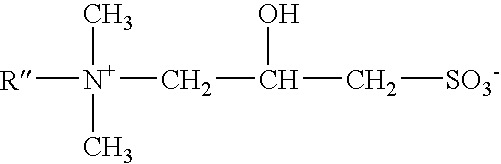
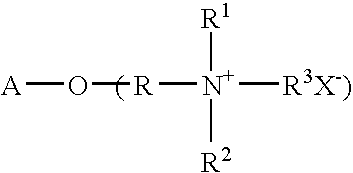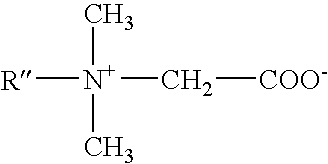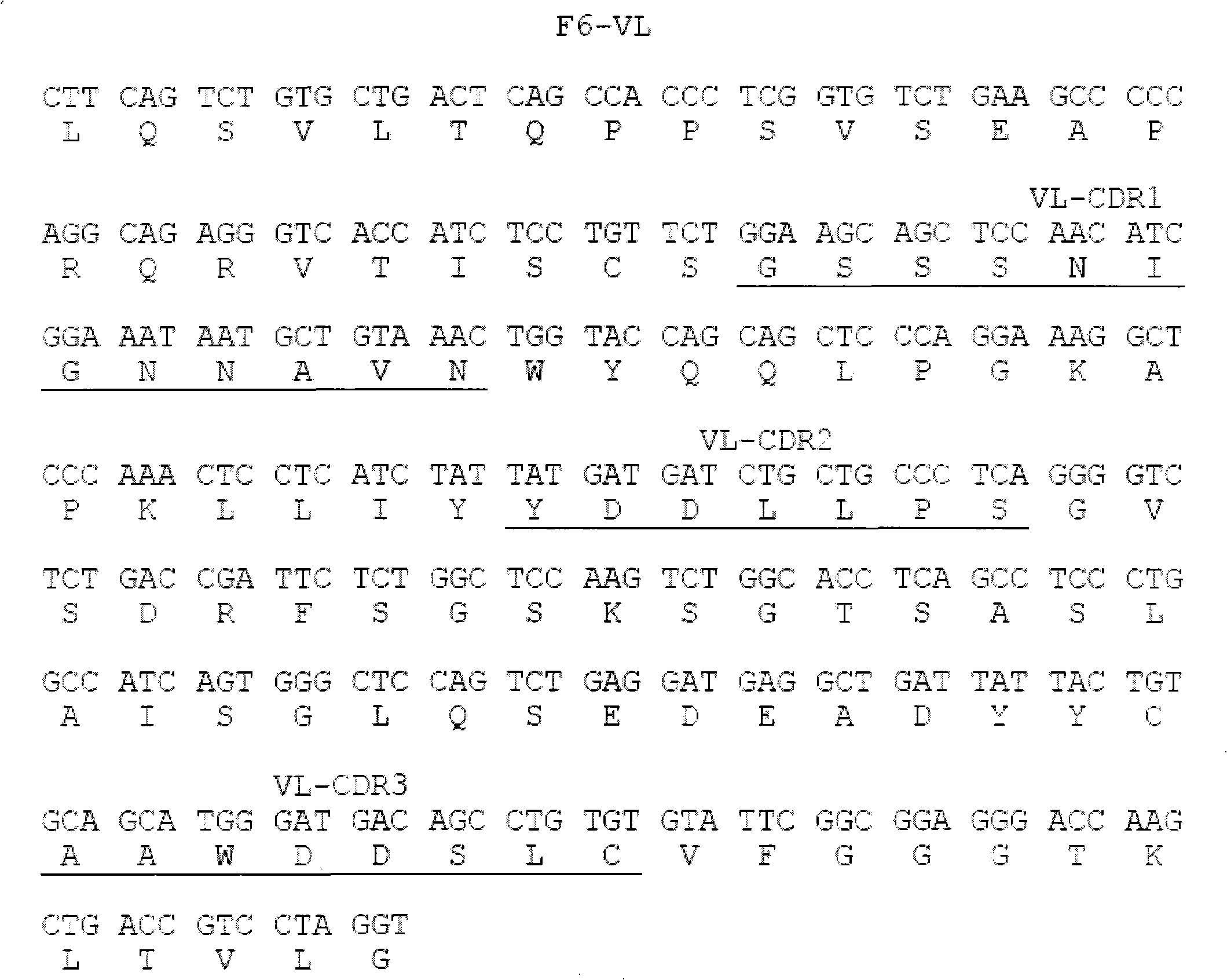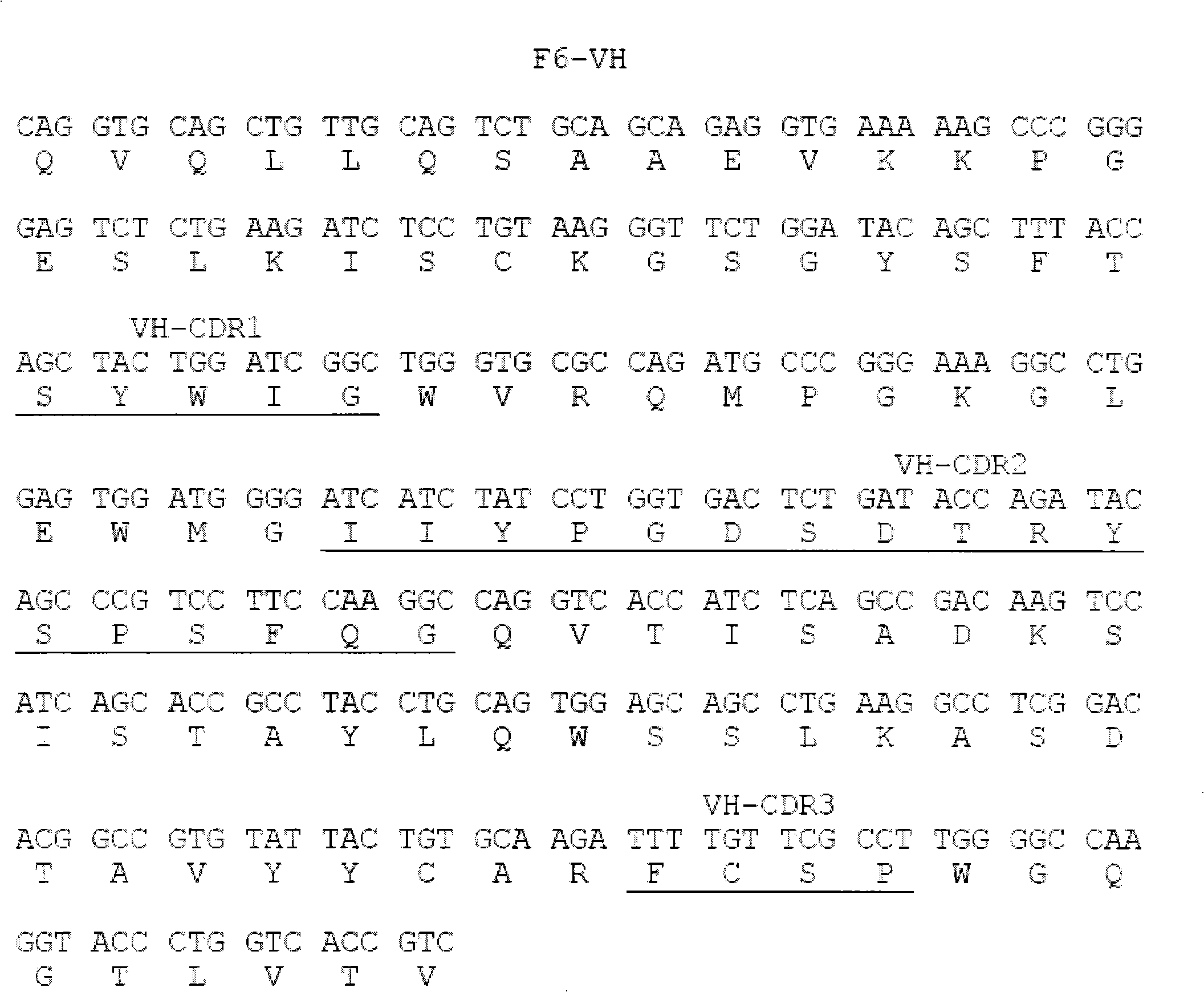Patents
Literature
390 results about "In vitro test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
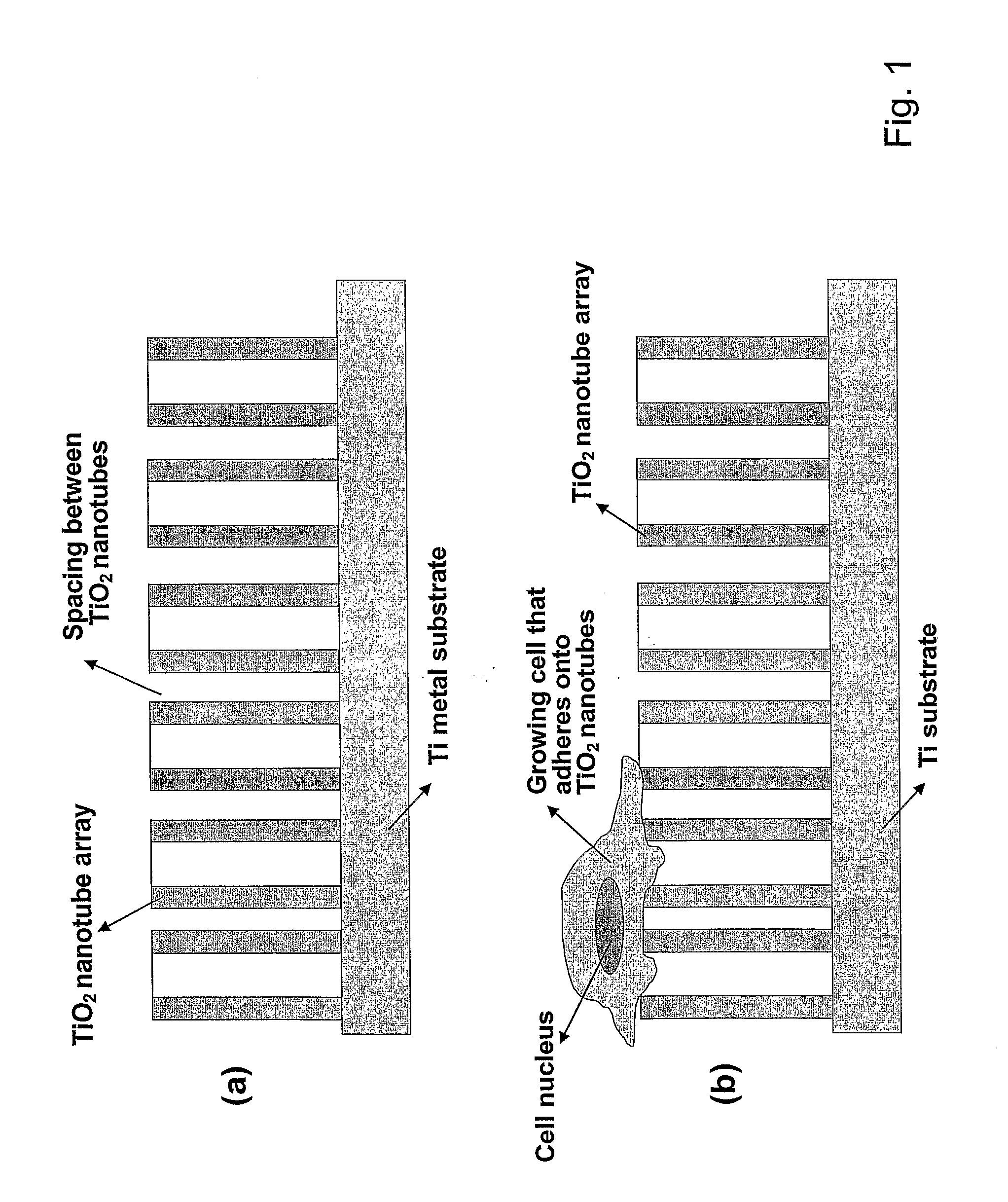
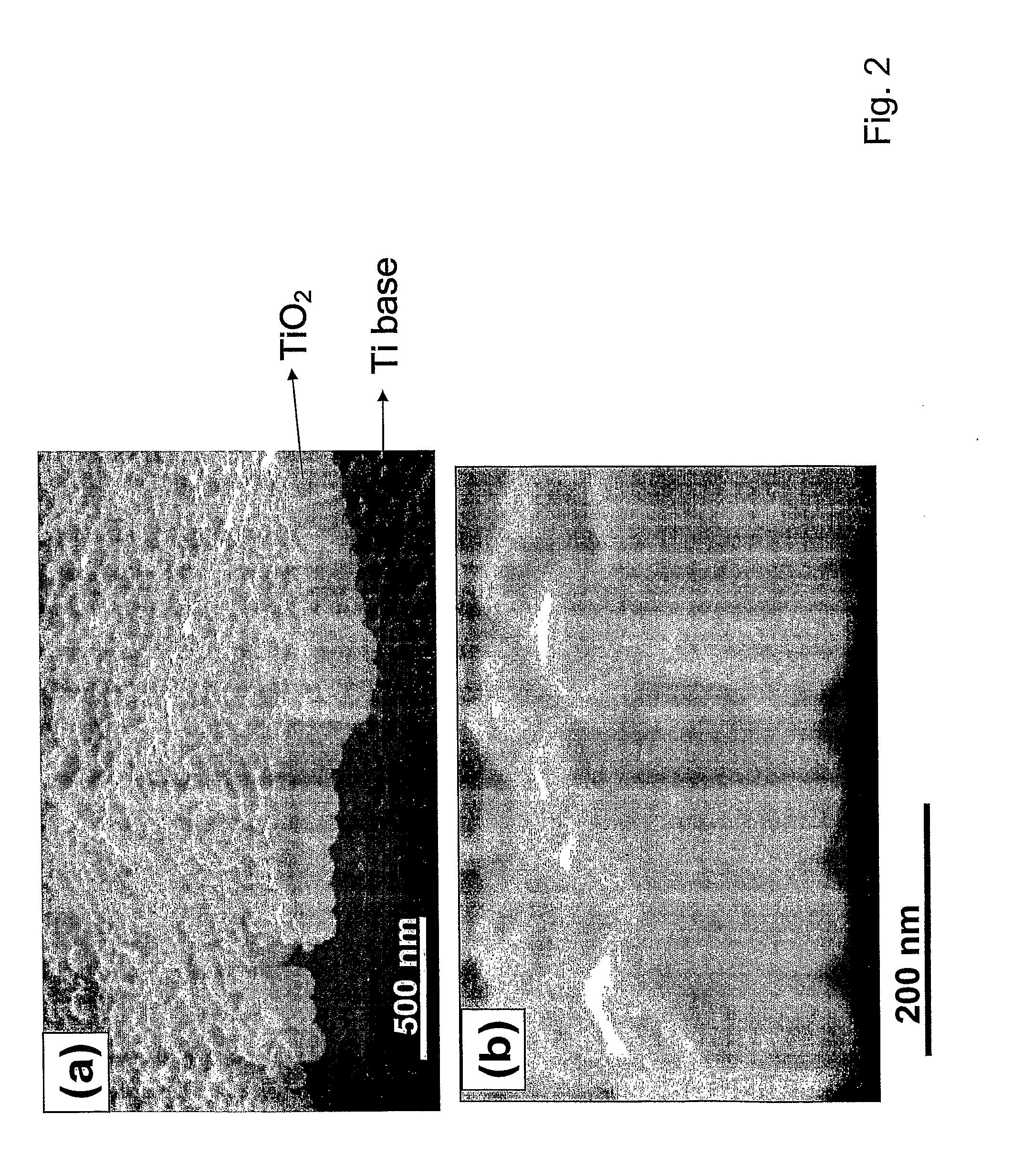
Articles comprising large-surface-area bio-compatible materials and methods for making and using them
ActiveUS20100303722A1Improve cell adhesionAccelerated cell growth characteristicImmobilised enzymesBioreactor/fermenter combinationsCell culture mediaBone growth
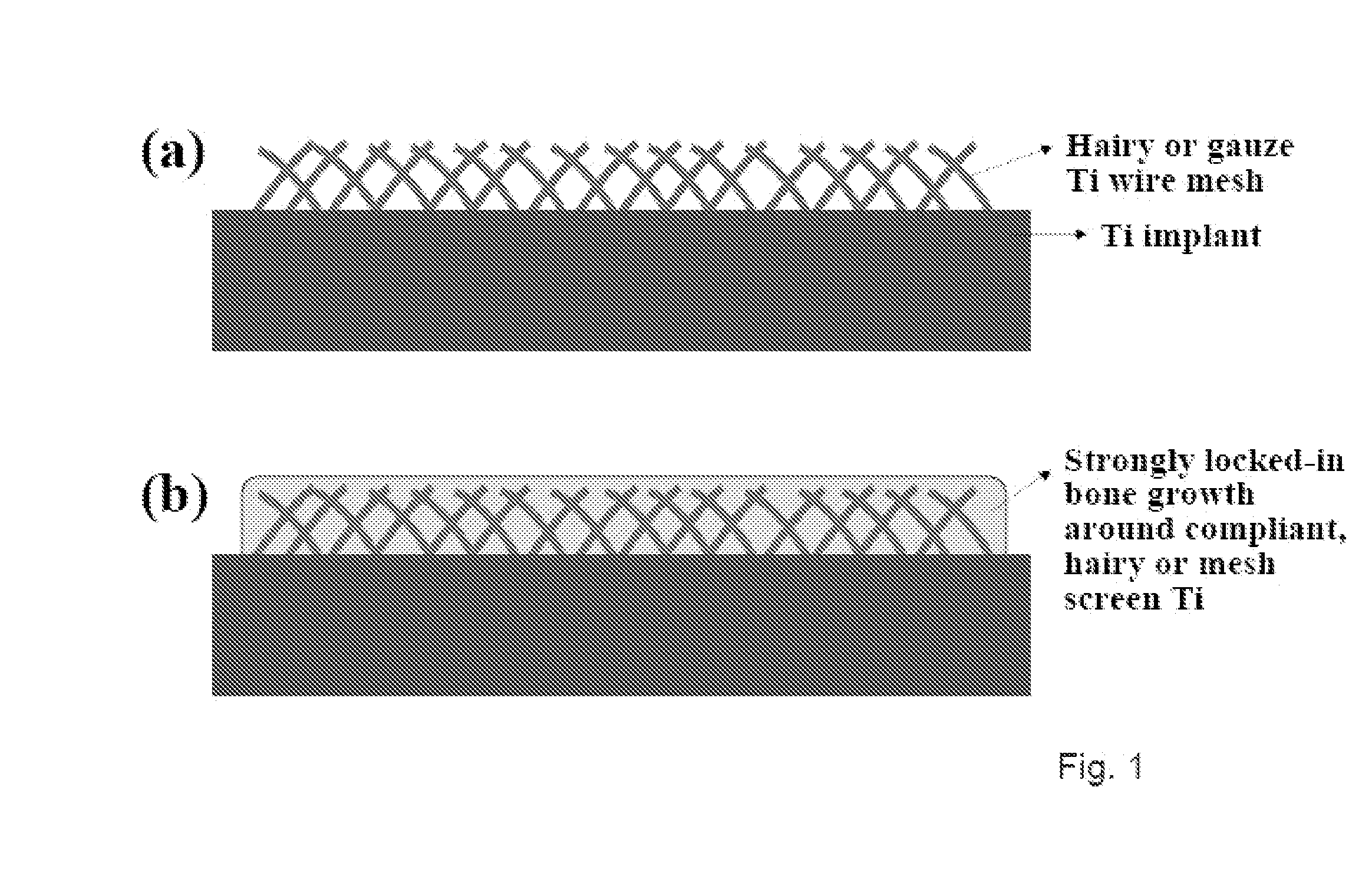
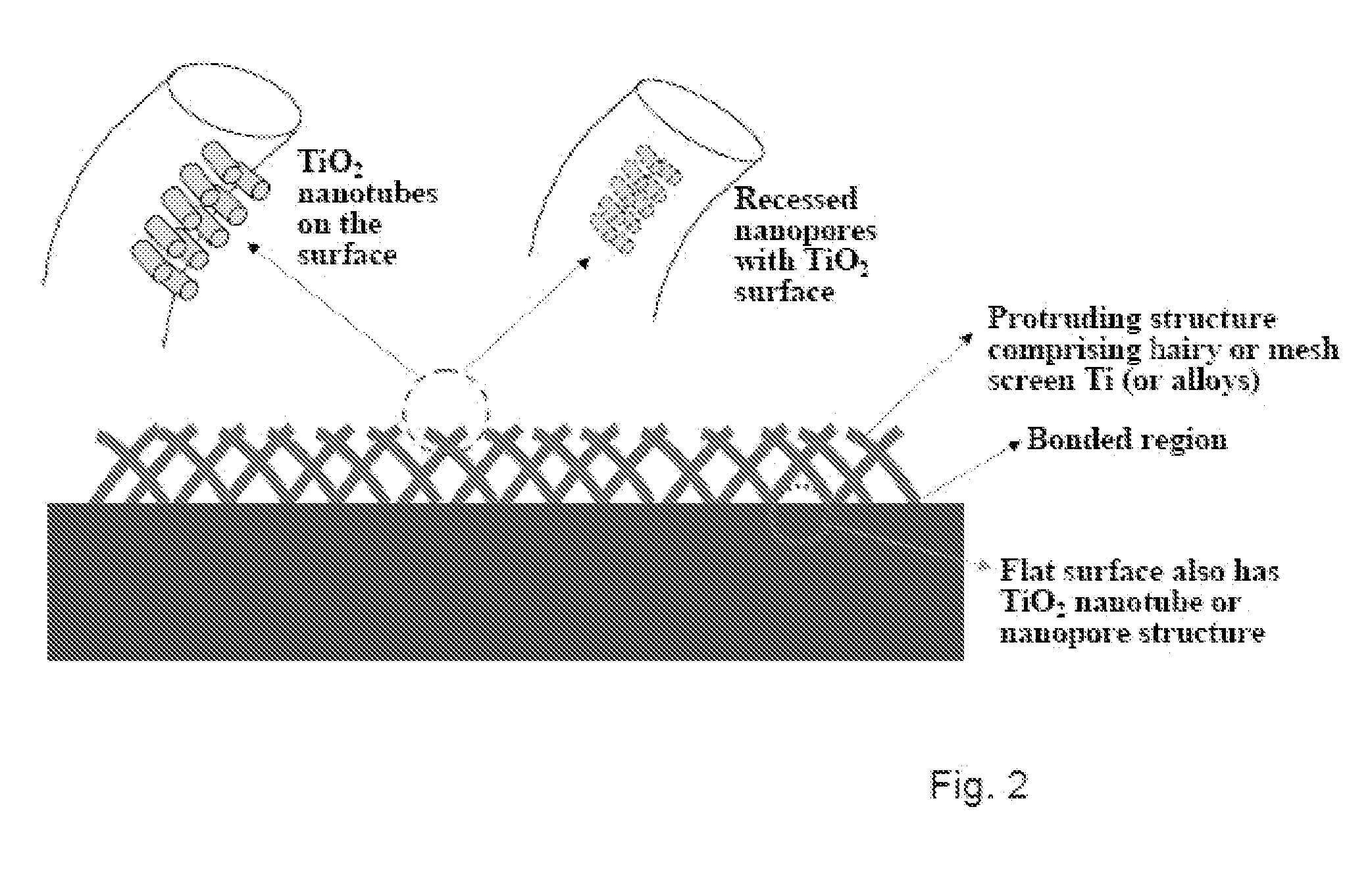
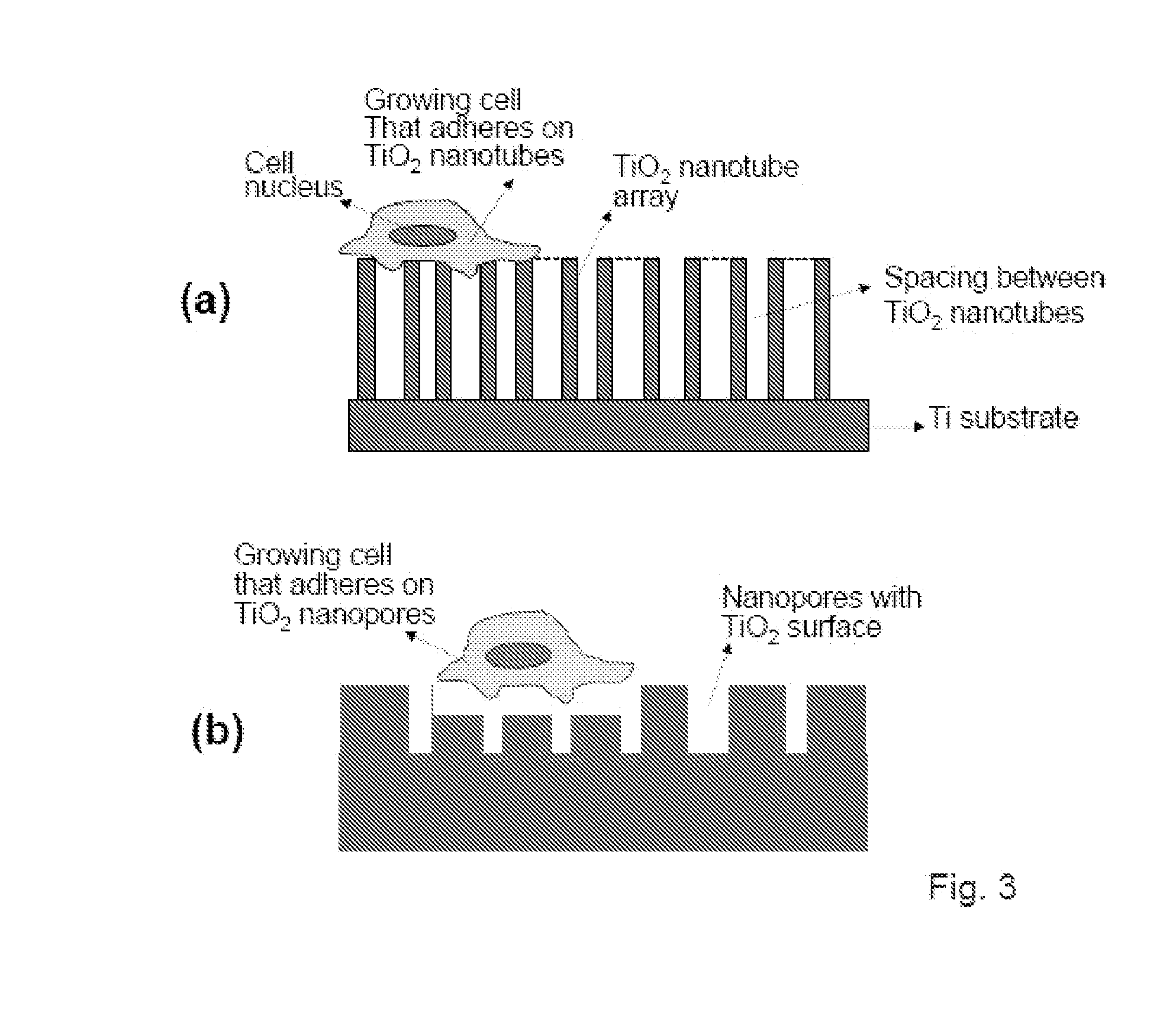
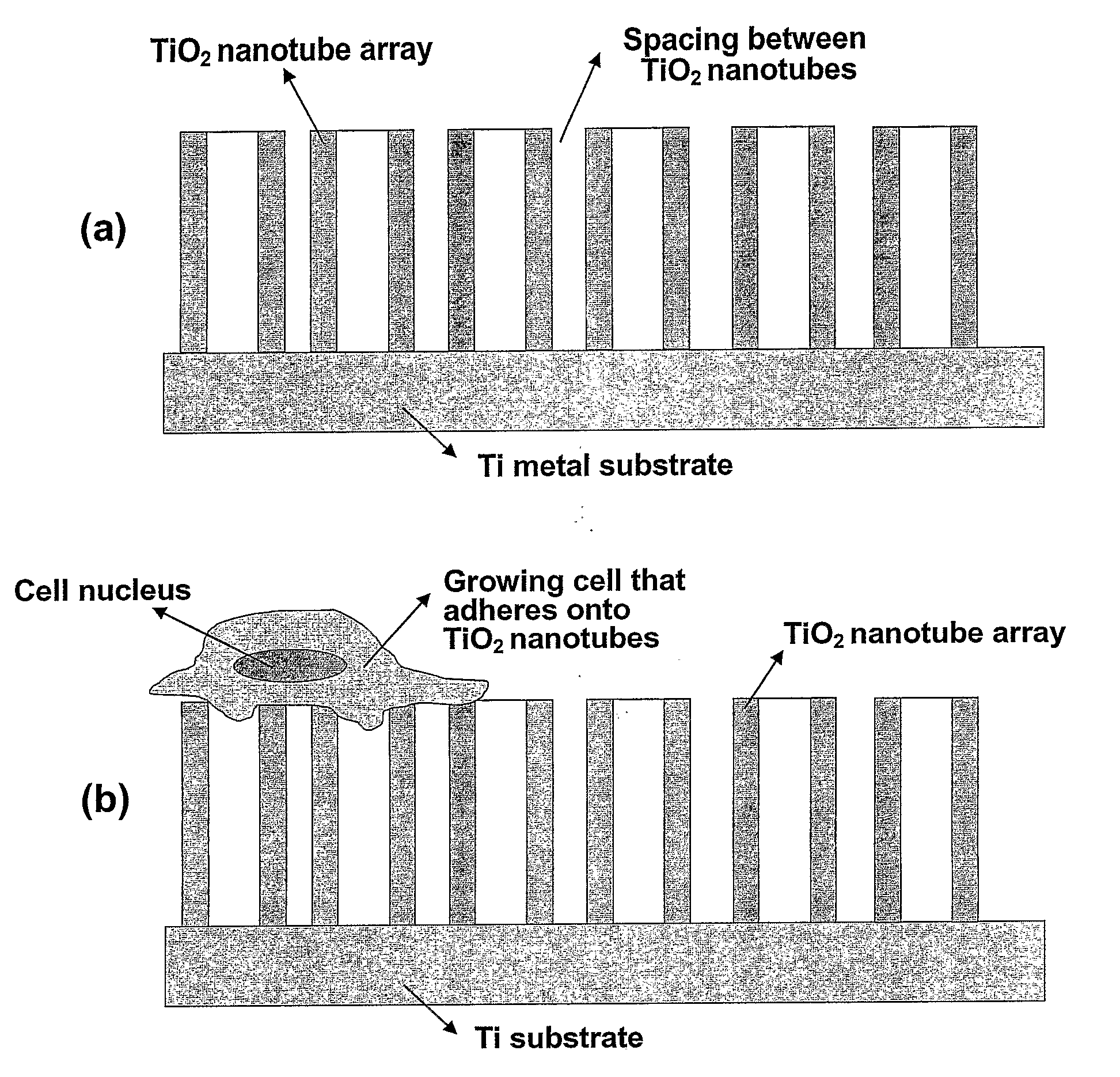
The present invention provides articles of manufacture comprising biocompatible nanostructures comprising significantly increased surface area for, e.g., organ, tissue and / or cell growth, e.g., for bone, tooth, kidney or liver growth, and uses thereof, e.g., for in vitro testing of drugs, chemicals or toxins, or as in vivo implants, including their use in making and using artificial tissues and organs, and related, diagnostic, screening, research and development and therapeutic uses, e.g., as drug delivery devices. The present invention provides biocompatible nanostructures with significantly increased surface area, such as with nanotube and nanopore array on the surface of metallic, ceramic, or polymer materials for enhanced cell and bone growth, for in vitro and in vivo testing, cleansing reaction, implants and therapeutics. The present invention provides optically transparent or translucent cell-culturing substrates. The present invention provides biocompatible and cell-growth-enhancing culture substrates comprising elastically compliant protruding nanostructure substrates coated with Ti, TiO2 or related metal and metal oxide films.
Owner:RGT UNIV OF CALIFORNIA
Compositions comprising nanostructures for cell, tissue and artificial organ growth, and methods for making and using same
ActiveUS20090220561A1Improve bone formationIncreased durabilityBioreactor/fermenter combinationsElectrolysis componentsIn vivoNanostructure
The invention provides articles of manufacture comprising biocompatible nanostructures comprising nanotubes and nanopores for, e.g., organ, tissue and / or cell growth, e.g., for bone, kidney or liver growth, and uses thereof, e.g., for in vitro testing, in vivo implants, including their use in making and using artificial organs, and related therapeutics. The invention provides lock-in nanostructures comprising a plurality of nanopores or nanotubes, wherein the nanopore or nanotube entrance has a smaller diameter or size than the rest (the interior) of the nanopore or nanotube. The invention also provides dual structured biomaterial comprising micro- or macro-pores and nanopores. The invention provides biomaterials having a surface comprising a plurality of enlarged diameter nanopores and / or nanotubes.
Owner:RGT UNIV OF CALIFORNIA
Embossed cell analyte sensor and methods of manufacture
ActiveUS7312042B1Eliminate needEasy to controlMicrobiological testing/measurementMaterial analysis by electric/magnetic meansAnalyteEngineering
An analyte measurement system is provided having sensors with embossed test chamber channels. In one embodiment, the sensors are elongate test strips for in vitro testing, each test strip having a substrate, at least one electrode, an embossed channel in the electrode, and lidding tape covering at least a portion of the embossed channel. Methods of manufacture are also disclosed for filling the sensor channels with reagent, and for trimming the ends of the sensors to eliminate the need for a calibration code during use of the sensors with a meter.
Owner:ABBOTT DIABETES CARE INC
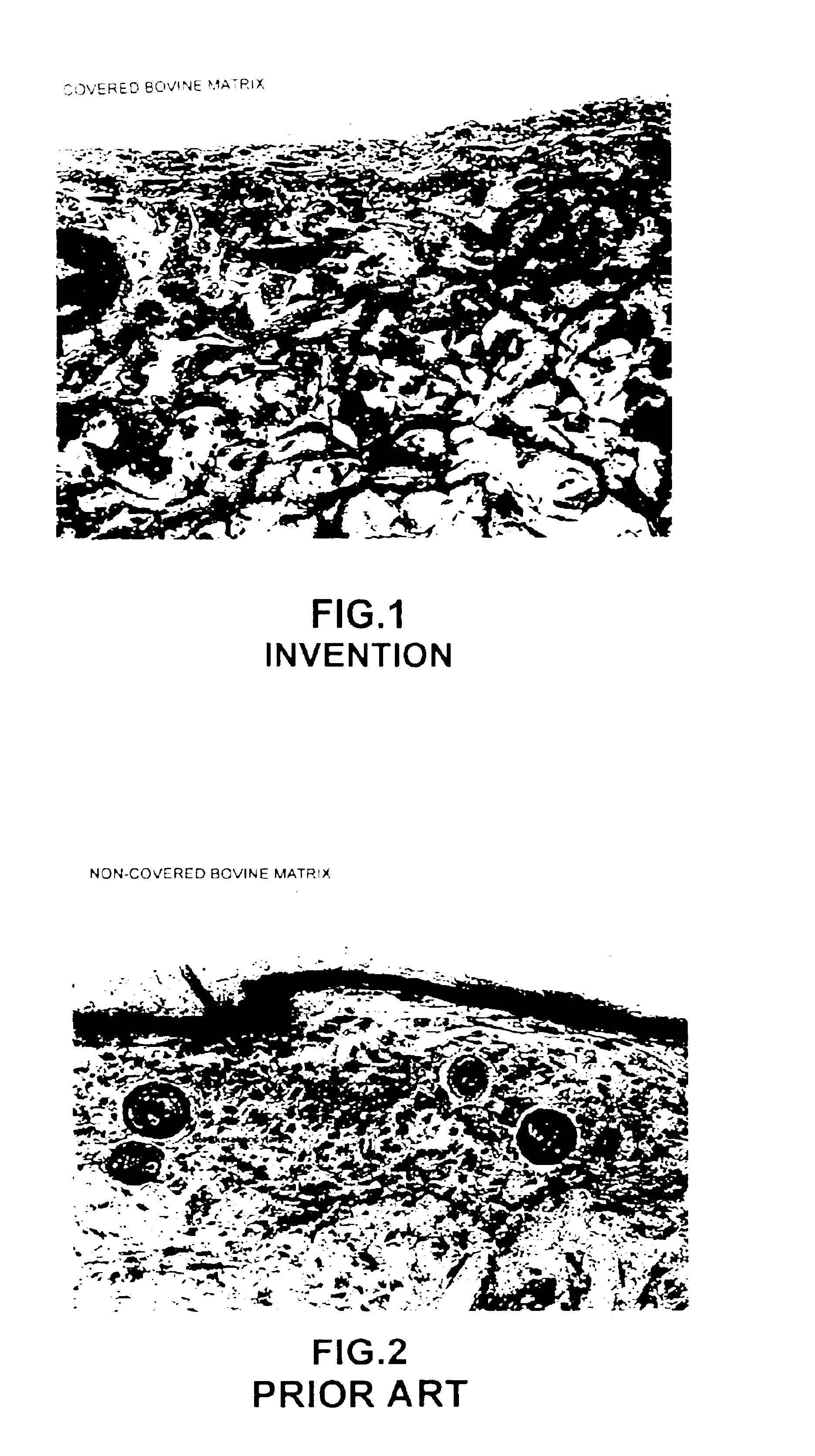
Processes for the preparation of novel collagen-based supports for tissue engineering, and biomaterials obtained
A composite product is disclosed as a collagen support comprising at least one porous collagen layer covered on at least one side with an essentially compact collagen membrane consisting either of a collagen film prepared by drying a collagen gel, preferably in air or a gaseous fluid, or of a very highly compressed collagen sponge. At least one of the two layers, i.e. the porous layer and the essentially compact membrane, may comprise normal, genetically modified or malignant living cells originating particularly from young or elderly subjects. This composite product is used as a collagen support for the manufacture of artificial skin intended especially for performing in vitro tests on the efficacy of potentially active substances or for reconstructing damaged areas of skin in vivo.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Mild, viscous cleansing composition with versatile compatibility and enhanced conditioning
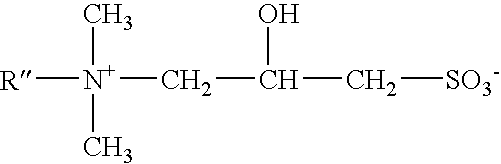
ActiveUS7541320B2Maintain good propertiesHighly efficient thickening/suspending agentsCosmetic preparationsNon-ionic surface-active compoundsWater insolubleAdditive ingredient
Mild shampoo compositions are described that are economical, have excellent in-use properties, and provide enhanced conditioning benefits. The compositions have low ocular irritation potential and are particularly suitable for use by children. The compositions include a surfactant system composed on an alkyl ethoxy sulfate having at least 3 ethylene oxide groups, a betaine surfactant, and an hydroxysultaine surfactant in specific ratios; and a non-volatile water-insoluble silicone. In-vitro tests are described that allows selection of optional ingredients that do not compromise the mildness or hair conditioning performance of the composition.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
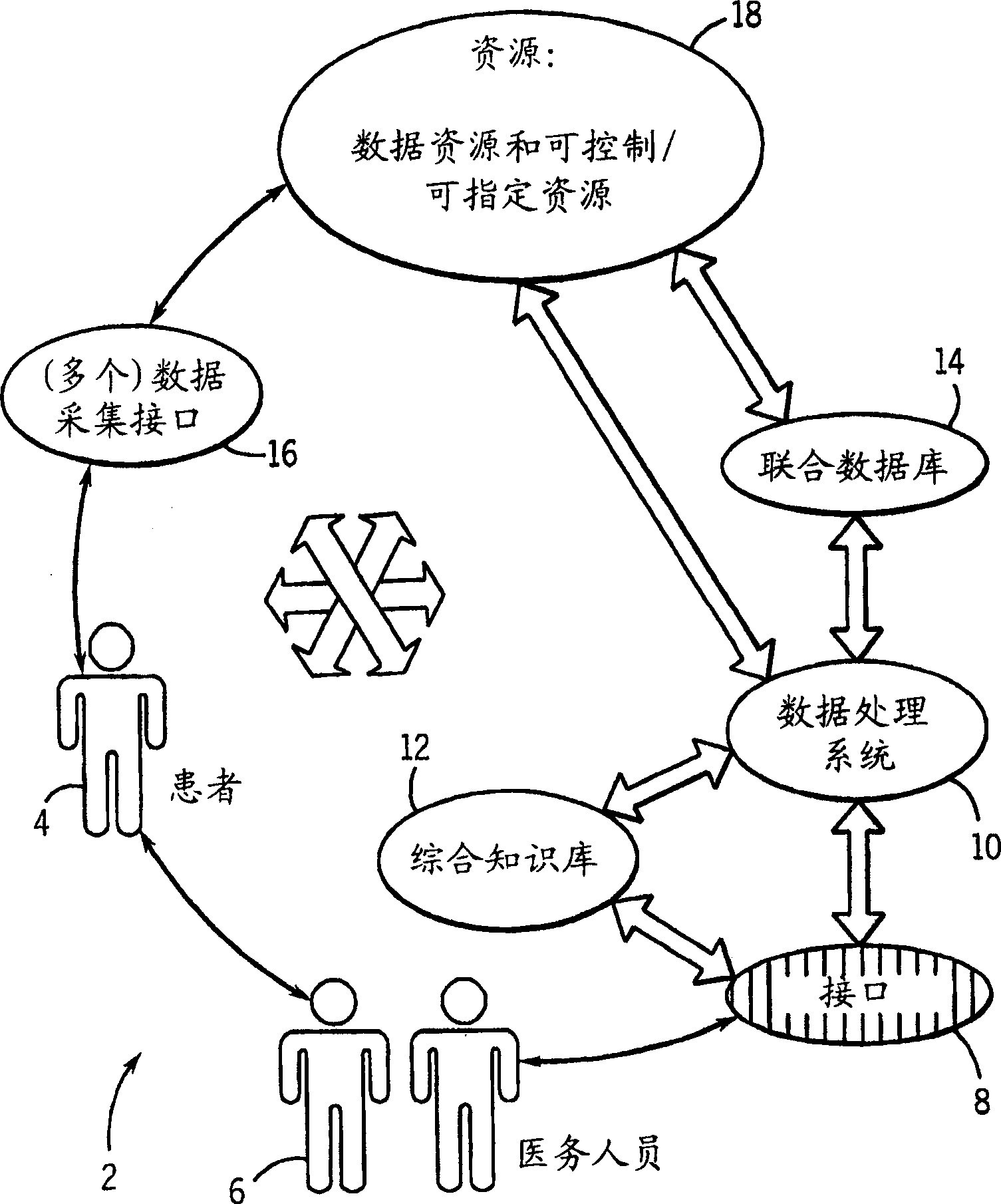
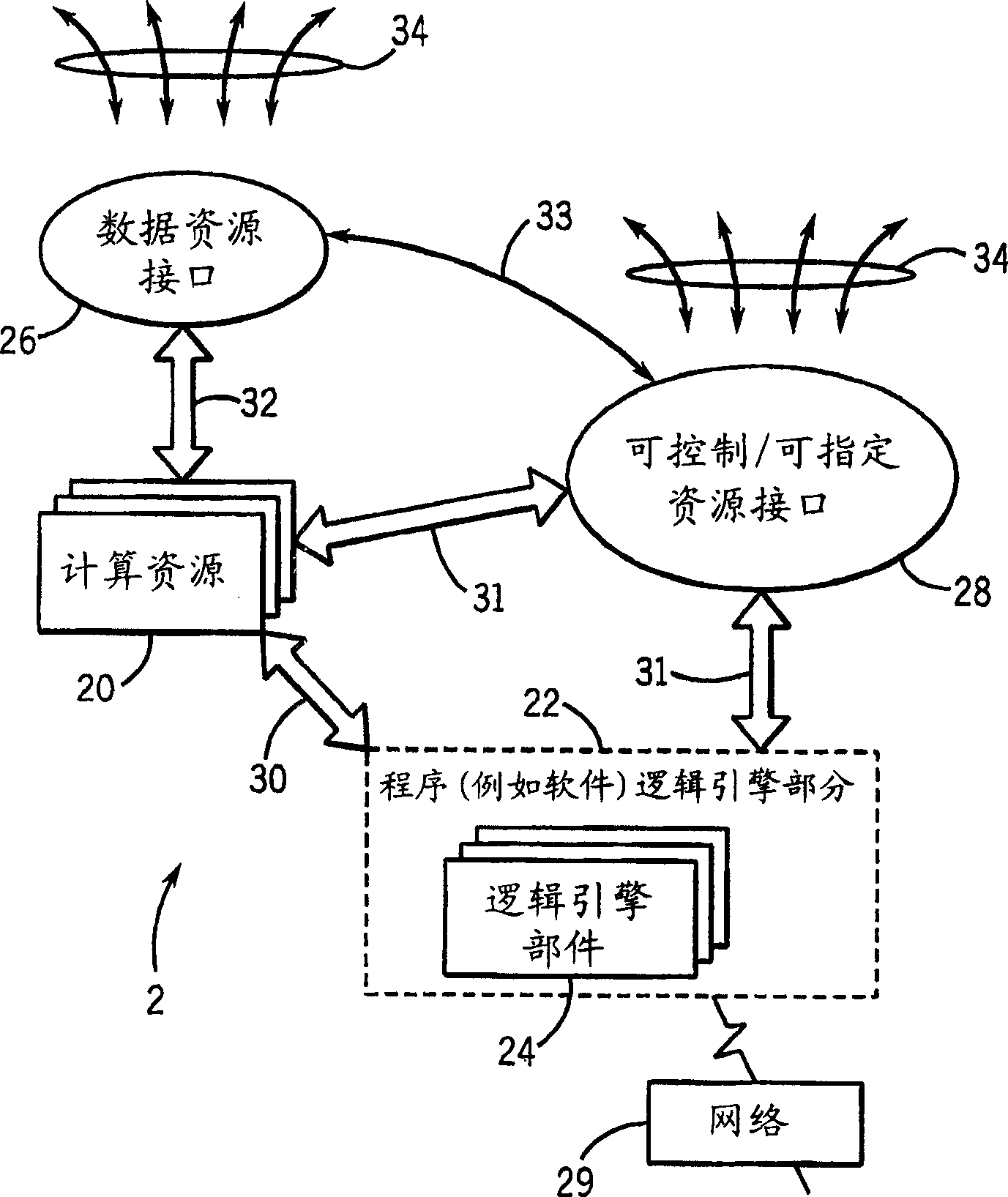
Medical data analysis method and apparatus incorporating in vitro test data
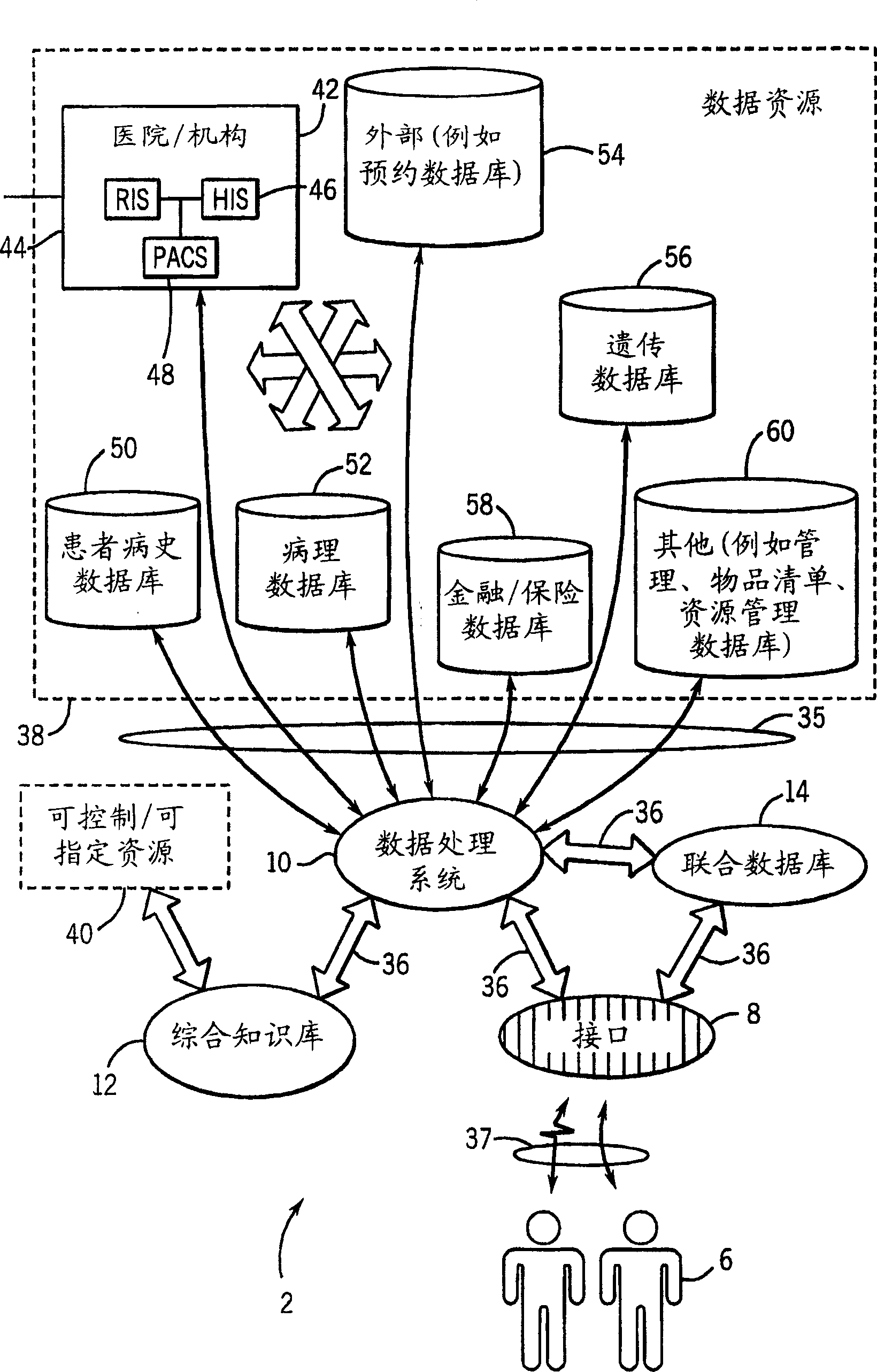
InactiveCN1751309AConvenient careInformativeComputer-assisted medical data acquisitionMedical automated diagnosisTest sampleIn vitro test
Techniques are provided for processing in vitro test sample data. The in vitro test sample data is accessed and additional data may be accessed, such as from an integrated knowledge base (12), from a plurality of controllable and prescribable data resources (40). Analysis (440; 448) of the data is performed, such as via an automated computer-assisted data operating algorithm (22) to identify features of interest available or possibly available from the in vitro test data. Processes (446; 454; 458; 460) are performed, either on the data or upon an in vitro sample based upon the analysis. Results of the processing may include acquisition (438) of additional test samples, modification (444; 452) of processing of test samples or of sample data, and so forth.
Owner:GE MEDICAL SYST GLOBAL TECH CO LLC
Mild, viscous cleansing composition with versatile compatibility and enhanced conditioning
ActiveUS20050164896A1Excellent latherExcellent Conditioning PropertiesCosmetic preparationsHair removalAdditive ingredientWater insoluble
Mild shampoo compositions are described that are economical, have excellent in-use properties, and provide enhanced conditioning benefits. The compositions have low ocular irritation potential and are particularly suitable for use by children. The compositions include a surfactant system composed on an alkyl ethoxy sulfate having at least 3 ethylene oxide groups, a betaine surfactant, and an hydroxysultaine surfactant in specific ratios; and a non-volatile water-insoluble silicone. In-vitro tests are described that allows selection of optional ingredients that do not compromise the mildness or hair conditioning performance of the composition.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Method for preparing medicine sustained-releasing bracket
ActiveCN101185779ANo oxidative decompositionThickness is easy to controlStentsSurgeryDrug release rateSustained release drug
A preparation method of a drug sustained-release stent includes a preparation stent, a drug sustained-release stent constituted by drug coating on the stent, and the drug coating of the stent is composed of the following steps sequentially: (1) the preparation of a sustained-release drug coating layer; (2) the coating of the sustained-release drug coating layer; (3) drying. The dosages of the drug components on the sustained-release drug stent of the invention are in line with the designed dosages, and the drug can be released according to the stipulated dosages in the stipulated time of the design requirements completely in vitro tests. The invention has good biocompatibility, low cost of spraying process, high yield, controllable thickness of the coating layer, and slow and stable drug release rate.
Owner:上海赢生医疗科技有限公司
Synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and composition and formulation thereof
ActiveCN101537020APromote growth and reproductionStrong antagonistic effectOrganic active ingredientsBacteria material medical ingredientsBacillus licheniformisSynbiotics
The invention relates to a synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and a composite and a formulation thereof, being characterized in that the synbiotics comprises bacillus licheniformis medical live bacterial powder and the oligosaccharide class prebiotics, wherein the bacillus licheniformis medical live bacterial powder contains live bacteria about 30 billion / gram; the weight ratio of the bacillus licheniformis medical live bacterial powder to the oligosaccharide class prebiotics is 1:1 to 76; the weight ratio of the medical live bacterial powder of the composite, the oligosaccharide class prebiotics and auxiliary materials is as follows: 1.25-5 percent of the bacillus licheniformis medical live bacterial powder, 5-95 percent of the oligosaccharide class prebiotics, 20-50 percent of preferable oligosaccharide class prebiotics, 30-65 percent of diluting agents, 1-20 percent of bonding agents, 1-15 percent of disintegrating agents, 0.1-3 percent of lubricating agents, 1-10 percent of coating agents, 0.01-0.1 percent of flavoring agents, 0.0001-0.001 percent of coloring agents, and 0.1-15 percent of suspending agents. The synbiotics composite is prepared into oral common tablets, oral cavity disintegration tablets, dispersing tablets, enteric-coated tablets, granular formulation, enteric-coated granular formulation, capsules, enteric-coated capsules, dry supensoid agents and external tablets according to a conventional process; and an in vitro test shows that each formulation can promote the growth and the reproduction of the synbiotics of bacillus licheniformis, restrain the growth of harmful bacteria, enhance the immunizing power of parasitifers, reduce diarrhea and enhance the health care.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Lactobacillus plantarum bb9 capable of adhering to gastrointestinal tract and cholesterol removal
InactiveUS20110117629A1Good acidGood bile toleranceBacteriaFood ingredient functionsTolerabilityIn vitro test
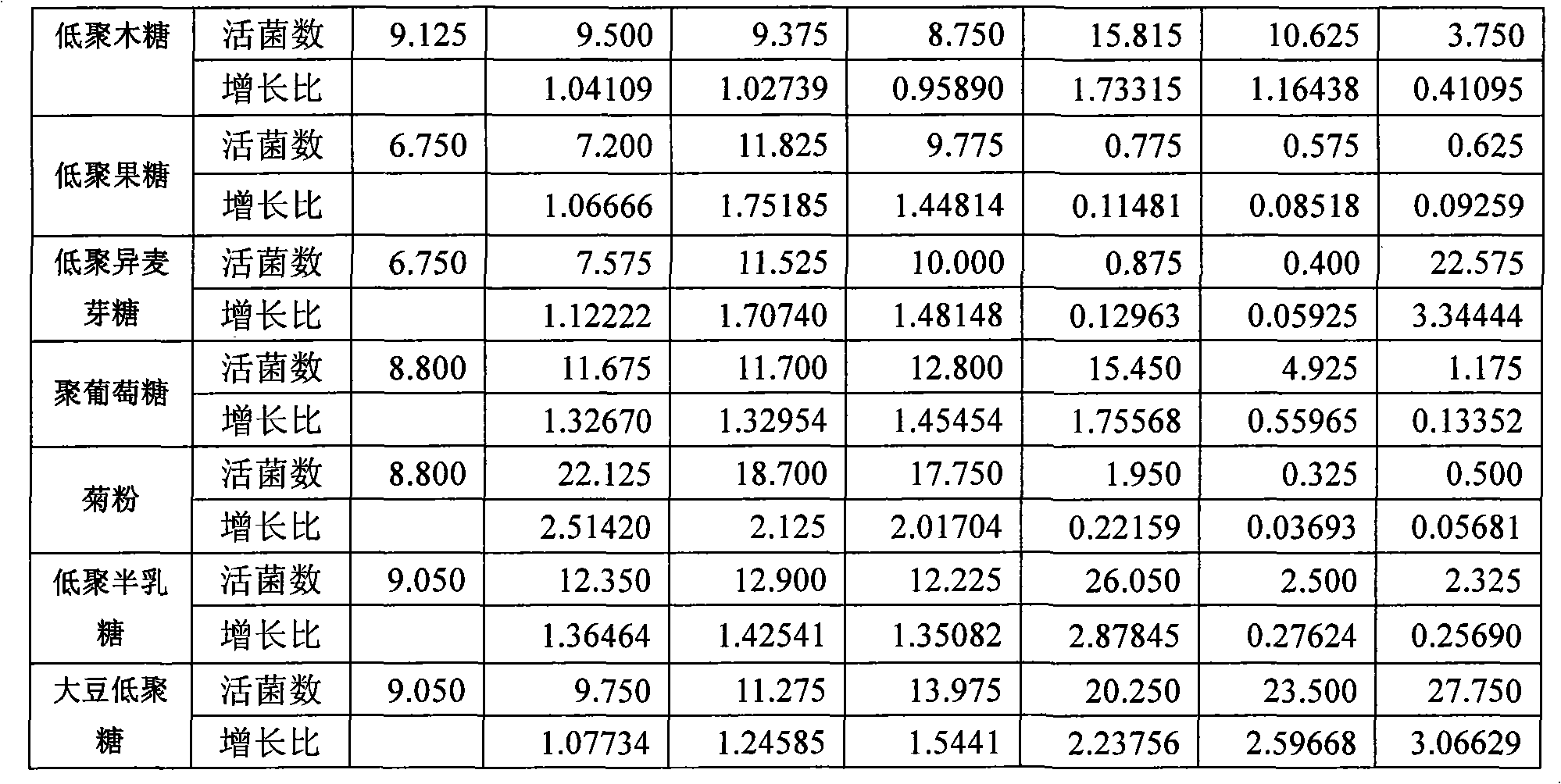
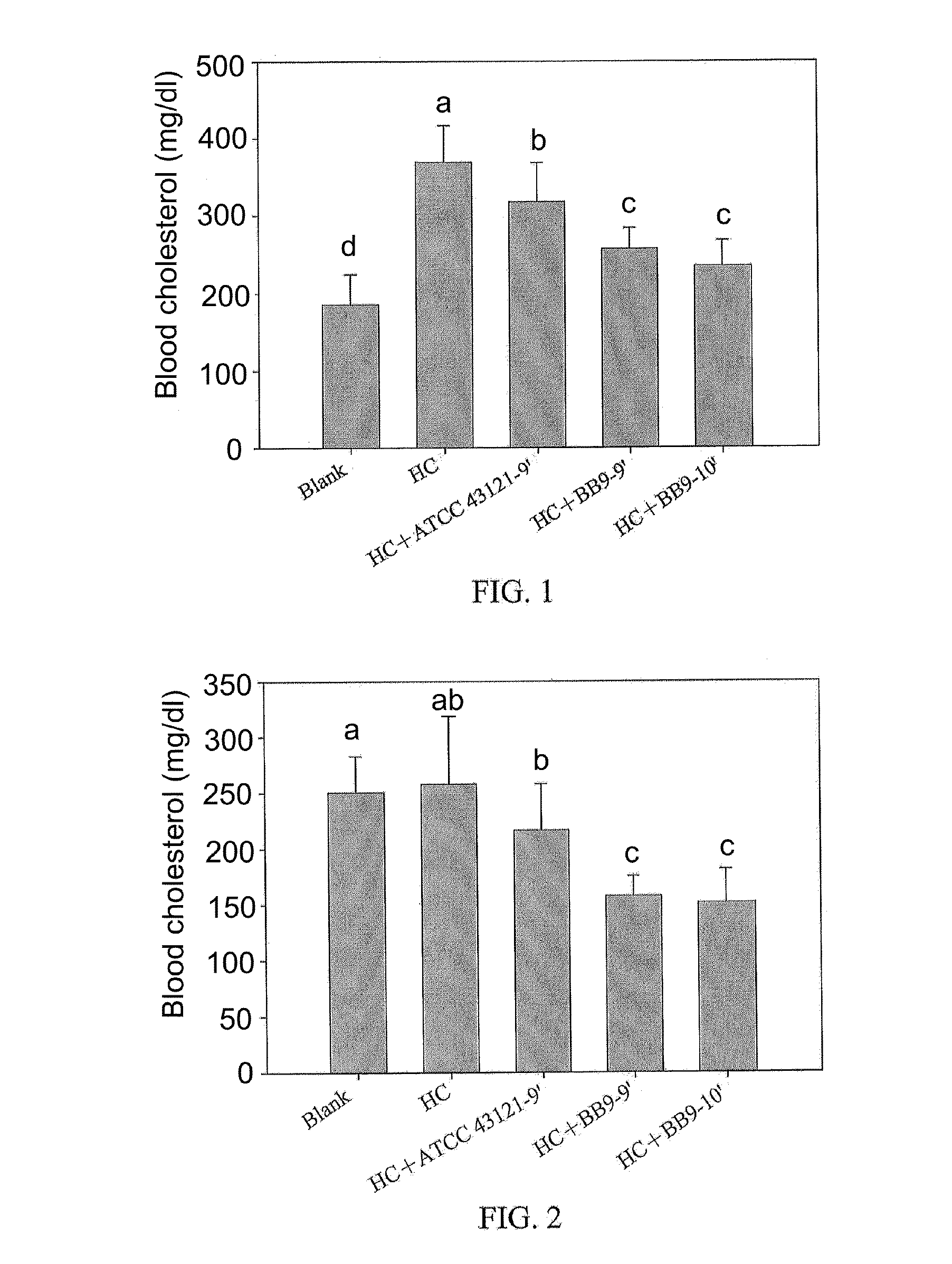
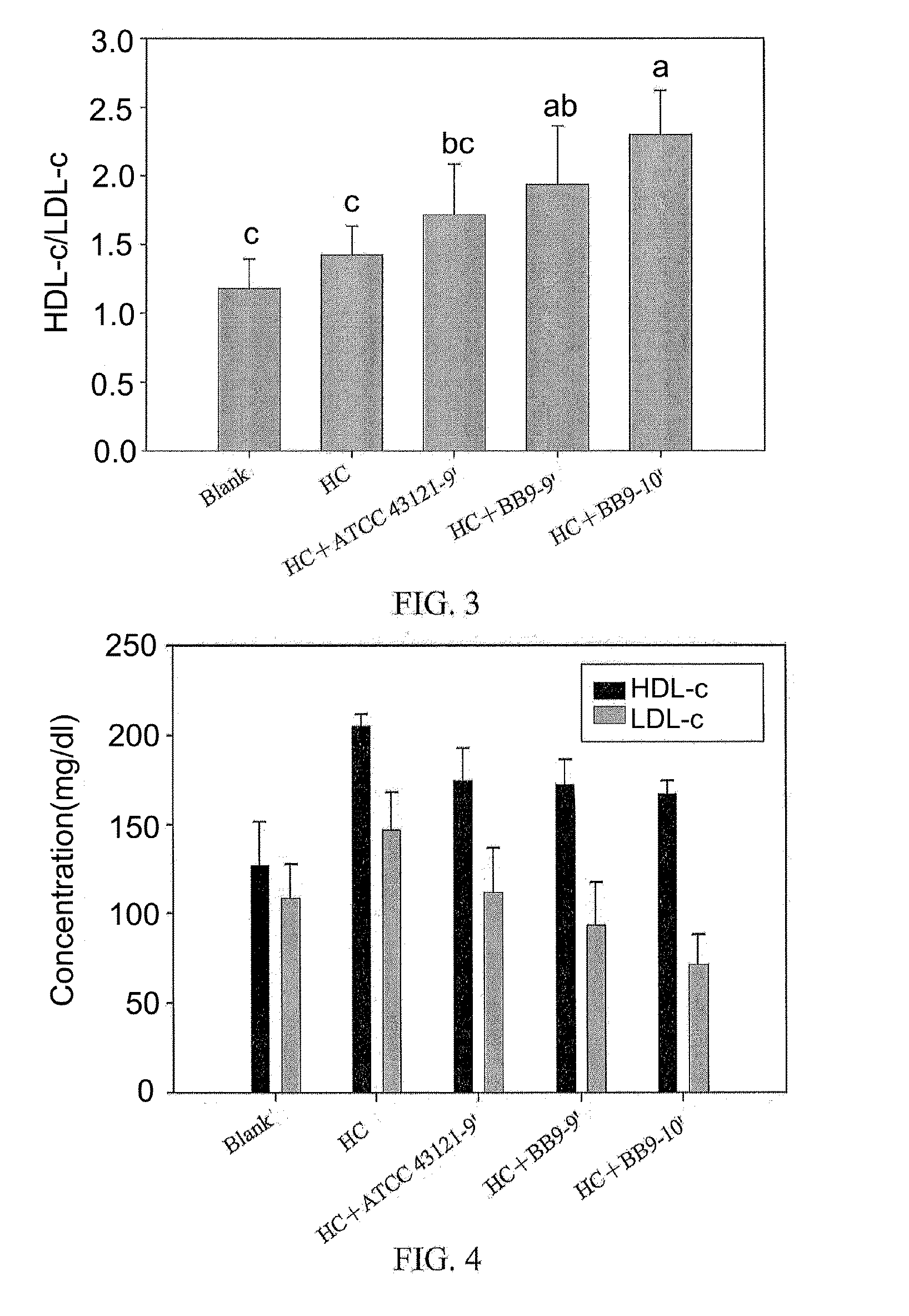
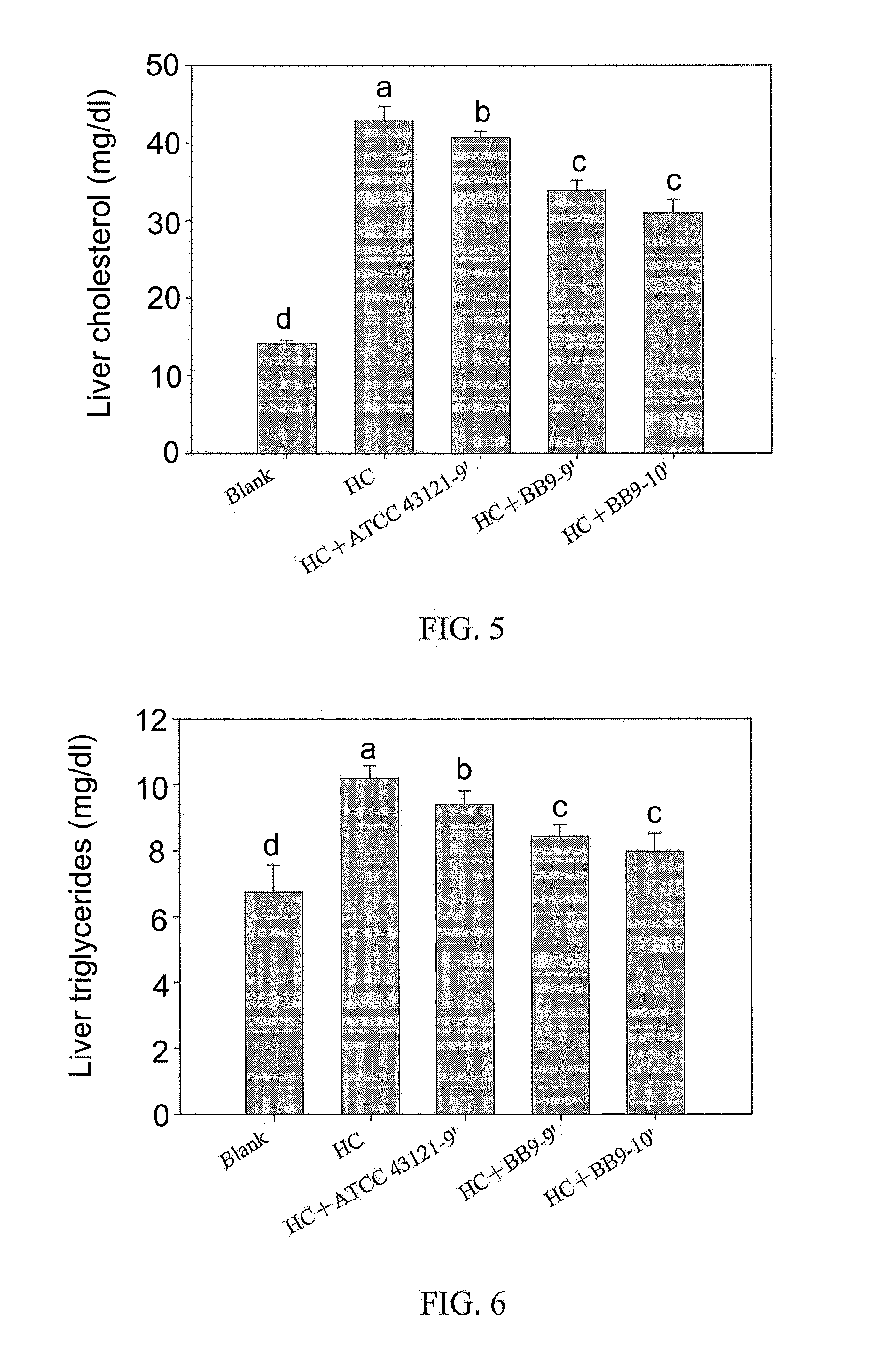
A Lactobacillus plantarum BB9 capable of adhering to gastrointestinal tract and cholesterol removal is isolated from fruits and exhibits high BSH activity. In-vitro tests demonstrate that Lactobacillus plantarum BB9 has good acid and bile tolerance, and strong ability to adhere to intestinal cells. In-vivo tests show that hamsters fed high cholesterol diets added with BB9 strain have cholesterol and triglycerides in blood and liver effectively reduced, and their HDL-c / LDL-c ratios in blood are significantly higher than those of hamsters fed Lactobacillus acidophilus ATCC 43121 strain. It is hoped that the excellent acid and bile tolerance and intestinal adherence of the lactobacillus strain provided herein could produce cholesterol-lowering effect in humans.
Owner:FAMILY MEDICINE INTERANTIONAL
Analyte monitoring device and methods of use
In aspects of the present disclosure, a no coding blood glucose monitoring unit including a calibration unit is integrated with one or more components of an analyte monitoring system to provide compatibility with in vitro test strip that do not require a calibration code is provided. Also disclosed are methods, systems, devices and kits for providing the same.
Owner:ABBOTT DIABETES CARE INC
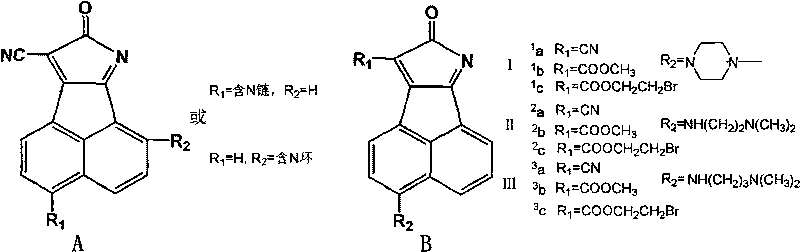
5- or 6-substited naphthoyl imines compounds and antineoplastic application
InactiveCN101323591AStrong cytotoxicityAvoid side effectsOrganic active ingredientsOrganic chemistrySide effectIn vitro test
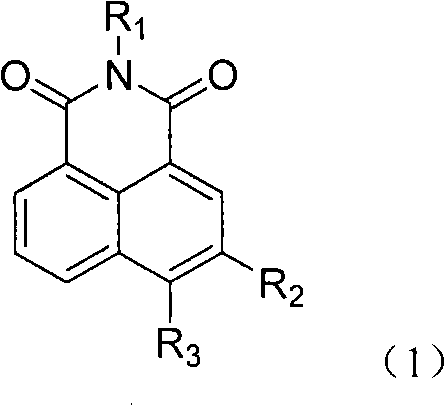
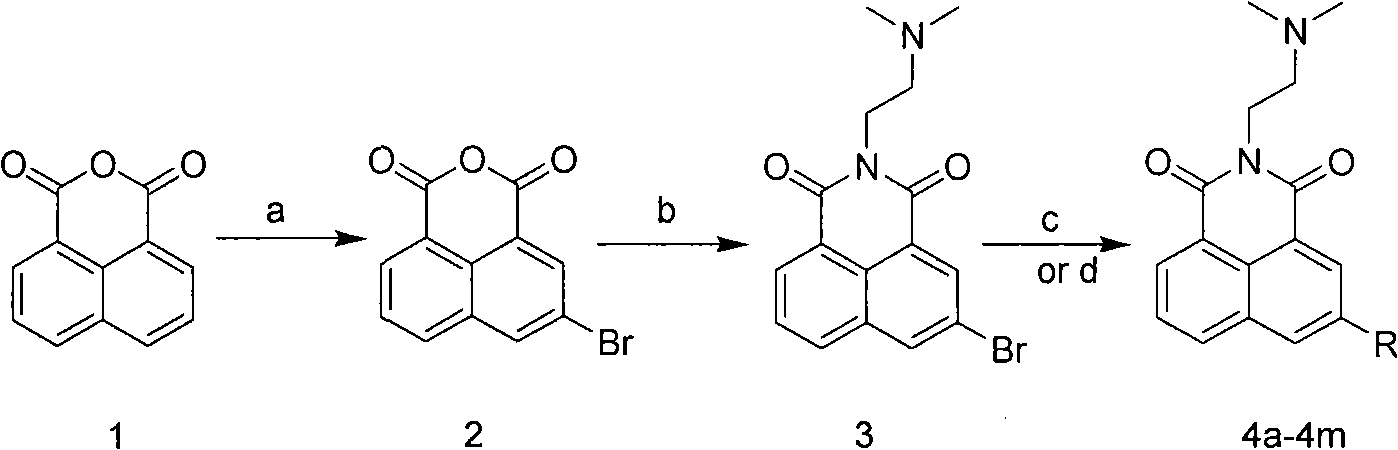
The invention relates to a 5-site or 6-site substituent naphthalimide compound and antitumor applications thereof, which belongs to the fine chemical field. The compound is characterized in that various aliphatic amidogen, heterocyclic amidogen, aryl and Ar aryl are respectively introduced into the 5-site or 6-site of the naphthalimide by a nucleophilic substitution reaction to obtain small molecule compound similar to a lead compound amonafide structure. The small molecule compound has obvious inhibiting activity in vitro test on Hela and P388D1, and wherein, some compounds have stronger cytotoxicity than the amonafide under the same test conditions. In addition, the structures of the compounds take on diversity; the compounds without acetylation sites can be prevented from having the side effect similar to the amonafide; the other compounds with the acetylation sites can be prevented from having the side effect by personalized administration, therefore, the compound has medical application prospect in curing diseases related to tumors.
Owner:DALIAN UNIV OF TECH
O-dicyano-acenaphtho pyrazine compound and anti-tumor application thereof
InactiveCN101723907AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryQuinonePyrazine
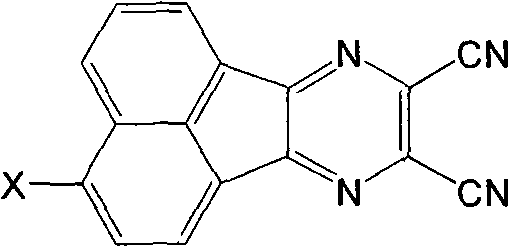
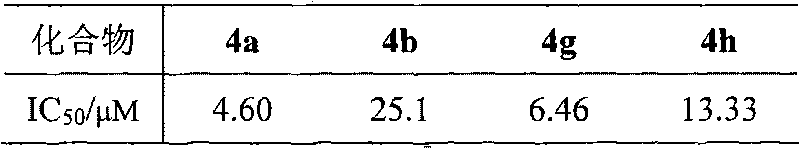
The invention relates to a DNA-targeted o-dicyano-acenaphtho pyrazine compound in the field of application of antineoplastic medicaments. The compound is prepared by taking acenaphthene quinone as a raw material and performing bromination, cyclization and aromatic nucleophilic substitution reaction. The compound is characterized in that: one end takes an amino straight chain of a flexible side chain or an annular group as an electron-donating group, while the other end takes two cyanogen groups as electron-withdrawing groups; the two ends form an electron deficient large-plane conjugated system; and the structure accords with an ideal DNA intercalator. The compound shows obvious inhibitory activity to an in vitro test of MCF-7 (human mammary cancer cells).
Owner:DALIAN UNIV OF TECH
Lactobacillus plantarum Grx16 and application thereof
ActiveCN105132318AExcellent ability to inhibit α-glucosidase activity in vitroStrong reducing activityMilk preparationBacteriaLactic acid bacteriumIn vitro test
The invention relates to separation, selection and identification of lactobacilli having a function of inhibiting activity of alpha-glucosidase, in particular to a lactobacillus plantarum and an application thereof. Lactobacilli are separated out from intestinal canals of longevous people in Bama of Guangxi; on the basis of comprehensive comparison of acid resistance, cholate resistance, in-vitro antioxidant ability and sensitivity to antibiotics, a strain having a best inhibition function to activity of alpha-glucosidase is selected out by means of in-vitro tests; the strain is identified as Lactobacillus plantarum Grx16 by means of physiological and biochemical tests, API reagent strips and 16s rDNA sequencing analysis. The lactobacillus plantarum Grx16 can be used for preparation of fermented milk having the function of inhibiting activity of alpha-glucosidase.
Owner:上海昊岳生物科技有限公司
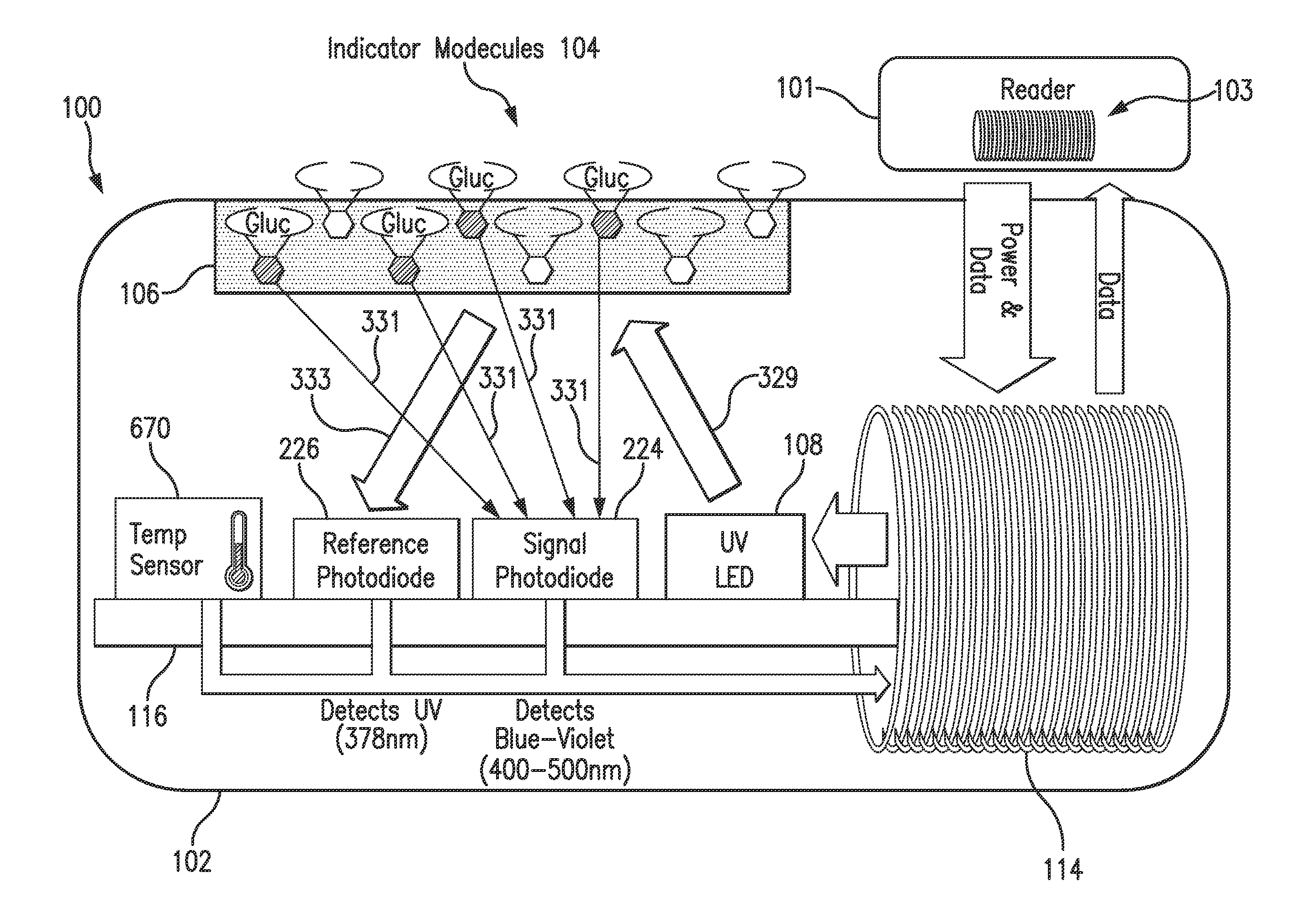
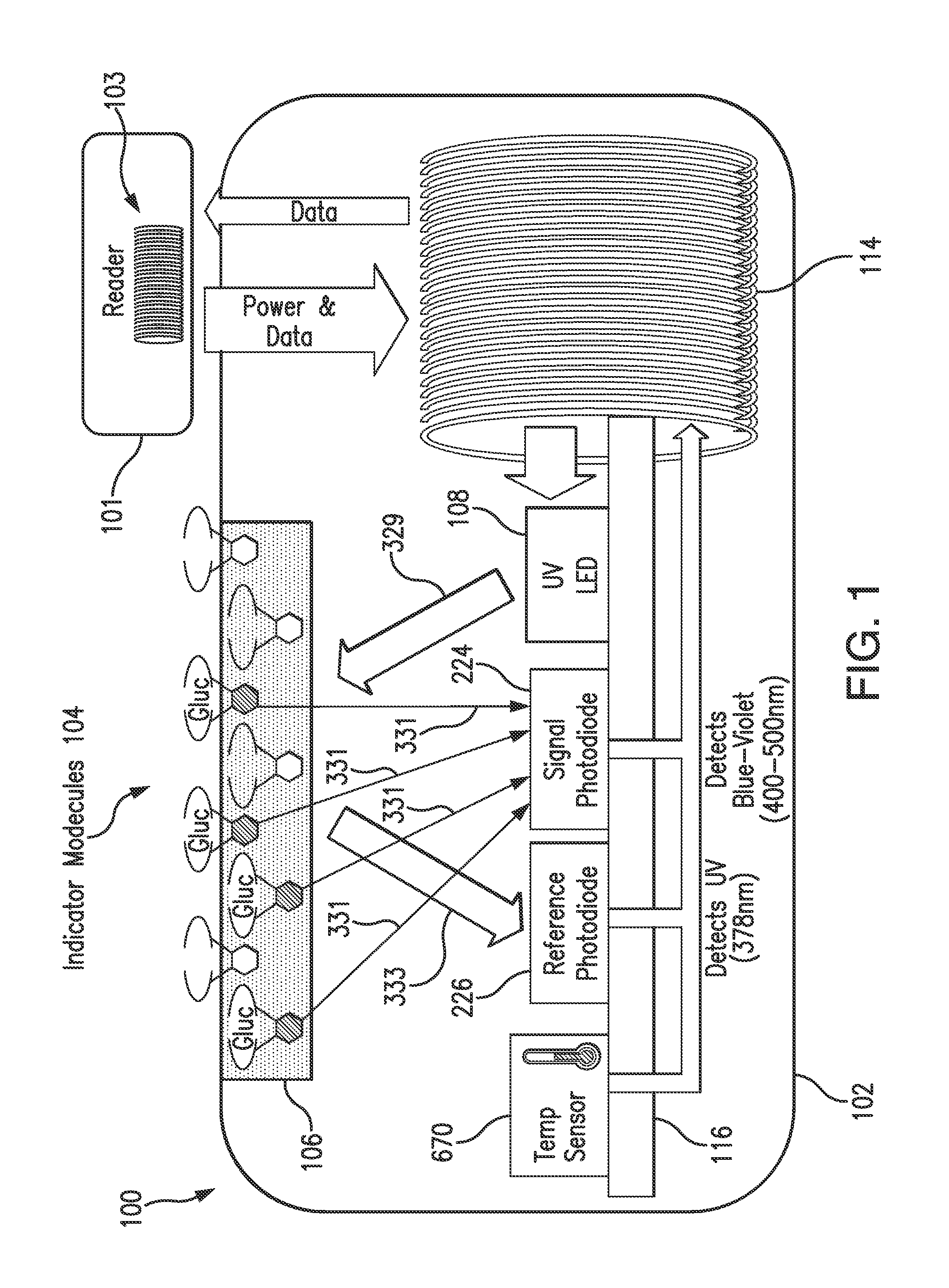
Purification of glucose concentration signal in an implantable fluorescence based glucose sensor
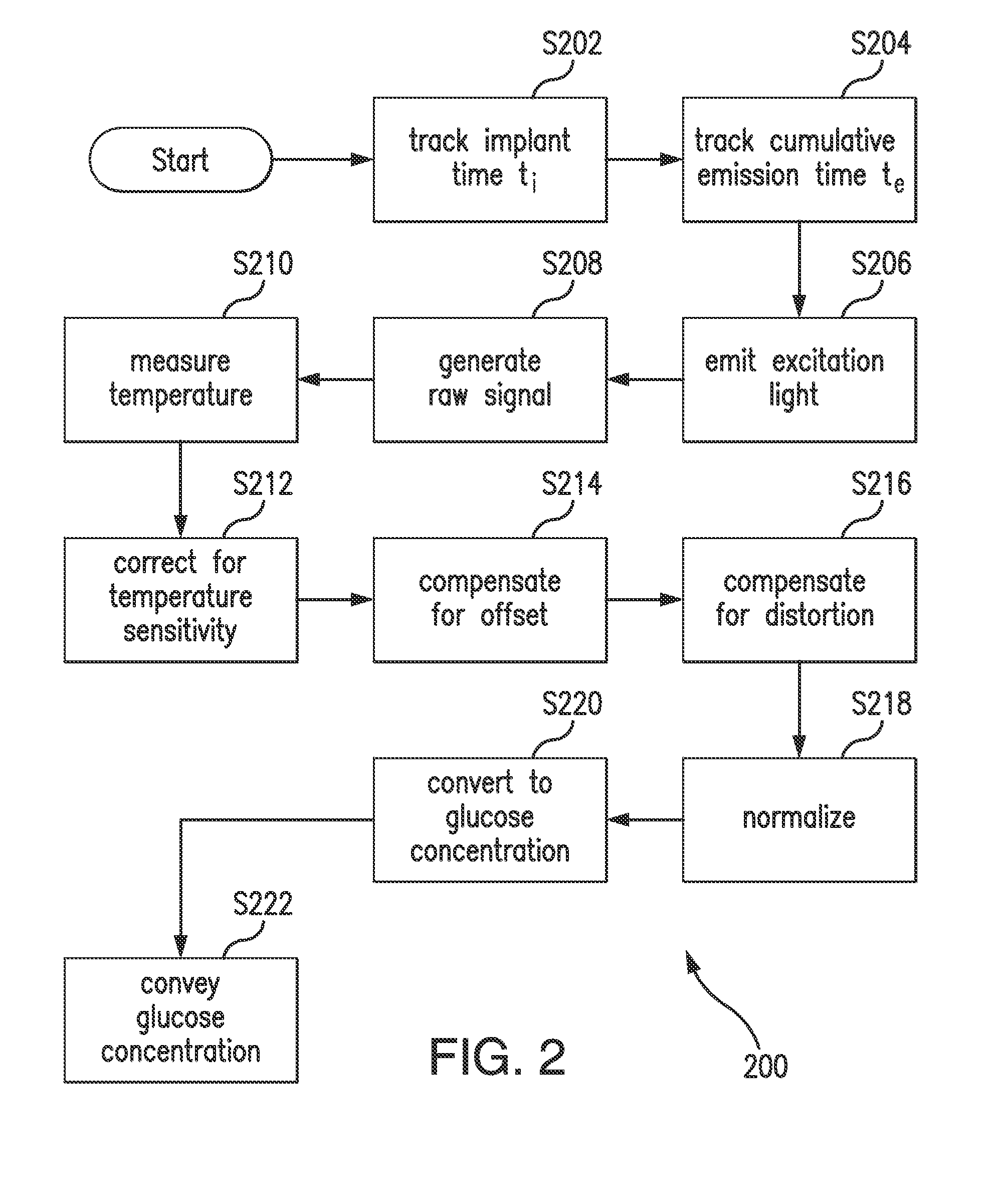
Methods, sensors, and systems for determining a concentration of glucose in a medium of a living animal are disclosed. Determining the glucose concentration may involve emitting excitation light from a light source to indicator molecules, generating a raw signal indicative of the amount of light received by a photodetector, purifying and normalizing the raw signal, and converting the normalized signal to a glucose concentration. The purification may involve removing noise (e.g., offset and / or distortion) from the raw signal. The purification and normalization may involve tracking the cumulative emission time that the light source has emitted the excitation light and tracking the implant time that has elapsed since the optical sensor was implanted. The purification and normalization may involve measuring the temperature of the sensor. The purification, normalization, and conversion may involve using parameters determined during manufacturing, in vitro testing, and / or in vivo testing.
Owner:SENSEONICS INC
In-vitro test stimulator
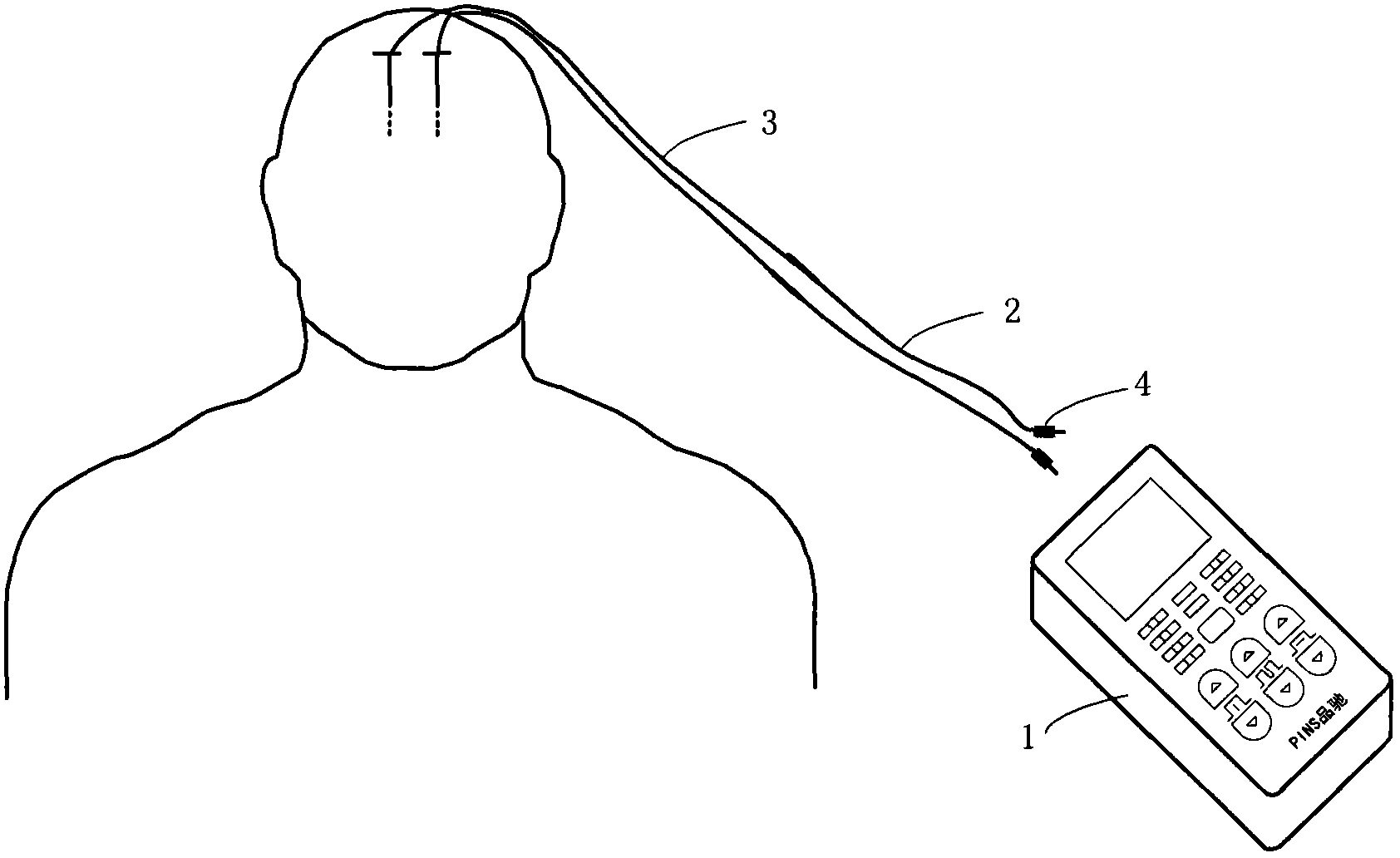
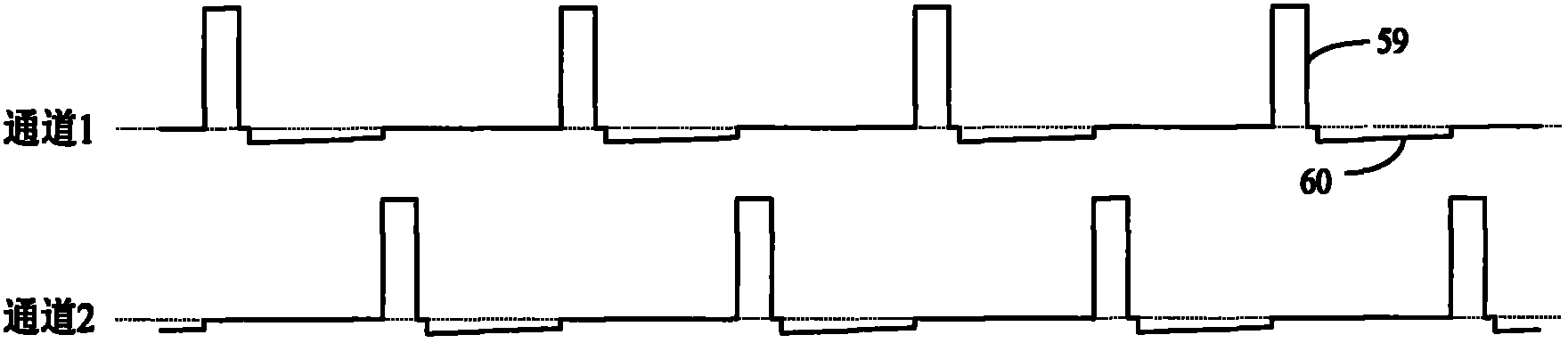
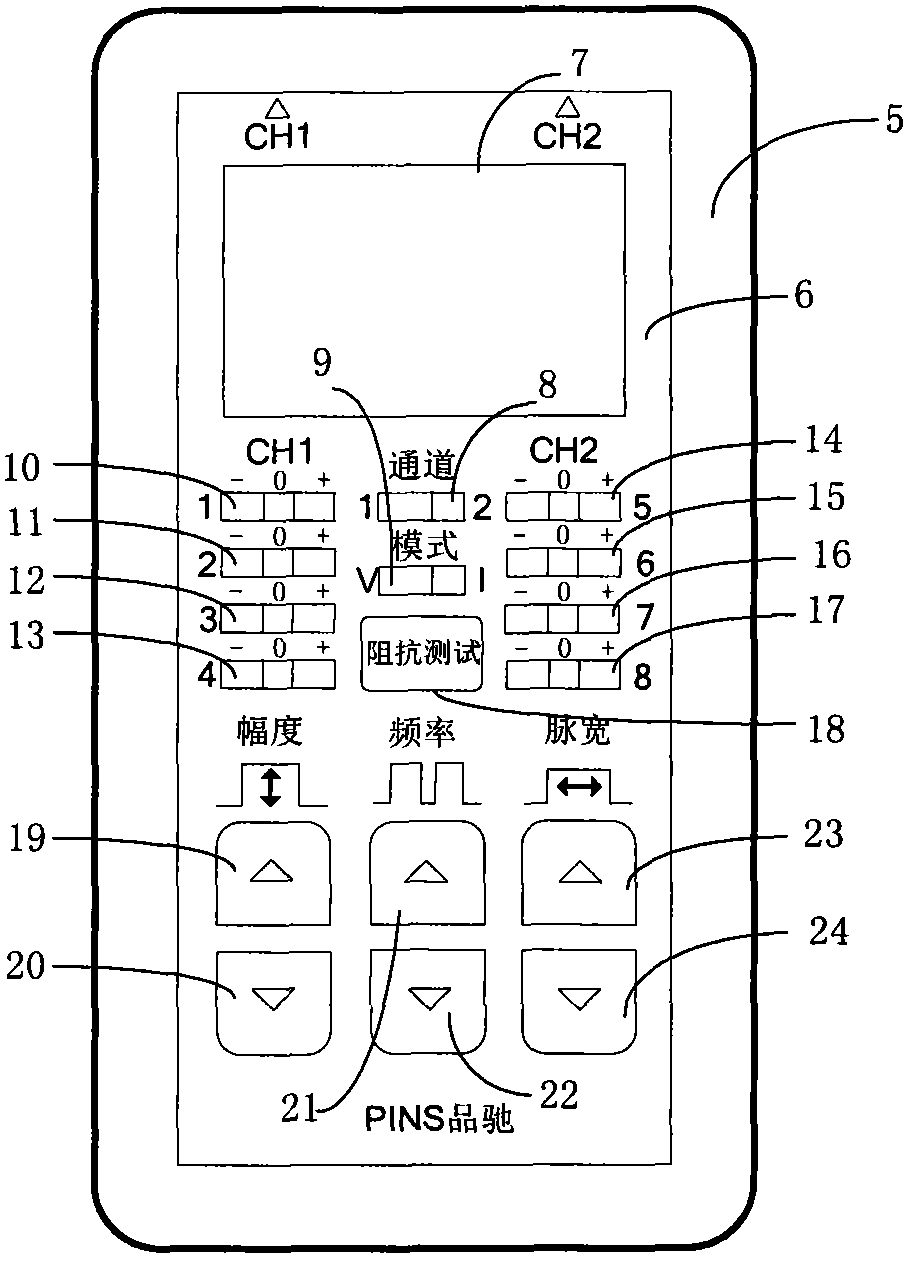
ActiveCN102921105ASmall and beautiful appearanceEasy to operateElectrotherapyDiagnostic recording/measuringPulse parameterIn vitro test
The invention discloses an in-vitro test stimulator, and belongs to the technical field of implanted medical instruments. The in-vitro test stimulator is mainly used for nervous electric stimulation therapy test. The double-channel pulse output in-vitro test stimulator with a user interface can output accurate parameter adjustable electrical stimulation pulse, and is used for treatment test or short-term electric stimulation therapy experience of patients in implanted nerve stimulator operation. The test stimulator is characterized in that the test stimulator adopts integrated structural design, and mainly comprises a shell, a toggle switch, a film key, a display screen, a battery, a printed circuit board and the like. The test stimulator is small and simple; through the key and the switch, pulse parameters and test electrode load impedance can be adjusted and the polarity of an electrode contact can be set, so the test stimulator is intuitive and convenient to operate; and through the design of self-locking function and error operation prevention, error operation of the patients in use can be effectively prevented, and the test stimulator is safe and reliable. The test stimulator can be widely applied to test assessment and experience of electric stimulation therapy.
Owner:BEIJING PINS MEDICAL +1
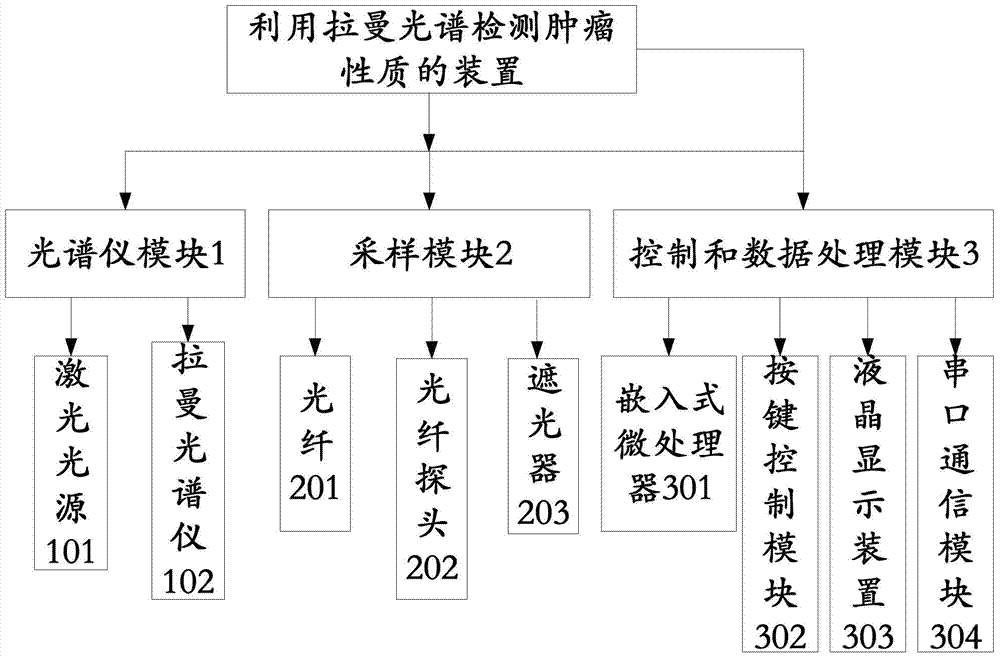
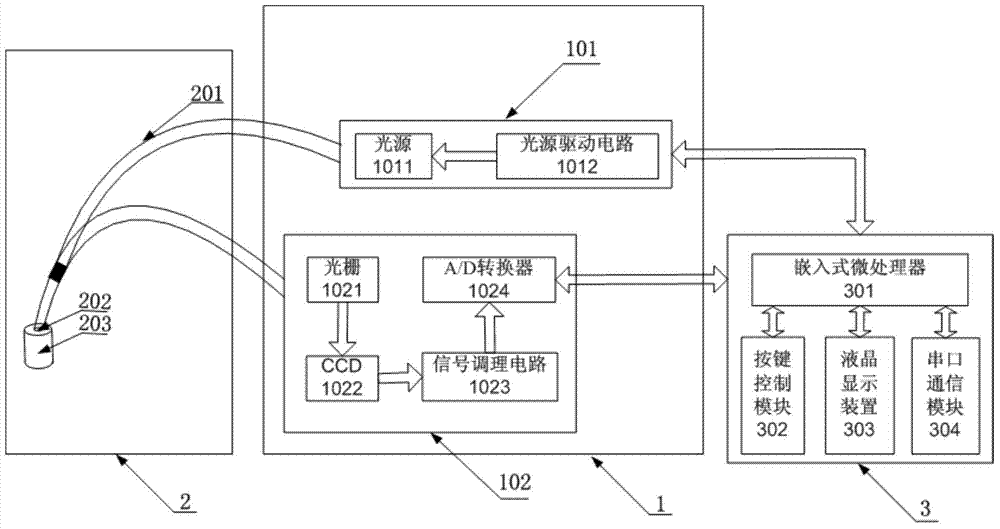
Device for detecting tumor characters by Raman spectrum
The invention discloses a device for detecting tumor character by Raman spectrum. The device comprises a spectrometer module, a sampling module and a control and data processing module, wherein the spectrometer module comprises a laser light source and a Raman spectrometer; the laser light source is used for providing the laser needed by detection; the Raman spectrometer is used for acquiring the Raman spectrum of a sample to obtain a digital signal containing sample molecular information; the sampling module comprises optical fibers and an optical fiber probe; the optical fiber probe is used for collecting the Raman spectrum; the laser light source is connected with the optical fiber probe by the optical fiber, and the optical fiber probe is connected with the Raman spectrometer by the optical fiber; the control and data processing module comprises an embedded microprocessor which is respectively connected with the laser light source and the Raman spectrometer, so that the Raman spectrometer and the laser light source can be controlled in the detection process; and the control and data processing module can be used for judging the tumor character by processing the spectral data after reading the digital signal acquired by the Raman spectrometer. The portable device supports in vivo or in vitro test and is capable of carrying out rapid clinical diagnosis on the tumor character.
Owner:BEIHANG UNIV
Novel piperazinoamide compound
ActiveCN101851211ASimple preparation processRaw materials are easy to getOrganic active ingredientsNervous disorderConvulsionIn vitro test
The invention relates to a novel piperazinoamide compound, a preparation method thereof, and application thereof in treating neurodegenerative disease, mental disease, epilepsy, convulsion, apoplexy, and the like. The structure of the piperazinoamide compound is shown as formula I. The in vitro tests verify that the piperazinoamide compound has an effect of inhibiting cells BV2 from secreting IL-1beta effectively, and has high safety. The formula I is shown in the description.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Analyte Monitoring Device and Methods of Use
ActiveUS20160228041A1Surgical needlesMaterial analysis by electric/magnetic meansAnalyteIn vitro test
In aspects of the present disclosure, a no coding blood glucose monitoring unit including a calibration unit is integrated with one or more components of an analyte monitoring system to provide compatibility with in vitro test strip that do not require a calibration code is provided. Also disclosed are methods, systems, devices and kits for providing the same.
Owner:ABBOTT DIABETES CARE INC
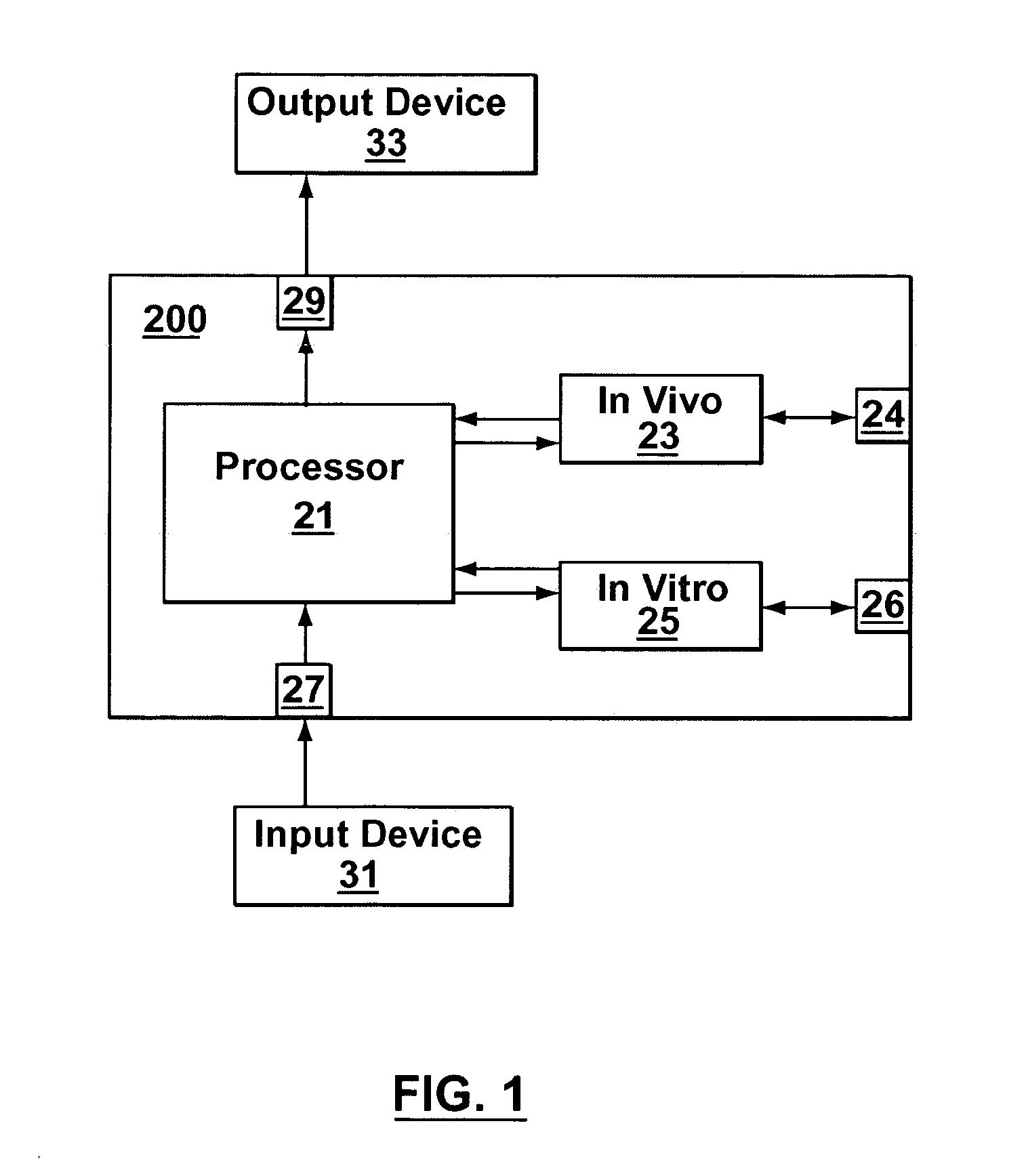
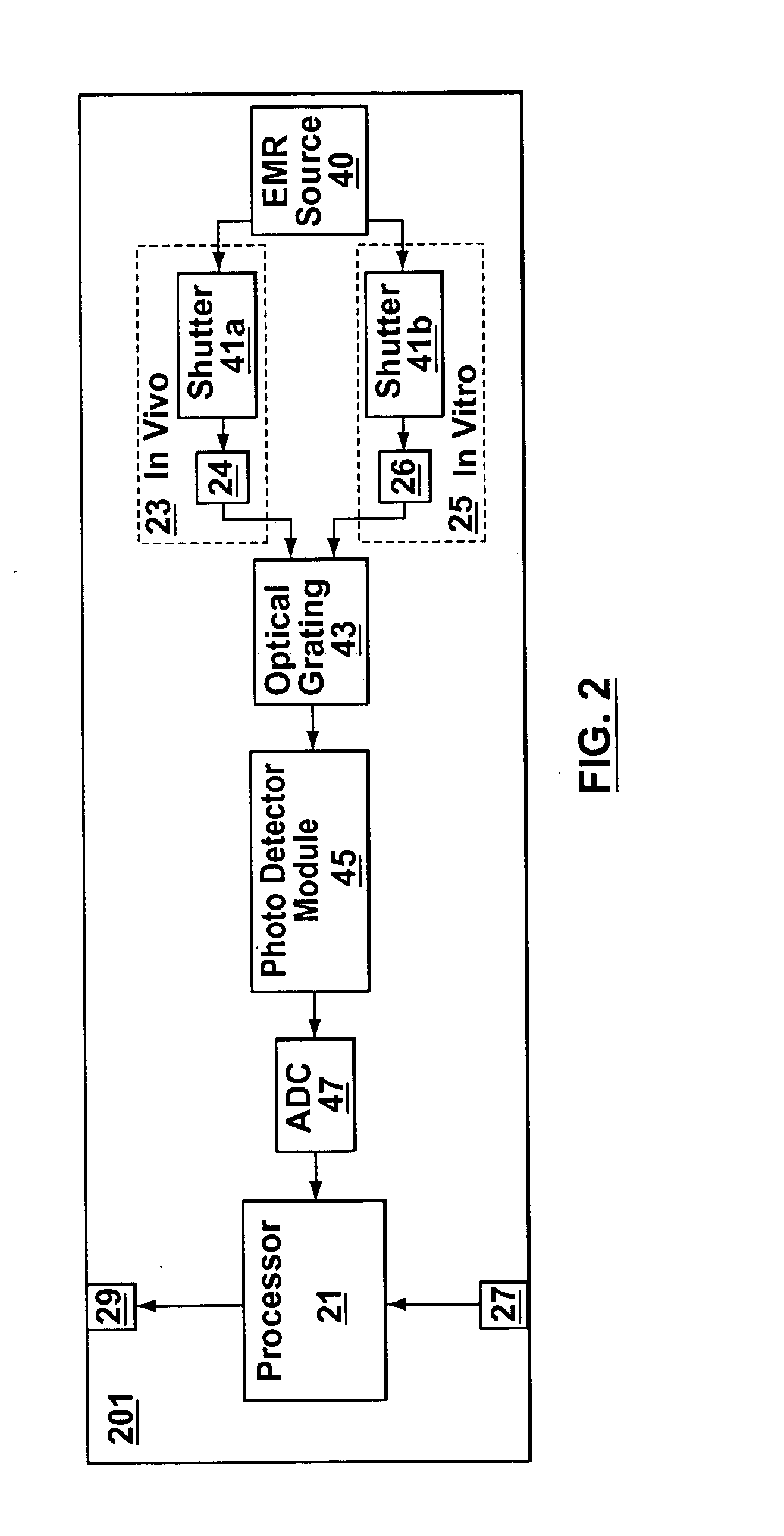
Joint-diagnostic in vivo & in vitro apparatus
InactiveUS20050203356A1Material analysis by observing effect on chemical indicatorColor/spectral properties measurementsAnalyteCombined use
In vivo testing for analytes in a life-form is an attractive concept because a biological sample does not have to be removed from the life-form. However, in vivo testing alone is unable to provide information that is accurate, complete and / or reliable enough to safely replace in vitro testing. In contrast to performing either in vivo or in vitro testing independently and alone, some embodiments of the present invention provide a joint-diagnostic apparatus for combined in vivo and in vitro testing. In some specific embodiments results from an in vitro measurement module are used in combination with subsequent in vivo measurements / observations obtained at a later time, and / or vice versa. Accordingly, in some embodiments in vitro measurements are used to compliment and / or partially compensate for some of the limitations of in vivo testing, and at the same time enabling some of the benefits of in vivo testing by reducing the number of biological samples taken.
Owner:TYCO HEALTHCARE GRP LP
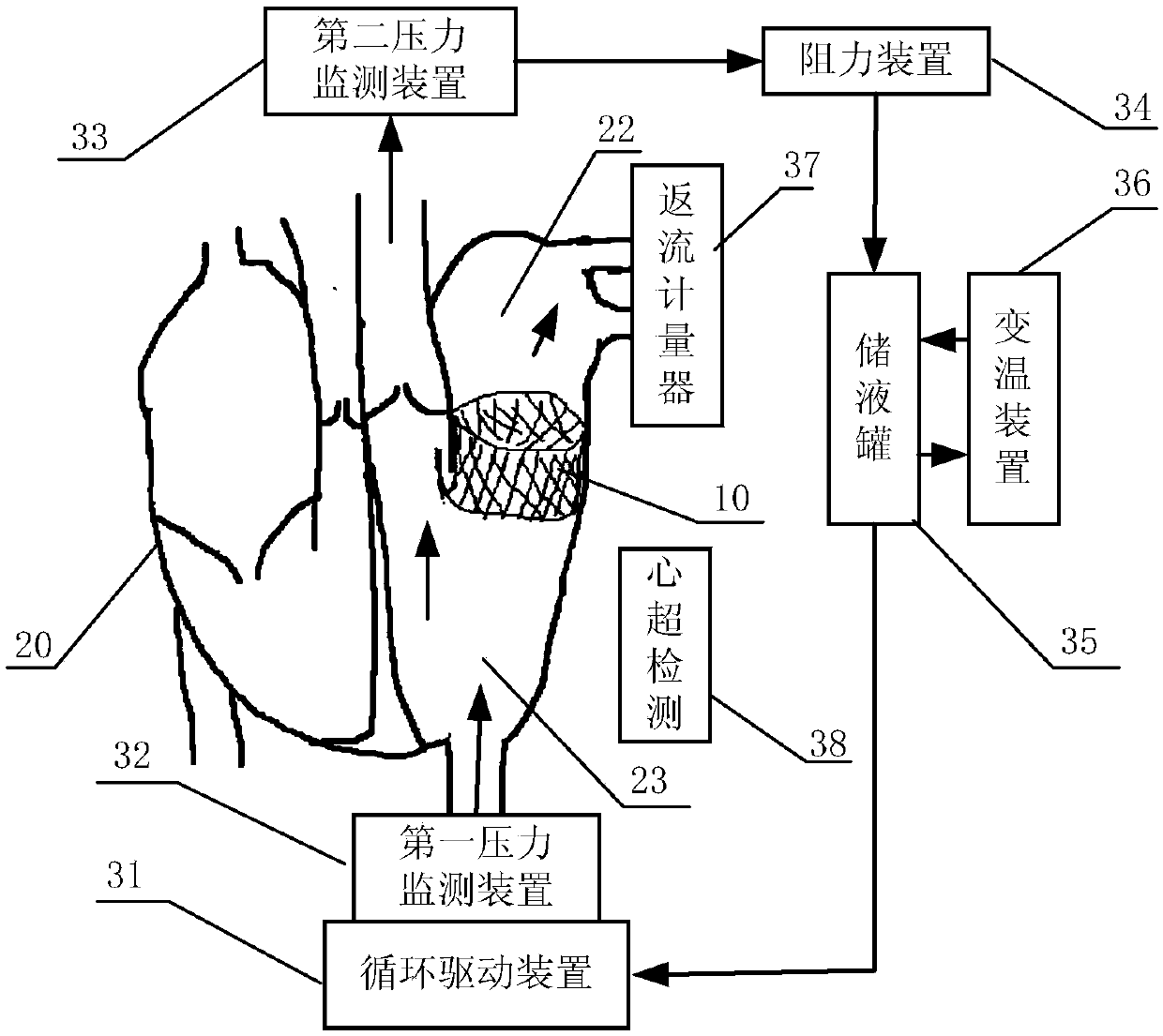
In-vitro performance testing system and testing method of transcatheter bicuspid valve valved stent
PendingCN107773328AAchieve hydrodynamic performanceEnables in vitro performance testingHeart valvesStationary stateIn vivo
The invention discloses an in-vitro performance testing system and testing method of a transcatheter bicuspid valve valved stent, and relates to prostheses capable of being implanted in vivo, in particular to a testing device and testing method applied to heart valve performance testing. The in-vitro performance testing system comprises a valve testing part which is internally provided with the bicuspid valve valved stent, a liquid circulation device and a monitoring device; a first testing part is a heart specimen of which the left ventricular apex is provided with a circulation inlet, and asecond testing part is a section of artificial blood vessel or a section of blood vessel specimen for sewing the tested bicuspid valve valved stent; the liquid testing device is connected with the first testing part to constitute a first testing circulating path for steady flow circulation and a second testing circulating path for simulating the ventricular beat of the human body; the liquid circulation device is connected with the second testing part to constitute a third testing circulating path which is connected with circulation lateral branches, in vitro tests of fluid mechanic performance, support fixing states, valve backflow, perivalvular leakage, and influences on surrounding important organization structures of the bicuspid valve valved stent can be achieved, and a durability experiment of the bicuspid valve valved stent is completed.
Owner:SHANGHAI TONGJI HOSPITAL
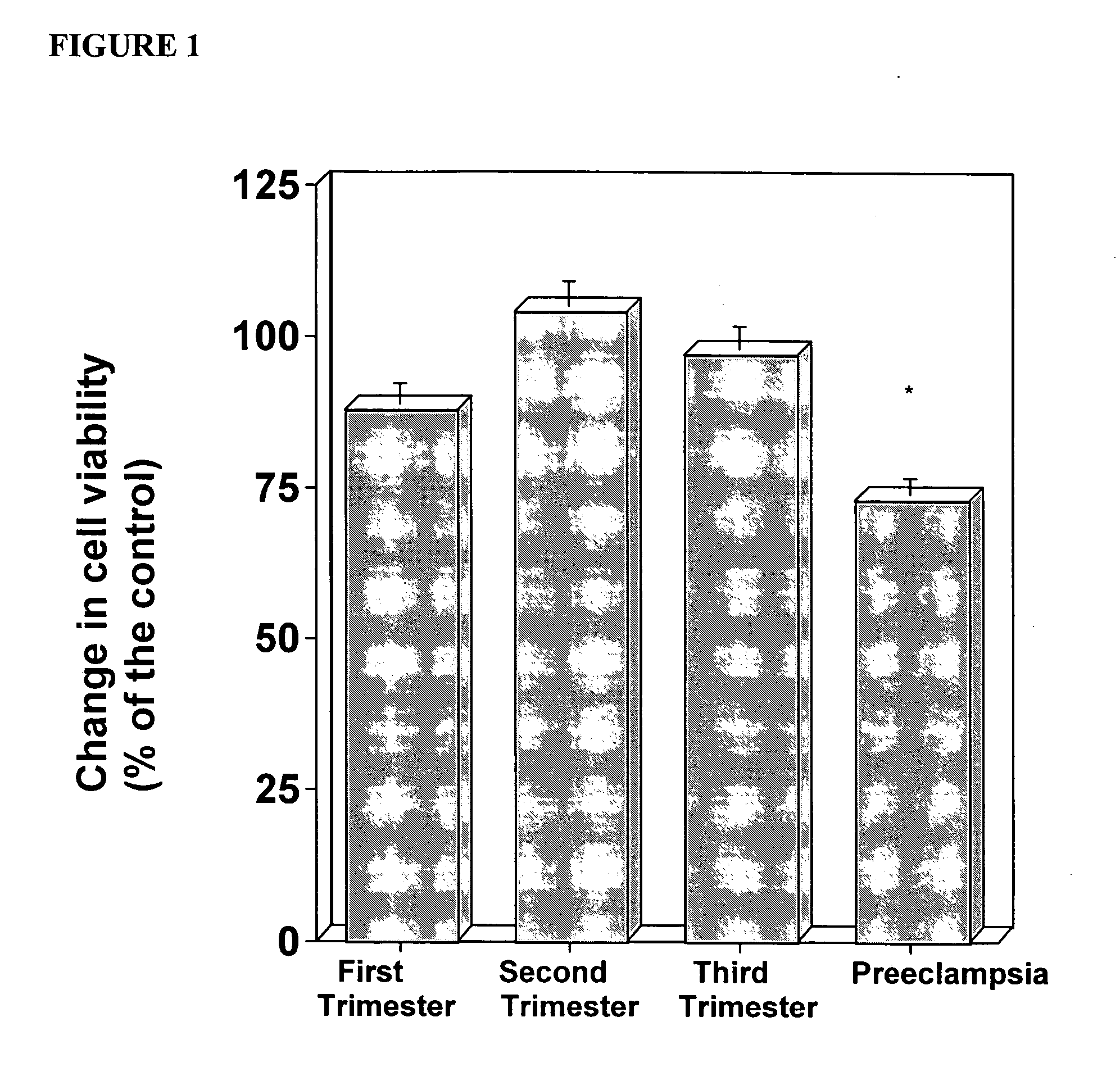
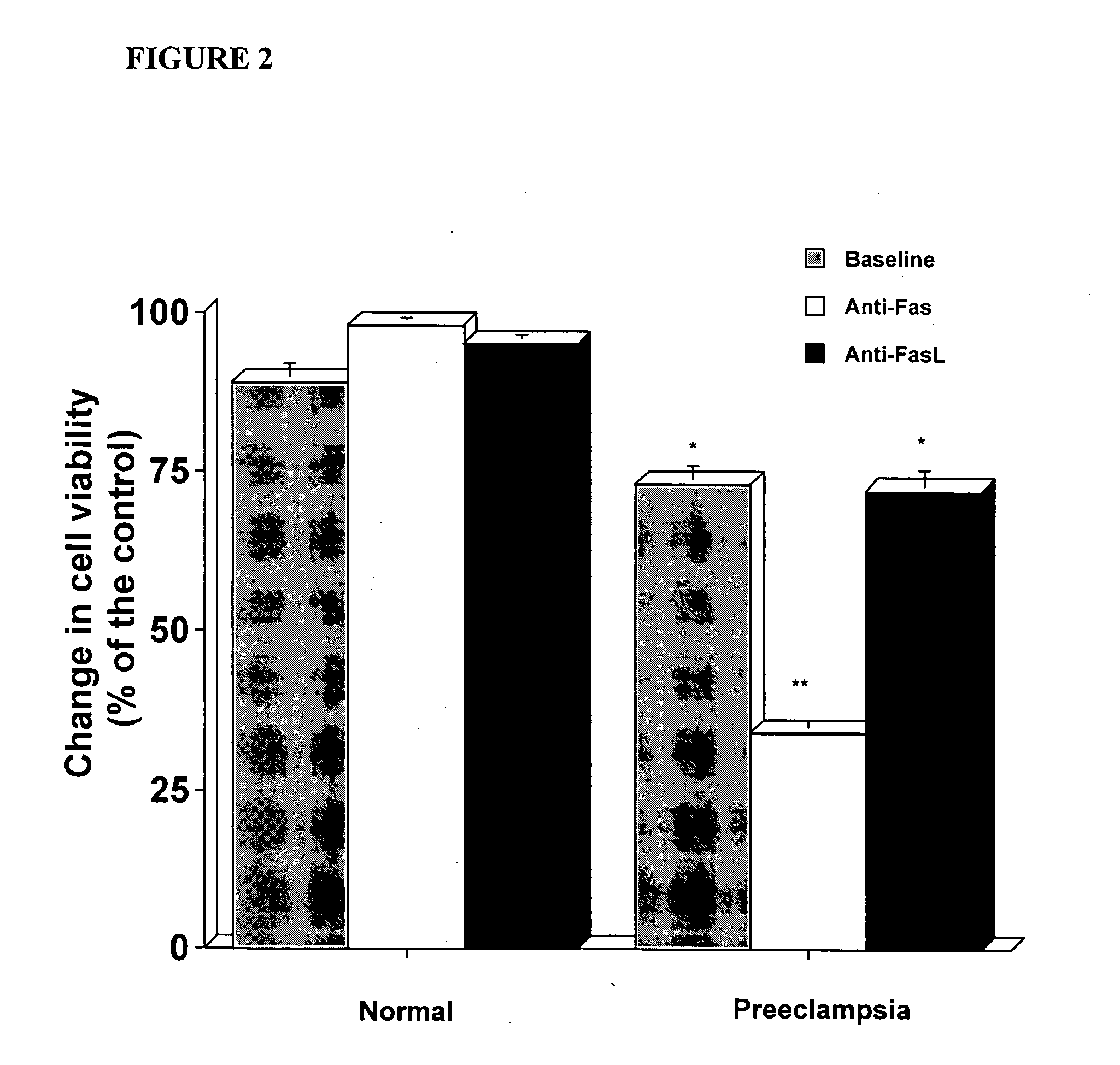
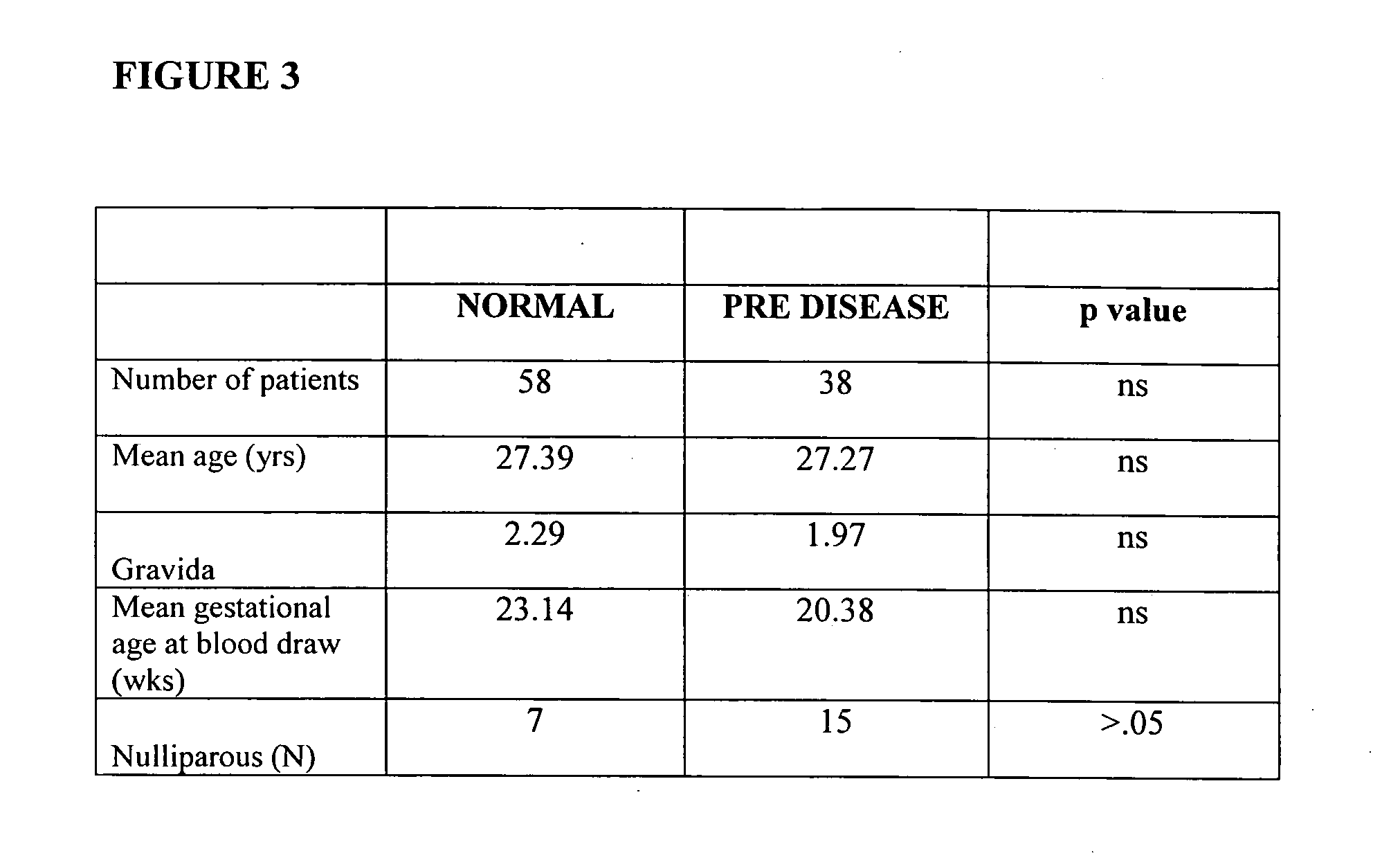
In vitro test to detect risk of preeclampsia
ActiveUS20050074746A1Reduction in trophoblast viabilityFunctional impairmentMicrobiological testing/measurementArtificial cell constructsObstetricsIn vitro test
The present invention provides methods for identifying patients at risk of developing preeclampsia. In further embodiments, the present invention relates to methods for the diagnosis of patients with preeclampsia.
Owner:YALE UNIV +1
Lactobacillus plantarum with oxidation resistance and application thereof
ActiveCN109182162AGood probiotic propertiesAvoid damageCosmetic preparationsMilk preparationIntestinal wallsSide effect
The invention discloses lactobacillus plantarum with oxidation resistance and preservation number of CGMCC15780, and application of the lactobacillus plantarum. The lactobacillus plantarum is a lactobacillus strain with high oxidation resistance, which is screened from traditional fermented yak yogurt and silage in the Qinghai-Tibet Plateau. In vivo and in vitro tests prove that the lactobacillusplantarum has relatively good oxidation resistance, and can be used for effectively promoting increase of the activity of antioxidative enzyme in a body. The lactobacillus plantarum having relativelygood hydrophobic performance can increase the opportunity of adhering to the intestinal walls and achieving the probiotic effect, does not have toxic or side effect, and can be used as health-protecting foods or medicines to enhance the body immunity, relieve senescence, beautify, regulate intestinal flora and the like.
Owner:哈尔滨美华生物技术股份有限公司
Human cytokine and use thereof
The invention discloses a new secretive cytokine NS128 of human beings, which relates to a coding amino acid sequence of the cytokine or the polynucleotide of the cytokine fragment, containing a genetic engineering carrier and a host cell of the polynucleotide. The invention also relates to a production method of the amino acid sequence of the cytokine or the polypeptide of the cytokine fragment and the production method of the polypeptide as well as a small interference RNA, which suppresses the expression of the cytokine. The invention also relates to a salt which contains the polynucleotide or the salt of acceptable drugs, or a medical composition of the polypeptide or the salt of acceptable drugs. The invention further relates to applications of the polynucleotide of the cytokine, the polypeptide or the small interference RNA in preparation of prevention and / or treatment medicines for tumor, infection, hematopoietic disorder and autoimmune disease, etc., in particular having clinical implications for tumor, inflammation and nervous system disease and so on related to MAPK signal pathway. The invention also relates to a method of in vitro testing whether the expression of the polynucleotide or the polypeptide changes. The invention also relates to a monoclonal or polyclonal antibody which is specifically bound with the polypeptide or an active fragment of the polypeptide.
Owner:SINOGENOMAX +1
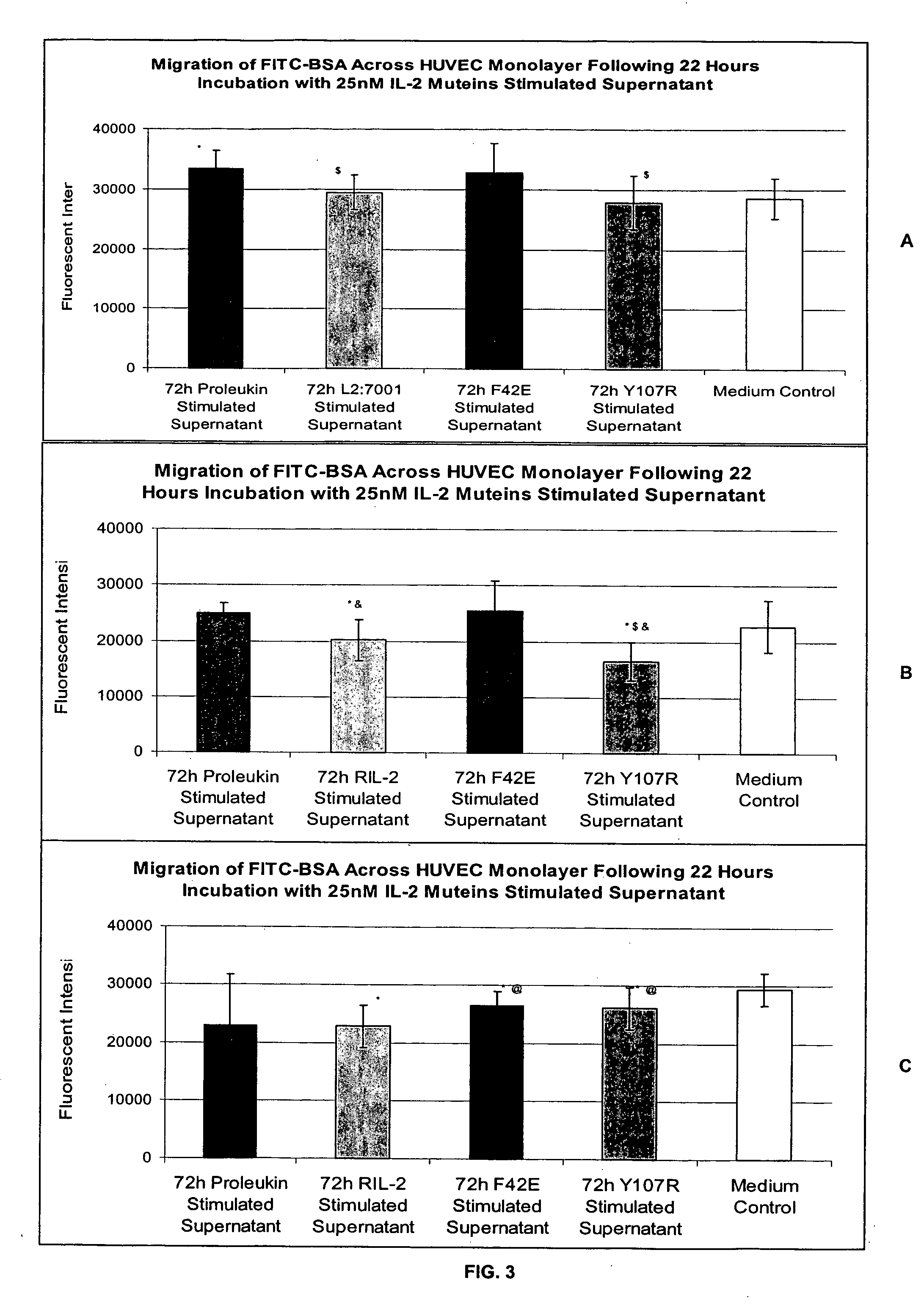
In vitro test system for predicting patient tolerability of therapeutic agents
InactiveUS20060234205A1Simple and efficacious in vitroPredict tolerabilityPeptide/protein ingredientsMicrobiological testing/measurementTolerabilityIn vitro test
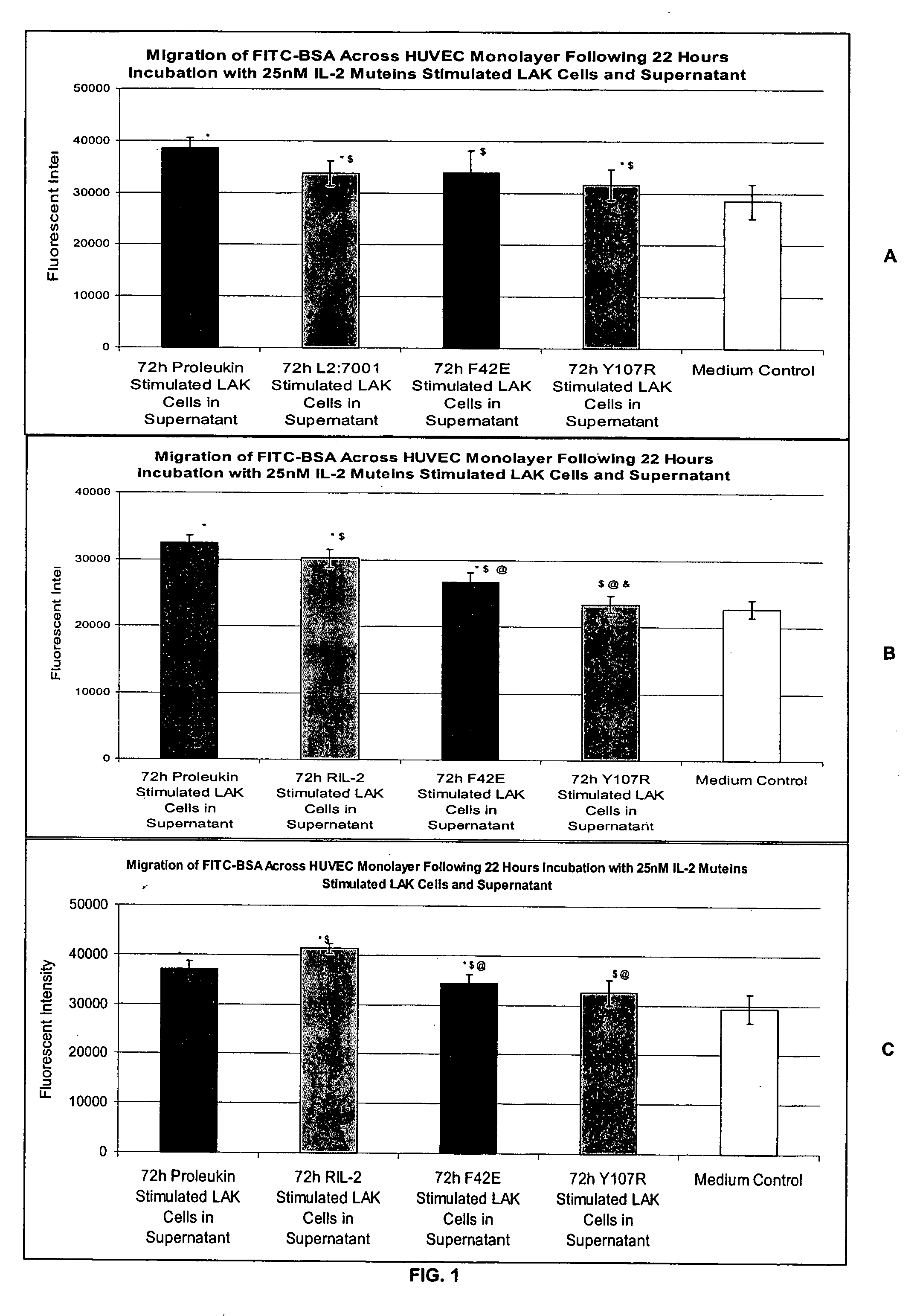
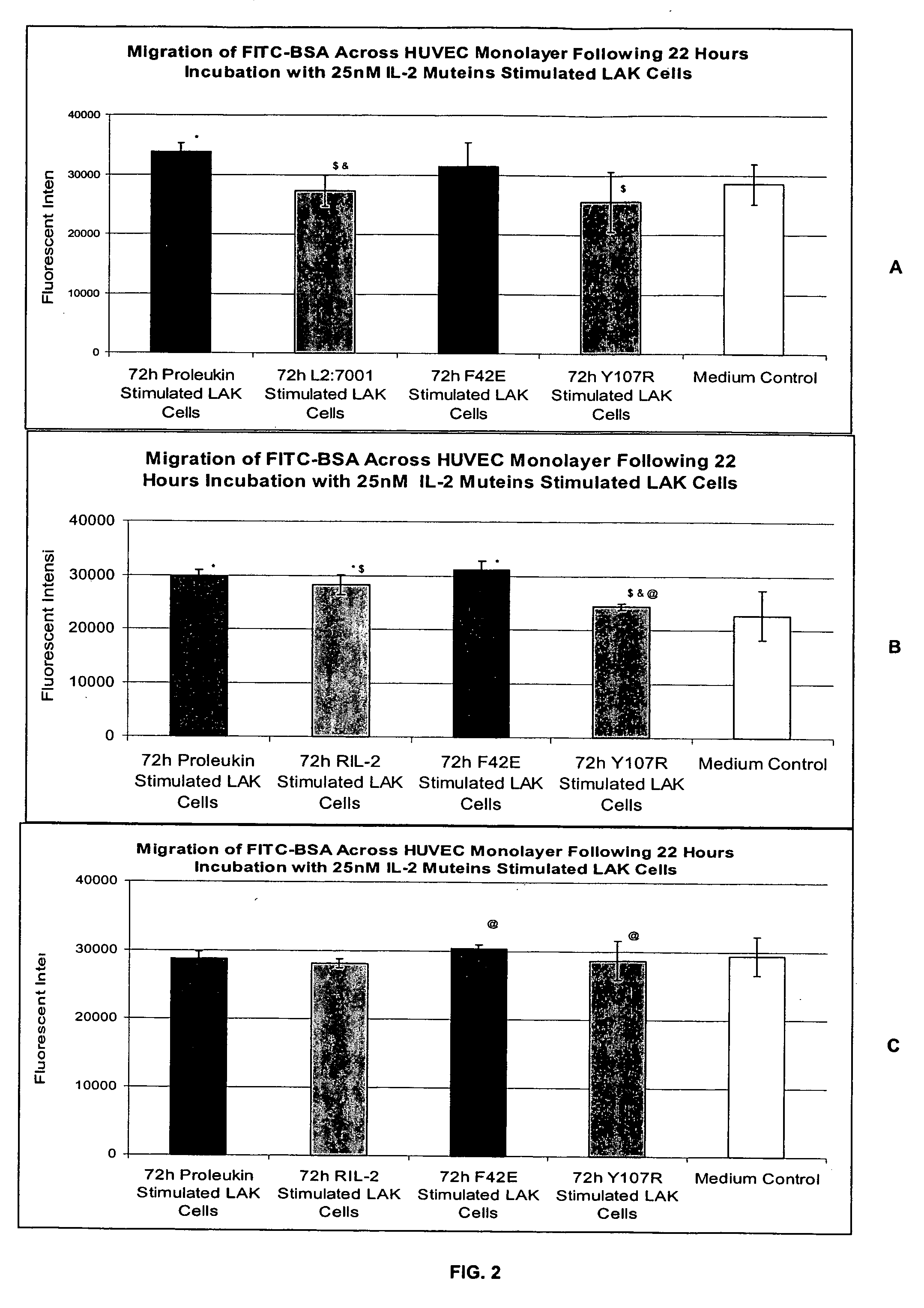
Methods for predicting patient tolerability to therapeutic agents, such as cytokines, lymphokines and immunotoxins, are disclosed. The methods utilize an in vitro model of vascular leak syndrome (VLS) to assess the effect of the agent in question on the permeability of large proteins across confluent monolayers of endothelial cells (EC).
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
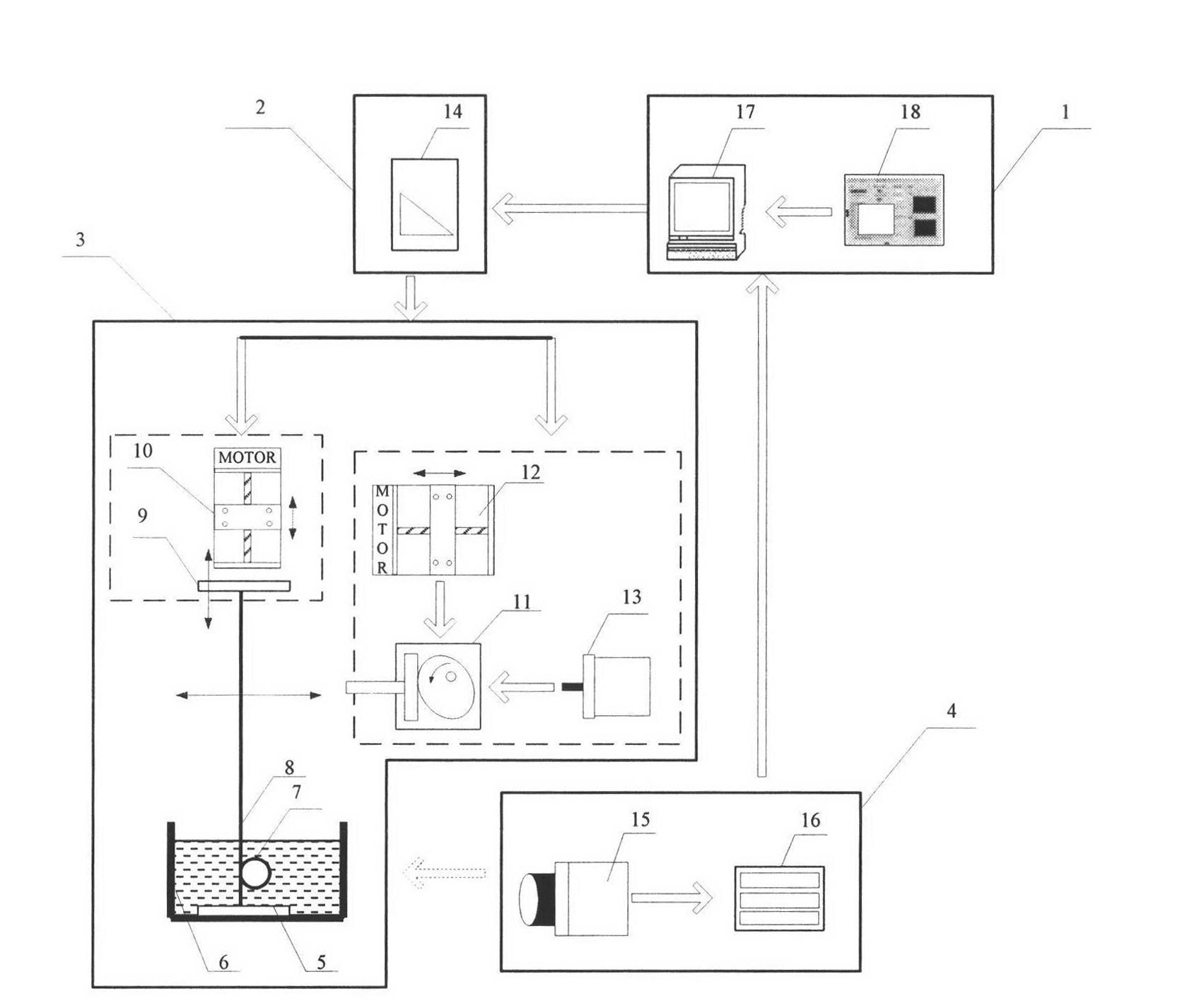
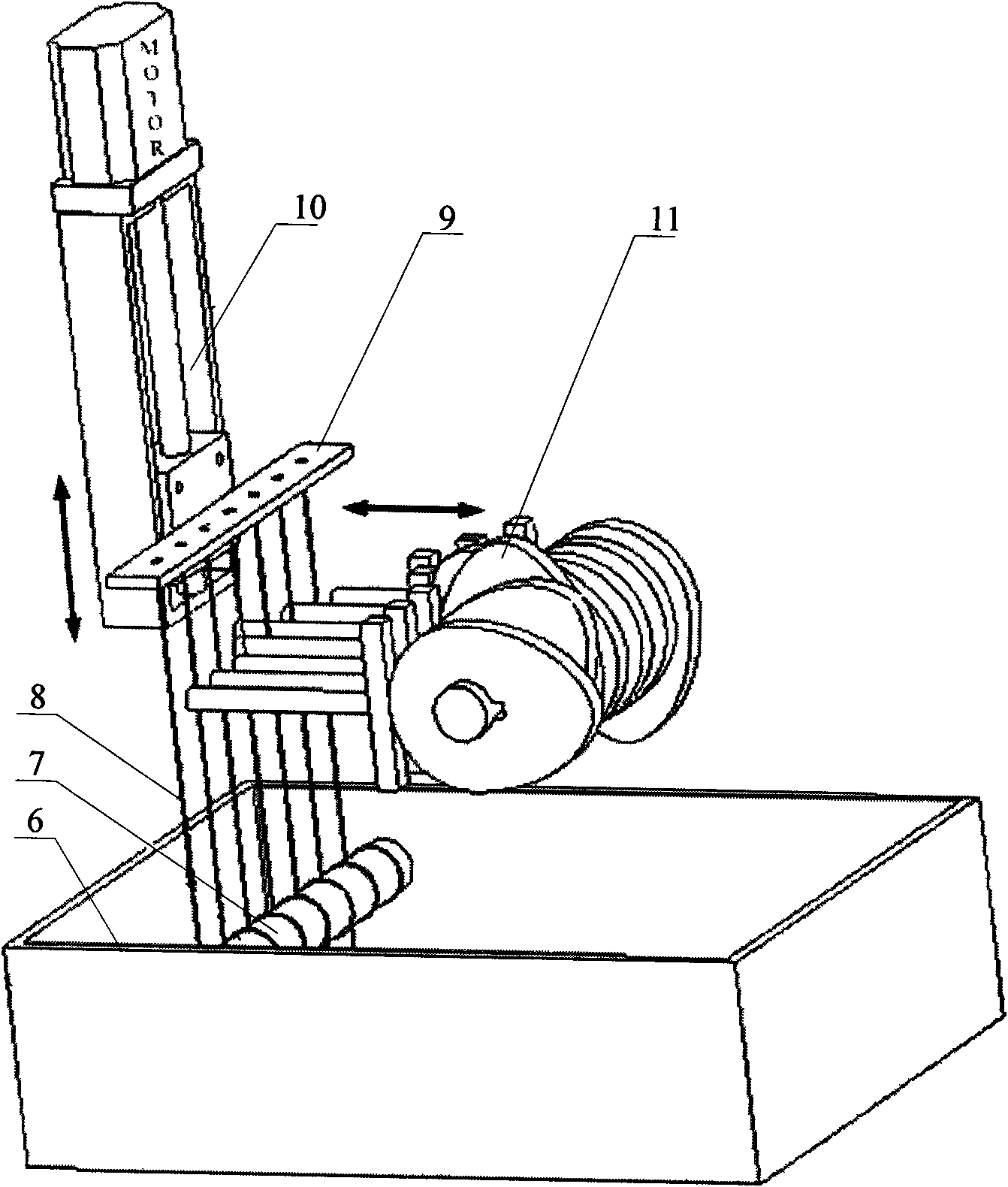
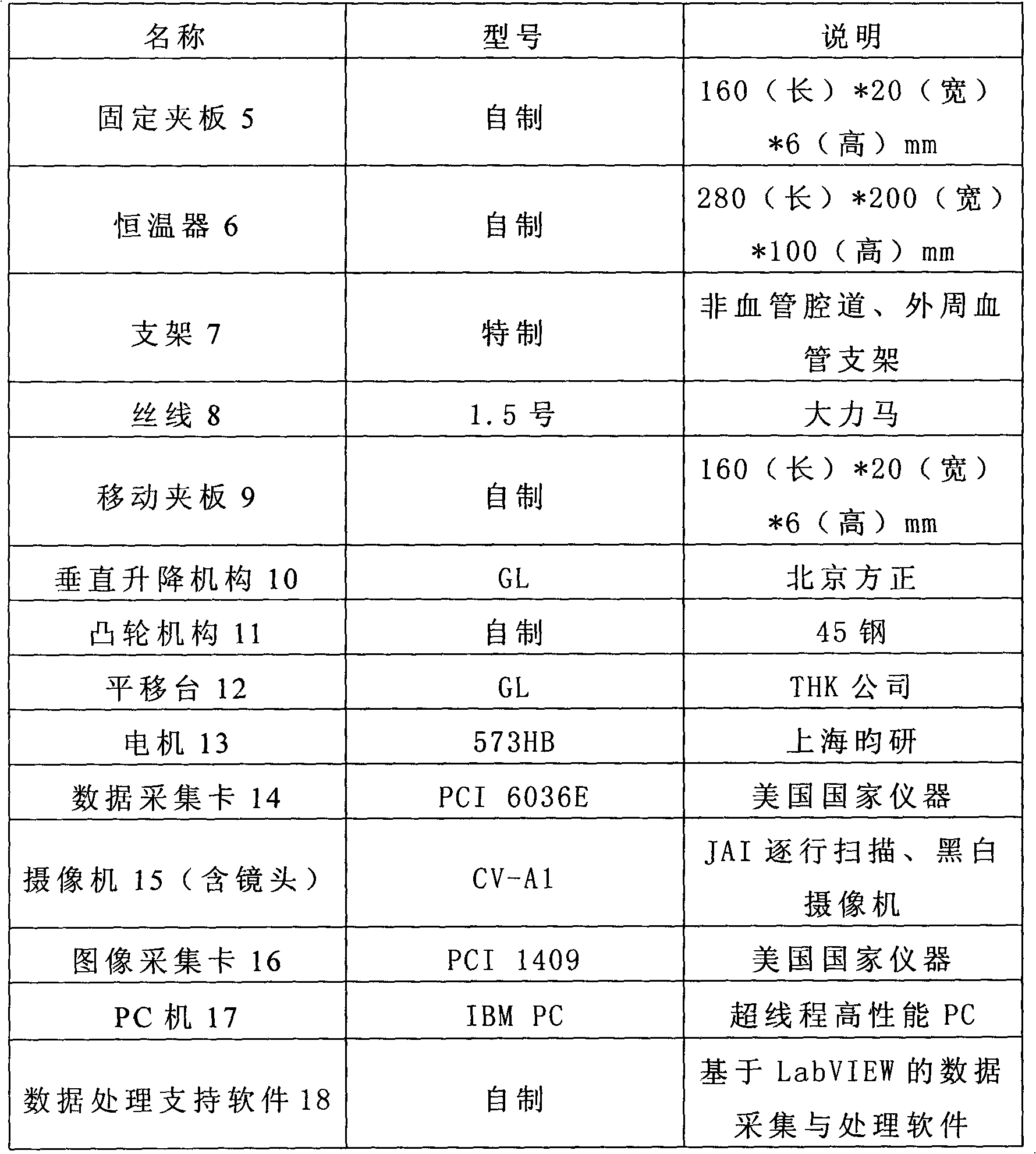
In vitro test device and test method for fatigue performance of self-expanding tunnel stent
InactiveCN102279098AEffective simulationRapid digitizationMachine part testingHuman–machine interfaceMachine vision
The in vitro test device and test method for the fatigue performance of the self-expanding cavity stent is a complete set of methods suitable for testing the fatigue performance parameters of the stent in vitro. The self-expanding cavity stent fatigue performance testing device is mainly composed of four parts: man-machine Interfaces, controls, actuators, and machine vision. The PC of the human-machine interface is connected to the output port of the data acquisition card and the input port of the image acquisition card. The data acquisition card controls the operation of the execution device. The execution device mainly includes a loading device, a peristaltic device and a thermostat. The loading device is arranged in parallel The wires are used to fix the stent, and the stent is loaded to simulate the long-term radial annular contraction pressure of various lumens on the stent.
Owner:SOUTHEAST UNIV
Predictive radiosensitivity network model
InactiveUS20080234946A1Help studyImprove abilitiesMedical simulationDigital computer detailsRadiation sensitivityIn vitro test
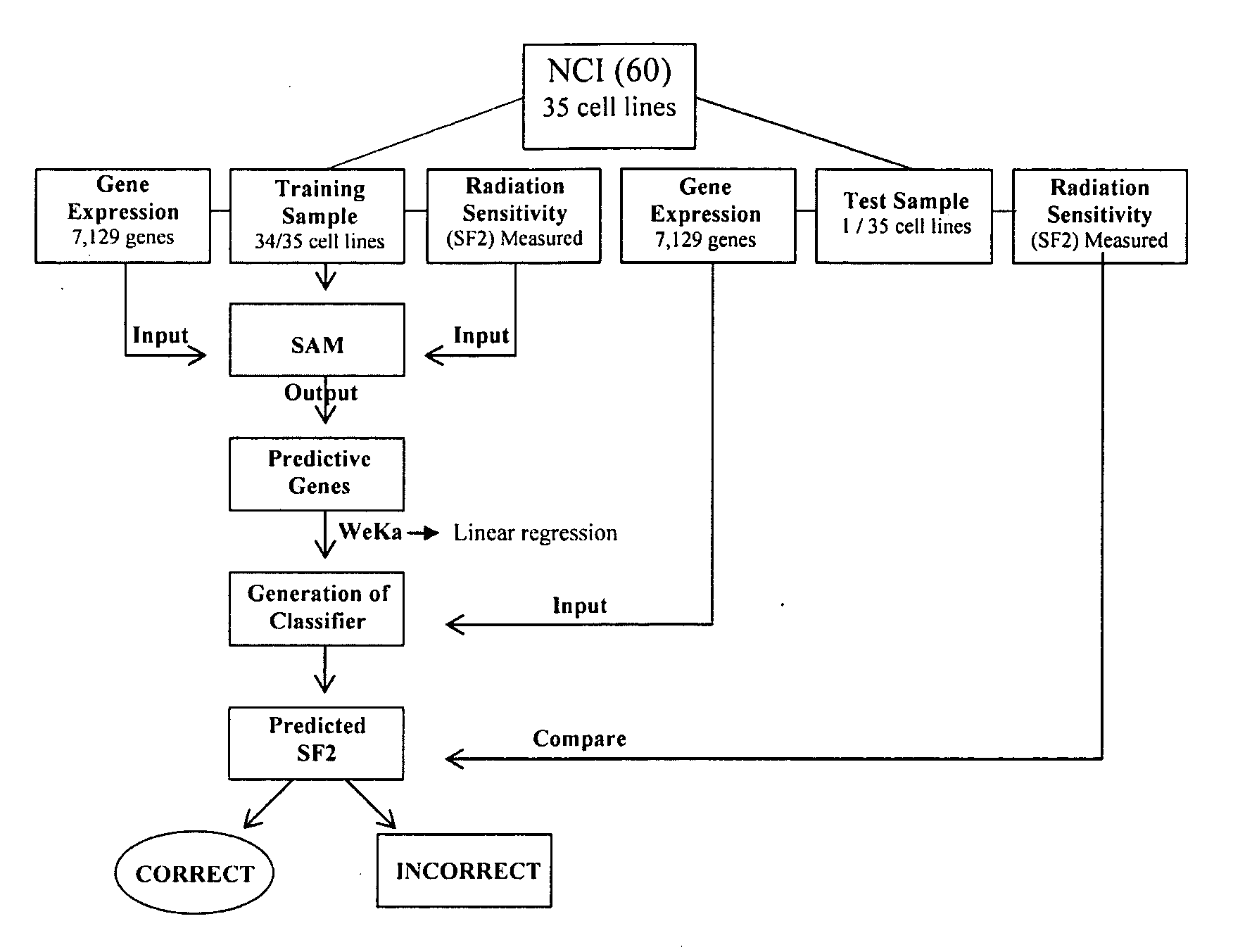
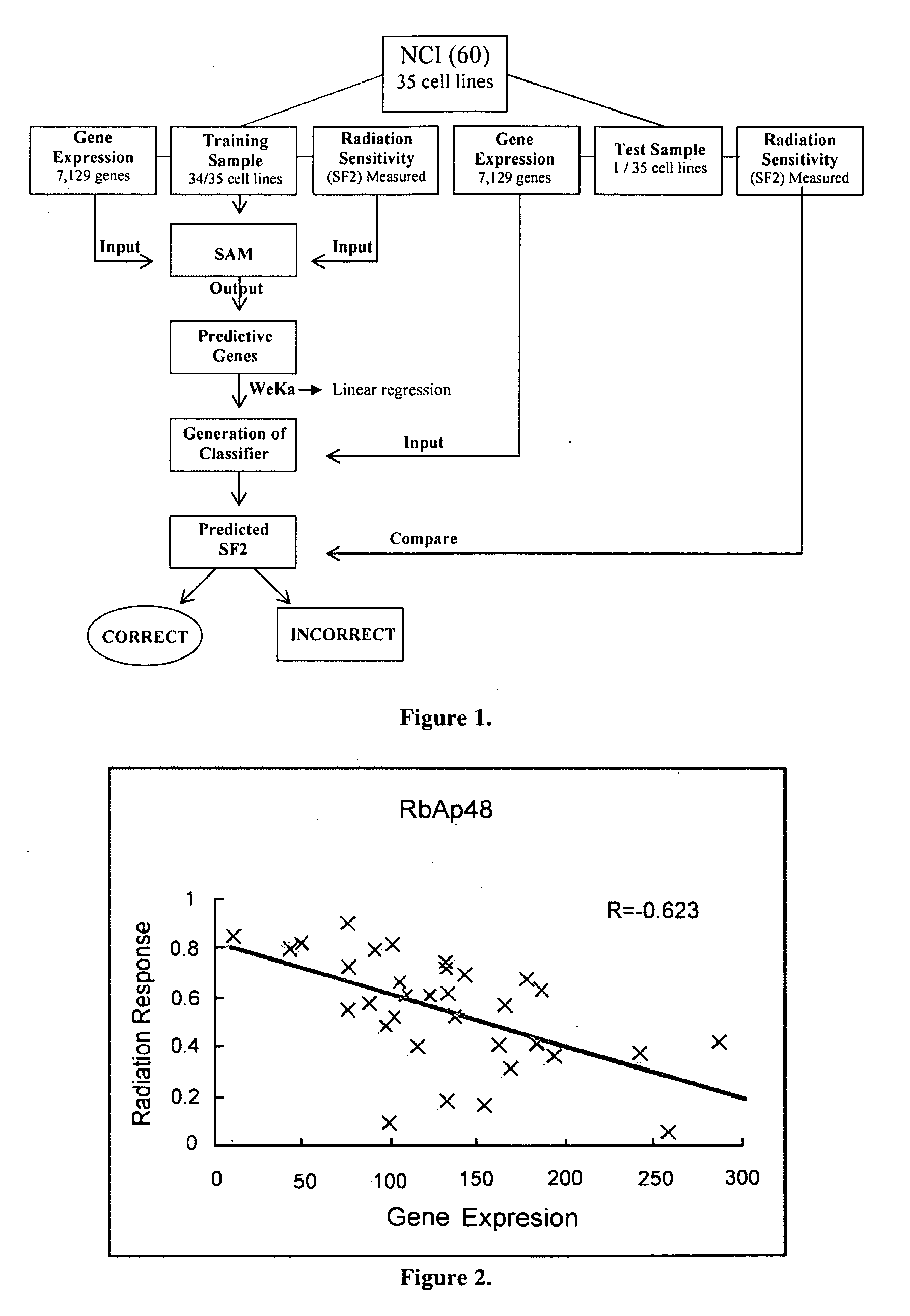
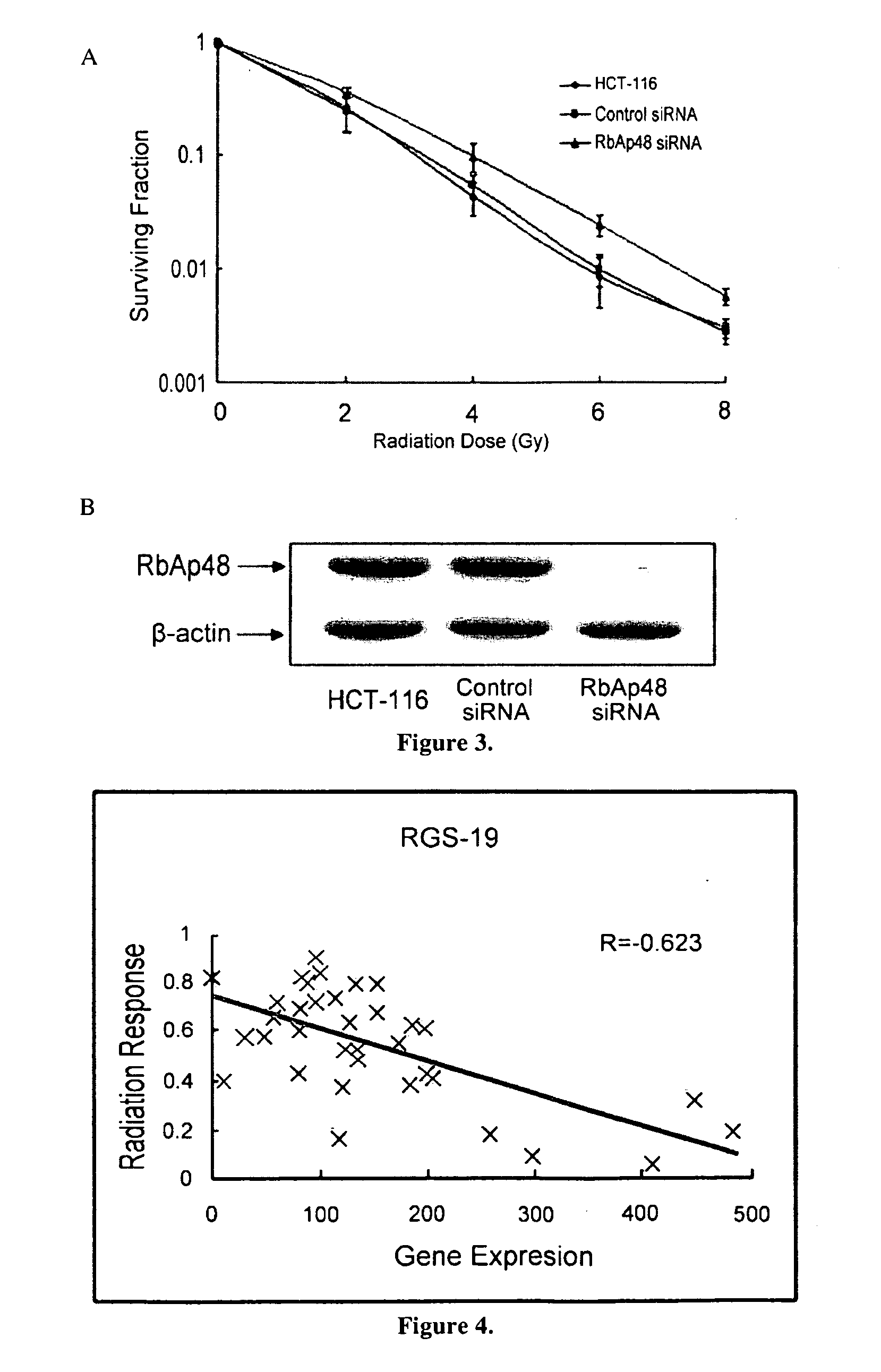
This invention is a model that simulates the complexity of biological signaling in a cell in response to radiation therapy. Using gene expression profiles and radiation survival assays in an algorithm, a systems model was generated of the radiosensitivity network. The network consists of ten highly interconnected genetic hubs with significant signal redundancy. The model was validated with in vitro tests perturbing network components, correctly predicting radiation sensitivity 2 / 3 times. The model's clinical relevance was shown by linking clinical radiosensitivity targets to the model network. Clinical applications were confirmed by testing model predictions against clinical response to preoperative radiochemotherapy in patients with rectal or esophageal cancer.
Owner:UNIV OF SOUTH FLORIDA +1
Composition with skin-whitening and anti-ageing effects, skin care product containing composition as well as preparation method and application of composition
InactiveCN103690450AGood long-lasting moisturizing effectHas whitening and anti-aging effectsCosmetic preparationsToilet preparationsBiotechnologySkin test
The invention discloses a composition with skin-whitening and anti-ageing effects, a skin care product containing the composition as well as a preparation method and an application of the composition. The composition is prepared from the following components: 0.2%-10.0% of L-ascorbic acid 2-glucoside, 0.1%-0.2% of superoxide dismutase, 0.1%-0.5% of a grape seed extract, 0.1%-0.5% of a soybean fermentation extract, 10%-30% of propanediol, 0.1%-0.2% of aloe powder and the balance amount of water. An experimental result shows that the composition has the skin-whitening and anti-ageing effects, the method is easy in operation, the production cost is low, and the product stability is high; an evaluation on an in vitro test and a human body skin test proves that the composition has good skin-whitening and anti-ageing effects and is safe and free from side effects; meanwhile, the composition and the skin care product containing the composition, provided by the invention, also have better long-lasting hydrating effects.
Owner:ZTE ENERGY TIANJIN CO LTD
Novel population of multipotent cardiac precursor cells derived from human blastocysts derived stem cells
A novel population of multipotent cardiac precursor (MCP) cells derived from human blastocysts derived stem cells is disclosed, methods for the preparation thereof and use of the cells for in vitro testing. Basement cells derived from hBS cells are also disclosed and method for the preparation of MCP cells from basement cells. The MCP cells have the following characteristicsi) at least 1% of the cells exhibit no antigen expression of one or more markers for undifferentiated cell, the marker being selected from the group consisting of SSEA-3, SSEA-4, TRA-1-60, TRA-1-81 and Oct-4,ii) at least 1% of the cells exhibit no protein expression of one or more of a neural marker including nestin or GFAPiii) at least 1% of the cells exhibit protein and / or gene expression of one or more of a mesodermal marker including brachyury, vimentin or desminiv) at least 1% of the cells exhibit protein and / or gene expression of Flk-1 (KDR).Furthermore, the MCP cells have a characteristic morphology. They grow as clusters of small, round and phase-bright cells; individual cells are 5-20 μm in diameter and each cluster is composed of 2-500 cells. They form clusters of round or elongated shape, that appear as loosely adherent cell clumps that as illustrated in FIG. 2 panel a, b and c. Furthermore, they have a relatively high nucleus-to-cytoplasma ratio, e.g. 1:2-1:64 of the total volume of the cell and / or appear as balloons on a string, as illustrated in FIG. 18, schematic sketch. Moreover, the MCP cells are non-contracting.
Owner:CELLARTIS AB (SE)
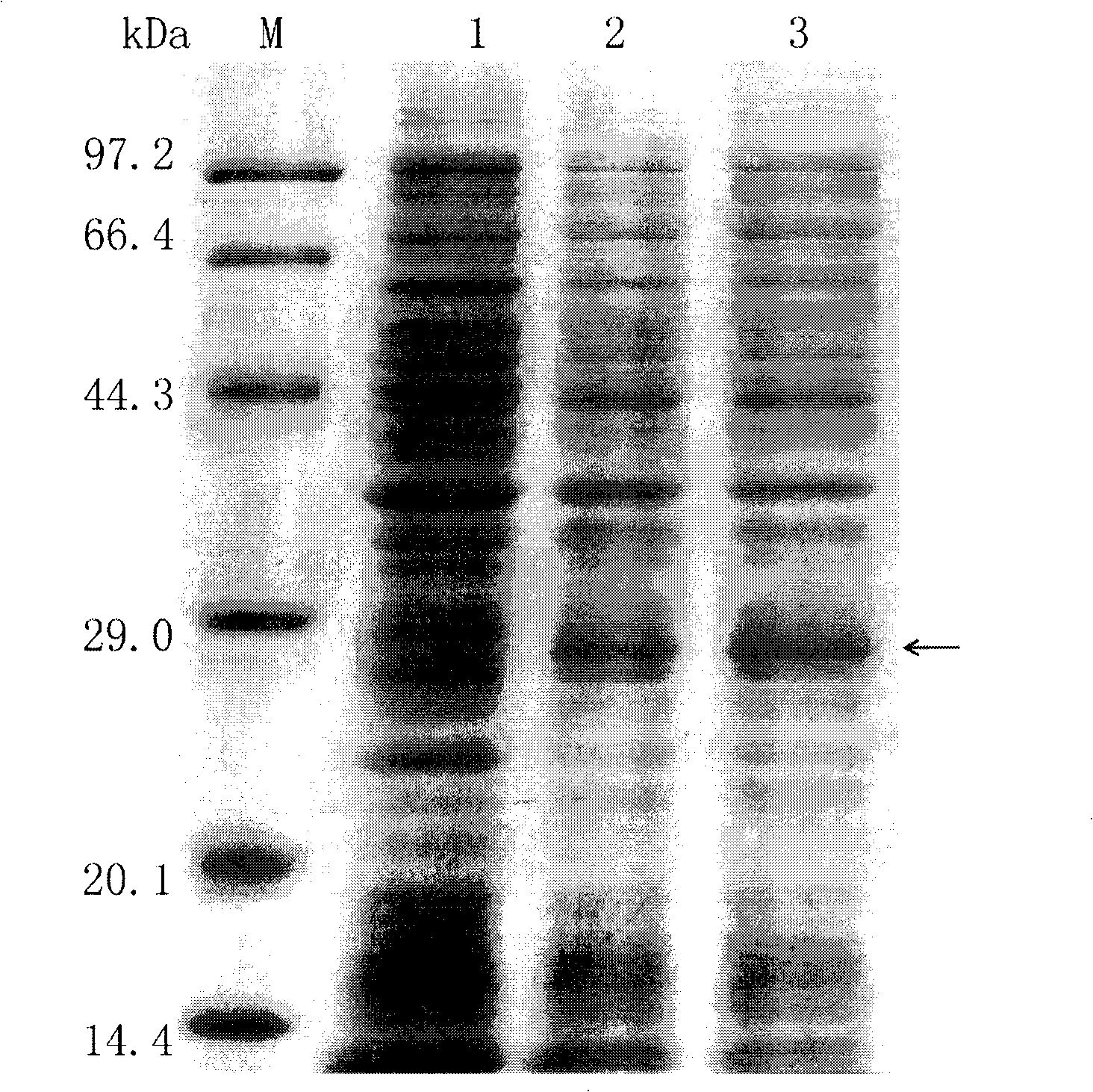
Human anti-human tumor necrosis factor-alpha single chain antibody
The invention discloses a whole human source single chain antibody combined with specificities of human tumor necrosis factor-Alpha (hTNF-Alpha). The antibody of the invention is formed by connecting the first end and the tail end of the variable area of a light chain and a heavy chain of the antibody by a flexible connecting peptide. The antibody can neutralize the activity of hTNF-Alpha in extracorporeal tests, and can be used for detecting the hTNF-Alpha or can be applicable to treating related diseases of the hTNF-Alpha, such as rheumatoid arthritis and Crohn's disease. The invention also comprises nucleogelase, carrier and host cell that express the whole human source single chain antibody of the invention.
Owner:CHINA PHARM UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com