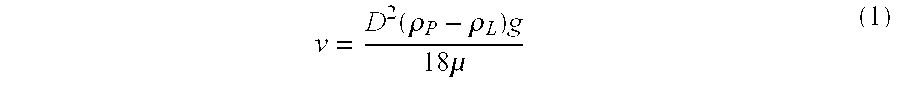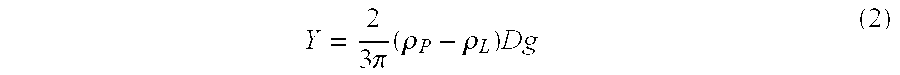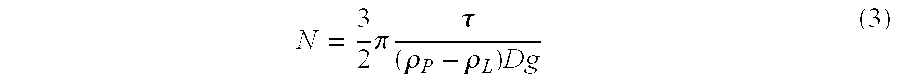Patents
Literature
580 results about "Intrathecal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
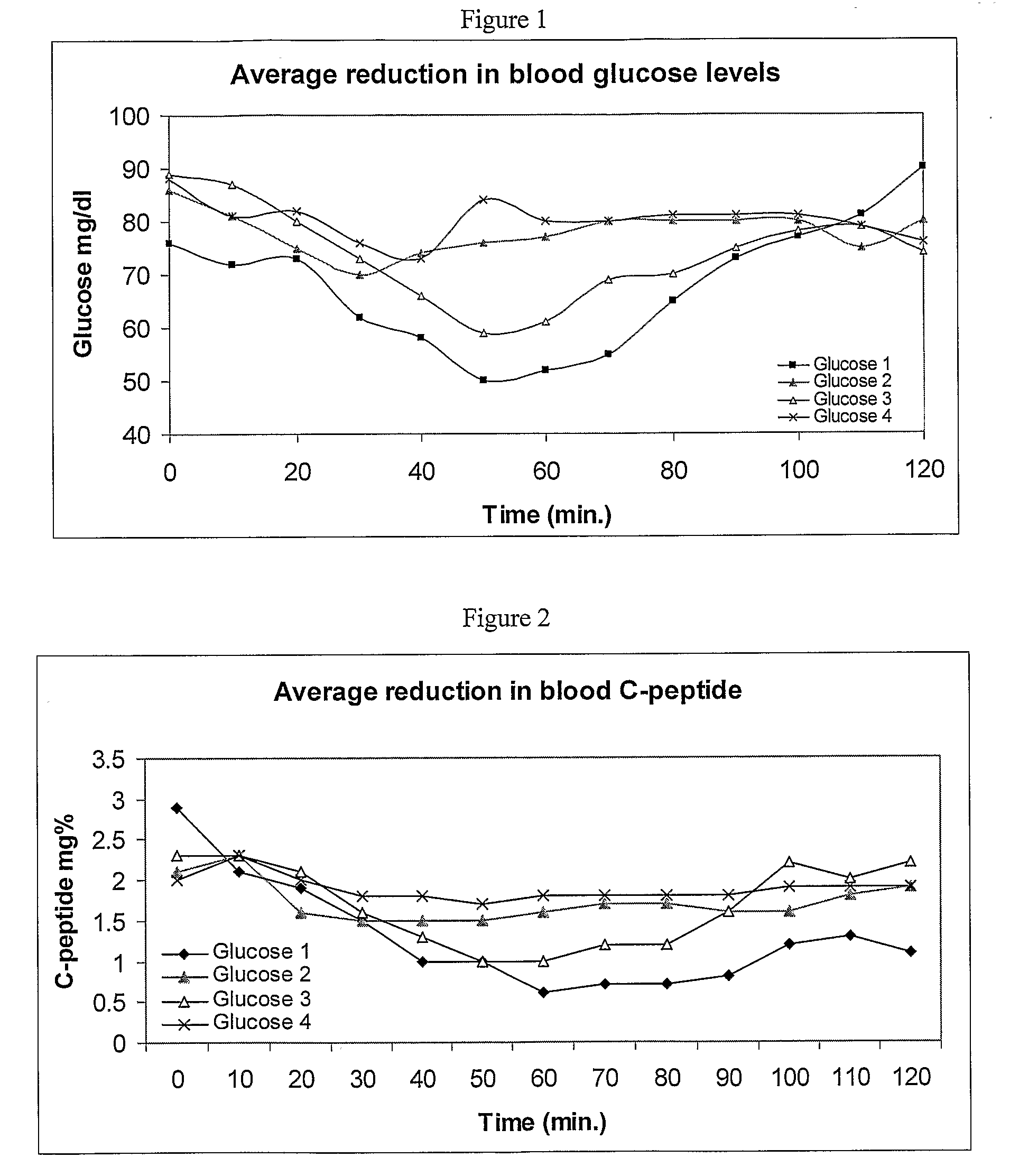
Intrathecal administration is a route of administration for drugs via an injection into the spinal canal, or into the subarachnoid space so that it reaches the cerebrospinal fluid (CSF) and is useful in spinal anesthesia, chemotherapy, or pain management applications. This route is also used to introduce drugs that fight certain infections, particularly post-neurosurgical. The drug needs to be given this way to avoid being stopped by the blood brain barrier. The same drug given orally must enter the blood stream and may not be able to pass out and into the brain. Drugs given by the intrathecal route often have to be compounded specially by a pharmacist or technician because they cannot contain any preservative or other potentially harmful inactive ingredients that are sometimes found in standard injectable drug preparations.
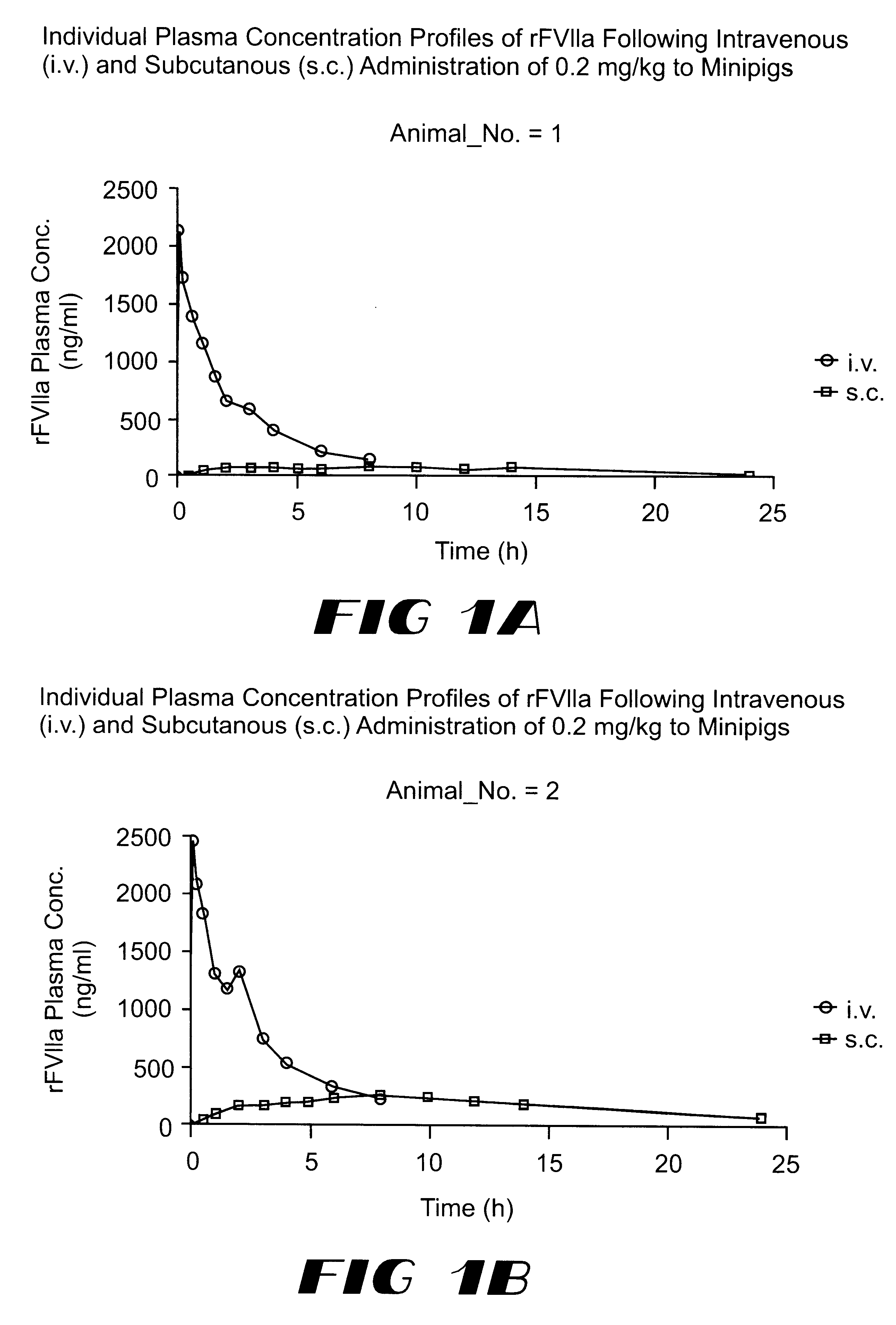
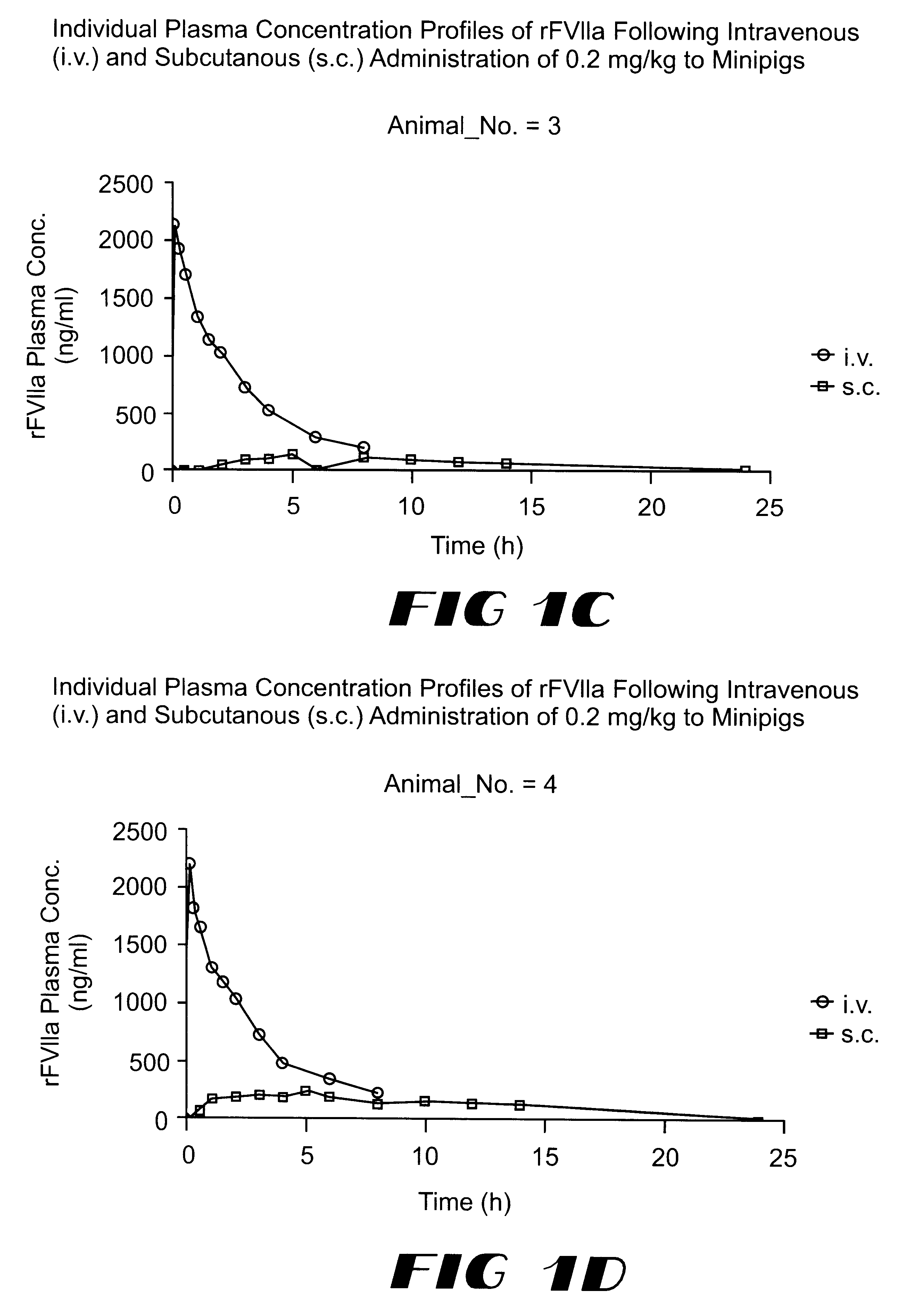
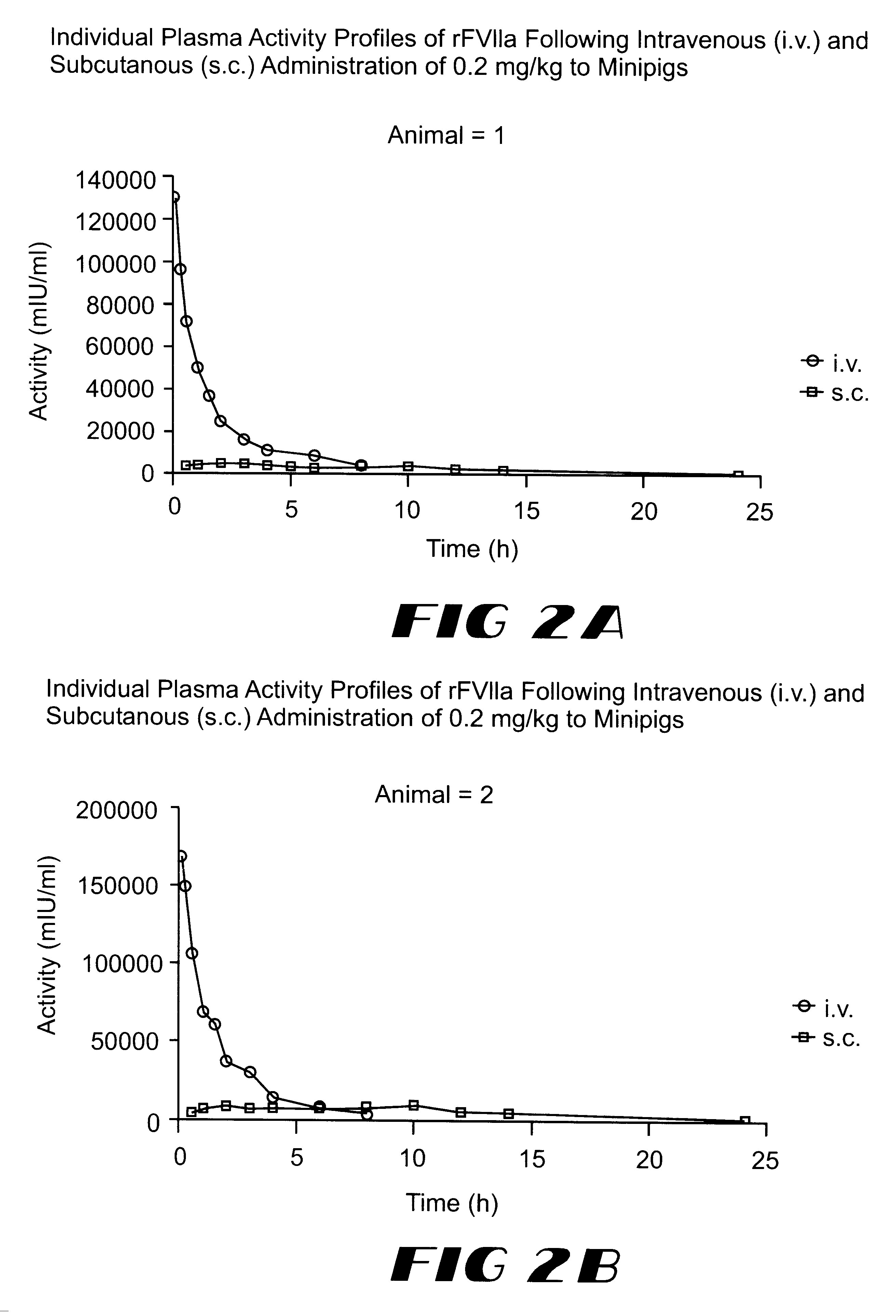
Coagulation factor VIIa composition
The invention relates to a pharmaceutical composition comprising Factor VIIa for subcutan, intramuscular or interdermal administration.Factor VIIa administered subcutanously, intramuscularly or intradermally shows a sufficient transport into the bloodstream in biologically active form and in adequate concentrations, and favorable pharmacokinetic properties.
Owner:NOVO NORDISK AS
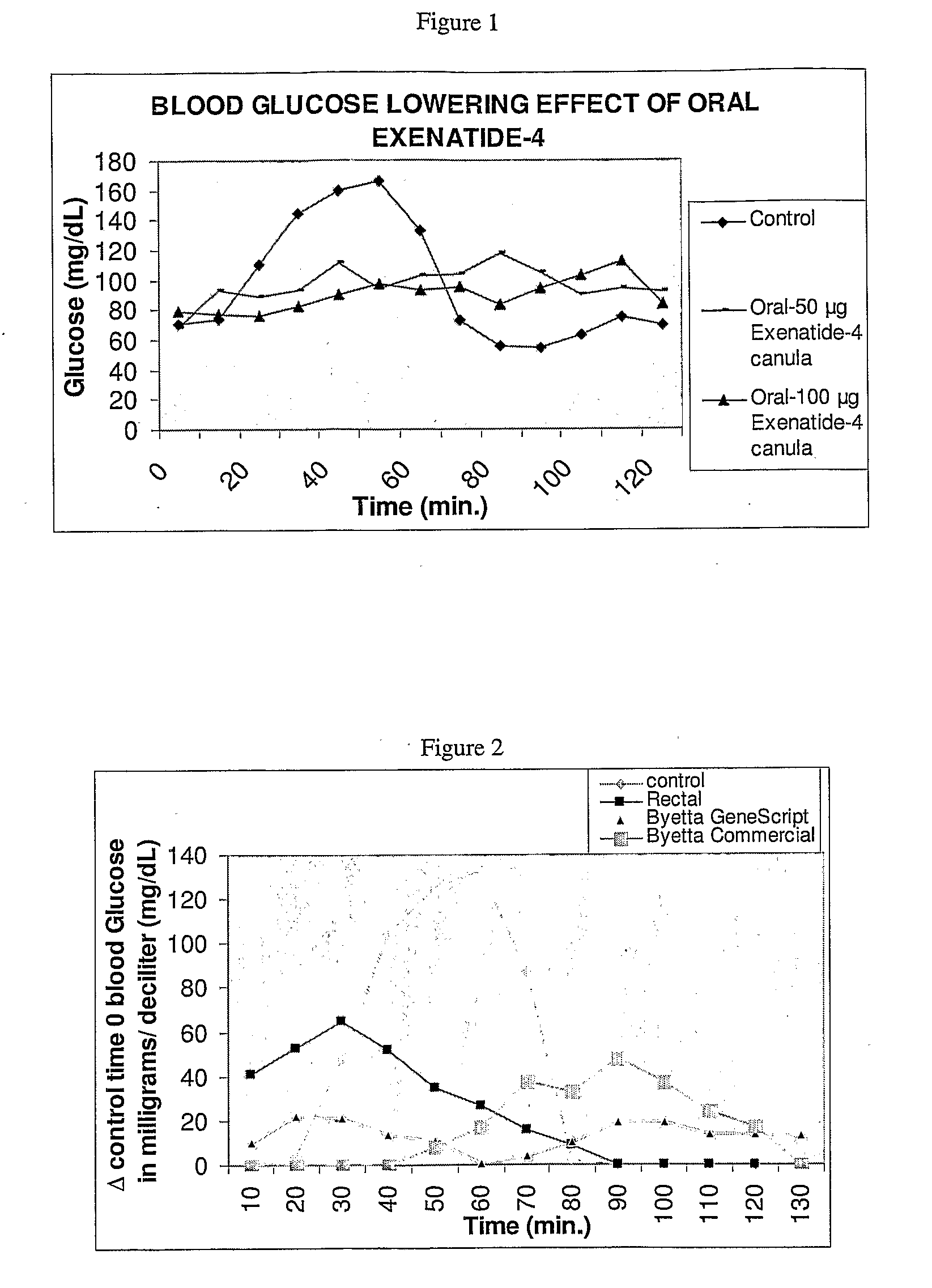
Methods and compositions for oral administration of exenatide
InactiveUS20110046053A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a byetta, fish oil, and a protease inhibitor, method for treating diabetes mellitus, comprising administering same, and methods for oral or rectal administration of a byetta.
Owner:ORAMED
Rapid acting drug delivery compositions
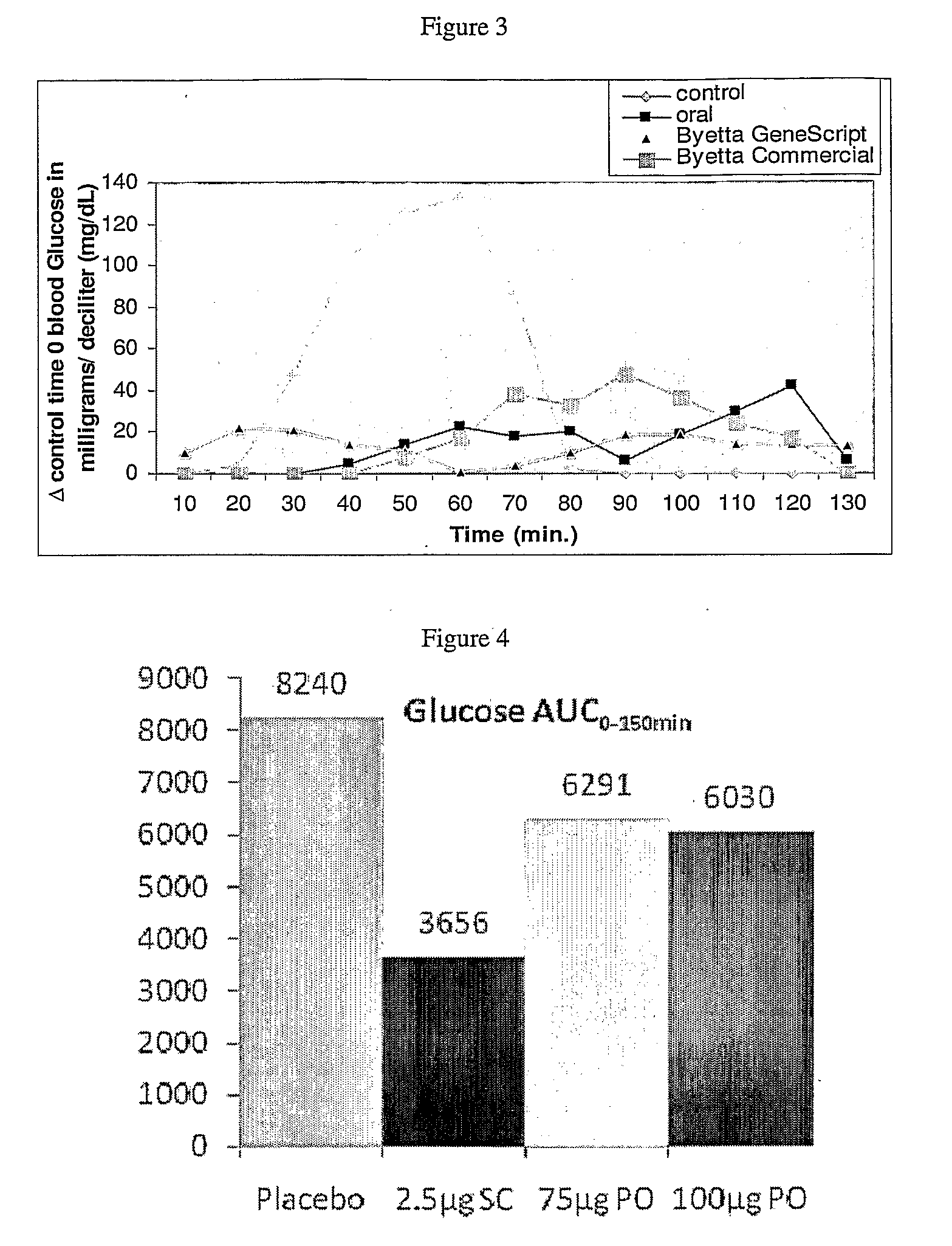
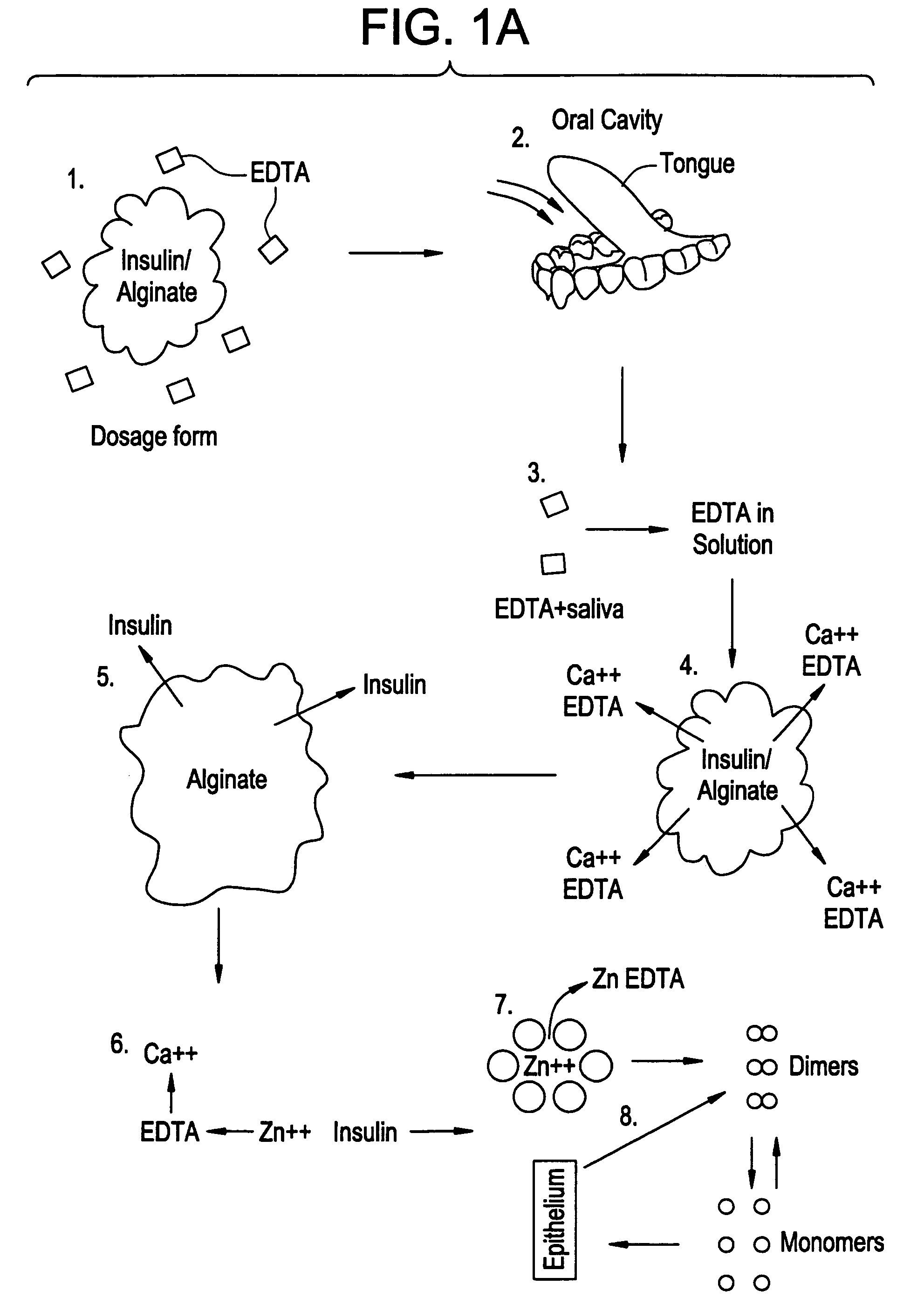
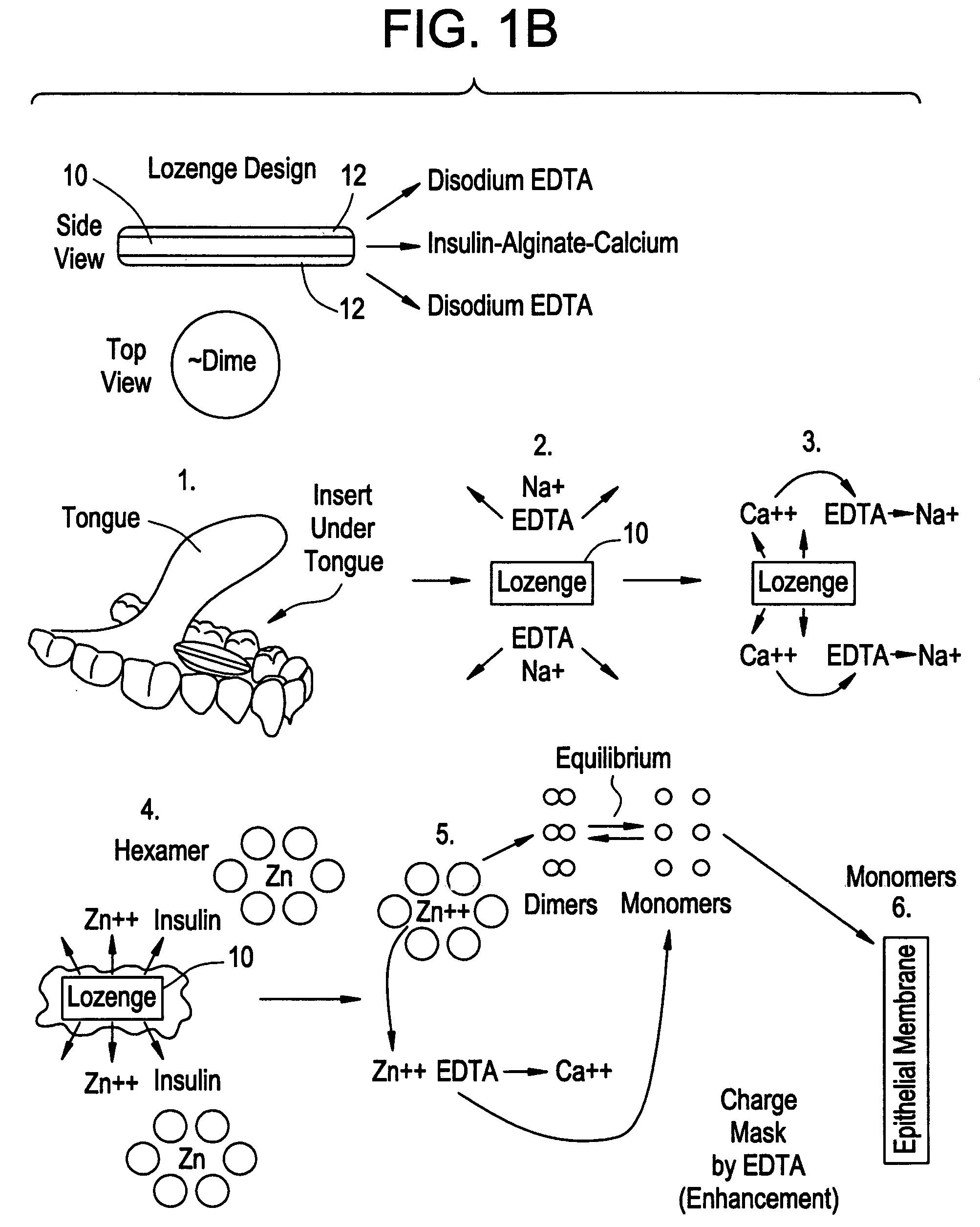
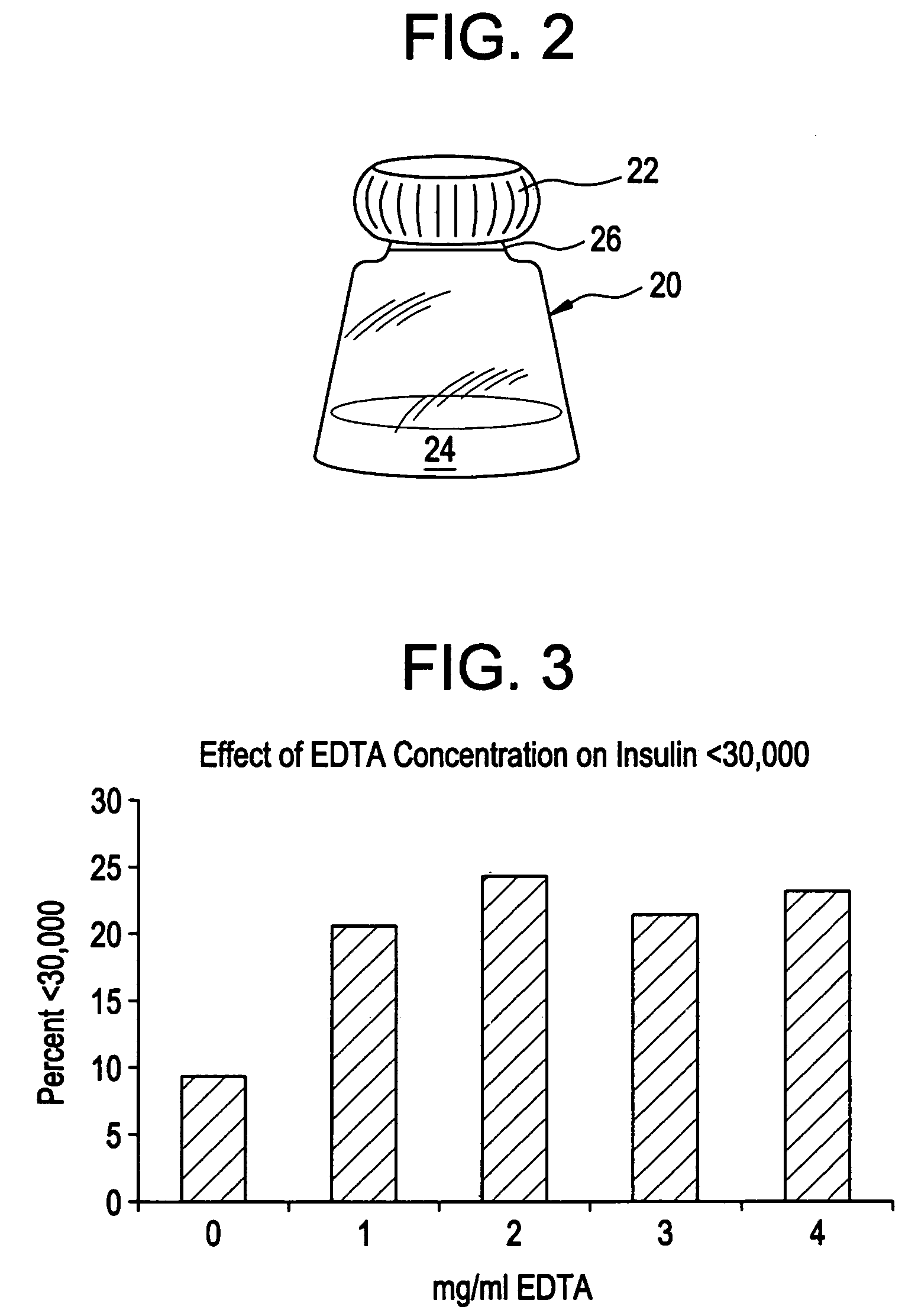
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
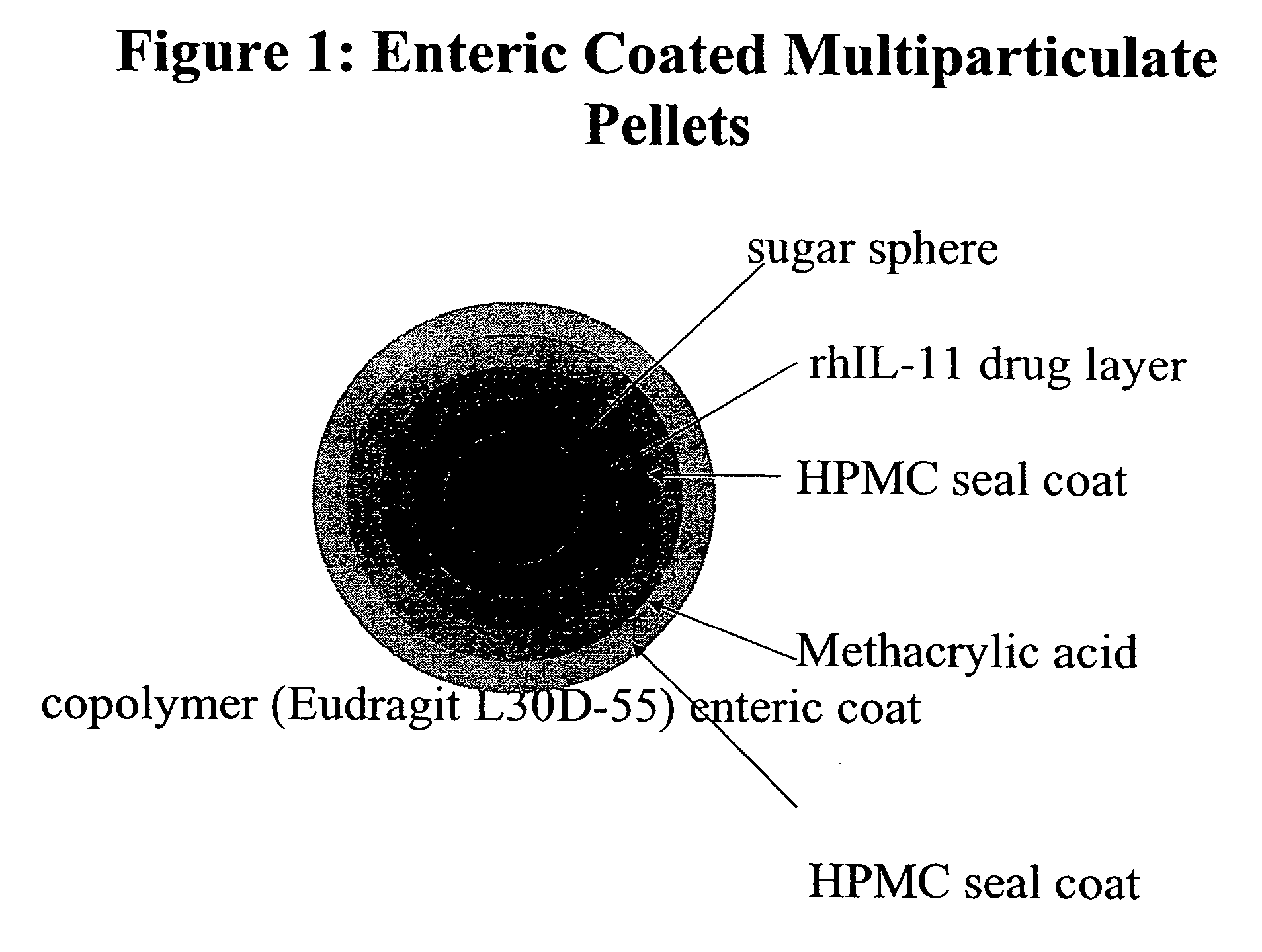
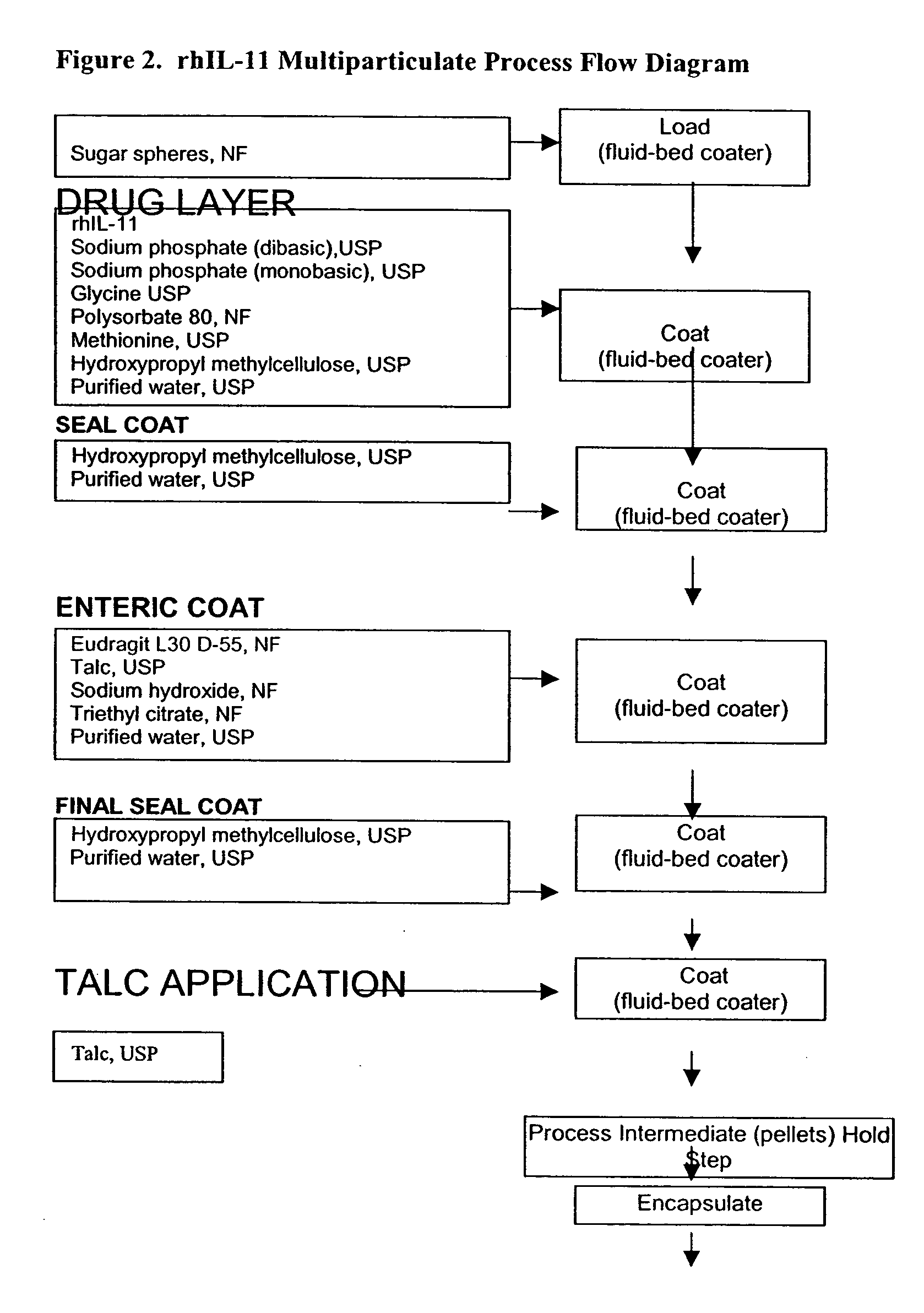
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
InactiveUS20040126358A1Increase ionic strengthReduced strengthAntipyreticAnalgesicsOral medicationWhite blood cell
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WYETH LLC
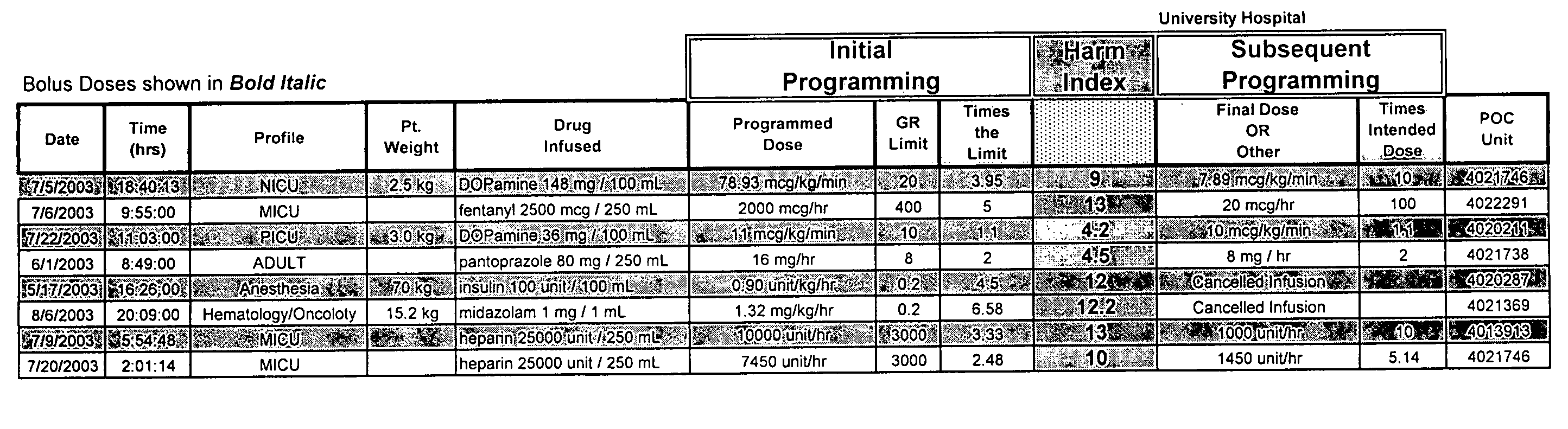
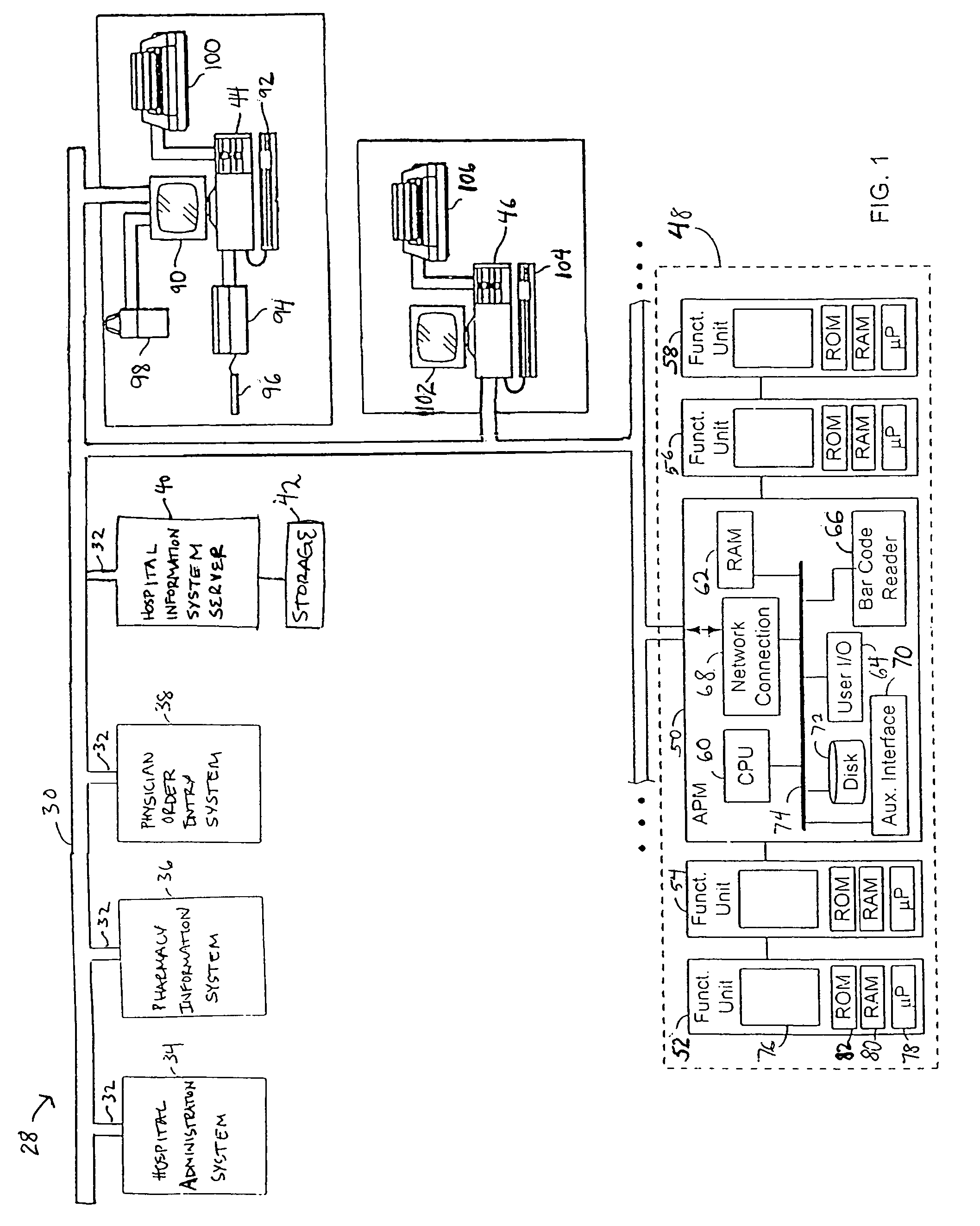
Intravenous medication harm index system
ActiveUS7657443B2Effective monitoringEffective trackingData processing applicationsHealth-index calculationVeinMedicine
Owner:CAREFUSION 303 INC
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
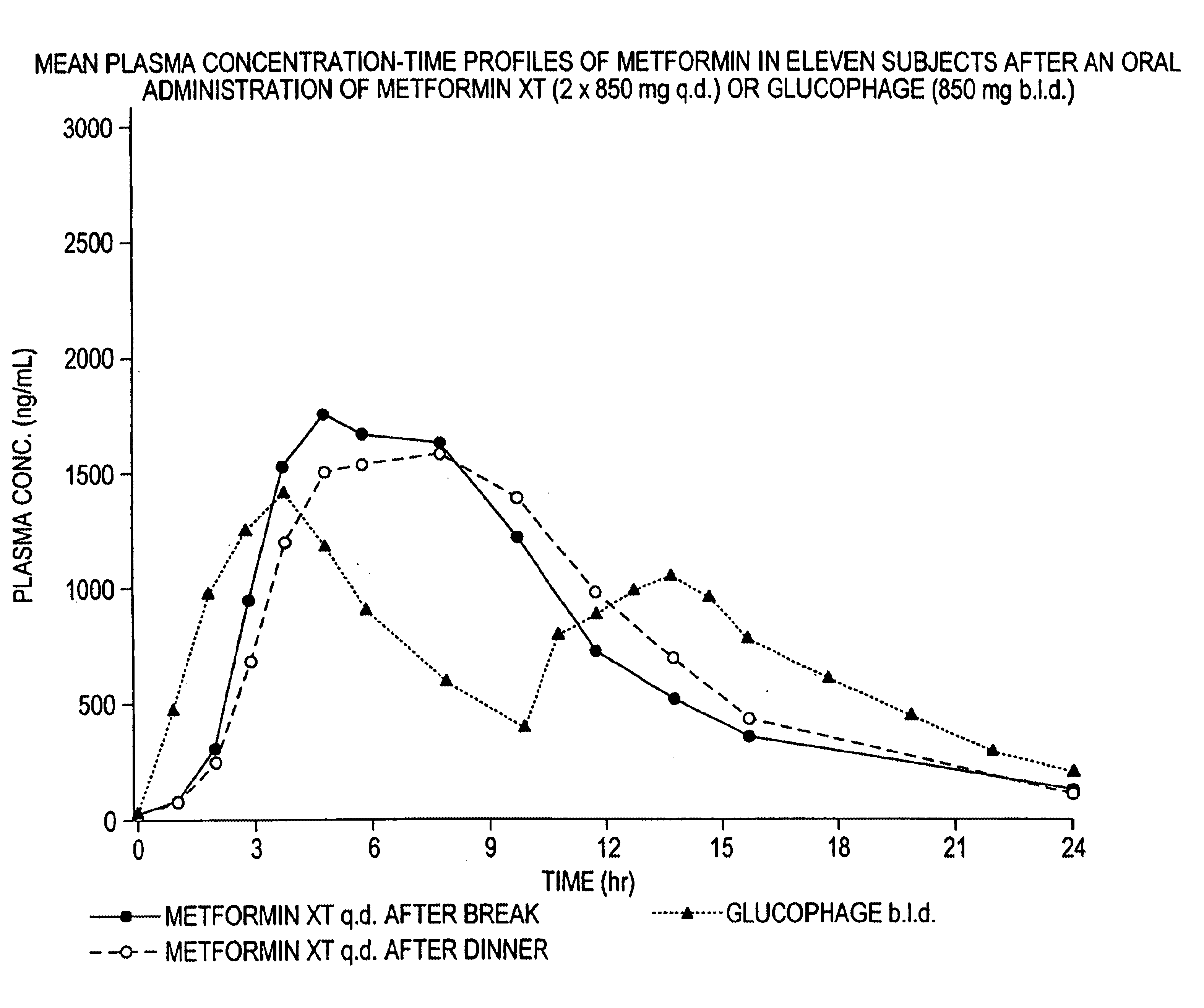
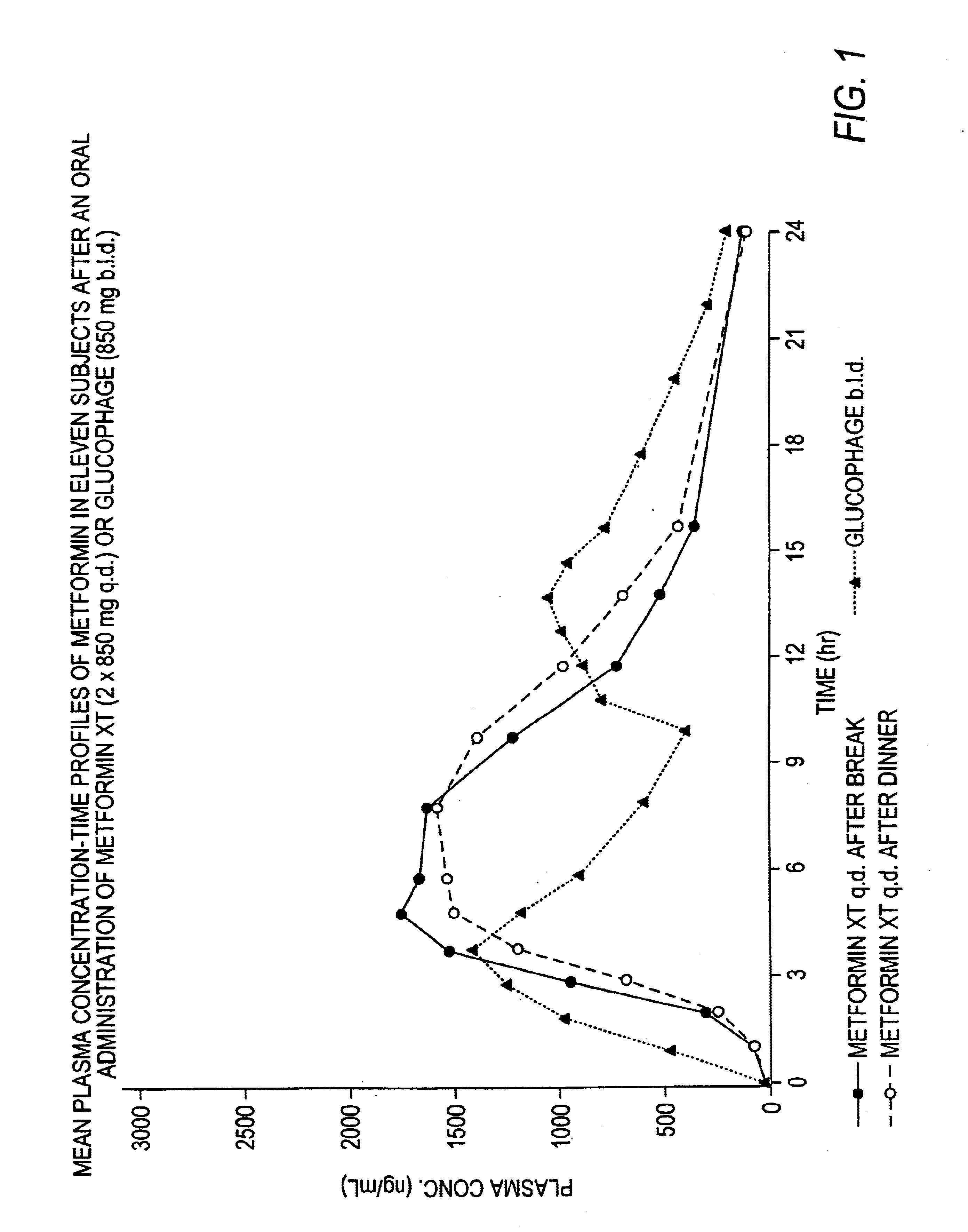
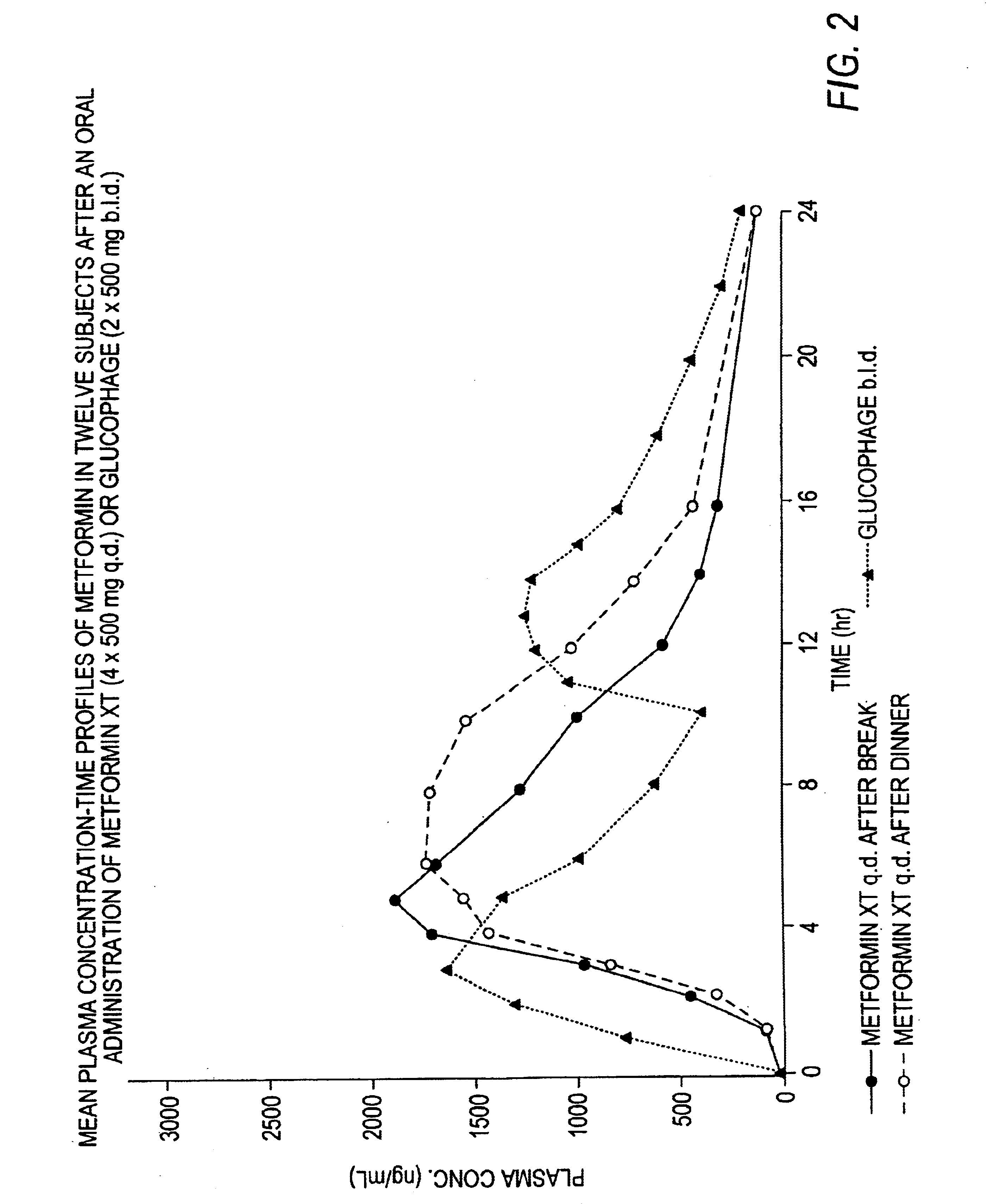
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
Oral administration form for an acid liable active proton pump inhibitor
Novel administration form for acid-labile active compounds are described. The novel administration forms have no enteric layers and are suitable for oral administration.
Owner:TAKEDA GMBH
Controlled Release Pharmaceutical Compositions Comprising a Fumaric Acid Ester
InactiveUS20080299196A1Decrease glass transition pointLow film forming temperatureBiocideSenses disorderDiseaseHigh concentration
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designed to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
Composition and method for inhibiting platelet aggregation
The present invention provides novel compounds of dinucleotide polyphosphates and the method of preventing or treating diseases or conditions associated with platelet aggregation. The method comprises administering systemically to a patient a pharmaceutical comprising a purinergic P2τ receptor antagonist, in an amount effective to elevate its extracellular concentration to bind to P2τ receptors and inhibit P2τ receptor-mediated platelet aggregation. Methods of systemic administration include injection by intravenous, intramuscular, intrasternal and intravitreal routes, infusion, transdermal administration, oral administration, rectal administration and intra-operative instillation.
Owner:INSPIRE PHARMA +1
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances
ActiveUS20100008985A1Disperse fastEfficient packagingBiocidePowder deliveryWater insolubleProphylactic treatment
One aspect of the invention relates to a pharmaceutical dosage unit for sublingual, buccal, pulmonary or oral administration, said dosage unit having a weight of 20-500 mg and comprising 1-80 Wt. % of a microgranulate that is distributed throughout a solid hydrophilic matrix; said microgranulate being characterised in that it: has a volume weighted average diameter of 5-100 m; contains at least 0.01 wt. %, preferably at least 0.1 wt. % of one or more water-insoluble pharmaceutically active substances; contains at least 10 wt. %, preferably at least 20 wt. % of an emulsifier component; and is capable of forming a micro-emulsion upon contact with saliva or water. The dosage units of the present invention achieve the inherent benefits of oral delivery whilst at the same time realising a high transmucosal absorption rate of the cannabinoids contained therein. Other aspects of the present invention relate to the use of the aforementioned dosage units in the therapeutic or prophylactic treatment and to a process for the manufacture of said dosage units.
Owner:ECHO PHARM BV (NL)
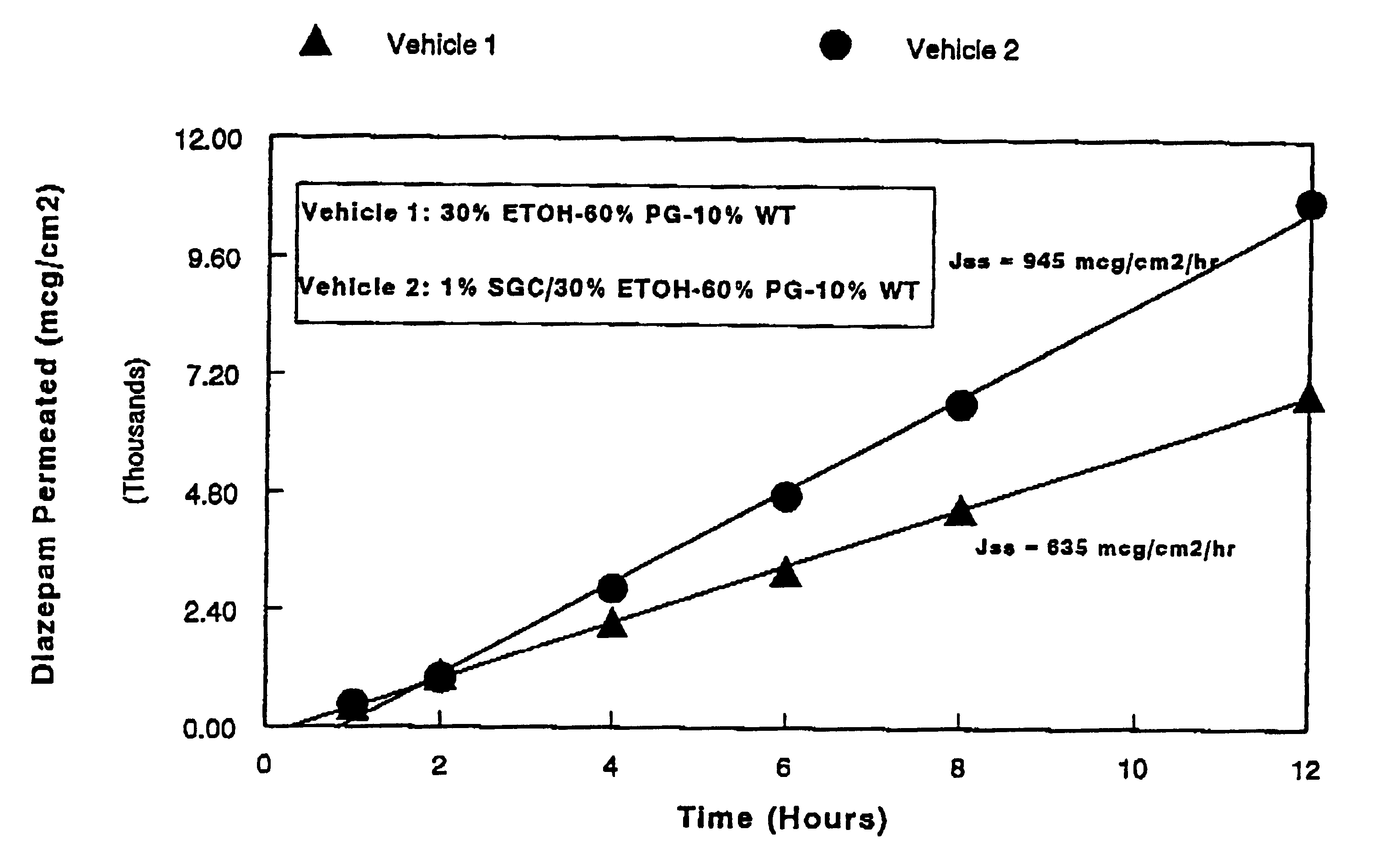
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
Transdiscal administration of high affinity anti-MMP inhibitors
ActiveUS7429378B2Prevent degradationReduced effectivenessPeptide/protein ingredientsSkeletal disorderMedicineIntervertebral disc
The present invention relates to injecting a high affinity antagonist of MMPs into a diseased intervertebral disc.
Owner:DEPUY SPINE INC (US)
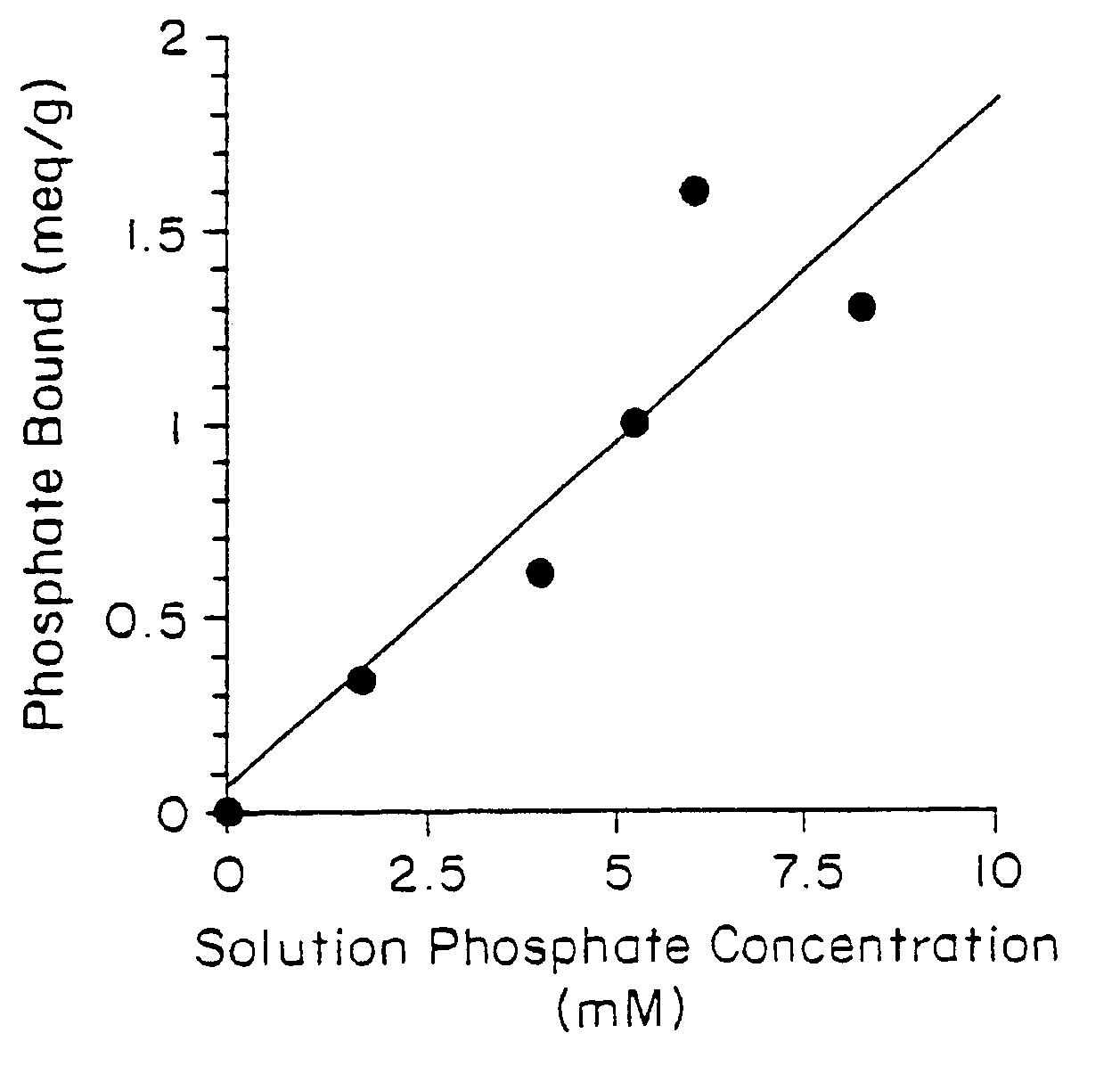
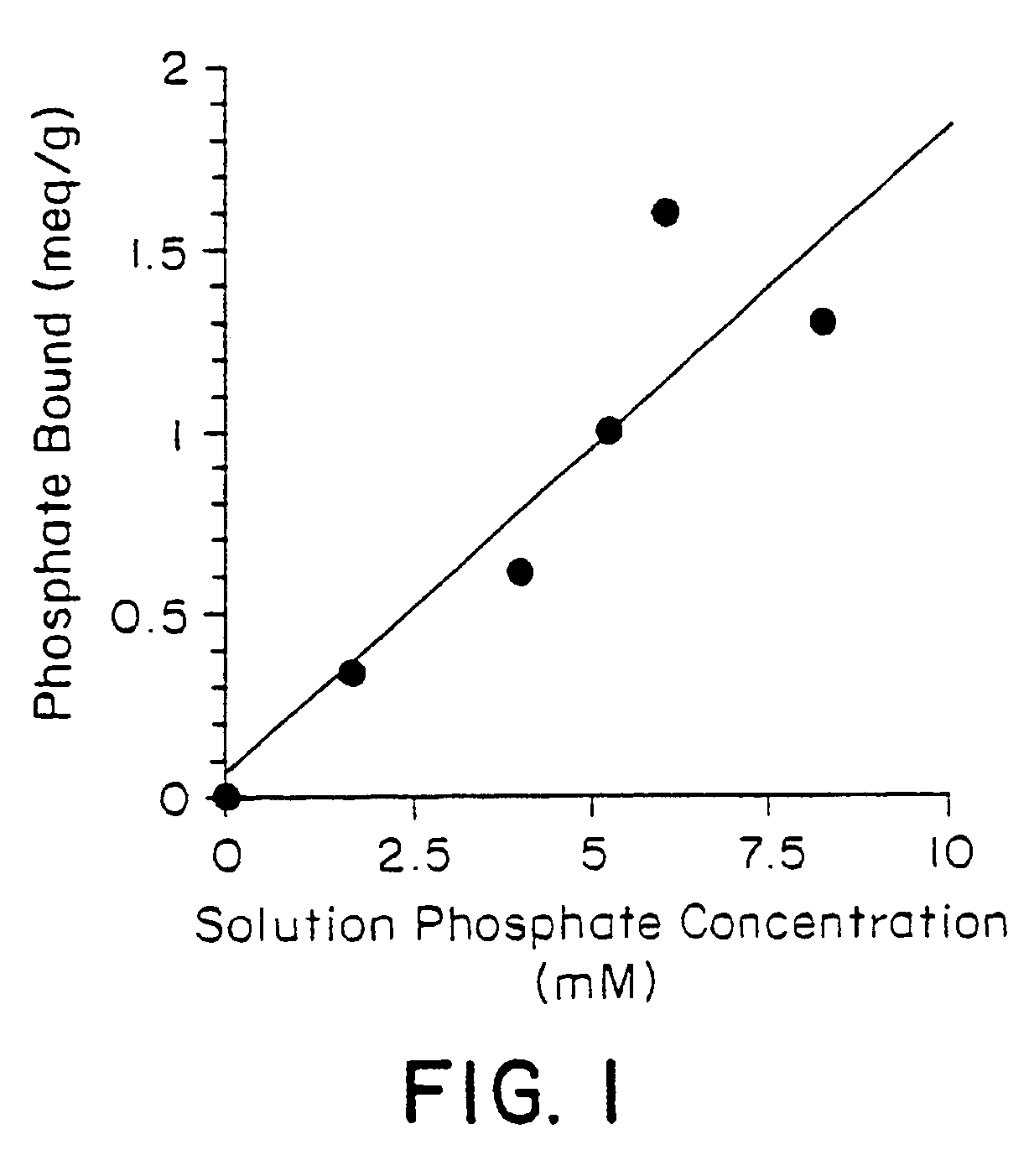
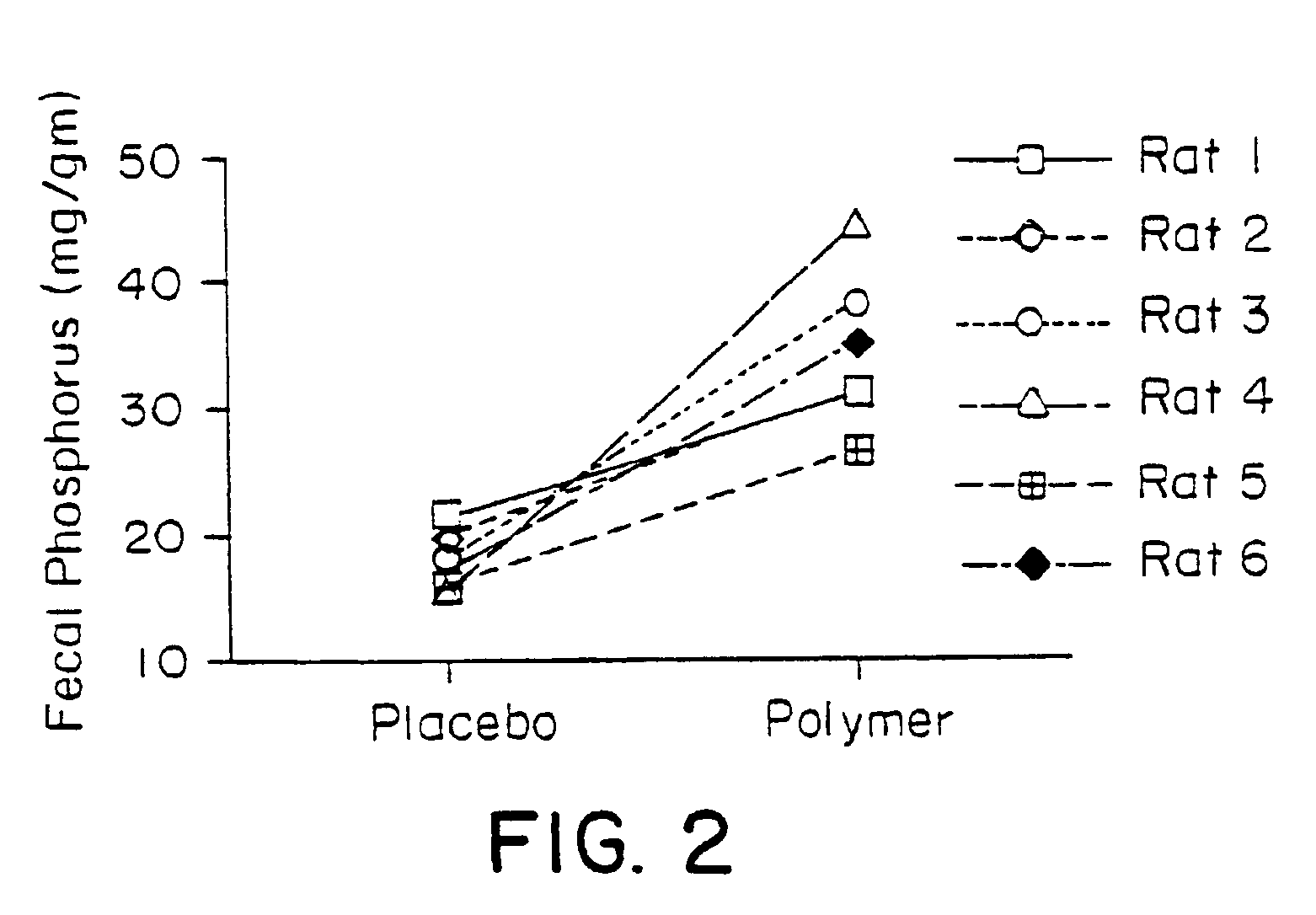
Phosphate-binding polymers for oral administration
InactiveUS7014846B2Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationBuccal administration
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
Transdiscal administration of cycline compounds
Owner:DEPUY SPINE INC (US)
Formulation for oral administration of apoptosis promoter
InactiveUS20100297194A1Improve oral bioavailabilityConvenient route of administrationOrganic active ingredientsBiocideDiseaseAntioxidant
An orally deliverable pharmaceutical composition comprises as a sole or first active ingredient a compound of Formula I defined herein or a pharmaceutically acceptable salt thereof, for example ABT-263 free base or ABT-263 bis-HCl salt, dispersed, in a free base equivalent amount of at least about 2.5% by weight of the composition, in a pharmaceutically acceptable carrier; wherein said active ingredient is in solid-state form and / or the composition further comprises, dispersed in the carrier, a pharmaceutically acceptable heavier-chalcogen antioxidant in an amount effective to inhibit oxidation of the active ingredient at a thioether linkage thereof. The composition is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBOTT LAB INC +1
Transdiscal administration of cycline compounds
ActiveUS20050038001A1Stop agingPrevent degradationBiocideNervous disorderIntervertebral discMedicine
Owner:DEPUY SPINE INC (US)
Coated filter bag material for oral administration of medicament in liquid and methods of making same
InactiveUS7090858B2Extended shelf lifeOrganic active ingredientsPowder deliveryOral medicationIntrathecal
Owner:JAYARAMAN SWAMINATHAN
Pharmaceutical Formulation Containing Opioid Agonist, Opioid Antagonist and Gelling Agent
InactiveUS20150031718A1Avoid abuseReduce releaseBiocidePharmaceutical non-active ingredientsOpioid antagonistPharmaceutical formulation
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Methods and compositions for oral administration of proteins
ActiveUS20110142800A1Promote absorptionPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a protein, an absorption enhancer, a protease inhibitor, methods for treating diabetes mellitus, comprising administering same, and methods for oral administration of a protein with an enzymatic activity, comprising orally administering same.
Owner:ENTERA BIO LTD
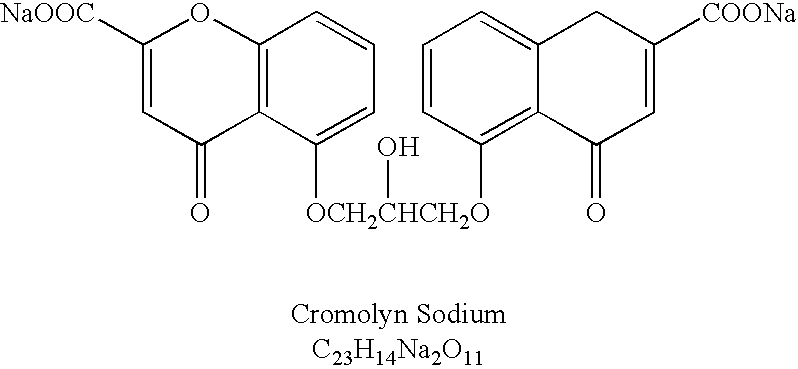
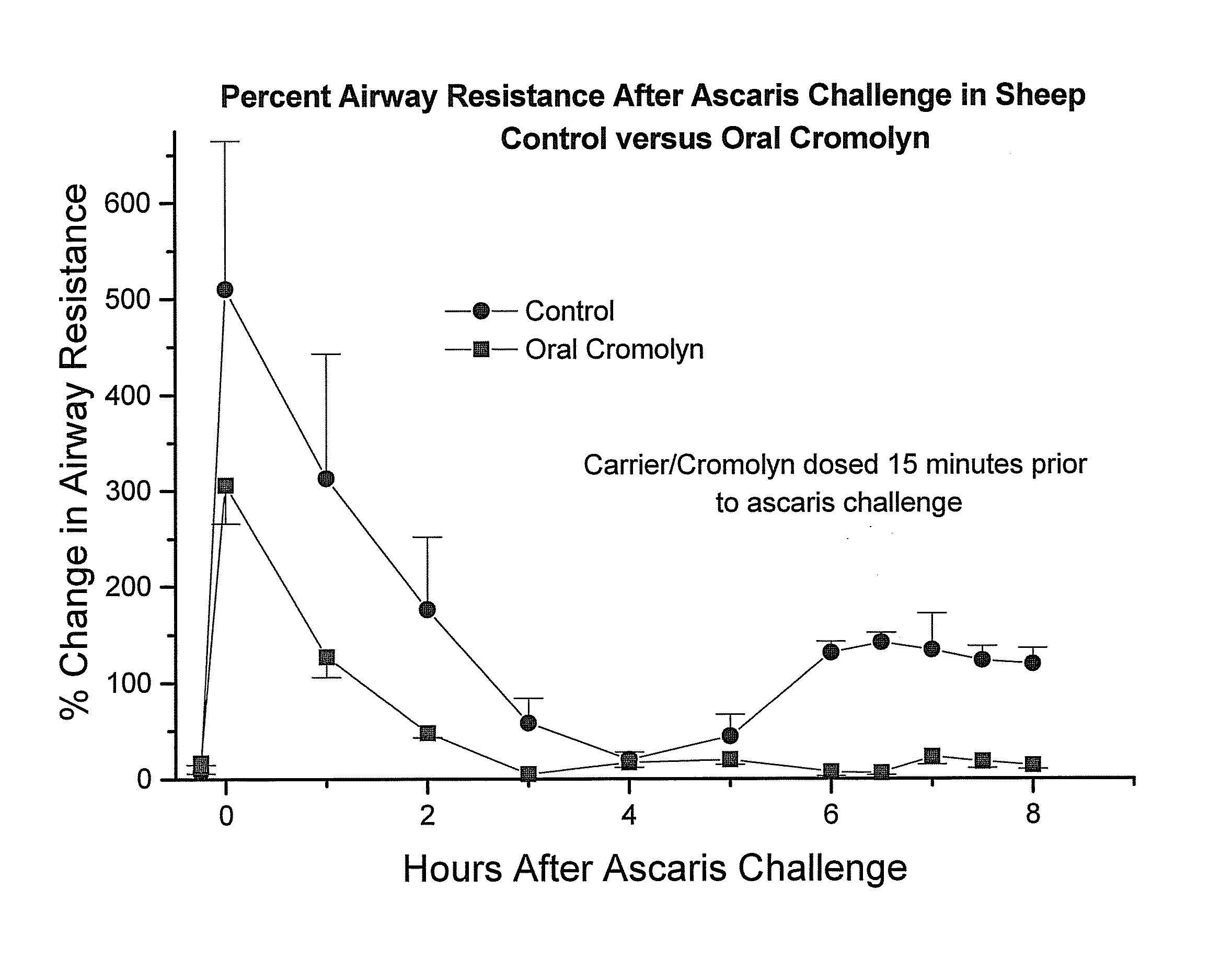
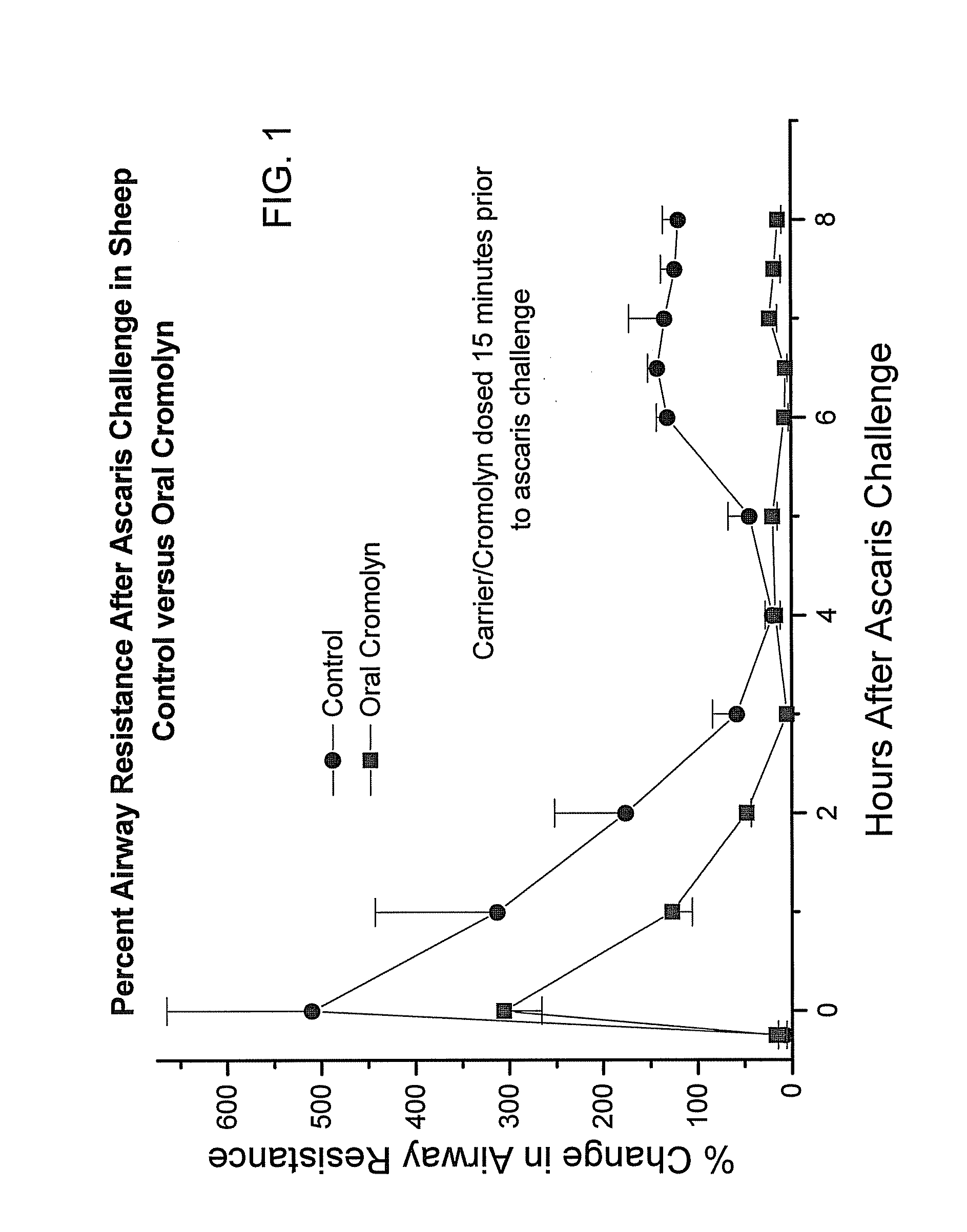
Formulations for oral administration of cromolyn sodium
InactiveUS20040259952A1High activityImprove absorption rateBiocideAntipyreticOral medicationCromolyn Sodium
An oral dosage form comprises cromolyn sodium (sodium or disodium cromoglycate), and an acylated amino acid delivery agent.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Oral suspension formulation
An aqueous pharmaceutical suspension for oral administration of a drug, which suspension maintains its content uniformity for prolonged period.
Owner:UNILAB PHARMATECH
Transdiscal administration of specific inhibitors of pro-inflammatory cytokines
ActiveUS7344716B2Prevent degradationFree from painPeptide/protein ingredientsHydrolasesCytokine SuppressionIntervertebral disc
The present invention relates to injecting a high specificity cytokine antagonist into a diseased intervertebral disc.
Owner:DEPUY SYNTHES PROD INC
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS20110318323A1Effective and less approachEffectively and extensivelyNervous disorderHydrolasesIduronate-2-sulfataseHunter syndrome
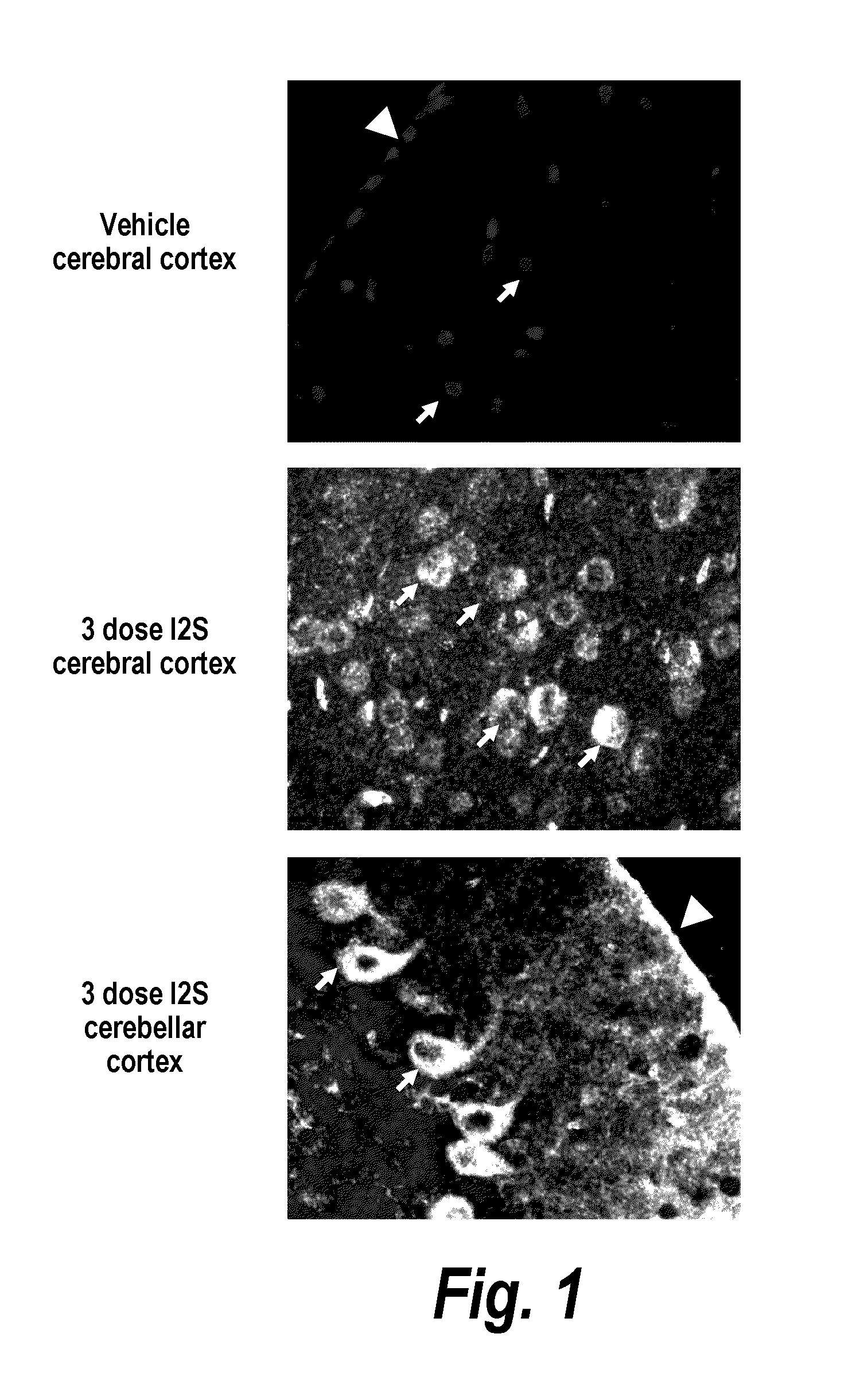
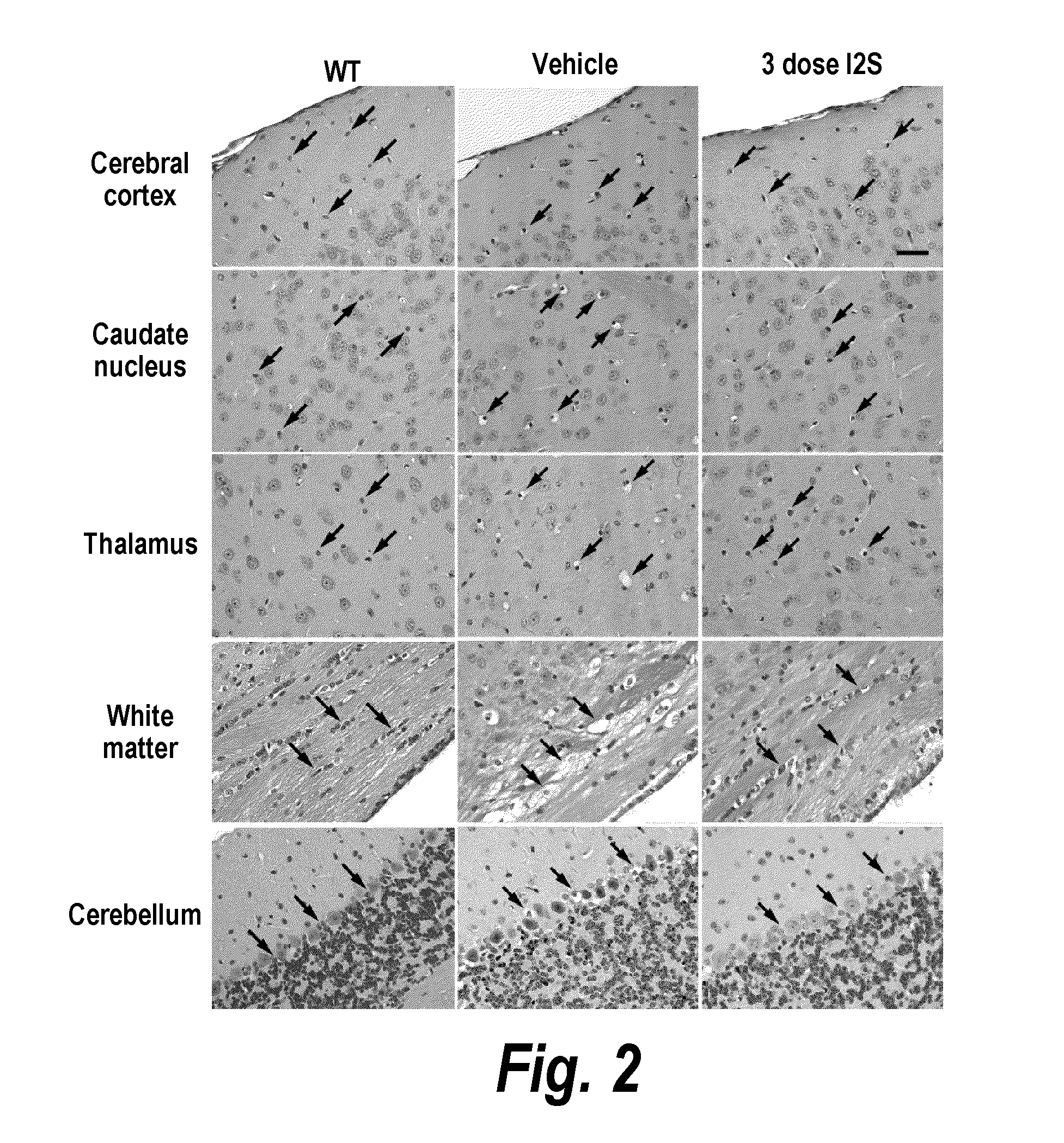
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
Formulations for oral administration of cromolyn sodium
InactiveUS20080194676A1Prevent and reduce incidencePrevent and reduce and severityBiocideAntipyreticOral medicationCromolyn Sodium
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Dosage forms for oral administration and methods of treatment using the same
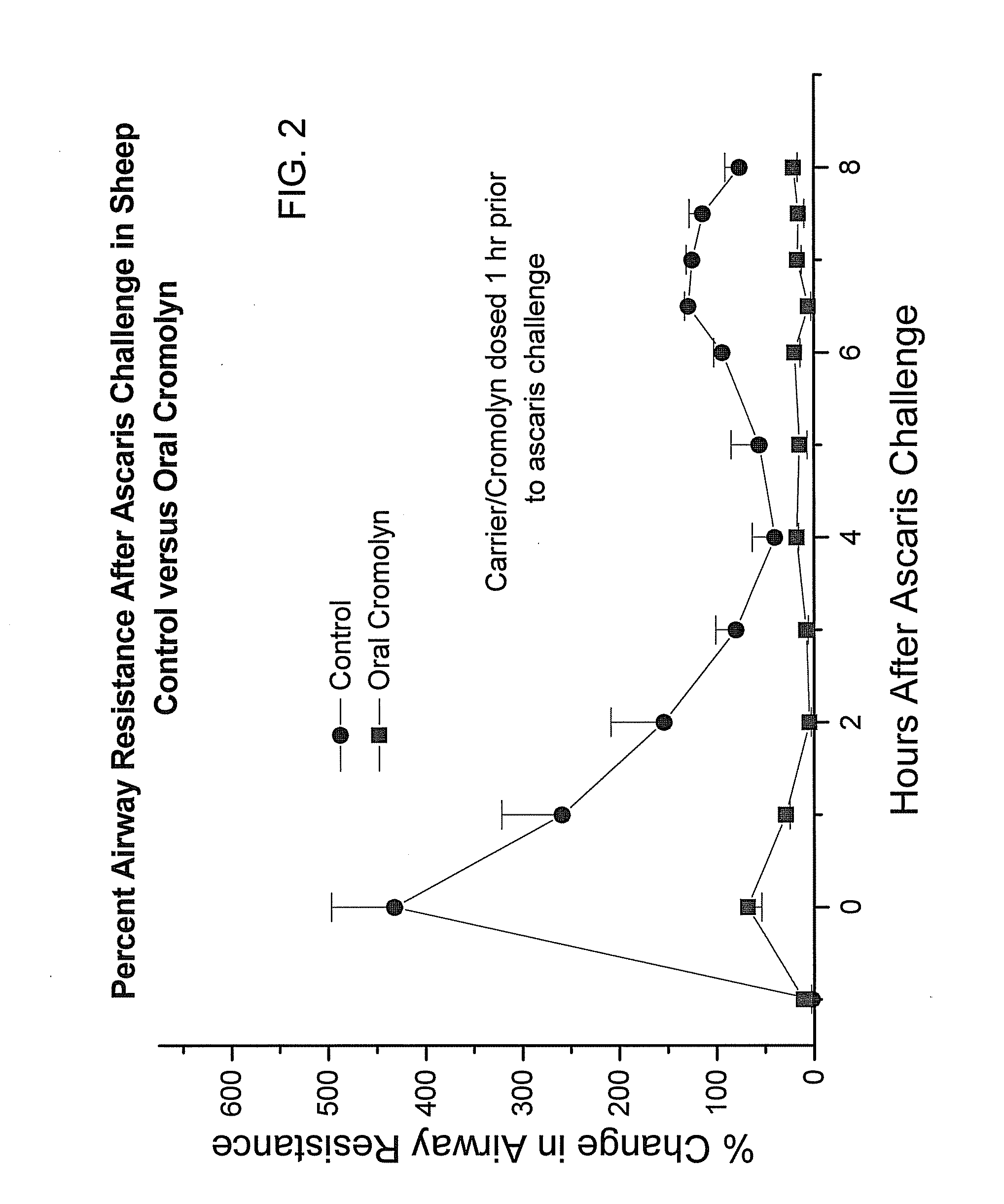
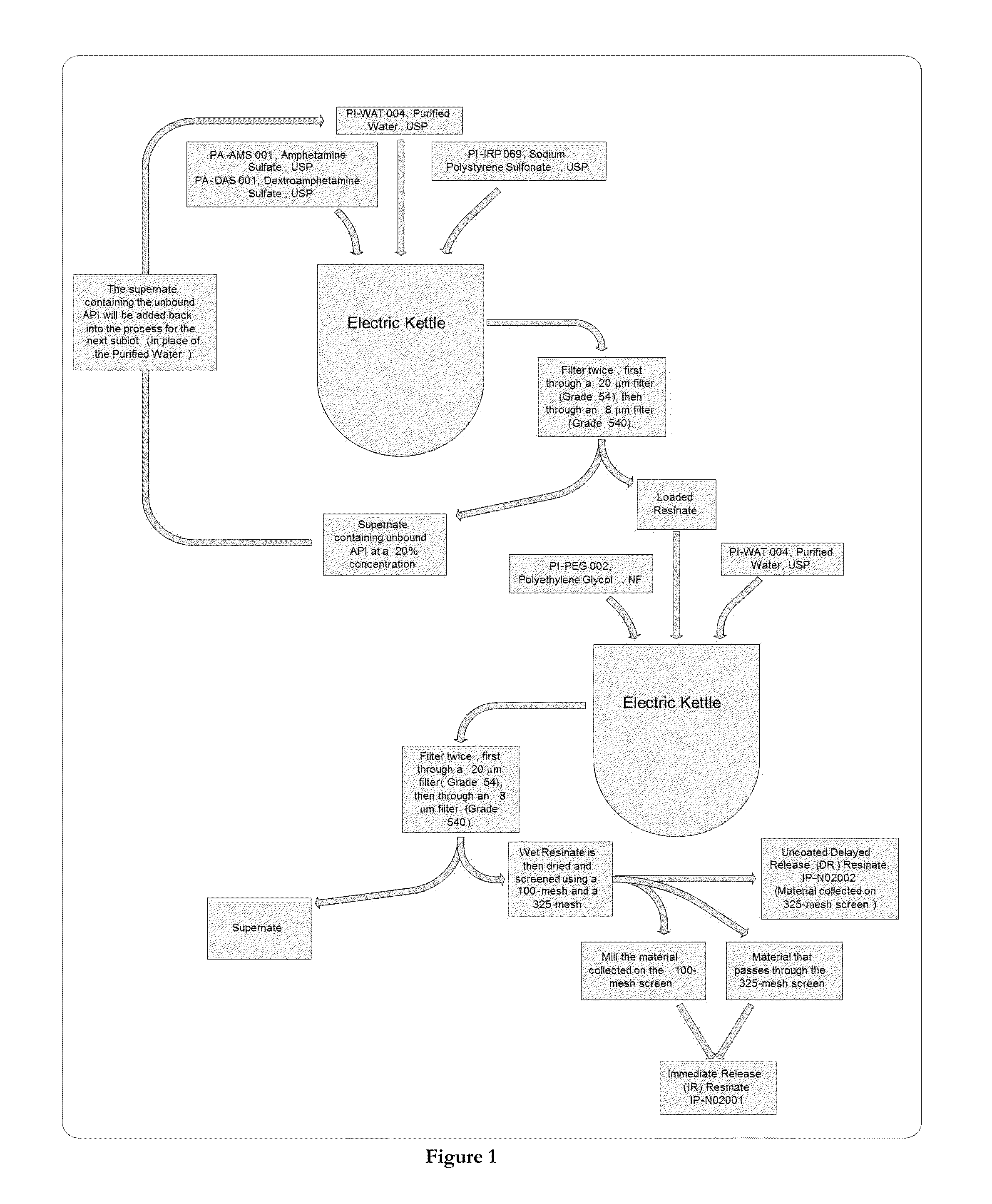
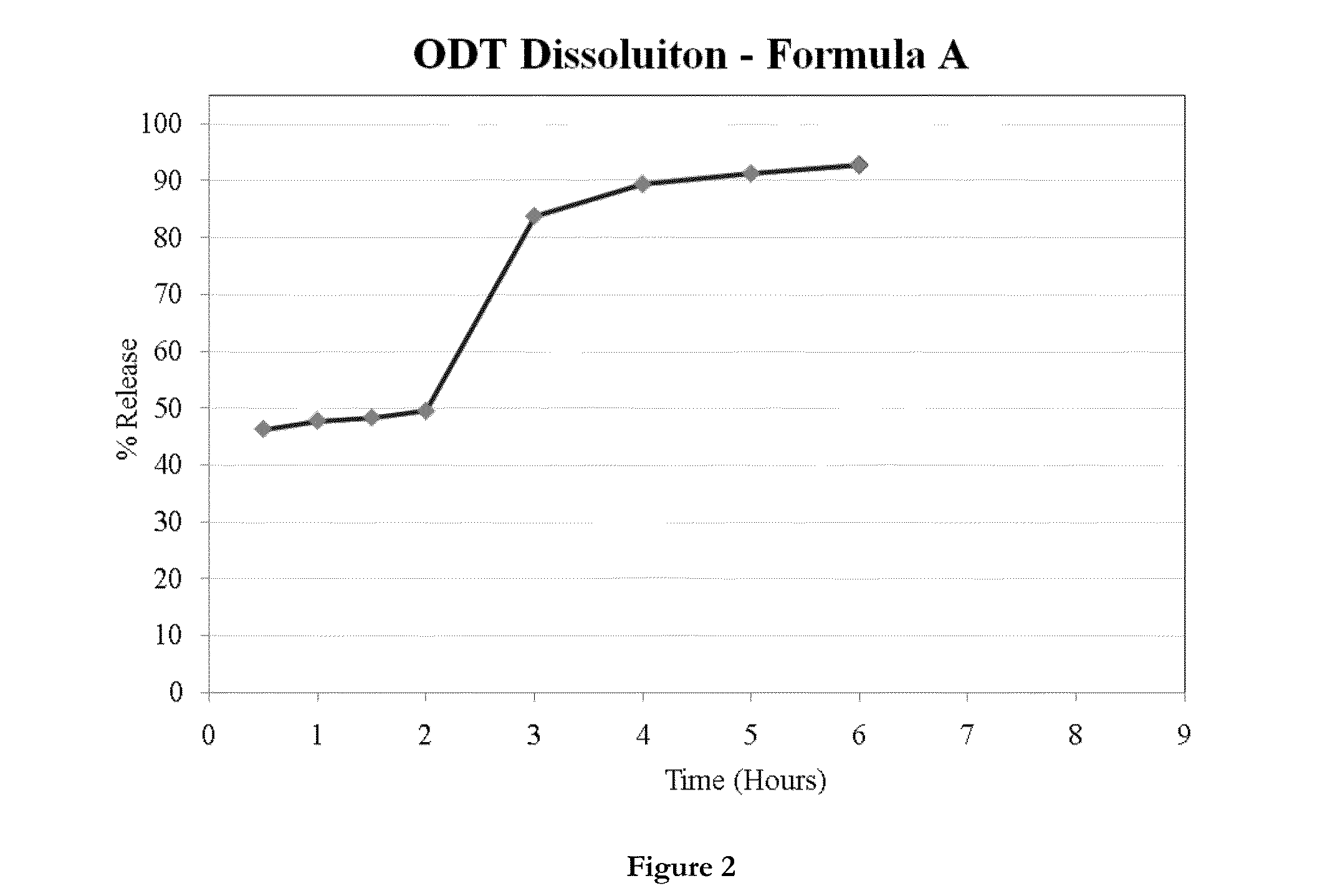
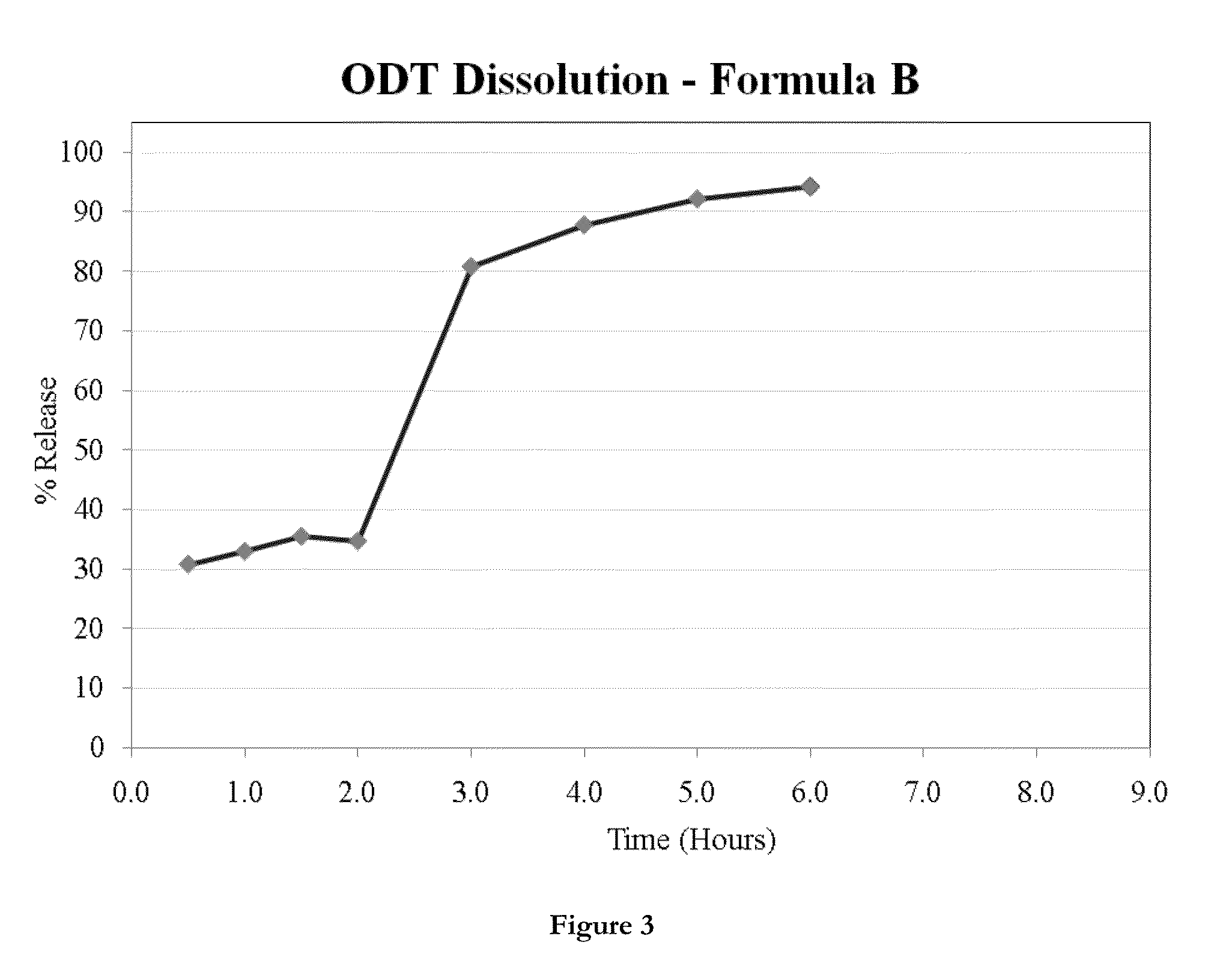
ActiveUS20130236554A1Increase intakeEffective and prolonged treatmentBiocideOrganic active ingredientsOral medicationIntrathecal
The invention relates to dosage forms that provide prolonged therapy. In particular, the invention relates to dosage forms including various pluralities of drug-containing resin particles. The invention also relates to methods of making these dosage forms and methods of treating using these dosage forms.
Owner:NEOS THERAPEUTICS LP
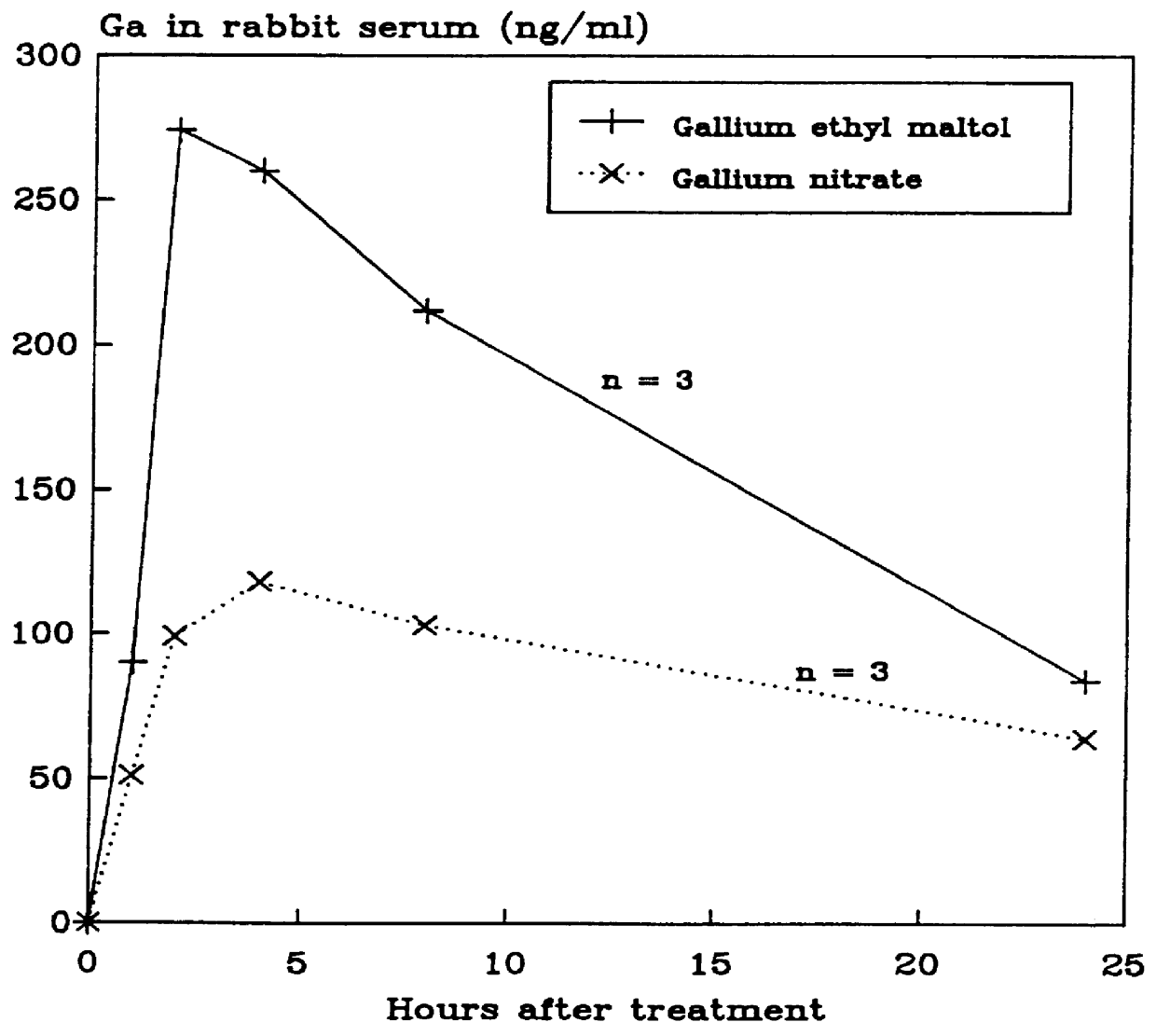
Solid pharmaceutical compositions for the oral administration of gallium
The subjects of this invention are pharmaceutical compositions that comprise gallium complexes of 3-hydroxy-4-pyrones. The compositions have been developed to provide pharmaceutically acceptable gallium bioavailability together with low toxicity, particularly for oral administration. Compositions included in this invention should be useful in providing gallium to humans and other animals for a wide variety of medical and veterinary applications, including the treatment, prevention, or diagnosis of certain bone diseases, certain cancers, and certain disorders of calcium homeostasis.
Owner:BERNSTEIN LAWRENCE RICHARD
Methods and compositions for oral administration of insulin
ActiveUS9186412B2Promote absorptionPeptide/protein ingredientsMetabolism disorderDiabetes mellitusIntrathecal
This invention provides compositions comprising a protein, an absorption enhancer, a protease inhibitor, methods for treating diabetes mellitus, comprising administering same, and methods for oral administration of a protein with an enzymatic activity, comprising orally administering same.
Owner:ENTERA BIO LTD
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833550B2Reduce morbidityNot impart bitter tastePowder deliverySenses disorderNasal cavityAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com