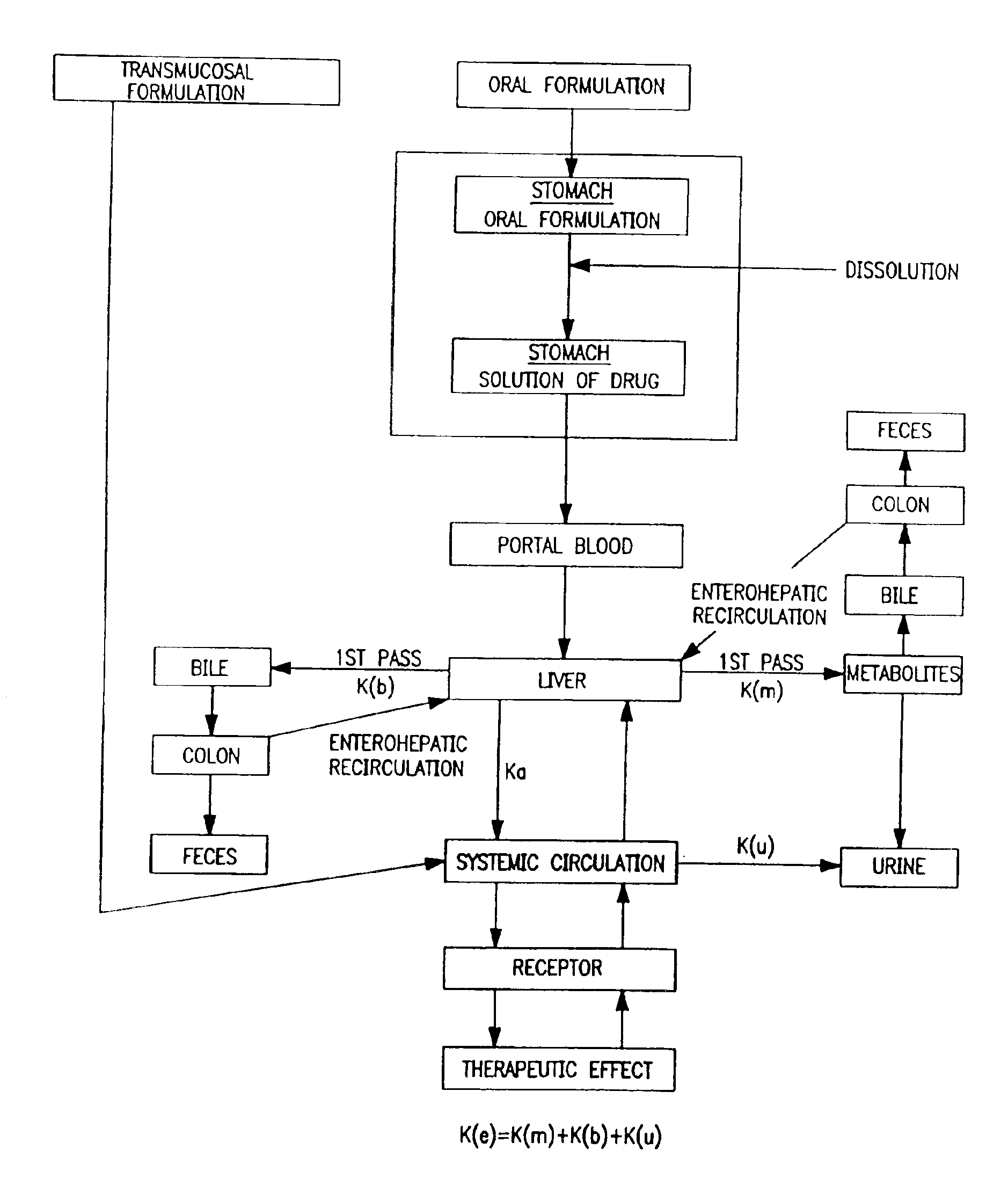
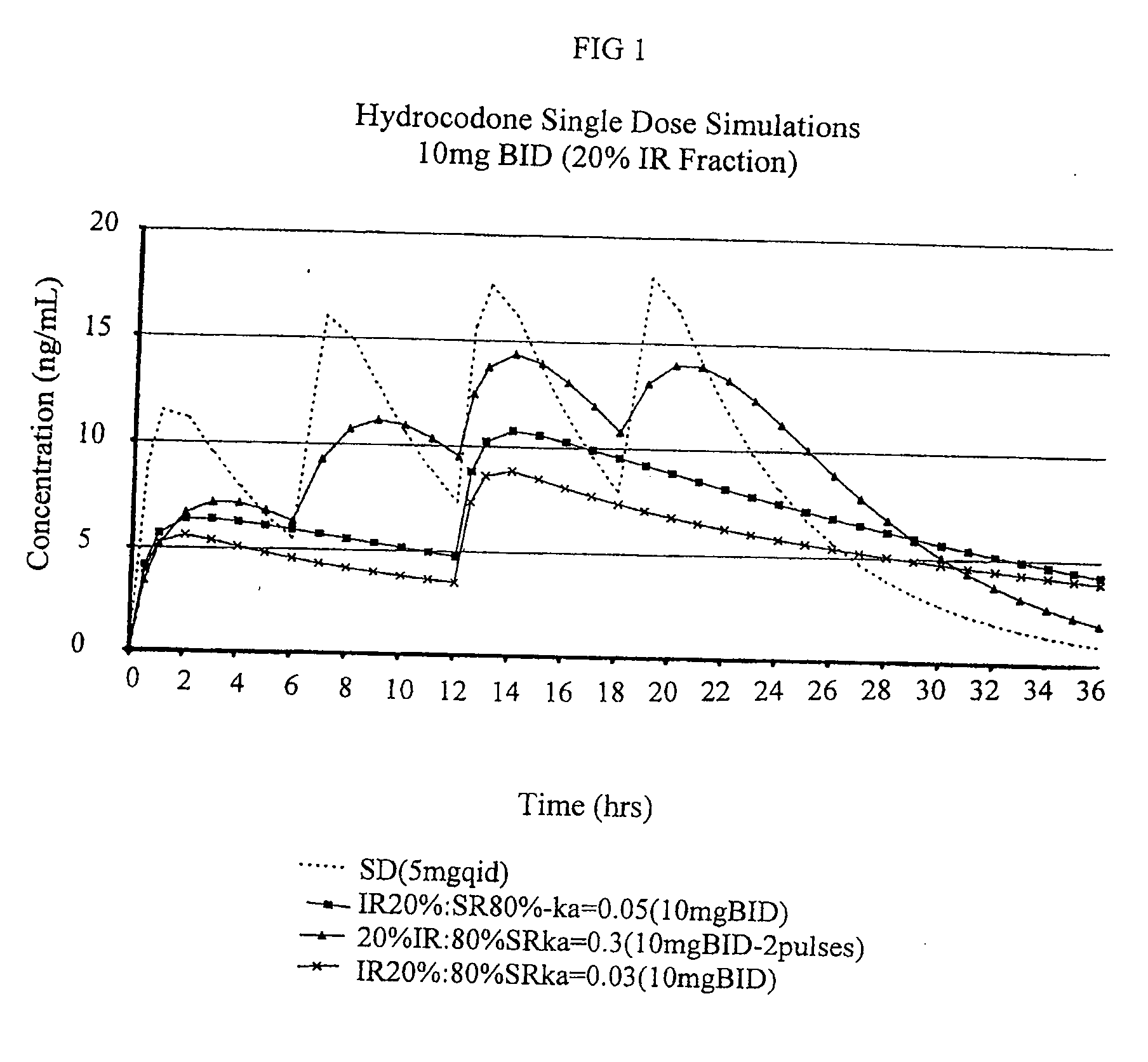
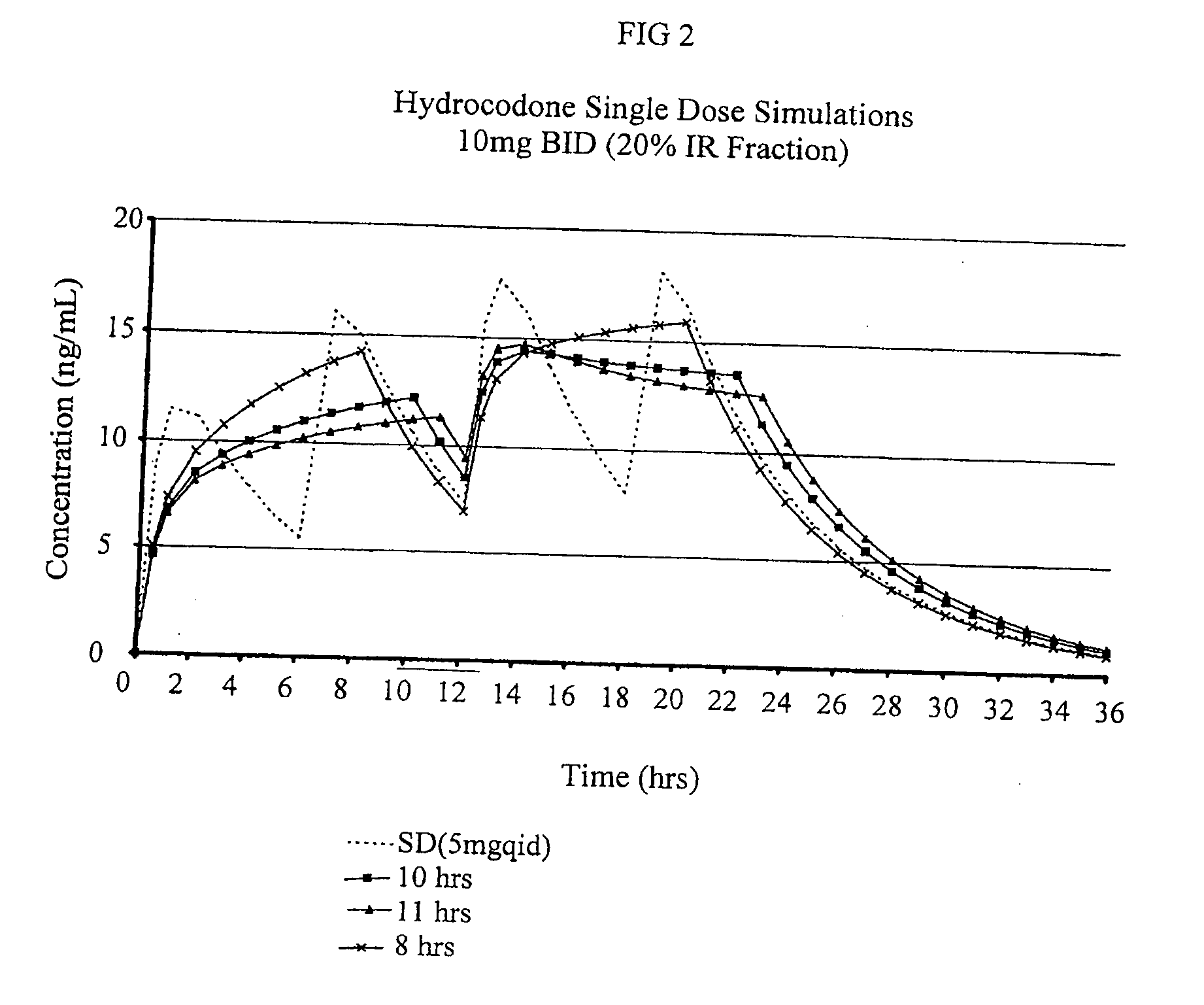
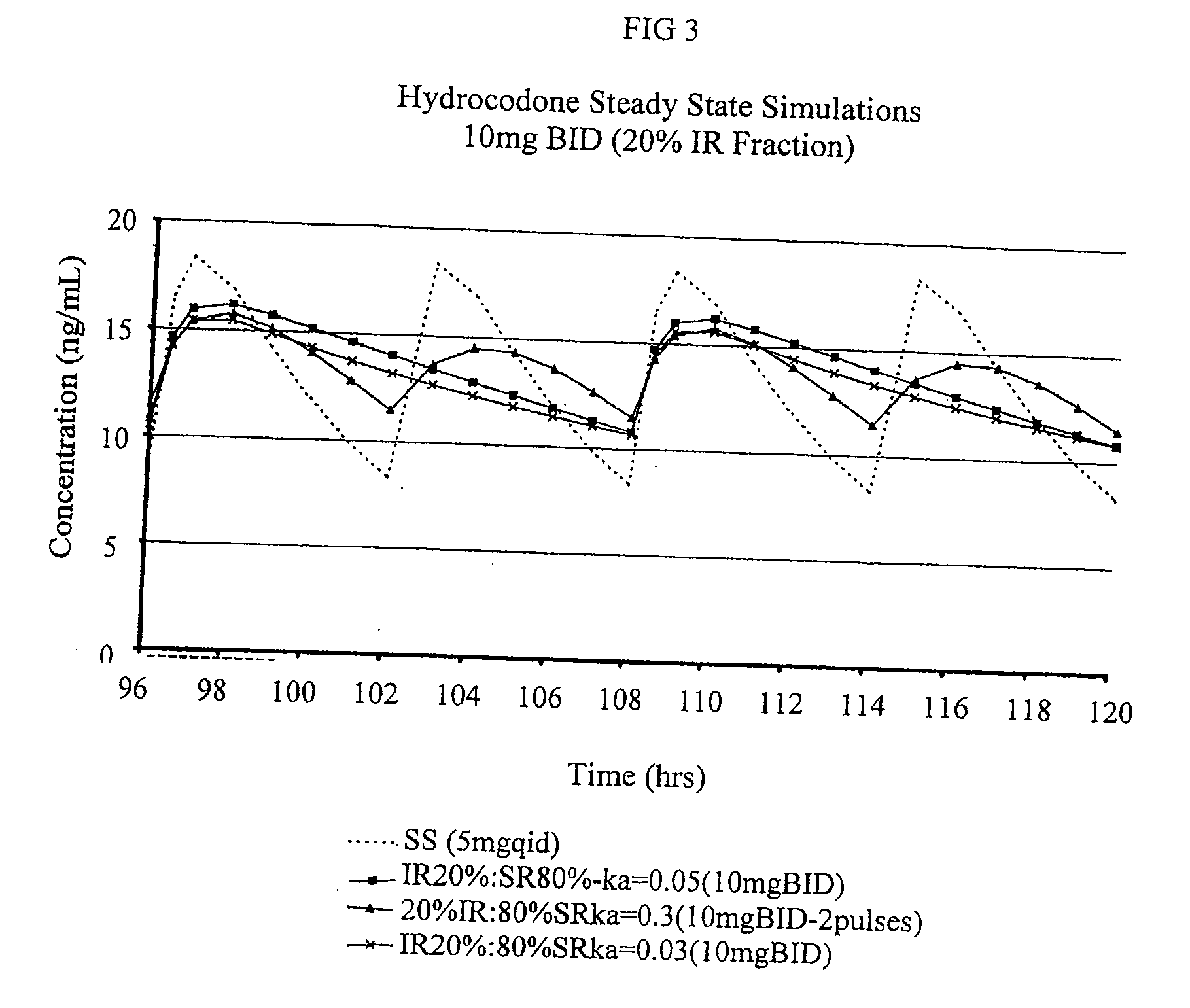
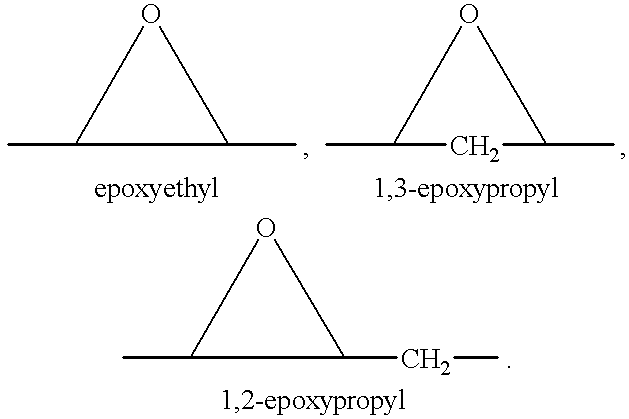Patents
Literature
284results about How to "Rapid onset" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
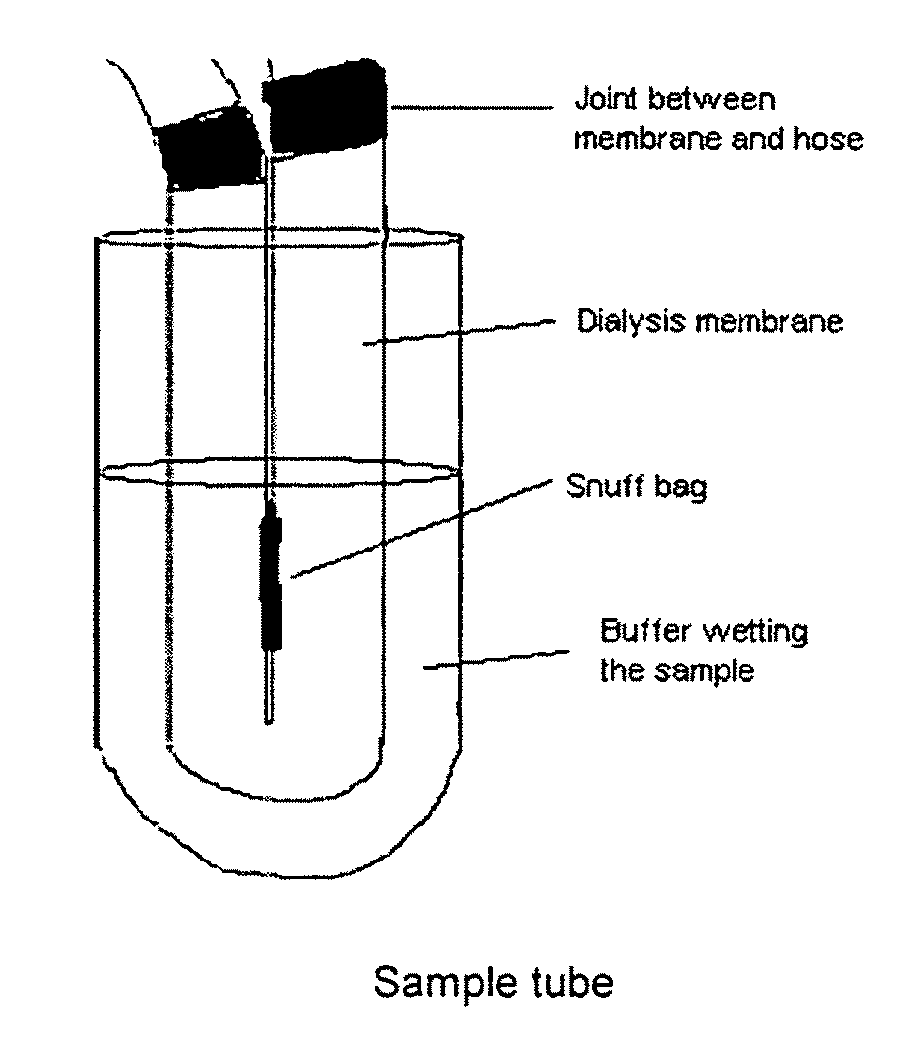
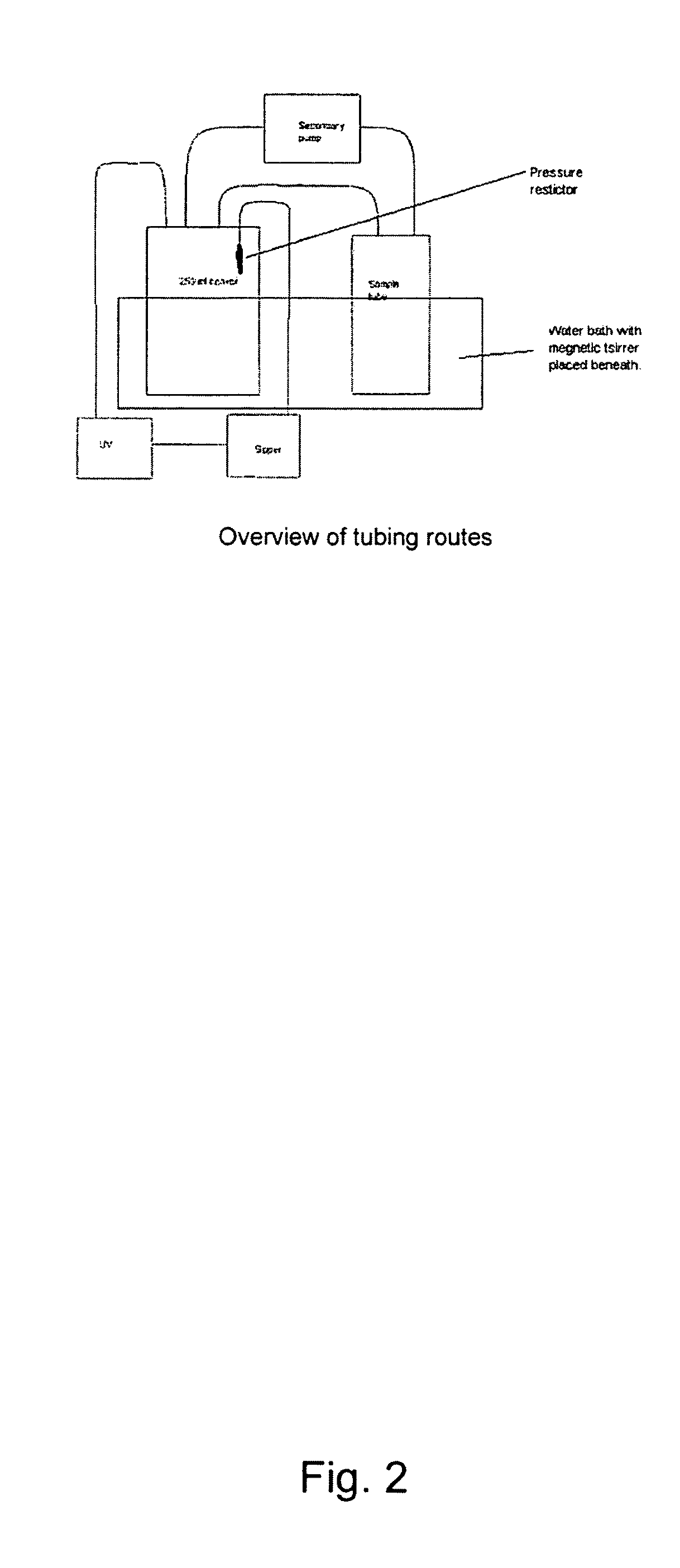
Snuff Composition
ActiveUS20090293895A1Quick effectRapid onsetPowder deliveryOrganic active ingredientsCellulosePharmacology
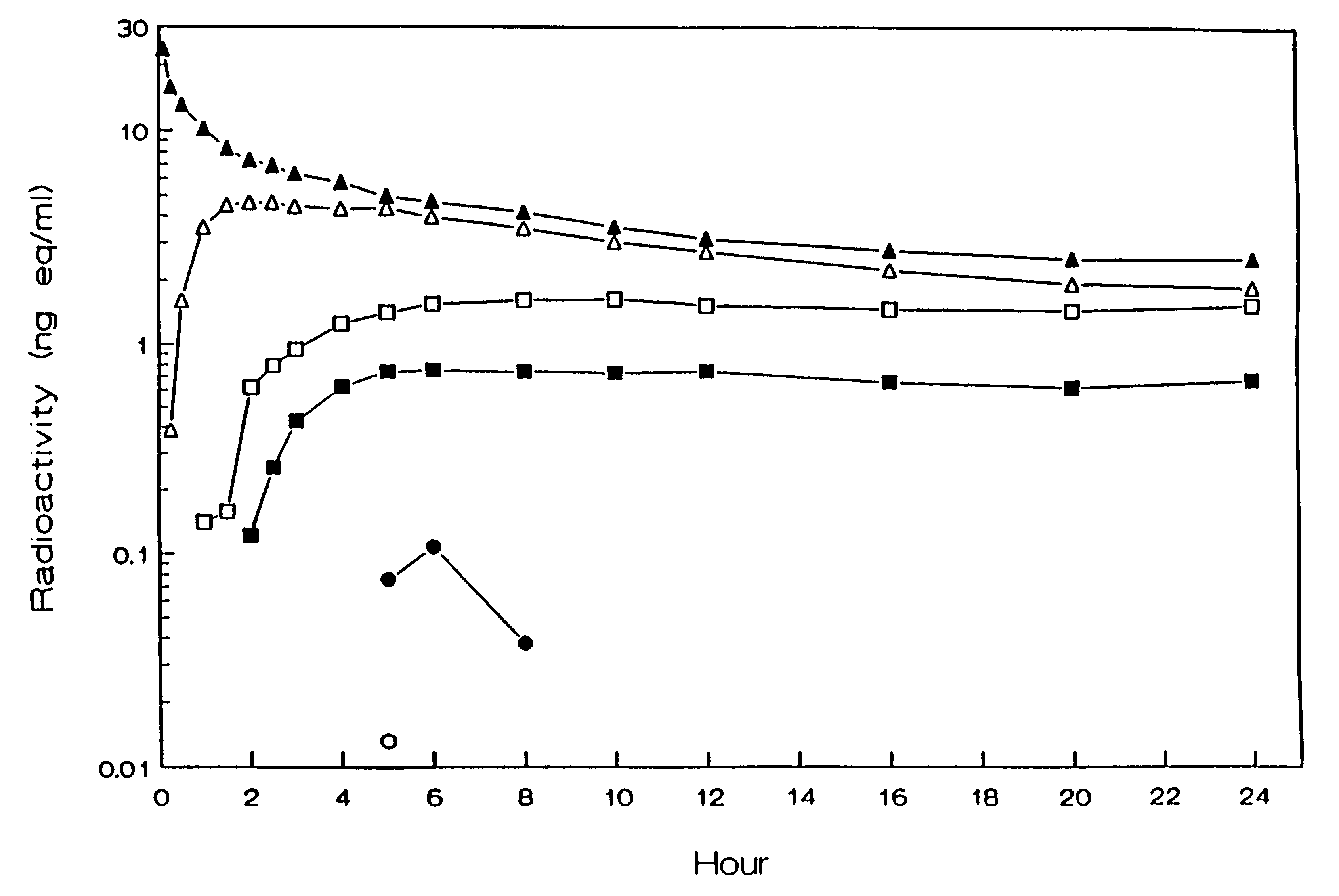
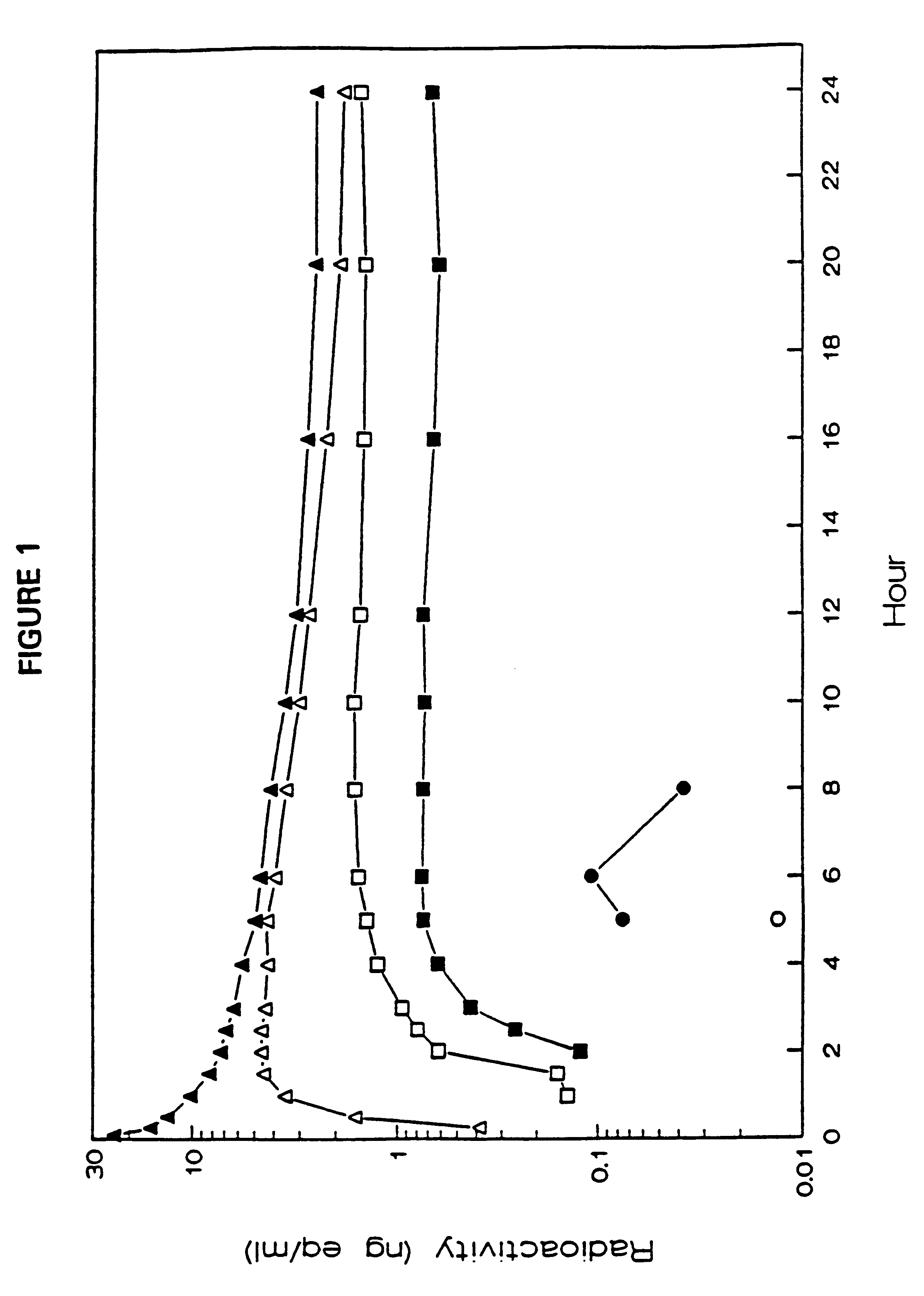
Use of a nicotine-cellulose combination for the preparation of a snuff composition for achievement of a fast onset of action of nicotine after application of the snuff composition to the oral cavity of a subject, wherein the composition has a high release rate so that when subjected to an in vitro dissolution test about 45% or more of the total content of nicotine is released within 30 minutes. Moreover, the invention relates to an improved snuff composition for application to the oral cavity.
Owner:MODORAL BRANDS INC
Use of mometasone furoate for treating airway passage and lung diseases
InactiveUS6057307AMaximize treating said rhinitisMinimize absorptionPowder deliveryBiocideDiseaseAerosolize
The administration of aerosolize particles of mometasone furoate in the form of dry powders, solutions, or aqueous suspension for treating corticosteroid-responsive diseases of the surfaces of upper and / or lower airway passages and / or lungs, e.g., allergic rhinitis and asthma is disclosed.
Owner:MERCK SHARP & DOHME CORP
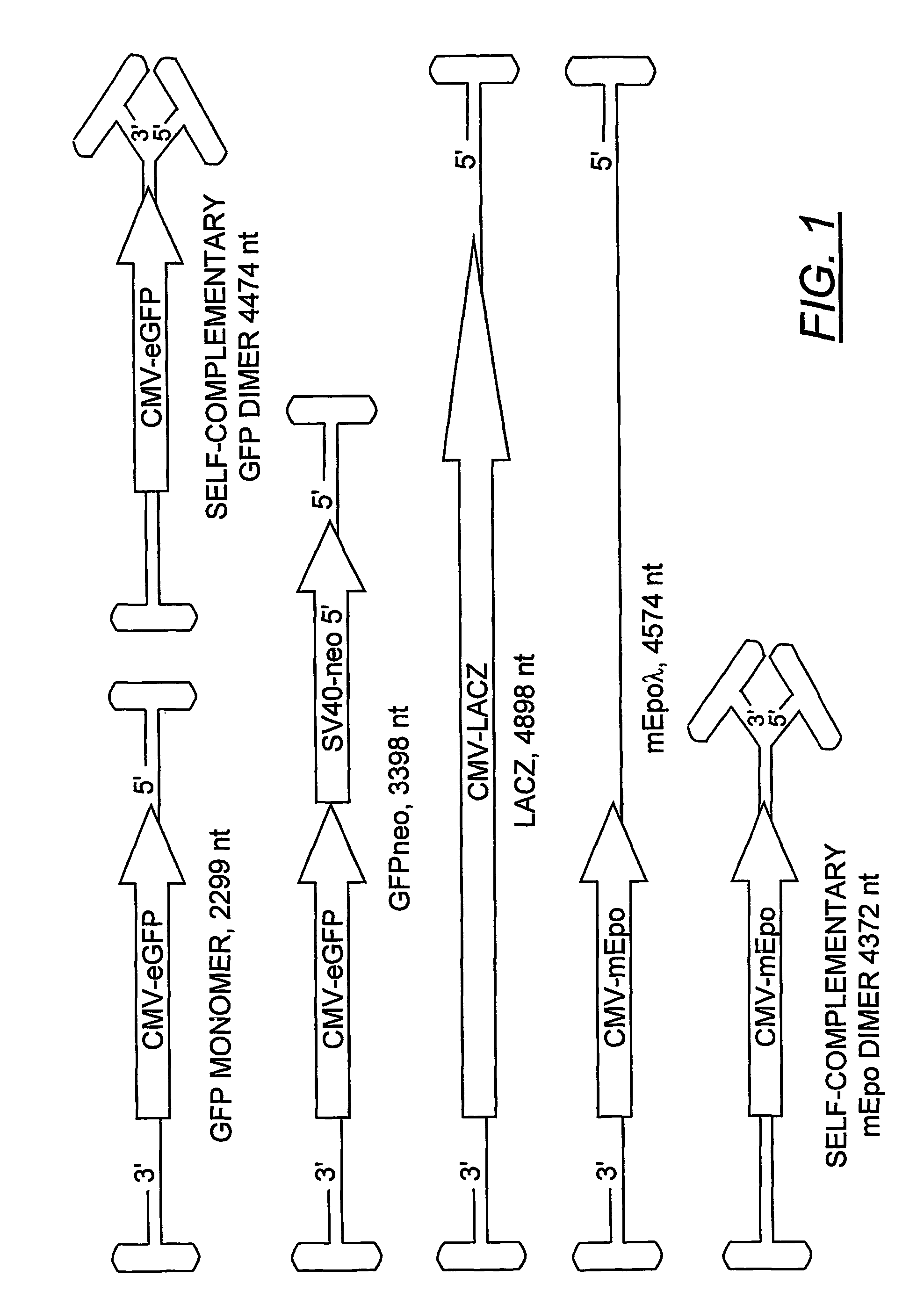
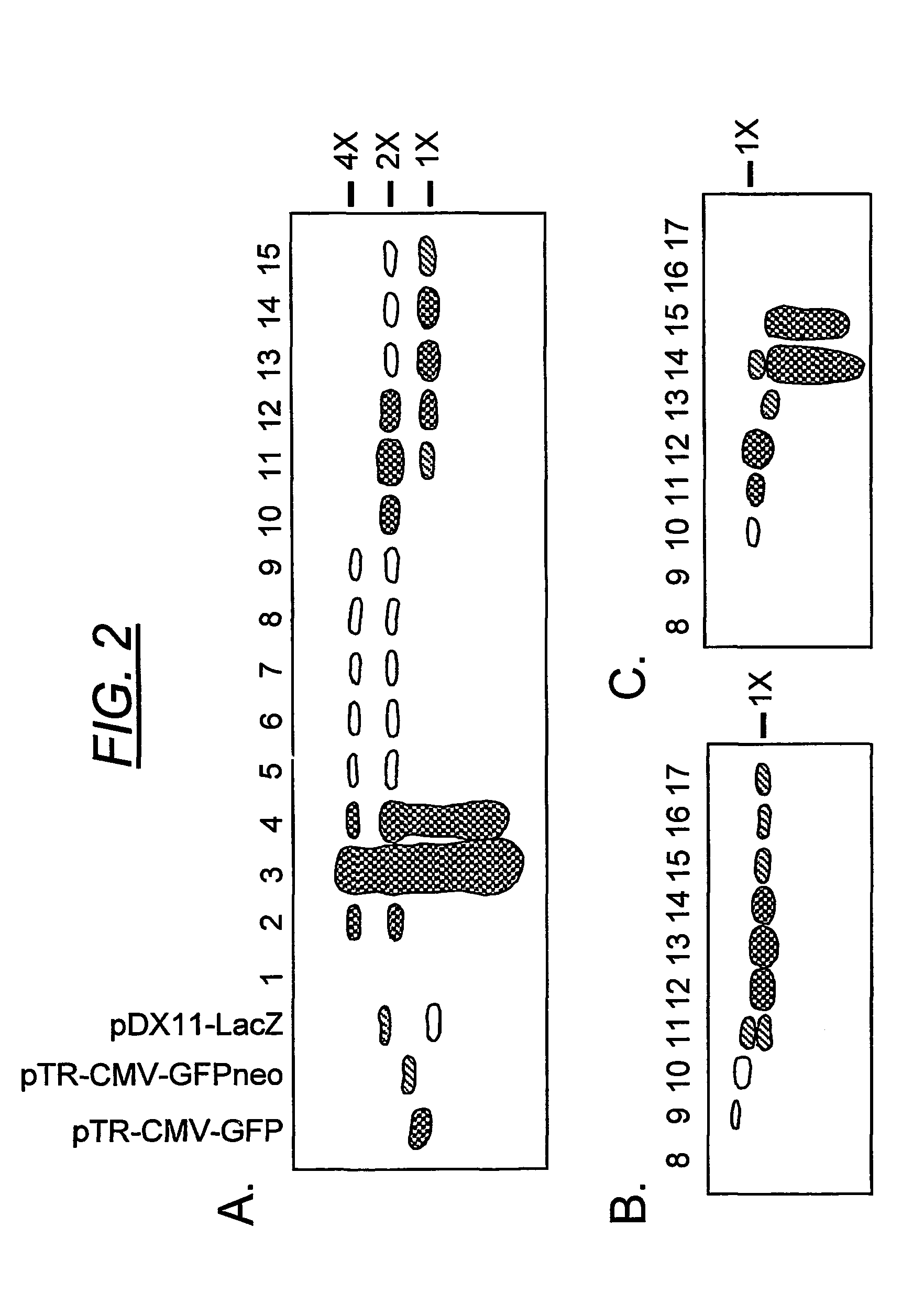
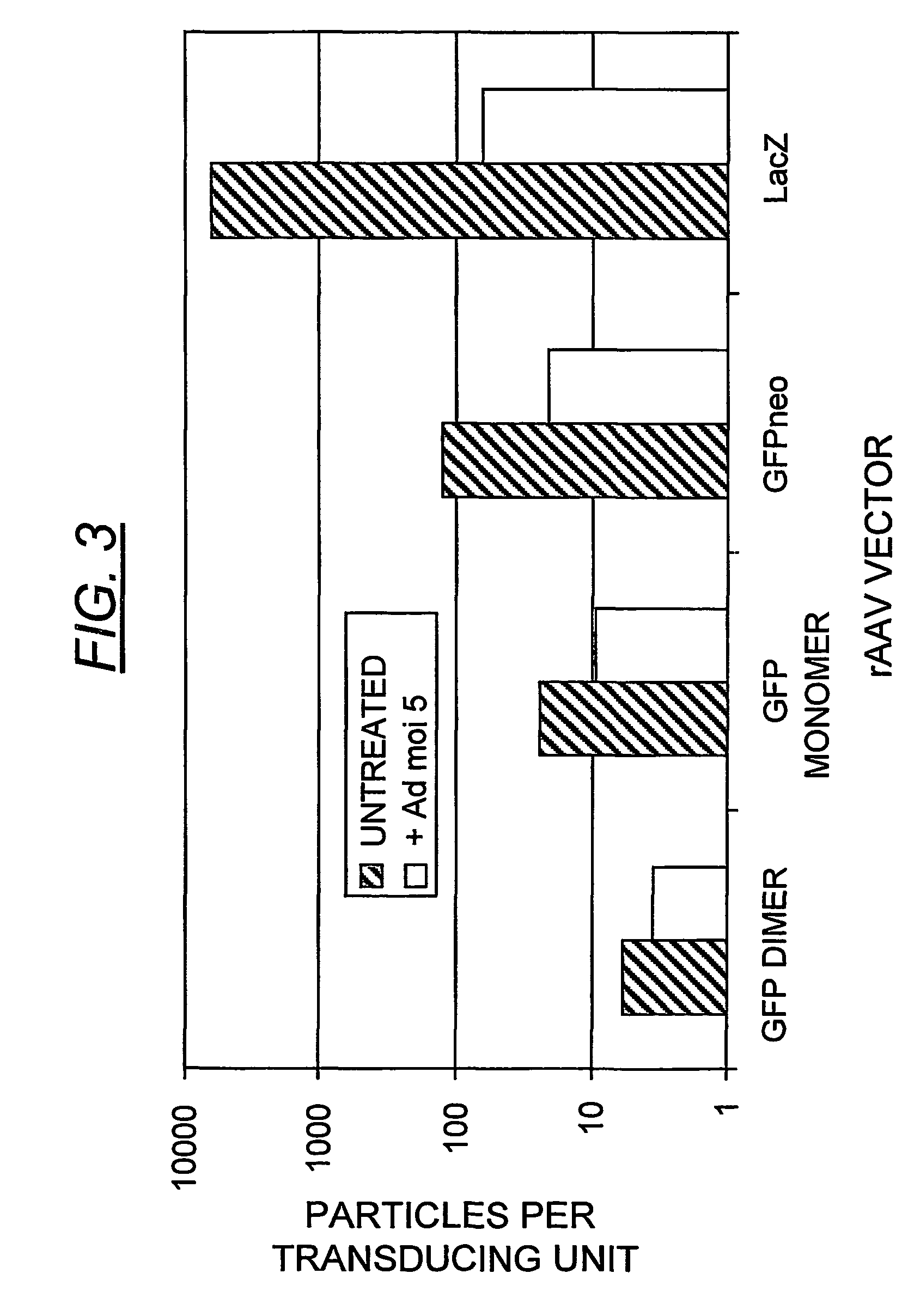
Duplexed parvovirus vectors
InactiveUS7465583B2High transduction efficiencyRapid onsetBiocidePeptide/protein ingredientsGeneticsViral vector
The present invention provides duplexed parvovirus vector genomes that are capable under appropriate conditions of forming a double-stranded molecule by intrastrand base-pairing. Also provided are duplexed parvovirus particles comprising the vector genome. Further disclosed are templates and methods for producing the duplexed vector genomes and duplexed parvovirus particles of the invention. Methods of administering these reagents to a cell or subject are also described. Preferably, the parvovirus capsid is an AAV capsid. It is further preferred that the vector genome comprises AAV terminal repeat sequences.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Delayed release pharmaceutical composition containing 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol
InactiveUS20050058706A1Good repeatabilityGood treatment effectOrganic active ingredientsBiocideHydrophobic polymerBULK ACTIVE INGREDIENT
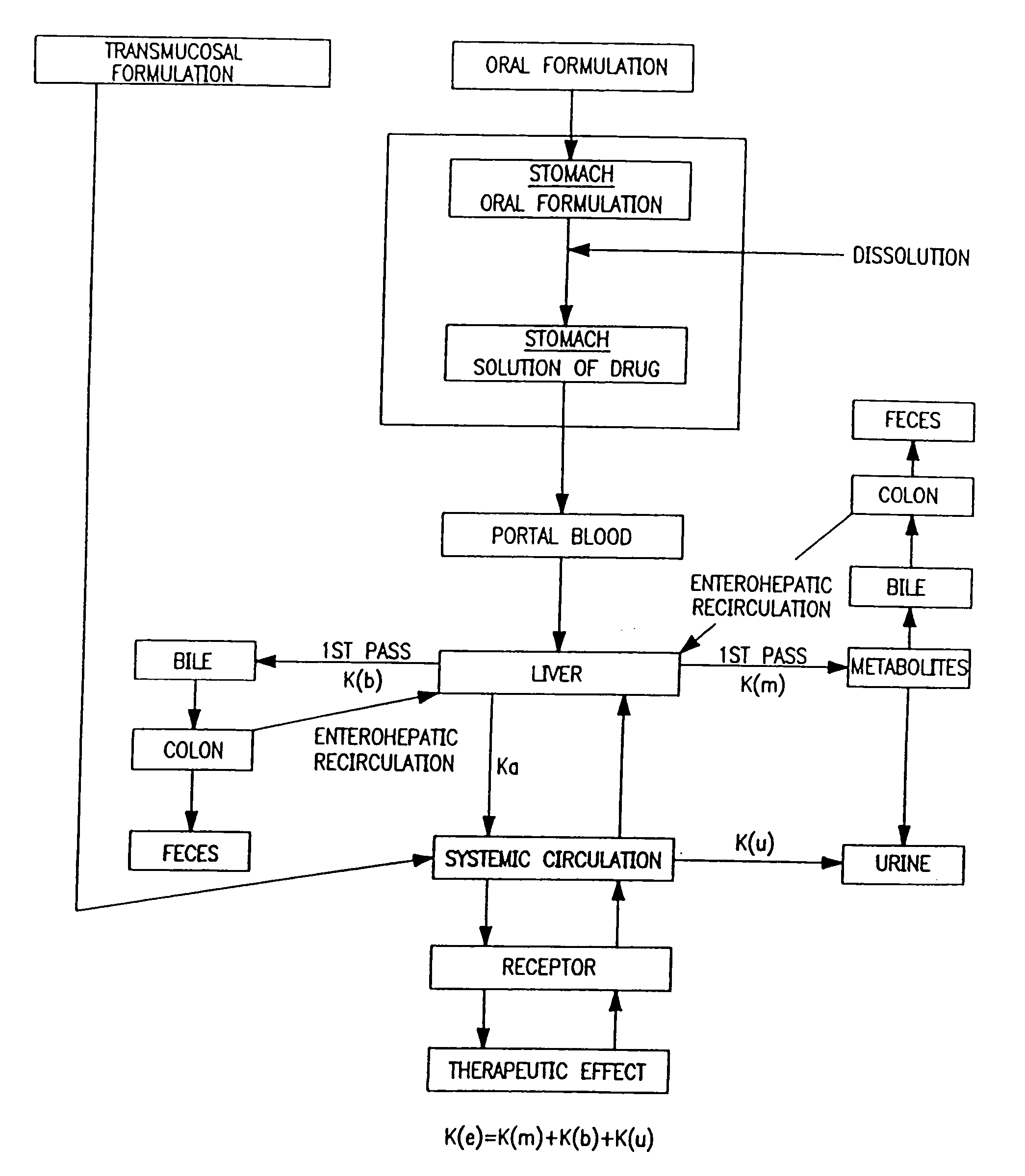
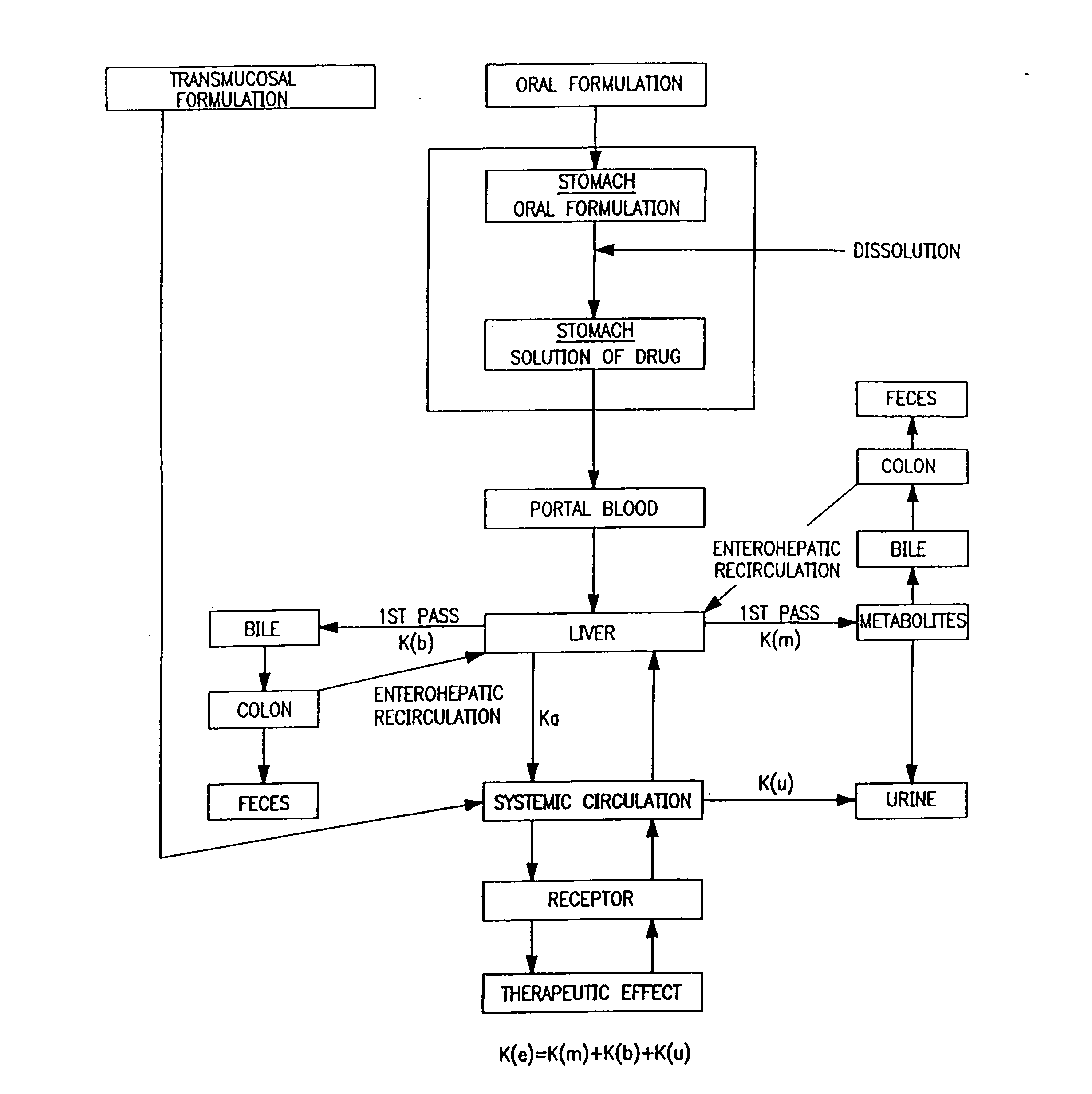
A pharmaceutical formulation for delayed release of the active ingredient 3-(3-dimethylamino-1-ethyl-2-methylpropyl)phenol or a pharmaceutically acceptable salt thereof in a matrix containing between 1 and 80 wt. % of at least one pharmaceutically acceptable hydrophilic or hydrophobic polymer as a matrix forming agent and exhibiting in vivo the following release rate: 3 to 35% by weight (based on 100% by weight active ingredient) 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 0.5 hours; 5 to 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 1 hour; 10 to 75% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 2 hours; 15 to 82% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 3 hours; 30 to 97% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 6 hours; more than 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 12 hours; more than 70% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 18 hours, and more than 80% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 24 hours.
Owner:GRUNENTHAL GMBH
Dosage Form For Insertion Into The Mouth
InactiveUS20100112050A1Efficient deliveryImproved bioavailability and deliveryPowder deliveryBiocideActive agentMigraine
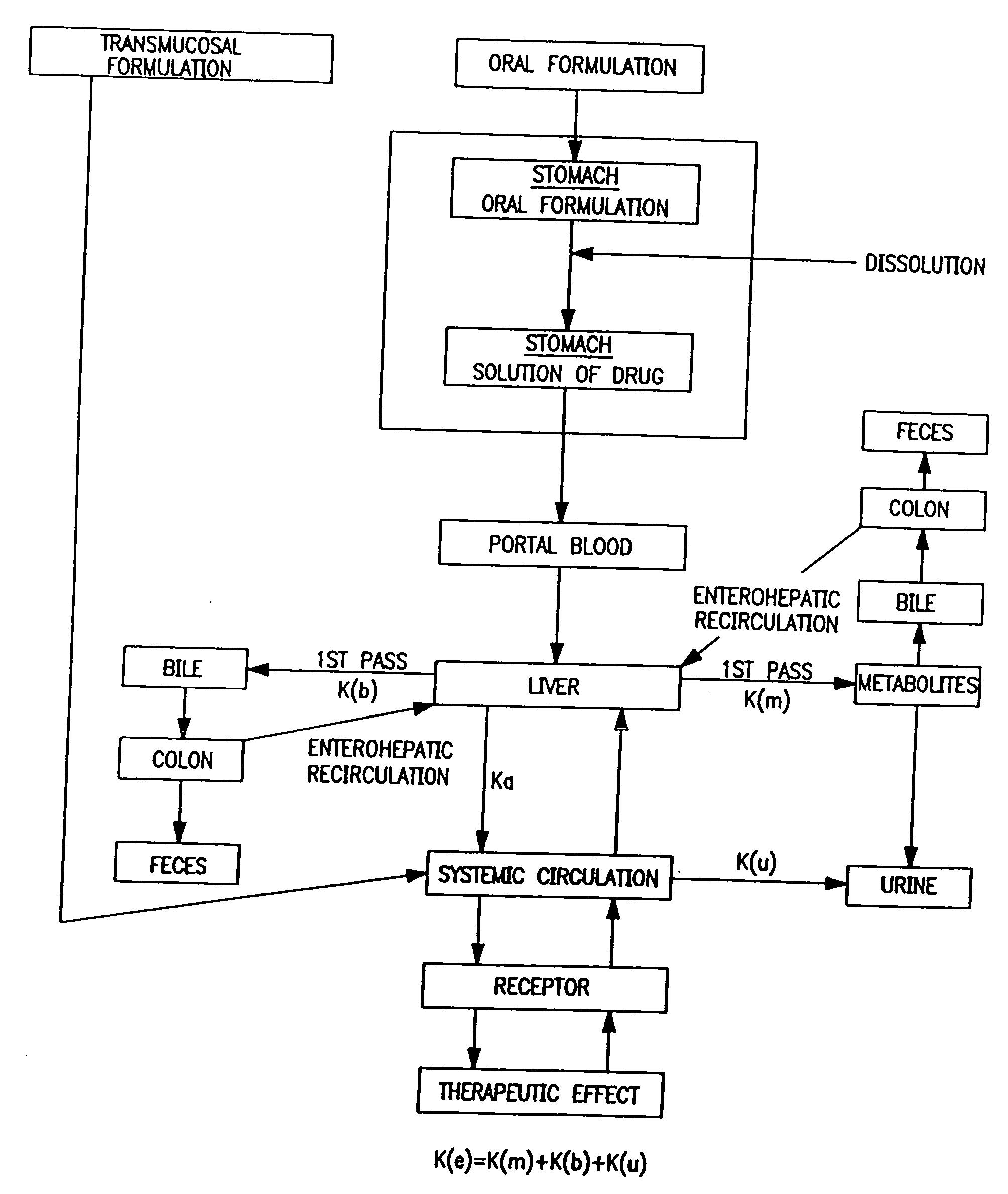
Oral dosage forms as a biodegradable, water soluble film for delivering pharmaceutically active agents, particularly anti-migraine agents to patients through insertion into the mouth of patient and methods for administering pharmaceutically active agents to patients by insertion into the mouth to provide selective uptake of said agents through the mucosa and thus avoiding the gastrointestinal tract.
Owner:NAL PHARM LTD
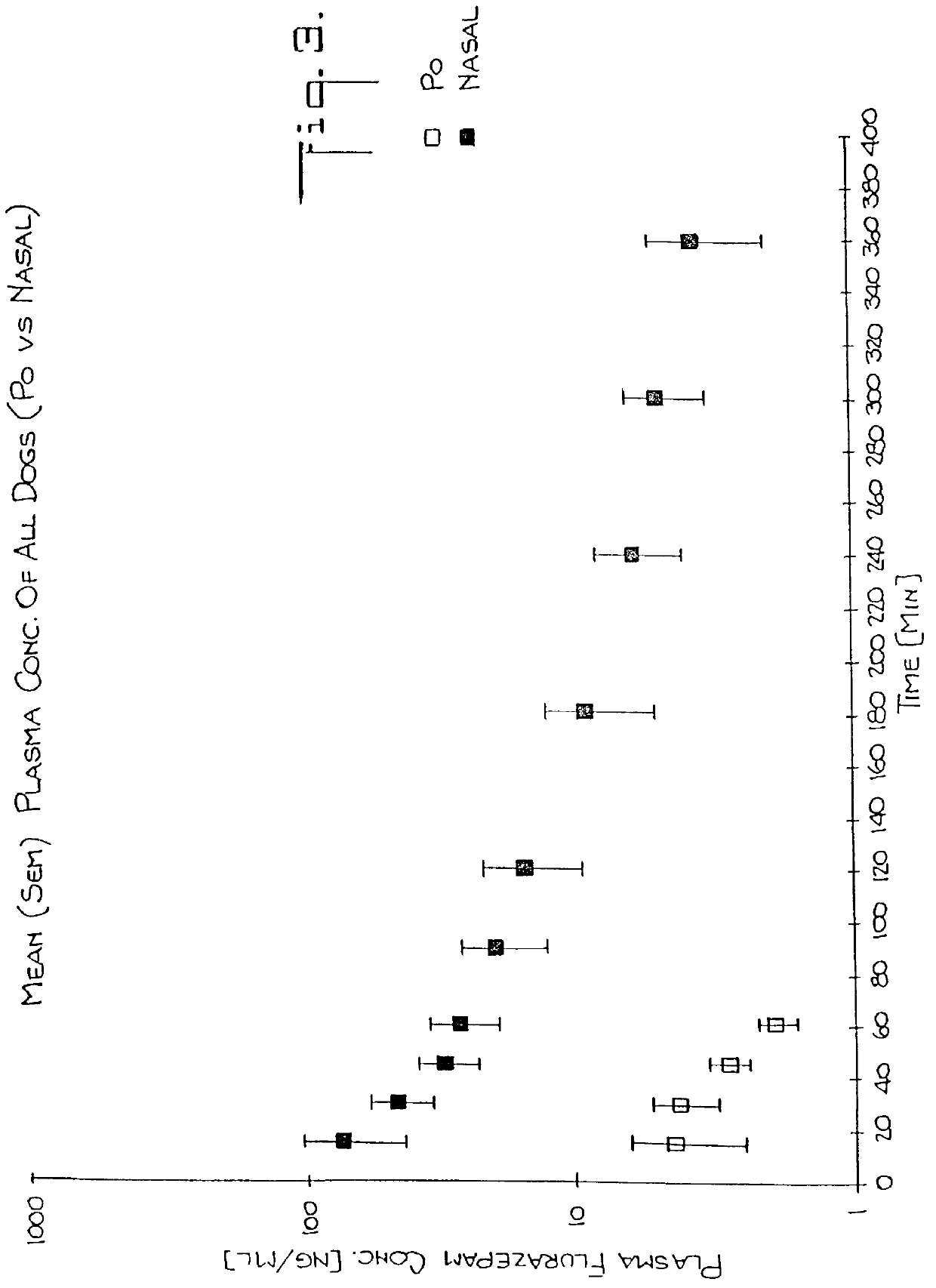
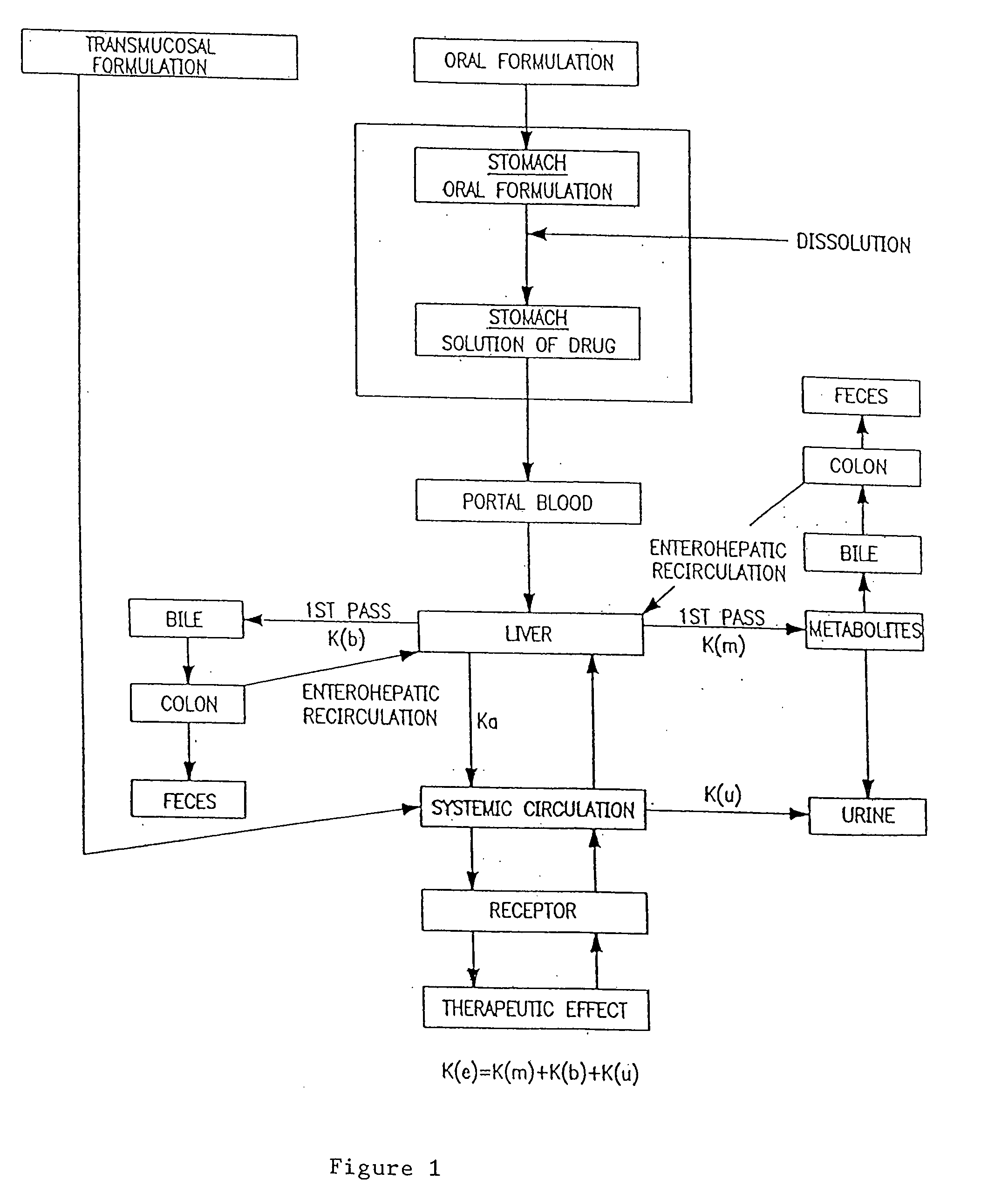
Nasal administration of benzodiazepine hypnotics
Nasal administration of benzodiazepines is described as providing improved therapeutic effects as compared to conventional delivery techniques. The compositions comprise a benzodiazepine hypnotic in a pharmaceutically acceptable nasal carrier.
Owner:QUESTCOR PHARMA
Effervescent oral fentanyl dosage form and methods of administering fentanyl
Fentanyl-containing dosage forms and methods using same are described. These dosage forms include substantially less fentanyl by weight than known oral formulation and have advantages in terms of reduced cost and reduced side effects. These dosage forms are intended for oral administration of fentanyl across the oral mucosa.
Owner:CEPHALON INC
Intradermal delivery of substances
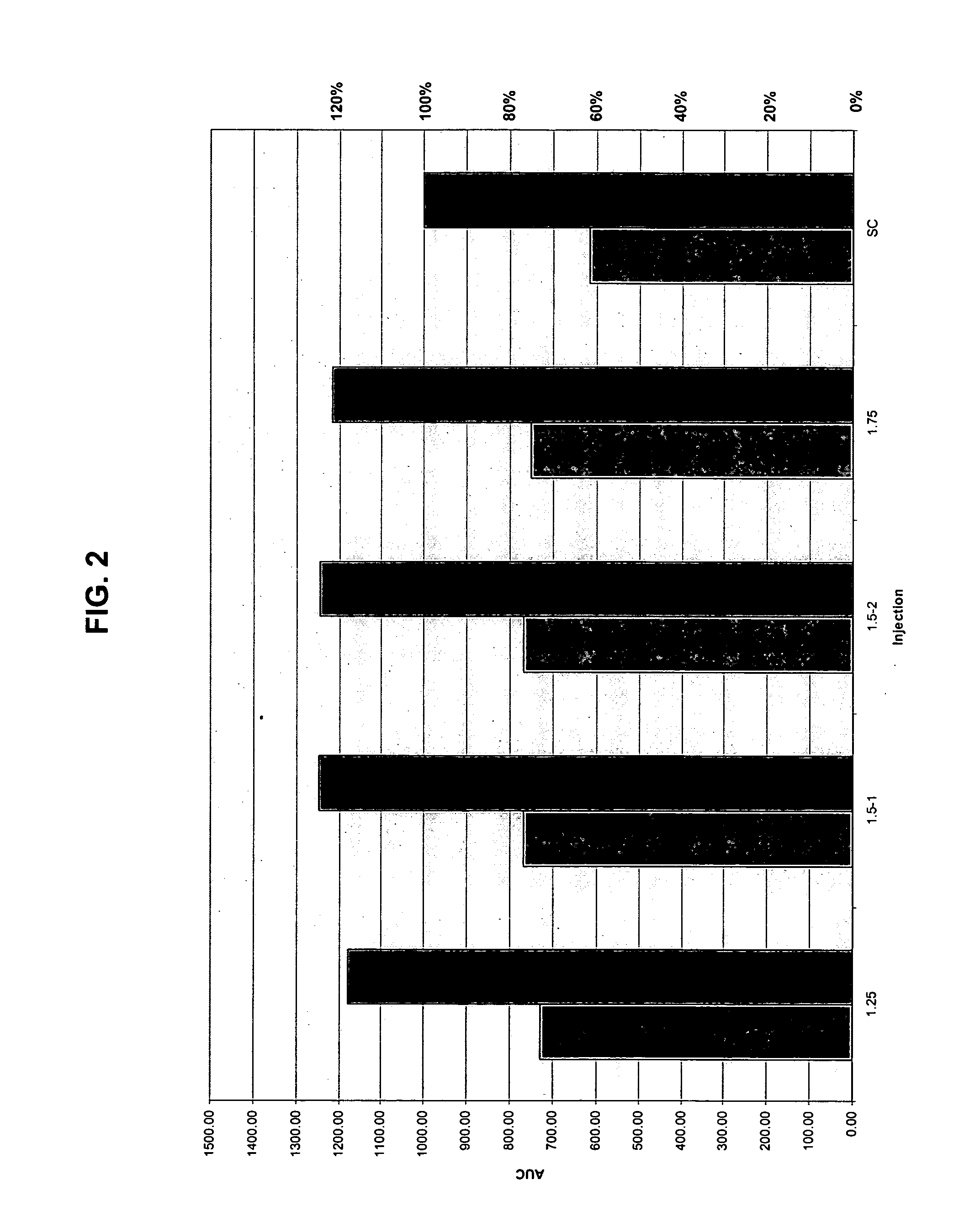
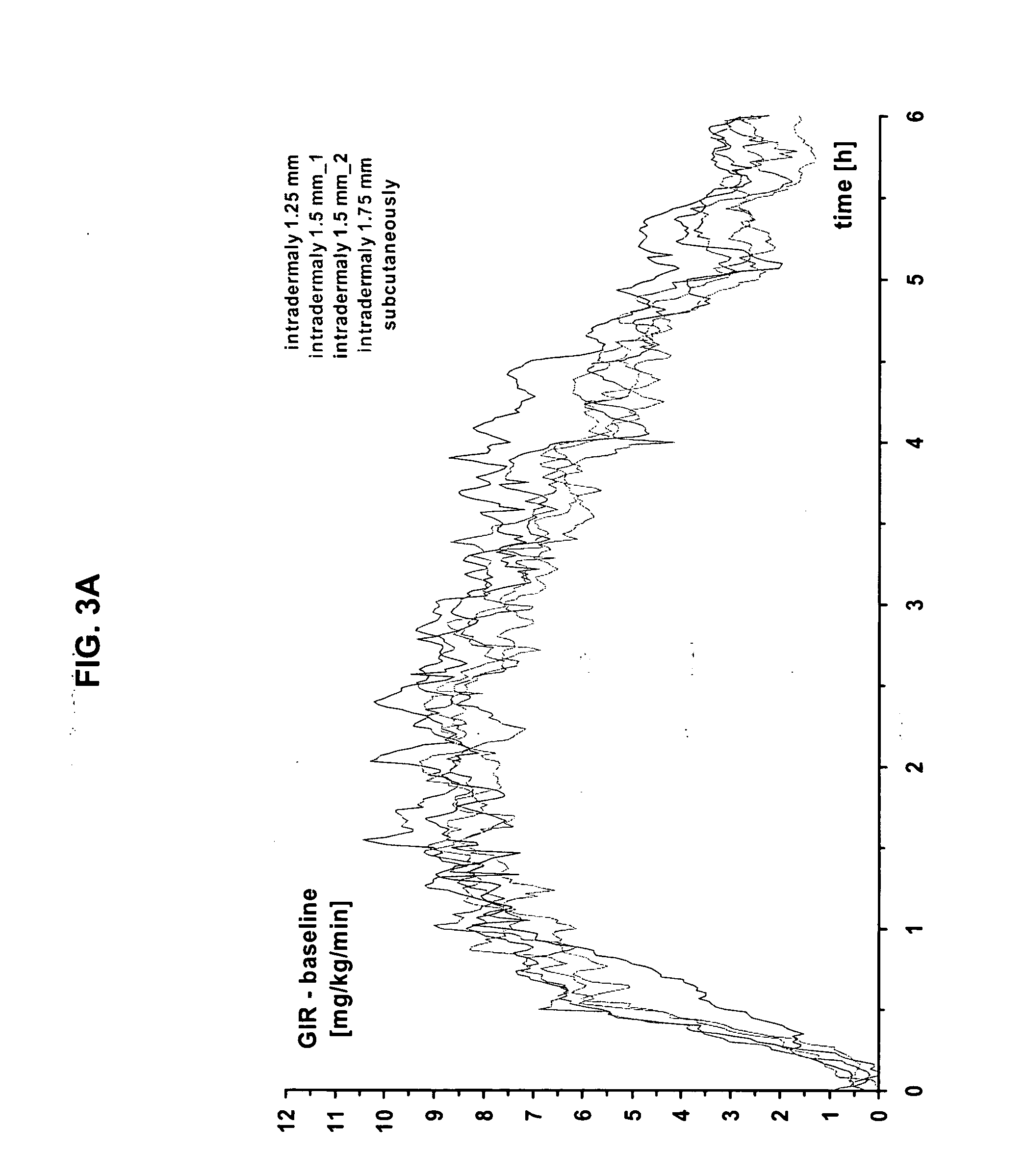
InactiveUS20040073160A1Increase uptakeRapid uptake rateJet injection syringesPeptide/protein ingredientsWhole bodyGrowth hormone
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
Buccal, polar and non-polar spray containing ondansetron
InactiveUS20060198790A1Effect be very fastRapid absorptionOrganic active ingredientsNervous disorderSolventOral mucosa
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide ondansetron for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, ondansetron, and optional flavoring agent; formulation II: aqueous polar solvent, ondansetron, optionally flavoring agent, and propellant; formulation III: non-polar solvent, ondansetron, and optional flavoring agent; formulation IV: non-polar solvent, ondansetron, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, ondansetron, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, ondansetron, optional flavoring agent, and propellant.
Owner:DUGGER HARRY A III +1
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption
InactiveUS20030100885A1Increase uptakeRapid onsetOrganic active ingredientsAmpoule syringesWhole bodySystemic absorption
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption.
Owner:BECTON DICKINSON & CO
Buccal, polar and non-polar sprays containing propofol
InactiveUS20050002867A1Fast absorptionRapid onsetBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray or capsule
InactiveUS6998110B2Rapid onsetFast absorptionBatteries circuit arrangementsAerosol deliverySolventPharmacology
Owner:SUDA
Compositions comprising nanoparticulate naproxen and controlled release hydrocodone
InactiveUS20080113025A1Reduces and eliminates developmentImprove complianceBiocidePowder deliveryControl releaseHydrocodone
The invention relates to a compositions comprising a nanoparticulate naproxen composition in combination with a multiparticulate modified release hydrocodone composition that, upon administration to a patient, delivers a hydrocodone in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of hydrocodone-comprising particles and the at least one subsequent component comprising a second population of hydrocodone-comprising particles, wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form comprising such a combination composition.
Owner:ELAN PHRMA INT LTD
Buccal, polar and non-polar spray or capsule containing drugs for treating pain
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:SUDA
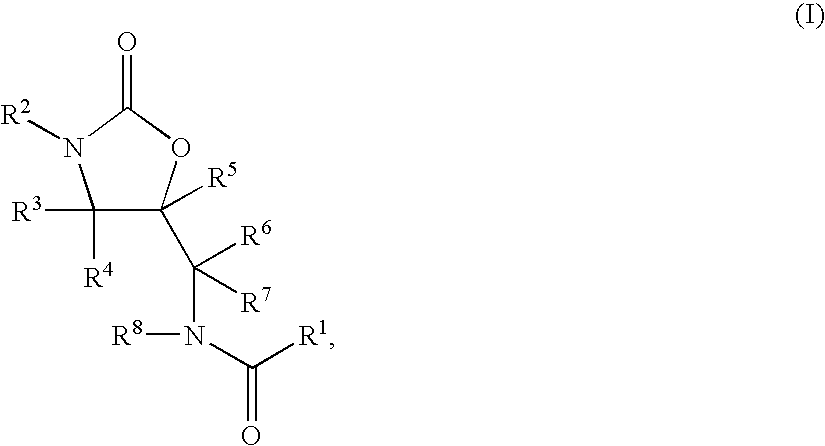
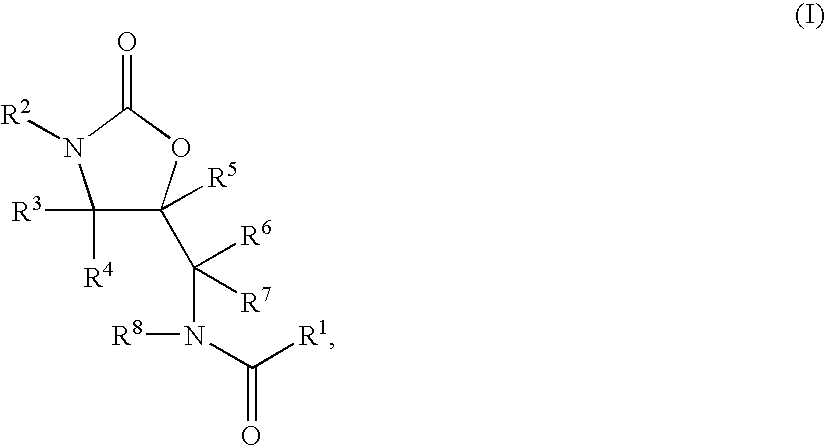
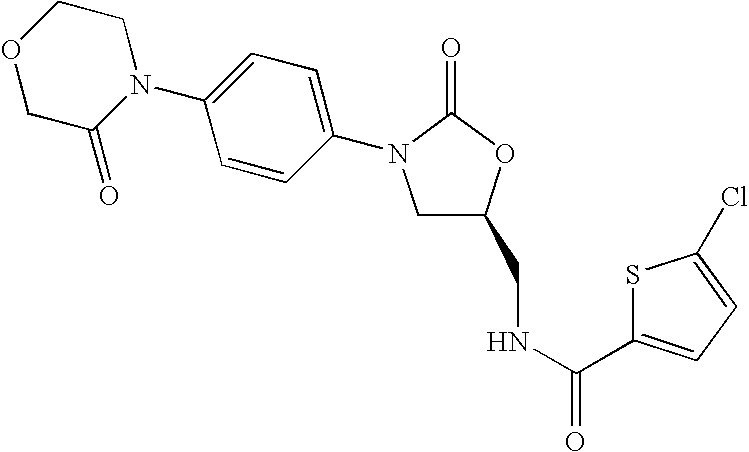
Substituted Oxazolidinones and Their Use in the Field of Blood Coagulation
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Use of D4 and 5-HT2A antagonists, inverse agonists or partial agonists
InactiveUS20050203130A1Augment effectEffect be very fastBiocideAnimal repellantsCompound (substance)Partial agonist
The present invention relates to the use of compounds and compositions of compounds having D4 and 5-HT2A antagonistic, partial agonistic or inverse agonistic activity for the treatment of the underlying dysregulation of the emotional functionality of mental disorders (i.e. affect instability-hypersensitivity-hyperaesthesia-dissociative phenomena-etc). The invention also relates to methods comprising administering to a patient diagnosed as having a neuropsychiatric disorder a pharmaceutical composition containing (i) compounds having D4 antagonistic, partial agonistic or inverse agonistic activity and (ii) compounds having 5-HT2A antagonistic, partial agonistic or inverse agonistic, and (iii) any known medicinal compound and compositions of said compounds. The combined D4 and 5-HT2A antagonistic, partial agonistic or inverse agonistic effects may reside within the same chemical or biological compound or in two different chemical and / or biological compounds.
Owner:PHARMANEUROBOOST
Buccal, polar and non-polar spray or capsule containing drugs for treating metabolic disorders
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Use of mometasone furoate for treating airway passage and lung diseases
InactiveUS6365581B1Maximize treating said rhinitisMinimize absorptionBiocideOrganic active ingredientsDiseaseAerosolize
Owner:MERCK SHARP & DOHME CORP
Intranasal Formulation of Epinephrine for the Treatment of Anaphylaxis
ActiveUS20150005356A1Fast onset timeSuitable for intranasal useBiocidePharmaceutical delivery mechanismBronchospasmInjection epinephrine
This invention relates to pharmaceutical compositions of epinephrine for delivery to the nasal mucosa and methods of treating a subject in acute severe anaphylaxis, bronchospasm or during cardiopulmonary resuscitation (CPR). The composition further comprising agents, that either prevent localized degradation of epinephrine or enhance its absorption in the nasal mucosa to counter anaphylactic effects, symptoms or complications in a subject.
Owner:G2B PHARMA
Buccal, polar and non-polar spray or capsule containing drugs for treating disorders of the gastrointestinal tract or urinary tract
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray or capsule containing drugs for treating muscular and skeletal disorders
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
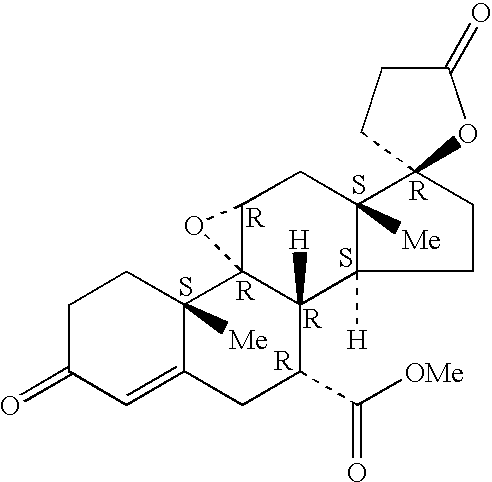
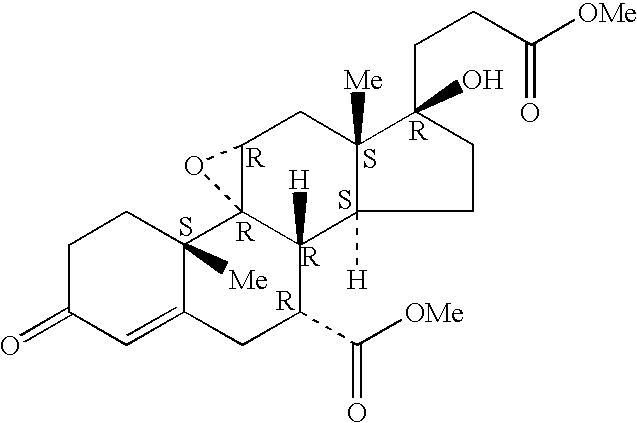
Epoxy-steroidal aldosterone antagonist and calcium channel blocker combination therapy for treatment of congestive heart failure
InactiveUS20020042405A1Reduce pathogenicityImprove the level ofMetabolism disorderBlood disorderCalcium channel blockerAldosterone
A combination therapy comprising a therapeutically-effective amount of an epoxy-steroidal aldosterone receptor antagonist and a therapeutically-effective amount of a calcium channel blocker is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, congestive heart failure, cirrhosis and ascites. Preferred calcium channel blockers are those compounds having high potency and bioavailability. Preferred epoxy-steroidal aldosterone receptor antagonists are 20-spiroxane steroidal compounds characterized by the presence of a 9alpha,11alpha-substituted epoxy moiety. A preferred combination therapy includes the calcium channel blocker verapamil HCl (Benzenacetonitrile, (±)-alpha[3[[2-(3,4-dimethoxyphenyl) ethyl]methylamino]propyl]-3,4-dimethoxy-alpha-(1-methylethyl)hydrochloride) and the aldosterone receptor antagonist epoxymexrenone.
Owner:SCHUH JOSEPH R
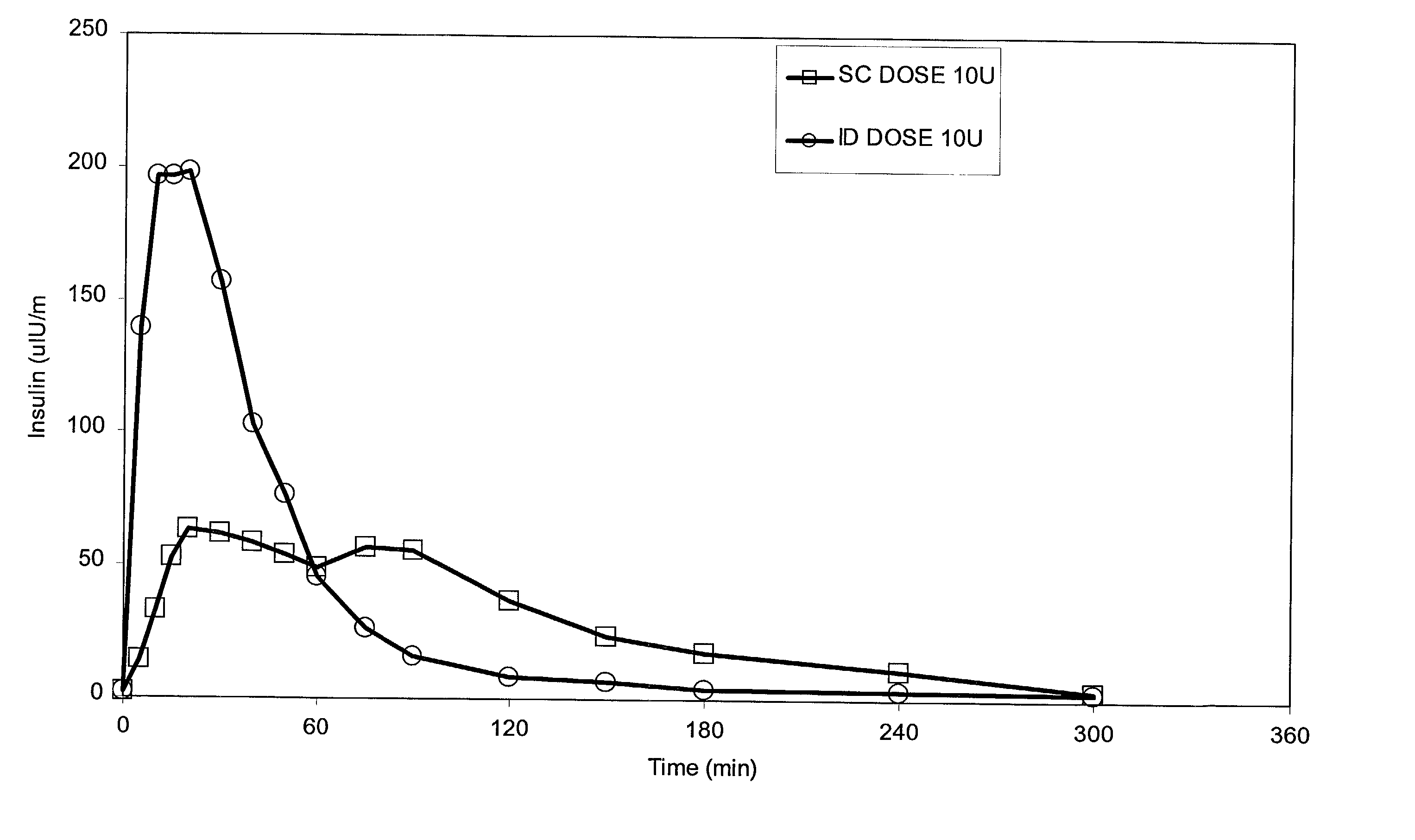
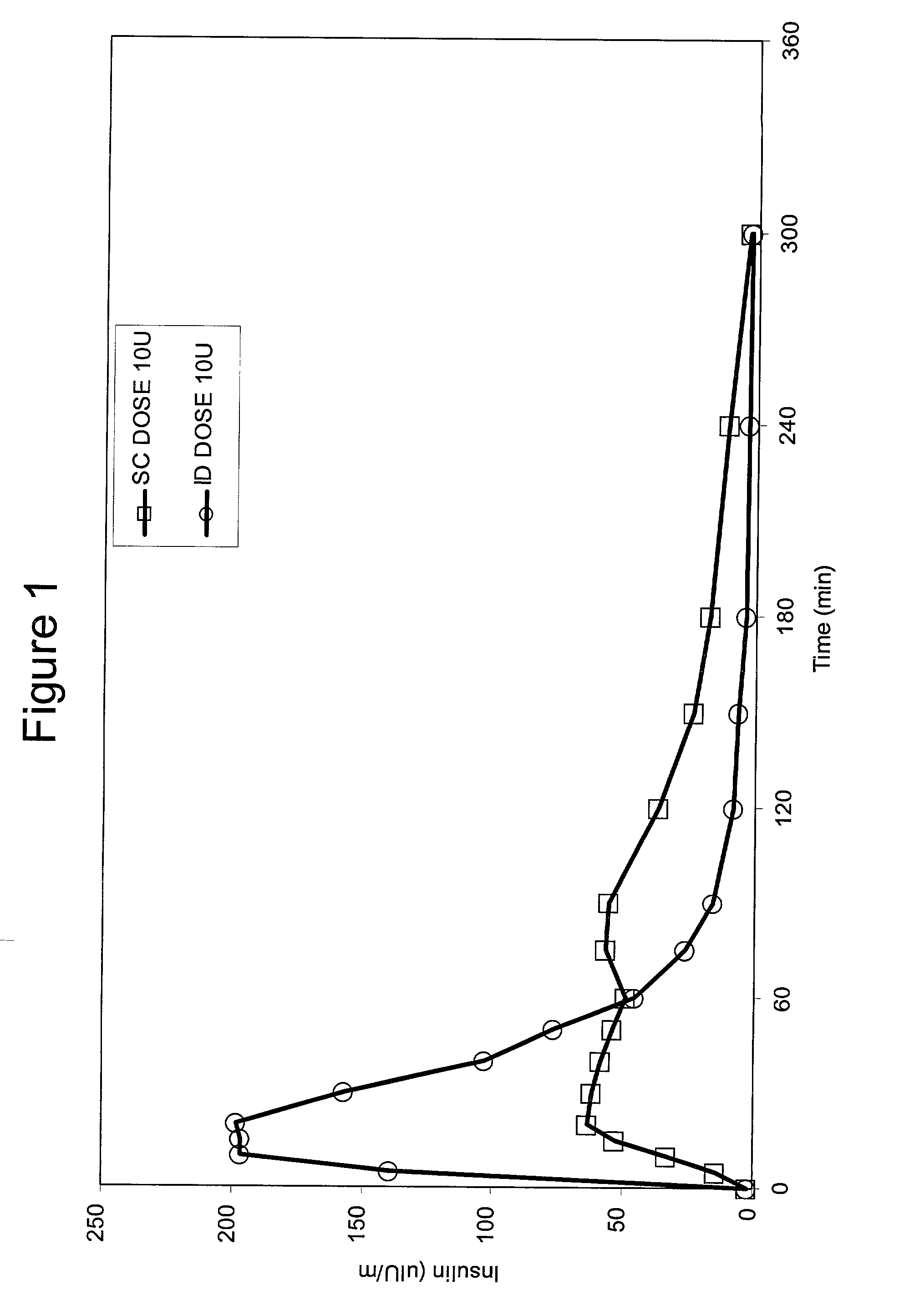
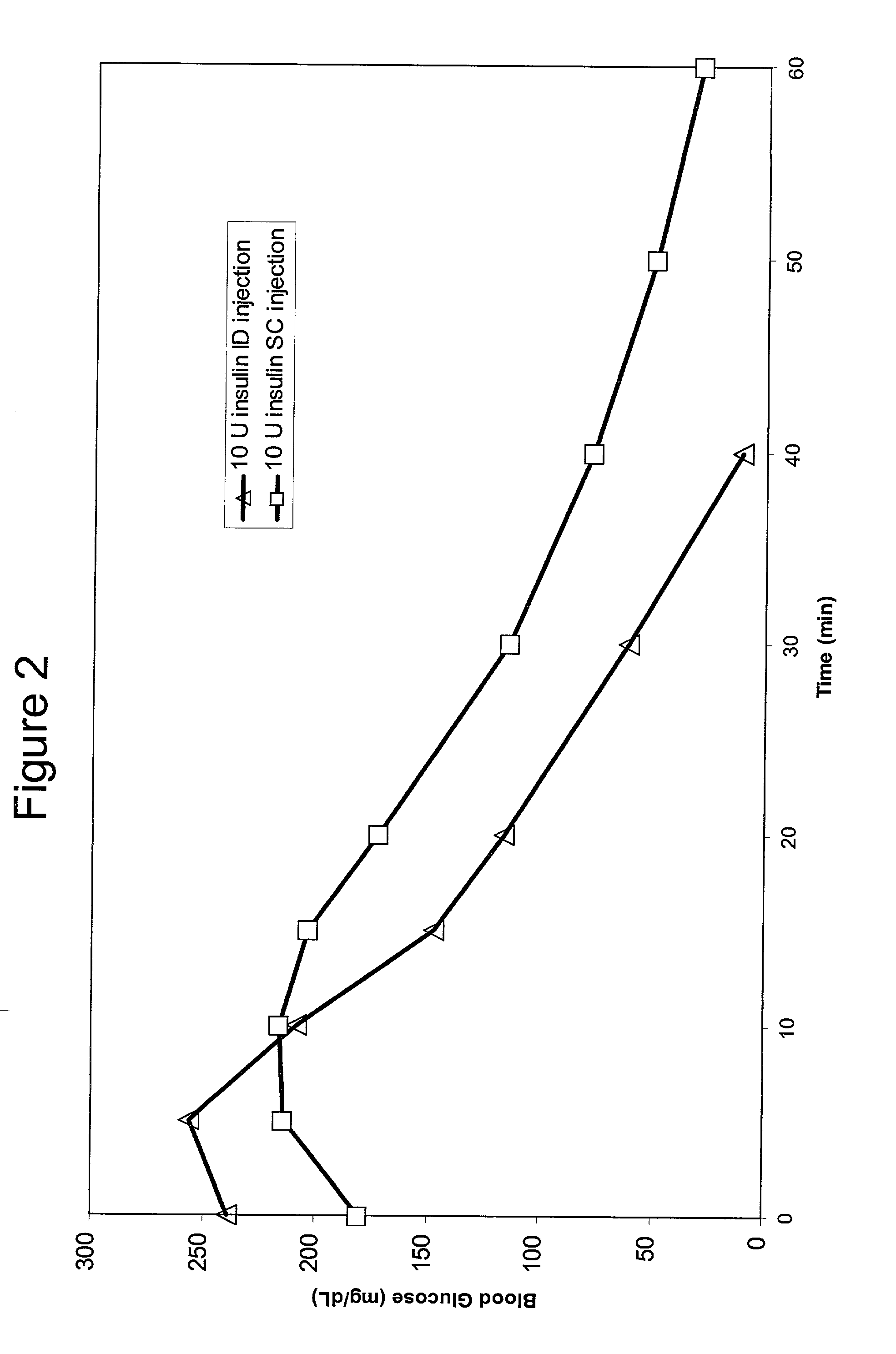
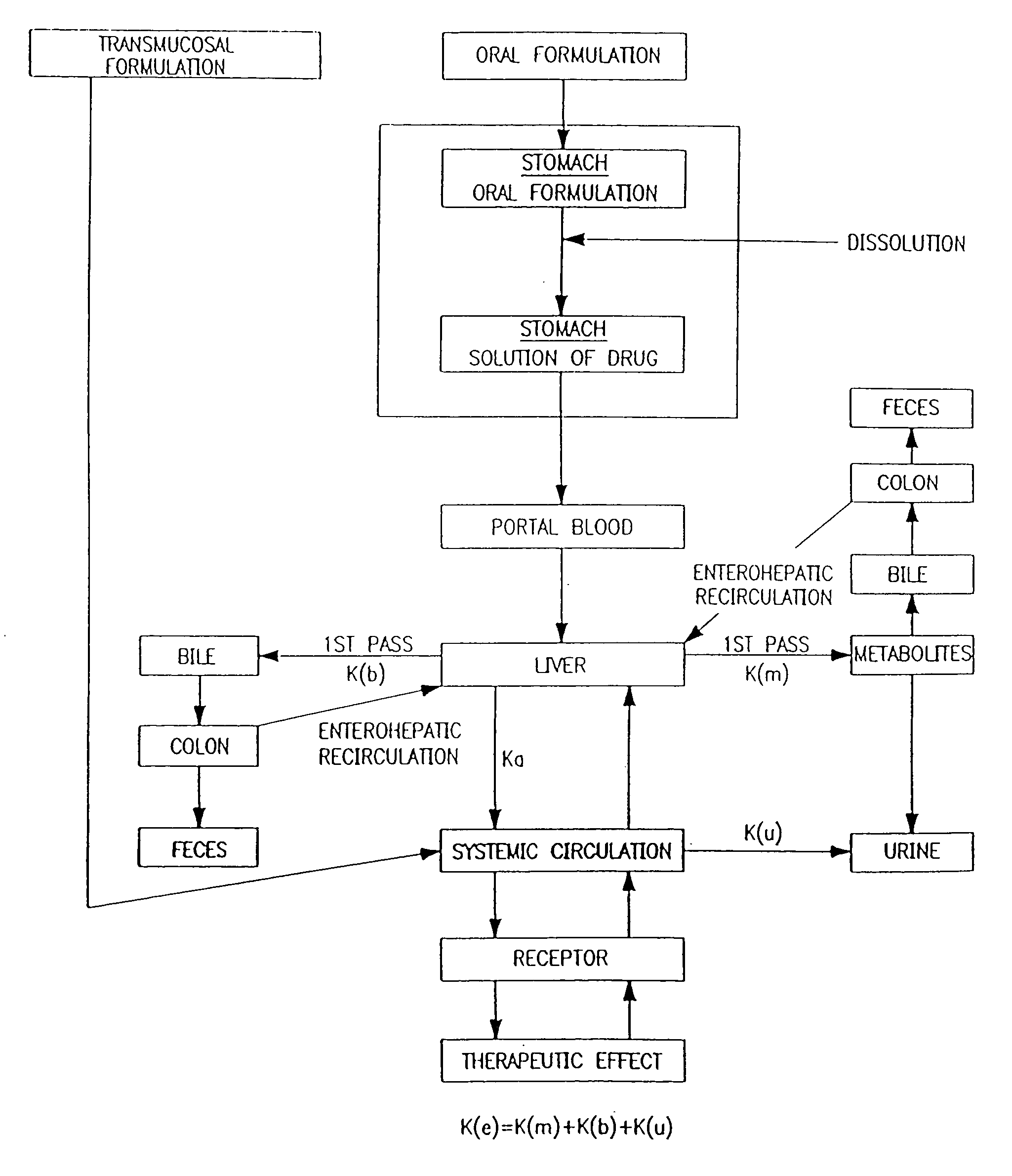
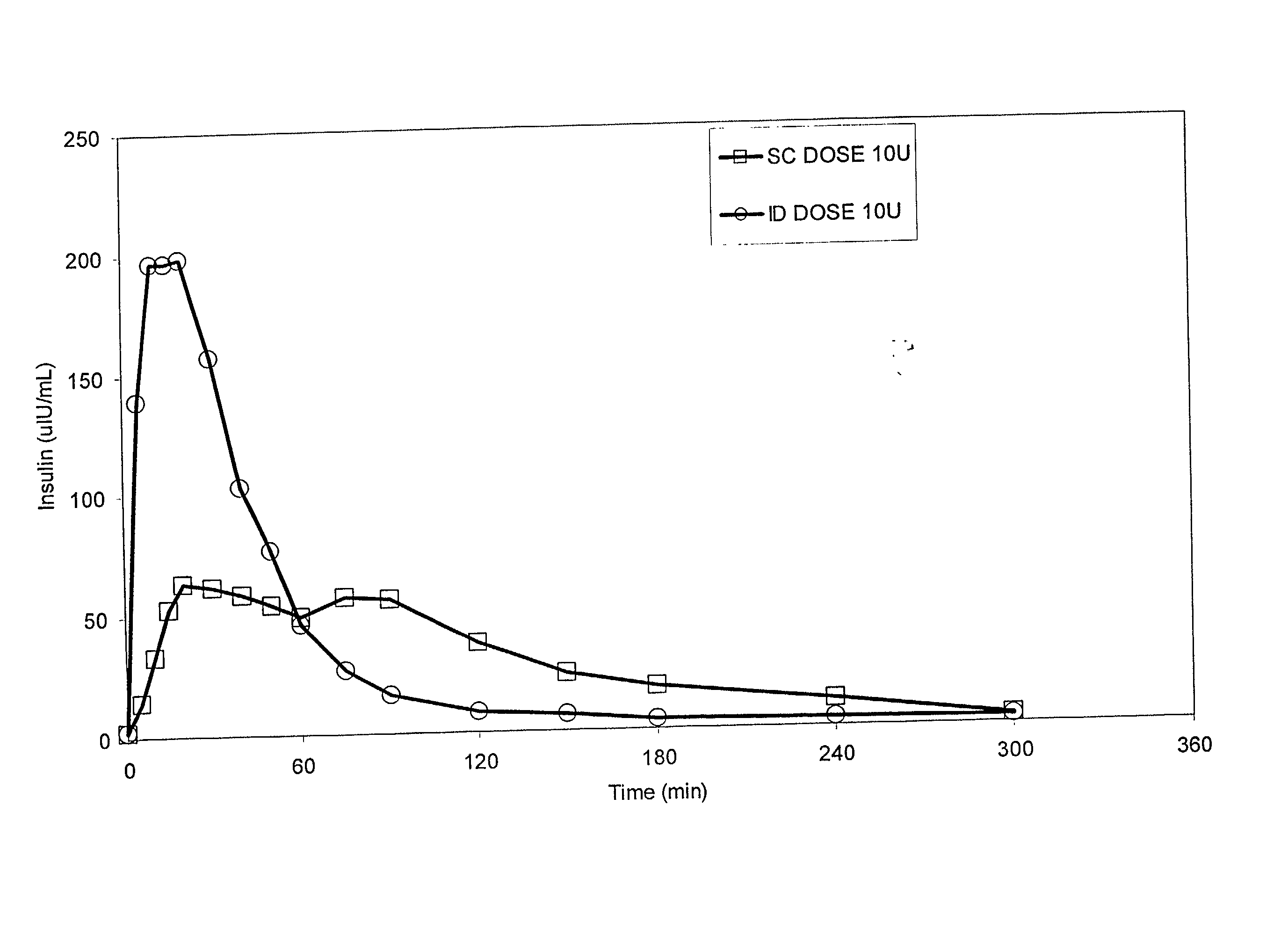
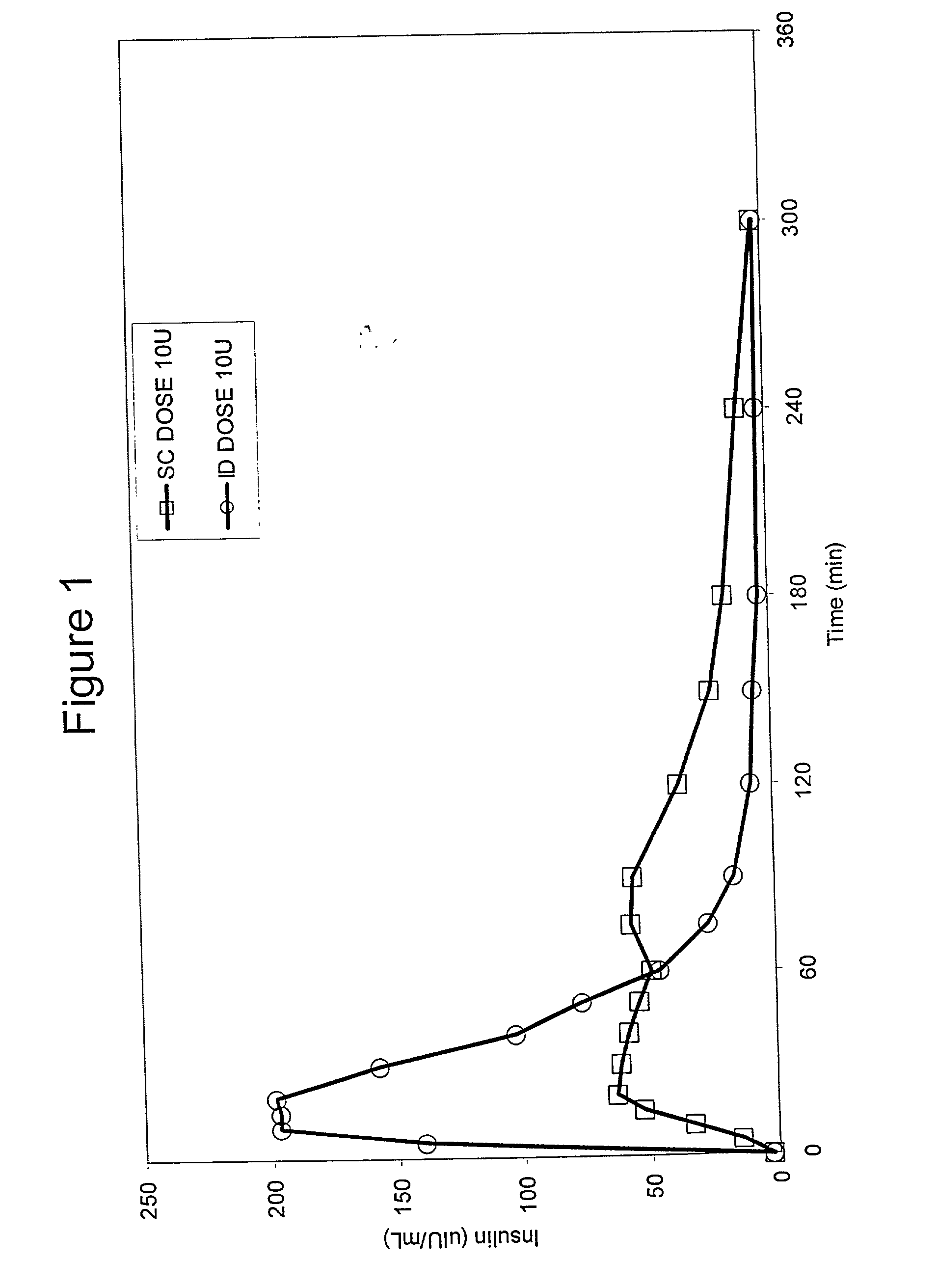
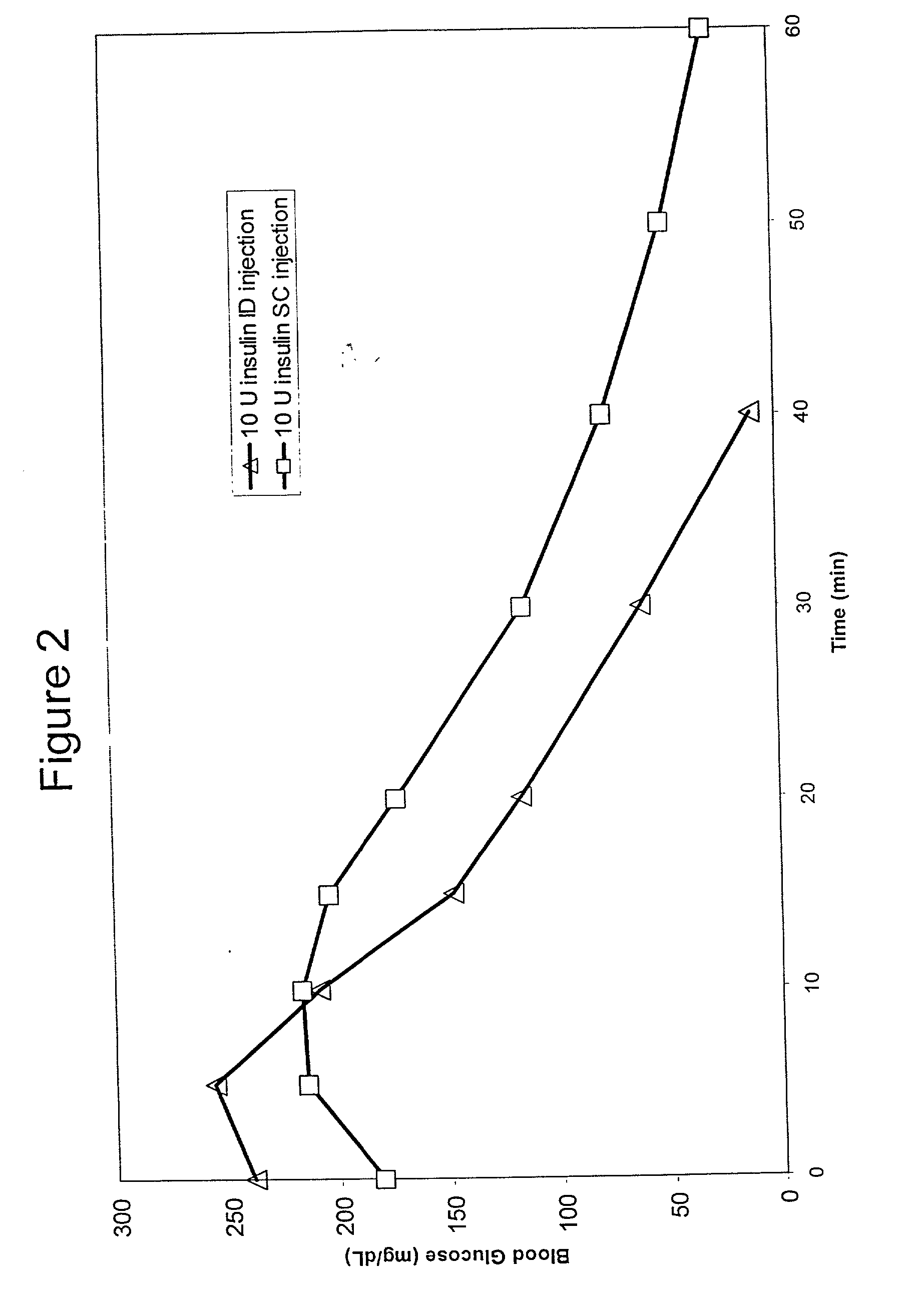
Method for altering insulin pharmacokinetics
InactiveUS20050055010A1Enhance bioavailabilityHigh bioavailabilityPeptide/protein ingredientsMetabolism disorderNon fastingClinical efficacy
The present invention relates to methods for administration of insulin into the intradermal compartment of subject's skin, preferably to the dermal vasculature of the intradermal compartment. The methods of the present invention enhance the pharmacokinetic and pharmacodynamic parameters of insulin delivery and effectively result in a superior clinical efficacy in the treatment and / or prevention of diabetes mellitus. The methods of the instant invention provide an improved glycemic control of both non-fasting (i.e., post-prandial) and fasting blood glucose levels and thus have an enhanced therapeutic efficacy in treatment, prevention and / or management of diabetes relative to traditional methods of insulin delivery, including subcutaneous insulin delivery.
Owner:BECTON DICKINSON & CO
Buccal, polar and non-polar spray or capsule containing drugs for treating allergies or asthma
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
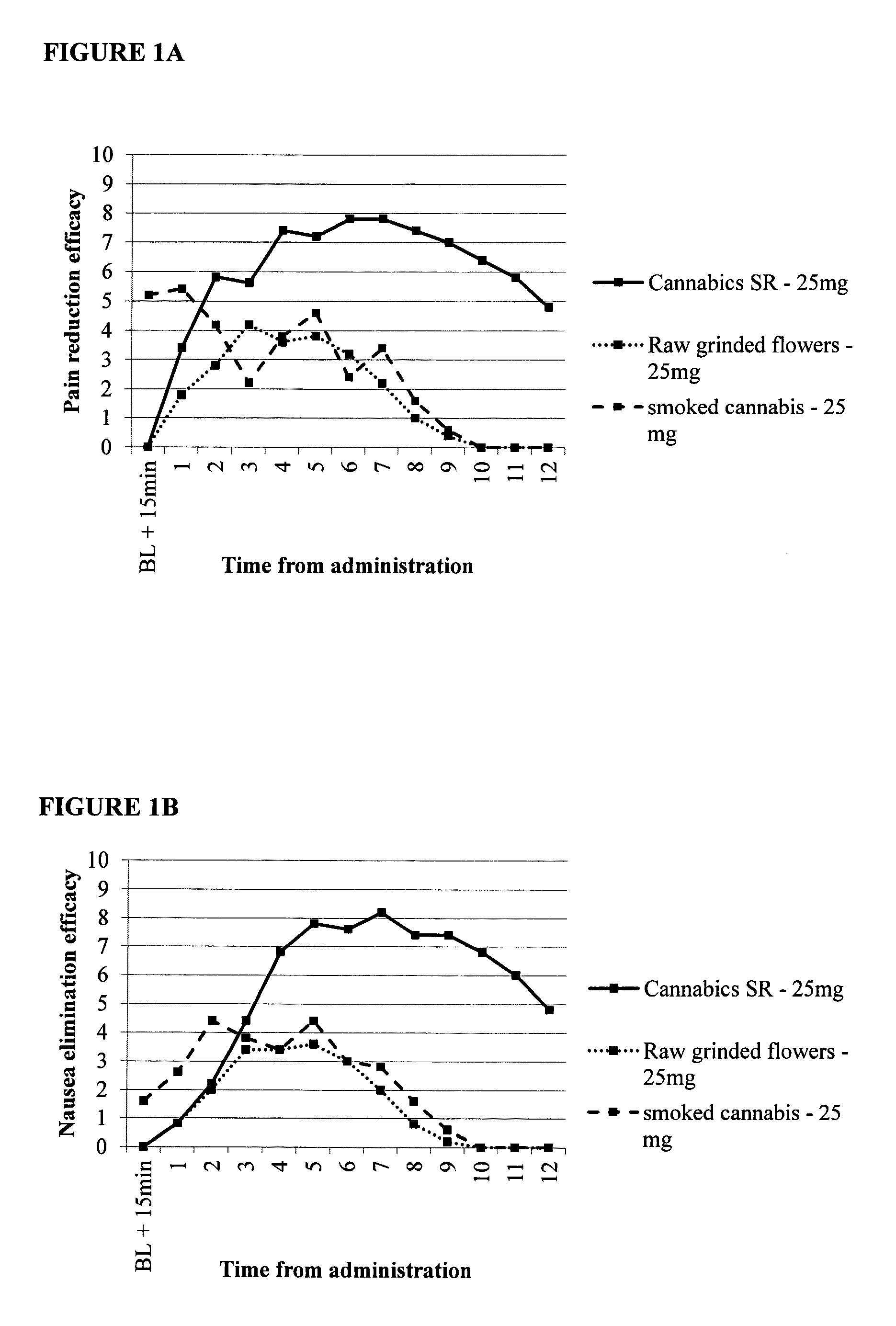
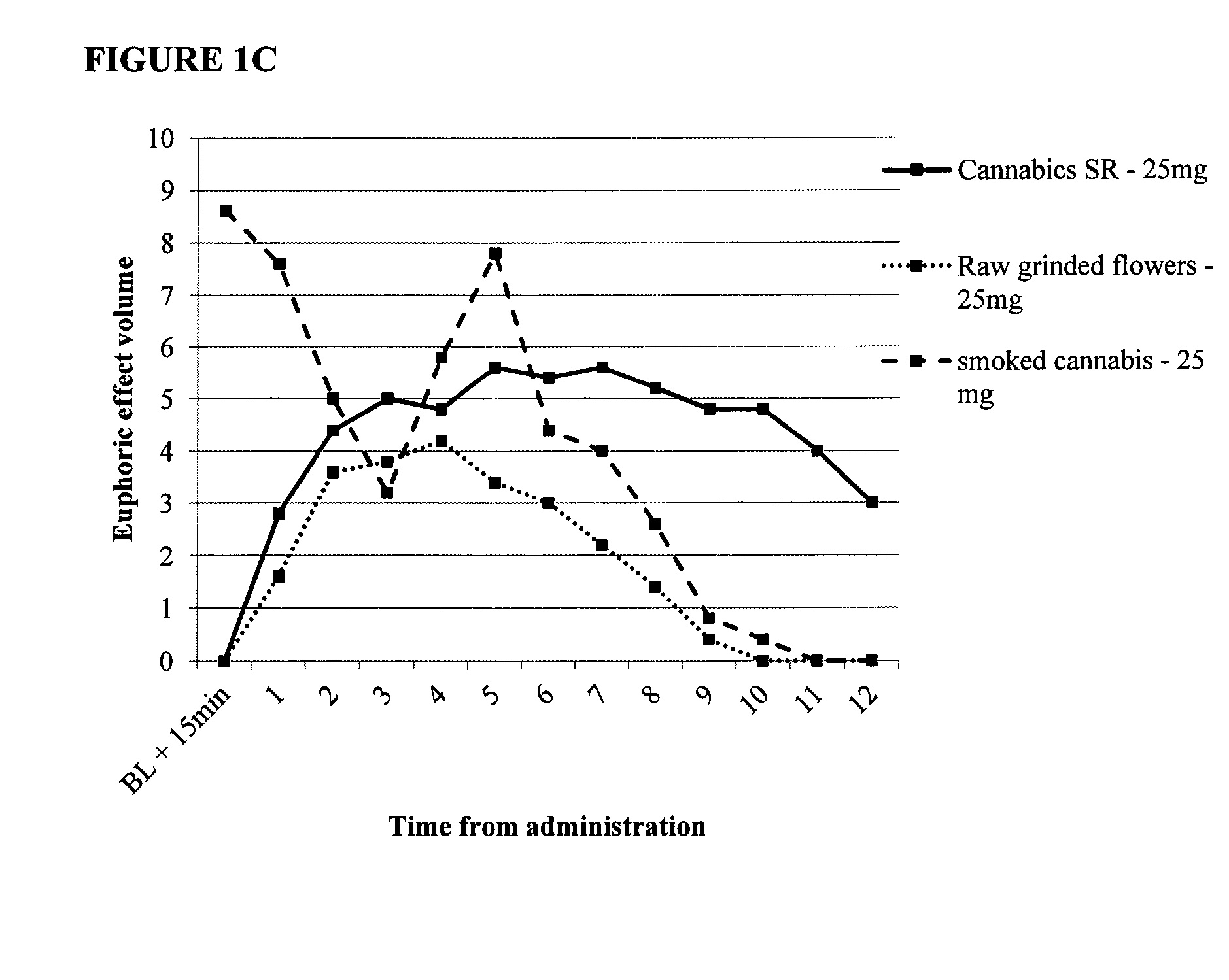
Compositions for combined immediate and sustained release of cannabinoids, methods of manufacture and use thereof
InactiveUS20150057342A1Long duration of activityBroad therapeutic windowBiocideHydroxy compound active ingredientsCannabinoidImmediate release
Provided are oral pharmaceutical compositions comprising sustained release or a combination of sustained and immediate release formulation of cannabinoids, a process for their preparation and methods of use thereof.
Owner:CANNABICS PHARMA
Analgesics for nasal administration
InactiveUS20050142072A1Rapid uptakeRapid onsetPowder deliveryBiocideNasal Cavity EpitheliumBlood plasma
An analgesic and a delivery agent are combined in a pharmaceutical composition such that, on introduction into the nasal cavity of a patient to be treated, the analgesic may be delivered to the bloodstream to produce within 30 minutes a therapeutic plasma concentration, Cther, of 0.2 ng / ml or greater which is maintained for a duration Tmaint of at least 2 hours. The analgesic may be an opioid analgesic or a non-steroidal anti-inflammatory drug.
Owner:IONIX PHARMA +1
Method of treating neurodegenerative diseases using D4 and 5-HT2A antagonists, inverse agonists or partial agonists
InactiveUS20050119249A1Augment effectGood treatment effectBiocideAnimal repellantsPartial agonistCompound (substance)
The present invention relates to methods of treating neurodegenerative diseases or disorders, such as Parkinson disease and related cognitive diseases or disorders with compounds and compositions of compounds having D4 and / or 5-HT2A antagonistic, partial agonistic or inverse agonistic activity in combination with other compounds for treating said diseases. The invention also relates to pharmaceutical compositions for administration to a patient diagnosed as having a neurodegenerative disease or disorder and / or a cognitive disease or disorder, said pharmaceutical composition containing (i) compounds having D4 antagonistic, partial agonistic or inverse agonistic activity and / or (ii) compounds having 5-HT2A antagonistic, partial agonistic or inverse agonistic, and / or (iii) any known medicinal compound or compositions of said compounds in combination with another compound for treating said diseases or disorders to augment the therapeutic effect or to provide a faster onset of the therapeutic effect of said other compound.
Owner:PHARMANEUROBOOST
Buccal, polar and non-polar spray containing zolpidem
InactiveUS20060216240A1Rapid onsetFast absorptionOrganic active ingredientsNervous disorderSolventFast onset
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide zolpidem for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, zolpidem, and optional flavoring agent; formulation II: aqueous polar solvent, zolpidem, optionally flavoring agent, and propellant; formulation III: non-polar solvent, zolpidem, and optional flavoring agent; formulation IV: non-polar solvent, zolpidem, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, zolpidem, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, zolpidem, optional flavoring agent, and propellant.
Owner:MAGNA PHARMA INC
Adhesive bioerodible ocular drug delivery system
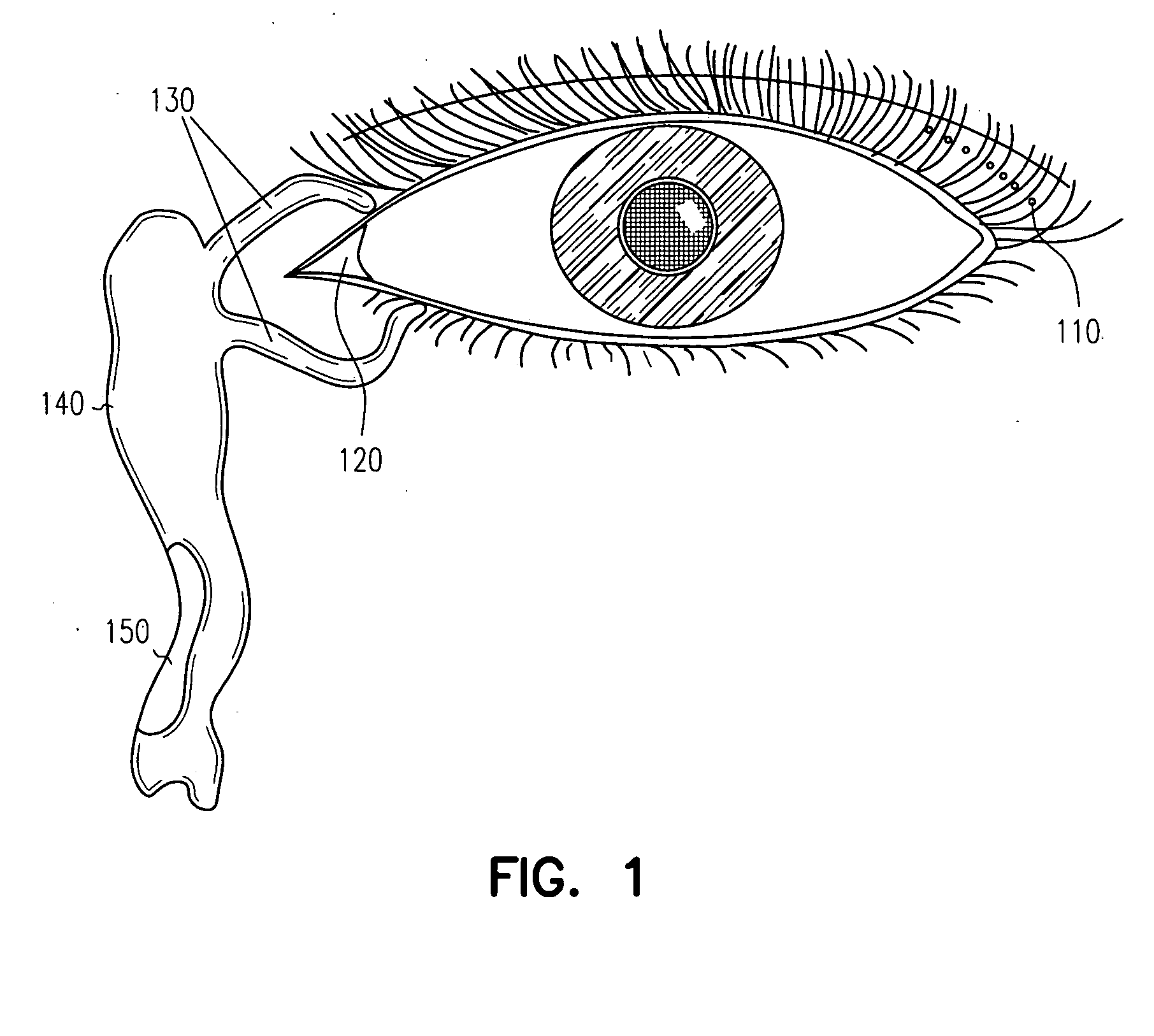
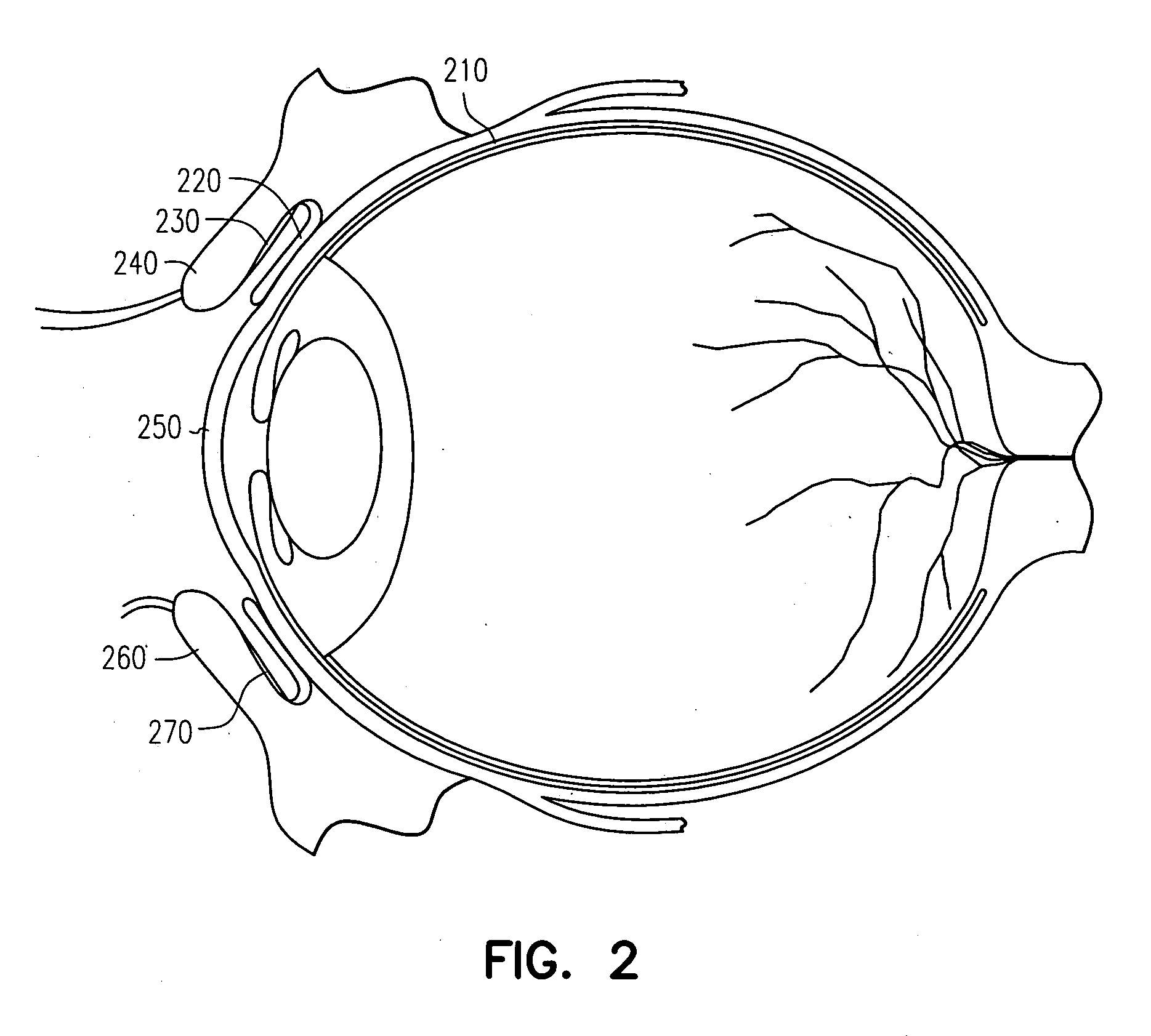
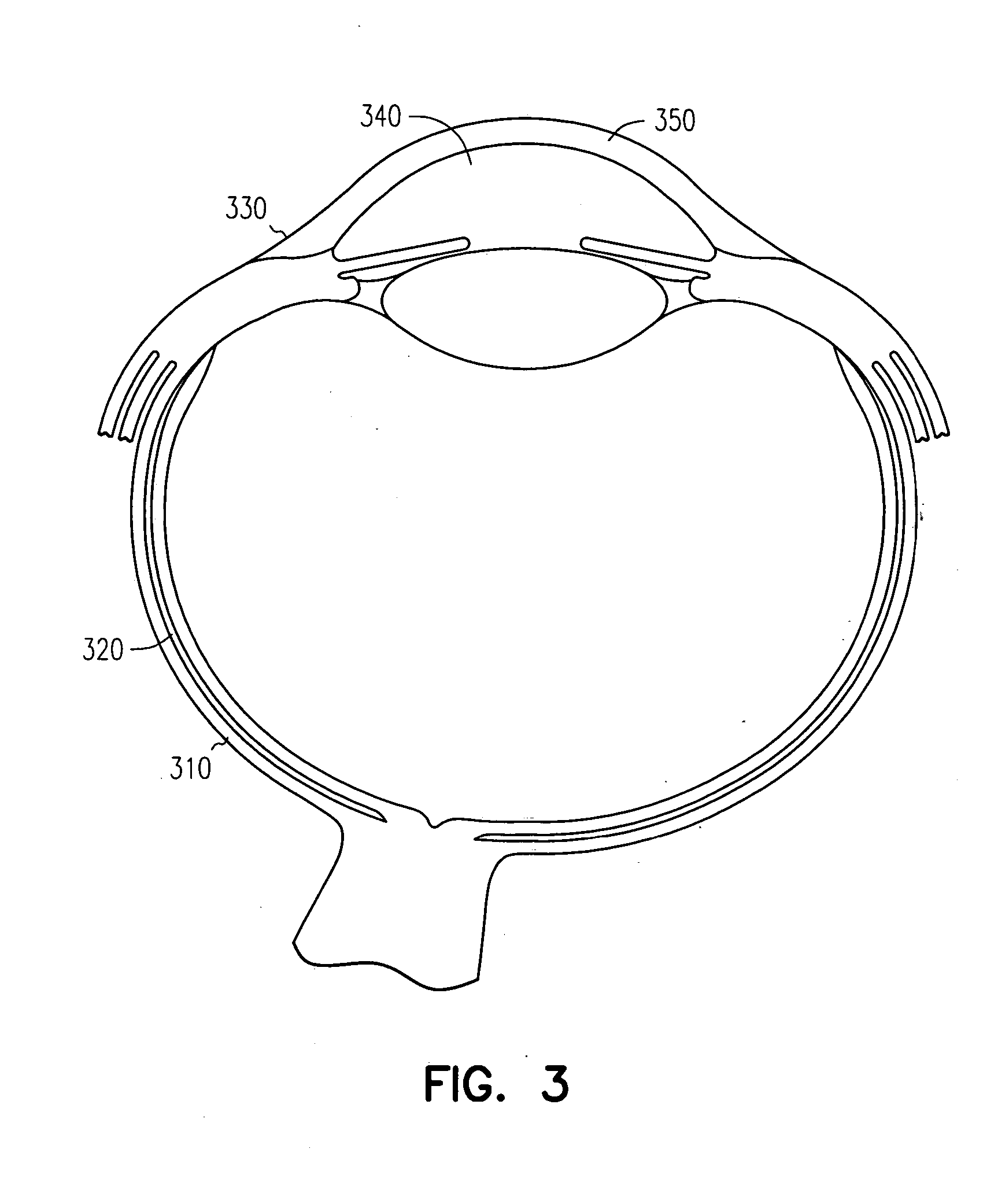
InactiveUS20050013845A1Suitable bioadhesive capabilityRapid onsetFemale contraceptivesSheet deliveryConjunctivaWhole body
Owner:ARIUS TWO
Buccal, polar and non-polar sprays containing propofol
InactiveUS20060222597A1Rapid onsetFast absorptionBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com