Method of simultaneously enhancing analgesic potency and attenuating dependence liability caused by exogenous and endogenous opioid agonists
a technology of exogenous and endogenous opioid agonists and analgesic potency, which is applied in the direction of biocide, instruments, peptide/protein ingredients, etc., can solve the problems of anti-analgesic effects, hyperexcitability, hyperalgesia, and other undesirable side effects, and achieves a wide range of effects. , the effect of reducing the dependence liability of endogenous opioid agonists and enhancing the analgesic po
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
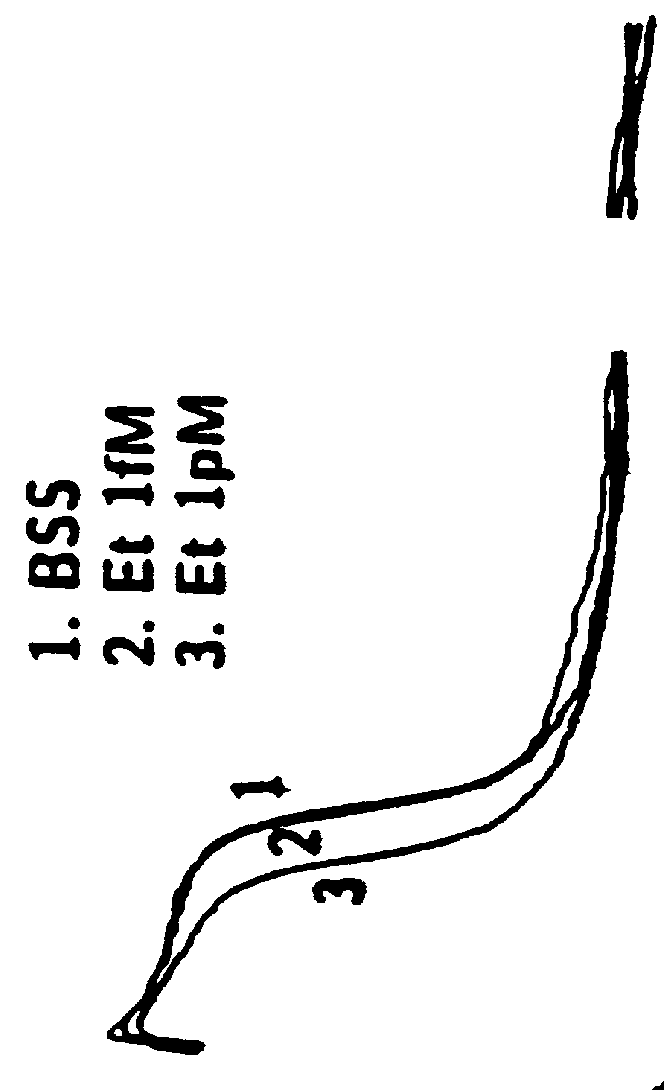
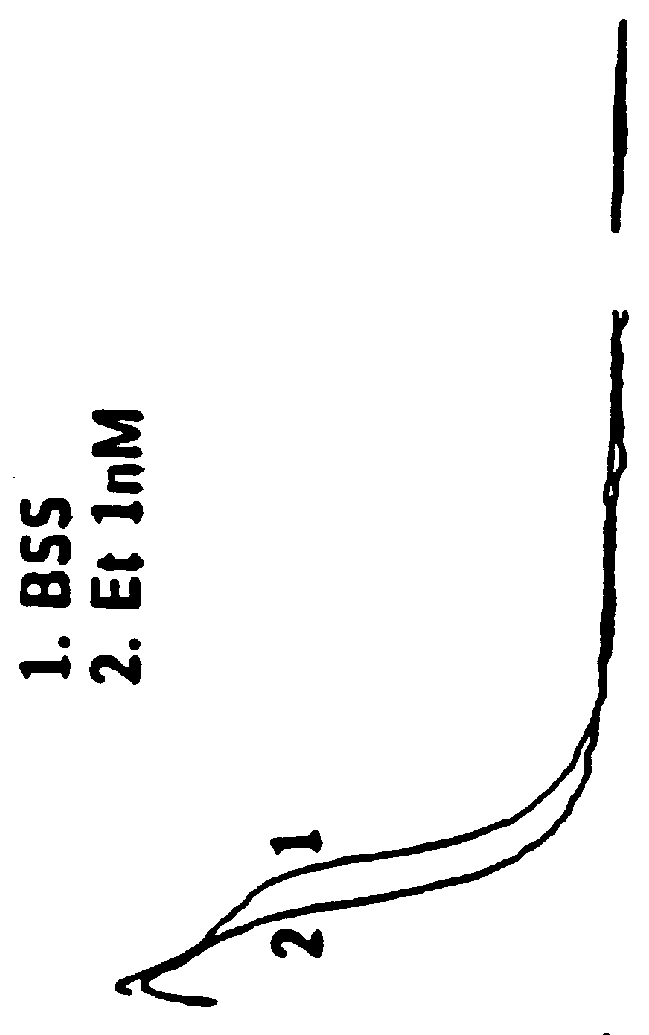
The effects of etorphine and dihydroetorphine on nociceptive types of DRG neurons in culture are described in Example 1. Etorphine and dihydroetorphine are the first compounds determined by the inventors by electrophysiologic analyses on DRG neurons to have specific antagonist action on excitatory opioid receptor functions when applied at ultra-low (pM) concentrations. This is in contrast to their well-known agonist action at inhibitory opioid receptors when applied at higher concentrations.
Etorphine and Dihydroetorphine Act as Potent Selective Antagonists at Excitatory Opioid Receptors on DRG Neurons Thereby Enhancing Inhibitory Effects of Bimodally-Acting Opioid Agonists
Methods (Used in This and Following Examples): The experiments described herein were carried out on dorsal root ganglion (DRG) neurons in organotypic explants of spinal cord with attached DRGs from 13-day-old fetal mice after 3 to 5 weeks of maturation in culture. The DRG-cord explants were grown on collagen-coated...
example 2
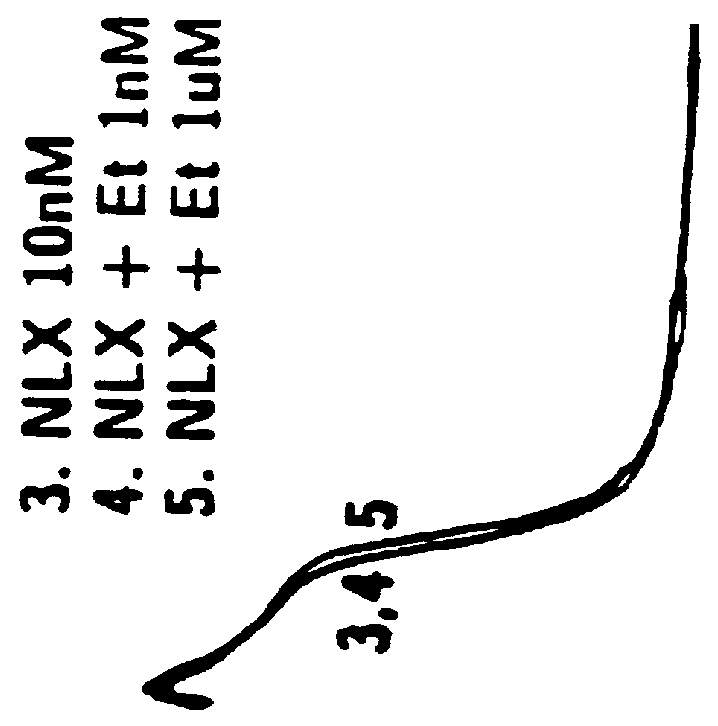
Diprenorphine, Naloxone and Naltrexone, at Low Concentrations, Show Potent Selective Antagonist Action at Excitatory Opioid Receptors
Drug tests: Mouse DRG-cord explants, grown for >3 weeks as described in Example 1, were tested with the opioid antagonists, diprenorphine, naltrexone and naloxone. Electrophysiological recordings were made as in Example 1.
Results: The opioid receptor antagonists naloxone and diprenorphine were previously shown to block, at nM concentrations, both inhibitory APD shortening of DRG neurons by .mu.M opioid agonists as well as excitatory APD prolongation by nM opioids. Tests at lower concentrations have revealed that pM diprenorphine, as well as pM naloxone or naltrexone, act selectively as antagonists at mu, delta and kappa excitatory opioid receptors, comparable to the antagonist effects of pM etorphine and dihydroetorphine. In the presence of pM diprenorphine, morphine (n=7) and DAGO (n=7) no longer elicited APD prolongation at low (pM-nM) concentrations...
example 3
Chronic Co-treatment of DRG Neurons with Morphine and Ultra-low-dose Naloxone or Naltrexone Prevents Development of Opioid Excitatory Supersensitivity ("Dependence") and Tolerance
Co-administration of ultra-low (pM) concentrations of naloxone or naltrexone during chronic treatment of DRG neurons with .mu.M levels of morphine was effective in preventing development of opioid excitatory supersensitivity and tolerance which generally occurs after sustained exposure to bimodally-acting opioids. Acute application of fM dynorphin A-(1-13) or fM morphine (n=21), as well as 1 nM naloxone (n=11), to DRG neurons chronically exposed to 1 .mu.M morphine together with 1 pM naloxone or naloxone or naltrexone (for 1-10 weeks) did not evoke the usual excitatory APD prolongation observed in chronic morphine-treated cells tested after washout with BSS (see FIG. 6). Furthermore, there was no evidence of tolerance to the usual inhibitory effects of .mu.M opioids (n=6) (FIG. 6).
These results are consonan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com