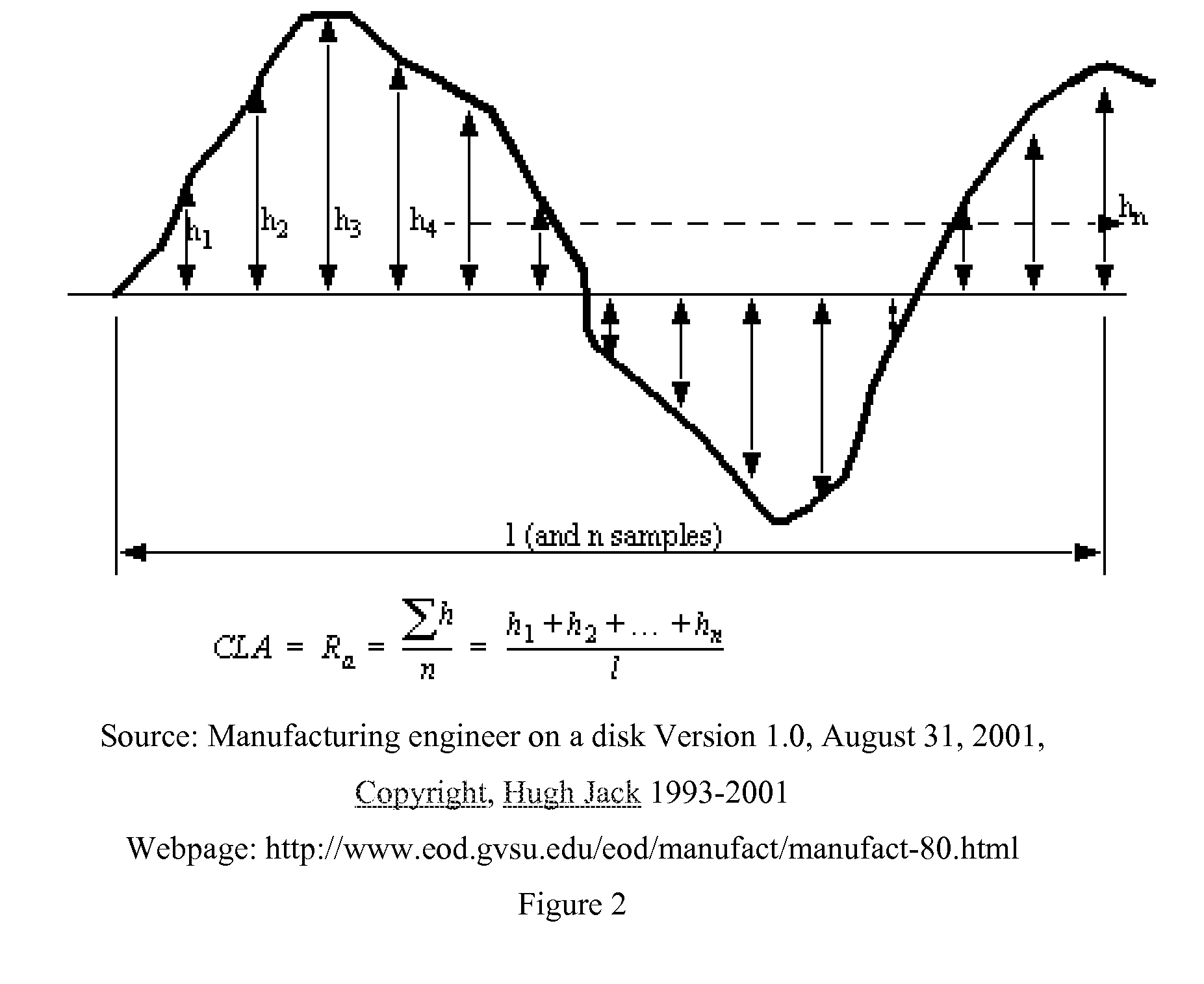
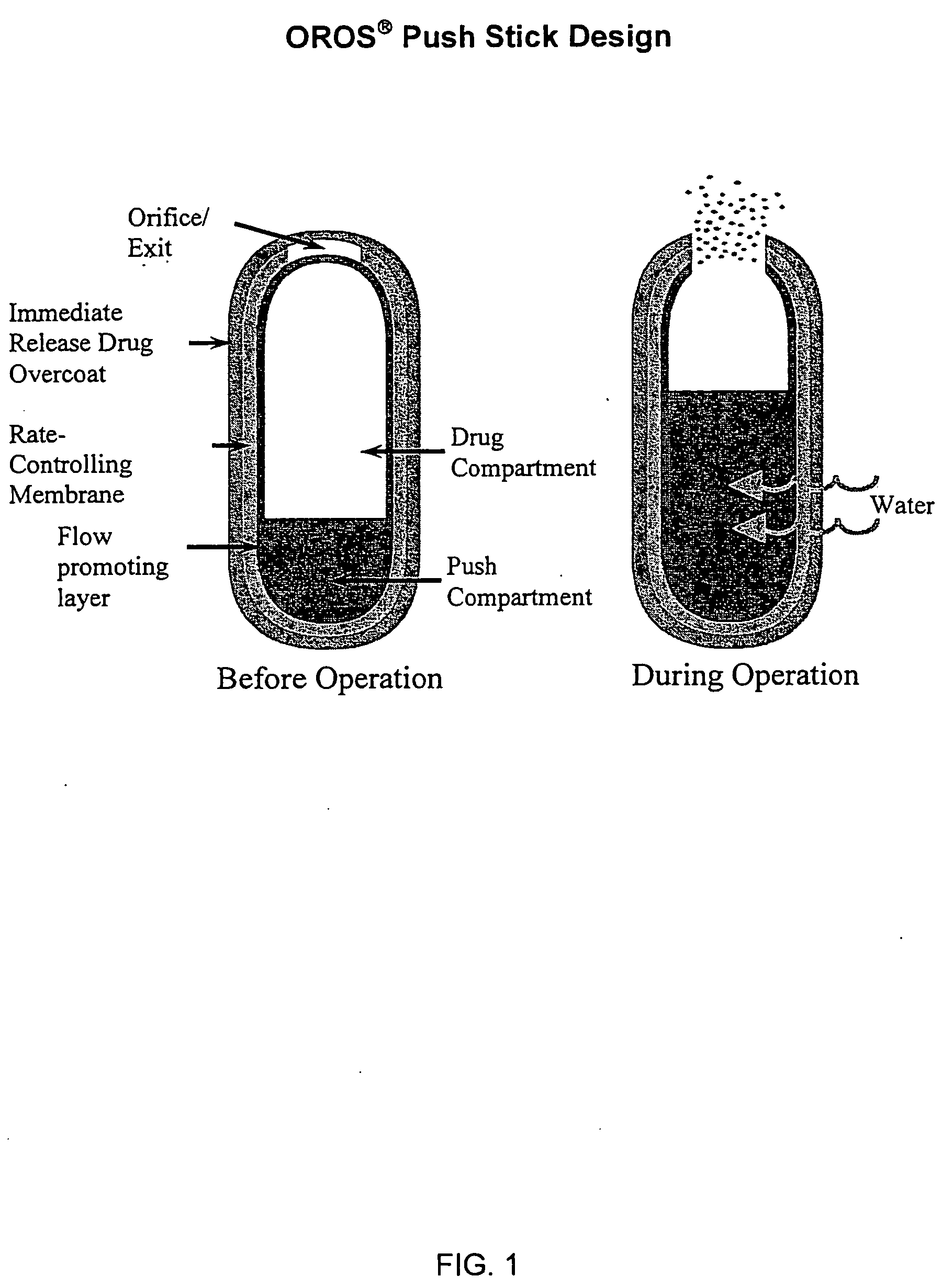
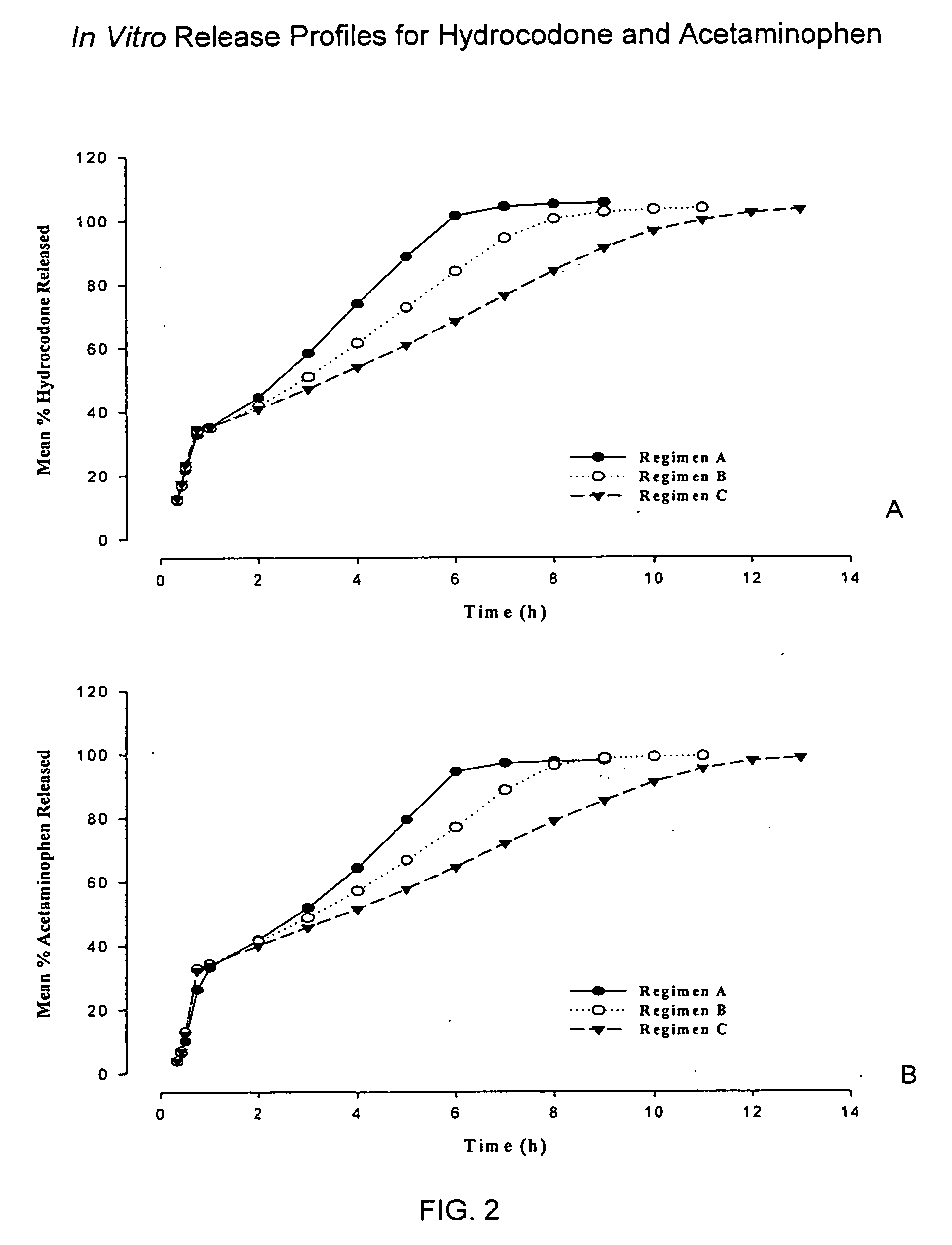
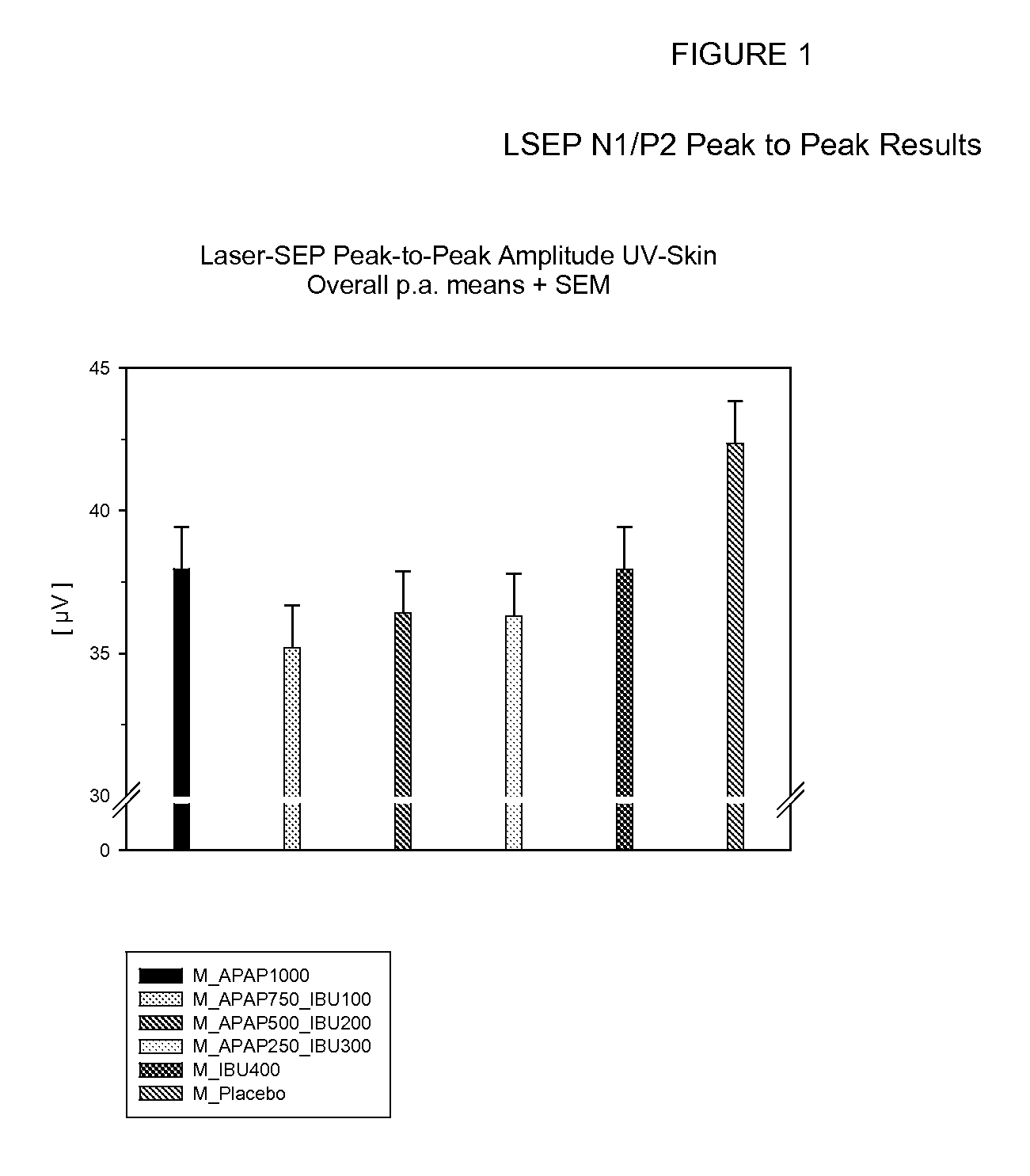
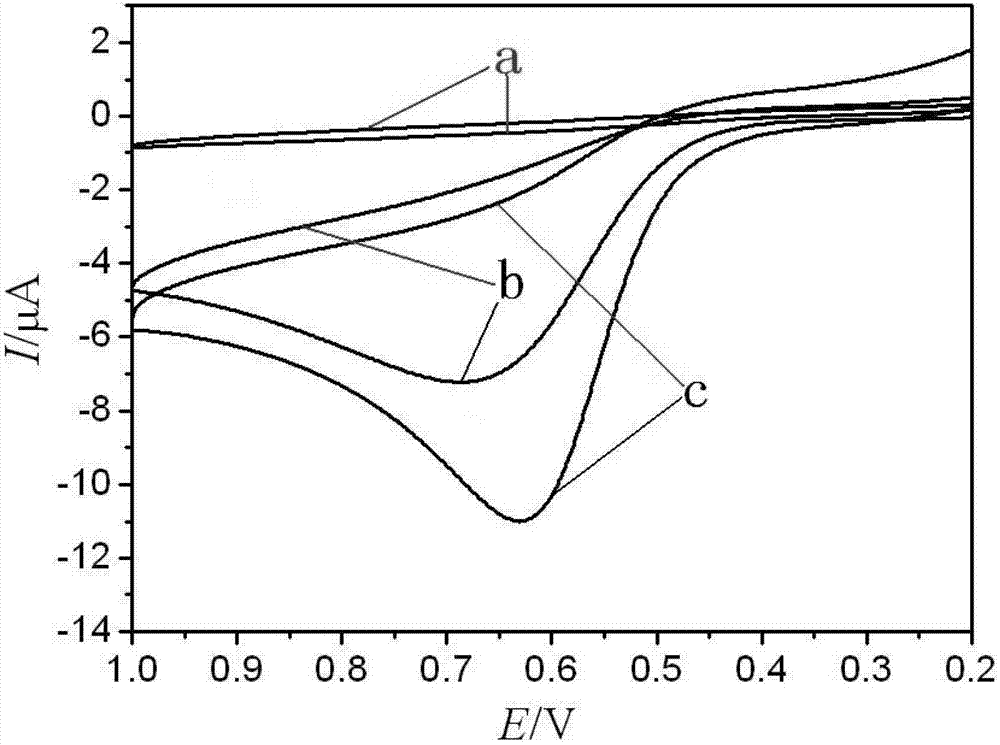
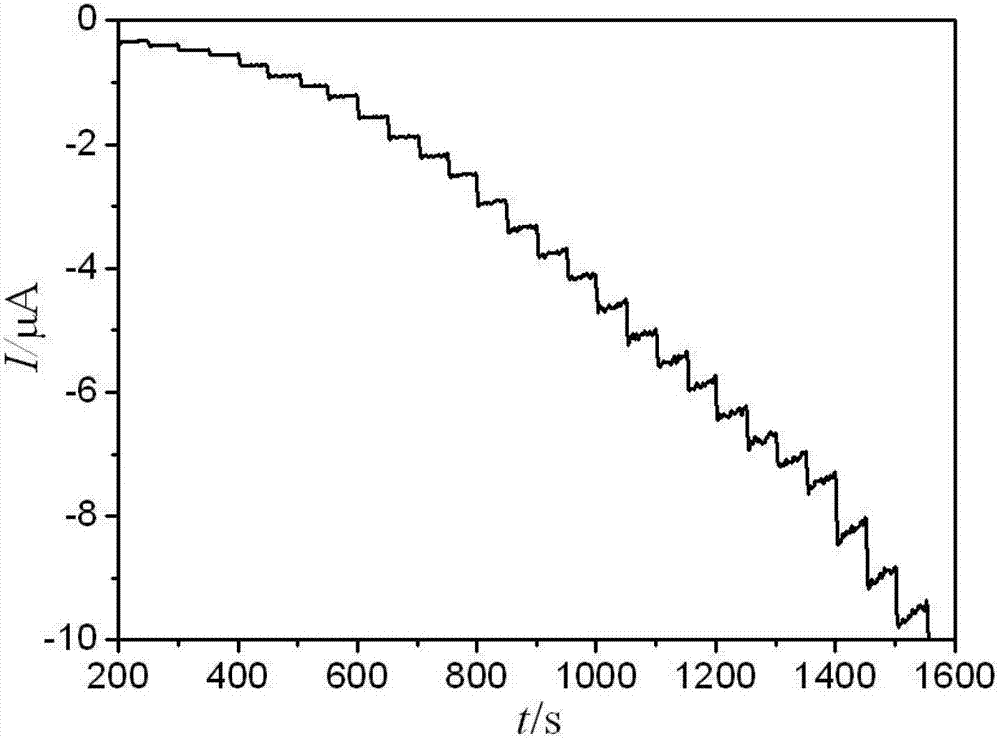
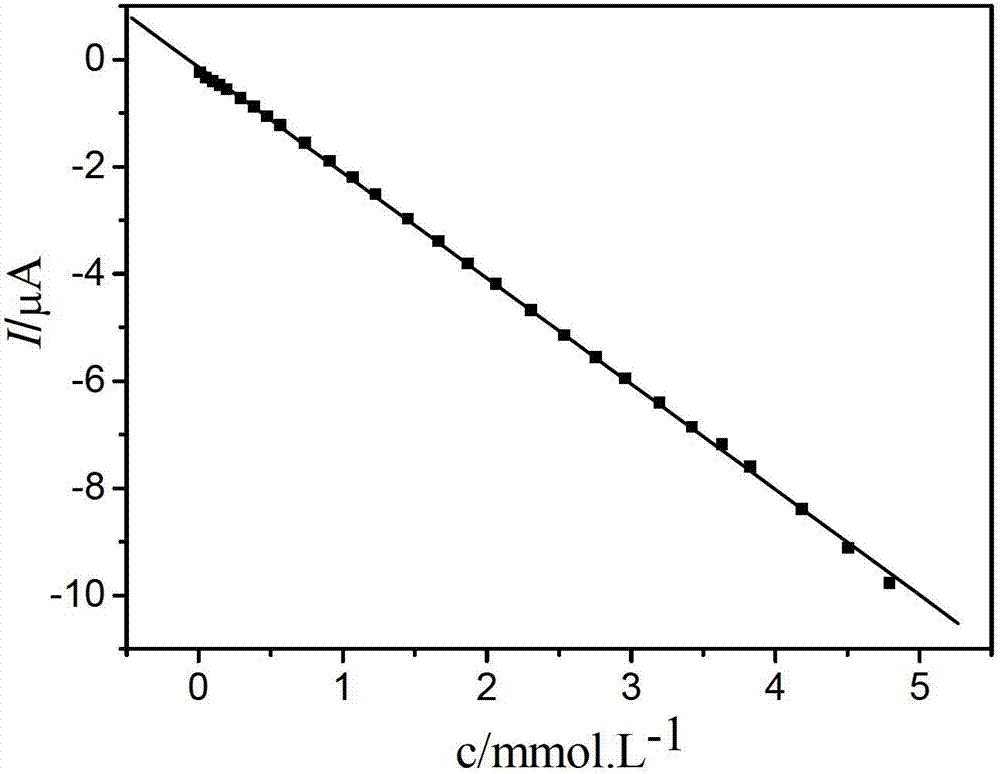
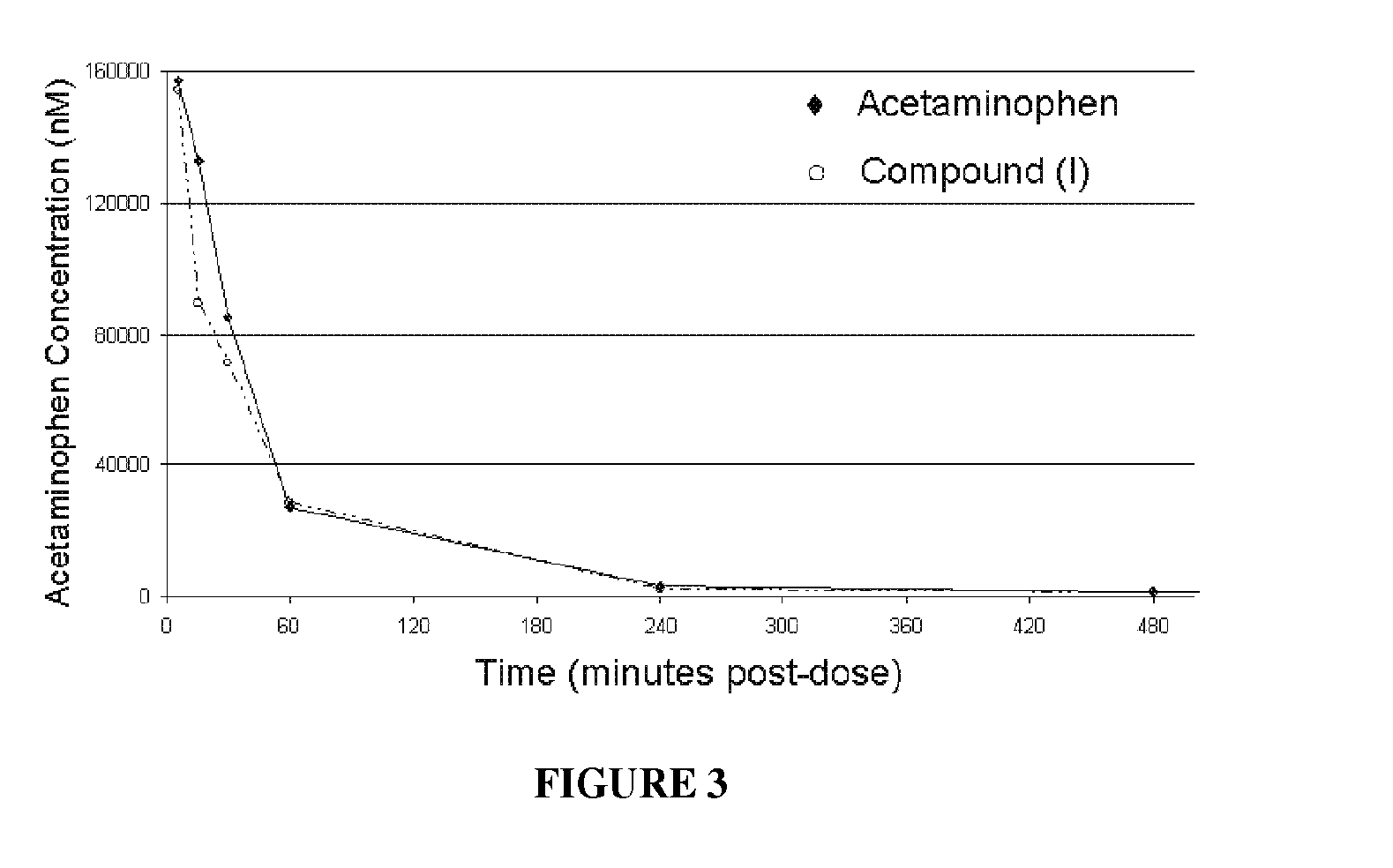
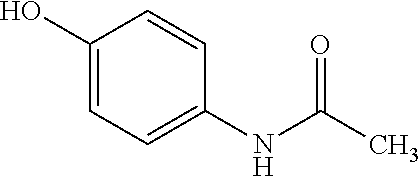
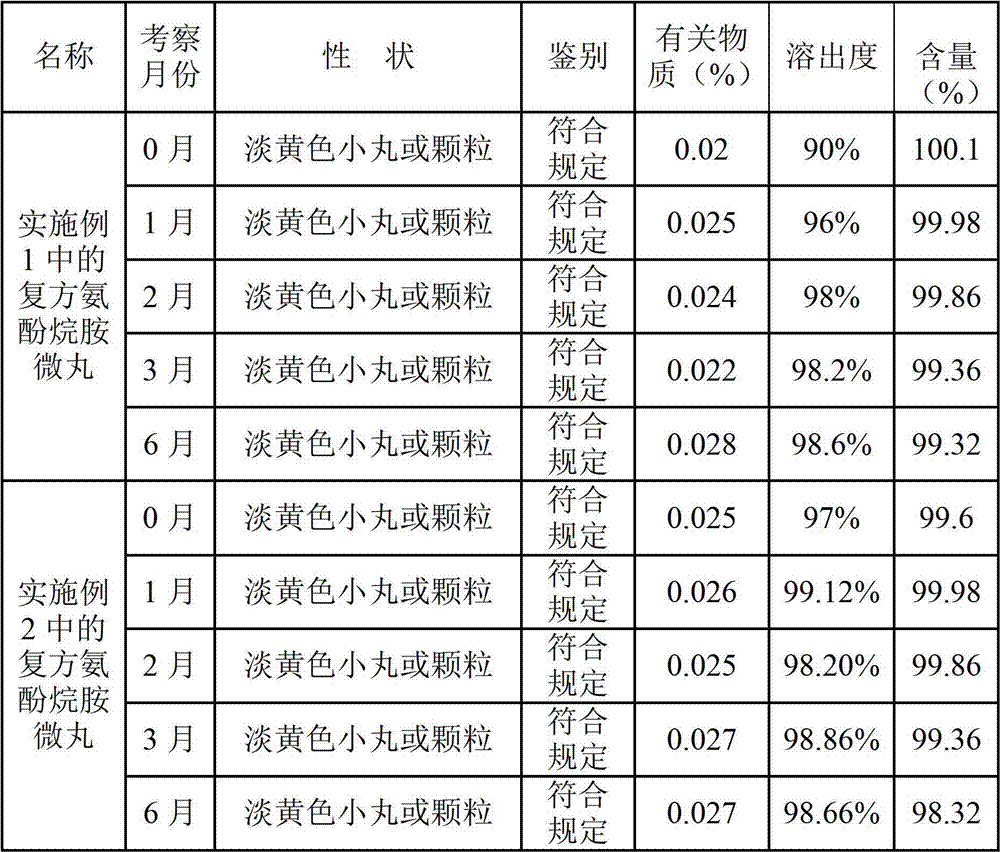
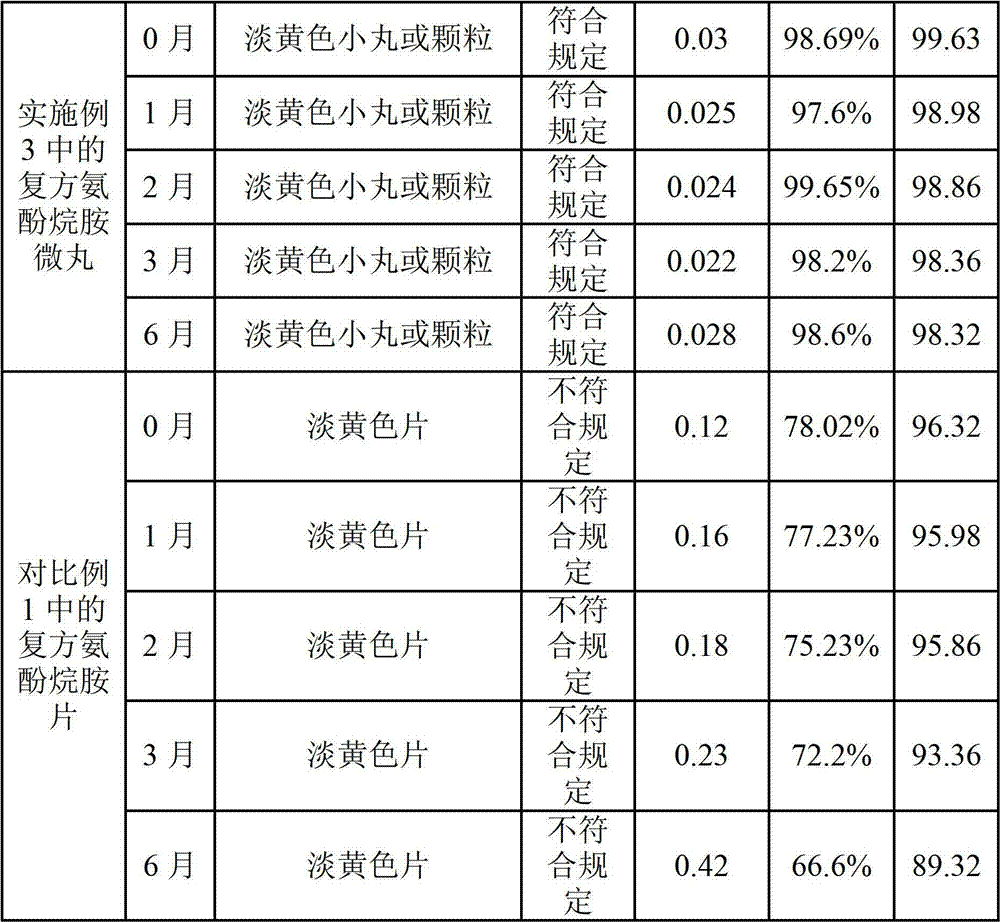
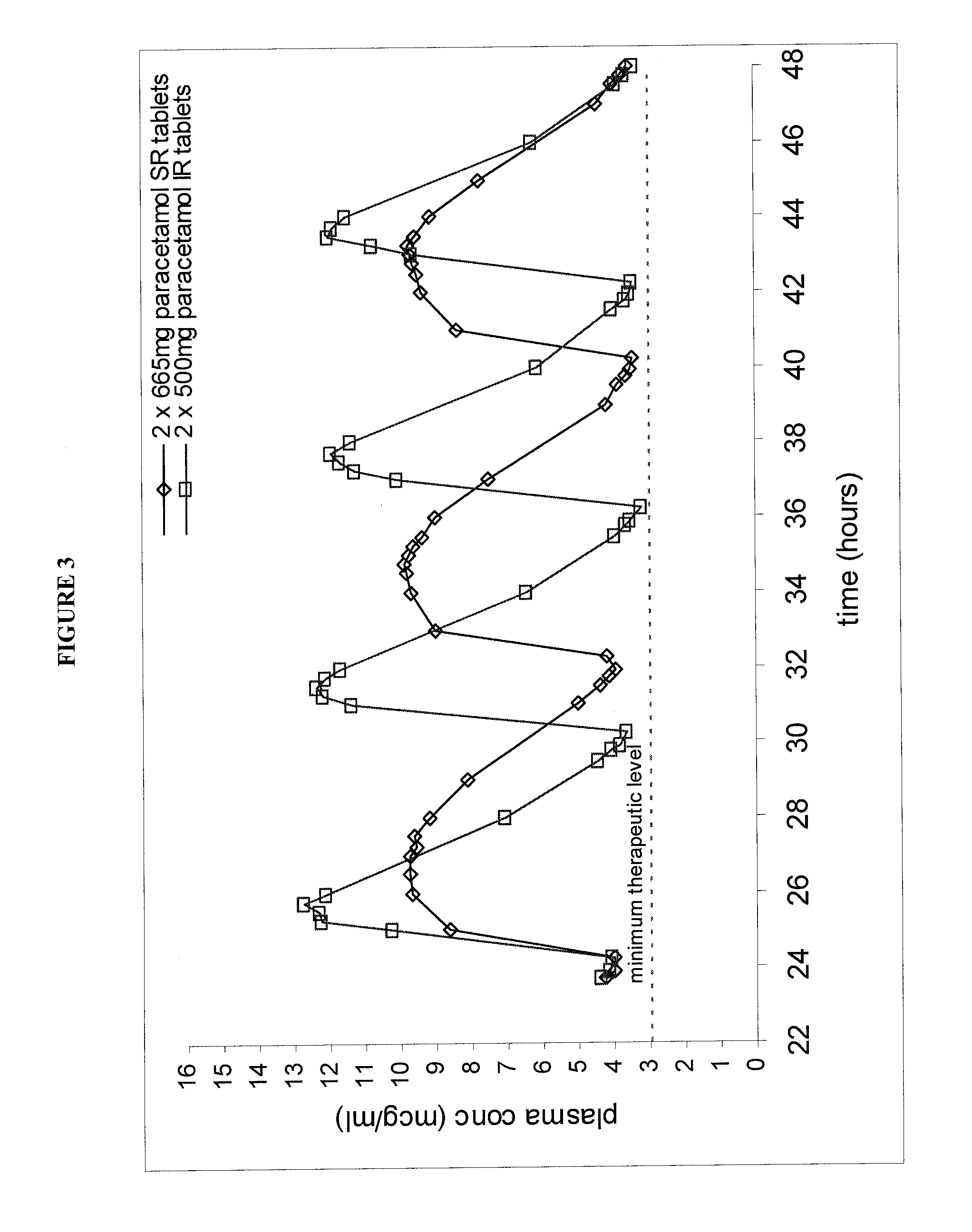
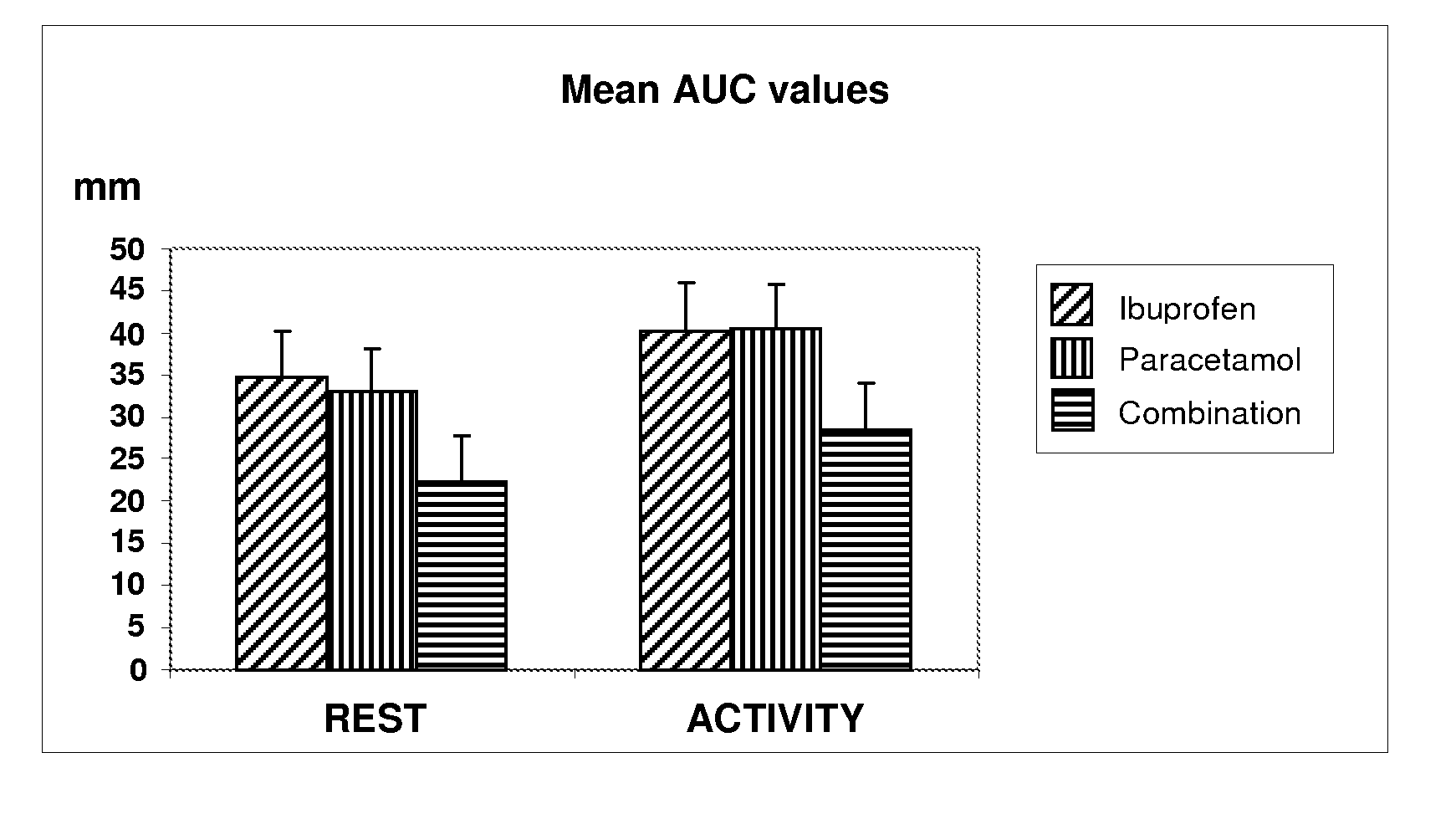
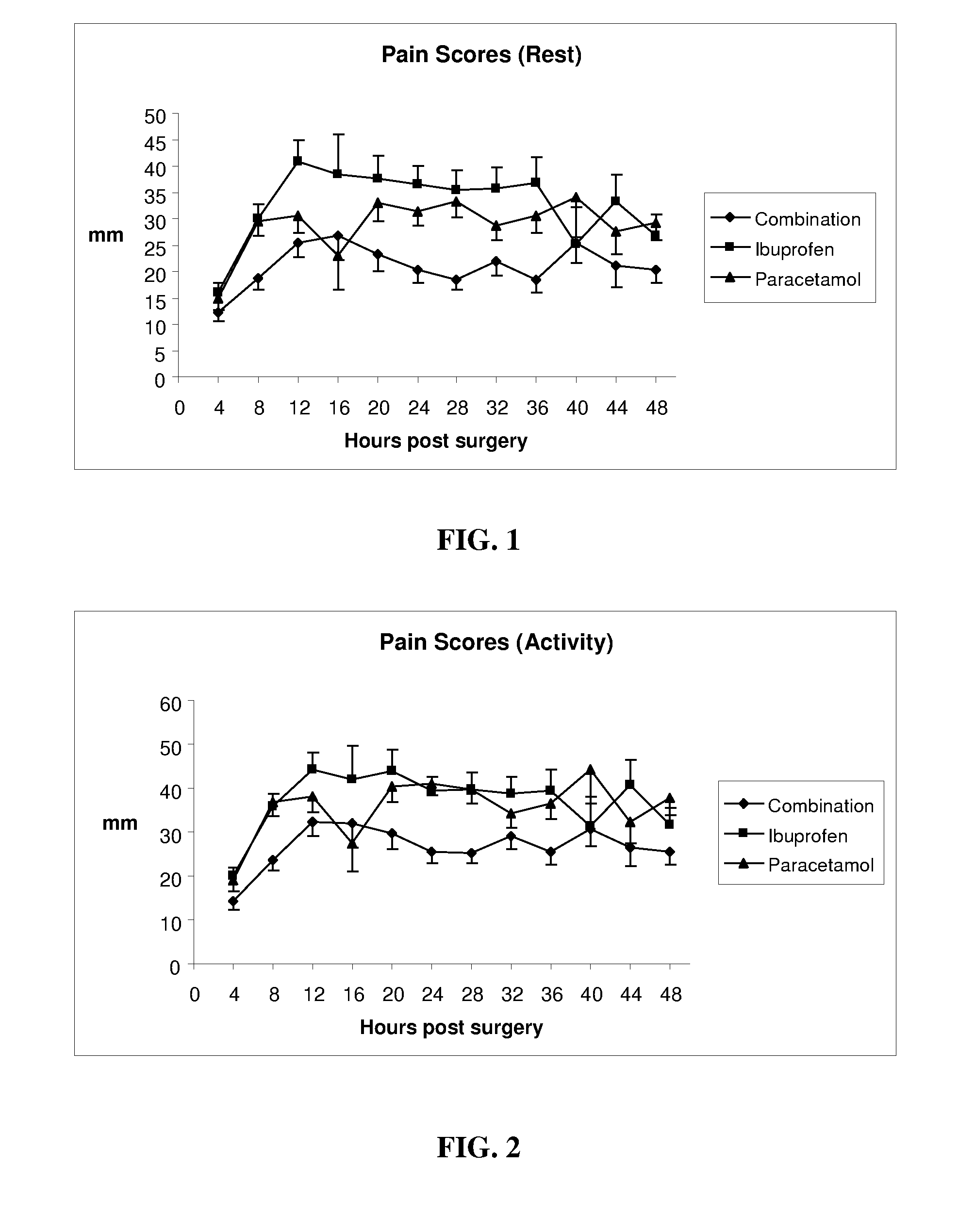
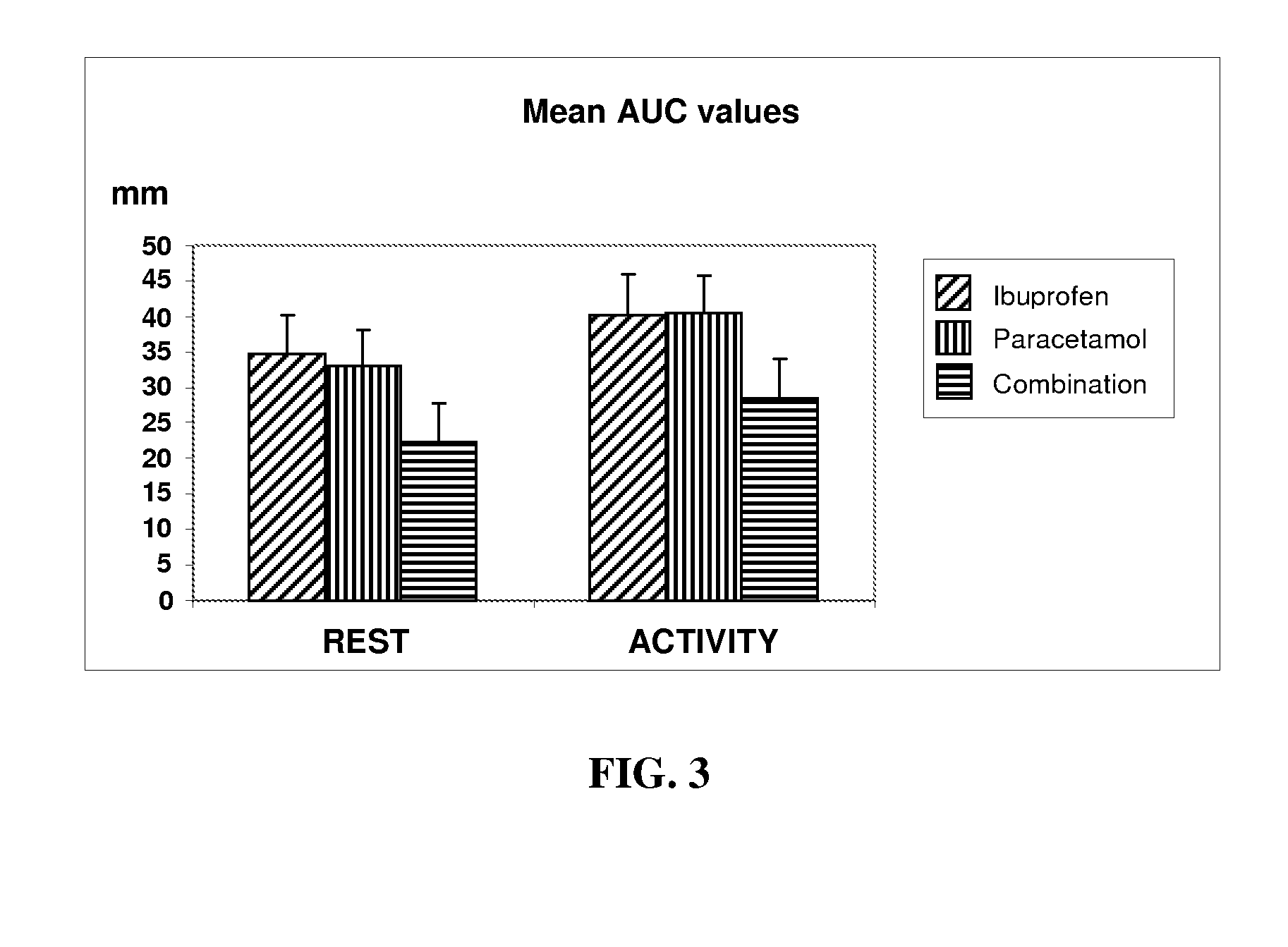
Patents
Literature
608 results about "Acetaminophen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This drug is used to treat mild to moderate pain (from headaches, menstrual periods, toothaches, backaches, osteoarthritis, or cold/flu aches and pains) and to reduce fever..
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
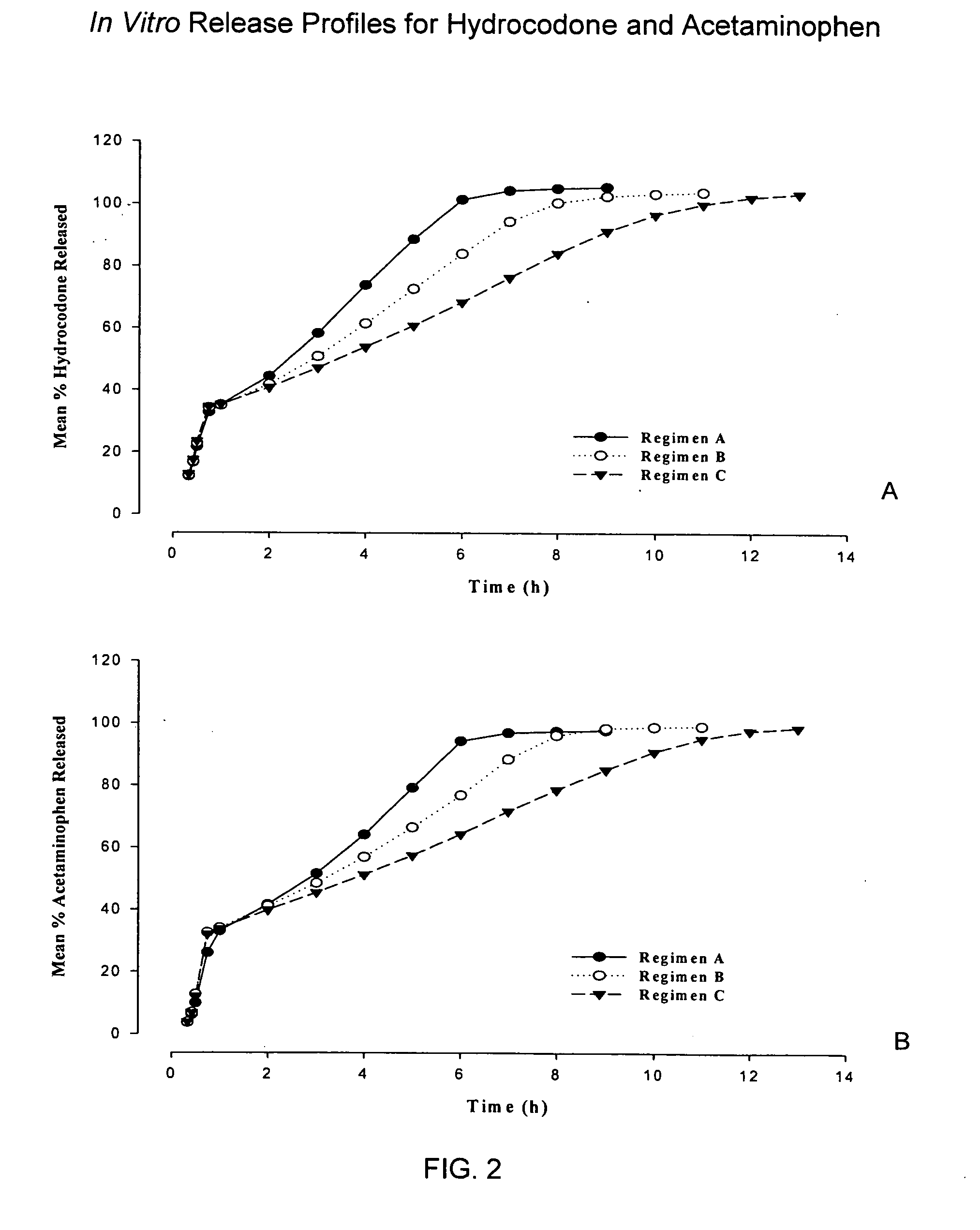
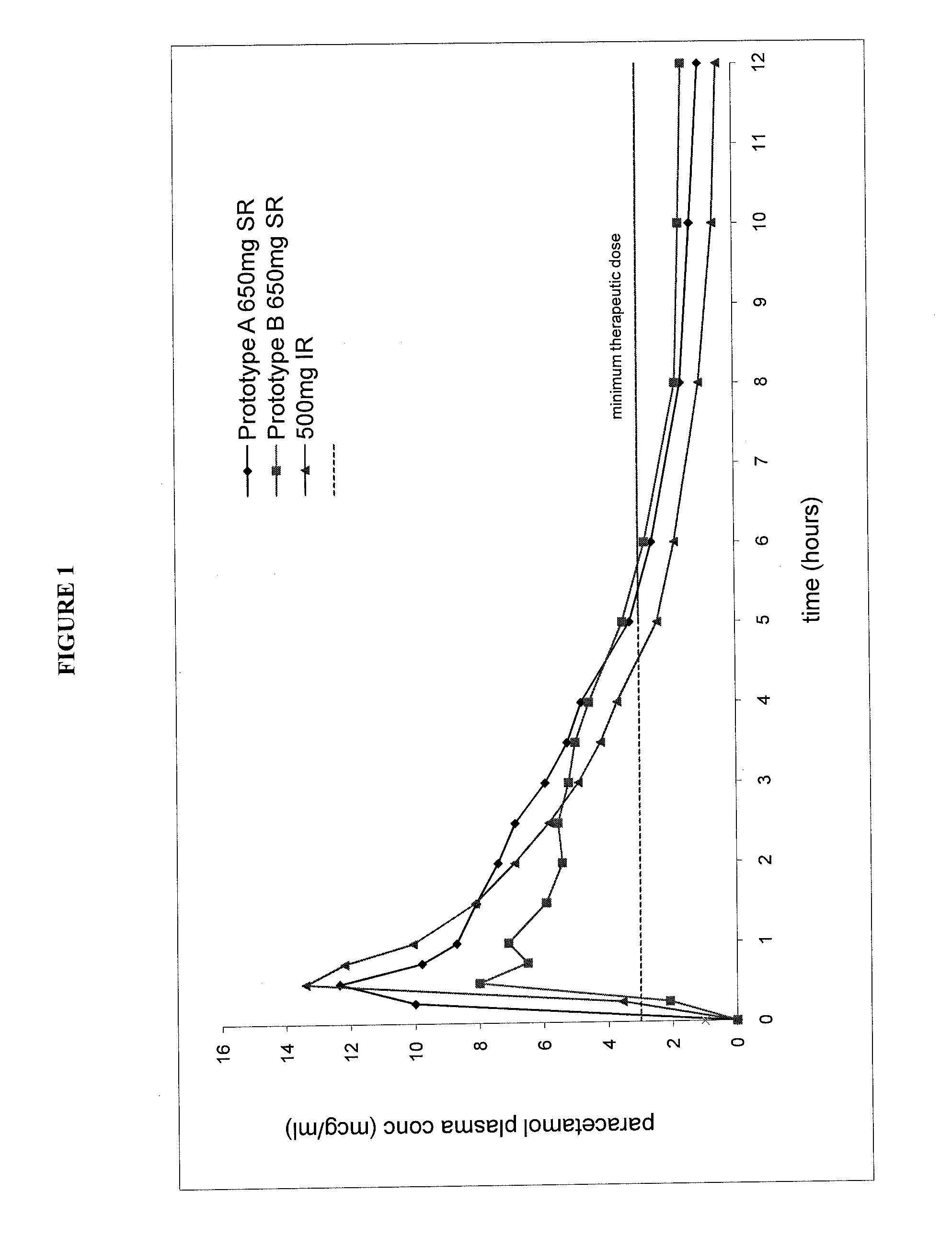
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
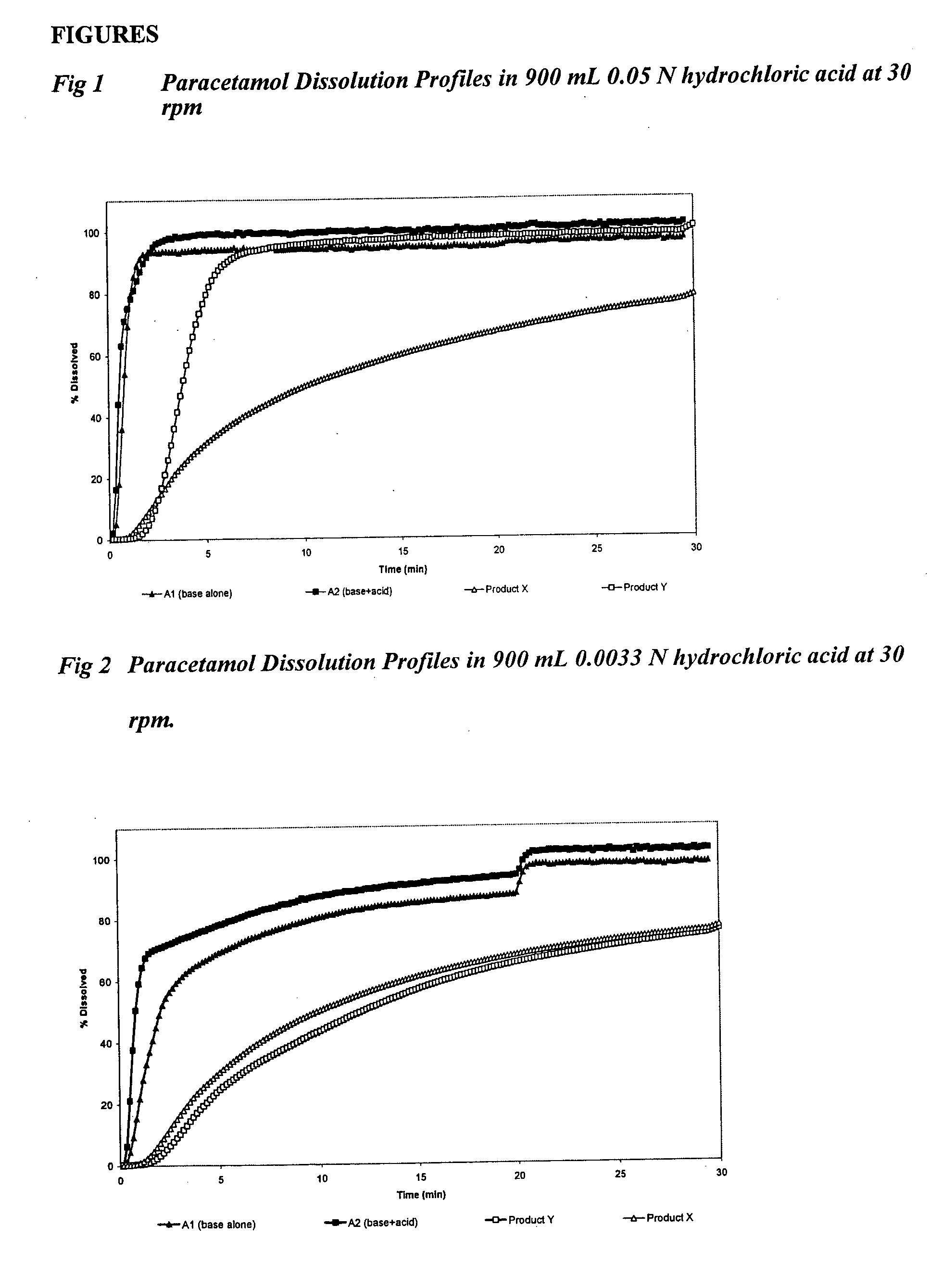
Stable liquid paracetamol compositions, and method for preparing same
PCT No. PCT / FR97 / 01452 Sec. 371 Date Jun. 5, 1998 Sec. 102(e) Date Jun. 5, 1998 PCT Filed Aug. 5, 1997 PCT Pub. No. WO98 / 05314 PCT Pub. Date Feb. 12, 1998Novel stable paracetamol compositions for use in therapeutic chemistry and specifically galenic pharmacy are disclosed. The compositions contain a solution of paracetamol in an aqueous solvent combined with a buffer having a pH of 4 to 8, and a free radical capturing agent. A water-insoluble inert gas is carefully bubbled through the aqueous solvent to remove oxygen from the medium. Said compositions may also be combined with a centrally or peripherally acting analgesic agent, and are provided as injectable compositions for relieving pain.
Owner:SCR PHARMATOP
Opioid agonist/opioid antagonist/acetaminophen combinations
InactiveUS20020058673A1Reducing oral abuse potential of dosage formImprove subjective experienceBiocideDrug compositionsOpioid antagonistOpioid Agonist
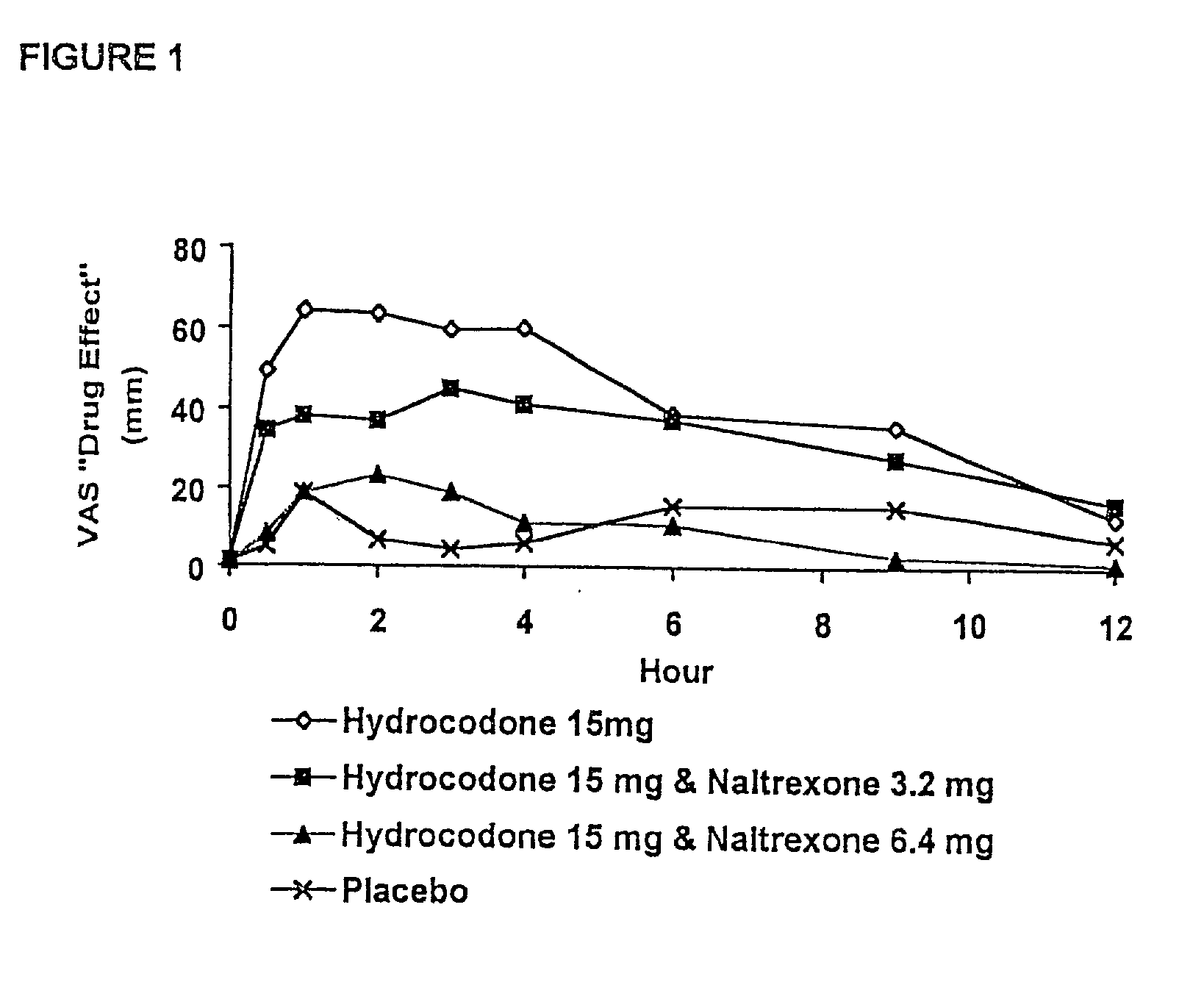
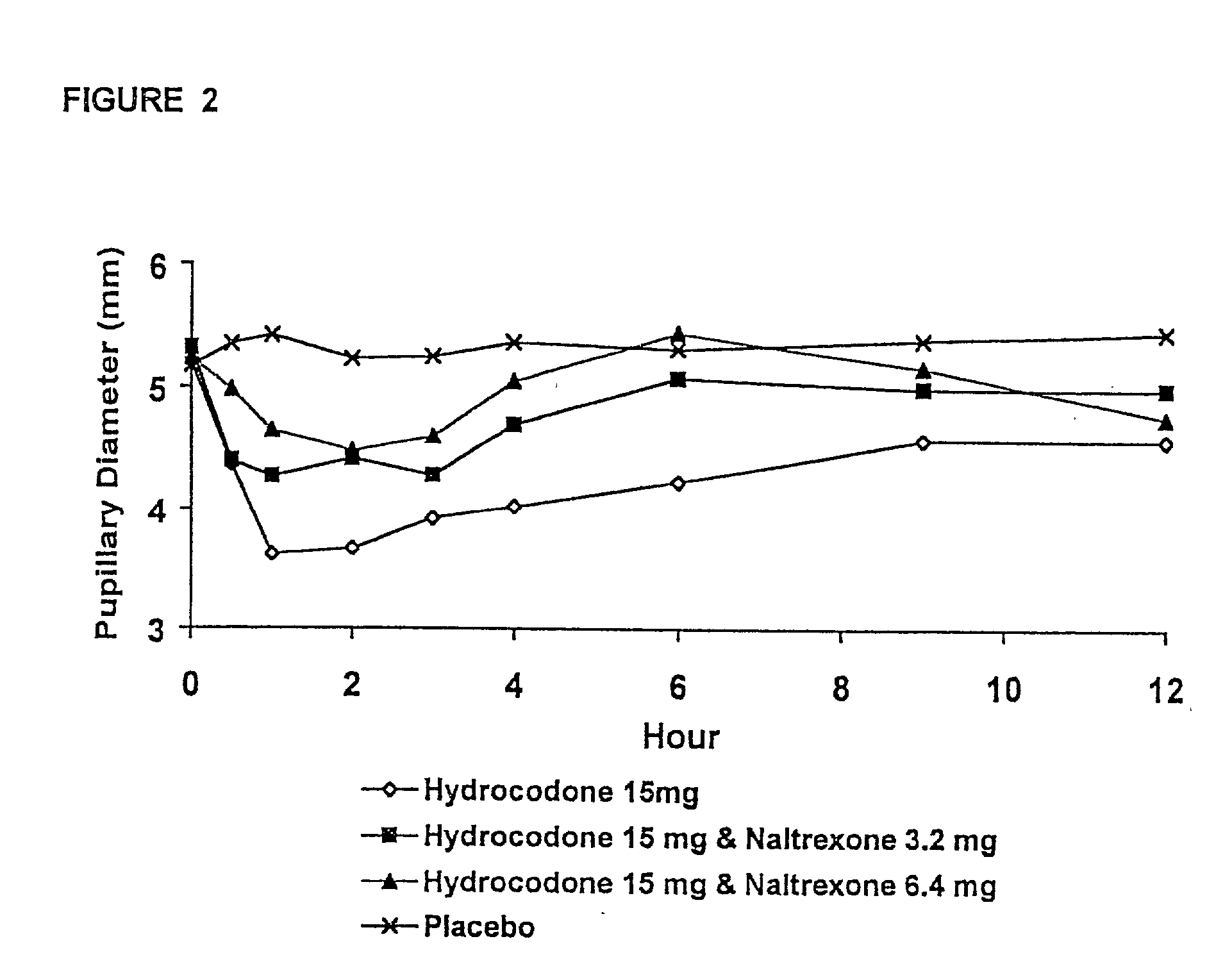
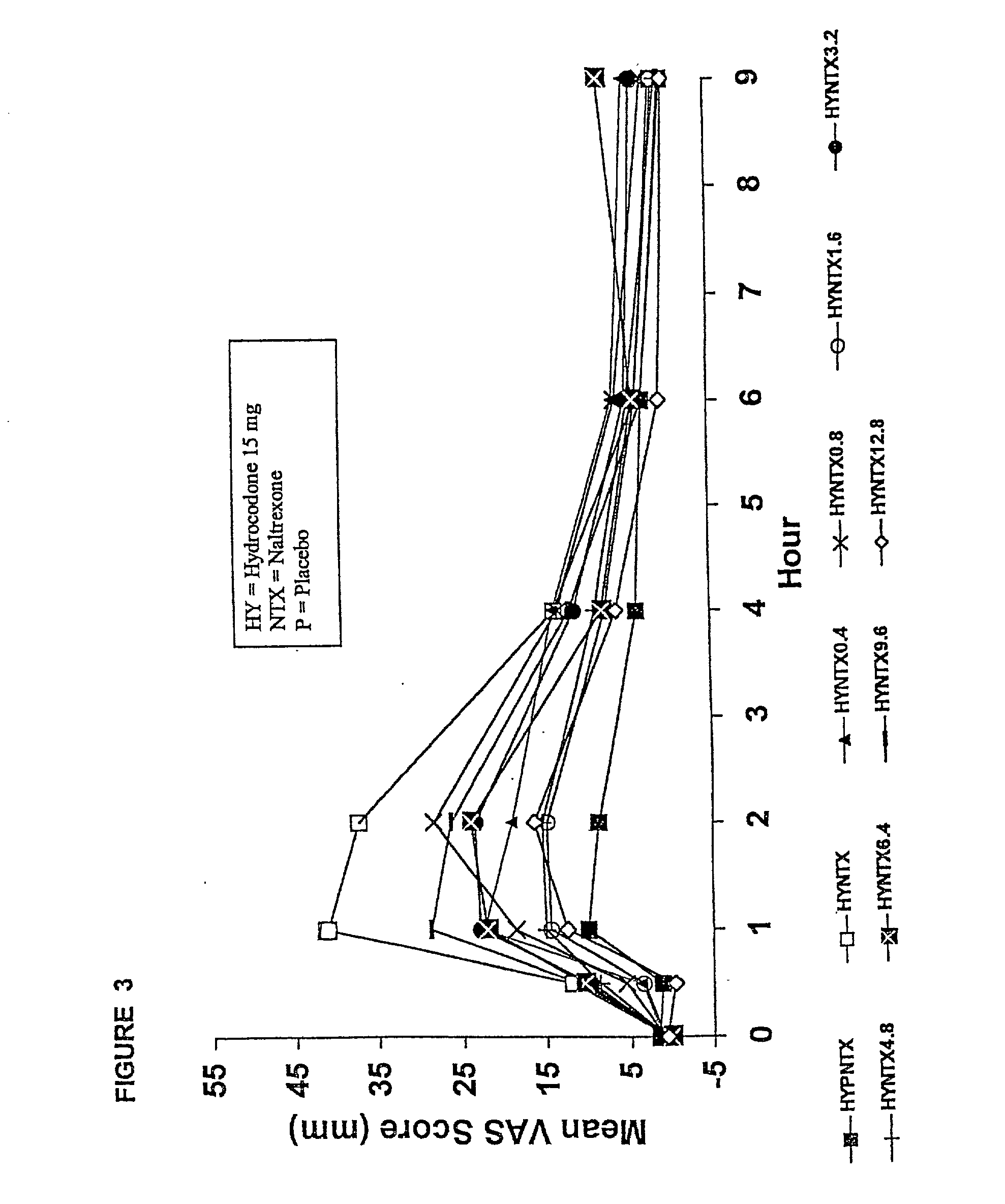
The invention is directed in part to oral dosage forms comprising a combination of an opioid agonist, acetaminophen and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Nanoparticulate acetaminophen formulations
InactiveUS20060292214A1Reduce feverRemissionOrganic active ingredientsPowder deliveryNanoparticleBioavailability
The invention is directed to compositions comprising a nanoparticulate acetaminophen composition, or a salt or derivative thereof, having improved bioavailability. The nanoparticulate acetaminophen particles of the composition have an effective average particle size of less than about 2000 nm and are useful in the treatment of aches and pain, and in the reduction of fever and related conditions.
Owner:ELAN PHRMA INT LTD
Sustained release monoeximic formulations of opioid and nonopioid analgesics
InactiveUS20080031901A1Improve abilitiesSimple compositionBiocideAntipyreticHydrocodoneAnalgesic agents
Owner:ABBOTT LAB INC
Composition and method for treating the effects of diseases and maladies
InactiveUS6841544B2Prevent relapseGood effectBiocideInorganic active ingredientsDiseaseNon steroidal anti inflammatory
A medicinal composition for treating pain resulting from an inflammatory response comprises at least one pain relieving and anti-inflammatory pharmaceutical and at least one nutraceutical in a pharmaceutically acceptable base. The pharmaceutical is preferably acetaminophen or a non-steroidal anti-inflammatory drug (NSAID). The nutraceutical is preferably an immune booster, an anti-oxidant, a liver protectant, or a joint relief agent. Methods of using these compositions to treat pain caused by inflammation are also disclosed.
Owner:BIOSELECT INNOVATIONS
Acetaminophen formulation for joint pain relief
The invention relates generally to a formulation which may comprise additional vitamins, minerals, herbs and supplements and methods for using the same for joint pain relief. The formulation may comprise supplements such as glucosamine, hyaluronic acid and methylsulfonylmethane (MSM) and acetaminophen for acute joint pain. The invention also encompasses methods for joint pain relief with the formulation described herein.
Owner:AJG BRANDS
Oral delivery system
ActiveUS20070141144A1Promote absorptionGood moisture absorptionBiocidePowder deliveryOral medicationAnalgesics effects
The present invention relates generally to formulations comprising paracetamol. More particularly, the present invention provides a swallow formulation comprising paracetamol which facilitates the rapid delivery of paracetamol into the circulatory system following oral administration. The present invention further relates to methods for inducing efficient pain relief including an analgesic effect by the administration of the paracetamol formulation.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Sustained release formulations of opioid and nonopioid analgesics
ActiveUS20070281018A1Improve abilitiesSimple compositionBiocideAnimal repellantsHydrocodoneSustained Release Formulations
The present invention relates to SRSR solid dosage forms for administering pharmaceutical agents, particularly Hydrocodone and acetaminophen, methods for preparing said dosage forms, and methods for providing therapeutic agents to patients in need of treatment.
Owner:ABBVIE INC
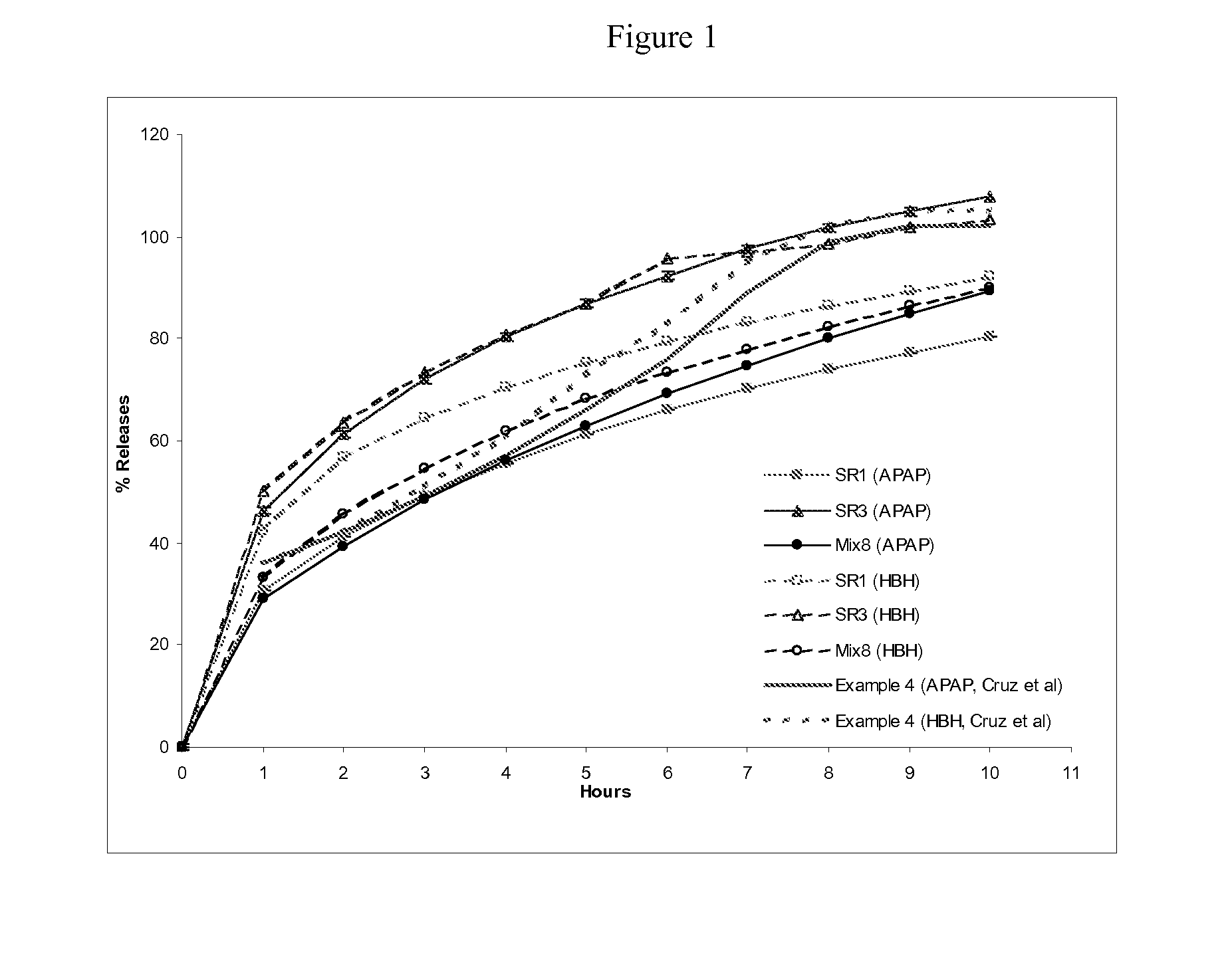
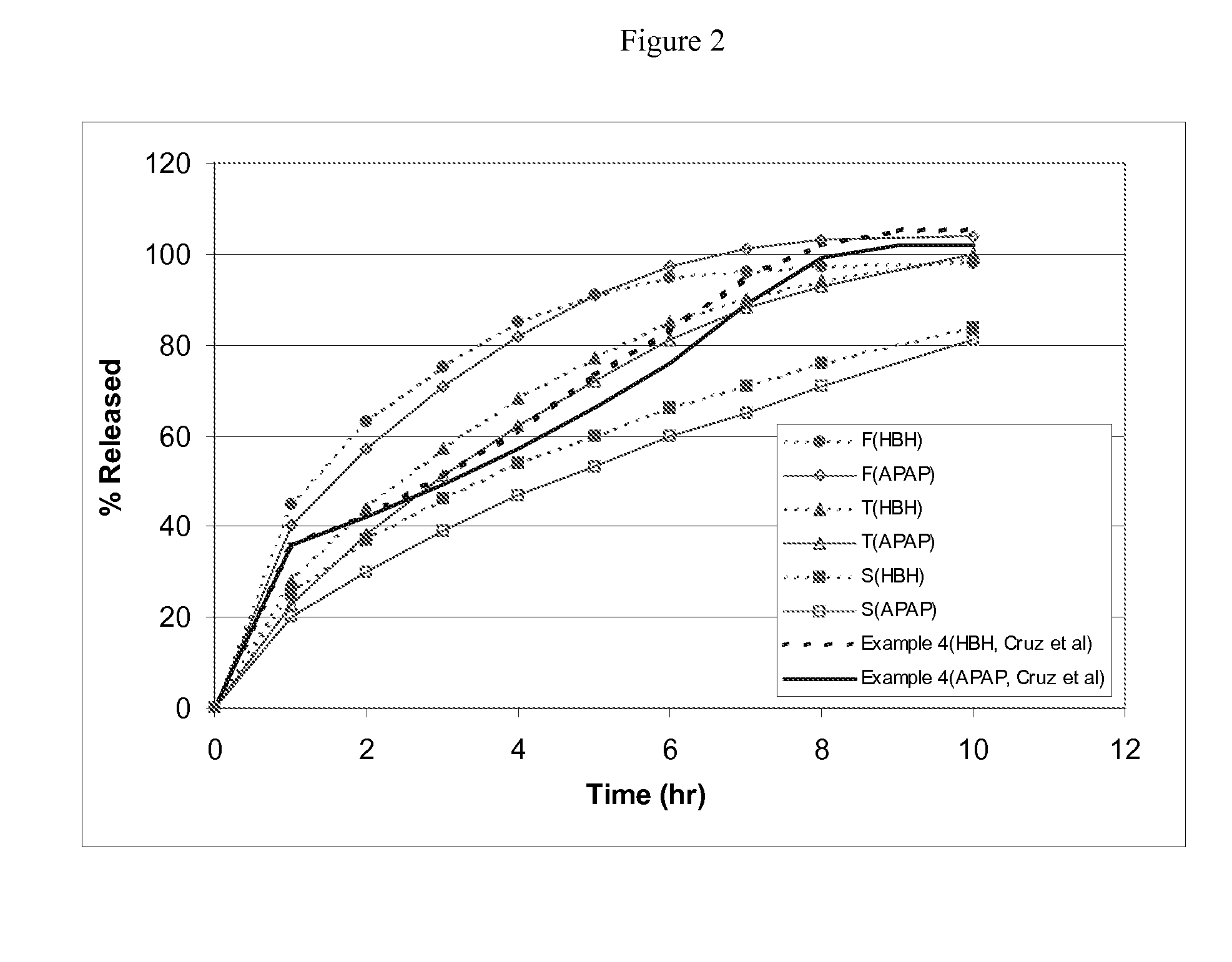
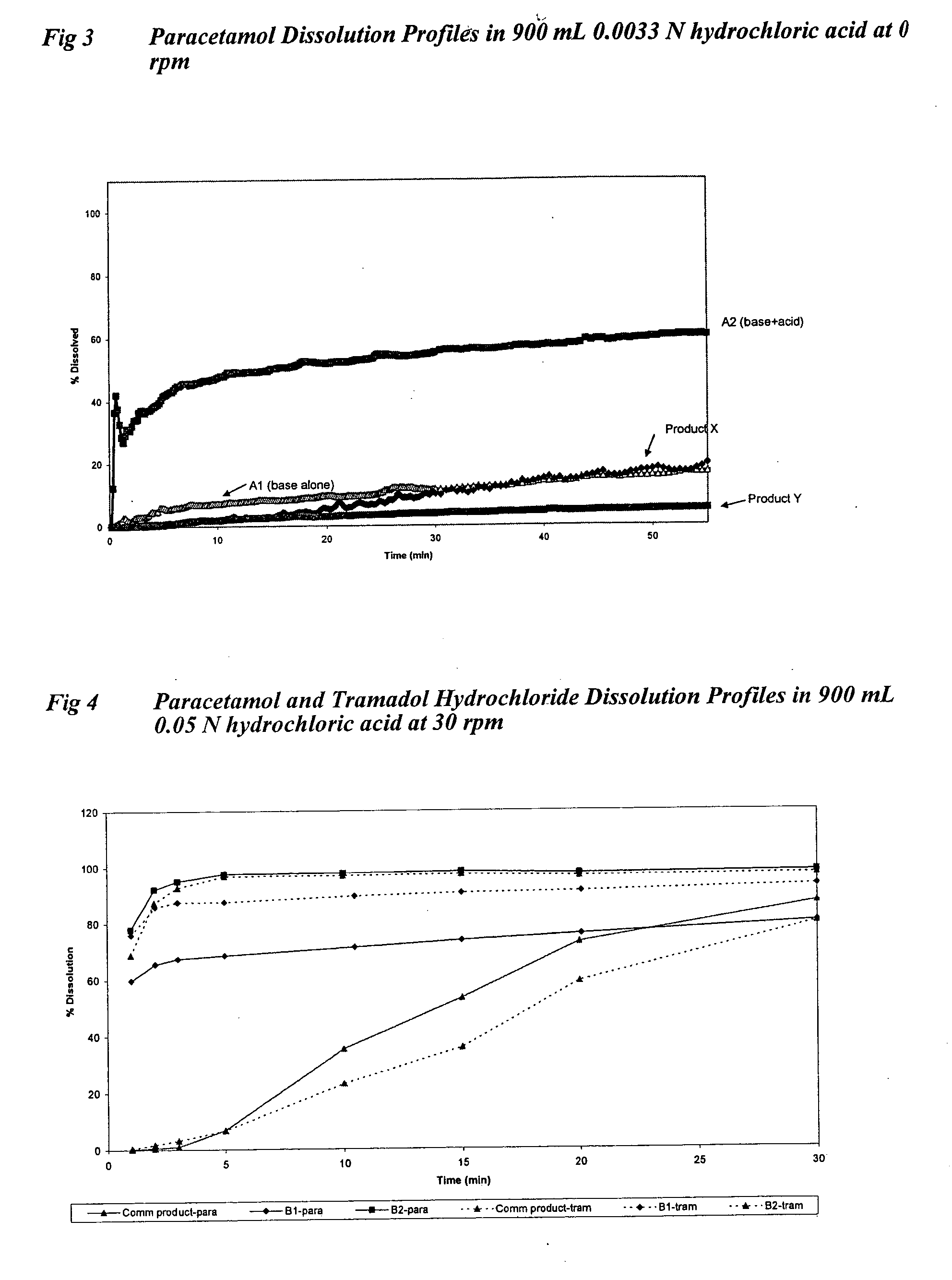
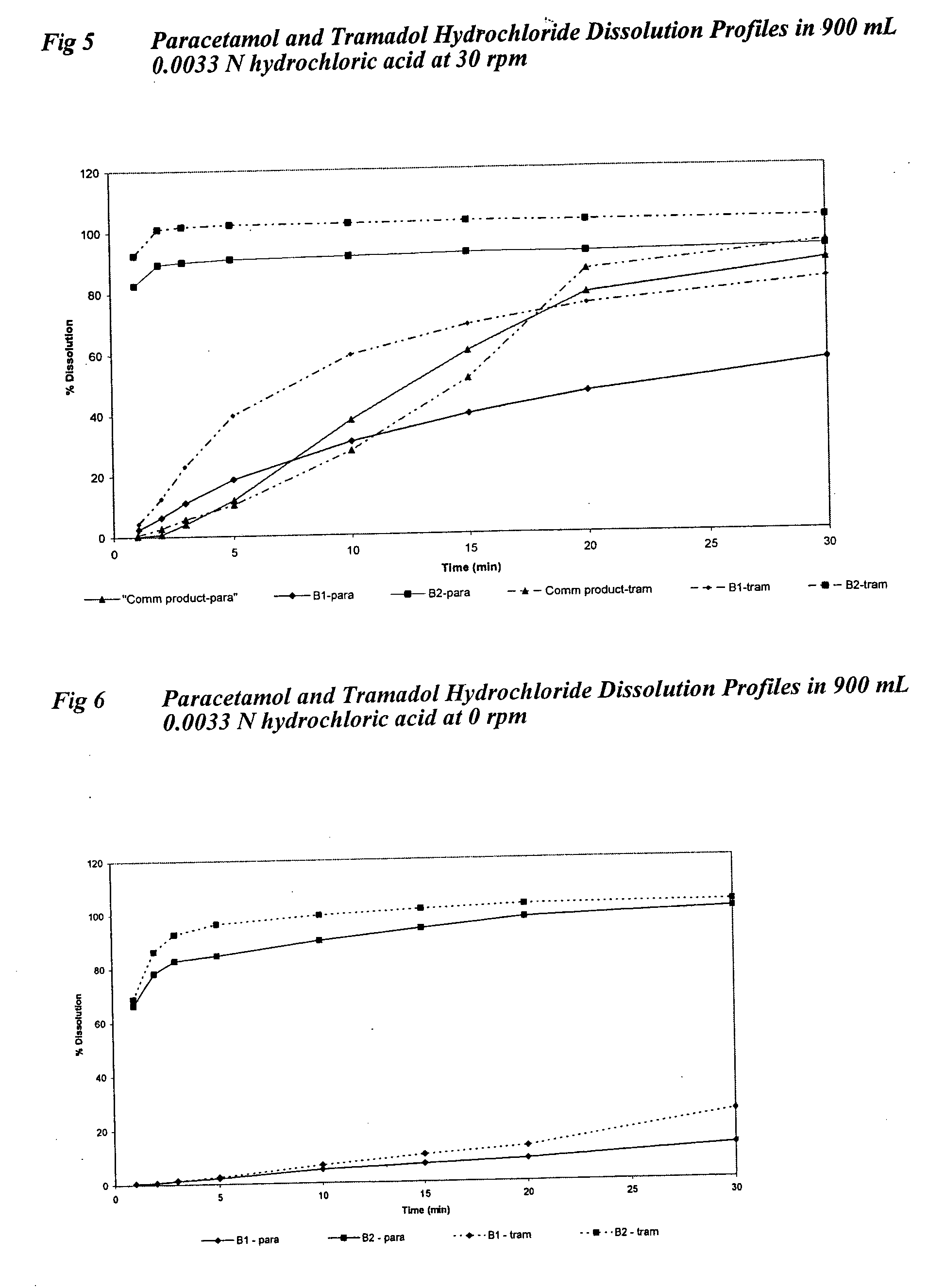
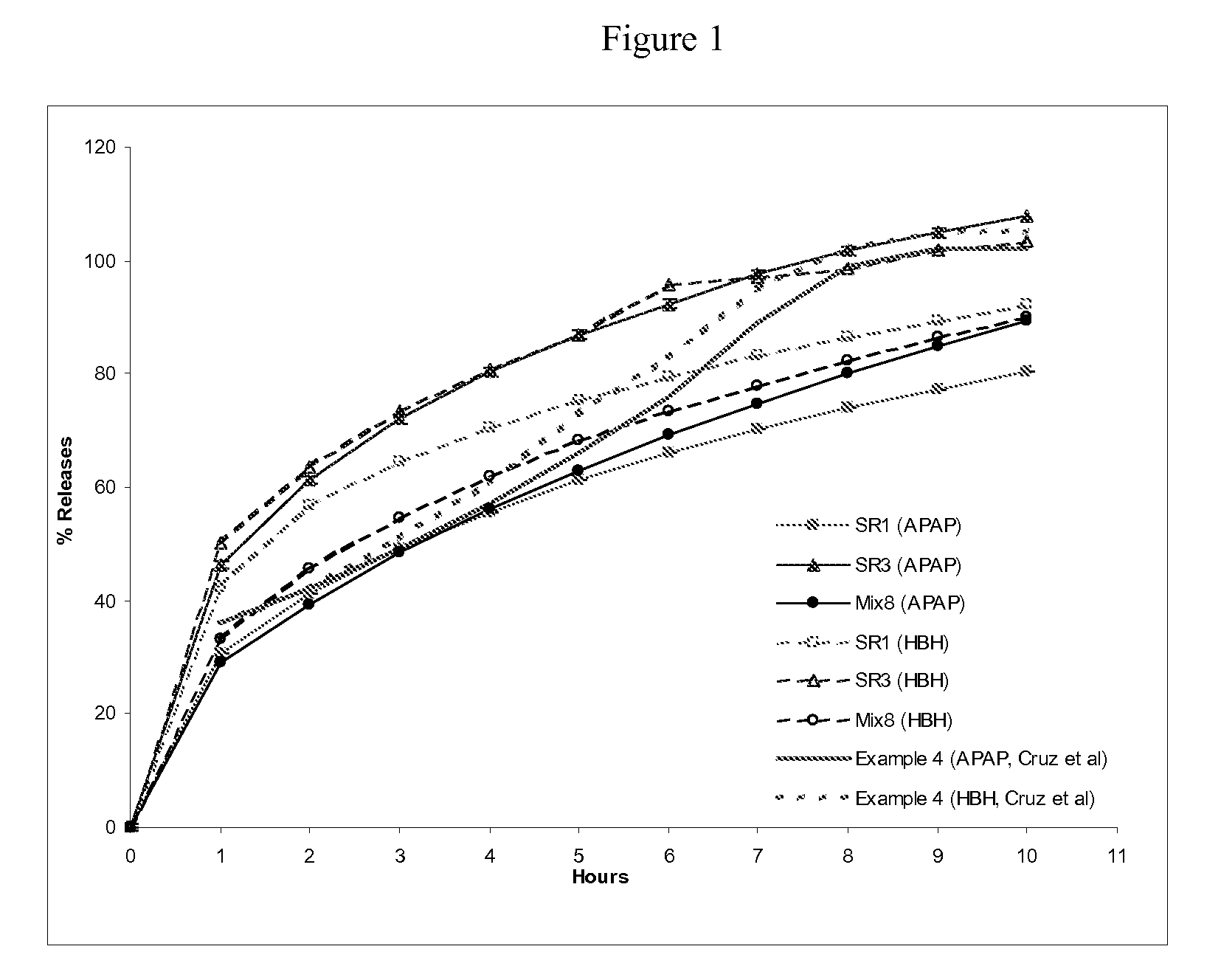
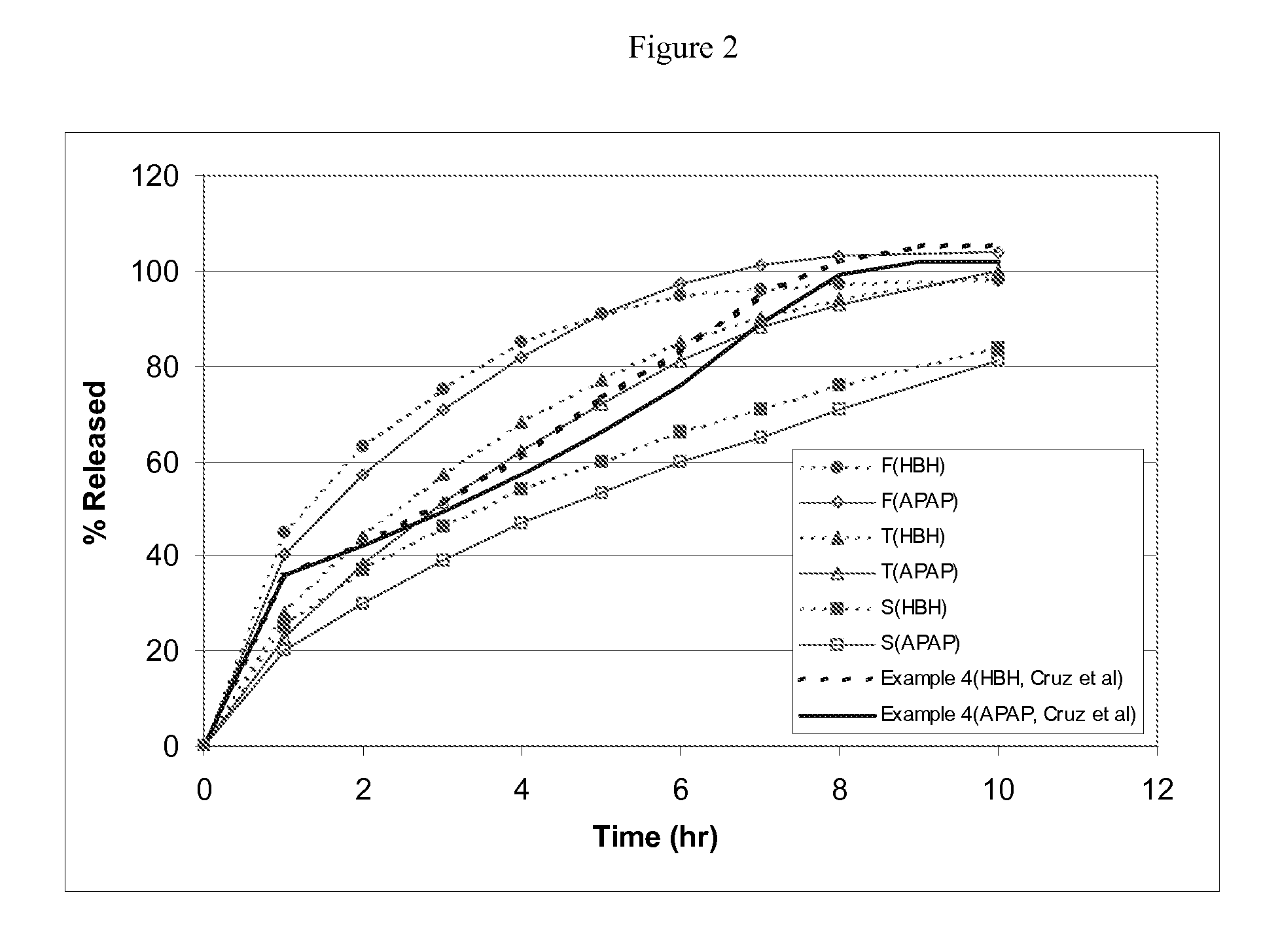
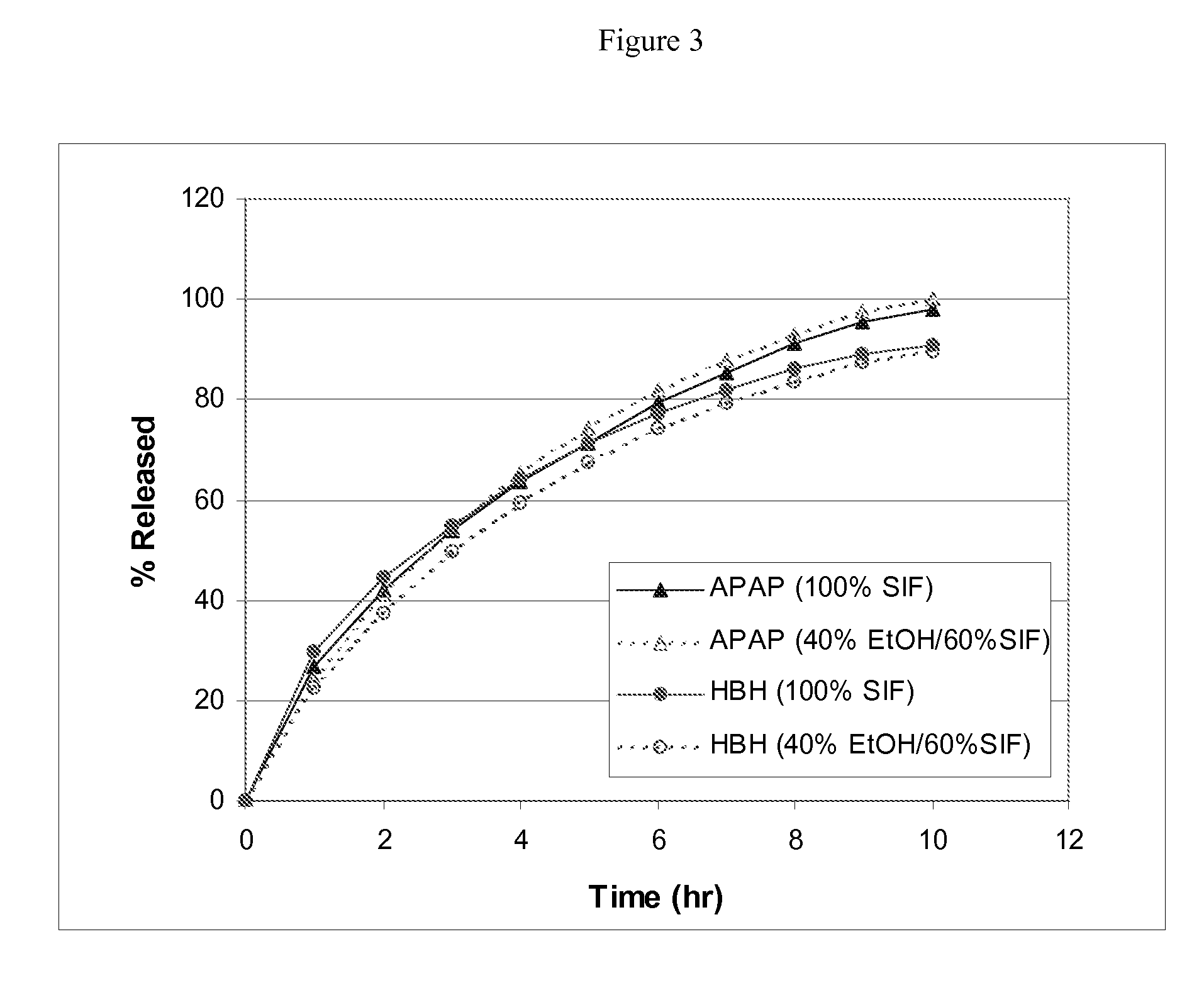
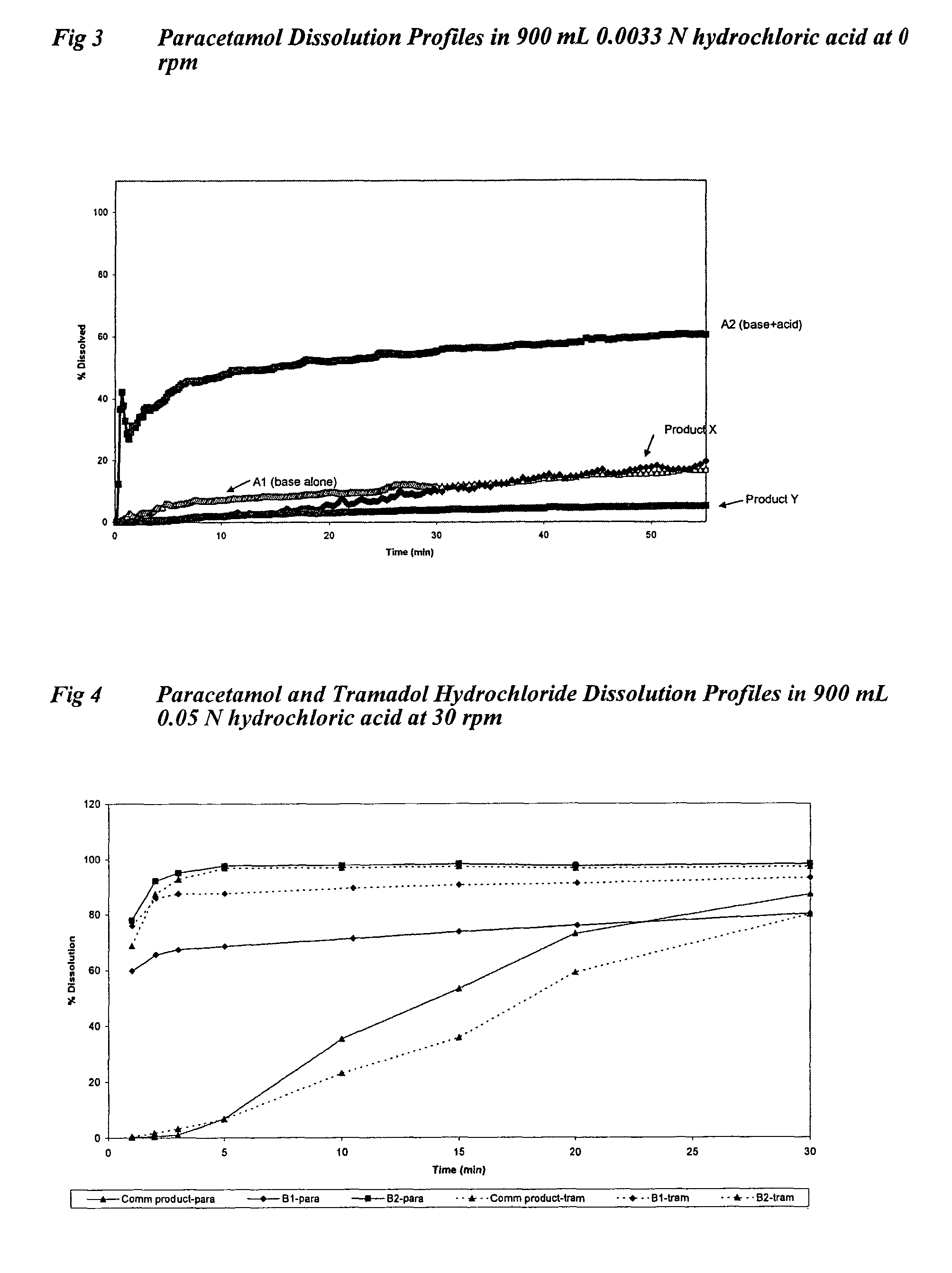
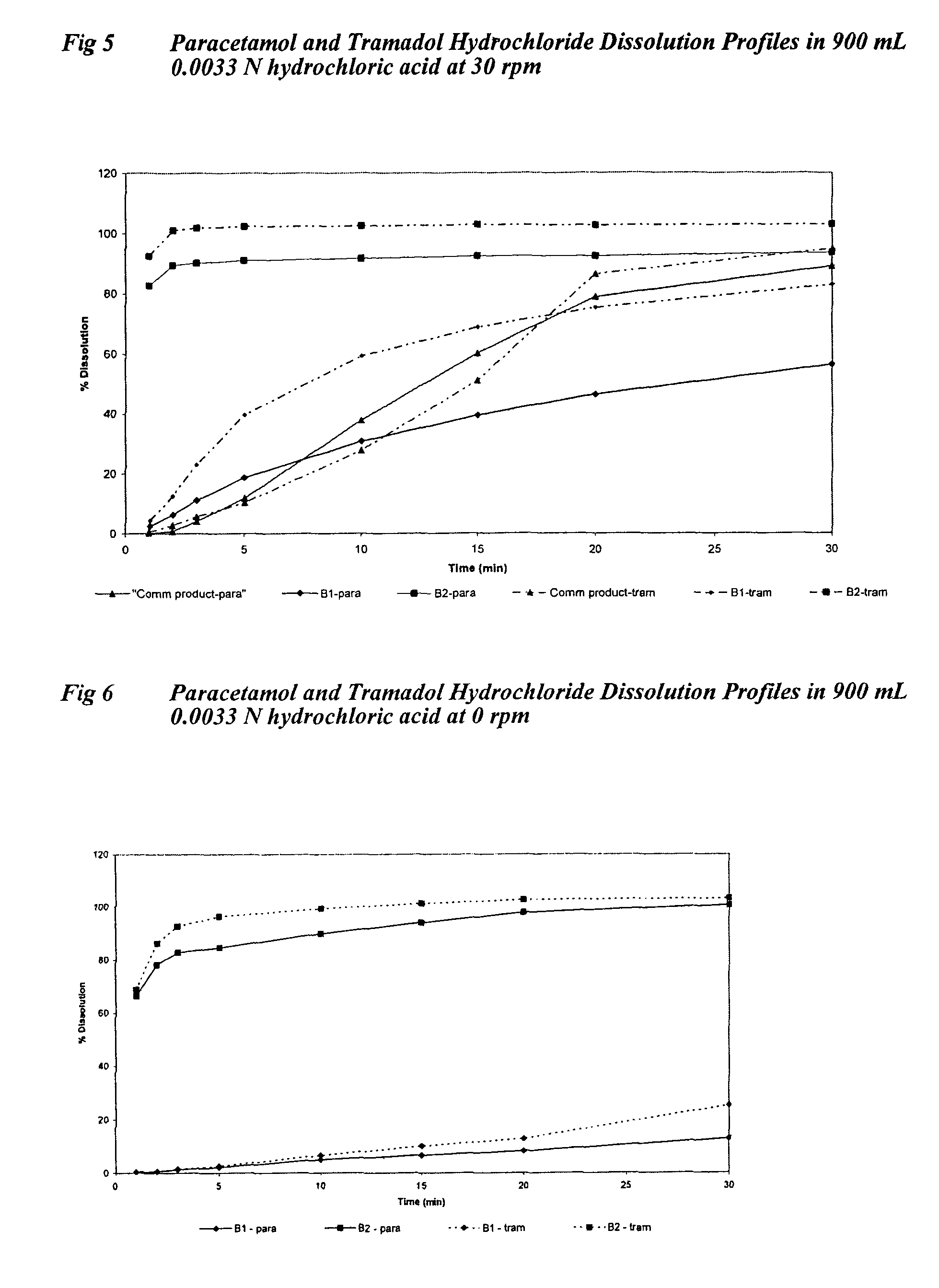
Sustained release formulations containing acetaminophen and tramadol
InactiveUS7374781B2Improve efficiency and qualityProvide clinical efficiencyOrganic active ingredientsNervous disorderDrugSustained Release Formulations
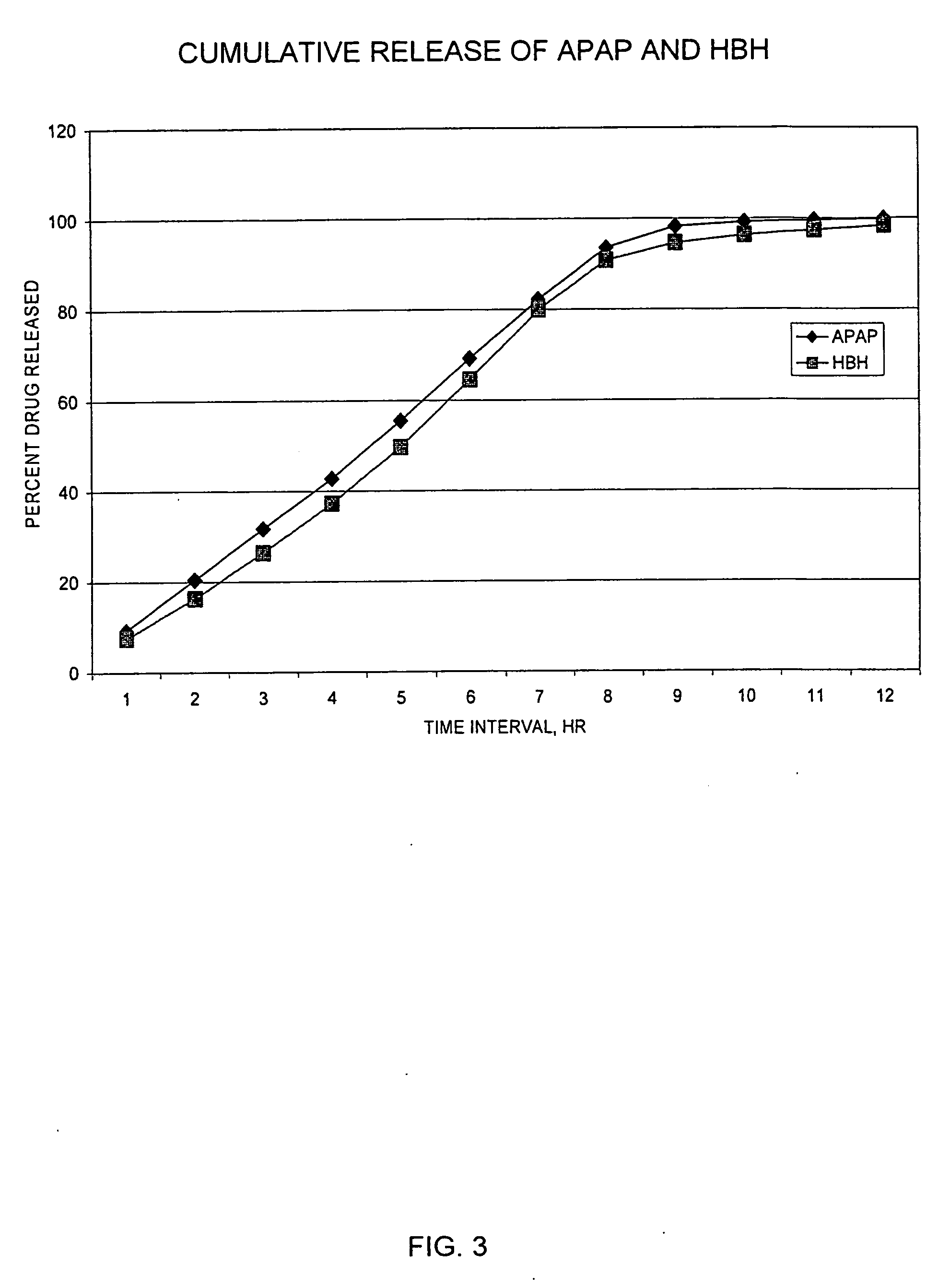
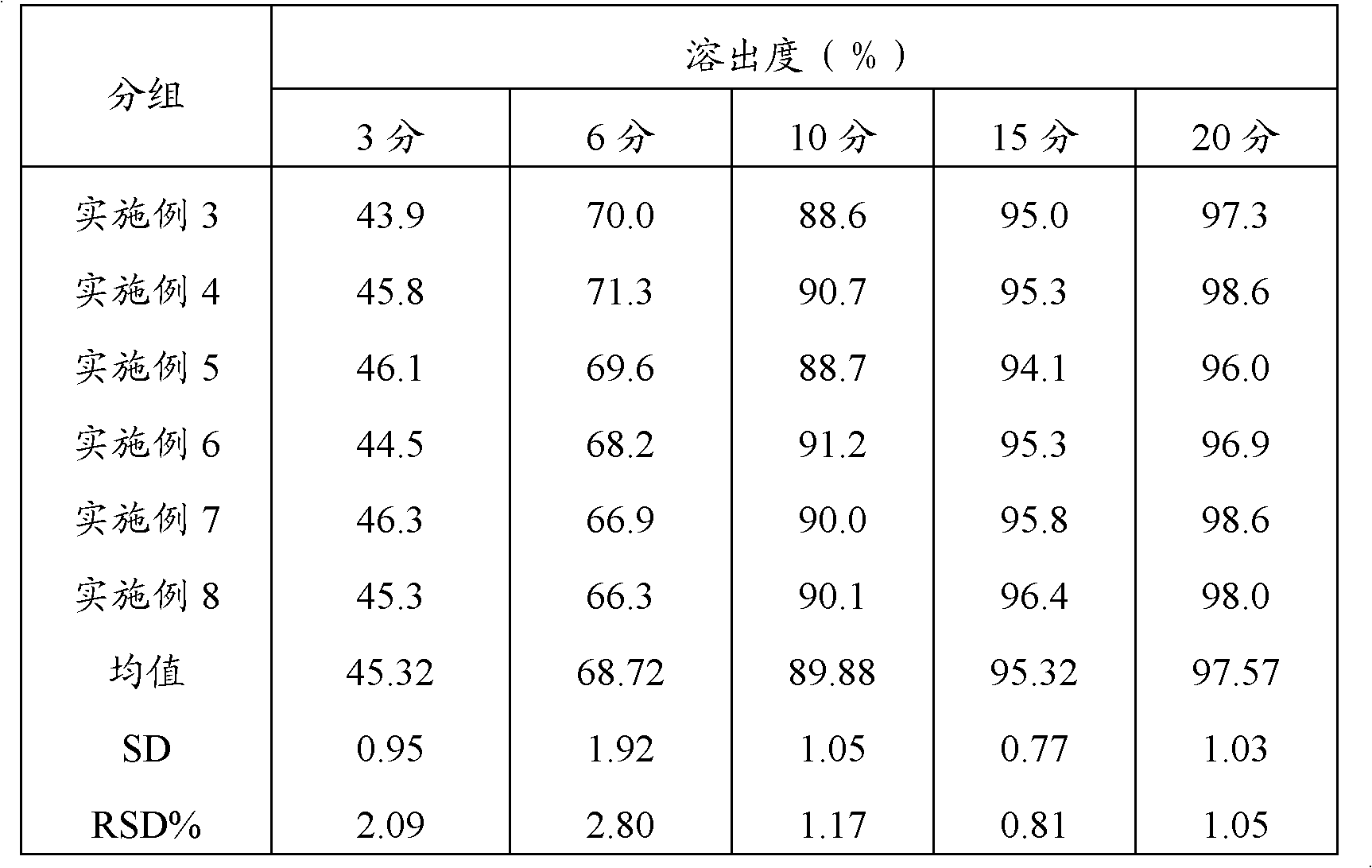
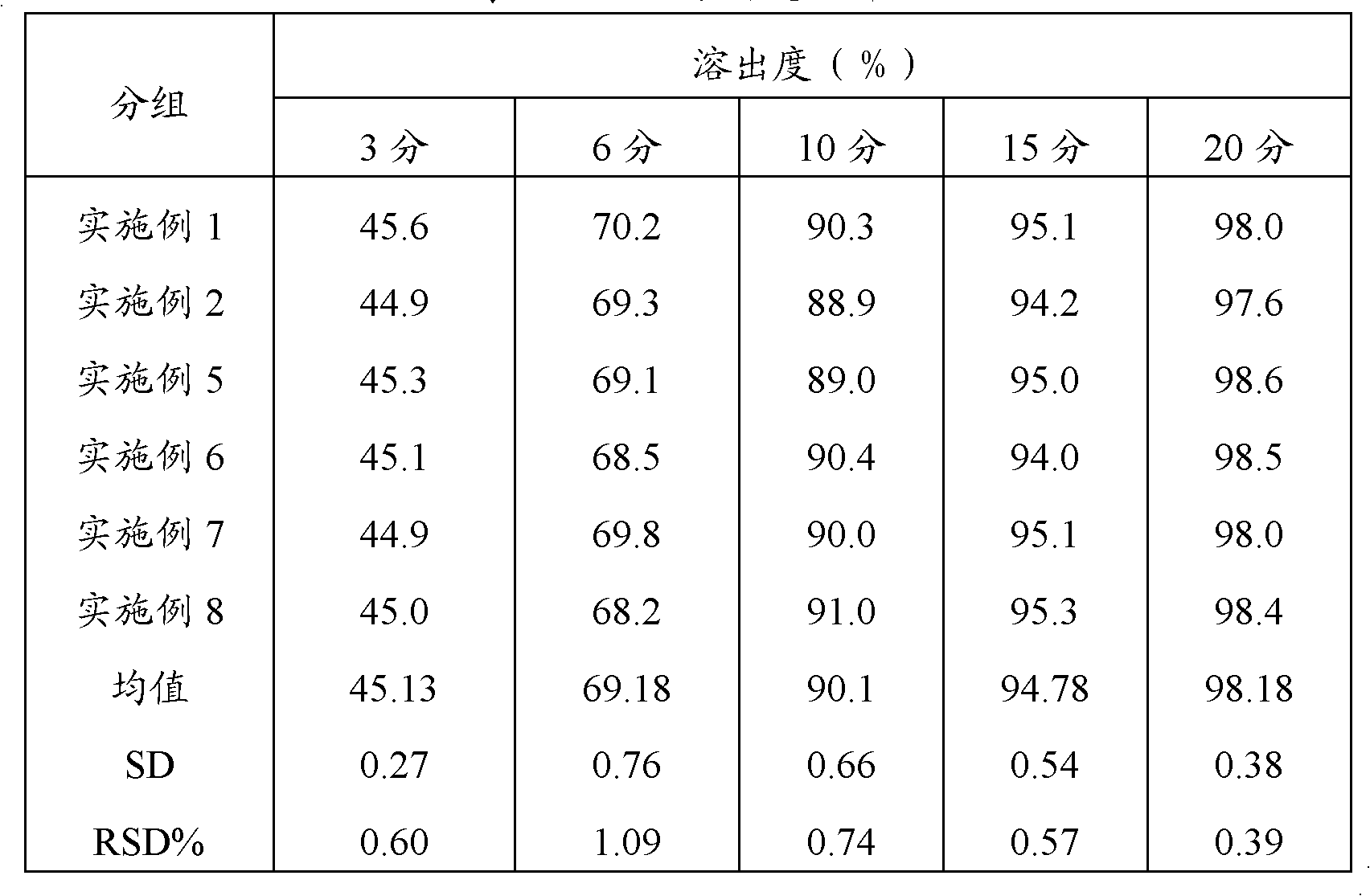
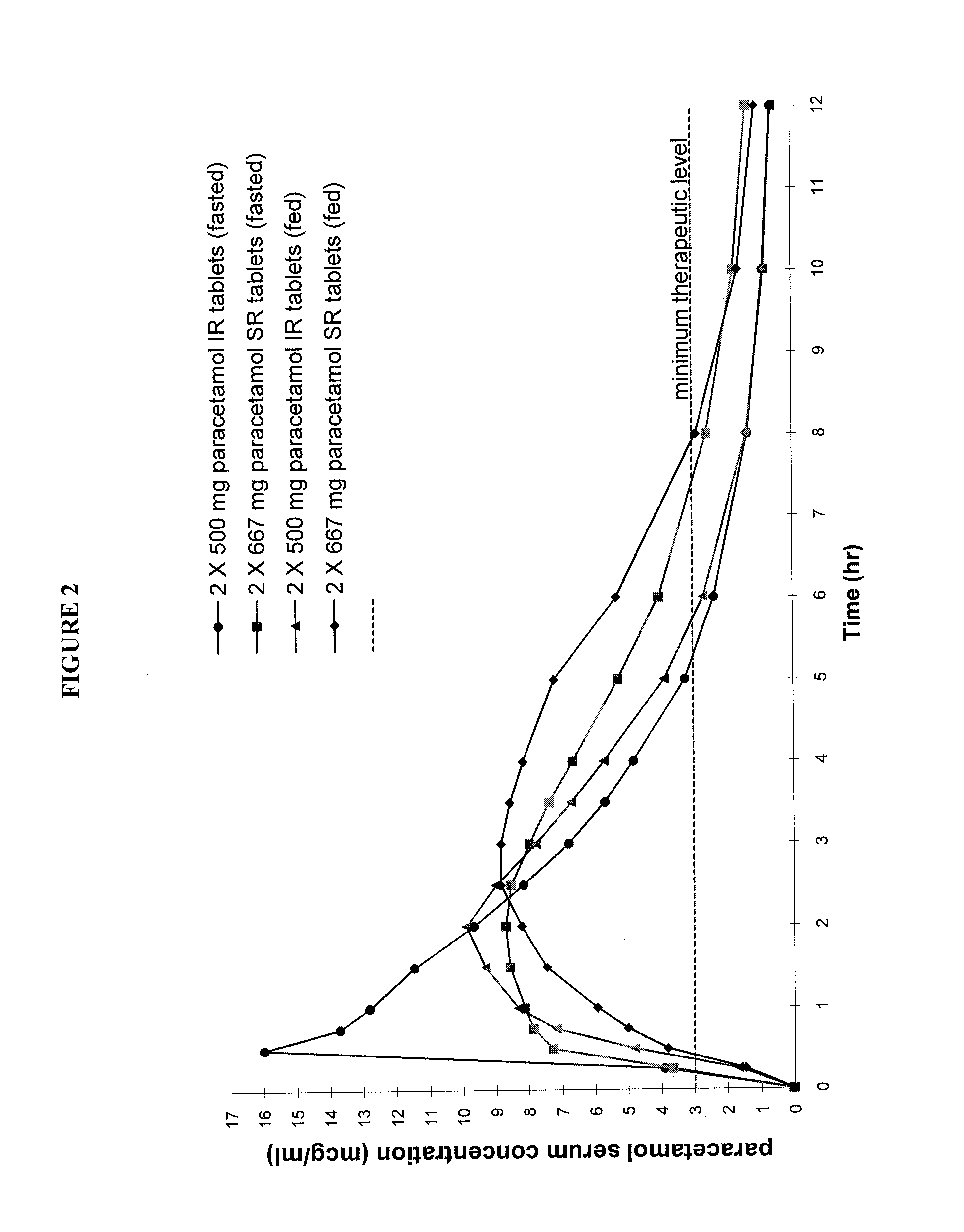
A sustained release formulation as a unit dose contains 100 mg-1000 mg of Acetaminophen and 15 mg-150 mg of tramadol hydrochloride, which comprises of 1) an immediate release portion comprising of 25%-75% of the total effective amount of drug in the dosage form and 2) a sustained release portion comprising of a) 25%-75% of the total effective amount of drugs in the dosage form; b) 6%-50% of gelling polymers of the total formulation, and c) optionally an enteric coating at a level of 5%-40% of the total formulation. The set forth formulation dissolves 25%-60% of the total drug in the first hour, 50%-90% of the total drug in the first four hours and not less than 80% of the total drug in the first 12 hours using USP dissolution method II at 50 rpm.
Owner:ZHANG SHUYI +1
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Acetaminophen / ibuprofen combinations and method for their use
A pharmaceutical dosage form is provided comprising a non-steroidal anti-inflammatory agent and acetaminophen, and methods for their use. In one embodiment, the dosage form is comprised of ibuprofen and acetaminophen as the sole pharmaceutically effective agents, wherein the ibuprofen and acetaminophen are in a weight ratio of about 12 parts:about 88 parts.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Electrochemical paracetamol molecular imprinting transducer and manufacturing method thereof
InactiveCN102735729AElectrochemically activeGood linear relationshipMaterial electrochemical variablesCross-linkFunctional monomer
The invention discloses an electrochemical paracetamol molecular imprinting transducer and a manufacturing method thereof. According to the transducer, a molecular imprinting technology and an electric analysis and chemical detection technology are combined. Paracetamol is taked as a template molecule, acrylamide is taken as a functional monomer, special resource rosin of Guangx is combined with paracetamol and acrylamide, and maleated rosin ethylene glycol dimethacrylate compounded by the rosin serving as a raw material is used as a cross-linking agent. The electrochemical paracetamol molecular imprinting transducer can be used for measuring the content of paracetamol, and has favorable selecting recognition performance. The electrochemical analysis method for detecting the paracetamol, which is provided by the invention, is simple and practical, and meanwhile, can be used for overcoming the defect of the complexity of the traditional method.
Owner:GUANGXI UNIV FOR NATITIES
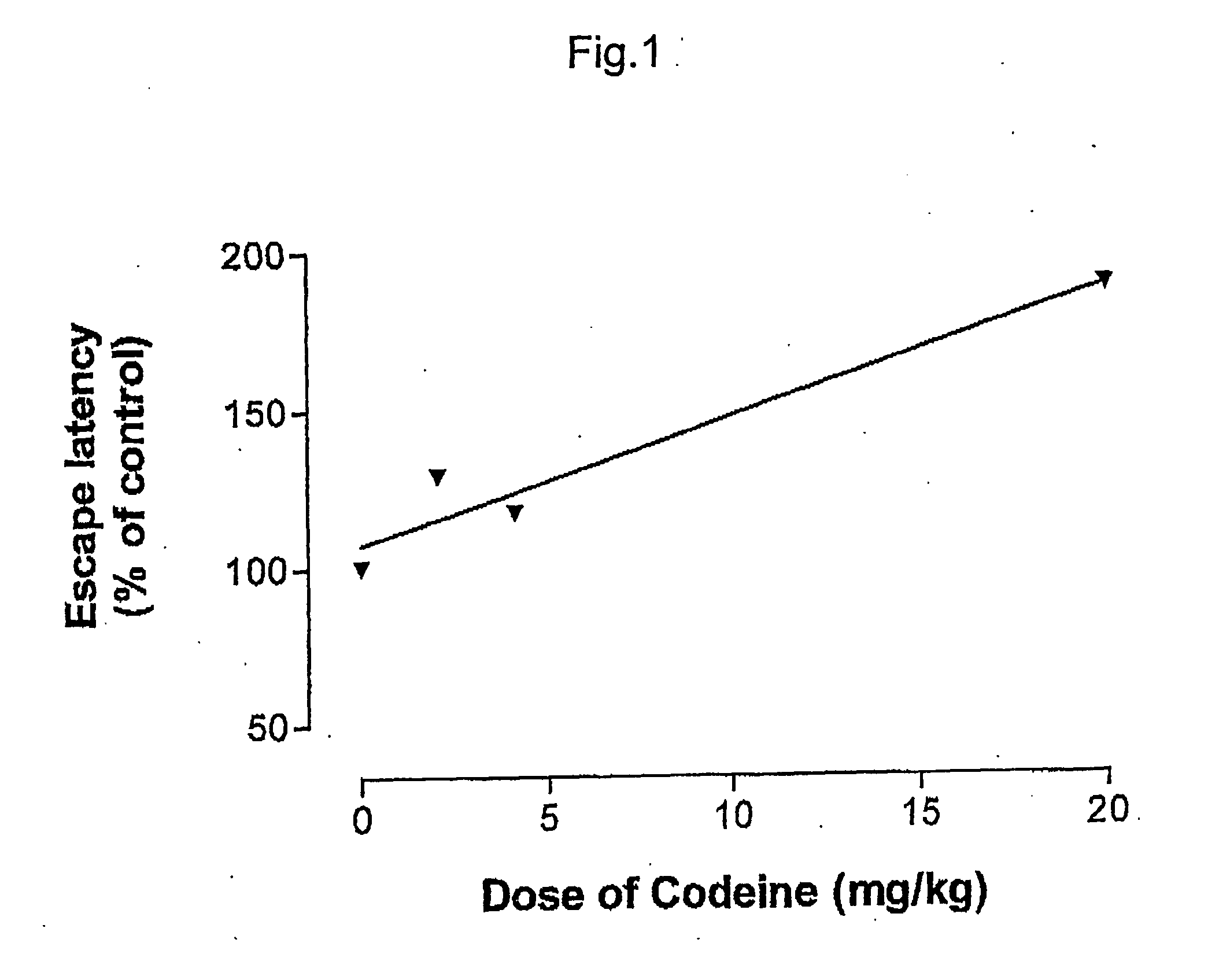
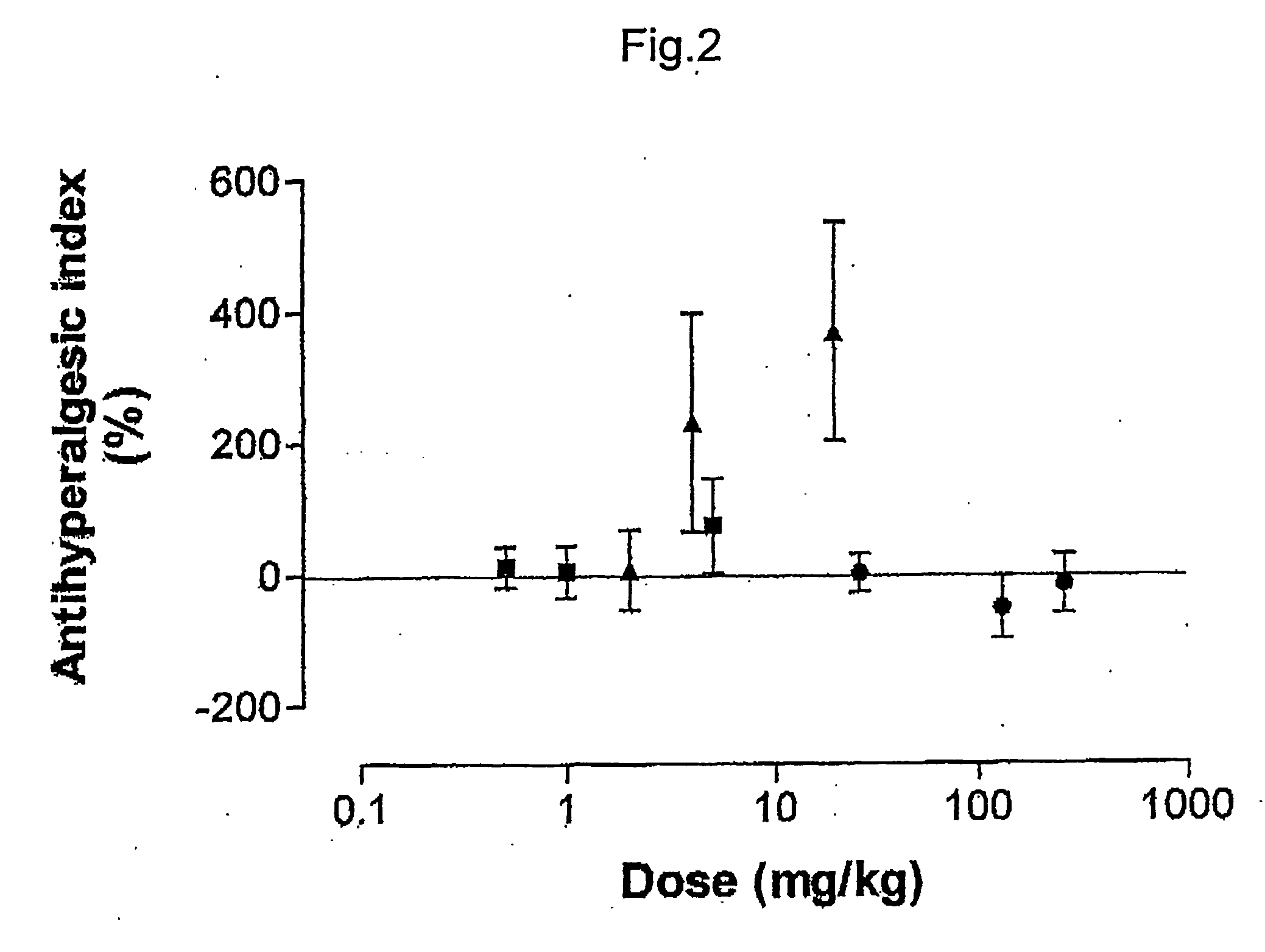
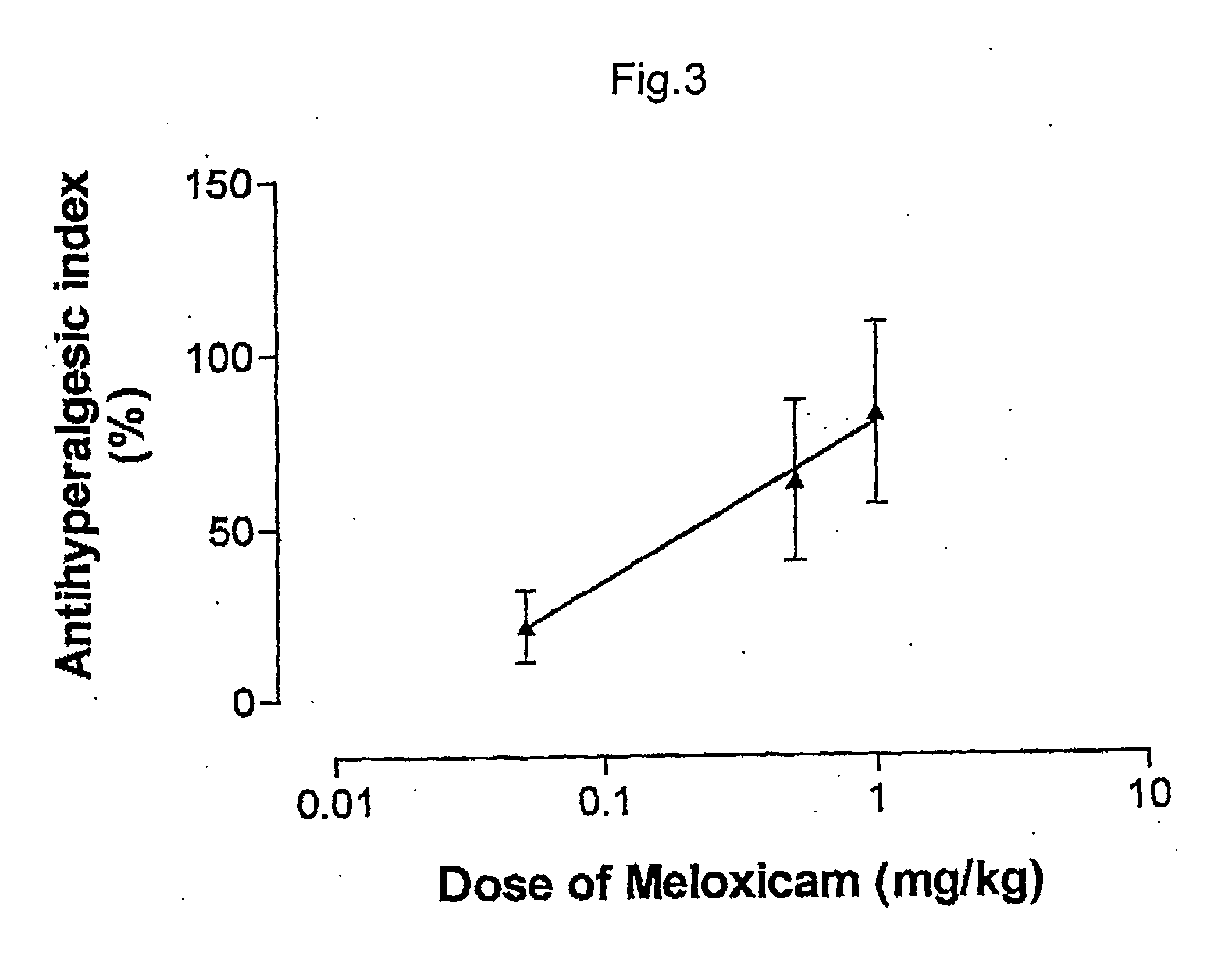
Pharmaceutical combinations of cox-2 inhibitors and opiates
ABSTRACTA pharmaceutical composition comprises a combination of a selective or specific COX 2 inhibitor or a pharmaceutically acceptable salt or derivative thereof and an opiate or a pharmaceutically acceptable salt or derivative thereof, for example a combination of meloxicam and codeine, as active ingredients, and a pharmaceutically acceptable carrier. It may include a centrally-acting cyclo-oxygenase inhibitor such as paracetamol or its pharmaceutically acceptable salts or derivatives. The pharmaceutical compositions are used in methods of providing symptomatic relief or treatment of pain, in an algesic and / or hyperalgesic state, with or without fever, in particular that associated with inflammation such as that associated with trauma, osteoarthritis, rheumatoid arthritis, non-inflammatory myalgia or dysmenorrhoea
Owner:ADCOCK INGRAM LTD
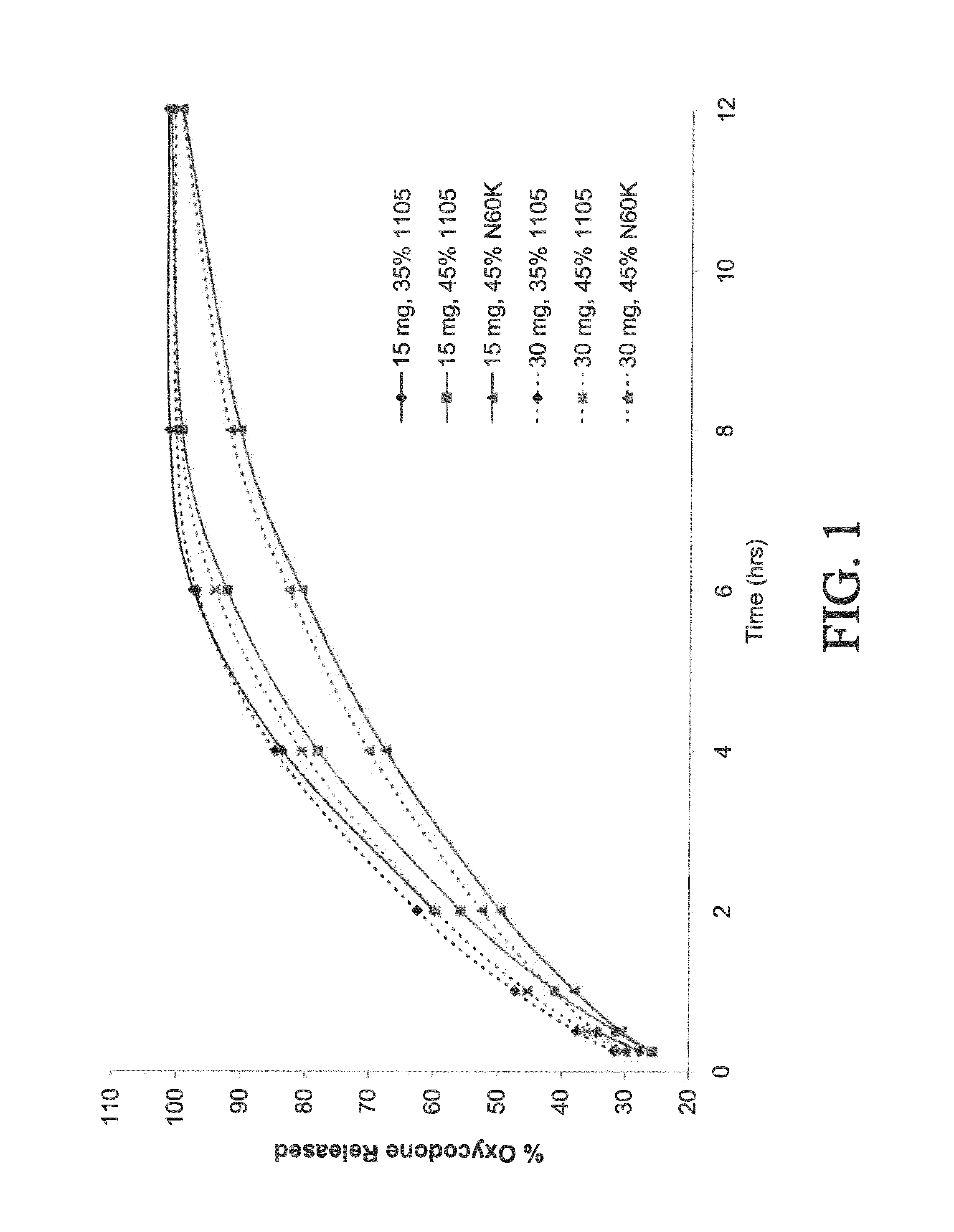
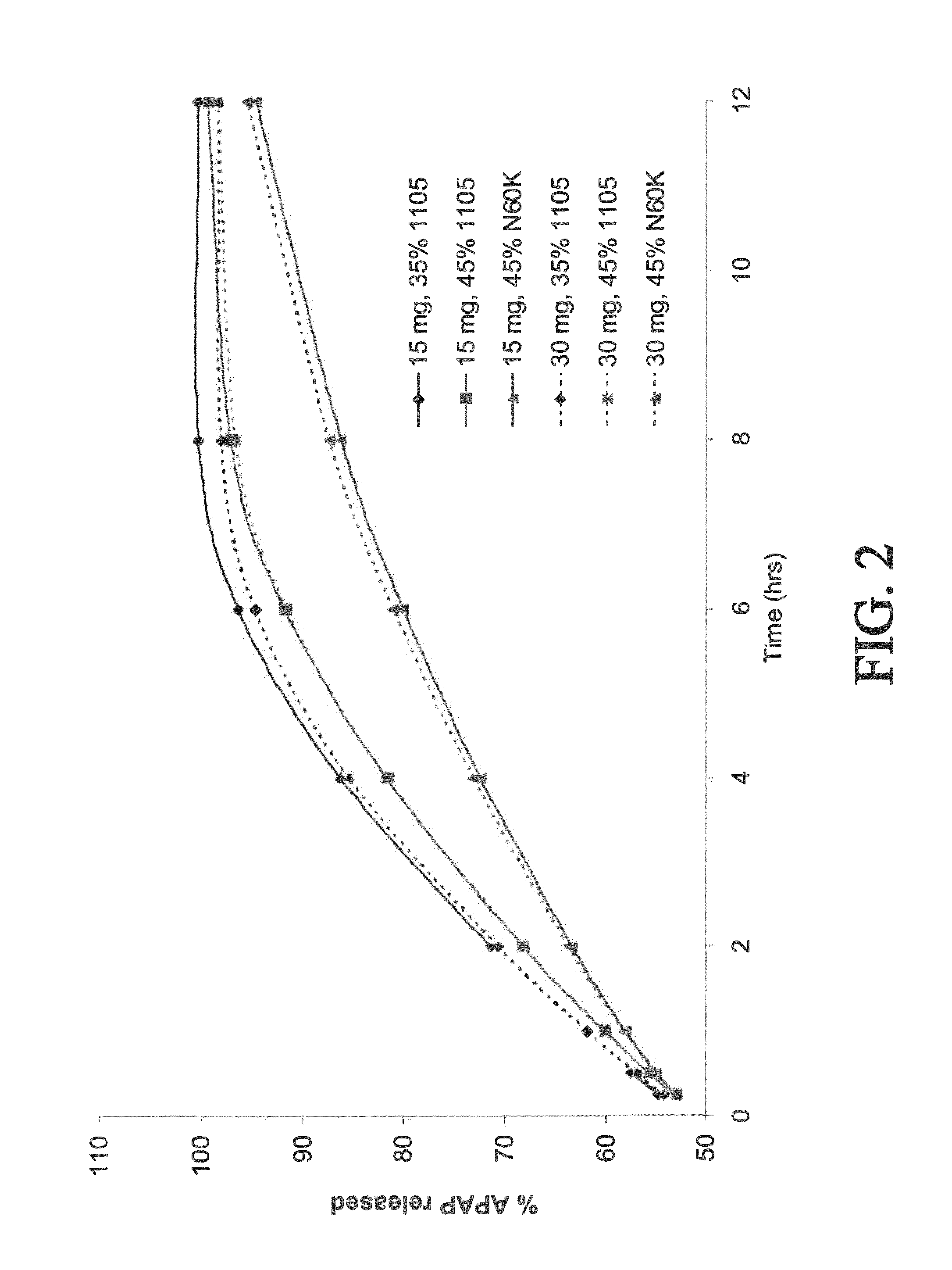
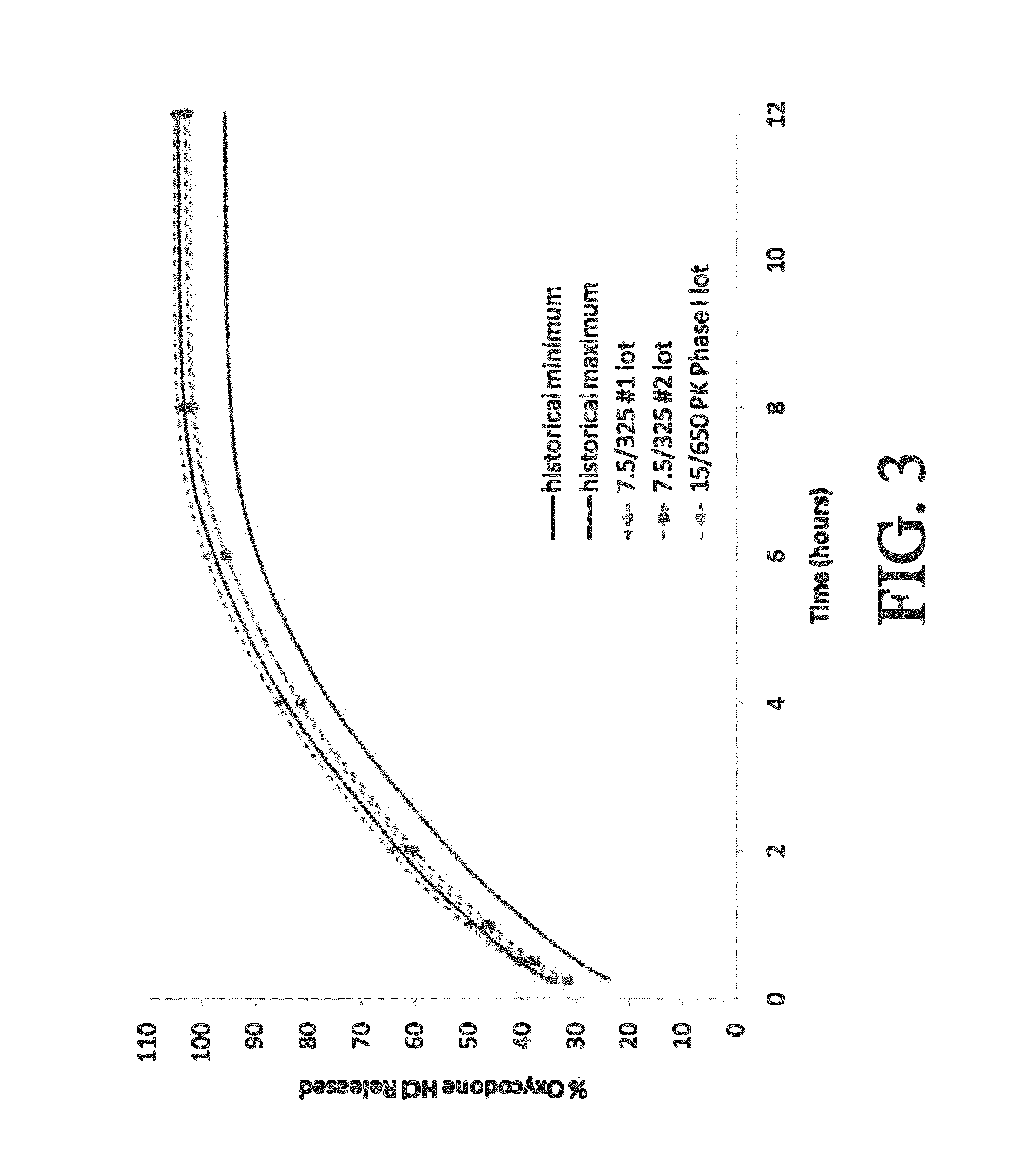
Combination composition comprising oxycodone and acetaminophen for rapid onset and extended duration of analgesia
The present disclosure provides an extended release pharmaceutical composition comprising oxycodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
Application of fructus forsythiae aglycone in preparing medicament for preventing or treating liver injury or liver failure
InactiveCN103989668ASignificant preventionGood treatment effectDigestive systemHeterocyclic compound active ingredientsSide effectAglycone
The invention discloses application of fructus forsythiae aglycone in preparing a medicament for preventing or treating liver injury or liver failure, and belongs to the medical field. The fructus forsythiae aglycone is a traditional Chinese medicine monomer obtained by extracting from the conventional fructus forsythiae, and is shown to have better treatment effect for the liver injury caused by acetaminopben, the livery injury caused by anti-tumor drug cis-platinum, acute or chronic livery injury caused by carbon tetrachloride, the livery injury caused by D-galactosamine and the liver failure caused by the D-galactosamine and lipopolysaccharide according to an animal test, has effect, which is better than that of fructus forsythiae and fructus forsythiae aglycone, in treating liver injury or liver failure. The fructus forsythiae aglycone is exact in curative effect and low in side effect for treating the liver injury and the liver failure, and has wide medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Compositions and methods useful for treatment of respiratory illness
Disclosed are compositions including phenylephrine, its salts, and mixtures thereof, in combination with acetaminophen; and optionally in combination with additional pharmaceutical actives. The compositions have a pH of about 6.5 to about 7.5 and may be substantially free of aldehydes. The invention also provides a method of stabilizing phenylephrine. Also disclosed are methods of treating respiratory illness through administration of a composition comprising phenylephrine, its salts, and mixtures thereof, in combination with acteaminophen; and optionally in combination with additional pharmaceutical actives, wherein the composition has a pH of from about 6.5 to about 7.5 and may be substantially free of aldehydes.
Owner:THE PROCTER & GAMBLE COMPANY
Sustained release monoeximic formulations of opioid and nonopioid analgesics
InactiveUS20110166171A1Improve abilitiesSimple compositionBiocideAntipyreticHydrocodoneSolid Dose Form
The present invention relates to monoeximic solid dosage forms for administering pharmaceutical agents, particularly Hydrocodone and acetaminophen, methods for preparing said dosage forms, and methods for providing therapeutic agents to patients in need of treatment.
Owner:ABBOTT LAB INC
Water-soluble acetaminophen analogs
InactiveUS20110212926A1Slow onsetDelaying the onset of acetaminophen actionBiocideNervous disorderDiseaseTreatment fever
The present invention provides water-soluble acetaminophen prodrugs and formulations which may be suitable for parenteral administration. Methods of treating a disease or condition responsive to acetaminophen (such as fever and / or pain) using the acetaminophen prodrugs, as well as kits, unit dosages, and combinations with additional pharmaceutical agent(s) are also provided.
Owner:ACORDA THERAPEUTICS INC
Liquid pharmaceutical formulation containing paracetamol
The present invention relates to a sugar-free liquid pharmaceutical formulation comprising an aqueous solution of paracetamol, a solubilizing agent containing polyethylene glycol, a thickening agent containing xanthan gum, and a sweetening system containing sucralose and a mixture of polyols containing glycerol, sorbitol and xylitol in a total amount between approx. 15% and 35% w / v relative to the total volume of said pharmaceutical formulation.
Owner:AZIENDE CHIMHE RIUNITE ANGELINI FRANCESCO A C R A F
Preparation method of compound paracetamol and amantadine pellets
ActiveCN102861106AEvenly distributedEasy to fillNervous disorderAntipyreticDissolutionCalculus bovis
The invention discloses a preparation method of compound paracetamol and amantadine pellets. The compound paracetamol and amantadine pellet is mainly prepared from the following raw materials: chlorpheniramine maleate, calculus bovis factitious, caffeine, amantadine hydrochloride, acetaminophen and dextrin, and the mass ratio of the above raw materials is 1 to 5 to 7.5 to 50 to 125 to (4-5); the preparation method comprises the following steps: adding the chlorpheniramine maleate and the caffeine in the form of solution into a dextrin aqueous solution, spraying and packing the mixed solution on the mixed masterbatches consisting of the acetaminophen, the amantadine hydrochloride and the calculus bovis factitious, and then spraying the rest of dextrin and the rest of mixed powder to obtain the compound paracetamol and amantadine pellet. The method is simple to operate, liable to control and suitable for industrial production, and the obtained pellet has the advantages of low related substances, good content uniformity and dissolution rate and the like.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Oral paracetamol formulations
The present invention relates generally to formulations comprising paracetamol. More particularly, the present invention provides a swallow formulation comprising paracetamol which facilitates the rapid delivery of paracetamol into the circulatory system following oral administration. The present invention further relates to methods for inducing efficient pain relief including an analgesic effect by the administration of the paracetamol formulation.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Complex acetaminophen vitamin C dispersion tablet and preparing method thereof
InactiveCN1507862AQuick effectDefinite curative effectOrganic active ingredientsAntipyreticSorbyl alcoholCross-link
The present invention relates to compound paracetamol vitamin C disperser tablet and its preparation method. It is formed from paracetamol, vitamin C, disintegrant, filling agent, correctives, adhesive and lubricating agent according to the ratio of 200-400:200-400:1-60:1-100:1-60:2-50:1-10. The disintegrant is carboxy methyl starch sodium, hydroxypropyl starch, low-substituted hydroxypropyl cellulose, calcium cellulose glycollate, cross-linked sodium cellulose glycollate, sodium lauryl sulfate, and the filling agent is lactose, mannitol, sorbyl alcohol, starch, modified starch, beta-dextrin, calcium sulfate dihydrate, microcrystal cellulose, and the corrective is wintergreen oil, peperitol, aspatan, citric acid, cane sugar, sterioside, the adhesive is polyvidone, hydroxypropyl cellulose, gelling starch and ethyl cellulose, and the lubricating agent is magnesium stearate, calcium stearate, polyglycol 6000 and talcum powder.
Owner:严洁
Oral disintegrant of compound paracetamol
InactiveCN1679525ASimple preparation processDisintegration has little adverse effectOrganic active ingredientsAntipyreticChlorphenamine maleateOrally disintegrating tablet
An oral disintegrating tablet of compound paracetamol is prepared from paracetamol, pseudoephedrine hydrochloride, dextromethorphan HBr, chlorphenamine maleate, filler, disintegrant, adhesive or moistening agent, lubricant, flavouring and pigment through direct tabletting.
Owner:FUDAN UNIV
Liquid dosage form of acetaminophen
The present invention relates to a liquid dosage form comprising pharmaceutically insoluble and unpleasant active drug and liquid excipient base. In particular, the invention relates to a liquid dosage form comprising Acetaminophen and liquid excipient base as a solubilizer.
Owner:AUROBINDO PHARMA LTD
Method for preparing compound paracetamol and amantadine hydrochloride tablet
InactiveCN102488711AHigh dissolution rateAntiviralsUnknown materialsQuality standardAmantadine Hydrochloride
The invention particularly relates to a method for preparing a compound paracetamol and amantadine hydrochloride tablet, which belongs to the field of medicines. According to the method, submicron powder and ordinary fine powder which has been sieved with a 100-mesh sieve are mixed in proportion, a proper amount of accessories are added, and a prepared finished product is enabled to have better dissolvability, more than 90% on average. After six-month accelerated normal temperature stability experiments, the dissolvability of the finished product prepared by using the method is about 90%, and all the other indexes of the finished product accord with quality standard requirements.
Owner:吉林省吴太感康药业有限公司 +1
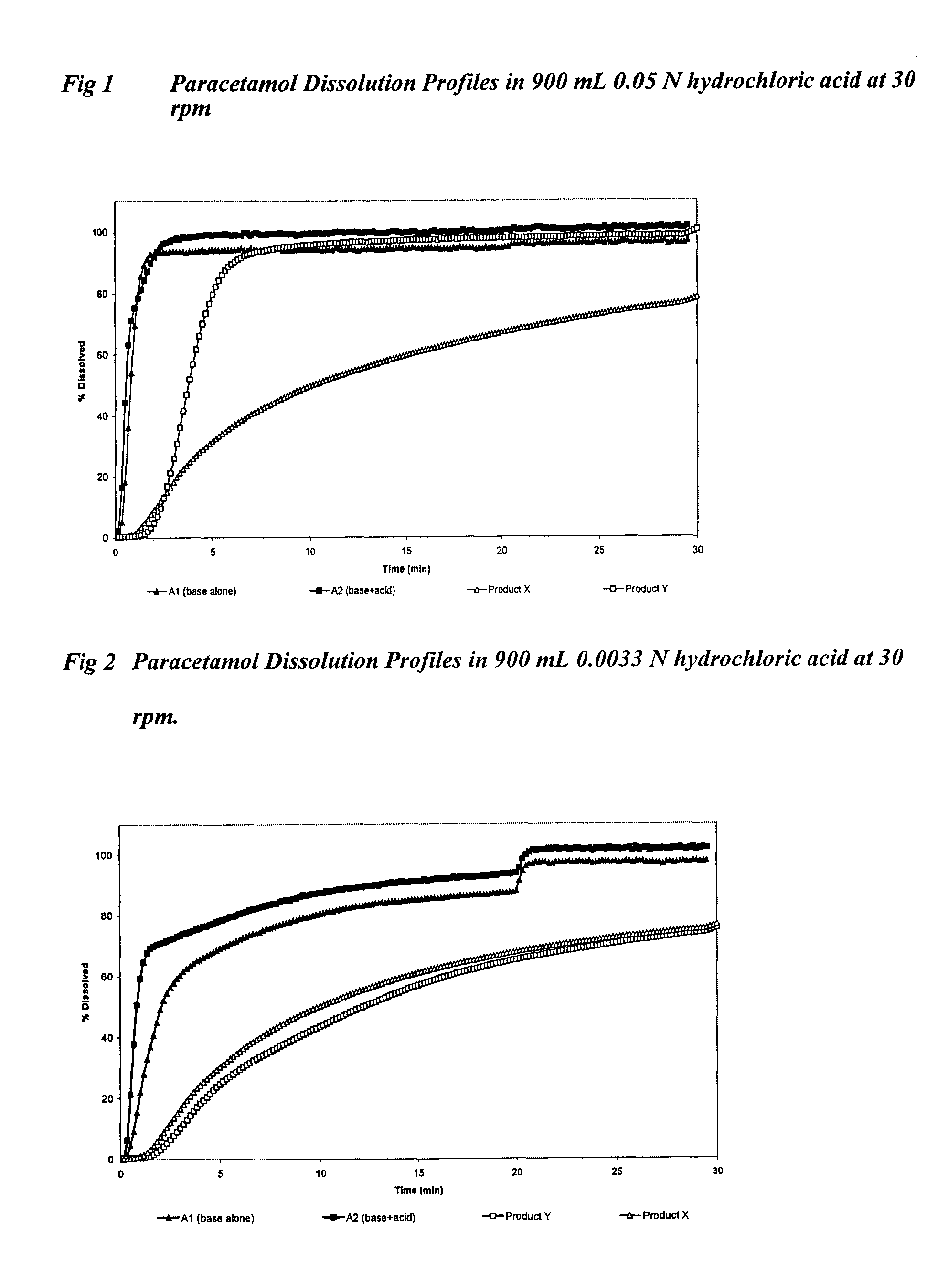
Composition
A pharmaceutical composition comprising an immediate release phase and a sustained release phase of paracetamol is described which has a unique in vitro dissolution profile resulting in advantageous pharmacokinetic properties.
Owner:SMITHKLINE BEECHAM LTD
Pharmaceutical composition of ibuprofen and paracetamol and methods of using the same
A combination pharmaceutical composition for the treatment of pain comprising between about 125 mg and about 150 mg ibuprofen and between about 475 mg and about 500 mg paracetamol, and a method for alleviating pain in a patient comprising administering to the patient a pharmaceutical composition comprising between about 125 mg and about 150 mg ibuprofen and between about 475 mg and about 500 mg paracetamol.
Owner:AFT PHARM LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com