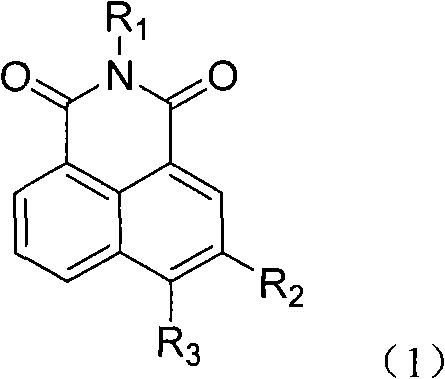
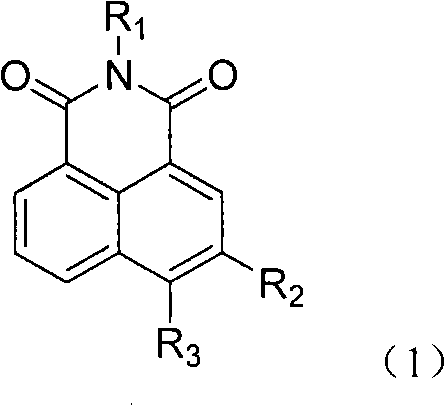
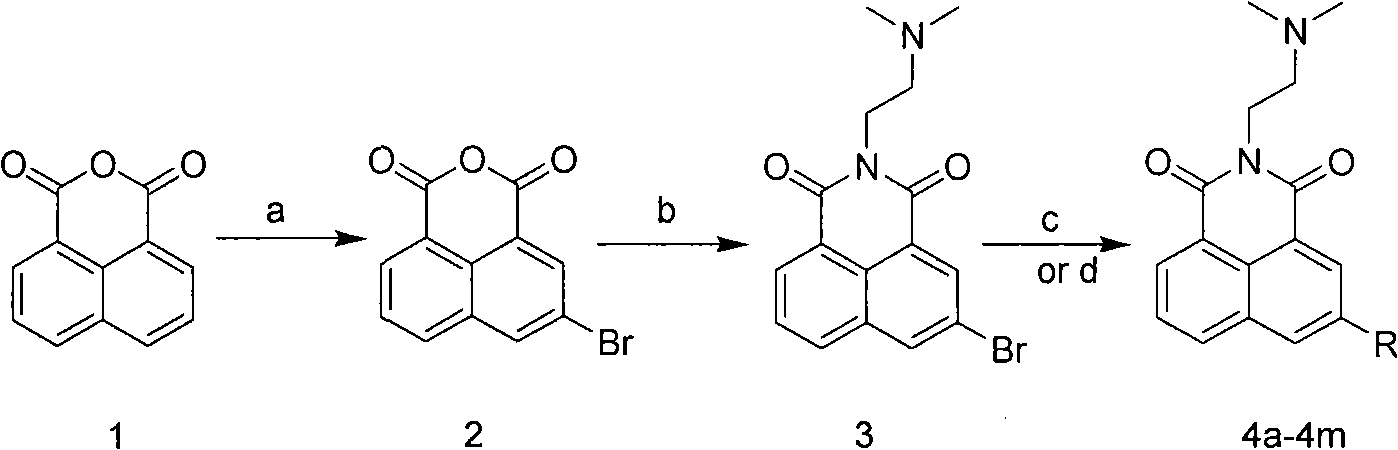
5- or 6-substited naphthoyl imines compounds and antineoplastic application
A technology of naphthalene imide and compound, applied in the field of naphthalimide compound and anti-tumor application, can solve the problems of toxic and side effects, unpredictable and serious toxic and side effects, and achieve the effects of strong cytotoxicity and avoiding toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 3-bromo-1,8-naphthalene anhydride (2):
[0033] Liquid bromine (8.2g, 51.3mmol) was added dropwise to a mixed solution of 1,8-naphthalene anhydride (10g, 50.5mmol) and 70% nitric acid (200mL) at 25°C. After the mixture was stirred at 70°C for 2 hours, it was cooled, filtered, washed with water, and dried to obtain the target compound as a solid.
Embodiment 2
[0035] Preparation of 5-bromo-[2-(dimethylamino)ethyl]-1hydro-benzo[de]isoquinoline-1,3(2hydro)-dione (3):
[0036] A mixture of 3-bromo-1,8-naphthalene anhydride (2) (277mg, 1mmol) and N,N-dimethylethylenediamine (1.2mmol) was heated to reflux in ethanol (20mL) for 2 hours, and the solvent was removed, Obtain the target product.
[0037] Yield 92%, light brown solid, mp 77.2-78.6°C; 1 H NMR (400MHz CDCl 3 )δ (ppm) 8.65 (s, 1H) 8.59 (dd, 1H, J = 1.0 and 7.2Hz) 8.35 (s, 1H) 8.11 (dd, 1H, J = 0.7 and 8.3Hz) 7.77 (t, 1H, J =7.8Hz) 4.34(t, 2H, J=6.9Hz) 2.70(t, 2H, J=6.9Hz) 2.39(s, 6H)
Embodiment 3
[0039] 5-(dimethylamino-ethylamine)-2-[2-(dimethylamino)ethyl]-1 hydrogen-benzo[de]isoquinoline-1,3(2 hydrogen)-dione ( 4a) Preparation:
[0040] Compound 3 (174mg, 0.5mmol), CuI (9.6mg, 0.05mmol), Proline (11.5mg, 0.1mmol), Cs 2 CO 3 (247.5mg, 0.6mmol) and N,N-dimethylethylenediamine (0.75mmol) in DMSO (2mL), stirred at 110°C for 8 hours under nitrogen protection. The crude product was purified by column chromatography using dichloromethane: methanol as the developing solvent to obtain the pure target compound.
[0041] Yield 52%, orange solid, mp 97.2-98.6°C; 1 H NMR (400MHz CDCl 3 )δ (ppm) 8.265 (dd, 1H, J = 0.8 and 7.2Hz) 8.02 (s, 1H) 7.93 (d, 1H, J = 7.6Hz) 7.58 (t, 1H, J = 7.2Hz) 7.11 (s, 1H) 4.94 (t, 1H, J = 4.4Hz) 4.32 (t, 2H, J = 7.2Hz) 3.32-3.28 (m, 2H) 2.67-2.64 (m, 4H) 2.37 (s, 6H) 2.30 (s, 6H); 13 C NMR (100MHz CDCl 3 )δ (ppm) 164.6, 164.4, 147.1, 133.8, 131.6, 127.1, 126.6, 123.3, 122.4, 122.0, 121.9, 110.0, 57.4, 57.0, 45.7, 45.1, 41.0, 38.1; IR (KBr cm -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com