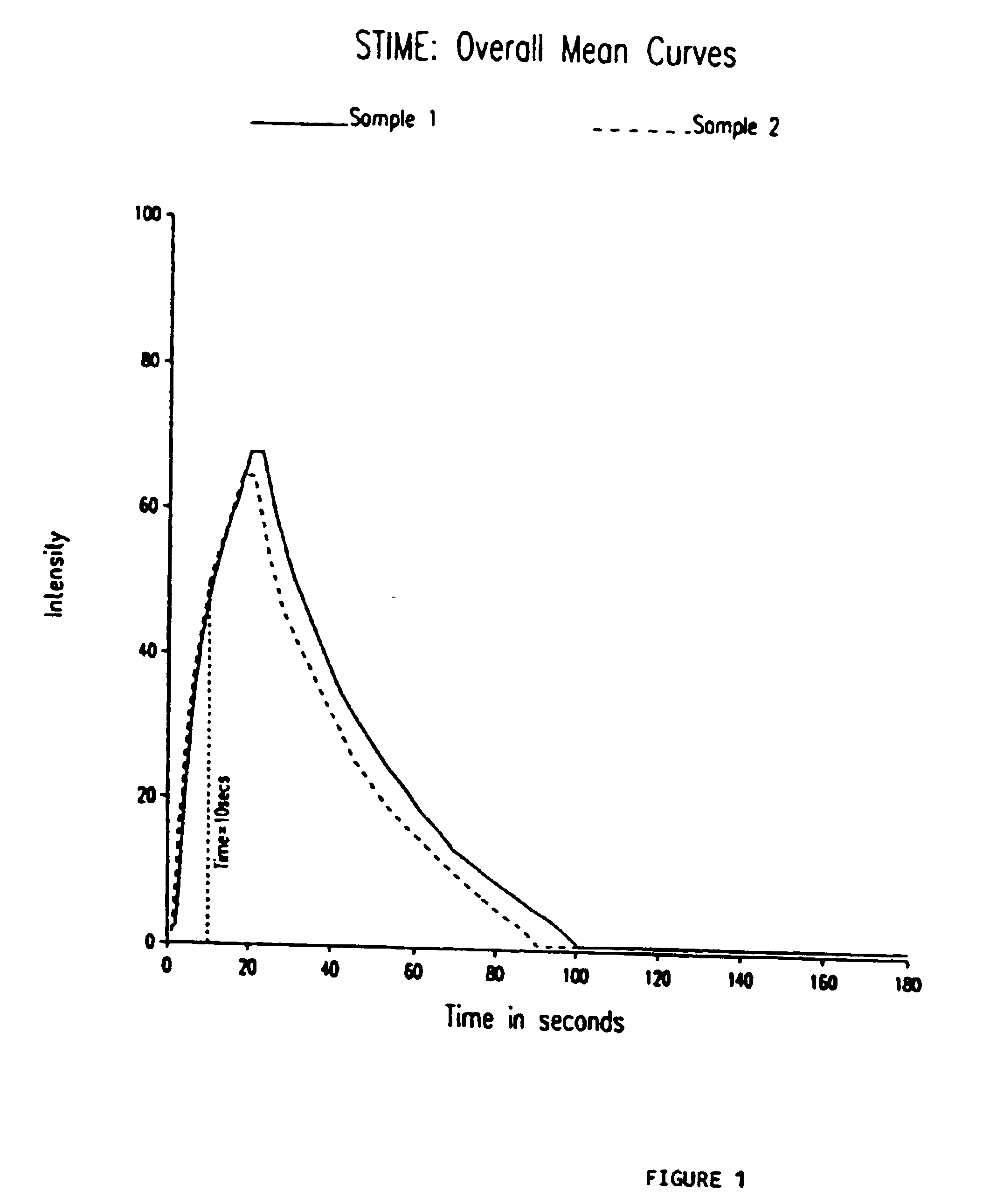
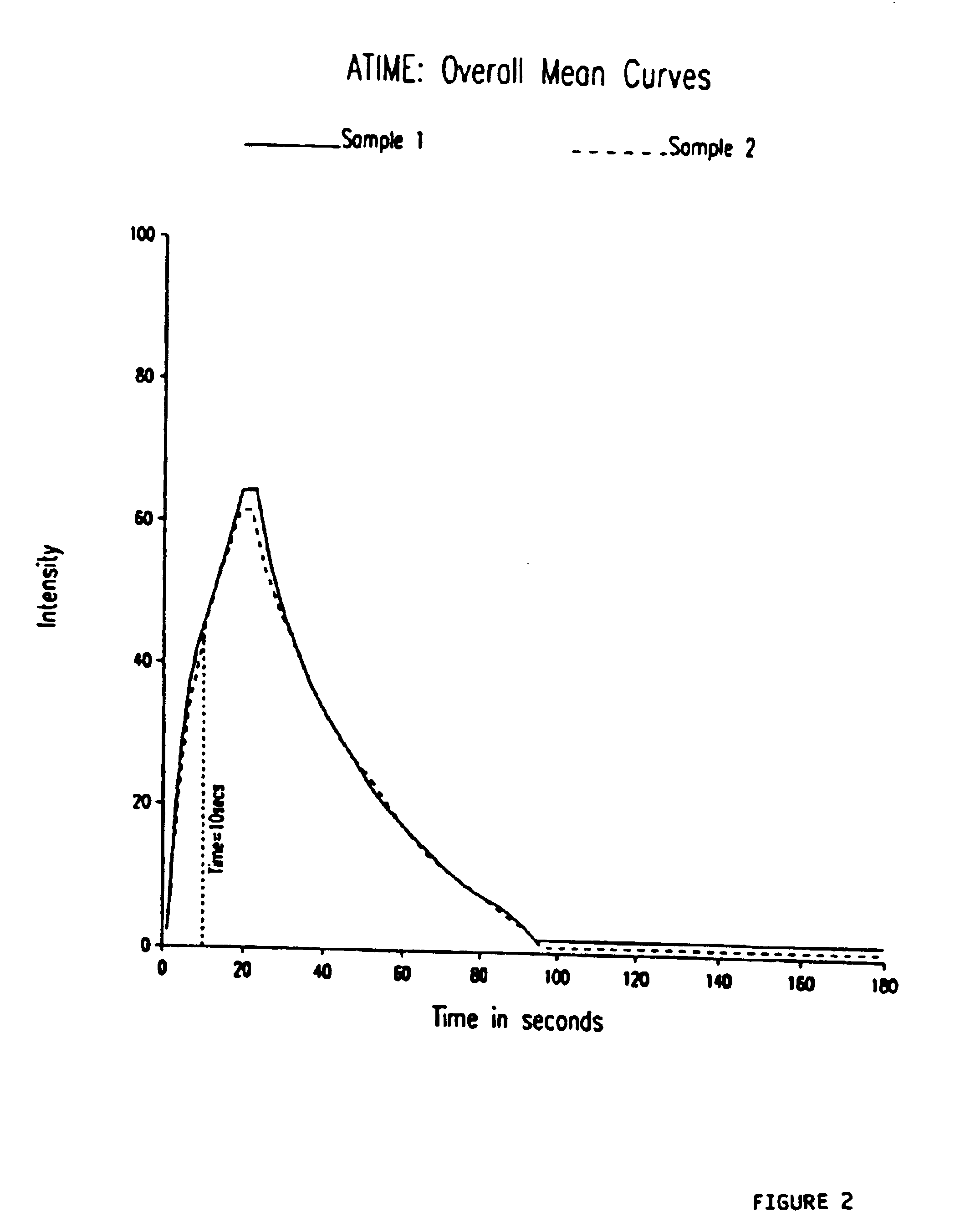
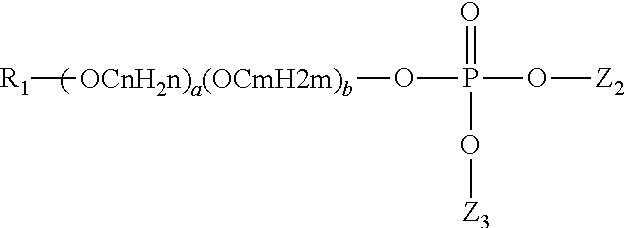Patents
Literature
1540 results about "Sucralose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sucralose is an artificial sweetener and sugar substitute. The majority of ingested sucralose is not broken down by the body, so it is noncaloric. In the European Union, it is also known under the E number E955. It is produced by chlorination of sucrose. Sucralose is about 320 to 1,000 times sweeter than sucrose, three times as sweet as both aspartame and acesulfame potassium, and twice as sweet as sodium saccharin. Evidence of benefit is lacking for long-term weight loss with some data supporting weight gain and heart disease risks.
Sucralose-containing composition and edible products containing the composition
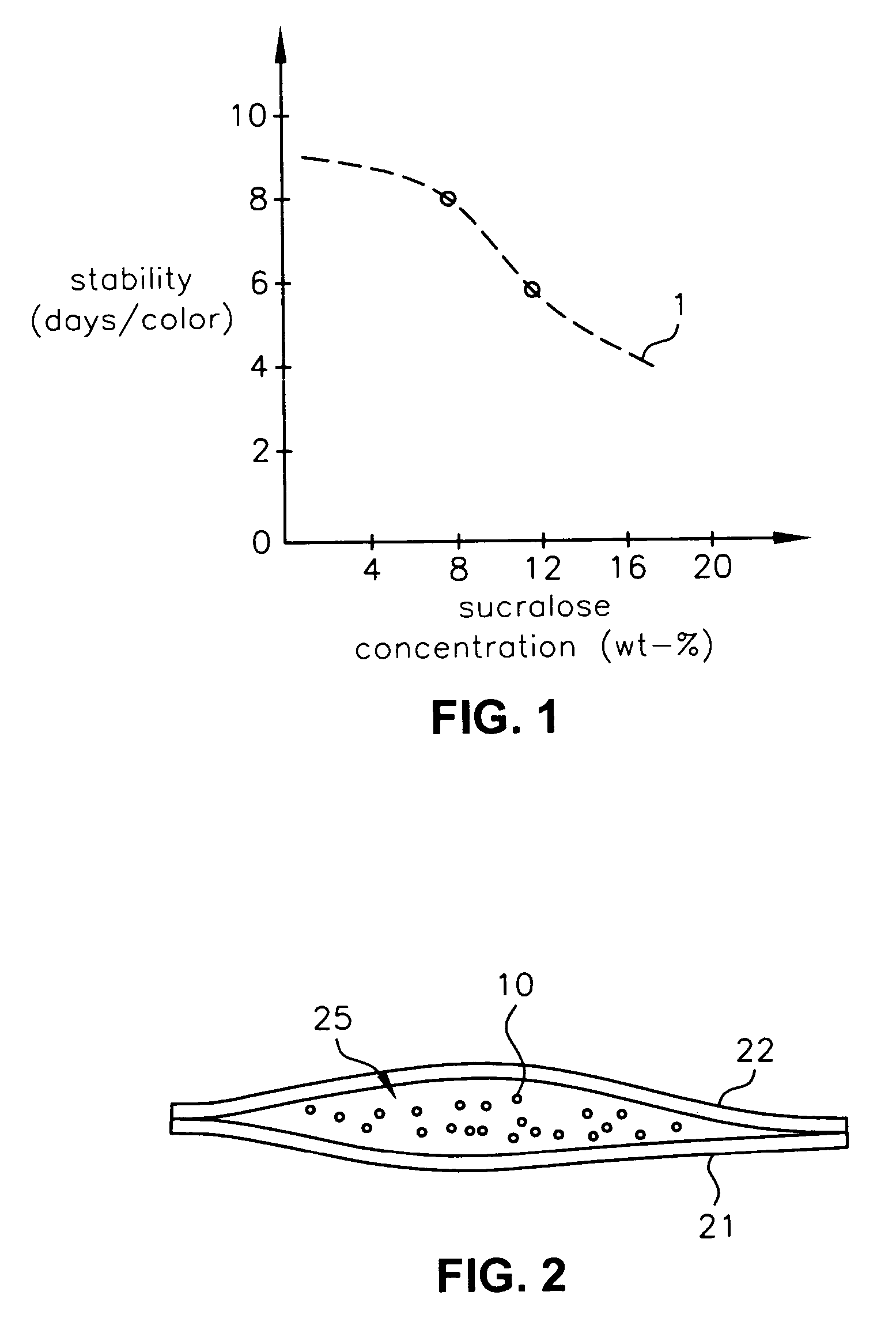
A composition which is obtained by causing a specific compound to be present together with sucralose. The composition provides a sucralose in a stable form, more particularly, a sucralose which is still stable and thus is significantly suppressed with respect to the decrease in sweetness and discoloration (browning blackening), even when it is subjected to a warming treatment under a condition wherein temperature is high and especially water content is low and / or pH is low. The stabilized sucralose-containing composition can be used a sweetener by itself and as a compound with a food or a drug.
Owner:SAN EI GEN F F I
Dental bleaching compositions containing sucralose
Dental bleaching compositions that include sucralose. The bleaching agent is dispersed within a carrier, which is optimally sticky and viscous such as a mixture of propylene glycol and silica fume. Anhydrous propylene glycol and / or anhydrous glycerin are especially useful in order to maintain the desired degree of hydration of the perborate being used. Flavorants may be added to enhance the taste of the dental compositions, since they will be used within a person's mouth. For best results, a flexible, thin-walled, comfortable-fitting, custom dental tray is used with the dental bleaching compositions. The dental compositions are sufficiently sticky and viscous so as to adhere and retain a dental tray against a person's teeth which is designed so as to not exert significant mechanical pressure onto the person's teeth.
Owner:ULTRADENT PROD INC
Dental bleaching compositions containing sucralose
InactiveUS6322774B1Effectively disguise the bitter taste of such agents over timeAccurate quantityCosmetic preparationsImpression capsFlavouring agentGlycerol
Dental bleaching compositions that include sucralose as a non-nutritive sweetener. The bleaching agent is dispersed within a carrier, which is optimally sticky and viscous, such as a mixture of a liquid or solvent carrier and a tackifying agent. Propylene glycol and / or glycerin are especially useful liquid or solvent carriers. Flavorants may be added to enhance the taste of the dental compositions, since they will be used within a person's mouth. For best results, a flexible, thin-walled, comfortable-fitting, custom dental tray is used with the dental bleaching compositions. The dental compositions are preferably sufficiently sticky and viscous so as to adhere and retain a dental tray against a person's teeth which is designed so as to not exert significant mechanical pressure onto the person's teeth.
Owner:ULTRADENT PROD INC
Oral Care Compositions With Improved Sweetness
The present invention is directed to improved sweetener compositions and oral care compositions, especially those in the form of a toothpaste or oral / dental rinse, comprising the same. The sweetener composition comprises a combination of saccharin, sucralose and a rebaudioside, or sweeteners of similar sucrose equivalence and type, preferably in a ratio of about 1:about 1:about 2. The sweetener composition of the invention was found to significantly improve the taste profile, long lasting freshness and clean feel of oral care compositions and to deliver an in-use sweetness that was more natural and pleasant than artificial sweeteners alone.
Owner:THE PROCTER & GAMBLE COMPANY
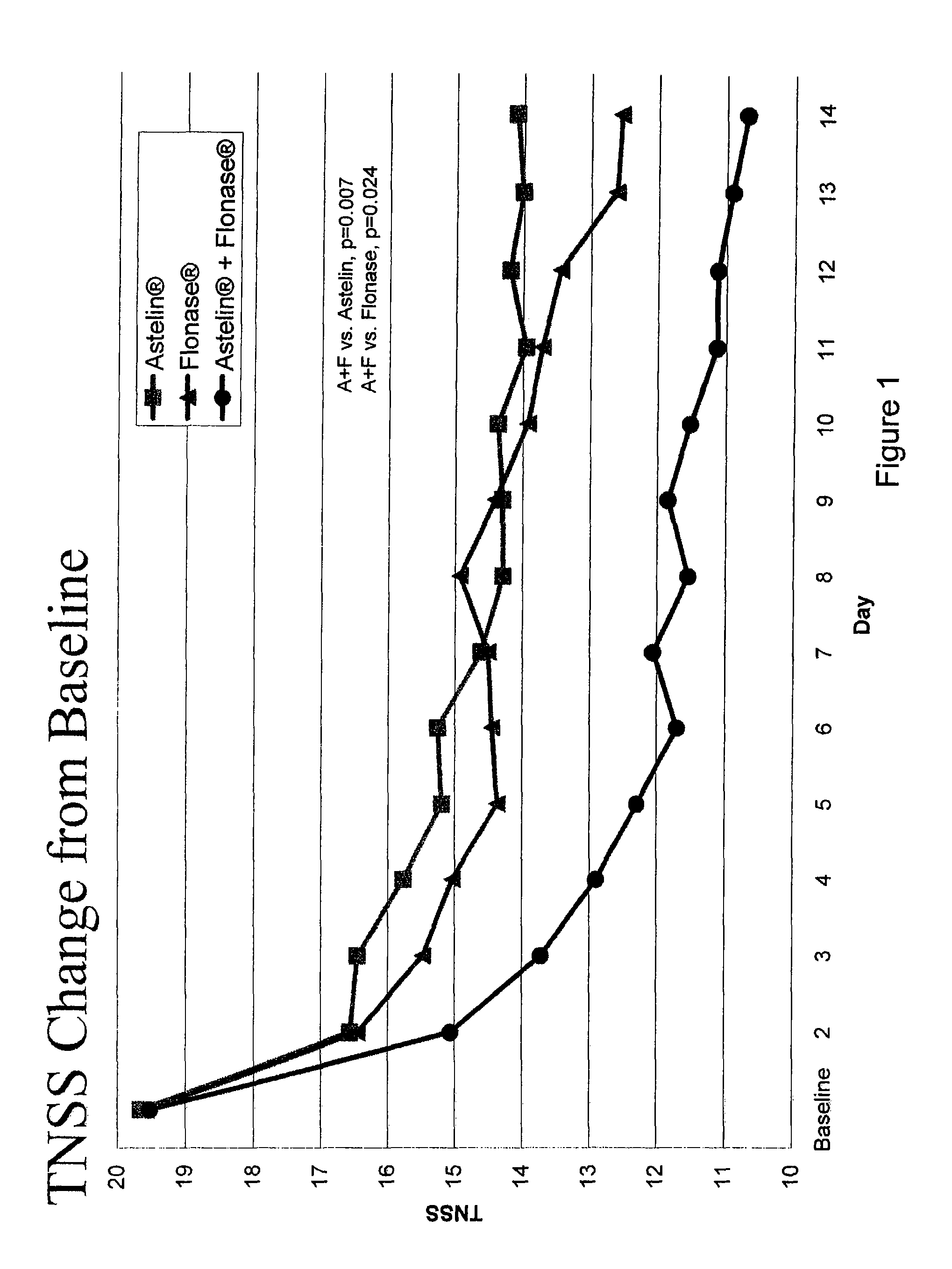
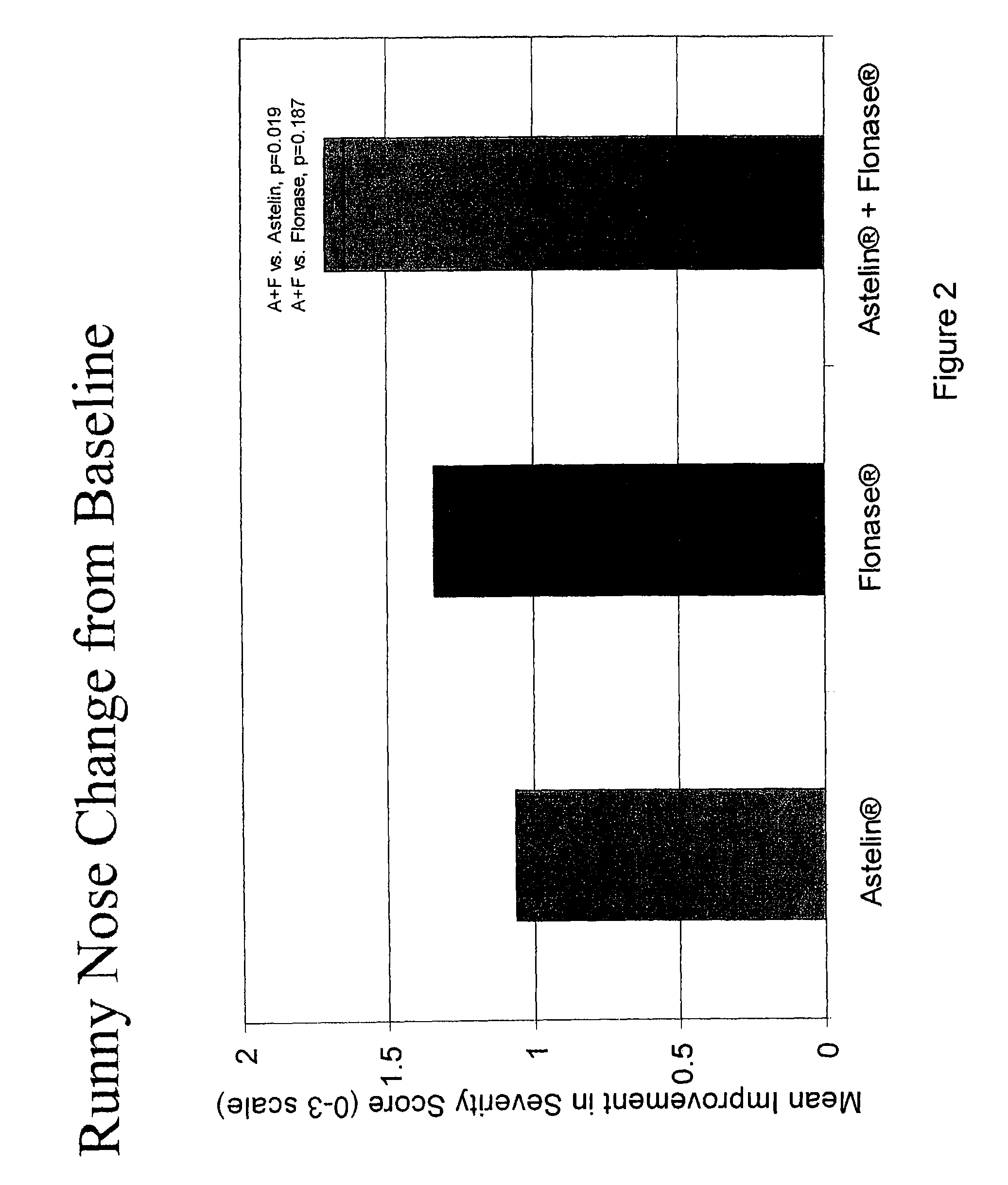
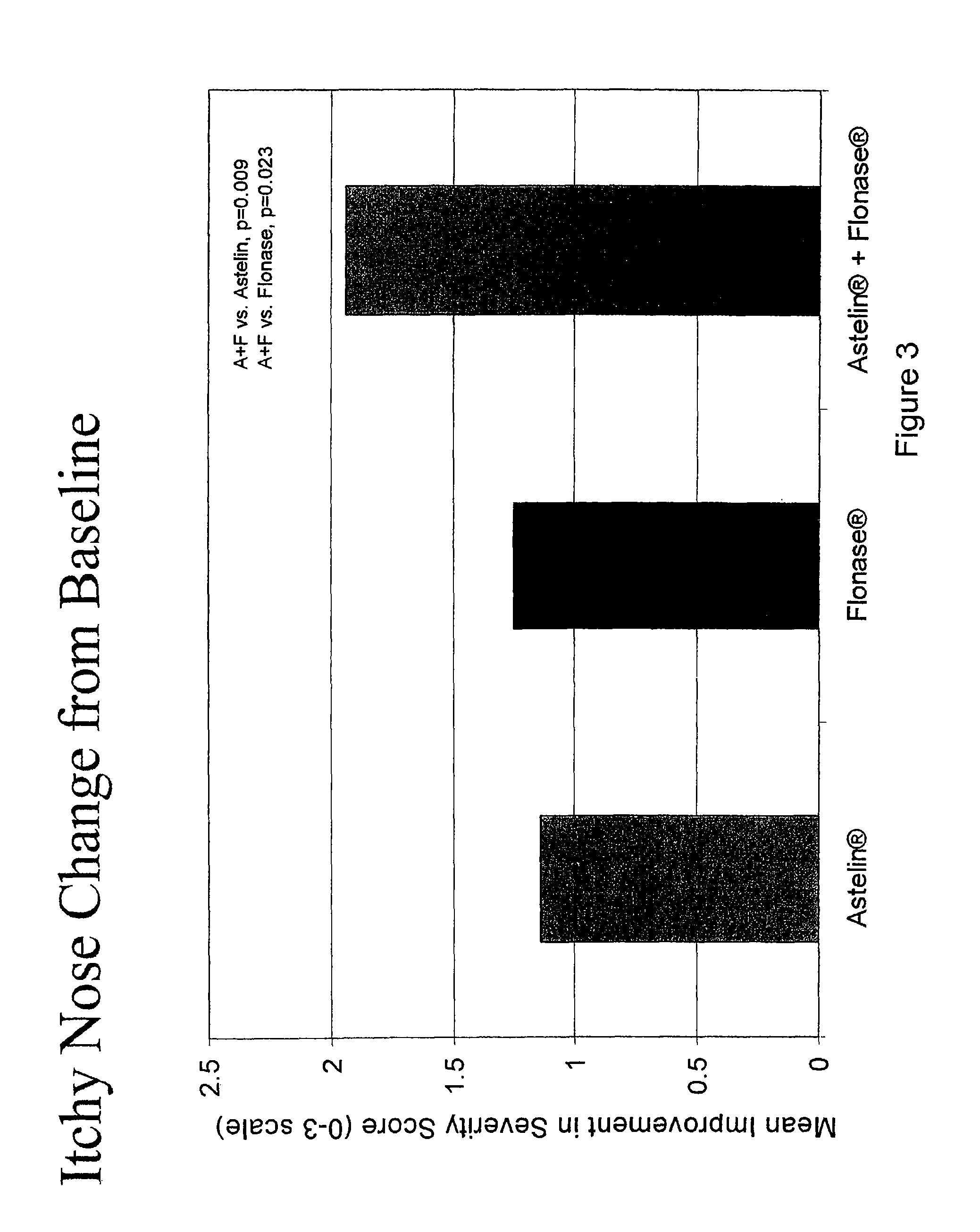
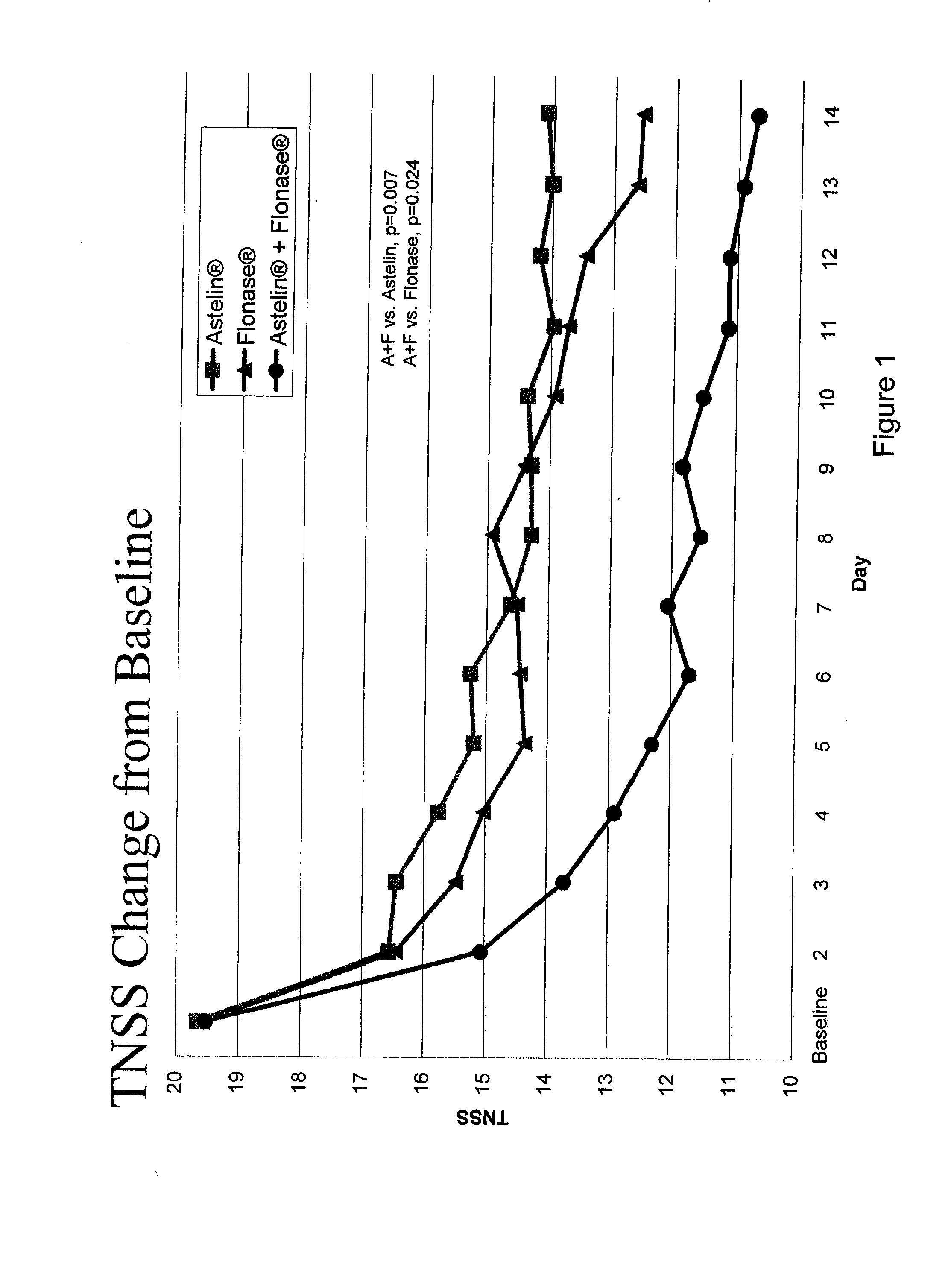
Compositions comprising azelastine and methods of use thereof
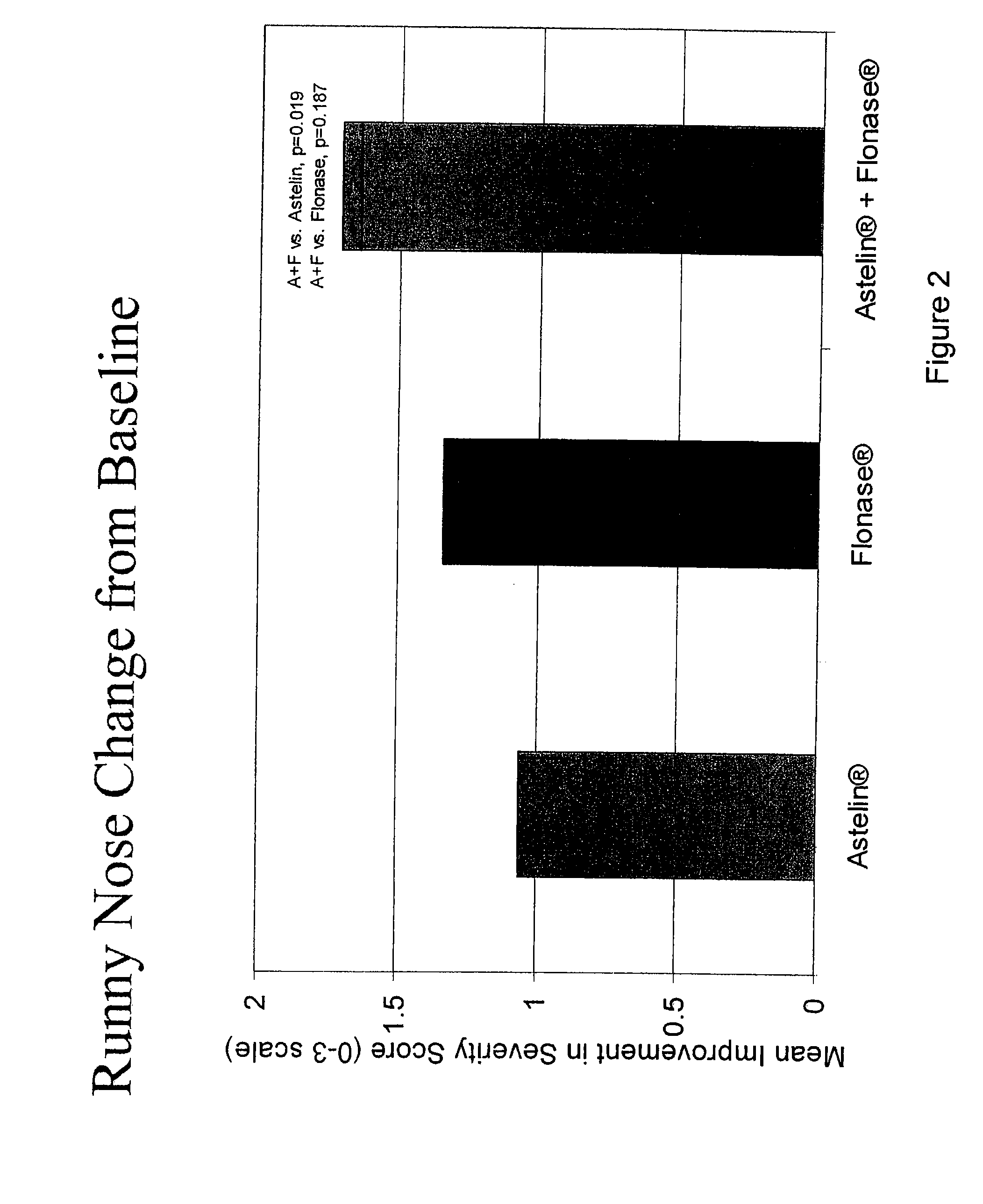
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
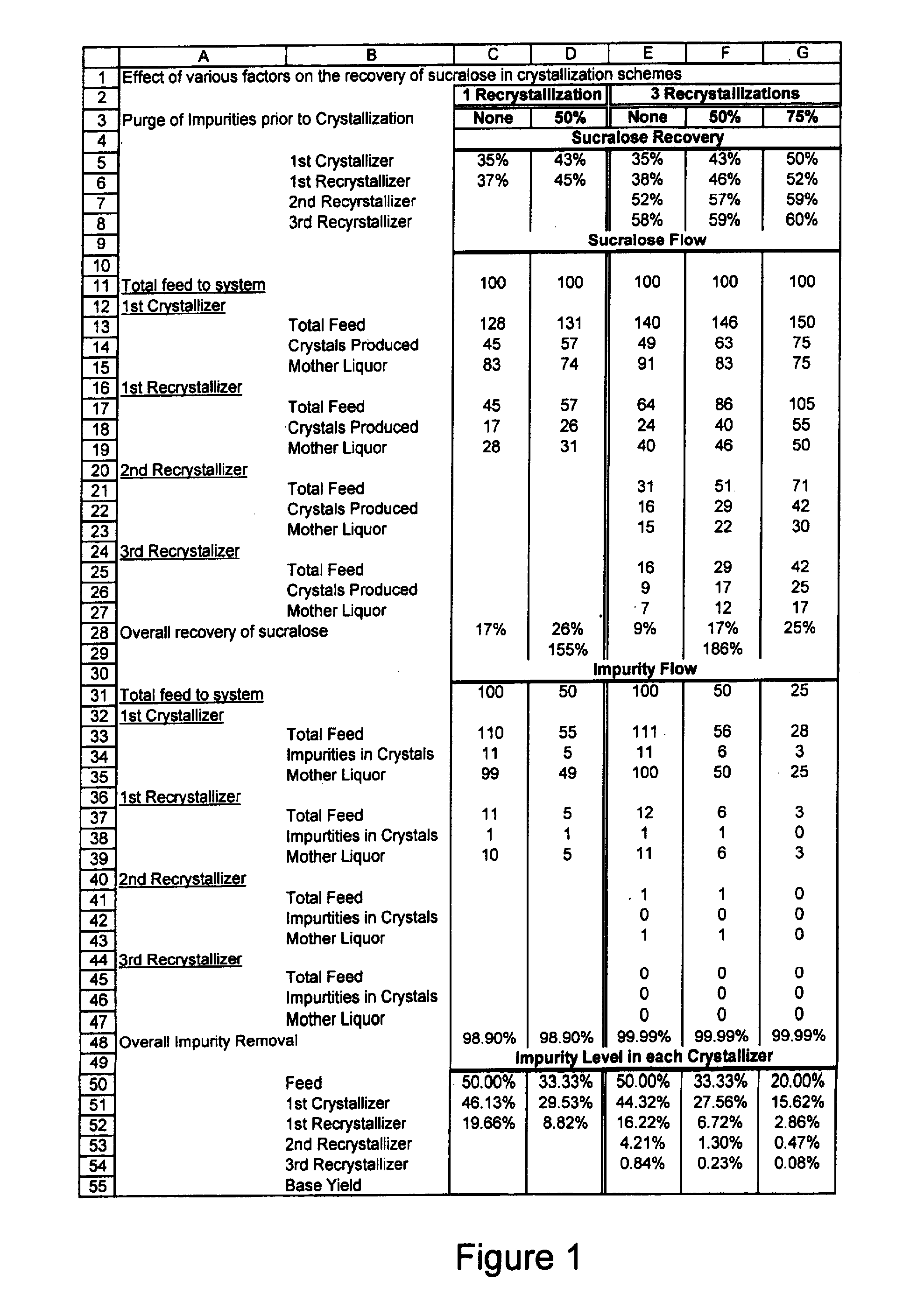
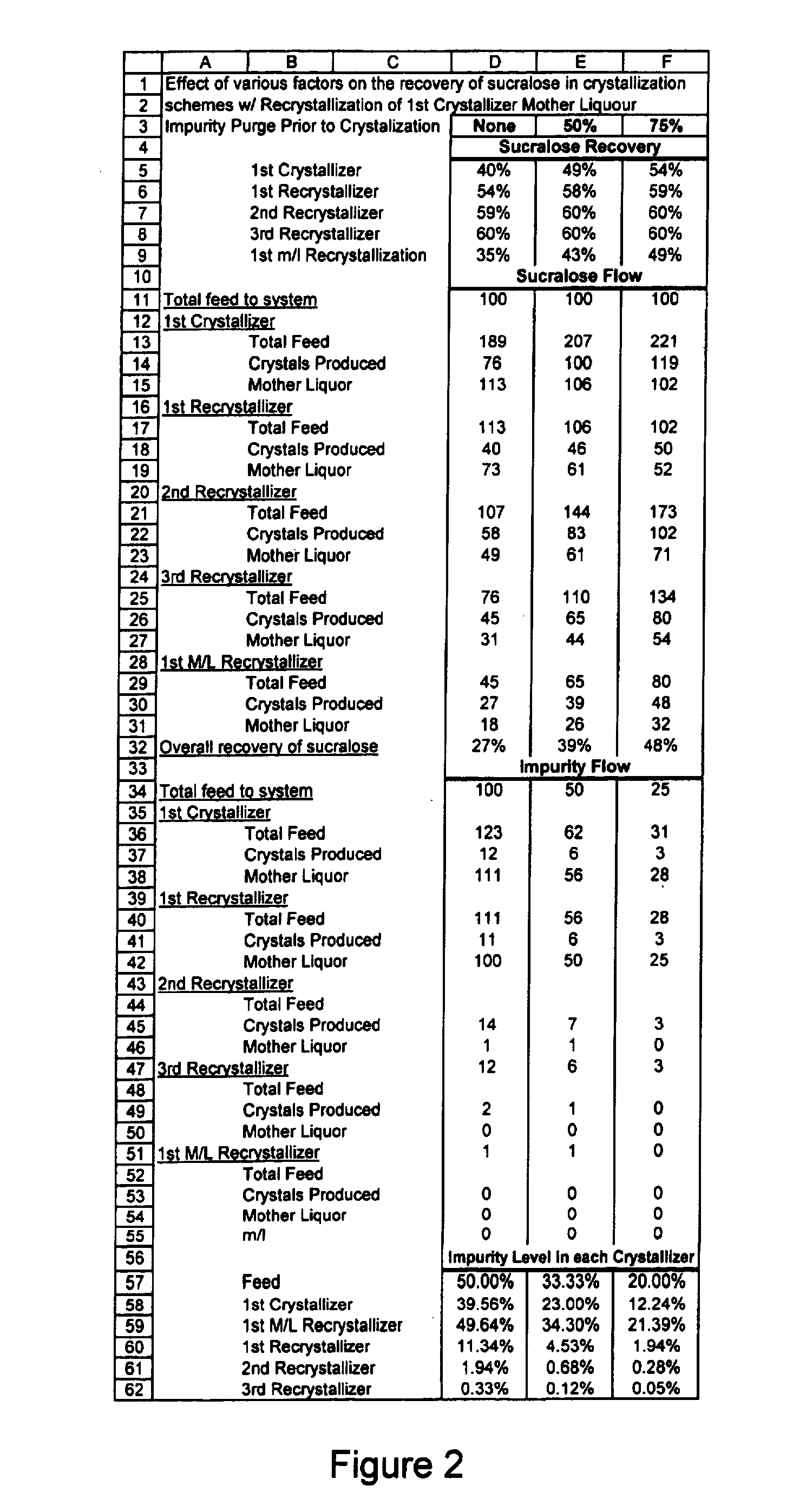
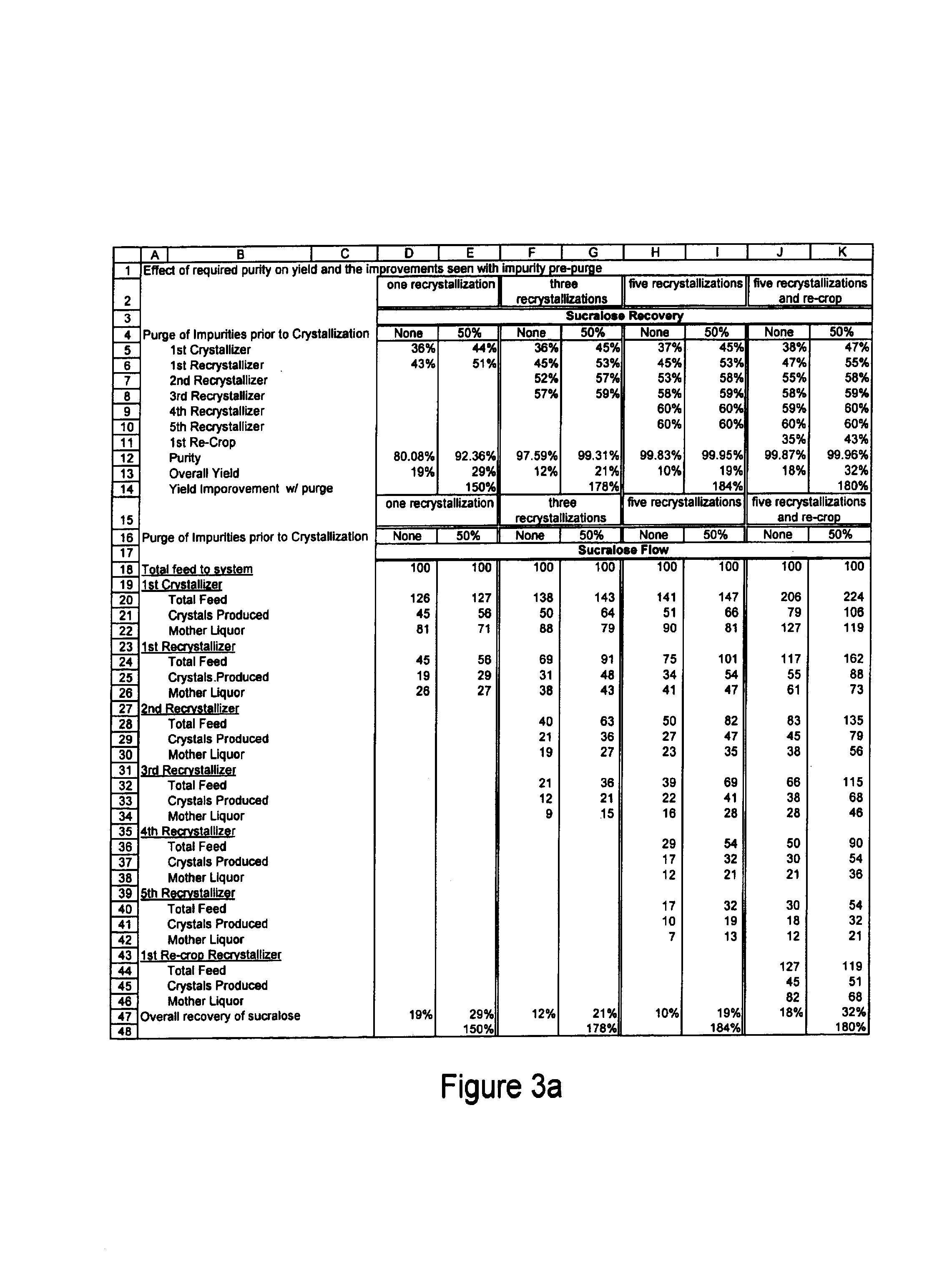
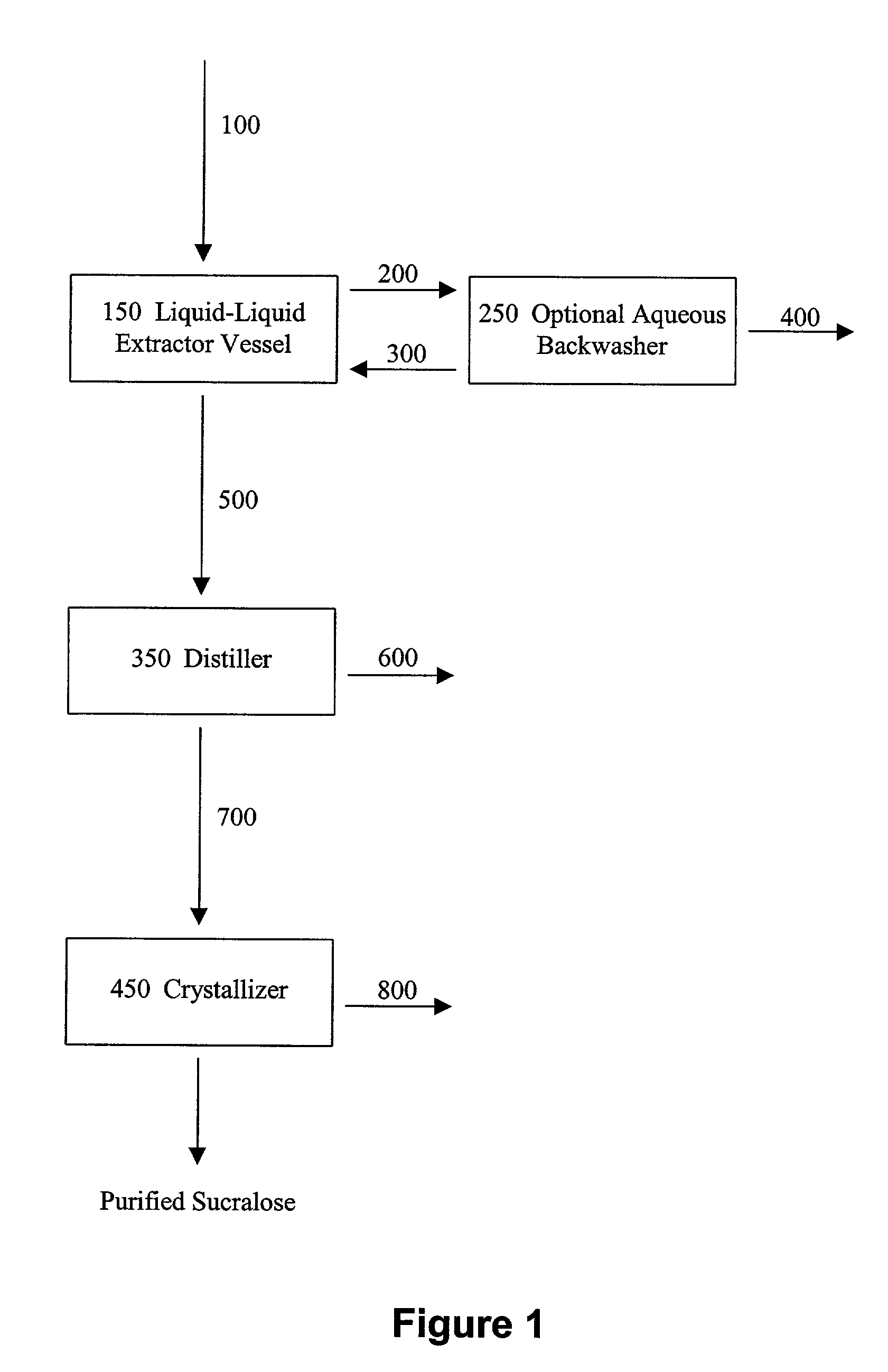
Process for improving sucralose purity and yield
InactiveUS6998480B2Improve efficiencyHigh purityEsterified saccharide compoundsSugar derivativesSucraloseCrystallization Purification
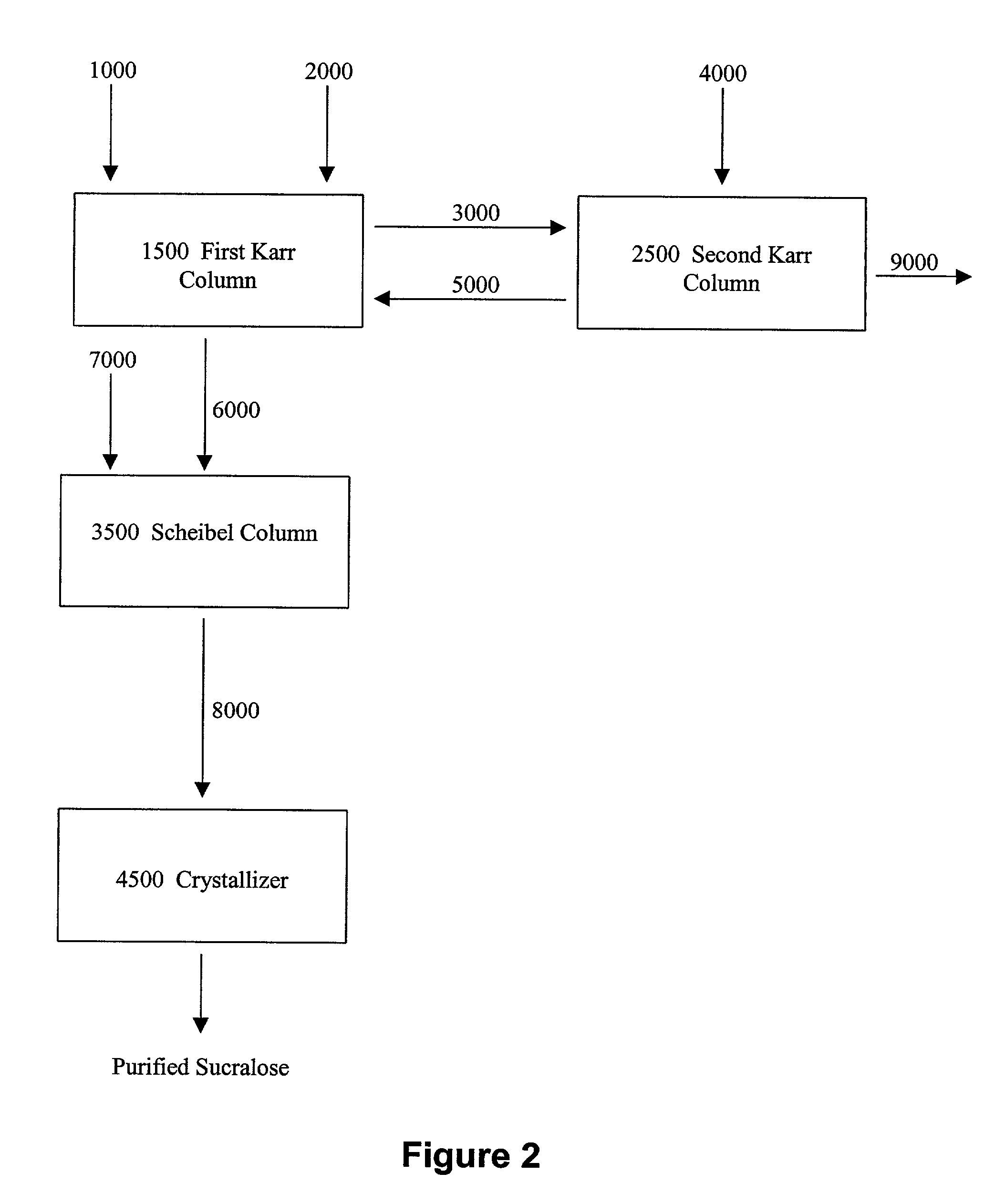
This invention relates to processes for purifying sucralose by the use of an initial non-crystallization purification procedure followed by three or more sequential crystallization steps and recycle of the mother liquor remaining from each crystallization step to the feed of another crystallization or purification step. This invention also relates to sucralose compositions as well as compositions comprising the sucralose compositions of the present invention. These compositions may be highly pure and have a superior taste profile.
Owner:TATE & LYLE TECH LTD
Crystalline form of sucralose, and method for producing it
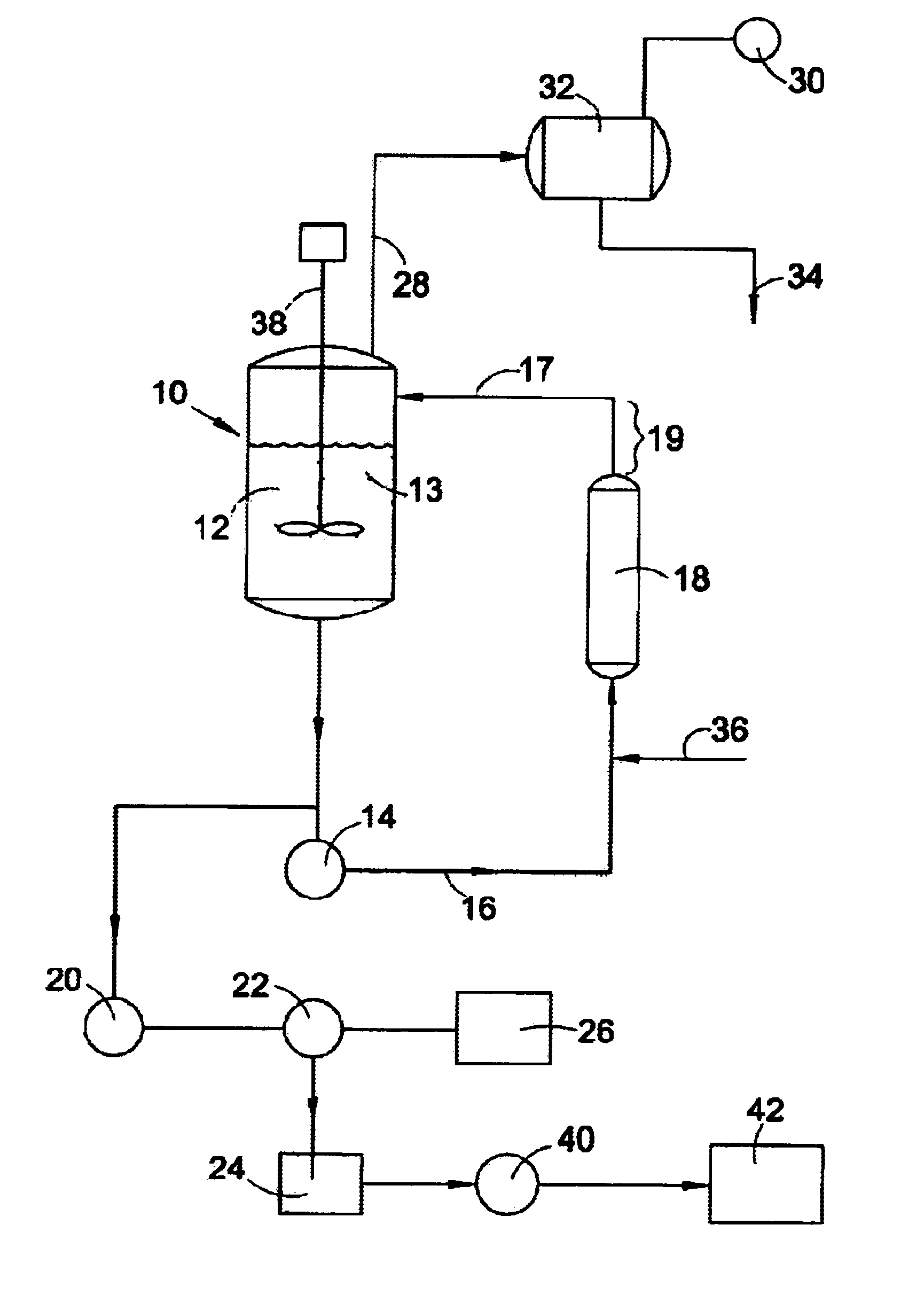
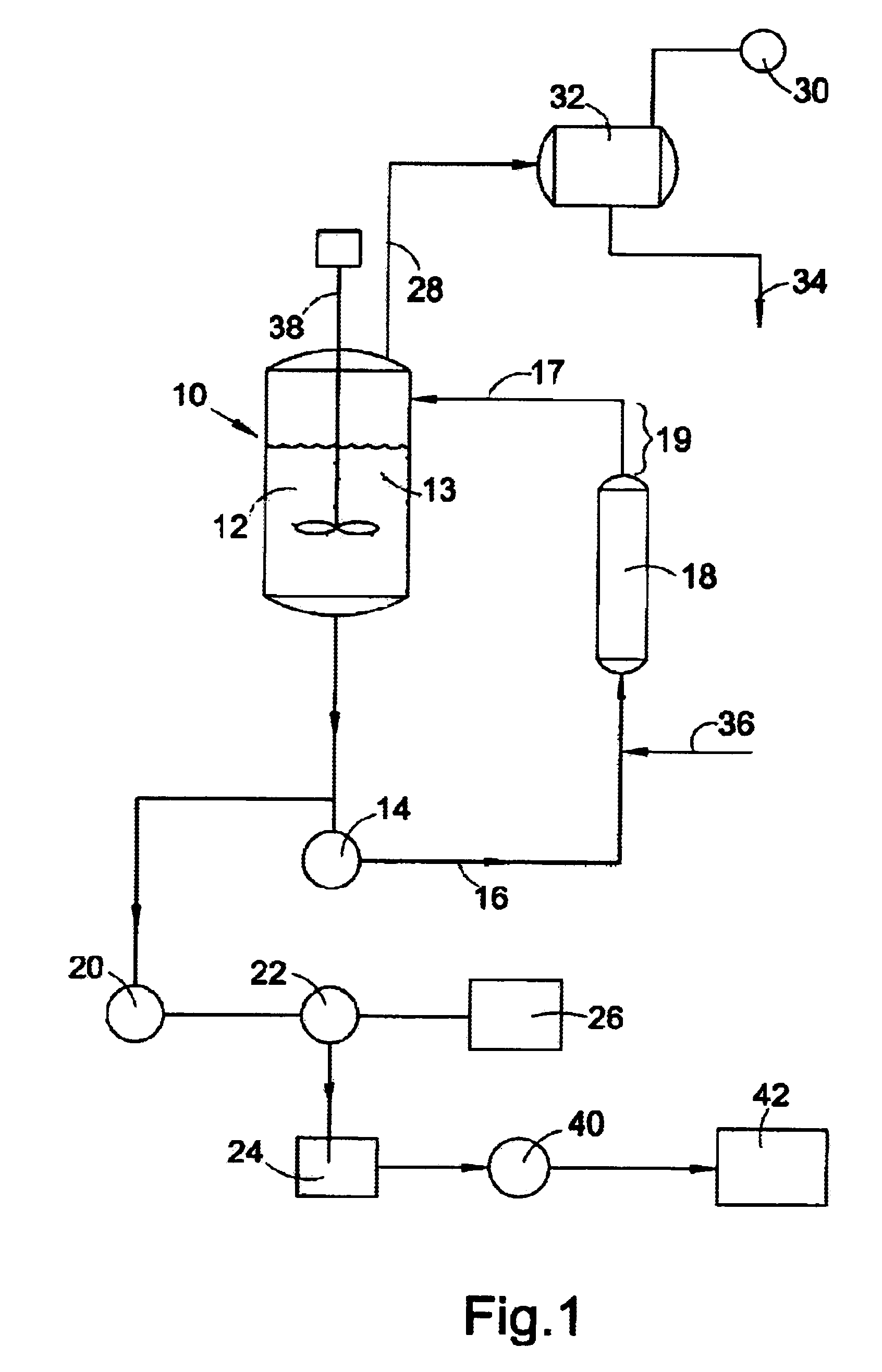
A crystalline form of sucralose, and a method of making it. The method involves continuously crystallizing sucralose from an aqueous solution by a process providing continuous removal and recirculation of the vessel contents, and providing a long residence time for sucralose in the system. The crystals thus formed are of a relatively low length / diameter ratio, have an unsymmetrical shape, and exhibit good stability. The larger crystals in particular are tapered as compared to the rod-like larger crystals in prior art product.
Owner:TATE & LYLE TECH LTD
Methods for buffer stabilized aqueous deacylation
The present invention relates to novel methods for stabilizing aqueous deacylation, via use of buffers in the production of sucralose. The present invention provides a process for producing sucralose from an acyl-sucralose compound whereby the acyl-sucralose compound is deacylated in the presence of a buffering agent, which stabilizes the pH of the feed mixture and decreases the accumulation of undesired anhydro compounds. Further, the present invention provides a process whereby the acyl-sucralose compound is deacylated directly either prior to or after removal of the tertiary amide reaction vehicle from the neutralized chlorination feed mixture. An aqueous solution of sucralose including salts and other compounds is produced, from which sucralose is recovered by extraction and purified by crystallization.
Owner:TATE & LYLE TECH LTD
Process for the preparation of sucralose
ActiveUS20070227897A1Economical and efficientHigh yieldEsterified saccharide compoundsElectrolysis componentsSucroseElectrolysis
A process for preparing sucrose-6-ester is provided, which comprises electrolyzing an electrolyte solution containing sucrose, an acylating reagent and a halide catalyst. Also disclosed is a process for preparing sucralose, which involves the preparation and chlorination of sucrose-6-ester followed by deacylation of the molecule. The process of the invention can be more readily performed with a higher yield than those in the art.
Owner:TECHNO (FUJIAN) FOOD INGREDIENTS CO LTD
Carbonated drinks
A carbonated drink having both the rich taste of a plant-derived component(s) such as fruit juice(s) and the stimulating and refreshing feel of carbonic acids is provided. The carbonated drink contains 10 to 80% by weight of the plant-derived component(s) and 2% by volume or, more of carbon dioxide, has a soluble solids content of not more than 8 degree as indicated by a refractive saccharometer, contains high intensity sweetener(s), the sweetness due to which is not less than 25% by weight (on the sucrose equivalent basis), and has an entire sweetness equivalent to the sweetness of a sucrose content of 8 to 14% by weight. Furthermore, it contains sucralose as at least one high intensity sweetener, with the sweetness due to sucralose accounting for not less than 50% of the sweetness due to all high intensity sweetener(s) on the sucrose equivalent basis.
Owner:SAN EI GEN F F I
High-intensity sweetener-polyol compositions
InactiveUS20050196503A1Improve sweetness qualityImprove taste qualityConfectionerySweetmeatsCyclamatesMANNITOL/SORBITOL
The present invention provides a sweetener composition and methods for improving the taste of a sweetener composition. The sweetener composition includes a mixture of a high-intensity sweetener such as aspartame, encaspsulated aspartame, neotame, encapsulated neotame, cyclamate, sucralose, saccharin or Acesulfame-K, with polyols such as maltitol, sorbitol, mannitol, erythritol, xylitol, lactitol, or palatinit, wherein the high-intensity sweetener is present in the mixture in an amount from about 0.0001% to 15% by weight.
Owner:RICHMOND CHEM CORP
Sucralose formulations to mask unpleasant tastes
InactiveUS20060121066A1Unexpected synergy of bitter taste masking effectivenessBiocideOrganic active ingredientsSweetnessExcipient
The present invention is directed to a pharmaceutically acceptable taste masking liquid excipient base for administration of a relatively large amount of unpleasant tasting medicines. More particularly, the enhanced sweetness and taste masking effect are produced by the addition of sucralose to the excipient base with maintenance of a pH from about 2 to about 5. The invention is further directed to medicinal compositions comprising such a liquid excipient base and unpleasant tasting medicines. Still further, the invention is directed to a method for taste masking unpleasant tasting medicines through their incorporation into the claimed liquid excipient bases.
Owner:WYETH LLC
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Method of improving sweetness delivery of sucralose
InactiveUS6998144B2Improving sweetness delivery profileImproved profileBiocideConfectioneryMedicineSweetness
Owner:HEARTLAND CONSUMER PROD
Process for purification of sucralose
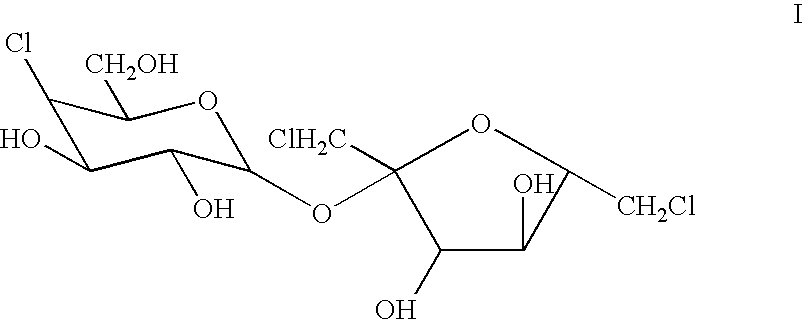
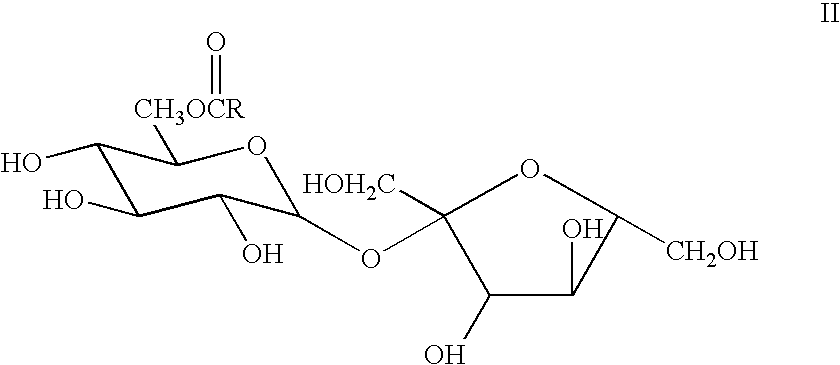
InactiveUS20070160732A1Easy to handleFeasible at commercial scaleSugar derivativesSugar derivatives preparationAcetylationSucralose
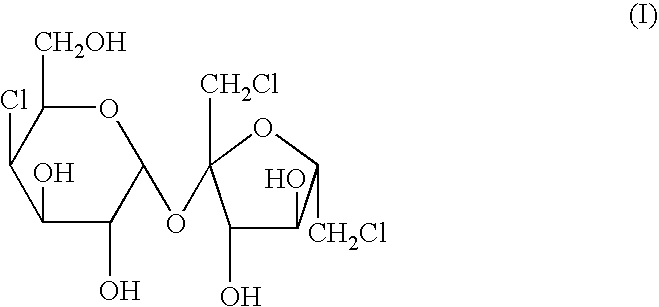
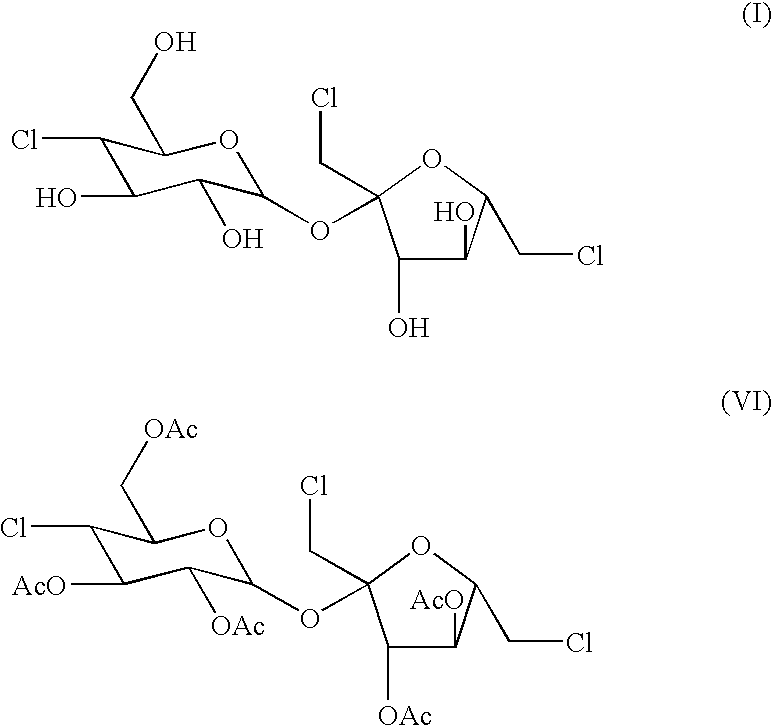
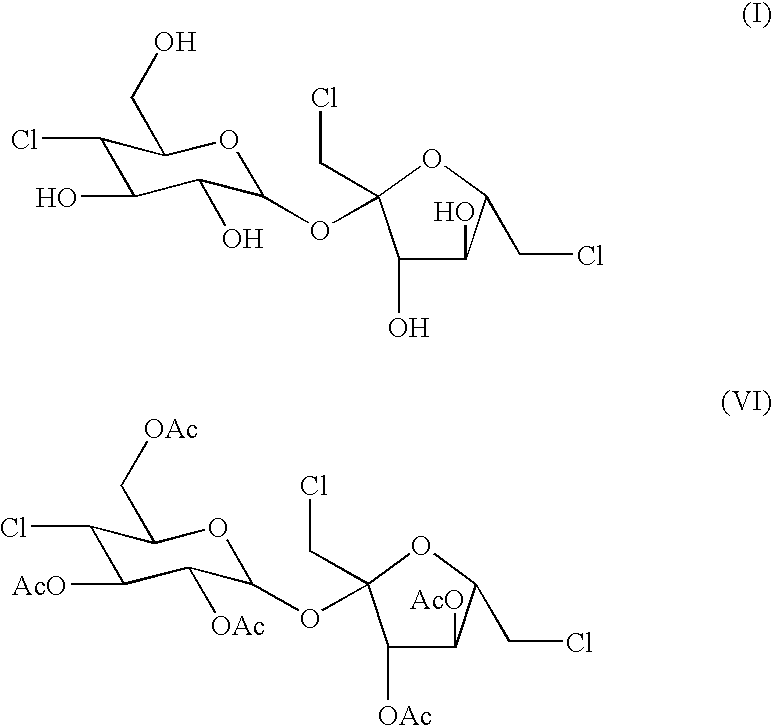
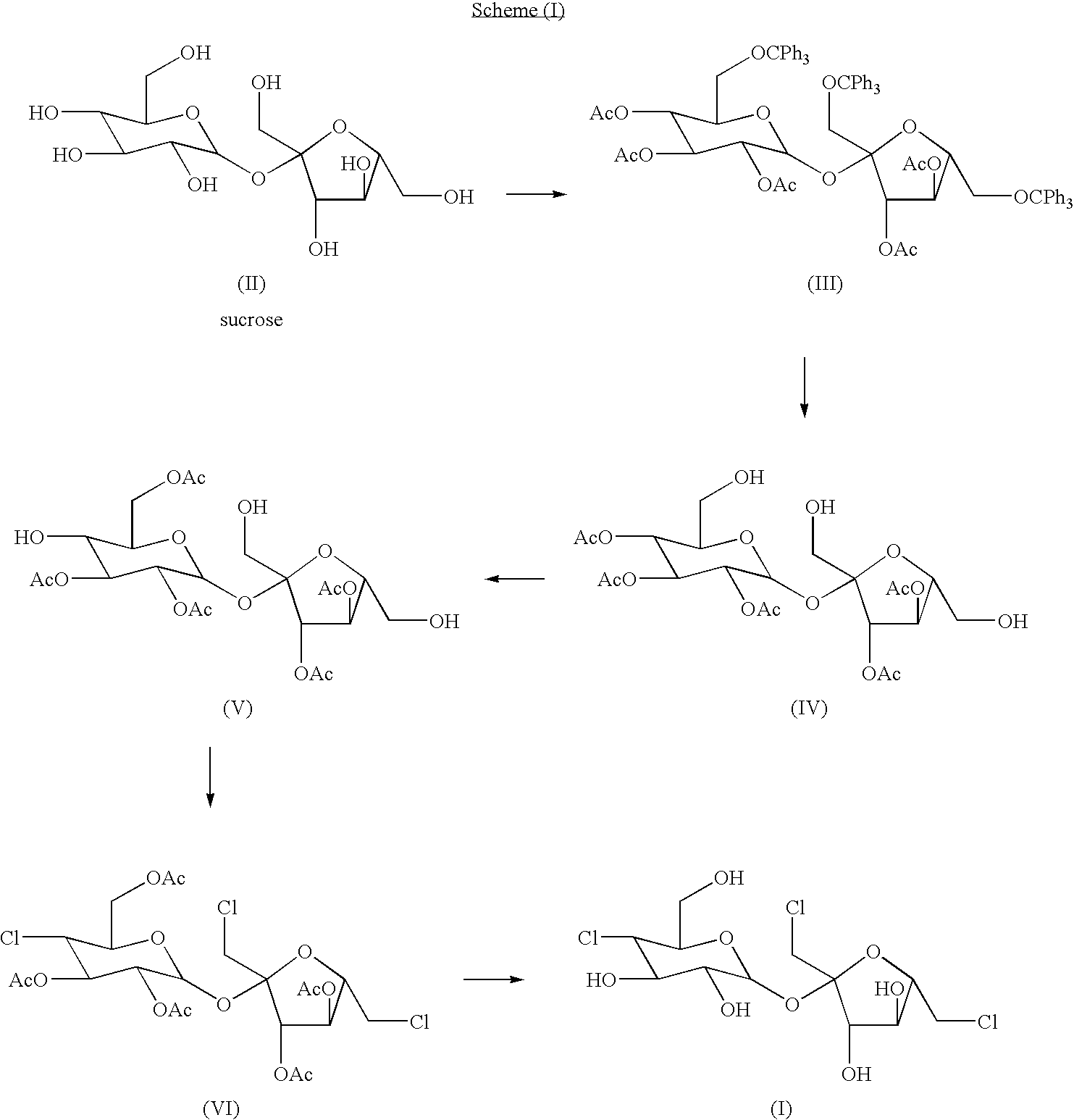
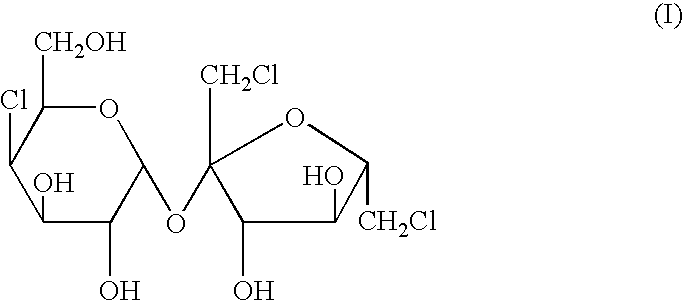
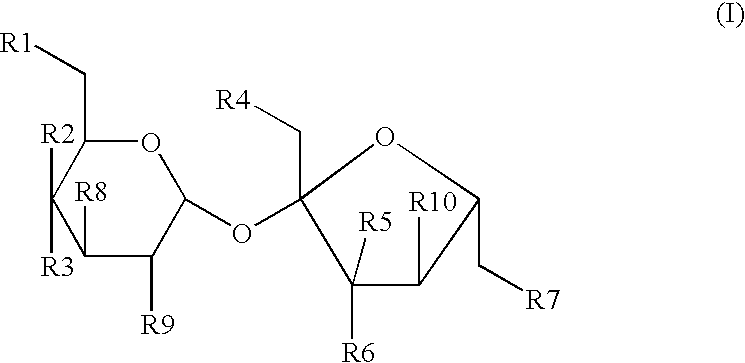
The present invention relates to a process for the purification of Sucralose of formula (I) which comprises acetylation of substantially impure Sucralose to its penultimate intermediate 4,1',6'-trichloro-4,1',6'-trideoxy galactosucrose penta-acetate (TOPSA) of formula (VI) followed by purification of TOPSA and then deacetylation of purified TOPSA.
Owner:ALEMBIC LTD
Peach gum jelly and preparation method thereof
InactiveCN104256228ATransparent appearanceIncrease elasticityFood ingredient functionsFood preparationCarrageenanSucrose
The invention relates to a peach gum jelly and a preparation method thereof. The preparation method of the peach gum jelly comprises the following steps: (1) preparing peach gum hydrolysate; (2) boiling 100-300 parts of water, 0.1-1.0 part of carrageenan, 0.2-1.2 parts of konjac glucomannan, 5-25 parts of peach gum hydrolysate, 5-15 parts of peach blossom aqueous extract, 3-12 parts of saccharose, 0.002-0.01 part of stevioside, 0.004-0.03 part of sucralose and 0.1-0.5 part of potassium chloride at high temperature; and (3) cooling, adding auxiliary materials, sterilizing and packaging. The jelly prepared by the preparation method has favorable mouthfeel and diversified flavors due to the addition of the peach gum and the peach blossom aqueous extract while the health function of the jelly is increased and is used for reinforcing the physical health of a human body. Compared with a traditional peach gum food, the peach gum jelly prepared by the preparation method can be stored for a long time at normal temperature, has the characteristics of transparent appearance, favorable elasticity, smooth and mellow mouthfeel, sweet and sour taste, diversified flavors and the like and is capable of meeting of different crowds.
Owner:无锡康顿生物科技有限公司
Sweetener composition
A sweetener composition containing less than 1 gram of carbohydrates includes a monosaccharide derive polyol such as erythritol, a reduced calorie sweetener such as tagatose, a bulking agent such as polydextrose or maltodextrin and a high intensity, non-nutritive sweetener such as sucralose. The sweetness profile of the sweetener composition may be customized by adjusting the level of high intensity, non-nutritive sweetener included in the compositon.
Owner:MILES LOREN
Sucralose-containing composition and edible products containing the composition
A composition which is obtained by causing a specific compound to be present together with sucralose. The composition provides a sucralose in a stable form, more particularly, a sucralose which is still stable and thus is significantly suppressed with respect to the decrease in sweetness and discoloration (browning, blackening), even when it is subjected to a warming treatment under a condition wherein temperature is high and especially water content is low and / or pH is low. The stabilized sucralose-containing composition can be used as a sweetener by itself and as a compound with a food or a drug.
Owner:SAN EI GEN F F I
Reduction of saltiness with sweeteners
InactiveUS20070082061A1Improve palatabilityReduce repetition rateBiocideOrganic active ingredientsBowel cleansingSucrose
The present invention provides compositions for bowel cleansing that have improved palatability through the inclusion of a sweetener, such as a chlorinated sucrose isomer. The invention also provides methods of reducing the saltiness of an orally consumed substance, including phosphate salt and PEG / salt bowel cleansers, through the use of a sweetener. Utilizing a sweetener including Sucralose to reduce the saltiness of a substance unexpectedly contradicts the conventional belief that sweeteners amplify saltiness.
Owner:C B FLEET CO INC
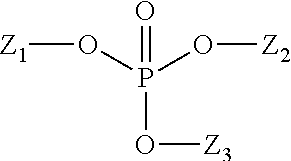
Methods and compositions for altering the sweetness delivery profile of sucralose
Abstract of the Disclosure The present invention teaches novel compositions and methods for altering the sweetness delivery profile of 4,1',6'-trichloro-4,1',6'-trideoxygalactosucrose. This invention also teaches novel uses for compositions comprising 4,1',6'-trichloro-4,1',6'-trideoxygalactosucrose.
Owner:TATA & LYLE TECH LTD (GB)
Dietary nutritional supplements for heal thcare
InactiveUS20090004334A1Pleasant tasteBiocideAnimal feeding stuffNutrition supplementationSOY LECITHIN
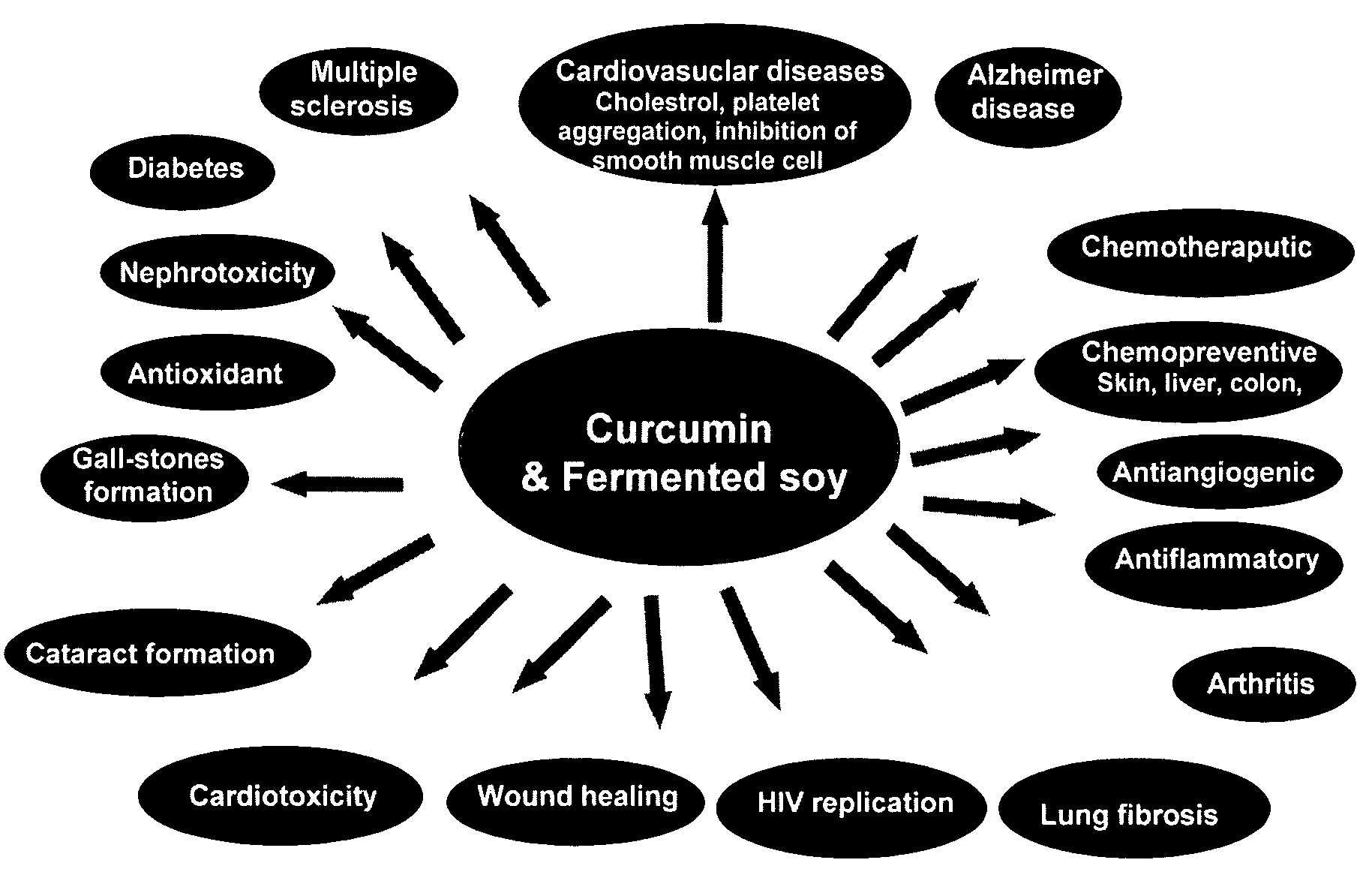
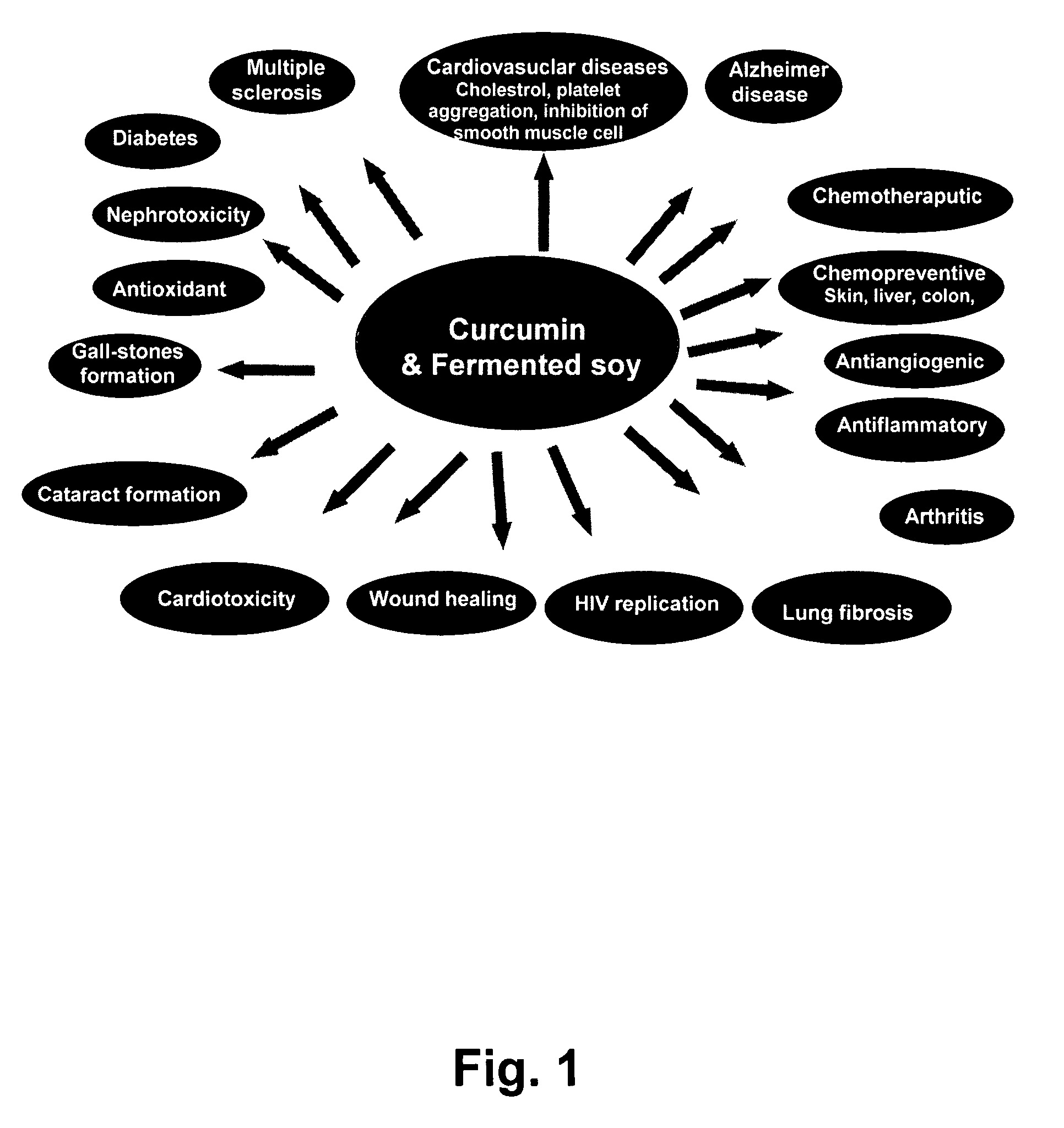
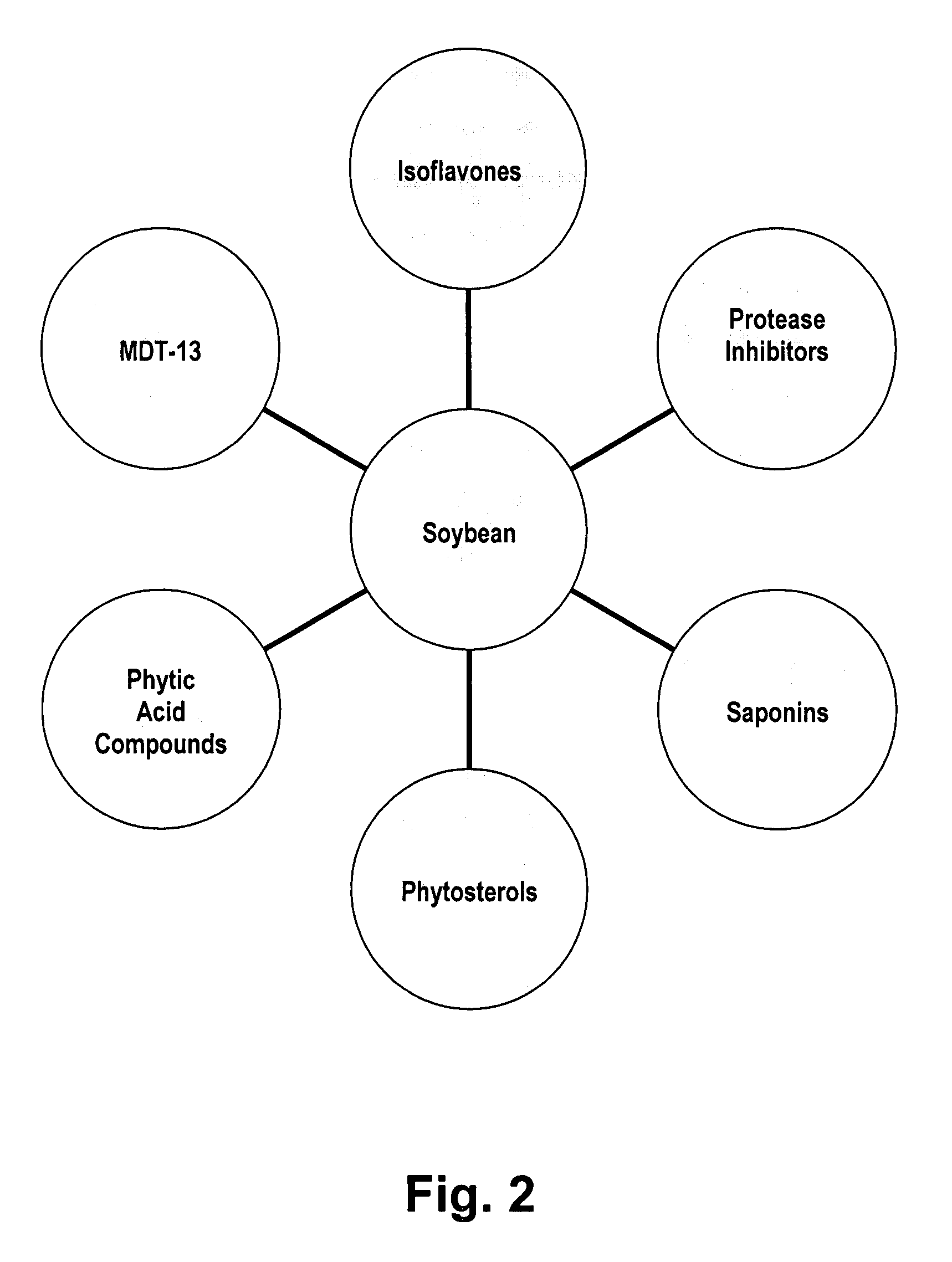
Disclosed are dietary nutritional supplements providing health benefits. Supplements provide various formulations of fermented soybean in combinations with sweetener(s), curcumin, and various other herbs and spices. Compositions are preferably in liquid or solid forms. In preferred embodiments of the invention, supplements have Haelan 951® or dehydrated fermented soy made from Haelan 951®, Curcumin C3 Complex®, sucralose, stevia, soy lecithin, barley malt, natural chocolate flavor, cocoa, bioperine, Piper longum, ginger, cardamom long, and cinnamon.
Owner:ESSENCE OF LIFE
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Oral care compositions with improved sweetness
The present invention is directed to improved sweetener compositions and oral care compositions, especially those in the form of a toothpaste or oral / dental rinse, comprising the same. The sweetener composition comprises a combination of saccharin, sucralose and a rebaudioside, or sweeteners of similar sucrose equivalence and type, preferably in a ratio of about 1:about 1: about 2. The sweetener composition of the invention was found to significantly improve the taste profile, long lasting freshness and clean feel of oral care compositions and to deliver an in-use sweetness that was more natural and pleasant than artificial sweeteners alone.
Owner:PROCTER & GAMBLE CO
No-carb tabletop sweeteners substitute
InactiveUS20060073254A1High strengthFacilitate compositionFood ingredient functionsFood preparationLow Carbohydrate DietsHigh intensity
The invention describes no-carbohydrate tabletop sweetener compositions including a combination of a high intensity sweetener, such as sucralose and fillers such as amino acids and proteins. Amino acids combinations improve the taste quality of the sweetener substitute. The composition is suitable for use as tabletop sweeteners and ideal for low-carbohydrate diet purposes.
Owner:MCNEIL NUTRITIONALS
High-intensity sweetener composition and delivery of same
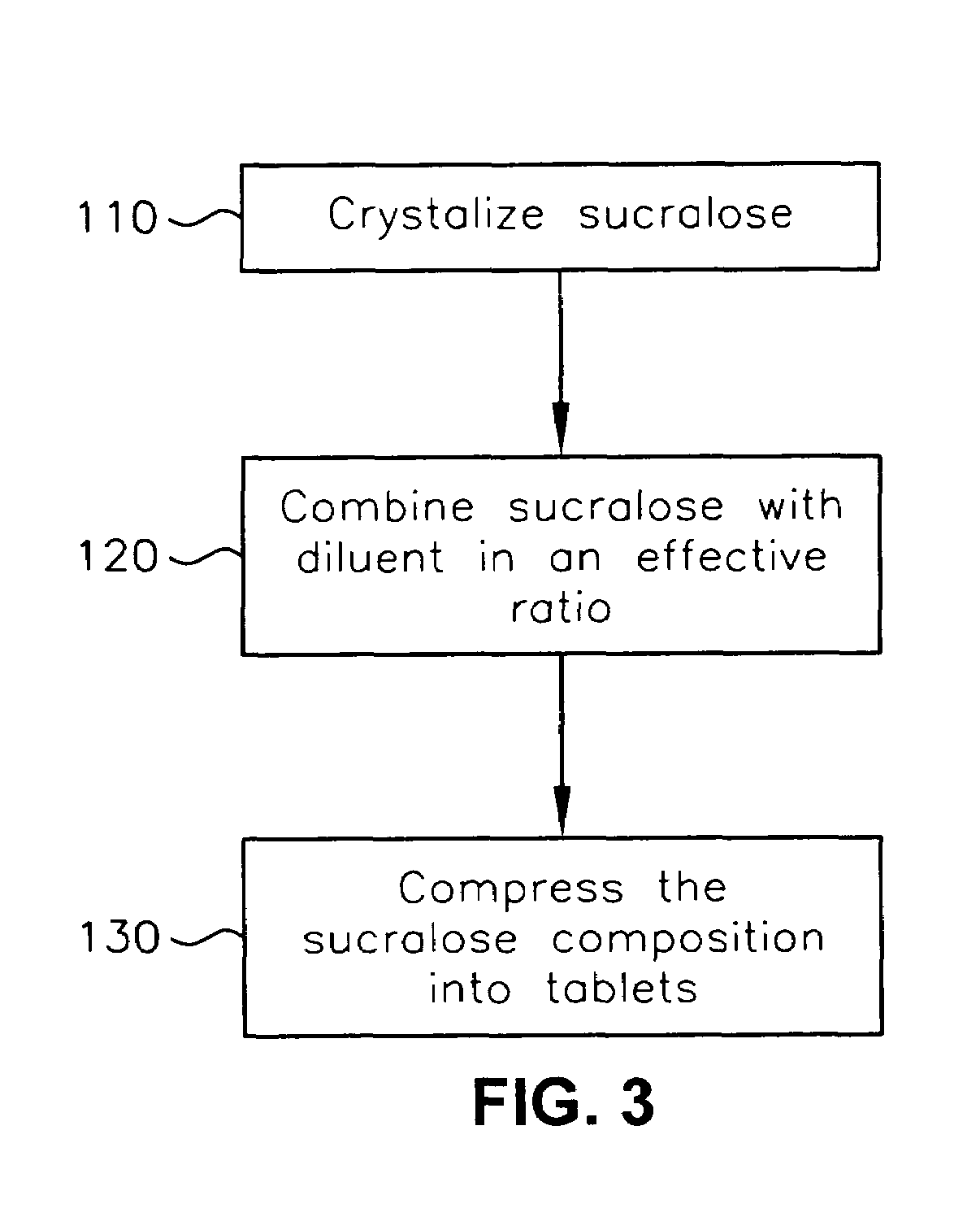
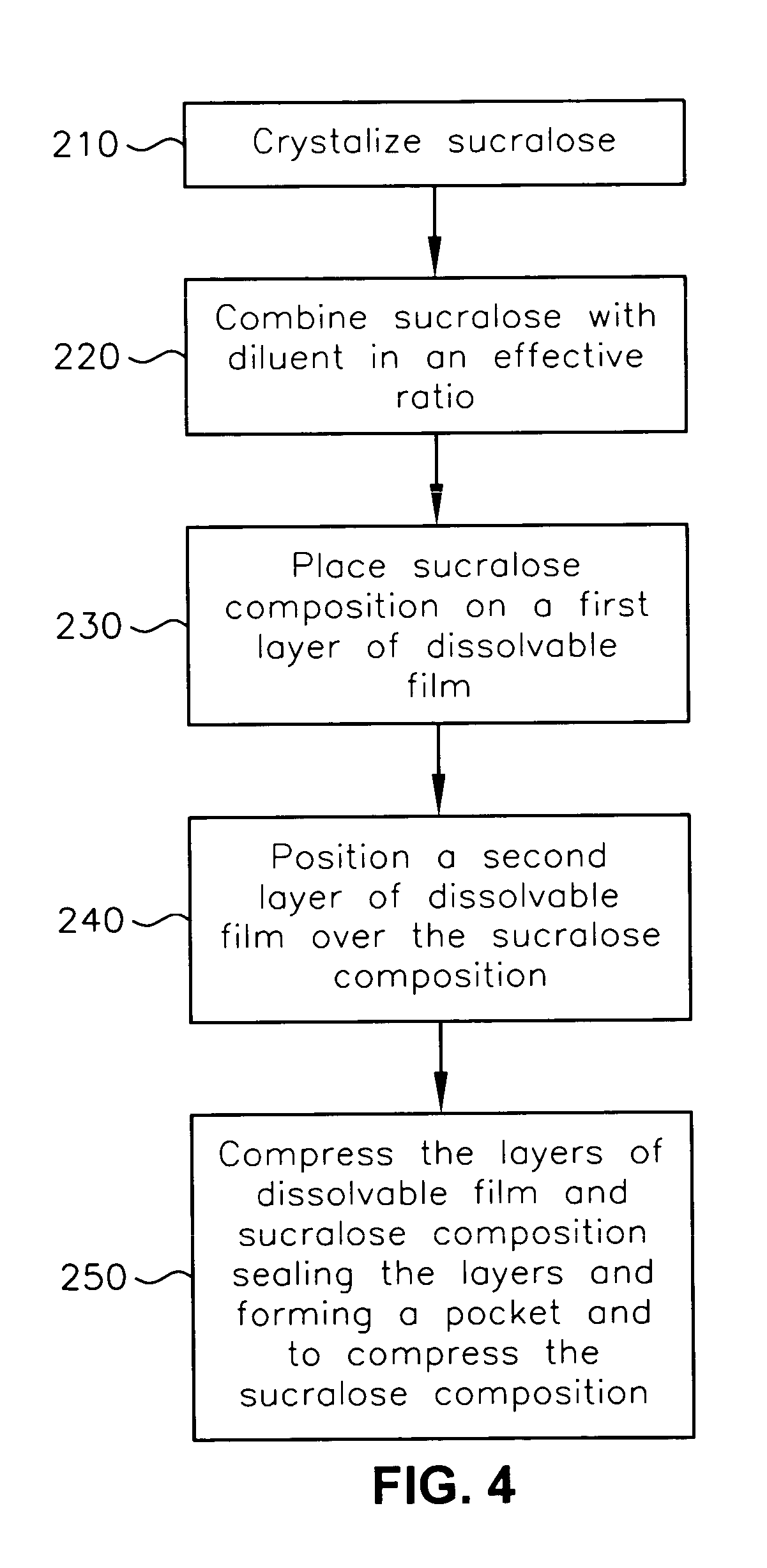
A composition for sweetening ingestable solids or liquids. The composition is compressed and comprises less than about 7.5 weight-% sucralose and one or more diluents. The composition is compressed at a pressure of greater than 2 pounds per square inch. A method of forming the composition and a system for delivering the composition are also provided.
Owner:HEARTLAND CONSUMER PROD
Tablet candy and producing method thereof
ActiveCN101658233AEnhance memoryImprove thinkingConfectionerySweetmeatsFlavoring essencesGamma-Aminobutyric acid
The invention provides a tablet candy and a producing method thereof. The tablet candy is made of the following raw materials according to weight percent: 73.9-85% of sorbitol, 2-5% of flaxseed powder, 0.1-0.3% of L-theanine, 0.1-0.5% of gamma-aminobutyric acid, 0.5-2% of phosphatidylserine, 5-10% of xylitol, 0.1-0.3% of sucralose, 0-5% of flavoring essence, 0-0.1% of colorant, and 1-3% of magnesium stearate. In the tablet candy of the invention, flaxseed powder, L-theanine, gamma-aminobutyric acid and phosphatidylserine interwork according to certain proportion and in synergy, promote mutually, can effectively relieve pressure, tranquillize mood, calm sentiments, and improve memory and thinking capability, so that the tablet candy has health care functions of relieving pressure, relaxingmood, enhancing memory and the like, and is suitable to be eaten by common people as well as diabetics, obese people or people hoping to reduce weight.
Owner:汕头市合优食品有限公司
Beverage products with non-nutritive sweetener and bitterant
Aspects of the invention relate to beverage compositions, including, for example, concentrated and ready-to-drink formulations sweetened with at least one non-nutritive sweetener and further including a bitterant compound in an amount sufficient to reduce the lingering sweet aftertaste of the non-nutritive sweetener(s). In certain illustrative embodiments, the non-nutritive sweetener(s) may be one or more of the following: a steviol glycoside, Lo Han Guo, thaumatin, monatin, monellin, brazzein, sucralose. Another aspect of the invention relates to a method that combines a non-nutritive sweetener having a lingering sweet aftertaste with a bitterant compound to create a mixture such that when the mixture is contained in a beverage, the bitterant compound is present in an amount sufficient to reduce the lingering sweet aftertaste of the non-nutritive sweetener.
Owner:CONCENTRATE MFG OF IRELAND
Method for reaction of sucralose esterified single solvent
ActiveCN106349300AEliminate recyclingReduce consumptionEsterified saccharide compoundsTin organic compoundsAcetic anhydrideSucrose
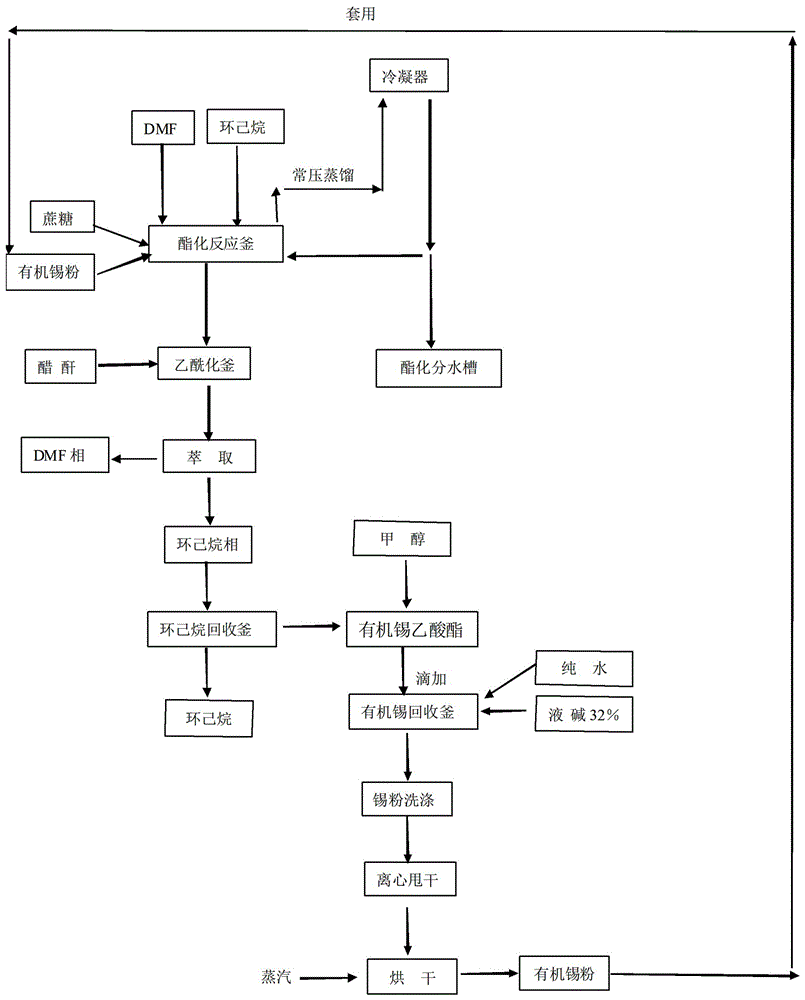
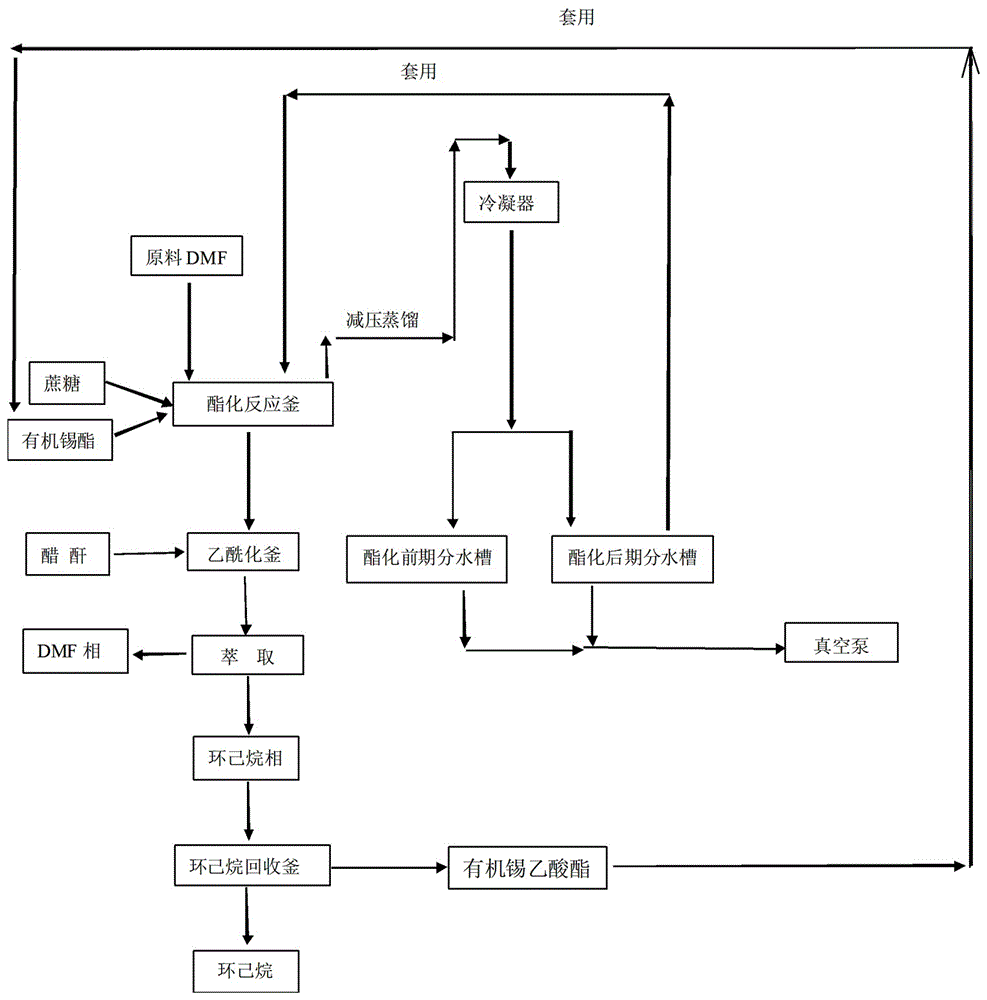
The invention relates to a method for the reaction of sucralose esterified single solvent, characterized by: 1) DMF, sucrose and organotin acetate are subjected to negative pressure esterification in an esterification kettle while DMF is recovered to the bypass channels of earlier stage by negative pressure distillation and condensing, and the recovered DMF whose water content is larger than 0.9-1.0% ppm is to be delivered into the recovery system; 2) Negative pressure recovery of DMF continues until the water content is less than 0.6% ppm, then which is returned to the esterification kettle for the next batch of esterification reaction; 3) The esterification reaction material is added into the esterification kettle, and acetic anhydride is added dropwisely in; water is added after adding dropwisely, and sucrose 6 ester and organotin acetate are extracted and separated with cyclohexane; organic tin acetate present in the cyclohexane phase, which is produced through evaporating of the cyclohexane solvent. The advantages of the method for the reaction of sucralose esterified single solvent is shortening the production cycle, eliminating the original tin recovery process, reducing the consumption of raw materials and energy and maximizing the savings in all costs.
Owner:ANHUI JINGHE IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com