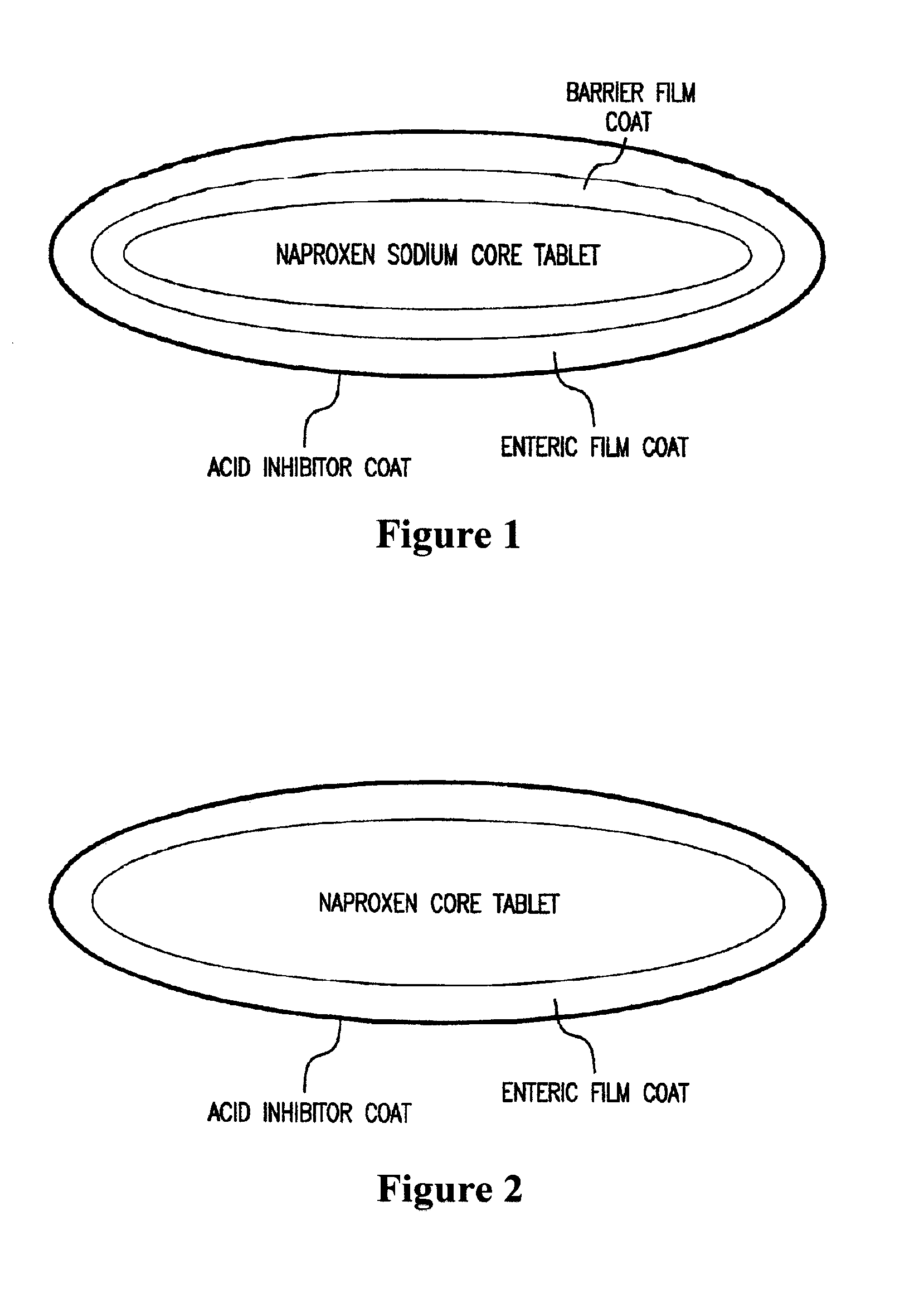
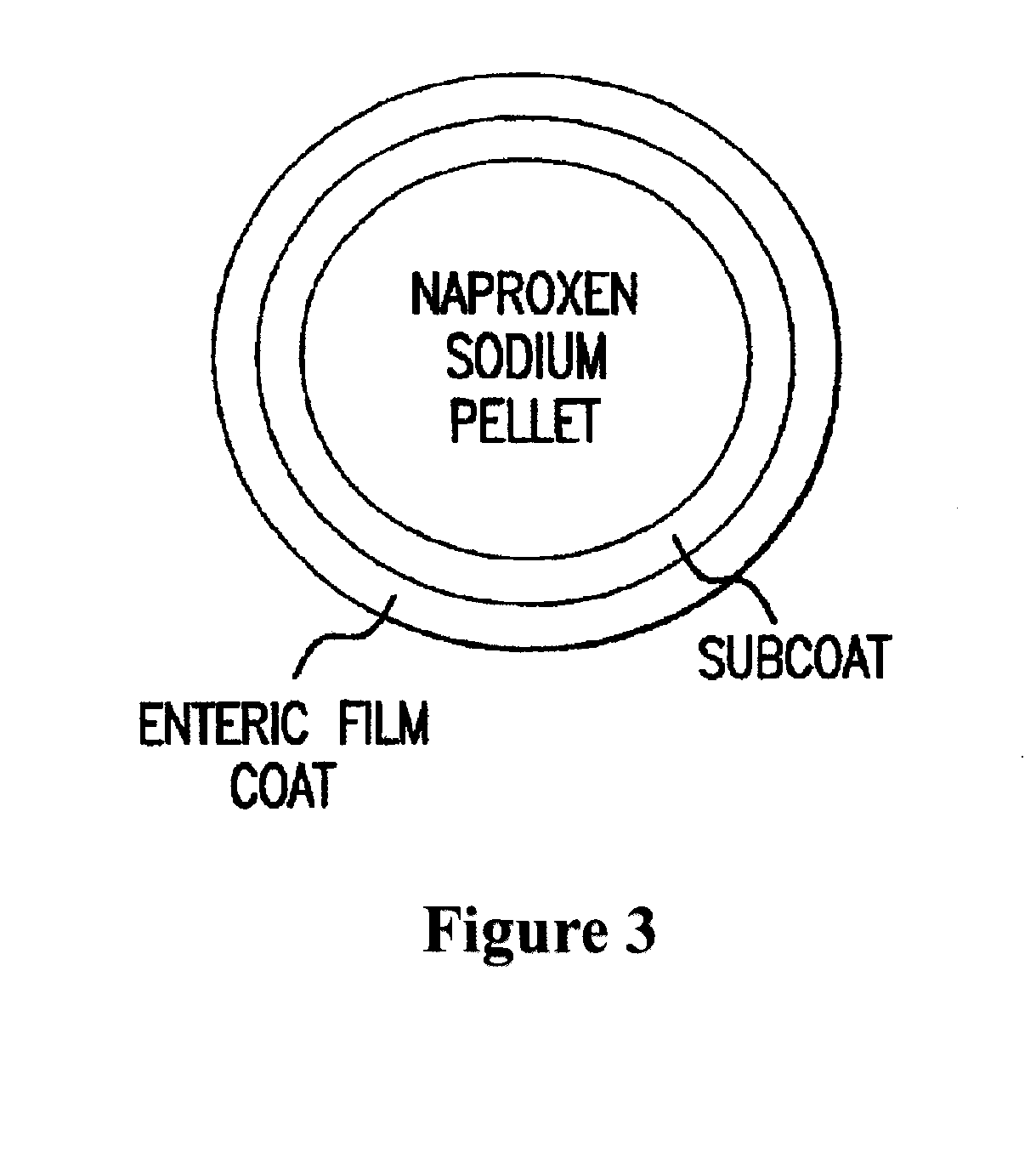
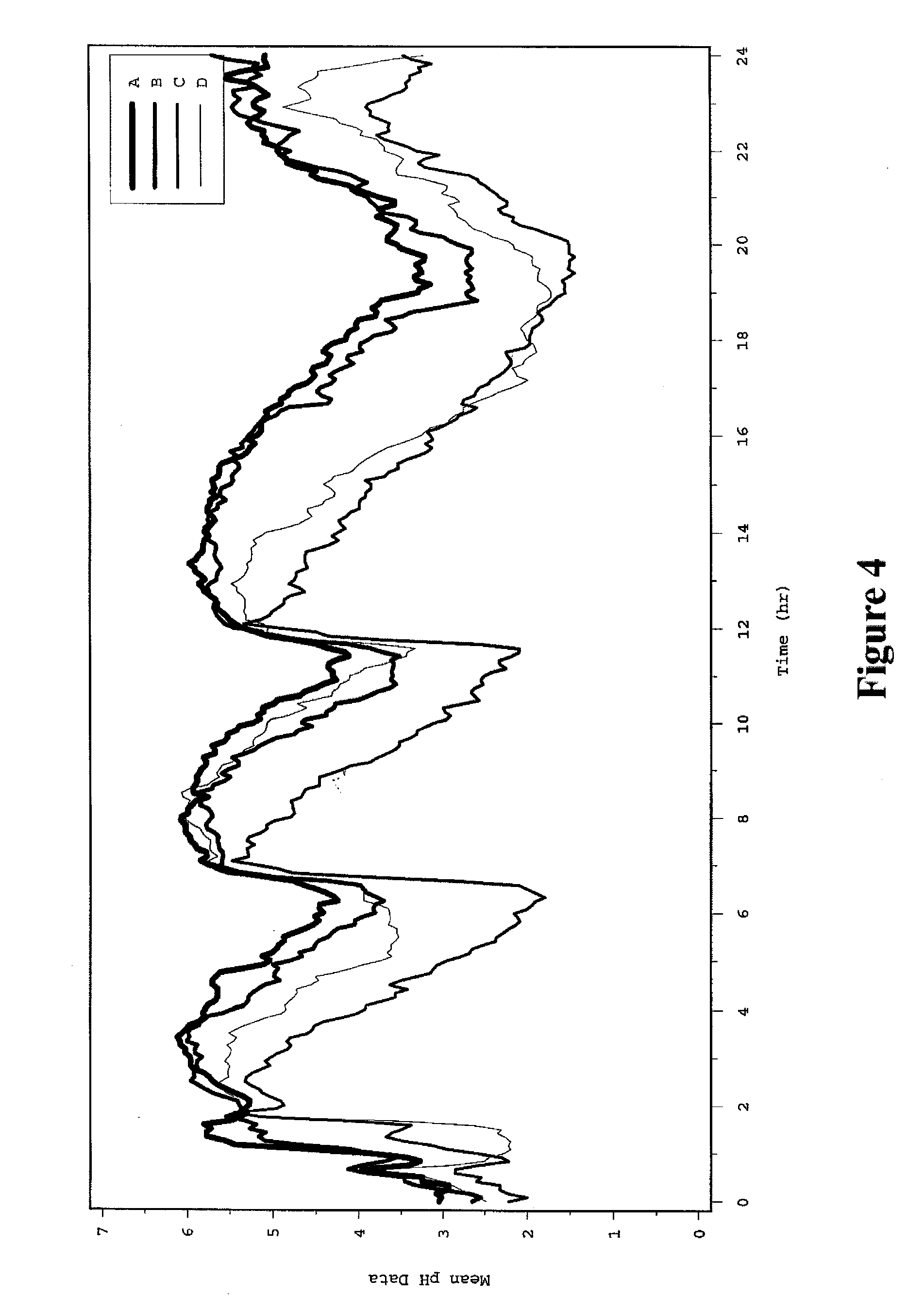
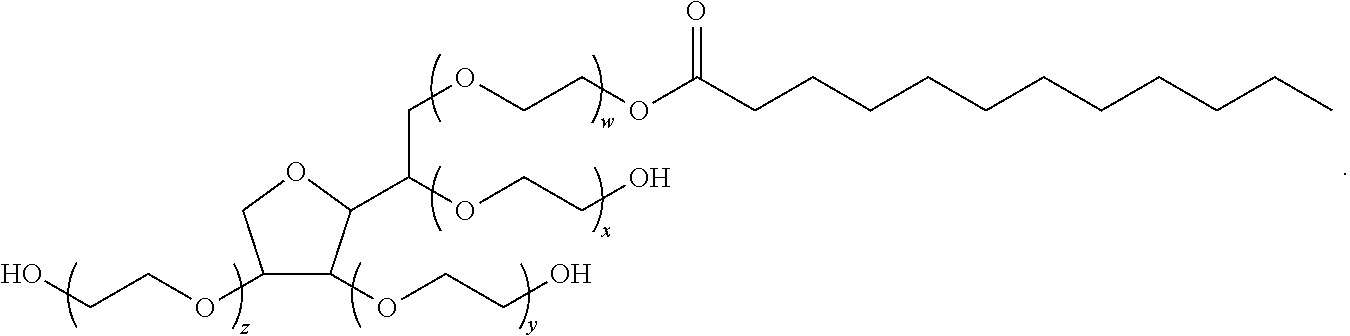
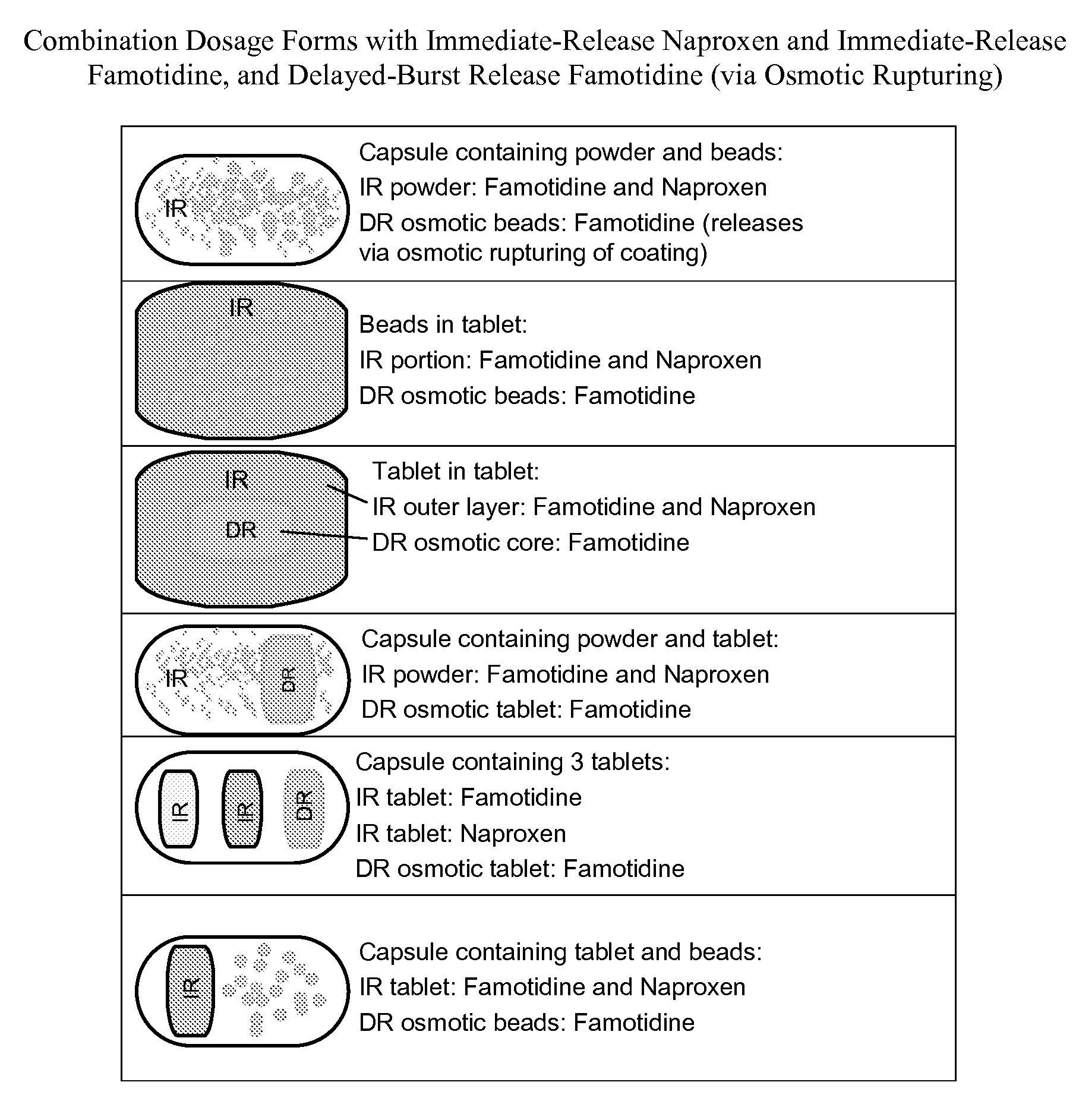
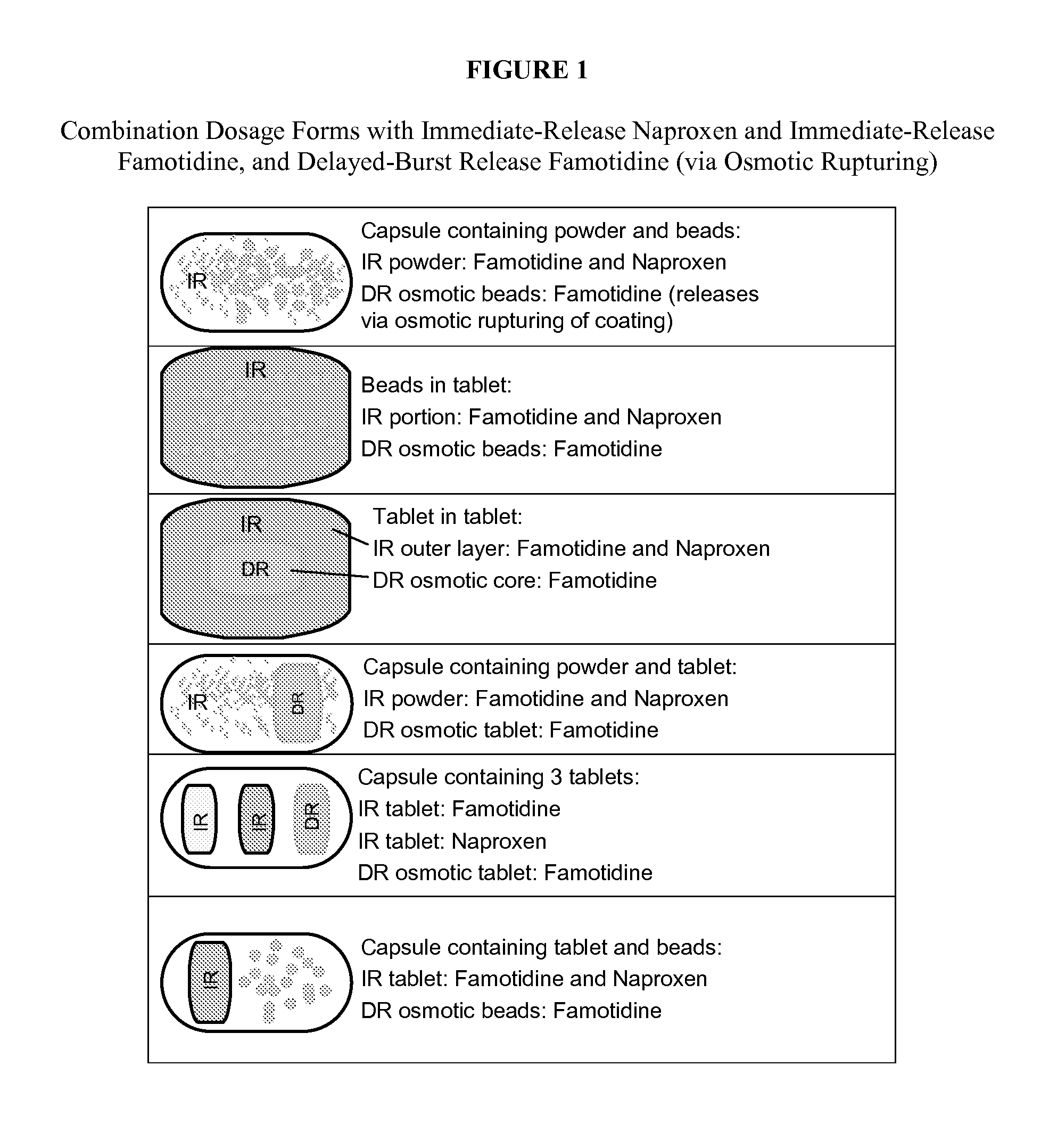
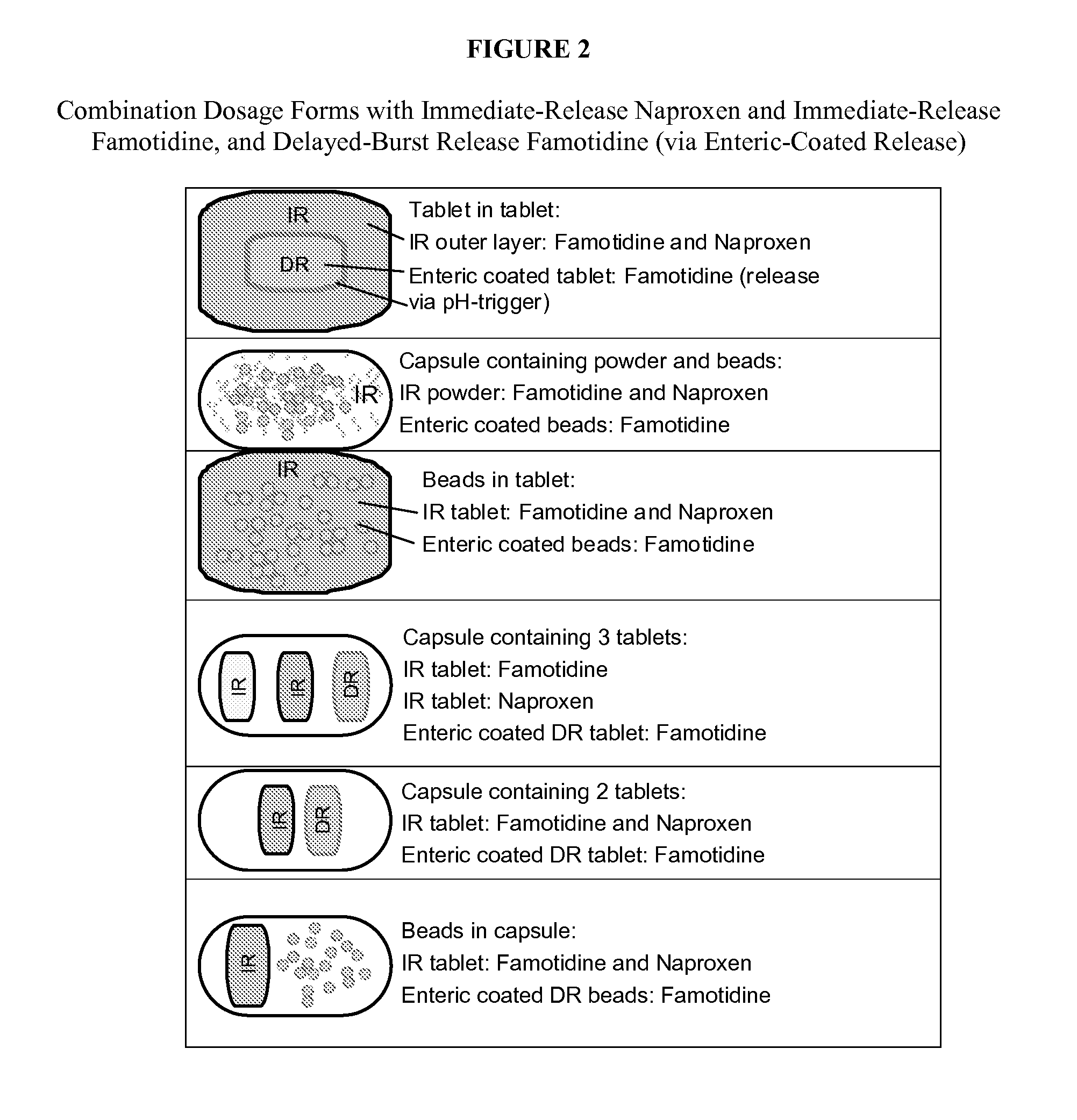
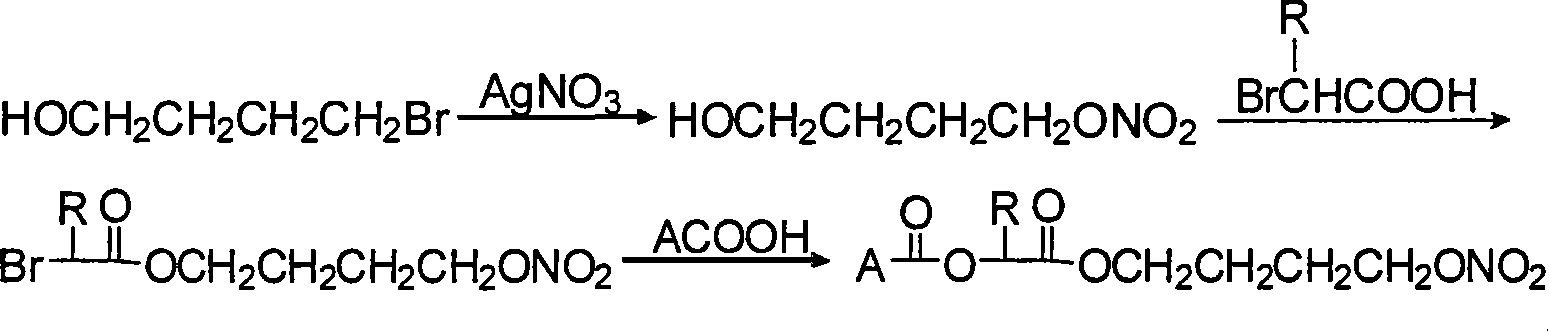Patents
Literature
201 results about "Naproxen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Naproxen is used to relieve mild to moderate pain from various conditions.
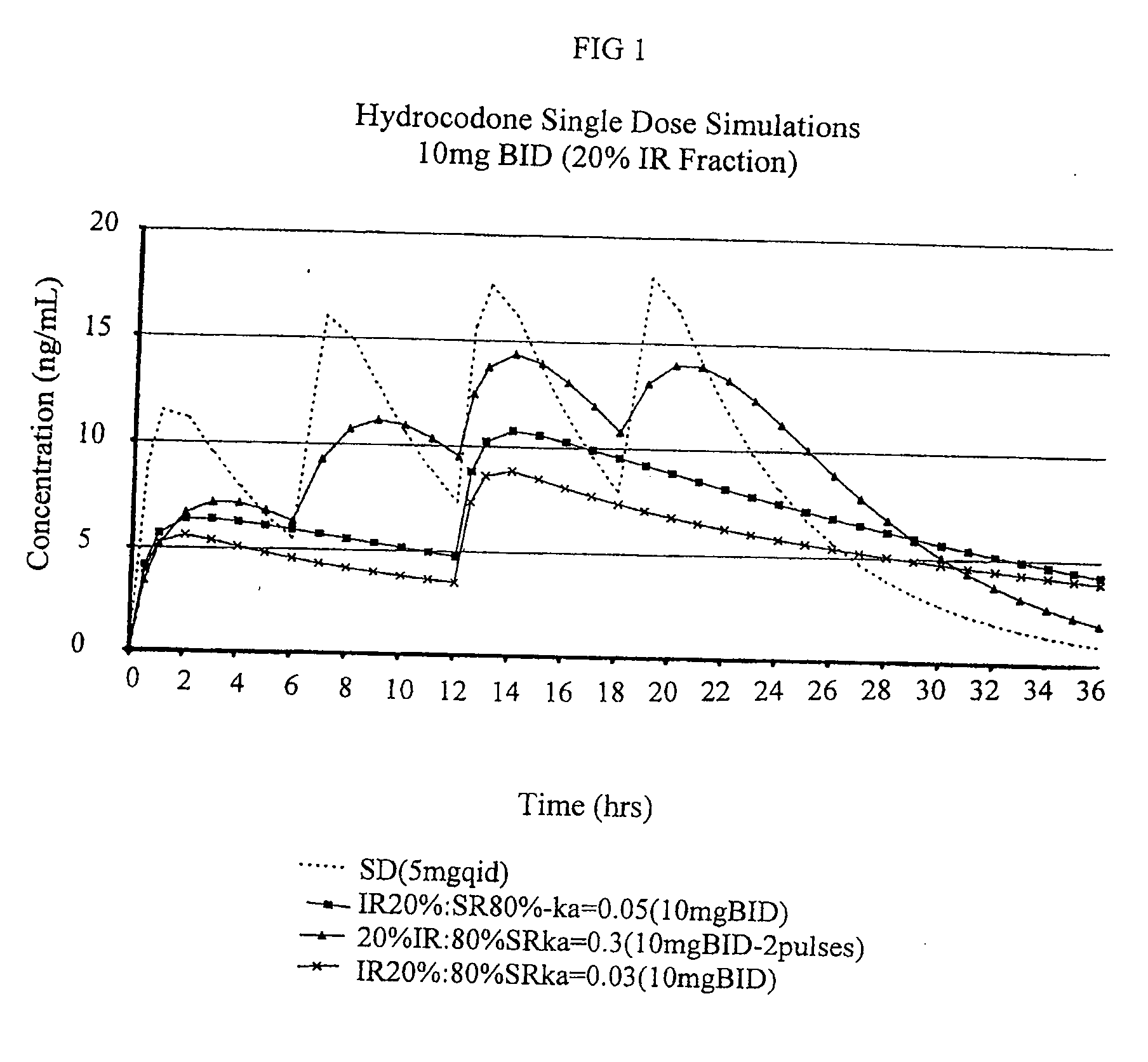
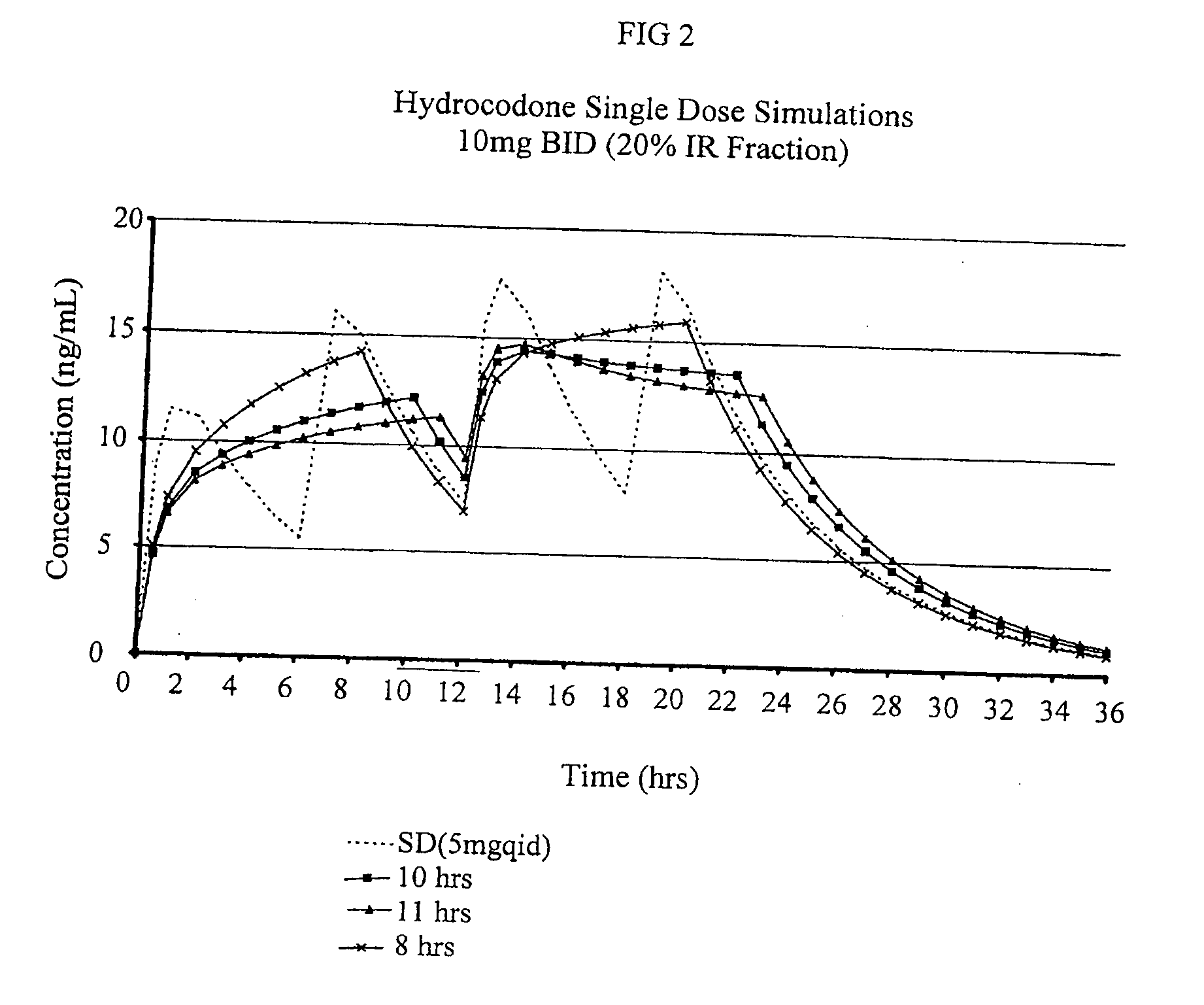
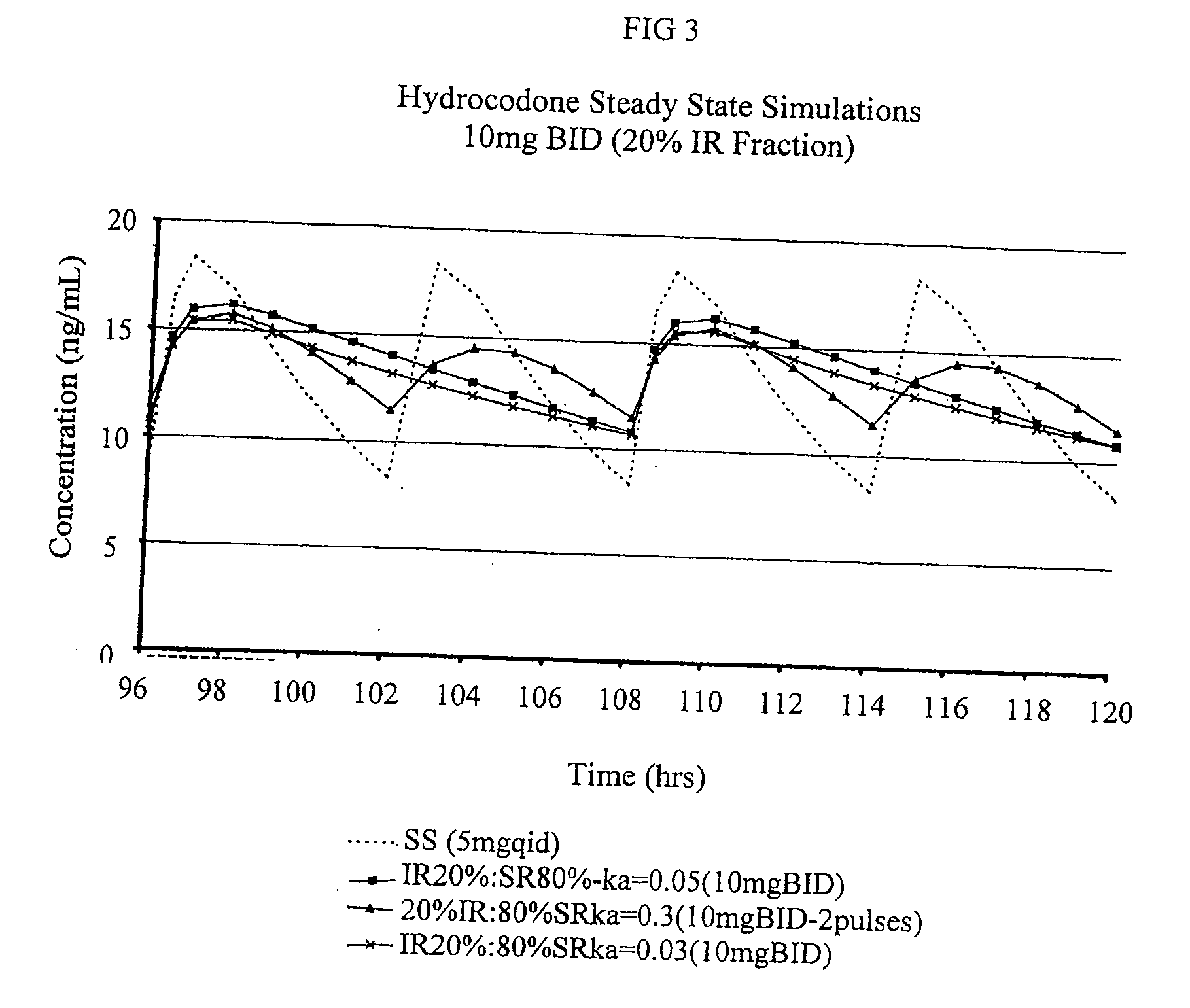
Compositions comprising nanoparticulate naproxen and controlled release hydrocodone
InactiveUS20080113025A1Reduces and eliminates developmentImprove complianceBiocidePowder deliveryControl releaseHydrocodone
The invention relates to a compositions comprising a nanoparticulate naproxen composition in combination with a multiparticulate modified release hydrocodone composition that, upon administration to a patient, delivers a hydrocodone in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of hydrocodone-comprising particles and the at least one subsequent component comprising a second population of hydrocodone-comprising particles, wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form comprising such a combination composition.
Owner:ELAN PHRMA INT LTD
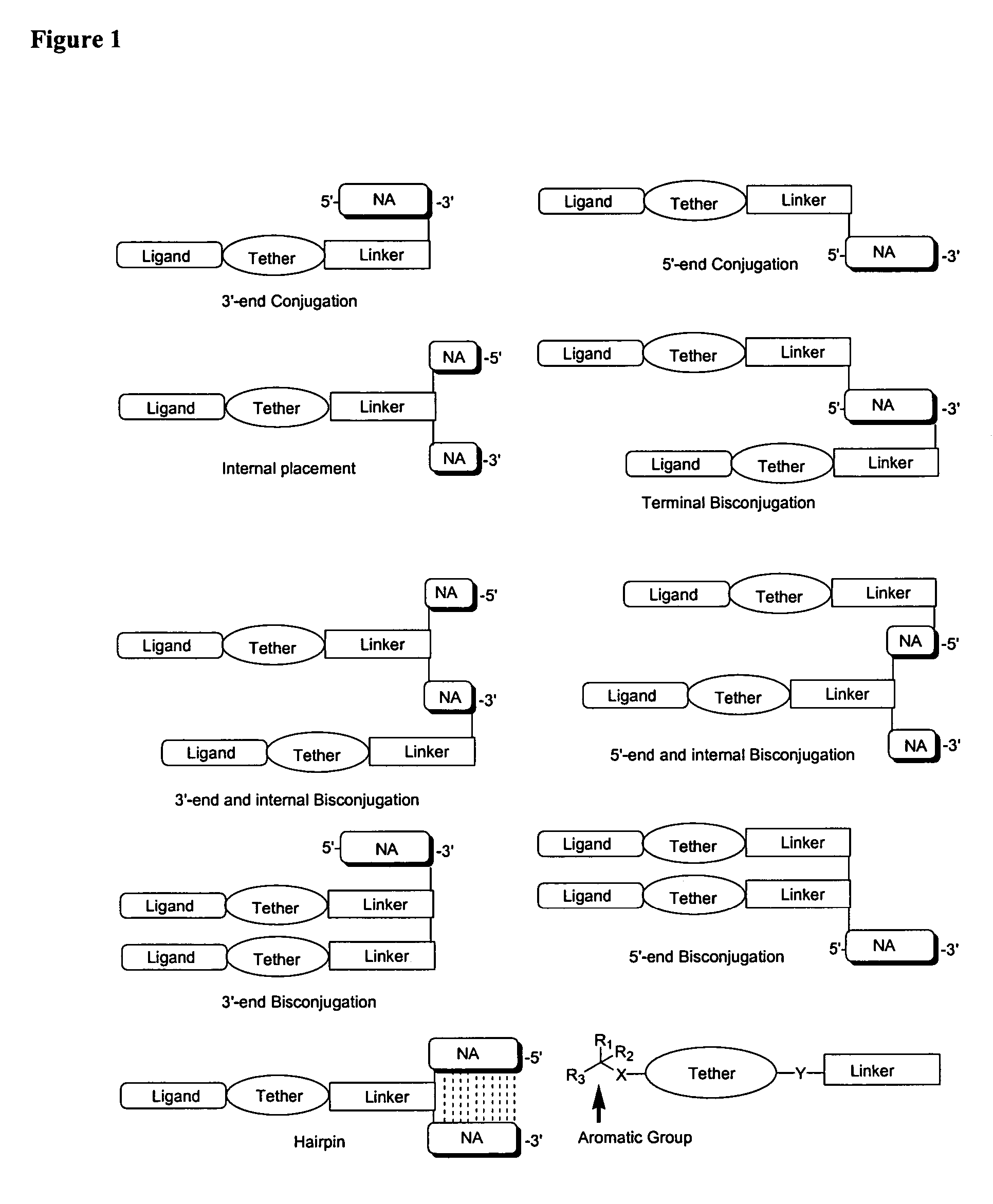
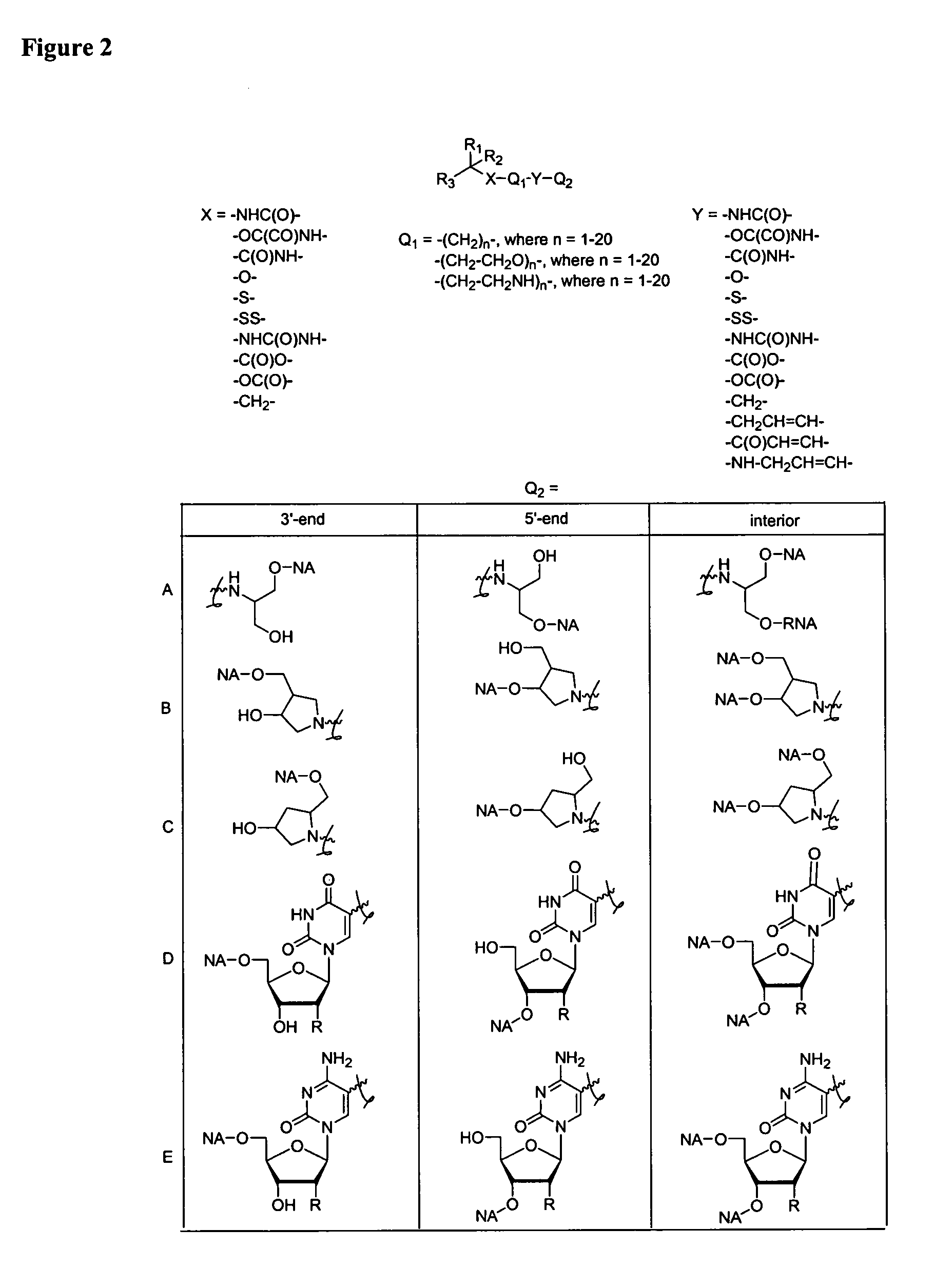
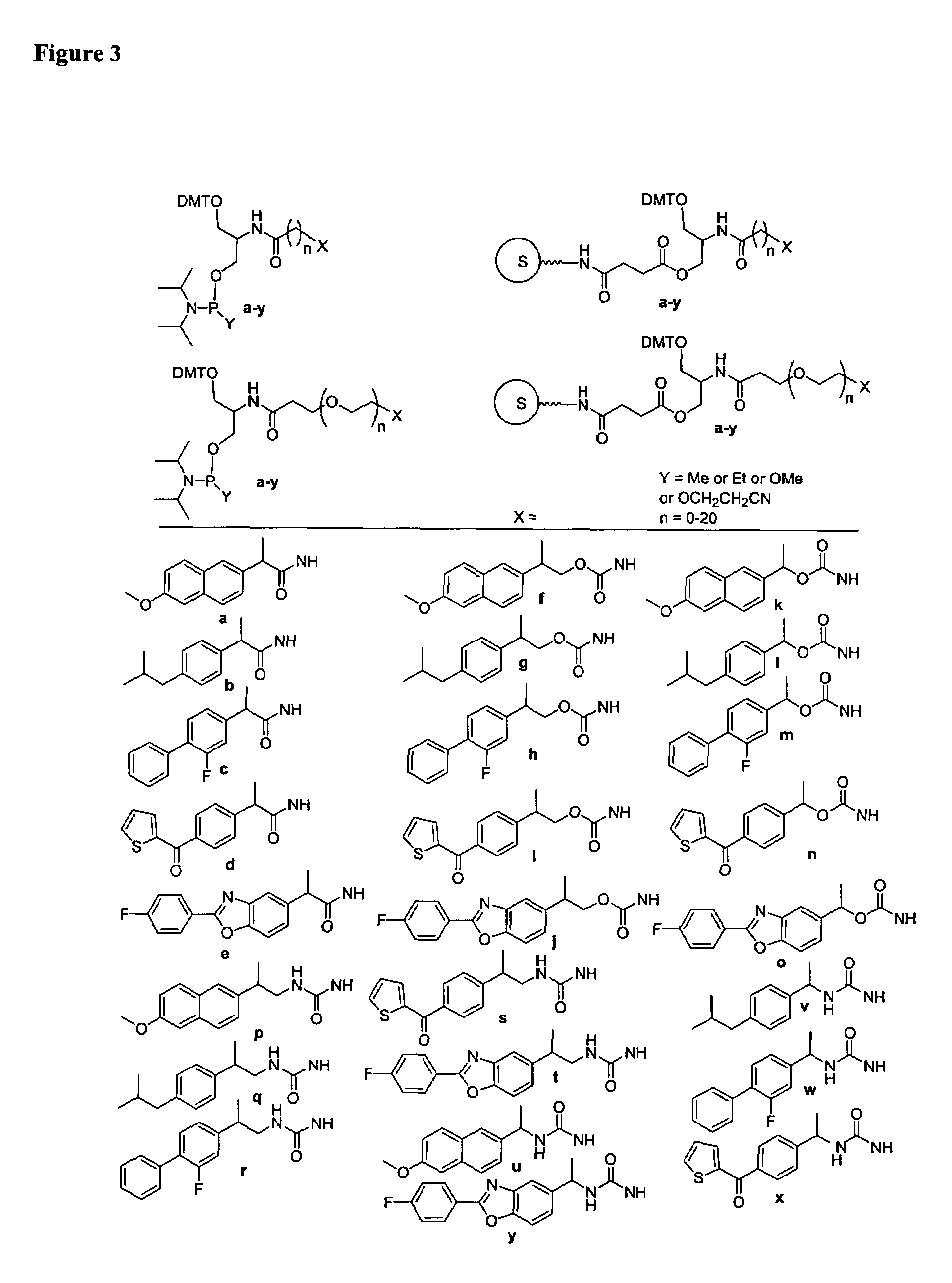
Single-stranded and double-stranded oligonucleotides comprising a 2-arylpropyl moiety
ActiveUS7626014B2Improved pharmacokinetic propertiesAntibacterial agentsSenses disorderSingle strandDouble strand
The present invention provides single-stranded and double-stranded oligonucleotides comprising at least one aralkyl ligand that improves the pharmacokinetic properties of the oligonucleotides. The aralkyl ligands of the present invention include naproxen, ibuprofen and derivatives thereof. The present invention also provides methods for modulating gene expression using the modified oligonucleotide compounds and compositions comprising those modified oligonucleotides.
Owner:ALNYLAM PHARMA INC
Compositions for Preventing and Reducing Delayed Onset Muscle Soreness
InactiveUS20080317886A1Inhibit inflammationSpeed up recoveryBiocideSugar food ingredientsSucroseL glutamate
The present invention relates to the compositions that enhance post-exercise recovery processes to increase both strength and muscle mass, replace glycogen stores, and prevent inflammation, resulting in the prevention and / or reduction of delayed onset muscle soreness. Additionally, it provides a feeling of muscle relaxation as well as a feeling of mental tranquility immediately following exercise. The composition consists of any or all high-glycemic sugars and / or polysaccharides (e.g., sucrose, glucose, maltodextrin), all essential amino acids and beta-hydroxy-beta-methylbutyrate and can include other amino acids sources (e.g. whey protein), performance enhancing agents (e.g., caffeine, L-glutamate), anti-inflammatory agents (e.g., ginger, boswellia, curcumen), antioxidants (vitamin C, vitamin E, selenium, polyphenols,), insulin-mimicking agents (cinnamon, Banaba), analgesics (e.g. aspirin, ibuprofen, naproxen, acetaminophen), and to methods of treating humans and animals by administration of these novel compositions to humans and animals in need thereof.
Owner:SOUTHWEST IMMUNOLOGY
Non-steroidal antiinflammatory drug formulations for topical application to the skin
InactiveUS20030082226A1Improve performanceSignificant positive effectOrganic active ingredientsAntipyreticSkin penetrationAntiinflammatory drug
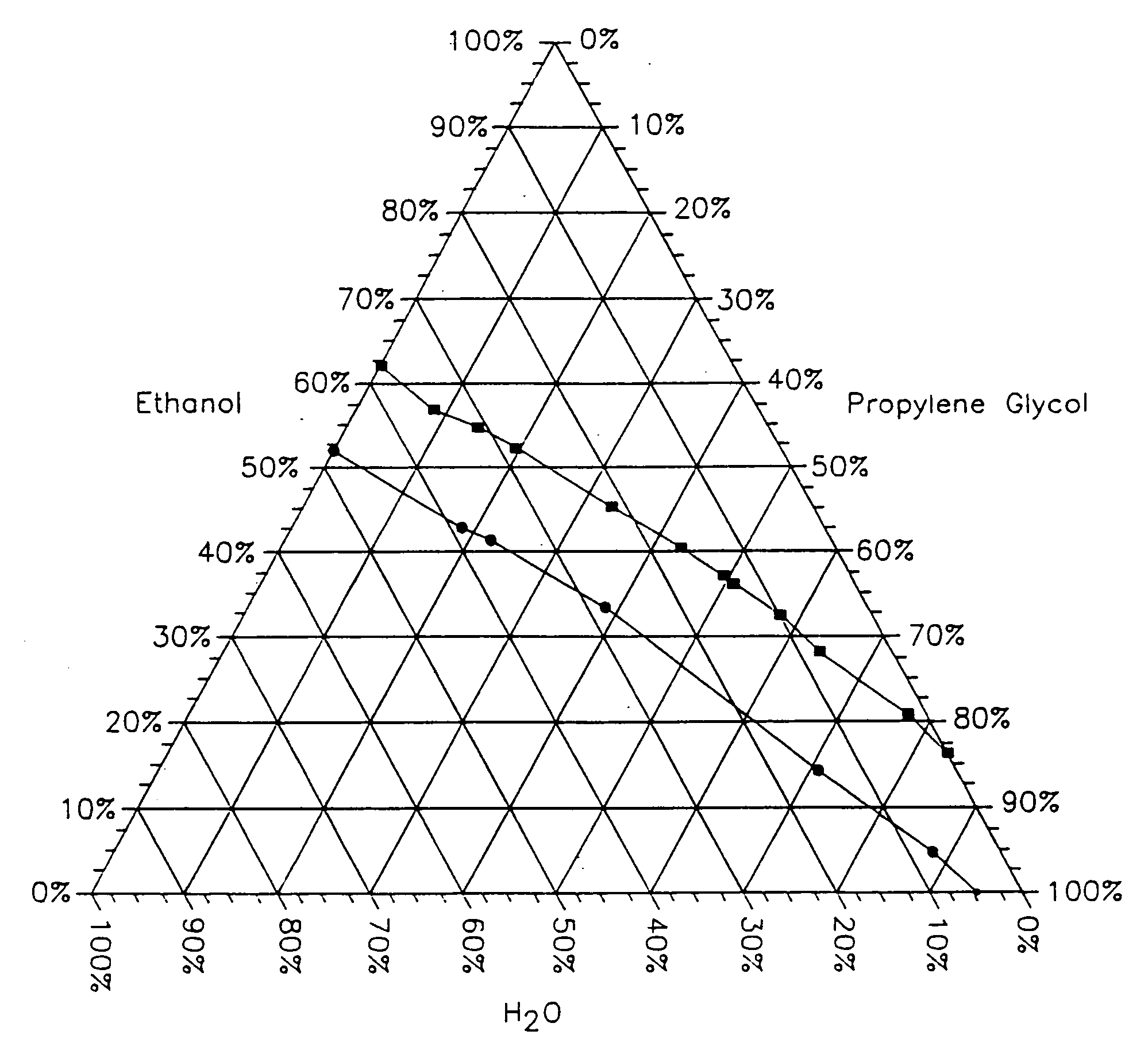
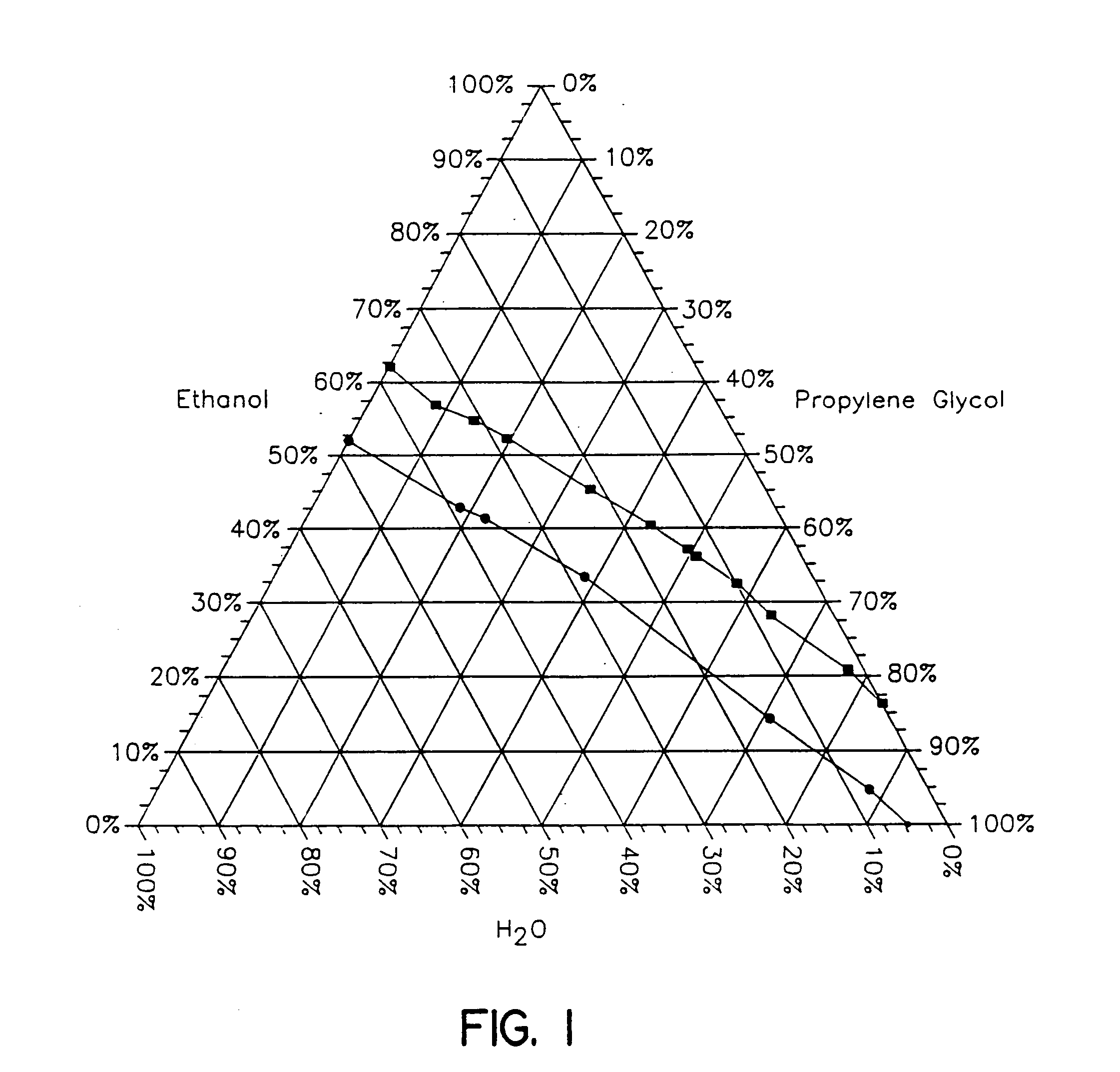
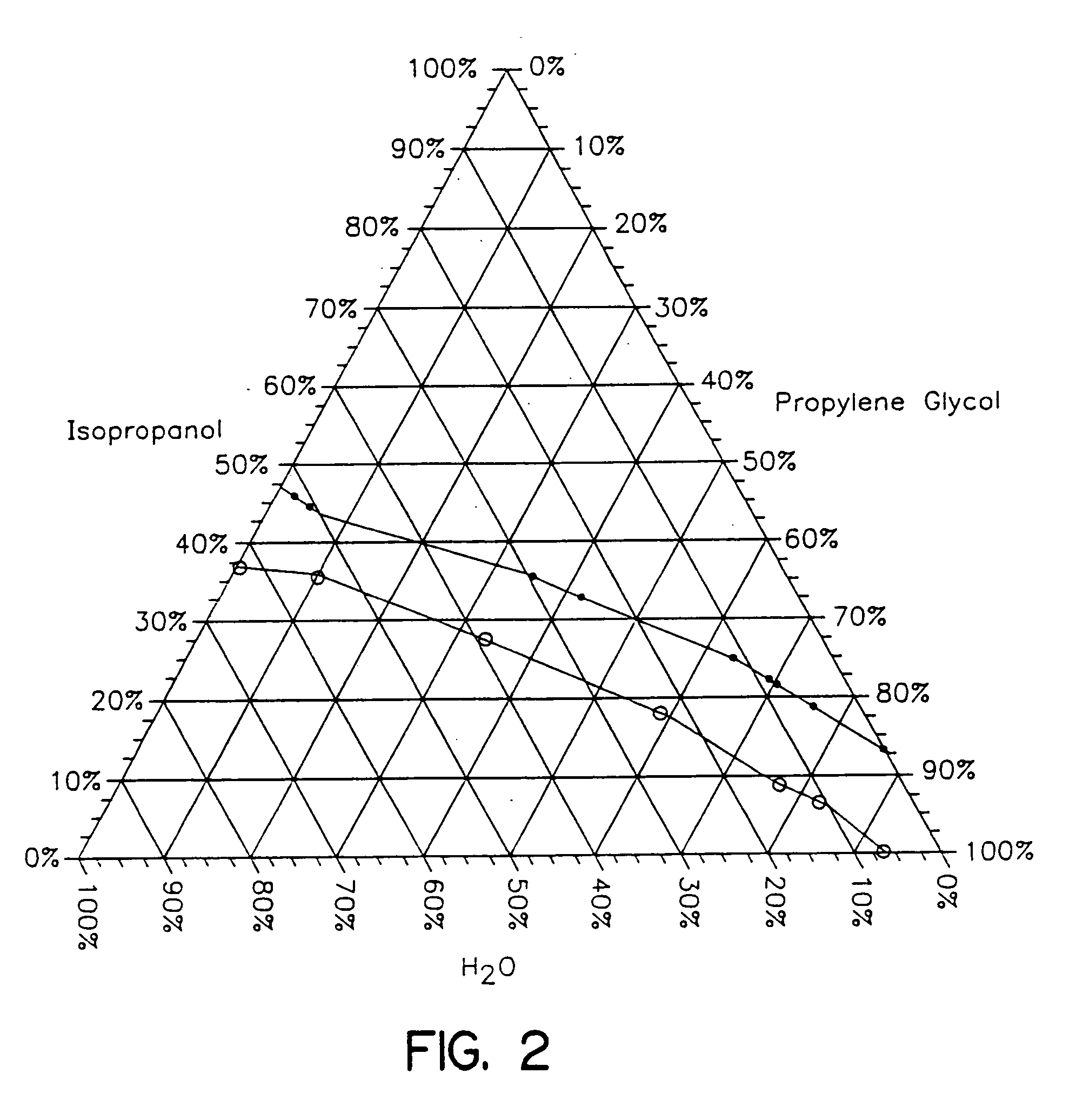
Topical alcoholic or aqueous alcoholic gels containing ibuprofen or other NSAIDs, such as, naproxen, in substantially neutral salt form, have enhanced penetration through skin and may provide rapid pain / inflammation relief by including in the formulation 2-n-nonyl-1,3-dioxolane or other hydrocarbyl derivative of 1,3-dioxolane-or 1,3-dioxane or acetal, as skin penetration enhancing compound. The amount of propylene glycol may be varied to adjust the initial flux of the NSAID through the skin, especially for ibuprofen, naproxen, and ketorolac.
Owner:SAMOUR CARLOS M +2
Pharmaceutical Compositions for the Coordinated Delivery of Naproxen and Esomeprazole
InactiveUS20100172983A1Reduce riskMinimize adverse effectsBiocideAntipyreticAnti arthriticUlcer care
The present disclosure is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The disclosure also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:POZEN INC
Topical composition containing naproxen
The present invention generally relates to the transdermal delivery of various compositions. In some aspects, the transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising ibuprofen and / or an ibuprofen salt, a nitric oxide donor, and optionally, a hostile biophysical environment. In some cases, the composition may be stabilized using a stabilization polymer such as xanthan gum, KELTROL® BT and / or KELTROL® RD; propylene glycol; and a polysorbate surfactant such as Polysorbate 20, which unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Nsaid dose unit formulations with h2-receptor antagonists and methods of use
InactiveUS20110313009A1Reduce releaseReduce riskOrganic active ingredientsBiocideImmediate releaseReceptor antagonist
The present invention generally relates to unit dosage forms of naproxen and H2-receptor antagonists, comprising an immediate-release formulation of naproxen; an immediate-release formulation of an H2-receptor antagonist, and a delayed-burst release formulation of an H2-receptor antagonist.
Owner:HORIZON PHARMA USA
Solid oral formulations for combination therapy
InactiveUS20080085314A1Reduce gastric acid secretionPromote gastric acid secretionBiocideDrug compositionsRanitidineOmeprazole
A first, solid oral pharmaceutical composition includes an extended release acetaminophen, a non-steroidal anti-inflammatory drug, such as naproxen or ibuprofen, and a third drug capable of reducing gastric acid secretion, such as ranitidine or omeprazole. A second, solid oral pharmaceutical composition includes a non-steroidal anti-inflammatory drug and an agent for reducing gastric acid secretion.
Owner:POLY MED
Transdermal plaster of aryl propionic non-steroid antiphlogistic
InactiveCN1387842AImprove adhesionImprove stabilityOrganic active ingredientsAntipyreticTransdermal patchWhole body
The present invention relates to medicine technology and is especially one kind of new preparation form. The transdermal plaster is noe kind of antiphlogistic containing Flubiprofen, Ketoprofen, Ibuprofen, Rosorolfen, Naproxan and other aryl propionic non-steroid. It has three parts including non-sticking layer, medicine layer and lining layer. The medicine layer incldues medicine dispersed in matrix, and the matrix consists of non-polar polymer and plasticizer and may contains tackifier, transdermal promoter and oxidant. It has accurate admistration amount, no stimulation to gastrointestinaltract, relatively higher local medicine density in the affected part and controllable medicine release.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
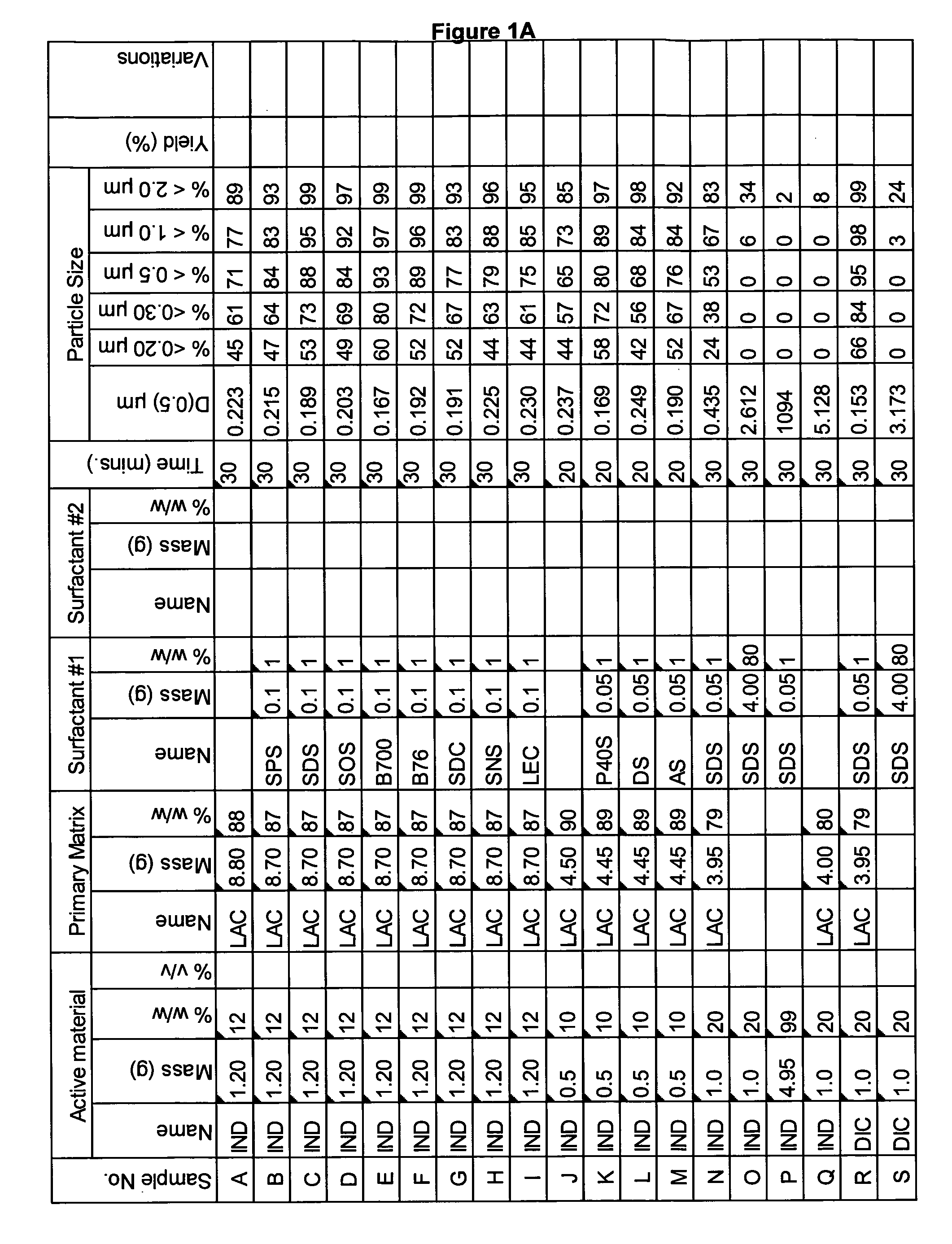
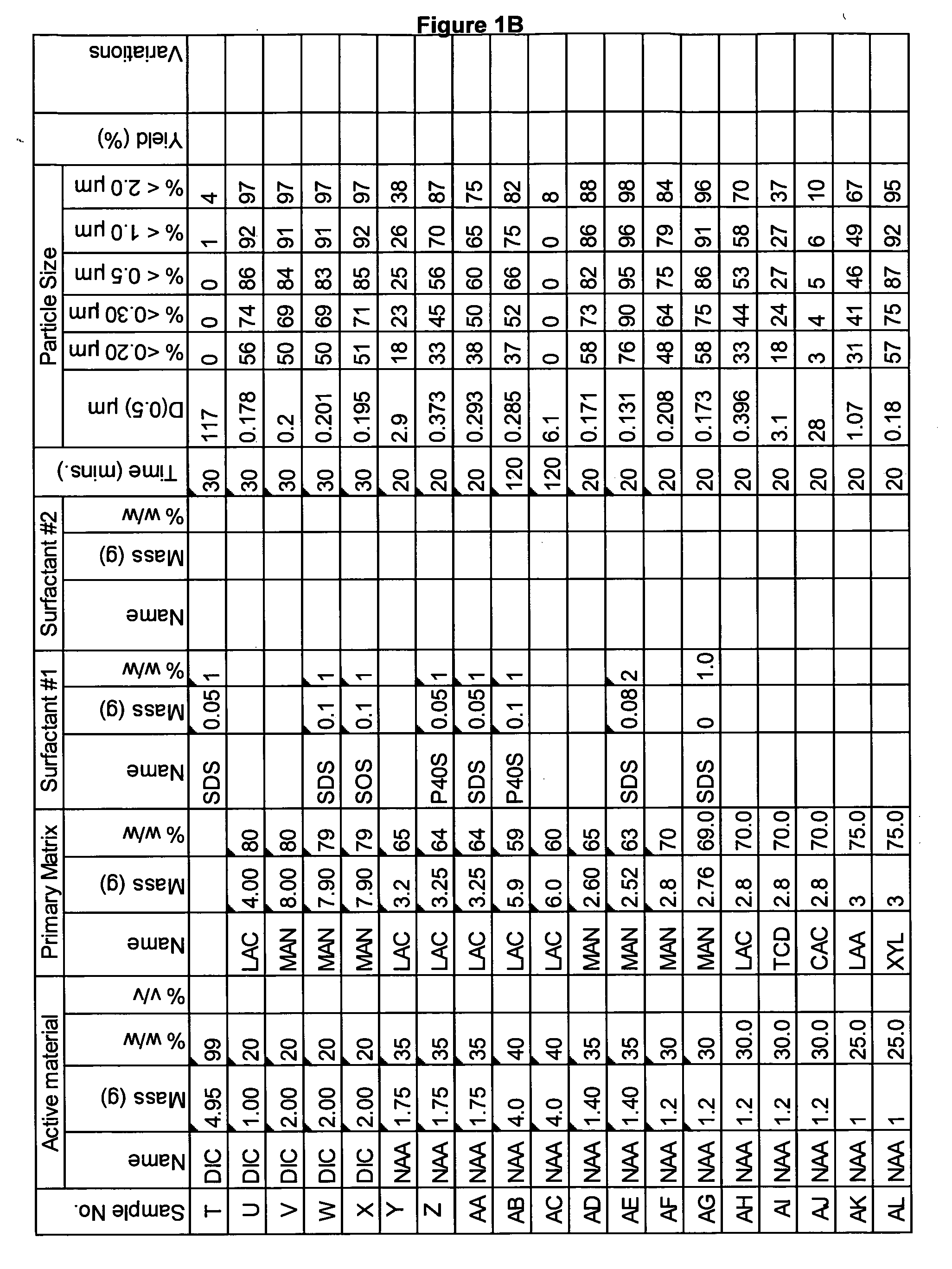
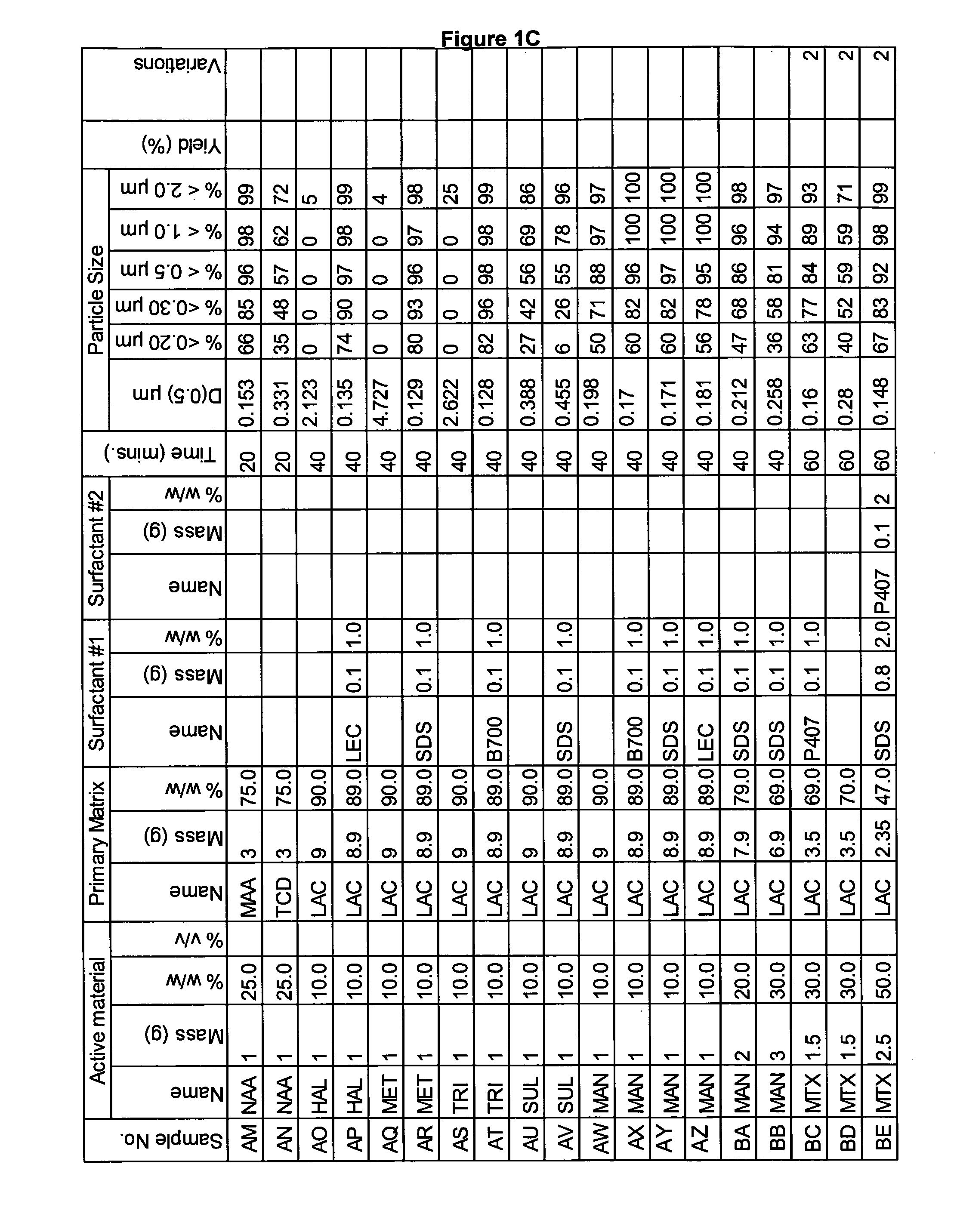
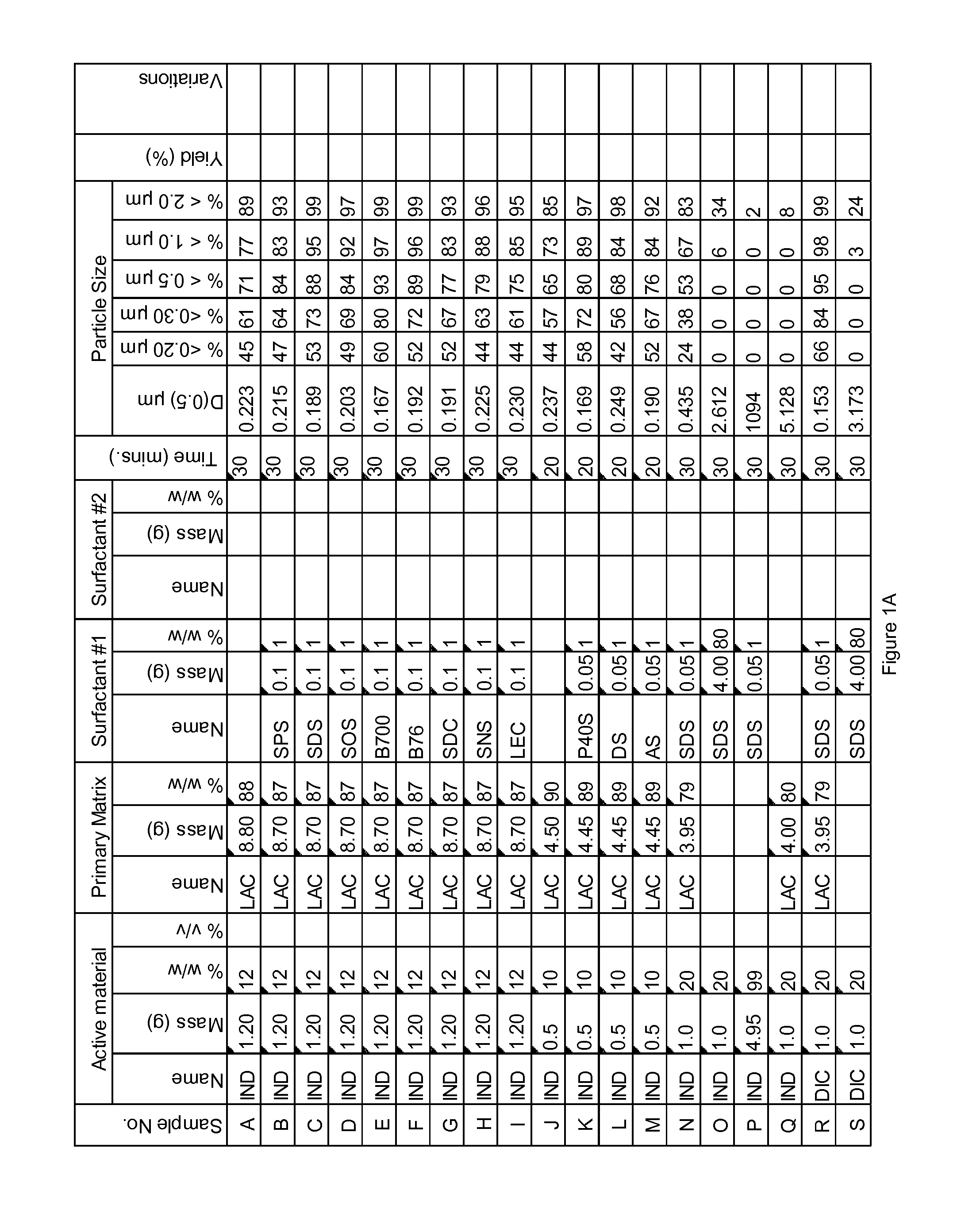
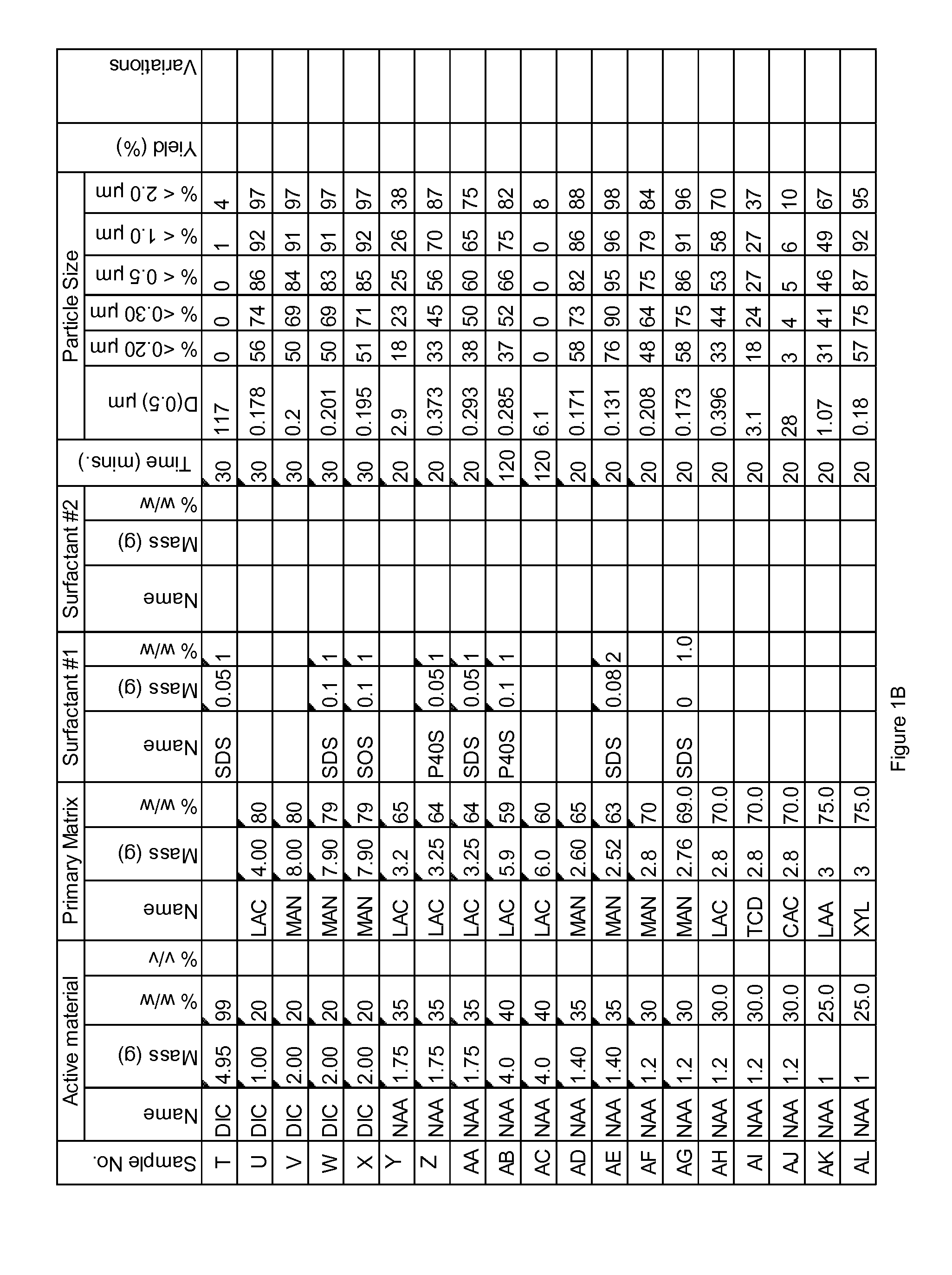
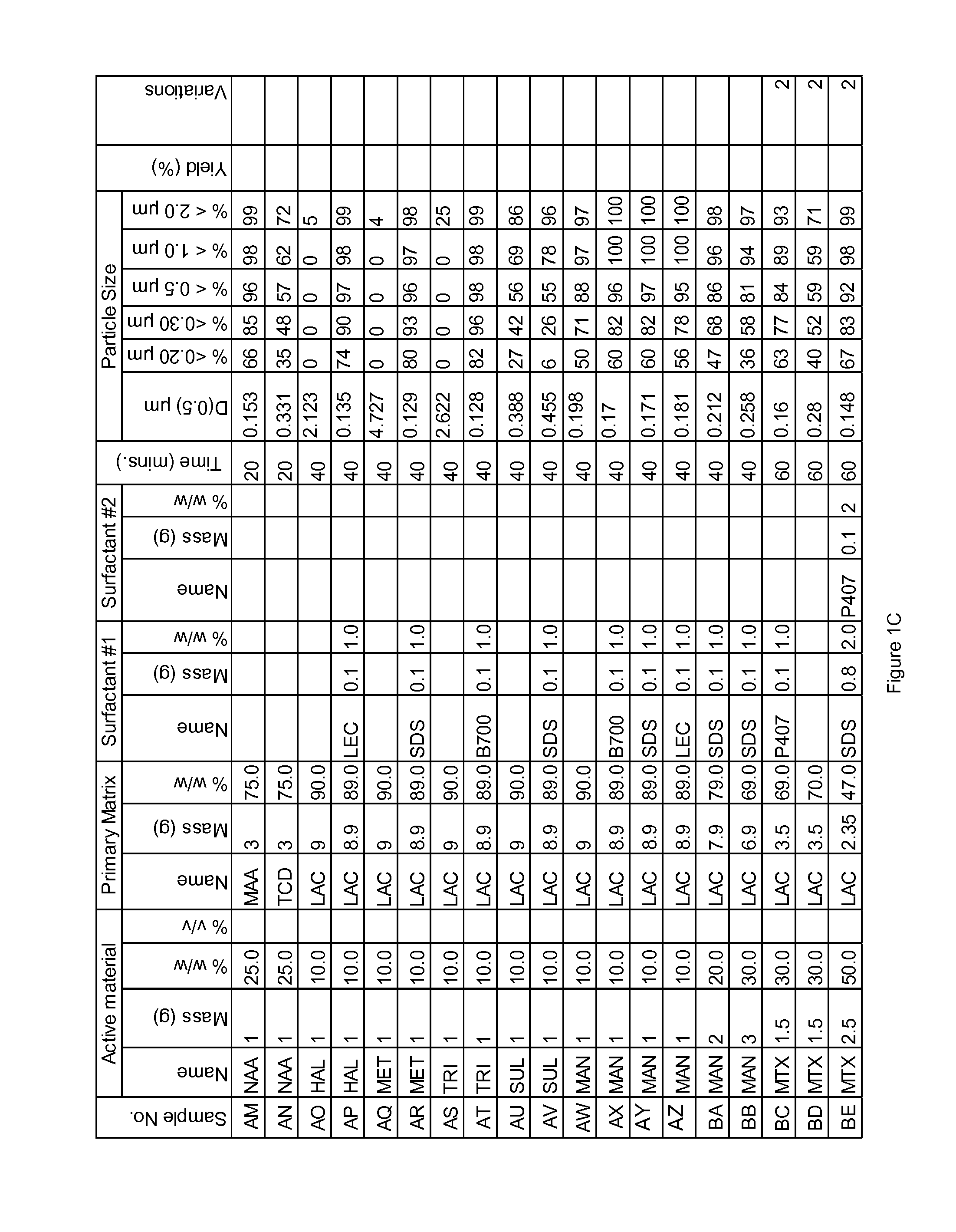
Novel formulation of naproxen
The present invention relates to methods for producing particles of naproxen using dry milling processes as well as compositions comprising naproxen, medicaments produced using naproxen in particulate form and / or compositions, and to methods of treatment of an animal, including man, using a therapeutically effective amount of naproxen administered by way of said medicaments.
Owner:ICEUTICA PTY LTD
Pharmaceutical formulations comprising nsaid and proton pump inhibitor drugs
InactiveUS20100305163A1Increased riskAvoid erosionBiocideAntipyreticTherapeutic intentPharmaceutical formulation
Aspects of the invention relate to pharmaceutical formulations comprising an NSAID and acid reducer drug for therapeutic purposes, and methods of preparing the same. Further aspects of the invention relate to fixed dose pharmaceutical formulations comprising naproxen, or pharmaceutically acceptable salts thereof, and esomeprazole, or pharmaceutically acceptable salts thereof.
Owner:DR REDDYS LAB LTD +1
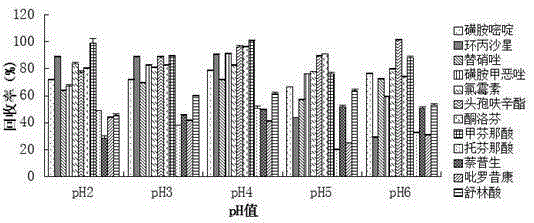
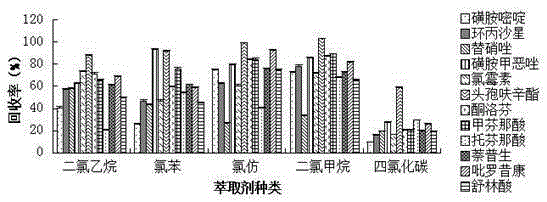
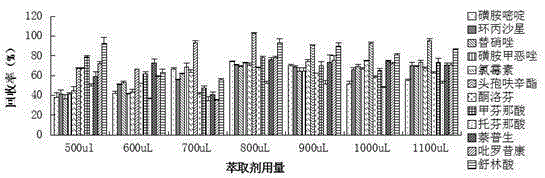
Method for measuring 12 types of remaining medicine in water environment through separation and enrichment
ActiveCN105424825ASimplified processing stepsImprove extraction efficiencyComponent separationWater dischargePretreatment method
The invention relates to a method for measuring 12 types of remaining medicine in a water environment through separation and enrichment at the same time, and belongs to the field of safety detection of a trace of organic pollutant residue in the water environment. The content of 12 types of frequently-used medicine in the water environment (drinking water, faucet water, river water and water discharged into and out of sewage treatment plants) is directly measured with an ultra performance liquid-chromatography-mass spectrometer (UPLC-MS / MS) as a detection tool after a water sample is subjected to solid phase extraction combined with ultrasonic-assisted dispersion liquid-liquid micro-extraction (UA-DLLME) separation and enrichment. The 12 types of antibiotic include ketoprofen, ciprofloxacin, tinidazole, tolfenamic acid, sulfadiazine, sulindac, naproxen, sulfamethoxazole, chloramphenicol, cefuroxime axetil, piroxicam and mefenamic acid. Inspection and optimization are conducted on a sample pretreatment method and instrument detection conditions of the water sample, and the optimal UA-DLLME method is established and is successfully applied to practical sample detection. Compared with a traditional method, the method has the advantages of being high in sensitivity, high in extraction and recycle rate, wide in suitable object, friendly to the environment, and the like.
Owner:SHENYANG PHARMA UNIVERSITY +1
Industrial synthesis technique for DL-naproxen
InactiveCN101234963AHigh purityReduce generationOrganic compound preparationCarboxylic compound preparationAlcoholKetone
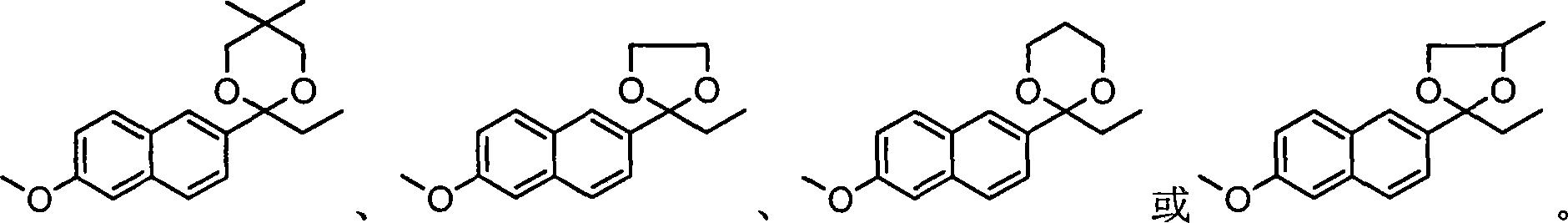
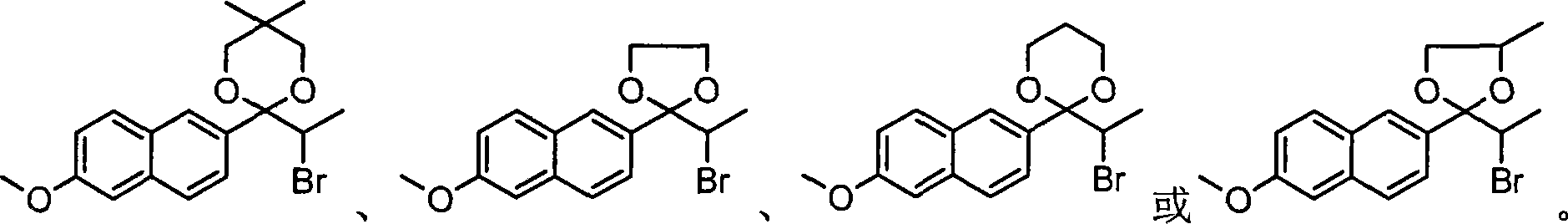
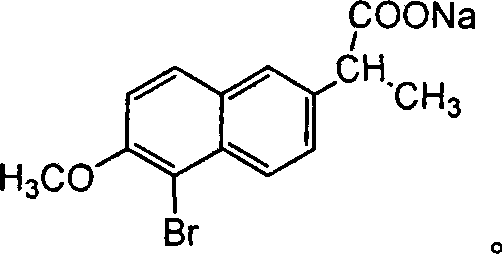
The invention relates to an industrial synthesis technique of DL-naproxen, which uses 2-propinonyl-6-methoxynaphthalene is reacted with neopentyl glycol, ethylene alcohol, 1, 3-methyl glycol or 1, 2-methyl glycol via ketol reaction to give ketone I, then processes a-bromo-reaction which is subjected to rearrangement to open ring, at last processes alkali hydrolytic reaction and acidification reaction to obtain DL-naproxen. The industrial synthesis technique of DL-naproxen has simple operation, high yield and low cost, which is fit for commercial production.
Owner:江苏八巨药业有限公司
Topical composition containing naproxen
The present invention generally relates to the transdermal delivery of various compositions. In some aspects, the transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising ibuprofen and / or an ibuprofen salt, a nitric oxide donor, and optionally, a hostile biophysical environment. In some cases, the composition may be stabilized using a stabilization polymer such as xanthan gum, KELTROL® BT and / or KELTROL® RD; propylene glycol; and a polysorbate surfactant such as Polysorbate 20, which unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Combined nsaid and acid blocker formulation and method
InactiveUS20090233970A1Reduce gastrointestinal irritationGood pain reliefBiocideNervous disorderNaproxenPain relief
The present invention is directed to co-administration of a non-steroidal anti-inflammatory agent (NSAID) and acid blocking agent for the treatment of pain and inflammation with reduced gastrointestinal irritation. A pharmaceutical composition suitable for the co-administration contains a therapeutically effective amount of at least one non-steroidal anti-inflammatory agent, and a therapeutically effective amount of at least one acid blocking agent. A ratio of the non-steroidal anti-inflammatory agent to acid blocking agent in the composition is within a range that provides greater pain relief and reduction of inflammation with less gastrointestinal irritation than that obtainable by the administration of the non-steroidal anti-inflammatory agent or acid blocking agent alone. Examples of pharmaceutical compositions for co-administration of the agents are those containing ibuprofen and ranitidine (“ibudine”), as well as naproxen and ranitidine (“naprodine”).
Owner:NICKELL ROBERT P
Process
At the present invention relates to a new process for the preparation of the (S)-naproxen 4-nitrooxybutyl ester and to new intermediates obtained and used therein. The invention further relates to the use of the new intermediates for the manufacturing of pharmaceutically active compounds such as (S)-naproxen 4-nitrooxybutyl ester. The invention also relates to the use of (S)-naproxen 4-nitrooxybutyl ester prepared according to the process of the present invention for the manufacturing of a medicament for the treatment of pain.
Owner:NICOX SA
Non-steroidal anti-inflammatory drug formulations for topical applications to the skin
Topical alcoholic or aqueous alcoholic gels containing ibuprofen or other NSAIDs, such as, naproxen, in substantially neutral salt form, have enhanced penetration through skin and may provide rapid pain / inflammation relief by including in the formulation 2-n-nonyl-1,3-dioxolane or other hydrocarbyl derivative of 1,3-dioxolane or 1,3-dioxane or acetal, as skin penetration enhancing compound. The amount of propylene glycol may be varied to adjust the initial flux of the NSAID through the skin, especially for ibuprofen, naproxen, and ketorolac.
Owner:MACROCHEM CORP
Combinations of proton pump inhibitors, sleep aids, buffers and pain relievers
Pharmaceutical compositions comprising a proton pump inhibitor, one or more buffering agent, a sleep aid and acetaminophen, ibuprofen, aspirin or naproxen are described. Methods are described for treating gastric acid related disorders and inducing sleep, using pharmaceutical compositions comprising a proton pump inhibitor, a buffering agent, a sleep aid and a pain reliever.
Owner:SANTARUS
Medicine composition containing matrine class alkaloid, preparation method and pharmaceutical application
The invention provides a medicine composition containing matrine class alkaloid, a preparation method and pharmaceutical applications. The medicine composition is the combination of the matrine class alkaloid and inflammation-resisting pain-relieving class medicines. The inflammation-resisting pain-relieving medicine comprises the non-steroidal inflammation-resisting medicines of aspirin, acetaminophen, indometacin, ibuprofen, oxyphenbutazone, naproxen, mefenamic acid, diclofenac sodium, celecoxib, rofecoxib, valdecoxib and the like and also comprises the vegetable inflammation-resisting pain-relieving class medicines of escin, ferulic acid, berberine, wilfordine, ephedrine and the like and the pain-relieving class medicines of morphia, demerol and the like. The matrine class alkaloid and one or various of the inflammation-resisting pain-relieving class medicines can form a medicine composition used for the pharmaceutical applications of resisting cold, allaying a fever and treating the swelling and pain of the bone joint and the muscle, rheumatic diseases, cardiovascular diseases, arteriosclerotic diseases, tumors, anaphylactic diseases, senile dementia, mosquito bite, insect bite and the like.
Owner:QINGDAO QIYUAN BIO TECH CO LTD
Long-acting viscose dispersing type transdermal patch and preparing process thereof
InactiveCN105250243AHigh transdermal efficiencyModerate viscosityOrganic active ingredientsAntipyreticTransdermal patchTG - Triglyceride
The invention discloses a long-acting viscose dispersing type transdermal patch and a preparing process thereof and belongs to the medical technical field. The transdermal patch is mainly prepared from bulk pharmaceutical chemicals (such as non-steroid anti-inflammatory drug ibuprofen and salt form thereof, naproxen and salt form thereof, ketoprofen, indometacin and salt form thereof), a penetration enhancer (menthol, oleic acid, medium chain triglyceride, propylene glycol monolaurate, azone, propylene glycol and the like), a dispersing solvent (water, acetone, ethanol, carbinol, ethyl acetate and the like), a polyacrylate pressure-sensitive adhesive (crylic acid, butyl acrylate, crylic acid 2-ethylhexyl ester and the like), a backing layer and a release liner. The long-acting viscose dispersing type transdermal patch and the preparing process thereof have the advantages that drugs can be released from a matrix continuously for 12-48 h, the number of drug residues is low, transdermic absorption property is excellent, and dosing frequency and dosing amount can be reduced; skin irritation is avoided, and adhesion and compliance are high; main drugs and additives are stable in a viscose mechanism; preparing technology is simple, and pollution is avoided.
Owner:CHINA PHARM UNIV
Nonsteroidal antiinflammatories with nitric oxide donors and its preparation method
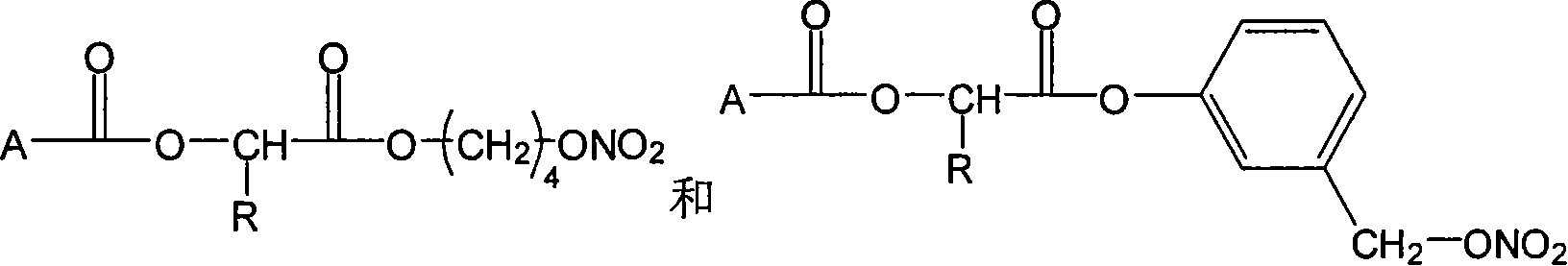
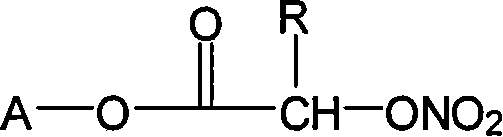
InactiveCN101053662ALittle side effectsMild reaction conditionsAntipyreticAnalgesicsIndometacinSide effect
The invention relates to a non-steroidal anti-inflammatory drug with nitrogen oxide donor and a method for preparing same, which can be used to eliminate inflammation, relieve fever and stop pain, and can decrease the frequently seen side effect thereof on the gastrointestinal tract. The structure of which is A-O(X)-CO-O(y)-B-ONO2,wherein, A is the non-steroidal anti-inflammatory group; B is the connection group, when x=0, y=1; when x=1 then y=0. The the non-steroidal anti-inflammatory groups includes aspirin, diclofenac, indometacin, lumiracoxib, brufen, ketoprofen, naproxen, piroxicam, and meloxicam. The preparation process are that the bromhydrin (or hydroxybenzene) reacts with silver nitrate into hydroxy nitrate, then reacts with bromo acid into the connection group of nitrogen oxide donor, then connects with the non-steroidal anti-inflammatory drug; or the non-steroidal anti-inflammatory drug condensates with bromo acid into a bromide intermediate, then reacts with silver nitrate into the non-steroidal anti-inflammatory drug with nitrogen oxide donor.
Owner:江苏吴中苏药医药开发有限责任公司
Process for preparing pharmaceutical compositions for use with soft gelatin formulations
InactiveCN1477953AIncrease concentrationImprove comfortOrganic active ingredientsAntipyreticMedicineAdditive ingredient
The invention disclosed herein is a process for increasing the achievable concentration of a pharmaceutically active ingredient relative to fill composition viscosity for dosage units. The process is particularly useful in the preparation of soft gelatin capsules containing ibuprofen, naproxen, indomethacin, and acetaminophen, as the pharmaceutically active ingredient. As a result of the process, lesser quantities of composition ingredients other than the pharmaceutically active ingredient are needed to accomplish the same therapeutically effective dosage, thereby significantly increasing the concentration of the pharmaceutically active ingredient resulting in either a reduction in overall fill volume and dosage unit size or an increase in concentration of pharmaceutically active ingredient per dosage form.
Owner:R P SCHERER TECH INC
Method for preparing (S)-naproxen by enzyme resolution of racemic naproxen ester
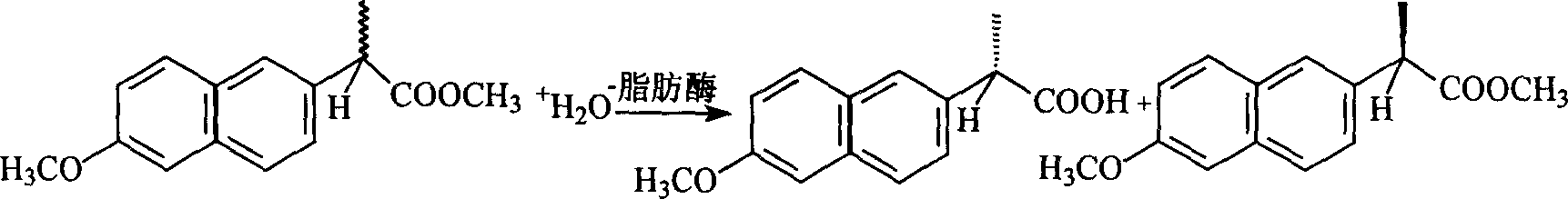
InactiveCN101880695AEffective dispersionEffective response speedChemical recyclingFermentationOrganic solventSlurry
The invention discloses a method for preparing (S)-naproxen by enzyme resolution of racemic naproxen ester. The method comprises the following steps of: resolving and hydrolyzing the racemic naproxen ester to form the (S)-naproxen by using esterase or lipase in an aqueous phase medium, wherein the racemic naproxen ester is mechanically disrupted, and dispersed in the aqueous phase medium to form suspended slurry, and the esterase or lipase is added to carry out hydrolysis reaction. In the method, the (R)-naproxen ester is subjected to racemization in the presence of sodium alcoholate serving as a base catalyst so as to reduce production cost and improve reaction yield. The method realizes effective dispersion of the naproxen ester in the single aqueous phase reaction medium, greatly improves the concentration of reactants, quickens reaction speed and improves the reaction yield. The comprehensive yield of the (S)-naproxen can reach 86 percent, and the optical purity can reach over 97 percent. The method has the advantages of simple process, no need of organic solvent or surfactant, and less environmental pollution.
Owner:EAST CHINA UNIV OF SCI & TECH
Synthesizing method and use of bone-targeted antiphlogistic medicament
InactiveCN101475484AIncreased SOD contentImprove cartilage surface symptomsOrganic active ingredientsOrganic compound preparationSynthesis methodsArthritis
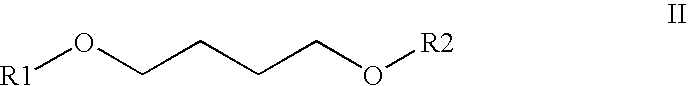
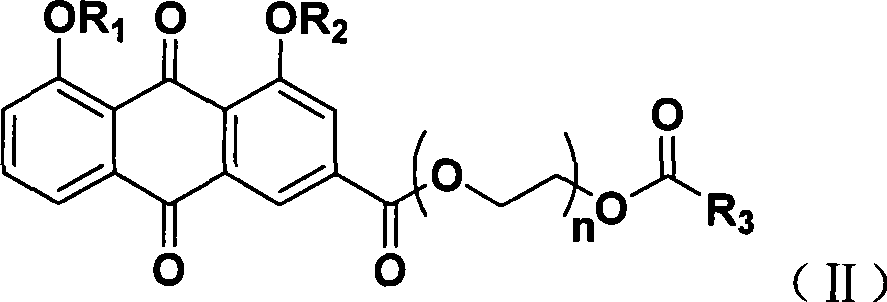
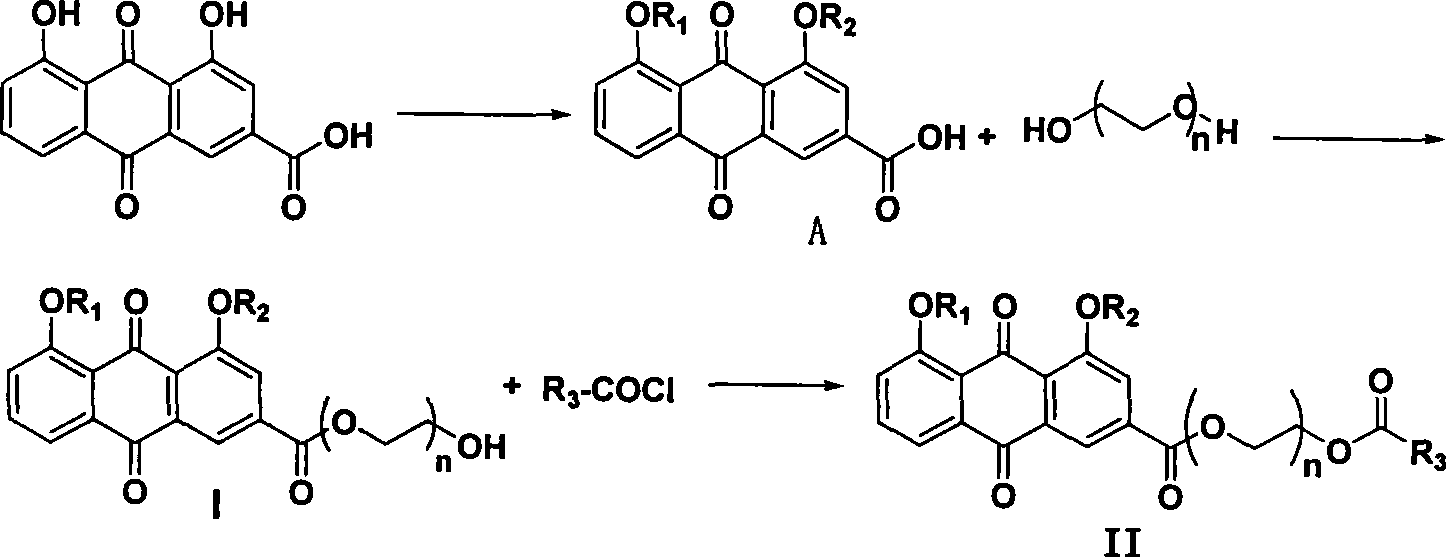
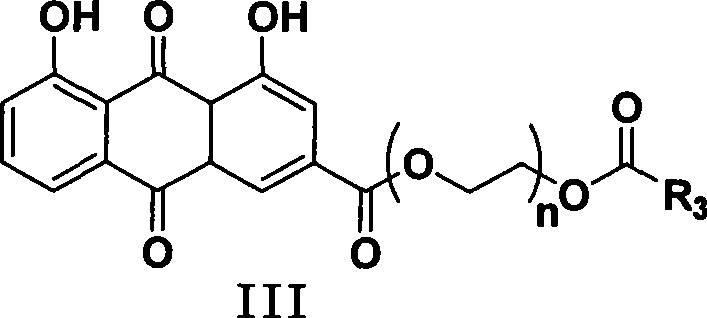
The invention discloses a bone-targeted anti-inflammatory agent, a synthesis method and application thereof, and in particular relates to compounds having a structure in a formula (II), wherein R1 or R2 respectively independently refers to hydrogen or acyl between C1 and C8; R3 refers to aspirin, ibuprofen, naproxen, indometacin or carboxyl residue of diclofenac; and n refers to an integer between 1 and 10. The application of the compounds is to prepare the bone-targeted antiinflammatory agent, in particular medicines for treating osteoarthritis or rheumatic arthritis.
Owner:SOUTHEAST UNIV
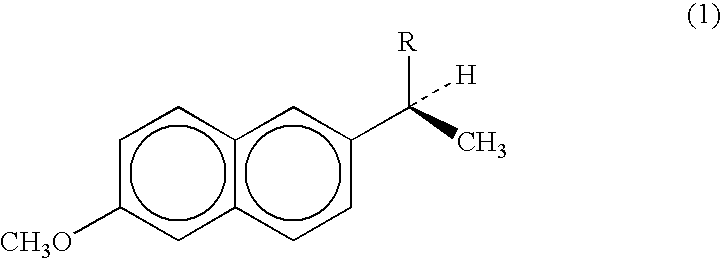
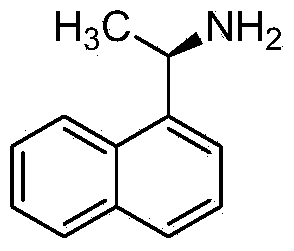
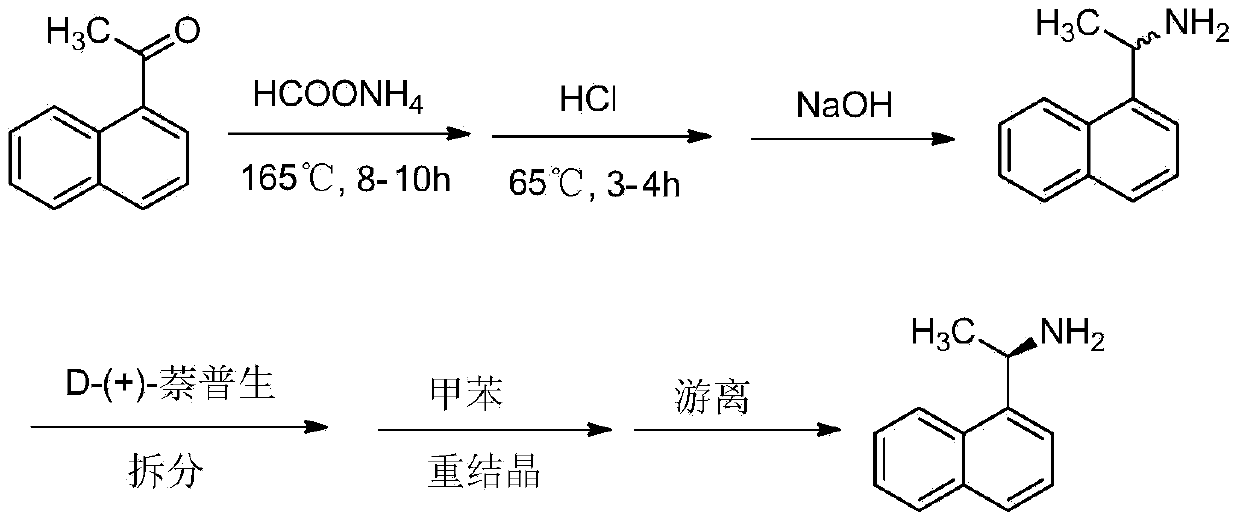
Method for preparing cinacalcet intermediate R-(+)-1-(1-naphthyl)ethamine
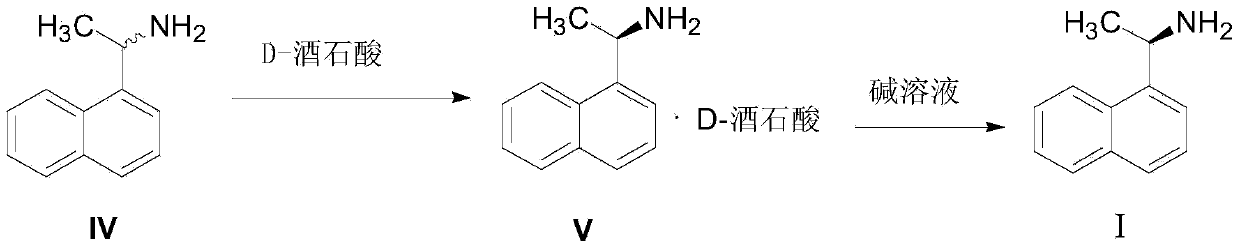
ActiveCN103420845ALow costMeet the requirements of green chemistryAmino compound purification/separationDrugs synthesisCinacalcet
The invention relates to the field of drug synthesis, in particular to a method for preparing a cinacalcet intermediate R-(+)-1-(1-naphthyl)ethamine. The method is characterized in that D-tartaric acid which is low in price and easy to obtain is selected to replace D-(+)-naproxen to serve as a resolving agent, e.e. of an obtained product is up to 99.9%, and the method has the advantages of low cost, environmental friendliness and the like.
Owner:CHINA PHARM UNIV +1
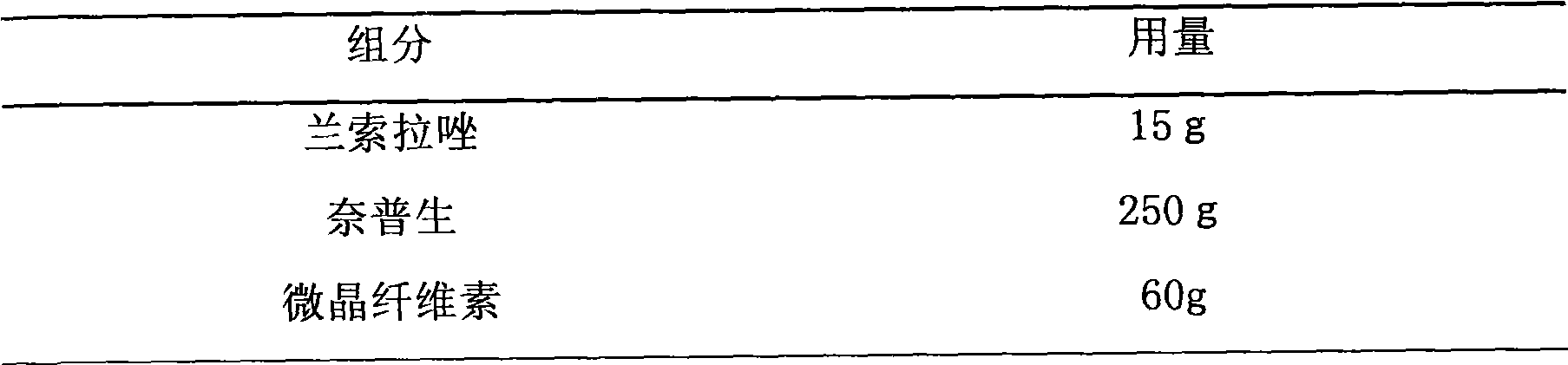
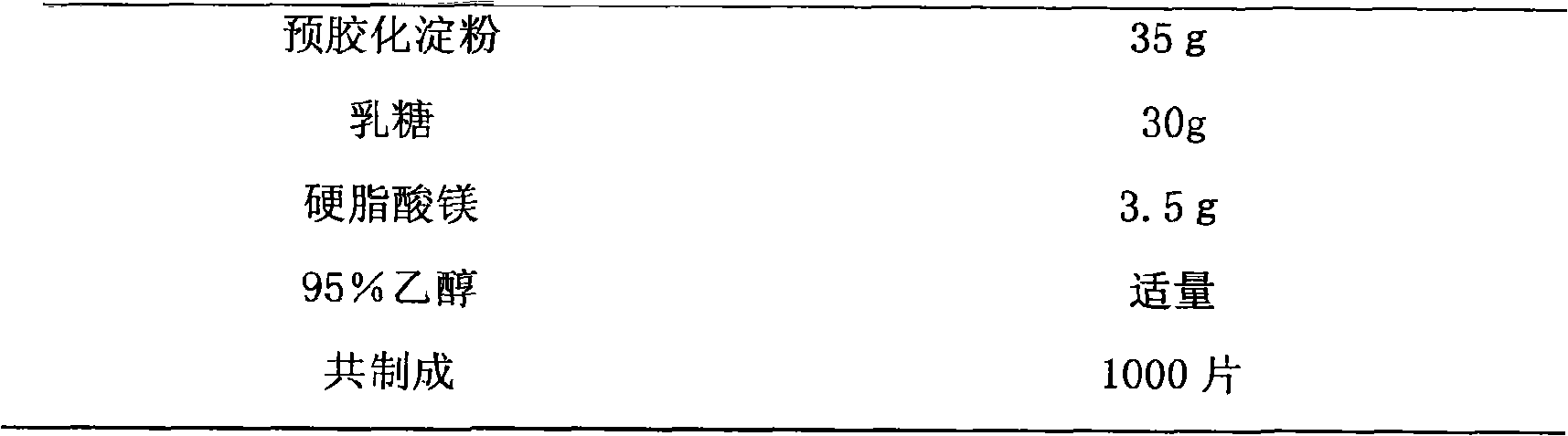
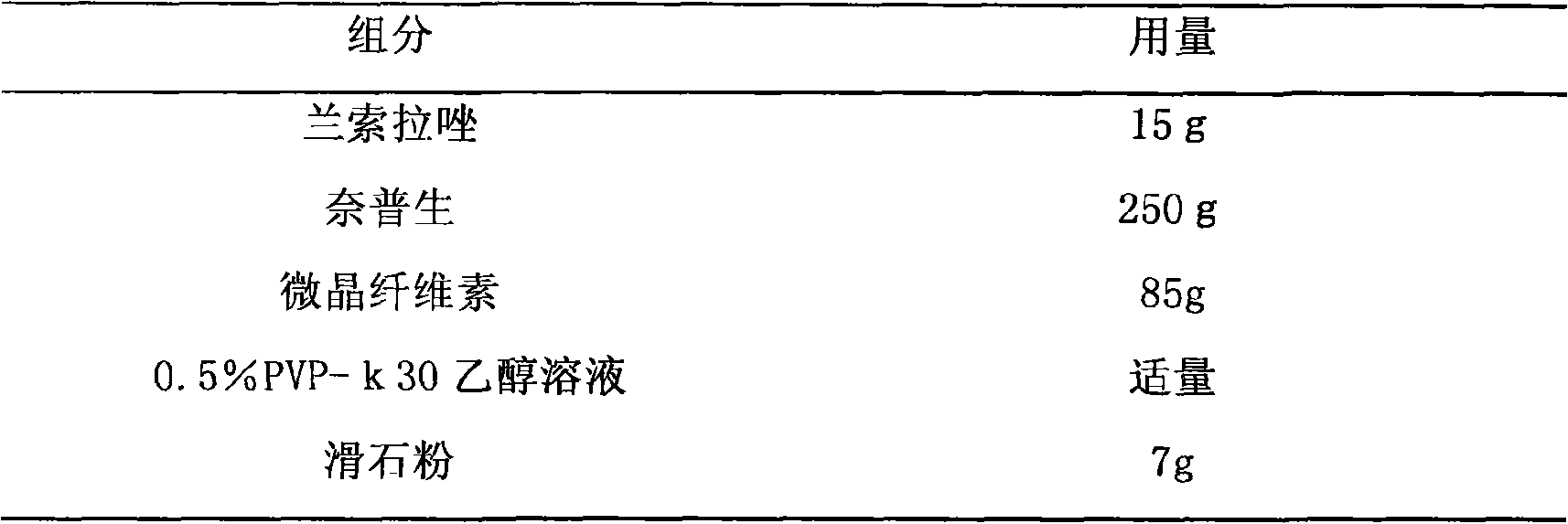
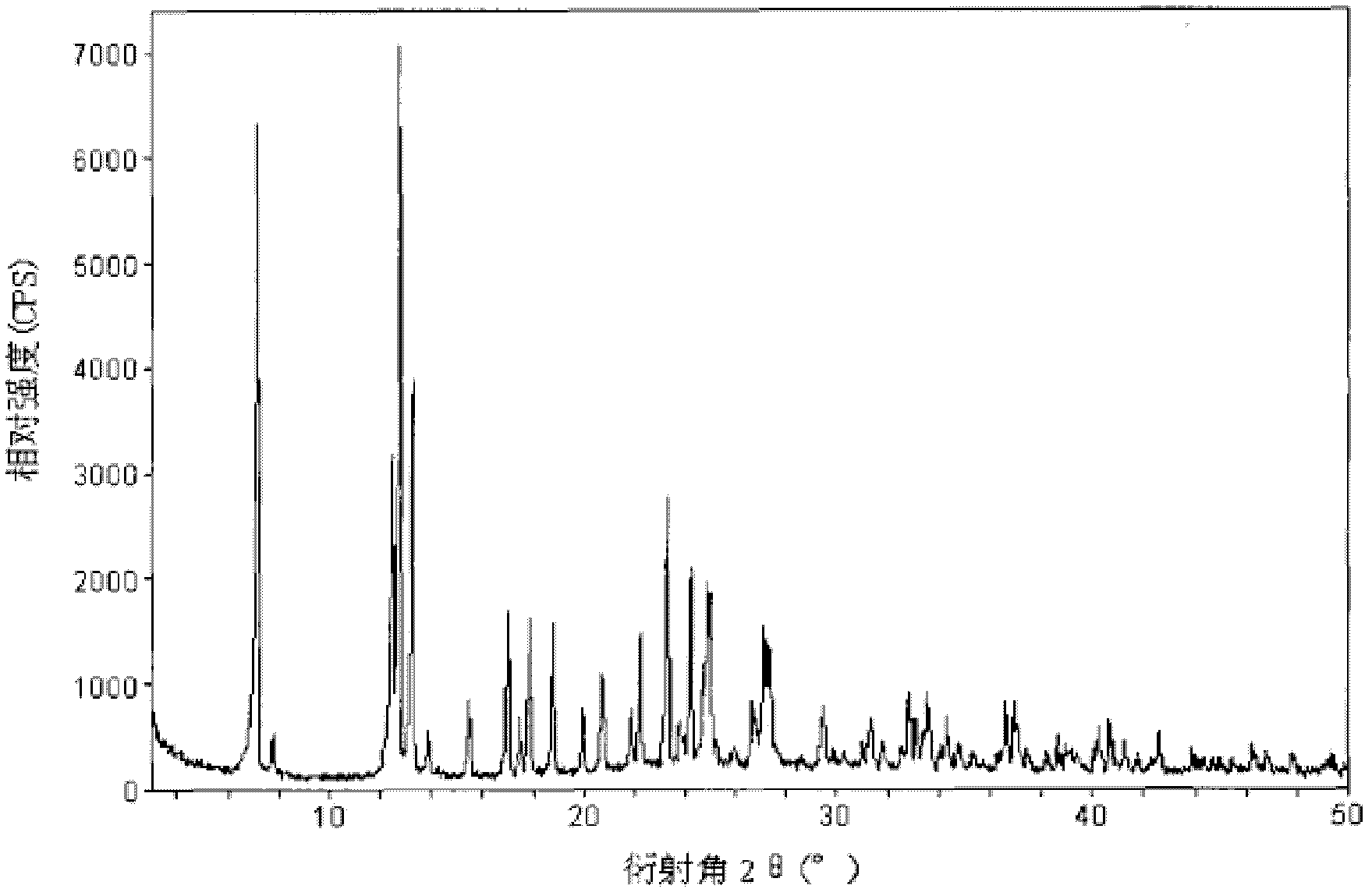
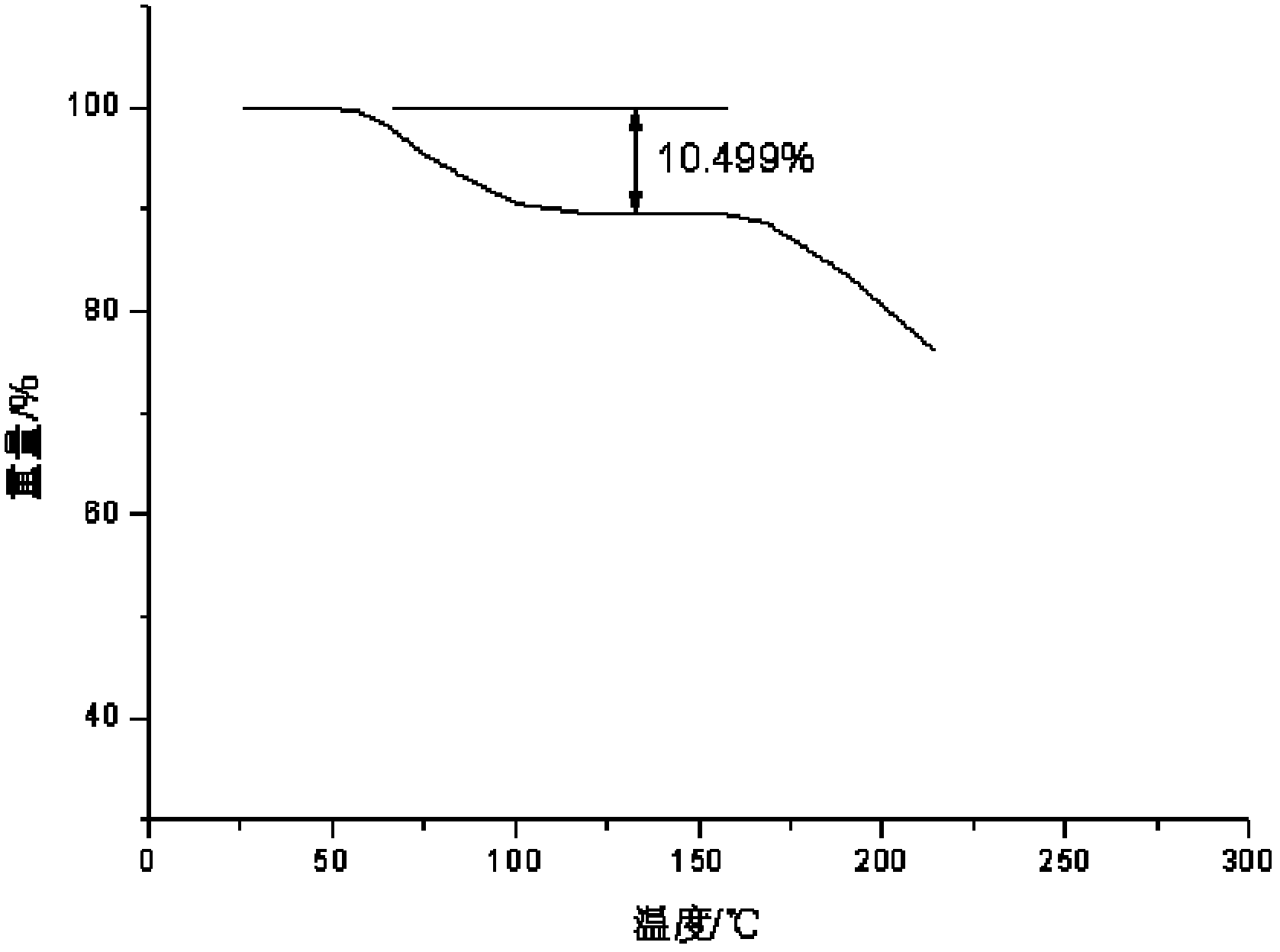
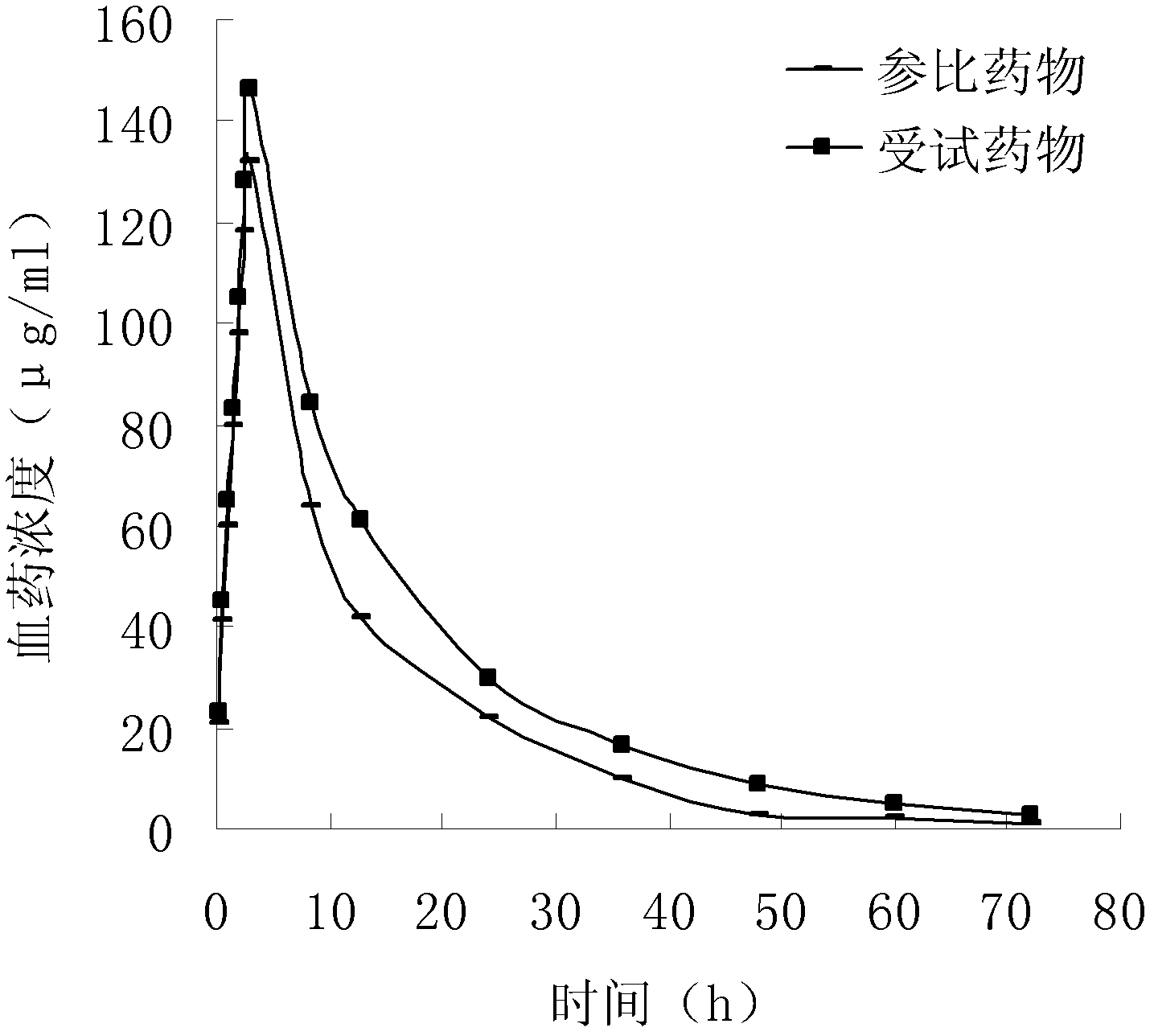
Drug composite containing lansoprazole and naproxen
The invention relates to a drug composite containing lansoprazole and naproxen, which takes lansoprazole and naproxen as active constituents and forms the drug composite by mixing acceptable accessories pharmaceutically; in the composite, the weight ratio of the lansoprazole and naproxen is 1;3-1:33, 1:17 is preference. The drug composite can be prepared into oral preparation and is used for treating rheumatoid arthritis, osteoarthritis and ankylosing spondylitis patients who use non-steroidal anti-inflammatory drugs to cause gastrohelcosis danger possibly.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
Method for recycling naproxen resolution solvent, resolving agent and by-product by overgravitational field
InactiveCN101514156AHigh recovery rateSimple processCarboxylic compound separation/purificationSocial benefitsResource saving
The invention discloses a method for recycling naproxen resolution solvent, resolving agent and by-product by the overgravitational field. A solution containing a resolution solvent, a resolving agent such as N-n-octylglucamine, and by-product such as L-naproxen and L-naproxen N-n-octylglucamine salt after the separation of D- naproxen N-n-octylglucamine salt is firstly added in a overgravitational revolving bed for recycling resolution solvent, then the residual solution is diluted by water, the pH value is adjusted to 7 to 12, the resolving agent is recycled, the mother liquor is concentrated, the alkali C is added, the D,L- naproxen salt is obtained by the racemization reaction. The method adopts the overgravitational revolving bed to recycle the resolution solvent, the process is simple, the whole process is safe, steady and reliable, the operation and the maintenance are simple, the runing cost is greatly reduced, at the same time the production efficiency is improved; the obtained methanol is uniform, the recovery rate of the resolving agent and the D,L- naproxen salt is high, the obtained resolution solvent, resolving agent and D,L- naproxen salt can be directly reused, thus being resource-saving and having obvious environment benefit and social benefit.
Owner:ZHEJIANG CHARIOTEER PHARMA
Naproxen hydrate crystal, preparation method thereof and pharmaceutical composition containing the crystal and sumatriptan
InactiveCN102276447AImprove solubilitySimple processOrganic active ingredientsNervous disorderCross-linkSodium bicarbonate
Owner:HAINAN JINRUI PHARMA
Method for biological catalytic preparing naprosyn
InactiveCN1584037AEasy to separateSolve the problem of slow responseFermentationMedicinal chemistryIonic liquid
A method for preparing optical pure naproxen by lipase chiral resoluted naproxen methoxycarbonyl in water-ionic liquid two-phase system is disclosed. It is carried out by taking lipase cylindrical candidiasis L-1754 as catalyst and taking racemic naproxen methoxycarbonyl as reactive substratum. It achieves three-dimensional selectivity and activity.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Novel formulation of naproxen
The present invention relates to methods for producing particles of naproxen using dry milling processes as well as compositions comprising naproxen, medicaments produced using naproxen in particulate form and / or compositions, and to methods of treatment of an animal, including man, using a therapeutically effective amount of naproxen administered by way of said medicaments.
Owner:ICEUTICA PTY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com