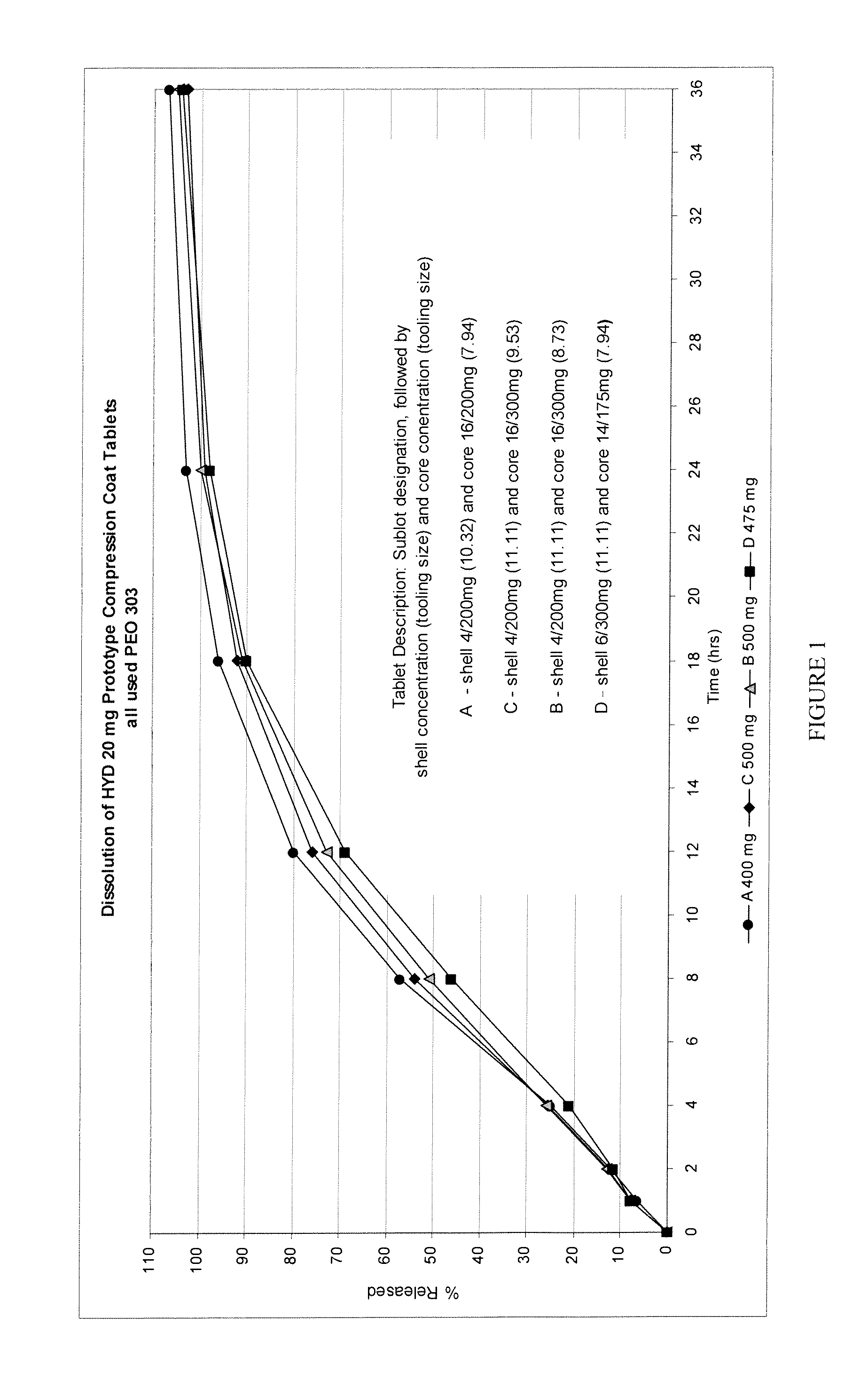
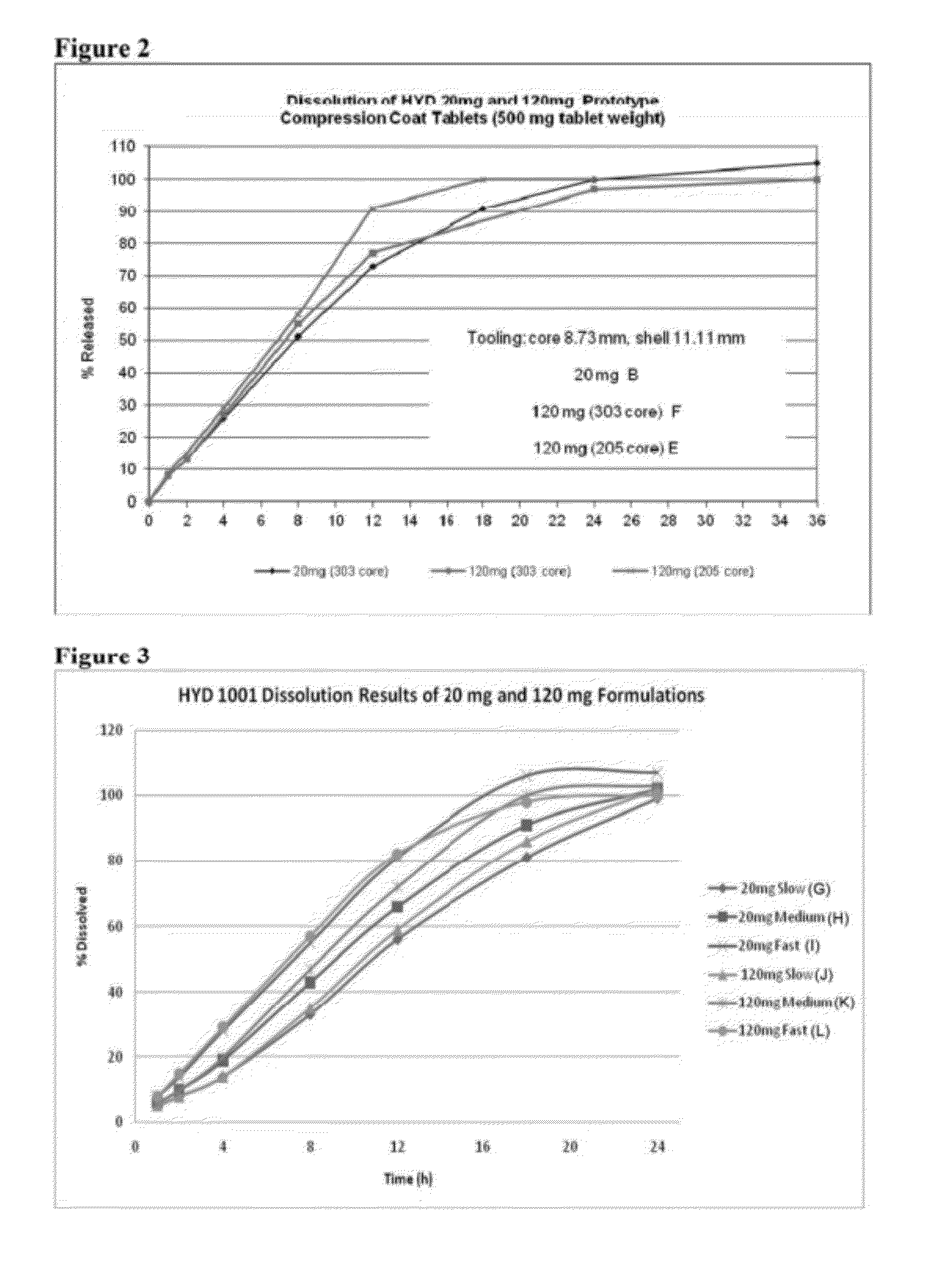
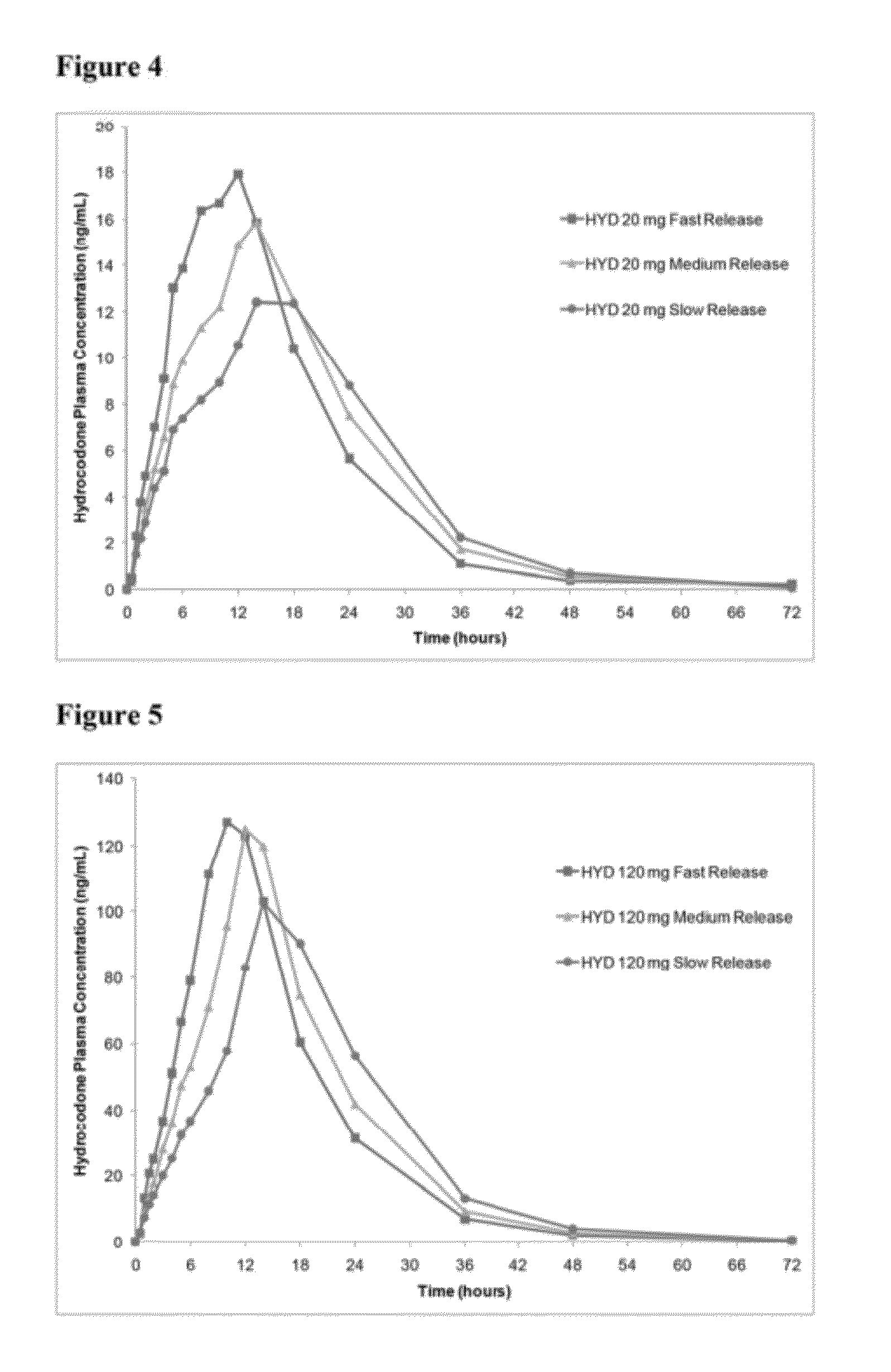
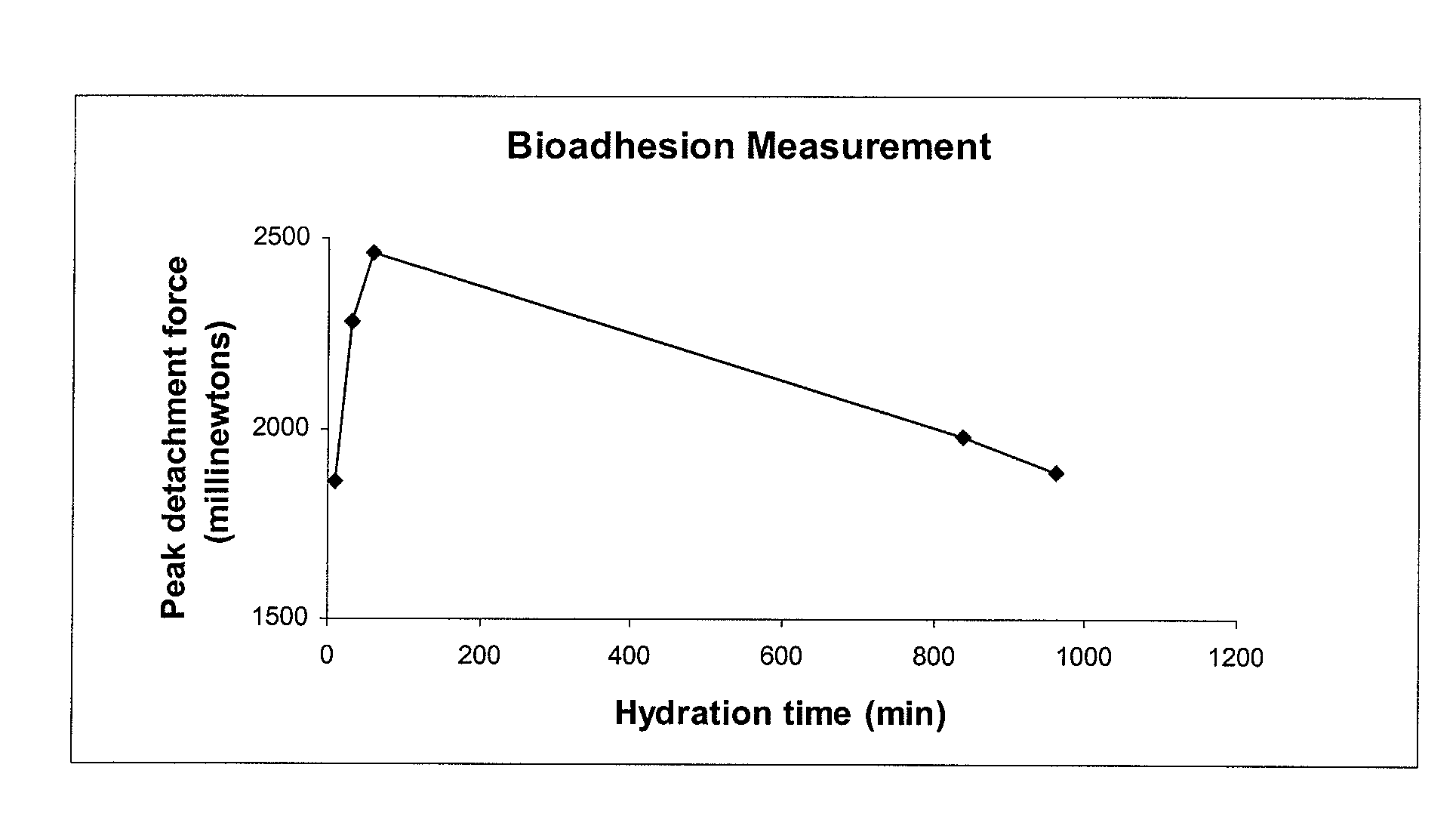
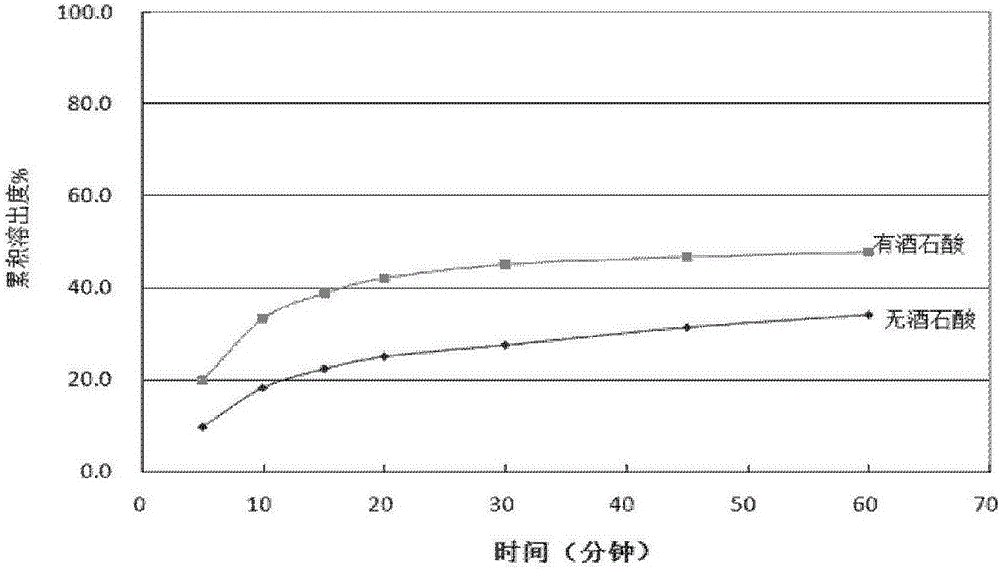
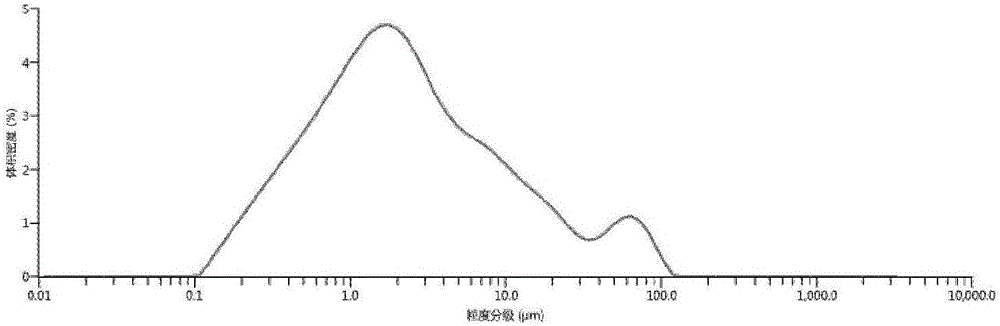
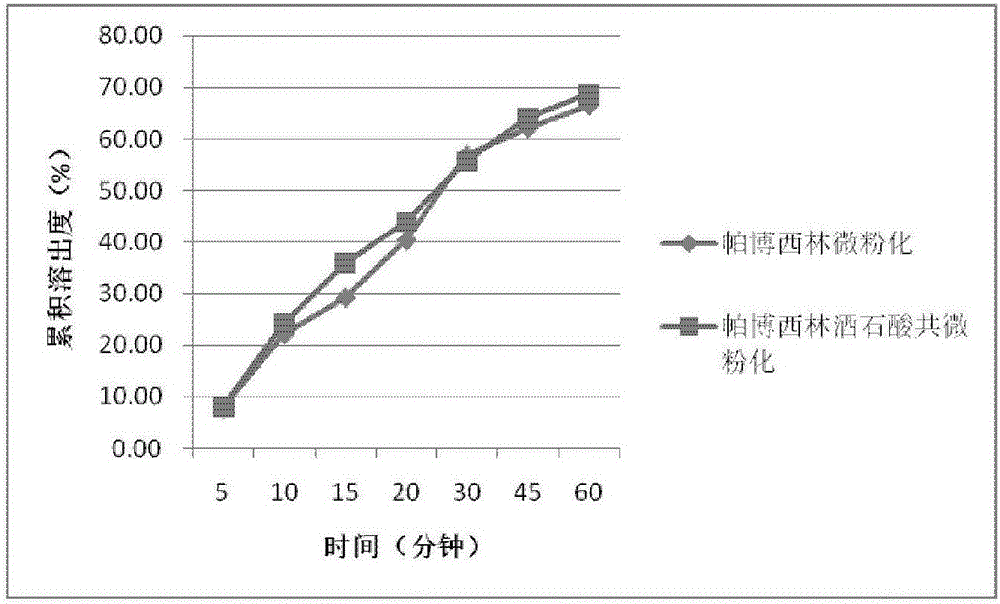
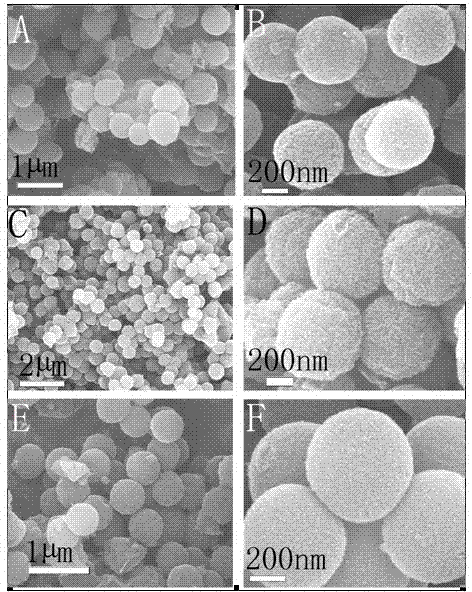
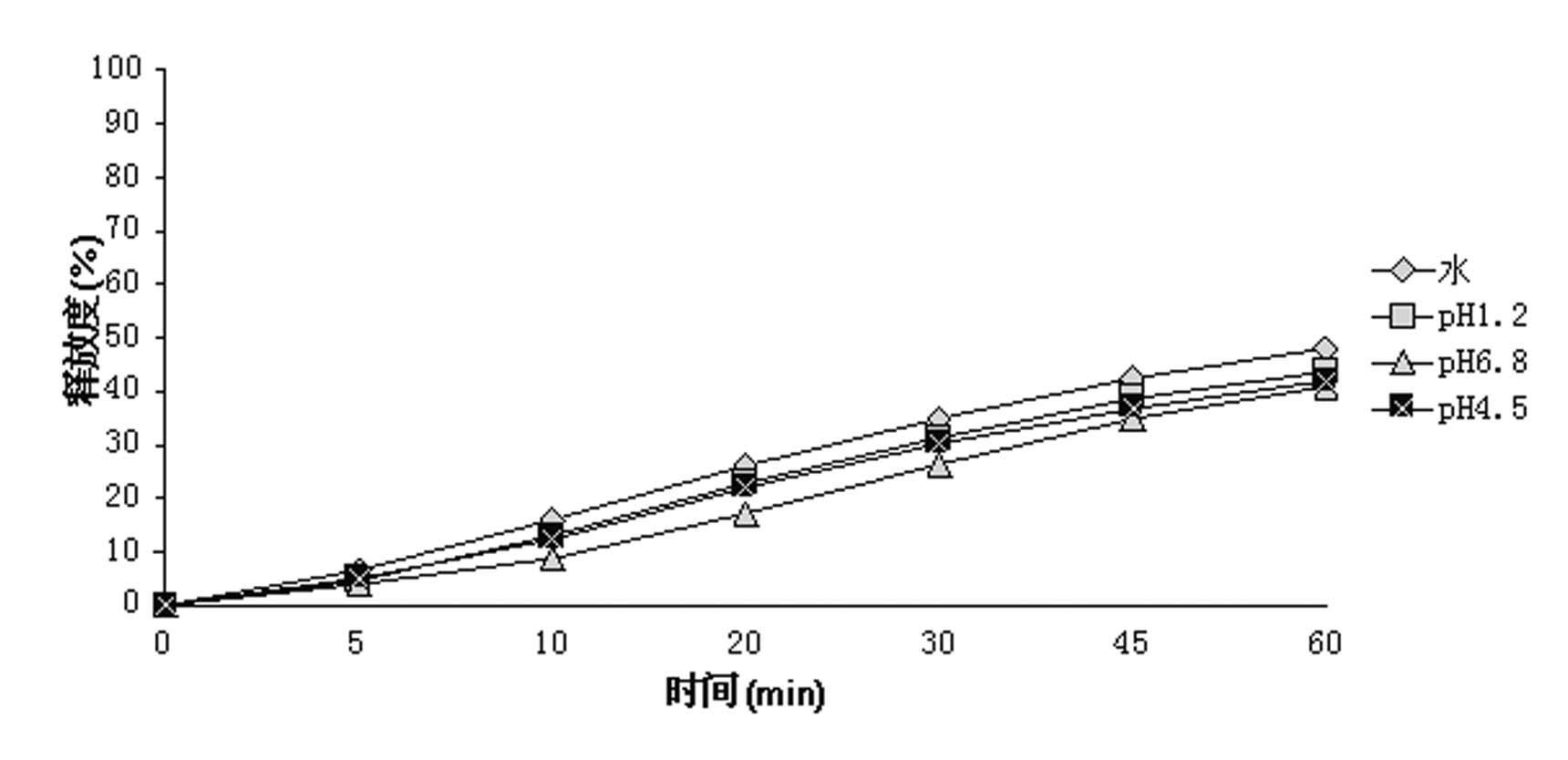
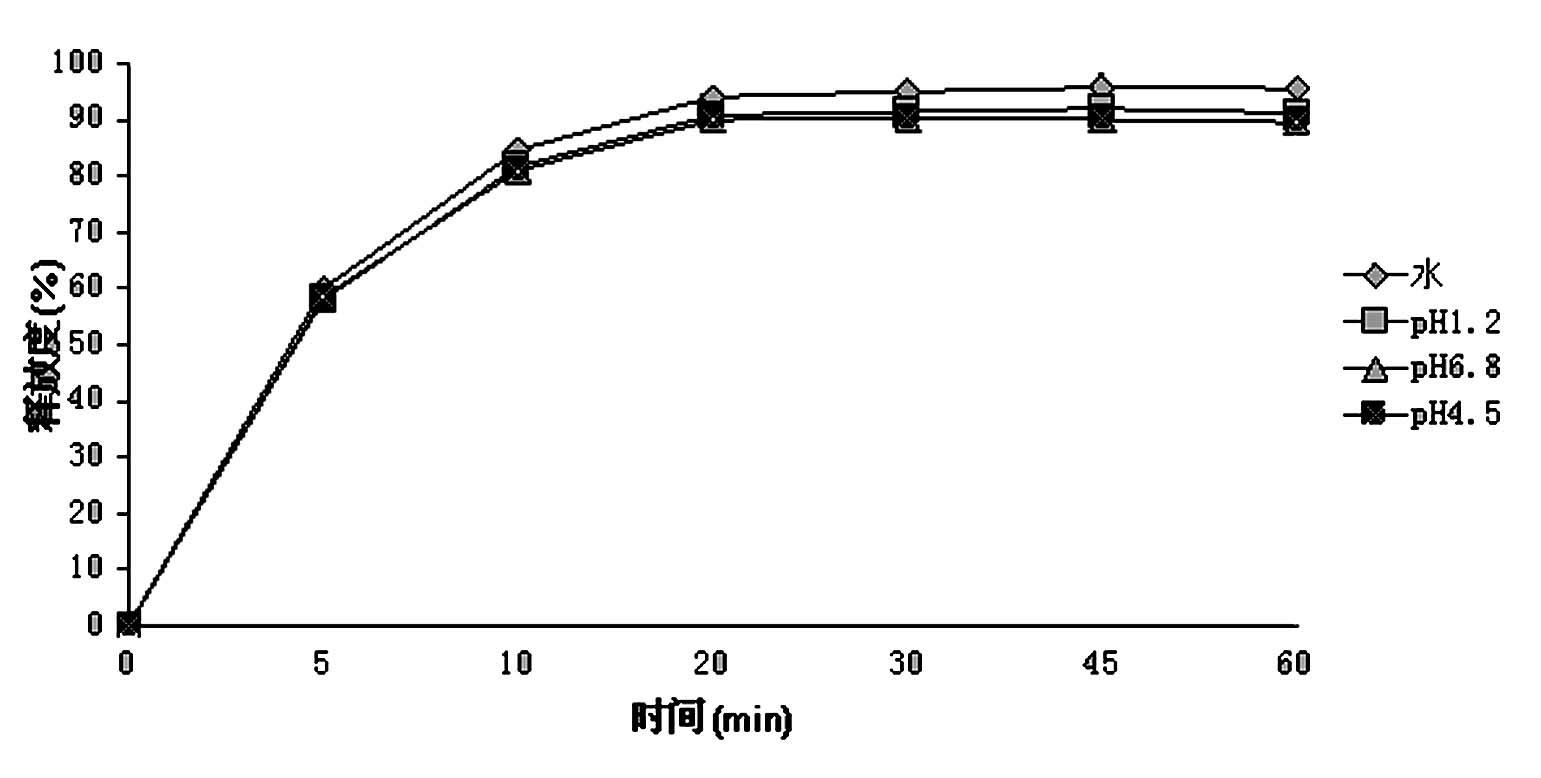
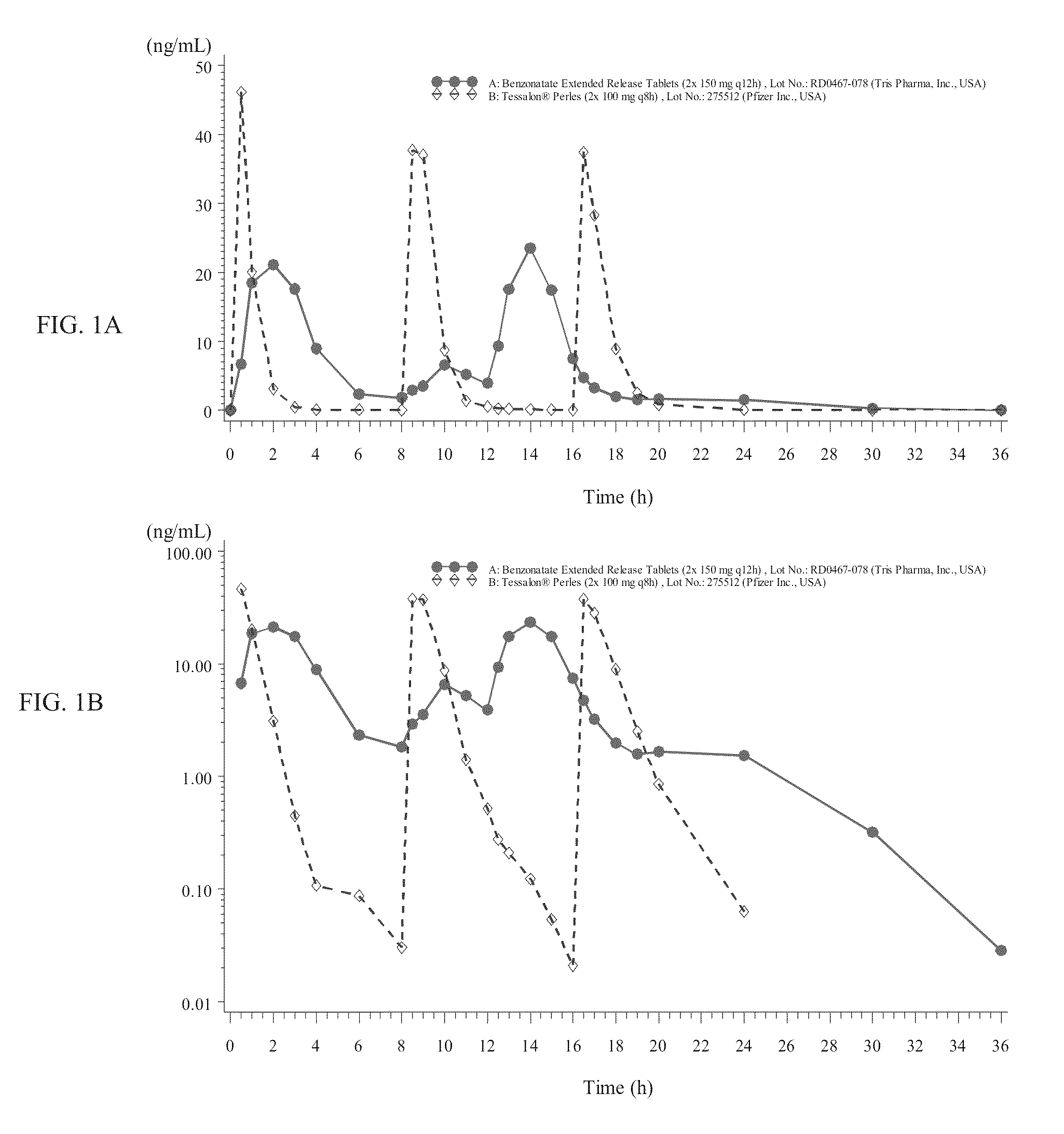Patents
Literature
398 results about "In vitro dissolution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In Vitro Dissolution Absorption System (IDAS) The In Vitro Dissolution Absorption System (IDAS TM) combines traditional dissolution testing with a means to determine and quantify interactions with a bio-relevant membrane.
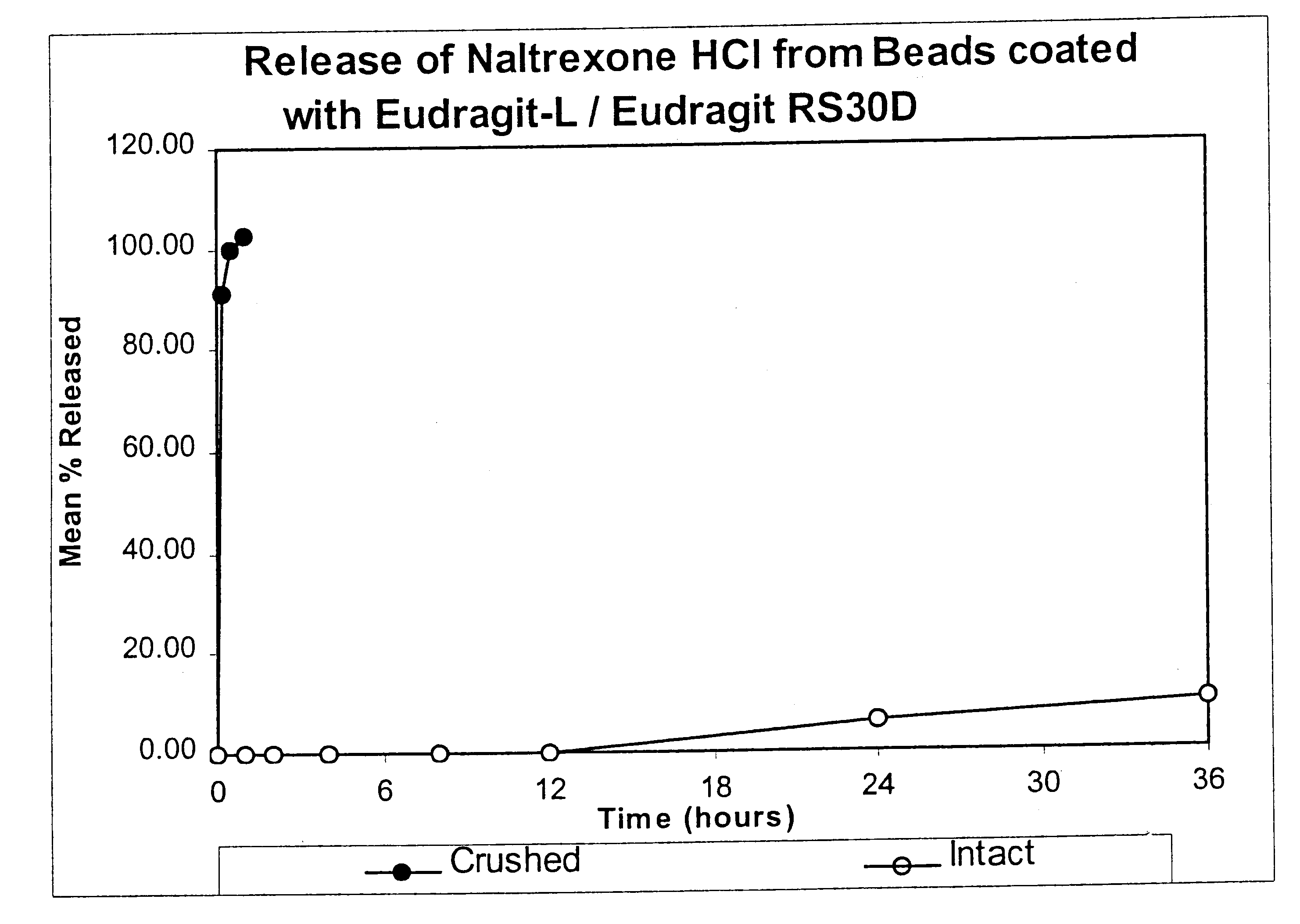
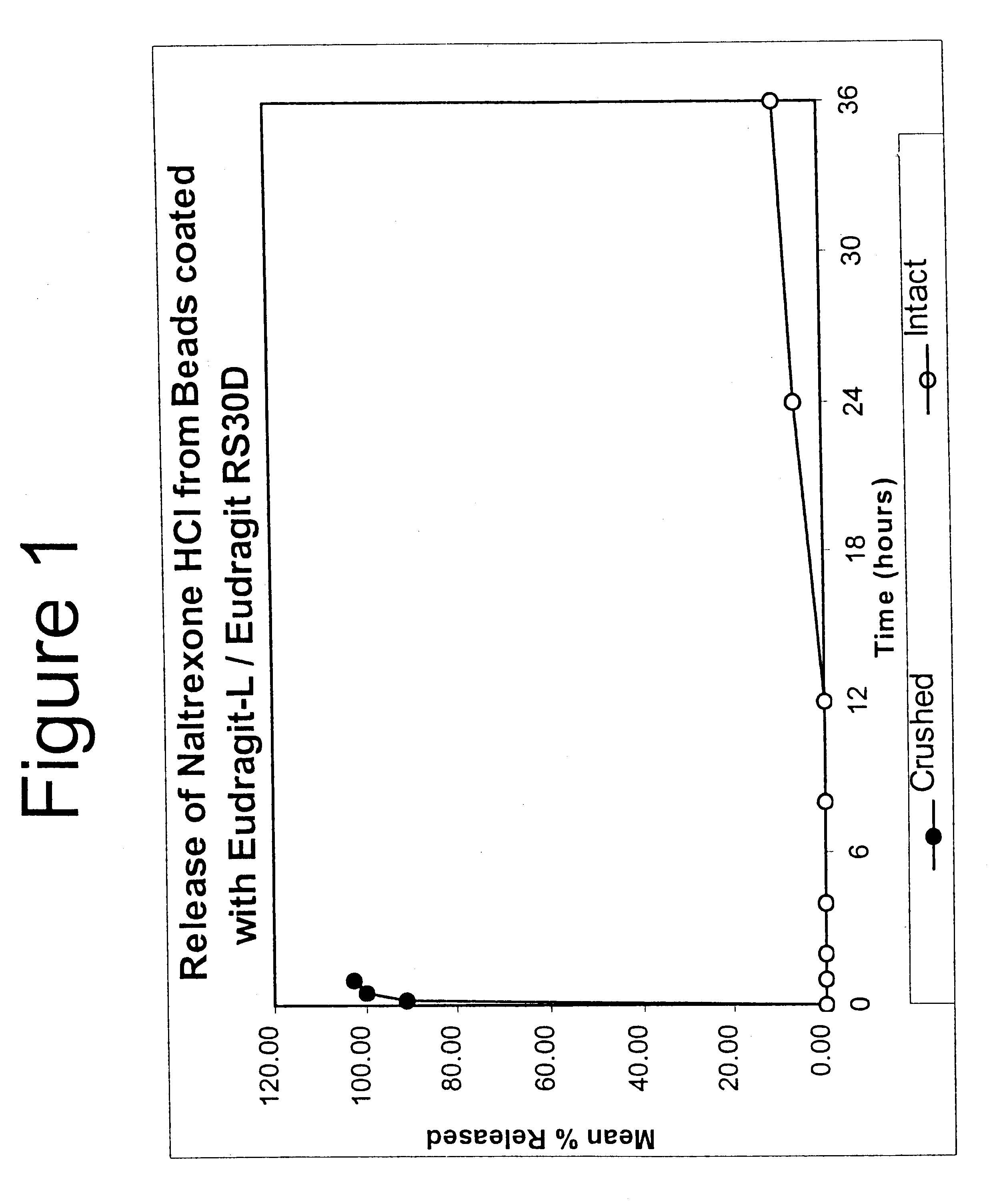
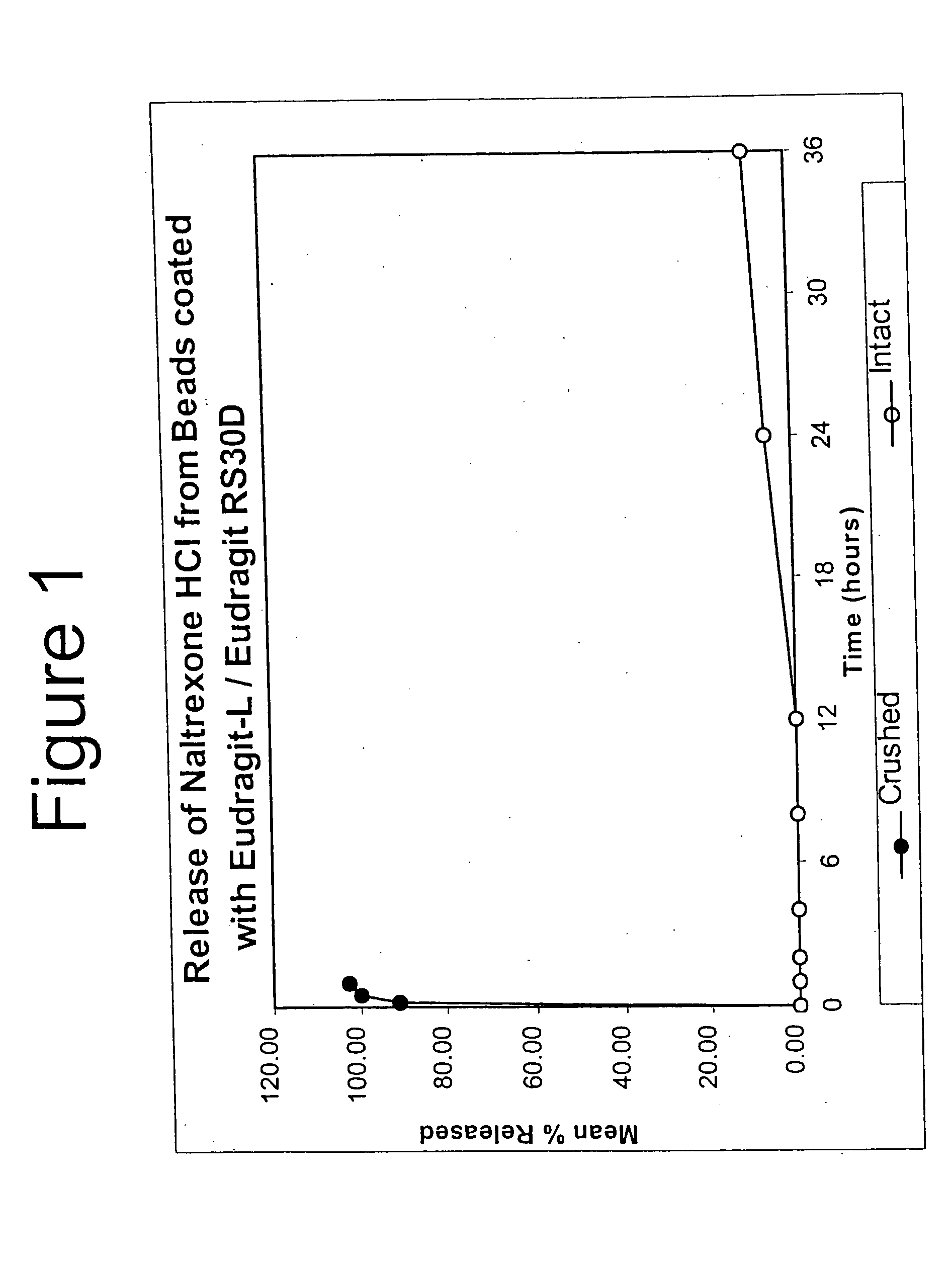
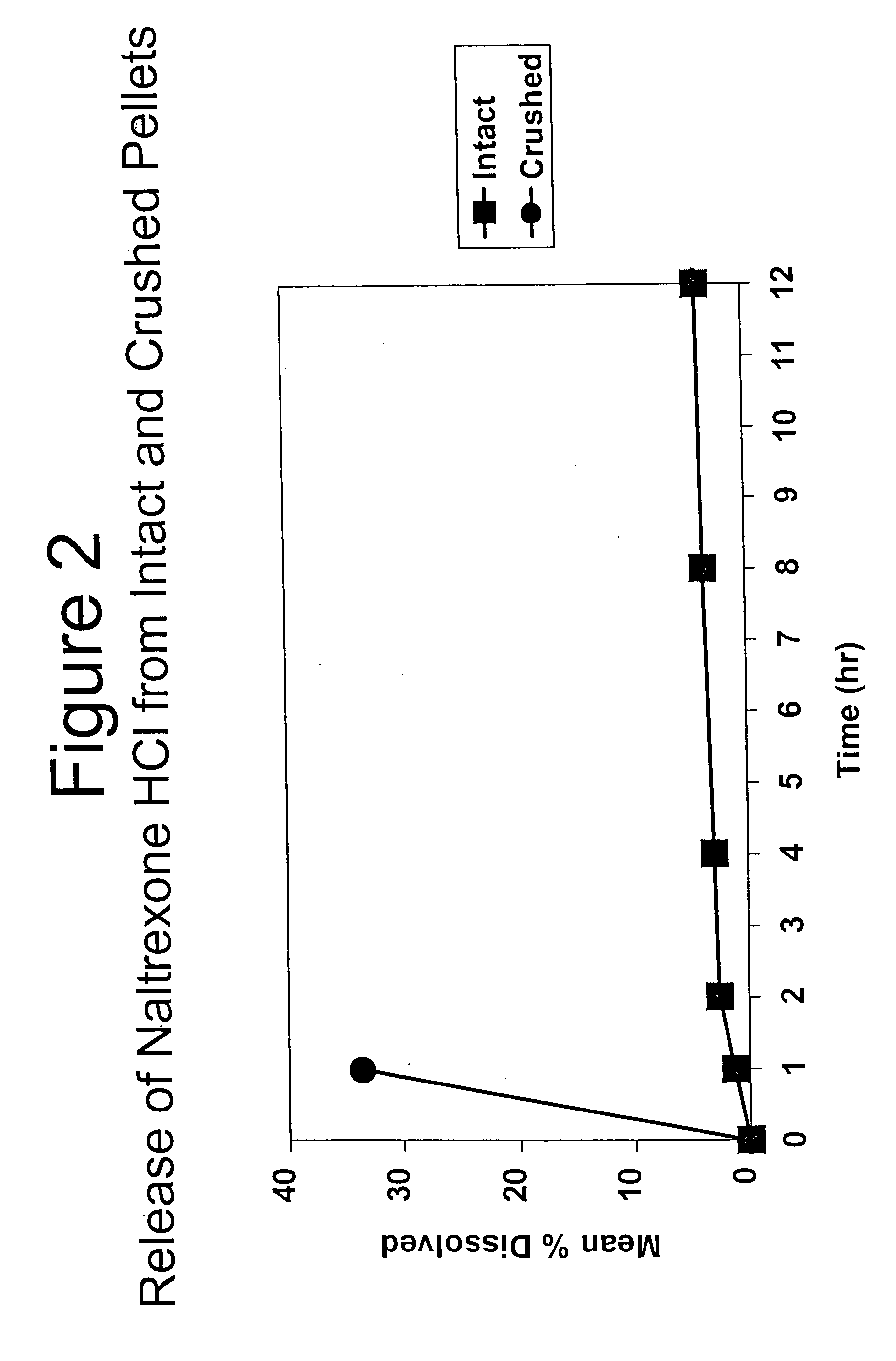
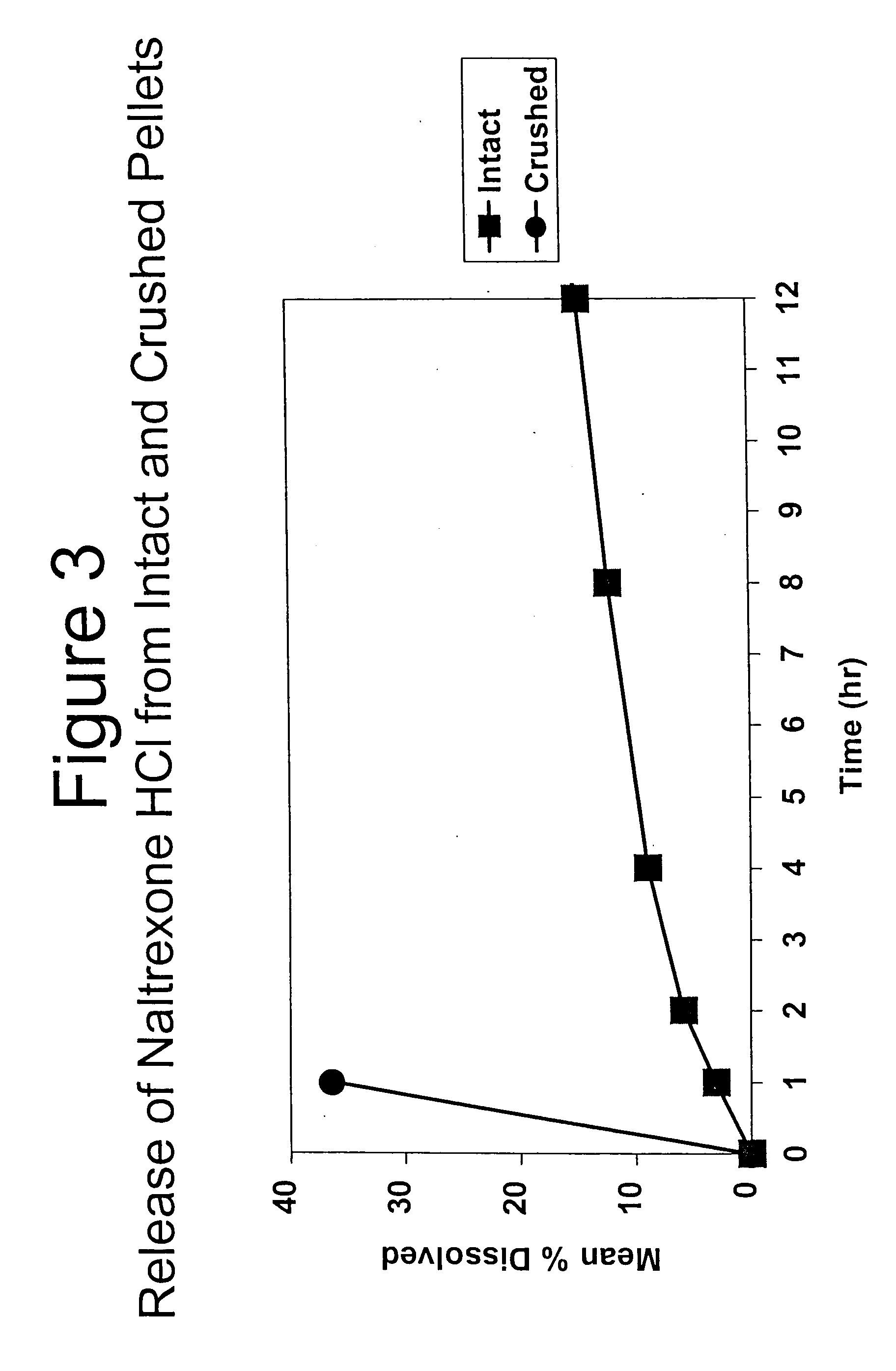
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
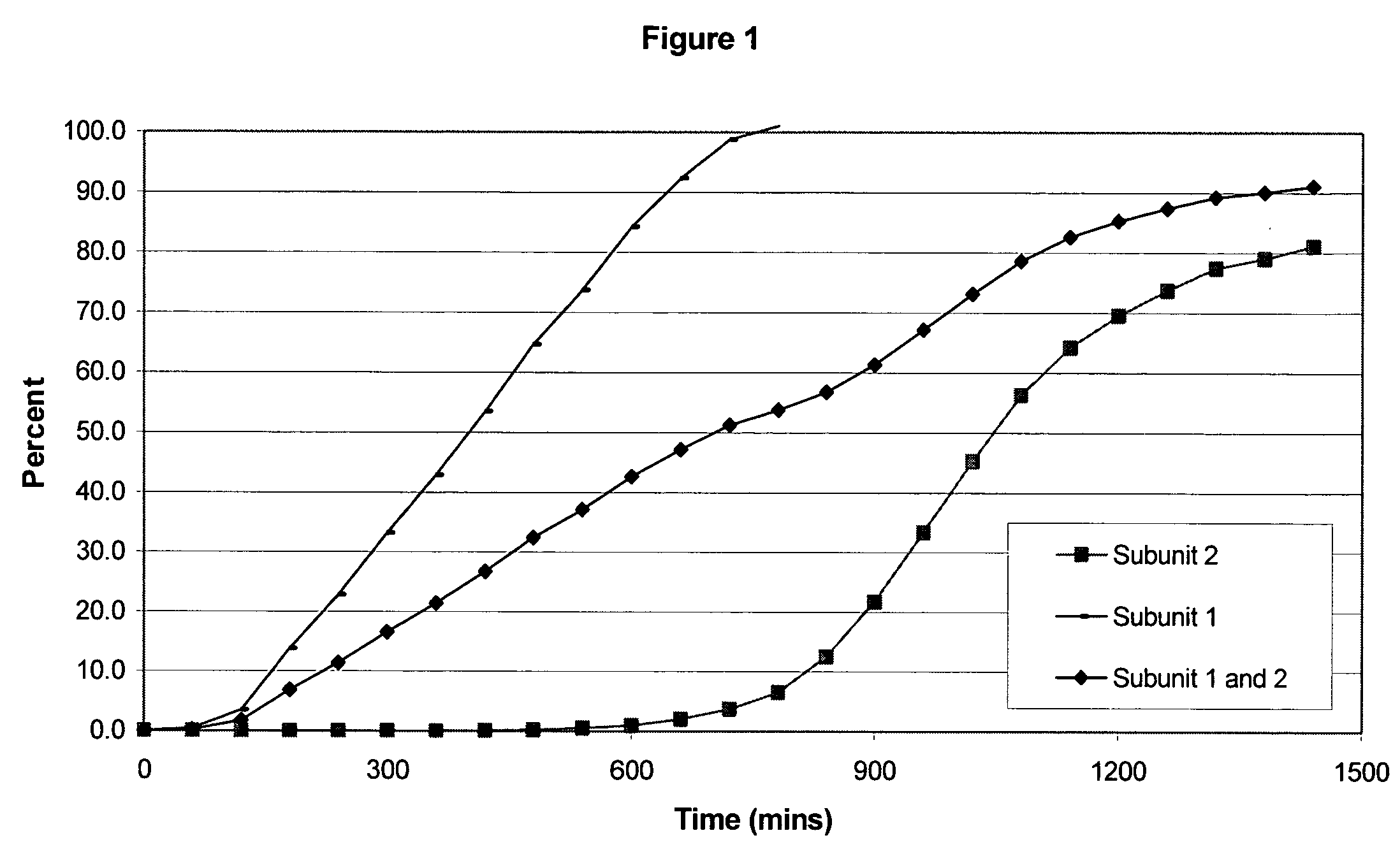
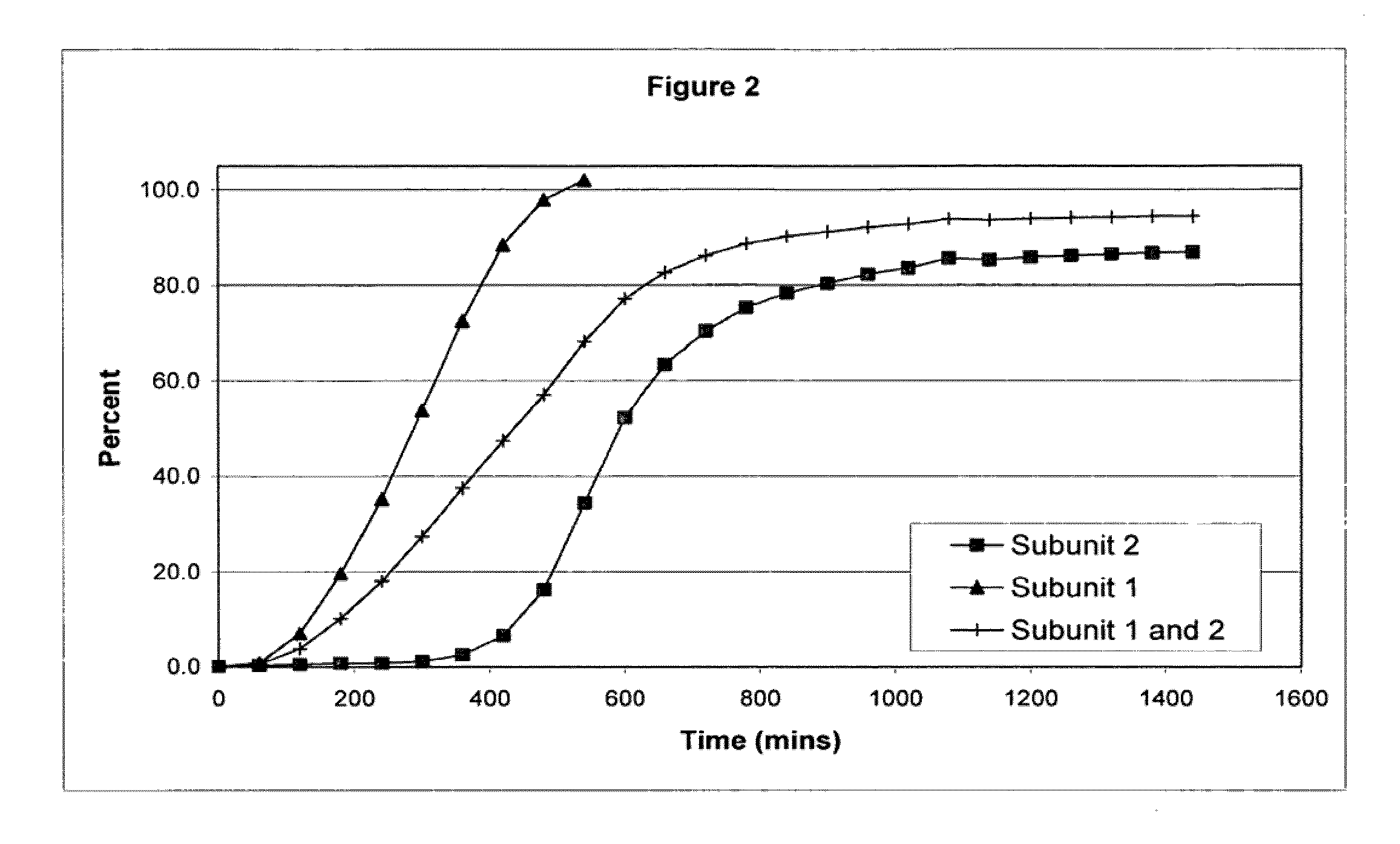
Sustained release opioid formulations and method of use
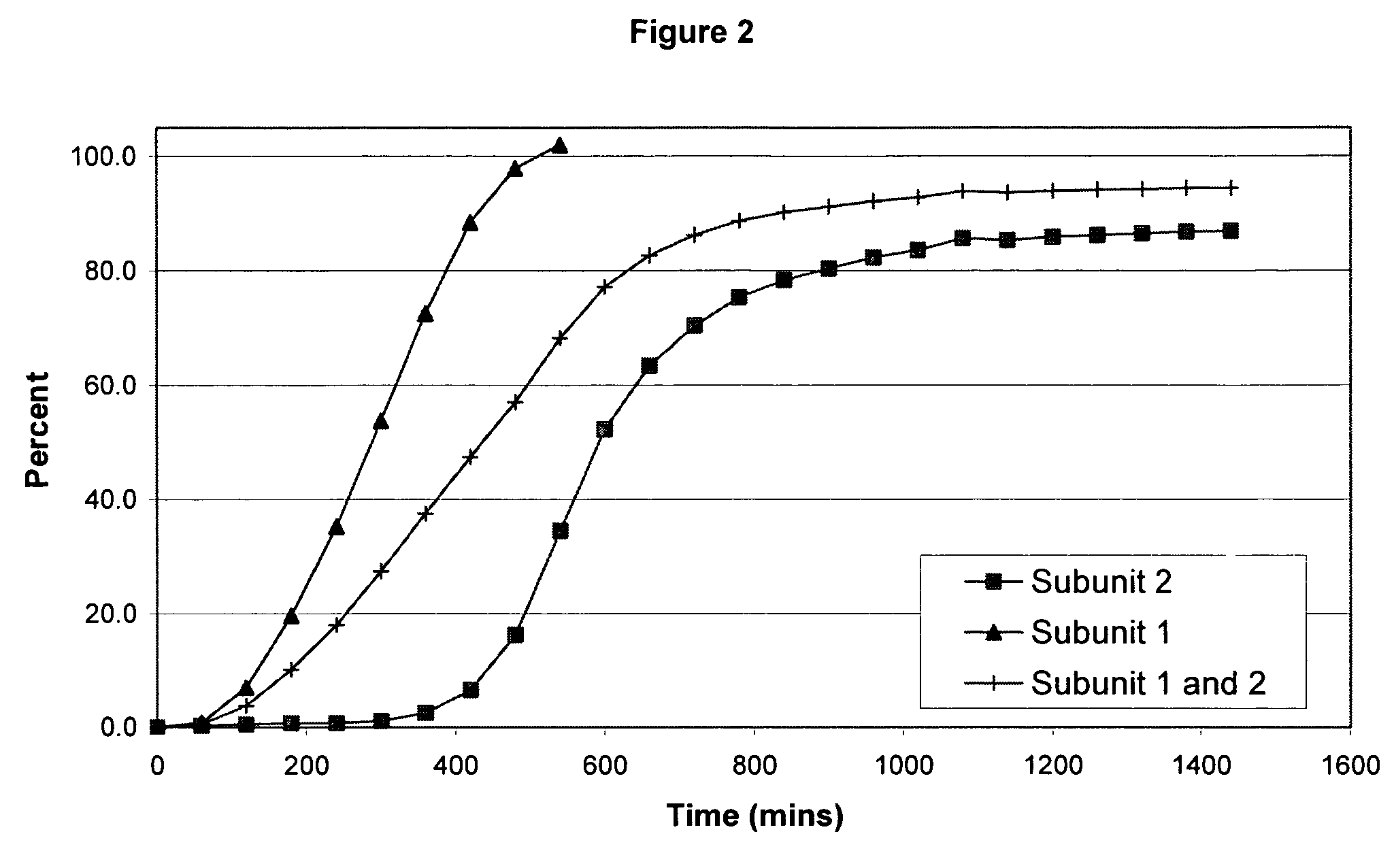
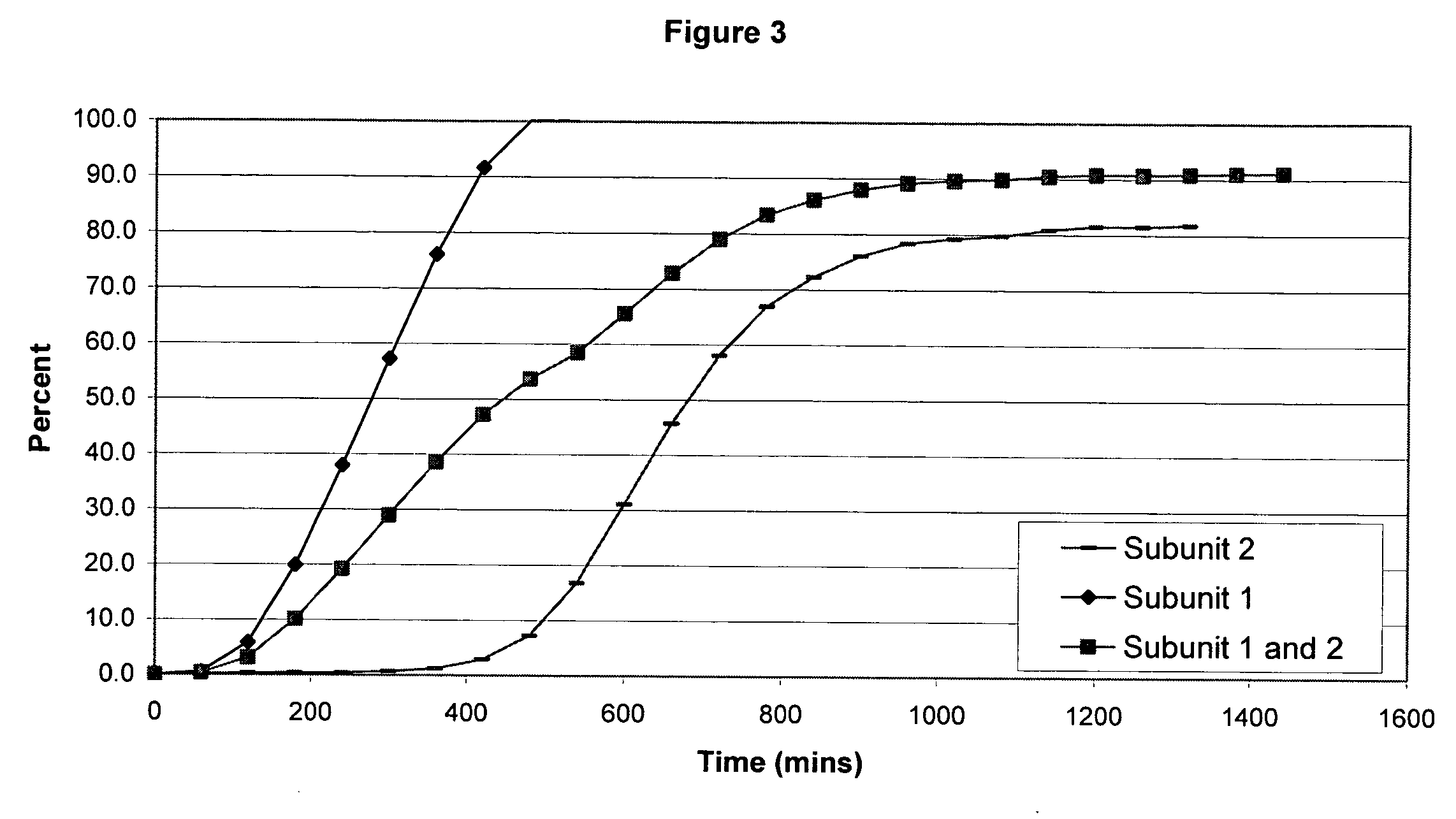
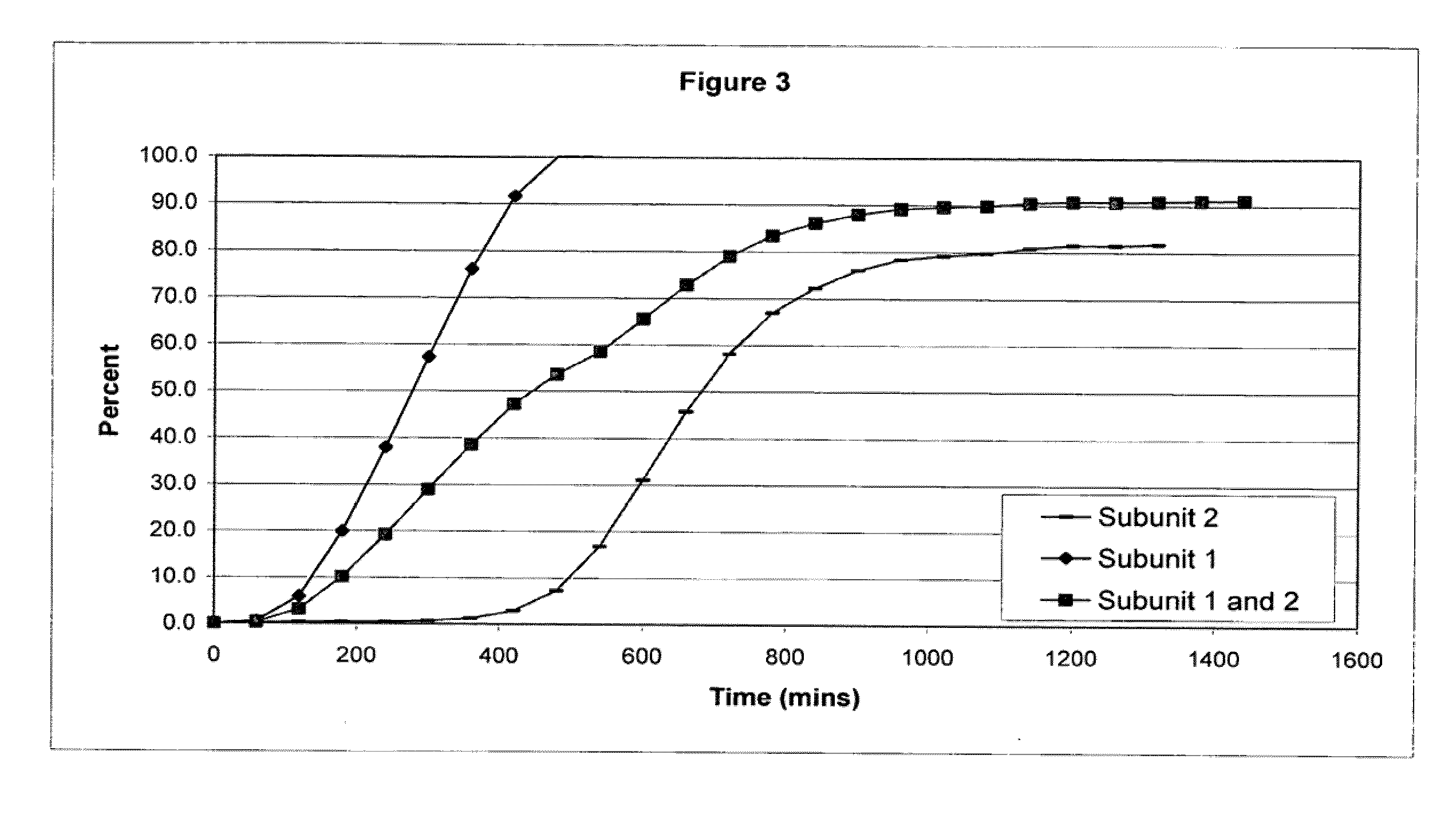
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; less than about 10% within about 10 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
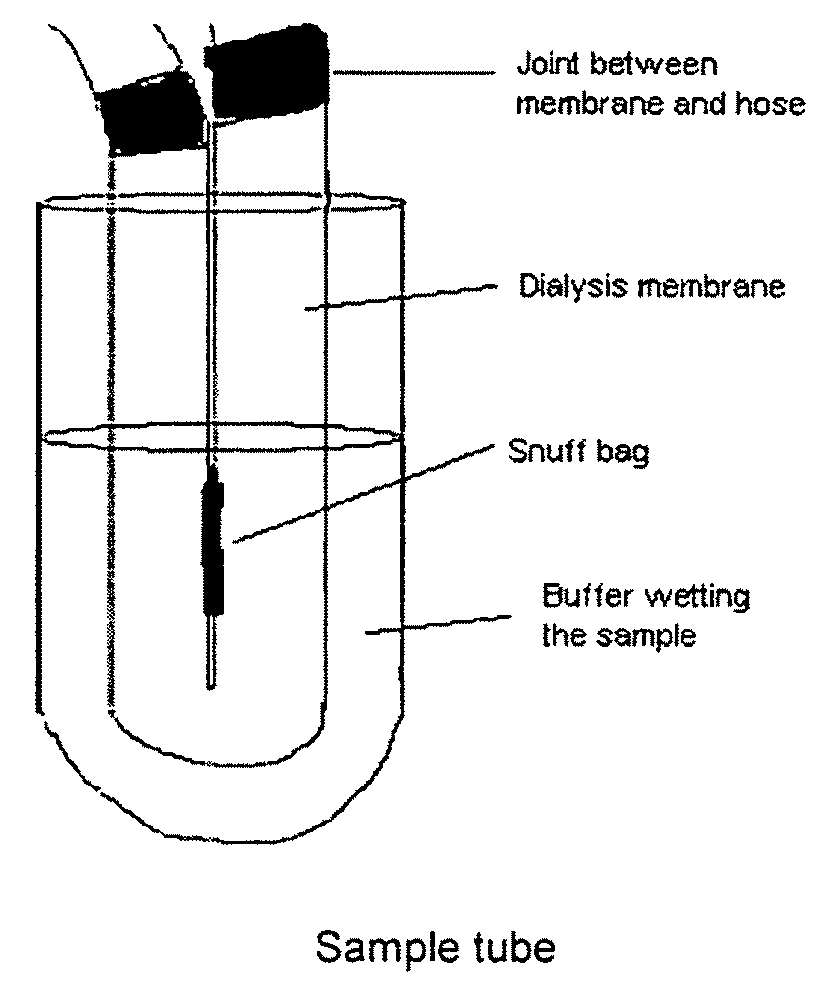
Snuff Composition
ActiveUS20090293895A1Quick effectRapid onsetPowder deliveryOrganic active ingredientsCellulosePharmacology
Use of a nicotine-cellulose combination for the preparation of a snuff composition for achievement of a fast onset of action of nicotine after application of the snuff composition to the oral cavity of a subject, wherein the composition has a high release rate so that when subjected to an in vitro dissolution test about 45% or more of the total content of nicotine is released within 30 minutes. Moreover, the invention relates to an improved snuff composition for application to the oral cavity.
Owner:MODORAL BRANDS INC
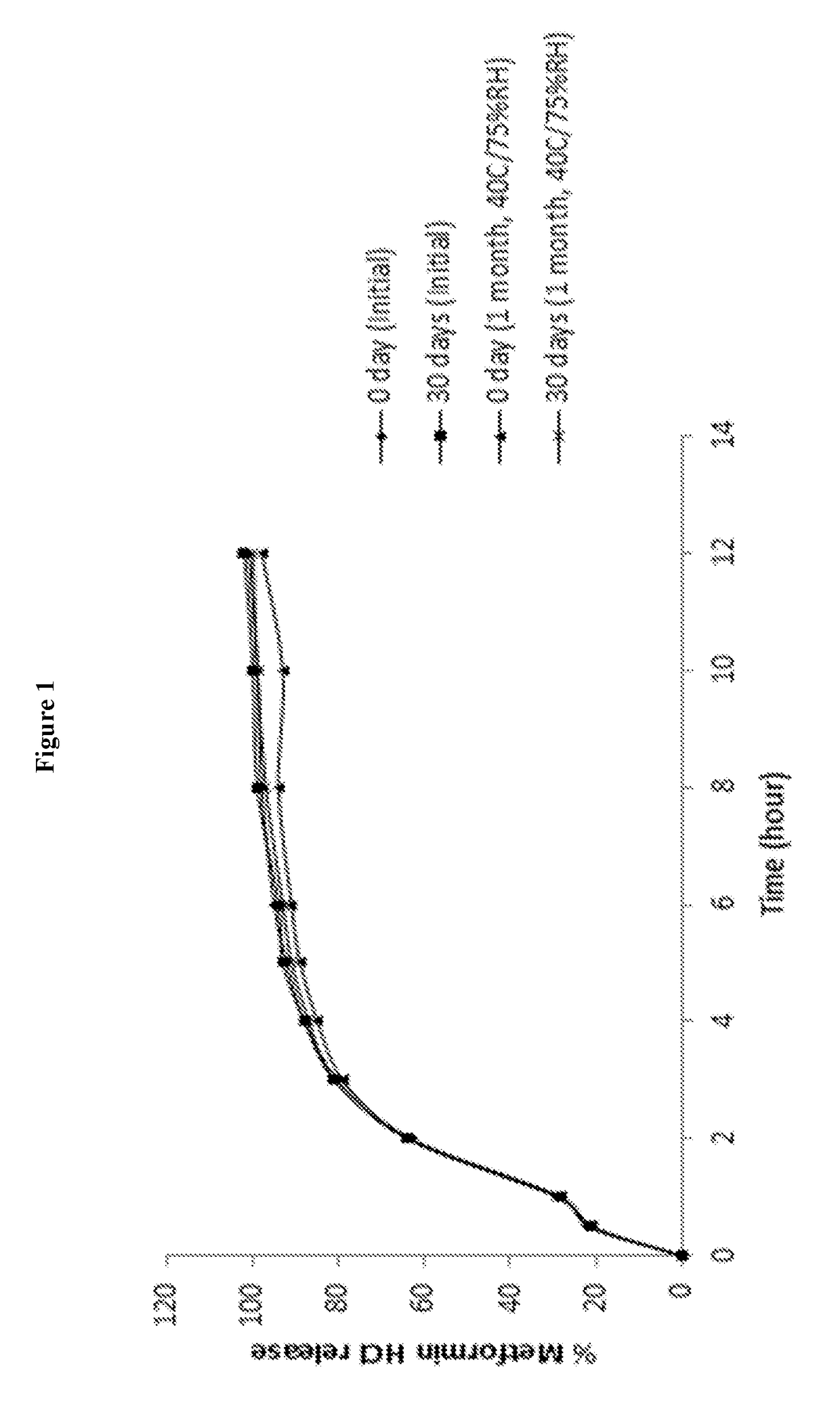
Direct compression metformin hydrochloride tablets
InactiveUS6117451AGood compressibilityImproved flowabilityPowder deliveryBiocideMetformin HydrochlorideHigh doses
Metformin Hydrochloride (herein referred to as metformin HCl) that may be 98.5%-100% pure is a high dose drug capable of being directly compressed with specific excipients into tablets having desired, hardness, disintegrating ability, and acceptable dissolution characteristics. Metformin HCl is not inherently compressible and thus presents formulation problems. Excipients used in the formulation enhance the flow and compaction properties of the drug and tableting mix. Optimal flow contributes to uniform die fill and weight control. The binder used ensures sufficient cohesive properties that allow metformin HCl to be compressed using the direct compression method. The tablets produced provide an acceptable in-vitro dissolution profile.
Owner:PHARMALOGIX
Encased Tamper Resistant Controlled Release Dosage Forms
ActiveUS20120164220A1Reducing abuse potential of dosage formBiocideNervous disorderGastric fluidEnzyme
In certain embodiments, the present invention is directed to a solid controlled release dosage form comprising: a core comprising a first portion of an opioid analgesic dispersed in a first matrix material; and a shell encasing the core and comprising a second portion of the opioid analgesic dispersed in a second matrix material; wherein the amount of opioid analgesic released from the dosage form is proportional within 20% to elapsed time from 8 to 24 hours, as measured by an in-vitro dissolution in a USP Apparatus 1 (basket) at 100 rpm in 900 ml simulated gastric fluid without enzymes (SGF) at 37 C.
Owner:PURDUE PHARMA LP
Pharmaceutical compositions of rifaximin
ActiveUS20090028940A1Extended stayImprove complianceAntibacterial agentsBiocideImmediate releasePharmaceutical medicine
A pharmaceutical composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s) and release controlling agent(s). Pharmaceutical composition of rifaximin comprising: at least two entities wherein one entity is an immediate release or fast release and the other is controlled release. The pharmaceutical composition in the form of multilayer tablet comprising, at least one layer comprising, therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s); said layer providing controlled release rifaximin; and at least one layer which provides increased residence time of the dosage form in the gastrointestinal tract. The pharmaceutical formulation comprising rifaximin having an in vitro dissolution profile, wherein about 70% of rifaximin is released in about 24 hours. The composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt(s) or enantiomer(s) or polymorph(s) thereof, one or more release controlling agent(s) and pharmaceutically acceptable excipient(s) causing pathogenic eradication.
Owner:LUPIN LTD
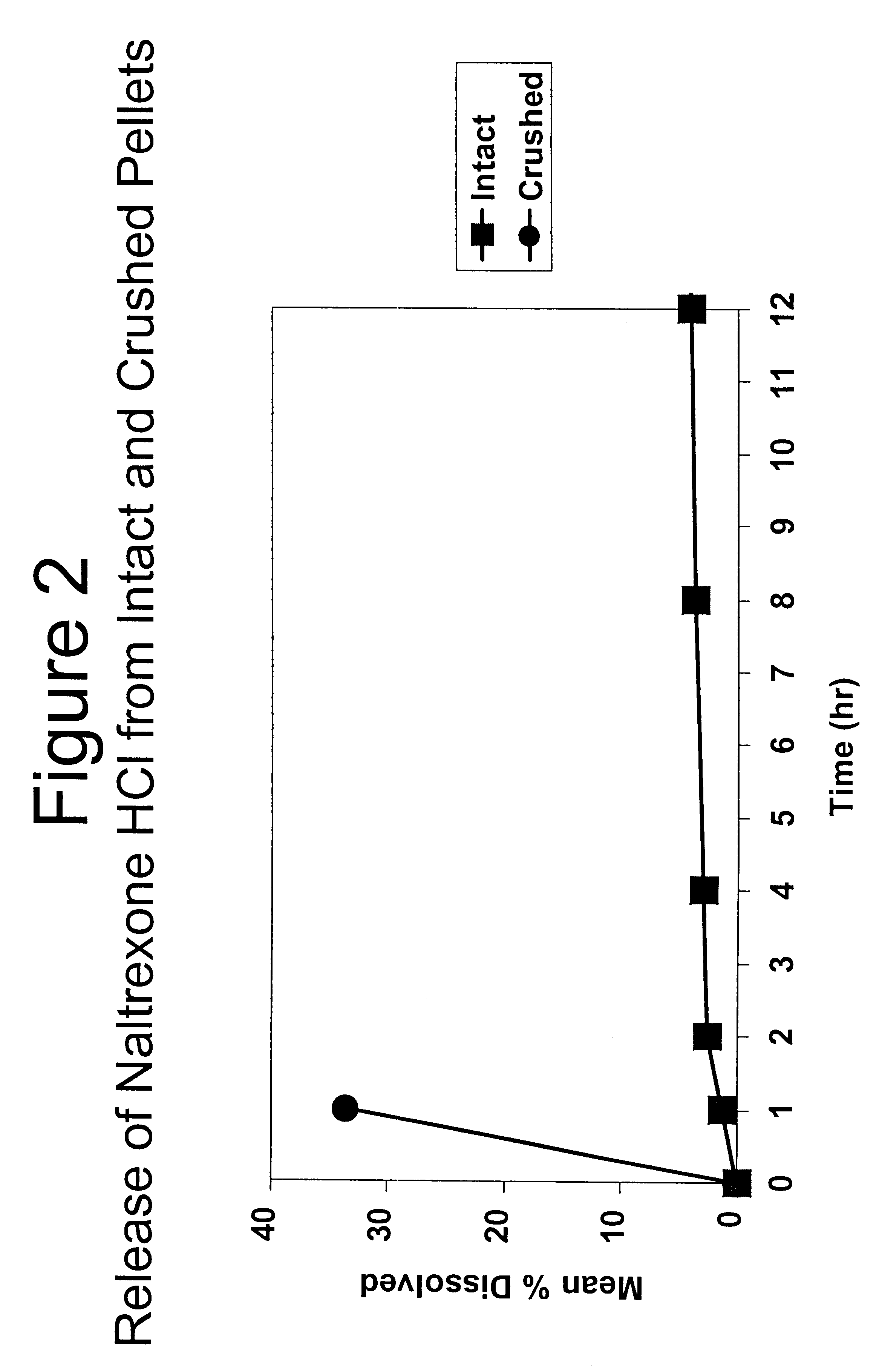
Alcohol Resistant Dosage Forms
Disclosed in certain embodiments is a controlled release dosage form comprising a matrix comprising a pharmaceutically acceptable salt of an opioid analgesic in a controlled release material; wherein less than 25% of the opioid salt is released after 1 hour of in-vitro dissolution of the dosage form in 900 ml of Simulated Gastric Fluid with 20% ethanol using a USP Apparatus I (basket) apparatus at 100 rpm at 37 degrees C.°.
Owner:PURDUE PHARMA LP
Tamper-resistant oral opioid agonist formulations
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
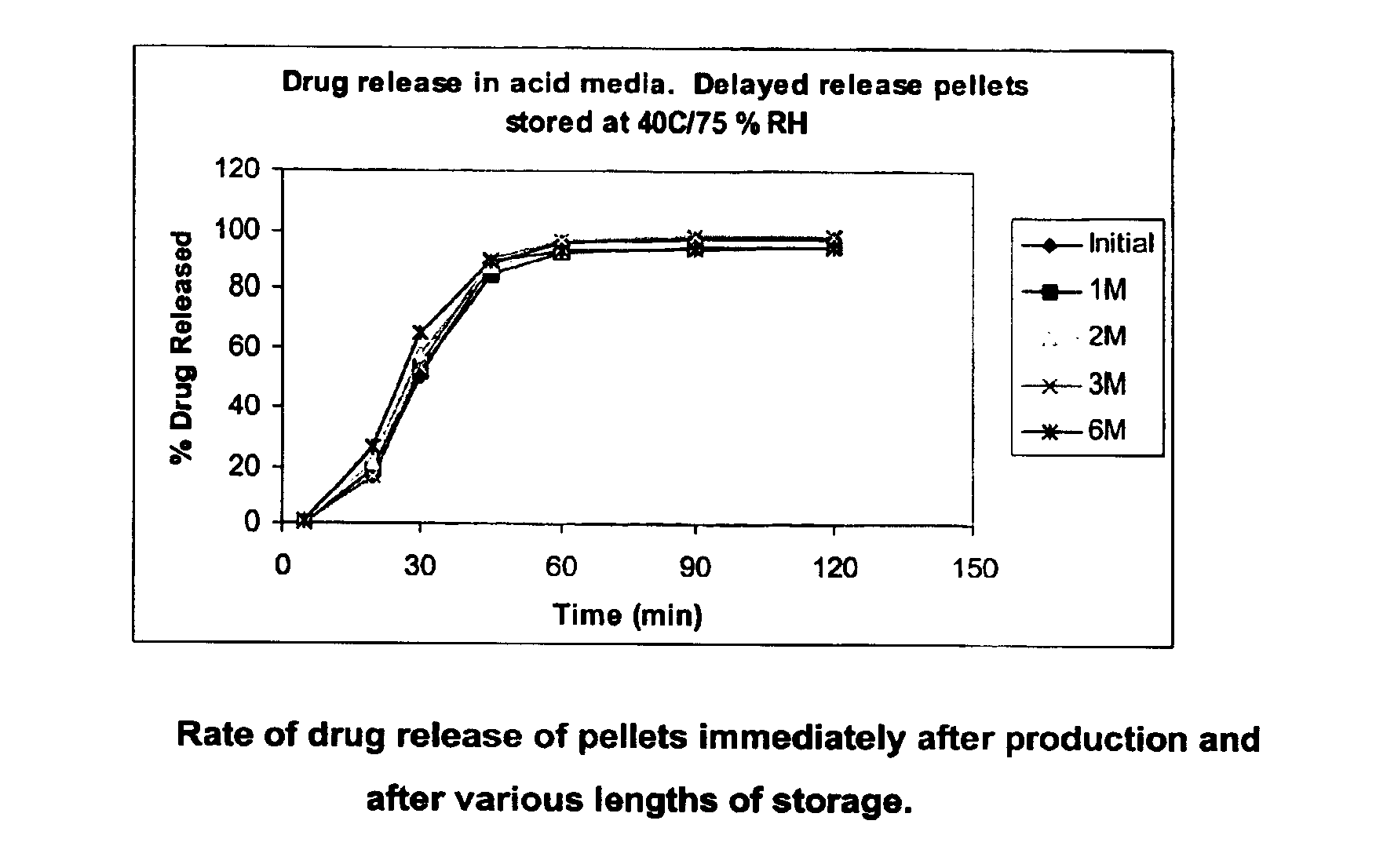
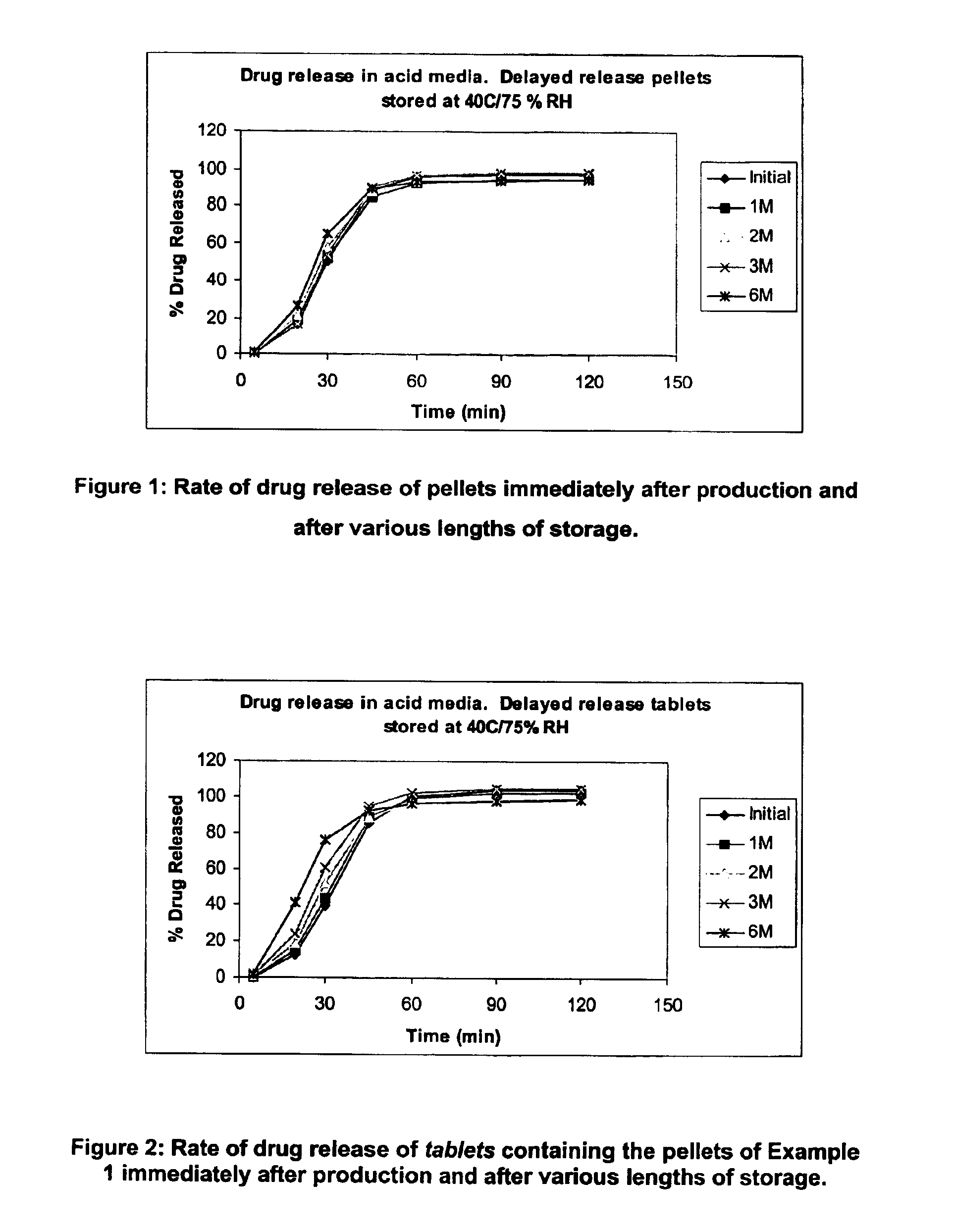
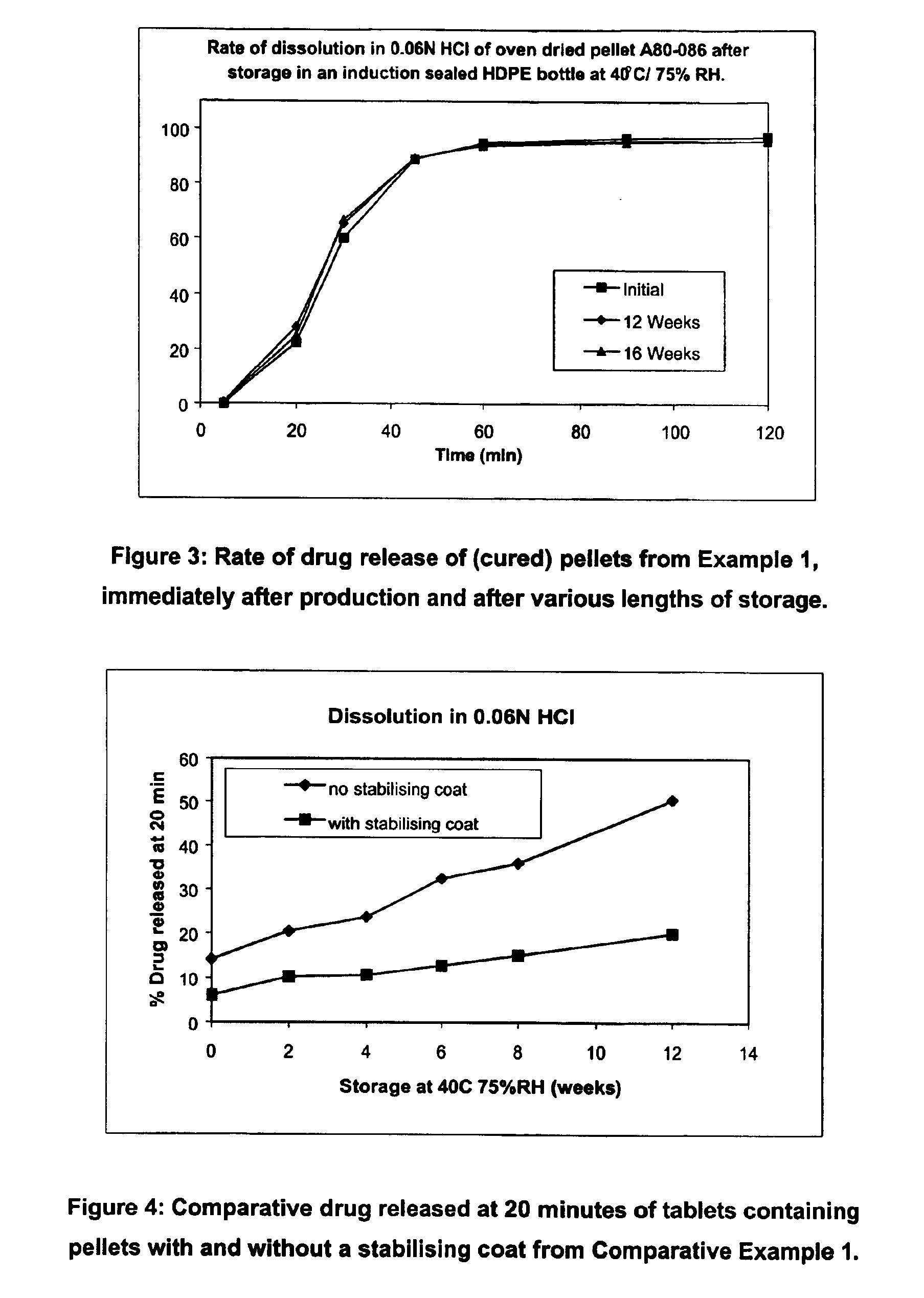
Modified release coated drug preparation
InactiveUS6958161B2Extension of timeImprove trustBiocideTetracycline active ingredientsDissolutionBULK ACTIVE INGREDIENT
A modified release preparation having one or more coated core elements, each core element including an active ingredient and having a modified release coating, wherein a stabilising coat is provided between each core element and its modified release coating so that, upon in vitro dissolution testing, the amount of active ingredient released at any time on a post-storage dissolution profile is within 40 percentage points of the amount of active ingredient released at any time on a pre-storage dissolution profile.
Owner:MAYNE PHARMA INT
Microencapsulated and controlled-release formulations of isoflavone from enriched fractions of soy and other plants
InactiveUS6890561B1Facilitate user complianceMaintain activityPowder deliveryOrganic active ingredientsAdditive ingredientBULK ACTIVE INGREDIENT
There is provided an orally-administrable formulation for the controlled release or stable storage of a granulated isoflavone-enriched fraction or mixture of such fractions, comprising at least one granulated isoflavone-enriched fraction and at least one carrier, diluent or excipient therefor. Preferably, the formulation is characterized in that the total in vitro dissolution time of said formulation required for release of 75% of the active ingredients available from the formulation is between about 4 and about 18 hours, as determined by the U.S.P. XXIII paddle method at a paddle speed of 75 rpm, using simulated intestinal fluid without the digestive enzymes normally found in intestinal fluid, at pH 6.8, and a temperature of 37° C. A process for the preparation of such a formulation is also provided.
Owner:LYCORED BIO
Sustained release opioid formulations and methods of use
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
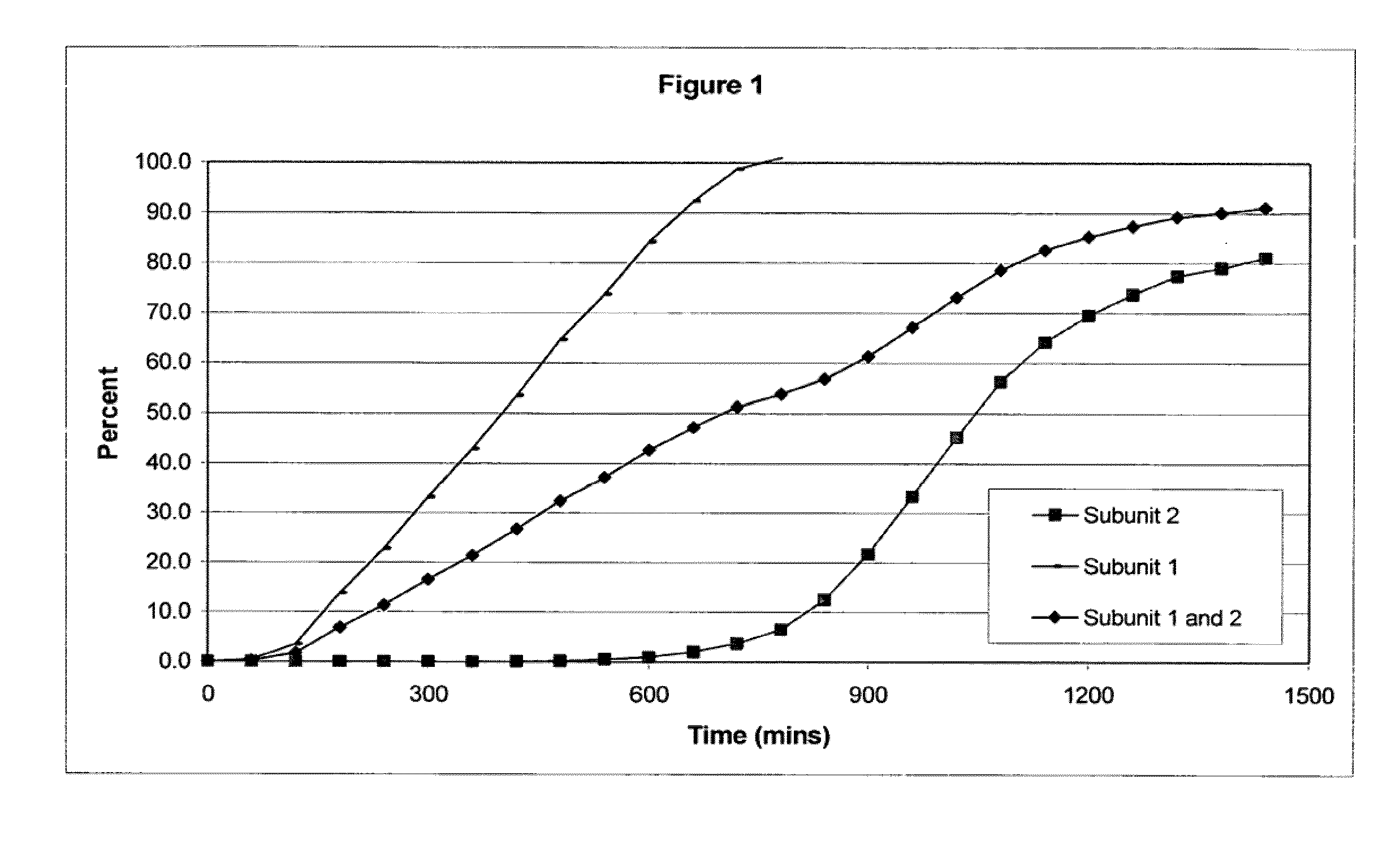
Stabilized sustained release tramadol formulations
InactiveUS6645527B2Stable dissolutionAvoid throughputOrganic active ingredientsNervous disorderWaxDissolution
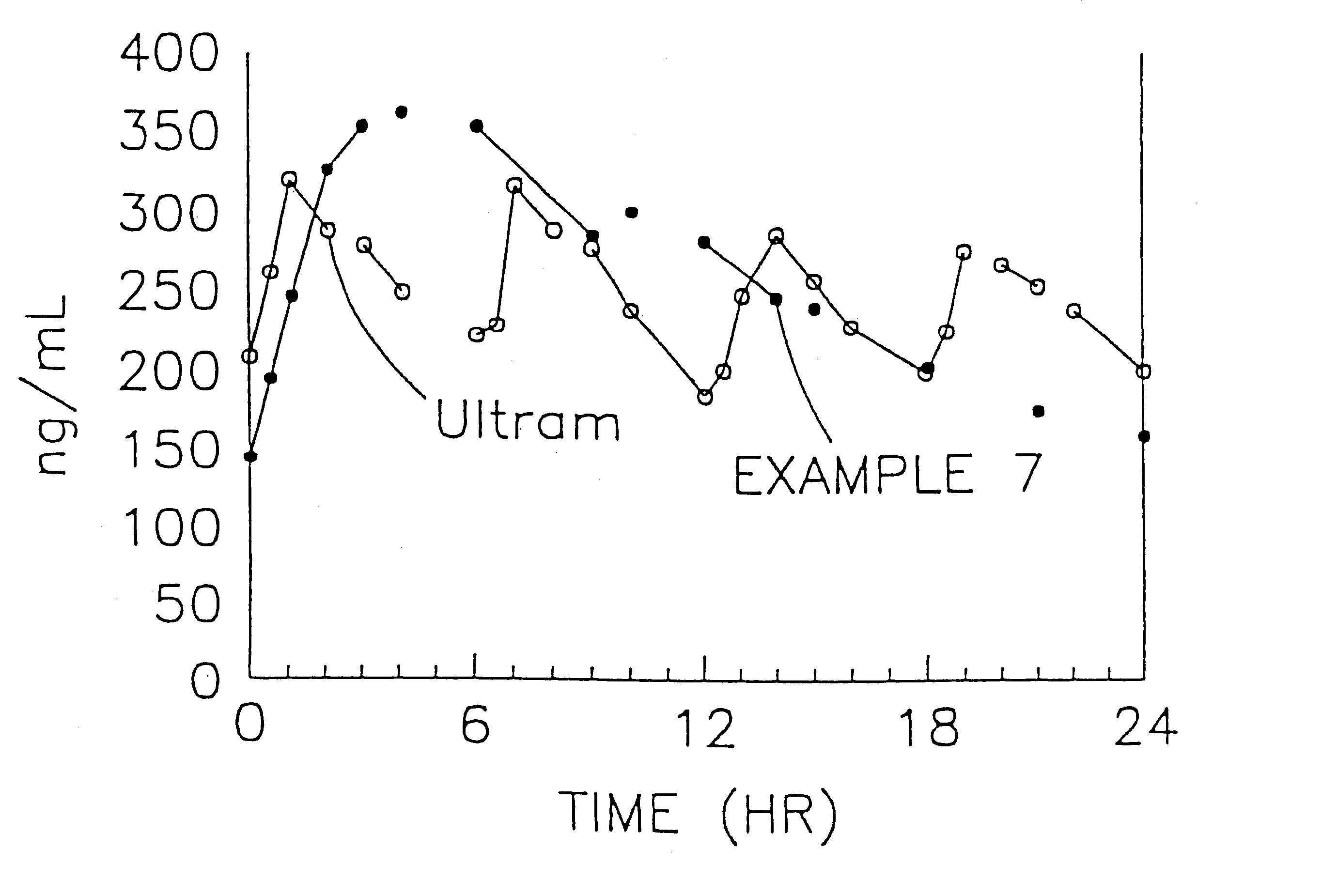
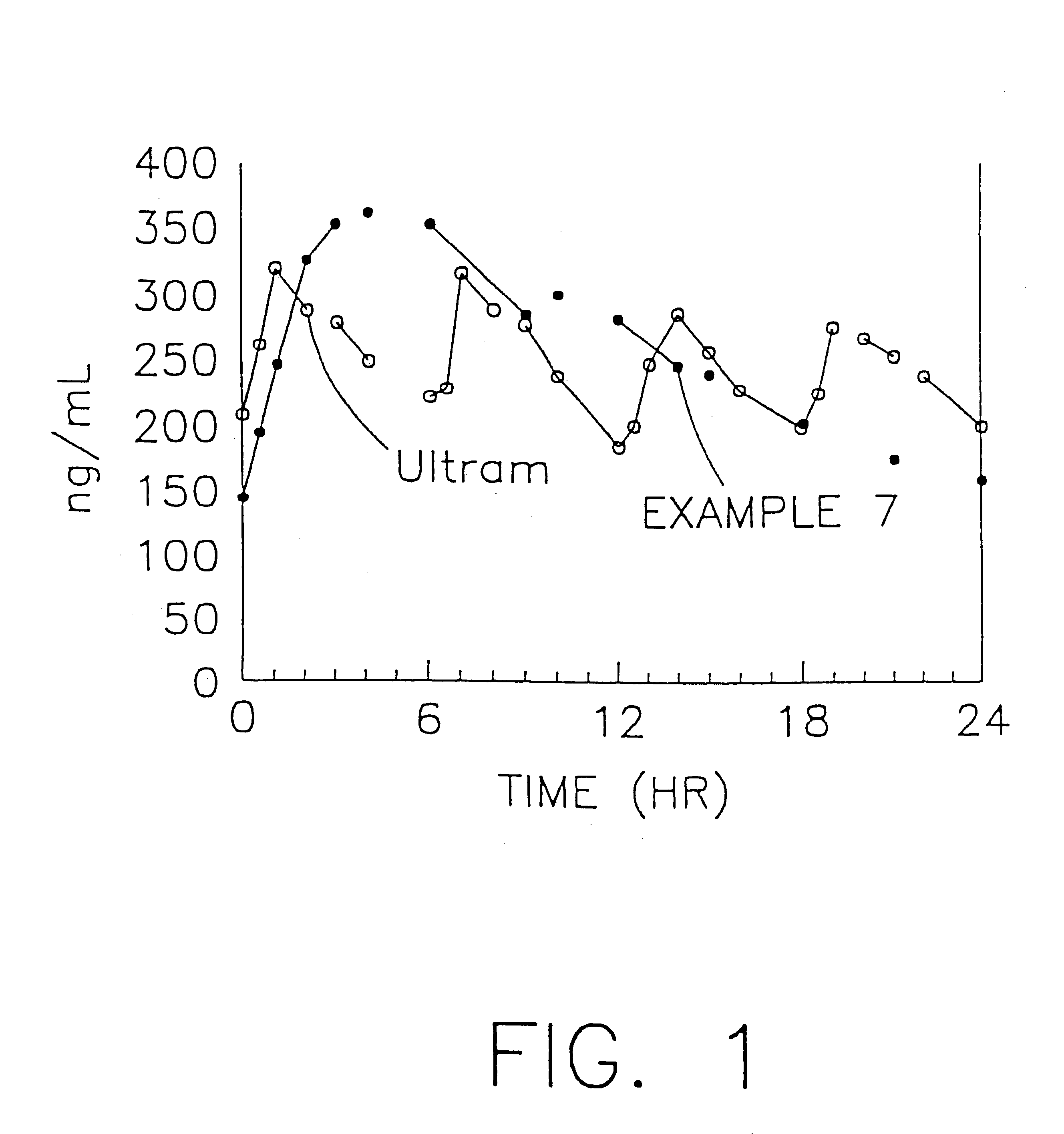
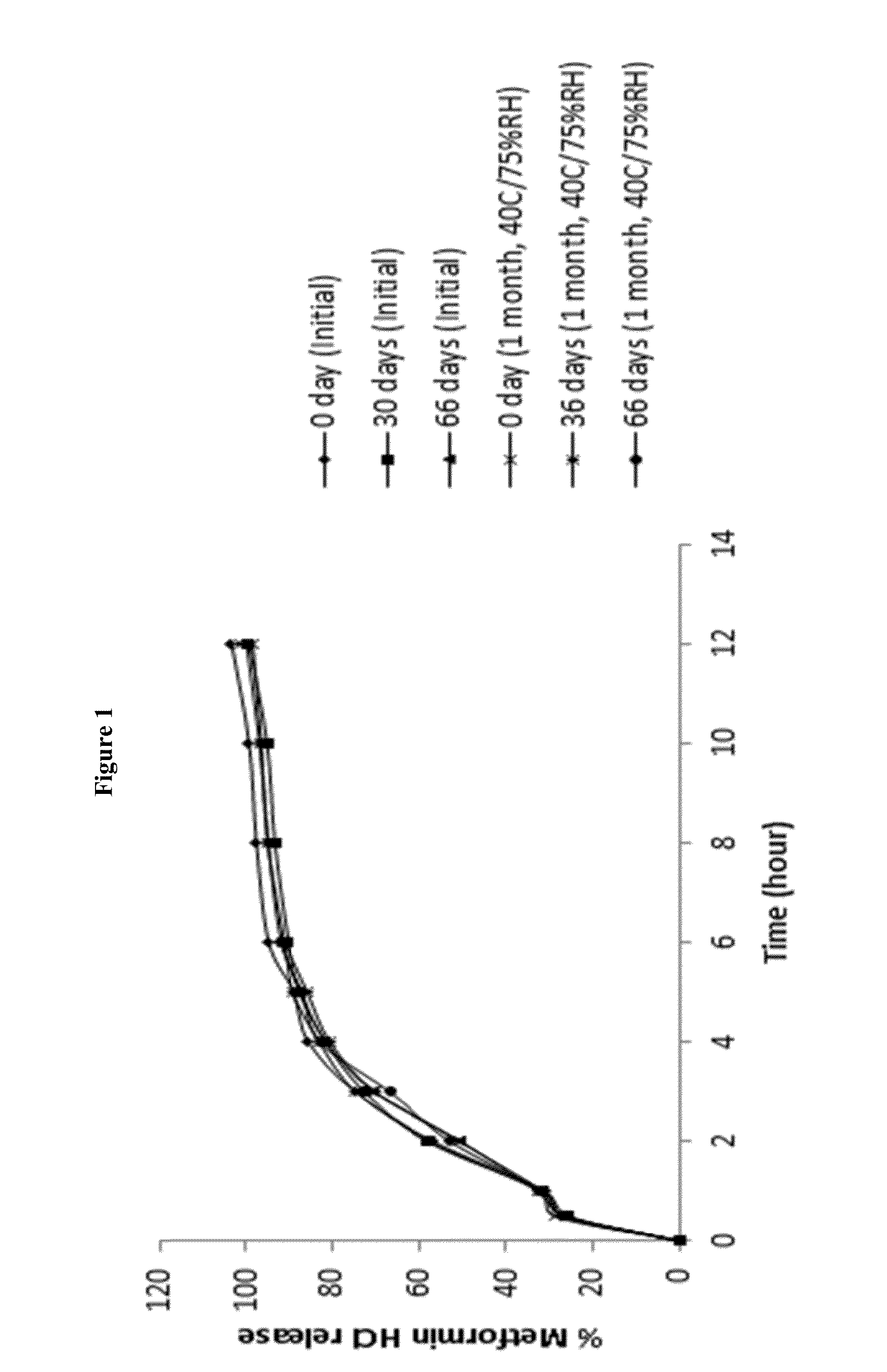
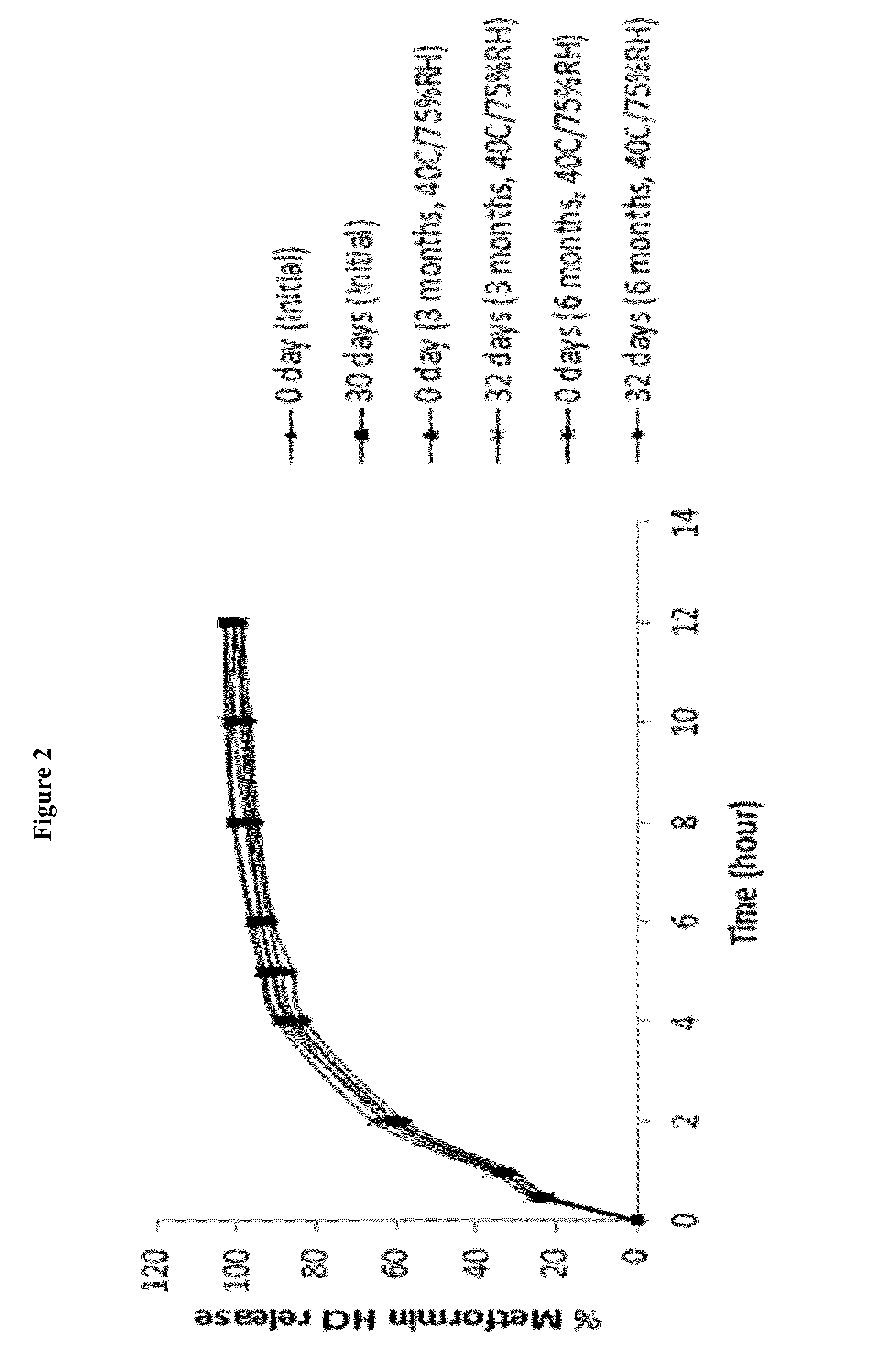
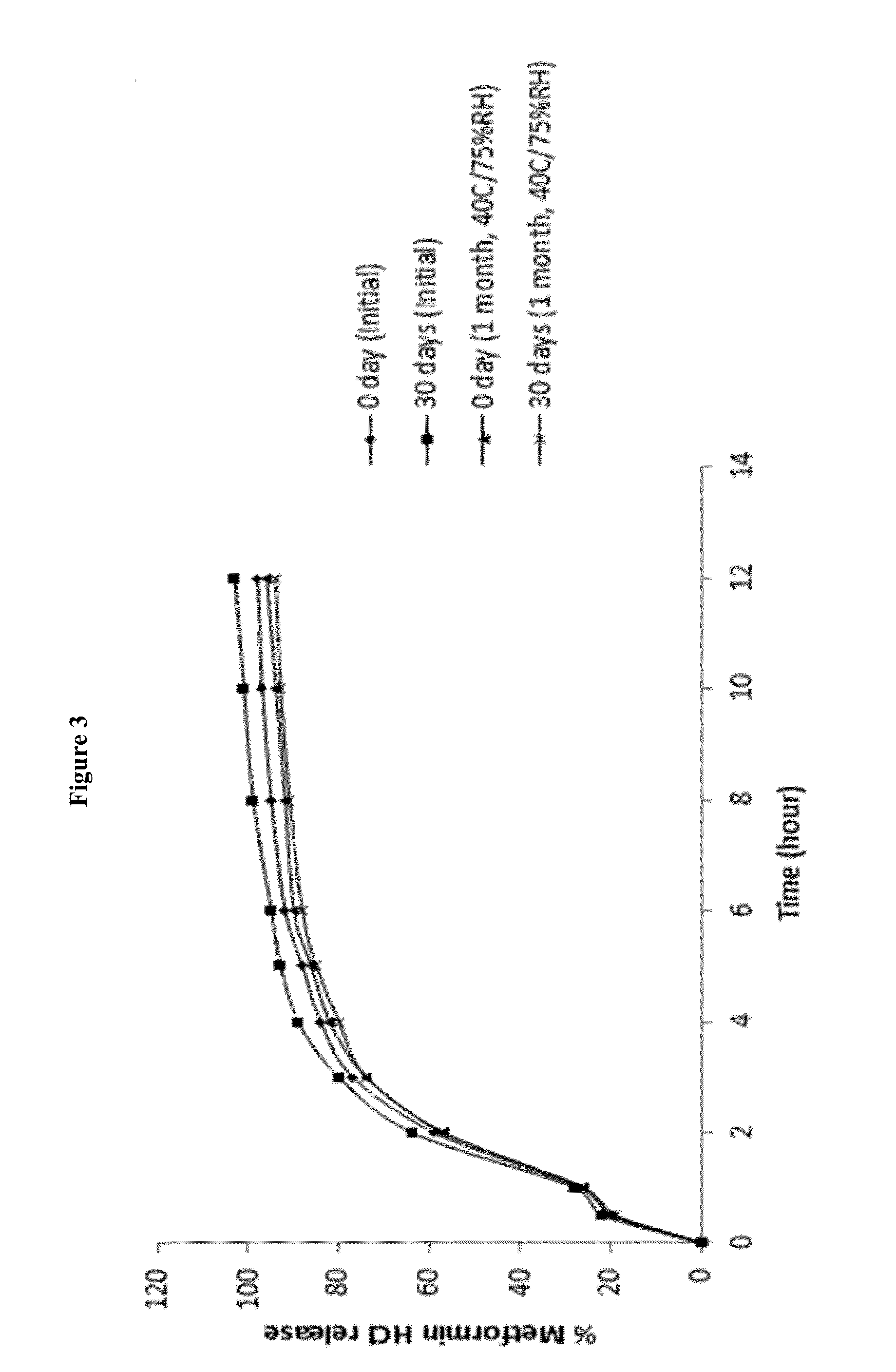
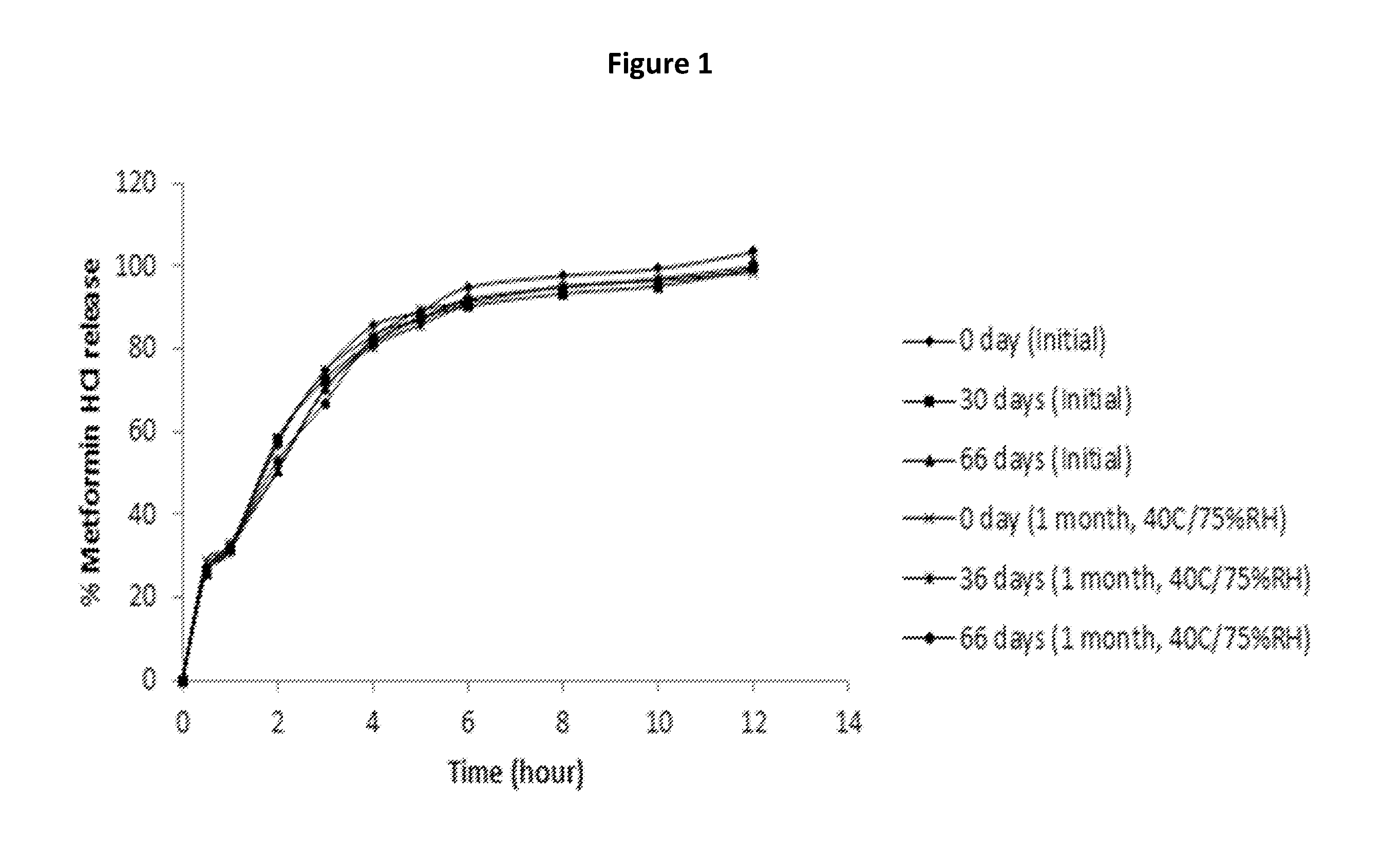
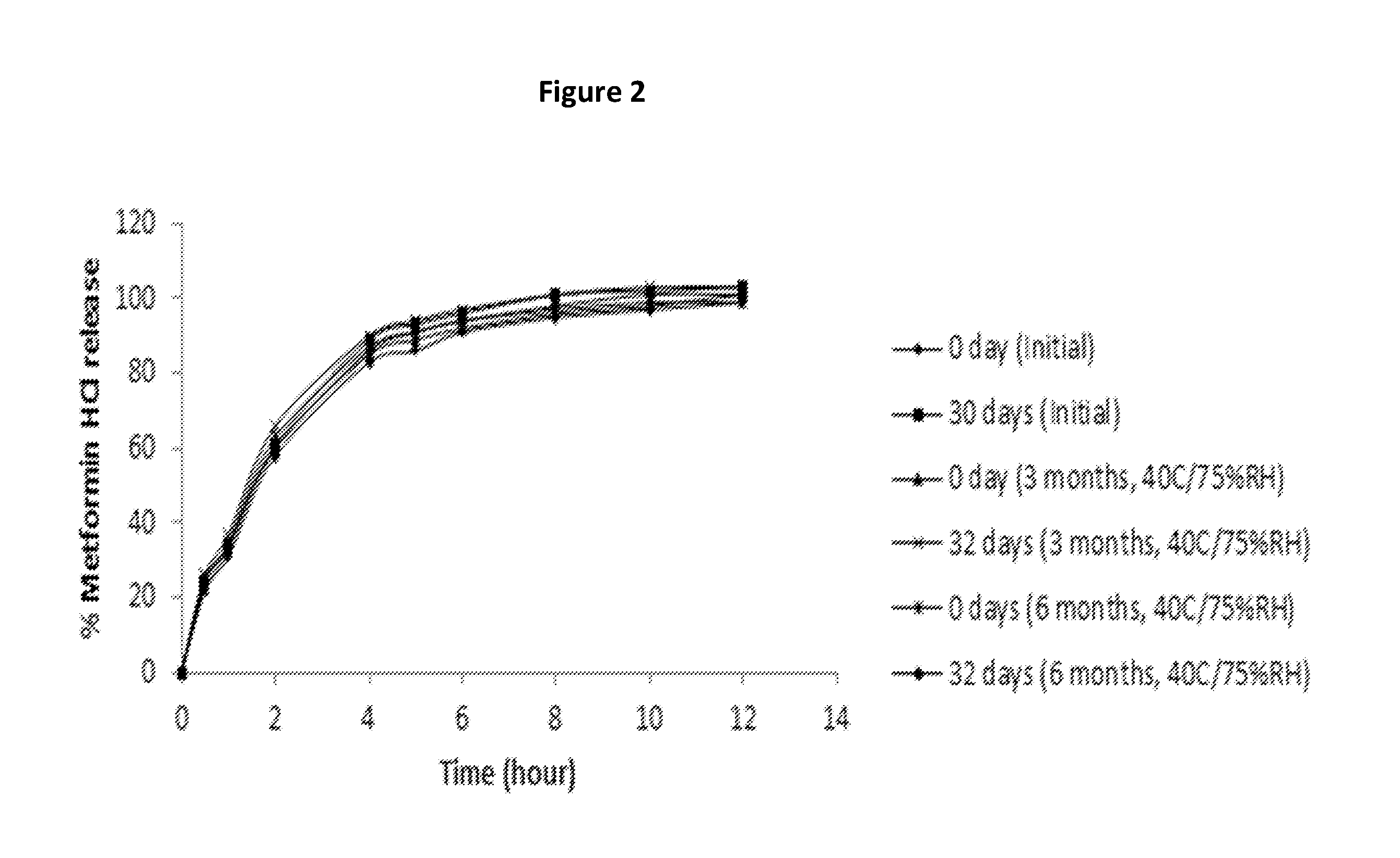
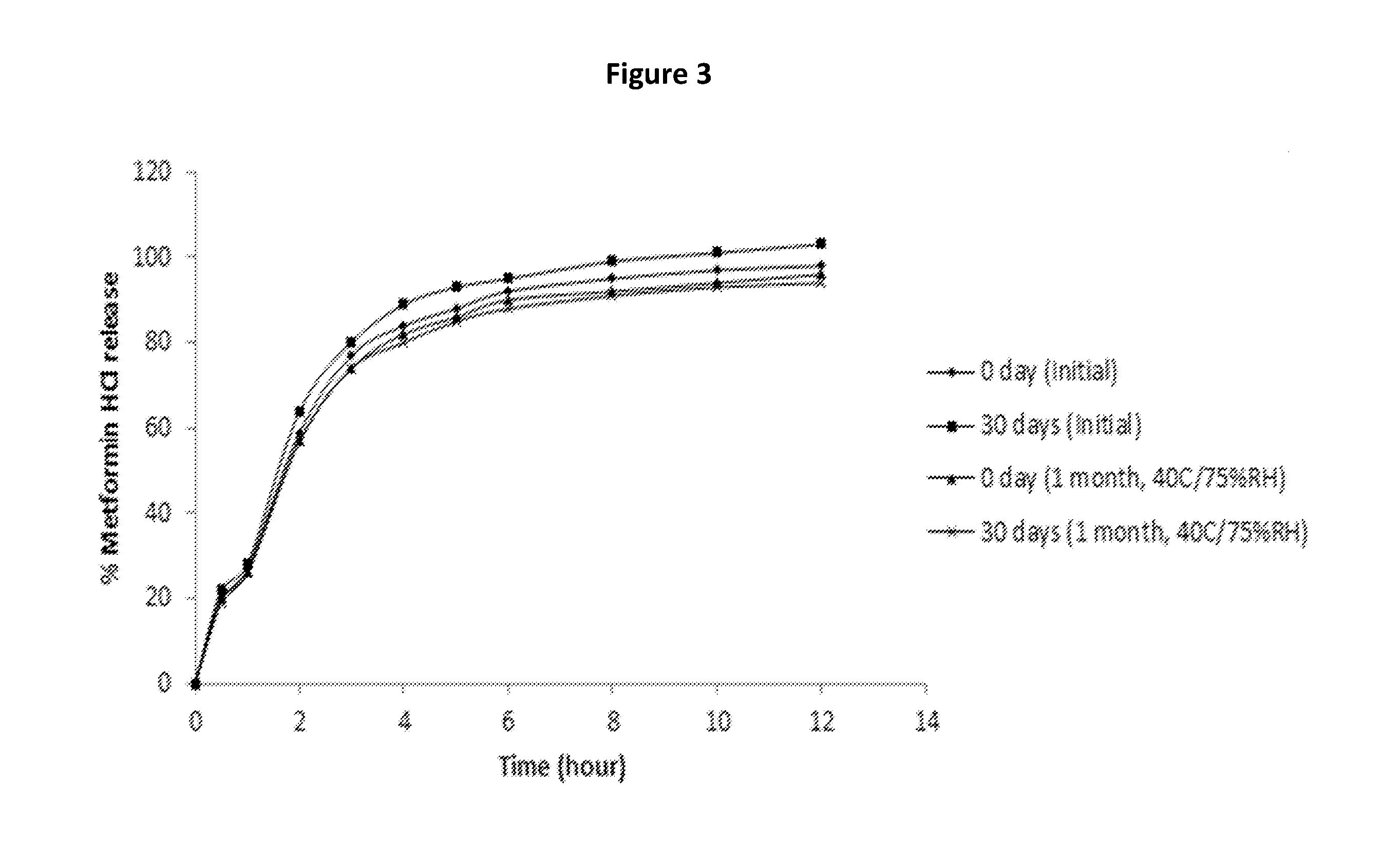
A stabilized sustained release oral solid dosage form which includes an effective amount of tramadol or a pharmaceutically acceptable salt thereof dispersed in a matrix of a hydrophobic material comprising a wax-like substance which was melted or softened during the preparation of the matrix, is cured at a temperature from about 35° C. to about 65° C. for a time period from about 4 to about 72 hours, such that the formulation, when subjected to in-vitro dissolution after exposure to accelerated storage conditions of at least one month at 40° C. / 75% RH, releases an amount of tramadol which does not vary at any given dissolution time point by more than about 20% of the total amount of tramadol released when compared to in-vitro dissolution conducted prior to subjecting the dosage form to the accelerated storage conditions.
Owner:PURDUE PHARMA LP
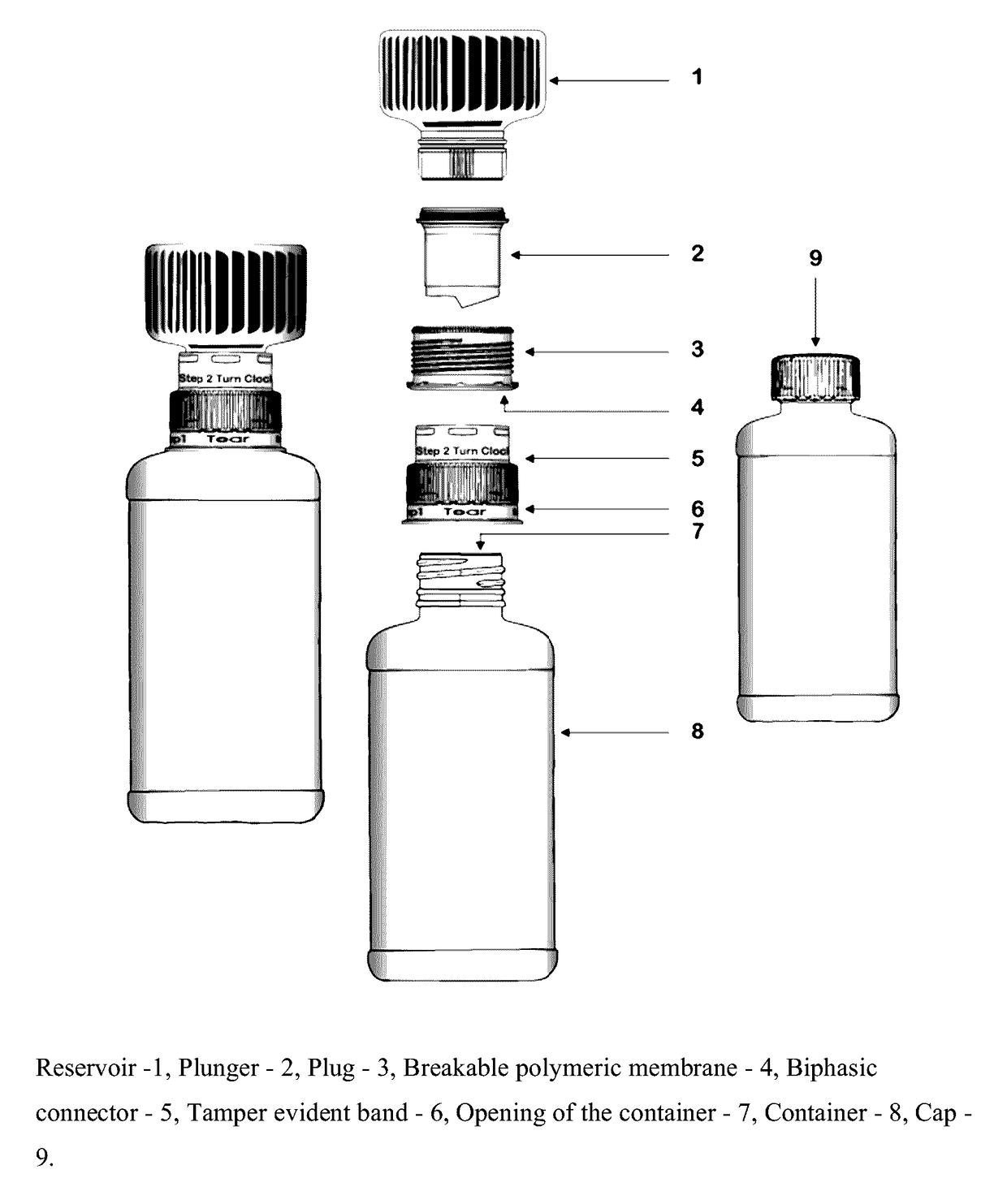
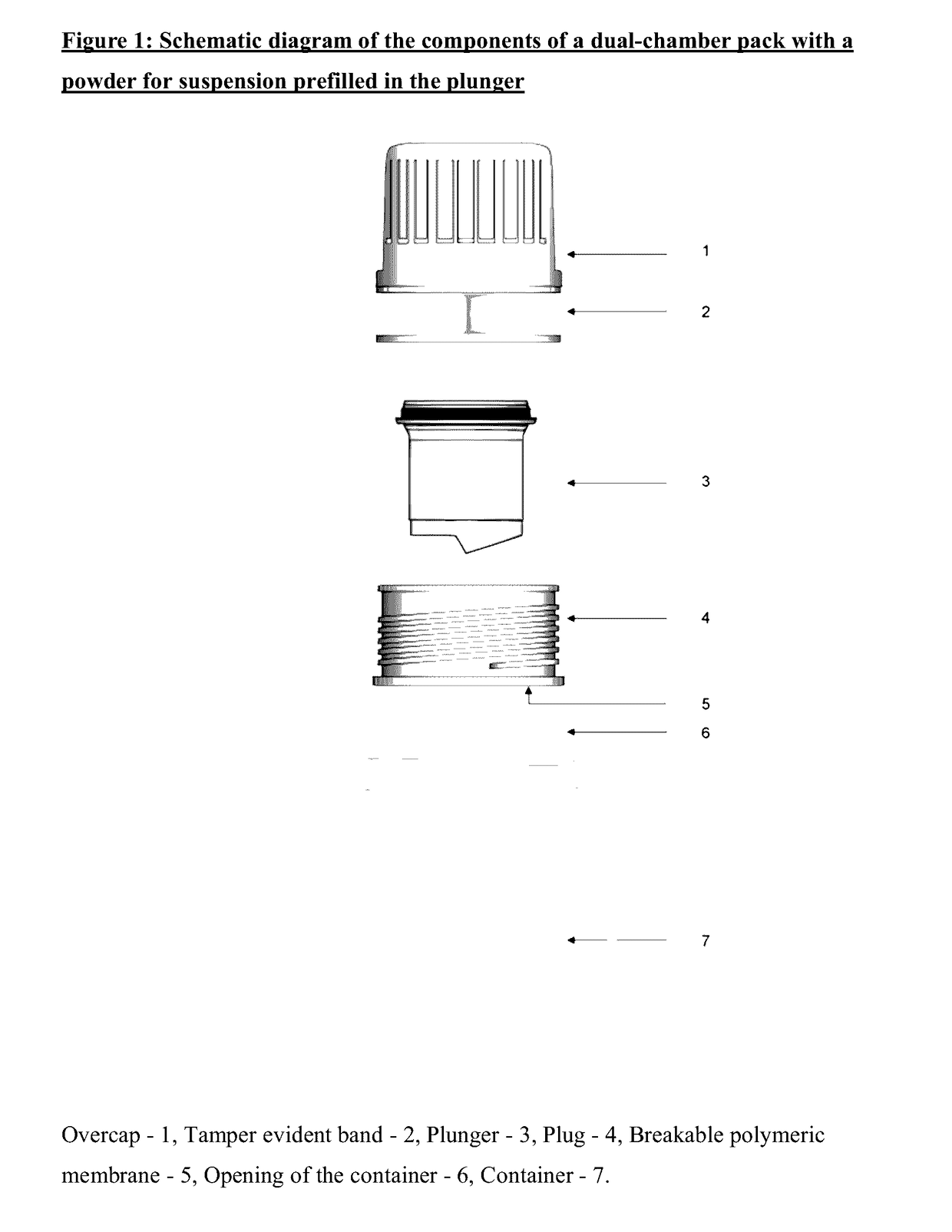
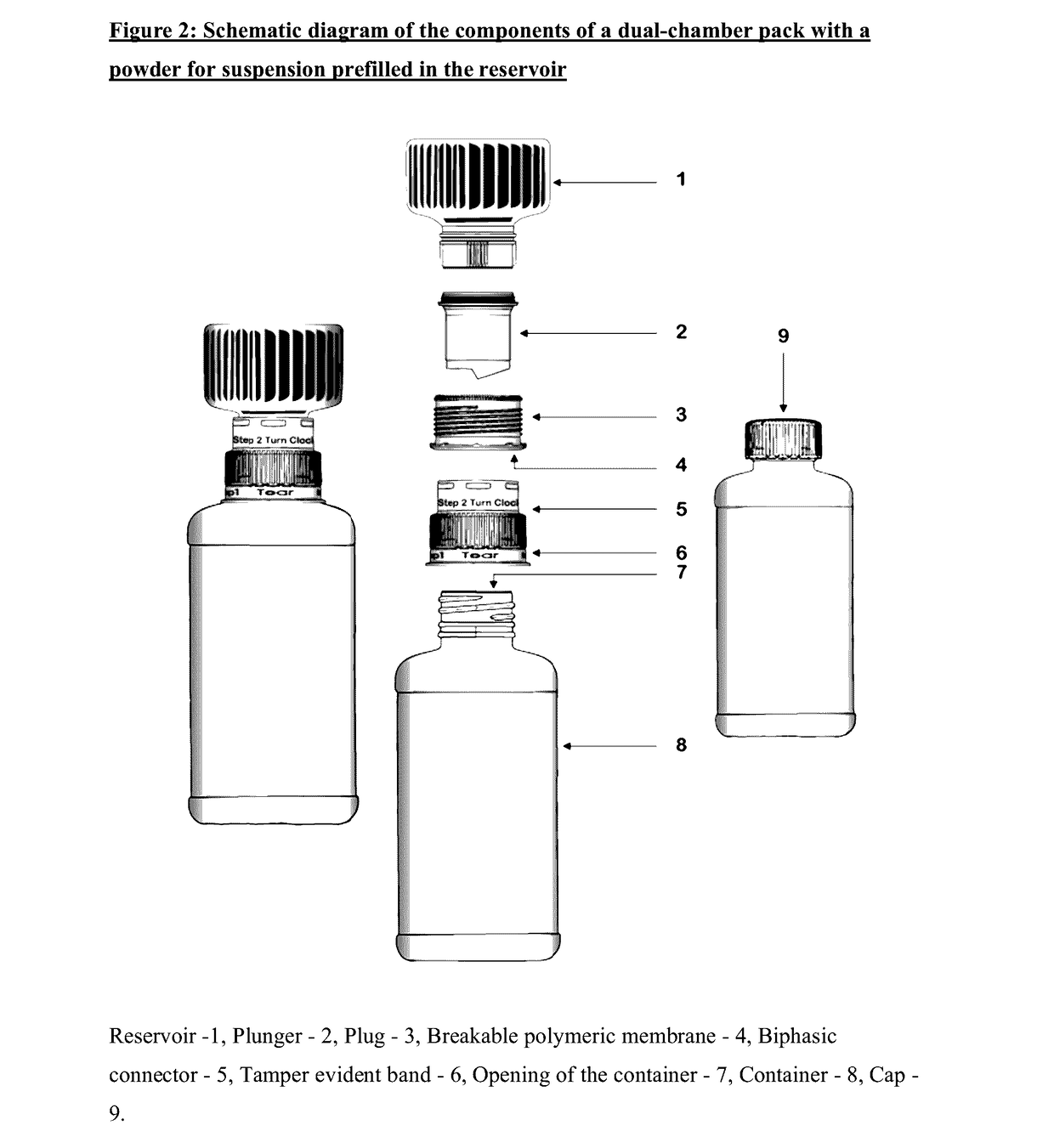
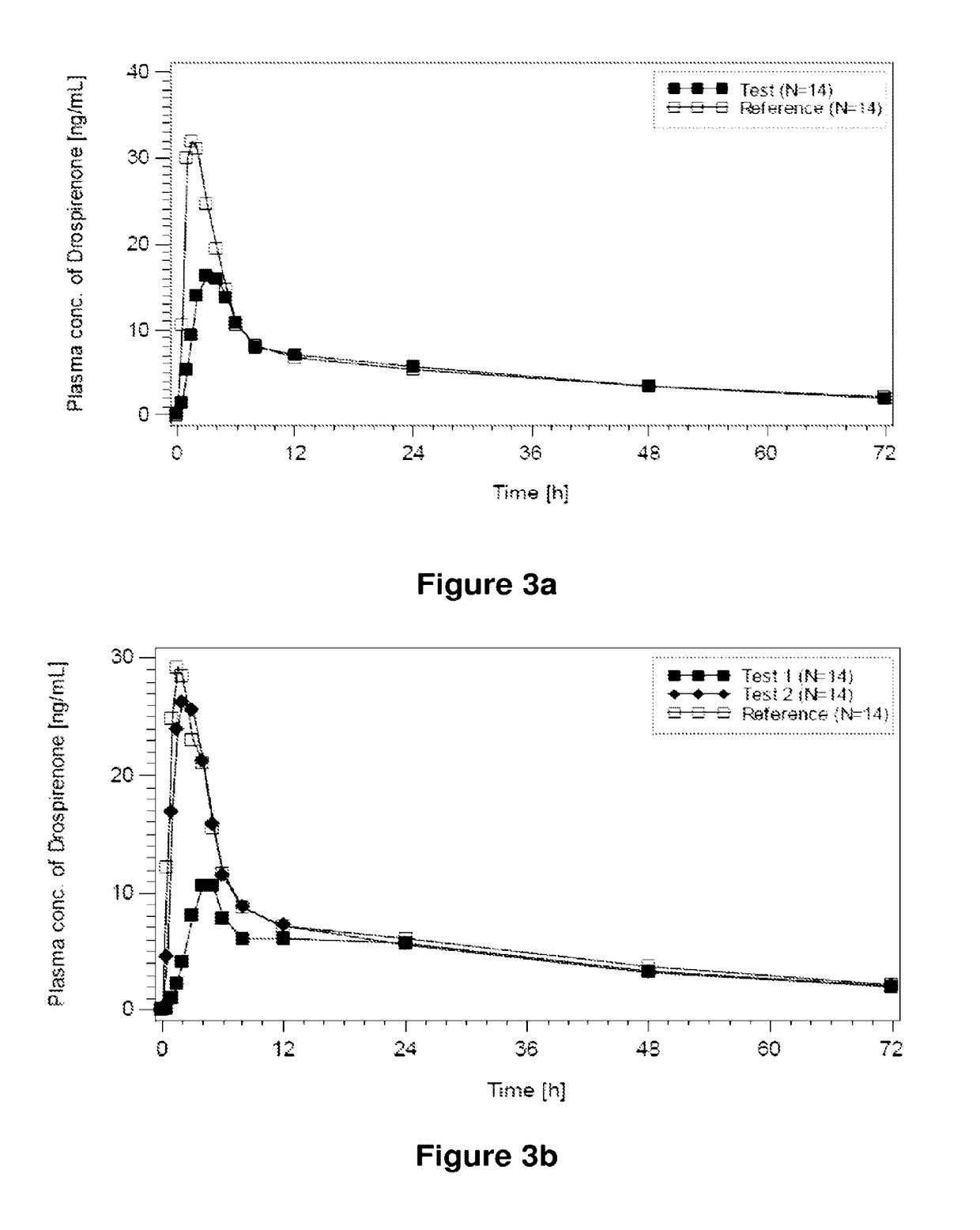
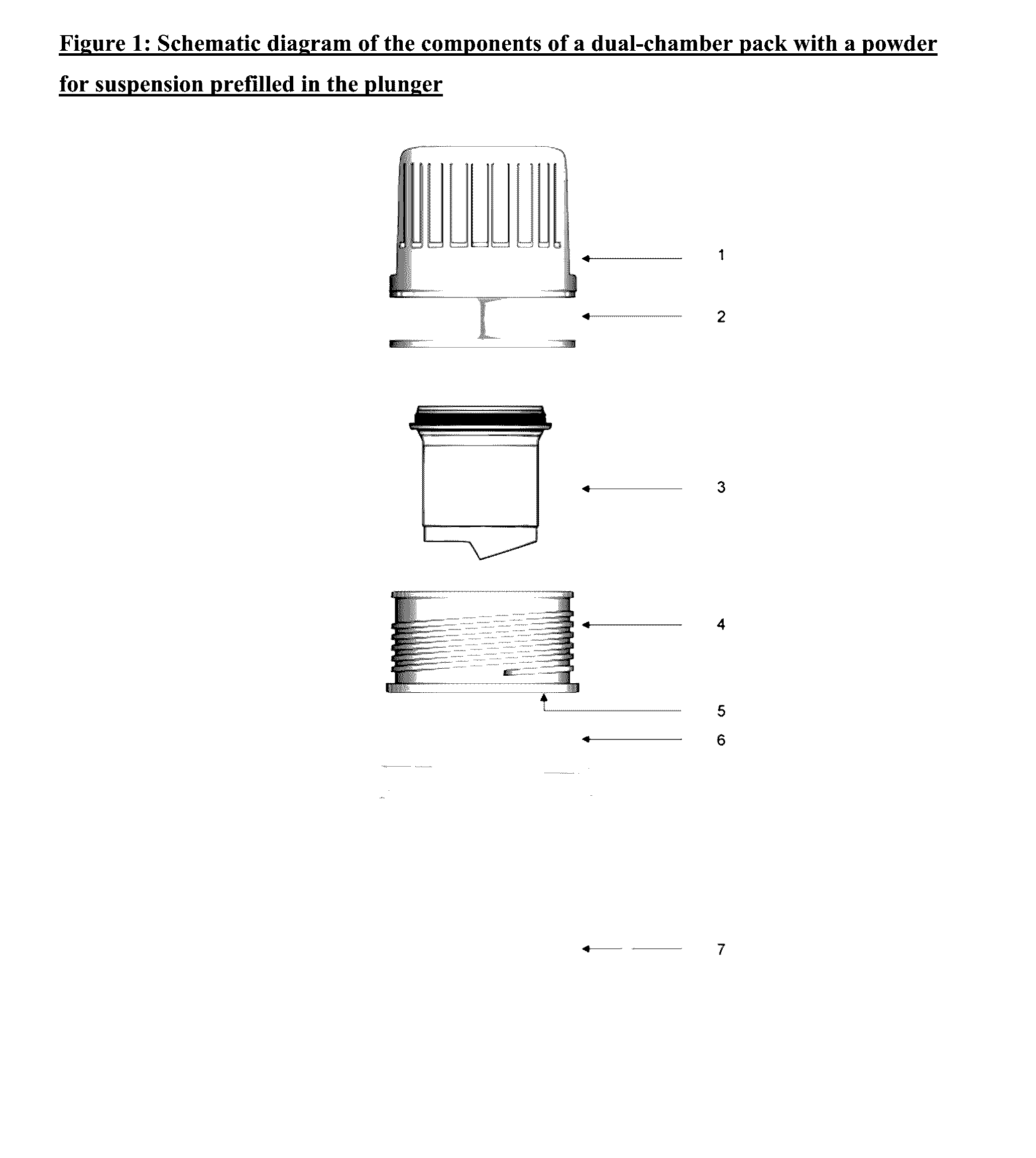
Dual-chamber pack for extended release suspension compositions
InactiveUS20170119627A1Allows the end-users ease of dispensingEnsures stability of active ingredient during storagePowder deliveryDispersion deliveryBULK ACTIVE INGREDIENTActive ingredient
Owner:SUN PHARMA INDS
Extended release suspension compositions
InactiveUS20160228379A1Easy to managePatient compliance is goodAntibacterial agentsPowder deliveryPolymer scienceBULK ACTIVE INGREDIENT
The present invention relates to extended release suspension compositions of an active ingredient. Said extended release suspension compositions comprise multiple coated cores of the active ingredient and a suspension base, wherein the suspension base generates a hypertonic condition such that there is no substantial change in the in-vitro dissolution release profile of the extended release suspension compositions upon storage for at least seven days. The invention also relates to processes for the preparation of said extended release suspension compositions.
Owner:SUN PHARMA INDS
Extended release suspension compositions
ActiveUS20160271070A1Easy to managePatient compliance is goodPowder deliveryDispersion deliveryBULK ACTIVE INGREDIENTActive ingredient
The present invention relates to a method for preparing a stable extended release suspension composition comprising multiple coated cores of an active ingredient by using a suspension base, wherein the suspension base ensures substantially similar in-vitro dissolution release profile of the active ingredient upon storage of the suspension compositions for at least seven days.
Owner:SUN PHARMA INDS
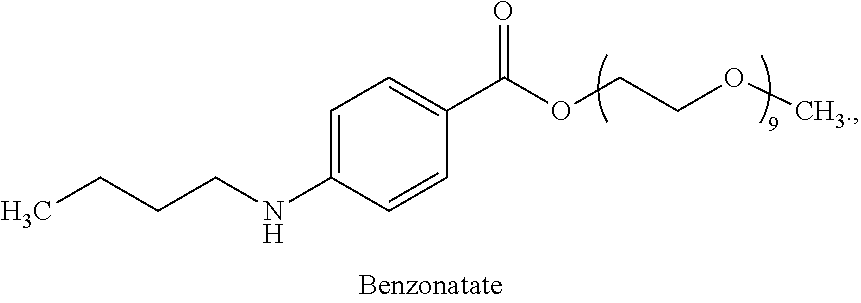
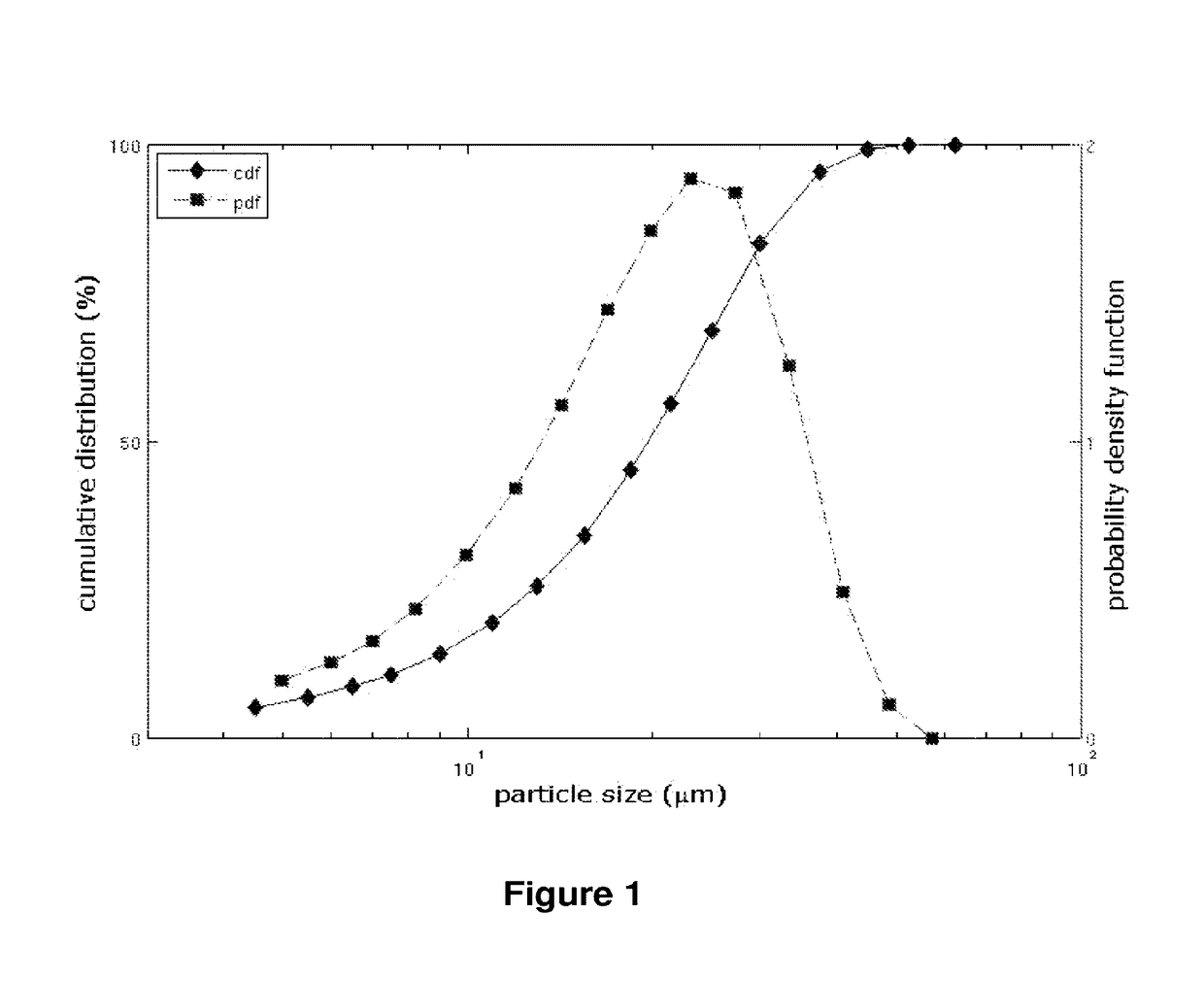
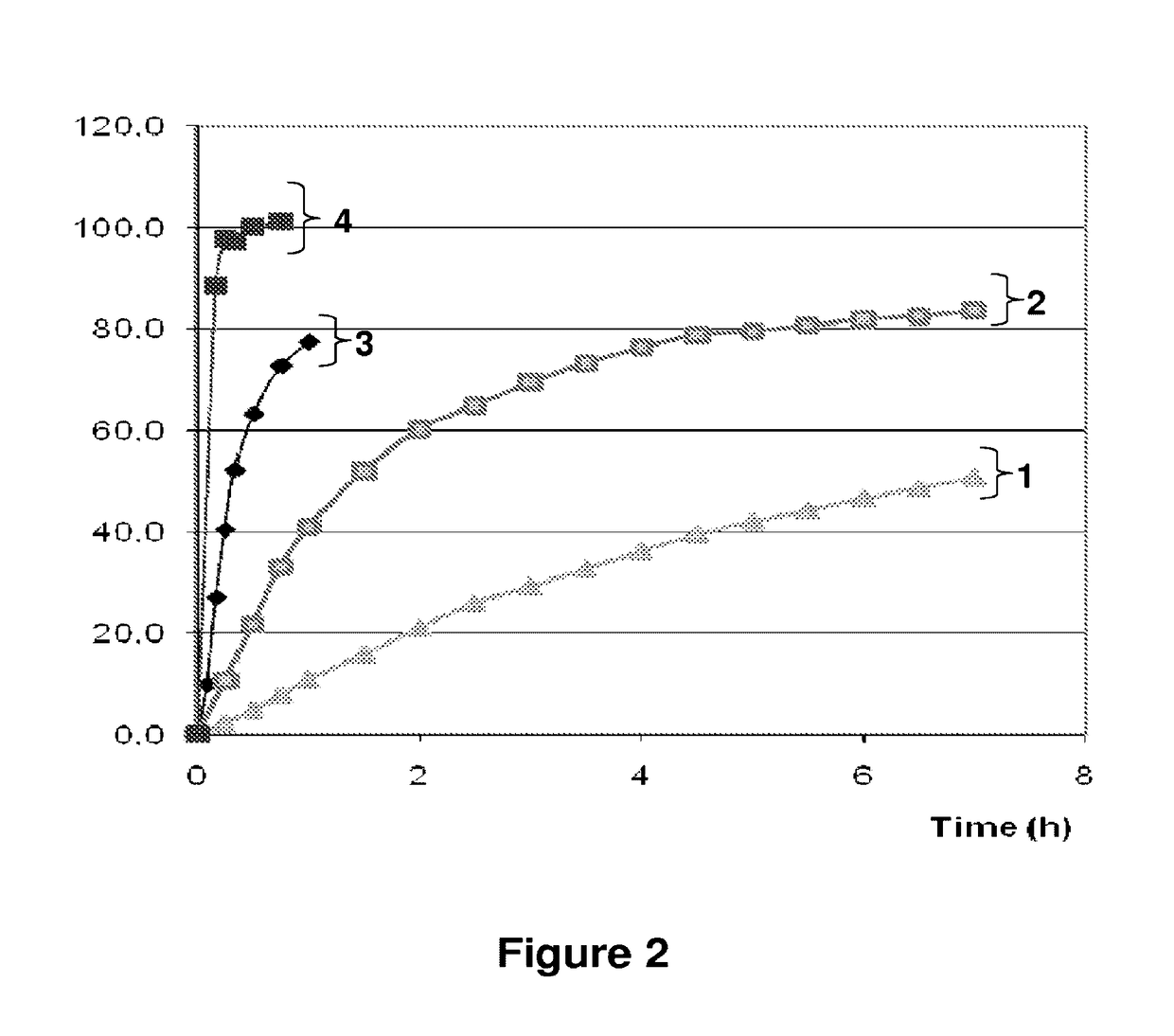
Benzonatate modified release solid tablets and capsules
ActiveUS9180104B2Avoiding undesirable and potentially serious side effectStable rateEther/acetal active ingredientsEster active ingredientsCelluloseWax
A 12-hour anti-tussive modified release solid tablet or capsule is described which comprises a benzonatate in a matrix with a sufficient amount of one or more pharmaceutically acceptable modified release pH-independent, substances to provide a 12-hour modified release profile to the benzonatate, wherein there is substantially no benzonatate release from the tablet or capsule in the buccal cavity and no more than about 25% release of the benzonatate within 1 hour as determined in an in vitro dissolution assay. The modified release may be provided by (a) a high melt temperature, water-insoluble wax or waxy substance, (b) a low viscosity hydrophilic polymer such a hydroxypropyl methylcellulose, (c) a reverse enteric coating, or combinations thereof. The benzonatate may be in an adsorbate with a silico or silicate or in a complex with a weak acidic ion exchange resin complex.
Owner:TRIS PHARMA
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
ActiveUS9603860B2Inhibit ovulationPowder deliveryOrganic active ingredientsInitial treatmentPharmaceutical drug
A pharmaceutical composition comprising an active contraceptive drug and one or more pharmaceutically-acceptable excipients. The pharmaceutical composition, when subjected to an in vitro dissolution test according to the USP XXIII Paddle Method, results in no more than 50% of said active drug initially present being dissolved within 30 minutes, and at least 50% of the active drug being dissolved in a time range from about 3 hours to about 4 hours. The pharmaceutical composition is administered daily to a patient having a BMI of about 25 kg / m2 or more for at least a portion of a treatment cycle. The pharmaceutical composition does not cause a number of days of bleeding events in the patient exceeding an average of 15% per treatment cycle in consecutive treatment cycles of administration after an initial treatment cycle of administration.
Owner:LAB LEON FARMA
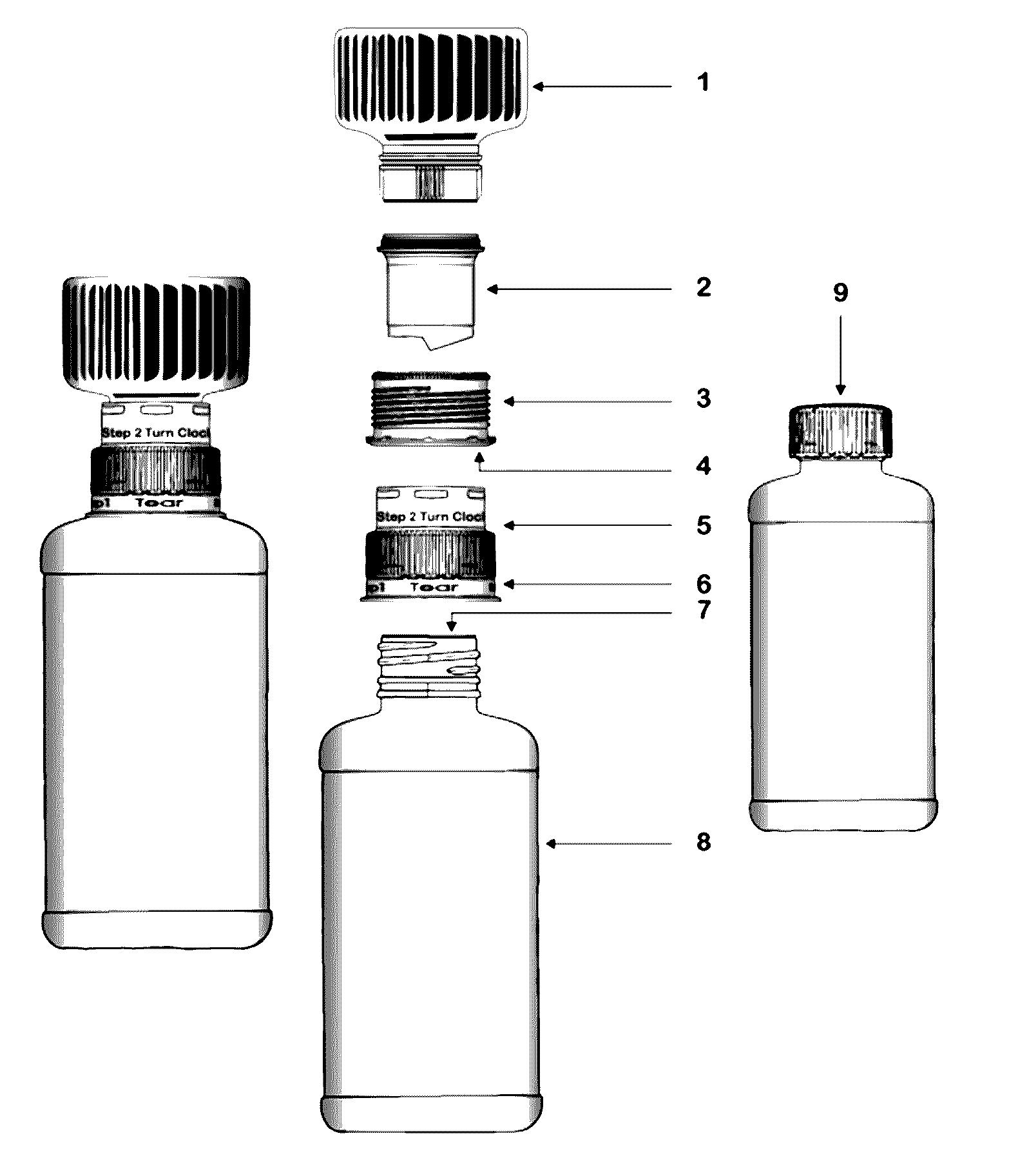
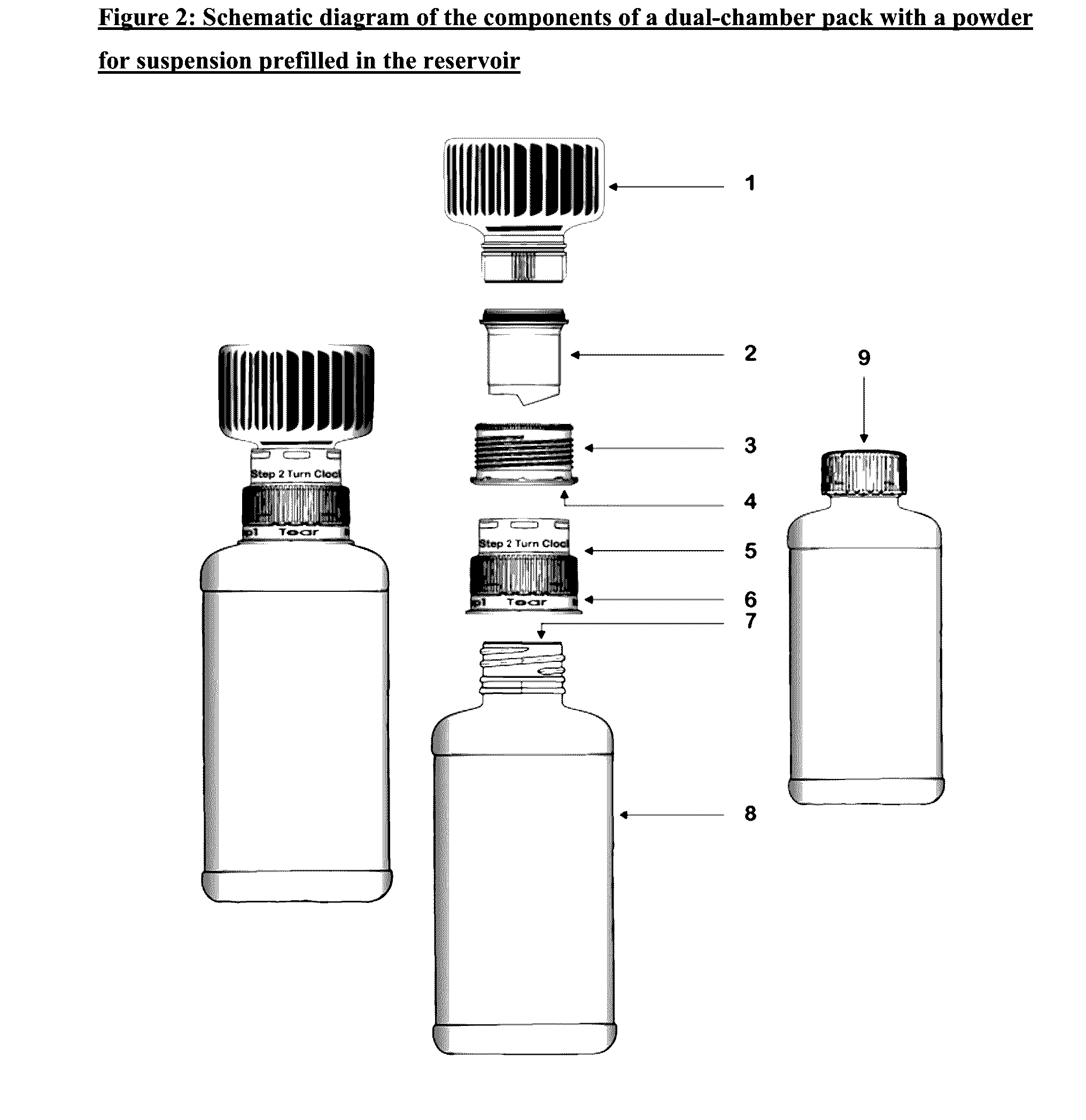
Dual-chamber pack for extended release suspension compositions
InactiveUS20160317388A1Maintaining content uniformityDispensed with easePowder deliveryDispersion deliveryBULK ACTIVE INGREDIENTBiological activation
The present invention relates to a dual-chamber pack comprising a first chamber prefilled with a suspension base and a second chamber prefilled with a powder for suspension comprising an active ingredient, wherein upon activation of the dual-chamber pack, the contents of both the chambers are mixed to form an extended release suspension composition which is characterized by having no substantial change in the in-vitro dissolution release profile of the active ingredient upon storage for at least seven days.
Owner:SUN PHARMA INDS
Medicinal preparation of palbociclib and preparing method thereof
ActiveCN105816437AImprove in vitro dissolutionReduce drug particle sizePowder deliveryOrganic active ingredientsPharmacyAdjuvant
The invention belongs to the field of pharmacy and particularly relates to a medicinal preparation of palbociclib and a preparing method thereof. The medicinal preparation comprises palbociclib, an acidic adjuvant and an optional hydrophily high-molecular material. Compared with a conventional preparation, the medicinal preparation has more excellent solubility and in-vitro dissolution property, and can be used for enhancing in-vivo adsorption and bioavailability of palbociclib.
Owner:SHENZHEN PHARMACIN CO LTD
Preparation method and applications of mesoporous carbon
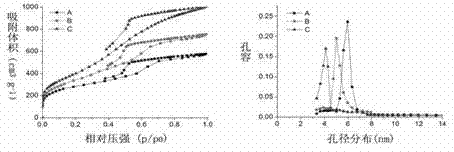
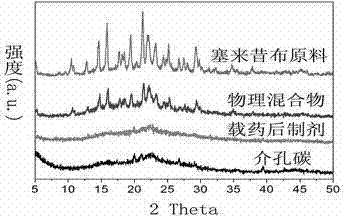
InactiveCN103086346ALarge specific surface areaUniform particle sizePowder deliveryCarbon preparation/purificationSucroseSide effect
The invention belongs to the technical field of medicine, and relates to mesoporous carbon and a preparation process thereof, and an application of the mesoporous carbon in insoluble drug delivery systems. According to the process, a hard template method is adopted to prepare the mesoporous carbon, wherein mesoporous silica is adopted as a template, sucrose is adopted as a carbon source, and sulfuric acid is adopted as a catalyst to prepare the mesoporous carbon, wherein the prepared mesoporous carbon has characteristics of large specific surface area, stable property, no toxic-side effect and good biocompatibility, and can be adopted as a insoluble drug carrier. According to the present invention, a solvent method and a melting method are adopted to carry out drug embedding and adsorption so as to achieve uniform dispersion of the drug in the pores and on the surface of the carrier; with the drug delivery system, water solubility of insoluble drugs can be significantly enhanced, and in vitro dissolution rate and oral bioavailability can be improved.
Owner:SHENYANG PHARMA UNIVERSITY
Gastroretentive extended release suspension compositions
InactiveUS20180008539A1Conducive to commercial productionPatient compliance is goodOrganic active ingredientsDispersion deliveryIn vitro dissolutionExtended release
The present invention relates to a gastroretentive extended release suspension composition, wherein the composition is characterized by having no substantial change in the in-vitro dissolution release profile upon storage for at least seven days. The invention also relates to processes for the preparation of said gastroretentive extended release suspension compositions.
Owner:SUN PHARMA INDS
Snuff composition
ActiveUS9402809B2Quick effectRapid onsetPowder deliveryOrganic active ingredientsCelluloseTraditional medicine
Use of a nicotine-cellulose combination for the preparation of a snuff composition for achievement of a fast onset of action of nicotine after application of the snuff composition to the oral cavity of a subject, wherein the composition has a high release rate so that when subjected to an in vitro dissolution test about 45% or more of the total content of nicotine is released within 30 minutes. Moreover, the invention relates to an improved snuff composition for application to the oral cavity.
Owner:MODORAL BRANDS INC
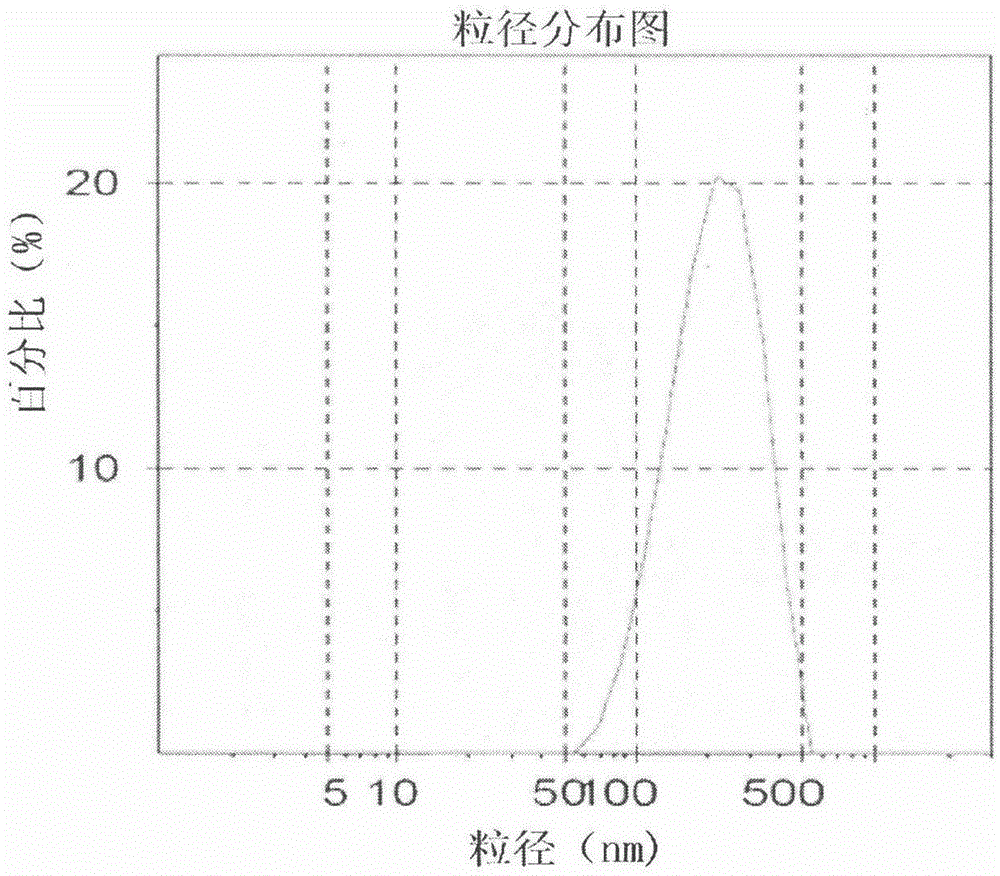
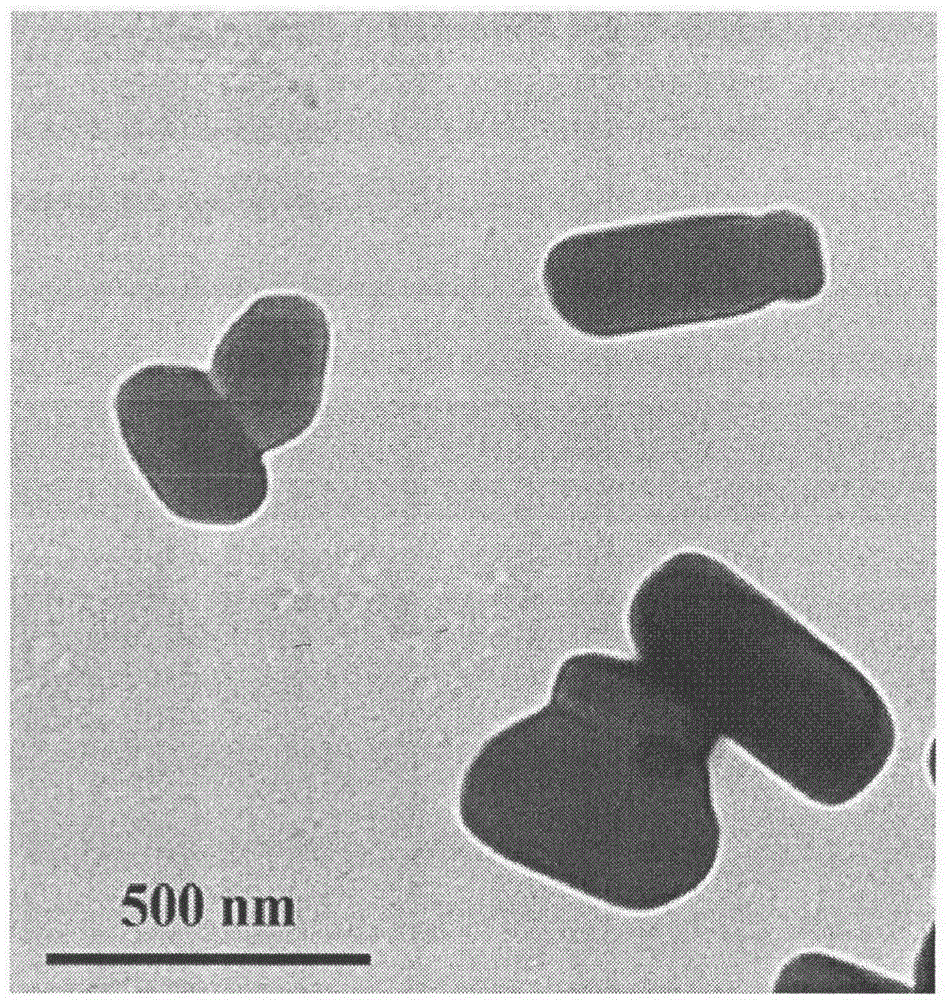
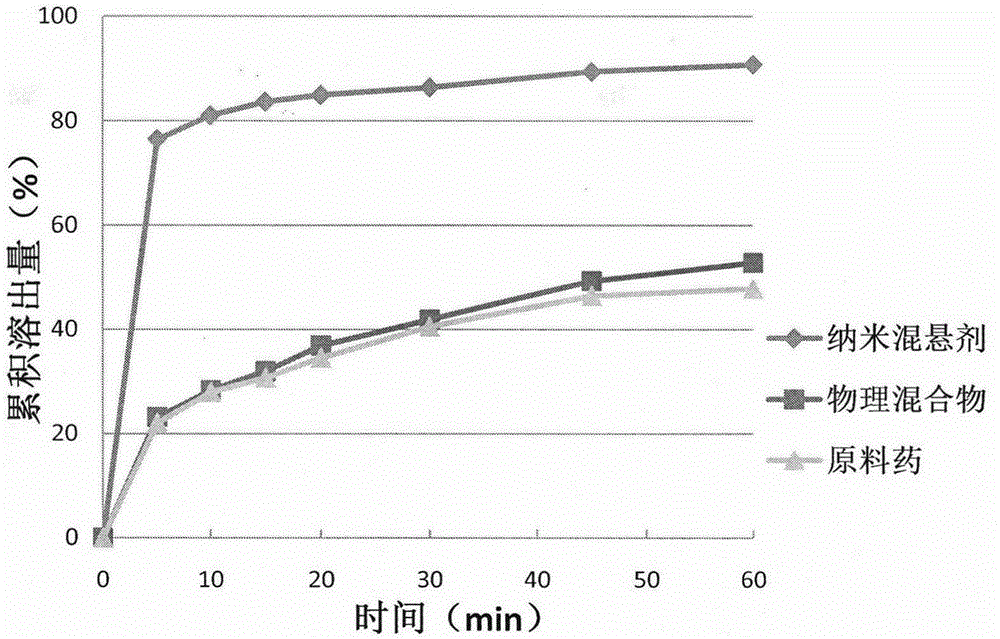
Celecoxib nanosuspension and preparation method thereof
InactiveCN105147607ASimple prescriptionSimple processOrganic active ingredientsAntipyreticSolubilityDissolution
The invention belongs to the field of pharmaceutical preparations and provides a Celecoxib nanosuspension and a preparation method thereof. Celecoxib is a novel non-steroidal anti-inflammatory drug, inhibits cyclooxygenase -2(COX-2) is through specificity, has anti-inflammatory and pain-easing effects and is clinically used for treating osteoarthritis and rheumatoid arthritis. However, the Celecoxib has very low solubility and in-vivo bioavailability. In order to increase the dissolution and the bioavailability of the drug, a high-speed shearing combined high-pressure homogenizing method is adopted to prepare the Celecoxib nanosuspension, the particle size and the polydispersion index PI are used as indicators for formulation technology optimization, a laser particle analyzer and a transmission electron microscope are adopted to study the particle size and form of the drug, the dissolution of the drug is evaluated through in-vitro dissolution experiments, and rat in-vivo pharmacokinetic study is conducted. Measurement results prove that the drug particle size of the nanosuspension is 50-500 nm, and the dissolution and the in-vivo bioavailability of the drug are increased obviously.
Owner:CHINA PHARM UNIV
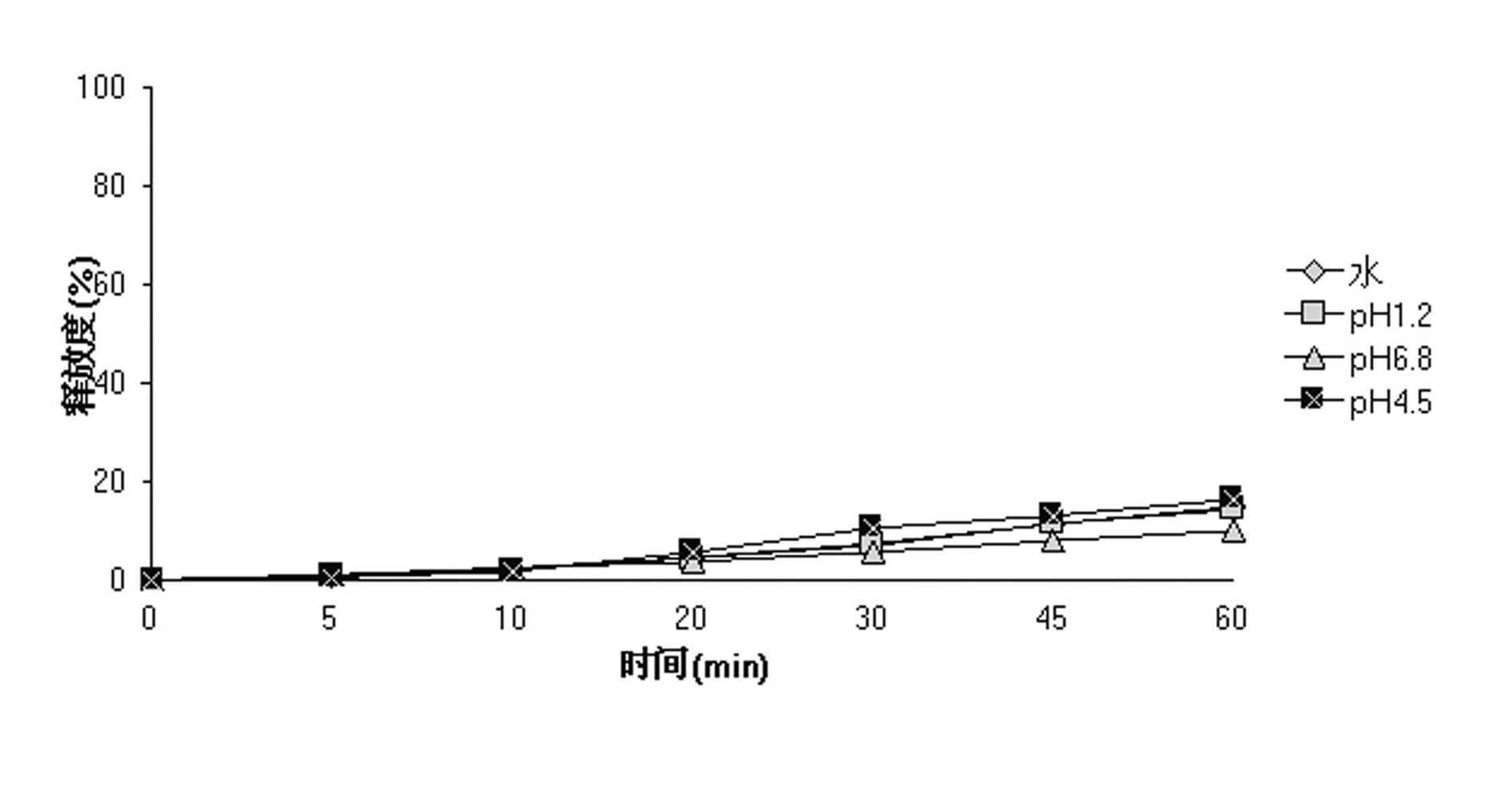
Andrographolide ground suspending liquid, preparation method thereof, and application of pharmaceutical preparation
InactiveCN102614133AImprove stabilityDissolution rate is fastAntibacterial agentsOrganic active ingredientsImmediate releaseDrugs preparations
The invention relates to andrographolide ground suspending liquid, a preparation method thereof, and the application of pharmaceutical preparation, which belongs to the field of pharmaceutical preparation. The preparation method comprises the steps of adding andrographolide into hydrophilic accessory solution with certain concentration, and grinding the andrographolide in a basket grinder to prepare suspending liquid with particle sizes smaller than 3000 nm. Liquid layers of pharmaceutical suspending liquid are laminated onto blank pellet cores with certain particle size range, to prepare andrographolide immediate-release pellets. After grinding, by reducing pharmaceutical particle sizes, increasing particle surface areas and improving the wettability of pharmaceutical particles, the dissolution in vitro of the drug is improved, and hydrophilic carriers are adopted to effectively prevent the aggregation of pharmaceutical particles so as to improve the stability of the pharmaceutical preparation. The preparation method is simple and easy for industrialized production, and the dissolving-out speed of the prepared andrographolide immediate-release pellets is high, so that the bioavailability is obviously improved.
Owner:SHENYANG PHARMA UNIVERSITY
Water-soluble drug sustained-release tablet and preparation method thereof
InactiveCN101288652ASimple preparation processSimple processPill deliveryPharmaceutical non-active ingredientsProlonged-release tabletWater soluble drug
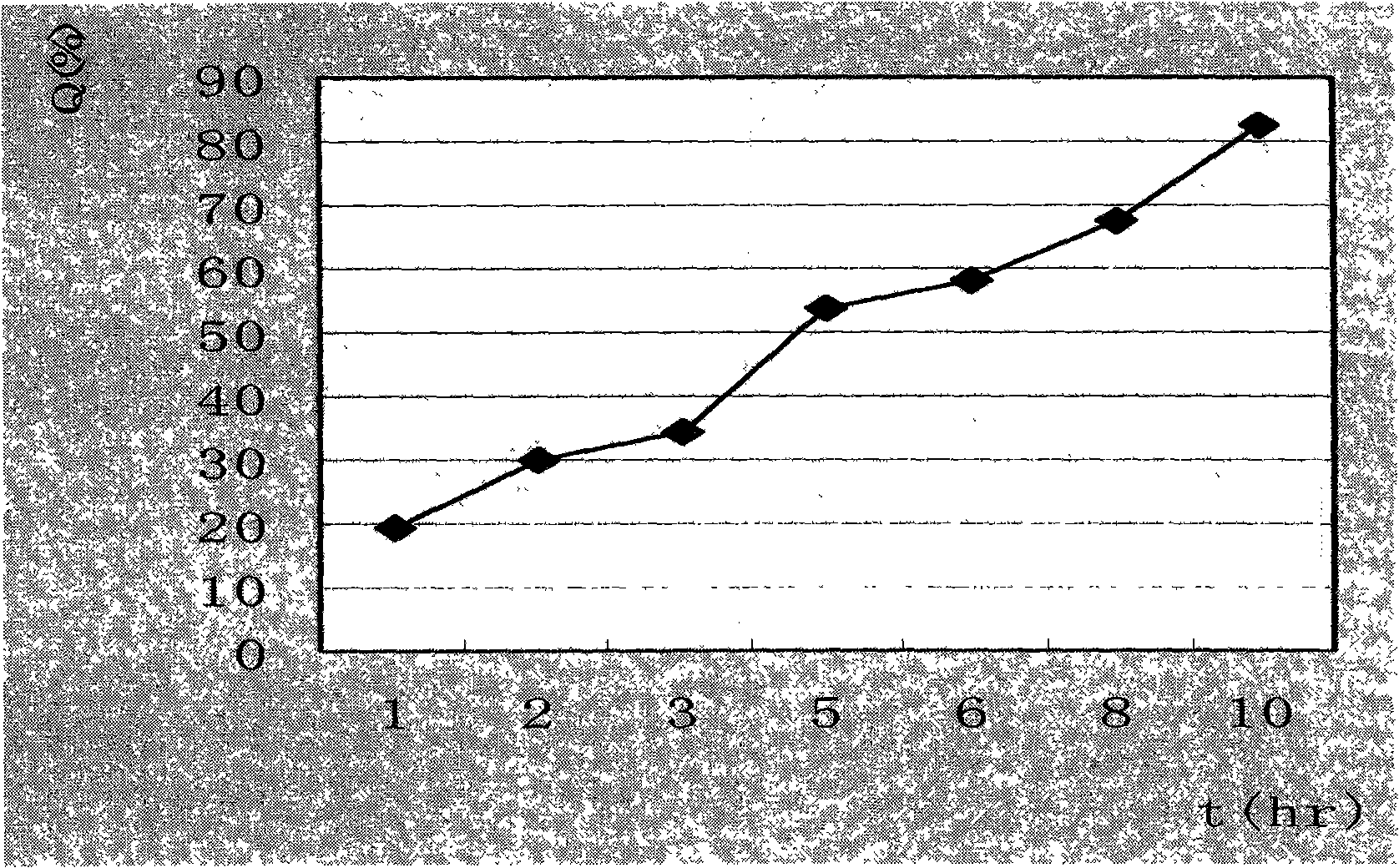
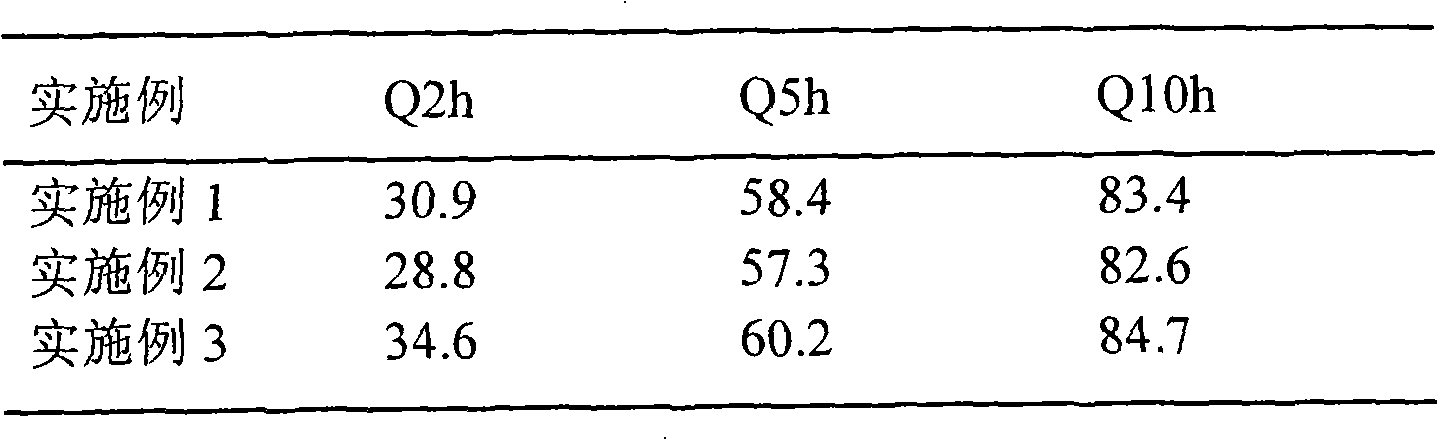
The invention relates to a water-soluble drug sustained-release tablet and a preparation method thereof, the technology is characterized in that: the components and the weight percentages are as follows: 1 to 20 percent of water-soluble drug, 20 to 70 percent of hydrophilic gel, 30 to 50 of percent waxy material and the rest of filler, lubricant and other excipients that are commonly used for ordinary tablets. The method takes the hydrophilic gel sustained-release material and the waxy sustained-release material as a mixed matrix, the water-soluble drug is added to change the features of fast dissolution and short half life of the drug release, thus achieving the purpose of slow and smooth release. The drug of the invention has good stability, the needed equipment is simple, the manufacturing process is simple and easy, the operatability is strong, the in vitro dissolution test shows that the release rate is 25 to 35 percent in 2 hours, 55 to 65 percent in 5 hours and more than 80 percent in 10 hours, thus having good sustained-release effect. In addition, the sustained-release tablet is less affected by the gastrointestinal tract environment, the absorption is stable, and the individual difference is small, thus being the ideal sustained-release tablet.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Valsartan spray-dried nanosuspension and preparation method of valsartan spray-dried nanosuspension
InactiveCN102920654AUniform particle sizeLarge particle sizePowder deliverySolution deliveryValsartanAnti solvent
The invention discloses a valsartan nanosuspension, a spray-dried power, and a preparation method of the valsartan nanosuspension and the spray-dried power. A valsartan spray-dried nanosuspension and a preparation method of the valsartan spray-dried nanosuspension belong to the field of nano-drug preparations. The valsartan spray-dried nanosuspension consists of 1 part of valsartan, 0.1-1 part of a stabilizing agent and 10-100 parts of a spray-drying protecting agent by mass; the valsartan spray-dried nanosuspension is prepared by an anti-solvent precipitation method and / or a high-pressure homogenizing method and / or an acid-alkali neutralization method in combination with a spray-drying technology. The drug particles in the spray-dried powder are reduced to a nano-grade, and the hydrophilicity, the in vitro dissolution rate and the bioavailability of the valsartan are greatly improved. The powder can be directly processed or be processed together an excipient into tablets, capsules and the like. The valsartan spray-dried nanosuspension has the advantages of simple preparation process, stable product quality and easy implementation of productization.
Owner:SHENYANG PHARMA UNIVERSITY
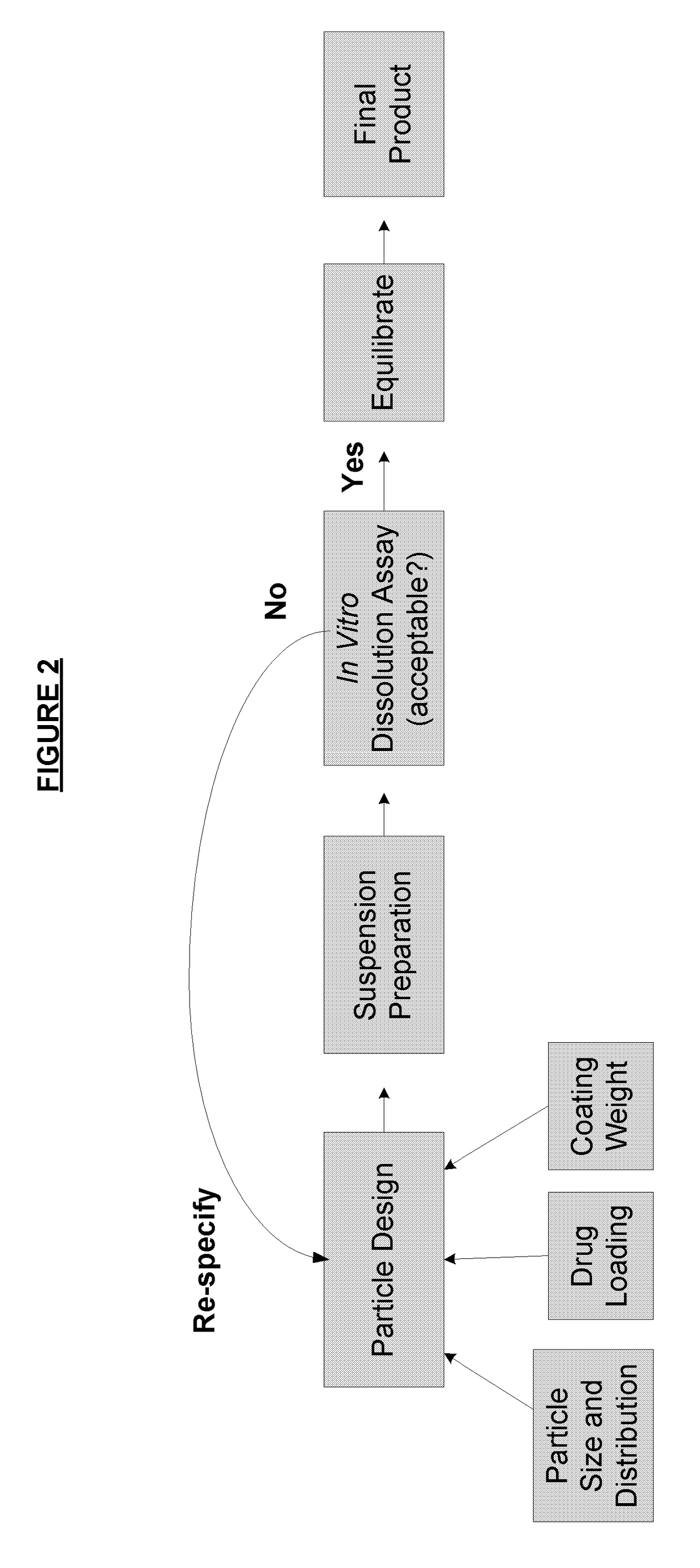
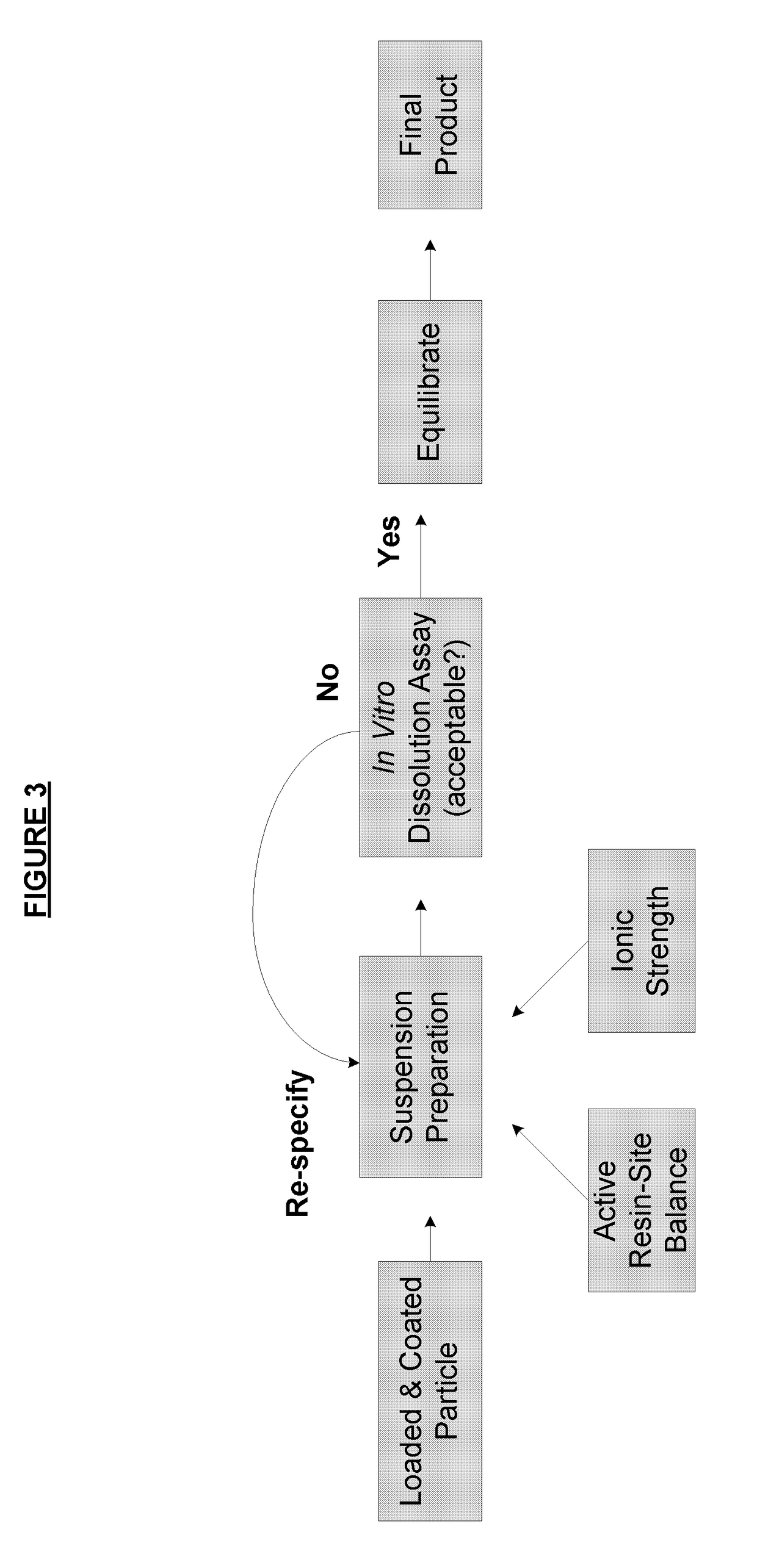
Method of formulating and designing liquid drug suspensions containing ion exchange resin particles
ActiveUS8470375B1Maintaining bioequivalenceMaintaining bioavailabilityDispersion deliverySolution deliveryQuality controlDissolution
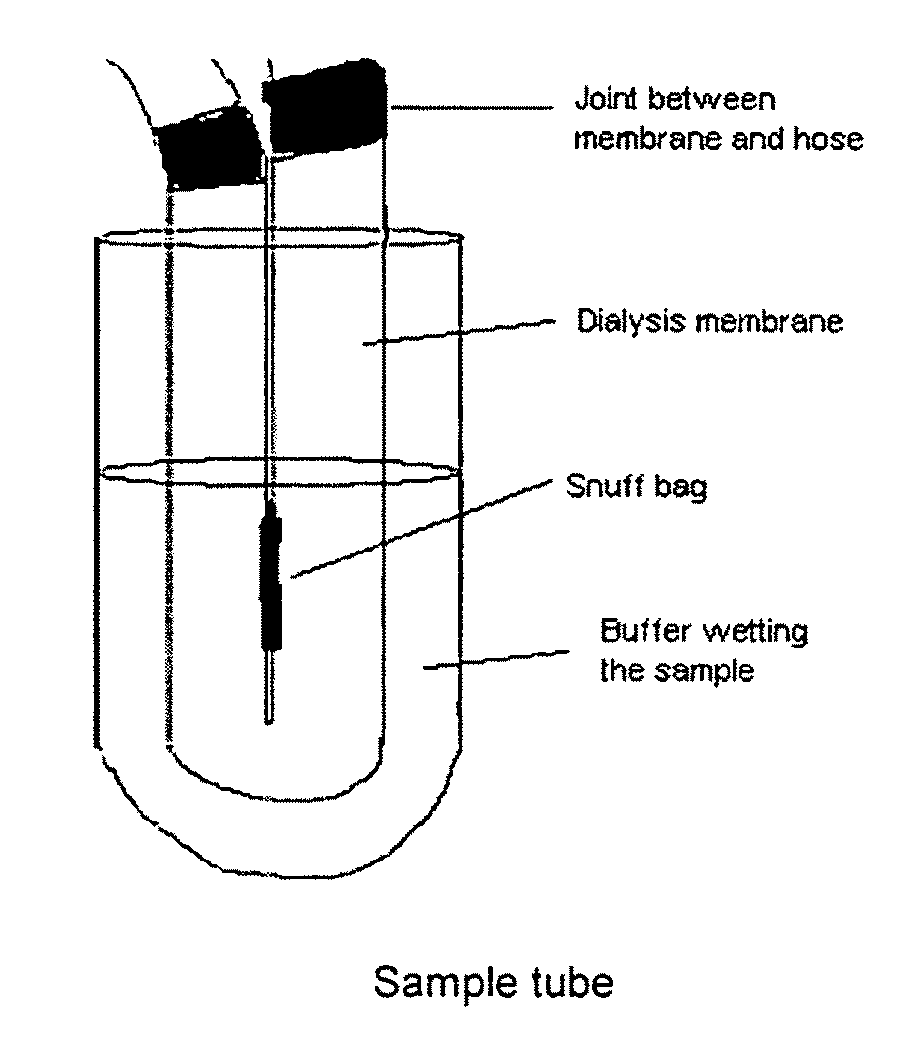
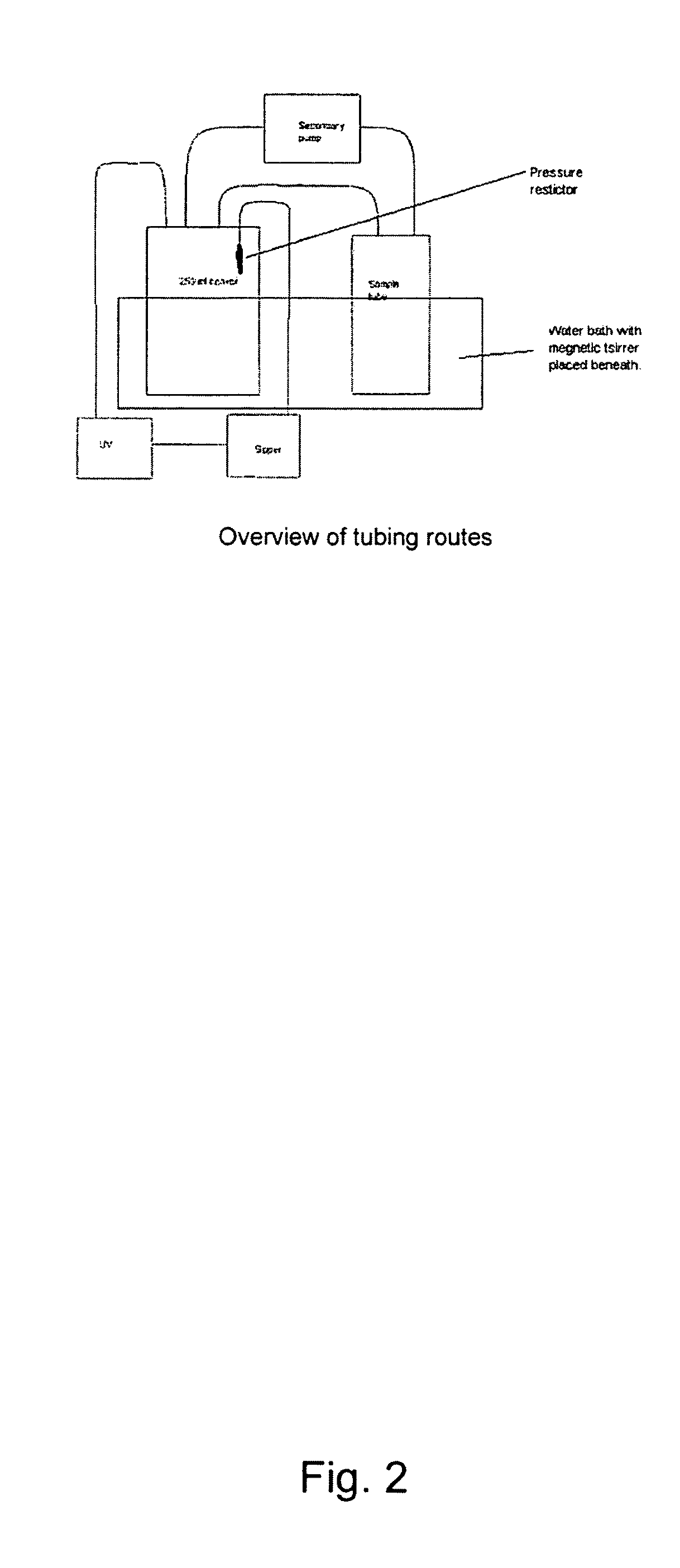
The invention relates to the formulation and quality control of liquid drug suspensions. In particular, the invention relates to methods of formulating liquid suspensions comprising drug-containing resin particles. The invention also relates to methods of confirming the acceptability of drug-containing resin particles for use in formulating liquid drug suspensions. The invention further relates to methods of formulating liquid suspensions in which drug-containing resin particles, the liquid suspension, or both are modified to achieve a desired in vitro dissolution profile. The invention also relates to a novel dissolution method and methods of predicting in vivo bioequivalence based on in vitro dissolution methods.
Owner:NEOS THERAPEUTICS LP
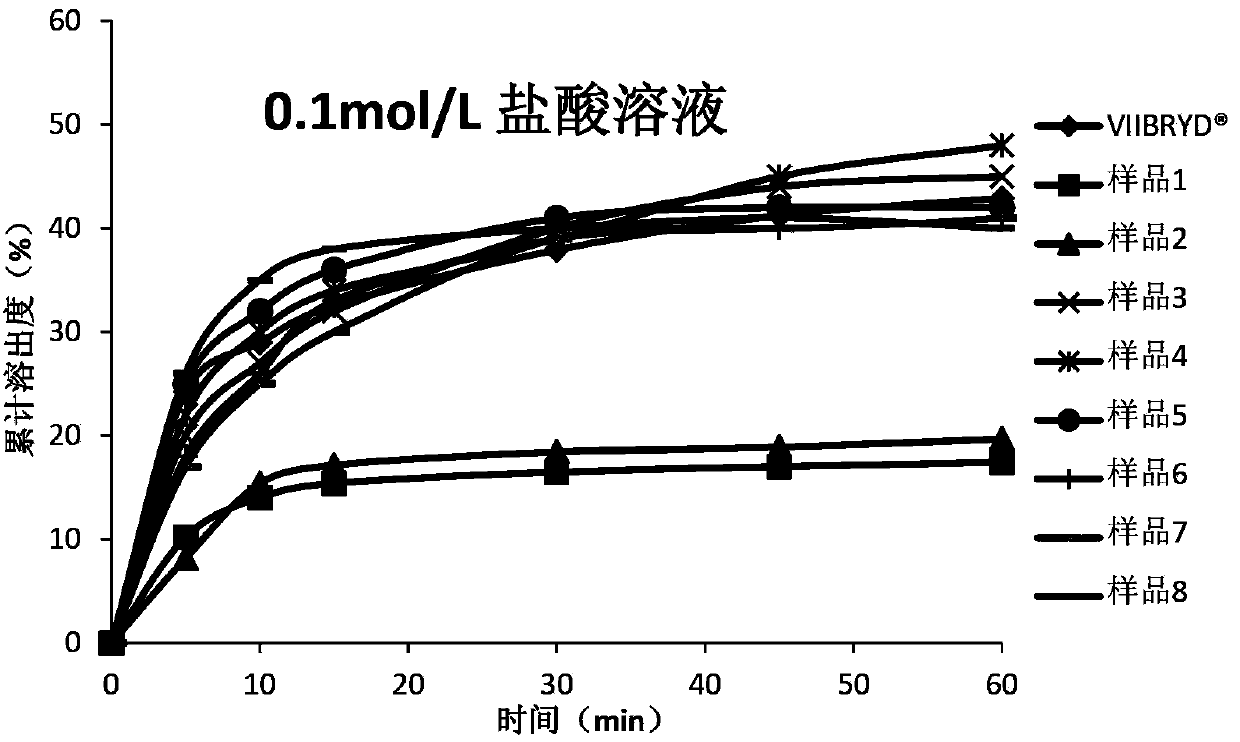
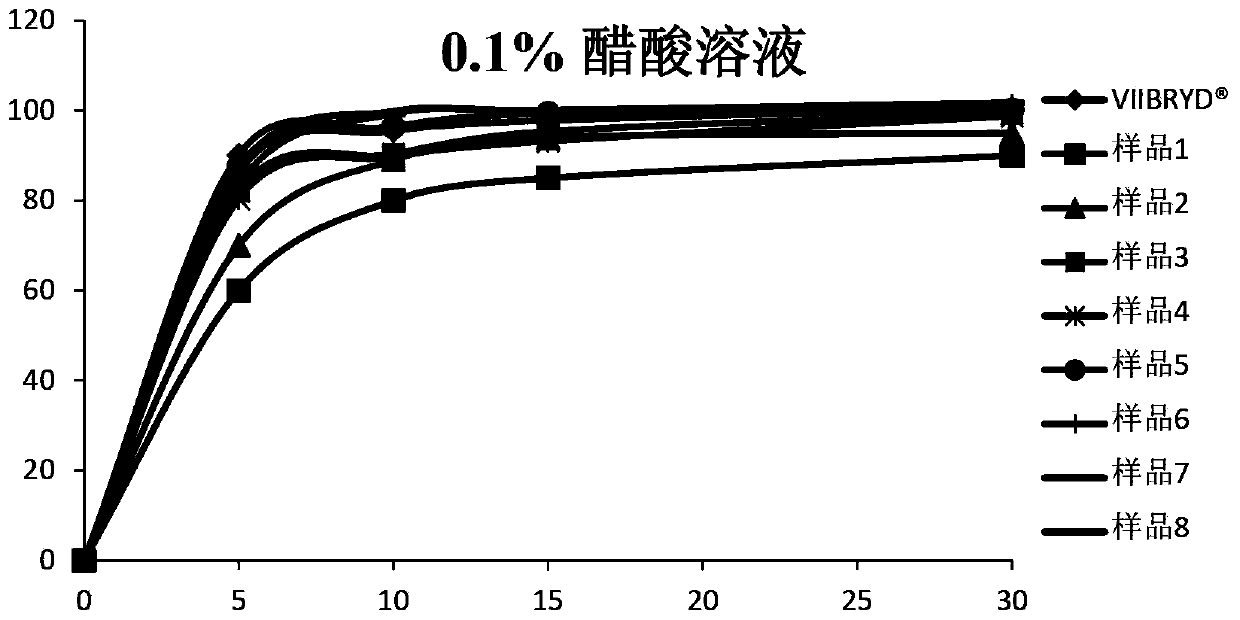
Vilazodone hydrochloride composition and preparation method thereof
ActiveCN104116741ASolving In Vitro Dissolution ProblemsImprove bioavailabilityOrganic active ingredientsNervous disorderPharmaceutical medicineMicrocarrier
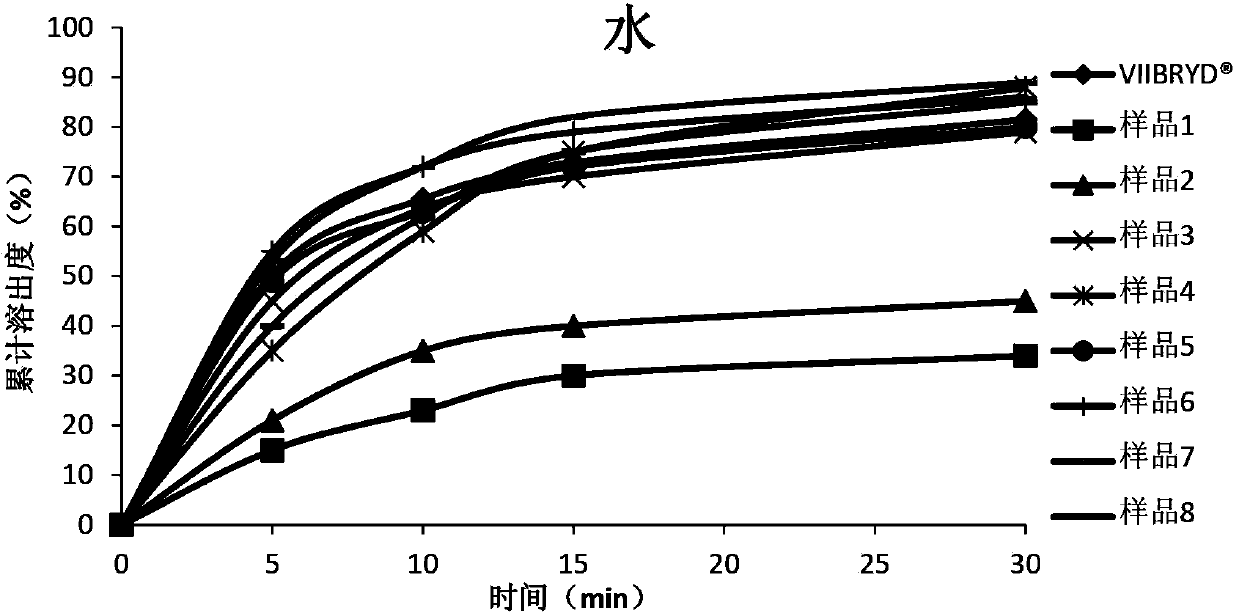
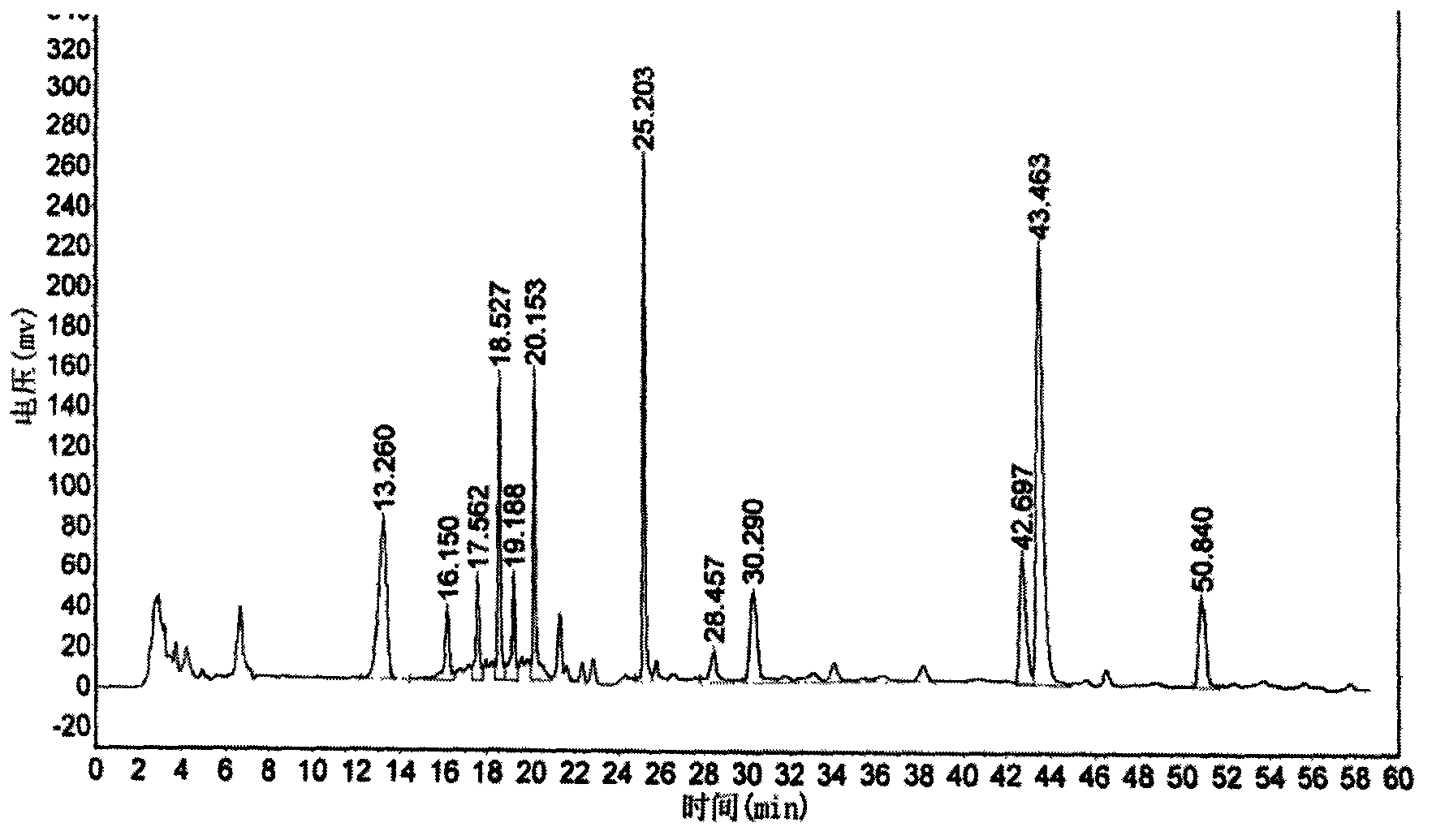
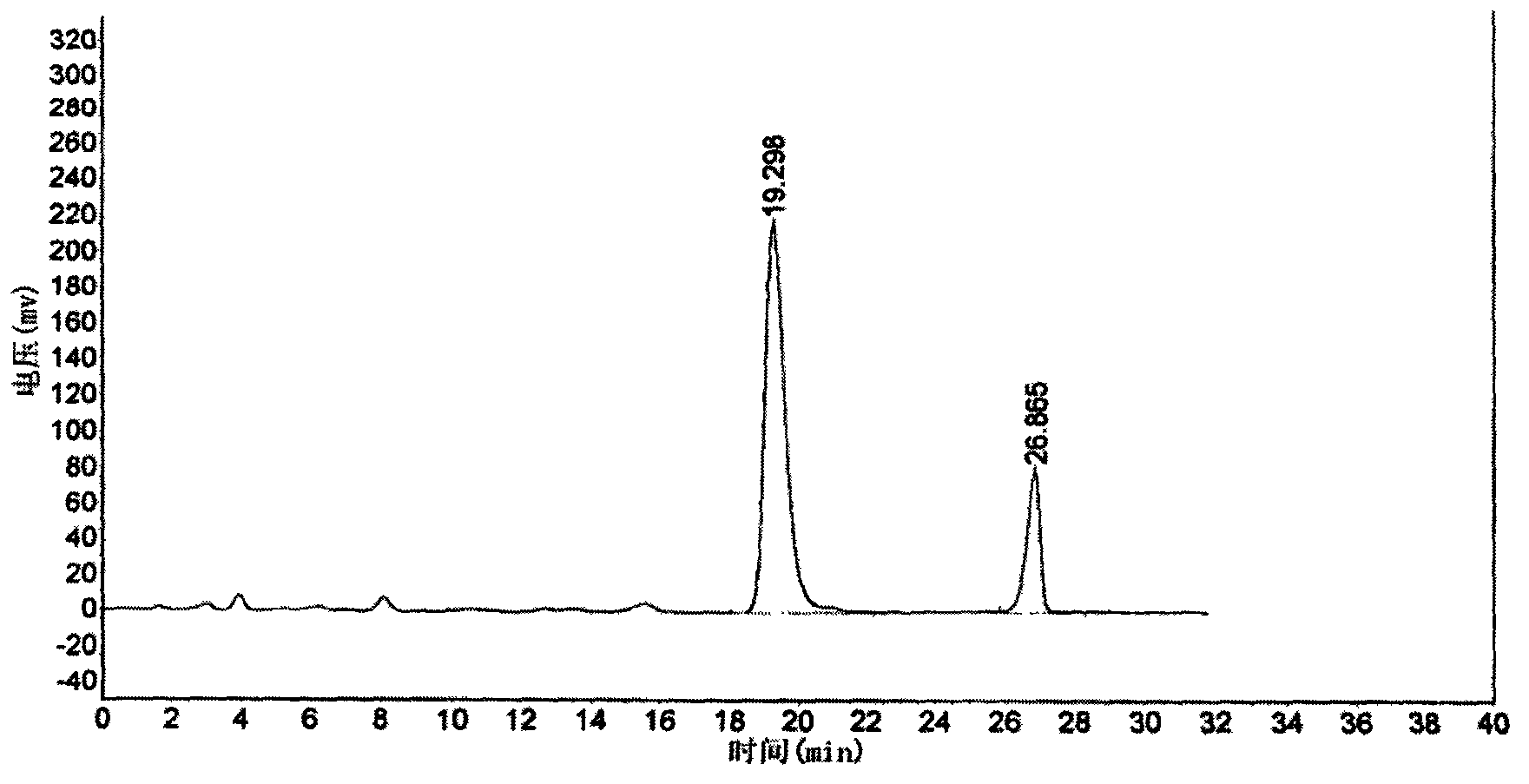
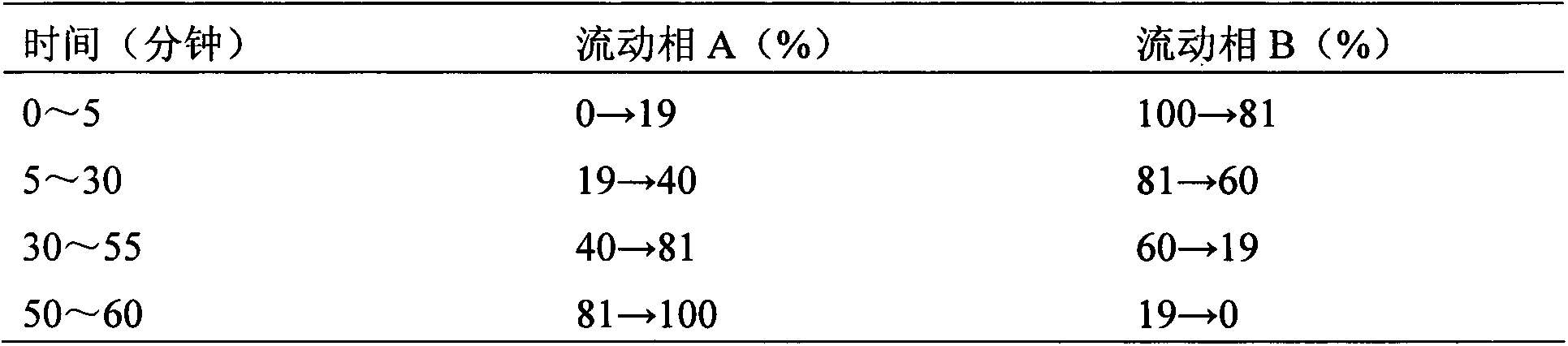
The invention relates to a vilazodone hydrochloride composition and a preparation method thereof. The composition includes vilazodone hydrochloride, a microcarrier and pharmaceutically acceptable excipients for an oral solid preparation. The preparation method is as below: mixing and crushing vilazodone hydrochloride and the microcarrier, and evenly mixing the mixture with pharmaceutically acceptable excipients for oral solid preparation. The obtained composition preparation has greatly improved dissolution in vitro, and in vivo bioavailability reaching bioequivalence to VIIBRYD.
Owner:JIANGSU SIMCERE PHARMA
Six-flavor hematinic capsule, its quality control method and application thereof
ActiveCN103381217ARealize authenticationAccurate measurementSenses disorderComponent separationBlurred visionLithospermum
The invention discloses a six-flavor hematinic capsule, its quality control method and an application thereof. The six-flavor hematinic capsule is prepared by adding auxiliary materials into raw materials consisting of Chinese angelica, Ligusticum wallichii, Radix Astragali, prepared rehmannia root, lithospermum and white peony root. According to the quality control method of the six-flavor hematinic preparation, whether the six-flavor hematinic capsule contains white peony root, Chinese angelica, Radix Astragali, prepared rehmannia root, lithospermum, Ligusticum wallichii and components is identified by thin layer chromatography; by high-performance liquid chromatography, in vitro dissolution behavior of the six-flavor hematinic capsule is determined, and effective component groups are identified by fingerprint; verbascoside and calycosin glucoside are used as reference substances to simultaneously determine contents in the six-flavor hematinic capsule; and the content of a volatile component ligustilide in the six-flavor hematinic capsule is determined by a gas chromatographic method. The method provided by the invention is simple to operate, is accurate and advanced, has good linear relation, reappearance, precision, stability and recovery rate, can be adopted to effectively control product quality and guarantee curative effect of the product. The invention also provides an application in the preparation of a medicine for treating eye damage and blurred vision.
Owner:GUANGDONG GUOYUAN GUOYAO PHARMA CO LTD
Dexibuprofen slow release pellet and preparation method thereof
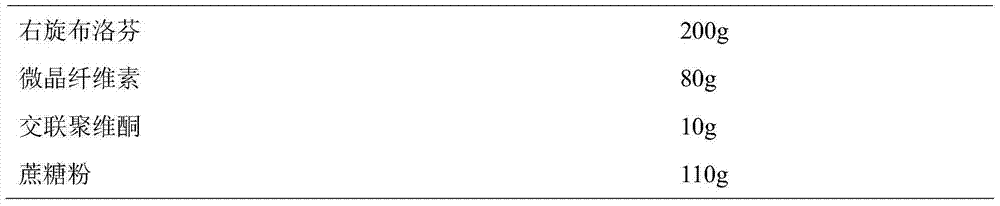
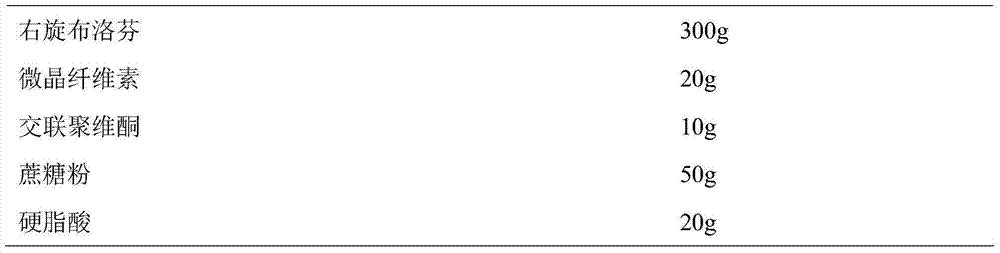
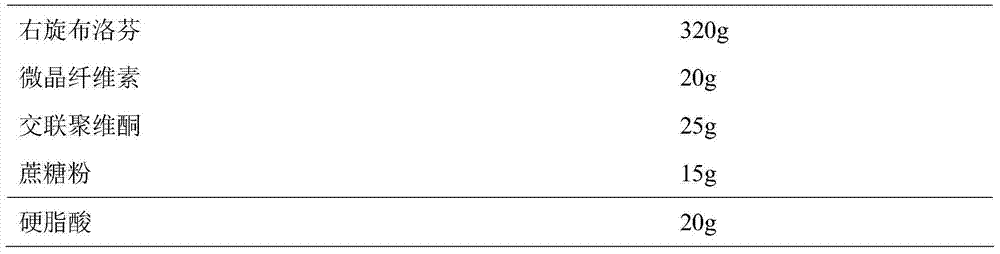
ActiveCN104739773AReduce the number of dosesImprove complianceOrganic active ingredientsAntipyreticAdhesiveIn vitro dissolution
The invention discloses a dexibuprofen slow release pellet and a preparation method thereof, and belongs to the field of medicinal preparations. The dexibuprofen slow release pellet comprises 50-80% of dexibuprofen, 0-5% of a slow release material, 0-2.5% of an adhesive, 8.75-47.5% of a filler, and 1.25-6.25% of a disintegrating agent, and all above percentages are based on the total weight of the pellet. The dexibuprofen slow release pellet has the advantages of good stability, stable quality, simple device and process, strong operationality, and suitableness for industrial large scale production. In vitro dissolution test shows that the accumulative release rate within 1h is 10-35%, the accumulative release rate within 2h is 25-55%, the accumulative release rate within 4h is 50-80%, and the accumulative release rate within 7h is 75% or above, so the dexibuprofen slow release pellet has a good release curve.
Owner:LUNAN BETTER PHARMA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com