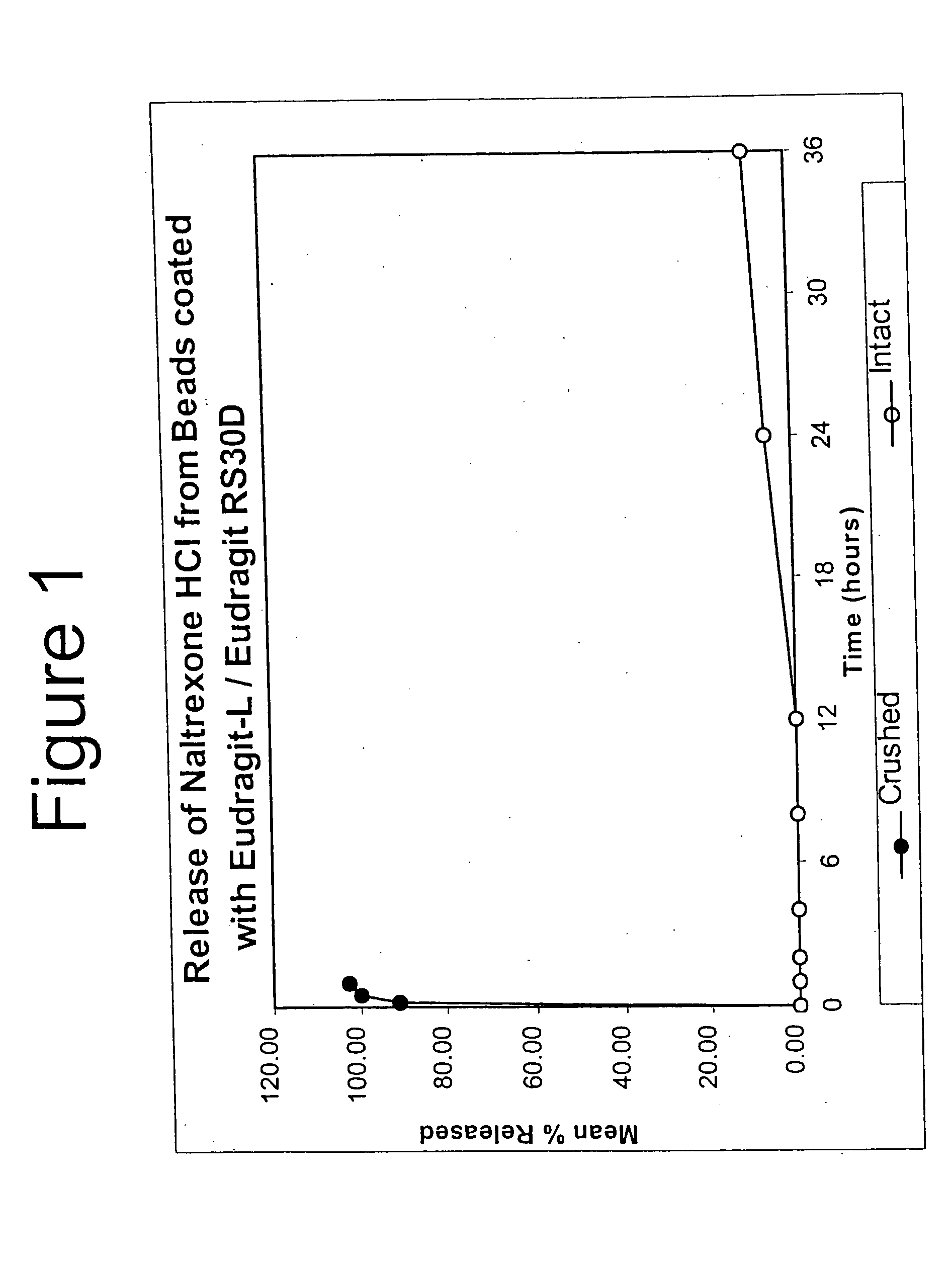
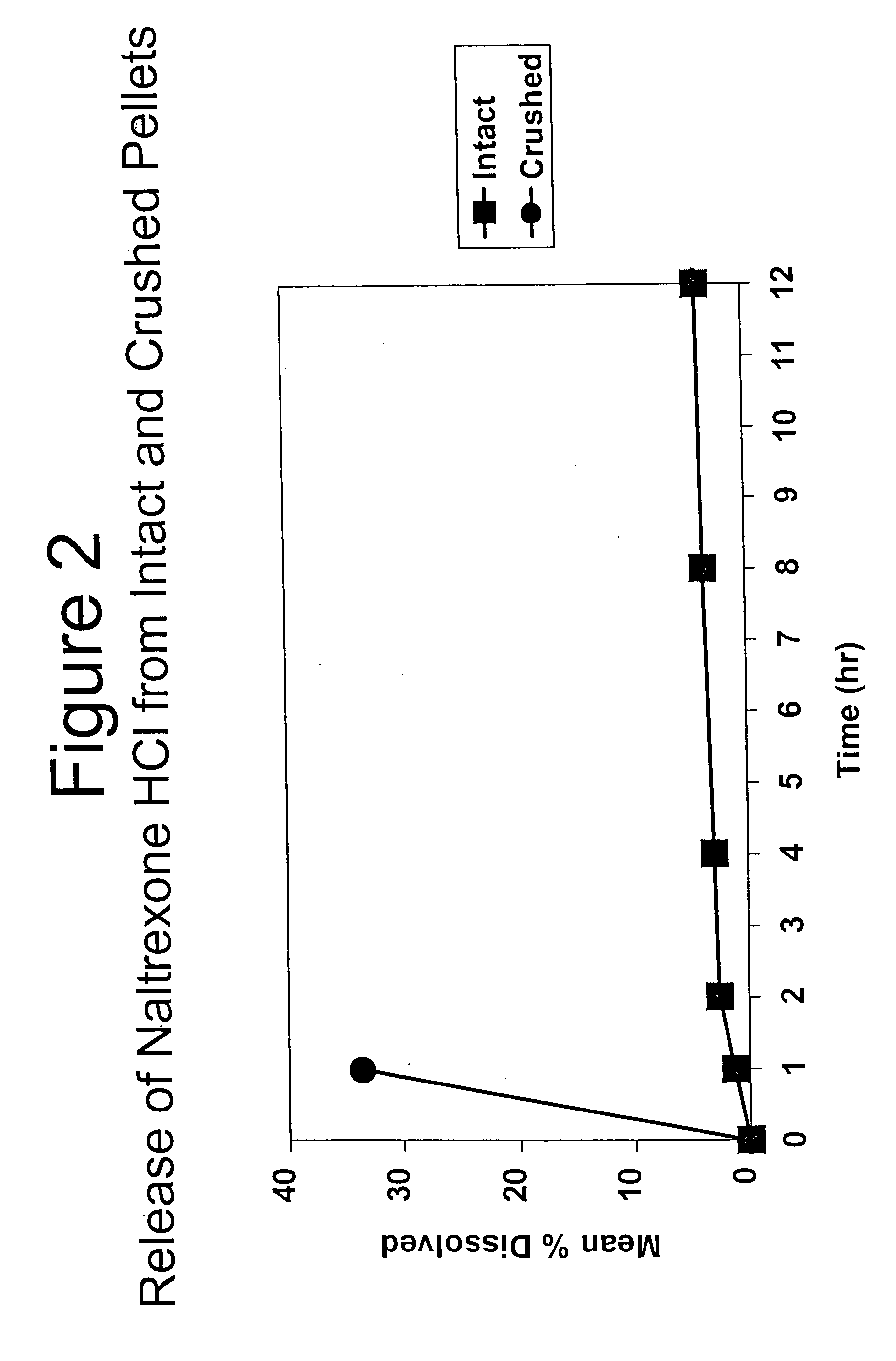
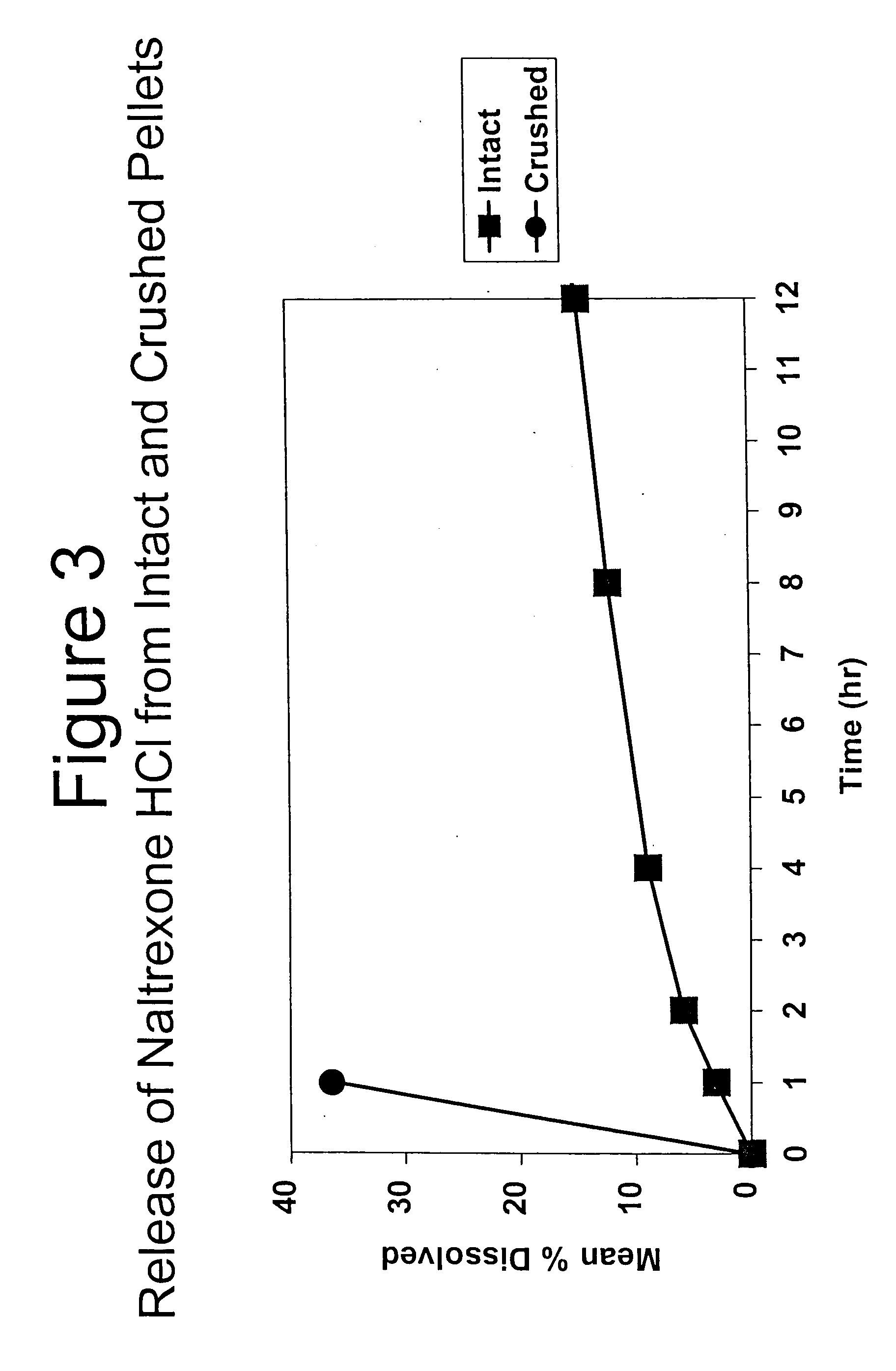
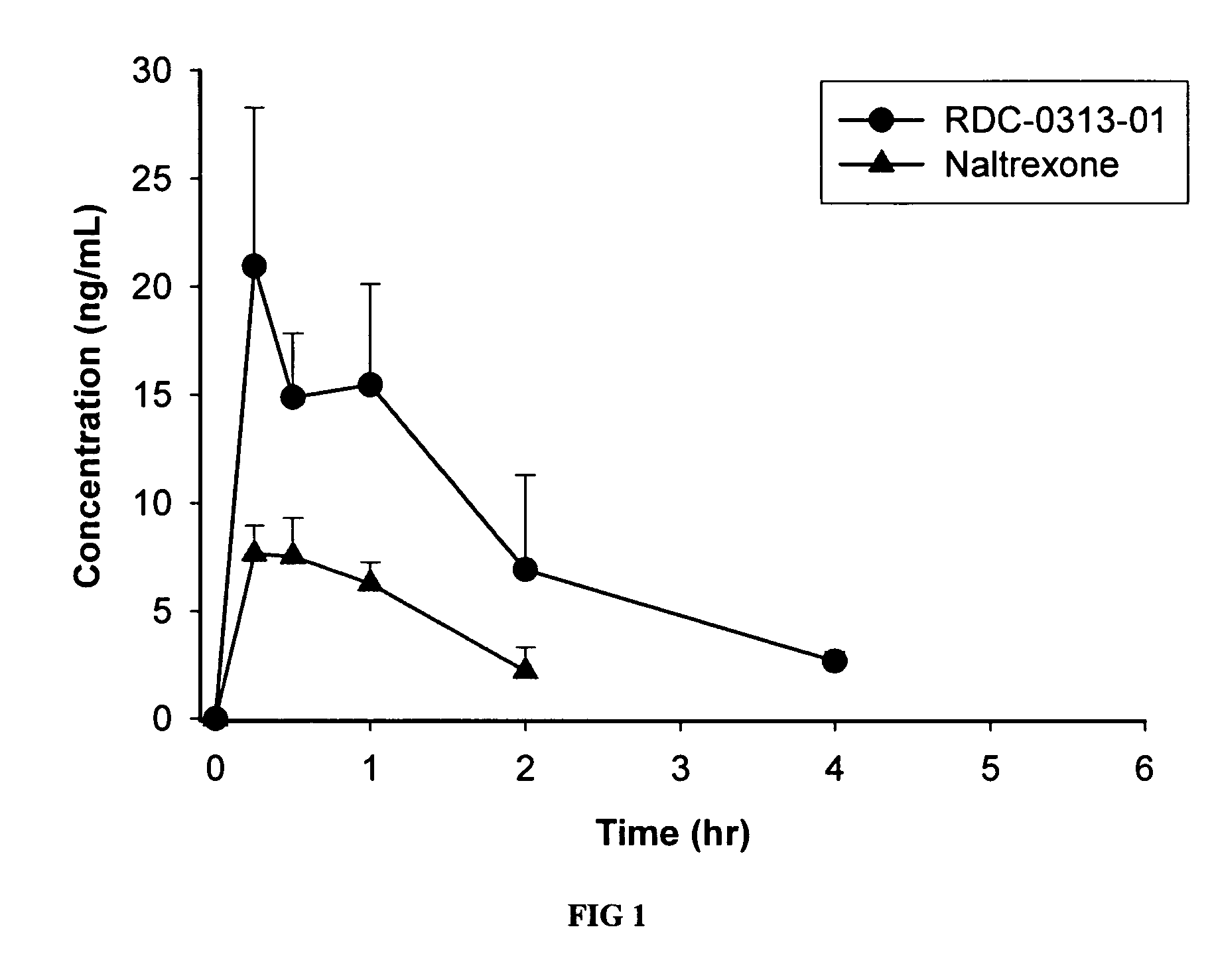
Patents
Literature
200 results about "Opioid antagonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
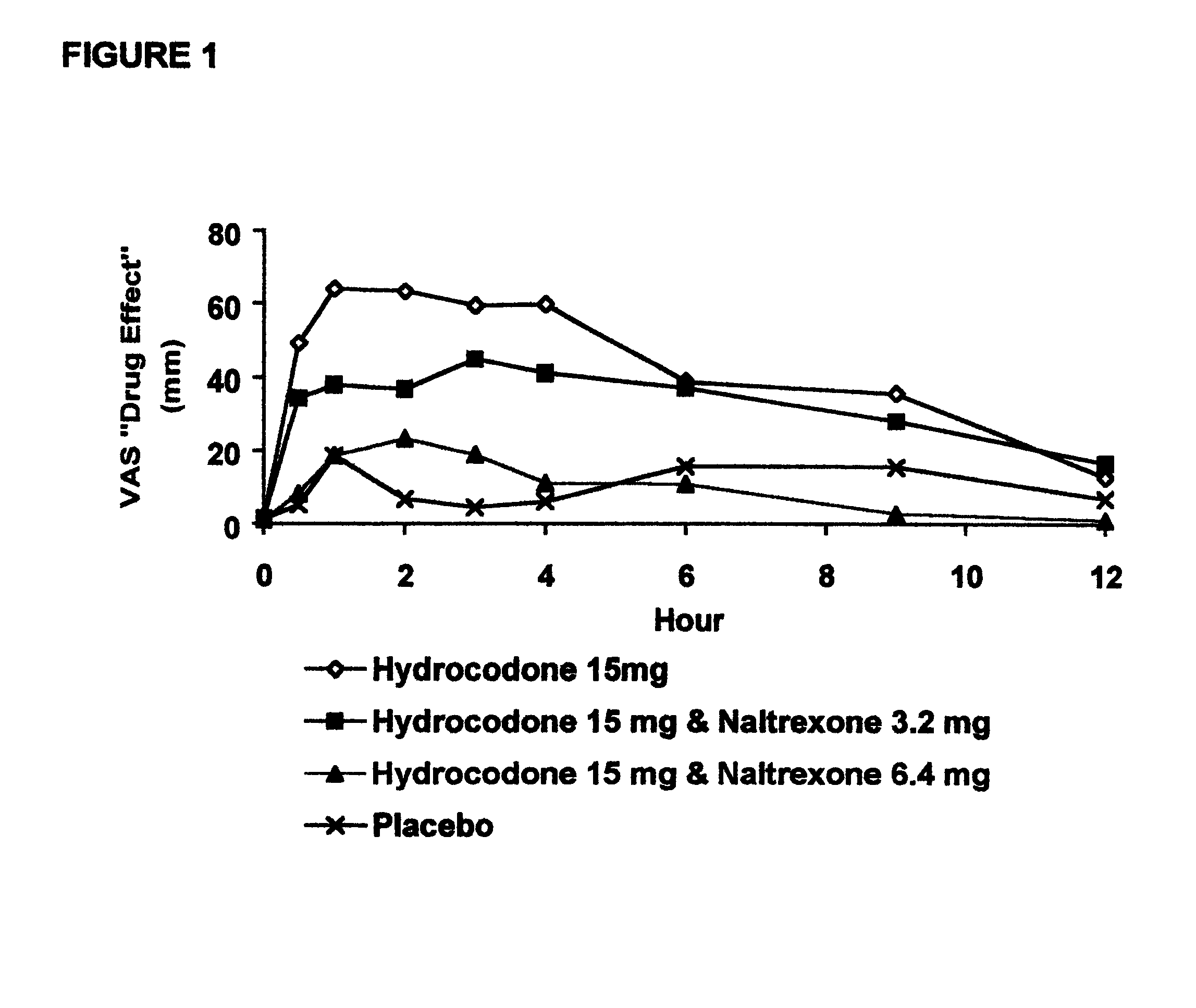
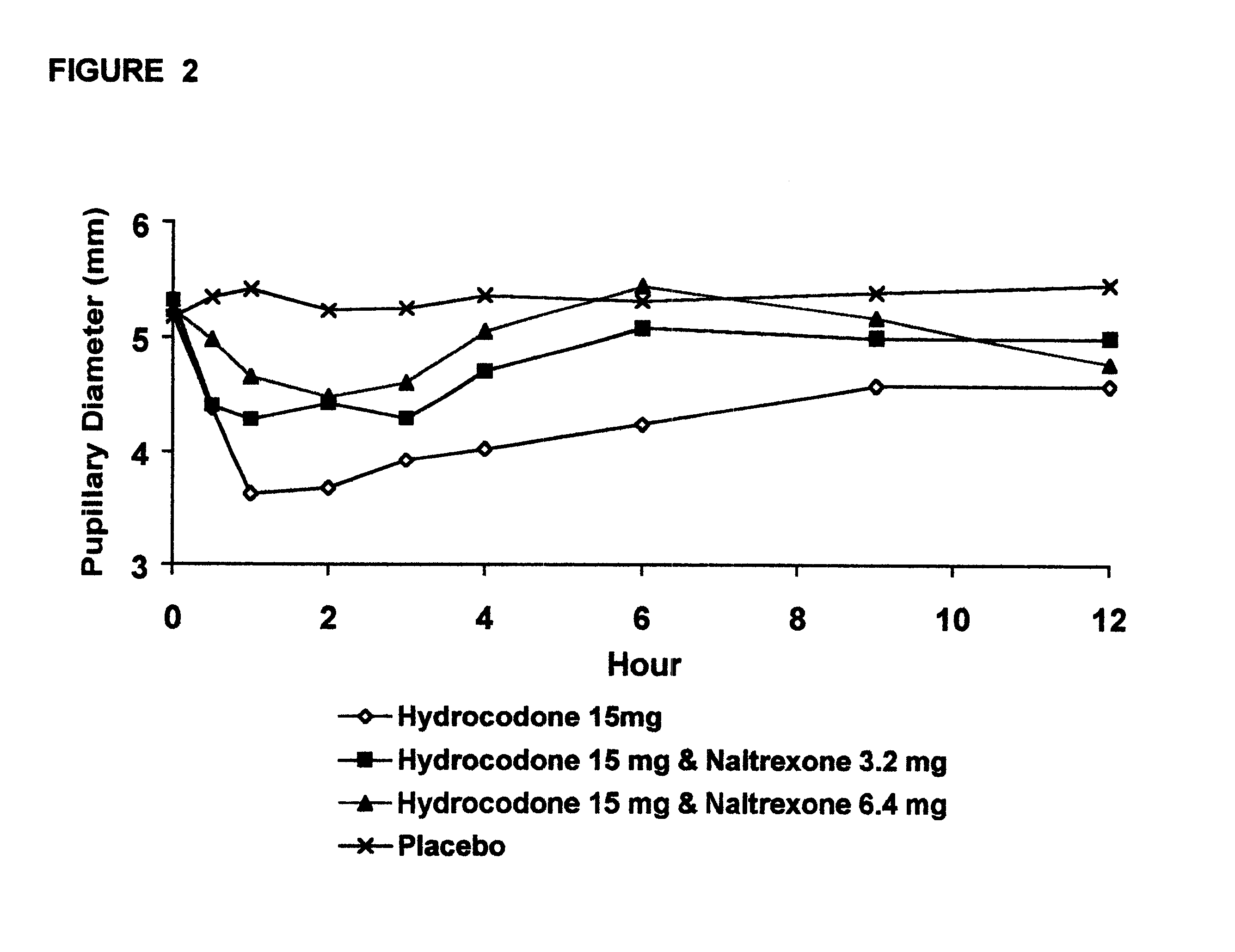
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors. Naloxone and naltrexone are commonly used opioid antagonist drugs which are competitive antagonists that bind to the opioid receptors with higher affinity than agonists but do not activate the receptors. This effectively blocks the receptor, preventing the body from responding to opioids and endorphins.
Method of preventing abuse of opioid dosage forms
InactiveUS6228863B1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:PURDUE PHARMA LP
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Autonomous drug delivery system
An autonomous drug delivery system advantageously utilizes physiological monitor outputs so as to automatically give a bolus of a rescue drug or other necessary medication when certain criteria and confidence levels are met. An emergency button is provided to manually trigger administration of the rescue drug. The rescue drug may be an opioid antagonist in response to an analgesia overdose, a hypotensive drug to avert an excessive drop in blood pressure or an anti-arrhythmia drug to suppress abnormal heartbeats, to name a few.
Owner:MASIMO CORP
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS7144587B2Reducing abuse potential of dosage formLower potentialPowder deliveryPill deliveryOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Method for treating emotional or mental illness and emotional or mental illness concomitant with seizures
Disclosed herein is a method for treating depression associated with alcoholism in a patient comprising administering to the patient a pharmacologically effective dose of an opioid antagonist, and a pharmacologically effective dose of at least one drug compound selected from the group consisting of a tricyclic antidepressant, an a-typical antidepressant, and lithium.
Owner:DANTE LEE G
Use of methylnaltrexone to treat irritable bowel syndrome
Methods of treating irritable bowel syndrome with peripheral opioid antagonists, such as methylnaltrexone, are provided. Formulations comprising peripheral opioid antagonists, such as methylnaltrexone, and irritable bowel syndrome therapeutic agents are also provided.
Owner:PROGENICS PHARMA INC
Abuse-resistant controlled-release opioid dosage form
InactiveUS20030065002A1High oral : parenteral potency ratioEliminate the effects ofBiocideNervous disorderControlled releaseOpioid antagonist
Abuse-resistant, controlled release opioid tablets are a combination containing an opioid antagonist such as naloxone at a level that needed to suppress the euphoric effect of the opioid, if the combination were crushed to break the controlled release properties causing the opioid and opioid antagonist to be released as a immediate release product as a single dose. The controlled release nature of the table prevents the accumulation of orally effective amounts of opioid antagonist when taken normally. The opioid antagonist is contained in a controlled-release matrix and released, over time, with the opioid.
Owner:PURDUE PHARMA LP
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
Sequestered antagonist formulations
InactiveUS20060182801A1Lower potentialIncurs riskBiocidePowder deliveryOpioid antagonistOpioid Agonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the mean Cmax of the antagonist after single dose oral administration of the dosage form after tampering to the mean Cmax of antagonist after single dose oral administration of an intact dosage form is at least 1.5:1.
Owner:PURDUE PHARMA LP
Smoking cessation treatments using naltrexone and related compounds
InactiveUS6541478B1Reduce weight gainPrevent relapseBiocideNervous disorderOpioid antagonistAntianxiety Agent
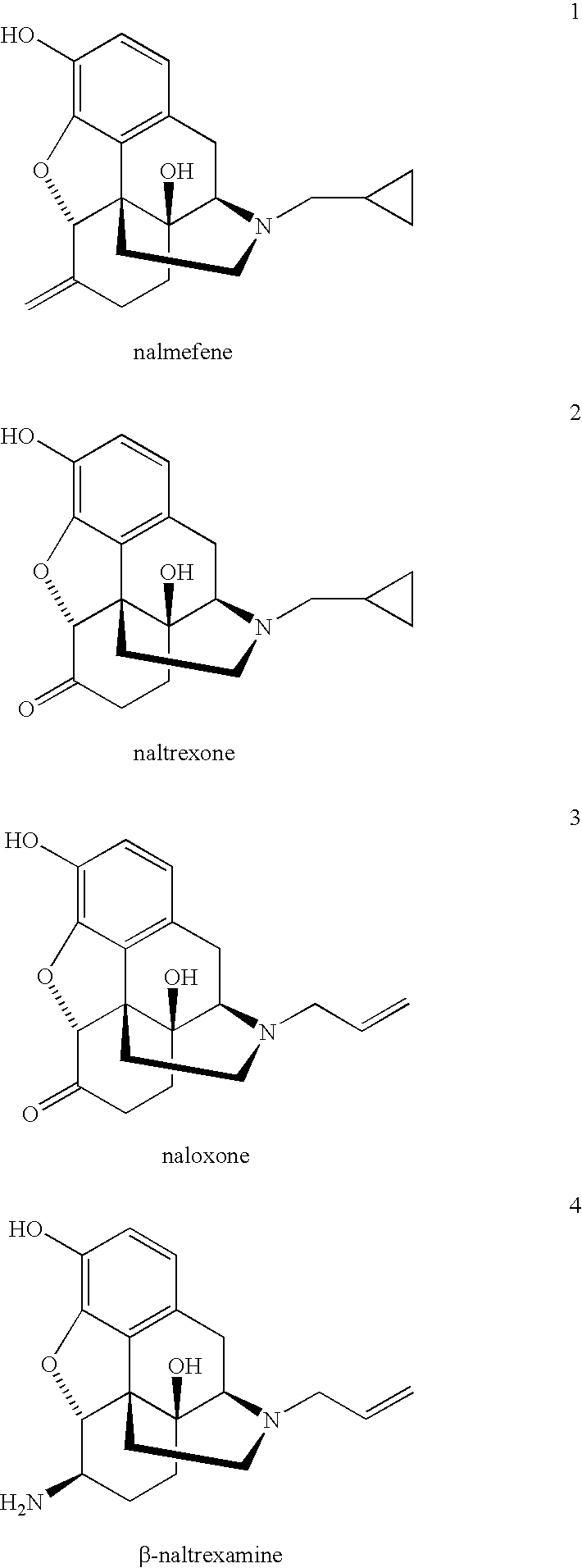
Nicotine dependency is treated by administration of an opioid antagonist. In some embodiments, rapid or ultra rapid detoxification techniques include using a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone or naltrexone or a mixture of any one of these, and either clonidine or related compounds either while awake, or while under sedation or anesthesia, followed by continued administration of an effective amount of an opioid antagonist with or without agents that enhance nicotine dependency treatment. Persons are also treated for nicotine dependency with more gradual detoxification methods using administration of a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone, naltrexone, or a mixture of any of these, and an effective amount of agents used to treat nicotine withdrawal including nicotine, such as that delivered by a nicotine patch, nicotine chewing gum, nicotine inhaler or other methods for delivering nicotine, antidepressants and antianxiety agents, and / or clonidine and related compounds. Administration of an effective amount of an opioid antagonist to prevent relapse, attenuate craving, and reduce weight gain during and after treatment for nicotine dependency is continued in some embodiments.
Owner:YALE UNIV
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant agent
InactiveUS20070014732A1Reducing abuse potential of dosage formLower potentialOrganic active ingredientsNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Methods and materials for the treatment of pain comprising opioid antagonists
InactiveUS20050038062A1Enhance neuropathic pain-alleviating potencyEnhancing the potency of opioid agonistsBiocideNervous disorderOpioid AgonistOpioid antagonist
Methods and compositions for treating subjects with pain, including neuropathic pain, using opioid antagonists or combinations of opioid antagonists and opioid agonists, including, for example, wherein the amount of an opioid antagonist enhances the neuropathic pain-alleviating potency of an opioid agonist.
Owner:PAIN THERAPEUTICS INC
Opioid agonist/opioid antagonist/acetaminophen combinations
InactiveUS20020058673A1Reducing oral abuse potential of dosage formImprove subjective experienceBiocideDrug compositionsOpioid antagonistOpioid Agonist
The invention is directed in part to oral dosage forms comprising a combination of an opioid agonist, acetaminophen and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Tamper-resistant oral opioid agonist formulations
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Methods and compositions for the treatment of brain reward system disorders by combination therapy
The present invention is directed to a combination treatment of an opioid antagonist e.g., naltrexone and a second compound selected from the group consisting of a GABA B agonist, an NMDA antagonist, a serotonin antagonist, and a cannabinoid antagonist is the key to the successful treatment of a brain reward system disorder. A brain reward system, include but are not limited, to pathological gambling, compulsive alcohol consumption, compulsive over-eating and obesity, compulsive smoking, and drug addiction. The compounds and methods of the present invention effectively reduce the cravings, withdrawal symptoms and negative drug side effects associated with a monotherapy. As such, patient compliance is greatly increased, thereby decreasing relapse of a brain reward system disorder.
Owner:ALKERMES INC
Methods and materials useful for the treatment of arthritic conditions, inflammation associated with a chronic condition or chronic pain
InactiveUS20050245557A1Good pain reliefBetter pain controlBiocideAnimal repellantsOpioid AgonistOpioid antagonist
Methods and materials, including novel compositions, dosage forms and methods of administration, useful for treating arthritic conditions, inflammation associated with a chronic condition, and / or chronic pain, including pain from arthritis and inflammation, using opioid antagonists, including combinations of opioid antagonists and opioid agonists. Methods and materials comprising opioid antagonists or combinations opioid antagonists and agonists may optionally include one or more additional therapeutic agents.
Owner:PAIN THERAPEUTICS INC
Hydrophilic opioid abuse deterrent delivery system using opioid antagonists
InactiveUS20080075771A1Resistant to opioid abuseReduce abuse potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed herein are oral dosage forms of opioid therapeutic agents that are resistant to abuse and methods of their formulation. In particular, oral dosage forms that are resistant to dissolution in aqueous solutions of ethanol are described. The oral dosage forms may include one or more opioid antagonists that are sequestered from the opioid therapeutic agent such that the opioid antagonist has no substantial effect on the activity of the opioid therapeutic agent when the dosage form is taken orally as prescribed, but the opioid antagonist is released in an amount that reduces the effectiveness of the opioid therapeutic agent contained in the dosage form when the dosage form is crushed.
Owner:LAB INT
Compositions for affecting weight loss
InactiveUS20070275970A1Appetite suppressantReduce weightBiocideMetabolism disorderOpioid antagonistMelanocortin 3 receptor
Disclosed are compositions for affecting weight loss comprising a first compound and a second compound, where the first compound is an opioid antagonist and the second compound causes increased agonism of a melanocortin 3 receptor (MC3-R) or a melanocortin 4 receptor (MC4-R) compared to normal physiological conditions. Also disclosed are methods of affecting weight loss, increasing energy expenditure, increasing satiety in an individual, or suppressing the appetite of an individual, comprising identifying an individual in need thereof and treating that individual to antagonize opioid receptor activity and to enhance α-MSH activity.
Owner:OREXIGEN THERAPEUTICS INC
Transdermal dosage form comprising an active agent and a salt and a free-base form of an adverse agent
InactiveUS20080020028A1Sufficient amountInhibition effectBiocideNervous disorderOpioid antagonistPreventing pain
This invention relates to a tamper-resistant transdermal dosage form comprising an active agent, such as an opioid, or a pharmaceutically acceptable salt thereof, a free base of an adverse agent, such as an opioid antagonist, and a pharmaceutically acceptable salt of an adverse agent, such as an opioid antagonist. The transdermal dosage form allows an effective amount of the active agent, or a pharmaceutically acceptable salt thereof, to be transdermally administered to an animal. The invention further relates to methods for treating or preventing pain in an animal comprising contacting the skin of an animal in need thereof with the transdermal dosage form of the invention for an amount of time sufficient to treat or prevent pain.
Owner:PURDUE PHARMA LP
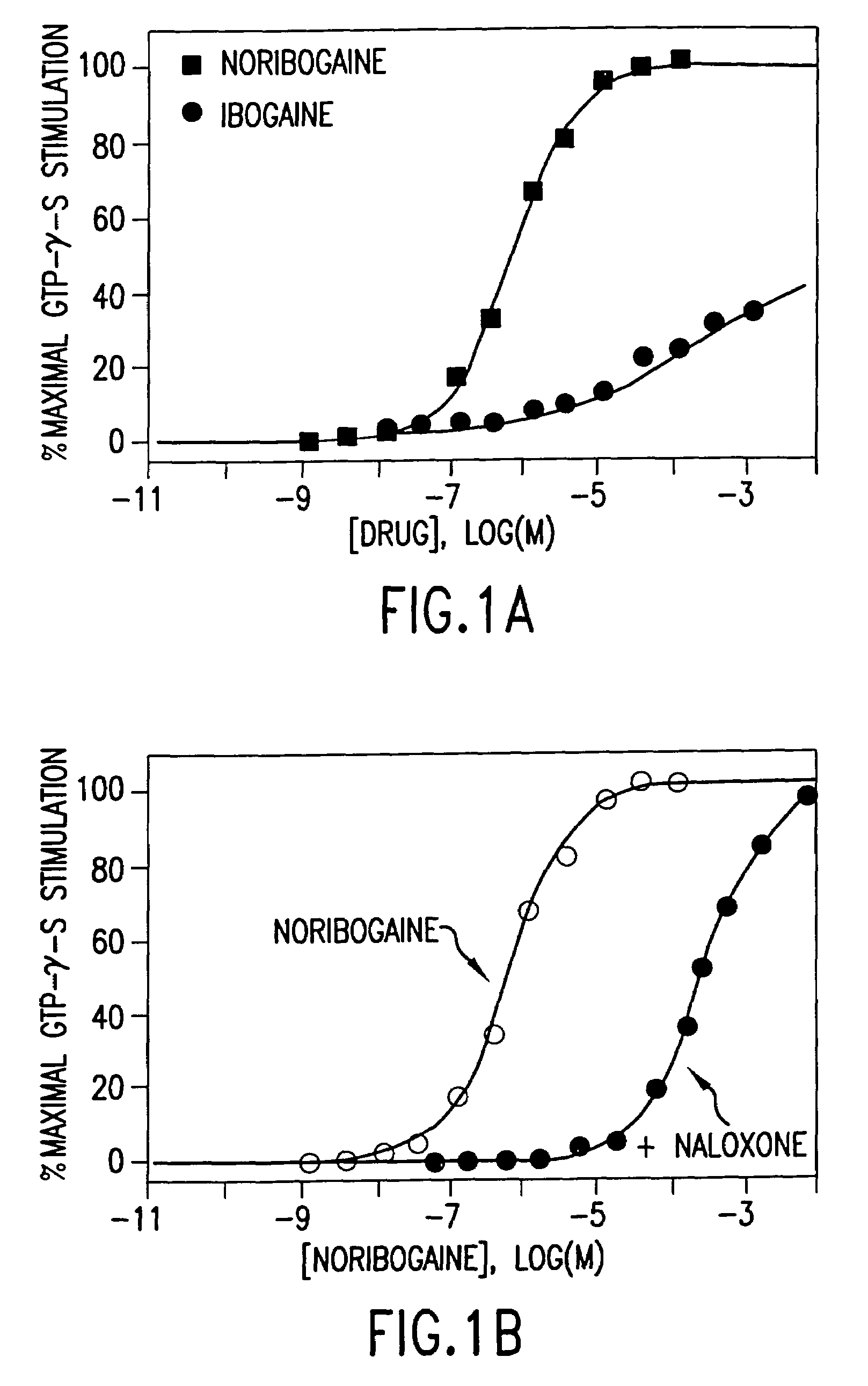
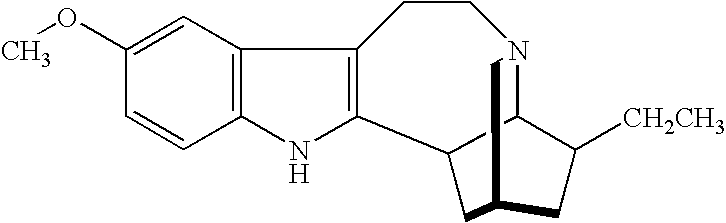
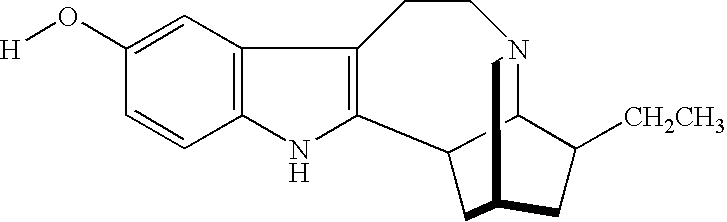
Noribogaine in the treatment of pain and drug addiction
InactiveUS7220737B1Eliminate symptomsConveniently preparedBiocideAnimal repellantsOpioid antagonistTreatment pain
The present invention is directed to methods of treating patients for pain by administering noribogaine. Noribogaine may also be used to treat patients for the symptoms associated with withdrawal from drug dependency. In the latter case, the noribogaine treatment should be supplemented with the administration of an opioid antagonist such as naloxone.
Owner:DEMERX
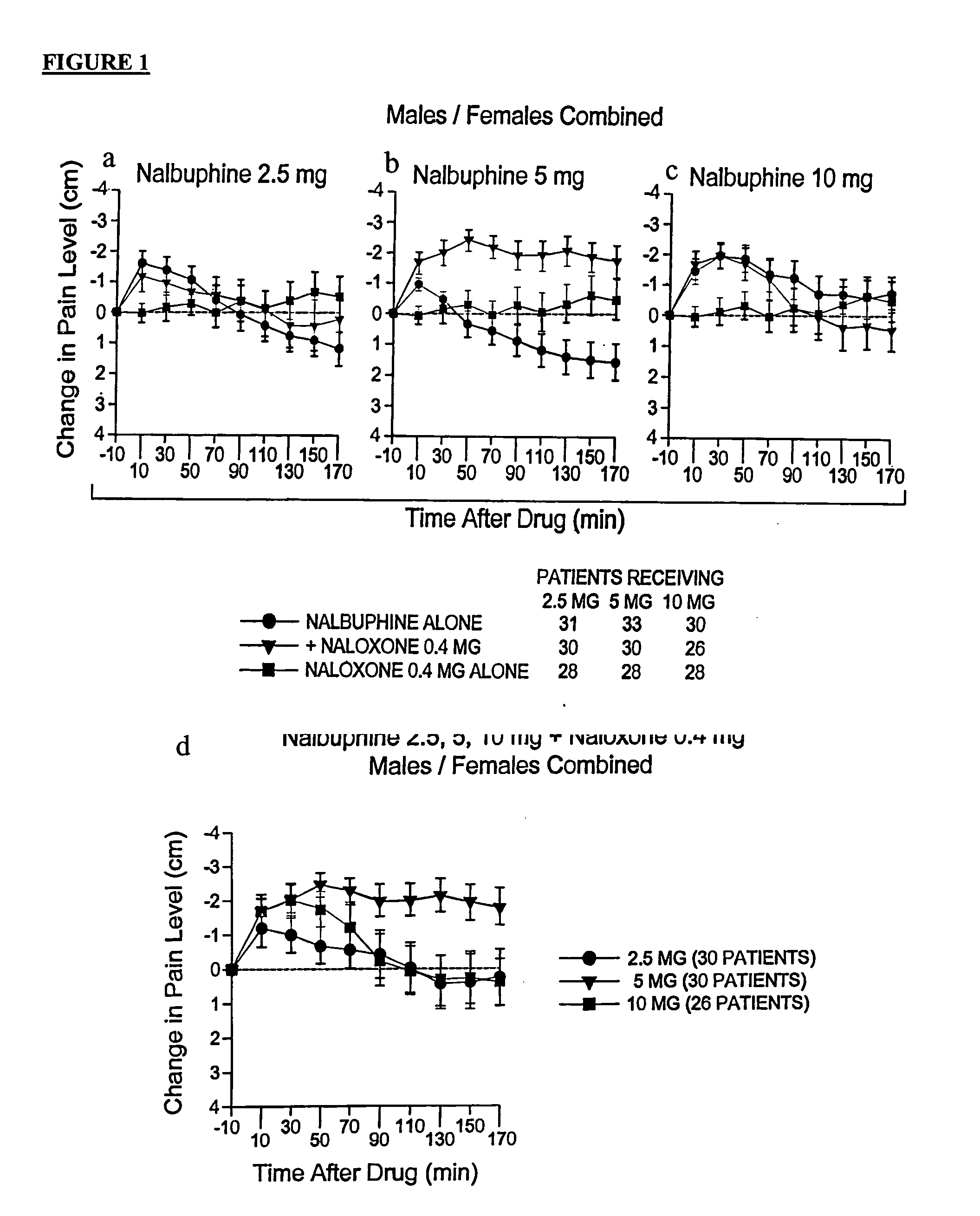
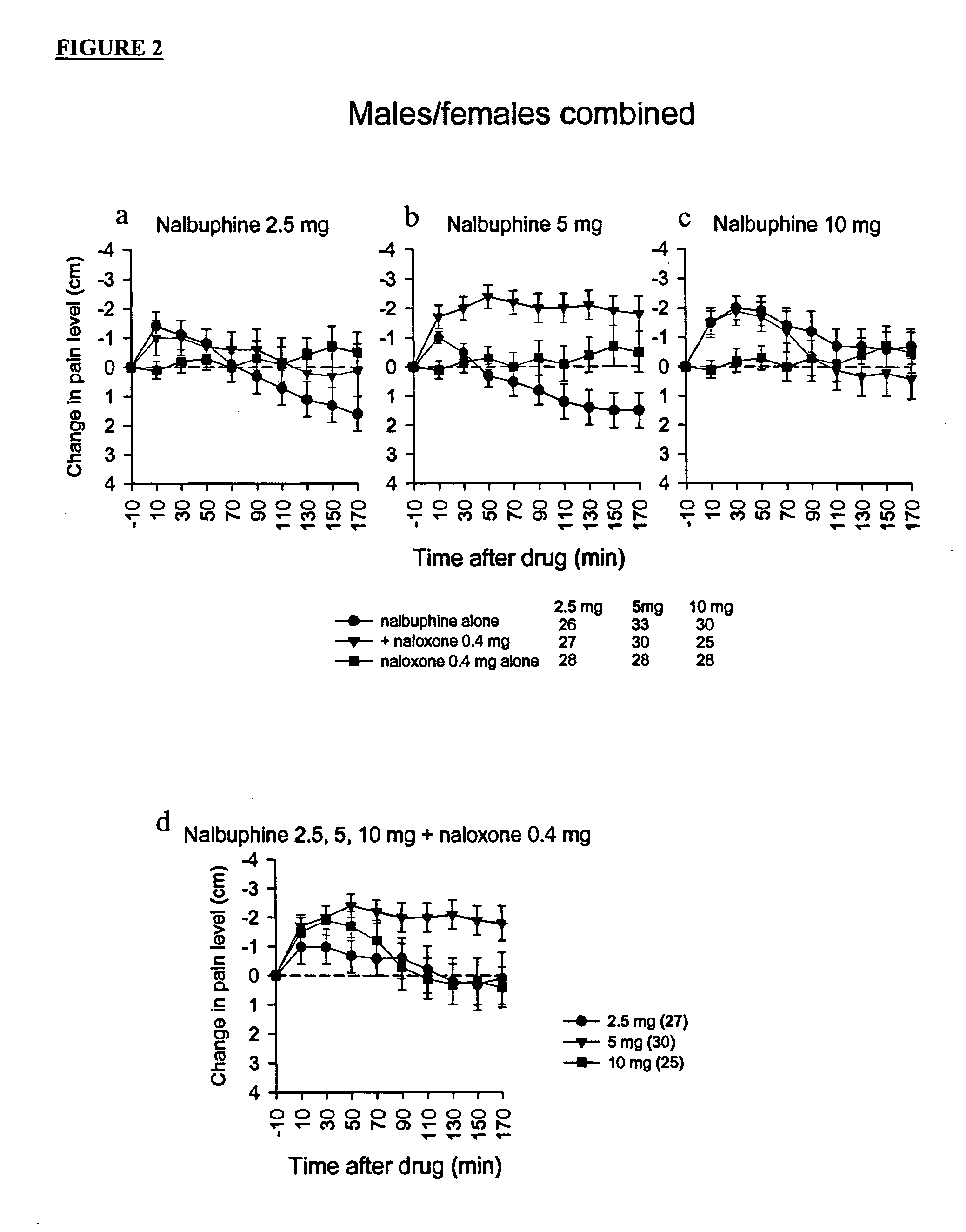
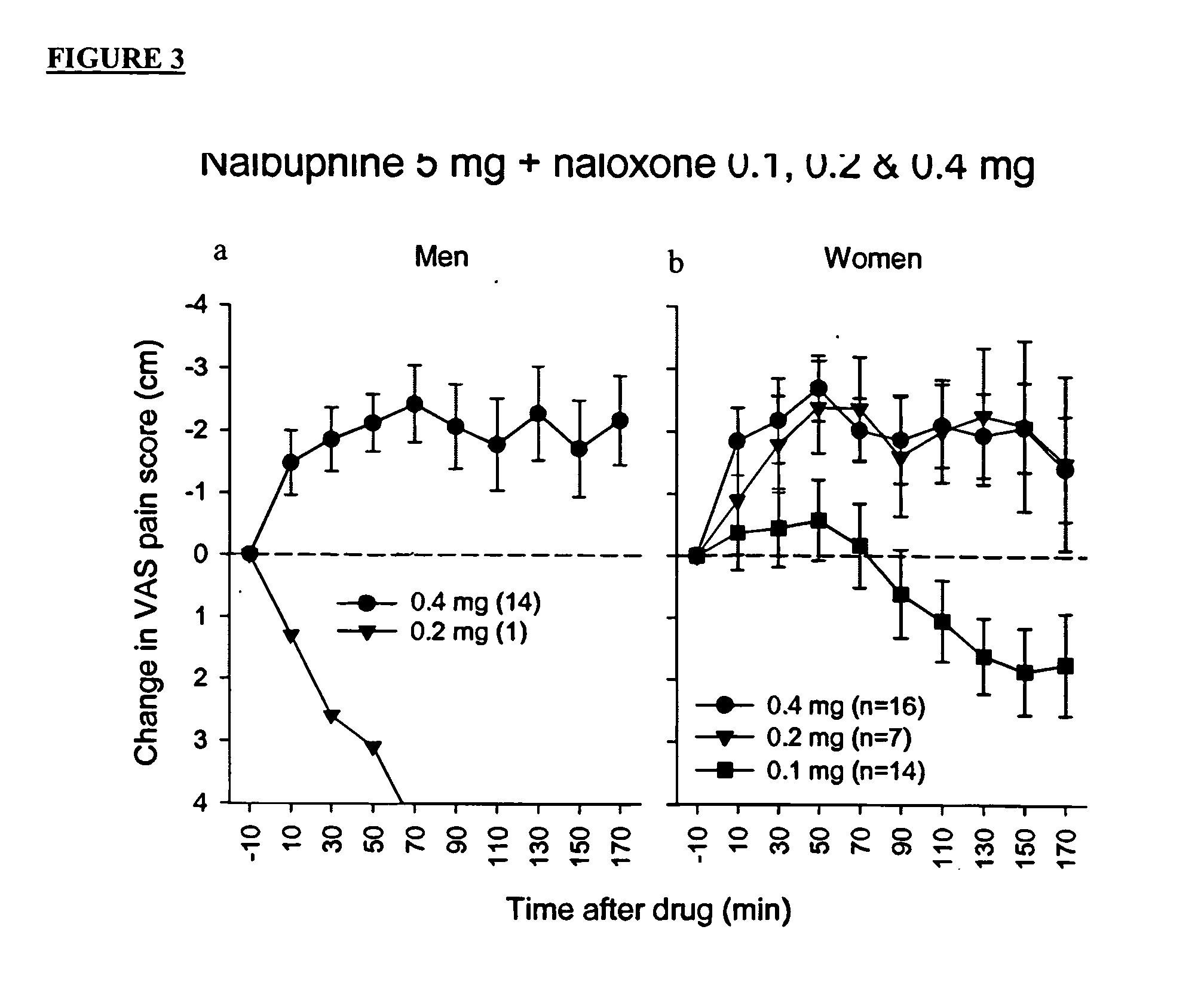
Treatment of pain with combinations of nalbuphine and other kappa-opioid receptor agonists and opioid receptor antagonists
InactiveUS20040180916A1Enhances nalbuphine analgesiaMinimize ToxicityBiocideNervous disorderOpioid antagonistPentazocine
Methods and compositions for treating, managing or ameliorating pain in a subject (preferably, a mammal, and more preferably, a human) comprising administration of a centrally acting (i.e., crosses the blood brain barrier) agonist of a kappa-opioid receptor and a centrally acting opioid antagonist such that the analgesia achieved by this administration is greater than with administration of either the kappa-opioid receptor agonist or the opioid antagonist alone. Preferably the kappa-opioid receptor is nalbuphine or a salt or prodrug of nalbuphine and the opioid antagonist is naloxone or a salt or prodrug of naloxone. Preferred methods of administration include mucosal (e.g. intranasal or pulmonary) and intravenous. Other kappa-opioid receptors include pentazocine and butorphanol.
Owner:RGT UNIV OF CALIFORNIA
Abuse Resistant Opioid Transdermal Delivery Device Containing Opioid Antagonist Microspheres
InactiveUS20080233178A1Reduced potential for abuseLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Owner:PURDUE PHARMA LP
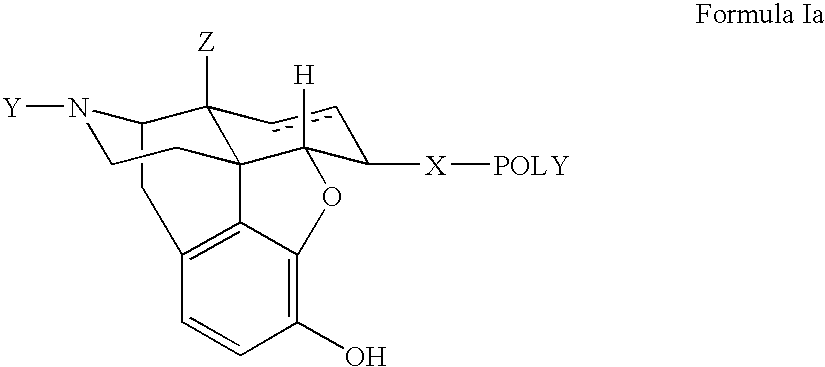
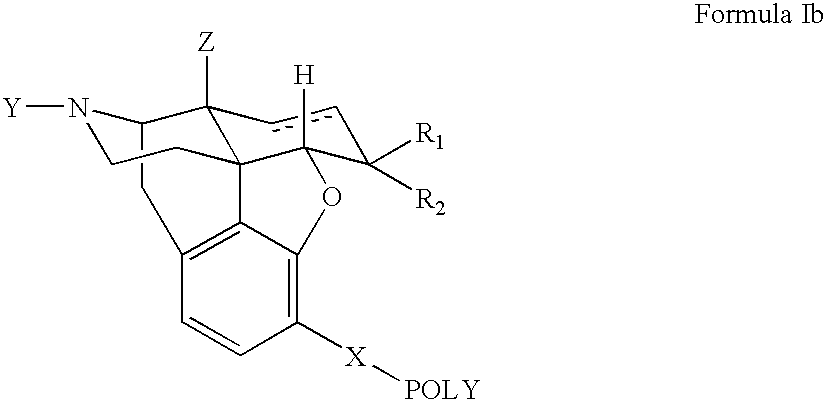
Polymer conjugates of opioid antagonists
The invention provides polymer conjugates of opioid antagonists comprising a polymer, such as poly(ethylene glycol), covalently attached to an opioid antagonist. The linkage between the polymer and the opioid antagonist is preferably hydrolytically stable. The invention also includes a method of treating one or more side effects associated with the use of opioid analgesics, such as constipation, nausea, or pruritus, by administering a polymer conjugate of the invention.
Owner:NEKTAR THERAPEUTICS INC
Methods and compositions for treating distress dysfunction and enhancing safety and efficacy of specific medications
InactiveUS20110159048A1Good treatment effectEliminate side effectsBiocideNervous disorderDiseaseNeurotransmitter systems
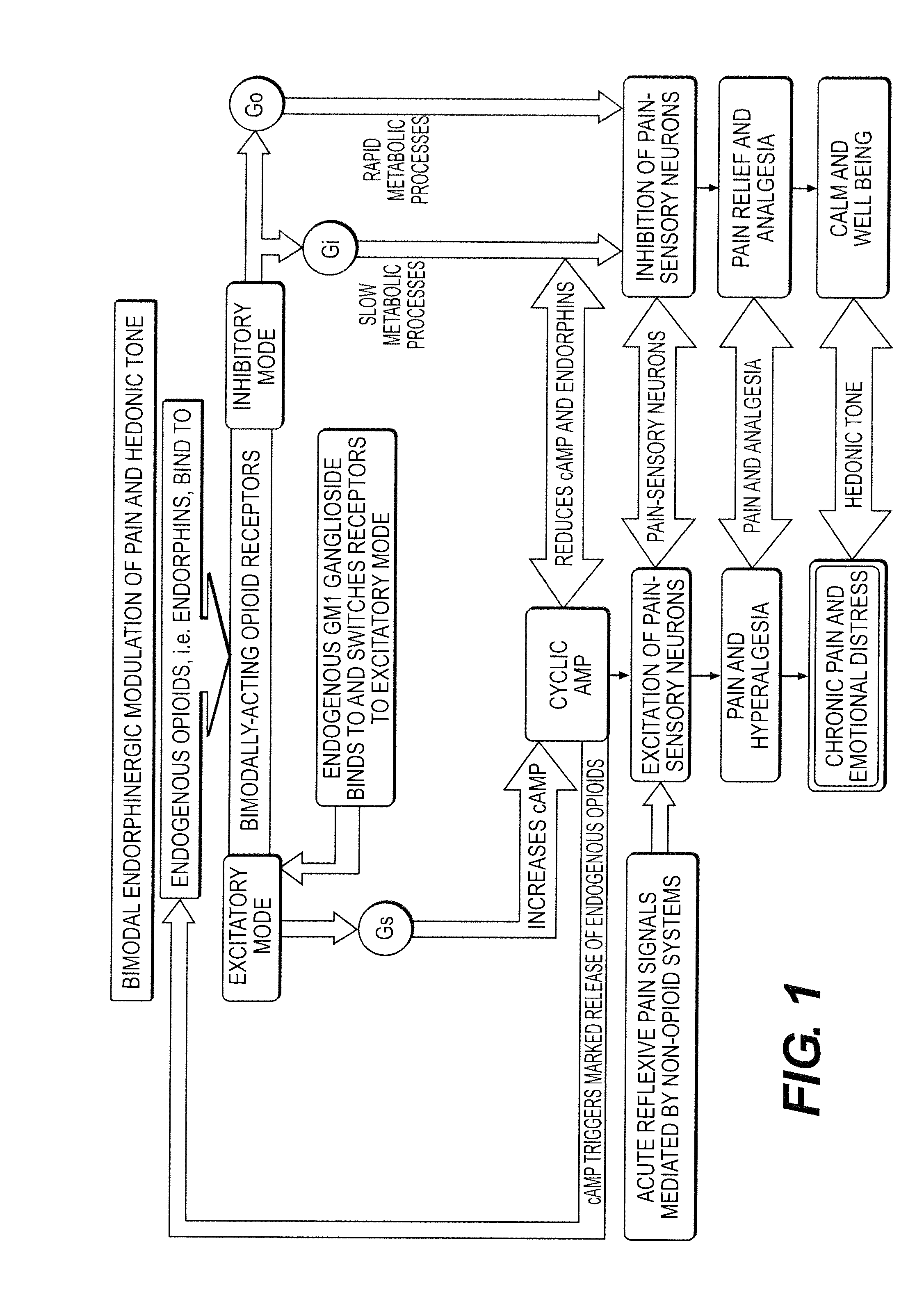
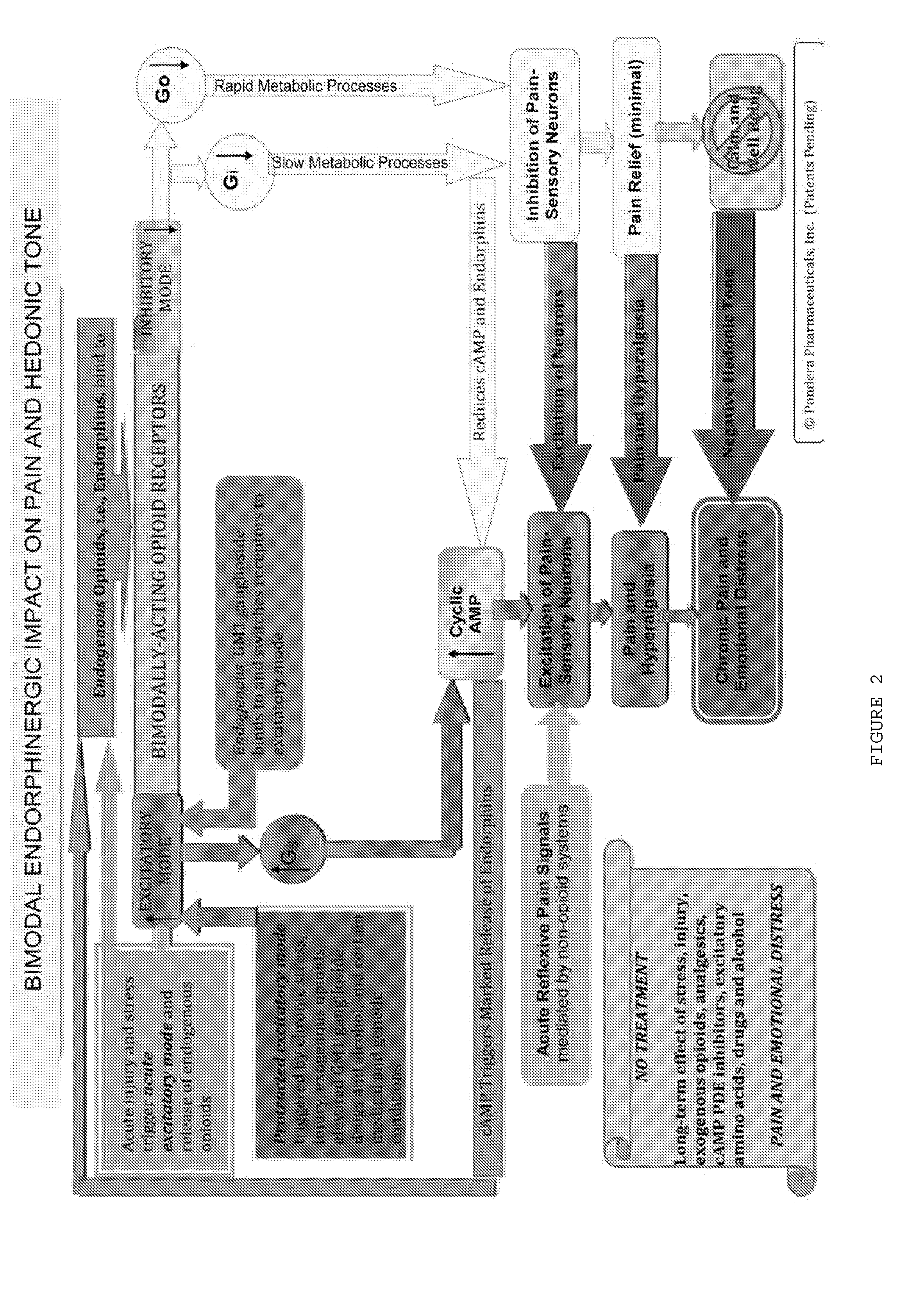
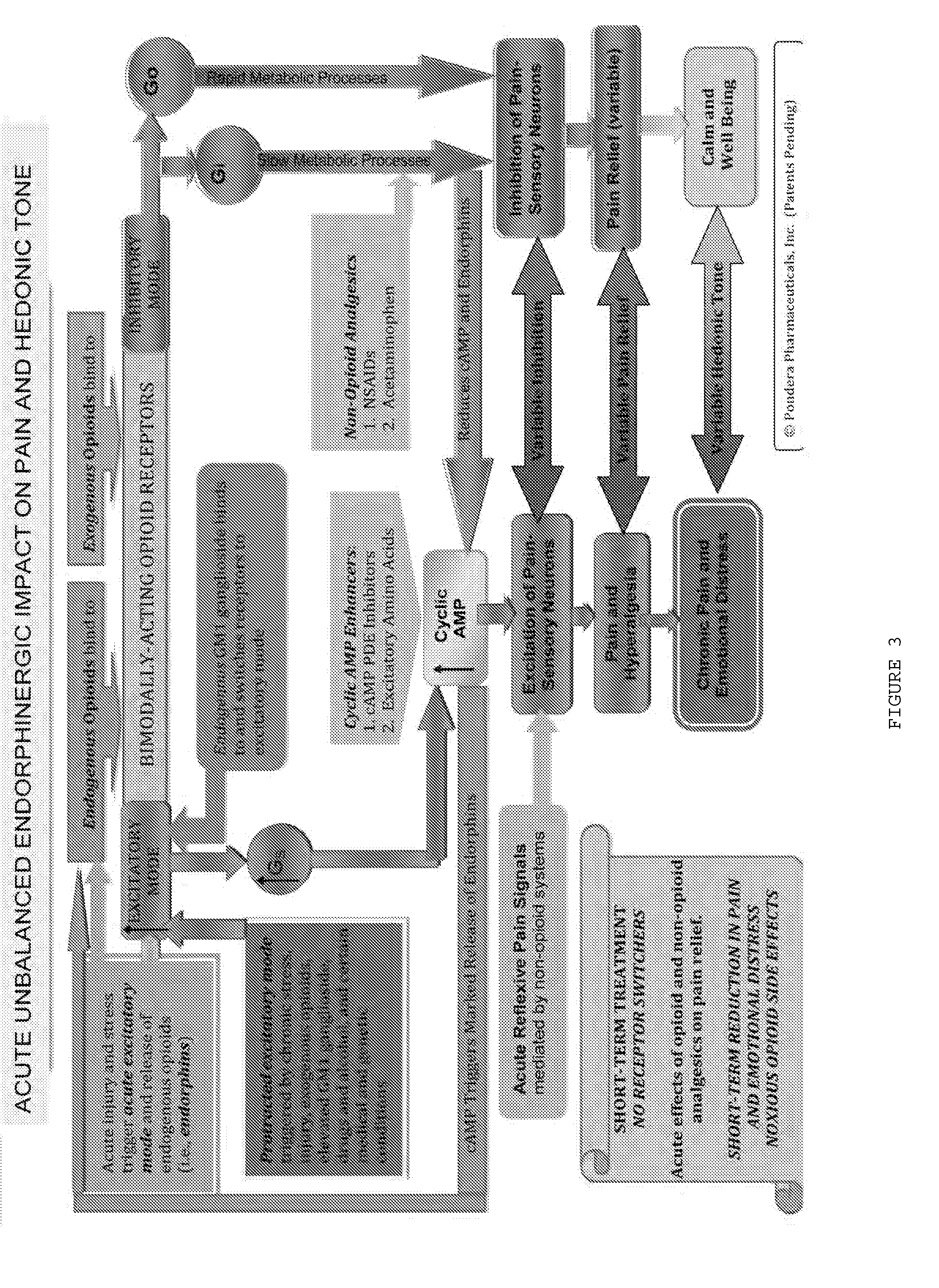
The present invention relates to methods and compositions for reducing Distress Dysfunction by restoring and maintaining homeostatic balance in the neurotransmitter systems underlying the Stress Response and the experience of distress and hedonic tone. Distress Dysfunction refers to the experience of dysfunctional emotional and physical distress that interferes with the individual's quality of life and functioning. A novel understanding of the bimodal opioid modulation of pain, and its impact, through serotonergic, dopaminergic, epinephrinergic, and norepinephrinergic processes, on hedonic tone, leads directly to new generation pharmaceutical formulations that are remarkably safe and effective for the treatment of a wide variety of Distress Dysfunctions, including anxiety, depression, anger, insomnia, mood disorders, eating disorders, sexual problems, pain, substance and behavioral addictions, gastrointestinal disorders, autistic spectrum disorders, attention-deficit and hyperactivity disorders, and other emotional and physical distress disorders. The foundation of this discovery is the power of Receptor Switchers, such as ultra-low-dose and very-low-dose opioid antagonists and GM1 ganglioside attenuators, in blocking acute and protracted excitatory opioid receptor signaling. Co-administration of Receptor Switchers with Endorphin Enhancers, such as specific cAMP PDE inhibitors and excitatory amino acids, is an excellent formulation for restoring healthy homeostatic balance to the endogenous opioid system, using the body's endorphins to reduce emotional and physical distress, and through synergistic and homeostatic processes, restoring positive hedonic tone. The addition of Synergistic Enhancers, such as amino acids, SSRI and SNRI agents, and non-opioid analgesics, as well as Exogenous Opioids, enhances and prolongs these therapeutic benefits. The novel principles discovered by this invention also teach a new generation of safe and effective formulations for the treatment of respiratory conditions, neuropathy, and nociceptive pain.
Owner:PONDERA BIOTECH
Compositions and methods for increasing insulin sensitivity
ActiveUS20070128298A1Modulating blood glucose levelImprove insulin resistanceBiocidePeptide/protein ingredientsOpioid antagonistD-Glucose
Methods and compositions for treating a blood glucose condition involve identifying a suitable subject and administering an effective amount of a composition that contains one or more of an opioid antagonist, an anticonvulsant, and a psychotherapeutic agent. The compositions can include insulin. In some embodiments, such methods and compositions can be used to modulate a blood glucose level. In preferred embodiments, such methods and compositions are useful for increasing a subject's sensitivity to insulin.
Owner:OREXIGEN THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com