Patents
Literature
161 results about "Cyrtanthus elatus virus A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
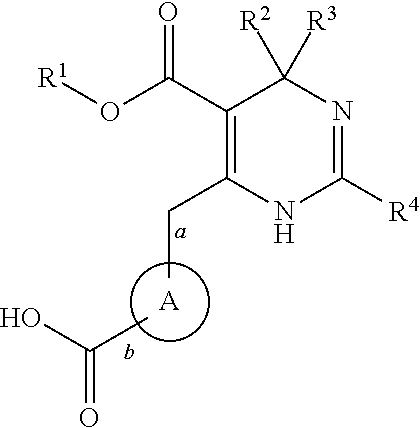
Novel 4-methyl-dihydropyrimidines for the treatment and prophylaxis of hepatitis b virus infection
Owner:GUO LEI +8
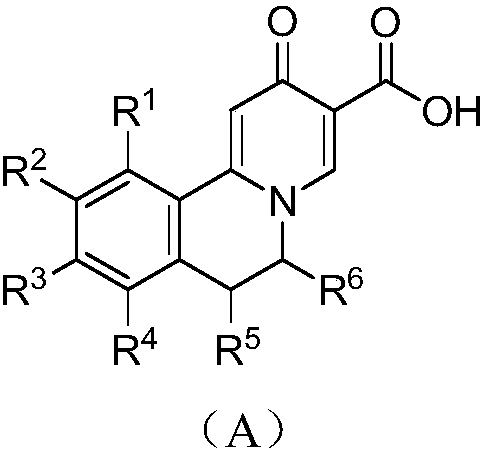
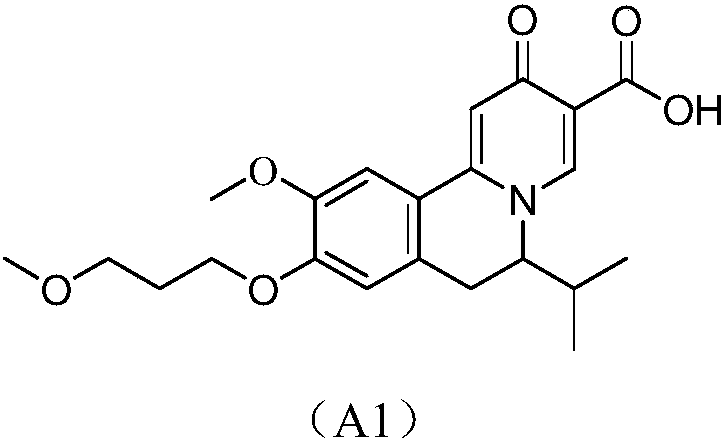
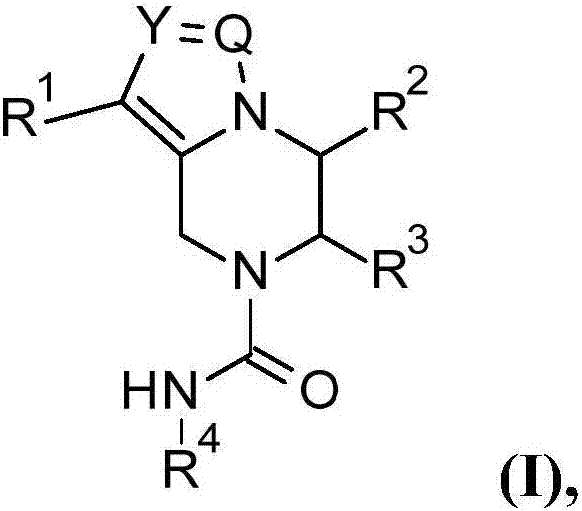
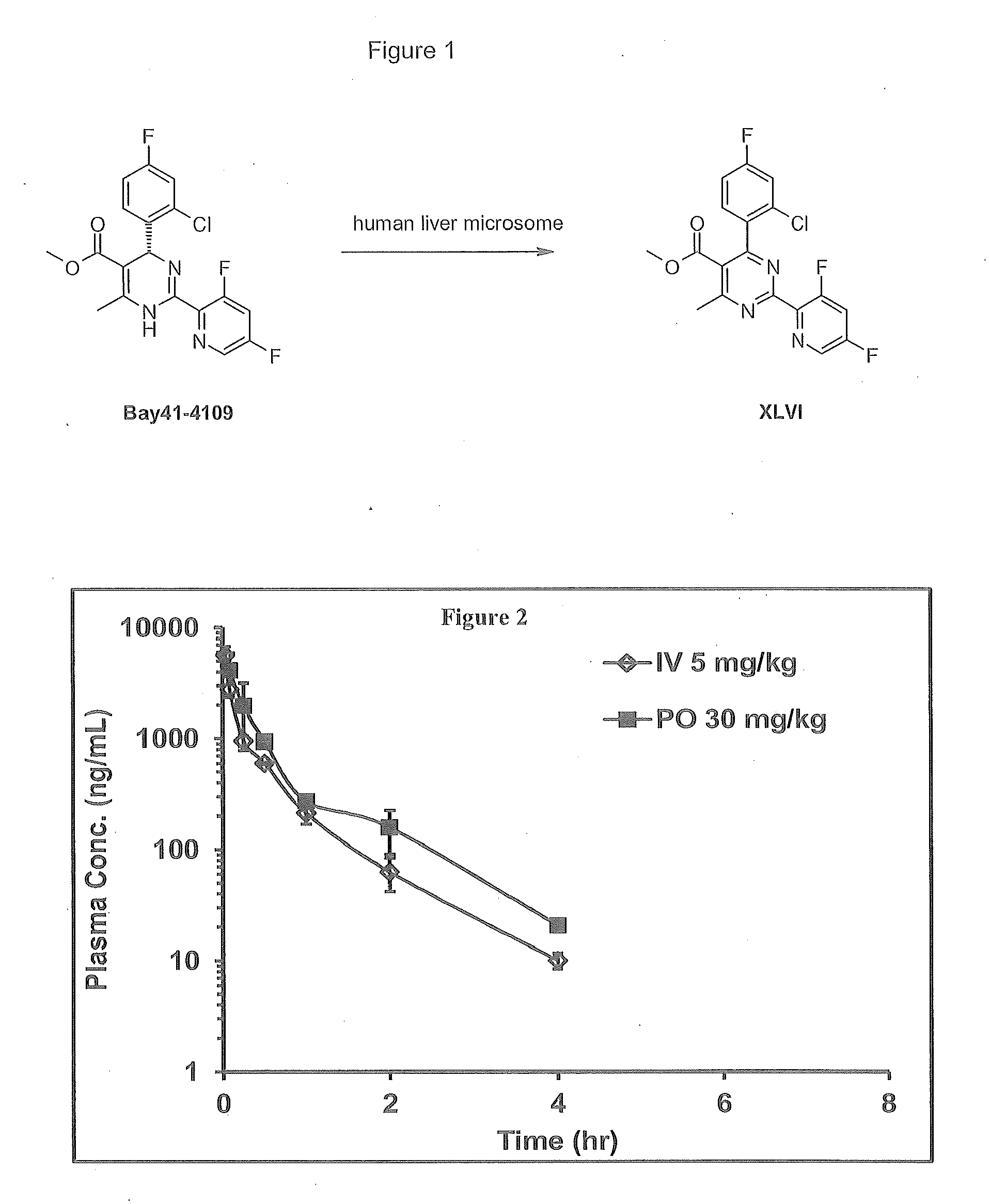
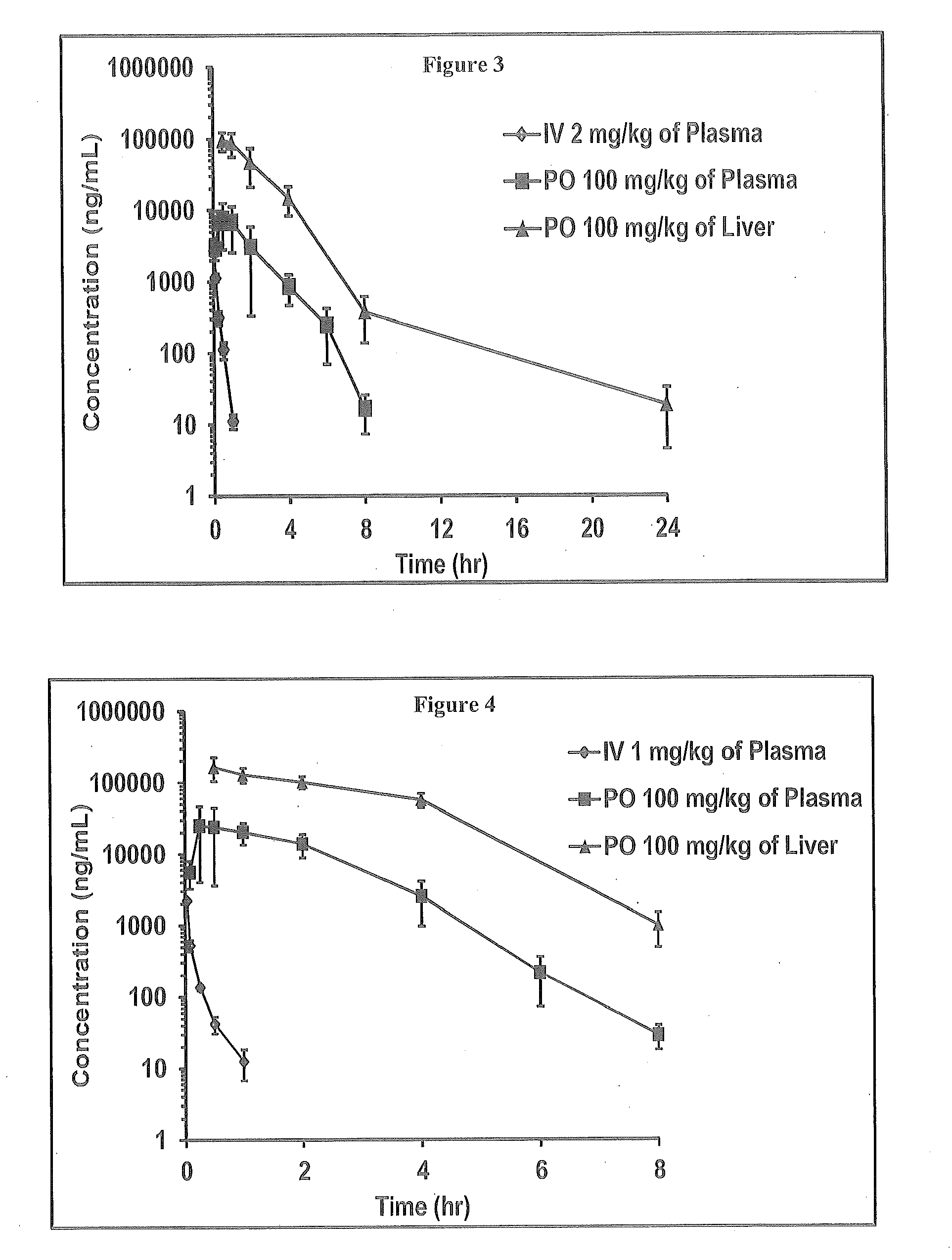
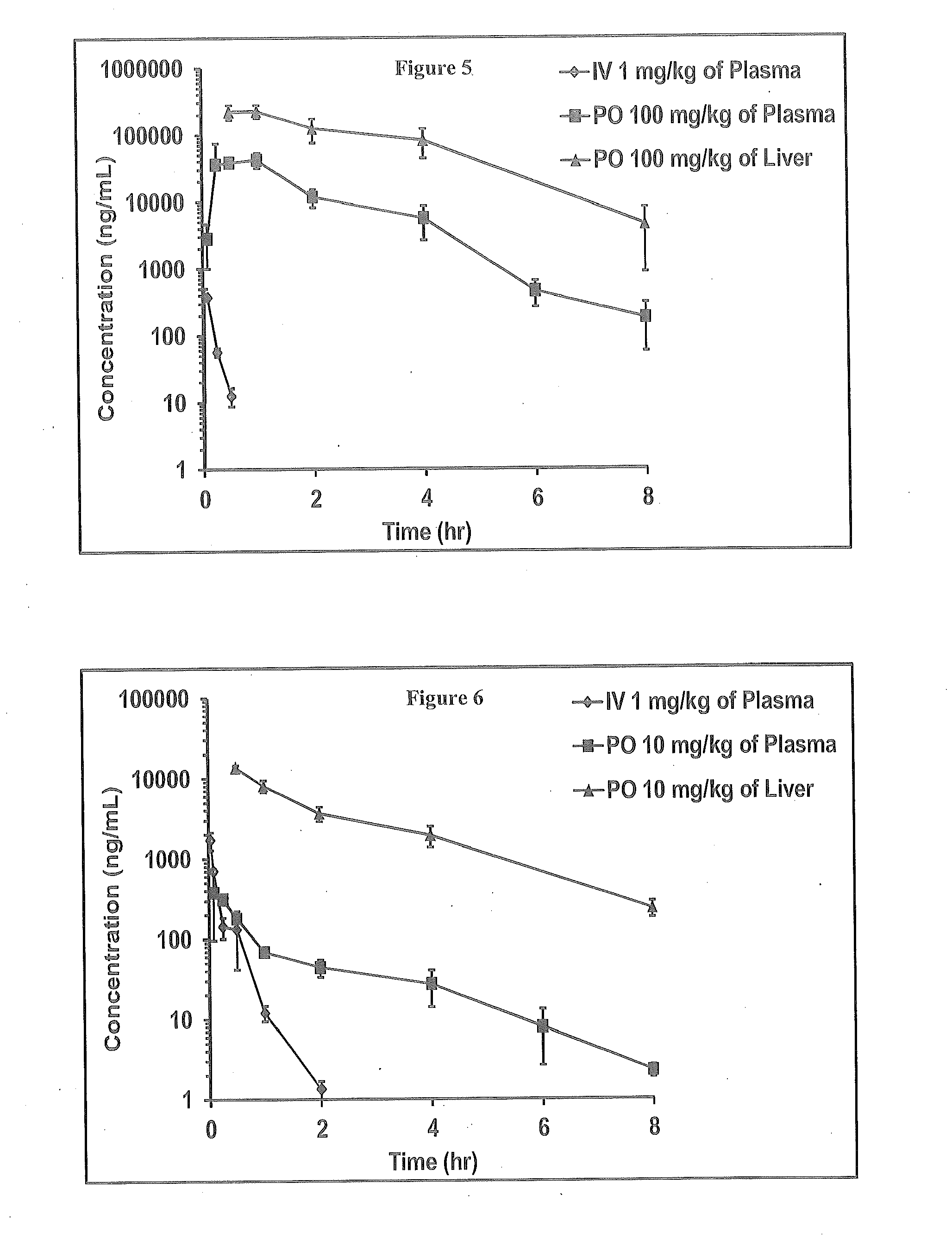
Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection
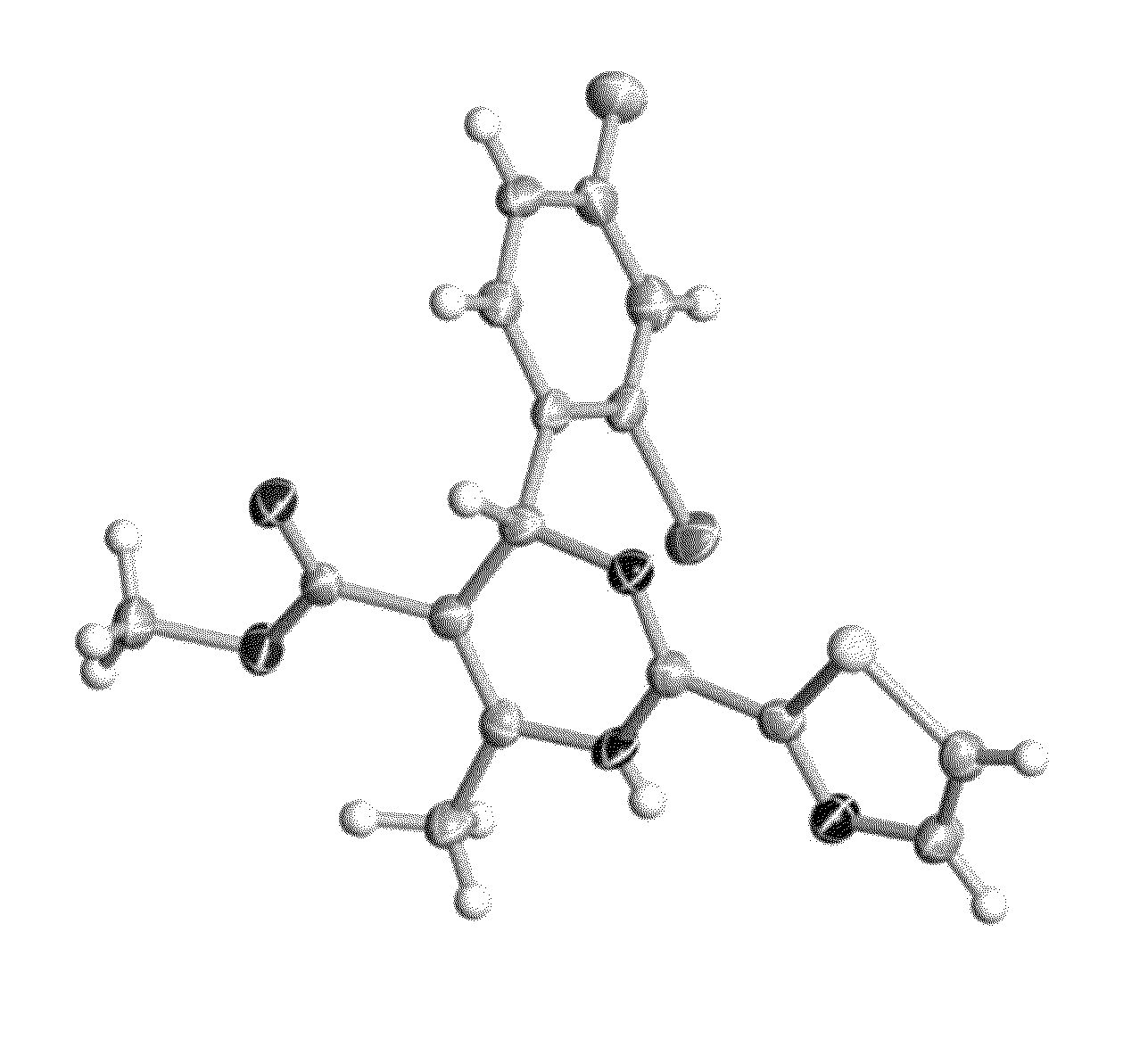
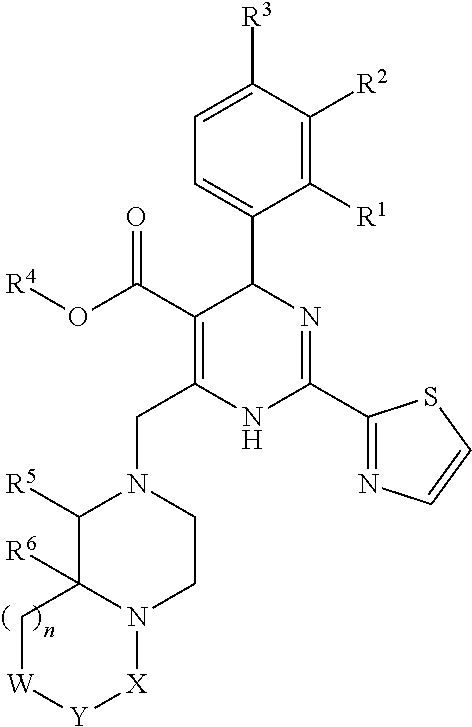
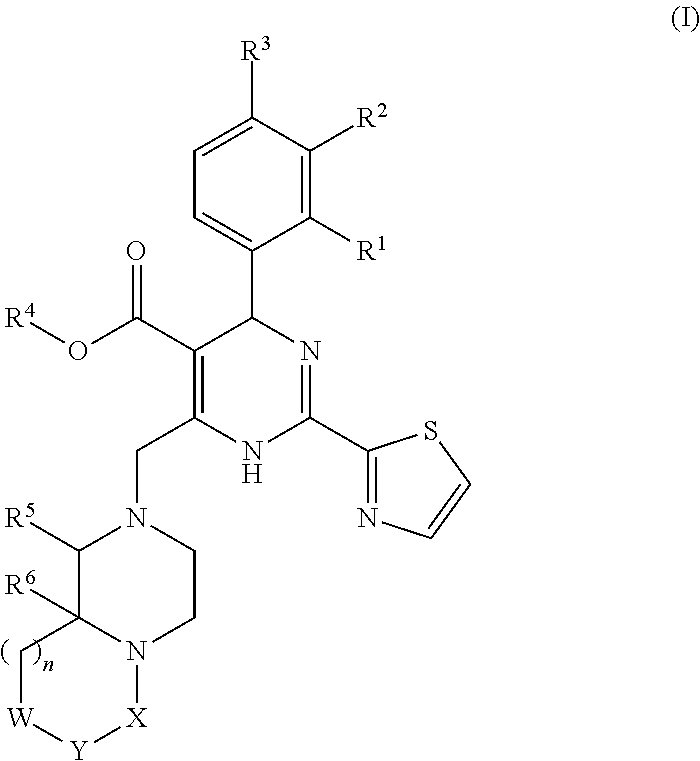
The invention provides novel compounds having the general formula:wherein R1 to R6, W and X are as described herein and their pharmaceutically acceptable salt, enantiomer or diastereomer thereof, and compositions including the compounds and methods of using the compounds.
Owner:F HOFFMANN LA ROCHE INC
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
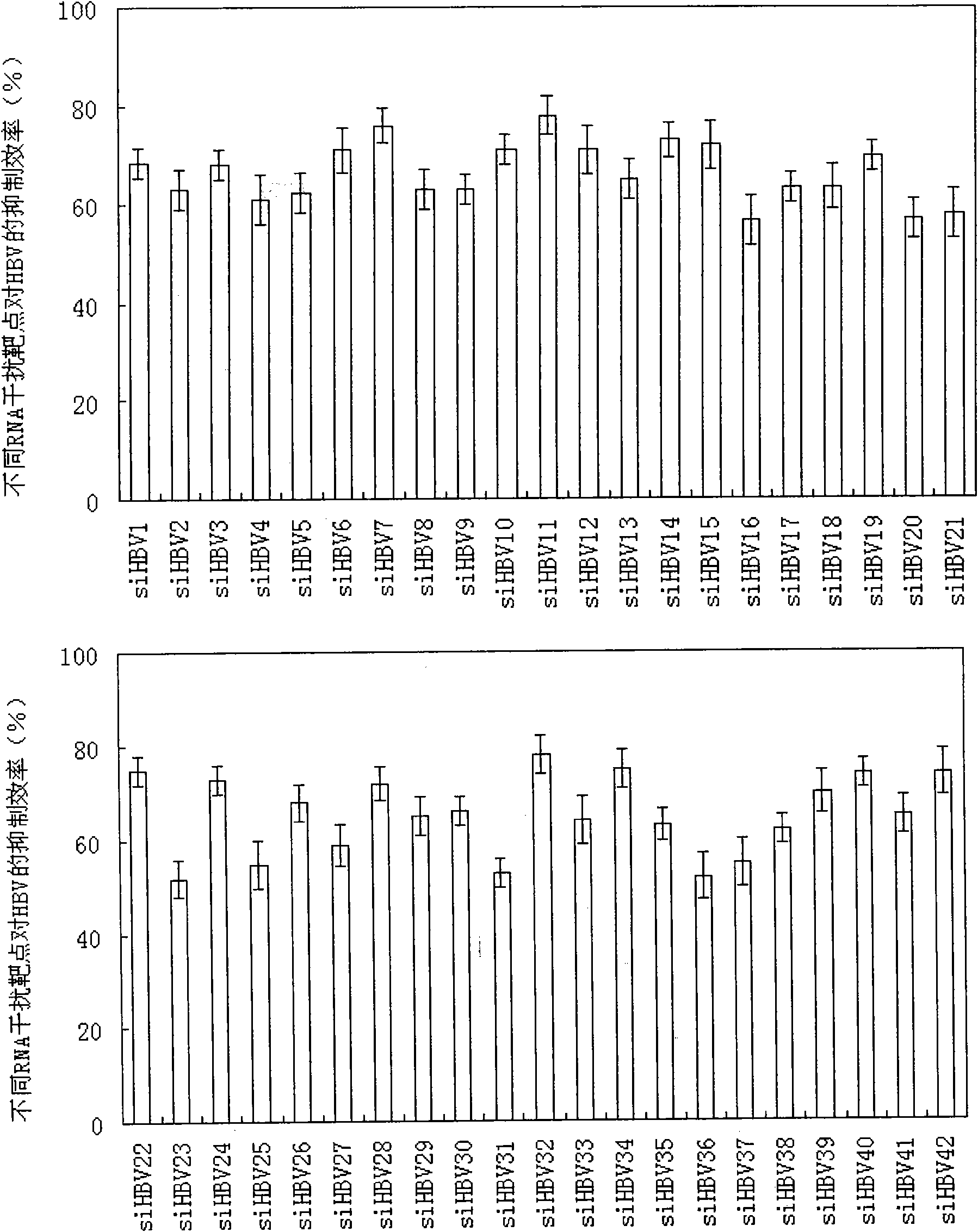
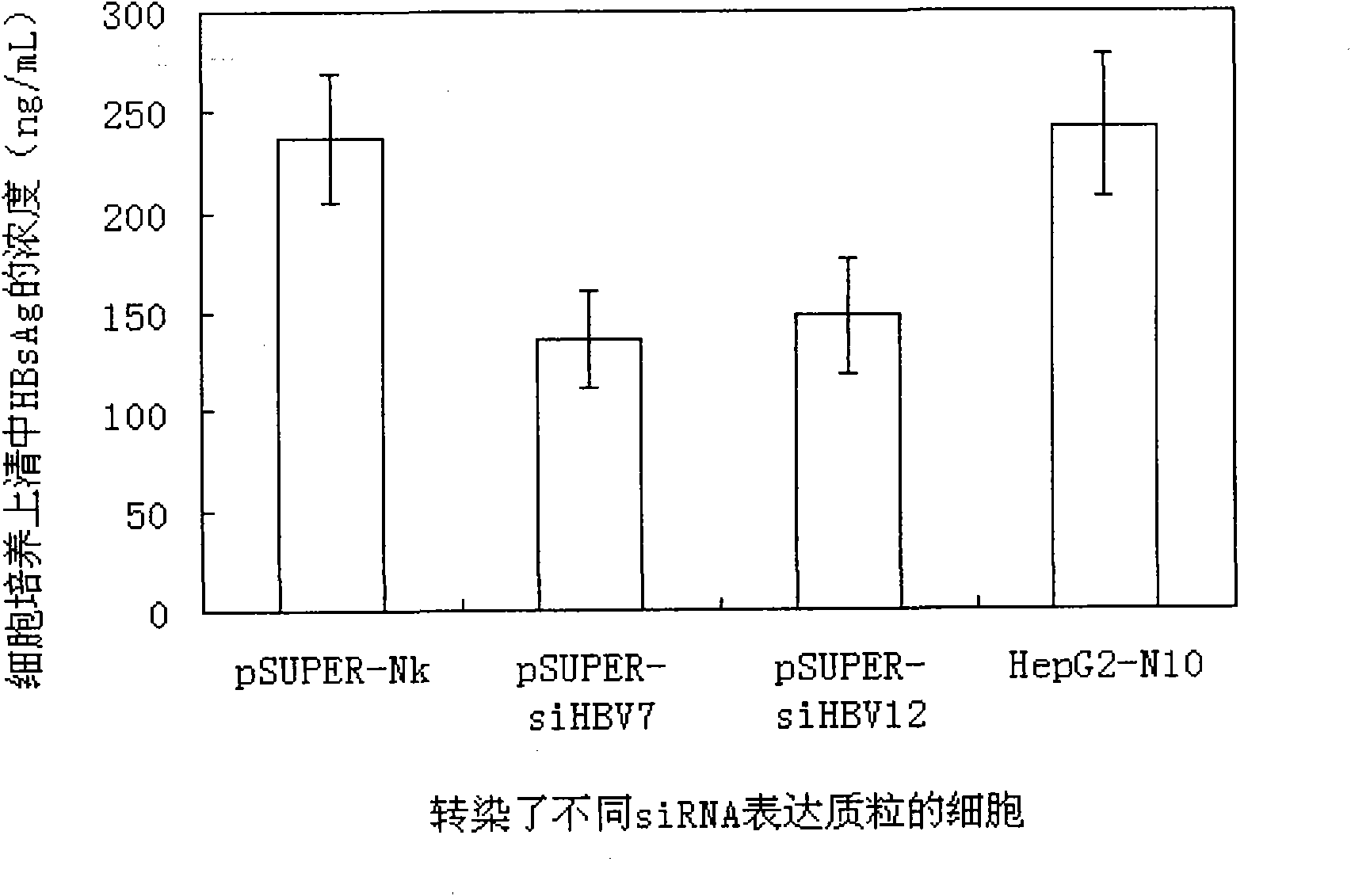
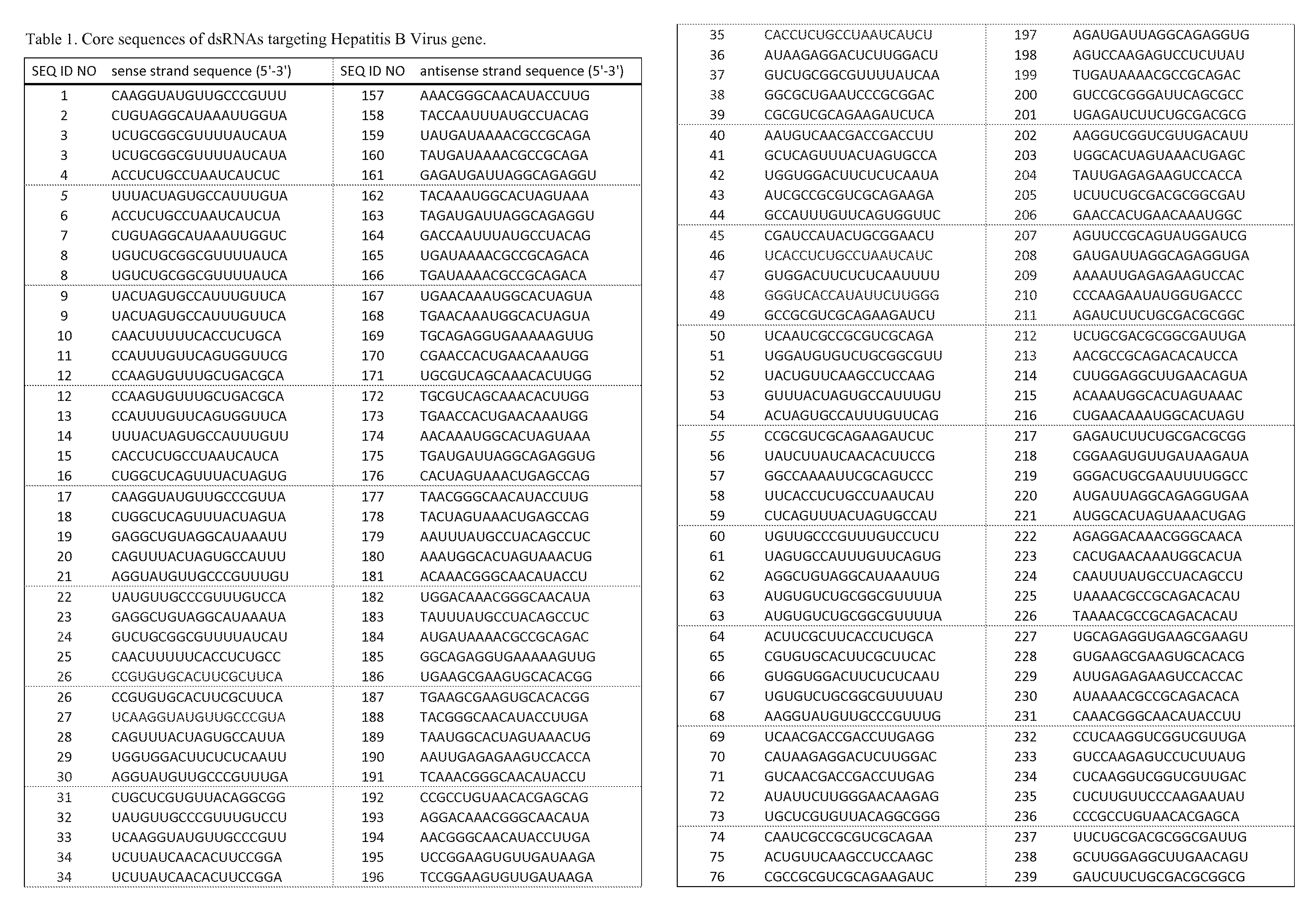
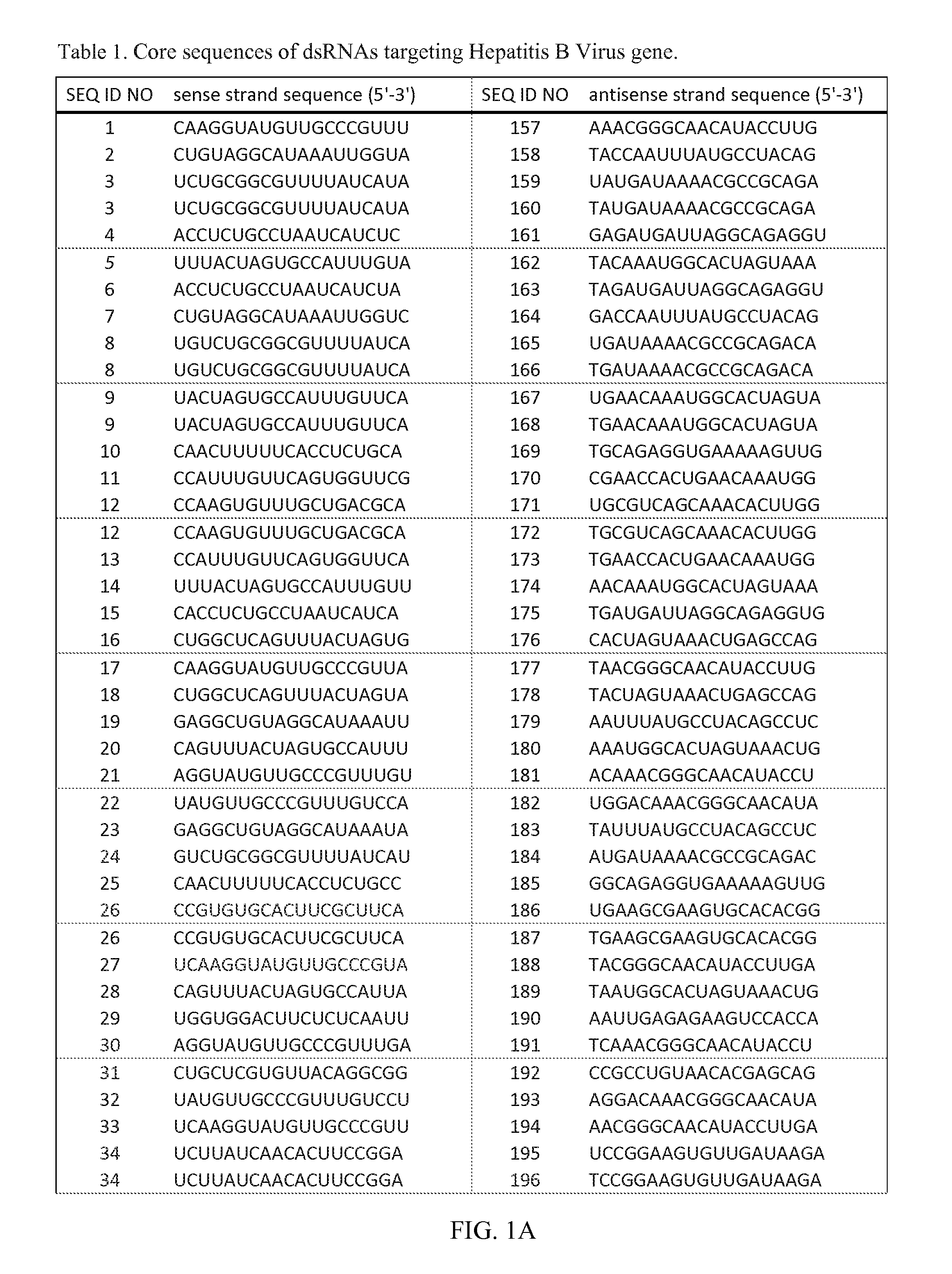
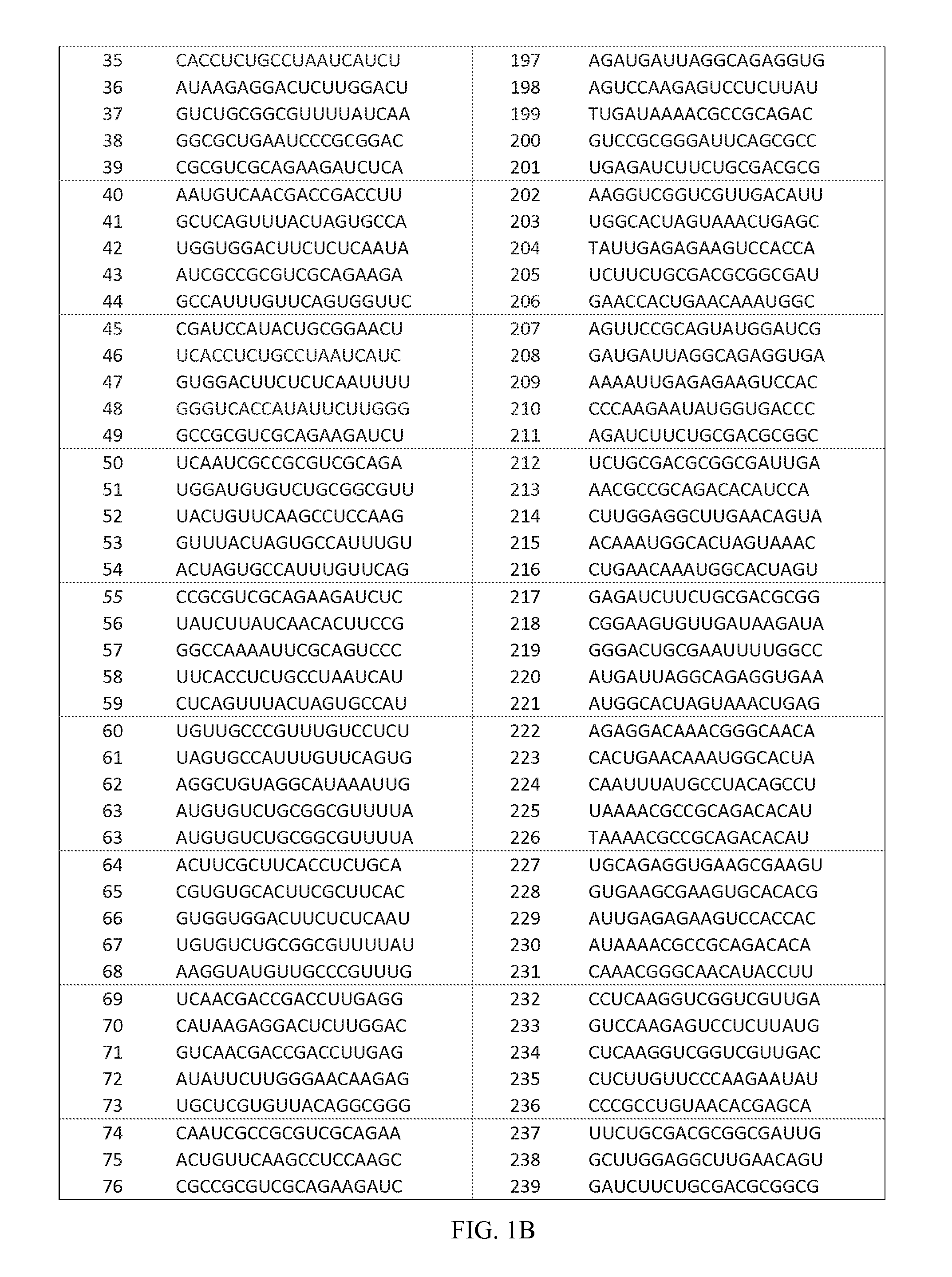
RNA interference target for treating hepatitis B virus infection
The invention relates to an RNA interference target for treating hepatitis B virus infection with 42 different targeting hepatitis B viruses (HBV), which can be used for preparing a medicament for treating the hepatitis B virus infection. The invention provides a recombinant expression vector of siRNA and / or miRNA and / or ribozyme and / or antisense oligonucleotide, which can be used for expressing targeting HBV. The invention relates to a cell which has the function of inhibiting the expression of HBV virus gene and can express and / or is induced with the siRNA and / or the miRNA and / or the ribozyme and / or the antisense oligonucleotide and / or the medication obtained according to the RNA interference target.
Owner:XIAMEN UNIV +1
Compositions and methods for inhibiting gene expression of hepatitis B virus
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of a Hepatitis B Virus gene. The invention also relates to a pharmaceutical composition comprising the dsRNA or nucleic acid molecules or vectors encoding the same together with a pharmaceutically acceptable carrier; methods for treating diseases caused by Hepatitis B Virus infection using said pharmaceutical composition; and methods for inhibiting the expression of a Hepatitis B Virus gene in a cell.
Owner:ARROWHEAD PHARMA INC
Nucleic acid-based compounds and methods of use thereof
The invention provides compounds capable of treating against hepatitis infections, particularly hepatitis B viral infections. Compounds of the invention are nucleic acid-based and preferably comprise 2, 3, 4, 5 or 6 nucleoside units.
Owner:MIGENIX INC (CA)
Novel 6-amino acid heteroaryldihydropyrimidines for the treatment and prophylaxis of hepatitis b virus infection
ActiveUS20160237078A9High selectivity indexTreatment or prophylaxis of HBV infectionOrganic active ingredientsOrganic chemistryMedicineHepatitis B virus
Owner:F HOFFMANN LA ROCHE & CO AG
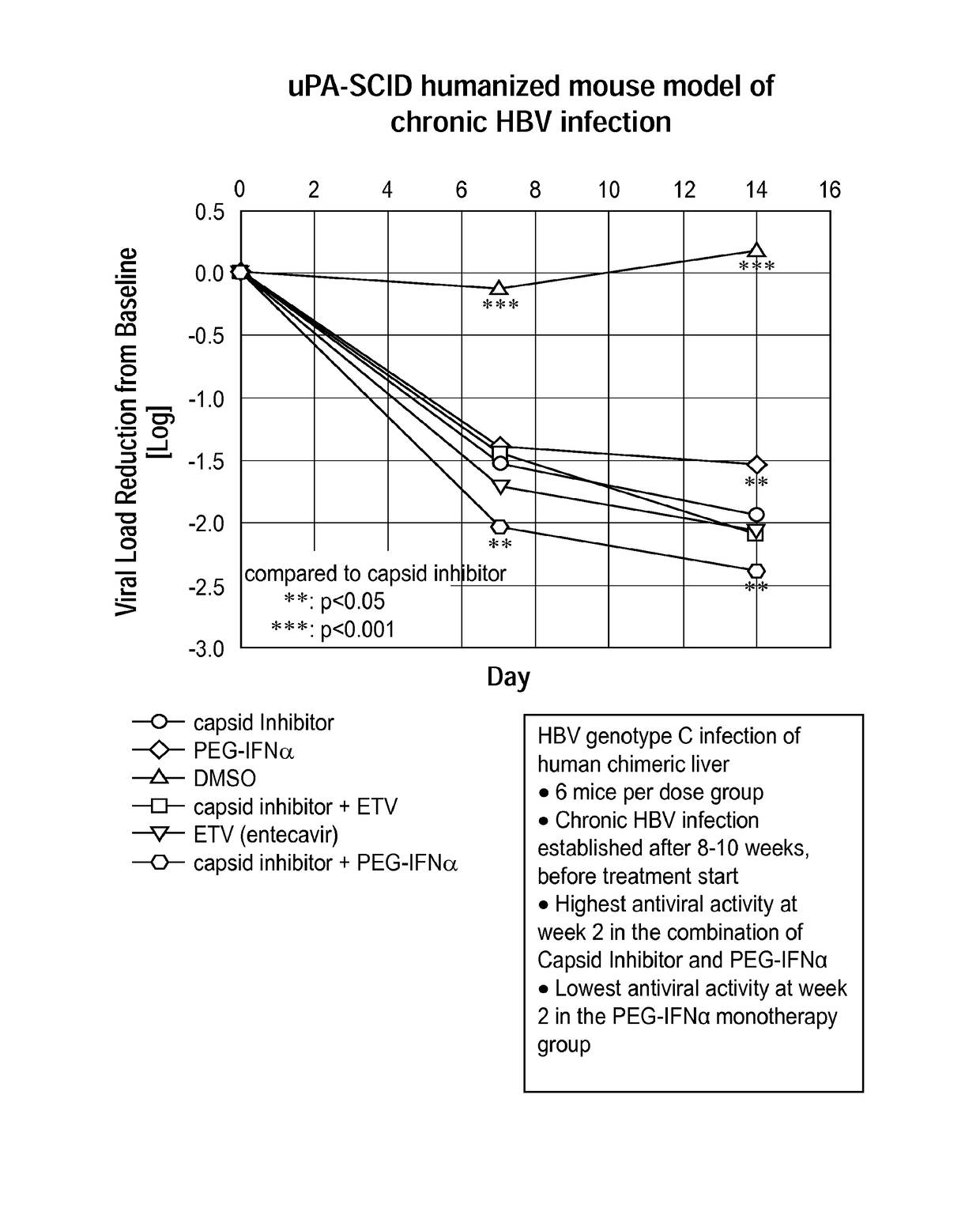
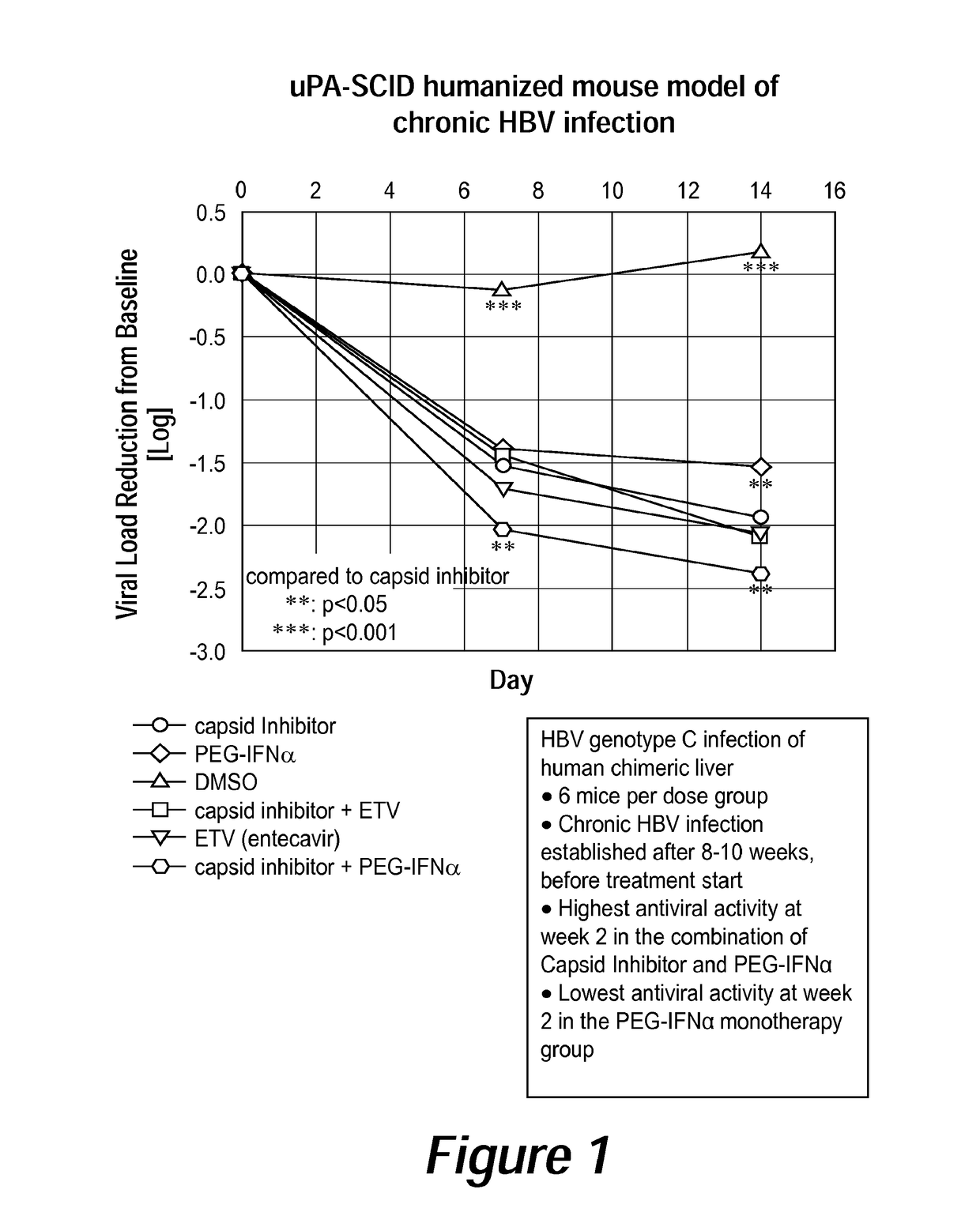
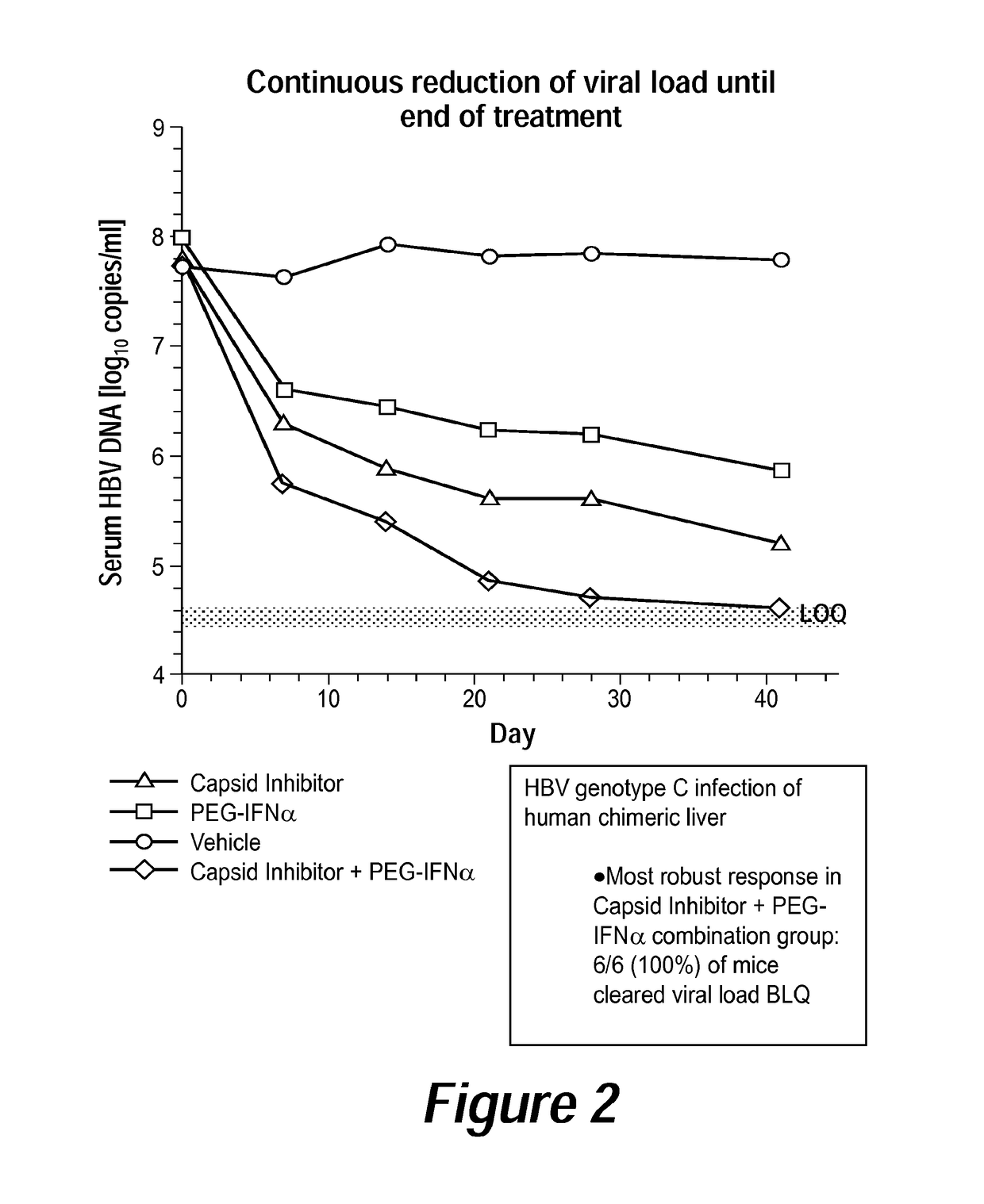
Combination therapy for treatment of hbv infections
InactiveUS20170182021A1Additional HBV virus replication suppression efficacyUseful in treatmentPeptide/protein ingredientsAntiviralsCombined Modality TherapyPeginterferon alfa-2a
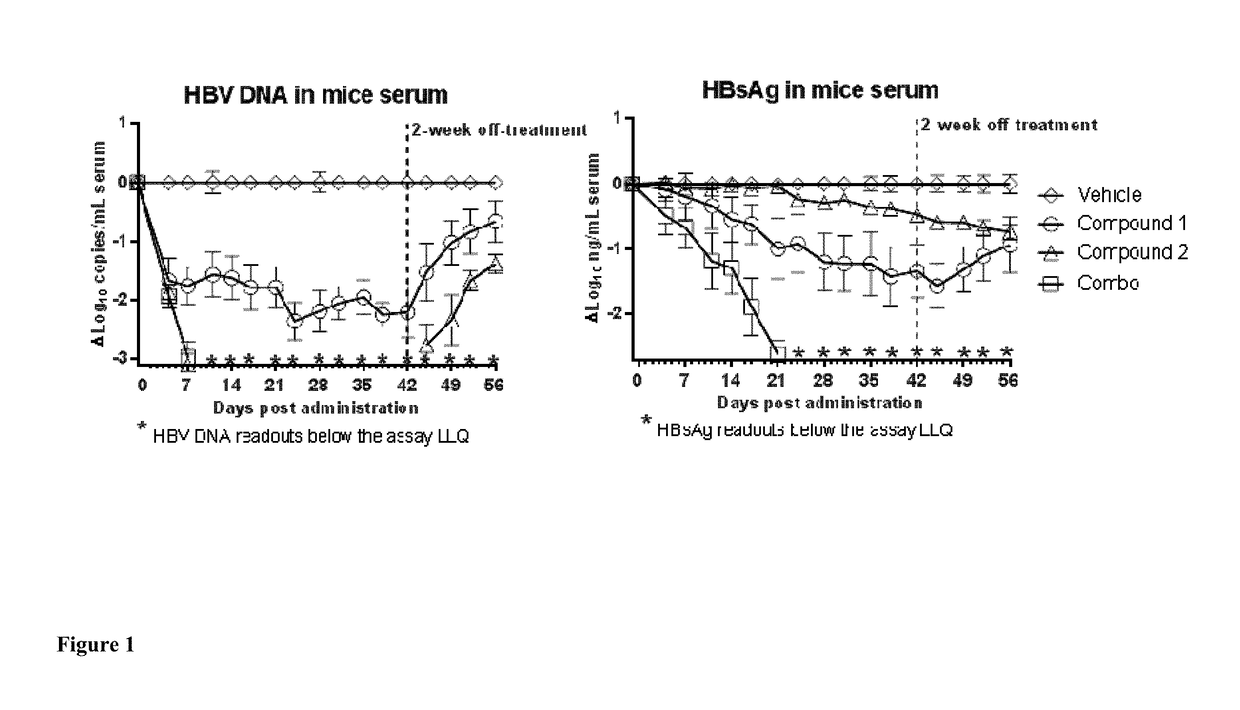
Provided herein is a combination therapy comprising a compound of Formula I and peginterferon alfa-2a, or another interferon analog. The combination therapy is useful for the treatment of HBV infection. Also provided herein are compositions comprising a compound of Formula I and peginterferon alfa-2a, or another interferon analog.
Owner:NOVIRA THERAPEUTICS
Methods of treating hbv and hcv infection
InactiveUS20110150836A1Symptoms improvedReduction in rate of progressBiocidePeptide/protein ingredientsHepatitis c viralPurine
This application relates to a purine derivative and pharmaceutical compositions which are useful for treating a hepatitis B viral infection or a hepatitis C viral infection.
Owner:GILEAD SCI INC
HBV antisense inhibitors
ActiveUS8598334B2Promoting seroconversionReduce the amount requiredOrganic active ingredientsSugar derivativesOligomerMethylene bridge
Antisense oligomers useful for modulating hepatitis B virus infections, and for the treatment of hepatitis B virus (HBV) and hepatitis B virus-related conditions in animals including humans. More particularly, antisense oligomers with modified nucleotides for treatment of HBV in animals, more particularly antisense oligomers comprising 2′O-4′C-methylene-bridged sugars, or nucleotides with other 2′O-4′C bridged sugars, also known as locked nucleic acids (LNA), for treatment of HBV in animals, and more particularly for treatment of HBV in humans.
Owner:GLAXO GRP LTD
6-fused heteroaryldihydropyrimidines for the treatment and prophylaxis of hepatitis B virus infection
Owner:F HOFFMANN LA ROCHE & CO AG
Novel 4-methyl-dihydropyrimidines for the treatment and prophylaxis of hepatitis b virus infection
Owner:F HOFFMANN LA ROCHE & CO AG
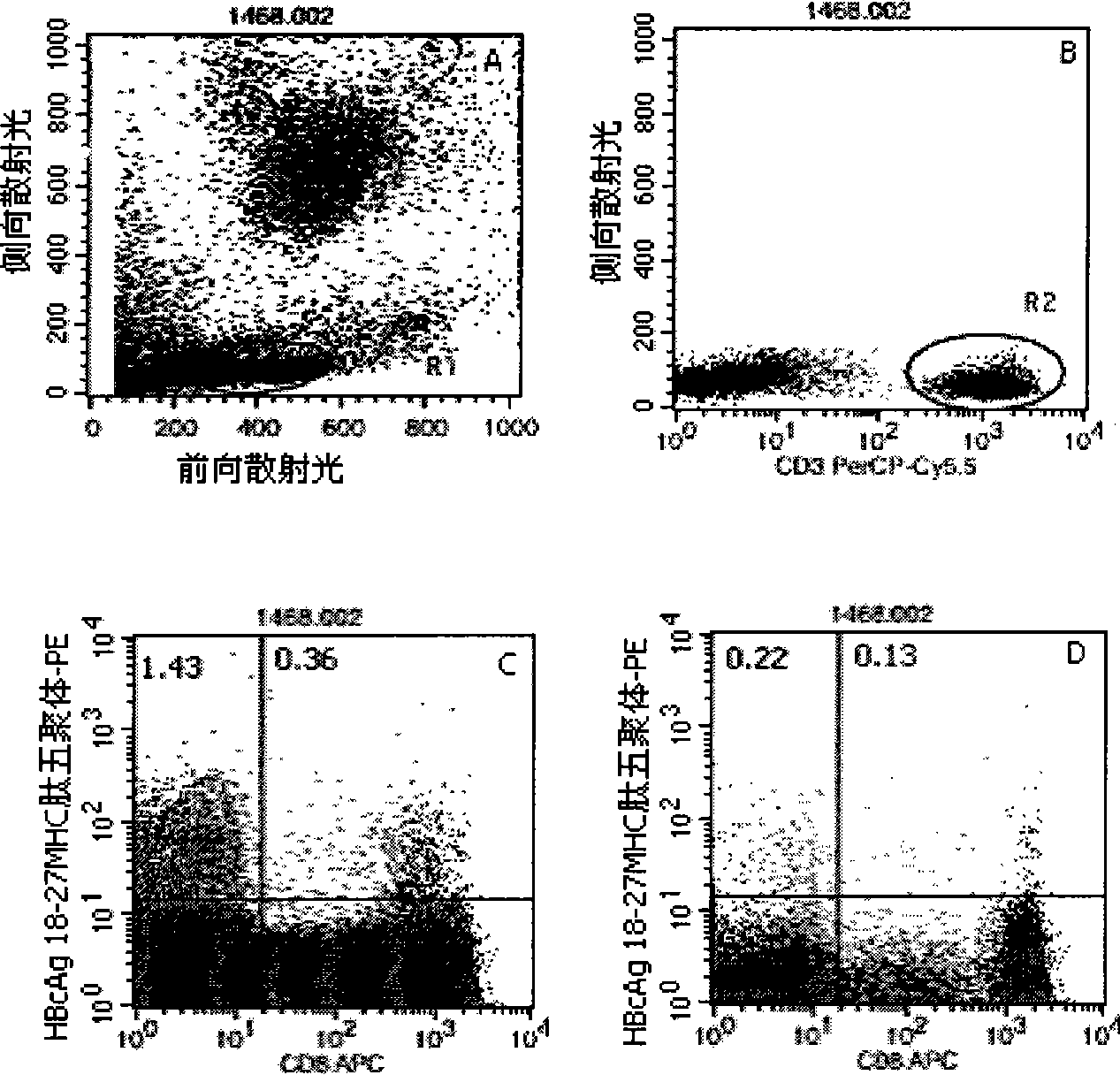
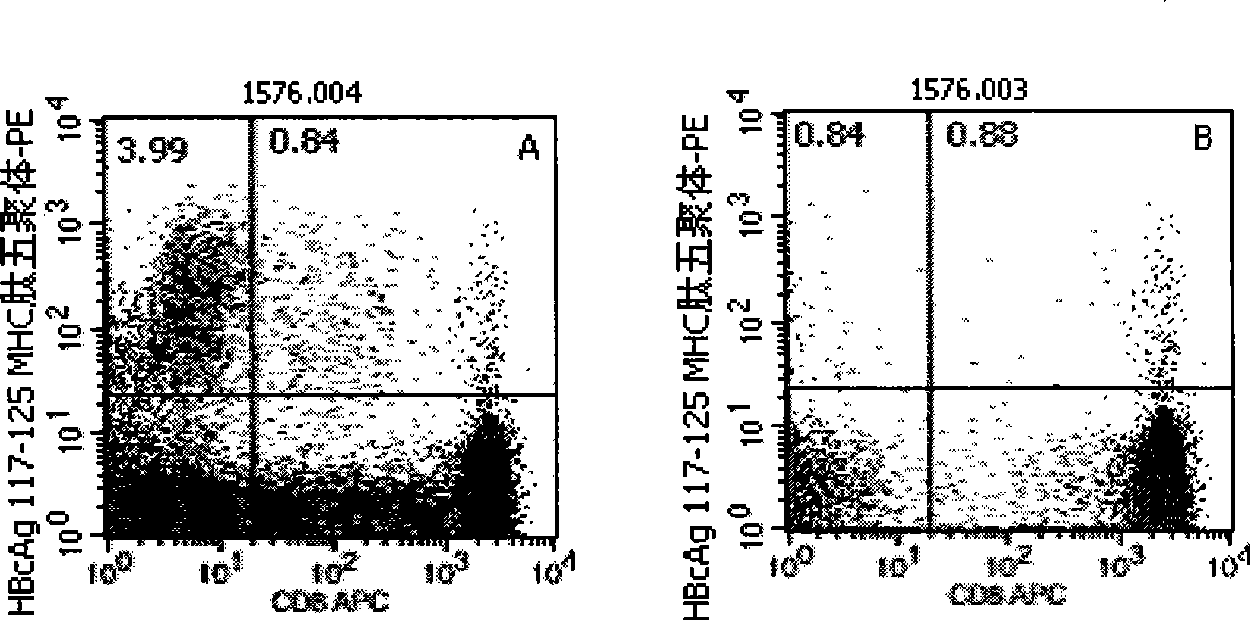
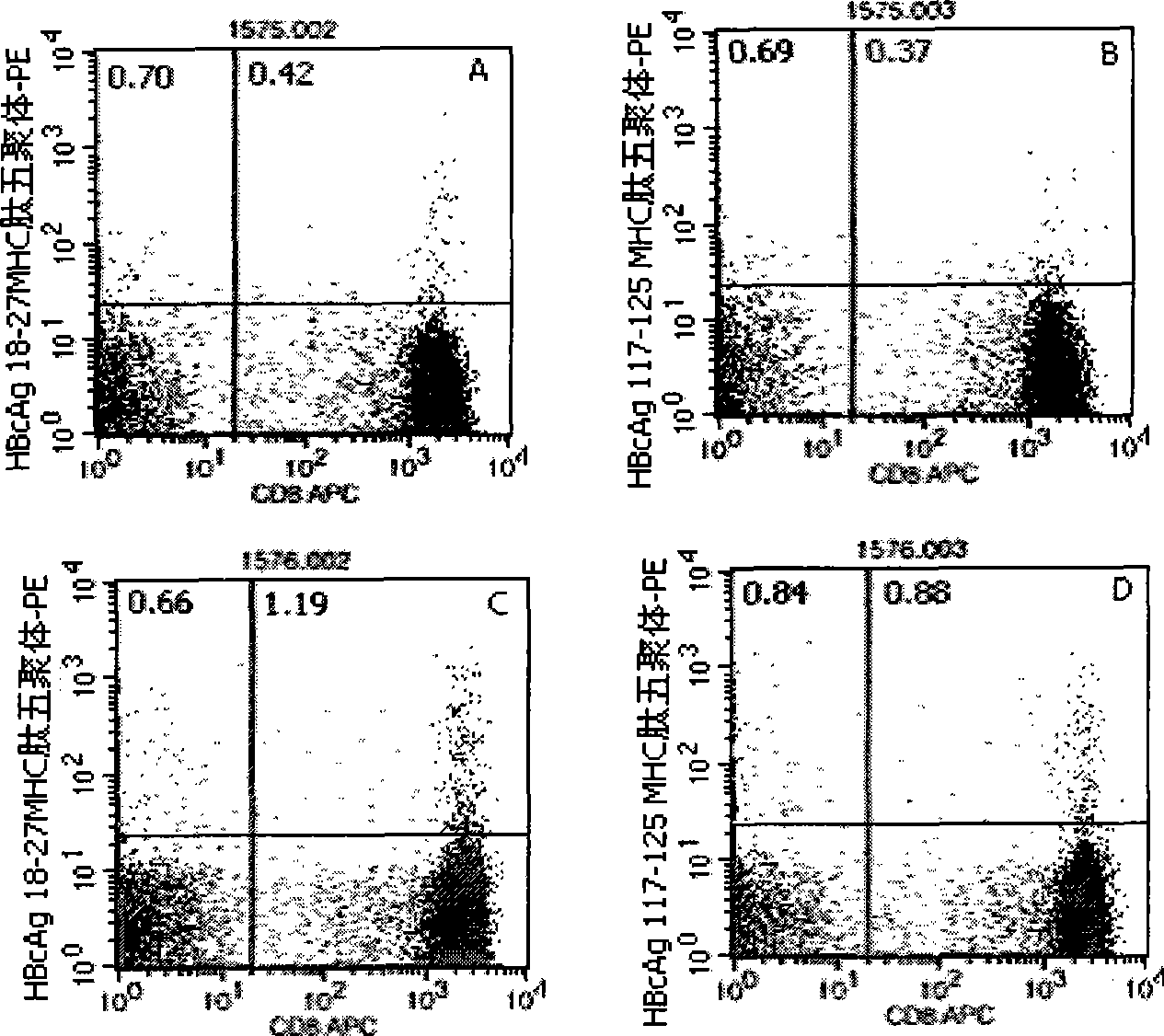
Quantitative determination method for hepatitis b virus specificity cell toxicity T lymphocyte
The invention belongs to the field of immunodetection and discloses a method for the quantitative determination of specific cytotoxicityT lymphocyte of hepatitis B virus. The method adopts the technical proposal that the major histocompatibility compound of antigen peptide pentamer, a mouse anti-human CD3 monoclonal antibody and a mouse anti-human CD8 monoclonal antibody which are labeled by different fluoresceins are incubated with the anticoagulant peripheral blood of an HLA-A0201 or HLA-A2402 positive masculine hepatitis B virus carrier for a proper period; and after the operations of hematid schizolysis, centrifugal washing and cell fixation are completed, the CD3 gating technique is used for the quantitative determination of the specific cytotoxicityT lymphocyte of hepatitis B virus on a flow cytometry. The invention can increase the determination of the specificity, the sensitivity and the stability of the specific CTL.
Owner:JIANGSU PROVINCE HOSPITAL
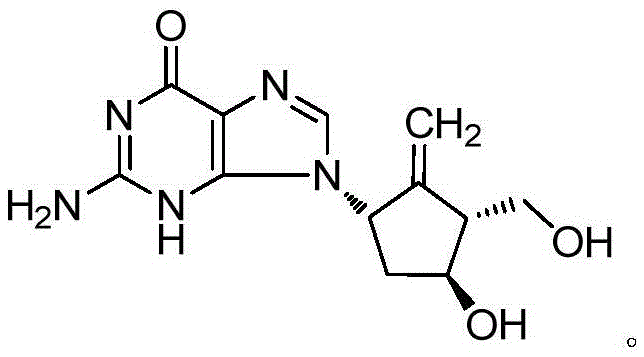
Entecavir fatty acid derivatives and pharmaceutical composition thereof
ActiveCN105585569AImprove bioavailabilityRelieve headacheOrganic chemistryAntiviralsSide effectAcid derivative
The invention provides entecavir fatty acid derivatives and pharmaceutical composition thereof and further relates to preparation methods of the entecavir fatty acid derivatives. The entecavir fatty acid derivatives had higher bioavailability and smaller toxic and side effects, and the pharmaceutical composition of the entecavir fatty acid derivatives is safer and more efficient when used for treating hepatitis B virus infection.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
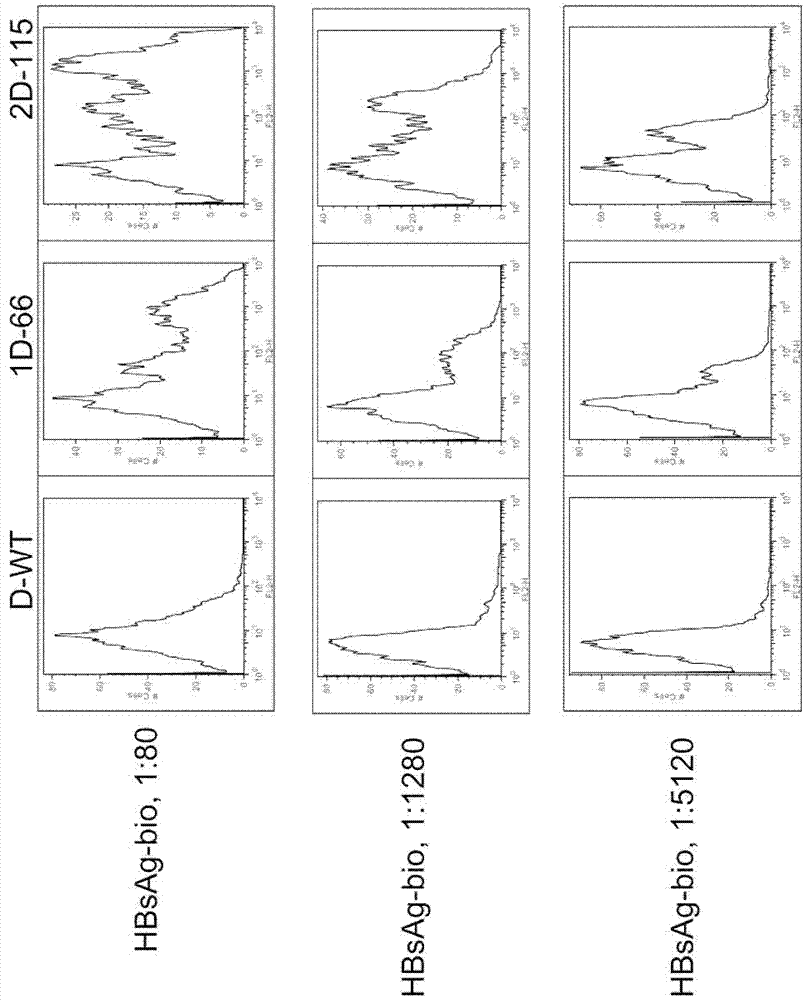
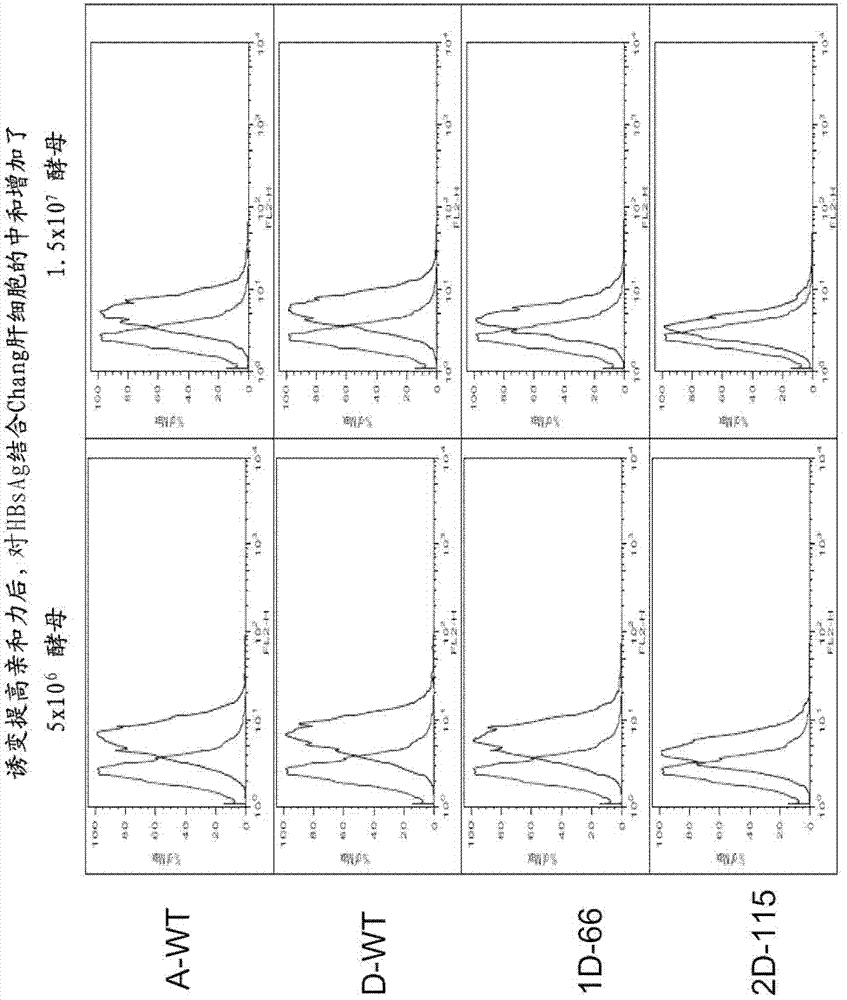
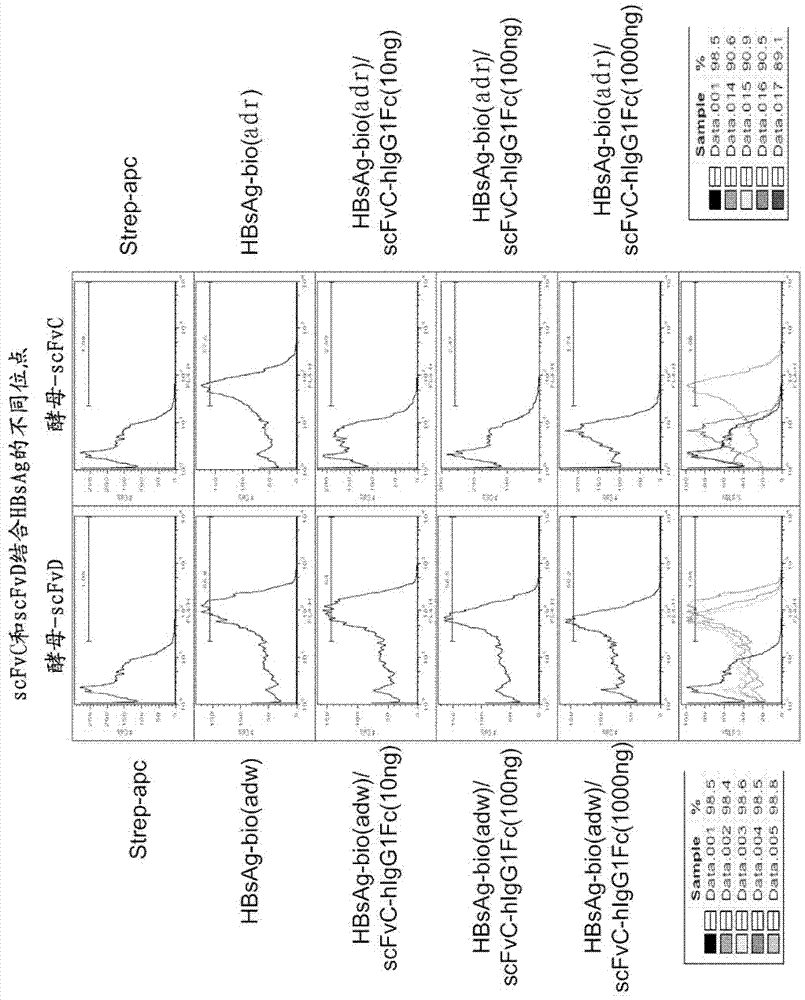
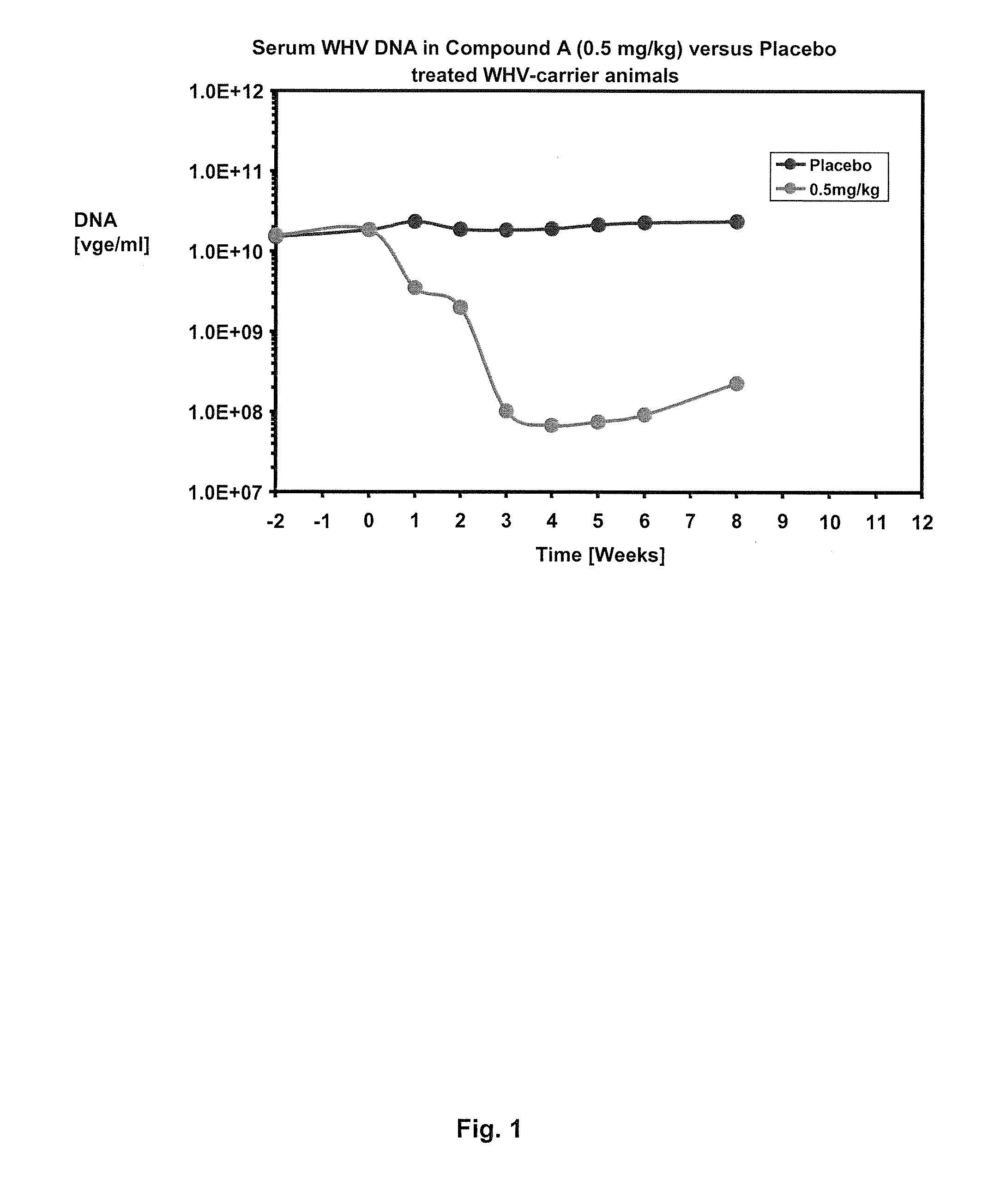
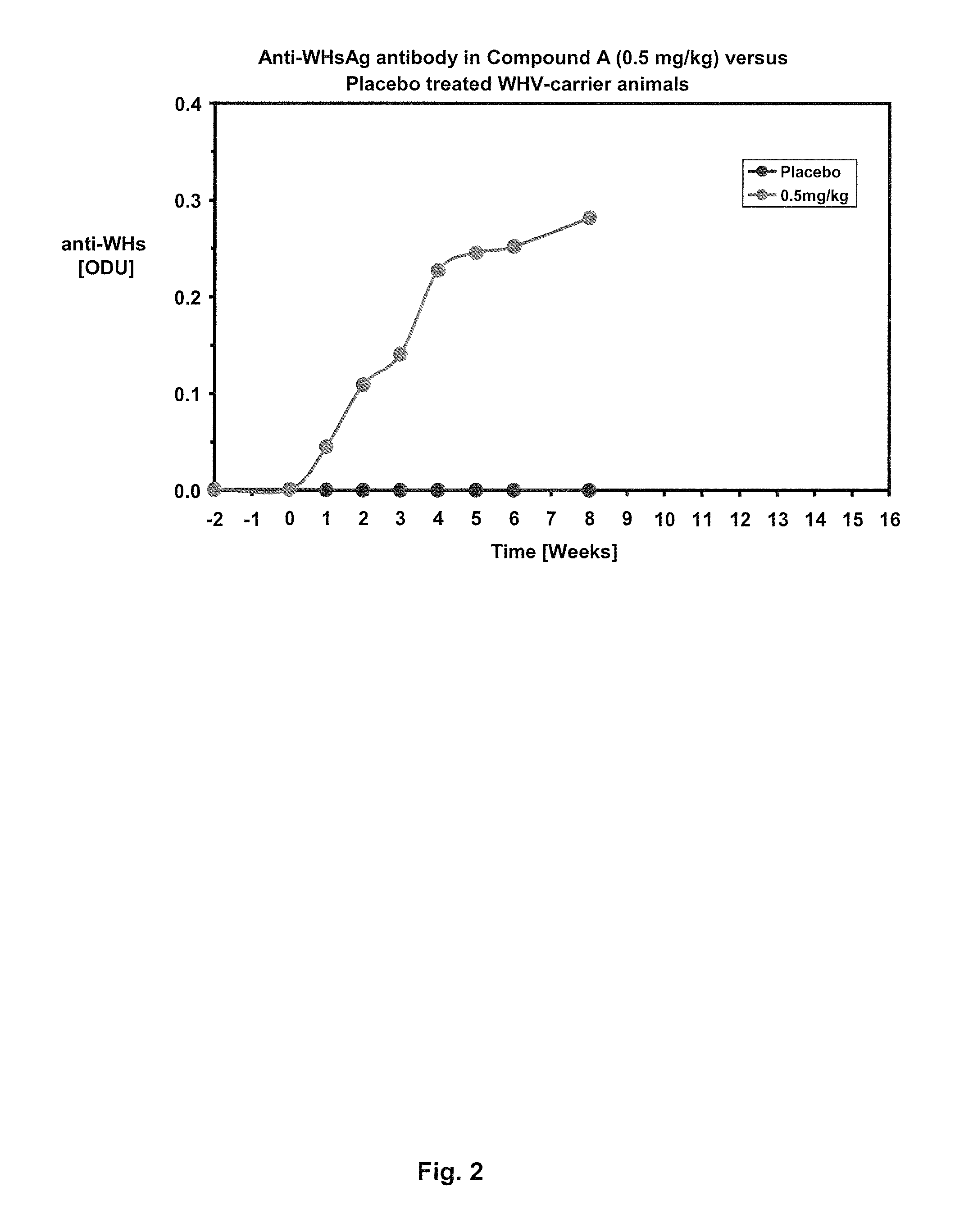
Treatment of hepatitis B virus infection with human monoclonal antibodies
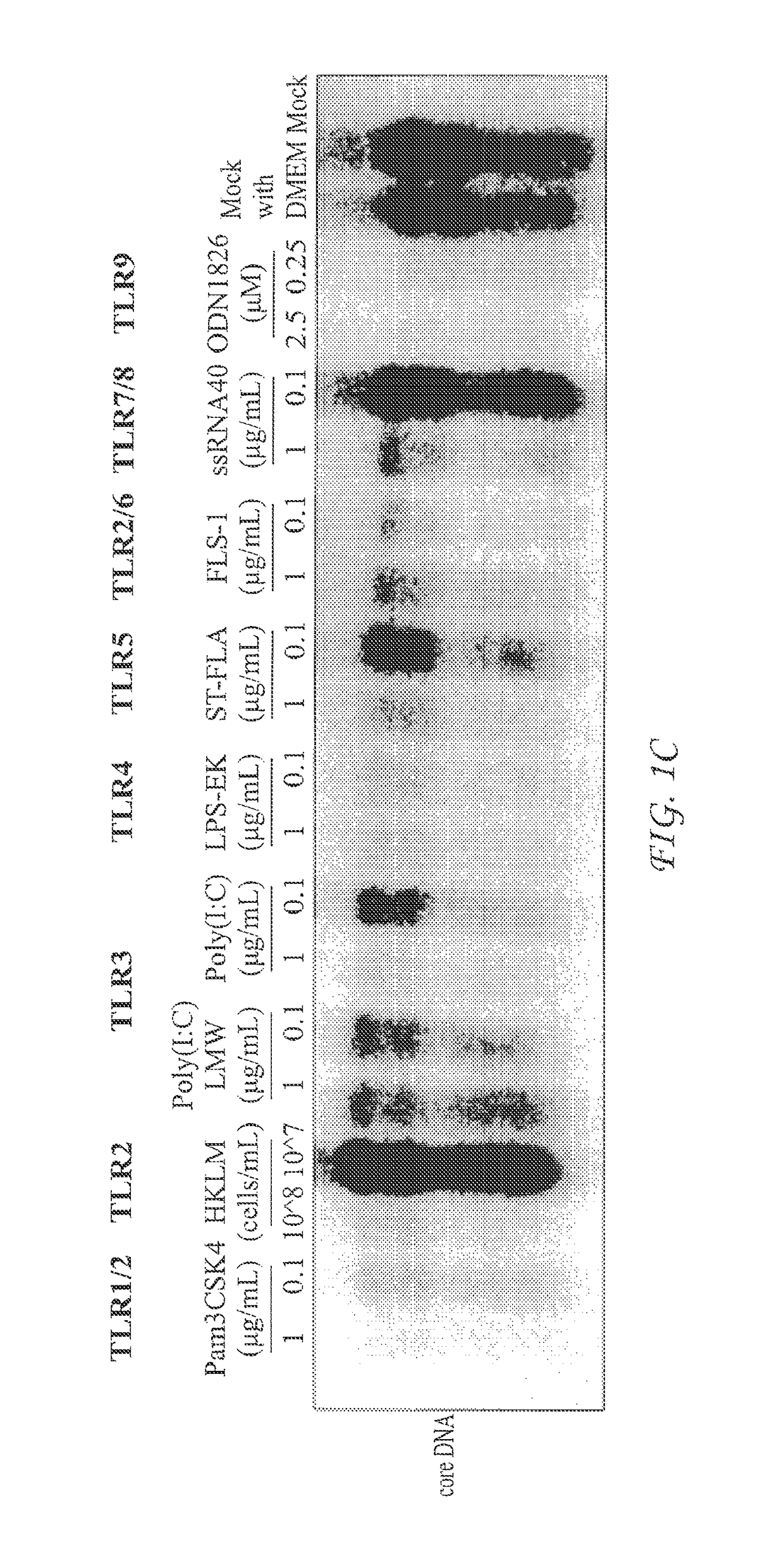
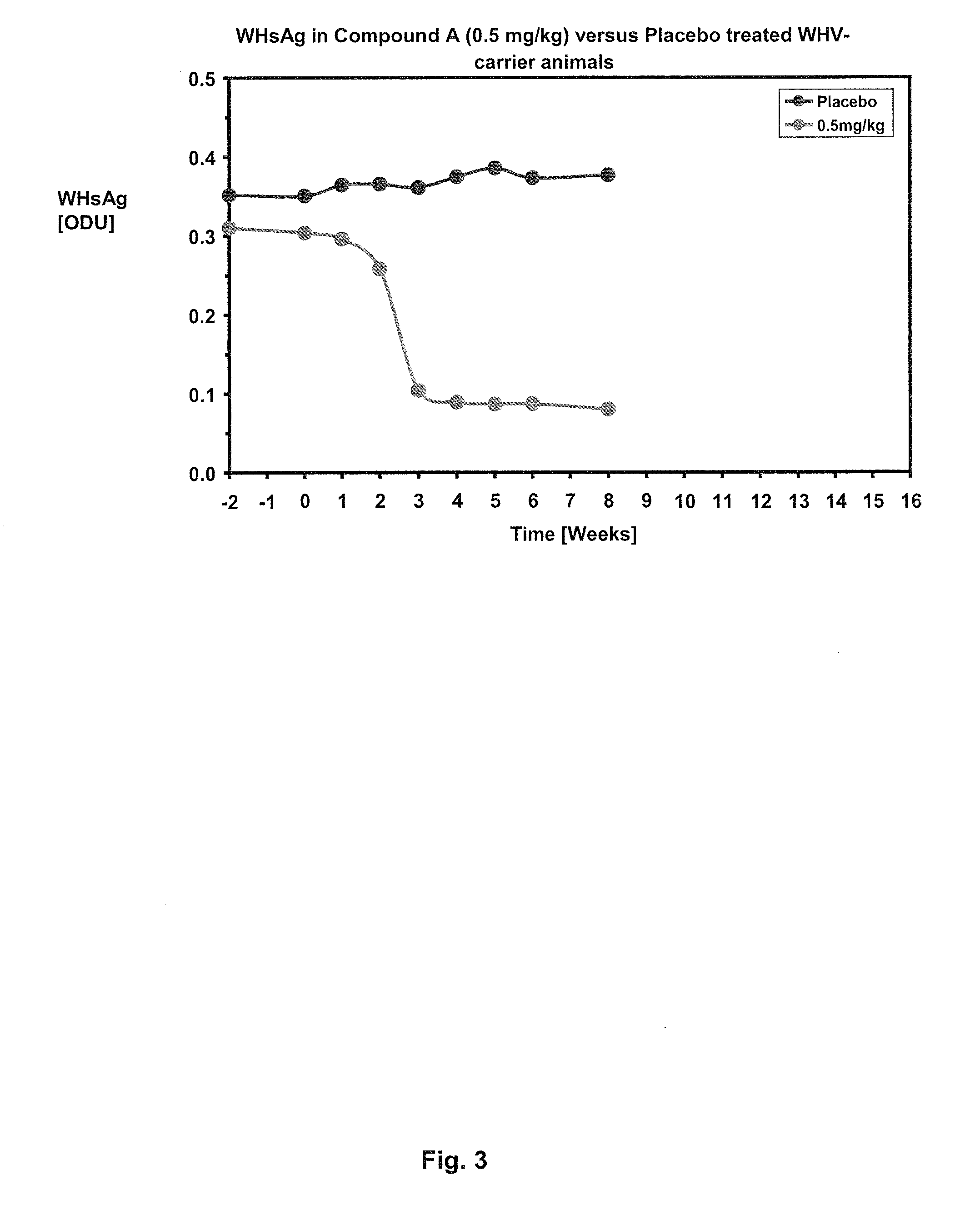
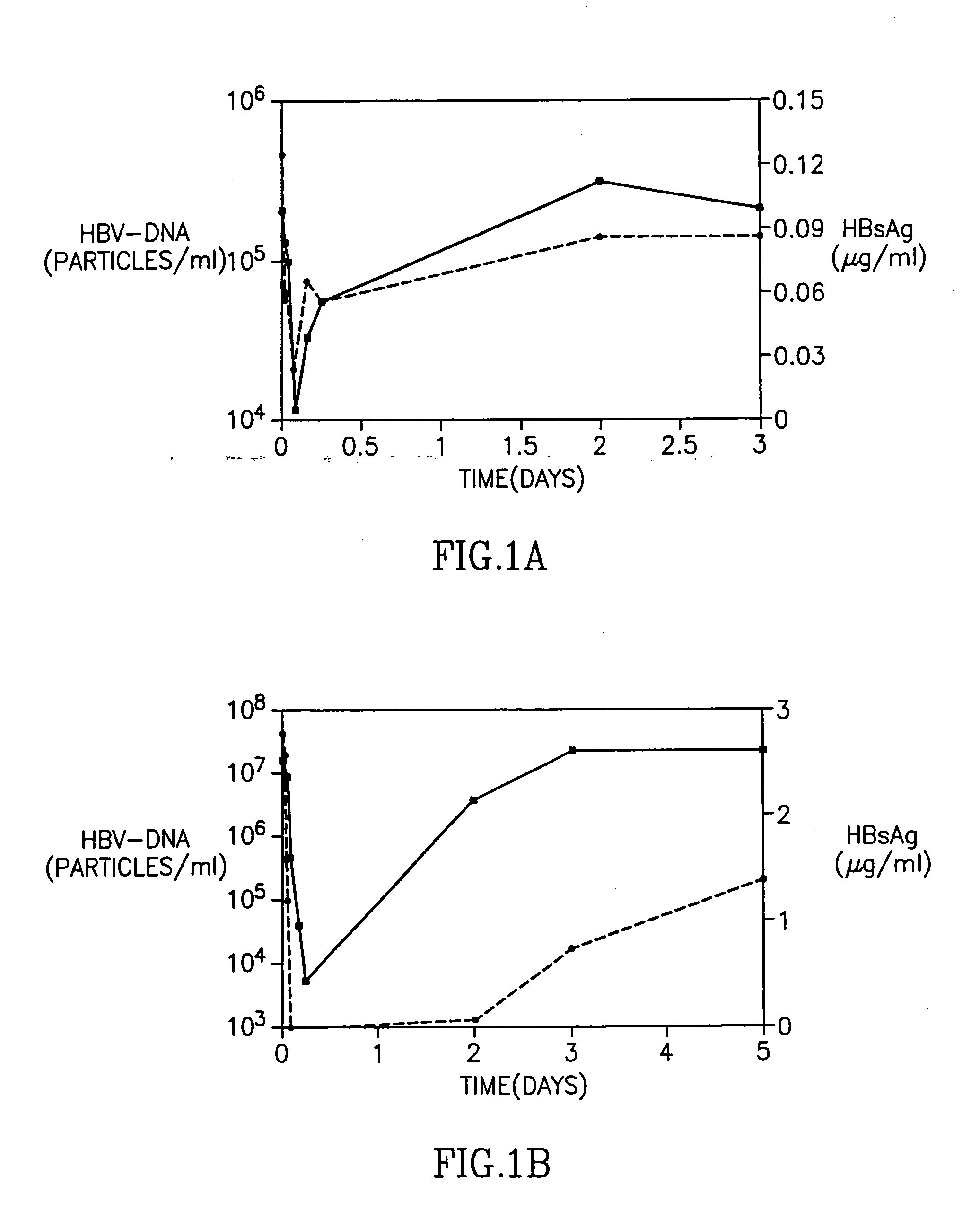
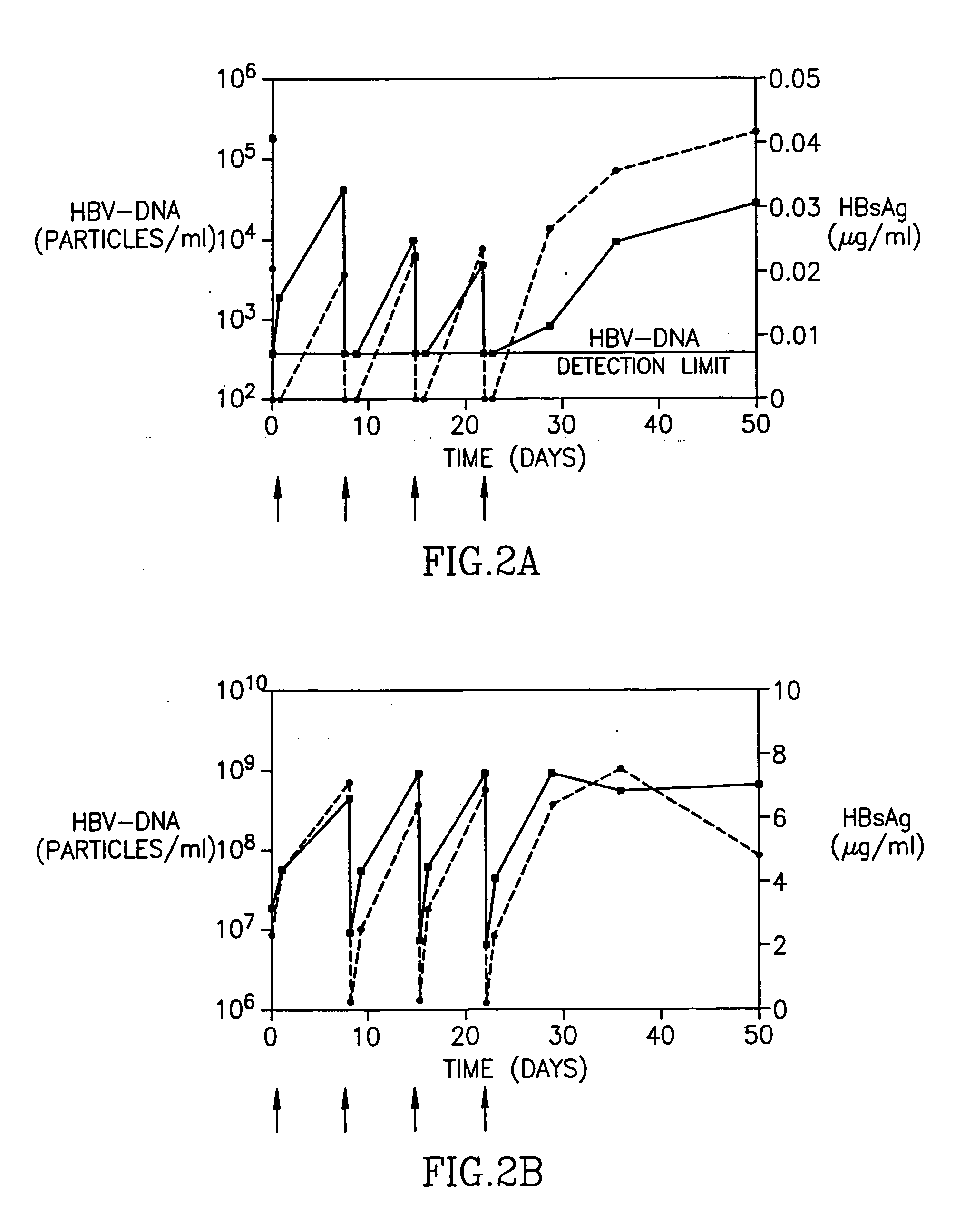
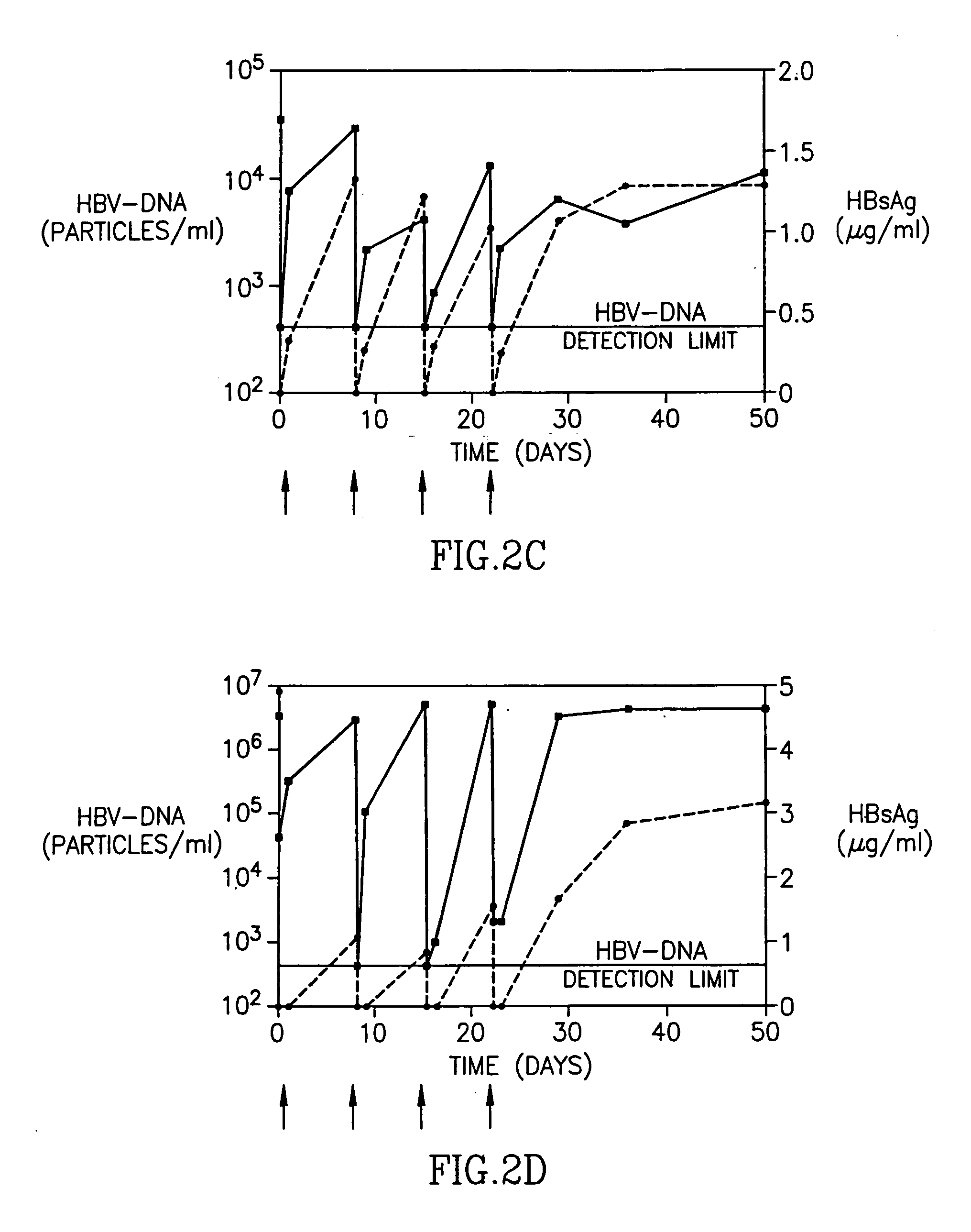
InactiveUS20050260195A1Eliminate infectionMicrobiological testing/measurementImmunoglobulins against virusesHBsAgCyrtanthus elatus virus A
Disclosed is a pharmaceutical composition for the treatment or prevention of hepatitis B virus infection, comprising a 1:3 mixture of two fully human anti HBsAg monoclonal antibodies 19.79.5 and 17.1.41. Also disclosed are preferred modes of administration. The pharmaceutical composition can be given as a monotherapy or in combination with other anti viral agents.
Owner:XTL BIOPHARMLS
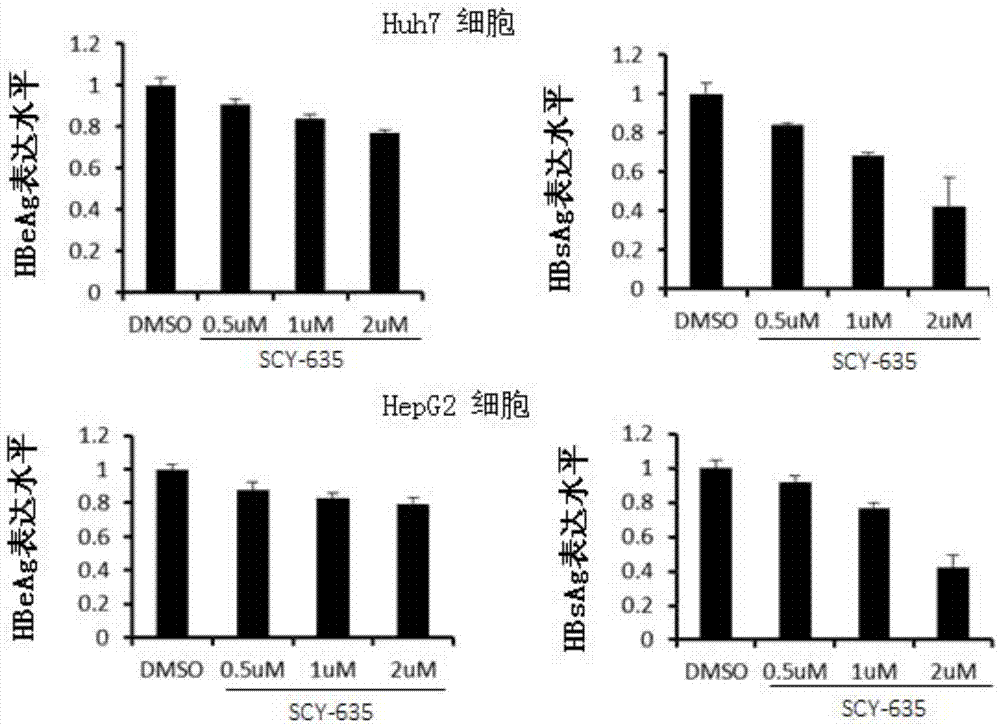
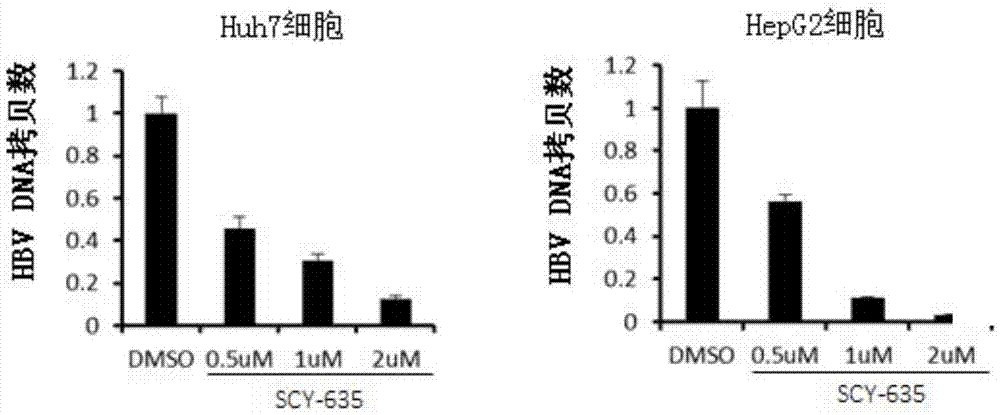
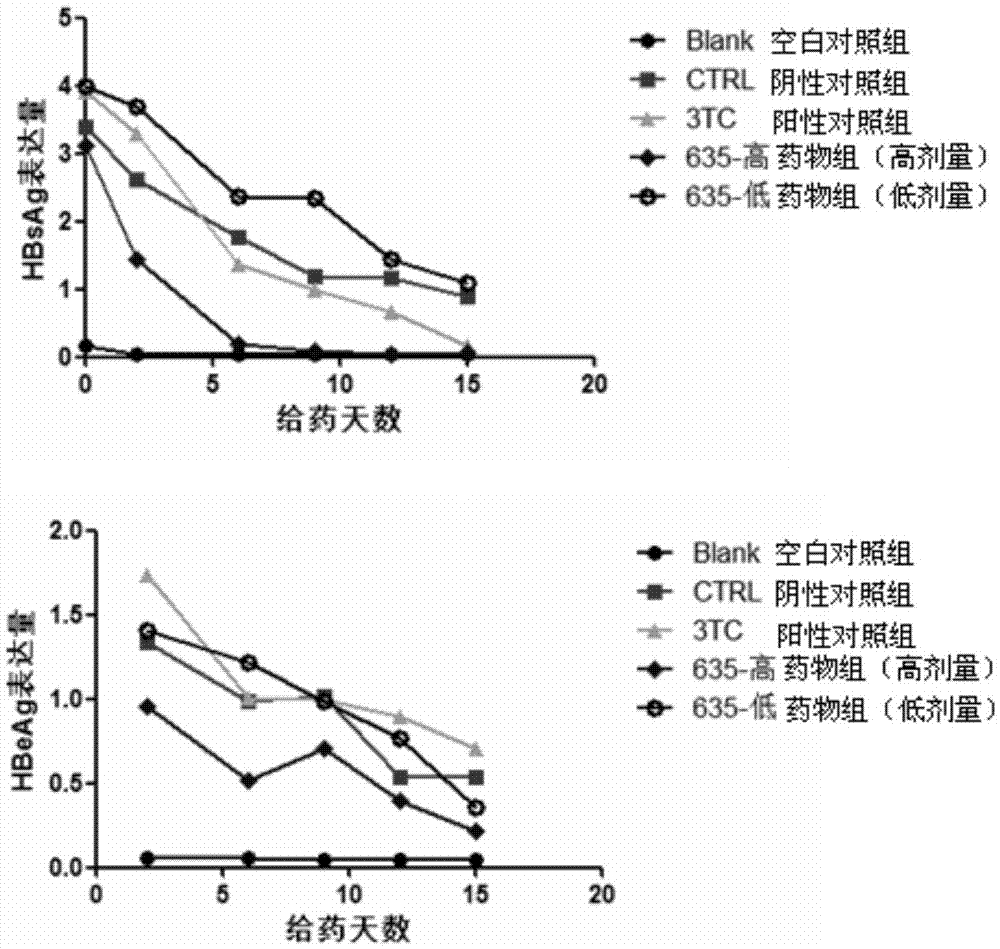
Application of cyclophilin inhibitor
The invention relates to application of a cyclophilin inhibitor, and particularly provides application of a cyclophilin inhibitor or pharmaceutically acceptable salt or solvate thereof in preparation of medicines. The medicines are used for treating and / or preventing diseases caused by hepatitis B virus infection.
Owner:WATERSTONE PHARMA WUHAN
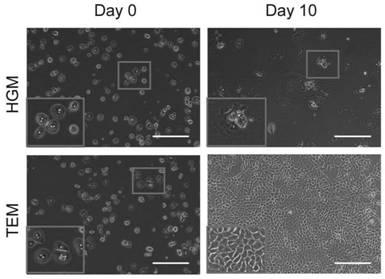
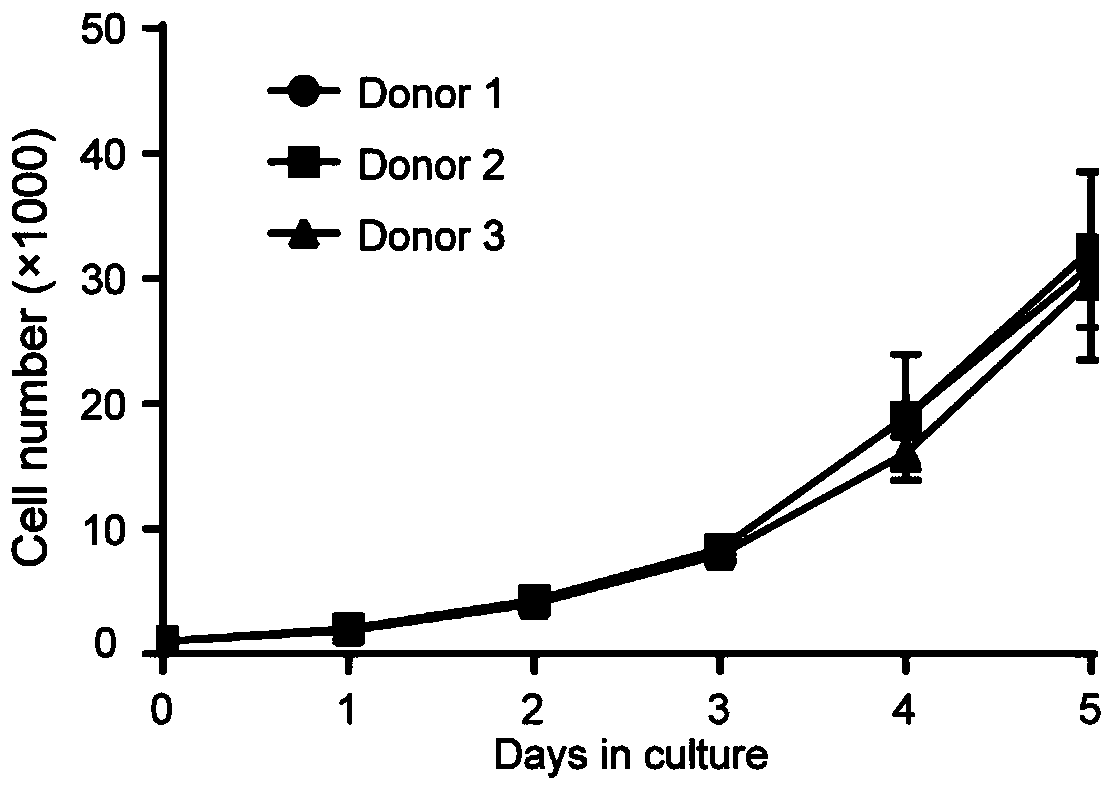
Liver precursor-like cell model derived from primary hepatocyte for hepatitis B virus infection, preparation method and application
ActiveCN109337858ANew sourceChromosomal stabilityCompound screeningApoptosis detectionIn vitro transformationInfected cell
The invention belongs to the field of hepatitis B virus infected cell models in life sciences and medicine, and provides a liver precursor-like cell model derived from a primary hepatocyte for hepatitis B virus infection, a preparation method and application. The liver precursor-like cell model consists of functional liver cells after three-dimensional differentiation, and the functional liver cells after three-dimensional differentiation are obtained by liver precursor-like cells obtained after in vitro conversion and cultivation of human primary hepatocytes after three-dimensional cultivation and liver maturation cultivation. Through experimental verification, after the liver precursor-like cell model is infected with HBV, the related genes of HBV infections such as RXRA, HNF4A, NTCP andthe like can be expressed highly, and the cell model can be used in hepatitis B virus infection research or can be co-cultured with HBV for preparing a hepatitis B virus infection cell model.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
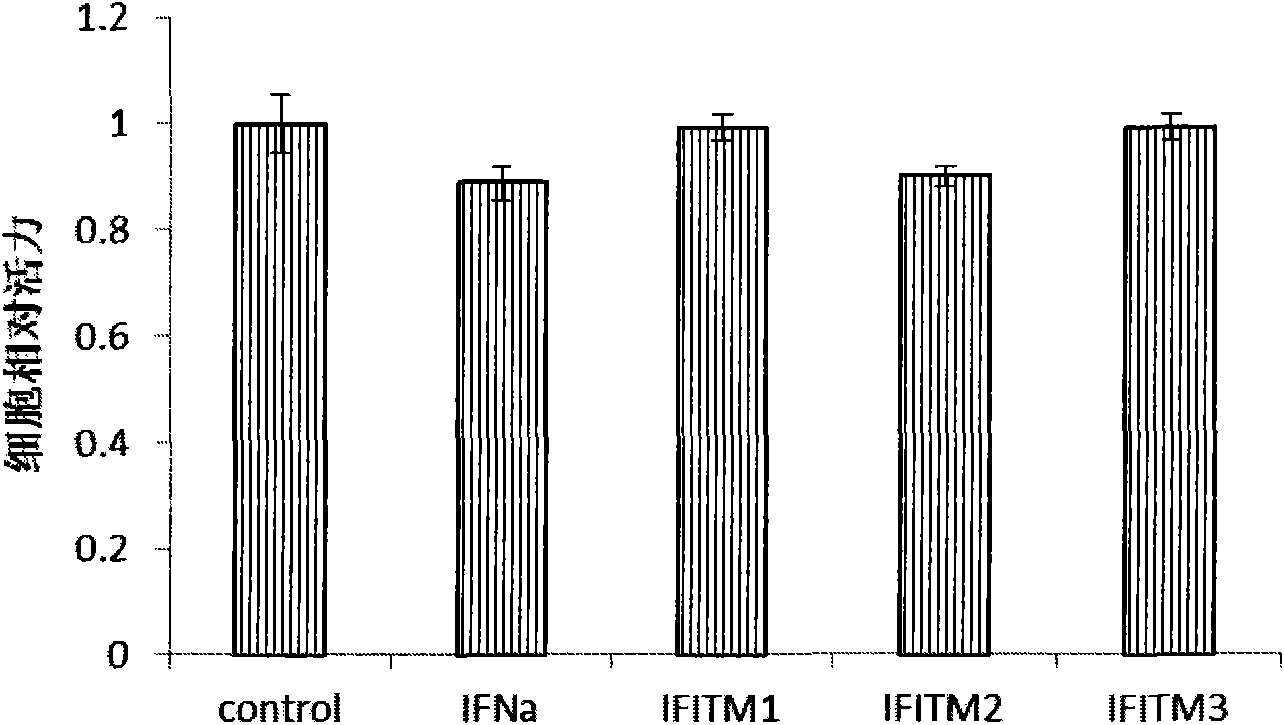
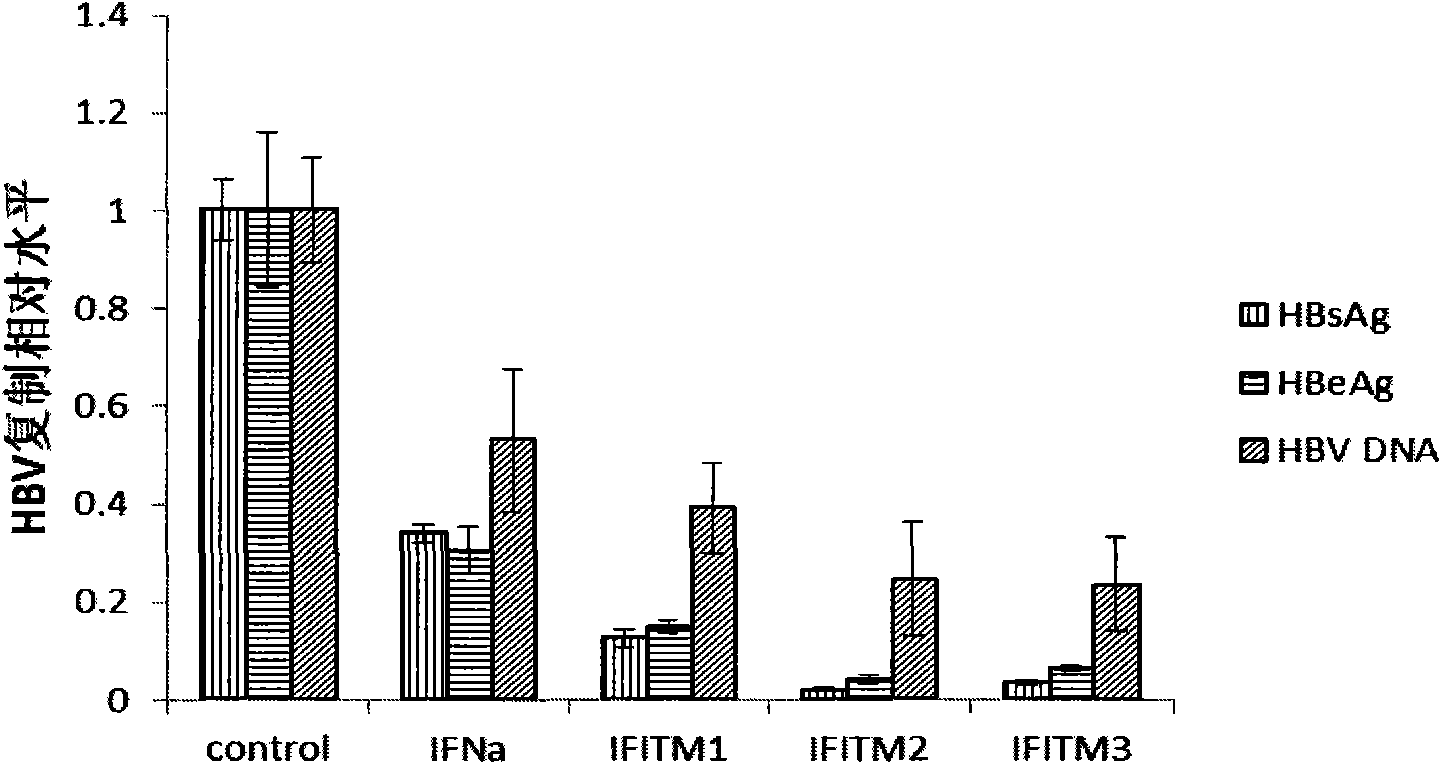
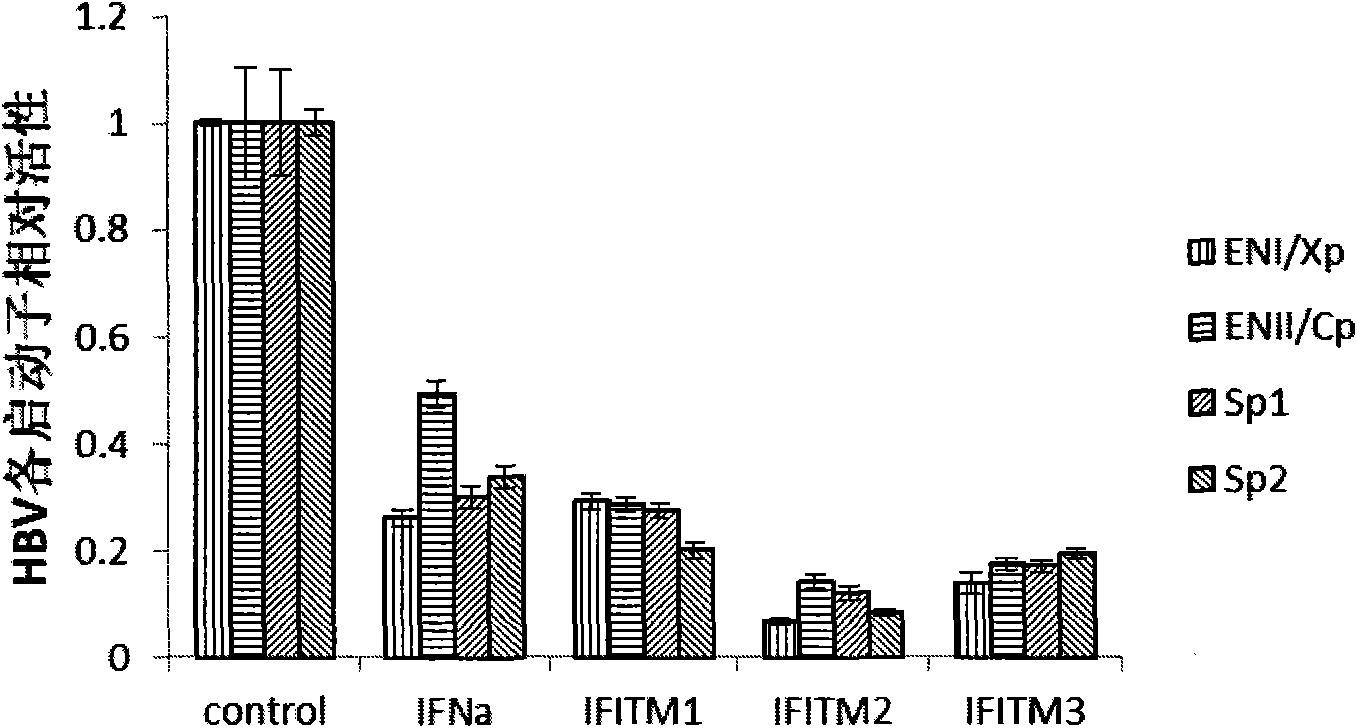
Application of interferon-induced transmembrane protein 3 (IFITM 3) for preparing medicament against hepatitis B virus (HBV) infection
The invention relates to new application of interferon-induced transmembrane proteins (IFITMs) in pharmaceutical engineering, in particular to application of IFITM 3 in preparing a medicament for treating diseases related to hepatitis B virus (HBV) infection. Eukaryotic expression vectors of IFITM 1, IFITM2 and the IFITM 3 are respectively constructed by a molecular biological method in a lab; and in vivo-in vitro tests prove that the IFITM 1, the IFITM2 and the IFITM 3 can obviously inhibit HBV replication, wherein, the IFITM 3 has the most remarkable effect. Therefore, the IFITM 3 and an encoding gene thereof are expected to become novel medicaments for treating HBV-related diseases and reducing hazard of HBV-related diseases.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
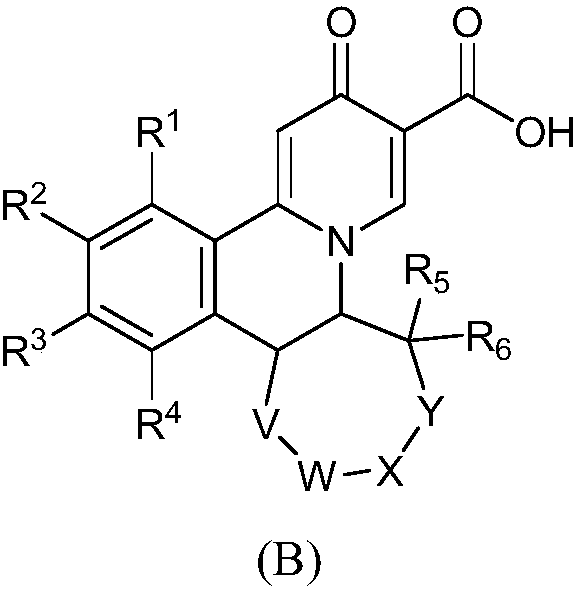
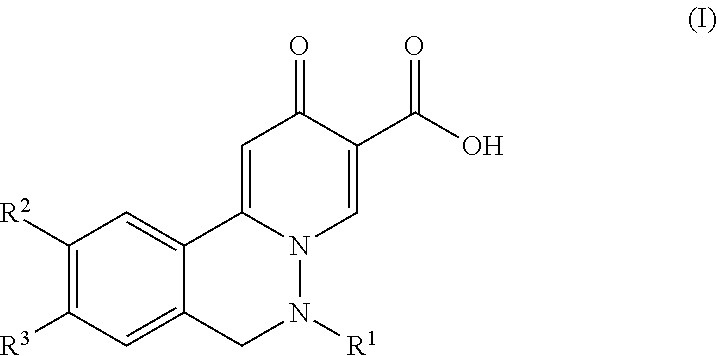
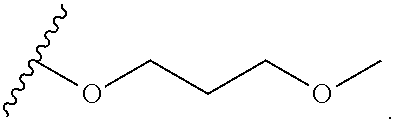
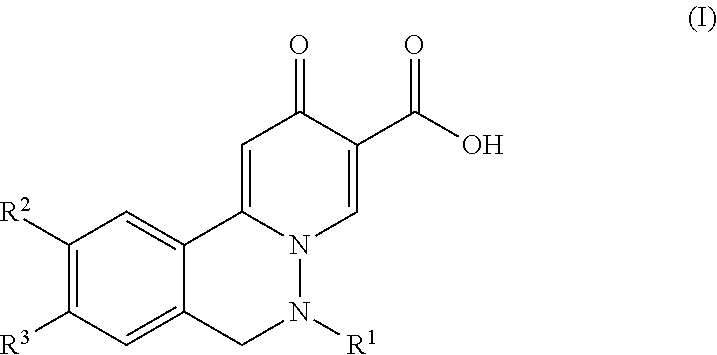
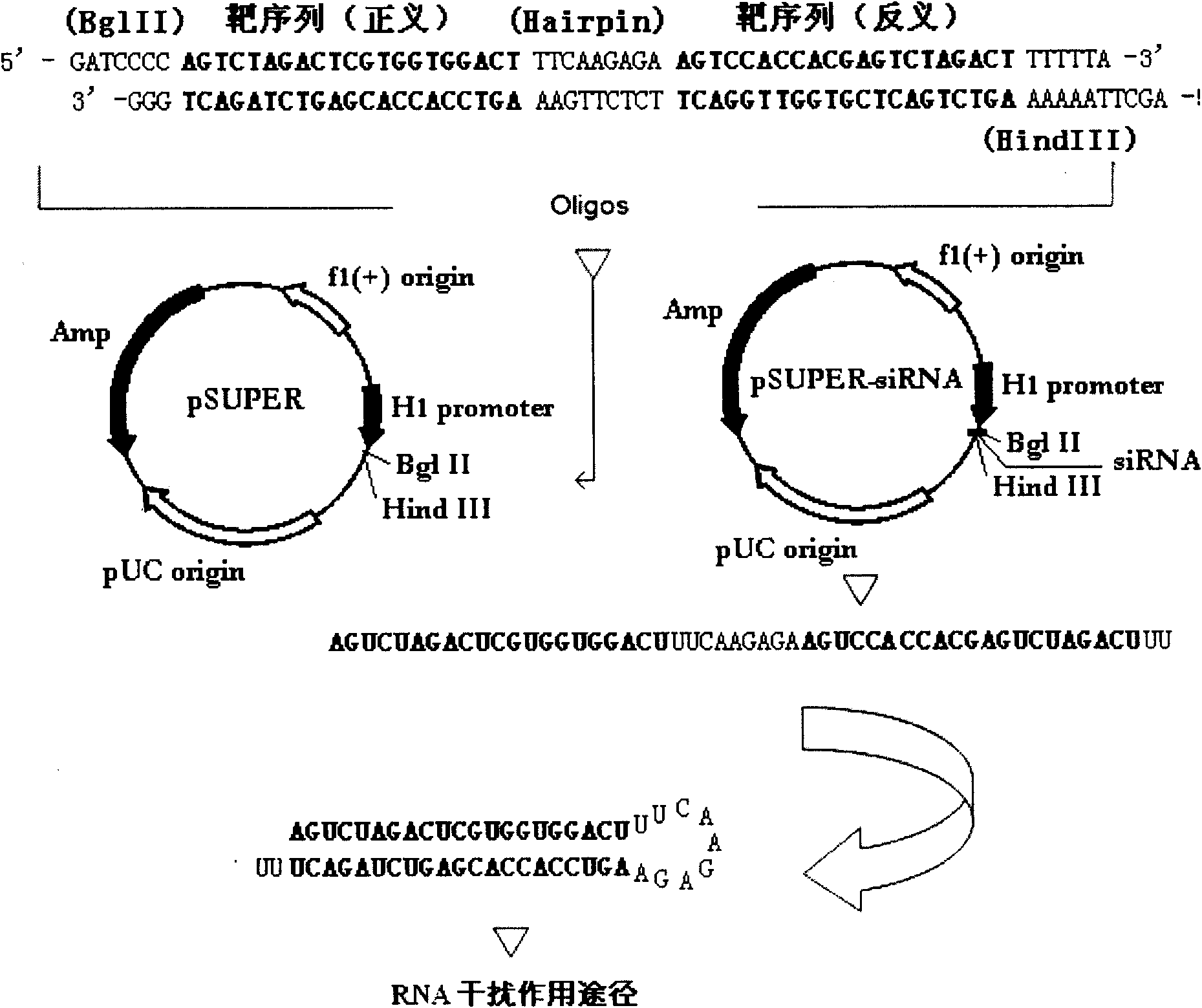
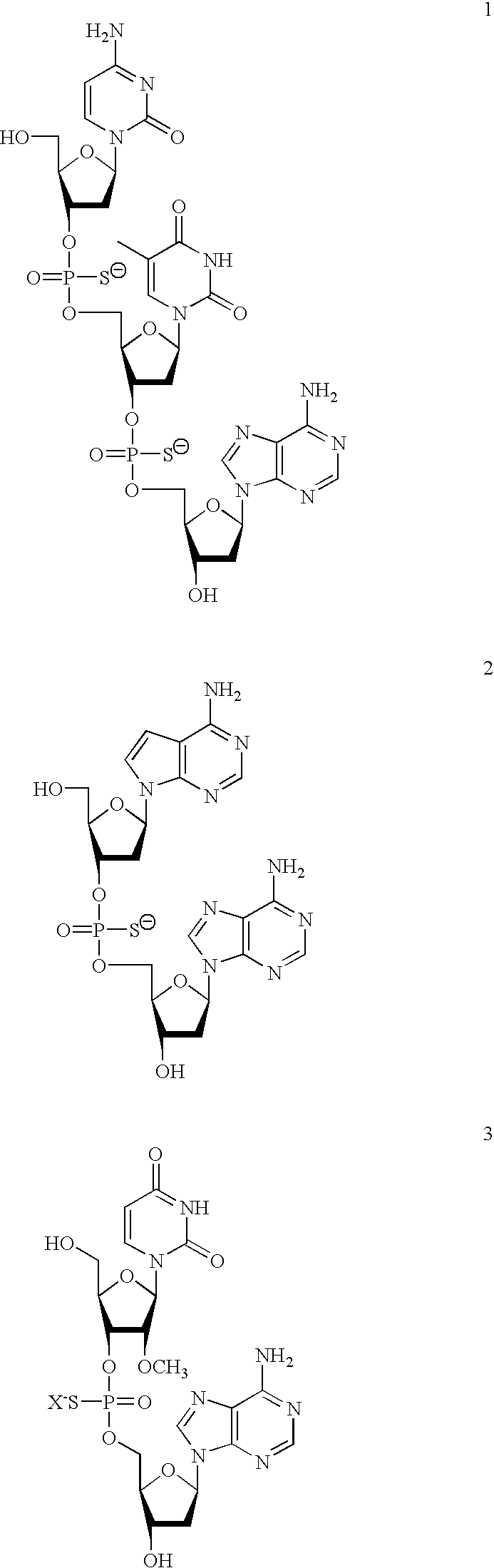
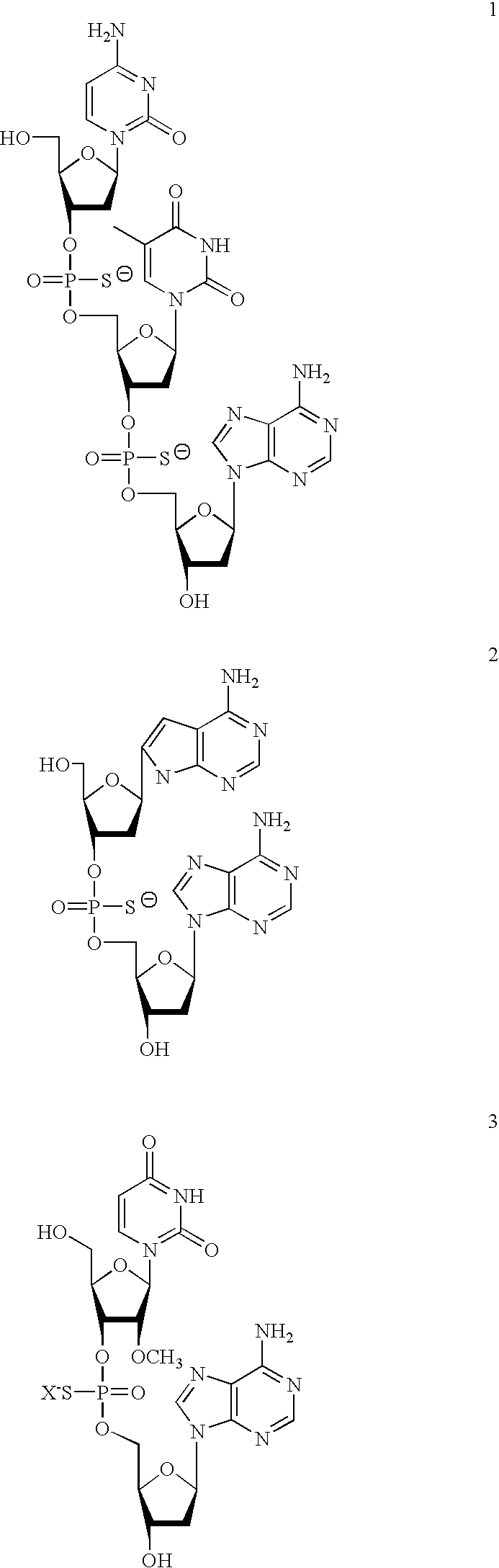
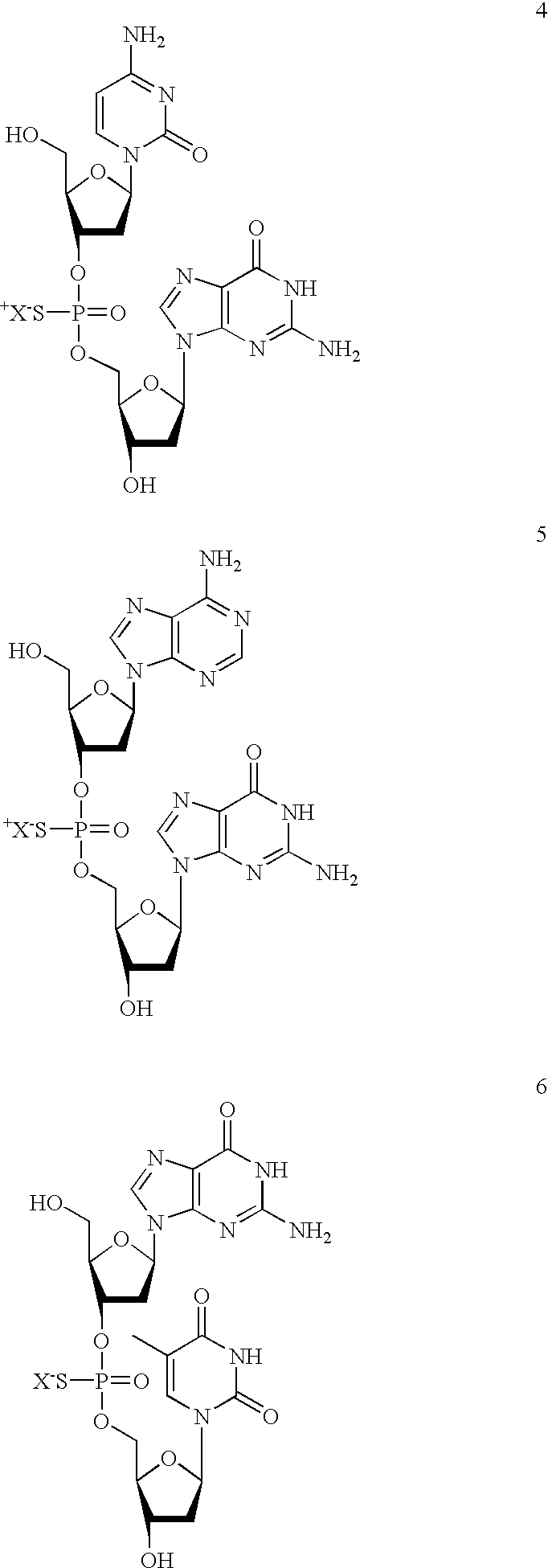
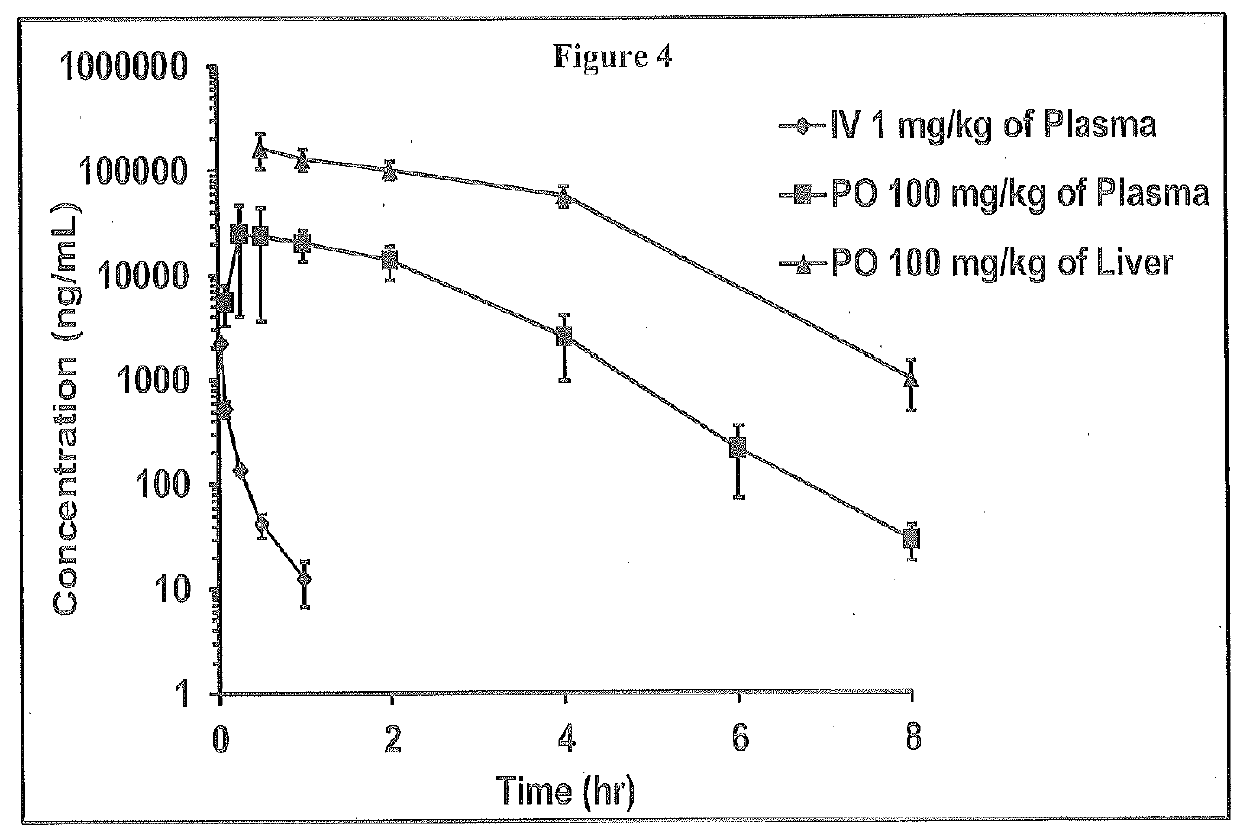
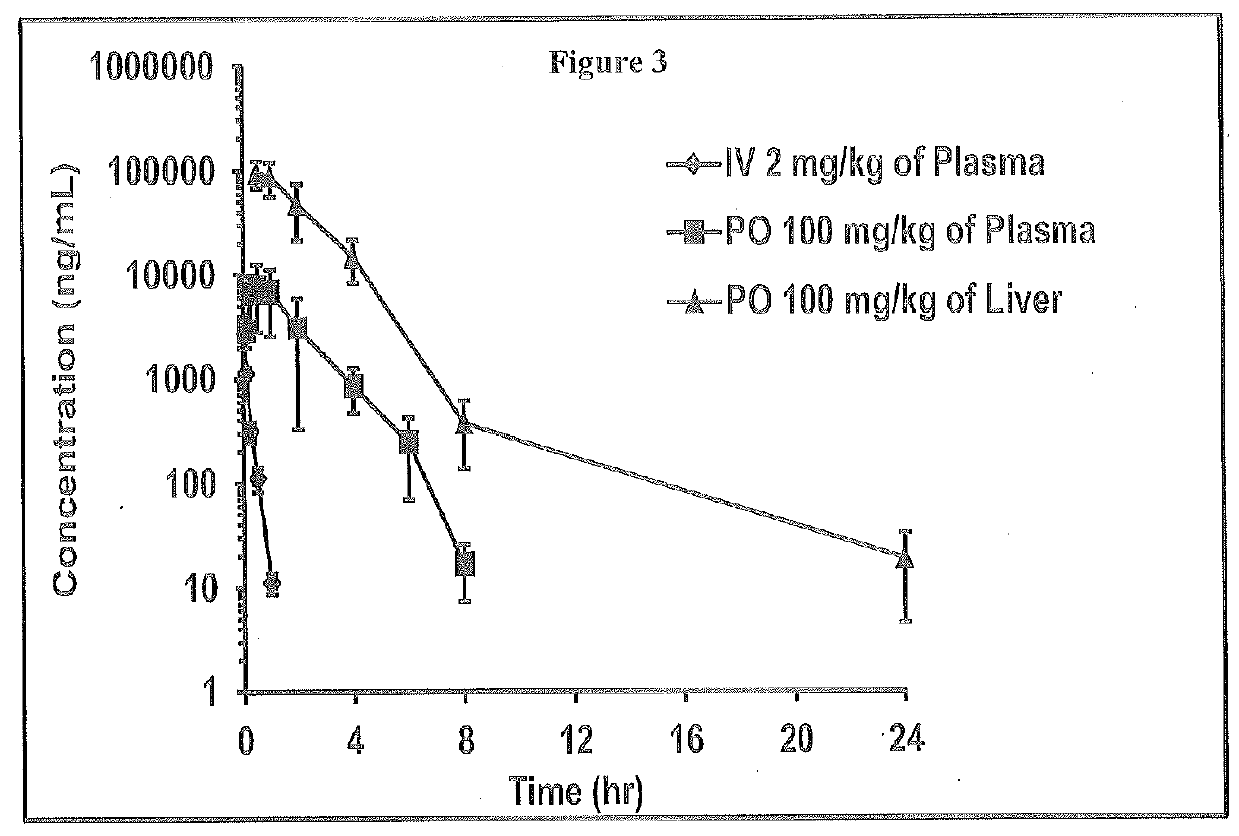
2-oxo-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis B virus infection
Owner:F HOFFMANN LA ROCHE & CO AG
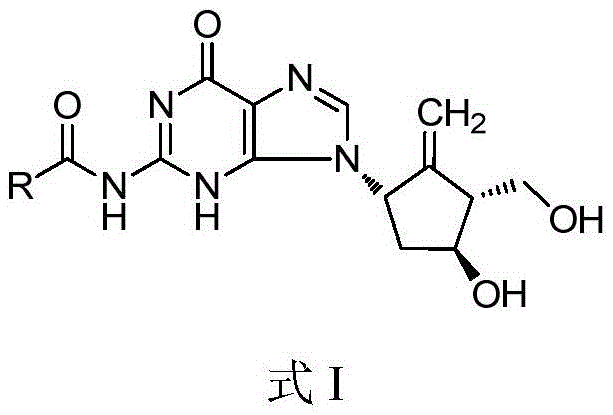
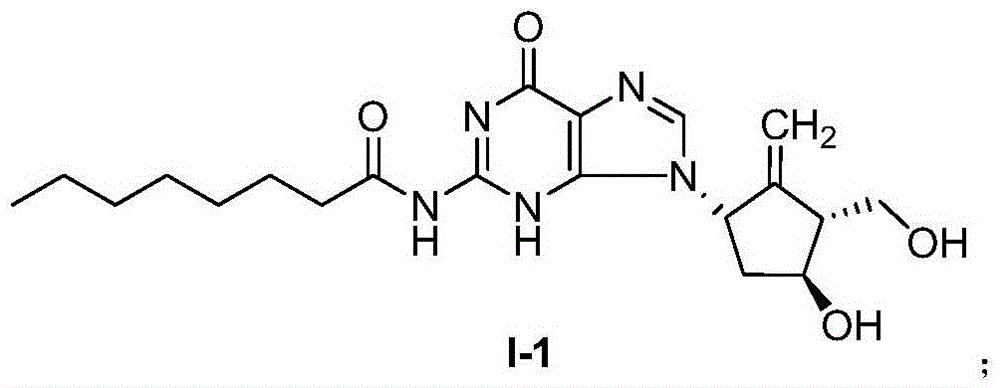
Compound for treating or preventing hepatitis B virus infection and preparation method and application thereof
ActiveCN108530449AInhibition of replicationImprove clearanceOrganic chemistryAntiviralsClearance rateHBsAg
The invention discloses a compound taking quinolizine ketone as a mother nucleus and for treating or preventing hepatitis B virus infection. The compound comprises optical isomer, raceme, cis-trans-isomer and any combination thereof or medicinal salt thereof. The invention further discloses a preparation method and application of the compound. The compound can remarkably lower in-vivo HBsAg leveland inhibit duplication of HBV, has good medicinal attribute and is low in toxicity, and pharmacokinetic and pharmacodynamic functions are improved; efficiency of combining with the HBV can be improved greatly, and clearance rate of in-vivo HBV can be further increased.
Owner:河南春风医药科技有限公司
Combined treatment with a tlr7 agonist and an hbv capsid assembly inhibitor
The present invention is directed to compositions and methods for treating hepatitis B virus infection. In particular, the present invention is directed to a combination therapy comprising administration of a TLR7 agonist and an HBV capsid assembly inhibitor for use in the treatment of chronic hepatitis B patient.
Owner:F HOFFMANN LA ROCHE INC
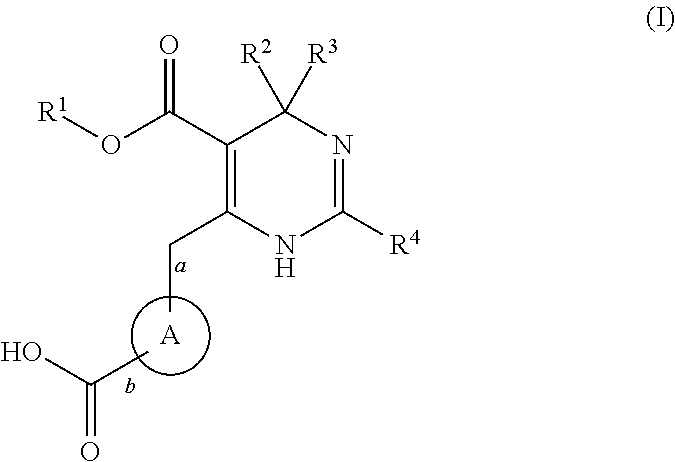
Pyrazine compounds for the treatment of infectious diseases
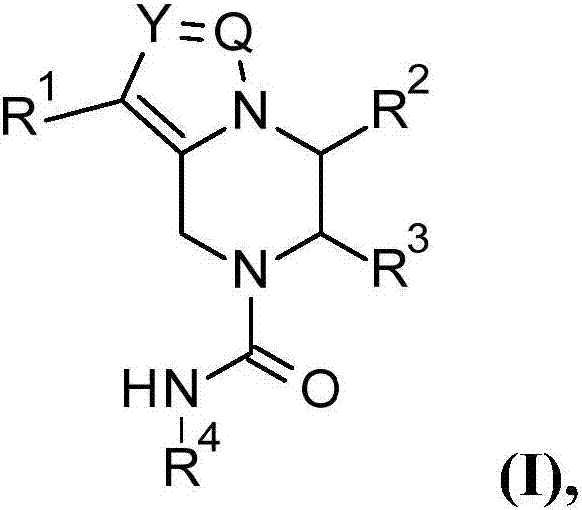
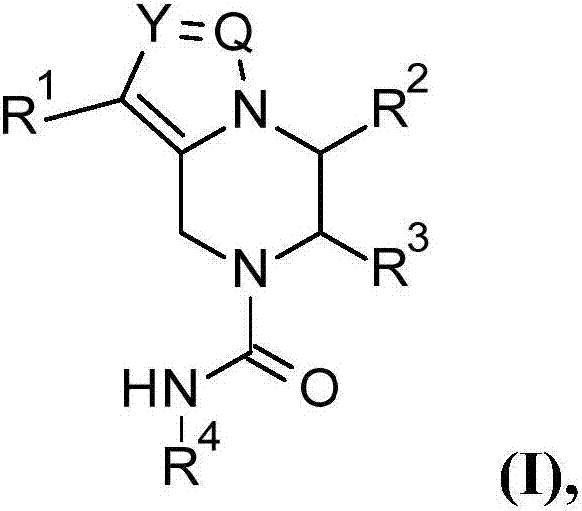
The present invention relates to compounds of the formula (I), or pharmaceutically acceptable salts, enantiomer or diastereomer thereof, wherein R1 to R4 are as described above. The compounds may be useful for the treatment or prophylaxis of hepatitis B virus infection.
Owner:F HOFFMANN LA ROCHE & CO AG
Compounds for the treatment of hepatitis b virus infection
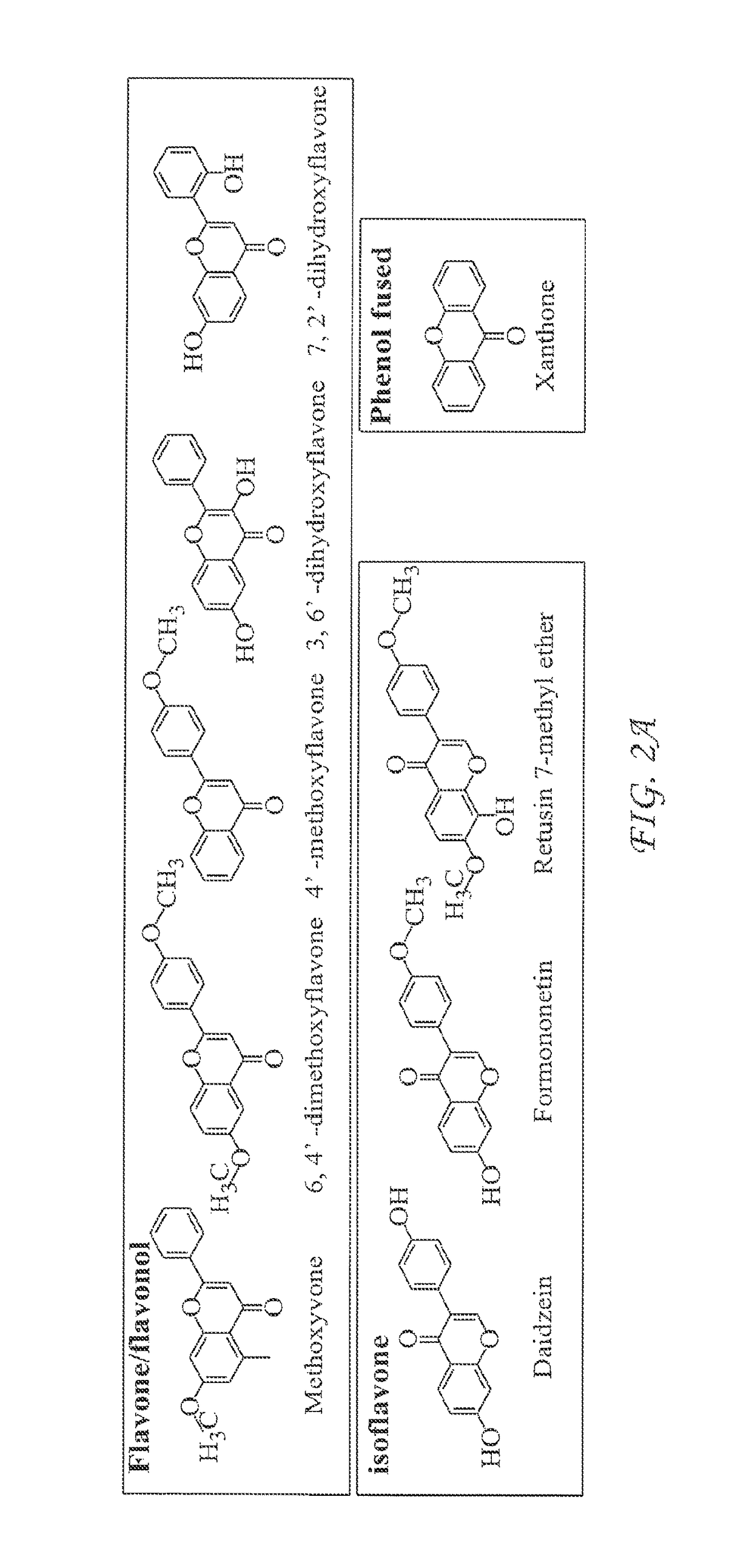
InactiveUS20170342068A1Inhibition productionInhibition of secretionOrganic chemistryAntiviralsHepatitis B virusCyrtanthus elatus virus A
Owner:GILEAD SCI INC
Anti-hepatitis B virus X protein peptide medicament
InactiveCN103992388AObvious pharmacodynamic effectInhibit biological activityFungiBacteriaFunctional activityMolecular level
The invention relates to the field of polypeptide medicament, and particularly relates to an anti-hepatitis B virus X protein peptide and polypeptide coding the peptide, and application thereof. Specifically, the invention relates to a polypeptide with functional activity to inhibit hepatitis B virus X protein (HBx) at the molecular level, cellular level and animal level, and therefore can inhibit hepatitis caused by hepatitis B virus infection of, cirrhosis caused by repeated attack of hepatitis, and the liver cancer occurred on the basis of cirrhosis. The polypeptide and peptide analogs thereof including their functional fragments and functional variants, and the genes encoding these peptides, peptide analogs or their functional fragments and functional variants can be widely used for the prevention and control of hepatopathy after hepatitis B infection including hepatitis, cirrhosis and liver cancer.
Owner:TIANJIN TOPTECH BIO SCI & TECH
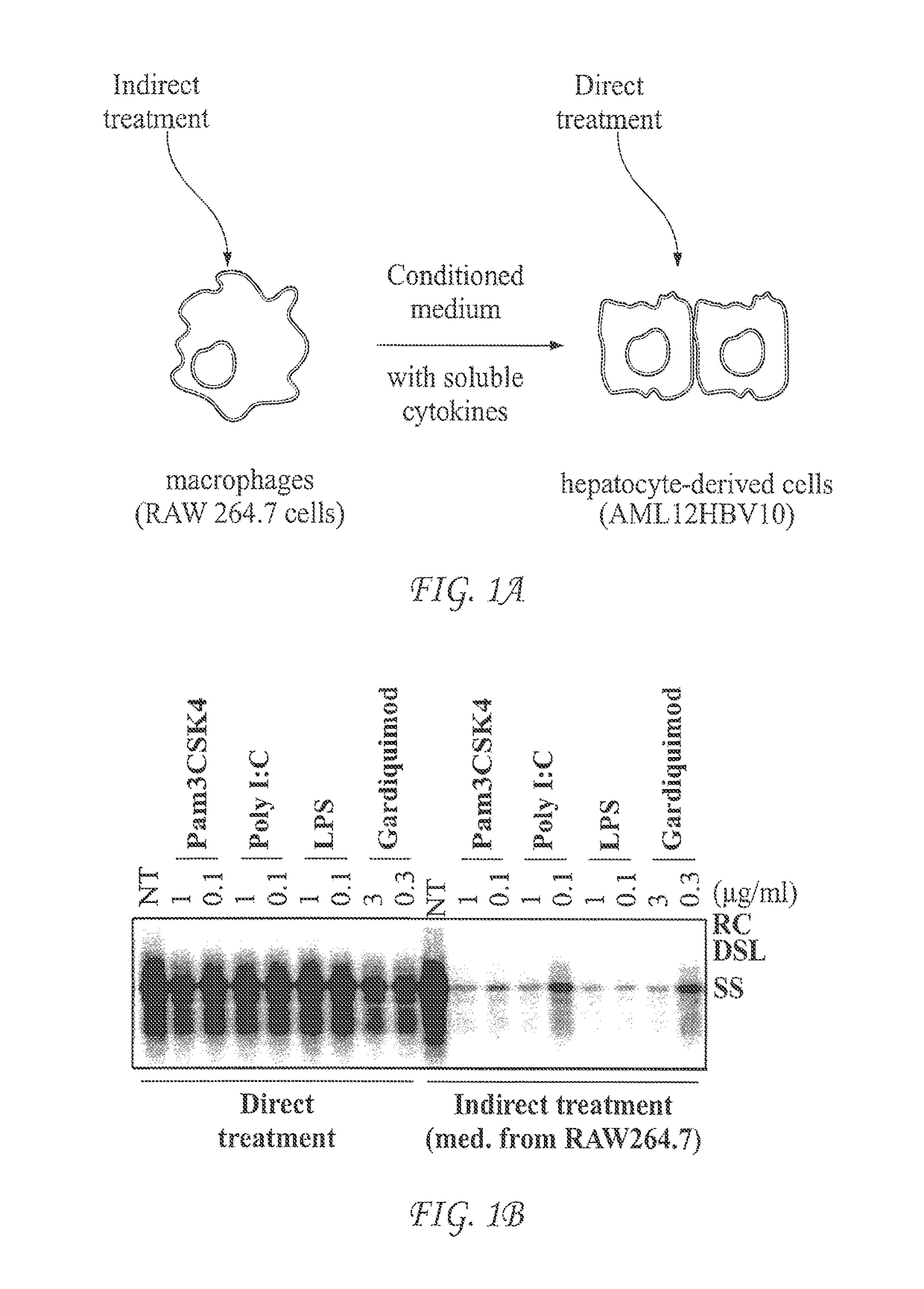
Use of sting agonists to treat hepatitis B virus infection
The invention includes methods of treating a subject having hepatitis B viral (HBV) infection. In certain embodiments, the method of the invention comprises stimulating the innate cytokine response in macrophages, dendritic cells and / or liver non-parenchymal cells with small molecular STING agonists, thus suppressing HBV replication in hepatocytes. In other embodiments, the method of the invention can be used to treat chronic HBV infections. The invention further provides methods of identifying compounds useful in treating HBV infection in a subject.
Owner:DREXEL UNIV
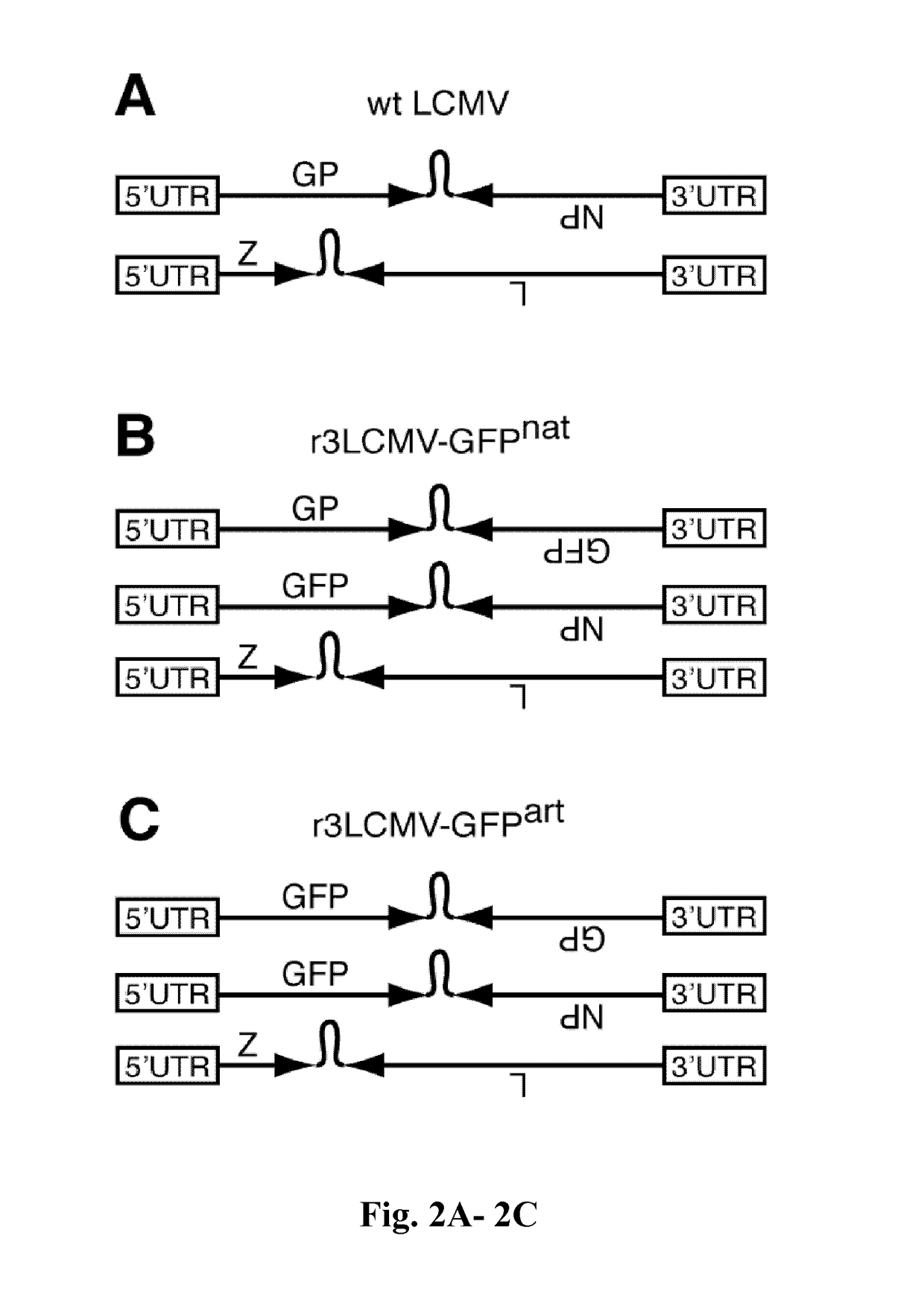
Vaccines against hepatitis b virus
ActiveUS20180319845A1SsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B virusImmunotherapy
The present application provides immunotherapies for Hepatitis B virus infections. Provided herein are genetically modified arenaviral vectors suitable as vaccines for prevention and treatment of Hepatitis B virus infections. Also provided herein are pharmaceutical compositions and methods for the treatment of Hepatitis B virus infections. Specifically, provided herein are pharmaceutical compositions, vaccines, and methods of treating Hepatitis B virus infection.
Owner:HOOKIPA BIOTECH GMBH
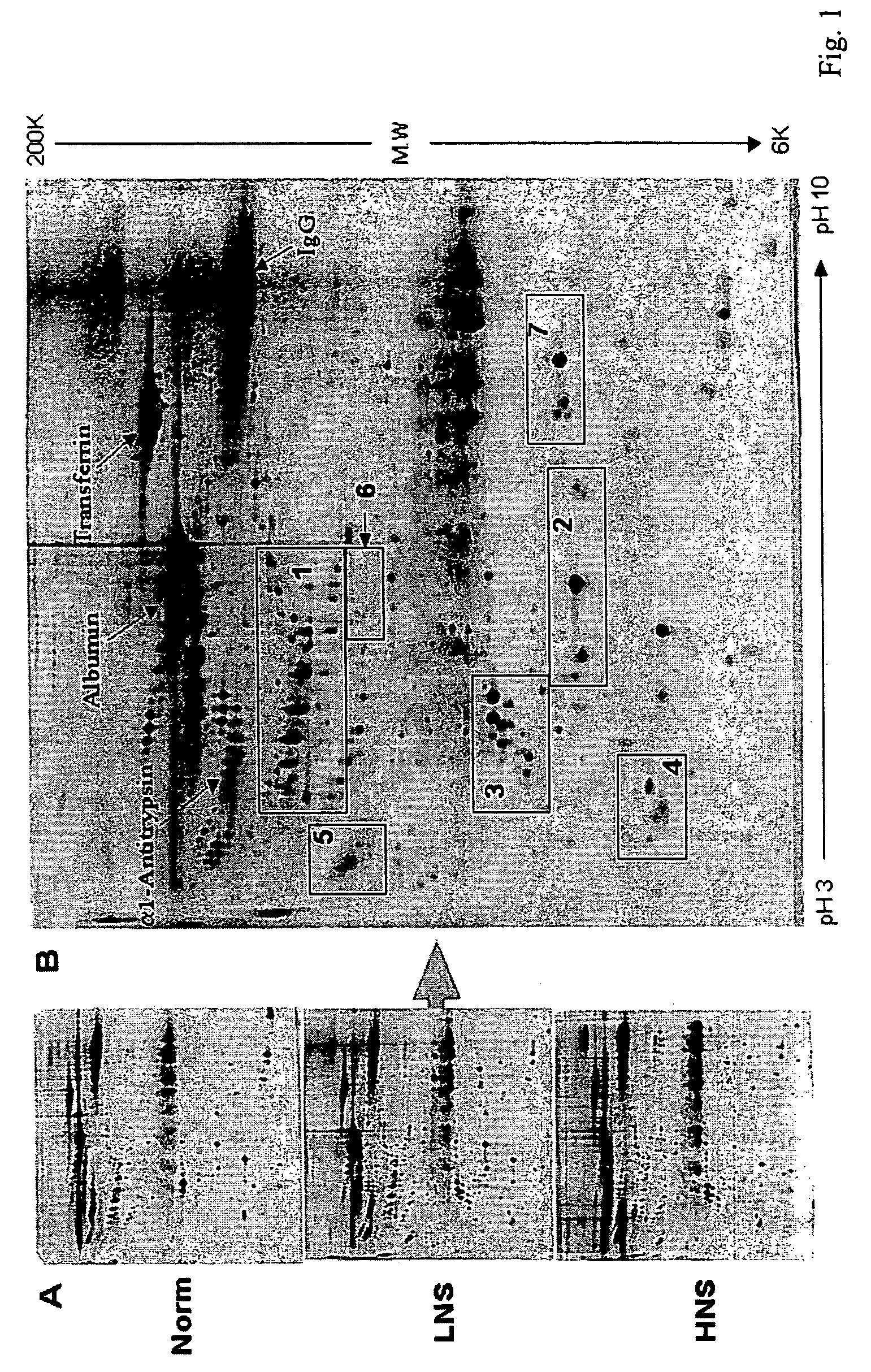
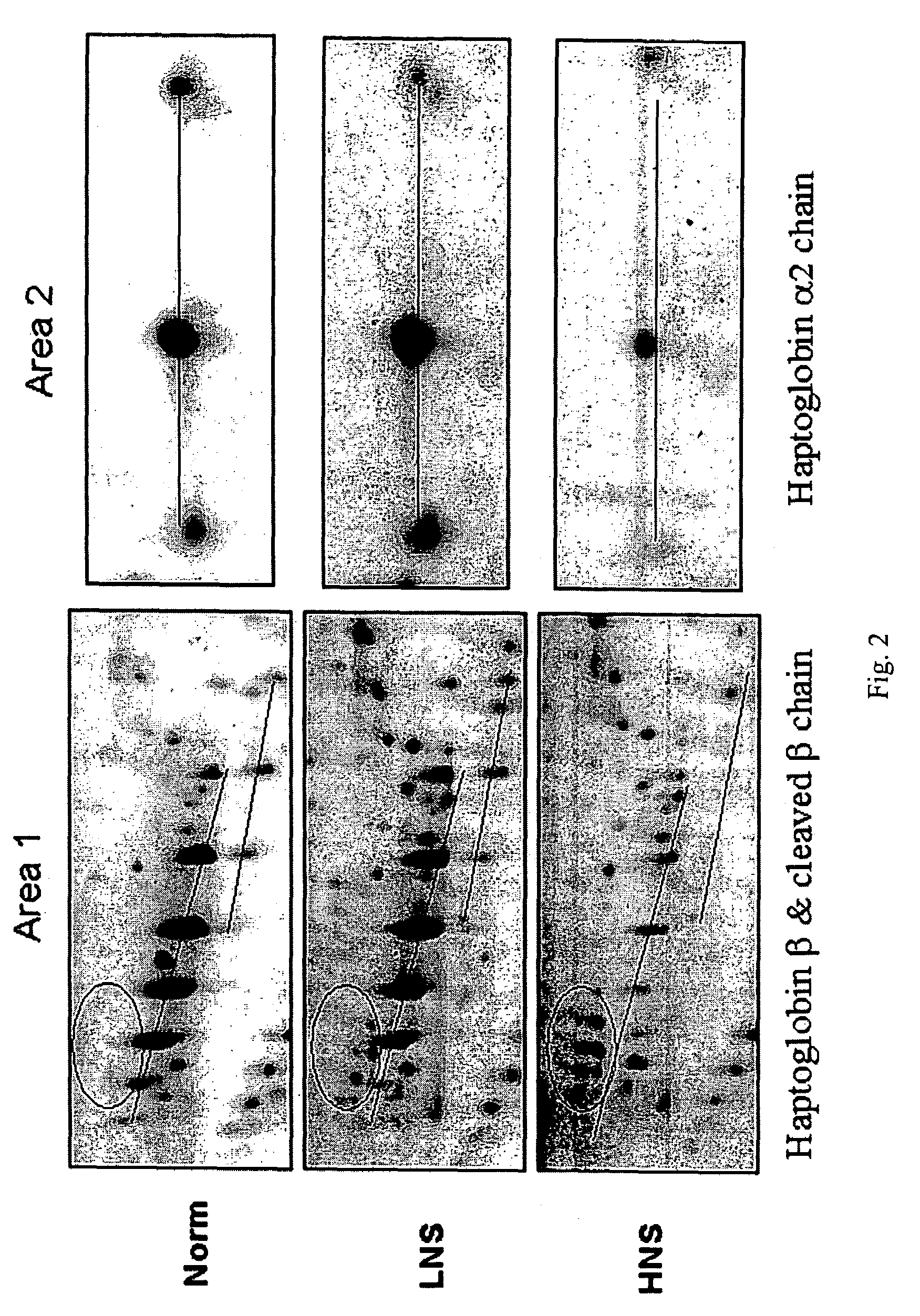
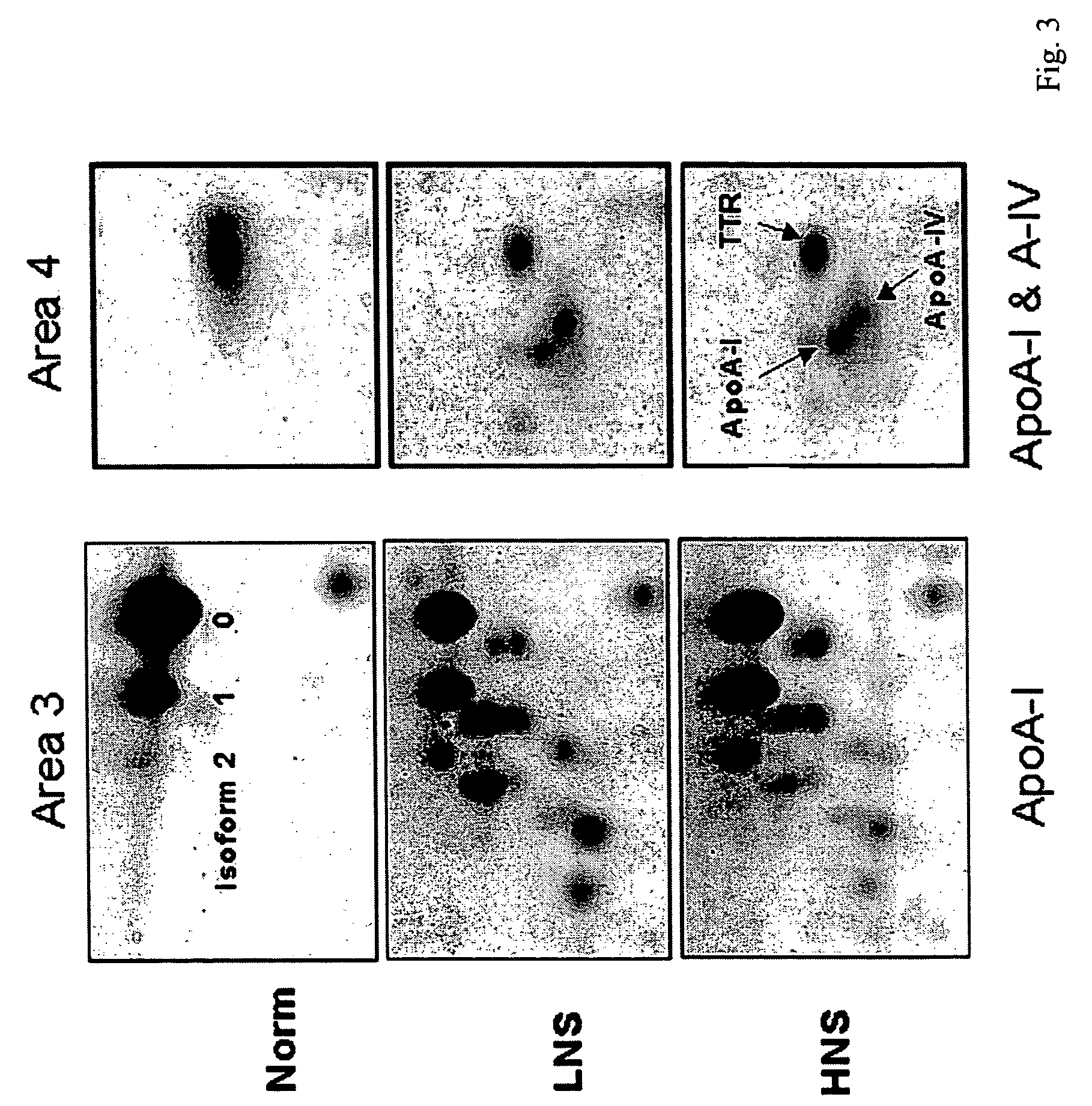
Serum biomarkers of Hepatitis B Virus infected liver and methods for detection thereof
ActiveUS7257365B2Cosmonautic condition simulationsWave based measurement systemsHepatitis B virusBiomarker (petroleum)
The invention provides a method for detecting the presence of altered serum proteins in an Hepatitis B Virus (HBV)-infected patient with liver inflammation, comprising: obtaining a sample of serum from the patient; subjecting the sample to protein gel electrophoresis to separate proteins contained therein; staining proteins separated on the electrophoresis gel with silver nitrate solution; scanning the images of stained proteins into an image analysis scanner to obtain gel images; comparing the gel images to control samples of electrophoresis gels prepared from serum of normal patient and serum of HBV-infected patient with liver inflammation to determine whether the sample of serum from the patient contains specific serum proteins. This invention also provides serum protein biomarkers for the diagnosis of patients with HBV infection and liver inflammation.
Owner:THE UNIVERSITY OF HONG KONG
Specific HBV antibody
The present invention provides a special high affinity anti-HBV antibody, an antigen-binding fragment and a composition thereof. The present invention provides isolated nucleic acid molecule encoding the antibody or the antigen-binding fragment. The present invention further provides a drug and / or a diagnostic composition containing the antibody, the antigen-binding fragment or the antibody composition, and uses and methods of the antibody or the antigen-binding fragment for prevention, treatment and / or diagnosis of HBV infections.
Owner:傅阳心
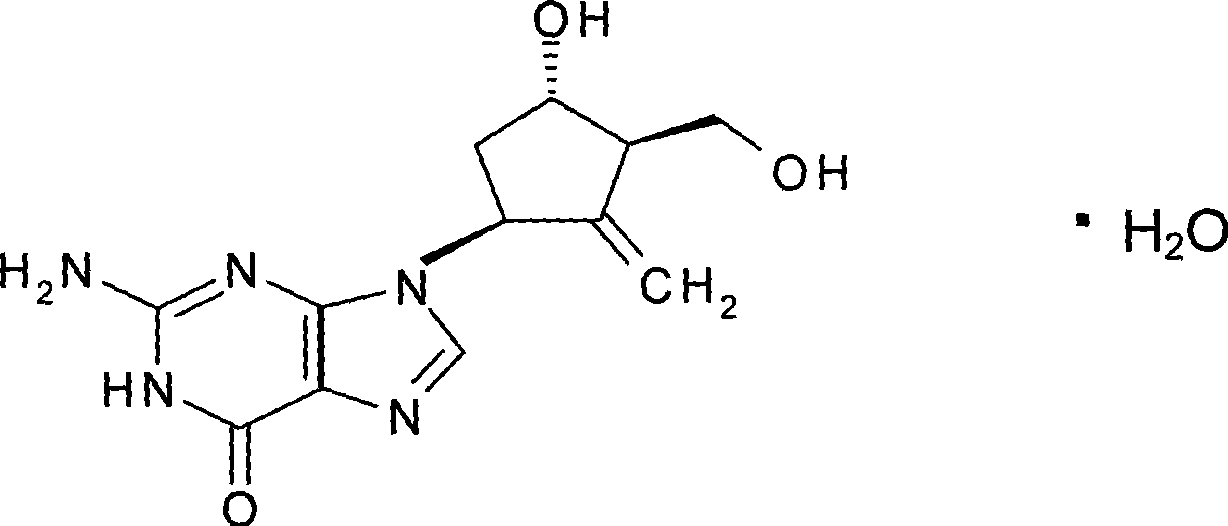
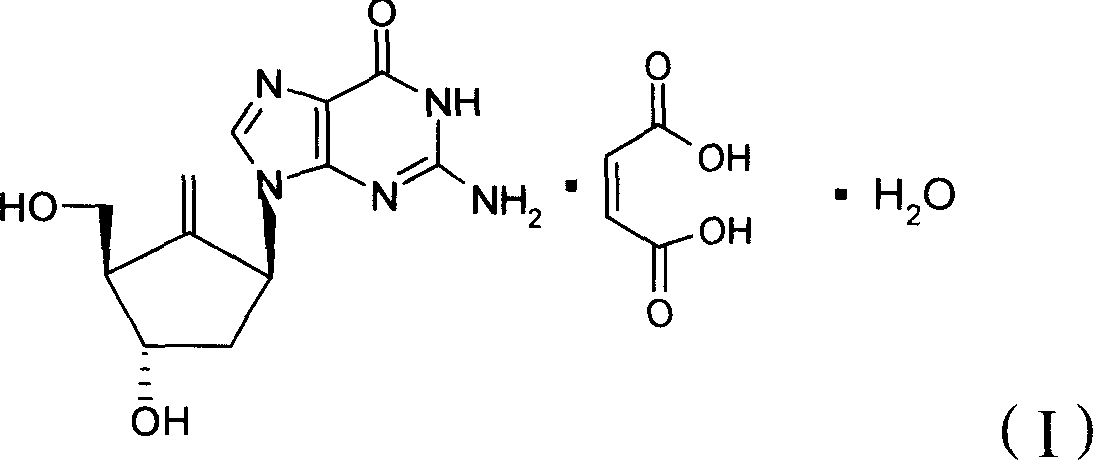
Crystallization type Entecavir formulation as well as preparation method and use thereof
InactiveCN101371841ASimple processSuitable for industrial productionOrganic active ingredientsDigestive systemDiseaseAdditive ingredient
The invention relates to a medical composition used for treating hepatitis B virus infective diseases; the composition comprises a medicinally active component, crystallized Entecavir, and a medicinal excipient. The tablets and capsules prepared by the medical composition is obviously superior in stability to similar medicinal preparations by using amorphous Entecavir as the active component under the conditions of illumination, high temperature, high moisture, and the like.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka.patsnap.com/patent_img/22766c19-01fe-40a6-8b2e-6bdb5566cfb0/US20160122344A1-20160505-C00001.PNG)
![Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka.patsnap.com/patent_img/22766c19-01fe-40a6-8b2e-6bdb5566cfb0/US20160122344A1-20160505-C00002.PNG)
![Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka.patsnap.com/patent_img/22766c19-01fe-40a6-8b2e-6bdb5566cfb0/US20160122344A1-20160505-C00003.PNG)

![2-oxo-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis B virus infection 2-oxo-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka.patsnap.com/patent_img/21cc2167-d20c-4363-a742-04a1b3928482/US10093671-C00001.png)
![2-oxo-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis B virus infection 2-oxo-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka.patsnap.com/patent_img/21cc2167-d20c-4363-a742-04a1b3928482/US10093671-C00002.png)
![2-oxo-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis B virus infection 2-oxo-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka.patsnap.com/patent_img/21cc2167-d20c-4363-a742-04a1b3928482/US10093671-C00003.png)