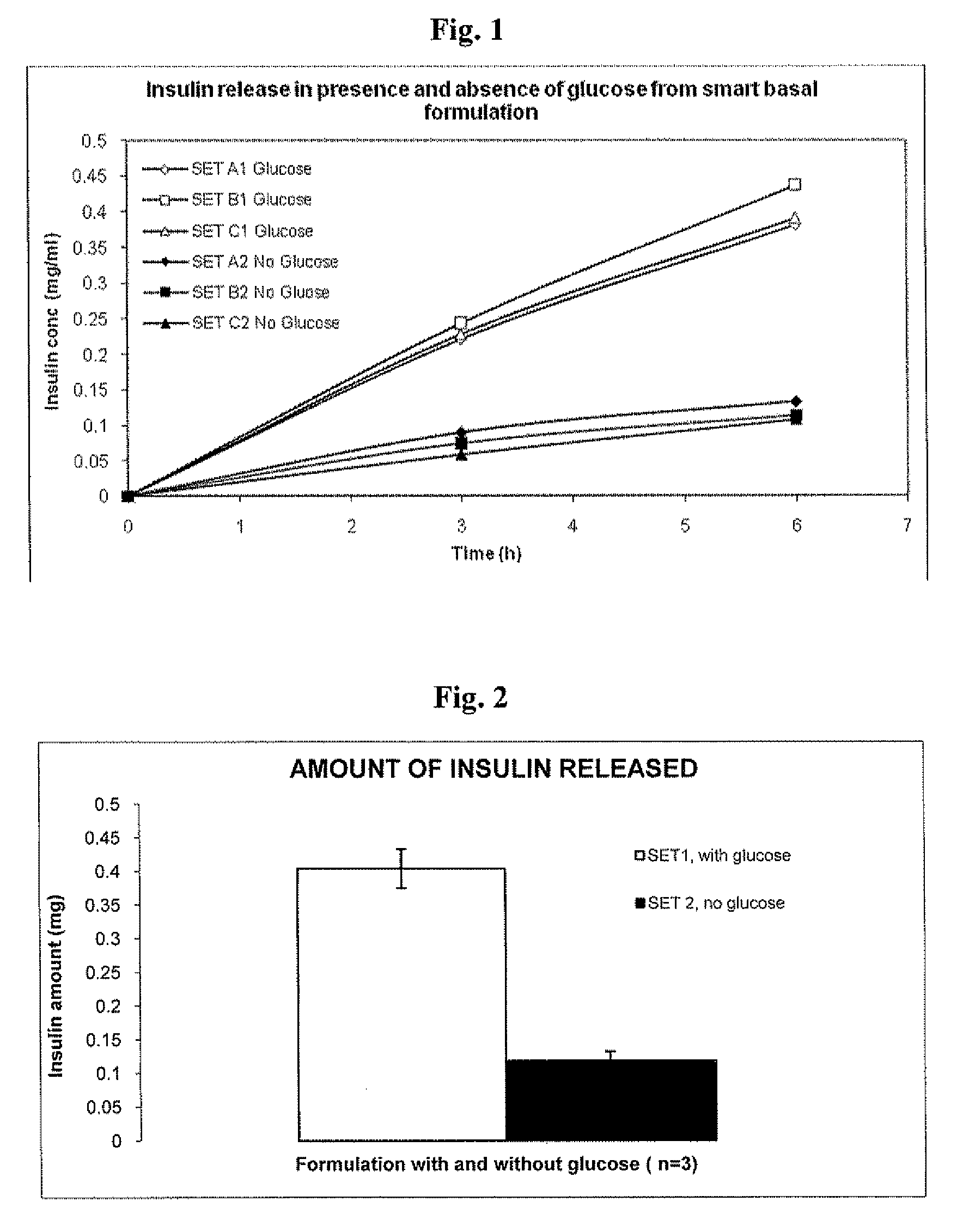
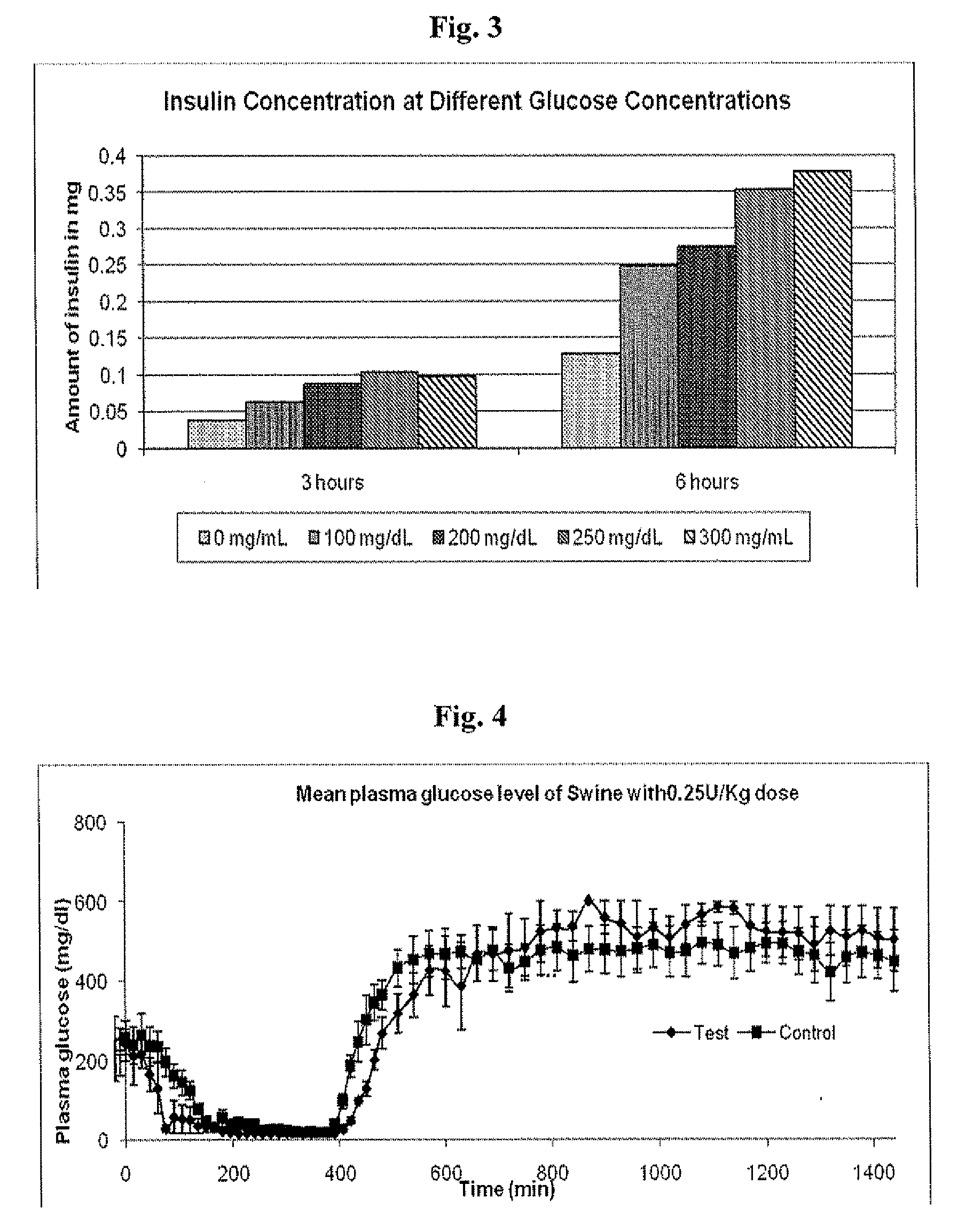
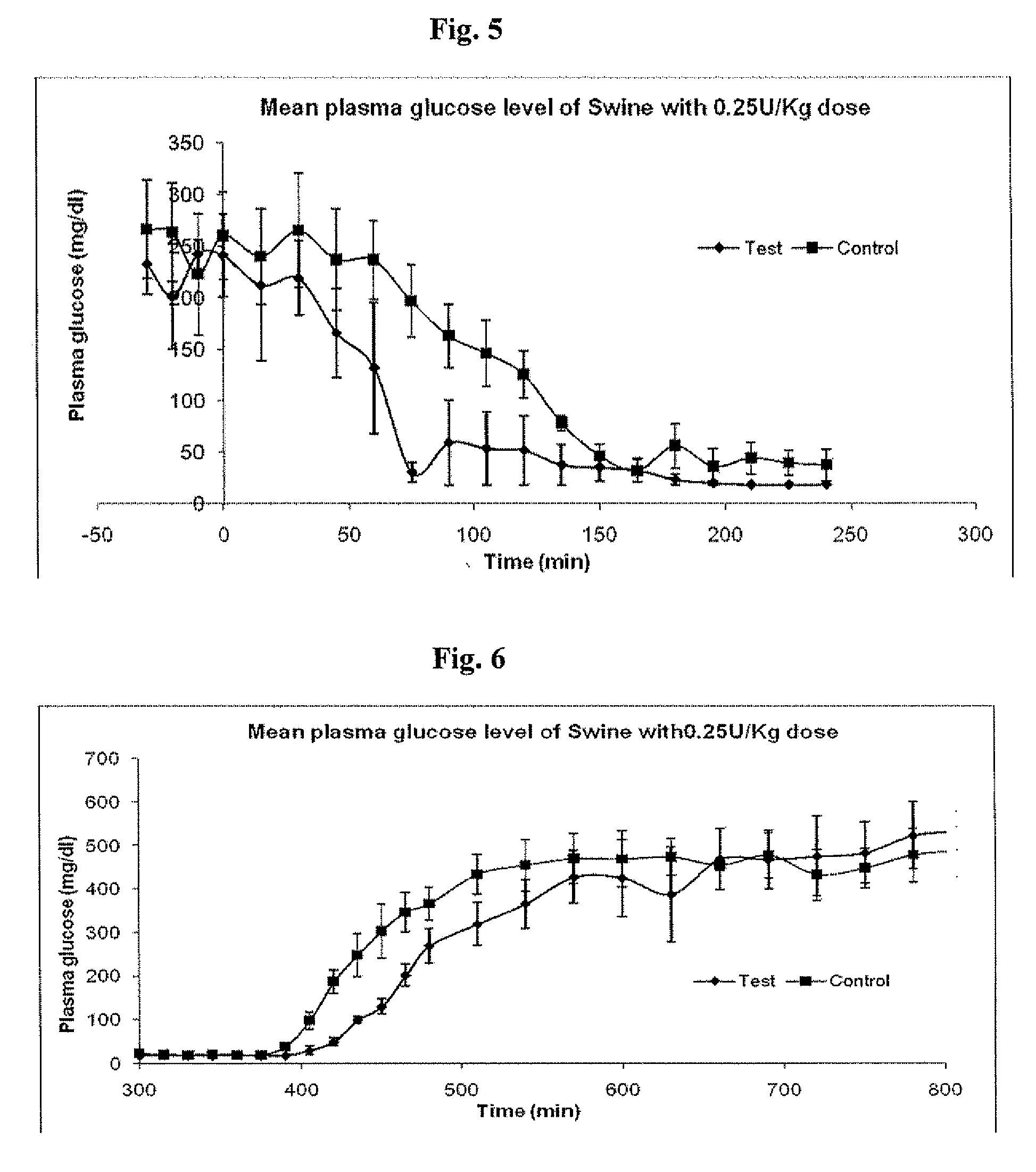
Injectable insulin formulations that are capable of modifying the amount of insulin released based on the patient's tissue glucose levels, methods for making and using these formulations are described herein. The formulation may be administered via subcutaneous, intradermal or intramuscular administration. In one preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin, an oxidizing agent or enzyme and a reducing agent or enzyme, a diluent and optionally one or more thickening agents. If a thickening agent is present in the formulation, the thickening agent increases the viscosity of the formulation following administration. Preferably the formulation contains an insulin, a diluent, glucose oxidase and peroxidase. Following administration to a patient, the insulin is released from the formulations as a function of the patient's tissue glucose level, which in turn maintains the patient's blood glucose level within an optimum range. The formulation is often referred to as a “smart” formulation since it modifies its release rate of insulin according to the patient's needs at a particular time. In a preferred embodiment, the formulation is designed to release insulin into the systemic circulation over time with a basal release profile following injection in a patient. In another embodiment, the formulation is designed to release insulin into the systemic circulation over time with a non-basal release profile following injection in a patient, such as a regular human insulin release profile or a prandial release profile.
Owner:BIODEL
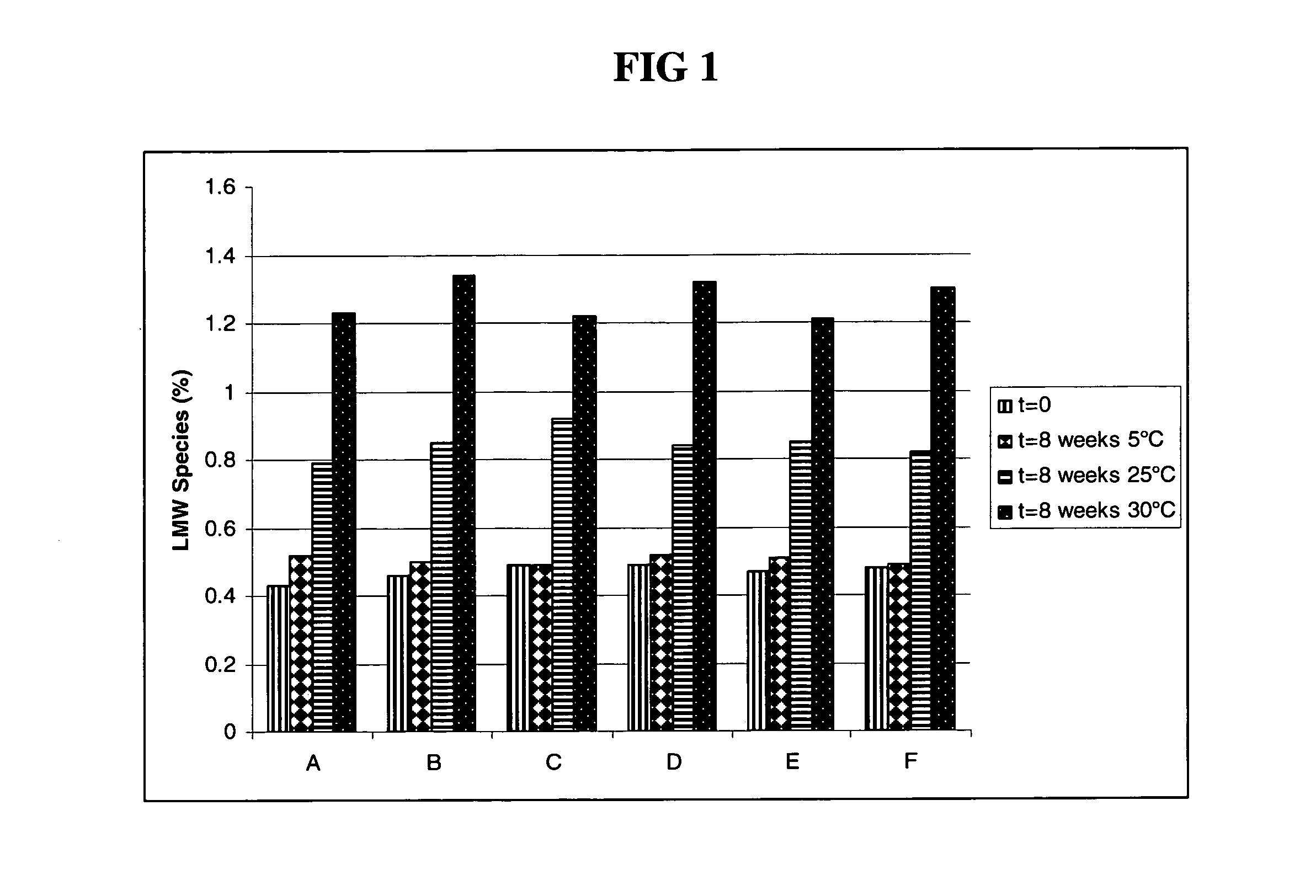
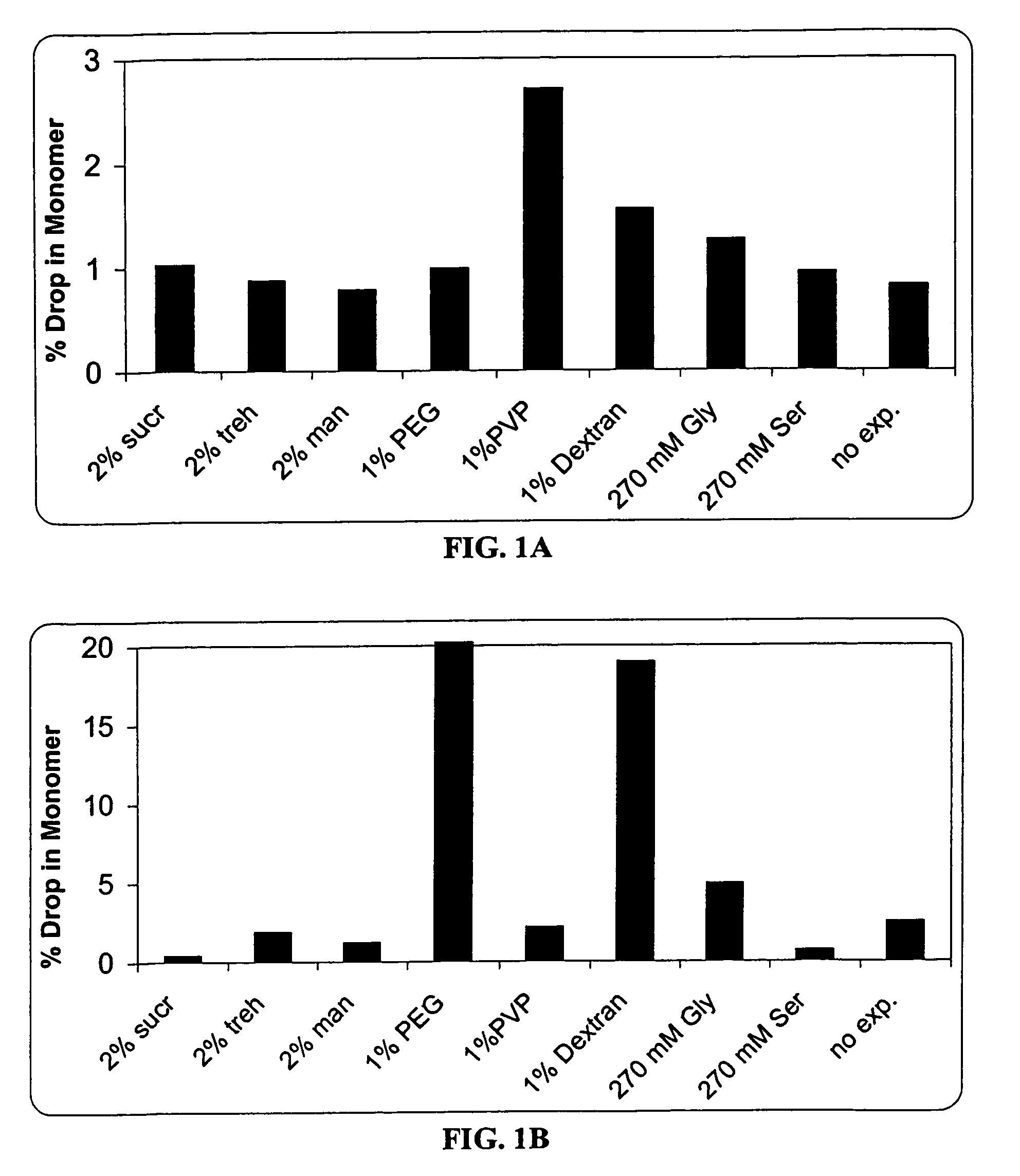
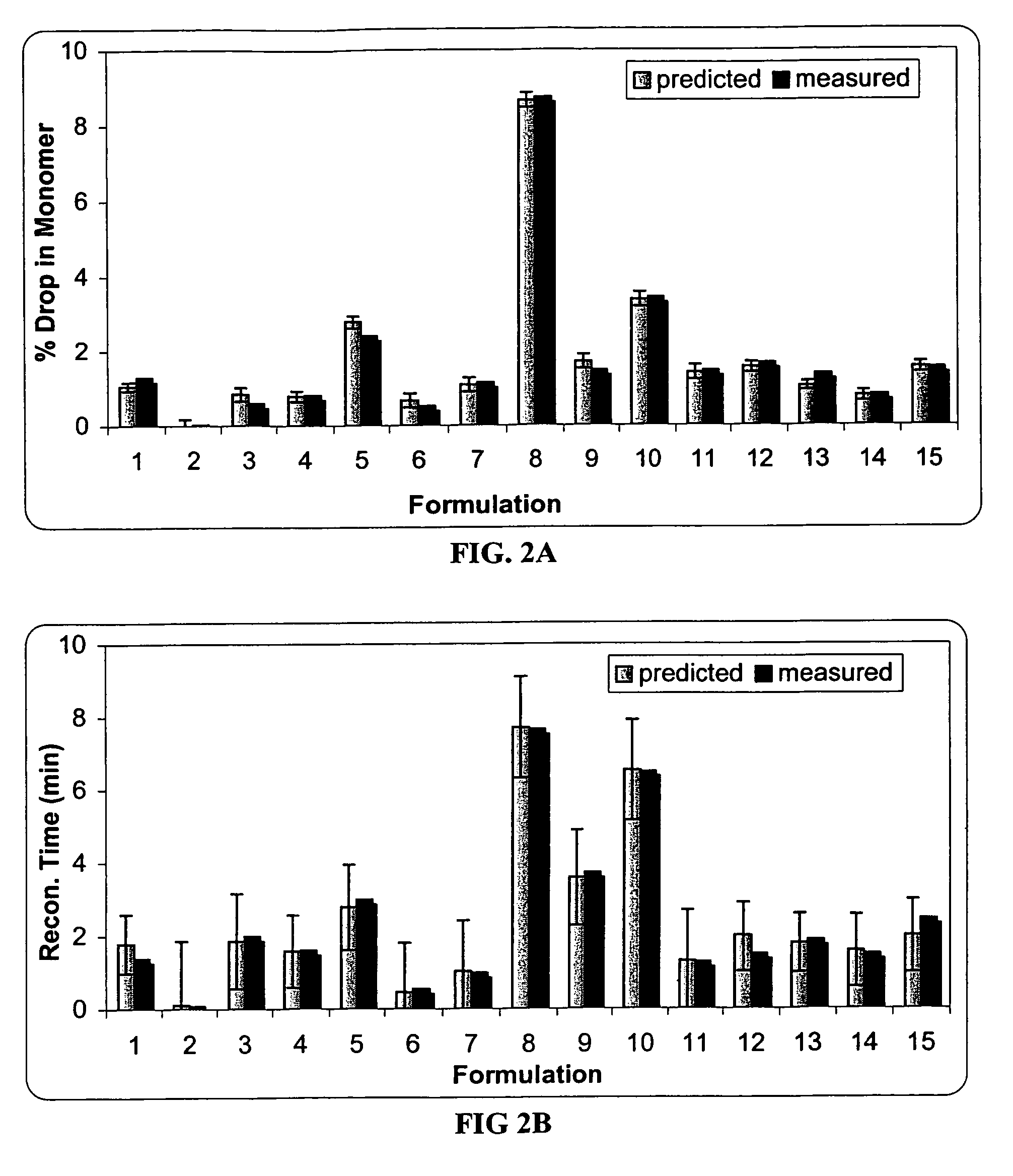
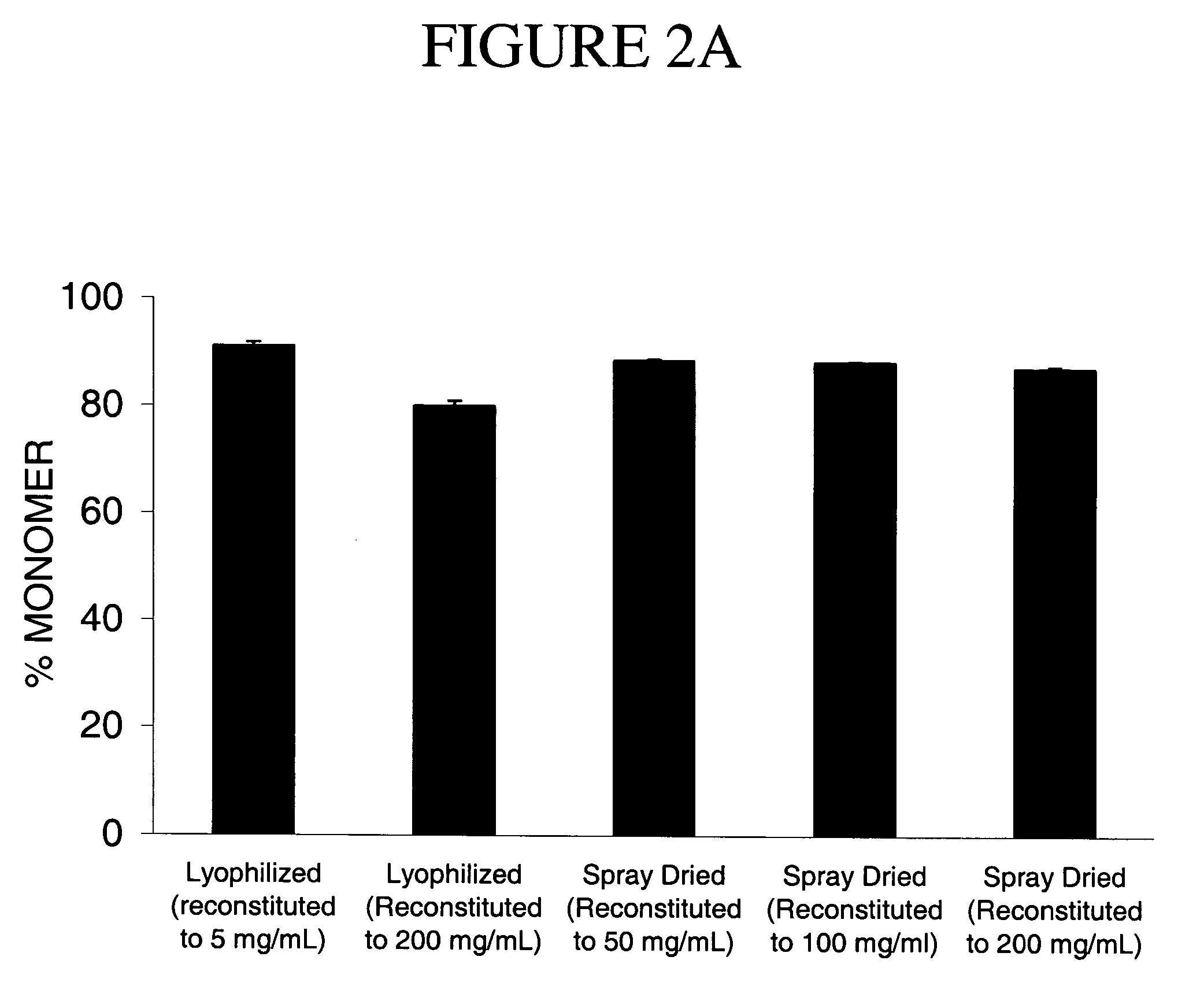
Methods for producing high concentration lyophilized pharmaceutical formulations
InactiveUS20120121580A1Contribute to physical structure and uniformity and stabilityPeptide/protein ingredientsImmunoglobulins against animals/humansHigh concentrationTherapeutic protein
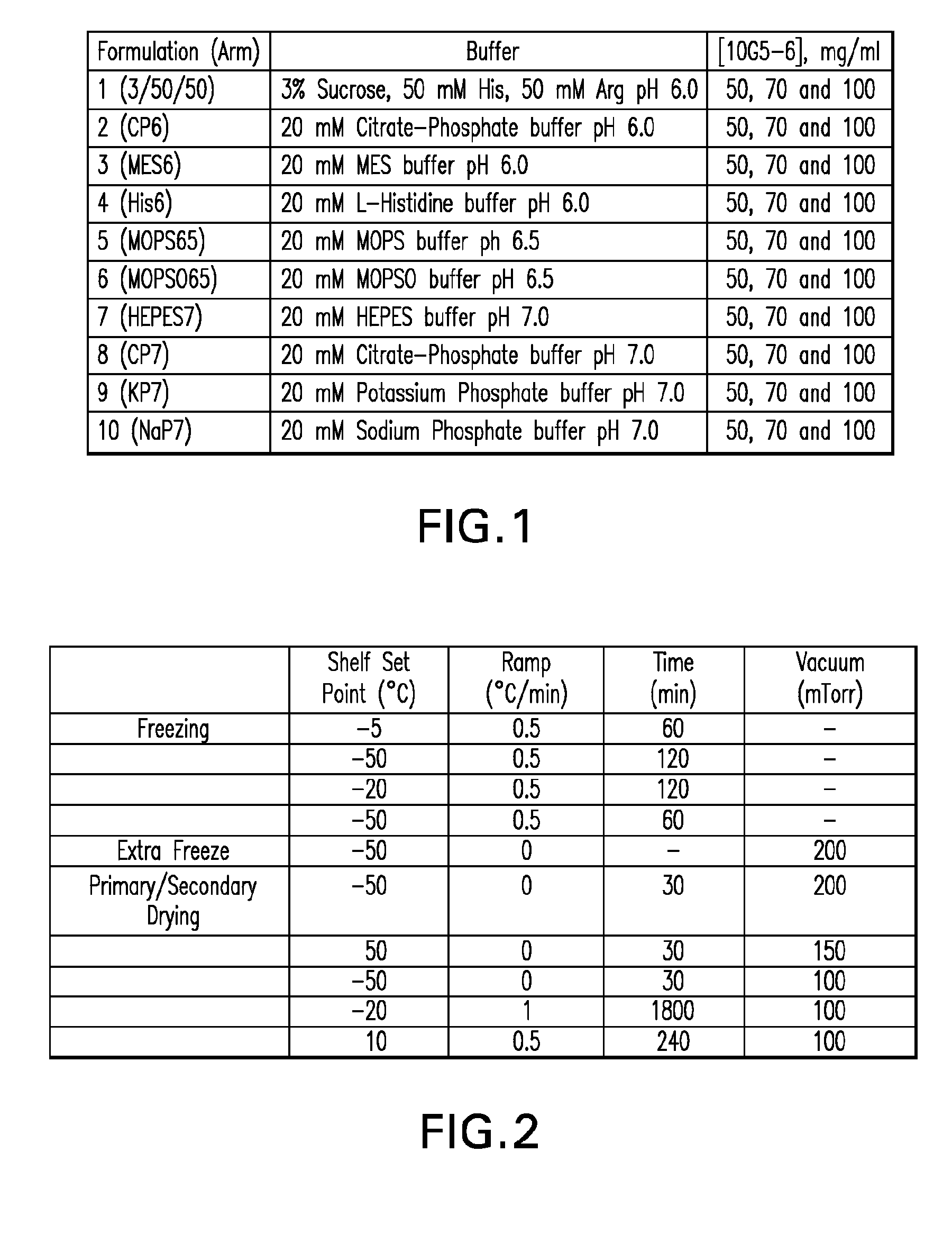
The present invention relates to methods of producing lyophilized pharmaceutical compositions comprising a high concentration of therapeutic protein or antibody prior to lyophilization, wherein the lyophilized formulation can be reconstituted with a diluent in about 15 minutes or less. The invention also relates to the high concentration lyophilized formulations produced by the methods described herein. The lyophilized formulations produced by the methods of the invention are stable and are suitable for veterinary and human medical use and are suitable for modes of administration including oral, pulmonary and parenteral, such as intravenous, intramuscular, intraperitoneal, or subcutaneous injection. Also provided by the invention are high concentration pharmaceutical compositions that have long term stability and can be reconstituted, following lyophilization, in a short period of time, preferably 15 minutes or less.
Owner:MERCK SHARP & DOHME CORP
Oligonucleotide chelate complexes
ActiveUS20120046348A1Reduce the impactSuppressing and reducingOrganic active ingredientsBiocideTolerabilityDivalent metal
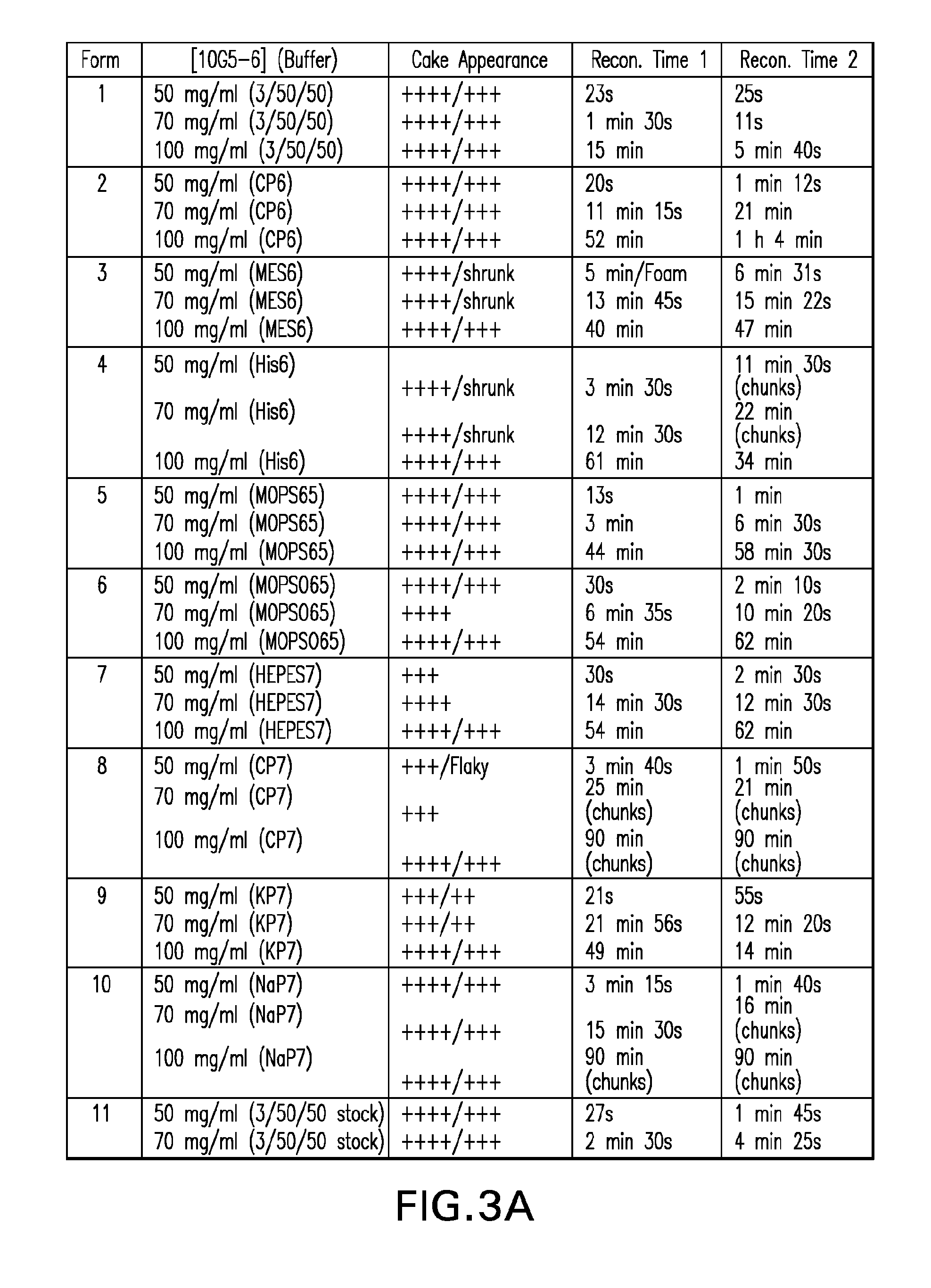
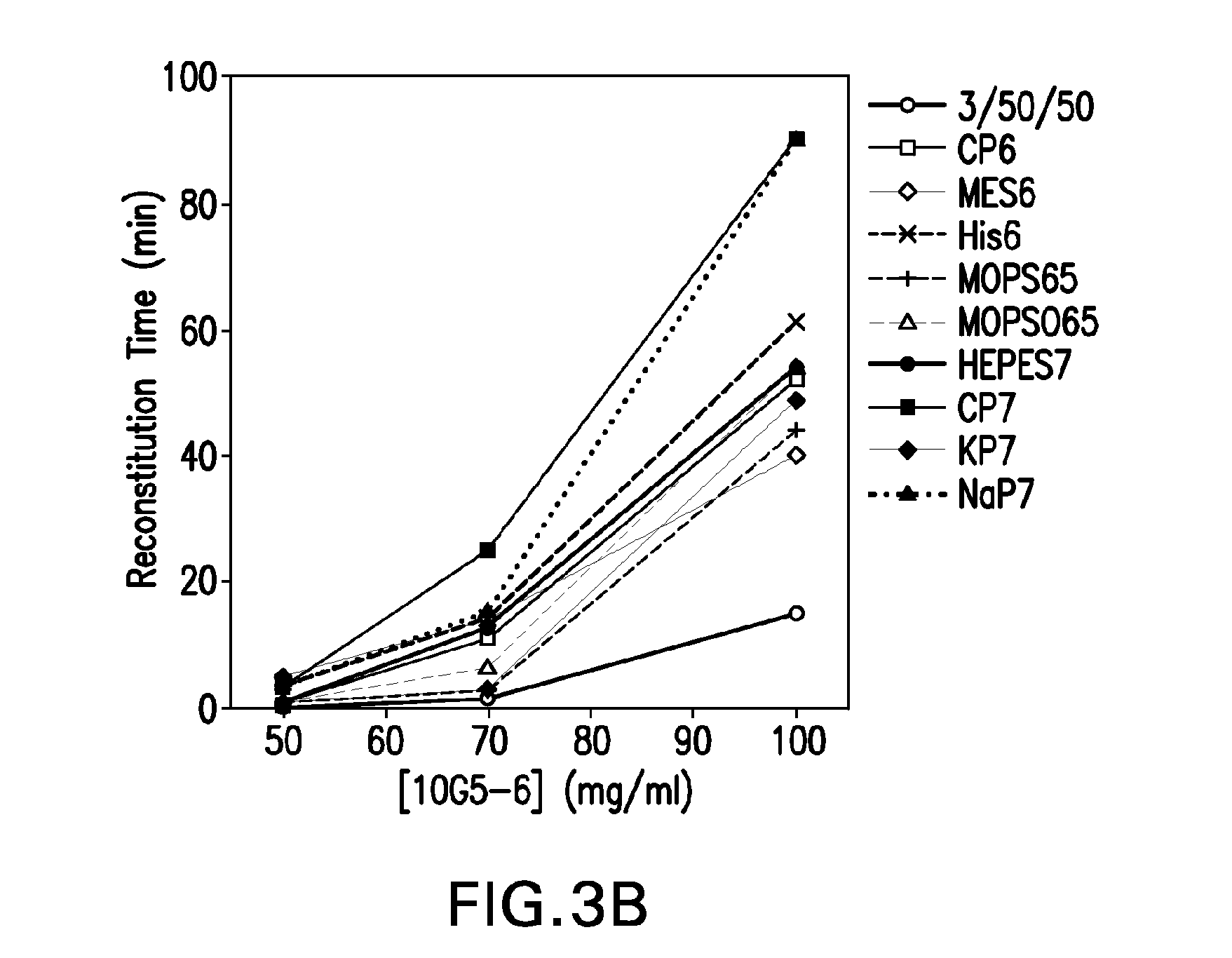
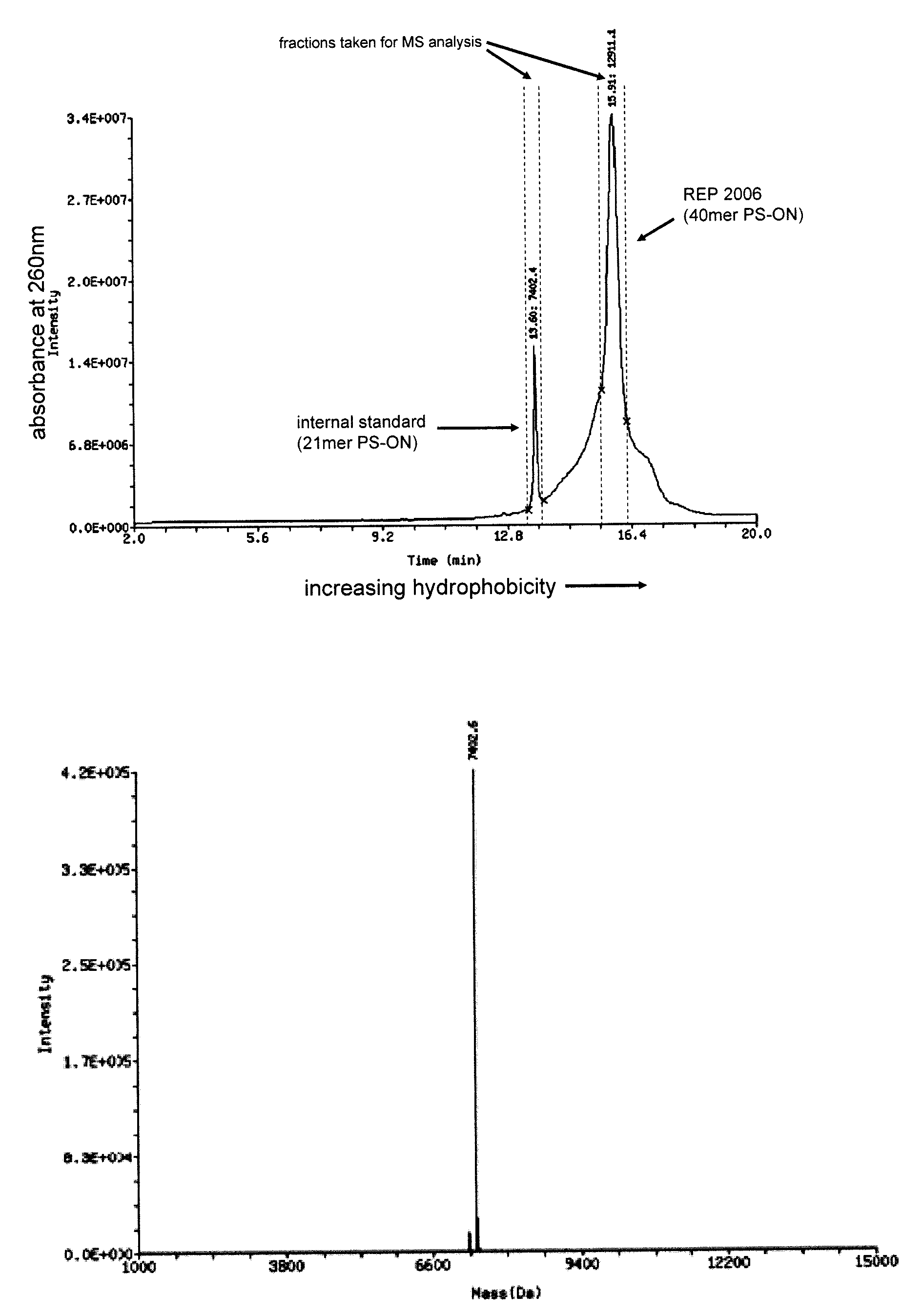
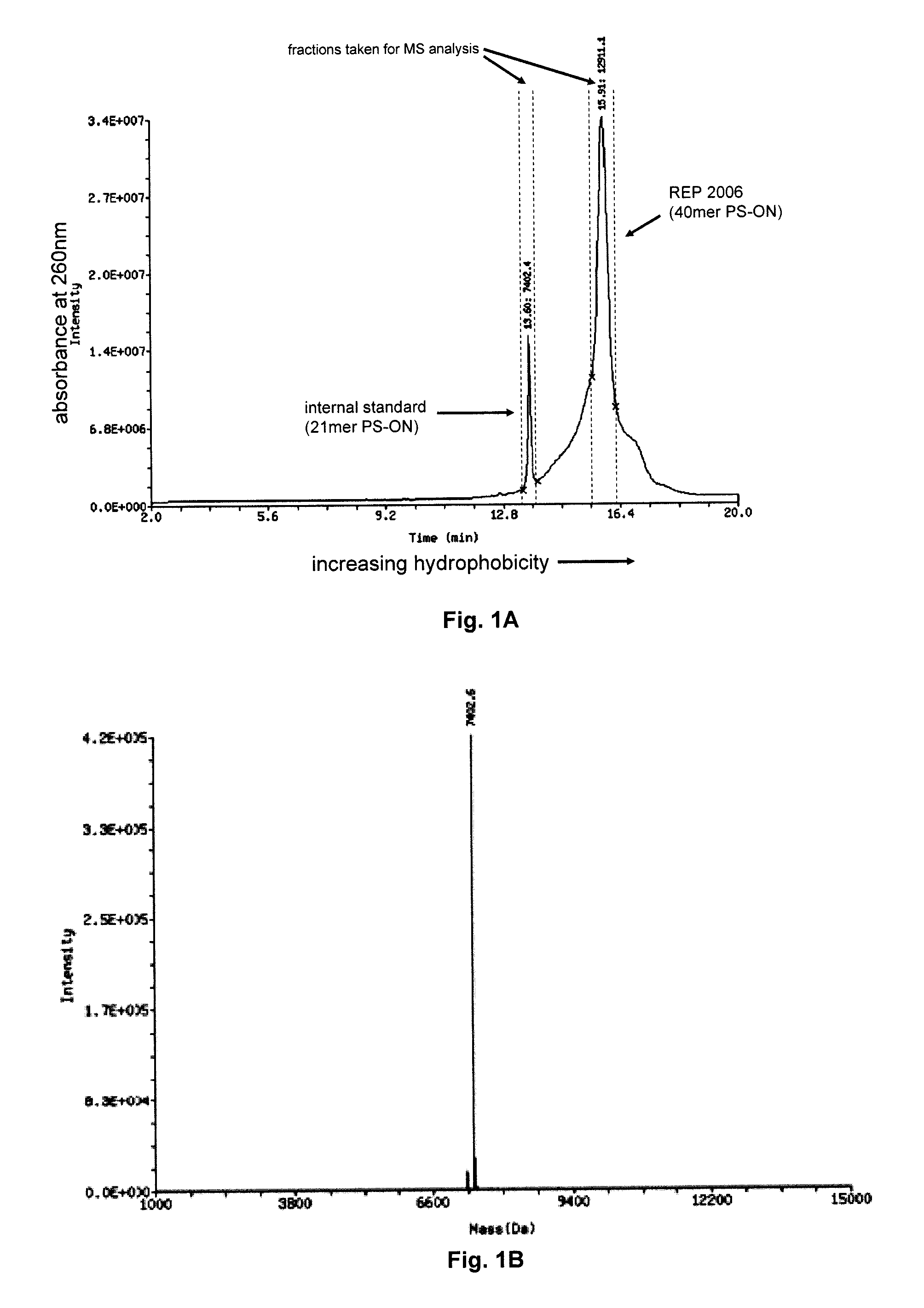
The present disclosure describes the broadly active chelation of diverse divalent 2+ metal cations by any oligonucleotide (ON), regardless of size or modification. This chelation effect is specific to cations which are divalent (or of higher valency) and results in the formation of oligonucleotide chelate complexes which do not behave like salts. It is described herein a novel composition of an ON chelate complex prepared using any ON and a divalent metal cation and methods for the suppression of anti-coagulation and or subcutaneous injection site reactions and or improved tolerability with oligonucleotides by the use of ON chelate complexes during oligonucleotide administration.
Owner:REPLICOR INC
Antibody-containing particles and compositions
InactiveUS20050053666A1Remove complexityShort timePowder deliveryAntibody ingredientsSubcutaneous injectionDiluent
A composition is provided comprising antibody-containing particles. These particles can be used to form antibody-containing powders useful for reconstitution with a suitable diluent. The reconstituted compositions, in turn, comprise an antibody in an amount suited for delivery by injection, such as subcutaneous injection. Methods for preparing the various compositions as well as methods of use are also provided.
Owner:NOVARTIS FARMA
Methods and compositions for reducing activity of the atrial natriuretic peptide receptor and for treatment of diseases
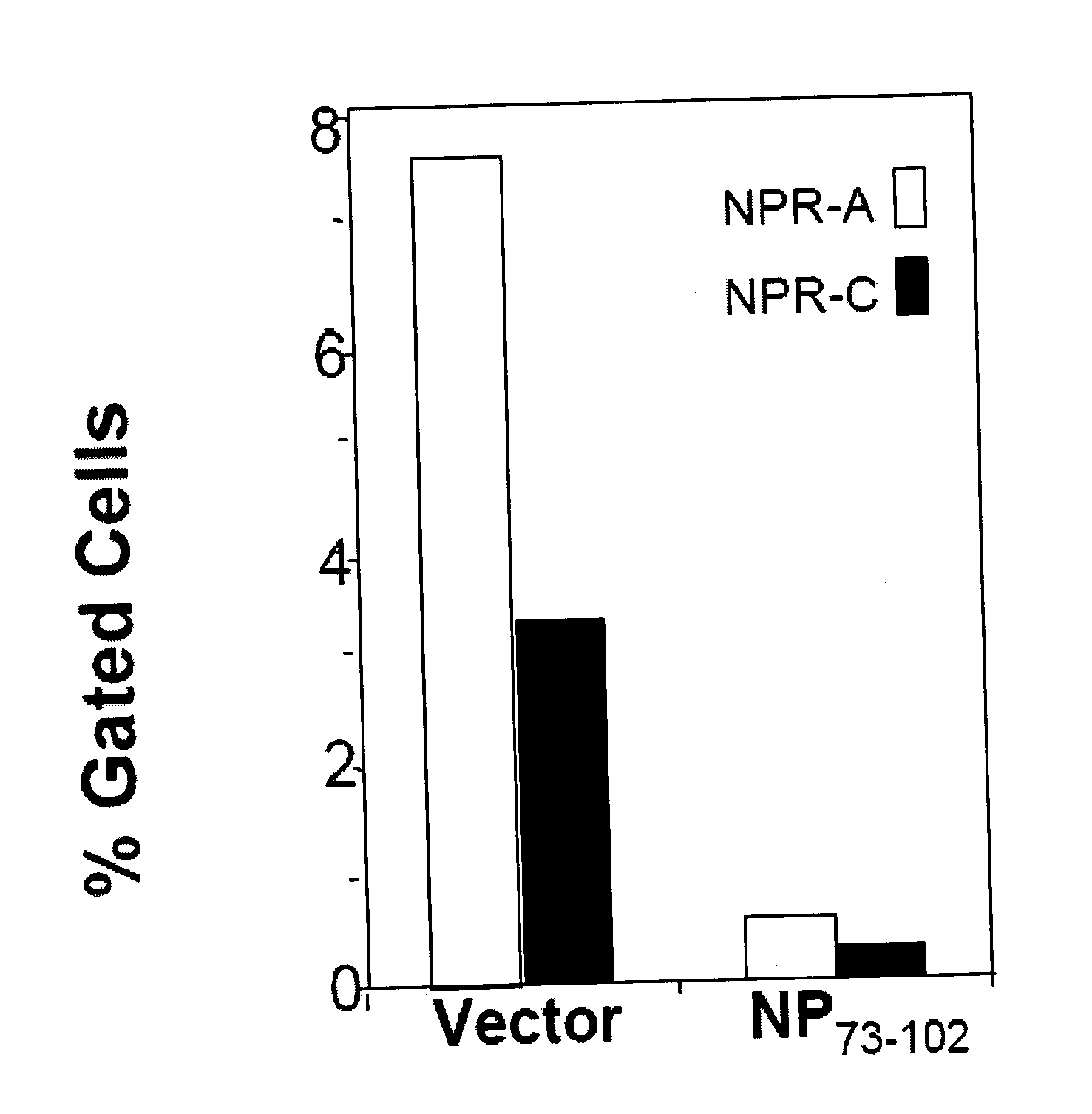
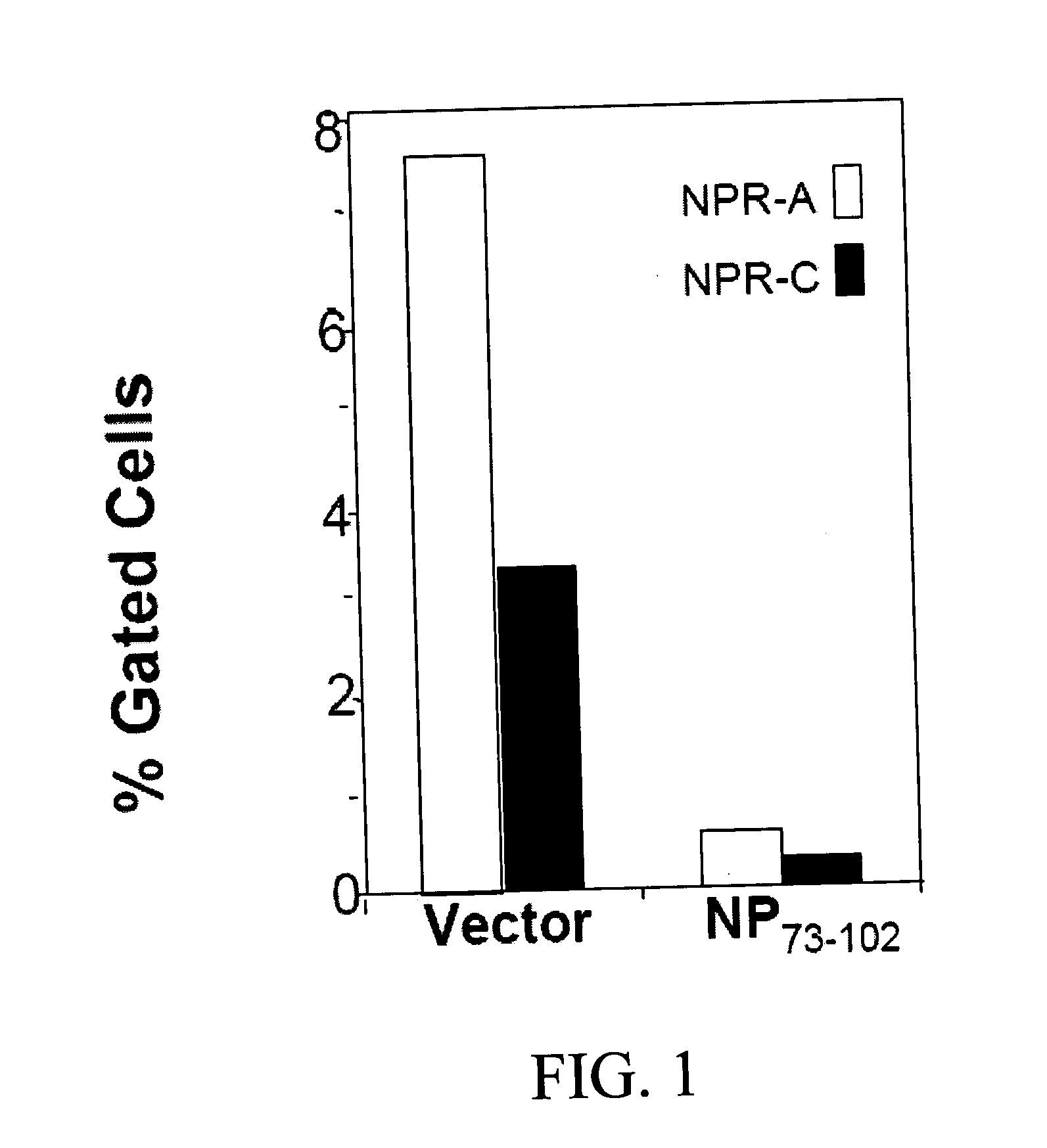
InactiveUS20080214437A1Reduction in formation of tumorIncreased apoptosisCompounds screening/testingOrganic active ingredientsLipid formationDisease
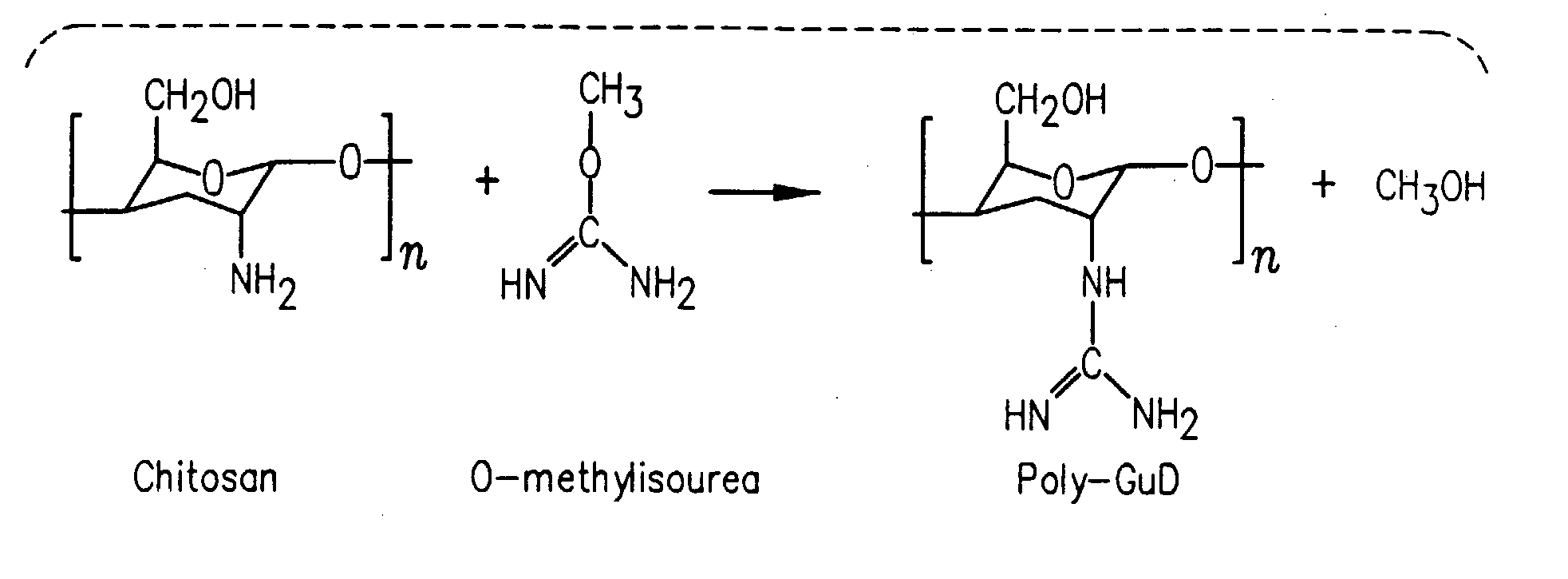
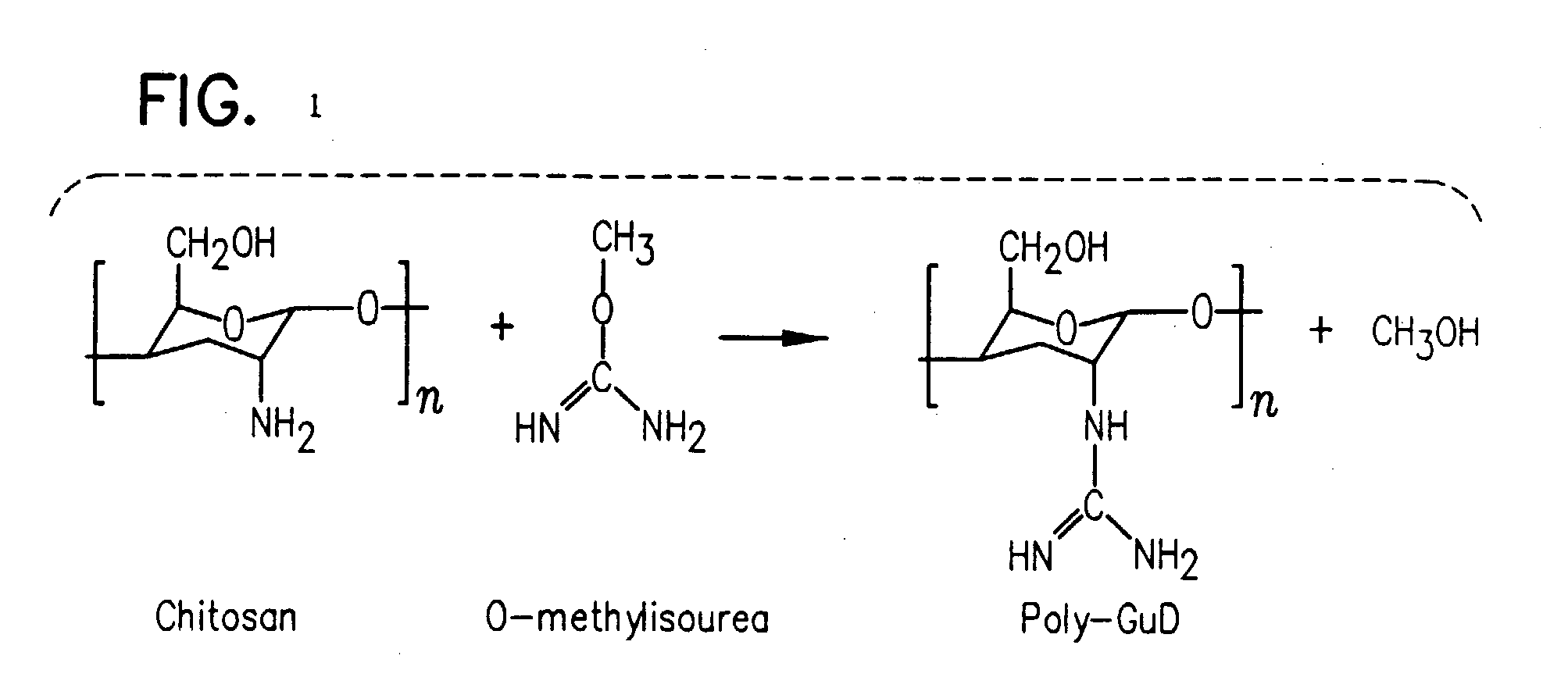
Methods, compositions and devices are provided by the present invention for reducing activity of a natriuretic peptide receptor and other signals. Therapeutic treatments are provided by use of polynucleotides encoding a natriuretic peptide or by regulating the expression of natriuretic peptide receptor, such as NPRA and NPRC, or combinations of these therapies. Routes used for delivering polynucleotides encoding a natriuretic peptide, or, for example, siRNA that down regulates natriuretic peptide receptor include subcutaneous injection, oral gavage, transdermal and intranasal delivery routes. Compositions can include chitosan, chitosan derivatives, and chitosan derivative and a lipid. Transdermal delivery can use a transdermal cream. Intranasal delivery can use a dropper or an aspirator for delivery of a mist. Oral gavage delivers equivalent to oral delivery. Delivery permits cell and tissue specific targeting of gene therapies resulting in expression of a natriuretic peptide or down regulation of natriuretic peptide receptor. A variety of cancers, asthma and viral diseases can be treated therapeutically using the methods and compositions of the present invention.
Owner:MOHAPATRA SHYAM S +4
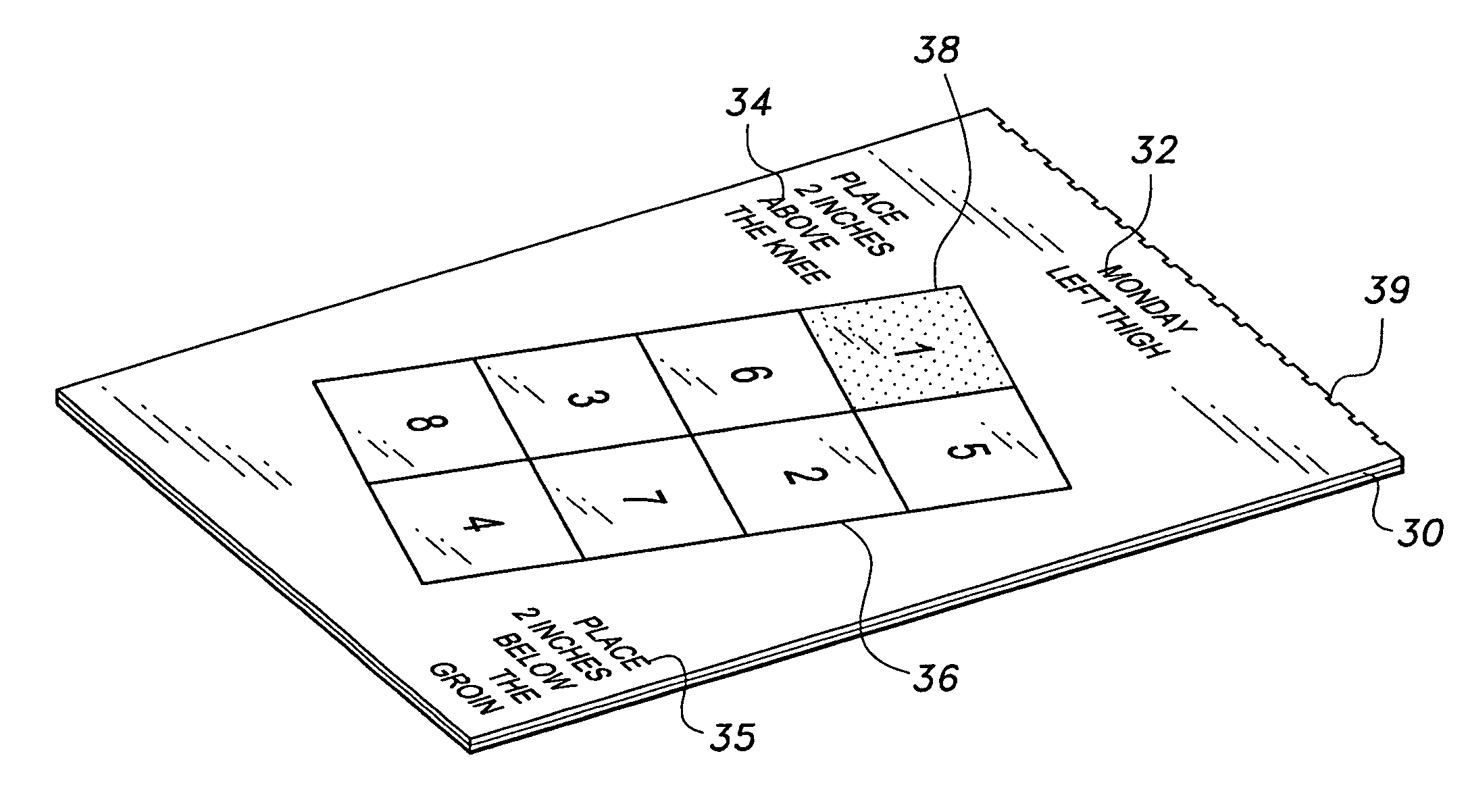
Self-injection guide tablet
InactiveUS7713234B2Oral administration deviceIntravenous devicesSubcutaneous injectionBiomedical engineering
The self-injection guide tablet for subcutaneous injections includes adhesively mounted templates with headings indicating a particular day and body injection area. Each template further includes a grid guide, designed to cover the body injection area, along with instructions for placing the grid guide on the particular body injection area. Each grid guide includes a plurality of injection grids, in column or row format, with indicia marking a predetermined injection grid. Upon removing the grid guide from the specific template, the predetermined injection grid remains on the template, so the vacated injection grid forms the injection area for the subcutaneous injection.
Owner:KARANZAS DOREEN A
Rapid Acting Injectable Insulin Compositions
InactiveUS20080090753A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsDissolutionExcipient
Injectable insulin formulations with improved stability and rapid onset of action are described herein. The formulations may be for subcutaneous, intradermal or intramuscular administration, In the preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. These formulations are rapidly absorbed into the blood stream when administered by subcutaneous injection. In the preferred embodiment, the insulin is provided as a dry powder in a sterile vial. This is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water, a zinc chelator such as EDTA and a dissolution agent such as citric acid shortly before or at the time of administration. In another embodiment, the insulin is stored as a frozen mixture, ready for use upon thawing.
Owner:BIODEL
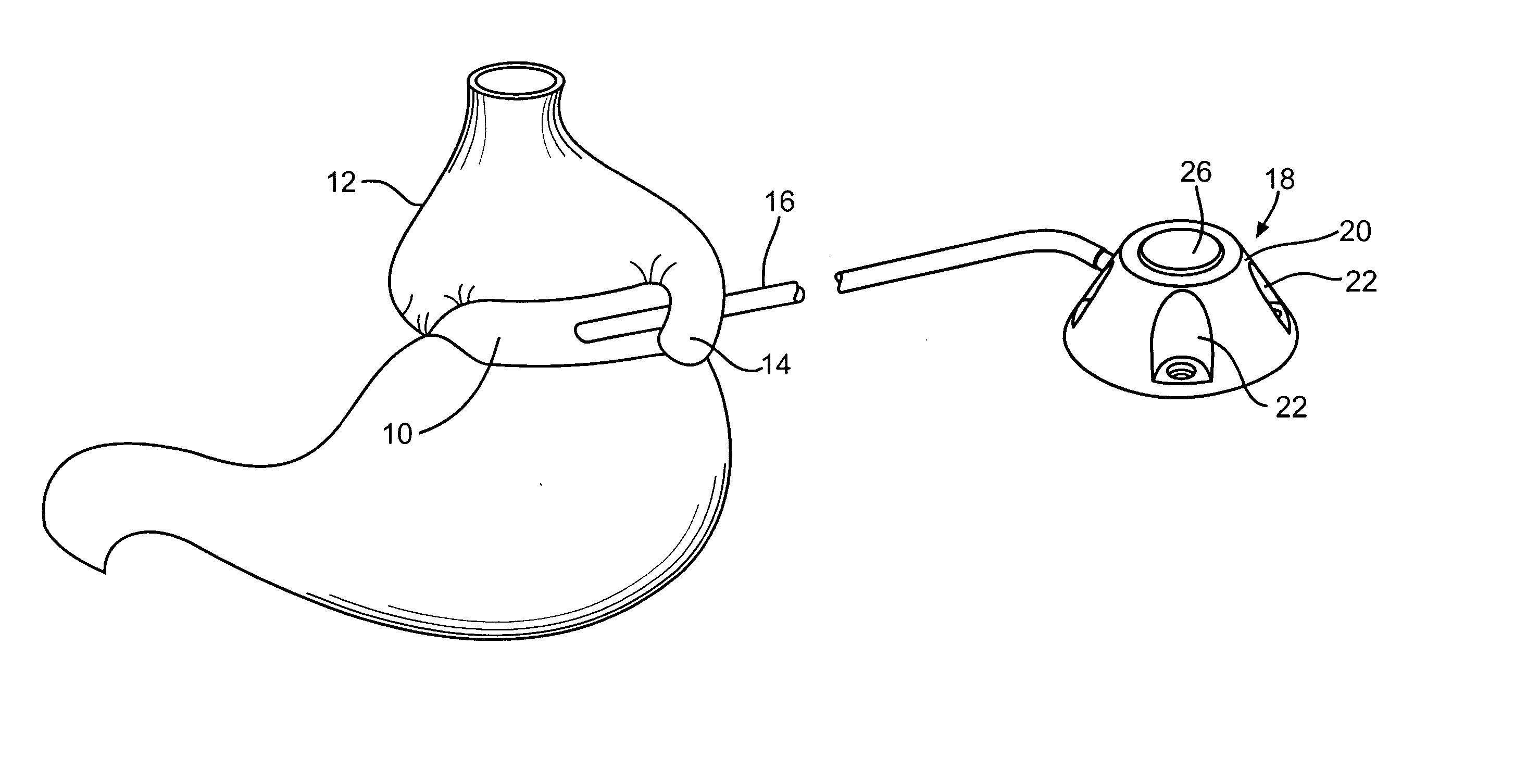
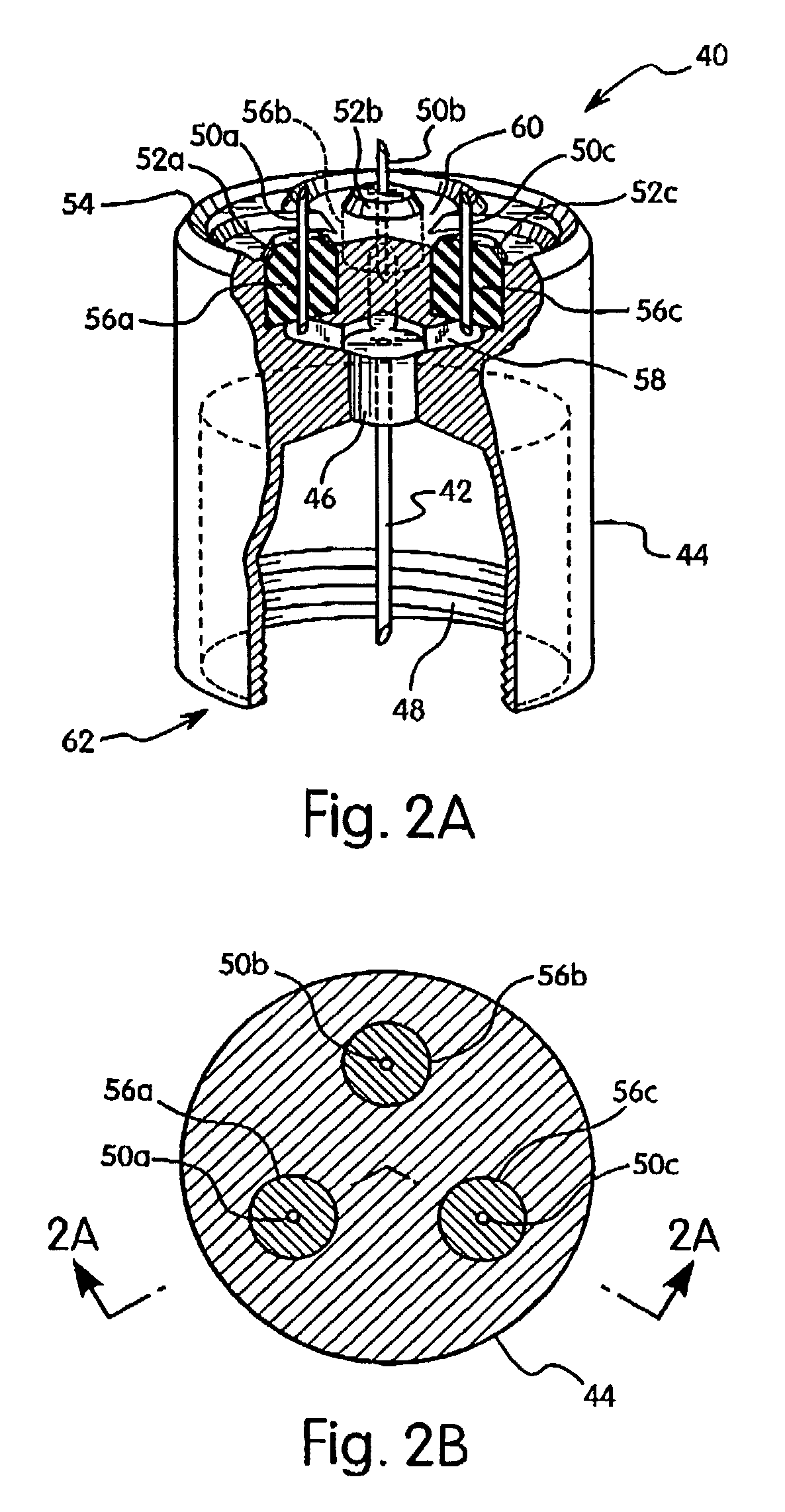
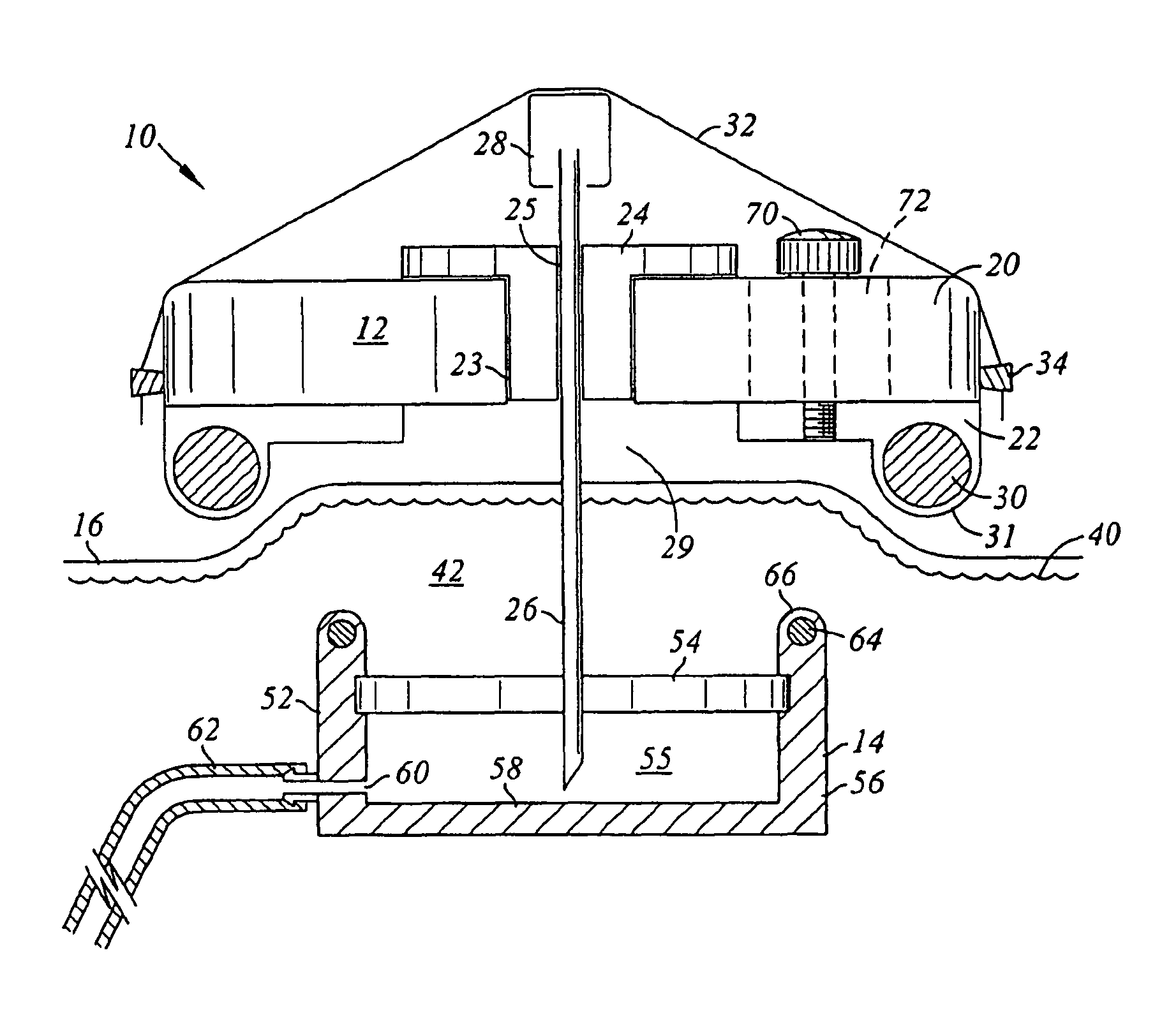
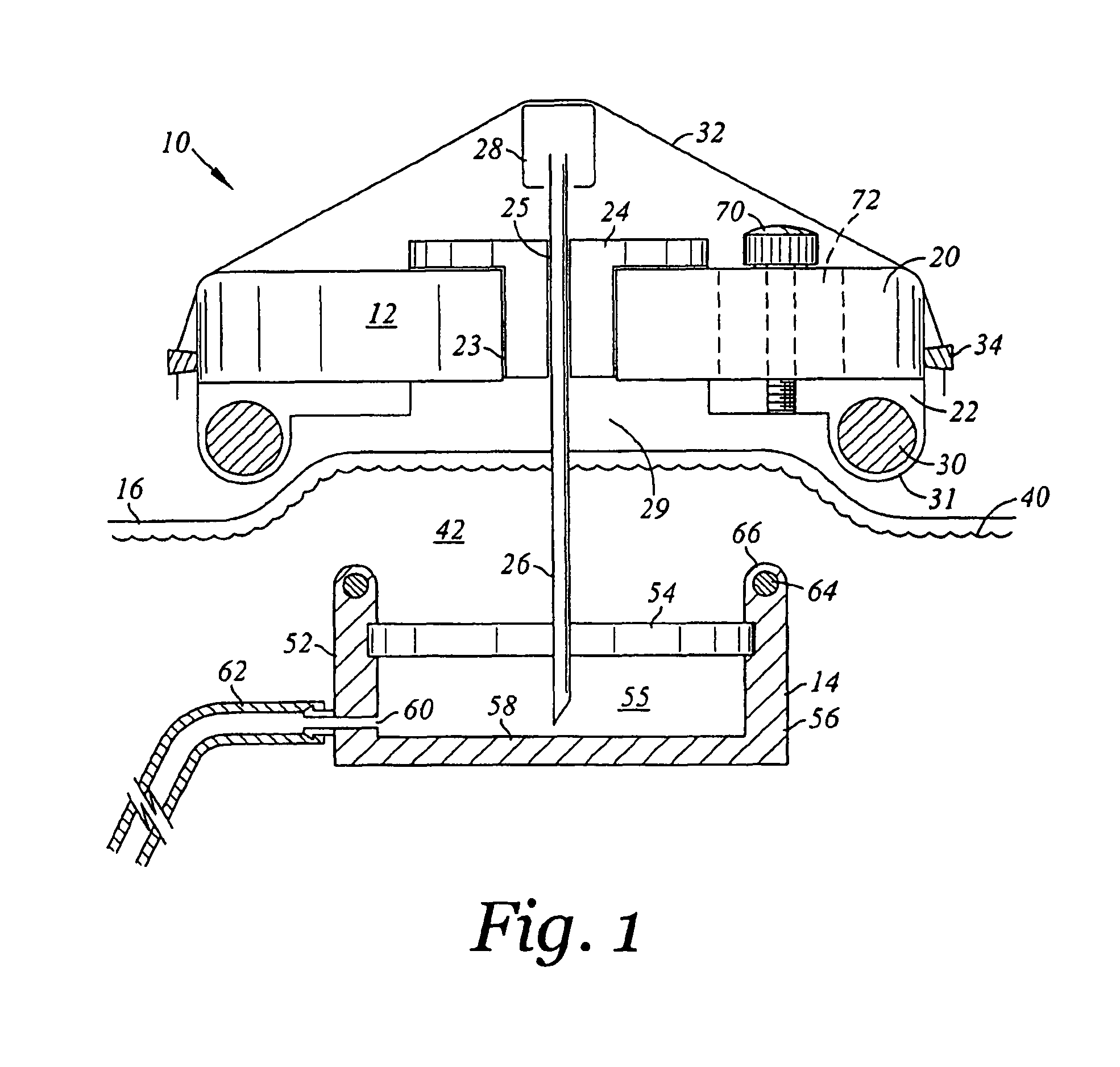
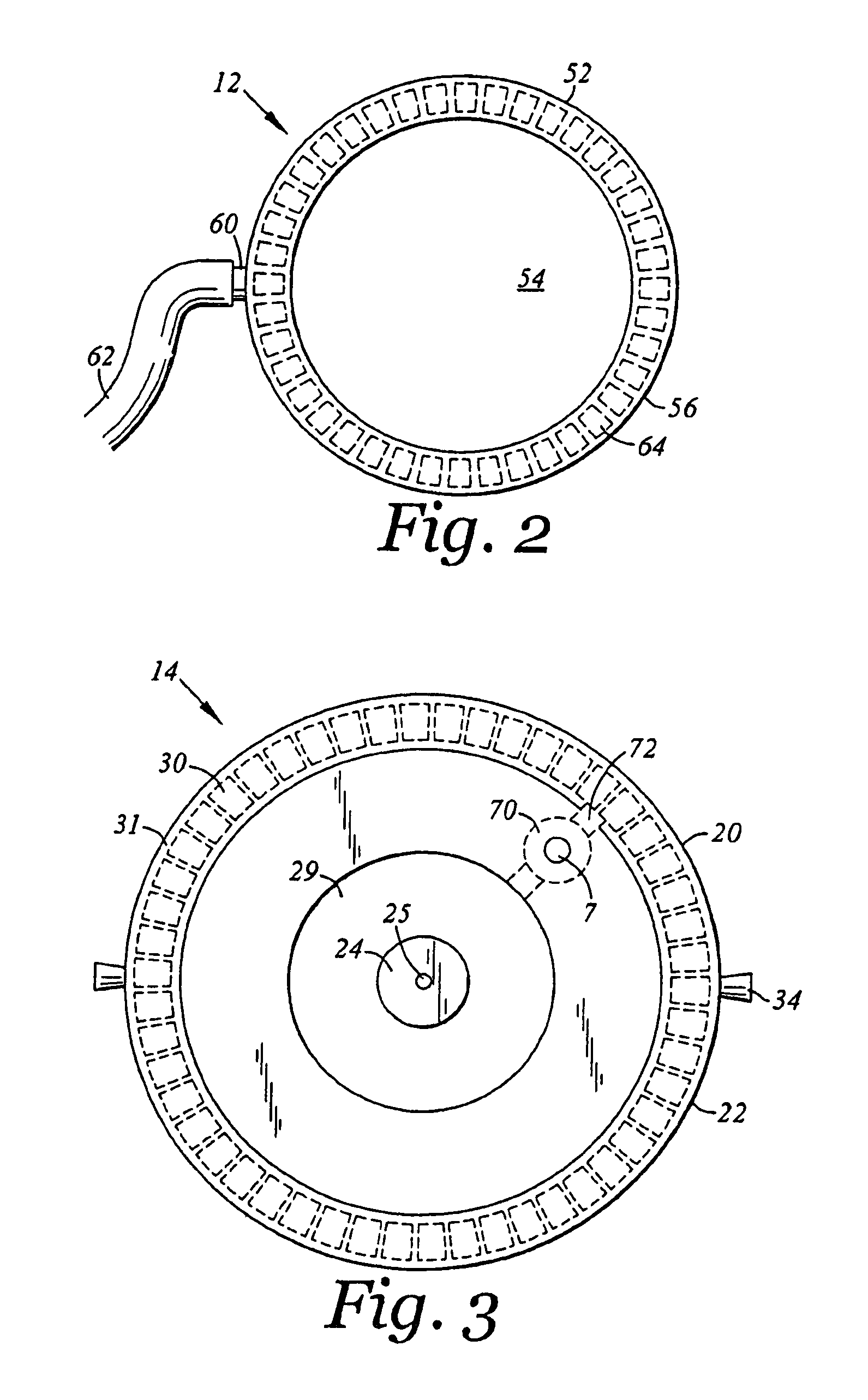
Implantable central venous injection port and external needle guide
An assembly for subcutaneous injections comprising an implantable injection port comprising a body with a plenum, an elastomeric self-sealing diaphragm sealed to the body or with a plenum, an outlet connecting the plenum to a conduit, and one or more ferromagnetic compatible elements positioned about the elastomeric self-sealing diaphragm and near the top side; and a needle support platform comprising a body having a top side and bottom side, with a needle guide extending from the top side to the bottom side, a needle secure; and an attachment base with a top side and bottom side, a center hollow open to the top and bottom sides, and one or more ferromagnetic compatible elements positioned about the center hollow and near the bottom side of the attachment base, the bottom side of the body of the needle support platform resting on the top side of the attachment base and slidable thereon, the position of the body on the attachment base adapted to be detachably secured.
Owner:CHUTER TIMOTHY A M +1
Liraglutide long-acting microsphere injection and preparation method thereof
ActiveCN102085355AReduce the burden of treatmentImprove Medication AdherencePowder deliveryPeptide/protein ingredientsTreatment burdenMicrosphere
The invention provides a long-acting microsphere injection as an antidiabetic medicament, the long-acting microsphere injection comprises liraglutide, PLGA (poly(lactic-co-glycolic acid)), excipients and a surfactant, and the invention simultaneously relates to a preparation method of the injection. Liraglutide long-acting sustained-release microspheres provided by the invention are designed to perform subcutaneous injection once every 28 days, thereby greatly reducing treatment burden on a patient, improving medication compliance and reducing treatment cost; simultaneously, results of in vitro release studies, animal experiments and the like prove that the obtained sustained-release microspheres can slowly release a medicament for a long time in vitro and in vivo.
Owner:蚌埠丰原涂山制药有限公司
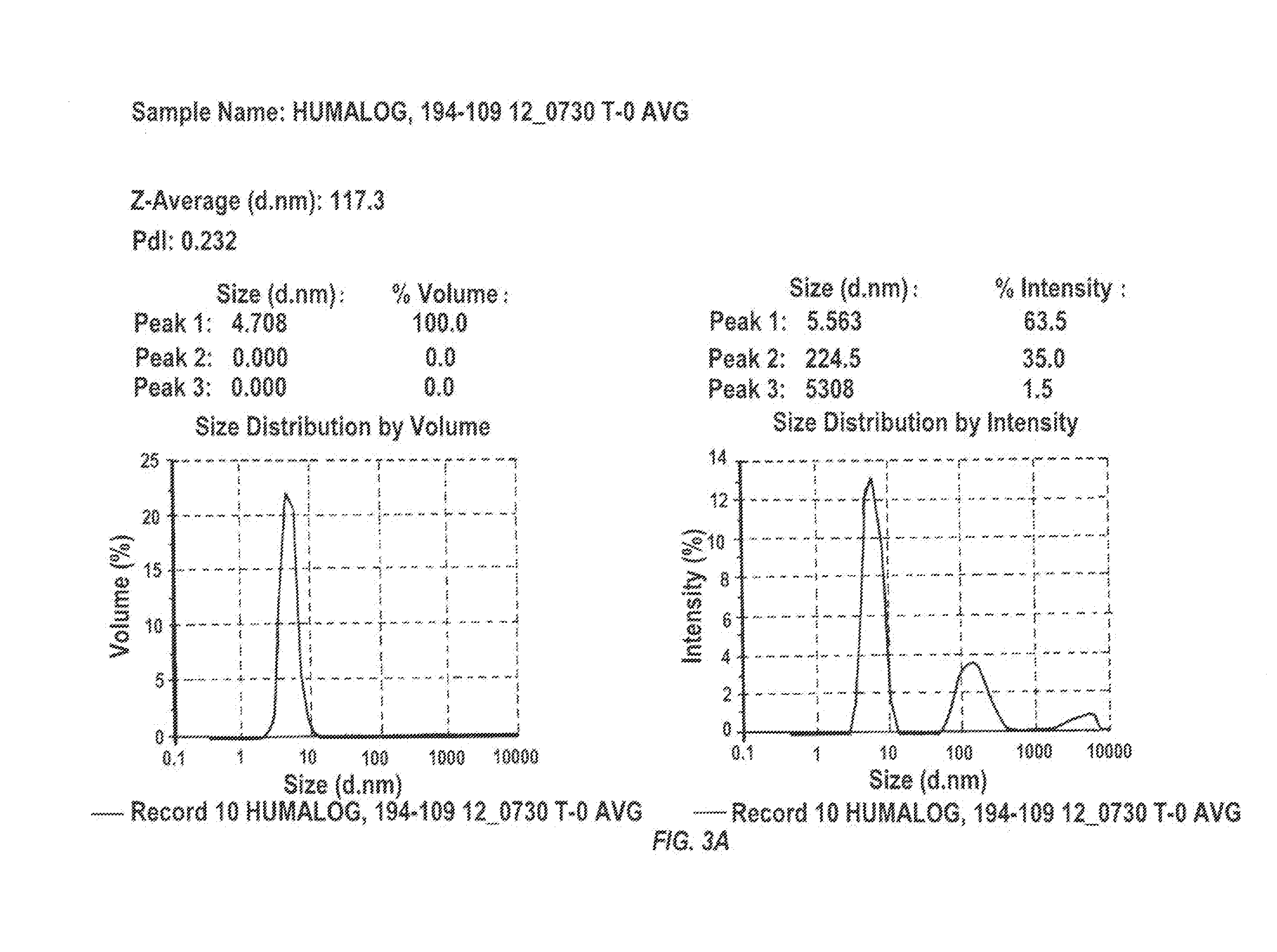
Stabilized ultra-rapid-acting insulin formulations
InactiveUS20150273022A1Quick effectPromote absorptionBiocidePeptide/protein ingredientsZinc compoundsMagnesium salt
Compositions and methods for enhancing the stability of rapid acting injectable insulin formulations have been developed for subcutaneous injection. The formulations contain insulin in combination with a zinc chelator such as ethylenediaminetetraacetic acid (“EDTA”), a dissolution / stabilization agent such as citric acid, a magnesium salt, a zinc compound and, optionally, additional excipients. New presentations include rapid acting concentrated insulin formulations and a way to enhance the absorption of commercially available rapid acting analog formulations while maintaining insulin stability.
Owner:ALBIREO PHARMA INC
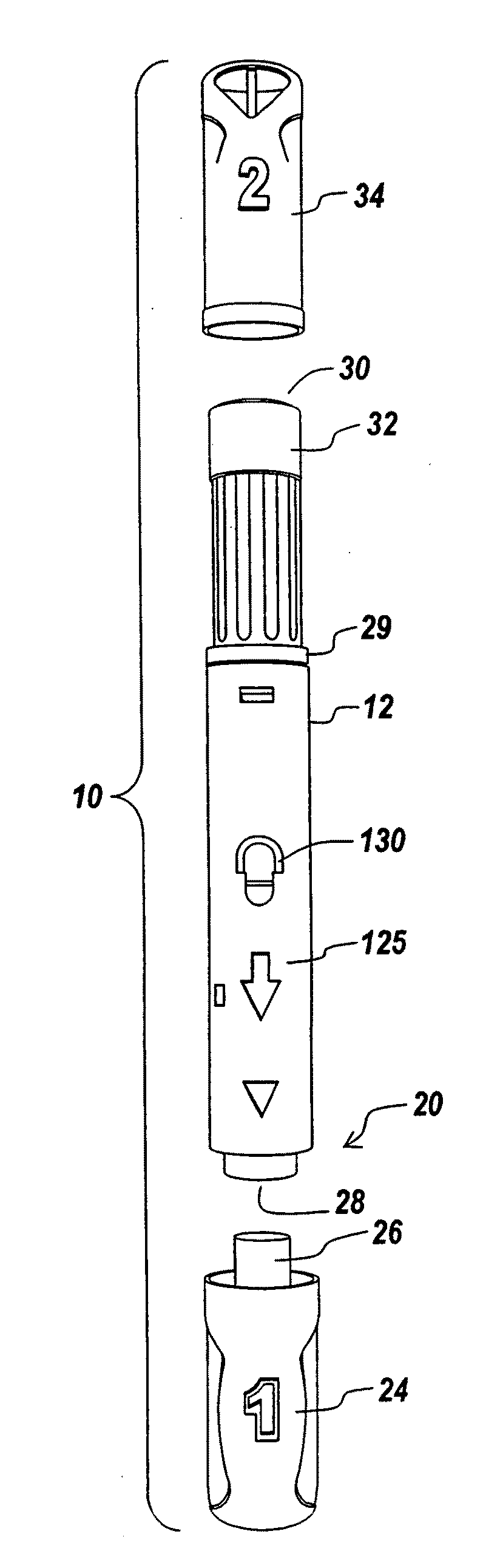
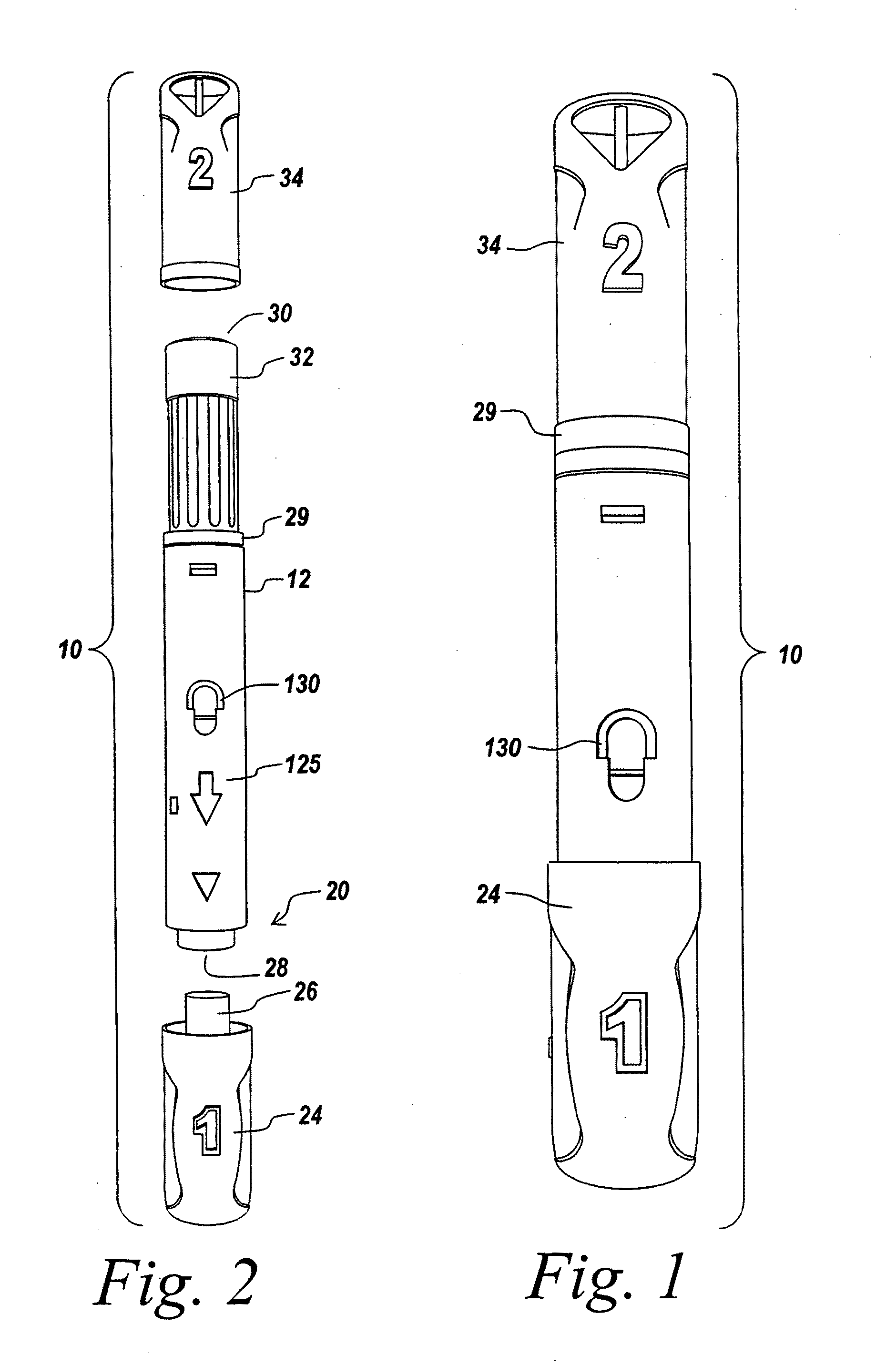
Method And Apparatus For Delivering A Therapeutic Substance Through An Injection Port
Adapters for utilizing a syringe or pen injector with a subcutaneous injection port to deliver a therapeutic substance through the injection port and methods of using the adapters are provided. A syringe adapter has a body having a first end and a second end. The first end of the body is configured to receive and engage the end of a syringe so that the cannula of the syringe is held at a fixed position with the respect to the adapter. The second end of the adapter configured to mate with a mating portion of the injection port. When the second end of the adapter engages the mating portion of the injection port, the adapter assures that the cannula of the syringe is properly aligned with the subcutaneous injection port and assures that the cannula penetrates the injection port to the proper depth. Adapters for use with pen style delivery systems are also disclosed. Additionally, an adapter to facilitate loading a syringe with a therapeutic substance from a vial is disclosed.
Owner:EMBECTA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com