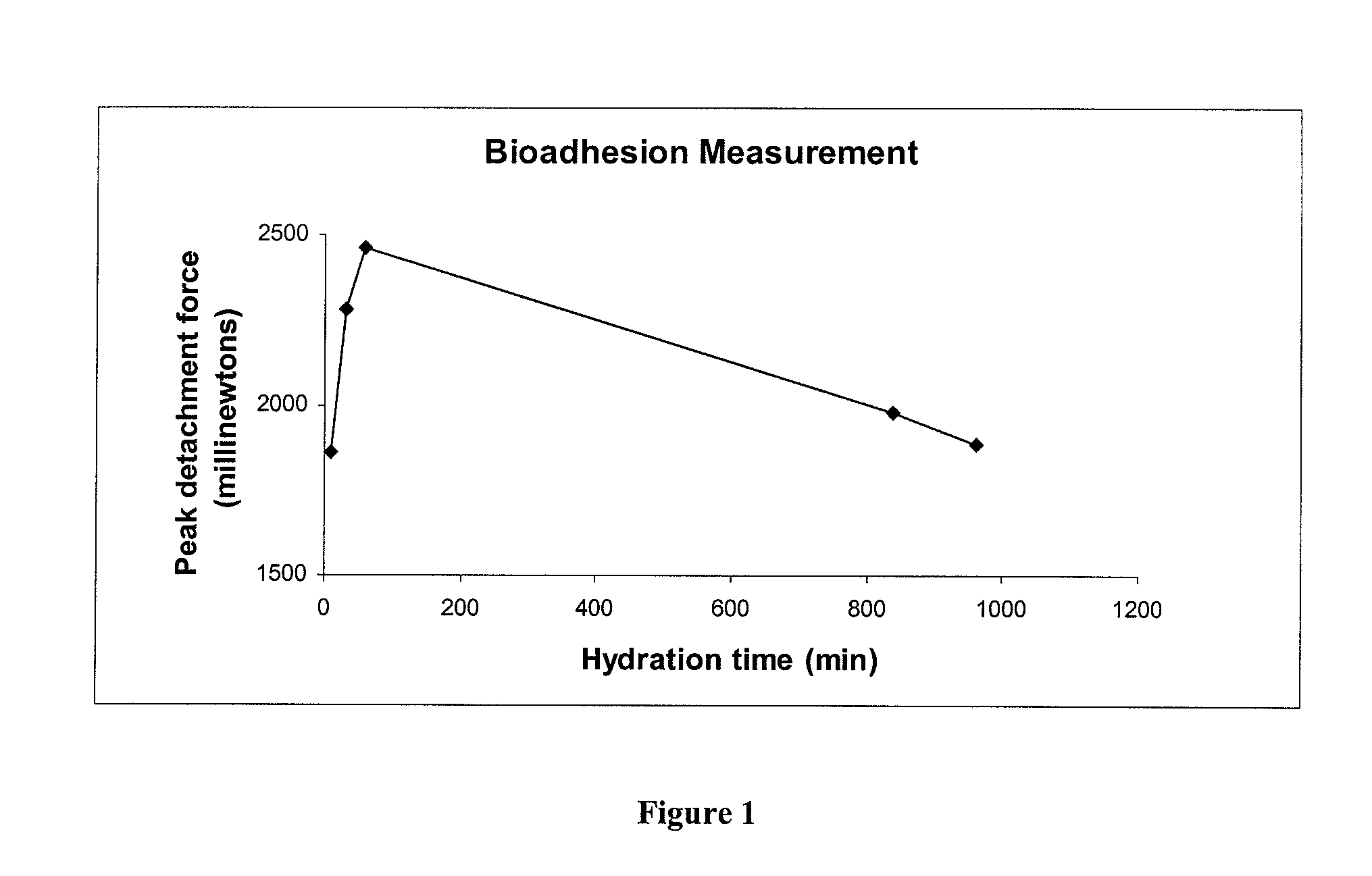
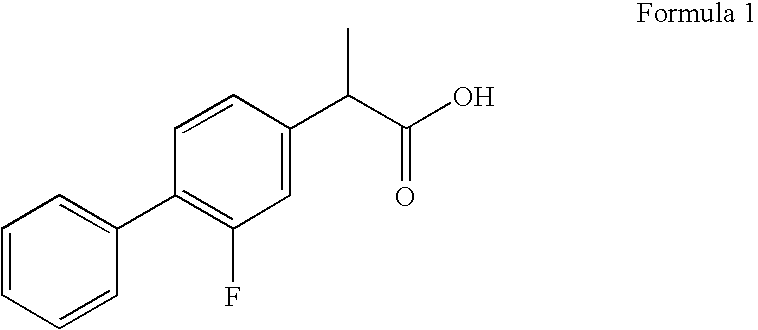
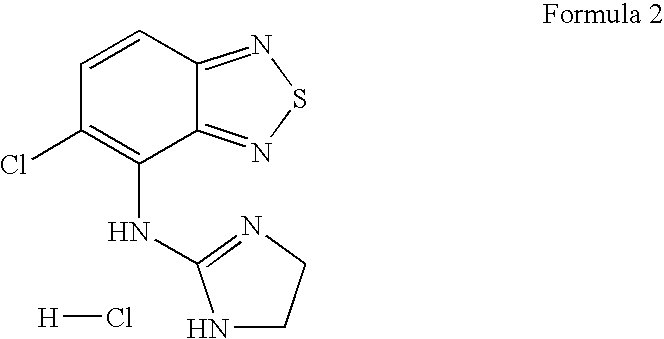
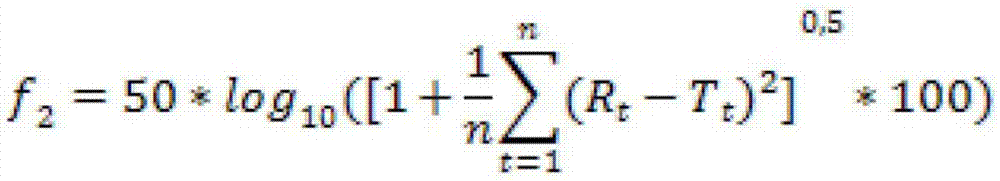Patents
Literature
54 results about "Multilayer tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
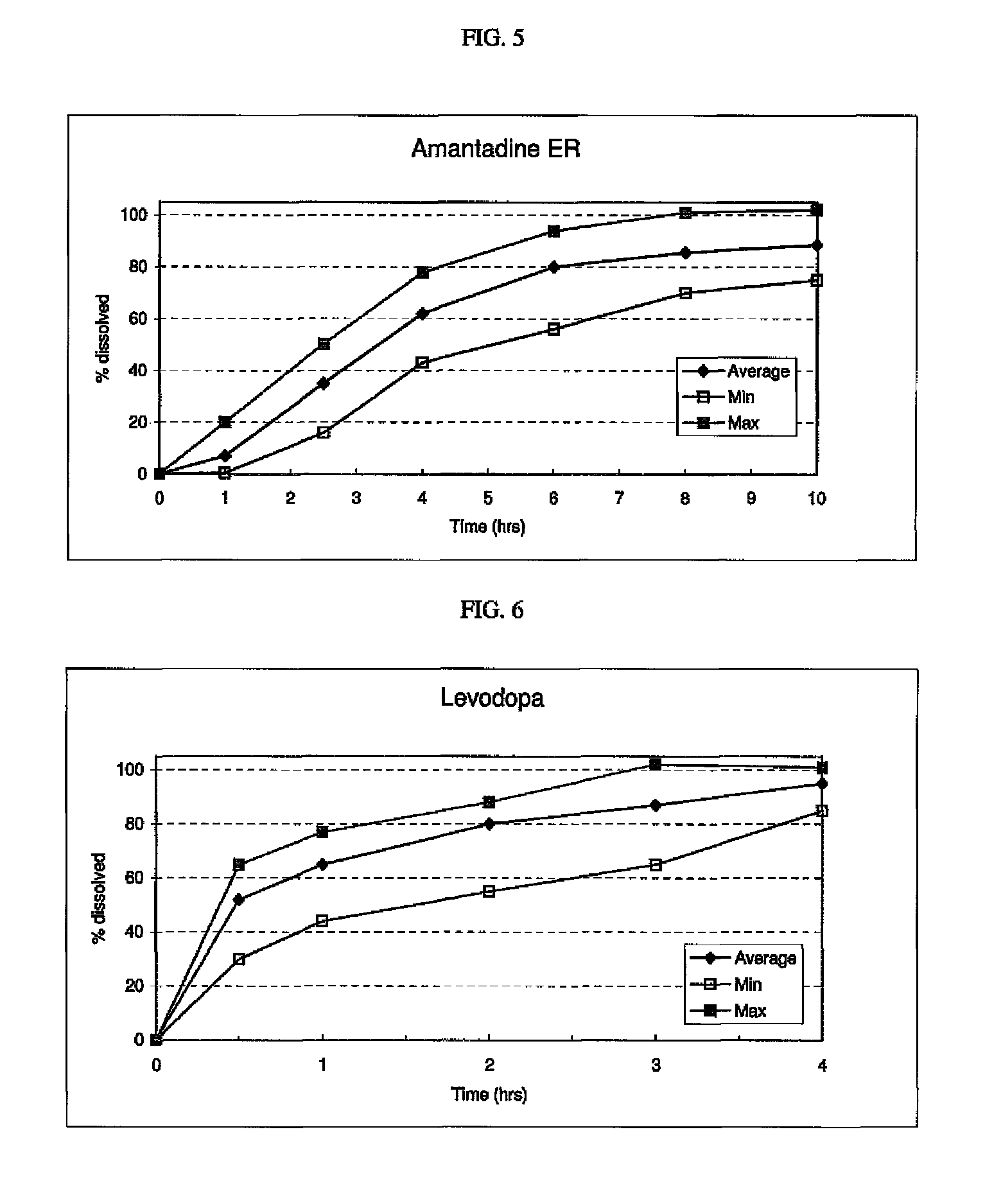
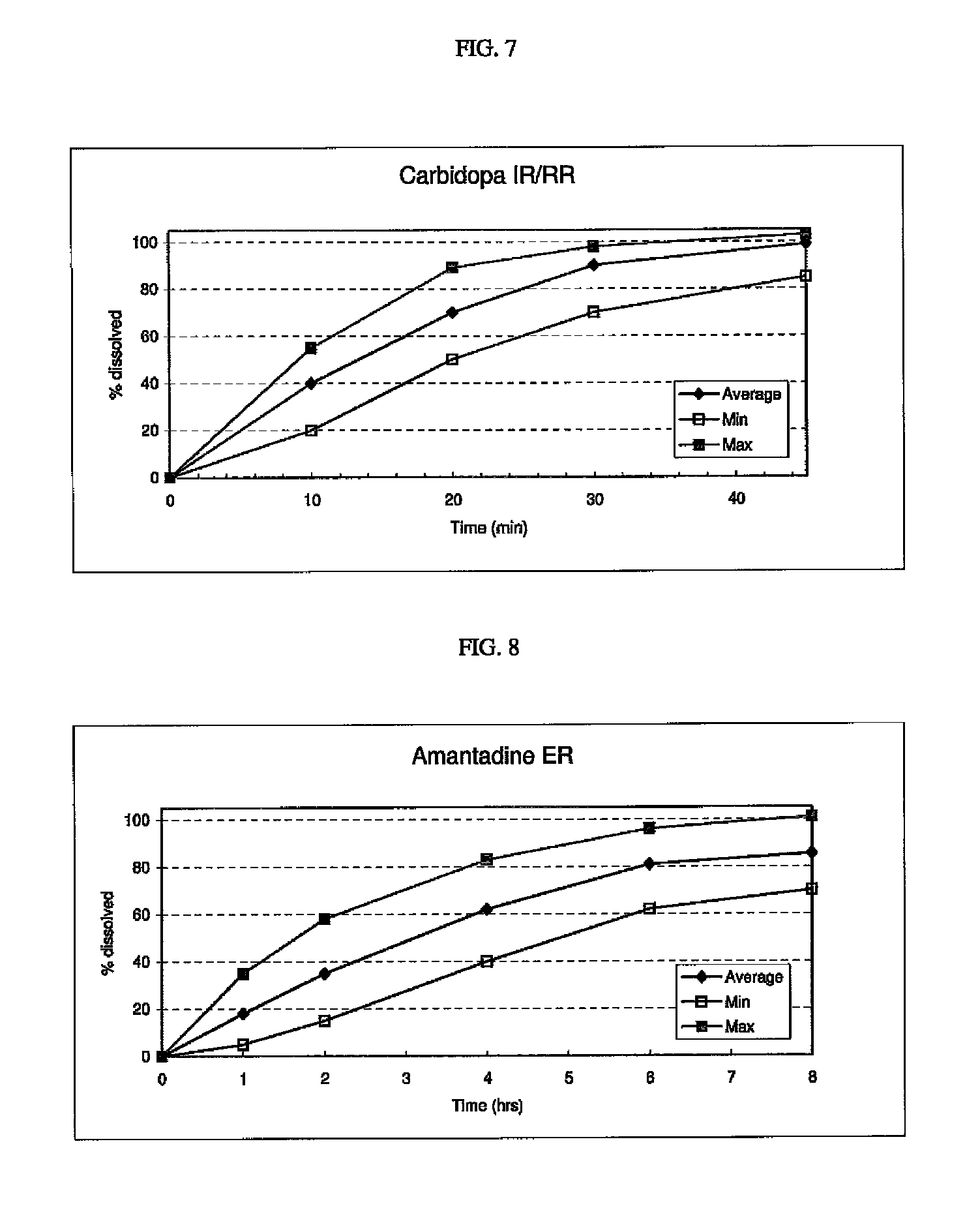
Triple Combination Release Multi-Layered Tablet
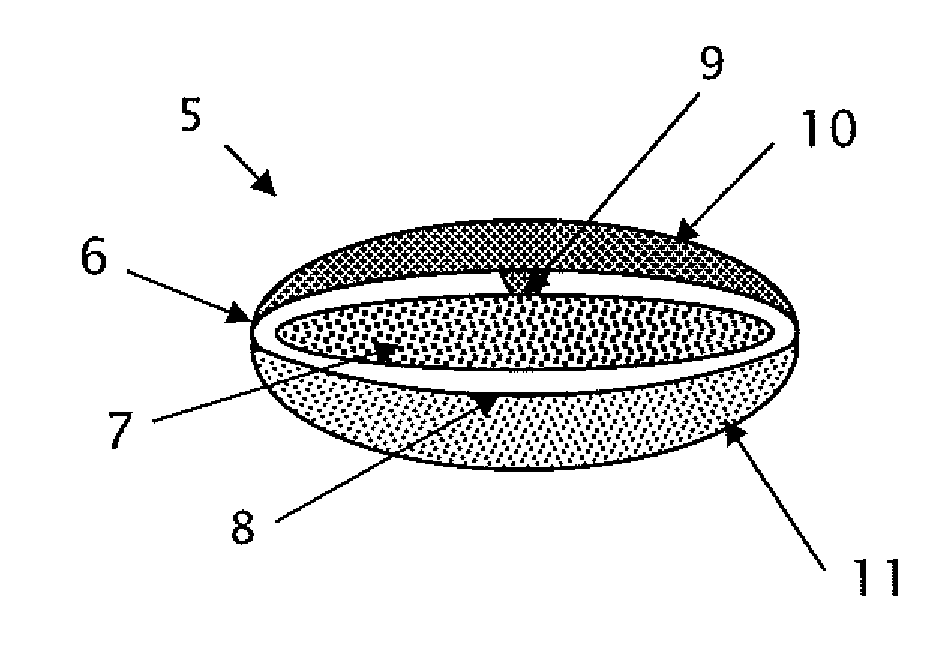
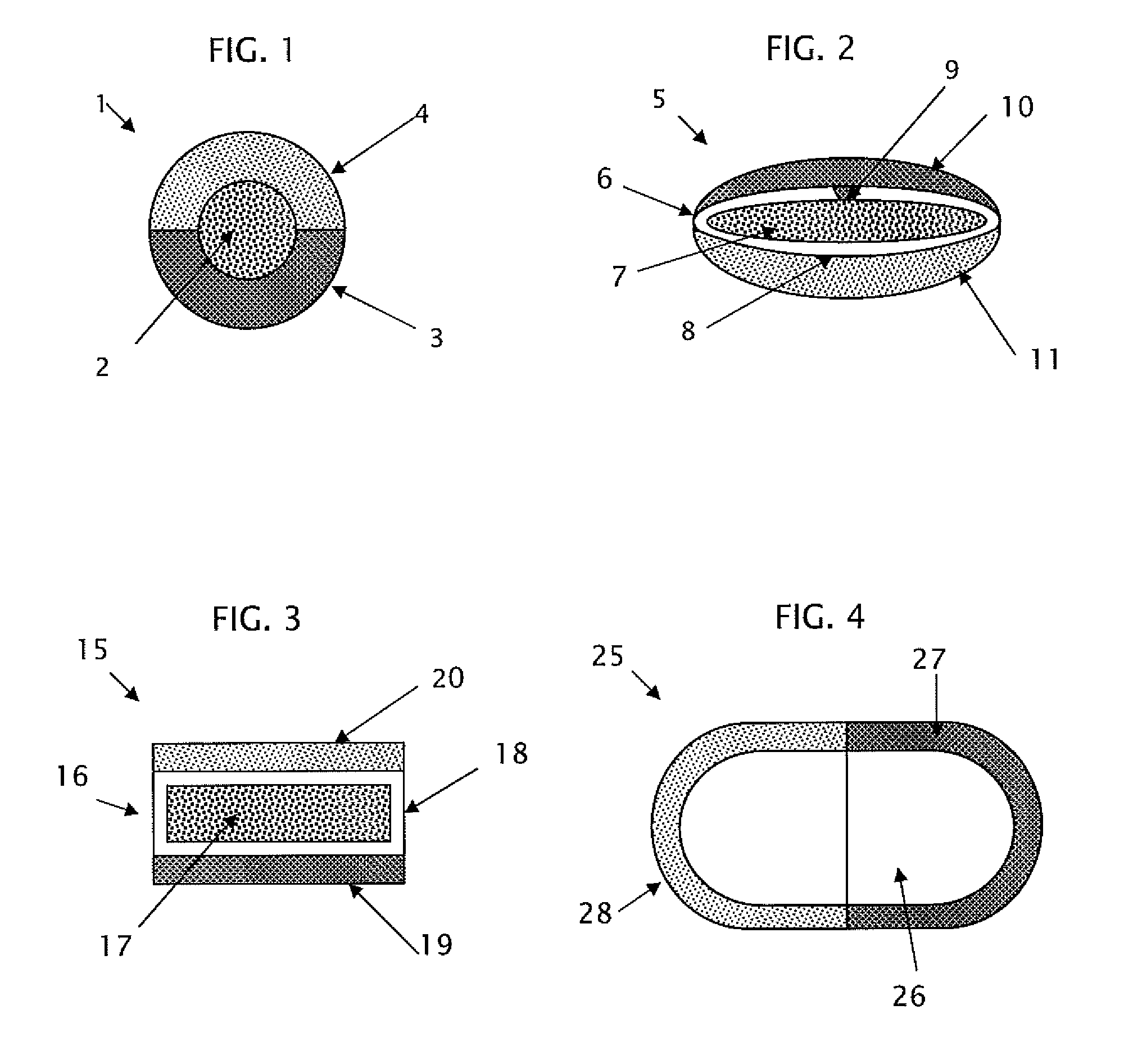
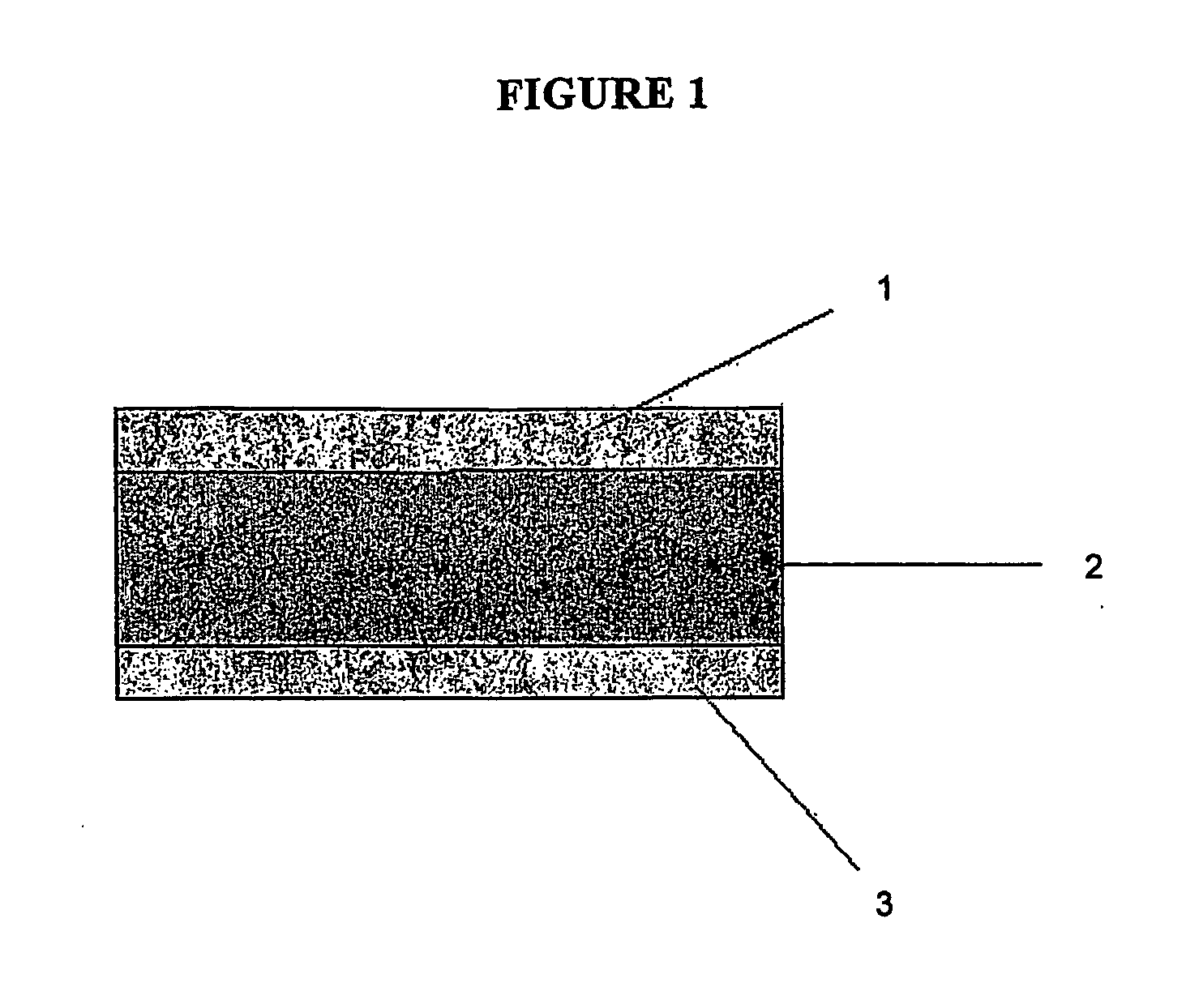
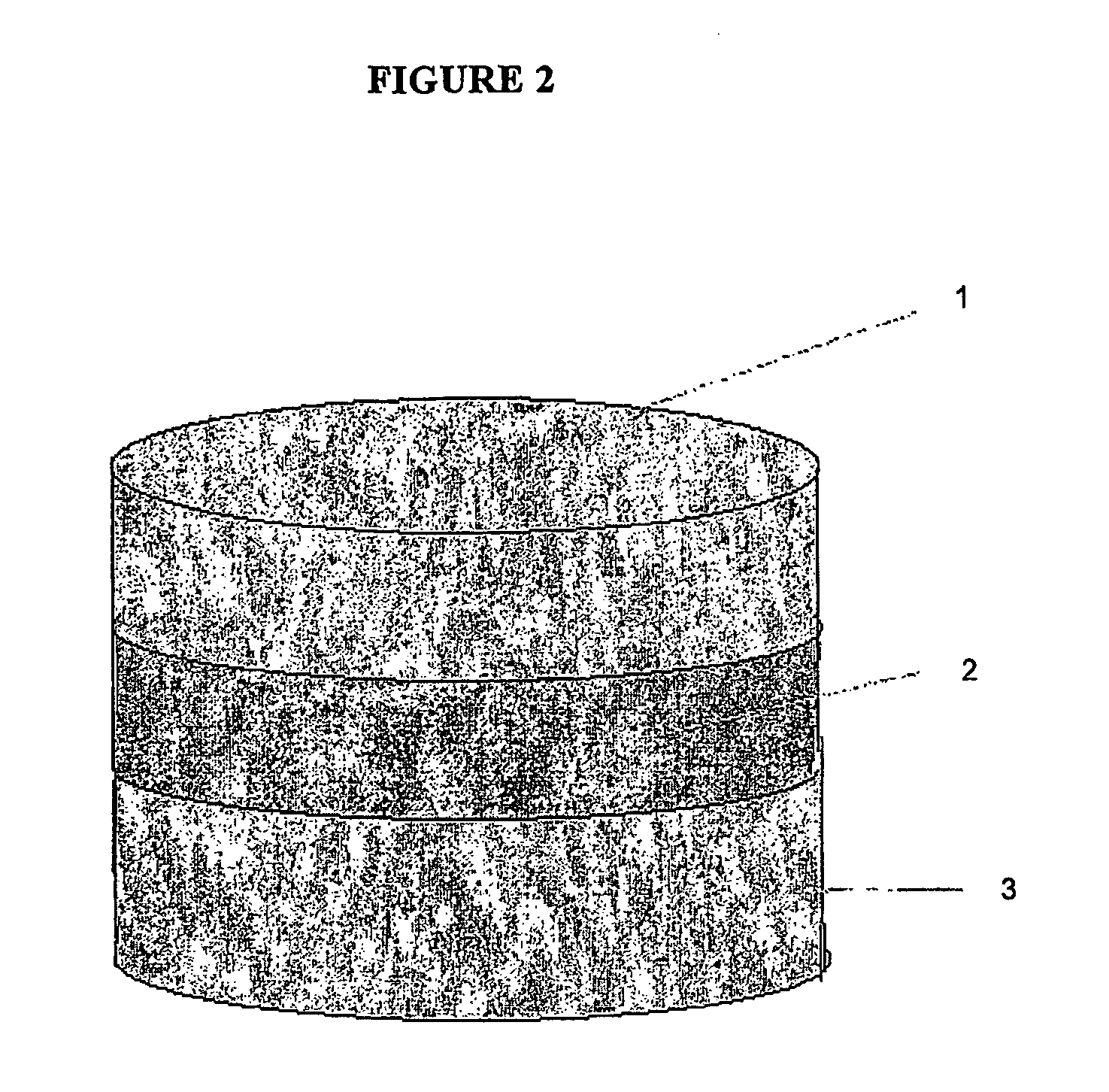
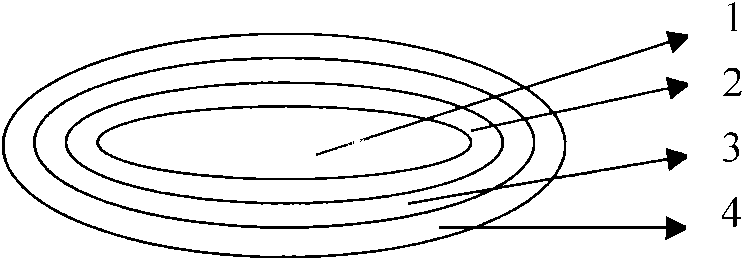
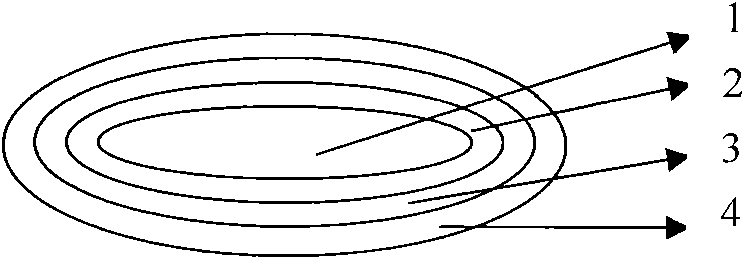
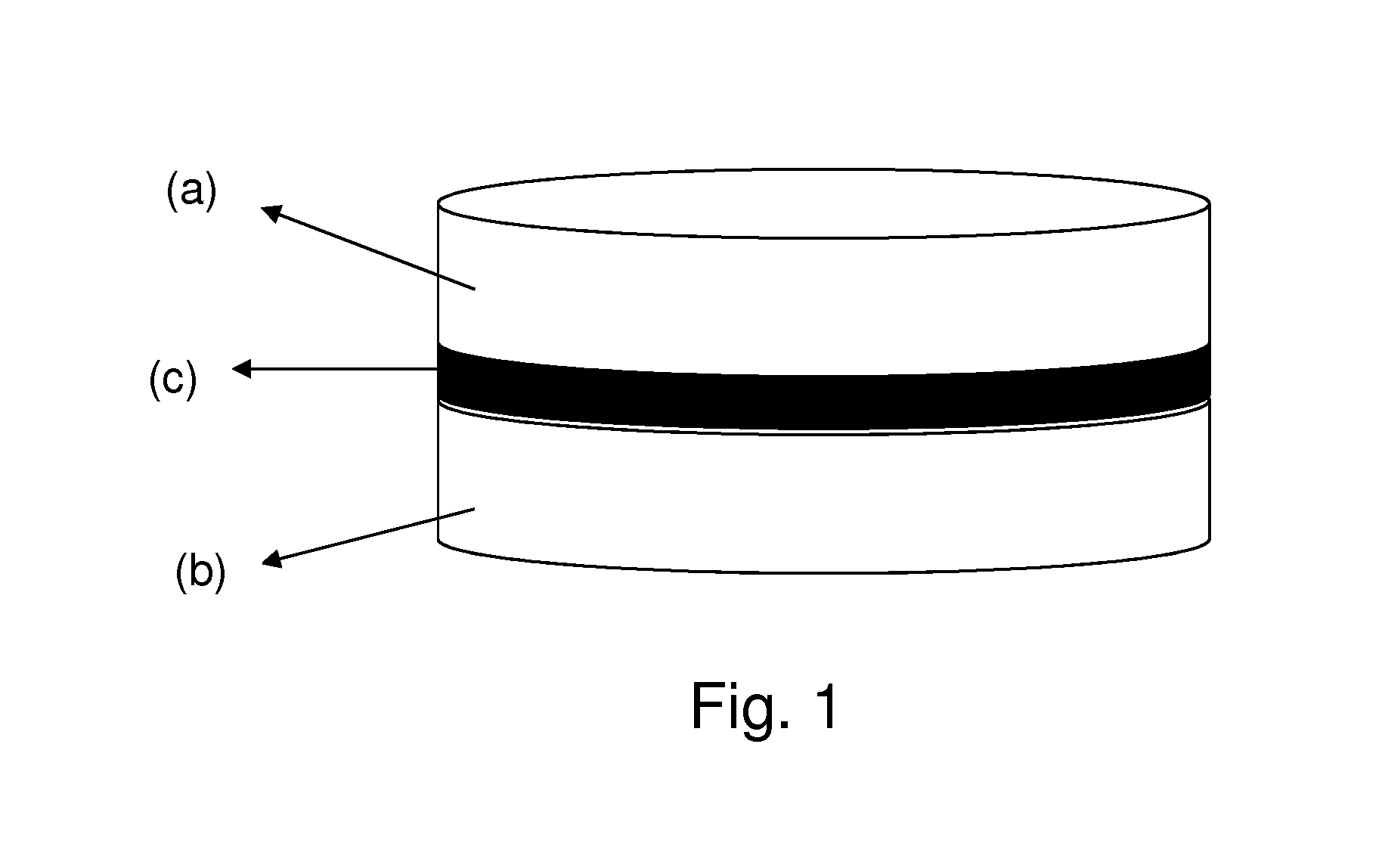
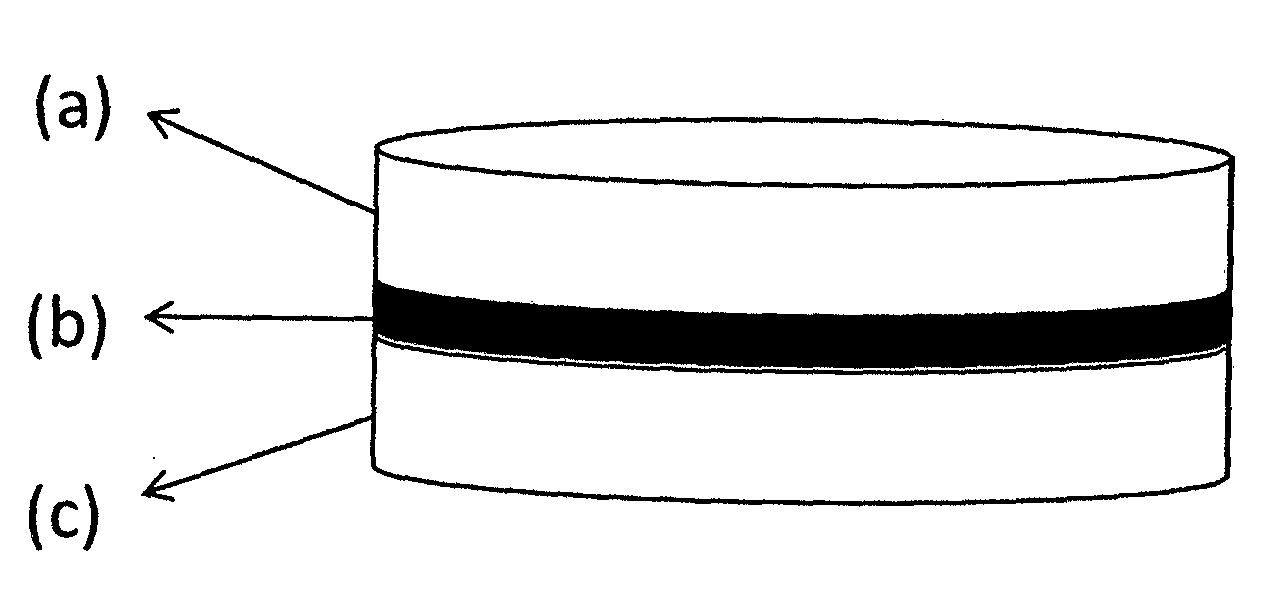
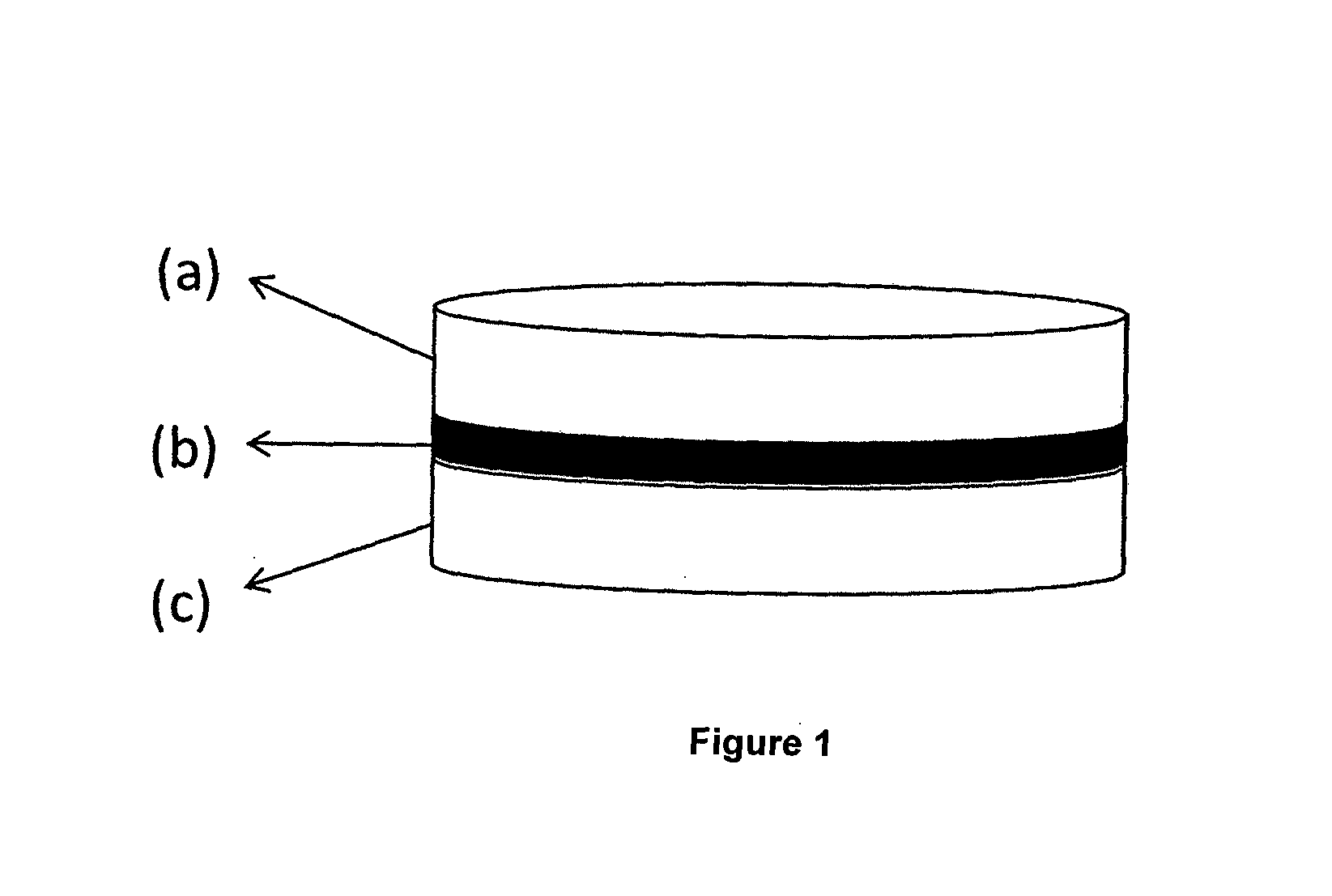
This invention pertains to a multi-layered tablet for a triple combination release of active agents to an environment of use. More particularly, the invention pertains to a multi-layered tablet (1) comprising two external drug-containing layers (2 and 3) in stacked arrangement with respect to and on opposite sides of an oral dosage form (4) that provides a triple combination release of at least one active agent. In one embodiment of the invention the dosage form is an osmotic device. In another embodiment of the invention the dosage form is a gastro-resistant coated core. In yet another embodiment of the invention the dosage form is a matrix tablet. In a different embodiment the dosage form is a hard capsule.
Owner:ACELLA HLDG LLC +1
Pharmaceutical compositions of rifaximin
ActiveUS20090028940A1Extended stayImprove complianceAntibacterial agentsBiocideImmediate releasePharmaceutical medicine
A pharmaceutical composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s) and release controlling agent(s). Pharmaceutical composition of rifaximin comprising: at least two entities wherein one entity is an immediate release or fast release and the other is controlled release. The pharmaceutical composition in the form of multilayer tablet comprising, at least one layer comprising, therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s); said layer providing controlled release rifaximin; and at least one layer which provides increased residence time of the dosage form in the gastrointestinal tract. The pharmaceutical formulation comprising rifaximin having an in vitro dissolution profile, wherein about 70% of rifaximin is released in about 24 hours. The composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt(s) or enantiomer(s) or polymorph(s) thereof, one or more release controlling agent(s) and pharmaceutically acceptable excipient(s) causing pathogenic eradication.
Owner:LUPIN LTD
Dual layer tablet, method of making and use thereof
InactiveUS6863830B1Easy to optimizeComprehensive treatmentOrganic chemistryOther chemical processesPotassium persulfateLithium hypochlorite
A method for treating a recirculating water system which comprises introducing into said water system a multifunctional, multilayer tablet, wherein the multilayer tablet comprises a fast dissolving layer and a slow dissolving layer, wherein said fast dissolving layer releases a combination of active ingredients including a member selected from the group consisting of lithium hypochlorite, calcium hypochlorite, trichloroisocyanuric acid (TCCA), anhydrous sodium dichloroisocyanurate, sodium persulfate, potassium persulfate, potassium monopersulfate, sodium monopersulfate, and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent, fragrance, or surfactant and, wherein said slow dissolving layer includes a member selected from the group consisting of trichloroisocyanuric acid (TCCA), calcium hypochlorite, 1,3-dichloro-5,5-dimethylhydantoin (DCDMH), 1,3-dibromo-5,5-dimethylhydantoin (DBDMH), 1-bromo-3-chloro-5,5-dimethylhydantoin (BCDMH), 1,3-dichloro-5-ethyl-5-methylhydantoin (DCEMH), 1,3-dibromo-5-ethyl-5-methylhydantoin (DBEMH), 1-bromo-3-chloro-5-methyl-5-ethylhydantoin (BCEMH), and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent or surfactant.
Owner:BIO LAB
Multilayer tablets containing thiazolidinedione and biguanides and methods for producing them
InactiveUS20060057202A1Facilitated releaseEfficient compressionBiocideOrganic active ingredientsAcute hyperglycaemiaImmediate release
A novel patient-convenient, cost effective pharmaceutical composition, comprising of thiazolidinediones and biguanide for controlling hyperglycemia manufactured as multilayer tablet and its process of manufacturing, for immediate release of thiazolidinediones or thiazolidinediones and biguanide and prolonged release of the biguanide only, the tablet comprising of minimum two layers wherein one outer layer comprises of a mixture of excipients and thiazolidinediones or thiazolidinediones and biguanide allowing immediate release of thiazolidinediones or thiazolidinediones and biguanide respectively and the other layer arranged in contact with the immediate release layer which comprises of a novel composition of excipients and a minimum one or more non-biodegradable, inert polymer(s) and the biguanide allowing pH independent prolonged release of the biguanide up to a period of 8-12 hours. The tablets are for once a day dosing. The tablets may optionally be film coated or enrobed by soft gelatin ribbons for additional protection against oxidation, photodegradation, identification, ease of swallowing, taste masking and for aesthetic appeal without altering the dissolution profile.
Owner:INVENTIA HEALTHCARE LTD
Dual layer tablet, method of making and use thereof
InactiveUS20050040116A1Easy to optimizeComprehensive treatmentOrganic chemistryOther chemical processesPotassium persulfateLithium hypochlorite
A method for treating a recirculating water system which comprises introducing into said water system a multifunctional, multilayer tablet, wherein the multilayer tablet comprises a fast dissolving layer and a slow dissolving layer, wherein said fast dissolving layer releases a combination of active ingredients including a member selected from the group consisting of lithium hypochlorite, calcium hypochlorite, trichloroisocyanuric acid (TCCA), anhydrous sodium dichloroisocyanurate, sodium persulfate, potassium persulfate, potassium monopersulfate, sodium monopersulfate, and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent, fragrance, or surfactant and, wherein said slow dissolving layer includes a member selected from the group consisting of trichloroisocyanuric acid (TCCA), calcium hypochlorite, 1,3-dichloro-5,5-dimethylhydantoin (DCDMH), 1,3-dibromo-5,5-dimethylhydantoin (DBDMH), 1-bromo-3-chloro-5,5-dimethylhydantoin (BCDMH), 1,3-dichloro-5-ethyl-5-methylhydantoin (DCEMH), 1,3-dibromo-5-ethyl-5-methylhydantoin (DBEMH), 1-bromo-3-chloro-5-methyl-5-ethylhydantoin (BCEMH), and mixtures thereof, and at least one of a clarifier, chelating agent, sequesterant, algaestat, water softener, algaecide, corrosion inhibitor, scale inhibitor, flocculent, disintegrant, dispersant, colorant, dissolution control agent or surfactant.
Owner:BIO LAB
Chewing gum in the form of multi-layer tablets
ActiveUS20060204451A1Improve featuresOvercome difficultiesBiocidePeptide/protein ingredientsSandwich likeAdjuvant
Disclosed are tablets having a sandwich-like structure comprising at least one inner layer of gum base containing one or more active pharmaceutical, dietetic or nutritional ingredients and two non-contigous outer layers comprising antiadhesion excipients and compression adjuvants preventing the adhesion to the punches of the tabletting machine and possibly active ingredients which are the same as or different from those present in the inner layer. Said tablets are obtainable by direct compression of mixtures or granulates of the various components of each layer.
Owner:JAGOTEC AG
Stable Pharmaceutical Composition Comprising a Fixed Dose Combination of Fenofibrate and an Hmg-Coa Reductase Inhibitor
InactiveUS20080131503A1Avoid interactionImprove stabilityBiocideDrug compositionsHMG-CoA reductaseAdditive ingredient
Owner:VELOXIS PHARMA
Triple combination release multi-layered tablet
This invention pertains to a multi-layered tablet for a triple combination release of active agents to an environment of use. More particularly, the invention pertains to a multi-layered tablet (1) comprising two external drug-containing layers (2 and 3) in stacked arrangement with respect to and on opposite sides of an oral dosage form (4) that provides a triple combination release of at least one active agent. In one embodiment of the invention the dosage form is an osmotic device. In another embodiment of the invention the dosage form is a gastro-resistant coated core. In yet another embodiment of the invention the dosage form is a matrix tablet. In a different embodiment the dosage form is a hard capsule.
Owner:ACELLA HLDG LLC +1
Pharmaceutical Multilayer Tablet for Controlled Release of Active Ingredients With Highly pH-Dependent Solubility
InactiveUS20070190146A1Improve solubilityFast drug release profilePill deliveryControlled releaseBULK ACTIVE INGREDIENT
The present invention relates to a pharmaceutical controlled release multilayer tablet comprising at least two layers, at least one active ingredient with highly pH-dependent solubility, at least one pharmaceutically acceptable pH maintaining excipient and at least one pharmaceutically acceptable matrix forming excipient, characterized in that said at least one active ingredient with highly pH-dependent solubility and said at least one pharmaceutically acceptable pH maintaining excipient are respectively comprised in at least one distinct layer.
Owner:SANOFI AVENTIS SA
Rate modulated delivery of drugs from a composite delivery system
InactiveUS20110195116A1Facilitated releaseInhibition releaseBiocidePeptide/protein ingredientsAdditive ingredientMedicine
This invention relates to a pharmaceutical dosage form for the delivery of at least one active pharmaceutical ingredient (API) or the pharmaceutically active salts and isomers thereof, to a desired absorption location of the human or animal body, preferably the gastrointestinal tract, in a predetermined rate-modulated manner. The dosage form is orally ingestible and is in the form of a multi-layered tablet preferably three layers and each layer includes an API or capsule containing a multiplicity of multi-layered granules. Each layer contains one or more APIs mixed or blended with at least one and preferably a matrix of polymers and, where appropriate, excipients, which, in use, inhibit release of an API in a region of the gastrointestinal tract other than the desired absorption location and, thus, facilitate release of the API in a rate controlled manner when in the desired absorption location. Methods of manufacturing said dosage form are further disclosed.
Owner:ADCOCK INGRAM INTPROP
Orally-dispersible multilayer tablet
The present invention relates to a multilayer orodispersible tablet and to the process for preparing it.
Owner:ETHYPHARM SA
Laminated tablet and manufacturing method therefor
ActiveUS20140023708A1Excellent adhesionSuppress layer separationPill deliveryNon-woven fabricsEngineeringMaterial Protrusion
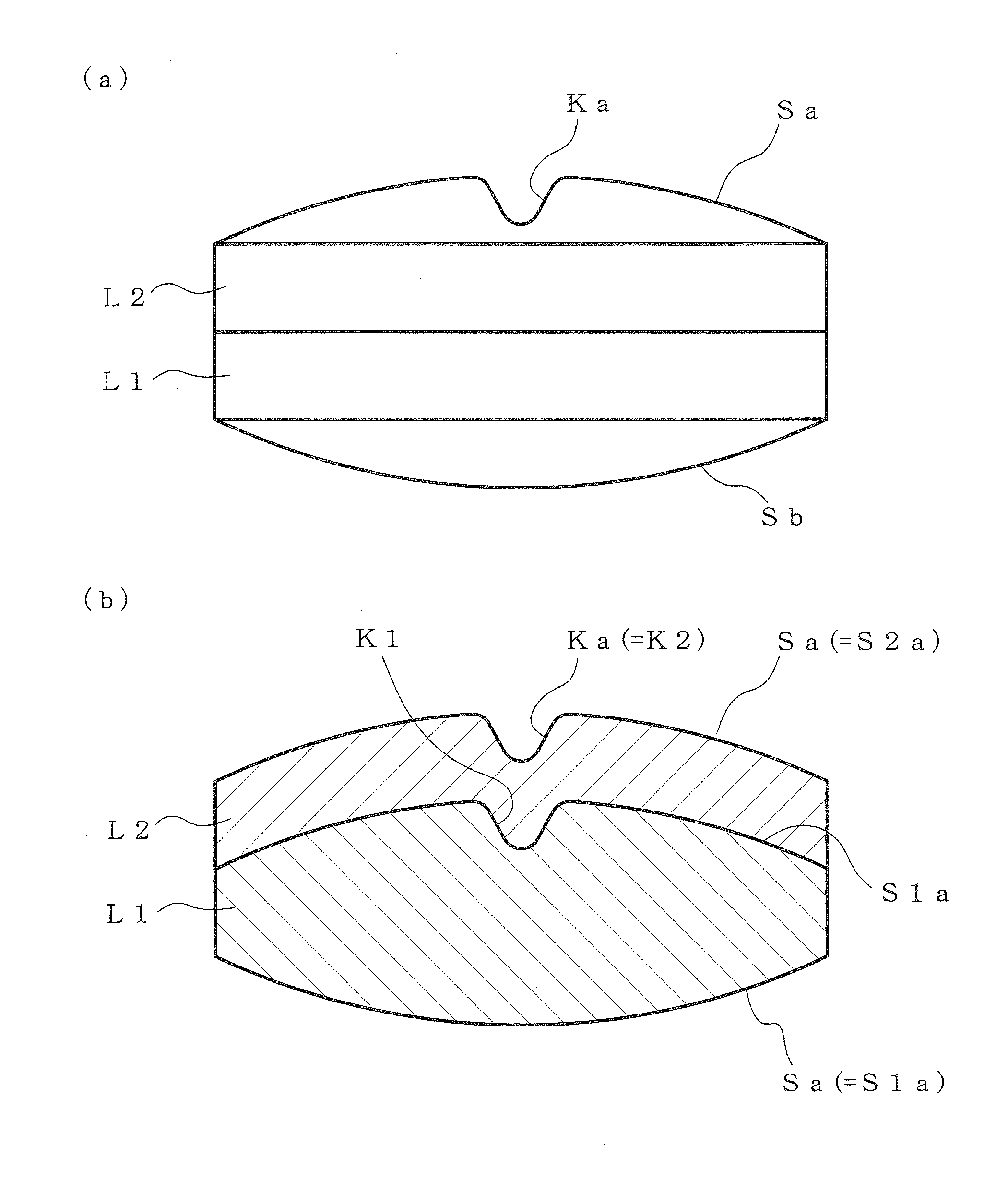
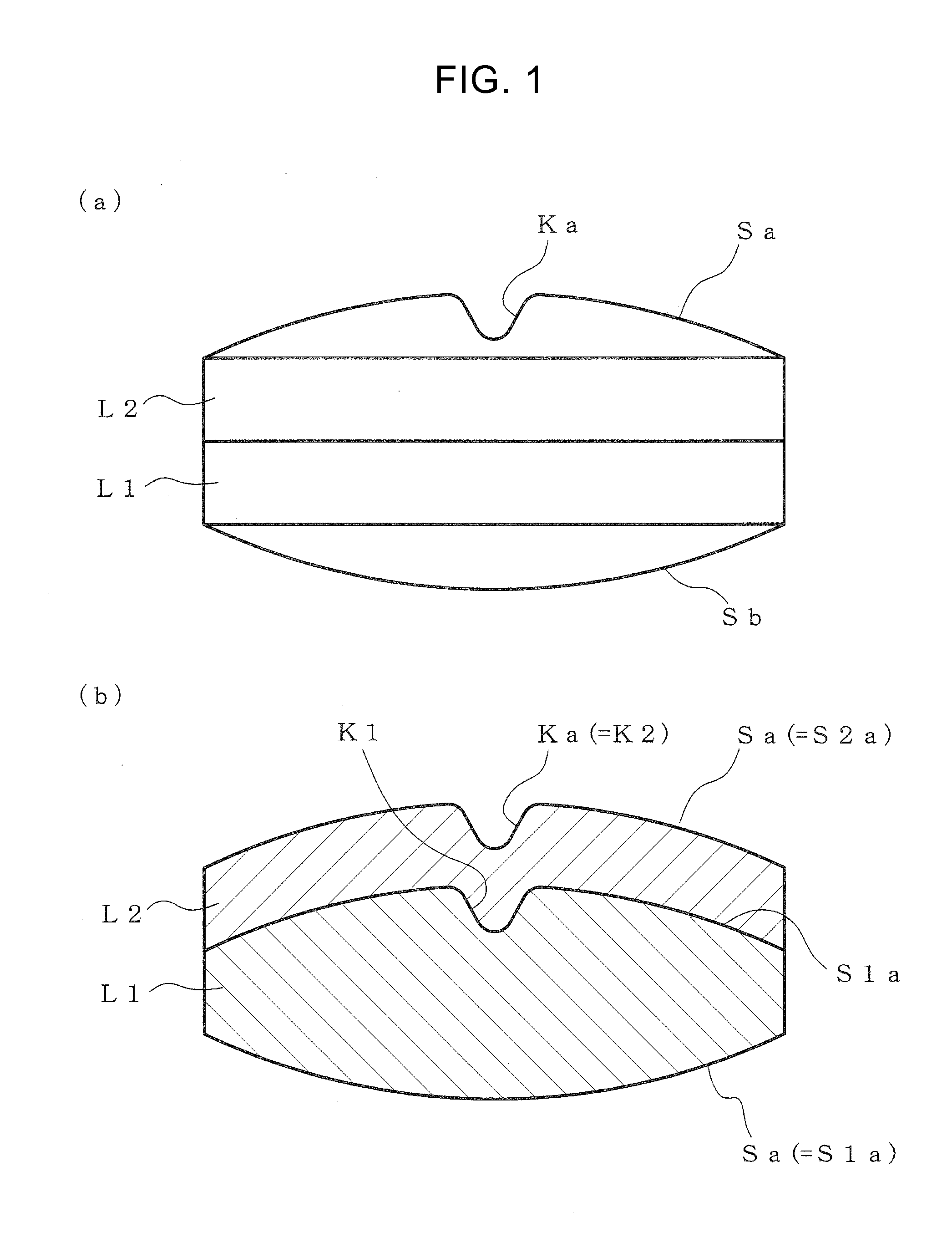
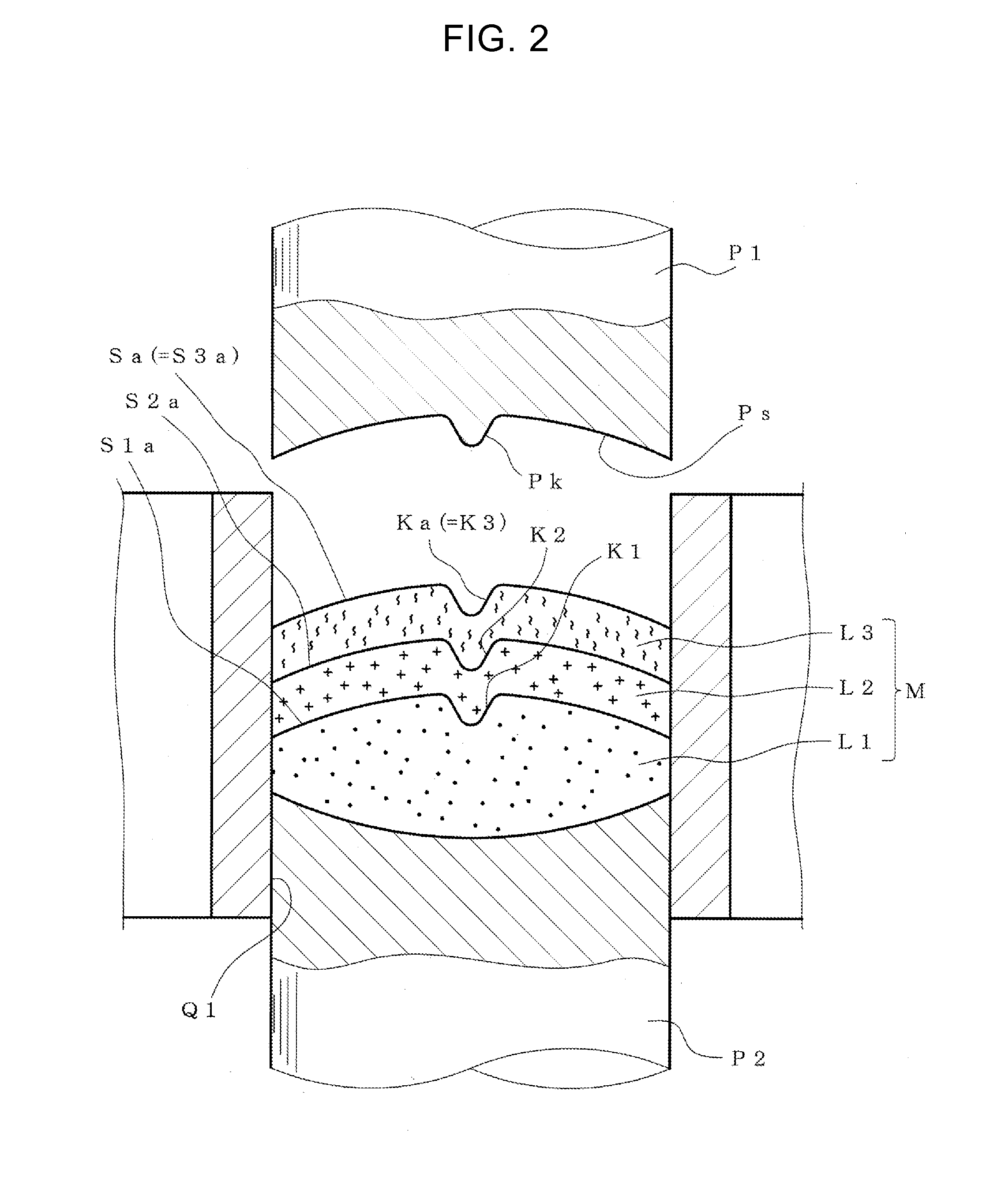
According to the present invention, a multilayer tablet showing suppressed layer separation and a production method thereof are provided. A concave portion having a depth of not less than 0.1 mm Ka is formed on at least one surface Sa of the both front and back surfaces (Sa, Sb) of a multilayer tablet. Particularly, a multilayer structure obtained by, in tableting, forming a convex portion for forming the concave portion on at least the upper punch, and preliminarily compressing all layers in the multilayer tablet with the upper punch to form a concave portion having the same shape with a depth of not less than 0.1 mm on the upper surface of all layers, wherein the powder materials of the next layer are protruding into the concave portion, is a preferable embodiment.
Owner:TAKEDA PHARMA CO LTD
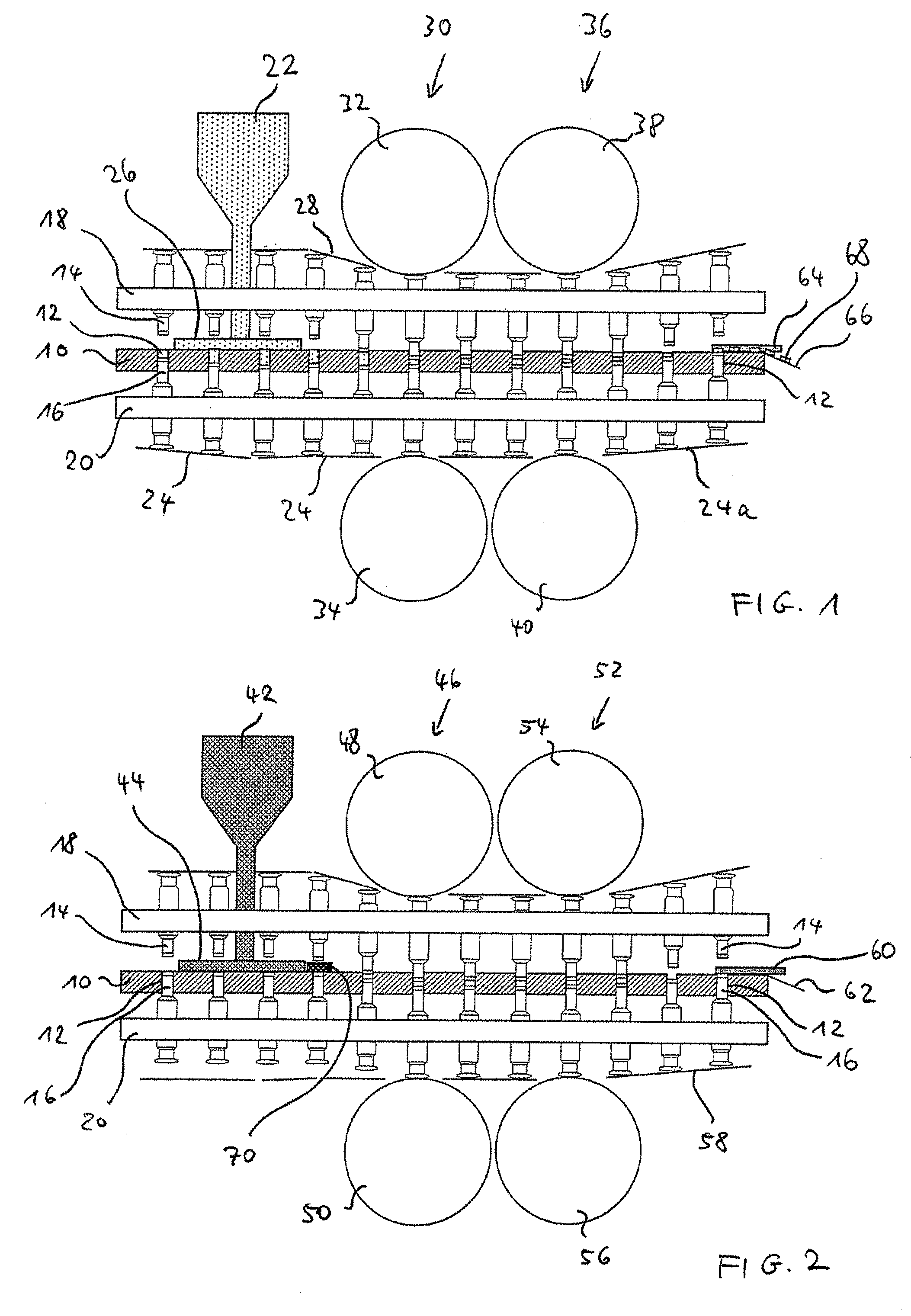
Method for testing multilayer tablets
ActiveUS20090152751A1Easy to compressStrong compressionMouldsWood working apparatusEngineeringMechanical engineering
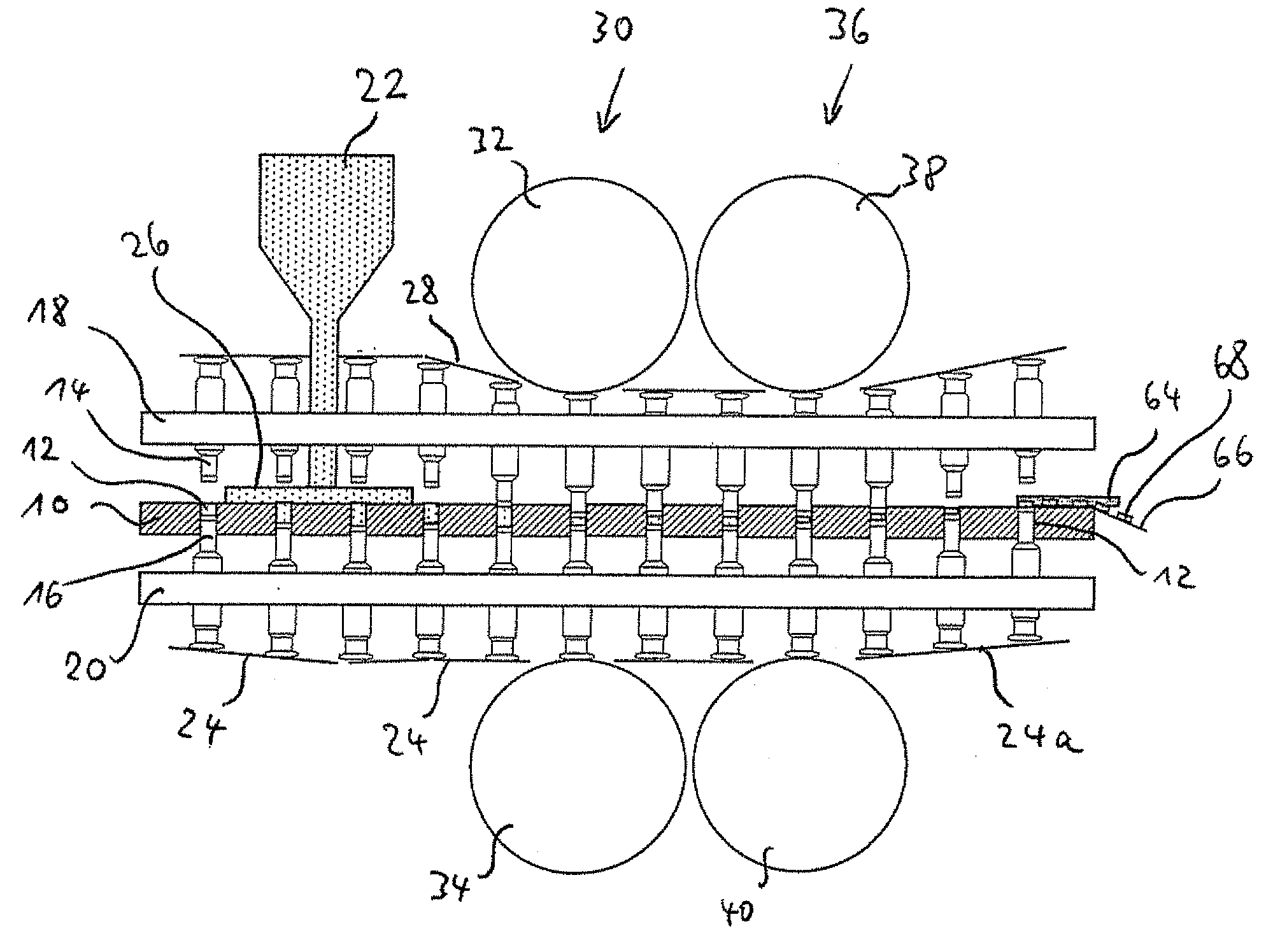
The present invention is related to a method for testing multilayer tablets in a multiple rotary press, in which die holes of a circulating die plate are successively filled with tablet material of different layers in succeeding filling devices, and the tablet material is compressed one layer after the foregoing layer into pressed articles having n layers by means of synchronously circulating compression punches, and the pressed articles are subsequently ejected in an unloading station and taken out, in which in a testing procedure, pressed articles with m layers are taken out after the compression in a respective unloading station and are conveyed to a testing station, wherein applies 1<=m<=n, wherein before taking out the pressed articles, at least the m-th layer is compressed more strongly than during the normal manufacture of the multilayer tablets, wherein applies m<n. According to the present invention it is provided that only pressed articles of die holes are supplied to the testing station, which had been completely filled with the tablet material of the m-th layer already before the initiation of the testing procedure.
Owner:FETTE
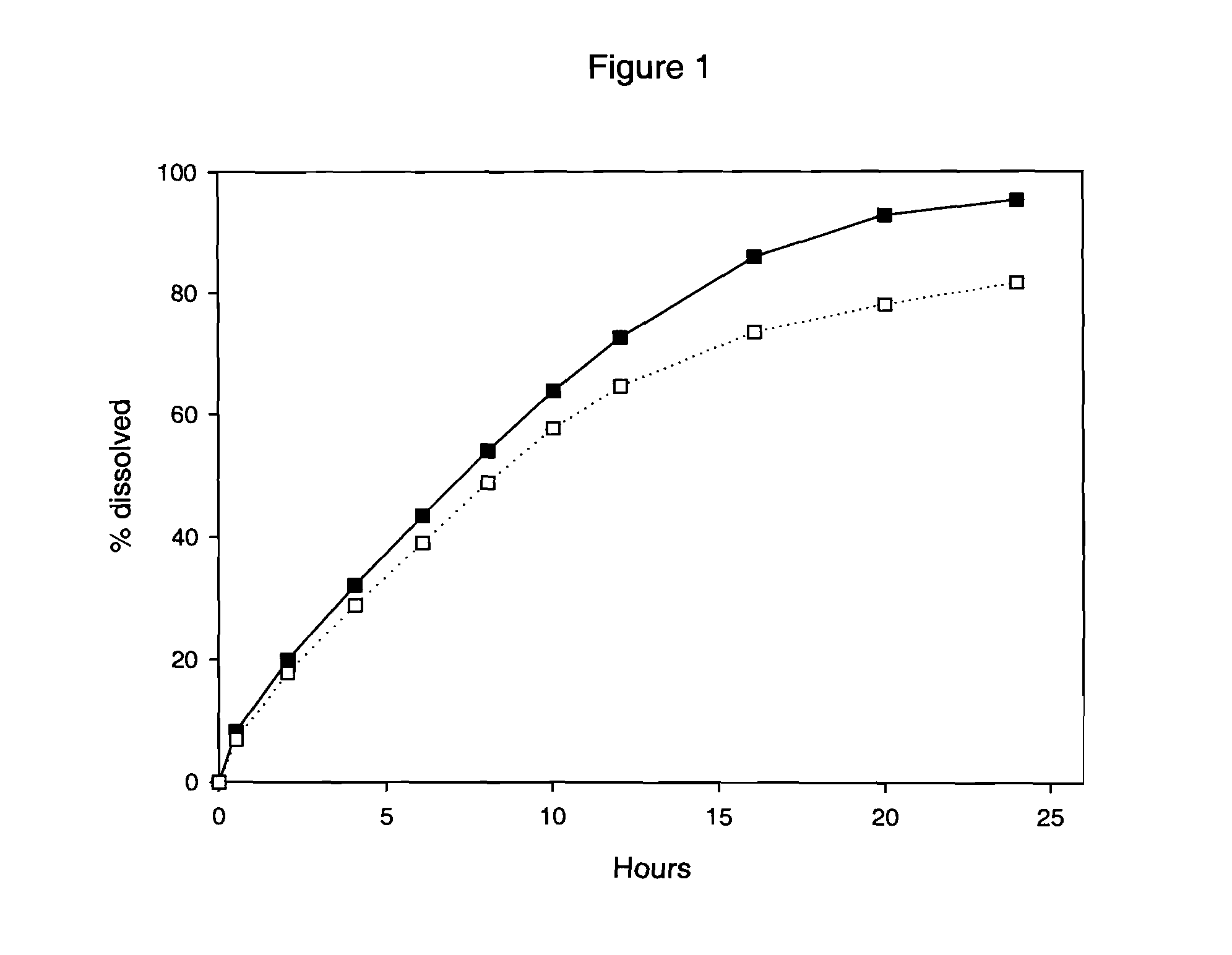
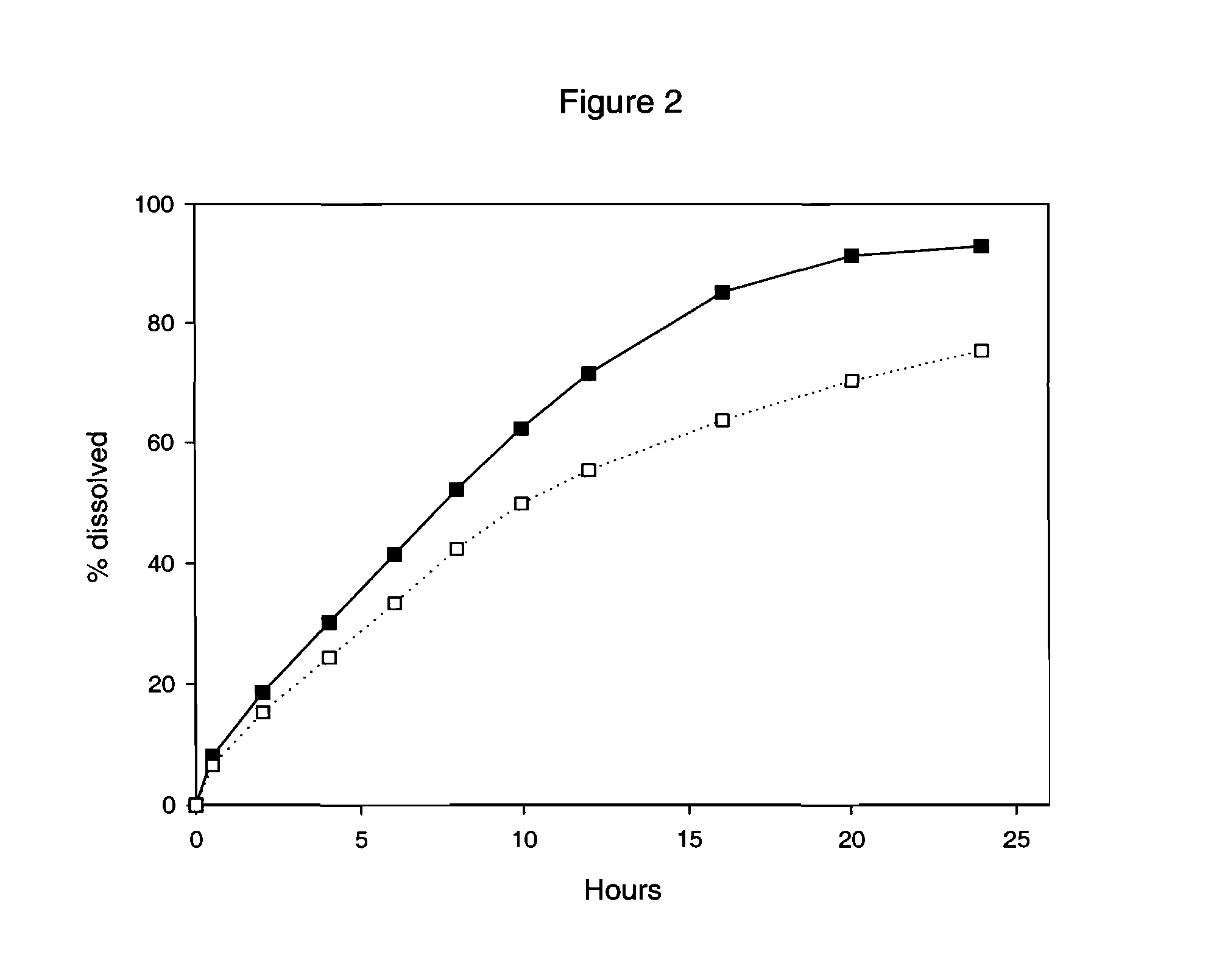
Multilayer Omeprazole Tablets
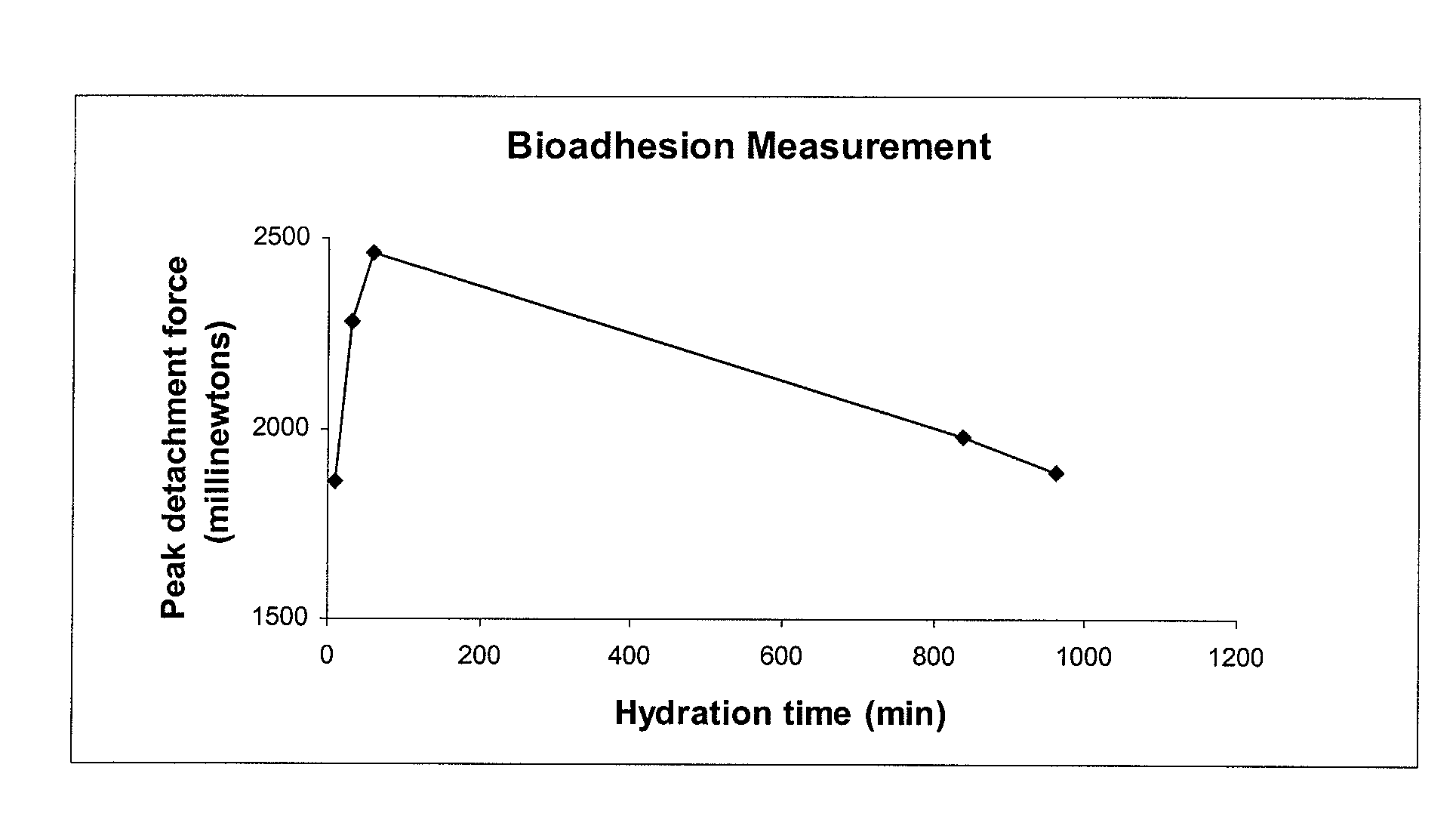
Multilayer tablets of Omeprazole and / or a salt thereof essentially bioequivalent in terms of plasma Omeprazole Cmax and AUC to Omeprazole capsules and / or Omeprazole Magnesium tablets consisting of multiple unit pellets are provided. Also provided are methods for production of these multilayer tablets and methods for their use in treating dyspepsia, peptic ulcer disease, gastroesophageal reflux disease and Zollinger-Ellison syndrome.
Owner:APTAPHARMA
Method for preparing aspirin and dipyridamole multilayer tablets
The invention provides a method for preparing aspirin and dipyridamole multilayer tablets, which is characterized by comprising: preparing dipyridamole sustained-release tablet cores by tabletting; and coating a stomach soluble insulation layer, an aspirin quick-release layer and a stomach soluble protective layer in turn. The method has the advantage that aspirin common release and dipyridamole sustained-release compound preparations are prepared by using conventional pharmaceutical equipment.
Owner:SHANDONG XINHUA PHARMA CO LTD
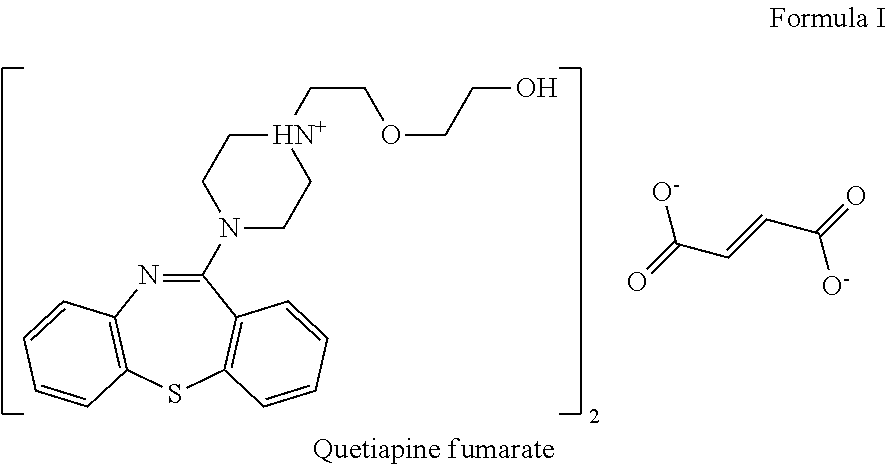
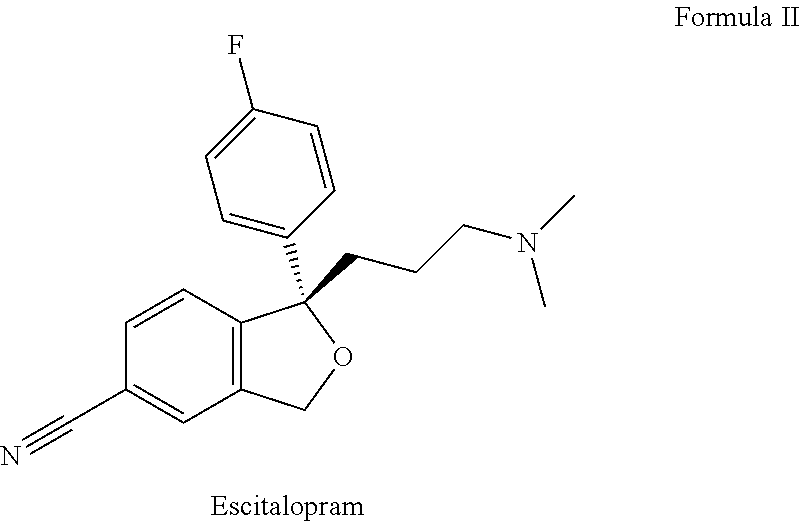
Pharmaceutical formulations comprising quetiapine and escitalopram
The present invention relates to a multilayer tablet formulation comprising a combination of quetiapine or a pharmaceutically acceptable salt thereof and escitalopram or a pharmaceutically acceptable salt thereof.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Controlled Release Flurbiprofen and Muscle Relaxant Combinations
This invention is a novel controlled release (CR) flurbiprofen and muscle relaxant combinations for oral administration with anti-inflammatory, analgesic, myorelaxant activity and methods of its manufacture. The pharmaceutical composition of the present invention is administered orally in tablet, multilayer tablet, multicoated tablet and capsule form.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Multilayer orodispersible tablet
Owner:ETHYPHARM SA
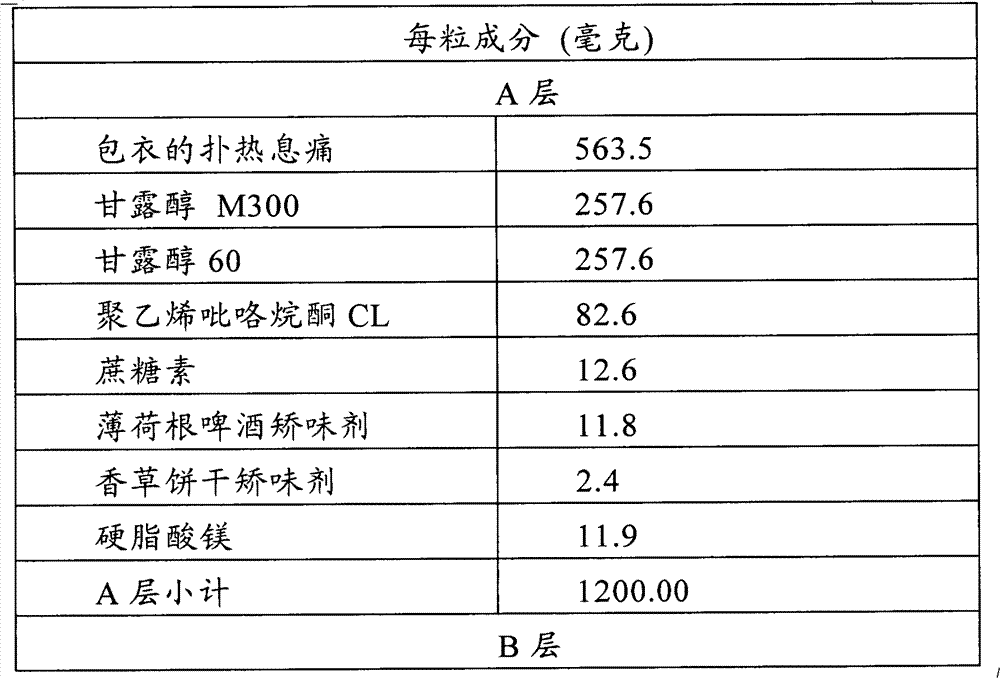
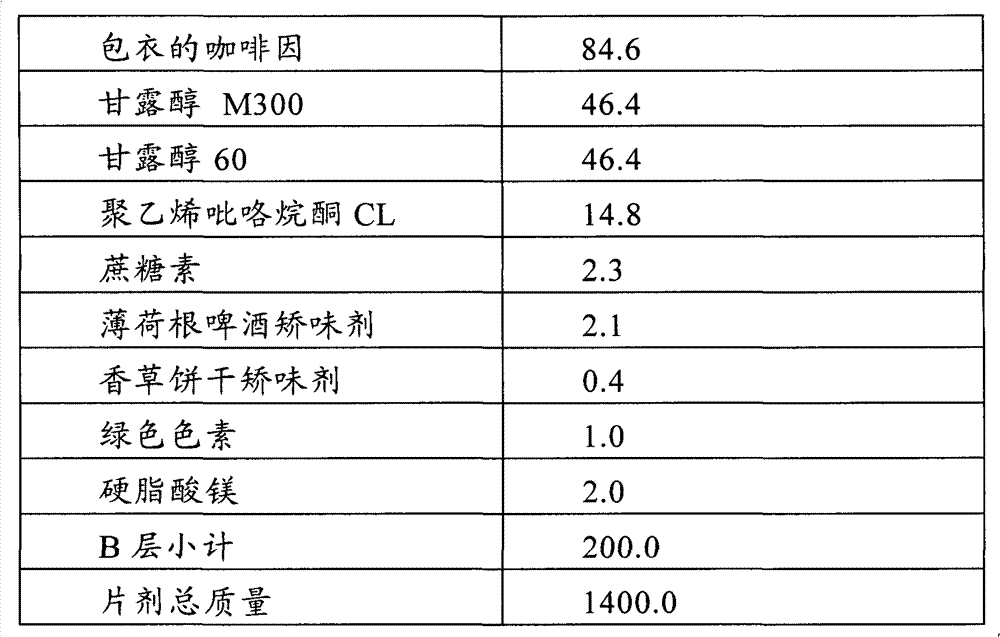
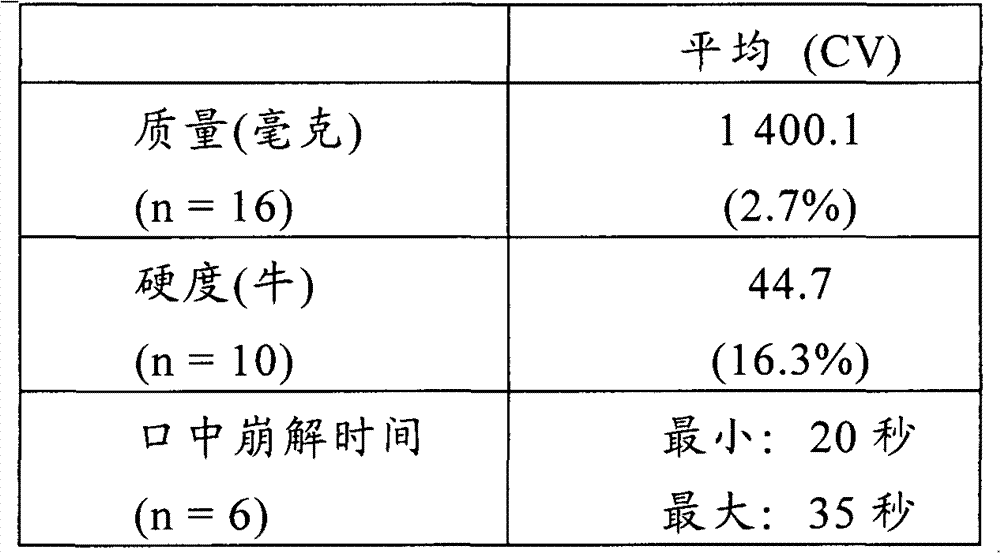
Multi-layer tablet containing calsium-path receptor retarder and ACE inhibitor, and preparing method
InactiveCN1814290AEasy to manufactureQuality improvementMetabolism disorderPill deliveryAdditive ingredientLevamlodipine
This invention is a multilayer tablet contains calcium channel acceptor retarder and ACE inhibitor. Its feature is that it contains calcium channel acceptor retarder pleated sheet and ACE inhibitor pleated sheet. The calcium channel acceptor retarder pleated sheet contains levamlodipine or amoldipine or salt that can be accepted on pharmacy of them. The ACE inhibitor pleated sheet contains methylphenidate or its pharmacy accepted salt. They can be produced by normal medicine equipment, and preparation is easy, the multilayer tablet made can be quickly disintegrated, main medicine component can be quickly stripped, and its quality stability is good.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
Pharmaceutical compositions of rifaximin
ActiveUS8383151B2Extended stayImprove complianceAntibacterial agentsBiocideImmediate releasePharmaceutical medicine
A pharmaceutical composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s) and release controlling agent(s). Pharmaceutical composition of rifaximin comprising: at least two entities wherein one entity is an immediate release or fast release and the other is controlled release. The pharmaceutical composition in the form of multilayer tablet comprising, at least one layer comprising, therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s); said layer providing controlled release rifaximin; and at least one layer which provides increased residence time of the dosage form in the gastrointestinal tract. The pharmaceutical formulation comprising rifaximin having an in vitro dissolution profile, wherein about 70% of rifaximin is released in about 24 hours. The composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt(s) or enantiomer(s) or polymorph(s) thereof, one or more release controlling agent(s) and pharmaceutically acceptable excipient(s) causing pathogenic eradication.
Owner:LUPIN LTD
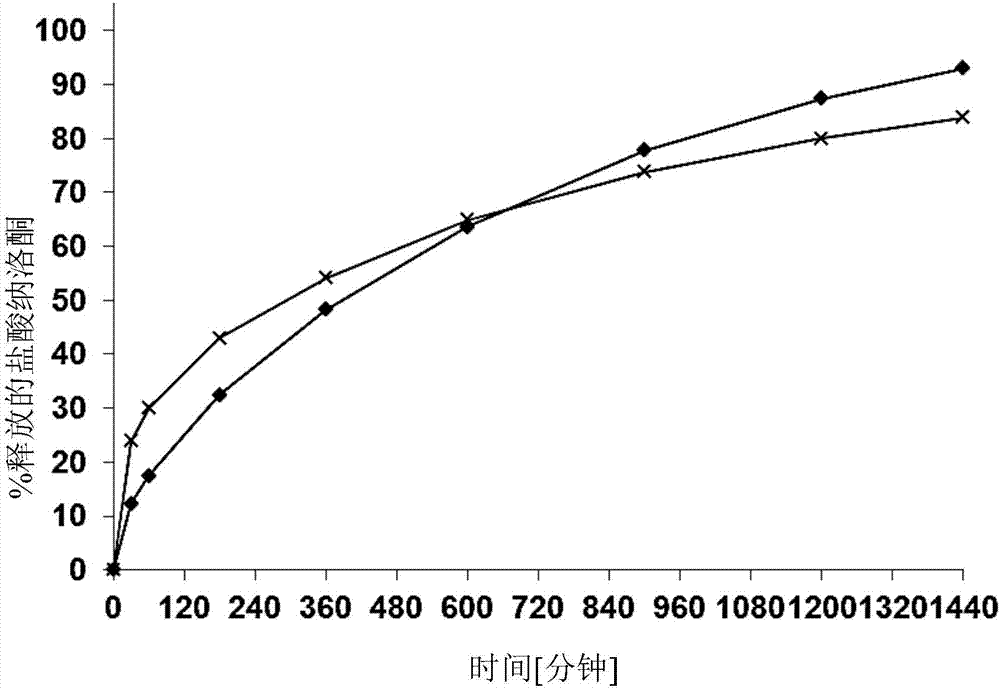
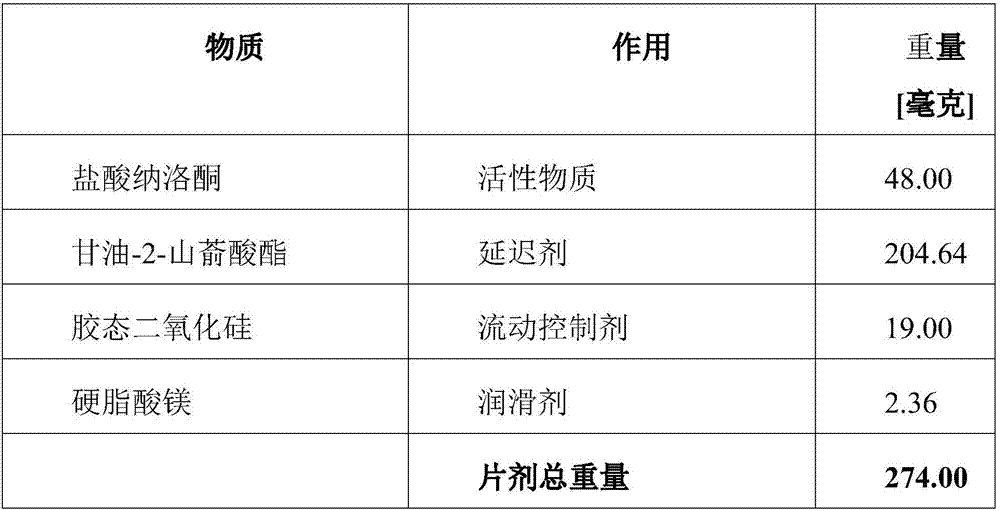
Naloxone monopreparation and multi-layer tablet
InactiveCN107205943AAchieve release kineticsOrganic active ingredientsNervous disorderGlycerolBULK ACTIVE INGREDIENT
The present invention relates to a solid, oral pharmaceutical composition comprising naloxone, or a pharmaceutically acceptable salt thereof as the active ingredient, the composition having a delayed release of said active ingredient. The composition can comprise a matrix containing glycerol di-behenic acid esters as matrix formers, with a mass ratio of naloxone to matrix former(s) of between 1:1 and 1:10, whereby the active ingredient naloxone has a delayed release. According to the invention, in order to provide a composition suitable for a dosage covering at least twelve hours for treating opioid-induced obstipation, the composition has an in-vitro release rate of the active ingredient, measured using a vane stirrer method according to Eur. Ph. at 75 U / min in 500 ml of 0.1 N hydrochloric acid at 37 DEG C, of 0 % to 75 % in 2 h, 3 % to 95 % in 4 h, 20 % to 100 % in 10 h, 30 % to 100 % in 16 h, 50 % to 100 % in 24 h and more than 80 % in 36 h, said composition having an IC50 / Cmax value of at least 40. Preferably, the composition comprises a value for tmax (naloxone) / tmax (naloxone-3-glucuronoid) of at least 5. In an alternative embodiment, the composition can take the form of a multi-layer tablet.
Owner:德威洛克制药有限公司
Multilayer Proton Pump Inhibitor Tablets
Multilayer tablets of a proton pump inhibitor essentially bioequivalent in terms of plasma Cmax and AUC to capsules and / or tablets consisting of multiple unit pellets of the proton pump inhibitor are provided. Also provided are methods for production of these multilayer tablets and methods for their use in treating dyspepsia, peptic ulcer disease, gastroesophageal reflux disease and Zollinger-Ellison syndrome.
Owner:APTAPHARMA
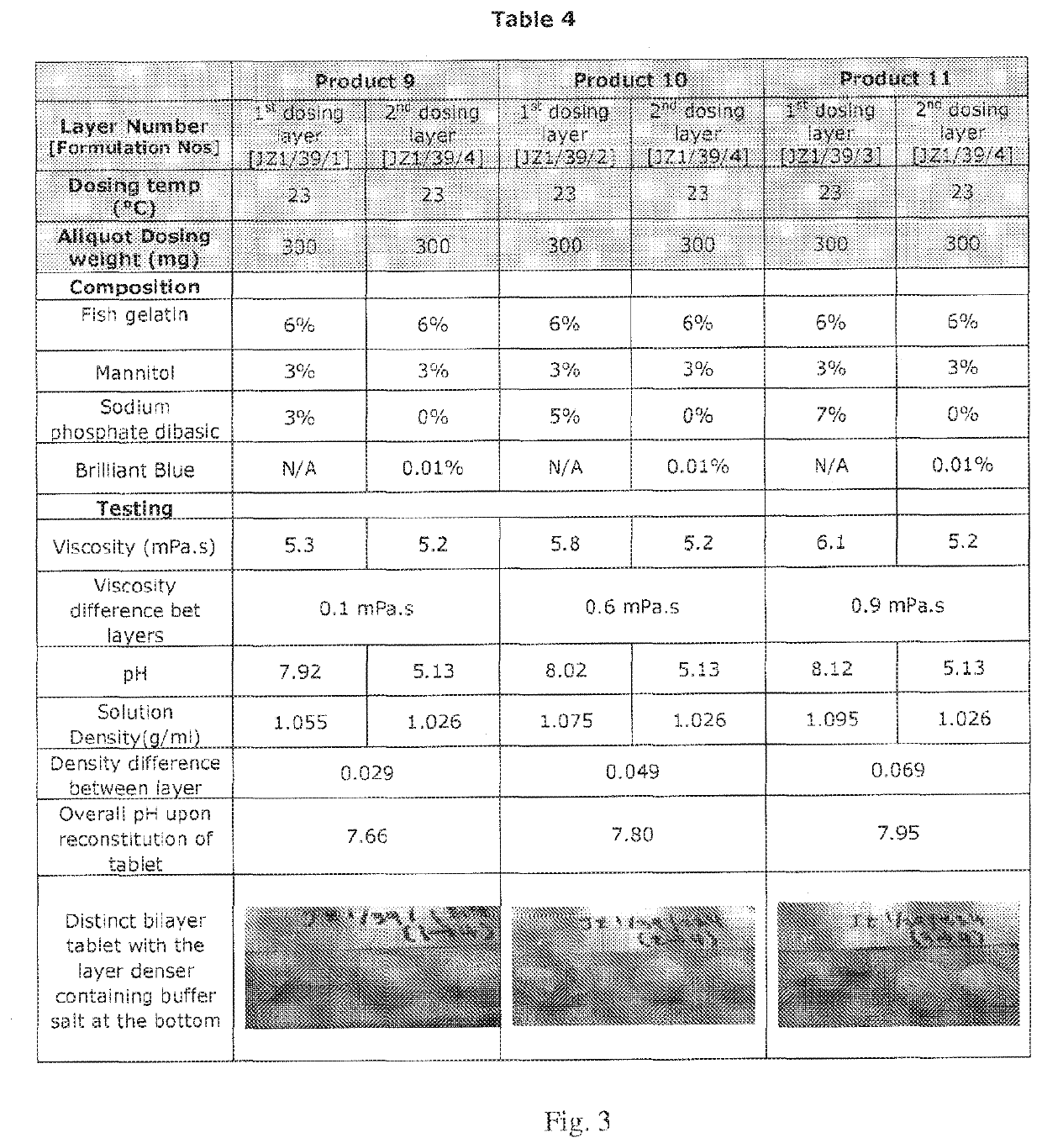
Compositions of different densities for fast disintegrating multi-layer tablet
Described herein is a method for forming multi-layer drug dosage forms having at least two layers. In the method, a first formulation comprising a non-gelling matrix forming agent and having a first density is dosed into a preformed mold. A second formulation comprising a non-gelling matrix former and having a second density not equal to the first density is subsequently dosed into the preformed mold. Then, the combination of the formulations dosed into the mold is freeze dried to form the multi-layer dosage form having at least two layers. The use of a density difference between the first and second formulations ensures formation of a product with two distinct layers.
Owner:CATALENT U K SWINDON ZYDIS LTD
Combined pharmaceutical formulation containing diacerein
InactiveUS20150004229A1Reduce the amount requiredEnhanced advantageBiocidePill deliveryPharmaceutical formulationCombination drug
The present invention relates to a combined pharmaceutical formulation having therapeutic anti-inflammatory, analgesic, antipyretic and osteoarthritis activities. From another perspective, the present invention relates to a new solid oral dosage form comprising a pharmaceutically effective amount of NSAID and a pharmaceutically effective amount of diacerein and the solid oral dosage form according to the present invention is preferably in the form of tablet and more preferably in the form of multi-layer tablet.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
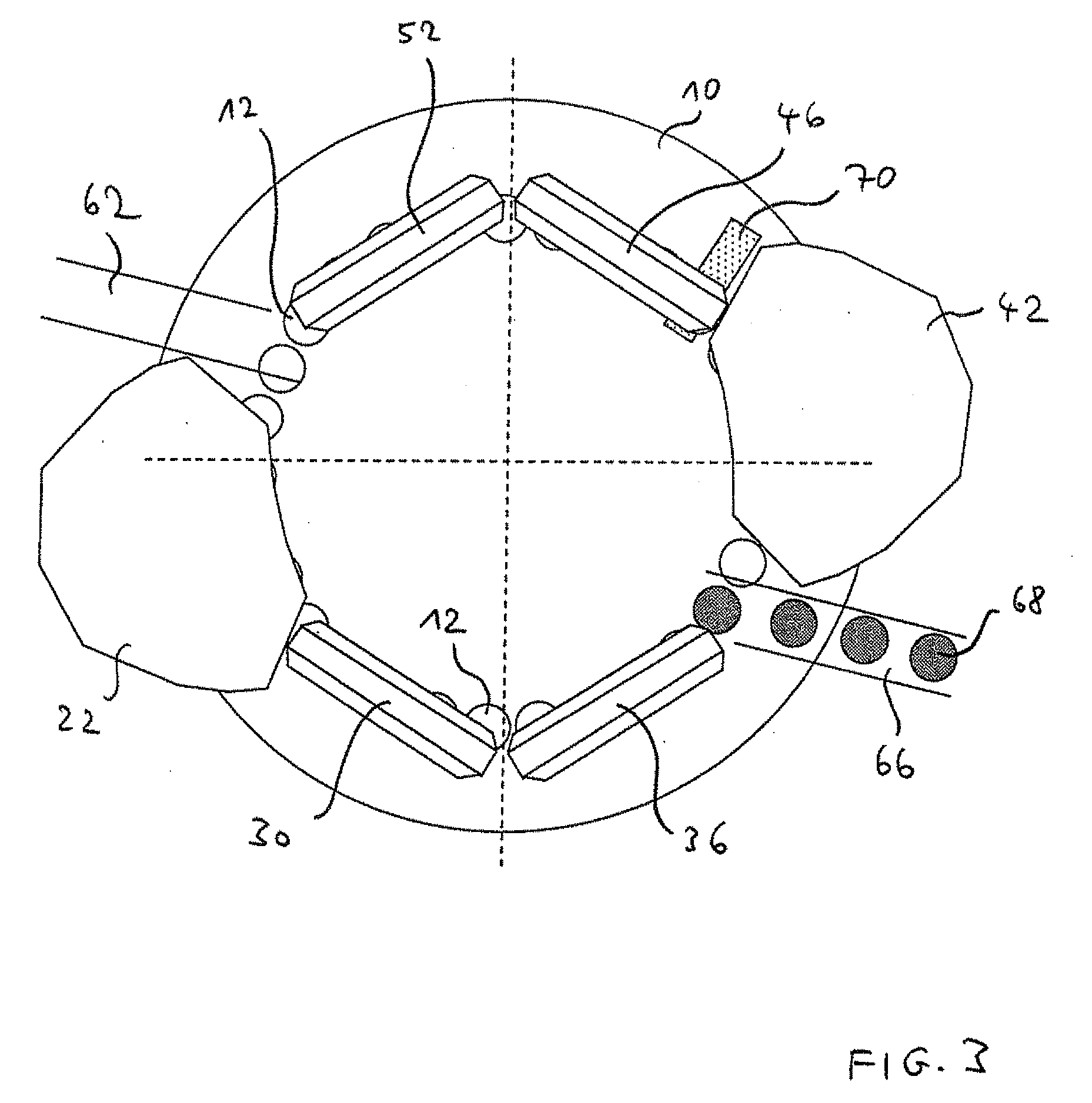
Method for testing multilayer tablets in a multiple rotary press, the tested tablets produced under normal operation, with m layers pressed and the m+1 layer suctioned off and the tablet fed to the testing station
ActiveUS8525050B2Accuracy testIncrease the number ofWithdrawing sample devicesAuxillary shaping apparatusMultilayer tabletFill device
The invention is related to a method for testing multilayer tablets in a multiple rotary press, in which die holes of a circulating die plate are successively filled with tablet material of different layers in consecutive filling devices, and the tablet material is compressed layer after layer to pressed articles having n layers by means of synchronously circulating compression punches in compression stations respectively associated to the filling devices, and the pressed articles are subsequently ejected and unloaded in a unloading station.
Owner:FETTE
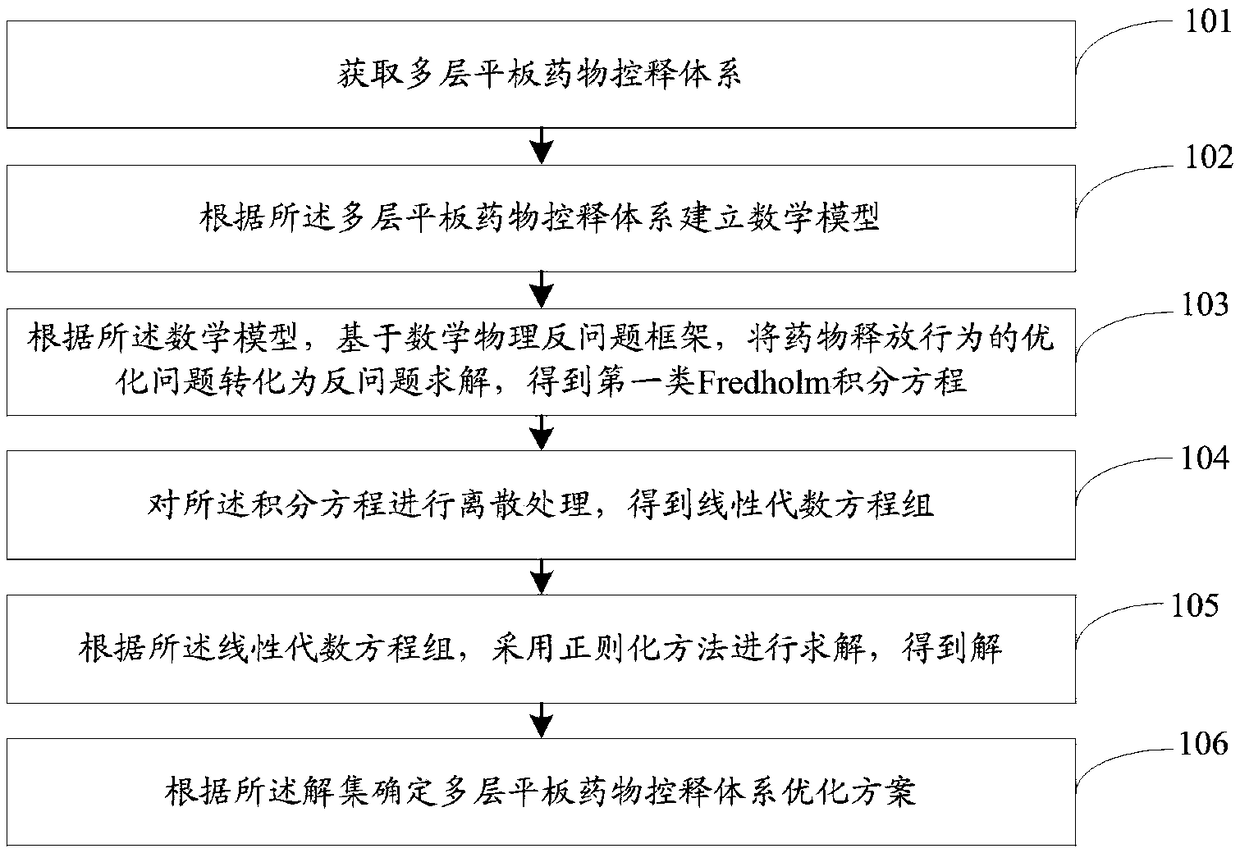
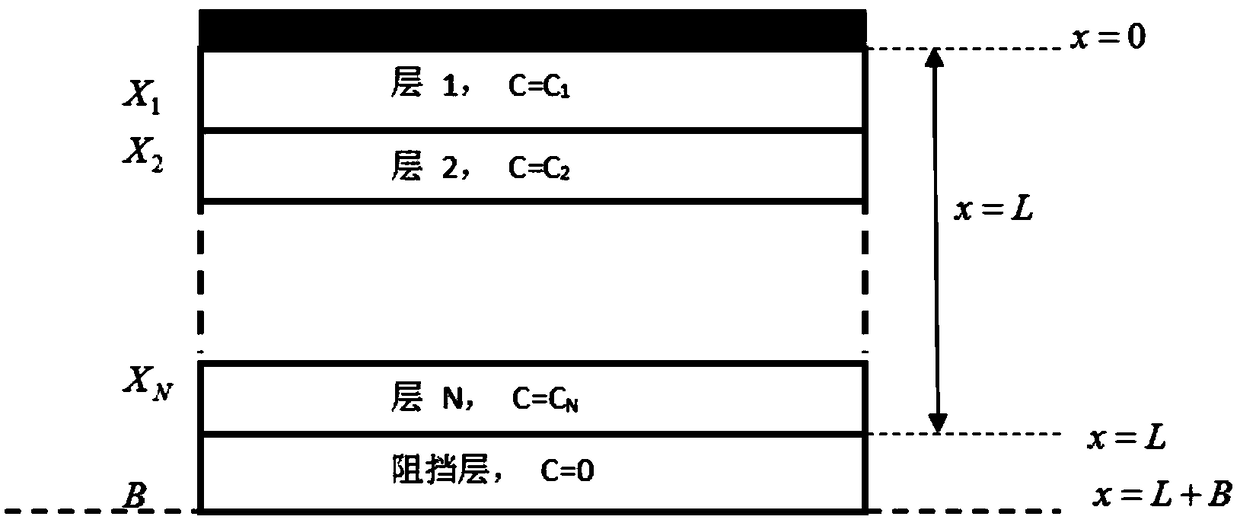
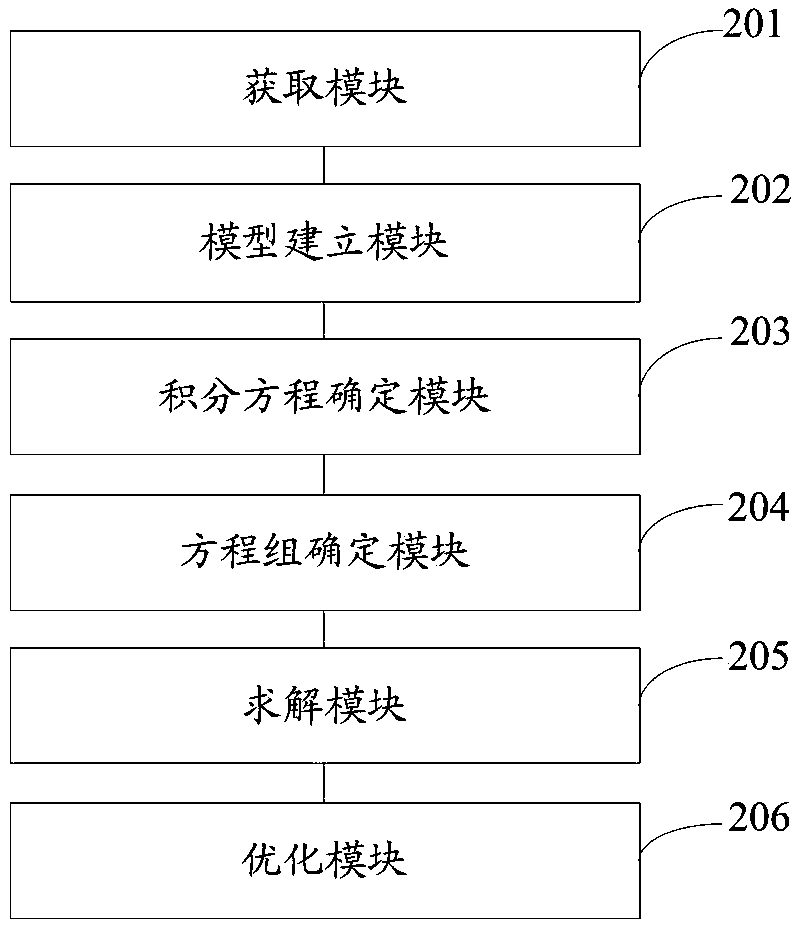
Multilayer tablet drug controlled release system optimization method and system
ActiveCN109473146AAccurate predictionChemical property predictionMolecular designControlled releaseMedicine
The invention discloses a multilayer tablet drug controlled release system optimization method and system. The method comprises the following steps: acquiring a multilayer tablet drug controlled release system; establishing a mathematical model according to the multilayer tablet drug controlled release system; transforming an optimization problem of a drug release behavior into inverse problem solving on the basis of a mathematical physics inverse problem framework according to the mathematical model so as to obtain a first type of Fredholm integral equations; performing discrete processing onthe integral equations so as to obtain a system of linear algebraic equations; solving by a regularization method to obtain a solution according to the system of linear algebraic equations; determining the multilayer tablet drug controlled release system optimization scheme according to the solution. According to the method or system disclosed by the invention, parameters can be accurately predicted.
Owner:HARBIN INST OF TECH SHENZHEN GRADUATE SCHOOL
Modified release multi-layer tablet cannabinoid formulations
ActiveUS10772837B2Nervous disorderHydroxy compound active ingredientsControlled drugsPharmaceutical drug
The present invention provides modified release pharmaceutical compositions, and methods for administering the compositions to a user, including humans. The composition may contain a combination of ingredients in proportions calculated to achieve therapeutic effect, including at least the following ingredients: one or more natural or synthetic cannabinoids, one or more release modifying agent(s), and one or more pharmaceutically acceptable excipient(s). The composition may be in a multi-layered solid dosage form to provide fast, controlled and also sustained release of specific ingredients. More specifically, the invention relates to modified release pharmaceutical compositions comprising cannabinoids and a process for preparation thereof. More specifically, the invention may control drug release in accordance with the therapeutic purpose and pharmacological properties of active substances.
Owner:CANNTAB THERAPEUTICS LTD
Process of manufacture of novel drug delivery system: multilayer tablet composition of thiazolidinedione and biguanides
InactiveUS8911781B2Facilitated releaseEfficient compressionOrganic active ingredientsBiocideAcute hyperglycaemiaImmediate release
A novel patient-convenient, cost effective pharmaceutical composition, comprising of thiazolidinediones and biguanide for controlling hyperglycemia manufactured as multilayer tablet and its process of manufacturing, for immediate release of thiazolidinediones or thiazolidinediones and biguanide and prolonged release of the biguanide only, the tablet comprising of minimum two layers wherein one outer layer comprises of a mixture of excipients and thiazolidinediones or thiazolidinediones and biguanide allowing immediate release of thiazolidinediones or thiazolidinediones and biguanide respectively and the other layer arranged in contact with the immediate release layer which comprises of a novel composition of excipients and a minimum one or more non-biodegradable, inert polymer(s) and the biguanide allowing pH independent prolonged release of the biguanide up to a period of 8-12 hours. The tablets are for once a day dosing. The tablets may optionally be film coated or enrobed by soft gelatin ribbons for additional protection against oxidation, photodegradation, identification, ease of swallowing, taste masking and for aesthetic appeal without altering the dissolution profile.
Owner:INVENTIA HEALTHCARE LTD
Fragrant monopersulfate compositions for water treatment and articles containing them
InactiveUS20070032397A1Good fragrance qualityQuality improvementSpecific water treatment objectivesNon-surface-active detergent compositionsSimple Organic CompoundsPersulfate
The invention relates to fragrant compositions for oxidizing organic compounds in pool or spa water, having an alkali metal monopersulfate, a fragrance, and an optional carrier, which do not have a multilayer tablet structure and which do not generate gas, fumes or significant heat on addition to water.
Owner:ZODIAC POOL CARE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com