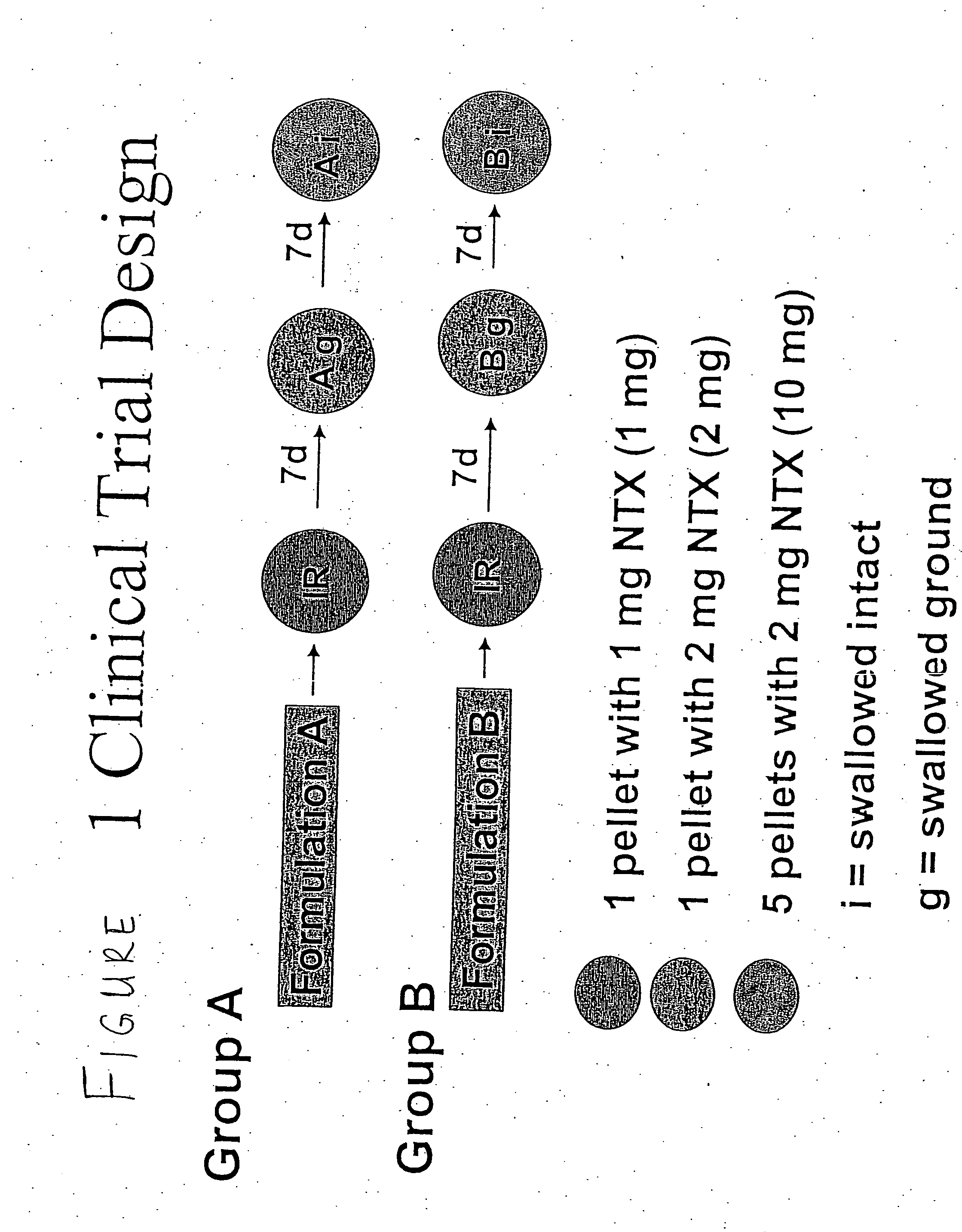
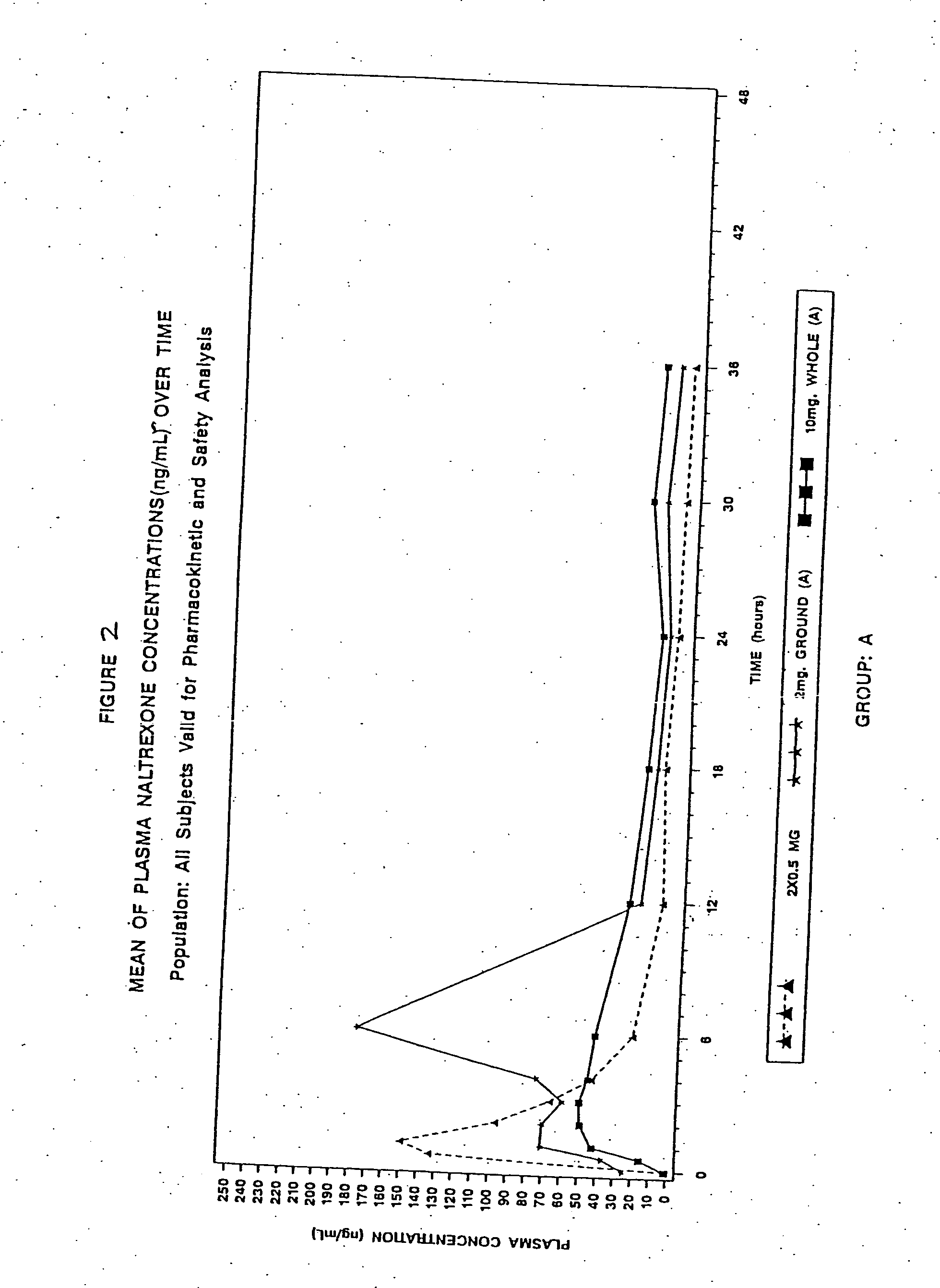
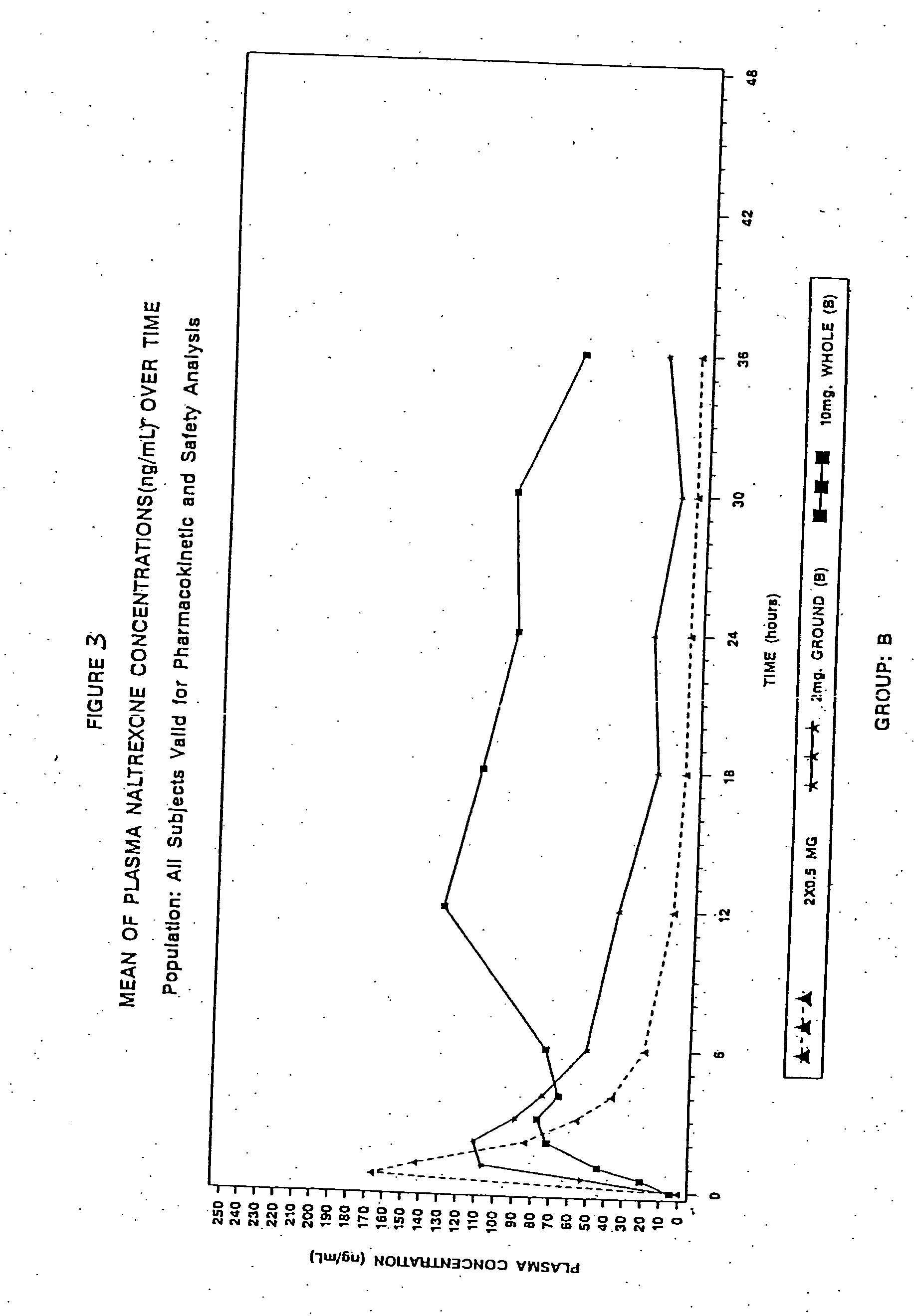
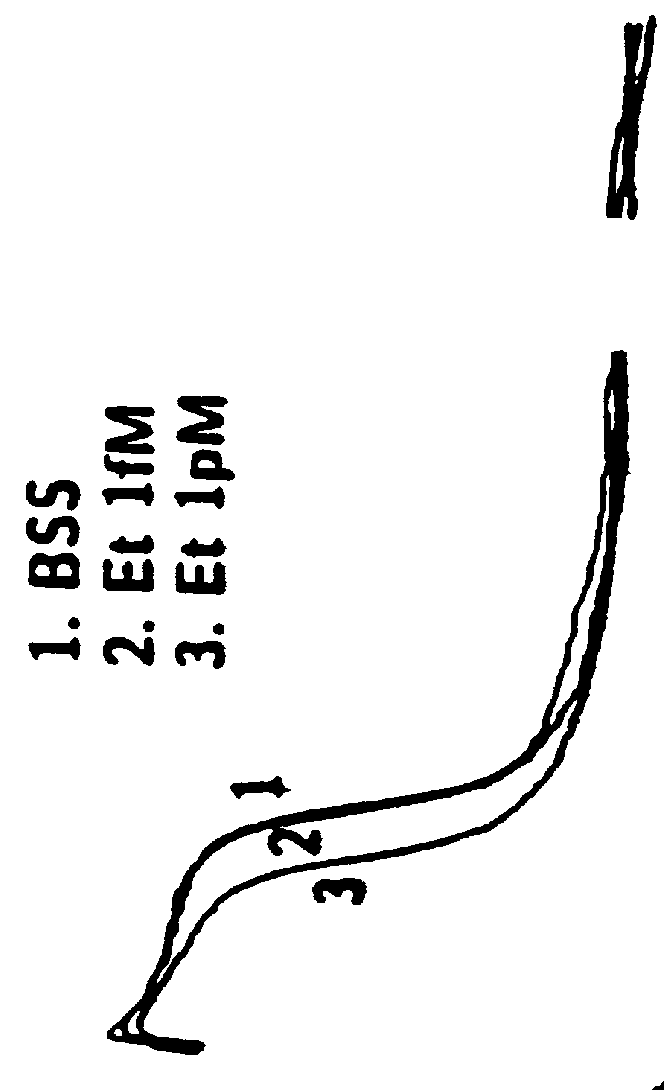
Patents
Literature
159 results about "Opioid Agonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
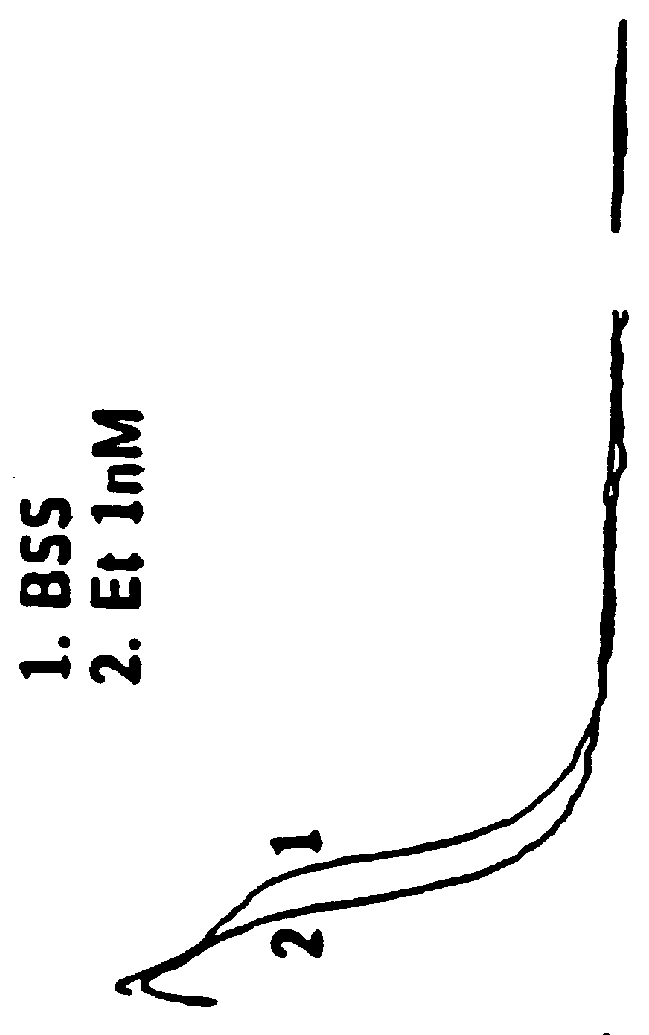
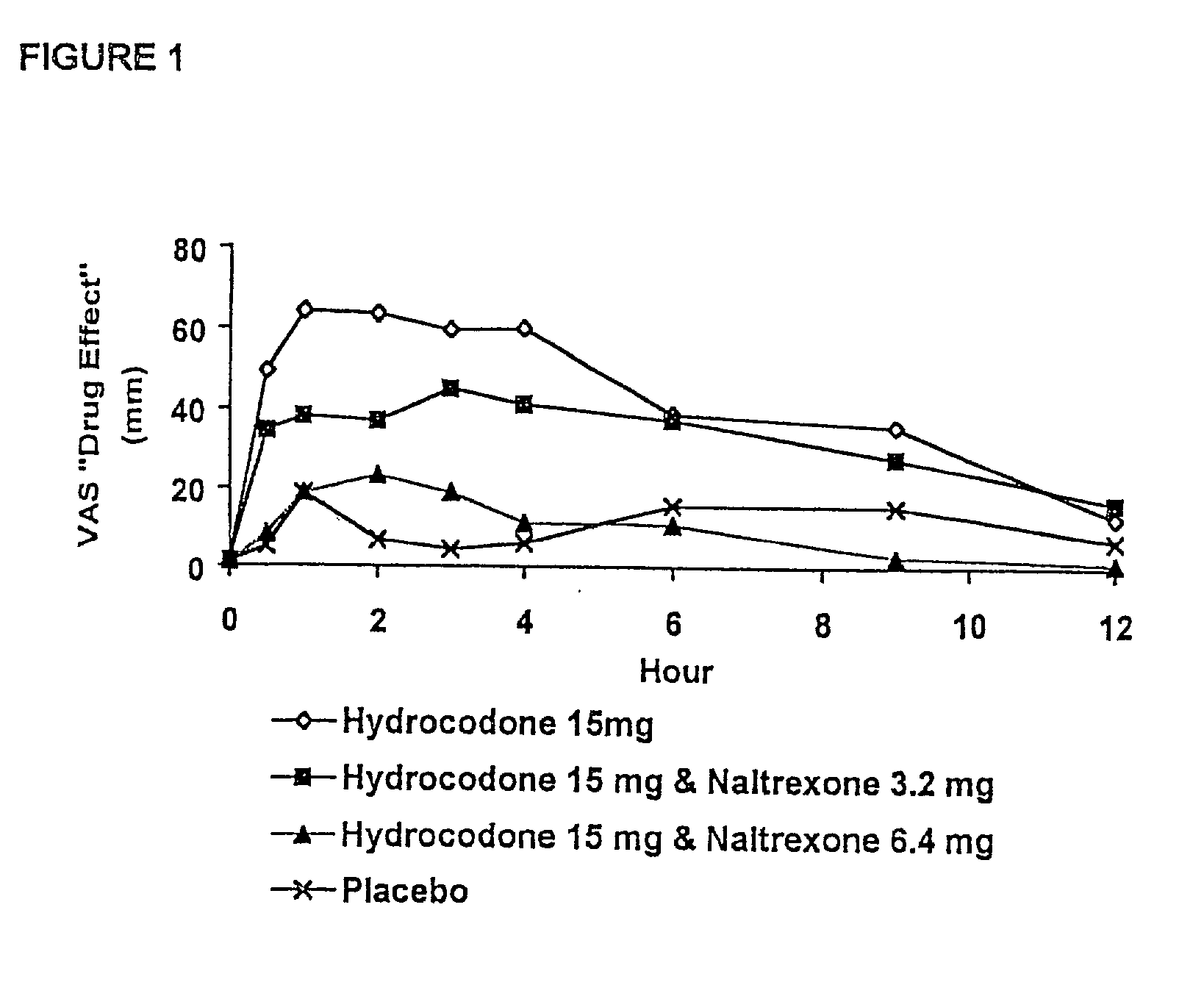
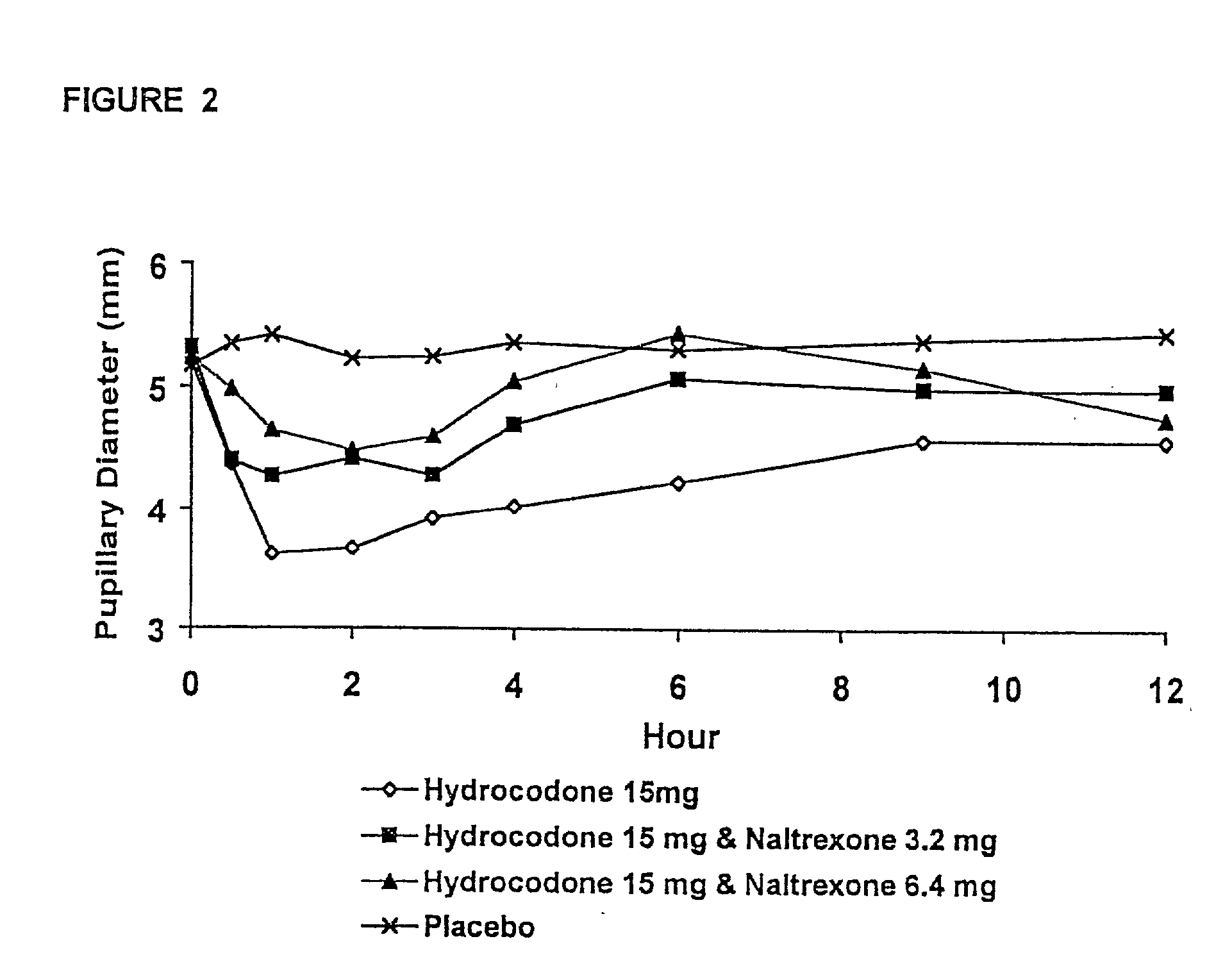
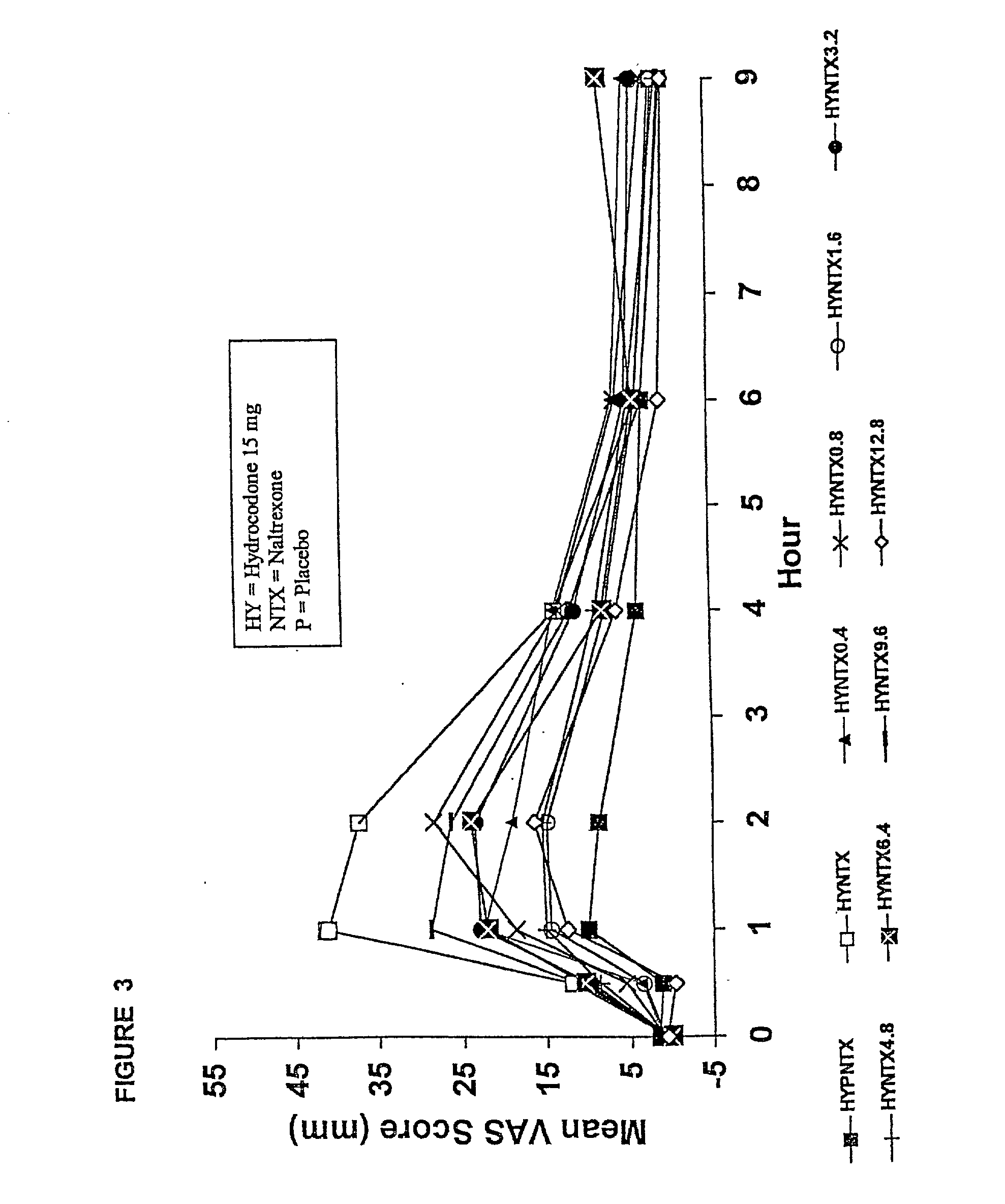
Responses (1) Opioid agonists bind to the opioid receptors and provide pain relief. Morphine is a pure opioid agonist whose principal therapeutic action is analgesia. Other members of the class known as opioid agonists include substances such as oxycodone, hydromorphone, fentanyl, codeine, and hydrocodone.
Method of preventing abuse of opioid dosage forms
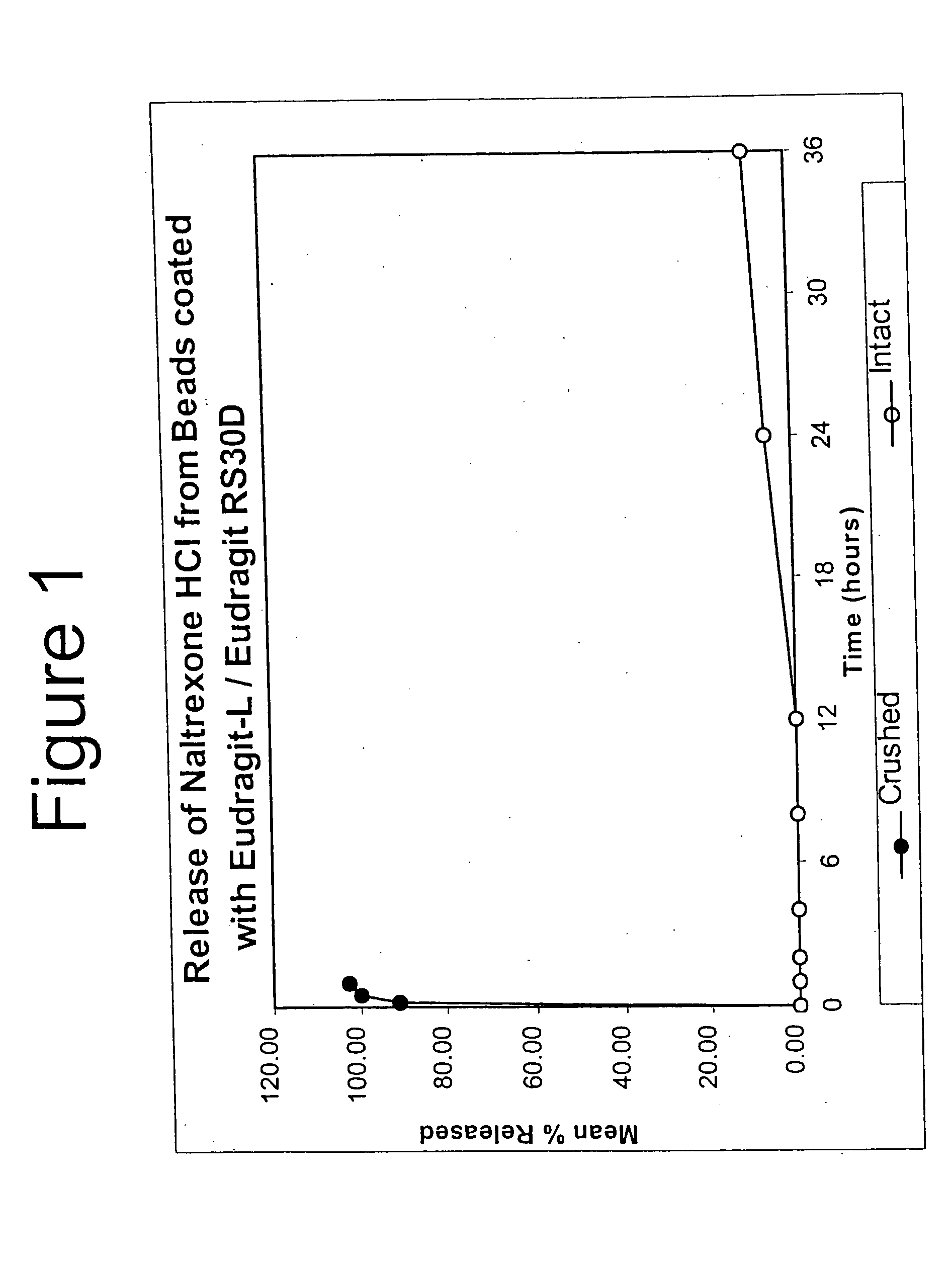
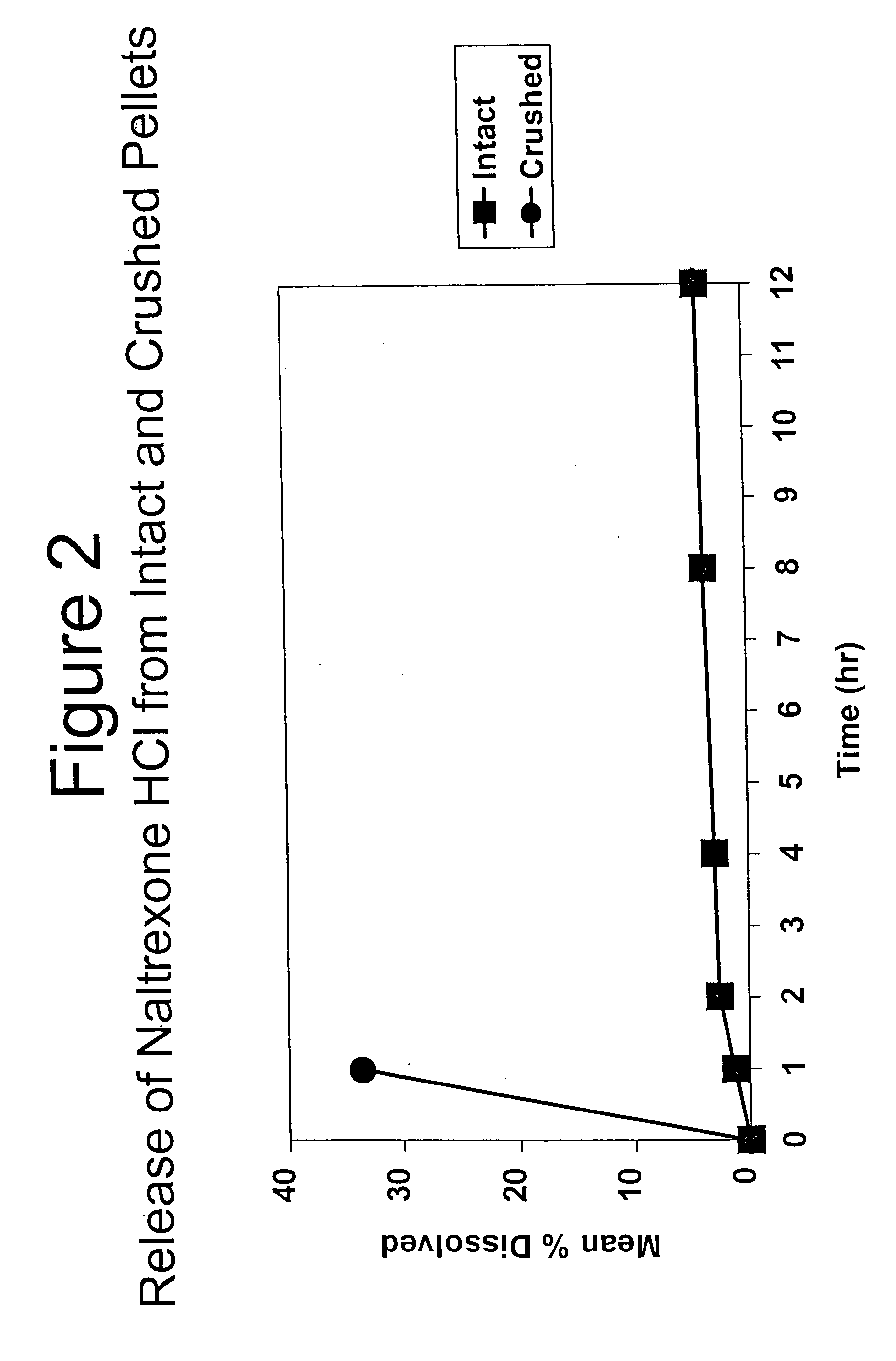
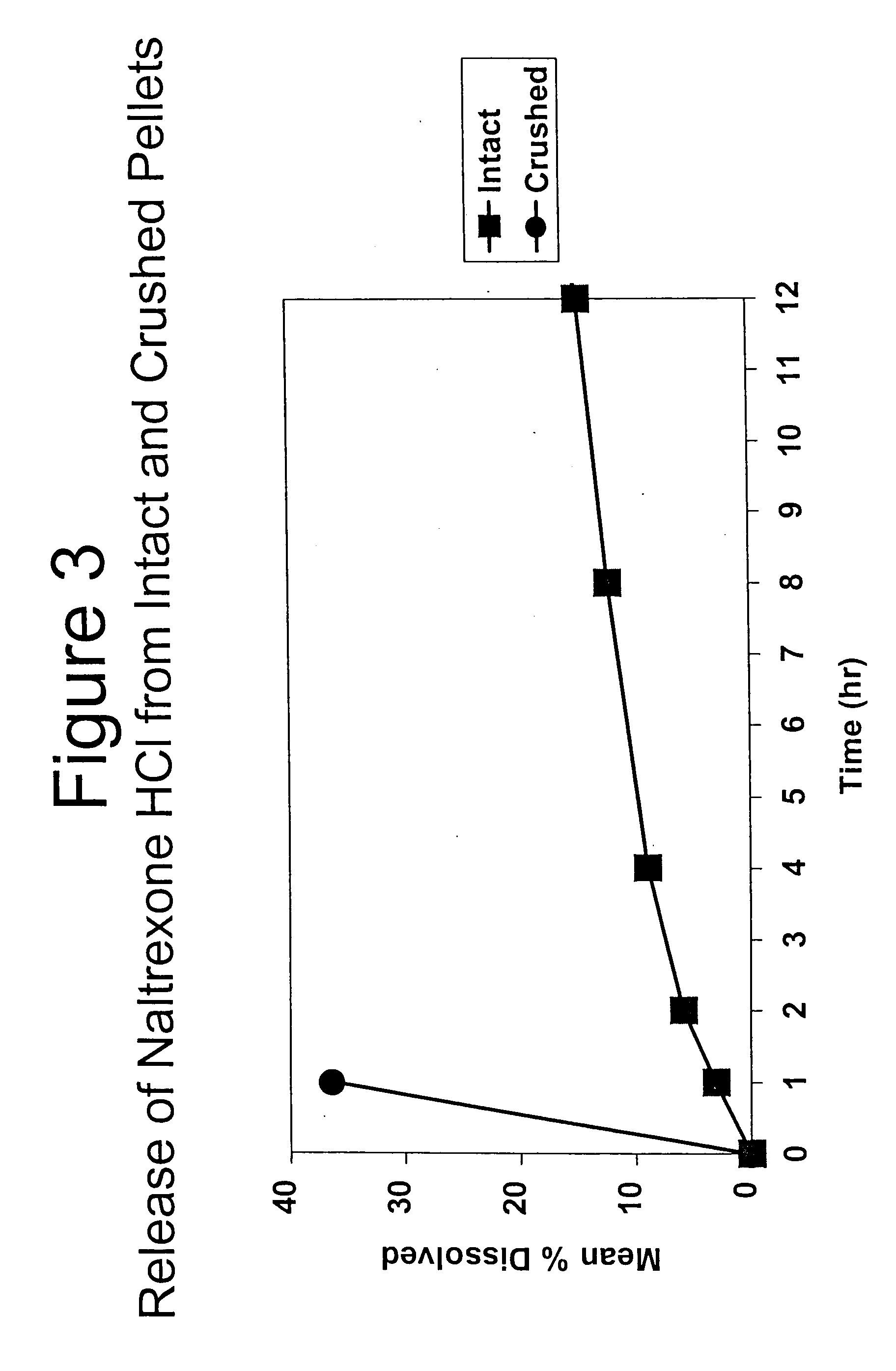
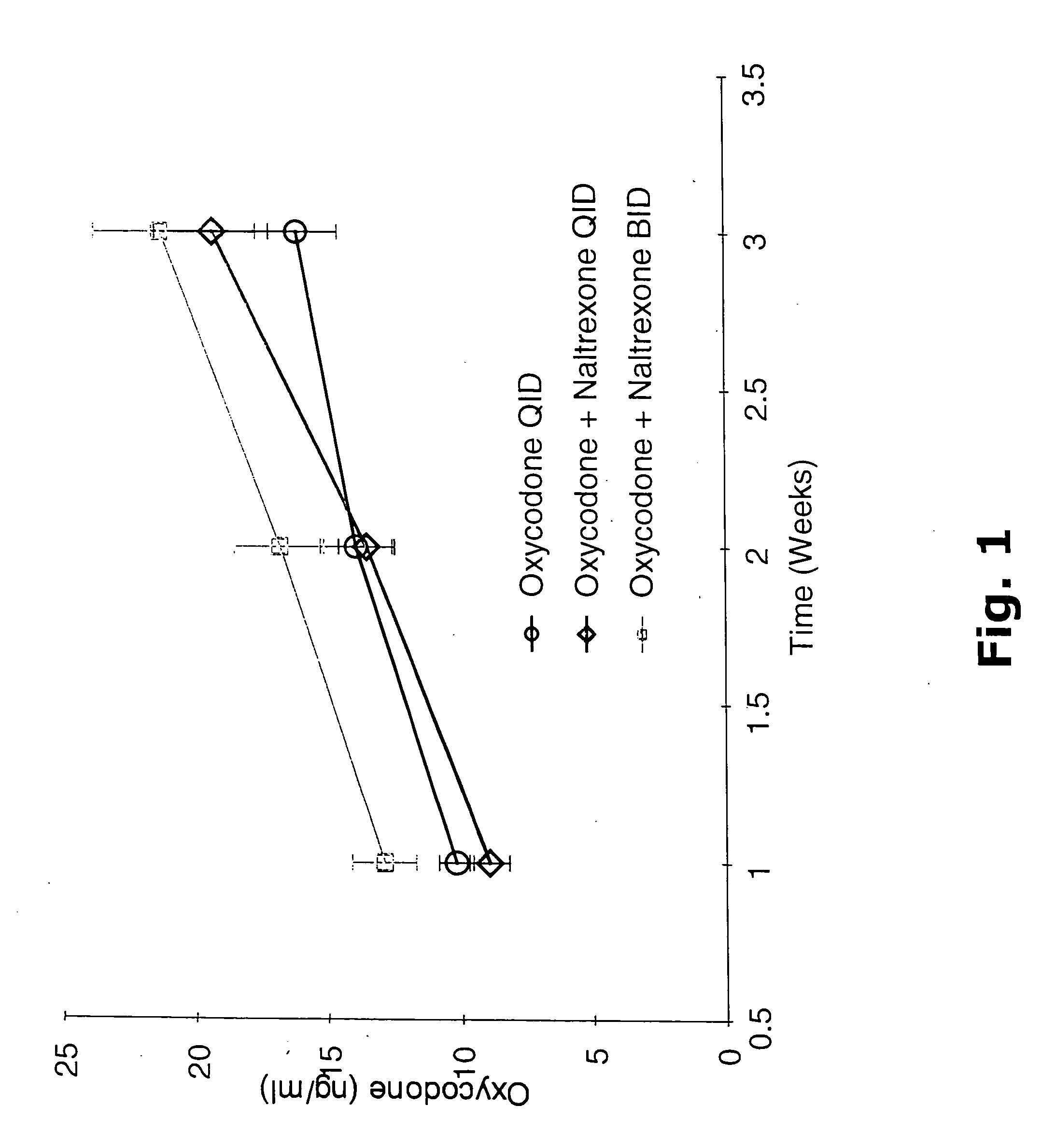
InactiveUS6228863B1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
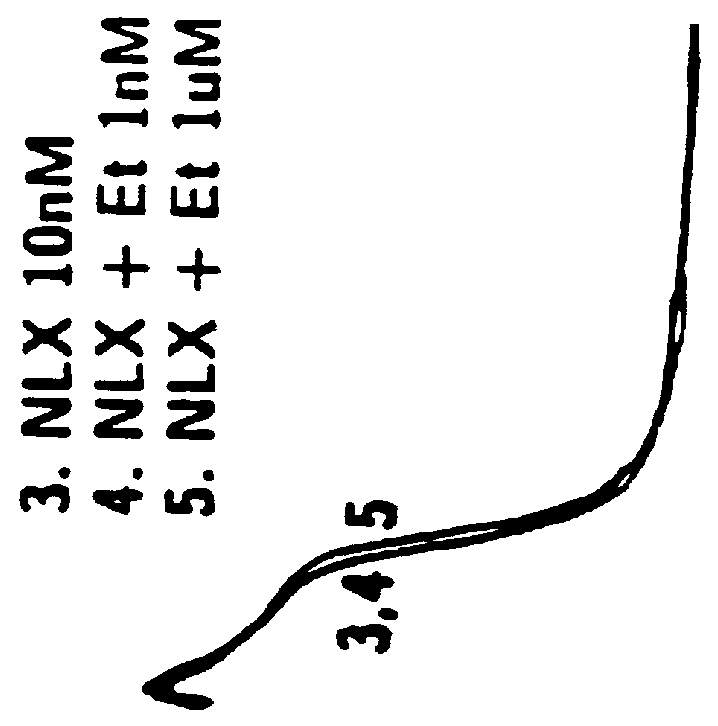
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:PURDUE PHARMA LP
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
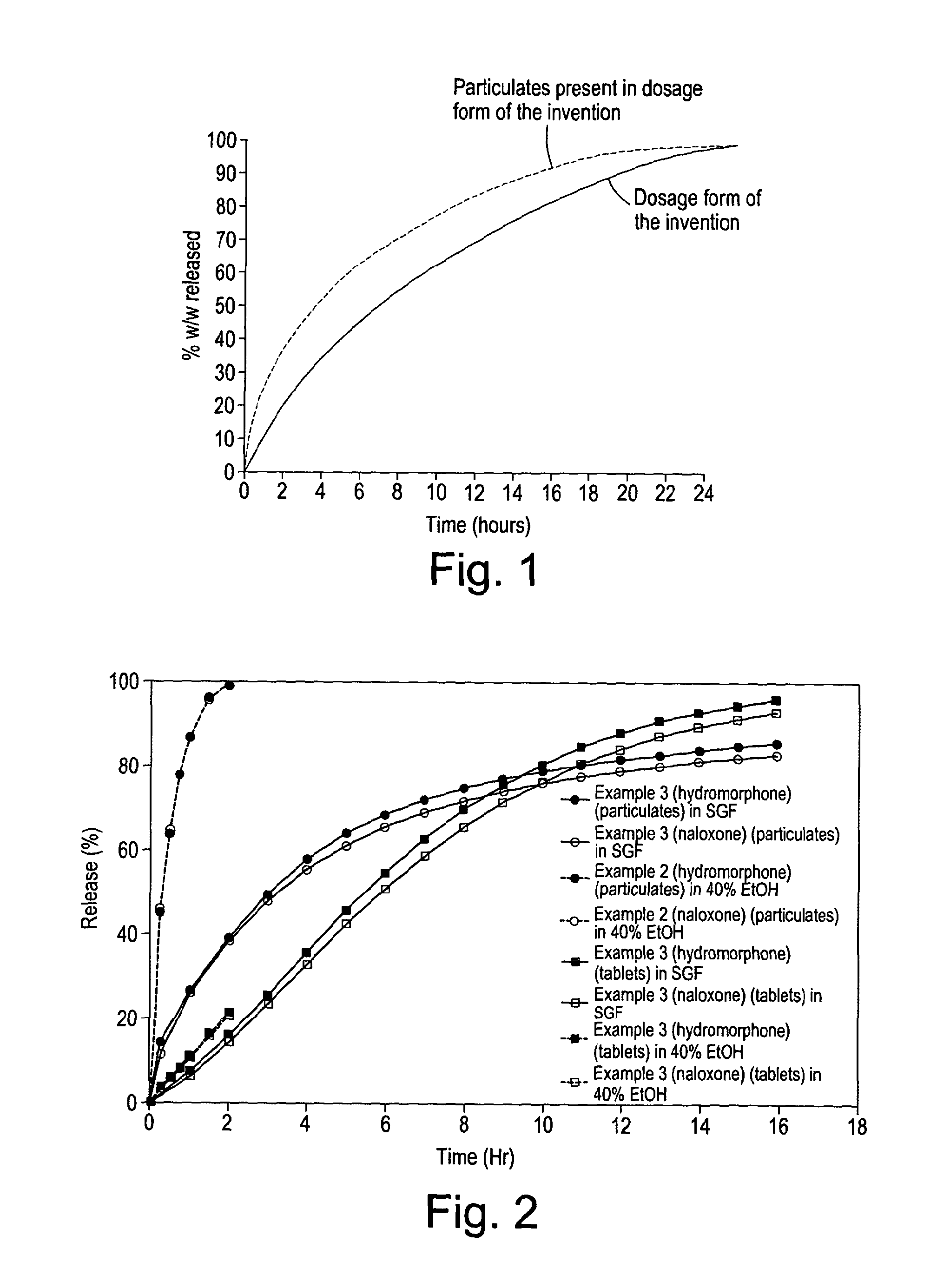
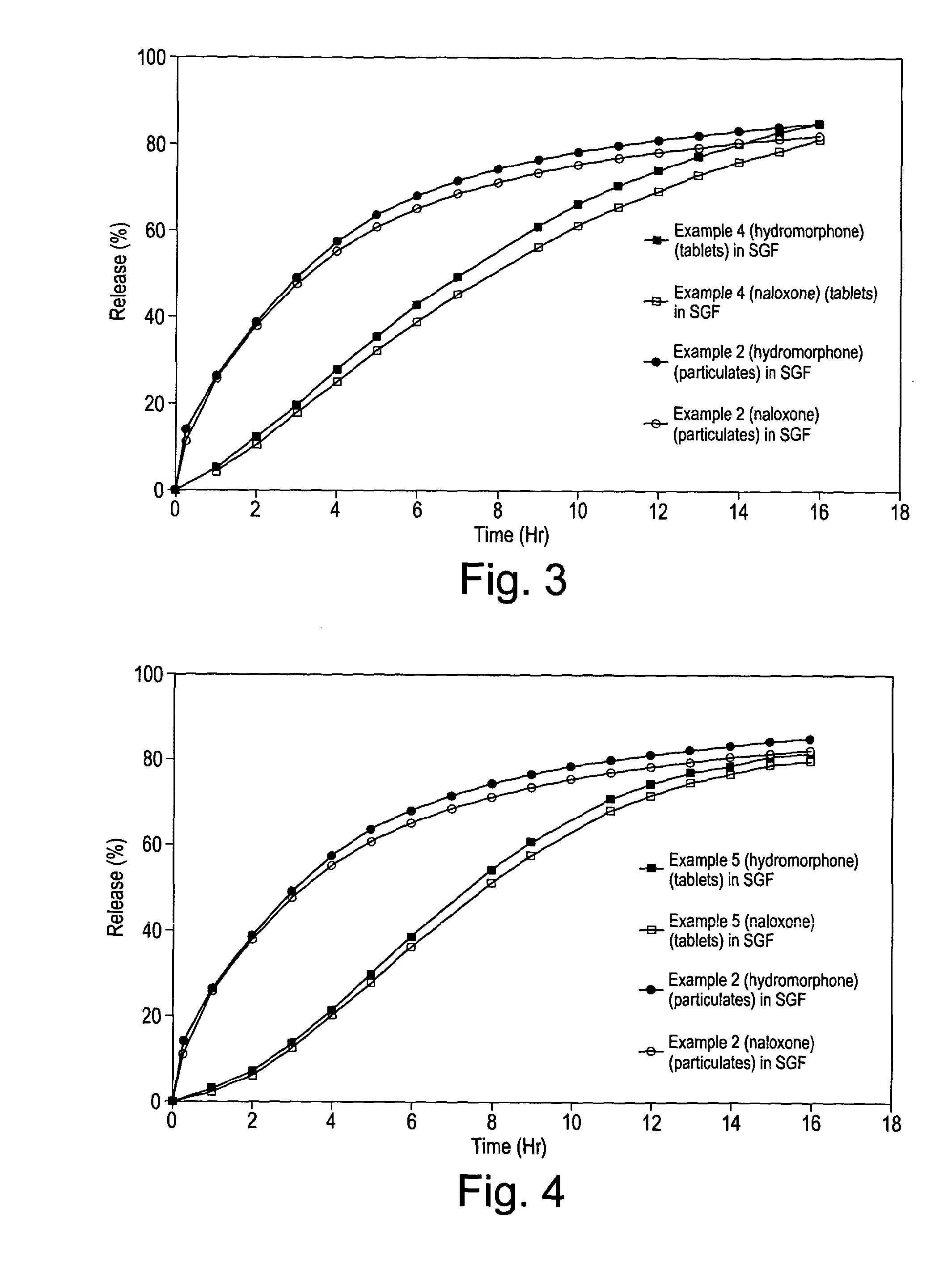
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS7144587B2Reducing abuse potential of dosage formLower potentialPowder deliveryPill deliveryOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Production of analgesic synergy by co-administration of sub-analgesic doses of a MU opioid agonist and a kappa-2 opioid agonist
InactiveUS6310072B1High analgesic potencyReduction tendencyBiocideNervous disorderOpioid AgonistCo administration
An analgesic composition is disclosed comprising a sub-analgesic dosage of a mu-opioid agonist, optionally in the form of a pharmaceutically acceptable salt, and a sub-analgesic dosage of a kappa2-opioid agonist, optionally in the form of a pharmaceutically acceptable salt. There is also disclosed a method for producing analgesia in humans and lower animals which comprises administering concurrently to a human or lower animal in need of such treatment an analgesic composition of the invention.
Owner:THE LYNX PROJECT
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
Multimodal Abuse Resistant and Extended Release Opioid Formulations
The present invention is in the field of oral, abuse resistant pharmaceutical compositions of opioid agonists, extended release pharmaceutical compositions of opioid agonists and extended release abuse resistant pharmaceutical compositions of opioid agonists and the use thereof. The present invention is also directed to extended release pharmaceutical compositions and the use thereof for preventing or minimizing the risk of abuse and / or toxicity from either intentional or unintentional tampering. The present invention is further directed at a method of preventing or minimizing the risk of abuse and / or toxicity from either intentional or unintentional tampering.
Owner:RELMADA THERAPEUTICS
Sequestered antagonist formulations
InactiveUS20060182801A1Lower potentialIncurs riskBiocidePowder deliveryOpioid antagonistOpioid Agonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the mean Cmax of the antagonist after single dose oral administration of the dosage form after tampering to the mean Cmax of antagonist after single dose oral administration of an intact dosage form is at least 1.5:1.
Owner:PURDUE PHARMA LP
Pain relief lollipop compositions and methods
InactiveUS20090191257A1Without pain and invasivenessFast pain reliefOrganic active ingredientsNervous disorderGabapentinOpioid Agonist
A pain relief lollipop comprises a candy matrix comprising (a) an opioid agonist, (b) an N-methyl-D-aspartate receptor antagonist different from the opioid agonist, (c) gabapentin, or a pharmaceutically acceptable salt thereof, and, optionally, a muscle relaxant, sedative, anxiolytic, and / or antidepressant. A patient can self-administer small amounts of the pain relief drug as needed by simply licking or sucking on the lollipop in response to his subjective experience of pain.
Owner:INNOVATIVE PHARMA
Method of simultaneously enhancing analgesic potency and attenuating dependence liability caused by exogenous and endogenous opioid agonists
InactiveUSRE36547E1Enhance analgesic potencyDecrease dependence liabilityCompound screeningBiocideEndogenous OpiatesNervous system
This invention relates to a method of selectively enhancing the analgesic potency of morphine and other clinically used bimodally-acting opioid agonists and simultaneously attenuating development of physical dependence, tolerance and other undesirable side effects caused by the chronic administration of said bimodally-acting opioid agonists comprising the co-administration of a bimodally-acting opioid agonist which activates both inhibitory and excitatory opioid receptor-mediated functions of neurons in the nociceptive (pain) pathways of the nervous system and an opioid receptor antagonist which selectively inactivates excitatory opioid receptor-mediated side effects. This invention also relates to a method of using excitatory opioid receptor antagonists alone to block the undesirable excitatory side effects of endogenous bimodally-acting opioid agonists which may be markedly elevated during chronic pain. This invention further relates to a method of long-term treatment of previously detoxified opiate, cocaine and alcohol addicts utilizing said excitatory opioid receptor antagonists, either alone or in combination with low-dose methadone, to prevent protracted physical dependence, and to compositions comprising an excitatory opioid receptor antagonist of the invention and a bimodally-acting opioid agonist.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant agent
InactiveUS20070014732A1Reducing abuse potential of dosage formLower potentialOrganic active ingredientsNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Compositions and methods for controlling abuse of medications
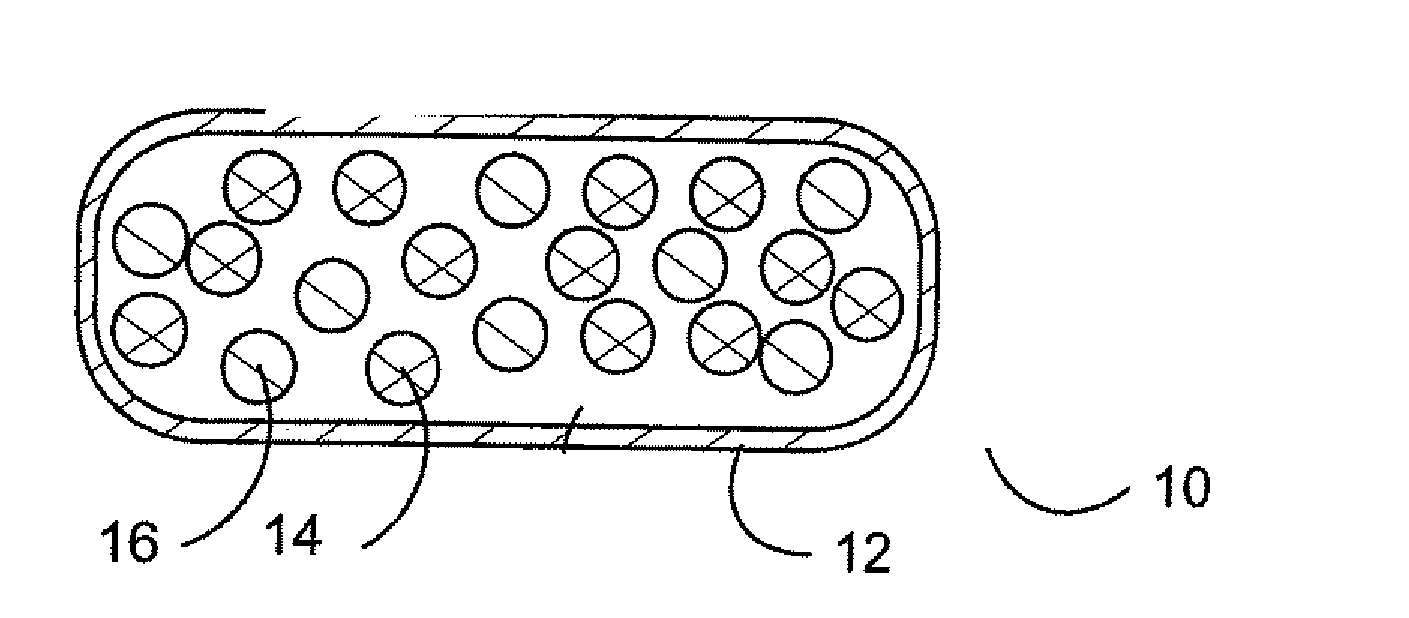
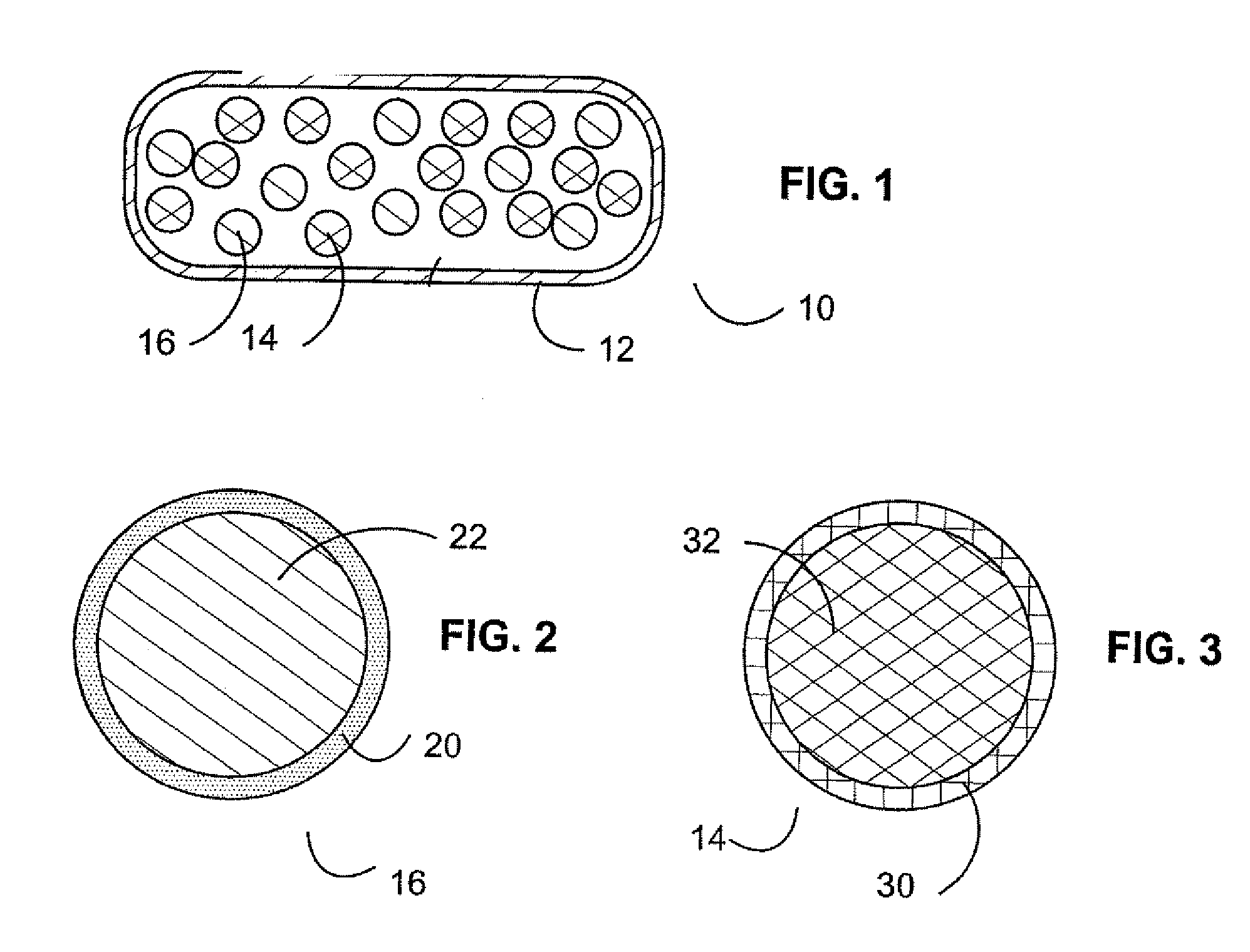
Pharmaceutical dosage forms are provided for use in deterring abuse of opioids or other medications, which help avoid harm to a patient dependent on the medication. In one case, a pharmaceutical oral dosage form is provided that includes a plurality of microcapsules, each microcapsule of the plurality containing an opioid agonist medication in a controlled release form, and a partial opioid agonist sequestered in the pharmaceutical dosage form, such that upon oral administration of the pharmaceutical oral dosage form the partial opioid agonist will pass through the gastrointestinal tract without uptake by the body.
Owner:PHARMORX THERAPEUTICS
Methods and materials for the treatment of pain comprising opioid antagonists
InactiveUS20050038062A1Enhance neuropathic pain-alleviating potencyEnhancing the potency of opioid agonistsBiocideNervous disorderOpioid AgonistOpioid antagonist
Methods and compositions for treating subjects with pain, including neuropathic pain, using opioid antagonists or combinations of opioid antagonists and opioid agonists, including, for example, wherein the amount of an opioid antagonist enhances the neuropathic pain-alleviating potency of an opioid agonist.
Owner:PAIN THERAPEUTICS INC
Opioid agonist/opioid antagonist/acetaminophen combinations
InactiveUS20020058673A1Reducing oral abuse potential of dosage formImprove subjective experienceBiocideDrug compositionsOpioid antagonistOpioid Agonist
The invention is directed in part to oral dosage forms comprising a combination of an opioid agonist, acetaminophen and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Tamper-resistant oral opioid agonist formulations
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Methods and materials useful for the treatment of arthritic conditions, inflammation associated with a chronic condition or chronic pain
InactiveUS20050245557A1Good pain reliefBetter pain controlBiocideAnimal repellantsOpioid AgonistOpioid antagonist
Methods and materials, including novel compositions, dosage forms and methods of administration, useful for treating arthritic conditions, inflammation associated with a chronic condition, and / or chronic pain, including pain from arthritis and inflammation, using opioid antagonists, including combinations of opioid antagonists and opioid agonists. Methods and materials comprising opioid antagonists or combinations opioid antagonists and agonists may optionally include one or more additional therapeutic agents.
Owner:PAIN THERAPEUTICS INC
Hydrophilic opioid abuse deterrent delivery system using opioid antagonists
InactiveUS20080075771A1Resistant to opioid abuseReduce abuse potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed herein are oral dosage forms of opioid therapeutic agents that are resistant to abuse and methods of their formulation. In particular, oral dosage forms that are resistant to dissolution in aqueous solutions of ethanol are described. The oral dosage forms may include one or more opioid antagonists that are sequestered from the opioid therapeutic agent such that the opioid antagonist has no substantial effect on the activity of the opioid therapeutic agent when the dosage form is taken orally as prescribed, but the opioid antagonist is released in an amount that reduces the effectiveness of the opioid therapeutic agent contained in the dosage form when the dosage form is crushed.
Owner:LAB INT
Abuse Resistant Opioid Transdermal Delivery Device Containing Opioid Antagonist Microspheres
InactiveUS20080233178A1Reduced potential for abuseLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Owner:PURDUE PHARMA LP
Tamper resistant lipid-based oral dosage form for opioid agonists
A tamper resistant drug delivery system made of at least one lipid, at least one gelling agent and at least one drug active, such as oxycodone, where the system gels rapidly in the presence of water or a solution containing water, and the drug active releases into the digestive system, wherein the weight ratio of gelling agent to lipid is less than 1:1.4.
Owner:SHEARKERSHMAN LAB
High drug loaded injectable microparticle compositions and methods of treating opioid drug dependence
InactiveUS7041320B1Reduce opioid consumptionRelieve symptomsPowder deliveryPharmaceutical non-active ingredientsOpioid AgonistMedicine
Owner:BIOTEK
Sequestered antagonist formulations
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the mean Cmax of the antagonist after single dose oral administration of the dosage form after tampering to the mean Cmax of antagonist after single dose oral administration of an intact dosage form is at least 1.5:1.
Owner:PURDUE PHARMA LP
Polymer conjugates of opioid antagonists
The invention provides polymer conjugates of opioid antagonists comprising a polymer, such as poly(ethylene glycol), covalently attached to an opioid antagonist. The linkage between the polymer and the opioid antagonist is preferably hydrolytically stable. The invention also includes a method of treating one or more side effects associated with the use of opioid analgesics, such as constipation, nausea, or pruritus, by administering a polymer conjugate of the invention.
Owner:NEKTAR THERAPEUTICS INC
Pharmaceutical Formulation Containing Opioid Agonist, Opioid Antagonist and Gelling Agent
InactiveUS20150031718A1Avoid abuseReduce releaseBiocidePharmaceutical non-active ingredientsOpioid antagonistPharmaceutical formulation
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Dosage Form
InactiveUS20130330409A1Improve stress resistanceSmall diameterPowder deliveryBiocideParticulatesSedative/hypnotic
The present invention provides a dosage form, particularly a tamper resistant dosage form, comprising; non-stretched melt extruded particulates comprising a drug selected from an opioid agonist, a tranquilizer, a CNS depressant, a CNS stimulant or a sedative hypnotic; and a matrix; wherein said melt extruded particulates are present as a discontinuous phase in said matrix.
Owner:EURO-CELTIQUE SA
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
Diversion-resistant opioid formulations
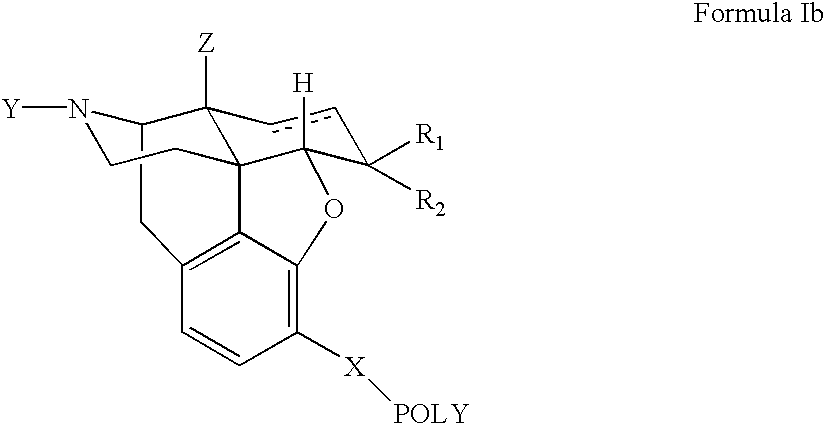
The present invention provides a composition comprising an opioid agonist, and a polymer-antagonist conjugate. The polymer-antagonist conjugate preferably does not hydrolyze upon administration to a patient, and does not bind to the opioid receptors. The covalent bond between the polymer and the antagonist in the conjugate is broken over a defined period of time to release the antagonist into the formulation. The released antagonist attenuates the liking of the agonist, thereby eliminating the incentive to the diversion of the medicines.
Owner:ELYSIUM THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com