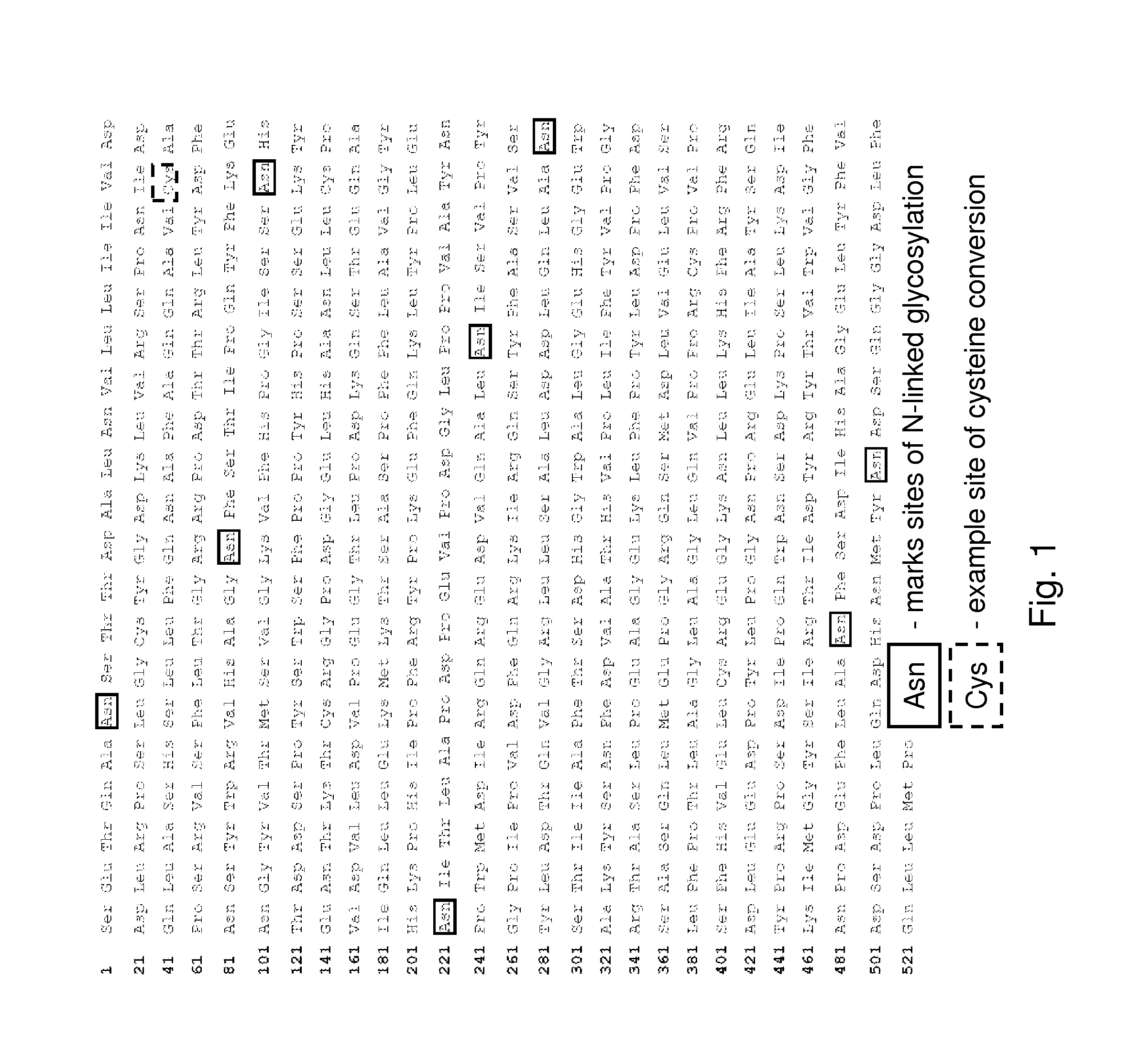
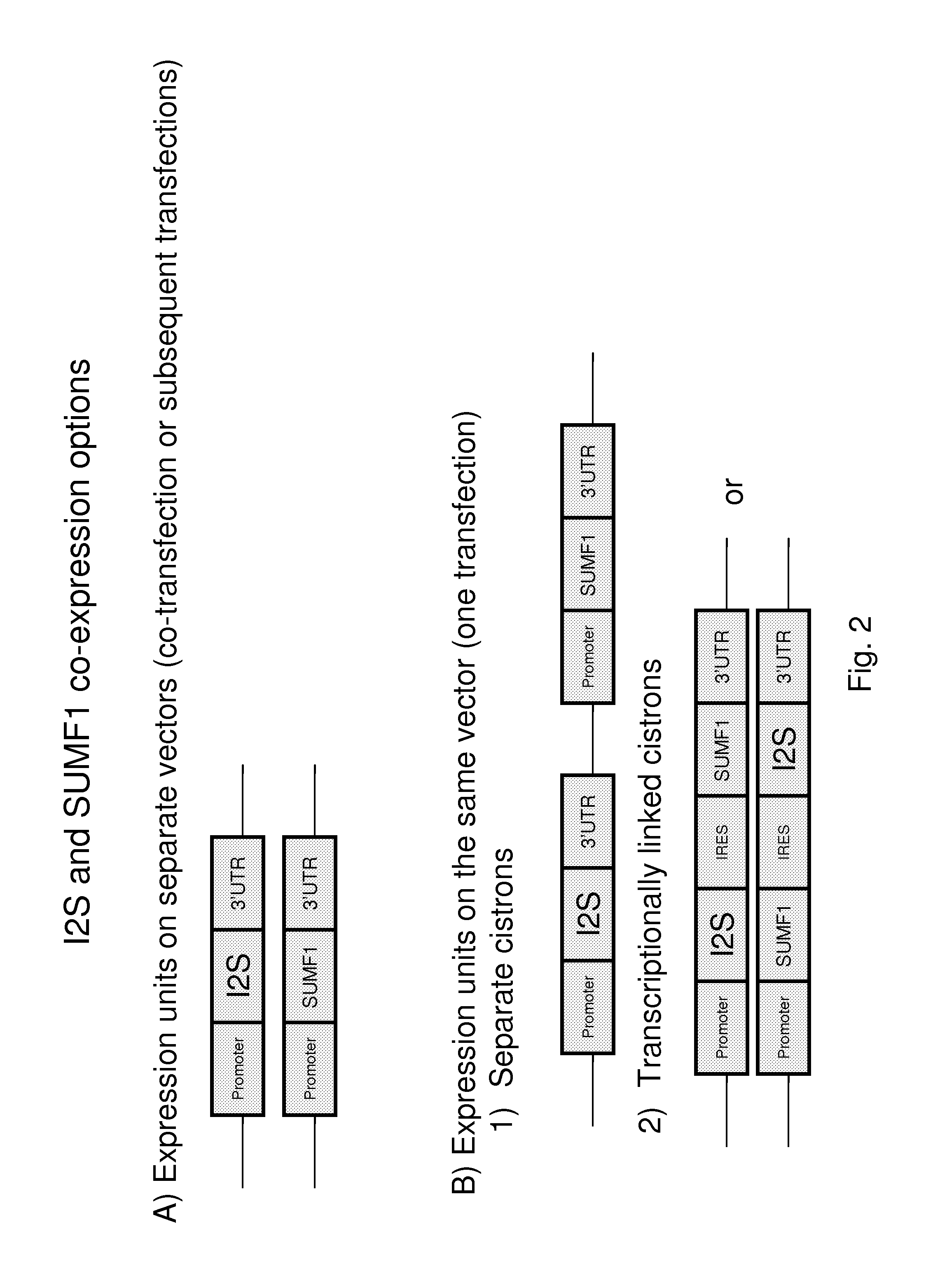
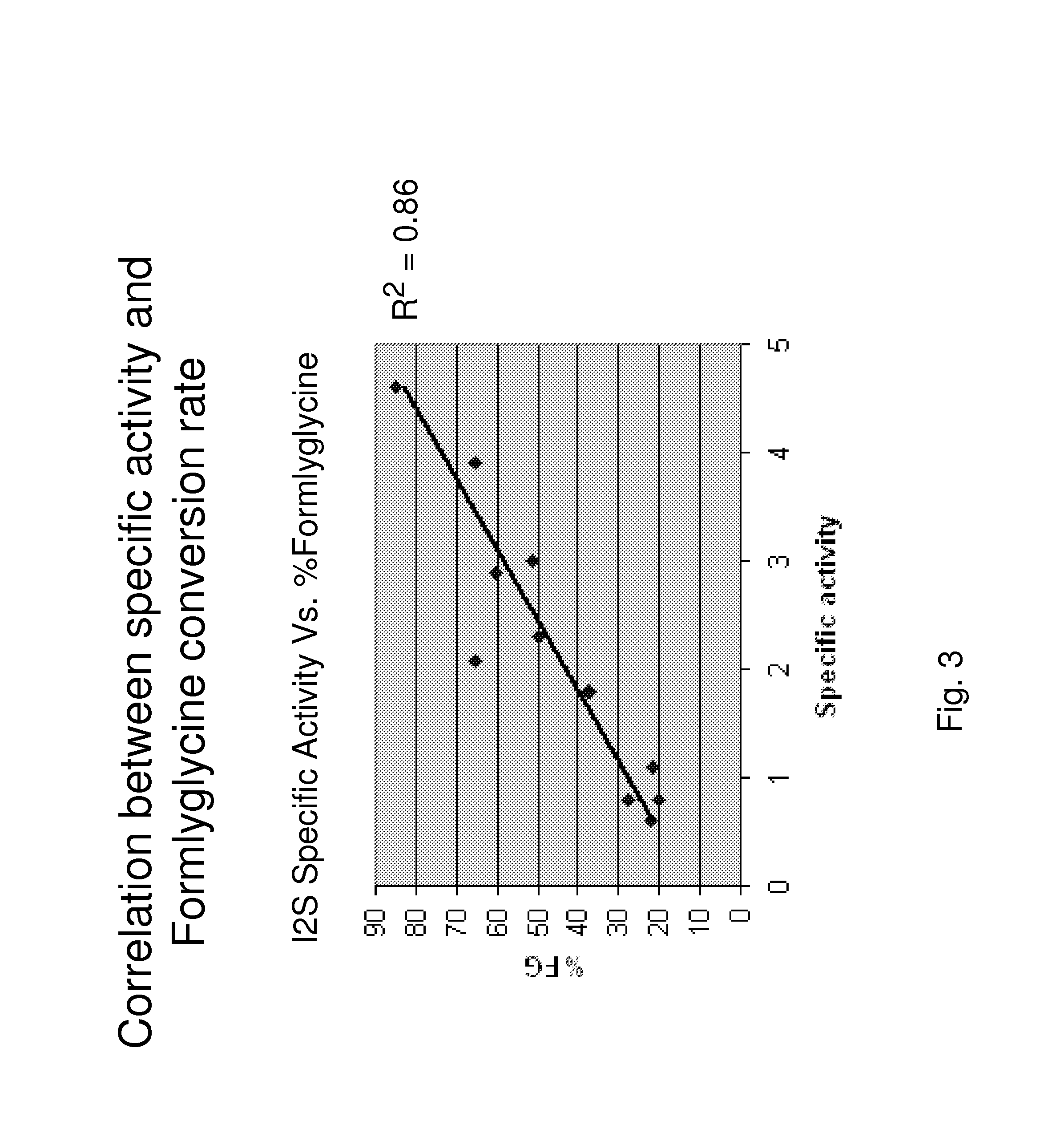
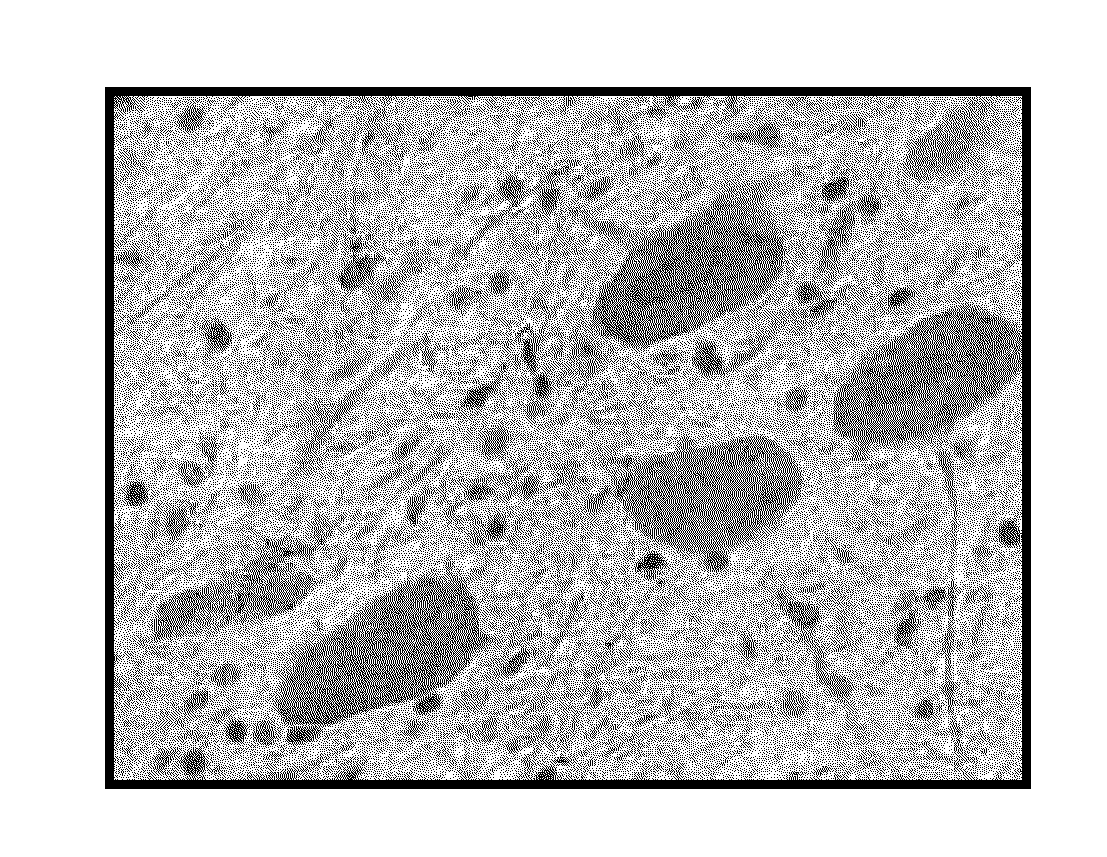
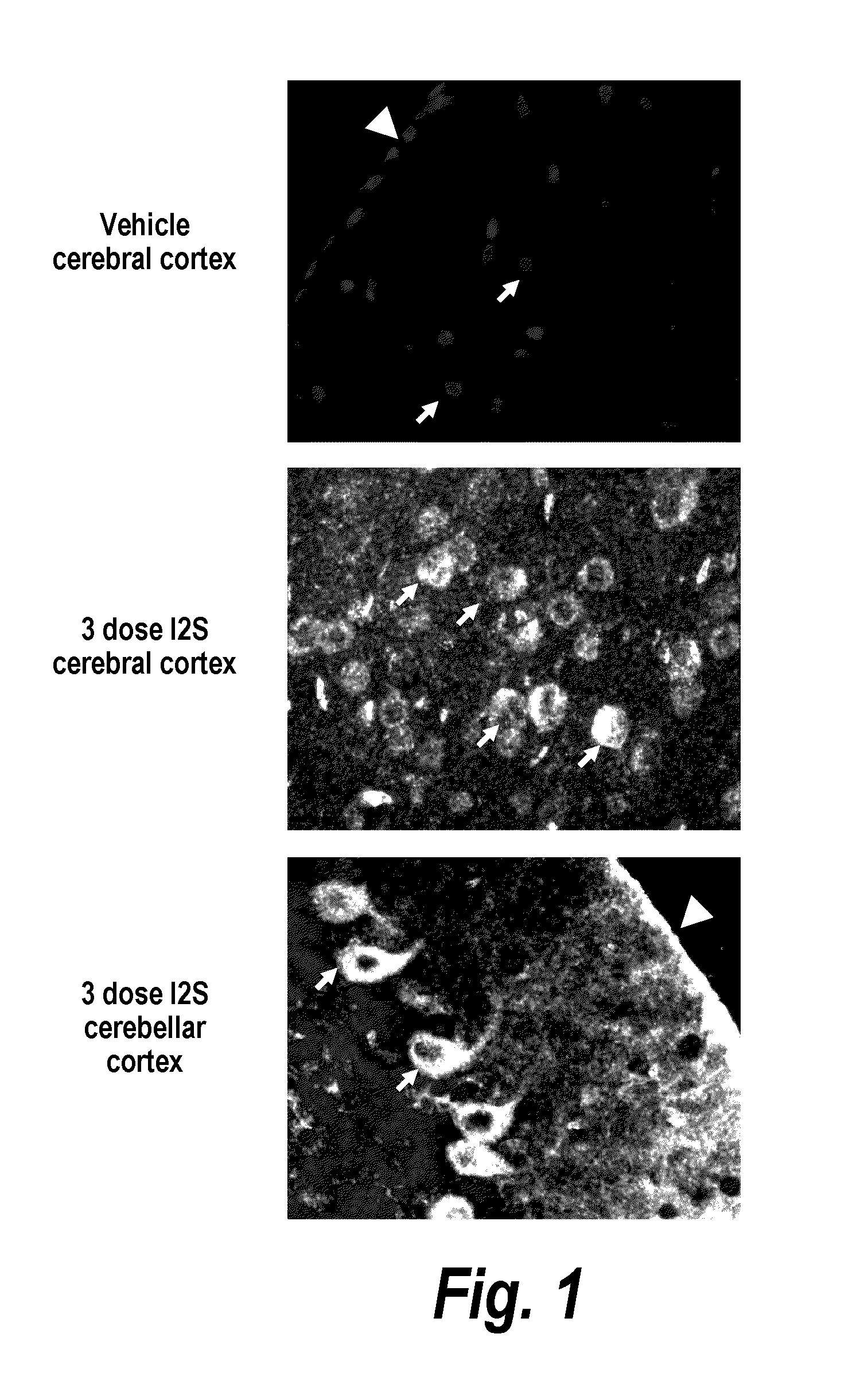
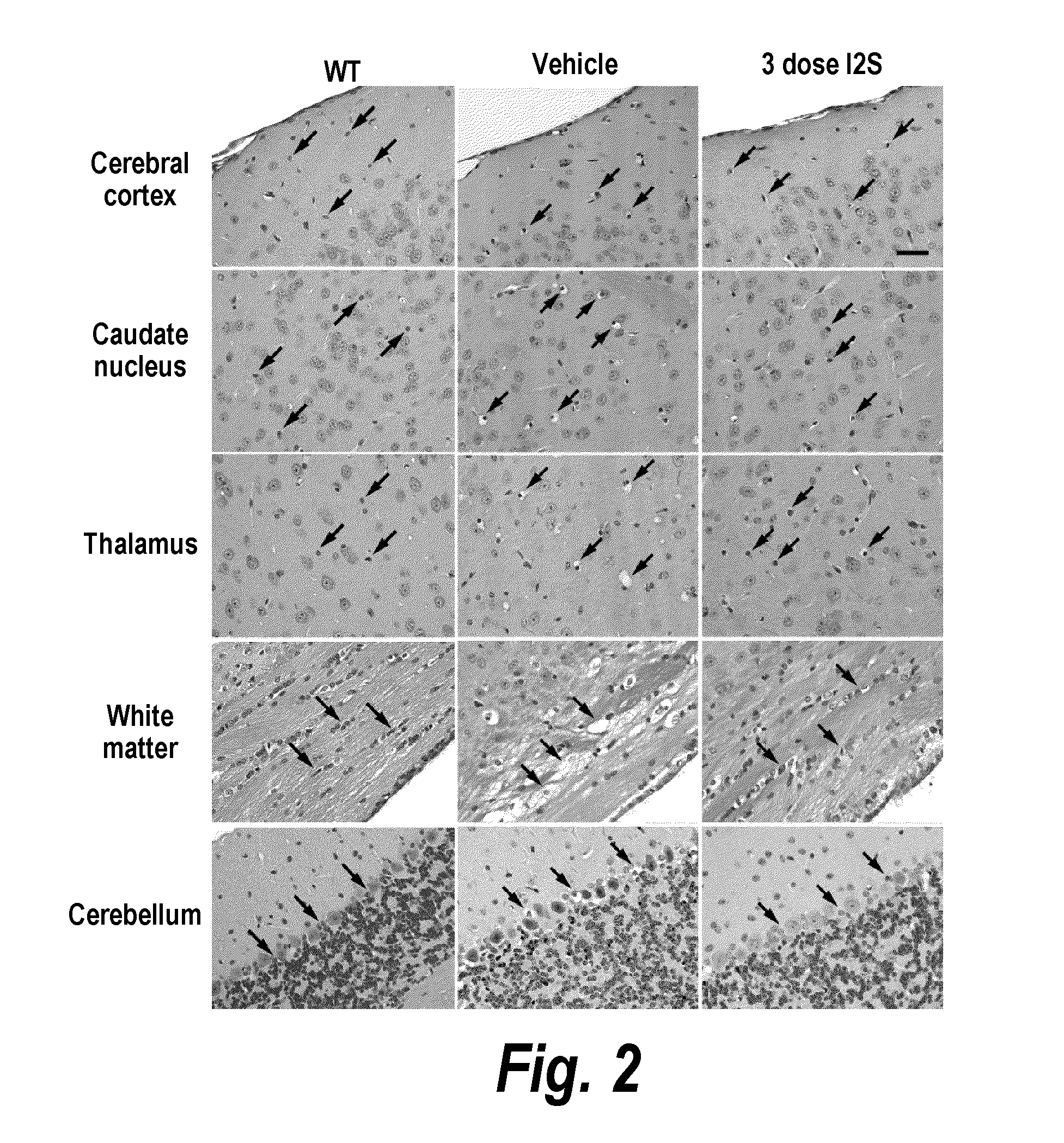
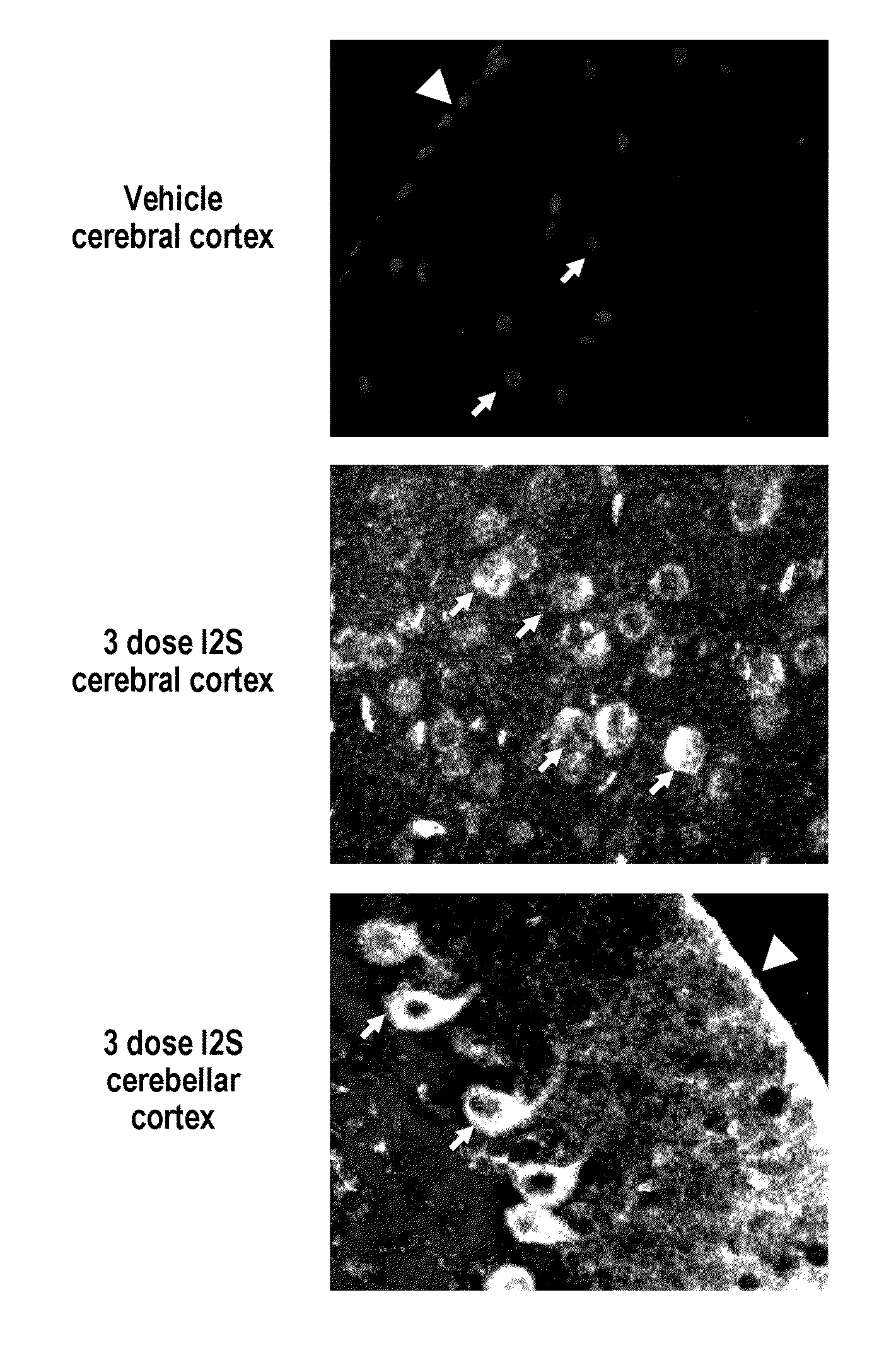
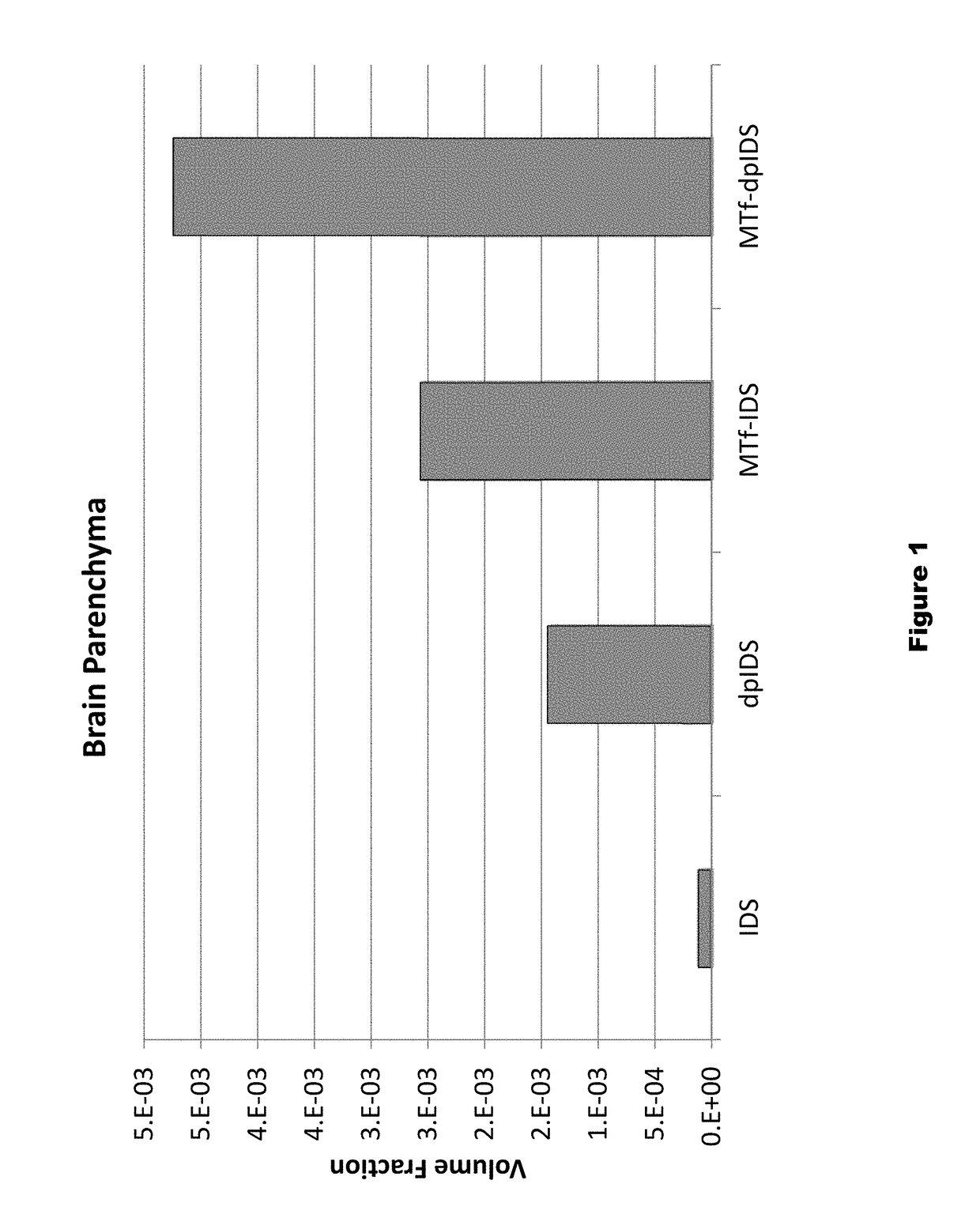
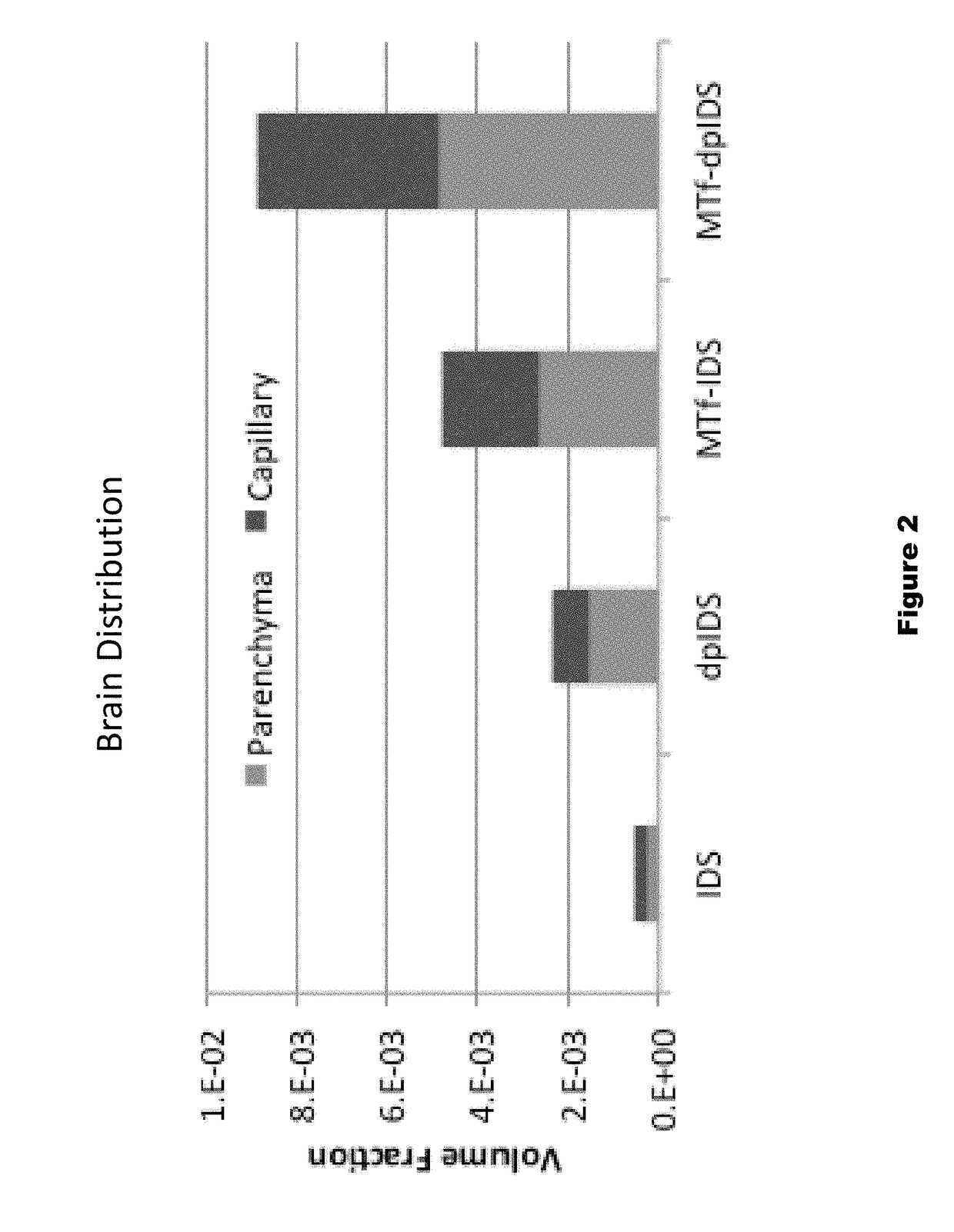
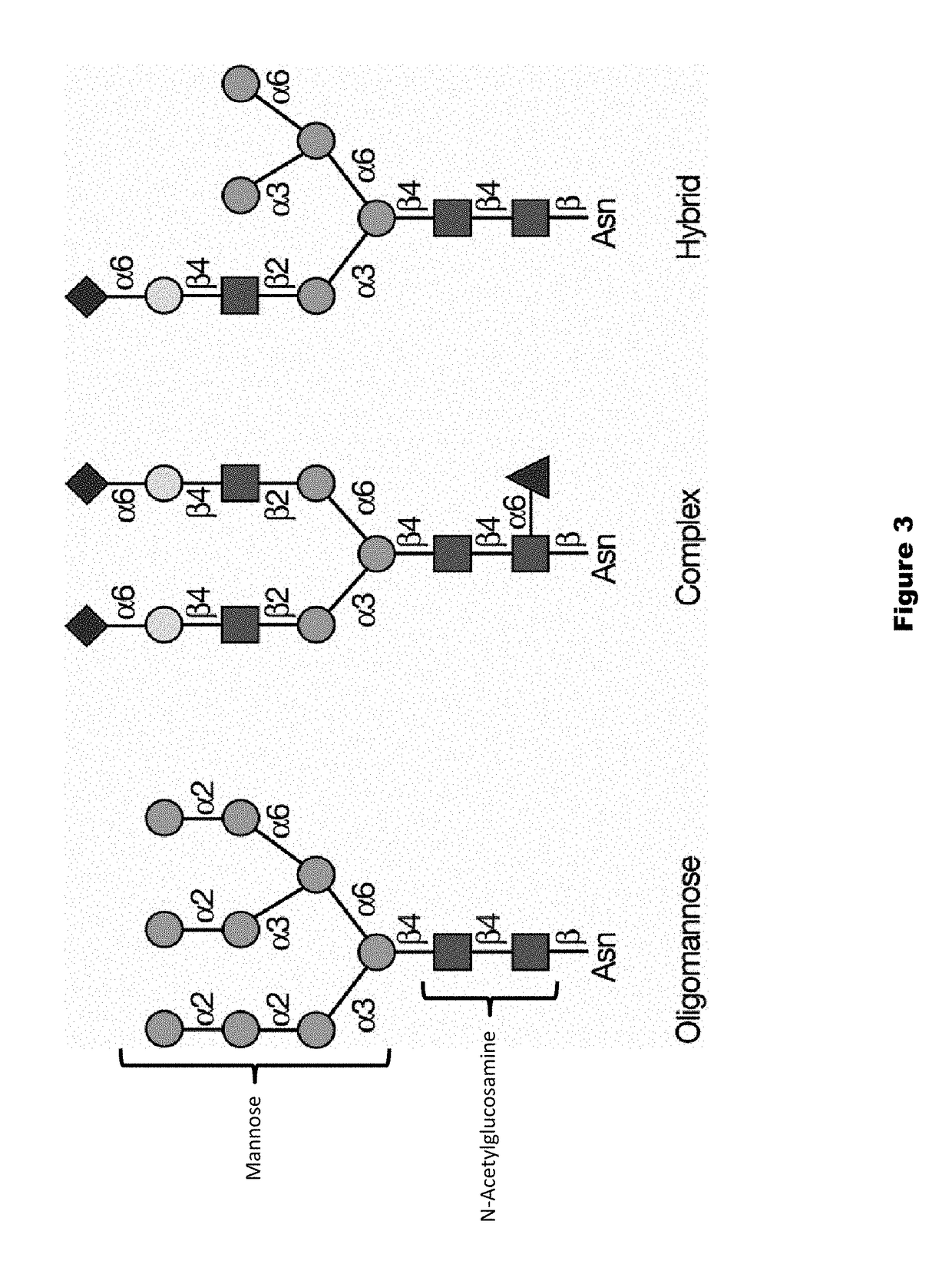
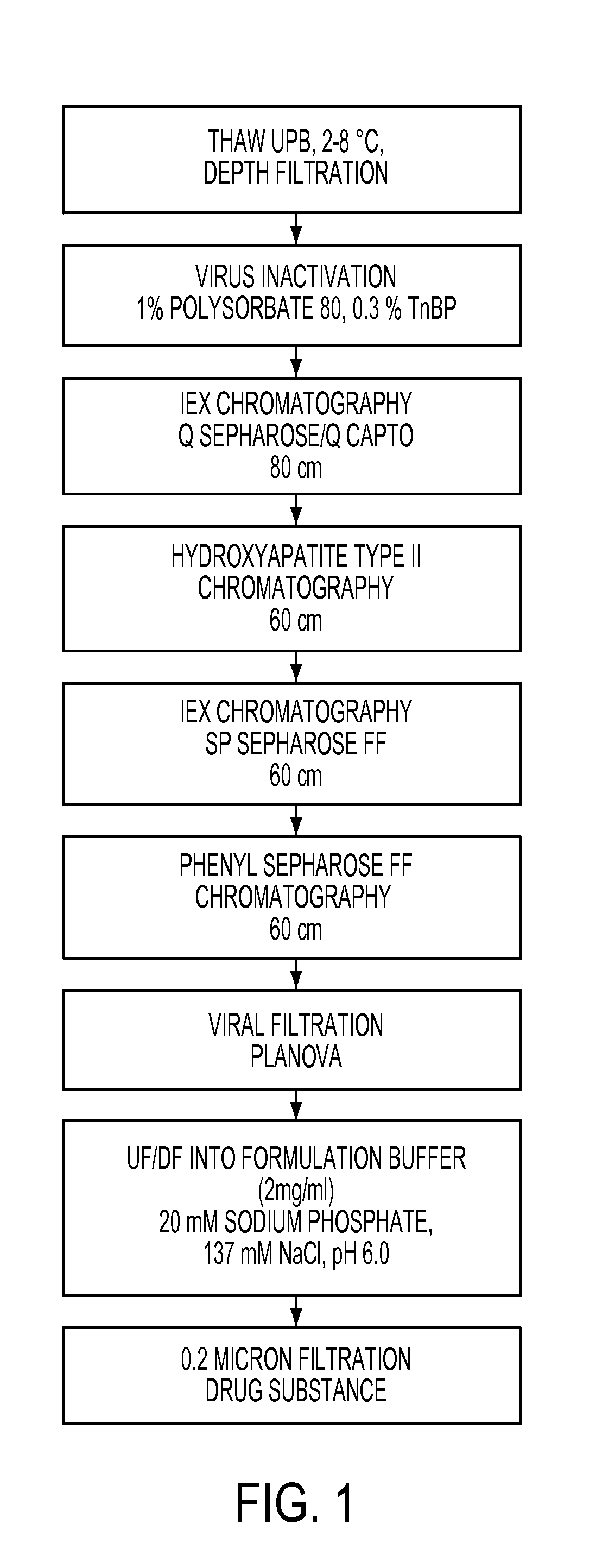
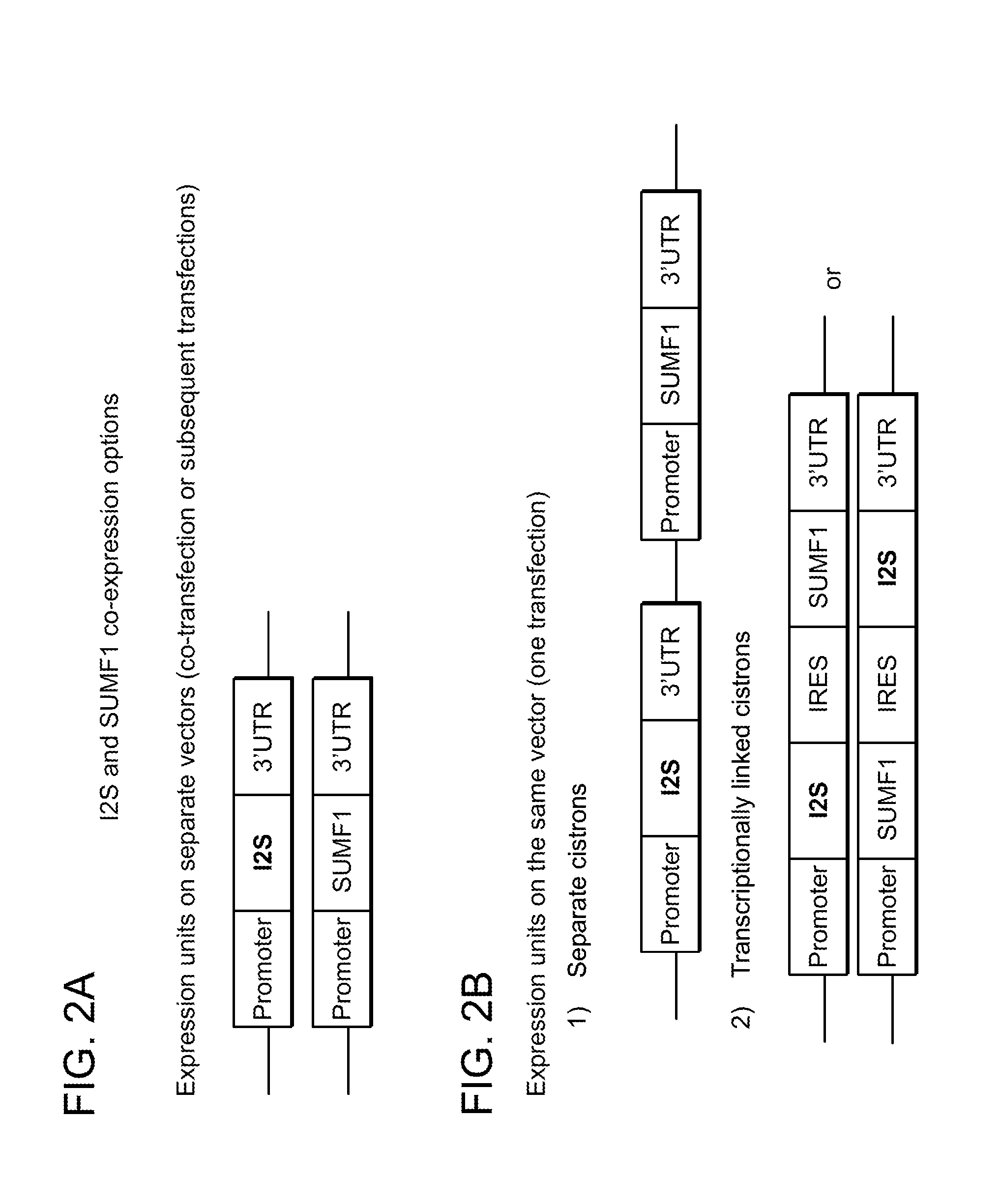
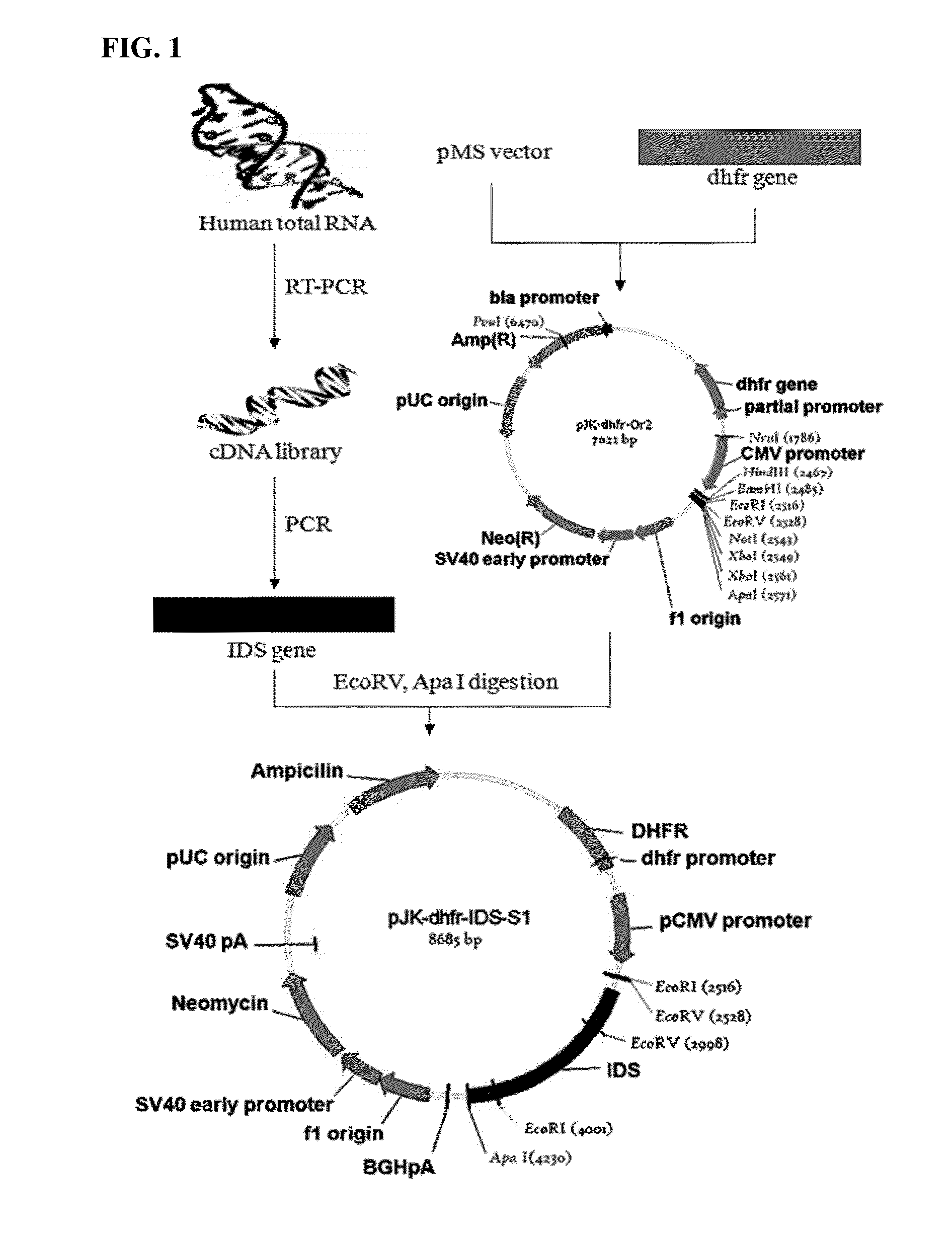
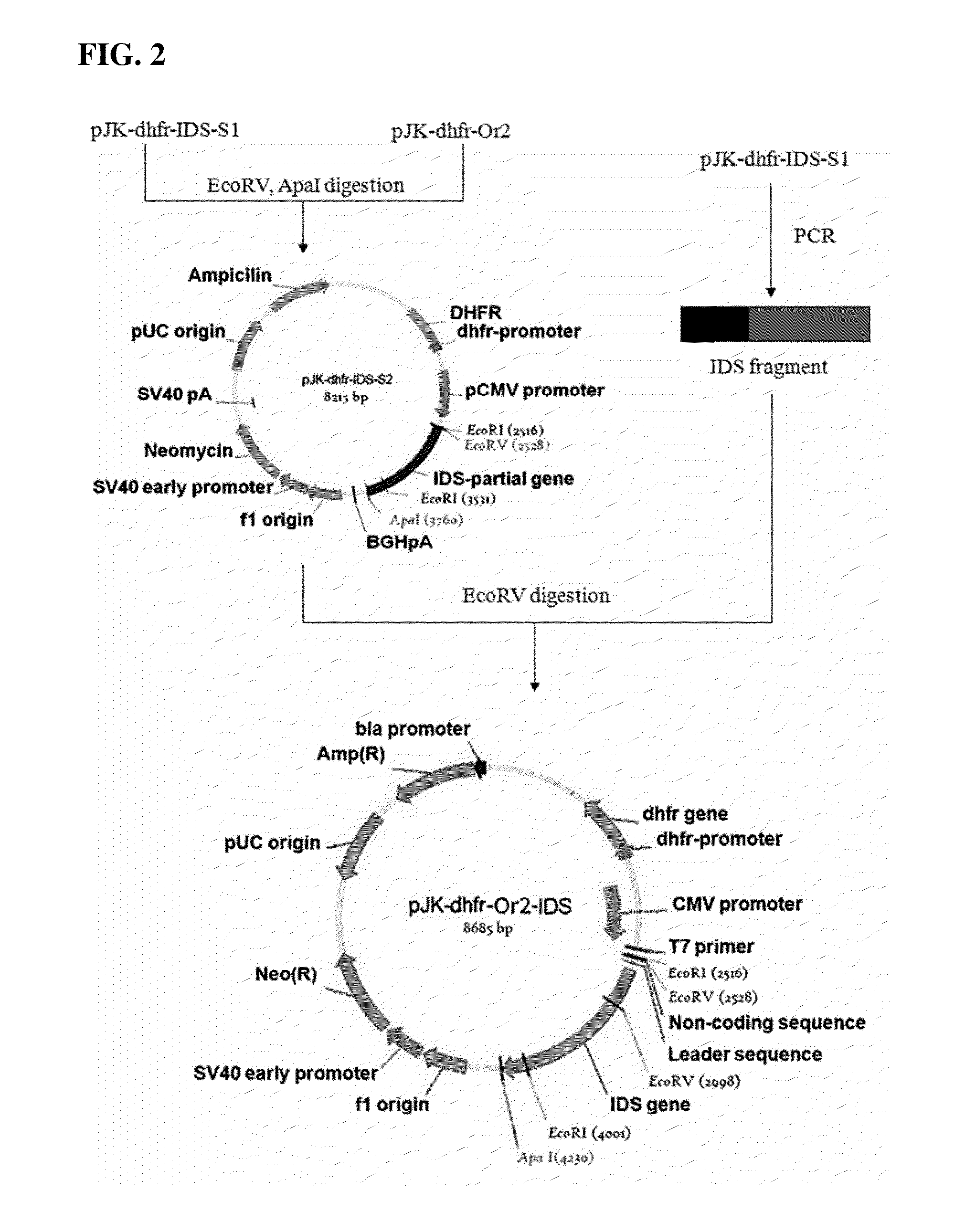
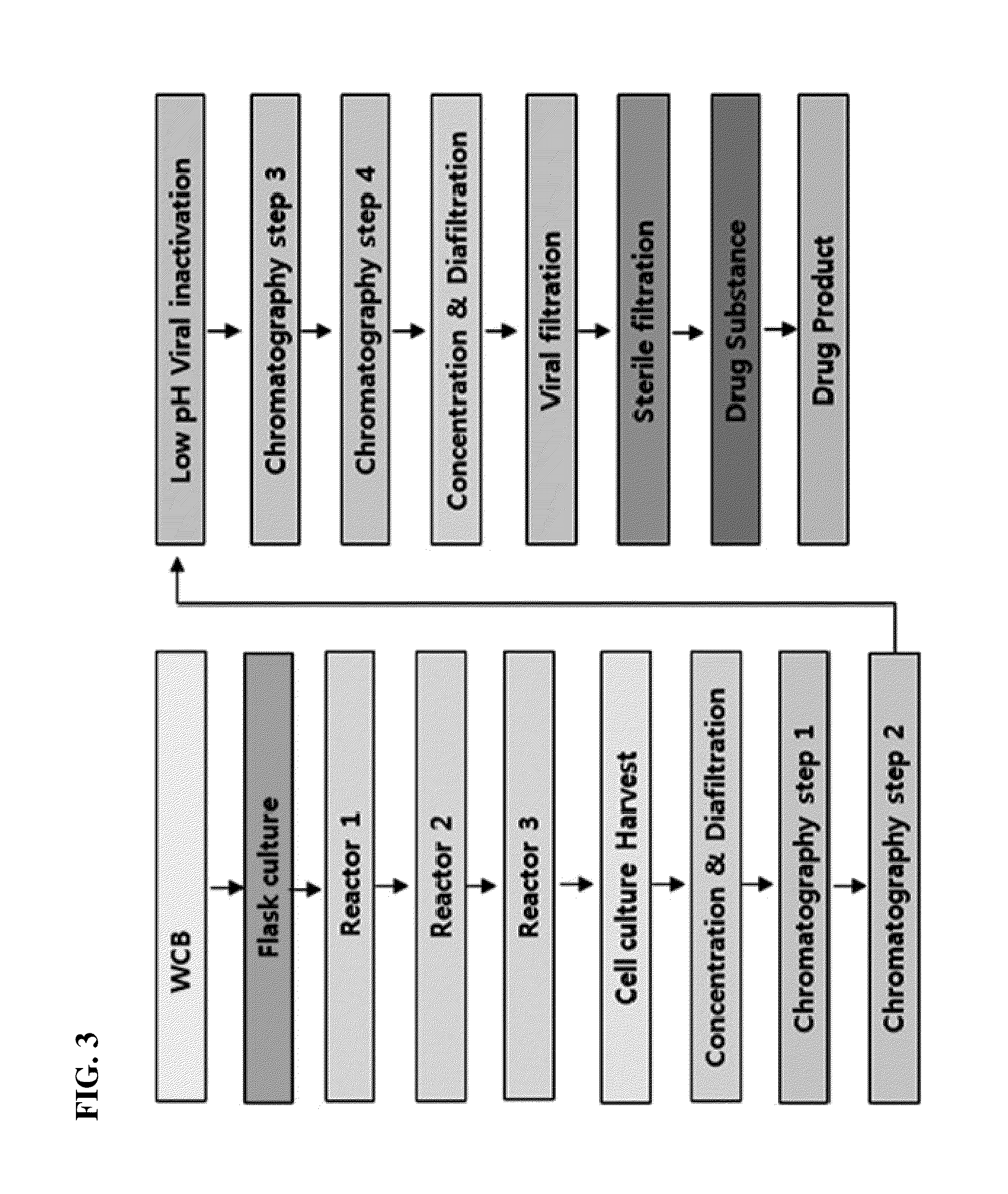
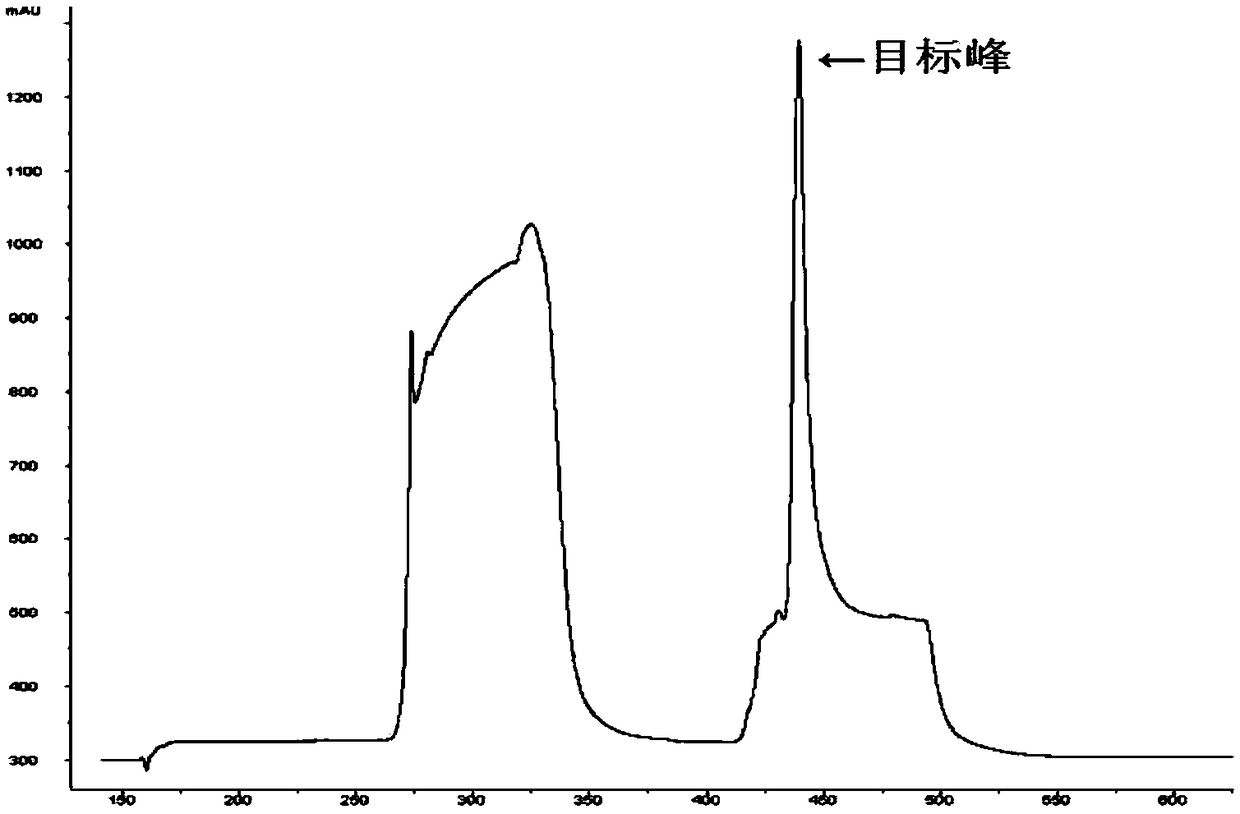
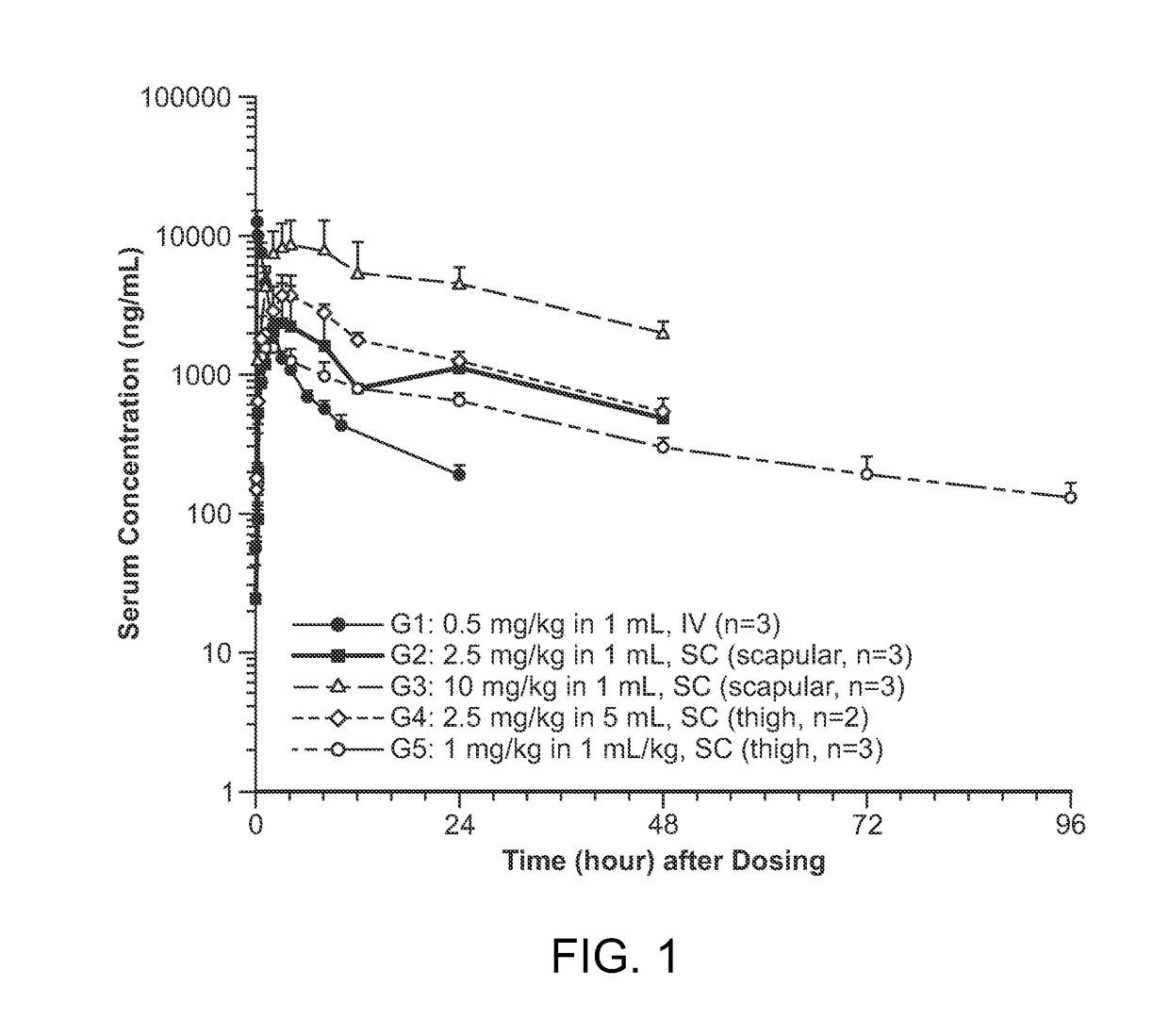
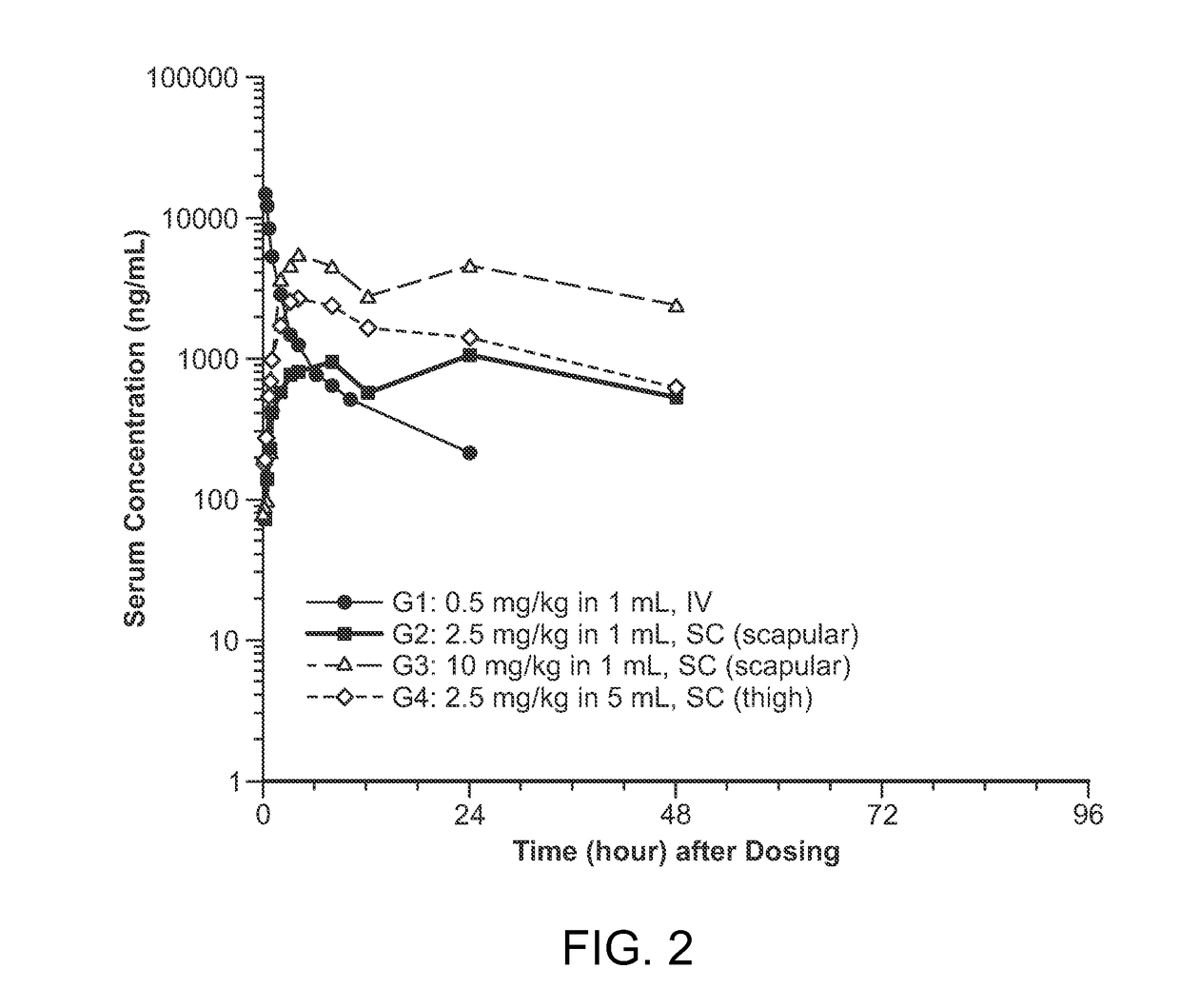
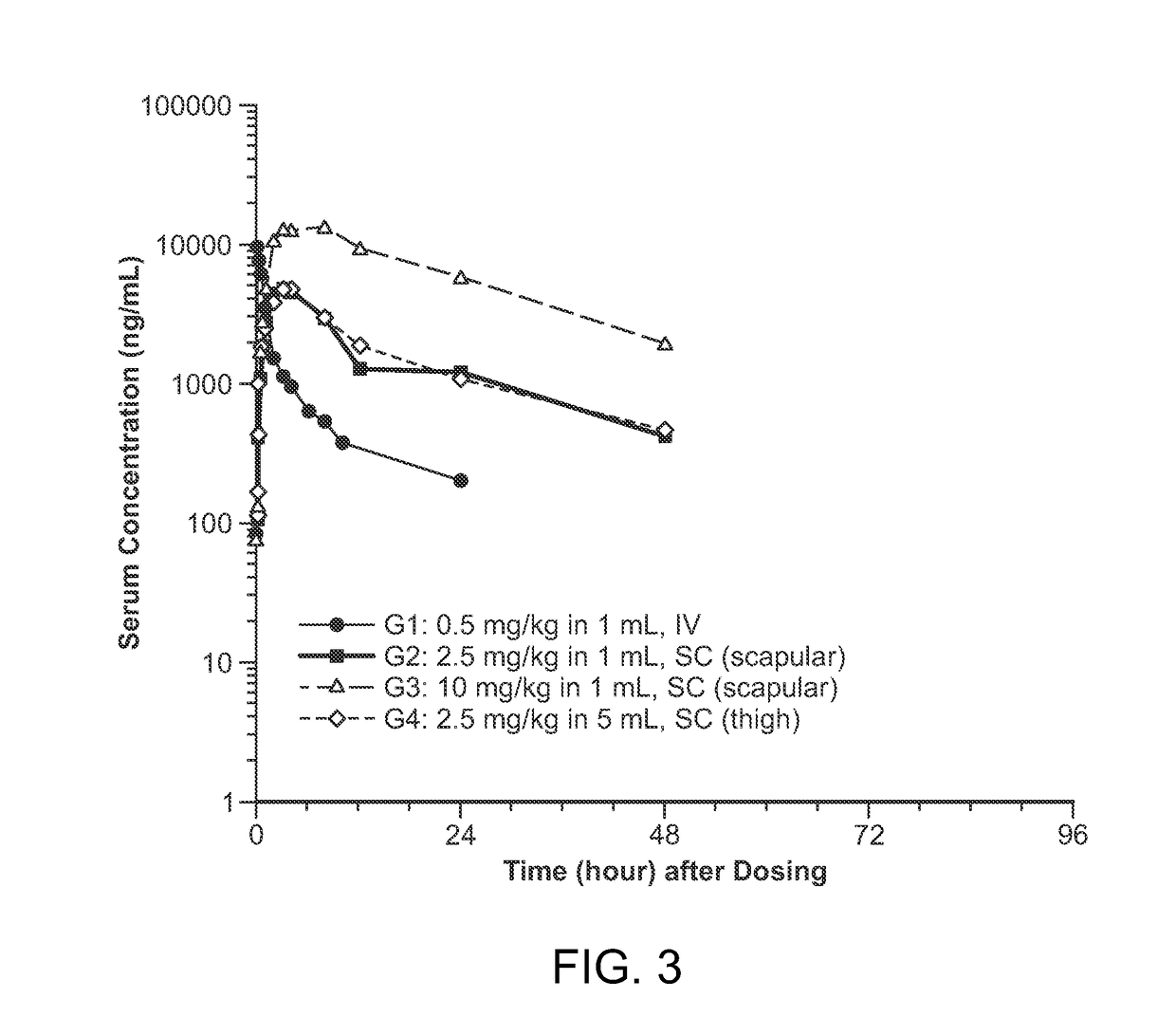
Patents
Literature
32 results about "Iduronate-2-sulfatase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
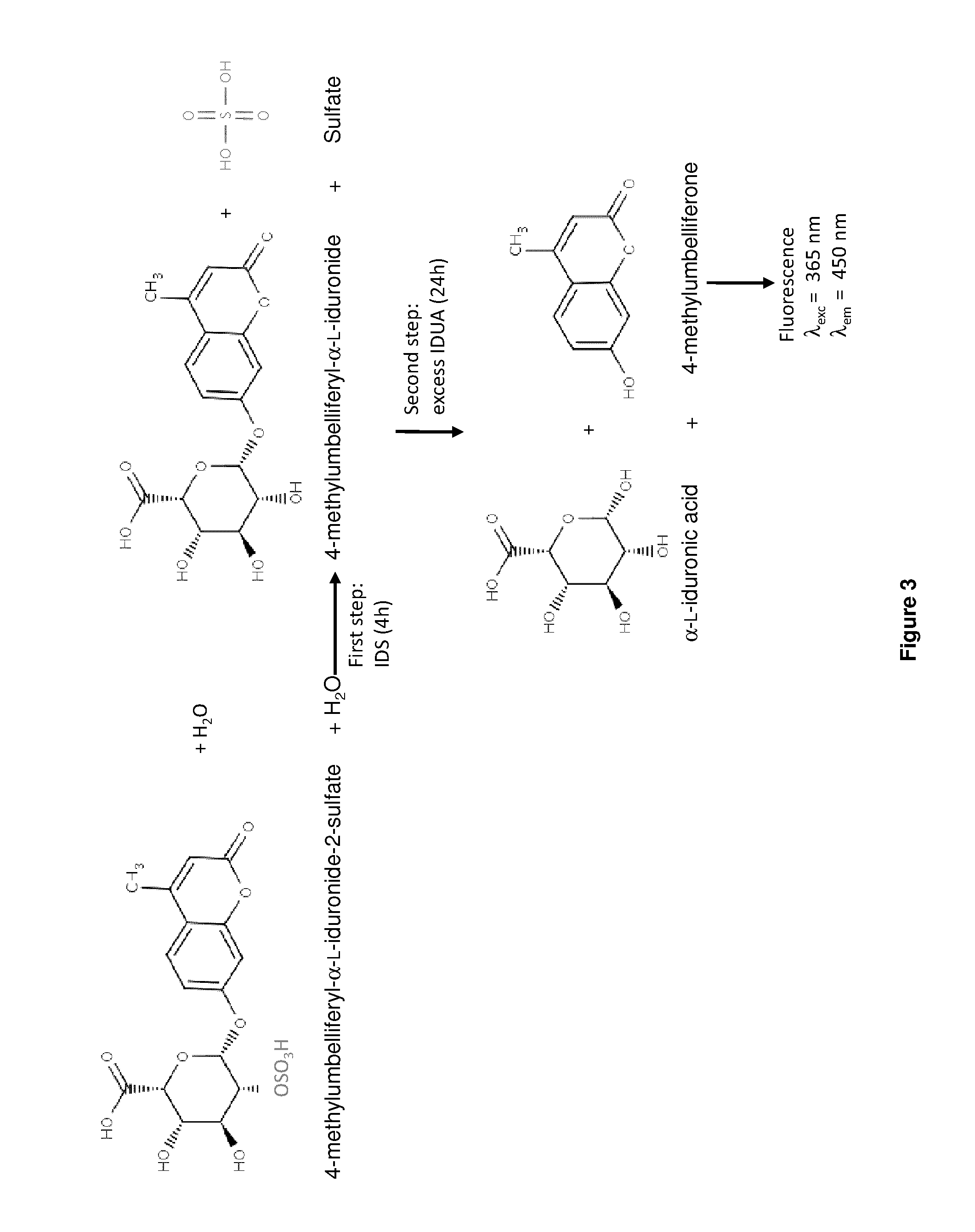
Iduronate 2-sulfatase (IDS) is a sulfatase enzyme associated with Hunter syndrome.
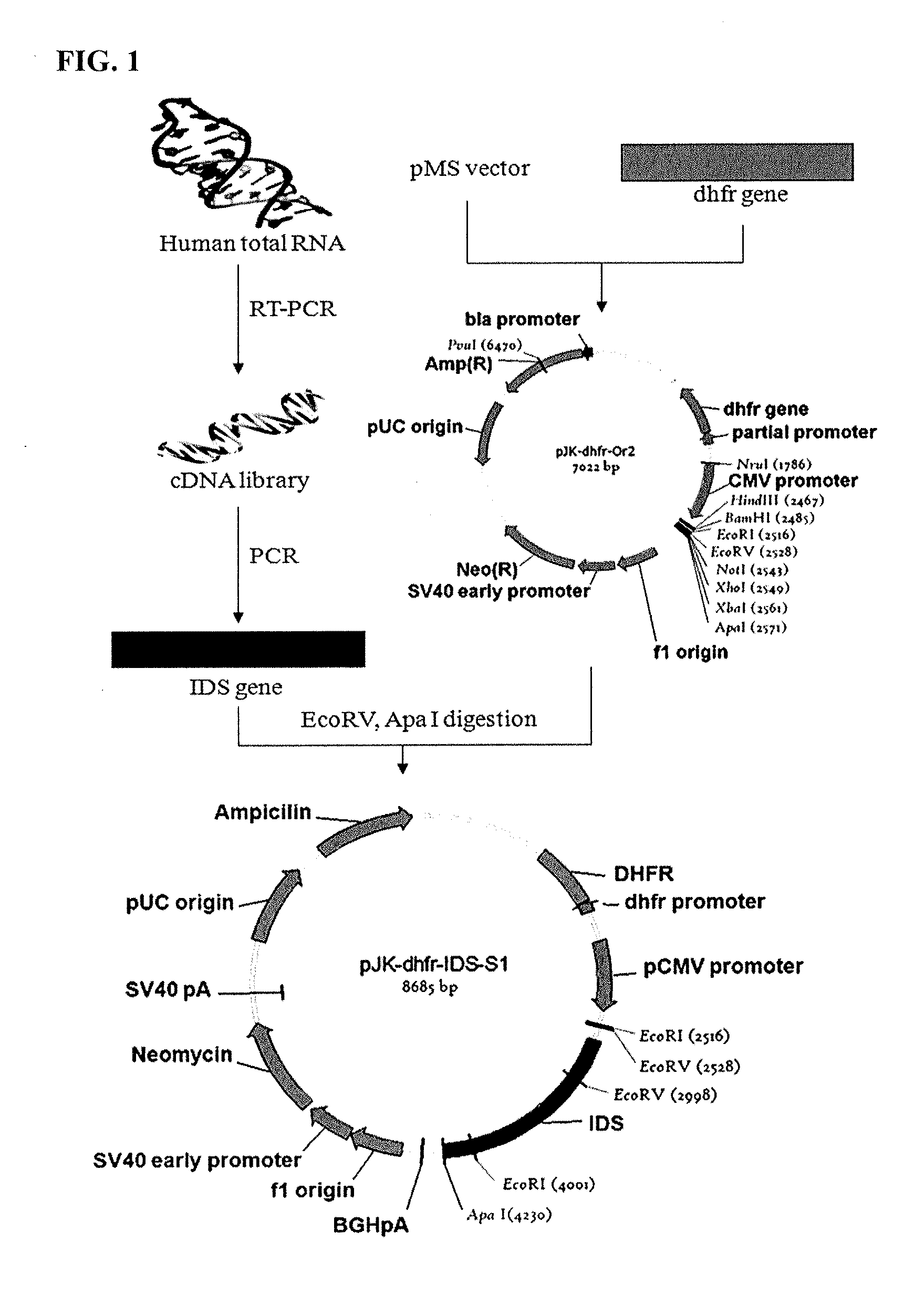
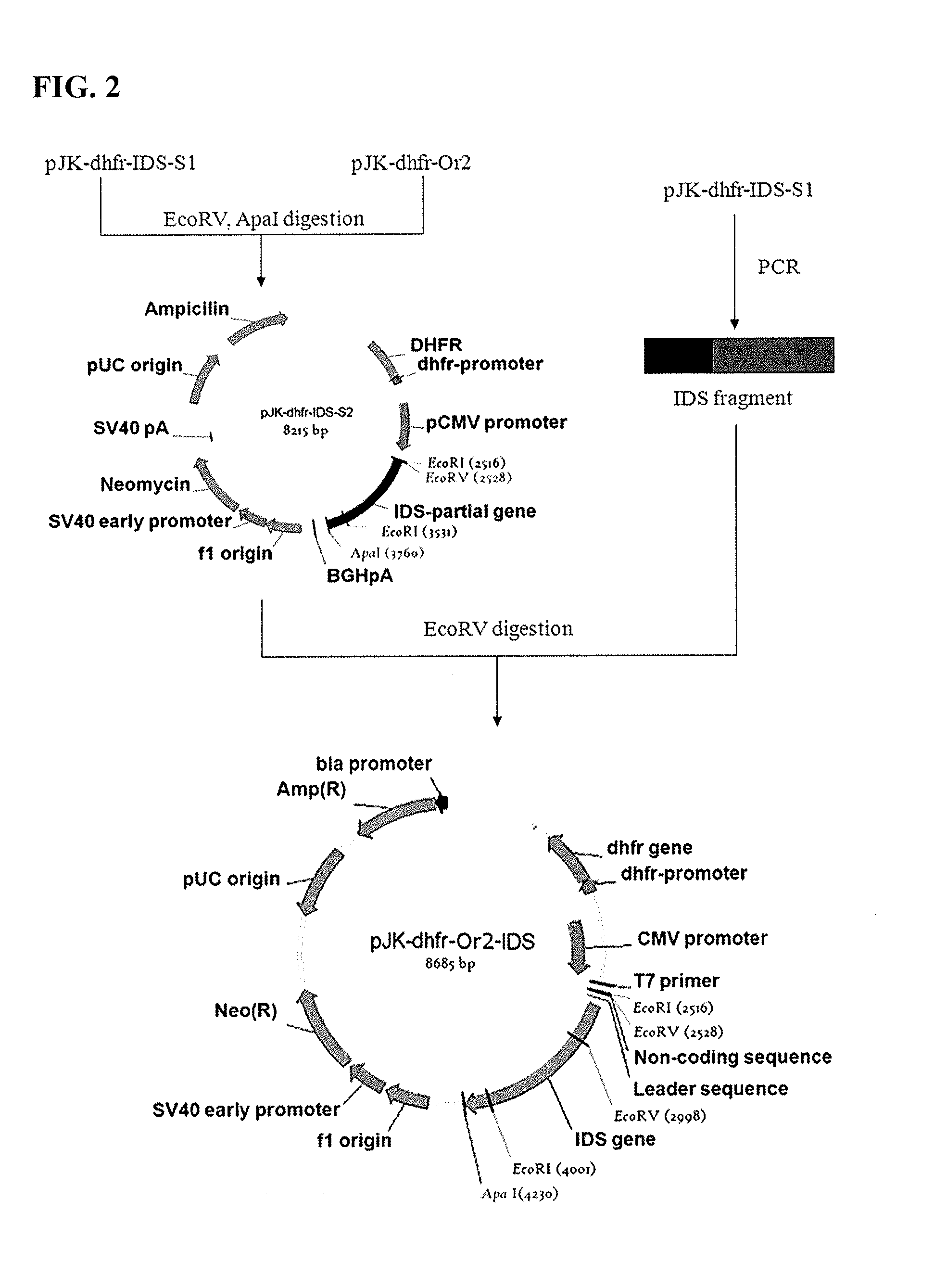
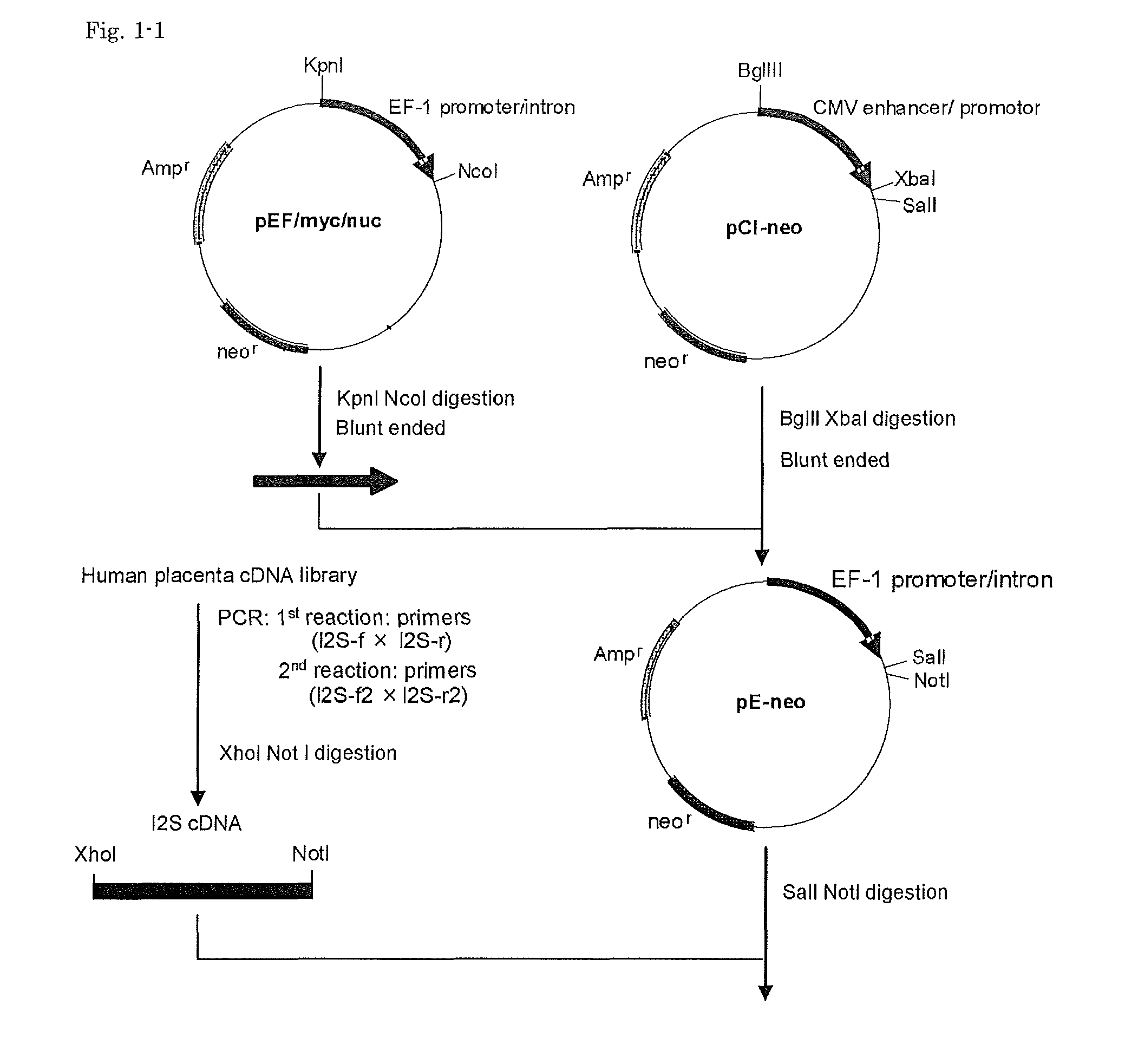
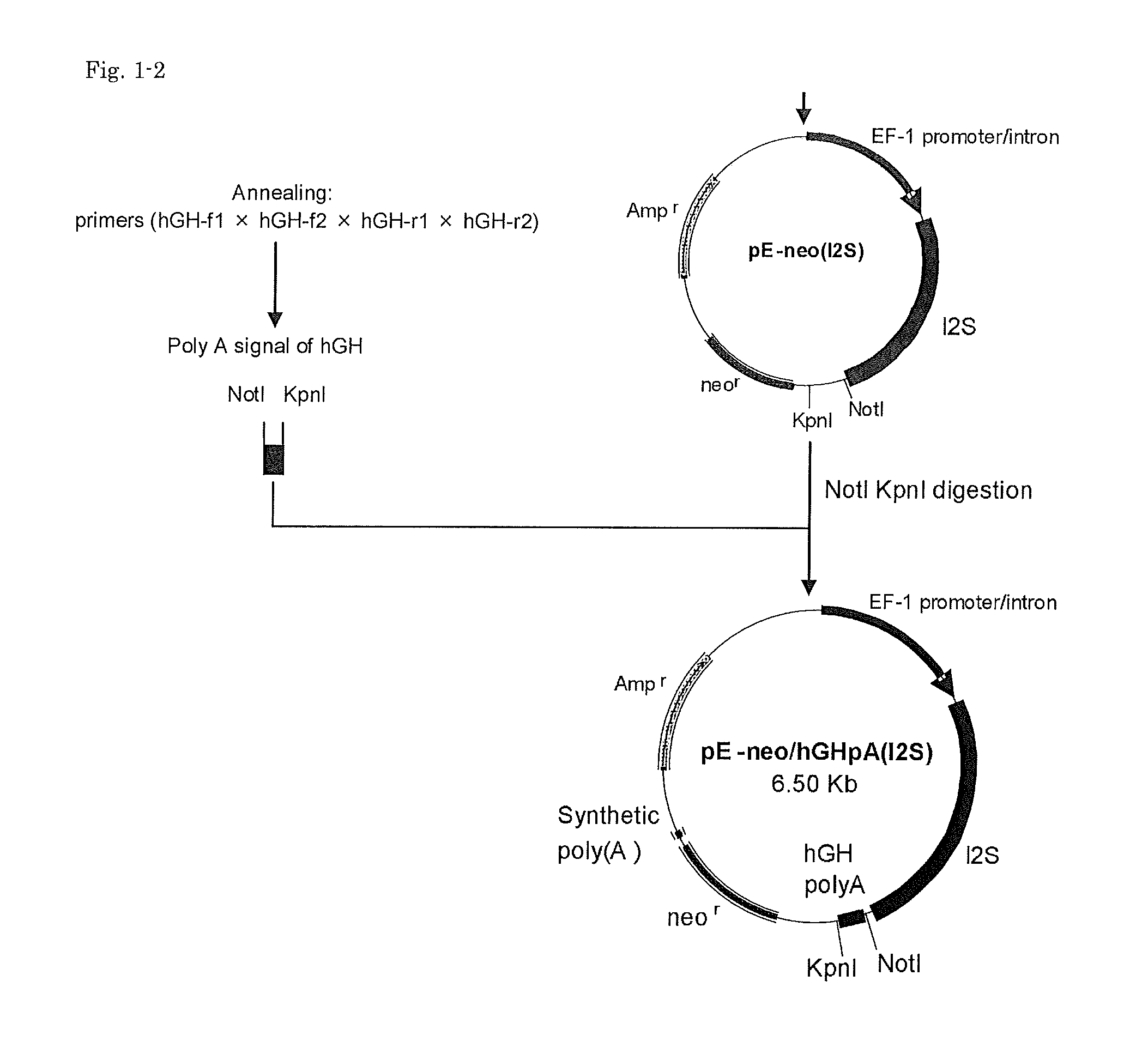
Cells for producing recombinant iduronate-2-sulfatase
ActiveUS20140004593A1Effective enzyme replacement therapyImprove the level ofAnimal cellsHydrolasesIduronate-2-sulfataseSerum free
The present invention provides, among other things, methods and compositions for production of recombinant I2S protein with improved potency and activity using cells co-express I2S and FGE protein. In some embodiments, cells according to the present invention are engineered to simultaneously over-express recombinant I2S and FGE proteins. Cells according to the invention are adaptable to various cell culture conditions. In some embodiments, cells of the present invention adaptable to a large-scale suspension serum-free culture.
Owner:TAKEDA PHARMA CO LTD
Glycosylation variants of iduronate 2-sulfatase
The present invention provides a highly glycosylated iduronate-2-sulfatase enzyme comprising an iduronate-2-sulfatase polypeptide with at least 5 kilodalton (kDa) more sugar than iduronate-2-sulfatase purified from a natural source, e.g. human liver. The present invention also provides an enzymatically active polypeptide fragment or variant of such a highly glycosylated iduronate-2-sulfatase. The present invention further provides an isolated nucleic acid encoding iduronate-2-sulfatase, as well as an expression vector, a host cell and a method for producing the present highly glycosylated iduronate-2-sulfatase enzyme. In one embodiment the present invention is directed to a method for producing a glycosylated iduronate-2-sulfatase enzyme which comprises culturing a host cell containing a nucleic acid encoding an enzymatically active iduronate-2-sulfatase polypeptide wherein the host cell glycosylates the polypeptide to a greater degree than a native iduronate-2-sulfatase polypeptide expressed by a natural human liver cell.
Owner:WOMENS & CHILDRENS HOSPITAL
Methods and Compositions for Increasing Iduronate 2-Sulfatase Activity in the CNS
Provided herein are methods and compositions for treating a subject suffering from a deficiency in iduronate 2-sulfatase in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody that crosses the blood brain barrier (BBB) and an iduronate 2-sulfatase.
Owner:JCR PHARMA +1
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS20110318323A1Effective and less approachEffectively and extensivelyNervous disorderHydrolasesIduronate-2-sulfataseHunter syndrome
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS8545837B2Effective and less invasiveEffectively and extensivelyNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseHunter syndrome
Owner:TAKEDA PHARMA CO LTD
Method of producing recombinant iduronate-2-sulfatase
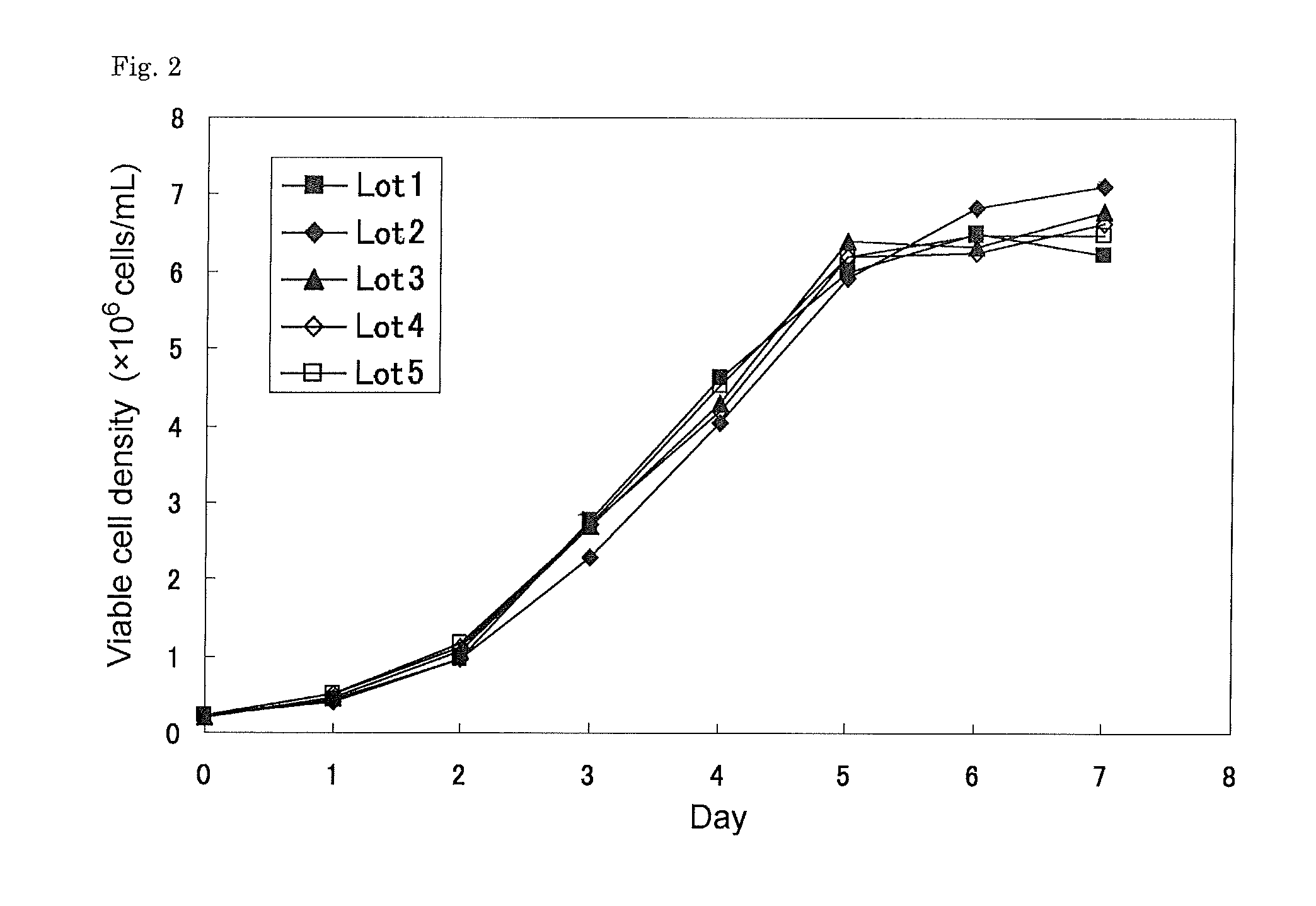
InactiveUS20140004097A1Conducive to effective treatmentEfficient productionHydrolasesPeptide/protein ingredientsSerum igeSerum free media
The present invention provides, among other things, methods and compositions for large-scale production of recombinant I2S protein using suspension culture of mammalian cells in serum-free medium. In particular, the present invention uses mammalian cells co-express a recombinant I2S protein and a formylglycine generating enzyme (FGE).
Owner:SHIRE HUMAN GENETIC THERAPIES INC
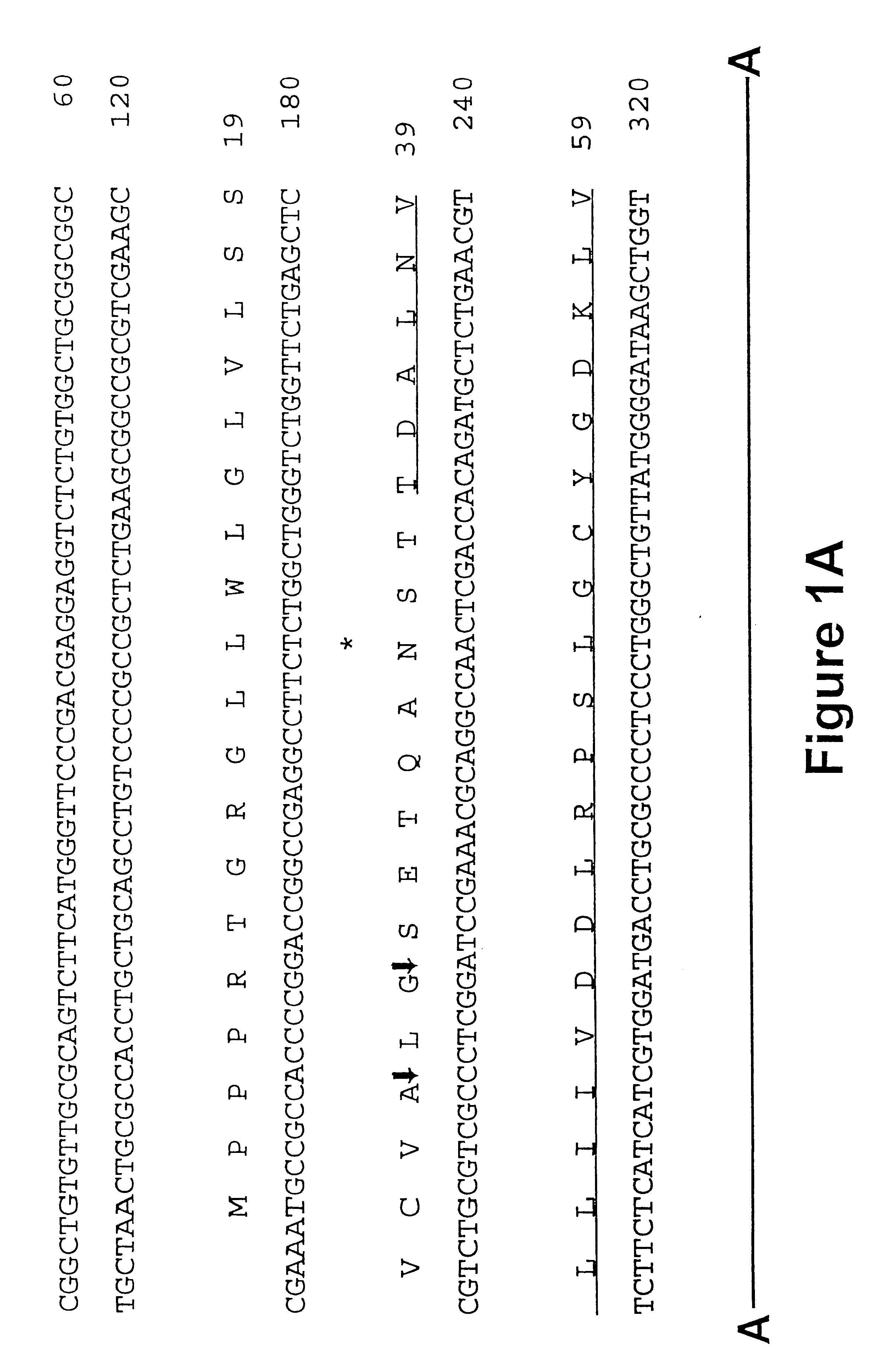
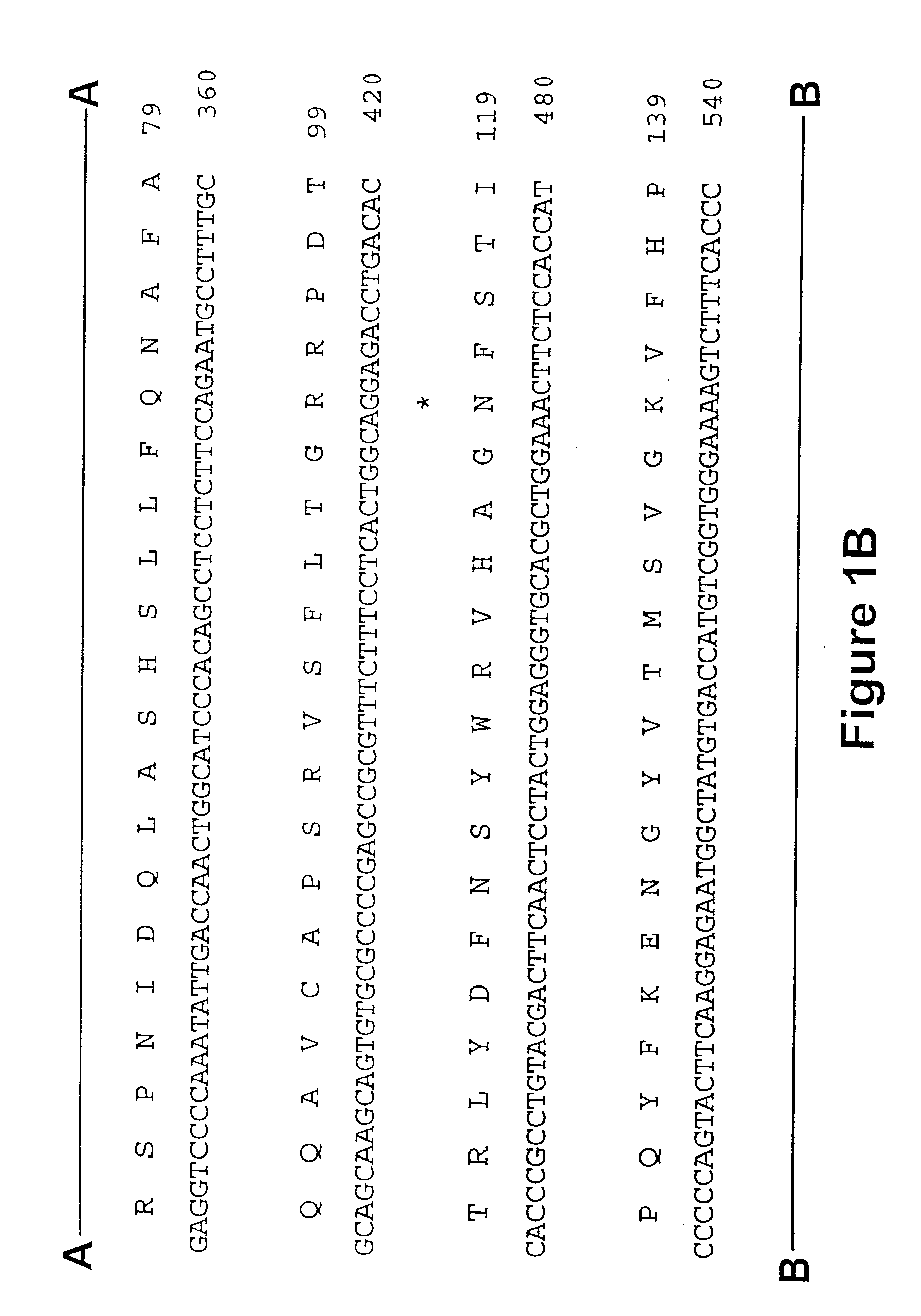
Iduronate-2-sulfatase and use thereof
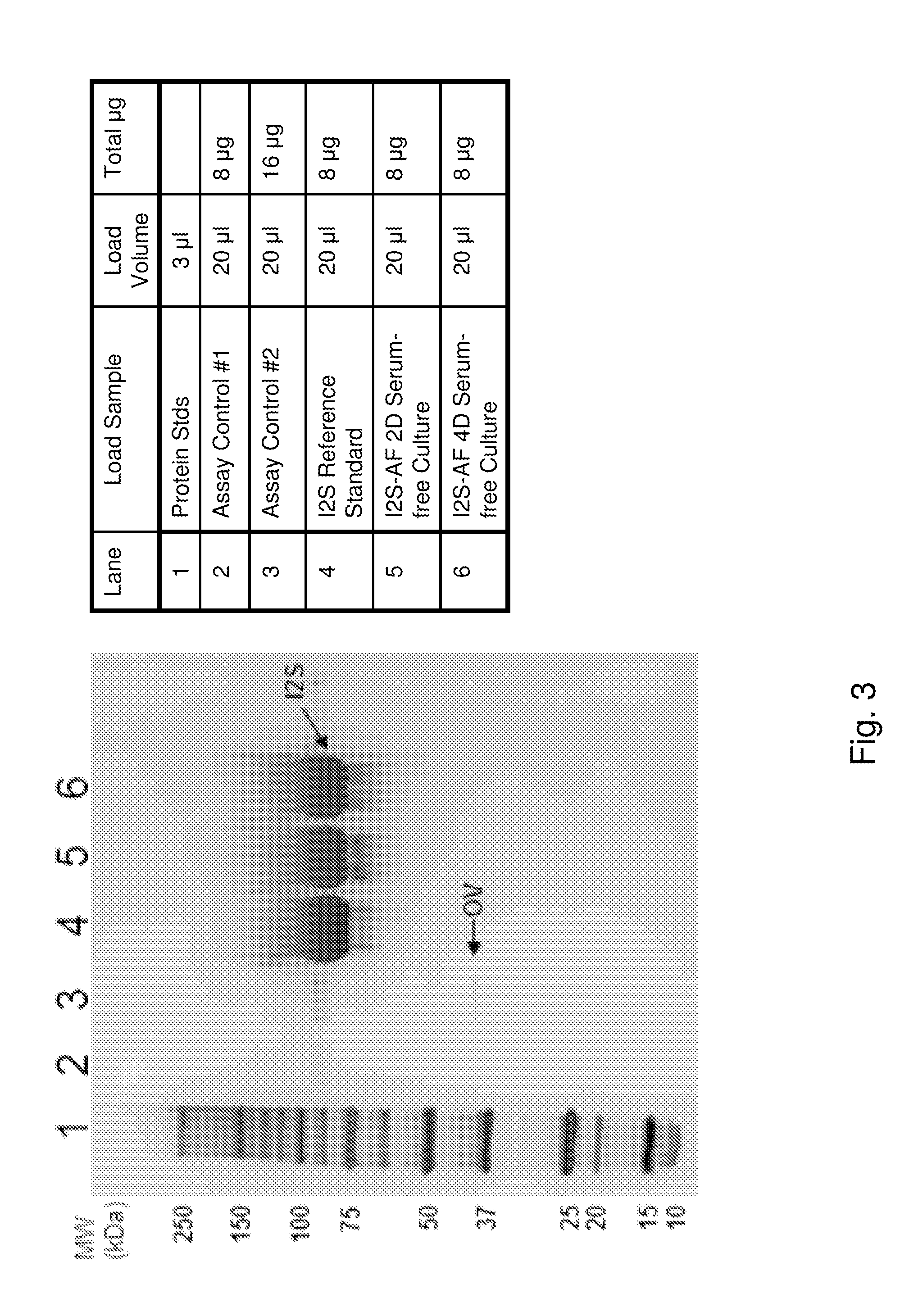
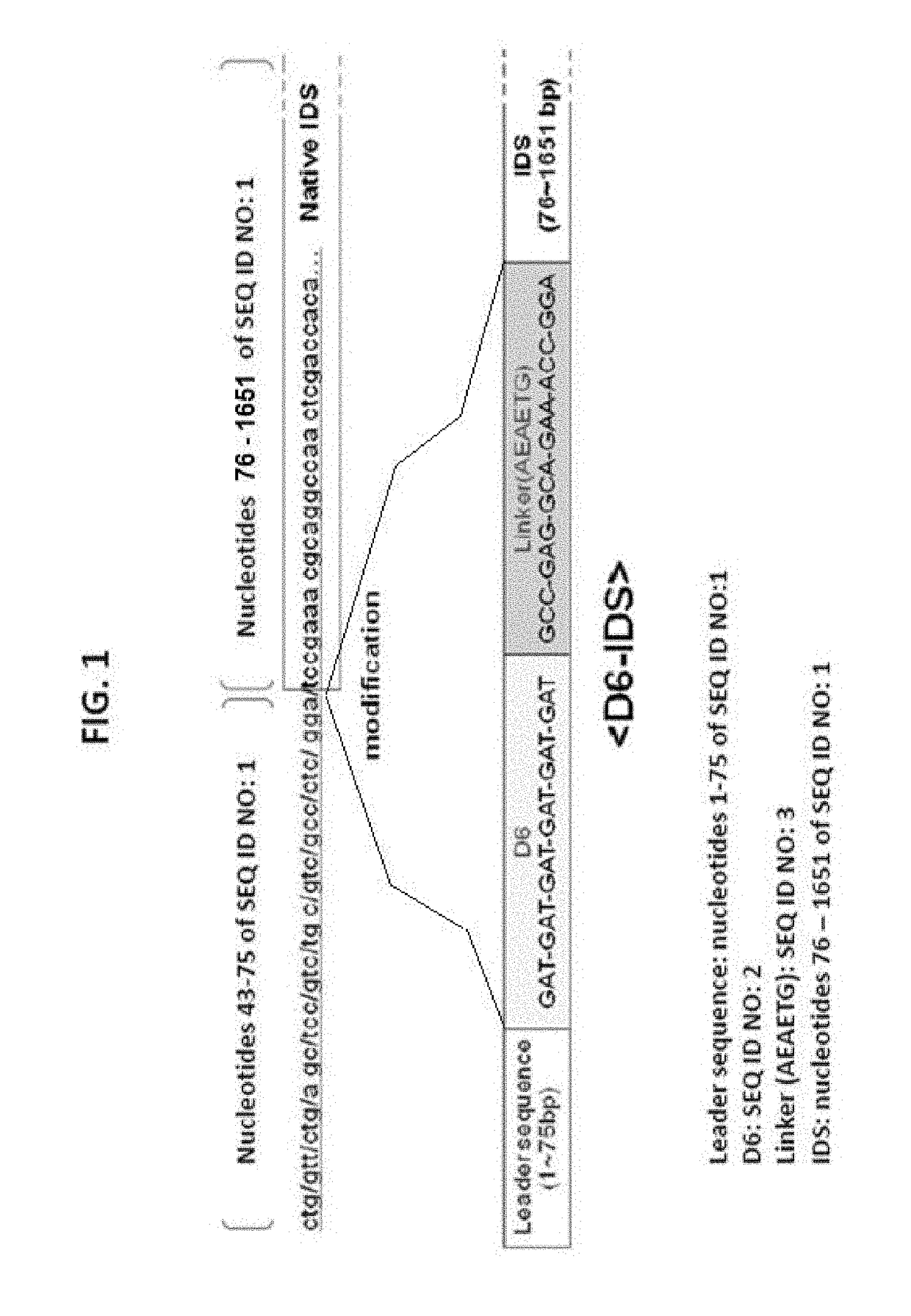
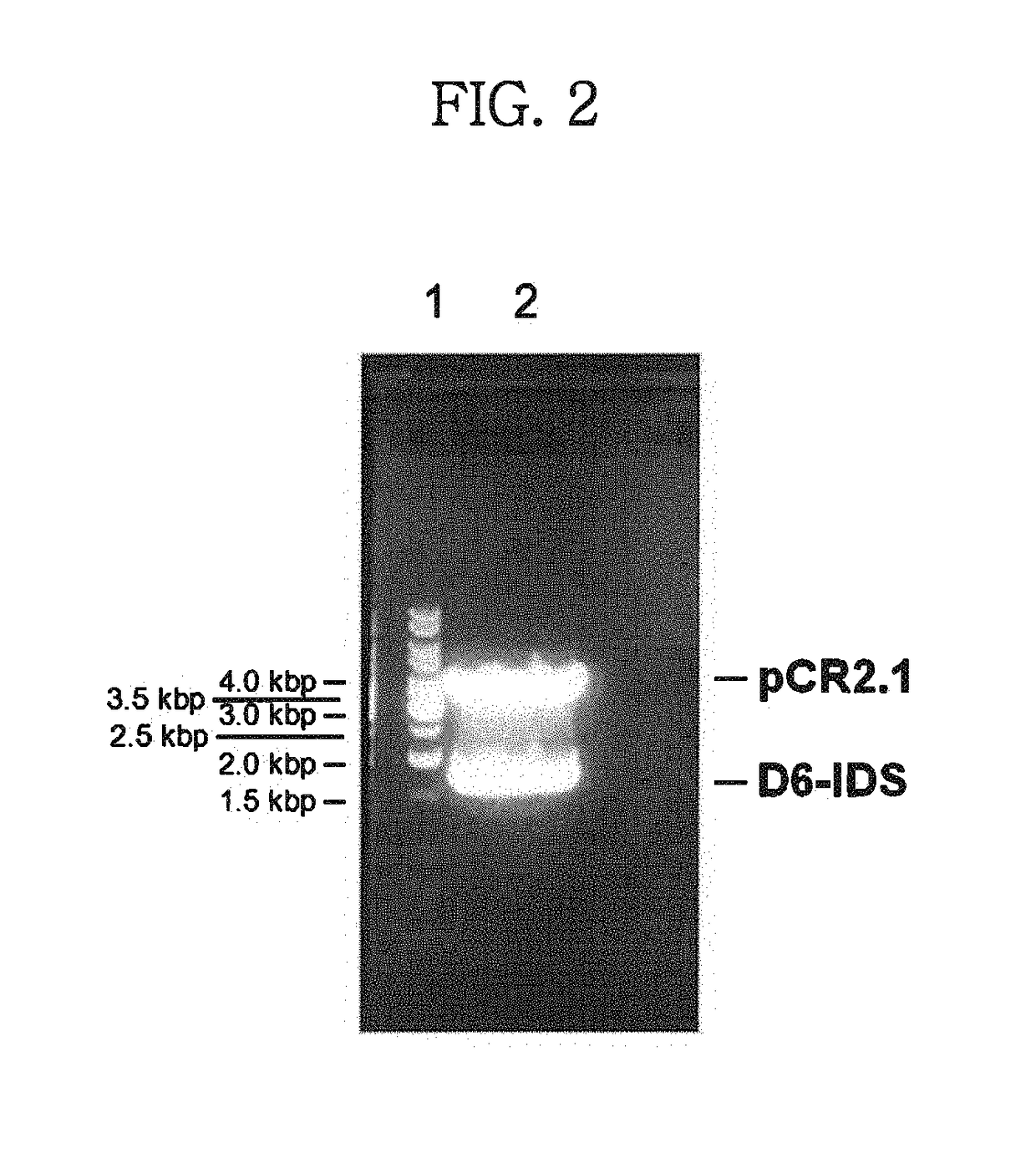
Disclosed is a modified iduronate-2-sulfatase (IDS) gene constructed by inserting the nucleotide of SEQ ID NO: 2 into a wild-type IDS gene. In addition to being negatively charged, the improved IDS enzyme encoded by the modified gene exhibits a sufficient retention time in blood to target the bone, so that it is more effective for treating Hunter syndrome.
Owner:THE GREEN CROSS CORP
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS20140271598A1Effective and less approachEffectively and extensivelySenses disorderNervous disorderIduronate-2-sulfataseHunter syndrome
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for increasing iduronate 2-sulfatase activity in the CNS
ActiveUS8834874B2Nervous disorderPeptide/protein ingredientsIduronate-2-sulfataseIduronate-2-sulfatase activity
Provided herein are methods and compositions for treating a subject suffering from a deficiency in iduronate 2-sulfatase in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody that crosses the blood brain barrier (BBB) and an iduronate 2-sulfatase.
Owner:JCR PHARMA +1
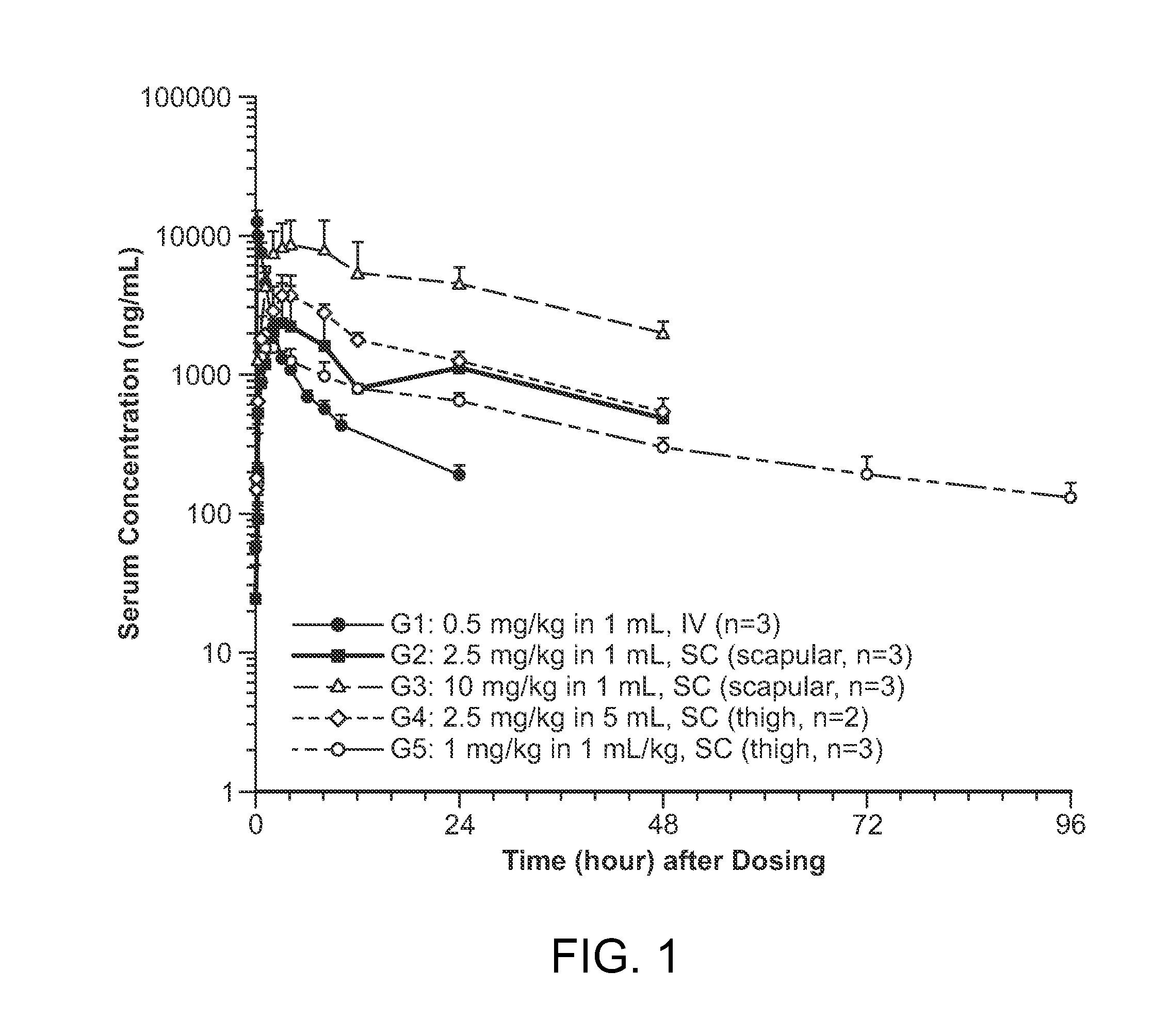
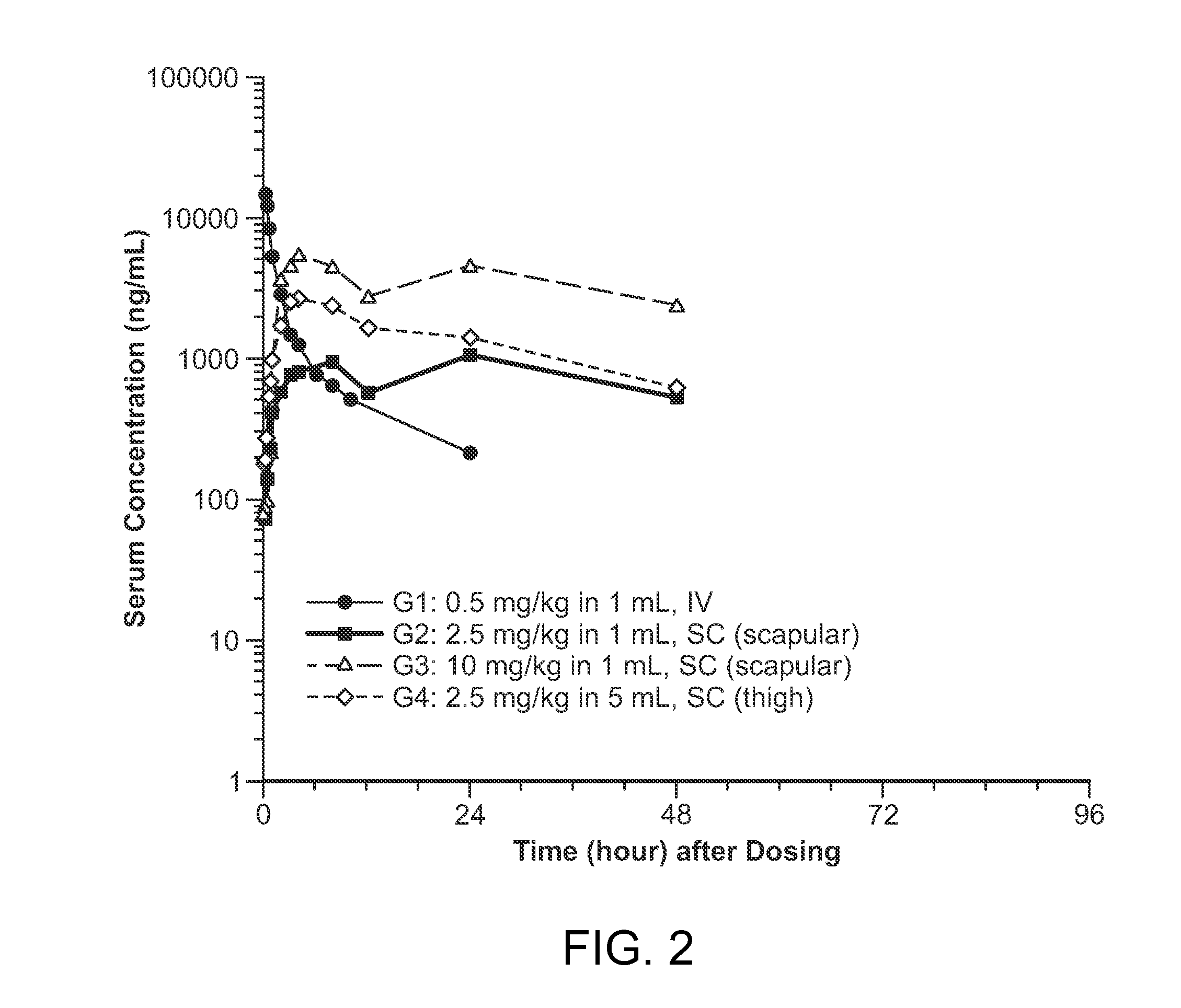
Subcutaneous administration of iduronate- 2-sulfatase
InactiveUS20150086526A1Improved enzyme replacement therapyImprove bioavailabilityNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseLysosome
The present invention provides, among other things, compositions, kits and methods for subcutaneous delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention provides methods for treating Hunter syndrome by subcutaneous administration of a replacement iduronate-2-sulfatase (I2S) protein. In some embodiments, the present invention provides a kit comprising an arrangement of components for subcutaneously administering iduronate-2-sulfatase (I2S) protein.
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Methods and compositions for CNS delivery of iduronate-2-sulfatase
ActiveUS9220677B2Effective and less invasiveEffectively and extensivelyPowder deliverySenses disorderIduronate-2-sulfataseHunter syndrome
The present invention provides, among other things, compositions and methods for CNS delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention includes a stable formulation for direct CNS intrathecal administration comprising an iduronate-2-sulfatase (I2S) protein, salt, and a polysorbate surfactant for the treatment of Hunters Syndrome.
Owner:TAKEDA PHARMA CO LTD
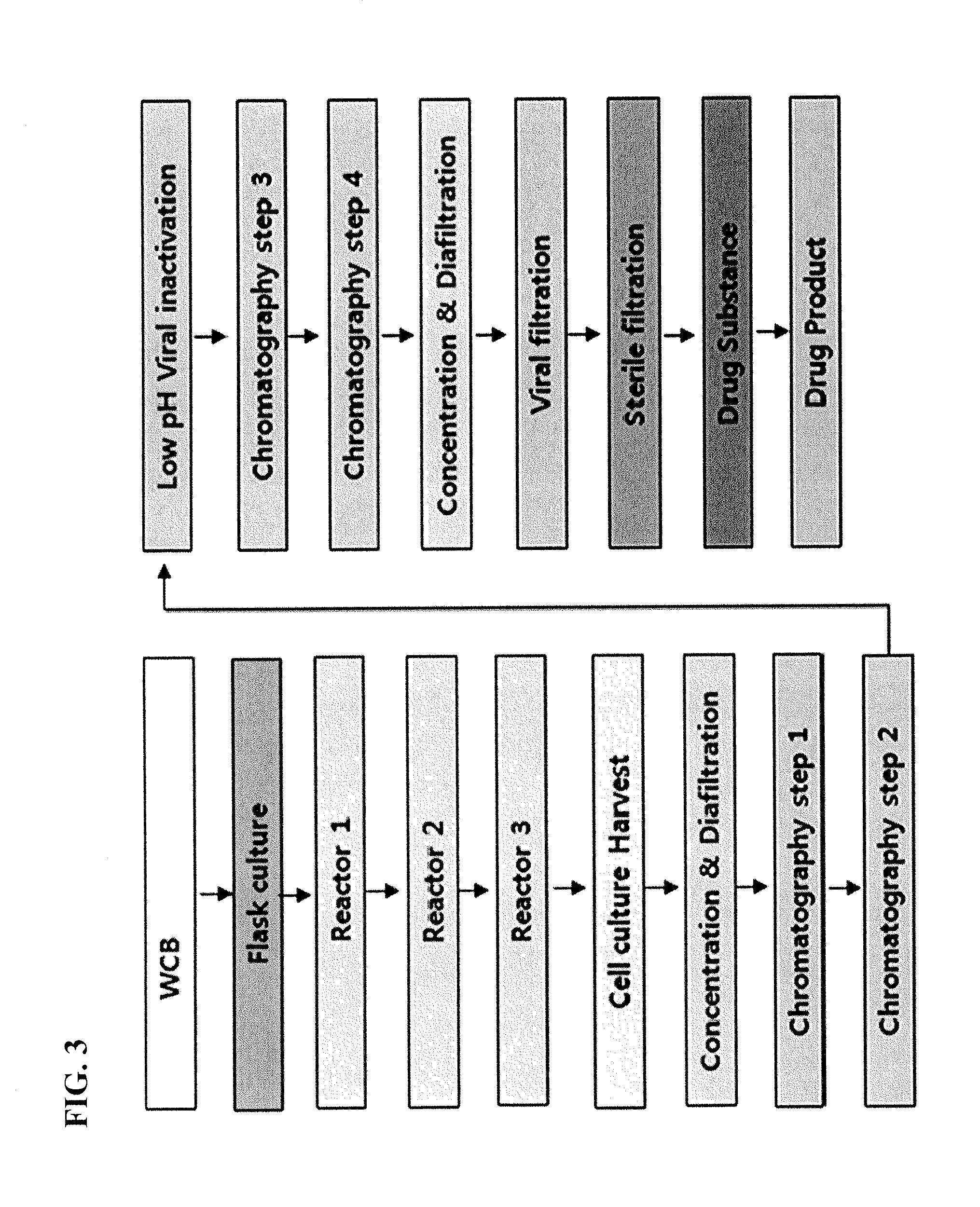
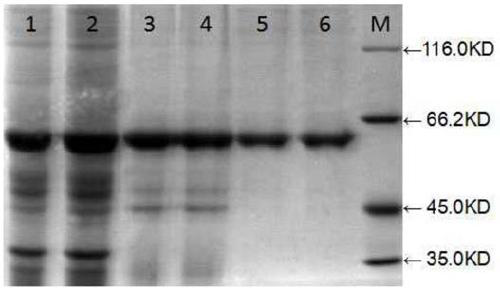
Purification of iduronate-2-sulfatase
ActiveUS9051556B2Improve bioavailabilityEffective and cheap and fast processNervous disorderBacteriaIduronate-2-sulfataseCell culture media
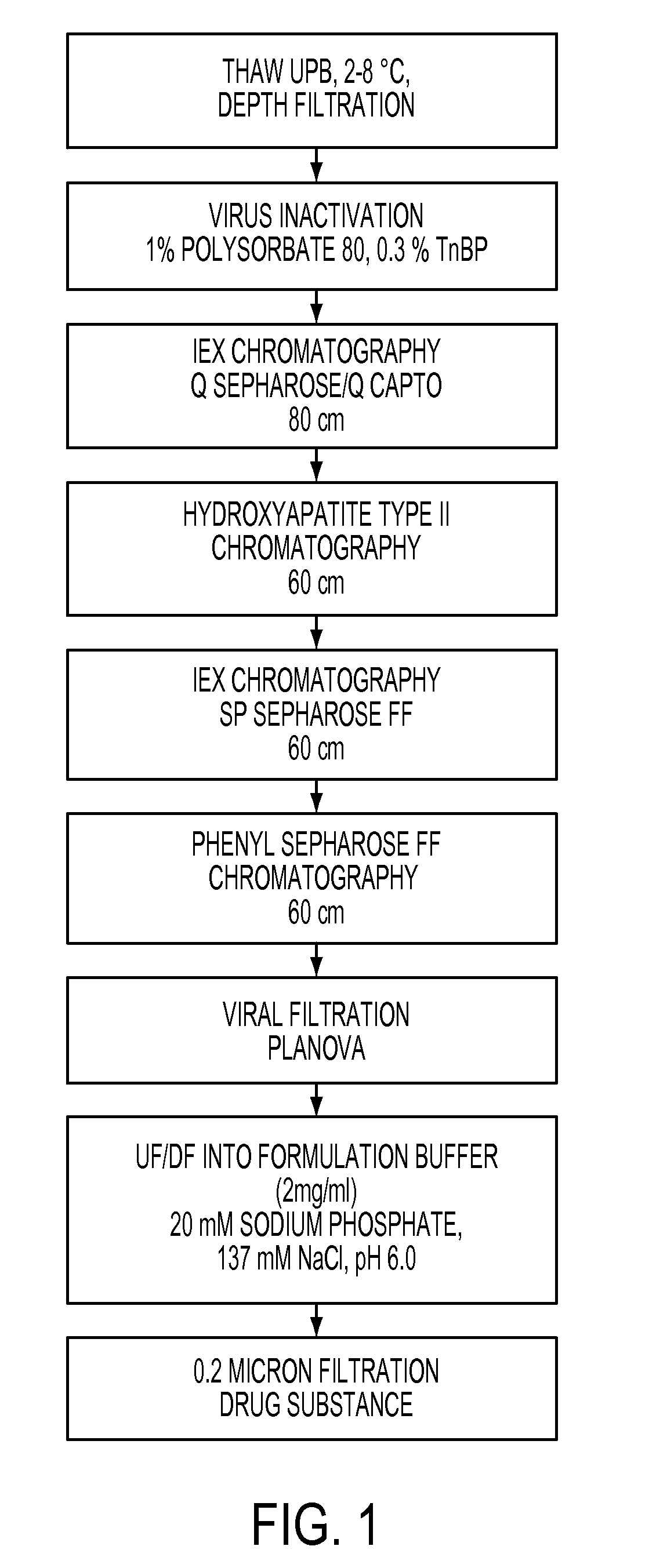
The present invention provides, among other things, improved methods for purifying I2S protein produced recombinantly for enzyme replacement therapy. The present invention is, in part, based on the surprising discovery that recombinant I2S protein can be purified from unprocessed biological materials, such as, I2S-containing cell culture medium, using a process involving as few as four chromatography columns.
Owner:TAKEDA PHARMA CO LTD
Composition and formulation comprising recombinant human iduronate-2-sulfatase and preparation method thereof
ActiveUS20140242059A1Superior in pharmaceutical efficacyImprove securityNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseHunter syndrome
Disclosed is a composition comprising recombinant iduronate-2-sulfatase (IDS). The glycosylation pattern and formylglycine content of the IDS composition are different from those of Elaprase and have superior pharmaceutical efficacy and are safer than the conventional agent and thus can be effectively used for the therapy of Hunter syndrome.
Owner:MEDIGENEBIO CORP +1
Glycosylation variants of iduronate 2-sulfatase
The present invention provides a highly glycosylated iduronate-2-sulfatase enzyme comprising an iduronate-2-sulfatase polypeptide with at least 5 kilodalton (kDa) more sugar than iduronate-2-sulfatase purified from a natural source, e.g. human liver. The present invention also provides an enzymatically active polypeptide fragment or variant of such a highly glycosylated iduronate-2-sulfatase. The present invention further provides an isolated nucleic acid encoding iduronate-2-sulfatase, as well as an expression vector, a host cell and a method for producing the present highly glycosylated iduronate-2-sulfatase enzyme. In one embodiment the present invention is directed to a method for producing a glycosylated iduronate-2-sulfatase enzyme which comprises culturing a host cell containing a nucleic acid encoding an enzymatically active iduronate-2-sulfatase polypeptide wherein the host cell glycosylates the polypeptide to a greater degree than a native iduronate-2-sulfatase polypeptide expressed by a natural human liver cell.
Owner:WOMENS & CHILDRENS HOSPITAL
Methods for sterilizing preparations of digestive enzymes
InactiveUS20060115376A1Effective sterilizationAvoid radiationPowder deliverySenses disorderIduronate-2-sulfataseFungal microorganisms
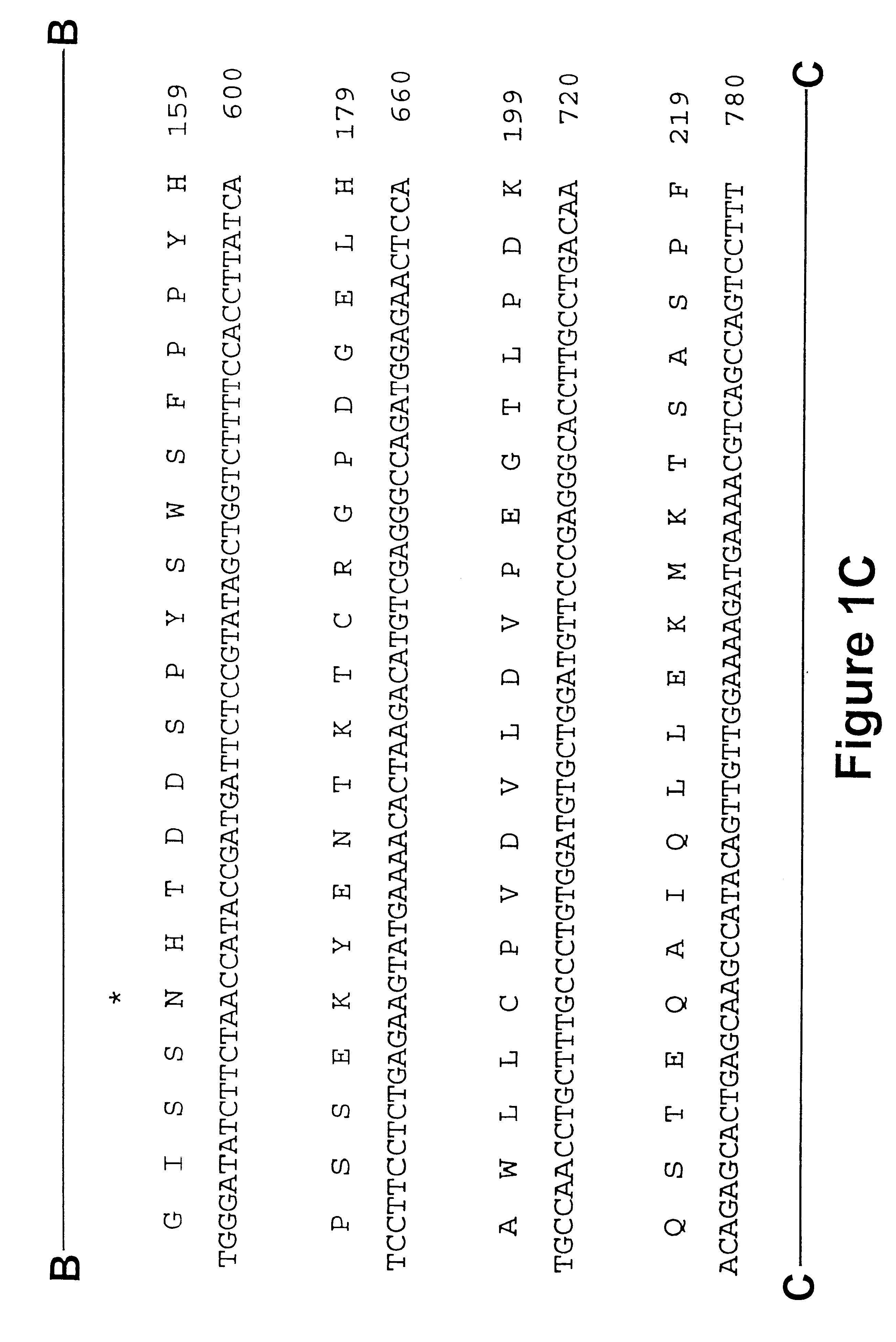
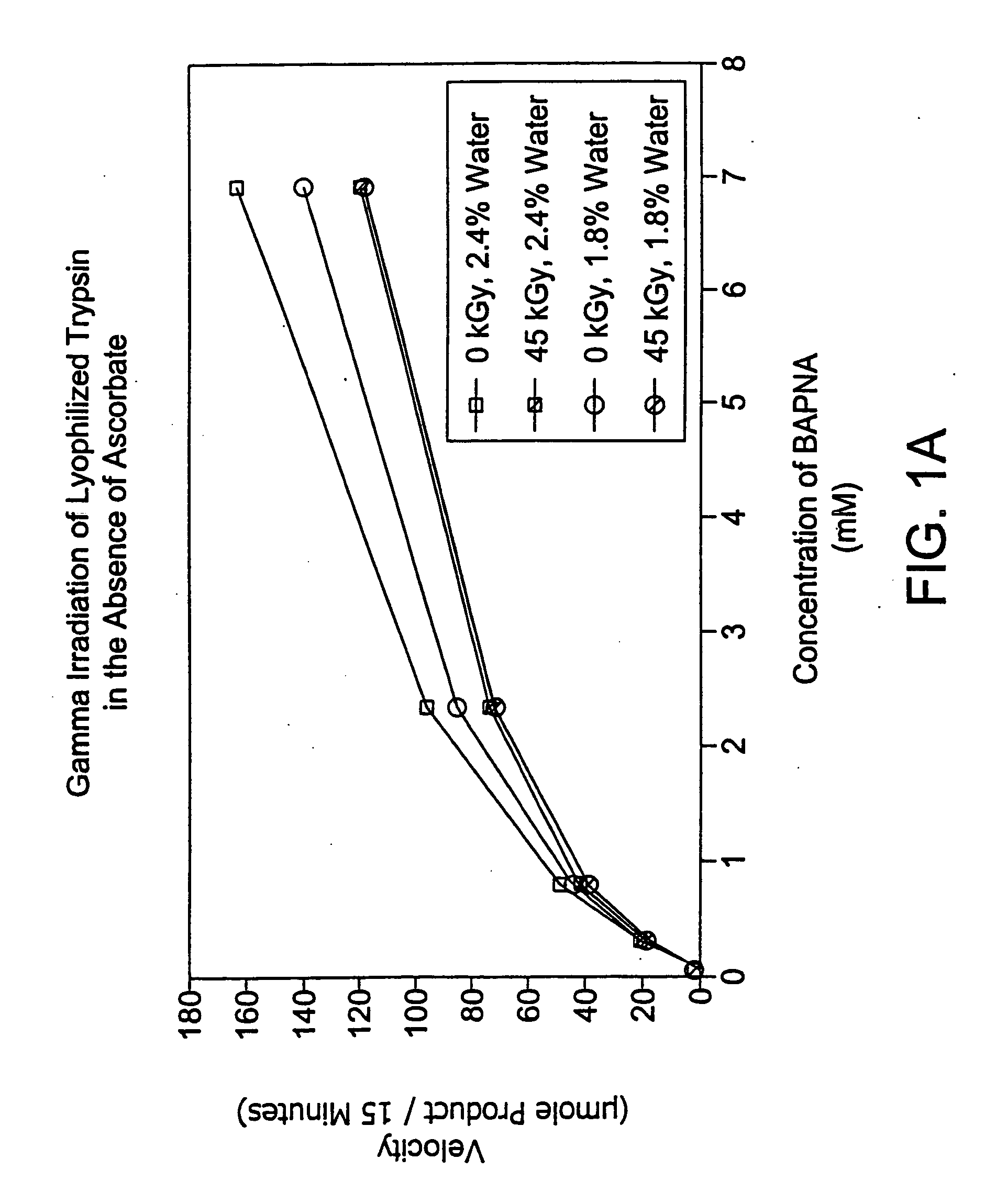
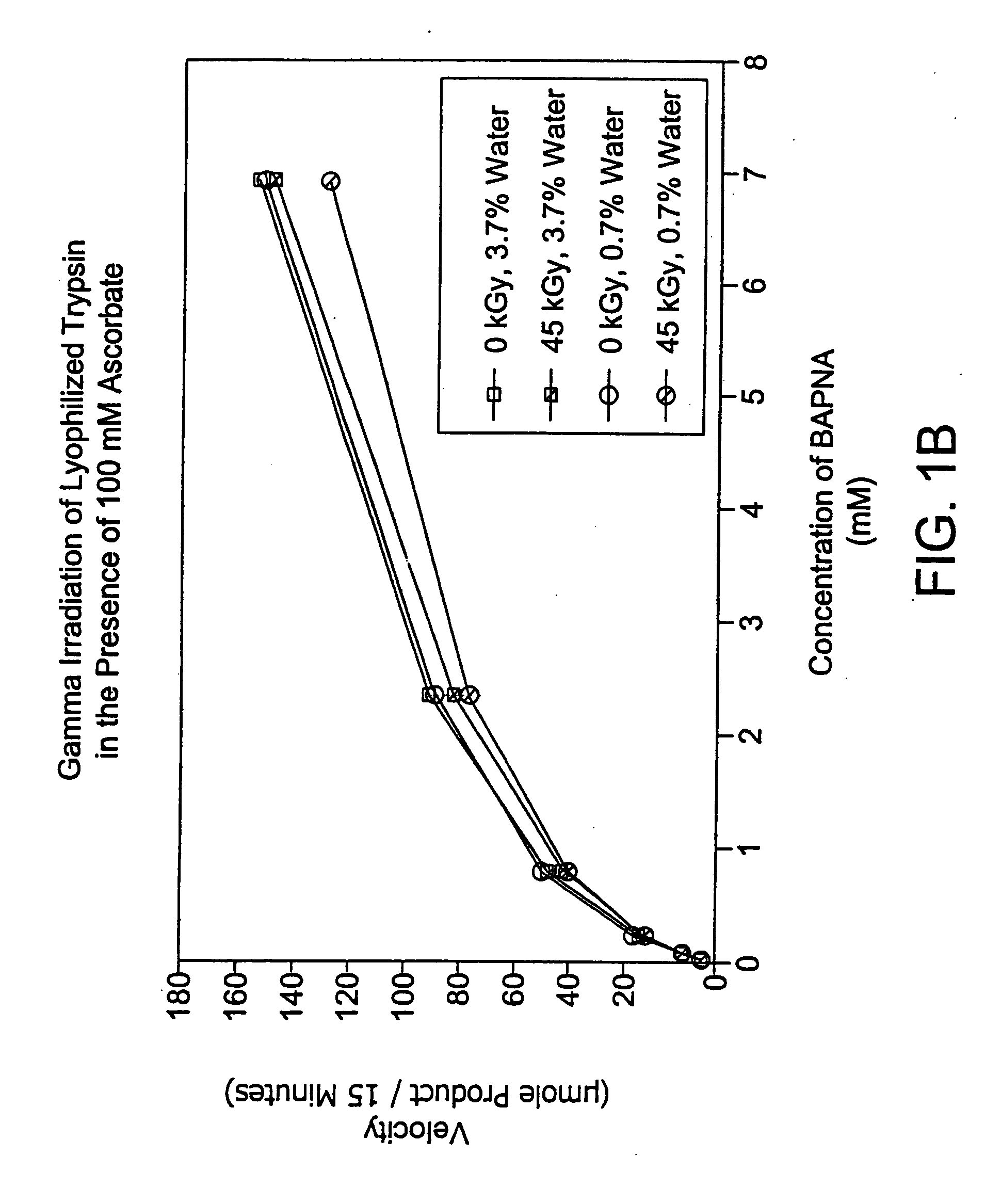
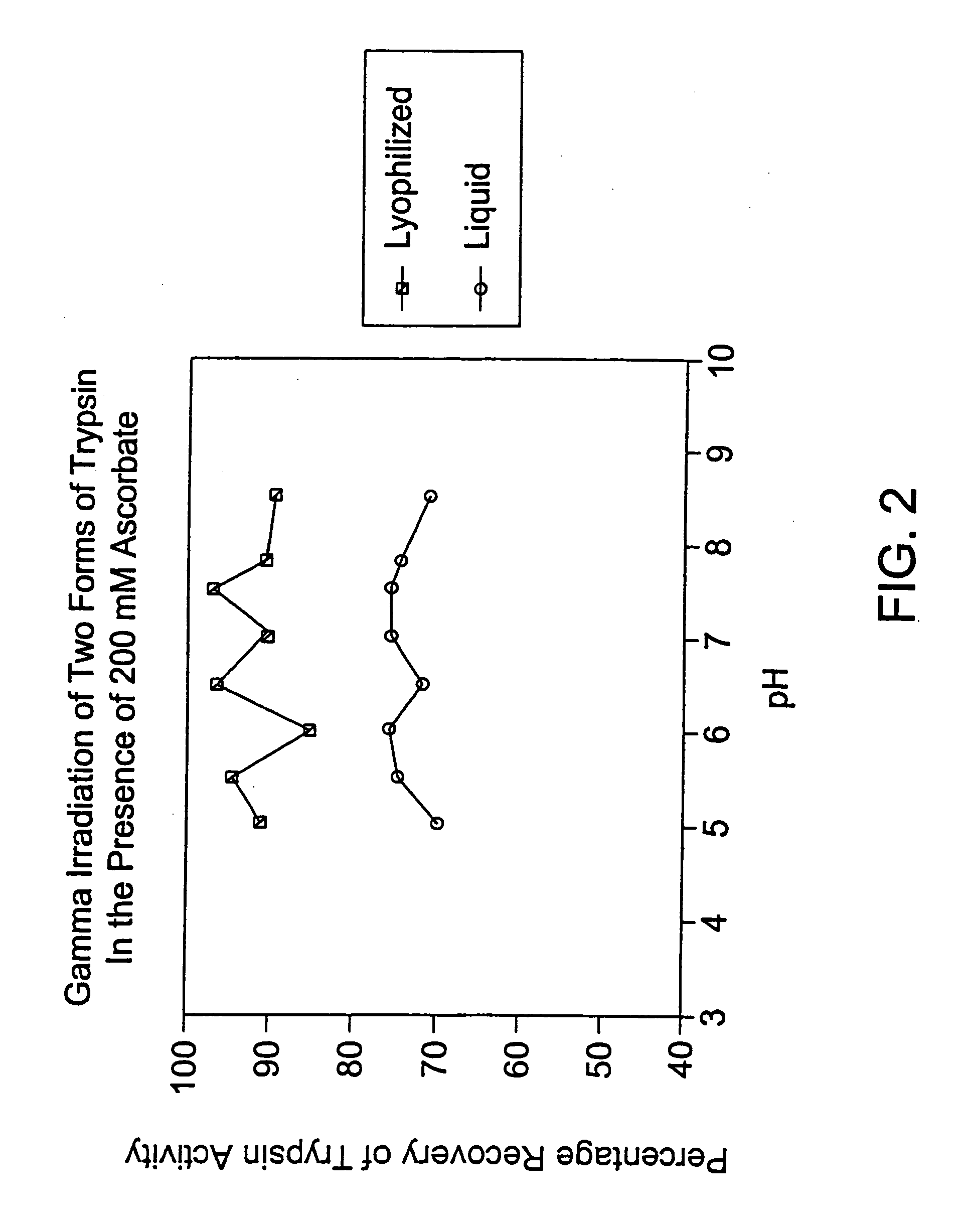
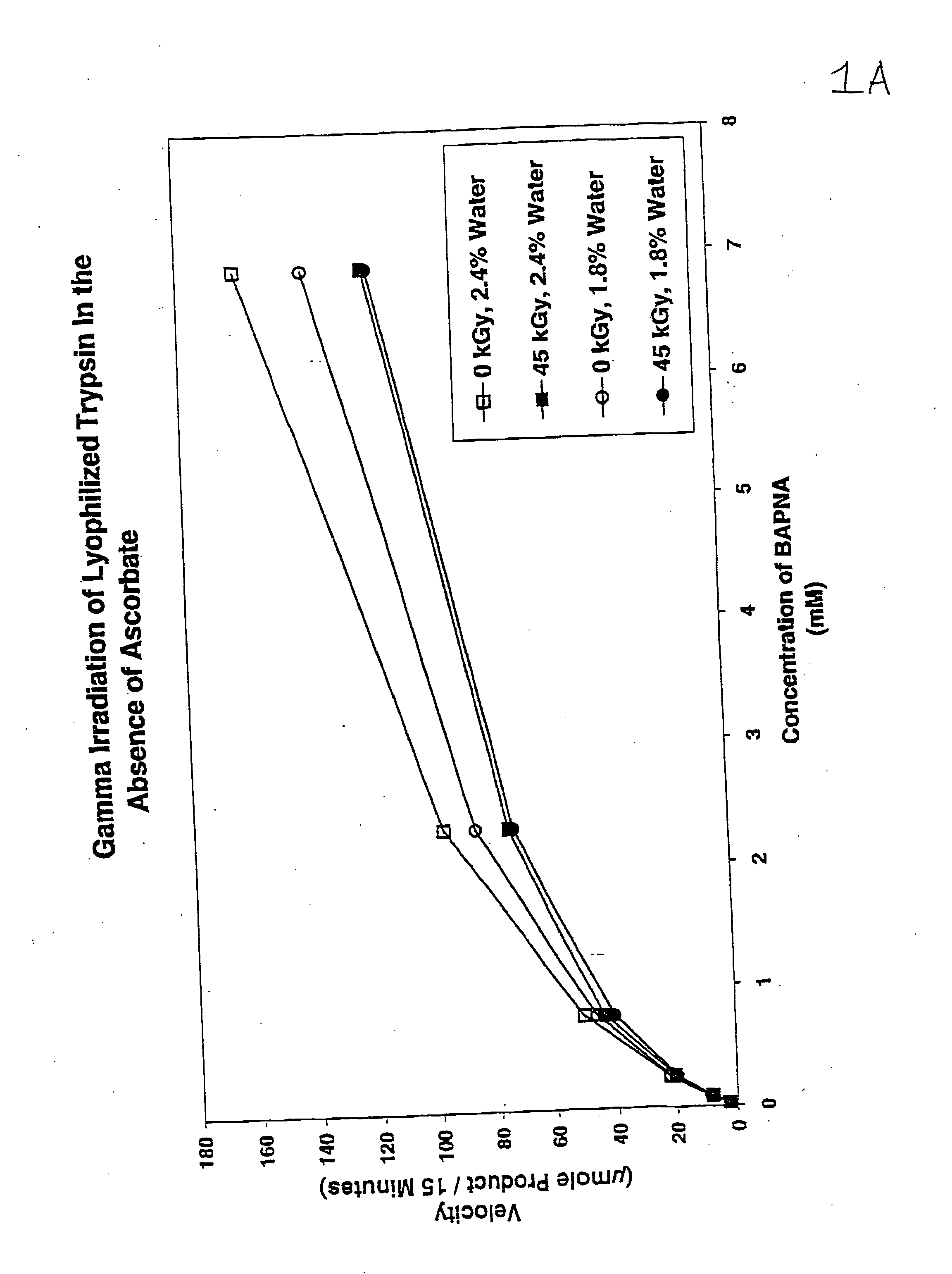
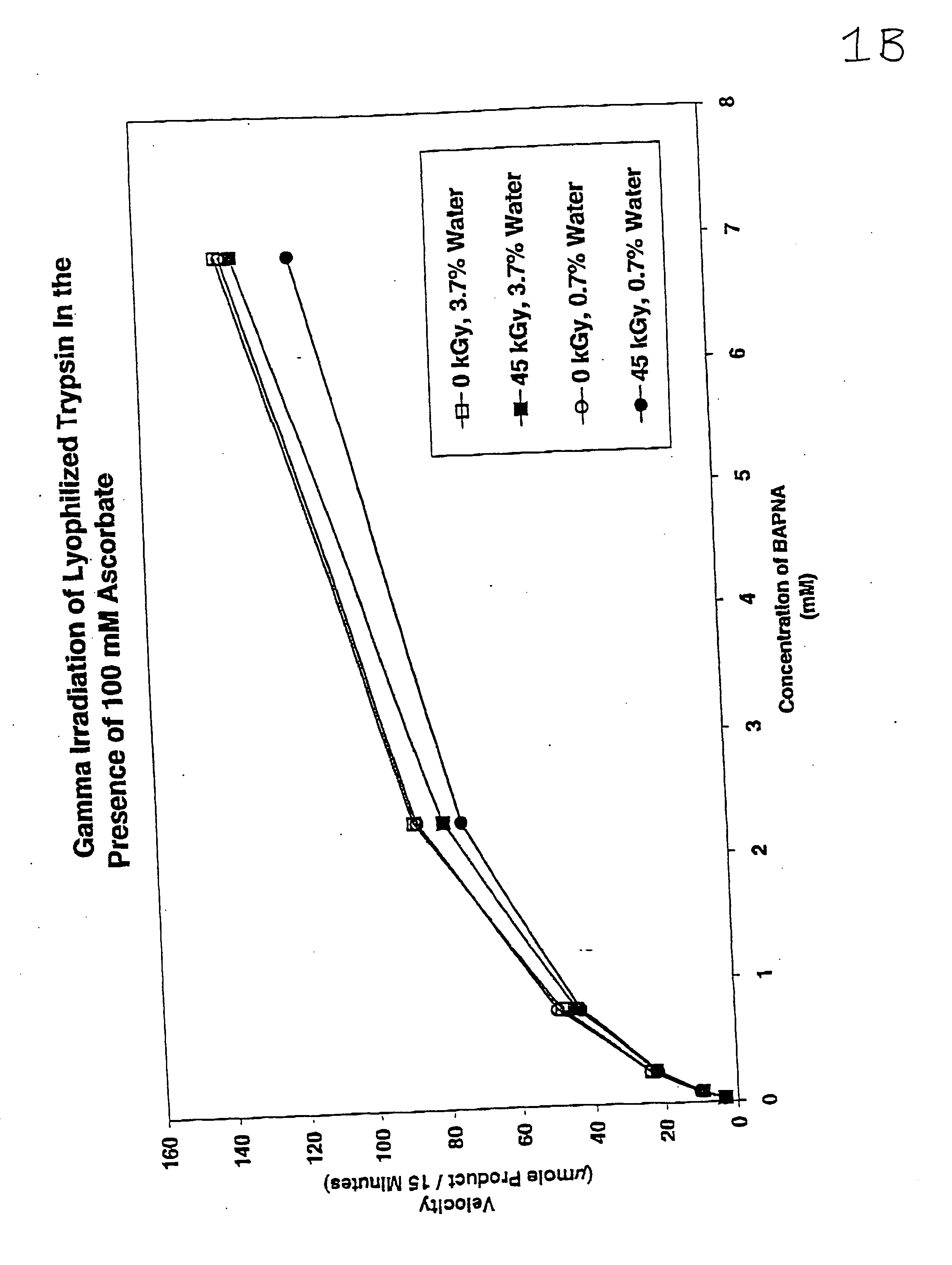
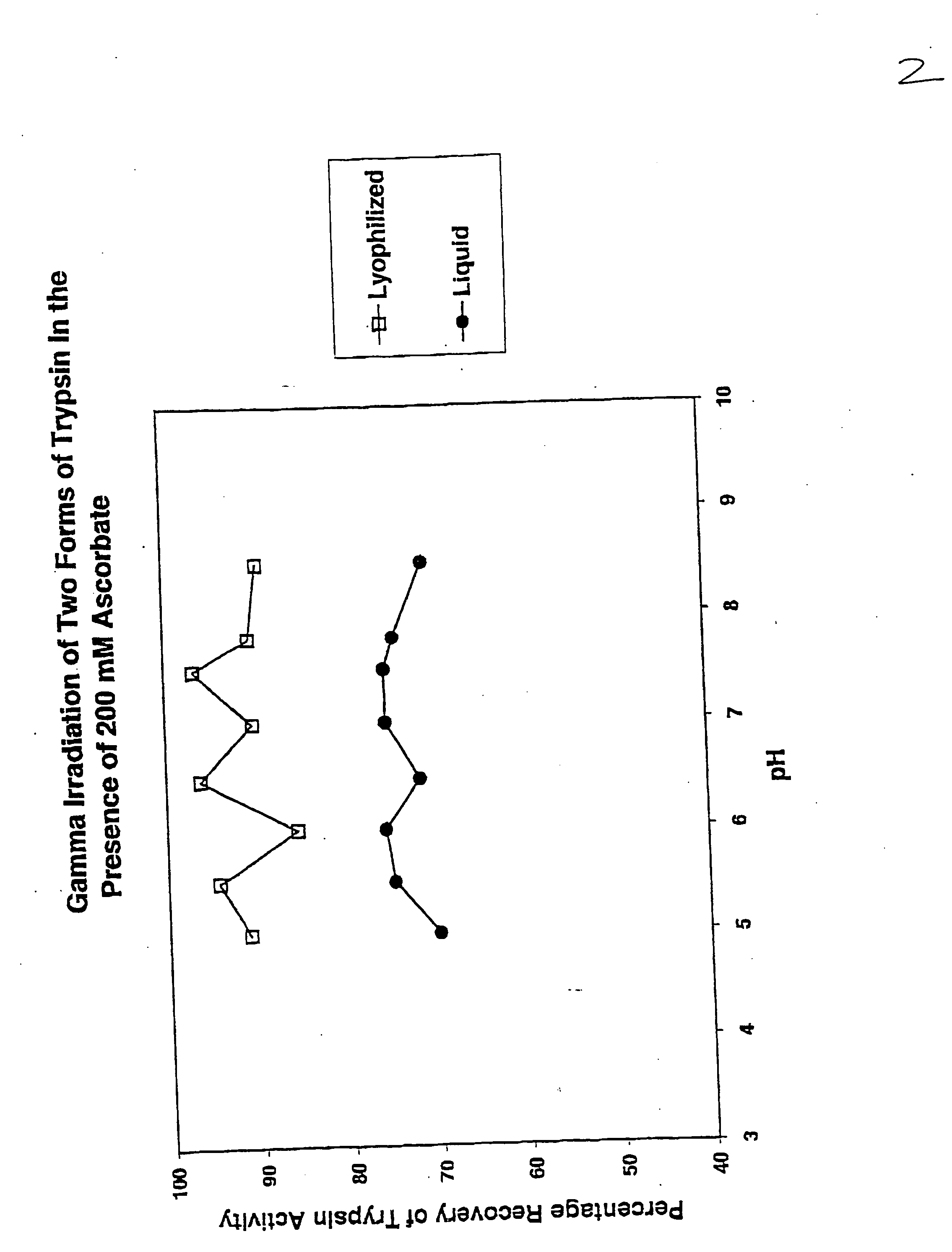
Methods are disclosed for sterilizing preparations of digestive enzymes to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. These methods involve sterilizing preparations of digestive enzymes, such as trypsin, α-galactosidase and iduronate-2-sulfatase, with irradiation.
Owner:CLEARANT
Dephosphorylated lysosomal storage disease proteins and methods of use thereof
Provided are substantially dephosphorylated forms of lysosomal storage disease (LSD) proteins, including dephosphorylated forms of iduronate-2-sulfatase (IDS, or I2D) and iduronidase (IDU), having increased ability to traverse or penetrate the blood brain barrier (BBB) relative to phosphorylated forms of the protein, and p97 conjugates thereof. Also provided are compositions comprising such dephosphorylated LSD proteins and p97 conjugates, and methods of use thereof, for instance, to treat any one or more lysosomal storage diseases, such as Hunter Syndrome (or MPS Type II).
Owner:BIOASIS TECH
Purification of iduronate-2-sulfatase
ActiveUS20140004096A1Improve bioavailabilityEffective and cheap and fast processNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseCell culture media
The present invention provides, among other things, improved methods for purifying I2S protein produced recombinantly for enzyme replacement therapy. The present invention is, in part, based on the surprising discovery that recombinant I2S protein can be purified from unprocessed biological materials, such as, I2S-containing cell culture medium, using a process involving as few as four chromatography columns.
Owner:TAKEDA PHARMA CO LTD
Cells for producing recombinant iduronate-2-sulfatase
ActiveUS9150841B2Effective enzyme replacement therapyImprove the level ofPeptide/protein ingredientsHydrolasesIduronate-2-sulfataseSerum free
The present invention provides, among other things, methods and compositions for production of recombinant I2S protein with improved potency and activity using cells co-express I2S and FGE protein. In some embodiments, cells according to the present invention are engineered to simultaneously over-express recombinant I2S and FGE proteins. Cells according to the invention are adaptable to various cell culture conditions. In some embodiments, cells of the present invention adaptable to a large-scale suspension serum-free culture.
Owner:TAKEDA PHARMA CO LTD
Composition and formulation comprising recombinant human iduronate-2-sulfatase and preparation method thereof
ActiveUS20150320843A1Improve efficacyImprove securityNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseHunter syndrome
Disclosed are a composition comprising recombinant iduronate-2-sulfatase (IDS) and a method for treating Hunter syndrome. The glycosylation pattern and formylglycine content of the IDS composition are different from those of ELAPRASE® and have superior pharmaceutical efficacy and are safer than the conventional agent and thus can be effectively used for the therapy of Hunter syndrome.
Owner:THE GREEN CROSS CORP +1
Method for production of recombinant human iduronate 2-sulfatase
ActiveUS9279109B2Eliminate riskReduce usageHydrolasesMetabolism disorderIduronate-2-sulfatasePhosphate
Disclosed is a method for production of recombinant human iduronate 2-sulfatase (rhI2S) (the 26th to 550th amino acids of SEQ ID NO: 10) in a large scale, with a high purity, and mannose 6-phosphate residues. The method comprises the steps of (a) culturing rhI2S (the 26th to 550th amino acids of SEQ ID NO: 10)-producing mammalian cells in a serum-free medium, (b) collecting culture supernatant, (c) subjecting the culture supernatant to cation-exchange column chromatography, (d) to dye affinity column chromatography, (e) to anion-exchange column chromatography, and (f) to a column chromatography employing as solid phase a material having affinity for phosphate group, and (g) to gel filtration column chromatography, in the order.
Owner:JCR PHARMA
Production method of escherichia coli expressed human iduronate-2-sulfatase
InactiveCN109022389ASimplify the washing stepsImprove efficiencyHydrolasesMicroorganism based processesIduronate-2-sulfataseEscherichia coli
The invention discloses a production method of escherichia coli expressed human iduronate-2-sulfatase. The method employs a 5L fermentation tank of escherichia coli recombinant bacteria for high-density culture and induction expression, the fermentation broth subjected to induction expression is centrifuged to collect thalli, high-pressure homogenization technique is employed to crush the recombinant bacteria to obtain a recombinant protein inclusion body, the recombinant protein inclusion body is dissolved and is then loaded to a Ni column for affinity chromatography purification, and after desalination and freeze-drying, the recombinant human iduronate-2-sulfatase can be obtained. The method provided by the invention simplifies the tedious washing steps of the inclusion body, explores appropriate ultrasonic conditions to transform insoluble inclusion body protein into soluble protein, avoids the denaturation and renaturation operations on the inclusion body brought about by use of 8Murea, 6M guanidine hydrochloride or other strong denaturing agents, and can achieve a protein purity of 95% or more and a recovery efficiency of 70% or above by Ni column affinity chromatography, thus reaching the effect of simple, effective and rapid purification.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Subcutaneous administration of iduronate-2-sulfatase
InactiveUS9603908B2Pharmacokinetic parameterEfficient deliveryNervous disorderPeptide/protein ingredientsIduronate-2-sulfataseLysosome
The present invention provides, among other things, compositions, kits and methods for subcutaneous delivery of lysosomal enzymes for effective treatment of lysosomal storage diseases. In some embodiments, the present invention provides methods for treating Hunter syndrome by subcutaneous administration of a replacement iduronate-2-sulfatase (I2S) protein. In some embodiments, the present invention provides a kit comprising an arrangement of components for subcutaneously administering iduronate-2-sulfatase (I2S) protein.
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Targeted iduronate-2-sulfatase compounds
InactiveUS20150290341A1Treat symptomsEfficient transportPolypeptide with localisation/targeting motifPeptide/protein ingredientsIduronate-2-sulfataseChemical reaction
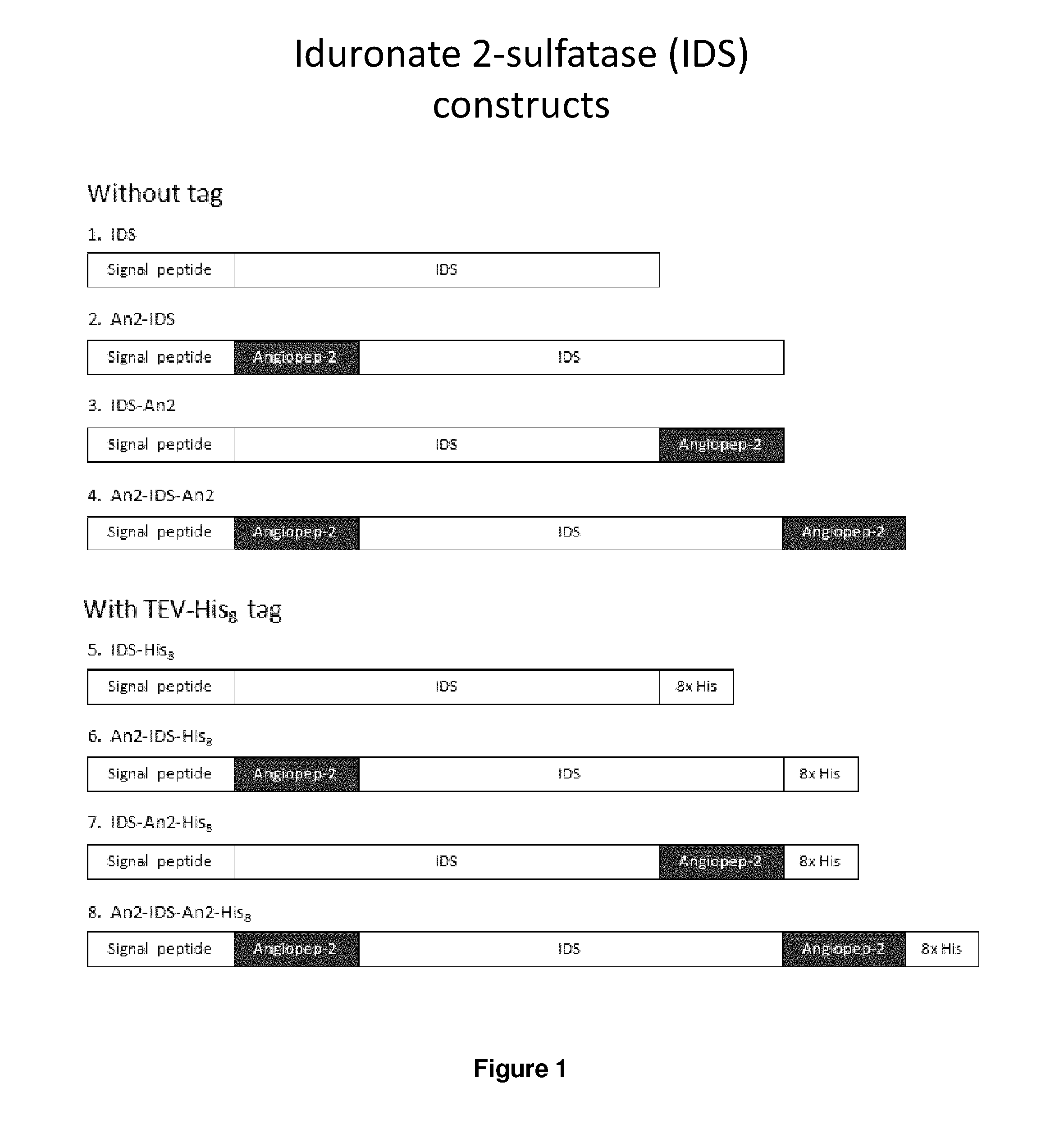
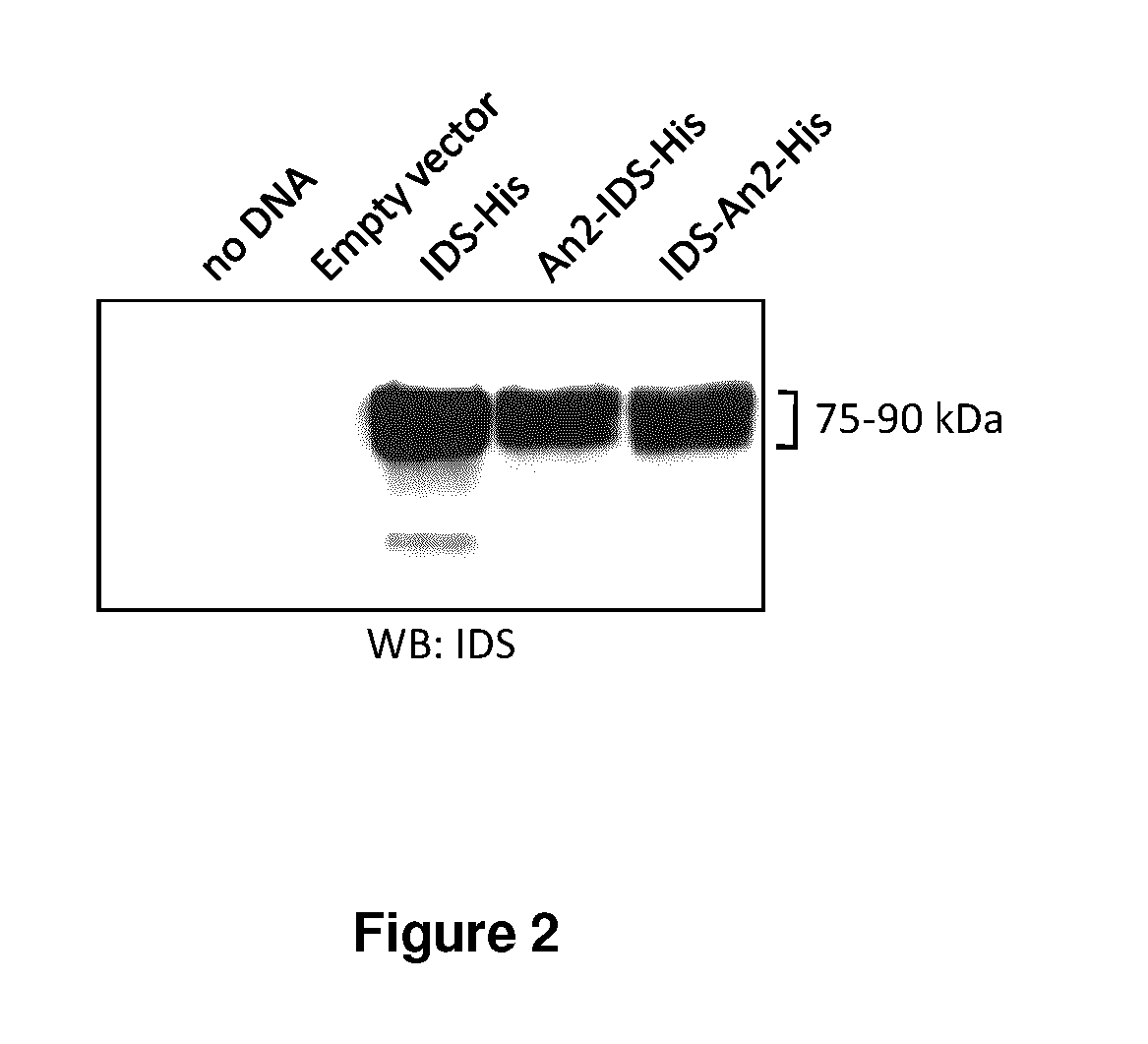
The present invention is related to a compound that includes a lysosomal enzyme and a targeting moiety, for example, a compound that includes iduronate-2-sulfatase conjugated to Angiopep-2 through a linker formed by specific click chemistry reactions. In certain embodiments, these compounds, owing to the presence of the targeting moiety, can cross the blood-brain barrier or accumulate in the lysosome more effectively than the enzyme alone. The invention also features pharmaceutical compositions containing such compounds and methods for treating lysosomal storage disorders (e.g., mucopolysaccharidosis Type II) using such compounds.
Owner:ANGLACHEM INC
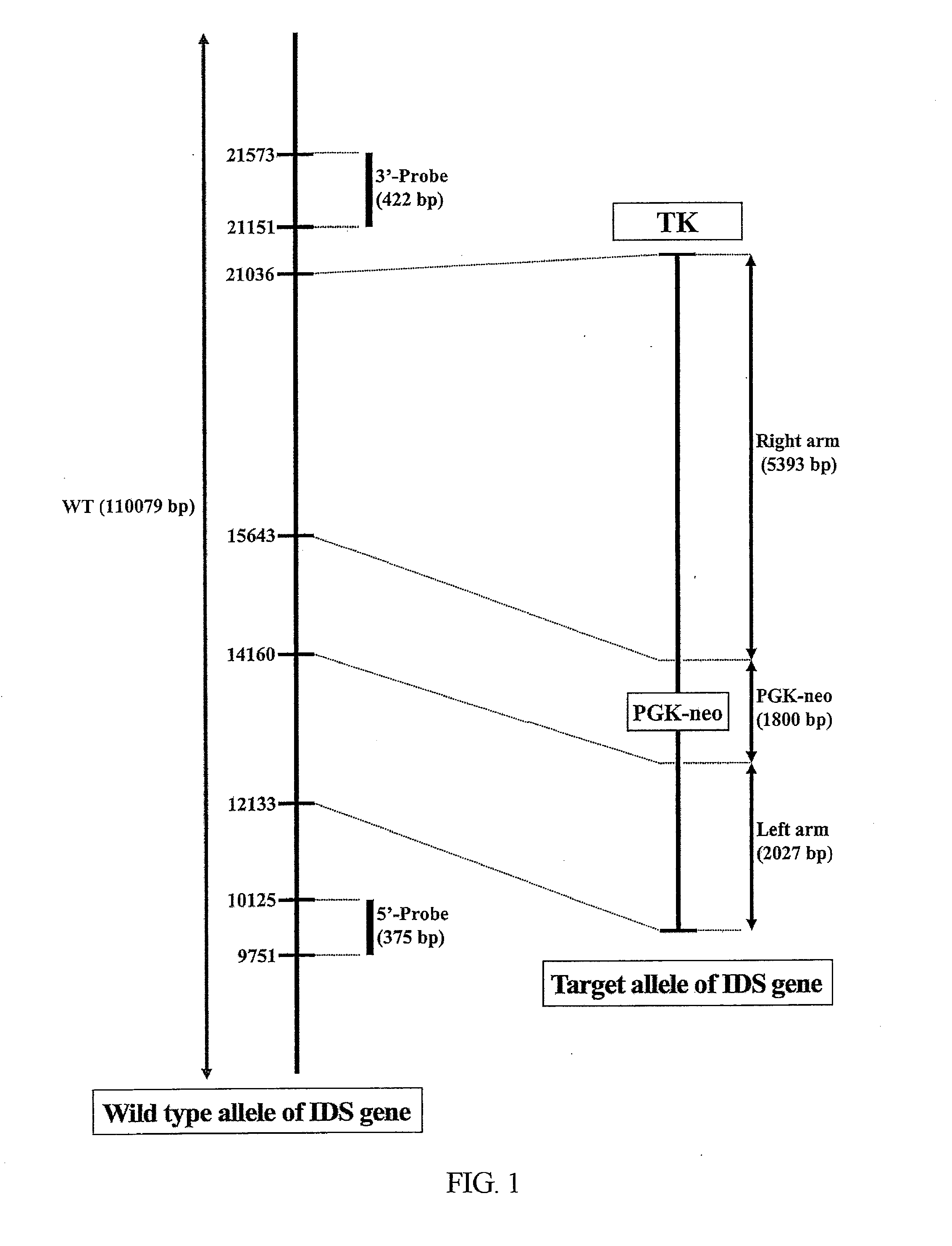
Transgenic mouse model for developing enzyme replacement therapy for iduronate-2-sulfatase deficiency syndrome
InactiveUS20120180148A1Improve adverse effectsMaximum efficacyAnimal husbandryIduronate-2-sulfataseRegimen
The present invention relates to a transgenic mouse model for developing enzyme replacement therapy for iduronate-2-sulfatase deficiency syndrome, for example, Hunter syndrome. More specifically, the present invention relates to a transgenic mouse to be used for developing enzyme replacement therapy for iduronate-2-sulfatase, wherein the immune response against injected enzyme, such as, recombinant iduronate-2-sulfatase has been minimized in transgenic mouse model in the course of treating in vivo iduronate-2-sulfatase replacement.
Owner:MEDIGENEBIO CORP
Targeted lysosomal enzyme compounds
InactiveCN104145015APolypeptide with localisation/targeting motifPowder deliveryIduronate-2-sulfataseLysosome
The present invention is related to a compound that includes a lysosomal enzyme and a targeting moiety, for example, where compound is a fusion protein including iduronate-2-sulfatase and Angiopep-2. In certain embodiments, these compounds, owning to the presence of the targeting moiety can crossing the blood-brain barrier or accumulate in the lysosome more effectively than the enzyme alone. The invention also features methods for treating lysosomal storage disorders (e.g., mucopolysaccharidosis Type II) using such compounds.
Owner:ANGLACHEM INC
Composition and formulation comprising recombinant human iduronate-2-sulfatase and preparation method thereof
InactiveUS20160145589A1Improve efficacyImprove securityHydrolasesPeptide/protein ingredientsIduronate-2-sulfataseMedicine
A composition comprising recombinant iduronate-2-sulfatase (IDS) and a method for producing a purified recombinant IDS are provided. The glycosylation pattern and formylglycine content of the IDS composition are different from those of ELAPRASE® and have superior pharmaceutical efficacy and are safer than the conventional agent and thus can be effectively used for the therapy of Hunter syndrome.
Owner:THE GREEN CROSS CORP +1
Lyophilized Preparation
ActiveUS20190336586A1Improve expression efficiencyHigh expressionPowder deliveryNervous disorderIduronate-2-sulfataseLysosome
[Problem] To provide a pharmaceutical composition containing a fusion protein comprising an antibody and a lysosomal enzyme as an active ingredient, which is stable enough to permit its distribution to the market.[Solution] A lyophilized formulation containing; a fusion protein comprising an antibody and a lysosomal enzyme as an active ingredient, and further containing a neutral salt, a disaccharide, a nonionic surfactant, and a buffer. Such a lyophilized formulation includes, for example, as an active ingredient, a fusion protein comprising an anti-transferrin receptor antibody and human iduronate-2-sulfatase, and further containing sodium chloride as the neutral salt, sucrose as the disaccharide, poloxamer as the nonionic surfactant, and phosphate buffer as the buffer.
Owner:JCR PHARMA
Methods for sterilizing preparations of digestive enzymes
InactiveUS20050047958A1Reducing residual solvent contentLow preparation temperaturePowder deliverySenses disorderIduronate-2-sulfataseFungal microorganisms
Methods are disclosed for sterilizing preparations of digestive enzymes to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. These methods involve sterilizing preparations of digestive enzymes, such as trypsin, α-galactosidase and iduronate-2-sulfatase, with irradiation.
Owner:CLEARANT
Iduronate-2-sulfatase and use thereof
InactiveUS20180030424A1Effective therapyPeptide/protein ingredientsHydrolasesIduronate-2-sulfataseNucleotide
Provided is a modified iduronate-2-sulfatase (IDS) gene constructed by inserting the nucleotide of SEQ ID NO: 2 into a wild-type IDS gene. In addition to being negatively charged, the improved IDS enzyme encoded by the modified gene exhibits a sufficient retention time in blood to target the bone, so that it is more effective for treating Hunter syndrome.
Owner:THE GREEN CROSS CORP
Iduronate-2-sulfatase and use thereof
InactiveUS20180127733A9Effective therapyPeptide/protein ingredientsHydrolasesIduronate-2-sulfataseNucleotide
Provided is a modified iduronate-2-sulfatase (IDS) gene constructed by inserting the nucleotide of SEQ ID NO: 2 into a wild-type IDS gene. In addition to being negatively charged, the improved IDS enzyme encoded by the modified gene exhibits a sufficient retention time in blood to target the bone, so that it is more effective for treating Hunter syndrome.
Owner:THE GREEN CROSS CORP
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com