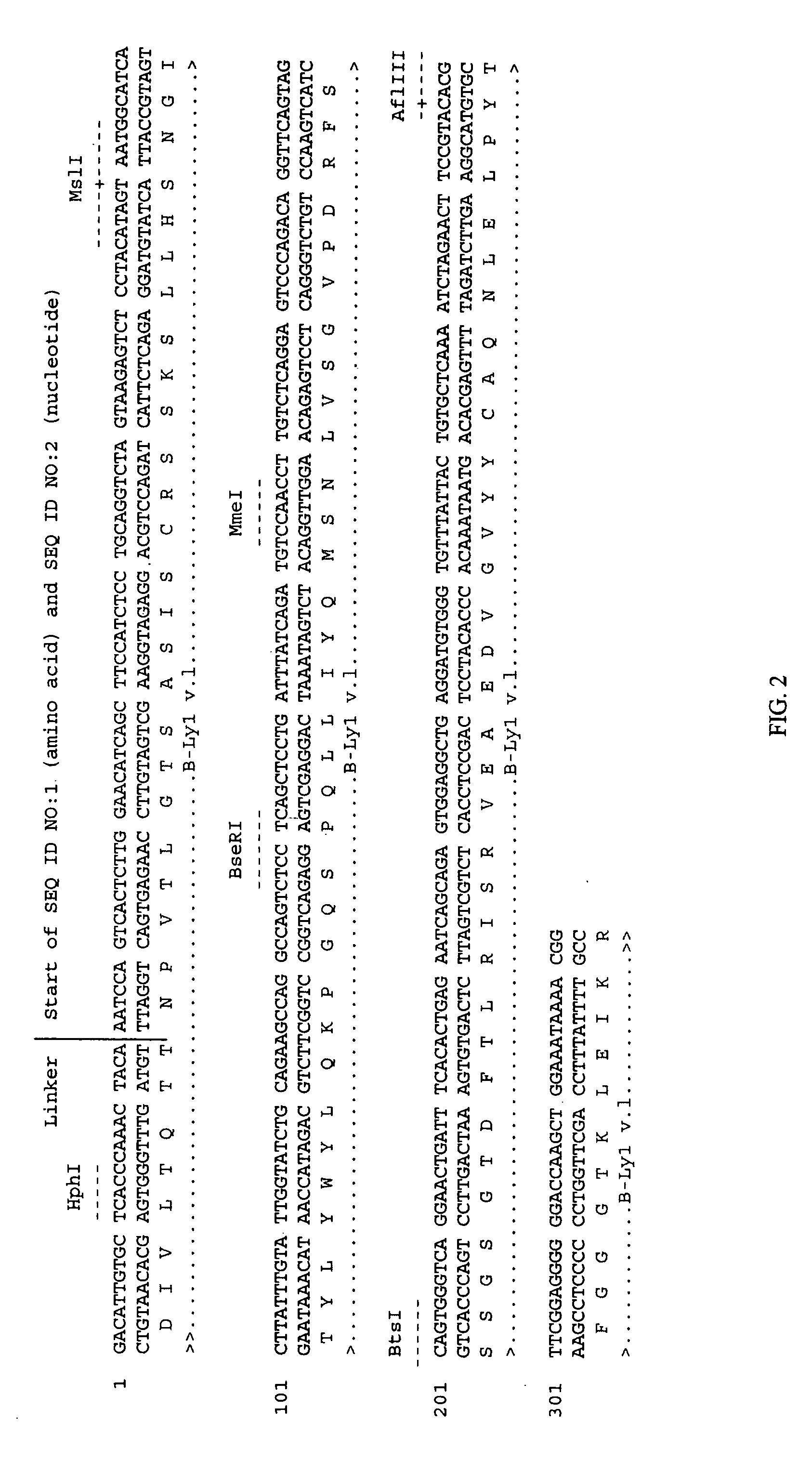
Patents
Literature
31312results about How to "Good curative effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antigen binding molecules with increased Fc receptor binding affinity and effector function
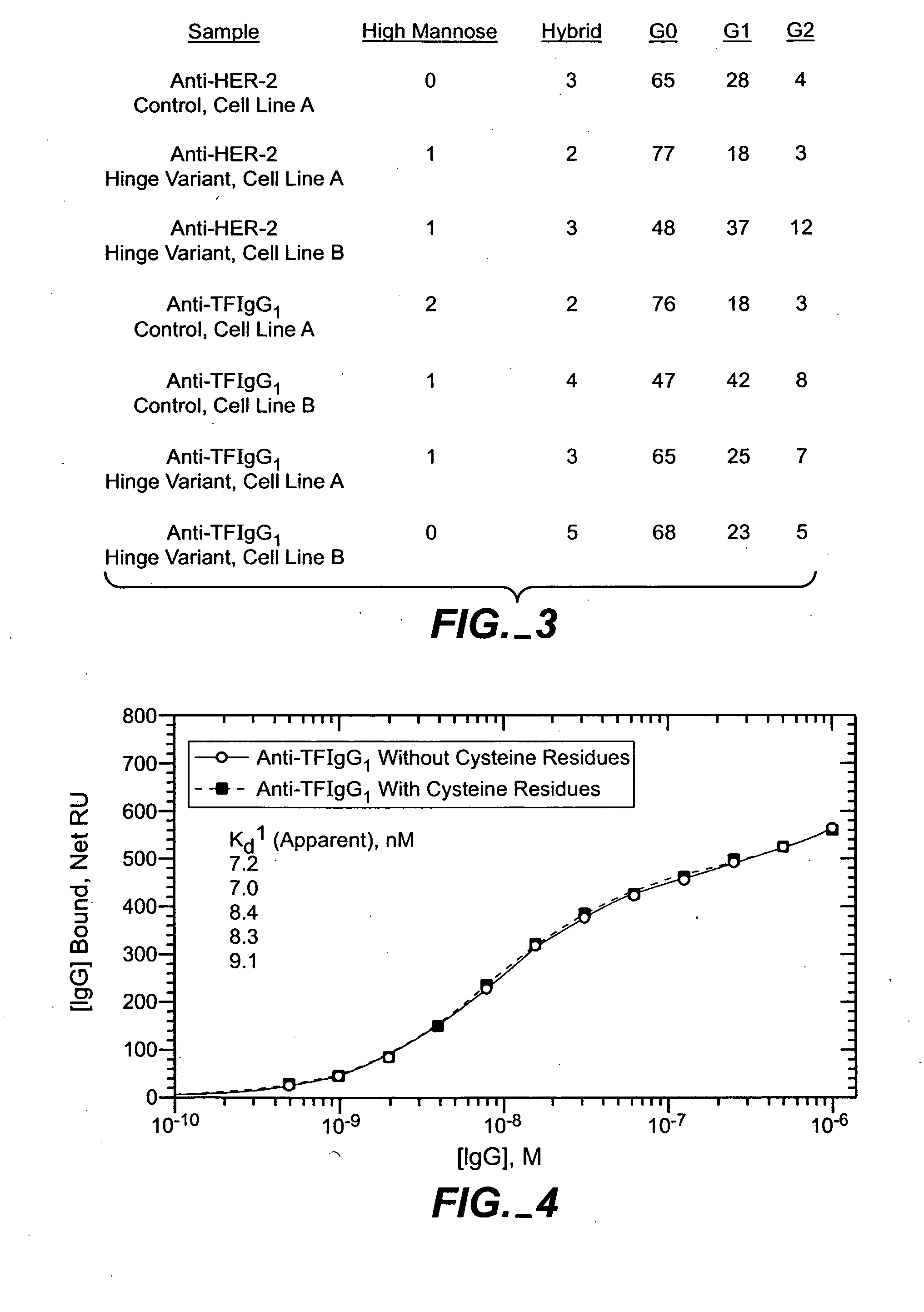
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies specific for human CD20. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
PD-1 binding proteins
ActiveUS8168757B2Regulating T cell responsesImprove immunityAntibody mimetics/scaffoldsAntibody ingredientsHost immunitySignalling pathways
The present invention features PD-1 binding proteins, a subset of which inhibits binding of PD-L1 to the PD-1 receptor. These binding proteins can be employed to modulate the immune system through the manipulation of the PD-1 signaling pathway, enhancing host immunity to treat infections and cancer.
Owner:MERCK SHARP & DOHME LLC
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
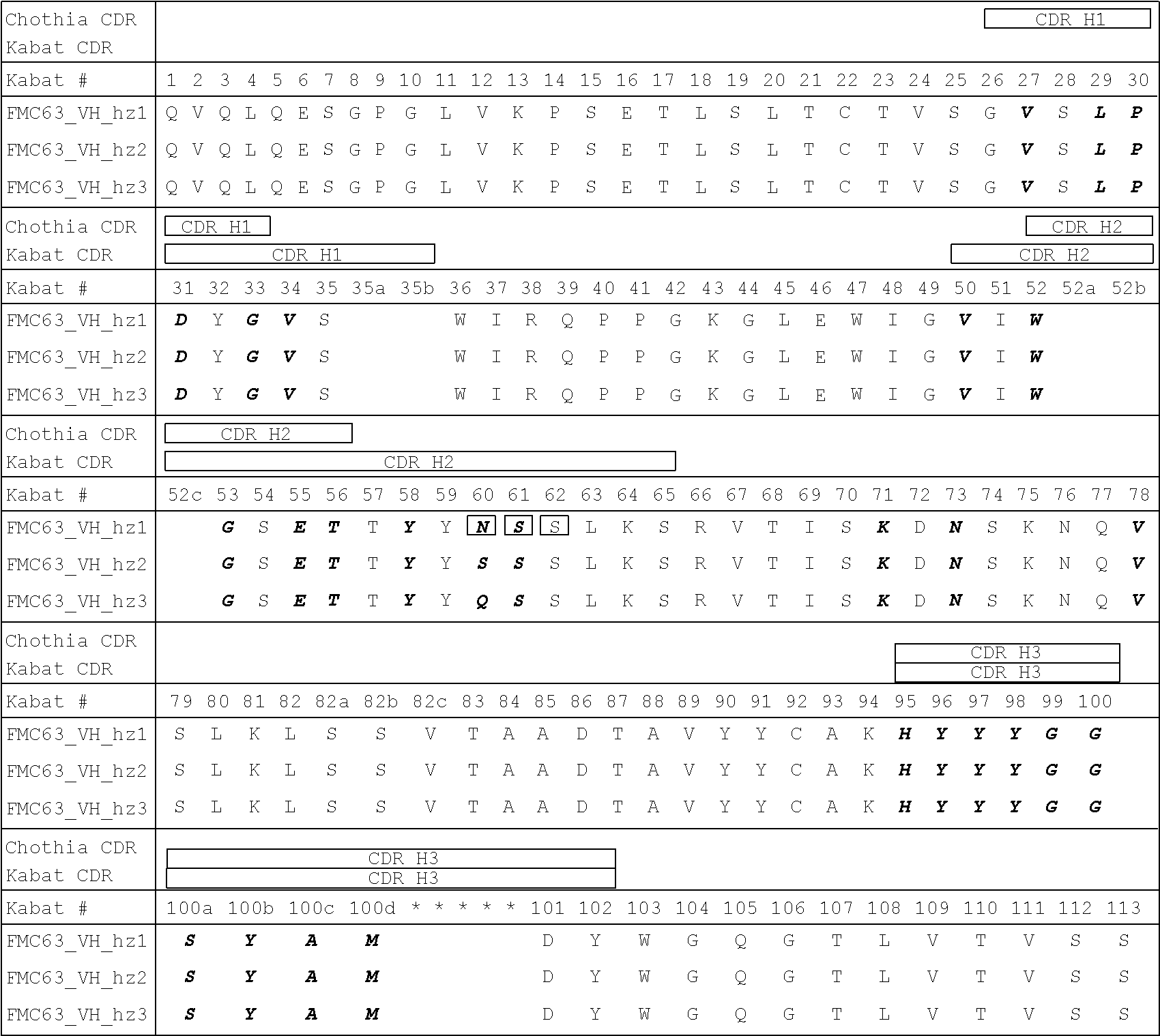
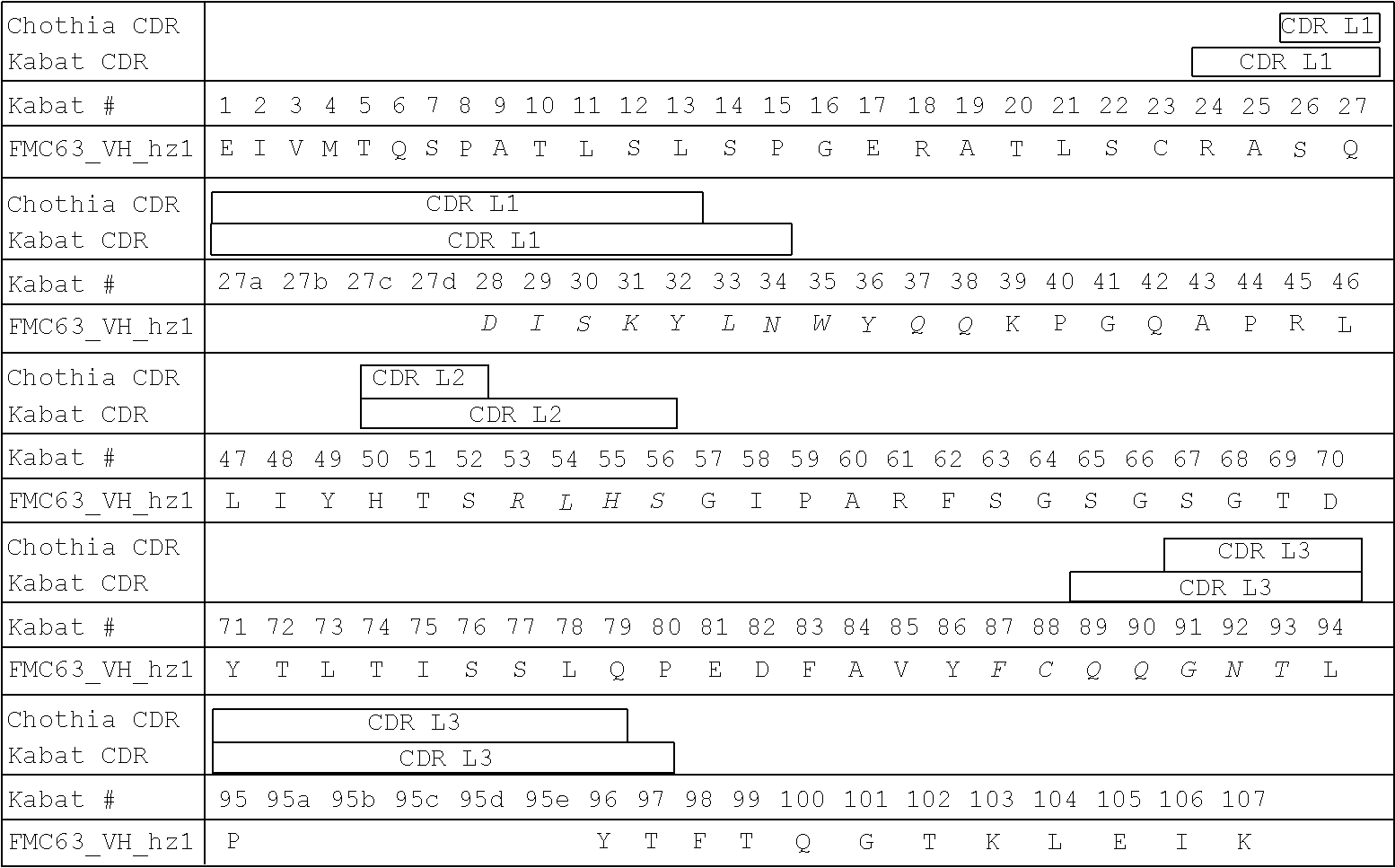
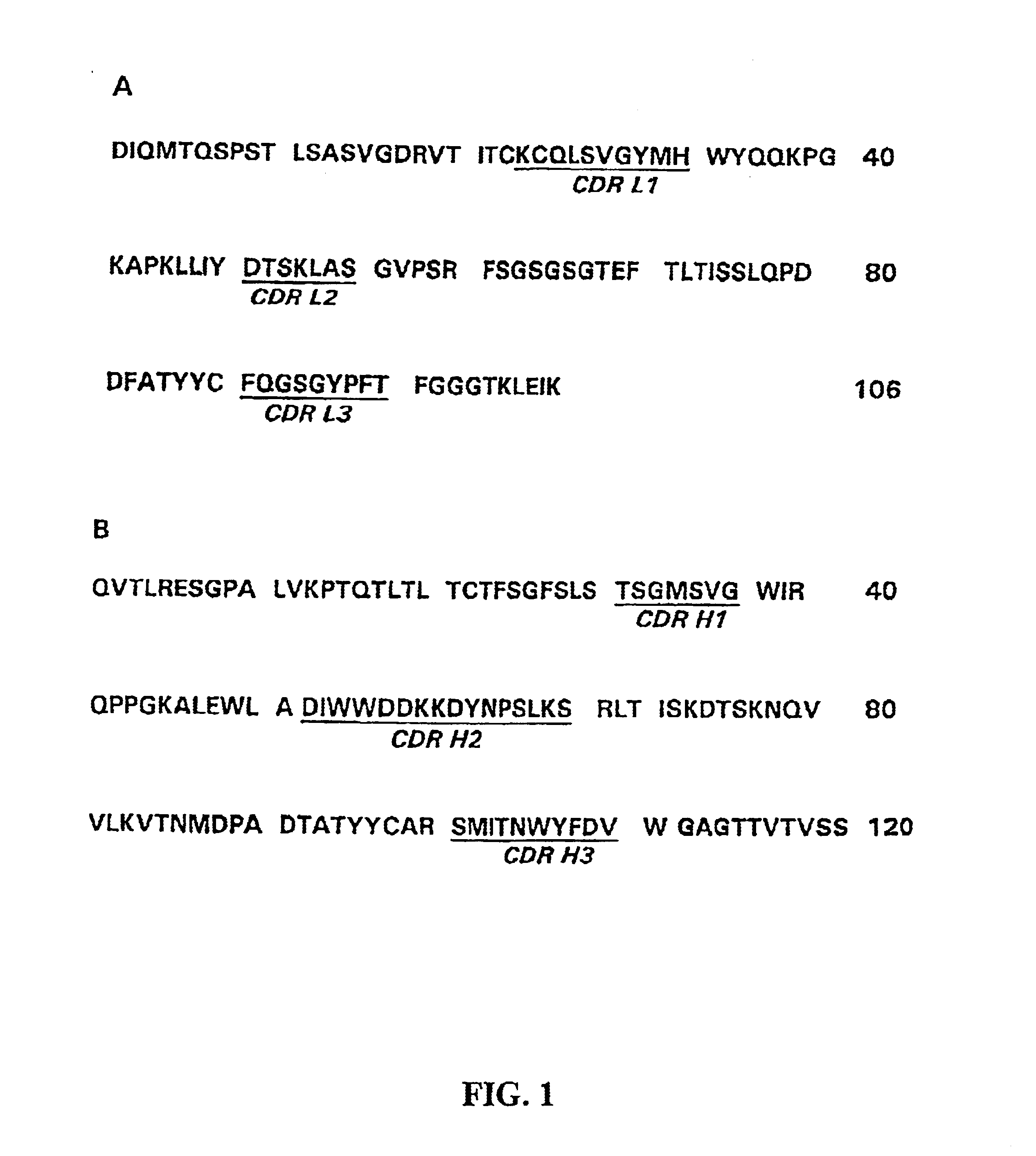
Humanization of antibodies
InactiveUS20050042664A1Limited diversityFast and less labor intensive productionHybrid immunoglobulinsMicrobiological testing/measurementAntigen bindingHumanized antibody
The present invention provides methods of re-engineering or re-shaping an antibody from a first species, wherein the re-engineered or re-shaped antibody does not elicit undesired immune response in a second species, and the re-engineered or re-shaped antibody retains substantially the same antigen binding-ability of the antibody from the first species. In accordance with the present invention, a combinatorial library comprising the CDRs of the antibody from the first species fused in frame with framework regions derived from a second species can be constructed and screened for the desired modified antibody. In particular, the present invention provides methods utilizing low homology acceptor antibody frameworks for efficiently humanizing an antibody or a fragment thereof. The present invention also provides antibodies produced by the methods of the invention.
Owner:MEDIMMUNE LLC
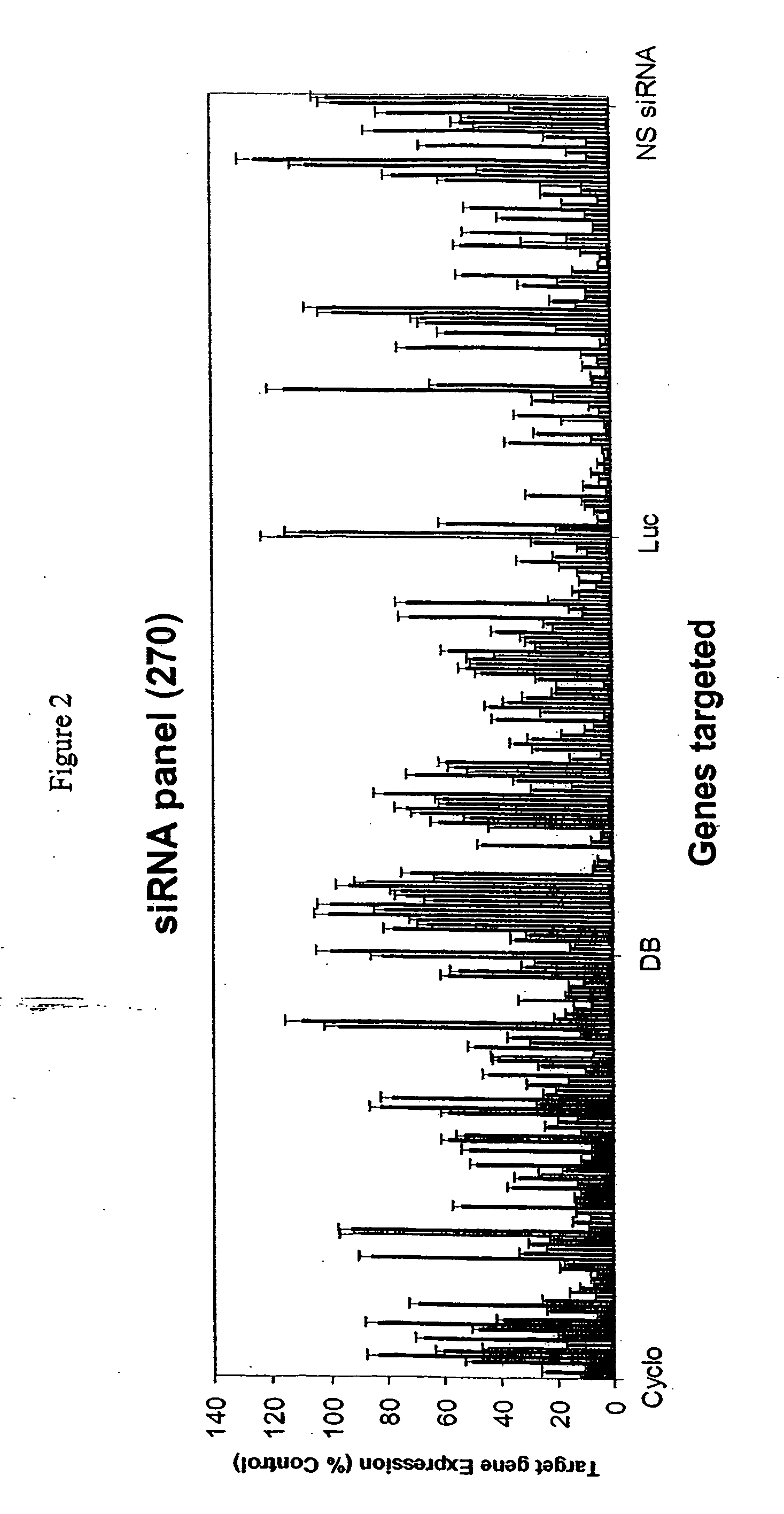
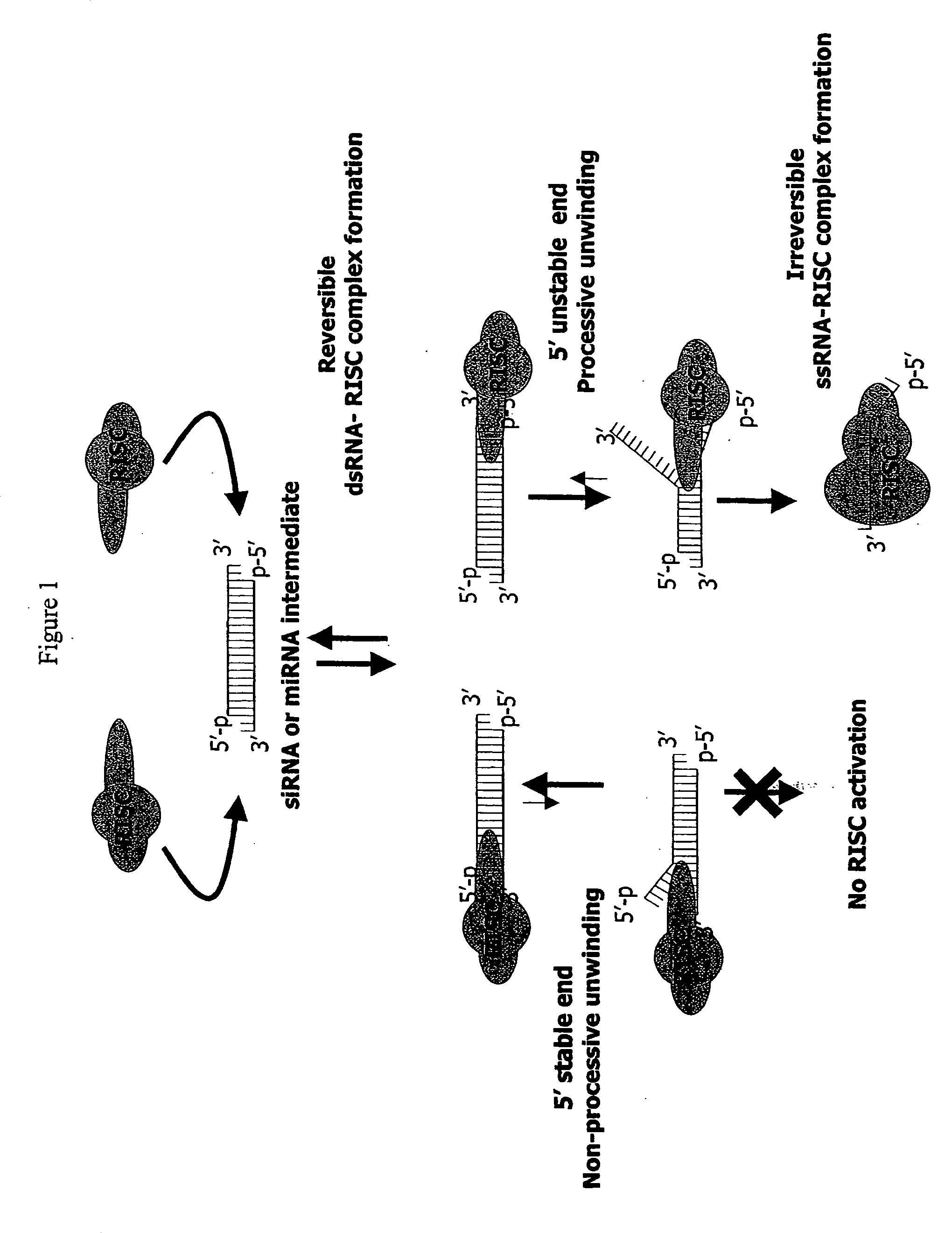
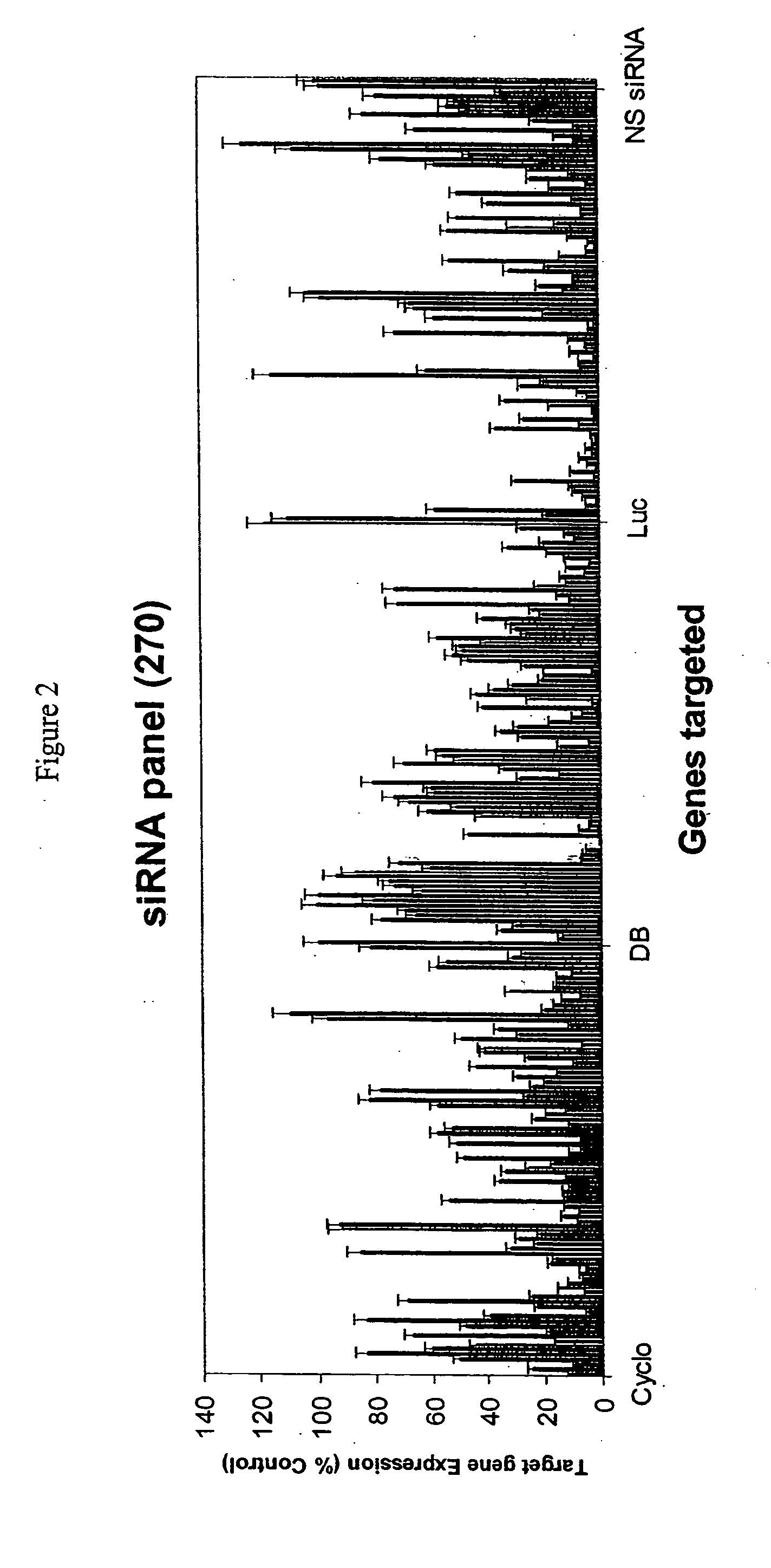
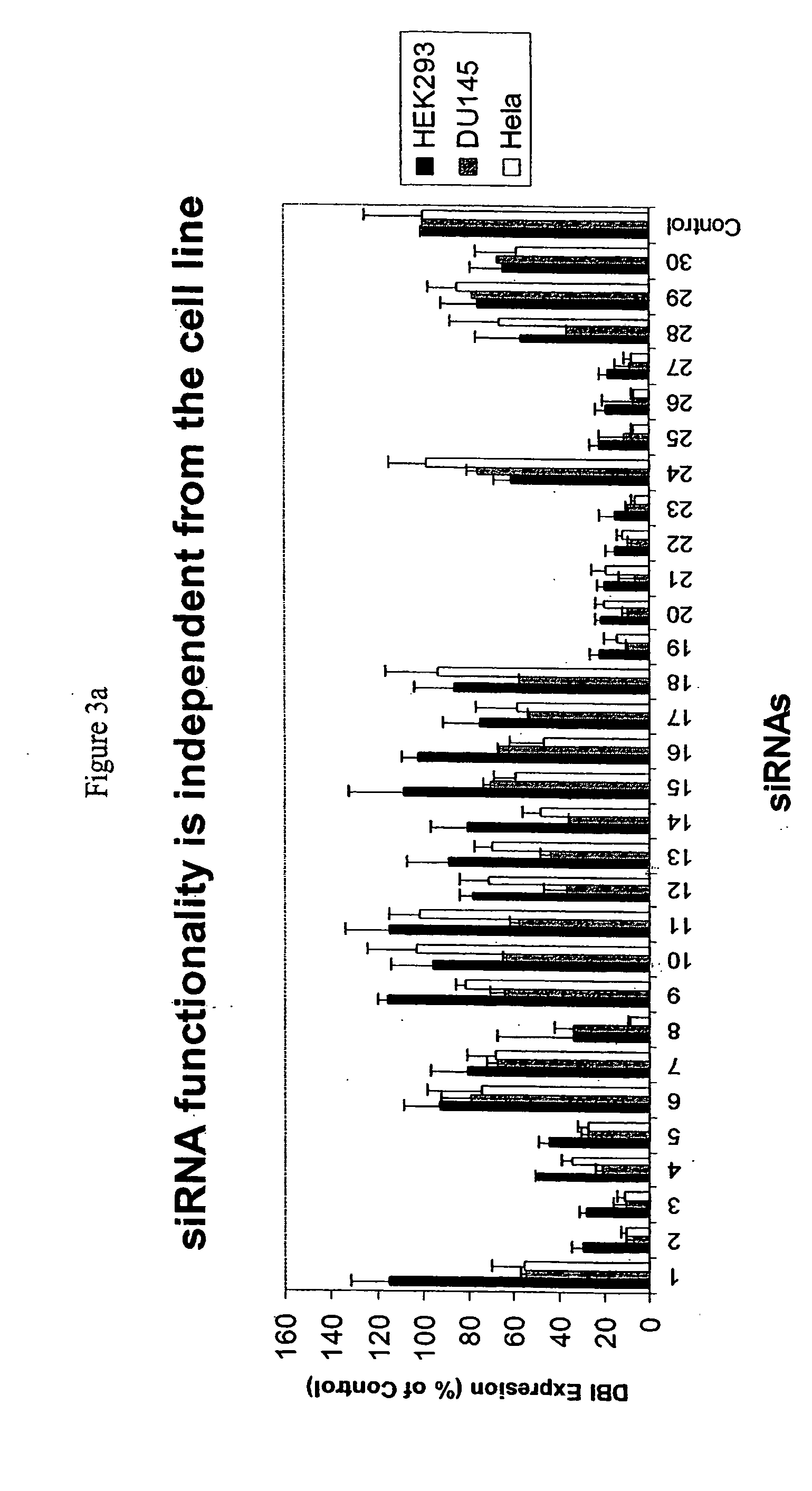
Methods and compositions for selecting siRNA of improved functionality
InactiveUS20050255487A1Improve efficiencyGood curative effectOrganic active ingredientsGenetic material ingredientsGene silencingSilencing gene
Efficient sequence specific gene silencing is possible through the use of siRNA technology. By selecting particular siRNAs by rational design, one can maximize the generation of an effective gene silencing reagent, as well as methods for silencing genes. Methods, compositions, and kits generated through rational design of siRNAs are disclosed.
Owner:THERMO FISHER SCIENTIFIC INC
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS7355008B2Function increaseGood curative effectAntibacterial agentsSenses disorderTherapeutic antibodyEffector cell
Owner:MARCOGENICS INC +1
Functional and hyperfunctional siRNA
ActiveUS20050246794A1Improve efficiencyGood curative effectOrganic active ingredientsGenetic material ingredientsSilent geneGene silencing
Owner:THERMO FISHER SCIENTIFIC INC
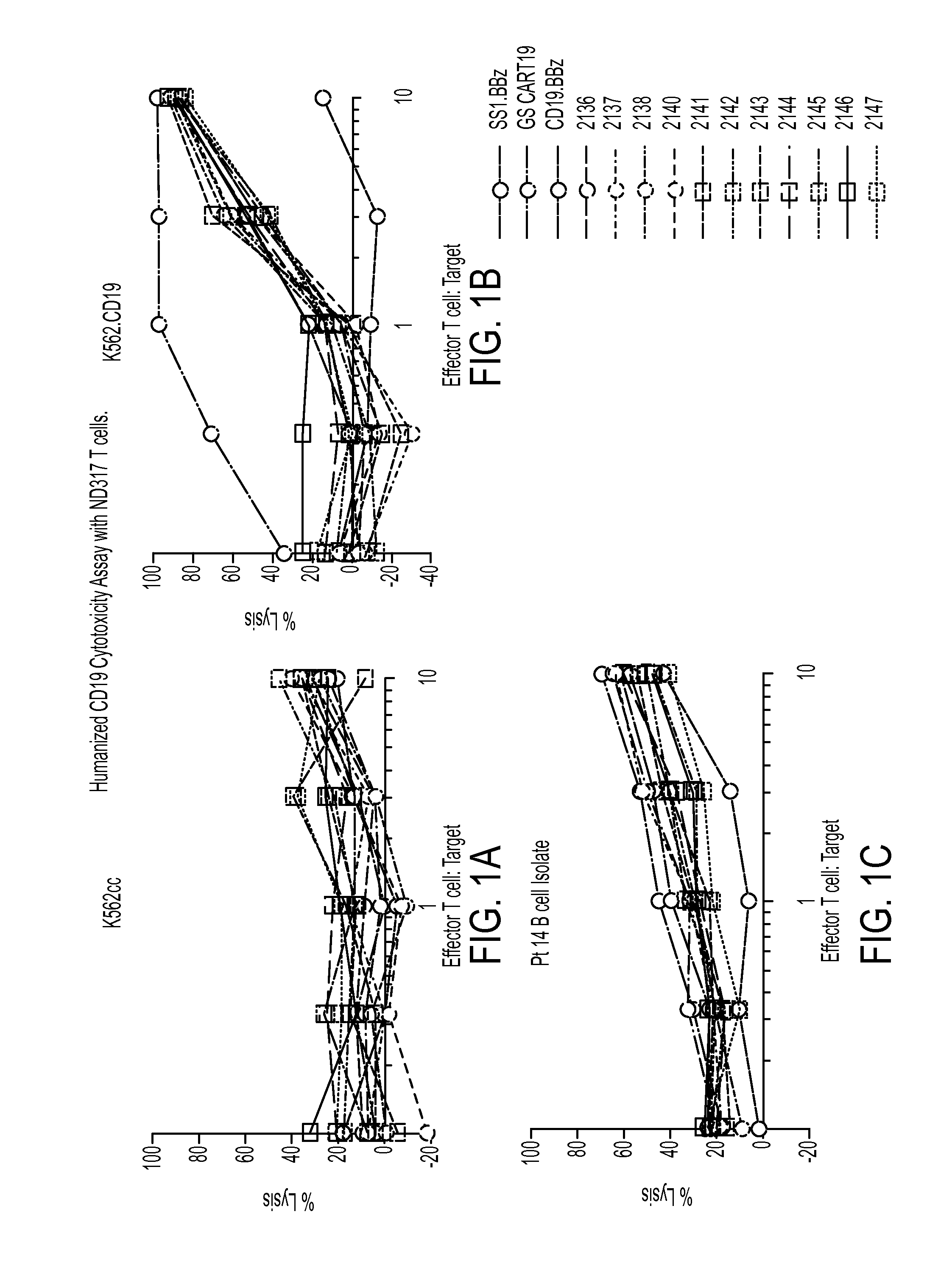
Treatment of cancer using humanized Anti-cd19 chimeric antigen receptor
ActiveUS20140271635A1Improve efficacyGood curative effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCancer researchAntigen receptor
The invention provides compositions and methods for treating diseases associated with expression of CD19. The invention also relates to chimeric antigen receptor (CAR) specific to CD19, vectors encoding the same, and recombinant T cells comprising the CD19 CAR. The invention also includes methods of administering a genetically modified T cell expressing a CAR that comprises a CD19 binding domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
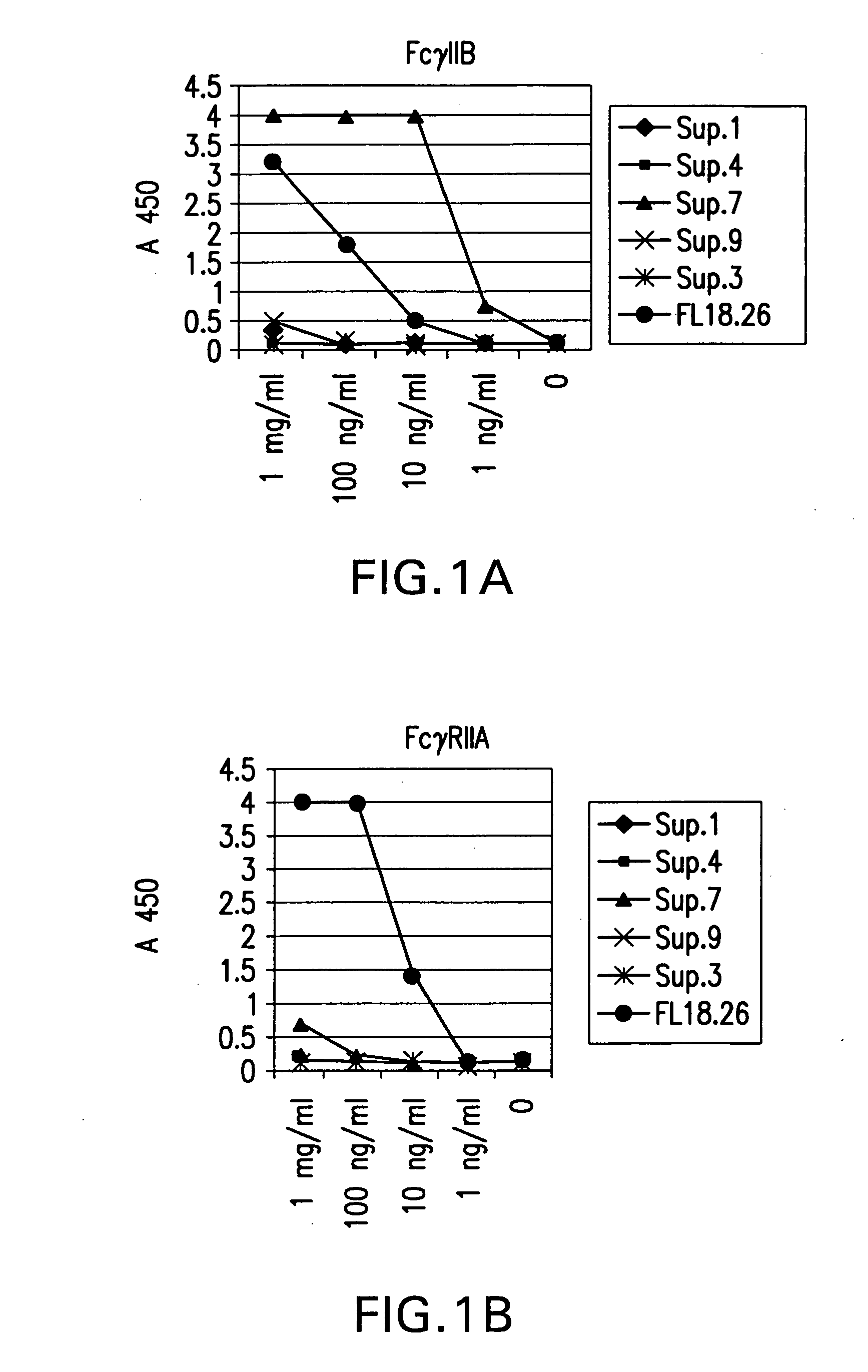
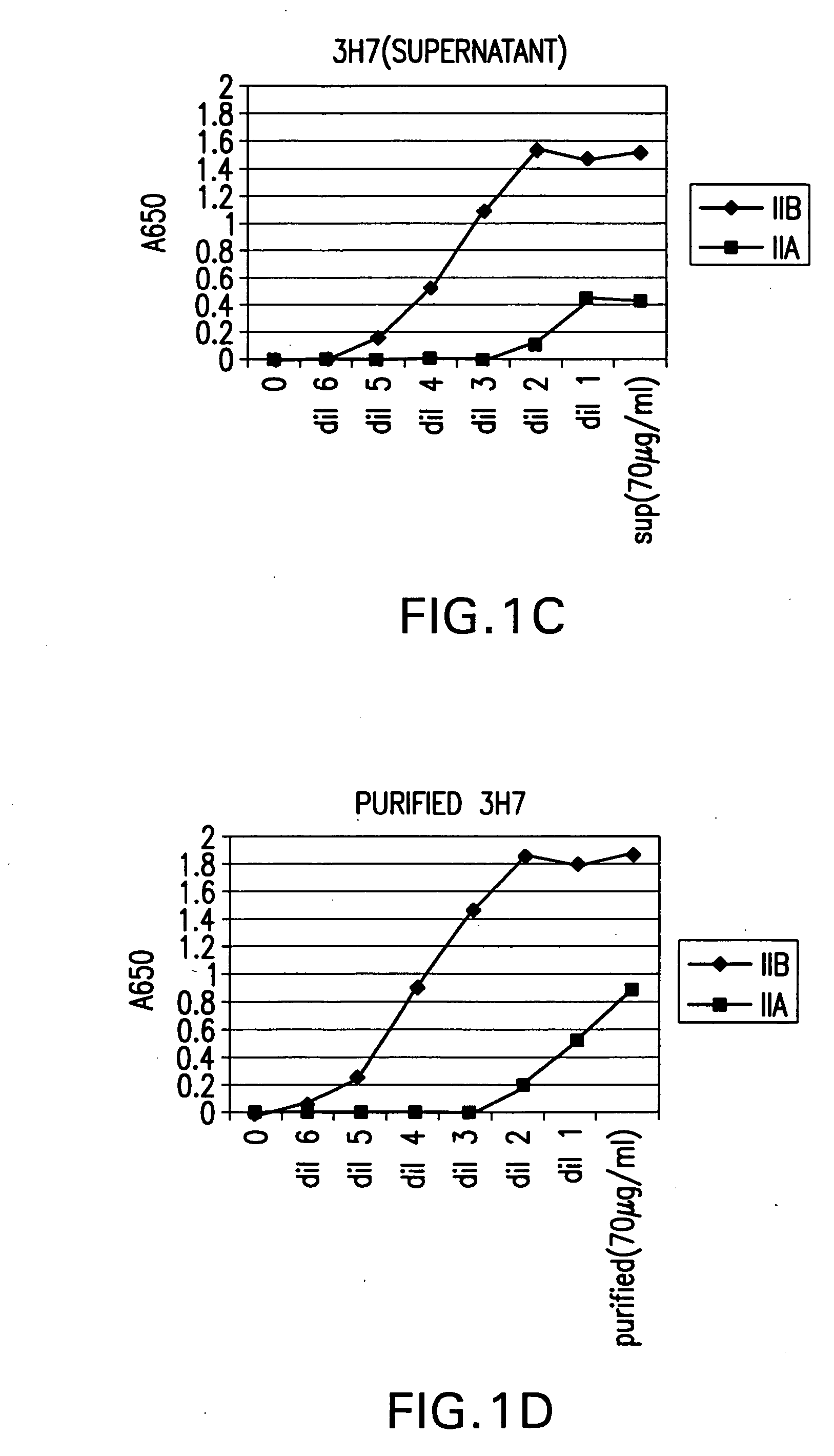
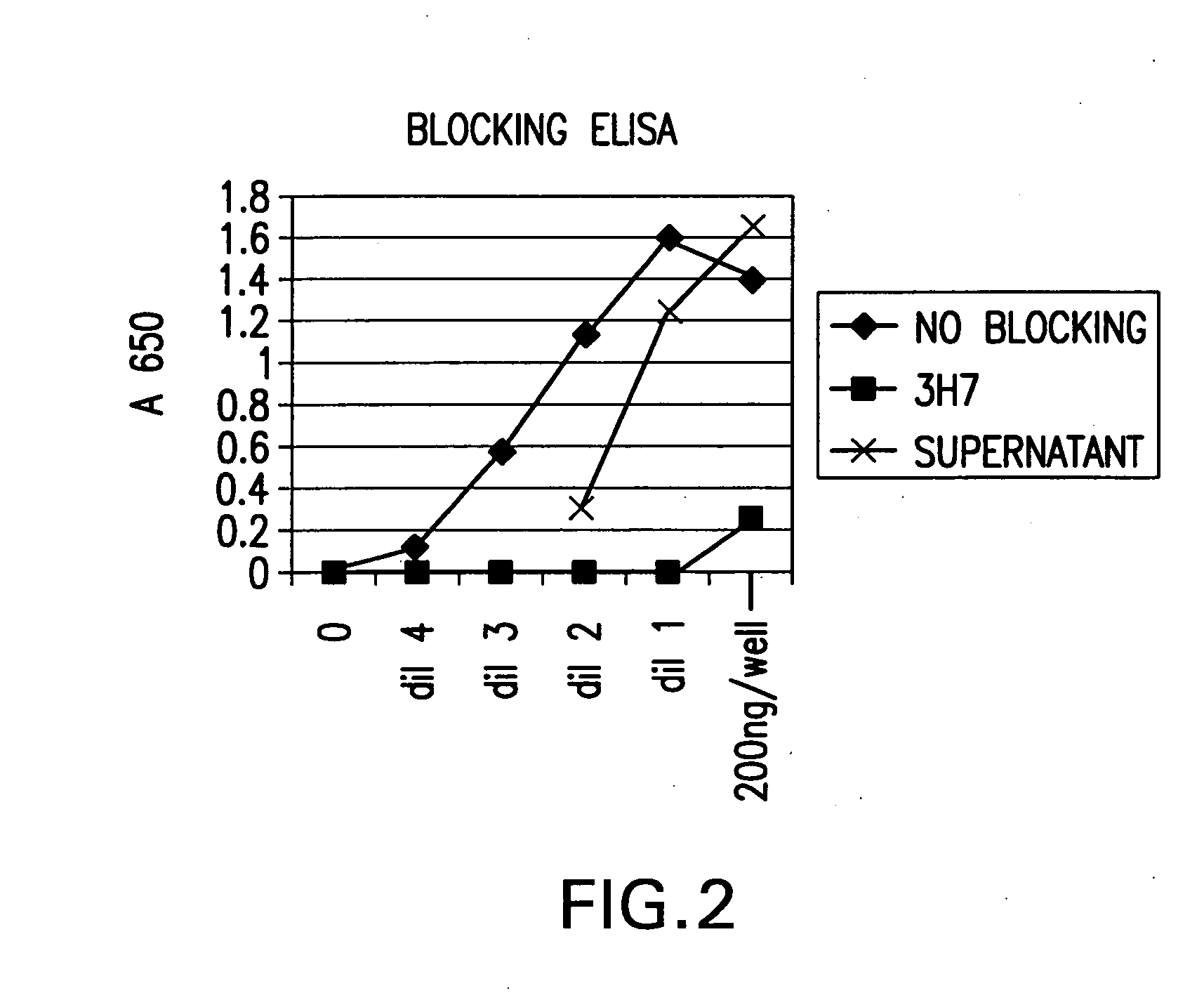
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20040185045A1Strong therapeutic activityEnhancing antibody-mediated effector functionSenses disorderAntipyreticTherapeutic antibodyTreatment effect
The present invention relates to antibodies or fragments thereof that specifically bind FcgammaRIIB, particularly human FcgammaRIIB, with greater affinity than said antibodies or fragments thereof bind FcgammaRIIA, particularly human FcgammaRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
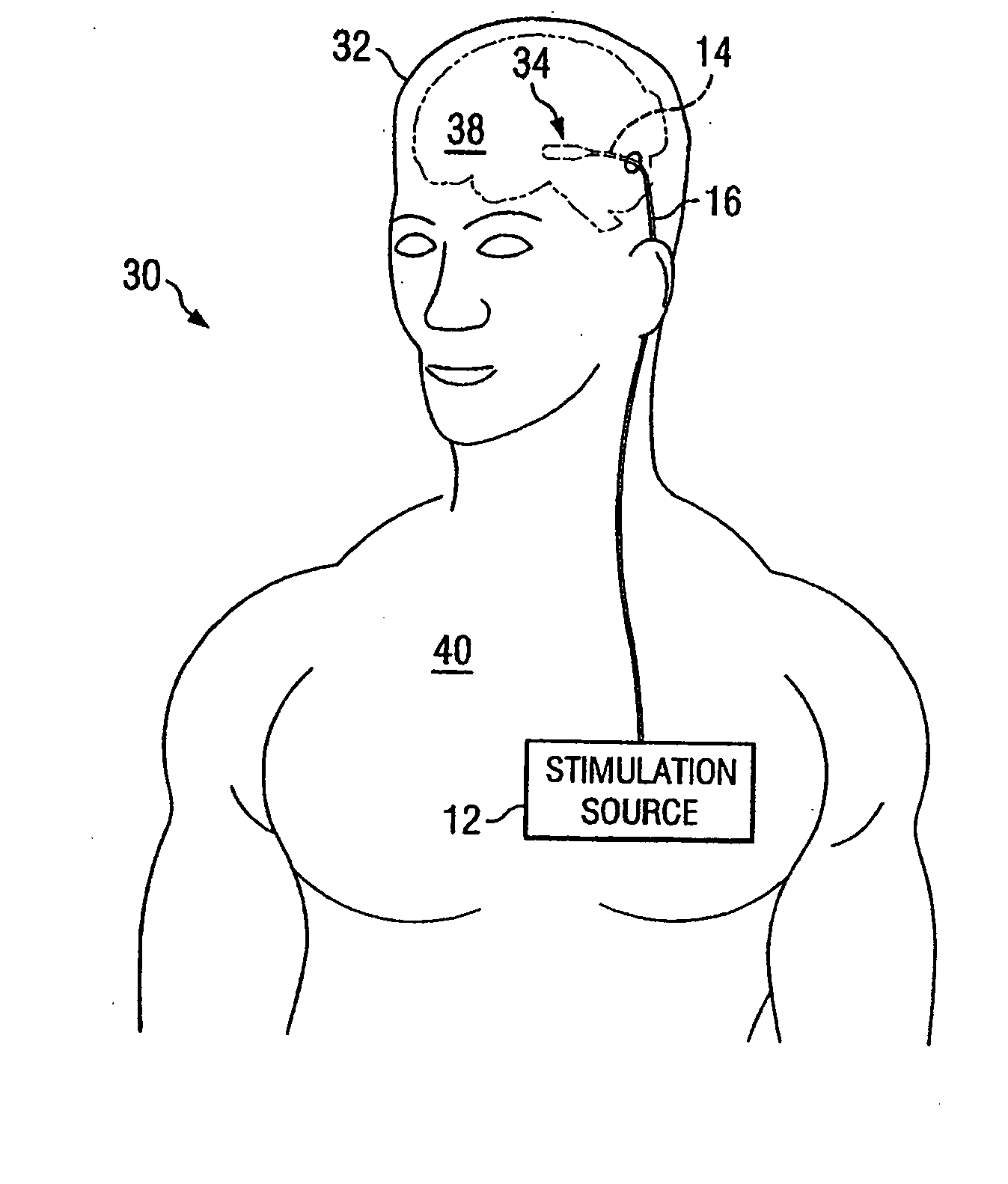
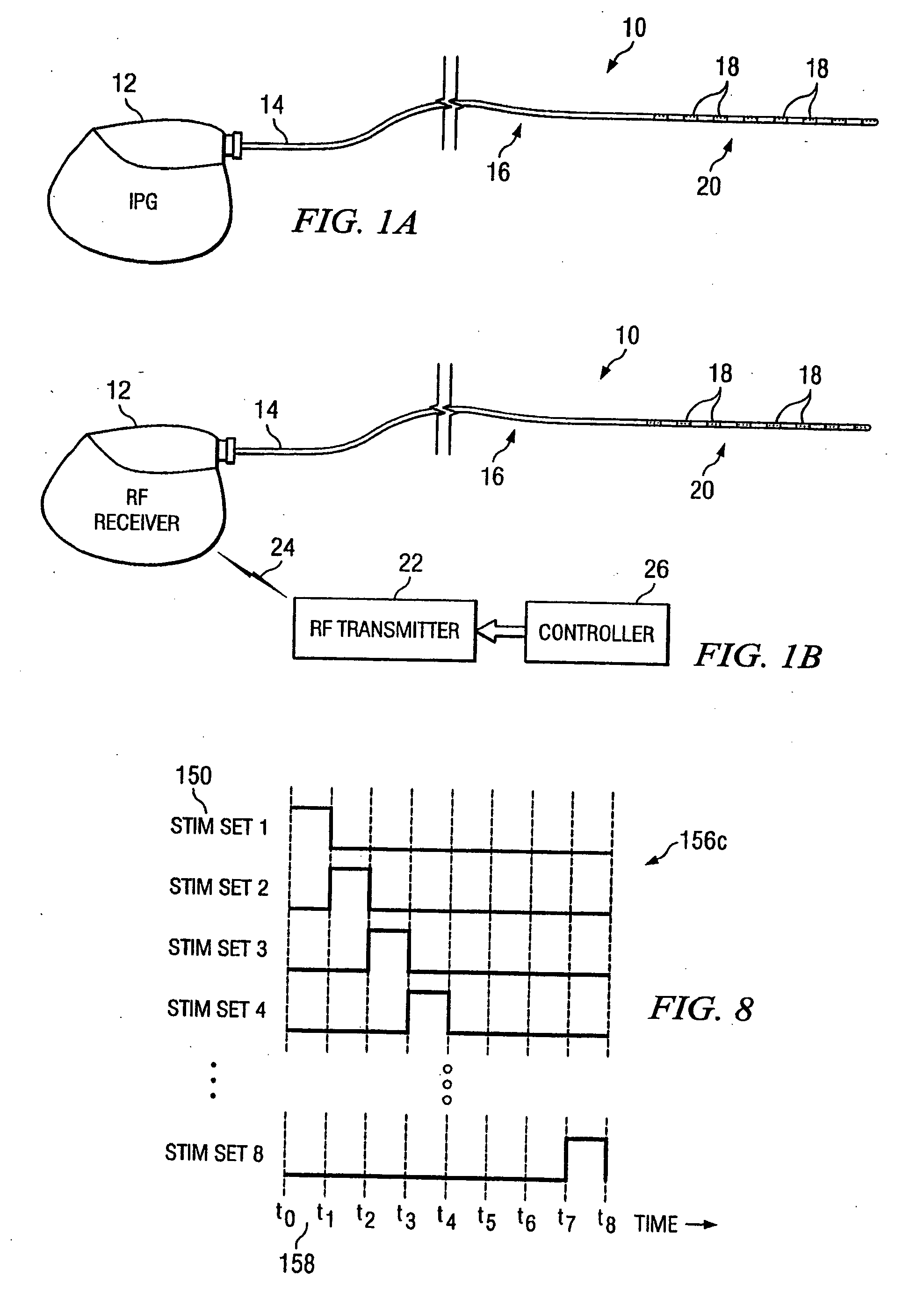
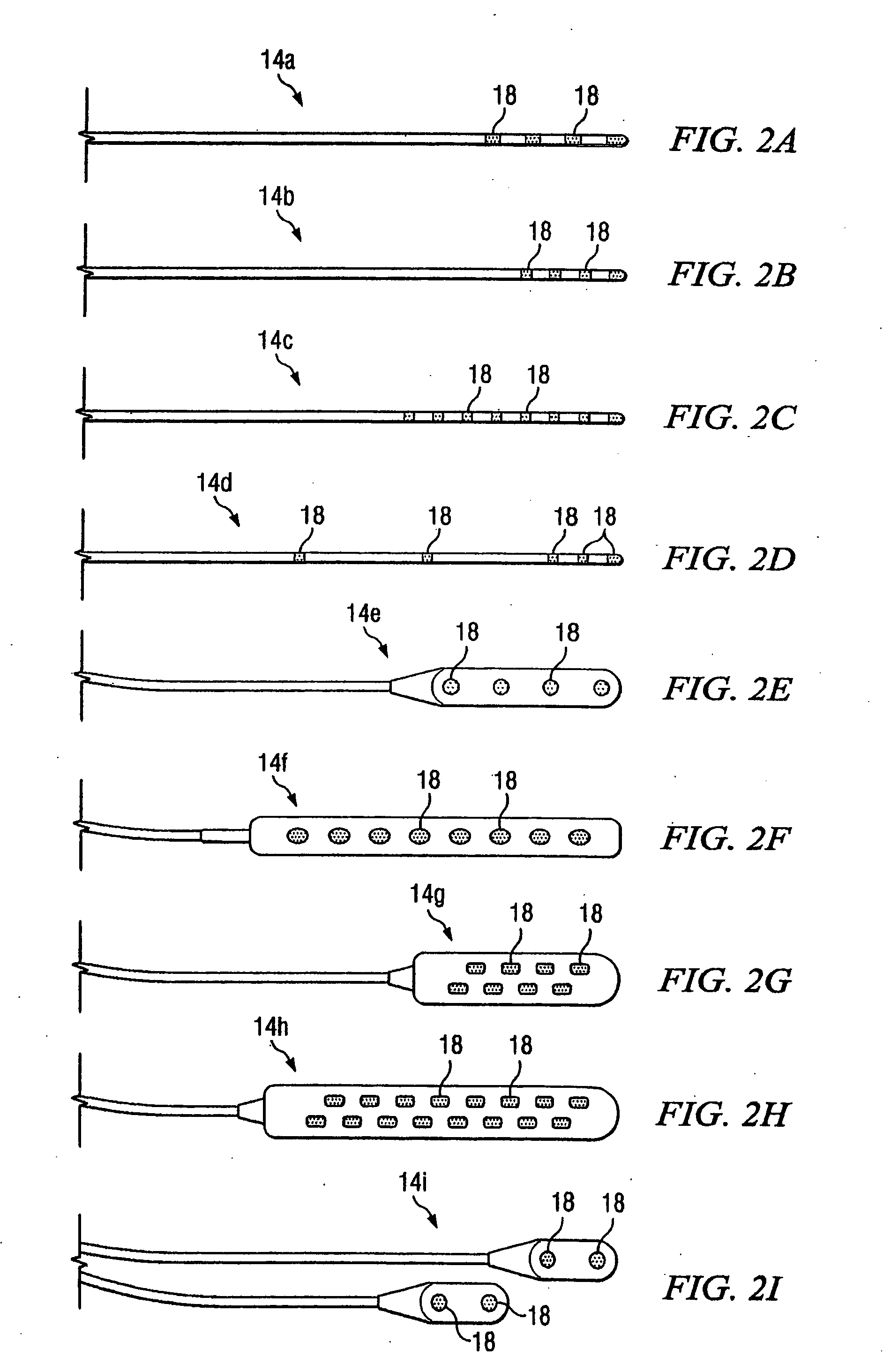
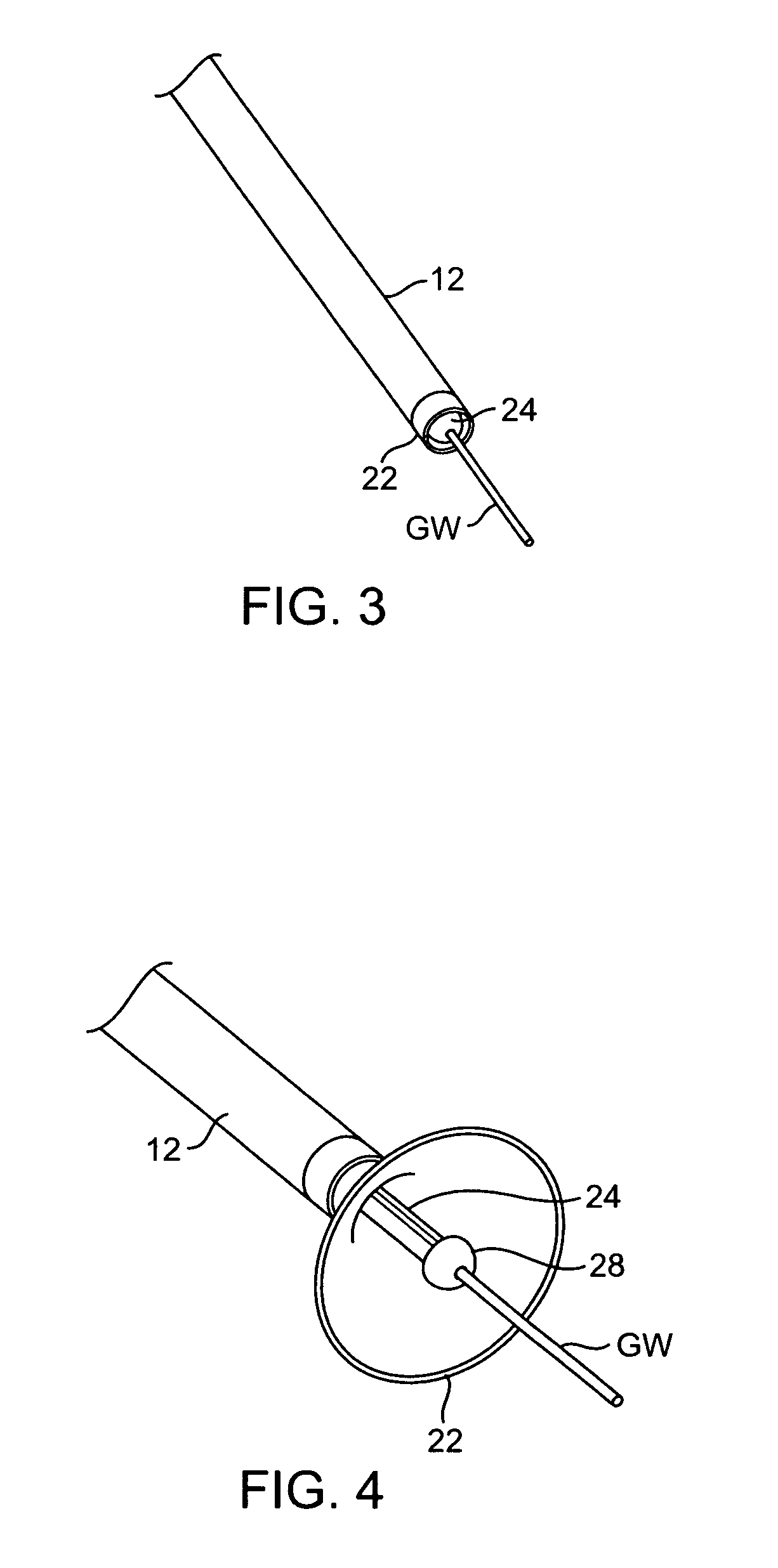
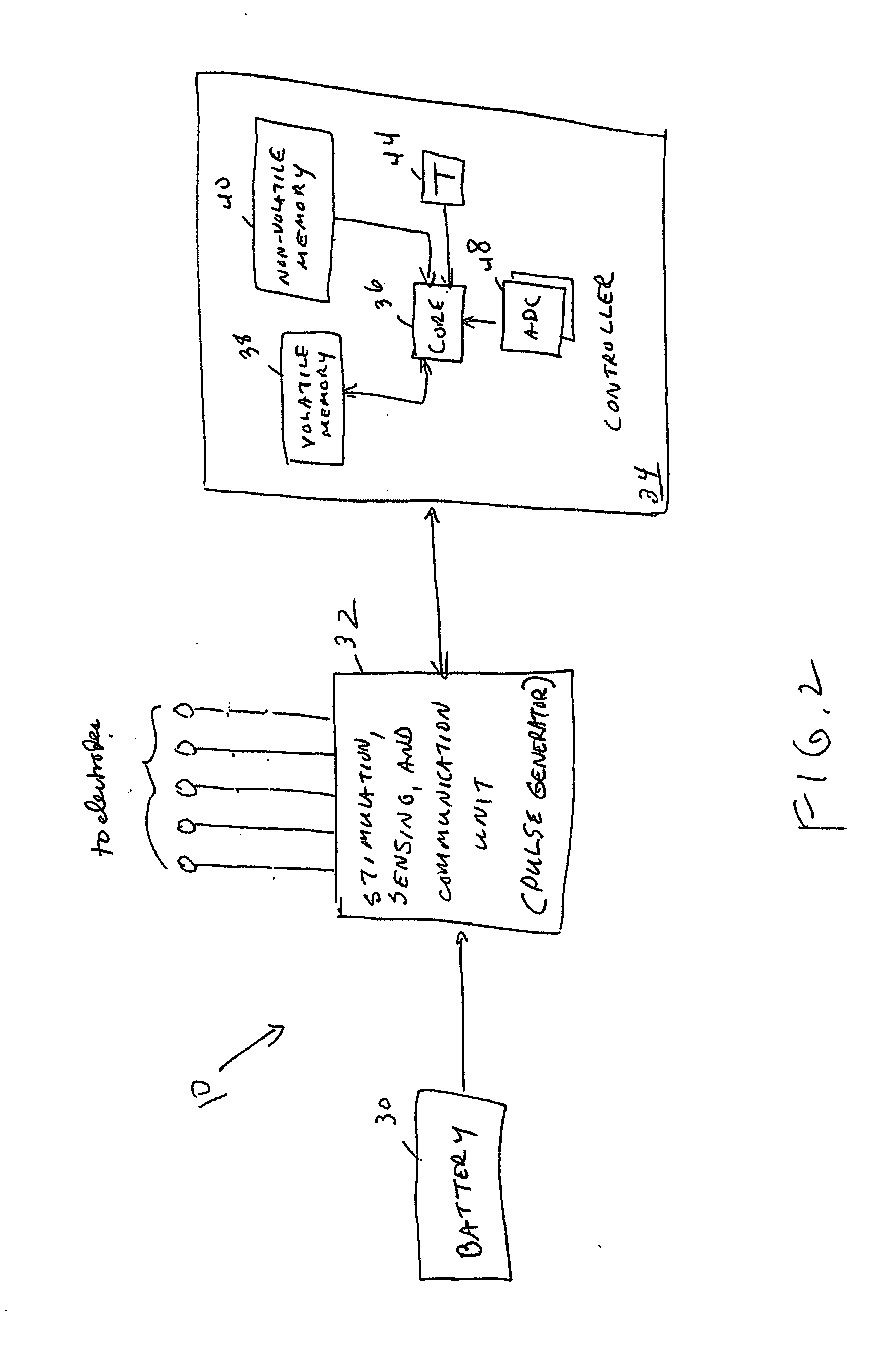
Electrical stimulation system and method for stimulating tissue in the brain to treat a neurological condition
InactiveUS20060004422A1Reduce and eliminate certain problem and disadvantageRemissionSpinal electrodesHead electrodesElectricityMedicine
According to one aspect, a stimulation system is provided for electrically stimulating a predetermined site to treat a neurological condition. The system includes an electrical stimulation lead adapted for implantation in communication with a predetermined site, wherein the site is brain tissue site. The stimulation lead includes one or more stimulation electrodes adapted to be positioned in the predetermined site. The system also includes a stimulation source that generates the stimulation pulses for transmission to the one or more stimulation electrodes of the stimulation lead to deliver the stimulation pulses to the predetermined site to treat a neurological disorder or condition.
Owner:ADVANCED NEUROMODULATION SYST INC
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050064514A1Enhanced antibody effector functionGood curative effectAntibacterial agentsSenses disorderEffector cellChemistry
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MACROGENICS INC
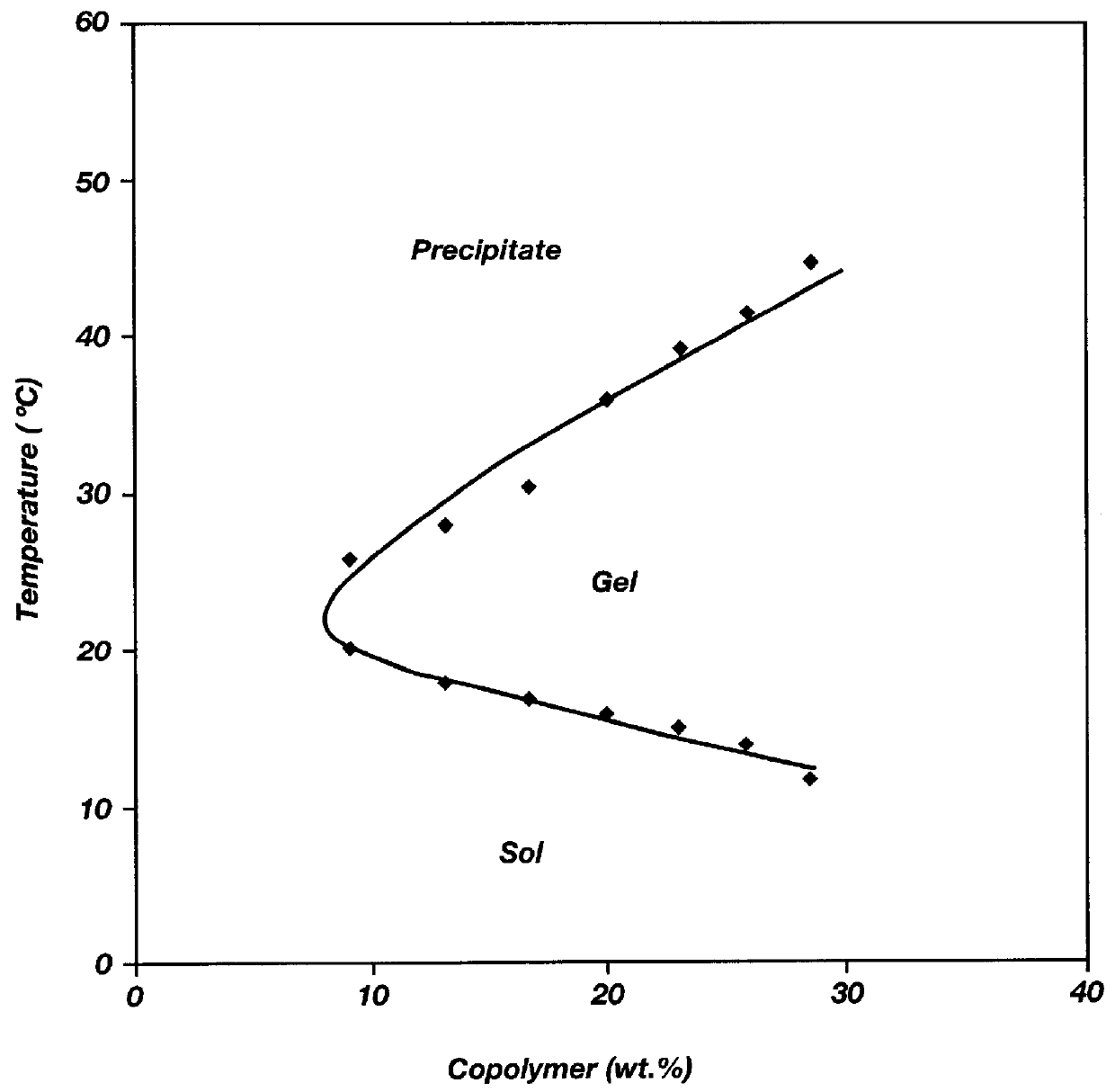
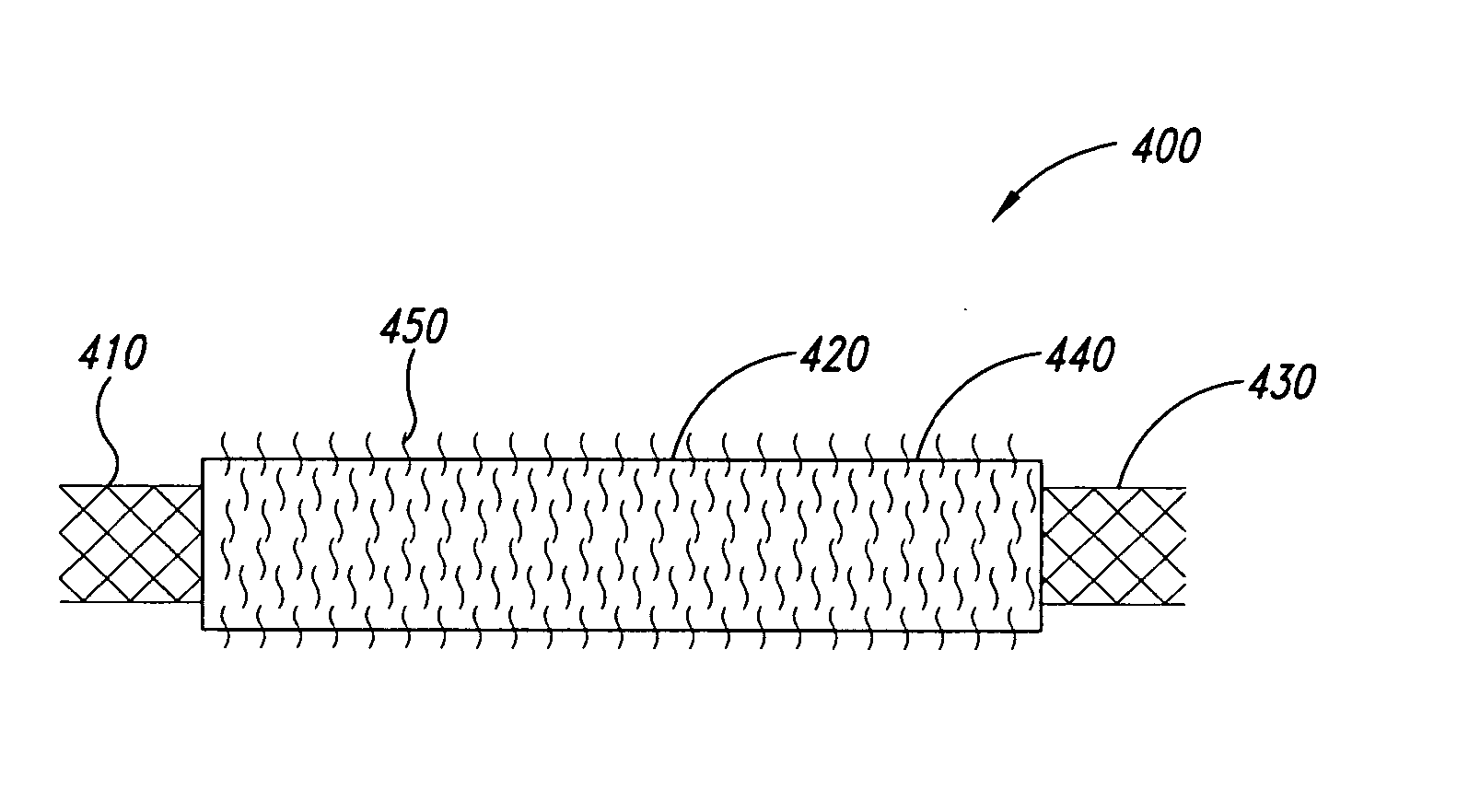
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
Aortic valve repair
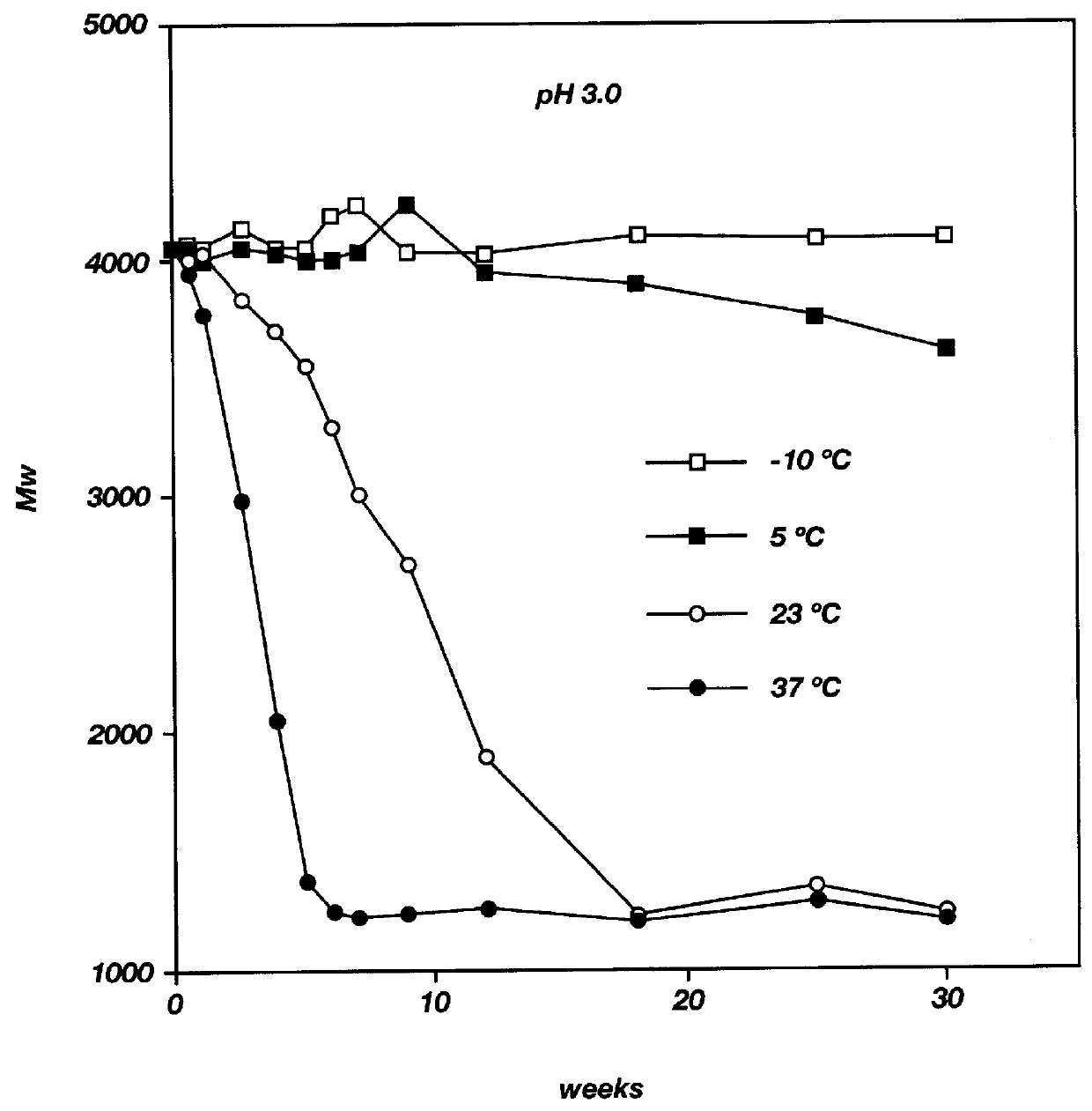
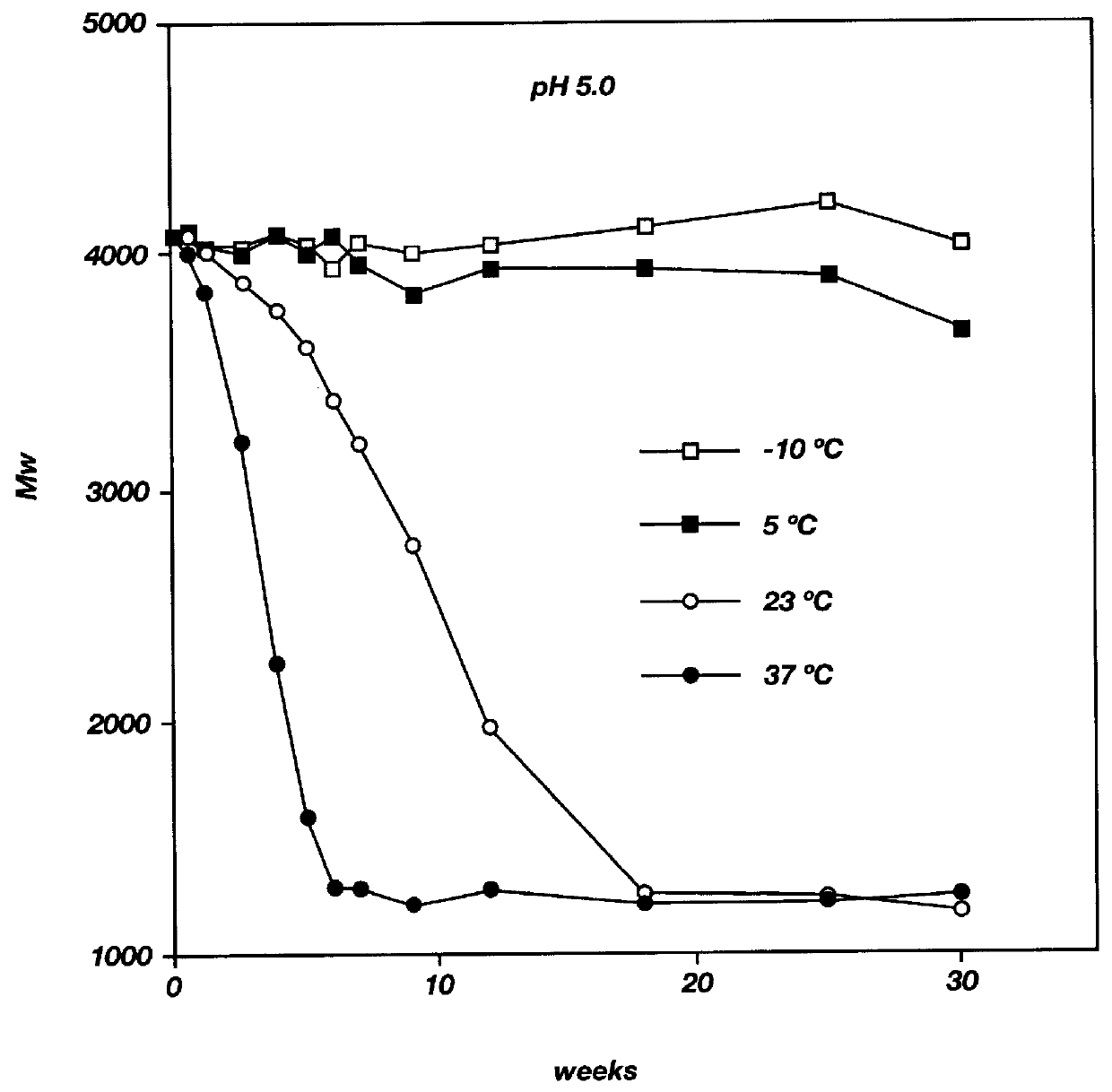
ActiveUS7803168B2Good curative effectReduce restenosisUltrasonic/sonic/infrasonic diagnosticsCannulasAortic valve repairNeoaortic valve
The present invention provides devices and methods for decalcifying an aortic valve. The methods and devices of the present invention break up or obliterate calcific deposits in and around the aortic valve through application or removal of heat energy from the calcific deposits.
Owner:TWELVE
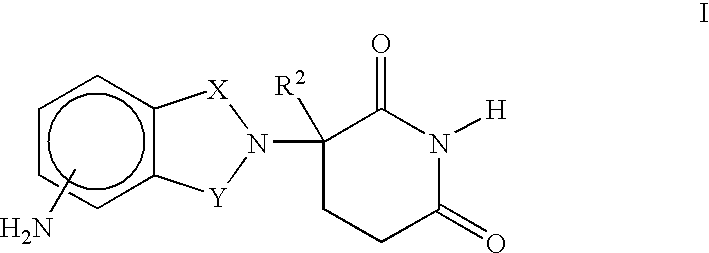
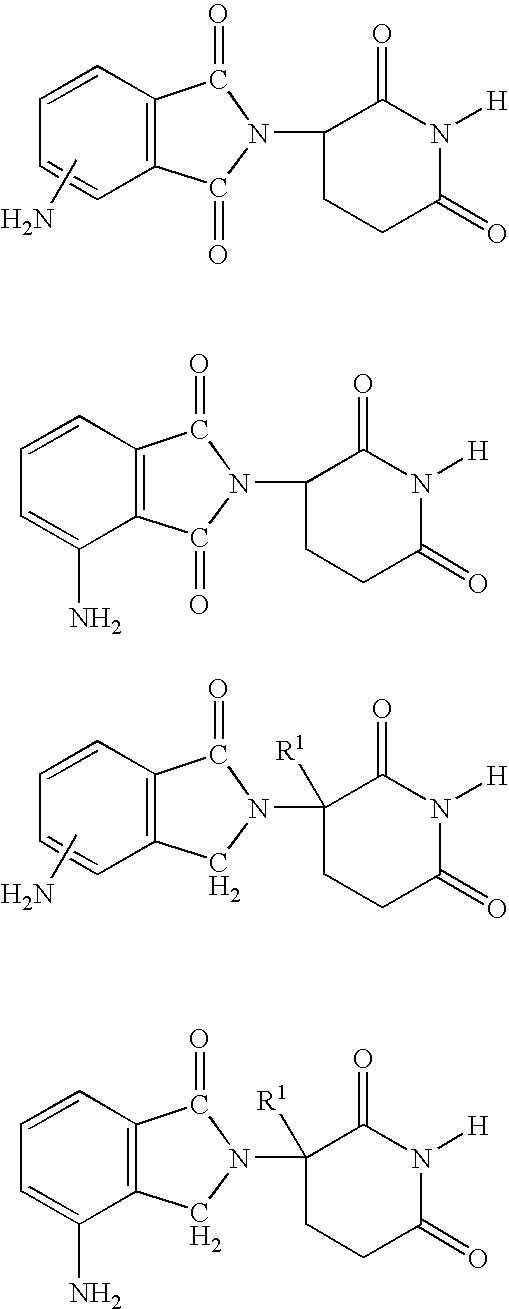
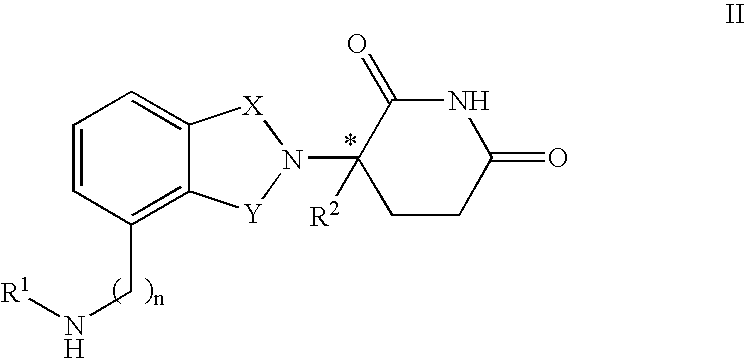
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myeloproliferative diseases
InactiveUS20040087546A1Reduce adverse effectsImprove toleranceBiocideNervous disorderActive agentMyeloproliferative disease
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent, and / or the transplantation of blood or cells. Particular second active agents are capable of suppressing the overproduction of hematopoietic stem cells or ameliorating one or more of the symptoms of a myeloproliferative disease. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Functional and hyperfunctional siRNA directed against Bcl-2
InactiveUS20050245475A1Improve efficiencyGood curative effectOrganic active ingredientsGenetic material ingredientsSilencing geneCancer research
Owner:THERMO FISHER SCIENTIFIC INC
Anti-RSV antibodies
InactiveUS6818216B2Reduce dosing frequencyReduce dosageAnimal cellsSugar derivativesSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection.
Owner:MEDIMMUNE LLC
Methods of synthesis and use
InactiveUS6235886B1Increase the number ofGood curative effectSugar derivativesMicrobiological testing/measurementNucleotideOrganic chemistry
Oligonucleotide and nucleotide amine analogs and methods of preparing and using these compounds are provided by the present invention.
Owner:IONIS PHARMA INC
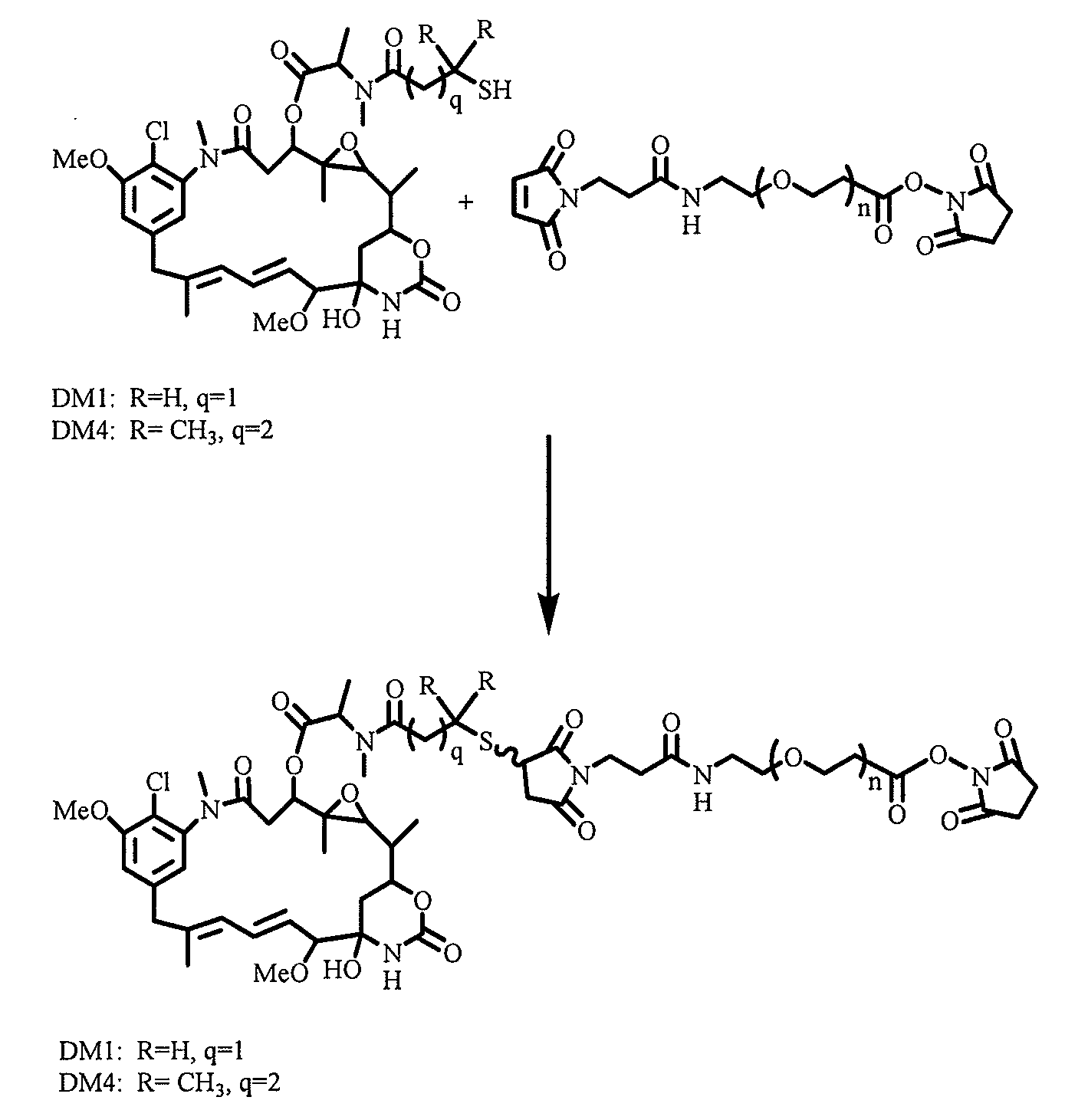
Potent conjugates and hydrophilic linkers
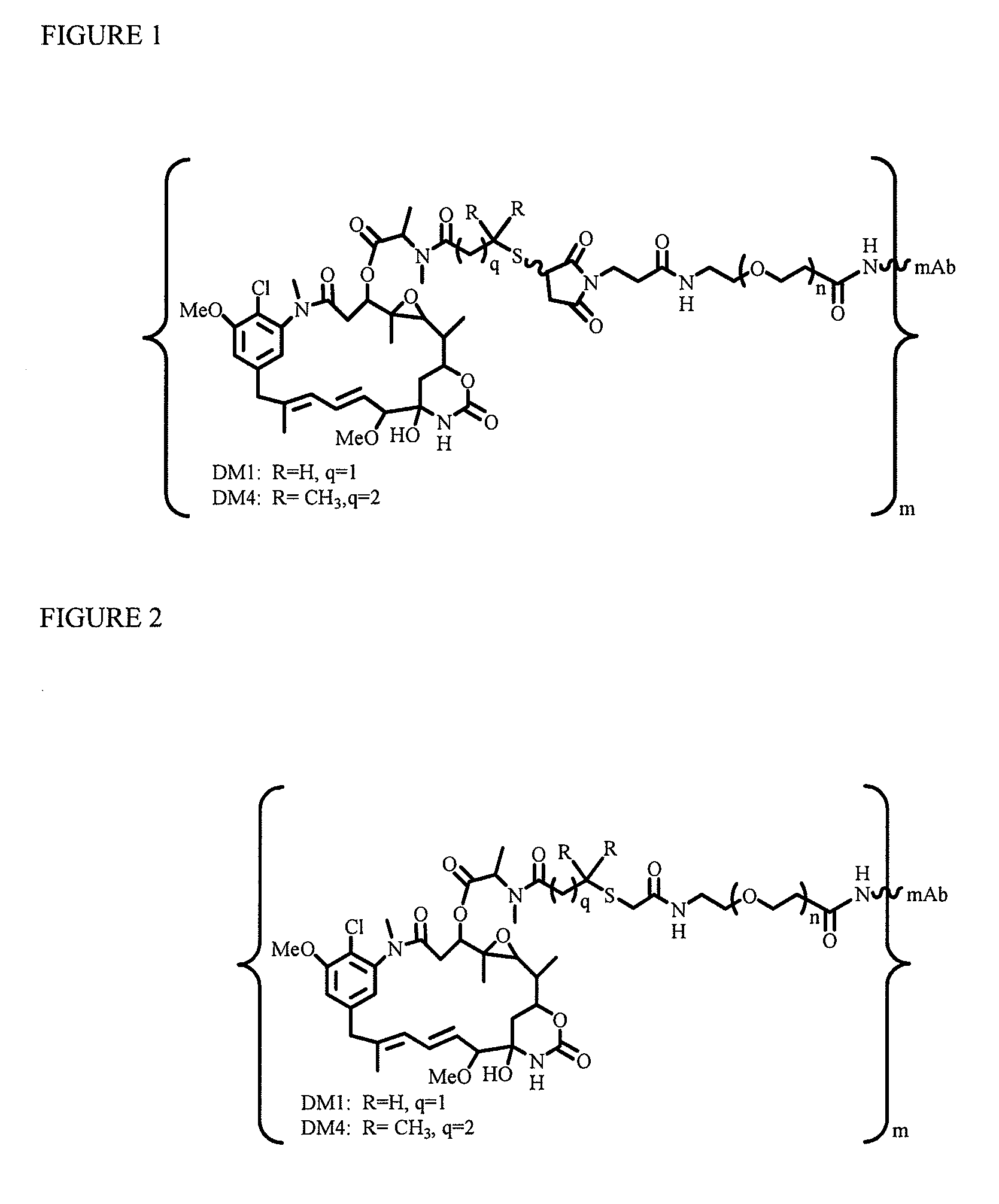
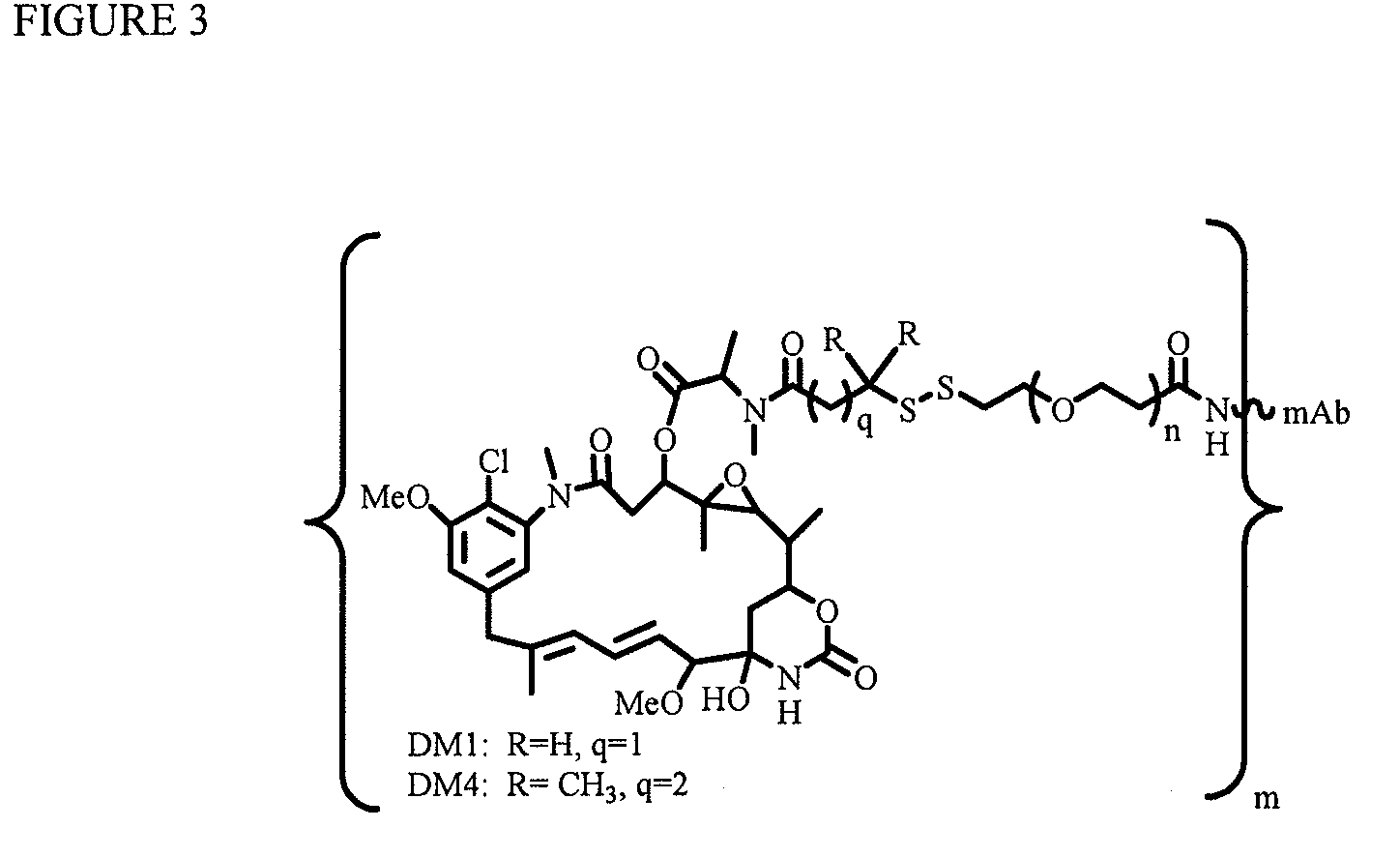
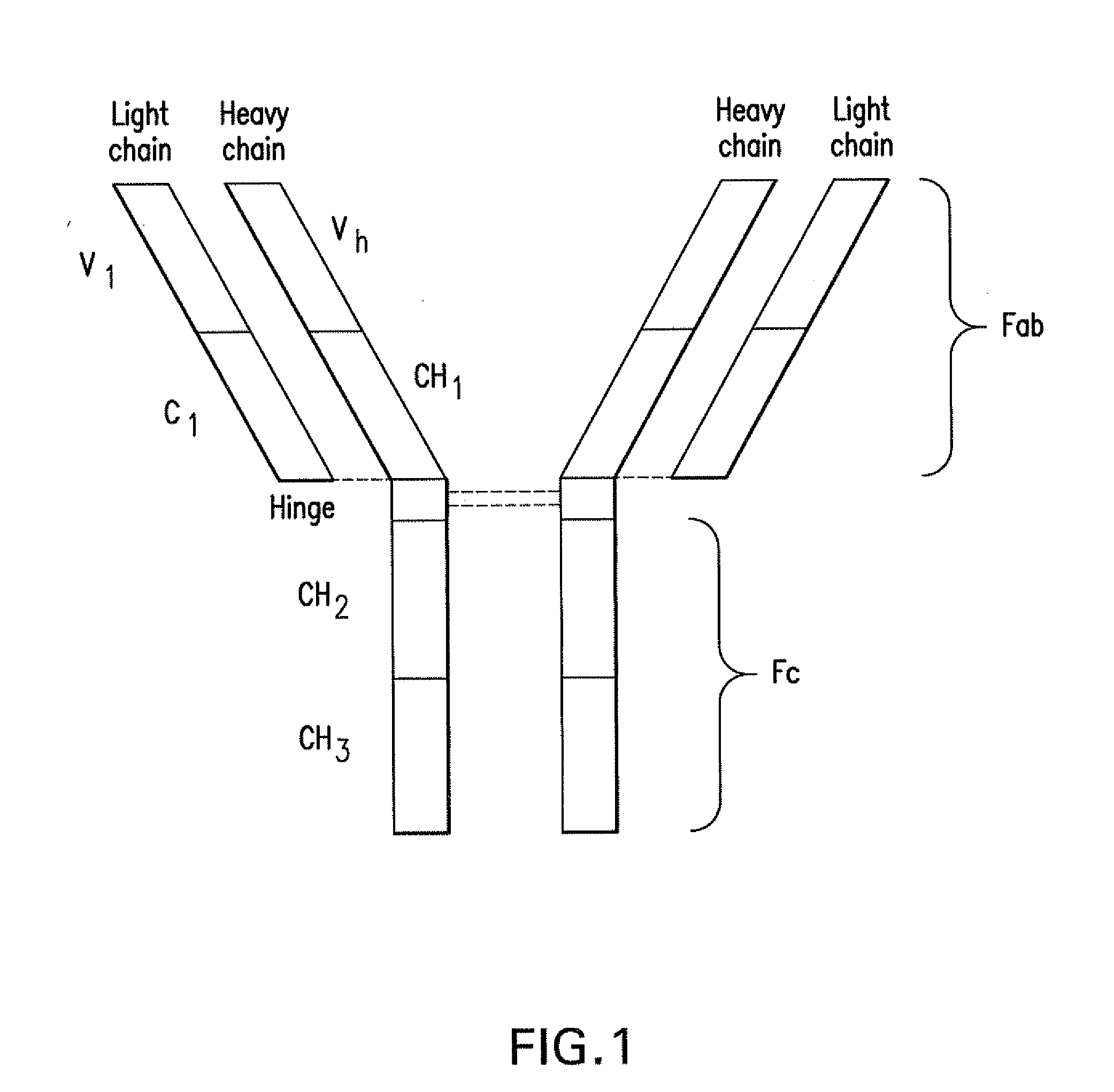
InactiveUS20100129314A1Increase activityImprove mannerPeptide/protein ingredientsAntiviralsAntigenThioether
Linkers for binding drugs to cell binding agents are modified to hydrophilic linkers by incorporating a polyethylene glycol spacer. The potency or the efficacy of the cell-binding agent-drug conjugates is surprisingly enhanced several folds in a variety of cancer cell types, including those expressing a low number of antigens on the cell surface or cancer cells that are resistant to treatment. A method for preparing maytansinoids bearing a thioether moiety and a reactive group which allows the maytansinoid to be linked to a cell-binding agent in essentially a single step is also provided.
Owner:IMMUNOGEN INC
Pd-1 binding proteins
ActiveUS20110008369A1Enhance host anti-microbial immunityImprove immunityAntibody mimetics/scaffoldsImmunoglobulins against animals/humansPD-L1Host immunity
The present invention features PD-1 binding proteins, a subset of which inhibits binding of PD-L1 to the PD-1 receptor. These binding proteins can be employed to modulate the immune system through the manipulation of the PD-1 signaling pathway, enhancing host immunity to treat infections and cancer.
Owner:MERCK SHARP & DOHME LLC
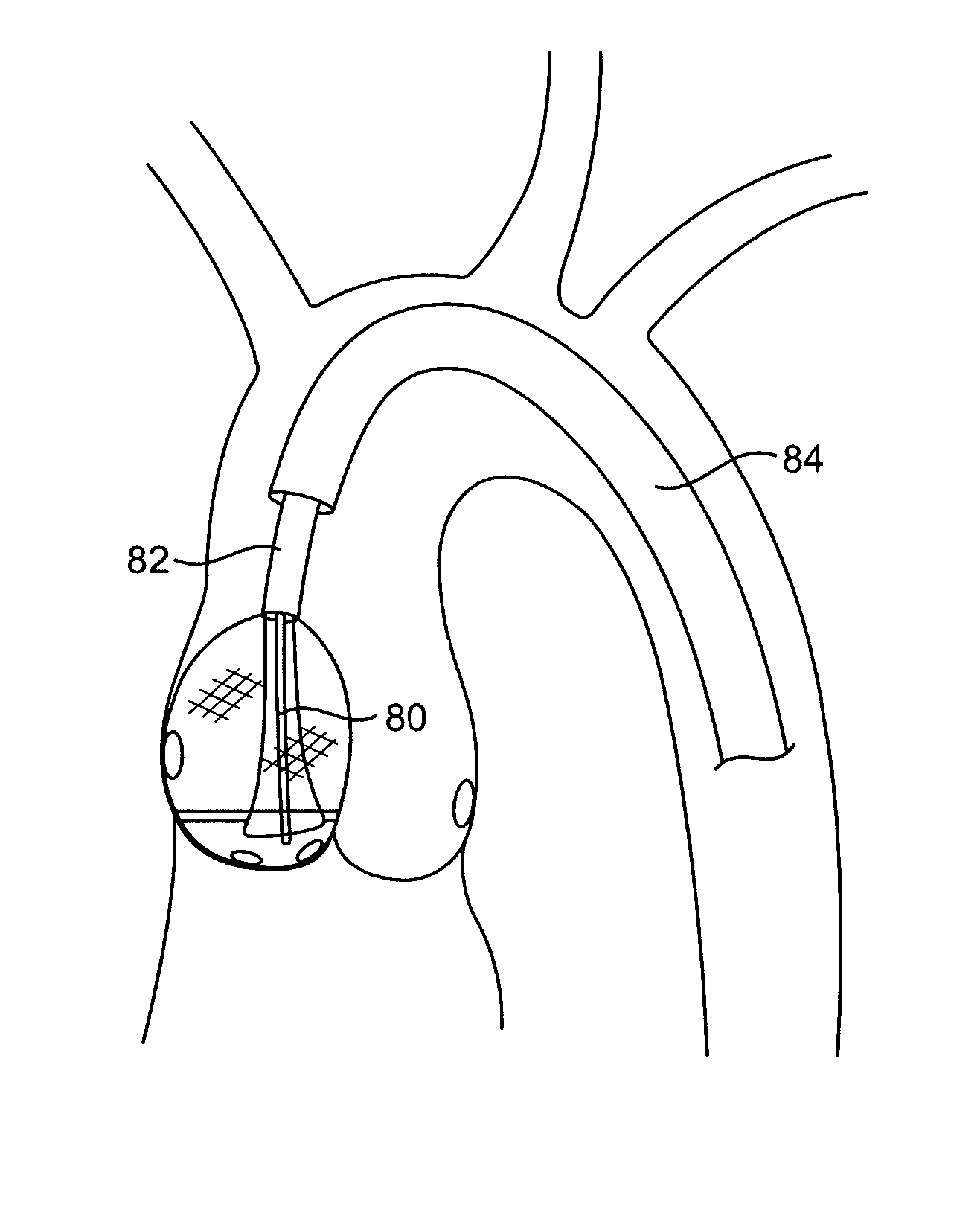
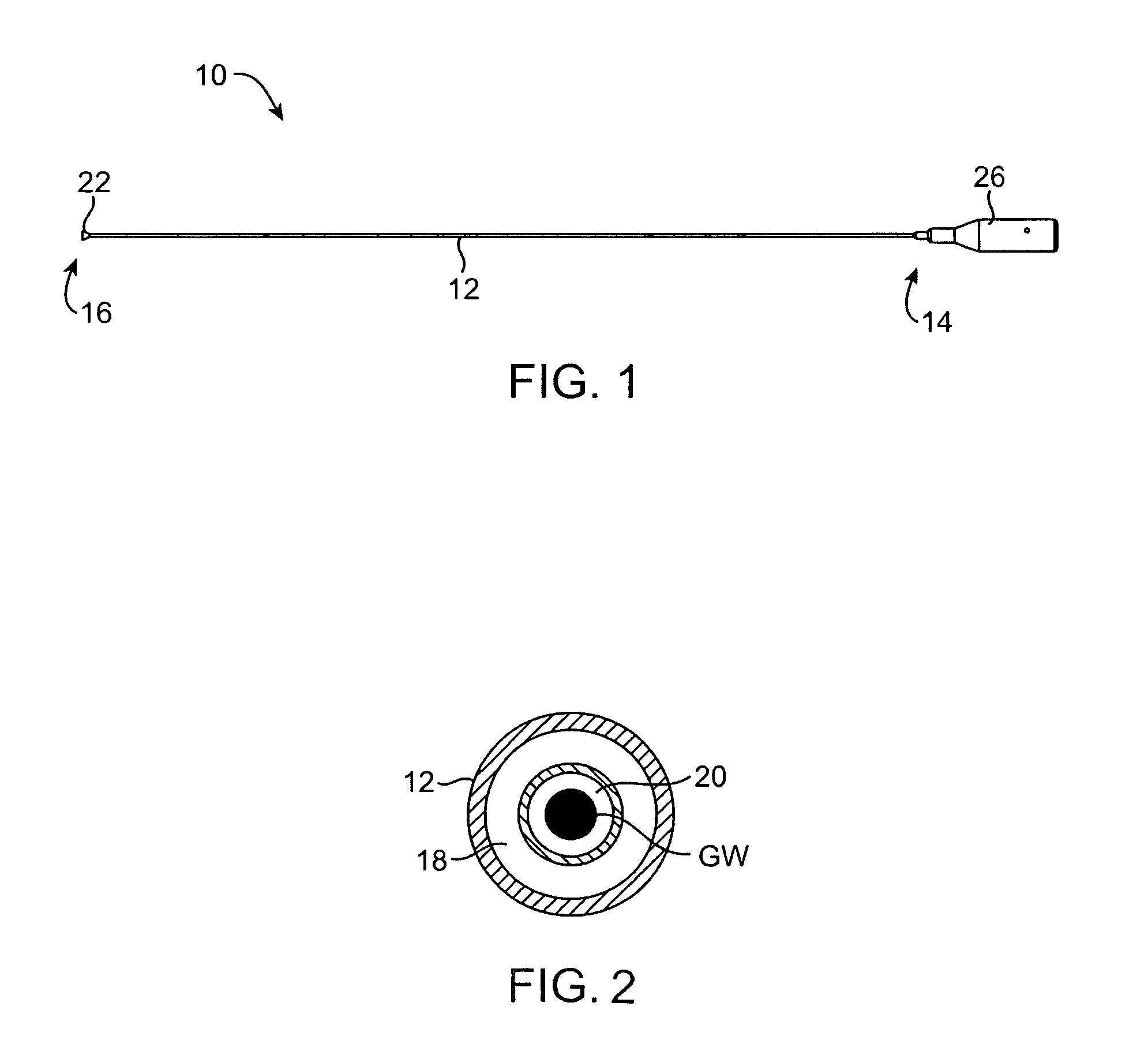
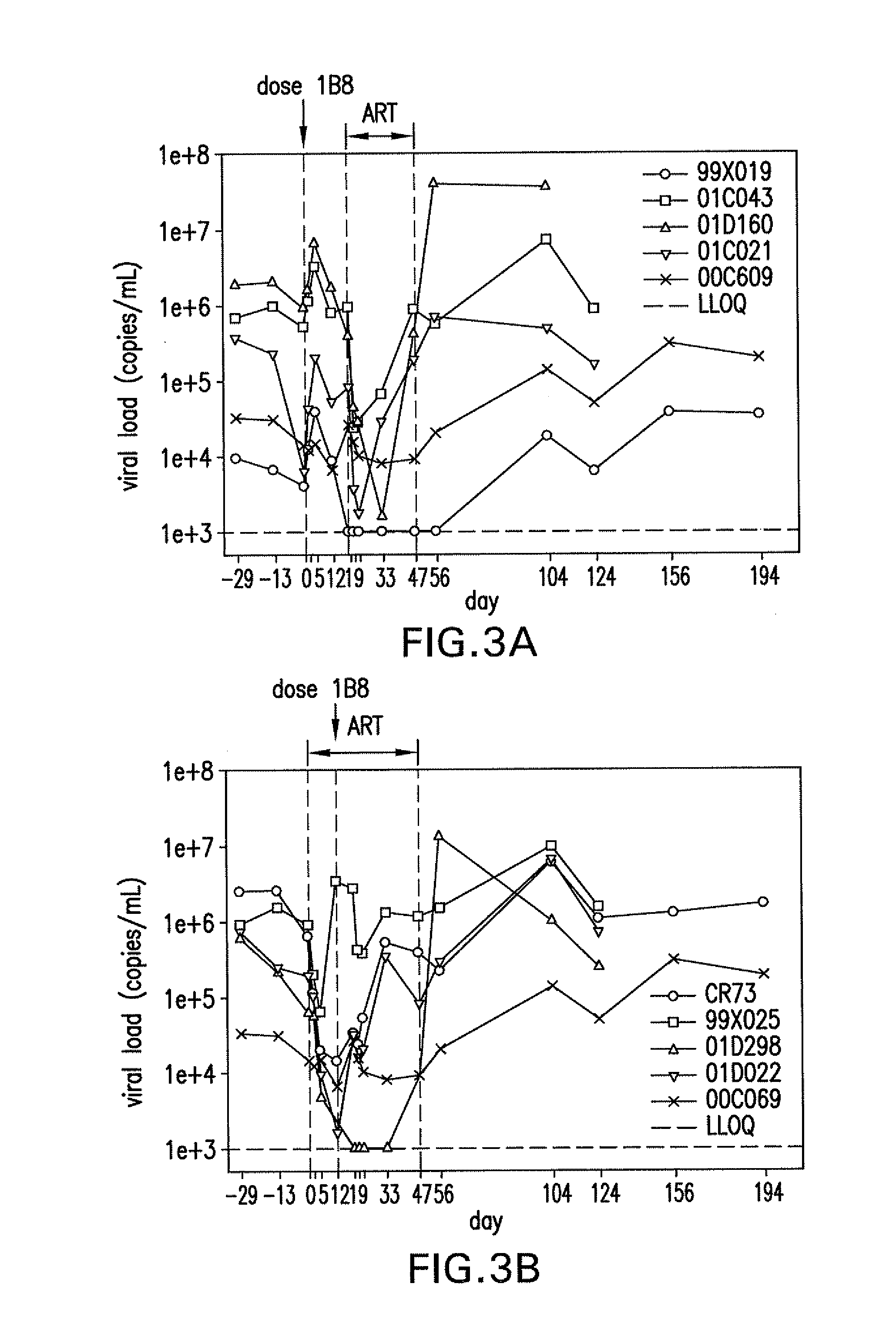
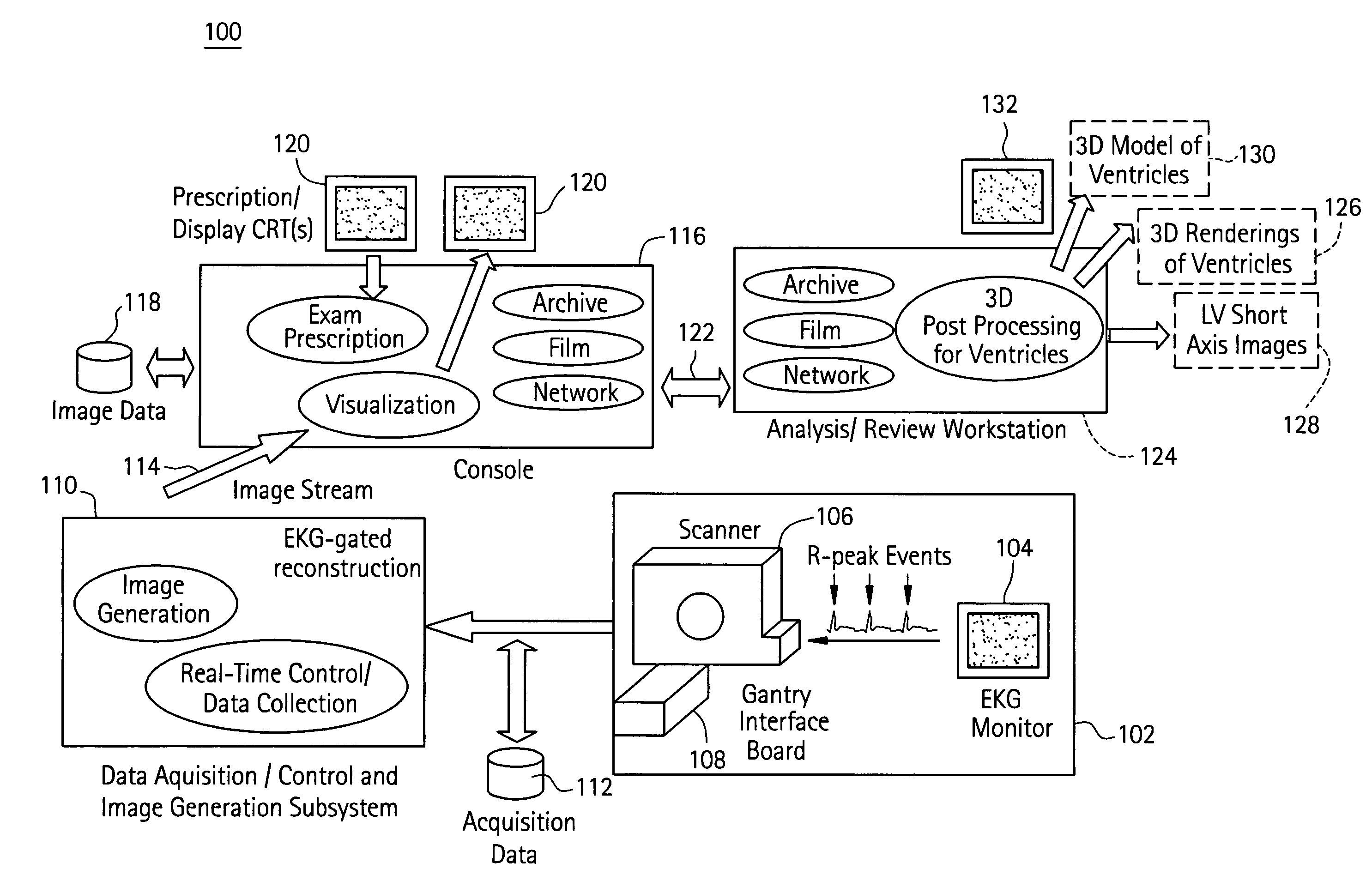
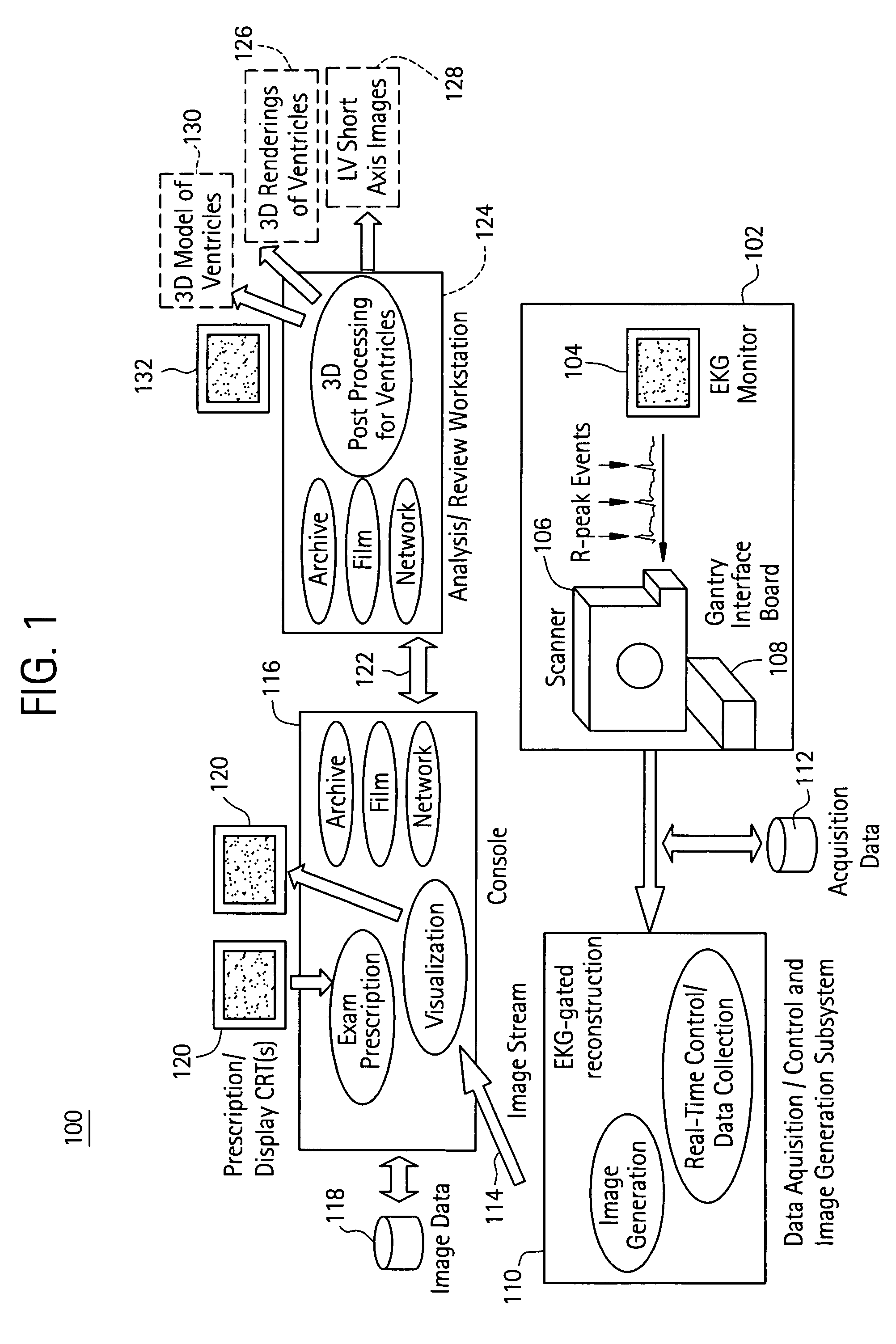
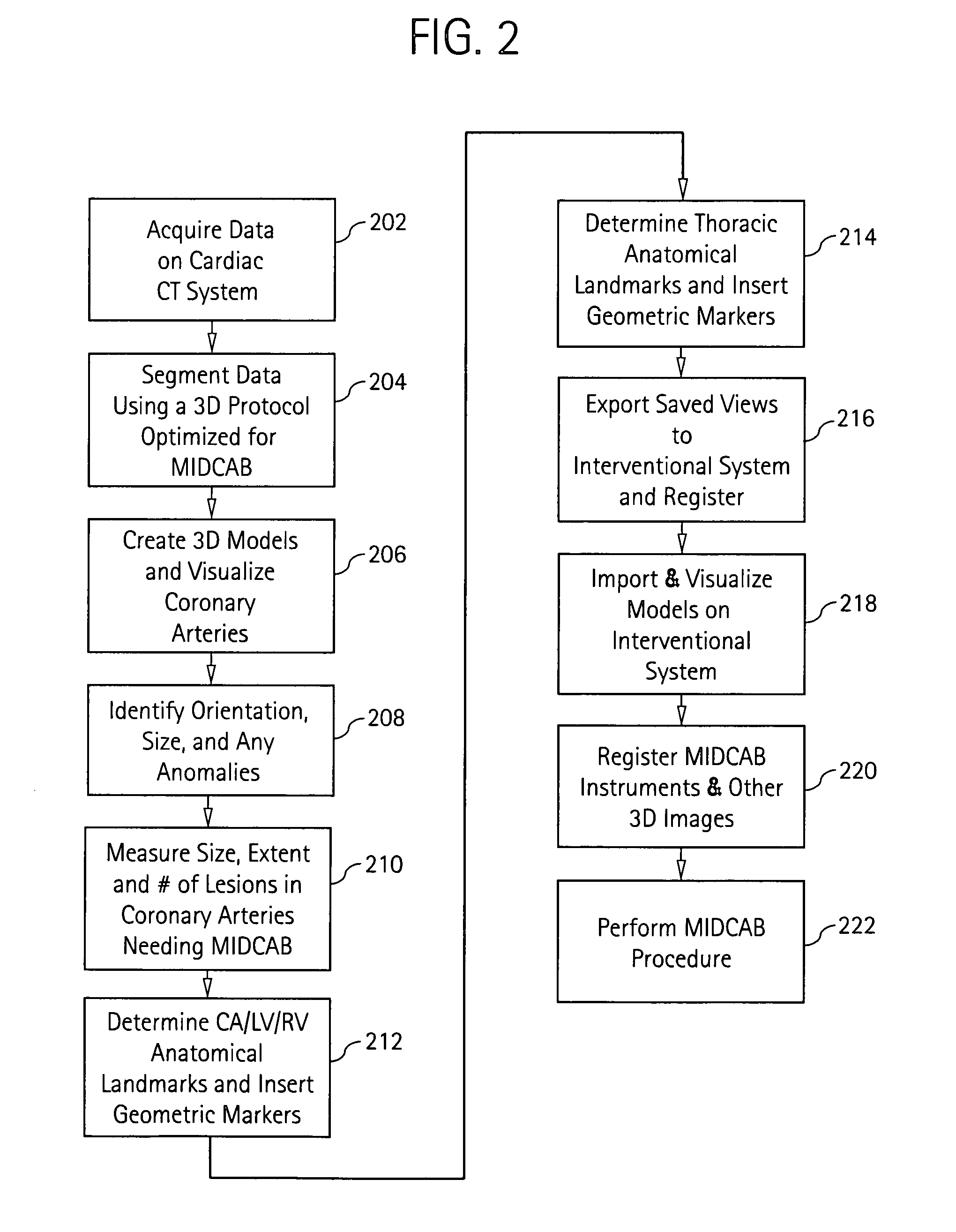
Cardiac imaging system and method for planning minimally invasive direct coronary artery bypass surgery
ActiveUS7813785B2High precisionShorten the construction periodUltrasonic/sonic/infrasonic diagnosticsMedical simulationAnatomical landmarkCoronary arteries
A method for planning minimally invasive direct coronary artery bypass (MIDCAB) for a patient includes obtaining acquisition data from a medical imaging system, and generating a 3D model of the coronary arteries and one or more cardiac chambers of interest. One or more anatomical landmarks are identified on the 3D model, and saved views of the 3D model are registered on an interventional system. One or more of the registered saved views are visualized with the interventional system.
Owner:APN HEALTH +1
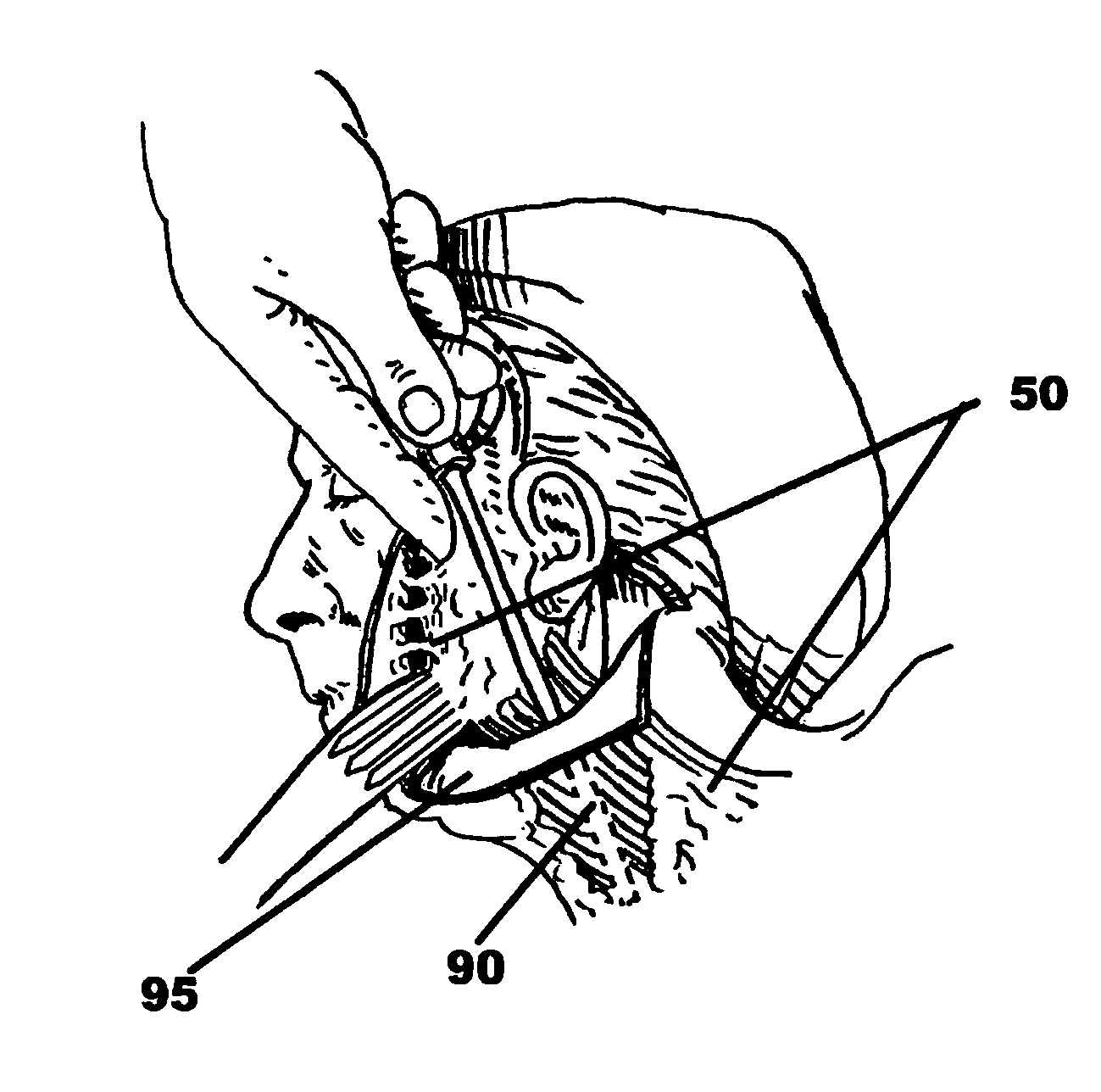
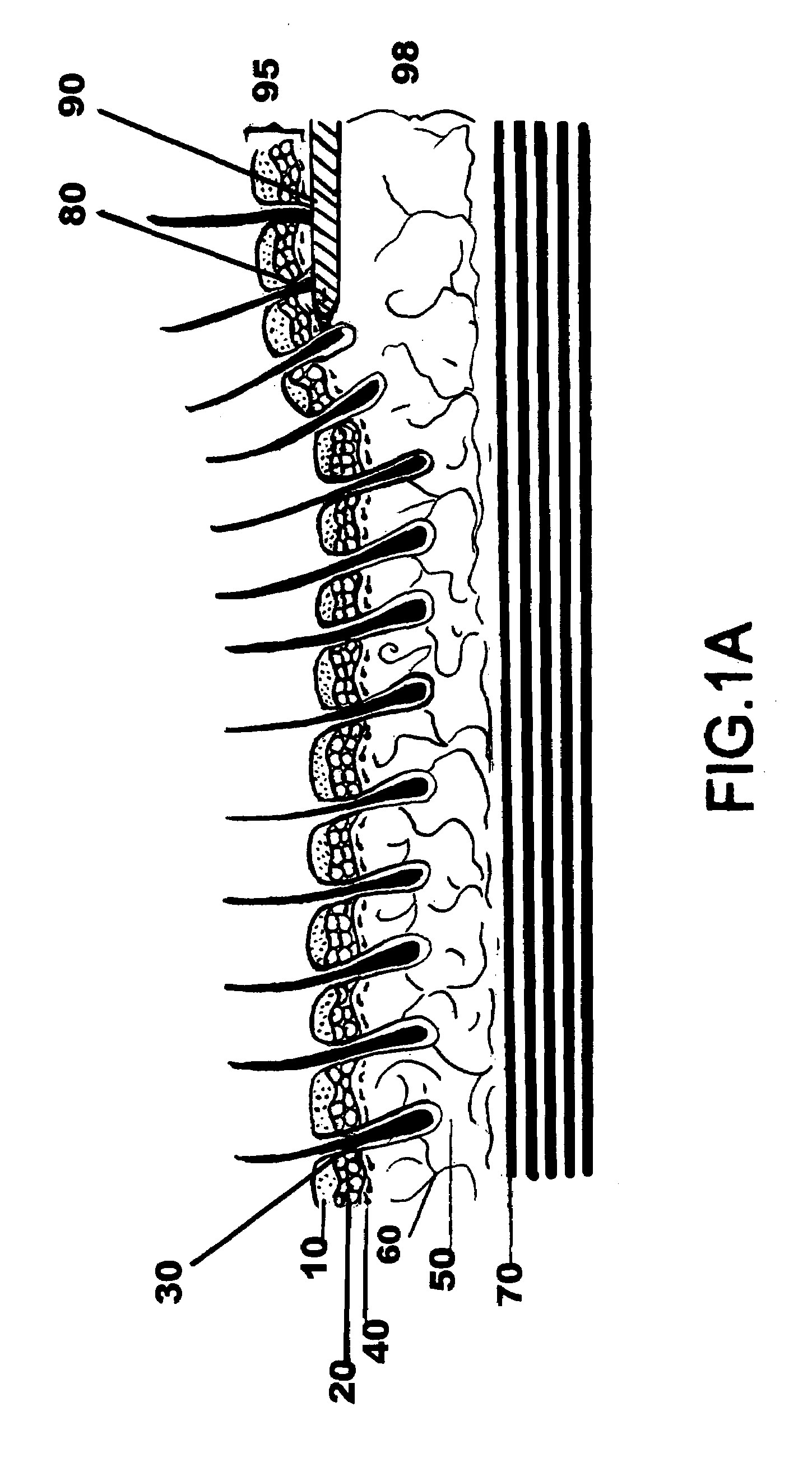
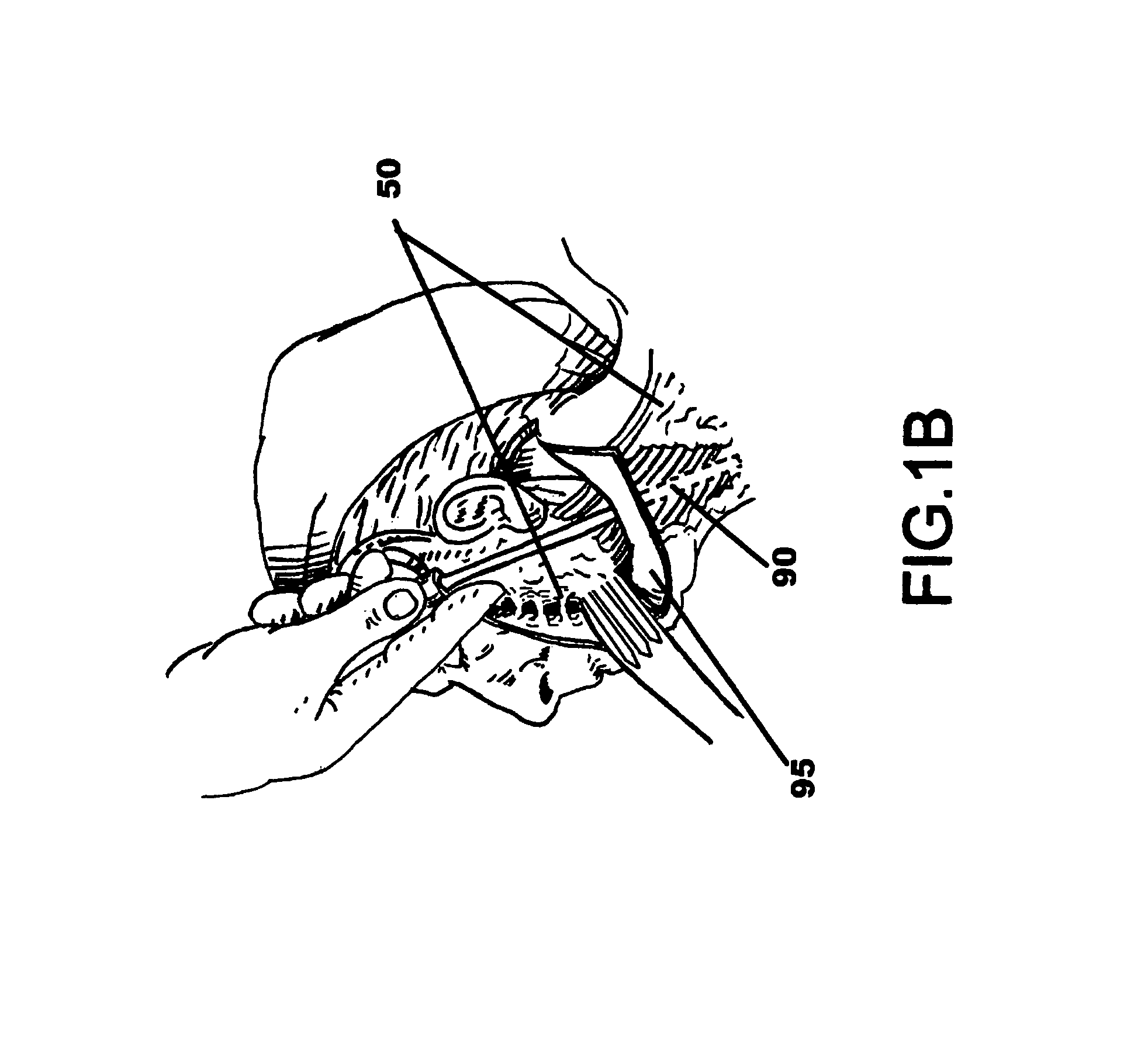
Facial tissue strengthening and tightening device and methods
InactiveUS7494488B2Improve efficacyImprove securityUltrasound therapyElectrotherapyForms of energyFacial tissue
A device is described that can be used quickly and accurately by surgeons to provide uniform facial tissue planes that are tunnel-free and wall-free thus optimizing face lifting, tightening, and implant delivery. The device is comprised of a shaft with a substantially planar tip further comprised of relative protrusions and energized relative recession lysing segments. Forward motion of the device precisely divides and energizes various tissue planes causing contraction, especially via the fibrous tissues. Other forms of energy and matter can be delivered down the shaft to further enhance desirable tissue modification and contraction.
Owner:WEBER PAUL J
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Intravascular devices and fibrosis-inducing agents
InactiveUS20050149173A1Reducing perigraft leakageFacilitate “anchoring”StentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Implantable medical device having multiple electrode/sensor capability and stimulation based on sensed intrinsic activity
ActiveUS20060167497A1BlockingGood curative effectElectrotherapyDiagnostic recording/measuringElectricityIntrinsic activity
In one embodiment, an implantable neurostimulator comprises a pulse generator that generates an electrical pulse signal to stimulate a neural structure in a patient, a stimulation lead assembly coupled to the pulse generator for delivering the electrical pulse signal to the neural structure, a plurality of sensors coupled to the pulse generator, and sensor select logic. Each sensor is individually selectable and the sensor select logic selects any two or more of the plurality of sensors for sensing a voltage difference between the selected sensors. In other embodiments, two or more physiologic parameters are sensed. In yet another embodiment, a method comprises sensing intrinsic electrical activity on a person's nerve and stimulating the nerve based on the sensed intrinsic electrical activity of the nerve.
Owner:LIVANOVA USA INC
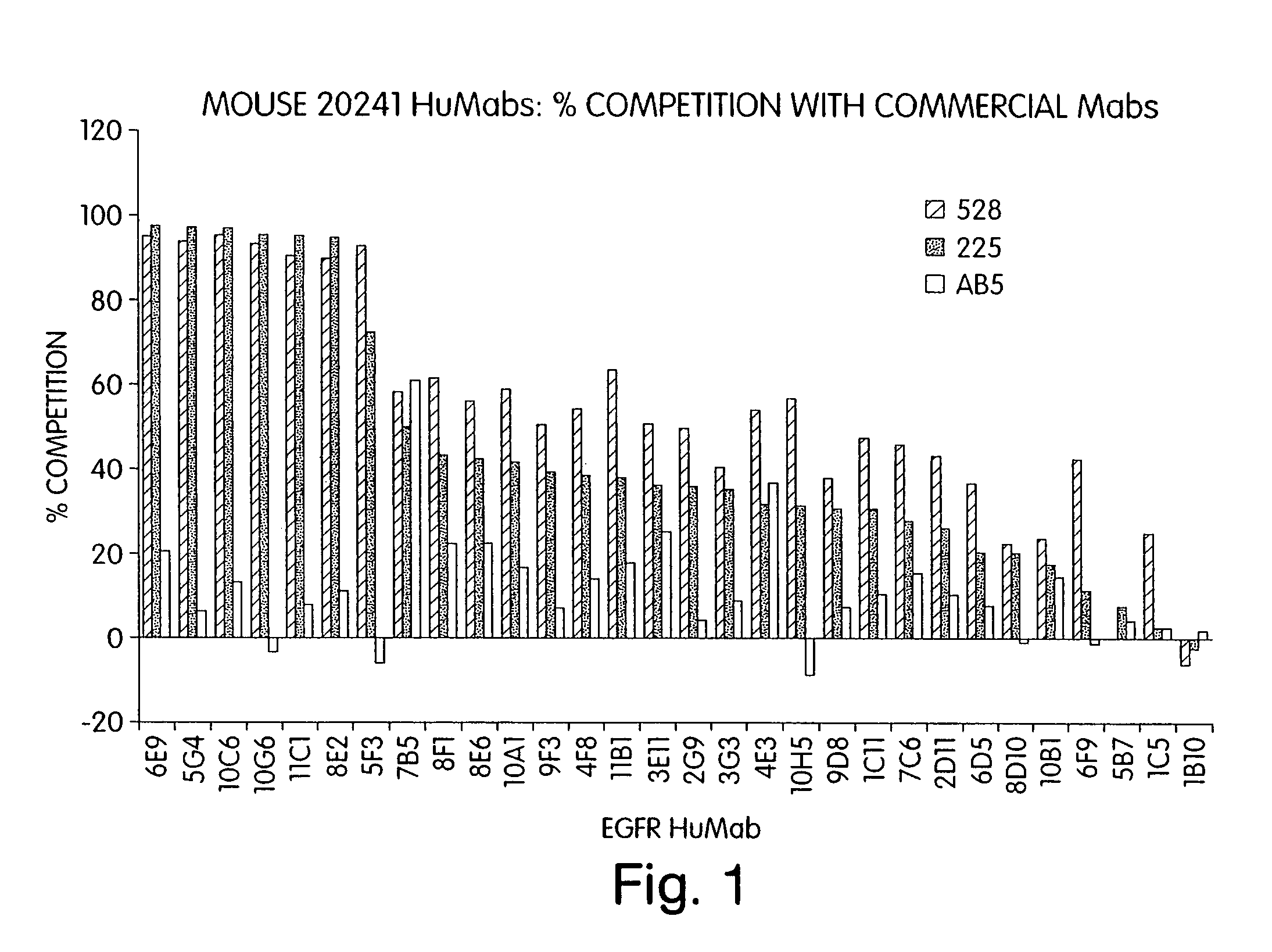
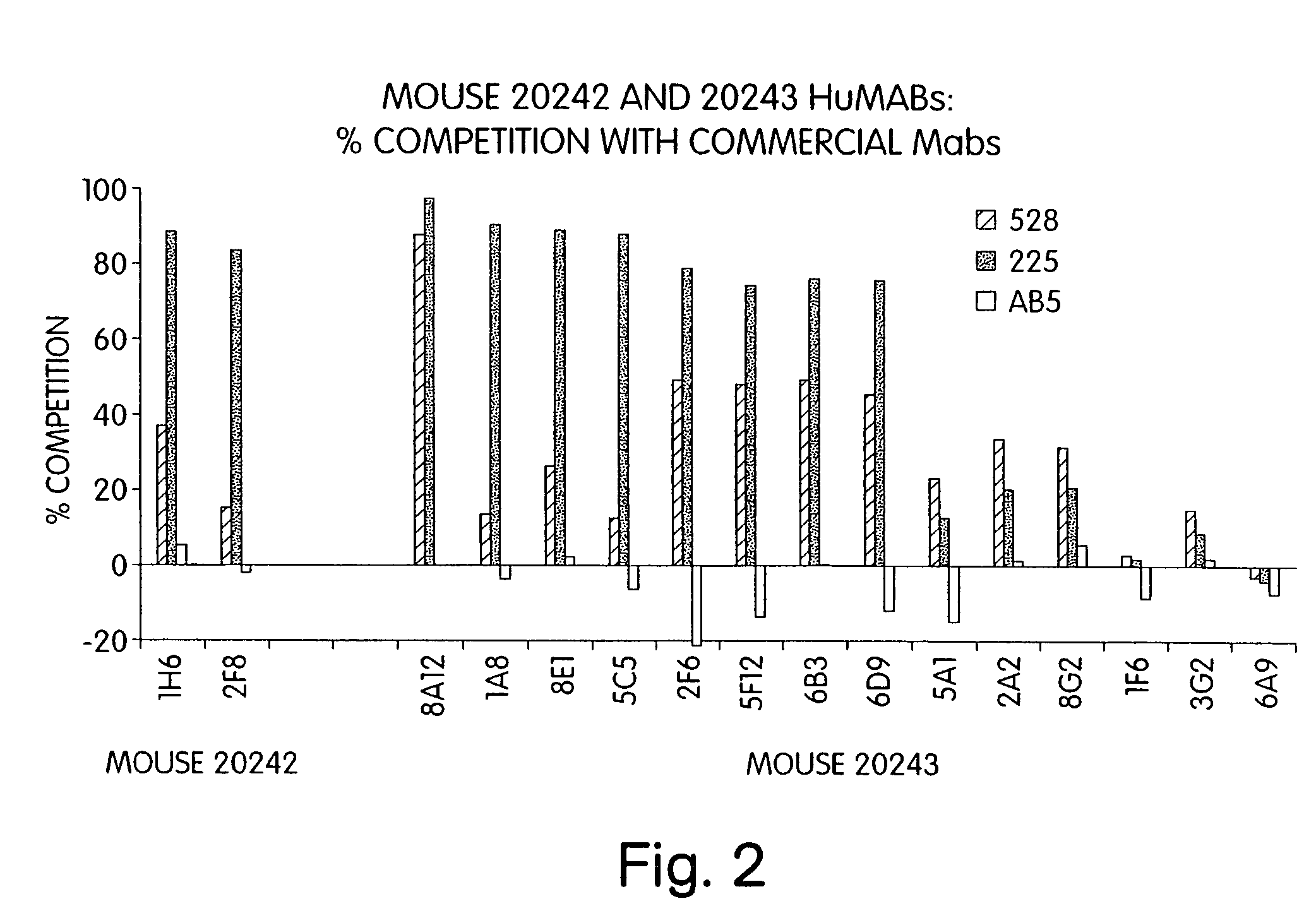
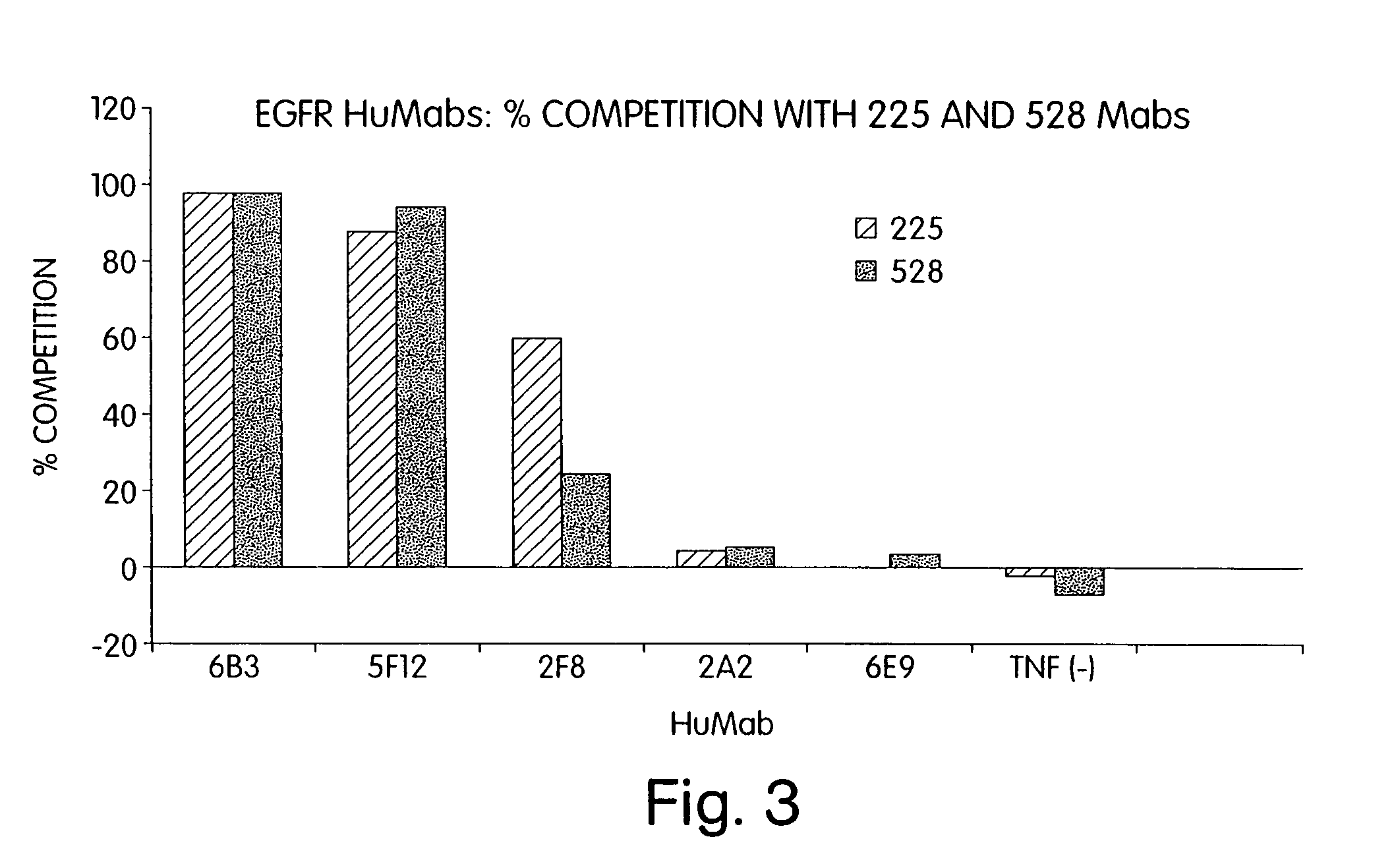
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
InactiveUS7247301B2Less immunogenicReduce adverse side effectsInorganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB INC
Heparin barrier coating for controlled drug release
ActiveUS20050004663A1Reduce frictional forceReduce forceSuture equipmentsStentsCompound (substance)Antioxidant
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations as well as other therapeutic agents may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:WYETH
Serum albumin binding peptides for tumor targeting
InactiveUS20050287153A1Altered pharmacodynamicsFacilitated DiffusionImmunoglobulins against blood coagulation factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthPeptide ligand
Peptide ligands having affinity for serum albumin are useful for tumor targeting. Conjugate molecules comprising a serum albumin binding peptide fused to a biologically active molecule demonstrate modified pharmacokinetic properties as compared with the biologically active molecule alone, including tissue (e.g., tumor) uptake, infiltration, and diffusion.
Owner:GENENTECH INC
Antibodies with altered effector functions
InactiveUS20050152894A1Good curative effectEffectively treating said mammalAntibacterial agentsSenses disorderDiseaseEffector functions
The invention provides antibodies with altered effector functions, and methods of using these antibodies in the treatment of various diseases. The invention further provides compositions, kits and articles of manufacture for practicing methods of the invention.
Owner:GENENTECH INC
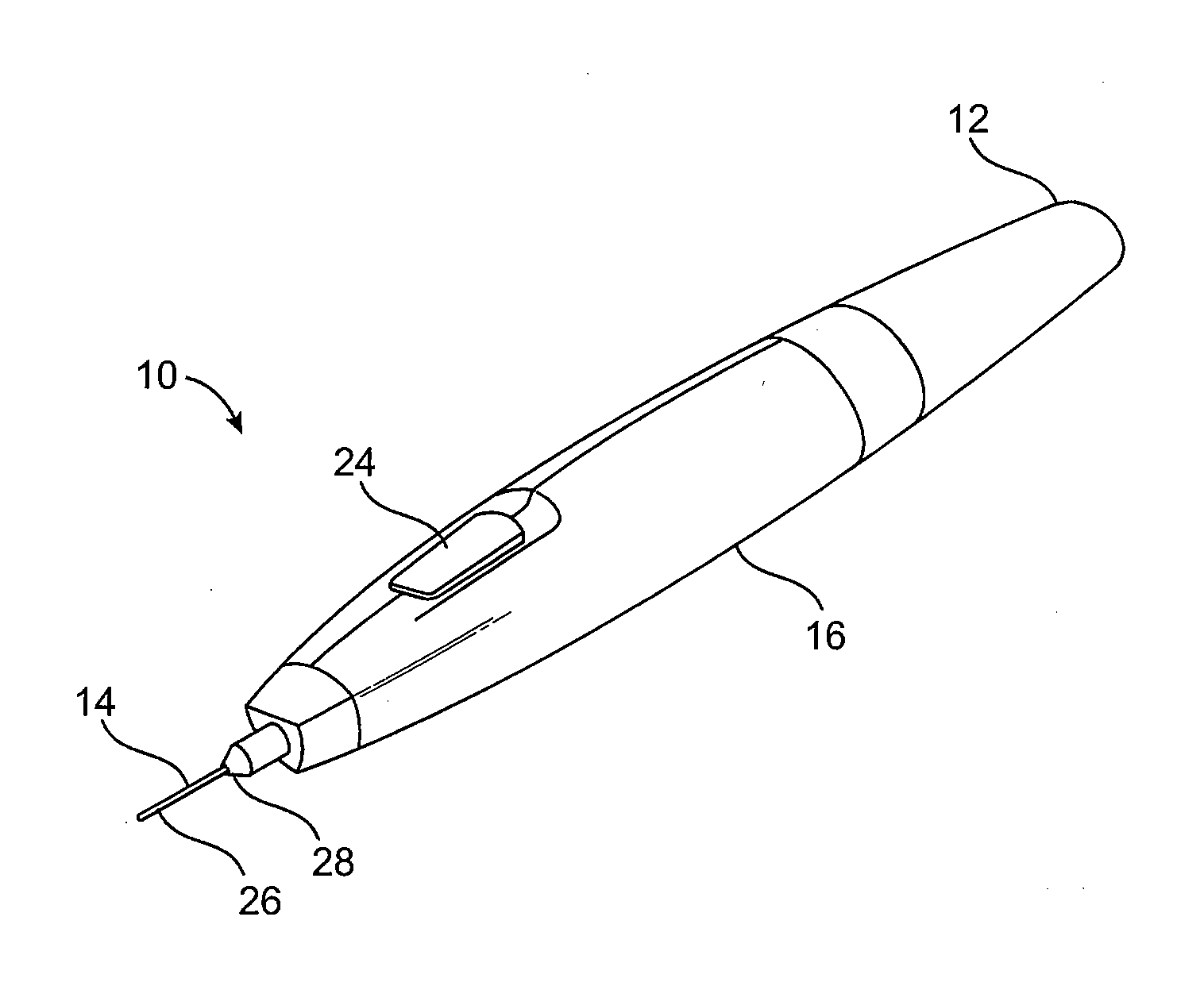
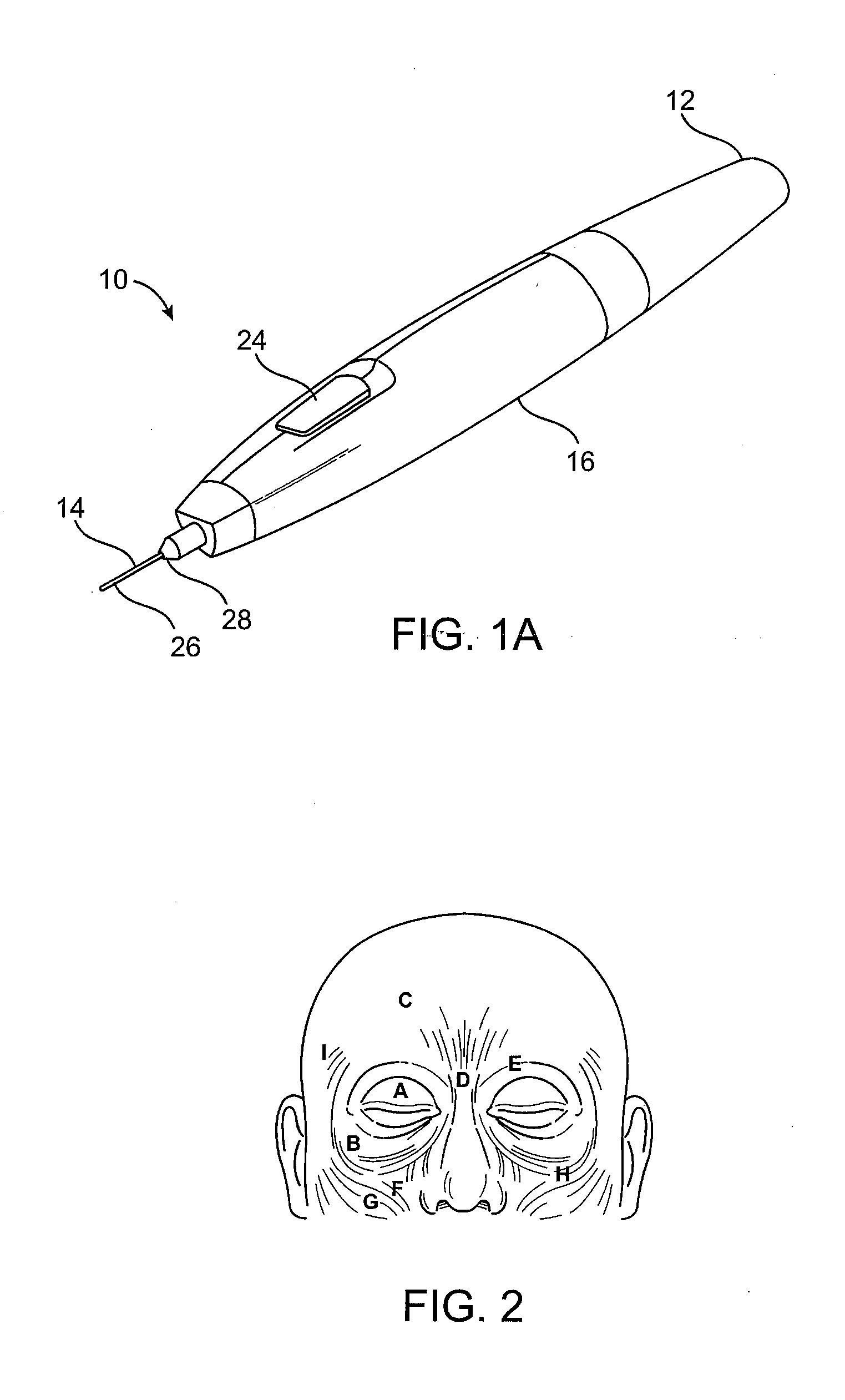
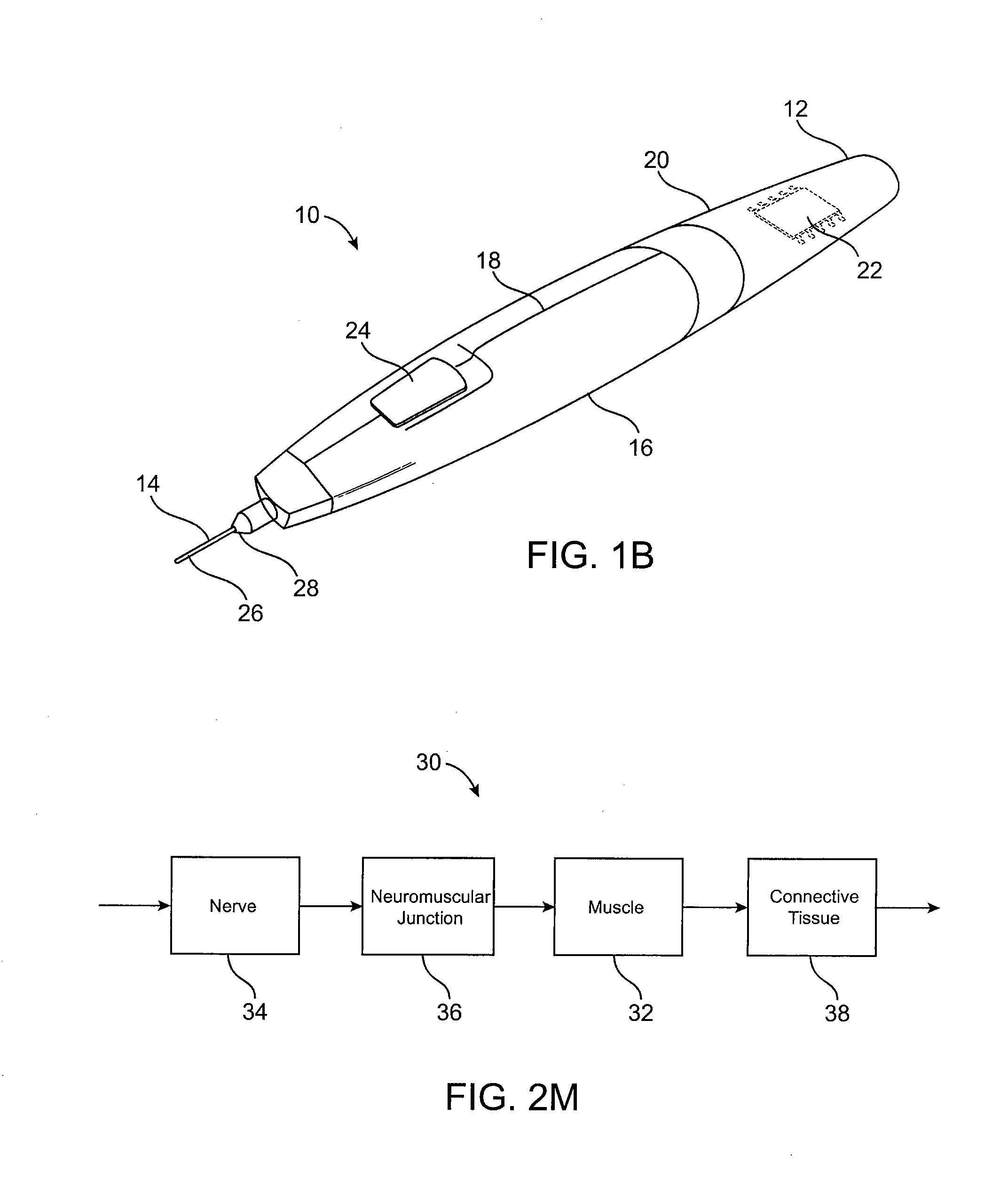
Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (FAT)
ActiveUS20080183164A1Reduce wrinklesAlter shapeDiagnostic recording/measuringSurgical instruments for heatingWrinkle skinMuscle contraction
Devices, systems, and methods treat cosmetic defects, and often apply cooling with at least one tissue-penetrating probe inserted through of the skin of a patient. The cooling may remodel one or more target tissue so as to effect a desired change in a composition of the target tissue and / or a change in its behavior. Exemplary embodiments of the cooling treatments will interfere with the nerve / muscle contractile function chain so as to mitigate wrinkles of the skin. Related treatments may be used therapeutically for treatment of back and other muscle spasms, chronic pain, and the like. Some embodiments may remodel subcutaneous adipose tissue so as to alter a shape or appearance of the skin surface.
Owner:PACIRA CRYOTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com