Patents
Literature
1662results about How to "Improved profile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
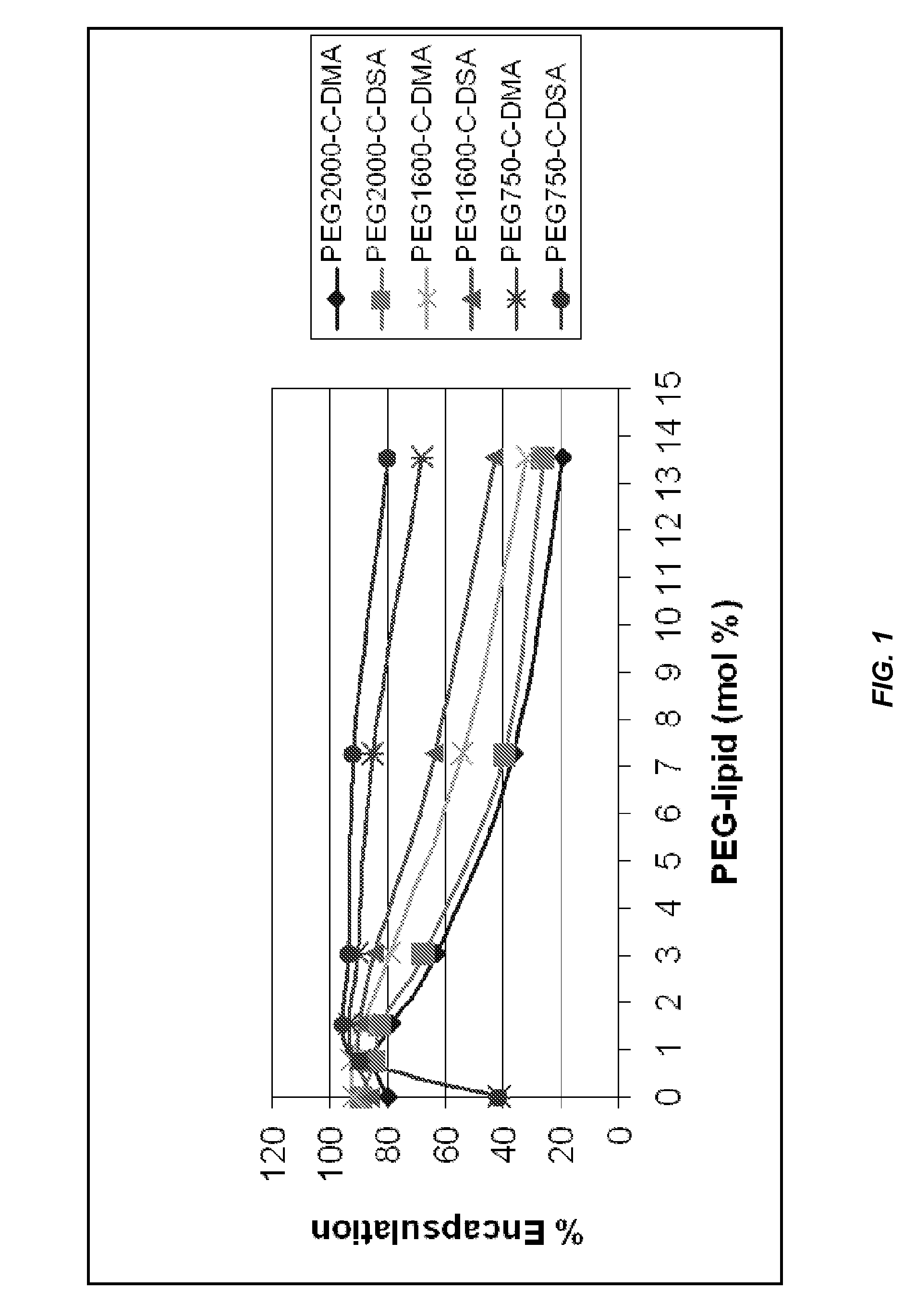
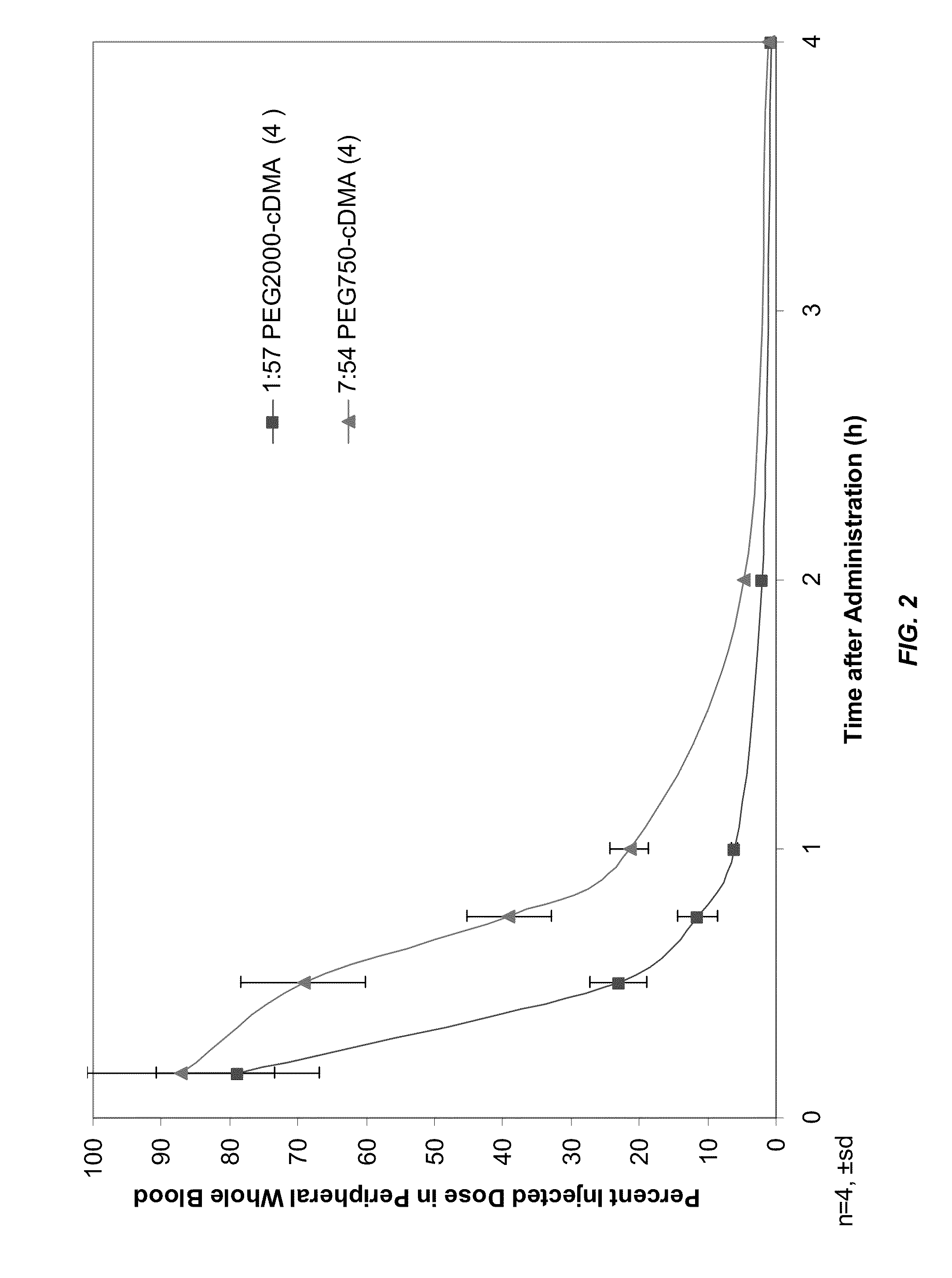
Lipid formulations for nucleic acid delivery
ActiveUS8283333B2Improve effectivenessHigh activityOrganic active ingredientsSugar derivativesLipid particleActive agent
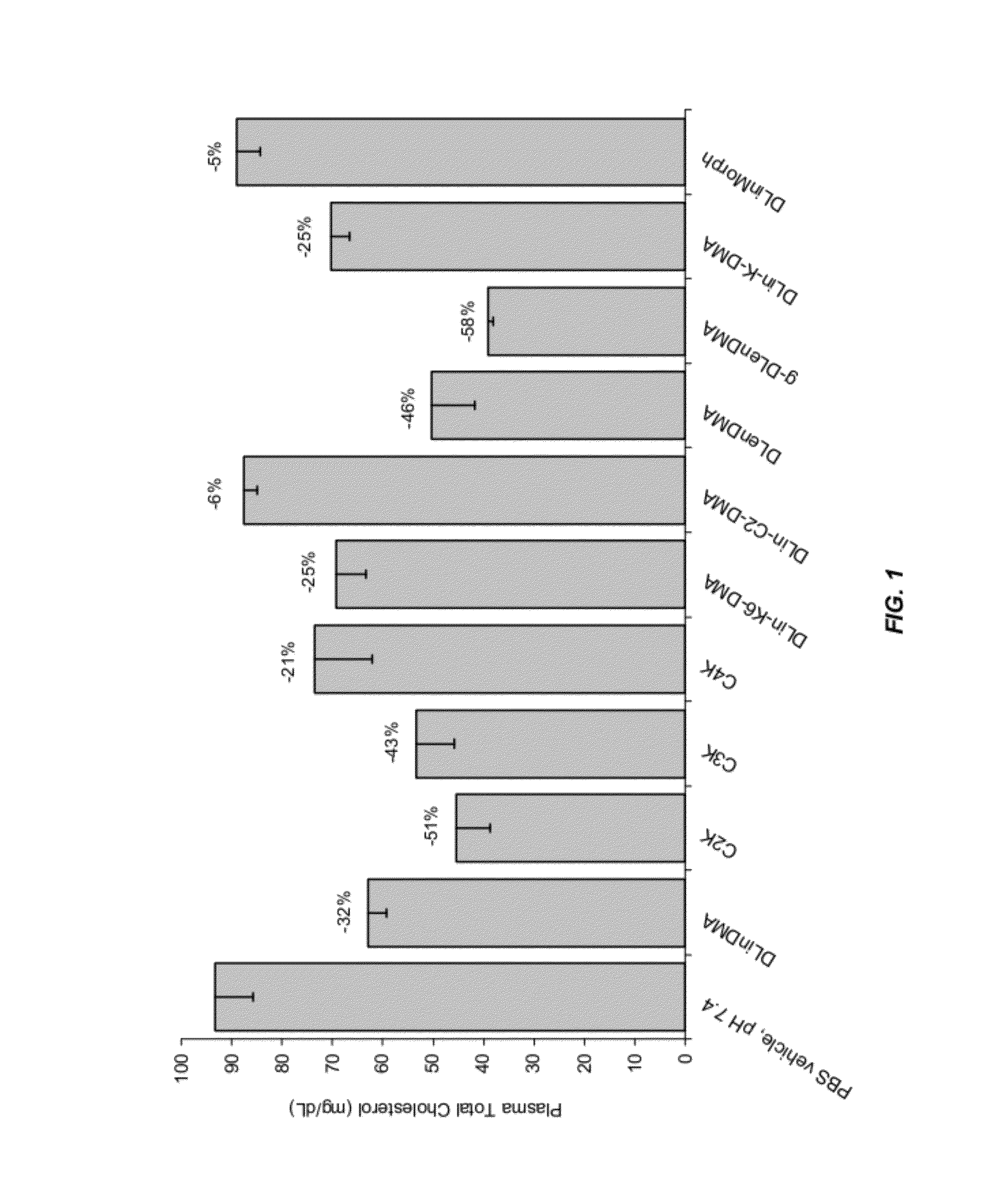
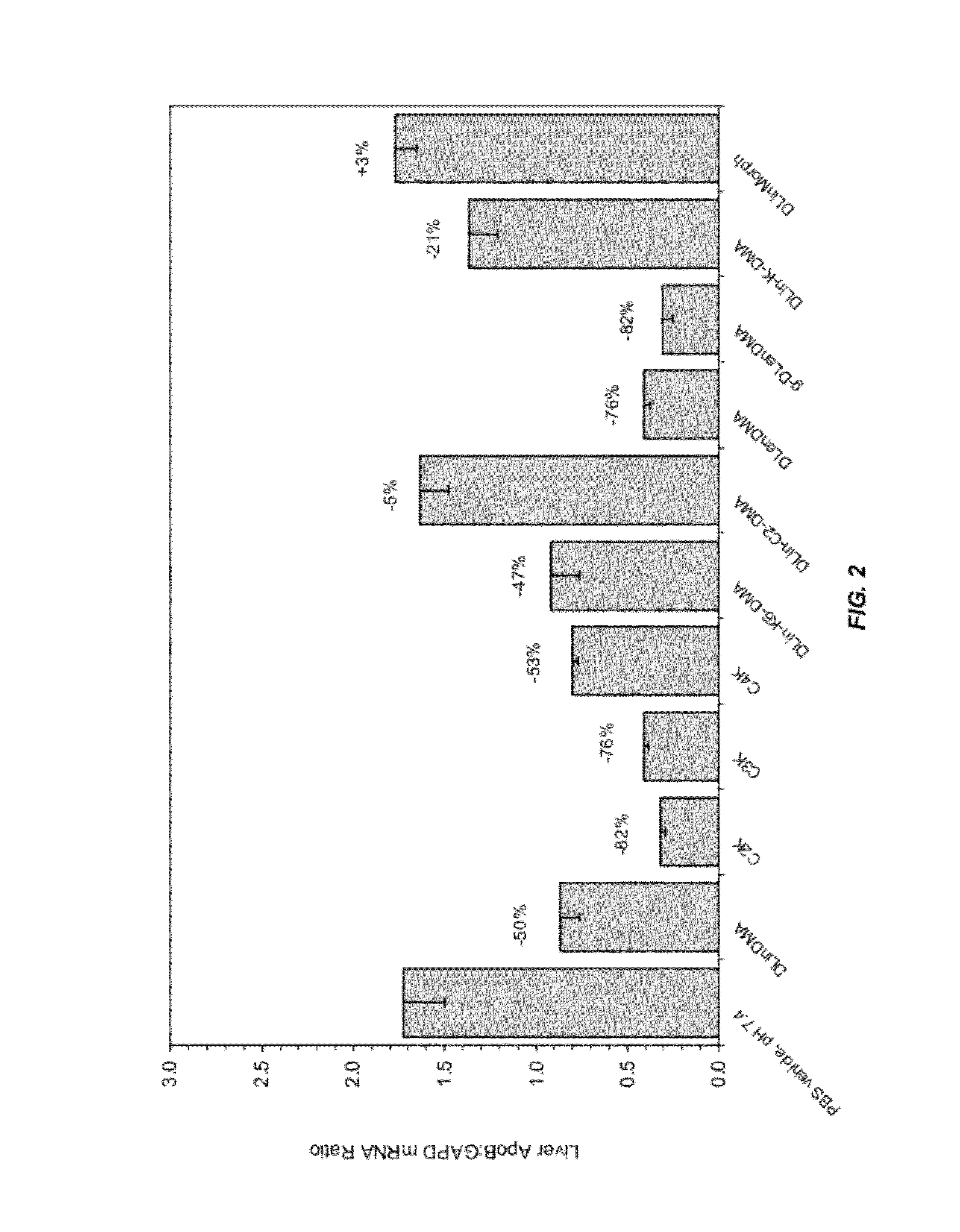
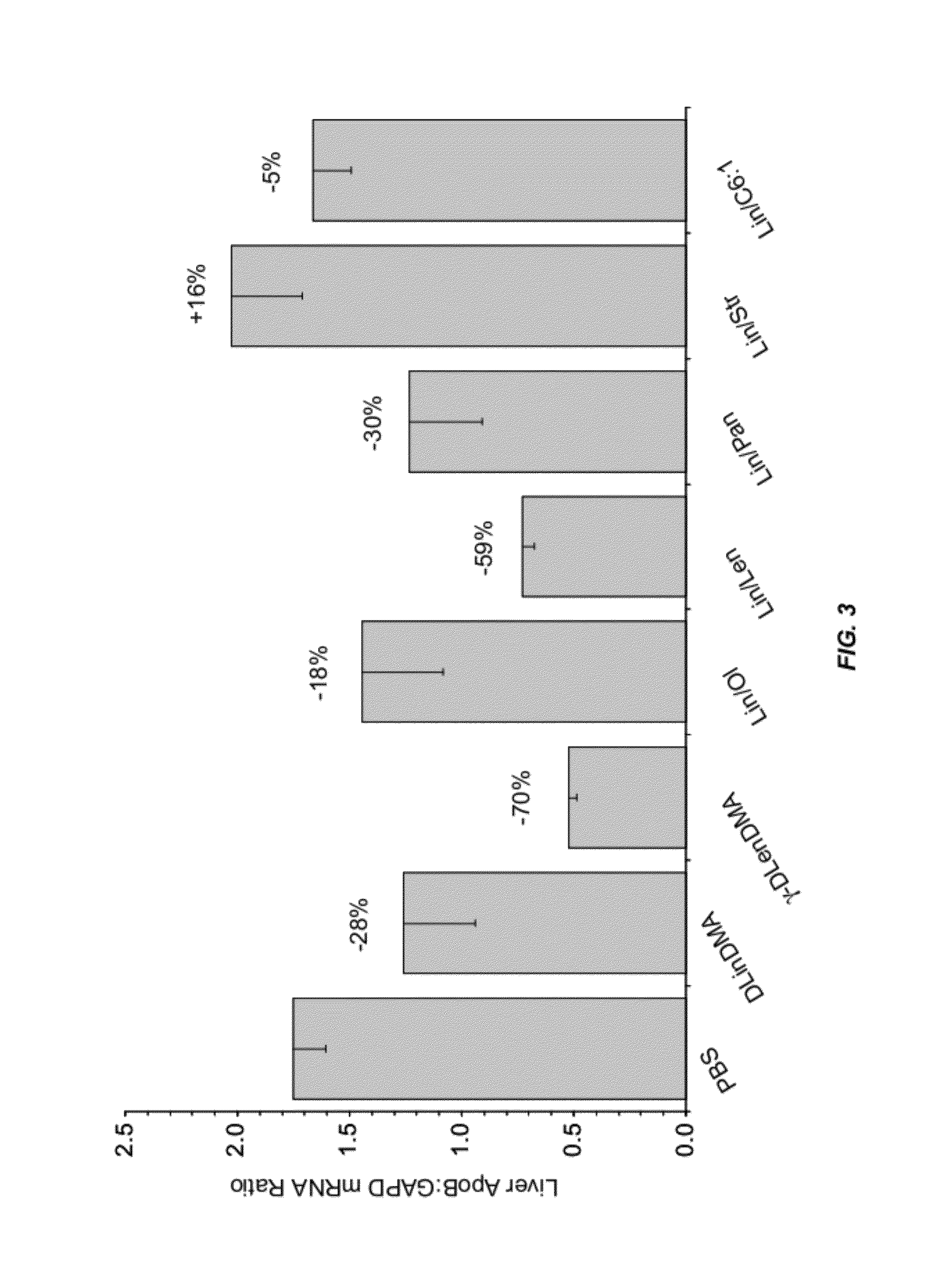
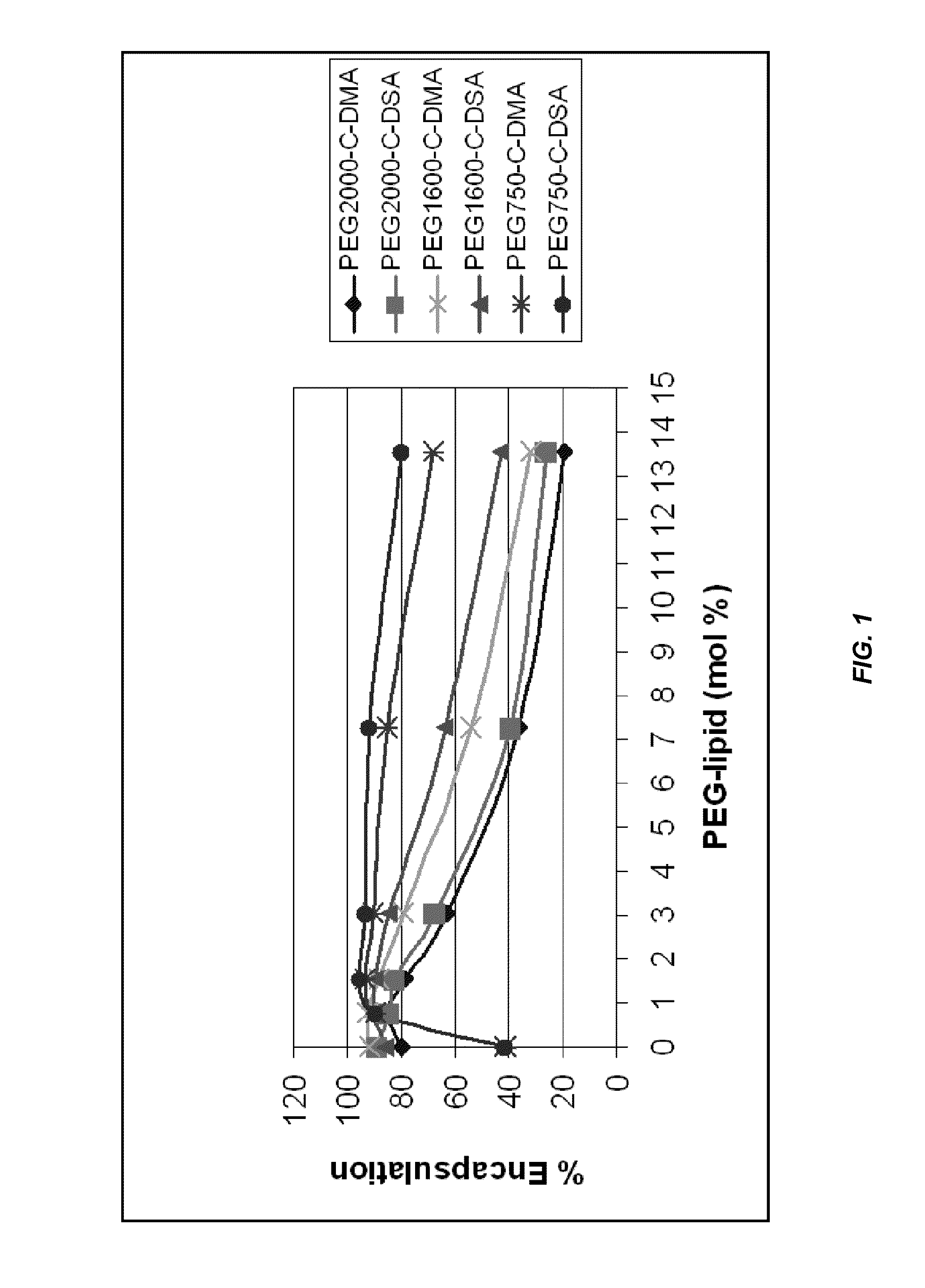
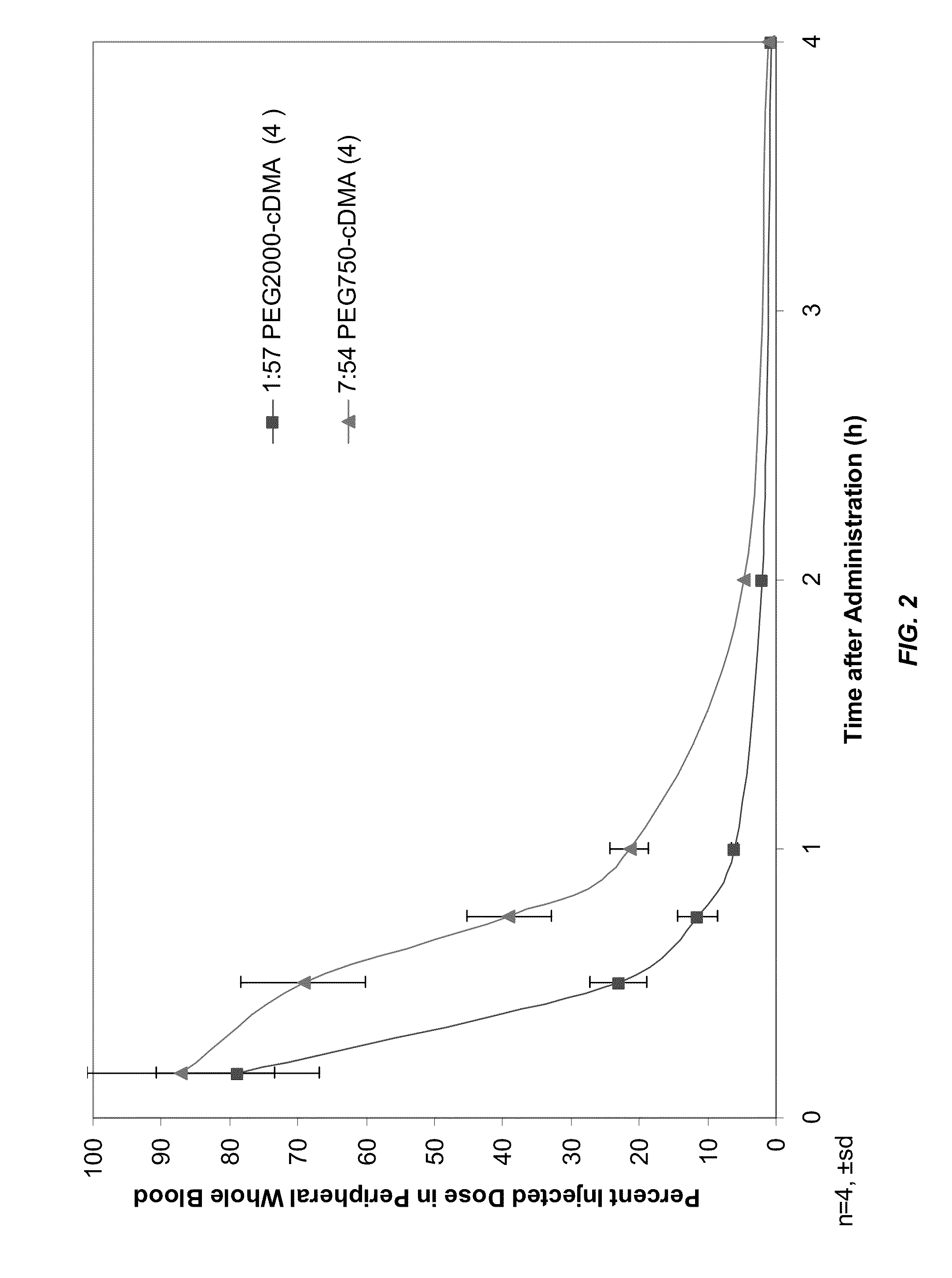
The present invention provides novel, serum-stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides serum-stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (e.g., one or more interfering RNA molecules), methods of making the SNALP, and methods of delivering and / or administering the SNALP (e.g., for the treatment of cancer). In particular embodiments, the present invention provides tumor-directed lipid particles that preferentially target solid tumors. The tumor-directed formulations of the present invention are capable of preferentially delivering a payload such as a nucleic acid to cells of solid tumors compared to non-cancerous cells.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
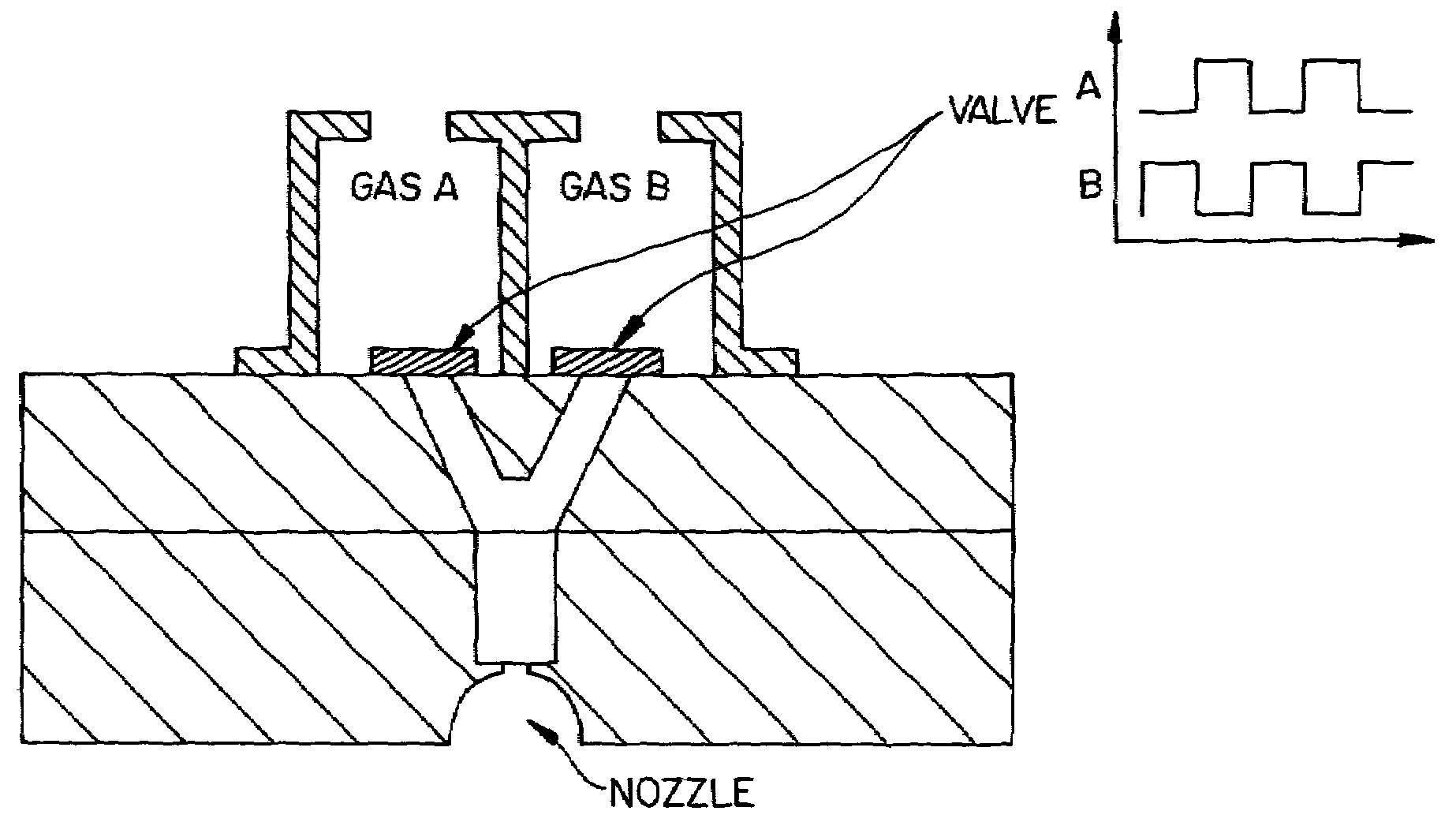
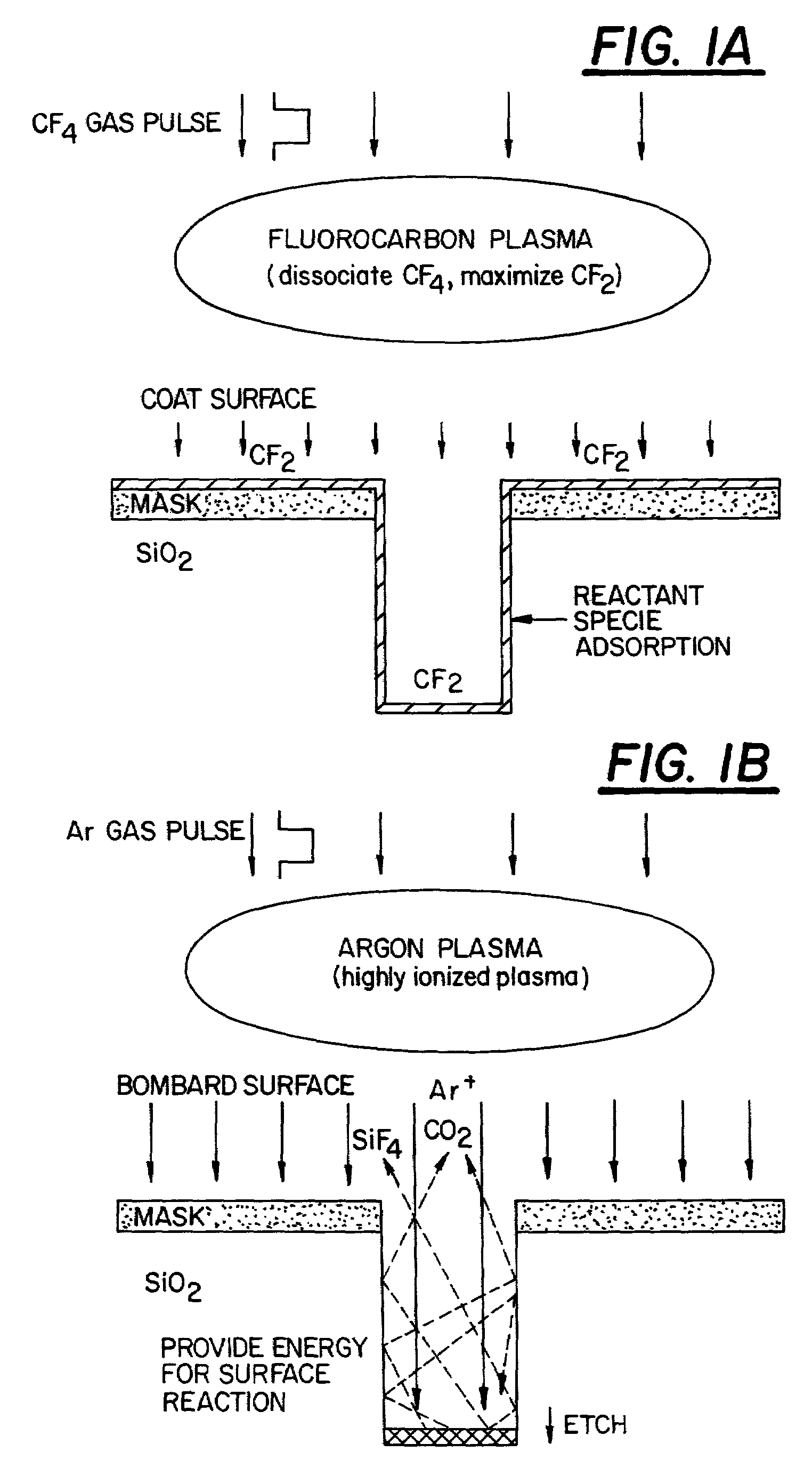
Pulsed plasma processing method and apparatus
InactiveUS7166233B2Remove restrictionsImproved profileDecorative surface effectsVacuum evaporation coatingEngineeringPlasma processing
In a method for performing a plasma-assisted treatment on a substrate in a reactor chamber by: introducing at least one process gas into the reactor chamber; and creating a plasma within the reactor chamber by establishing an RF electromagnetic field within the chamber and allowing the field to interact with the process gas, the electromagnetic field is controlled to have an energy level which varies cyclically between at least two values each sufficient to maintain the plasma, such that each energy level value is associated with performance of a respectively different treatment process on the substrate.
Owner:TOKYO ELECTRON LTD
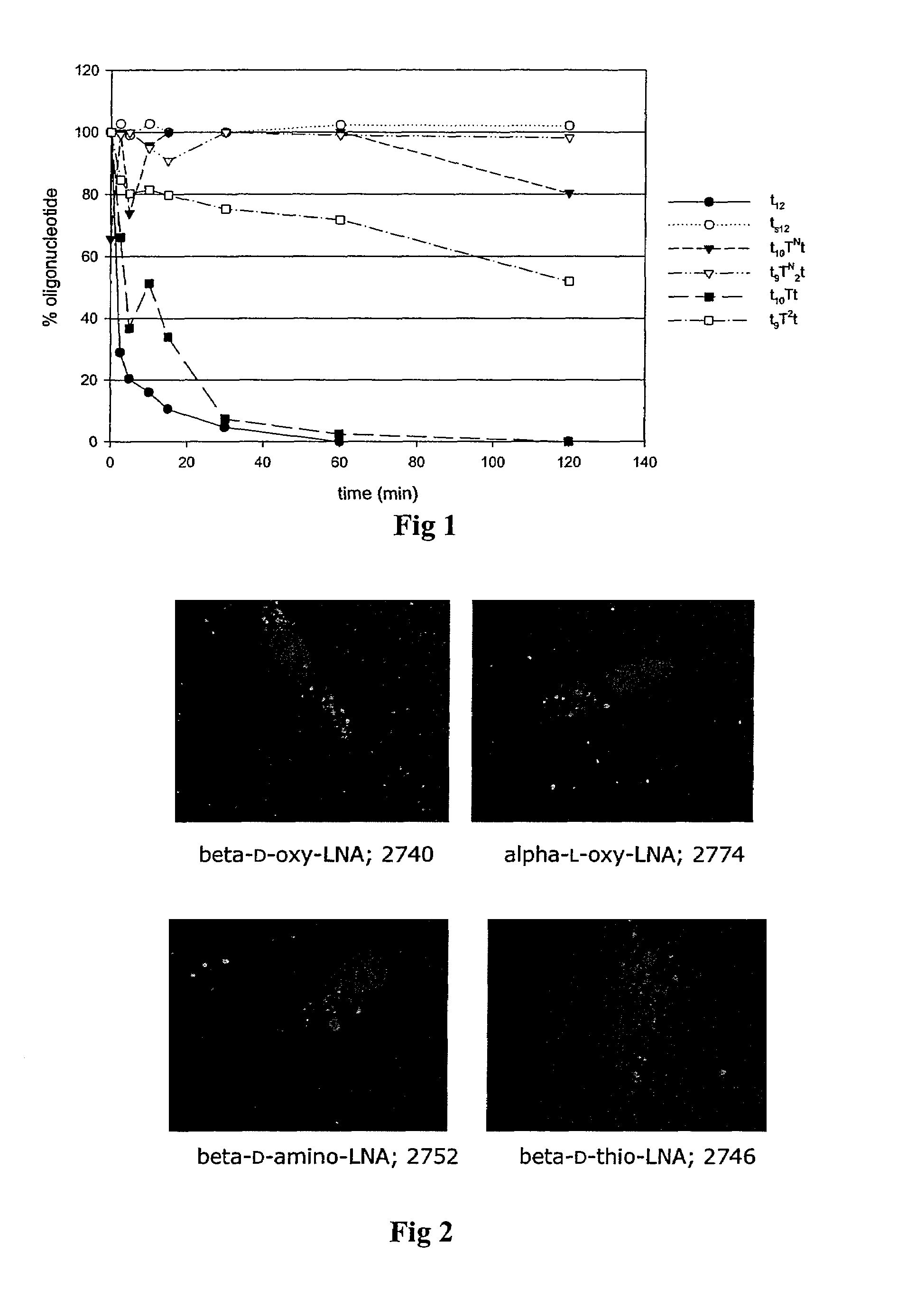
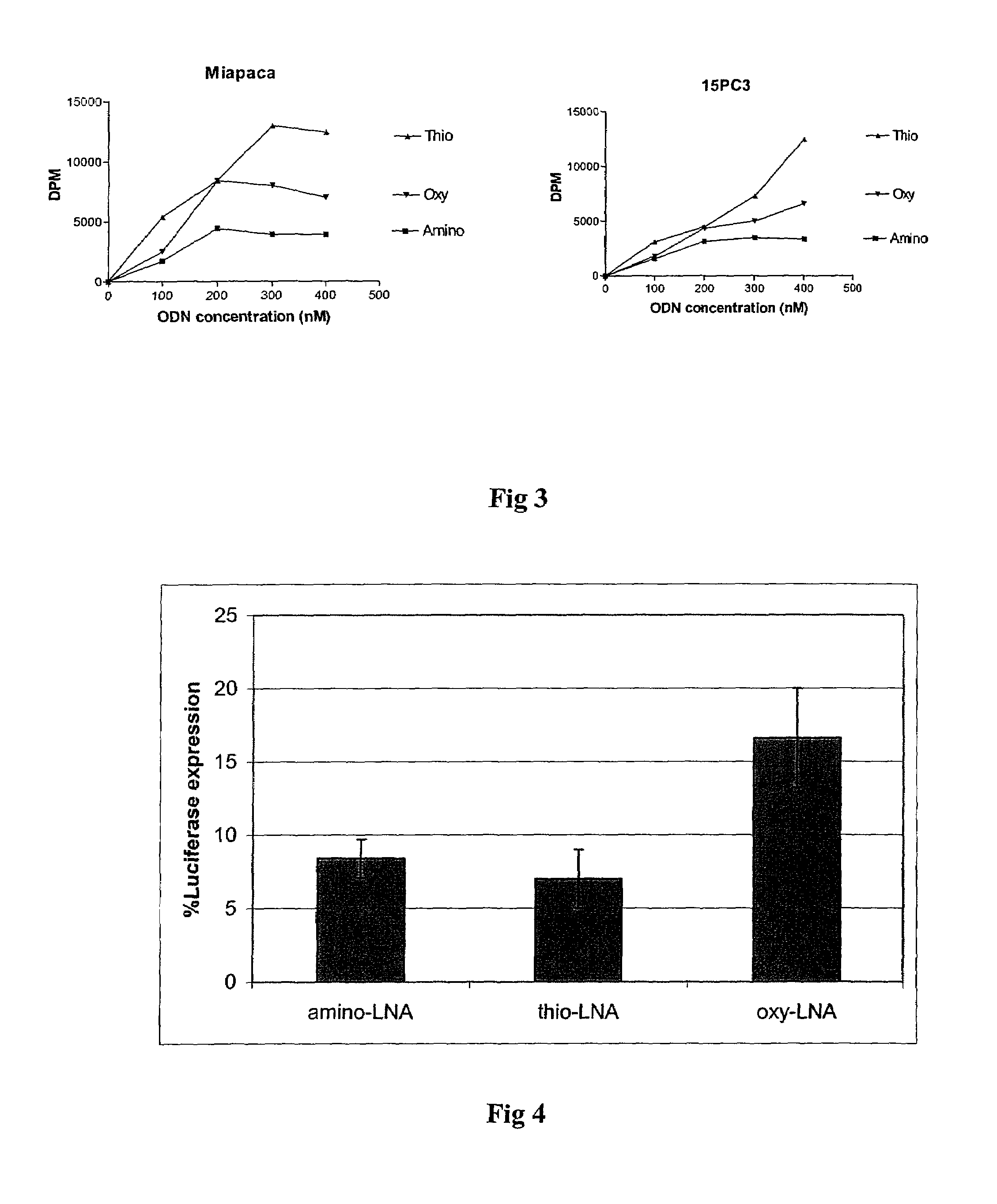
Oligonucleotides with alternating segments of locked and non-locked nucleotides
ActiveUS7687617B2Increased Design PossibilitiesEfficient substrateSugar derivativesActivity regulationNucleotideThio-
The present invention is directed to novel oligonucleotides with improved antisense properties. The novel oligonucleotides comprise at least one Locked Nucleic Acid (LNA) selected from beta-D-thio / amino-LNA or alpha-L-oxy / thio / amino-LNA. The oligonucleotides comprising LNA may also include DNA and / or RNA nucleotides. The present invention also provides a new class of pharmaceuticals which comprise antisense oligonucleotides and are useful in antisense therapy.
Owner:SANTARIS PHARMA AS
Impeller housing for percutaneous heart pump
Disclosed herein are heart pumps that include a catheter assembly and that can be applied percutaneously. Some embodiments include a locking device that prevents components of the catheter assembly from being separated when in use. The catheter assembly can include an expandable tip. In some embodiments, the catheter assembly includes a housing having a wall structure, a portion of which can have a bulbuous shape or can be deformable. In other embodiments, the housing can be configured to reduce fluttering or deflection of the housing and / or to maintain a gap between the housing and an impeller blade disposed therein.
Owner:PENN STATE RES FOUND +1
Compositions and methods for silencing apolipoprotein B
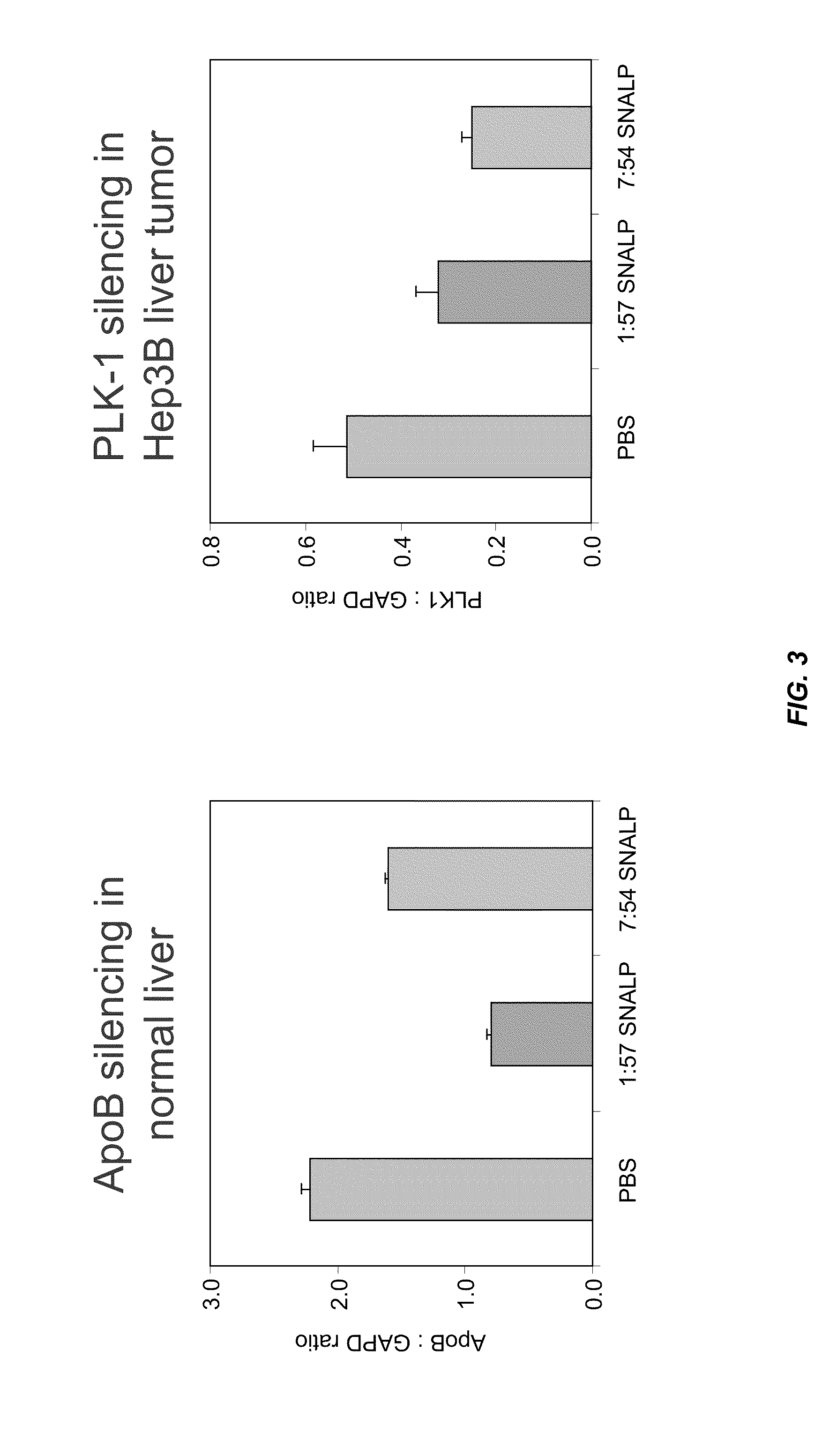
InactiveUS8236943B2Improve effectivenessHigh activityOrganic active ingredientsNanotechLipid particleApolipoproteins b
The present invention provides compositions and methods for the delivery of interfering RNAs that silence APOB expression to liver cells. In particular, the nucleic acid-lipid particles provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of APOB at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
High-potency sweetener for hydration and sweetened hydration composition
InactiveUS20070116823A1Improve flavor profileImproving temporal profile profileMetabolism disorderFood preparationAdditive ingredientSweetness
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic high potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as a hydration product. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
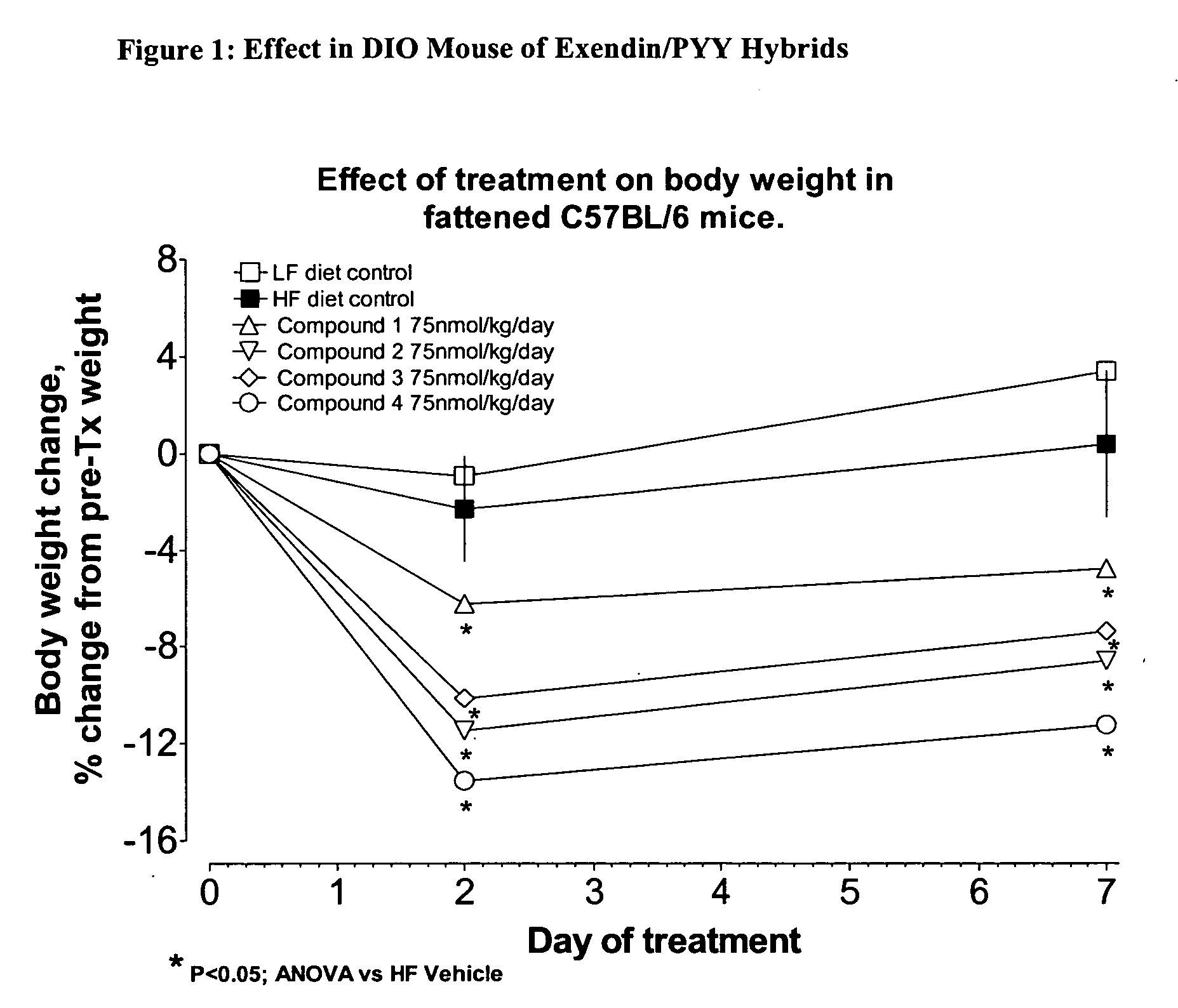
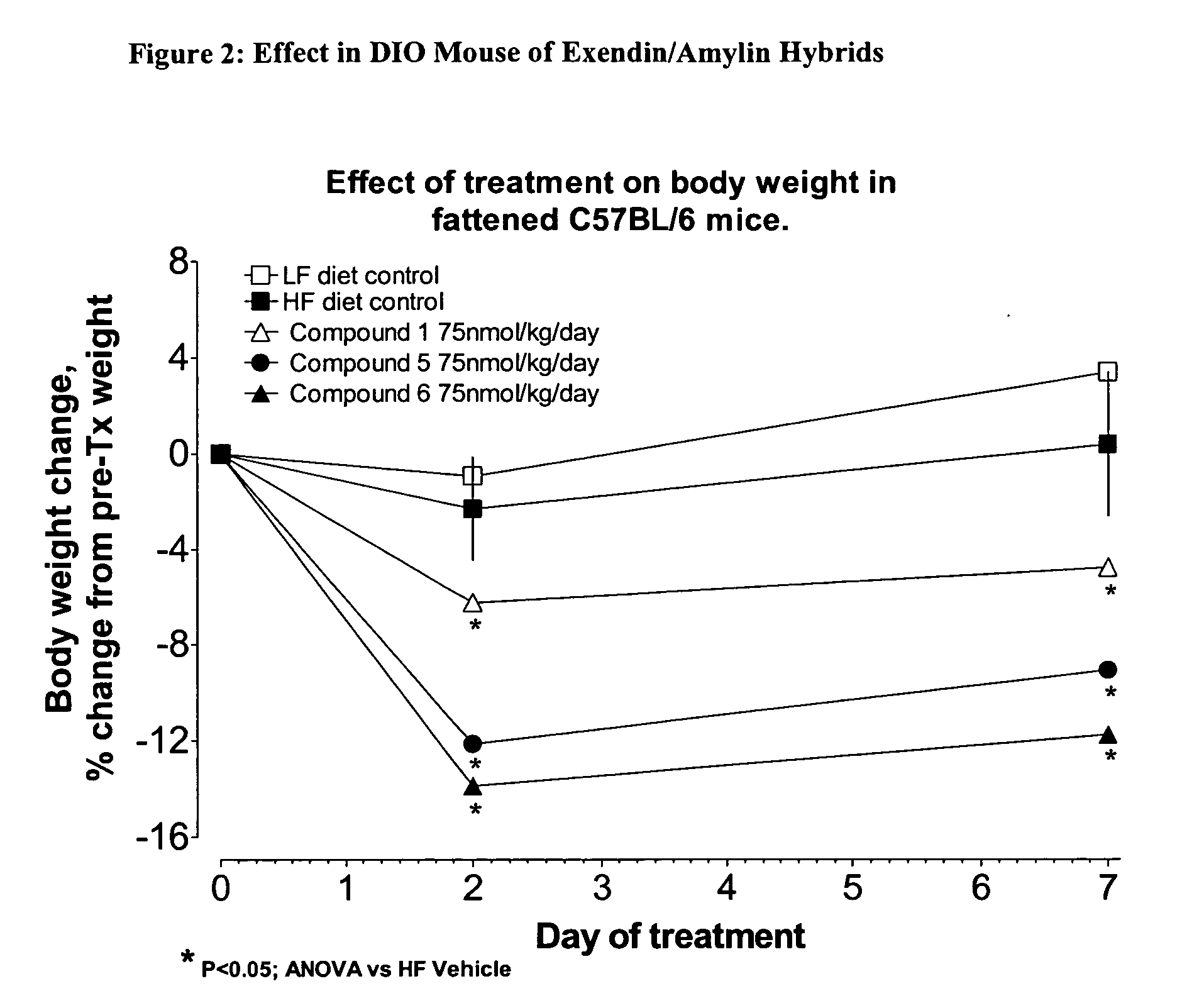
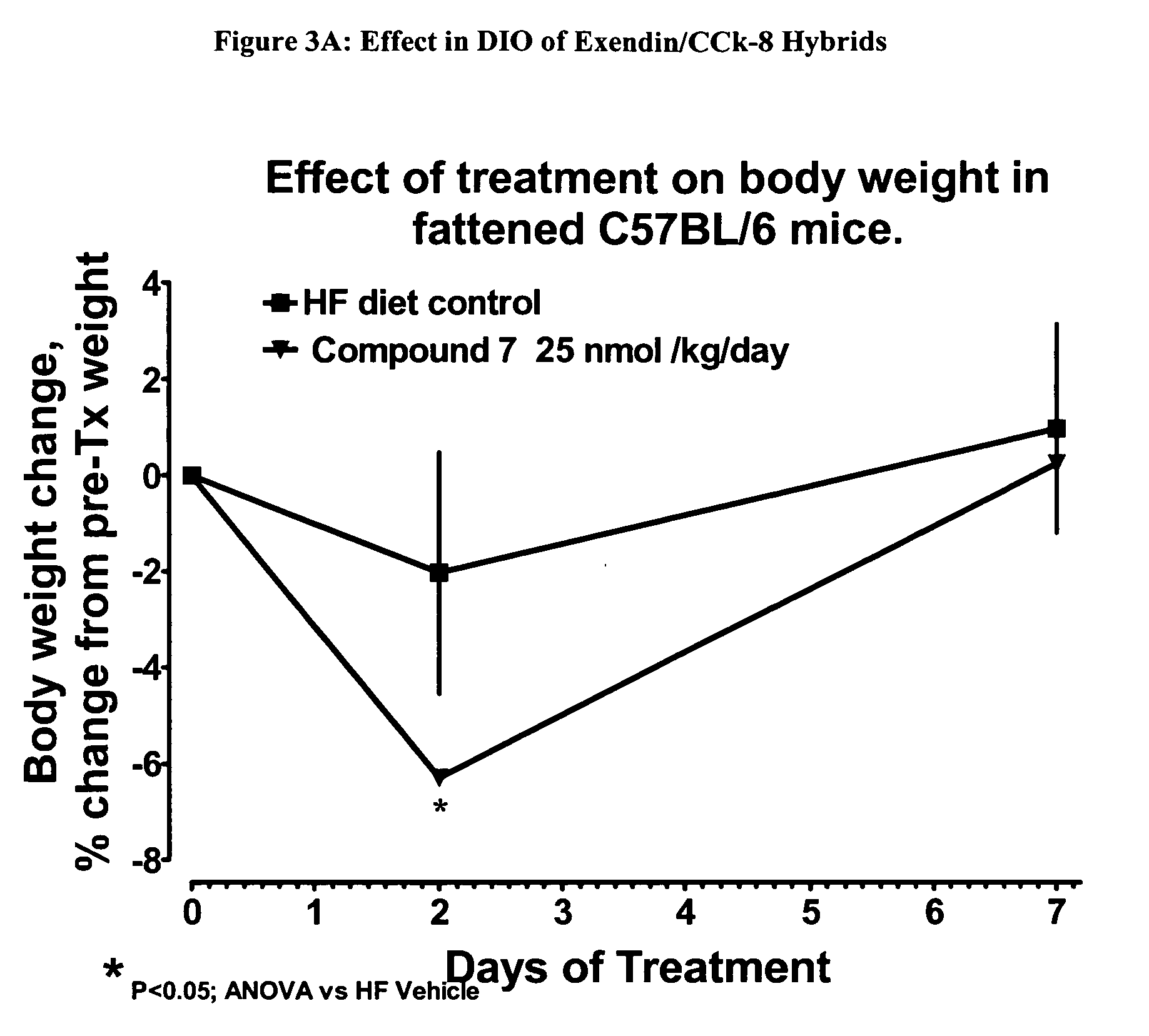
Hybrid polypeptides with selectable properties
ActiveUS20060293232A1Reverse glucose intoleranceIncrease massNervous disorderPeptide/protein ingredientsBlood lipidsGestational diabetes
The present invention relates generally to novel, selectable hybrid polypeptides useful as agents for the treatment and prevention of metabolic diseases and disorders which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, such as diabetes and diabetes-related conditions. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
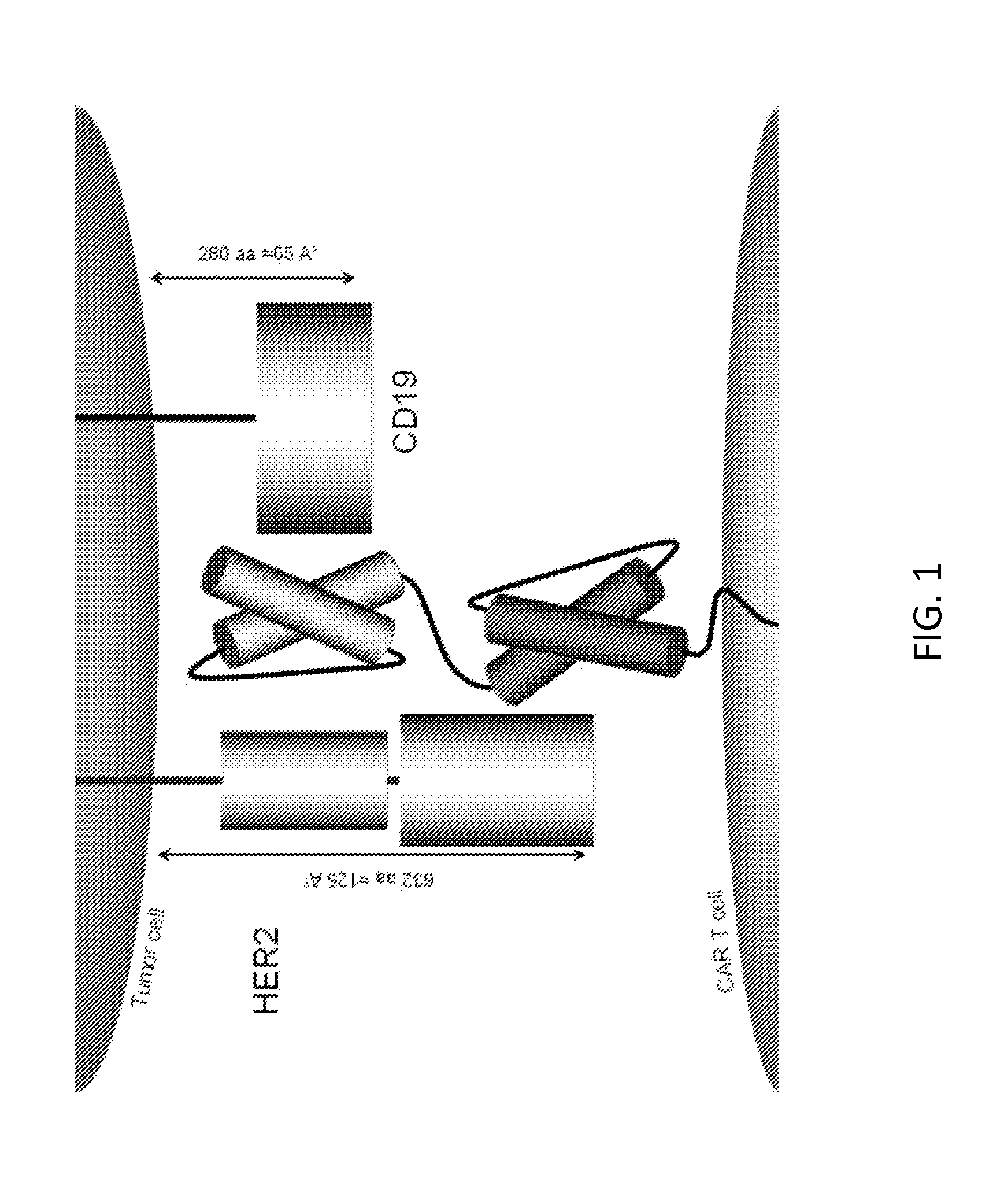
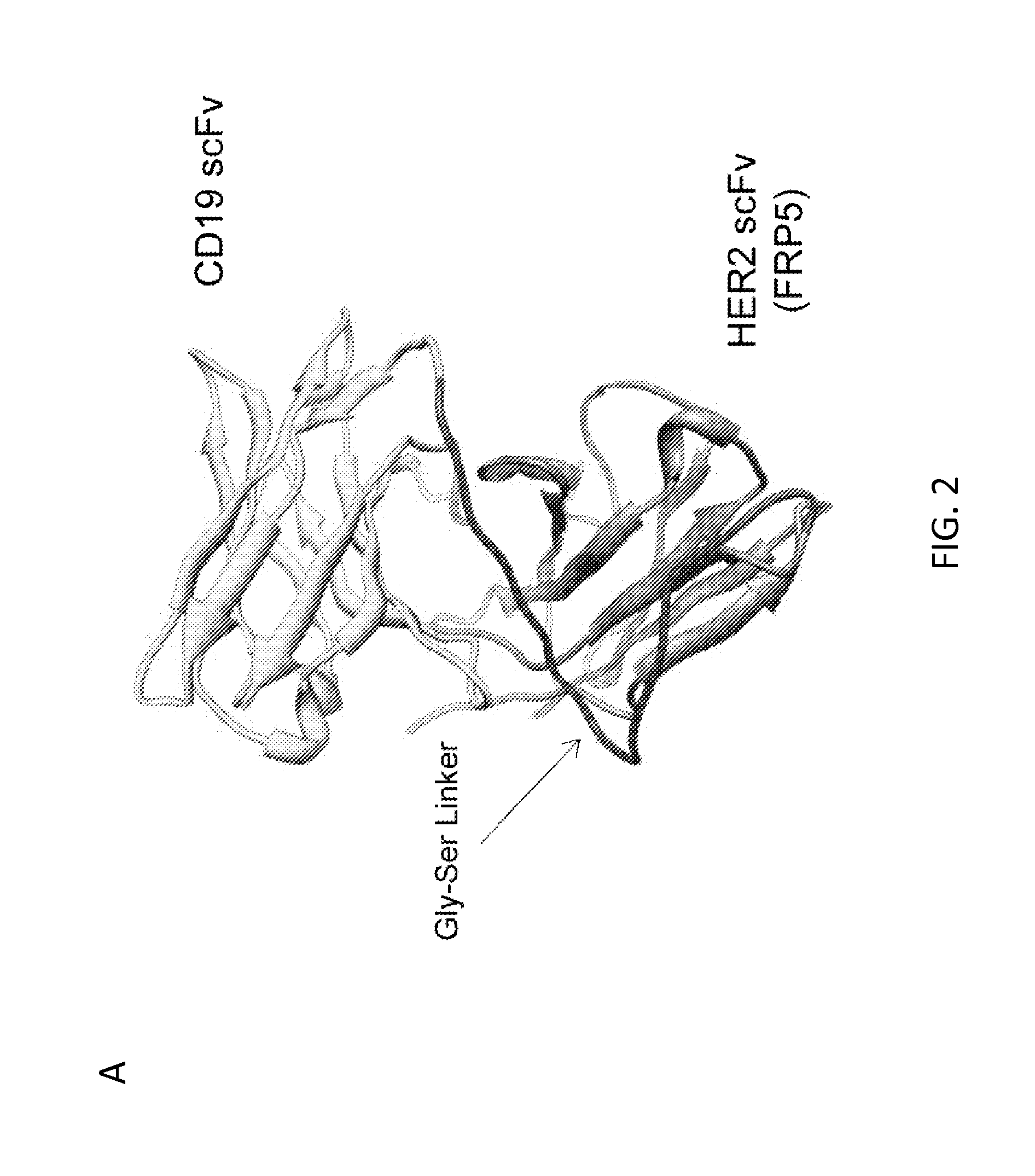
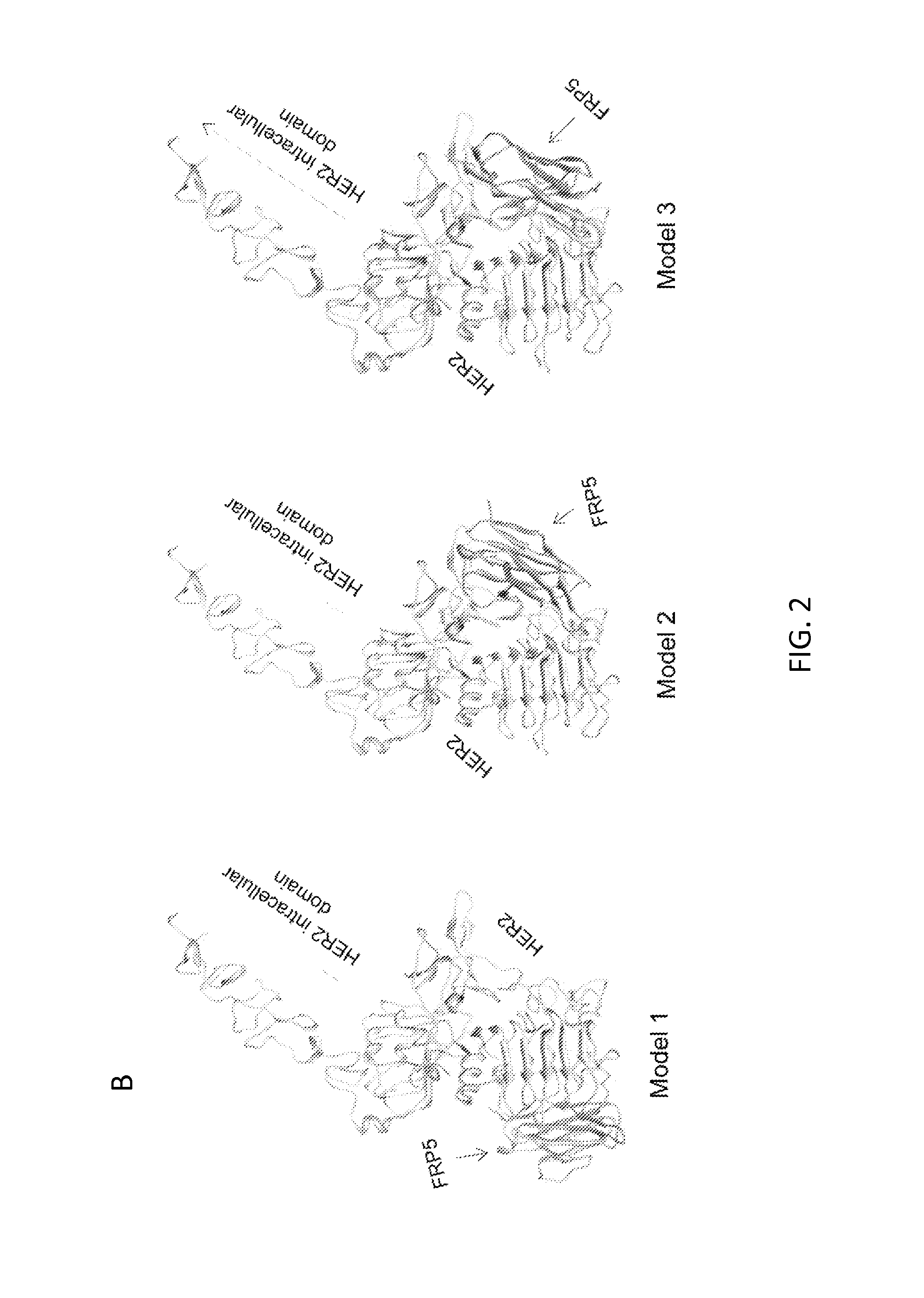
Chimeric antigen receptor for bispecific activation and targeting of t lymphocytes
InactiveUS20130280220A1Increase T cell activationEffectively offsetting tumor escapeBiocideGenetic material ingredientsLymphocyteT lymphocyte
Embodiments of the invention include methods and compositions related to improved cells encoding a chimeric antigen receptor that is specific for two or more antigens. In certain aspects the receptor encompasses two or more non-identical antigen recognition domains. The antigens are tumor antigens, in particular embodiments.
Owner:BAYLOR COLLEGE OF MEDICINE
High-Potency Sweetener Composition With Antioxidant and Compositions Sweetened Therewith
ActiveUS20070116838A1Improve flavor profileImproving temporal profile profileCosmetic preparationsDispersion deliveryAdditive ingredientAntioxidant
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic, high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic, high-potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as antioxidants. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
Natural High-Potency Tabletop Sweetener Compositions with Improved Temporal and/or Flavor Profile, Methods for Their Formulation, and Uses
ActiveUS20070116828A1Improving temporal profile and flavor profileImproved profileFood ingredientsFood preparationSweetening agentsFood flavor
The present invention relates generally to tabletop sweetener compositions comprising non-caloric or low-caloric natural high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different forms of tabletop sweetener compositions comprising at least one non-caloric or low-caloric natural high-potency sweeteners in combination with at least one bulking agent, or at least one sweet taste improving composition, or at least one anti-caking agent, or combinations thereof. The present invention also relates to tabletop compositions and methods that can improve the tastes of non-caloric or low-caloric natural high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the tabletop compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
Means for Controlled Sealing of Endovascular Devices
Expandable sealing means for endoluminal devices have been developed for controlled activation. The devices have the benefits of a low profile mechanism (for both self-expanding and balloon-expanding prostheses), contained, not open, release of the material, active conformation to the “leak sites” such that leakage areas are filled without disrupting the physical and functional integrity of the prosthesis, and on-demand, controlled activation, that may not be pressure activated.
Owner:ENDOLUMINAL SCI
Method for preventing a metal corrosion in a semiconductor device
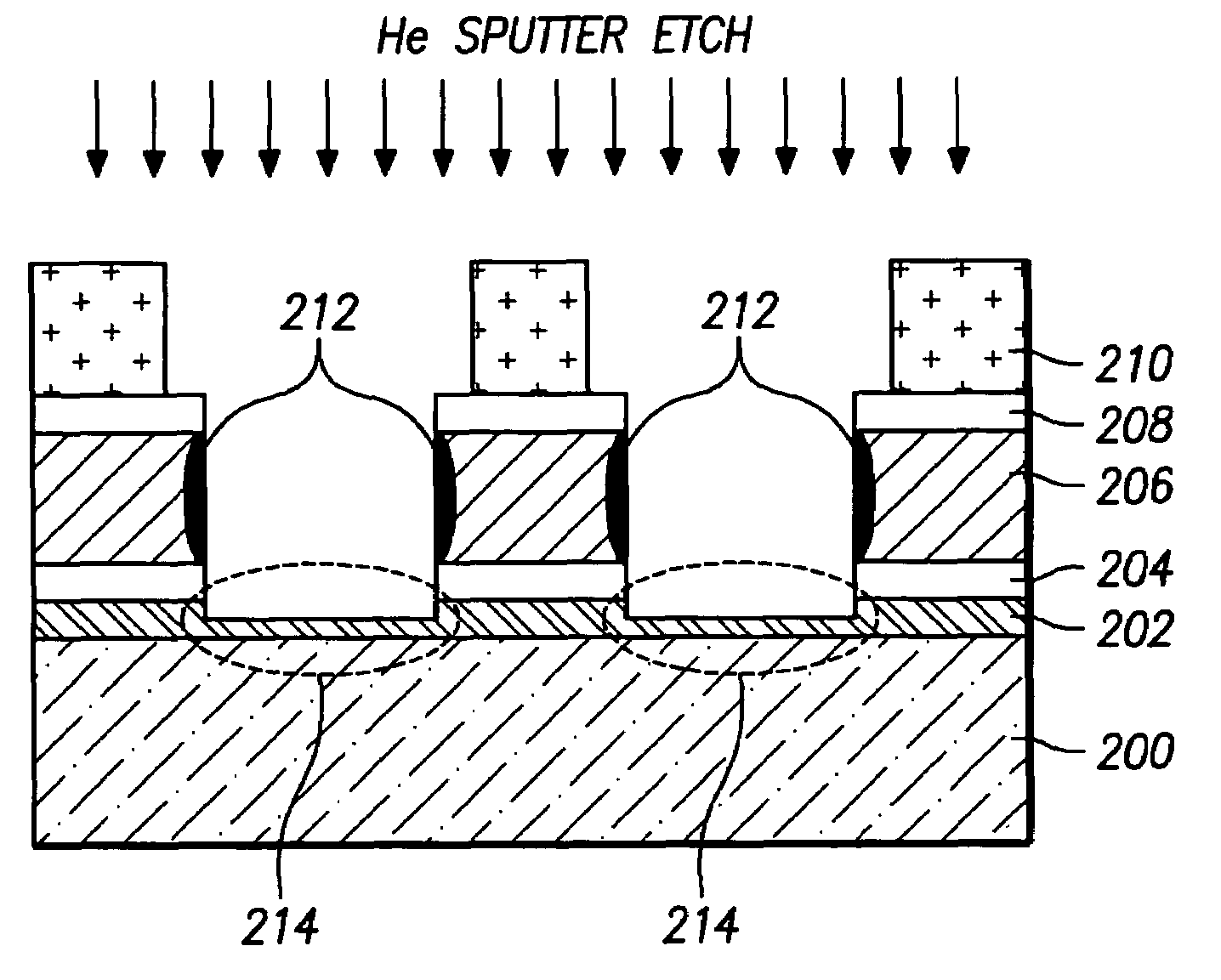
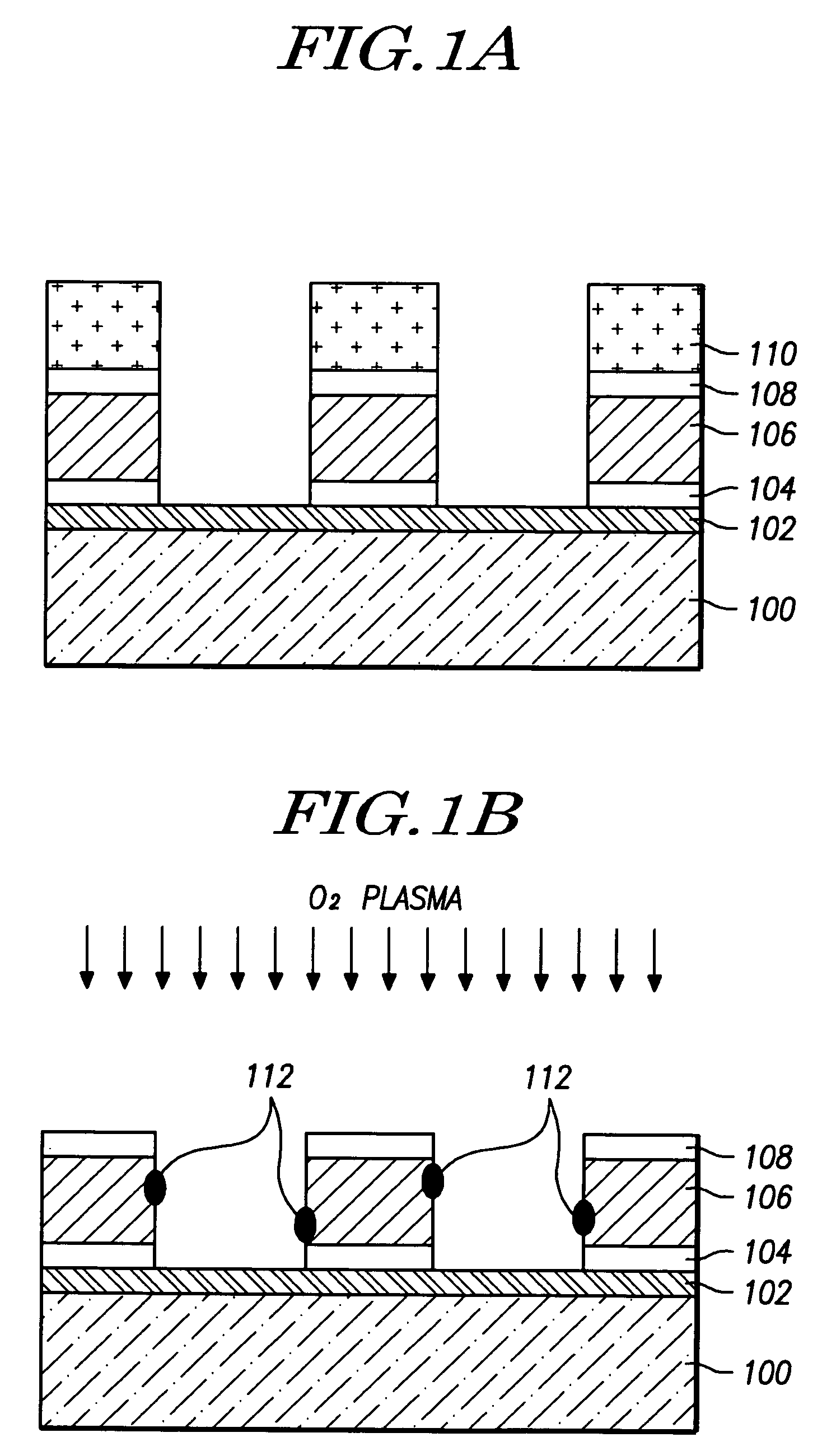
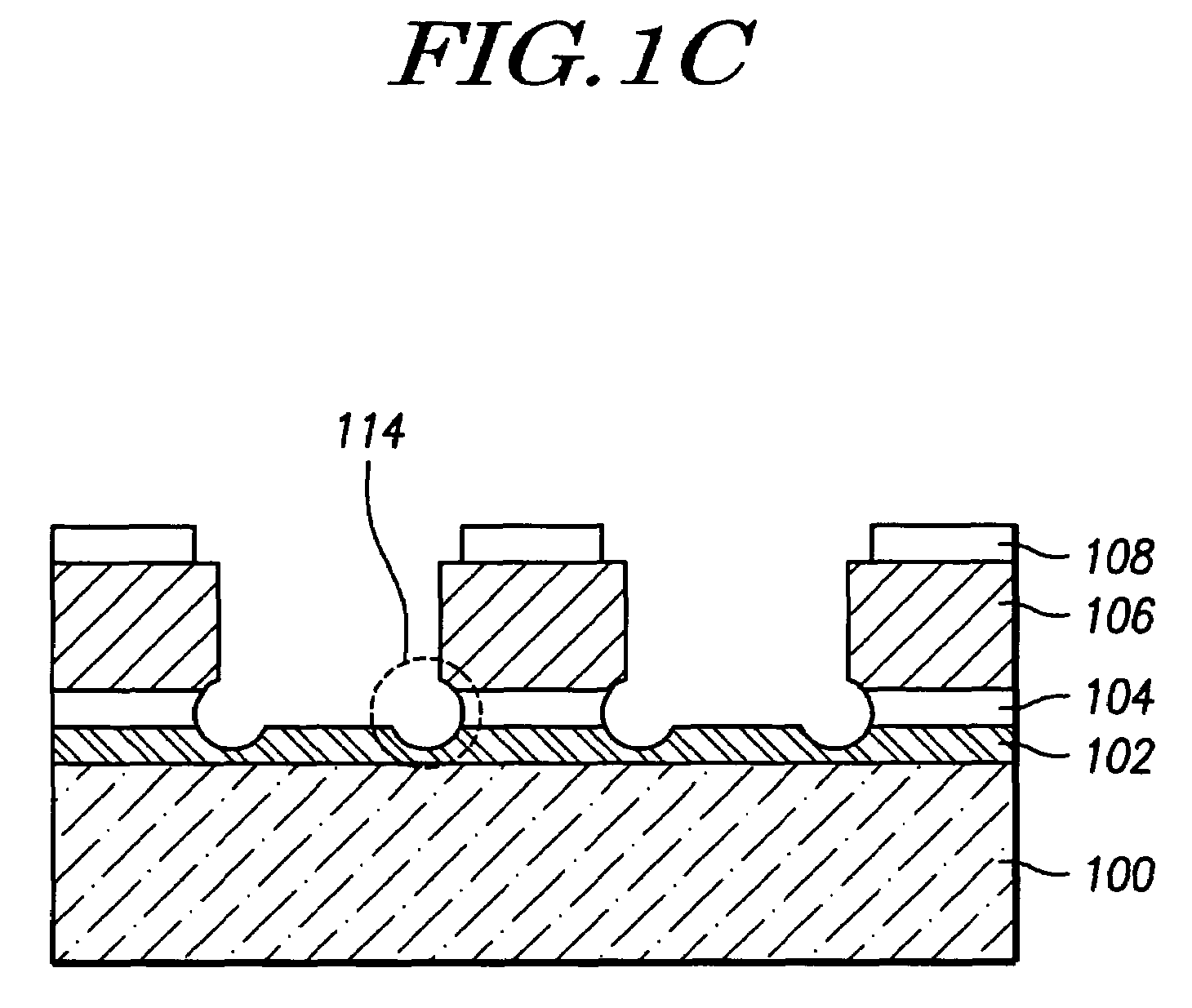
InactiveUS7468319B2Improved profileImprove reliabilitySemiconductor/solid-state device manufacturingPresent methodPhotoresist
Owner:DONGBU ELECTRONICS CO LTD
Novel lipid formulations for delivery of therapeutic agents to solid tumors
ActiveUS20110076335A1Improve effectivenessFavorable toxicity profileOrganic active ingredientsSpecial deliveryLipid formationLipid particle
The present invention provides novel, serum-stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides serum-stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (e.g., one or more interfering RNA molecules), methods of making the SNALP, and methods of delivering and / or administering the SNALP (e.g., for the treatment of cancer). In particular embodiments, the present invention provides tumor-directed lipid particles that preferentially target solid tumors. The tumor-directed formulations of the present invention are capable of preferentially delivering a payload such as a nucleic acid to cells of solid tumors compared to non-cancerous cells.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Hepatitis C inhibitor tri-peptides
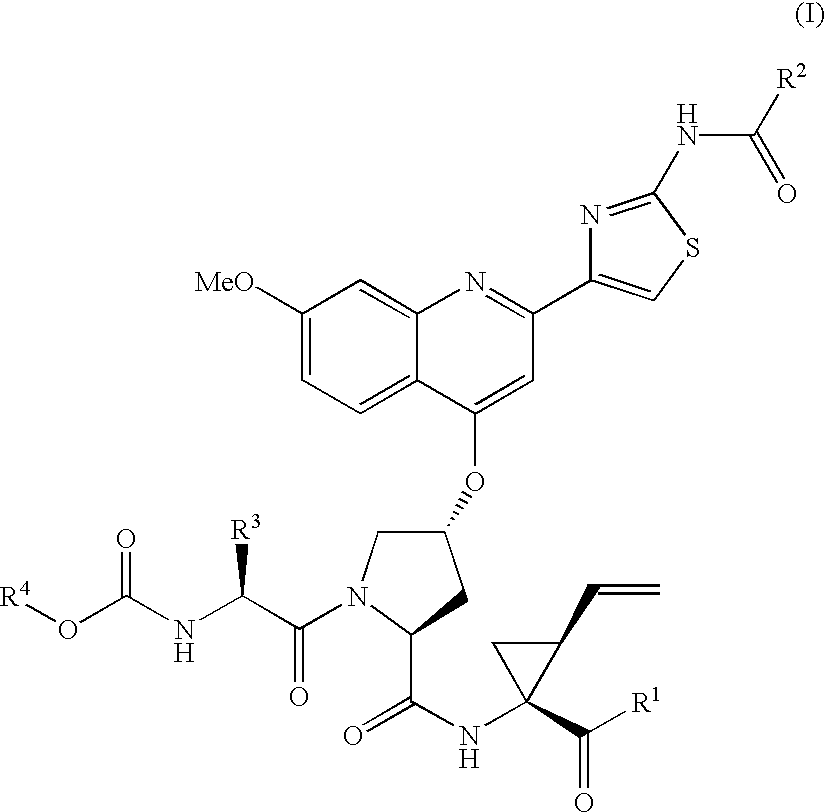
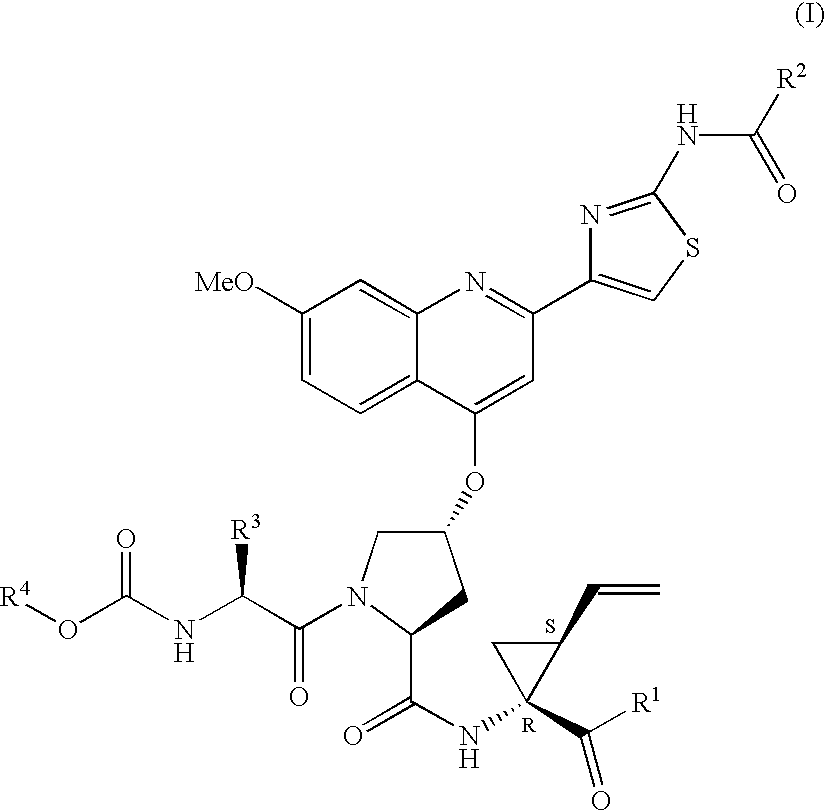
ActiveUS7091184B2Better pharmacokinetic profileNot significant inhibitory activityBiocideDipeptide ingredientsHcv ns3 proteaseHepatitis C
Compounds of formula (I):wherein R1 is hydroxyl or sulfonamide derivative; R2 is t-butyl or —CH2—C(CH3)3 or —CH2-cyclopentyl; R3 is t-butyl or cyclohexyl and R4 is cyclobutyl, cyclopentyl or cyclohexyl; or a pharmaceutically acceptable salt thereof, are described as useful as inhibitor of the HCV NS3 protease.
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical Composition with High-Potency Sweetener
InactiveUS20070116829A1Improve flavor profileImproving temporal profile profileSugar derivativesPharmaceutical delivery mechanismSweetnessFood flavor
The present invention relates generally to pharmaceutical compositions comprising non-caloric or low-caloric high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different pharmaceutical compositions comprising at least one non-caloric or low-caloric natural and / or synthetic high-potency sweetener, at least one sweet taste improving composition, and a pharmaceutically active substance. The present invention also relates to pharmaceutical compositions and methods that can improve the tastes of non-caloric or low-caloric natural and / or synthetic,, high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the pharmaceutical compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
Rapidly releasing and taste-masking pharmaceutical dosage form
InactiveUS6221402B1Inhibition releaseGood drug release profilePowder deliveryBiocideTaste maskingDosage form
A rapidly-releasing and taste-masking pharmaceutical dosage form and a process for preparing such oral dosage form are disclosed.
Owner:PFIZER PHAMACEUTICALS INC
Expandable trans-septal sheath
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system and has utility in the performance of procedures in the left atrium. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement to the right atrium and through the atrial septum into the left atrium. The distal end of the sheath is subsequently expanded using a radial dilatation device. In an exemplary application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of left atrial implants, mitral valve repair, or the like.
Owner:ONSET MEDICAL CORP
High-Potency Sweetener Composition With Dietary Fiber and Compositions Sweetened Therewith
InactiveUS20070116837A1Improve flavor profileImproving temporal profile profileMetabolism disorderConfectioneryChemistryDietary fibre
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic, high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic, high-potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as a dietary fiber source. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
Rotational intravascular ultrasound probe with an active spinning element
ActiveUS20100234736A1Improve image qualityAccurate diagnosis of medicalCatheterInfrasonic diagnosticsManufacturing cost reductionSonification
An intravascular ultrasound probe is disclosed, incorporating features for utilizing an advanced transducer technology on a rotating transducer shaft. In particular, the probe accommodates the transmission of the multitude of signals across the boundary between the rotary and stationary components of the probe required to support an advanced transducer technology. These advanced transducer technologies offer the potential for increased bandwidth, improved beam profiles, better signal to noise ratio, reduced manufacturing costs, advanced tissue characterization algorithms, and other desirable features. Furthermore, the inclusion of electronic components on the spinning side of the probe can be highly advantageous in terms of preserving maximum signal to noise ratio and signal fidelity, along with other performance benefits.
Owner:VOLCANO CORP
Nanoparticulate megestrol formulations
ActiveUS7101576B2Improved pharmacokinetic profileLess variabilityPowder deliveryOrganic active ingredientsNanoparticleMegestrol
Owner:ALKERMES PHARMA IRELAND LTD
Analyte sensors comprising blended membrane compositions and methods for making and using them
ActiveUS20110152654A1Promote hydrationImprove mechanical propertiesImmobilised enzymesBioreactor/fermenter combinationsAnalyteChemical reaction
Embodiments of the invention provide analyte sensors having elements designed to modulate their chemical reactions as well as methods for making and using such sensors. In certain embodiments of the invention, the sensor includes an analyte modulating membrane that comprises a blended mixture of a linear polyurethane / polyurea polymer, and a branched acrylate polymer.
Owner:MEDTRONIC MIMIMED INC
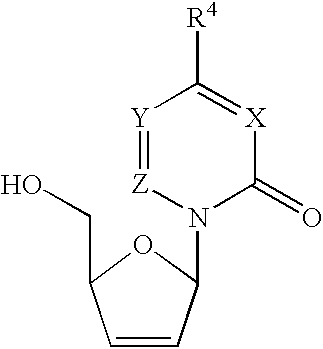
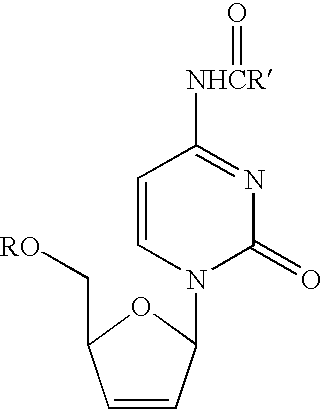
Method for the synthesis of 2′,3′-dideoxy-2′,3′-didehydronucleosides
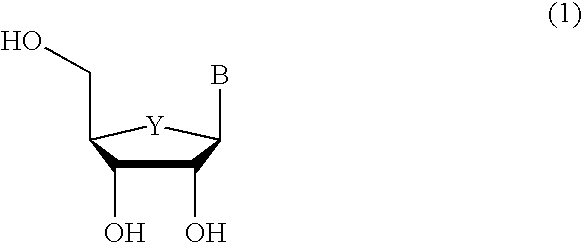
An efficient synthetic route to antiviral 2′,3′-dideoxy-2′,3′-didehydro-nucleosides, such as 2′,3′-dideoxy and 2′- or 3′-deoxyribo-nucleoside analogs, from available precursors is disclosed, with the option of introducing functionality as needed. In one embodiment, a method for the preparation of β-D and β-L-2′,3′-dideoxy-2′,3′-didehydro-nucleosides is described that includes: activating a compound of structure (1) wherein B is a pyrimidine or purine base and Y is O, S or CH2 with an acyl halide of the formula X—C(═O)R1, X—C(═O)C(R1)2OC(═O)R1 or X—C(═O)OR1 (wherein X is a halogen, and each R1 is independently hydrogen, lower alkyl, alkyl, aryl or phenyl); reducing the resulting compound with a reducing agent to form a 2′,3′-dideoxy-2′,3′-didehydro-nucleoside; and optionally deprotecting the nucleoside. The haloacylation of the first step can form the 2′-acyl-3′-halonucleoside, the 3′-acyl-2′-halonucleoside, or a mixture thereof.
Owner:GILEAD PHARMASSET LLC
High-Potency Sweetener Composition with Fatty Acid and Compositions Sweetened Therewith
ActiveUS20070116819A1Improve temporal profile and/or flavor profileImproved profileMilk preparationSugar food ingredientsAdditive ingredientSweetening agents
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic, high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic, high-potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as fatty acids. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
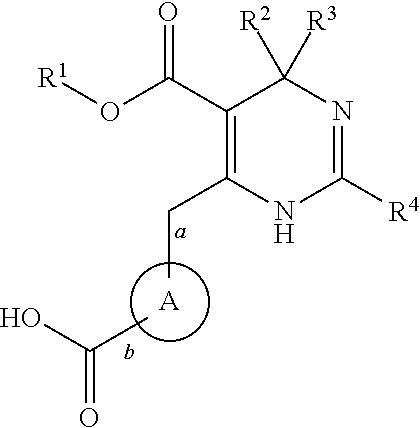
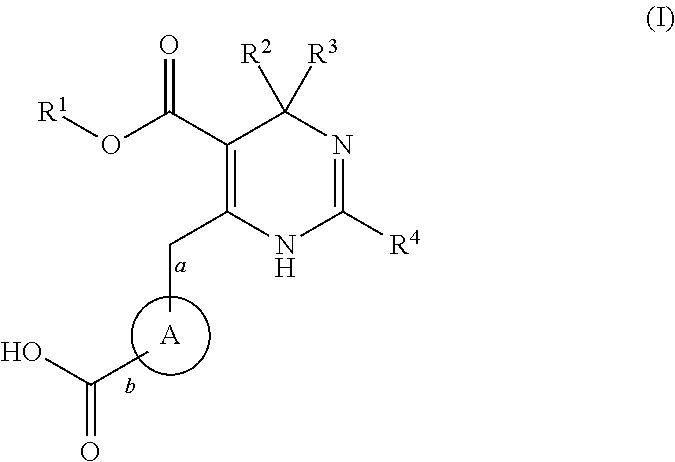
Novel 6-amino acid heteroaryldihydropyrimidines for the treatment and prophylaxis of hepatitis B virus infection
ActiveUS20150031687A1Superior anti-HBV activityHigh selectivity indexBiocideOrganic chemistryHenipavirus InfectionsCyrtanthus elatus virus A
Owner:F HOFFMANN LA ROCHE & CO AG
Sweetener Compositions Having Enhanced Sweetness and Improved Temporal and/or Flavor Profiles
The present invention relates generally to improving the taste of sweetener compositions having enhanced sweetness. In particular, the present invention relates to compositions that can improve the tastes of sweetness enhanced sweetener compositions including at least one sweetness enhancer and at least one sweetener by imparting a more sugar-like taste or characteristic. In particular, the compositions and methods provide at least one sweetness enhancer, at least one sweetener comprising a carbohydrate sweetener, a natural-high-potency sweetener, a synthetic high-potency sweetener, or a combination thereof, and at least one sweet taste improving composition.
Owner:THE COCA-COLA CO
High-Potency Sweetener Composition With Phytoestrogen and Compositions Sweetened Therewith
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic, high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic, high-potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as phytoestrogens. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
High-potency sweetener compositon with rubisco protein, rubiscolin, rubiscolin derivatives, ace inhibitory peptides, and combinations thereof, and compositions sweetened therewith
ActiveUS20080107775A1Improve flavor profileImproving temporal profile profileNervous disorderTobacco treatmentRubiscolinAdditive ingredient
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic high potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as rubisco protein, rubiscolin, rubiscolin derivatives, ACE inhibitory peptide, and combinations thereof. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
High-Potency Sweetener for Weight Management and Compositions Sweetened Therewith
ActiveUS20070116840A1Improve flavor profileImproving temporal profile profileMetabolism disorderAcidic food ingredientsAdditive ingredientSweetness
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic high potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as a weight management agent. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
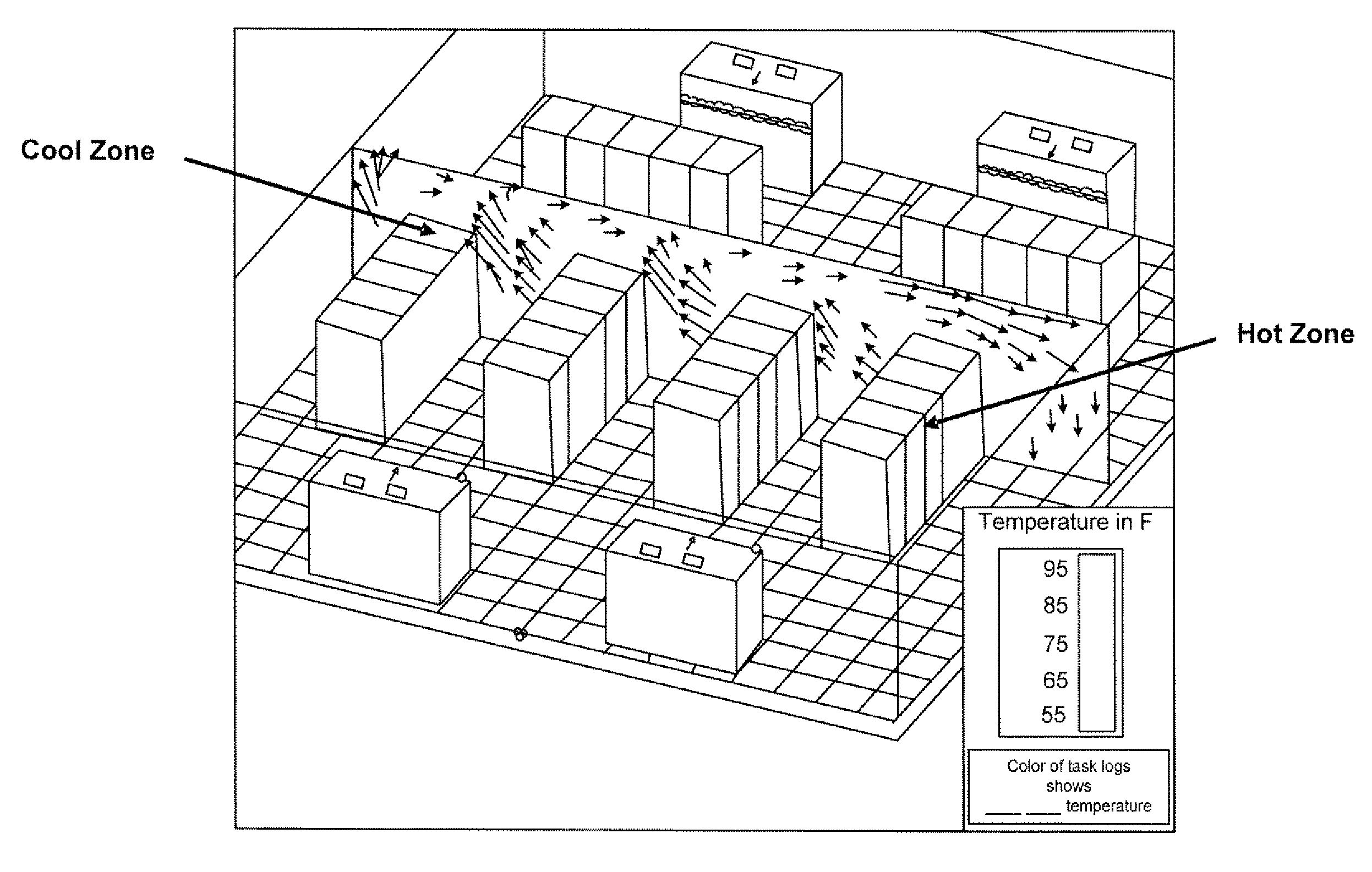
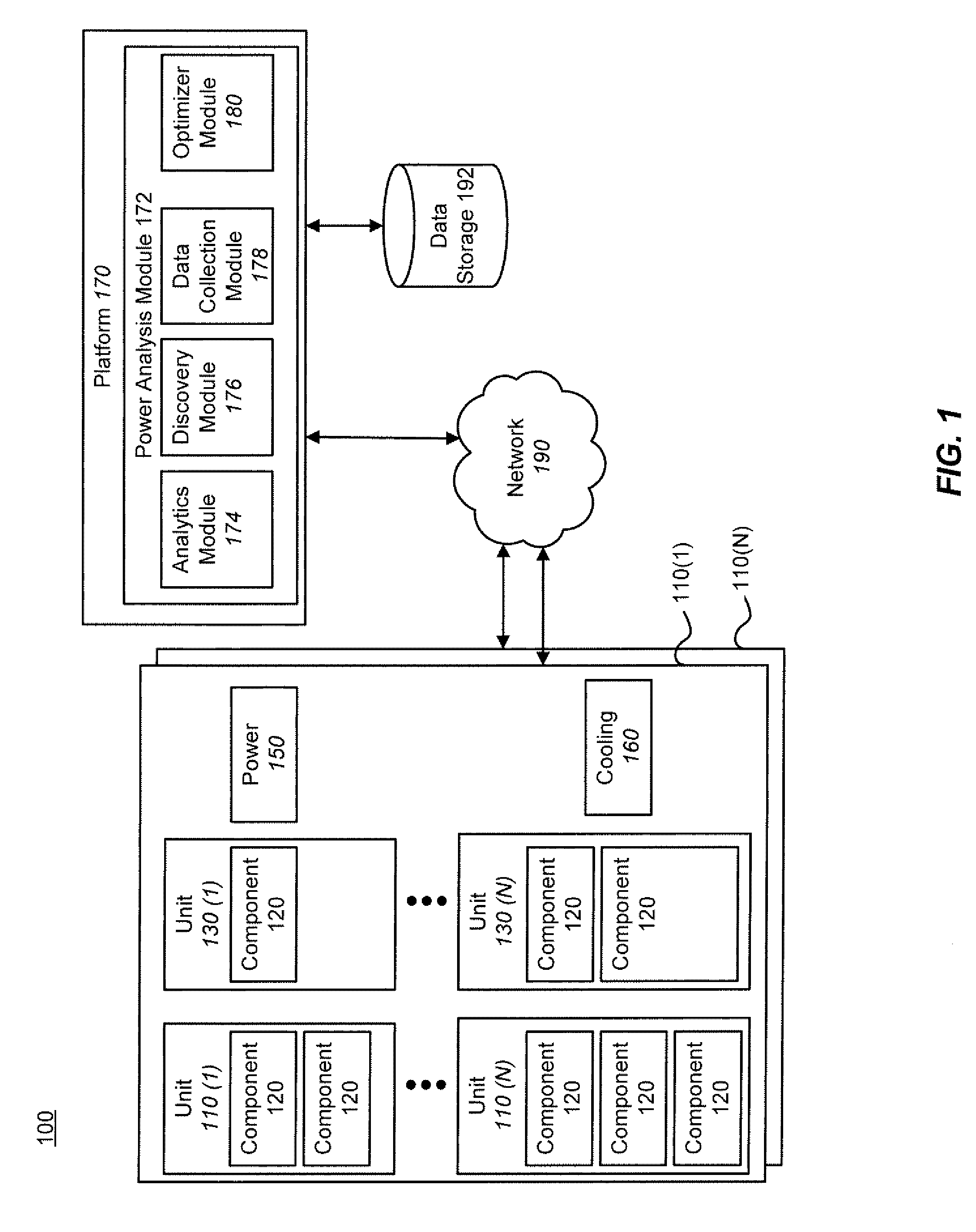
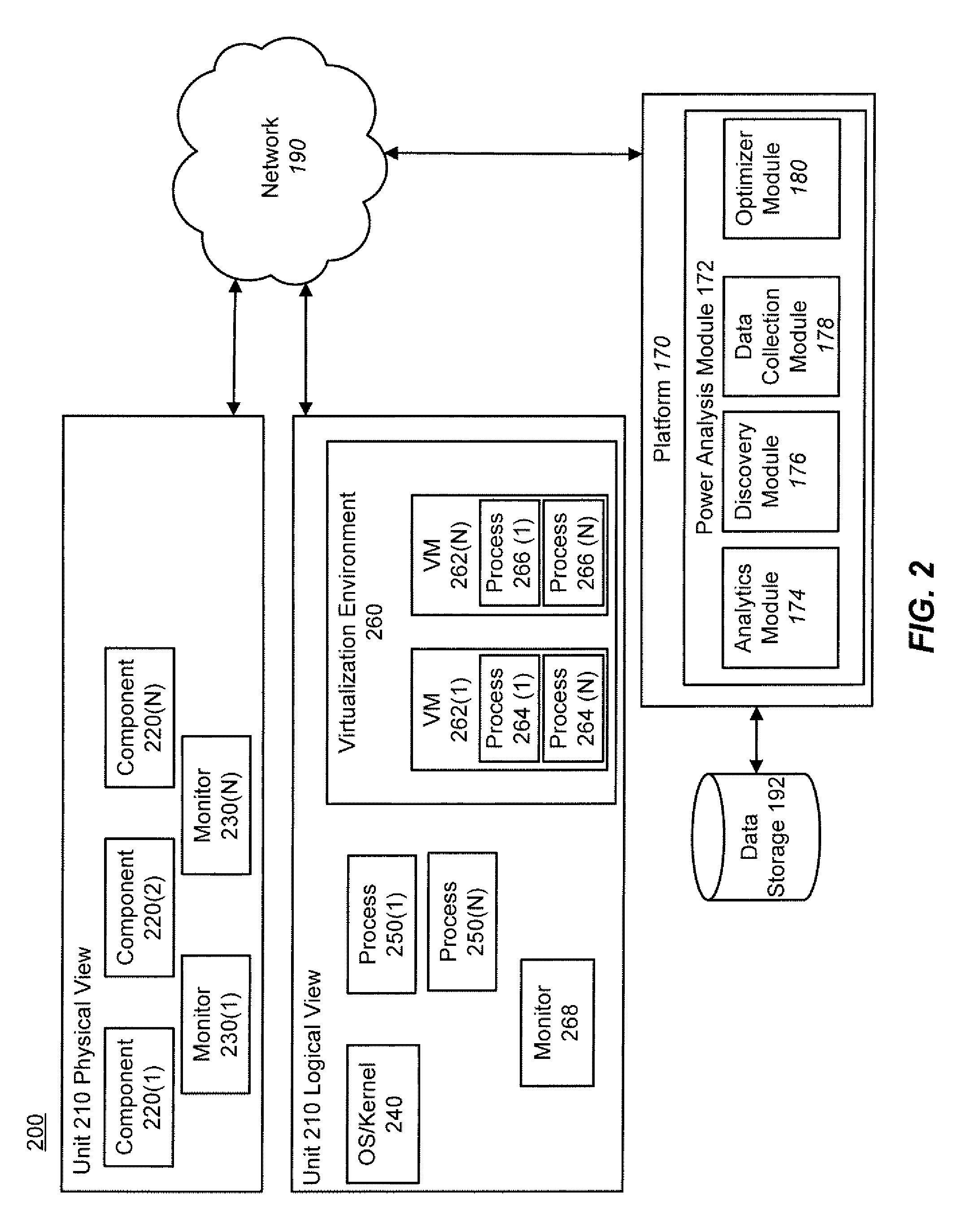
Techniques for power analysis
ActiveUS20110016342A1Enhanced thermal profileComponent can be removedHardware monitoringPower supply for data processingPower analysisData center
Techniques for power analysis for data centers are disclosed. In one particular exemplary embodiment, the techniques may be realized as a method for power analysis for a plurality of computing platform components comprising receiving information associated with a component, retrieving, using a computer processor, electronically stored data associated with the component, estimating power usage of the component based at least in part on the stored data, and outputting an indicator of power usage.
Owner:SCHNEIDER ELECTRIC IT CORP
Chewing Gum with High-Potency Sweetener
InactiveUS20070116800A1Improve temporal profile and/or flavor profileImproved profileChewing gumAcidic food ingredientsTime distributionSweetening agents
The present invention relates generally to chewing gum compositions comprising non-caloric or low-caloric high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different chewing gum compositions comprising at least one non-caloric or low-caloric natural and / or synthetic high-potency sweetener, at least one sweet taste improving composition, and a gum base. The present invention also relates to chewing gum compositions and methods that can improve the tastes of non-caloric or low-caloric natural and / or synthetic, high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the chewing gum composition and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com