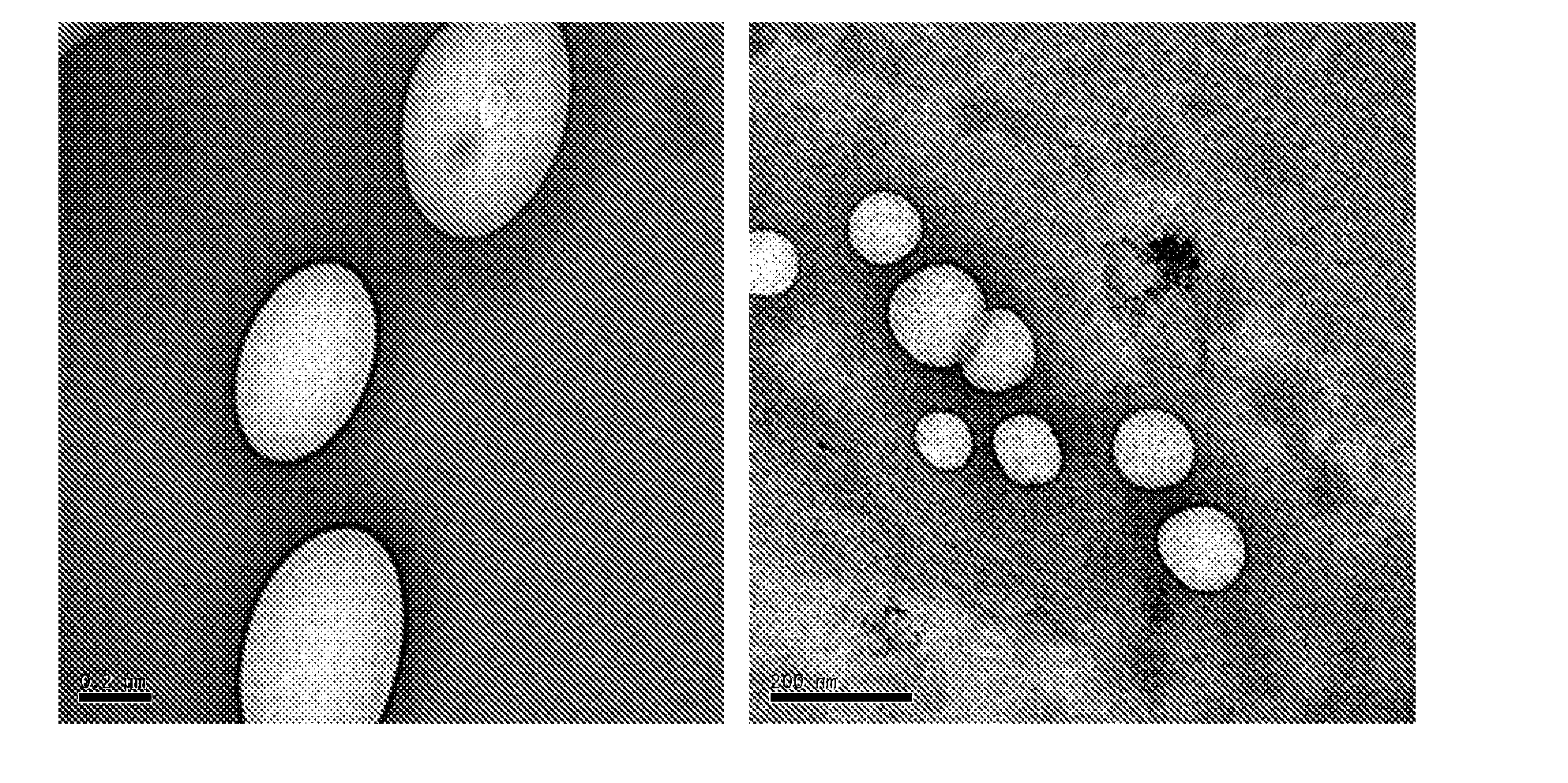
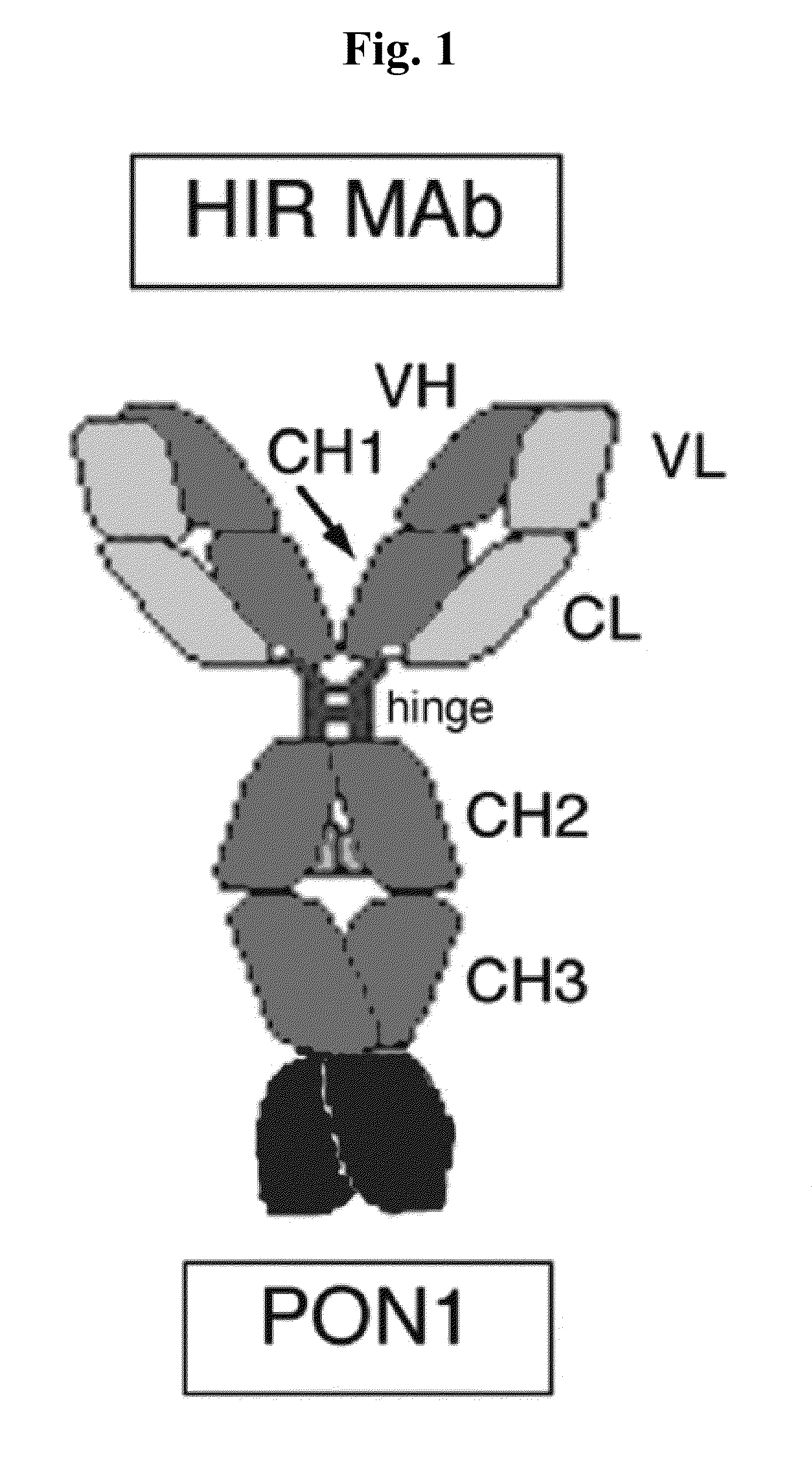Patents
Literature
352 results about "Systemic administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Systemic administration is a route of administration of medication, nutrition or other substance into the circulatory system so that the entire body is affected. Administration can take place via enteral administration (absorption of the drug through the gastrointestinal tract) or parenteral administration (generally injection, infusion, or implantation).
Methods for treatment and prevention of neuromuscular and muscular conditions by peripherally administered erythropoietin
InactiveUS7309687B1Improve cognitive functionAvoid damageNervous disorderPeptide/protein ingredientsDiseaseCentral neuron
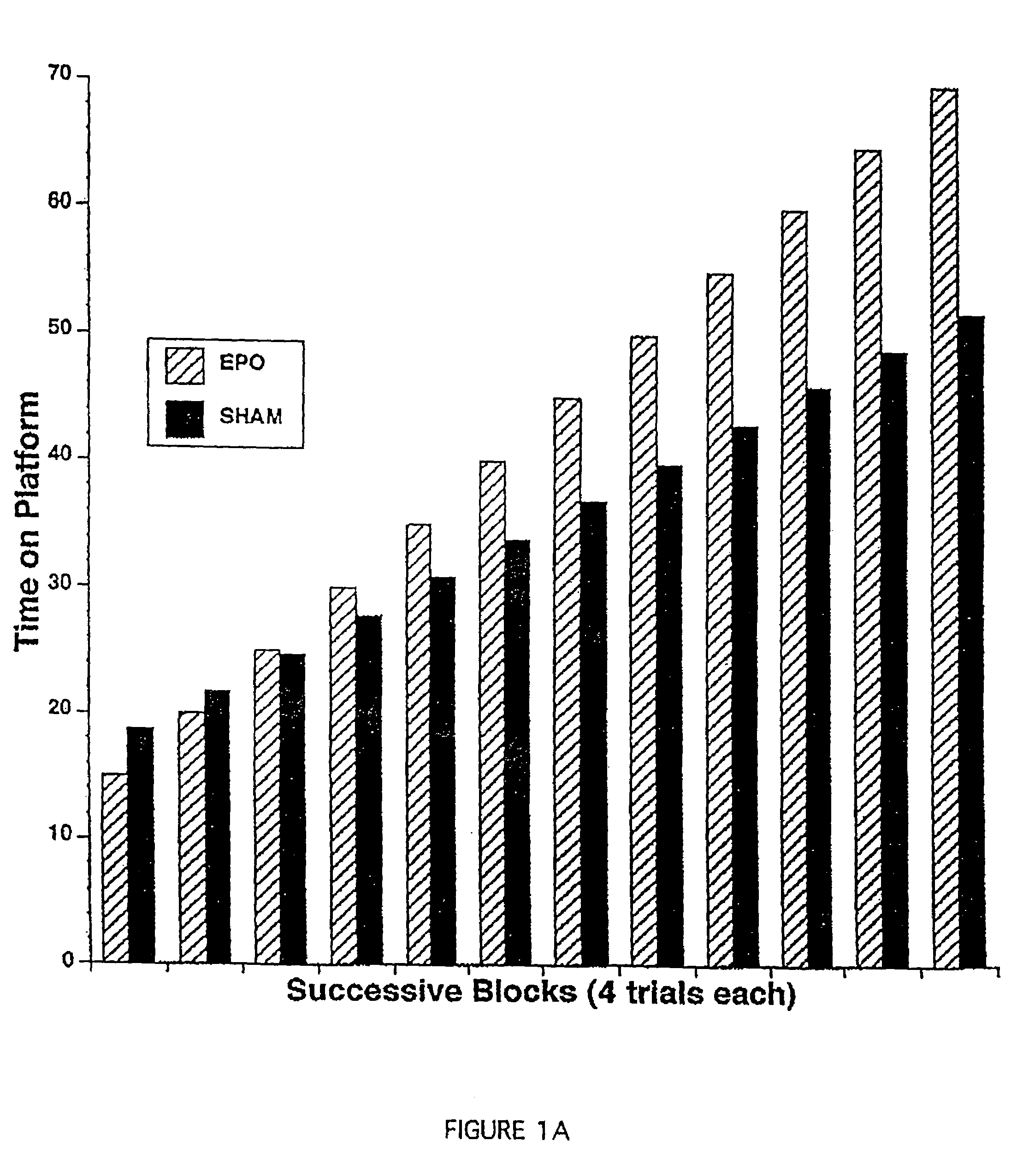
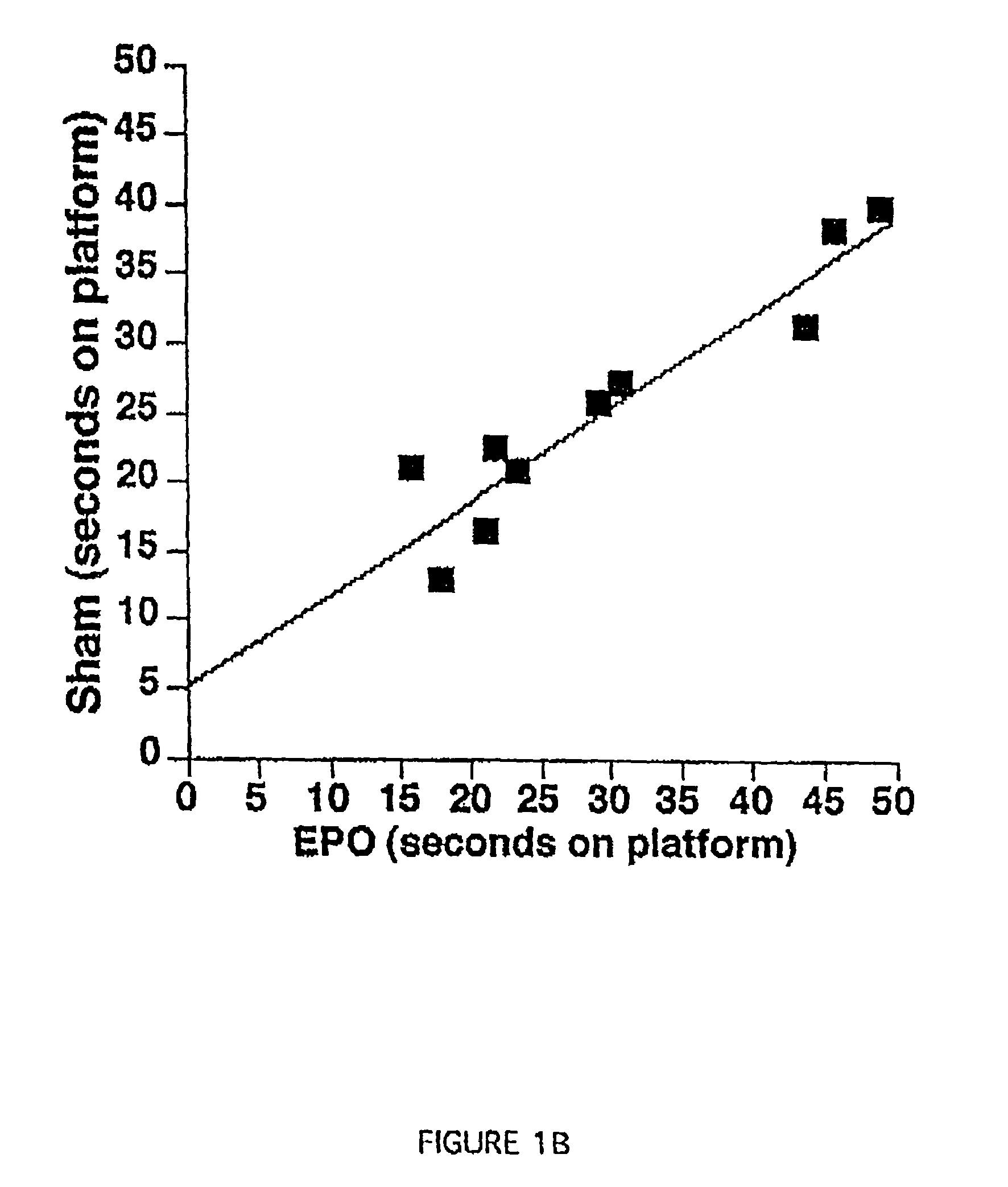
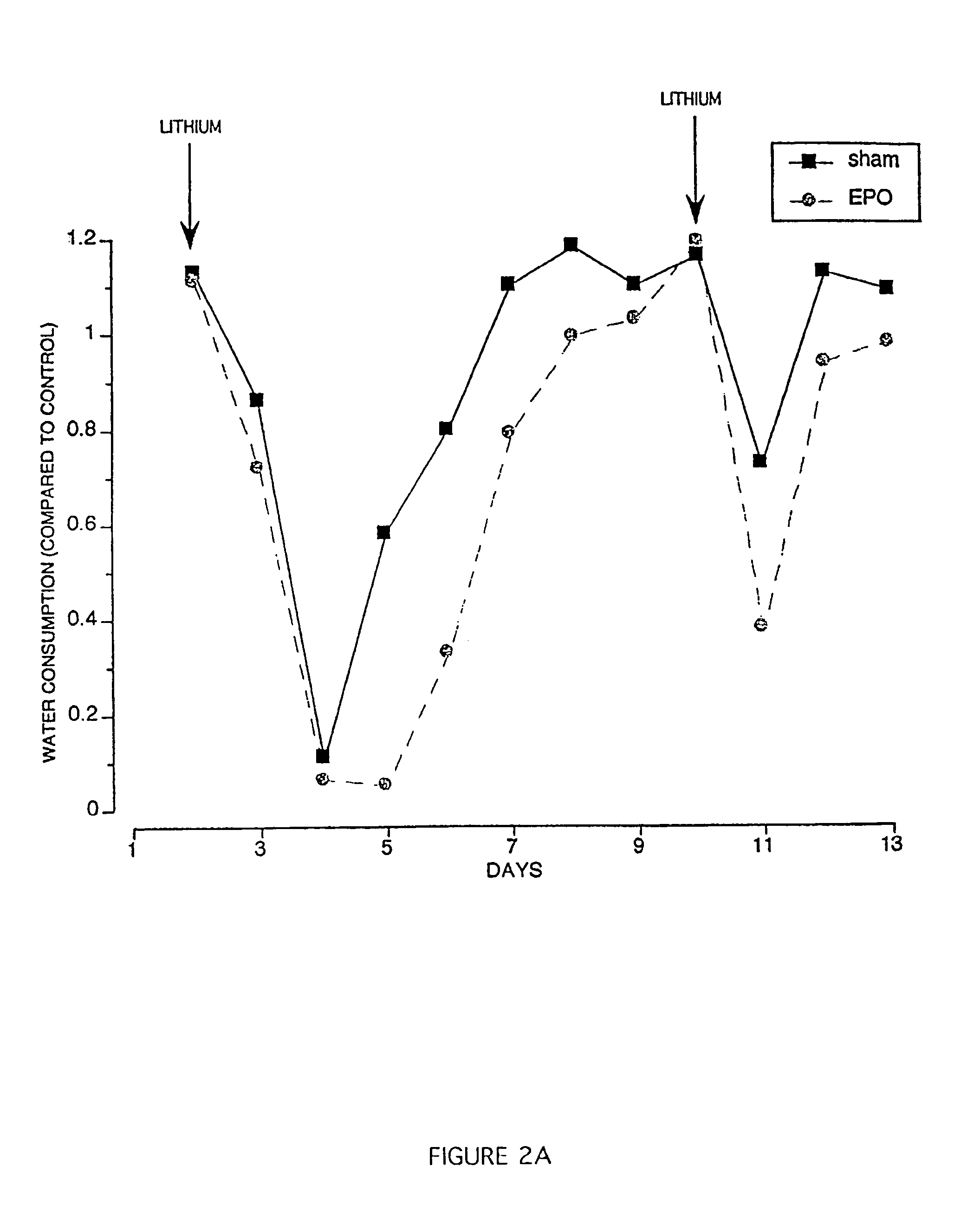
Methods and compositions are provided for protecting or enhancing excitable tissue function in mammals by systemic administration of an erythropoietin receptor activity modulator, such as erythropoietin, which signals via an EPO-activated receptor to modulate the function of excitable tissue. Excitable tissues include central neuronal tissues, such as the brain, peripheral neuronal tissues, retina, and heart tissue. Protection of excitable tissues provides treatment of hypoxia, seizure disorders, neurodegenerative diseases, hypoglycemia, and neurotoxin poisoning. Enhancement of function is useful in learning and memory. The invention is also directed to compositions and methods for facilitating the transport of molecules across endothelial cell tight junction barriers, such as the blood-brain barrier, by association of molecules with an erythropoietin receptor activity modulator, such as an erythropoietin.
Owner:THE KENNETH S WARREN INST
Composition and method for inhibiting platelet aggregation
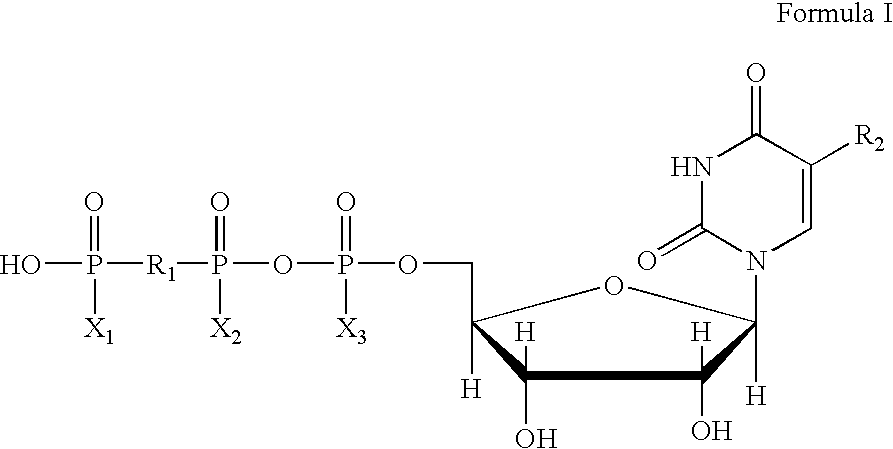
The present invention provides novel compounds of dinucleotide polyphosphates and the method of preventing or treating diseases or conditions associated with platelet aggregation. The method comprises administering systemically to a patient a pharmaceutical comprising a purinergic P2τ receptor antagonist, in an amount effective to elevate its extracellular concentration to bind to P2τ receptors and inhibit P2τ receptor-mediated platelet aggregation. Methods of systemic administration include injection by intravenous, intramuscular, intrasternal and intravitreal routes, infusion, transdermal administration, oral administration, rectal administration and intra-operative instillation.
Owner:INSPIRE PHARMA +1
Adhesive bioerodible transmucosal drug delivery system
InactiveUS20070207192A1Suitable bioadhesive capabilityRapid onsetAntibacterial agentsBiocideWhole bodyIrritation
The present invention is directed to a mucoadhesive delivery system for the local or systemic administration of a pharmaceutical agent. The delivery system of the invention effectively and facilely enables transport of the pharmaceutical agent through mucosal membranes and into the vasculattire of the mucosa. The delivery system includes an at least partially water soluble bioadhesive layer and an at least partially water soluble backing layer. Incorporated within either or both of these layers are the pharmaceutical agent and a mucosal penetration enhancing agent. The mucosal penetration enhancing agent displays localized tissue irritation properties. The mucoadhesive delivery system may be in the form of a gel, film, disc or patch. It may be applied to any mucosal membrane of a patient including but not limited to those of the buccal and nasal cavities, throat, eye, vagina, alimentary tract and peritoneum.
Owner:ARIUS TWO
High-density lipoprotein coated medical devices
InactiveUS20050175666A1Reduce the amount requiredPromote formationPeptide/protein ingredientsGenetic material ingredientsVulnerable plaqueWhole body
An implantable medical device is disclosed comprising a high-density lipoprotein (HDL), recombinant HDL, high-density lipoprotein mimics (HDLm), or a combination thereof. Method are also disclosed for local and systemic administration HDL, recombinant HDL or HDLm for the prevention, treatment, or amelioration of a vascular disorder, disease or occlusion such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Injectable pharmaceutical composition for systematic administration of pharmacologically active ingredients
InactiveUS7309497B2Quick eliminationReduce frequencyOrganic active ingredientsNervous disorderAdditive ingredientWhole body
The invention relates to novel pharmaceutical compositions for the systemic administration of pharmacologically active ingredients. The invention relates in particular to an injectable pharmaceutical composition comprising (a) a pharmacologically active ingredient in a solid phase, (b) a vehicle consisting substantially of polyol fatty acid esters with a degree of esterification of over 80%, and (c) a wetting agent consisting substantially of polyol fatty acid esters with a monoester proportion of over 60%. The inventive composition is used for the systemic administration of numerous pharmacologically active ingredients, whereby the ingredients are released from the pharmaceutical composition over a period of at least 12, preferably at least 24 hours.
Owner:UCB SA
Composition and method for treatment and prevention of traumatic synovitis and damage to articular cartilage
ActiveUS6979679B2Easy to produceReduce presenceBiocideSkeletal disorderWhole bodyInflammatory arthropathy
Owner:ARTHRODYNAMIC HLDG LLC
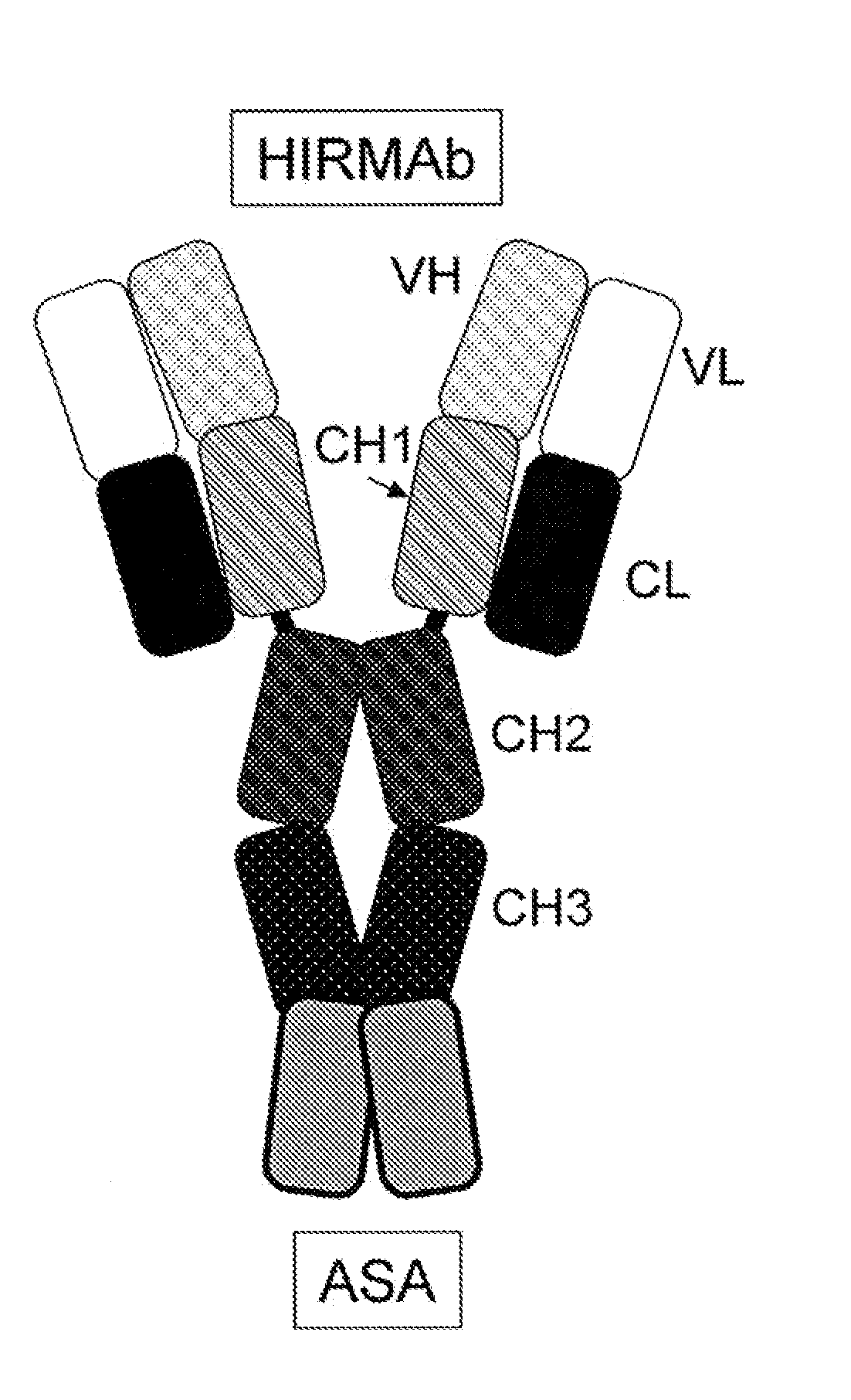
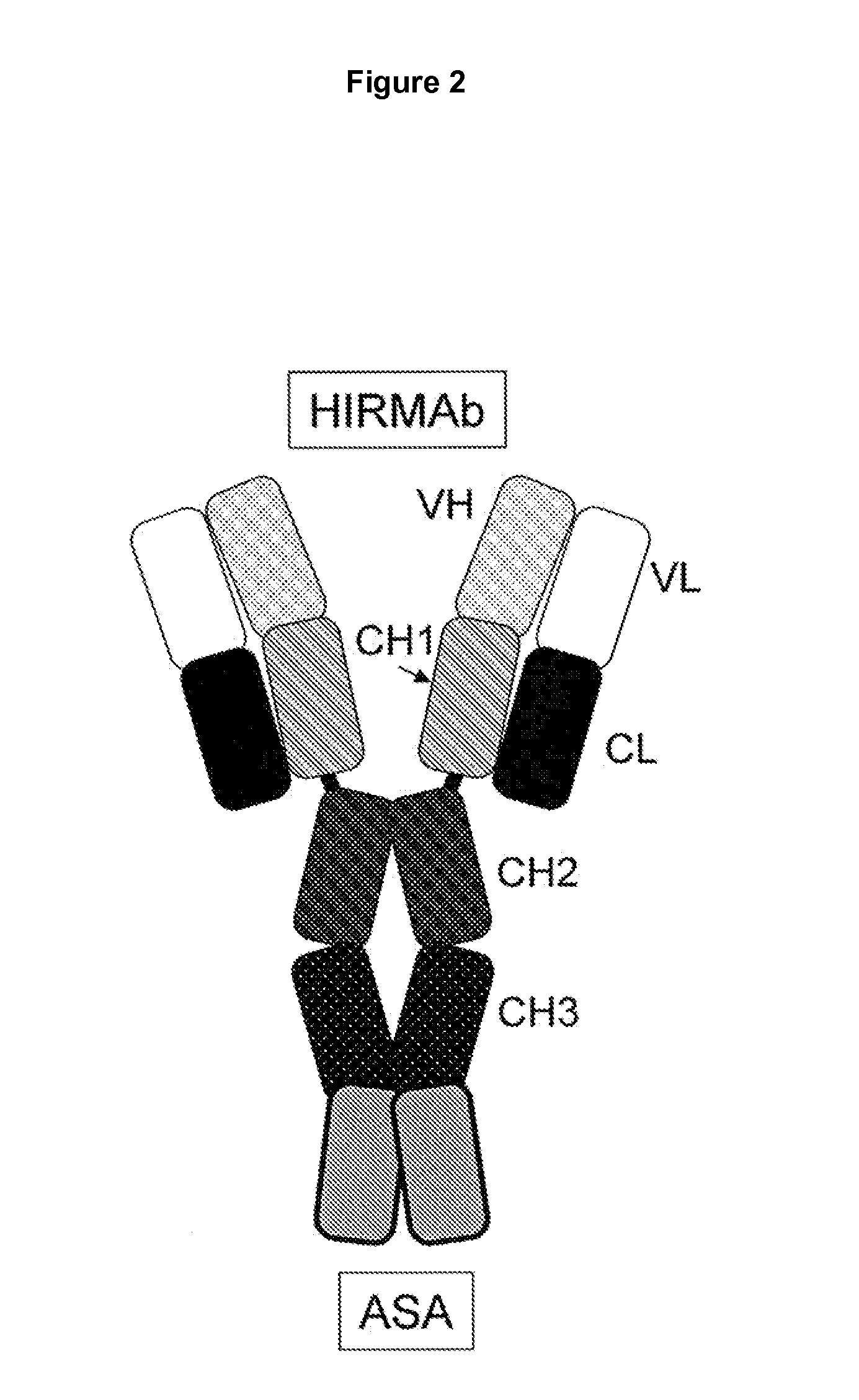
Methods and Compositions for Increasing Iduronate 2-Sulfatase Activity in the CNS
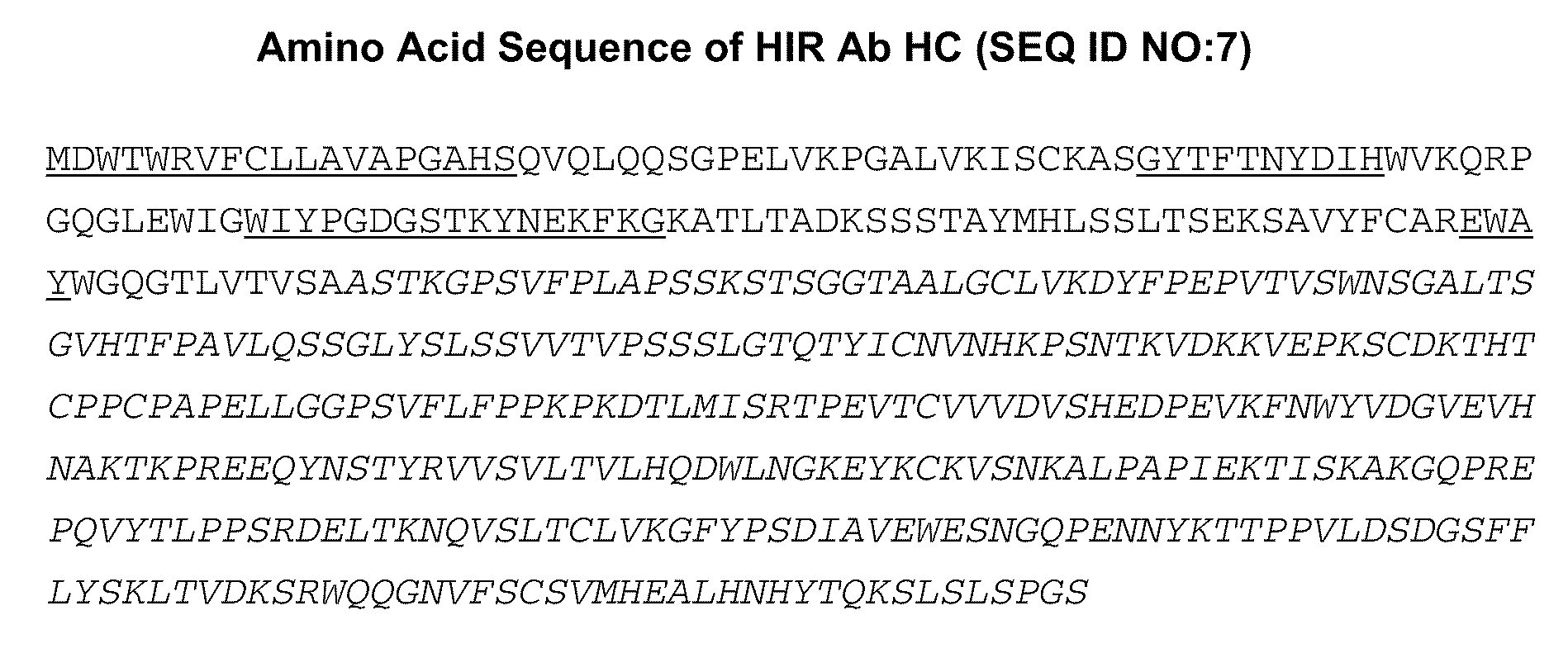
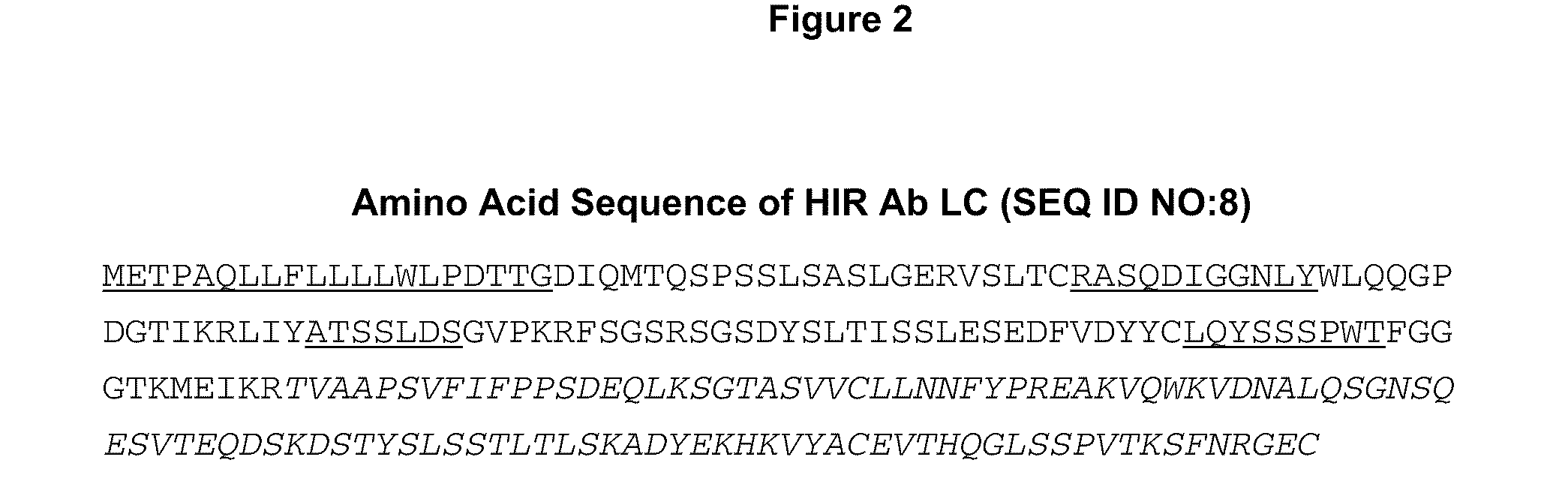
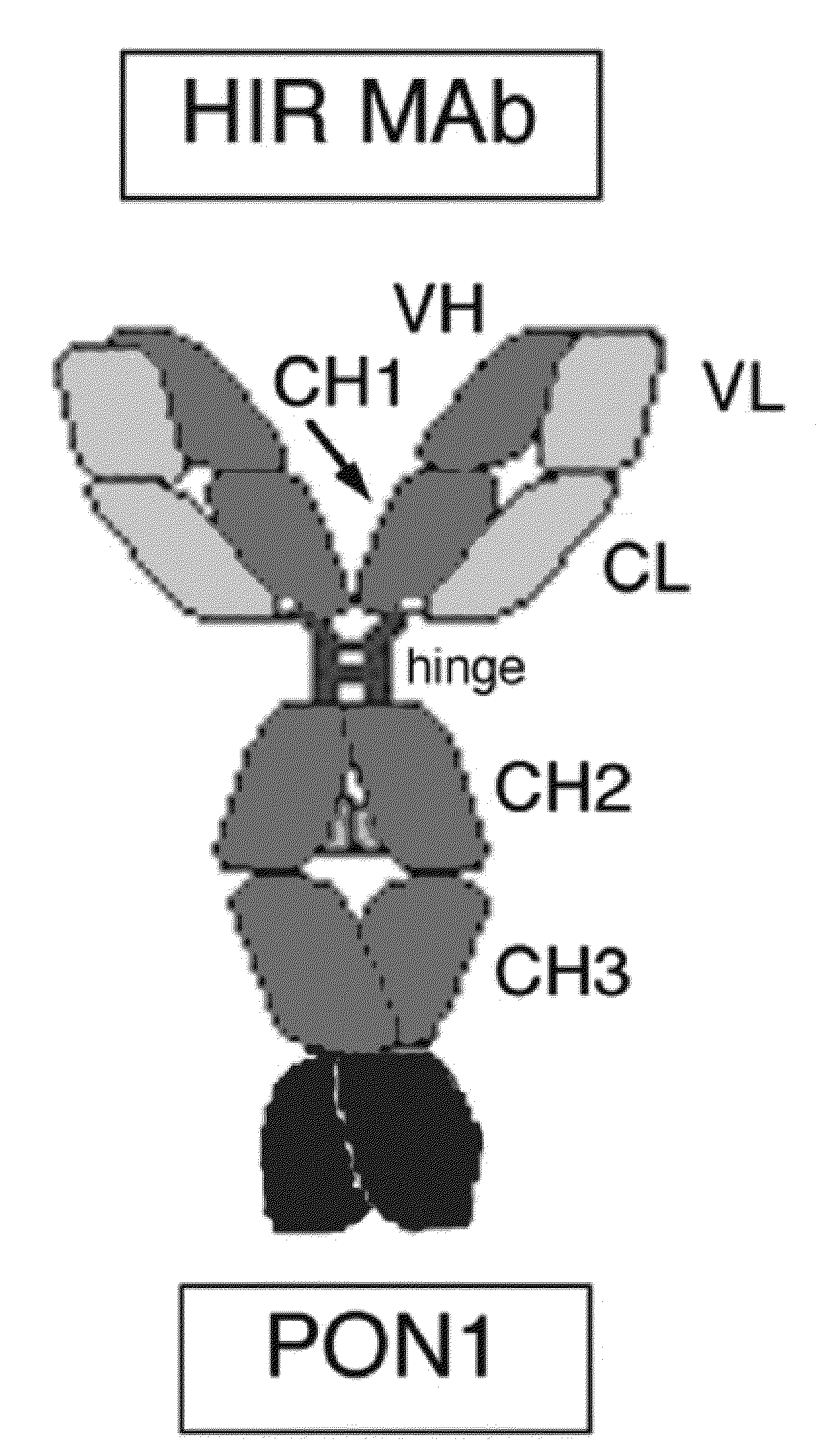
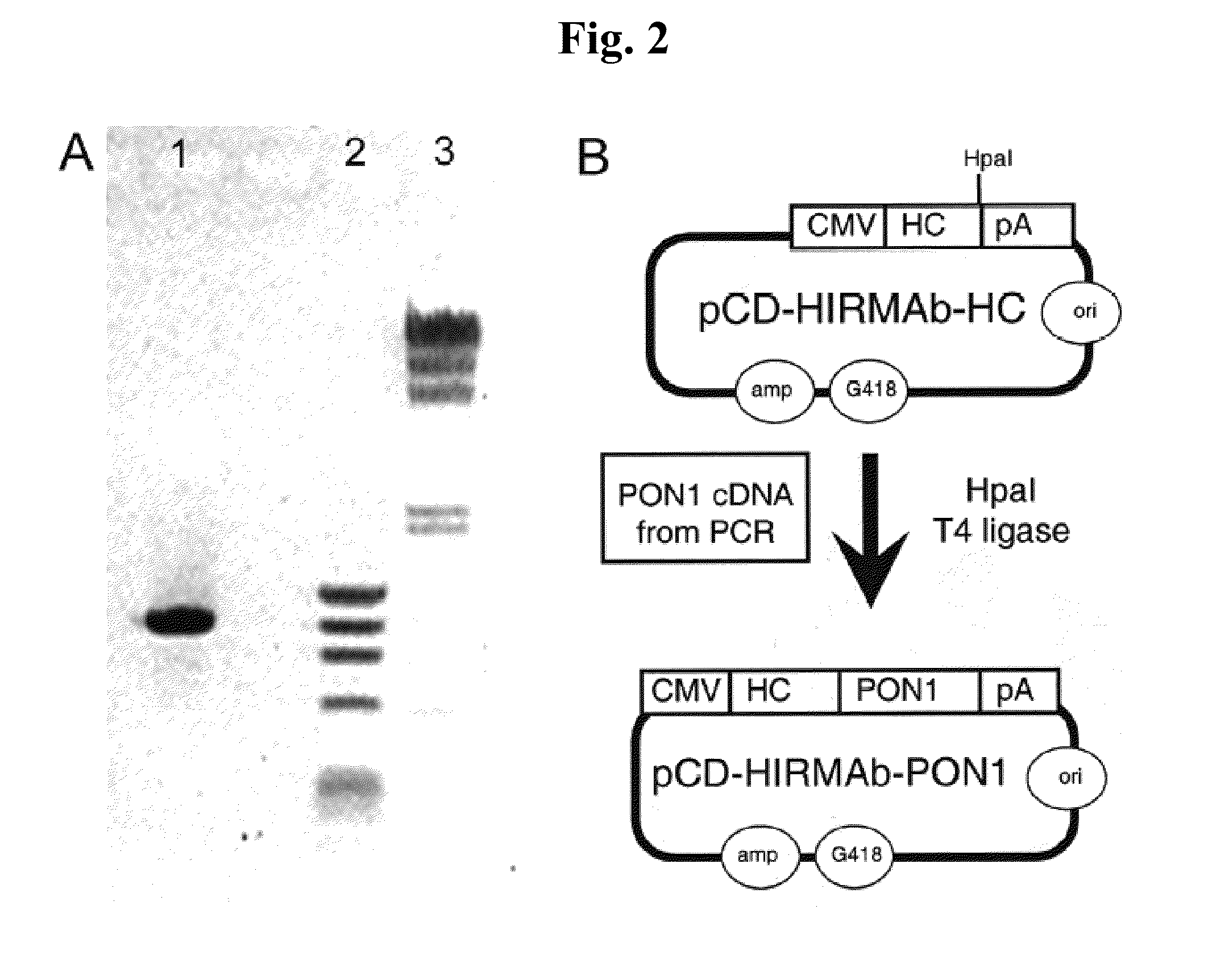
Provided herein are methods and compositions for treating a subject suffering from a deficiency in iduronate 2-sulfatase in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody that crosses the blood brain barrier (BBB) and an iduronate 2-sulfatase.
Owner:JCR PHARMA +1
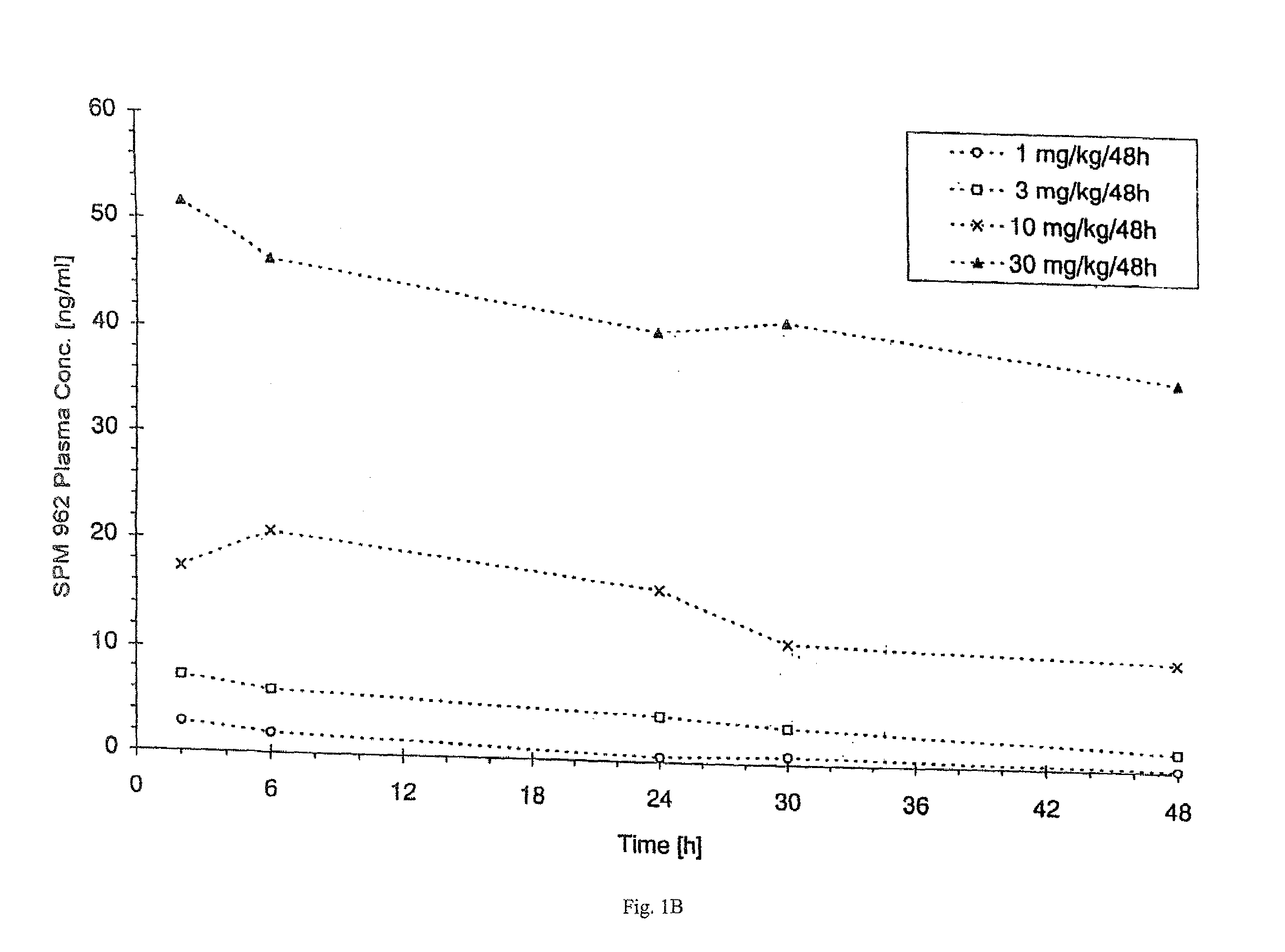
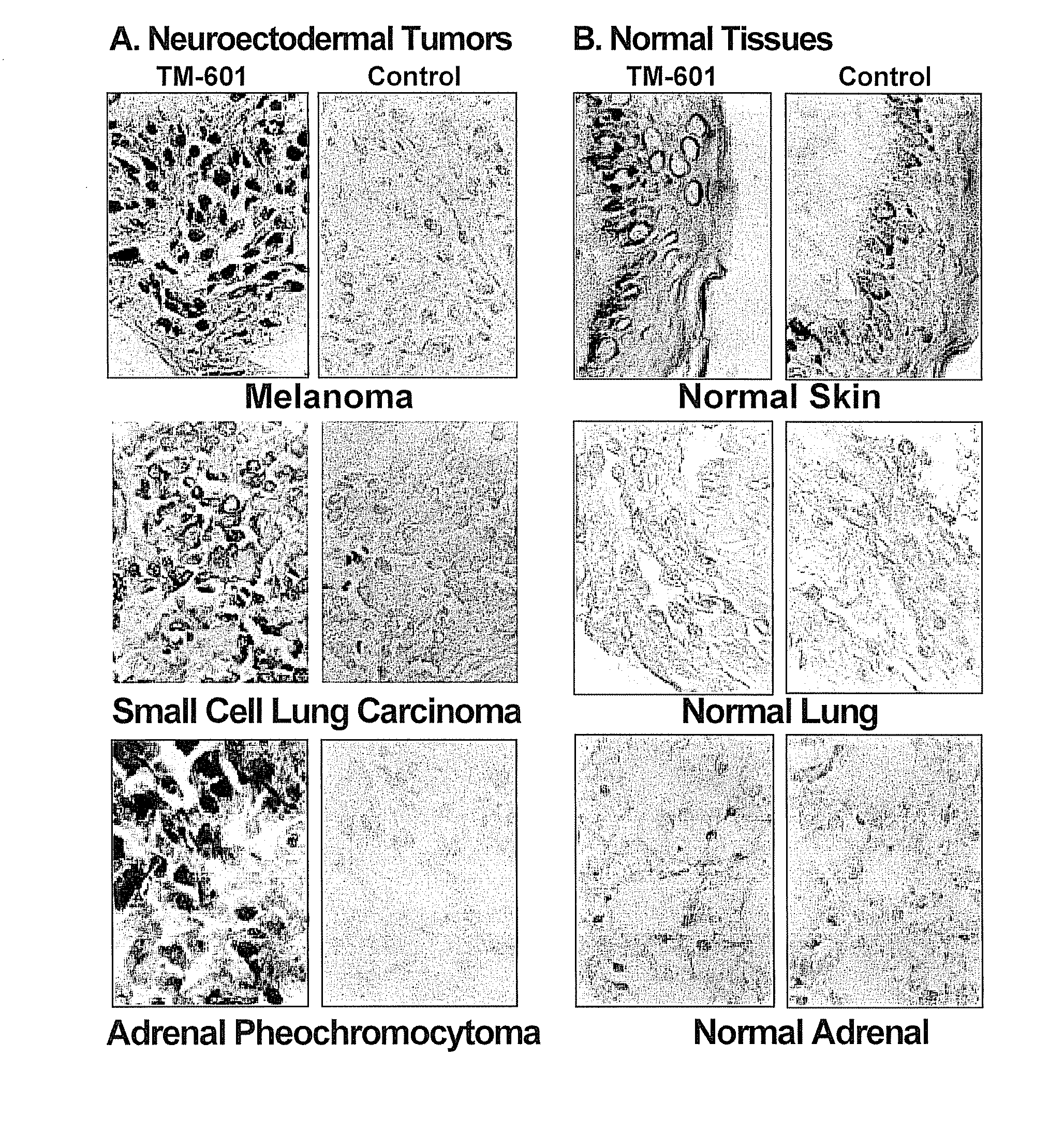
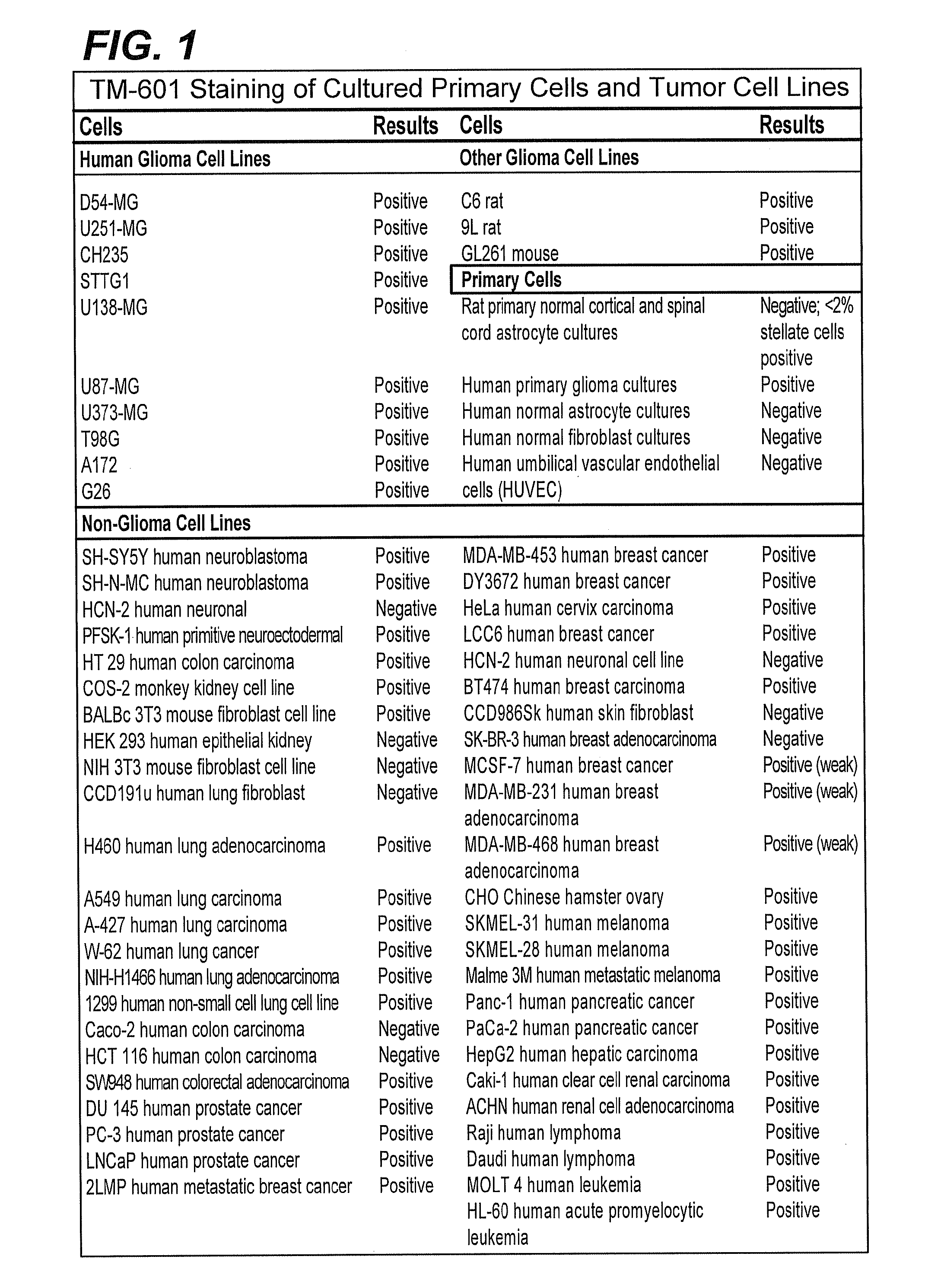
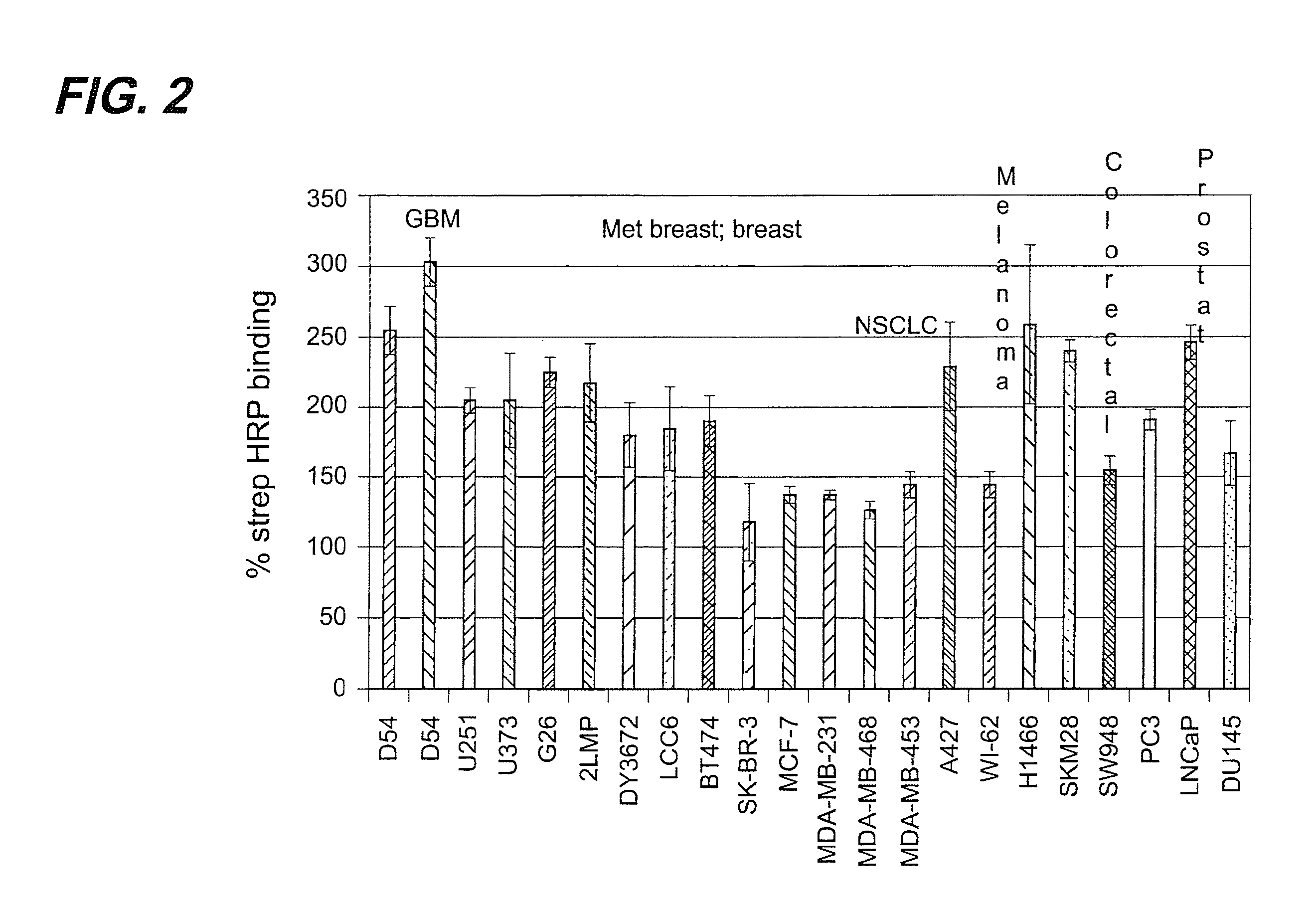
Systemic Administration of Chlorotoxin Agents for the Diagnosis and Treatment of Tumors
InactiveUS20100215575A1Efficient deliveryEffectively delivered intravenouslyUltrasonic/sonic/infrasonic diagnosticsSenses disorderWhole bodyOncology
Owner:MORPHOTEX INC
Drug-enhanced adhesion prevention
ActiveUS20050181023A1Inhibition formationOrganic active ingredientsBiocideSurgical operationPemirolast
The present invention includes methods for the inhibition of post-operative adhesion formation between tissue surfaces in a body cavity having been subjected to a surgical procedure, which methods involve administering Pemirolast, or an analog thereof, directly to tissue surfaces in the body cavity in amounts and under conditions effective to inhibit formation of adhesions, and to delivery vehicles and compositions suitable for use for local, non-systemic administration of a drug to the body and directly to tissue within a body cavity having been subjected to a surgical procedure.
Owner:ETHICON INC
Systemic administration of NAC as an adjunct in the treatment of bioterror exposures such as anthrax, smallpox or radiation and for vaccination prophylaxis, and use in combination with DHEA for the treatment of smallpox and other viruses
InactiveUS20040022873A1No toxicityWithout fear of compromisingBiocideTripeptide ingredientsWhole bodyPoisonous effects
The invention is for the combination and related methods of N-acetyl-cysteine oral, inhaled, or intravenous, or glutathione inhaled or intravenous, generally in combination with antibiotic and / or antiviral therapy to ameliorate the toxic effects of infection with materials used in Bioterror incidents such as Bacillus anthracis and smallpox virus, and alternatively, upon exposure to radiation, during testing, and vaccination, as treatment prior to treatment with antibiotic or antiviral therapy to ameliorate the toxic effects of infection and exposure with these organisms.
Owner:YOUR ENERGY SYST
Therapeutic agents—I
InactiveUS7449492B2Antibacterial agentsOrganic active ingredientsChemical fractionChemical synthesis
The present invention relates generally to chemical agents useful in the treatment and prophylaxis of infection by pathogenic or potentially pathogenic entities, or entities capable of opportunistic infection in mammals, including humans and primates, non-mammalian animals and avian species. More particularly, the present invention provides a chemical agent of the macrocyclic diterpene family obtainable from a member of the Euphorbiaceae family of plants or botanical or horticultural relatives thereof or derivatives or chemical analogues or chemically synthetic forms of the agents for use in the treatment or prophylaxis of infection by pathogenic entities in mammalian, animal and avian subjects. The present invention further contemplates a method for the prophylaxis and / or treatment in mammalian, animal or avian subjects of infection or potential infection by pathogenic entities by the topical or systemic administration of a macrocyclic diterpene obtainable from a member of the Euphorbiaceae family of plants or their botanical or horticultural derivatives or a derivative, chemical analogue or chemically synthetic form of the agent. The chemical agent of the present invention may be in the form of a purified compound, mixture of compounds, a precursor form of one or more of the compounds capable of chemical transformation into a therapeutically active agent or in the form of a chemical fraction, sub-fraction, preparation or extract of the plant.
Owner:AF 30 APRIL 2003 +1
Glycosaminoglycan composition and method for treatment and prevention of interstitial cystitis
ActiveUS20060234978A1Simple compositionTreatment and/or prevention of interstitial cystitisBiocideCarbohydrate active ingredientsN Acetyl D GlucosamineWhole body
The invention provides compositions and methods useful for the treatment and / or prevention of interstitial cystitis and / or a related urinary tract condition in man or in animals. Specifically, provided are compositions specially formulated for direct instillation into the bladder and / or parenteral use in the treatment and / or prevention of interstitial cystitis. Compositions adapted for direct instillation into the bladder and / or for systemic administration are provided comprised of therapeutic amounts of: chondroitin sulfate in combination with hyaluronan (hyaluronic acid) are provided. Compositions adapted for direct instillation into the bladder and / or for systemic administration are also provided comprised of therapeutic amounts of: chondroitin sulfate, hyaluronan (hyaluronic acid) and N-acetyl D-glucosamine.
Owner:ARTHRODYNAMIC HLDG LLC
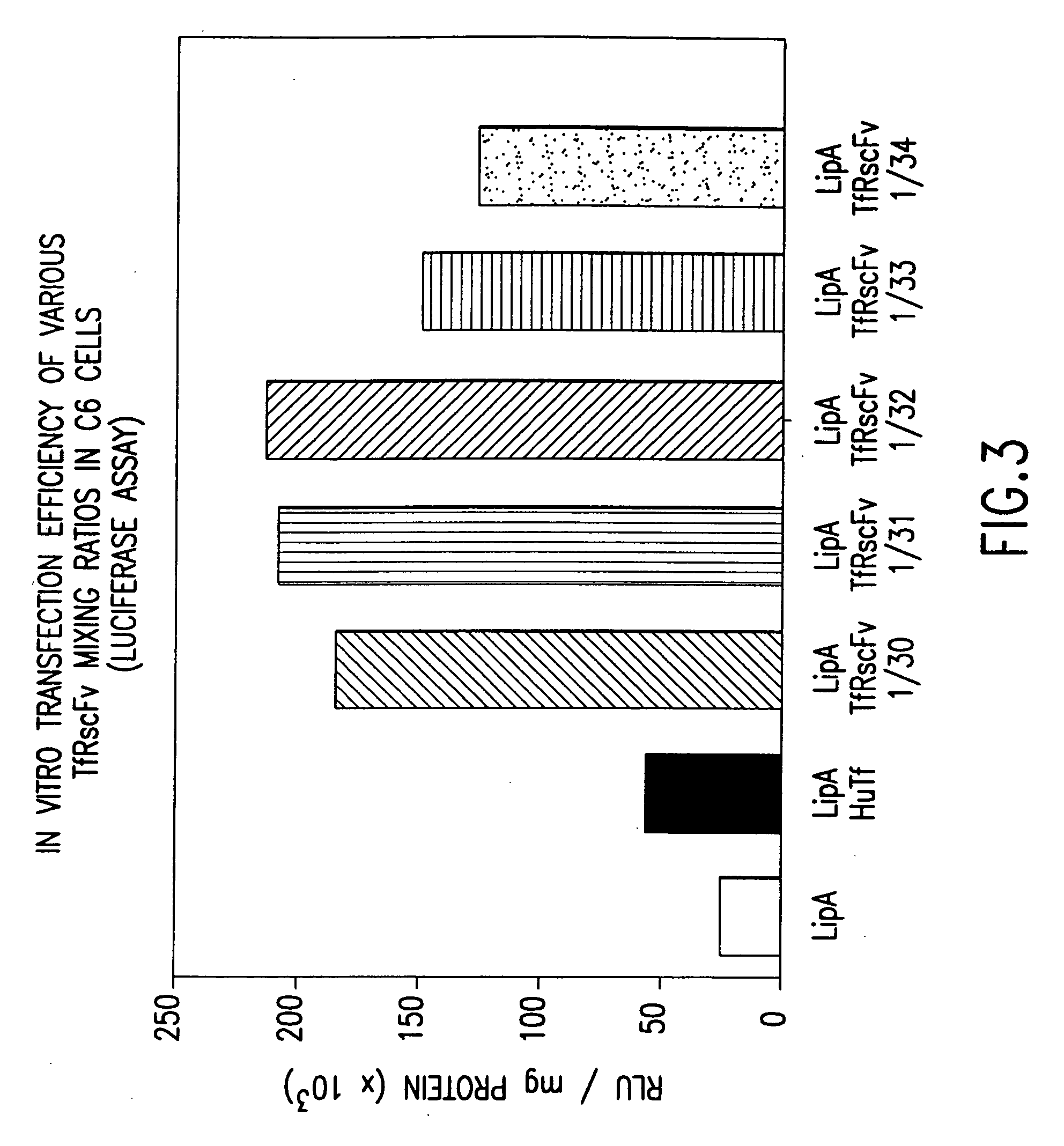
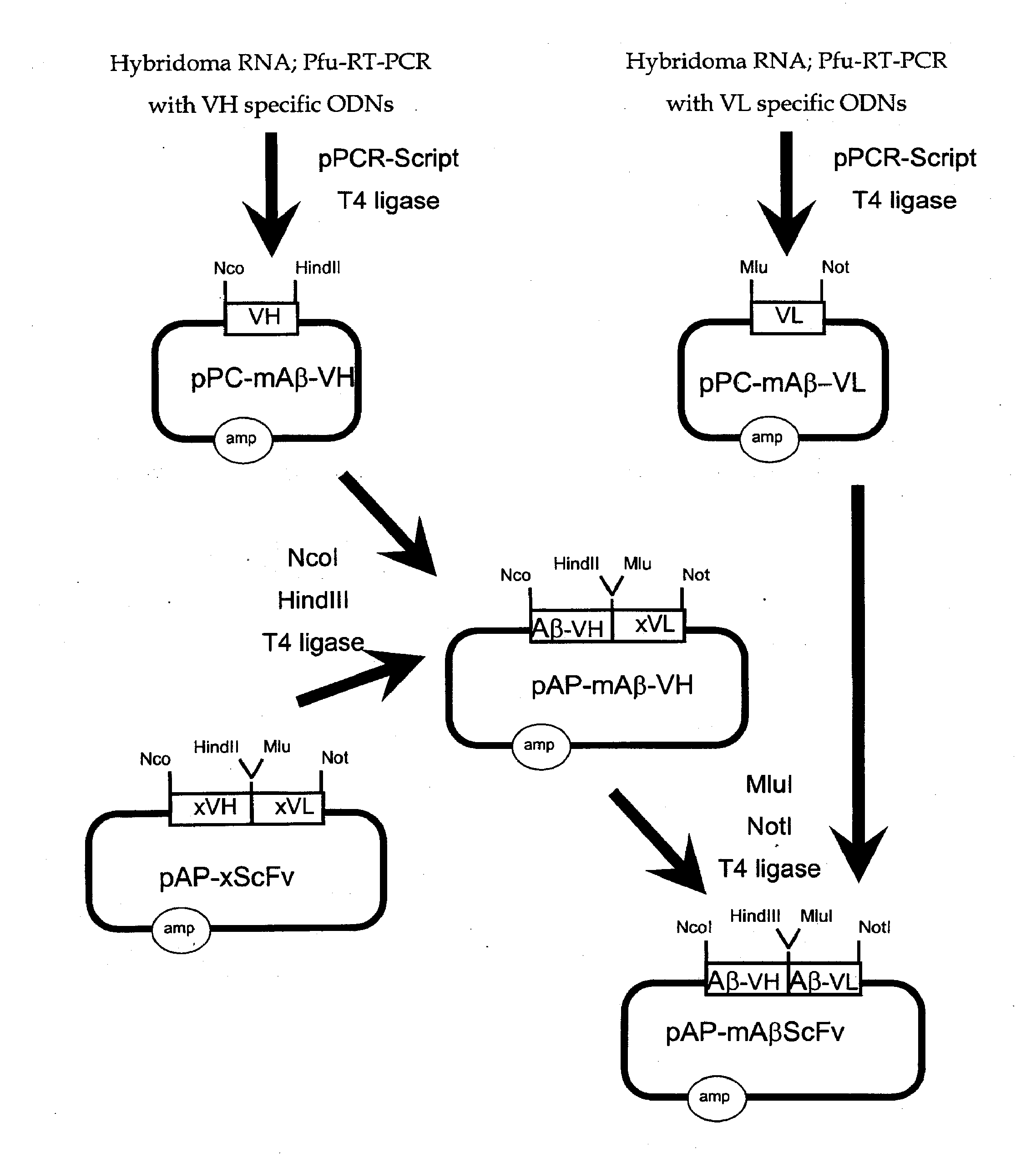
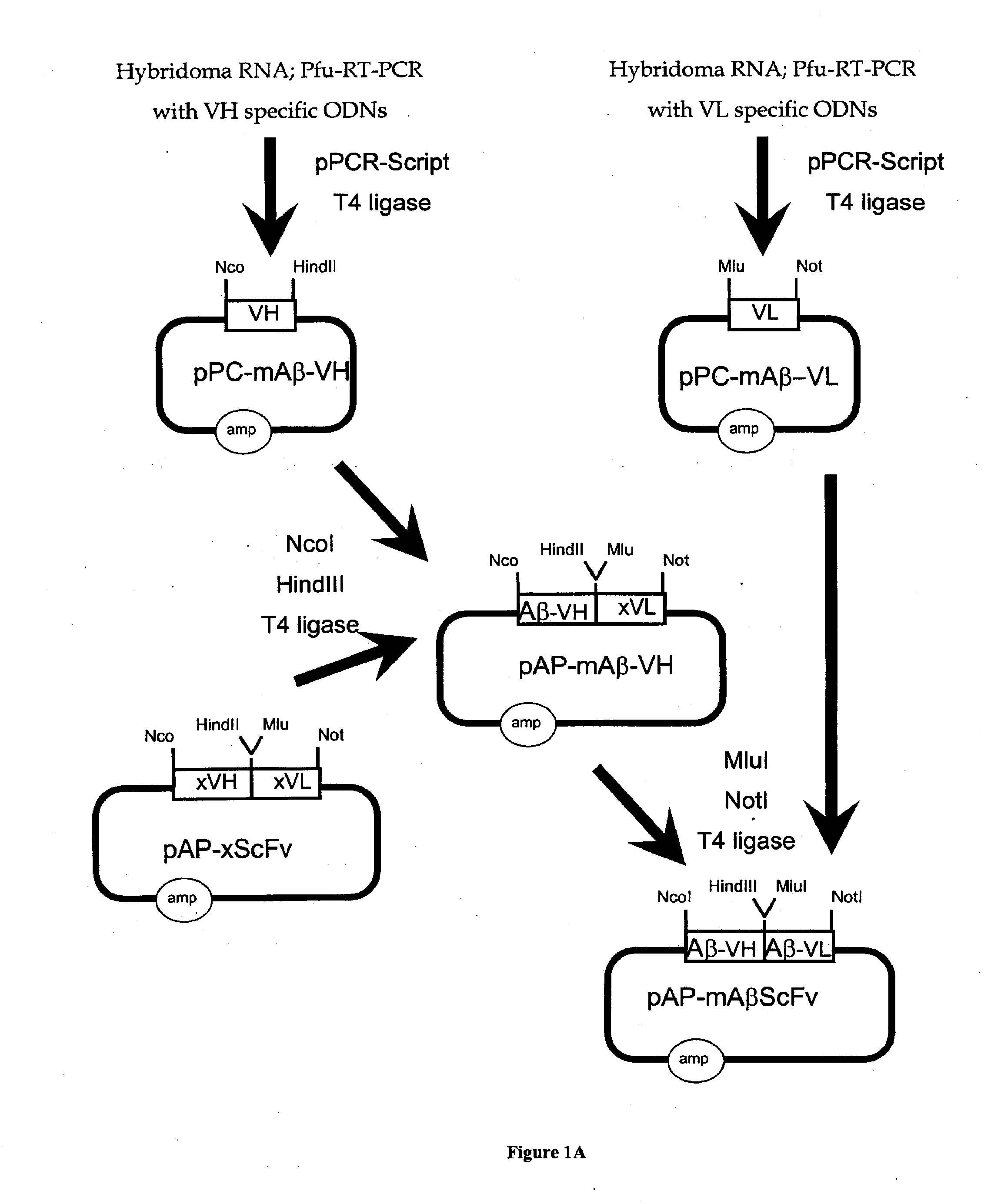
Simplified and improved method for preparing an antibody or an antibody fragment targeted immunoliposome for systemic administration of a therapeutic or diagnostic agent
InactiveUS7780882B2Low effective doseLessening severe side effectVectorsPeptide/protein ingredientsDiagnostic agentAntibody fragments
A method of preparing an antibody- or antibody fragment-targeted cationic immunoliposome or polymer complex comprises the steps of (a) preparing an antibody or antibody fragment; (b) mixing said antibody or antibody fragment with a cationic liposome to form a cationic immunoliposome or with a cationic polymer to form a polyplex; and (c) mixing said cationic immunoliposome or said polyplex with a therapeutic or diagnostic agent to form said antibody- or antibody fragment-targeted cationic immunoliposome or polymer complex.
Owner:GEORGETOWN UNIV
Systemic administration of therapeutic amino acids and N-acetylamino acids
Embodiments relate to compositions and use of compositions comprising amino acids and / or N-acetylamino acids for systemic administration to a mammal. Systemic administration is believed to alleviate or improve symptoms or syndromes associated with nervous, vascular, musculoskeletal, or cutaneous systems.
Owner:YU RUEY J +1
Methods and Compositions for Increasing Arylsulfatase A Activity in the CNS
ActiveUS20130142794A1Polypeptide with localisation/targeting motifNervous disorderArylsulfatase A activityInsulin receptor
Provided herein are methods and compositions for treating a subject suffering from a deficiency in arylsulfatase A in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody to a human insulin receptor and an arylsulfatase A.
Owner:JCR PHARMA +1
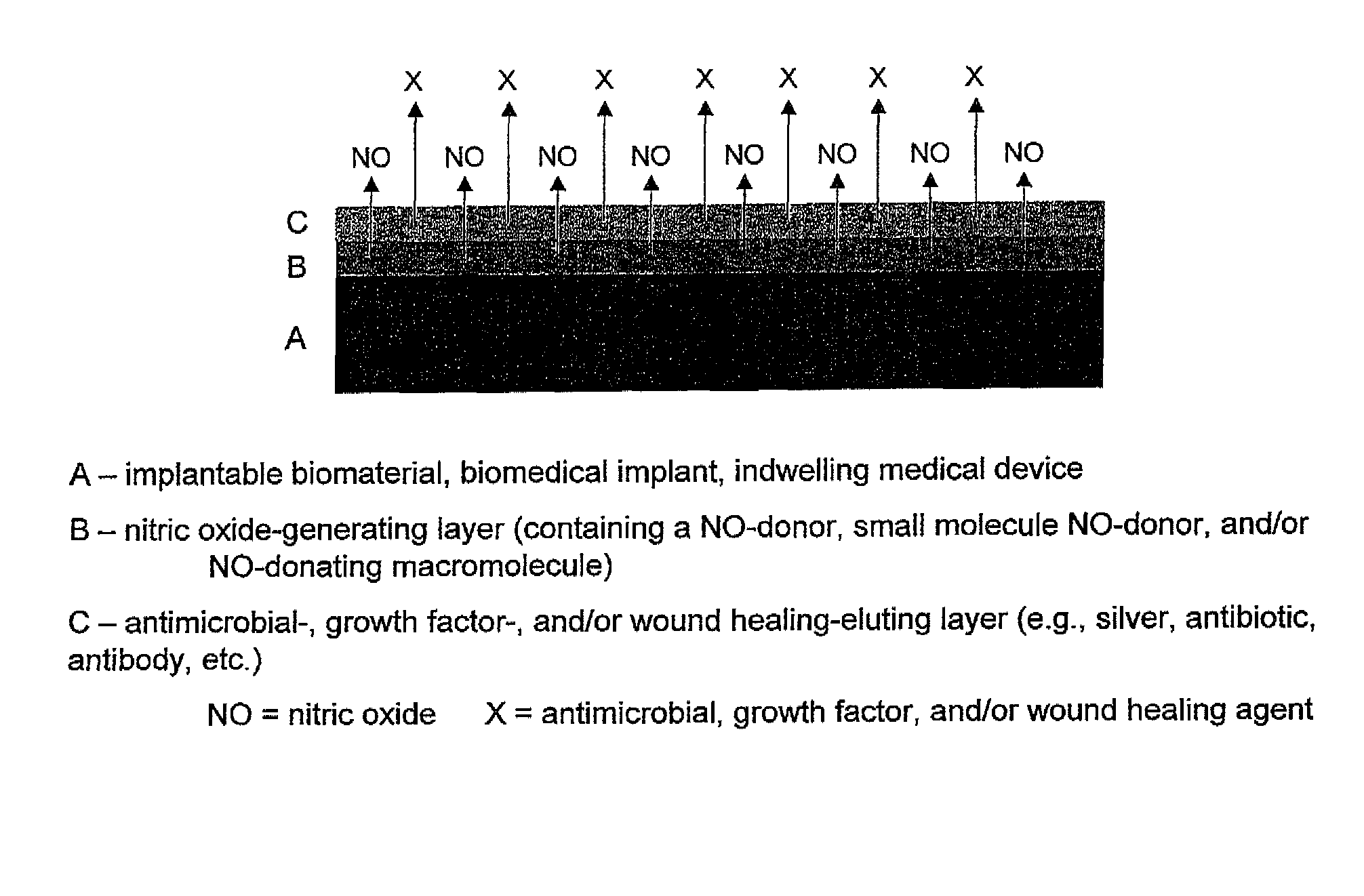
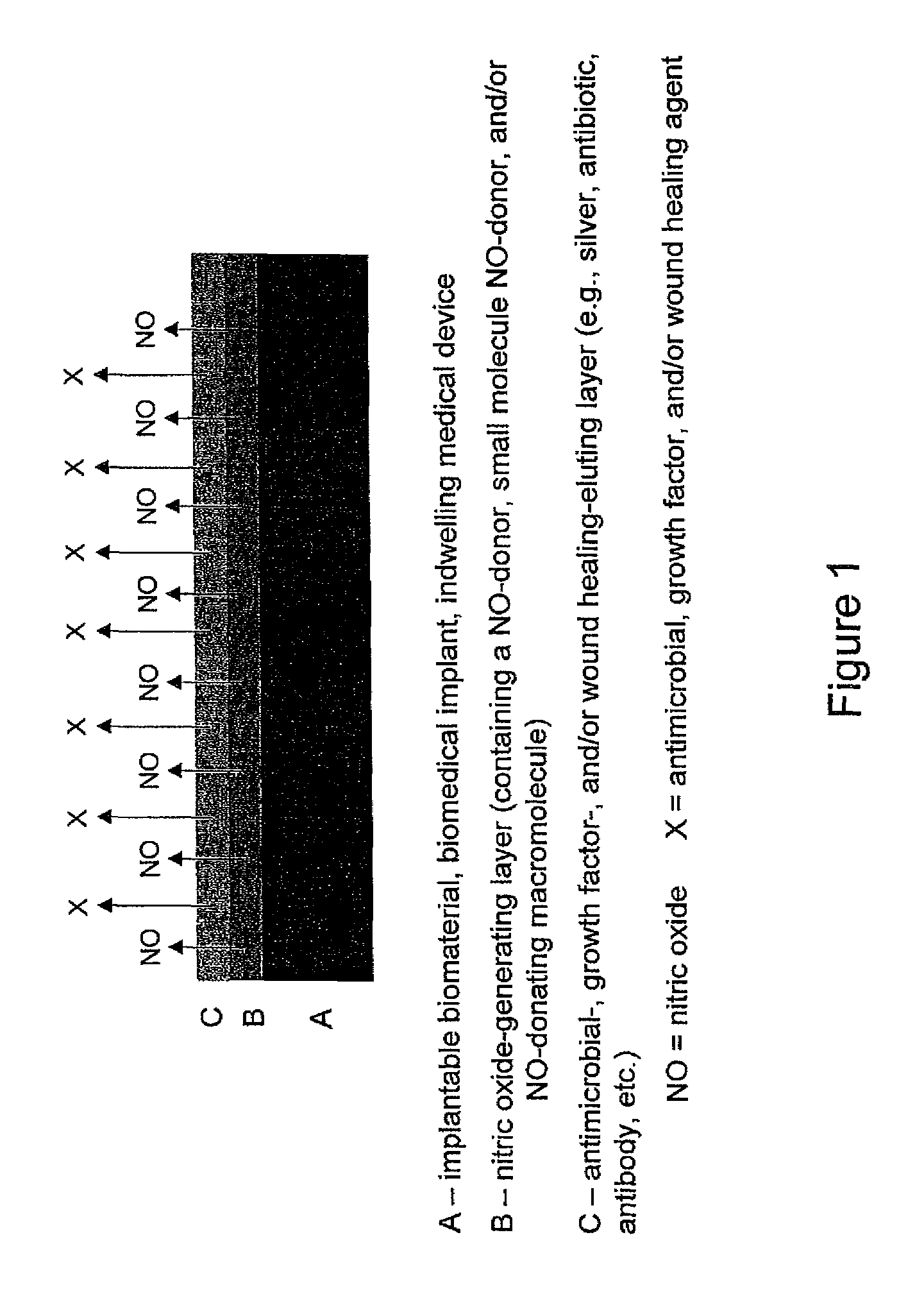
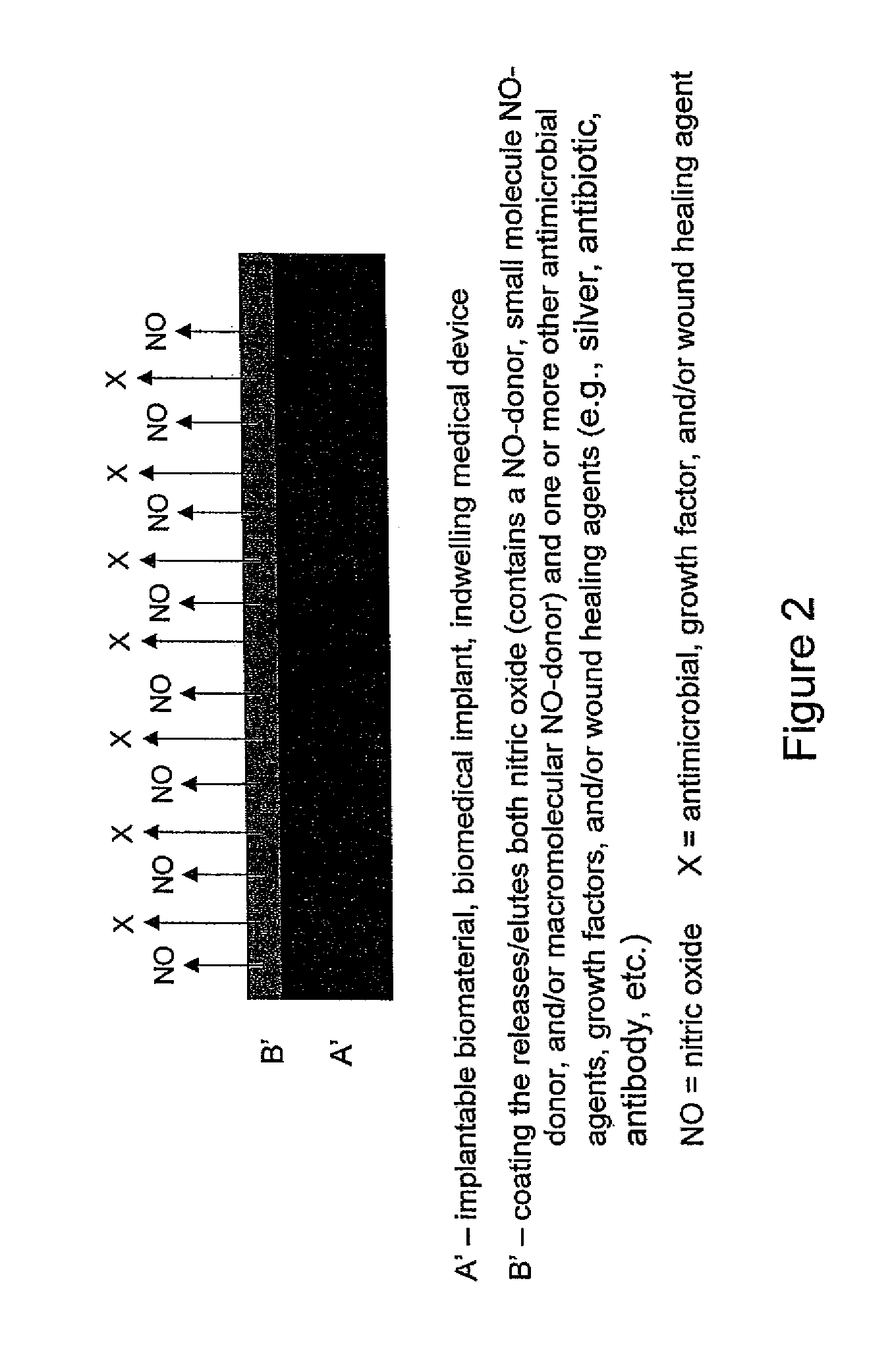
Use of nitric oxide to enhance the efficacy of silver and other topical wound care agents
ActiveUS8399005B2Meet cutting requirementsPromote wound healingAntibacterial agentsPowder deliveryAntibiotic resistanceActive agent
The present invention is directed to compositions comprising at least one nitric oxide donor and at least one second therapeutically active agent with antimicrobial or wound healing capability. In one embodiment, the nitric oxide donor is a nanoparticle which is designed to control for the amount and duration of release of nitric oxide. The nanoparticle may further comprise the additional therapeutically active agent. The composition is useful for enhancing wound healing and for treating and preventing microbial infection. In one embodiment, the composition is directed toward reducing oral bacteria or dental plaque. The combination of one or more nitric oxide donors and one or more additional therapeutically active agent results in unexpected synergistic effects, wherein both the antimicrobial efficacy of the nitric oxide and the antimicrobial or wound healing efficacy of the second therapeutically active agent are enhanced. As a result, a patient may benefit from reduced dosage requirements and a reduced likelihood of antimicrobial resistance. The composition may be formulated for local or systemic administration, for topical applications as well as for use in coatings for medical supplies and devices.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Preparation of antibody or an antibody fragment-targeted immunoliposomes for systemic administration of therapeutic or diagnostic agents and uses thereof
InactiveUS20070065499A1Improve efficiencySimple methodGenetic material ingredientsAntibody ingredientsDiagnostic agentAntibody fragments
The present invention provides methods of preparing an antibody- or antibody fragment-targeted cationic immunoliposome or polymer complex comprising (a) preparing an antibody or antibody fragment; (b) mixing said antibody or antibody fragment with a cationic liposome to form a cationic immunoliposome or with a cationic polymer to form a polyplex; and (c) mixing said cationic immunoliposome or said polyplex with a therapeutic or diagnostic agent to form said antibody- or antibody fragment-targeted cationic immunoliposome or polymer complex. The present invention also provides cationic immunoliposome or polymer complexes produced by such methods and compositions comprising such complexes. The present invention also provides methods for treating various diseases and disorders, including cancers, by administering the complexes and compositions of the invention to a patient.
Owner:GEORGETOWN UNIV
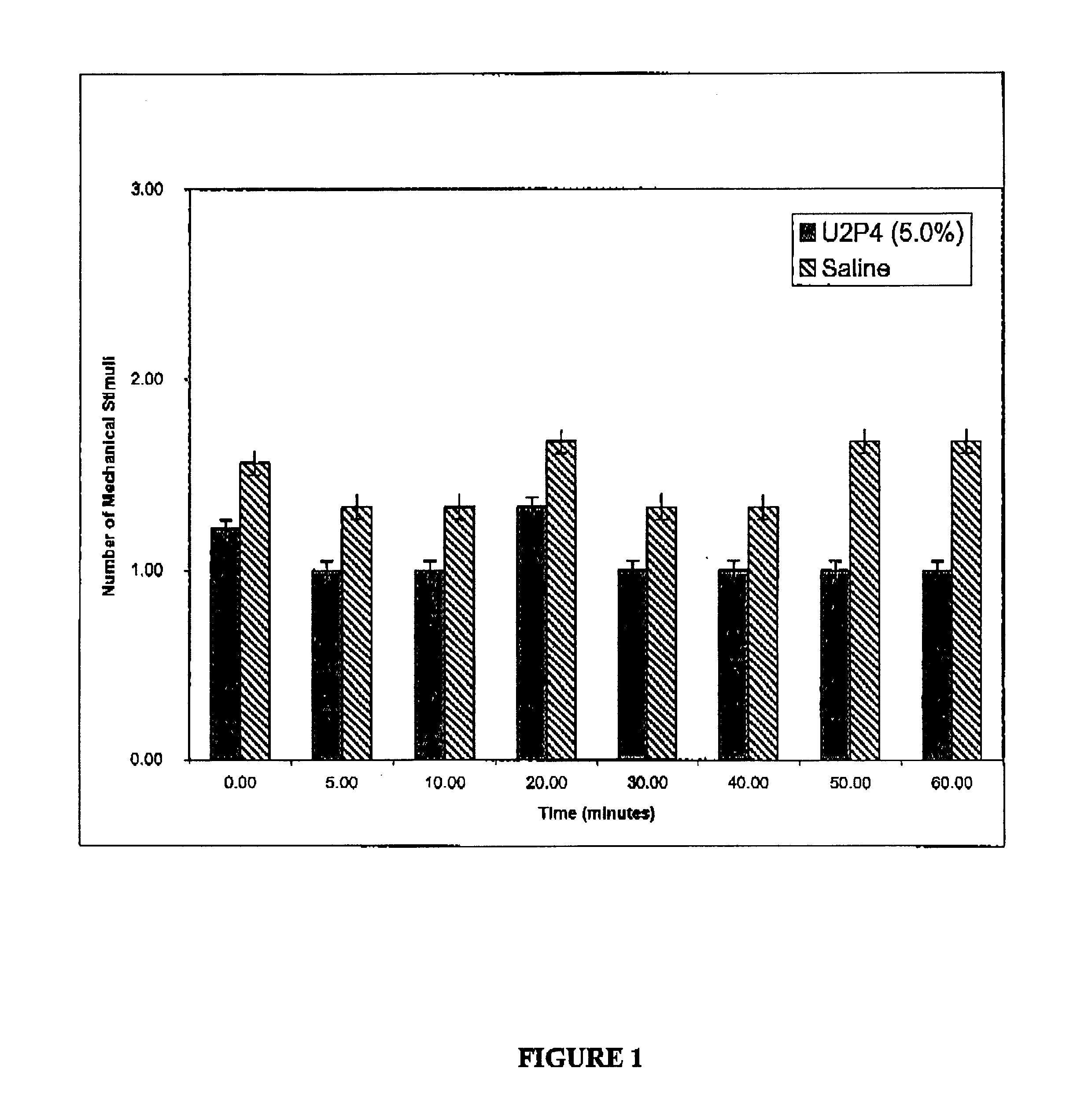
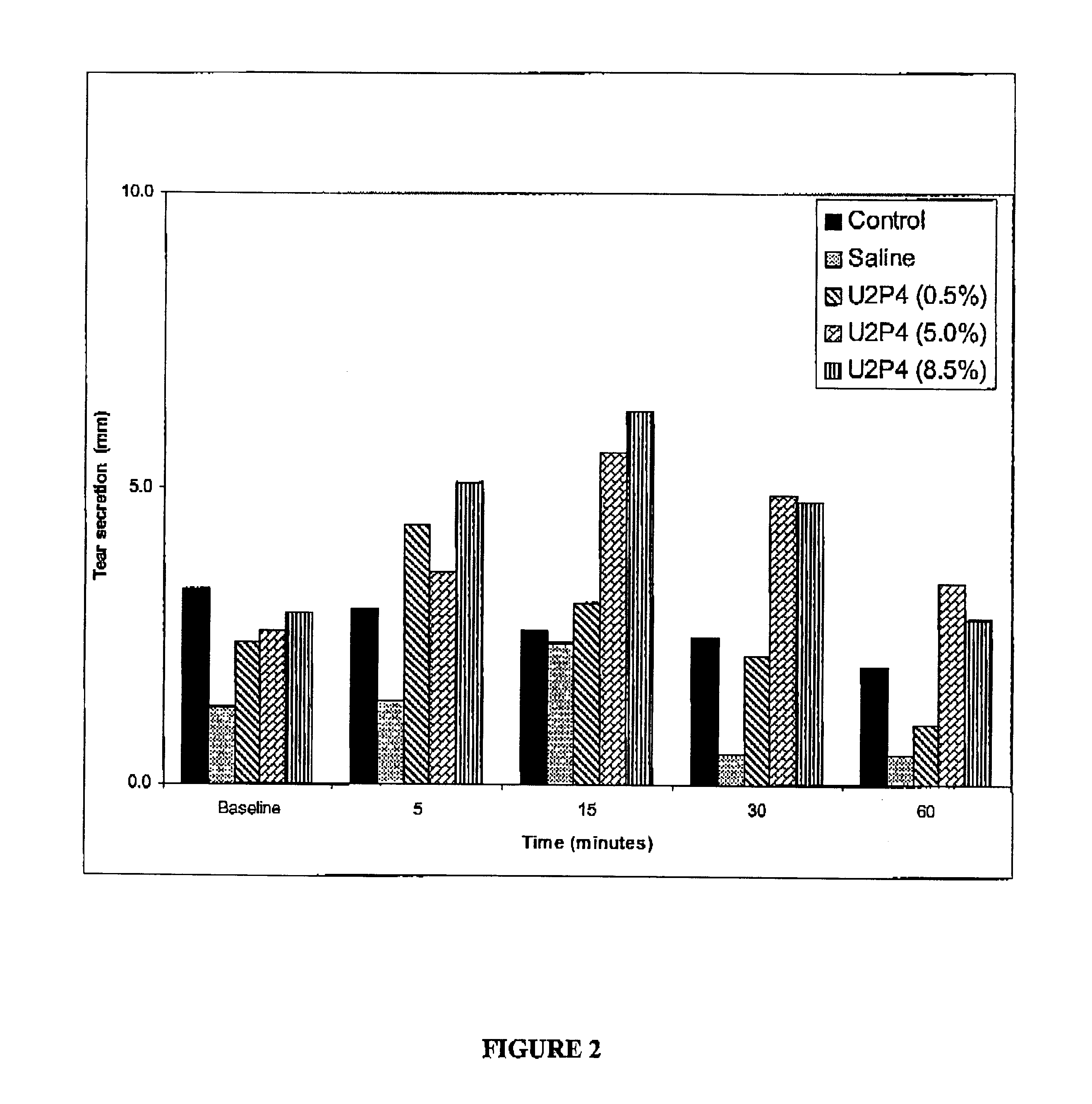
Method of treating dry eye disease with purinergic receptor agonists
A method and preparation for the stimulation of tear secretion in a subject in need of such treatment is disclosed. The method comprises administering to the ocular surfaces of the subject a purinergic receptor agonist such as uridine 5′-triphosphate [UTP], dinucleotides, cytidine 5′-triphosphate [CTP], adenosine 5′-triphosphate [ATP], or their therapeutically useful analogs and derivatives, in an amount effective to stimulate tear fluid secretion and enhance drainage of the lacrimal system. Pharmaceutical formulations and methods of making the same are also disclosed. Methods of administering the same would include: topical administration via a liquid, gel, cream, or as part of a contact lens or selective release membrane; or systemic administration via nasal drops or spray, inhalation by nebulizer or other device, oral form (liquid or pill), injectable, intra-operative instillation or suppository form.
Owner:MERCK SHARP & DOHME CORP
Methods and compositions for liver specific delivery of therapeutic molecules using recombinant AAV vectors
InactiveUS20010051611A1Prevent and limit expressionPrevents terminal glycosylationOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsDiseaseHepatic Diseases
Provided are methods for selectively expressing therapeutic molecules, such as secretory proteins, antisense molecules and ribozymes, in the liver. The methods find use in treating hepatic diseases or conditions. The methods also find use in treating any disease or condition in which systemic administration of the therapeutic substance, for example, a secretory protein, is desired. The methods involve administering to a mammalian patient having a need for liver expression of a therapeutic molecule an AAV vector containing a therapeutically effective amount of the therapeutic molecule. Also provided are novel vectors employable in these methods.
Owner:CHIRON CORP
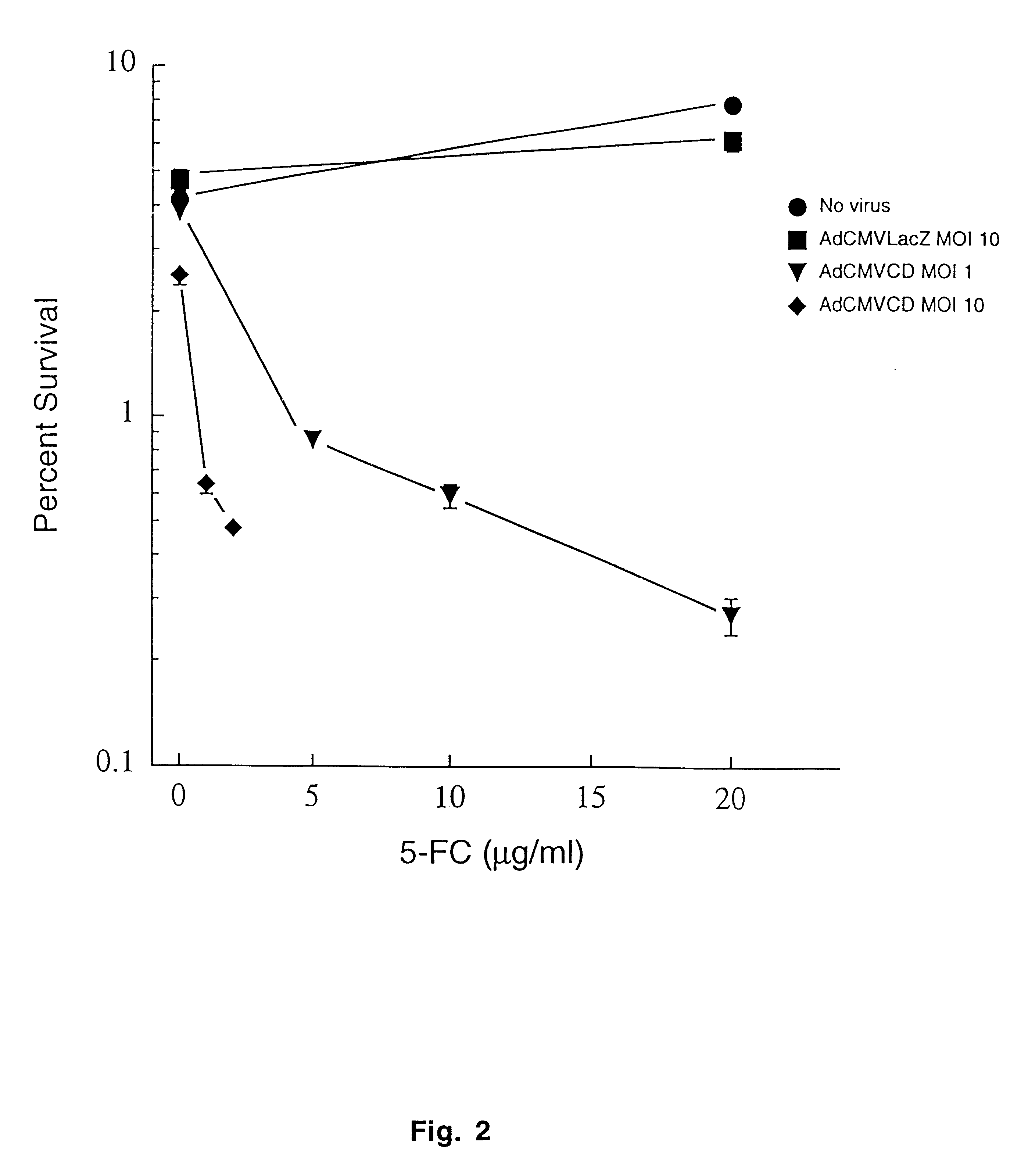
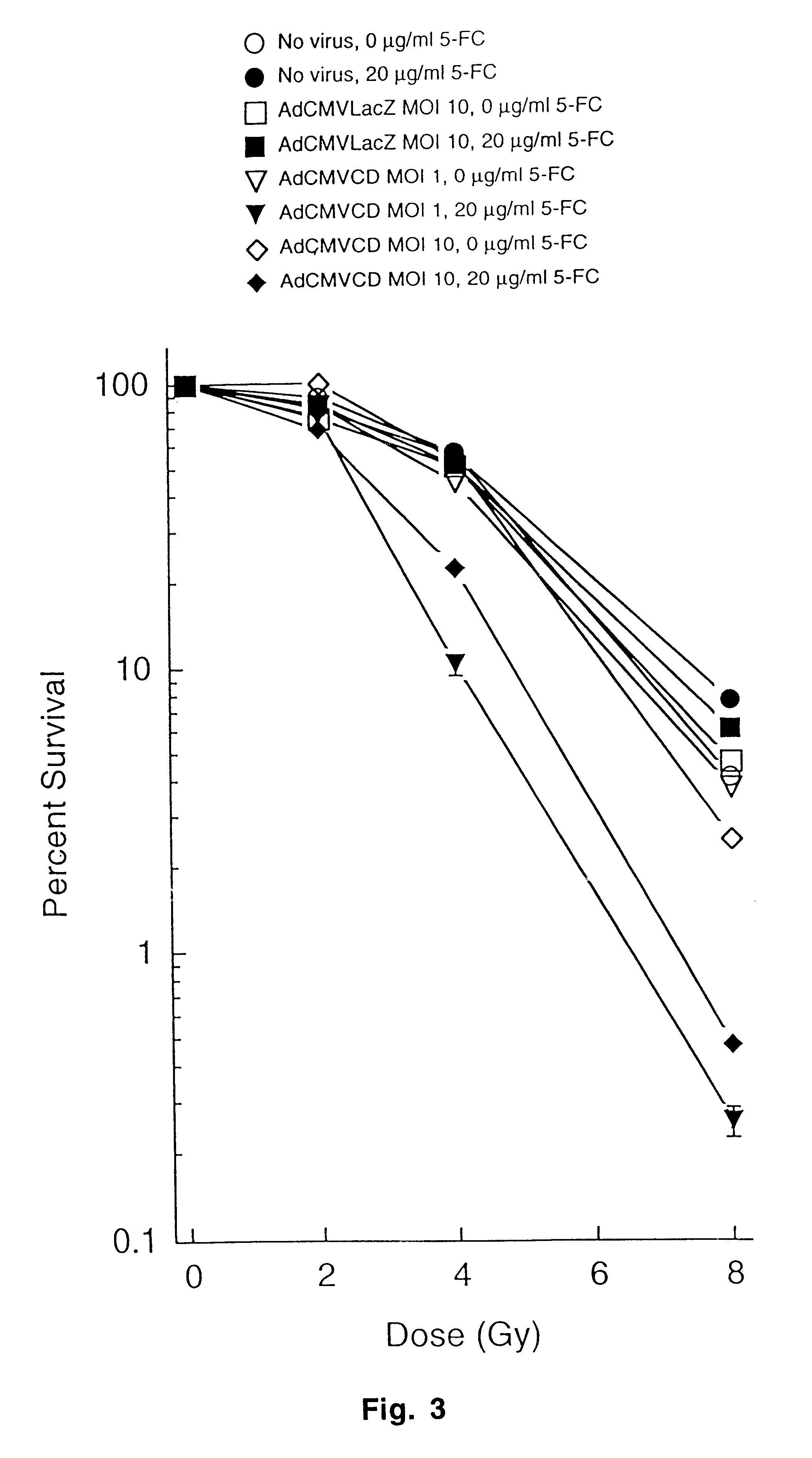
Molecular chemotherapy enhancement of radiotherapy
InactiveUS6552005B1Tumour growth inhibitionCurrent is limitedBiocidePeptide/protein ingredientsWhole bodyCytotoxicity
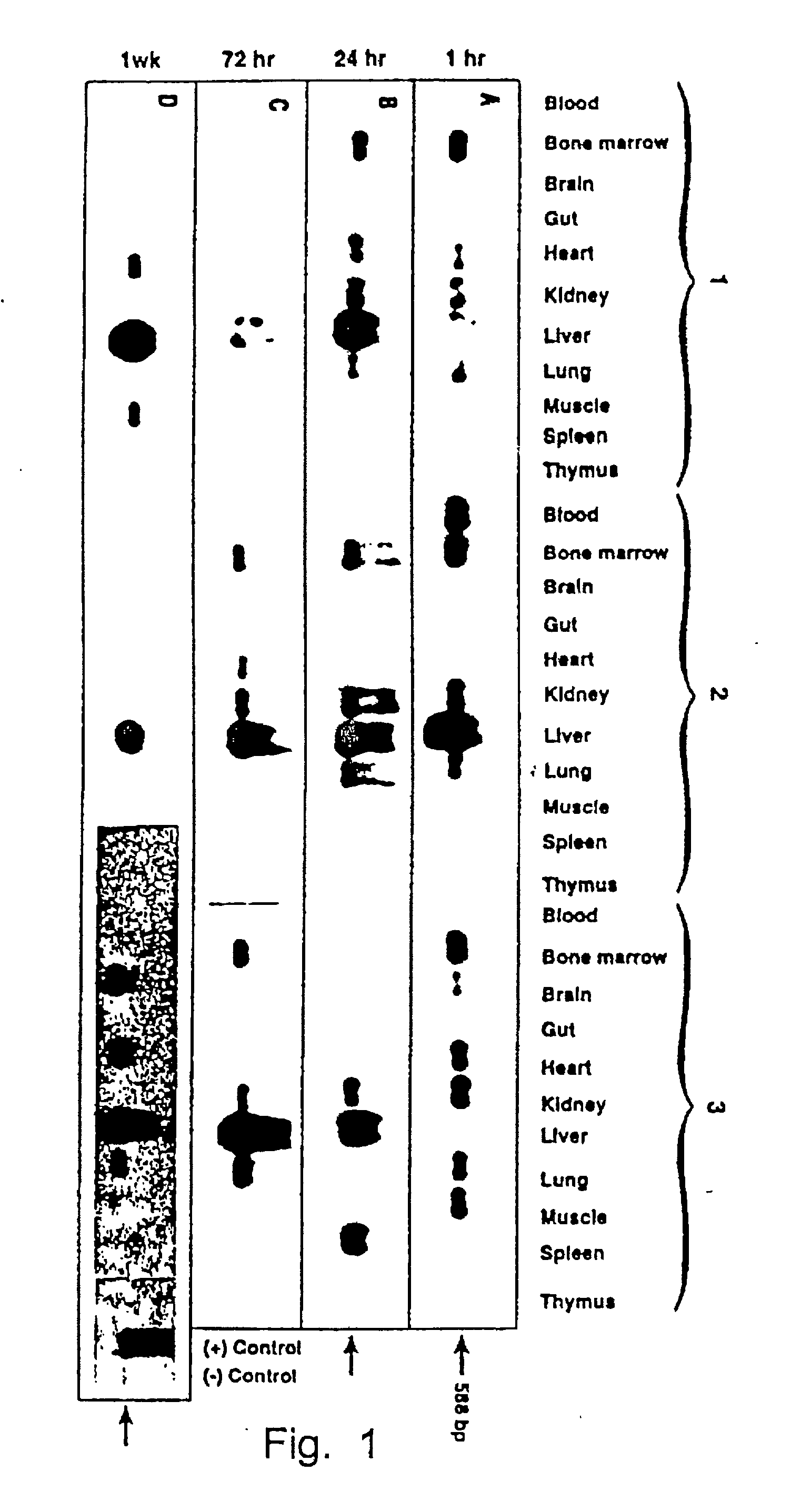
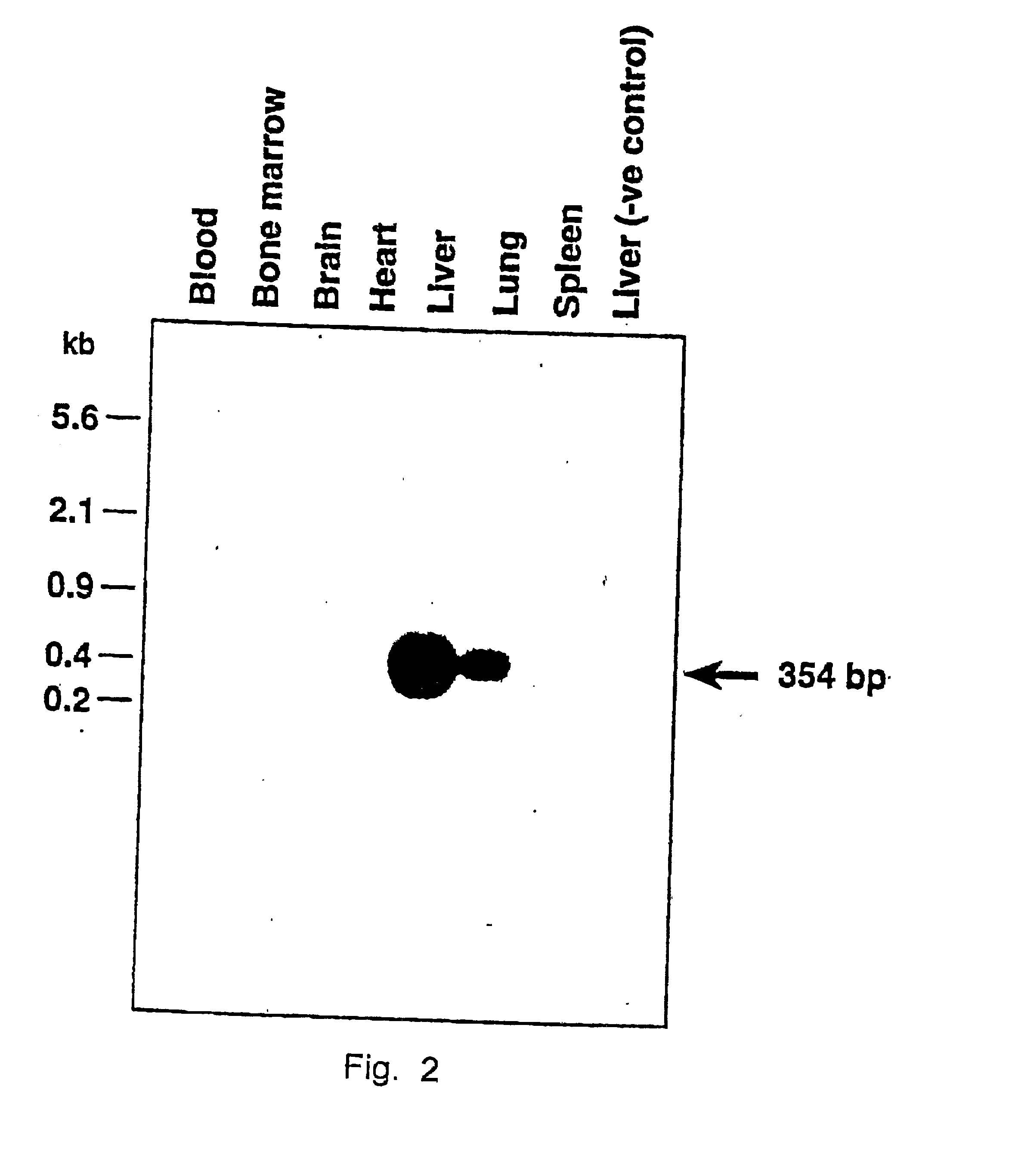
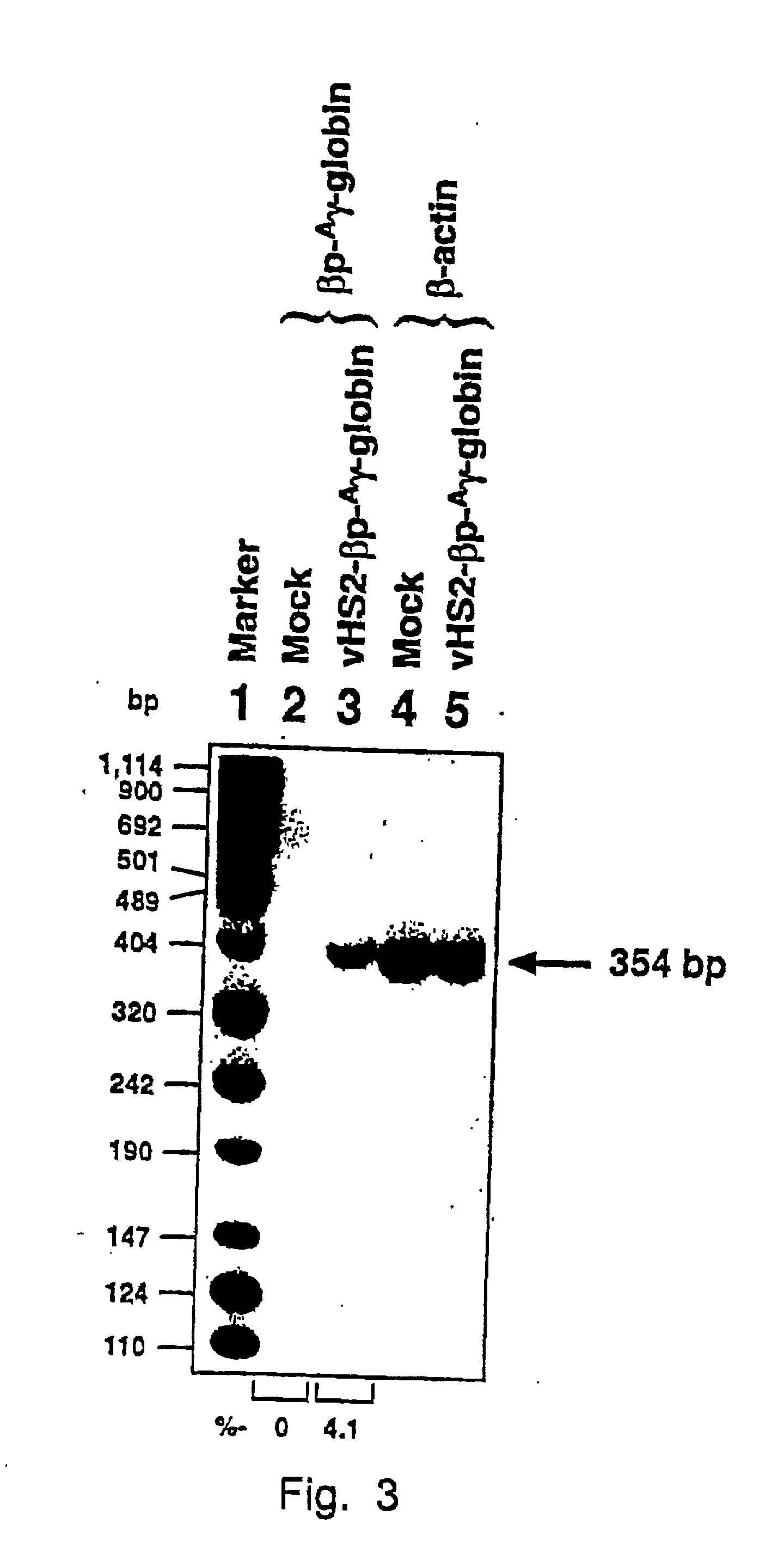
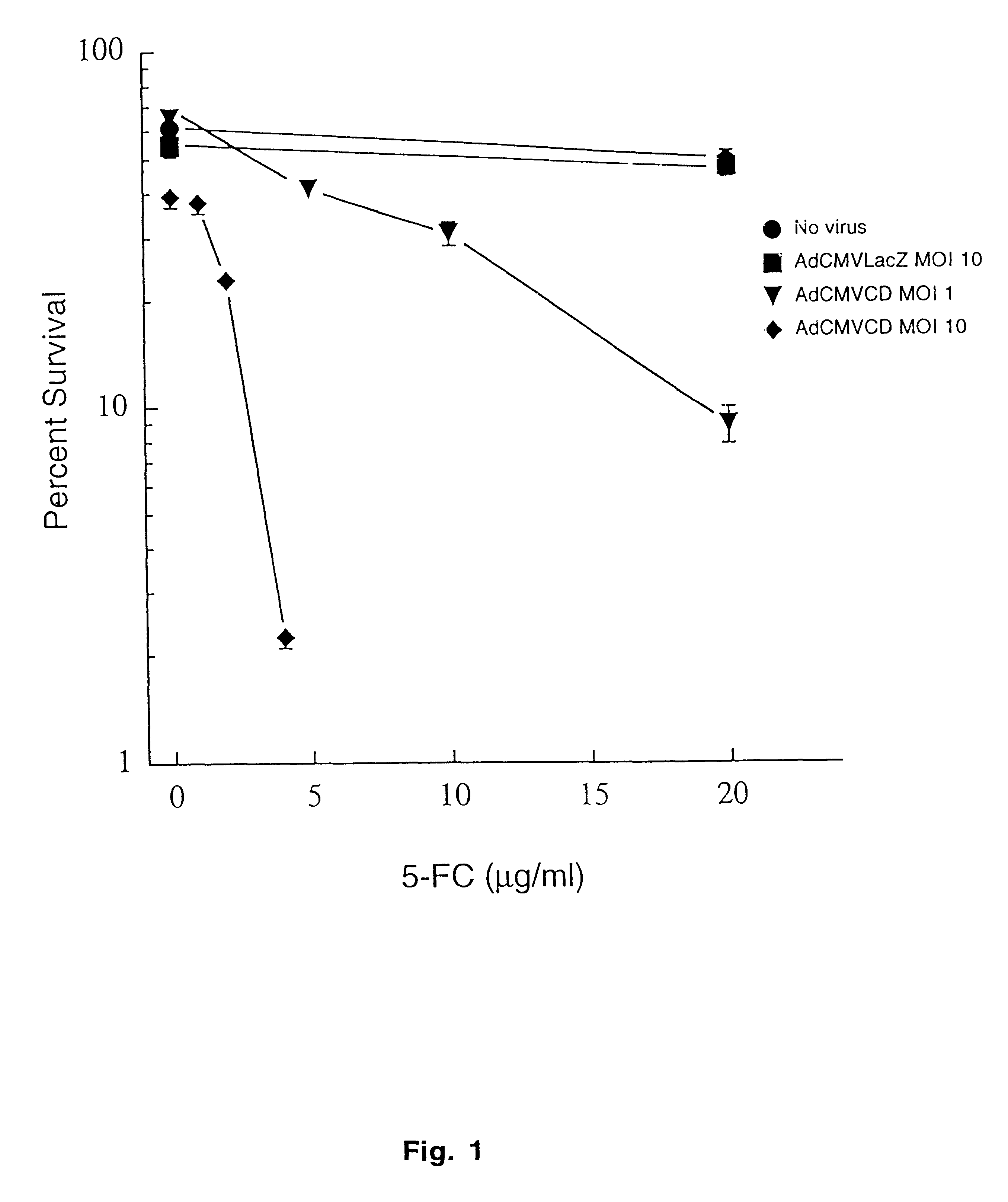
The present invention provides a new approach for cancer treatment by utilizing gene therapy combined with radiation therapy to enhance cytotoxicity in malignant cells. Specifically, the present invention demonstrates that molecular chemotherapy with the cytosine deaminase gene and 5-fluorocytosine is an effective radiosensitizing strategy which may lead to substantial improvement in tumor control, with less normal tissue toxicity than conventional systemic administration of 5-fluorouracil, that would translate into improved cure rates and better survival. A noninvasive method is described for continuous in vivo monitoring of 5-fluorouracil production via magnetic resonance spectroscopy. An adenovirus encoding cytosine deaminase gene which selectively replicates in tumor cells with a defective p53 pathway was constructed. Also provided is an adenovirus which encodes a fusion protein of cytosine deaminase and uracil phosphoribosyltransferase.
Owner:CDEPT
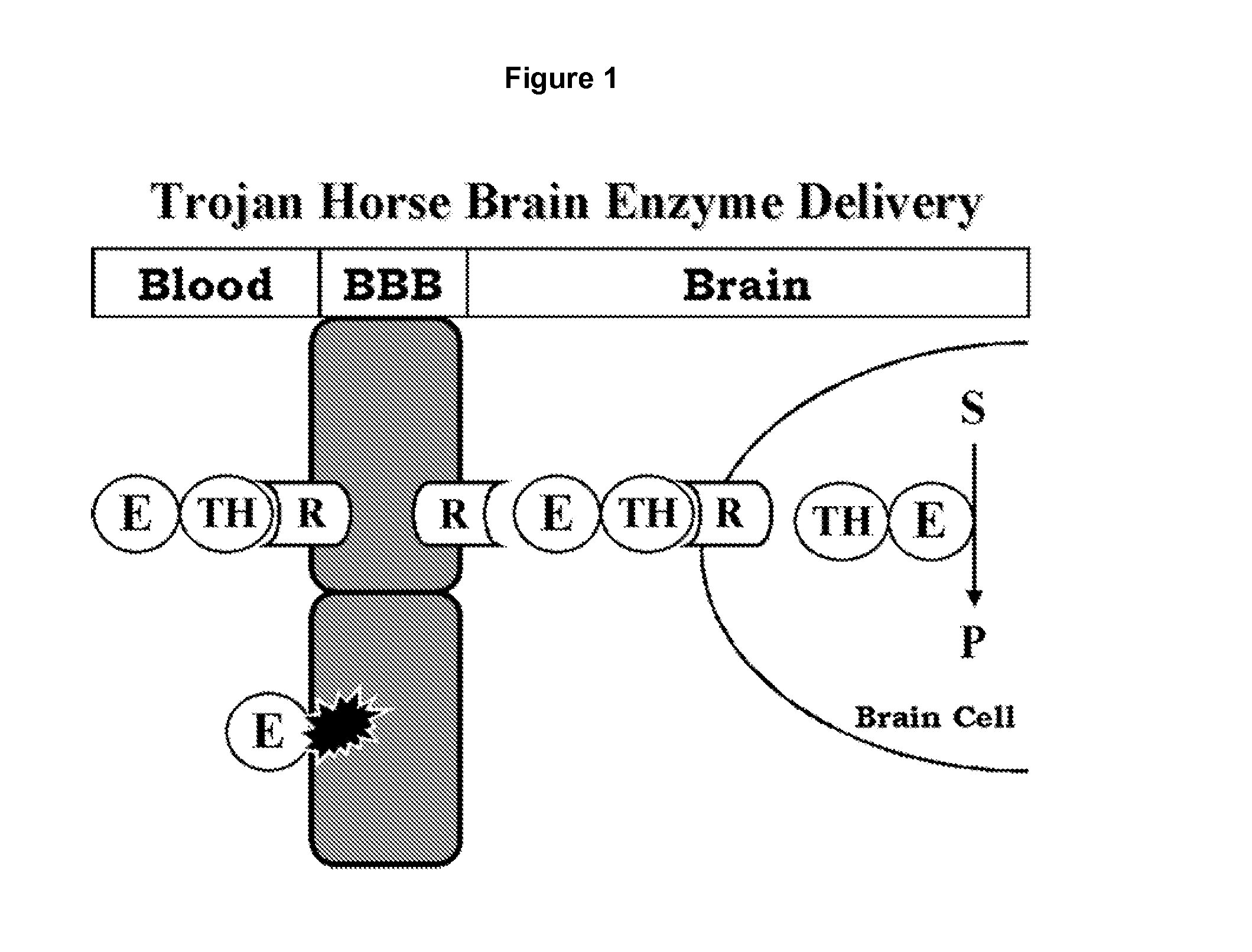
Methods for Diagnosing and Treating CNS Disorders by Trans-Blood-Brain Barrier Delivery of Protein Compositions
The invention provides methods for treating or diagnosing CNS disorders by systemic administration of therapeutic or diagnostic protein compositions that are capable of crossing the blood-brain barrier, in some embodiments in both directions, while allowing their activity once across the barrier to remain substantially intact. The agents are transported across the blood-brain barrier via one or more endogenous receptor-mediated transport systems. Also provided are methods for manufacturing the compositions used in the methods described herein.
Owner:JCR PHARMA +1
Drug-enhanced adhesion prevention
The present invention includes methods for the inhibition of post-operative adhesion formation between tissue surfaces in a body cavity having been subjected to a surgical procedure, which methods involve administering Pemirolast, or an analog thereof, directly to tissue surfaces in the body cavity in amounts and under conditions effective to inhibit formation of adhesions, and to delivery vehicles and compositions suitable for use for local, non-systemic administration of a drug to the body and directly to tissue within a body cavity having been subjected to a surgical procedure.
Owner:ETHICON INC
Local Administration of Retinoids to Treat Deficiencies in Dark Adaptation
InactiveUS20080275133A1Reduce of in dark adaptationIncrease intensityBiocideSenses disorderRetinoidSide effect
The present invention relates to improving, at least in part, a deficiency in dark adaptation for an individual. The therapy for dark adaptation includes local administration of a retinoid, such as a Vitamin A or a derivative thereof, such that deleterious side effects seen with systemic administration are avoided.
Owner:UNIV OF CALFORNIA SAN FRANCISCO
Compositions and methods for blood-brain barrier delivery of organophosphatases
InactiveUS20100098693A1Polypeptide with localisation/targeting motifNervous disorderWhole bodyOrganic phosphates
Provided herein are compositions and related methods for delivering an organophosphatase to the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody to a receptor expressed on the surface of the blood-brain barrier (BBB receptor) and an organophosphatase. In some embodiments, the compositions described herein are used to treat a subject suffering from or at high risk of exposure to an organophosphate (e.g., a nerve gas).
Owner:ARMAGEN TECH
Compositions and Methods for Inducing Nanoparticle-mediated Microvascular Embolization of Tumors
InactiveUS20140363496A1Improve permeabilityImprove retentionPowder deliveryPeptide/protein ingredientsNitric oxideWilms' tumor
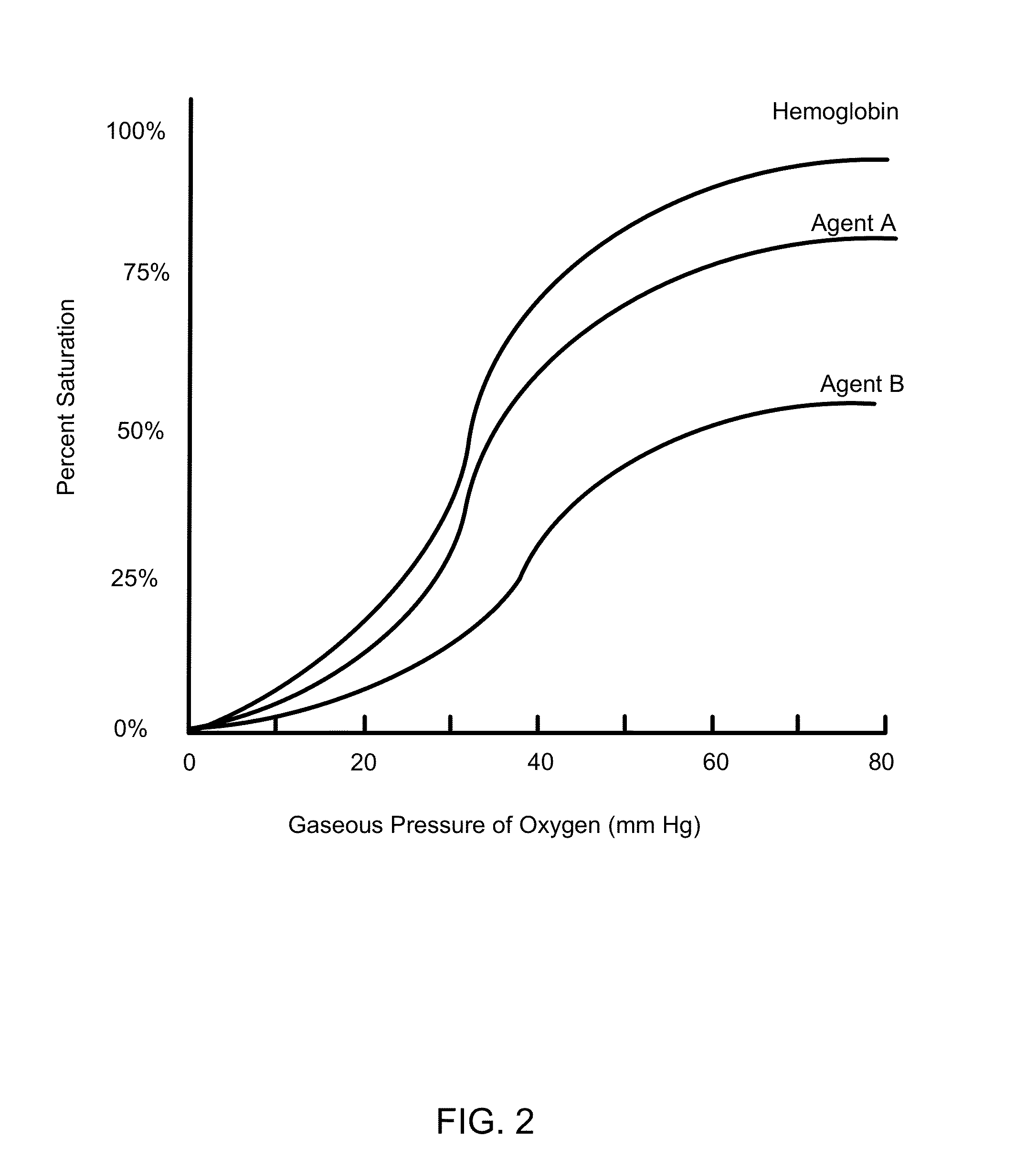
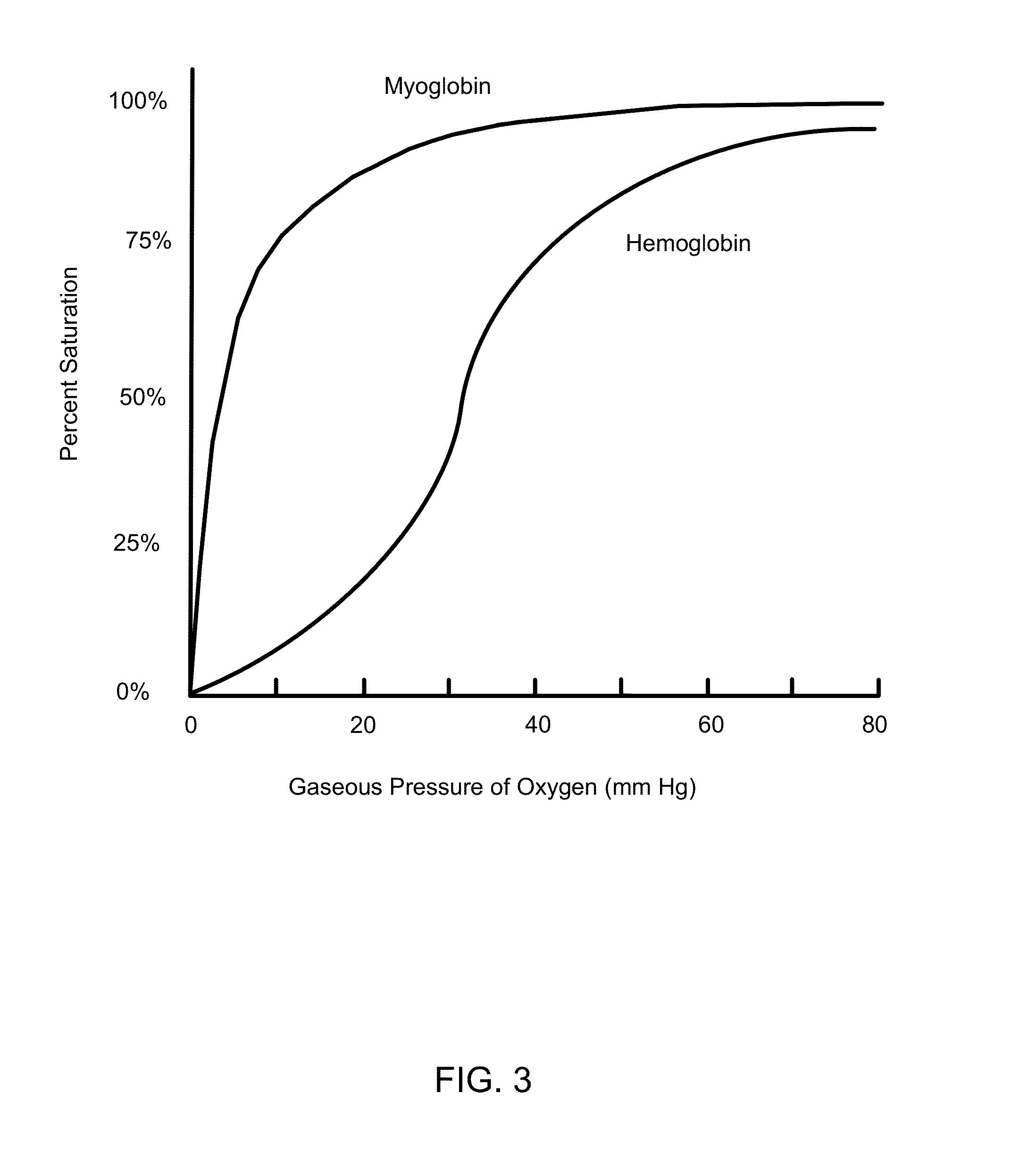
Nanoparticle mediated microvascular embolization (NME) of tumor tissue may occur after systemic administration of PEM, leading to widespread shutdown of vascular flow, hemorrhage, and necrosis. PEM constructs are developed that incorporate large amounts of iron-containing protein, possess high oxygen affinities, and demonstrate delayed nitric oxide binding. Such properties induce selective NME of tumors after extravasation, and will likely enhance the effect of VEGFR TKIs and / or mTOR inhibitors.
Owner:POSEIDA THERAPEUTICS INC
Treatment method against side-effects of chemotherapy
InactiveUS6979688B2Preserving anti-cancer systemic efficacyAdverse side effectBiocideKetone active ingredientsSide effectWhole body
A method and composition is provided for organ rescue wherein a specific counter-measure is applied locally to a tissue at risk for or exhibiting an adverse side effect of a cancer treatment. More particularly, the method and composition is directed at controlling Hand-Foot Syndrome, a painful redness and cracking of the skin of the hands and feet which can occur with systemic treatment with 5-fluorouracil or a precursor thereof. Uracil ointment is applied to the skin of the hands and feet to prevent Hand-Foot Syndrome which can occur from systemic administration of 5-fluorouracil (or precursor thereof) as cancer treatment.
Owner:ASYMMETRIC THERAPEUTICS
Methods and compositions for increasing alpha-l-iduronidase activity in the CNS
Provided herein are methods and compositions for treating a subject suffering from a deficiency in α-L-Iduronidase in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody to a human insulin receptor and an α-L-Iduronidase. A therapeutically effective systemic dose is based on the specific CNS uptake characteristics of human insulin receptor antibody-α-L-Iduronidase fusion antibodies as described herein.
Owner:ARMAGEN INC +1
Tranexamic acid skin externally applied nano-preparation, as well as preparation method and use thereof
InactiveCN103565743AIncrease percutaneous permeabilityGood curative effectPeptide/protein ingredientsEmulsion deliveryUse medicationMedicine
The invention relates to a tranexamic acid skin externally applied nano-preparation, as well as a preparation method and a use thereof. The nano-preparation is prepared from tranexamic acid, medicated oil, an emulsifier, pharmaceutical additives, a thickener and pure water. The tranexamic acid skin externally applied nano-preparation provided by the invention has good physiological skin compatibility and can effectively promote the percutaneous absorption of tranexamic acid, improve the effect of preventing or treating pigmentation of the tranexamic acid and avoid toxicity and side effects, which are caused by systemic administration of the tranexamic acid.
Owner:CENT HOSPITAL XUHUI DISTRICT SHANGHAI CITY +1
Composition and method for treatment of joint damage
ActiveUS7485629B2Facilitating nutrient transferPromote regenerationBiocideCarbohydrate active ingredientsWhole bodyInflammatory arthropathy
The invention provides compositions useful for the treatment and / or prevention of damage to diarthrodial (synovial) joints and, in particular, traumatic synovitis, inflammation of the synovial membrane, and damage to the articular cartilage of the joint. Specifically, provided are compositions specially formulated for intra-articular and / or parenteral use in the treatment and / or prevention of traumaticsynovitis and / or damage to articular cartilage. Compositions adapted specifically for post surgical joint lavage or treatment and / or prevention of inflammatory arthritis, osteoarthritis (OA) and / or degenerative joint disease (DJD) are also provided. Compositions adapted for intra-articular and / or systemic administration comprised of therapeutic amounts of: chondroitin sulfate and hyaluronan (hyaluronic acid) are provided.
Owner:ARTHRODYNAMIC HLDG LLC
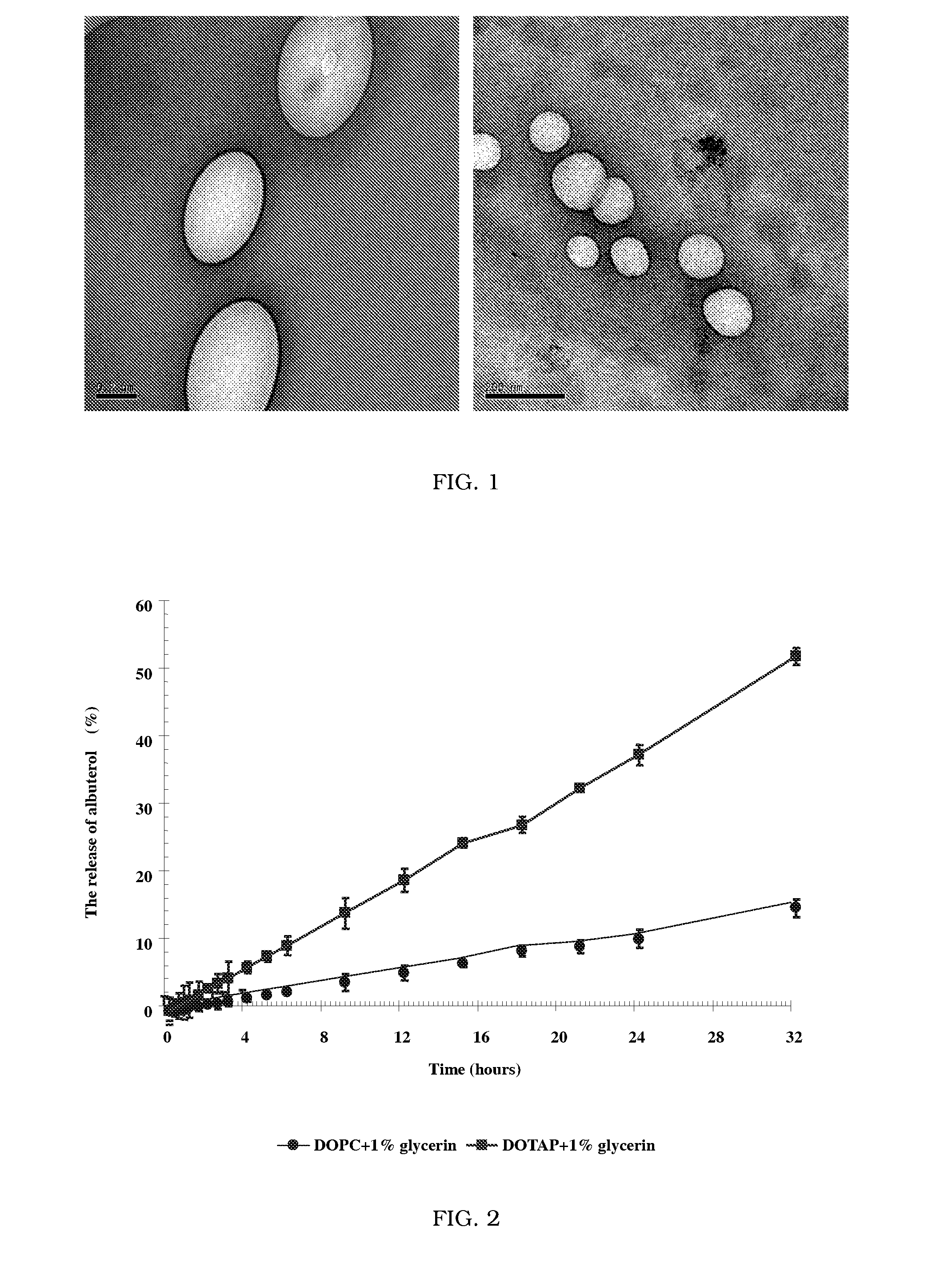
Novel formulation of dehydrated lipid vesicles for controlled release of active pharmaceutical ingredient via inhalation
InactiveUS20090047336A1Reduce systemic side effectsLow toxicityOrganic active ingredientsBiocideLipid formationSide effect
A new formulation of dehydrated lipid vesicles employs a vesicle preserver and permits the control of release and delivery of active pharmaceutical ingredients into the respiratory system for treatment in particular of asthma. The typical formulation provides controlled release of the active pharmaceutical ingredient from 0% to 100% from 0 to 72 hours after inhalation, changes the systemic administration to topical administration, allows prolonged therapeutic period for one administration, increased stability, with reduced dose, reduced systemic side effects, reduced toxicity.
Owner:HONG KONG BAPTIST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com