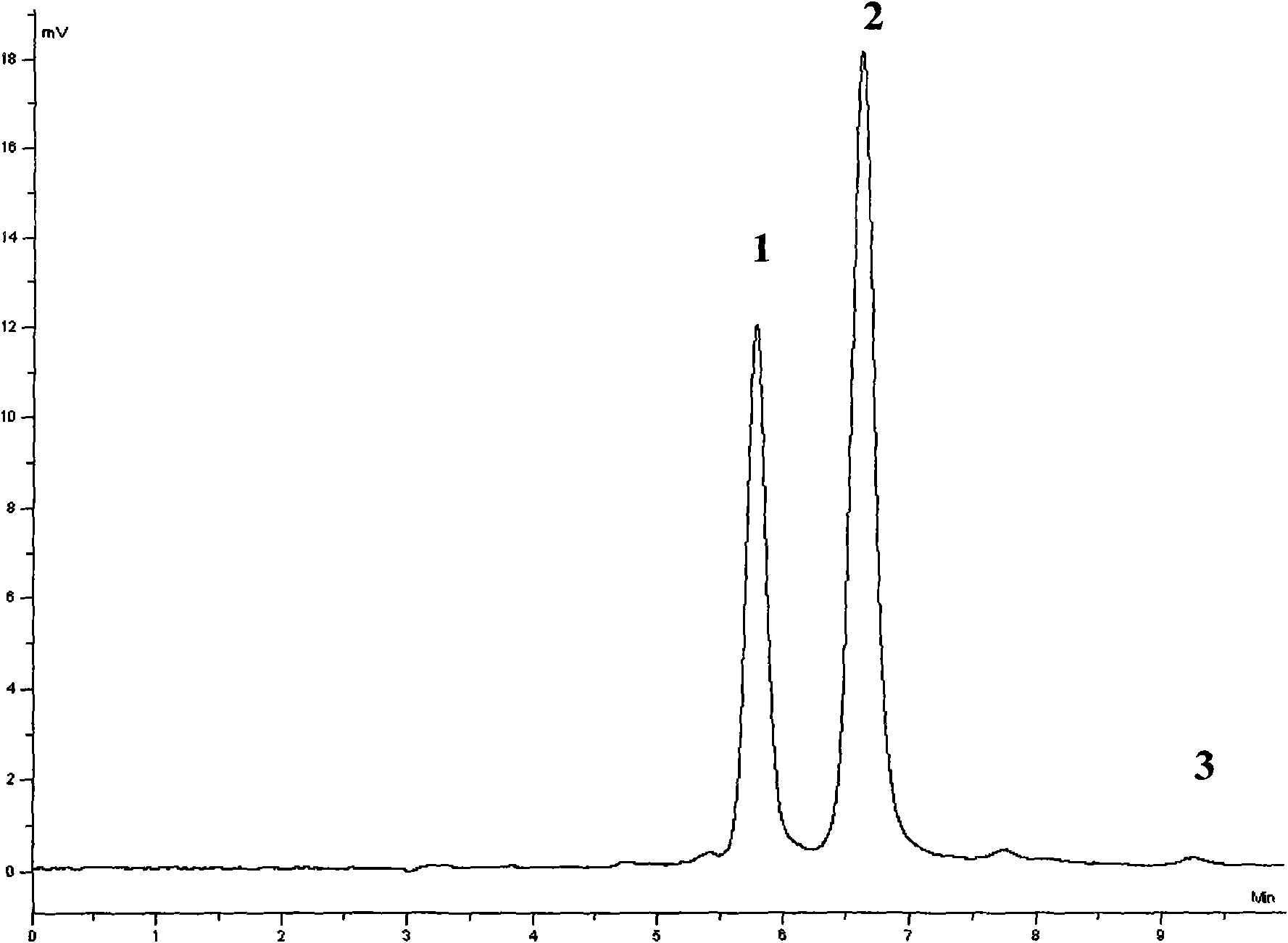
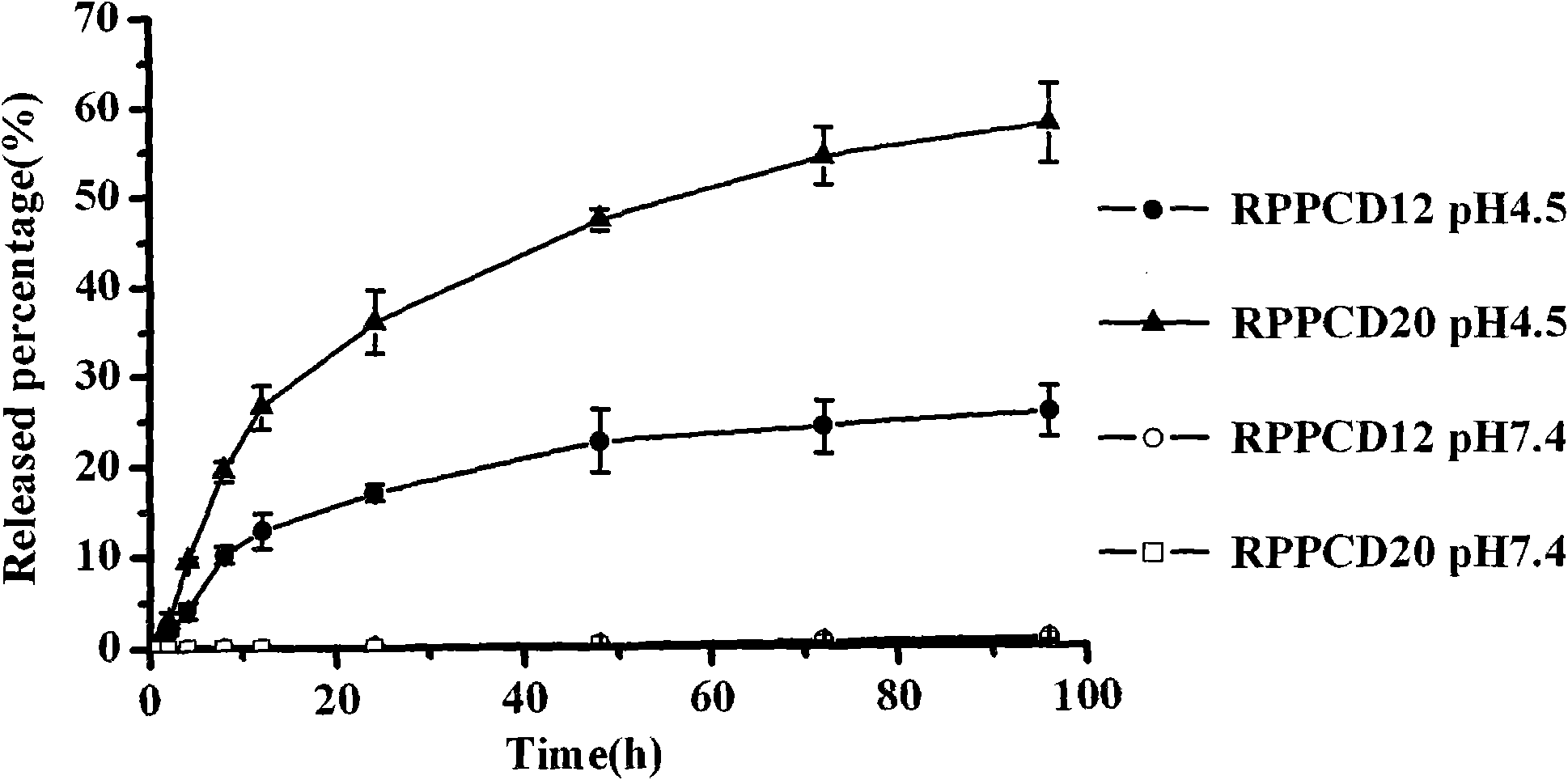
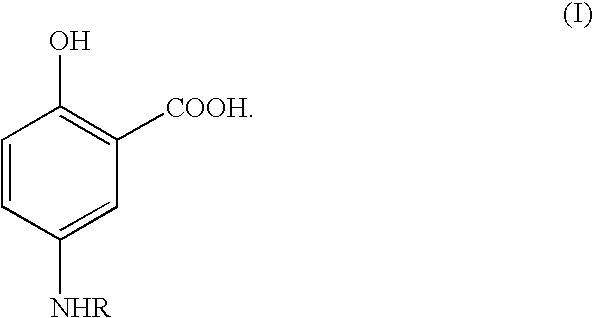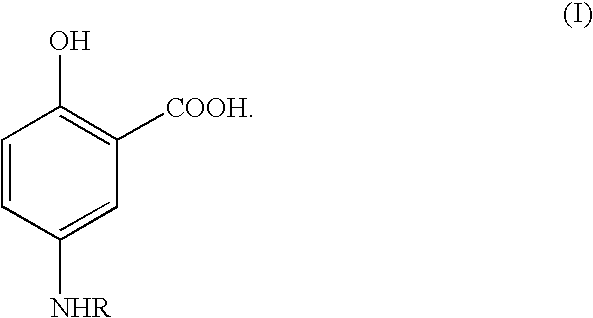Patents
Literature
245 results about "Systemic circulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Systemic circulation is the part of the cardiovascular system which carries oxygenated blood away from the heart to the body, and returns deoxygenated blood back to the heart. This physiologic theory of circulation was first described by Amato Lusitano, a Portuguese doctor working in Italy, in his work composed by seven volumes Curationum Medicinalium Centuriæ Septem 1st. edition in 1551. He was the first to describe venous valves. In 1628, William Harvey published his description of blood circulation in De motu cordis. This term is opposed and contrasted to the term pulmonary circulation first proposed by Ibn al-Nafis.
Methods and compositions for pulmonary administration of a TNFa inhibitor
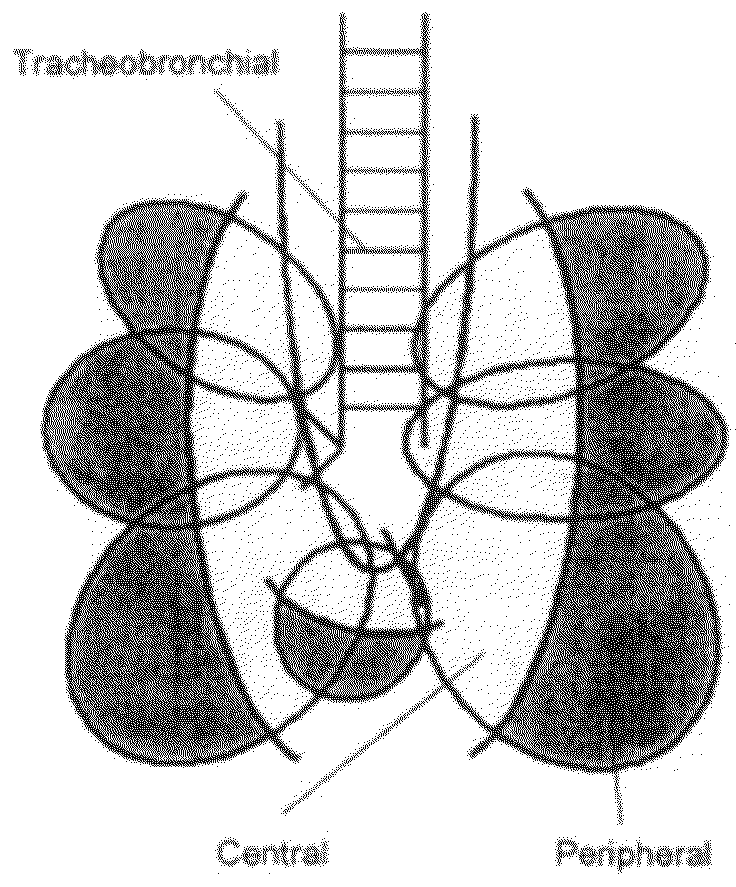
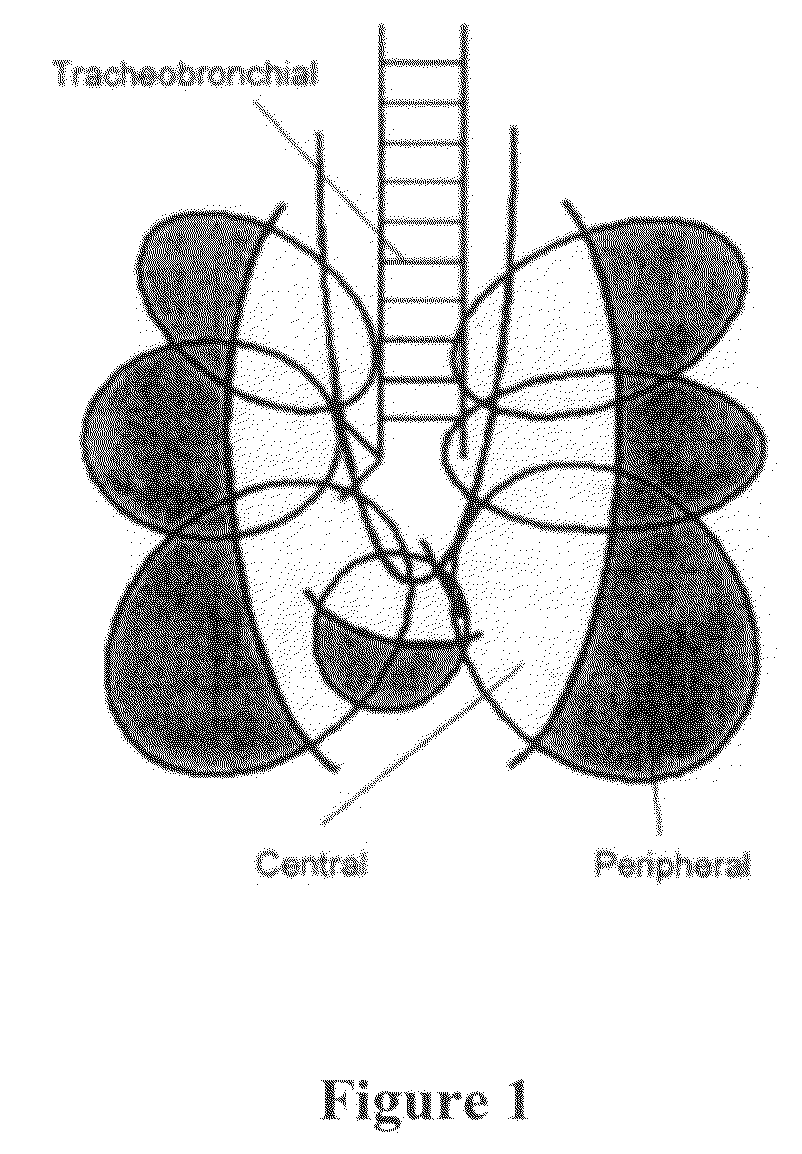
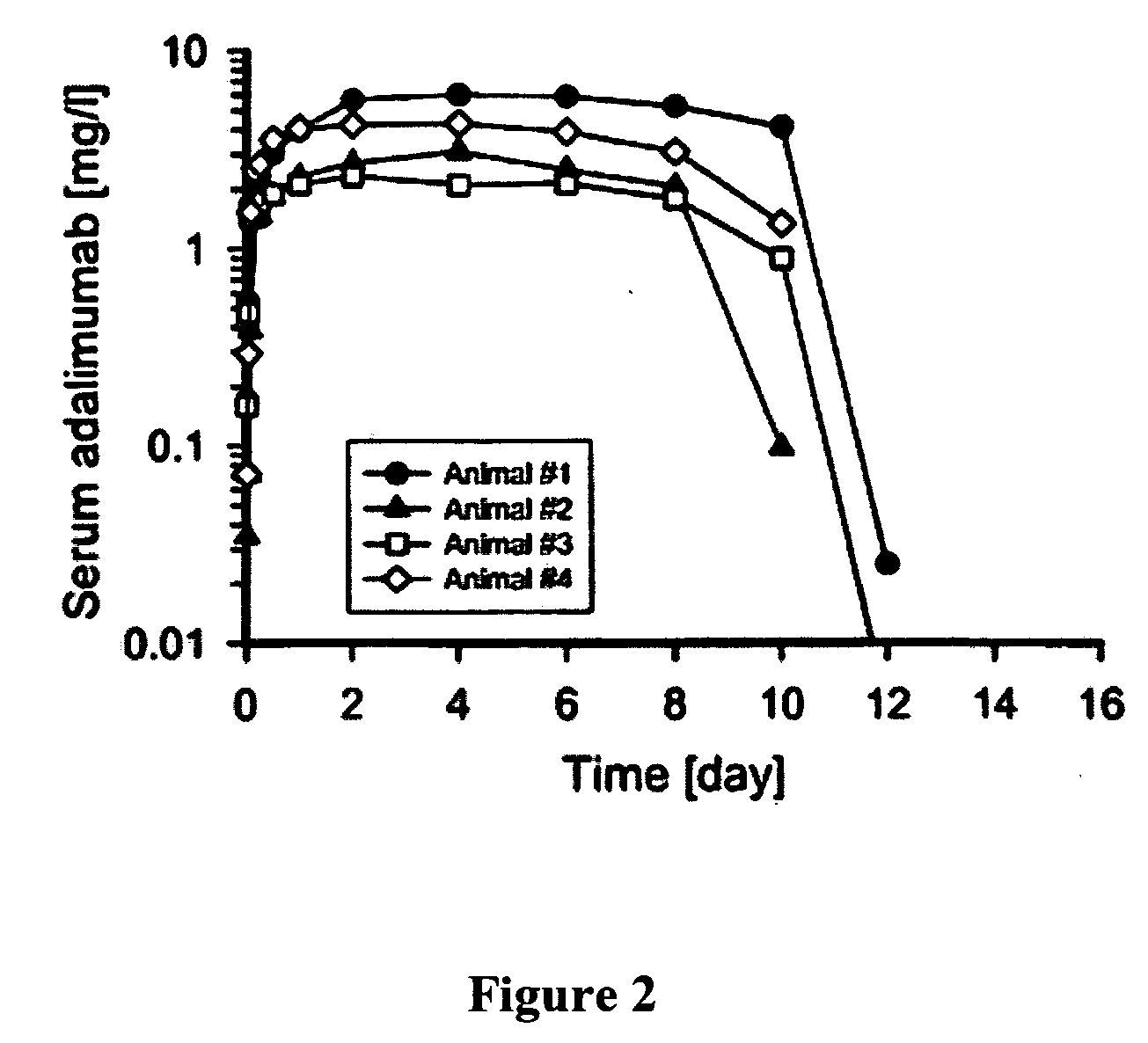
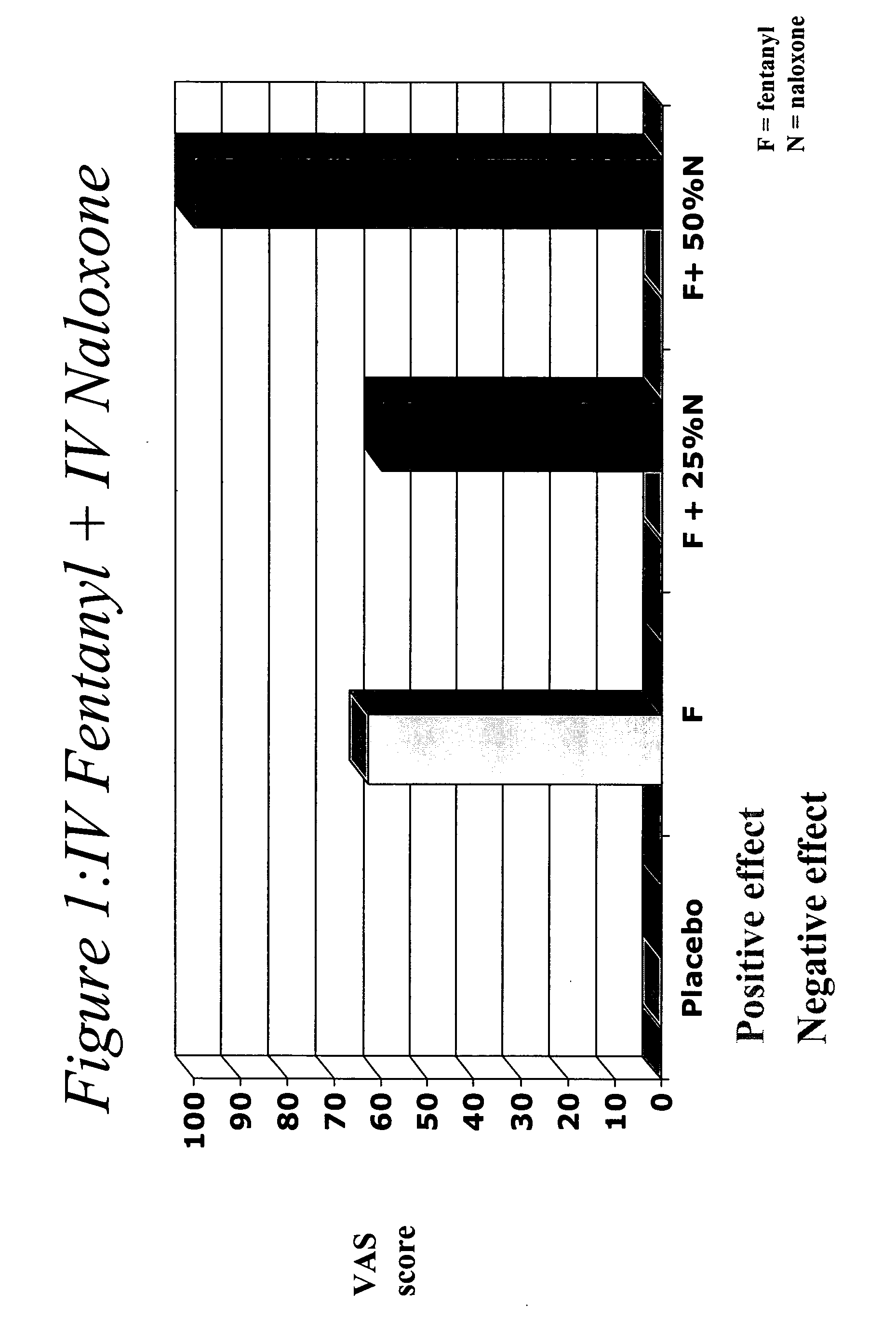
The invention describes methods of pulmonary delivery of a TNFα inhibitor to a subject having a disorder in which TNFα is detrimental, such that the disorder is treated. Also included is a method of achieving systemic circulation of a TNFα inhibitor in a subject comprising administering the TNFα inhibitor to the central lung region or the peripheral lung region of the subject via inhalation, such that systemic circulation of the TNFα inhibitor is achieved.
Owner:ABBVIE BIOTECHNOLOGY LTD
Abuse resistant transmucosal drug delivery device
ActiveUS20070148097A1Reduced illicit abuse potentialAbuse of the drug is impededOrganic active ingredientsNervous disorderDrug antagonistAbusable drug
The present invention relates to a solid pharmaceutical dosage form for abusable drug delivery with reduced illicit abuse potential. The dosage form is presented as a bioerodable transmucosal delivery device that includes an abusable drug and an antagonist to the abusable drug associated with an abuse-resistant matrix. The devices of the invention may be in the form of a layered film or a tablet. Upon application in a non-abusive manner, the device adheres to the mucosal surface, providing transmucosal drug delivery of the drug with minimal absorption of the antagonist into systemic circulation.
Owner:BIODELIVERY SCI
Transdermal device for administration through de-epithelialized skin
InactiveUS6264979B1Prevent proliferationImprove adhesionAdhesive dressingsAbsorbent padsCellular DebrisActive agent
A transdermal device is described suitable for delivery of a pharmaceutical to the systemic circulation through de-epithelialized skin. In its various embodiments the device includes means to prevent the attack of any protein or polypeptide active agent included therein by proteolytic enzymes which exude from the lesion, means to prevent the ingress of bacteria and other cellular debris from the lesion and means to ensure that substantially all said active agent is directed to the de-epithelialized lesion.
Owner:SVEDMAN PAL
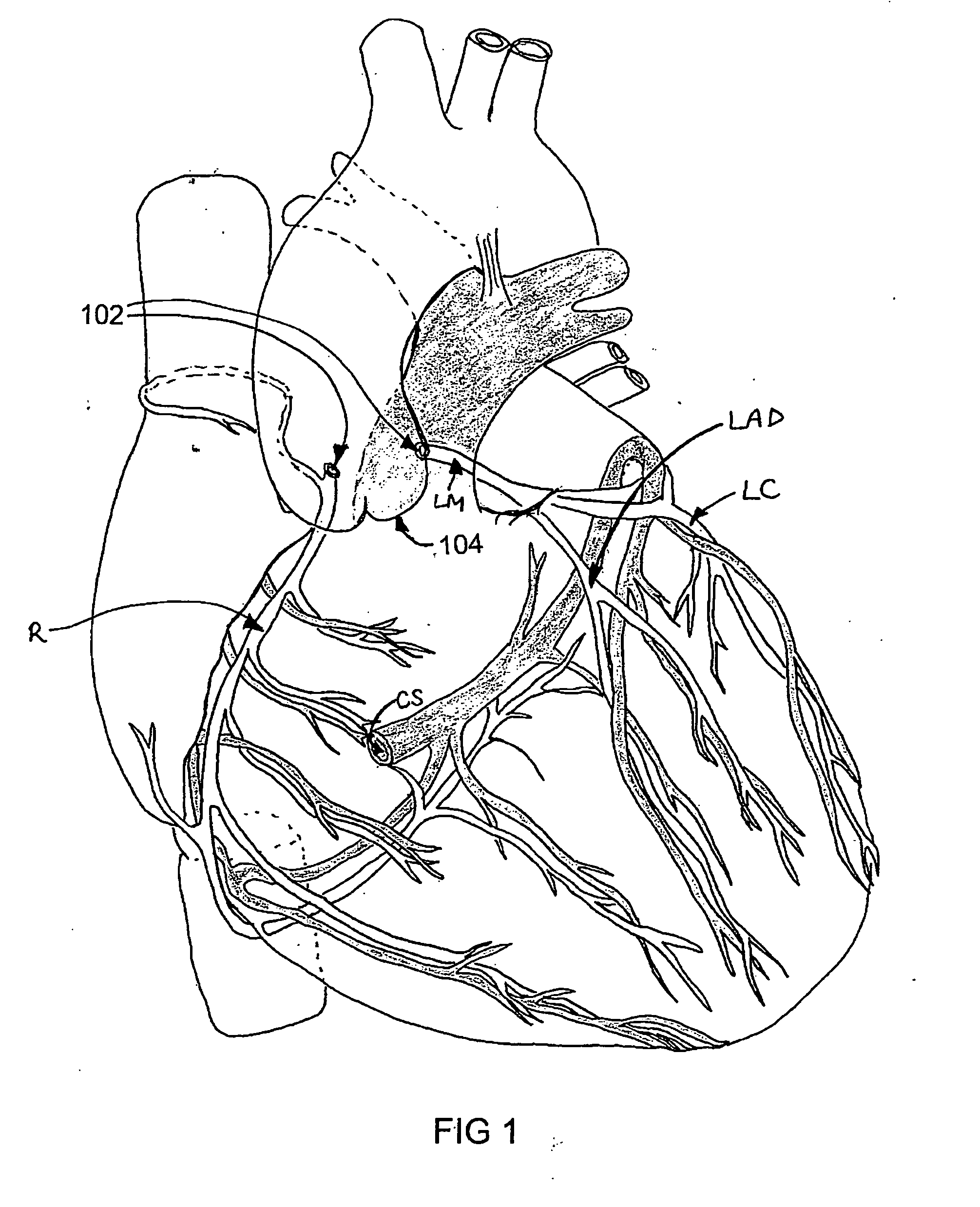
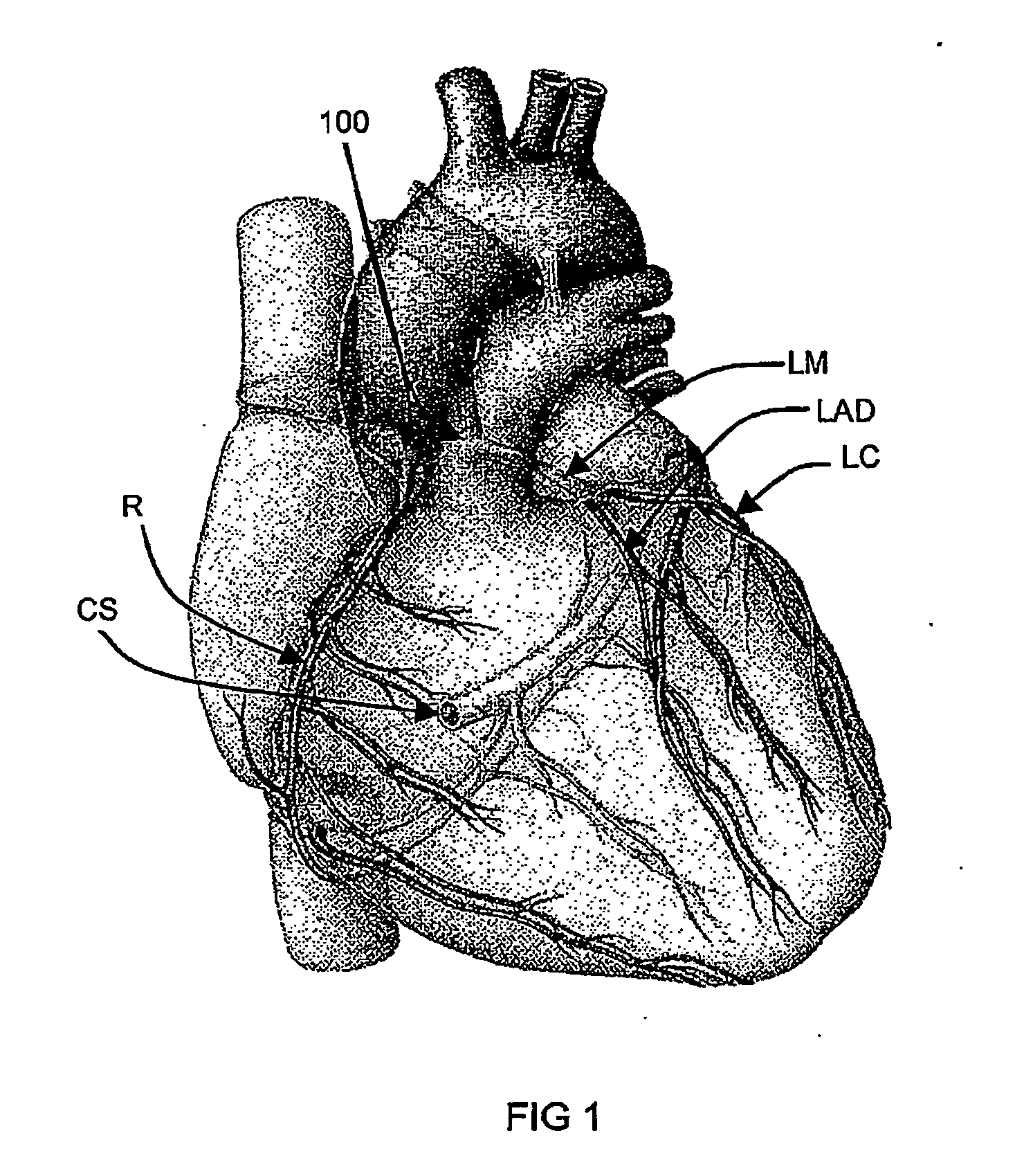
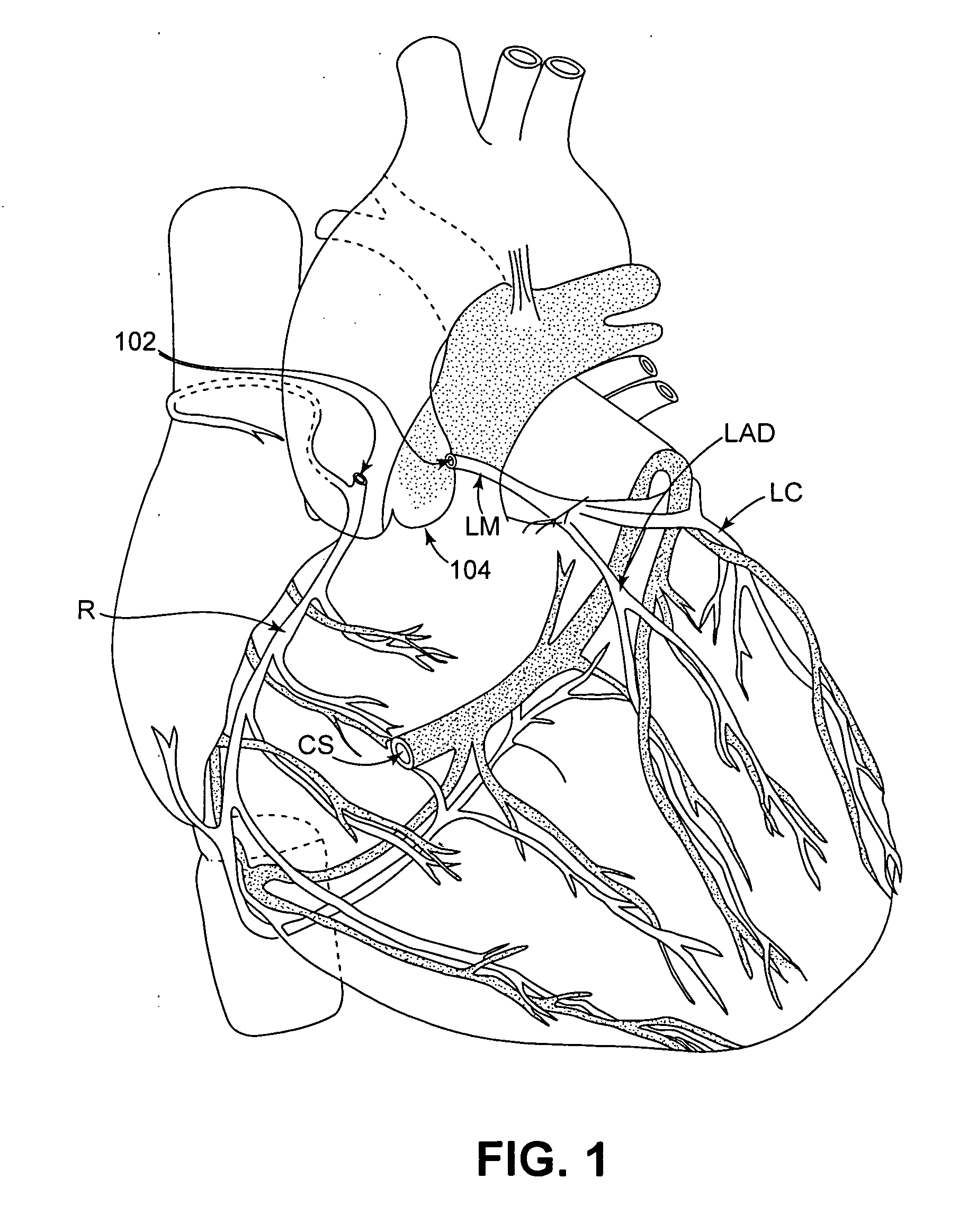
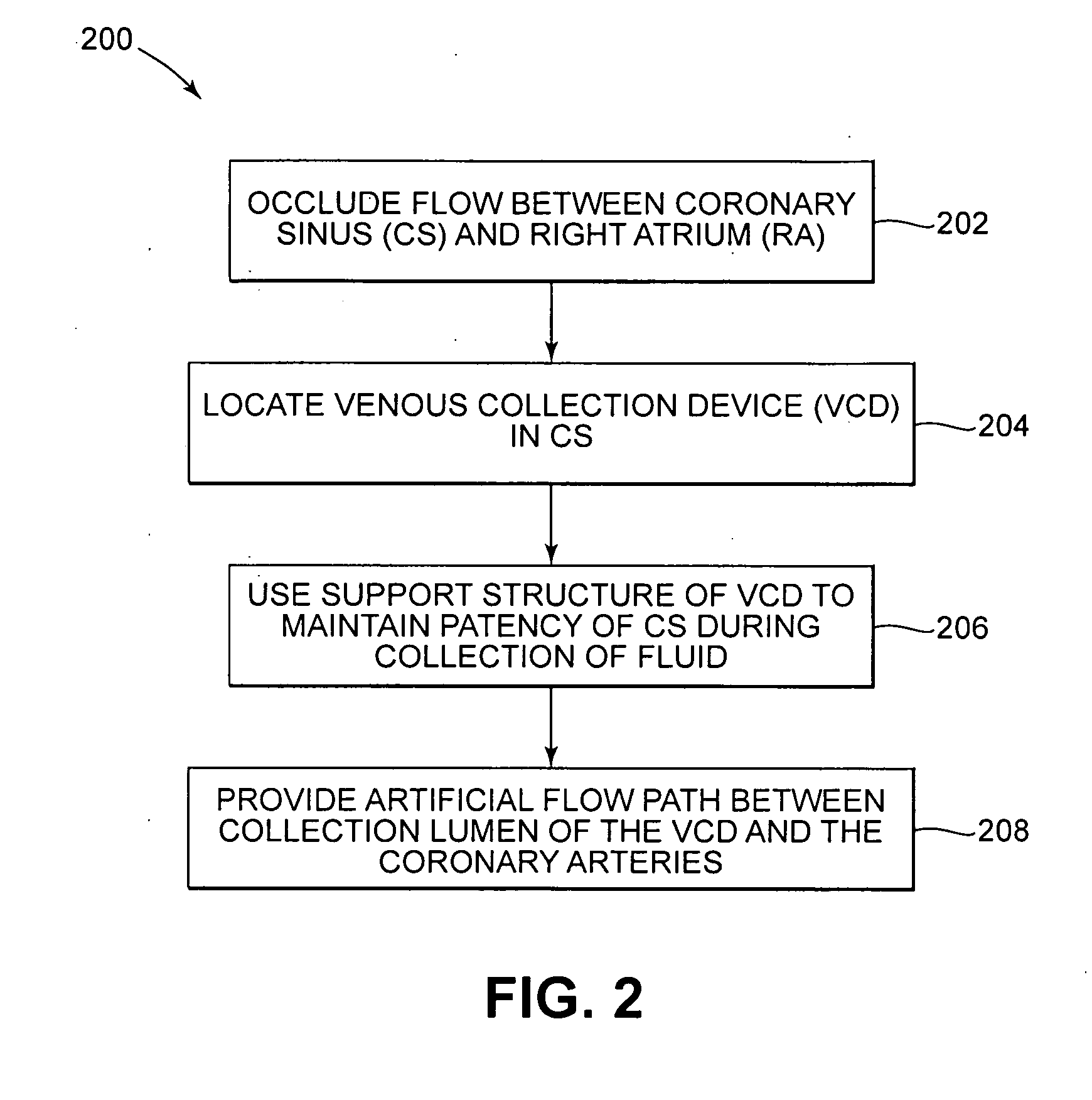
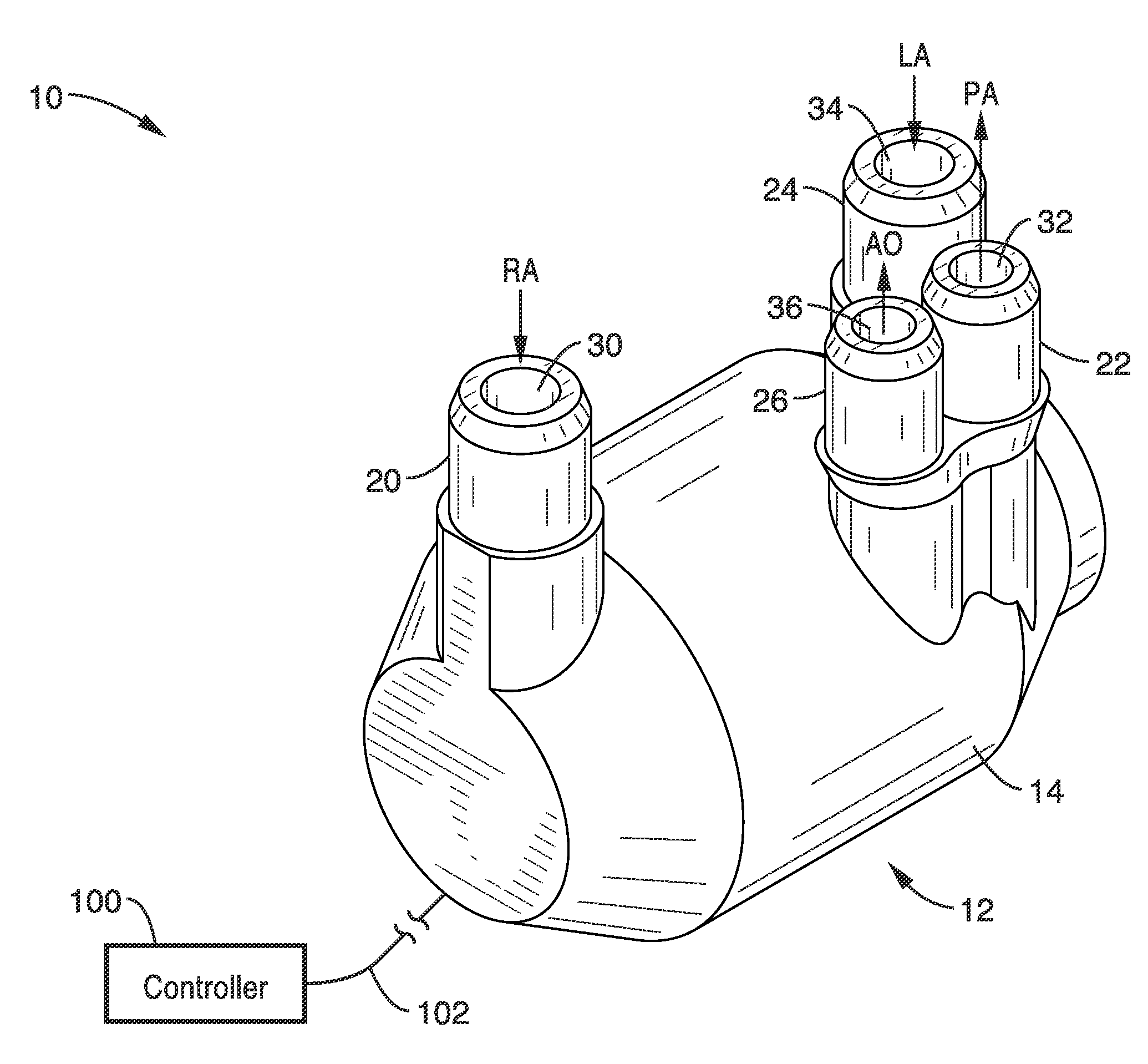
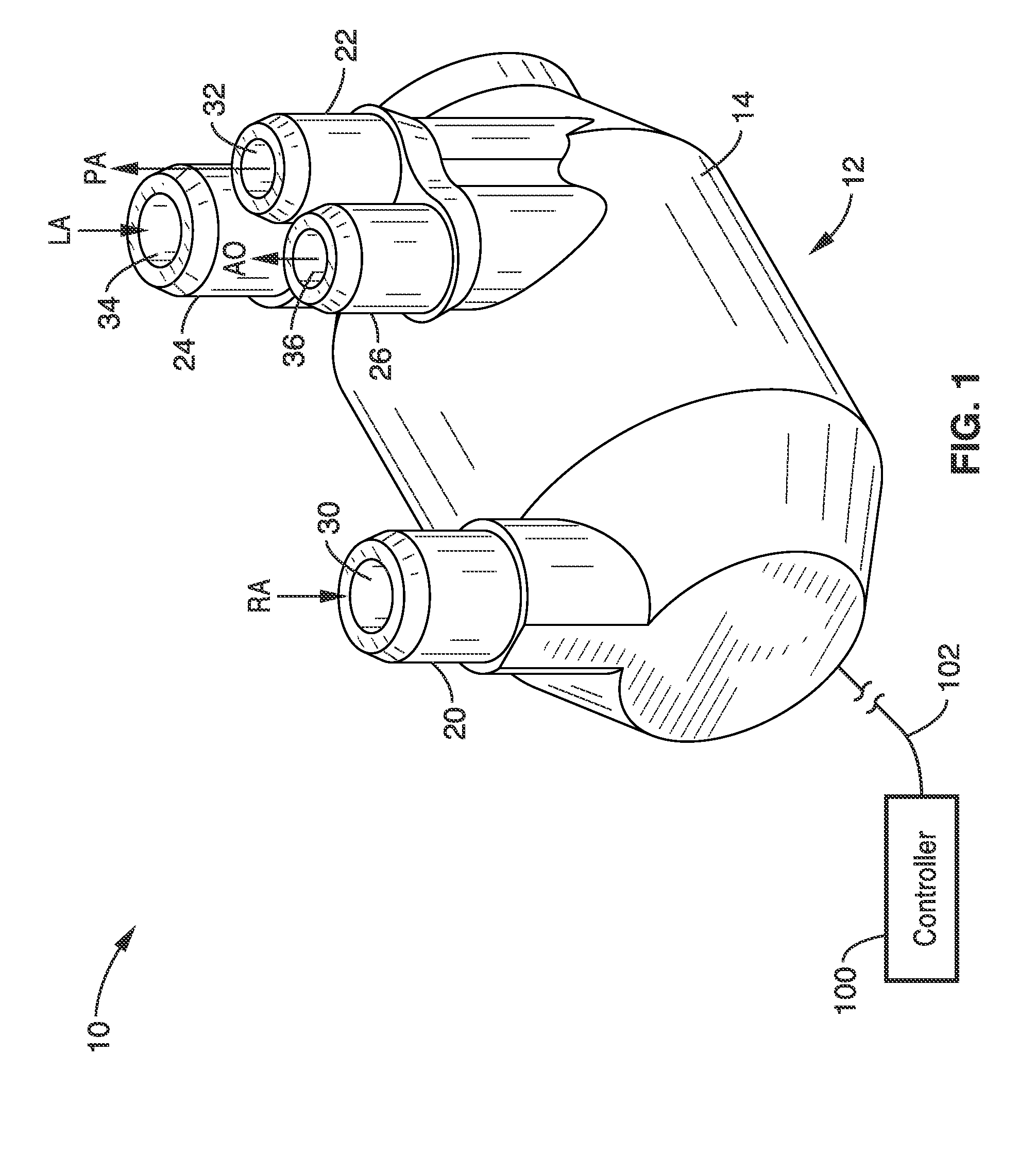
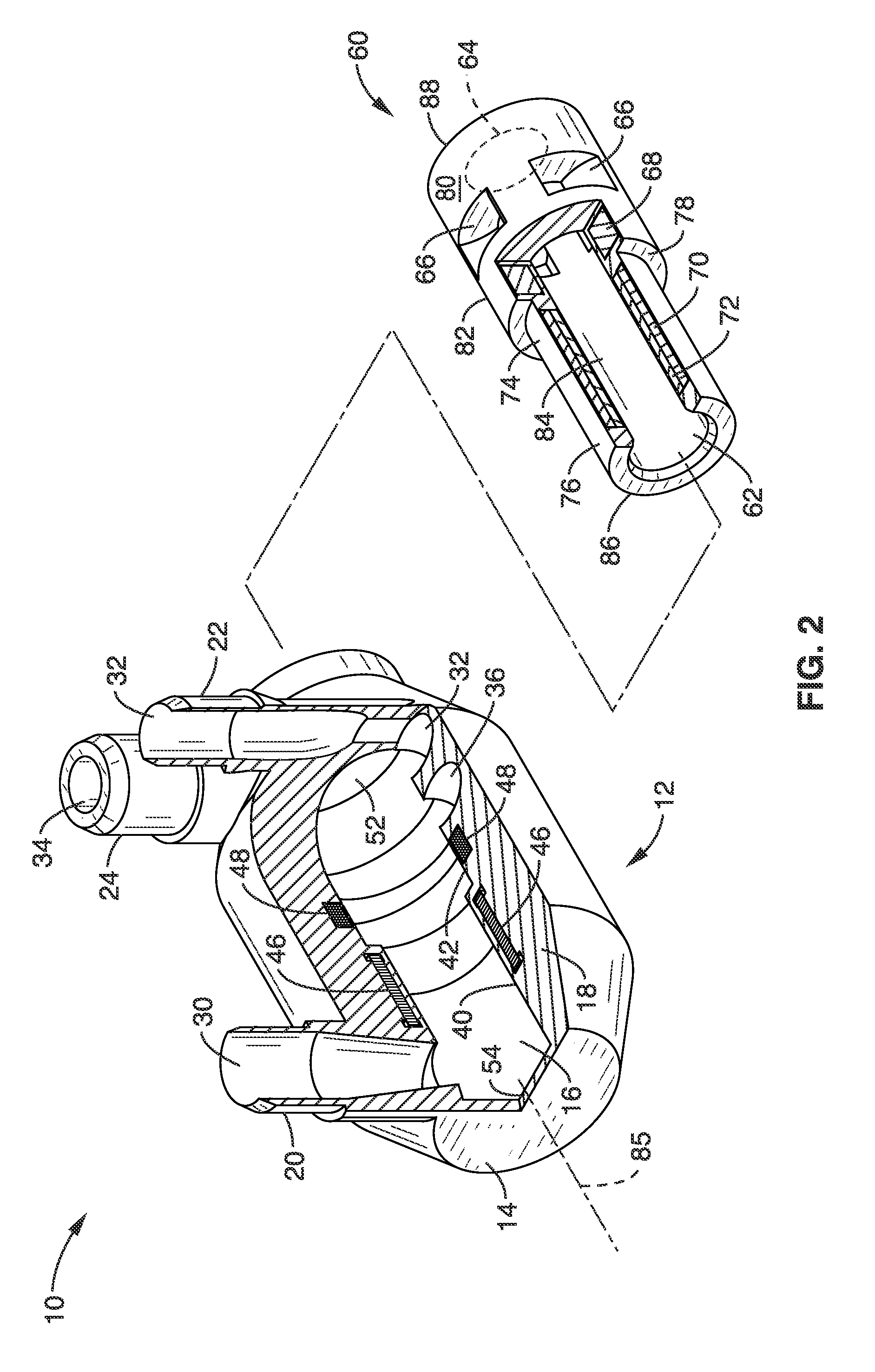
Isolating cardiac circulation
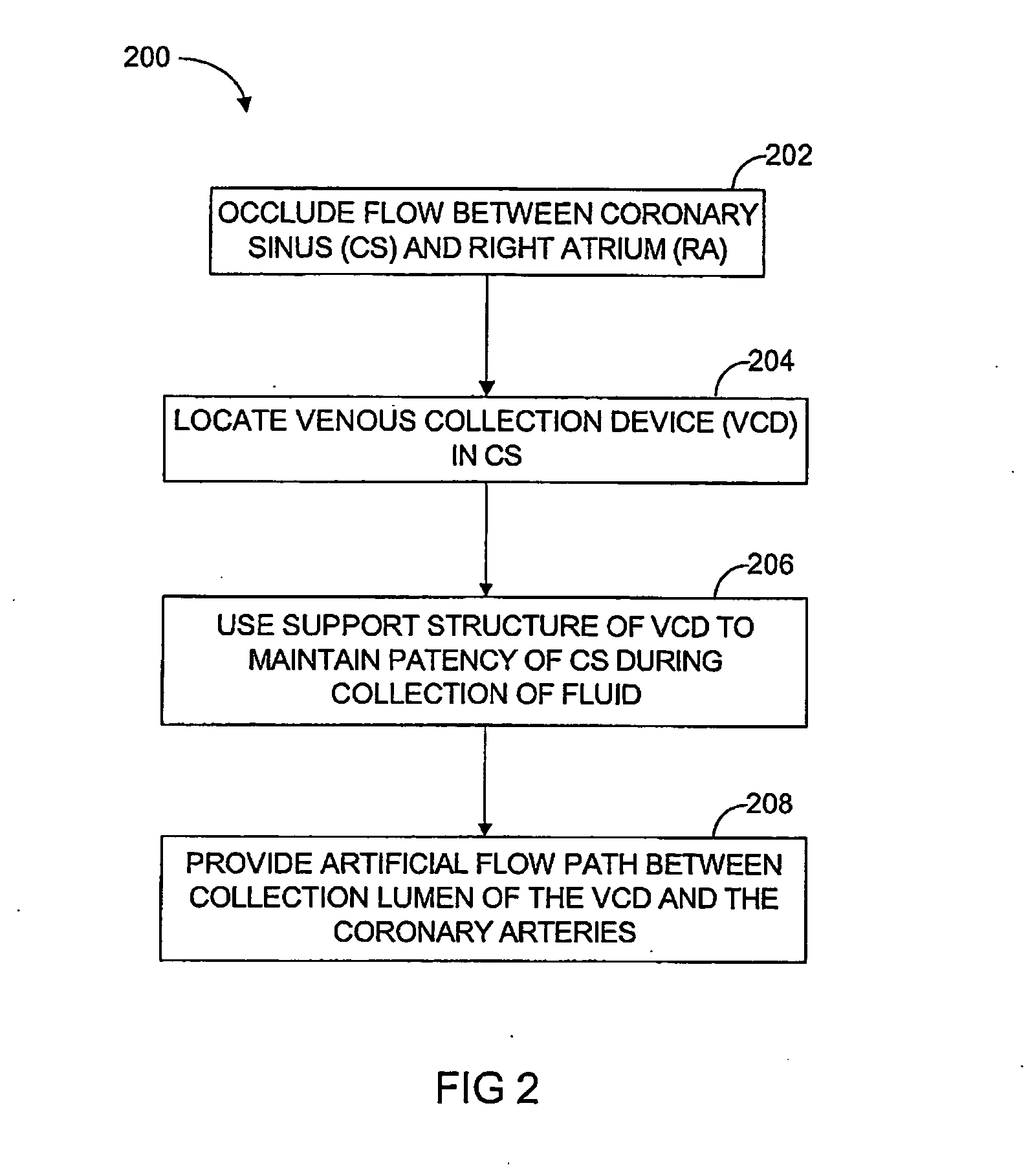
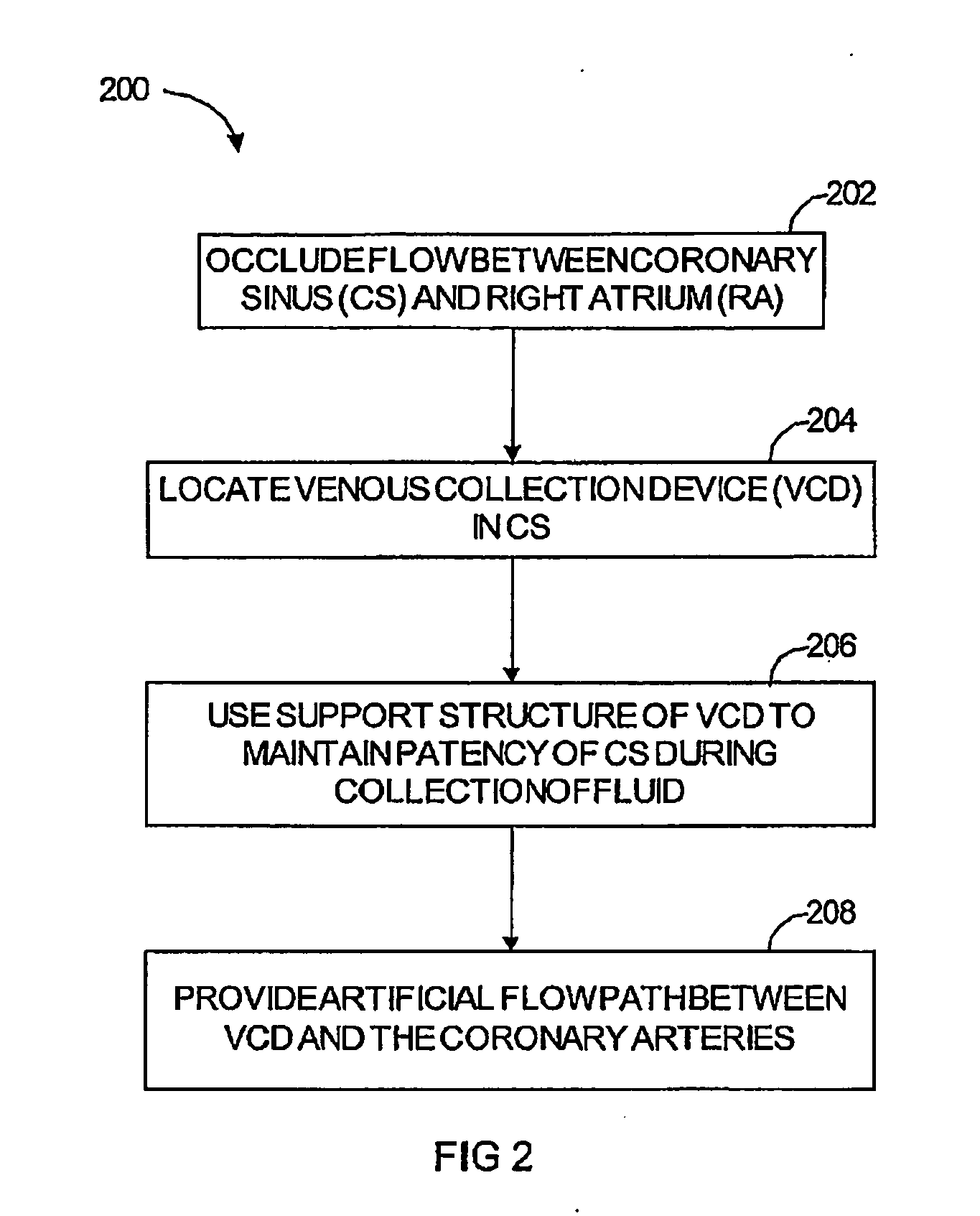
In a method for substantially isolating cardiac circulation from systemic circulation, flow between the coronary sinus and the right atrium is occluded. A venous collection device having a collection lumen and a support structure is located in the coronary sinus. The support structure is used to maintain patency of the coronary sinus during collection of fluid through the collection lumen. An artificial flow path is provided between the collection lumen and the one or more coronary arteries, thus isolating the cardiac circulation. According to the method, cardiac pumping for systemic circulation can be maintained during isolation of the cardiac circulation.
Owner:OSPREY MEDICAL
Polynucleotide delivery to cardiac tissue
InactiveUS20060148742A1Improve ejection fractionReduce probabilityVirusesBalloon catheterCoronary arteriesVein
A method for delivering a polynucleotide to cardiac tissue, including substantially isolating the coronary venous circulation from systemic circulation, and introducing a polynucleotide into the isolated coronary venous circulation to effect localized transfection of cardiac tissue. The polynucleotide advantageously produces a therapeutic effect, such as increasing or decreasing the expression level of a protein in the cardiac tissue.
Owner:OSPREY MEDICAL
Water-dispersible oral, parenteral, and topical formulations for poorly water soluble drugs using smart polymeric nanoparticles
ActiveUS8313777B2Control releaseImprove bioavailabilityPowder deliveryNervous disorderWater dispersibleSmart polymer
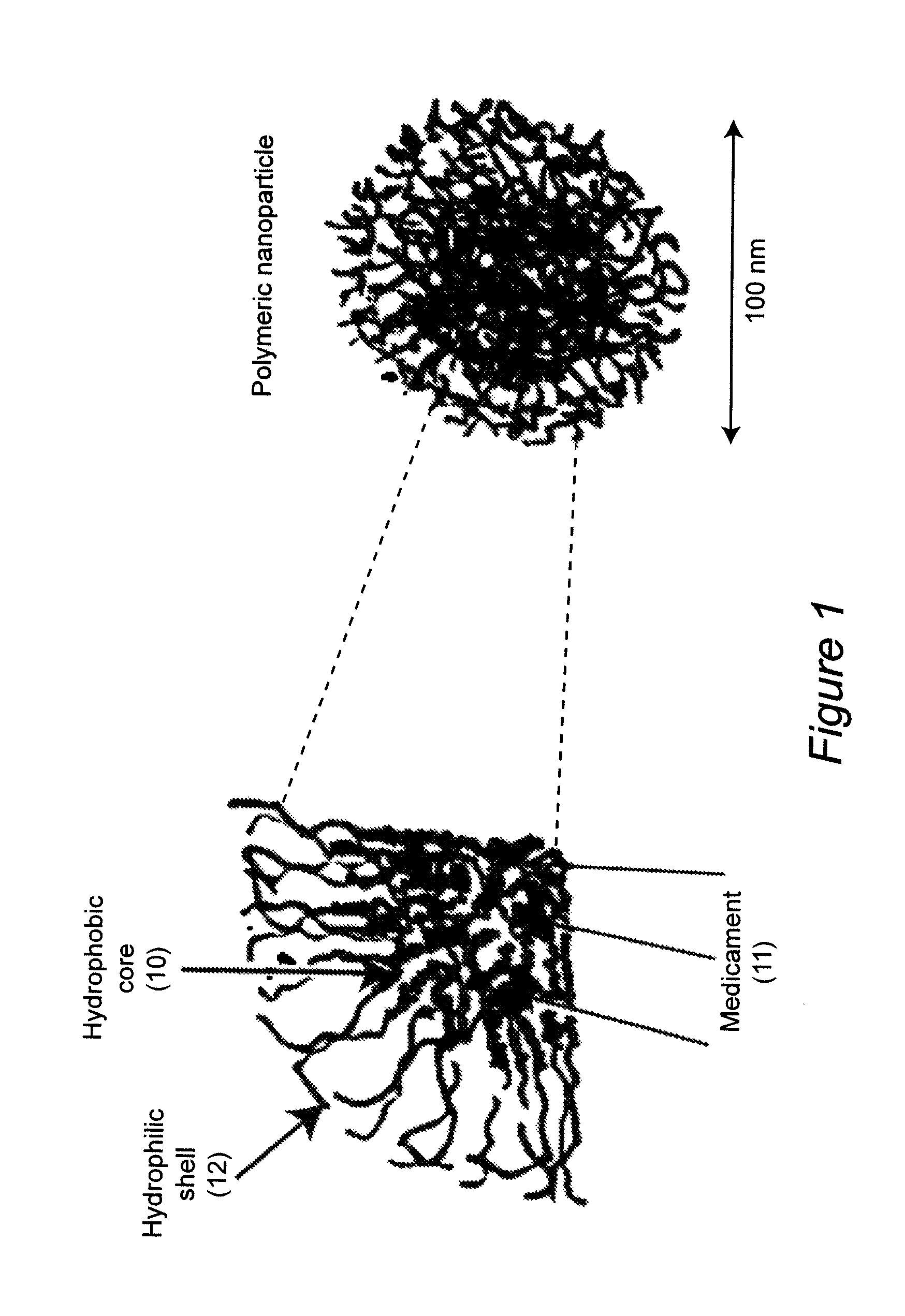
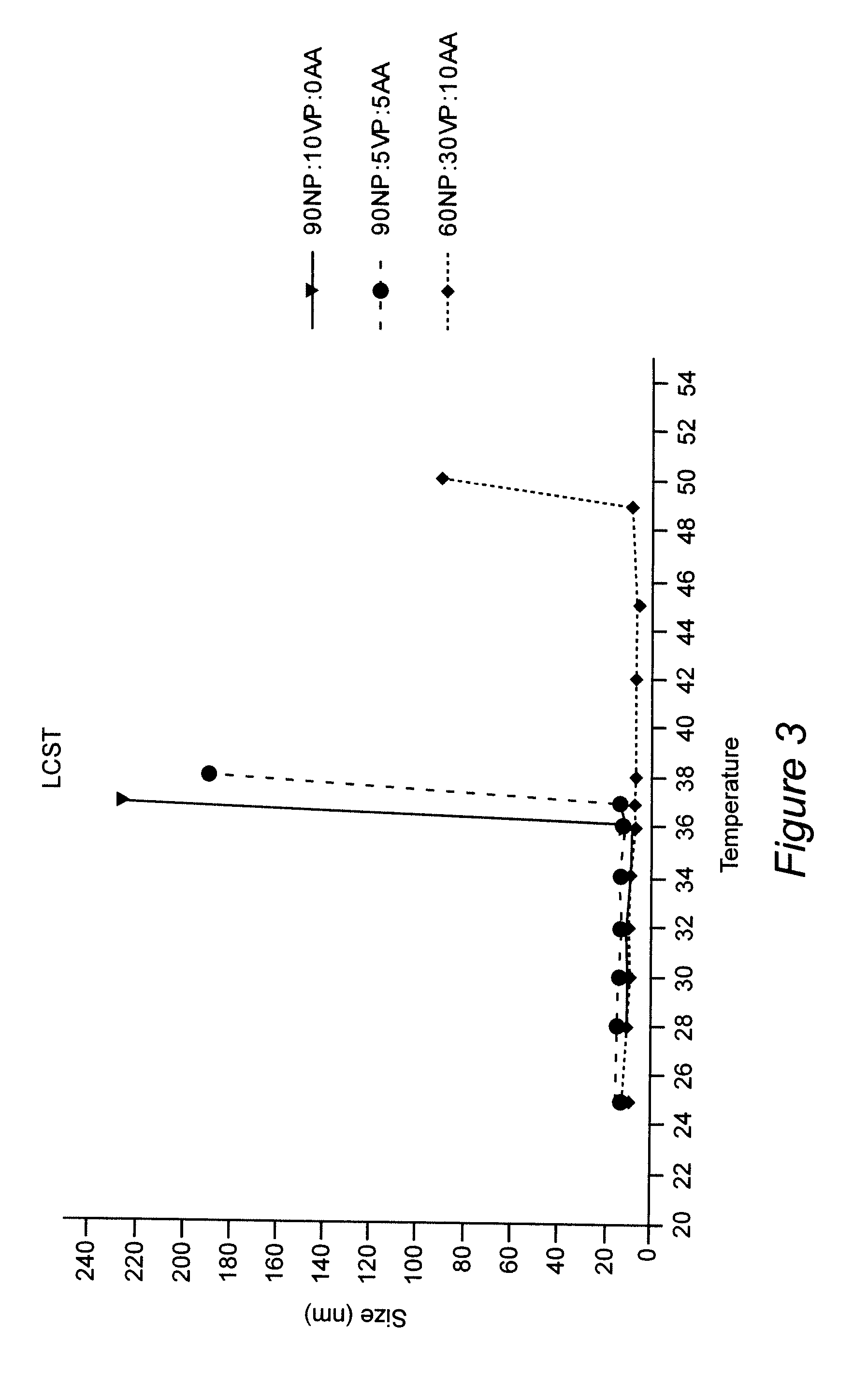
Polymeric nanoparticles with a hydrophobic core and a hydrophilic shell are formed from: 1) N-isopropyl acrylamide (NIPAAM), at a molar ratio of about 50% to about 90%, and preferably 60% for specific delivery routes such as oral or parenteral; either water-soluble vinyl derivatives like vinylpyrolidone (VP) or vinyl acetate (VA), or water insoluble vinyl derivatives like methyl methacrylate (MMA) or styrene (ST), at a molar ratio of about 10% to about 30%; and acrylic acid (AA), at a molar ratio of about 10% to about 30%. The formed nanoparticles may be optionally surface functionalized using reactive groups present in AA, including PEGylation, or conjugation of moieties such as chemotherapeutics, contrasting agents, antibodies, radionucleides, ligands, and sugars, for diagnostic, therapeutic, and imaging purposes. The polymeric nanoparticles are preferably dispersed in aqueous solutions. The polymeric nanoparticles incorporate one or more types of medicines or bioactive agents in the hydrophobic core; on occasion, the medicine or bioactive agent may be conjugated to the nanoparticle surface via reactive functional groups. The polymeric nanoparticles are capable of delivering the said medicines or bioactive agents through oral, parenteral, or topical routes. The polymeric nanoparticles allow poorly water soluble medicines or bioactive agents, or those with poor oral bioavailability, to be formulated in an aqueous solution, and enable their convenient delivery into the systemic circulation.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
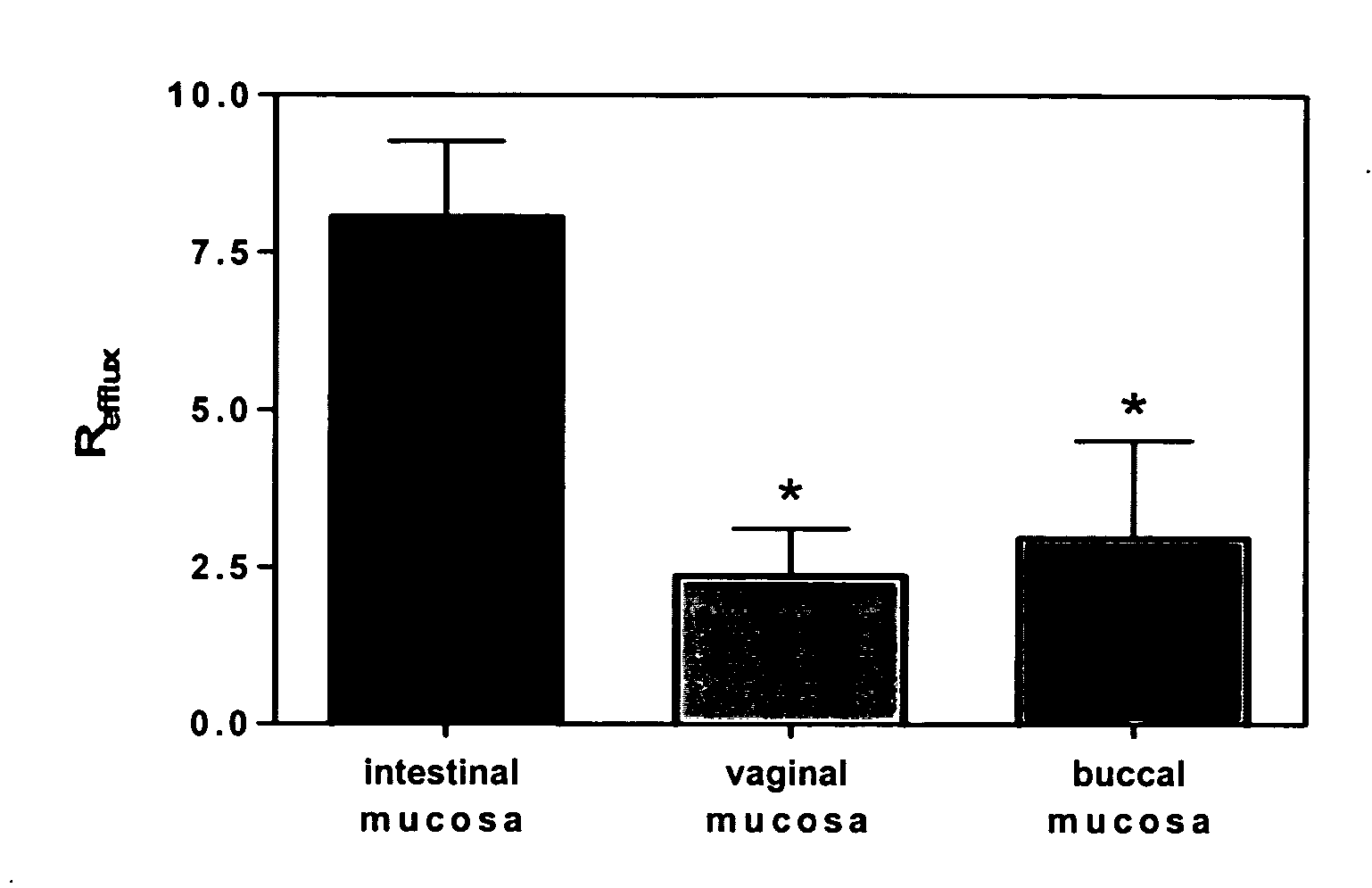
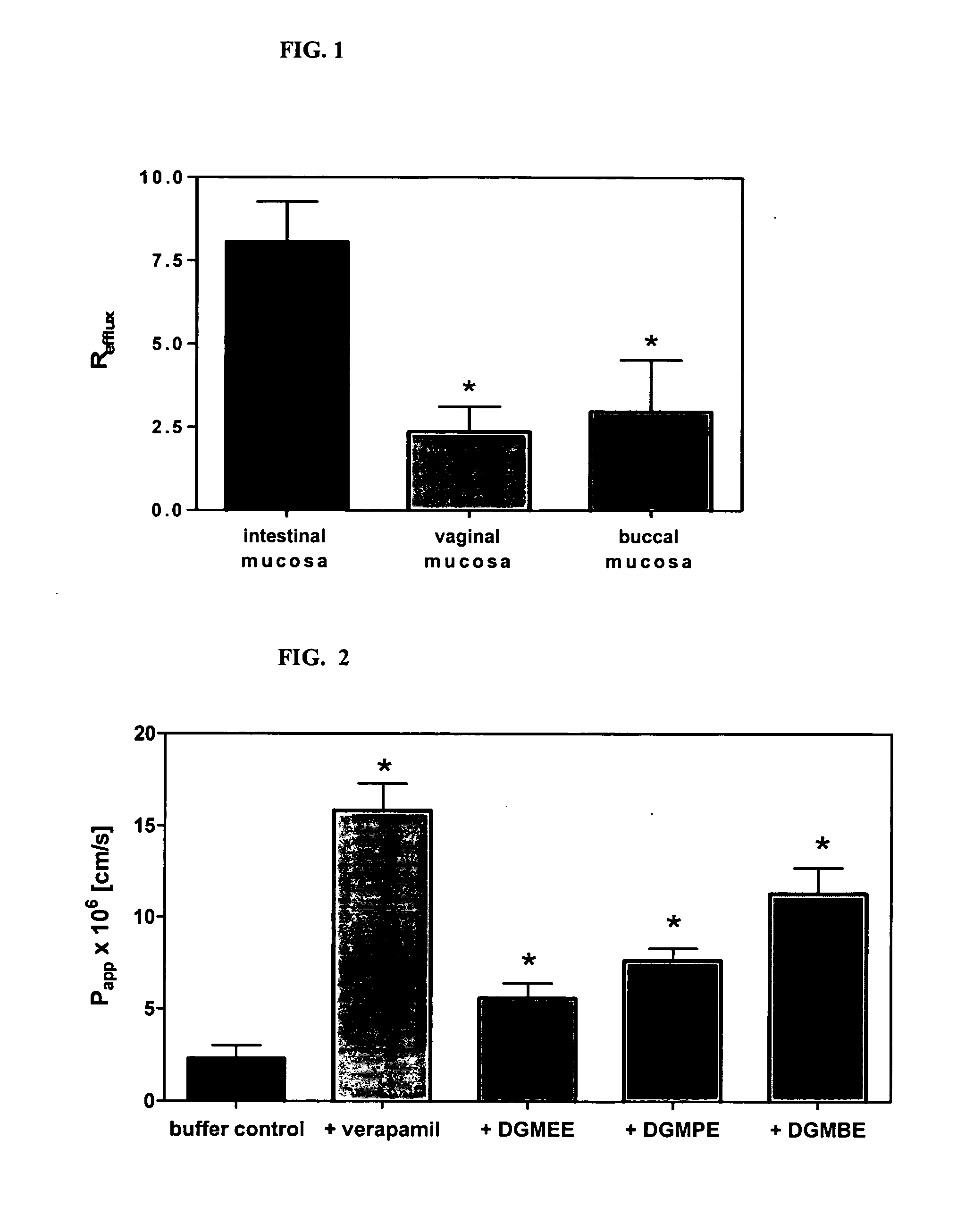
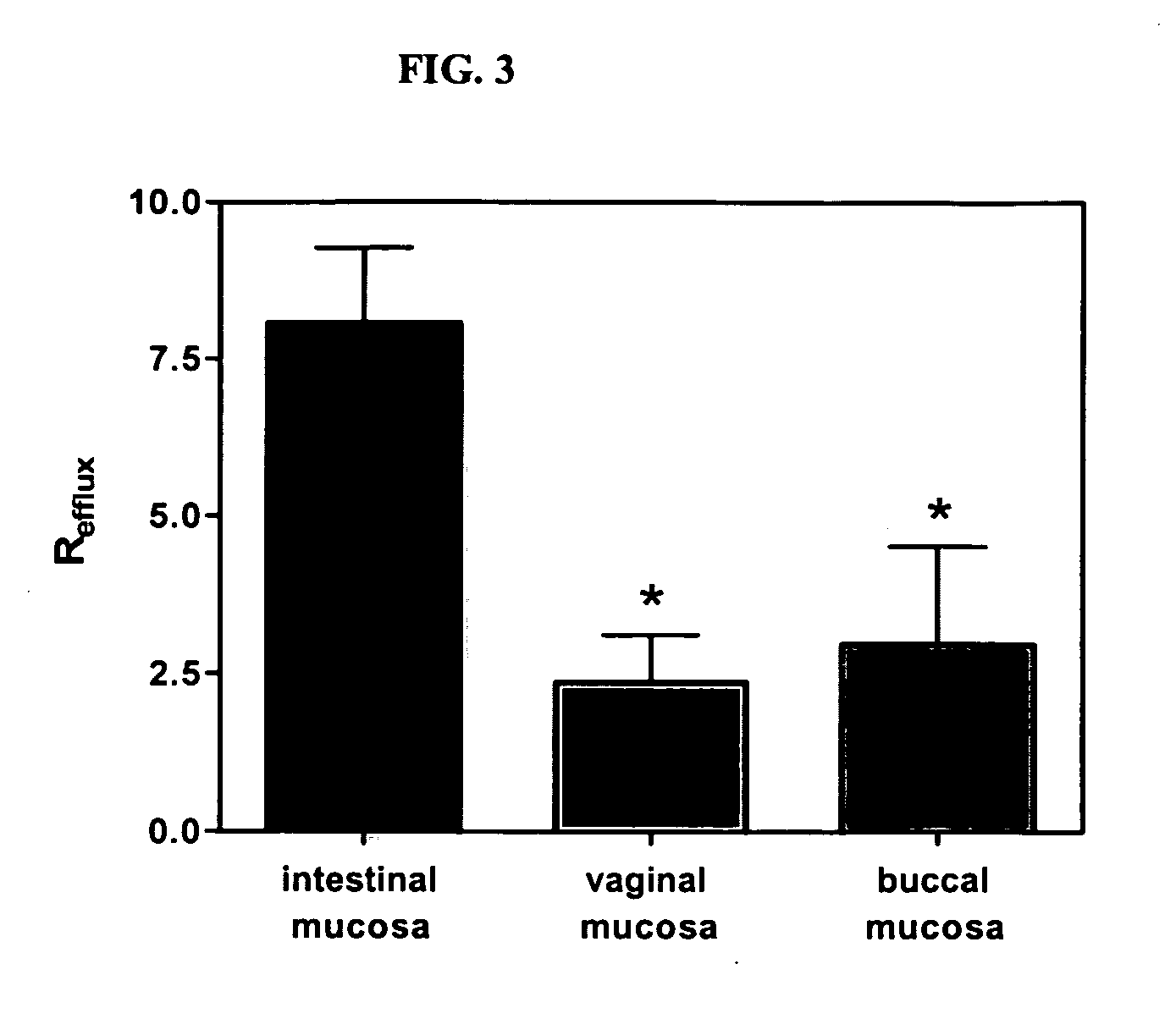
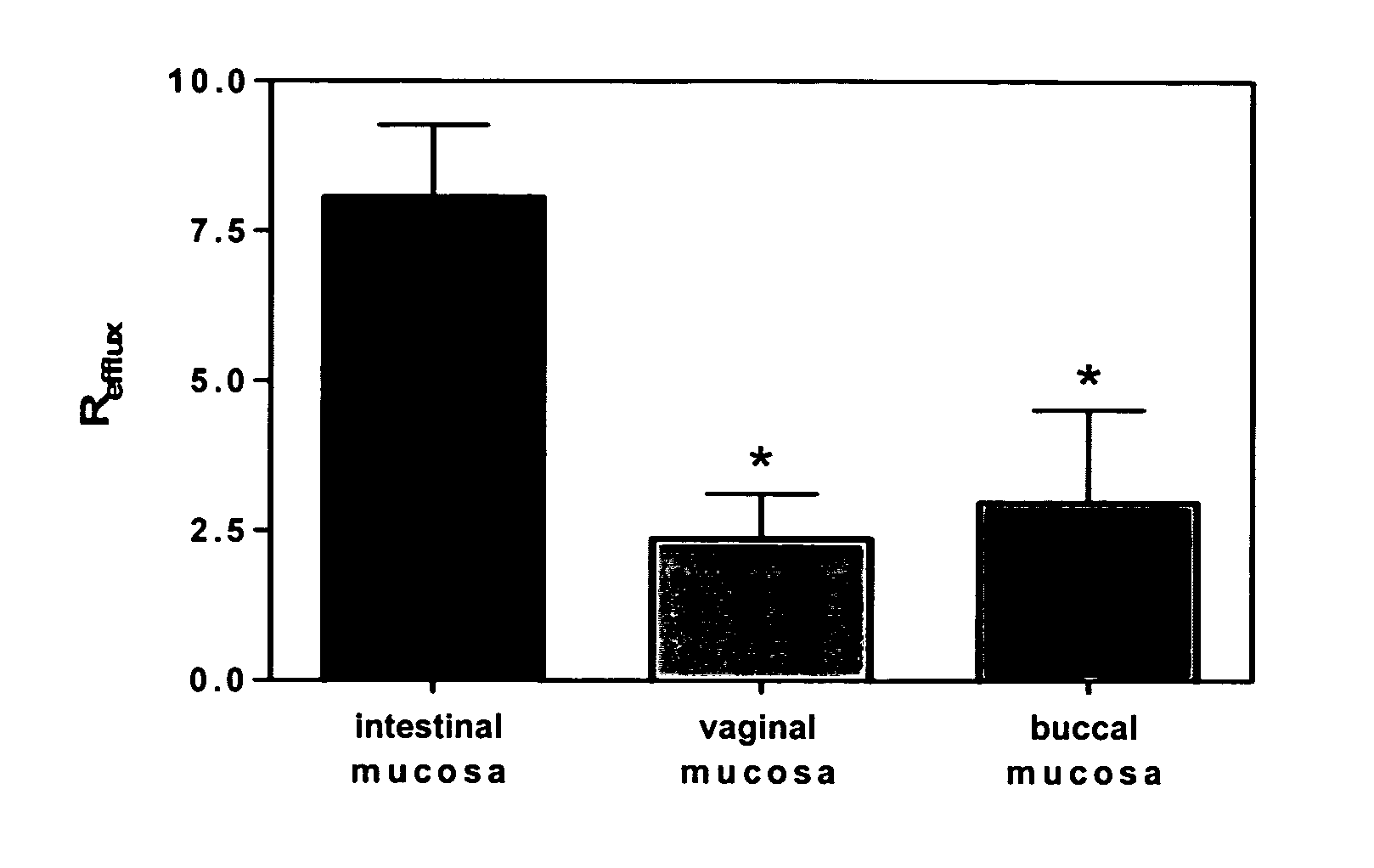
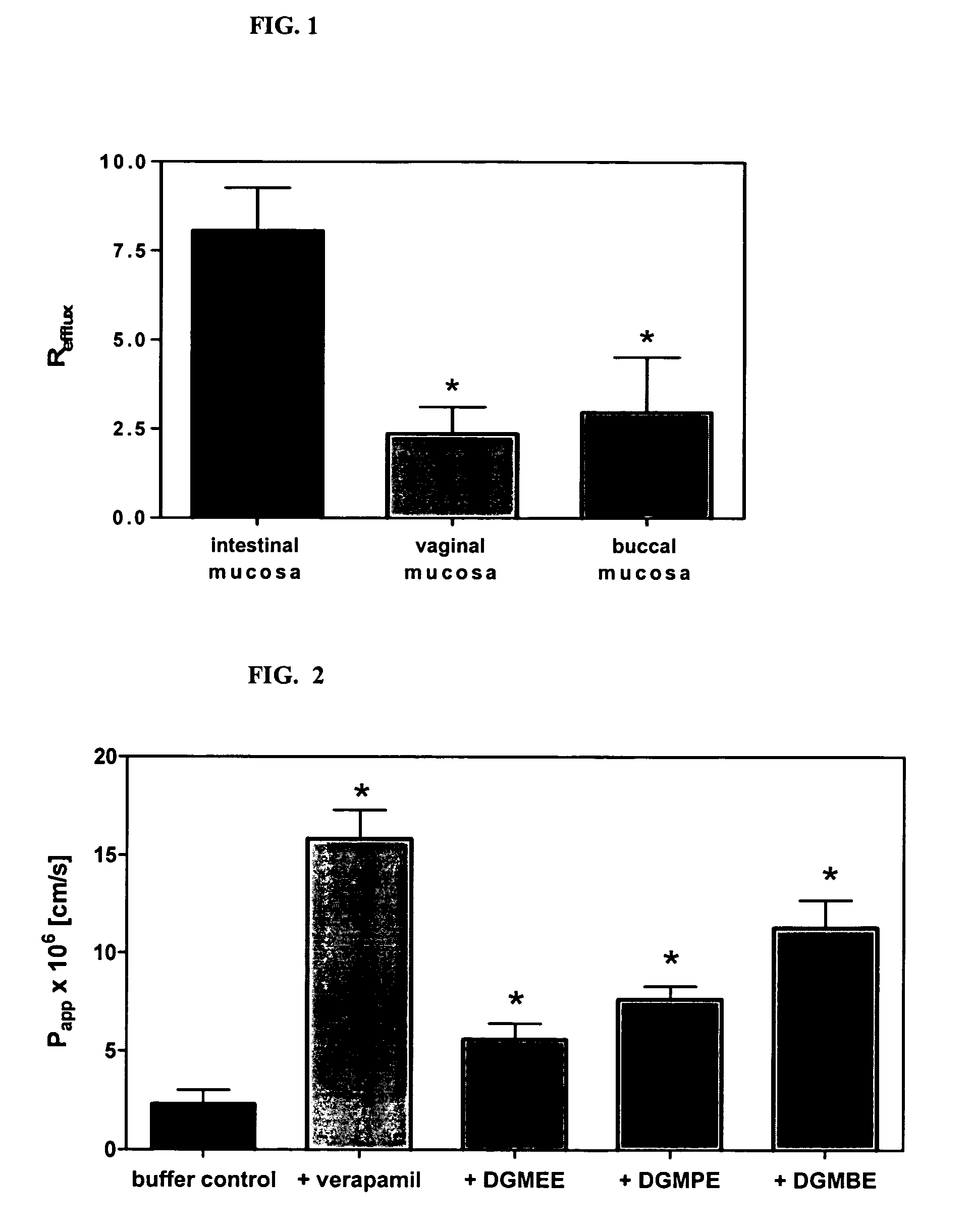
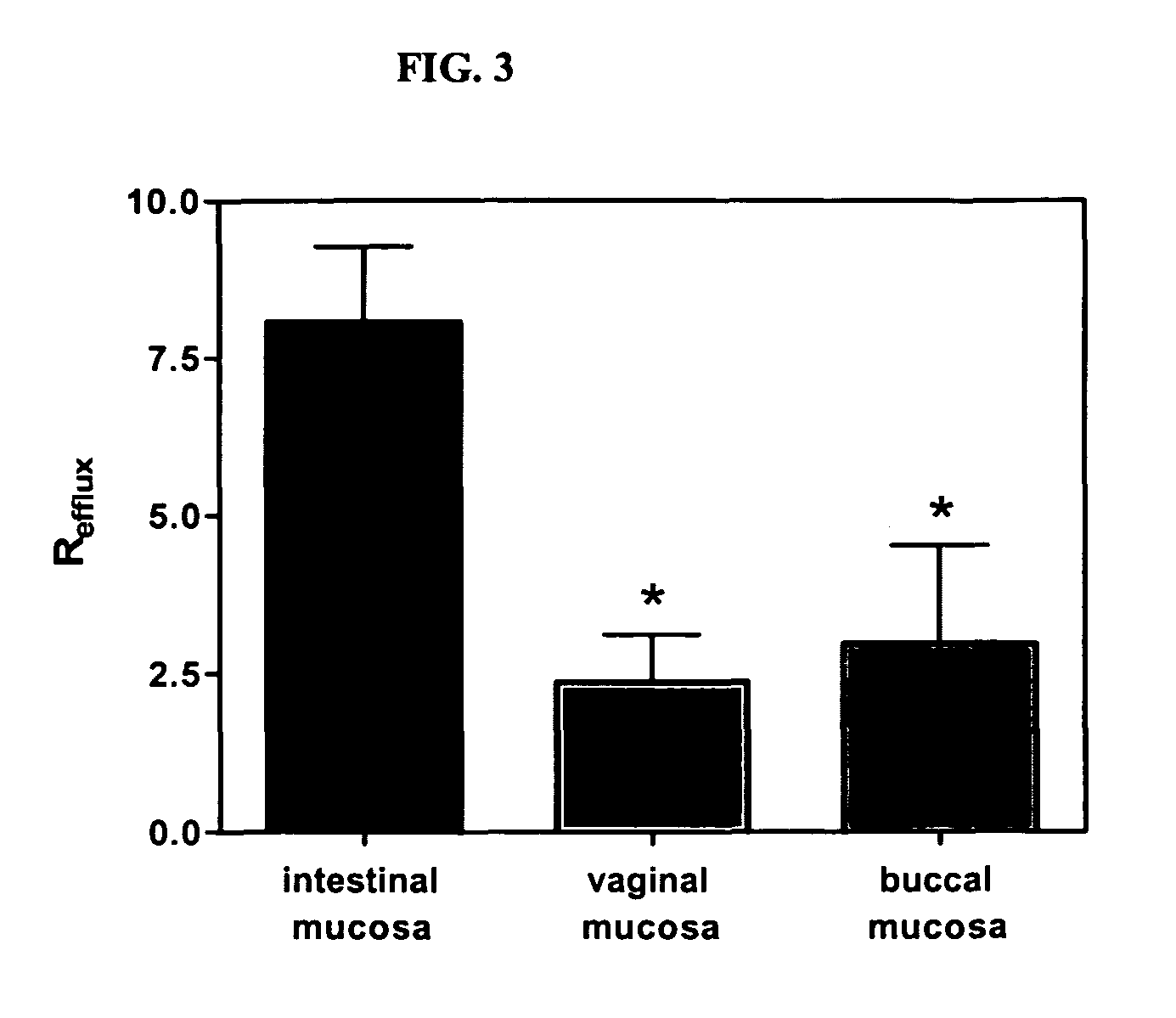
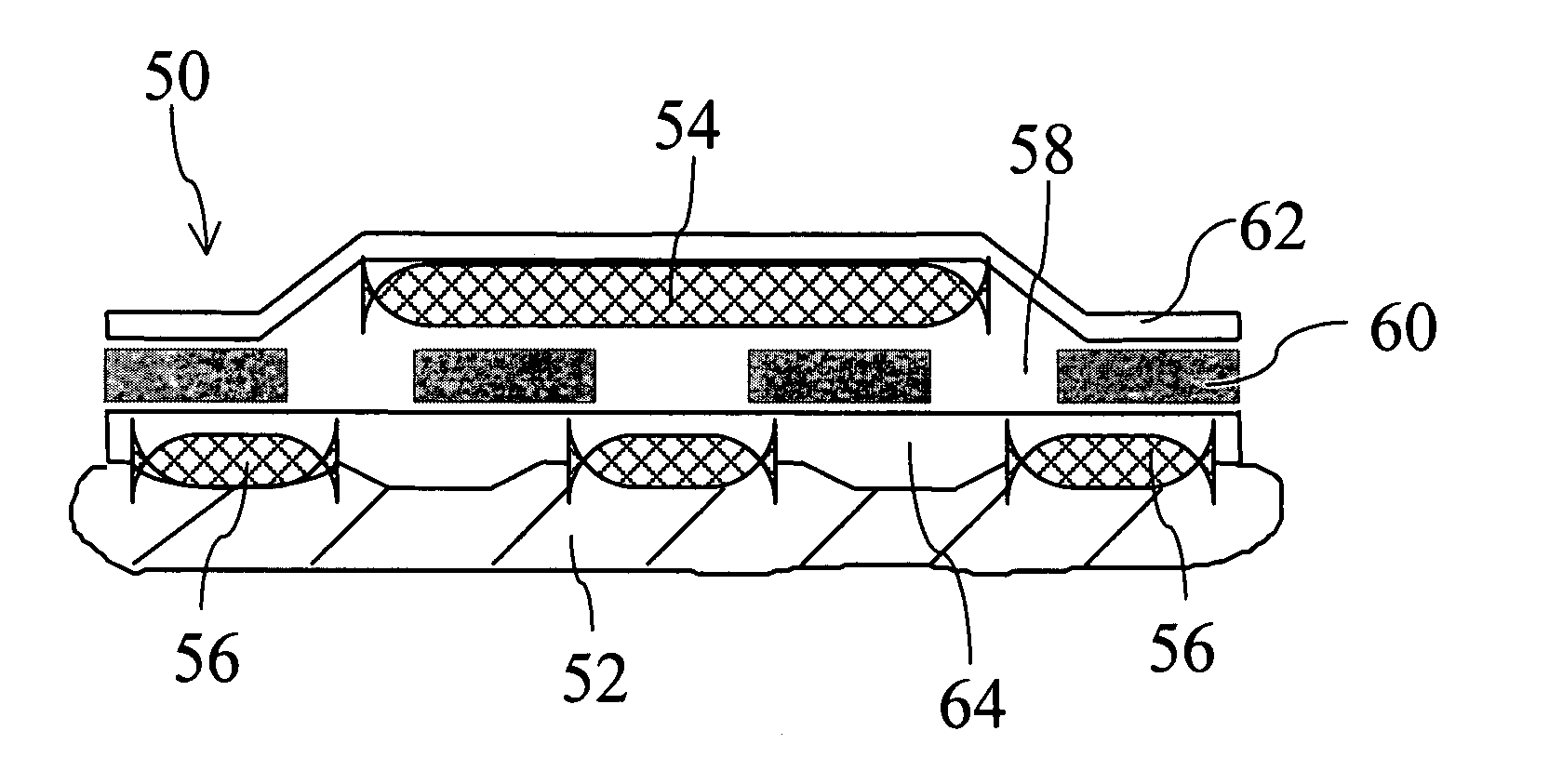
Method for augmentation of intraepithelial and systemic exposure of therapeutic agents having substrate activity for cytochrome P450 enzymes and membrane efflux systems following vaginal and oral cavity administration
InactiveUS20070036834A1Improve bioavailabilityImprove drug solubilityBiocidePowder deliveryWhole bodyMedicine
A vaginal or buccal delivery of therapeutic agents having a substrate affinity for metabolic cytochrome P-450 enzymes and membrane efflux transporter systems. A method for augmentation of systemic exposure to the therapeutic agents having a substrate affinity for cytochrome P-450 enzymes and membrane efflux transporter systems, by delivering said agents to the systemic circulation through vaginal or buccal mucosa.
Owner:FEMINA PHARMA +1
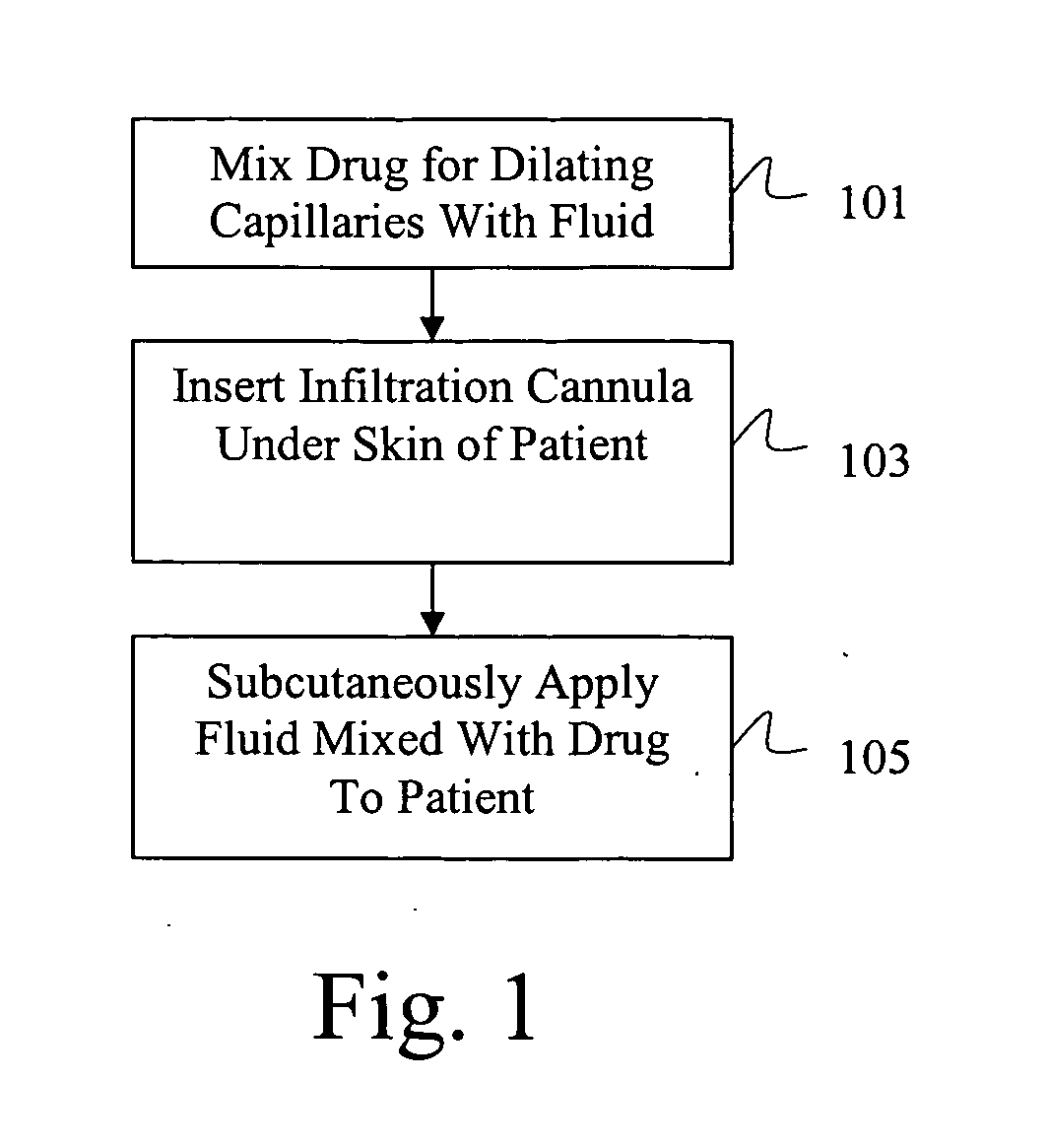
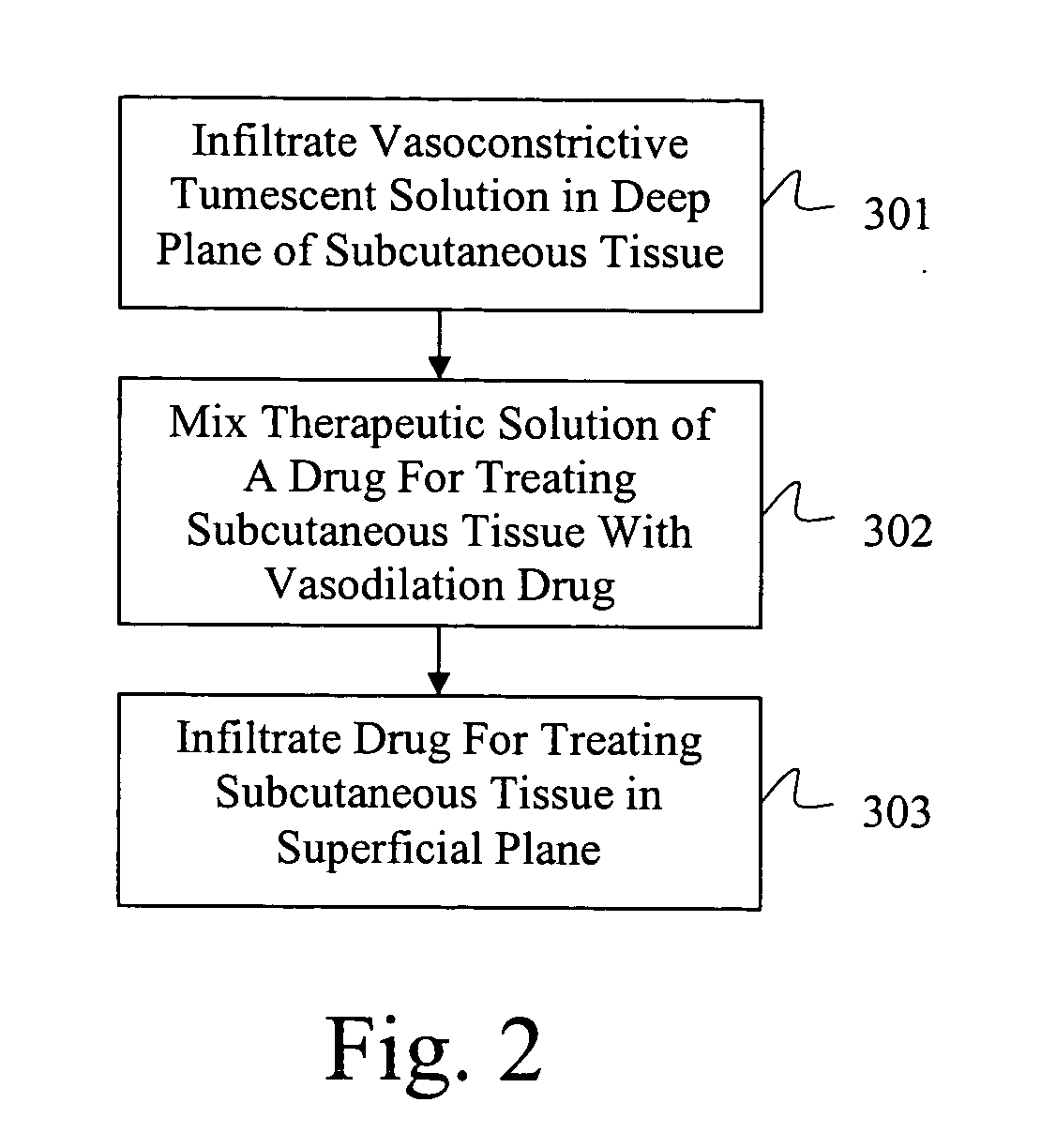
Drug delivery system for accelerated subcutaneous absorption
ActiveUS20050287134A1Prevent and delay absorptionImprove absorption rateBiocidePeptide/protein ingredientsWhole bodyVascular dilation
A method for accelerating subcutaneous absorption of a fluid or drug into the systemic circulation of a specific targeted tissue. A first drug operative to produce local capillary vasodilatation and / or increase the rate of bulk flow of solution through the interstitial space is mixed with a fluid. The fluid may contain a crystalloid solution or a dilute solution of a pharmacologic drug required for a routine or emergency therapeutic treatment for a patient. The first drug is substantially non-toxic to the patient. The fluid mixed with the first drug is then delivered subcutaneously or into deeper tissues of the patient, by use of a hyperdermic needle or infiltration cannula. As the capillaries are dilated, the fluid is efficiently absorbed and circulated systemically.
Owner:KLEIN JEFFREY A
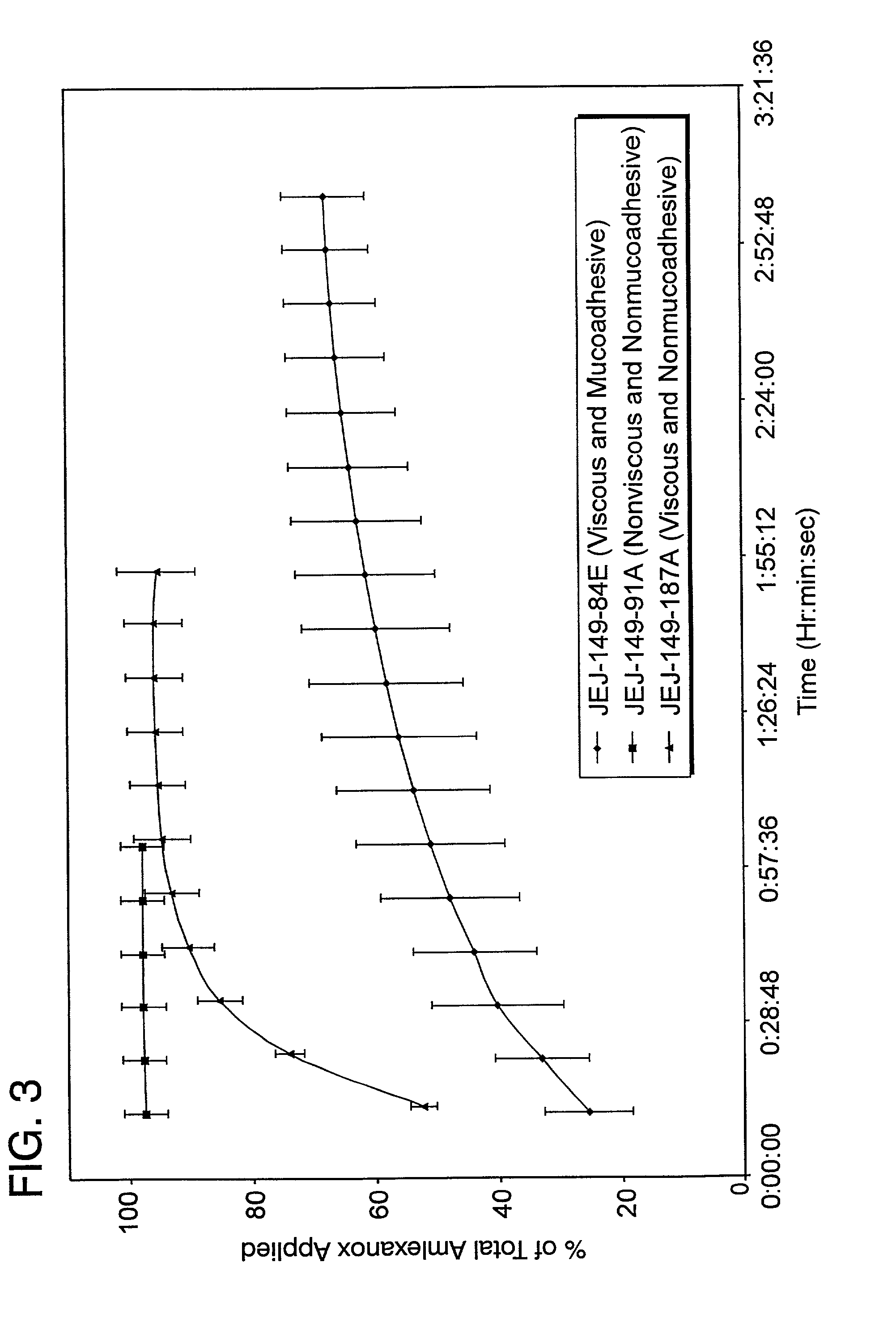
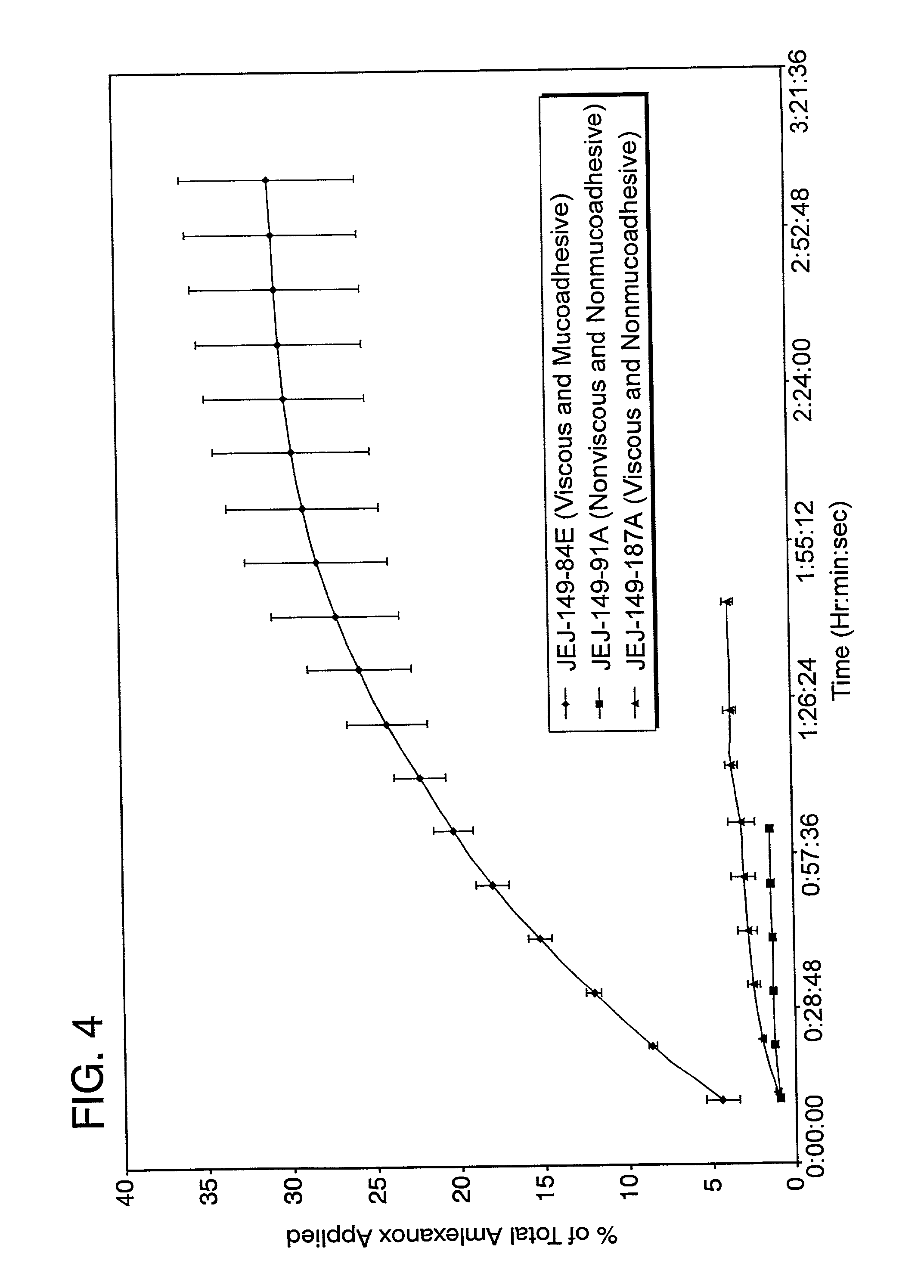
Liquid formulations for the prevention and treatment of mucosal diseases and disorders
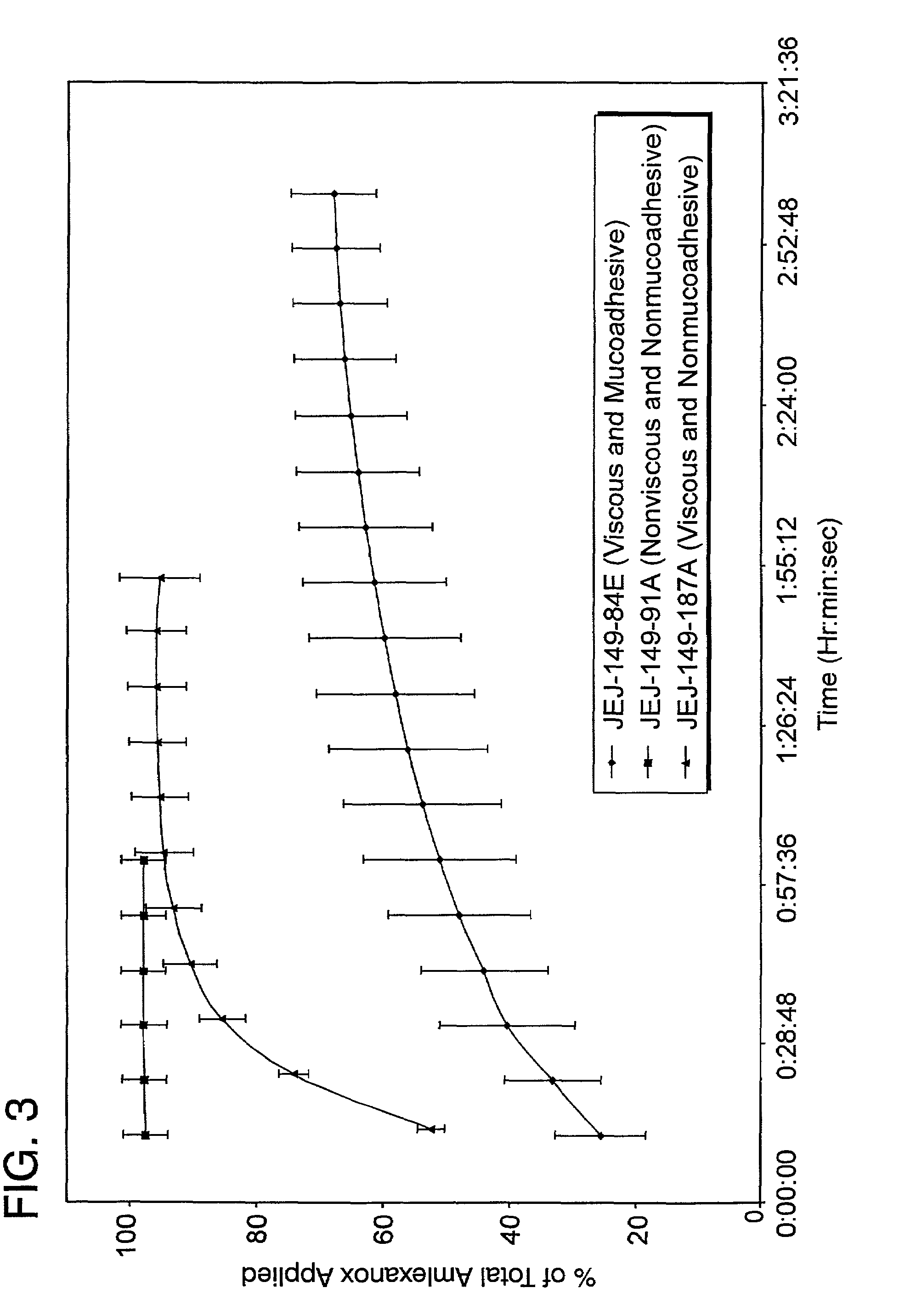
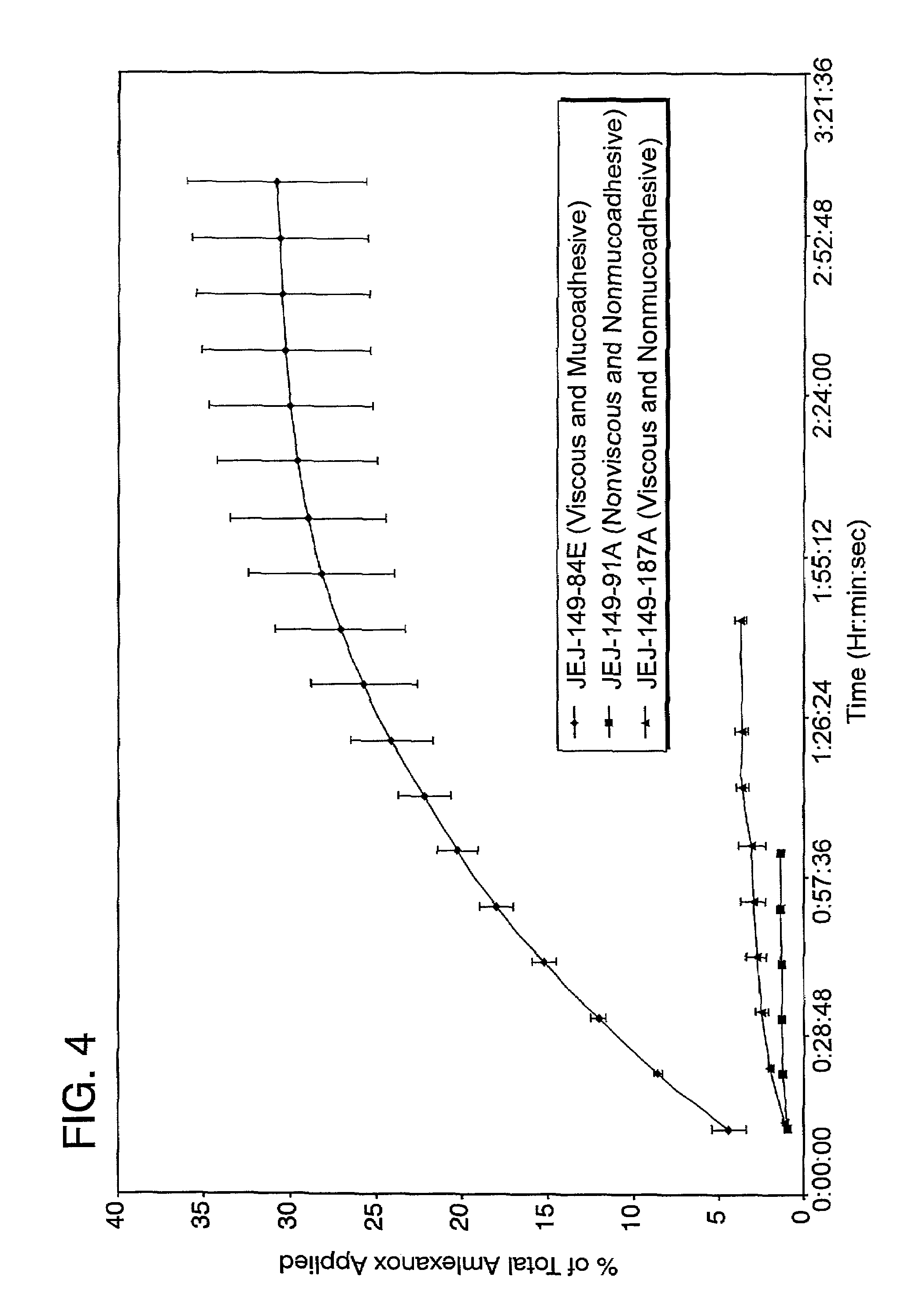
Stable, viscous, mucoadhesive aqueous compositions which are useful for the prevention and treatment of ulcerative, inflammatory, and / or erosive disorders of mucous membranes and / or the delivery of pharmaceutically active compounds to mucosal surfaces for topical treatment or transfer to the systemic circulation.
Owner:AMAG PHARMA
Controlled Release Delivery System for Nasal Applications and Method of Treatment
InactiveUS20070149454A1Improve bioavailabilityImproved profileOrganic active ingredientsBiocideFemale Sexual Arousal DisorderNasal cavity
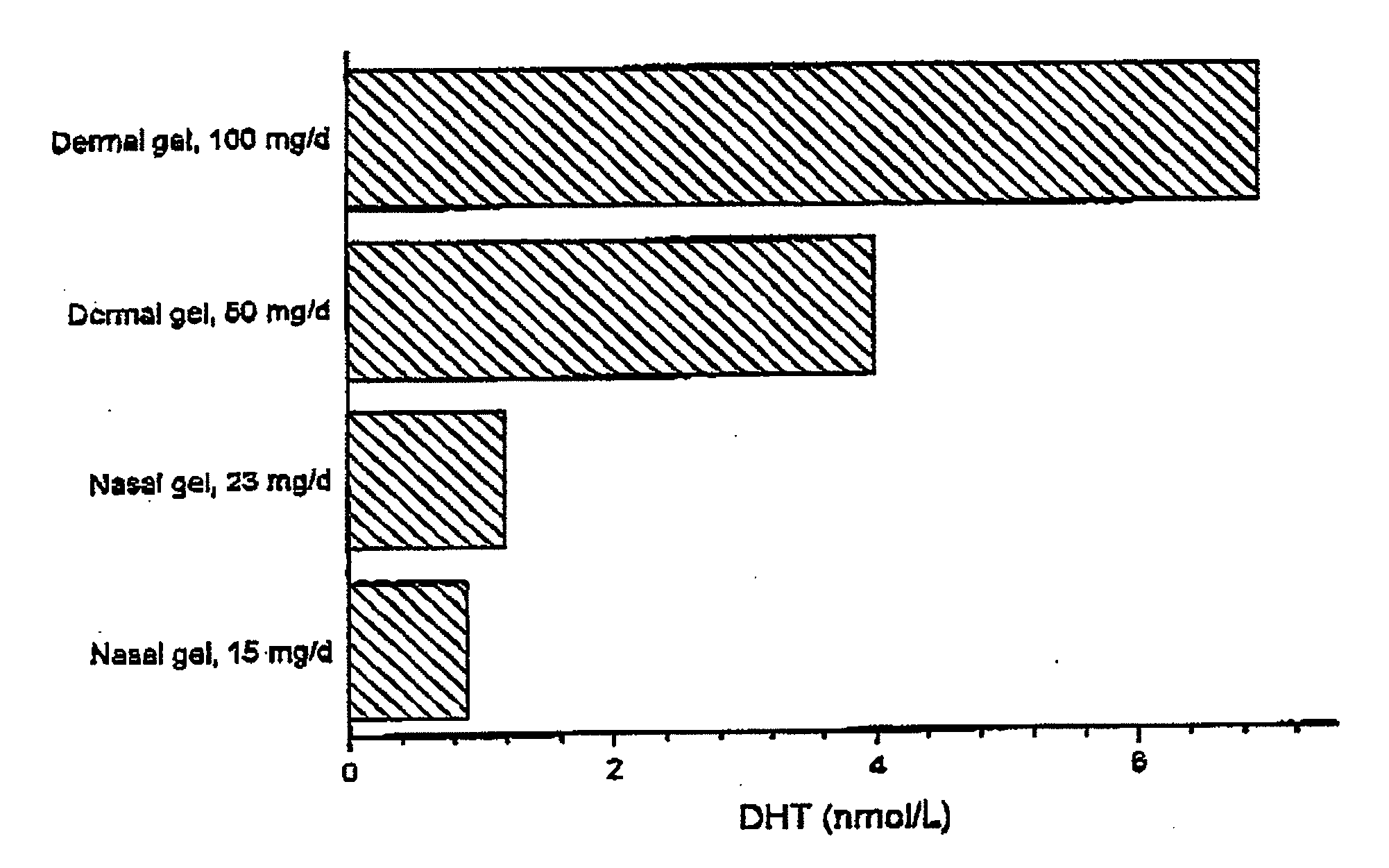
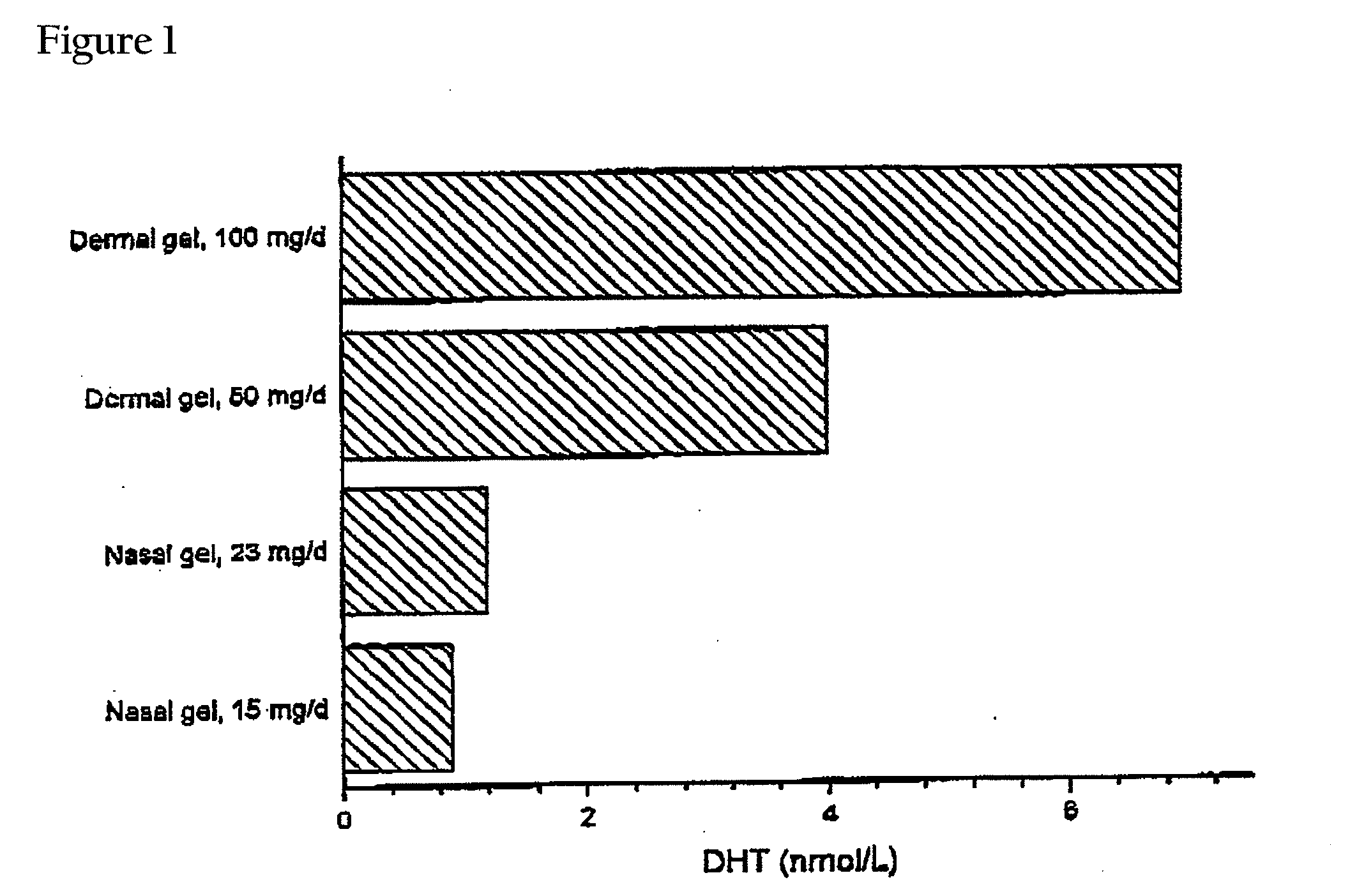
This invention relates to a gel formulation for nasal administration of a controlled release formulation of hormones to the systemic circulation and / or to the brain. The special lipophilic or partly lipophilic system of the invention leads to higher bioavailability of the active ingredient caused by sustained serum levels in plasma but also leads to a more favorable serum level profile. The special lipophilic or partly lipophilic system also allows for the modulation of brain functioning. The invention also relates to the nasal administration of steroid hormones for treatment of female sexual dysfunction (FSD) or female arousal disorder.
Owner:MATTERN PHARMA
Liquid formulations for the prevention and treatment of mucosal diseases and disorders
Stable, viscous, mucoadhesive aqueous compositions which are useful for the prevention and treatment of ulcerative, inflammatory, and / or erosive disorders of mucous membranes and / or the delivery of pharmaceutically active compounds to mucosal surfaces for topical treatment or transfer to the systemic circulation.
Owner:AMAG PHARMA
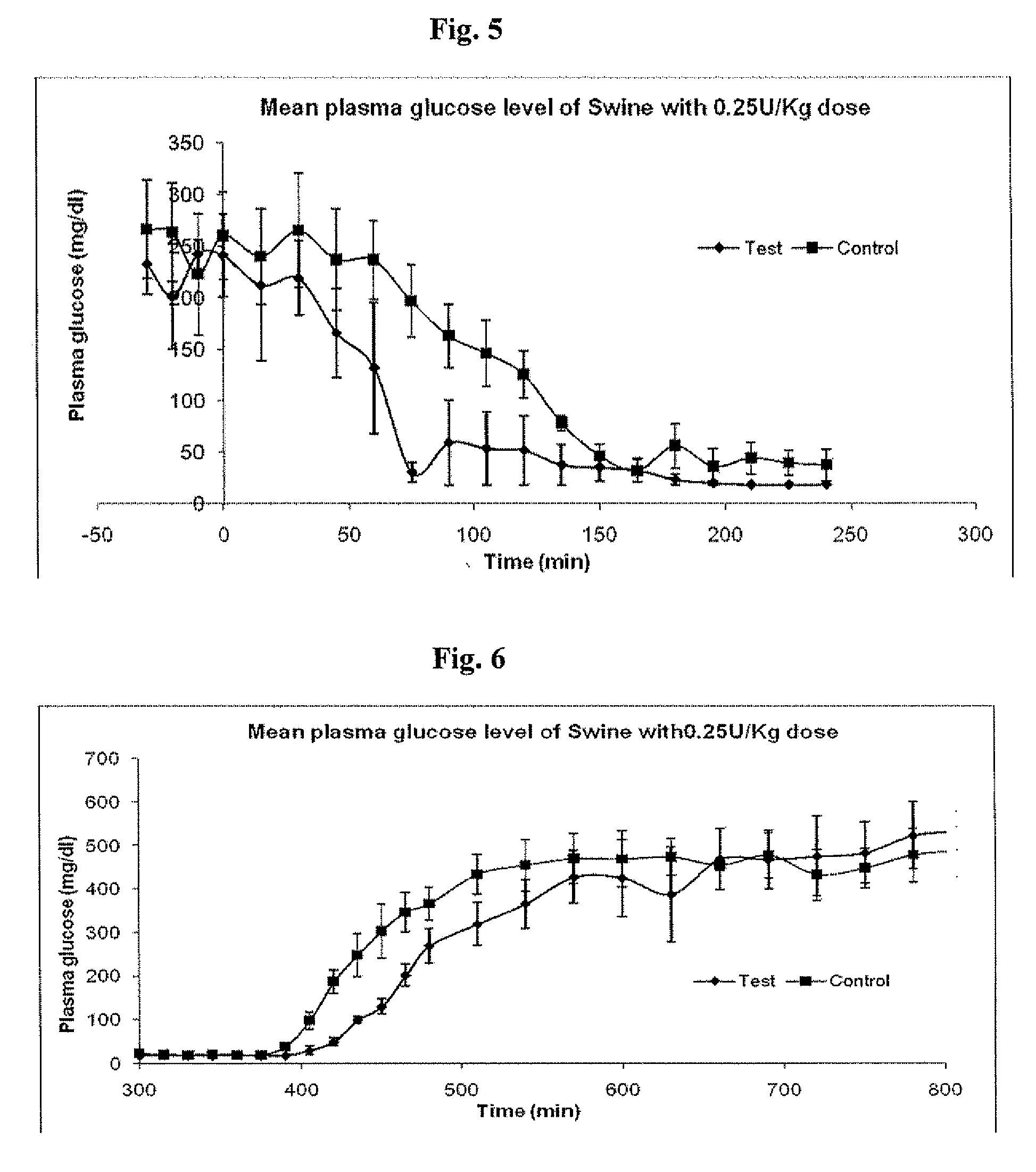
Insulin formulations for insulin release as a function of tissue glucose levels
InactiveUS20090175840A1Reduce productionRaise the pHPeptide/protein ingredientsMetabolism disorderInjections insulinReducing agent
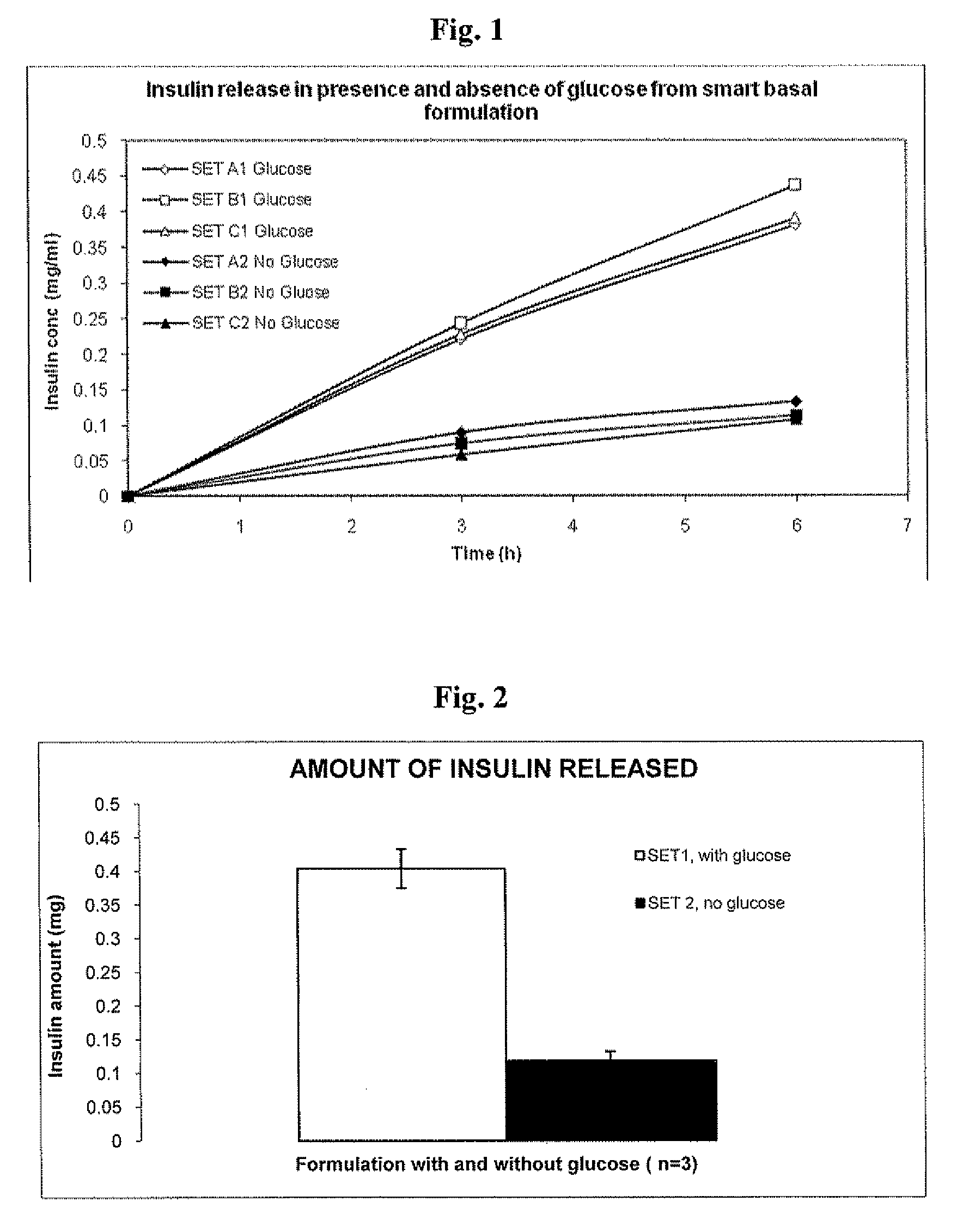
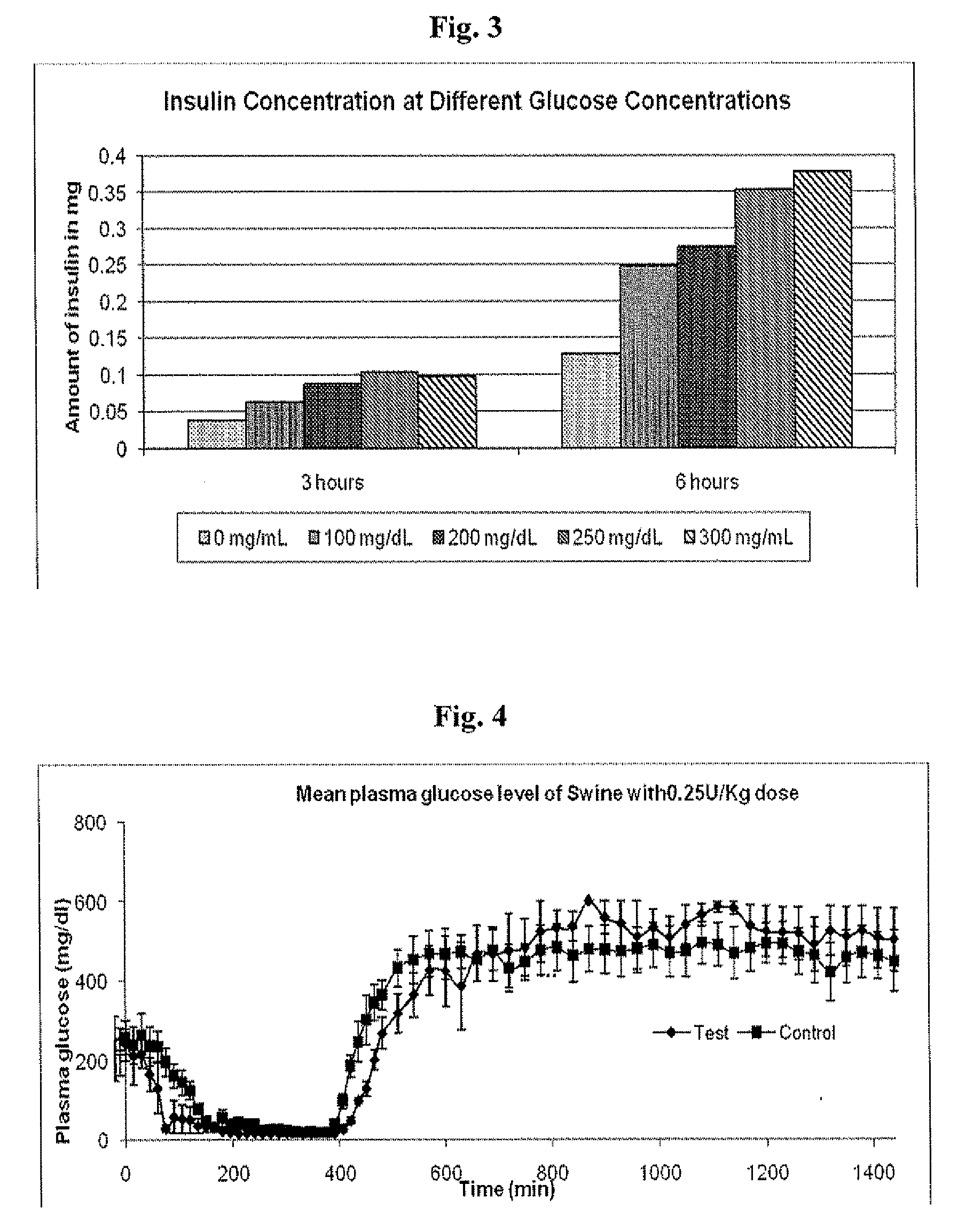
Injectable insulin formulations that are capable of modifying the amount of insulin released based on the patient's tissue glucose levels, methods for making and using these formulations are described herein. The formulation may be administered via subcutaneous, intradermal or intramuscular administration. In one preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin, an oxidizing agent or enzyme and a reducing agent or enzyme, a diluent and optionally one or more thickening agents. If a thickening agent is present in the formulation, the thickening agent increases the viscosity of the formulation following administration. Preferably the formulation contains an insulin, a diluent, glucose oxidase and peroxidase. Following administration to a patient, the insulin is released from the formulations as a function of the patient's tissue glucose level, which in turn maintains the patient's blood glucose level within an optimum range. The formulation is often referred to as a “smart” formulation since it modifies its release rate of insulin according to the patient's needs at a particular time. In a preferred embodiment, the formulation is designed to release insulin into the systemic circulation over time with a basal release profile following injection in a patient. In another embodiment, the formulation is designed to release insulin into the systemic circulation over time with a non-basal release profile following injection in a patient, such as a regular human insulin release profile or a prandial release profile.
Owner:BIODEL
Vertical air conditioner indoor unit and air conditioner
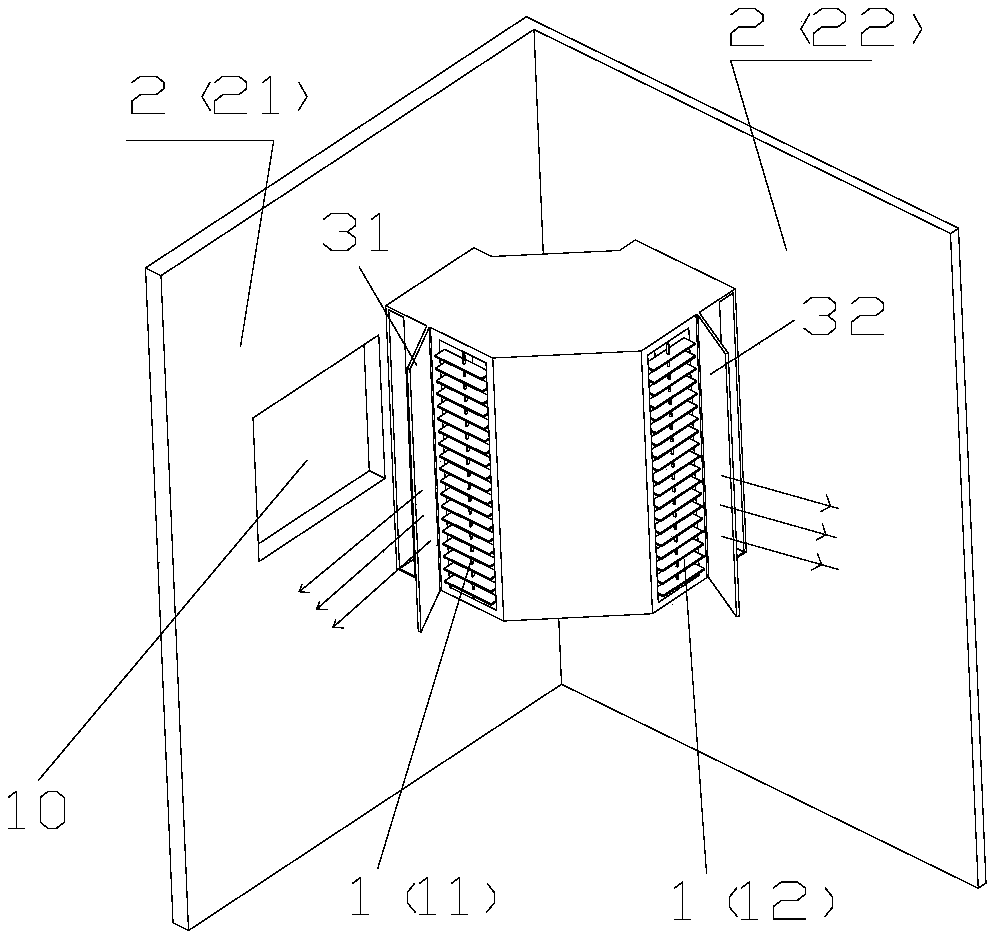
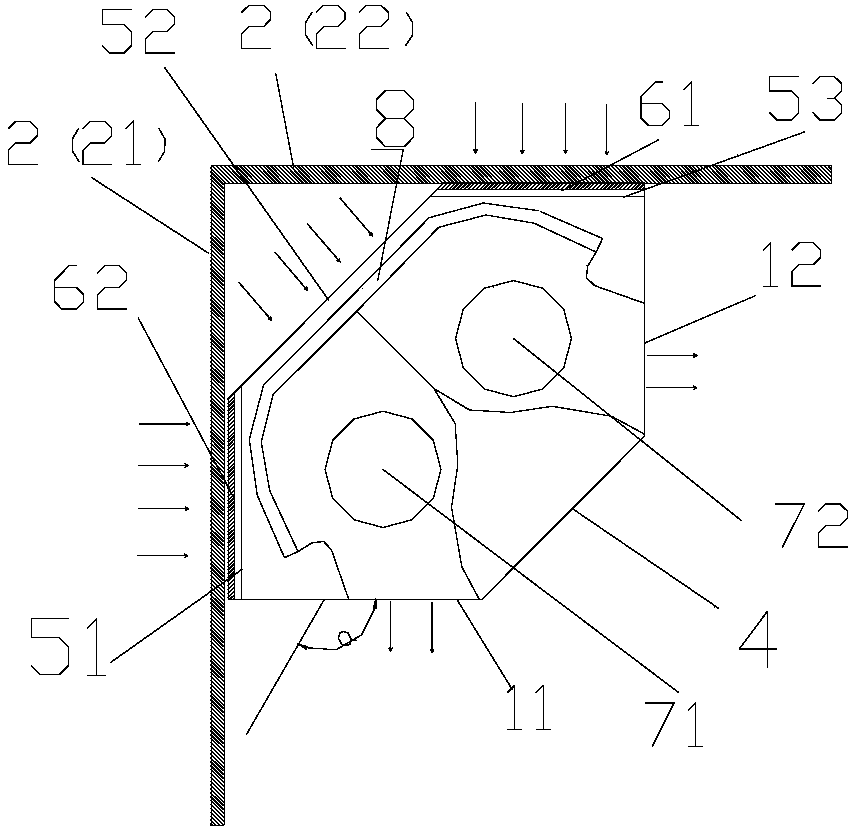
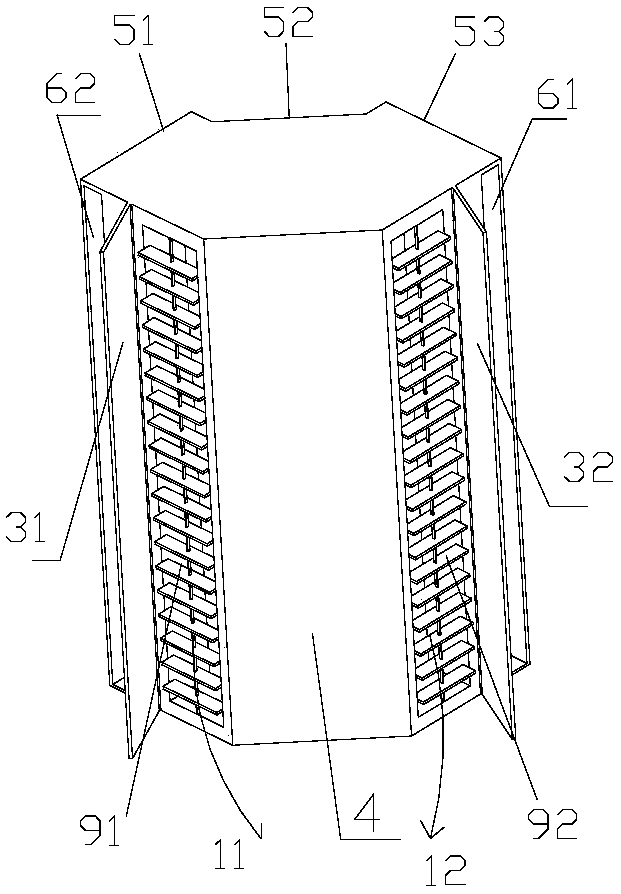
PendingCN108413499AFacilitates the formation of the Coanda effectImprove comfortLighting and heating apparatusHeating and ventilation casings/coversSystemic circulationWaste management
The invention provides a vertical air conditioner indoor unit and an air conditioner. The vertical air conditioner indoor unit (1) is installed at the position of an indoor wall corner (2) and comprises a first air outlet (11) and a second air outlet (12), the first air outlet (11) can conduct air outlet in the direction of a first wall face (21) of the wall corner (2), and the second air outlet (12) can conduct air outlet in the direction of a second wall face (22) of the wall corner (2). By means of the vertical air conditioner indoor unit and the air conditioner, airflow sent from the leftand right air outlets can flow along the wall, the air conditioner is prevented from blowing a person directly, the left and right air outlets achieve air supply along the wall, surrounding airflow can be achieved, the airflow achieves systemic circulation indoors, the coanda effect of the airflow is conveniently formed, even temperature distribution caused by uneven indoor airflow is prevented, the indoor temperature distribution is even, and the comfort of the user is improved.
Owner:GREE ELECTRIC APPLIANCES INC
Cartilage and Bone Repair Composition
InactiveUS20080031915A1Promote bone formationPromote cartilage formationBiocidePeptide/protein ingredientsCollagen iWhole body
The invention relates to a cartilage and bone repair composition comprising a group of human mesenchymal stem cells that are differentiated to the chondro-osteogenic lineage, by means of the amplification thereof with human serum and a transforming growth factor-beta 1 with a molecular domain for binding to collagen I (TGF-β1-DUC) obtained in eukaryotic expression systems, and a biocompatible material which is absorbed by the cells thus treated. The inventive composition can be employed using implants in the area to be repaired or it can be employed directly by injecting the cells in suspension either at the site of the injury or into the systemic circulation for the widespread distribution thereof.
Owner:UNIV DE MALAGA
Topical anesthetic composition and method of administration
InactiveUS20050014823A1Minimize occlusionRelieve painBiocidePharmaceutical delivery mechanismWhole bodyIntact skin
The present invention includes a topical anesthetic composition having one or more local anesthetic agents, a penetration enhancer, and an anhydrous, volatile solvent combined to provide a liquid homogenous solution. The present invention may also include water in the topical anesthetic composition as concentration levels low enough to avoid precipitation of the local anesthetic agent and penetration enhancer from solution and undesired enhanced delivery of the local anesthetic agent through the skin or mucosa to the systemic circulation. The topical anesthetic composition can be conveniently sprayed onto intact skin and can be absorbed percutaneously with greater efficacy in order to shorten the time needed for the anesthesia to take effect.
Owner:HAWKINS CHEM
Isolating cardiac circulation
In a method for substantially isolating cardiac circulation from systemic circulation, flow between the coronary sinus and the right atrium is occluded. A venous collection device having a collection lumen and a support structure is located in the coronary sinus. The support structure is used to maintain patency of the coronary sinus during collection of fluid through the collection lumen. An artificial flow path is provided between the collection lumen and the one or more coronary arteries, thus isolating the cardiac circulation. According to the method, cardiac pumping for systemic circulation can be maintained during isolation of the cardiac circulation.
Owner:OSPREY MEDICAL
Controlled release delivery system for nasal applications
InactiveUS20050100564A1Improve bioavailabilityFavourable serum level profileBiocideOrganic active ingredientsControl releaseSexual hormones
This invention relates to a pernasally administrable preparation for the controlled release of sexual hormones to the systemic circulation, in particular to a formulation which enables its active ingredient to be absorbed in a sustained manner providing a better bioavailability at very low doses and longer duration of action.
Owner:PRECISIOBIOTIX TECH INC +1
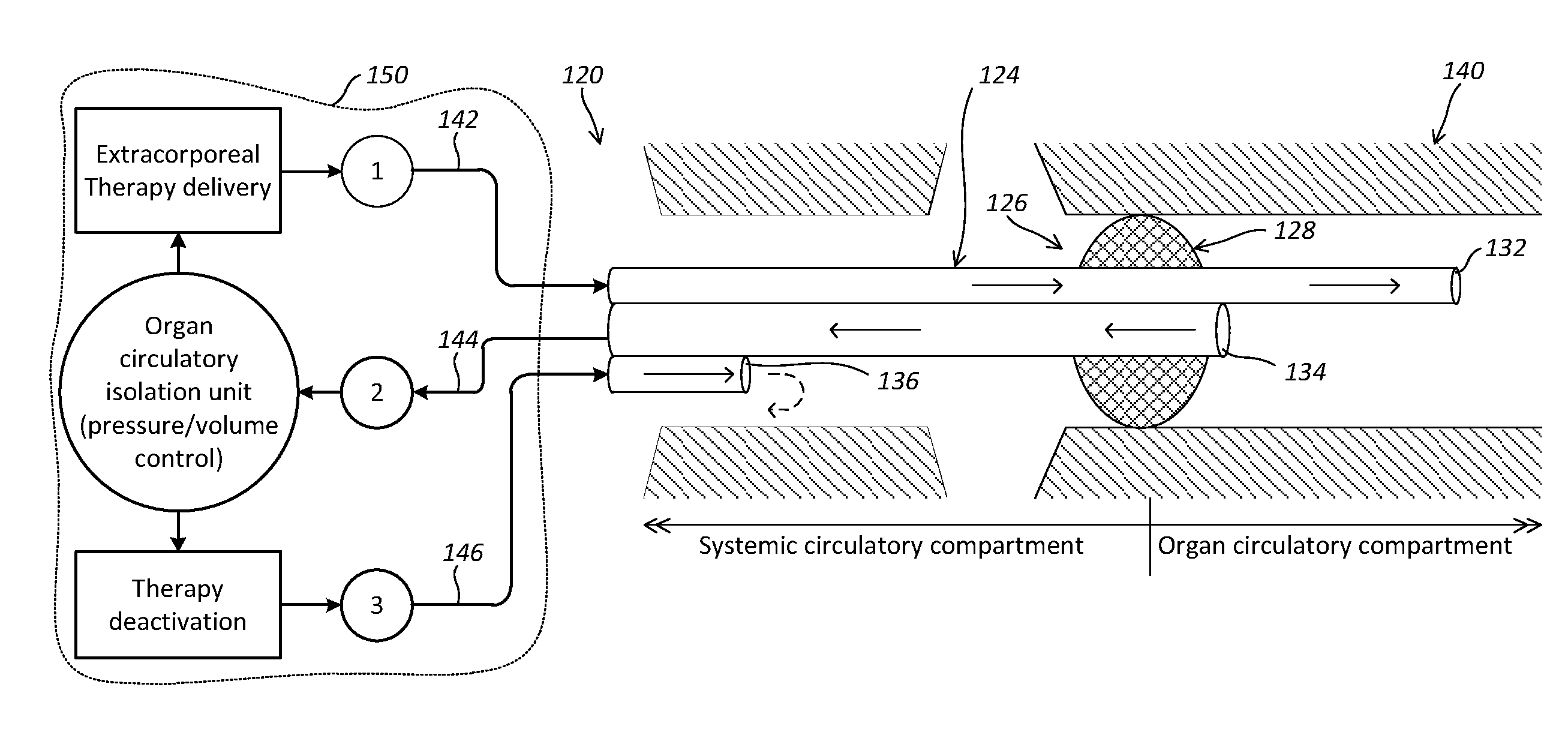
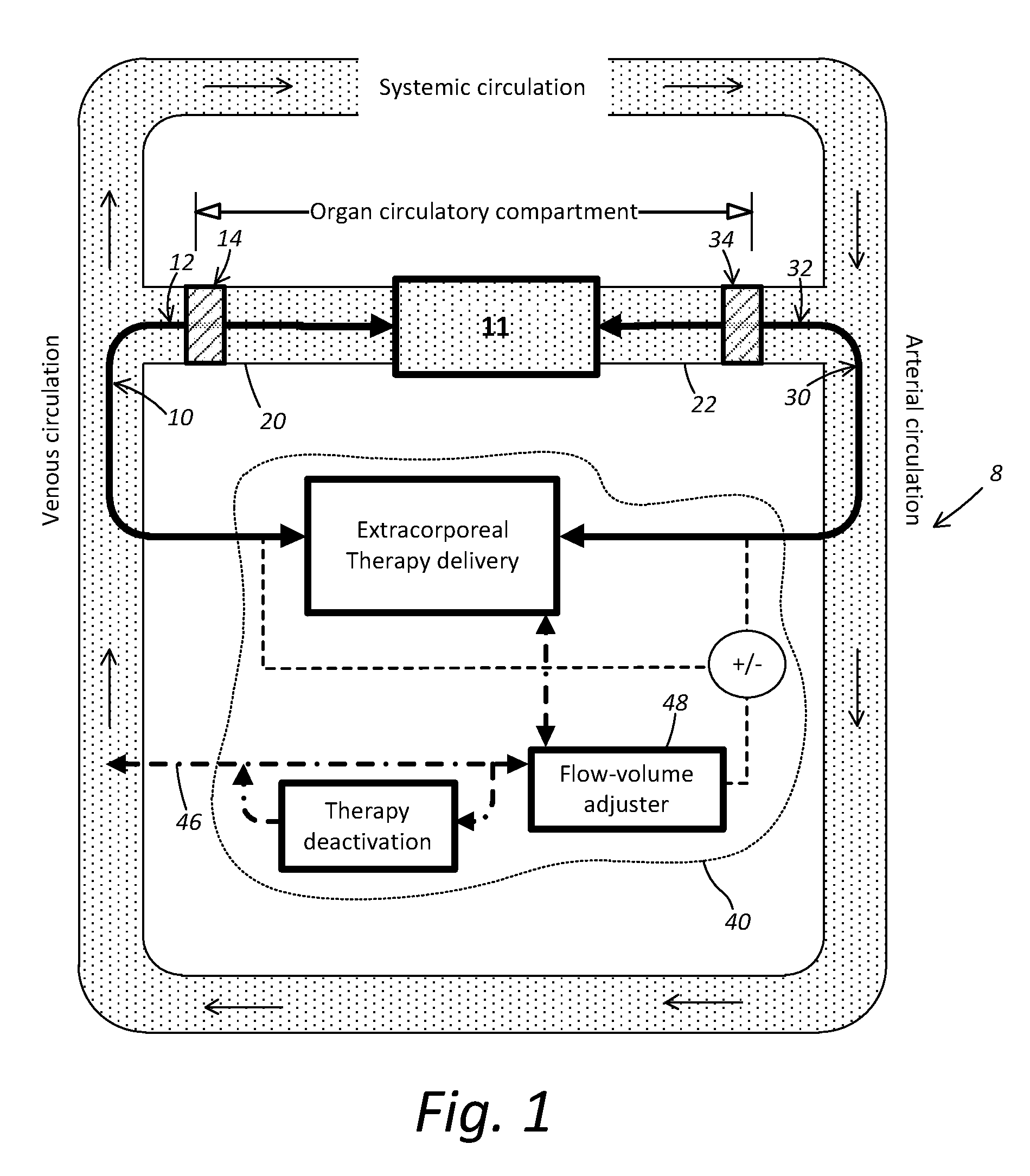
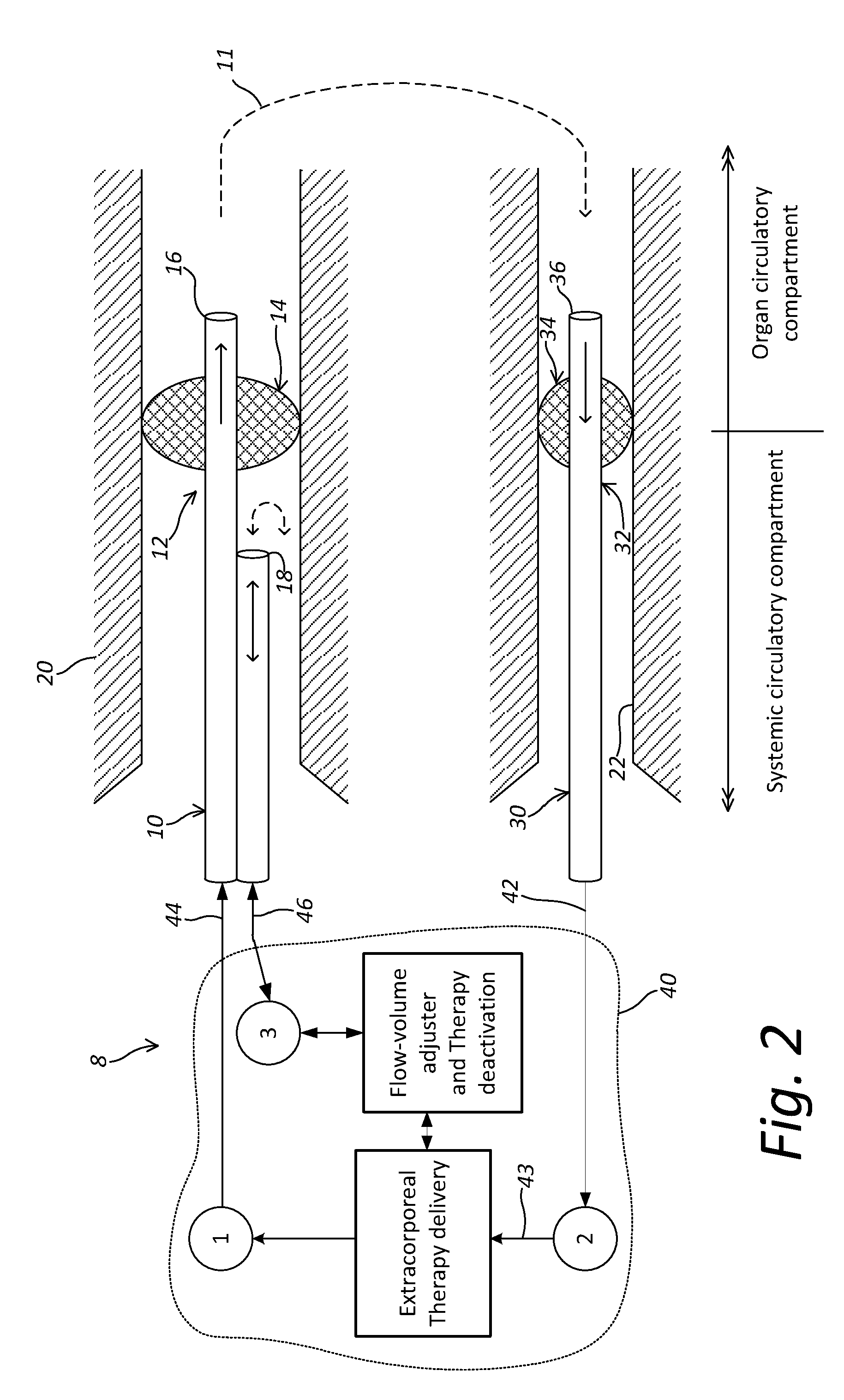
Localized Therapy Delivery and Local Organ Protection
ActiveUS20120302995A1Prevent and minimize systemic side effectLocalized treatmentBalloon catheterMulti-lumen catheterVenous accessVein
A system for perfusing a localized site within a body includes a catheter assembly having a venous access line that is adapted to deliver perfusate to the localized site, a venous or arterial drainage line adapted to drain perfusate from the localized site, and an occlusion device adapted to prevent some or substantially all physiological blood flow between the localized site and the systemic circulation of the body during and in the course of perfusing and draining perfusate to and from the localized site. The system may include a blood circuit associated with the catheter assembly to facilitate blood conditioning for use as the perfusate, in the course of a controlled perfusion and / or drainage of untreated, treated, or inactivated treated blood to and from the localized site. A delivery machine may control the blood circuit and catheter assembly in order to both deliver perfusate to, and drain some or all perfusate from, the localized site in a manner that provides perfusate to substantially only the localized site.
Owner:NIRVA MEDICAL
Intraoral delivery of nicotine for smoking cessation
InactiveUS20070298090A1Promote absorptionIncrease success rateBiocideNervous disorderMucoadhesionSmoking cessation
Dosage forms of a nicotine delivery system are disclosed in which a mucoadhesive film, made up of one or more non-microbial hydrocolloid(s) and an effective dose of nicotine, dissolves when applied intraorally to release the nicotine which is absorbed through the oramucosac and directly reaches systemic circulation. Methods for preparing various versions of the dosage forms are disclosed. Methods to assist smoking cessation or provide substitutes for smoking by administrating the dosage form are also provided.
Owner:THALLIUM HLDG CO LLC
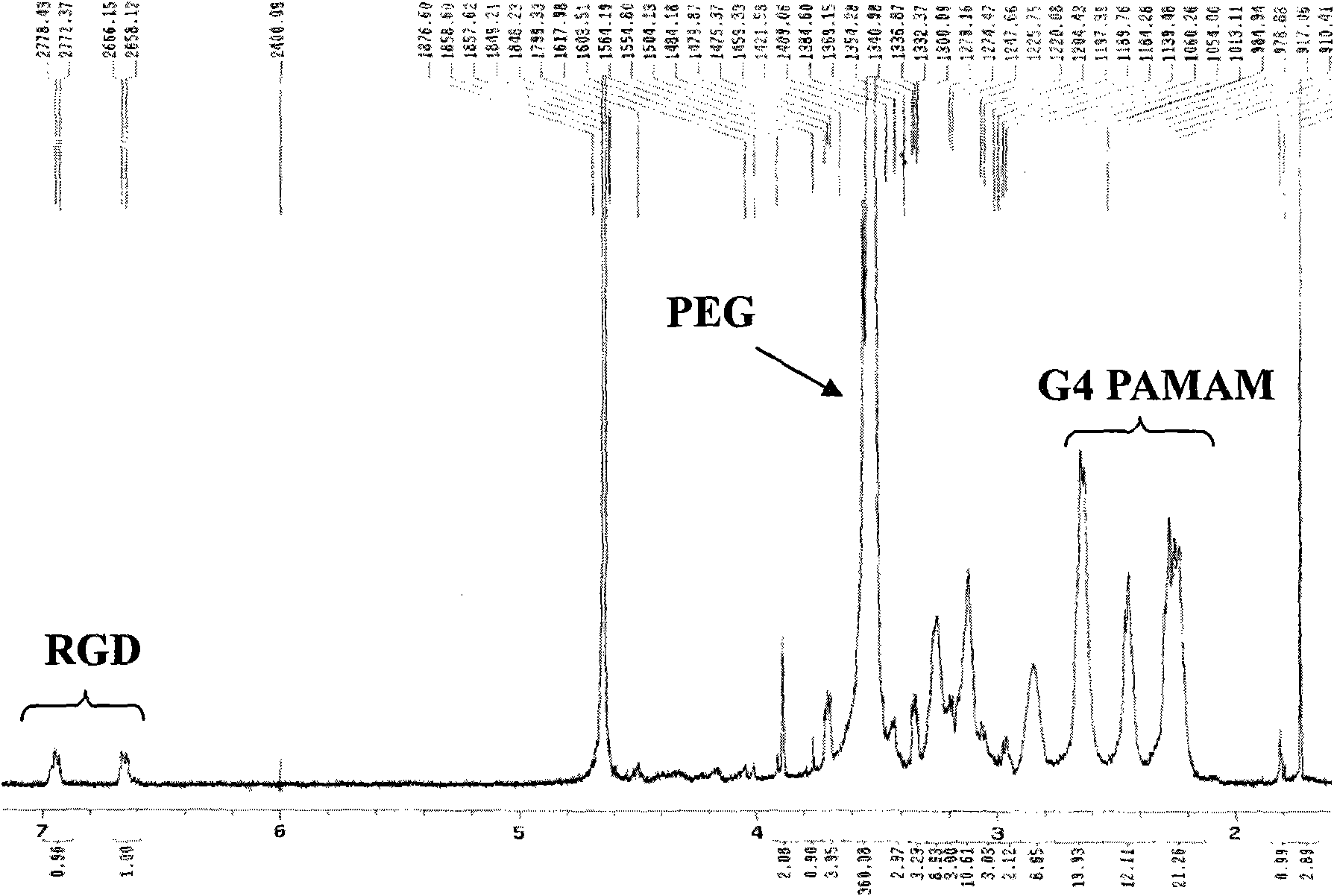
Anti-tumor nano prodrug system based on dendrimer and preparation method thereof
InactiveCN101879313AHigh molecular weightImprove hydrophilicityOrganic active ingredientsPharmaceutical non-active ingredientsDendrimerCyclic peptide
The invention relates to a multi-function nano prodrug system based on a dendrimer, which belongs to the technical field of biomedicines and nano medical science. The nano system comprises four function parts: an outermost layer RGD (arginyl-glycocoll-aspartate) cyclic peptide as an active targeting head group (1), a polyethylene glycol (PEG) hydrophilic chain segment (2) connected with the targeting head group and the dendrimer, an anti-tumour drug (3) connected with the dendrimer by an acid sensitive chemical bond and a dendrimer kernel (4). The general formula of the nano system is RGD-PEG-Dendrimer-DOX (doxorubicine), wherein the macromolecule attribute of a dendrimer vector makes a vector system passively target to tumor tissues through an EPR (Enhanced Permeability and retention) effect; the RGD cyclic peptide makes the vector system actively target to the tumor tissues through the interaction of a ligand and a receptor and promotes the endocytosis of the tumor tissues; the acid sensitive chemical bond ensures that a drug-carrying system is stable in systemic circulation and releases active drugs after arriving tumor positions and entering an acid organelle to perform the function of cytotoxicity.
Owner:FUDAN UNIV
Method for augmentation of intraepithelial and systemic exposure of therapeutic agents having substrate activity for cytochrome P450 enzymes and membrane efflux systems following vaginal and oral cavity administration
A vaginal or buccal delivery of therapeutic agents having a substrate affinity for metabolic cytochrome P-450 enzymes and membrane efflux transporter systems. A method for augmentation of systemic exposure to the therapeutic agents having a substrate affinity for cytochrome P-450 enzymes and membrane efflux transporter systems, by delivering said agents to the systemic circulation through vaginal or buccal mucosa.
Owner:FEMINA PHARMA +1
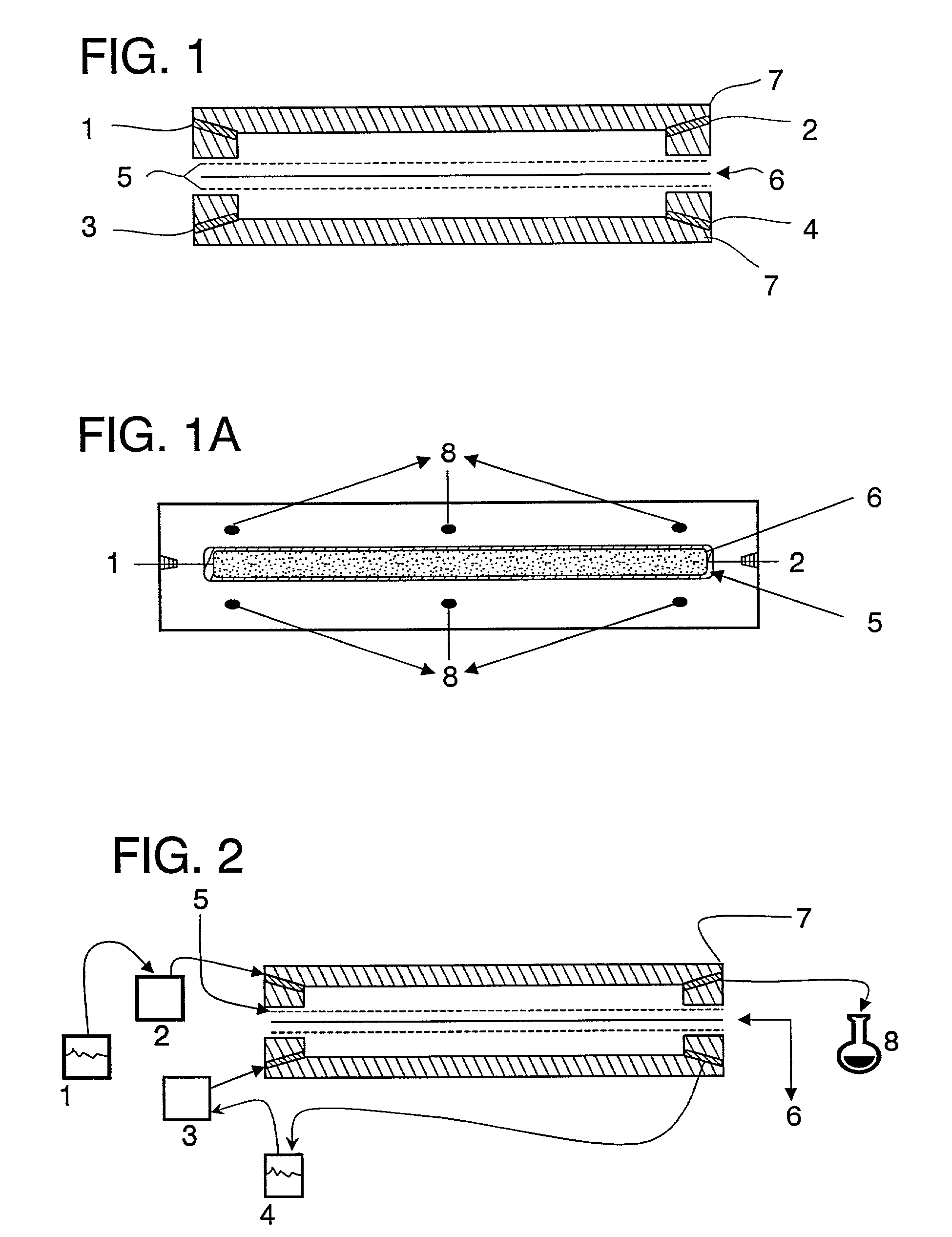
Electric energy auxiliary device of transdermal drug delivery patch
InactiveUS20060009731A1Reduce deliveryReduce polarizationElectrotherapyMagnetotherapyHuman bodyWhole body
An electric energy auxiliary device of transdermal drug delivery patch is provided which includes an upper electrode, a lower electrode, and a drug storage area between two electrodes, generating a kinetic energy in the pharmaceutical compound in the drug storage area toward the skin by a electric field between two electrodes. For the pharmaceutical compound with the same polarity, the pharmaceutical compound is pushed by the electric energy interacted with polar the pharmaceutical compound so as to deliver the drug the human body. The lower electrode generates an ion channel with electroporation on the surface of skin. By using the change of electric field on the surface of skin, a temporary ion channel is generated in the cells where the pharmaceutical compound enters the systemic circulation of human body via skin. This temporary ion channel allows large compound to pass into the skin of human body.
Owner:NATIONAL CHUNG CHENG UNIV
Human growth hormone formulations
InactiveUS20080095837A1Organic active ingredientsPeptide/protein ingredientsDiseaseBuccal administration
The present invention relates to dosage forms of human growth hormone, the use of an absorption enhancer to allow absorption of human growth hormone into the systemic circulation in a biologically active form, in particular after oral administration, as well as the use of oral dosage forms comprising human growth hormone and an absorption enhancer for the treatment of human growth hormone deficiencies and disorders associated therewith.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
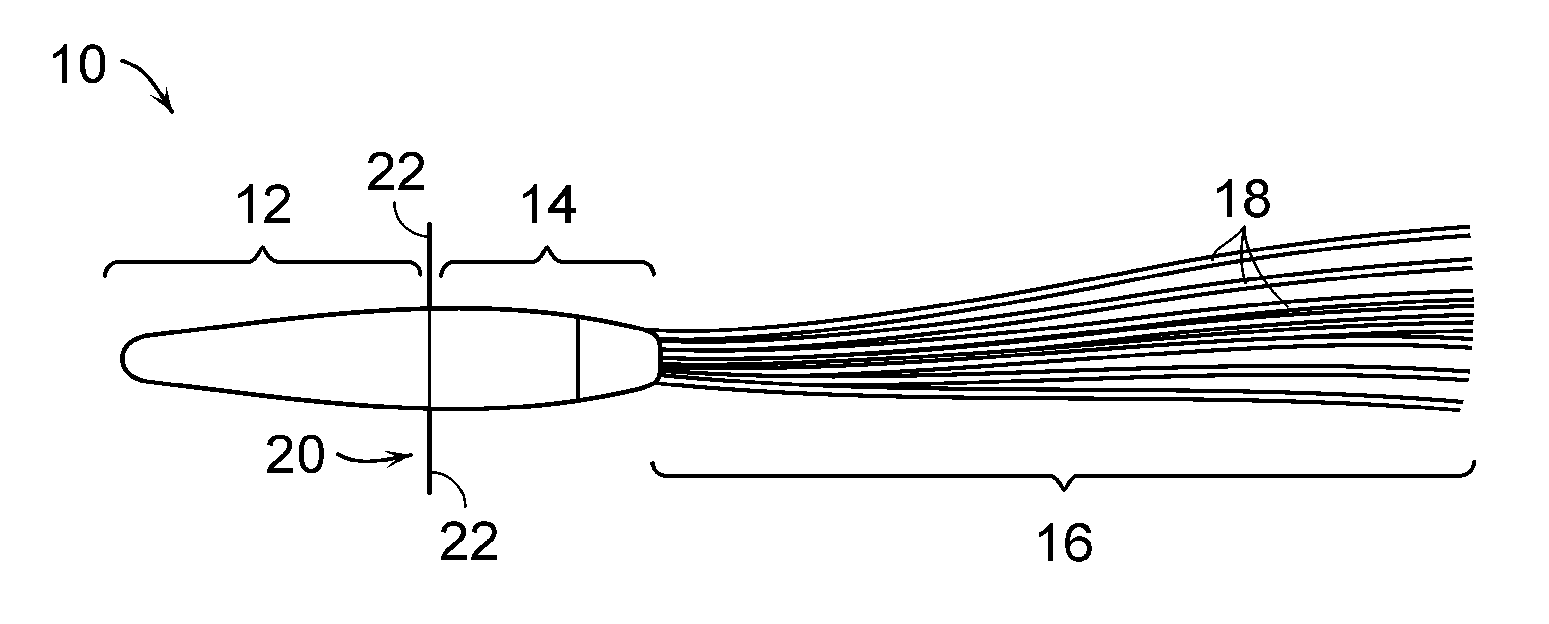
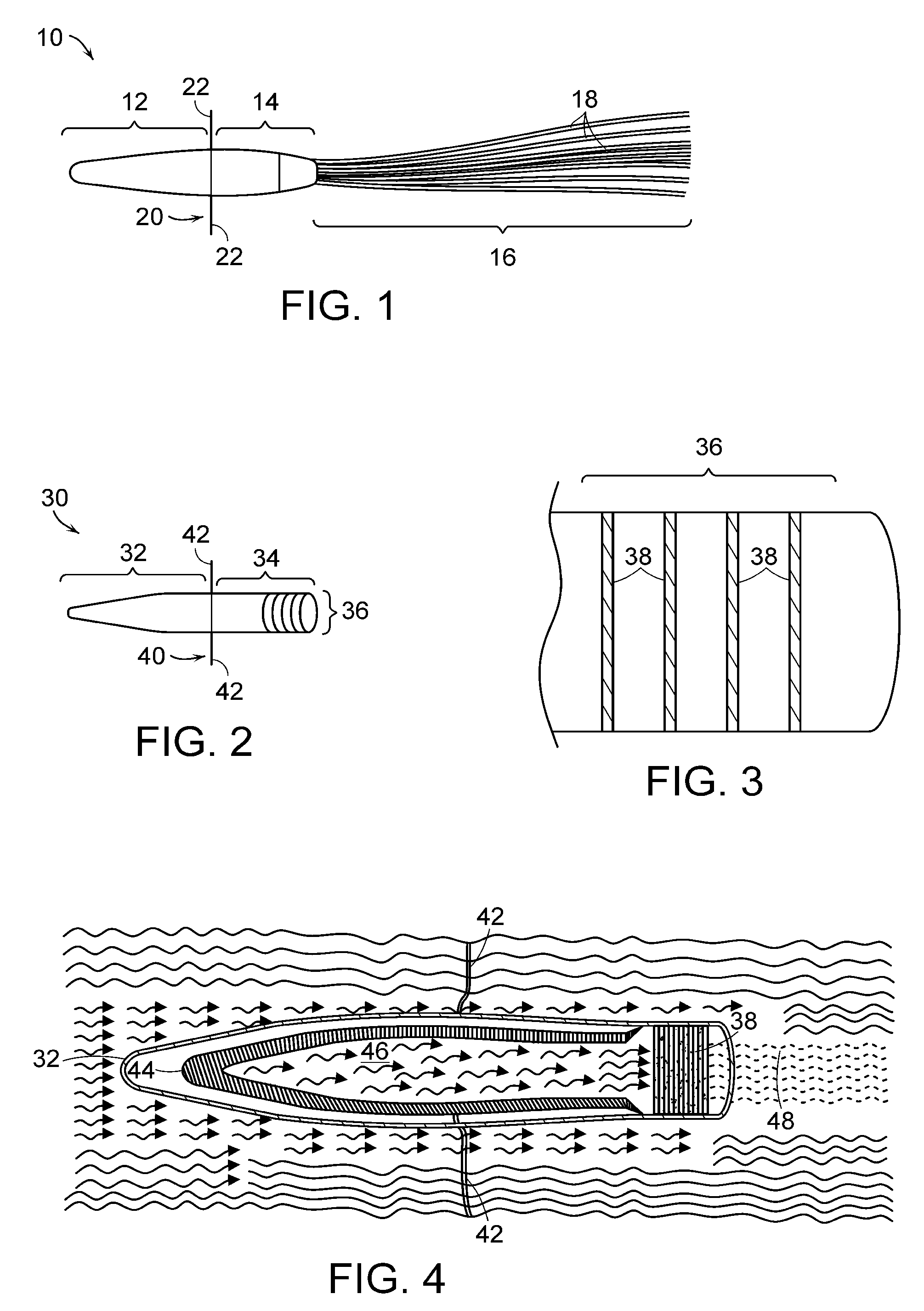
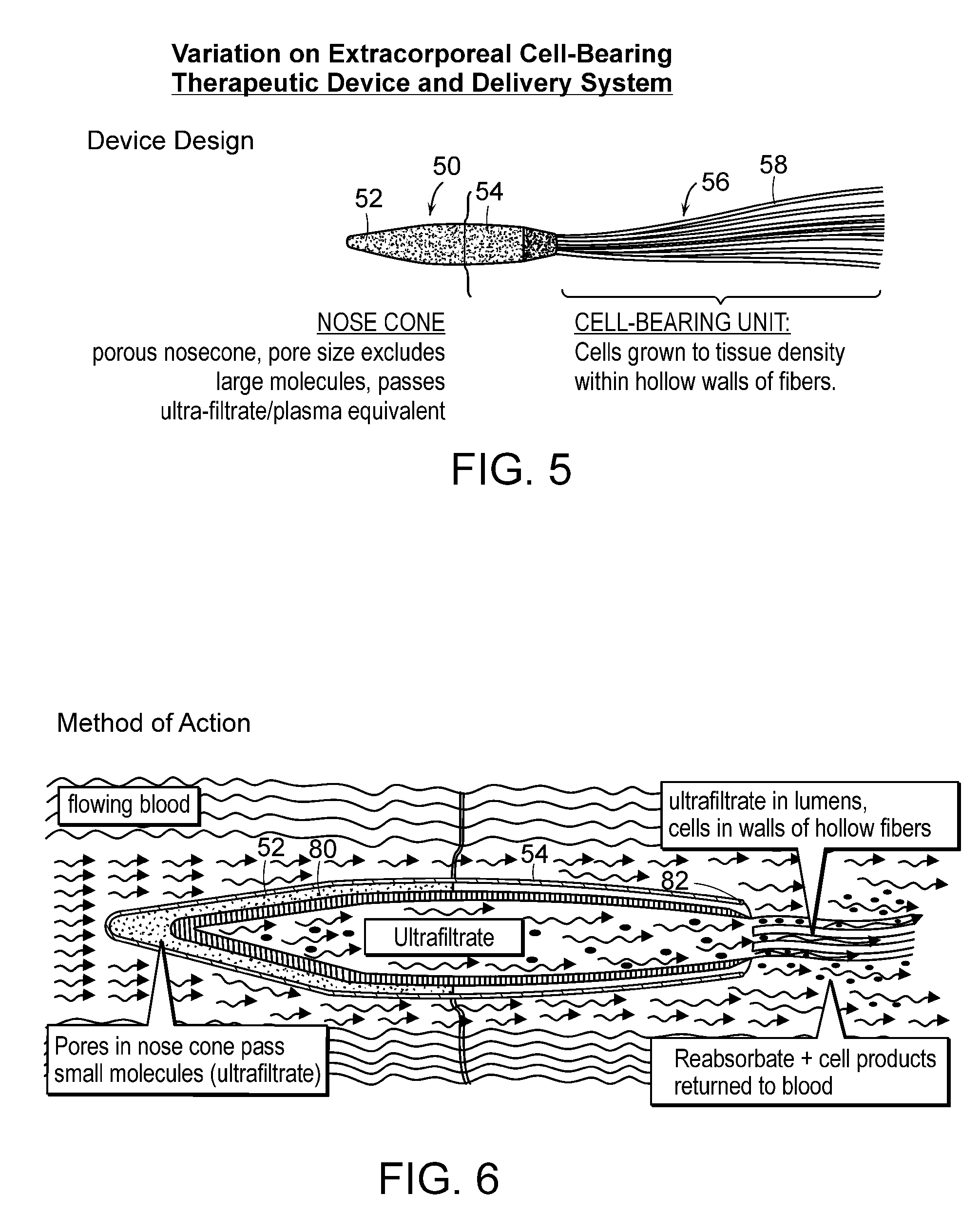
Extracorporeal cell-based therapeutic device and delivery system
InactiveUS20090081296A1Easily taken out of circulation systemReduce turbulencePowder deliveryBiocideHollow fibreFiber
Extracorporeal cell-based therapeutic devices and delivery systems are disclosed which provide a method for therapeutic delivery of biologically active molecules produced by living cells in response to a dynamic physiologic environment. One embodiment includes long hollow fibers in which a layer of cells are grown within the intraluminal volume or within a double hollow-filled chamber. Another embodiment includes a wafer or a series of wafers providing a substrate onto which cells are grown. The wafer(s) are inserted into a device. A device may deliver a pre-selected molecule, for example, a hormone, into a mammal's systemic circulation and / or may deliver a member of different cell products. The device is adapted to secure viable cells which produce and secrete the pre-selected molecule into blood or fluid. The invention also provides a minimally invasive method for percutaneously introducing into a preselected blood vessel or body cavity the device of the invention.
Owner:INNOVATIVE BIOTHERAPIES
Total artificial heart
ActiveUS20110144744A1Avoid stagnationInhibit the formation of thrombusControl devicesBlood pumpsImpellerEngineering
A total artificial heart having a rotor with an impeller, wherein the rotor is mounted within a pump housing on a hollow shaft. The rotor is magnetically driven to produce rotary motion of the impeller for pumping blood. The motor is disposed within the pump housing such that axial translation within the housing acts as a shuttle valve to alternate flow between pulmonary and systemic circulation.
Owner:OREGONHEART
Aminosalicylate derivatives for treatment of inflammatory bowel disease
InactiveUS7157444B2High retention rateReduce absorptionSalicyclic acid active ingredientsBiocideIntestinal structureOral medication
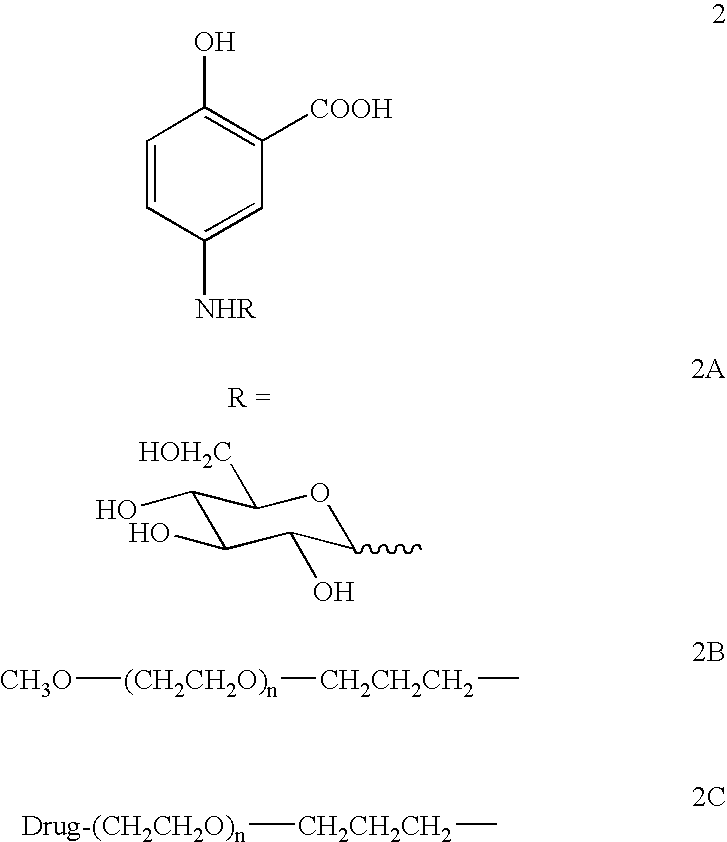
Therapeutic 5-aminosalicylic acid derivative compositions having general formula (I), wherein R is a 1-deoxy sugar residue or a poly(ethylene glycol) chain-containing residue, are provided. The compositions enable topical delivery of 5-aminosalicylic acid to the gastrointestinal tract following oral administration in pharmaceutical preparations. According to the invention, the compositions stabilize pharmaceutical compositions containing therapeutic 5-aminosalicylic acid derivatives in a manner that enhances the retention of said compositions in the intestine, decreases the cellular absorption thereof, and decreases the transfer of said compositions or the 5-aminosalicylic acid derived therefrom to the systemic circulation
Owner:NELSON DEANNA JEAN
Compounds with the biological activity of vasoactive intestinal peptide for the treatment of pulmonary and arteriolar hypertension
InactiveUS20080221041A1High activityPromote circulationPeptide/protein ingredientsMetabolism disorderDiseaseWhole body
The present invention relates to peptides which are highly biologically and pharmacologically active as therapeutic drug for the treatment of diseases related to hypertension, especially in medical interventions involving dilatation and remodeling of arterial blood vessels, either in the pulmonary or in the systemic circulation. The peptides which can be used according to the invention for the treatment of said diseases comprise at least one specific highly conservative amino acid residue sequence which seem to play an important role in connection with pulmonary and arteriolar hypertension events. It could be shown that the known naturally occurring peptides “vasoactive intestinal peptide (VIP)” and “pituitary adenylate cyclase-activating polypeptide (PACAP)”, having these specific sequences are potent drugs which can be successfully used for treatment of primary pulmonary hypertension (PPH), secondary pulmonary hypertension (SPH), and hypertension of the systemic circulation. Furthermore, the present invention discloses pharmaceutical compositions useful for treatment of PPH, SPH, and hypertension of the systemic circulation within said methods.
Owner:MONDOBIOTECH AG
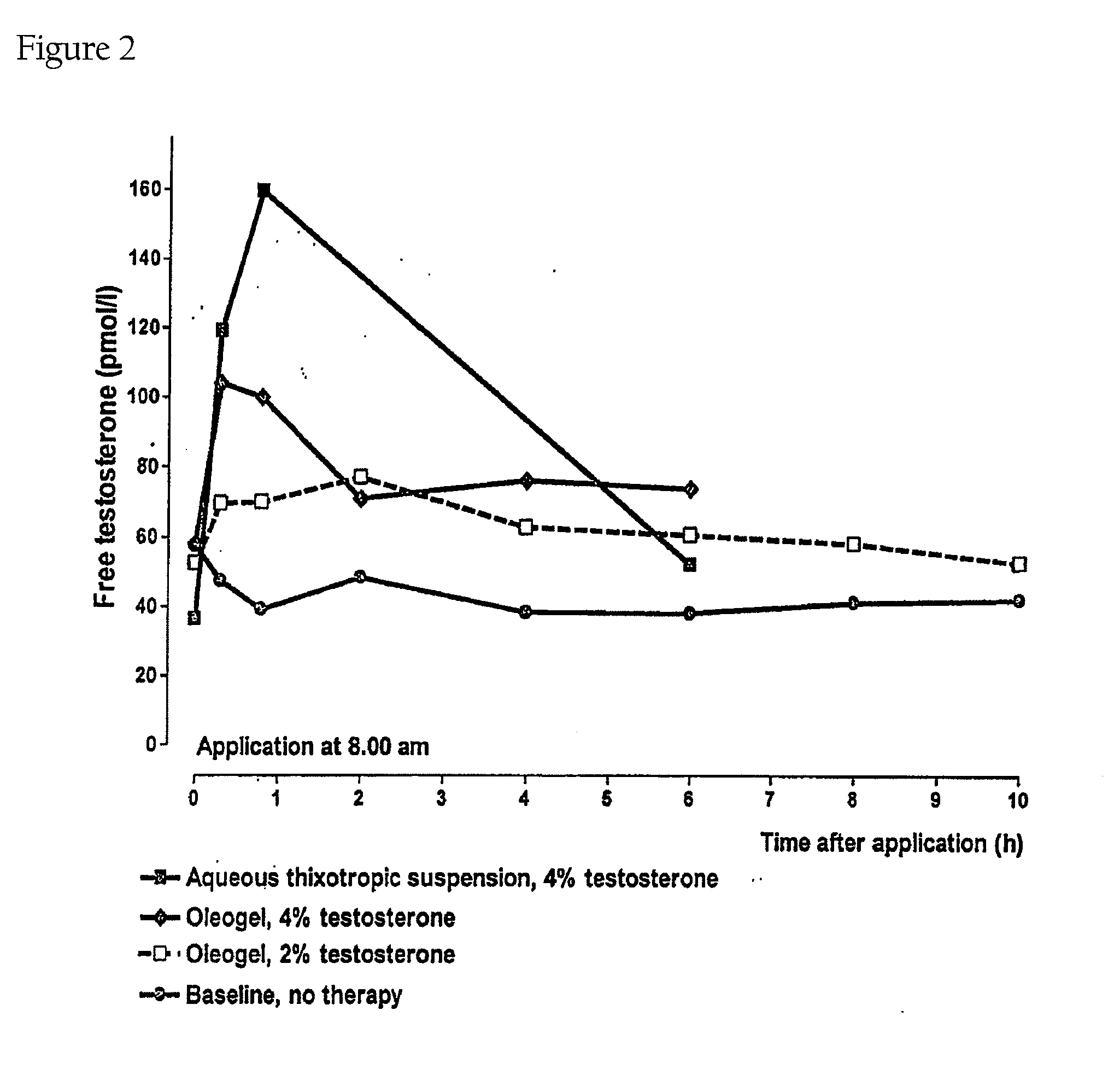
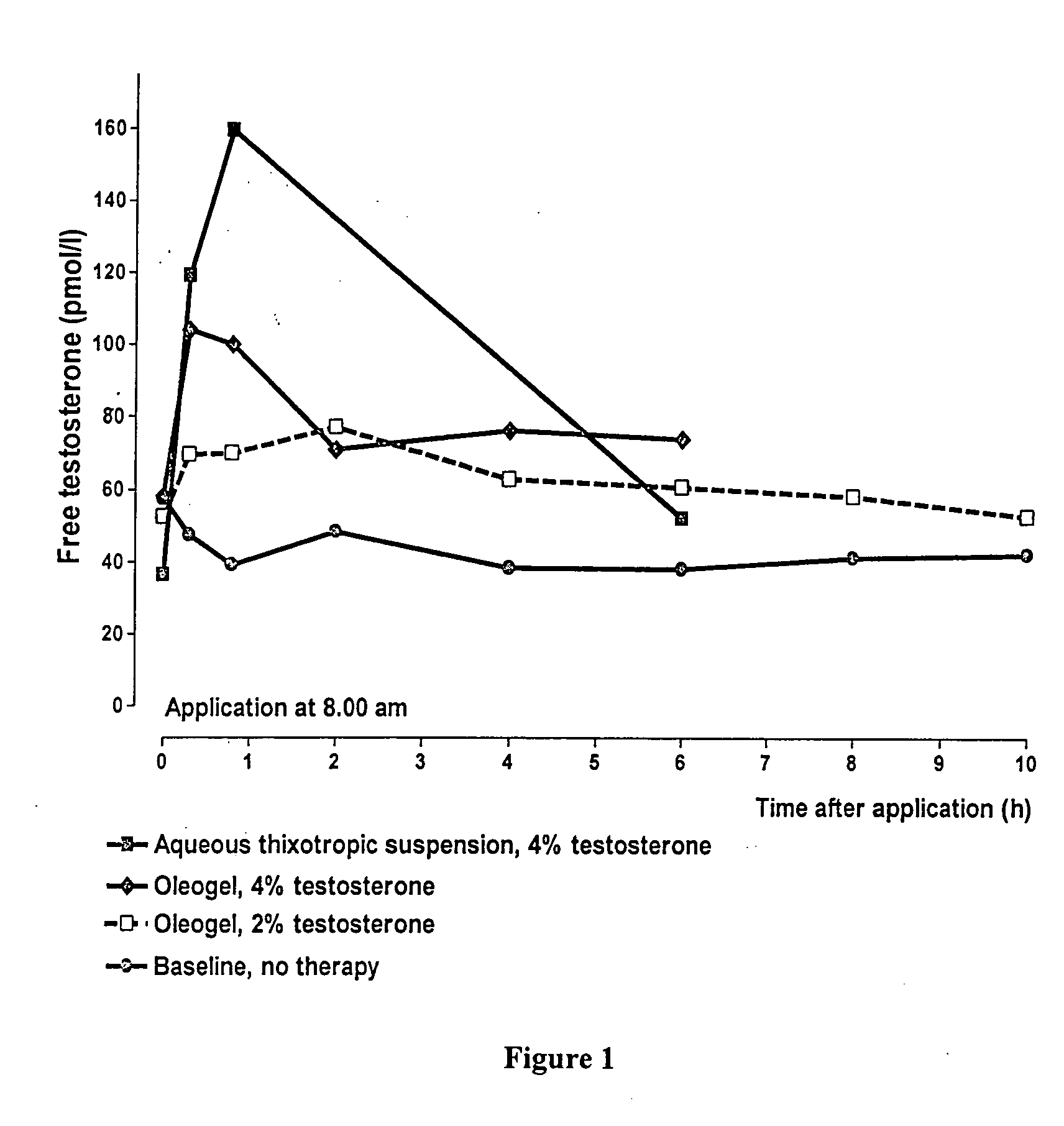
Controlled release topical testosterone formulations and methods
InactiveUS20150297733A1Constant effective testosterone blood levelEasy to useOrganic active ingredientsInorganic non-active ingredientsBlood levelControlled release
The present invention relates to testosterone topical formulations, especially high testosterone concentration formulations, such as between about 6% to about 15% w / w or higher, for the controlled release of testosterone into the systemic circulation of males and females for providing constant effective testosterone blood levels, without inducing undesired testosterone spike in blood levels or testosterone transference, following topical administration. The present invention also relates to methods and pre-filled multi-dose airless applicator systems for pernasal administration of the nasal testosterone gels of the present invention
Owner:ACERUS BIOPHARMA INC
Use of a VEGF Antagonist in Treating Retinopathy of Prematurity
InactiveUS20160159893A1Stopping abnormal blood vessel growthExtended half-lifeLaser surgerySenses disorderDiseaseRetina
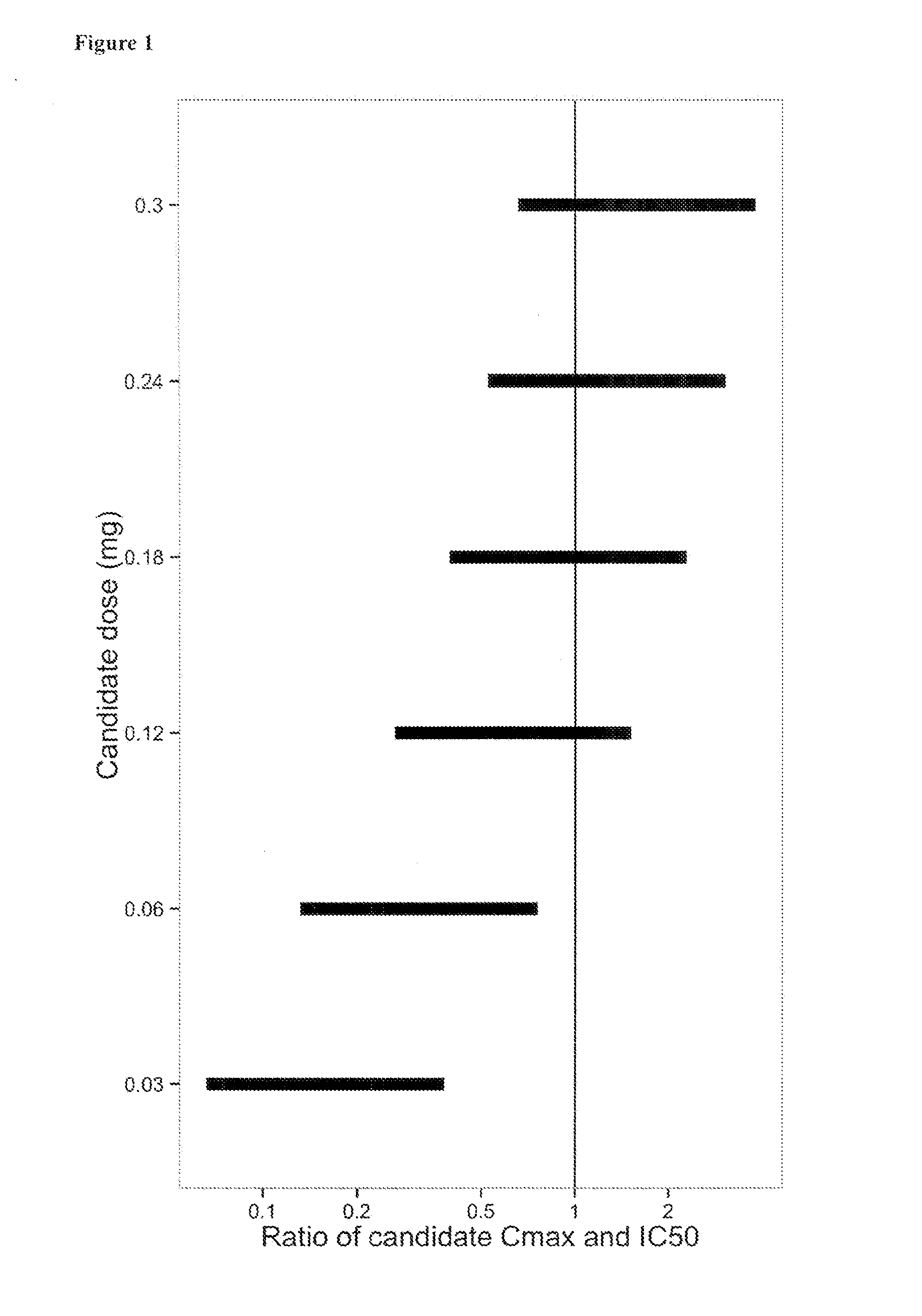
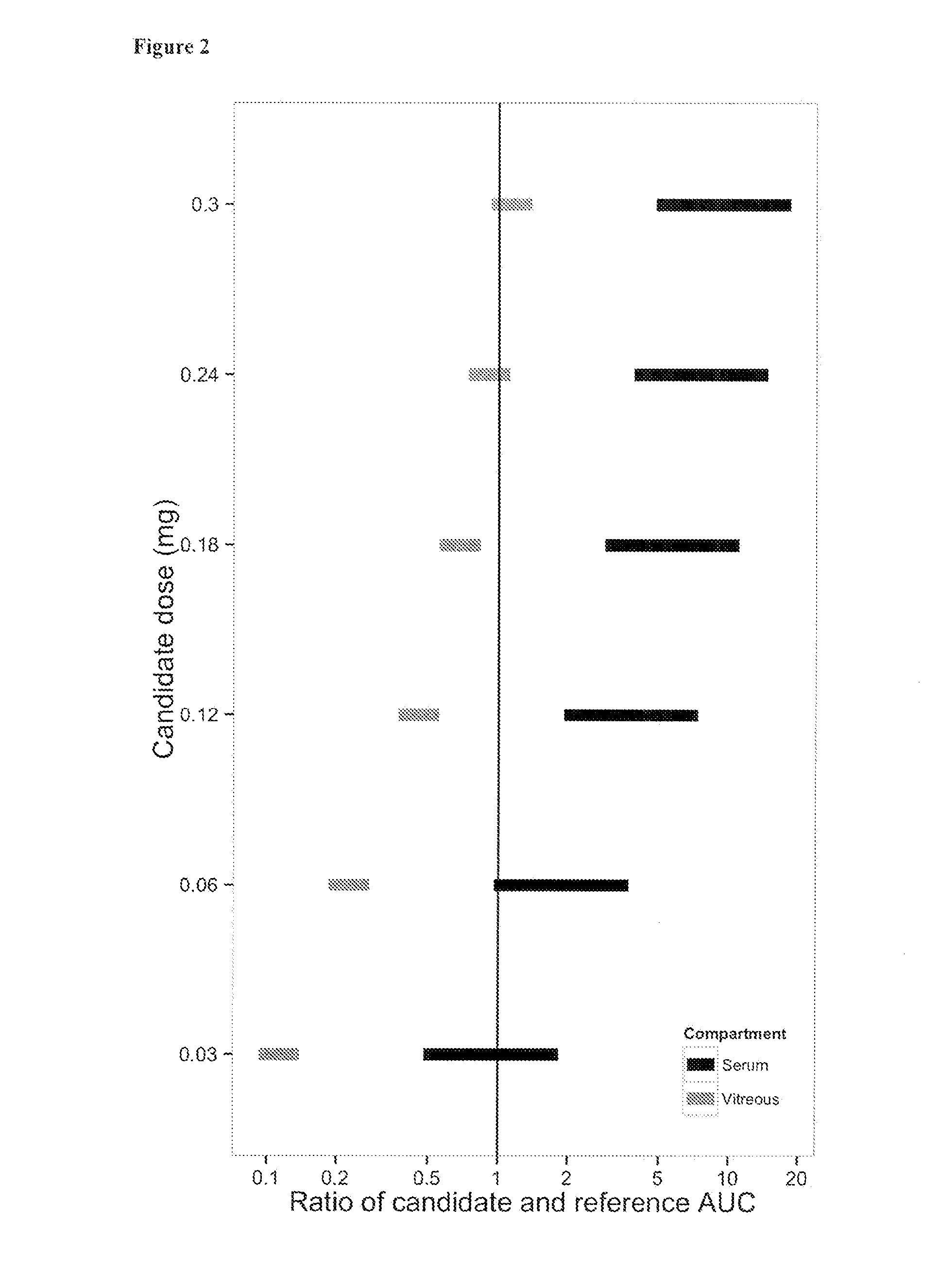
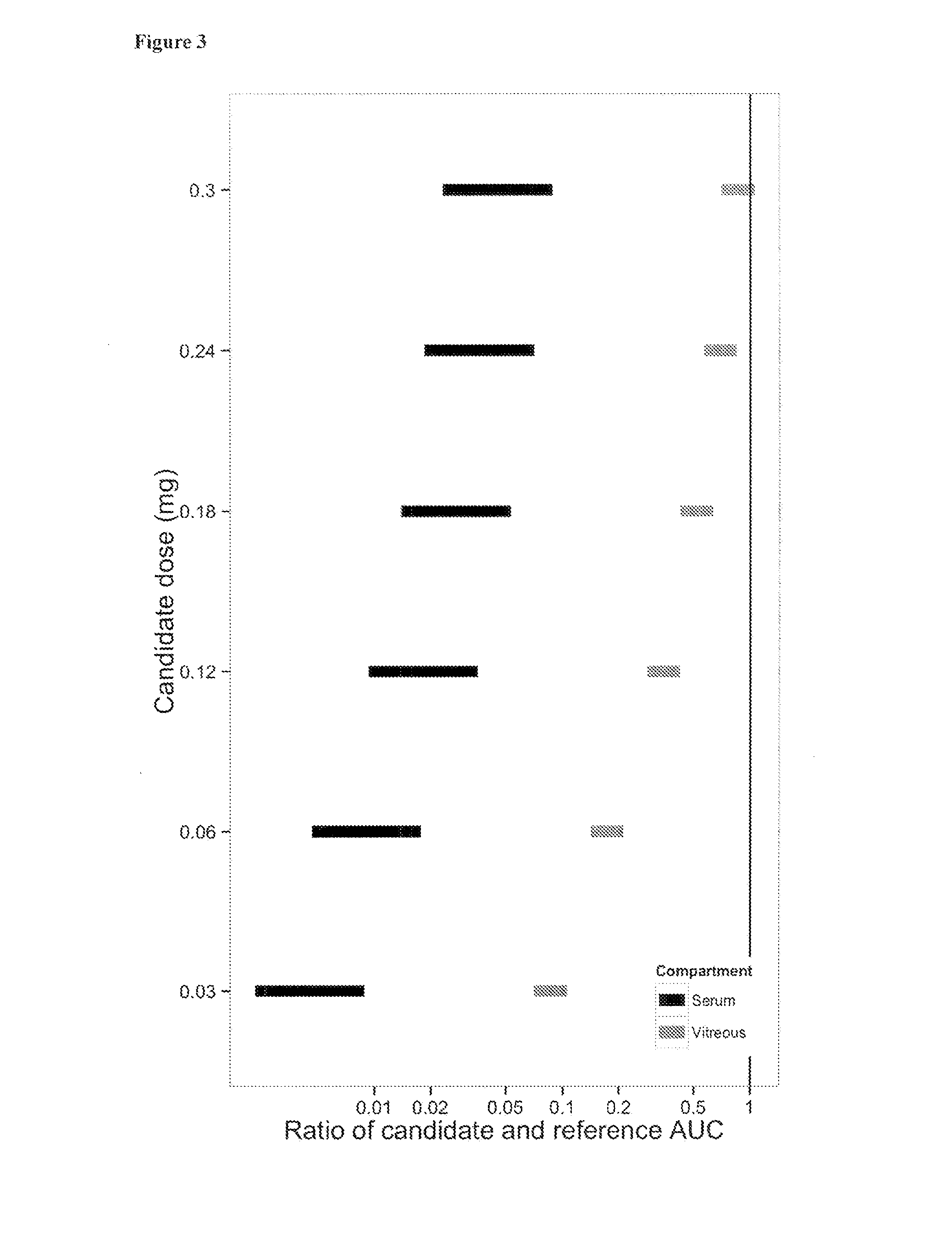
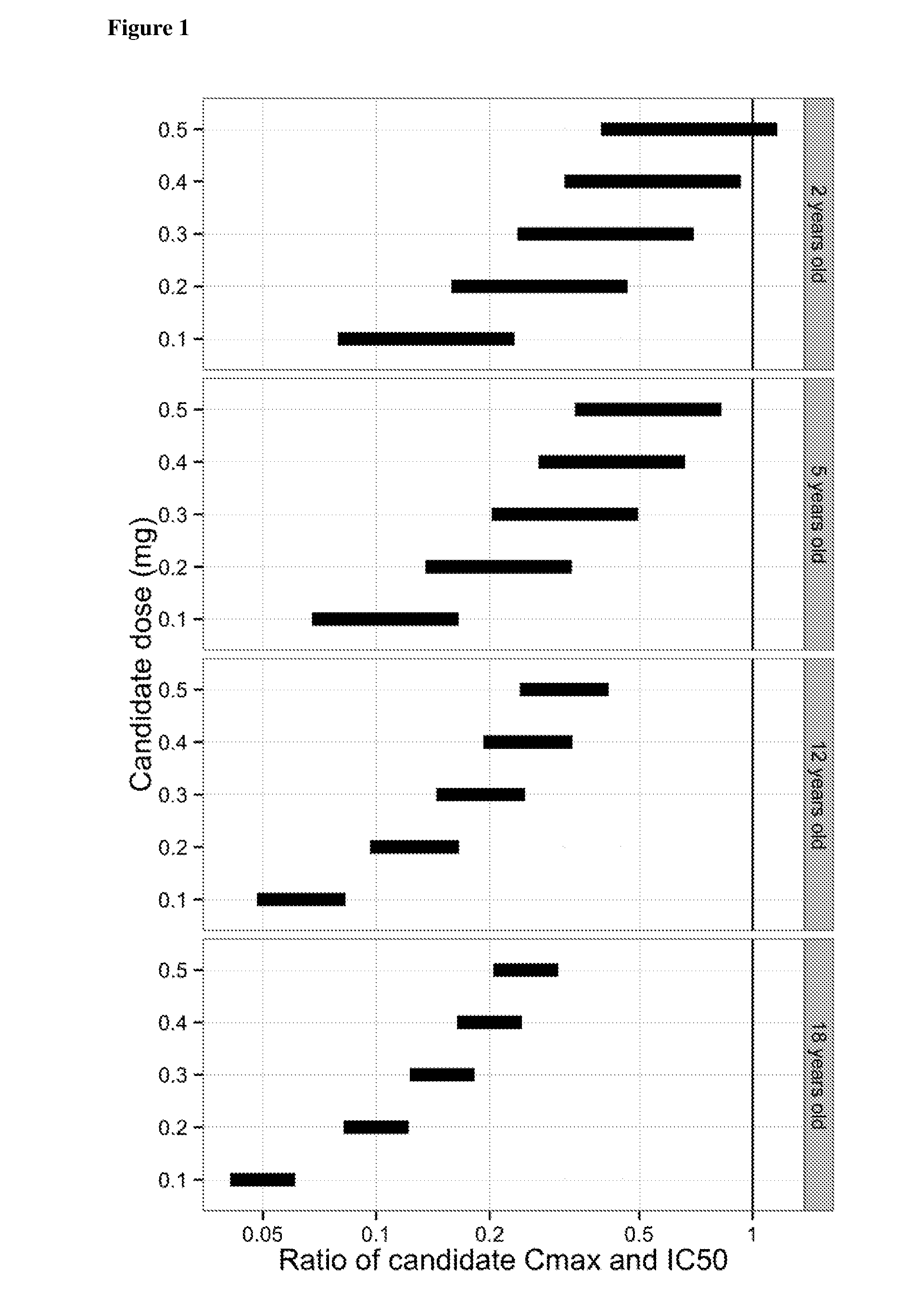
The present invention relates to the use of a VEGF antagonist in the treatment of retinal neovascular disorders in infants. In particular, the invention provides a method for treating an infant having retinopathy of prematurity (ROP), wherein said method comprises administering to the eye of an infant a VEGF antagonist that either does not enter or is rapidly cleared from the systemic circulation. The term “infant” is typically used to refer to young children from birth up to the age of 12 months. The VEGF antagonist may be administered intravitreally, e.g. through injection, or topically, e.g. in form of eye drops.
Owner:NOVARTIS AG
Use of VEGF antagonist in treating chorioretinal neovascular and permeability disorders in paediatric patients
InactiveUS20160168240A1Minimize handlingExtended maintenance periodLaser surgerySenses disorderDiseasePaediatric patients
The present invention relates to the use of a VEGF antagonist in the treatment of chorioretinal neovascular or permeability disorders in children. In particular, the invention provides a VEGF antagonist for use in a method for treating a child having CNV or ME, wherein said method comprises administering to the eye of a child a VEGF antagonist that either does not enter or is rapidly cleared from the systemic circulation. The VEGF antagonist may be administered intravitreally, e.g. through injection, or topically, e.g. in form of eye drops. The invention further provides the use of a VEGF antagonist in the manufacture of a medicament for treating a child having a chorioretinal neovascular or permeability disorder.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com